Abstract
Inbred salt-sensitive (SS) rats developed by John Rapp and distributed by Harlan (SS/JrHsd) were shown to model ovariectomy-induced hypertension because on a low-sodium (LS) diet, ovariectomized SS (SS-OVX) animals became hypertensive in contrast to their sham-operated (SS-SHAM) normotensive littermates. After Harlan merged with Envigo in 2015, inconsistencies in the LS normotensive phenotype were reported. To further investigate these inconsistencies, we studied the effects of ovariectomy on SS and salt-resistant (SR) rats purchased from Envigo (SS/JrHsd/Env) between 2015 and 2017. The mean arterial pressure (MAP) in SS rats on a LS diet exceeded 160 mmHg at 7 mo old. Ovariectomy at 3 mo had no detectable effect on MAP from 4 to 7 mo, nor did ovariectomy at 1.5 mo significantly affect MAP at 10 mo in either strain; only strain differences in MAP were observed [MAP: SR-SHAM (n = 7 rats), 102 ± 3 mmHg; SR-OVX (n = 6 rats), 114 ± 1 mmHg; SS-SHAM (n = 7 rats), 177 ± 6 mmHg; SS-OVX (n = 5 rats), 190 ± 12 mmHg; where P < 0.0001 vs. SR, same ovarian-status for SS-SHAM and SS-OVX, respectively]. Whole genome sequencing revealed more genomic variants of SS/JrHsd/Env, including single nucleotide and insertion deletion polymorphisms and higher heterozygous/homozygous ratios compared with the reference genome, than for SS/JrHsd/Mcwi and SS/Jr rats maintained in Milwaukee, WI and Toledo, OH, respectively, and which still exhibit normal blood pressure on a LS diet. These findings demonstrate that the female SS/JrHsd/Env rat has genetically diverged from the original phenotype, which was normotensive on a LS diet when the ovaries were intact but rapidly developed hypertension when the ovaries were removed. Nonetheless, the SS/JrHsd/Env rat could be a valuable model that complements other animal models of spontaneous hypertension used to investigate mechanisms of essential hypertension.
Keywords: genetic drift, ovariectomy, spontaneously hypertensive rat
INTRODUCTION
Dissecting the etiology of hypertension in humans has been a difficult task, largely because of the genetically heterogeneous nature of the human population (44). To address this heterogeneity, various experimental rat models have been developed to investigate the genesis or pathophysiology of hypertension (29, 43). Lewis Dahl selectively bred Sprague-Dawley rats based on susceptibility and resistance to a high-sodium diet (8% NaCl), which in 1962 gave rise to outbred salt-sensitive (SS) and salt-resistant (SR) rat strains (8, 19). Two decades later, John Rapp (Jr) at the University of Toledo (previously known as the Medical College of Ohio, Toledo) developed inbred SS and SR strains (42), which Harlan (Hsd) began distributing commercially in 1986 as SS/JrHsd and SR/JrHsd rats.
Many investigators have used SS/JrHsd and SR/JrHsd rats to study physiological, biochemical, and genetic mechanisms of susceptibility and resistance to sodium-dependent hypertension. Numerous studies showed that young 2- to 3-mo-oldSS/JrHsd rats exhibited normal though higher blood pressure than age-matched SR/JrHsd animals when maintained on a low-sodium (LS) diet (0.3% NaCl) (21, 52). In contrast, on a high-sodium diet (8% NaCl), blood pressure rapidly rose within 2 wk in the SS/JrHsd rats, but not in the SR/JrHsd animals. The SS/JrHsd on a high-sodium diet has also been used as a model of renal injury because these animals rapidly developed proteinuria, glomerulosclerosis, and tubulointerstitial damage (21, 28).
Other investigators, including us, have used the female SS/JrHsd rat maintained on a LS diet as a model to investigate ovarian hormone regulation of blood pressure. Within a month after ovariectomy, mean arterial pressure (MAP) increased by ~10 mmHg in 4-mo-old SS/JrHsd, and the effect of ovariectomy was prevented by concomitant treatment with 17β-estradiol (15). Furthermore, the ovariectomy-induced increase in MAP was magnified with age. At 7 mo, MAP in the sham-operated (SHAM) controls was 135 mmHg, whereas MAP in the ovariectomized (OVX) group surpassed 155 mmHg. Thus, the ovariectomized SS/JrHsd rat became a useful model to investigate mechanisms of hypertension associated with ovarian hormone loss (16, 31, 32, 45, 55, 57).
After Envigo was created in September 2015 through the integration of several companies, including Harlan Laboratories (https://www.envigo.com/; accessed June 13, 2018), the company began supplying SR/JrHsd and SS/JrHsd rats (40, 58). Recent studies on the SS/JrHsd rats from Envigo (SS/JrHsd/Env) have reported blood pressures on a LS diet, which were markedly higher than previously observed (47, 59). To further investigate this change in blood pressure phenotype in the SS/JrHsd/Env rat, we studied the effect of ovariectomy on blood pressure as a function of age in animals maintained on a LS diet since weaning.
METHODS
Animals.
Female SS/JrHsd/Env and SR/JrHsd/Env inbred rats (abbreviated SS and SR hereon in, respectively) were purchased between 2015 and 2017 from Envigo (Indianapolis, IN) at 4 wk old. Animals were maintained on a LS diet (0.1% NaCl) (no. 7034; Teklad; Madison, WI) since weaning at Envigo and on a purified phytoestrogen-free LS diet (0.128% NaCl) (no. 120597; Teklad) (15) once at Georgetown University. Rats were housed in temperature and humidity controlled rooms with a constant light/dark cycle (12/12 h). Food and water were provided ad libitum. Body weight and food and water intake were measured weekly. All methods were approved by the Georgetown University Animal Care and Use Committee.
Experimental design.
Different cohorts of SS and SR rats were studied. In one cohort, SS and SR rats were subjected to sham surgery or ovariectomy at 3 mo and their MAP was followed by radio telemetry from 4 to 8 mo. In a second cohort, ovariectomies or sham surgeries were conducted at 1.5 mo and MAP was measured terminally when they were 10 mo old. The third cohort consisted of 4-mo-old SS and SR rats whose right kidneys were used for renal histology studies. In a fourth cohort, juvenile SS rats were instrumented with radio transmitters and MAP was measured when they were 1 mo old.
Ovariectomy and sham surgeries.
SS and SR rats were randomly split into OVX or SHAM groups. In the OVX group, rats were anesthetized with 2.5% isoflurane at 1 l/min oxygen (isoflurane, USP; Piramal Healthcare; Medak, Andhra Pradesh, India) and ovaries were exposed via bilateral flank incisions and excised, as we described previously (15). In the SHAM group, bilateral flank incisions were made and the ovaries were manipulated but not removed. All rats were administered the analgesic carprofen (5 mg/kg; Rimadyl, no. 10000319; Zoetis; Parsippany, NJ) subcutaneously for up to 24 h aftersurgery and were allowed to recover for 7–14 days. Uterus and body weights were measured at the end of each experiment.
Mean arterial pressure and heart rate.
Blood pressure was measured acutely by indwelling catheters or by radio telemetry. Terminal blood pressure measurements were conducted as described (1). Briefly, animals were anesthetized with 2.5% isoflurane and placed on a heated table to maintain body temperature at 37°C. An indwelling vascular catheter was placed in the femoral artery and MAP was determined using a Blood Pressure Analyzer (Digi-Med, Louisville, KY). MAP and heart rate (HR) data were collected every 30 s and averaged over 15 min or more.
In 4-mo-old rats, radio transmitters (no. HD-S10; Data Sciences International; St. Paul, MN) were implanted in the left femoral artery, as we previously described (12). Carprofen was administered subcutaneously for up to 2 days postsurgery. After recovery from surgery (7–10 days), MAP and HR recordings were taken every 5 min for 10 s from 6 PM to 6 AM and presented as daily averages using a Data Acquisition and Analysis System (Dataquest ART v4.36 (now replaced by Ponemah v6.41); Data Sciences International). The active and dormant periods were defined as 6 PM to 6 AM and 6 AM to 6 PM, respectively. During this period, some transmitters started failing when the animals reached 7 mo; therefore, we present the MAP data up until 7 mo.
Four-week-old SS rats were implanted with radio transmitters (no. PA-C10; Data Sciences International) in a modified version of a previously described method for measuring MAP and HR in young rats (12). The catheter was implanted in the left femoral artery and the battery pack was placed subcutaneously in the left flank. Postsurgical analgesic was given as above and the rats were closely followed during the 1-wk recovery period. MAP and HR recordings were then averaged over the active period (6 PM to 6 AM) and also over the dormant period (6 AM to 6 PM) from measurements taken every 5 min for 10 s.
Tissue harvest.
Rats were euthanatized by isoflurane followed by cardiac puncture. At time of euthanization, the uterus, heart, and kidneys were harvested and wet weights were measured before tissues were frozen in liquid nitrogen until further processing.
Genomic DNA isolation and whole genome sequencing.
The left frozen kidney from 8-mo-old SS rats was cut up into small pieces over dry ice. Genomic DNA was isolated from 20 mg of kidney tissue by DNeasy Blood and Tissue Kit (no. 69504; Qiagen; Hilden, Germany) according to the manufacturer’s instructions. The quality of the isolated DNA was confirmed on a 1% agarose gel and quantification was done on a NanoDrop 2000 spectrophotometer (5). A DNA library was constructed using the NEBNext DNA Library Prep Kit (New England BioLabs, Ipswich, MA) according to the manufacturer’s instructions. Pair-end sequencing was performed on an Illumina sequencing platform, with the read length of PE150 base pairs at each end (Novogene; Chula Vista, CA). The average depth of sequencing was 35× and the error rate was 0.01%.
Whole genome sequence comparison.
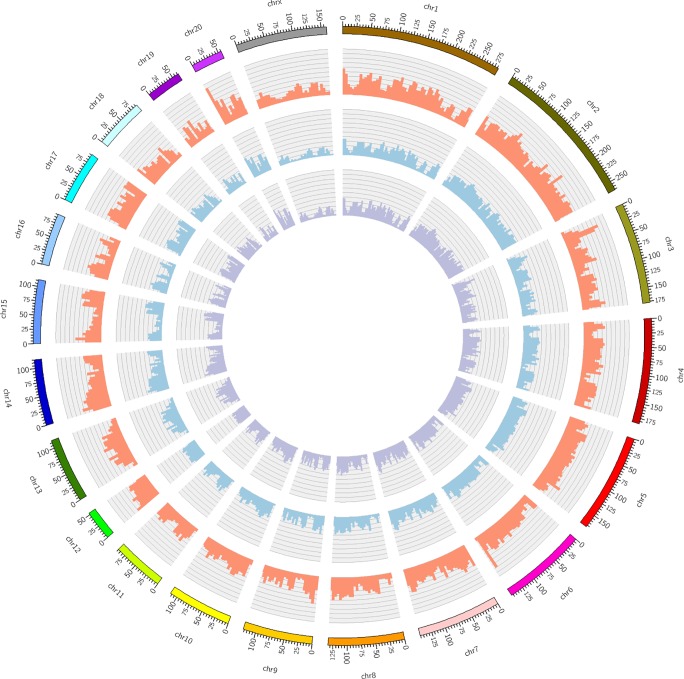
The raw next-generation sequencing data of the SS/JrHsd rat bred in Milwaukee (SS/JrHsd/Mcwi) and SS/Jr were obtained from European Bioinformatics Institute’s European Nucleotide Archive (SS/JrHsd/Mcwi: ERS207928; SS/Jr: ERS207927). The raw reads were mapped to the Rnor_6.0 reference genome using Bowtie2 (27). After BAM files were sorted and indexed, the variants were called using mpileup combined with bcftools from the SAMtools package (30). Vcftools (9) was used to calculate genomic variant densities in a 5-Mb sliding window. The Circos program (22) was then used to generate the Circos plot. Detailed statistical analyses and comparisons of genomic variants among different SS strains were performed using Ensembl Variant Effect Predictor (35) and Real Time Genomics tools (https://realtimegenomics.com/products/rtg-tools/; accessed June 1, 2018).
Histological analysis of kidneys.
The right kidney was harvested at 4 and 8 mo at time of euthanasia. After decapsulation, the kidney was weighed and dissected transversely. The middle section of each kidney was fixed in HistoChoice (Amresco; Solon, OH) for 16–24 h at room temperature. Fixed renal tissue was then stored in 70% ethanol before being paraffin-embedded. Masson’s trichrome staining of transverse tissue sections (3–5 µm) was performed by the Histopathology and Tissue Shared Resource (Georgetown University). Time duration of storage in 70% ethanol and time of staining was different for 4- and 8-mo-old rats, leading to differences in stain intensity. Slides were observed and photographed using an Olympus BX43 camera at ×4 and ×20 magnification using cellSens software (Olympus Lifesciences; Waltham, MA). A semiquantitative index was used to score glomeruli in each section (~100 per rat) from 0 (best, 0% glomerular damage) to 4 (worst, 75–100% glomerular damage) on the basis of severity of glomerulosclerosis, as described previously (39). One transverse kidney section was scored per rat. A weighted score was obtained by multiplying the degree of damage by the percentage of glomeruli affected, leading to a glomerular injury index (31). The grading of glomerular damage was performed in a blinded manner (C. Baylis).
Statistical analyses.
Prism 7.0 (GraphPad Software; La Jolla, CA) was used to analyze all data. Two-way analysis of variance (ANOVA) followed by Sidak’s or Tukey’s multiple comparison analysis was used as a post hoc test to analyze differences in the effects of treatments between rat groups (OVX vs. SHAM) and analyze the effect of strain (SS vs. SR). All data are expressed as scatter plots showing the means ± SE (where n = number of rats) unless otherwise described. The significance threshold was defined as 0.05. The G*Power 3.1.9.2 (Heinrich Heine University, Düsseldorf, Germany) statistical power analyses tool (11) was used to calculate sample size.
RESULTS
Effect of ovariectomy on organ and body weight in SR and SS rats.
Ovariectomy causes sizeable reductions in rat uterus wet weights due to the loss of 17β-estradiol (6, 56). Therefore, we measured uterine wet weights to confirm that surgical removal of the ovaries was complete. In all our OVX rats, there was more than a 50% reduction in uterine wet weight compared with SHAM controls (Tables 1 and 2). Uterine atrophy was greater in SR (70% reduction) compared with SS (50–60% reduction) rats. In contrast, ovariectomy had no effect on other tissue weights, including the heart and kidneys, when normalized to body weight (Table 1).
Table 1.
Body weight and tissue weight in SHAM and OVX 10-mo-old SS and SR rats
| Measurement | SR-SHAM (n = 7) | SR-OVX (n = 6) | SS-SHAM (n = 7) | SS-OVX (n = 5) |
|---|---|---|---|---|
| Age, mo | 10 | 10 | 10 | 10 |
| Initial body weight, g | 149 ± 5 | 136 ± 5 | 161 ± 5 | 158 ± 3* |
| Final body weight, g | 275 ± 5 | 351 ± 19# | 322 ± 8* | 337 ± 8 |
| Left kidney, g | 0.8 ± 0.02 | 0.8 ± 0.01 | 1 ± 0.01 | 1 ± 0.07# |
| Left kidney, g normalized to 100 g body wt | 0.3 ± 0.007 | 0.31 ± 0.007# | 0.3 ± 0.005 | 0.3 ± 0.03 |
| Right kidney, g | 0.8 ± 0.03 | 0.9 ± 0.01 | 1 ± 0.01 | 1 ± 0.07 |
| Right kidney, g normalized to 100 g body wt | 0.3 ± 0.008 | 0.3 ± 0.01# | 0.3 ± 0.007 | 0.3 ± 0.06 |
| Heart, g | 0.8 ± 0.02 | 0.9 ± 0.03 | 1 ± 0.03 | 1 ± 0.06 |
| Heart normalized to 100 g body wt, g | 0.3 ± 0.008 | 0.3 ± 0.01# | 0.3 ± 0.007 | 0.3 ± 0.03 |
| Uterus, g | 0.6 ± 0.02 | 0.2 ± 0.07# | 0.6 ± 0.04 | 0.4 ± 0.03# |
| Uterus normalized to 100 g body wt, g | 0.2 ± 0.01 | 0.06 ± 0.03# | 0.2 ± 0.02 | 0.1 ± 0.008# |
Data are expressed as means ± SE; n, number of rats. All rats were maintained on a low-sodium diet (<0.13% NaCl) since weaning. Ovariectomies (OVX) or sham surgeries (SHAM) were conducted at 6 wk of age. Shown are body and tissue weights of 10-mo-old salt-sensitive (SS) and salt-resistant (SR) rats.
P < 0.05 vs. SR, same gonadal status;
P < 0.05 vs. SHAM, same strain; two-way ANOVA and Sidak’s post hoc analysis.
Table 2.
Body weight and tissue weight in SHAM and OVX 1- and 8-mo-old SS and SR rats
| Measurement | SS (n = 13) | SR-SHAM (n = 3) | SR-OVX (n = 2) | SS-SHAM (n = 3) | SS-OVX (n = 4) |
|---|---|---|---|---|---|
| Age, mo | 1 | 8 | 8 | 8 | 8 |
| Initial body weight, g | 229 ± 3 | 221 ± 4 | 261 ± 6* | 261 ± 4* | |
| Final body weight, g | 125 ± 4 | 275 ± 7 | 308 ± 9 | 315 ± 13* | 361 ± 7*# |
| Left kidney, g | 0.5 ± 0.02 | 0.9 ± 0.04 | 0.8 ± 0.04 | 1 ± 0.05 | 1 ± 0.06* |
| Left kidney normalized to 100 g body wt, g | 0.4 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.01# | 0.3 ± 0.01 | 0.3 ± 0.02 |
| Right kidney, g | 0.6 ± 0.01 | 0.8 ± 0.02 | 0.8 ± 0.03 | 1 ± 0.04* | 1 ± 0.07 |
| Right kidney normalized to 100 g body wt, g | 0.5 ± 0.01 | 0.3 ± 0.002 | 0.3 ± 0.008 | 0.3 ± 0.02 | 0.3 ± 0.02 |
| Heart, g | 0.5 ± 0.01 | 0.8 ± 0.03 | 0.8 ± 0.02 | 1 ± 0.03* | 2 ± 0.05* |
| Heart normalized to 100 g body wt, g | 0.5 ± 0.01 | 0.3 ± 0.005 | 0.3 ± 0.004 | 0.5 ± 0.009* | 0.4 ± 0.01* |
| Uterus, g | 0.5 ± 0.09 | 0.2 ± 0.02# | 0.6 ± 0.08 | 0.3 ± 0.07# | |
| Uterus normalized to 100 g body wt, g | 0.2 ± 0.03 | 0.06 ± 0.007# | 0.2 ± 0.03 | 0.08 ± 0.02# |
Data are expressed as means ± SE; n, number of rats. All rats were maintained on a low-sodium diet (<0.13% NaCl) since weaning. Ovariectomies (OVX) or sham surgeries (SHAM) were conducted at 3 mo of age. Shown are body and tissue weights of 1-mo-old and 8-mo-old salt-sensitive (SS) and 8-mo-old salt-resistant (SR) rats.
P < 0.05 vs. SR, same gonadal status;
P < 0.05 vs. SHAM, same strain; two-way ANOVA and Sidak’s post hoc analysis.
Ovariectomy increased body weight in the SR rats by 27%. In comparison, the tendency to increase body weight after ovariectomy did not reach statistical significance in the SS rats (Tables 1 and 2). Regardless of gonadal status, SS rats were 7–14% heavier than SR rats in all age groups (Table 2). SS rats also ate more than SR animals; however, ovariectomy did not significantly affect food intake [Food: SR-SHAM (n = 7), 10.9 ± 0.5 g; SR-OVX (n = 6), 11.8 ± 0.4 g; SS-SHAM (n = 7), 14.4 ± 0.5 g; SS-OVX (n = 5), 13.9 ± 0.3 g; where P < 0.0001 vs. SR, same gonadal status for SS-SHAM and SS-OVX, respectively].
Mean arterial pressure and heart rate as a function of age in SS and SR rats ovariectomized at 3 mo.
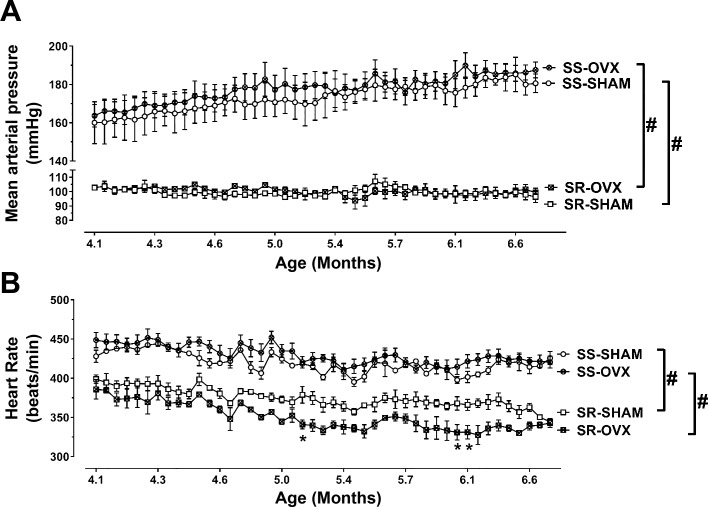
Removing the ovaries of sexually mature young adult SS rats at 3 mo had no effect on their MAP (Fig. 1A) or HR (Fig. 1B) between 4 to 7 mo of age. Based on the mean MAP ± SD of 160 ± 19 mmHg (n = 3) and 163.6 ± 12 mmHg (n = 3), respectively, in 4-mo-old SS-SHAM and SS-OVX rats, a power analysis suggested that a sample size of 420 rats/group would be required to observe significant differences in MAP between the SS-OVX and SS-SHAM animals. MAP (Fig. 1A) and HR (Fig. 1B) were markedly lower in SR compared with SS rats. Ovariectomy at 3 mo also had no effect on MAP in SR rats, although it did lower HR between 5 and 6 mo of age.
Fig. 1.
Mean arterial pressure (MAP) and heart rate (HR) in sham-operated (SHAM) or ovariectomized (OVX) salt-resistant (SR) and salt-sensitive (SS) rats from 4 to 7 mo of age. After being weaned, all rats were maintained on a low-sodium diet (<0.13% NaCl). Ovariectomies or sham surgeries were conducted at 3 mo. Shown is the MAP (A) and HR (B) from 4 to 7 mo of age measured by radio telemetry. The data in SR-SHAM (n = 3), SR-OVX (n = 2), SS-SHAM (n = 3), and SS-OVX (n = 4) are expressed as means ± SE; *P < 0.05 vs. SHAM, same strain or #P < 0.0001 vs. SR, same gonadal status, by Tukey’s multiple comparison in two-way ANOVA; SS/JrHsd/Env, salt-sensitive rats developed by John Rapp, distributed by Harlan, and purchased from Envigo; SR/JrHsd/Env, salt-resistant rats developed by John Rapp, distributed by Harlan, and purchased from Envigo.
Mean arterial pressure and heart rate in 10-mo-old SR and SS rats ovariectomized at 6 wk of age.
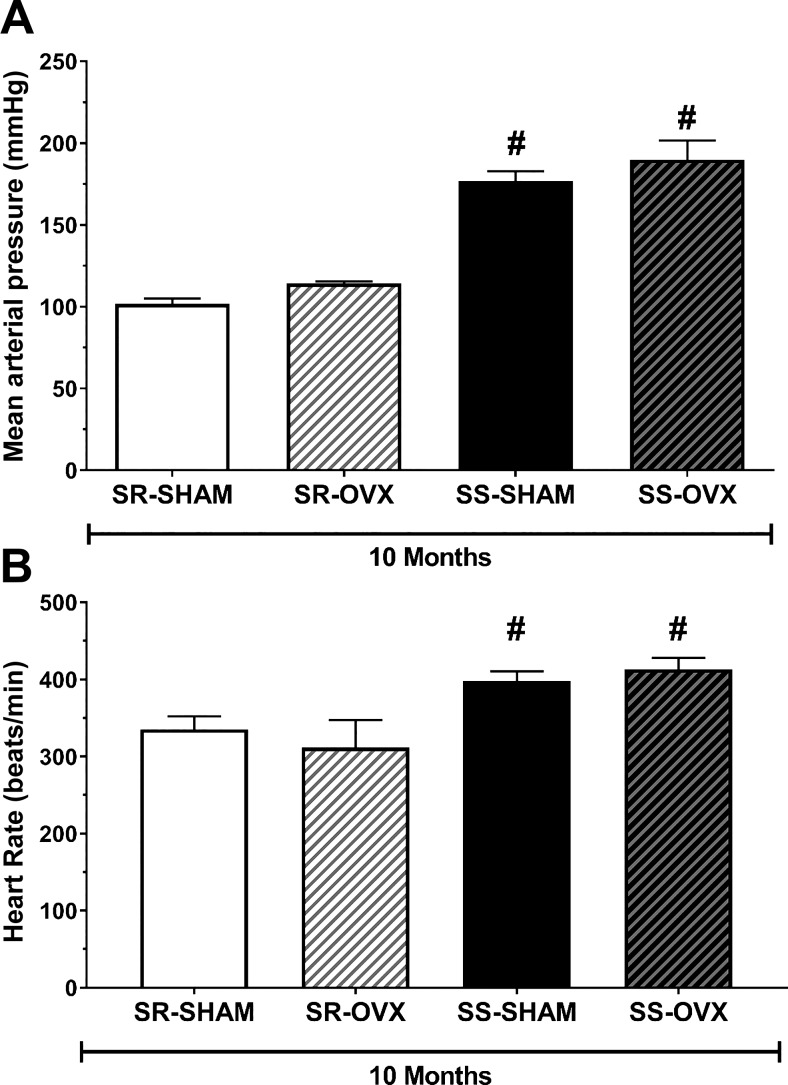
Ovariectomies were also conducted on sexually immature juvenile (6 wk old) SR and SS rats. At 10 mo of age, there was no detectable effect of ovariectomy on MAP (Fig. 2A) or HR (Fig. 2B) in either strain; however, both MAP and HR were substantially higher in SS compared with SR rats.
Fig. 2.
Mean arterial pressure (MAP) and heart rate (HR) in sham-operated (SHAM) and ovariectomized (OVX) salt-sensitive (SS) and salt-resistant (SR) rats at 10 mo of age. After being weaned, all rats were maintained on a low-sodium diet (<0.13% NaCl). Ovariectomies or sham surgeries were conducted at 6 wk old. Shown is the terminal MAP (A) and HR (B) measured at 10 mo. The data in SR-SHAM (n = 7), SR-OVX (n = 6), SS-SHAM (n = 7), and SS-OVX (n = 5) are expressed as means ± SE; *P < 0.05 vs. SHAM, same strain or #P < 0.0001 vs. SR, same gonadal status, by Tukey’s multiple comparison in two-way ANOVA; SS/JrHsd/Env, salt-sensitive rats developed by John Rapp, distributed by Harlan, and purchased from Envigo; SR/JrHsd/Env, salt-resistant rats developed by John Rapp, distributed by Harlan, and purchased from Envigo.
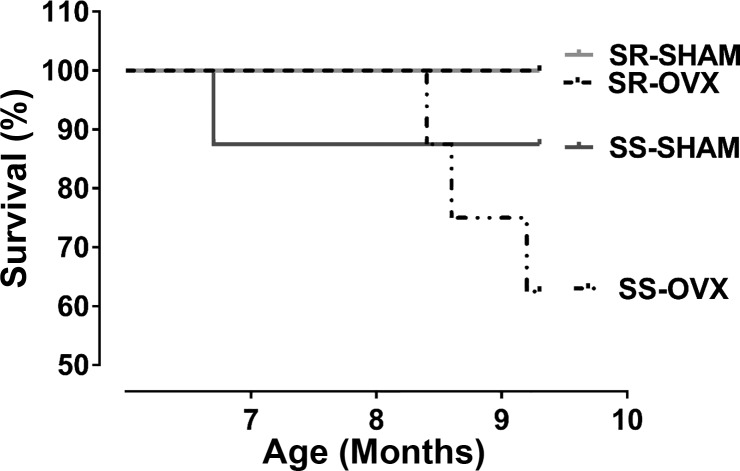
Effect of ovariectomy on survival in SS and SR rats.
All of the SR rats lived to 10 mo of age regardless of whether or not their ovaries were removed at 6 wk of age (Fig. 3). In contrast, survival rates in both SS animal groups were less than 100% at 10 mo of age. The survival rate was worst in the SS-OVX group (62%) compared with the SS-SHAM cohort (89%).
Fig. 3.
Survival curves of sham-operated (SHAM) and ovariectomized (OVX) salt-sensitive (SS) and salt-resistant (SR) rats. After being weaned, all rats were maintained on a low-sodium diet (<0.13% NaCl). Ovariectomies or sham surgeries were conducted at 6 wk old. Shown are survival curves of SR-SHAM (n = 7), SR-OVX (n = 6), SS-SHAM (n = 7), and SS-OVX (n = 5) cohorts expressed as the percentage of animals that survived through 10 mo of age; SS/JrHsd/Env, salt-sensitive rats developed by John Rapp, distributed by Harlan, and purchased from Envigo; SR/JrHsd/Env, salt-resistant rats developed by John Rapp, distributed by Harlan, and purchased from Envigo.
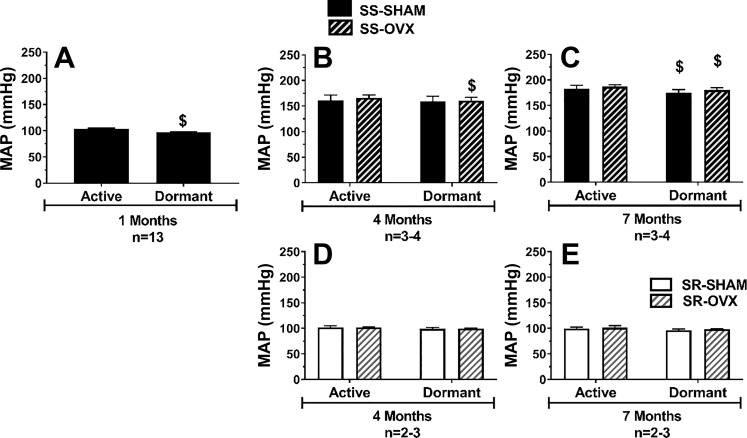
Mean arterial pressure during active and dormant phases in SS and SR rats as a function of ovariectomy.
The MAP in 1-mo-old normotensive SS rats was 7 mmHg lower during the dormant phase (daytime) compared with the active phase (nighttime); this amounted to a 7% dip in MAP during the dormant phase (Fig. 4A). To determine if this phase effect on MAP was affected by ovariectomy, we also measured MAP during the dormant and active phases at 4 and 7 mo in animals subjected to ovariectomy or sham operation at 3 mo. By 4 mo, the SS rats were hypertensive and there was no significant drop in MAP between the active and dormant phase in the SS-SHAM group, whereas the SS-OVX group exhibited a small (<4%) dip in MAP during the dormant phase (Fig. 4B). At 7 mo, MAP in both the SS-OVX and SS-SHAM rats slightly dipped during the dormant phase, but this drop was small (<4%) (Fig. 4C). Interestingly, there were no detectable differences in MAP between the dormant and active phases in the normotensive SR rats at both 4 and 7 mo regardless of whether or not their ovaries were removed.
Fig. 4.
Mean arterial pressure (MAP) during the active and dormant phases in salt-sensitive (SS) and salt-resistant (SR) rats as a function of age and ovariectomy. After being weaned, all rats were maintained on a low-sodium diet (<0.13% NaCl). Ovariectomies or sham surgeries were conducted at 3 mo. Shown is average MAP measured by radio telemetry in the active and dormant phases in SS (A–C) and SR (D and E) rats in SS intact (solid black bar) rats at 1 mo (A), in SS-SHAM (solid black bar) and SS-OVX (striped black bar) rats at 4 mo (B) and at 7 mo (C), and in SR-SHAM (solid white bar) and SR-OVX (striped white bar) rats at 4 mo (D) and 7 mo (E); $P < 0.0001 vs. active phase; SS/JrHsd/Env, salt-sensitive rats developed by John Rapp, distributed by Harlan, and purchased from Envigo; SR/JrHsd/Env, salt-resistant rats developed by John Rapp, distributed by Harlan, and purchased from Envigo; n, number of rats.
Genetic variation between SS/JrHsd/Env and other SS strains.
To investigate why young adult SS rats were no longer normotensive on a LS diet, whole genome sequences of the SS/JrHsd/Env strain were compared with the original stock of SS/Jr rats (from the University of Toledo) and the SS/JrHsd/Mcwi strain maintained at the Medical College of Wisconsin. Because direct comparisons are not feasible, genomic sequences of these three strains were each compared with the Rnor_6.0 rat reference genome for cataloging variants. The Circos plot indicates that at multiple genomic regions, there are more genomic variants of SS/JrHsd/Env compared with the Rnor_6.0 reference than for SS/JrHsd/Mcwi and SS/Jr (Fig. 5). Further statistical analysis showed that SS/JrHsd/Env also have more single nucleotide polymorphisms and insertion deletion polymorphisms compared with SS/JrHsd/Mcwi and SS/Jr (Table 3). Based on the Rnor_6.0 reference genome, SS/JrHsd/Env and SS/JrHsd/Mcwi shared ~4,518,847 variants, with ~2,980,649 genetic variants exclusively observed in SS/JrHsd/Env rats and ~916,979 variants found only in SS/JrHsd/Mcwi. SS/JrHsd/Env and SS/Jr shared ~4,738,391 variants, with ~2,761,105 genetic variants exclusively observed in SS/JrHsd/Env and ~886,810 genetic variants found only in SS/Jr. Additionally, SS/JrHsd/Env had much higher heterozygous/homozygous ratios compared with SS/Jr and SS/JrHsd/Mcwi (Table 3).
Fig. 5.
Genomic variant densities of SS/JrHsd/Env, SS/JrHsd/Mcwi, and SS/Jr rats. Shown is a Circos plot representing the genomic variant densities of the SS/JrHsd/Env, SS/JrHsd/Mcwi, and SS/Jr rat strains. Each colored bar on the outermost ring represents a single rat chromosome (chr) with each tick mark denoting a physical distance of 5 Mb. The inner whorls contain histograms, which represent the total number of genomic variants per 5 Mb of the SS/JrHsd/Env (red), SS/JrHsd/Mcwi (blue), and SS/Jr (purple) rat genomes compared with the Rnor_6.0 reference genome (Brown Norway strain; https://www.rgd.mcw.edu/; accessed June 1, 2018). The histograms in each of the three inner whorls are represented on the same scale (0–40K). SS/JrHsd/Env, salt-sensitive rats developed by John Rapp, distributed by Harlan, and purchased from Envigo; SS/JrHsd/Mcwi, salt-sensitive rats developed by John Rapp and bred in Milwaukee; SS/Jr, salt-sensitive rats developed by John Rapp and bred in Toledo.
Table 3.
Statistical summary of genomic variants in SS/JrHsd/Env, SS/JrHsd/Mcwi, and SS/Jr compared with the Rnor_6.0 reference genome
| Genomic Variants | SS/JrHsd/Env | SS/JrHsd/Mcwi | SS/Jr |
|---|---|---|---|
| Total variants | 7,499,496 | 5,435,826 | 5,625,201 |
| SNPs | 5,431,851 | 3,929,796 | 4,145,324 |
| Insertions | 1,029,983 | 838,117 | 833,806 |
| Deletions | 1,037,662 | 667,913 | 646,071 |
| Total heterozygous-to-homozygous ratio | 0.31 | 0.10 | 0.10 |
| SNP heterozygous-to-homozygous ratio | 0.30 | 0.08 | 0.08 |
| Insertion heterozygous-to-homozygous ratio | 0.35 | 0.17 | 0.16 |
| Deletion heterozygous-to-homozygous ratio | 0.30 | 0.13 | 0.10 |
Whole genome sequence for the salt-sensitive SS/JrHsd/Env rats was obtained from Novogene, and the raw next-generation sequencing data of SS/JrHsd/Mcwi and SS/Jr were obtained from European Bioinformatics Institute’s European Nucleotide Archive (SS/JrHsd/Mcwi: ERS207928; SS/Jr: ERS207927). Genomic sequences of these three strains were each compared with the Rnor_6.0 rat reference genome for cataloging variants. SNP, single-nucleotide polymorphism; SS/JrHsd/Env, salt-sensitive rats developed by John Rapp, distributed by Harlan, and purchased from Envigo; SS/JrHsd/Mcwi, salt-sensitive rats developed by John Rapp and bred in Milwaukee; SS/Jr, salt-sensitive rats developed by John Rapp and bred in Toledo.
Effect of age on glomerular injury in SS and SR rats.
To assess whether or not the hypertension in the SS rats was also associated with renal injury, the kidneys of SS and SR rats were examined by histology. Although the SS rat kidneys exhibited more glomerular damage compared with the normotensive SR rats at 4 mo, the amount of overall renal tubular damage observed was mild, with only minor focal tubule-interstitial fibrosis observed in the SS rats (Fig. 6, A and C, Table 4). By 8 mo, the SS rats exhibited more glomerular injury compared with 4-mo-old SS rats or the age-matched SR rats (Fig. 6, B and C, Table 4); however, the amount of overall renal tubular damage was moderate, with some focal tubule-interstitial fibrosis in 8-mo-old SS animals (Fig. 6B, Table 4). All SS rats had a higher number of casts around their renal tubules, whereas none were observed in the SR rats.
Fig. 6.
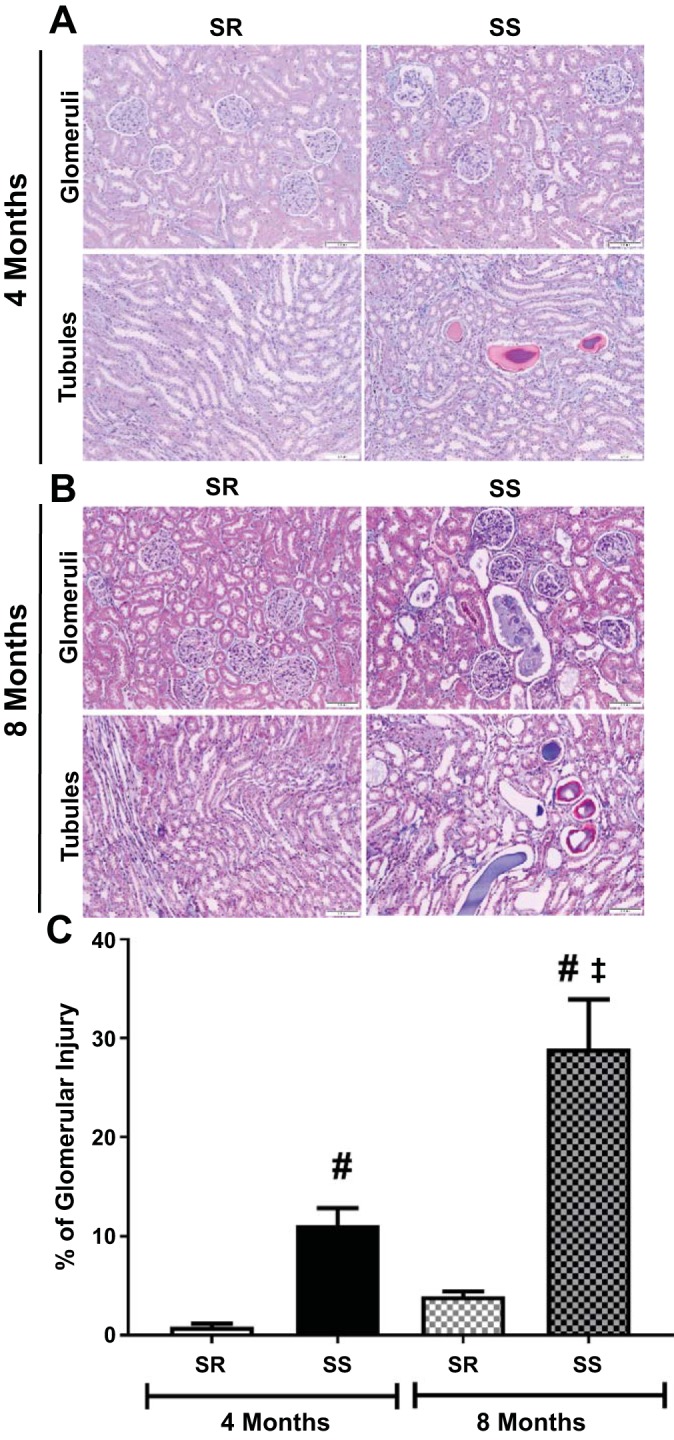
Renal pathology in SS and SR rats at 4 and 8 mo of age. After being weaned, all rats were maintained on a low-sodium diet (<0.13% NaCl). Shown are representative images of renal pathology (A) and quantification of glomerular injury (B) in transverse sections of right kidneys from 4-mo-old and 8-mo-old SS and SR rats; ~100 glomeruli were studied per section, and one section was analyzed per rat. Images were taken at ×4 and ×20 magnification of glomeruli and interstitial tubular space. Quantification of renal pathology in 4-mo-old SR (n = 8) and SS (n = 7) rats and 8-mo-old SR (n = 7) and SS (n = 6) animals is expressed as the means ± SE of the percentage of glomerular injury in each kidney; #P < 0.0001 vs. SR, same age; ‡P < 0.006 vs. 4 mo, same strain; two-way ANOVA and Sidak’s post hoc analysis; SS/JrHsd/Env, salt-sensitive rats developed by John Rapp, distributed by Harlan, and purchased from Envigo; SR/JrHsd/Env, salt-resistant rats developed by John Rapp, distributed by Harlan, and purchased from Envigo.
Table 4.
Renal histology in 4- and 8-mo-old SS and SR rats
| Injury Grade |
|||||||
|---|---|---|---|---|---|---|---|
| Age of Rats | 0 | 1+ | 2+ | 3+ | 4+ | Tubular Injury | Number of Casts |
| 4 Mo old | |||||||
| SR (n = 8) | 101 ± 2 | 1 ± 0.3 | 0 ± 0 | 0 ± 0 | 0 ± 0 | None | 0 ± 0 |
| SS (n = 7) | 88 ± 5 | 7 ± 1# | 0.1 ± 0.1# | 0.1 ± 0.1 | 0.8 ± 0.3# | Mild | 9 ± 1# |
| 8 Mo old | |||||||
| SR (n = 7) | 98 ± 1 | 4 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | Minimal | 0 ± 0 |
| SS (n = 7) | 92 ± 8 | 7 ± 1 | 1 ± 0.6#‡ | 0.7 ± 0.7 | 2 ± 0.7#‡ | Mild | 6 ± 0.3# |
All rats (n) were maintained on a low-sodium diet (<0.13% NaCl) since weaning. Masson’s trichrome stained transverse kidney sections were analyzed and scored based on severity of glomeruli injury; ~100 glomeruli were studied per section and one section analyzed per rat. Data are means ± SE and expressed using a 0–4 + glomerular injury scale: 0, no damage; 1+, focal damage involving < 25% of the glomerulus; 2+, focal damage, between 25 and 50% of the glomerulus; 3+, widespread damage involving 50–75% of the glomerulus; 4+, widespread damage involving 75–100% of the glomerulus and includes obsolescent glomeruli. Description of tubular injury and the number of casts are also tabulated. Area of casts were not taken into consideration because number of casts were not statistically compared. SR, salt-resistant; SS, salt-sensitive.
P < 0.05 vs. SR, same age group;
P < 0.05 vs. 4 mo, same strain; two-way ANOVA.
DISCUSSION
The major finding of this study is that female SS rats purchased between 2015 and 2017 from Envigo are a new accelerated model of spontaneous hypertension. In fact, the SS rat from Envigo more closely resembles the phenotype of the spontaneously hypertensive rat (SHR) than the inbred SS rat originated by John Rapp (40, 50, 58). Whole genome sequence comparison confirmed that the Envigo SS rat is genetically different from SS/Jr rats bred at the University of Toledo and SS/JrHsd/Mcwi rats maintained at the Medical College of Wisconsin (Fig. 5, Table 3). Although the genomic sequences of SS/JrHsd/Env, SS/JrHsd/Mcwi, and SS/Jr are each different from each other, there are greater differences between SS/JrHsd/Env and the other two strains than the differences between SS/JrHsd/Mcwi and SS/Jr (38). Similarly to the SHR, the Envigo SS rat is normotensive as a juvenile (Fig. 4A) but spontaneously develops accelerated hypertension on a LS diet within 4 mo of age. We previously showed that 4- to 5-mo-old female SS rats obtained from Harlan and maintained on a LS diet have MAPs lower than 125 mmHg (15). In this study, SS rats maintained on a LS diet had a MAP that exceeded 160 mmHg at 4 mo and over 180 mmHg at 7 mo while the SR remained normotensive up to 7 mo (Fig. 1A). By demonstrating the spontaneous development of accelerated hypertension in the female Envigo SS rat, this current work supports and extends a recent study by Zimmerman and Lindsey (59), who reported that female SS rats purchased from Envigo and maintained on a LS diet (0.1% NaCl) have blood pressures over 150 mmHg at 4 mo of age.
The second major finding of this study is that the Envigo SS rat maintained on a LS diet is not a useful model for studying ovarian hormone modulation of blood pressure. In 2004, we showed removal of the ovaries at 3 mo induced hypertension and 17β-estradiol replacement prevented this effect of ovariectomy in SS rats purchased from Harlan and maintained on a LS diet (15). Here we show that ovariectomy had no detectable effect on MAP in Envigo SS rats (Figs. 1A and 2A). In fact, a power analysis suggested that more than 400 rats per group would be necessary to observe a 5-mmHg higher MAP in the OVX compared with the SHAM animal group.
Despite the loss of the ovariectomy-associated hypertension phenotype, survival in the SS-OVX group was reduced to 62% compared with 89% in the SS-SHAM cohort or 100% in the SR-SHAM and SR-OVX animals (Fig. 3). This finding suggests that even though ovariectomy did not lead to higher blood pressures, the sudden loss of ovarian hormones altered pathways that contribute to death presumably via stroke or heart failure. Therefore, the SS-OVX animal may be worth investigating as a model of blood pressure-independent effects of ovarian hormone loss on mechanisms of stroke or heart failure. Interestingly, ovariectomy was not found to markedly increase MAP in the SHR (3), which further supports our supposition that the Envigo SS rat behaves more similarly to the SHR than the original Rapp SS rat.
Hypertension is often associated with chronic kidney disease. In this study, however, we found that glomerular injury in the SS rats was minor at 4 mo and only slightly greater compared with SR rats (Fig. 5C). There was only a small increase in the glomerular injury in the SS rats as they aged from 4 (11%) to 8 (29%) mo. Moreover, we only found mild evidence of tubular injury at 8 mo of age when they reached a MAP over 180 mmHg (Fig. 1A). In contrast, SS rats obtained from Harlan before 2004 and maintained on a high-sodium diet for 3 wk exhibited severe renal injury at similar levels of hypertension (180–190 mmHg) (34, 54) to what we observed in Envigo SS rats at 7 mo (Fig. 2A). In these previous studies, the SS rats purchased from Harlan exhibited severe renal injury, including blocked and dilated tubules in the outer medulla and significant glomerular and tubular damage (34, 54). Thus, the mild renal injury phenotype in the presence of significant hypertension in the Envigo SS rat lends further support to the premise that sodium rather than the hypertension drives renal injury in salt-sensitive hypertension. Even though blood pressure was markedly elevated, the modest level of renal pathology observed in the Envigo SS rat is similar to what is observed in the SHR. Sullivan et al. (49) showed that female SHR at 4 mo exhibited no evidence of overt renal injury or glomerulosclerosis although the MAP reached nearly 150 mmHg.
Arterial pressure in healthy individuals typically drops by 10–20% during sleep. Individuals who do not exhibit this blood pressure dipping pattern are called “non-dippers” and are at higher risk for developing kidney and cardiovascular diseases (46). Blood pressure has also been shown to drop ≥10% in rats during their dormant phase (i.e., daytime) (26). We found that MAP did not drop by ≥10% in either SS or SR rats during their dormant phase, regardless of whether or not their ovaries were removed. This suggests that SS and SR rats from Envigo may be useful for studying health risks associated with non-dippers (17).
Studies in animal models of spontaneous hypertension enable investigations into idiopathic hypertension, which is the major clinical form of hypertension. The TGR(mREN2)27 rat is a transgenic animal model that spontaneously develops malignant hypertension due to extra copies of the renin gene (53). The SHR is an example of a nontransgenic animal that spontaneously develops hypertension and is the most commonly studied animal model of hypertension. The SHR was developed by Okamoto and Aoki by selectively inbreeding male and female Wistar rats (37). Most SHR lines bred at various institutions do not exhibit renal pathology; however, the stroke-prone SHR does develop renal damage (2, 7). Fawn-hooded rats are another model of spontaneous hypertension (33). They are different from most SHRs in that they develop hypertension accompanied with focal and segmental glomerulosclerosis and proteinuria. Hypertension and proteinuria progressively increase with age in these animals, and the renal damage leads to premature death from end-stage renal failure (23, 24). Here, we report that the Envigo SS rat is distinct from the SS/Jr and SS/JrHsd/Mcwi rats and is a new model of spontaneous accelerated hypertension associated with only mild renal pathology when maintained on a LS diet. Hence, we recommend the Envigo SS rat be referred to as SS/JrHsd/Env and likewise that the normotensive Envigo SR rat be referred to as SR/JrHsd/Env. Renaming these animals will avoid the assumption that the Envigo SS rats display phenotypes similar to that of SS rats purchased from Harlan before 2004.
Changes in the blood pressure phenotype in the female SS rat have not only been observed with commercial vendors like Envigo and Charles River Laboratories (59) but also within academic colonies (14). Moreover, changes in the normotensive phenotype have not only been observed in females; male SS inbred rats have been reported to exhibit hypertension while maintained on a LS diet (4, 10, 20, 48). Robert Danziger (10) has suggested hypertension observed in Envigo rats on a LS diet could be due to the breeding strategy of Envigo. Envigo typically chooses breeders based on systolic blood pressure readings (by tail cuff) of over 160 mmHg (J. McClellan, Envigo, personal communication). According to Envigo, male and female SS rats have average systolic blood pressures over 150 mmHg by 7–8 wk (~2 mo). Envigo feeds their breeders a 0.5% NaCl diet (no. 2018S; Teklad) while the inventory or production colony is maintained on a LS diet (0.12% NaCl; no. 7034; Teklad), and these procedures are identical to the dietary conditions Harlan has used since 2002 (J. McClellan, Envigo, personal communication). It is well known that maternal diet and preexisting hypertension affect the offspring’s blood pressure (13, 14, 51). Thus, the diet of the breeders could have contributed to the change in blood pressure phenotype. Also, other environmental factors like the microbiome have been reported to modulate blood pressure in Dahl rats (36).
This is not the first time SS rats were reported to be resistant to salt-induced hypertension. In the 1990s, SS/JrHsd rats were found to be resistant to salt-induced hypertension due to contamination of the line (41). Genetic studies at that time confirmed that the SS/JrHsd rats had increased heterozygosity and allelic variants (47, 52). In this study, we show that the genomic sequences of SS/JrHsd/Env, SS/JrHsd/Mcwi and SS/Jr are each different from each other, with greater differences between SS/JrHsd/Env and the other two strains than the relatively smaller differences between SS/JrHsd/Mcwi and SS/Jr (Fig. 5, Table 3). Our findings also confirm previous observations regarding genetic differences between SS/JrHsd/Mcwi and SS/Jr (38). Because the salt-sensitivity phenotype is preserved in the SS/JrHsd/Mcwi and SS/Jr strains (25, 34), it is possible that these genotypic differences have contributed to the change in blood pressure phenotype in SS rats purchased from Envigo.
A particularly troubling observation is that the rate of heterozygosity is higher in the Envigo strain compared with the SS/JrHsd/Mcwi and SS/Jr strains (Table 3). This suggests that the Envigo strain is not an inbred strain to the same extent to which the SS/JrHsd/Mcwi and SS/Jr are inbred. Thus, it might be worth rederiving the SS/Jr line at Envigo. Furthermore, it is also possible that the Envigo SS rats have more genetic variation in their colony due to the segregating heterozygous alleles not being fixed for homozygosity.
Perspectives and Significance
Our findings emphasize the importance of employing scientifically sound breeding programs and keeping required checks in place to confirm an animal model has not drifted from its originally documented bonafide phenotype. Point mutations that occur in inbred strains are missed until they affect the phenotype of interest. We have recently reported one such example in the recombination activating gene 1 null mouse, which is no longer resistant to angiotensin II-induced hypertension (18). It is unlikely, however, that point mutations account for the change in phenotype in the SS/JrHsd/Env rat because sequencing has confirmed there is a large variation in sequence compared with the other SS rat strains (SS/Jr and SS/JrHsd/Mcwi). For these reasons and in the interest of preserving scientific rigor and reproducibility, it is imperative that investigators report explicit details concerning the experimental animals procured from commercial sources or bred in house and include the purchase date, breeding conditions, and husbandry environment.
GRANTS
This work was supported by National Institutes of Health Grants TL1-TR001431 (to A. V. Pai), UL1-TR001409 (to K. Sandberg), and R01-HL119380 (to K. Sandberg and H. Ji).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.V.P., C.A.W., and K.S. conceived and designed research; A.V.P., C.A.W., A.M.A.d.S., D.A.W., H.J., X.W., and C.B. performed experiments; A.V.P., C.A.W., X.C., D.A.W., H.J., and K.S. analyzed data; A.V.P., C.A.W., A.M.A.d.S., X.C., H.J., C.B., and K.S. interpreted results of experiments; A.V.P. and X.C. prepared figures; A.V.P., C.A.W., C.B., and K.S. drafted manuscript; A.V.P., C.A.W., A.M.A.d.S., X.C., D.A.W., H.J., X.W., C.B., and K.S. edited and revised manuscript; A.V.P., C.A.W., A.M.A.d.S., X.C., D.A.W., H.J., X.W., C.B., and K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Michelle L. Gumz for advice regarding circadian rhythms and Dr. Carolyn Ecelbarger for assistance with histology. We also thank Parnika S. Kadam, Emma J. Pollner, Jia Li, and Xin Liu for technical assistance.
REFERENCES
- 1.Basu P, Sen U, Tyagi N, Tyagi SC. Blood flow interplays with elastin: collagen and MMP: TIMP ratios to maintain healthy vascular structure and function. Vasc Health Risk Manag 6: 215–228, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun MC, Herring SM, Gokul N, Monita M, Bell R, Hicks MJ, Wenderfer SE, Doris PA. Hypertensive renal disease: susceptibility and resistance in inbred hypertensive rat lines. J Hypertens 31: 2050–2059, 2013. doi: 10.1097/HJH.0b013e328362f9a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinson KN, Rafikova O, Sullivan JC. Female sex hormones protect against salt-sensitive hypertension but not essential hypertension. Am J Physiol Regul Integr Comp Physiol 307: R149–R157, 2014. doi: 10.1152/ajpregu.00061.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandramohan G, Durham N, Sinha S, Norris K, Vaziri ND. Role of gamma melanocyte-stimulating hormone-renal melanocortin 3 receptor system in blood pressure regulation in salt-resistant and salt-sensitive rats. Metabolism 58: 1424–1429, 2009. doi: 10.1016/j.metabol.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Chen WC, Kerr R, May A, Ndlovu B, Sobalisa A, Duze ST, Joseph L, Mathew CG, Babb de Villiers C. The integrity and yield of genomic DNA isolated from whole blood following long-term storage at -30°C. Biopreserv Biobank 16: 106–113, 2018. doi: 10.1089/bio.2017.0050. [DOI] [PubMed] [Google Scholar]
- 6.Cheng CL, de Groat WC. Effects of agonists for estrogen receptor α and β on ovariectomy-induced lower urinary tract dysfunction in the rat. Am J Physiol Renal Physiol 306: F181–F187, 2014. doi: 10.1152/ajprenal.00298.2013. [DOI] [PubMed] [Google Scholar]
- 7.Churchill PC, Churchill MC, Griffin KA, Picken M, Webb RC, Kurtz TW, Bidani AK. Increased genetic susceptibility to renal damage in the stroke-prone spontaneously hypertensive rat. Kidney Int 61: 1794–1800, 2002. doi: 10.1046/j.1523-1755.2002.00321.x. [DOI] [PubMed] [Google Scholar]
- 8.Dahl LK, Heine M, Tassinari L. Effects of chronia excess salt ingestion. Evidence that genetic factors play an important role in susceptibility to experimental hypertension. J Exp Med 115: 1173–1190, 1962. doi: 10.1084/jem.115.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST, McVean G, Durbin R; 1000 Genomes Project Analysis Group . The variant call format and VCFtools. Bioinformatics 27: 2156–2158, 2011. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danziger R. Need to measure cardiac size with Dahl salt-sensitive rats. J Proteomics 74: 2220, 2011. doi: 10.1016/j.jprot.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 11.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191, 2007. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 12.Fekete A, Sasser JM, Baylis C. Chronic vasodilation produces plasma volume expansion and hemodilution in rats: consequences of decreased effective arterial blood volume. Am J Physiol Renal Physiol 300: F113–F118, 2011. doi: 10.1152/ajprenal.00478.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geurts AM, Mattson DL, Liu P, Cabacungan E, Skelton MM, Kurth TM, Yang C, Endres BT, Klotz J, Liang M, Cowley AW Jr. Maternal diet during gestation and lactation modifies the severity of salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Hypertension 65: 447–455, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillis EE, Williams JM, Garrett MR, Mooney JN, Sasser JM. The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am J Physiol Regul Integr Comp Physiol 309: R62–R70, 2015. doi: 10.1152/ajpregu.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension 44: 405–409, 2004. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- 16.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension 35: 484–489, 2000. doi: 10.1161/01.HYP.35.1.484. [DOI] [PubMed] [Google Scholar]
- 17.Hoshide S, Kario K, Hoshide Y, Umeda Y, Hashimoto T, Kunii O, Ojima T, Shimada K. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community-dwelling normotensives. Am J Hypertens 16: 434–438, 2003. doi: 10.1016/S0895-7061(03)00567-3. [DOI] [PubMed] [Google Scholar]
- 18.Ji H, Pai AV, West CA, Wu X, Speth RC, Sandberg K. Loss of resistance to angiotensin II-induced hypertension in the Jackson Laboratory recombination-activating gene null mouse on the C57BL/6J background. Hypertension 69: 1121–1127, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joe B. Dr Lewis Kitchener Dahl, the Dahl rats, and the “inconvenient truth” about the genetics of hypertension. Hypertension 65: 963–969, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson RJ, Gordon KL, Giachelli C, Kurth T, Skelton MM, Cowley AW Jr. Tubulointerstitial injury and loss of nitric oxide synthases parallel the development of hypertension in the Dahl-SS rat. J Hypertens 18: 1497–1505, 2000. doi: 10.1097/00004872-200018100-00019. [DOI] [PubMed] [Google Scholar]
- 21.Kassab S, Miller MT, Novak J, Reckelhoff J, Clower B, Granger JP. Endothelin-A receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertension 31: 397–402, 1998. doi: 10.1161/01.HYP.31.1.397. [DOI] [PubMed] [Google Scholar]
- 22.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res 19: 1639–1645, 2009. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuijpers MH, de Jong W. Relationship between blood pressure level, renal histopathological lesions and plasma renin activity in fawn-hooded rats. Br J Exp Pathol 68: 179–187, 1987. [PMC free article] [PubMed] [Google Scholar]
- 24.Kuijpers MH, Gruys E. Spontaneous hypertension and hypertensive renal disease in the fawn-hooded rat. Br J Exp Pathol 65: 181–190, 1984. [PMC free article] [PubMed] [Google Scholar]
- 25.Kumarasamy S, Waghulde H, Cheng X, Haller ST, Mell B, Abhijith B, Ashraf UM, Atari E, Joe B. Targeted disruption of regulated endocrine-specific protein (Resp18) in Dahl SS/Mcw rats aggravates salt-induced hypertension and renal injury. Physiol Genomics 50: 369–375, 2018. doi: 10.1152/physiolgenomics.00008.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo TB, Chen CY, Wang YP, Lan YY, Mak KH, Lee GS, Yang CC. The role of autonomic and baroreceptor reflex control in blood pressure dipping and nondipping in rats. J Hypertens 32: 806–816, 2014. doi: 10.1097/HJH.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 27.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359, 2012. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leibowitz A, Volkov A, Voloshin K, Shemesh C, Barshack I, Grossman E. Melatonin prevents kidney injury in a high salt diet-induced hypertension model by decreasing oxidative stress. J Pineal Res 60: 48–54, 2016. doi: 10.1111/jpi.12287. [DOI] [PubMed] [Google Scholar]
- 29.Leong XF, Ng CY, Jaarin K. Animal models in cardiovascular research: hypertension and atherosclerosis. BioMed Res Int 2015: 1–11, 2015. doi: 10.1155/2015/528757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R; 1000 Genome Project Data Processing Subgroup . The sequence alignment/map format and SAMtools. Bioinformatics 25: 2078–2079, 2009. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maric C, Sandberg K, Hinojosa-Laborde C. Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. J Am Soc Nephrol 15: 1546–1556, 2004. doi: 10.1097/01.ASN.0000128219.65330.EA. [DOI] [PubMed] [Google Scholar]
- 32.Maric C, Xu Q, Sandberg K, Hinojosa-Laborde C. Age-related renal disease in female Dahl salt-sensitive rats is attenuated with 17 beta-estradiol supplementation by modulating nitric oxide synthase expression. Gend Med 5: 147–159, 2008. doi: 10.1016/j.genm.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattson DL, Kunert MP, Roman RJ, Jacob HJ, Cowley AW Jr. Substitution of chromosome 1 ameliorates L-NAME hypertension and renal disease in the fawn-hooded hypertensive rat. Am J Physiol Renal Physiol 288: F1015–F1022, 2005. doi: 10.1152/ajprenal.00374.2004. [DOI] [PubMed] [Google Scholar]
- 34.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol 304: R407–R414, 2013. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, Flicek P, Cunningham F. The Ensembl variant effect predictor. Genome Biol 17: 122, 2016. doi: 10.1186/s13059-016-0974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics 47: 187–197, 2015. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J 27: 282–293, 1963. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- 38.Padmanabhan S, Joe B. Towards precision medicine for hypertension: a review of genomic, epigenomic, and microbiomic effects on blood pressure in experimental rat models and humans. Physiol Rev 97: 1469–1528, 2017. doi: 10.1152/physrev.00035.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137–143, 1984. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- 40.Rapp JP. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension 4: 753–763, 1982. doi: 10.1161/01.HYP.4.6.753. [DOI] [PubMed] [Google Scholar]
- 41.Rapp JP. Genetic analysis of inherited hypertension in the rat. Physiol Rev 80: 135–172, 2000. doi: 10.1152/physrev.2000.80.1.135. [DOI] [PubMed] [Google Scholar]
- 42.Rapp JP, Dene H. Development and characteristics of inbred strains of Dahl salt-sensitive and salt-resistant rats. Hypertension 7: 340–349, 1985. [PubMed] [Google Scholar]
- 43.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ 3: 7, 2012. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanders PW. Salt-sensitive hypertension: lessons from animal models. Am J Kidney Dis 28: 775–782, 1996. doi: 10.1016/S0272-6386(96)90265-6. [DOI] [PubMed] [Google Scholar]
- 45.Sartori-Valinotti JC, Venegas-Pont MR, Lamarca BB, Romero DG, Yanes LL, Racusen LC, Jones AV, Ryan MJ, Reckelhoff JF. Rosiglitazone reduces blood pressure in female Dahl salt-sensitive rats. Steroids 75: 794–799, 2010. doi: 10.1016/j.steroids.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solocinski K, Holzworth M, Wen X, Cheng KY, Lynch IJ, Cain BD, Wingo CS, Gumz ML. Desoxycorticosterone pivalate-salt treatment leads to non-dipping hypertension in Per1 knockout mice. Acta Physiol (Oxf) 220: 72–82, 2017. doi: 10.1111/apha.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.St Lezin EM, Pravenec M, Wong A, Wang JM, Merriouns T, Newton S, Stec DE, Roman RJ, Lau D, Morris RC. Genetic contamination of Dahl SS/Jr rats. Impact on studies of salt-sensitive hypertension. Hypertension 23: 786–790, 1994. doi: 10.1161/01.HYP.23.6.786. [DOI] [PubMed] [Google Scholar]
- 48.Sterzel RB, Luft FC, Gao Y, Schnermann J, Briggs JP, Ganten D, Waldherr R, Schnabel E, Kriz W. Renal disease and the development of hypertension in salt-sensitive Dahl rats. Kidney Int 33: 1119–1129, 1988. doi: 10.1038/ki.1988.120. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 293: R1573–R1579, 2007. doi: 10.1152/ajpregu.00429.2007. [DOI] [PubMed] [Google Scholar]
- 50.Sustarsic DL, McPartland RP, Rapp JP. Developmental patterns of blood pressure and urinary protein, kallikrein, and prostaglandin E2 in Dahl salt-hypertension-susceptible rats. J Lab Clin Med 98: 599–606, 1981. [PubMed] [Google Scholar]
- 51.Thrift AG, Srikanth V, Fitzgerald SM, Kalyanram K, Kartik K, Hoppe CC, Walker KZ, Evans RG. Potential roles of high salt intake and maternal malnutrition in the development of hypertension in disadvantaged populations. Clin Exp Pharmacol Physiol 37: e78–e90, 2010. doi: 10.1111/j.1440-1681.2009.05266.x. [DOI] [PubMed] [Google Scholar]
- 52.Walder RY, Morgan DA, Haynes WG, Sigmund RD, McClain AM, Stokes JB, Mark AL. Genetic characterization of the “new” Harlan Sprague Dawley Dahl salt-sensitive rats. Hypertension 27: 546–551, 1996. doi: 10.1161/01.HYP.27.3.546. [DOI] [PubMed] [Google Scholar]
- 53.Whitworth CE, Fleming S, Cumming AD, Morton JJ, Burns NJ, Williams BC, Mullins JJ. Spontaneous development of malignant phase hypertension in transgenic Ren-2 rats. Kidney Int 46: 1528–1532, 1994. doi: 10.1038/ki.1994.437. [DOI] [PubMed] [Google Scholar]
- 54.Wu Y, Takahashi H, Suzuki E, Kruzliak P, Soucek M, Uehara Y. Impaired response of regulator of Gαq signaling-2 mRNA to angiotensin II and hypertensive renal injury in Dahl salt-sensitive rats. Hypertens Res 39: 210–216, 2016. doi: 10.1038/hr.2015.132. [DOI] [PubMed] [Google Scholar]
- 55.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 296: F771–F779, 2009. doi: 10.1152/ajprenal.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yue TL, Vickery-Clark L, Louden CS, Gu JL, Ma XL, Narayanan PK, Li X, Chen J, Storer B, Willette R, Gossett KA, Ohlstein EH. Selective estrogen receptor modulator idoxifene inhibits smooth muscle cell proliferation, enhances reendothelialization, and inhibits neointimal formation in vivo after vascular injury. Circulation 102, Suppl 3: III281–III288, 2000. doi: 10.1161/01.CIR.102.suppl_3.III-281. [DOI] [PubMed] [Google Scholar]
- 57.Zheng W, Ji H, Maric C, Wu X, Sandberg K. Effect of dietary sodium on estrogen regulation of blood pressure in Dahl salt-sensitive rats. Am J Physiol Heart Circ Physiol 294: H1508–H1513, 2008. doi: 10.1152/ajpheart.01322.2007. [DOI] [PubMed] [Google Scholar]
- 58.Zicha J, Dobešová Z, Vokurková M, Rauchová H, Hojná S, Kadlecová M, Behuliak M, Vaněčková I, Kuneš J. Age-dependent salt hypertension in Dahl rats: fifty years of research. Physiol Res 61, Suppl 1: S35–S87, 2012. [DOI] [PubMed] [Google Scholar]
- 59.Zimmerman MA, Lindsey SH. Inconsistent blood pressure phenotype in female Dahl salt-sensitive rats. Am J Physiol Renal Physiol 311: F1391–F1392, 2016. doi: 10.1152/ajprenal.00454.2016. [DOI] [PubMed] [Google Scholar]