Abstract
Purpose of review:
novel immunotherapies such as checkpoint inhibitors, bispecific and chimeric antigen receptor T cells are leading to promising responses when treating solid tumors and hematological malignancies. T cell neoplasms include leukemia and lymphomas that are derived from T cells. These are rare diseases with generally poor clinical outcomes. This review describes the rational and preliminary results of these approaches for people with T cell lymphoma and leukemia.
Recent findings:
for T cell neoplasms, despite significant research effort, only few agents, such as monoclonal antibodies and allogeneic stem cell transplantation, showed some clinical activity. One of the major hurdles to targeting T cell neoplasms is that activation or elimination of T cells, either normal or neoplastic, can cause significant toxicity. A need to develop novel safe and effective immunotherapies for T cell neoplasms exists.
Summary:
In this review, we will discuss the rationale for immunotherapy of T cell leukemia and lymphoma and present the most recent therapeutic approaches.
Keywords: Immunotherapy, T cell lymphoma, T-ALL, T cell lymphoma, CART cell therapy
Introduction
In the last few years, immunotherapy has revolutionized the treatment of cancer. After the first wave of clinical responses with monoclonal antibodies, a new generation of immunotherapies has become available (1-4). Checkpoint inhibitors, bispecific antibodies, and chimeric antigen receptor T cells (CART) have led to unprecedented responses in patients with relapsed and refractory neoplasms (1-4). After being validated in the most common cancer subtypes, some of these approaches are now being tested in rare neoplasms such as T cell lymphoma (TCL) and leukemia (T-ALL). The need for effective and safe new therapies is urgent, as the overall prognosis for these rare diseases is generally poor (5, 6).
T cell lymphomas and leukemias represent a broad group of disorders, characterized by clonal growth and dysfunction of T cells at different stages of maturation and commitment. Traditionally, T cell lymphomas have been divided into cutaneous TCL (CTCL) and peripheral TCL (PTCL). Overall, TCL represent about 10% of all non-Hodgkin’s lymphomas (NHL) (7). PTCL-not otherwise specified (PTCL-NOS) is the most common subtype (26%), followed by angioimmunoblastic TCL (AITL, 19%), anaplastic large cell lymphoma (ALCL) divided into anaplastic lymphoma kinase (ALK) positive (7%), and ALK negative (6%) and enteropathy-associated TCL (EATL; <5%) (5, 8). The majority of PTCL originate from the CD4+ helper cells while only a minority of PTCL is derived from the CD8+ cytotoxic cells. Additionally, some PTCL, like AITL, share a similar phenotype with the T follicular helper cells (TFH) (9, 10). CTCLs are a group of mature TCLs that present primarily in the skin, but can progress to blood, lymph nodes and visceral organs. Mycosis Fungoides (MF) is the most common subtype of CTCL, while Sezary Syndrome (SS) is more rare. In 2016, it was estimated that MF was diagnosed in 1620 subject and SS in 70 in the United States (11). Extranodal NK/T cell lymphoma (ENKL) is a distinct TCL that is frequently localized in the nasal area (nasal cavity, nasopharynx, paranasal sinus and palate in 68%) or extra-nasal (in 26%). In an analysis of the International T-cell Lymphoma Project, over 1153 patients, 136 (12%) had ENKL, and the frequency is higher in Asia than in the Western countries (22% vs 5%) (12). Adult T-cell leukemia/lymphoma (ATLL), T large granular lymphocytic leukemia (T-LGL) and T-prolymphocytic leukemia (T-PLL) are rare, mature leukemic T-cell lymphoproliferative disorders. ATLL is extremely aggressive and is promoted by human T-cell lymphotropic virus type 1 (HTLV1). T-LGL accounts for 2-5% of all chronic lymphoproliferative disorders in North America and Europe. It is usually an indolent disease which can be associated with autoimmune disorders. T-PLL accounts for approximately 2% of mature lymphoid malignancies, and usually presents with diffuse hepatosplenomegaly and leukocytosis (13). Precursor T-cell lymphoblastic lymphoma/leukemia instead arises from early T cell progenitors. It is a rapidly growing, aggressive disease (14, 15), occurring more frequently in late childhood, adolescence and young adults. T-ALL represents 10-15% of pediatric and 20-25% of adult cases of ALL in the western world and Japan.
This large and heterogeneous group of diseases varies in clinical behavior and overall prognosis. In general, there are limited effective treatment options for T cell lymphoproliferative disorders in frontline and even more so in the relapse/refractory setting, where median survival is poor. As an example, the survival for PTCL is 5 months for refractory patients and 11 months for relapsed patients (16). Other TCL subtypes that are generally considered more indolent, such as CTCL, are extremely difficult to treat when they progress with tumor involvement and/or systemic disease. In the last few years, new treatment options have been proposed and are in early stage of development. Given the success of checkpoint inhibition and CART in other lymphomas and leukemias, investigating the effects of immunotherapy for T cell neoplasms has become a topic of research interest. Here we present a broad view of the known immunotherapy strategies for T cell lymphoma and leukemia and discuss their possible future development toward successful clinical results.
Immune system in T cell neoplasms – rationale for immunotherapy
Tumor microenvironment (TME) and chronic inflammation play an important role in the development of slow-growing subtypes of TCL, such as MF and SS. In CTCL, normal tumor-infiltrating T cells (TIL) (mostly CD8+) have been demonstrated to have an exhausted/anergic phenotype, thus being unable to exert an effective anti-tumor response. These T cells are characterized by the expression of predominantly immunoinhibitory molecules, like programmed death-1 (PD1) and other checkpoint molecules such as ICOS, Tim-3 and LAG-3. The T cell neoplastic clone seems to be anergic as well, without consistent upregulation of immune checkpoint ligand (17). T cell exhaustion has also been associated with disease progression in an extremely aggressive disease like ATLL {Miyatake, 2013 #146}. In vitro interaction with epithelial and fibroblastic cell-lines was shown to induce apoptosis resistance in primary ATLL cells and ATLL cell lines(18), as well as HTLV1 latency(19). In ATLL models, fibroblasts acquire an activated pro-inflammatory phenotype enhancing tumorigenesis(20). Interestingly, MF and SS cells have the potential to inhibit normal T-cell proliferation and suppress dendritic cells maturation by secreting Th2-type cytokines (21). Similarly, skin fibroblasts in advanced CTCL promote a Th2-dominant microenvironment with excess of CCL26/eotaxin-3 (22). Therefore, T cell neoplasms possess a T cell immune infiltrate but immune suppression is likely preventing potentially tumor-specific T cells to efficiently kill neoplastic cells (23, 24).
Another important population found in the microenvironment of CTCL is the tumor-associated macrophages (TAM). TAM are important components of the innate immune response and can be activated in response to microenvironment. These responses can range between anti-tumorigenic (pro-inflammatory and phagocytic towards tumor cells) or pro-tumorigenic (promoting tumor cell survival, metastasis, angiogenesis, as well as suppression of surrounding immune cells) (25). Immunosuppressive macrophage (M2) infiltration has been associated with poor clinical outcomes in several cancer types (3, 26-28). The presence of TAM in patients with MF has been correlated to a worse outcome (29, 30), and the presence of M2 macrophages with more advanced stage (31). This suggests that TAM in MF play an important role in both the pathogenesis and progression of disease.
Similarly, PTCL cells are known to have many interactions with the microenvironment. PTCL cells produce soluble and surface molecules like the programmed death ligand 1 (PD-L1) that allow them to escape the antitumor cytotoxic T cells. They also recruit eosinophils from the bloodstream by releasing IL-5 and IL-13. Intratumor eosinophils secrete high amounts of IL-10 and IL-4, which sustain macrophage differentiation into the tumor-favoring (M2) phenotype(32).
Therefore, at least for certain subsets of TCL, the reinvigoration of exhausted TIL or the inhibition of immunosuppressive macrophages could represent rational therapeutic strategies.
The Epstein-Barr virus (EBV) plays an important role in the pathogenesis of some TCL subtypes like ENKL. The virus is more frequently found in B-cell clones associated with TCL, but interestingly, it can also infect T and NK cells, despite the usual lack of the EBV receptor CD21(33, 34). EBV-infected B-cell clones are often found in PTCL-NOS and AITL. Latent EBV infection has transformative properties on infected cells, but it can also cause inflammation in the tumor microenvironment(35). EBV seems to trigger the infiltration of CD8+ T cells and cytotoxic CD8+ cells in the EBV infected germinal centers(36). In other EBV-related cancers, such as Hodgkin lymphoma (HL) and nasopharyngeal carcinoma (NPC), a larger number of dendritic cells and TAM has been detected in EBV+ disease but not in EBV- (37-39). In PTCL, the DNA of EBV in peripheral blood correlates with a worse prognosis(40). In ENKL, one of the main oncogenic driver, NF-kB, is driven by the EBV protein LMP1. LMP1 and NF-kB activate a series of genes that lead to the production of Th1 cytokines TNF-α and IFN-γ(34, 41). Moreover, in ENKL EBV infection promotes the upregulation on infected lymphoma cells of the PD-L1. Binding of PD-L1 on lymphoma cells by PD1 on effector T cells suppresses T cell toxicity, allowing ENKL to escape this regulation(34, 41). This might explain the good activity of checkpoint inhibitors in ENKL.
In consideration of this, it would be expected that viral-driven TCL might respond to checkpoint inhibitors. Another example of a virus-enhanced disease is ATLL. This subtype of T cell lymphoma is driven by HTLV1. In this case though, the anti-PD1 study led to activation of a milder presentation of the disease and frank progression of regular cases: the phase 2 protocol was closed right after the first 3 patients enrolled (42). Unfortunately, there are not many preclinical models of TCL treated with immunotherapy. Warteig and colleaguesfound that PD-1 was a tumor suppressor for TCL in TCL cell lines and in a mouse model. PD-1 blockade in these models seems to promote PI3K/AKT activity and tumor growth. PI3K/AKT is the target of PI3K-inhibitors like duvelisib, that have recently demonstrated good activity in TCL (43, 44). It seems evident that the use of checkpoint inhibitors in TCL with oncogenically activated TCR pathways needs to be preceded by some special considerations(43).
Immunotherapeutic strategies for T cell leukemia and lymphoma
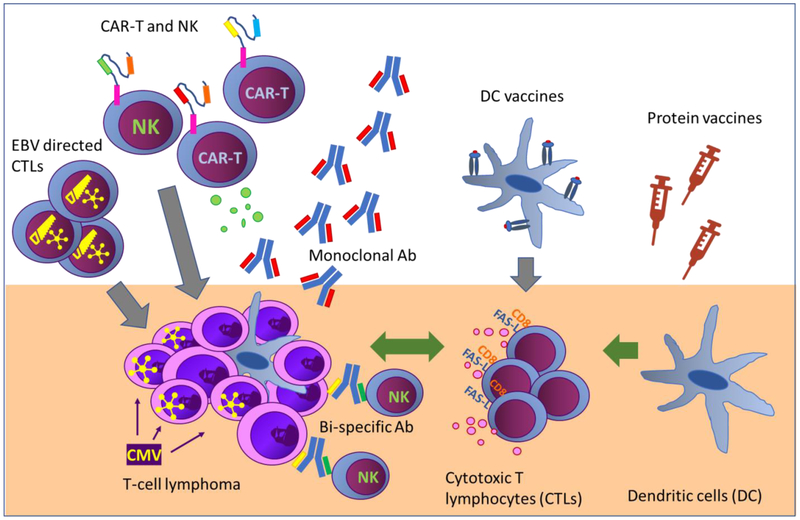
As discussed in the previous section, the development of immunotherapies for certain TCL subsets is supported by preclinical and correlative evidence. However, the role of immunotherapy for TCL in the clinic is still controversial. There are several strategies to use the immune system against TCL (figure 1) can be classified into five main categories: I. complete substitution of the patient immune system, i.e. allogeneic stem cell transplantation (allo-HCT); II. killing of tumor cells by targeting them with monoclonal antibodies or immunoconjugates; III. reinvigoration of exhausted anti-tumor T cells, i.e. immune checkpoint inhibitors (ICI); IV. induce an immune response against tumor antigens, i.e. vaccines or the stimulation of the immune system via the toll-like receptors molecules; V. adoptive cell transfer of tumor specific lymphocytes; VI. additional approaches. In the following paragraphs, we will discuss these approaches in detail and present the latest results for each of them (table 1).
Figure 1.
Diagram showing the different published approaches to target T cell leukemia and lymphoma.
All approaches in the figure and references:
Monoclonal Ab and ICI: (42, 56 – 65, 67, 71 – 73, 77, 80, 82, 98 – 102)
Table 1.
Results of immunotherapy in clinical trials
| Strategy | Disease | Response | Reference |
|---|---|---|---|
| Monoclonal antibodies | |||
| CD30; brentuximab vedotin | ALCL | ORR 86% (CR 57%) | Pro, JCO 2012 |
| PTCL, AITL | ORR 41%(PTCL), 54%(AITL) | Horwitz, Blood 2014 | |
| Prince, Lancet 2017 | |||
| CD30+ CTCL | TTNT 14m (BV) vs 6m (PC) | Horwitz, Blood 2017 | |
| CD52; alemtuzumab | PTCL | ORR 36% (CR 21%) | Enblad 2004 |
| MF/SS | ORR 55% (CR 32%) | Lundin, Blood 2003 | |
| T-PLL | ORR 90%, med. OS 17 – 33 mo | Dearden, Blood 2001 | |
| CCR4; mogamulizumab | ATLL | ORR 50%, med. PFS 5.2 mo | Ishida, JCO 2012 |
| CCR4+ PTCL/CTCL | ORR 35%, med. PFS 3 mo | Ogura, JCO 2014 | |
| CTCL | ORR 28%, PFS 7.7 mo | Kim, Blood 2017 | |
| CD38; daratumumab | ENKT | NA-ongoing | NCT02927925 |
| CD38; isatuximab | T-ALL | Closed-no response | NCT02999633 |
| CD25; camidanlumab tesirine (ADCT301) | PTCL | ORR 33% (all PR) | Horwitz, Blood 2017 |
| CD30/CD16A; bispecific (AFM13) | CD30+ CTCL | NA-ongoing | NCT03192202 |
| Checkpoint inhibition | |||
| pembrolizumab | ENKL | ORR 100% (5/7 CR) | Kwong, Blood 2017 |
| MF/SS | ORR 38% (1/8 CR) | Khodadoust, Blood 2016 | |
| nivolumab | PTCL | ORR 40% (all PR) | Lesokhin, JCO 2016 |
| ATLL | Closed-disease progression | NCT02631746 | |
| BV + nivolumab | CD30+ PTCL, CTCL | NA-ongoing | NCT02581631 |
| Macrophages activation | |||
| - CD47 Hu5F9-G4 | CTCL, PTCL | NA-ongoing | NCT02216409 |
| - CD47 CC-90002 | TCL | NA-ongoing | NCT02663518 |
| - CD47 TTI-621 | TCL | NA-ongoing | NCT03013218 |
| EBV-CTLs | EBV+ ENKL in CR after RT | 4/6 maintained CR | Bollard, JCO 2014 |
| EBV+ ENKL and PTCL active disease | 4/7 ORR (3 CR) |
I. Allogeneic stem-cell transplantation
One of the first immunotherapies used for T cell lymphoma and leukemia is allogeneic stem cell transplantation. In allo-HCT the entire hemopoietic and immune system of the patient is substituted with one derived from an HLA-matched donor(45). This strategy has demonstrated to be the only curative option for a subset of TCL(46-48), however treatment-related mortality and late graft versus host complications could impact long-term quality of life (46-48). For eligible patients with aggressive TCL subtypes, allo-HCT represents an option in the relapsed/refractory setting (46-48).
Results from the international BMT registries have shown that 31% of the patients with TCL remain disease free 3 years after allo-HCT. A recent report on the largest series of TCL patients (n=284) that underwent allo-HCT showed overall survival (OS) and progression-free survival (PFS) rates at 2 years of 61.1% and 47.8%, respectively (48). The rate of transplant-related mortality (TRM) at 1 year was 13.2% (95% CI: 8.3 – 18.1). Acute graft-versus-host disease (GVHD) of any grade was reported in 42.3%, and 34.5% of the patients developed chronic GVHD. Response to the last therapy prior to transplant impacted on PFS. The median PFS reached in patients who received allo-HCT while in CR and was 36.8, 19.2 and 4.9 months for patients in PR, stable disease or progressive disease, respectively. The type of transplant donor impacted on TRM: cumulative TRM at 6 months was 2.9% for patients with a match-related donor, 7.8% with a matched-unrelated donor, and 14.8% with mismatched donors. The majority of the relapses after allo-HCT occurred within 12 months (48). For T lymphoblastic lymphoma/leukemia, allo-HCT is still an important treatment strategy. In a recent retrospective report, 37 patients (median age 21y, range 8 – 47) were transplanted with different techniques (27% from a matched sibling, 65% from a haploidentical family donor, 5% from matched unrelated donor and 3% from cord blood). OS and PFS at 3 years were 71.7% and 69.5%. TRM was 13.5% and grade III-IV acute graft-versus-host disease (aGVHD) 16%. Allo-HCT done in first complete remission (CR) was associated to improved OS (3 year OS 79.1% vs 39.1%, p=0.001)(47).
Unfortunately, lymphoma or leukemia relapses after allo-SCT are common and donor lymphocyte infusion (DLI) is an option, in particular for T-ALL, where there is extensive experience (49-51). The rational of DLI is the adoptive transfer of tumor (recipient)-reactive T cell clones to boost graft-versus-lymphoma/leukemia effect. However, efficacy of this procedure in this context is rather limited, especially in highly aggressive diseases, with ORR of 15-29% (52). In PTCL, DLI has demonstrated anecdotal activity, but in some cases at the cost of high-grade GVHD (46).
II. Monoclonal antibodies and immunoconjugates
Monoclonal antibody immunotherapy has been one of the most explored modality of immunotherapy for TCL. So far three alemtuzumab, brentuximab vedotin (BV) and mogamulizumab have been approved by the Food and Drug Administration (FDA) for TCL.
Alemtuzumab is a humanized anti-CD52 monoclonal antibody. CD52 is a surface co-stimulator of T cell activation and proliferation(53) and is expressed on both T and B lymphocytes and monocytes. However, monocytes and natural killer (NK) cells appear to be more resistant than lymphocytes to alemtuzumab-mediated lysis (54). The reason for this seems to be the lower density of CD52 on these cells(54). CD52 is expressed in 40% of PTCL-NOS(55), as well as on MF/SS and T-PLL. A phase II Swedish and German trial for PTCL in the relapsed/refractory setting reported an overall response rate (ORR) of 36% (5/14 patient responded, CR 3, 21%). However, a high rate of opportunistic infections occurred. Five patients died from infectious complications and an unexpected level of hematological toxicity prompted early termination of the trial. Another study conducted in MF/SS heavily pre-treated patients showed an ORR in 12/22 (55%) with 32% CR rate. In this trial several patients also experienced infective complications (50%)(56). Alemtuzumab demonstrated response rate that can exceed 90% in T-PLL, but in patients without a consolidation with allogeneic stem-cell transplantation the median OS for those achieving CR is short (17-33 months) (57-59). Based on these results, Alemtuzumab is now considered the standard first line therapy for T-PLL. In a phase II study on patients with HTLV1 associated ATLL, an objective response was achieved in 15 of 29 patients, but duration of response was only 14.5 months (60).
Another target that has been evaluated for T cell neoplasms is CD30. CD30 is a cell membrane protein of the tumor necrosis factor receptor family. It is expressed on activated T and B cells. Brentuximab vedotin (BV) is a CD30-targeted immunoconjugate and was the first to demonstrate activity in patients with anaplastic large cell lymphoma (ALCL). In a phase II trial of 58 patients with relapsed disease, BV was administered intravenously at a dose of 1.8 mg/kg every 3 weeks. ORR was 86% with 57% CR, and a median PFS of 13.3 months. However, the median duration of response was 12.6 months (61). The phase II study conducted in patients with relapsed/refractory PTCL enrolled 22 PTCL-NOS and 13 AITL. ORR was 41% for PTCL-NOS and 54% for AITL (62). BV has also demonstrated activity in CD30+ CTCL. A large phase III international randomized study of BV versus physician’s choice (PC) in CD30+ CTCL, showed superiority of BV in achieving an objective global response lasting at least 4 months (56.3% vs 12.5%, CI 18.4 – 26.1, p<0.0001) (63). A longer-term update of this study showed a time-to-next-treatment (TTNT) significantly longer for BV versus PC (median 14.2 vs 6.1 months respectively). Peripheral neuropathy associated with BV was one of the biggest concerns in this study, but 86% of the patients experienced a complete resolution or improvement of this symptom after stopping the drug (64). These studies led to a broad FDA approval of BV for relapsed/refractory CD30+ systemic ALCL in the US in 2011 and for CD30+ CTCL and primary cutaneous ALCL in 2017.
Mogamulizumab (KW-0761) is a humanized anti-CC chemokine receptor-4 (CCR4) monoclonal antibody (65-67). CCR4 is normally expressed on type 2 helper T cells and by the neoplastic T cell clones of PTCL (in 34%)(68), CTCL(in 31-100%)(69) and ATLL (in 88.3%)(70). In ATLL, CD4+, CD25+, CCR4+ T cells are the main HTLV1 reservoir. CCR4 is also a poor prognostic factor as CCR4+ PTCL-NOS have demonstrated a shorter survival compared to CCR4-negative ones (71, 72). A multicenter phase II study with mogamulizumab for relapsed/refractory CCR4+ PTCL and CTCL showed an ORR of 35% (95% CI 20% - 53%) with 14% CR. In this study, limited responses were recorded in PTCL-NOS (16 patients, ORR 19%, 6% CR), better in AITL (12 patients, ORR 50%, 25% CR) and in CTCL (8 patients, ORR 37%, 0% CR) (67). A phase III study of mogamulizumab vs vorinostat, the universally available standard of care in CTCL, named MAVORIC is ongoing (NCT01728805), preliminary results show ORR of 28.0% for mogamulizumab vs 4.8% for vorinostat. Median PFS was superior in the mogamulizumab arm (7.7 vs 3.1 months). Patient-reported outcomes showed greater symptom reduction and improved functional status, favoring mogamulizumab. The most common adverse events were infusion reactions and skin rash (73). In relapsed/refractory ATLL (65), this drug has demonstrated a response rate of 31%, with 13% complete responses. This is a promising result for ATLL, considering the extremely low responses to conventional chemotherapy (74-76). Mogalizumab was approved in 2014 for ATLL in Japan, and most recently in 2018 also by the FDA.
Daratumumab is a monoclonal antibody directed against CD38, a type II-transmembrane glycoprotein mediating signaling transduction in immune cells. CD38 is broadly expressed in leukocytes, including thymocytes, activated T and B cells and plasma cells. Some subsets of TCL, as well as T-ALL express CD38 (77). In ENKT lymphoma, high CD38 expression has been associated to inferior outcome (78). Preclinical data on NKTL cell lines show induction of antibody-dependent cytotoxicity (ADCC) by daratumumab. Investigators also found that all-trans retinoid acid (ATRA) given before daratumumab could enhance CD38 expression on T cells, leading to improved results (79). A report of the use of daratumumab for post-allotransplant ENKT (80) has demonstrated a complete remission with associated normalization of EBV titers. A phase II international study of daratumumab for relapsed/refractory ENKL is ongoing in Asia (NCT02927925). Preclinical data on patient-derived xenografts (PDX) models of T-ALL demonstrated efficacy in 14 of 15 murine models (77). However, a phase II trial in T-ALL evaluating another CD38-directed antibody isatuximab (NCT02999633) was terminated early due to unsatisfactory benefit/risk balance.
ADCT-301 (camidanlumab tesirine) is an anti-CD25 (IL-2Rα) immunoconjugate, promoting the delivery into target cells of the cytotoxin SG3199. CD-25 is normally expressed in on activated T cells, activated B cells, some thymocytes, myeloid precursors, and oligodendrocytes(81). Expression of CD25 can be detected on 42-50% of PTCL, 54% of CTCL, as well as on 58-78% of HL and 40% of DLBCL. An international phase I study is ongoing in the relapse/refractory setting for all these disorders. Interim results for 12 heavily pretreated T-cell lymphoma patients showed an ORR of 33%, however all responses were PR (82).
When compared to B cell lymphomas, TCL seem to lose more easily their surface markers with time and probably with treatment (83, 84). Thus, since the treatment with monoclonal antibodies eliminates the T cell clones expressing a specific marker, re-treatment with the same drug must be carefully evaluated.
III. Immune checkpoint inhibition
The PD1 receptor on T cells and its ligands PD-L1 and PD-L2 expressed on tumor cells or myeloid cells, inhibit T-cells activation and proliferation (85). The PD-1 pathway is critical in regulating effector T-cells response in tissues by suppressing T-cell activity and limiting tissue damage (86). By upregulating the ligands for PD1, tumor cells block the antitumor immune responses sent by the microenvironment(86).
ICI like anti-PD1 or anti-CTLA4 antibodies have led to promising responses in solid tumors(87-90) and in HL (91-93). In 2010, the FDA approved the anti CTLA4 ipilimumab for melanoma(86). Since 2014, the FDA has approved the anti-PD1 pembrolizumab and nivolumab, and the anti-PD-L1 atezolizumab, avelumab, and durvalumab for several solid tumors (in particular melanoma, lung cancer, urothelial carcinoma, colorectal cancer, hepatic carcinoma, Merkel-cell carcinoma)(94). For hematologic malignancies, nivolumab was approved in 2016 for HL, pembrolizumab in 2017 for HL, and in 2018 for primary mediastinal large B-cell lymphoma(94). Therefore, broadening the use of ICI for T cell neoplasms is a desirable potential treatment option. The activity of targeting the PD1 pathway has been demonstrated in T-cell lymphomas with increased PD-L1 expression in the tumor microenvironment (95, 96). However, as ICI can also target the PD1+ or PD-L1+ neoplastic T cells together with TIL, there is the concern that they can also activate tumor cells. Nevertheless, ICI have been tested in TCL. Recently, Lee Ratner and colleagues related in a letter to the New England Journal of Medicine about the activating effect of the checkpoint inhibitor nivolumab on ATLL. Nivolumab was being studied in a phase 2 clinical trial (NCT02631746), but after the first three patients enrolled, it was evident that the drug was causing progression of disease. PD1 in these cases could have worked as a tumor suppressor, and its inhibition might have enhanced disease progression (42). Some additional perplexities for the use of PD1 inhibition arise in the context of bridging therapy to allo-HCT. Investigators observed higher incidence and severity of graft versus host disease in B-cell lymphoma patients treated with checkpoint inhibitors prior to allo-HCT(97). However, in other TCL subset cases, like the ENKL, treatment with the anti-PD1 monoclonal antibody pembrolizumab led to promising efficacy with limited toxicities (98, 99). Patient numbers are still very small: in the pembrolizumab study, 7 relapsed/refractory patients were treated, with an ORR of 100% with 5/7 patients achieving CR (99). In a recent report, 3 refractory patients with high tumor load treated with nivolumab also achieved a response, in one case response was seen after just one dose (100). As mentioned, ENKL is classically associated with EBV infection, that upregulates the expression on lymphoma cells of PD-L1. Binding of PD-L1 on lymphoma cells by PD1 on effector T cells suppresses T cell toxicity, allowing ENKL to escape this regulation. This mechanism can explain the favorable activity of this class of drugs in ENKL. Based on these results, a protocol using pembrolizumab in frontline for patients with localized stage disease is opening at the MSKCC. For these patients, the classical chemotherapy will be given only if not achieving CR after pembrolizumab and localized radiotherapy.
Despite the good response seen in ENKL, nivolumab has demonstrated modest activity in other types of TCL. A phase I trial included, among other histologies, 5 relapsed/refractory PTCL patients, that showed an ORR of 40% (all were PR). The patients who responded seemed to achieve durable control of disease (101). In a phase II multicenter study, 24 relapsed/refractory MF and SS patients were treated with pembrolizumab. The ORR was 38% with 1 CR and 8 partial responses (PR), also in this case, patients with response achieved durable response (8/9 of those patients sustained responses for a median of 32 weeks). As for adverse events, skin flare was observed in some SS patients. There were 3 immune-related toxicities. These included 2 pneumonitis and one grade 3 diarrhea(102). At the MSKCC, a new combination of BV and nivolumab is under investigation for the relapsed-refractory population of PTCL and CTCL, and for other CD30+ lymphomas in a phase I-II study (NCT02581631).
IV. Vaccines
Dendritic cell-based vaccines have been extensively investigated in B-cell lymphomas while experience in T-cell lymphomas has been limited to CTCL, and with varying results (103). Intratumoral injection of autologous lysate of dendritic cells (104) or of a toll-like receptor-9 (TRL9) agonist combined with radiation (103), have demonstrated to induce a T-cell immune response against the lymphoma, a response that has been seen in other cancers. Also, some patients could benefit from an abscopal effect, with the reduction of lesions of the body not directly infiltrated with the lysate.
V. Adoptive cell transfer of tumor specific lymphocytes
EBV-directed cytotoxic CTL
Immunotherapy using antigen-specific cytotoxic T cells (CTL) targeted against Epstein-Barr virus (EBV) is another promising immunologic strategy that can be used in EBV-driven diseases. The major clinical experience with EBV-CTLs has been in EBV-associated post-transplant lymphoproliferative disease (PTLD) and HL (105-107). In recent years, EBV-CTLs therapy has also been applied to ENKL as consolidation post chemo-radiotherapy or in the relapsed/refractory setting (108). Responses to this approach were observed in 13 of 21 patients with EBV+ lymphoma (11 CR). Six T-cell lymphomas (5 ENKT, 1 PTCL) received EBV-CTLs as consolidation, 2 patients resulted to be primary refractory, and all the others maintained a continuous CR. Other 7 T-cell lymphoma patients were treated with active disease (5 ENKT and 2 PTCL). Responses were seen for 4/7 patients (3 patients achieving CR)(108). At MSKCC a phase I-II trial with EBV-CTLs for all EBV-driven diseases is currently ongoing (NCT00002663).
CAR-T cells
In 2017 the FDA approved the first gene-therapy for cancer, Kymriah (Novartis), an anti CD19 chimeric antigen receptor T cell (CART) product. Few months later another CART19 product, Yescarta (Kite/Gilead), was also approved by the FDA. A chimeric antigen receptor is a synthetic protein that is developed by the fusion of an extracellular antigen-recognizing domains (typically a single-chain variable fragment derived from a monoclonal antibody) and intracellular signaling domains (CD3ζ and CD28 or 4-1BB). (28) Anti-CD19 CART led to impressive clinical responses in CD19+ B-cell acute lymphoblastic leukemia and non-Hodgkin lymphoma (109). There is therefore great interest in developing CART therapy for T cell neoplasm. However, one of the issues of using CART to target TCL and T-ALL is, the potential targets that are expressed in tumor cells might also be expressed also in CART cells, potentially causing fratricide. Several strategies are under preclinical development and a limited number of these approaches have already reached clinical trials.
Multiple preclinical studies have evaluated different antigens as the target for CART: CD30 (110, 111), CD5(112), CD3(113), CD4(114), CCR4(115) and more recently CD7(116, 117). Interestingly the two reports on CART7 used CRISPR-Cas9 to knock-out CD7 on CART cells and avoid CART-CART fratricide. A possible drawback of these approaches is that in clinical trials, if effective, these CART products will cause profound T cell aplasia could put the patient at serious risk of potentially fatal infections. To overcome this issue, an interesting approach was proposed by Maciocia et al(118). The authors developed CART cells that can specifically target one of the 2 variants of the constant regions of the TCR: C1 or C2. Therefore, once the tumor-specific constant region is known, a specific CART product could be administered killing tumor cells and normal T cells with the same TCR constant region but sparing the ones with the other variant. A potential limitation of this approach is that a subset of TCL and several T-ALL lack surface TCR expression (119). This approach could potentially reduce toxicity and will be evaluated in clinical trials in the future.
The groups at Baylor College of Medicine and at the University of North Carolina have tested anti-CD30 CART in patients with HL and CD30+ ALCL. The reported results in 2 patients with ALCL(120) showed 1 CR that persisted 9 months after the fourth infusion of CD30 CAR-Ts. Although CD30 may also be expressed by normal activated T cells, no patients developed impaired virus-specific immunity. Other centers in China are evaluating similar approaches in clinical trials (NCT02274584, NCT02958410, NCT02259556). Anti-CD5 CART cells are now evaluated in a clinical trial at Baylor College of Medicine (NCT03081910). Other groups are using CAR NK cells for TCL/T-ALL, for example, anti-CD7 CAR-pNK (NCT02742727).
VI. Additional approaches
Tumor-associated macrophage activation
Typically, TAM infiltration in tumor is correlated with poor prognosis(121). Increasing CD47 expression is a way for tumor cells to escape phagocytosis as CD47 binds a signal-regulatory-protein (SIRP-α) on the surface of macrophages sending a “do-not-eat-me” signal (122). Targeting and blocking this molecule promotes tumor cell phagocytosis by IFN-γ-primed macrophages (123). At present time, three phase I clinical trials are testing CD47 antagonists (Hu5F9-G4, CC-90002, and TTI-621) enrolling T and B cell lymphomas. Some early reports have shown activity of macrophage activation in B cell lymphoma and solid cancer (124), but for T cell lymphoma early phase trials are still ongoing (NCT02663518, NCT03013218, NCT02953509). The modulation of TAM has been also demonstrated to be the underlying mechanism of IFN-α and INF-γactivity in CTCL (especially MF), suppressing the recruitment of T-regulatory cells and lymphoma cells in the skin, and decreasing the production of cytokines by TAMs. IFN-α has been used for CTCL since 1984, and evidences of the clinical efficacy are impaired due to heterogeneity in treatment schedule, patient selection and methodology. As a result, ORR ranges from 0 to 80%, without a clear correlation between dose and response(125-128)
Bispecific antibodies
Bispecific antibodies anti-CD30/CD16A (AFM13) have been developed to redirect NK cells against CD30+ diseases (129). CD16A is a receptor for the IgG Fc domain, with an activating function on NK cells and macrophages. Direct engagement of NK cells towards CD30 with AFM13 is supposed to induce lymphoma cell killing trough NK cell-mediated and T cell-mediated cytotoxicity. This strategy is under evaluation for HL with some encouraging preliminary results(130). In TCL this idea could very interesting to investigate because of the stimulation of NK cells and not T towards the tumor. For CD30+ T-cell lymphoma, a phase I/II protocol is ongoing, enrolling PTCL and cutaneous-ALCL (NCT03192202).
Conclusions and future directions
T cell neoplasms represent a group of diseases with an unmet medical need. Currently, there are limited effective treatment options with overall poor prognostic outcomes for patients with these diseases. The overall survival for most patients with T cell leukemia and lymphoma is poor, and there is a dire need for novel therapies. Immunotherapy has shown unprecedented responses in cancer patients, and several approaches are now being also evaluated for T cell leukemia and lymphoma. At present time, the immunotherapeutic treatment options include BV, alemtuzumab, mogalizumab, IFNγ for SS, pembrolizumab for systemic MF and refractory ENKL and allogeneic transplantation. However, these approaches are usually reserved for selected cases with relapsed and refractory disease and responses are far from optimal.
One of the main issues in the development of effective immunotherapies with monoclonal antibodies for T cell neoplasms is the fact that normal T cells also share the potential tumor targets. Effective anti-T cell therapies like alemtuzumab in TCL, anti-CD3 (OKT3)(131) or anti-CD-2 siplizumab(132) in T-ALL have led to significant toxicity in clinical trials, mostly infections and infusion-related reactions, due to T cell depletion and/or activation. An exception to this paradigm is targeting CD30 with BV that is leading to manageable toxicity and clinical responses in CD30+ ALCL; however, responses are not durable. On this regard, the use of CART for T cell neoplasm will necessarily include strategies to reduce toxicity or specifically recognize tumor cells, like CART targeting the tumor-specific TCR constant region.
Immune checkpoint inhibitors show promising results in several cancers however, in some subset of T cell neoplasms like ATLL, ICI can lead to tumor activation. Using ICI for T cell neoplasms is challenging because both immune effector cells and tumor cells are T lymphocytes and can potentially express PD-1 or other exhaustion markers. Studies of the PD-1 expression in tumor cells and the TME need to be performed in order to support the rationale to test new T-cell immunotherapies. As an example, virus-driven neoplasm like ENKL are exquisitely sensitive to ICI.
It is likely that each T cell leukemia and lymphoma subset will require tailored immunotherapy based on a deep understanding of the tumor characteristics but also of the patient immune system and tumor-microenvironment.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748, the Gabrielle’s Angel Foundation grant (PI: M.R.), the Parker Institute for Cancer Immunotherapy (PI: M.R.), the ASH Scholar Award (PI: M.R.) and the NCI CDA (K99 CA212302-01A1, PI: M.R.).
Footnotes
Conflict of Interest:
Steven M. Horwitz reports grants and personal fees from ADC Therapeutics, grants and personal fees from Aileron, grants and personal fees from Seattle Genetics, grants and personal fees from Takeda, grants and personal fees from Kyowa Hakka Kirin, grants and personal fees from Verastem, personal fees from Portola, personal fees from Corvus, grants from Celgene, grants from Spectrum, grants from Forty-Seven outside the submitted work. Alison J. Moskowitz reports grants from Merck, grants from BMS, grants from Incyte, grants and personal fees from Seattle Genetics, personal fees from Bristol-Meyers Squibb, personal fees from Cell Medica, personal fees from Kyowa Hakko kirin Pharma outside the submitted work. Paola Ghione, Nadia E.K. De Paola, and Marco Ruella declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science (New York, NY). 2018;359(6382):1361–5. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 2.Maloney DG. Anti-CD20 antibody therapy for B-cell lymphomas. The New England journal of medicine. 2012;366(21):2008–16. doi: 10.1056/NEJMct1114348. [DOI] [PubMed] [Google Scholar]
- 3.Brody J, Kohrt H, Marabelle A, Levy R. Active and passive immunotherapy for lymphoma: proving principles and improving results. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(14):1864–75. doi: 10.1200/jco.2010.33.4623. [DOI] [PubMed] [Google Scholar]
- 4.Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131(1):68–83. doi: 10.1182/blood-2017-07-740993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horwitz SM, Ansell SM, Ai WZ, Barnes J, Barta SK, Choi M, et al. NCCN Guidelines Insights: T-Cell Lymphomas, Version 2.2018. Journal of the National Comprehensive Cancer Network : JNCCN. 2018;16(2):123–35. doi: 10.6004/jnccn.2018.0007. [DOI] [PubMed] [Google Scholar]
- 6.Mehta-Shah N, Ratner L, Horwitz SM. Adult T-Cell Leukemia/Lymphoma. Journal of oncology practice. 2017;13(8):487–92. doi: 10.1200/jop.2017.021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89(11):3909–18. [PubMed] [Google Scholar]
- 8.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(25):4124–30. doi: 10.1200/jco.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 9.Piccaluga PP, Agostinelli C, Califano A, Rossi M, Basso K, Zupo S, et al. Gene expression analysis of peripheral T cell lymphoma, unspecified, reveals distinct profiles and new potential therapeutic targets. The Journal of clinical investigation. 2007;117(3):823–34. doi: 10.1172/jci26833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piccaluga PP, Agostinelli C, Califano A, Carbone A, Fantoni L, Ferrari S, et al. Gene expression analysis of angioimmunoblastic lymphoma indicates derivation from T follicular helper cells and vascular endothelial growth factor deregulation. Cancer research. 2007;67(22):10703–10. doi: 10.1158/0008-5472.can-07-1708. [DOI] [PubMed] [Google Scholar]
- 11.Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA: a cancer journal for clinicians. 2016. doi: 10.3322/caac.21357. [DOI] [PubMed] [Google Scholar]
- 12.Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113(17):3931–7. doi: 10.1182/blood-2008-10-185256. [DOI] [PubMed] [Google Scholar]
- 13.Matutes E, Brito-Babapulle V, Swansbury J, Ellis J, Morilla R, Dearden C, et al. Clinical and laboratory features of 78 cases of T-prolymphocytic leukemia. Blood. 1991;78(12):3269–74. [PubMed] [Google Scholar]
- 14.Drobna M, Szarzynska-Zawadzka B, Dawidowska M. T-cell acute lymphoblastic leukemia from miRNA perspective: Basic concepts, experimental approaches, and potential biomarkers. Blood reviews. 2018. doi: 10.1016/j.blre.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Belver L, Ferrando A. The genetics and mechanisms of T cell acute lymphoblastic leukaemia. Nature reviews Cancer. 2016;16(8):494–507. doi: 10.1038/nrc.2016.63. [DOI] [PubMed] [Google Scholar]
- 16.Bellei M, Foss FM, Shustov AR, Horwitz SM, Marcheselli L, Kim WS, et al. The outcome of peripheral T-cell lymphoma patients failing first line therapy: a report from the prospective, international T-Cell project. Haematologica. 2018. doi: 10.3324/haematol.2017.186577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray D, Eldershaw SA, Pearce H, Davies N, McMurray J, Scarisbrick JJ, et al. T Cell Versus T Cell; A Study of the Immune Checkpoint Landscape in Cutaneous T Cell Lymphoma. Blood. 2017;130(Suppl 1):2754. [Google Scholar]
- 18.Miyatake Y, Oliveira AL, Jarboui MA, Ota S, Tomaru U, Teshima T, et al. Protective roles of epithelial cells in the survival of adult T-cell leukemia/lymphoma cells. The American journal of pathology. 2013;182(5):1832–42. doi: 10.1016/j.ajpath.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Kinpara S, Hasegawa A, Utsunomiya A, Nishitsuji H, Furukawa H, Masuda T, et al. Stromal cell-mediated suppression of human T-cell leukemia virus type 1 expression in vitro and in vivo by type I interferon. Journal of virology. 2009;83(10):5101–8. doi: 10.1128/jvi.02564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vicario M, Mattiolo A, Montini B, Piano MA, Cavallari I, Amadori A, et al. A Preclinical Model for the ATLL Lymphoma Subtype With Insights Into the Role of Microenvironment in HTLV-1-Mediated Lymphomagenesis. Frontiers in microbiology. 2018;9:1215. doi: 10.3389/fmicb.2018.01215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thumann P, Luftl M, Moc I, Bagot M, Bensussan A, Schuler G, et al. Interaction of cutaneous lymphoma cells with reactive T cells and dendritic cells: implications for dendritic cell-based immunotherapy. The British journal of dermatology. 2003;149(6):1128–42. [DOI] [PubMed] [Google Scholar]
- 22.Miyagaki T, Sugaya M, Fujita H, Ohmatsu H, Kakinuma T, Kadono T, et al. Eotaxins and CCR3 interaction regulates the Th2 environment of cutaneous T-cell lymphoma. The Journal of investigative dermatology. 2010;130(9):2304–11. doi: 10.1038/jid.2010.128. [DOI] [PubMed] [Google Scholar]
- 23.Rubio Gonzalez B, Zain J, Rosen ST, Querfeld C. Tumor microenvironment in mycosis fungoides and Sezary syndrome. Current opinion in oncology. 2016;28(1):88–96. doi: 10.1097/cco.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 24.Querfeld C, Curran SA, Leung S, Myskowski PL, Horwitz SM, Halpern AC, et al. T Cells in CTCL Have an Exhausted Phenotype While Cutaneous Dendritic Cells Display a Normally Activated Mature Phenotype. Blood. 2014;124(21):1695. [Google Scholar]
- 25.Russ A, Hua AB, Montfort WR, Rahman B, Riaz IB, Khalid MU, et al. Blocking "don't eat me" signal of CD47-SIRPalpha in hematological malignancies, an in-depth review. Blood reviews. 2018. doi: 10.1016/j.blre.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kulpa DA, Lawani M, Cooper A, Peretz Y, Ahlers J, Sekaly RP. PD-1 coinhibitory signals: the link between pathogenesis and protection. Seminars in immunology. 2013;25(3):219–27. doi: 10.1016/j.smim.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruella M, Kalos M. Adoptive immunotherapy for cancer. Immunological reviews. 2014;257(1):14–38. doi: 10.1111/imr.12136. [DOI] [PubMed] [Google Scholar]
- 29.Sugaya M, Miyagaki T, Ohmatsu H, Suga H, Kai H, Kamata M, et al. Association of the numbers of CD163(+) cells in lesional skin and serum levels of soluble CD163 with disease progression of cutaneous T cell lymphoma. Journal of dermatological science. 2012;68(1):45–51. doi: 10.1016/j.jdermsci.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Assaf C, Hwang ST. Mac attack: macrophages as key drivers of cutaneous T-cell lymphoma pathogenesis. Experimental dermatology. 2016;25(2):105–6. doi: 10.1111/exd.12894. [DOI] [PubMed] [Google Scholar]
- 31.Tada K, Hamada T, Asagoe K, Umemura H, Mizuno-Ikeda K, Aoyama Y, et al. Increase of DC-LAMP+ mature dendritic cell subsets in dermatopathic lymphadenitis of mycosis fungoides. European journal of dermatology : EJD. 2014;24(6):670–5. doi: 10.1684/ejd.2014.2437. [DOI] [PubMed] [Google Scholar]
- 32.Pizzi M, Margolskee E, Inghirami G. Pathogenesis of Peripheral T Cell Lymphoma. Annual review of pathology. 2018;13:293–320. doi: 10.1146/annurev-pathol-020117-043821. [DOI] [PubMed] [Google Scholar]
- 33.Fox CP, Shannon-Lowe C, Gothard P, Kishore B, Neilson J, O'Connor N, et al. Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in adults characterized by high viral genome load within circulating natural killer cells. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;51(1):66–9. doi: 10.1086/653424. [DOI] [PubMed] [Google Scholar]
- 34.Shannon-Lowe C, Rickinson AB, Bell AI. Epstein-Barr virus-associated lymphomas. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2017;372(1732). doi: 10.1098/rstb.2016.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan GW, Visser L, Tan LP, van den Berg A, Diepstra A. The Microenvironment in Epstein-Barr Virus-Associated Malignancies. Pathogens (Basel, Switzerland). 2018;7(2). doi: 10.3390/pathogens7020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Beek J, zur Hausen A, Snel SN, Berkhof J, Kranenbarg EK, van de Velde CJ, et al. Morphological evidence of an activated cytotoxic T-cell infiltrate in EBV-positive gastric carcinoma preventing lymph node metastases. The American journal of surgical pathology. 2006;30(1):59–65. [DOI] [PubMed] [Google Scholar]
- 37.Kamper P, Bendix K, Hamilton-Dutoit S, Honore B, Nyengaard JR, d'Amore F. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin's lymphoma. Haematologica. 2011;96(2):269–76. doi: 10.3324/haematol.2010.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barros MH, Hassan R, Niedobitek G. Tumor-associated macrophages in pediatric classical Hodgkin lymphoma: association with Epstein-Barr virus, lymphocyte subsets, and prognostic impact. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(14):3762–71. doi: 10.1158/1078-0432.ccr-12-0129. [DOI] [PubMed] [Google Scholar]
- 39.Barros MH, Segges P, Vera-Lozada G, Hassan R, Niedobitek G. Macrophage polarization reflects T cell composition of tumor microenvironment in pediatric classical Hodgkin lymphoma and has impact on survival. PloS one. 2015;10(5):e0124531. doi: 10.1371/journal.pone.0124531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YR, Kim SJ, Cheong JW, Chung H, Jang JE, Kim Y, et al. Pretreatment Epstein-Barr virus DNA in whole blood is a prognostic marker in peripheral T-cell lymphoma. Oncotarget. 2017;8(54):92312–23. doi: 10.18632/oncotarget.21251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chuang HC, Lay JD, Hsieh WC, Wang HC, Chang Y, Chuang SE, et al. Epstein-Barr virus LMP1 inhibits the expression of SAP gene and upregulates Th1 cytokines in the pathogenesis of hemophagocytic syndrome. Blood. 2005;106(9):3090–6. doi: 10.1182/blood-2005-04-1406. [DOI] [PubMed] [Google Scholar]
- 42. *.Ratner L, Waldmann TA, Janakiram M, Brammer JE. Rapid Progression of Adult T-Cell Leukemia-Lymphoma after PD-1 Inhibitor Therapy. The New England journal of medicine. 2018;378(20):1947–8. doi: 10.1056/NEJMc1803181. [DOI] [PubMed] [Google Scholar]; In this letter, Ratner and colleagues report the unfortunate experience of anti-PD1 for ATLL: the drug had probably an activating role on the lymphoma cells. The trial was closed after only three patients enrolled.
- 43.Wartewig T, Kurgyis Z, Keppler S, Pechloff K, Hameister E, Ollinger R, et al. PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature. 2017;552(7683):121–5. doi: 10.1038/nature24649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horwitz SM, Koch R, Porcu P, Oki Y, Moskowitz A, Perez M, et al. Activity of the PI3K-delta,gamma inhibitor duvelisib in a phase 1 trial and preclinical models of T-cell lymphoma. Blood. 2018;131(8):888–98. doi: 10.1182/blood-2017-08-802470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bourlon C, Lacayo-Lenero D, Inclan-Alarcon SI, Demichelis-Gomez R. Hematopoietic Stem Cell Transplantation for Adult Philadelphia-Negative Acute Lymphoblastic Leukemia in the First Complete Remission in the Era of Minimal Residual Disease. Current oncology reports. 2018;20(4):36. doi: 10.1007/s11912-018-0679-9. [DOI] [PubMed] [Google Scholar]
- 46.Cudillo L, Cerretti R, Picardi A, Mariotti B, De Angelis G, Cantonetti M, et al. Allogeneic hematopoietic stem cell transplantation in Primary Cutaneous T Cell Lymphoma. Annals of hematology. 2018;97(6):1041–8. doi: 10.1007/s00277-018-3275-z. [DOI] [PubMed] [Google Scholar]
- 47.Li C, Yang D, Chen J, Wang P, Zhang Y, Wu D. Outcome of Allogeneic Stem Cell Transplantation in T Cell Lymphoblastic Lymphoma. Blood. 2017;130(Suppl 1):5535. [Google Scholar]
- 48.Mehta-Shah N, Teja S, Tao Y, Cashen AF, Beaven A, Alpdogan O, et al. Successful Treatment of Mature T-Cell Lymphoma with Allogeneic Stem Cell Transplantation: The Largest Multicenter Retrospective Analysis. Blood. 2017;130(Suppl 1):4597. [Google Scholar]
- 49.Inamoto Y, Fefer A, Sandmaier BM, Gooley TA, Warren EH, Petersdorf SH, et al. A phase I/II study of chemotherapy followed by donor lymphocyte infusion plus interleukin-2 for relapsed acute leukemia after allogeneic hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(9):1308–15. doi: 10.1016/j.bbmt.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyamoto T, Fukuda T, Nakashima M, Henzan T, Kusakabe S, Kobayashi N, et al. Donor Lymphocyte Infusion for Relapsed Hematological Malignancies after Unrelated Allogeneic Bone Marrow Transplantation Facilitated by the Japan Marrow Donor Program. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2017;23(6):938–44. doi: 10.1016/j.bbmt.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Slavin S, Naparstek E, Nagler A, Ackerstein A, Samuel S, Kapelushnik J, et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse after allogeneic bone marrow transplantation. Blood. 1996;87(6):2195–204. [PubMed] [Google Scholar]
- 52.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–50. [PubMed] [Google Scholar]
- 53.Zhao Y, Su H, Shen X, Du J, Zhang X, Zhao Y. The immunological function of CD52 and its targeting in organ transplantation. Inflammation research : official journal of the European Histamine Research Society [et al]. 2017;66(7):571–8. doi: 10.1007/s00011-017-1032-8. [DOI] [PubMed] [Google Scholar]
- 54.Rao SP, Sancho J, Campos-Rivera J, Boutin PM, Severy PB, Weeden T, et al. Human peripheral blood mononuclear cells exhibit heterogeneous CD52 expression levels and show differential sensitivity to alemtuzumab mediated cytolysis. PloS one. 2012;7(6):e39416. doi: 10.1371/journal.pone.0039416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karlin L, Coiffier B. The changing landscape of peripheral T-cell lymphoma in the era of novel therapies. Seminars in hematology. 2014;51(1):25–34. doi: 10.1053/j.seminhematol.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Lundin J, Hagberg H, Repp R, Cavallin-Stahl E, Freden S, Juliusson G, et al. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood. 2003;101(11):4267–72. doi: 10.1182/blood-2002-09-2802. [DOI] [PubMed] [Google Scholar]
- 57.Dearden CE, Matutes E, Cazin B, Tjonnfjord GE, Parreira A, Nomdedeu B, et al. High remission rate in T-cell prolymphocytic leukemia with CAMPATH-1H. Blood. 2001;98(6):1721–6. [DOI] [PubMed] [Google Scholar]
- 58.Krishnan B, Else M, Tjonnfjord GE, Cazin B, Carney D, Carter J, et al. Stem cell transplantation after alemtuzumab in T-cell prolymphocytic leukaemia results in longer survival than after alemtuzumab alone: a multicentre retrospective study. British journal of haematology. 2010;149(6):907–10. doi: 10.1111/j.1365-2141.2010.08134.x. [DOI] [PubMed] [Google Scholar]
- 59.Hopfinger G, Busch R, Pflug N, Weit N, Westermann A, Fink AM, et al. Sequential chemoimmunotherapy of fludarabine, mitoxantrone, and cyclophosphamide induction followed by alemtuzumab consolidation is effective in T-cell prolymphocytic leukemia. Cancer. 2013;119(12):2258–67. doi: 10.1002/cncr.27972. [DOI] [PubMed] [Google Scholar]
- 60.Sharma K, Janik JE, O'Mahony D, Stewart D, Pittaluga S, Stetler-Stevenson M, et al. Phase II Study of Alemtuzumab (CAMPATH-1) in Patients with HTLV-1-Associated Adult T-cell Leukemia/lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(1):35–42. doi: 10.1158/1078-0432.ccr-16-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. *.Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(18):2190–6. doi: 10.1200/jco.2011.38.0402. [DOI] [PubMed] [Google Scholar]; Provides the first evidence of the activity of the monoclonal antibody immunoconjugate brentuximab vedotin in patients with relapsed/refractory ALCL.
- 62.Horwitz SM, Advani RH, Bartlett NL, Jacobsen ED, Sharman JP, O'Connor OA, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123(20):3095–100. doi: 10.1182/blood-2013-12-542142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prince HM, Kim YH, Horwitz SM, Dummer R, Scarisbrick J, Quaglino P, et al. Brentuximab vedotin or physician's choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet (London, England). 2017;390(10094):555–66. doi: 10.1016/s0140-6736(17)31266-7. [DOI] [PubMed] [Google Scholar]
- 64.Horwitz SM, Scarisbrick JJ, Dummer R, Duvic M, Kim YH, Walewski J, et al. Updated Analyses of the International, Open-Label, Randomized, Phase 3 Alcanza Study: Longer-Term Evidence for Superiority of Brentuximab Vedotin Versus Methotrexate or Bexarotene for CD30-Positive Cutaneous T-Cell Lymphoma (CTCL). Blood. 2017;130(Suppl 1):1509. [Google Scholar]
- 65.Yamamoto K, Utsunomiya A, Tobinai K, Tsukasaki K, Uike N, Uozumi K, et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(9):1591–8. doi: 10.1200/jco.2009.25.3575. [DOI] [PubMed] [Google Scholar]
- 66.Ishida T, Utsunomiya A, Jo T, Yamamoto K, Kato K, Yoshida S, et al. Mogamulizumab for relapsed adult T-cell leukemia-lymphoma: Updated follow-up analysis of phase I and II studies. Cancer science. 2017;108(10):2022–9. doi: 10.1111/cas.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ogura M, Ishida T, Hatake K, Taniwaki M, Ando K, Tobinai K, et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(11):1157–63. doi: 10.1200/jco.2013.52.0924. [DOI] [PubMed] [Google Scholar]
- 68.Asano N, Suzuki R, Ohshima K, Kagami Y, Ishida F, Yoshino T, et al. Linkage of expression of chemokine receptors (CXCR3 and CCR4) and cytotoxic molecules in peripheral T cell lymphoma, not otherwise specified and ALK-negative anaplastic large cell lymphoma. International journal of hematology. 2010;91(3):426–35. doi: 10.1007/s12185-010-0513-0. [DOI] [PubMed] [Google Scholar]
- 69.Capriotti E, Vonderheid EC, Thoburn CJ, Bright EC, Hess AD. Chemokine receptor expression by leukemic T cells of cutaneous T-cell lymphoma: clinical and histopathological correlations. The Journal of investigative dermatology. 2007;127(12):2882–92. doi: 10.1038/sj.jid.5700916. [DOI] [PubMed] [Google Scholar]
- 70.Ishida T, Utsunomiya A, Iida S, Inagaki H, Takatsuka Y, Kusumoto S, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9(10 Pt 1):3625–34. [PubMed] [Google Scholar]
- 71.Ishida T, Inagaki H, Utsunomiya A, Takatsuka Y, Komatsu H, Iida S, et al. CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(16):5494–500. doi: 10.1158/1078-0432.ccr-04-0371. [DOI] [PubMed] [Google Scholar]
- 72.Al-Zahrani M, Savage KJ. Peripheral T-Cell Lymphoma, Not Otherwise Specified: A Review of Current Disease Understanding and Therapeutic Approaches. Hematology/oncology clinics of North America. 2017;31(2):189–207. doi: 10.1016/j.hoc.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 73.Kim YH, Bagot M, Pinter-Brown L, Rook AH, Porcu P, Horwitz SM, et al. Anti-CCR4 Monoclonal Antibody, Mogamulizumab, Demonstrates Significant Improvement in PFS Compared to Vorinostat in Patients with Previously Treated Cutaneous T-Cell Lymphoma (CTCL): Results from the Phase III MAVORIC Study. Blood. 2017;130(Suppl 1):817.28576878 [Google Scholar]
- 74.Tobinai K, Uike N, Saburi Y, Chou T, Etoh T, Masuda M, et al. Phase II study of cladribine (2-chlorodeoxyadenosine) in relapsed or refractory adult T-cell leukemia-lymphoma. International journal of hematology. 2003;77(5):512–7. [DOI] [PubMed] [Google Scholar]
- 75.Tsukasaki K, Tobinai K, Shimoyama M, Kozuru M, Uike N, Yamada Y, et al. Deoxycoformycin-containing combination chemotherapy for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study (JCOG9109). International journal of hematology. 2003;77(2):164–70. [DOI] [PubMed] [Google Scholar]
- 76.Ohno R, Kobayashi T, Tanimoto M, Hiraoka A, Imai K, Asou N, et al. Randomized study of individualized induction therapy with or without vincristine, and of maintenance-intensification therapy between 4 or 12 courses in adult acute myeloid leukemia. AML-87 Study of the Japan Adult Leukemia Study Group. Cancer. 1993;71(12):3888–95. [DOI] [PubMed] [Google Scholar]
- 77.Bride KL, Vincent TL, Im SY, Aplenc R, Barrett DM, Carroll WL, et al. Preclinical efficacy of daratumumab in T-cell acute lymphoblastic leukemia. Blood. 2018;131(9):995–9. doi: 10.1182/blood-2017-07-794214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang L, Wang H, Li PF, Lu Y, Xia ZJ, Huang HQ, et al. CD38 expression predicts poor prognosis and might be a potential therapy target in extranodal NK/T cell lymphoma, nasal type. Annals of hematology. 2015;94(8):1381–8. doi: 10.1007/s00277-015-2359-2. [DOI] [PubMed] [Google Scholar]
- 79.Mustafa N, Nee HFA, Lee XTJ, Jin W, Yu Y, Chen Y, et al. Daratumumab Efficiently Targets NK/T Cell Lymphoma with High CD38 Expression. Blood. 2017;130(Suppl 1):2814.29284611 [Google Scholar]
- 80.Hari P, Raj RV, Olteanu H. Targeting CD38 in Refractory Extranodal Natural Killer Cell-T-Cell Lymphoma. The New England journal of medicine. 2016;375(15):1501–2. doi: 10.1056/NEJMc1605684. [DOI] [PubMed] [Google Scholar]
- 81.Triplett TA, Curti BD, Bonafede PR, Miller WL, Walker EB, Weinberg AD. Defining a functionally distinct subset of human memory CD4+ T cells that are CD25POS and FOXP3NEG. European journal of immunology. 2012;42(7):1893–905. doi: 10.1002/eji.201242444. [DOI] [PubMed] [Google Scholar]
- 82.Horwitz SM, Hamadani M, Fanale MA, Feingold J, Spira AI, Fields PA, et al. Interim Results from a Phase 1 Study of ADCT-301 (Camidanlumab Tesirine) Show Promising Activity of a Novel Pyrrolobenzodiazepine-Based Antibody Drug Conjugate in Relapsed/Refractory Hodgkin/Non-Hodgkin Lymphoma. Blood. 2017;130(Suppl 1):1510. [Google Scholar]
- 83.Dhandha MM, Sufficool KE, Vidal CI, Robbins KJ, Fesler MJ, Batanian JR, et al. Immunophenotype Expression Change From CD52+ to CD52- on Erythrodermic Peripheral T-cell Lymphoma, Not Otherwise Specified After Treatment With Alemtuzumab. The American Journal of dermatopathology. 2018;40(7):547–50. doi: 10.1097/dad.0000000000001000. [DOI] [PubMed] [Google Scholar]
- 84.Tuset E, Matutes E, Brito-Babapulle V, Morilla R, Catovsky D. Immunophenotype changes and loss of CD52 expression in two patients with relapsed T-cell prolymphocytic leukaemia. Leukemia & lymphoma. 2001;42(6):1379–83. doi: 10.3109/10428190109097766. [DOI] [PubMed] [Google Scholar]
- 85.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annual review of immunology. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. The New England journal of medicine. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 89.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. The New England journal of medicine. 2017;377(19):1824–35. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 90.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. The New England journal of medicine. 2017;377(20):1919–29. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 91.Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(19):2125–32. doi: 10.1200/jco.2016.72.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. The New England journal of medicine. 2015;372(4):311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. The Lancet Oncology. 2016;17(9):1283–94. doi: 10.1016/s1470-2045(16)30167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. International immunopharmacology. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 95.Wilcox RA, Feldman AL, Wada DA, Yang ZZ, Comfere NI, Dong H, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood. 2009;114(10):2149–58. doi: 10.1182/blood-2009-04-216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Phillips T, Devata S, Wilcox RA. Challenges and opportunities for checkpoint blockade in T-cell lymphoproliferative disorders. Journal for immunotherapy of cancer. 2016;4:95. doi: 10.1186/s40425-016-0201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Merryman RW, Armand P. Immune Checkpoint Blockade and Hematopoietic Stem Cell Transplant. Current hematologic malignancy reports. 2017;12(1):44–50. doi: 10.1007/s11899-017-0362-5. [DOI] [PubMed] [Google Scholar]
- 98.Li X, Cheng Y, Zhang M, Yan J, Li L, Fu X, et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. Journal of hematology & oncology. 2018;11(1):15. doi: 10.1186/s13045-018-0559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. *.Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129(17):2437–42. doi: 10.1182/blood-2016-12-756841. [DOI] [PubMed] [Google Scholar]; In this publication, Kwong and colleagues report the positive experience (100% ORR) of PD1 blockade in relapsed/refractory NKTL nasal type.
- 100.Chan TSY, Li J, Loong F, Khong PL, Tse E, Kwong YL. PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing L-asparaginase: efficacy and safety. Annals of hematology. 2018;97(1):193–6. doi: 10.1007/s00277-017-3127-2. [DOI] [PubMed] [Google Scholar]
- 101.Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(23):2698–704. doi: 10.1200/jco.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khodadoust M, Rook AH, Porcu P, Foss FM, Moskowitz AJ, Shustov AR, et al. Pembrolizumab for Treatment of Relapsed/Refractory Mycosis Fungoides and Sezary Syndrome: Clinical Efficacy in a Citn Multicenter Phase 2 Study. Blood. 2016;128(22):181. [Google Scholar]
- 103.Kim YH, Gratzinger D, Harrison C, Brody JD, Czerwinski DK, Ai WZ, et al. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood. 2012;119(2):355–63. doi: 10.1182/blood-2011-05-355222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maier T, Tun-Kyi A, Tassis A, Jungius KP, Burg G, Dummer R, et al. Vaccination of patients with cutaneous T-cell lymphoma using intranodal injection of autologous tumor-lysate-pulsed dendritic cells. Blood. 2003;102(7):2338–44. doi: 10.1182/blood-2002-08-2455. [DOI] [PubMed] [Google Scholar]
- 105.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–35. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet (London, England). 1995;345(8941):9–13. [DOI] [PubMed] [Google Scholar]
- 107.Morales O, Mrizak D, Francois V, Mustapha R, Miroux C, Depil S, et al. Epstein-Barr virus infection induces an increase of T regulatory type 1 cells in Hodgkin lymphoma patients. British journal of haematology. 2014;166(6):875–90. doi: 10.1111/bjh.12980. [DOI] [PubMed] [Google Scholar]
- 108.Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(8):798–808. doi: 10.1200/jco.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ruella M, June CH. Chimeric Antigen Receptor T cells for B Cell Neoplasms: Choose the Right CAR for You. Curr Hematol Malig Rep. 2016;11(5):368–84. doi: 10.1007/s11899-016-0336-z. [DOI] [PubMed] [Google Scholar]
- 110.Savoldo B, Rooney CM, Di Stasi A, Abken H, Hombach A, Foster AE, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110(7):2620–30. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113(25):6392–402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mamonkin M, Rouce RH, Tashiro H, Brenner MK. A T-cell-directed chimeric antigen receptor for the selective treatment of T-cell malignancies. Blood. 2015;126(8):983–92. doi: 10.1182/blood-2015-02-629527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen KH, Wada M, Firor AE, Pinz KG, Jares A, Liu H, et al. Novel anti-CD3 chimeric antigen receptor targeting of aggressive T cell malignancies. Oncotarget. 2016;7(35):56219–32. doi: 10.18632/oncotarget.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pinz K, Liu H, Golightly M, Jares A, Lan F, Zieve GW, et al. Preclinical targeting of human T-cell malignancies using CD4-specific chimeric antigen receptor (CAR)-engineered T cells. Leukemia. 2016;30(3):701–7. doi: 10.1038/leu.2015.311. [DOI] [PubMed] [Google Scholar]
- 115.Perera LP, Zhang M, Nakagawa M, Petrus MN, Maeda M, Kadin ME, et al. Chimeric antigen receptor modified T cells that target chemokine receptor CCR4 as a therapeutic modality for T-cell malignancies. American journal of hematology. 2017;92(9):892–901. doi: 10.1002/ajh.24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gomes-Silva D, Srinivasan M, Sharma S, Lee CM, Wagner DL, Davis TH, et al. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood. 2017;130(3):285–96. doi: 10.1182/blood-2017-01-761320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cooper ML, Choi J, Staser K, Ritchey JK, Devenport JM, Eckardt K, et al. An "off-the-shelf" fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Leukemia. 2018. doi: 10.1038/s41375-018-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maciocia PM, Wawrzyniecka PA, Philip B, Ricciardelli I, Akarca AU, Onuoha SC, et al. Targeting the T cell receptor beta-chain constant region for immunotherapy of T cell malignancies. Nature medicine. 2017;23(12):1416–23. doi: 10.1038/nm.4444. [DOI] [PubMed] [Google Scholar]
- 119.Langerak AW, van Den Beemd R, Wolvers-Tettero IL, Boor PP, van Lochem EG, Hooijkaas H, et al. Molecular and flow cytometric analysis of the Vbeta repertoire for clonality assessment in mature TCRalphabeta T-cell proliferations. Blood. 2001;98(1):165–73. [DOI] [PubMed] [Google Scholar]
- 120.Ramos CA, Ballard B, Zhang H, Dakhova O, Gee AP, Mei Z, et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. The Journal of clinical investigation. 2017;127(9):3462–71. doi: 10.1172/jci94306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. The New England journal of medicine. 2010;362(10):875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annual review of immunology. 2014;32:25–50. doi: 10.1146/annurev-immunol-032713-120142. [DOI] [PubMed] [Google Scholar]
- 123.Lin GHY, Chai V, Lee V, Dodge K, Truong T, Wong M, et al. TTI-621 (SIRPalphaFc), a CD47-blocking cancer immunotherapeutic, triggers phagocytosis of lymphoma cells by multiple polarized macrophage subsets. PloS one. 2017;12(10):e0187262. doi: 10.1371/journal.pone.0187262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ansell S, Chen RW, Flinn IW, Maris MB, Connor OA, Johnson LDS, et al. A Phase 1 Study of TTI-621, a Novel Immune Checkpoint Inhibitor Targeting CD47, in Patients with Relapsed or Refractory Hematologic Malignancies. Blood. 2016;128(22):1812. [Google Scholar]
- 125.Trautinger F, Eder J, Assaf C, Bagot M, Cozzio A, Dummer R, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sezary syndrome - Update 2017. European journal of cancer (Oxford, England : 1990). 2017;77:57–74. doi: 10.1016/j.ejca.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 126.Bunn PA Jr., Foon KA, Ihde DC, Longo DL, Eddy J, Winkler CF, et al. Recombinant leukocyte A interferon: an active agent in advanced cutaneous T-cell lymphomas. Annals of internal medicine. 1984;101(4):484–7. [DOI] [PubMed] [Google Scholar]
- 127.Knobler RM, Trautinger F, Radaszkiewicz T, Kokoschka EM, Micksche M. Treatment of cutaneous T cell lymphoma with a combination of low-dose interferon alfa-2b and retinoids. Journal of the American Academy of Dermatology. 1991;24(2 Pt 1):247–52. [DOI] [PubMed] [Google Scholar]
- 128.Olsen EA, Bunn PA. Interferon in the treatment of cutaneous T-cell lymphoma. Hematology/oncology clinics of North America. 1995;9(5):1089–107. [PubMed] [Google Scholar]
- 129.Wu J, Fu J, Zhang M, Liu D. AFM13: a first-in-class tetravalent bispecific anti-CD30/CD16A antibody for NK cell-mediated immunotherapy. Journal of hematology & oncology. 2015;8:96. doi: 10.1186/s13045-015-0188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ansell SM, Chen RW, Forero-Torres A, Armand P, Lossos IS, Reeder CB, et al. A Phase 1 Study Investigating the Combination of AFM13 and the Monoclonal Anti-PD-1 Antibody Pembrolizumab in Patients with Relapsed/Refractory Hodgkin Lymphoma after Brentuximab Vedotin Failure: Data from the Dose Escalation Part of the Study. Blood. 2017;130(Suppl 1):1522. [Google Scholar]
- 131.Gramatzki M, Burger R, Strobel G, Trautmann U, Bartram CR, Helm G, et al. Therapy with OKT3 monoclonal antibody in refractory T cell acute lymphoblastic leukemia induces interleukin-2 responsiveness. Leukemia. 1995;9(3):382–90. [PubMed] [Google Scholar]
- 132.O'Mahony D, Morris JC, Stetler-Stevenson M, Matthews H, Brown MR, Fleisher T, et al. EBV-related lymphoproliferative disease complicating therapy with the anti-CD2 monoclonal antibody, siplizumab, in patients with T-cell malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(7):2514–22. doi: 10.1158/1078-0432.ccr-08-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]