Abstract
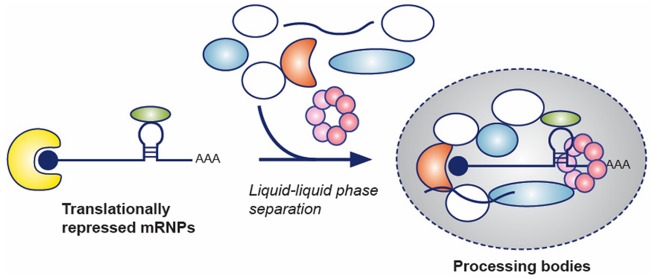
Processing bodies (P-bodies) are cytoplasmic ribonucleoprotein (RNP) granules primarily composed of translationally repressed mRNAs and proteins related to mRNA decay, suggesting roles in post-transcriptional regulation. P-bodies are conserved in eukaryotic cells and exhibit properties of liquid droplets. However, the function of P-bodies in translational repression and/or mRNA decay remains contentious. Here we review recent advances in our understanding of the molecular composition of P-bodies, the interactions and processes that regulate P-body liquid–liquid phase separation (LLPS), and the cellular localization of mRNA decay machinery, in the context of how these discoveries refine models of P-body function.
Processing bodies (P-bodies) are cytoplasmic ribonucleoprotein (RNP) granules comprised primarily of mRNAs in complex with proteins associated with translation repression and 5′-to-3′ mRNA decay. These RNP granules are conserved in eukaryotes and bear similarities to other RNP granules, such as Cajal bodies, nucleoli, and stress granules, in that they depend on complex networks of protein–RNA interactions, low-complexity protein sequences, and liquid–liquid phase separation (LLPS) for their formation.12 Despite their similarities, each of these RNP granules is distinct in its molecular composition and function. For example, stress granules and P-bodies share some protein components, they can come into contact with each other, and both can be induced by cellular stress;15 however, stress granules uniquely contain translation initiation factors. Similarly, while P-bodies and GW-bodies, which are associated with miRNA/siRNA silencing, were originally conflated, AGO2 and GW182 were found to localize to P-bodies only in metazoans,16−20 and GW-bodies have more recently been shown to colocalize with multivesicular bodies, not P-bodies, in higher eukaryotes as well.21 Therefore, despite the nonmembrane-bounded nature of these RNP granules, each has a unique molecular composition that is likely related to its function.
P-bodies were discovered during the investigation of the localization of proteins associated with the 5′-to-3′ mRNA decay pathway, and the additional observation of mRNA decay intermediates in these structures led to the initial hypothesis that P-bodies were cellular sites of mRNA decay. However, it was subsequently demonstrated that macroscopically observable P-bodies are not required for mRNA decay to occur22,23 and that mRNAs can recycle from P-bodies to translating polysomes.24 More recently, mRNA decay has been observed despite a lack of P-bodies in yeast strains lacking functional edc3 and lsm4 genes.25 An alternative, though not necessarily mutually exclusive model, has thus emerged positing that P-bodies are storage sites for translationally repressed mRNAs and inactive mRNA decay enzymes, which undergo LLPS (vide infra) as a result of the dense network of protein–protein interactions that form when mRNA decay factors accumulate on polysome-free transcripts.26,27 The function of P-bodies in mRNA decay, therefore, is still an open question, largely due to the challenge of directly visualizing mRNA degradation in diffraction-limited structures within living cells,28 as well as the difficulty of biochemically purifying labile liquid droplets from cells.
Many membraneless RNP granules, including Cajal bodies, nucleoli, and mammalian stress granules, have recently been described as having properties of liquid droplets (reviewed in refs (12, 29, and 30)). At the same time, in vitro studies have shown the propensity of RNA-binding proteins and low-sequence-complexity proteins to undergo LLPS either alone or in the presence of RNA.31−34 The physical basis of LLPS has attracted a great deal of attention in recent years because of the critical role that proper messenger ribonucleoprotein (mRNP) assembly plays in pathogenesis33,35−37 and in stress responses.38 Liquid droplet formation has been reconstituted using intrinsically disordered regions (IDRs) and protein fragments, low-complexity sequences, or SLiMs from RNA-binding and RNA granule associated proteins.12,37,39 It has been suggested, by extension, that P-bodies must also be liquid droplets, especially considering the frequent occurrence of low-complexity domains (LCDs) in P-body components.40 However, it is only recently that direct evidence has accumulated that P-bodies and their constituent proteins undergo LLPS.
In this review, we describe recent advances in our understanding of the properties and composition of P-bodies, with a focus on advances since the last major overview of the field.41 First, we provide an update on both targeted and high-throughput methods to identify protein and RNA components of P-bodies. Second, we review evidence that P-bodies and their constituents have the ability to undergo LLPS, considered in context of the regulation of P-body assembly. Finally, we re-examine models of P-body function in light of recent investigations into mRNA decay in cells and in liquid droplets.
P-Body Composition
A majority of proteins constitutively associated with P-bodies are involved in translational repression and/or RNA decay (reviewed in refs (27 and 41)). One major class of proteins is associated with mRNA deadenylation and 5′-to-3′ decay (reviewed in refs (27)), including the deadenylation complex Ccr4-Not, Lsm1-7, the decapping coactivator and enzyme Dcp1/Dcp2, various decapping activators such as Edc3, Pat1, DDX6 (Rck/p54, Dhh1p in yeast), and EDC4, and the 5′-to-3′ exoribonuclease Xrn1. Another class includes RNA-binding proteins that facilitate translational repression such as 4E-T42,43 and CPEB1.44 A detailed, though not necessarily comprehensive, summary of P-body proteins and their functions has already been discussed in various previous reviews, to which we refer the reader.26,27,41 These constituents have been verified, and a number of additional P-body-associated factors have been recently identified, through mass spectrometry-based proteomics and/or targeted studies with support from subcellular imaging. The newly identified P-body proteins described in this section are summarized in Table 1, and two examples are presented Figure 1.
Table 1. Recently Identified P-Body Proteins.
| Protein | Function | Reference |
|---|---|---|
| NoBody | mRNA decay | D’Lima et al. 201750 |
| YTHDF2 | mRNA stability | Wang et al. 201453 |
| CCHCR1 | Regulation of mRNA metabolism | Lin et al. 201454 |
| K63 | P-body assembly | Tenekeci et al. 201655 |
| TRAF6 | Regulation of phosphorylation | Tenekeci et al. 201655 |
| Tob1 | Post-transcriptional regulation | Shapouri et al. 201556 |
| TUT4 | Uridyltransferase | Hubstenberger et al. 201748 |
| APOBEC3F | Cytidine deaminase | Hubstenberger et al. 201748 |
| HNRNPU | RNA binding protein | Hubstenberger et al. 201748 |
| SYNCRIP/HNRNPQ | RNA binding protein | Hubstenberger et al. 201748 |
| IGF2BP1 | RNA binding protein | Hubstenberger et al. 201748 |
| DHX9 | RNA binding protein | Hubstenberger et al. 201748 |
| IGF2BP1 | RNA binding protein | Hubstenberger et al. 201748 |
| IGF2BP3 | RNA binding protein | Hubstenberger et al. 201748 |
| PUM1 | Translation repression | Hubstenberger et al. 201748 |
| PUM2 | Translation repression | Hubstenberger et al. 201748 |
| STAU2L | Translation repression | Hubstenberger et al. 201748 |
| ILF3 | Translation repression | Hubstenberger et al. 201748 |
| MYO1C | Myosin | Hubstenberger et al. 201748 |
| MYO1D | Myosin | Hubstenberger et al. 201748 |
| MYO6 | Myosin | Hubstenberger et al. 201748 |
| MYH10 | Myosin | Hubstenberger et al. 201748 |
Figure 1.
Recently identified P-body proteins. (A) Proposed interactions between inhibitory microprotein NBDY and the mRNA decapping complex. (B) m6A-dependent mRNA localization to P-bodies and mRNA degradation mediated by YTHDF2.
P-body proteomics and transcriptomics
Mass spectrometry-based proteomics has been applied to global identification of proteins that copurify with P-body components, many of which have been confirmed to localize to P-bodies. Several studies have identified interaction partners of the RNA helicase DDX6 (Dhh1p in yeast). Two studies of human DDX61,45 identified a largely overlapping DDX6 interaction network consisting of P-body proteins including DCP1A/B, EDC3, EDC4, LSM14A, PATL1, and 4E-T/EIF4ENIF1. Cary et al.46 purified a GFP-Dhh1p construct from yeast and compared copurified proteins under basal and stress conditions. This study again confirmed previously identified P-body components and putative interactors and also showed that the previously identified stress granule component47 Ydj1, a chaperone, was enriched under stress conditions and was required for assembly of some proteins into glucose depletion induced P-bodies. However, because DDX6/Dhh1p is also distributed throughout the cytoplasm, each of these studies also identified interaction partners of DDX6/Dhh1p that are not associated with P-bodies.
A recent report by Hubstenberger et al.48 described P-body purification via fluorescence-activated particle sorting rather than affinity purification, followed by transcriptomic and proteomic analysis with immunofluorescence-based confirmation. This study design eliminated non-P-body-associated protein–protein interactions in addition to identifying novel P-body proteins including RNA modifying enzymes (e.g., TUT4, APOBEC3F), RNA binding proteins (e.g., PUM1/2, STAU2, HNRNPU), and, surprisingly, myosins (e.g., MYO6), potentially suggesting a link between P-bodies and the cytoskeleton (see Table 1).49 Remarkably, concurrent sequencing of the sorted P-body-associated RNAs identified approximately 1/5 of cellular RNAs, many of which were intact, translationally repressed mRNAs associated with regulatory processes. These results support enrichment of mRNA decay factors and RNA binding proteins in P-bodies, an association of P-bodies with translational repression for a large number of transcripts, and suggest that chaperones and myosins could play roles in their formation or localization.
Other recently identified P-body proteins
NoBody (NBDY),50 a mammalian 69-amino acid microprotein translated from a previously annotated long noncoding RNA (LINC01420), was recently found to copurify with the human mRNA decapping complex (Figure 1A). Importantly, this protein would not have been identified through standard affinity proteomics approaches, because it was not previously represented in annotated protein databases. Epitope-tagged NBDY was found to colocalize with P-bodies via immunofluorescence imaging, and modulation of NBDY expression levels led to changes in P-body numbers, which is a general property of P-body proteins.26,51 Silencing of NBDY expression was shown to destabilize a reporter of nonsense-mediated decay, suggesting that NBDY is an inhibitor of mRNA turnover. While its specific molecular interaction network remains to be fully elucidated, NBDY forms a specific photo-cross-link to EDC450 and contains a C-terminal polyproline sequence with the potential to interact with EVH1 domains52 such as that found in Dcp1A.
YTHDF253 is a recently discovered mRNA modification “reader” protein that localizes to P-bodies (Figure 1B). YTHDF2 specifically binds to N6-methyladenosine and promotes destabilization of mRNAs bearing this modification. The C-terminal domain of this protein is the “reader” domain that binds the mRNA modification, and the P/Q/N-rich N-terminal domain is required for colocalization with its RNA targets in P-bodies. Du et al. showed that YTHDF2 recruits the CCR4-NOT complex through a direct interaction between the YTHDF2 N-terminal region and the SH domain of the CNOT1 subunit, which is essential for destabilizing m6A mRNAs.57 These results are of particular interest because they expand the association of P-bodies to mRNA decay through a newly discovered mRNA-modification-dependent mechanism.
Finally, CCHCR1,54 a protein of unknown function, was recently shown to interact with the core P-body component EDC4 in an immunoprecipitation-based proteomics study. Recombinant GFP-CCHCR1 was shown to colocalize with P-body markers in mammalian cells, and its overexpression produced enlarged P-bodies—again, a common property of P-body resident proteins.
Together, recent global and targeted studies of P-body composition continue to underscore the relationship of mRNA decay factors to P-bodies, as well as the relationship of P-body protein expression levels to P-body morphology and numbers. It remains possible, that, due to the rapid exchange of P-body factors with the cytoplasm,58,59 additional P-body-associated proteins may remain to be identified.
Liquid–Liquid Phase Separation
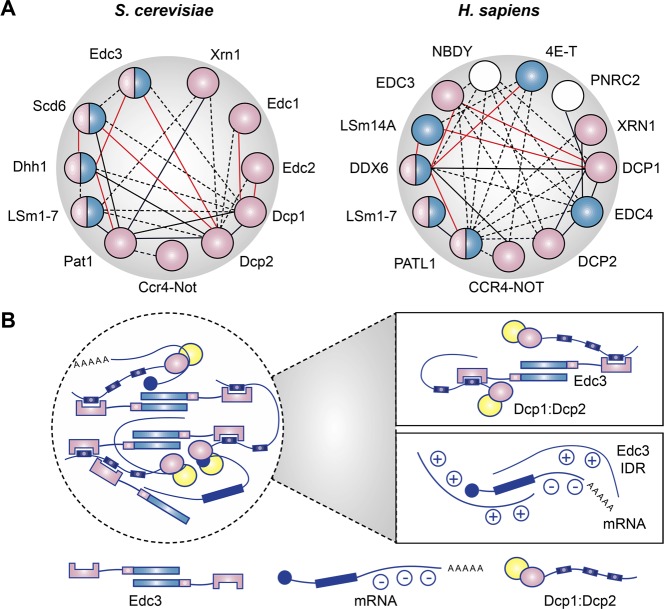
LLPS has been reported to depend on several factors: (1) a dense network of redundant interactions between proteins and RNA, (2) low-complexity sequences, or short linear motifs (SLiMs) within which scaffolds can bind to folded domains of other client proteins, and (3) weak to intermediate interactions, including polyelectrolyte–polyelectrolyte interactions (complex coacervation), between proteins and RNA (reviewed in refs (12)). A summary of the P-body protein–protein and protein–RNA interaction network is provided in Figure 2, illustrating these LLPS-promoting properties.
Figure 2.
Factors that drive P-Body assembly and liquid–liquid phase separation (LLPS). (A) Interactions between P-body proteins. Components critical to P-body assembly are colored in blue, which include P-body proteins required for the maintenance of pre-existing P-bodies and factors that affect the induction of new P-bodies by arsenite or vinblastine treatment, cold shock,1 or glucose depletion.2 Components colored in pink directly interact with RNA.3−8 Solid black lines indicated direct protein–protein interactions, and red lines indicate mutual exclusiveness. Dotted black lines suggest indirect or putative interactions. Only interactions with at least two sources of evidence derived from BioGRID are included (except for PatL1, whose interaction with many P-body components is not well covered in the database9,10). (B) Intermolecular interactions that promote LLPS. Upper box: simultaneous protein–protein interactions between intrinsically disordered regions (IDRs) and dimerization of P-body components.11 Lower box: association between positively charged IDRs of P-body components such as Edc313 with RNA transcripts promotes phase separation, possibly through electrostatic14 and cation−π interactions.
Fluorescence microscopy has provided direct evidence for the liquid droplet nature of P-bodies in living cells. Now-classic fluorescence recovery after photobleaching (FRAP) studies58,59 showed rapid exchange of P-body proteins and RNA with the cytoplasm, as well as P-body/stress granule fusion events,59 supporting dynamic and liquid-like properties. A recent report by Kroschwald and co-workers verified both yeast and mammalian P-bodies as liquids in living cells based on close observations of their dynamics.60 First, the spherical morphology of P-bodies is consistent with a liquid-like state as caused by surface tension. Second, two P-bodies can relax and fuse together to a new larger body that maintained its round shape, suggesting the dynamic nature of these structures. Third, P-bodies can be reversibly dissolved by hexanediol, which interferes with weak intermolecular interactions and serves as an indicator of LLPS. Therefore, cellular P-bodies exhibit key features of a liquid droplet.
A number of studies have reconstituted LLPS, as well as the related phenomenon of protein hydrogel formation in vitro using purified proteins,31,33,34,61,62 demonstrating that this phenomenon is common among proteins containing low-complexity or prion-like sequences and that it is common among RNA-binding proteins such as FUS and hnRNPA1/2. These results have recently been extended to P-body proteins. For example, the purified intrinsically disordered region of Lsm4 forms liquid droplets that rapidly mature into amyloid-like fibrils,34 a consequence of liquid droplet metastability.63 The mRNA decapping complex, centering on the enzyme Dcp2, which catalyzes the first step in 5′-to-3′ mRNA decay, and its coactivators, can also undergo LLPS. Purified Edc3 (protein enhancer of decapping 3) and a fragment of Dcp2 from fission yeast can phase separate when mixed in vitro, as can a mixture of Pdc1, the yeast ortholog of metazoan Edc4/Hedls,64 fused to glutathione S-transferase and Edc311 and a ternary system of Dcp1, Dcp2, and Edc313 (Figure 2B, upper box). This process depended on multivalent interactions mediated by helical leucine-rich repeats (HLMs) in yeast Dcp2 and Pdc111 and was enhanced by the presence of IDRs in Edc3, addition of RNA, or decreased salt concentrations.13 Importantly, these in vitro studies have permitted the systematic investigation of parameters controlling phase separation, such as salt and protein concentrations. The phase diagram obtained by Sprangers and colleagues13 suggested that the cellular concentrations of many P-body proteins are close to the LLPS phase boundary, suggesting that small perturbations to cellular expression levels or interactions could drive the system to either state. Taken together, these results are consistent with a propensity of P-body proteins, in isolation or in combination, to form liquid droplets.
Regulation of P-Body Assembly and Disassembly
Interactions responsible for P-body assembly
P-body assembly in vivo has been studied in the context of both maintenance of existing P-bodies and de novo formation of new P-bodies, e.g. in response to stress, and both are considered here. P-Body maintenance depends on the concentrations of many P-body resident proteins, which have been previously extensively reviewed (reviewed in refs (26)). Specifically, the Yjef-N domain of yeast Edc3, the C-terminal Q/N-rich domain of yeast Lsm4,65,66 and the RGG domain of human Lsm467 and DDX61,43 have been shown to be important for P-body maintenance (Figure 2A, colored in blue). These domains are of particular interest because they contain low-complexity sequences and/or RNA binding activities that are implicated in LLPS, suggesting that phase separation is likely important in formation and maintenance of P-bodies in cells. Beyond these domains, additional protein factors affect P-body maintenance in metazoans, including EDC4,22 CPEB1,68 and 4E-T,58 depletion of which leads to a loss of P-bodies, while depletion of Pat1b causes a decrease in P-body numbers.69 P-body maintenance is also dependent on the presence of translationally repressed mRNA, as treatment with cycloheximide, which traps mRNA in polysomes, causes loss of P-bodies,71 and depletion of Dcp2, Dcp1, and Xrn1 leads to increased P-body numbers in yeast, likely due to accumulation of mRNA decay intermediates.26,70 Consistent with cross-talk between polysomes and P-bodies, polysome-associated RNA binding proteins including SCP160 have recently been shown to regulate P-body maintenance in yeast.72De novo P-body formation also increases under specific stress conditions,71 such as glucose starvation73 and osmotic stress,25 and P-bodies disappear during mitosis,74 suggesting that their formation is stimulus-responsive. For de novo P-body formation, DDX6, 4E-T, and Lsm14a are required, while EDC4 and Pat1b depletion lead to minor defects in assembly.1,75
Importantly, some interactions are redundant in P-body assembly. For example, Rao et al. showed that yeast edc3Δ lsm4Δ strain deficient in P-body maintenance and de novo assembly under glucose starvation could still form P-bodies in the stationary phase.2 Overexpression of Dhh1 could partially rescue P-body assembly during glucose starvation, and Psp2 and Pby1, components of yeast P-bodies, were also able to restore P-bodies under normal growth conditions in this strain, suggesting that alternative multivalent protein–protein interactions could drive P-body assembly in the absence of Edc3 and Lsm4. These results suggest that the network of multivalent protein–protein and protein–RNA interactions (with the exception of a few essential factors) is more important than individual components or interactions for P-body assembly. This is consistent with a major role for multivalent interactions in LLPS.
Active remodeling and post-translational modifications
Recent reports also suggest important roles of ATPase activity in maintaining P-body integrity,76,77 which implies the likelihood that the regulation of P-body fluidity is an energy-consuming process. However, a fine control of the ATPase activity must be achieved since hyperactivity of ATPases could lead to dissipation of P-bodies.77 The observation that phase-separated liquid droplets can mature in vitro to amyloid-like aggregates34 may be consistent with a role for energy-dependent remodeling processes in the maintenance of P-body liquidity and the prevention of toxic aggregate formation.
Post-translational protein modifications (PTMs) play a significant role in modulating P-body formation. For example, many P-body proteins are phosphorylated, including yeast Dcp2, which requires Ste20-mediated phosphorylation for P-body localization.78 Dcp1A is phosphorylated during 3T3-L1 differentiation, which stabilizes its interaction with Dcp279 and is hyperphosphorylated during mitosis,80 coincident with loss of P-bodies during cell division. More recently, Dcp1A has been shown to be both mono- and polyubiquitylated on many of its lysine residues.81 Indeed, this study showed that polyubiquitylation of Dcp1A induces phosphorylation at S315, suggesting that these modifications may be functionally linked. Mutation of a series of ubiquitylated lysines in the C-terminal portion of Dcp1A to arginine was associated with a defect in P-body size, Dcp2 binding, and decapping activity. Global repression of K63-linked polyubiquitin chains via expression of a dominant negative mutant ubiquitin led to a complete loss of P-bodies. These results implicate ubiquitin as a key regulator of P-body assembly, either independently or through modulation of other PTMs. A pair of studies demonstrated that arginine dimethylation in the C-terminal R/G-rich domain of Lsm482 is important for the formation of P-bodies in human cells.67 Cells expressing Lsm4 lacking this domain were deficient in observable P-bodies, though mRNA decay and translational repression were unaffected, and depletion of the arginine methyltransferase PRMT5 resulted in a defect in P-body assembly, decoupling its structural and functional roles in the P-body. It is tempting to speculate that the location of the modification within a low-complexity sequence region suggests that it may participate in liquid phase separation. While the mechanisms by which these PTMs regulate P-body formation remain to be fully elucidated, it is reasonable to speculate that PTMs are likely to affect the protein–protein and protein–RNA interaction network that is required for P-body assembly and LLPS.
New Insights into mRNA Decay in Cells and in Liquid Droplets
Models of P-body function have been difficult to differentiate due to the challenge of imaging RNA decay in cells in real time as well as the challenge of purifying or reconstituting P-bodies in vitro, but significant advances have now been made in the detection of mRNA decay inside living cells, as well as analysis of mRNA within P-bodies. Very recently, a single-molecule reporter of 5′-to-3′ mRNA decay has been described, in which 5′ and 3′ end binding of two different fluorescent proteins is directed by orthogonal stem loops, while an Xrn1 endonuclease-resistant motif is included between these sites.83 Therefore, intact mRNAs emit two colors, but stable 3′ ends that report on 5′-to-3′ decay emit only one color. Imaging of these reporters in living human cells revealed mRNA decay products throughout the cytoplasm with no accumulation inside P-bodies. Even more recently, an mRNA single-molecule imaging system based on the MS2 stem loop and MS2-binding coat protein was re-engineered to allow labeled mRNA to decay with kinetics similar to the endogenous, unlabeled transcript.84 This system revealed mRNA decay events in real time that also occurred throughout the yeast cytoplasm rather than in cytoplasmic granules. Along the same lines, in fluorescence-sorted P-bodies, while a greater variance in polyA tail length relative to total cellular mRNA was observed, no accumulation of 5′-end degraded transcripts was detected, suggesting that most P-body-resident mRNA is intact.48 Finally, in vitro reconstitution of an LLPS system including the decapping enzyme Dcp2, which catalyzes the first step in 5′-to-3′ mRNA decay, dramatically decreased the activity of the enzyme; a similar effect was observed on the activity of RNase A, which is not normally present in P-bodies, consistent with general inhibition of enzymatic activity within liquid droplets, though the mechanism of this inhibition was not delineated.13 While it remains impossible to completely exclude either model of P-body function, accumulating evidence is most consistent with P-body as a storage granule containing translationally repressed mRNAs and inactive decapping enzymes, with catalysis of mRNA decay occurring throughout the cytoplasm.
Outlook
P-bodies are unique among RNP granules in their composition, which includes translationally repressed mRNA and protein factors associated with mRNA decay. However, since these factors partition between P-bodies and the cytoplasm, it has remained unresolved whether mRNA decay occurs inside P-bodies or in the cytoplasm, although they are clearly not required for mRNA decay.22 Increasing evidence for regulation of P-body assembly in cells by chaperones, protein PTMs, and energy-dependent remodeling supports an important cellular role for these structures but does not differentiate functional hypotheses. Supporting an association of P-bodies with mRNA decay, a majority of previously known and recently discovered P-body-associated proteins are involved in mRNA degradation. In contrast, the lack of localization of mRNA decay events to P-bodies in live cell imaging studies, the failure to observe 5′-end-truncated mRNA in biochemically purified P-bodies, and the apparent inhibition of Dcp2 and RNase A in liquid droplets in vitro are inconsistent with mRNA decay occurring within these RNP granules.
Several key questions remain to be addressed in order to fully support either model of P-body function. If P-bodies are mRNA storage granules, the activities of mRNA decapping and decay enzymes, which are plentiful and associated with RNA in these RNP granules, must be inhibited such that a majority of transcripts are protected from decay. The mechanism by which this inhibition occurs would need to be established. By extension, this model would predict that mRNA decay would be activated in the cytoplasm, so the mechanism by which enzymatic activity is enhanced outside the P-body must also be established. It is reasonable to expect that further biochemical characterization of mRNA decay inside and outside liquid droplets could shed light on these questions. It is also possible that, due to the liability and rapid exchange of P-body factors, additional P-body-resident proteins may not yet have been successfully purified and could remain to be discovered. Continuing efforts to elucidate the full P-body composition could potentially improve our understanding of their biochemical activity or inactivity. Finally, it is possible that P-bodies fulfill some other cellular role unrelated to mRNA decay, though the molecular mechanism by which mRNA is protected from enzymatic degradation within the P-body would remain of interest in this case.
Glossary
List of Abbreviations
- FRAP
fluorescence recovery after photobleaching
- HLM
helical leucine-rich repeats
- IDR
intrinsically disordered regions
- LCD
low-complexity domains
- LLPS
liquid–liquid phase separation
- P-bodies
bodies processing
- PTM
post-translational protein modifications
- RNP
granules ribonucleoprotein granules
- mRNP
messenger ribonucleoprotein
- RRM
RNA recognition motifs
- SLiMs
short linear motifs
Author Contributions
† Y.L. and Z.N. contributed equally.
This work was supported by the Searle Scholars Program, the NIH (R01GM122984), and Yale University West Campus start-up funds (to S.A.S.). Y.L. was supported in part by a Yale Chemistry Department Dox Fellowship.
The authors declare no competing financial interest.
References
- Ayache J.; Benard M.; Ernoult-Lange M.; Minshall N.; Standart N.; Kress M.; Weil D. (2015) P-body assembly requires DDX6 repression complexes rather than decay or Ataxin2/2L complexes. Mol. Biol. Cell 26, 2579–2595. 10.1091/mbc.E15-03-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao B. S.; Parker R. (2017) Numerous interactions act redundantly to assemble a tunable size of P bodies in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 114, E9569–E9578. 10.1073/pnas.1712396114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajyaguru P.; She M.; Parker R. (2012) Scd6 targets eIF4G to repress translation: RGG motif proteins as a class of eIF4G-binding proteins. Mol. Cell 45, 244–254. 10.1016/j.molcel.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F.; Li C.; Roy B.; Jacobson A. (2014) Yeast Edc3 targets RPS28B mRNA for decapping by binding to a 3′ untranslated region decay-inducing regulatory element. Mol. Cell. Biol. 34, 1438–1451. 10.1128/MCB.01584-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Meng X.; Li D.; Chen S.; Luo J.; Zhu L.; Singer R. H.; Gu W. (2017) Binding of DEAD-box helicase Dhh1 to the 5′-untranslated region of ASH1 mRNA represses localized translation of ASH1 in yeast cells. J. Biol. Chem. 292, 9787–9800. 10.1074/jbc.M117.776492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgur S.; Basquin J.; Kamenska A.; Filipowicz W.; Standart N.; Conti E. (2015) Structure of a Human 4E-T/DDX6/CNOT1 Complex Reveals the Different Interplay of DDX6-Binding Proteins with the CCR4-NOT Complex. Cell Rep. 13, 703–711. 10.1016/j.celrep.2015.09.033. [DOI] [PubMed] [Google Scholar]
- Chowdhury A.; Kalurupalle S.; Tharun S. (2014) Pat1 contributes to the RNA binding activity of the Lsm1–7-Pat1 complex. RNA 20, 1465–1475. 10.1261/rna.045252.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D.; Decker C. J.; Parker R. (2003) The enhancer of decapping proteins, Edc1p and Edc2p, bind RNA and stimulate the activity of the decapping enzyme. RNA 9, 239–251. 10.1261/rna.2171203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J. E.; Truffault V.; Boland A.; Huntzinger E.; Chang C. T.; Haas G.; Weichenrieder O.; Coles M.; Izaurralde E. (2012) A direct interaction between DCP1 and XRN1 couples mRNA decapping to 5′ exonucleolytic degradation. Nat. Struct. Mol. Biol. 19, 1324–1331. 10.1038/nsmb.2413. [DOI] [PubMed] [Google Scholar]
- Ozgur S.; Chekulaeva M.; Stoecklin G. (2010) Human Pat1b connects deadenylation with mRNA decapping and controls the assembly of processing bodies. Mol. Cell. Biol. 30, 4308–4323. 10.1128/MCB.00429-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm S. A.; Kamenz J.; Noldeke E. R.; Neu A.; Zocher G.; Sprangers R. (2014) In vitro reconstitution of a cellular phase-transition process that involves the mRNA decapping machinery. Angew. Chem., Int. Ed. 53, 7354–7359. 10.1002/anie.201402885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani S. F.; Lee H. O.; Hyman A. A.; Rosen M. K. (2017) Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298. 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz S.; Noldeke E. R.; Sprangers R. (2017) A synergistic network of interactions promotes the formation of in vitro processing bodies and protects mRNA against decapping. Nucleic Acids Res. 45, 6911–6922. 10.1093/nar/gkx353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumiller W. M. Jr.; Keating C. D. (2016) Phosphorylation-mediated RNA/peptide complex coacervation as a model for intracellular liquid organelles. Nat. Chem. 8, 129–137. 10.1038/nchem.2414. [DOI] [PubMed] [Google Scholar]
- Stoecklin G.; Kedersha N. (2013) Relationship of GW/P-bodies with stress granules. Adv. Exp. Med. Biol. 768, 197–211. 10.1007/978-1-4614-5107-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G.; Blau H. (2005) Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat. Cell Biol. 7, 633–U628. 10.1038/ncb1265. [DOI] [PubMed] [Google Scholar]
- Jakymiw A.; Lian S.; Eystathioy T.; Li S.; Satoh M.; Hamel J. C.; Fritzler M. J.; Chan E. K. (2005) Disruption of GW bodies impairs mammalian RNA interference. Nat. Cell Biol. 7, 1267–1274. 10.1038/ncb1334. [DOI] [PubMed] [Google Scholar]
- Ding L.; Spencer A.; Morita K.; Han M. (2005) The developmental timing regulator AIN-1 interacts with miRISCs and may target the argonaute protein ALG-1 to cytoplasmic P bodies in C. elegans. Mol. Cell 19, 437–447. 10.1016/j.molcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Rehwinkel J.; Behm-Ansmant I.; Gatfield D.; Izaurralde E. (2005) A crucial role for GW182 and the DCP1:DCP2 decapping complex in miRNA-mediated gene silencing. RNA 11, 1640–1647. 10.1261/rna.2191905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Rivas F. V.; Wohlschlegel J.; Yates J. R.; Parker R.; Hannon G. J. (2005) A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 7, 1261–1266. 10.1038/ncb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbings D. J.; Ciaudo C.; Erhardt M.; Voinnet O. (2009) Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat. Cell Biol. 11, 1143–1149. 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- Eulalio A.; Behm-Ansmant I.; Schweizer D.; Izaurralde E. (2007) P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol. Cell. Biol. 27, 3970–3981. 10.1128/MCB.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker C. J.; Teixeira D.; Parker R. (2007) Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae. J. Cell Biol. 179, 437–449. 10.1083/jcb.200704147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brengues M.; Teixeira D.; Parker R. (2005) Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science 310, 486–489. 10.1126/science.1115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch S.; Nissan T. (2017) An mRNA decapping mutant deficient in P body assembly limits mRNA stabilization in response to osmotic stress. Sci. Rep. 7, 44395. 10.1038/srep44395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks T. M.; Lykke-Andersen J. (2008) The control of mRNA decapping and P-body formation. Mol. Cell 32, 605–615. 10.1016/j.molcel.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R.; Sheth U. (2007) P bodies and the control of mRNA translation and degradation. Mol. Cell 25, 635–646. 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Pavani S. R.; Thompson M. A.; Biteen J. S.; Lord S. J.; Liu N.; Twieg R. J.; Piestun R.; Moerner W. E. (2009) Three-dimensional, single-molecule fluorescence imaging beyond the diffraction limit by using a double-helix point spread function. Proc. Natl. Acad. Sci. U. S. A. 106, 2995–2999. 10.1073/pnas.0900245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A. A.; Weber C. A.; Julicher F. (2014) Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 30, 39–58. 10.1146/annurev-cellbio-100913-013325. [DOI] [PubMed] [Google Scholar]
- Uversky V. N. (2017) Intrinsically disordered proteins in overcrowded milieu: Membrane-less organelles, phase separation, and intrinsic disorder. Curr. Opin. Struct. Biol. 44, 18–30. 10.1016/j.sbi.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Boeynaems S., Bogaert E., Kovacs D., Konijnenberg A., Timmerman E., Volkov A., Guharoy M., De Decker M., Jaspers T., Ryan V. H., Janke A. M., Baatsen P., Vercruysse T., Kolaitis R. M., Daelemans D., Taylor J. P., Kedersha N., Anderson P., Impens F., Sobott F., Schymkowitz J., Rousseau F., Fawzi N. L., Robberecht W., Van Damme P., Tompa P., and Van Den Bosch L. (2017) Phase Separation of C9orf72 Dipeptide Repeats Perturbs Stress Granule Dynamics, Mol. Cell 65, 1044–1055, e1045. 10.1016/j.molcel.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M. T.; Elbaum-Garfinkle S.; Holehouse A. S.; Chen C. C.; Feric M.; Arnold C. B.; Priestley R. D.; Pappu R. V.; Brangwynne C. P. (2017) Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nat. Chem. 9, 1118–1125. 10.1038/nchem.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliex A.; Temirov J.; Lee J.; Coughlin M.; Kanagaraj A. P.; Kim H. J.; Mittag T.; Taylor J. P. (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133. 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.; Protter D. S.; Rosen M. K.; Parker R. (2015) Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol. Cell 60, 208–219. 10.1016/j.molcel.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S.; Parker R. (2016) Hypo- and Hyper-Assembly Diseases of RNA-Protein Complexes. Trends Mol. Med. 22, 615–628. 10.1016/j.molmed.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protter D. S.; Parker R. (2016) Principles and Properties of Stress Granules. Trends Cell Biol. 26, 668–679. 10.1016/j.tcb.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabretta S.; Richard S. (2015) Emerging Roles of Disordered Sequences in RNA-Binding Proteins. Trends Biochem. Sci. 40, 662–672. 10.1016/j.tibs.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Riback J. A., Katanski C. D., Kear-Scott J. L., Pilipenko E. V., Rojek A. E., Sosnick T. R., and Drummond D. A. (2017) Stress-Triggered Phase Separation Is an Adaptive, Evolutionarily Tuned Response, Cell 168, 1028–1040, e1019. 10.1016/j.cell.2017.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott T. J.; Petsalaki E.; Farber P.; Jervis D.; Fussner E.; Plochowietz A.; Craggs T. D.; Bazett-Jones D. P.; Pawson T.; Forman-Kay J. D.; Baldwin A. J. (2015) Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell 57, 936–947. 10.1016/j.molcel.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas S.; Izaurralde E. (2013) The role of disordered protein regions in the assembly of decapping complexes and RNP granules. Genes Dev. 27, 2628–2641. 10.1101/gad.227843.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker C. J.; Parker R. (2012) P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harbor Perspect. Biol. 4, a012286. 10.1101/cshperspect.a012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraiuolo M. A.; Basak S.; Dostie J.; Murray E. L.; Schoenberg D. R.; Sonenberg N. (2005) A role for the eIF4E-binding protein 4E-T in P-body formation and mRNA decay. J. Cell Biol. 170, 913–924. 10.1083/jcb.200504039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenska A.; Simpson C.; Vindry C.; Broomhead H.; Benard M.; Ernoult-Lange M.; Lee B. P.; Harries L. W.; Weil D.; Standart N. (2016) The DDX6–4E-T interaction mediates translational repression and P-body assembly. Nucleic Acids Res. 44, 6318–6334. 10.1093/nar/gkw565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczynska A.; Aigueperse C.; Kress M.; Dautry F.; Weil D. (2005) The translational regulator CPEB1 provides a link between dcp1 bodies and stress granules. J. Cell Sci. 118, 981–992. 10.1242/jcs.01692. [DOI] [PubMed] [Google Scholar]
- Bish R.; Cuevas-Polo N.; Cheng Z.; Hambardzumyan D.; Munschauer M.; Landthaler M.; Vogel C. (2015) Comprehensive Protein Interactome Analysis of a Key RNA Helicase: Detection of Novel Stress Granule Proteins. Biomolecules 5, 1441–1466. 10.3390/biom5031441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary G. A.; Vinh D. B.; May P.; Kuestner R.; Dudley A. M. (2015) Proteomic Analysis of Dhh1 Complexes Reveals a Role for Hsp40 Chaperone Ydj1 in Yeast P-Body Assembly. G3: Genes, Genomes, Genet. 5, 2497–2511. 10.1534/g3.115.021444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R. W.; Muhlrad D.; Garcia J.; Parker R. (2015) Differential effects of Ydj1 and Sis1 on Hsp70-mediated clearance of stress granules in Saccharomyces cerevisiae. RNA 21, 1660–1671. 10.1261/rna.053116.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubstenberger A., Courel M., Benard M., Souquere S., Ernoult-Lange M., Chouaib R., Yi Z., Morlot J. B., Munier A., Fradet M., Daunesse M., Bertrand E., Pierron G., Mozziconacci J., Kress M., and Weil D. (2017) P-Body Purification Reveals the Condensation of Repressed mRNA Regulons, Mol. Cell 68, 144–157, e145. 10.1016/j.molcel.2017.09.003 [DOI] [PubMed] [Google Scholar]
- Aizer A.; Brody Y.; Ler L. W.; Sonenberg N.; Singer R. H.; Shav-Tal Y. (2008) The dynamics of mammalian P body transport, assembly, and disassembly in vivo. Mol. Biol. Cell 19, 4154–4166. 10.1091/mbc.E08-05-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Lima N. G.; Ma J.; Winkler L.; Chu Q.; Loh K. H.; Corpuz E. O.; Budnik B. A.; Lykke-Andersen J.; Saghatelian A.; Slavoff S. A. (2017) A human microprotein that interacts with the mRNA decapping complex. Nat. Chem. Biol. 13, 174–180. 10.1038/nchembio.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenger-Gron M.; Fillman C.; Norrild B.; Lykke-Andersen J. (2005) Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping. Mol. Cell 20, 905–915. 10.1016/j.molcel.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Peterson F. C.; Volkman B. F. (2009) Diversity of polyproline recognition by EVH1 domains. Front. Biosci., Landmark Ed. 14, 833–846. 10.2741/3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Lu Z.; Gomez A.; Hon G. C.; Yue Y.; Han D.; Fu Y.; Parisien M.; Dai Q.; Jia G.; Ren B.; Pan T.; He C. (2014) N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120. 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y. H.; Wong C. C.; Li K. W.; Chan K. M.; Boukamp P.; Liu W. K. (2014) CCHCR1 interacts with EDC4, suggesting its localization in P-bodies. Exp. Cell Res. 327, 12–23. 10.1016/j.yexcr.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Tenekeci U.; Poppe M.; Beuerlein K.; Buro C.; Muller H.; Weiser H.; Kettner-Buhrow D.; Porada K.; Newel D.; Xu M.; Chen Z. J.; Busch J.; Schmitz M. L.; Kracht M. (2016) K63-Ubiquitylation and TRAF6 Pathways Regulate Mammalian P-Body Formation and mRNA Decapping. Mol. Cell 63, 540. 10.1016/j.molcel.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Shapouri F.; Saeidi S.; de Iongh R. U.; Casagranda F.; Western P. S.; McLaughlin E. A.; Sutherland J. M.; Hime G. R.; Familari M. (2016) Tob1 is expressed in developing and adult gonads and is associated with the P-body marker, Dcp2. Cell Tissue Res. 364, 443–451. 10.1007/s00441-015-2328-z. [DOI] [PubMed] [Google Scholar]
- Du H.; Zhao Y.; He J. Q.; Zhang Y.; Xi H. R.; Liu M. F.; Ma J. B.; Wu L. G. (2016) YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 7, 12626. 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei M. A.; Ingelfinger D.; Heintzmann R.; Achsel T.; Rivera-Pomar R.; Luhrmann R. (2005) A role for eIF4E and eIF4E-transporter in targeting mRNPs to mammalian processing bodies. RNA 11, 717–727. 10.1261/rna.2340405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N.; Stoecklin G.; Ayodele M.; Yacono P.; Lykke-Andersen J.; Fritzler M. J.; Scheuner D.; Kaufman R. J.; Golan D. E.; Anderson P. (2005) Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 169, 871–884. 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschwald S.; Maharana S.; Mateju D.; Malinovska L.; Nuske E.; Poser I.; Richter D.; Alberti S. (2015) Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. eLife 4, e06807 10.7554/eLife.06807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M.; Han T. W.; Xie S.; Shi K.; Du X.; Wu L. C.; Mirzaei H.; Goldsmith E. J.; Longgood J.; Pei J.; Grishin N. V.; Frantz D. E.; Schneider J. W.; Chen S.; Li L.; Sawaya M. R.; Eisenberg D.; Tycko R.; McKnight S. L. (2012) Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767. 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani S. F.; Rice A. M.; Peeples W. B.; Lin Y.; Jain S.; Parker R.; Rosen M. K. (2016) Compositional Control of Phase-Separated Cellular Bodies. Cell 166, 651–663. 10.1016/j.cell.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y.; Brangwynne C. P. (2017) Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 10.1126/science.aaf4382. [DOI] [PubMed] [Google Scholar]
- Wang C. Y.; Chen W. L.; Wang S. W. (2013) Pdc1 functions in the assembly of P bodies in Schizosaccharomyces pombe. Mol. Cell. Biol. 33, 1244–1253. 10.1128/MCB.01583-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker C.; Teixeira D.; Parker R. (2007) Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae. J. Cell Biol. 179, 437–449. 10.1083/jcb.200704147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijns M. A.; Alexander R. D.; Spiller M. P.; Beggs J. D. (2008) A role for Q/N-rich aggregation-prone regions in P-body localization. J. Cell Sci. 121, 2463–2472. 10.1242/jcs.024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribas-Layton M.; Dennis J.; Bennett E. J.; Damgaard C. K.; Lykke-Andersen J. (2016) The C-Terminal RGG Domain of Human Lsm4 Promotes Processing Body Formation Stimulated by Arginine Dimethylation. Mol. Cell. Biol. 36, 2226–2235. 10.1128/MCB.01102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serman A.; Le Roy F.; Aigueperse C.; Kress M.; Dautry F.; Weil D. (2007) GW body disassembly triggered by siRNAs independently of their silencing activity. Nucleic Acids Res. 35, 4715–4727. 10.1093/nar/gkm491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnef A.; Maldonado M.; Bugaut A.; Balasubramanian S.; Kress M.; Weil D.; Standart N. (2010) Distinct functions of maternal and somatic Pat1 protein paralogs. RNA 16, 2094–2107. 10.1261/rna.2295410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U.; Parker R. (2003) Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 300, 805–808. 10.1126/science.1082320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira D.; Sheth U.; Valencia-Sanchez M. A.; Brengues M.; Parker R. (2005) Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA 11, 371–382. 10.1261/rna.7258505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner J.; Wang C.; Prescianotto-Baschong C.; Estrada A. F.; Spang A. (2014) The polysome-associated proteins Scp160 and Bfr1 prevent P body formation under normal growth conditions. J. Cell Sci. 127, 1992–2004. 10.1242/jcs.142083. [DOI] [PubMed] [Google Scholar]
- Teixeira D.; Parker R. (2007) Analysis of P-body assembly in Saccharomyces cerevisiae. Mol. Biol. Cell 18, 2274–2287. 10.1091/mbc.E07-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.; Jakymiw A.; Wood M. R.; Eystathioy T.; Rubin R. L.; Fritzler M. J.; Chan E. K. (2004) GW182 is critical for the stability of GW bodies expressed during the cell cycle and cell proliferation. J. Cell Sci. 117, 5567–5578. 10.1242/jcs.01477. [DOI] [PubMed] [Google Scholar]
- Kamenska A.; Simpson C.; Vindry C.; Broomhead H.; Benard M.; Ernoult-Lange M.; Lee B. P.; Harries L. W.; Weil D.; Standart N. (2016) The DDX6–4E-T interaction mediates translational repression and P-body assembly. Nucleic Acids Res. 44, 6318–6334. 10.1093/nar/gkw565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.; Myong S. (2016) RNA Remodeling Activity of DEAD Box Proteins Tuned by Protein Concentration, RNA Length, and ATP. Mol. Cell 63, 865–876. 10.1016/j.molcel.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugler C. F.; Hondele M.; Heinrich S.; Sachdev R.; Vallotton P.; Koek A. Y.; Chan L. Y.; Weis K. (2016) ATPase activity of the DEAD-box protein Dhh1 controls processing body formation. eLife 5, e18746 10.7554/eLife.18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. H.; Choi E. J.; Parker R. (2010) Dcp2 phosphorylation by Ste20 modulates stress granule assembly and mRNA decay in Saccharomyces cerevisiae. J. Cell Biol. 189, 813–827. 10.1083/jcb.200912019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang P. Y.; Shen Y. F.; Su Y. L.; Kao C. H.; Lin N. Y.; Hsu P. H.; Tsai M. D.; Wang S. C.; Chang G. D.; Lee S. C.; Chang C. J. (2013) Phosphorylation of mRNA decapping protein Dcp1a by the ERK signaling pathway during early differentiation of 3T3-L1 preadipocytes. PLoS One 8, e61697 10.1371/journal.pone.0061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizer A.; Kafri P.; Kalo A.; Shav-Tal Y. (2013) The P body protein Dcp1a is hyper-phosphorylated during mitosis. PLoS One 8, e49783 10.1371/journal.pone.0049783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenekeci U.; Poppe M.; Beuerlein K.; Buro C.; Muller H.; Weiser H.; Kettner-Buhrow D.; Porada K.; Newel D.; Xu M.; Chen Z. J.; Busch J.; Schmitz M. L.; Kracht M. (2016) K63-Ubiquitylation and TRAF6 Pathways Regulate Mammalian P-Body Formation and mRNA Decapping. Mol. Cell 62, 943–957. 10.1016/j.molcel.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Brahms H.; Meheus L.; de Brabandere V.; Fischer U.; Luhrmann R. (2001) Symmetrical dimethylation of arginine residues in spliceosomal Sm protein B/B’ and the Sm-like protein LSm4, and their interaction with the SMN protein. RNA 7, 1531–1542. 10.1017/S135583820101442X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvathova I.; Voigt F.; Kotrys A. V.; Zhan Y.; Artus-Revel C. G.; Eglinger J.; Stadler M. B.; Giorgetti L.; Chao J. A. (2017) The Dynamics of mRNA Turnover Revealed by Single-Molecule Imaging in Single Cells. Mol. Cell 68, 615–625. 10.1016/j.molcel.2017.09.030. [DOI] [PubMed] [Google Scholar]
- Tutucci E.; Vera M.; Biswas J.; Garcia J.; Parker R.; Singer R. H. (2018) An improved MS2 system for accurate reporting of the mRNA life cycle. Nat. Methods 15, 81–89. 10.1038/nmeth.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]