Abstract
Morphine remains widely used in clinical settings due to its potent analgesic properties. However, one of the gravest risks of all opioids is their ability to induce respiratory depression and subsequent brain hypoxia that can lead to coma and death. Due to these life-threatening effects, our goal was to examine the effects of intravenous morphine at a wide range of doses (0.1–6.4 mg/kg) on changes in brain oxygen levels in freely moving rats. We used oxygen sensors coupled with high-speed amperometry and conducted measurements in the nucleus accumbens (NAc) and subcutaneous (SC) space, the latter serving as a proxy for blood oxygen levels that depend on respiratory activity. We also examined the effects of morphine on NAc, muscle, and skin temperature. Morphine induced dose-dependent decreases in SC oxygen levels, suggesting respiratory depression, but differential effects on NAc oxygen: increases at low and moderate doses (0.1–1.6 mg/kg) and decreases at the highest dose tested (6.4 mg/kg). Morphine also increased brain temperature at low and moderate doses but induced a biphasic, down-up change at high doses. The oxygen increases appear to result from a neurovascular coupling mechanism via local vasodilation and enhanced oxygen entry into brain tissue to compensate for blood oxygen drops caused by modest respiratory depression. At high morphine doses, this adaptive mechanism is unable to compensate for the enhanced respiratory depression, resulting in brain hypoxia. Hence, morphine appears to be safe when used as an analgesic at clinically relevant doses but poses great risks at high doses, likely to be abused by drug users.
NEW & NOTEWORTHY With the use of oxygen sensors coupled with amperometry, we show that morphine induces differential effects on brain oxygen levels, slightly increasing them at low doses and strongly decreasing them at high doses. In contrast, morphine dose dependently decreases oxygen levels in the SC space. Therefore, morphine engages opposing mechanisms affecting brain oxygen levels, enhancing them through neurovascular coupling at low, clinically relevant doses and decreasing them due to dramatic respiratory depression at high doses, likely to be abused.
Keywords: metabolism, neurovascular coupling, nucleus accumbens, opioids, oxygen electrochemistry, rats
INTRODUCTION
Respiratory depression with subsequent brain hypoxia is the most threatening effect of opioid drugs (Dahan et al. 2005; Pattinson 2008; Yeadon and Kitchen 1989). Individual opioids differ in their ability to induce respiratory depression, an effect that is dose dependent and especially strong when opioids are delivered intravenously. Whereas respiratory depression is usually minor following therapeutic use of opioid drugs and can be easily controlled in the hospital environment, this effect can be dramatic, resulting in coma and death, when highly potent opioids are used as drugs of abuse at high doses (Jaffe et al. 1997; Simon 1997). Respiratory depression appears to be the leading cause of the dramatic rise in lethality induced by heroin, fentanyl, and synthetic opioids in recent years (Compton et al. 2016).
In our previous study (Solis et al. 2017b), we used oxygen sensors coupled with amperometry to examine the changes in brain [nucleus accumbens (NAc)] oxygen induced by heroin in freely moving rats. Through intravenous delivery at a relatively low dose (0.1 mg/kg), within the range optimal for maintaining self-administration (Wise 1989), heroin strongly but transiently decreased NAc levels of oxygen, indicative of brain hypoxia. The rats injected with heroin displayed slowed and shallow breathing, suggesting respiratory depression, and this mechanism was confirmed by the monitoring of oxygen levels in the subcutaneous (SC) space—a densely vascularized area with no or minimal metabolic activity of its own. At this location, heroin strongly decreased oxygen levels, suggesting that the drop in brain oxygen is caused by a decrease in blood oxygen levels as a consequence of respiratory depression. Whereas heroin and its active metabolites reach the brain very rapidly (Gottås et al. 2013; Oldendorf et al. 1972), determining the rapidity and strength of its hypoxic effects, different changes in NAc oxygen were found with oxycodone, a less potent opioid drug with slower transport into brain tissue and more prolonged effects (Kokki et al. 2014; Lofwall et al. 2012). In contrast to heroin, oxycodone at low intravenous doses that maintain self-administration (0.15–0.3 mg/kg) modestly increased NAc oxygen levels, but at high doses (0.6–1.2 mg/kg), it strongly decreased these levels (Solis et al. 2018). Whereas these decreases are an obvious result of the drop in blood oxygen levels following respiratory depression, the mechanism underlying increases in brain oxygen induced by low- and moderate-dose oxycodone injections remains unclear. Oxygen diffusion from the arterial blood into the brain tissue is gradient dependent, thus following blood oxygen concentration changes, but oxygen entry also increases due to cerebral vasodilation induced by neural activation (Attwell et al. 2010; Fox and Raichle 1986). This latter gradient-independent mechanism appears to be responsible for phasic increases in NAc glucose induced by heroin and oxycodone (Solis et al. 2017b, 2018), and this mechanism could also explain moderate increases in oxygen induced by oxycodone at low, behaviorally relevant doses.
The primary goal of this study was to examine changes in brain oxygen in freely moving rats induced by morphine, the oldest opiate analgesic that is still widely used and can cause respiratory depression, coma, and lethality at higher doses. Similar to our previous studies, our recordings were conducted in the NAc, a deep brain structure critically involved in sensorimotor integration and a part of the brain’s motivation-reinforcement circuit (Di Chiara 2002; Wise 1989). Morphine was administered intravenously at a wide range of doses (0.1–12.8 mg/kg), from low doses, within the range of analgesia and self-administration, to large doses, within the range of possible overdose. To assess the contribution of respiratory depression in mediating morphine-induced NAc oxygen responses, we examined how morphine affects oxygen levels in the SC space, a proxy of oxygen levels in arterial blood. Finally, to clarify possible mechanisms underlying morphine-induced oxygen responses, we examined the effects of this drug on temperature recorded from the NAc, temporal muscle, and skin. This three-point temperature-recording paradigm makes it possible to evaluate the effects of drugs on brain metabolic activity assessed by intrabrain heat production and on peripheral vascular tone (Kiyatkin 2010, 2014).
MATERIALS AND METHODS
Subjects.
Twenty-three adult male Long-Evans rats (Charles River Laboratories, Wilmington, MA), weighing 460 ± 40 g at the time of surgery, were used in this study. Rats were individually housed in a climate-controlled animal colony maintained on a 12:12-h light-dark cycle with food and water available ad libitum. All procedures were approved by the National Institute on Drug Abuse, Intramural Research Program, Animal Care and Use Committee, and complied with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication 865-23).
Overview of the study.
This study describes the results of three experiments conducted in freely moving rats. In the first two experiments, we used oxygen sensors coupled with amperometry to examine how intravenous morphine affects extracellular levels of oxygen in the NAc and SC space. In the third experiment, we used the three-point thermorecording technique to examine how intravenous morphine affects temperature in the NAc, temporal muscle, and skin.
Surgical preparations.
Surgical procedures for the electrochemical experiments have been described in detail in Solis et al. (2017a). In brief, under general anesthesia (Equithesin; a mixture of sodium pentobarbital and chloral hydrate), each rat was unilaterally implanted in the right NAc (experiment 1) or SC space (experiment 2) with a guide cannula (Bioanalytical Systems, West Lafayette, IN), into which an electrochemical sensor was later inserted. Target coordinates of the recordings in the right NAc shell were the following: anterior-posterior, +1.2 mm; mediolateral, ±0.8 mm; and dorsoventral, +7.6 mm from the skull surface, according to coordinates of the rat brain atlas (Paxinos and Watson 1998). The guide cannula was secured with dental acrylic in a head mount anchored to the skull. During the same surgical procedure, rats were also implanted with a chronic jugular catheter, which ran to the head mount and was secured to the same head assembly. Rats were allowed a minimum of 4 days of postoperative recovery and at least three daily habituation sessions (~6 h each) in the environment of future recordings.
Surgical procedures for thermorecording experiments have been described in detail in Kiyatkin et al. (2014). In brief, under the same general anesthesia protocol, rats were implanted with a jugular catheter and three copper-constantan thermocouple electrodes in the NAc shell (anterior-posterior, +1.2 mm; mediolateral, ±0.8 mm; dorsoventral, +7.4 mm), temporal muscle, and subcutaneously along the nasal ridge, with the tip ~15 mm anterior to bregma. The probes were secured with dental cement to the three stainless-steel screws threaded into the skull. As shown in our previous studies (Kiyatkin 2010), by simultaneously recording temperature from these three locations, it is possible to assess the effect of a physiological or drug stimulus on intrabrain heat production, due to metabolic brain activation, and heat loss or retention, due to changes in skin vascular tone (vasoconstriction/vasodilation).
Electrochemical detection of oxygen.
In the experiments assessing oxygen, we used commercially produced oxygen sensors (7002-02; Pinnacle Technology, Lawrence, KS). These sensors consist of an epoxy-sheathed disk electrode that is grounded to a fine surface using a diamond-lapping disk. These sensors are prepared from Pt-Ir wire, 180 μm in diameter, with a sensing area of 0.025 mm2 at the sensor’s tip. The active electrode is incorporated with an integrated Ag/AgCl reference electrode. Dissolved oxygen is reduced on the active surface of these sensors, which is held at a stable potential of −0.6 V vs. the reference electrode, producing an amperometric current. The current from the sensor is relayed to a computer via a potentiostat (Model 3104; Pinnacle Technology) and recorded at 1-s intervals using the Performance Analysis of Logs software utility (version 1.5.0; Pinnacle Technology).
Oxygen sensors were calibrated at 37°C by the manufacturer (Pinnacle Technology), according to a standard protocol described in Bolger et al. (2011). The sensors produced incremental current changes with increases in oxygen concentrations within the wide range of previously reported brain oxygen concentrations (0–50 μM). Substrate sensitivity of oxygen sensors varied from 1.03 to 2.31 nA/μM (mean = 1.55 nA/μM). Oxygen sensors were also tested by the manufacturer for their selectivity toward other electroactive substances, including dopamine (0.4 μM) and ascorbate (250 μM), none of which had significant effects on reduction currents.
Experimental procedures.
At the beginning of each electrochemical experiment, rats were minimally anesthetized (<2 min) with isoflurane, and the sensor was inserted through the guide cannula into the NAc (experiment 1) or SC space (experiment 2). The rat was then placed in the testing chamber, and the sensor was connected to the potentiostat via an electrically shielded, flexible cable and a multichannel electrical swivel. The injection port of the jugular catheter on the head mount was connected to a plastic catheter extension that allowed drug delivery from outside of the cage, thus minimizing detection of intravenous drug injections by the rat. Testing began a minimum of 2 h after insertion of the sensor when the baseline current stabilized.
In the electrochemical experiments (n = 15 rats), we examined changes in oxygen in the NAc and SC space induced by intravenous morphine (morphine sulfate; obtained from the National Institute on Drug Abuse, Intramural Research Program Pharmacy) at 0.1, 0.4, 1.6, and 6.4 mg/kg doses over the course of 8- to 12-h recording sessions. To minimize the volume of the injected solution, we used two catheter extensions. Morphine was delivered in ascending dose order in 0.1 (0.1 and 1.6 mg/kg) and 0.4 (0.4 and 6.4 mg/kg) ml volumes at a speed of 0.1 ml/10 s. Time intervals between injections were 90–150 min, typically long enough for the restoration of the current baselines. At the end of each session, rats were anesthetized with intravenous Equithesin and disconnected from the potentiostat, and the sensors were carefully removed. Typically, rats underwent only one recording session, and upon completion, they were euthanized by decapitation under deep isoflurane anesthesia. The rat brains were then removed, stored in 10% formalin, sectioned for verification of sensor placement using a rat stereotaxic atlas (Paxinos and Watson 1998), and assessed for possible brain tissue damage. In several rats, electrochemical recordings with oxygen sensors were repeated 2–4 days later.
The experimental protocol for the thermorecording experiments (n = 8 rats) was similar to that of the electrochemical experiments, with some exceptions, due to multisession recordings. Each rat that underwent temperature recording received several morphine injections in ascending order (one injection per session) with 1 free day between drug sessions. Doses administered to the rats were the same as those used in the oxygen experiments (0.1–6.4 mg/kg), with the exception of a higher 12.8-mg/kg dose, which was administered to the rats during their last recording session. Tests with the 12.8-mg/kg dose were conducted only in five rats to avoid possible overdose. In these experiments, we also measured locomotor activity using an infrared photobeam array (Med-PC IV; Med Associates, Fairfax, VT), with four infrared motion detectors (four transmitters and four receivers) placed at regular distances on outer walls of the test chambers at 2.0 cm above the cage-floor level. A count was recorded whenever a break in the signal occurred, and measurements were stored as cumulative values of breaks for each subsequent 1-min bin.
Data analysis.
Electrochemical data were sampled at 1 Hz (i.e., mean current over 1 s) and analyzed as raw currents with 1-min time resolution. Because each individual sensor differed slightly in background current and substrate sensitivity in vitro, currents were transformed into concentrations and then normalized and expressed as relative percent concentration changes, with the prestimulus baseline current set to 100%. Basal electrochemical currents and their concentration equivalents were used to estimate basal levels of oxygen in the NAc and SC space. One-way repeated-measures ANOVA, followed by Fishers least-significant difference post hoc tests, was used to evaluate the statistical significance of drug-induced changes in oxygen.
Temperature data were sampled with 10 s time resolution and analyzed in terms of absolute changes in each recording location (i.e., NAc, temporal muscle, skin), relative changes with respect to baseline (set to 0°C), as well as changes in NAc-muscle and skin-muscle temperature differentials. Since the brain and temporal muscle receive arterial blood from the same common carotid artery and are equally exposed to blood-delivered heat from the body, NAc-muscle temperature differentials provide the source of heat production, yielding a measure of drug-induced metabolic brain activation. Skin temperature is determined by the state of peripheral vessels, but it also depends on the temperature of arterial blood inflow. Therefore, the skin-muscle temperature differential serves as an accurate measure of peripheral vascular tone, another important determinant of brain temperature changes (Kiyatkin 2010). We used one-way ANOVA with repeated measures, followed by Fishers least-significant difference post hoc tests, to evaluate the effects of morphine on these different dependent measures.
The effects of morphine on locomotor activity were analyzed with 1-min time resolution. Time-dependent correlation analyses were also used to examine the relationship between changes in oxygen levels in the NAc and SC space, as well as between changes in NAc levels and temperature parameters.
RESULTS
Changes in oxygen levels in the NAc and SC space.
Morphine induced distinct changes in NAc oxygen levels depending on the drug dose (Fig. 1A). At three lower doses, oxygen levels increased (0.1 mg/kg: F6,96 = 2.40, P < 0.01; 0.4 mg/kg: F5,465 = 1.27, P = 0.059; 1.6 mg/kg: F7,1260 = 3.40, P < 0.001), and the effect was stronger and more prolonged with each higher dose. The oxygen increase was small and transient at the 0.1 mg/kg dose, more prolonged at 0.4 mg/kg, and longest at the 1.6 mg/kg dose. Morphine at the highest dose induced an opposite response, a strong and prolonged drop in NAc oxygen (F6,1440 = 13.92, P < 0.001). Relative to baseline NAc oxygen levels, estimated by current changes and prerecording calibration values (13.34 ± 0.87 μM; SD = 4.67 μM), morphine-induced oxygen increases were much smaller in amplitude (5–15%) than the oxygen decrease induced by the 6.4 mg/dose (~50%).
Fig. 1.
Mean (±SE) changes in oxygen levels in the nucleus accumbens (NAc; A) and subcutaneous (SC) space (B) induced by intravenous administration of morphine at a wide range of doses. Closed symbols show values significantly different from preinjection baseline values. Vertical, dotted lines (at 0 min) show the moment of drug injection; n = number of cases averaged to produce mean. Horizontal, dotted lines (at 100%) show preinjection oxygen levels. C: the relationships between changes in NAc and SC oxygen levels. Red symbols show initial values, and purple symbols show values up to the peak of the oxygen response.
Morphine induced distinct changes in oxygen levels in the SC space (Fig. 1B). In contrast to the NAc, where morphine at low and moderate doses increased oxygen levels, in the SC space, morphine induced significant oxygen decreases at each dose (F7,175 = 1.66, F7,651 = 2.95, F6,1080 = 17.96, and F6,1440 = 46.89 for 0.1, 0.4, 1.6, and 6.4 mg/kg, respectively; each at least P < 0.05). As shown in Fig. 1B and indicated by the F values of the ANOVA, the strength of the effect and its duration was clearly dose dependent. Basal levels of oxygen in this location (18.84 ± 1.58 μM; SD = 1.58 μM) were significantly larger (t = 3.06; P < 0.01) and more variable than in the NAc.
Next, we used time-dependent correlation analyses to examine the relationships between morphine-induced oxygen changes in the brain and SC space (Fig. 1C). Changes in these two parameters were opposite in direction at the three lower doses, increasing in the NAc and decreasing in the SC space; these changes negatively correlated. The correlation coefficient did not reach significance for the 0.1 mg/kg morphine dose (r = −0.29, not significant) but was significant and stronger for the 0.4 mg/kg dose (r = −0.50, P < 0.01) and maximal for the 1.6 mg/kg dose (r = −0.76, P < 0.001). In the latter case, after reaching nadir in the SC space, oxygen levels in this location slowly returned to baseline, but NAc oxygen levels continued to increase and reached their peaks at ~90 min postinjection, whereas SC oxygen levels were already at baseline. During this time interval (32–90 min), both parameters positively correlated (r = 0.94, P < 0.0001).
In contrast to the lower doses, changes in oxygen levels in the NAc and SC space induced by morphine at the 6.4 mg/kg dose strongly and positively correlated both at the descending (0–16 min) and ascending (16–132 min) curves of the brain response; the correlation in both cases was exceptionally strong (r = 0.97 and = 0.94, respectively).
Figure 2 shows original examples of changes in electrochemical currents induced by intravenous morphine in the NAc and SC space. The primary current data were collected with 1-s time resolution, inverted in polarity, and calibrated in concentration values based on previously determined sensor sensitivity. Note the differences in basal values of oxygen, which were typically larger in the SC space than in the NAc.
Fig. 2.
Original records of changes in electrochemical currents detected in the nucleus accumbens (NAc) and subcutaneous (SC) space by oxygen sensors during intravenous injections of morphine (1.6 and 6.4 mg/kg) in freely moving rats (identified as ES117, -121, and -123). Original data were collected with 1 s time resolution, inverted in polarity, and calibrated in concentration values (in micromolars), based on sensor sensitivity determined in vitro. Note that basal current values and respective oxygen concentrations in the SC space are larger than those in the NAc.
Changes in temperature parameters and locomotor activity.
Morphine at all doses tested induced significant temperature changes in all recording locations (Fig. 3). The absolute baseline temperatures were largest in the NAc (37.08 ± 0.16°C) and significantly lower in the temporal muscle (36.12 ± 0.13°C; t = 4.66, P < 0.001), but their relative changes displayed a similar pattern, showing a strong positive correlation (r = 0.990 for 1.6 mg/kg, P < 0.0001). Skin temperature had the lowest basal value (35.09 ± 0.14°C, P < 0.01 vs. muscle and NAc), and its changes differed from those seen in the NAc and muscle. At the lowest dose tested (0.1 mg/kg), morphine induced monophasic and moderate increases in NAc and muscle temperature that became progressively larger at higher doses. At the 6.4 mg/kg dose, the temperature increases in the NAc, and muscle developed with a prolonged latency, and at the highest dose tested (12.8 mg/kg), there were prominent decreases prior to the increases. These temperature decreases were not evident at the smallest morphine dose but became stronger and more prolonged at higher doses (Fig. 3).
Fig. 3.
Mean (±SE) changes in temperature in the nucleus accumbens (NAc), temporal muscle, and skin (top row), temperature differentials (middle row), and locomotor activity (bottom row) induced by intravenous administration of morphine at a wide range of doses. Closed symbols show values significantly different from preinjection baseline values. Vertical, dotted lines (at 0 min) show the moment of drug injection; n = number of cases averaged to produce mean. Horizontal, dotted lines show baseline values of temperature or locomotion.
The NAc-muscle differential shows changes in intrabrain heat production; it increases during metabolic brain activation and decreases during metabolic inhibition. As shown in Fig. 3, this parameter did not change at the 0.1 mg/kg dose but increased at the 0.4, 1.6, and 6.4 mg/kg doses. The increase in the NAc-muscle differential was evident within the first 60 min postinjection at the 0.4 and 1.6 mg/kg doses but was delayed in onset following the 6.4 mg/kg injection. In contrast, the NAc-muscle differential showed a biphasic response at the highest drug dose (12.8 mg/kg), significantly decreasing for ~120 min postinjection and then significantly increasing above the preinjection baseline.
The skin-muscle temperature differential is an index reflecting changes in skin vascular tone; its decreases represent skin vasoconstriction, whereas its increases represent skin vasodilation (see Kiyatkin 2010). As shown in Fig. 3, this parameter was monophasically decreased by morphine at the three lower doses. The decrease in skin-muscle differential was minimal at the 0.1 mg/kg and clearly larger at the 0.4 and 1.6 mg/kg doses. In contrast to the lower morphine doses, the two highest doses induced biphasic, up-down changes in this parameter, indicating initial skin vasodilation, followed by skin vasoconstriction. The initial rise in this parameter was clearly larger at the 12.8 mg/kg dose than the 6.4 mg/kg dose.
Increases in brain temperature depend on two primary factors: 1) the increase in intrabrain heat production and 2) the decrease in heat loss from the brain to the rest of the body and then to the cooler external environment. As seen in Fig. 3, decreases in skin-muscle differentials were much larger in amplitude than increases in brain-muscle differentials, indicating that peripheral vasoconstriction provides a much stronger contribution to the morphine-induced brain temperature increases than metabolic brain activation. This is illustrated in Fig. 4A, which shows the relationship between the NAc temperature increase induced by morphine at the 1.6 mg/kg dose and the two temperature differentials. Whereas the brain temperature increases tightly and linearly correlated with decreases in the skin-muscle differential (r = −0.988), the correlation was much weaker between NAc temperature and the NAc-muscle differential, which rapidly increased within 8–10 min after the injection and then remained at a stable plateau, while brain temperature gradually increased. More complex relationships between brain temperature and temperature differentials were found for the largest morphine dose that induced a biphasic change in brain temperature (Fig. 4B). In this case, the NAc-muscle differential rapidly decreased when the brain temperature decreased but then inverted into a slow increase, while the NAc temperature increased. On the other hand, the skin-muscle differential increased rapidly after morphine injection, while NAc temperature started to increase, and the differential reached a plateau, while NAc temperature continued to decrease. After this, the direction of the relationship inverted, in which the differential gradually decreased below its baseline, while brain temperature increased, showing a tight correlation up to the peak of NAc temperature elevation (r = 0.938).
Fig. 4.

Time-dependent correlative relationships between changes in nucleus accumbens (NAc) temperature and NAc-muscle and skin-muscle temperature differentials following intravenous morphine administration at 1.6 (A) and 12.8 (B) mg/kg doses. Each graph shows coefficients of correlation and regression lines calculated for different time intervals following the onset of morphine injection (black circles). Closed symbols show values up to the nadir of NAc temperature response.
Relationships between morphine-induced changes in NAc oxygen and temperature parameters.
Oxygen arriving to the brain from arterial blood is essential for metabolic activity of neural cells. Since brain temperature depends on intrabrain heat production and heat loss from brain tissue, certain relationships should exist between morphine-induced changes in NAc oxygen and the three temperature parameters (NAc temperature and NAc-muscle and skin-muscle temperature differentials). To clarify this issue, we examined correlative relationships between these parameters for the two morphine doses (1.6 and 6.4 mg/kg) that induced opposite changes in brain oxygen (Fig. 5).
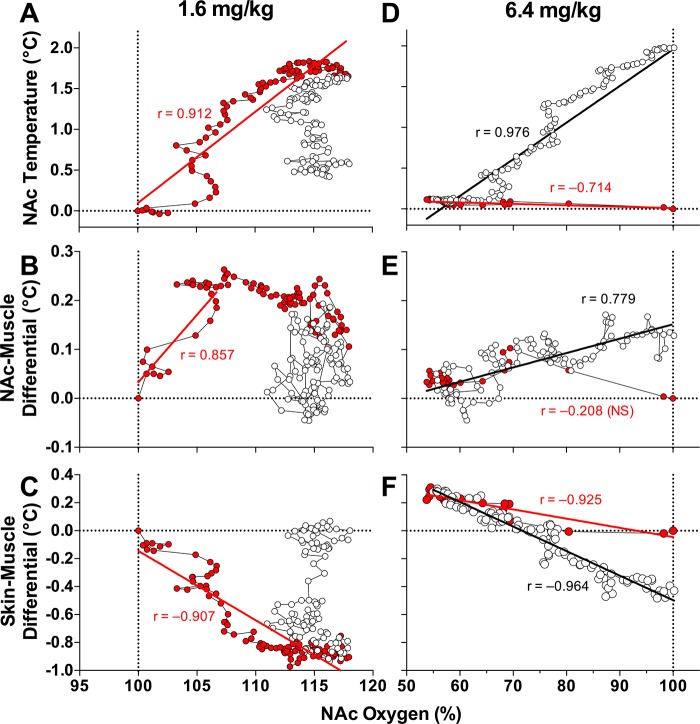
Fig. 5.
Time-dependent correlative relationships between changes in nucleus accumbens (NAc) oxygen and 3 temperature parameters following intravenous morphine administration at 1.6 and 6.4 mg/kg doses. A and D: NAc temperature; B and E: NAc-muscle differential; C and F: skin-muscle differential. Values up to the peak of the oxygen increase (A–C) or peak of oxygen decrease (D–F) are shown as red symbols. Each graph shows coefficients of correlation and regression lines calculated for different time intervals following the onset of morphine injection. See text for additional explanations.
When morphine was injected at the 1.6 mg/kg dose (Fig. 5, A–C), both NAc oxygen levels and NAc temperature increased, showing a tight correlation until both parameters peaked (r = 0.912). Within the first 10 min postinjection, the increase in NAc oxygen was more rapid than the increase in NAc temperature. Then, the brain temperature decreased toward baseline, but NAc oxygen remained at increased levels (Fig. 5A). Both oxygen and the brain-muscle differential increased rapidly after morphine was injected (r = 0.857), but from ~15 min, the brain-muscle differential peaked, while NAc oxygen continued to increase (Fig. 5B). Then, the brain-muscle differential decreased, but oxygen levels remained elevated. Brain oxygen levels and the skin-muscle differentials showed a strong negative correlation (r = −0.907) until the peak of the oxygen response (Fig. 5C).
Different relationships between oxygen levels and the temperature parameters were found for the 6.4 mg/kg morphine dose (Fig. 5, D–F). In this case, oxygen levels dropped rapidly before any changes in brain temperature, but then, they returned toward baseline, displaying a tight positive correlation with increases in NAc temperature (Fig. 5D). Oxygen changes were also independent of changes in the brain-muscle differential, which increased slowly, while oxygen levels returned to baseline after the initial drop (Fig. 5E). Both descending and ascending curves of the NAc oxygen decrease negatively correlated with the skin-muscle differential, which increased while oxygen dropped and decreased while oxygen levels returned to baseline (Fig. 5F).
DISCUSSION
This study produced several new findings. We showed that intravenous morphine induces opposite changes in NAc oxygen levels depending on its dose. Morphine at a large dose strongly decreases oxygen levels but modestly increases them when administered at low and moderate doses. Morphine also induced distinct changes in brain temperature, eliciting increases at low and moderate doses and a biphasic, down-up change at a higher dose. This dose-dependent dissociation of morphine-induced oxygen and temperature responses suggests that different mechanisms underlie these contrasting changes.
As an analgesic drug, morphine is used in humans at low doses, varying from 5 to 20 mg for a 70-kg individual (or 0.07–0.3 mg/70 kg) (Paris and Yealy 2006; Zimmer 2004). Within this dose range (0.1–0.3 mg/kg), morphine is also effective in rats in sensitive nociceptive tests reflecting “affective components of pain” and during experimental noxious states (Kayser and Guilbaud 1983, 1990; Kiyatkin 1989). As shown here, morphine at a 0.1 mg/kg dose induces a very small and transient NAc oxygen increase coupled with an equally weak and transient oxygen drop in the SC space. Since the SC space is metabolically inactive, it is deducible that oxygen decreases in this location reflect decreases in blood oxygen levels due to a modest decrease in respiratory activity. Similar but gradually larger decreases in SC oxygen were induced by morphine at the 0.4 and 1.6 mg/kg doses, reflecting strengthening of respiratory depression. However, oxygen levels in the NAc were moderately increased by morphine at these doses, suggesting normal or even slightly increased oxygenation of brain tissue under conditions of moderate hypoxia in peripheral tissues. At the largest dose (6.4 mg/kg), morphine induced robust oxygen decreases in both the brain and SC space that correlated tightly. This dose-dependent dissociation between central and peripheral changes in oxygen suggests that morphine engages different and opposing mechanisms to regulate the brain’s extracellular oxygen levels.
Oxygen entry from arterial blood vessels into the extracellular environment in any tissue depends on its levels in arterial blood (Attwell et al. 2010). However, the entry of oxygen into brain tissue can be independently enhanced via neurovascular coupling, where neuronal activation induces cerebral vasodilation and increases local cerebral blood flow (Attwell et al. 2010; Fox and Raichle 1986; Martin et al. 2006; Paulson et al. 2010). This gradient-independent mechanism is the likely cause for the rapid NAc oxygen increases elicited in awake rats by arousing stimuli (Solis et al. 2017a). Therefore, it appears that by activating this adaptive mechanism, morphine at low and moderate doses is able to increase oxygen entry into brain tissue even when breathing is slightly depressed, and oxygen levels in arterial blood slightly decreased. When the dose of morphine exceeds certain levels (~4 mg/kg as a one-half sum of 1.6 and 6.4 mg/kg), this neurovascular coupling mechanism is unable to compensate for the strong oxygen decreases in arterial blood, and thus its levels in brain tissue drop, inducing brain hypoxia that is greatly enhanced with higher drug doses.
Whereas neurovascular coupling appears to be the most evident mechanism for the increases in NAc oxygen found in this study, it is more difficult to define the neural source triggering local cerebral vasodilation. Particularly, it is unknown whether intravenous morphine at low, behaviorally active doses (0.1–1.6 mg/kg) could excite accumbal neurons. Paradoxically, most neuronal studies with morphine were conducted in anesthetized animal preparations, which dramatically affect neuronal responses to physiological and drug stimuli (Windels and Kiyatkin 2006), and morphine was usually administered intraperitoneally or subcutaneously at large or very large doses. Moreover, most of these studies were focused on brain structures that are involved in pain, nociception, and the functions of endogenous nociceptive and antinociceptive mechanisms. Morphine also induced c-fos expression, an index of metabolic neural activation, in multiple brain structures, including the dorsal and ventral striatum (Chang et al. 1988; Garcia et al. 2003; Liu et al. 1994). It is possible to speculate that cerebral vasodilation induced by morphine is mediated via its direct action on opioid receptors expressed on brain vessels. However, the direct vasomotor effects of opioid drugs on cerebral vessels under in vitro and in vivo conditions appear to be weak or absent (Benyó and Wahl 1996). Finally, it is also unlikely that changes in arterial blood pressure and cerebral perfusion pressure could explain increases in NAc oxygen induced by morphine at doses used in this study. Intravenous morphine injected at these low and moderate doses in freely moving rats has weak effects on arterial blood pressure, which slightly decreases but does not increase (Shanazari et al. 2011; Thurston et al. 1993). However, a rapid transient drop in arterial blood pressure induced by intravenous morphine at high doses could contribute to decreases in NAc oxygen, but this effect appears to be minor compared with that induced by respiratory depression.
Whereas direct evidence that intravenous morphine induces activation of accumbal neurons is limited, our temperature experiment provided support for this effect. First, we found that morphine, even at the lowest dose, rapidly but transiently increases the NAc-muscle differential, suggesting a rise in intrabrain heat production. Since all energy spent for neuronal activity is finally transformed into heat (Hodgkin 1967; Ritchie 1973; Siesjö 1978), changes in intrabrain heat production can be viewed as a reliable and dynamic index of metabolic brain activation. With the use of time-dependent correlative analyses, we found that brain oxygen increases were more rapid than the increases in brain-muscle differentials that are consistent with the time delay between neuronal activation as an electrophysiological event and the heat-producing metabolic activation that always follows spiking activity (Ritchie 1973).
In contrast to hyperthermia induced by morphine at low and moderate doses, high-dose morphine injections induced hypothermia that occurred due to decreased intrabrain heat production and peripheral vasodilation that promotes heat loss to the external environment. Importantly, this hypothermic effect occurred immediately after the injection, had a relatively short duration, and was followed by robust temperature elevation. Similar to the lower doses, this secondary temperature increase occurred due to a rise in the NAc-muscle differential and decrease in the skin-muscle differential. Therefore, consistent with previous work using oxygen consumption and glucose-utilization techniques (Cohen et al. 1991; London et al. 1990; Moyer et al. 1957), morphine at large doses (>2–4 mg/kg) decreases brain metabolism. However, at the same high doses, morphine decreases NAc oxygen levels, suggesting a possible relationship between these phenomena. The brain oxygen drop induced by large-dose morphine injection was very rapid and occurred before any changes in brain temperature and both temperature differentials. Thus whereas respiratory depression is traditionally viewed as an effect mediated via the direct action of the drug on μ-receptors expressed in brain neurons, the rapidity of this effect may suggest a peripheral contribution.
Conclusions and human implications.
The present study confirms the unique properties of morphine as a highly safe analgesic drug with an exceptionally large therapeutic window. When used in rats under quiet resting conditions at a low, analgesic dose (0.1 mg/kg), intravenous morphine had only weak and transient effects on brain and SC oxygen levels (<±5%), slightly increased brain and body temperatures and had negligible effects on locomotor activity. Whereas morphine at the 12.8-mg/kg (128-fold higher) dose induced much stronger physiological and behavioral effects, this dose did not induce lethality in any rat tested. However, as shown by our oxygen measurements in the SC space, morphine inhibits respiratory activity at all doses tested in this study, but NAc oxygen levels notably decreased only at the 6.4 mg/kg dose, which is 64-fold higher than analgesic doses. In addition to the appearance of brain hypoxia, this dose range was associated with other potentially adverse effects, such as powerful hypoactivity, muscular rigidity, skin vasodilation, decreased brain metabolic activity, and brain hypothermia. The same pattern of temperature response occurs during general anesthesia induced by pentobarbital (Kiyatkin and Brown 2005).
The effects of morphine on brain oxygen differed from the effects of heroin and fentanyl, which decreased NAc oxygen at low intravenous doses that maintain drug self-administration in rats (0.1 and 0.03 mg/kg, respectively). Therefore, based on its ability to induce brain hypoxia in rats, in terms of dose, morphine is ~28-fold weaker than heroin and ~4,800-fold weaker than fentanyl. This suggests that heroin and especially fentanyl are considerably more dangerous drugs than morphine.
Morphine acts on opioid receptors naturally activated by endogenous opioid peptides released under potentially dangerous conditions (Frederickson and Geary 1982; Millan 1981; Simon 1991). Therefore, the effects of morphine at low doses could mimic manifestations of a generalized adaptive syndrome (analgesia, hypoactivity, diminished responsiveness to sensory stimuli, peripheral vasoconstriction, hyperthermia) aimed to adapt to and provide life support under these dangerous conditions. However, excessive and selective activation of this system is maladaptive and underscores the danger of high-dose morphine used as an addictive drug.
Overall, our study produced the notable findings that although morphine induces dose-dependent decreases in respiratory activity within a wide dose range, it induces differential effects on NAc oxygen levels, depending on dose, through distinct and opposing underlying mechanisms. At low, clinically relevant doses, morphine slightly increases NAc oxygen levels, presumably due to physiologically adaptive mechanisms, but morphine decreases NAc oxygen levels at higher doses that are likely to be abused. Such oxygen decreases thus indicate that at higher morphine doses, the neural mechanisms regulating oxygen entry into brain tissue are unable to compensate for the enhanced respiratory depression and subsequent decreases in blood oxygen, thus leading to brain hypoxia. Therefore, whereas morphine appears to be safe at clinically relevant and analgesic doses, when abused at high doses, it poses great health risks due to respiratory depression and the development of brain hypoxia, which can lead to coma and lethality.
GRANTS
Support for this study is provided by the National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.A.K. conceived and designed research; E.S., A.A., and E.A.K. performed experiments; E.S., A.A., and E.A.K. analyzed data; E.S., A.A., and E.A.K. interpreted results of experiments; E.S. and A.A. prepared figures; E.A.K. drafted manuscript; E.S., A.A., and E.A.K. edited and revised manuscript; A.A., E.S., and E.A.K. approved final version of manuscript.
REFERENCES
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 468: 232–243, 2010. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyó Z, Wahl M. Opiate receptor-mediated mechanisms in the regulation of cerebral blood flow. Cerebrovasc Brain Metab Rev 8: 326–357, 1996. [PubMed] [Google Scholar]
- Bolger FB, McHugh SB, Bennett R, Li J, Ishiwari K, Francois J, Conway MW, Gilmour G, Bannerman DM, Fillenz M, Tricklebank M, Lowry JP. Characterisation of carbon paste electrodes for real-time amperometric monitoring of brain tissue oxygen. J Neurosci Methods 195: 135–142, 2011. doi: 10.1016/j.jneumeth.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Chang SL, Squinto SP, Harlan RE. Morphine activation of c-fos expression in rat brain. Biochem Biophys Res Commun 157: 698–704, 1988. doi: 10.1016/S0006-291X(88)80306-1. [DOI] [PubMed] [Google Scholar]
- Cohen SR, Kimes AS, London ED. Morphine decreases cerebral glucose utilization in limbic and forebrain regions while pain has no effect. Neuropharmacology 30: 125–134, 1991. doi: 10.1016/0028-3908(91)90195-H. [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT. Nonmedical prescription-opioid use and heroin use. N Engl J Med 374: 1296, 2016. doi: 10.1056/NEJMra1508490. [DOI] [PubMed] [Google Scholar]
- Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, Olofsen E, Danhof M. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth 94: 825–834, 2005. doi: 10.1093/bja/aei145. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res 137: 75–114, 2002. doi: 10.1016/S0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA 83: 1140–1144, 1986. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson RC, Geary LE. Endogenous opioid peptides: review of physiological, pharmacological and clinical aspects. Prog Neurobiol 19: 19–69, 1982. doi: 10.1016/0301-0082(82)90020-X. [DOI] [PubMed] [Google Scholar]
- Garcia MM, Anderson AT, Edwards R, Harlan RE. Morphine induction of c-fos expression in the rat forebrain through glutamatergic mechanisms: role of non-n-methyl-D-aspartate receptors. Neuroscience 119: 787–794, 2003. doi: 10.1016/S0306-4522(02)00975-2. [DOI] [PubMed] [Google Scholar]
- Gottås A, Øiestad EL, Boix F, Vindenes V, Ripel Å, Thaulow CH, Mørland J. Levels of heroin and its metabolites in blood and brain extracellular fluid after i.v. heroin administration to freely moving rats. Br J Pharmacol 170: 546–556, 2013. doi: 10.1111/bph.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL. The Conduction of the Nervous Impulse. Liverpool, UK: Liverpool University Press, 1967. [Google Scholar]
- Jaffe JH, Knapp CM, Ciraulo DA. Opiates: clinical aspects. In: Substance Abuse, edited by Lowinson JH, Ruiz P, Millman RB, Langrod JG. Baltimore, MD: Williams & Wilkins, 1997, p. 158–166. [Google Scholar]
- Kayser V, Guilbaud G. Differential effects of various doses of morphine and naloxone on two nociceptive test thresholds in arthritic and normal rats. Pain 41: 353–363, 1990. doi: 10.1016/0304-3959(90)90012-3. [DOI] [PubMed] [Google Scholar]
- Kayser V, Guilbaud G. The analgesic effects of morphine, but not those of the enkephalinase inhibitor thiorphan, are enhanced in arthritic rats. Brain Res 267: 131–138, 1983. doi: 10.1016/0006-8993(83)91046-6. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. Brain temperature homeostasis: physiological fluctuations and pathological shifts. Front Biosci (Landmark Ed) 15: 73–92, 2010. doi: 10.2741/3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA. Morphine: some puzzles of well-known substance. Int J Neurosci 45: 231–246, 1989. doi: 10.3109/00207458908986236. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. State-dependent and environmental modulation of brain hyperthermic effects of psychoactive drugs of abuse. Temperature (Austin) 1: 201–213, 2014. doi: 10.4161/23328940.2014.969074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Brain and body temperature homeostasis during sodium pentobarbital anesthesia with and without body warming in rats. Physiol Behav 84: 563–570, 2005. doi: 10.1016/j.physbeh.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Kim AH, Wakabayashi KT, Baumann MH, Shaham Y. Critical role of peripheral vasoconstriction in fatal brain hyperthermia induced by MDMA (Ecstasy) under conditions that mimic human drug use. J Neurosci 34: 7754–7762, 2014. doi: 10.1523/JNEUROSCI.0506-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokki M, Välitalo P, Kuusisto M, Ranta VP, Raatikainen K, Hautajärvi H, Kokki H. Central nervous system penetration of oxycodone after intravenous and epidural administration. Br J Anaesth 112: 133–140, 2014. doi: 10.1093/bja/aet337. [DOI] [PubMed] [Google Scholar]
- Liu J, Nickolenko J, Sharp FR. Morphine induces c-fos and junB in striatum and nucleus accumbens via D1 and N-methyl-D-aspartate receptors. Proc Natl Acad Sci USA 91: 8537–8541, 1994. doi: 10.1073/pnas.91.18.8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall MR, Moody DE, Fang WB, Nuzzo PA, Walsh SL. Pharmacokinetics of intranasal crushed OxyContin and intravenous oxycodone in nondependent prescription opioid abusers. J Clin Pharmacol 52: 600–606, 2012. doi: 10.1177/0091270011401620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Broussolle EP, Links JM, Wong DF, Cascella NG, Dannals RF, Sano M, Herning R, Snyder FR, Rippetoe LR, Toung TJ, Jaffe JH, Wagner HN Jr. Morphine-induced metabolic changes in human brain. Studies with positron emission tomography and [fluorine 18]fluorodeoxyglucose. Arch Gen Psychiatry 47: 73–81, 1990. doi: 10.1001/archpsyc.1990.01810130075010. [DOI] [PubMed] [Google Scholar]
- Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage 32: 33–48, 2006. doi: 10.1016/j.neuroimage.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Stress and endogenous opioid peptides: a review. Mod Probl Pharmacopsychiatry 17: 49–67, 1981. doi: 10.1159/000402406. [DOI] [PubMed] [Google Scholar]
- Moyer JH, Pontius R, Morris G, Hershberger R. Effect of morphine and n-allylnormorphine on cerebral hemodynamics and oxygen metabolism. Circulation 15: 379–384, 1957. doi: 10.1161/01.CIR.15.3.379. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH, Hyman S, Braun L, Oldendorf SZ. Blood-brain barrier: penetration of morphine, codeine, heroin, and methadone after carotid injection. Science 178: 984–986, 1972. doi: 10.1126/science.178.4064.984. [DOI] [PubMed] [Google Scholar]
- Paris PM, Yealy DM. Pain management. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice, edited by Marx JA, Hockberger RS, Walls RM. St. Louis, MO: Mosby Elsevier, 2006. [Google Scholar]
- Pattinson KT. Opioids and the control of respiration. Br J Anaesth 100: 747–758, 2008. doi: 10.1093/bja/aen094. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Hasselbalch SG, Rostrup E, Knudsen GM, Pelligrino D. Cerebral blood flow response to functional activation. J Cereb Blood Flow Metab 30: 2–14, 2010. doi: 10.1038/jcbfm.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1998. [Google Scholar]
- Ritchie JM. Energetic aspects of nerve conduction: the relationships between heat production, electrical activity and metabolism. Prog Biophys Mol Biol 26: 147–187, 1973. doi: 10.1016/0079-6107(73)90019-9. [DOI] [PubMed] [Google Scholar]
- Shanazari AAP, Aslani Z, Ramshini E, Alaei H. Acute and chronic effects of morphine on cardiovascular system and the baroreflexes sensitivity during severe increase in blood pressure in rats. ARYA Atheroscler 7: 111–117, 2011. [PMC free article] [PubMed] [Google Scholar]
- Siesjö BK. Brain Energy Metabolism. Chichester, UK: Wiley, 1978. [Google Scholar]
- Simon EJ. Opiates: neurobiology. In: Substance Abuse, edited by Lowinson JH, Ruiz P, Millman RB, Langrod JG. Baltimore, MD: Williams & Wilkins, 1997, p. 148–158. [Google Scholar]
- Simon EJ. Opioid receptors and endogenous opioid peptides. Med Res Rev 11: 357–374, 1991. doi: 10.1002/med.2610110402. [DOI] [PubMed] [Google Scholar]
- Solis E Jr, Afzal A, Kiyatkin EA. Changes in brain oxygen and glucose induced by oxycodone: relationships with brain temperature and peripheral vascular tone. Neuropharmacology 133: 481–490, 2018. doi: 10.1016/j.neuropharm.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr, Cameron-Burr KT, Kiyatkin EA. Rapid physiological fluctuations in nucleus accumbens oxygen levels induced by arousing stimuli: relationships with changes in Brain glucose and metabolic neural activation. Front Integr Nuerosci 11: 9, 2017a. doi: 10.3389/fnint.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr, Cameron-Burr KT, Shaham Y, Kiyatkin EA. Intravenous heroin induces rapid brain hypoxia and hyperglycemia that precede brain metabolic response. eNeuro 4: ENEURO.0151-17.2017, 2017b. doi: 10.1523/ENEURO.0151-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston CL, Starnes A, Randich A. Changes in nociception, arterial blood pressure and heart rate produced by intravenous morphine in the conscious rat. Brain Res 612: 70–77, 1993. doi: 10.1016/0006-8993(93)91645-9. [DOI] [PubMed] [Google Scholar]
- Windels F, Kiyatkin EA. General anesthesia as a factor affecting impulse activity and neuronal responses to putative neurotransmitters. Brain Res 1086: 104–116, 2006. doi: 10.1016/j.brainres.2006.02.064. [DOI] [PubMed] [Google Scholar]
- Wise RA. The brain and reward. In: The Neuropharmacological Basis of Reward, edited by Liebman JM, Cooper SJ. Oxford, UK: Oxford University Press, 1989, p. 377–424. [Google Scholar]
- Yeadon M, Kitchen I. Opioids and respiration. Prog Neurobiol 33: 1–16, 1989. doi: 10.1016/0301-0082(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Zimmer G. Acute pain management. In: Emergency Medicine: A Comprehensive Study Guide, edited by Tintinalli JE, Kelen GD, Stapczynski JS. New York: McGraw-Hill, 2004, p. 257–264. [Google Scholar]