Abstract
Purpose
In phase 1 testing, alisertib tablets with irinotecan and temozolomide showed significant antitumor activity in patients with neuroblastoma. The current study sought to: confirm activity of this regimen; evaluate an alisertib oral solution (OS); and evaluate biomarkers of clinical outcomes.
Methods
We conducted a two-stage phase 2 trial of alisertib tablets (60 mg/m2/dose × 7 days), irinotecan (50 mg/m2/dose IV × 5 days), and temozolomide (100 mg/m2/dose orally × 5 days) in patients with relapsed or refractory neuroblastoma. The primary endpoint was best objective response. A separate cohort was treated with alisertib at 45 mg/m2 using OS instead of tablets. Exploratory analyses sought to identify predictors of toxicity, response, and progression-free survival (PFS) using pooled data from phase 1, phase 2, and OS cohorts.
Results
Twenty and 12 eligible patients were treated in the phase 2 and OS cohorts, respectively. Hematologic toxicities were the most common adverse events. In phase 2, 4 partial responses were observed in 19 evaluable patients (21%). The estimated PFS at 1 year was 34%. In the OS cohort, 3 patients (25%) had first cycle dose-limiting toxicity (DLT). Alisertib OS at 45 mg/m2 had significantly higher median Cmax and exposure compared to tablets at 60 mg/m2. Higher alisertib trough concentration was associated with first cycle DLT, while MYCN amplification was associated with inferior PFS.
Conclusion
This combination shows antitumor activity, particularly in patients with MYCN non-amplified tumors. Data on an alisertib oral solution expand the population able to be treated with this agent.
Keywords: Alisertib, Aurora A kinase, Irinotecan, Temozolomide, Neuroblastoma, Oral Solution
Introduction
Children with advanced neuroblastoma require novel approaches to improve their outcomes.1 Preclinical and clinical data suggest a role for Aurora A kinase inhibition in this disease.2–6 The combination of irinotecan and temozolomide is a common regimen utilized in the management of patients with recurrent neuroblastoma.7 We previously conducted a phase 1 study of escalating doses of the oral Aurora A kinase inhibitor alisertib (formerly known as MLN8237) together with fixed doses of irinotecan and temozolomide in children with relapsed and refractory neuroblastoma.2 We demonstrated a maximum tolerated dose (MTD) of 60 mg/m2 alisertib as enteric-coated tablets, with hematologic toxicities being the most common adverse events. We observed significant antitumor activity in this context, with a 31.8% response rate and a 52% estimated progression-free survival (PFS) rate at 2 years.
Given that children with neuroblastoma are most commonly diagnosed as toddlers and infants, development of an alisertib liquid formulation is of interest for this indication. An oral solution had previously been evaluated in adult subjects and compared to a capsule formulation.8 This experience demonstrated a relative bioavailability of 1.26 and higher peak plasma concentration for the solution compared to capsule. Based upon these data, we hypothesized that an alisertib dose of 45 mg/m2 using the oral solution would yield similar exposure to the alisertib MTD of 60 mg/m2 obtained with enteric-coated tablets.
Our experience in the phase 1 setting as well as prior publications suggested potential predictors of toxicity and response to this regimen. For example, there appeared to be a higher rate of first cycle dose limiting toxicity (DLT) in patients with higher alisertib exposure (AUC).2 In addition, no irinotecan-pretreated patients had an objective response. Given the importance of glucuronidation in irinotecan metabolism,9–11 we also hypothesized that UGT1A1 genotype would be associated with toxicity of this regimen. As Aurora A plays a role in stabilizing Myc proteins,3,6,12 we predicted that tumors characterized by MYCN amplification or increased Myc expression13,14 would be particularly sensitive to alisertib. Other potential markers of clinical benefit include Aurora A protein expression,15 AURKA single nucleotide polymorphisms,16 and LIN28B expression.17
We therefore conducted this study with the following goals. First, we sought to confirm the tolerability and antitumor activity of alisertib, irinotecan, and temozolomide at the MTD in a phase 2 trial. Second, we evaluated a cohort of patients treated with an alisertib oral solution formulation to describe toxicity and pharmacokinetic parameters in the context of this combination regimen. Third, we performed a detailed assessment of potential clinical, biologic, pharmacogenomic, and pharmacokinetic predictors of response and toxicity to this regimen using pooled data from phase 1, phase 2, and oral solution cohorts.
Methods
Patient Eligibility Criteria
Eligibility criteria included: age 1–30 years; high-risk neuroblastoma; relapsed or refractory disease; and presence of evaluable disease by bone marrow morphology, CT/MRI scan, and/or MIBG scans obtained within 4 weeks of enrollment. In addition, patients were required to have adequate performance score (Lansky or Karnofsky score ≥ 50) and to meet the following required washout periods: 3 weeks from last systemic therapy; 12 weeks from prior myeloablative therapy; 2 weeks from prior small port radiation; 6 weeks from prior 131I-MIBG therapy; and 3 months from large field radiation.
Organ function requirements for entry were: absolute neutrophil count (ANC) ≥ 1000/µL; unsupported platelet count ≥ 100,000/µL; serum creatinine ≤ 1.5 times the upper limit of age-adjusted normal value; total bilirubin ≤ 1.5 times upper limit of normal; and ALT < 135 U/L.
Exclusion criteria included: pregnant; breast feeding; prior allogeneic stem cell transplant; need for hemodialysis; active infection; known history of HIV or hepatitis B or C infection; known active intraparenchymal brain metastasis; and prior treatment with alisertib. Patients previously treated with irinotecan and/or temozolomide were eligible if they did not have prior disease progression while treated on a regimen containing those agents. Patients unable to swallow intact pills were excluded from the phase 2 cohort, but were eligible for the oral solution cohort.
The study was conducted by the New Approaches to Neuroblastoma Therapy (NANT) consortium following ethical principles of the Declaration of Helsinki. Each NANT site’s institutional review board approved the study. Patients and/or legal guardians provided written informed consent, with assent obtained as appropriate.
Protocol Therapy
Patients received irinotecan 50 mg/m2/dose intravenously (IV) over 60 minutes and temozolomide 100 mg/m2/dose orally one hour prior to irinotecan on Days 1–5. Alisertib was administered orally once daily on Days 1–7 (at time of temozolomide on Days 1–5). Patients on the phase 2 cohort received alisertib 60 mg/m2/dose as enteric coated tablets. Patients on the oral solution cohort received alisertib 45 mg/m2/dose as the oral solution. All patients received mandatory myeloid growth factor support (short- or long-acting at the discretion of the treating investigator) starting on Day 8 and oral cefixime or cefpodoxime diarrhea prophylaxis for a minimum of 10 days starting two days prior to each cycle.18 Cycles repeated every 21 days for up to 34 cycles. Patients with dose-limiting toxicity (DLT; defined in next section) were able to receive subsequent cycles with defined dose modifications.
Toxicity Assessment
Adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 4. The definition of DLT was initially identical to that used in the phase 1 portion of the study.2 The definition was amended during the phase 2 and OS portions of the study to remove prolonged neutropenia or thrombocytopenia from the definition unless these toxicities resulted in > 14 day delay in starting subsequent cycle. Post-amendment, only failure to meet neutrophil or platelet criteria to start a subsequent course by day 36 remained as a criterion for hematologic DLT. For the purposes of the current report, hematologic toxicities observed prior to the amendment were reassessed to be consistent with amended definition for the summaries of toxicity and associations with biomarkers.
Response Assessment
Patients underwent disease staging at baseline and then after cycles 2, 4, and then every 4 cycles. Response was graded according to version 1.2 of the NANT response criteria that classifies patients as having one of the following overall response categories based upon underlying response at soft tissue sites, MIBG positive sites, and bone marrow disease: complete response (CR); CR with minimal residual disease (CR-MRD); partial response (PR); minor response (MR); stable disease (SD); and progressive disease (PD). These criteria utilize RECIST criteria for measurable tumors19, Curie score for MIBG scan response20, and bone marrow (BM) morphology.21 BM response was graded as CR (required two time points to confirm), CR unconfirmed (one time point only), CR-MRD (bone marrow involvement ≤ 5% at study entry with negative follow-up biopsies), SD, or PD. Patients with at least SD or better underwent central review of MIBG scans, CT scans, and bone marrow pathology slides. Overall responses of CR, CR-MRD, or PR were considered objective responses.
Pharmacokinetic Studies
Submission of serial plasma samples for alisertib and irinotecan pharmacokinetic testing was required for patients in the oral solution cohort and optional for patients in the phase 2 cohort. The sampling schedule, sample analysis, and pharmacokinetic analysis were identical to that described for the phase 1 study2 and included detailed testing around cycle 1, day 4 dosing.
Correlative Biomarker Studies
The study included optional pharmacogenomics and tissue biomarkers aims. Patients consenting to the pharmacogenomics aim provided whole blood in EDTA tubes. DNA was extracted using a QIAamp DNA Blood Mini Kit (QIAGEN) per manufacturer’s instructions. UGT1A1*28 (rs8175347) genotyping was performed as described previously22 using a modified method of Akaba et al.23 AURKA was genotyped at two SNPs. Genotyping for the G>A polymorphism (rs1047972 in codon 57) and T>A polymorphism (rs2273535 in codon 31) was performed by amplification and detected on a Bio-Rad CFX384 Real-Time PCR detection system (Hercules CA). The real time PCR methods were validated against a standard PCR reaction with sequence detection of the polymorphisms. Primer and probe sequences were provided by Takeda (Cambridge, MA). The forward and reverse primer sequences for rs227353 were CTGGCCACTATTTACAGGTAATGGA and TGGAGGTCCAAAACGTGTTCTC, respectively with probe/reporter 1 (VIC-labeled) sequence ACTCAGCAATTTCCTT and probe/reporter 2 (FAM-labeled) sequence CTCAGCAAATTCCTT. The forward and reverse primer sequences for rs1047972 were CGGCTTGTGACTGGAGACA and GGGTCTTGTGTCCTTCAAATTCTTC, respectively with probe/reporter 1 (VIC-labeled) sequence CAGCGCGTTCCTT and probe/reporter 2 (FAM-labeled) sequence CAGCGCATTCCTT.
Patients consenting to the tissue biomarkers aim provided archival paraffin-embedded tumor tissue. Immunohistochemistry was performed for Mycn, Myc, Aurora A, and Lin28B (see Supplemental Figures 1 and 2 for representative images). Assays for Mycn and Myc were performed as previously described.14 We utilized these results as well as results of MYCN amplification status collected at baseline to create a composite variable that coded a patient with MYCN amplification or positivity for Mycn or Myc protein expression into a composite “MYCN/Myc” positive group. Patients with MYCN non-amplified tumors that were also negative for Mycn and for Myc protein expression were coded as “MYCN/Myc” negative. Aurora A and Lin28B immunostaining was performed using Leica BondTM Epitope Retrieval Solution 1 and BondTM Polymer Refine Detection Kit. The Aurora A primary antibody was obtained from Sigma-Aldrich (St. Louis, MO) and used at a concentration of 1:400. The Lin28B primary antibody was obtained from Cell Signaling (Darmstadt, Germany) and used at a concentration of 1:160.
Study Design and Statistical Methods
The phase 2 cohort utilized a two-stage design intended to exclude a null objective response rate < 15%. This design included 14 patients in the first stage and the addition of 6 patients in a second stage if at least one objective response was observed among the first 14 patients. Under the hypothesis of a true response rate that is ≥ 15%, there was a 90% chance that at least 1 of the 14 patients in the first stage would experience an objective response. Conversely, zero responders in the first 14 would lead us to conclude that the true response rate is less than 15%.
The oral solution cohort was intended to follow the rolling 6 dose escalation design24 starting at the 45 mg/m2 dose level, with pharmacokinetic data and DLTs in the first cycle of therapy impacting dose escalation decisions. Dose escalation was allowed only if pharmacokinetic data demonstrated alisertib levels were below those predicted based upon adult bioavailability data. The protocol included a provision to expand a dose level to 12 patients to obtain additional toxicity and pharmacokinetic data before deciding to escalate.
Progression-free survival (PFS) was calculated as the time from start of treatment until progression or death, whichever occurred first. Patients who were alive and free of progression were censored at the date that their status was last documented. Patients who started another therapy prior to progression were censored at that time. The Kaplan-Meier product limit method was used to display the PFS pattern over time. One-year PFS probabilities were based on Kaplan-Meier plots; associated standard errors for one-year PFS were based on Greenwood’s formula. Potential associations between binary biomarkers and occurrence of DLT or objective response were assessed using Fisher’s exact tests. Potential associations between ordered groups defined by increasing number of copies of variant alleles for UGT1A1 or AURKA were assessed using an exact logistic regression test for trend. Potential associations between groups defined by biomarkers and PFS were assessed using logrank tests. Differences in pharmacokinetic parameters between alisertib oral solution and tablet formulations utilized Wilcoxon rank sum tests. All p-values were two-sided and there was no adjustment for multiple testing. Analyses were performed using SAS version 9.4 software (SAS Institute, Inc., Cary, North Carolina) and figures were generated using R version 3.4.2 (R Core Team, Vienna, Austria).
Results
Patient Characteristics
Thirty-four patients were enrolled to both cohorts (phase 2 and OS) from May 2014–May 2015. Results presented in this report are current through the January 10, 2018 data cut-off. One patient in the OS cohort developed rising hepatic transaminase levels after receiving cefixime prophylaxis only and did not receive anticancer therapy prior to removal from study. Another patient in the OS cohort completed one cycle of therapy before molecular profiling of his tumor revealed the presence of an EWSR1 translocation diagnostic of Ewing sarcoma instead of neuroblastoma. He was declared ineligible and removed from study after one cycle. The remaining 32 patients comprise the main analytical cohort of patients for this report (Table 1).
Table 1.
Patient characteristics of 20 patients in phase 2 cohort and 12 patients in oral solution cohort.
| Characteristic | Phase 2 (n = 20) |
Oral Solution (n = 12) |
|---|---|---|
| Median Age (Range), years | 10.7 (4.3–19.4) | 3.0 (1.6–7.2) |
| Male : Female | 14 : 6 | 6 : 6 |
| MYCN Amplified : Non-Amplified | 3 : 14 | 6 : 5 |
| Prior Irinotecan | 11 (55%) | 2 (17%) |
| Prior Temozolomide | 10 (50%) | 2 (17%) |
| Median Number of Prior Regimens (Range) | 3 (1–10) | 1 (1–2) |
| > 3 Prior Regimens | 8 (40%) | 0 (0%) |
Patients in the phase 2 cohort were heavily pre-treated with 40% having had more than 3 prior regimens, 55% with prior irinotecan, and 50% with prior temozolomide. Six of 11 patients in the OS cohort with known MYCN status had MYCN amplified tumors. Patients received a median of 4.5 (range 1–34) and 2 cycles (range 1–24) in the phase 2 and OS cohorts, respectively.
Antitumor Activity
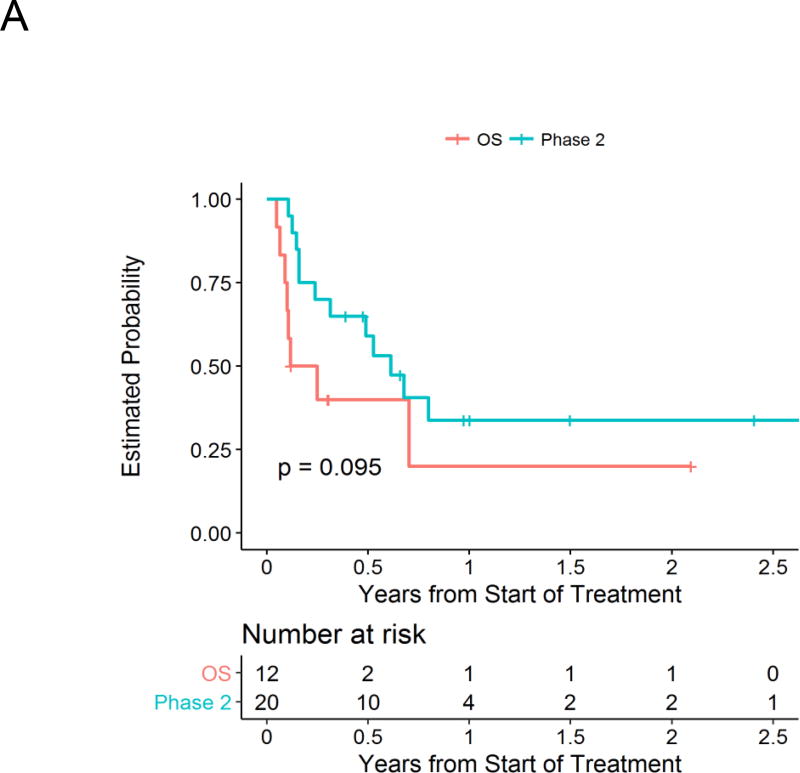
Responses are summarized in Table 2 according to cohort. In phase 2, one patient enrolled with site-reported evaluable disease at baseline that was not observed/confirmed on central review, leaving 19 patients evaluable for response. Of these 19 patients, 4 patients had an objective response (21.1%; all partial responses). Two of these patients had previously received irinotecan and temozolomide. In the OS cohort, all 12 patients were evaluable for response, with one patient with CR-MRD for a response rate of 8.3%. The estimated 1-year PFS was 34% (±12%) in the phase 2 cohort and 20% (±16%) in the OS cohort (Figure 1A).
Table 2.
Best response for patients in the Phase 2 and Oral Solution cohorts.
| Response Category | Phase 2 (n = 20) |
Oral Solution (n = 12) |
|---|---|---|
| Complete Response (CR) | 0 | 0 |
| CR-Minimal Residual Disease (MRD) | 0 | 1 |
| Partial Response (PR) | 4 | 0 |
| Minor Response | 2* | 2** |
| Stable Disease | 8 | 3 |
| Progressive Disease | 5 | 6 |
| Not Evaluable | 1 | 0 |
| Response Rate (CR + CR-MRD + PR) | 4 / 19 = 21.1% | 1 / 12 = 8.3% |
Minor responses in the Phase 2 consisted of one patient with bone marrow CR in setting of SD by MIBG scan and one patient with bone marrow CR-MRD in setting of SD by MIBG and CT scans.
Minor responses in the Oral Solution cohort consisted of one patient with an MIBG CR in setting of SD by CT scan and bone marrow criteria and one patient with a PR by CT scan in setting of SD by MIBG scan and bone marrow criteria.
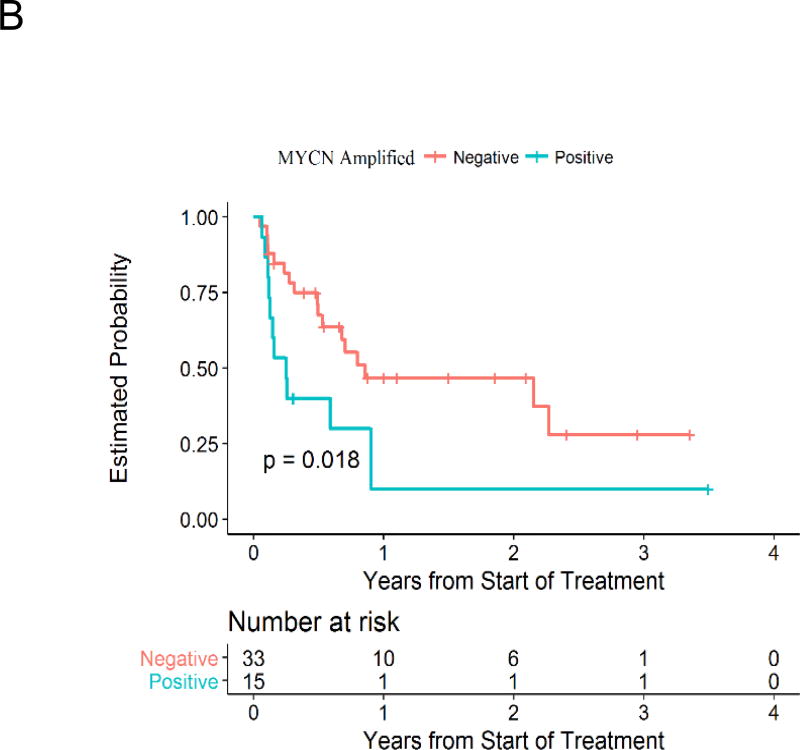
Figure 1.
Estimated progression-free survival for patients on Phase II and Oral Solution cohorts (A) and for groups defined by MYCN amplification status (B).
Toxicity
Details of first course toxicity according to cohort are shown in Table 3. In the phase 2 cohort, hematologic adverse events were the most common grade 3+ toxicities. Fourteen (70%) patients had first cycle grade 3+ neutropenia, 11 (55%) patients had first cycle grade 3+ thrombocytopenia, and 15 (75%) had either Grade 3+ thrombocytopenia or Grade 3+ neutropenia. Ten patients (50%) had a delay of any duration in starting the second cycle due to delayed hematologic recovery. Non-hematologic toxicities were generally lower grade, though twenty percent of patients in the phase 2 cohort had grade 3 diarrhea in the first cycle. Four of the 20 patients (20%) who were evaluable for toxicity had first cycle DLT [1 DLT was non-hematologic (grade 3 dehydration and grade 3 vomiting) and 3 were hematologic]. One patient had first cycle hematologic toxicity that was initially classified as DLT but was then reclassified as non-DLT according to the amended definition of hematologic toxicity. Of the 4 patients with first cycle DLT, 3 had DLT in subsequent cycles (cycles 2, 2, and 3). Among the 19 patients who received > 1 cycle, 10 (53%) had DLT in subsequent cycles. Among the 7 patients who had first DLT occurring in a subsequent cycle beyond the first cycle, these first DLTs occurred in cycles 2, 2, 3, 4, 4, 8, and 15.
Table 3.
Grade 3 and higher hematologic and non-hematologic adverse events reported as at least possibly related to protocol therapy in the first cycle in Phase 2 and Oral Solution cohorts.
| Adverse Event | Phase 2 (n = 20) | Oral Solution (n = 12) | ||
|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Hematologic | ||||
| Neutropenia | 50% | 20% | 17% | 42% |
| Thrombocytopenia | 15% | 40% | 33% | |
| Lymphopenia | 25% | 10% | 8% | 17% |
| Leukopenia | 25% | 15% | 17% | 25% |
| Anemia | 15% | 33% | ||
| Non-hematologic | ||||
| Diarrhea | 20% | 8% | ||
| AST elevation | 5% | 8% | ||
| ALT elevation | 5% | |||
| Nausea | 8% | |||
| Hypoalbuminemia | 8% | |||
| Pleural effusion | 8% | |||
| Febrile neutropenia | 8% | |||
| Vomiting | 5% | |||
| Dehydration | 5% | |||
For the OS cohort, 1 of the first 6 patients treated with alisertib OS at 45 mg/m2/dose had first cycle DLT (grade 3 diarrhea qualifying as DLT, and grade 4 neutropenia > 7 days – with the neutropenia DLT based on the initial DLT definition but not based on the amended definition). This dose level was expanded to a total of 12 patients to obtain additional tolerability and pharmacokinetic data (see below). In the second group of 6 patients, 2 patients had first cycle DLT (one with grade 3 hypoalbuminemia, and one with grade 3 pleural effusion who also had prolonged neutropenia and prolonged thrombocytopenia that had not resolved by Day 36), such that 3 of 12 patients (25%) had first cycle DLT. As exposure was not below levels observed with tablets at 60 mg/m2 (see below), the dose was not escalated and 45 mg/m2 was determined to be the recommended dose of oral solution in this combination. First cycle grade 3+ thrombocytopenia and grade 3 diarrhea were seen in 33% and 8% of OS cohort patients, respectively (Table 3). Two patients (16.7%) had a delay of any duration in starting the second cycle due to delayed hematologic recovery. Among 10 patients who received > 1 cycle, 2 (20%) had first DLT (1 non-hematologic and 1 hematologic) in subsequent cycles (cycles 3 and 16).
Alisertib and Irinotecan Pharmacokinetics
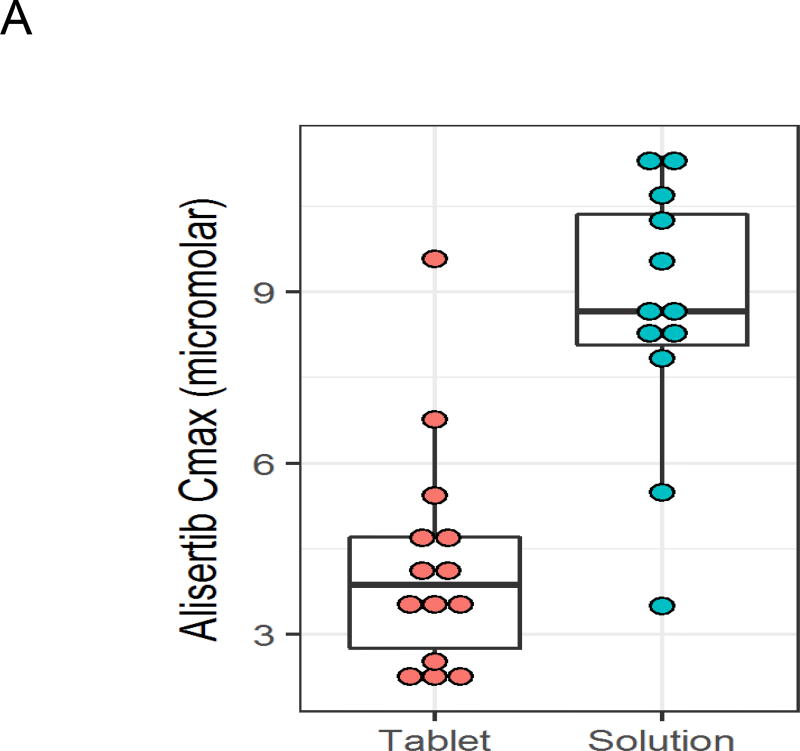
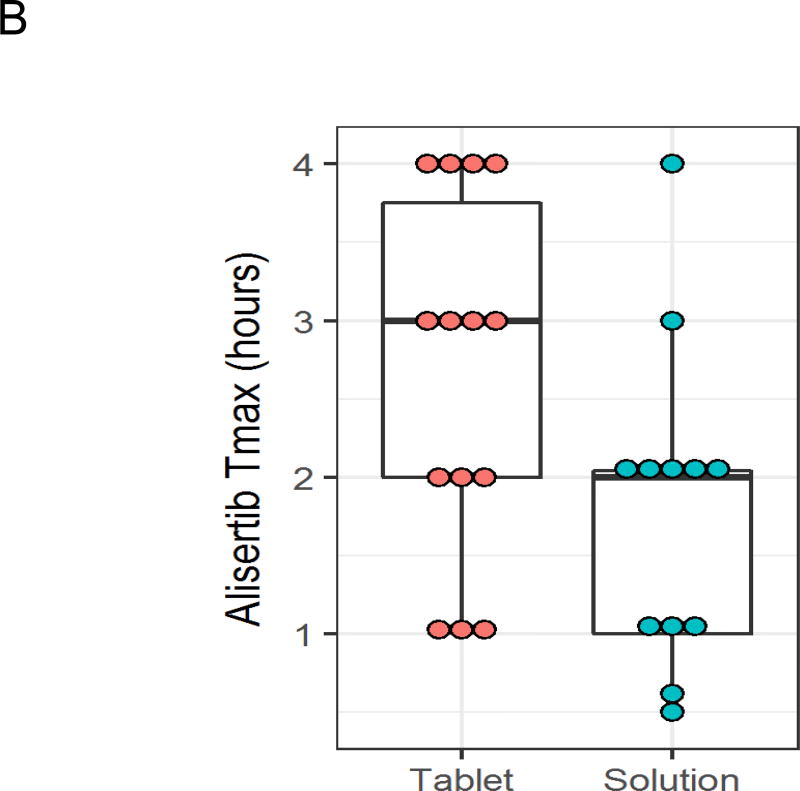
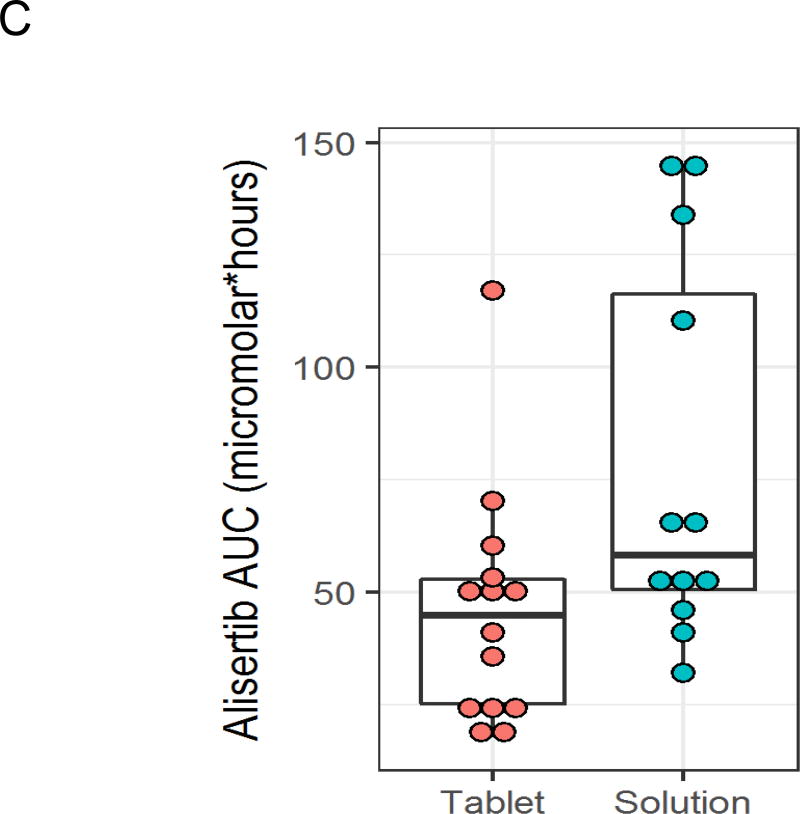
Detailed alisertib pharmacokinetic data were available for all 12 patients in the OS cohort treated with 45 mg/m2 (Supplemental Table 1). In addition, alisertib pharmacokinetic data were available for 8 patients in the phase 2 cohort and for 6 patients treated with alisertib tablets at the 60 mg/m2 dose level from our previously published phase 1 study.2 Compared to data from these 14 patients treated with 60 mg/m2 tablets, the OS yielded higher peak plasma concentrations (Figure 2A; median 8.67 vs. 3.86 µM; p<0.001) and higher exposure (AUC; Figure 2B; median 58 vs. 45 µM·hour; p=0.025). There was a trend to suggest earlier time to peak concentration with the OS (Figure 2C; median 2 vs. 3 hours; p=0.081). There were no differences in half-life (median 8.3 vs. 10.4 hours; p=0.57) or trough concentration (median 0.58 vs. 0.72 µM; p=0.72) between formulations.
Figure 2.
Alisertib peak plasma concentration (Cmax; A), time to peak plasma concentration (B), and exposure (C) after Cycle 1, Day 4 dosing for patients treated with alisertib oral solution at 45 mg/m2 (n = 12) or enteric coated tablets at 60 mg/m2 (n = 14).
Detailed irinotecan pharmacokinetic data were available for 8 patients in the OS cohort, with 4 patients without samples for irinotecan pharmacokinetics due to blood volume considerations in smaller patients. Irinotecan pharmacokinetic data were also available for 6 patients in the phase 2 and for 6 patients treated with alisertib tablets at the 60 mg/m2 dose level from our previously published phase 1 study.2 Irinotecan exposure and clearance were qualitatively similar for patients receiving alisertib as oral solution or as enteric-coated tablets (Supplemental Table 2). Likewise, SN-38 and SN-38G peak plasma concentration and exposure appeared similar between groups treated with alisertib oral solution or enteric-coated tablet.
Predictors of Toxicity and Antitumor Activity
We next pooled data from phase 1, phase 2, and OS cohorts to identify potential predictors of DLT, response, and PFS in 54 patients (22 previously reported and 32 patients in this report; Table 4). We examined the association between first course DLT and any DLT in any cycle with predictors of interest. Only alisertib Day 5 trough in the first cycle was significantly associated with first cycle DLT (5% with trough below median of 0.46 µM vs. 38% with trough ≥ 0.46 µM; p=0.045). Only 1 (14%) of the 7 patients < 4 years old experienced grade 2+ diarrhea at any time, compared to 28 (60%) of 47 patients aged 4 or older (p=0.041). No other variables were significantly associated with grade 2+ diarrhea (data not shown).
Table 4.
Association between biomarkers and measures of toxicity and clinical benefit in a pooled dataset consisting of patients treated on the Phase 12 (n = 22), Phase 2 (n = 20), and Oral Solution (n = 12) cohorts.
| Measures of Toxicity | Measures of Clinical Benefit | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Biomarker | N | First Cycle DLT* (n=11) |
p | DLT in Any Cycle* (n=27) |
p | Objective Response (n=12)** |
p | 1-Yr PFS | p |
|
| |||||||||
| Age < 4 years | 7 | 14% | 1.0 | 29% | 0.42 | 0% | 0.33 | 29% | 0.002 |
| Age ≥ 4 years | 47 | 21% | 53% | 26% | 42% | ||||
|
| |||||||||
| Age < 8 years | 28 | 18% | 0.74 | 39% | 0.17 | 18% | 0.52 | 31% | 0.16 |
| Age ≥ 8 years | 26 | 23% | 62% | 27% | 47% | ||||
|
| |||||||||
| Prior irinotecan | 18 | 28% | 0.48 | 50% | 1.0 | 11% | 0.30 | 30% | 0.41 |
| No prior irinotecan | 36 | 17% | 50% | 28% | 43% | ||||
|
| |||||||||
| Prior temozolomide | 17 | 35% | 0.081 | 53% | 1.0 | 12% | 0.30 | 32% | 0.56 |
| No prior temozolomide | 37 | 14% | 49% | 27% | 41% | ||||
|
| |||||||||
| ≤ 2 Prior regimens | 37 | 14% | 0.081 | 43% | 0.24 | 22% | 1.0 | 42% | 0.97 |
| > 2 Prior regimens | 17 | 35% | 65% | 24% | 32% | ||||
|
| |||||||||
| Alisertib Cmax> median | 21 | 24% | 0.70 | 48% | 1.0 | 19% | 1.0 | 28% | 0.09 |
| Alisertib Cmax ≤ median | 21 | 14% | 43% | 24% | 55% | ||||
|
| |||||||||
| Alisertib Day 5 trough > median | 21 | 33% | 0.045 | 57% | 0.21 | 24% | 1.0 | 45% | 0.26 |
| Alisertib Day 5 trough ≤ median | 21 | 5% | 33% | 19% | 33% | ||||
|
| |||||||||
| Alisertib exposure> median | 21 | 24% | 0.70 | 52% | 0.54 | 19% | 1.0 | 41% | 0.97 |
| Alisertib exposure ≤ median | 21 | 14% | 38% | 24% | 38% | ||||
|
| |||||||||
| UGT1A1 genotype 6 / 6 | 17 | 12% | 0.094 | 47% | 0.63 | 35% | 0.24 | 57% | 0.62 |
| UGT1A1 genotype 6 / 7 | 17 | 18% | 35% | 6% | 26% | ||||
| UGT1A1 genotype 7 / 7 | 5 | 60% | 80% | 20% | 40% | ||||
|
| |||||||||
| AURKA Codon 31 wild type | 27 | 22% | 0.96 | 48% | 0.82 | 19% | 0.90 | 32% | 0.33 |
| AURKA Codon 31 heterozygote | 12 | 17% | 42% | 25% | 54% | ||||
| AURKA Codon 31 homozygote | 1 | 0% | 0% | 0% | 100% | ||||
|
| |||||||||
| AURKA Codon 57 wild type | 27 | 15% | 0.40 | 41% | 0.51 | 19% | 1.0 | 37% | 0.56 |
| AURKA Codon 57 heterozygote | 13 | 21% | 54% | 23% | 43% | ||||
| AURKA Codon 57 homozygote | 0 | -- | -- | -- | -- | ||||
|
| |||||||||
| MYCN amplified | 15 | 13% | 0.47 | 33% | 0.22 | 7% | 0.14 | 10% | 0.018 |
| MYCN non-amplified | 33 | 24% | 55% | 27% | 47% | ||||
|
| |||||||||
| MYCN amplified or Myc/Mycn + | 18 | 17% | 1.0 | 39% | 1.0 | 11% | 0.21 | 12% | 0.019 |
| MYCN non-amplified and Myc/Mycn − | 16 | 19% | 44% | 31% | 56% | ||||
|
| |||||||||
| Aurora A protein + | 12 | 25% | 0.36 | 50% | 0.46 | 17% | 1.0 | 69% | 0.078 |
| Aurora A protein − | 18 | 11% | 33% | 22% | 31% | ||||
|
| |||||||||
| Lin28B protein + | 17 | 18% | 1.0 | 47% | 0.47 | 12% | 0.36 | 56% | 0.37 |
| Lin 28B protein − | 13 | 15% | 31% | 31% | 34% | ||||
Utilizing amended DLT definition (see Methods).
Includes patients with complete response, complete response with minimal residual disease, or partial response.
None of the variables were significantly associated with response. Patients with tumors with evidence of Mycn or Myc activity (analyzed either as MYCN amplification or who were positive for the composite variable with positivity for MYCN amplification, Mycn protein expression, and/or Myc protein expression) had numerically lower objective response rates (Table 4). MYCN status did result in statistically significant differences in terms of PFS. The 1-year PFS rate for patients with MYCN amplification was 10% (±9%) vs. 47% (±10%) for patients without MYCN amplification (p=0.018; Figure 2B). A similar pattern was seen using the composite of MYCN amplification or Mycn or Myc protein expression. The only other significant association with PFS included lower 1-year PFS rate for patients < 4 years old [29% (±0%)] compared to older patients [42% (±8%); p=0.002]. Patients with positive Aurora A protein expression had higher 1-year PFS rate than patients with negative Aurora A protein (69% vs. 31%), though the difference was not statistically significant (p=0.078). We also investigated PFS using the combination of Aurora A protein expression and the composite of MYCN amplification or Mycn or Myc protein expression. Only three patients had tumors that were positive for both Aurora A expression and positive for the composite of MYCN amplification or Mycn or Myc protein expression. These 3 patients had a 100% 1-year PFS compared to 0% for the 8 patients who had tumors that were negative for Aurora A expression but positive for the composite of MYCN amplification or Mycn or Myc protein expression (p=0.002).
Discussion
In this study, we have extended our prior phase 1 experience to understand more fully the antitumor activity and toxicity of the combination of alisertib with irinotecan and temozolomide. We present phase 2 data that highlight the activity of this regimen in neuroblastoma. Our evaluation of an alisertib oral solution provides important new data that will be critical in the clinical development of this agent in an indication that preferentially occurs in young children. Our investigation of biomarkers across the full cohort of patients treated on the phase 1, phase 2, and oral solution portions of the study has improved our understanding of patients most likely to experience severe toxicity with this regimen, another key set of data needed to advance the pediatric clinical development of this agent.
Our phase 2 experience demonstrates antitumor activity greater than might be expected based upon the published experience with irinotecan and temozolomide alone.7,25,26 Specifically, a prior phase 2 trial of irinotecan and temozolomide in patients with first relapsed neuroblastoma reported an objective response rate of 16% and 1-year PFS rate of approximately 20%.7 Nevertheless, our phase 2 response rate and estimated 1-year PFS are lower than observed in our published phase 1 experience. We note that the phase 2 population was paradoxically more heavily pretreated (median 3 prior regimens; range 1–10) than our phase 1 population (median 1 prior regimen; range 1–7) and more heavily pretreated than the prior phase 2 trial of irinotecan and temozolomide alone. Our biomarker studies complement these clinical findings and demonstrate that patients with MYCN / Myc-driven tumors have poor outcomes despite treatment with this regimen. This finding is contrary to preclinical rationale demonstrating a role for Aurora A kinase inhibitors in destabilizing Myc proteins, though newer Aurora A kinase inhibitors may have a greater effect on Myc protein stability.3 Importantly, the poor outcomes seen in this population may also simply reflect the known adverse prognostic effect of MYCN amplification in the setting of recurrent high-risk neuroblastoma.27 Our non-randomized study design is not able to differentiate a prognostic marker from a predictive marker that might enable patient selection for this trial.28
Evaluation of an alisertib oral solution in adults demonstrated higher exposure with the use of an oral solution.8 Based upon a relative bioequivalence of 1.26, we investigated the oral solution at a dose of 45 mg/m2, which represents 75% of the dose established for use with the tablet formulation in the context of this combination.2 We show that this dose level is tolerable and, as expected based upon other comparisons of liquid vs. tablet formulations of other agents, we observed higher Cmax and a trend towards earlier Tmax with the oral solution. We hypothesized that exposures would be comparable between solution at 45 mg/m2 and tablet at 60 mg/m2. We observed extensive interpatient variability in both groups and a slightly higher median exposure with the oral solution. The wide range of exposures with both formulations allowed us to investigate exposure as a potential predictor of toxicity and antitumor activity, with no associations seen. Despite higher exposure, patients on the oral solution cohort had a low response rate, which may have been due to the high incidence of MYCN amplification (55%) in this cohort.
As in our phase 1 experience, cumulative toxicity was substantial. In the Phase 2 cohort, one patient who experienced Grade 3 diarrhea (not a DLT) discontinued therapy after the 1st course; of the 19 patients who received more than 1 course, 10 (53%) required a dose reduction of at least one agent in subsequent courses of therapy. We designed our biomarker studies to provide new insights into subgroups at higher risk of severe toxicity. The most promising marker was higher trough concentration on Day 5 of the first cycle. Whether this or other biomarkers can be used to modify drug doses in this regimen will require further investigation.
In conclusion, we have further defined the antitumor activity of this regimen in a heavily pretreated population. Our investigation of an alisertib oral solution now allows younger children and older patients unable to swallow pills to be able to receive this agent. Our findings suggest a potential role for Aurora A kinase inhibition in the management of patients with advanced neuroblastoma, though the toxicity profile is more consistent with regimens utilized during the frontline management of patients with high-risk neuroblastoma. Future studies should focus on evaluating this combination in a more homogeneous population earlier in the course of their illness (e.g., first relapsed disease or during initial induction chemotherapy). Moreover, testing novel Aurora A kinase inhibitors with greater effects on Mycn protein stability3 as well as novel combinations with Aurora A kinase inhibitors is a high priority.
Supplementary Material
Statement of Translational Relevance.
Preclinical data have demonstrated activity of the Aurora A kinase inhibitor, alisertib, as monotherapy or in combination with conventional chemotherapy in models of neuroblastoma. A phase 1 study of alisertib, irinotecan, and temozolomide in children with relapsed neuroblastoma defined the recommended dosing for this combination and showed promising response and progression-free survival rates. In order to extend that observation, the current report describes the results of a phase 2 trial of this combination and also provides data on the use of an alisertib oral solution that allowed younger patients to be treated. Companion biomarker studies from all patients treated with this combination provide potential tools to predict response and toxicity following treatment with this regimen. These results provide a fuller understanding of the toxicity, anti-neuroblastoma activity, and potential biomarkers in patients treated with this combination.
Acknowledgments
The authors gratefully acknowledge the NANT Operations Center, NANT institutions, research nurses, research assistants, participating patients and families, as well as the alisertib team at Millennium. Supported by National Cancer Institute P01 81403 (S. Groshen, N. Boucher, S. Czarnecki, C. Luo, D. Tsao-Wei, K. Matthay, A. Marachelian); P30 CA15083 (R. Kudgus, J. Reid, R. McGovern); NIH/NCRR UCSF-CTSI UL1 RR024131; Alex’s Lemonade Stand Foundation (K. Matthay, S. DuBois); and Millennium Pharmaceuticals, Inc.
Disclosures: Millennium Pharmaceuticals, Inc. provided alisertib and research funding to support this trial.
Footnotes
Previous Presentation: Presented at the 2016 American Society of Clinical Oncology Annual Meeting and the 2016 Advances in Neuroblastoma Research Meeting.
Clinicaltrials.gov Trial Number: NCT01601535
References
- 1.Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J Clin Oncol. 2015;33:3008–17. doi: 10.1200/JCO.2014.59.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DuBois SG, Marachelian A, Fox E, et al. Phase I Study of the Aurora A Kinase Inhibitor Alisertib in Combination With Irinotecan and Temozolomide for Patients With Relapsed or Refractory Neuroblastoma: A NANT (New Approaches to Neuroblastoma Therapy) Trial. J Clin Oncol. 2016;34:1368–75. doi: 10.1200/JCO.2015.65.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gustafson WC, Meyerowitz JG, Nekritz EA, et al. Drugging MYCN through an allosteric transition in Aurora kinase A. Cancer Cell. 2014;26:414–427. doi: 10.1016/j.ccr.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maris JM, Morton CL, Gorlick R, et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP) Pediatr Blood Cancer. 2010;55:26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosse YP, Lipsitz E, Fox E, et al. Pediatric phase I trial and pharmacokinetic study of MLN8237, an investigational oral selective small-molecule inhibitor of Aurora kinase A: a Children's Oncology Group Phase I Consortium study. Clin Cancer Res. 2012;18:6058–64. doi: 10.1158/1078-0432.CCR-11-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otto T, Horn S, Brockmann M, et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell. 2009;15:67–78. doi: 10.1016/j.ccr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Bagatell R, London WB, Wagner LM, et al. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: a Children's Oncology Group study. J Clin Oncol. 2011;29:208–13. doi: 10.1200/JCO.2010.31.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falchook GS, Venkatakrishnan K, Sarantopoulos J, et al. Relative bioavailability of a prototype oral solution of the Aurora A kinase inhibitor alisertib (MLN8237) in patients with advanced solid tumors. Int J Clin Pharmacol Ther. 2015;53:563–72. doi: 10.5414/CP202359. [DOI] [PubMed] [Google Scholar]
- 9.Deeken JF, Slack R, Marshall JL. Irinotecan and uridine diphosphate glucuronosyltransferase 1A1 pharmacogenetics: to test or not to test, that is the question. Cancer. 2008;113:1502–10. doi: 10.1002/cncr.23777. [DOI] [PubMed] [Google Scholar]
- 10.Hu ZY, Yu Q, Zhao YS. Dose-dependent association between UGT1A1*28 polymorphism and irinotecan-induced diarrhoea: a meta-analysis. Eur J Cancer. 2010;46:1856–65. doi: 10.1016/j.ejca.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 11.Stewart CF, Panetta JC, O'Shaughnessy MA, et al. UGT1A1 promoter genotype correlates with SN-38 pharmacokinetics, but not severe toxicity in patients receiving low-dose irinotecan. J Clin Oncol. 2007;25:2594–600. doi: 10.1200/JCO.2006.10.2301. [DOI] [PubMed] [Google Scholar]
- 12.Richards MW, Burgess SG, Poon E, et al. Structural basis of N-Myc binding by Aurora-A and its destabilization by kinase inhibitors. Proc Natl Acad Sci U S A. 2016;113:13726–13731. doi: 10.1073/pnas.1610626113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang LL, Suganuma R, Ikegaki N, et al. Neuroblastoma of undifferentiated subtype, prognostic significance of prominent nucleolar formation, and MYC/MYCN protein expression: a report from the Children's Oncology Group. Cancer. 2013;119:3718–26. doi: 10.1002/cncr.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang LL, Teshiba R, Ikegaki N, et al. Augmented expression of MYC and/or MYCN protein defines highly aggressive MYC-driven neuroblastoma: a Children's Oncology Group study. Br J Cancer. 2015;113:57–63. doi: 10.1038/bjc.2015.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shang X, Burlingame SM, Okcu MF, et al. Aurora A is a negative prognostic factor and a new therapeutic target in human neuroblastoma. Mol Cancer Ther. 2009;8:2461–9. doi: 10.1158/1535-7163.MCT-08-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan JY, Ajani JA, Gu J, et al. Association of Aurora-A (STK15) kinase polymorphisms with clinical outcome of esophageal cancer treated with preoperative chemoradiation. Cancer. 2012;118:4346–53. doi: 10.1002/cncr.26581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnepp RW, Khurana P, Attiyeh EF, et al. A LIN28B-RAN-AURKA Signaling Network Promotes Neuroblastoma Tumorigenesis. Cancer Cell. 2015;28:599–609. doi: 10.1016/j.ccell.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner LM, Crews KR, Stewart CF, et al. Reducing irinotecan-associated diarrhea in children. Pediatr Blood Cancer. 2008;50:201–7. doi: 10.1002/pbc.21280. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Matthay KK, Shulkin B, Ladenstein R, et al. Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: a report for the International Neuroblastoma Risk Group (INRG) Task Force. Br J Cancer. 2010;102:1319–26. doi: 10.1038/sj.bjc.6605621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villablanca JG, Ji L, Shapira-Lewinson A, et al. Predictors of response, progression-free survival, and overall survival using NANT Response Criteria (v1.0) in relapsed and refractory high-risk neuroblastoma. Pediatr Blood Cancer. 2018;65:e26940. doi: 10.1002/pbc.26940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goetz MP, McKean HA, Reid JM, et al. UGT1A1 genotype-guided phase I study of irinotecan, oxaliplatin, and capecitabine. Invest New Drugs. 2013;31:1559–67. doi: 10.1007/s10637-013-0034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akaba K, Kimura T, Sasaki A, et al. Neonatal hyperbilirubinemia and mutation of the bilirubin uridine diphosphate-glucuronosyltransferase gene: a common missense mutation among Japanese, Koreans and Chinese. Biochem Mol Biol Int. 1998;46:21–6. doi: 10.1080/15216549800203512. [DOI] [PubMed] [Google Scholar]
- 24.Skolnik JM, Barrett JS, Jayaraman B, et al. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26:190–5. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- 25.Kushner BH, Kramer K, Modak S, et al. Irinotecan plus temozolomide for relapsed or refractory neuroblastoma. J Clin Oncol. 2006;24:5271–6. doi: 10.1200/JCO.2006.06.7272. [DOI] [PubMed] [Google Scholar]
- 26.Wagner LM, Crews KR, Iacono LC, et al. Phase I trial of temozolomide and protracted irinotecan in pediatric patients with refractory solid tumors. Clin Cancer Res. 2004;10:840–8. doi: 10.1158/1078-0432.ccr-03-0175. [DOI] [PubMed] [Google Scholar]
- 27.London WB, Castel V, Monclair T, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from the International Neuroblastoma Risk Group project. J Clin Oncol. 2011;29:3286–92. doi: 10.1200/JCO.2010.34.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballman KV. Biomarker: Predictive or Prognostic? J Clin Oncol. 2015;33:3968–71. doi: 10.1200/JCO.2015.63.3651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.