Abstract
We examined the control of breathing by O2 and CO2 in deer mice native to high altitude to help uncover the physiological specializations used to cope with hypoxia in high-altitude environments. Highland deer mice (Peromyscus maniculatus) and lowland white-footed mice (P. leucopus) were bred in captivity at sea level. The first and second generation progeny of each population was raised to adulthood and then acclimated to normoxia or hypobaric hypoxia (12 kPa O2, simulating hypoxia at ~4,300 m) for 6–8 wk. Ventilatory responses to poikilocapnic hypoxia (stepwise reductions in inspired O2) and hypercapnia (stepwise increases in inspired CO2) were then compared between groups. Both generations of lowlanders appeared to exhibit ventilatory acclimatization to hypoxia (VAH), in which hypoxia acclimation enhanced the hypoxic ventilatory response and/or made the breathing pattern more effective (higher tidal volumes and lower breathing frequencies at a given total ventilation). In contrast, hypoxia acclimation had no effect on breathing in either generation of highlanders, and breathing was generally similar to hypoxia-acclimated lowlanders. Therefore, attenuation of VAH may be an evolved feature of highlanders that persists for multiple generations in captivity. Hypoxia acclimation increased CO2 sensitivity of breathing, but in this case, the effect of hypoxia acclimation was similar in highlanders and lowlanders. Our results suggest that highland deer mice have evolved high rates of alveolar ventilation that are unaltered by exposure to chronic hypoxia, but they have preserved ventilatory sensitivity to CO2.
Keywords: generational effects, hypercapnic ventilatory response, hypoxia acclimation, hypoxic ventilatory response
INTRODUCTION
Animals native to high altitude can provide insight into the evolution of complex physiological systems because they have often adapted to the stressors associated with this challenging environment. High altitude is both cold and hypoxic, which challenges the ability of endotherms to maintain O2 supply for thermoregulation and exercise. However, many human, other mammal, and bird populations live, reproduce, and exercise at high altitude, and the emerging evidence suggests that they have overcome the challenges of this environment through evolved changes in the O2 transport cascade (32, 59). The function of this cascade, composed of ventilation, pulmonary diffusion, circulation, tissue diffusion, and cellular O2 utilization, relies on adequate rates of ventilation to maintain tissue O2 supply; therefore, increasing breathing is critical for O2 uptake in hypoxic environments (59).
Breathing is stimulated by reductions in arterial O2 levels at high altitude. Ventilation increases in response to acute hypoxia challenge, a process termed the hypoxic ventilatory response (HVR). Peripheral chemoreceptors in the carotid bodies sense reductions in the partial pressure of O2 (Po2) in arterial blood, which initiates the hypoxic chemoreflex that results in the HVR (12, 46). Breathing and ventilatory O2 chemosensitivity is further enhanced with prolonged exposure to hypoxia over days to weeks, a process termed ventilatory acclimatization to hypoxia, (VAH). VAH is believed to result from increases in chemosensitivity of the carotid bodies and from increases in central gain of the afferent signals transmitted from the carotid bodies to the brainstem (40, 41, 48, 62). The resulting increases in ventilation improve O2 uptake by increasing alveolar and arterial Po2 (17).
Breathing at high altitude is also modulated by arterial CO2 levels. The increases in ventilation that act to minimize the fall in arterial Po2 also lead to a decline in the partial pressure of CO2 (Pco2) (respiratory hypocapnia) (44, 52). This can reduce the CO2 chemoreflex drive to breathe, acting as feedback that inhibits the ventilatory response to environmental hypoxia (44). As a result, the HVR measured in poikilocapnic (uncontrolled CO2) conditions is generally lesser in magnitude than when the HVR is measured under isocapnic conditions (when arterial Pco2 is experimentally maintained) (34, 53). Furthermore, exposure to chronic hypoxia can increase ventilatory CO2 sensitivity (9, 10, 29, 49, 57). Therefore, CO2 sensitivity can have a strong influence on breathing and O2 uptake in individuals at high altitude.
How has the control of breathing been adjusted in high-altitude natives? The isocapnic HVR as a measure of O2 chemosensitivity has been examined in some studies of highland human populations, but the HVR of most other highland species has been examined in poikilocapnic conditions. Nevertheless, the literature suggests that highland natives can differ from lowland natives in divergent ways, with some highlanders exhibiting similar or enhanced ventilatory responses (24, 42, 53) and others exhibiting a blunted HVR (20, 23, 50). However, in these studies, it has often been difficult to distinguish uniquely evolved differences in highland taxa from developmental or multigenerational effects of exposure to hypoxia (2, 33). Much less is known about CO2 sensitivity of breathing in highland taxa. Highland humans appear to have a reduced ventilatory sensitivity to CO2 (54, 55), but it is unclear whether other high-altitude taxa exhibit a similar or distinct pattern of CO2 sensitivity.
The objective of this study was to examine how the control of breathing by O2 and CO2 has evolved in high-altitude populations of deer mice (Peromyscus maniculatus). Deer mice are broadly distributed across North America and can be found from sea level to over 4,300 m elevation in the Rocky Mountains (16, 38, 56). High-altitude populations must sustain high metabolic rates in the wild (13), and they have evolved a higher aerobic capacity (V̇o2max) in hypoxia than their low-altitude counterparts (3, 4, 27, 60) in association with changes in hemoglobin-O2 affinity, cardiac function, muscle capillarity and metabolic phenotype, and tissue gene expression (6, 27, 28, 39, 51, 56, 58, 60, 61). We recently found that the control of breathing also differs in high-altitude native deer mice compared with a congeneric species from low altitude (white-footed mouse, P. leucopus) in a study of animals that were born and raised in captivity at sea level but were the first generation progeny of wild parents (18). Specifically, we found that highlanders do not appear to exhibit VAH in contrast with the robust VAH exhibited by lowlanders but that highlanders have a fixed breathing pattern that is similar to hypoxia-acclimated lowlanders (18). However, because these observations were made in a first generation progeny, it was unclear whether they resulted from an evolved difference in highlanders or from persistent multigenerational effects of the wild parents being born and raised in different native environments. Here, we sought to examine these possibilities by studying mice from both the first and second generations raised in captivity. We also sought to determine whether variation in CO2 sensitivity has evolved in high-altitude mice and whether this might contribute to the apparent differences in breathing during poikilocapnic hypoxia and in VAH.
MATERIALS AND METHODS
Mouse populations.
Wild adult mice were live-trapped at low altitude on the Great Plains (Nine Mile Prairie, Lancaster County, NE, at 40°52′12″ N, 96″48′20.3″ W, 430 m above sea level) (P. leucopus) and at high altitude on the summit of Mount Evans (Clear Creek County, CO, at 39°35′18″N, 105°38′38″ W, 4,350 m above sea level) (P. maniculatus rufinus) and were then transported to McMaster University (Hamilton, Canada; ~50 m above sea level) and held in captivity. Mice were bred within each population in common conditions to produce a first generation (G1) progeny. This was accomplished by putting breeding pairs in individual cages, removing the male when the female was visibly pregnant, and weaning the born pups and moving them to a separate cage at 21 days after birth. G1 mice were similarly bred within each population to produce a second generation (G2) progeny. The experiments here were conducted on several independent families of G1 mice (3 lowland and 5 highland families) and G2 mice (4 lowland and 4 highland families). We did not determine the relatedness of the wild mice used to establish our breeding colonies, but deer mice were abundant and population sizes are large at each trapping site, so it is likely that the breeders in our colony were unrelated and represented a good proportion of the genetic diversity of the wild parental populations. All captive-born progenies were held in standard holding conditions (24°C–25°C, 12:12 light-dark photoperiod) with unlimited access to food and water and were raised in ambient conditions (sea level normoxia) until 6 mo of age before experiments were conducted. All animal protocols followed guidelines established by the Canadian Council on Animal Care and were approved by the McMaster University Animal Research Ethics Board.
Acclimation groups.
To assess the influence of the native population and acclimation environment, mice were chronically exposed to 1) standard normobaric normoxia or 2) hypobaric hypoxia, simulating the pressure at an elevation of ~4,300 m (barometric pressure of 60 kPa, Po2 ~12.5 kPa). Specially designed hypobaric chambers were used for exposure to chronic hypoxia, as previously described (18, 30). Mice in hypobaric hypoxia were temporarily returned to normobaric conditions twice per week for <20 min for cage cleaning. Ventilatory measurements were carried out after 6–8 wk of exposure.
Acute hypoxia responses.
We examined the effects of hypoxia acclimation on the response to acute hypoxia in both G1 and G2 mice from each population. Breathing and O2 consumption rates (V̇o2) were measured in unrestrained mice using barometric plethysmography and respirometry techniques that are consistent with our previous studies (18, 19). Mice were placed in a whole body plethysmograph with normoxic air (21 kPa O2, balance N2) supplied at a rate of 600 ml/min and were given 20–60 min to adjust to the chamber until relaxed and stable breathing and metabolism were observed. Measurements were then recorded for an additional 20 min at 21 kPa O2, after which mice were exposed to stepwise reductions in inspired Po2 at 16, 12, 10, 9, and 8 kPa for 20 min at each step. Incurrent gas composition was set by mixing dry compressed gases using precision flow meters (Sierra Instruments, Monterey, CA) and a mass flow controller (MFC-4, Sable Systems, Las Vegas, NV), such that the desired Po2 was delivered to the chamber at a constant rate of 600 ml/min. Body temperature (Tb) was measured at the end of the experiment using a mouse rectal probe (RET-3-ISO, Physitemp). Tb was also measured exactly 24 h later to determine normoxic Tb (this was used as a proxy for the normoxic Tb at the start of the experiment, which was not measured to prevent stress to the animal).
Breathing and V̇o2 were determined during the last 10 min at each Po2. Incurrent and excurrent air flows were subsampled at 200 ml/min. For incurrent air, O2 fraction was continuously measured using a galvanic fuel cell O2 analyzer (FC-10, Sable Systems). For excurrent air, we first measured water vapor using a thin-film capacitive water vapor analyzer (RH-300, Sable Systems) then dried the gas stream with prebaked drierite and measured O2 fraction as above and CO2 fraction using an infrared CO2 analyzer (CA-10, Sable Systems). These data were used to calculate V̇o2, expressed at standard temperature and pressure, using appropriate equations for dry air as described by Lighton (26). Chamber temperature was continuously recorded with a thermocouple (TC-2000, Sable Systems). Breathing frequency and tidal volume were measured from changes in flow across a pneumotachograph in the plethysmograph wall, detected using a differential pressure transducer (Validyne DP45, Cancopass, Mississauga, Canada). Tidal volume was calculated using established equations (7, 21) assuming a constant rate of decline in Tb with declining Po2, which we have previously shown results in similar tidal volumes to those calculated using direct Tb measurements at each Po2 (19). Total ventilation was determined as the product of breathing frequency and tidal volume. Total ventilation and tidal volume data are expressed at standard temperature and pressure. Ventilatory O2 equivalent is the quotient of total ventilation and V̇o2. All data was acquired using a PowerLab 16/32 and Labchart 8 Pro software (ADInstruments, Colorado Springs, CO).
Mice were returned to their acclimation environment after completing the above protocol and allowed at least 2 days to recover and were then subjected to one of two protocols to measure either the acute hypoxia response in the presence of elevated inspired CO2 or acute hypercapnia responses, as described below.
Acute hypoxia responses with elevated inspired CO2.
We measured responses to acute hypoxia in the presence of modestly elevated levels of inspired CO2 in G1 mice to examine the HVR under conditions in which respiratory hypocapnia was reduced. We used the same whole body plethysmograph and the same stepwise hypoxia conditions as were used for the acute hypoxia responses that are described above, except that mice were also exposed to a constant incurrent Pco2 of 2 kPa across all acute hypoxia steps. Breathing and metabolism were determined as described above, except that CO2 fraction was measured in dry incurrent air for a few minutes at the beginning of each step (to assure a constant incurrent CO2 baseline at the desired level), after which it was measured in dry excurrent air for the remaining time at each step.
Acute hypercapnia responses.
We measured responses to acute stepwise hypercapnia in G2 mice to assess ventilatory CO2 sensitivity. We used the same whole body plethysmograph and the same conditions as were used for the measurements of acute hypoxia response that are described above, except that breathing and metabolism were measured during acute step-wise increases in environmental Pco2 at 0, 2, 4, and 6 kPa CO2. These measurements were made once in normoxia (21 kPa O2) and once in hypoxia (12 kPa O2) in two separate experiments that were conducted in a random order and separated by at least 2 days. Breathing and metabolism were determined as described above, except that CO2 fraction was continuously measured in dry incurrent air, and incurrent and excurrent air was dried and scrubbed free of CO2 with soda lime and ascarite before O2 fraction was measured. We therefore calculated V̇o2 using appropriate equations for dry and CO2-free air as described by Lighton (26).
Statistical analysis.
Two-factor ANOVA and Holm-Sidak posttests were used throughout. The main effects of acclimation environment (normoxia vs. hypoxia) and inspired gas composition (repeated measure) were evaluated within each population to determine the impacts of hypoxia acclimation on O2 or CO2 sensitivity of breathing. Values are reported as means ± SE. All statistical analysis was conducted with SigmaStat software (version 3.5) with a significance level of P < 0.05.
RESULTS
Acute hypoxia responses.
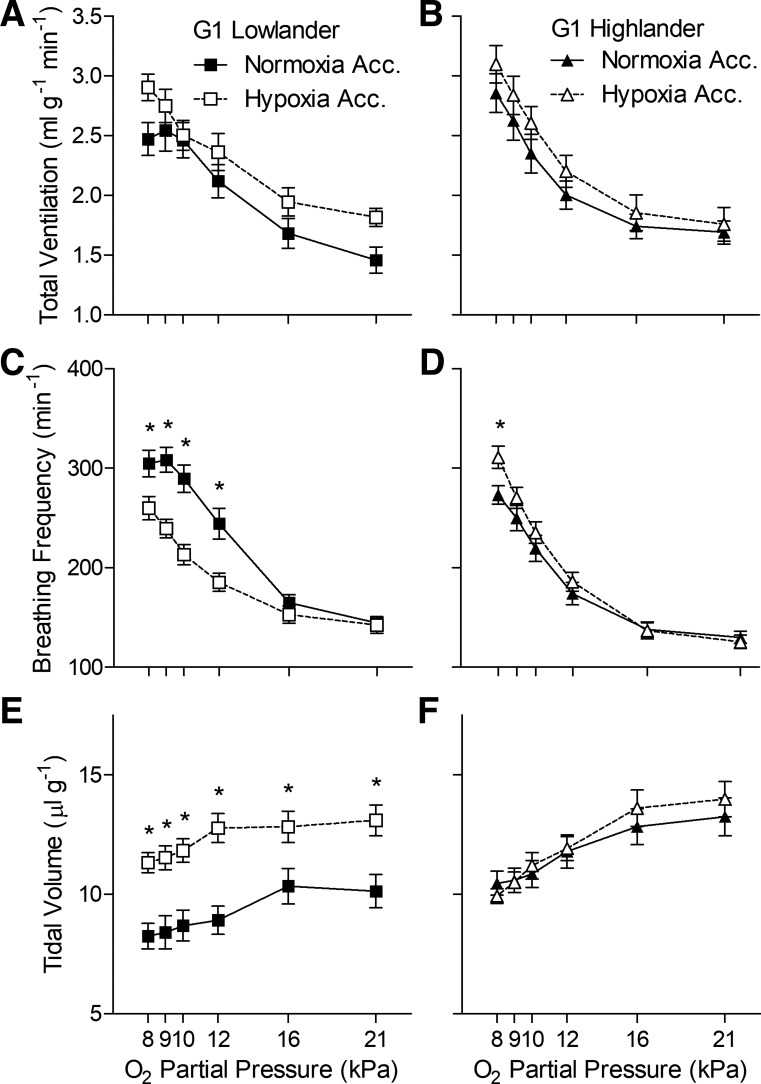
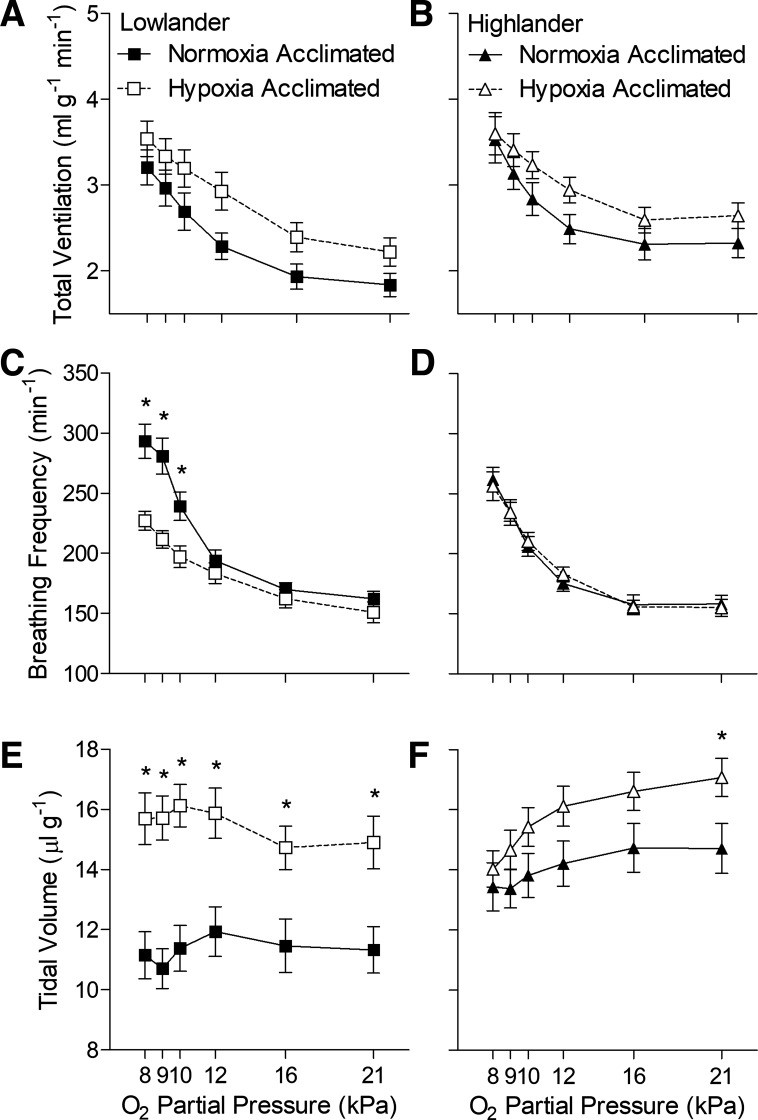
Hypoxia acclimation altered breathing in lowland mice but not in highland mice (Figs. 1 and 2, Tables 1 and 2). Among G1 mice, lowlanders exhibited a robust ventilatory response to hypoxia that was primarily driven by increases in breathing frequency, offset slightly by small decreases in tidal volume, and led to a rise in the ventilatory O2 equivalent (Fig. 1, A, C, and E, Tables 1 and 2). Hypoxia acclimation had an appreciable effect on breathing, reflected primarily by strong increases in tidal volume and reductions in breathing frequency (Fig. 1, C and E, Table 1), which offset each other such that there was no significant change in total ventilation and only a decrease ventilatory O2 equivalent at 8 kPa O2 after hypoxia acclimation (Fig. 1A, Tables 1 and 2). V̇o2 and Tb declined in response to hypoxia challenge, but hypoxia acclimation reduced the declines in Tb (Tables 1 and 2). In contrast, hypoxia acclimation had very little effect on breathing or metabolism in G1 highland mice (Fig. 1, B, D, and F, Tables 1 and 2).
Fig. 1.
Hypoxia acclimation has very little effect on breathing in highland deer mice (B, D, and F) from the first generation (G1) raised in captivity, unlike G1 mice from low altitude (A, C, and E). *Significant pairwise difference between acclimation groups within each Po2 using Holm-Sidak posttests (n as follows: 13 normoxia-acclimated lowlanders, 15 hypoxia-acclimated lowlanders, 15 normoxia-acclimated highlanders, 13 hypoxia-acclimated highlanders). Acc, acclimation.
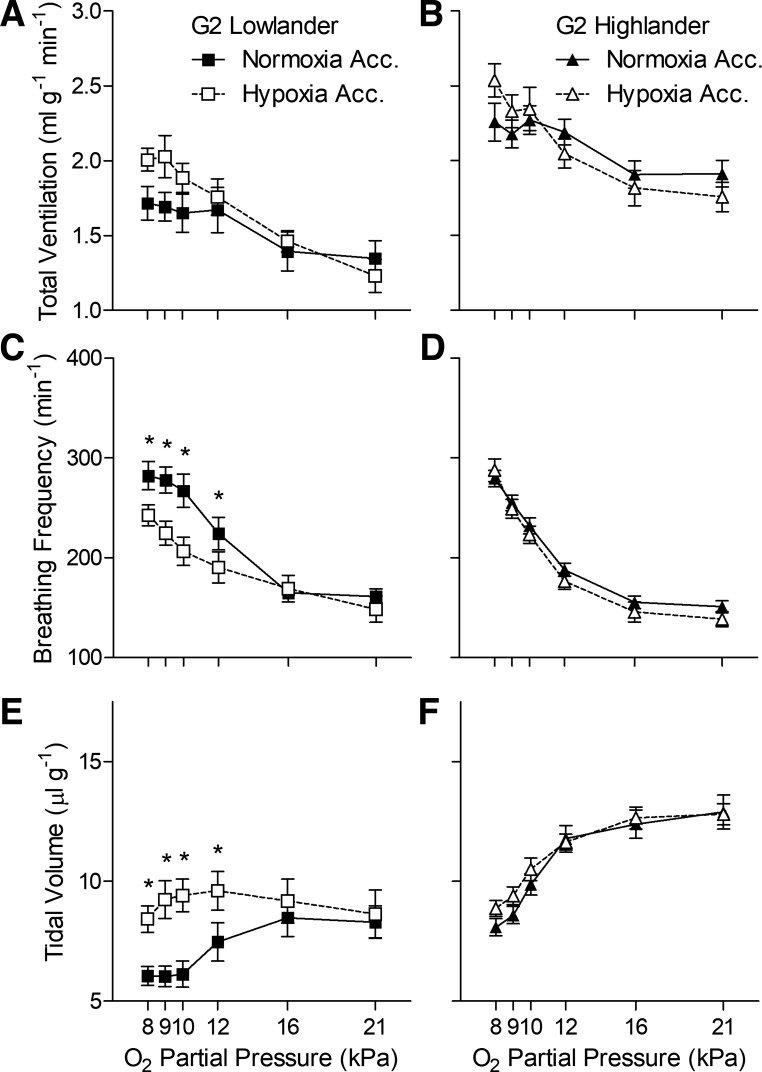
Fig. 2.
Hypoxia acclimation has no effect on breathing in highland deer mice (B, D, and F) from the second-generation (G2) raised in captivity, unlike G2 mice from low altitude (A, C, and E). *Significant pairwise difference between acclimation groups within each Po2 using Holm-Sidak posttests (n as follows: 10 normoxia-acclimated lowlanders, 9 hypoxia-acclimated lowlanders, 14 normoxia-acclimated highlanders, 14 hypoxia-acclimated highlanders). Acc, acclimation.
Table 1.
Statistical results of two-way ANOVA of acute hypoxia responses
| Acclimation Environment |
Acute Po2 |
Interaction |
||||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| First generation mice | ||||||
| Total ventilation | ||||||
| Lowlander | 2.612 | 0.118 | 78.61 | <0.001 | 1.889 | 0.101 |
| Highlander | 1.149 | 0.294 | 74.62 | <0.001 | 0.416 | 0.837 |
| Breathing frequency | ||||||
| Lowlander | 11.67 | 0.002 | 179.9 | <0.001 | 12.07 | <0.001 |
| Highlander | 1.160 | 0.291 | 286.4 | <0.001 | 3.841 | 0.003 |
| Tidal volume | ||||||
| Lowlander | 18.74 | <0.001 | 10.12 | <0.001 | 0.555 | 0.735 |
| Highlander | 0.092 | 0.764 | 31.17 | <0.001 | 0.954 | 0.449 |
| O2 consumption | ||||||
| Lowlander | 1.233 | 0.277 | 12.4 | <0.001 | 1.989 | 0.084 |
| Highlander | 0.794 | 0.381 | 22.92 | <0.001 | 2.148 | 0.064 |
| Body temperature | ||||||
| Lowlander | 6.047 | 0.021 | 196.8 | <0.001 | 8.878 | 0.006 |
| Highlander | 12.92 | 0.001 | 61.55 | <0.001 | 6.532 | 0.017 |
| Ventilatory O2 equivalent | ||||||
| Lowlander | 0.065 | 0.801 | 101.3 | <0.001 | 4.133 | 0.002 |
| Highlander | 0.015 | 0.904 | 161.9 | <0.001 | 1.168 | 0.328 |
| Second generation mice | ||||||
| Total ventilation | ||||||
| Lowlander | 1.418 | 0.251 | 17.78 | <0.001 | 2.212 | 0.061 |
| Highlander | 0.043 | 0.838 | 24.67 | <0.001 | 3.467 | 0.006 |
| Breathing frequency | ||||||
| Lowlander | 5.799 | 0.028 | 59.74 | <0.001 | 5.089 | <0.001 |
| Highlander | 0.486 | 0.492 | 236.6 | <0.001 | 1.111 | 0.358 |
| Tidal volume | ||||||
| Lowlander | 4.830 | 0.043 | 6.258 | <0.001 | 6.296 | <0.001 |
| Highlander | 0.562 | 0.460 | 71.05 | <0.001 | 0.979 | 0.433 |
| O2 consumption | ||||||
| Lowlander | 0.827 | 0.377 | 17.84 | <0.001 | 0.945 | 0.457 |
| Highlander | 0.016 | 0.900 | 27.12 | <0.001 | 4.900 | <0.001 |
| Body temperature | ||||||
| Lowlander | 0.057 | 0.815 | 143.0 | <0.001 | 2.486 | 0.134 |
| Highlander | 6.126 | 0.020 | 32.53 | <0.001 | 4.640 | 0.041 |
| Ventilatory O2 equivalent | ||||||
| Lowlander | 0.079 | 0.782 | 95.14 | <0.001 | 0.366 | 0.871 |
| Highlander | 0.012 | 0.913 | 85.07 | <0.001 | 0.562 | 0.729 |
One degree of freedom for acclimation environment, 5 degrees of freedom for acute Po2 and their interaction, 130 degrees of freedom for the residuals of first generation lowland and highland mice and second generation highland mice, and 80 degrees of freedom for the residuals of second generation lowland mice. Boldface denotes significant P values.
Table 2.
Rate of O2 consumption and body temperature during acute hypoxia exposure in first generation laboratory-raised mice
| Lowland Peromyscus leucopus |
Highland Peromyscus maniculatus |
|||
|---|---|---|---|---|
| Acute Po2, kPa | Normoxia-acclimated | Hypoxia-acclimated | Normoxia-acclimated | Hypoxia-acclimated |
| O2 consumption rate, ml·g−1·min−1 | ||||
| 21 | 0.045 ± 0.004 | 0.045 ± 0.003 | 0.048 ± 0.003 | 0.045 ± 0.003 |
| 16 | 0.043 ± 0.004 | 0.040 ± 0.002 | 0.039 ± 0.003 | 0.039 ± 0.004 |
| 12 | 0.039 ± 0.003 | 0.041 ± 0.003 | 0.035 ± 0.002 | 0.037 ± 0.002 |
| 10 | 0.035 ± 0.003 | 0.037 ± 0.002 | 0.032 ± 0.002 | 0.038 ± 0.002 |
| 9 | 0.031 ± 0.002 | 0.038 ± 0.002 | 0.031 ± 0.002 | 0.037 ± 0.002 |
| 8 | 0.027 ± 0.002 | 0.036 ± 0.002 | 0.030 ± 0.002 | 0.033 ± 0.002 |
| Ventilatory O2 equivalent, ml air/ml O2 | ||||
| 21 | 33.97 ± 2.58 | 41.20 ± 1.79 | 35.94 ± 1.49 | 39.16 ± 2.22 |
| 16 | 40.43 ± 2.09 | 48.95 ± 2.23 | 44.93 ± 1.95 | 47.77 ± 2.27 |
| 12 | 55.86 ± 2.74 | 58.28 ± 2.24 | 57.13 ± 2.66 | 59.31 ± 1.77 |
| 10 | 71.97 ± 3.32 | 67.79 ± 3.18 | 74.18 ± 2.68 | 70.28 ± 3.96 |
| 9 | 81.87 ± 4.53 | 73.46 ± 2.51 | 84.98 ± 2.69 | 79.22 ± 4.17 |
| 8 | 92.26 ± 5.50 | 82.31 ± 2.81* | 97.92 ± 3.84 | 95.21 ± 5.16 |
| Body temperature, °C | ||||
| 21 | 37.29 ± 0.29 | 37.25 ± 0.24 | 36.08 ± 0.24 | 36.49 ± 0.23 |
| 8 | 33.52 ± 0.21 | 34.80 ± 0.21* | 33.60 ± 0.23 | 35.20 ± 0.34* |
Values are means ± SE. Po2, partial pressure of O2.
Significant pairwise difference from normoxia-acclimated mice of the same population.
The diminished effects of hypoxia acclimation in G1 highland mice were also observed in G2 highland mice (Fig. 2, Tables 1 and 3). Hypoxia acclimation had a strong effect on breathing in G2 lowlanders, as it did in G1 lowlanders, as reflected by significant increases in tidal volume (Fig. 2E) and reductions in breathing frequency (Fig. 2C) during acute hypoxia. Interestingly, there appeared to be some variation in breathing across generations in lowlanders, which tended to have lower total ventilation, tidal volume, and ventilatory O2 equivalent in G2 than in G1. Nevertheless, even though there was some subtle variation in the magnitude of breathing and the HVR across generations, the effects of hypoxia acclimation on breathing pattern in lowlanders was generally preserved. In contrast, hypoxia acclimation had no effect on breathing or metabolism in G2 highlanders (Fig. 2, Table 2), as was observed in G1 highlanders, and the magnitude of breathing and the HVR was very similar across generations.
Table 3.
Rate of O2 consumption and body temperature during acute hypoxia exposure in second-generation laboratory-raised mice
| Lowland Peromyscus leucopus |
Highland Peromyscus maniculatus |
|||
|---|---|---|---|---|
| Acute Po2, kPa | Normoxia-acclimated | Hypoxia-acclimated | Normoxia-acclimated | Hypoxia-acclimated |
| O2 consumption rate, ml·g−1·min−1 | ||||
| 21 | 0.045 ± 0.004 | 0.044 ± 0.004 | 0.061 ± 0.002 | 0.051 ± 0.003 |
| 16 | 0.040 ± 0.003 | 0.039 ± 0.003 | 0.055 ± 0.003 | 0.052 ± 0.003 |
| 12 | 0.038 ± 0.003 | 0.039 ± 0.004 | 0.051 ± 0.003 | 0.049 ± 0.003 |
| 10 | 0.032 ± 0.003 | 0.037 ± 0.003 | 0.045 ± 0.002 | 0.049 ± 0.004 |
| 9 | 0.031 ± 0.002 | 0.035 ± 0.003 | 0.039 ± 0.001 | 0.043 ± 0.003 |
| 8 | 0.028 ± 0.002 | 0.031 ± 0.002 | 0.037 ± 0.001 | 0.042 ± 0.002 |
| Ventilatory O2 equivalent, ml air/ml O2 | ||||
| 21 | 31.03 ± 2.28 | 29.97 ± 3.99 | 31.87 ± 1.73 | 34.78 ± 1.71 |
| 16 | 35.75 ± 1.49 | 38.67 ± 2.47 | 34.84 ± 1.51 | 35.82 ± 2.12 |
| 12 | 44.96 ± 1.58 | 45.53 ± 2.53 | 43.67 ± 1.75 | 42.85 ± 2.40 |
| 10 | 53.45 ± 1.72 | 52.37 ± 2.10 | 51.37 ± 2.64 | 48.84 ± 1.81 |
| 9 | 57.85 ± 1.88 | 58.20 ± 1.56 | 55.67 ± 2.76 | 55.31 ± 3.10 |
| 8 | 64.07 ± 2.32 | 64.62 ± 2.06 | 61.38 ± 3.47 | 62.34 ± 4.55 |
| Body temperature, °C | ||||
| 21 | 37.63 ± 0.33 | 37.45 ± 0.22 | 36.23 ± 0.29 | 36.58 ± 0.30 |
| 8 | 34.30 ± 0.19 | 34.70 ± 0.30 | 34.50 ± 0.23 | 35.80 ± 0.31* |
Values are means ± SE. Po2, partial pressure of O2.
Significant pairwise difference from normoxia-acclimated mice of the same population.
Hypercapnic ventilatory response.
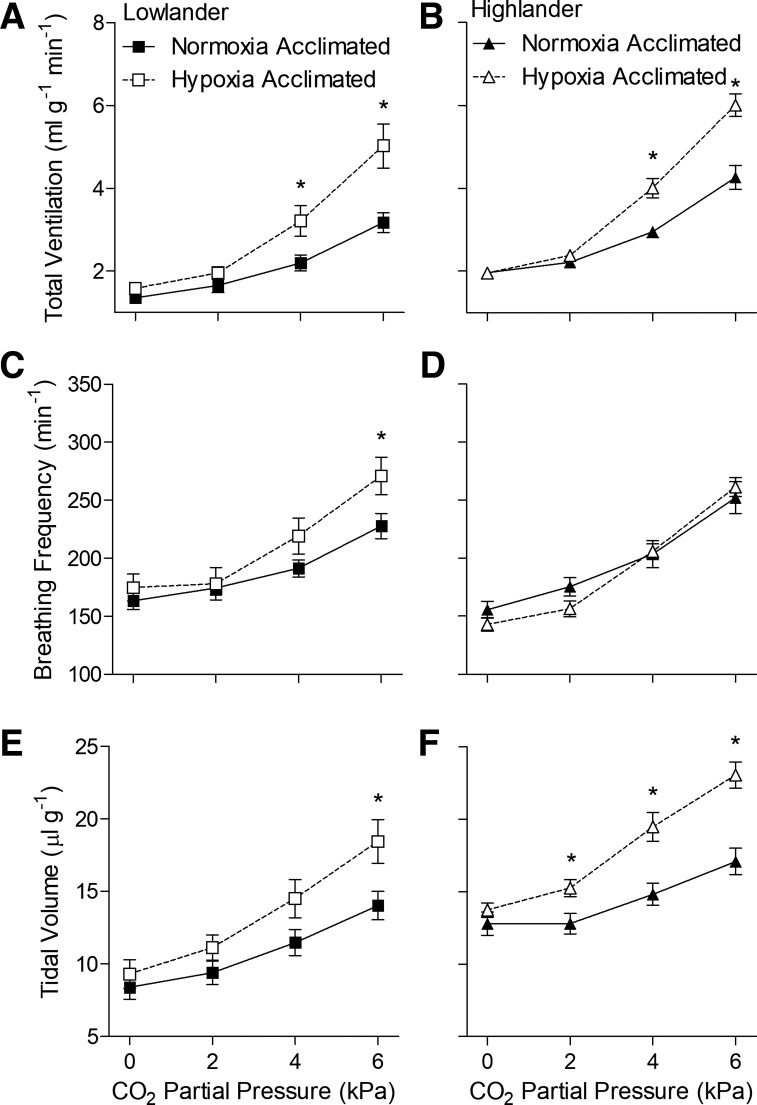
We next sought to determine whether hypoxia acclimation increases CO2 sensitivity of breathing and whether this effect of hypoxia acclimation is altered in highlanders. We examined these possibilities by measuring the ventilatory response to stepwise hypercapnia in normoxia and hypoxia. We found that hypoxia acclimation enhanced the hypercapnic ventilatory response in both lowland and highland mice when tested in normoxic conditions (Fig. 3, Table 4). Both lowlanders and highlanders exhibited similar robust ventilatory responses to increasing CO2 that were driven by increases in both tidal volume and breathing frequency (Fig. 3, Table 4). This response was augmented similarly after hypoxia acclimation in both populations, particularly at higher CO2 levels, because of further increases in tidal volume in both populations and breathing frequency in lowlanders. V̇o2 and Tb were not altered by acute hypercapnia or by hypoxia acclimation in either population (Table 4, data not shown).
Fig. 3.
Hypoxia acclimation increased ventilatory sensitivity to CO2 in both lowland (A, C, and E) and highland (B, D, and F) mice when measured in normoxic conditions (21 kPa O2). Measurements were made on second generation (G2) mice. *Significant pairwise difference between acclimation groups within each Pco2 using Holm-Sidak posttests (n as follows: 10 normoxia-acclimated lowlanders, 9 hypoxia-acclimated lowlanders, 13 normoxia-acclimated highlanders, 14 hypoxia-acclimated highlanders).
Table 4.
Statistical results of two-way ANOVA of acute hypercapnia responses, measured in normoxia or hypoxia
| Acclimation Environment |
Acute Pco2 |
Interaction |
||||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| Normoxic conditions (21 kPa O2) | ||||||
| Total ventilation | ||||||
| Lowlander | 7.437 | 0.014 | 92.07 | <0.001 | 9.496 | <0.001 |
| Highlander | 11.68 | 0.002 | 249.3 | <0.001 | 20.26 | <0.001 |
| Breathing frequency | ||||||
| Lowlander | 2.535 | 0.130 | 51.60 | <0.001 | 3.006 | 0.039 |
| Highlander | 0.223 | 0.641 | 181.2 | <0.001 | 3.477 | 0.020 |
| Tidal volume | ||||||
| Lowlander | 3.451 | 0.081 | 106.0 | <0.001 | 5.843 | 0.002 |
| Highlander | 12.55 | 0.002 | 120.8 | <0.001 | 15.61 | <0.001 |
| O2 consumption | ||||||
| Lowlander | 3.022 | 0.100 | 1.741 | 0.170 | 0.755 | 0.524 |
| Highlander | 0.579 | 0.454 | 1.404 | 0.248 | 0.315 | 0.815 |
| Body temperature | ||||||
| Lowlander | 0.045 | 0.835 | 5.147 | 0.037 | 0.572 | 0.460 |
| Highlander | 0.055 | 0.817 | 39.52 | <0.001 | 0.374 | 0.546 |
| Hypoxic conditions (12 kPa O2) | ||||||
| Total ventilation | ||||||
| Lowlander | 9.610 | 0.007 | 87.13 | <0.001 | 11.52 | <0.001 |
| Highlander | 4.372 | 0.047 | 118.6 | <0.001 | 4.831 | 0.004 |
| Breathing frequency | ||||||
| Lowlander | 0.088 | 0.770 | 16.40 | <0.001 | 8.088 | <0.001 |
| Highlander | 1.925 | 0.178 | 45.09 | <0.001 | 0.723 | 0.541 |
| Tidal volume | ||||||
| Lowlander | 4.916 | 0.041 | 195.6 | <0.001 | 5.279 | 0.003 |
| Highlander | 14.87 | <0.001 | 210.6 | <0.001 | 14.00 | <0.001 |
| O2 consumption | ||||||
| Lowlander | 0.001 | 0.991 | 1.020 | 0.392 | 1.262 | 0.297 |
| Highlander | 0.095 | 0.761 | 3.722 | 0.015 | 1.835 | 0.148 |
| Body temperature | ||||||
| Lowlander | 1.087 | 0.312 | 9.683 | 0.006 | 0.184 | 0.673 |
| Highlander | 0.444 | 0.511 | 24.77 | <0.001 | 0.776 | 0.387 |
One degree of freedom for acclimation environment, 3 degrees of freedom for acute Pco2 and their interaction, and 51 and 75 degrees of freedom for the residuals of lowland and highland mice, respectively, in normoxic and hypoxic conditions. Boldface denotes significant P values.
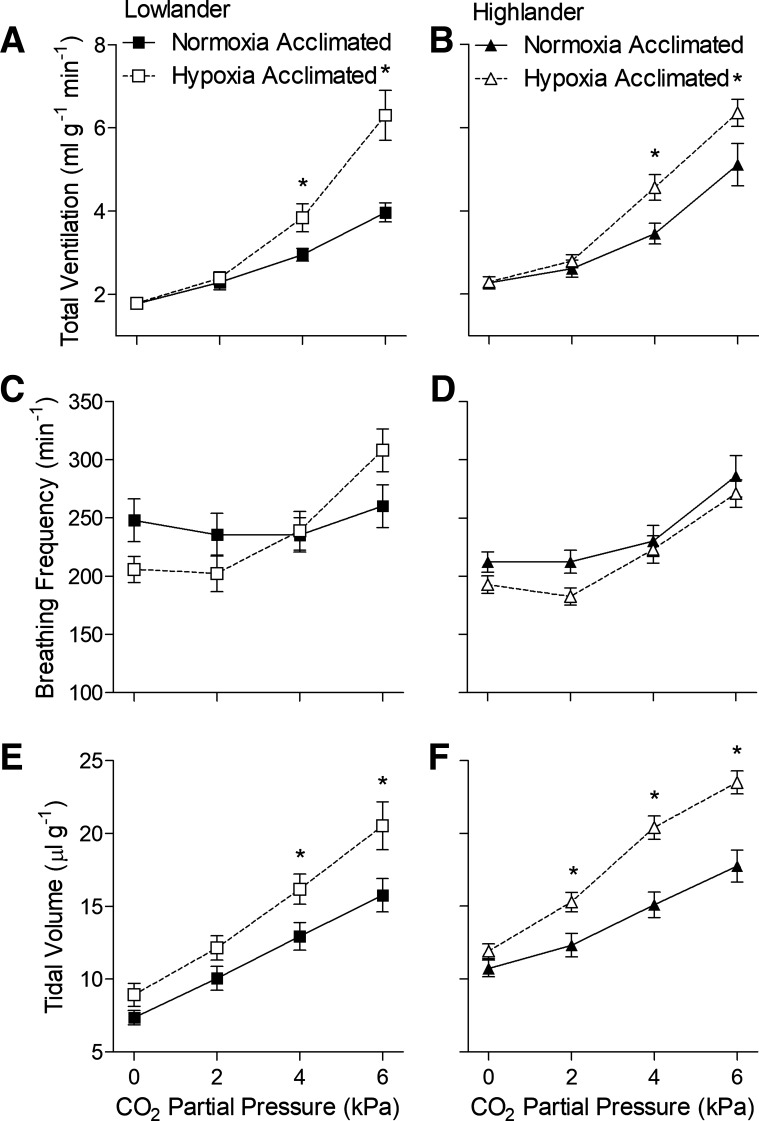
We found that hypoxia acclimation resulted in comparable increases in the hypercapnic ventilatory response when it was tested in hypoxic conditions (12 kPa O2) (Fig. 4, Table 4). The response to acute hypercapnia was similar to that observed in normoxic conditions, except that ventilation was higher overall due to hypoxia. Hypoxia acclimation augmented the hypercapnic ventilatory response measured in hypoxia in both populations, as observed for the hypercapnic ventilatory response measured in normoxia, but in this case, the increases in ventilation were entirely caused by increases in tidal volume (Fig. 4, E and F). These findings suggest that hypoxia acclimation increases the CO2 sensitivity of breathing in both highland and lowland mice, but in general, there were no apparent differences in the hypercapnic ventilatory response of highlanders compared with lowlanders.
Fig. 4.
Hypoxia acclimation increased ventilatory sensitivity to CO2 in both lowland (A, C, and E) and highland (B, D, and F) mice, when measured in hypoxic conditions (12 kPa O2). Measurements were made on second generation (G2) mice. *Significant pairwise difference between acclimation groups within each Pco2 using Holm-Sidak posttests (n as in Fig. 3).
Acute hypoxia responses in 2 kPa CO2.
We sought to examine whether the apparent lack of VAH in highlanders could be a by-product of increases in ventilatory sensitivity to respiratory hypocapnia after chronic hypoxia. Given that hypoxia acclimation appears to augment CO2 sensitivity (Figs. 3 and 4), this could foreseeably augment the restraining influence of respiratory hypocapnia on the poikilocapnic HVR and thus offset other effects of chronic hypoxia that stimulate breathing and would tend to cause VAH. We examined this possibility by measuring the HVR during moderately elevated inspired CO2 (2 kPa CO2) to offset respiratory hypocapnia with the prediction that increases in CO2 would amplify the effects of chronic hypoxia on breathing. There was some modest support for this prediction, as reflected by the apparent increase in the magnitude of the effects of hypoxia acclimation on average in both populations (compare the variation in tidal volume in Fig. 5 with Fig. 1). In highlanders in particular, hypoxia acclimation increased tidal volume at the higher Po2 values when measured in the presence of 2 kPa CO2 (Fig. 5F, Table 5). However, hypoxia acclimation still had a much smaller effect on breathing in highlanders than in lowlanders, and there were still no significant main effects of hypoxia acclimation on total ventilation, breathing frequency, or tidal volume in highlanders (Table 5). Otherwise, the effects of acute hypoxia on breathing and metabolism in the presence of 2 kPa CO2 were quite similar to those observed without CO2 in the inspired gas (Tables 5 and 6). Therefore, the apparent lack of VAH in highlanders cannot be explained by variation in the CO2 sensitivity of breathing.
Fig. 5.
Hypoxic ventilatory responses measured in the presence of moderately elevated levels of inspired CO2 (2 kPa). Measurements were made on first generation (G1) lowlander (A, C, and E) and highlander (B, D, and F) mice. *Significant pairwise difference between acclimation groups within each Po2 using Holm-Sidak posttests (n as in Fig. 1).
Table 5.
Statistical results of two-way ANOVA of acute hypoxia responses, measured with elevated inspired CO2
| Acclimation Environment |
Acute Po2 |
Interaction |
||||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| Total ventilation | ||||||
| Lowlander | 4.082 | 0.054 | 41.97 | <0.001 | 0.451 | 0.812 |
| Highlander | 1.672 | 0.207 | 37.58 | <0.001 | 0.800 | 0.552 |
| Breathing frequency | ||||||
| Lowlander | 10.09 | 0.004 | 93.50 | <0.001 | 10.64 | <0.001 |
| Highlander | 0.001 | 0.987 | 130.7 | <0.001 | 0.428 | 0.828 |
| Tidal volume | ||||||
| Lowlander | 17.93 | <0.001 | 1.419 | 0.222 | 1.454 | 0.209 |
| Highlander | 3.223 | 0.084 | 11.74 | <0.001 | 1.422 | 0.220 |
| O2 consumption | ||||||
| Lowlander | 0.656 | 0.425 | 1.226 | 0.300 | 0.237 | 0.946 |
| Highlander | 3.637 | 0.068 | 10.99 | <0.001 | 0.898 | 0.484 |
| Body temperature | ||||||
| Lowlander | 7.929 | 0.009 | 97.30 | <0.001 | 0.244 | 0.625 |
| Highlander | 7.939 | 0.009 | 36.55 | <0.001 | 8.415 | 0.007 |
One degree of freedom for acclimation environment, 5 degrees of freedom for acute Po2 and their interaction, and 130 degrees of freedom for the residuals of lowland and highland mice. Boldface denotes significant P values.
Table 6.
Rate of O2 consumption and body temperature during acute hypoxia exposure, measured with elevated inspired CO2
| Lowland Peromyscus leucopus |
Highland Peromyscus maniculatus |
|||
|---|---|---|---|---|
| Acute Po2, kPa | Normoxia-acclimated | Hypoxia-acclimated | Normoxia-acclimated | Hypoxia-acclimated |
| O2 consumption rate, ml·g−1·min−1 | ||||
| 21 | 0.039 ± 0.003 | 0.041 ± 0.003 | 0.052 ± 0.005 | 0.054 ± 0.003 |
| 16 | 0.037 ± 0.003 | 0.038 ± 0.002 | 0.042 ± 0.003 | 0.046 ± 0.002 |
| 12 | 0.037 ± 0.002 | 0.041 ± 0.002 | 0.039 ± 0.003 | 0.048 ± 0.003 |
| 10 | 0.039 ± 0.003 | 0.039 ± 0.002 | 0.039 ± 0.002 | 0.046 ± 0.003 |
| 9 | 0.037 ± 0.002 | 0.040 ± 0.002 | 0.039 ± 0.002 | 0.047 ± 0.004 |
| 8 | 0.035 ± 0.002 | 0.038 ± 0.002 | 0.036 ± 0.002 | 0.044 ± 0.004 |
| Body temperature, °C | ||||
| 21 | 36.94 ± 0.35 | 37.92 ± 0.28 | 36.49 ± 0.22 | 36.36 ± 0.17 |
| 8 | 34.11 ± 0.35 | 34.79 ± 0.24 | 34.30 ± 0.25 | 35.60 ± 0.23* |
Values are means ± SE. Po2, partial pressure of O2.
Significant pairwise difference from normoxia-acclimated mice of the same population.
DISCUSSION
Effective control of ventilation is critically important for small endotherms in the O2-limited environment at high altitude to maintain adequate tissue O2 supply for thermogenesis and exercise. Previously, we observed that hypoxia acclimation had little effect on breathing and the HVR of high-altitude native deer mice at levels of chronic hypoxia that did induce a VAH response in lowland mice (18). Here, we show that the apparent blunting of VAH is observed across multiple generations of laboratory-raised highland mice, suggesting that this blunting has evolved in response to the challenges of life at high altitude. This blunting was not associated with any evolved change in the effects of hypoxia acclimation on CO2 sensitivity of breathing in highland mice. As a consequence, variation in the ventilatory sensitivity to respiratory hypocapnia does not appear to contribute to the attenuation of VAH in highlanders.
VAH is attenuated in high-altitude native deer mice.
Our findings suggest that the apparent lack of VAH in high-altitude native deer mice may result from an evolved change in the magnitude of hypoxia-induced plasticity of breathing. In previous studies, it has been challenging to establish whether changes in the control of breathing in highland taxa are evolved and genetically based because it has often been difficult to exclude the influence of developmental and/or parental exposure to different environments (2, 33). The blunted VAH we previously reported in first generation highlanders raised in captivity suggested that this blunting cannot be explained by differences in the developmental environment (18). However, it was possible that these differences between populations of first generation mice could be explained by exposure of parents and/or germ cells to different environments. For example, exposure of parents and their germline cells to hypoxia has persistent effects on hypoxia tolerance in offspring in zebrafish (15). However, the persistent lack of VAH in the second generation of highlanders raised in captivity cannot be attributed to the exposure of parents and germline cells to the high-altitude environment. There might have been some effect of the native low-altitude environment in lowlanders, based on our observations that breathing tended to decline slightly from G1 to G2, but this did not affect the expression of VAH in lowlanders. The attenuation of VAH in highlanders is more likely to be an evolved trait and could also be influenced by transgenerational epigenetic effects.
Our understanding of the potential importance of transgenerational epigenetic effects on cardiorespiratory physiology at high altitude is still in its infancy (1, 17, 37). There is known to be epigenetic regulation of cardiorespiratory physiology and carotid body function in response to chronic exposure to intermittent hypoxia in adulthood or early development (35, 36), but it is unknown whether such effects can persist across generations. Effects of some other environmental stressors are known to persist across multiple generations [e.g., transgenerational effects of pollutants on survival and development in zebrafish (5)], but it is unclear whether chronic hypoxia can have a similarly persistent effect. Disentangling the relative importance of genetically and epigenetically based changes in adaptive phenotypes at high altitude will be an exciting area for future research.
What are the mechanisms that account for blunting of the VAH in high-altitude native deer mice? In lowlanders, VAH arises from adjustments in the carotid bodies and the central nervous system. Chronic hypoxia enhances O2 chemosensitivity of the carotid bodies, which appears to be associated with neovascularization and growth of the organ, and changes in O2 signaling by O2-sensitive glomus cells (22, 47, 62). Some of these adjustments appear to be attenuated in highland deer mice, as reflected by our previous observation that highlanders do not exhibit carotid body growth in response to chronic hypoxia (18). Chronic hypoxia also leads to increases in central gain of the afferent signals from the carotid body in lowlanders (e.g., changes in glutamatergic signaling in the nucleus of the solitary tract) (40, 48), and it is possible that these mechanisms are also attenuated in highland mice. However, before carrying out the current study, we could not exclude the possibility that the apparent blunting in VAH arose from a variation in the effects of CO2 on breathing because the HVR was measured under poikilocapnic conditions. Our results here suggest that this is not the case because highlanders still exhibited a blunted VAH when respiratory hypocapnia was alleviated by exposure to moderately elevated, inspired CO2 (Fig. 5). This likely implies that VAH and its underlying peripheral and/or central mechanisms are indeed blunted in highland mice. This does not appear to have altered the effects of chronic hypoxia on hypoxic anapyrexia because the magnitude of Tb depression in response to acute hypoxia was still reduced after hypoxia acclimation in highland mice (Tables 2 and 3).
Effects of chronic hypoxia on the CO2 sensitivity of breathing.
Hypoxia acclimation increased the CO2 sensitivity of breathing, driven primarily by larger increases in tidal volume in response to high CO2. This observation is consistent with previous observations in humans in which chronic hypoxia increases ventilatory CO2 sensitivity and/or lowers the recruitment threshold above which blood CO2 stimulates ventilation (9, 10, 54). Chronic hypoxia is well known to induce mechanisms of acid-based compensation to counter respiratory hypocapnia and alkalosis (45), so it is possible that apparent changes in the CO2 sensitivity of breathing arise from changes in the relationship between CO2 and pH or in pH buffering of the blood (9, 10). It is also possible that increases in ventilatory CO2 sensitivity in response to chronic hypoxia arise from increases in the chemosensitivity of peripheral or central CO2/pH chemoreceptors, as suggested by previous studies in humans (11). If this is also the case in deer mice, then the mechanisms likely do not depend upon hypoxia-induced growth of the carotid bodies, which occurs in lowlanders but not in highlanders (18).
Ventilatory sensitivity to CO2 appeared to be similar in high-altitude mice and their low-altitude counterparts, both before and after hypoxia acclimation. This contrasts previous findings in some other high-altitude taxa. Ventilatory sensitivity to CO2 and ventilatory recruitment threshold are lower in Himalayans residing at high altitude than in lowlanders at sea level (55). Similarly, ventilatory sensitivity to CO2 is lower in Andeans residing at high altitude than in lowlanders acclimatized to the same altitude for 10 days (54). In bar-headed geese, a species that flies over the Himalayas during its biannual migration, ventilatory sensitivity to hypercapnia is unaltered, but sensitivity to respiratory hypocapnia is reduced, such that bar-headed geese breathe more than low-altitude birds when exposed to poikilocapnic hypoxia (53). Therefore, there appears to be differences across highland taxa, with ventilatory sensitivity to CO2 having been either unaltered or reduced.
Perspectives and Significance
The emerging evidence suggests that there are a number of changes in the control of breathing by hypoxia in high-altitude deer mice. Our results here and in previous studies suggest that VAH and hypoxia-induced growth of the carotid bodies are attenuated in highlanders, which may represent an evolved loss of plasticity associated with high-altitude adaptation. Highlanders instead exhibit a fixed breathing pattern, characterized by deep but less frequent breaths, which should improve effective (alveolar) ventilation and thus help increase arterial O2 saturation in hypoxia (18). These changes exist without any apparent alterations in ventilatory CO2 sensitivity. An intriguing question to consider is why these putatively evolved changes have taken place. VAH increases ventilation and thus improves respiratory gas exchange, so why have high-altitude mice not maintained the VAH response that is typical of lowlanders? One possibility is that highland mice have undergone the evolutionary process of genetic assimilation, in which a phenotype that originally exhibits adaptive plasticity becomes genetically fixed (assimilated) (8, 25, 43). Another possibility that we and others have discussed previously is that there may have been an overall restructuring of the hypoxic chemoreflex in high-altitude deer mice (18, 31). It is possible that by fixing a breathing pattern that is beneficial for O2 uptake, highlanders may avoid some costs associated with plasticity in response to chronic hypoxia at high altitude (e.g., chronic sympathetic activation, etc.). Given the harshness of high-altitude environments and the correspondingly strong selection favoring respiratory performance (14), evolved changes in control of breathing may help safeguard O2 uptake and contribute to the success and high abundance of deer mice in high-altitude environments.
GRANTS
The equipment and operational costs of this research were supported by funds from McMaster University, the Canadian Foundation for Innovation, the Ontario Ministry of Research and Innovation, and a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to G. R. Scott. C. M. Ivy was supported by a NSERC Postgraduate Scholarship and an Ontario Graduate Scholarship. G. R. Scott is supported by the Canada Research Chairs Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.M.I. and G.R.S. conceived and designed research; C.M.I. performed experiments; C.M.I. analyzed data; C.M.I. and G.R.S. interpreted results of experiments; C.M.I. and G.R.S. prepared figures; C.M.I. drafted manuscript; C.M.I. and G.R.S. edited and revised manuscript; C.M.I. and G.R.S. approved final version of manuscript.
REFERENCES
- 1.Brown CJ, Rupert JL. Hypoxia and environmental epigenetics. High Alt Med Biol 15: 323–330, 2014. doi: 10.1089/ham.2014.1016. [DOI] [PubMed] [Google Scholar]
- 2.Brutsaert T. Why are high altitude natives so strong at high altitude? Nature vs. nurture: Genetic factors vs. growth and development. Adv Exp Med Biol 903: 101–112, 2016. doi: 10.1007/978-1-4899-7678-9_7. [DOI] [PubMed] [Google Scholar]
- 3.Cheviron ZA, Bachman GC, Connaty AD, McClelland GB, Storz JF. Regulatory changes contribute to the adaptive enhancement of thermogenic capacity in high-altitude deer mice. Proc Natl Acad Sci USA 109: 8635–8640, 2012. doi: 10.1073/pnas.1120523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheviron ZA, Bachman GC, Storz JF. Contributions of phenotypic plasticity to differences in thermogenic performance between highland and lowland deer mice. J Exp Biol 216: 1160–1166, 2013. doi: 10.1242/jeb.075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corrales J, Thornton C, White M, Willett KL. Multigenerational effects of benzo[a]pyrene exposure on survival and developmental deformities in zebrafish larvae. Aquat Toxicol 148: 16–26, 2014. doi: 10.1016/j.aquatox.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson NJ, Lyons SA, Henry DA, Scott GR. Effects of chronic hypoxia on diaphragm function in deer mice native to high altitude. Acta Physiol (Oxf) 223: e13030, 2018. doi: 10.1111/apha.13030. [DOI] [PubMed] [Google Scholar]
- 7.Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–87, 1955. [PubMed] [Google Scholar]
- 8.Ehrenreich IM, Pfennig DW. Genetic assimilation: a review of its potential proximate causes and evolutionary consequences. Ann Bot 117: 769–779, 2016. doi: 10.1093/aob/mcv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan J-L, Burgess KR, Basnyat R, Thomas KN, Peebles KC, Lucas SJE, Lucas RA, Donnelly J, Cotter JD, Ainslie PN. Influence of high altitude on cerebrovascular and ventilatory responsiveness to CO2. J Physiol 588: 539–549, 2010. doi: 10.1113/jphysiol.2009.184051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan J-L, Subudhi AW, Evero O, Bourdillon N, Kayser B, Lovering AT, Roach RC. AltitudeOmics: enhanced cerebrovascular reactivity and ventilatory response to CO2 with high-altitude acclimatization and reexposure. J Appl Physiol (1985) 116: 911–918, 2014. doi: 10.1152/japplphysiol.00704.2013. [DOI] [PubMed] [Google Scholar]
- 11.Fatemian M, Robbins PA. Selected contribution: chemoreflex responses to CO2 before and after an 8-h exposure to hypoxia in humans. J Appl Physiol (1985) 90: 1607–1614, 2001. doi: 10.1152/jappl.2001.90.4.1607. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829–898, 1994. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- 13.Hayes JP. Field and maximal metabolic rates of deer mice (Peromyscus maniculatus) at low and high altitudes. Physiol Zool 62: 732–744, 1989. doi: 10.1086/physzool.62.3.30157924. [DOI] [Google Scholar]
- 14.Hayes JP, O’Connor CS. Natural selection on thermogenic capacity of high-altitude deer mice. Evolution 53: 1280–1287, 1999. doi: 10.1111/j.1558-5646.1999.tb04540.x. [DOI] [PubMed] [Google Scholar]
- 15.Ho DH, Burggren WW. Parental hypoxic exposure confers offspring hypoxia resistance in zebrafish (Danio rerio). J Exp Biol 215: 4208–4216, 2012. doi: 10.1242/jeb.074781. [DOI] [PubMed] [Google Scholar]
- 16.Hock R. Physiological responses of deer mice to various native altitudes. In: The Physiological Effects of High Altitude, edited by Weihe W. New York: Macmillan, 1964, p. 59–72. doi: 10.1016/B978-1-4831-6699-5.50013-9. [DOI] [Google Scholar]
- 17.Ivy CM, Scott GR. Control of breathing and the circulation in high-altitude mammals and birds. Comp Biochem Physiol A Mol Integr Physiol 186: 66–74, 2015. doi: 10.1016/j.cbpa.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Ivy CM, Scott GR. Control of breathing and ventilatory acclimatization to hypoxia in deer mice native to high altitudes. Acta Physiol (Oxf) 221: 266–282, 2017. doi: 10.1111/apha.12912. [DOI] [PubMed] [Google Scholar]
- 19.Ivy CM, Scott GR. Ventilatory acclimatization to hypoxia in mice: methodological considerations. Respir Physiol Neurobiol 235: 95–103, 2017. doi: 10.1016/j.resp.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Ivy CM, York JM, Lague SL, Chua BA, Alza L, McCracken KG, Milsom WK, Scott GR. Validation of a pulse oximetry system for high-altitude waterfowl by examining the hypoxia responses of the Andean goose (Chloephaga melanoptera). Physiol Biochem Zool 91: 859–867, 2018. doi: 10.1086/697053. [DOI] [PubMed] [Google Scholar]
- 21.Jacky JP. Barometric measurement of tidal volume: effects of pattern and nasal temperature. J Appl Physiol Respir Environ Exerc Physiol 49: 319–325, 1980. doi: 10.1152/jappl.1980.49.2.319. [DOI] [PubMed] [Google Scholar]
- 22.Kusakabe T, Powell FL, Ellisman MH. Ultrastructure of the glomus cells in the carotid body of chronically hypoxic rats: with special reference to the similarity of amphibian glomus cells. Anat Rec 237: 220–227, 1993. doi: 10.1002/ar.1092370209. [DOI] [PubMed] [Google Scholar]
- 23.Lague SL, Chua B, Alza L, Scott GR, Frappell PB, Zhong Y, Farrell AP, McCracken KG, Wang Y, Milsom WK. Divergent respiratory and cardiovascular responses to hypoxia in bar-headed geese and Andean birds. J Exp Biol 220: 4186–4194, 2017. doi: 10.1242/jeb.168799. [DOI] [PubMed] [Google Scholar]
- 24.Lague SL, Chua B, Farrell AP, Wang Y, Milsom WK. Altitude matters: differences in cardiovascular and respiratory responses to hypoxia in bar-headed geese reared at high and low altitudes. J Exp Biol 219: 1974–1984, 2016. doi: 10.1242/jeb.132431. [DOI] [PubMed] [Google Scholar]
- 25.Lande R. Evolution of phenotypic plasticity in colonizing species. Mol Ecol 24: 2038–2045, 2015. doi: 10.1111/mec.13037. [DOI] [PubMed] [Google Scholar]
- 26.Lighton JRB. Measuring Metabolic Rates : A Manual for Scientists. New York: Oxford University, 2008, p. 1–200. doi: 10.1093/acprof:oso/9780195310610.001.0001. [DOI] [Google Scholar]
- 27.Lui MA, Mahalingam S, Patel P, Connaty AD, Ivy CM, Cheviron ZA, Storz JF, McClelland GB, Scott GR. High-altitude ancestry and hypoxia acclimation have distinct effects on exercise capacity and muscle phenotype in deer mice. Am J Physiol Regul Integr Comp Physiol 308: R779–R791, 2015. doi: 10.1152/ajpregu.00362.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahalingam S, McClelland GB, Scott GR. Evolved changes in the intracellular distribution and physiology of muscle mitochondria in high-altitude native deer mice. J Physiol 595: 4785–4801, 2017. doi: 10.1113/JP274130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathew L, Gopinath PM, Purkayastha SS, Sen Gupta J, Nayar HS. Chemoreceptor sensitivity in adaptation to high altitude. Aviat Space Environ Med 54: 121–126, 1983. [PubMed] [Google Scholar]
- 30.McClelland GB, Hochachka PW, Weber JM. Carbohydrate utilization during exercise after high-altitude acclimation: a new perspective. Proc Natl Acad Sci USA 95: 10288–10293, 1998. doi: 10.1073/pnas.95.17.10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milsom WK. Different solutions to restoring oxygen delivery at altitude. Acta Physiol (Oxf) 222: e12926, 2018. doi: 10.1111/apha.12926. [DOI] [PubMed] [Google Scholar]
- 32.Monge C, León-Velarde F. Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol Rev 71: 1135–1172, 1991. doi: 10.1152/physrev.1991.71.4.1135. [DOI] [PubMed] [Google Scholar]
- 33.Moore LG. Measuring high-altitude adaptation. J Appl Physiol (1985) 123: 1371–1385, 2017. doi: 10.1152/japplphysiol.00321.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore LG, Harrison GL, McCullough RE, McCullough RG, Micco AJ, Tucker A, Weil JV, Reeves JT. Low acute hypoxic ventilatory response and hypoxic depression in acute altitude sickness. J Appl Physiol (1985) 60: 1407–1412, 1986. doi: 10.1152/jappl.1986.60.4.1407. [DOI] [PubMed] [Google Scholar]
- 35.Nanduri J, Makarenko V, Reddy VD, Yuan G, Pawar A, Wang N, Khan SA, Zhang X, Kinsman B, Peng Y-J, Kumar GK, Fox AP, Godley LA, Semenza GL, Prabhakar NR. Epigenetic regulation of hypoxic sensing disrupts cardiorespiratory homeostasis. Proc Natl Acad Sci USA 109: 2515–2520, 2012. doi: 10.1073/pnas.1120600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nanduri J, Peng YJ, Wang N, Khan SA, Semenza GL, Kumar GK, Prabhakar NR. Epigenetic regulation of redox state mediates persistent cardiorespiratory abnormalities after long-term intermittent hypoxia. J Physiol 595: 63–77, 2017. doi: 10.1113/JP272346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nanduri J, Semenza GL, Prabhakar NR. Epigenetic changes by DNA methylation in chronic and intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol 313: L1096–L1100, 2017. doi: 10.1152/ajplung.00325.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natarajan C, Inoguchi N, Weber RE, Fago A, Moriyama H, Storz JF. Epistasis among adaptive mutations in deer mouse hemoglobin. Science 340: 1324–1327, 2013. doi: 10.1126/science.1236862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikel KE, Shanishchara NK, Ivy CM, Dawson NJ, Scott GR. Effects of hypoxia at different life stages on locomotory muscle phenotype in deer mice native to high altitudes. Comp Biochem Physiol B Biochem Mol Biol 224: 98–104, 2018. doi: 10.1016/j.cbpb.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Pamenter ME, Carr JA, Go A, Fu Z, Reid SG, Powell FL. Glutamate receptors in the nucleus tractus solitarius contribute to ventilatory acclimatization to hypoxia in rat. J Physiol 592: 1839–1856, 2014. doi: 10.1113/jphysiol.2013.268706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pardal R, Ortega-Sáenz P, Durán R, López-Barneo J. Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell 131: 364–377, 2007. doi: 10.1016/j.cell.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 42.Pichon A, Zhenzhong B, Favret F, Jin G, Shufeng H, Marchant D, Richalet J-P, Ge R-L. Long-term ventilatory adaptation and ventilatory response to hypoxia in plateau pika (Ochotona curzoniae): role of nNOS and dopamine. Am J Physiol Regul Integr Comp Physiol 297: R978–R987, 2009. doi: 10.1152/ajpregu.00108.2009. [DOI] [PubMed] [Google Scholar]
- 43.Pigliucci M, Murren CJ, Schlichting CD. Phenotypic plasticity and evolution by genetic assimilation. J Exp Biol 209: 2362–2367, 2006. doi: 10.1242/jeb.02070. [DOI] [PubMed] [Google Scholar]
- 44.Powell FL. The influence of chronic hypoxia upon chemoreception. Respir Physiol Neurobiol 157: 154–161, 2007. doi: 10.1016/j.resp.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powell FL, Fedde MR, Gratz RK, Scheid P. Ventilatory response to CO2 in birds. I. Measurements in the unanesthetized duck. Respir Physiol 35: 349–359, 1978. doi: 10.1016/0034-5687(78)90008-7. [DOI] [PubMed] [Google Scholar]
- 46.Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998. doi: 10.1016/S0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- 47.Prabhakar NR, Jacono FJ. Cellular and molecular mechanisms associated with carotid body adaptations to chronic hypoxia. High Alt Med Biol 6: 112–120, 2005. doi: 10.1089/ham.2005.6.112. [DOI] [PubMed] [Google Scholar]
- 48.Reid SG, Powell FL. Effects of chronic hypoxia on MK-801-induced changes in the acute hypoxic ventilatory response. J Appl Physiol (1985) 99: 2108–2114, 2005. doi: 10.1152/japplphysiol.01205.2004. [DOI] [PubMed] [Google Scholar]
- 49.Schoene RB, Roach RC, Hackett PH, Sutton JR, Cymerman A, Houston CS. Operation Everest II: ventilatory adaptation during gradual decompression to extreme altitude. Med Sci Sports Exerc 22: 804–810, 1990. doi: 10.1249/00005768-199012000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Schwenke DO, Bolter CP, Cragg PA. Are the carotid bodies of the guinea-pig functional? Comp Biochem Physiol A Mol Integr Physiol 146: 180–188, 2007. doi: 10.1016/j.cbpa.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 51.Scott GR, Elogio TS, Lui MA, Storz JF, Cheviron ZA. Adaptive modifications of muscle phenotype in high-altitude deer mice are associated with evolved changes in gene regulation. Mol Biol Evol 32: 1962–1976, 2015. doi: 10.1093/molbev/msv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott GR, Milsom WK. Flying high: a theoretical analysis of the factors limiting exercise performance in birds at altitude. Respir Physiol Neurobiol 154: 284–301, 2006. doi: 10.1016/j.resp.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 53.Scott GR, Milsom WK. Control of breathing and adaptation to high altitude in the bar-headed goose. Am J Physiol Regul Integr Comp Physiol 293: R379–R391, 2007. doi: 10.1152/ajpregu.00161.2007. [DOI] [PubMed] [Google Scholar]
- 54.Slessarev M, Mardimae A, Preiss D, Vesely A, Balaban DY, Greene R, Duffin J, Fisher JA. Differences in the control of breathing between Andean highlanders and lowlanders after 10 days acclimatization at 3850 m. J Physiol 588: 1607–1621, 2010. doi: 10.1113/jphysiol.2009.186064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slessarev M, Prisman E, Ito S, Watson RR, Jensen D, Preiss D, Greene R, Norboo T, Stobdan T, Diskit D, Norboo A, Kunzang M, Appenzeller O, Duffin J, Fisher JA. Differences in the control of breathing between Himalayan and sea-level residents. J Physiol 588: 1591–1606, 2010. doi: 10.1113/jphysiol.2009.185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snyder LR, Born S, Lechner AJ. Blood oxygen affinity in high- and low-altitude populations of the deer mouse. Respir Physiol 48: 89–105, 1982. doi: 10.1016/0034-5687(82)90052-4. [DOI] [PubMed] [Google Scholar]
- 57.Somogyi RB, Preiss D, Vesely A, Fisher JA, Duffin J. Changes in respiratory control after 5 days at altitude. Respir Physiol Neurobiol 145: 41–52, 2005. doi: 10.1016/j.resp.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Storz JF, Runck AM, Sabatino SJ, Kelly JK, Ferrand N, Moriyama H, Weber RE, Fago A. Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc Natl Acad Sci USA 106: 14450–14455, 2009. doi: 10.1073/pnas.0905224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Storz JF, Scott GR, Cheviron ZA. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J Exp Biol 213: 4125–4136, 2010. doi: 10.1242/jeb.048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tate KB, Ivy CM, Velotta JP, Storz JF, McClelland GB, Cheviron ZA, Scott GR. Circulatory mechanisms underlying adaptive increases in thermogenic capacity in high-altitude deer mice. J Exp Biol 220: 3616–3620, 2017. doi: 10.1242/jeb.164491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Velotta JP, Ivy CM, Wolf CJ, Scott GR, Cheviron ZA. Maladaptive phenotypic plasticity in cardiac muscle is attenuated in high-altitude deer mice. Evolution, 2018. doi: 10.1111/evo.13626. [DOI] [PubMed] [Google Scholar]
- 62.Wang Z-Y, Olson EB Jr, Bjorling DE, Mitchell GS, Bisgard GE. Sustained hypoxia-induced proliferation of carotid body type I cells in rats. J Appl Physiol (1985) 104: 803–808, 2008. doi: 10.1152/japplphysiol.00393.2007. [DOI] [PubMed] [Google Scholar]