Abstract
Physical activity plays an essential role in maintaining a healthy body, yet it also provides unique benefits for the vascular and cellular systems that sustain a healthy brain. While the benefit of exercise has been observed in humans of all ages, the availability of preclinical models has permitted systematic investigations into the mechanisms by which exercise supports and protects the brain. Over the past twenty-five years, rodent models have shown that increased physical activity elevates neurotrophic factors in the hippocampal and cortical areas, facilitating neurotransmission throughout the brain. Increased physical activity (such as by the voluntary use of a running wheel or regular, timed sessions on a treadmill) also promotes proliferation, maturation and survival of cells in the dentate gyrus, contributing to the process of adult hippocampal neurogenesis. In this way, rodent studies have tremendous value as they demonstrate that an ‘active lifestyle’ has the capacity to ameliorate a number of age–related changes in the brain, including the decline in adult neurogenesis. Moreover, these studies have shown that greater physical activity may protect the brain health into advanced age through a number of complimentary mechanisms: in addition to upregulating factors in pro-survival neurotrophic pathways and enhancing synaptic plasticity, increased physical activity promotes brain health by supporting the cerebrovasculature, sustaining the integrity of the blood–brain barrier, increasing glymphatic clearance and proteolytic degradation of amyloid beta species, and regulating microglia activation. Collectively, preclinical studies demonstrate that exercise initiates diverse and powerful neuroprotective pathways that may converge to promote continued brain health into old age. This review will draw on both seminal and current literature that highlights mechanisms by which exercise supports the functioning of the brain, and aids in its protection.
INTRODUCTION
Regular exercise plays essential role in maintaining a healthy lifestyle. It bolsters the cardiovascular, and immune and metabolic systems and in addition, modulates the internal milieu of the brain [1–6]. Both acute, high-intensity activity and regular, moderate aerobic exercise have been reported to increase levels of circulating neurotrophic factors and enhance neurotransmission, exerting beneficial effects on mood and cognitive functions in individuals of all ages [7–16]. In young adults, an acute bout of high-intensity cycling induced an increase in serum levels of brain-derived neurotrophic factor (BDNF) that correlated with blood lacate [7], as did long-term exercise of moderate intensity (i.e. 5 weeks of cycling, 3 times per week, at intensities below the ventilatory threshold) [8]; both regimens correlated with improved cognitive performance. Adults over 65 years of age that participated in regular exercise (e.g. walking, hiking, biking, weight-training, stretching, or others) score higher on common memory tests and have been shown to be at a reduced risk for dementia and impairment in executive functions associated with advancing age [17–21]. These benefits often correlated with measures such as frequency and/or intensity of exercise, and caloric expenditure. Exercise such as walking, aerobic exercise, and strength training, was also shown to improve executive functions and cognition in individuals already suffering from early stages of dementia [18, 22–24]. To this end, the importance of exercise in supporting continued brain health in the aging brain, even individuals affected by the early stages of neurodegeneration, is gaining recognition. Moreover, because studies suggest that physical activity can reduce the risk of cognitive decline, an active lifestyle can be thought of as a ‘preventative’ strategy to ameliorate the deterioration of brain health much as it is with cardiovascular dysfunction.
In humans, age-related changes in the brain commonly underlie cognitive decline; these may include progressive reductions in neurotrophic levels, alterations in the cerebrovasculature, and declining neurogenesis [25–34]. The hippocampus is particularly vulnerable to age-related morphological and functional alterations [34–39]. Similar age-related alterations have been observed in rodents, making them an ideal model for identifying changes that occur on a cellular level and for investigating interventional or preventative strategies [40–43]. As in humans, rodent models have shown age–related reductions in hippocampal volume [38, 44, 45]. This is, in part, a consequence of a decline in cell proliferation [46, 47] and morphologic changes in the rodent brain, such as reductions in dendritic branching, spine densities, and fibres projecting into the hippocampus [38, 42, 43, 45, 48–51]. In addition, advanced age in both rodents and humans have been associated with a reduction in trophic support to the brain, such as declining levels of BDNF and its full-length receptor, tropomyosin receptor kinase B (TrkB) [35, 37, 42]. The loss of neurotrophic support is concomitant with attenuated neuronal survival and synaptic plasticity [29, 36, 43, 48, 50, 52, 53]. Importantly, age-related neurological changes correlate with functional decline, particularly in hippocampus-dependent learning and memory tasks, and navigational strategies [5, 54–59].
Notably, though the hippocampus is known to be a principle site of mammalian adult neurogenesis [60], there has been some discrepancy as to whether this occurs in humans [61–65]. A recent paper by Sorrells and colleagues found no evidence for neurogenesis beyond youth [64], while Boldrini et al. published a paper shortly thereafter showing that neurogenesis did in fact persist into adulthood, with a small age-related decline [61], in keeping with previous studies [62, 65]. Indeed, numerous studies have suggested that adult-born neural stem cells may be stimulated to form distinct populations of neurons and though these cells may lose proliferative potential and become quiescent with age, they can be reactivated upon stimulation, including running[63, 66–71].
Over two decades ago, mice living in enriched cages were showed to have elevated neurogenesis in the dentate gyrus, an effect that is most remarkable in young rodents; these cages were larger, housed many mice and contained a number of accessories (including a running wheel) [56, 72, 73]. Subsequent studies dissociated the different elements of the enriched environment, and demonstrated that voluntary running alone is sufficient to increase cell proliferation and survival in the hippocampus, enhance synaptic plasticity and improve cognitive performance [54, 56, 57, 74, 75]. In young mice, treadmill training and voluntary wheel running has been consistently associated with enhanced performance on hippocampal-dependent memory tasks such as the Y-Maze and Morris water maze [54, 56, 57, 74, 76], as well as tasks that do not specifically depend on the learning or consolidation of spatial memory (i.e. contextual fear conditioning, passive avoidance learning, and novel object recognition) [75, 77–81]. In aging rodents, exercise attenuates the age-related decline in cell proliferation and differentiation [41, 54, 57]. Moreover, just one month of voluntary running ameliorates many neurological and behavioural deficits associated with aging [41, 54, 57, 82, 83]. Notably, even short bouts of mild-intensity exercise (4–6 minutes on 5 consecutive days, for 5 weeks) initiates pro-survival pathways in aged rats [82]. In addition, mice that underwent long-term voluntary running throughout middle-age exhibited improved performance on spatial memory tasks later in life, which correlated with an increase in BDNF levels and enhanced neurogenesis in the dentate gyrus [41, 54, 76].
Rodent models have shown that short-term periods of increased physical activity are sufficient to upregulate central and peripheral factors that support the brain, and promote synaptic plasticity [4, 5, 12, 84–86] (Table 1). Nerve growth factor (Ngf), Bdnf and TrkB messenger RNA (mRNA) levels are upregulated in the hippocampus of adult rats after 3 days of free access to a running wheel, as are genes related to synaptic trafficking and signal transduction [85]. In addition, exercise promotes trophic support to the vasculature, including increased central and circulating levels of insulin-like growth factor 1 (IGF-1), which has also been shown to support neurotransmission and neuronal survival [1, 2, 56, 87–90]. The greater, systemic benefits of exercise and their relation to brain health cannot be understated, as the neuroprotective effects of exercise extend beyond the promotion of neurotrophic pathways and hippocampal neurogenesis. Voluntary wheel running has been shown to upregulate tight-junction (TJ)–associated proteins of the blood-brain barrier (BBB), which protects the brain from circulating molecules that might contribute to toxicity, inflammation, or pathogenesis [91–93]. Running has also been shown to reduce microglia activation and cytokine levels in the hippocampus of aged mice, representing another mechanism by which exercise may protect the neurovascular unit and in turn, the brain from insult [6, 94, 95]. In models of advanced aging and Alzheimer’s disease (AD), exercise reduces soluble amyloid-beta (Aβ) load and plaque formation, in part mediated by glymphatic clearance and proteolytic degradation [76, 94, 96–99]. Voluntary exercise has also been shown to delay white matter atrophy and protect cerebral vasculature in mouse models of AD, correlating with improved performance on spatial memory tasks [100–102].
Table 1.
Summary of the effects of increased physical activity on the rodent brain. The neuroprotective benefits of physical activity are noted in a variety of ages; ‘age at onset’ describes the age, if specified, at which the activity paradigm (whether it be acute or chronic) is commenced. (The specific effects on vasculature are not included in this table.) Please see abbreviation listed below the table
| System affected by increased activity | Age at onset, Species, Sex | ’Exercise’ duration and protocol | Details |
| Blood-brain barrier/Neurovascular unit | 7-8 weeks; 13 weeks, mice (male) | 5 weeks; 2 weeks (respectively), VWR | Reduced pro-inflammatory cytokines and methamphetamine-induced oxidative stress in cerebral vasculature; enhanced expression and/or co-localisation of tight junction proteins (e.g. claudin-5, occludin and ZO-1) [91, 93] |
| 12 weeks, mice (male) | 5 weeks, VWR | Maintained BBB integrity (enhanced expression and/or co-localisation of tight junction proteins claudin-5, occludin and ZO-1) in a mouse model of early brain metastasis; limited tumour extravasation [132] | |
| adult, rats (male) | 3 consecutive days, TR•30 min, speeds increasing from 5 m/min to 12 m/min, 0 incline | Reduced the expression of MMP, and mitigated BBB disruption (specifically, the reduction of occludin) following middle cerebral arterial occlusion in ischemia model of stroke (TR within 24 hrs) [130] | |
| 12 months, mice (female) | 6 months, VWR | VWR from middle to early old-age attenuated age-related deterioration of neurovascular structures and vascular leakage (shown by extravasation of fibrin[ogen]), microglial activation, and decline in astrocytic ApoE; the benefit to the NVU was not seen in exercised ApoE deficient mice [92] | |
| Glymphatic System | 9 weeks, mice (female) | 5 weeks, VWR (averaged 6.7 km per night) | Glymphatic influx (as measured by fluorescent tracer) increased in awake, exercised mice as compared to mice that had been sedentary; overall tracer influx was impaired during acute running (measured in awake running mice); history of daily running increased CSF flux in widespread brain regions (primarily in hypothalamus and ventral parts of the cortex) [145] |
| 14–16 months, mice (male) | 6 weeks, VWR | Increased expression of AQP4 on astrocytic endfeet; accelerated clearance of tracer and decreased Aβ accumulation; increased dendrites, dendritic spines and postsynaptic density protein (PSD95); improved spatial memory [94] | |
| Neurotrophins | |||
| BDNF | 8 weeks, mice (male) | Acute session, TR•120 min, at 15 m/min, with an incline of 5% •mice were acclimated to motorized TR running for 3 days (5 min/day,15 m/min, 5%); acute session began 72 hrs after acclimatisation | Obese, glucose-intolerant mice (fed high-fat diet) shown to have a reduction in BDNF levels, as well as reduction in TrkB phosphorylation and CREB activation in the prefrontal cortex; 2 hrs following an acute session of TR, levels of phosphorylated TrkB and CREB significantly elevated over sedentary mice fed the same high fat diet [347] |
| 2 – 5 months, mice and rats | 3 days – 4 weeks, VWR | BDNF consistently upregulated with VWR, along with proteins associated with BDNF signalling cascade; most notably increased in dentate gyrus, CA1, CA3, and CA4 of the hippocampus, and in the most caudal third of cortex (examples: [4, 5, 85, 86, 186, 190, 348, 349]) | |
| 2 months, rats (male) | 3 days, VWR (against 100 g resistance) | Positive correlation between exercise and hippocampal protein levels of [synaptic proteins] synapsin I and synaptophysin mRNA; synapsin I correlated with the amount of exercise (i.e. running distance); blocking TrkB (BDNF signalling) abolished the upregulation of both proteins [86] | |
| 3 months, rats (male) | 1 week, VWR (against 100 g resistance)•minimum of 100 m/day | Increased BDNF, concomitant with improved performance on MWM; inhibiting the action of BDNF blocked cognitive enhancement following exercise, as well as downstream proteins involved in synaptic plasticity (CREB and synapsin I); best performance (learning and recall) associated with highest expression of BDNF and CREB mRNA levels [5] | |
| 9 months, mice (female) | 8 months, VWR (note: same animals underwent behavioural testing at 1 and 6-months) | VWR throughout middle-age (i.e. 8 months total) increased BDNF protein levels in the hippocampus of 15 month-old runners compared to age-matched controls; reduced age-related cognitive decline [48] | |
| 8 or 12 months, mice (male) | 5 or 8 weeks, TR•began at 10 m/min, 20 min for the first day and increased 10 min/day until 60 min/day reached; intensity increased to maintain 70% of animals’ VO2 max; speed increased to 11 m/min in final week | Increased proliferation and maturation in dentate gyrus, and restored age-dependent decline in BDNF and TrkB [350] | |
| 24 months, rats (female) | 1 week habituation + 4 weeks exercise regimen, TR (4 consecutive days/week)•daily regimen: warm-up (3 min, 2 m/min), two bouts of running (4–6 min, 10 m/min) with 1 min interval between | In aging rats, shorts bouts of mild-intensity exercise increased muscle oxygen consumption by soleus and heart; lactate levels remained stable throughout 4 weeks (levels indicative of mild-moderate intensity exercise); reversed age-related spatial learning and memory impairment; increased Bdnf mRNA and protein in hippocampus; increased levels of phosphorylated AKT and phosphorylated CREB protein in the hippocampus; results suggest even short bouts of exercise effective at facilitating hippocampal plasticity [82] | |
| 4 weeks, mice (male) | 4 weeks | Increased production of DBHB, a ketone body produced in the liver and capable of crossing the BBB, which in turn induced activity of BDNF promotors in the hippocampus via HDAC2/HDAC3 inhibition; increase in neurotransmitter release, dependent upon TrkB signalling [192] | |
| NGF | 3 – 4 months, rats (male) | 2, 4 or 7 days, VWR•mice acclimated to wheel for 3 days, then removed for 10 before testing [3, 190] | NGF is increased in the acute/short-term phase, reported in the hippocampus at 2 – 3 days and in the cortex, from 2 – 7 days [3, 85, 190] |
| 3 – 4 months, rats (male) | 4, 7 and 28 days, VWR•1 week acclimation•at least 5 km/day [85] | ||
| IGF-1 | adult, rats (male) | 5 days, VRW (against 100 g resistance) | 5 days VWR increased hippocampal levels of IGF-1, but not IGF-2; improved performance on MWMBlocking hippocampal IGF-1 receptors during 5-day exercise period blocked the uptake of circulating IGF-1 into the hippocampus and in turn, diminished the upregulation of BDNF and its precusor, pro-BDNF; diminished rate of acquisition and abolished preference for probe quadrant in MWM; eliminated exercise-facilitated upregulation of synapsin I mRNA and protein in the hippocampus [1] |
| adult, rats (male) | 2 weeks, TR•60 minutes, 17 m/min•rats assigned to both sedentary group and exercise familiarised to treadmill | In response to exercise, IGF-1 participates in neuronal stimulation and c-fos expression in hippocampus; subcutaneous administration of IGF-1 to sedentary animals increased number of new neurons in the hippocampus; infusion of IGF-1 anti-serum blocked uptake of circulating IGF-1 into hippocampus, which abolished increase in new neurons; infusion of IGF-1 anti-serum throughout exercise period abolished both short-term and long-term survival of new cells in hippocampus [89] | |
| Note: Physical activity produces increase in IGF-1 levels in circulation and hippocampus; uptake of circulating IGF-1 into hippocampus involved in both exercise-induced elevation in BDNF and neurogenesis; infusion of IGF-1 increases glutamate receptor subunits (particularly NR2A and NR2B) in aged mice; IGF-1 levels correlated with vascular density [1, 29, 43, 200, 215, 219, 220] | |||
| Neurotransmitters | |||
| DA | young, rats (male) | young rats: 6 months ‘endurance training’, TR•progressive treadmill test performed on an 18° incline, correlated to peak oxygen consumption [254] | Increased radioligand ([3H]SP) binding to D2 receptors in the striatum and increased “synaptic coupling ratio” (defined as the “specific DA binding/DOPAC concentration”) [254]; in aged rats, regular exercise attenuated age-associated increases in DA metabolites (which can be associated oxidative stress), and increased D2 receptor binding [351] |
| ►18 months, rats (male) ►aged rats: 12 weeks, TR [351] | |||
| 8 weeks, mice (males) | 2 or 4 weeks, TR•10 m/min for 20–60 min per day (increased at an increment of 10 min per day), 5 days/week the first week; 60 min/day at the same speed, 10 m/min, 5 days/week for additional 1 or 3 weeks•both mice assigned to exercise and sedentary groups underwent 1 week of habituation training on the treadmill (9 m/min, 10 min/day for 5 days) | TR regimen (meant to replicate moderate exercise in humans) completely protected against LPS-induced dopaminergic neuronal loss in the substantia nigra and attenuated motor impairment following 4 weeks of running; neuroprotection in the nigrostriatal pathway was dependent on the activation of the BDNF-TrkB signalling pathway rather than modulation of microglial activation or cytokine/chemokine levels; intrastriatal perfusion of BDNF alone was sufficient to counteract LPS-induced DA neuron loss; protection not observed after 2 weeks of running [246] | |
| 3 months vs 23 months, rats (female) | 9 weeks, TR•5 days/week, intensity adjusted to approximately 70% of their peak oxygen consumption (time, grade and speed increased as weeks progressed, and differed for each age group) | TH mRNA, TH immunoreactivity, and TH activity showed age-related decline in the hypothalamus; endurance training significantly elevated all TH parameters in the hypothalamus of old animals (p < 0.05), but there was no significant change in young animals following training [259] | |
| Note: DA neurotransmission has been shown to increase BDNF production, as well as surface expression and phosphorylation of TrkB (in vitro and in vivo); TrkB signalling shown to increase expression of D1 and D3 receptors (in vitro and in vivo) [246–248, 259] | |||
| NA | 3 months, rats (male) | 3 days, VWR | BDNF mRNA upregulated in CA1, CA2, CA3, CA4 and detate gyrus; modest increase in BDNF mRNA with antidepressant, tranylcypromine, CA3 and dentate gyrus only; β-adrenergic receptor blockade significantly blunted BDNF mRNA elevations in response to exercise, and inhibited modest elevations resulting from antidepressant treatment in CA3 [189] |
| young, rats (sex not specified)•age not specified, 146 + /–2 g at start of study | 5 days/week, 2 weeks (TR) (1 hour, 25 m/min, 3% slope)•workload corresponded to 70% VO2 max•4 day break, in which microdialysis probe implanted + recovery•Acute session of 1 or 2 hrs | Increased levels of NA centrally and peripherally following 1 and 2 hr exercise sessions; the peak of NA concentration in the cortex is higher with 2 hours of exercise, and levels remain elevated for longer periods as compared to a 1-hour session [266] (see also [265]) | |
| adult, rats (male)•age not specified, 220 g on arrival | 5 days, VWR (against 100 g resistance) | Blocking the β-adrenergic receptors with propranolol (β-blocker that crosses the BBB) but not nadolol (peripherally acting, does not cross the BBB) before each of five consecutive nights of exercise reversed the exercise-induced improvement in learning and memory in rat [271] (see also [270]) | |
| 5-HT | adult,rats (male)•age not specified, 300 +/–15 g at start of study | Acute session, 120 min, TR•trained 6 – 7 times prior to experiment day, gradually accustomed to run at 25 m/min; 2 days before experimentation, ran 30 min at a speed of 25 m/min | Hippocampal and cortical 5-HT levels significantly increased by 90 min of intense aerobic exercise (collected by microdialysis); maximal levels in the cortex, 30 min after exercise cessation and in the hippocampus, 60 min after cessation; hippocampal levels remained elevated at least 90 minutes after cessation; cortical 5-HT levels rapidly decrease when hippocampal levels still maximal [352] |
| 6 weeks vs 3 months vs 1 year, mice (female)•Tph2 -/- and controls | 6 days, VWR | Tph2-deficient mice have normal baseline hippocampal neurogenesis but impaired proliferation in response to increased physical activity; serotonergic deficits results in alterations in Sox2-positive precusor cells; serotonergic signalling required for proproliferative effect on physical activity, in young and aged animals [292] (see also: [293]) | |
| Glutamate | 28 – 40 days, mice (sex not specified) | 7 – 10 days, VWR averaged 4 km/day | Synaptic plasticity in dentate gyrus examined in vitro: LTP significantly greater in slices prepared from runners than control animals; LTP significantly reduced by NR2B subunit antagonists in both groups; LTP blocked by an antagonist with higher affinity for NR2A (NVP-AAM077) in running groups, with only slight depression in controls; NVP-AAM077 completely blocked LTD in runners but not controls [236] |
| 3 months, rats (male) | 3, 7 or 28 days, VWR (against 100 g resistance)•VWR followed 1 week habituation at least 5 km/day•Similar results seen with TR, 3, 7, 15 or 30 days (40 minutes daily, 10 m/min) | Increased synaptic glutamate receptors (mRNA and/or protein levels) in several brain regions including, but not limited to, the hippocampus, motor cortex, sensory cortex, and striatum; mRNA expression of NMDAR subunits modulated in hippocampus after 3 days of VMR (NR2A and NR2B); AMPA receptors modulated after 10 days of VWR or 30 days of TR [85, 186, 353] | |
| 3 months, rats (male) | 3 or 7 days, VWR at least 5 km/day (against 100 g resistance) | Blocking NMDAR (MK-801, delivered unilaterally by microsphere into hippocampus) was sufficient to fully abrogate exercise-induced increases in Bdnf, TrkB, CREB, and Synapsin I, suggesting an interaction between BDNF and glutamate signalling may be necessary for increased transcription of genes modulating synaptic plasticity [4] | |
| GABA | 5–6 weeks, mice (male) | 10 days, VWR | GABAergic transmission excitatory in the first two weeks and becomes inhibitory as granule cells mature and integrate into networks; involved in initial integration of adult-born neurons [354]Young, 1-week old progenitor cells receive input from inhibitory GABAergic interneurons and cholinergic input from the septum, as well as multiple intra-hippocampal glutamatergic cells types, all of which have been implicated in the maturation and integration of new neurons [296]; exercise enhances new dentate granule cell number, arborization and morphological complexity but only NMDA-mediated glutamatergic inputs shown to be modified by running [296] Electrophysiological recordings from slices taken from exercised mice suggest that alternations to glutamatergic inputs, rather than GABAergic inputs, are predominately responsible for increased morphological complexity in new neurons [296] |
| 5–6 weeks, mice (male) | 40 days, VWR | In new neurons, ratio of interneuron inputs to new neurons was reduced, but GABAergic inhibitory synaptic transmission was not changed by running; in mature granular cells in the outer molecular layer, synaptic inhibition was strongly increased, (possibly due to interneuron sprouting of axonal collaterals onto these cells) [297] | |
| young, rats (males),•age not specified, 140 – 160 g on arrival | 4 weeks, VWR•ran 5 – 9 km per night•began running 1 week after arrival | Gene expression of various GABAA receptor subunits as well as the GABA-synthesising enzyme glutamic acid decarboxylase-67 (GAD67) altered in the forebrain of runners (in situ hybridisation histochemistry): region-specific decreases in mRNA expression of α2, β3 and γ2 GABAAR subunits and region-specific increases in β1 subunit; α5 and δ subunits showed differential increases in mRNA expression levels; GAD67 mRNA increased in many forebrain regions, including all hippocampal cell layers, peri-paraventricular nucleus, bed nucleus stria terminalis, nucleus accumbens core and motor cortex [241] | |
| 6 weeks, mice (male) | 6 weeks | During cold water swim stress, increased expression of the protein products of the immediate early genes c-fos and arc in granular neurons (new and mature) of sedentary mice but not runnersRunners showed enhanced local inhibitory mechanisms in the hippocampus during stress test: increased in stress-induced activation of hippocampal interneurons, expression of vesicular GABA transporter, and extracellular GABA release [355] | |
| ACh | adult, rats (male) | 5 minutes, walking on treadmill (2.3 m/min, 0 incline) | Increased ACh (as well as NA and 5-HT) levels in cerebral cortex, sampled from freely-behaving animals by microdialysis [226, 227, 356] |
| 3–4 months, rats (male) | 30 seconds or 3 minutes, walking on treadmill (4 cm/s, 0 incline) | Increased ACh in hippocampus; increased regional blood flow; abolished by AChR antagonists; various degrees of physical activity shown to elevate ACh levels in cortex and hippocampus [357] | |
| ►26–29 months, ‘healthy aged’ rats (male) | ► 30 seconds or 3 minutes, walking on treadmill (2, 4, or 8 cm/s, 0 incline) | ►Similarly, increased ACh release in hippocampus of aged rats (likely cholinergic fibres that originate in the septal complex of forebrain and project to hippocampus); increased regional blood flow [237]. | |
| adult, mice (male) | 15 consecutive days, TR (30 min, 5 m/min, 0 incline) | In scopolamine-treated mice, a pharmacological model of amnesia, treadmill exercise ameliorated short-term memory impairment, suppressed AChE expression, and enhanced angiogenesis [239] | |
| Note: An increased concentration of ACh in the hippocampus supports the generation of theta oscillations, which serves to facilitate synaptic plasticity, learning and memory [240, 324, 325]. | |||
| Neurogenesis | 3 weeks, mice (female) | 40 days in enriched environment (tunnels, toys and running wheel; 3 mice per cage)►second group survived 68 days total (after 40 days in enriched or control environments, tested on MWM for 5 consecutive days, then returned to assigned environments for an additional 23 days) | Housing in enriched environments, which included running wheels, induced neurogenesis: when mice were sacrificed 1 day after final BrdU injection (daily, 12 days), no significant difference between two groups suggesting little influence on proliferative activity of progenitor cells in dentate gyrus; when mice sacrificed 4 weeks after last injection, a significantly higher number of BrdU+ cells in the dentate gyrus of mice living in enriched environments, suggesting a survival-promoting effect on proliferating neuronal precursors; studies in mice of an alternative background (129/SvJ rather than C57BL/6) did show a significant increase in the number of progenitor cells under similar conditions [72, 73] |
| 3 months, mice (female) | 12 days, 40 days, VWR (vs other conditions in enriched environments) | Running is sufficient to increase hippocampal neurogenesis: an increase in both proliferating cells (measured 1 day after last BrdU injection, injected daily for 12 days) as well surviving neurons, after an additional 4 weeks of VWR, seen in running group and enriched environment groups only LTP and spatial learning in mice [56] | |
| 3 months, mice (female) | 2 months or 4 months, VWR 1+ months | Improved performance on MWM, increased cell proliferation (as measured by BrdU+ cells) and enhanced LTP in the dentate gyrus. | |
| 3 months vs 19 months, mice (male) | 45 days, VWR | Faster acquisition and better retention on MWM than sedentary age-matched controls; age-related decline in neurogenesis ameliorated (compared to young and aged controls); fine morphology of new neurons did not differ between young and aged runners; perimeter and surface area of blood vessel increased in young runners but not aged mice; angiogenesis was not a rate-limiting factor for neurogenesis (angiogenesis not increased in this study, although reported to be increase in motor cortex, cerebellum and hippocampus following running in other studies) [57] | |
| 3 months vs, 12 and24 months, mice (male) | 6 months VWR (young mice) vs 10 days VWR (aged) | Chronic (i.e. long-term) running starting at 3 months of age attenuated age-dependent decline in precursor cell proliferation measured at 9 months; short-term running reduced age-related decline in cell proliferation at 12 and 24 months, but did not return net neurogenesis to ‘young levels’ in this study [41] |
ACh – acetylcholine; AChE – acetylcholinesterase; AKT – Protein kinase B; BDNF – brain derived neurotrophic factor; BrdU – bromodeoxyuridine; CA – cornu Ammonis (i.e. hippocampal subfield); CREB – cAMP response element-binding protein; CSF – cerebrospinal fluid; DA – dopamine; DCX – doublecortin; DOPAC – 3,4-Dihydroxyphenylacetic acid (a dopamine metabolite); DRN – dorsal raphe nucleus; HDAC – histone deacetylase; 5-HT – serotonin; IGF-1 – insulin growth factor 1; LPS – Lipopolysaccharide; LTD – long-term depression; LTP – long-term potentiation; MMP – matrix metalloproteinases; MWM – Morris Water Maze; NA – noradrenaline; NMDAR – N-methyl-D-aspartate receptor; NR2A/2B – NMDAR subunits 2A and 2B; NGF – neurotrophic growth factor; TR – treadmill running (controlled for duration and speed); VWR – voluntary wheel running (animals are freely behaving); ZO-1 – zonula occludens 1.
The remainder of the review will survey preclinical literature reporting the neuroprotective benefits of exercise, with special attention paid to the mechanisms by which exercise supports a healthy aging brain*. Exercise, as it pertains to these animal studies, will be defined as a greater degree of physical activity than is otherwise permitted in a control home cage either by granting free access to a running wheel, or through timed session on a treadmill. Drawing from both seminal and recent literature, the mechanisms by which exercise promotes the maintenance of BBB integrity, clearance of Aβ peptides, and upregulation of neurotransmitters and neurotrophic factors (principally, BDNF) will be discussed.
EXERCISE MAINTAINS THE BBB AND SUPPORTS THE NEUROVASCULAR UNIT
The BBB in health and disease
The BBB is a highly selective semi-permeable membrane at the interface between the circulatory system and brain parenchyma, formed of specialised endothelial cells that compose the walls of all cerebral blood vessels (Fig. 1). The endothelial cells are in turn encased by a layer of pericytes, astrocytic endfoot processes and neurons. Together, these cells comprise the neurovascular unit, communicating with one another to tightly regulate cerebral blood-flow and maintain a homeostatic chemical environment within the brain [103, 104].
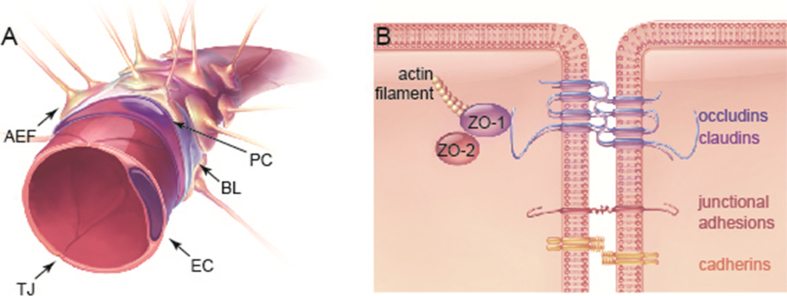
Fig.1.
The anatomy of the blood–brain barrier (BBB). (A) The BBB is comprised of astrocytic endfeet (AEF) and pericytes (PC), which surround a single layer of endothelial cells. Endothelial cells secrete extracellular matrix proteins, forming the basal lamina (BL). Endothelial cells are connected by tight junctions (TJ) that form a highly selective semi-permeable membrane, separating the brain’s parenchyma from the circulatory system. (B) TJs anchor endothelial cells close together through a number of transmembrane proteins (e.g. occludin, claudins and junctional adhesion molecules). These TJ proteins interact with cytoplasmic scaffolding proteins such as zonula occludens (ZO), as well as with the actin cytoskeleton. Other integral membrane proteins and cell adhesion molecules, such as cadherins and integrins, are also found at cell-cell junctions [105]. Exercise has been shown to increase the expression of claudins, as well as the co-expression of proteins from the ZO and occludin family at the plasma membrane.
TJs between adjacent endothelial cells set the brain’s vasculature apart from most other blood vessels in the body. They are primarily formed by transmembrane proteins (occludins, claudins, and junctional adhesion molecules) that interact with cytoplasmic scaffolding proteins (i.e. zonula occludens [ZO] proteins and the actin cytoskeleton), and other membrane-associated proteins (i.e. kinases, GTPases, and G proteins) [105–108]. The integrity of TJs is critical to forming the protective barrier of the BBB, which selectively limits the passage of molecules between the circulating blood and the extracellular fluid in the brain [104, 108] (Fig. 1). Both meta-analyses of human subjects and animal models have revealed an association between normal aging and BBB deterioration [109–111]. Injury or disease may contribute to BBB dysfunction, and can be associated with the presence inflammatory markers; if this state is prolonged or persistent, a compromised BBB can permit the extravasation of circulating molecules, including those that are neurotoxic [112–120].
Exercise protects the neurovascular unit
In animal models of aging, habitual long-term (or, ‘chronic’) aerobic activity has been shown to protect the neurovascular unit and maintain the integrity of the BBB. Soto and colleagues demonstrated that daily voluntary running (at an average of 3 km per night) from midlife (12 months of age) to early old age (18 months of age) reduced age-related neurovascular dysfunction in mice, leading to a ‘younger-like’ brain. Long-term voluntary running, representing an ‘active’ lifestyle, was shown to preserve the basement membrane of theneurovascular unit and to prevent the neurovascular deterioration and leakage observed in sedentary mice at 18 months: a significant reduction in fibrin levels was found in aged runners compared to sedentary age-matched controls, with levels more closely resembling those seen in 12-month-old mice [92]. Aged runners also showed higher levels and coverage of cortical and hippocampal pericytes, as well as a reduction in astrocytic and microglial activation. Notably, running prevented an age-related decline in astrocytic ApoE, an anti-inflammatory protein important in cerebrovascular maintenance and pericytic signalling [92, 121, 122]. Running was not, however, associated with a significant effect in aging ApoE-deficient mice, suggesting that ApoE may partially mediate the beneficial effect of exercise on the neurovascular unit [92]. As allelic variations in APOE are associated with decreased longevity and the development of familial forms of AD [123, 124], this is a relevant observation for translational research. It is possible that exercise may offer greater BBB protection in some individuals than others, however this would not diminish the other neuroprotective and health benefits promoted by exercise. For instance, voluntary running and treadmill training were also shown to decrease microglial and astrocytic activation in aged rodents (with some sex- and region-specific effects), which may aid in the protection of the neurovascular unit from damage [90, 94, 95].
Exercise maintains the BBB
Animal models of disease and toxicity, with injury to the BBB, can provide additional insight into the benefits of an active lifestyle. Spontaneously hypertensive rats (SHR) have been shown to have greater BBB permeability and increased angiotensin II (Ang II) in the brain parynchema (both locally-produced and circulating), which not only alters neurovascular coupling and dysregulates cerebral perfusion, but permits the extravasation of circulating inflammatory cells [125]. In a recent study, the maximal aerobic capacities of individual SHR and Wistar Koyoto (control) rats were determined at the start of experimentation, and used to ensure rats with identical capacities were allocated to both the trained and sedentary groups [125]. Rats in the exercise group then underwent aerobic treadmill exercise for 2 months, set to 50–60% of their own maximal exercise capacity (performed 5 days/week, 1 hour/day). The marked fluorescein isothiocyanate dextran (FITC, 10 kD) leakage seen in the autonomic areas of the sedentary SHR group was significantly diminished after just 2 weeks of treadmill training. Although regional Ang II content was not directly measured, authors of the study suggest that regular treadmill running may reduce Ang II content in autonomic areas, as simultaneous intracerebroventricular Ang II infusion abrogated training effects on both FITC leakage and microglia activation. This interpretation is supported by studies demonstrating that pharmacological blockage of the Ang II receptor, AT1, reduced BBB permeability in autonomic regions of hypertensive rats; AT1 receptor blockade has also been shown to reduce BBB permeability in the hippocampus and cortex [125–129].
Physical activity may support or strengthen the BBB by modulating the expression of TJ-proteins, and in the same way, aid in its recovery following injury. Started 24 hours following middle cerebral artery occlusion in rats, short-term treadmill running (30 minutes per day for 3 days) attenuated an ischemia-induced reduction in occludin, reduced disruption of the BBB, and diminished expression of matrix metalloproteinases (MMPs) [130]. Similar results were observed in rats undergoing a pre-ischemia treadmill program (30 min per day, 5 days/week, 3 weeks) [131]. Rats that had been exercised prior to ischema showed reduced infarction size and reduced Evan’s Blue extravasation; moreover, exercised rats show enhanced expression of proteins associated with the basal lamina such as collagen IV, a major component of the basement membrane of cerebral microvessel. In a murine model of brain metastasis, mice that ran for 5 weeks prior to tumor cell infusion showed enhanced expression of claudin-5 and occludin [132]. Furthermore, while tumor cells target ZO-1 to increase BBB permeability, prior running resulted in a reduction in tumor cell extravasation at both 48-hour and 3-week time points.
As an interventional strategy, exercise was reported to be protective in a mouse model of drug–induced damage to the BBB. Methamphetamine (METH) exposure has been shown to reduce the expression of claudin-5, occludin and ZO-1, and disrupt the co-localisation of occludin and ZO-1 in the plasma membrane [91, 93]. Two weeks of voluntary running following exposure to METH enhanced the expression of TJ proteins, stabilised the BBB, and attenuated a METH-induced increase in systemic pro-inflammatory cytokines. Furthermore, exercise significantly increased the number cells co-labelled with bromodeoxyuridine (BrdU) and NeuN, a neuronal nuclear antigen, compared to sedentary mice following METH administration. This demonstrated that, in addition to its effects on the BBB, exercise can attenuated the suppression of neurogenesis associated with METH-toxicity [91, 93].
Together, these studies indicate that engagement in regular sessions of aerobic physical activity has the capacity to strengthen the BBB by upregulating TJ-associated proteins, and in this way, protects the brain from circulating insults. Reductions in BBB permeability were reported whether exercise programs was administered prior to or following an injury (such as that imposed by ischemia or drug toxicity), with a rapid increase in TJ proteins observed after just 3–14 days of scheduled treadmill running or voluntary wheel running (respectively) [91, 130].
EXERCISE PROMOTES GLYMPHATIC CLEARANCE IN AGED MICE
The glymphatic system is defined by a space between the vessel basement membrane and glial limitans formed around pre- and post-capillary vessels, in which the cerebrospinal fluid exchanges with interstitial fluid. The astrocytic endfeet that line the paravascular space express astrocyte water channel aquaporin 4 (AQP4), a bidirectional channel believed to facilitate influx and efflux of molecules between these fluids. Glympathic influx delivers glucose and signalling molecules to the cerebrospinal fluid; efflux, conversely, has an important role in the clearance of waste products and compounds in the interstitial fluid (including Aβ and tau), and eventually drains into the subarachnoid space and cervical lymph nodes [133]. Glymphatic activity has been found to be increased in sleep, and decreased in traumatic brain injury, stroke, and subarachnoid hemorrhage [134–136]. In addition, glymphatic transport is reduced with age and certain diseases, such as small vessel disease [134, 137–141]. Furthermore, glymphatic dysfunction has implications in disorders characterised by accumulation of abnormal proteins, such as AD. For example, the accumulation of Aβ40 and cerebral amyloid angiopathy (CAA) can result in Aβ deposits in the basement membrane drainage pathways, in turn impeding the glymphatic elimination of Aβ and interstitial fluid from the brain [141–143]. Since the glymphatic system is believed to be under immune surveillance by blood-borne macrophage and T cells, it serves as an important bridge between the brain and rest of the body [138]. Nonetheless, it is worth noting that our understanding of the mechanisms of the glymphatic system, or a similar sort of fluid filtration system, still requires refinement [144].
In middle-aged mice, 6 weeks of voluntary running has been shown to accelerate efficiency of glymphatic clearance, assessed using intracisternal injections of a paravascular tracer. The expression of AQP4 was increased in runners, as was its distribution to astrocytic endfeet, while BBB permeability was unaltered [94, 145]. Running mice also showed reduced intraneuronal Aβ1 - 40 accumulation in the cortex as well as reduced Aβ1 - 42 deposition outward of vessel. Furthermore, access to a voluntary wheel running reduced activation of astrocytes and microglial [94], an important observation given recent focus on the relationship between the BBB and a glymphatic system [133, 139, 144]. Although research pertaining to glymphatic clearance of neurotoxins is relatively young, these studies suggest that such a system may participate in the neuroprotective benefits of increased physical activity.
EXERCISE FACILITATES CLEARANCE AND DEGRADATION OF SOLUBLE Aβ
Regular, long-term aerobic activity that commences in the pre-plaque stage has been shown to inhibit hippocampal and cortical Aβ deposition in several mouse models [76, 99, 146, 147]. TgCRND8 mice who underwent voluntary running for 3–5 months have shown reduced Aβ load, and CAA pathology [76, 99, 146]. Similar reductions in the number of plaques have been reported in Tg2576 mice [98, 148]. Ten weeks to 5 months of treadmill training in APP/PS1 trangenic mice (beginning at 3 months of age) also inhibited the expression of AD-like pathology, including robust reductions in both Aβ deposition and tau phosphorylation [147, 149, 150]. Recent work has shown that housing animals in enriched environments (equipped with activity wheels) is effective at ameliorating memory deficits related to Aβ toxicity, as induced by intra-hippocampus infusions of Aβ25 - 35 [151]. When housed in an enriched environment prior to the introduction of Aβ, which provides both voluntary access to physical activity and cognitive stimulation, rats showed improved performance on short–and long–term novel object recognition challenges. Exercised rats also presented lower lipid peroxidation, showing that physical activity may protect against Aβ-induced cellulardamage [151].
Recent studies have focused on the mechanisms by which exercise attenuates the build-up of Aβ in the brain [96, 97, 101, 150, 152–154]. The supportive role of exercise in glymphatic clearance of toxic molecules, including Aβ, was described in the previous section. A 2017 study showed that exercise can also decrease Aβ production in NSE/APPsw mice, by biasing the processing of amyloid precursor protein (APP) toward a non-amyloidogenic pathway [96]. This effect was mediated by enhancement of the sirtuin-1 pathway. Authors demonstrated that treadmill running reduced levels of the β-site APP cleaving enzyme (BACE-1), which is responsible for the first cleavage step in the transformation of APP into Aβ. In addition, exercise enhanced expression of a disintegrin and metalloproteinase domain-containing protein 10 (ADAM-10), which cleaves APP in a nonamyloidogenic pathway [96]. These changes correlated with reductions in cortical Aβ40 and Aβ42, reduced cortical markers of cell death, and improved performance on the Morris water maze [96].
Moore and colleagues used Tg2576 mice, which normally develop amyloid plaques around 9 months of age, to determine if the preventative effects of exercise are ‘dose dependent’ using two different exercise regimens [97]. Animals engaged in either low-intensity training or high-intensity treadmill training, where soleus muscle citrate synthesis was increased by 39% and 71% relative to sedentary mice, respectively. In both in the cortex and hippocampus, treadmill training from 3 to 6 months of age reduced soluble Aβ40 and Aβ42 in an exercise-training dose-dependent manner. Moreover, there was an upregulation of 5 proteins involved in Aβ clearance (or efflux) and degradation: neprilysin, insulin-degrading enzyme (IDE), matrix metallopeptidase 9 (MMP9), low-density lipoprotein-related receptor 1 (LRP1) and heat-shock protein 70 (HSP70). Upregulation of these proteins was also dependent on training intensity [97]. The intensity-dependent reduction of Aβ is an interesting observation, as it is consistent with the positive correlation between duration and/or intensity of exercise and BDNF production noted in both humans and animals.
The studies described here demonstrate that exercise protects against Aβ build-up when commenced in a pre-plaque period and in this way, it can be considered in the context of a greater preventative strategy. In genetic animal models, the effects on Aβ load could not be recapitulated when exercise was commenced after plaque onset [155], however studies of AD patients in nursing homes have reportedpositive cognitive effects on cognition and behaviour that can likely be attributed to some of the other factors positively affected by exercise [22, 156, 157].
EXERCISE PRODUCES A RAPID UPREGULATION OF NEUROTROPHIC FACTORS
BDNF
Growth and neurotrophic factors, as well as hormones produced both in the brain and periphery, are essential for the growth and maturation, survival, and function of cells in the brain [1, 20, 89, 158–160]. BDNF, the most widely distributed neurotrophin in the brain, participates in neurogenesis, synaptogenesis and dendritogenesis, and also initiates signalling cascades that upregulate transcription of pro–survival genes [55, 161–168]. In addition, BDNF-mediated pathways promote the induction of hippocampal long-term potentiation (LTP) [161, 169]. Conditional TrkB knockout mice show age and region–specific reductions in spine density as well as altered spine morphologies in the hippocampus, which are linked to impaired LTP [38, 45]. Moreover, disruption of hippocampal BDNF/TrkB signalling — genetical or pharmacological — has been shown to result in a variety of memory deficits in rodent models, demonstrating the functional relationship between BDNF and cognition [5, 153, 159, 169–172]. In humans, BDNF and full-length TrkB are reduced with age and in early stages of AD, where decreased BDNF/TrkB signalling has associated with both cognitive decline and the presence of oligomeric Aβ1 - 42 [35, 37, 173–178]. Reductions in BDNF and full-length TrkB have also been described in aging rodents, and correlate to AD-like pathology, aberrant spine morphology, and impaired signalling pathways [42, 45, 174, 179].
BDNF is known to be elevated by physical activity. In healthy human participants, BDNF has been reported to be elevated in blood serum both after one acute session of moderate to intense (anaerobic) exercise as well as by chronic aerobic training, such as 5 weeks of training on a stationary bike, 3 times per week, where workload was gradually increased to maintain 60% of maximal oxygen consumption (VO2 max) for 60 minutes [8, 9, 180, 181]. However, meta-analyses revealed that regular exercise augments the effect of any single exercise session on serum levels of BDNF, and that only aerobic exercise had an effect on resting BDNF levels [182, 183]. And while BDNF is robustly up-regulated in contractingmuscle fibres, analyses of the arterial-to-internal jugular venous difference in plasma BDNF levels suggest that neurotrophin produced and released from the brain contributes 70–80% of circulating levels following 4 hours of aerobic exercise [9].
As in humans, increased physical activity in rodents elicits a rapid elevation in the transcription and translation of BDNF and TrkB in the hippocampus. Indeed, a positive correlation between levels of hippocampal BDNF and the activation of gene transcription pathways has been described after only 6 hours of voluntary running in mice (during which they ran approximately 6 km), mimicking an acute session of exercise in humans [184]. (Note: Six days earlier, mice had been exposed to the wheel for 48 hours to habituate them to the wheel). In rats, the upregulation of Bdnf mRNA has been well-characterised after 2 days of voluntary wheel running and TrkB, after 7 days [3–5, 85, 185–190]. The upregulation of Bdnf mRNA is region-specific, demonstrated by in situ hybridisation in rats aged 3 and 22 months; changes are most notable in the dentate gyrus, CA1, CA3, and CA4 of the hippocampus, and in the most caudal third of cortex, though less robust in the aged cohort [188, 191].
The uptake of peripherally circulating factors, released in greater quantities during exercise, contribute to central increases in BDNF production [1, 85, 192] (Fig. 3). Blocking hippocampal uptake of circulating IGF-1 or the IGF-1 receptor significantly blunts the exercise-induced increase in Bdnf mRNA and protein, as well as that of it precursor protein [1, 75, 193]. Recently, β-hydroxybutyrate (DBHB), a BBB-permeable ketone body produced in the liver, has been shown to accumulate and stimulate production of BDNF in the hippocampus following exercise [192]. There, it acts as both an energy source and an inhibitor of class I histone deacetylases to enhance BDNF expression. It has also been shown to attenuate stress-induced behavioural and hippocampal neuroinflammation [194]. Notably, exercise-associated increases of hippocampal BDNF are replicated by direct ventricular application of DBHB [192]. And while there is an age–related decline in the transport of DBHB into the brain, studies have suggested that exercise has the capacity to regulate the expression of the monocarboxylate transporters that mediate its passage [195–199]. Together, these studies demonstrate that the production of BDNF, and in turn its effects upon neuronal survival/plasticity, can be modulated by circulating factors. As many of these factors decline with age, exercise may serve to enhance their presence and, in turn, their ability to reach the brain [29, 43, 192, 198, 200].
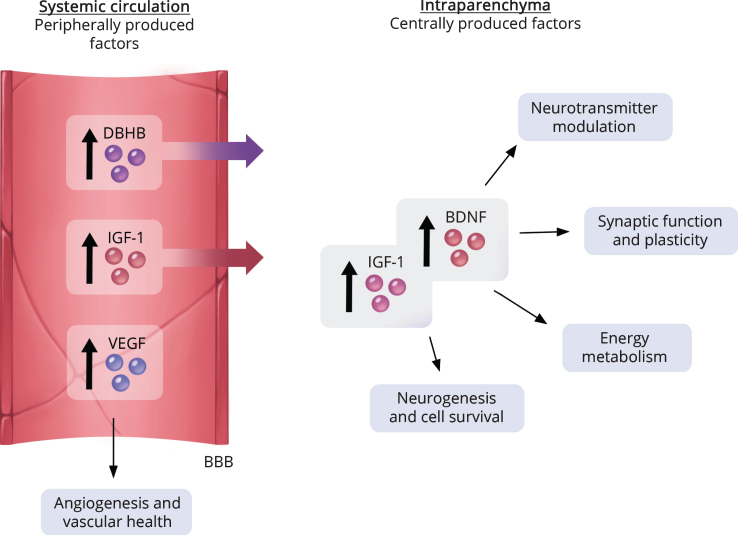
Fig.3.
Both circulating and central factors participate in exercise-facilitated protection of the brain. Exercise can increase both peripheral and central factors that co-operate in sustaining brain health. Systemic circulating factors that are elevated by exercise include beta-hydroxybutyrate (DBHB), vascular epithelial growth factor (VEGF) and insulin growth factor-1 (IGF-1). Some circulating factors, such as DBHB and IGF-1, are capable of crossing the blood-brain barrier (BBB) and may contribute to the upregulation of BDNF [16, 19, 20, 192, 209].
Notably, prolonged periods of exercise (i.e. 3 weeks to 8 months of voluntary running) produce progressive elevations of Bdnf transcription and protein translation in rodents [54, 57, 185, 201–203] (Fig. 2). In one experiment, mice who ran daily for 2, 4, 7, 14, 28 and 90 days showed a progressive increase in BDNF protein levels in the hippocampus; the results were mirrored in mice who ran on alternating days rather than daily [201, 202]. Furthermore, protein levels have been shown to remain elevated over control levels beyond the period of exercise: following a 3 week period of daily voluntary running, hippocampal levels of BDNF were reported to be elevated for at least 2 weeks [201]. Moreover, a history of exercise has also been describe as a “molecular primer” for BDNF production, where a brief exposure to running can rapidly increase hippocampal levels following a period of rest [202]. For example, in rats returning to exercise after a week-long sedentary period, 2 days of running produced BDNF levels comparable to those requiring several weeks of running in mice who were exercise naïve. The potentiation of BDNF upregulation was comparable between animals that ran daily and those who ran on alternate days (though the post-exercise decay was shorter in the latter group), suggesting that alternative exercise regimens can provide similar benefits [202]. Recent studies have addressed whether workload, in the absence of added stress, leads to greater Bdnf transcription by comparing rats assigned to 7 days of no exercise, voluntary running, or voluntary running on a loaded ‘resistance’ wheel [204]. One week of running on a loaded wheel demonstrated a positive correlation between BDNF production and “workload”, as measured by energy expenditures calculated by distance, resistance and body weight; no change was seen in TrkB between groups. These animal studies are valuable as they are consistent with observations in human studies of acute high-intensity exercise and aerobic exercise, and establish similarities between species in the required ‘work’ to produced elevated BDNF levels. More specifically, they demonstrate a variety of exercise regimens are capable of producing increased BDNF levels, on a similar time-course, in both rodents and humans, and that regular exercise is required for prolonged elevations. This provides good rationale for using rodents to establish the molecular effectors downstream of activity-induced elevations in BDNF.
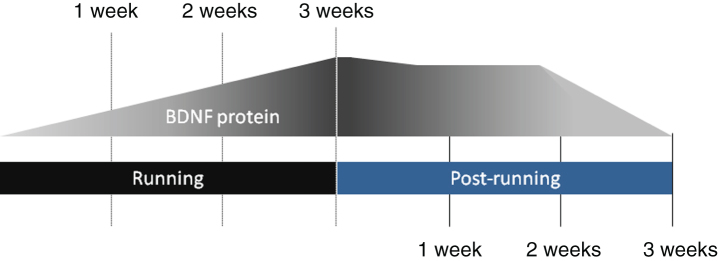
Fig.2.
Regular physical activity produces a progressive and sustained increase in the expression of brain-derived neurotrophic factor (BDNF). Elevation of intraparenchymal BDNF protein levels are graded with the duration (or total period) of regular sessions of physical activity, with profound mRNA increases detectable starting from 2–3 days. Cessation of exercise is followed by a gradual decline of BDNF protein level though it has been shown to remain significantly higher than baseline levels for up to 2 weeks. Notably, a return to exercise results in a rapid reinstatement of elevated protein levels, more quickly than is typical of exercise-naïve rodents [201, 202].
Neural growth factor
While some studies have reported upregulation of Ngf mRNA after physical activity, its role in supporting exercise-mediated neurogenesis and neuroprotection is less well-defined. A study of middle cerebral artery stroke in rats showed that forced treadmill running for 12 weeks (but not 4 or 8 weeks) increased cortical Ngf expression and reduced the volume of infarction [205]. In contrast, when mice were exposed to 10 days of treadmill ‘rehabilitation’ intervention beginning 4 days after stroke induction, NGF/Trk-A signally was not elevated; instead, BDNF/TrkB signalling was increased and associated with repair processes [206]. Previously, analyses had shown that voluntary running mediated only a transient increase in Ngf expression in the hippocampus, with levels elevated in after 3 days but not beyond [85], though it remained elevated in the caudal cortex when measured after 7 days of running [190]. Topographical differences within the hippocampus were found with in situ hybridisation, with a modest but significant increase in Ngf mRNA after 7 days of running in the dentate gyrus and CA4 regions, as well as in the caudal cortex [190]. The local and temporal differences in NGF versus BDNF upregulation in response to exercise suggests that they likely play different roles in the neuroprotection of the aging brain.
Insulin-like growth factor-1
The protective effects of exercise also involves trophic factors that are produced peripherally, such as such as IGF-1 and vascular endothelial growth factor (VEGF) [1, 2, 16, 147, 207–209]. Peripheral factors can aid in neuroprotection by supporting angiogenesis and modulation of energy metabolism [1, 6, 89, 180, 181, 210]. Stimulated by circulating growth hormone (GH), IGF-1 is produced by the liver before being released into the circulatory system [211]. It is also derived and stored in peripheral tissue, such as skeletal muscle, from which it can be rapidly released; transient increases in circulating IGF-1 following acute, high-intensity (anaerobic) exercise are believed to be muscle-derived, and are independent of GH levels [29, 193, 212–214]. However, studies have suggested that muscle-derived IGF-1 may also be an important source of circulating levels following periods of more moderate, regular exercise [193, 213, 215].
Circulating IGF-1 is BBB-permeable and thus able to reach the brain parenchyma. It is widely expressed centrally, where it is believed to modulate and support both cells and cerebrovasculature. In the brain, glial cells are known to have a role in mediating neuroprotective properties of IGF-1 and have been particularly well-studied in models of hypoxia, where IGF-1 treatment reduced oligodendrocyte loss and neuronal death [216–219]. Importantly, both GH and IGF-1 levels decline with age, coincident with neurological and vascular changes [28, 29, 43, 211, 220]. In 32 month-old rats, 2–4 weeks of IGF-1 treatment enhanced both neurogenesis and vascular density and ameliorated age–related declines in the performance of tasks involving working memory (e.g. repeated acquisition task and novel object recognition) [29, 88, 200, 220]. In 28 month-old rats, 4 weeks of continuous [mini-pump] IGF-1 infusion into the lateral ventricle reversed an age-related decreases in hippocampal glutamatergic N-methyl-D-aspartate ionotropic (NMDA) receptor subunits NR2A and NR2B (NMDAR2A and NMDAR2B, respectively), subunits that are well-connected to learning and memory [200, 220]. Recent studies have also shown IGF-1 to protect cultured hippocampal cells (under stressful conditions) against NMDA-mediated excitotoxicity by modifying the phosphorylation of NMDA2B [221].
Rats undergoing 15 days of treadmill running show that regular training elevated the level of circulating IGF-1 taken up into the hippocampus, where it is believed to modulate the effects of exercise [89]. Blocking hippocampal IGF-1 receptors during exercise significantly attenuated elevations in the transcription and translation of BDNF as well as that of its precursor, pro-BDNF. It also abrogated the signalling cascade downstream of BDNF/TrkB, including the phosphorylation of CaMKII and MAPK-II, paralleled by a dampened upregulation of proteins regulating synaptic function, such as synapsin I [1, 20]. In addition, inhibiting the uptake of circulating IGF-1 with subcutaneous infusion of anti-serum blocked exercised-induced increases in the number of new granular cells in rats undergoing treadmill training [89]. Finally, while blocking IGF-1 did not affect exercise-enhanced learning acquisition, it attenuated the benefit to memory recall [1]. These studies suggest that while IGF-1 and BDNF co-operate to facilitate neurogenesis and synaptic plasticity, they may each play unique roles [1, 2, 89, 200].
EXERCISE INCREASES NEUROTRANSMITTER LEVELS IN THE CENTRAL NERVOUS SYSTEM
Alterations in neurotransmission as a result of age or pathology is a well-known contributor to impairments in learning and memory. Acute bouts of moderate to high intensity aerobic exercise (20 to 120 minutes) have been shown to influence a number of different neurotransmitter systems implicated in cognitive function and mood, including dopamine (DA), serotonin (5-HT), noradrenaline (NA), acetylcholine (ACh), glutamate, and gamma-aminobutyric acid (GABA) (Table 1; see reviews: [16, 222]). In this way, acute sessions of physical activity may enhance cognitive performance by increasing both blood flow and tissue content of neurotransmitters associated with cortical arousal, such as glutamate, NA and ACh [223–231]. Moreover, evidence suggests that increases in central neurotransmitters may co-operate with neurotrophins to promote beneficial long-term neuronal adaptations [222]. Indeed, a reciprocal relationship exists between several neurotransmitters and BDNF in the hippocampus [189, 203, 232–235]. These studies suggest that increases in neurotransmission and BDNF/TrkB signalling may converge to enhance neuronal survival, synaptic plasticity and neurogenesis, such as that observed to be coincident with increased physical activity [4, 5, 236]. The following discussion of neurotransmitters is limited to those that have been most closely linked to BDNF in the pro-survival and signal transduction pathways; however, it should be noted that other transmitters, including ACh and GABA, have also been linked to the neuronal adaptations, cerebrovascular effects, and cognitive improvements associated with exercise [166, 227, 228, 237–241].
Dopamine
Positron emission tomography (PET) and post-mortem biochemical analyses have revealed declines in DA synthesis, receptor binding, and receptor levels in the aging human brain [242–245]. These alterations may hold significance not only for mood and motor functions, well-known to be under dopaminergic governance, but from the perspective of neuroprotection in the aging brain.
Dopaminergic neurotransmission has been associated with enhanced BDNF signalling, a relationship well-described in vitro. In embryonic neurons, D1 receptor agonism transactivates TrkB and several factors in its signalling pathway, accelerating morphological maturation, differentiation, and neurite outgrowth [248]. Similarly, pharmacological stimulation of the D1-D2 receptor hetero-oligomer was shown to increase BDNF production via a calcium signalling cascade in cultured striatal neurons and the adult rat brain [247]. In turn, BDNF originating from cortical neurons can elicit long-term adaptations by influencing the response of targets cells to DA, in part by controlling the expression of select DA receptor subtypes. This has been demonstrated in vivo using lesioning and gene–targeting experiments: mice lacking TrkB showed a global reduction in D1 mRNA [249], while BDNF-null mice and 6-hydroxydopamine (6-OHDA) lesioning of cortical dopaminergic neurons show BDNF to be necessary for normal expression of D3 receptor in the nucleus accumbens [250].
In both human and animals studies, moderate aerobic exercise such as treadmill running has been reported to increase DA synthesis, DA receptor expression and neurotransmission in a variety of structures, including the striatum, hippocampus,midbrain, pons-medulla and prefrontal cortex [210, 225–227, 251–259]. Some of these effects may be more profound in aged rodents, or under pathological conditions. For example, 8 weeks of was shown to upregulate the expression and activity of tyrosine hydroxylase, the rate-limiting enzyme in the synthesis of catecholamines, in aged mice but not in young mice, representing a reversal of its age-related decline [259]. In a neurotoxicity model of Parkinsonian dopaminergic cell loss, 28-days of intensive treadmill running enhanced DA bioavailability and neurotransmission both by increasing DA release and, by modulating the expression of its transporter, the time DA resides in the extracellular space [260]. These studies demonstrate that regular physical activity has the capacity to promote adaptive changes that facilitate dopaminergic transmission which might otherwise be compromise dueto age or disease.
By enhancing both DA neurotransmission and BDNF production, exercise may promote neuroprotection of dopaminergic cells via a co-operative activation of pro-survival pathways. A 4-week history of treadmill running at speeds that incrementally built to the equivalent of a moderate intensity session (60 minutes/day, 5 days per week) was shown to completely protect against inflammation-induced dopaminergic neuronal loss in the substantia nigra and attenuated motor impairment in freely-behaving mice [246]. Neuroprotection in the nigrostriatal pathway was dependent on the activation of the BDNF-TrkB signalling pathway rather than modulation of microglial activation or cytokine/chemokine levels [246]. In contrast to the 4-week regimen, 2 weeks of exercise prior to the inflammatory challenge was insufficient to provide significant protection, which could be indicative of a minimal requirement for adequate exercise-facilitated BDNF translation to rescue neuronal loss in the striatum.
Noradrenaline
NA modulates a wide variety of brain functions, including attention, memory consolidation and memory retrieval [261–263]. Following both session of both moderate and intense exercise, tissue content of NA and its metabolites has been reported to be widely elevated throughout the brain (i.e. striatum, cortex, hippocampus, pons-medulla, and amygdala) as well as in the blood [226, 264–268]. Early microdialysis experiments showed that following 2 weeks of training on a treadmill (5 days per week, 1 hour at 25 m/min, 3% slope; workload equivalent to 70% of VO2 max), rats that exercised for 1 to 2 hours showed increased levels of NA in both brain microdialysate and blood plasma (note: there was a 4 day rest after the initial, 2-week training for surgery and recovery) [266]. While both 1 and 2 hours were sufficient to significantly increase cortical NA levels within 40 minutes of the onset of treadmill running, the magnitude and duration of change was not equivalent: the maximal increase seen in a 1 hour session was fivefold over baseline, still evident 30 minutes after running cessation, while 2 hours of running increased NA levels 6.5 times over basal levels, with significant elevations lasting for 70minutes [266].
Notably, exercise-induced upregulation of Bdnf is dependent on the activation of the transcription factor, cyclic AMP response element binding protein (CREB), which is in part facilitated by increased neurotransmission via the cAMP second messenger pathway [184, 269]. Selectively blocking β-adrenergic receptors throughout 3 days of voluntary wheel running abolishes Bdnf mRNA elevations within the hippocampus, suggesting noradrenergic signalling plays an important role in its upregulation [189]. Noradrenergic receptor antagonism was also found to blunt the exercise-mediated enhancement of learning and memory in rodents, as assessed by performance in contextual fear conditioning [270] and the Morris water maze [271]. In addition, activation of noradrenergic G protein-coupled receptors has been shown to transactivate BDNF/TrkB pathways, representing another means by which NA signalling participates in neuronal adaptations associated with exercise (Fig. 4A) [184, 189, 272–274].
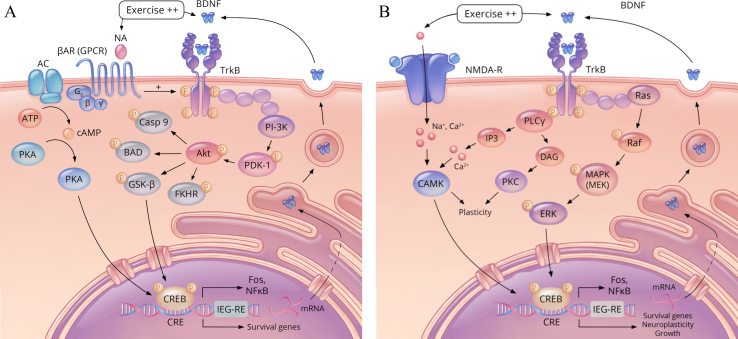
Fig.4.
Exercise-activated pathways regulating growth, survival, and neuroplasticity. (A) Schematic representation of the effect of increased noradrenaline (NA)–signalling and brain-derived neurotrophic factor (BDNF) / tropomyosin receptor kinase B (TrkB) signalling. Noradrenaline binds to the β-adrenergic receptor (βAR), a G protein–coupled receptor (GPCR) which can initiate a cyclic adenosine monophosophate (cAMP) pathway to phosphorylate (and activate) the transcription factor cAMP response element-binding protein (CREB). In addition, NA–mediated signalling can augment BDNF signalling pathways by transactivating its receptor, TrkB. Phosphorylation of TrkB initiates the phosphatidylinositol-3 kinase (PI-3K) pathway to activate protein kinase B (Akt), which phosphorylates glycogen synthase kinase 3-β (GSK-β), deactivating it, in turn increasing CREB activity. Phosphorylated CREB transcribes a number of pro-survival genes, such as Immediate-Early Gene-Regulatory Element (IEG-RE), and nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB). In addition, it acts to further promote the transcription of BDNF and TrkB. Proteins that are deactivated by Akt include a number of pro-apoptotic factors (i.e. Casp 9, FKHR, and BAD) (shown in dark grey). Activated proteins and kinases are shown in pinks and blue, and activated CREB, in yellow. Additional abbreviations: adenosine triphosphate (ATP); Bcl-2-associated death promoter (BAD); cAMP response element (CRE); caspase 9 (Casp 9); forkhead transcription factor (FKHR); protein kinase A (PKA); phosphoinositide-dependent protein kinase-1 (PDK-1). (B) Two additional exercise-facilitated pathways involved in the growth, survival and plasticity pathways are the mitogen-activated protein kinases/extracellular signal-regulated kinases pathway (MAPS/ERK) and the phospholipase Cγ/Ca2 + /calmodulin-dependent protein kinase II (PLCγ/CaMKII) pathway. Glutamate NMDA receptors activity also facilitates the effect of exercise on CREB activation. These pathways, like the PI-3K pathway, have also been shown to be involved BDNF/TrkB activation in promoting genes involved in survival and neuroplasticity. Activated proteins and kinases are shown in pinks and blue, and activated CREB, in yellow. Additional abbreviations: Ca2 + /calmodulin-dependent protein kinase (CAMK), diacyl-glycerol (DAG), Mitogen-activated protein kinase kinase (MEK), inositol trisphosphate (IP3), protein kinase C (PKC) [184, 287, 345, 346].
Interestingly, noradrenergic signalling has also been shown to protect against Aβ toxicity, achieved both through the transactivation of TrkB [275] and by facilitating increased expression of Bdnf [269]. It is possible that this represents an additional mechanism by which exercise-facilitated alterations in neurotransmission can confer neuroprotection. The NA system may also protect the brain by modulating the central inflammatory response, including that in response to Aβ, via adrenergic receptors on astrocytes and microglia [224, 276–278]. Selective lesioning of NA fibres has been shown to impair microglial migration and phagocytosis, reducing clearance of Aβ in the hippocampus and frontal cortex [277]. This is a particularly interesting observation considering that degeneration of noradrenergic cells within the locus coeruleus has been associated not only with the severity of cognitive disturbance but with the cortical correlates of AD, including Aβ plaque load and neurofibrillary tangles [277, 279, 280]. Noradrenergic lesioning also exacerbates lipopolysaccharide (LPS)-induced systemic inflammation, which is positively correlated to levels of hippocampal pro-inflammatory cytokines and microglial activation in aged rats [281]. Serum and BDNF levels were reduced in rats who were pre-treated with a selective noradrenergic toxin prior to receiving LPS [281]. Collectively, NA has been shown to play a number of important neuroprotective roles, both by transactivating TrkB and participating in the clearance of toxic peptides by microglia. It is possible that exercise may support these roles by facilitating NA neurotransmission.
Serotonin
Declines in the serotonergic system have been described in the aging human brain, and include decreased levels of 5-HT2 receptor in the prefrontal cortex as well as reduced binding capacity [245, 282]. In addition, post-mortem analyses of patients with mild cognitive impairment have revealed a global reduction in 5-HT1A and 5-HT2A receptors [283]. Activation of 5-HT1A receptors has been shown to be associated with decreased NMDAR-mediated currents in pyramidal neurons of the prefrontal cortex, which in turn destabilises NMDA2B receptors; in contrast, activation of 5-HT2A/2C receptors increases NMDA receptor-mediated currents by activating intracellular signalling cascades [284–286]. These observations are of relevance when considering the role of NMDA-mediated signalling in LTP and synaptic plasticity. Indeed, exercise-enhanced learning has been associated with the downregulation of 5-HT1A receptors and, conversely, upregulation of 5-HT2A receptors in the hippocampus [287–289]. The inhibition of 5-HT1A (a Gi protein–coupled receptor) and activation of 5-HT2A (a Gs protein–coupled receptor) results in elevated levels of cAMP, which in turn is involved in intracellular kinases and transcriptional factors supporting learning and memory [290]. Moreover, different 5-HT receptor subtypes have been demonstrated to play acute and opposing roles during various stages of neuronal development in vitro: where 5-HT1A receptors promote self-renewal of precursor cells, 5-HT2A/C receptors effect both proliferation and promote neuronal differentiation [291].
Recent studies demonstrate 5-HT to be required for activity-induced neurogenesis in both young and aged mice [292, 293]. Mice deficient in tryptophan hydroxylase 2 (Tph2-/-), the enzyme mediating de novo 5-HT production in the raphe nucleus, exhibit selective depletion of brain-derived 5-HT. While Tph2-/- mice show normal baseline hippocampal neurogenesis, the proproliferative effect of voluntary running (6 days) was shown to required the release of central 5-HT [292]. Similarly, running-stimulated neurogenesis was reported to be impaired in mice deficient in Ang-converting enzyme 2 (ACE2), an enzyme which converts Ang II to Ang-(1–7); this modification transforms Ang signalling from a vasoconstrictive and pro-inflammatory signal to one that is vasodilatory and anti-inflammatory. ACE2 is also involved in the intestinal absorption of tryptophan, the precursor to serontonin, with ACE2-/- mice showing significant reductions in both blood and brain 5-HT levels [293]. Worth noting is that there is great variation in the literature pertaining to the tissue content of 5-HT following periods of physical activity, signifying that the duration and intensity of both acute sessions and chronic training impacts the synthesis of 5-HT, and the expression of its receptors and transporters, likely in a region-specific manner.
Glutamate
Glutamatergic neurotransmission, altered in normal aging and disease, is critically involved in facilitating neuronal plasticity and modulating both LTP and long-term depression (LTD) [294–297]. Aging has been associated with a decline of glutamate content in the prefrontal cortex and hippocampus, a reduction in high–affinity glutamate transporters and glutamatergic receptors, and reduced glutamate uptake capacity [298–300]. Expression of the NMDA receptor class is particularly influenced by advanced age [200, 298, 301, 302]. As neuronal plasticity is in large part dependent on synaptic NMDAR activation, a decrease in synaptic NMDAR expression may functionally contribute to the age-related in memory and cognition [295, 303–305].
In rodents, short-term periods of voluntary running have been shown to modulate expression of glutamate receptors, upregulating NR2A and NR2B NMDA subunits within 3 and 7 days (respectively) [85, 236, 303] and glutamate receptor 5 (GlutR5), within 10 days [186]. Glutamatergic signalling has been shown to promote transcription of several genes involved in synaptic transmission as well as neuronal survival, via CREB activation. In this way, NMDA-mediated transmission has also been shown to contribute to the upregulation of BDNF and TrkB following exercise, further facilitating its effects [4]. Site-specific blockade of hippocampal NMDARs with MK-801 prevented increases in the transcription of Bdnf, TrkB, CREB, and synapsin I mRNA observed in control animals following 3 days of wheel running (against 100 g resistance) [4, 5]. In addition, rats given unrestricted access to a running wheel for 10 days had a significantly lower threshold for the induction of LTP in the dentate gyrus in vivo, as well as a potentiated LTP response to tetanic stimulation [186]. Similar results were demonstrated in vitro using hippocampal slices from running and non-running mice, showing that following 7–10 days, changes in NR2A and [to a lesser] extent NR2B were responsible for the augmentation of LTP and LTD [236]. More recent research shows that, together with GABA, exercise-mediated increases in glutamatergic transmission also influences the integration of young adult-born neurons into neural networks, which may underlie improvements to specific cognitive tasks [296].
Notably, activation of extracellular NMDARs induces excitatory neurotoxicity that has been implicated in neurodegeneration, including that in AD. Studies have suggested that an inverse relationship exists between excessive glutamate levels in the brain and neurogenesis and/or cell survival, possibly due to extrasynaptic calcium influx. Activation of extracellular NMDARs has recently been linked to accelerated transcription of tau, concomitant with a suppression of the mitogen-activated protein kinases/extracellular signal-regulated kinasespathway (MAP/ERK) signalling pathway [306]. Rodent studies suggest that increased physical activity may ameliorate excessive glutamatergic activity, in part by regulating glutamate release and oxidation, as well as through the BDNF-mediated activation of the ERK pathway [163, 164, 307–309]. In the latter respect, it shares mechanistic similarities to treatment with low doses of antidepressants such as ketamine, an NMDAR antagonist that dampens spontaneous glutamate signalling [163]. Mice given free access to a running wheel for 6–8 weeks show a decrease hippocampal glutamate levels that correlated with increased hippocampal volume on magnetic resonance (MR) imaging [307]. These studies reveal that exercise-induced alterations to glutamatergic transmission play an important role in promoting the transcription of genes involved in synaptic plasticity, as well as in promoting cell survival.
EXERCISE AND BDNF-MEDIATED PATHWAYS: NEUROPROTECTION AND SYNAPTIC PLASTICITY
Neurogenic and pro-survival pathways
Exercise promotes neuronal differentiation, survival and plasticity through the phosphorylation of CREB by BDNF [310], with likely contributions by excitatory noradrenergic and glutamatergic signalling pathways. Foundational experiments demonstrated BDNF to be neuroprotective using hippocampal slices and cortical neurons in vitro: under apoptotic conditions, BDNF activates the phosphatidylinositol-3 kinase/protein kinase B pathway (PI-3k/AKT) as well as MAPS/ERK to stimulate the transcription of survival-promoting genes via CREB (Fig. 4A and B, respectively) [171, 287, 310–313]. The phospholipase Cγ/Ca2 + /calmodulin-dependent protein kinase II (PLCγ/CaMKII) pathway is also activated by BDNF-TrkB signalling, again resulting in gene transcription of pro-survival genes [314]. In vivo studies have revealed notable mechanistic similarities between antidepressant treatments and exercise in rodents, with both activating excitatory pro-survival pathways. These studies offer insight into how exercise may mediate proliferative and/or neurogenic processes in the dentate gyrus via a combination of neuronal activation (i.e. synaptic transmission) and BDNF/TrkB signalling[184, 188, 189, 191, 315].
Recently, Ma et al. (2017) showed that BDNF/TrkB signalling is responsible for the persistent adult neurogenesis in the dentate gyrus stimulated by chronic treatment with antidepressants, such as low-dose ketamine [163]. Ketamine treatment produced a rapid increase in BDNF levels in the dentate gyrus that coincided with accelerated differentiation of doublecortin positive (DCX+) progenitor cells into functionally mature neurons. Ablation of TrkB in progenitor cells or disruption of TrkB-dependent ERK signalling blocked the generation of newborn neurons as well as the anti-depressant behavioural effects of the drug [163]. In addition, both antidepressants and voluntary exercise are known to activate the PI-3k/AKT pathway in the hippocampus, which promotes neuronal survival and inhibit apoptosis [287, 316, 317]. Chen et al. (2005) showed that 2 weeks of voluntary running in rats produced a robust increase in the phosphorylation of the full-length TrkB receptor, a result that was potentiated when animals were concurrently treated with an antidepressant [188–190, 287]. The study reported that levels of active (i.e. phosphorylated) forms of PI-3k, protein-dependent kinase-1 (PDK-1) and Akt were increased in the hippocampus, as were levels of phosphorylated glycogen synthase kinase-3β (GSK3β, inactive form), which ultimately led to a significant enhancement of CREB [287]. Chen and colleagues further showed that phosphorylation of CREB was augmented when antidepressant treatment was combined with voluntary running (beyond either treatment alone) suggesting they work synergistically to modulate the PI-3k/AKT neuroprotective pathway, possibly a result of the transactivation of TrkB or a consequence of increased NA and/or 5-HT–mediated neuronal stimulation [287].
Another mechanism by which BDNF may faciliate neuroprotection is through the initiation of a synaptic NMDAR-associated intracellular cascade that attenuates the excitotoxicity that results from extrasynaptic NMDAR-mediated calcium influx [164, 303, 318]. Lau et al. (2015) found that BDNF/TrkB signalling induces protective adaptations, termed “adaptogenomics”, by enhancing the nuclear calcium-inhibin β-A pathway [164]. In turn, inhibin β-A (or ‘activin A’, as a heterodimer) was shown to reduce extrasynaptic calcium influx and mitochondrial dysfunction, both of which contribute to excitotoxicity. Physical activity may therefore contribute to the neuroprotective adaptogenomic pathway by upregulating BDNF by enhancing synaptic NMDA activity while reducing extrasynaptic NMDA activity [164].
Exercise, signal transduction and synaptic plasticity
BDNF is known to alter gene transcription both pre–and post–synaptically [81, 160, 165, 319]. Quantitative analyses have revealed that elevated BDNF/TrkB signalling, resulting from short-term increases in physical activity, positively correlates with mRNA expression of several proteins involved in vesicular trafficking and neurotransmitter release. For instance, in freely running rats, syntaxin and synaptotagmin mRNA levels were shown to be upregulated by 3 days (continuing for at least 28 days), while synapsin I was elevated at 3 and 7 days [4, 5, 85, 86]. Site-specific blockade of hippocampal TrkB was sufficient to abolish the exercise-induced increases in Bdnf, TrkB, CREB, synapsin I and synaptophysin reported after 3 days of voluntary running [4, 86]. Blocking CaMKII had similar effects as TrkB inhibition, demonstrating its downstream involvement in upregulating both signalling molecules and Bdnf and TrkB expression in response to physical activity [4]. Together, these results demonstrate that short-term exercise has the capacity to regulate synaptic transmission under the direction of BDNF [4, 5, 86, 320–322]. The effects of extended-periods of running are equally dependent on BDNF/TrkB signalling. After having undergone chronic treadmill running for a total of 4 weeks, mice showed increased hippocampal protein levels of BDNF, total and phosphorylated TrkB, and synaptotagmin. These molecular changes were associated with behavioural outcomes, as exercised mice showed improved performance on the passive avoidance test as compared to sedentary controls [81]. Again, TrkB inhibition abolished the exercise-induced increases in BDNF and synaptotagmin levels, and impaired performance on the same test of aversion memory [81].
In vitro studies suggest that alterations in protein synthesis in response to BDNF can occur on the order of hours and are regionally localised to dendrites and axons, with protein synthesis machinery residing in pre–and post–synaptic terminals [158, 171, 319, 323]. The rapidly increased production of signal transduction molecules may be in part responsible for the relatively quick effects of exercise on mood and cognitive performance. By modulating the transcription of synaptic proteins such as synapsin I, synaptophysin, and synaptotagmin, exercise puts in place pre-synaptic structures to support enhanced neurotransmission. A complimentary increase in the expression of neurotransmitter receptor proteins, such as subunit-specific increases in NMDARs, further facilitates the enhancement of neurotransmission. As selective blockade of NMDARs and BDNF both abolished exercise-facilitated gene transcription, both neuronal activity and BDNF/TrkB signalling seem to be an essential part of both the pro-survival and plastic response.
BDNF has been shown to be involved in several forms of LTP. Using site-specific gene deletion, Zakharenko and colleagues dissociated the effects of BDNF released from pre-synaptic neurons in the C1 and C3 regions from that released post-synaptically at C1, finding that presynaptic release of BDNF from C3 was required to modulate a pre-synaptic component of LTP [319]. In particular, BDNF was shown to be required for a pre-synaptic component of theta burst LTP, which is thought to be of relevance for behavioural activity, as well as for the full expression of 200 Hz LTP. Exercise may then influence LTP by way of BDNF but also through its governance of neurotransmitter release, such as ACh, which is found to be elevated in the hippocampus and cortex with increased physical activity [226, 227, 237]. An increased concentration of ACh in the hippocampus supports the generation of theta oscillations, which in turn, serves to facilitate synaptic plasticity, learning and memory [240, 324, 325]. Acute physical activity has also been reported to produce immediate and robust neuronal activation of the hippocampal formation, as measured by c-fos positive cells [315]. On-going movements (i.e. wheel running, walking, swimming, etc.) generate theta oscillations that are positively correlated to the force or intensity of these movements and remain elevated the duration of activity. These theta oscillations have been associated with glutamatergic inputs originating from the medial septal nucleus and/or the entorhinal cortex, as lesioning experiments have eliminated movement-related theta [315]. Recalling that physical activity is known to modulate the expression of NMDAR, it is interesting to note that changes in the levels of BDNF, ACh, and NMDA-mediated signalling likely work synergistically to mediated enhancement of synaptic plasticity.
CLOSING REMARKS
In this review, we highlighted the many contributions of an active lifestyle to continued brain health. First, as discussed, the BDNF/TrkB pathways initiated by physical activity are involved in neuroprotection, synaptic plasticity, and neurogenesis. Knockout mice have demonstrated that BDNF is required for the enhancement of hippocampal neurogenesis following housing in enriched environments [326]. Moreover, intracerebroventricular injections of BDNF have mimicked the effects of 1 week of treadmill running on spatial and non-spatial learning [187], and chronic intrahippocampal administration of BDNF has been shown to rescue cognitive deficits following a ventricular injection of Aβ in rats [327]. Of note, only enriched environments that include a running wheel were shown to increase BDNF levels, showing it may have a unique role in neuroprotection [74, 75, 328]. Indeed, much past literature has focused on the link between physical activity and neurogenesis, showing that physical activity alone is sufficient for increasing cell proliferation and promoting cell survival [54, 56, 57]. However some studies suggest that cognitive stimulation is an essential part of differentiating proliferating cells into neurons and integrating them into networks [41, 329]. Considerable efforts are now being made to better understand the complexities of adult neural stem cells and the dynamic range of states in which they can exist (for excellent review, see [63]). Moreover, though a greater proportion of adult neural stem cells have entered quiescence, current research centres on how certain stimuli may transition these cells from quiescence to a proliferative state. Nonetheless, mitigating the age-related decline in neurogenesis is only one way by which an active lifestyle can keep the brain ‘young’ [330]. Several decades of literature demonstrate its benefit to the neurons, glia and cerebrovaculature of the brain. In animal models of aging, disease and toxicity, physical exercise has been shown to upregulate TJs of the BBB and to protect the neurovascular unit. Exercise has also been shown to reduce markers of microglia activation in the brain of aged mice, further mitigating damage to the neurovascular unit [90, 95, 331–333]. Finally, exercise may also aid in the protection of an aging brain by increasing glymphatic clearance, and promoting the clearance and degradation of Aβ [76, 94, 96–99].
Both animal and human literature suggest that many of the protective benefits of exercise are orchestrated by elevations in central and peripheral neurotrophic factors. Increases in neurotrophic levels have been reported to be associated with both acute sessions of high-intensity exercise and chronic aerobic exercise. In healthy, young human adults, BDNF levels in the serum were shown to increase during acute sessions of high-intensity anaerobic cycling (correlating with lactate levels), but not at workloads below the ventilatory threshold [7].However, BDNF in plasma were shown to be elevate during moderate-intensity endurance exercise, such as a 4-hour row at a workload 10-15% below the lactate threshold [9]. Other studies still show that both acute high-intensity exercise and regular, moderate aerobic exercise increases serum levels of BDNF and IGF-1, and improves some measures of memory and cognition in young participants [8, 13, 334, 335]. Notably, a recent meta-analysis of 29 studies showed that aerobic exercise, but not resistance training, increased resting concentrations of peripheral BDNF [183]. As circulating BDNF receives contributions from both the brain and other sources, including contracting muscle, this discrepancy may reflect a difference in the relative contribution of these sources during aerobic and anaerobic exercise.
Many studies demonstrate a correlation between BDNF and/or work levels and improved performance in various tests of memory and cognition (with the support of many meta-analyses). Still, the degree to which exercise specifically affects memory and cognition in young participants, independent of mood or acute effects on attention, lacks firm consensus [16, 183, 335–338]. To-date, clinic research has been somewhat limited by study size and is not often sex-balance; it also shows great variability in experimental and sampling protocols. It is possible that performance on certain memory testing may be more malleable to acute exercise sessions than others and augmented via different mechanisms, such as rapid changes in blood flow or the release of neurotransmitters that modulate attention. In this way, it is possible that acute cognitive changes may be independent of elevated BDNF and more reliant on other rapid physiological processes. In contrast, improvements to memory, cognition or executive function that correlate with regular exercise may be a result of long-term neuronal adaptations. Cognitive improvements associated with exercise are most striking in aged participants, and possibly even more so in the eldest of participants due to an age-dependent decline in baseline BDNF levels relative to young individuals [18, 23]. Incognitively-unimpaired aging participants as well as those with mild cognitive impairment, aerobic activity correlates with a reduced risk of developing deficits in executive functions and a reduced incidence of dementia [18, 21, 22]. In these prospective studies, the intensity, duration and frequency of exercise correlate with improved scores on tests of memory and cognition, though it is worth noting that sex differences may emerge. Moreover, while these parameters correlate to performance, it is important to recognise the feasibility of intense exercise, even with supervision, in some elderly populations, particularly following an event such as a stroke. To this end, even light and moderate exercise, with regularity, has been shown to be beneficial.
Together, human and preclinical studies shape a powerful story demonstrating that physical activity can counteract many aspects of decline in the brain’s environment associated with aging. Because ‘exercise regimens’ of various work-levels and durations seem to produce similar effects in animals and humans, we are able to gain valuable insight into the downstream mechanisms by which physical activity might contribute to continued brain health. It is important to recognise that the topic addressed in this review cover only a fraction of the potential benefits of exercise — particularly regular, aerobic exercise — to adaptive changes in the hippocampus. The objective of this review was to highlight the protective effects of neurotropic factors to the brain and how, in addition to the well-established enhancement of synaptic plasticity, adaptive effects may include changes in support for the BBB, clearance of toxic species and reduced neuroinflammation. These rest heavily on brain-derived alterations driving gene transcription. The relative contributions that centrally vs peripherally-derived trophic factors have to a single cognitive test score is likely dependent on whether testing follows an acute, isolated session or a history of regular exercise. Still, recent literature provides evidence to support a strong (and, perhaps underappreciated) contribution from peripheral neurotrophins and hormones to hippocampus adaptations, such as those that are muscle- or liver-derived [1, 2, 89, 192, 339–344]. In addition, it is possible that acute changes in cortical activity during exercise may contribute to long-term adaptations (for an excellent recent review, see [315]). Therefore, the lasting impression should be one that emphasizes that different durations, intensities, and forms of exercise may contribute unique benefits to the brain, though long-term exercise provides the greatest and longest-lasting benefits. Individual persons may have individual limitations to the types of exercise they do, possibly restricted by age or mobility. It is also possible that sex and polymorphics differences may influence the degree to which exercise influences the different systems discussed here. Indeed, a limitation of animal and human research alike is the lack of well-powered, sex-balanced studies. Nonetheless, there is a great body of evidence that demonstrates that an active lifestyle bestows a diverse set of benefits that mitigate the age-related changes in the brain that contribute to cognitive decline.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
We thank our medical illustrator, Hang Yu Lin, for her wonderful contributions to this paper.
Footnotes
Please note that the specific effects of exercise on the cerebrovasculature, and on neurogenesis in animal models of AD, are the subjects of other focused reviews found within this issue (see papers by Barnes, J.N. and Corkery, A.T.,“Exercise improves vascular function, but does this translate to the brain”; Llorens-Martin, M. “Exercising new neurons to vanquish Alzheimer’s Disease”). While these topics are of relevance to the discussion here and will be briefly mentioned, please refer to those articles for greater detail. In this review, general topics surrounding AD will be discussed as they pertain to the neuroprotective effect of exercise, such as exercise-facilitated clearance of Aβ species.
REFERENCES
- [1]. Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–33. doi: 10.1016/j.neuroscience.2006.02.084 [DOI] [PubMed] [Google Scholar]
- [2]. Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci. 2006;24:1265–76. doi: 10.1111/j.1460-9568.2006.05026.x [DOI] [PubMed] [Google Scholar]
- [3]. Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0 [DOI] [PubMed] [Google Scholar]
- [4]. Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122:647–57. doi: 10.1016/j.neuroscience.2003.08.001 [DOI] [PubMed] [Google Scholar]
- [5]. Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–90. doi: 10.1111/j.1460-9568.2004.03720.x [DOI] [PubMed] [Google Scholar]
- [6]. Speisman RB, Kumar A, Rani A, Foster TC, Ormerod BK. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav Immun. 2013;28:25–43. doi: 10.1016/j.bbi.2012.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. 2007;39:728–34. doi: 10.1249/mss.0b013e31802f04c7 [DOI] [PubMed] [Google Scholar]
- [8]. Griffin EW, Mullally S, Foley C, Warmington SA, O’Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104:934–41. doi: 10.1016/j.physbeh.2011.06.005 [DOI] [PubMed] [Google Scholar]
- [9]. Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94:1062–9. doi: 10.1113/expphysiol.2009.048512 [DOI] [PubMed] [Google Scholar]
- [10]. Tang SW, Chu E, Hui T, Helmeste D, Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci Lett. 2008;431:62–5. doi: 10.1016/j.neulet.2007.11.019 [DOI] [PubMed] [Google Scholar]
- [11]. Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res. 2013;2013:657508. doi: 10.1155/2013/657508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Gomez-Pinilla F, Hillman C. The influence of exercise on cognitive abilities. Compr Physiol. 2013;3:403–28. doi: 10.1002/cphy.c110063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Jeon YK, Ha CH. Expression of brain-derived neurotrophic factor, IGF-1 and cortisol elicited by regular aerobic exercise in adolescents. J Phys Ther Sci. 2015;27:737–41. doi: 10.1589/jpts.27.737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Lange-Asschenfeldt C, Kojda G. Alzheimer’s disease, cerebrovascular dysfunction and the benefits of exercise: from vessels to neurons. Exp Gerontol. 2008;43:499–504. doi: 10.1016/j.exger.2008.04.002 [DOI] [PubMed] [Google Scholar]
- [15]. Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: A unifying theory. Clin Psychol Rev. 2001;21:33–61. doi: 10.1016/S0272-7358(99)00032-X [DOI] [PubMed] [Google Scholar]
- [16]. Basso JCS, WA. The effects of acute exercise on mood, congition, neurophysiology and neurochemical pathways: A review. Brain Plasticity. 2017;2:127–52. doi: 10.3233/BPL-160040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–30. doi: 10.1111/1467-9280.t01-1-01430 [DOI] [PubMed] [Google Scholar]
- [18]. Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004 [DOI] [PubMed] [Google Scholar]
- [19]. Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/S0166-2236(02)02143-4 [DOI] [PubMed] [Google Scholar]
- [20]. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–72. doi: 10.1016/j.tins.2007.06.011 [DOI] [PubMed] [Google Scholar]
- [21]. Clark S, Parisi J, Kuo J, Carlson MC. Physical activity is associated with reduced risk of executive function impairment in older women. J Aging Health. 2016;28:726–39. doi: 10.1177/0898264315609908 [DOI] [PubMed] [Google Scholar]
- [22]. Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–704. doi: 10.1016/j.apmr.2004.03.019 [DOI] [PubMed] [Google Scholar]
- [23]. Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, et al. BDNF mediates improvements in executive function following a 1-year exercise intervention. Front Hum Neurosci. 2014;8:985. doi: 10.3389/fnhum.2014.00985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. de Assis GG, de Almondes KM. Exercise-dependent BDNF as a modulatory factor for the executive processing of individuals in course of cognitive decline. A systematic review. Front Psychol. 2017;8:584. doi: 10.3389/fpsyg.2017.00584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Carlsson A. Brain neurotransmitters in aging and dementia: similar changes across diagnostic dementia groups. Gerontology. 1987;33:159–67. doi: 10.1159/000212870 [DOI] [PubMed] [Google Scholar]
- [26]. Peters R. Ageing and the brain. Postgrad Med J. 2006;82:84–8. doi: 10.1136/pgmj.2005.036665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behavioural Brain Research. 2011;221:555–63. doi: 10.1016/j.bbr.2010.11.058 [DOI] [PubMed] [Google Scholar]
- [28]. Sonntag WE, Eckman DM, Ingraham J, Riddle DR. Regulation of Cerebrovascular Aging In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL) 2007. [Google Scholar]
- [29]. Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001 [DOI] [PubMed] [Google Scholar]
- [30]. Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–94. doi: 10.1038/nrn1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer’s disease–lessons from pathology. BMC Med. 2014;12:206. doi: 10.1186/s12916-014-0206-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Laske C, Stransky E, Leyhe T, Eschweiler GW, Maetzler W, Wittorf A, et al. BDNF serum and CSF concentrations in Alzheimer’s disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41:387–94. doi: 10.1016/j.jpsychires.2006.01.014 [DOI] [PubMed] [Google Scholar]
- [33]. Ota M, Yasuno F, Ito H, Seki C, Nozaki S, Asada T, et al. Age-related decline of dopamine synthesis in the living human brain measured by positron emission tomography with l-[β-11C]DOPA. Life Sci. 2006;79:730–6. doi: 10.1016/j.lfs.2006.02.017 [DOI] [PubMed] [Google Scholar]
- [34]. He J, Farias S, Martinez O, Reed B, Mungas D, Decarli C. Differences in brain volume, hippocampal volume, cerebrovascular risk factors, and apolipoprotein E4 among mild cognitive impairment subtypes. Arch Neurol. 2009;66:1393–9. doi: 10.1001/archneurol.2009.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Michalski B, Corrada MM, Kawas CH, Fahnestock M. Brain-derived neurotrophic factor and TrkB expression in the “oldest-old,” the 90+ Study: correlation with cognitive status and levels of soluble amyloid-beta. Neurobiol Aging. 2015;36:3130–9. doi: 10.1016/j.neurobiolaging.2015.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, et al. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–51. doi: 10.1093/cercor/bhg081 [DOI] [PubMed] [Google Scholar]
- [37]. Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30:5368–75. doi: 10.1523/JNEUROSCI.6251-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. von Bohlen und Halbach O. Involvement of BDNF in age-dependent alterations in the hippocampus. Front Aging Neurosci. 2010;2. doi: 10.3389/fnagi.2010.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Mathews KJ, Allen KM, Boerrigter D, Ball H, Shannon Weickert C, Double KL. Evidence for reduced neurogenesis in the aging human hippocampus despite stable stem cell markers. Aging Cell. 2017;16:1195–9. doi: 10.1111/acel.12641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–71. doi: 10.1016/j.expneurol.2005.05.014 [DOI] [PubMed] [Google Scholar]
- [41]. Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27:1505–13. doi: 10.1016/j.neurobiolaging.2005.09.016 [DOI] [PubMed] [Google Scholar]
- [42]. Silhol M, Bonnichon V, Rage F, Tapia-Arancibia L. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience. 2005;132:613–24. doi: 10.1016/j.neuroscience.2005.01.008 [DOI] [PubMed] [Google Scholar]
- [43]. Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–20. doi: 10.1210/endo.138.8.5330 [DOI] [PubMed] [Google Scholar]
- [44]. O’Shea A, Cohen RA, Porges EC, Nissim NR, Woods AJ. Cognitive aging and the hippocampus in older adults. Front Aging Neurosci. 2016;8:298. doi: 10.3389/fnagi.2016.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. von Bohlen und Halbach O, Krause S, Medina D, Sciarretta C, Minichiello L, Unsicker K. Regional- and age-dependent reduction in trkB receptor expression in the hippocampus is associated with altered spine morphologies. Biol Psychiatry. 2006;59:793–800. doi: 10.1016/j.biopsych.2005.08.025 [DOI] [PubMed] [Google Scholar]
- [46]. Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging. 2010;31:151–61. doi: 10.1016/j.neurobiolaging.2008.03.002 [DOI] [PubMed] [Google Scholar]
- [47]. Daynac M, Morizur L, Chicheportiche A, Mouthon MA, Boussin FD. Age-related neurogenesis decline in the subventricular zone is associated with specific cell cycle regulation changes in activated neural stem cells. Sci Rep. 2016;6:21505. doi: 10.1038/srep21505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Rex CS, Lauterborn JC, Lin CY, Kramar EA, Rogers GA, Gall CM, et al. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. 2006;96:677–85. doi: 10.1152/jn.00336.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Greene E, Naranjo JN. Degeneration of hippocampal fibers and spatial memory deficit in the aged rat. Neurobiol Aging. 1987;8:35–43. [DOI] [PubMed] [Google Scholar]
- [52]. Granger R, Deadwyler S, Davis M, Moskovitz B, Kessler M, Rogers G, et al. Facilitation of glutamate receptors reverses an age-associated memory impairment in rats. Synapse. 1996;22:332–7. doi: [DOI] [PubMed] [Google Scholar]
- [53]. Lynch G, Rex CS, Gall CM. Synaptic plasticity in early aging. Ageing Res Rev. 2006;5:255–80. doi: 10.1016/j.arr.2006.03.008 [DOI] [PubMed] [Google Scholar]
- [54]. Marlatt MW, Potter MC, Lucassen PJ, van Praag H. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev Neurobiol. 2012;72:943–52. doi: 10.1002/dneu.22009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379 [DOI] [PubMed] [Google Scholar]
- [56]. van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2014;76 Pt C:639–56. doi: 10.1016/j.neuropharm.2013.04.005 [DOI] [PubMed] [Google Scholar]
- [59]. Leal G, Comprido D, de Luca P, Morais E, Rodrigues L, Mele M, et al. The RNA-binding protein hnRNP K mediates the effect of BDNF on dendritic mRNA metabolism and regulates synaptic NMDA receptors in hippocampal neurons. eNeuro. 2017;4. doi: 10.1523/ENEURO.0268-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Bond AM, Ming GL, Song H. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell. 2015;17:385–95. doi: 10.1016/j.stem.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22:589–99 e5. doi: 10.1016/j.stem.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7. doi: 10.1038/3305 [DOI] [PubMed] [Google Scholar]
- [63]. Katsimpardi L, Lledo PM. Regulation of neurogenesis in the adult and aging brain. Curr Opin Neurobiol. 2018;53:131–8. doi: 10.1016/j.conb.2018.07.006 [DOI] [PubMed] [Google Scholar]
- [64]. Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–81. doi: 10.1038/nature25975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–27. doi: 10.1016/j.cell.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Encinas JM, Fitzsimons CP. Gene regulation in adult neural stem cells. Current challenges and possible applications. Adv Drug Deliv Rev. 2017;120:118–32. doi: 10.1016/j.addr.2017.07.016 [DOI] [PubMed] [Google Scholar]
- [67]. Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–56. doi: 10.1016/j.stem.2010.03.017 [DOI] [PubMed] [Google Scholar]
- [68]. Noguchi H, Kimura A, Murao N, Matsuda T, Namihira M, Nakashima K. Expression of DNMT1 in neural stem/precursor cells is critical for survival of newly generated neurons in the adult hippocampus. Neurosci Res. 2015;95:1–11. doi: 10.1016/j.neures.2015.01.014 [DOI] [PubMed] [Google Scholar]
- [69]. Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–4. doi: 10.1126/science.1251141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Katsimpardi L, Rubin LL. Young systemic factors as a medicine for age-related neurodegenerative diseases. Neurogenesis (Austin). 2015;2:e1004971. doi: 10.1080/23262133.2015.1004971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–63. doi: 10.1038/nm.3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72]. Kempermann G, Brandon EP, Gage FH. Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr Biol. 1998;8:939–42. doi: 10.1016/S0960-9822(07)00377-6 [DOI] [PubMed] [Google Scholar]
- [73]. Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–5. doi: 10.1038/386493a0 [DOI] [PubMed] [Google Scholar]
- [74]. Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18:605–9. doi: 10.1101/lm.2283011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Vivar C, Potter MC, van Praag H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci. 2013;15:189–210. doi: 10.1007/7854_2012_220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76]. Maliszewska-Cyna E, Xhima K, Aubert I. A comparative study evaluating the impact of physical exercise on disease progression in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2016;53:243–57. doi: 10.3233/JAD-150660 [DOI] [PubMed] [Google Scholar]
- [77]. Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behav Neurosci. 2004;118:1123–7. doi: 10.1037/0735-7044.118.5.1123 [DOI] [PubMed] [Google Scholar]
- [78]. Falls WA, Fox JH, MacAulay CM. Voluntary exercise improves both learning and consolidation of cued conditioned fear in C57 mice. Behav Brain Res. 2010;207:321–31. doi: 10.1016/j.bbr.2009.10.016 [DOI] [PubMed] [Google Scholar]
- [79]. O’Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176:362–6. doi: 10.1016/j.bbr.2006.10.018 [DOI] [PubMed] [Google Scholar]
- [80]. Chen HI, Lin LC, Yu L, Liu YF, Kuo YM, Huang AM, et al. Treadmill exercise enhances passive avoidance learning in rats: the role of down-regulated serotonin system in the limbic system. Neurobiol Learn Mem. 2008;89:489–96. doi: 10.1016/j.nlm.2007.08.004 [DOI] [PubMed] [Google Scholar]
- [81]. Liu YF, Chen HI, Yu L, Kuo YM, Wu FS, Chuang JI, et al. Upregulation of hippocampal TrkB and synaptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol Learn Mem. 2008;90:81–9. doi: 10.1016/j.nlm.2008.02.005 [DOI] [PubMed] [Google Scholar]
- [82]. Aguiar AS Jr, Castro AA, Moreira EL, Glaser V, Santos AR, Tasca CI, et al. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech Ageing Dev. 2011;132:560–7. doi: 10.1016/j.mad.2011.09.005 [DOI] [PubMed] [Google Scholar]
- [83]. Albeck DS, Sano K, Prewitt GE, Dalton L. Mild forced treadmill exercise enhances spatial learning in the aged rat. Behav Brain Res. 2006;168:345–8. doi: 10.1016/j.bbr.2005.11.008 [DOI] [PubMed] [Google Scholar]
- [84]. Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–87. doi: 10.1111/j.1460-9568.2008.06524.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85]. Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–16. doi: 10.1046/j.1460-9568.2002.02158.x [DOI] [PubMed] [Google Scholar]
- [86]. Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070:124–30. doi: 10.1016/j.brainres.2005.11.062. [DOI] [PubMed] [Google Scholar]
- [87]. van Praag H, Fleshner M, Schwartz MW, Mattson MP. Exercise, energy intake, glucose homeostasis, and the brain. J Neurosci. 2014;34:15139–49. doi: 10.1523/JNEUROSCI.2814-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88]. Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559–69. doi: 10.1016/S0306-4522(98)00143-2 [DOI] [PubMed] [Google Scholar]
- [89]. Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–34. doi: 10.1523/JNEUROSCI.21-05-01628.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Gomes da Silva S, Simoes PS, Mortara RA, Scorza FA, Cavalheiro EA, da Graca Naffah-Mazzacoratti M, et al. Exercise-induced hippocampal anti-inflammatory response in aged rats. J Neuroinflammation. 2013;10:61. doi: 10.1186/1742-2094-10-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91]. Park M, Levine H, Toborek M. Exercise protects against methamphetamine-induced aberrant neurogenesis. Sci Rep. 2016;6:34111. doi: 10.1038/srep34111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92]. Soto I, Graham LC, Richter HJ, Simeone SN, Radell JE, Grabowska W, et al. APOE stabilization by exercise prevents aging neurovascular dysfunction and complement induction. PLoS Biol. 2015;13:e1002279. doi: 10.1371/journal.pbio.1002279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93]. Toborek M, Seelbach MJ, Rashid CS, Andras IE, Chen L, Park M, et al. Voluntary exercise protects against methamphetamine-induced oxidative stress in brain microvasculature and disruption of the blood-brain barrier. Mol Neurodegener. 2013;8:22. doi: 10.1186/1750-1326-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94]. He XF, Liu DX, Zhang Q, Liang FY, Dai GY, Zeng JS, et al. Voluntary exercise promotes glymphatic clearance of amyloid beta and reduces the activation of astrocytes and microglia in aged mice. Front Mol Neurosci. 2017;10:144. doi: 10.3389/fnmol.2017.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. Kohman RA, Bhattacharya TK, Wojcik E, Rhodes JS. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J Neuroinflammation. 2013;10:114. doi: 10.1186/1742-2094-10-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96]. Koo JH, Kang EB, Oh YS, Yang DS, Cho JY. Treadmill exercise decreases amyloid-beta burden possibly via activation of SIRT-1 signaling in a mouse model of Alzheimer’s disease. Exp Neurol. 2017;288:142–52. doi: 10.1016/j.expneurol.2016.11.014 [DOI] [PubMed] [Google Scholar]
- [97]. Moore KM, Girens RE, Larson SK, Jones MR, Restivo JL, Holtzman DM, et al. A spectrum of exercise training reduces soluble Abeta in a dose-dependent manner in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;85:218–24. doi: 10.1016/j.nbd.2015.11.004 [DOI] [PubMed] [Google Scholar]
- [98]. Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in TgAlzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation. 2008;5:13. doi: 10.1186/1742-2094-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99]. Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–21. doi: 10.1523/JNEUROSCI.0496-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100]. Zhang Y, Chao FL, Zhou CN, Jiang L, Zhang L, Chen LM, et al. Effects of exercise on capillaries in the white matter of transgenic AD mice. Oncotarget. 2017;8:65860–75. doi: 10.18632/oncotarget.19505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101]. Kyrtsos CR, Baras JS. Modeling the Role of the Glymphatic pathway and cerebral blood vessel properties in Alzheimer’s disease pathogenesis. PLoS One. 2015;10:e0139574. doi: 10.1371/journal.pone.0139574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102]. Morrone CD, Liu M, Black SE, McLaurin J. Interaction between therapeutic interventions for Alzheimer’s disease and physiological Abeta clearance mechanisms. Front Aging Neurosci. 2015;7:64. doi: 10.3389/fnagi.2015.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103]. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–38. doi: 10.1038/nrn3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104]. Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–96. doi: 10.1038/nm.3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105]. Luissint AC, Artus C, Glacial F, Ganeshamoorthy K, Couraud PO. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012;9:23. doi: 10.1186/2045-8118-9-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106]. Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–94. doi: 10.1083/jcb.147.1.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107]. Jiao H, Wang Z, Liu Y, Wang P, Xue Y. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J Mol Neurosci. 2011;44:130–9. doi: 10.1007/s12031-011-9496-4 [DOI] [PubMed] [Google Scholar]
- [108]. Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–57. doi: 10.1016/S0306-4522(98)00058-X [DOI] [PubMed] [Google Scholar]
- [109]. Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–52. doi: 10.1016/j.neurobiolaging.2007.07.015 [DOI] [PubMed] [Google Scholar]
- [110]. Elahy M, Jackaman C, Mamo JC, Lam V, Dhaliwal SS, Giles C, et al. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun Ageing. 2015;12:2. doi: 10.1186/s12979-015-0029-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111]. Erdo F, Denes L, de Lange E. Age-associated physiological and pathological changes at the blood-brain barrier: A review. J Cereb Blood Flow Metab. 2017;37:4–24. doi: 10.1177/0271678X16679420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112]. Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031 [DOI] [PubMed] [Google Scholar]
- [113]. Hartz AM, Bauer B, Soldner EL, Wolf A, Boy S, Backhaus R, et al. Amyloid-beta contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke. 2012;43:514–23. doi: 10.1161/STROKEAHA.111.627562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114]. Zenaro E, Piacentino G, Constantin G. The blood-brain barrier in Alzheimer’s disease. Neurobiol Dis. 2017;107:41–56. doi: 10.1016/j.nbd.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115]. Kalaria RN. Cerebrovascular degeneration is related to amyloid-beta protein deposition in Alzheimer’s disease. Ann N Y Acad Sci. 1997;826:263–71. doi: 10.1111/j.1749-6632.1997.tb48478.x [DOI] [PubMed] [Google Scholar]
- [116]. Kook SY, Hong HS, Moon M, Ha CM, Chang S, Mook-Jung I. Abeta(1)(-)(4)(2)-RAGE interaction disrupts tight junctions of the blood-brain barrier via Ca(2)(+)-calcineurin signaling. J Neurosci. 2012;32:8845–54. doi: 10.1523/JNEUROSCI.6102-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117]. Kook SY, Seok Hong H, Moon M, Mook-Jung I. Disruption of blood-brain barrier in Alzheimer disease pathogenesis. Tissue Barriers. 2013;1:e23993. doi: 10.4161/tisb.23993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118]. Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–50. doi: 10.1038/nrneurol.2017.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119]. Ulrich JD, Huynh TP, Holtzman DM. Re-evaluation of the Blood-Brain Barrier in the Presence of Alzheimer’s Disease Pathology. Neuron. 2015;88:237–9. doi: 10.1016/j.neuron.2015.10.008 [DOI] [PubMed] [Google Scholar]
- [120]. Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68:930–41. doi: 10.1016/j.biopsych.2010.06.012 [DOI] [PubMed] [Google Scholar]
- [121]. Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–6. doi: 10.1038/nature11087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122]. Zlokovic BV. Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease. JAMA Neurol. 2013;70:440–4. doi: 10.1001/jamaneurol.2013.2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123]. Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124]. Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–18. doi: 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125]. Buttler L, Jordao MT, Fragas MG, Ruggeri A, Ceroni A, Michelini LC. Maintenance of Blood-Brain Barrier Integrity in Hypertension: A Novel Benefit of Exercise Training for Autonomic Control. Front Physiol. 2017;8:1048. doi: 10.3389/fphys.2017.01048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126]. Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension. 2014;63:572–9. doi: 10.1161/HYPERTENSIONAHA.113.01743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127]. Biancardi VC, Stern JE. Compromised blood-brain barrier permeability: novel mechanism by which circulating angiotensin II signals to sympathoexcitatory centres during hypertension. J Physiol. 2016;594:1591–600. doi: 10.1113/JP271584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128]. Pelisch N, Hosomi N, Mori H, Masaki T, Nishiyama A. RAS inhibition attenuates cognitive impairment by reducing blood- brain barrier permeability in hypertensive subjects. Curr Hypertens Rev. 2013;9:93–8. [DOI] [PubMed] [Google Scholar]
- [129]. Pelisch N, Hosomi N, Ueno M, Nakano D, Hitomi H, Mogi M, et al. Blockade of AT1 receptors protects the blood-brain barrier and improves cognition in Dahl salt-sensitive hypertensive rats. Am J Hypertens. 2011;24:362–8. doi: 10.1038/ajh.2010.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130]. Zhang Y, Zhang P, Shen X, Tian S, Wu Y, Zhu Y, et al. Early exercise protects the blood-brain barrier from ischemic brain injury via the regulation of MMP-9 and occludin in rats. Int J Mol Sci. 2013;14:11096–112. doi: 10.3390/ijms140611096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131]. Guo M, Cox B, Mahale S, Davis W, Carranza A, Hayes K, et al. Pre-ischemic exercise reduces matrix metalloproteinase-9 expression and ameliorates blood-brain barrier dysfunction in stroke. Neuroscience. 2008;151:340–51. doi: 10.1016/j.neuroscience.2007.10.006 [DOI] [PubMed] [Google Scholar]
- [132]. Wolff G, Davidson SJ, Wrobel JK, Toborek M. Exercise maintains blood-brain barrier integrity during early stages of brain metastasis formation. Biochem Biophys Res Commun. 2015;463:811–7. doi: 10.1016/j.bbrc.2015.04.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133]. Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci (Lond). 2017;131:2257–74. doi: 10.1042/CS20160381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134]. Gaberel T, Gakuba C, Goulay R, Martinez De Lizarrondo S, Hanouz JL, Emery E, et al. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke. 2014;45:3092–6. doi: 10.1161/STROKEAHA.114.006617 [DOI] [PubMed] [Google Scholar]
- [135]. Siler DA, Gonzalez JA, Wang RK, Cetas JS, Alkayed NJ. Intracisternal administration of tissue plasminogen activator improves cerebrospinal fluid flow and cortical perfusion after subarachnoid hemorrhage in mice. Transl Stroke Res. 2014;5:227–37. doi: 10.1007/s12975-014-0329-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136]. Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi: 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137]. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38. doi: 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138]. Wuerfel J, Haertle M, Waiczies H, Tysiak E, Bechmann I, Wernecke KD, et al. Perivascular spaces–MRI marker of inflammatory activity in the brain? Brain. 2008;131:2332–40. doi: 10.1093/brain/awn171 [DOI] [PubMed] [Google Scholar]
- [139]. Verheggen ICM, Van Boxtel MPJ, Verhey FRJ, Jansen JFA, Backes WH. Interaction between blood-brain barrier and glymphatic system in solute clearance. Neurosci Biobehav Rev. 2018;90:26–33. doi: 10.1016/j.neubiorev.2018.03.028 [DOI] [PubMed] [Google Scholar]
- [140]. Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845–61. doi: 10.1002/ana.24271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141]. Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;93:215–25. doi: 10.1016/j.nbd.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142]. Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–44. doi: 10.1111/j.1365-2990.2007.00926.x [DOI] [PubMed] [Google Scholar]
- [143]. Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra11. doi: 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144]. Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG. The role of brain barriers in fluid movement in the CNS: is there a ’glymphatic’ system? Acta Neuropathol. 2018;135:387–407. doi: 10.1007/s00401-018-1812-4 [DOI] [PubMed] [Google Scholar]
- [145]. von Holstein-Rathlou S, Petersen NC, Nedergaard M. Voluntary running enhances glymphatic influx in awake behaving, young mice. Neurosci Lett. 2018;662:253–8. doi: 10.1016/j.neulet.2017.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146]. Ambree O, Leimer U, Herring A, Gortz N, Sachser N, Heneka MT, et al. Reduction of amyloid angiopathy and Abeta plaque burden after enriched housing in TgCRND8 mice: involvement of multiple pathways. Am J Pathol. 2006;169:544–52. doi: 10.2353/ajpath.2006.051107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147]. Tapia-Rojas C, Aranguiz F, Varela-Nallar L, Inestrosa NC. Voluntary running attenuates memory loss, decreases neuropathological changes and induces neurogenesis in a mouse model of Alzheimer’s disease. Brain Pathol. 2016;26:62–74. doi: 10.1111/bpa.12255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148]. Yuede CM, Zimmerman SD, Dong H, Kling MJ, Bero AW, Holtzman DM, et al. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tgmouse model of Alzheimer’s disease. Neurobiol Dis. 2009;35:426–32. doi: 10.1016/j.nbd.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149]. Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004 [DOI] [PubMed] [Google Scholar]
- [150]. Liu HL, Zhao G, Zhang H, Shi LD. Long-term treadmill exercise inhibits the progression of Alzheimer’s disease-like neuropathology in the hippocampus of APP/PS1 transgenic mice. Behav Brain Res. 2013;256:261–72. doi: 10.1016/j.bbr.2013.08.008 [DOI] [PubMed] [Google Scholar]
- [151]. Prado Lima MG, Schimidt HL, Garcia A, Dare LR, Carpes FP, Izquierdo I, et al. Environmental enrichment and exercise are better than social enrichment to reduce memory deficits in amyloid beta neurotoxicity. Proc Natl Acad Sci U S A. 2018. doi: 10.1073/pnas.1718435115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152]. Um HS, Kang EB, Leem YH, Cho IH, Yang CH, Chae KR, et al. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22:529–39. [PubMed] [Google Scholar]
- [153]. Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–806. doi: 10.1523/JNEUROSCI.5654-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154]. Revilla S, Sunol C, Garcia-Mesa Y, Gimenez-Llort L, Sanfeliu C, Cristofol R. Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology. 2014;81:55–63. doi: 10.1016/j.neuropharm.2014.01.037 [DOI] [PubMed] [Google Scholar]
- [155]. Richter H, Ambree O, Lewejohann L, Herring A, Keyvani K, Paulus W, et al. Wheel-running in a transgenic mouse model of Alzheimer’s disease: protection or symptom? Behav Brain Res. 2008;190:74–84. doi: 10.1016/j.bbr.2008.02.005 [DOI] [PubMed] [Google Scholar]
- [156]. Rolland Y, Abellan van Kan G, Vellas B. Physical activity and Alzheimer’s disease: from prevention to therapeutic perspectives. J Am Med Dir Assoc. 2008;9:390–405. doi: 10.1016/j.jamda.2008.02.007 [DOI] [PubMed] [Google Scholar]
- [157]. Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, et al. Exercise program for nursing home residents with Alzheimer’s disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55:158–65. doi: 10.1111/j.1532-5415.2007.01035.x [DOI] [PubMed] [Google Scholar]
- [158]. Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–62. doi: 10.1126/science.7886457 [DOI] [PubMed] [Google Scholar]
- [159]. Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–64. doi: 10.1016/S0896-6273(00)80378-5 [DOI] [PubMed] [Google Scholar]
- [160]. Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci U S A. 1995;92:8074–7. doi: 10.1073/pnas.92.17.8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161]. Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702–11. doi: 10.1038/nn1903 [DOI] [PubMed] [Google Scholar]
- [162]. Bednarek E, Caroni P. beta-Adducin is required for stable assembly of new synapses and improved memory upon environmental enrichment. Neuron. 2011;69:1132–46. doi: 10.1016/j.neuron.2011.02.034 [DOI] [PubMed] [Google Scholar]
- [163]. Ma Z, Zang T, Birnbaum SG, Wang Z, Johnson JE, Zhang CL, et al. TrkB dependent adult hippocampal progenitor differentiation mediates sustained ketamine antidepressant response. Nat Commun. 2017;8:1668. doi: 10.1038/s41467-017-01709-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164]. Lau D, Bengtson CP, Buchthal B, Bading H. BDNF Reduces Toxic Extrasynaptic NMDA Receptor Signaling via Synaptic NMDA Receptors and Nuclear-Calcium-Induced Transcription of inhba/Activin A. Cell Rep. 2015;12:1353–66. doi: 10.1016/j.celre2015.07.038 [DOI] [PubMed] [Google Scholar]
- [165]. Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166]. Waterhouse EG, An JJ, Orefice LL, Baydyuk M, Liao GY, Zheng K, et al. BDNF promotes differentiation and maturation of adult-born neurons through GABAergic transmission. J Neurosci. 2012;32:14318–30. doi: 10.1523/JNEUROSCI.0709-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167]. Danzer SC, Crooks KR, Lo DC, McNamara JO. Increased expression of brain-derived neurotrophic factor induces formation of basal dendrites and axonal branching in dentate granule cells in hippocampal explant cultures. J Neurosci. 2002;22:9754–63. doi: 10.1523/JNEUROSCI.22-22-09754.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168]. Russo-Neustadt AA, Chen MJ. Brain-derived neurotrophic factor and antidepressant activity. Curr Pharm Des. 2005;11:1495–510. doi: 10.2174/1381612053764788 [DOI] [PubMed] [Google Scholar]
- [169]. Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–9. doi: 10.1038/381706a0 [DOI] [PubMed] [Google Scholar]
- [170]. Ma YL, Wang HL, Wu HC, Wei CL, Lee EH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82:957–67. doi: 10.1016/S0306-4522(97)00325-4 [DOI] [PubMed] [Google Scholar]
- [171]. Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27:265–77. doi: 10.1016/S0896-6273(00)00035-0 [DOI] [PubMed] [Google Scholar]
- [172]. Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–70. doi: 10.1038/sj.m4001957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173]. Fahnestock M. Brain-derived neurotrophic factor: the link between amyloid-beta and memory loss. Future Neurology. 2011;6:627–39. doi: 10.2217/fnl.11.44 [DOI] [Google Scholar]
- [174]. Peng S, Garzon DJ, Marchese M, Klein W, Ginsberg SD, Francis BM, et al. Decreased brain-derived neurotrophic factor depends on amyloid aggregation state in transgenic mouse models of Alzheimer’s disease. J Neurosci. 2009;29:9321–9. doi: 10.1523/JNEUROSCI.4736-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175]. Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J Neurochem. 2005;93:1412–21. doi: 10.1111/j.1471-4159.2005.03135.x [DOI] [PubMed] [Google Scholar]
- [176]. Webster MJ, Herman MM, Kleinman JE, Shannon Weickert C. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr Patterns. 2006;6:941–51. doi: 10.1016/j.modge2006.03.009 [DOI] [PubMed] [Google Scholar]
- [177]. Garzon D, Yu G, Fahnestock M. A new brain-derived neurotrophic factor transcript and decrease in brain-derived neurotrophic factor transcripts 1, 2 and 3 in Alzheimer’s disease parietal cortex. J Neurochem. 2002;82:1058–64. doi: 10.1046/j.1471-4159.2002.01030.x [DOI] [PubMed] [Google Scholar]
- [178]. Garzon DJ, Fahnestock M. Oligomeric amyloid decreases basal levels of brain-derived neurotrophic factor (BDNF) mRNA via specific downregulation of BDNF transcripts IV and V in differentiated human neuroblastoma cells. J Neurosci. 2007;27:2628–35. doi: 10.1523/JNEUROSCI.5053-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179]. Devi L, Ohno M. TrkB reduction exacerbates Alzheimer’s disease-like signaling aberrations and memory deficits without affecting beta-amyloidosis in 5XFAD mice. Transl Psychiatry. 2015;5:e562. doi: 10.1038/t2015.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180]. Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25:89–98. doi: 10.1016/j.tem.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181]. Rothman SM, Griffioen KJ, Wan R, Mattson MP. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann N Y Acad Sci. 2012;1264:49–63. doi: 10.1111/j.1749-6632.2012.06525.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182]. Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183]. Dinoff A, Herrmann N, Swardfager W, Liu CS, Sherman C, Chan S, et al. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS One. 2016;11:e0163037. doi: 10.1371/journal.pone.0163037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184]. Chen MJ, Russo-Neustadt AA. Running exercise-induced up-regulation of hippocampal brain-derived neurotrophic factor is CREB-dependent. Hippocampus. 2009;19:962–72. doi: 10.1002/hipo.20579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185]. Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363:43–8. doi: 10.1016/j.neulet.2004.03.058 [DOI] [PubMed] [Google Scholar]
- [186]. Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–9. doi: 10.1016/j.neuroscience.2003.09.029 [DOI] [PubMed] [Google Scholar]
- [187]. Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampal-dependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus. 2009;19:973–80. doi: 10.1002/hipo.20631 [DOI] [PubMed] [Google Scholar]
- [188]. Garza AA, Ha TG, Garcia C, Chen MJ, Russo-Neustadt AA. Exercise, antidepressant treatment, and BDNF mRNA expression in the aging brain. Pharmacol Biochem Behav. 2004;77:209–20. doi: 10.1016/j.pbb.2003.10.020 [DOI] [PubMed] [Google Scholar]
- [189]. Ivy AS, Rodriguez FG, Garcia C, Chen MJ, Russo-Neustadt AA. Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacol Biochem Behav. 2003;75:81–8. doi: 10.1016/S0091-3057(03)00044-3 [DOI] [PubMed] [Google Scholar]
- [190]. Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. doi: 10.1016/0006-8993(96)00273-9 [DOI] [PubMed] [Google Scholar]
- [191]. Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21:679–82. doi: 10.1016/S0893-133X(99)00059-7 [DOI] [PubMed] [Google Scholar]
- [192]. Sleiman SF, Henry J, Al-Haddad R, El Hayek L, Abou Haidar E, Stringer T, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife. 2016;5. doi: 10.7554/eLife.15092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193]. Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21:5678–84. doi: 10.1523/JNEUROSCI.21-15-05678.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194]. Yamanashi T, Iwata M, Kamiya N, Tsunetomi K, Kajitani N, Wada N, et al. Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci Rep. 2017;7:7677. doi: 10.1038/s41598-017-08055-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195]. Nehlig A. Brain uptake and metabolism of ketone bodies in animal models. Prostaglandins Leukot Essent Fatty Acids. 2004;70:265–75. doi: 10.1016/j.plefa.2003.07.006 [DOI] [PubMed] [Google Scholar]
- [196]. Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x [DOI] [PubMed] [Google Scholar]
- [197]. Portela LV, Brochier AW, Haas CB, de Carvalho AK, Gnoato JA, Zimmer ER, et al. Hyperpalatable Diet and Physical Exercise Modulate the Expression of the Glial Monocarboxylate Transporters MCT1 and 4. Mol Neurobiol. 2017;54:5807–14. doi: 10.1007/s12035-016-0119-5 [DOI] [PubMed] [Google Scholar]
- [198]. Regen DM, Callis JT, Sugden MC. Studies of cerebral beta-hydroxybutyrate transport by carotid injection; effects of age, diet and injectant composition. Brain Res. 1983;271:289–99. doi: 10.1016/0006-8993(83)90291-3 [DOI] [PubMed] [Google Scholar]
- [199]. Shima T, Matsui T, Jesmin S, Okamoto M, Soya M, Inoue K, et al. Moderate exercise ameliorates dysregulated hippocampal glycometabolism and memory function in a rat model of type 2 diabetes. Diabetologia. 2017;60:597–606. doi: 10.1007/s00125-016-4164-4 [DOI] [PubMed] [Google Scholar]
- [200]. Sonntag WE, Bennett SA, Khan AS, Thornton PL, Xu X, Ingram RL, et al. Age and insulin-like growth factor-1 modulate N-methyl-D-aspartate receptor subtype expression in rats. Brain Res Bull. 2000;51:331–8. doi: 10.1016/S0361-9230(99)00259-2 [DOI] [PubMed] [Google Scholar]
- [201]. Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588–97. doi: 10.1016/j.neuroscience.2010.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202]. Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–61. doi: 10.1016/j.neuroscience.2005.03.026 [DOI] [PubMed] [Google Scholar]
- [203]. Berchtold NC, Kesslak JP, Cotman CW. Hippocampal brain-derived neurotrophic factor gene regulation by exercise and the medial septum. J Neurosci Res. 2002;68:511–21. doi: 10.1002/jnr.10256 [DOI] [PubMed] [Google Scholar]
- [204]. Lee M, Soya H. Effects of acute voluntary loaded wheel running on BDNF expression in the rat hippocampus. J Exerc Nutrition Biochem. 2017;21:52–7. doi: 10.20463/jenb.2017.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205]. Ang ET, Wong PT, Moochhala S, Ng YK. Neuroprotection associated with running: is it a result of increased endogenous neurotrophic factors? Neuroscience. 2003;118:335–45. doi: 10.1016/S0306-4522(02)00989-2 [DOI] [PubMed] [Google Scholar]
- [206]. Chen J, Qin J, Su Q, Liu Z, Yang J. Treadmill rehabilitation treatment enhanced BDNF-TrkB but not NGF-TrkA signaling in a mouse intracerebral hemorrhage model. Neurosci Lett. 2012;529:28–32. doi: 10.1016/j.neulet.2012.09.021 [DOI] [PubMed] [Google Scholar]
- [207]. Maliszewska-Cyna E, Lynch M, Oore JJ, Nagy PM, Aubert I. The benefits of exercise and metabolic interventions for the prevention and early treatment of Alzheimer’s disease. Curr Alzheimer Res. 2017;14:47–60. doi: 10.2174/1567205013666160819125400 [DOI] [PubMed] [Google Scholar]
- [208]. Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163:1064–78. doi: 10.1016/j.cell.2015.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209]. Pan W, Kastin AJ. Interactions of IGF-1 with the blood-brain barrier in vivo and in situ. Neuroendocrinology. 2000;72:171–8. doi: 10.1159/000054584 [DOI] [PubMed] [Google Scholar]
- [210]. Baek SS. Role of exercise on the brain. J Exerc Rehabil. 2016;12:380–5. doi: 10.12965/jer.1632808.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211]. Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, et al. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. doi: 10.3389/fnagi.2013.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212]. Berg U, Bang P. Exercise and circulating insulin-like growth factor I. Horm Res. 2004;62 Supp1 1:50–8. doi: 10.1159/000080759 [DOI] [PubMed] [Google Scholar]
- [213]. Cappon J, Brasel JA, Mohan S, Cooper DM. Effect of brief exercise on circulating insulin-like growth factor I. J Appl Physiol (1985) 1994;76:2490–6. doi: 10.1152/jappl.1994.76.6.2490 [DOI] [PubMed] [Google Scholar]
- [214]. Prior SJ, Jenkins NT, Brandauer J, Weiss EP, Hagberg JM. Aerobic exercise training increases circulating insulin-like growth factor binding protein-1 concentration, but does not attenuate the reduction in circulating insulin-like growth factor binding protein-1 after a high-fat meal. Metabolism. 2012;61:310–6. doi: 10.1016/j.metabol.2011.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215]. Park JS, Hoke A. Treadmill exercise induced functional recovery after peripheral nerve repair is associated with increased levels of neurotrophic factors. PLoS One. 2014;9:e90245. doi: 10.1371/journal.pone.0090245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216]. Cao Y, Gunn AJ, Bennet L, Wu D, George S, Gluckman PD, et al. Insulin-like growth factor (IGF)-1 suppresses oligodendrocyte caspase-3 activation and increases glial proliferation after ischemia in near-term fetal sheep. J Cereb Blood Flow Metab. 2003;23:739–47. doi: 10.1097/01.WCB.0000067720.12805.6F [DOI] [PubMed] [Google Scholar]
- [217]. Guan J, Gunn AJ, Sirimanne ES, Tuffin J, Gunning MI, Clark R, et al. The window of opportunity for neuronal rescue with insulin-like growth factor-1 after hypoxia-ischemia in rats is critically modulated by cerebral temperature during recovery. J Cereb Blood Flow Metab. 2000;20:513–9. doi: 10.1097/00004647-200003000-00010 [DOI] [PubMed] [Google Scholar]
- [218]. Guan J, Miller OT, Waugh KM, McCarthy DC, Gluckman PD. Insulin-like growth factor-1 improves somatosensory function and reduces the extent of cortical infarction and ongoing neuronal loss after hypoxia-ischemia in rats. Neuroscience. 2001;105:299–306. doi: 10.1016/S0306-4522(01)00145-2 [DOI] [PubMed] [Google Scholar]
- [219]. Sizonenko SV, Sirimanne ES, Williams CE, Gluckman PD. Neuroprotective effects of the N-terminal tripeptide of IGF-1, glycine-proline-glutamate, in the immature rat brain after hypoxic-ischemic injury. Brain Res. 2001;922:42–50. doi: 10.1016/S0006-8993(01)03148-1 [DOI] [PubMed] [Google Scholar]
- [220]. Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197 Pt 4:575–85. doi: 10.1046/j.1469-7580.2000.19740575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221]. Li Y, Sun W, Han S, Li J, Ding S, Wang W, et al. IGF-1-involved negative feedback of NR2B NMDA subunits protects cultured hippocampal neurons against NMDA-Induced excitotoxicity. Mol Neurobiol. 2017;54:684–96. doi: 10.1007/s12035-015-9647-7 [DOI] [PubMed] [Google Scholar]
- [222]. Lin T-W, Kuo Y-M. Exercise benefits brain function: The monoamine connection. Brain Sciences. 2013;3:39–53. doi: 10.3390/brainsci3010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223]. Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog Neurobiol. 1996;50:335–62. doi: 10.1016/S0301-0082(96)00033-0 [DOI] [PubMed] [Google Scholar]
- [224]. Engleton RP, Abbruscato T. Drug abuse and the neurovascular unit In: Davis TP, editor. Advances in pharmacology Volume seventy one, Pharmacology of the blood brain barrier targeting CNS disorders. First edition ed. London, England: Academic Press; 2014. p. 451–80. doi: 10.1016/bs.apha.2014.06.019 [DOI] [PubMed] [Google Scholar]
- [225]. McMorris T. Developing the catecholamines hypothesis for the acute exercise-cognition interaction in humans: Lessons from animal studies. Physiology & Behavior. 2016;165:291–9. doi: 10.1016/j.physbeh.2016.08.011 [DOI] [PubMed] [Google Scholar]
- [226]. Meeusen R, De Meirleir K. Exercise and brain neurotransmission. Sports Med. 1995;20:160–88. doi: 10.2165/00007256-199520030-00004 [DOI] [PubMed] [Google Scholar]
- [227]. Meeusen R, Piacentini MF, De Meirleir K. Brain microdialysis in exercise research. Sports Med. 2001;31:965–83. doi: 10.2165/00007256-200131140-00002 [DOI] [PubMed] [Google Scholar]
- [228]. Elhusseiny A, Hamel E. Muscarinic–but not nicotinic–acetylcholine receptors mediate a nitric oxide-dependent dilation in brain cortical arterioles: a possible role for the M5 receptor subtype. J Cereb Blood Flow Metab. 2000;20:298–305. doi: 10.1097/00004647-200002000-00011 [DOI] [PubMed] [Google Scholar]
- [229]. Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol (1985). 2006;100:1059–64. doi: 10.1152/japplphysiol.00954.2005 [DOI] [PubMed] [Google Scholar]
- [230]. Serrador JM, Freeman R. Enhanced cholinergic activity improves cerebral blood flow during orthostatic stress. Front Neurol. 2017;8:103. doi: 10.3389/fneur.2017.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231]. Smith KJ, Ainslie PN. Regulation of cerebral blood flow and metabolism during exercise. Exp Physiol. 2017;102:1356–71. doi: 10.1113/EP086249 [DOI] [PubMed] [Google Scholar]
- [232]. Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–47. doi: 10.1523/JNEUROSCI.15-11-07539.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233]. O’Dell TJ, Connor SA, Guglietta R, Nguyen PV. Adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn Mem. 2015;22:461–71. doi: 10.1101/lm.031088.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234]. Garcia C, Chen MJ, Garza AA, Cotman CW, Russo-Neustadt A. The influence of specific noradrenergic and serotonergic lesions on the expression of hippocampal brain-derived neurotrophic factor transcripts following voluntary physical activity. Neuroscience. 2003;119:721–32. doi: 10.1016/S0306-4522(03)00192-1 [DOI] [PubMed] [Google Scholar]
- [235]. Kellner Y, Godecke N, Dierkes T, Thieme N, Zagrebelsky M, Korte M. The BDNF effects on dendritic spines of mature hippocampal neurons depend on neuronal activity. Front Synaptic Neurosci. 2014;6:5. doi: 10.3389/fnsyn.2014.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236]. Vasuta C, Caunt C, James R, Samadi S, Schibuk E, Kannangara T, et al. Effects of exercise on NMDA receptor subunit contributions to bidirectional synaptic plasticity in the mouse dentate gyrus. Hippocampus. 2007;17:1201–8. doi: 10.1002/hipo.20349 [DOI] [PubMed] [Google Scholar]
- [237]. Uchida S, Suzuki A, Kagitani F, Hotta H. Responses of acetylcholine release and regional blood flow in the hippocampus during walking in aged rats. J Physiol Sci. 2006;56:253–7. doi: 10.2170/physiolsci.SC001706 [DOI] [PubMed] [Google Scholar]
- [238]. Knipper M, da Penha Berzaghi M, Blochl A, Breer H, Thoenen H, Lindholm D. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur J Neurosci. 1994;6:668–71. doi: 10.1111/j.1460-9568.1994.tb00312.x [DOI] [PubMed] [Google Scholar]
- [239]. Heo YM, Shin MS, Lee JM, Kim CJ, Baek SB, Kim KH, et al. Treadmill exercise ameliorates short-term memory disturbance in scopolamine-induced amnesia rats. Int Neurourol J. 2014;18:16–22. doi: 10.5213/inj.2014.18.1.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240]. Giocomo LM, Hasselmo ME. Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Mol Neurobiol. 2007;36:184–200. doi: 10.1007/s12035-007-0032-z [DOI] [PubMed] [Google Scholar]
- [241]. Hill LE, Droste SK, Nutt DJ, Linthorst AC, Reul JM. Voluntary exercise alters GABA(A) receptor subunit and glutamic acid decarboxylase-67 gene expression in the rat forebrain. J Psychopharmacol. 2010;24:745–56. doi: 10.1177/0269881108096983 [DOI] [PubMed] [Google Scholar]
- [242]. Ota M, Yasuno F, Ito H, Seki C, Nozaki S, Asada T, et al. Age-related decline of dopamine synthesis in the living human brain measured by positron emission tomography with l-[β-11C]DOPA. Life Sci. 2006;79:730–6. doi: 10.1016/j.lfs.2006.02.017 [DOI] [PubMed] [Google Scholar]
- [243]. Iyo M. The detection of age-related decrease of dopamine D1, D2 and serotonin 5-HT2 receptors in living human brain. Prog Neuropsychopharmacol Biol Pschiatry. 2002;17(3):415–21. [DOI] [PubMed] [Google Scholar]
- [244]. Peters R. Ageing and the brain. Postgrad Med J. 2006;82:84–8. doi: 10.1136/pgmj.2005.036665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245]. Wong DF, Wagner HN Jr, Dannals RF, Links JM, Frost JJ, Ravert HT, et al. Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science. 1984;226:1393–6. doi: 10.1126/science.6334363 [DOI] [PubMed] [Google Scholar]
- [246]. Wu SY, Wang TF, Yu L, Jen CJ, Chuang JI, Wu FS, et al. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun. 2011;25:135–46. doi: 10.1016/j.bbi.2010.09.006 [DOI] [PubMed] [Google Scholar]
- [247]. Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O’Dowd BF, et al. Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci U S A. 2009;106:21377–82. doi: 10.1073/pnas.0903676106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248]. Iwakura Y, Nawa H, Sora I, Chao MV. Dopamine D1 receptor-induced signaling through TrkB receptors in striatal neurons. J Biol Chem. 2008;283:15799–806. doi: 10.1074/jbc.M801553200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249]. Do T, Kerr B, Kuzhikandathil EV. Brain-derived neurotrophic factor regulates the expression of D1 dopamine receptors. J Neurochem. 2007;100:416–28. doi: 10.1111/j.1471-4159.2006.04249.x [DOI] [PubMed] [Google Scholar]
- [250]. Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411:86–9. doi: 10.1038/35075076 [DOI] [PubMed] [Google Scholar]
- [251]. Fisher BE, Li Q, Nacca A, Salem GJ, Song J, Yip J, et al. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. NeuroReport. 2013;24:509–14. doi: 10.1097/WNR.0b013e328361dc13 [DOI] [PubMed] [Google Scholar]
- [252]. Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27:5291–300. doi: 10.1523/JNEUROSCI.1069-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253]. Gilliam PE, Spirduso WW, Martin TP, Walters TJ, Wilcox RE, Farrar RP. The effects of exercise training on [3H]-spiperone binding in rat striatum. Pharmacol Biochem Behav. 1984;20:863–7. doi: 10.1016/0091-3057(84)90008-X [DOI] [PubMed] [Google Scholar]
- [254]. MacRae PG, Spirduso WW, Cartee GD, Farrar RP, Wilcox RE. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolite levels. Neurosci Lett. 1987;79:138–44. doi: 10.1016/0304-3940(87)90686-0 [DOI] [PubMed] [Google Scholar]
- [255]. Mathes WF, Nehrenberg DL, Gordon R, Hua K, Garland T Jr, Pomp D. Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity. Behav Brain Res. 2010;210:155–63. doi: 10.1016/j.bbr.2010.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256]. Sutoo De, Akiyama K. Regulation of brain function by exercise. Neurobiol Dis. 2003;13:1–14. doi: 10.1016/S0969-9961(03)00030-5 [DOI] [PubMed] [Google Scholar]
- [257]. Arnold JC, Salvatore MF. Exercise-mediated increase in nigral tyrosine hydroxylase is accompanied by increased nigral GFR-alpha1 and EAAC1 expression in aging rats. ACS Chem Neurosci. 2016;7:227–39. doi: 10.1021/acschemneuro.5b00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258]. Baek DJ, Lee CB, Baek SS. Effect of treadmill exercise on social interaction and tyrosine hydroxylase expression in the attention-deficit/ hyperactivity disorder rats. J Exerc Rehabil. 2014;10:252–7. doi: 10.12965/jer.140162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259]. Tumer N, LaRochelle JS, Yurekli M. Exercise training reverses the age-related decline in tyrosine hydroxylase expression in rat hypothalamus. J Gerontol A Biol Sci Med Sci. 1997;52:B255–9. [DOI] [PubMed] [Google Scholar]
- [260]. Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27:5291–300. doi: 10.1523/JNEUROSCI.1069-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261]. Groch S, Wilhelm I, Diekelmann S, Sayk F, Gais S, Born J. Contribution of norepinephrine to emotional memory consolidation during sleep. Psychoneuroendocrinology. 2011;36:1342–50. doi: 10.1016/j.psyneuen.2011.03.006 [DOI] [PubMed] [Google Scholar]
- [262]. Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–43. doi: 10.1016/S0092-8674(04)00259-4 [DOI] [PubMed] [Google Scholar]
- [263]. O’Dell TJ, Connor SA, Guglietta R, Nguyen PV. beta-Adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn Mem. 2015;22:461–71. doi: 10.1101/lm.031088.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264]. Brown BS, Payne T, Kim C, Moore G, Krebs P, Martin W. Chronic response of rat brain norepinephrine and serotonin levels to endurance training. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:19–23. doi: 10.1152/jappl.1979.46.1.19 [DOI] [PubMed] [Google Scholar]
- [265]. Dunn AL, Reigle TG, Youngstedt SD, Armstrong RB, Dishman RK. Brain norepinephrine and metabolites after treadmill training and wheel running in rats. Med Sci Sports Exerc. 1996;28:204–9. doi: 10.1097/00005768-199602000-00008 [DOI] [PubMed] [Google Scholar]
- [266]. Pagliari R, Peyrin L. Norepinephrine release in the rat frontal cortex under treadmill exercise: a study with microdialysis. J Appl Physiol (1985). 1995;78:2121–30. doi: 10.1152/jappl.1995.78.6.2121 [DOI] [PubMed] [Google Scholar]
- [267]. Sarbadhikari SN, Saha AK. Moderate exercise and chronic stress produce counteractive effects on different areas of the brain by acting through various neurotransmitter receptor subtypes: a hypothesis. Theor Biol Med Model. 2006;3:33. doi: 10.1186/1742-4682-3-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268]. Semenova TP, Ivanov VA, Tret’yak TM. Brain levels of noradrenalin, dopamine, and serotonin in rats with different levels of motor activity. Neurosci Behav Physiol. 1981;11:153–5. doi: 10.1007/BF01182373 [DOI] [PubMed] [Google Scholar]
- [269]. Counts SE, Mufson EJ. Noradrenaline activation of neurotrophic pathways protects against neuronal amyloid toxicity. J Neurochem. 2010;113:649–60. doi: 10.1111/j.1471-4159.2010.06622.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270]. Van Hoomissen JD, Holmes PV, Zellner AS, Poudevigne A, Dishman RK. Effects of β-Adrenoreceptor Blockade During Chronic Exercise on Contextual Fear Conditioning and mRNA for Galanin and Brain-Derived Neurotrophic Factor. Behav Neurosci. 2004;118:1378–90. doi: 10.1037/0735-7044.118.6.1378 [DOI] [PubMed] [Google Scholar]
- [271]. Ebrahimi S, Rashidy-Pour A, Vafaei AA, Akhavan MM. Central ß-adrenergic receptors play an important role in the enhancing effect of voluntary exercise on learning and memory in rat. Behav Brain Res. 2010;208:189–93. doi: 10.1016/j.bbr.2009.11.032 [DOI] [PubMed] [Google Scholar]
- [272]. Chen MJ, Nguyen TV, Pike CJ, Russo-Neustadt AA. Norepinephrine induces BDNF and activates the PI-3K and MAPK cascades in embryonic hippocampal neurons. Cell Signal. 2007;19:114–28. doi: 10.1016/j.cellsig.2006.05.028 [DOI] [PubMed] [Google Scholar]
- [273]. Di Lieto A, Rantamaki T, Vesa L, Yanpallewar S, Antila H, Lindholm J, et al. The responsiveness of TrkB to BDNF and antidepressant drugs is differentially regulated during mouse development. PLoS One. 2012;7:e32869. doi: 10.1371/journal.pone.0032869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274]. Rantamaki T, Vesa L, Antila H, Di Lieto A, Tammela P, Schmitt A, et al. Antidepressant drugs transactivate TrkB neurotrophin receptors in the adult rodent brain independently of BDNF and monoamine transporter blockade. PLoS One. 2011;6:e20567. doi: 10.1371/journal.pone.0020567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275]. Liu X, Ye K, Weinshenker D. Norepinephrine Protects against Amyloid-beta Toxicity via TrkB. J Alzheimers Dis. 2015;44:251–60. doi: 10.3233/JAD-141062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276]. Heneka MT, Galea E, Gavriluyk V, Dumitrescu-Ozimek L, Daeschner J, O’Banion MK, et al. Noradrenergic depletion potentiates beta -amyloid-induced cortical inflammation: Implications for Alzheimer’s disease. J Neurosci. 2002;22:2434–42. doi:20026222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277]. Heneka MT, Nadrigny F, Regen T, Martinez-Hernandez A, Dumitrescu-Ozimek L, Terwel D, et al. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci U S A. 2010;107:6058–63. doi: 10.1073/pnas.0909586107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278]. Lockrow J, Boger H, Gerhardt G, Aston-Jones G, Bachman D, Granholm AC. A noradrenergic lesion exacerbates neurodegeneration in a Down syndrome mouse model. J Alzheimers Dis. 2011;23:471–89. doi: 10.3233/JAD-2010-101218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279]. Bondareff W, Mountjoy CQ, Roth M, Rossor MN, Iversen LL, Reynolds GP, et al. Neuronal degeneration in locus ceruleus and cortical correlates of Alzheimer disease. Alzheimer Dis Assoc Disord. 1987;1:256–62. doi: 10.1097/00002093-198701040-00005 [DOI] [PubMed] [Google Scholar]
- [280]. Matthews KL, Chen CP, Esiri MM, Keene J, Minger SL, Francis PT. Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol Psychiatry. 2002;51:407–16. [DOI] [PubMed] [Google Scholar]
- [281]. Bharani KL, Derex R, Granholm AC, Ledreux A. A noradrenergic lesion aggravates the effects of systemic inflammation on the hippocampus of aged rats. PLoS One. 2017;12:e0189821. doi: 10.1371/journal.pone.0189821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282]. Iyo M, Yamasaki T. The detection of age-related decrease of dopamine D1, D2 and serotonin 5-HT2 receptors in living human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:415–21. [DOI] [PubMed] [Google Scholar]
- [283]. Hasselbalch SG, Madsen K, Svarer C, Pinborg LH, Holm S, Paulson OB, et al. Reduced 5-HT2A receptor binding in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1830–8. doi: 10.1016/j.neurobiolaging.2007.04.011 [DOI] [PubMed] [Google Scholar]
- [284]. Lesch KP, Waider J. Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron. 2012;76:175–91. doi: 10.1016/j.neuron.2012.09.013 [DOI] [PubMed] [Google Scholar]
- [285]. Yuen EY, Jiang Q, Chen P, Feng J, Yan Z. Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of n-methyl-D-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J Biol Chem. 2008;283:17194–204. doi: 10.1074/jbc.M801713200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286]. Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005;25:5488–501. doi: 10.1523/JNEUROSCI.1187-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287]. Chen MJ, Russo-Neustadt AA. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res Mol Brain Res. 2005;135:181–93. doi: 10.1016/j.molbrainres.2004.12.001 [DOI] [PubMed] [Google Scholar]
- [288]. Chennaoui M, Grimaldi B, Fillion MP, Bonnin A, Drogou C, Fillion G, et al. Effects of physical training on functional activity of 5-HT 1B receptors in rat central nervous system: role of 5-HT-moduline. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2000;361:600–4. doi: 10.1007/s002100000242 [DOI] [PubMed] [Google Scholar]
- [289]. Renoir T, Pang TYC, Zajac MS, Chan G, Du X, Leang L, et al. Treatment of depressive-like behaviour in Huntington’s disease mice by chronic sertraline and exercise. British Journal of Pharmacology. 2012;165:1375–89. doi: 10.1111/j.1476-5381.2011.01567.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290]. Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–94. doi: 10.1016/j.tins.2004.08.001 [DOI] [PubMed] [Google Scholar]
- [291]. Klempin F, Babu H, De Pietri Tonelli D, Alarcon E, Fabel K, Kempermann G. Oppositional effects of serotonin receptors 5-HT1a, 2, and 2c in the regulation of adult hippocampal neurogenesis. Front Mol Neurosci. 2010;3. doi: 10.3389/fnmol.2010.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292]. Klempin F, Beis D, Mosienko V, Kempermann G, Bader M, Alenina N. Serotonin is required for exercise-induced adult hippocampal neurogenesis. J Neurosci. 2013;33:8270–5. doi: 10.1523/JNEUROSCI.5855-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293]. Klempin F, Mosienko V, Matthes S, Villela DC, Todiras M, Penninger JM, et al. Depletion of angiotensin-converting enzyme 2 reduces brain serotonin and impairs the running-induced neurogenic response. Cell Mol Life Sci. 2018. doi: 10.1007/s00018-018-2815-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294]. Blandini F, Greenamyre JT, Fancellu R, Nappi G. Blockade of subthalamic glutamatergic activity corrects changes in neuronal metabolism and motor behavior in rats with nigrostriatal lesions. Neurol Sci. 2001;22:49–50. doi: 10.1007/s100720170041 [DOI] [PubMed] [Google Scholar]
- [295]. Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0 [DOI] [PubMed] [Google Scholar]
- [296]. Sah N, Peterson BD, Lubejko ST, Vivar C, van Praag H. Running reorganizes the circuitry of one-week-old adult-born hippocampal neurons. Sci Rep. 2017;7:10903. doi: 10.1038/s41598-017-11268-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297]. Vivar C, Peterson BD, van Praag H. Running rewires the neuronal network of adult-born dentate granule cells. Neuroimage. 2016;131:29–41. doi: 10.1016/j.neuroimage.2015.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298]. Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev. 2001;122:1–29. doi: 10.1016/S0047-6374(00)00225-6 [DOI] [PubMed] [Google Scholar]
- [299]. Blandini F, Greenamyre JT, Nappi G. The role of glutamate in the pathophysiology of Parkinson’s disease. Funct Neurol. 1996;11:3–15. [PubMed] [Google Scholar]
- [300]. Francis PT. Glutamatergic systems in Alzheimer’s disease. Int J Geriatr Psychiatry. 2003;18:S15–21. doi: 10.1002/gps.934 [DOI] [PubMed] [Google Scholar]
- [301]. Mora F, Segovia G, del Arco A. Aging, plasticity and environmental enrichment: Structural changes and neurotransmitter dynamics in several areas of the brain. Brain Research Reviews. 2007;55:78–88. doi: 10.1016/j.brainresrev.2007.03.011 [DOI] [PubMed] [Google Scholar]
- [302]. Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998;54:369–415. doi: 10.1016/S0301-0082(97)00055-5 [DOI] [PubMed] [Google Scholar]
- [303]. Suen PC, Wu K, Levine ES, Mount HT, Xu JL, Lin SY, et al. Brain-derived neurotrophic factor rapidly enhances phosphorylation of the postsynaptic N-methyl-D-aspartate receptor subunit 1. Proc Natl Acad Sci U S A. 1997;94:8191–5. doi: 10.1073/pnas.94.15.8191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304]. Fox CJ, Russell KI, Wang YT, Christie BR. Contribution of NR2A and NR2B NMDA subunits to bidirectional synaptic plasticity in the hippocampus in vivo. Hippocampus. 2006;16:907–15. doi: 10.1002/hipo.20230 [DOI] [PubMed] [Google Scholar]
- [305]. Luscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol. 2012;4:a005710–a. doi: 10.1101/cshperspect.a005710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306]. Sun XY, Tuo QZ, Liuyang ZY, Xie AJ, Feng XL, Yan X, et al. Extrasynaptic NMDA receptor-induced tau overexpression mediates neuronal death through suppressing survival signaling ERK phosphorylation. Cell Death Dis. 2016;7:e2449. doi: 10.1038/cddis.2016.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307]. Biedermann S, Fuss J, Zheng L, Sartorius A, Falfan-Melgoza C, Demirakca T, et al. In vivo voxel based morphometry: detection of increased hippocampal volume and decreased glutamate levels in exercising mice. Neuroimage. 2012;61:1206–12. doi: 10.1016/j.neuroimage.2012.04.010 [DOI] [PubMed] [Google Scholar]
- [308]. Herbst EA, Holloway GP. Exercise increases mitochondrial glutamate oxidation in the mouse cerebral cortex. Appl Physiol Nutr Metab. 2016;41:799–801. doi: 10.1139/apnm-2016-0033 [DOI] [PubMed] [Google Scholar]
- [309]. Mourao FA, Leite HR, de Carvalho LE, Ferreira EVTH, Pinto MC, de Castro Medeiros D, et al. Neuroprotective effect of exercise in rat hippocampal slices submitted to in vitro ischemia is promoted by decrease of glutamate release and pro-apoptotic markers. J Neurochem. 2014;131:65–73. doi: 10.1111/jnc.12786 [DOI] [PubMed] [Google Scholar]
- [310]. Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–47. doi: 10.1016/S0896-6273(00)80395-5 [DOI] [PubMed] [Google Scholar]
- [311]. Hetman M, Kanning K, Cavanaugh JE, Xia Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:22569–80. doi: 10.1074/jbc.274.32.22569 [DOI] [PubMed] [Google Scholar]
- [312]. Behrens MM, Strasser U, Koh JY, Gwag BJ, Choi DW. Prevention of neuronal apoptosis by phorbol ester-induced activation of protein kinase C: blockade of p38 mitogen-activated protein kinase. Neuroscience. 1999;94:917–27. doi: 10.1016/S0306-4522(99)00212-2 [DOI] [PubMed] [Google Scholar]
- [313]. Pizzorusso T, Ratto GM, Putignano E, Maffei L. Brain-derived neurotrophic factor causes cAMP response element-binding protein phosphorylation in absence of calcium increases in slices and cultured neurons from rat visual cortex. J Neurosci. 2000;20:2809–16. doi: 10.1523/JNEUROSCI.20-08-02809.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314]. Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70:304–22. doi: 10.1002/dneu.20765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315]. Rendeiro C, Rhodes JS. A new perspective of the hippocampus in the origin of exercise-brain interactions. Brain Struct Funct. 2018;223:2527–45. doi: 10.1007/s00429-018-1665-6 [DOI] [PubMed] [Google Scholar]
- [316]. Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, et al. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–43. doi: 10.1038/sj.cdd.4401662 [DOI] [PubMed] [Google Scholar]
- [317]. Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–7. doi: 10.1016/S0092-8674(00)81883-8 [DOI] [PubMed] [Google Scholar]
- [318]. Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2007;35:208–19. doi: 10.1016/j.mcn.2007.02.019 [DOI] [PubMed] [Google Scholar]
- [319]. Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, et al. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–90. doi: 10.1016/S0896-6273(03)00543-9 [DOI] [PubMed] [Google Scholar]
- [320]. Rosahl TW, Geppert M, Spillane D, Herz J, Hammer RE, Malenka RC, et al. Short-term synaptic plasticity is altered in mice lacking synapsin I. Cell. 1993;75:661–70. doi: 10.1016/0092-8674(93)90487-B [DOI] [PubMed] [Google Scholar]
- [321]. Cesca F, Baldelli P, Valtorta F, Benfenati F. The synapsins: key actors of synapse function and plasticity. Prog Neurobiol. 2010;91:313–48. doi: 10.1016/j.pneurobio.2010.04.006 [DOI] [PubMed] [Google Scholar]
- [322]. Chin LS, Li L, Ferreira A, Kosik KS, Greengard P. Impairment of axonal development and of synaptogenesis in hippocampal neurons of synapsin I-deficient mice. Proc Natl Acad Sci U S A. 1995;92:9230–4. doi: 10.1073/pnas.92.20.9230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323]. Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–6. doi: 10.1126/science.273.5280.1402 [DOI] [PubMed] [Google Scholar]
- [324]. Lawson VH, Bland BH. The role of the septohippocampal pathway in the regulation of hippocampal field activity and behavior: analysis by the intraseptal microinfusion of carbachol, atropine, and procaine. Exp Neurol. 1993;120:132–44. doi: 10.1006/exnr.1993.1047 [DOI] [PubMed] [Google Scholar]
- [325]. Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–47. doi: 10.1016/0306-4522(94)90341-7 [DOI] [PubMed] [Google Scholar]
- [326]. Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–6. doi: 10.1111/j.1460-9568.2006.05059.x [DOI] [PubMed] [Google Scholar]
- [327]. Zhang L, Fang Y, Lian Y, Chen Y, Wu T, Zheng Y, et al. Brain-derived neurotrophic factor ameliorates learning deficits in a rat model of Alzheimer’s disease induced by abeta1-42. PLoS One. 2015;10:e0122415. doi: 10.1371/journal.pone.0122415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328]. Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience. 2012;219:62–71. doi: 10.1016/j.neuroscience.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329]. Fahey B, Barlow S, Day JS, O’Mara SM. Interferon-alpha-induced deficits in novel object recognition are rescued by chronic exercise. Physiol Behav. 2008;95:125–9. doi: 10.1016/j.physbeh.2008.05.008 [DOI] [PubMed] [Google Scholar]
- [330]. Jackson PA, Pialoux V, Corbett D, Drogos L, Erickson KI, Eskes GA, et al. Promoting brain health throughexercise and diet in older adults: a physiological perspective. J Physiol. 2016;594:4485–98. doi: 10.1113/JP271270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331]. Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–22. doi: 10.1016/j.neurobiolaging.2005.03.013 [DOI] [PubMed] [Google Scholar]
- [332]. Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–72. doi: 10.1111/j.1471-4159.2006.04165.x [DOI] [PubMed] [Google Scholar]
- [333]. Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–7. doi: 10.1002/glia.440070111 [DOI] [PubMed] [Google Scholar]
- [334]. Whiteman AS, Young DE, Budson AE, Stern CE, Schon K. Entorhinal volume, aerobic fitness, and recognition memory in healthy young adults: A voxel-based morphometry study. Neuroimage. 2016;126:229–38. doi: 10.1016/j.neuroimage.2015.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335]. Whiteman AS, Young DE, He X, Chen TC, Wagenaar RC, Stern CE, et al. Interaction between serum BDNF and aerobic fitness predicts recognition memory in healthy young adults. Behav Brain Res. 2014;259:302–12. doi: 10.1016/j.bbr.2013.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336]. Chang YK, Labban JD, Gapin JI, Etnier JL. The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res. 2012;1453:87–101. doi: 10.1016/j.brainres.2012.02.068 [DOI] [PubMed] [Google Scholar]
- [337]. Cox EP, O’Dwyer N, Cook R, Vetter M, Cheng HL, Rooney K, et al. Relationship between physical activity and cognitive function in apparently healthy young to middle-aged adults: A systematic review. J Sci Med Sport. 2016;19:616–28. doi: 10.1016/j.jsams.2015.09.003 [DOI] [PubMed] [Google Scholar]
- [338]. Tsai CL, Pan CY, Chen FC, Wang CH, Chou FY. Effects of acute aerobic exercise on a task-switching protocol and brain-derived neurotrophic factor concentrations in young adults with different levels of cardiorespiratory fitness. Exp Physiol. 2016;101:836–50. doi: 10.1113/EP085682 [DOI] [PubMed] [Google Scholar]
- [339]. Wrann CD. FNDC5/irisin - their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plast. 2015;1:55–61. doi: 10.3233/BPL-150019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340]. Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, et al. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013;18:649–59. doi: 10.1016/j.cmet.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341]. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. doi: 10.1038/nature10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342]. Briken S, Rosenkranz SC, Keminer O, Patra S, Ketels G, Heesen C, et al. Effects of exercise on Irisin, BDNF and IL-6 serum levels in patients with progressive multiple sclerosis. J Neuroimmunol. 2016;299:53–8. doi: 10.1016/j.jneuroim.2016.08.007 [DOI] [PubMed] [Google Scholar]
- [343]. Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–22. doi: 10.1172/JCI27794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344]. Papp C, Pak K, Erdei T, Juhasz B, Seres I, Szentpeteri A, et al. Alteration of the irisin-brain-derivedneurotrophic factor axis contributes to disturbance of mood in COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2023–33. doi: 10.2147/COPD.S135701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345]. Chen M, Do H. Wnt Signaling in Neurogenesis during Aging and Physical Activity. Brain Sci. 2012;2:745–68. doi: 10.3390/brainsci2040745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346]. Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35:47–56. doi: 10.1016/j.tins.2011.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347]. Baranowski BJ, MacPherson REK. Acute exercise induced BDNF-TrkB signaling is intact in the prefrontal cortex of obese glucose intolerant male mice. Appl Physiol Nutr Metab. 2018. doi: 10.1139/apnm-2018-0108 [DOI] [PubMed] [Google Scholar]
- [348]. Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behav Brain Res. 2001;120:87–95. doi: 10.1016/S0166-4328(00)00364-8 [DOI] [PubMed] [Google Scholar]
- [349]. Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–12. doi: 10.1016/S0306-4522(00)00349-3 [DOI] [PubMed] [Google Scholar]
- [350]. Wu CW, Chang YT, Yu L, Chen HI, Jen CJ, Wu SY, et al. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J Appl Physiol (1985). 2008;105:1585–94. doi: 10.1152/japplphysiol.90775.2008 [DOI] [PubMed] [Google Scholar]
- [351]. MacRae PG, Spirduso WW, Walters TJ, Farrar RP, Wilcox RE. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolites in presenescent older rats. Psychopharmacology (Berl). 1987;92:236–40. doi: 10.1007/BF00177922 [DOI] [PubMed] [Google Scholar]
- [352]. Gomez-Merino D, Bequet F, Berthelot M, Chennaoui M, Guezennec CY. Site-dependent effects of an acute intensive exercise on extracellular 5-HT and 5-HIAA levels in rat brain. Neurosci Lett. 2001;301:143–6. doi: 10.1016/S0304-3940(01)01626-3 [DOI] [PubMed] [Google Scholar]
- [353]. Real CC, Ferreira AFB, Hernandes MS, Britto LRG, Pires RS. Exercise-induced plasticity of AMPA-type glutamate receptor subunits in the rat brain. Brain Research. 2010;1363:63–71. doi: 10.1016/j.brainres.2010.09.060 [DOI] [PubMed] [Google Scholar]
- [354]. Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–15. doi: 10.1016/j.neuron.2005.08.023 [DOI] [PubMed] [Google Scholar]
- [355]. Schoenfeld TJ, Rada P, Pieruzzini PR, Hsueh B, Gould E. Physical exercise prevents stress-induced activation of granule neurons and enhances local inhibitory mechanisms in the dentate gyrus. J Neurosci. 2013;33:7770–7. doi: 10.1523/JNEUROSCI.5352-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [356]. Kurosawa M, Okada K, Sato A, Uchida S. Extracellular release of acetylcholine, noradrenaline and serotonin increases in the cerebral cortex during walking in conscious rats. Neurosci Lett. 1993;161:73–6. doi: 10.1016/0304-3940(93)90143-9 [DOI] [PubMed] [Google Scholar]
- [357]. Nakajima K, Uchida S, Suzuki A, Hotta H, Aikawa Y. The effect of walking on regional blood flow and acetylcholine in the hippocampus in conscious rats. Auton Neurosci. 2003;103:83–92. doi: 10.1016/S1566-0702(02)00263-1 [DOI] [PubMed] [Google Scholar]