Abstract
A voluntary contraction of muscles with one arm increases the excitability of corticospinal projections to the contralateral resting arm, a phenomenon known as crossed facilitation. Although many motor tasks engage simultaneous activation of the arm and trunk, interactions between corticospinal projections targeting these segments remain largely unknown. Using transcranial magnetic stimulation over the trunk representation of the primary motor cortex, we examined motor-evoked potentials (MEPs) in the resting erector spinae (ES) muscle when the contralateral arm remained at rest or performed 20% of isometric maximal voluntary contraction (MVC) into index finger abduction, thumb abduction, elbow flexion, and elbow extension. We found that MEP size in the ES increased during all voluntary contractions, with greater facilitation occurring during elbow flexion and index finger abduction. To further examine the origin of changes in MEP size, we measured short-interval intracortical inhibition (SICI) and cervicomedullary MEPs (CMEPs) in the ES muscle during elbow flexion and index finger abduction and when the arm remained at rest. Notably, SICI decreased and CMEPs remained unchanged in the ES during both voluntary contractions compared with rest, suggesting a cortical origin for the effects. Our findings reveal crossed facilitatory interactions between trunk extensor and proximal and distal arm muscles, particularly for elbow flexor and index finger muscles, likely involving cortical mechanisms. These interactions might reflect the different role of these muscles during functionally relevant arm and trunk movements.
NEW & NOTEWORTHY Many of the tasks of daily life involve simultaneous activation of the arm and trunk. We found that responses in the erector spinae muscles evoked by motor cortical stimulation increased in size during elbow flexion and extension and during index finger abduction and thumb abduction. Crossed facilitation with the trunk was more pronounced during elbow flexion and index finger abduction. These results might reflect the different role of these muscles during arm and trunk movements.
Keywords: back muscles, corticospinal pathway, erector spinae, intracortical inhibition, motor-evoked potentials, subcortical pathways
INTRODUCTION
Interactions between arm and trunk muscles are evident in a number of activities of daily living. For example, trunk muscles are activated before or concurrent with voluntary arm movements (Aruin and Latash 1995; Hodges and Richardson 1997a, 1997b) and when individuals reach for objects beyond arm’s length (Kaminski et al. 1995; Levin 1996). Trunk muscles are involved in keeping the center of mass over the support surface while arm muscles are more involved in countering reaction forces generated by limb movement onset (van der Fits et al. 1998). Indeed, deficits in trunk control (Cacho et al. 2011; Reft and Hasan 2002) and afferent input from the trunk movement (Adamovich et al. 2001) can alter the trajectory of arm movements. Despite this evidence, the effect of voluntary contraction of distal and proximal arm muscles on corticospinal excitability of trunk muscles, and its mechanisms of action, remains largely unexplored.
Several lines of evidence suggest that physiological pathways controlling arm and trunk muscles interact. Electrophysiological studies using transcranial magnetic stimulation (TMS) over the primary motor cortex showed that the size of motor-evoked potentials (MEPs; reflecting changes in corticospinal excitability) in the erector spinae (ES) muscle increases during contralateral shoulder abduction in standing and lying postures (Davey et al. 2002). MEPs in the ES muscle also increase during a rapid shoulder flexion task that requires postural control (Chiou et al. 2016). Note that the nature of these interactions can be influenced by the task. For example, dynamic elbow flexion but not elbow extension changes MEP size in trunk muscles (Christmas et al. 2016). When muscles close to the trunk play a postural role, corticospinal responses in a hand muscle increase when the hand is involved in precise force control (Schieppatti et al. 1996). Furthermore, studies showed that electromyographic (EMG) activity in the ES muscle increases according to activation of different arm muscles during functional motor tasks involving the arm and trunk (Marcolin et al. 2015). Even the onset of muscle activity in the ES has been shown to depend on the direction of the arm movement (Hodges and Richardson 1997b). Crossed facilitatory effects also differ when proximal and distal arm muscles are active. Evidence showed that voluntary activation of elbow flexor muscles increased MEP size in hand muscles and that contractions of hand muscles increased MEP size in homologous muscles on the contralateral side (Bunday and Perez 2012; Bunday et al. 2013). Indeed, voluntary activation of elbow flexors and extensor muscles has a different effect on pathways controlling contralateral homologous and heteronymous muscles (Perez et al. 2014). Thus we hypothesized that voluntary activation of proximal and distal arm muscles would result in different corticospinal facilitation in a trunk muscle. Evidence has shown that crossed corticospinal facilitation can occur at the level of the primary motor cortex or spinal motoneurons or at both sites (Bunday et al. 2012; Perez and Cohen 2008). Therefore, we tested short-interval intracortical inhibition (SICI) and MEPs elicited by TMS at the primary motor cortex and cervicomedullary junction (CMEPs) respectively, to examine cortical and subcortical mechanisms contributing to changes in MEP size in the ES muscle in intact humans.
METHODS
Subjects.
Sixteen healthy volunteers (8 female, 8 male; 1 left handed) with a mean (±SD) age of 29.7 ± 10.9 yr participated in the study. All subjects gave informed consent to the experimental procedures, which were approved by the local ethics committee at the University of Pittsburgh. The study was performed in accordance with the Declaration of Helsinki. Subjects were preselected out of a total of 25 subjects who were screened to ensure that they showed visible MEPs elicited by TMS in the ES muscle across conditions tested. All subjects confirmed that they were not taking any prescription drugs on a regular basis.
EMG recordings.
EMG was recorded bilaterally from the ES and unilaterally from the first dorsal interosseous (FDI), abductor pollicis brevis (APB), and biceps (BB) and triceps (TB) brachii of the dominant arm (Fig. 1A) through surface electrodes (Ag-AgCl; 10-mm diameter) secured on the skin over the belly of each muscle. The signals were amplified (×1,000), filtered (30–1,000 Hz), and sampled at 2 kHz for offline analysis (CED 1401 with Signal software; Cambridge Electronic Design, Cambridge, UK).
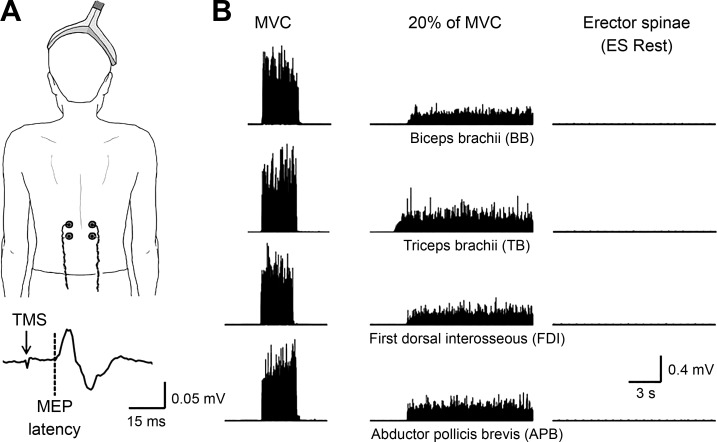
Fig. 1.
A: schematic illustration of the coil position (when the cone coil was used) and the electrodes over the erector spinae (ES) muscle at the 12th thoracic vertebral level. Raw motor-evoked potential (MEP) trace elicited in the ES muscle by transcranial magnetic stimulation (TMS) over the primary motor cortex from a representative subject (20 stimuli, averaged unrectified). The arrow indicates the TMS pulse, and the dotted line indicates the onset of the MEP. B: raw rectified electromyographic (EMG) activity from each of the muscles tested during 20% of maximal voluntary contraction (MVC) into elbow flexion [recording from the biceps brachii (BB)], elbow extension [recording from the triceps brachii (TB)], index finger abduction [recording from the first dorsal interosseous (FDI)], and thumb abduction [recording from the abductor pollicis brevis (APB)].
Experimental setup.
Subjects were seated in an armchair with head supported by a headrest. At the beginning of the experiment, all subjects performed two to three unilateral isometric maximal voluntary contractions (MVCs) for 3–5 s into index finger and thumb abduction, and elbow flexion and extension, separated by 30 s of rest. During maximal contractions subjects received verbal encouragement to perform maximally. MVCs for the ES were collected in a prone position with subjects’ pelvis and legs secured by the investigators. Testing was completed with the trunk resting on a chair (conditioned referred here as “rest”) and when subjects performed index finger abduction, thumb abduction, elbow flexion, and elbow extension in a pseudorandomized order. During index finger and thumb abduction, subjects were instructed to press with their index finger or thumb against a custom lever in the abduction direction with the forearm pronated and the wrist restrained by straps. During elbow flexion and extension testing, subjects were seated with both shoulders and elbows flexed to 90° and the forearm supinated. Here, a custom-built arm device was used to maintain the position of the arm. Since a voluntary contraction of arm muscles can generate EMG activity in the ES, in a preliminary study (n = 8) we tested the effects of 10, 20, and 30% of MVC with all arm muscles tested on background EMG activity in the ES muscle. We found that subjects were able to maintain 20% of MVC with each of the muscles tested without eliciting additional background EMG activity in the ES muscle. Thus testing was performed at rest and when the contralateral arm remained at rest or when performing 20% of MVC into index finger and thumb abduction and elbow flexion and extension. EMG activity in the ES and in the arm muscle tested were continuously displayed on an oscilloscope, and verbal feedback was provided to subjects to ensure that physiological measurements were acquired at similar levels of background EMG activity. A total of 3.4 ± 2.0% trials in which mean rectified EMG activity exceeded 2 SD of the mean average rectified EMG, measured 100 ms before the stimulus artifact, were excluded from further analysis (Bunday et al. 2012, 2013).
TMS.
TMS pulses were delivered via a Magstim 2002 monophasic stimulator (Magstim Company) through a bat-wing (loop diameter, 90 mm; handle pointing backward and 45° away from the midline) or a double-cone coil (loop diameter, 110 mm; handle pointing vertically upwards). In individuals in whom an MEP could not be elicited in the ES muscle with a bat-wing coil, the double-cone coil was used. We determined the optimal position for eliciting a MEP in the ES muscle (hot spot) by moving the coil in small steps along the area corresponding to the primary motor cortex. The hot spot was defined as the region where the largest MEP in the ES could be evoked with the minimum intensity (Rothwell et al. 1999). With this coil position the current flowed in a posterior-anterior direction and probably produced D-wave and early I-wave activation (Sakai et al. 1997). The TMS coil was held to the head of the subject with a custom coil holder, while the head was firmly secured to a headrest by straps. TMS was used to elicit MEPs, resting motor threshold (RMT), and SICI.
MEPs.
RMT [78.8 ± 18.2% of the maximal stimulator output (MSO)] was defined as the minimal stimulus intensity required to induce MEPs ≤50 μV peak-to-peak amplitude in at least three to five consecutive trials in the relaxed ES muscle (Rothwell et al. 1999). Based on our previous results (Chiou et al. 2018), we used a stimulus intensity needed to elicit an MEP with a peak-to-peak amplitude of ~0.1 mV (89.7 ± 12.7% MSO) in the ES muscle. Single TMS pulses were delivered at 4-s intervals in sets of 10 separated by rest periods as needed. Twenty MEPs were tested during each voluntary contraction.
SICI.
We observed that voluntary contraction into elbow flexion and index finger abduction increased MEP size in the ES muscle to a larger extent than elbow extension and thumb abduction. Therefore, we examined the contribution from the primary motor cortex to changes in ES MEP size by testing SICI using a previously described method (Kujirai et al. 1993) at rest first and when subjects performed 20% of MVC into elbow flexion and index finger abduction in a randomized order (n = 8). A conditioning stimulus (CS) was set at an intensity needed to elicit ~50% of SICI, which corresponded to ~70% of active motor threshold (55.2 ± 13.1% MSO). This low-intensity stimulus allowed us to assess SICI independently of the effects on short-intracortical facilitation at low contraction levels (Ortu et al. 2008). The same stimulus intensity was used for the CS across conditions. The test stimulus (TS) was set at an intensity needed to elicit an MEP with a peak-to-peak amplitude of ~0.1 mV (86.4 ± 15.2% MSO). The CS was delivered 2.5 ms before the test stimulus. Previous studies showed that the size of the test MEP can influence the magnitude of SICI (Roshan et al. 2003). Since our results from the single-pulse TMS showed that ES MEPs became larger during the elbow flexion and index finger abduction compared with rest, we adjusted the size of the test MEP by decreasing the TMS stimulus intensity to match the size of the test MEP at rest. SICI was also tested by adjusting the size of the test MEP to match that of the resting test MEP. SICI was calculated by expressing the size of the conditioned MEP as a percentage of the size of the test MEP. Twenty test MEPs and 20 conditioned MEPs were tested in each condition.
CMEPs.
Since voluntary contraction into elbow flexion and index finger abduction increased MEP size in the ES muscle to a larger extent than elbow extension and thumb abduction, we examined subcortical contributions to changes in ES MEP size during elbow flexion and index finger abduction by stimulating the corticospinal tract at the cervicomedullary junction using a circular magnetic coil (diameter, 90 mm) located over one side of the neck, lateral, or near the inion with current flowing downward in the coil (Bunday et al. 2014; Chiou et al. 2018; Taylor and Gandevia 2004). The position of the coil was marked on the subjects using a removable marker pen once the optimal coil position for evoking the largest CMEP was identified. The coil was held firmly to the back of the neck of the subject by one of the experimenters, and since our voluntary contractions were isometric, there was very little head displacement observed during the contractions. The latency of CMEPs was also monitored frame-by-frame to ensure that the stimulation was consistent and accurate across trials. Cervical root activation was investigated by increasing the intensity until an abrupt decrement in latency occurred and then decreasing the intensity and verifying that the response was potentiated by a small background contraction (Taylor 2006). The latency of CMEPs was significantly shorter than MEPs elicited by TMS (CMEP = 10.2 ± 1.2 ms and MEP = 16.7 ± 1.9 ms; P < 0.001) indicating that the stimulation activated corticospinal axons directly. CMEPs were tested at rest and during 20% of MVC into elbow flexion or index finger abduction with the contralateral arm (n = 8) using an intensity needed to elicit a CMEP with a peak-to-peak amplitude of ~0.1 mV (intensity: 92.2 ± 6.7% MSO). Ten CMEPs were tested in each condition.
Data analysis.
Data were analyzed using SigmaPlot software (version 12.5; Systat Software, San Jose, CA). Normal distribution and homogeneity of variances were tested by the Shapiro-Wilk's test and by the equal variance test, respectively. If the data failed the normality test (P < 0.05), nonparametric tests were used. Repeated-measures ANOVA was performed to determine the effect of CONDITION (rest, elbow flexion, elbow extension, index finger abduction, and thumb abduction) on MEP size and mean rectified EMG in the ES muscle and the effect of MUSCLE (BB, TB, FDI, and APB) on the level of muscle activity. Repeated-measures ANOVA was also used to examine the effect of SUBCONDITION (rest, elbow flexion, and index finger abduction) on SICI adjusted and unadjusted and CMEPs in the ES muscle. Paired t-tests were employed to compare the latencies of MEPs elicited by TMS over the primary motor cortex and the cervicomedullary junction. Holm-Sidak post hoc test was used to test for significant comparisons. Significance was set at P < 0.05. Group data are presented as the means ± SD in the text.
RESULTS
EMG.
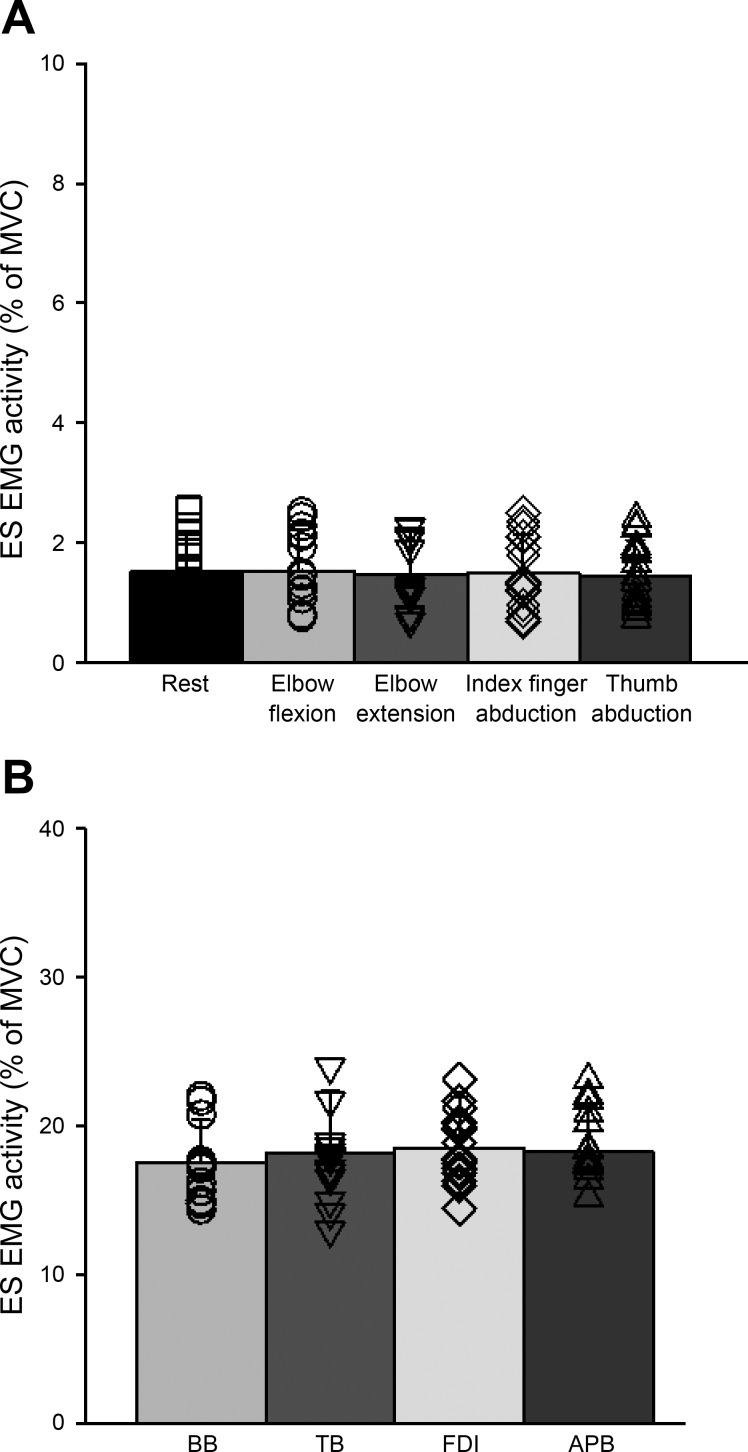
Repeated-measures ANOVA showed no effect of CONDITION (F4,60 = 0.9, P = 0.47, n = 16; Fig. 2A) on mean rectified EMG activity in the contralateral ES muscle. This result indicates that mean rectified ES EMG activity remains constant when the right arm was at rest or performed 20% of MVC into index finger abduction, thumb abduction, elbow flexion and elbow extension. We also found no effect of MUSCLE (F3,45 = 2.1, P = 0.1, n = 16; Fig. 2B) on the level of muscle contraction exerted by each muscle tested across conditions. Furthermore, repeated-measures ANOVA showed no effect of CONDITION (F4,60 = 0.65, P = 0.63, n = 16) on mean rectified EMG activity in the ipsilateral ES muscle.
Fig. 2.
Electromyography (EMG). A: group data showing background EMG in erector spinae (ES) across voluntary contractions (n = 16). Testing was completed with the trunk resting on a chair (conditioned referred here as “rest”) and when subjects performed index finger abduction, thumb abduction, elbow flexion, and elbow extension in a pseudorandomized order. The abscissa shows the condition tested (rest, elbow flexion, elbow extension, index finger abduction, and thumb abduction), and the ordinate shows the mean background EMG activity in the ES muscle [as a %maximal voluntary contraction (MVC)]. B: group data showing the background EMG in each muscle tested during 20% of MVC (n = 16). The abscissa shows the muscle tested [biceps brachii (BB), triceps brachii (TB), first dorsal interosseous (FDI), and abductor pollicis brevis (APB)] and the ordinate shows the contraction level (as a % of MVC). Note that individual data are shown for each condition. Error bars indicate the SD. *P < 0.05.
MEPs.
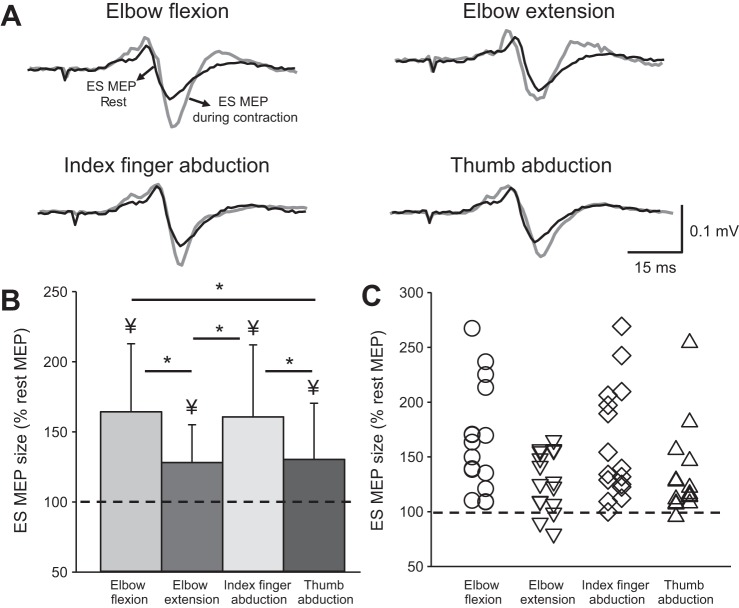
Figure 3A illustrates traces of averaged MEPs elicited by TMS over the primary motor cortex in the ES muscle from a representative subject. Note that the size of MEPs in the ES muscle increased during all voluntary contractions compared with rest but to a larger extent during elbow flexion and index finger abduction.
Fig. 3.
Motor-evoked potentials (MEPs). A: MEPs traces recorded from the erector spinae (ES) muscle of a representative subject. Traces show the average of 20 MEPs in the ES muscle at rest (black traces) and during 20% of maximal voluntary contraction (MVC; gray traces). B: group data (n = 16) showing MEPs in ES across conditions. The abscissa shows the condition tested (elbow flexion, elbow extension, index finger abduction, and thumb abduction) and the ordinate shows the size of the ES MEP during 20% of MVC (as a % of the ES MEP obtained at rest). The horizontal dashed line represents the size of the ES MEP at rest. Note that the amplitudes of MEPs in the ES muscle increased during all voluntary contractions, with greater facilitation observed during elbow flexion and index finger abduction. C: note that that majority of participants show increases in ES MEPs during all voluntary contractions compared with rest. Error bars indicate the SD. *P < 0.05, comparison between voluntary contractions. ¥P < 0.05, comparison between rest and all voluntary contractions.
Repeated-measures ANOVA revealed an effect of CONDITION (F4,60 = 11.29; P < 0.001) on ES MEP size (n = 16; Fig. 3B). Post hoc tests showed that ES MEP amplitude increased during elbow flexion (164.31 ± 48.58%, P < 0.001; 16/16), elbow extension (128.07 ± 27.03%, P = 0.04; 13/16), index finger abduction (160.73 ± 51.39%, P < 0.001; 15/16), and thumb abduction (130.36 ± 40.10%, P = 0.03; 14/16) compared with rest. Note that changes in MEP size in the ES muscle were also larger during elbow flexion compared with elbow extension (P = 0.01) and thumb abduction (P = 0.02). In addition, changes in MEP size in the ES muscle were larger during index finger abduction compared with elbow extension (P = 0.02) and thumb abduction (P = 0.04). The majority of subjects showed larger ES MEP size during elbow flexion (16/16) and during index finger flexion (15/16; Fig. 3C). No difference was found in the amplitudes of the ES MEP between elbow flexion and index finger abduction (P = 0.9) or between elbow extension and thumb abduction (P = 0.8).
SICI.
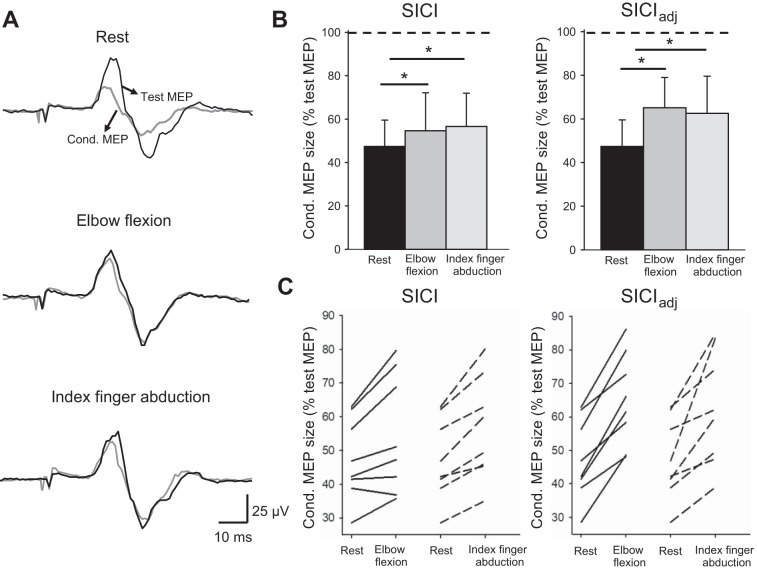
Figure 4A illustrates averaged data from SICI measurements in a representative subject. Note that the magnitude of SICI decreased during elbow flexion and index finger abduction compared with rest. Repeated-measures ANOVA revealed an effect of SUBCONDITIONS (F2,14 = 11.8, P < 0.001, n = 8) on ES MEP size (Fig. 4B). Post hoc tests showed that SICI in the ES decreased during elbow flexion (P = 0.008) and during index finger abduction (P = 0.001; Fig. 4B, left). Since MEP size increased during voluntary contraction, SICI was also tested with an adjusted test stimulus intensity. Similarly, there was a decrease in SICIadj in the ES during elbow flexion (P = 0.002) and during index finger abduction (P = 0.005) compared with rest (Fig. 4B, right). Note that SICIadj in the ES was reduced in all participants (8/8) during elbow flexion and during index finger abduction compared with rest (Fig. 4C). Mean background EMG in the ES was similar across the conditions tested (F2,14 = 1.2, P = 0.3, n = 8).
Fig. 4.
Short-interval intracortical inhibition (SICI). A: SICI recorded from the erector spinae (ES) muscle of a representative subject. Traces show the average of 20 test motor-evoked potentials (Test MEP, black traces) and conditioned MEPs (Cond. MEP, gray traces) indicated by arrows. B: group data showing SICI in the ES (n = 8). The abscissa shows the subconditions tested in the unadjusted (rest, elbow flexion and index finger abduction) and adjusted (rest, elbow flexion and index finger abduction) conditions. The ordinate shows the size of the conditioned MEP expressed as a % of the test MEP. The horizontal dotted line shows SICI at rest. Note that SICI decreased (increased conditioned MEP size) during elbow flexion and index finger abduction when SICI was tested with an adjusted and unadjusted test MEP size. C: note that all participants show reduction in SICIadj during elbow flexion (solid lines) and index finger abduction (dotted lines) compared with rest. Error bars indicate the SD. *P < 0.05, comparison between subconditions.
CMEPs.
Figure 5A illustrates examples of averaged CMEPs in the ES muscle in a representative subject. Note that ES CMEP size remained similar during contralateral elbow flexion and index finger abduction compared with rest. Repeated-measures ANOVA revealed no effect of SUBCONDITIONS (F2,14 = 2.36; P = 0.13, n = 8) on ES CMEP size, suggesting that the amplitude of CMEPs in the ES muscles remain the same at rest, during elbow flexion and index finger abduction. Mean background EMG in the ES was similar across the conditions tested (F2,14 = 2.07; P = 0.16, n = 8).
Fig. 5.
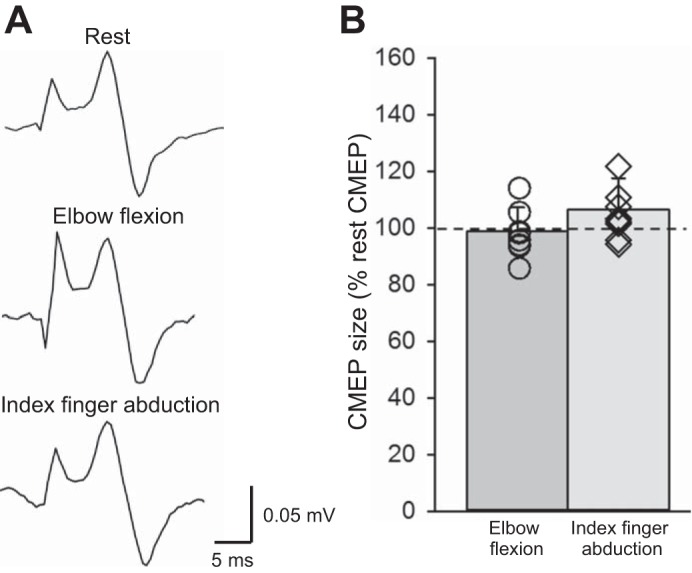
Cervicomedullary motor-evoked potentials (CMEPs). A: CMEPs recorded from the erector spinae (ES) muscle of a representative subject. Traces show the average of 10 CMEPs. B: group data (n = 8). The abscissa shows the conditions tested (elbow flexion and index finger abduction). The ordinate shows the size of the CMEPs (as % of the rest CMEP). The horizontal dashed line represents the size of the CMEP at rest. Note that individual data are shown for each condition. Error bars indicate the SD. *P < 0.05, comparison between subconditions.
DISCUSSION
Our findings demonstrate that corticospinal excitability of a trunk muscle increases during voluntary activation of proximal and distal arm muscles in intact humans. Specifically, we found that MEP size in the ES muscle increased during elbow flexion and extension and during index finger abduction and thumb abduction. Note that the ES MEP facilitation was greater during elbow flexion and index finger abduction compared with the other voluntary contractions. SICI decreased and CMEPs remained unchanged in the ES during elbow flexion and index finger abduction compared with rest, suggesting that crossed facilitatory interactions are mediated at the level of the primary motor cortex. We argue that these findings might reflect the role of proximal and distal arm muscles during functionally relevant arm and trunk movements.
Crossed facilitation of a trunk muscle during arm voluntary contraction.
Our results agree with a previous study showing that MEP size of the voluntarily active ES muscle increases during strong levels of contralateral shoulder abduction when tested in standing and lying postures (Davey et al. 2002). We extended these results and for the first time examined crossed corticospinal facilitation between the trunk and different proximal and distal arm muscles and the mechanisms contributing to this effect. We found that MEPs in the ES muscle increased in size during elbow flexion and extension and during index finger abduction and thumb abduction, with greater facilitation observed during elbow flexion and index finger abduction. This agrees with topographical studies of the primary motor cortex showing that motor cortical zones controlling various forelimb segments are largely interconnected (Capaday et al. 1998; Huntley and Jones 1991). This is also consistent with evidence showing that crossed corticospinal facilitatory effects are present not only between contralateral homologous muscles but also between bilateral nonhomologous muscles (Chiou et al. 2013; Hortobâgyi et al. 2003; Perez and Cohen 2008; Zijdewind and Kernell 2001). An intriguing question is why ES MEPs were more facilitated by elbow flexion than elbow extension. If the primary motor cortex controls different forelimb segments as a whole rather than individually (Devanne et al. 2002), one might expect that all proximal muscles will exert similar facilitatory effects on the size of MEPs in the ES muscle. Although the representations of elbow flexor and extensor muscles in the primary motor cortex are close in monkeys (Kwan et al. 1978) and humans (Penfield and Boldrey 1937), some differences exist in the neural control of these muscles. For example, the intrinsic properties of human elbow flexor and extensor motor units differ (Wilson et al. 2015) and phase-dependent modulation of MEPs is present in elbow flexors but not in elbow extensors during arm cycling (Spence et al., 2016), supporting the view that both muscles are subject to different motor control principles. In addition, evidence showed that ipsilateral MEPs tested by TMS over the primary motor cortex are frequently elicited in elbow flexors while they are not present in elbow extensors (Ziemann et al. 1999). Thus it is possible that elbow flexors might be better suited to contribute to the stronger interaction with the back extensors observed in this study. This is also consistent with evidence showing that dynamic elbow flexion but not elbow extension changes MEP size of the ES muscle (Christmas et al. 2016). It is important to note that crossed facilitatory effects are more pronounced during strong levels of voluntary activity (Muellbacher et al. 2000; Perez and Cohen 2008). However, it is less likely that this factor contributed to our results since we found that the level of EMG activity exerted during elbow flexion and extension was similar across voluntary contractions.
Another important question is why ES MEPs were more facilitated by index finger abduction compared with thumb abduction. Electrophysiological and biomechanical studies suggest that the control of index finger and thumb muscles differ. In monkeys, a single corticomotoneuronal cell does not facilitate the FDI and APB muscles simultaneously (Buys et al. 1986), which might contribute to relatively independent movements of these digits. In humans, the size of MEPs in the FDI increased in accordance with the posture of the hand during grasping but it remained unchanged in the APB (Perez and Rothwell 2015). Hand trajectory during pointing is affected during reaching movements involving the trunk (Adamovich et al. 2001). During a postural task involving the whole arm, MEPs in the FDI were greater when the task involved precise force control with the hand (Schieppatti et al. 1996). Biomechanical studies also showed that during grasping the APB has a more stabilizing role (Chao et al. 1976) while the FDI contributes to the fine grading of forces (Maier and Hepp-Reymond 1995). Therefore, it is possible that these physiological and biomechanical features make the FDI more suitable to have stronger facilitatory interactions with the ES muscle during arm movements.
Neuronal mechanisms.
We found a decrease in intracortical inhibition in the ES muscle during contralateral elbow flexion and index finger abduction. These results agree with previous findings suggesting that intracortical circuits contribute to modulate crossed corticospinal facilitation between arm muscles (Chiou et al. 2013; Perez and Cohen 2008). This agrees with lesion experiments and single-unit recordings in monkeys suggesting that the primary motor cortex is involved in the coordination of limb segments (Kalaska and Drew 1993). Since ~50% of corticospinal neurons project to both proximal and distal arm muscles (McKiernan et al. 1998), it is possible that extensive intraspinal branching of corticospinal axons might also contribute to interactions found in our study. Indeed, it could also be argued that changes in spinal excitability might contribute to crossed facilitation in the ES muscle, since subcortical mechanisms have been shown to be involved in some crossed corticospinal facilitatory effects in intact humans (Muellbacher et al. 2000; Stedman et al. 1998). Since we found no changes in the size of CMEPs, it is less likely that our results reflect changes in corticospinal transmission or motoneuron excitability (Taylor and Gandevia 2004; Ugawa et al. 1994). The latencies of CMEPs in the ES muscle were shorter than the latency of MEPs elicited by TMS over the primary motor cortex, supporting the view that corticospinal axons were stimulated directly. In addition, the location of magnetic stimulation was distant from the root outflow for the ES at vertebral level T12; thus it is unlikely that any direct stimulation of the relevant ventral roots innervating ES T12 occurred. Our results are also consistent with a previous study showing a lack of contribution of subcortical pathways to crossed facilitation when similarly low levels of voluntary contraction were performed (Stedman et al. 1998). However, others have shown that crossed facilitatory effects also involve changes in spinal reflexes (Hortobâyi et al. 2003); therefore, this possibility cannot be completely excluded.
Functional significance.
It is possible that the observed crossed facilitatory effects are relevant to limb and trunk interactions during unimanual and bimanual actions (Carson et al. 2008; Lee et al. 2010). Functional interactions between the trunk and arm muscles are well recognized with ample evidence showing that when the arms are moved, trunk muscle activity increases concurrently (Aruin and Latash 1995; Benvenuti et al. 1997; Bouisset and Zattara 1987; Hodges and Richardson 1997b). Studies showed greater activation of the ES muscle when different arm muscles are active during functional motor tasks such as pushups (Marcolin et al. 2015). Even the onset of muscle activity in the ES has been shown to depend on the direction of the arm movement (Hodges and Richardson 1997b). The greater facilitation from elbow flexors to the ES can be used to support postural perturbations since arm flexion movements are likely to cause anterior displacement of the center of mass that requires activation of the trunk extensors (i.e., ES) to minimize the postural displacement (Aruin and Latash 1995; Hodges and Richardson, 1997b). The more pronounced facilitatory effects of the FDI to the ES can also be related to anticipatory postural adjustments needed to stabilize the whole arm before upcoming finger activation (Caronni and Cavallari 2009). Indeed, the earlier onset of anticipatory postural adjustments for proximal arm muscles has been associated with higher precision of pointing movements (Bruttini et al. 2016). Altogether, our findings support the view of strong interactions between the neural control of trunk and proximal and distal hand muscles in intact humans.
These results may have clinical relevance since previous evidence suggests that crossed corticospinal facilitatory effects might be beneficial in improving arm function in patients with specific neurological disorders (Hamzei et al. 2012; Kowalczewski et al. 2011). Since many patients with stroke (Verheyden et al. 2006) or spinal cord injury (Field-Fote and Ray 2010) have reduced trunk control, the use of the arms to increase corticospinal excitability of projections to trunk muscles may increase neural interactions, which could contribute to improve functional outcomes. As such, crossed facilitation between arm and trunk muscles might represent an opportunity for trunk rehabilitation and its effect on functionally relevant motor tasks remain to be tested.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant 1-R01-NS-090622 and Department of Veterans Affairs Grants I01RX000815 and I01RX002472.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.-Y.C., P.H.S., and M.A.P. conceived and designed research; S.-Y.C., P.H.S., and M.A.P. performed experiments; S.-Y.C., P.H.S., and M.A.P. analyzed data; S.-Y.C., P.H.S., and M.A.P. interpreted results of experiments; S.-Y.C., P.H.S., and M.A.P. prepared figures; S.-Y.C., P.H.S., and M.A.P. drafted manuscript; S.-Y.C., P.H.S., and M.A.P. edited and revised manuscript; S.-Y.C., P.H.S., and M.A.P. approved final version of manuscript.
REFERENCES
- Adamovich SV, Archambault PS, Ghafouri M, Levin MF, Poizner H, Feldman AG. Hand trajectory invariance in reaching movements involving the trunk. Exp Brain Res 138: 288–303, 2001. doi: 10.1007/s002210100694. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. Directional specificity of postural muscles in feed-forward postural reactions during fast voluntary arm movements. Exp Brain Res 103: 323–332, 1995. doi: 10.1007/BF00231718. [DOI] [PubMed] [Google Scholar]
- Benvenuti F, Stanhope SJ, Thomas SL, Panzer VP, Hallett M. Flexibility of anticipatory postural adjustments revealed by self-paced and reaction-time arm movements. Brain Res 761: 59–70, 1997. doi: 10.1016/S0006-8993(97)00260-6. [DOI] [PubMed] [Google Scholar]
- Bouisset S, Zattara M. Biomechanical study of the programming of anticipatory postural adjustments associated with voluntary movement. J Biomech 20: 735–742, 1987. doi: 10.1016/0021-9290(87)90052-2. [DOI] [PubMed] [Google Scholar]
- Bruttini C, Esposti R, Bolzoni F, Cavallari P. Higher precision in pointing movements of the preferred vs. non-preferred hand is associated with an earlier occurrence of anticipatory postural adjustments. Front Hum Neurosci 10: 365, 2016. doi: 10.3389/fnhum.2016.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Oudega M, Perez MA. Aberrant crossed corticospinal facilitation in muscles distant from a spinal cord injury. PLoS One 8: e76747, 2013. doi: 10.1371/journal.pone.0076747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Tazoe T, Rothwell JC, Perez MA. Subcortical control of precision grip after human spinal cord injury. J Neurosci 34: 7341–7350, 2014. doi: 10.1523/JNEUROSCI.0390-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Perez MA. Impaired crossed facilitation of the corticospinal pathway after cervical spinal cord injury. J Neurophysiol 107: 2901–2911, 2012. doi: 10.1152/jn.00850.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys EJ, Lemon RN, Mantel GW, Muir RB. Selective facilitation of different hand muscles by single corticospinal neurones in the conscious monkey. J Physiol 381: 529–549, 1986. doi: 10.1113/jphysiol.1986.sp016342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacho EW, de Oliveira R, Ortolan RL, Varoto R, Cliquet A Jr.. Upper limb assessment in tetraplegia: clinical, functional and kinematic correlations. Int J Rehabil Res 34: 65–72, 2011. doi: 10.1097/MRR.0b013e32833d6cf3. [DOI] [PubMed] [Google Scholar]
- Capaday C, Devanne H, Bertrand L, Lavoie BA. Intracortical connections between motor cortical zones controlling antagonistic muscles in the cat: a combined anatomical and physiological study. Exp Brain Res 120: 223–232, 1998. doi: 10.1007/s002210050396. [DOI] [PubMed] [Google Scholar]
- Caronni A, Cavallari P. Anticipatory postural adjustments stabilise the whole upper-limb prior to a gentle index finger tap. Exp Brain Res 194: 59–66, 2009. doi: 10.1007/s00221-008-1668-2. [DOI] [PubMed] [Google Scholar]
- Carson RG, Kennedy NC, Linden MA, Britton L. Muscle-specific variations in use-dependent crossed-facilitation of corticospinal pathways mediated by transcranial direct current (DC) stimulation. Neurosci Lett 441: 153–157, 2008. doi: 10.1016/j.neulet.2008.06.041. [DOI] [PubMed] [Google Scholar]
- Chao EY, Opgrande JD, Axmear FE. Three-dimensional force analysis of finger joints in selected isometric hand functions. J Biomech 9: 387–396, 1976. doi: 10.1016/0021-9290(76)90116-0. [DOI] [PubMed] [Google Scholar]
- Chiou SY, Gottardi SE, Hodges PW, Strutton PH. Corticospinal excitability of trunk muscles during different postural tasks. PLoS One 11: e0147650, 2016. doi: 10.1371/journal.pone.0147650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SY, Hurry M, Reed T, Quek JX, Strutton PH. Cortical contributions to anticipatory postural adjustments in the trunk. J Physiol 596: 1295–1306, 2018. doi: 10.1113/JP275312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SY, Wang RY, Liao KK, Wu YT, Lu CF, Yang YR. Co-activation of primary motor cortex ipsilateral to muscles contracting in a unilateral motor task. Clin Neurophysiol 124: 1353–1363, 2013. doi: 10.1016/j.clinph.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Christmas D, Dave S, Chiou SY, Strutton PH. Effects of dynamic arm cycling on crossed-corticospinal facilitation to trunk muscles (Abstract). 46th Annual Meeting of the Society for Neuroscience San Diego, CA, November 12–16, 2016. [Google Scholar]
- Davey NJ, Lisle RM, Loxton-Edwards B, Nowicky AV, McGregor AH. Activation of back muscles during voluntary abduction of the contralateral arm in humans. Spine 27: 1355–1360, 2002. doi: 10.1097/00007632-200206150-00019. [DOI] [PubMed] [Google Scholar]
- Devanne H, Cohen LG, Kouchtir-Devanne N, Capaday C. Integrated motor cortical control of task-related muscles during pointing in humans. J Neurophysiol 87: 3006–3017, 2002. doi: 10.1152/jn.2002.87.6.3006. [DOI] [PubMed] [Google Scholar]
- Field-Fote EC, Ray SS. Seated reach distance and trunk excursion accurately reflect dynamic postural control in individuals with motor-incomplete spinal cord injury. Spinal Cord 48: 745–749, 2010. doi: 10.1038/sc.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzei F, Läppchen CH, Glauche V, Mader I, Rijntjes M, Weiller C. Functional plasticity induced by mirror training: the mirror as the element connecting both hands to one hemisphere. Neurorehabil Neural Repair 26: 484–496, 2012. doi: 10.1177/1545968311427917. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Contraction of the abdominal muscles associated with movement of the lower limb. Phys Ther 77: 132–142, 1997a. doi: 10.1093/ptj/77.2.132. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Feedforward contraction of transversus abdominis is not influenced by the direction of arm movement. Exp Brain Res 114: 362–370, 1997b. doi: 10.1007/PL00005644. [DOI] [PubMed] [Google Scholar]
- Hortobágyi T, Taylor JL, Petersen NT, Russell G, Gandevia SC. Changes in segmental and motor cortical output with contralateral muscle contractions and altered sensory inputs in humans. J Neurophysiol 90: 2451–2459, 2003. doi: 10.1152/jn.01001.2002. [DOI] [PubMed] [Google Scholar]
- Huntley GW, Jones EG. Relationship of intrinsic connections to forelimb movement representations in monkey motor cortex: a correlative anatomic and physiological study. J Neurophysiol 66: 390–413, 1991. doi: 10.1152/jn.1991.66.2.390. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Drew T. Motor cortex and visuomotor behavior. Exerc Sport Sci Rev 21: 397–436, 1993. doi: 10.1249/00003677-199301000-00013. [DOI] [PubMed] [Google Scholar]
- Kaminski TR, Bock C, Gentile AM. The coordination between trunk and arm motion during pointing movements. Exp Brain Res 106: 457–466, 1995. doi: 10.1007/BF00231068. [DOI] [PubMed] [Google Scholar]
- Kowalczewski J, Chong SL, Galea M, Prochazka A. In-home tele-rehabilitation improves tetraplegic hand function. Neurorehabil Neural Repair 25: 412–422, 2011. doi: 10.1177/1545968310394869. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519, 1993. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan HC, MacKay WA, Murphy JT, Wong YC. Spatial organization of precentral cortex in awake primates. II. Motor outputs. J Neurophysiol 41: 1120–1131, 1978. doi: 10.1152/jn.1978.41.5.1120. [DOI] [PubMed] [Google Scholar]
- Lee M, Hinder MR, Gandevia SC, Carroll TJ. The ipsilateral motor cortex contributes to cross-limb transfer of performance gains after ballistic motor practice. J Physiol 588: 201–212, 2010. doi: 10.1113/jphysiol.2009.183855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MF. Interjoint coordination during pointing movements is disrupted in spastic hemiparesis. Brain 119: 281–293, 1996. doi: 10.1093/brain/119.1.281. [DOI] [PubMed] [Google Scholar]
- Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip. I. Contribution of 15 finger muscles to isometric force. Exp Brain Res 103: 108–122, 1995. doi: 10.1007/BF00241969. [DOI] [PubMed] [Google Scholar]
- Marcolin G, Petrone N, Moro T, Battaglia G, Bianco A, Paoli A. Selective activation of shoulder, trunk, and arm muscles: a comparative analysis of different push-up variants. J Athl Train 50: 1126–1132, 2015. doi: 10.4085/1062-6050-50.9.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan BJ, Marcario JK, Karrer JH, Cheney PD. Corticomotoneuronal postspike effects in shoulder, elbow, wrist, digit, and intrinsic hand muscles during a reach and prehension task. J Neurophysiol 80: 1961–1980, 1998. doi: 10.1152/jn.1998.80.4.1961. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol 111: 344–349, 2000. doi: 10.1016/S1388-2457(99)00243-6. [DOI] [PubMed] [Google Scholar]
- Ortu E, Deriu F, Suppa A, Tolu E, Rothwell JC. Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. J Physiol 586: 5147–5159, 2008. doi: 10.1113/jphysiol.2008.158956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield WG, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60: 389–443, 1937. doi: 10.1093/brain/60.4.389. [DOI] [Google Scholar]
- Perez MA, Butler JE, Taylor JL. Modulation of transcallosal inhibition by bilateral activation of agonist and antagonist proximal arm muscles. J Neurophysiol 111: 405–414, 2014. doi: 10.1152/jn.00322.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci 28: 5631–5640, 2008. doi: 10.1523/JNEUROSCI.0093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Rothwell JC. Distinct influence of hand posture on cortical activity during human grasping. J Neurosci 35: 4882–4889, 2015. doi: 10.1523/JNEUROSCI.4170-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res 151: 330–337, 2003. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W; The International Federation of Clinical Neurophysiology . Magnetic stimulation: motor evoked potentials. Electroencephalogr Clin Neurophysiol Suppl 52: 97–103, 1999. [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res 113: 24–32, 1997. doi: 10.1007/BF02454139. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Trompetto C, Abbruzzese G. Selective facilitation of responses to cortical stimulation of proximal and distal arm muscles by precision tasks in man. J Physiol 491: 551–562, 1996. doi: 10.1113/jphysiol.1996.sp021239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence AJ, Alcock LR, Lockyer EJ, Button DC, Power KE. Phase- and workload-dependent changes in corticospinal excitability to the biceps and triceps brachii during arm cycling. Brain Sci 6: E60, 2016. doi: 10.3390/brainsci6040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman A, Davey NJ, Ellaway PH. Facilitation of human first dorsal interosseous muscle responses to transcranial magnetic stimulation during voluntary contraction of the contralateral homonymous muscle. Muscle Nerve 21: 1033–1039, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- Taylor JL. Stimulation at the cervicomedullary junction in human subjects. J Electromyogr Kinesiol 16: 215–223, 2006. doi: 10.1016/j.jelekin.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. Noninvasive stimulation of the human corticospinal tract. J Appl Physiol (1985) 96: 1496–1503, 2004. doi: 10.1152/japplphysiol.01116.2003. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation of corticospinal pathways at the foramen magnum level in humans. Ann Neurol 36: 618–624, 1994. doi: 10.1002/ana.410360410. [DOI] [PubMed] [Google Scholar]
- Verheyden G, Vereeck L, Truijen S, Troch M, Herregodts I, Lafosse C, Nieuwboer A, De Weerdt W. Trunk performance after stroke and the relationship with balance, gait and functional ability. Clin Rehabil 20: 451–458, 2006. doi: 10.1191/0269215505cr955oa. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Thompson CK, Miller LC, Heckman CJ. Intrinsic excitability of human motoneurons in biceps brachii versus triceps brachii. J Neurophysiol 113: 3692–3699, 2015. doi: 10.1152/jn.00960.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Ishii K, Borgheresi A, Yaseen Z, Battaglia F, Hallett M, Cincotta M, Wassermann EM. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol 518: 895–906, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdewind I, Kernell D. Bilateral interactions during contractions of intrinsic hand muscles. J Neurophysiol 85: 1907–1913, 2001. doi: 10.1152/jn.2001.85.5.1907. [DOI] [PubMed] [Google Scholar]