Acute myeloid leukemia (AML) is the most common adult leukemia with ≈21,000 cases diagnosed annually in the U.S.A. This genetically heterogeneous malignancy is characterized by aberrant proliferation of myeloid progenitor cells and a partial block in differentiation, which results in a build-up of immature blast cells in the bone marrow and peripheral blood of patients, causing physical symptoms such as bleeding, infection and anemia, eventually leading in death. The FMS-like tyrosine kinase-3 receptor tyrosine kinase (FLT3), normally involved in the growth, differentiation and survival of hematopoietic and dendritic cells, is dysregulated in ≈30% of AML patients as a result of somatic mutations and imparts a poor prognosis. The most common mutations, found in ≈25% of AML patients and <5% of myelodysplastic syndrome (MDS) patients, result in internal tandem duplications (ITD) in the juxtamembrane domain of the receptor; rarer mutations occur within the “activation loop” of FLT3 and have been identified in ≈7% of AML patients.1 The contribution of oncogenic, constitutively activated FLT3 to cellular transformation, combined with its prevalence, suggested that targeting FLT3 could provide therapeutic benefit in AML. Of the ensuing FLT3 kinase inhibitors investigated in pre-clinical studies and clinical trials, the broad-spectrum kinase inhibitor, midostaurin (CGP41251; PKC412; Rydapt®), is the first health authority-approved targeted therapy for the treatment of mutant FLT3-positive AML. Here, we outline the early design of midostaurin, the preclinical discovery of its activity against oncogenic FLT3, and its subsequent clinical development as a therapeutic agent for FLT3 mutant-positive AML and several rare blood disorders.
Midostaurin (Figure 1) was identified in a drug discovery effort aimed towards optimizing the protein kinase C inhibitory activity of staurosporine, a natural product isolated from Streptomyces staurosporeus, which had been shown to inhibit the growth of leukemia and melanoma cell lines.
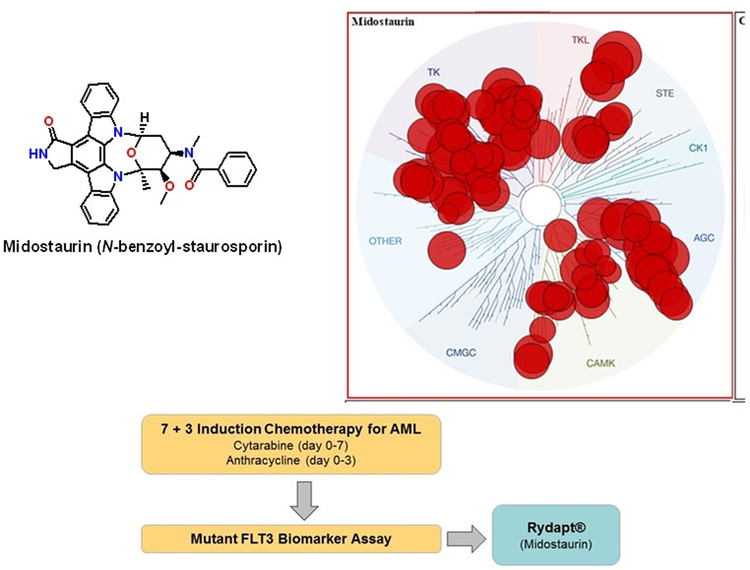
Figure 1.
Schematic for midostaurin as a multi-targeted clinical therapeutic for AML. Shown is the structure for midostaurin and a representation of its effects on the human kinome (upper panel). Midostaurin is used in combination with 7+3 induction chemotherapy for patients that have tested positive for mutant FLT3 (lower panel). The kinase dendrogram is adapted and is reproduced with permission from Cell Signaling Inc. Reprinted with permission from Zarrinkar et al. Blood 2009;114:2984–2992.
Based upon supportive antiproliferative activity in tumor cell lines and murine xenograft models, midostaurin progressed into clinical trials, both as single agent and in combination with chemotherapy in patients bearing solid tumors or having lymphoproliferative disorders, but although a well-tolerated dosing regimen was identified, drug efficacy was insufficient to warrant further clinical development.2 Midostaurin was subsequently shown to inhibit the activity of several additional protein kinases, including the PDGF and VEGF receptor kinases and the drug was evaluated as an angiogenesis inhibitor the treatment of diabetic retinopathy.2
In 2001, a collaborative effort between the Dana-Farber Cancer Institute and Novartis Pharmaceuticals was conducted to identify inhibitors of mutant FLT3-positive AML in cell-based assays. At low nanomolar concentrations, midostaurin was found to potently inhibit the proliferation of murine hematopoietic cells that had been transfected with constructs encoding either an ITD or a D835Y point mutation in the FLT3 kinase to render them growth factor independent3. In these early studies, midostaurin was then demonstrated to suppress growth and induce apoptosis in many mutant FLT3-positive AML cells, as well as to inhibit cell cycle progression, via inhibiting FLT3 kinase activity.3 Furthermore, oral administration of midostaurin to mice transplanted with marrow transduced to induce an AML-like disease, substantially prolonged survival.3
The understanding that activating mutations in FLT3 conferred a poor prognosis in AML, spurred the clinical investigation of FLT3 inhibition as a therapeutic approach for the disease, and as such midostaurin was tested in relapsed patients at doses determined to be well-tolerated in earlier clinical studies. Although clinical efficacy as a single agent was found to be limited, co-administration of midostaurin with standard chemotherapy in advanced patients led to improved clinical responses. Based upon these encouraging results, midostaurin was investigated in a large, randomized phase III trial where it was added to standard induction therapy (cytarabine and daunorubicin induction and cytarabine consolidation, also known as 7+3 regimen) in patients with newly-diagnosed FLT3-positive AML, where it was found to significantly enhance patient survival. 4
Midostaurin was approved by the FDA in April, 2017 (by the EMA in September), for newly diagnosed, mutant FLT3-positive, adult AML patients, as part of a combination therapy approach with cytarabine and daunorubicin induction and cytarabine consolidation; in parallel the LeukoStrat CDx FLT3 Mutation Assay was approved as a companion diagnostic (Figure 1). At the same time, midostaurin was approved as monotherapy for adult patients with rare blood disorders, including mast cell leukemia (MCL), aggressive systemic mastocytosis (ASM), and systemic mastocytosis with associated haematological neoplasm (SM-AHN). This resulted from a concomitant development, based upon midostaurin potently inhibiting the D816V-mutated KIT receptor tyrosine kinase, which plays a significant role in the pathogenesis of mast cell malignancies.5
Prior to year 2000, because of the many kinases known to be crucial for intracellular signaling and the knowledge that most bound ATP within a highly homologous binding site, there was much skepticism that it would be possible to design ATP-competitive protein kinase inhibitors with adequate selectivity to be efficacious at tolerated doses. However, the success of imatinib, a relatively selective type-2 ATP-competitive kinase inhibitor, in treating chronic myeloid leukemia (CML) allayed many of these concerns. In contrast, midostaurin inhibits a large number of protein kinases through binding within the catalytic site in a type-1, ATP-competitive fashion. However, this might be an attribute, since it is now appreciated that the clinical profile of midostaurin in AML is distinct from that of other agents targeting FLT3,4leading to speculation that the efficacy results from a combination of inhibitory effects of midostaurin on multiple kinases important for leukemic cell viability. Abnormal signaling independent of FLT3 may contribute to the growth and viability of transformed cells, despite the presence of a FLT3 inhibitor. Adding to a survival advantage is the cross-talk between components of major signaling pathways that function downstream of oncogenic FLT3. Examples of kinases that might be involved include IGF1R, JAK2, KIT, LYN, PDK1, PDGFR, RET, SYK and VEGFR, implicated in intracellular signaling in leukemic cells as well as stromal support for cell viability (Figure 1). It is thus believed that the efficacy of midostaurin in patients may be at least in part attributed to its polypharmacology, which might effectively inhibit those factors allowing for survival of AML in the presence of small molecule FLT3 inhibition.
Acknowledgments
FUNDING: This work was funded by the NIH grant P01CA66996. P.W.M. is an employee of Novartis Pharma AG.
References
- (1).Weisberg E, Barrett R, Liu Q, Stone R, Gray N, Griffin JD (2009) FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist Updat 12, 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Fabbro D, Buchdunger E, Wood J, Mestan J, Hofmann F, Ferrari S, Mett H, O’Reilly T, and Meyer T (1999) Inhibitors of protein kinases: CGP 41251, a protein kinase inhibitor with potential as an anticancer agent. Pharmacol. Ther 82, 293–301. [DOI] [PubMed] [Google Scholar]
- (3).Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, Gilliland DG, Griffin JD (2002) Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell 1, 433–443. [DOI] [PubMed] [Google Scholar]
- (4).Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, Thiede C, Prior TW, Döhner K, Marcucci G, Lo-Coco F, Klisovic RB, Wei A, Sierra J, Sanz MA, Brandwein JM, de Witte T, Niederwieser D, Appelbaum FR, Medeiros BC, Tallman MS, Krauter J, Schlenk RF, Ganser A, Serve H, Ehninger G, Amadori S, Larson RA, Döhner H (2017) Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 377, 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, Awan FT, Hexner E, Mauro MJ, Sternberg DW, Villeneuve M, Huntsman Labed A., Stanek EJ, Hartmann K, Horny HP, Valent P, Reiter A (2016) Efficacy and safety of midostaurin in advanced systemic mastocytosis. N. Engl. J. Med 374, 2530–2541. [DOI] [PubMed] [Google Scholar]