Abstract
It is well established that repeated social defeat stress can induce negative long-term consequences such as increased anxiety-like behavior and enhances the reinforcing effect of psychostimulants in rodents. In the current study, we evaluated how the immune system may play a role in these long-term effects of stress. A total of 148 OF1 mice were divided into different experimental groups according to stress condition (exploration or social defeat) and pre-treatment (saline, 5 or 10 mg/kg of the anti-inflammatory indomethacin) before each social defeat or exploration episode. Three weeks after the last social defeat, anxiety was evaluated using an elevated plus maze paradigm. After this test, conditioned place preference (CPP) was induced by a subthreshold dose of cocaine (1 mg/kg). Biological samples were taken four hours after the first and the fourth social defeat, 3 weeks after the last defeat episode, and after the CPP procedure. Plasma and brain tissue (prefrontal cortex, striatum and hippocampus) were used to determine the levels of the pro-inflammatory cytokine interleukin 6 (IL-6). Results showed an increase of peripheral and brain IL-6 levels after the first and fourth social defeat that was reverted three weeks later. Intraperitoneal administration of the anti-inflammatory drug indomethacin before each episode of stress prevented this enhancement of IL-6 levels and also reversed the increase in the rewarding effects of cocaine in defeated mice. Conversely, this protective effect was not observed with respect to the anxiogenic consequences of social stress. Our results confirm the hypothesis of a modulatory proinflammatory contribution to stress-induced vulnerability to drug abuse disorders and highlight anti-inflammatory interventions as a potential therapeutic tool to treat stress-related addiction disorders.
Introduction
Scientific evidence suggests that alterations of inflammatory parameters are linked to a vulnerability to mental illnesses [1], including depression [2, 3, 4], bipolar disorder [5, 6, 7], schizophrenia [8, 9, 10] and autism [11]. In addition, substance use disorders (SUD) are assumed to be related to changes in the immune system activity [12, 13]. Addiction can be considered a multifactorial mental disorder caused by an interaction between biological and environmental factors [14, 15, 16]. Although the exact mechanisms of the genesis of addiction have not been completely unraveled, a growing body of evidence relates alterations of the immune response to a possible cause of vulnerability to SUD [17, 18]. Neuroinflammation could help to explain some of the effects of drugs, including toxicity, deleterious cognitive effects [17, 19, 20, 21] and reward modulation, particularly taking into account that the immune signal significantly modulates the mesolimbic dopamine system [18, 22].
Both clinical and preclinical studies have shown that psychostimulants such as cocaine or methamphetamine activate the central and peripheral components of the innate immune system [12, 23, 24, 25, 26]. Repeated consumption of psychostimulants promotes a neuroinflammatory pattern characterized by an enhanced activation of glial and microglial cells [12] and increased release of glial cytokines with potential neurotoxic effects [27]. Moreover, plasmatic cytokines levels are under consideration by some researchers as possible biomarkers for cocaine users [24, 28].
Another cornerstone of our understanding of drug addiction is stress, with emotional stressors representing the main source of stress in humans [29]. Life-threatening situations induce a physiological response to stress which is adaptive and crucial for survival, and a failure to end that response may induce deleterious effects [30, 31]. In pre-clinical research, social defeat in an agonistic encounter is a rodent model with ecological validity that mimics real-life situations of social stress [32, 33, 34]. It has repeatedly been reported that different models of social defeat stress enhance the unconditioned and conditioned rewarding response to psychostimulant drugs and precipitate the reinstatement of drug seeking in the self-administration and conditioned place preference (CPP) paradigms [29, 34, 35, 36]. Alterations in the corticotrophin-releasing factor (CRF) neurotransmission system [35, 37, 38], epigenetic forms of plasticity [39] or inflammatory processes appear to be central to stress-induced vulnerability [4, 40, 41].
Recent basic research shows that stress induces activation of the immune system, thereby promoting the stimulation of microglia and leukocytes, altering the levels of peripheral and brain cytokines and leading to monocytes trafficking into the brain [40, 42]. Moreover, pro-inflammatory markers alter the permeability of the blood brain barrier (BBB) [43], which is also affected by social stress procedures [44]. When the BBB is compromised, peripheral immune cells can penetrate the central nervous system (CNS), thus causing or enhancing existing neuro-inflammation [45]. In this way, inflammatory processes are being posited as a link between stress and disease, by altering behavioral and neuroendocrine functions and leading to the vulnerability and enhanced sensibility to drugs that is reported after social stress procedures.
In response to this link between immune response, vulnerability to addiction and stress, some researchers have employed anti-inflammatory agents such as non-steroidal anti-inflammatory drugs (NSAIDs) as a therapeutic approach. For instance, the inflammatory potential of ethanol is well established [18, 20, 21, 46, 47]; it produces an inflammatory response via activation of microglia and astrocytes, contributing to neurodegeneration and the deleterious effects reported in alcoholics [48, 49] and in animal studies [20, 21, 50]. Pascual and coworkers [46] found that administration of indomethacin prior to ethanol binge-drinking prevented ethanol-induced brain damage in adolescent rats, as it blocked neural cell death and also attenuated short and long-term detrimental effects on cognitive and motor processes. Other researchers found that anti-inflammatory treatments also reverse cognitive impairments induced by social stress in animal models [42, 51].
Based on the above-mentioned observations, we hypothesize that the long-term sensitization to the rewarding properties of cocaine, constantly reported in experimental animals after social defeat stress, is mediated by a pro-inflammatory state. Likewise, we hypothesize that this inflammatory mechanism is also underlying the long-term anxiety-like behavior displayed by social defeated animals. In order to test these hypotheses, we first determined whether there is an increase in peripheral and brain inflammatory response after social stress by measuring plasmatic and brain levels of interleukin 6 (IL-6). We then determined if anti-inflammatory treatment with indomethacin before each stress episode blocks the inflammatory response induced by social stress, and therefore the enhanced cocaine response and anxiety-like behavior expected after social stress experiences.
Material and methods
Animals
A total number of 148 OF1 adult mice (Charles River, France) were used in this study. The animals were housed as previously described in detail [35] in groups of four in plastic cages (27 × 27 × 14 cm) during the entire experimental procedure. To reduce their stress levels in response to experimental manipulations, mice were handled for 5 minutes per day on each of the 3 days prior to initiation of social defeat experiences. Aggressive opponents were individually housed in plastic cages (21 × 32 × 20 cm) for a month prior to initiation of the experiments in order to heighten aggression [52]. All mice were housed under the following conditions: constant temperature; a reversed light schedule (white light on 8:00–20:00 hours); and food and water available ad libitum, except during behavioral tests. The experimental protocol has been approved by an Institutional Review Committee for the use of animal subjects (Comité d'Ètica d’Experimentació i Benestar Animal, number A1426847710979). Procedures involving mice and their care were conducted according to national, regional and local laws and regulations, which are in compliance with the Directive 2010/63/EU. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Drugs
The anti-inflammatory indomethacin (Sigma-Aldrich, Spain) was dissolved in 5% DMSO (dimethyl sulfoxide) and injected intraperitoneally (i.p.) at a dose of 5 or 10 mg/kg 30 minutes before each social defeat. For CPP, a dose of 1 mg/kg of cocaine hydrochloride (Alcaliber laboratory, Spain) was employed. This dose of cocaine was selected on the basis of previous CPP studies showing 1 mg/kg to be a sub-threshold dose [53, 54, 55]. All the treatments were adjusted to a volume of 0.01 ml/g of weight. Control groups were injected with physiological saline (NaCl 0.9%), which was also used to dissolve the drugs.
Experimental design
The experimental design is depicted in Table 1. We studied whether social stress could induce an inflammatory response and if the anti-inflammatory indomethacin could modulate the effects of social defeat on the conditioned rewarding effects of cocaine (1 mg/kg). Mice received physiological saline, or a 5 or 10 mg/kg dose of indomethacin prior to each social defeat (RSD) (RSD-SAL, RSD-INDO5, RSD-INDO10) or exploration (EXP) (EXP-SAL, EXP-INDO10). Subsequently, 19 days after the last social defeat, anxiety was evaluated in the elevated plus maze (EPM) test. One day afterwards, the CPP procedure was initiated.
Table 1. Experimental design.
| RSD / Exploration | |||||||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | ||||
| 1st set of mice | SAL/ INDO5 / INDO 10 | SAL/ INDO5 / INDO 10 | SAL/ INDO5 / INDO 10 | SAL/ INDO5 / INDO 10 | EPM | 1 mg/kg cocaine CPP | Brain extraction and Blood samples |
| 2nd set of mice | Brain extraction and Blood samples | Brain extraction and Blood samples | Brain extraction and Blood samples | ||||
| Experimental day | 1 | 4 | 7 | 10 | 29 | 31–40 | 60–70 |
Biological samples were taken four hours after the first and fourth social defeats, 3 weeks after the last social defeat, and following the CPP procedure. In the case of the control group (EXP), samples were also taken four hours after the first exploration session and after the CPP procedure.
Apparatus and procedures
Procedure of social defeat
The social defeat protocol performed in this study was validated and described in detail in previous research papers from our research group [35, 36, 44]. Each of the social defeat episodes consisted of three phases, each of which began by introducing the “intruder” (the experimental animal) into the home cage of the “resident” (the aggressive opponent) for 10 minutes [56]. During this initial phase, the intruder was protected from attack, but the wire mesh walls of the cage allowed for social interactions and species-typical threats from the male aggressive resident, thus leading to instigation and provocation [57]. The wire mesh was then removed from the cage to allow confrontation between the two animals for a 5-minute period. In the third phase, the wire mesh was returned to the cage to separate the two animals once again for another 10 minutes to allow for social threats by the resident. Intruder mice were exposed to a different aggressor during each episode of social defeat. The criterion used to define an animal as defeated was the adoption of a specific posture signifying defeat, characterized by an upright submissive position, limp forepaws, upwardly angled head, and retracted ears [52]. In order to minimize the physical wounding during social defeats, the 5-minute direct encounters were finished earlier in the intruder displayed submissive supine posture for more than 8 seconds or if it was bitten by the aggressor more than 12 times. All agonistic encounters were videotaped to confirm social defeat.
Conditioned place preference-CPP
Place conditioning consisted of three phases and took place during the dark cycle [58], following an unbiased procedure in initial spontaneous preference terms as previously described in detail [35, 59]. For place conditioning, twelve identical Plexiglas boxes with black and white equal-sized compartments (30.7 × 31.5 × 34.5 cm) separated by a gray central area (13.8 × 31.5 × 34.5 cm) were used. The CPP protocol consists of three phases, the first one being the pre-conditioning (Pre-C). In Pre-C phase, mice were given access to both compartments of the CPP box for 15 min (900 s) for 3 consecutive days. For the evaluation of the initial/natural preference, we use the data for the time spent by the animal in each compartment registered during the third (last) day of the pretest. Animals showing a strong, unconditioned aversion (less than 33% of the session time, i.e., 300 s) or preference (more than 67%, i.e., 600 s) for any compartment were excluded for the experiment. After this initial analysis of the natural preferences, a CPP box compartment (black or white) was chosen to be paired with the drug and the other one with the vehicle, taking into account that, in each group, half the animals received the treatment in the most preferred compartment and the other half in the least preferred one. Additionally, we statistically confirmed that there were no significant differences between the time spent in the drug-paired and the saline-paired compartments to avoid any preference bias before conditioning.
In the second phase (conditioning), animals underwent two pairings per day. First, they received an injection of physiological saline before being confined to the vehicle-paired compartment for 30 minutes. After a 4-hour interval, they received cocaine immediately before being confined to the drug-paired compartment for 30 minutes. In the third phase or post-conditioning (Post-C), the time spent by the untreated mice in each compartment during a 15-minute observation period was recorded. The difference in seconds between the time spent in the drug-paired compartment in the Post-C test and that spent in the Pre-C test is a measure of the degree of conditioning induced by the drug. If this difference is positive, then the drug is considered to have induced a preference for the drug-paired compartment, whereas the opposite indicates the induction of an aversion.
All groups in which a preference for the drug-paired compartment was established underwent an extinction session every 72 hours, which consisted of placing the mice in the apparatus for 15 minutes. This was repeated until the time spent in the drug-paired compartment by each group was similar to that of the Pre-C.
The effects of non-contingent administration of a priming dose of cocaine were evaluated 24 hours after the confirmation of extinction. Reinstatement tests were the same as those for the Post-C (free ambulation for 15 minutes), except for the fact that mice were tested 15 minutes after administration of the drug (half of the dose used for conditioning). This procedure was repeated with progressively lower priming doses until a non-effective priming injection was determined.
Elevated plus maze-EPM
The elevated plus maze (EPM) test was carried out essentially following the procedure previously described by Daza-Losada and coworkers [59]. The maze consisted of two open arms (30 × 5 × 0.25 cm) and two enclosed arms (30 × 5 × 15 cm), and the junction of the four arms formed a central platform (5 × 5 cm). The floor of the maze was made of black Plexiglas and the walls of the enclosed arms were made of clear Plexiglas. The open arms had a small edge (0.25 cm) to provide the animals with additional grip. The entire apparatus was elevated 45 cm above floor level. In order to facilitate adaptation, mice were transported to the dimly illuminated laboratory 1 hour prior to testing. At the beginning of each trial, subjects were placed on the central platform so that they were facing an open arm and were allowed to explore for 5 minutes. The maze was thoroughly cleaned with a damp cloth after each trial. The measurements recorded during the test period were number of entries and time and percentage of time spent in each section of the apparatus (open arms, closed arms, central platform). An arm was considered to have been visited when the animal placed all four paws on it. The time and percentage of time spent in the open arms and the number of open arm entries are generally used to characterize the anxiolytic effects of drugs. In addition, the number of closed and total entries indicates motor activity.
Tissue sampling
To obtain blood and tissue samples, unperfused mice were sacrificed by cervical dislocation and then decapitated. Blood was collected from the neck into a Microvette CB 300 capillary tube (Sarstedt, Germany). Blood samples were kept on ice, and plasma was separated from whole blood by centrifugation (5 minutes, 5000G) and transferred to sterile 0.2 ml microcentrifugue tubes. Plasma samples were stored at -80°C until IL-6 concentration determination.
Brains were rapidly removed and the prefrontal cortex (PFC), striatum (STR) and hippocampus (HIP) were dissected following the procedure described by Heffner and coworkers [60] and kept on dry ice until storage at -80°C. Prior to IL-6 determination, brains were homogenized and prepared following the procedure described by Alfonso-Loeches and coworkers [61]. Frozen brain cortices were homogenized in 250 mg of tissue/0.5 ml of cold lysis buffer (1% NP-40, 20 mM Tris-HCl pH 8, 130 mM NaCl, 10 mM NaF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 40 mM DTT, 1 mM Na3VO4, and 10 mM PMSF). Brain homogenates were kept on ice for 30 minutes and centrifuged at maximum speed for 15 minutes, after which the supernatant was collected and protein levels were determined by the Bradford assay from ThermoFisher (Ref: 23227).
IL-6 ELISA assay
To determine IL-6 concentration in plasma and tissues, we used a Mouse IL-6 ELISA Kit obtained from Abcam (Ref: ab100712) following the manufacturer’s instructions. To determine absorbance, we employed an iMark microplate reader (Bio-RAD) controlled by Microplate Manager 6.2 software. The optical density was read at 450nm and the final results were calculated using a standard curve following the manufacturer’s instructions, and were expressed as pg/ml for plasma, and pg/mg for tissue samples.
Statistical analyses
For the CPP data, the time spent in the drug-paired compartment during Pre-C and Post-C tests was analyzed with a mixed three-way ANOVA, with two between-subjects variables—Pre-treatment, with three levels (Saline, Indomethacin 5 or 10 mg/kg), and Stress, with two levels (RSD and EXP)—and a within-subjects variable—Days, with two levels (Pre-C and Post-C). For the EPM data, a two-way ANOVA, with two between-subjects variables—Pre-treatment, with two levels (Saline or Indomethacin 10), and Stress, with two levels (RSD and EXP)—was employed. In all cases, post-hoc comparisons were performed with Bonferroni tests. In addition, the groups showing CPP, extinction and reinstatement values were analyzed by a Student’s t-test.
Data concerning IL-6 concentration were analyzed using a single factor analysis ANOVA, with a between-subjects variable: Stress, with 4 levels (Exploration, first social defeat, fourth social defeat and 3 weeks after the last social defeat). For the IL-6 levels measure performed after the CPP procedure, we used an ANOVA with a between-subjects variable: Stress, with 3 levels (Exploration, RSD and RSD plus Indomethacin). Data are presented as mean ± SEM. A p-value < 0.05 was considered statistically significant. Analyses were performed using SPSS v22.
Results
Indomethacin blocks the increase in the conditioned rewarding effects of cocaine (1 mg/kg) induced by social defeat stress
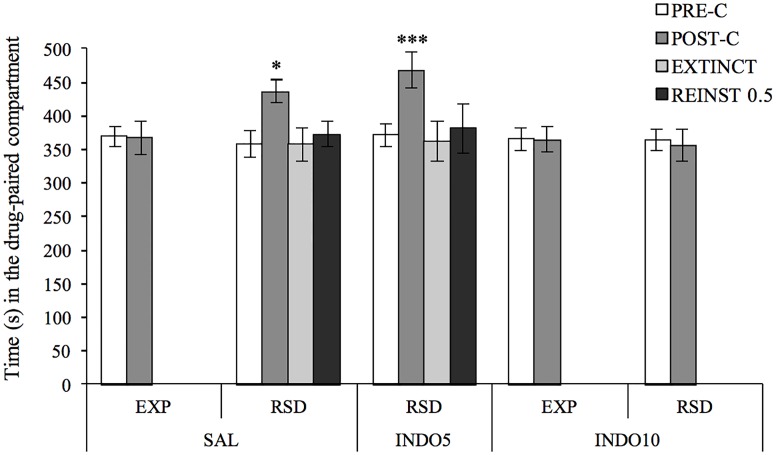
ANOVA of the CPP data (Fig 1) showed a significant effect of the variable Days F(1,68) = 10.905, p <0.01 and the interactions Days x Pre-treatment F(2,68) = 4.657, p <0.05 and Days x Pre-treatment x Stress F(1,68) = 3.862, p <0.05. As expected, socially defeated animals pretreated with saline (RSD-SAL) developed CPP, since they spent more time in the drug-paired compartment in the Post-C than in the Pre-C test (p <0.05), a preference that was not observed in defeated mice treated with the highest dose of indomethacin (RSD-INDO10), in contrast with the CPP developed in defeated mice treated with the lowest dose of indomethacin (RSD-INDO5 p <0.001). Neither of the groups that developed preference for the drug-paired compartment showed reinstatement after a priming dose of 0.5 mg/kg cocaine.
Fig 1. Administration of the highest indomethacin dose before each social defeat blocked acquisition of the CPP induced by 1 mg/kg of cocaine in defeated mice.
Before the social stress protocol animals were randomly assigned to the following groups according to the pre-treatment they received: saline (EXP-SAL n = 13; RSD-SAL n = 14); 5 (RSD-INDO5 n = 16) or 10 mg/kg (EXP-INDO10 n = 13; RSD-INDO10 n = 17) of indomethacin. Bars represent the time (s) spent in the drug-paired compartment before conditioning sessions in the PRE-C test (white bars), after conditioning sessions in the POST-C test (dark grey bars), in the last extinction (EXTINCT) session (light gray bars), and in the reinstatement (REINST 0.5) test (black bars). Data presented as mean values ± SEM *p <0.05, ***p <0.001 significant difference in the time spent in the drug-paired compartment versus PRE-C.
Indomethacin fails to prevent the long-term anxiogenic effects of social defeat
The data of the EPM test are presented in Table 2. ANOVA for the time spent in the open arms F(1,59) = 18.360, p <0.001, the percentage of time spent in the open arms F(1,59) = 15.751, p <0.001, the number of entries into the open arms F(1,59) = 4.594, p <0.05, the percentage of entries into the open arms F(1,59) = 15.793, p <0.001, the time spent in the closed arms F(1,59) = 5.267, p <0.05, the number of entries into the closed arms F(1,59) = 19.641, p <0.001 and the total number of entries F(1,59) = 19.887, p <0.001 revealed a significant effect of the variable Stress. Post-hoc analyses showed that socially defeated animals spent less time, a lower percentage of time and a lower percentage of entries into the open arms (p <0.001 in all cases), while they spent more time in the closed arms (p <0.05), made more total entries (p <0.001), and more entries into the open (p <0.05) and closed arms (p <0.001) than control non-stressed animals.
Table 2. Administration of indomethacin before each SD fails to prevent the long-lasting anxiogenic effect of stress in the EPM.
| EXP | RSD | ||||
|---|---|---|---|---|---|
| SAL | INDO10 | SAL | INDO5 | INDO10 | |
| Time OA (s) | 88 ± 9 | 96 ± 13 | 60 ± 7 * | 56 ± 12 * | 47 ± 7 * |
| % time OA | 38 ± 3 | 37 ± 5 | 25 ± 3 * | 24 ± 5 * | 20 ± 3 * |
| Open entries | 17 ± 4 | 23 ± 4 | 33 ± 6 *** | 31.3 ± 6 *** | 26 ± 4 *** |
| % entries OA | 39 ± 4 | 45 ± 2 | 29 ± 4 * | 28 ± 5 * | 26 ± 4 * |
| Time in CA (s) | 146 ± 10 | 172 ± 18 | 181 ± 9 *** | 186 ± 17 *** | 193 ± 10 *** |
| Closed entries | 28 ± 6 | 25 ± 2 | 86 ± 17 * | 89 ± 21 * | 80 ± 14 * |
| Total entries | 45 ± 9 | 48 ± 5 | 119 ± 20 * | 120 ± 24 * | 105 ± 16 * |
Data are presented as mean values ±S.E.M.
*p <0.05;
***p <0.001 significant difference with the exploration groups.
Social defeat increases brain and peripheral IL-6 levels
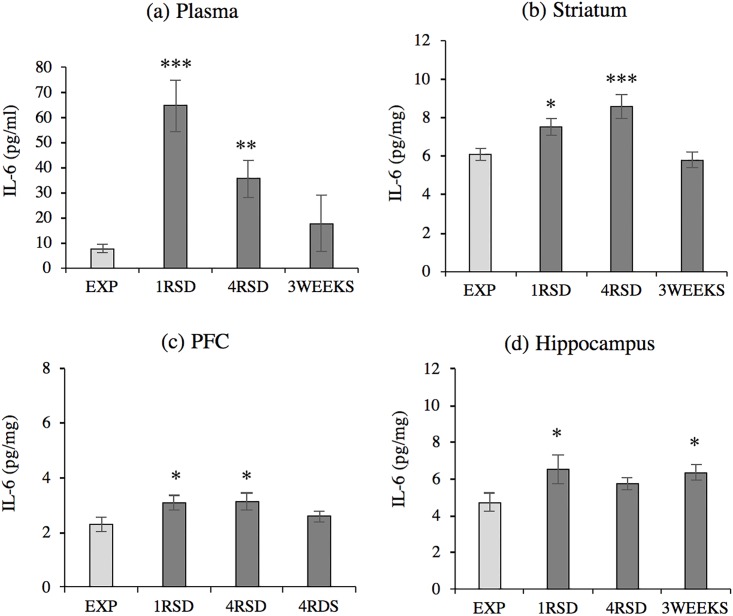
Regarding plasmatic circulating IL-6 levels (Fig 2a), the ANOVAF F(3,37) = 10.321, p <0.001 showed that levels were significantly increased in defeated mice after the first (p <0.001) and the fourth (p <0.01) social defeat compared with non-stressed animals. However, no differences were detected in plasma three weeks after the final exposure to stress.
Fig 2. Social defeat increases IL-6 levels in the plasma, STR, PFC and hippocampus.
Groups defined by stress condition and social defeat episode: (a) Plasma (EXP n = 11, 1RSD n = 11, 4RSD n = 11, 3WEEKS n = 8); (b) Striatum (EXP n = 12, 1RSD n = 12, 4RSD n = 12; 3WEEKS n = 8); (c) PFC (EXP n = 7, 1RSD n = 7, 4RSD n = 7; 3WEEKS n = 7); (d) Hippocampus (EXP n = 8, 1RSD n = 8, 4RSD n = 8; 3WEEKS n = 8). Data are presented as mean values ± SEM (pg/ml in plasma and pg/mg in brain tissue) *p <0.05; **p <0.01; ***p < 0.001 vs. exploration (EXP) group.
Similar results were observed in the three brain areas studied. The ANOVA revealed a significant increase in IL-6 protein levels in the STR F(3,40) = 7.878, p <0.001 (Fig 2b); PFC F(3,24) = 2.991, p <0.05 (Fig 2c) and hippocampus F(3,28) = 2.891, p <0.05 (Fig 2d) after the first social defeat (ps <0.05), in the STR (p <0.001) and the PFC (p <0.05) after the fourth defeat, and only in the hippocampus three weeks after the last social defeat (p <0.05).
Indomethacin blocks the increase induced by social defeat in IL-6 levels
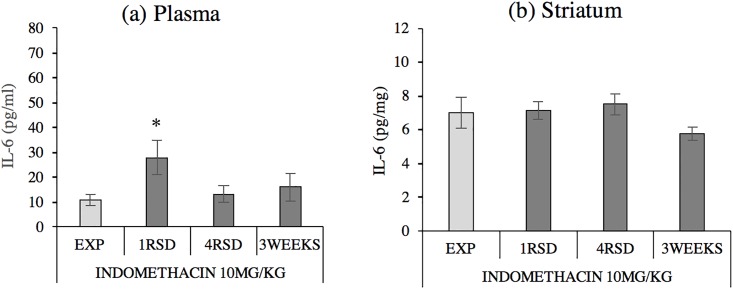
The ANOVA F(3,27) = 2.971, p <0.05 of plasmatic IL-6 levels in animals pretreated with indomethacin before social stress (Fig 3) showed that plasmatic IL-6 protein levels were significantly increased with respect to controls in defeated mice only after the first social defeat (p <0.05). No differences were detected in the STR F(3,25) = 1.969, p >0.05.
Fig 3. Indomethacin decreases plasma and striatum (STR) IL-6 levels in defeated mice.
Groups defined by stress condition and social defeat episode, all pretreated with indomethacin 10 mg/kg: (a) Plasma (EXP n = 7, 1RSD n = 8, 4RSD n = 8, 3WEEKS = 8); (b) Striatum (EXP n = 6, 1RSD n = 7, 4RSD n = 8, 3WEEKS n = 8). Data are presented as mean values ± SEM (pg/mg) *p <0.05 vs. exploration (EXP) group.
Increased plasma and brain IL-6 levels after cocaine-induced CPP in defeated mice
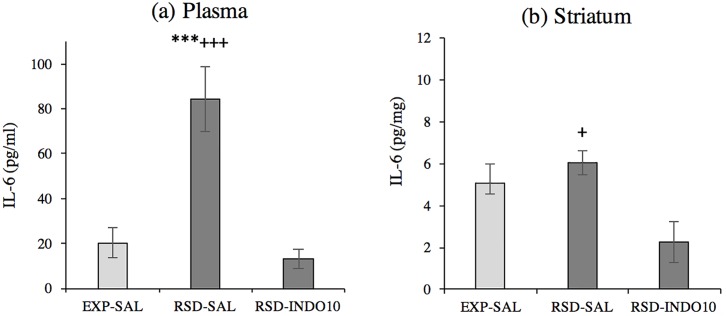
The ANOVA F(2,21) = 19.279, p <0.001 of plasmatic IL-6 levels after cocaine CPP (Fig 4) revealed significantly increased IL-6 protein levels in defeated mice compared to the exploration group (p <0.001). Pre-treatment with indomethacin before each social stress episode prevented this increase, as no significant differences were detected in plasmatic levels of socially defeated animals pretreated with indomethacin versus non-stressed controls, while a significant difference was observed when compared to socially defeated animals without anti-inflammatory treatment (p < 0.001).
Fig 4. Social defeat increases plasma and striatal IL-6 levels after cocaine-induced CPP.
Groups defined by stress condition and pre-treatment: (a) Plasma (EXP-SAL n = 8, RSD-SAL n = 8, RSD-INDO10 n = 8); (b) Striatum (EXP-SAL n = 6, RSD-SAL n = 6, RSD-INDO10 n = 10). Data are presented as mean values ± SEM (pg/mg); ***p < 0.001 vs. exploration (EXP) group.; +++p< 0.001; +p <0.05 vs. pretreated defeated (RSD-INDO10) group.
Similar results were obtained in the STR, as ANOVA F(2,19) = 5.644, p <0.01 showed increases in IL-6 protein levels in defeated animals after CPP when compared with stressed mice previously treated with indomethacin (p <0.05).
Discussion
In the current study, we explored the role of the immune system in the long-term effects of social stress on anxiety-like behavior and the conditioned rewarding effects of cocaine in mice. Social defeat induced a long-term increase in anxiety when evaluated with the EPM test and produced a significant increase in the conditioned reinforcing effect of cocaine in the CPP paradigm. With the aim of determining a possible role of the immune response in the genesis of these stress effects, we first verified that social defeat increased levels of the proinflammatory cytokine IL-6. Pre-treatment with the anti-inflammatory drug indomethacin before each stress episode prevented this enhancement of IL-6 levels and reversed the increase in the rewarding effects of cocaine in defeated mice. Conversely, this protective effect was not observed with respect to the anxiogenic consequences of social stress.
It has been widely demonstrated, in humans and animal models, that stressful experiences have a modulatory effect on the behavioral and physiological response to drugs [62]. In general, social stress induces a sensitization of the reward system that makes mice more sensitive to the effects of drugs [63]. In this regard, the CPP paradigm is a useful tool to study how stress can modify sensitivity to the secondary motivational properties and hedonic valence of drugs [64, 65]. In the present experiments, we observed that socially defeated animals developed CPP with a sub-threshold dose of cocaine (1 mg/kg), while this conditioned preference was not observed in animals under the exploration condition (non-stressed). These results are in agreement with previous data reported by our group for cocaine [35, 36, 44] and other substances such as alcohol or MDMA [66]. We also found that defeated animals displayed a long-term increase in anxiety-like behavior, spending less time and a lower percentage of time, and performing fewer entries and a lower percentage of entries into the open arms of the EPM than their non-stressed counterparts. This result has also been consistently replicated in the literature [66, 67, 68, 69, 70, 71].
We hypothesized that these behavioral consequences of social stress are somehow mediated by a neuroinflammatory immune response. To validate this hypothesis, we first determined if social stress could trigger an inflammatory response. We observed increased levels of the cytokine IL-6 in defeated mice four hours after social defeat episodes. Socially defeated animals displayed significantly higher plasmatic and brain (STR, PFC and hippocampus) IL-6 levels after the first and fourth social defeat when compared with exploration mice. This is not surprising, as other researchers have also reported increased levels of proinflammatory cytokines in response to social stressors [40, 68, 72]. For example, Hodes and coworkers [40] found that the higher responsiveness of the immune system to stress—characterized by higher levels of pro-inflammatory cytokines—was correlated with a higher vulnerability of mice to a stress-induced depressive-like phenotype.
However, most of these previous reports only dealt with the acute inflammatory consequences of social stress. We have focused on long term-effects in the present study by extending the timeframe of the IL-6 profile and determining its levels three weeks after the stress episode, immediately before performing the behavioral tests. Three weeks after the last social defeat encounter a significant increase in IL-6 levels was registered only in the hippocampus of socially defeated mice, which is considered a central structure in CPP establishment [73, 74]. After continuous exposure to the aggressor stimulus for 10 days, other researchers have reported an up-regulated plasmatic IL-6 levels up to 35 days after the last defeat episode [40]. We believe that these discrepancies may be a result of our shorter and intermittent social stress protocol, while the other model can be considered chronic. It should be stressed that social defeat involves physical contact during the aggressive encounter and can sometimes incur physical wounding as a consequence, which can confound the interpretation of the inflammatory measures in the brain or the blood. While some researchers did not report alterations in inflammatory markers after RSD when physical wounding is completely suppressed [75], other investigators find alterations in cytokines and in the immune response after non-physical social stress models, such as social threat exposure in juvenile mice [76] or vicarious social defeat in adults [40, 77], findings that corroborate the immune response to social stressors. We are aware of the possible interference of physical injuring in the immune response and, therefore, our protocol of RSD has been designed to minimize the physical wounding following the indications of Burke and co-workers [78].
Once we confirmed the existence of an acute immune reaction triggered by social stress episodes, we aimed to determine if the increased sensitivity to the rewarding properties of cocaine and anxiety-like behavior is somehow modulated by this pro-inflammatory response. Considering that cytokine IL-6 levels were generally similar in stressed and non-stressed mice (with the exception of the hippocampus) when they performed the anxiety and CPP tests, the different behavior of defeated mice can be explained by an initial role of the pro-inflammatory response by which long-term adaptations are promoted. For this reason, we decided to block the development of an inflammatory response by administering the anti-inflammatory indomethacin before each social stress episode. At the highest dose of indomethacin (10 mg/kg), we registered a general assuaging of the increase in IL-6 levels after the first and fourth social defeat compared with levels displayed in non-treated animals, although significantly higher levels of IL-6 in plasma continued to be measured after the first defeat when compared with the exploration group.
We also detected increased levels of IL-6 after the CPP procedure. Administration of four daily low doses of cocaine (1mg/kg) induced an enhancement of plasmatic IL-6 cytokine levels in all animals. This enhanced immune signaling was more pronounced in animals under the stress condition. Socially defeated animals presented increased IL-6 levels in plasma, with these levels proving to be statistically higher than in non-stressed animals. Again, pretreatment with indomethacin reversed this enhancement in the effect of stress, and socially defeated animals pretreated with the anti-inflammatory displayed similar IL-6 levels to the exploration group after cocaine CPP. The potential of cocaine as a xenobiotic that can activate proinflammatory central immune signaling is well documented [See revisions 17, 19]. We have found that the inflammatory potential of cocaine is exacerbated by previous stress experience, whereas an anti-inflammatory pre-treatment before stress can reverse it. It is known that previous stress experiences can sensitize peripheral and central components of the immune system [68, 69, 70]. We hypothesized that our social stress paradigm would induce long-term changes in the immune response of our experimental mice, making their immune system more reactive to insults. Indeed, indomethacin administration before each social defeat blocked the proinflammatory response induced by social stress and avoided the development of sensitization of the neuroimmune axis.
Once we had demonstrated that indomethacin was capable of reducing the release of cytokine induced by social defeat, we set out to evaluate if this decrease was related to the behavioral consequences of stress. Administration of the higher dose of indomethacin (10 mg/kg) before each social defeat completely reversed the stress-induced increase in the rewarding properties of cocaine, since defeated mice treated with this anti-inflammatory did not develop CPP induced by cocaine. One possible mechanism by which neuroinflammation can enhance the rewarding properties of cocaine is the activation of the hypothalamus hypothalamic-pituitary-adrenal (HPA) axis. It had been demonstrated that IL-6 promotes the activation the HPA axis at the hypothalamic level [79, 80], leading to the release of CRF. CRF modulates dopamine function due to its interaction within the ventral tegmental area, a key structure for drug reward effects [81, 82]. Elevated CRF levels would alter the function of the dopaminergic neurons, causing long-term neuroadaptations along this pathway [83, 84]. In this sense, increased levels of CRF in the ventral tegmental area have recently been related to increased rewarding properties of cocaine in the self-administration paradigm [85, 86].
Conversely, the anti-inflammatory treatment failed to prevent anxiety-like behavior in our socially defeated animals. While there is general agreement in the literature about the link between an enhancement of IL-6 levels and the induction of anxiety (See revision on [87]), the decrease of IL-6 levels was not effective in blocking the anxiogenic consequences of social defeat in our study. Hodes and collaborators [40] reported similar results in an experiment carried out with socially stressed mice pretreated with an IL-6 monoclonal antibody (mAbs), a pharmacological intervention that neutralizes circulating IL-6 cytokine, thereby preventing it from binding to IL-6 receptors in cell membranes. The authors found that chronic administration of IL-6 mAb prevented the development of social avoidance, which they considered a marker of susceptibility to stress consequences, while it failed to reduce anxiety-like behavior induced by stress in the EPM. One possible explanation of this discrepancy between the depressive and anxiogenic consequences of social stress may be different mechanisms for their genesis. Regarding this, Wohleb and collaborators [68, 69, 70] proposed a neuroinflammatory mechanism for the genesis of anxiety that is in line with our present results. They theorized that the pro-inflammatory profile induced by stress alters the function of vascular endothelial cells in the BBB, allowing peripheral monocytes to traffic into the brain, leading to the development of anxiety. They confirmed this hypothesis using transgenic knockdown mice for the proinflammatory interleukin-1 receptor (IL-1R1) in endothelial cells. These transgenic mice are protected from increased permeability of the inflammatory signals between brain resident microglia and peripheral blood monocytes, and, as a result, have a decreased central inflammation and did not develop anxiety-like behavior after social stress when compared with wild type mice [70]. Additionally, Wohleb and collaborators reported that this monocyte infiltration into the brain occurs in regions specifically associated with fear, anxiety and threat appraisal such as the prefrontal cortex, the hippocampus, or the amygdala while it is not registered in other regions such as the motor or somatosensory cortex, and the STR [88]. This region specificity of monocyte infiltration could explain why indomethacin failed to block the genesis of anxiety while it was effective in blocking the stress-induced increase in the rewarding properties of cocaine. As the indomethacin treatment attenuated but did not completely block the increase of peripheral IL-6 after the first RSD, we hypothesize that this increase was enough to start the mechanism that led to increases in the permeability to BBB. The decreased integrity of the BBB let monocytes traffic into the more permeable brain regions, while other regions less susceptible for this peripheral infiltration, such as the striatum (key in reward drug response), were not affected.
Conclusions
The results of this research surpass current basic knowledge and take on a clear translational relevance, since IL-6 levels have been found to be altered in humans under conditions of social stress [83], and even more so in those with cocaine use disorders [17]. The confirmation of the contribution of inflammation to stress-induced vulnerability to mental-disorders provides new research opportunities, especially in the field of drug abuse disorders; from considering inflammatory parameters as a possible biomarker for diagnosis, to developing anti-inflammatory strategies as preventive or therapeutic interventions.
Supporting information
(XLSX)
(XLSX)
(XLSX)
Data Availability
All the data are available in the supplementary data.
Funding Statement
This work was supported by the Ministerio de Economía y Competitividad (MINECO), Dirección General de Investigación, PSI2014-51847-R and PSI 2017-83023-R; Instituto de Salud Carlos III, Red de Trastornos Adictivos (RTA) (RETICS RD12/0028/0005 and RD16/0017/0007) and Unión Europea, Fondos FEDER “A way to build Europe”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Reus G, Fries G, Stertz L, Badawy M, Passos I, Barichello T, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 2015;300:141–154. 10.1016/j.neuroscience.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 2.Alavi M, Grebely J, Matthews GV, Petoumenos K, Yeung B, Day C, et al. Effect of pegylated interferon-α‐2a treatment on mental health during recent hepatitis C virus infection. J Gastroenterol Hepatol 2012;27(5):957–965. 10.1111/j.1440-1746.2011.07035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birerdinc A, Afendy A, Stepanova M, Younossi I, Baranova A, Younossi ZM. Gene expression profiles associated with depression in patients with chronic hepatitis C (CH-C). Brain and behavior 2012;2(5):525–531. 10.1002/brb3.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ménard C, Pfau ML, Hodes GE, Russo SJ. Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology 2017;42(1):62 10.1038/npp.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiedorowicz JG, Prossin AR, Johnson CP, Christensen GE, Magnotta VA, Wemmie JA. Peripheral inflammation during abnormal mood states in bipolar I disorder. J Affect Disord 2015. November 15;187:172–178. 10.1016/j.jad.2015.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein BI, Collinger KA, Lotrich F, Marsland AL, Gill M, Axelson DA, et al. Preliminary findings regarding proinflammatory markers and brain-derived neurotrophic factor among adolescents with bipolar spectrum disorders. J Child Adolesc Psychopharmacol 2011;21(5):479–484. 10.1089/cap.2011.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalelioglu T, Akkus M, Karamustafalioglu N, Genc A, Genc ES, Cansiz A, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratios as inflammation markers for bipolar disorder. Psychiatry Res 2015. August 30;228(3):925–927. 10.1016/j.psychres.2015.05.110 Epub 2015 Jun 28. [DOI] [PubMed] [Google Scholar]
- 8.Khandaker GM, Dantzer R. Is there a role for immune-to-brain communication in schizophrenia? Psychopharmacology (Berl) 2016;233(9):1559–1573. 10.1007/s00213-015-3975-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull 2013;39(6):1174–1179. 10.1093/schbul/sbt141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C] PK11195 positron emission tomography study. Biol Psychiatry 2008;64(9):820–822. [DOI] [PubMed] [Google Scholar]
- 11.Morgan JT, Chana G, Abramson I, Semendeferi K, Courchesne E, Everall IP. Abnormal microglial–neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain Res 2012;1456:72–81. 10.1016/j.brainres.2012.03.036 [DOI] [PubMed] [Google Scholar]
- 12.Clark KH, Wiley CA, Bradberry CW. Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotoxicity research 2013;23(2):174–188. 10.1007/s12640-012-9334-7 [DOI] [PubMed] [Google Scholar]
- 13.Cui C, Shurtleff D, Harris RA. Neuroimmune mechanisms of alcohol and drug addiction. International review of neurobiology: Elsevier; 2014. p. 1–12. 10.1016/B978-0-12-801284-0.00001-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 2005;162(8):1403–1413. 10.1176/appi.ajp.162.8.1403 [DOI] [PubMed] [Google Scholar]
- 15.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010;35(1):217 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volkow N, Morales M. The brain on drugs: from reward to addiction. Cell 2015;162(4):712–725. 10.1016/j.cell.2015.07.046 [DOI] [PubMed] [Google Scholar]
- 17.Hutchinson MR, Watkins LR. Why is neuroimmunopharmacology crucial for the future of addiction research? Neuropharmacology 2014;76:218–227. 10.1016/j.neuropharm.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodrigues LC, Gobira PH, de Oliveira AC, Pelição R, Teixeira AL, Moreira FA, et al. Neuroinflammation as a possible link between cannabinoids and addiction. Acta neuropsychiatrica 2014;26(6):334–346. 10.1017/neu.2014.24 [DOI] [PubMed] [Google Scholar]
- 19.Coller JK, Hutchinson MR. Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther 2012;134(2):219–245. 10.1016/j.pharmthera.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 20.Montesinos J, Pascual M, Pla A, Maldonado C, Rodríguez-Arias M, Miñarro J, et al. TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain Behav Immun 2015;45:233–244. 10.1016/j.bbi.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 21.Montesinos J, Pascual M, Rodríguez-Arias M, Miñarro J, Guerri C. Involvement of TLR4 in the long-term epigenetic changes, rewarding and anxiety effects induced by intermittent ethanol treatment in adolescence. Brain Behav Immun 2016;53:159–171. 10.1016/j.bbi.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 22.Harricharan R, Abboussi O, Daniels WM. Addiction: A dysregulation of satiety and inflammatory processes. Prog Brain Res 2017; 235:65–91. 10.1016/bs.pbr.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 23.Araos P, Pedraz M, Serrano A, Lucena M, Barrios V, García-Marchena N, et al. Plasma profile of pro-inflammatory cytokines and chemokines in cocaine users under outpatient treatment: influence of cocaine symptom severity and psychiatric co-morbidity. Addict Biol 2015;20(4):756–772. 10.1111/adb.12156 [DOI] [PubMed] [Google Scholar]
- 24.Moreira FP, Medeiros JRC, Lhullier AC, de Mattos Souza Luciano Dias, Jansen K, Portela LV, et al. Cocaine abuse and effects in the serum levels of cytokines IL-6 and IL-10. Drug & Alcohol Dependence 2016;158:181–185. 10.1016/j.drugalcdep.2015.11.024 [DOI] [PubMed] [Google Scholar]
- 25.Xu E, Liu J, Liu H, Wang X, Xiong H. Role of microglia in methamphetamine-induced neurotoxicity. International journal of physiology, pathophysiology and pharmacology 2017;9(3):84 [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities. Ann N Y Acad Sci 2010;1187(1):101–121. 10.1111/j.1749-6632.2009.05141.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moratalla R, Khairnar A, Simola N, Granado N, García-Montes JR, Porceddu PF, et al. Amphetamine-related drugs neurotoxicity in humans and in experimental animals: main mechanisms. Prog Neurobiol 2017;155:149–170. 10.1016/j.pneurobio.2015.09.011 [DOI] [PubMed] [Google Scholar]
- 28.Maza-Quiroga R, García-Marchena N, Romero-Sanchiz P, Barrios V, Pedraz M, Serrano A, et al. Evaluation of plasma cytokines in patients with cocaine use disorders in abstinence identifies transforming growth factor alpha (TGFα) as a potential biomarker of consumption and dual diagnosis. PeerJ 2017;5:e3926 10.7717/peerj.3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miczek KA, Yap JJ, Covington III, HE. Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther 2008;120(2):102–128. 10.1016/j.pharmthera.2008.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charney DS. Psychobiological mechanisms of resilience and vulnerability. Focus 2004, 2(3):368–391 [DOI] [PubMed] [Google Scholar]
- 31.Goldstein DS, McEwen B. Allostasis, homeostats, and the nature of stress. Stress 2002;5(1):55–58. 10.1080/102538902900012345 [DOI] [PubMed] [Google Scholar]
- 32.Björkqvist K. Social defeat as a stressor in humans. Physiol Behav 2001;73(3):435–442. 10.1016/S0031-9384(01)00490-5 [DOI] [PubMed] [Google Scholar]
- 33.Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neuroscience & Biobehavioral Reviews 2003;27(5):457–491. [DOI] [PubMed] [Google Scholar]
- 34.Neisewander J, Peartree N, Pentkowski N. Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacology (Berl) 2012;224(1):33–56. 10.1007/s00213-012-2853-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrer-Perez C, Reguilón MD, Manzanedo C, Aguilar MA, Miñarro J, Rodríguez-Arias M. Antagonism of corticotropin-releasing factor CRF1 receptors blocks the enhanced response to cocaine after social stress. Eur J Pharmacol 2018;823:87–95. 10.1016/j.ejphar.2018.01.052 [DOI] [PubMed] [Google Scholar]
- 36.Montagud-Romero S, Reguilon M, Roger-Sanchez C, Pascual M, Aguilar M, Guerri C, et al. Role of dopamine neurotransmission in the long-term effects of repeated social defeat on the conditioned rewarding effects of cocaine. Prog Neuro-Psychopharmacol Biol Psychiatry 2016;71:144–154. 10.1016/j.pnpbp.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 37.Logrip ML, Koob GF, Zorrilla EP. Role of corticotropin-releasing factor in drug addiction. CNS drugs 2011;25(4):271–287. 10.2165/11587790-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zorrilla EP, Logrip ML, Koob GF. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocrinol 2014;35(2):234–244. 10.1016/j.yfrne.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montagud-Romero S, Montesinos J, Pascual M, Aguilar M, Roger-Sanchez C, Guerri C, et al. Up-regulation of histone acetylation induced by social defeat mediates the conditioned rewarding effects of cocaine. Prog Neuro-Psychopharmacol Biol Psychiatry 2016;70:39–48. 10.1016/j.pnpbp.2016.04.016 [DOI] [PubMed] [Google Scholar]
- 40.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A 2014. November 11;111(45):16136–16141. 10.1073/pnas.1415191111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, et al. Social stress increases the susceptibility to endotoxic shock. J Neuroimmunol 2001. April 2;115(1–2):36–45. 10.1016/S0165-5728(01)00273-9 [DOI] [PubMed] [Google Scholar]
- 42.Pfau ML, Russo SJ. Neuroinflammation regulates cognitive impairment in socially defeated mice. Trends Neurosci 2016;39(6):353–355. 10.1016/j.tins.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O’Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci 2007. August 29;27(35):9301–9309. 10.1523/JNEUROSCI.1418-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodríguez-Arias M, Montagud-Romero S, Rubio-Araiz A, Aguilar MA, Martín-García E, Cabrera R, et al. Effects of repeated social defeat on adolescent mice on cocaine-induced CPP and self-administration in adulthood: integrity of the blood–brain barrier. Addict Biol 2017;22(1):129–141. 10.1111/adb.12301 [DOI] [PubMed] [Google Scholar]
- 45.Bhattacharya A, Derecki NC, Lovenberg TW, Drevets WC. Role of neuro-immunological factors in the pathophysiology of mood disorders. Psychopharmacology (Berl) 2016;233(9):1623–1636. 10.1007/s00213-016-4214-0 [DOI] [PubMed] [Google Scholar]
- 46.Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci 2007;25(2):541–550. 10.1111/j.1460-9568.2006.05298.x [DOI] [PubMed] [Google Scholar]
- 47.Pascual M, Montesinos J, Guerri C. Role of the innate immune system in the neuropathological consequences induced by adolescent binge drinking. J Neurosci Res 2018;96(5):765–780. 10.1002/jnr.24203 [DOI] [PubMed] [Google Scholar]
- 48.Crews FT, Vetreno RP. Neuroimmune basis of alcoholic brain damage. International review of neurobiology. 2014;1(118)315–357. 10.1016/B978-0-12-801284-0.00010-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez-Reimers E, Santolaria-Fernandez F, Martin-Gonzalez MC, Fernandez-Rodriguez CM, Quintero-Platt G. Alcoholism: a systemic proinflammatory condition. World J Gastroenterol 2014. October 28;20(40):14660–14671. 10.3748/wjg.v20.i40.14660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vallés SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain pathology 2004;14(4):365–37. 10.1111/j.1750-3639.2004.tb00079.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duque A, Vinader-Caerols C, Monleón S. Indomethacin counteracts the effects of chronic social defeat stress on emotional but not recognition memory in mice. PloS one 2017;12(3):e0173182 10.1371/journal.pone.0173182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodriguez-Arias M, Minarro J, Aguilar MA, Pinazo J, Simon VM. Effects of risperidone and SCH 23390 on isolation-induced aggression in male mice. Eur Neuropsychopharmacol 1998. May;8(2):95–103. 10.1016/S0924-977X(97)00051-5 [DOI] [PubMed] [Google Scholar]
- 53.Arenas MC, Daza-Losada M, Vidal-Infer A, Aguilar MA, Miñarro J, Rodríguez-Arias M. Capacity of novelty-induced locomotor activity and the hole-board test to predict sensitivity to the conditioned rewarding effects of cocaine. Physiol Behav 2014;133:152–160. 10.1016/j.physbeh.2014.05.028 [DOI] [PubMed] [Google Scholar]
- 54.Montagud-Romero S, Daza-Losada M, Vidal-Infer A, Maldonado C, Aguilar MA, Miñarro J, et al. The novelty-seeking phenotype modulates the long-lasting effects of intermittent ethanol administration during adolescence. PloS one 2014;9(3):e92576 10.1371/journal.pone.0092576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vidal-Infer A, Arenas MC, Daza-Losada M, Aguilar MA, Miñarro J, Rodríguez-Arias M. High novelty-seeking predicts greater sensitivity to the conditioned rewarding effects of cocaine. Pharmacology Biochemistry and Behavior 2012;102(1):124–132. 10.1016/j.pbb.2012.03.031 [DOI] [PubMed] [Google Scholar]
- 56.Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav 1993;53(5):983–993. [DOI] [PubMed] [Google Scholar]
- 57.Covington HE, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Psychopharmacology (Berl) 2001;158(4):388–398. 10.1007/s002130100858 [DOI] [PubMed] [Google Scholar]
- 58.Maldonado C, Rodriguez-Arias M, Castillo A, Aguilar MA, Minarro J. Gamma-hydroxybutyric acid affects the acquisition and reinstatement of cocaine-induced conditioned place preference in mice. Behav Pharmacol 2006. March;17(2):119–131. 10.1097/01.fbp.0000190685.84984.ec [DOI] [PubMed] [Google Scholar]
- 59.Daza-Losada M, Rodriguez-Arias M, Maldonado C, Aguilar M, Guerri C, Minarro J. Acute behavioural and neurotoxic effects of MDMA plus cocaine in adolescent mice. Neurotoxicol Teratol 2009;31(1):49–59. 10.1016/j.ntt.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 60.Heffner TG, Hartman JA, Seiden LS. A rapid method for the regional dissection of the rat brain. Pharmacology Biochemistry and Behavior 1980;13(3):453–456. 10.1016/0091-3057(80)90254-3 [DOI] [PubMed] [Google Scholar]
- 61.Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci 2010. June 16;30(24):8285–8295. 10.1523/JNEUROSCI.0976-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koob GF. A role for brain stress systems in addiction. Neuron 2008;59(1):11–34. 10.1016/j.neuron.2008.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacNicol B. The biology of addiction. Canadian Journal of Anesthesia/Journal canadien d’anesthésie 2017;64(2):141–148. 10.1007/s12630-016-0771-2 [DOI] [PubMed] [Google Scholar]
- 64.Aguilar MA, Rodríguez-Arias M, Miñarro J. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Rev 2009;59(2):253–277. 10.1016/j.brainresrev.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 65.Tzschentke TM. Review on CPP: Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 2007;12(3‐4):227–462. 10.1111/j.1369-1600.2007.00070.x [DOI] [PubMed] [Google Scholar]
- 66.García-Pardo M, Blanco-Gandía M, Valiente-Lluch M, Rodríguez-Arias M, Miñarro J, Aguilar M. Long-term effects of repeated social stress on the conditioned place preference induced by MDMA in mice. Prog Neuro-Psychopharmacol Biol Psychiatry 2015;63:98–109. 10.1016/j.pnpbp.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 67.Iñiguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, et al. Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress 2014;17(3):247–255. 10.3109/10253890.2014.910650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci 2011. April 27;31(17):6277–6288. 10.1523/JNEUROSCI.0450-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci 2013. August 21;33(34):13820–13833. 10.1523/JNEUROSCI.1671-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci 2014. February 12;34(7):2583–2591. 10.1523/JNEUROSCI.3723-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci 2006. September 27;26(39):9967–9974. 10.1523/JNEUROSCI.2384-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iwata M, Ota KT, Li X, Sakaue F, Li N, Dutheil S, et al. Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biol Psychiatry 2016;80(1):12–22. 10.1016/j.biopsych.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 73.Chen C, Liu H, Guan X. Changes in microRNA expression profile in hippocampus during the acquisition and extinction of cocaine-induced conditioned place preference in rats. J Biomed Sci 2013;20(1):96 10.1186/1423-0127-20-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meyers RA, Zavala AR, Neisewander JL. Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport 2003;14(16):2127–2131. [DOI] [PubMed] [Google Scholar]
- 75.Hueston CM, Barnum CJ, Eberle JA, Ferraioli FJ, Buck HM, Deak T. Stress-dependent changes in neuroinflammatory markers observed after common laboratory stressors are not seen following acute social defeat of the Sprague Dawley rat. Physiol Behav. 2011; 104(2):187–198 10.1016/j.physbeh.2011.03.013 [DOI] [PubMed] [Google Scholar]
- 76.Lo Iacono L, Catale C, Martini A, Valzania A, Viscomi MT, Chiurchiù V, et al. From Traumatic Childhood to Cocaine Abuse: The Critical Function of the Immune System. Biol Psychiatry. 2018. 10.1016/j.biopsych.2018.05.022 [DOI] [PubMed] [Google Scholar]
- 77.Finnell JE, Muniz BL, Padi AR, Lombard CM, Moffitt CM, Wood CS, et al. Essential Role of Ovarian Hormones in Susceptibility to the Consequences of Witnessing Social Defeat in Female Rats. Biol Psychiatry. 2018; 84(5) 372–382. 10.1016/j.biopsych.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burke AR, DeBold JF, Miczek KA. CRF type 1 receptor antagonism in ventral tegmental area of adolescent rats during social defeat: prevention of escalated cocaine self-administration in adulthood and behavioral adaptations during adolescence. Psychopharmacology (Berl). 2016; 233(14) 2727–2736. 10.1007/s00213-016-4336-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silverman MN, Miller AH, Biron CA, Pearce BD. Characterization of an interleukin-6-and adrenocorticotropin-dependent, immune-to-adrenal pathway during viral infection. Endocrinology 2004;145(8):3580–3589. 10.1210/en.2003-1421 [DOI] [PubMed] [Google Scholar]
- 80.Wang J, Dunn AJ. Mouse interleukin-6 stimulates the HPA axis and increases brain tryptophan and serotonin metabolism. Neurochem Int 1998;33(2):143–154. 10.1016/S0197-0186(98)00016-3 [DOI] [PubMed] [Google Scholar]
- 81.Borgland SL, Ungless MA, Bonci A. Convergent actions of orexin/hypocretin and CRF on dopamine neurons: emerging players in addiction. Brain Res 2010;1314:139–144. 10.1016/j.brainres.2009.10.068 [DOI] [PubMed] [Google Scholar]
- 82.Boyson CO, Miguel TT, Quadros IM, DeBold JF, Miczek KA. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl) 2011;218(1):257–269. 10.1007/s00213-011-2266-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haass-Koffler CL, Bartlett SE. Stress and addiction: contribution of the corticotropin releasing factor (CRF) system in neuroplasticity. Frontiers in molecular neuroscience 2012;5:91 10.3389/fnmol.2012.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wanat M, Hopf F, Stuber G, Phillips P, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol (Lond) 2008;586(8):2157–2170. 10.1113/jphysiol.2007.150078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han X, DeBold JF, Miczek KA. Prevention and reversal of social stress-escalated cocaine self-administration in mice by intra-VTA CRFR1 antagonism. Psychopharmacology (Berl) 2017;234(18):2813–2821. 10.1007/s00213-017-4676-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Holly EN, Boyson CO, Montagud-Romero S, Stein DJ, Gobrogge KL, DeBold JF, et al. Episodic Social Stress-Escalated Cocaine Self-Administration: Role of Phasic and Tonic Corticotropin Releasing Factor in the Anterior and Posterior Ventral Tegmental Area. J Neurosci 2016. April 6;36(14):4093–4105. 10.1523/JNEUROSCI.2232-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hodes GE, Ménard C, Russo SJ. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiology of stress 2016;4:15–22. 10.1016/j.ynstr.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wohleb ES, McKim DB, Sheridan JF, Godbout JP. Monocyte trafficking to the brain with stress and inflammation: A novel axis of immune-to-brain communication that influences mood and behavior. Frontiers in Neuroscience. 2015; 8:447 10.3389/fnins.2014.00447 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All the data are available in the supplementary data.