Abstract
This is the fifth in the series of reviews developed as part of the Biomarkers of Nutrition for Development (BOND) program. The BOND Iron Expert Panel (I-EP) reviewed the extant knowledge regarding iron biology, public health implications, and the relative usefulness of currently available biomarkers of iron status from deficiency to overload. Approaches to assessing intake, including bioavailability, are also covered. The report also covers technical and laboratory considerations for the use of available biomarkers of iron status, and concludes with a description of research priorities along with a brief discussion of new biomarkers with potential for use across the spectrum of activities related to the study of iron in human health.
The I-EP concluded that current iron biomarkers are reliable for accurately assessing many aspects of iron nutrition. However, a clear distinction is made between the relative strengths of biomarkers to assess hematological consequences of iron deficiency versus other putative functional outcomes, particularly the relationship between maternal and fetal iron status during pregnancy, birth outcomes, and infant cognitive, motor and emotional development. The I-EP also highlighted the importance of considering the confounding effects of inflammation and infection on the interpretation of iron biomarker results, as well as the impact of life stage. Finally, alternative approaches to the evaluation of the risk for nutritional iron overload at the population level are presented, because the currently designated upper limits for the biomarker generally employed (serum ferritin) may not differentiate between true iron overload and the effects of subclinical inflammation.
Keywords: iron, iron nutrition, iron status assessment, iron biology, iron biomarkers
Introduction
The Biomarkers of Nutrition for Development (BOND) project is designed to provide evidence-based advice to anyone with an interest in the role of nutrition in health. Specifically, the BOND program provides state-of-the-art information with regard to the selection, use, and interpretation of biomarkers of nutrient exposure, status, function, and effect (1). To accomplish this objective, Iron Expert Panel (I-EP) members were recruited to evaluate the literature and draft comprehensive reports on the current state of the science with regard to specific nutrient biology and available biomarkers for assessing nutrient status at clinical and population levels.
Phase I of the BOND project included the evaluation of biomarkers for six nutrients: iodine, iron, zinc, folate, vitamin A, and vitamin B12. The reviews for iodine, zinc, vitamin A, and folate have been published previously (2–5). Readers interested in obtaining information on iodine or folate biomarkers that might be of use to their specific needs are encouraged to utilize the interactive BOND Query-Based System located on the BOND website: http://www.nichd.nih.gov/global_nutrition/programs/bond/Pages/index.aspx
This review represents the fifth in the series of reviews and covers all aspects of iron biology relevant to the discovery, selection, use, and interpretation of biomarkers. The paper is organized to provide the reader with a full appreciation of the background history of iron as a public health issue, its biology, and an overview of available biomarkers. Specific considerations for the use and interpretation of biomarkers of iron nutrition across a range of clinical and population-based applications are described. The paper also highlights priority research needs for moving this important global health agenda forward.
The biological importance of iron
Iron is the fourth most abundant element in the earth's crust and an essential component of almost all biological systems. Humans require iron for energy production, oxygen transport and utilization, cellular proliferation, and pathogen destruction. Some of the seminal events in the evolution of our understanding of iron in human health are outlined in Text Box 1 (6–21).
Text Box 1.
Historical landmarks in iron biology
• Hippocrates (c 460 BC–c 370 BC) is credited as the first physician to use iron as a topical therapeutic styptic.
• 1574: Nicolas Bautista Monardes (1493–1588) published on the use of iron to treat a variety of systemic illnesses (http://www.library.nd.edu/rarebooks/exhibits/durand/indies/monardes.html) (6, 7).
• 1681: Sydenham is credited with first recognizing the value of iron therapy for treatment of chlorosis (“green sickness”), a commonly recognized disorder prevalent among young women in in the 18th and 19th centuries (6, 7).
• 1713: Nicolas Lemery and Etienne Geoffroy first demonstrated the presence of iron in blood.
• 1831: Pierre Blaud, reported successful treatment of the chlorosis with a combination of ferrous sulfate and potassium carbonate (“Blaud's pill”) (8).
• Amand Trousseau (1801–67) documented the use of oral iron compounds and was the first to report the potential risk of giving therapeutic doses of iron to patients suffering from tuberculosis (9, 10).
• 1919: George Washington Carey recognized the importance of iron for oxygen transport: “It is not simply the heat that causes distress in a fever patient, but it is the lack of oxygen in the blood due to a deficiency in iron, the carrier of oxygen” (11).
• 1925: Fontes and Thivolle discovered that there are small quantities of iron in the plasma and the concentration is decreased in experimentally induced anemia.
• 1932: Heath, Strauss and Castle provided incontrovertible evidence that parenterally administered iron improved hemoglobin levels in patients with hypochromic anemia. They also recognized the importance of iron for the normal function of other tissues although most of their attention was focused on what could be observed clinically, i.e., abnormalities of the nails, skin and mucosae (12).
• 1925: The plasma iron transport protein transferrin was identified during the subsequent two decades (13).
• 1937: Laufberger crystalized the iron storage protein ferritin from a horse's spleen (14).
• 1937: McCance and Widdowson (15) concluded that: 1) iron is stored in the body and there are significant sex differences; 2) healthy adults maintain iron balance despite differences in dietary intake; 3) unlike some other minerals very little iron is excreted in the urine; 4) the amount of iron in the feces varies but is approximately equal to the amount consumed in food, implying that only a small proportion of dietary iron is absorbed; however, 5) because their methods were insufficiently sensitive to demonstrate that iron absorption is highly regulated, they concluded that there was no mucosal control.
• 1943: Hahn and Whipple demonstrated that: 1) iron absorption is highly regulated and affected by anemia, hypoxia, and recent antecedent intake (16); and 2) to account for the regulation of iron balance they proposed that a “mucosal block” occurs when the absorbing surface is exposed to large quantities of iron limiting further absorption. Subsequent experiments revealed that the mucosal block is not absolute and that body iron status has an important regulatory role in modulating iron absorption (17).
• 1963: Conrad and Crosby (18) demonstrated that: 1) radioactive iron administered to rats enters mucosal cells; and 2) when iron requirements are high, the iron is exported to the portal circulation; when low, the iron remains in the mucosal cells, as these cells are sloughed from the villi after ∼48 h taking the iron with them and thereby limiting absorption.
• 1997–2000: A mucosal iron transporter, responsible for nonheme iron absorption, divalent metal transporter 1 (DMT-1, also known as divalent cation transporter), natural resistance-associated macrophage protein (NRAMP2) (19), and the cellular iron exporter, ferroportin (20) were identified.
• 2001: Park et al. (21) coined the name “hepcidin” to describe a peptide that we now understand to be a major regulator of iron absorption and homeostasis.
The unique biological properties of iron stem from the marked variability of the Fe2+/Fe3+ redox potential which allows iron sites to have redox potentials from ∼–0.5 V to ∼+0.6 V, encompassing virtually the entire biologically relevant range. Protein ligands adapt these redox potentials to meet biological requirements (22). The Micronutrient Genomics Project (23) provides a comprehensive list of iron-requiring proteins, including enzymes, controlling factors, transporters, and storage proteins, with the relevant genetic information. The GWAS website (24) catalogues single nucleotide polymorphisms associated with iron overload and iron deficiency (22, 25). A classification system for iron-containing proteins based on biochemical function in humans was proposed by Crichton (22) and is outlined in Text Box 2.
Text Box 2.
Classification scheme for functional iron-containing proteins in human biology
Hemoproteins
The iron atoms in hemoproteins are bound to the four pyrrole rings of protoporphyrin IX (heme) and to one or two axial ligands in the protein. There are three types of hemoproteins:
• Oxygen carriers:
-Hemoglobin is the oxygen transporter in red blood cells. Hemoglobin binds oxygen in the lung and transports it throughout the body where it is used in aerobic metabolic pathways.
-Myoglobin is found in muscle tissue where it temporarily stores oxygen making it readily available during episodes of oxygen deprivation.
• Activators of molecular oxygen:
-Cytochrome oxidase
-Peroxidases
-Catalases
-Cytochrome P450s
• Electron transport proteins:
-Cytochromes transfer electrons from substrate oxidation to cytochrome c oxidase.
Iron-sulfur proteins
• Iron-sulfur proteins mediate one electron redox processes as integral components of the respiratory chain in mitochondria.
• They are also involved in many other metabolic processes including the control of gene expression, DNA damage recognition and repair, oxygen and nitrogen sensing, and the control of cellular iron acquisition and storage.
Other iron-containing proteins
• Mononuclear non-heme iron enzymes
• Dinuclear non-heme iron enzymes
• Proteins involved in iron transport and storage
Food sources and iron bioavailability
Foods that have relatively high iron content include liver, red meat, beans, nuts, green leafy vegetables, and fortified breakfast cereals, but iron absorption is very variable. For the purposes of this review, bioavailability is the term used to describe the extent to which iron is absorbed from the diet and used for normal body functions, which include incorporation into hemoglobin, ferritin, and iron enzymes (26, 27). Throughout this review we always use the nutritional definition of iron bioavailability. This includes both the absorption and utilization of iron. Iron, unlike other minerals, has no regulated excretion pathway, so absorbed iron is more or less completely utilized for functional or storage proteins. Bioavailability includes the iron used for storage, and absorption and bioavailability are used synonymously. The history of the landmark observations that provided the basis for our understanding of bioavailability is summarized in Text Box 3 (28–46).
Text Box 3.
Landmarks in the study of iron bioavailability
• McCance, Edgecombe and Widdowson initiated the study of dietary zinc and iron bioavailability and the inhibitory effect of phytate (28).
• Iron bioavailability studies were facilitated in the late 1940s and 1950s by the availability of two radioisotopes of iron, 55Fe and 59Fe (29, 30). Iron absorption and bioavailability are synonymous and were estimated based on the measured incorporation of iron isotopes into hemoglobin.
• Observations by Dubach et al. (31) and Moore and Dubach (30) simplified the methodical approach to measuring food iron absorption and bioavailability, demonstrating that 2 wk after the ingestion of radio iron, a mean of 80% of the amount absorbed is present in the circulating red blood cells in healthy human volunteers, making it unnecessary to undertake whole body counting.
• Layrisse et al. (32) demonstrated that absorption and bioavailability from a food item in a meal is a property of the composition of the meal, not of the specific food item, which led to the “common pool” concept of iron absorption and removed the necessity to tag individual food items (intrinsic labeling) when measuring nonheme iron absorption. A soluble isotopic tracer can be added to the meal. Its absorption and bioavailability will reflect the overall absorption and bioavailability from the meal (33, 34).
• This model was expanded to recognize the independent absorption of heme iron and the existence of contaminant iron, i.e., insoluble nonfood iron derived from dust, processing machinery, and cooking utensils (35) in the diet that is not available for absorption (36).
• Radio-isotope methodology has now been largely replaced by stable isotopes (37).
• The important enhancers and inhibitors of iron absorption have been identified.
-Ascorbic acid (38), and, to a lesser extent, other organic acids (39) and animal tissue (40, 41) are the most effective enhancers of nonheme iron absorption.
-Phytate (42, 43) and polyphenols (44, 45) are the most important inhibitors; calcium reduces iron absorption in single-meal studies (46).
The two major forms of food iron are heme iron in meat products (10–15% of daily dietary iron intake in populations that eat meat) and non-heme iron in both plant foods and animal source foods, including meat (47). Ferritin iron, present at fairly high levels in liver and legume seeds such as beans, and the various forms of iron used for food fortification, are important sources of non-heme iron. Heme iron is always well absorbed. On the other hand, the absorption of non-heme iron, including ferritin iron, depends on the iron status of the individual consuming the meal as well as its composition. Text Box 4 (44, 47–55) contains some key points regarding the various sources of iron.
Text Box 4.
Relative absorption of dietary sources of iron
Heme iron
• Muscle tissue contains heme iron in the form of hemoglobin and myoglobin.
• Heme iron is estimated to contribute 10–15% of the total iron in meat-eating populations, but because of its higher and more uniform absorption (estimated at 15–35%), it may contribute up to 40% or more of the total absorbed iron (47).
• The proportion of heme iron in lean meats ranges from ∼30% in white meats to ∼70% in red meats (48).
Ferritin iron
• Liver is rich in ferritin iron.
• Legume seeds such as beans may also contain up to 30% of their iron as ferritin iron (49).
• Recent studies indicate that iron in plant ferritin is readily released during cooking and digestion (50).
• In humans, ferritin iron is absorbed to the same extent as ferrous sulfate when consumed in a meal (51).
Nonheme iron
• Present in plant and animal foods.
• Varies widely in absorption from <1% to >90% depending on the iron status of the consumer and the presence of iron absorption inhibitors or enhancers in the food.
-The iron in beans and leafy vegetables, for example, is poorly absorbed due to the presence of phytate and phenolic compounds (44, 52).
-When these foods are consumed in composite meals together with foods providing iron absorption enhancers, such as ascorbic acid and muscle tissue, absorption may be increased to nutritionally useful levels (53, 54).
Fortification iron (e.g., ferrous sulfate, ferrous fumarate; for full list see below)
• Added to foods including cereal products, infant foods, condiments, milk and dairy products, and meal replacements.
• Iron compounds that are soluble in water or dilute acid enter the common nonheme iron pool in the gastrointestinal tract and are absorbed to the same extent as native nonheme iron compounds in the meal.
• Some iron fortification compounds, however, are not soluble in the gastric acid, so do not fully enter the common iron pool, and are poorly absorbed (48, 55).
Factors which influence non-heme iron bioavailability
Food components influence iron bioavailability by influencing iron absorption but have no influence on iron utilization. Food components that are inhibitors of non-heme iron absorption in general bind iron in the gastrointestinal tract and prevent its absorption, whereas enhancers of iron absorption are food components that weaken or prevent iron binding by inhibitory compounds by reducing the more reactive ferric iron to its less reactive ferrous state or additionally by binding iron in bioavailable complexes, thus preventing its binding to the inhibitor. Table 1 contains descriptions of the most common factors affecting iron absorption (26, 42, 44, 46, 52, 56–75).
TABLE 1.
Common factors affecting iron absorption
| Factor | Description |
|---|---|
| Inhibitors | |
| Phytic acid (myo-inositol hexaphosphate) | • The main inhibitor of nonheme iron absorption from plant-based diets |
| • Relatively high levels are found in whole grain cereals and legume seeds | |
| • A dose-dependent effect on iron absorption that starts at very low concentrations (42, 56) | |
| • At phytic acid:iron molar ratios >6, iron absorption is greatly inhibited from composite meals containing small amounts of enhancing components, whereas in cereal or soy meals with no enhancers, iron absorption is greatly inhibited by a molar ratio >1 (26) | |
| • Food-processing methods, such as milling, germination, fermentation, and the addition of phytase enzymes, can be used to degrade phytic acid and improve iron absorption from traditional or processed foods (57) | |
| • Ascorbic acid reverses the inhibitory effect of phytate | |
| • Ascorbic acid:iron molar ratio of 2:1 or 4:1 is recommended to overcome phytic acid inhibition of iron absorption in cereal foods that can be packaged to avoid ascorbic acid losses during storage (58) | |
| • EDTA will also overcome phytate inhibition in fortified foods such as wheat flour (59) | |
| Polyphenols | • Inhibit iron absorption in a dose-dependent way, although the strength of the binding depends on the structure of the phenolic compound |
| • Sources include: beverages (tea, coffee, cocoa, red wine) (60), vegetables (spinach, aubergine) (61), legumes (colored beans) (52), and cereals such as sorghum (44) | |
| • Polyphenol compounds vary widely in structure and extent of polymerization | |
| • The gallate-containing tea polyphenols appear to be most inhibitory (60) | |
| • Sorghum polyphenols are also very inhibitory | |
| • Although colored bean and sorghum varieties containing high levels of phytate and polyphenols are strongly inhibitory (48), the polyphenol inhibition is small relative to phytate inhibition (62). | |
| • Ascorbic acid, and to a lesser extent EDTA, will overcome the polyphenol inhibition of iron absorption (63) | |
| Calcium | • Calcium is a relatively weak iron absorption inhibitor causing a dose-dependent inhibition in simple meals but little or no inhibition in complex meals containing absorption enhancers (46) |
| • In a small bread meal, inhibition of iron absorption was dose related ≤ 300 mg Ca, with 165 mg Ca causing ∼50% inhibition whether added as calcium chloride or 150 mL milk (64) | |
| • The same quantity of milk added to a meal of steak, carrots, French fries, Camembert cheese, apple, bread, and water had no effect (65) | |
| • Ascorbic acid readily overcomes the calcium inhibition of iron absorption (66) | |
| Protein | • Peptides from partially digested food proteins can inhibit iron absorption depending on their nature |
| • Peptides from legume proteins and some milk proteins are inhibitory | |
| • The inhibitory nature of soy protein may be due to the peptides formed on digestion of the conglycinin fraction (67) | |
| • The inhibitory nature of casein is thought to be due to nonabsorbable complexes formed between iron and casein phosphopeptides (68) | |
| Enhancers | |
| Ascorbic acid (vitamin C) | • Ascorbic acid is the best-known and most potent enhancer of iron absorption either when present in fruits and vegetables (69) or added to fortified foods as the pure compound |
| • Its facilitating effect is due to its ability to convert ferric to ferrous iron at low pH and to its chelating properties (70) | |
| • Its effect is dose dependent and it can overcome much or all of the inhibition related to other food components as well as enhance the absorption of all currently available iron fortification compounds (71) except NaFeEDTA (72) | |
| • Its main disadvantage is that it is sensitive to losses during food processing, storage, and cooking because of oxidation | |
| Muscle tissue | • Muscle tissue from beef, lamb, chicken, pork, and fish, as well as liver tissue, enhance iron absorption from inhibitory meals (73) |
| • The mechanism is currently presumed to be linked to partially digested peptides | |
| • Cysteine-containing peptides could potentially reduce ferric iron to the ferrous form and chelate iron in the same way as ascorbic acid; the facilitating effect of enzymatically digested beef extract can be removed by oxidizing the cysteine residues (74) | |
| • Unlike other food proteins, muscle proteins are rapidly digested by pepsin; conceivably, the infusion of small peptides in the jejunum could be responsible for solubilizing iron and improving absorption (75) | |
Fortification iron
Iron bioavailability in relation to fortification compounds refers to both absorption and utilization, although the properties of the iron fortification compounds influence only iron absorption. The properties, including relative bioavailability, of iron compounds used in food fortification have been extensively reviewed (76). The order of preference for use in food fortification is as follows (77): 1) ferrous sulfate; 2) ferrous fumarate; 3) encapsulated ferrous sulfate or encapsulated ferrous fumarate; 4) electrolytic iron (a pure form of small particle size iron powder produced by an electrolytic processe) or ferric pyrophosphate; 5) sodium iron ethylene diamine tetraacetic acid (NaFeEDTA) (78) is preferred for phytic-acid-containing foods; 6) iron amino acid chelates, particularly iron-glycinate chelates, have also been used as iron supplements and as food fortificants for liquid milk, dairy products, wheat rolls, and multinutrient beverages (79, 80). However, more rigorous trials are required to establish their potential utility.
Text Box 5 (57, 58, 76–79, 81–83) contains some general caveats about the use of iron fortificants and enhancers of iron absorption).
Text Box 5.
General caveats regarding commonly used iron fortificants
• WHO/FAO (2006) provided recommendations for iron compounds to be added to specific foods, including cereal products, condiments, milk, and cocoa products (78).
• Iron absorption from electrolytic iron and ferric pyrophosphate added to foods is only half the iron absorption from ferrous sulfate. In order to ensure an adequate iron absorption from a fortified food, the iron fortification level when using electrolytic iron and ferric pyrophosphate should be twice the fortification level when using ferrous sulfate (76, 77).
• Particular care is recommended when adding elemental iron powders; only electrolytic iron powder is judged useful.
• Other commonly used iron powders, such as atomized or hydrogen-reduced iron powders, are judged to be too poorly absorbed and are not recommended.
• Ascorbic acid, phytase treatment, NaFeEDTA, and amino acid chelates can be used to enhance iron absorption from food vehicles rich in inhibitors.
-Ascorbic acid is recommended to be added at a 2:1 molar ratio in relation to fortification iron for low-phytate products and 4:1 for high-phytate products (58).
-Ascorbic acid's instability during processing, storage, and cooking is its main disadvantage.
-Phytases can be used to degrade phytic acid in high-phytate foods either during processing (57) or during digestion by addition to in-home fortification powders added to cereal gruels at the time of consumption (81).
-NaFeEDTA is specifically recommended for high phytate cereals such as whole-grain wheat flour (82) as well as for sauces rich in peptides such as soy and fish sauces.
-NaFeEDTA is particularly useful in the presence of phytate.
-Na2EDTA is also effective in combination with ferrous sulfate at molar ratios <1 with low-phytate cereals such as rice (83).
-Na2EDTA does not appear to increase the absorption of ferrous fumarate or ferric pyrophosphate.
-The amino acid chelate ferrous bisglycinate also protects iron from inhibitors and is especially useful in liquid products such as milk (79).
Nutrient-nutrient interactions
Iron, iodine, and vitamin A are the most common micronutrient deficiencies and often occur concurrently in infants, women, and children in resource-constrained settings. Deficiencies are most often due to higher requirements of these risk groups, low dietary intake, and poor bioavilability in food sources (84). In addition to the multiple exposure scenarios, it is also important to recognize that nutrients do not function in isolation and often interact within common pathways and biological systems. Examples of these types of interactions include the influence of iron deficiency on iodine utilization and the role of vitamin A, riboflavin, folic acid, vitamin B12, and ascorbic acid as potential causes of anemia (85). These complex interactions demand a more integrated approach to nutritional assessment in the global health context.
Text Box 6 (84, 86–96) contains some examples of iron-nutrient interactions. It is not a comprehensive list, but focuses on some of the best characterized of these interactions. Although uncertainty remains with regard to the implications of these interactions, the intent is to highlight both the need for more research and for awareness of their relationships, particularly in the context of nutritional assessment at both individual and population levels.
Text Box 6.
Examples of iron-nutrient interactions
Vitamin A
• An interaction of vitamin A in iron metabolism which results in less incorporation of iron into red blood cells is supported by animal studies in which long-term administration of vitamin A-deficient, but iron-sufficient diets, leads to anemia which can be corrected with vitamin A (86).
• In chronically inflamed/infected populations in low-resource settings, it is also possible that vitamin A deficiency induces anemia via its negative influence on the immune defense, leading to more infections and more anemia of infection and inflammation (87).
• The science regarding the interaction of vitamin A and iron at the metabolic level is evolving. Some examples of the impact of vitamin A deficiency include: impaired erythropoiesis, poor red blood cell differentiation, impaired incorporation of iron into hemoglobin, increased breakdown of malformed red blood cells, and impaired mobilization of iron from reticuloendothelial macrophages and liver iron stores (84).
• There is no agreement on the influence of vitamin A on iron absorption. Although there was a report of studies demonstrating enhanced iron absorption by Venezuelan peasants when vitamin A was added to iron-fortified wheat and maize breads (88), the results could not be confirmed in Swiss and Swedish students (89). The addition of vitamin A to an iron-fortified maize porridge resulted in a reduction in iron absorption by vitamin A-deficient Ivorian children (90).
Iodine
• Extensive data from animal studies indicate that iron deficiency with or without anemia impairs thyroid metabolism. This is supported by two recent intervention studies which showed that provision of both iron and iodine to iron-deficient, goitrous Ivorian and Moroccan children decreased goiter rates more effectively than did the provision of iodine alone (91, 92).
• It has been suggested that iron deficiency can lead to alterations in the thyroid hormone feedback system, reduce deiodinase activity and lower the transformation of thyroxine to triiodothyronine in the peripheral tissue, and reduce thyroid hormone synthesis.
• Thyroperoxidase, a heme enzyme that plays a key role in thyroid hormone synthesis by catalyzing the iodination of thyroglobulin, is markedly decreased in iron-deficient rats (93).
Zinc
• Due to similarities in absorption and transport, there has been a long-standing concern about the potential negative interaction between iron and zinc (94).
• The study of this interaction is complicated not only by shared chemistry, absorption, and transport but also study design (e.g., single supplements compared with food matrices, presence or absence of common enhancers and inhibitors).
• A debate exists with regard to the implications of this potential interaction to public health interventions with an emerging consensus that obviates concerns about joint administration of these essential elements (95, 96).
Criteria for categorizing bioavailable iron intake (dietary reference intakes)
Nutritional requirements for iron are markedly affected by the life stage of the individual (age, sex, and in the case of women, pregnancy). Various reference values for iron intake at the population level have been published, taking bioavailability into consideration (27, 47, 97). Several categories that have specific applications are summarized in Text Box 7. Detailed guidelines describing the appropriate application of these parameters are available (47, 97).
Text Box 7.
Dietary Reference Intake categories
Estimated average requirement or average requirement:
• The average daily intake needed to meet the estimated requirements of 50% of the individuals in the population being evaluated.
• The Institute of Medicine values for the United States and Canada assume a bioavailability of 18%.
RDA
• The average daily intake needed to meet the estimated requirements of 97.5% of the individuals in the population being studied.
• The Institute of Medicine values for the United States and Canada assume a bioavailability of 18%.
Adequate intake
• For populations in which the estimated average requirement cannot be specified and is usually the average daily intake based on observed or experimentally determined approximations in apparently healthy individuals who are assumed to have an adequate iron intake, e.g. full term during the first six months of life.
Upper level
• The highest continuous daily iron intake considered unlikely to pose any risk of adverse health effects for almost all individuals in the specified life stage and sex group.
WHO/FAO: recommended nutrient intake (RNI)
• Conceptually equivalent to the RDA, the RNI is the value used by WHO/FAO.
• Expanded stipulated values make adjustments for bioavailability providing separate levels for 15%, 10%, and 5% bioavailability.
Population reference intake
• Conceptually equivalent to the RDA, this is the value used by the European Food Safety Authority (EFSA).
• EFSA uses an absorption value of 16% for men and 18% for women to convert physiological requirements into dietary intakes.
Iron homeostasis
The requirement for iron in a multitude of biological processes emphasizes the importance of an uninterrupted iron supply for cellular turnover. This demand and the need to avoid the potential toxic effects of free iron are met by a rigorously regulated system that controls the rate of iron absorption, maintains a store of readily available iron and recycles iron derived from cells at the end of their life spans.
The cells that constitute the various body organs are being renewed constantly. Iron requirements change dramatically during cell growth and maturation. The overall requirement also changes because of physical growth in children, pathophysiological changes in organ function, and pregnancy. Iron importation must be controlled precisely and continuously to supply physiological needs and avoid potential toxicity. This is achieved by maintaining a readily available, highly regulated iron store. Considerable quantities of iron are rapidly mobilized if there is a sudden increase in physiological requirements. An adult with a 1000-mg store can extract 40 mg daily (98). On the other hand, maximal bioavailability from a high bioavailability Western diet is only 2–4 mg/d. It is convenient to review iron biology relevant to the selection of biomarkers of nutritional iron status by first dealing with systemic and cellular iron homeostasis. This is followed by a description of absorption and finally sections dealing with placental iron uptake and iron transfer into the nervous system.
Iron homeostasis is achieved by the coordinated operation of two systems. Iron supply is regulated by keeping the plasma iron level within a fairly narrow range (systemic iron homeostasis). Individual cells have the ability to adjust the amount of iron they import and to store any excess (cellular iron homeostasis). Almost two-thirds of body iron is found in the erythroid compartment (circulating red blood cells). Alterations in erythropoiesis therefore have a dominant effect on the regulation of iron absorption, transport, and storage (99).
Systemic iron homeostasis
Iron is transported through the systemic circulation and extracellular fluid bound to transferrin. Text Box 8 (22, 98, 100–107) contains a summary of some key elements of systemic iron transport.
Text Box 8.
Key elements of systemic iron transport
Transferrin
Transferrin is the major vehicle for iron delivery to cells and is present in the circulating plasma and extravascular fluid, and has the following characteristics:
• Apotransferrin (transferrin without attached iron) is a single-chain glycoprotein with two lobes, each of which can bind one ferric ion. It is synthesized in the liver and has a half-life of 8 d.
• Iron is tightly bound under physiological conditions in the plasma with an effective stability constant of 1026–1030 M−1. Iron bound to transferrin remains soluble, but is prevented from generating toxic free radicals.
• Binding is markedly affected by pH, being maximal above pH 7.0. Dissociation of the iron occurs if the pH is lowered, becoming virtually complete below pH 4.5 (22, 100, 101).
• Duodenal enterocytes, macrophages in the spleen, liver, and bone marrow, and hepatocytes are the major sources of iron that binds to transferrin.
• About 35% of the iron-binding sites on plasma transferrin are occupied at any one time; it is customarily expressed as percentage of transferrin saturation (TSAT); this corresponds to a plasma or serum iron concentration of ∼115 µg/L.
• A diurnal variation exists in both the plasma iron concentration and the TSAT, with higher levels in the morning in most individuals (100, 102). The pattern is usually reversed in in people who are awake at night and sleep during the day.
• The iron is removed by target cells and the apotransferrin returned to the plasma or extracellular fluid.
• This cycle is completed >10–15 times each day. Thus, the circulating transferrin pool contains only ∼3 mg Fe at any one time, but 10 times as much iron (∼35 mg), most of it destined for developing red blood cells (∼24 mg), moves through this transport system each day in a normal adult (98). However, the potential capacity of the system to respond to an increased demand for iron is remarkable. It is exemplified by patients suffering from thalassemia major with severe ineffective erythropoiesis. Rapid iron recycling may supply sufficient iron to sustain erythroid marrow production levels that are 6–10 times normal (103).
Ferroportin
Ferroportin is expressed on the surfaces of cells and is the only known cellular iron exporter (104, 105), transporting iron across the plasma membrane for its subsequent binding by transferrin in the plasma and extracellular fluid, and has the following traits:
• It is a transmembrane protein that transports ferrous iron.
• It is encoded by the SCL40A1 gene (solute carrier family 40 member 1).
• It binds ferrous iron. Iron transfer to transferrin requires oxidation by copper oxidases, ceruloplasmin in macrophages and hepatocytes, hephaestin in duodenal enterocytes (106), and zyklopen in the placenta (107).
DMT1
• DMT1 represents a large family of orthologous metal ion transporter proteins that are highly conserved from bacteria to humans (22).
• DMT1 can bind a variety of divalent metals, but is primarily an iron transporter in mammals.
Most of the iron entering the plasma pool (∼22 mg/d) is derived from the reprocessing of heme in red blood cells that have reached the end of their 100–120-d life spans by specialized macrophages in the spleen, liver (Kupffer cells), and bone marrow (98, 100, 108). These old red blood cells are phagocytosed and the heme is rapidly catabolized by cytosolic heme oxygenase-1 to yield biliverdin, carbon monoxide, and iron. The iron is either returned to the plasma via ferroportin within a mean transit time of ∼86 min or stored in ferritin (109, 110). When iron status is in the normal range, ∼64% is transferred to transferrin. In iron-deficient individuals, almost all the iron is released immediately, but as much as 80% may be retained and incorporated into ferritin in patients suffering from aplastic anemia when requirements are minimal because erythropoiesis is severely impaired. This intracellular ferritin iron pool is in dynamic equilibrium with the iron circuit. The half-time of residence in the pool is ∼6 d in an individual with a normal iron store. Smaller quantities of iron are exported by other cells, particularly hepatocytes. Absorption from the diet contributes only ∼1 mg/d in an iron-sufficient adult man. During their childbearing years, women absorb a little more, ∼1.5 mg/d, to compensate for menstrual blood losses (100).
Regulation of systemic iron homeostasis
Systemic iron homeostasis depends on the regulation of the rate of iron delivery to circulating transferrin. This is achieved by adjustments to the amount of ferroportin on cell membranes through the action of circulating hepcidin (111). Hepcidin binds to ferroportin, causing the complex to be ubiquinated, internalized and degraded (112, 113). Hepcidin is therefore the central regulator that controls iron absorption, iron recycling, and the size of the iron store in adults and children >6 mo (114–116). While hepcidin is detectable in the newborn infant (117), further studies are necessary to determine whether its regulation is similar to that demonstrated in older children and adults. Text Box 9 (111, 118–120) contains some salient features of hepcidin biology. The following is a brief summary of the key interacting pathways involved in control of hepcidin and iron concentrations.
Text Box 9.
Salient features of hepcidin
• Synthesized primarily in hepatocytes as an 84-amino-acid propeptide that is processed to the active 25-amino-acid peptide in the Golgi apparatus before being secreted into the circulation.
• Subsequent amino-terminal processing produces two smaller peptides with 22 and 20 amino acids that can be measured in urine, but are not detectable in plasma or present at only very low concentrations in healthy humans.
• These smaller peptides appear to lack ferroportin regulatory function.
• Circulating hepcidin is bound to α2-macroglobulin (118).
• Unregulated renal excretion is the major pathway for hepcidin clearance from the circulation, in addition to the quantity removed by receptor-mediated endocytosis primarily in hepatocytes and the macrophages of the liver, spleen, and bone marrow (119, 120).
• Circulating hepcidin concentrations are primarily regulated by the interaction of four interrelated pathways:
-hepatocellular iron stores [bone morphogenic protein (BMP)/sons of mothers against decapentaplegic (SMAD)]
-erythropoietic rate
-circulating iron concentration [human hemochromatosis (HME)/transferrin receptor (TfR)2]
-inflammatory cytokines [the janus kinase signal transducer and activator of transcription 3 (JAK/STAT)] (111)
Hepatocellular iron stores
The regulation by hepatocellular iron is a multifactorial process. Increasing hepatocellular iron stores promote the expression of BMP-6. In the presence of hemojuvelin (HJV), a membrane-bound protein, BMP-6 acts as an autocrine signal by binding to hepatocyte cell surface BMP-6 receptors. In this scenario, HJV acts as a co-receptor augmenting BMP-6 binding (39, 43). Transcription of the gene encoding hepcidin (HAMP) is regulated by the SMAD and signal transducer and activator of transcription 3 (STAT3) pathways (121).
HJV expression is regulated by iron and hypoxia; both act by inducing cleavage of HJV by furin to yield a soluble product (sHJV). sHJV acts as an antagonist to BMP-6-induced hepcidin synthesis. In response to acute iron deprivation, HJV is cleaved by matriptase-2 (type-two transmembrane serine protease, TTSP, also known as TMPRSS6), thereby attenuating the BMP-6 signal, which then leads to decreased hepcidin production. Hypoxia exerts control on hepcidin production by stabilizing liver-specific hypoxia inducible factor 1 (HIF-1) which increases the synthesis of matriptase-2. Hypoxia therefore reduces hepcidin synthesis by decreasing BMP-6 by two mechanisms: HJV antagonism by sHJV, and augmentation of martriptase-2 cleavage (122).
Erythropoiesis
The rate of production of red blood cells (erythropoiesis) exerts an important effect on hepcidin production. Increased erythropoiesis suppresses hepcidin synthesis. The effect usually overrides the control exerted by iron stores. It is therefore an important contributor to the iron overload, which may be severe, in patients suffering from conditions such as thalassemia major (122, 123). A putative regulator called erythroferrone which suppresses hepcidin expression was identified recently by Kautz et al. (124) in a mouse model. It is produced by erythroblasts in response to erythropoietin. Hepcidin suppression appears to require activation of the JAK2-STAT5 signaling pathway and to be independent of the canonical BMP-SMAD pathway (124). In patients who require blood transfusions, iron overload is a consequence of both the additional iron administered parenterally in the transfused blood and excessive absorption from the diet.
Circulating iron
Hepatocytes and developing erythrocytes express transferrin receptor 1 as well as a second transferrin receptor that is encoded by a separate gene, TfR2 (105, 125, 126). The function of both TfR1 and TfR2 are affected by HFE protein. Transferrin carrying iron binds to both TfR1 and TfR2. It displaces the HFE molecule from TfR1. The HFE molecule is then available to interact with TfR2, producing a complex that induces hepcidin transcription by BMP/SMAD signaling. TfR2 functions as a sensor of iron bound to transferrin. TfR2 is expressed in hepatocytes, regulating hepcidin expression, and in erythroid precursors, coordinating erythropoiesis with iron availability (105, 127).
Iron, inflammation, and hepcidin
The reciprocal relationships between nutrition and iron homeostasis, and inflammation have been described recently (128). Text Box 10 (105, 111, 129, 130) provides a brief outline of the specifics of this relationship as it pertains to iron homeostasis regulated by hepcidin.
Text Box 10.
Iron homeostasis, hepcidin, and inflammation
• Hepcidin contributes to innate immunity and is a major component of the anemia of chronic disease (ACD) and inflammation (129).
• Inflammatory cytokines, IL-2 and IL-6, stimulate hepcidin synthesis.
• IL-6 activates the JAK/STAT which stimulates the hepcidin promoter (105, 111).
• Endoplasmic reticulum stress also increases hepcidin expression.
• ACD is characterized by:
-moderate severity
-stability over the course of the illness
-hypoproliferativ, and morphologically normocytic erythrocytes without an increase in the red blood cell distribution width (RDW) (130)
-a modest decrease in erythrocyte survival
-sequestration of iron in the reticuloendothelial cells associated with a low serum iron and low total iron-binding capacity (TIBC), considered to reflect the primary role of increased hepcidin production (111)
Figure 1 is a graphic representation of our current understanding of the factors regulating hepcidin (131). Recent studies have found that yet other signal transduction pathways are involved in the control of hepcidin synthesis, including mammalian target of rapamycin (mTOR) and proliferative rat sarcoma/rapidly accelerated fibrosarcoma mitogen-activated protein kinase (RAS/RAF MAPK) signaling, linking hepcidin regulation to nutrient metabolism, cytokines, growth factors, and cellular proliferation (132).
FIGURE 1.
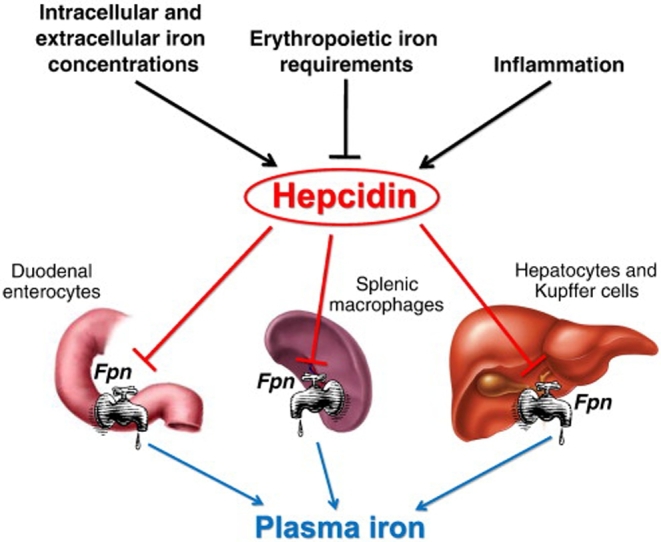
Factors regulating hepcidin. Fpn, ferroportin. Reproduced with permission from reference 131.
It is evident from this brief summary of our current understanding of systemic iron homeostasis that hepcidin is the final common pathway for signals that orchestrate the control of iron absorption and the delivery from stores. It is also clear that hepcidin secretion is regulated by a complex, finely tuned set of processes with multiple compensating factors, which becomes particularly relevant in pregnancy and development (see below).
Cellular iron homeostasis
Individual cells possess an independent complex system for regulating iron import and export, and intracellular iron economy and storage (99, 105, 129, 133). Iron uptake closely matches physiological requirements. The steps for getting iron into and transported within cells are outlined in Text Box 11 (99, 105, 126, 129, 130, 133–136).
Text Box 11.
Iron transport at the cellular level
Getting in
• Transferrin-receptor 1 (TfR1) provides high-affinity uptake of diferric transferrin by cells and is the dominant route of iron uptake in immature erythroid cells and perhaps most other cells (124, 126).
o Low-affinity cellular uptake of diferric transferrin by other mechanisms in nonerythroid tissues has also been described and its relative importance remains under investigation.
o Transferrin binds to TfR1, with diferric transferrin having a higher binding constant (105, 126, 129, 130) than the monoferric form at the pH of extracellular fluid (7.4).
• Apotransferrin does not compete significantly.
• The transferrin-TfR1 complex enters the cell by endocytosis in clathrin-coated vesicles.
• The endosome is acidified by an ATP-dependent proton pump, reducing the pH to ∼5.5.
• The iron is released from transferrin and reduced by 6-transmembrane epithelial antigen of the prostate 3 (STEAP3).
• The ferrous iron is transported across the endosomal membrane into the cell by the DMT1.
• The apotransferrin-TfR1 complex is returned to the plasma membrane where apotransferrin dissociates from TfR1 at the extracellular pH of 7.4 and re-enters the plasma pool.
• Hepatocytes also import iron by TfR1-independent pathways (99).
Once in
• Most of the cytosolic iron is transported across mitochondrial membranes by the transmembrane protein mitoferrin encoded by SLC25A37, for synthesis of heme by incorporation into protoporphyrin IX (134).
• Smaller quantities enter iron-sulfur clusters in both the mitochondrion and the cytosol.
• Iron is also incorporated into nonheme cytosolic enzymes or stored as ferritin.
• Emerging evidence indicates that there are complex mechanisms that facilitate the distribution of iron within the cell (135, 136).
• Some experimental evidence indicates that iron is transferred from endosomes to the mitochondria by direct contact rather than transfer through the cytoplasmic compartment (133).
Regulation of cellular iron homeostasis
Erythroid precursors have been the focus of much research on cellular iron uptake. However, it is assumed that all cells employ similar regulatory mechanisms (137), although erythroid cells express additional controlling factors that are related specifically to hemoglobin synthesis (137). Iron acquisition is matched to cellular requirements by control of cell surface TfR1 expression by the iron regulatory protein (IRP)–iron responsive element (IRE) system. IRP 1 and 2 sense the cell's immediate iron requirements. When there is a need for additional iron they become bound to stem loop structures (IREs) in the 3′ untranslated region of TfR mRNA, thereby preventing constitutive mRNA degradation and increasing the quantity of TfR expressed on the cell surface. More iron is imported. At the same time ferritin synthesis is suppressed by the binding of the IRPs to IREs on the 5′ untranslated region of ferritin mRNA. Less iron is incorporated into the cellular ferritin store. When iron in the cell is sufficient, the process is reversed with downregulation of TfR expression and increased storage in ferritin. Additional details on iron homeostasis in erythroid cells are provided in Text Box 12 (138–141).
Text Box 12.
Iron homeostasis in erythroid cells: iron supply and hemoglobin synthesis
• The precise matching of iron supply to erythropoietic requirements is a critical element of red blood cell maturation presumably because of the potential for iron-induced oxidative toxicity. Protoporphyrin IX synthesis is coordinated with iron availability by the IRPs.
• The mRNA for δ-aminolevulinic acid synthetase 2, the initial and rate-limiting enzyme in the heme synthetic pathway, has an IRE in the 5′ region of its mRNA; IRP binding inhibits heme synthesis.
• Globin synthesis is coordinated with heme synthesis through translational control by the heme-regulated transitional inhibitor.
• Additional mechanisms [e.g., effect of TfR2 on erythropoiesis (138), aconitase-associated control of erythropoieisis (139)] may have important roles.
• Reduced responsiveness of erythroid progenitors to erythropoietin in iron deficiency (139).
• Developing erythroblasts synthesize ferroportin (FPN) and can export iron. There are two FPN transcripts (FPN1A and FPN1B).
o Unlike FPN1A, the transcript that is expressed by all cells, FPN1B lacks an IRE in its 5′ untranslated region.
o FPN derived from both transcripts are responsive to hepcidin and systemic iron requirements.
o It has been hypothesized that FPN1B expression enhances sensing of systemic iron status and facilitation of restricted erythropoiesis in response to systemic iron deficiency (140, 141). FPN1A predominates once cells begin to produce hemoglobin.
• The developing red blood cell's requirements are prioritized. Ferroportin expression is suppressed and an adequate iron supply for heme synthesis is ensured (140).
Specific role of macrophages
Dedicated macrophages in the spleen, liver (Kupffer cells), and bone marrow have a specialized role in the body's internal iron economy (142). After a life span of ∼100–120 d in the circulation, red blood cells are removed from the circulation by these macrophages. This process involves the following two steps: 1) entry into an erythrophagolysosome where the red blood cell membrane is lysed; and 2) catabolism of the heme by an enzymatic complex containing NADPH-cytochrome c reductase, heme oxygenase 1, and biliverdin reductase to yield iron, carbon monoxide, and bilirubin.
Depending on the body's immediate needs, iron is transported across the plasma membrane by FPN, oxidized from the ferrous to the ferric state by ceruloplasmin, and then bound to apotransferrin in the plasma, or incorporated into ferritin in the cytosol for temporary storage.
Some erythrocytes are damaged in the circulation (intravascular hemolysis) even in healthy individuals. This intravascular hemolysis may be markedly accelerated in hemolytic anemias and diseases that cause ineffective erythropoiesis. Hemoglobin released into the plasma is rapidly bound to haptoglobin, a glycoprotein synthesized in the liver to form a complex that is too large to be filtered by the kidneys. The iron is conserved. The hemoglobin-haptoglobin complex binds to CD163 receptors on macrophages and hepatocytes, and is then endocytosed and degraded in lysosomes, releasing heme that is catabolized as described above. Heme may be separated from globin in the plasma. If this occurs, it is bound to another plasma protein, hemopexin. The complex is again too large to be filtered by the kidney. It is removed by macrophages in the liver and spleen expressing the CD91 receptor and catabolized (143).
Iron storage and recycling by hepatocytes
In the liver, hepatocytes have a central role in controlling the body's iron economy and are the main site for iron storage. They acquire iron from both the systemic and the portal (newly absorbed iron) circulations. Moreover they have the capacity to obtain iron via both TfR1- and TfR1-independent pathways (99). In addition, iron derived from hemoglobin-haptoglobin, heme-hemopexin, ferritin, lactoferrin, or non-transferrin-bound iron is recycled through hepatocytes. The liver is also the primary source of hepcidin.
Iron absorption
Body iron is controlled rigorously by the regulation of absorption in the duodenum and proximal jejunum. Iron excretion is minimal and unregulated in human beings. It results from loss from the gastrointestinal tract (putatively due to biliary iron excretion, cellular desquamation, and perhaps microscopic bleeding), in the urine and by the desquamation of skin cells (144). In women additional losses occur as a result of menstruation and the demands of pregnancy. The usual North American diet contains ∼7 mg Fe/1000 kcal (98). A healthy man with adequate iron stores utilizes ∼1 mg/d, but this can be increased to ∼2–4 mg/d in iron deficiency and reduced to 0.5 mg/d if iron stores are high. Much more iron can be utilized if supplemental iron is consumed (98). The rapidity with which these adjustments occur is remarkable. Absorption of a second dose of iron is blocked within 2–4 h of an initial dose (145). It is convenient to describe the process of absorption by considering four interrelated stages, which are described in Text Box 13 (18, 32, 34, 50, 104, 146–153).
Text Box 13.
Stages of iron absorption
1. The luminal phase
• Most of the dietary iron is present in one of three forms:
-Heme derived from hemoglobin and myoglobin in meat and fish
-Soluble nonheme iron that is derived from all the other iron in food and behaves as a common pool for absorption
-Iron that is insoluble in gastric juice and therefore not absorbed (32, 34)
• Heme iron is well absorbed and enters mucosal cells as the intact heme moiety.
• The absorption of all other forms of dietary iron present in both meat and plant foods depends on their solubilization in gastric juice and reduction from the ferric to the ferrous state.
• Absorption is markedly affected by meal composition. Unidentified components of meat (thought to be partially digested peptides), ascorbic acid, and to some extent other organic acids promote absorption, whereas phytates, certain polyphenols, and some plant and milk proteins are inhibitory.
• The mechanism of absorption for two types of iron, ferritin and nanoparticulate iron, remains uncertain.
-Some investigators have suggested that ferritin iron, an important iron source in both meat and vegetables, crosses the brush border membrane as an intact and is highly bioavailable molecule (146).
-Other studies suggest that iron is released from ferritin in the stomach to join the nonheme common pool (40).
• Food fortification with iron nanoparticles could enter the mucosal cell by mechanisms not available to the common nonheme pool iron (147).
2. Enterocyte uptake
• All forms of iron are predominantly absorbed in the duodenum and upper jejunum.
• Heme iron crosses the brush-border membrane as the intact iron porphyrin, although the transporter has not yet been identified.
• Almost all of the nonheme iron is taken up by the DMT1, which is a transmembrane protein encoded by the Slc11a2 gene that mediates proton-coupled ferrous iron uptake (148).
• Because most of the iron in the diet is ferric, reduction to the ferrous form is required for binding to DMT1.
-Dietary components, such as ascorbic acid, promote absorption in part by this mechanism.
-In addition, a brush-border membrane ferrireductase, duodenal cytochrome b (DCYTB), may play some role in facilitating reduction by electron transfer from intracellular ascorbate (149). DCYTB is markedly upregulated in iron deficiency and hypoxia and is thought to play a major role under these conditions.
• Observations in both rodents and humans indicate that DMT1 transports most of the nonheme iron that enters mucosal cells (148, 150). However, other systems may exist, because inactivation of intestine-specific Slc11a2 causes severe iron deficiency, but is not lethal.
3. Storage within the enterocyte and transport to the basolateral membrane
• The processes responsible for the transport of intracellular enterocyte iron are unknown.
• However, it is evident that all absorbed iron enters a common pathway after the iron in heme is released by heme oxygenase (151).
• The iron is then distributed as follows:
-to meet the requirements of intracellular compartments, such as mitochondria, transferred to the basolateral membrane for absorption into the portal circulation; or
-to be stored within the enterocyte as ferritin when the body's demands are low. Most of the iron ferritin is lost due to exfoliation of enterocytes (18).
4. Efflux across the basolateral membrane and binding to transferrin
• Ferrous iron is transported out of the enterocyte across the basolateral membrane by FPN1 (encoded by the Slc40a1 gene). Like DMT1, intestine-specific inactivation of this gene in mice causes severe iron deficiency but is not lethal (104).
• Binding to transferrin in the interstitial fluid requires that the iron be in the ferric form. Oxidation is mediated largely by hephaestin, a membrane-bound multicopper ferroxidase (152). Ceruloplasmin in the interstitial fluid also appears to play a role in this process (153).
Systemic regulators of iron absorption
In the absence of infection or inflammation, iron absorption is stimulated when body stores are low or erythropoiesis is increased. Finch (123) coined the terms “store regulator” and “erythroid regulator” to describe these two processes. The “erythroid regulator” has the dominant role and accounts for the nontransfusional iron overload that occurs in such conditions as thalassemia major in which absorption may continue to be enhanced despite a progressive increase in the size of the iron store. Both mechanisms are now known to be mediated by circulating hepcidin. The relationship between iron stores, hepcidin concentrations, and physiologic control of iron status by the “store regulator” are described elsewhere. Hepcidin production is suppressed by increasing storage iron. Hepcidin binds to FPN1 on the basolateral surface of duodenal enterocytes, leading to its internalization and degradation (113).
A new hormone named erythroferrone (ERFE) has been identified that increases iron absorption by suppressing hepcidin during stress erythropoiesis in mice (124). ERFE is secreted by human erythroblasts in response to erythropoietic stimulation by erythropoietin and appears to be a component of the “erythroid regulator.”
The overriding importance of the hepcidin/FPN1 axis in preventing excessive iron absorption is illustrated by two sets of observations. Virtually all inherited primary iron overload disorders result from mutations that affect hepcidin or ferroportin (154). The recent discovery of ERFE suggests that iron-loading anemias are also the result of hepcidin suppression in the face of iron overload. Hepcidin has also been shown to reduce enterocyte iron uptake by inhibiting DMT1 expression (155) through ubiquitin-dependent proteasome degradation of DMT1 in Caco-2 cells (156). In experimental animal models, iron absorption has been shown to be increased in response to oxygen deprivation (157). Hypoxia inducible factor 2α (HIF2α) has been identified as a key transcription factor in this response (158, 159). DMT1, Dcytb, and FPN1 have hypoxia response elements within their promoters that activate transcription in response to hypoxia (158–160).
Cellular regulators of iron absorption
Enterocytes also have the complex cellular machinery found in erythroid precursors that control their internal iron economy. These mechanisms ensure the safe handling of iron necessary for cellular metabolism and regulate the transfer of iron to the systemic circulation, and in particular afford protection against sudden iron surpluses in the duodenal lumen. This protection may occur by downregulation of DMT1 and incorporation of iron that crosses the brush border membrane into ferritin (161). The control of iron import and storage by the IRE/IRP system is described in detail elsewhere in this review.
The regulatory mechanisms that control iron absorption, storage, and export described for erythroid cells,are similar in enterocytes but with the additional involvement of some specific proteins, as follows: 1) the 3′ IRE of DMT1 mRNA stabilizes the mRNA, leading to increased protein levels in iron deficiency (162); 2) the IRE in the 5′ region of FPN 1 mRNA binds IRPs, thereby inhibiting protein translation (145, 161); and 3) DcytB and hephaestin mRNAs do not appear to contain IREs.
Control mechanisms are, however, even more complicated as two splice variants of both the FPN 1 and DMT1 mRNAs are present, one with and one without an IRE. Furthermore, transcriptional regulation is also important. For a more detailed description see references (145, 163).
There is debate regarding how well regulated iron absorption is in the neonate and infant. Animals models suggest that DMT-1 and FPN-1 are developmentally regulated (164) with achievement of full expression at the time of weaning (in rodents). Whether this is present in humans is unknown. Several stable isotope studies of iron absorption in term and preterm infants have shown a surprisingly consistent 35–40% rate, a finding that would suggest relatively limited active regulation given the wide range of iron status found in this population (114–116).
Placental iron transport
The placenta is a complex organ, which has many roles to play, including nutrient transfer and waste disposal, and endocrine functions that are integral to its role as a regulator of fetal growth and differentiation. The study of the placenta is complicated by the available resources that can be used. Most studies in humans utilize placentas from full-term infants. The use of animal models presents a range of challenges, from species differences in anatomy to differences in iron transport and metabolism. These differences have been reviewed recently (165). Text Box 14 (107, 148, 166–172) contains a summary of the current understanding of placental iron transport and potential adaptations to iron deficiency.
Text Box 14.
Mechanisms of placental iron transport
• Diferric transferrin binds to the TfR on the apical surface of the placental syncytiotrophoblast membrane (166). This is a high-affinity binding process, with the Kd for diferric transferrin being ∼10−9 M−1 (167).
• Following binding, the complex is internalized and is incorporated into coated vesicles.
• The vesicles are acidified (168) and the affinity of diferric transferrin for iron decreases.
• Although in most cells, iron is released and exits the endosome through DMT1, in the placenta, DMT1 may not be essential for this purpose (148).
• Recent studies suggest that other metal transporters, such as ZIP8, may fulfill this function (169).
• Following release into the cytoplasm of the syncytiotrophoblast, iron is transferred to the basolateral side. How this is accomplished is not known. It is unlikely to be as a freely diffusible moiety, since ferrous (III) iron is very insoluble at neutral pH, and ferric (II) iron is biologically very active. Specific iron chaperones, e.g., PCBP1 which is reportedly highly expressed in the placenta, have been suggested.
• At the basolateral membrane of the syncytium, iron effluxes through ferroportin in a similar fashion to that described for the duodenal cell. It moves through the membrane as Fe(II) and is oxidized by a copper ferroxidase termed zyklopen (107).
• It then binds to fetal transferrin, and is carried to the fetal liver and to the erythropoietic tissues.
• Irreversible changes in brain occur following prenatal iron deficiency (170).
Adaptation to iron deficiency
• An increase in TfR expression at the apical surface of the placental syncytium membrane results in increased iron uptake.
-Increases have been observed in DMT1 and zyklopen expression, but no change in ferroportin mRNA levels. The mechanism of regulation appears to be governed by the fetus. Fetal liver hepcidin correlates strongly with iron levels, and also with TfR levels in the placenta.
• In contrast, maternal liver iron and hepcidin do not correlate (171), suggesting that another signaling system may be operating between the placenta and the maternal liver.
• Recent suggestions for the signaling system have included GDF15 and soluble hemojuvelin (172), but much work remains to be done to demonstrate this.
Fetal iron
Fetal iron requirements change throughout pregnancy. In the early stages of pregnancy, iron is needed to maintain growth and differentiation of the fetus, so that although the amounts of iron may not be very high, iron deficiency can result in significant changes. It has been shown that the mid-pregnancy period is particularly important (173). Using cultured embryos, iron deficiency resulted in delayed growth and differentiation, and an increase in malformations. Providing iron over the 10.5- to 12.5-d period in rats reversed these changes (term is ∼22.5 d in the rat). Extending the work to in vivo studies, it was demonstrated that restoring iron status in the mother following iron deficiency in the early part of pregnancy only partly reversed the developmental effects (174).
During early development, the fetus is entirely dependent on maternal supplies to meet its iron requirements. In order to maintain iron status in the fetus, at least to the maximum extent possible, the placenta has developed a series of adaptive mechanisms, regulated partly by the same system as operates in other cell types. In humans, most of the iron delivered to the fetus comes from maternal transferrin (175), though recent data suggest that heme may also be a source (176). Because of its importance in the delivery of iron during pregnancy, the placenta has been studied fairly extensively, so that the transfer has been reasonably well elucidated.
The fetus will accumulate iron to the detriment of the mother (176, 177). For example, in early studies, reducing iron in the maternal diet of rats resulted in a significantly lower maternal iron status as reflected by lower hemoglobin and hematocrit (Hct) compared with control animals receiving iron-adequate diets. In contrast, levels in the fetal liver dropped only by ∼30% (174). Interestingly, this effect is strain specific, and other breeds of rat are less capable of maintaining iron levels in the face of nutritional stress (178). Other studies have shown different windows of sensitivity for other physiological parameters. For example, postnatal iron deficiency has been shown to result in permanent changes in hippocampal function in mice (179, 180), although restoration at the end of pregnancy can reverse the changes.
Iron homeostasis in the developing brain and nervous system
Iron is essential for normal brain development and function (181). The core functions of iron in the brain are listed in Text Box 15 (182–188). Disruption of these processes by iron deficiency lead to predictable and consistent structural, electrophysiological, and behavioral abnormalities both during the period of iron deficiency and long after iron repletion (170, 188–191).
Text Box 15.
Roles of Iron in the Brain
• Energy metabolism (182)
• Neuronal and oligodendroglial cell migration (183)
• Myelination (184)
• Monoamineregic and glutamatergic neurotransmitter metabolism (185, 186)
• Regulation of genes related to myelin, synaptic plasticity, and growth factors (187, 188)
Brain iron transport
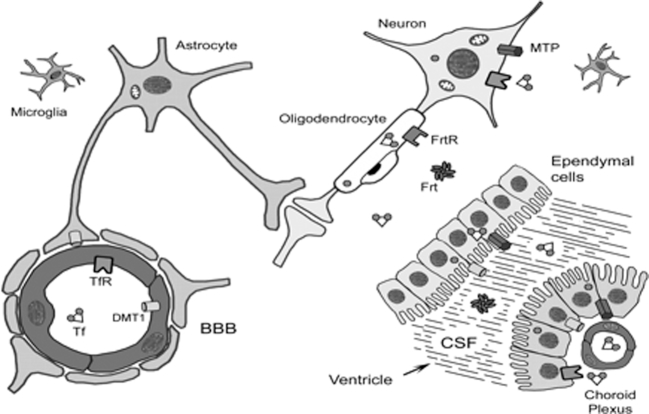
The transport of iron across the blood-brain barrier is not completely understood. Figure 2 shows an overview of iron transport in the brain (192).
FIGURE 2.
Diagrammatic portrayal of the BBB and the iron transport proteins believed to play a role in iron movement into the brain. BBB, blood-brain barrier; CSF, cerebrospinal fluid; DMT1, divalent metal transporter 1; Frt, ferritin; FrtR, ferritin receptor; MTP, metal transport protein or ferroportin; Tf, transferrin; TfR, transferrin receptor. Reproduced with permission from reference 192.
Some of the details of our current understanding of iron transport in the brain are outlined in Text Box 16 (193–196).
Text Box 16.
Evolving view of brain iron transport
Early view
• Early studies suggested that the transferrin-iron complex was transcytosed intact across the vascular endothelium (193).
• By this mechanism, ferric iron bound to transferrin would be available to bind TfR-1 located on the neuronal membrane with uptake via the Tfr-1/DMT-1 mechanism present in red blood cells (and any other “terminal” cell).
• In this mechanism, one or two molecules of ferric (Fe3+) iron are bound to serum transferrin.
• This mono- or diferric saturated transferrin binds to TfR-1, a transmembrane homodimer, whose binding affinity is greater for saturated holo(diferric)transferrin than for unsaturated apotransferrin.
Evolving view
• Ferric iron is reduced and then transferred via the classical TfR-DMT-1 mechanism into the endothelial cell and then extruded across the basal membrane via a ferroportin and the multicopper oxidase mechanism to transferrin synthesized in the brain (194, 195).
• At the basal (brain-facing) surface, ferrous iron is transported to the brain via ferroportin.
• A multicopper oxidase (e.g., ceruloplasmin or hephaestin) is needed to convert ferrous iron back to its ferric state so that endogenous brain transferrin will bind it and deliver it to TfR-1 located on the neuronal membrane for incorporation via the TfR-1-DMT-1 uptake mechanism described above.
• The possibility that oligodendrocytes do not obtain their iron via a transferrin mediated mechanism, but rather by via ferritin binding of the Tim-2 receptor, has been proposed (194, 196).
The mechanisms of iron transport to the central nervous system remain areas of intensive investigation to better understand the etiologies of deficiency as well as overload states (197). Even as our understanding of neuronal iron transport evolves, it is important to note that the uptake of iron via TfR-1-DMT-1 is developmentally controlled during the late fetal/early neonatal time period. For example, in the rat, hippocampal TfR-1 expression is minimal at postnatal day 5 (human developmental equivalent of 32 wk of gestation) and increases to adult levels by postnatal day 15 (human equivalent of 2–4 mo postnatal age) (198). This increased expression coincides with a burst of hippocampal metabolic activity characterized by increased glucose uptake, neuronal differentiation (199), maturation of electrophysiology (190), and upregulation of growth factors (200). The finding of increased iron uptake during this time period underscores the importance of iron in supporting neuronal metabolism and mitochrondrial integrity, most likely through its direct role in cytochromes (201).
Iron needs during critical periods of brain development
The brain consists of multiple regions that have different developmental trajectories (202). The risk to any brain region or nutrient-dependent process will be a function of the timing of the nutritional insult (203, 204). The following will summarize what is known about iron's role in brain development from the perspective of 1) brain monoamine metabolism, 2) brain energy metabolism, and 3) myelin formation.
Iron and monoamine metabolism
In humans, the monoamine neurotransmitter system begins its development in mid-gestation and continues to have a period of rapid development until ∼3 y of age. The multiple feedback loops in the dopamine and serotonin systems make it difficult to determine primary compared with secondary effects of iron. A summary of the impact of iron on monoamine metabolism is found in Text Box 17 (170, 205, 206). The observations outlined point to a critical period during which iron is necessary for normal brain monoamine metabolism development that occurs at the developmental equivalent of late gestation in the human. If correct, the information strongly supports the notion that maintenance of maternal iron status is key to offspring brain health and that post-hoc treatment of the iron-deficient newborn or child will not result in normal development of this neurotransmitter system, which is important for reward and mood/affect.
Text Box 17.
Iron, monoamine metabolism, and brain development
• The most direct effect of iron is likely to be on the synthesis of all three monoamine neurotransmitters, i.e., dopamine, serotonin and norepinephrine.
• Iron acts through alteration of the iron-containing enzymes tyrosine hydroxylase and tryptophan hydroxylase (205).
• Gestational and lactational iron deficiency alters aspects of dopamine and serotonin metabolism acutely during the period of iron deficieny and long term after iron repletion (170).
• Specifically, alterations are seen in the following:
-The neurotransmitters themselves
-Neuronal postsynaptic neurotransmitter receptors
-Neuronal presynaptic neurotransmitter reuptake mechanisms (170)
• Timing is important in terms of preventing the long-term sequelae.
Iron and brain energy metabolism
Iron is found at the active enzymatic core of cytochromes, which mediate electron transport and oxidative phosphorylation. Energy availability is essential for normal neuronal dendritic growth and differentiation, as evidenced by increases in glucose metabolism and iron uptake by brain regions during their growth spurt. A summary of the evidence for iron's role in brain energy metabolism is provided in Text Box 18 (179, 182, 185, 189–191, 200, 207–210).
Text Box 18.
Iron and brain energy metabolism
• Maintenance of brain energy status is affected by iron deficiency via reduced cytochrome c concentrations (182) and reduced cytochrome c oxidase activity (207), a reliable marker of neuronal activity (208).
• Gestational/lactational iron deficiency anemia as well as nonanemic neuronal iron deficiency alters phosphorus utilization for ATP and lactate metabolism in the developing hippocampus (185, 209), suggesting significant effects on neuronal energy metabolic balance.
• Disruption of energy metabolism in iron deficiency results in abnormal dendritic arborization, synapse formation, and expression of synaptic plasticity and growth factor genes (185, 189, 200, 209).
• Form follows function in that these abnormal dendritic arbors (in the hippocampus) have reduced electrophysiologic function (190) and the animals exhibit altered learning and memory behavior (179, 191, 209, 210).
As with the role of iron in monoamine development, a critical period exists when iron is essential for hippocampal neuronal development (postnatal day 10–25), after which provision of iron does not completely prevent long-term gene, metabolism, or structural and behavioral abnormalities (179). The hippocampus has been extensively studied from the perspective of neuronal metabolism as a way of understanding the role of iron sufficiency in learning and memory behavior. However, it is likely that other areas of the brain have different developmental trajectories and critical periods.
Iron and myelination
Myelination in the human begins in the late fetal period and extends at a rapid pace through the first 2–3 y (202). Myelin is the fatty sheath found on nerves that increases conduction speed and efficiency, properties that are in turn related to speed of processing for behavioral tasks. Hypomyelination increases the latency between peaks of electrophysiological activity in the auditory and visual systems. The role of iron can be summarized as follows (211): 1) iron is present in delta 9-desaturase as well as fatty acid elongases, important enzymes in the synthesis of fatty acids found in the myelin sheath; and 2) oligodendrocytes are highly active metabolic cells when they are synthesizing myelin and thus are dependent on adequate cytochrome activity.
In the rat model, iron-deficiency anemia (IDA) during gestation/lactation results in hypomyelination with significant alterations in the fatty acid profile of the myelin and reductions in myelin basic protein expression (187, 211).
Disorders of iron balance
Iron deficiency
The causes of iron deficiency are summarized in Text Box 19. An inadequate intake of bioavailable iron (nutritional iron deficiency) is the most common cause of iron deficiency and the most prevalent disorder of iron balance worldwide. It occurs primarily in individuals with increased requirements, especially in early childhood, the adolescent growth spurt, the childbearing years, and pregnancy. Pathological blood or hemoglobin loss (which may be undetected unless appropriate laboratory tests are carried out) and malabsorption due to diseases of the stomach and proximal small bowel account for most of the other cases (100). Genetic mutations of the TMPRSS6 gene are rare causes, giving rise to the clinical entity called iron-refractory iron-deficiency anemia (IRIDA), which is reviewed in reference (212). At the individual level, especially in populations where the prevalence of nutritional iron deficiency is low, establishing the cause is essential (e.g., occult bleeding in the gastrointestinal tract). In populations with a high prevalence of iron deficiency, evaluation of bioavailable iron intake is most important, although it may be necessary to assess the risk of exposure to parasitic helminths that cause bleeding such as hookworm and schistosomiasis.
Text Box 19.
Causes of iron deficiency
• Inadequate intake due to:
-Habitual/discretionary inadequate intake of bioavailable iron
-Nutritional iron “insecurity,” i.e., inadequate access or availability of bioavailable dietary iron (e.g., poor dietary diversity)
• Iron malabsorption due to:
-Celiac disease (hluten enteropathy)
-Chronic Helicobacter pylori gastritis
-Autoimmune atrophic gastritis
-Some surgical procedures involving the stomach or the upper small bowel
-Iron-refractory iron-deficiency anemia (IRIDA)
• Accelerated physiological requirements.
• Increased requirements for growth:
-Premature infants
-Early childhood
-Adolescent growth spurt
• Menstruation.
• Pregnancy.
• Pathological blood loss:
-Bleeding from the gastrointestinal and genitourinary tracts
-Parasitic infections notable helminth infections such as hookworm and schistosomiasis
-Menorrhagia
-Intravascular hemolysis
• Blood donation.
The primary focus of the following sections will be nutritional iron deficiency, which occurs when an individual consumes insufficient bioavailable iron on a daily basis over an extended period to meet requirements, despite maximal upregulation of the physiological mechanisms for accelerating absorption. Iron deficiency is the only disorder of iron balance in which nutrition has the primary role. Systemic iron overload occurs as a result of pathological disorders that impair the regulation of iron absorption, parenteral iron administration, or repeated blood transfusion. Conditions that lead to organ-specific iron accumulation are beyond the scope of this review.
Anemia
Anemia is the most evident consequence of iron deficiency. It is associated with significant morbidity and has been the focus for evaluating iron status. Pallor is often noted as a primary sign of anemia and patients may complain of symptoms that result from the diminished oxygen-carrying capacity of the blood, including weakness, fatigue, decreased physical work capacity, shortness of breath, and palpitations (100). However, the body's compensatory mechanisms for improving oxygen delivery to tissues are very effective if the onset of anemia is gradual and symptoms may not be noticeable until the hemoglobin is <80 g/L (213). Nevertheless, anemia is associated with significant morbidity. Iron-deficiency anemia is correlated with an increased risk for preterm delivery, low birth weight, and maternal and child mortality (214). Mental, motor, and emotional development is adversely affected in children, and IDA may be a contributory factor to heart failure (215).
The risk is particularly high in low-resource settings (Table 2) (216). However, iron deficiency remains a public health challenge in parts of the population even in the United States. The data in Table 3 are based on NHANES (2003–2006) (217). The global prevalence of anemia in 2010 was estimated to be 32.9%. Iron deficiency was the most prevalent cause (215).
TABLE 2.
Prevalence of anemia in infants and young children (birth to 5 y of age) by region, 20051
| Global region | Prevalence (%) |
|---|---|
| Africa | 64.6 |
| Asia | 47.7 |
| Europe | 16.7 |
| Latin America | 39.5 |
| North America | 3.4 |
| Oceania | 28.0 |
1Adapted with permission from reference 216.
TABLE 3.
Percentage of persons with iron deficiency (based on the body iron model) from NHANES (2003-06) (217)
| Children (age 1–2 y) | 14% |
| Children (age 3–5 y) | 4% |
| Females (age 12–19 y) | 9% |
| Females (age 20–49 y) | 9% |
The historically accepted model of nutritional iron deficiency (218) proposed by Bothwell et al. (100) is based on the severity of iron deficiency categorized by its impact on the erythron. It emphasizes the need to recognize the early stages of iron deficiency—before the appearance of frank anemia. In the event of a sudden increase in iron requirements—e.g., as the result of pathological blood loss—iron in stores can be mobilized within a few days. On the other hand, if there is little storage iron available it may take months to repair the deficit by increasing absorption from a nonsupplemented diet. Three stages in the evolution of uncomplicated nutritional iron deficiency are described as follows (100):
— Storage iron depletion: iron stores are exhausted, but there are no erythropoietic consequences, absent stainable bone marrow iron, low serum ferritin (SF); normal values for TSAT, red blood cell protoporphyrin, serum transferrin receptor (sTfR), and hemoglobin.
— Iron-deficient erythropoiesis: there is evidence of inadequacy in the iron supply for erythropoiesis, but no decrease in the hemoglobin concentration sufficient to be detected by the standards used to differentiate normal from anemic states, absent stainable bone marrow iron, low SF, low TSAT, increased red blood cell protoporphyrin and sTfR, and normal hemoglobin
— Iron-deficiency anemia: measurable functional iron deficiency (reduced circulating red blood cell mass), absent stainable bone marrow iron, low SF, low TSAT, increased red blood cell protoporphyrin and sTfR, and low hemoglobin.
This model has played a critical role in developing our approach to improved iron nutrition and the prevention of iron-deficiency anemia. It appropriately focuses on anemia as the overriding functional consequence of progressive iron deficiency. The currently available biomarkers have been selected to characterize the three stages in the evolution of uncomplicated nutritional iron-deficiency anemia. They provide a means of establishing an accurate assessment of the severity of iron deficiency at both the individual and population level. However, they may fail to identify other potentially important functional consequences of iron deficiency that may or may not be associated with anemia. Such nonspecific symptoms as mucocutaneous clinical findings such as koilonychia, angular stomatitis, and glossitis, as well as sideropenic dysphagia, have been described in the past (100). However, these symptoms are rarely encountered now and may well have resulted from the combined effect of iron deficiency and other nutritional or environmental factors. Pica is still encountered in some populations and iron deficiency may be a contributing factor in patients suffering from restless legs syndrome (219, 220).
The following sections are reviews of five other high-priority functional outcomes including: 1) pregnancy outcome; 2) fetal and infant neurologic development; 3) exercise capacity; 4) thyroid function; and 5) morbidity related to infectious disorders.
The effect of iron deficiency on the maturation of the central nervous system has been considered in the greatest detail because of the potential long-term consequences of iron deprivation during this critical period of the life cycle.
Effects of iron deficiency on pregnancy outcome
The evaluation of maternal iron status during pregnancy relies on the same biomarkers described in the preceding section, but their interpretation requires consideration of the evolving changes in the amount and distribution of body iron during the course of gestation. In this section, the underlying changes in iron homeostasis during pregnancy will be summarized for each trimester and the corresponding effects on the principal biomarkers of iron status described.
During pregnancy, iron is needed for the following: 1) the growth of the fetus and placenta; 2) the increase in the maternal red blood cell mass; 3) replenishing blood losses at parturition; and 4) restoring basal iron losses.
The total iron requirement during pregnancy is of the order of 1000 mg, with the exact amounts depending, in part, on body weight, fetal and placental size, and the extent of the expansion of red blood cell mass (221, 222).
The mother may remain iron replete if the iron requirement can be met from the combination of the stores present at conception and the amounts of dietary iron that can be absorbed during pregnancy. Iron requirements in excess of these amounts can lead first to maternal iron depletion, then to iron-deficient erythropoiesis and finally to IDA. To avoid the development of iron deficiency, a storage iron reserve of some 300–500 mg is likely to be needed, in conjunction with a diet with abundant bioavailable iron (223, 224). After delivery, the iron that was used in the expansion of the red blood cell mass, ∼400–500 mg, gradually becomes available for erythropoiesis or for replenishment of stores.
Most of the evidence for the relation of biomarkers to body iron status is indirect; bone marrow examination is seldom used to assess iron status during pregnancy. However, one study examined marrow iron stores in Swedish women with uncomplicated pregnancies who were not anemic during their first trimester. At about 12 wk of gestation, marrow iron stores were present in almost 90% of the women. By ∼35 wk of pregnancy, marrow iron stores were absent or present in only trace amounts in >60% of the women who had been given 200 mg Fe as ferrous sulfate daily and in all the women who had been given placebo (225).
The patterns of changes in the principal biomarkers of iron status during pregnancy and postpartum are shown graphically in Figure 3, derived from a study of apparently healthy Dutch women (226). It should be noted that because pregnancy is an inflammatory state (227–229), the potential confounding effects of inflammation on the interpretation of these biomarkers must be considered. In addition, the influence of obesity and weight gain during pregnancy on iron homeostasis and biomarkers of iron status has not been well characterized (230, 231). Generally, studies in initially iron-replete women given adequate iron supplementation during pregnancy have been used to assess the effects of pregnancy on biomarkers in the absence of iron deficiency and to estimate iron requirements (223, 225, 232).
FIGURE 3.
Biomarkers of iron status during pregnancy and 6 wk postpartum in 31 apparently healthy Dutch women. The participants in this study were >18 y old with an expected normal pregnancy based on a normal hematologic blood count, renal function, and liver enzymes at their first visit. Iron supplements were not routinely prescribed and were reserved for those with hemoglobin concentrations <105 g/L and reduced mean cell volume (<80 fL) in accordance with national guidelines. Data are shown as medians with the IQR. Asterisks represent the significance in relation to first trimester values; analyzed with linear mixed models, *P < 0.05, **P < 0.001, ***P < 0.000. sTfR, soluble transferrin receptor; sTfR-index, sTfR divided by log ferritin; TIBC, total iron-binding capacity. Reproduced with permission from reference 226.
First trimester
A number of changes occur that affect iron physiology, absorption, and status after conception and during the first trimester. Text Box 20 (223, 233) contains a list of some the key events. Using data derived from the Dutch study described above (226), median concentrations of the principal biomarkers of iron status in healthy women during the first trimester of pregnancy are shown in Figure 3. The following highlights what is known about specific biomarkers.
Text Box 20.
Basics of iron biology in pregnancy: first trimester
• After conception, the arrest of menstruation decreases iron losses to a basal level for much of the first trimester (Figure 1).
• The iron requirement falls to ∼0.8 mg/d (223) and iron absorption may decline.
• With a highly bioavailable diet, total (heme and nonheme) iron absorption has been estimated at only ∼0.4 mg/d (233).
• Initially, a reduction in erythropoiesis may slightly reduce the circulating red blood cell mass and increase iron stores (223).
• In the latter portion of the first trimester, fetal and placental growth accelerate and expansion of the plasma volume begins.
Hemoglobin
— In the absence of iron deficiency, the circulating hemoglobin concentration declines by ∼10 g/L during the first trimester (234).
— The threshold for anemia decreases from 120 to 110 g/L in guidelines from the CDC (235) and WHO (236).
— Modifications to the threshold are needed to account for the effects of altitude and smoking (236).
— Other corrective adjustments (e.g., adjusting cut-offs, different intervention strategies) have been proposed (237).
— The optimal hemoglobin concentrations for functional outcomes have not been determined (221, 222, 238).
Serum iron, ferritin, sTfR, and iron stores
— During the first trimester, with expansion of the plasma volume, the serum iron begins to fall whereas the serum transferrin concentration, shown as TIBC in Figure 3, begins a steady increase, resulting in a decrease in the serum transferrin saturation (226).
— By the end of the first trimester, the SF concentration, an indicator of body iron stores, begins a steady decline (239).
— SF concentrations are reduced as a result of hemodilution in pregnancy and exhibit considerable day-to-day intraindividual variability (240).
— An SF concentration >70 µg/L before pregnancy or early in the first trimester has been proposed as a means of identifying women who will not need iron supplementation during gestation (241). However, a variety of guidelines have been published with respect to the concentration of SF that is best to identify iron deficiency (242).
— The sTfR concentration remains stable (243–246) whereas the sTfR index (the sTfR concentration divided by log SF) begins to increase. The distribution of body iron stores, estimated from SF and serum transferrin receptor concentrations (247, 248) among pregnant women examined in NHANES, 1999–2006, was reported by Mei et al. (249). During the first trimester, 6.9% of these women were iron deficient, using the criterion of body iron index <0 mg/kg. (Body iron index is defined as the logarithm of the ratio of sTfR/ferritin. It provides a quantitative estimate of the size of the body iron store; discussed in greater detail later in this review.)
Erythrocyte protoporphyrin
— Zinc protoporphyrin (ZPP) is reputed to be “the most established biomarker of iron-deficient erythropoiesis” (250).
— Measured as the ZPP/heme ratio (ZPP/H) by a hematofluorometer, erythrocyte protoporphyrin is unaffected by the hemodilution of pregnancy (251).
— With an adequate iron supply, erythrocyte protoporphyrin, unlike sTfR, is also unaffected by increased erythropoiesis during pregnancy (252).
— Erythrocyte protoporphyrin remains within the reference range in iron-replete women during the first trimester (235, 246, 251–253).
Second trimester
A number of changes occurring in iron homeostasis as gestation progresses are highlighted in Text Box 21 (223, 233). Median concentrations of the principal biomarkers of iron status during the second trimester of pregnancy are again shown in Figure 3.
Text Box 21.
Basics of iron biology in pregnancy: second trimester
• Fetal and placental growth increase throughout the second trimester.
• Both the red blood cell mass and plasma volume expand steadily with a greater proportional increase in the plasma volume.
• Iron requirements steadily increase to ∼4 mg/d by the end of the second trimester (223).
• Total iron absorption rises to an estimated 1.9 mg/d from a highly bioavailable diet (233).
Hemoglobin
Serum iron, ferritin, sTfR and iron stores
— During the second trimester the serum iron continues to fall as the transferrin concentration rises, and a further decrease in the serum transferrin saturation is the result (Figure 3) (226).
— Serum ferritin concentrations continue to decrease during the second trimester in British women not receiving iron supplements (232).
— In the available studies of sTfR during the second trimester, concentrations remained stable or rose somewhat (226, 243, 244, 246). Because both functional iron deficiency and increased erythropoiesis raise sTfR concentrations, the relative contribution of the two processes during pregnancy is uncertain.
— In some studies, the increase in sTfR during pregnancy seems more closely associated with erythropoietic activity (244), but others suggest that iron deficiency is the predominant factor (243, 246). With the decrease in SF, the sTfR index increases during the second trimester (226, 246). In the pregnant women examined in NHANES 1999–2006, the proportion with body iron index <0 mg/kg increased to 14.3% in the second trimester (249).
— In iron-replete women, erythrocyte protoporphyrin remained within the reference range during the second trimester (235, 246, 250–253).
Third trimester
As pregnancy moves toward its conclusion, a number of processes continue with regard to the maternal and fetal physiology to meet iron needs; these are highlighted in Text Box 22 (223, 233). Again, median concentrations of the principal biomarkers of iron status in Dutch women during the third trimester of pregnancy are shown in Figure 3. The following describes aspects of physiology relative to specific iron biomarkers.
Text Box 22.
Basics of iron biology in pregnancy: third trimester
• Fetal and placental growth, together with expansion of the red blood cell mass and plasma volume, steadily increase to a peak in the latter portion of the third trimester.
• The overall increase in the red blood cell mass is ∼35% and in the plasma volume, ∼50%.
• The iron requirement rises to 6–7 mg/d and may be even higher, 10 mg/d, during the last 6–8 wk of pregnancy (223).
• Total iron absorption from a highly bioavailable diet also increases to an estimated 5.0 mg/d (233), but is still far below the calculated daily iron requirement.
Hemoglobin
— The circulating hemoglobin threshold for anemia during the third trimester is 110 g/L in guidelines from both the CDC (235) and the WHO (236).
— In women given iron supplements, the hemoglobin concentration in the third trimester rises from a nadir near the end of the second trimester but is appreciably lower in those who receive no supplemental iron (254).
Serum iron, ferritin, sTfR and iron stores
— During the third trimester, the serum iron falls further as the transferrin concentration continues to rise, resulting in a continued decline in the serum transferrin saturation (Figure 3) (226).
— In the Dutch study, SF decreased during the third trimester (Figure 3) (226) as sTfR increased, resulting in a marked increase in the TfR index.
— As in the second trimester, the relative contributions of functional iron deficiency and erythropoietic activity to the increase in sTfR have not been determined (226, 243, 244, 246).
— In the pregnant women examined in NHANES, 1999–2006, the proportion with body iron index <0 mg/kg rose to 29.5% in the third trimester (249).
— In women supplemented with iron, erythrocyte protoporphyrin remains within the reference range during the third trimester but increases in those with iron deficiency (251, 252).
Postpartum
Median concentrations of the principal biomarkers of iron status in Dutch women postpartum are shown in Figure 3. Key points relative to iron biomarkers include the following: 1) at 24 h postpartum, the median hemoglobin concentration was little changed from the third-trimester level but both fluid shifts and the extent of blood loss at delivery will determine individual values; 2) Delivery-associated inflammation is associated with increased concentrations of C-reactive protein (CRP), hepcidin, ferritin, and the sTfR index (226, 228, 255–257); 3) At 24 h postpartum, serum iron, transferrin, transferrin saturation, sTfR, and erythrocyte protoporphyrin are little changed from values in the third trimester (Figure 3); and 4) by 6 wk postpartum, all the biomarkers of iron status in Figure 3 had returned to levels not significantly different from those in the first trimester, with the exceptions of sTfR and the sTfR index, which remained elevated (226).
Potential biomarkers for maternal and fetal iron status
In summary, the interpretation of iron status biomarkers at various stages of pregnancy are derived from measurements in healthy Western women considered to be iron sufficient. In general they reflect maternal rather than infant iron status (177, 258). Determining iron needs by using functional criteria based on the health of the infant and mother has been advocated as an alternative approach (221, 222, 238). It is important to note that the putative beneficial effects of improved iron status for the infant have been based on comparisons between mothers receiving supplemental iron (usually with folic acid) and those given a placebo. The results of this series of observations have been inconsistent in different settings. Nevertheless some studies have shown critical functional benefits for the infant (259, 260). It will be important to correlate the results of current biomarker measurements during the various stages of pregnancy with the survival and health of the baby to determine whether they have predictive value. The evaluation of newer, currently experimental biomarkers such as serum hepcidin and novel red blood cell parameters will also be very valuable.
Effects of iron deficiency on fetal and infant neurological development
Three periods of pediatric development are at increased risk for iron deficiency; the fetus/newborn, children aged between 6 mo and 2.5 y, and female adolescents, particularly if they become pregnant. Although each show a wide range of motor and cognitive deficits, the first two are the most vulnerable, with iron deficiency resulting in neurodevelopmental alterations that persist despite iron repletion (261). Early studies demonstrated that deficits occur in iron deficiency without anemia (262) or by fetal conditions that are characterized by a shift of available iron into a polycythemic red blood cell mass at the expense of maintaining brain iron sufficiency (263). These findings in humans, supported by animal research in sheep, rats, and monkeys, suggest that iron is prioritized to red blood cells over other organs, including the brain, during fetal and early postnatal life (264–266). Thus, screening for iron deficiency by assessing anemia likely means that the brain has already been affected prior to diagnosis (267).
In humans, behavioral deficits map directly onto the abnormal brain processes (and their interactions) elucidated from the animal models (181). These include general reductions in intelligence, motor abnormalities, including activity levels and coordination, disrupted sleep patterns, slower speed of processing, altered affect and social interactions, and reduced learning and memory capacity (181). Long-term persistence of some of these abnormalities has been documented to adulthood (268). Because these long-term abnormalities exist in spite of relatively prompt diagnosis and treatment of anemia, biomarkers that index brain health independent of red blood cell iron status are critically needed.
Establishing the functional consequences of the effects of a nutrient deficiency on brain development can be challenging for the following reasons: 1) nutrient deficiencies frequently have their most profound effects on the brain when it is in its most rapid growth trajectory, during the late fetal period and from 0 to 3 y of postnatal age (202, 203, 269); 2) functional assessment of the brain at these ages is difficult because of the limited behavioral repertoire of the fetus and young child; 3) the effects can be relatively subtle, often affecting specific neurologic domains and behaviors rather than global function; and 4) common nutrient deficiencies are rarely fatal and thus tissue-level “proof of effect” is often not an option.
The features of the two general approaches typically used to assess the brain consequences of early-life nutrient deficiencies are highlighted in Text Box 23.
Text Box 23.
Approaches to evaluating the impact of nutritional insults on development
• Use of multidisciplinary assessments ranging from molecular neuroscience through behavior to construct a plausible biological proof.
-This approach can be driven top-down (i.e., from behavior to bench science) or bottom up.
-In either case the following are required: 1) developmentally appropriate preclinical models; 2) physiologically appropriate degrees of nutrient deficiency; and 3) a certainty as to the equivalency of animal behaviors to human behaviors.
• Clinical studies: can be either observational/treatment or prevention/supplementation studies.
-Observational studies evaluate developmental outcome when a population with a specific nutrient deficit is compared to a population without that specific nutrient deficit.
• The assumption is that all confounding variables that can affect brain development (including other nutrient deficiencies) will be equal between groups.
• Given that nutrient deficiencies tend to co-occur, this goal can be difficult to achieve.
-Prevention/supplementation studies: the nutrient in question is supplemented in one group and outcomes are compared to a nonsupplemented control.
• This approach can be problematic due to issue affecting rigor and reproducibility including, lack of baseline status assessment, lack of sensitive and specific nutrient biomarkers, and types and expectations regarding outcome measures and their relevance to nutrition or nutrient-brain interactions.
Human and animal studies strongly support the hypothesis that early-life iron deficiency alters the brain, both acutely, as long as iron deficiency persists, and chronically, well after iron repletion. A comprehensive review of this topic is well beyond the scope of this paper. However, several reviews of the subject are worth considering in order to appreciate the body of evidence that early-life iron deficiency is detrimental to long-term brain health (77, 181, 270–272).
In considering nutritional interventional studies in children, the following four key developmental neuroscience principles must be followed in order to optimize the chances of obtaining accurate information: 1) timing of the intervention; 2) clarity regarding level of risk in target populations; 3) use of age-appropriate testing tools; and 4) ensuring linkage between interventions and assessment. The key features of these principles are summarized in Text Box 24 (273–274). The following is a summary of some additional details about the implications of iron deficiency during development.
Text Box 24.
Key principles for evaluating neurological outcomes
Timing
• The nutritional intervention must occur at a time when there is a demonstrably high demand for the nutrient in the developing brain or brain region.
• The optimal timing for a particular nutrient is most often elucidated from developmentally appropriate preclinical models.
• Must consider whether administration of a supplement to a mother eventually results in accretion in the developing fetal brain (e.g., in considering iron supplementation during pregnancy, the driving force would be demonstration of an effect on iron-dependent fetal regional brain development) rather than influencing maternal hematologic status (although the two may well coincide).
Level of risk in target population
• The target population in the study must have a significant rate or risk of deficiency.
• There is no evidence in developmental neuroscience that further supplementation of any environmental factor (including nutrients) beyond a state of sufficiency results in “enhanced” brain development.
• Study populations cannot have a degree of deficiency that is so great that a relatively low dose intervention has no effect (273)
• Study populations should not have deficits of multiple nutrients that affect brain development such that it is impossible to assess the role of a single nutrient such as iron (274).
Age-appropriate testing tools
• Use neurodevelopmental test batteries that are appropriate to the proposed nutrient's role in the brain at the time that the supplement was administered.
• Global tests (i.e., Bayley Scales of Infant Development, IQ tests) are often too generic and fail to detect neurodevelopmentally important differences in higher brain processing, speed of performance, and subtle memory problems.
• Nevertheless, global tests are typically utilized in large-scale studies because they are easily performed across multiple enrollment sites.
• More specific neural domain tests tend to be technically difficult and may also be expensive even though they may be better designed to assess the specific nutritional deficit-induced pathology.
• The degree of neurodevelopmental deficit must be consistent with the effect size seen in the developmentally appropriate preclinical models in order not to misattribute a large effect size to a nutrient that is likely, in fact, to have a small effect.
Linkage between interventions and assessment
• Neurodevelopmental assessment batteries must be administered as close to the intervention as possible in order to avoid post-treatment confounding factors. These include the following:
-Negative environmental (including nutritional) factors
-Positive factors such as neural plasticity in the brain areas that were initially negatively impacted by the deficiency
The vulnerable period of pregnancy, through infancy and toddlerhood (181) will be considered separately because the etiologies of iron deficiency and neurodevelopmental consequences differ. In each case the pathophysiology leading to increased risk of iron deficiency, the animal models that support the hypothesized effects, and the human evidence for the deleterious neurodevelopmental effects will be examined.
Pathophysiology of iron deficiency in the fetus and neonate
As detailed above, much is known about the maternal-fetal transfer of iron. Evidence in humans points to prioritization of iron to the fetus at the expense of the mother (176, 177). In the fetus, evidence from humans (275, 276) and animal models (264) indicate that interorgan prioritization favors red blood cells over other organs, including the brain, the heart, and the liver—in that order (264, 276).
A number of gestational conditions lead to a disruption of the balance of fetal iron supply and demand and thus to fetal brain iron deficiency (277–279). These include the following: 1) severe maternal iron deficiency; 2) placental insufficiency (usually due to maternal hypertension); 3) maternal diabetes mellitus; and 4) maternal smoking.
The first two reduce available iron to the fetus, whereas the chronic fetal hypoxemia associated with poorly controlled maternal diabetes increases fetal iron demand for compensatory erythropoeisis beyond the capacity of the placenta's transport ability (166, 275, 280); for a review, see Nold and Georgieff (281).
Studies have shown that brain iron concentrations are reduced by 40% in newborn iron-deficient infants of diabetic mothers (276) and by 33% in newborn intrauterine growth-restricted infants (282). Looking at ferritin concentrations, 65% of infants of diabetic mothers and 50% of growth-restricted newborns have a cord SF concentration <60 µg/L (277).
Iron in the liver is predominantly in the form of ferritin and serves as a storage buffer for the fetus during periods of reduced iron supply. Nevertheless, in humans, once liver iron concentrations are <1500 µg/g dry tissue weight, brain iron concentration falls precipitously (276). Based on data presented by Saarinen and Siimes (283), a cord SF concentration of ∼40 µg/L in the child (275, 284) would reflect liver iron concentration indicative of potential risk for fetal/neonatal brain risk.
Preclinical models of gestational/lactational iron deficiency
The degree of brain iron deficiency seen in human autopsy specimens has been achieved in rat models of gestational/neonatal dietary iron deficiency (186, 187, 191, 285, 286). Similar evidence has been provided by genetic mutant nonanemic mouse models that isolate the iron deficiency to single brain regions (209). The totality of this evidence demonstrates the negative effects of gestational/neonatal iron deficiency on regional brain anatomy and function and is summarized in Text Box 25 (170, 184–191, 209, 210, 285, 287–292).
Text Box 25.
Preclinical evidence of effect of iron deficiency on the brain
• Electrophysiology (190).
• Neurotransmitter concentrations (170, 185, 186, 285, 290, 291).
• Myelination (184).
• Gene expression (187, 188, 209, 292).
• These regional cellular abnormalities are particularly concentrated in the developing hippocampus and striatum and result in abnormalities in behaviors dependent on those regions (170, 191, 209, 210).
The affected behaviors of gestational/lactational iron deficiency include abnormalities in hippocampus-based learning and memory (170, 191, 209, 210, 293) and dopaminergically driven behaviors (170, 294) as would be predicted by the time/dose/duration paradigm (203). These preclinical studies have demonstrated that the hippocampal, dopaminergic, and myelin effects persist beyond the initial neonatal deficiency into adulthood. It is also clear that only treatment at a time that is developmentally consistent with the third trimester of pregnancy is effective in preventing those long-term effects.
Gestational/lactational dietary iron-deficiency anemia in the rat has provided a convincing pathophysiologic model of the human condition. Although the pathology evinced by this model is due to reduced iron exposure, these models have been unable to specifically distinguish the role of iron from that of anemia (or the combination) in neurodevelopment (209).
The essentiality of iron to neurodevelopment was elucidated recently through generation of two nonanemic genetic mouse models: 1) transgenic mice that express tetracycline transactivator-regulated, dominant negative transferrin receptor (DNTfR1) in hippocampal neurons; 2) nonanemic Slc11a2(hipp/hipp) [double mutant, hippocampal neuron-specific knockout of Slc11a2(hipp/hipp)] mice (72, 91). In these models iron uptake is disrupted in specific sites (hippocampus) and at specific time (late gestation). The use of these models demonstrated both a structural vulnerability in the hippocampus and a critical period for the provision of iron, which if missed, results in irreversible long-term neurocognitive, structural, and genomic abnormalities (179, 209). Functionally, these models show that the loss of learning and memory capacity results specifically from the loss of hippocampal neuronal iron uptake (179, 209). The fundamental role of iron availability in learning and memory is also supported by a dose-dependent increase in expression of hippocampal neuronal iron transporters with increasingly difficult memory tasks (209). These models have also been used to demonstrate that nonanemic iron deficiency, which is more prevalent in humans than IDA, also disrupts brain development. Thus, the available evidence indicates that iron is essential for fetal and early postnatal brain development and function, and that iron deficiency leads to abnormal brain development and function.
Human studies
The abnormalities documented in preclinical animal models map directly onto the human behavioral and electrophysiological abnormalities in iron-deficient newborns, thereby providing a multilayered plausible biological proof for the important role of iron in neurodevelopment. The challenge of such studies in humans is linking measures of the myriad of structural and function components of the developing nervous system to biomarkers of iron nutrition. The former may be referred to as “bioindicators,” reflecting perturbations of specific structures/neural systems as compared to sensitive and specific biomarkers of iron nutrition/status (295).
Although data on the behavior of the iron-deficient newborn humans is limited, the existing literature strongly implicates nonanemic and anemic iron deficiency in the fetal and neonatal period as neurodevelopmental risks to precisely the systems that were rapidly developing at the time of the deficiency (284, 296–299). Text Box 26 (177, 273, 294, 296, 297–307) highlights some of the extant evidence as well as some of the bioindicators that have been used.
Text Box 26.
Impaired newborn iron status and neurodevelopment
• Infants with low cord ferritin (<76 µg/L) were almost 5-fold more likely to score poorly on fine motor skills, and almost 3-fold more likely to have poorer tractability, poorer language ability, and score worse on every subtest than children with normal ferritin concentrations at age 5 y (296).
• Suggests a critical period for multiple brain processes in the fetus and neonate.
• Several studies have evaluated the impact of maternal iron intervention on general developmental outcome.
-Multiple studies show significant positive findings, e.g., (299–302).
-Null studies have been reported with iron supplementation in pregnancy (273).
• Positive findings highlight the following:
-The importance of an appropriate dose of iron for the degree of iron deficiency of the maternal-fetal dyad (302).
-The importance of timing of the intervention was apparent in a subsequent large placebo-controlled supplementation trial in Nepal (302).
• Children of women originally randomized to placebo during pregnancy had poorer outcomes (300). They subsequently received supplemental iron/folic acid (with or without zinc) from 1 to 3 y of age.
• No effect was seen at age 7–9 y of age on neurocognitive (including frontal lobe) testing (302). Thus, postnatal iron supplementation was unable to counteract the prenatal effects of lower iron status.
• Studies of targeted brain areas and functions have used several “bioindicators” (i.e., high-end techniques to target functions in rapidly developing iron-dependent neural circuitry) including the following:
-Development of the hippocampus [rapidly developing in the late fetal/early neonatal period in humans and subserves recognition, e.g., discriminative memory behavior (303)], ID was associated with poor vocal recognition (304).
-The persistence of learning and memory problems at age 3.5 y. Performance was inversely proportional to iron status at birth (305).
-Monoamine metabolism as reflected by increased risk to infant temperament, potentially mediated by the developing dopaminergic system, has been documented (297). The findings are important because temperament is closely related to monoamine status (306). At birth, infants with iron deficiency without anemia (294) show profound short- and long-term changes in monoamine metabolism.
-Myelin formation/function: auditory brainstem responses were assessed as a reflection of speed of processing and a potential index of myelination in infants at birth (298, 307). Results indicated that iron deficiency early in the period of myelination of the central nervous system causes slower speed of processing along myelinated circuits.
Effects of iron deficiency on infants and toddlers
According to the 2007–2010 NHANES survey, in the United States, ∼7% of children aged 1–5 y have iron deficiency (308) compared to rates of IDA in regions of sub-Saharan Africa and Southeast Asia that exceed 50%. The high rate of infant and toddler iron deficiency and IDA around the world is due to multiple coinciding and mutually exacerbating factors (181). As with the fetus and neonate, iron requirements are high because of the rapid rate of somatic growth. The red blood cell mass continues to increase with somatic growth and accounts for the vast majority of total body iron demand (as hemoglobin), and also myoglobin in growing muscles. In addition, any rapidly growing and differentiating organ, such as the brain, will continue to have high demands. To meet this demand, the child relies on mobilizing iron present as ferritin and the (relatively) expanded red blood cell mass at birth, and from postnatal dietary iron intake. Infants with low iron stores at birth are at greater risk for lower stores at 9 mo of age; that risk is directly proportional to the rate of growth between birth and 9 mo (309). The gestational conditions that lead to low neonatal iron stores are discussed elsewhere.
During the postnatal and infancy period the sources of dietary iron include various infant feeding options (e.g., human milk, formula, animal milk), complementary foods (e.g., animal meat protein, wet- and dry-pack cereals), leading eventually to a mixed diet as available within the household. Throughout this period infants/children may also be exposed to iron supplements with or without micronutrients (310).
As in adults, iron utilization from these sources can be enhanced by ascorbic acid and inhibited by phytates and polyphenolic compounds (e.g., tea, coffee) in the diet. Worldwide, two significant pathologies contribute to the common occurrence of negative iron balance: blood loss from intestinal (usually parasitic) infections and the hepcidin-mediated ACD/inflammation which limits iron absorption during periods of infection and inflammation (181).
Unlike the fetus and neonate, there are no studies that quantify the degree of brain iron deficiency in iron-deficient anemic infants or toddlers. Similarly, no studies address whether iron is prioritized to red blood cells over the developing brain as occurs in the fetus and neonate. The presence of behavioral abnormalities in nonanemic iron-deficient children suggests, however, that this may well be the case (262).
Preclinical models of postnatal dietary iron deficiency
Virtually all preclinical studies that induce iron deficiency in the postweaning period (i.e., 21 d after birth in the rat) are designed to simulate IDA in humans after 1 y of age. These models have been used to demonstrate long-term effects on the monoaminergic and myelin systems that persist into adulthood even after remediation of iron deficiency. Alterations in these systems have been linked to functional abnormalities in neural circuits that regulate mood, affect, social behavior, motor behavior, and speed of processing (181). In summary, preclinical models provide mechanistic evidence to explain how postnatal dietary iron deficiency affects brain development and related functional outcomes. Text Box 27 (181–183, 186, 189–191, 298, 307, 311–325) contains a summary of knowledge gained from preclinical studies of iron deficiency.
Text Box 27.
Preclinical evidence of functional consequences: infants and toddlers
Monoamine
• Through its role in the activity of tyrosine hydroxylase and tryptophan hydroxylase, iron is responsible for the synthesis of dopamine and serotonin, respectively (186, 311).
• Iron deficiency reduces the concentration of dopamine D2 receptors in the ventral midbrain and prefrontal cortex (312).
• Studies have suggested that nonanemic iron deficiency is a risk to brain development and function (313).
• Behavioral abnormalities in preclinical models are consistent with disruptions of dopaminergic pathways and map well onto the behavioral findings in humans (181, 191, 312, 314, 315).
• Studies in nonhuman primate models have documented the following differences in outcomes based on the timing of iron deficiency:
-Prenatal iron deficiency in the rhesus monkey results in a behavioral phenotype of hyperactivity and lack of inhibitory control.
-Postnatal iron deficiency results in a hesitant, wary phenotype (316–318).
• Given the role of specific iron-sensitive areas of the brain and the role of the dopamine system in mediating activity and anxiety (319), the preclinical studies strongly support the hypothesis that an iron-dopamine link underlies the motor activity and anxiety behaviors seen in iron-deficient children (315, 320, 321).
Myelin
• Iron is an essential factor in myelination and oligodendrocyte function via its role in:
• Iron deficiency may affect oligodendroglia synthesis of myelin via cellular mitochondrial dysfunction caused by reduction of cytochrome concentrations (182, 323).
• Iron deficiency in the preweaning and postweaning periods alters myelination in the rat, as demonstrated by:
-Reduced 2,3-cyclic nucleotide-3-phophohydrolase (CNPase) activity
-25–35% reduction in myelin basic protein concentrations (324)
• Abnormalities in myelination observed in these rat models may be the underlying abnormalities in neural speed of processing that can explain the auditory brainstem evoked responses (298, 307) and visual evoked responses (325) reported in iron-deficient children.
Cellular energetics and mitochondrial health
• Studies have established that iron exerts its influence through its incorporation into cytochromes (182).
• Neurons (and glia) have high metabolic rates during development, and thus are sensitive to reductions in iron supply (286, 323).
• Neuronal iron deficiency results in changes in dendrite structure (189) and lower electrical output (190)—both are associated with poorer synaptic efficacy and memory deficits. As noted above, iron-deficient oligodendrocytes generate less myelin and also myelin with abnormal fatty acid profiles (323, 324).
Human studies
A number of systematic review and data analyses have been reported recently (270–272). Of particular relevance is the series on risk factors to early childhood development in The Lancet, originally published in 2007 and updated in 2011 (271, 272).
Studies of the effect of postnatal iron deficiency's effects on neurodevelopment fall into two categories;
- Larger-scale population-based studies that typically assess general outcome of observation/treatment trials and prevention/supplementation trials irrespective of initial iron status.
- Prevention/supplementation trials are more likely to have null or small effect-size results with respect to answering the question of whether early life iron deficiency causes neurodevelopmental abnormalities because the sample populations are unselected.
- Small effect-sizes in such an unselected group of subjects should be considered as evidence for a biologically relevant effect.
Smaller-scale studies designed to elucidate the role of iron in specific neurobehavioral abnormalities predicted from preclinical models.
Overall, 20 of the 22 studies reviewed in the two Lancet papers showed poorer functioning on general tests of cognition, motor, and social-emotional capacity in children with iron-deficiency anemia (271–272). The consistency of this finding across many study designs from multiple populations around the world suggests a robust effect.
In infants, toddlers, and young children, general functioning is typically assessed with broad-based standardized tests such as the following: 1) Bayley Scales of Infant Development—these provide information about mental and motor outcomes, each normed to a mean of 100 with an SD of 15; 2) neuropathologically specific tests can be behavioral or involve some type of neuroimaging, and can include electrophysiology to assess social-emotional function, speed of central neural processing, learning and memory, and complex behaviors.
Both approaches can assess acute (i.e., while iron deficient) and long-term (ie, after treatment and resolution) effects.
A number of studies have explored the effect of IDA on general function. Some of the key findings are summarized as follows: 1) six observational/treatment studies of otherwise well-nourished infants with IDA showed that iron-deficient infants scored 6–15 points lower on mental developmental indexes compared to iron-sufficient, nonanemic controls (326–331); 2) five of the studies showed a lower motor index with a similar effect size (326–329, 331); 3) recovery of neurobehavioral function with treatment was seen in two of the six studies (326, 328), but not in the others; 4) in one of the cohorts followed for the longest period of time (19 y), loss of IQ was noted over time from 1 y to age 19. The slope of this loss was greater in children who were chronically iron deficient between 12 mo and 3 y or who did not correct their hematology within 3 mo of therapy (181); 5) the results imply that critical periods of neurodevelopment exist that are dependent on iron sufficiency, a finding supported by the preclinical models.
Selected studies of the effect of acute IDA on pathophysiologically plausible regional brain circuitry are highlighted in Text Box 28 (209, 211, 262, 332–336).
Text Box 28.
Effect of iron deficiency and IDA on regional brain circuitry
• Dopaminergic systems: spontaneous eye blink rate, a dopaminergic function, was assessed in 19 iron-deficient anemic 10-mo-olds (332).
-Compared to iron-sufficient controls and nonanemic iron-deficient infants, the iron-deficient anemic infants had significantly slower eye blink rates, consistent with reduced dopamine function.
-A significant dose-response relationship between social-emotional behavioral abnormalities and the degree of iron deficiency exists. Infants with IDA have poorer soothability, less positive affect, and less social engagement than iron-sufficient infants, with nonanemic iron-deficient infants demonstrating intermediate degrees of abnormalities (262).
• Hippocampus: electrophysiological evidence of hippocampal dysfunction was provided in a study of 9- to 12-mo-old infants with IDA and iron-sufficient controls (333).
-In a maternal face recognition paradigm, iron-deficient anemic infants showed a maturational delay in recognition memory (333).
-Part of the effect was mediated by social-emotional status of the infants, consistent with the role of dopamine in hippocampus-dependent memory function. These effects suggest that iron deficiency causes hippocampal dysfunction during a period of rapid hippocampal development that extends from birth to 18 mo of age in the human.
-These observations are consistent with preclinical model studies that demonstrated that iron is essential for normal hippocampal neuronal development, and normal learning and memory function (209).
• Behavioral tasks were used to assess recognition memory.
-In 28 IDA, 28 iron-deficient and 21 iron-sufficient 9-mo-olds (334).
-IDA infants showed poorer object permanence and short-term memory than iron-sufficient infants.
-Iron-deficient infants demonstrated intermediate performance to the other two groups, suggesting a dose-response effect based on iron status.
• Electrophysiology: auditory brainstem evoked responses were utilized to assess the effect of IDA on speed of central neural processing in 29 IDA 6-mo-olds and 26 iron-sufficient controls (325).
Central conduction time was longer in the iron-deficient infants, indicating slower processing speed.
Because the delays were more prominent at 1-, 2-, and 4-y follow up (325), the authors concluded that the effect likely was a permanent alteration in a primary process such as myelination, rather than a developmental delay.
• Electrophysiology: visual evoked potentials were used in 25 IDA compared to 25 iron-sufficient toddlers aged 6 to 24 mo (335).
Demonstrated longer latencies on visual evoked responses in IDA infants
A dose-response effect was shown between the duration of latency delay and the degree of IDA. The data from the preclinical studies confirm significant alterations in the myelination process with iron deficiency (211).
Because a period of rapid myelination exists between 36 wk gestation and 2 y in humans, the findings suggest a vulnerable period precisely at the time that infants are at risk of iron deficiency (336).
Prevention and supplementation trials
Results from prevention/supplementation trials indicate a modest, but positive effect of iron supplementation on neurodevelopment. As noted above, a systematic review of these types of trials would be expected to show variability in response because of several factors that determine nutrient-brain interactions during development. When enrolling unselected (for iron deficiency) subjects, the baseline rate of iron deficiency, the severity of iron deficiency, the timing of intervention, the dose of the intervention relative to the degree of deficiency and the selection of the proper bioindicator of neurodevelopment relative to the stage of brain development when the deficiency occurred, all become relevant factors in whether an effect will or will not be seen.
At least seven trials of postnatal iron supplementation offer useful data and are characterized as follows: 1) two were conducted in developed countries (337, 338); 2) five were conducted in developing countries (339–343); 3) the trials did not have the same study designs, in that other risk factors to neurodevelopment (stunting, other micronutrient deficiencies, etc.) were present in four of the five trials set in developing countries; 4) supplementation in some trials was with a multimicronutrient preparation, making isolation of the role of iron in any findings more problematic; and 5) the risk of iron deficiency also varied widely, even in the two trials in the developed country settings.
In spite of these differences, all the trials in developing countries (where in some cohorts the risk of iron deficiency was high and the degree of iron deficiency when present was large) reported improved motor outcomes with iron supplementation (339–343), as did one trial in a high-risk population in a developed country, England (338). Better cognitive/language outcomes were present in two studies (342, 343), whereas improved social-emotional development was seen in three (340, 341, 343). Long-term follow-up of these cohorts will determine whether these benefits are sustained.
Given its important roles in cellular metabolic processes, it should not be surprising that an iron-deficient brain does not perform optimally. Of greater concern are the long-term morbidities conferred by early-life iron deficiency and the consistent finding that prompt treatment of early-life iron deficiency does not prevent long-term disability (in humans or in animal models). This failure to completely recover from brain iron deficiency is unlike the finding in red blood cells, where treatment resolves anemia with no apparent long-term consequences. Additional aspects of the impact of early iron deficiency on brain biology and human behavior are addressed below.
From a neurodevelopment perspective, the failure to achieve normal development after early iron deficiency suggests that critical periods of brain development have been missed and structure is permanently altered. Moreover, long-term dysregulation of critical synaptic and myelin gene expression in damaged areas suggests the possibility that early-life iron deficiency may exert epigenetic effects (187, 292). The long-term behavioral abnormalities of formerly iron-deficient humans are complex and confirm the ultimate interaction of altered major central nervous system processes and brain regions affected by iron status during development.
Iron is likely involved in many more biological processes in the developing brain than are currently recognized. One example might be the role of iron in the regulation of long-term gene expression (187, 292). While future research will undoubtedly add further evidence to the importance of iron, our current understanding presents an undeniable picture of its essentiality to healthy brain development. The key task from both a clinical and programmatic perspective is to use this knowledge to develop better tools for providing care and programs that address the importance of iron not just from a survival perspective but with an acknowledgment of its critical role in human development (344). “There is no convincing evidence that iron treatment of young children with IDA has an effect on psychomotor development or cognitive function within 30 days after commencement of therapy. The effect of longer-term treatment remains unclear” (344). The lack of a consistent response to iron therapy once IDA is present is consistent with the premise that prevention of IDA in the first place or earlier identification and treatment may be necessary to recover neural function acutely and prevent long-term effects.
Potential biomarkers for iron status during fetal and infant development
As discussed in detail above, iron distribution is markedly different in early human development compared to toddlers, children, and adults. Some key features of fetal/neonatal iron as they pertain specifically to assessment are highlighted in Text Box 29 (278, 345, 346).
Text Box 29.
Key aspects of iron status during fetal and neonatal periods
• Transplacental iron transport to the fetus is highest in the third trimester, averaging 1.35 mg/kg fetal body weight per day (346).
• Fetal body iron reaches 75 mg/kg (70–80% in hemoglobin, 10% in myoglobin and tissue enzymes, and 10–15% in stores).
Evidence for biomarkers
• A rise in SF from a median value of ∼45 µg/L at 14–16 wk to >100 µg/L at 39 wk has been observed (346).
• Serum iron and TIBC values also rise whereas ZPP/H is inversely correlated with gestational age.
• On the other hand, sTfR levels do not appear to be correlated with gestational age (278).
• Fetal hemoglobin is replaced by adult hemoglobin during the first 6 mo of life.
• Iron is transferred from RBC containing fetal hemoglobin to the storage compartment and then reutilized for the synthesis of adult hemoglobin.
• In a study of 573 normal infants and children, a median SF concentration of 101 µg/L was reported at birth, rising to 356 µg/L at 1 mo and then rapidly falling to ∼30 µg/L at 6–11 mo (345).
The biomarkers employed for evaluating iron status in adults have been measured in the umbilical cord blood of premature and full-term infants (Table 4) (277, 278, 346–348). However, the studies contain relatively limited sample sizes and the values reported, although qualitatively similar, are generally not consistent. Thus, infants are relatively polycythemic and have high iron stores at birth, but become critically dependent on an adequate supply of dietary iron by 4–6 mo. This dynamic flux between the red blood cells and stores makes it very difficult to estimate iron status precisely.
TABLE 4.
Reported biomarker values for umbilical cord blood1
| Biomarker | 26–28 wk | 29–31 wk | 32–36 wk | 37–41 wk | Reference |
|---|---|---|---|---|---|
| SF, µg/L | 75 (44–117) | — | 90 (45–142) | 171 (121–259) | Sweet et al. (278) |
| 131 (90–238) | Sweet et al. (347) | ||||
| 134 (40–310) | Chockalingam et al. (277) | ||||
| 101 | Siimes et al. (346) | ||||
| sTfR, µg/dL | 10.3 (7.5–16.5) | — | 8.2 (5.4–12.5) | 8.4 (6.4–10.6) | Sweet et al. (347) |
| 9.4 (6.7–10.8) | Sweet et al. (278) | ||||
| ZPP/H, µmol/mol | 122 ± 34 | 103 ± 18 | 122 ± 34 | — | Juul et al. (348) |
1Values are medians (ranges) or means ± SDs. SF, serum ferritin; sTfR, serum transferrin receptor; ZPP/H, zinc protoporphyrin/heme ratio.
Screening for iron deficiency in the neonate and infant has, in the past, been focused on the relationship between maternal and infant iron status and the prevention of anemia in early childhood. Although these studies do address important aspects of iron nutrition, they may fail to identify critical facets of neurologic dysfunction because anemia is the end-stage state of iron deficiency.
The developing brain can suffer the consequences of iron deficiency in the absence of anemia. Prioritization of available iron to developing red blood cells over other tissues during negative iron balance has been reported in developing humans and animal models as well as infants born to diabetic mothers and after intrauterine hypoxemia and erythropoietin administration (264, 275, 276, 349, 350). Brain iron deficiency, independent of anemia, is responsible for long-term neurological deficits (179, 209, 351). Thus, early detection (and prompt treatment) of iron deficiency-induced brain dysfunction should be the primary goal of screening for early-life iron deficiency (Table 5) (181, 268, 352).
TABLE 5.
Assessment tools for neurological function1
| Assessment | Modality | Iron-dependent neurologic process | Behavior construct | Other nutrients | Applicability for field use | Comment |
|---|---|---|---|---|---|---|
| Sensory evoked response (ABR, VEP) | Electrophysiology | Myelination, synaptic efficacy | Speed of processing | Oxygen, iodine (infection) | No | Sensitive; rapid; need equipment |
| Event-related potentials | Electrophysiology | Myelination, hippocampal integrity, synaptic efficacy | Speed of processing; recognition memory; implicit memory | Glucose, protein, oxygen, zinc, iodine | No | Very sensitive, not portable |
| T2-weighted MRI | Neuroimaging | Iron content | None | None | No | Detects overload, but not ID |
| Standardized tests (e.g., Bayley scales) | Behavior | Myelination, synaptic efficacy, monoamine status | Global function (integration of effects of brain-wide iron deficiency) | Protein, fat, oxygen, glucose, iodine | Yes | Nonspecific; not sensitive |
| A, not B | Behavior | Synaptic efficacy; monoamines | Working memory | Zinc, iodine | Yes | Trained tester |
| Actigraph | Physiology | Monoamines | Spontaneous motor activity | Protein, copper, iodine | Yes | Trained interpreter |
| Social-emotional behavior scale | Behavior; survey | Monoamines | Frontal lobe | Copper, iodine | Yes | Trained interpreter |
| INFANIB | Physical exam | Myelination | Global | Oxygen, iodine (infection) | Yes | — |
| Peabody developmental motor scale | Behavior | Myelination monoamines | Gross and fine motor | Protein, fat, oxygen, glucose, iodine | Yes | Nonspecific |
| Optotrak | Behavior | Myelination monoamines | Motor response | Oxygen, iodine | No | Equipment and trained interpreter |
1ABR, auditory brainstem response; ID, iron deficiency; VEP, visual evoked potential.
A need exists to generate serum biomarkers indexing brain dysfunction in the pre-anemic stage of iron deficiency when it is still possible to reverse it with iron treatment. Development of such tools relies on knowing the relationship between currently available biomarkers and brain iron status, or bioindicators reflecting iron-dependent brain function. Such tools do not currently exist (other than perhaps in the neonate) because brain iron status is typically unknown in at-risk children. MRI, while sensitive enough to measure brain pathology and relate it to iron overload (353), is not sensitive or specific enough to detect brain iron deficiency.
The application of various “-omic” approaches (e.g., proteomic, metabalomic analyses) have enabled identification of potential indicators in the cerebrospinal fluid that can index brain iron deficiency in nonhuman primates (354, 355), but no studies have assessed such potential biomarkers in cerebrospinal fluid in humans. Because of the obvious risk and invasive nature of such an approach it is unlikely that using lumbar puncture to diagnose brain iron deficiency in humans will become a clinical tool.
Given the current inability to directly assess brain iron status in infants and children, attempts have been made to relate classical hematologic [e.g., hemoglobin, Hct, mean corpuscular volume (MCV)] and nonhematologic (e.g., ferritin, TSAT, ZPP, sTfR) indicators to putative brain iron status (as indexed by abnormal neurobehavior).
To date, two types of approaches have been used to link iron to brain function/outcomes: 1) population-based iron biomarkers linked to neurological outcomes; or 2) studies in neonates that attempt to more directly link peripheral iron biomarkers to brain iron content. Examples of these approaches are included in Text Box 30 (262, 276, 283, 284, 296, 343, 349, 356–358).
Text Box 30.
Studies linking population-based biomarker cut-offs to neurological outcomes
• Population cut-offs for hemoglobin, Hct, and MCV were used in seminal early papers that demonstrated abnormal brain function as a function of IDA (343, 356).
• Lozoff et al. (262) chose to define nonanemic iron deficiency as ≥2 abnormal nonhematologic values in the context of a normal hemoglobin concentration and related nonanemic iron deficiency to poorer neurobehavioral performance.
• In none of these studies was it clear whether the relationship of these peripherally measured biomarkers to brain iron is linear or reflects a threshold for adverse outcomes.
• No studies have assessed the positive or negative predictive value of specific cut-offs for these markers.
Studies in neonates: peripheral biomarkers and neurological outcomes
• An autopsy study of nonanemic newborn infants demonstrated that a threshold response of brain iron concentration as a function of liver iron concentration occurred when liver iron was <10% of normal (276), a finding recently confirmed in the neonatal lamb (349).
• Calculations were made from a nomogram published by Saarinen and Siimes (283) relating SF concentrations to liver iron content to predict that the threshold ferritin concentration for brain iron deficiency in the neonate was 35 µg/L (284).
• This value was tested as a cut-off to determine whether it predicted brain function in neonates and was found to differentiate those with normal recognition memory from those with impaired recognition memory (284).
• Abnormalities in acute and long-term brain function have also been documented in neonates with cord blood ferritin concentrations <76 µg/L (i.e., the lowest quartile) (296, 357), suggesting that even these neonatal ferritin cut-offs are not reliable. The data also serve to emphasize that the 5th percentile cut-off for a population biomarker may not represent the level at which neurologic dysfunction occurs.
• Recently, ZPP/H > 118 µmol/mol cut-off in cord blood was associated with abnormal recognition memory task at 2 mo (358).
• No other hematologic or nonhematologic marker measured in the peripheral blood or serum has been assessed with respect to brain iron content.
• Whether a similar association, either linear or threshold, with any of the common hematological indexes or iron panel measures is present beyond the newborn period has yet to determined.
Future research should concentrate on matching currently available biomarkers of iron status with the time frames for the development of iron-dependent neural systems for which functional tests are available, e.g. myelination and monoamine-driven behaviors. Examples of potentially relevant tests of neurological function are listed in Table 5. Because prevention of brain iron deficiency is the goal, the changes in the screening tool ideally should occur before the onset of brain iron deficiency and the clinical diagnosis of neurobehavioral effects due to brain iron deficiency. Changes in these bioindicators of brain function would likely occur well before the onset of anemia. Diagnosis of brain iron deficiency can currently only be inferred clinically by alterations to behaviors that are dependent on iron deficiency (Table 5). Although these behaviors are not specific to iron deficiency, if they are present within the context of supporting evidence of IDA, they can be presumed to be due to iron-deficiency effects on the brain (Table 5). Myelination affects the speed of neurological processing and can be measured through sensory evoked responses (typically auditory brain stem responses or visual evoked potentials). A continued search for novel biomarkers and alternative approaches to understanding the relationship between nutritional iron status during pregnancy in infancy and early childhood is also essential.
Effect of iron deficiency on work/exercise capacity
The relationship between iron deficiency and work capacity was analyzed in depth in two critical reviews that included both animal experiments and studies in human volunteers written by Haas and Brownlie (359) and McClung and Murray-Kolb (360). They evaluated aerobic capacity, endurance, energetic efficiency, voluntary activity, and economic productivity. The following is a brief summary of their conclusions.
Aerobic capacity
Laboratory experiments in animals demonstrated that hemoglobin values were inversely correlated with aerobic capacity. The severity of the anemia had a direct proportional effect, that was greater once the hemoglobin fell below 70 g/L. Laboratory studies in human volunteers yielded similar results, demonstrating a strong causal relationship between the severity of IDA and aerobic capacity. Iron deficiency without anemia did not affect aerobic capacity. Field studies provided further support for a causal relationship between IDA and aerobic capacity in manual laborers (359, 360).
Endurance capacity (maximum length of time a given workload can be sustained)
Studies using the rat as the experimental model suggest that although aerobic capacity is primarily dependent on hemoglobin concentration, effects on endurance are mediated at least in part by reduced oxidative capacity due to tissue iron deficiency (361, 362). A small number of human experiments were, however, inconsistent and failed to provide convincing evidence of an effect of tissue iron deficiency on endurance capacity. Haas and Brownlie (359) suggest that the discrepancy between animal and human studies may well be attributed to experimental design.
Energetic efficiency (amount of physiological energy required to perform a specified amount of work)
In the laboratory, energy expenditure is measured by indirect calorimetry and work by the use of a treadmill or a cycle ergometer. Field studies employ practical surrogate parameters, such as heart rate and tasks completed. Haas and Brownlie (359) concluded that two of three human laboratory studies demonstrated that iron deficiency impairs energetic efficiency even in the absence of anemia.
Voluntary activity and economic productivity
A limited number of human laboratory experiments, as well as two field studies, suggested that iron deficiency with or without anemia might have an important influence on human behavior that could impact critical social activities such as childcare. Based on published models (363–365), food fortification with iron is predicted to have an important beneficial economic influence at the population level, but this has yet to be tested in human trials.
At least one report published after the reviews of Haas and Brownlie and McClung and Murray-Kolb supports their conclusions (366). Most of the studies involved trained athletes. Iron supplementation of nonanemic iron-deficient women (SF < 20 µg/L) improved muscle function (attenuation of the rate of decrease in maximal voluntary static quadriceps contractions) (367). Iron deficiency (sTfR > 8.0 mg/L) without anemia impaired aerobic adaptation and endurance capacity in untrained women who participated in an aerobic training program employing cycle ergometers (368, 369). Iron-depleted (SF < 20 µg/L) competitive nonanemic female rowers were reported to be slower than those with normal iron stores (370). Iron supplementation improved energetic efficiency (371).
In summary, IDA has a profound negative effect on work capacity. Fortification with iron and other micronutrients may provide significant economic benefits for individuals and societies (363, 365).
Biomarkers of iron deficiency for evaluating effects on work capacity
It is evident that iron deficiency associated with anemia may impair physical activity by affecting oxygen delivery and oxidative capacity resulting from iron deficiency at the tissue level. The reported studies have used the described below biomarkers that are applied to the evaluation of anemia. The principles that have been discussed in evaluating anemia should be applicable to exercise capacity. It will be necessary to develop specific criteria that ensure both adequate hemoglobin levels and tissue iron sufficiency.
Iron deficiency in relation to iodine utilization and thyroid function
Iodine deficiency, like iron deficiency, is a major global public health problem and many women and children, especially in the developing world, are at risk of both goiter and iron-deficiency anemia. Iron deficiency with or without anemia can have adverse effects on thyroid metabolism and can decrease the production of thyroid hormones. As discussed in the BOND iodine review (2), iron deficiency is likely to exacerbate iodine-deficiency disorders that include goiter, hypothyroidism, impaired mental function, and cretinism.
The influence of iron deficiency has been evaluated in relation to goiter and poor tolerance to cold (a symptom of hypothyroidism). In iron-deficient populations, it has been shown that the efficacy of iodized oil or iodized salt programs to prevent goiter can be blunted, and that the regulation of body temperature on cold exposure can be impaired. Zimmermann (372) has reviewed in depth the influence of iron status on iodine utilization and thyroid function. A brief summary follows.
Mechanism of impairment in thyroid function in iron deficiency
Humans (373) and rats (374) with IDA have decreased plasma T3 and T4 concentrations due to decreased levels of thyroperoxidase, a heme enzyme that catalyzes the iodination of thyroglobulin and the coupling of the iodotyrosine residues (93). These are the two initial steps of thyroid hormone synthesis. Other mechanisms explaining how iron deficiency could impair thyroid status have also been proposed. They include alterations to the thyroid hormone feedback system (375), which responds to low plasma levels of T3 and T4, lower transformation of T4 to T3 in the peripheral tissue due to decreased 5′-hepatic deiodinase activity (376), and nonspecifically through anemia and lower oxygen transport (377).
Iron deficiency decreases the efficacy of iodine supplementation
Supplemental iodine is less efficiently utilized for thyroid hormone synthesis when a population has a high prevalence of iron deficiency. Zimmermann et al. (378) evaluated the response to supplemental iodine in 109 goitrous children aged 6–12 y in the western Cote d'Ivoire. Fifty-three were not anemic at the start of the study whereas 56 suffered from IDA [hemoglobin (Hb) <110 g/L and SF <12 µg/L or sTfR >8.5 mg/L and ZPP >40 µmol/mol heme]. Each child received an oral daily dose of 200 mg I as iodized oil for 30 wk. The prevalence of goiter at 30 wk was 12% in the nonanemic group and 64% in those with iron-deficiency anemia. T4 levels were also significantly higher in the nonanemic group at 30 wk. This study was extended by providing an iron supplement (60 mg Fe as FeSO4 4 times/wk for 12 wk) to the children who suffered from iron-deficiency anemia at baseline. The iron supplement corrected the IDA in 50/56 children with IDA and the prevalence of goiter at 65 wk was reduced to 20% (91). Similarly, in a 9-mo fortification trial in goitrous Moroccan children comparing dual fortified salt containing both iodine and iron with iodized salt alone (379), a significantly greater reduction in thyroid volume and improvement in thyroid function was found in the group receiving iron with the iodine. The prevalence of goiter and hypothyroidism was significantly decreased in the children consuming the dual-fortified salt compared to those receiving the iodized salt. These results suggest that a high prevalence of iron deficiency in populations with endemic goiter will decrease the effectiveness of many national iodized salt programs.
Iron deficiency decreases thermoregulation in response to cold exposure
The initial studies on thyroid metabolism in iron-deficient rats focused on thermoregulation. Rats with iron deficiency anemia were shown to have lower T3 and T4 levels than controls, and the normal increase in T3 and T4 seen in iron-sufficient rats exposed to cold (4°C) was not found in rats with IDA (374). Lower T3 and T4 levels, and lower rectal temperatures, were also reported in anemic women (373); however, when nonanemic and anemic women were subjected to a cold stress, involving moving from a bath at 35°C to one at 28°C for 100 min, there was no difference in the response of thyroid hormones or rectal temperature. Treating the anemic women for 12 wk with iron supplements corrected the anemia, improved the rectal temperature at 100 min, and partially corrected the thyroid hormone levels. It is likely therefore that in severe cold, individuals with iron deficiency anemia will have more difficulty in maintaining body temperature and a reduced ability to tolerate cold.
Biomarkers of iron deficiency for evaluating effects on iodine utilization and thyroid function
The reported studies used the biomarkers that are applied to the evaluation of anemia as discussed elsewhere. The limited evidence available at the present time suggests that they will be suitable for evaluating the effects of iron status on thyroid function. However, further research is needed to define adequate and optimal iron status for normal iodine utilization and thyroid function.
Morbidity related to infectious diseases
As highlighted in Text Box 31, infectious diseases continue to be a major scourge of the global community (380). Although a comprehensive discussion of all infectious disease is beyond the scope of this review, it is clear that the current landscape with regard to iron and global health is complicated by a number of core issues.
Text Box 31.
| Annual deaths (in millions) | |
|---|---|
| Disease | Deaths |
| Respiratory Infections | 3.9 |
| Malaria | 1.3–3.0 |
| HIV/AIDS | 2.5 |
| Diarrheal diseases | 1.8 |
| Tuberculosis | 1.7 |
| Neglected tropical diseases | 0.5 |
Source: Center for Strategic and International Studies (CSIS) (380).
While HIV/AIDS, tuberculosis (TB), and malaria account for ∼16% of global mortality (381), a significant amount of attention has been given to the relationships between iron status, interventions, and malaria, primarily stimulated by a large randomized clinical trial of iron supplementation in children (382). The core issues are as follows: 1) the need to understand the mechanisms to explain increased risk for morbidity and mortality associated with iron supplementation; 2) how to assess nutritional iron status in the context of infection and inflammation; and 3) the relative risk-benefit associated with various intervention options to prevent and treat nutritional iron deficiency in the context of infections including malaria (383, 384). The challenges associated with malaria exemplify some core issues common to all infections and are illustrative of the key issues with regard to biomarkers of iron in this context.
A review of the literature related to iron nutrition and these diseases reveals several challenges to our ability to draw conclusions about a specific role of iron nutrition, or to develop and implement safe and effective interventions to address iron nutrition in these conditions.
Several recent studies have explored the connection between HIV infection, anemia, and nutritional iron status (385–388). Kerkhoff et al. (385) assessed iron status, including the measurement of hepcidin levels, and reported that patients surveyed suffered predominantly from ACD rather than IDA. Diouf et al. (386) reported that diets containing micronutrient-fortified lipid-based supplement improved anemia based on measurement of hemoglobin; however, they did not include specific biomarkers of nutritional iron status. Zinc deficiency was highly prevalent in this study cohort but the intervention did not improve status despite reaching intake levels comparable to DRI. Widen et al. (387) reported that antiretroviral therapy was associated with anemia and changes in iron biomarkers (sTfR). In a large observational study of HIV-infected adults in Tanzania, Petraro et al. (388) reported a high prevalence of anemia and identified numerous independent risk factors including BMI, CD4 count, and antiretroviral therapy. These authors discussed “iron-deficiency anemia,” although the only measures of anemia were hemoglobin and MCV, with no other iron-specific biomarkers nor any assessment of iron exposure.
In sum, these results reinforce the challenges of addressing iron nutrition in the context of infectious diseases. Fundamentally, there are several core concerns that can be categorized into the following questions:
Our ability to determine whether biomarkers used to assess iron nutritional status reflect iron nutrition or a physiological response to the condition(s) in question. How does the presence of inflammation (acute or chronic) in these conditions affect the selection, use, and interpretation of biomarkers used to assess iron status?
Anemia is a common comorbidity of these infections: how can we better understand the interactions among disease/comorbidities, treatment, and iron status?
If, in fact, evidence indicates nutritional iron deficiency, what is the safest and most efficacious intervention to use clinically or at scale?
How does the role of public health approaches to addressing iron deficiency at population levels impact on care of individuals with infectious diseases?
There is no question that nutritional iron deficiency is commonly observed in populations with infectious diseases and their comorbidities. The core issue is how to assess iron in the context of infection, treatment, food insecurity/malnutrition, and to develop safe and effective strategies to address these complex problems. A concerted effort has begun to develop solutions to these issues, particularly the relationships among nutrition, disease, and inflammation. These efforts are focused on improving approaches to iron status assessment, and more specifically, the distinction between nutritional iron status and anemia (48, 231). One application of these improved approaches would be their use by WHO to develop new guidelines for both iron assessment and interventions.
Iron overload
Although much of the focus of this report has been on the factors associated with and biomarkers employed to assess iron deficiency, the I-EP concluded that iron overload was an issue of sufficient public health importance to warrant specific coverage. The following section is an overview of the current understanding of the causes, public health significance, and assessment approaches related to iron overload.
Causes of systemic iron overload
A classification of the causes of systemic iron overload is shown in Text Box 32 (389, 390). The following section deals only with systemic iron overload which may potentially have a nutritional component. Rare genetic disorders and conditions that cause localized iron overload are beyond the scope of this review.
Text Box 32.
Systemic iron overload [adapted from Bacon et al. ( 389 ) and Pietrangelo ( 390 )]
Hereditary hemochromatosis
• HFE-related hemochromatosis (Type 1).
• Non-HFE-related:
-Juvenile hemochromatosis (Types 2A, 2B)
-TfR related hemochromatosis (Type 3)
-Ferroportin disease (Types 4A, 4B)
Secondary iron overload
Iron-loading anemias
• Thalassemia syndromes.
• Sideroblastic anemia.
• Chronic hemolytic anemia.
• Pyruvate kinase deficiency.
• Pyridoxine-responsive anemia.
Parenteral iron overload
• Transfusional iron overload (multiple red blood cell transfusions).
• Parenteral iron injections.
Chronic liver disease
• Porphyria cutanea tarda.
• Viral hepatitis, especially hepatitis C.
• Alcoholic liver disease.
• Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis.
• Following portacaval shunt.
The ability of the gastrointestinal tract to limit absorption even when large quantities of bioavailable iron are ingested is remarkable. However, acute iron poisoning may occur in young children who accidentally consume adult iron supplements (∼3 g is lethal in a 2-y-old) (391). On the other hand, there are very few reports of chronic iron overload as the result of the consumption of excessive quantities of iron in adults (392, 393). Furthermore, in most cases it would be difficult to exclude other factors such as liver disease, alcohol consumption, or an unrecognized genetic component resulting from a polymorphism affecting either hepcidin or FPN1 (392). Hepcidin suppression due to accelerated erythropoiesis (thalassemias and other hemolytic disorders; see Text Box 32) would also have to be considered. Despite the frequent inclusion of diet as a potential primary cause of chronic systemic iron overload in scholarly articles, there is very little observational evidence to support this view, with the sole exception of “African iron overload”, discussed below. The types of hereditary hemochromatosis are summarized in Table 6 (390). HFE hemochromatosis is by far the most prevalent; 10–15% of Caucasian Americans are carriers of the C282Y allele and 1 in 200–250 are homozygous for this autosomal recessive condition. Another HFE mutation, H63D, occurs in ∼20% individual worldwide (212). However, penetrance is low. Clinically significant consequences of iron overload are observed in only 28% of male and 1% of female C282Y homozygotes (394, 395).
TABLE 6.
Hereditary systemic iron-overload disorders1
| Disorder | Inheritance | Laboratory markers | Clinical phenotype |
|---|---|---|---|
| HFE hemochromatosis (type 1) | Autosomal recessive | TSAT ↑ early | Primary hepatocyte iron loading, hepatic fibrosis/cirrhosis, liver cancer |
| SF ↑ | Cardiomyopathy | ||
| Diabetes mellitus | |||
| Hypogonadism | |||
| Arthropathy | |||
| Skin pigmentation | |||
| Juvenile HJV hemochromatosis (type 2A) | Autosomal recessive | TSAT ↑ early | Similar to HFE hemochromatosis, but early onset and accelerated course, endocrine and cardiac disease more prominent than hepatic involvement |
| Juvenile HAMP hemochromatosis (type 2B) | Autosomal recessive | SF ↑ | |
| TFR2 hemochromatosis (type 3) | Autosomal recessive | TSAT ↑ early | Similar to HFE hemochromatosis |
| SF ↑ | |||
| FPN1 loss of function (type 4A) | Autosomal dominant | SF ↑ | Predominant macrophage iron loading in the liver, spleen, and bone marrow |
| TSAT often normal | Later parenchymal iron accumulation with mild liver disease | ||
| May have mild anemia | |||
| FPN1 gain of function (Type 4B) | Autosomal dominant | TSAT ↑ early | Similar to HFE |
| SF ↑ |
1 FPN1, ferroportin 1; HAMP, gene encoding hepcidin; HFE, human hemochromatosis; HJV, hemojuvelin; SF, serum ferritin; TFR2, transferrin receptor 2; TSAT, percentage transferrin saturation; ↑, increase. Adapted with permission from reference 390.
One ethnic group merits further consideration. An iron overload syndrome that occurs in South African Bantu men (originally called “Bantu siderosis”, also referred to as “African iron overload”, “sub-Saharan iron overload”) was first described in 1929 (396). An excessive iron intake due to the consumption of traditional beer brewed in iron drums was considered to be the primary etiological factor (100). The beer had an average iron concentration of 40 mg/L in a highly bioavailable form. The alcohol content was low and many individuals would regularly drink several liters a day. Fecal analyses demonstrated that 50–100 mg Fe/d was ingested from beer alone. However, subsequent studies conducted by Gordeuk et al. (397) suggested that genetic factors are equally important in the people of southern and central Africa. The most common phenotype appears to be loss of function in SCL40A1 (FPN1) acting in an autosomal dominant fashion (397, 398). The Q248H allele is the most prevalent polymorphism in sub-Saharan Africa and in African Americans. However, among African Americans the increased risk of iron overload appears to be minimal (399), perhaps because the consumption of excessive bioavailable iron is a necessary cofactor. It is nevertheless important to emphasize that the putative genetic component of sub-Saharan iron overload has not been identified with certainty at the present time.
Consequences of systemic iron overload
Iron is a highly reactive metal. Free iron can alternate between the Fe3+ and Fe2+ redox states, resulting in the gain or loss of free electrons. As a result harmful free radicals may be generated, causing damage to lipid membranes, DNA, and various cellular organelles (400). In addition, pathogens compete with body cells for iron; available iron may lead to increased pathogen virulence. The clinical consequences of systemic iron overload that are most conspicuous in patients with untreated HFE hemochromatosis are listed in Text Box 33.
Text Box 33.
HFE hemochromatosis: clinical phenotype
Nonspecific systemic symptoms
• Fatigue, malaise, lethargy.
• Weakness, weight loss.
Liver complications
• Fibrosis.
• Cirrhosis.
• Liver failure, ascites, encephalopathy.
• Hepatocellular carcinoma.
Heart complications
• Congestive cardiac failure due to iron deposition in the myocardium.
• Cardiac arrhythmias.
Pancreas
• Diabetes mellitus.
Other endocrine complications
• Disruption of the hypothalamic-pituitary axis.
• Hypogonadism, amenorrhea.
• Hypothyroidism.
Joint Problems
• Arthropathy.
• Typically pseudogout in the metacarpophalangeal joints of the hands.
• Other joints, especially hips and knees, may also be affected.
Skin
• Increased pigmentation.
• Porphyria cutanea tarda.
The clinical manifestations of secondary iron overload resulting from an iron-loading anemia such as thalassemia major include those observed in severely affected patients with hereditary hemochromatosis. Children with untreated β-thalassemia also suffer from severe anemia, marked hepatosplenomegaly, and skeletal abnormalities due to expansion of the erythroid elements in the bone marrow. Intercurrent infections, heart failure, and liver cirrhosis are the major causes of death in the first two decades of life (401).
Current controversy related to the possible role of iron in various conditions
Iron status and vascular disease
Sullivan (402) postulated in 1981 that ischemic heart disease occurs less frequently among premenopausal women than in men of the similar age because of differences in iron status. Interest in the possibility that iron had a causative role was stimulated by the observations of Salonen et al. (403) who reported that high SF concentrations in eastern Finnish men were associated with an increased risk for myocardial infarction. Numerous subsequent studies have failed to resolve the issue. In most cases iron status was defined by SF assays. Vascular disease is known to have an inflammatory component (404). Raised SF values may therefore merely reflect its properties as an acute-phase protein (APP), a conclusion that is supported by the absence of a significant association between vascular disease and either HFE hemochromatosis or “African iron overload.”
Iron and the metabolic syndrome
Diabetes mellitus is one of the most common complications of all iron overload states. The etiology is complex. Genetic factors, iron deposition in the liver, direct damage to the pancreas, and insulin resistance may all play a role (405). Patients need appropriate clinical management. The relationship between iron status, and both obesity and the metabolic syndrome has been characterized using various iron biomarkers. Increased SF values are commonly encountered in the presence of the metabolic syndrome (406). However, transferrin saturation is not significantly increased and hepatic iron overload, if present, is mild based on MRI (407). Iron is sequestered in macrophages. Hepcidin levels are raised and iron absorption decreased (408–412). The interaction between iron homeostasis and obesity has been reviewed recently (413, 414). Hyperferritinemia and hypoferremia is considered to be a consequence of hepcidin-induced iron sequestration in the reticuloendothelial system and the acute-phase ferritin response to inflammation. There is still considerable controversy about the potential role iron as a causative factor, but high-intensity blood donation which would be expected to markedly reduce the iron load was not associated with a decrease in the prevalence of the metabolic syndrome (415). Higher SF levels are also associated with type 2 diabetes. Once again, serum iron and transferrin saturation are not increased, suggesting that the rise in SF is an inflammatory response (416). Nevertheless, the relationship with iron status and the potential role of iron needs further study.
Iron and cancer
The overall risk for hepatocellular carcinoma in patients suffering from hemochromatosis is 6–10%. It is preceded by the development of liver cirrhosis and is responsible for 25–45% of all premature deaths associated with hemochromatosis (417). There is some evidence to suggest that iron status may be related to other cancers, but more research is needed to establish a causal role (418). The most convincing evidence suggests a relationship between high red meat intake and colon cancer. However, if there is a causal relationship, whether the critical factor is iron, heme, or some other meat ingredient has not been established.
Iron and infection
With the exception of Lactobacilli and Borrelia burgdorferi, all microorganisms require iron to survive (419). They compete with their hosts for available iron. The body's ability to withhold iron is considered to be a component of the innate immune response (420). Recent studies suggest that mild iron deficiency may provide a modest degree of protection against falciparum malaria, but more research is needed to establish the significance of these observations. On the other hand, several investigators have concluded that “African iron overload” increases the risk of chronic infectious disorders, particularly tuberculosis and HIV disease. These observations deal with patients who have significant organ damage, making it difficult to be sure of the specific role of iron overload. Moreover, SF, which may be confounded by the presence of inflammation, has often been used as the critical biomarker (421). Nevertheless, it seems likely that iron toxicity is a contributory factor. Similarly, iron overload is considered to be a contributory risk factor for bacterial infections which are a major cause of death in patients with thalassemia (422). On the other hand, infections are not a characteristic complication for most patients suffering from HFE hemochromatosis although the virulence of certain organisms may be increased; these include Vibrio vulnificus, Yersinia enterocolitica, Escherichia coli, and Listeria monocytogenes (423–426).
Biomarkers of iron overload
The diagnosis of iron overload is made in the clinical setting in patients who exhibit characteristic symptoms and signs or individuals considered to be at risk because of a family history of hemochromatosis or a hematological disorder that leads to secondary iron overload. Stainable bone marrow iron is an important research tool and also occasionally employed in the clinical setting. It is, however, an invasive procedure and not applicable to population surveys or the evaluation of intervention strategies. Moreover, it is important to note that bone marrow iron evaluation may be misleading in HFE hemochromatosis where the iron excess is present primarily in parenchymal cells, particularly hepatocytes.
SF and TSAT are used in combination by clinicians in the workup of individuals with clinical findings or a family history suggestive of hemochromatosis (389, 427, 428). However, screening of the general population is not recommended by either the American College of Physicians or the American Association for the Study of Liver Disease.
Investigators whose main focus is nutrition have usually employed upper levels for SF as the criterion of iron overload. The values chosen have varied, but one commonly quoted source gives levels of >200 µg/L for adult males and >150 µg/L for adult females as constituting “severe risk for iron overload” (218). Based on these criteria, >5% of men in the United States over the age of 30 y would be at risk [SF: 382 µg/L (95% CI: 344, 406 µg/L), 500 µg/L (95% CI: 434, 574 µg/L), and 552 µg/L (95% CI: 493, 574 µg/L) respectively for men aged 20–39, 40–59, and ≥60 y]. Similarly, >5% of apparently healthy women over the age of 40 y would be at risk [SF: 228 µg/L (95% CI: 201, 243 µg/L) and 391 µg/L (95% CI: 349, 445 µg/L) respectively for women aged 40–59 and ≥60 y] [Table 7 (429)].
TABLE 7.
SF and TSAT levels: 50th and 95th percentile values (95% CI) for SF and 50th and 90th percentile values (95% CI) for TSAT for selected age groups for the total US population (NHANES 1999–2002)1
| Sex, age (y) | SF 50th (µg/L) | SF 95th (µg/L) | TSAT 50th | TSAT 90th |
|---|---|---|---|---|
| F 12–19 | 26.0 (24.0, 27.0) | 76.0 (70.0, 85.0) | 19.8 (18.4, 20.9) | 32.9 (31.0, 36.6) |
| F 20–39 | 32.0 (30.0, 35.0) | 126 (110, 146) | 21.4 (19.8, 23.3) | 38.4 (35.0, 31.1) |
| F 40–59 | 53.0 (46.0, 57.0) | 228 (201, 243) | 21.5 (20.5, 22.5) | 34.8 (32.0, 37.6) |
| F ≥60 | 86.0 (80.0, 93.0) | 391 (349, 445) | 22.3 (21.1, 23.6) | 35.4 (31.8, 37.9) |
| M 12–19 | 42.0 (40.0, 54.0) | 142 (125, 154) | 25.8 (24.3, 26.9) | 42.8 (36.3, 50.4) |
| M 20–39 | 134 (125, 142) | 382 (344, 406) | 27.1 (25.7, 28.4) | 45.9 (43.7, 48.8) |
| M 40–59 | 150 (138, 168) | 500 (434, 574) | 26.8 (25.4, 28.9) | 41.6 (39.8, 43.2) |
| M ≥60 | 134 (125, 147) | 552 (493, 623) | 25.9 (24.7, 27.3) | 41.7 (39.4, 43.8) |
1SF, serum ferritin; TSAT, percentage transferrin saturation. Adapted with permission from reference 429.
It is highly unlikely that a sizeable proportion of middle-aged and older Americans are at risk for clinically significant iron overload. McKinnon et al. (430) reinforced this conclusion based on a survey of 1188 adults living in Busselton, Australia and also reported a direct correlation with BMI and a significant increase in SF levels between 1995 and 2005. They proposed a change in reference ranges to accommodate demographic and biomedical influences. The North American Hemochromatosis and Iron Overload Screening (HEIRS) Study also employed higher cut-off values (>300 µg/L for men and >200 µg/L for women) (431).
The I-EP has concluded that if the recommendations for cutoff levels published in the 2001 UNICEF/UNU/WHO guide for program managers (218) are used, SF values employed as the sole measure of iron status are of limited to value as a marker of increased iron stores in population surveys and the planning of intervention strategies. It is possible that higher SF cutoff levels would improve the evaluation of the risk of iron overload in population studies. However, the I-EP proposes that an alternative tactic based on the approach used by clinicians be considered. The first step would be to determine the prevalence of a genotype or phenotype (based on biomarkers) that is likely to explain the high SF values in the target population. The following groups are identified in Text Box 34.
Text Box 34.
True iron overload
Hereditary Hemochromatosis
Phenotype: SF↑, % TSAT↑ (with the exception of FPN1 loss of function, SF ↑, % TSAT often normal).
Iron-loading anemias
Abnormal globin genotype(s) causing thalassemia syndromes in most cases.
Phenotype: SF↑, % TSAT ↑.
Inflammation
Infection and inflammation.
Phenotype: SF↑, % TSAT ↓.
Obesity/insulin resistance/type 2 diabetes
Phenotype: SF ↑, % TSAT ↓.
Older men and postmenopausal women.
Phenotype: SF ↑, % TSAT, no trend or ↓.
Liver disease
Complex phenotypes: SF ↑, % TSAT ↑, abnormal liver enzymes.
True iron overload
An increase in TSAT preceding the progressive rise in SF is characteristic of the clinical phenotype of HFE hemochromatosis, juvenile HJV hemochromatosis, juvenile HAMP hemochromatosis, TFR2 hemochromatosis, and FPN1 gain-of-function ferroportin disease. The one exception is FPN1 loss-of-function ferroportin disease, which is characteristic of “African iron overload.” TSAT is often normal in the face of increased SF. Iron intake does not appear to play a significant role in the rate of iron accumulation in the common form of iron overload in Caucasians. In the HEIRS study, dietary iron intake was analyzed in 213 patients who were homozygous for HFE C282Y. No significant relationships between SF and dietary heme iron content, dietary nonheme iron content, or supplemental iron use were discovered (432). On the other hand, as described above, iron intake is considered to be an important factor in “African iron overload.”
Thalassemia syndromes are prevalent in many parts of the world, including the Mediterranean region, Africa, and Southeast Asia. Patients with severe anemia who may or may not be transfusion dependent require management by healthcare professionals. However, the carrier states for these disorders that are far more prevalent in the population are of most concern to nutritionists. The potential risk for inducing iron overload in thalassemia carriers depends on the type of thalassemia. Consequently, information about the prevalence of the various thalassemia syndromes must be taken into account when considering fortification or supplementation interventions.
Urinary and serum hepcidin levels are markedly decreased in β-thalassemia major and β-thalassemia intermedia (433, 434). They are also suppressed in hemoglobin E β-thalassemia, which is the most common severe thalassemia syndrome in Asia and β-thalassemia trait (435). On the other hand, Zimmermann et al. (436) measured iron absorption in 78 Thai women who were heterozygous for α-thalassemia 1, β-thalassemia, hemoglobin E, or were compound heterozygotes for hemoglobin E (HbE)/β-thalassemia. Twenty-five women with a normal globin genotype served as controls (Table 8). SF levels were significantly higher in women with α-1 thalassemia trait, β-thalassemia trait, and HbE/β-thalassemia. The increase was likely to be clinically important only for HbE/β-thalassemia. Absorption was appropriately downregulated in those with α-1 thalassemia or β-thalassemia trait, but not in HbE/β-thalassemia volunteers. The I-EP differs to some extent with the conclusion drawn by Zimmermann et al. (436) (that absorption was not adequately downregulated), and suggests that a more plausible conclusion would be that absorption was appropriately downregulated in α-1 thalassemia and β-thalassemia heterozygotes, but that the balance between stores and absorption was reached at a slightly higher level of storage iron in the case of the β-thalassemia trait. Nevertheless, the report does support our proposal that the potential risk for iron overload in populations where thalassemia syndromes are prevalent should be based on biomarkers that describe the prevalence of carrier status, particularly for β-thalassemia as well as compound heterozygosity.
TABLE 8.
Iron absorption in Thai women who are heterozygotes for HbE, α-Thal 1, or B-Thal and in compound heterozygotes for HbE/B-Thal1
| Normal Hb | HbE Trait | α-Thal Trait | Β-Thal Trait | HbE/β-Thal | |
|---|---|---|---|---|---|
| n | 25 | 26 | 18 | 27 | 9 |
| Age, y | 32.7 ± 7.8 | 32.1 ± 8.4 | 32.8 ± 11.3 | 36.7 ± 9.8 | 36 ± 10.3 |
| Weight, kg | 47.8 ± 7.3 | 53.5 ± 6.3 | 50.8 ± 8.8 | 50.4 ± 8.4 | 45.4 ± 10.0 |
| Hb | 13.0 ± 1.0 | 12.4 ± 1.2 | 11.6 ± 1.0 | 11.1 ± 0.7 | 6.9 ± 1.4 |
| SF, µg/L | 15 (1–148) | 23 (3–112) | 28 (1–142) | 52 (2–236) | 877 (206–4040) |
| TSAT | 30.7 ± 7.8 | 34.1 ± 10.2 | 30.4 ± 6.8 | 32.5 ± 7.0 | 77.8 ± 16.6 |
| % absorption2 | 8.7 (3.2, 23.4) | 7.5 (3.4, 16.7) | 5.6 (2.8, 11.3) | 4.5 (2.0, 9.7) | 10.5 (4.6, 23.9) |
1Values are means ± SDs or means (ranges) unless otherwise indicated. α-Thal, α-thalassemia; B-Thal, β-thalassemia; Hb, hemoglobin; HbE, hemoglobin E; SF, serum ferritin; TSAT, percentage transferrin saturation. Adapted with permission from reference 436.
2Geometric mean (–SD, +SD).
Inflammation
The inflammatory phenotype is not indicative of iron overload. It is induced by the combination of two pathological processes. SF is increased directly, independent of iron storage status, because it behaves as an acute-phase reactant. The quantitative relationship between SF and iron stores is disturbed. In addition, the release of iron from stores is downregulated, resulting in increased storage iron and, if of sufficient severity and duration, anemia. The inflammatory component of obesity, insulin resistance and type 2 diabetes, and aging may be important contributors to the demographic and biomedical variation for which McKinnon et al. (430) recommend reference range adjustments.
The anemia of infection/inflammation, often referred to as the “anemia of chronic disease,” is an acquired condition in which disordered iron homeostasis, due primarily to increased hepcidin secretion, has a central role. Increased SF, and a fall in the levels of serum iron, serum transferrin and TSAT are characteristic laboratory findings (437).
The relationship between iron status and obesity/insulin resistance/type 2 diabetes has been discussed previously in the section dealing with the consequences of iron overload. The preponderance of evidence suggests the increased SF is a manifestation of the associated subclinical inflammatory state and therefore not necessarily indicative of increased risk for the consequences of iron overload.
The phenotype in older men and postmenopausal women is also characteristic of a low-grade inflammatory state affecting a significant proportion of apparently healthy individuals. SF and TSAT values abstracted from a report by the US CDC based on data collected in NHANES 1999–2002 are shown in Table 7. It is noteworthy that SF values rise progressively in the 95th percentile for men and postmenopausal women whereas there is no significant trend in TSAT. At the 50th percentile there is little, if any, increase in SF.
Liver disease
The assessment of liver disease is complex and beyond the scope of this review. Evaluation by health professionals is required. However, there is one disorder that could affect the design of nutrition interventions. Hepatitis C is prevalent in some countries such as Mongolia. Patients with hepatitis C may accumulate excessive quantities of iron in the liver. Some studies have demonstrated that hepcidin production is downregulated by the hepatitis C virus (438, 439) and that iron removal by phlebotomy (440–442) may be beneficial and improve the response to treatment with interferon. More research is needed.
In summary, information about the prevalence of genotypes that put individuals at risk for iron overload may provide the most useful information when considering dietary manipulation or supplementation to improve iron status at the population level. Careful analysis of the impact of the inflammatory phenotypes that lead to increased SF levels in the absence of significant iron accumulation will also be essential.
Currently available biomarkers for evaluating iron nutrition
Biomarkers of iron nutrition and nutritional iron deficiency may be categorized as follows based on the aspects of iron nutrition that they address:
1) Intake (exposure) and excretion: Several established methods for measuring daily iron consumption are described below. The greater challenge is the determination of bioavailability. As outlined above, the diet always contains more iron than is required to meet the requirements of almost everyone. However, for myriad reasons discussed previously, the majority of the iron is not absorbed. Biomarkers of bioavailability therefore play a critical role in determining the adequacy of exposure. Biomarkers of exposure and bioavailability are discussed in the following section.
Iron excretion is not regulated in human beings. Excessive iron loss is most commonly the result of diseases that cause blood loss, the excretion of heme in the urine as consequence of intravascular hemolysis, or bleeding into the lung. Iron excretion has been measured experimentally in adults using both radio-isotopes (144, 443) and in toddlers and pregnant women using stable iron isotopes (444).
2) Status: Biomarkers of iron status were developed to characterize the stages of increasingly severe degrees of iron deficiency that would occur in an individual whose iron intake does not meet his or her requirements. As indicated above, three stages are generally recognized: storage iron depletion, iron-deficient erythropoiesis, and IDA. Currently available biomarkers make it possible to categorize the severity of iron deficiency in individuals. They are also employed in population studies to determine the prevalence of iron deficiency and to differentiate between iron-deficiency anemia and anemia due to infection and other factors.
Estimation of iron intake
The only available biomarker for iron exposure is the measurement of dietary (and supplement) iron intake. The information can be used to assess the risk of iron deficiency, and data may also be collected in order to compare the average iron intakes of different groups or to compare intakes between areas/countries. Other reasons for measuring iron intake are to rank individuals within a group and to assess the individual's usual intake, information that is often used in studies of nutritional epidemiology.
Accurate measurement of dietary iron intake is hampered by several factors, including the quality of food composition data, food fortification practices, supplement use, contamination iron, and inappropriate choice of methodology. Text Box 35 (445) provides the summary of the systematic review conducted by the EURRECA (European Recommendations Aligned) project on dietary intake methodology related to micronutrients.
Text Box 35.
Findings from systematic review on dietary intake methodology conducted by EURRECA ( 445 )
• The systematic review identified 79 studies that met the inclusion/exclusion criteria.
• FFQ was the most frequently used intake assessment method.
• Dietary record (DR) or 24-h recall were used as reference methods.
• Serum ferritin (biomarker of status, for validating intake data) was used as a reference method in 2 studies.
• When the FFQ was compared with the DR:
-Long-term intake (DR ≥7-d) correlation 0.49.
-Short-term intake (DR <7-d) correlation 0.45.
• Using a weighted DR as the reference method improves the correlation with FFQ.
• When the FFQ was compared with the 24-h recall:
-Long-term intake (7 × 24 h recall) correlation 0.45.
-Short-term intake (<7d × 24h recall) correlation 0.48.
A recent consultation sponsored by EURRECA (445) concluded that although obtaining an accurate measurement of dietary iron intake is challenging, a 7- to 10-d weighed food records (i.e., where the respondent is asked to weigh and record all foods and beverages at the time of consumption) including both weekdays and weekends (when dietary patterns may differ) is the “gold standard” for estimating iron intake.
In recent decades considerable effort has been invested in developing FFQs that give a reasonably accurate measure of average long-term intakes of nutrients. This information is required for nutritional epidemiology where associations between diet and various health endpoints are sought. Furthermore, as it can take many months for biomarkers of iron status to reach a steady state in response to changes in dietary intake, short-term (acute) measures of intake may not be appropriate and measures of usual intake may be the preferred approach. Supplemental Table 1 is a summary of selected studies used by the I-EP to evaluate the relative utility of available methods for assessing dietary intake in specific population groups.
Conclusions
The method of choice for determining iron intake depends on the question(s) being asked. Key considerations include the following: 1) day-to-day and seasonal variability; 2) between-subject and within-subject variance; 3) quality of food composition tables; 4) skills and experience of fieldworkers and individuals tasked with coding food diaries; 5) selection of foods and validation of FFQs; 6) respondent burden, compliance; 7) changes in dietary patterns initiated by dietary assessment; and 8) resources available
Duplicate diets (i.e., when a duplicate portion of all food and drink consumed throughout the day is weighed and put aside for chemical analysis) are the most accurate method as they avoid any errors introduced from food table calculations. However, they are generally only used for metabolic balance and other small-scale research studies on iron metabolism, as they do not necessarily generate representative data. A 7-d weighed record is the next most accurate option but requires good compliance from the subjects, and significant resources, primarily for fieldworkers to organize data collection and subsequent coding of the diaries, so this method is not suitable for large-scale studies. If the mean iron intake of a population, rather than that of individuals, is required, then FFQs are an acceptable tool. They sometimes overestimate (446–448) and sometimes underestimate (447, 449) iron intake, and this is possibly a function of the number of food items included since Heath (450) reported a less close agreement between diet record and FFQs when using 206 compared with 630 food items (451). They should be validated and, if necessary, calibrated against another (more accurate) method. Portion sizes need to be established, and tailored for the population group (452). Typical serving sizes can be found in reference databases [e.g. UK (453), USA (454), Europe (455, 456)]
Translating food consumption data into iron intake estimates
Whenever possible, local or regional food composition databases should be used to convert food intake into iron intake. The quality of the data is of overriding importance in that it should reflect the average and up-to-date iron content of the majority of foods in the diet. In industrialized countries, the consumption of processed and fortified foods has dramatically increased over the last 2 decades and this introduces many potential errors into the calculation of iron intake. The skill of food coders is critical as they often need to select codes for foods that closely represent any that are not listed in the food database, including assumptions about recipes and ingredients. Iron-fortified foods do not always contain the quantity of iron on the label as an “overage” may be added during processing to ensure the concentration in the final product meets the label declaration.
The EuroFIR (European Food Information Resource) FoodEXplorer (457) is an innovative interface, which allows its users the simultaneous search of more than 25 national food composition databases. Users have access to a wide range of data, linking foods and nutrients through harmonized data description (LanguaL), standardized component and value description through the use of thesauri (standard vocabularies), and associated nutrient value information. The search facility includes options to search for food name, food groups, and the most commonly used LanguaL food description, as well as the powerful and unique ability to compare the component values between foods from the several countries. The results can be downloaded as a standard Food Data Transport Package. Data in the EuroFIR FoodEXplorer includes >25 European countries as well as datasets from Australia, Canada and the United States, but access is limited to EuroFIR members. Other publications/databases include the following: United States (458) and Australia/New Zealand (459).
Estimation of iron bioavailability
As discussed previously, for the purposes of this review, bioavailability is defined as the extent to which iron is absorbed from the diet and used for normal body functions. In nutritional studies, it is customarily estimated by using an iron isotope to label the test food or iron compound and then determining the percentage of the ingested dose present in the circulating red blood cell compartment after 14 d. At this time red blood cell utilization of iron for hemoglobin synthesis is 80% in adults and 90% in infants and young children (460). The appropriate correction is made to derive an estimate of absorption. The term “relative bioavailability” is often applied to iron compounds being evaluated as potential food fortificants or dietary supplements. Customarily, this is a direct comparison between the test substance and ferrous sulfate given in the same meal (and ferrous sulfate is assumed to have a value of 100%). Adequate bioavailability is generally considered to be a vital component of iron nutrition, and poor bioavailability a critical contributor to the worldwide prevalence of nutritional iron deficiency. The majority of people consume sufficient iron to meet their physiological needs. However, absorption is limited because of the effects of dietary components, in particular phytates and polyphenols in cereal- and legume-based diets. Biomarkers of bioavailability therefore have considerable practical importance for the design of intervention strategies aimed at improving iron nutrition. Heme iron is virtually always well absorbed. However, most food iron is present as non-heme forms. Bioavailability is markedly affected by meal composition. Several indirect methods for estimating bioavailability have been developed over the past half century and calibrated against human isotopic absorption studies (461).
In vitro methods
Dialyzability: Miller et al. (462) demonstrated that there was a direct correlation between nonheme iron absorption measured by isotopic methods in human volunteers and the meal content of soluble low-molecular-weight iron complexes after simulated digestion in vitro. Briefly, mixtures of foods are homogenized and exposed at 37°C to pepsin at pH 2 (simulated gastric digestion). After 2 h, dialysis (6000–8000 molecular weight cutoff semipermeable membrane) is used to adjust the pH to intestinal levels, pancreatin and bile salts are added, and digestion continues for another 2 h. Finally the quantity of iron that diffuses across the membrane is measured.
The model provides important information about factors that affect food iron absorption. However, although it makes it possible to screen large numbers of samples rapidly, it has several limitations. Results are based on the assumption that iron destined for absorption is always bound to small-molecular-weight complexes. There are exceptions to this general rule. The results have qualitative value, but are unreliable for determining the magnitude of such effects. Furthermore, it has proven difficult to achieve uniformity between results for different laboratories. Therefore this biomarker is no longer widely used as a screening test for potential bioavailability, and has been superseded by the Caco-2 cell model.
Caco-2 cell assays: Caco-2 cells are a commercially available immortal cultured cell line that was isolated from an adenocarcinoma in a 72-y-old Caucasian man. The cells proliferate rapidly in vitro and can be induced to undergo spontaneous differentiation to develop some of the characteristics of small intestinal enterocytes. Under appropriate culture conditions they form a monolayer with tight junctions and brush border microvilli that express a number of digestive enzymes as well as the known iron transporters. The measurement of in vitro iron bioavailability has been refined by exposing monolayer cultures to food digestates prepared in a manner similar to that described above for dialysis (463). Large numbers of samples can be tested over periods of a few days. Although the results derived from this biomarker may be more precise and consistent than those from dialysis, they also have several limitations. The results predict the direction of response for all major nonheme iron absorption modifiers, but not necessarily the magnitude. Importantly, good quality cell culture facilities and experienced operators are essential. The Caco-2 cell method should be considered as an initial screen for new potential iron fortification products. However, it will always be necessary to test promising compounds in human absorption trials. The usefulness of in vitro models to predict the bioavailability of iron and zinc is reviewed in a consensus statement from the HarvestPlus expert consultation (461).
Animal models
Although several animal models have been used for studying iron absorption, the only standardized method that has seen widespread use is the Association of Official Analytical Chemists (AOAC) Rat Hemoglobin Repletion Assay (464–466). Briefly, weanling rats are fed an iron-deficient diet until they develop IDA and are then switched to the test diets. Iron absorption is derived from the increase in red blood cell mass calculated from the change in body weight and hemoglobin concentration of the experimental animal.
The method has most often been used to evaluate iron compounds being considered as food fortificants and gives a similar classification of iron compound bioavailability as in humans (467). It should however be noted that the rat does not respond to inhibitors and enhancers in the same way as humans and is not a good model to measure iron bioavailability from human diets (468).
Algorithms
Algorithms have been developed to estimate dietary iron absorption based on dietary data, including the form of the iron and the meal content of known enhancers and inhibitors of iron absorption. Monsen et al. (469) were the first to suggest this approach. However, they only considered the form of the iron (heme or nonheme) and the content of the two major enhancing factors, ascorbic acid and animal tissue (meat, fish or poultry). Nevertheless their original models are the basis for the bioavailability values employed by the FAO/WHO in calculating RNIs (78). Since this time several more comprehensive algorithms that consider the effect of important inhibitors have been published. One of the most comprehensive was developed by Hallberg and Hulthen (470). They utilize all the dietary factors that have been shown to affect nonheme iron absorption in a quantitative model that includes interactions. In addition, adjustments are made for iron status, so that bioavailability is estimated for an individual with a 40% reference absorption. Although beyond the scope of this review, the importance of estimated bioavailability in calculating iron requirements has been covered in detail elsewhere (471, 472). Armah et al. (473) published a similar algorithm based on the US diet in 2013. These newer algorithms attain greater precision, but the methods still have several important shortcomings. It is frequently difficult to obtain accurate semiquantitative data for the consumption of iron and the major enhancers and inhibitors of absorption, and the data upon which they have been based is nonheme iron, so assumptions about heme iron intake (and absorption) need to be added to the model. Furthermore, results of algorithms developed for one population group may not be representative when applied in a different setting. Finally, the results tend to underestimate bioavailability and there may be a 3-fold variation in estimates using different algorithms (474). Therefore most program planners employ the FAO/WHO absorption levels based on a qualitative description of the diet.
Human bioavailability measurements
Studies using radiolabeled iron in human volunteers are regarded as the gold standard for determining iron bioavailability and estimating absorption. Several methods, categorized according to their increasing complexity and precision, are available as described below.
Iron tolerance measurements
The method lacks sensitivity and can only be used for high doses (≥10 mg) of iron. The plasma iron concentration is measured at intervals (usually six samples at hourly intervals) after the ingestion of the iron compound of interest following an overnight fast. The rise in the plasma iron level is used as a measure of absorption by calculating the area under the curve (100, 475) or applying a compartmental model (476). Results are most often expressed as relative bioavailability with respect to FeSO4 (or another well-absorbed iron compound) by repeating the procedure with an equivalent quantity of this iron on a different day.
Isotopic absorption studies
Data obtained from isotopic studies are the basis of our understanding of food and fortification iron bioavailability. These iron bioavailability studies measure the utilization of the absorbed iron for hemoglobin synthesis and use this to estimate iron absorption. Earlier experiments utilized radioisotopes, but they have been supplanted by stable isotopes to eliminate the potential risk of exposure to radiation (477). Radioisotopes are added in trace amounts. On the other hand, milligram quantities of stable isotopes are required. As a consequence, stable isotopic methods are most suited to iron fortification studies. When used to investigate dietary factors, multiple meals are essential to avoid large increases in iron intake. The major limitation of the isotopic approach in general is the dependence on assays based on single meals or in some cases a limited number of meals on different days (478). Investigators may select meals that exaggerate the effects of bioavailability modifiers. It may therefore be difficult to predict dietary bioavailability because the effects of the absorption modifiers are often attenuated when the whole diet consumed over extended periods of time and the interaction between modifiers are considered.
Field trials
Field trials are costly and time consuming, but they provide the most accurate and reliable information about the potential impact of proposed fortification or supplementation programs. It is essential that they include a control arm because the iron status of the target group may change over time. This is particularly important for young children. The trial period should be ≥6 mo (60, 479). Iron status biomarkers, including hemoglobin, SF, sTfR, and ZPP, are used to measure the change in iron status between baseline and the end of the trial. Recent reports suggest that the most accurate estimates can be obtained by using the sTfR/SF ratio to calculate body iron index (248, 480). If this is done, it is also possible to estimate the bioavailability of the iron fortificant or supplement based on the change in body iron and the quantity of supplementary iron consumed.
Estimation of dietary bioavailability in a population
Bioavailability is a critical factor for estimating recommended nutrient intakes for iron and zinc (78). It has, however, proven difficult to obtain accurate values at the population level. As outlined above, algorithms may be misleading or difficult to apply because of incomplete dietary data. The WHO/FAO therefore employed a pragmatic qualitative approach to calculating dietary bioavailability by assigning one of three bioavailability levels depending on a general description of the diet (5%, 10%, or 15%) (78). On the basis of intake data and isotope studies, iron bioavailability has been estimated to be in the range of 14–18% for mixed diets and 5–12% for vegetarian diets in subjects with no iron stores, and these values have been used to generate dietary reference values for all population groups (26). Dainty et al. (481) recently proposed a new methodology. A full probability approach was used to estimate dietary iron bioavailability based on calculated requirements, estimated daily intake, and the distribution of SF values in the population sample. Subsequently, further refinements were made to the model using data from 2 nationally representative studies of adults in the UK and Ireland and a trial in elderly people in Norfolk, UK, and an interactive tool was published (482). An accompanying editorial (483) concluded that the model is likely to be widely used in populations for whom reliable dietary iron intake and status data are available. The method has several potential advantages. Results are based on direct observations in the target population sample. There is no need to analyze dietary data for enhancing and inhibiting factors or heme content: total iron intake is the only value that is required. The fortification level necessary to achieve a designated SF level can be estimated. There are, however, some significant constraints. The iron requirements and dietary intake must be in a steady state for at least a year. The method is therefore not suitable for children, pregnant women, or immediately after the onset of menopause. Finally, the investigators only analyzed data from adults eating a Western diet and employed SF as the biomarker for iron storage status. The approach should be evaluated in populations traditionally considered to be consuming low bioavailability diets. It would also be desirable to examine the use of body iron or the sTfR/SF ratio instead of SF alone.
Biomarkers of iron status and anemia risk
Iron stores and storage depletion
The bone marrow is a major iron storage site. All of this iron is available for erythropoiesis. The lack of iron only becomes a limiting factor once this supply is exhausted. The absence of stainable bone marrow iron is generally regarded as the gold standard for the diagnosis of iron deficiency. The invasive nature of the procedure makes it unsuitable for most epidemiological studies. It has, however, been an invaluable biomarker for the definitive identification of iron deficiency in a few well-designed field investigations in settings where the etiology of anemia is complex. It also remains an important option for diagnosing complicated anemias in hospital patients (484).
The source of erythropoiesis in the fetus is the liver until late in the third trimester when there is a liver-bone marrow “switch.” At approximately the same time, a liver-kidney “switch” for erythropoietin synthesis occurs. Thus, the interpretation of stainable iron in the neonate depends on gestational age and the timing of the switches. Consequently, due to both the changing physiology and the invasiveness of the procedure, stainable bone marrow iron is not typically utilized to determine neonatal iron status.
Serum ferritin
Although SF is an important biomarker of iron status, it represents only a minute fraction of the body's ferritin pool. Most of the ferritin iron is intracellular, with the highest concentrations being found in in cells that store iron and can also process heme (hepatocytes and specialized macrophages).
Structure and function of ferritin
The iron storage protein ferritin has a similar structure in animals and plants. The ferritin molecule consists of a protein shell with a molecular mass of ∼500 kDa composed of 24 subunits. The protein shell encloses a core of ferric-hydroxy-phosphate that can hold up to 4500 atoms of iron (485). Ferritin molecules acquire and release iron in concert with physiological needs. The mechanism of uptake and release of iron is still unclear. It is known that iron uptake requires an oxidizing agent and release a reducing agent. A range of isoferritins is found in various human tissues depending on the specific combinations of the 2 types of subunit, H and L (486). H-Rich isoferritins predominate in heart muscle and in red and white blood cells, whereas L-rich isoferritins are found primarily in liver, spleen, and placenta.
Ferritin is a soluble protein, but can degrade into insoluble hemosiderin. Both ferritin and hemosiderin provide a store of iron that is readily available to meet functional requirements. Normally most of the iron stored in the body (∼1 g in men and less in children and premenopausal women) is stored as ferritin but the proportion as hemosiderin increases with iron overload.
The uptake of iron into ferritin serves a second vital function. It prevents oxidative damage to tissues. Free iron could generate highly reactive free radicals that could damage DNA, oxidize cholesterol, or damage tissue proteins. Ferritin synthesis is induced by the presence of iron. The initial response is regulated by translation rather than by transcription (487). The translational control mechanism involves the 5′ IRE of ferritin mRNA. The eventual degradation of ferritin remains largely a mystery although studies with rat liver cells indicate that the half-life of the ferritin molecule is ∼72 h (487).
SF is a measure of the amount of iron in body stores if there is no concurrent infection or inflammation. When the SF concentration is >15 µg/L, iron stores are present. Values <12–15 µg/L are generally considered to be indicative of depleted iron stores. The SF measurement is widely available, well standardized and, in a subject with anemia, a low SF is diagnostic of IDA (488). In otherwise healthy individuals, SF concentration is directly proportional to the size of the iron store, with 1 µg/L SF corresponding to 8–10 mg storage iron in an adult (488). Although SF has been shown to give a larger and more consistent response to iron interventions than ZPP or sTfR (489), it is an APP and is increased independent of iron status by acute or chronic inflammation. The SF level is therefore difficult to interpret in settings where infectious diseases are common. It is also unreliable in patients with malignancy, hyperthyroidism, liver disease, and heavy alcohol intake (490).
SF resembles liver or spleen ferritin immunologically and is recognized and quantified by monoclonal or polyclonal antibodies raised against these ferritins. The extent to which SF is saturated with iron is variable and can be influenced by physiological conditions such as inflammation and iron overload. Iron saturation of ferritin in normal serum was reported to be 24% (491) but it is much less in iron overload. Ferritin appears to enter the blood plasma by secretion from hepatocytes or macrophages after synthesis on membrane-bound polysomes and glycosylation, or via direct release from damaged cell membranes. The 3 factors controlling SF levels are synthesis, release from the cells, and clearance from the plasma. Abnormalities occur in synthesis and release, but not in clearance. Many cells contain ferritin-binding proteins and injected spleen ferritin is rapidly taken up by the liver (492).
Normal ranges of serum ferritin
It was only after the development of sensitive immunoradiometric assays that ferritin was detected in the serum or plasma of normal individuals (493, 494). These early assays have been supplanted by enzyme-linked immunoassays using colorimetric and fluorescent substrates or by antibodies with chemiluminescent labels. The SF assay is available for routine analysis using automated immunoassay analyzers.
SF concentrations are normally within the range 15–300 µg/L and are lower in children than in adults. Mean values in women before menopause are lower than in men, reflecting women's lower iron stores caused by menstruation and childbirth. In women after menopause, SF concentration increases but remains lower than in men. The changes in SF concentration from birth to old age reflect changes in the amount of iron stored in the tissues (495). In normal individuals, there is a close relationship between the total amount of iron removed by phlebotomy and the SF concentration (496). The SF concentration is relatively stable in healthy persons and a reduction in the level of storage iron is the only known biological cause of low SF concentrations. In patients with IDA, a number of studies have been used to establish the threshold of 12–15 µg/L as indicative of iron deficiency (497) and this cut-off is the cornerstone for using SF in clinical practice to identify iron deficiency . Higher concentrations of SF may be related to infection, inflammation, adiposity, and alcohol consumption (490).
Iron supply
Serum iron, TIBC, percent transferrin saturation
Iron in the circulating plasma and extracellular fluid is bound to transferrin. Serum iron and transferrin saturation values have a circadian rhythm with most individuals exhibiting a morning peak and evening nadir. In the morning, transferrin saturation is ∼35% (range 20–55%). The pattern may be reversed in people who are awake at night and sleep during the day. When absorption and release from stores is insufficient to meet functional requirements, the serum iron level falls and the transferrin concentration (customarily expressed as the TIBC) is increased (100). The serum iron level, TIBC, and transferrin saturation are therefore all indicators of the adequacy of the iron supply to developing red blood cells and other tissues. The iron supply to developing erythrocytes is suboptimal when the transferrin saturation falls below 15% in iron-deficient, but otherwise healthy, individuals (139, 498). However, unlike iron deficiency, in infection and inflammation both plasma iron and the TIBC are reduced. Transferrin saturation is the biomarker that is employed most widely in nutrition studies as an indicator of iron deficiency; it is also one of the indicators in the “ferritin model” employed by the NHANES surveys in the United States (the “ferritin model” comprises three indicators, SF, transferrin saturation, and red blood cell protoporphyrin) (499).
As with adults, serum iron concentrations vary throughout the day in newborns, infants, and toddlers. Because of the high variation in serum iron and transferrin concentrations, percent transferrin saturation is not used as a biomarker of iron status in newborns. In the past it was employed once postnatal enteral feedings and growth had been established; percent transferrin saturation can then be useful in infants with parameters similar to those for adults indicating the presence of deficiency.
Erythrocyte protoporphyrin
Ferrochetalase catalyses the terminal step in heme synthesis, which converts protoporphyrin IX into heme by the insertion of ferrous iron. Zinc is an alternative metal substrate and ZPP is a normal metabolite that is formed in trace amounts during normal heme biosynthesis (500, 501). Erythrocyte ZPP is not increased with simple iron depletion, the stage of iron deficiency when iron delivery to the erythroid marrow is preserved. When the iron supply can no longer meet erythropoietic requirements, erythrocyte ZPP rises progressively, providing an index of the severity of the functional iron deficiency.
Using results of bone marrow examinations as the criteria for stages of iron deficiency, erythrocyte ZPP has been shown to provide a sensitive and specific measure of uncomplicated functional iron deficiency (iron-deficient erythropoiesis and IDA) (502). ZPP levels are established while red blood cells are maturing and then remain unaltered for the mature red blood cells lifespan (∼3–4 mo). Consequently, red blood cell ZPP is a measure the average iron availability to the erythroid marrow during the preceding 3–4 mo. While increased erythrocyte protoporphyrin is an early indicator of iron deficiency, the result is not specific. Levels are also increased with chronic lead poisoning, the anemia of inflammation, β-thalassemia trait, α-thalassemia trait, hemoglobin E disease, and some sickle cell carriers (503–505) and other less widely discussed xenobiotic compounds (e.g., lead and other metals, hexachlorobenzene, halogenated aromatic hydrocarbons, pesticides, sulfides, and dihydropyridines) that disrupt heme synthesis (506). Nevertheless, values >150 µmol/mol heme are highly suggestive of iron deficiency, usually with anemia, rather than thalassemia (504). Other cut-off values to distinguish between functional iron deficiency and thalassemia or hemoglobinopathy carrier status have been proposed (502).
ZPP levels have been assessed in studies of preterm infants as a marker of potential iron deficiency (348, 507). Standards for preterm and term newborns have not been formally established. A value >80 µmol/mol heme has been used as an indicator of iron deficiency in multiple studies of toddlers (262).
Reticulocyte hemoglobin and proportion of hypochromic circulating red blood cells
Reticulocyte hemoglobin content (reported as CHr, Ret-He, RHE, RHCc by different hematology analyzers), the proportion of hypochromic red blood cells, the ratio of microcytic to hypochromic red blood cells, as well as various parameters of reticulocyte volume can be measured by modern hematology analyzers (508, 509). These measures may be helpful in the evaluation of anemia in the clinical setting. For example, CHr is considered an optional approach to establish iron status in young children with anemia (510, 511). This group of methods has also been applied extensively to the management of iron status and the prediction of responsiveness to intravenous iron and erythropoiesis-stimulating agents in patients with chronic kidney disease (512). However, their application to the evaluation of nutritional anemia, particularly in limited-resource countries, is constrained by the cost of purchasing and maintaining the necessary analytical equipment.
Serum transferrin receptor
Kohgo et al. (513) were the first to identify TfR1 in the plasma by immunoassay. It is a single polypeptide chain with a molecular mass of 85 kDa, being a truncated fragment of the TfR1 monomer produced from cleavage by a serine protease (514). It circulates in the plasma bound to transferrin and is usually called the soluble or sTfR. It can also be called the plasma transferrin receptor. sTfR concentration is closely linked to cellular iron demands and the erythroid proliferation rate (515). When intracellular iron supply is reduced, cell surface TfR1 expression is upregulated in order to acquire more iron. It is downregulated when there is sufficient iron. Parallel changes in the concentration of sTfR occur.
The main source of serum sTfR is bone marrow erythroid precursors (516). Once iron stores are exhausted, soluble TfR values rise progressively as hemoglobin concentrations fall in adults undergoing phlebotomy (517). By calculating the induced iron deficit, Skikne et al. (517) demonstrated that the sTfR level provides a quantitative estimate of the size of the induced iron deficit in iron-deficient adults. sTfR concentrations have also been shown to correlate with the severity of iron deficiency in young children (518) and with the evaluation of iron status based on stainable bone marrow iron (519–521). However, sTfR concentrations are also affected by the rate of erythopoiesis (515, 522, 523). When iron supply is not limiting, the sTfR level can be used to monitor bone marrow erythropoietic activity (524).
When sTfR was measured in 12 nonanemic women aged 23–30 y on 15 consecutive days under standardized conditions the day-to-day variation was 8.1%, and there were no significant differences across the menstrual cycle. The conclusion of this study was that one blood sample was sufficient to measure sTfR (525). Although not typically used in newborns, infants, and toddlers, the developmental trajectory of plasma TfR has been studied (526, 527). Serum TfR increases during the first 4 mo of life and then remains relatively stable during the first year in breastfed infants (528).
A number of lifestyle and environmental factors have been reported to affect sTfR. Lower sTfR values were observed among smokers in a cross-sectional study (529). Although high consumption of alcohol is associated with liver damage and changes in iron metabolism, sTfR values in alcoholics were reported to be independent of weekly alcohol intake, age, duration of consumption, length of abstinence, time of last drink, and liver function tests (530). sTfR and sTfR/log ferritin index were unaffected by oral contraceptive use (531). In a study examining iron status and nutrient intake in 28 highly active (>12 h purposeful physical activity per week) and 28 sedentary young women it was reported that although the active women had higher iron and similar heme iron intakes, they had higher sTfR, sTfR/log ferritin index and lower ferritin concentrations than the sedentary women (532). It is not possible to draw conclusions about the etiology of the raised sTfR from this study but other published literature in the field indicates that high levels of physical activity are associated with lower iron status. In a cross-sectional study that compared the iron status of 234 obese adults with that of 172 nonobese adults attending an outpatient clinic, the obese patients had a higher prevalence of iron deficiency defined by sTfR and serum iron but not by ferritin (533). The authors suggest that the hypoferraemia of obesity is due to both true iron deficiency and inflammatory-mediated functional iron deficiency. By measuring serum hepcidin in obese premenopausal women, Tussing-Humphreys et al. (534) were able to investigate the reason for iron depletion in obesity. They postulated that hepcidin is expressed in response to obesity-related inflammation rather than changes in iron status, and concluded that the iron deficiency of obesity is a true body iron deficit rather than maldistribution of iron due to inflammation. These observations suggest that sTfR is an accurate biomarker of iron status in obese and overweight individuals. Finally, a systematic review of the relationship between obesity and hypoferraemia (535) included 25 studies of which 10 examined iron status in free-living obese individuals and 15 reported baseline iron biomarkers from bariatric surgery candidates. Nonobese subjects were used as controls in only 40% of the studies and sTfR was measured in only a few of the more recent studies. When reported, sTfR was slightly (and hepcidin was markedly) elevated in the obese subjects, but it was not possible to draw definitive conclusions due to insufficient data.
A large (n = 221) cross-sectional study demonstrated that men with altered glucose tolerance had significantly increased sTfR and ferritin values compared with normal controls (536). There was a negative correlation between SF and sTfR. Insulin sensitivity and glucose tolerance status were negatively correlated with sTfR.
sTfR concentrations are affected by erythropoietic rate. When iron supply is not limiting, sTfR levels are proportional to bone marrow erythropoietic activity (524). The evaluation of suspected IDA in the clinical setting must include information on conditions that impact erythropoiesis. The prevalence of some thalassemia traits may complicate the interpretation of sTfR levels in population surveys. sTfR concentrations are raised in carriers of α0- and β-thalassemia trait. Nevertheless, sTfR may still be informative if diagnostic criteria are adjusted to differentiate between iron deficiency and thalassemia traits (537–539).
sTfR/SF ratio
The sTfR/SF ratio has been shown to be more reliable than either parameter alone for the identification of iron deficiency (540). The sTfR/SF ratio proved to be the best predictor of absent bone marrow iron in a large population of schoolchildren with severe anemia and a wide variety of diseases including bacteremia, hookworm infection, HIV disease, and vitamin A and B12 deficiencies (541). However, the practical utility of the sTfR/SF ratio for detecting iron deficiency in the presence of inflammation is still unclear. The sTfR/SF ratio has also been shown to be the most sensitive indicator of a change in iron status following iron supplementation (542).
Iron-deficiency anemia: hemoglobin, Hct, MCV, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, RDW
The circulating red blood cells represent the largest and most accessible functional compartment for iron. The measurement of hemoglobin is both an important screening tool for detection of iron deficiency and an important criterion for determining severity. The Hct or packed cell volume was fairly widely employed in the past. However, it provides no additional information if hemoglobin values are available. The sensitivity of hemoglobin as a screening test for iron deficiency is low because there is considerable overlap in the in the hemoglobin concentrations of healthy individuals and those with mild or moderate iron deficiency. Specificity is also poor since other causes of anemia are prevalent in Africa, Asia, the Mediterranean region, and to some extent South America. The most important are other nutritional deficiencies, particularly vitamin A deficiency, infectious diseases (particularly malaria, HIV disease, and tuberculosis), and inherited red blood cell disorders (particularly the thalassemia syndromes and hemoglobin E disease).
Changes in the red blood cell indexes [reduced MCV and mean corpuscular hemoglobin (MCH) and increased RDW] are characteristic, but relatively late indicators of iron-deficient erythropoiesis. Reliable results are provided by automated instrumentation. However, they lack specificity. Several algorithms that allow iron deficiency to be distinguished from other causes of microcytic hypochromic anemia have been published (543, 544). They have not, however, gained widespread acceptance. Measurement of reticulocyte indexes, including the hemoglobin content of reticulocytes (CHr) and the reticulocyte hemoglobin equivalent (Ret He) provide earlier indicators of functional iron deficiency, but require specific models of hematology analyzer and are generally not used in field studies.
Hemoglobin concentrations are higher in newborn infants (130–180 g/L) than in older infants, toddlers, and children (>110 g/L) (545). A newborn with a hemoglobin concentration <130 g/L is considered anemic, although the etiology of anemia is much more likely to be due to blood loss or hemolysis than to iron deficiency. Iron-deficiency anemia at birth is extremely rare because of the active transport of iron from mother to fetus even in the face of significant maternal deficiency (177). Infants undergo a “physiologic anemia” in the first 6–8 wk. By 6 mo of age, hemoglobin norms are the same as older children and adults (>110 g/L). The MCV is higher in newborn infants due to the presence of fetal hemoglobin. Thus, the utilization of low MCV as an indicator of iron deficiency is problematic. The RDW is also elevated in neonates as the relatively larger red blood cells containing fetal hemoglobin (t1/2 = 60 days) are replaced by smaller cells containing hemoglobin A. Standards for hemoglobin, Hct, MCV, MCH concentration, and RDW after 6 mo of age have been published by the CDC (234, 546).
Implications related to inflammation for the interpretation of biomarkers of iron status
The evaluation of iron status in countries where infectious disorders, particularly recurrent childhood infections, parasitic illnesses, HIV disease, and tuberculosis, are prevalent is complicated by the behavior of SF as an acute-phase protein. Low-grade inflammation is also common in older people in all countries, confounding the use of SF as a biomarker of iron status. On the other hand, sTfR concentrations are less influenced by inflammation than SF (547), making it potentially a more robust measure of iron deficiency than SF in individuals suffering from infectious and inflammatory disorders, the ACD, and in the elderly (540, 548–551). However, some prevalent infectious disorders, particularly malaria, may be associated with increased sTfR levels because of changes in eythropoietic rate (552).
The systemic response to infection or tissue damage is a series of events known as the acute-phase response (APR). The local reaction is inflammation. The systemic APR may be induced by infection, inflammatory disorders (e.g., obesity, diabetes, malignancy) as well as physical trauma. At the metabolic level, the APR comprises the increased production (positive) or reduction (negative) of a numerous APPs prior to the full activation of the immune response. The main purpose of the APR is to prevent damage to the tissues by removing harmful molecules and pathogens. The changes in the levels of APPs reflect changes in their production by hepatocytes, which in turn are regulated by cytokines such as IL-1, IL-6, and tumor necrosis factor α acting in a complex network (553). The implications of the APR and inflammatory response to nutrition and vice versa was the focus of a collaboration between NICHD and BMGF called Inflammation and Nutrition Science for Program/Policy and Interpretation for Research Evidence (INSPIRE) (128).
For the purposes of this review, it is important to acknowledge that biomarkers used to assess iron nutrition, particularly SF (increased in inflammation) and transferrin (decreased in inflammation), are APPs influenced by the APR, which thus affects their selection, use, and interpretation. The case of SF offers an opportunity to explore the current efforts to address this challenge. The patterns by which APP rise and fall with inflammation have been studied and evaluated with the aim of using concentrations of other relevant markers of inflammation to account for the influence of inflammation on SF values to yield a value that would reflect only nutritional iron status. The timing of the increase, the extent of the rise and the period over which the concentrations of different APPs are elevated varies considerably depending on the specific APP and the type of infection or inflammatory disorder (128, 554, 555).
Northrop-Clewes (554) reviewed several studies to compare the responses reported for SF and inflammatory markers, including α-1-acid glycoprotein (AGP), CRP, α1-antichymotrypsin (ACT), haptoglobin, and fibrinogen, to infection or trauma (556). SF increased from its initial value by an average of ∼2.5-fold, peaked at 4 d and was still elevated at 6 d. AGP had a similar pattern to SF and increased on average 2-fold, peaked between 2 and 4 d and was still elevated at 6 d. On the other hand, both CRP and ACT rise and fall more rapidly than SF. The average increase in CRP was ∼3-fold, the peak value occurred at day 2. After 6 d the CRP was approaching baseline. ACT increased ∼2-fold, peaked at 2 d and had decreased somewhat at day 6, but less so than CRP. Haptoglobin and fibrinogen were still rising at day 6.
The potential of using SF to quantify iron deficiency in populations with widespread infections and inflammation has been the focus of considerable recent effort. A partnership between the WHO and CDC has convened a working group to address the use of SF as a biomarker for population surveillance. Those deliberations are being informed by collaboration between NICHD, CDC, Bill & Melinda Gates Foundation, and the Global Alliance for Improved Nutrition (GAIN) called the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project. Both projects have been informed by the INSPIRE Project. INSPIRE included an effort to identify potential approaches to account for the impact of the APR/inflammation on selection and interpretation of biomarkers.
Text Box 36 (294, 490, 557–559)outlines the primary approaches that were identified by the INSPIRE consultancy and are now being evaluated by both BRINDA and the WHO/CDC efforts.
Text Box 36.
Potential approaches to account for inflammation
• Ignore inflammation
Comment: the redistribution of the biomarker of interest without a real change in the total body content of the micronutrient results in a distorted measurement of the micronutrient status.
• Exclusion of the sample with elevated biomarkers of inflammation
Comment: not useful in areas of high infection burden and other acute and chronic inflammatory disorders, e.g., parts of sub-Saharan Africa. as this approach could substantially decrease sample size and could lead to an atypical residual sample which would bias the results especially if subjects with iron deficiency are more susceptible to infection.
• Change cut-off values
Comment: as the rise and fall of ferritin in response to infections and inflammation is variable and time dependent, the sensitivity of diagnosis would be expected to be low (490).
• Standardization (to calculate the prevalence of micronutrient deficiency in those with and without inflammation, then calculate a weighted prevalence estimate using a “standard” prevalence estimate of inflammation).
Comment: too many uncertainties regarding prevalence and type of inflammation and differences in biomarker/APP response: temporal, direction (up or down), and magnitude.
• Correction factor approach, e.g., Thurnham et al. (557)
Concerns: differences in inflammatory response in terms of developmental biology (infants compared with adults, pregnancy, etc.), temporal changes in context of acute compared with chronic infection (APP selection; CRP compared with AGP), and differences in relationships of nutrient biomarkers to inflammatory markers (294, 558, 559).
• Statistical options/regression modeling
Comment: current focus of both WHO and BRINDA projects.
These projects involve both systematic reviews of the relevant extant literature and de novo analyses of existing datasets to explore the biomarker/inflammation relationship. The Text Box 37 (560–565) summarizes some of the key findings of the BRINDA project specific to the intersection of iron biomarkers and inflammation.
Text Box 37.
Summary of BRINDA Aims and Key Findings ( 560 – 565 )
Aim 1: Identify risk factors of inflammation as defined by the positive APPs): CRP and AGP
• Inflammation as indicated by elevated CRP and AGP is common in population-based nutrition surveys of women of reproductive age (WRA) and preschool children (PSC).
• Factors associated with elevated CRP differ from those associated with AGP.
-A consistent positive relationship was observed between CRP and obesity among WRA
-A consistent positive relationship was observed between AGP and stunting in PSC
• Variability in the factors associated with CRP or AGP suggest the need to measure these APPs directly to understand inflammation in populations.
• Elevated CRP or AGP could not be predicted by sociodemographic covariates
Aim 2: Assess the relationships between inflammation and biomarkers of iron status and compare adjustment approaches in pursuit of more accurate assessment of micronutrient status of populations
Ferritin
• The association with CRP/AGP was consistent in almost all data sets, and in both PSC and WRA.
• The strength of correlation ranged widely between countries and tended to be stronger in children than in women.
• No clear cutoff (threshold) for CRP or AGP at which there was a change in the relationship between inflammation and ferritin was found.
BRINDA conclusion
• The regression correction is proposed as an improvement to the correction factor approach to account for the full range and severity of inflammation.
• Adjusting for malaria in addition to CRP and AGP did not significantly change the estimated prevalence of depleted iron stores.
sTfR:
• Association with AGP was stronger and more consistent than association with CRP in both PSC and WRA.
BRINDA conclusion
• The regression correction approach should be used to adjust TfR for AGP to account for the full range and severity of inflammation.
• The effect of adjusting the prevalence of iron-deficient erythropoiesis (i.e., elevated TfR) for CRP was minimal and inconsistent across surveys and therefore not recommended.
• In most countries, adjusting for malaria in addition to AGP did not significantly change the estimated prevalence of iron-deficient erythropoiesis.
Body iron index
• There was a slight positive association with CRP and AGP in both PSC and WRA.
BRINDA recommendation
• Body iron index is affected by inflammation and should be adjusted when estimating the prevalence of low body iron index, particularly in children living in areas with a high prevalence of inflammation and infections.
• Malaria does not appear to have an additional effect on body iron index that is independent of inflammation.
Aim 3: To assess factors associated with anemia amongst PSC and WRA, and to estimate the proportion of anemia associated with iron deficiency
• In both PSC and WRA, the proportion of anemia associated with iron deficiency depended on the underlying prevalence of infection/inflammation.
• The proportion of anemic individuals with concomitant iron deficiency varied by the burden of infections in the country and ranged from 30% to 58% in PSC and from 35% to 71% in WRA. Inflammation was associated with anemia in countries with high infection burdens.
Analytical Considerations
The physiologic aspects of the biomarkers of iron deficiency were described in the previous section. This section provides an overview of the analytical aspects, of the tools and methods used to ensure the quality of the measurement, and of preanalytical considerations that are relevant to sample collection, processing, and storage because they may affect the measurement results for these biomarkers. In addition to the presence of stainable iron in the bone marrow, iron status biomarkers can be categorized into hematological (red blood cell parameters) and biochemical indicators (mainly serum-based parameters, but also erythrocyte protoporphyrin). Most analytical methods that measure these biomarkers are well established and widely used in clinical practice, program evaluation, and research settings. They are relatively simple analytical techniques compared to techniques used to measure other nutrients in biological samples, yet there are remaining issues with regards to assay comparability and standardization. This section summarizes the advantages and disadvantages of common analytical methods used to measure whole-blood-based (Table 9) and serum-based (Table 10) iron status indicators.
TABLE 9.
Advantages and disadvantages of the main methods used to measure whole-blood-based iron status indicators1
| Indicator | Method | Advantages | Disadvantages |
|---|---|---|---|
| Hemoglobin | HemoCue | • Battery-operated, hand-held instrument | • Requires freshly collected blood |
| • Point of contact result can be reported to the participant | • Capillary blood sampling technique needs to be “standardized” to avoid considerable variability | ||
| • Requires one drop of blood | |||
| • Can be performed from a finger stick | |||
| • Inexpensive | |||
| • Suited for low-resource settings | |||
| Automated flow cytometry | • Fully automated instrument | • Restricted to laboratory setting | |
| • Available in most hospitals | • Usually requires venous blood from which the analyzer aspirates a small amount of specimen through a small-bore tube | ||
| • Good precision | • Instrumentation requires regular maintenance and periodic technical service | ||
| • Different blood cells can be counted; results can provide insight on general health status | |||
| Hematocrit or packed cell volume | Microcentrifuge | • Simple and inexpensive | • Requires freshly collected blood |
| • Instrument is portable | • Requires stable power supply | ||
| • Requires <100 µL blood | • Poor reproducibility | ||
| • Suitable for low-resource settings | • Blood may spill into centrifuge if capillaries are not properly sealed or if they break | ||
| Automated flow cytometry | • Fully automated instrument | • Restricted to laboratory setting | |
| • Available in most hospitals | • Usually requires venous blood from which the analyzer aspirates a small amount of specimen through a small-bore tube | ||
| • Good precision | • Instrumentation requires regular maintenance and periodic technical service | ||
| • Different blood cells can be counted; results can provide insight on general health status | |||
| Erythrocyte protoporphyrin | Hematofluorometer (ZPP, ZPP/H) | • Simple instrumentation | • Requires freshly collected blood |
| • Can be used in the field, if set up in one location (do not place in direct sunlight or in a draft) | • Needs stable power supply (voltage regulator) | ||
| • Point of contact result can be reported to the participant | • Moderate precision | ||
| • Requires one drop of blood | • Fluorescent interferences possible | ||
| • Can be performed from a finger stick | • Technique is important (oxygenate blood sample if not freshly collected) | ||
| • Inexpensive | • Instrument requires readjustment after moving (optics get misaligned easily) | ||
| • Calibration has to be done at the factory | |||
| Chemical extraction with conventional fluorometry (free erythrocyte protoporphyrin) | • Older or frozen EDTA anticoagulated blood can be used | • Manual multistep assay | |
| • Dried blood spots can be used | • Requires chemical fume hood due to the use of ethyl acetate/acidic acid and diluted hydrochloric acid; fumes are irritating | ||
| • Requires only 20-µL specimen per test | • Moderate precision (need for duplicates) | ||
| • Requires relatively inexpensive fluorometer | • Requires complete hemolysis of cells (either through freezing or dilution of fresh whole blood 1:5 with saline) | ||
| • Can be done with washed erythrocytes (when using fresh blood) to remove potential fluorescent interferences | • External fluorescent contamination possible (from wooden applicator sticks, glassware with soap residues, some hand lotions) | ||
| • Nondisposable actinic glassware needs to be washed with 10% (v/v) hydrochloric acid and thoroughly rinsed with deionized water, because concentrated acids quench fluorescence | |||
| • Work under subdued light conditions, particularly when handling calibrators | |||
| • Requires Hct to correct for packed cells |
1Hct, hematocrit; ZPP, zinc protoporphyrin; ZPP/H, zinc protoporphyrin/heme ratio.
TABLE 10.
Advantages and disadvantages of the main methods used to measure serum-based iron status indicators1
| Indicator | Method | Advantages | Disadvantages |
|---|---|---|---|
| Serum iron, TIBC, or UIBC | Colorimetric assay | • Requires relatively inexpensive photometric instrument | • Manual multistep assay |
| • Relatively inexpensive | • Requires fairly high sample volume (≥500 µL) | ||
| • Rigorous elimination of iron contamination | |||
| • Moderate precision | |||
| Clinical chemistry analyzer (colorimetric) | • High sample throughput | • Typically requires sample volume of ≥150 µL | |
| • Quick turnaround time to first result | • No control over lot-to-lot variability or assay recalibration/reformulation | ||
| • Available in commercial kit form for several instrument platforms | • Moderately expensive instrumentation | ||
| • Minimum operator involvement | • Instrumentation requires regular maintenance and periodic technical service | ||
| • Good precision | |||
| • Relatively low reagent cost | |||
| Atomic absorption spectrophotometry | • Relatively simple processing | • Relatively expensive instrumentation | |
| • Quick analysis time | • Instrumentation requires regular maintenance and periodic technical service | ||
| • Good precision | |||
| Serum transferrin | Immunoassay on analyzer | • High sample throughput | • Typically requires sample volume of ≥150 µL |
| • Quick turnaround time to first result | • No control over lot-to-lot variability or assay recalibration/reformulation | ||
| • Available in commercial kit form for several instrument platforms | • Moderately expensive instrumentation | ||
| • Minimum operator involvement | • Instrumentation requires regular maintenance and periodic technical service | ||
| • Good precision | |||
| Serum ferritin | ELISA assay | • Requires small to moderate sample volume (50–100 µL) | • Manual assay; several pipeting steps; adherence to strict timing |
| • Requires relatively inexpensive microplate reader (450 ± 10 nm filter) | • Plate washer is recommended; insufficient washing may result in poor precision | ||
| • Available in commercial kit form from several manufacturers | • Moderate precision (duplicates recommended) | ||
| • Reagent cost can be moderately high | |||
| Immunoassay on analyzer | • High sample throughput | • Typically requires sample volume of ≥150 µL | |
| • Quick turnaround time to first result | • No control over lot-to-lot variability or assay recalibration/reformulation | ||
| • Available in commercial kit form for several instrument platforms | • Moderately expensive instrumentation | ||
| • Minimum operator involvement | • Instrumentation requires regular maintenance and periodic technical service | ||
| • Good precision | |||
| • Relatively low reagent cost | |||
| Serum soluble transferrin receptor | ELISA assay | • Requires small sample volume (≤50 µL) • Requires relatively inexpensive microplate reader (450 ± 10 nm filter plus second filter for 550–650 nm) • Available in commercial kit form from few manufacturers | • Manual assay; several pipetting steps; adherence to strict timing • Plate washer is recommended; insufficient washing may result in poor precision • Moderate precision (duplicates recommended) • Reagent cost can be moderately high |
| Immunoassay on analyzer | • High sample throughput | • Typically requires sample volume of ≥150 µL | |
| • Quick turnaround time to first result | • No control over lot-to-lot variability or assay recalibration/reformulation | ||
| • Available in commercial kit form for several instrument platforms | • Moderately expensive instrumentation | ||
| • Minimum operator involvement | • Instrumentation requires regular maintenance and periodic technical service | ||
| • Good precision | • Moderately high reagent cost |
1TIBC, total iron-binding capacity; UIBC, unsaturated iron-binding capacity.
Stainable bone marrow iron
The Prussian blue stain (potassium ferrocyanide) is used to stain bone marrow aspirates or biopsies. Most of the stainable iron is hemosiderin located in macrophages. Roughly half of the nucleated erythroid precursors also contain small cytoplasmic iron granules (sideroblasts). The amount and distribution of the stainable iron provides a semiquantitative estimate of the size of body iron stores (566). The evaluation of bone marrow requires an experienced observer and careful attention to detail. Absent bone marrow iron may be reported in aspirates that contain insufficient bone marrow stroma for adequate evaluation (567). Iron contamination may occur if acid-washed glassware and slides are not used. Iron may be leached out of biopsy specimens during decalcification. Finally patients who have received some types of parenteral iron such as iron dextran that are processed in macrophages may have stainable bone marrow iron despite being iron deficient because the iron is in a form that is not easily mobilized (568). In malarial regions, hemozoin (malarial pigment) in the bone marrow may be misidentified as storage iron (521).
Hematological parameters
Hb
Hb measurements, used to assess anemia in an individual or in populations, are well standardized and provide good precision (Tables 9 and 11). The accepted reference method for the determination of Hb in human blood is the photometric determination of hemiglobincyanide (cyanmethemoglobin) (569). This method reliably measures all Hb variants except sulfhemoglobin. Red blood cells are lysed and potassium ferricyanide, which oxidizes Hb to methemoglobin, is added. Methemoglobin combines with potassium cyanide to form cyanmethemoglobin. The brown color is measured spectrophotometrically. The end point of the reaction is stable and the reaction is linear to ≥20 g/dL. However, reagents for cyanmethemoglobin are light sensitive and poisonous. This method is the benchmark against which all other methods are evaluated. It is the basis for Hb measurements on most automated cell counter analyzers and for hand-held “screening” devices. In this field-appropriate technology, whole blood is collected into a disposable cuvette containing reagent in dried form for Hb measurements and cyanmethemoglobin or azidemethemoglobin is measured with a simple, portable, dedicated photometer.
TABLE 11.
Available reference materials and proficiency testing programs for iron status indicators1
| Indicator | Reference materials | Selected list of PT8 programs |
|---|---|---|
| Hemoglobin | NIBSC IS 98/708 (dilute solution of hemiglobincyanide produced from bovine blood; 49.8 µmol/L [803.3 mg/L]; consensus value) | CAP Hematology and Clinical Microscopy Survey |
| Serum iron | NIST SRM 937 (iron metal); NIST SRM 3126A (iron standard solution) | CAP Chemistry Survey and Cal V/L Survey; UK NEQAS |
| Serum ferritin | NIBSC RM 94/572 (human plasma, freeze-dried; recombinant; 6.3 µg/ampoule; consensus value) | CAP Chemistry Survey and Cal V/L Survey; UK NEQAS |
| Serum soluble transferrin receptor | NIBSC RR 07/202 (human serum, freeze-dried; recombinant; 21.7 mg/L and 303 nmol/L [free sTfR monomer]; gravimetric/spectrophotometric value assignment) | CAP Soluble Transferrin Receptor Survey |
1Reference materials found online (577, 578). Cal V/L, Calibration Verification and Linearity Survey; CAP, College of American Pathologists; IS, international standard; NIST, National Institute of Standards and Technology; PT, proficiency testing; RM, reference material; RR, round robin; SRM, standard reference material; sTfR, serum transferrin receptor; UK NEQAS, United Kingdom National External Quality Assessment Service. Adapted with permission from reference 579.
The HemoCue is one such portable device that has been used worldwide to assess anemia rates in various populations. For tips on how to use the HemoCue instrument, how to collect a valid specimen, and how to avoid common mistakes, the reader is referred to the Micronutrient Survey Toolkit (570) and a manual published by Helen Keller International (571). Different HemoCue models are available (B-Hb, Hb-201, and Hb-301); the two newer models (Hb-201+ and Hb-301) no longer require the use of a control cuvette due to an internal self-test. The Hb-301 additionally offers an extended temperature range (10–40°C) for the storage of cuvettes compared to the previous models (15–30⁰C). This latest model measures absorbance of whole blood at an Hb/BbO2 isobestic point with turbidity compensation and does not require hemolysis of the sample. The instrument is factory calibrated against the reference method from the International Council for Standardization in Haematology (ICSH), needs no further calibration, and has no active reagents in the cuvette. Using venous blood from adult volunteer donors, good comparability of the B-Hb and Hb-201 models was demonstrated (572), but systematic differences between the Hb-301 (2.6% higher) and Hb-201 models were found (573). The exposure of cuvettes for all three models to elevated temperature (37°C) for up to 3 wk had only minimal effects (<1%) on Hb results (572). However, exposing the Hb-201 cuvettes to moisture and elevated temperature caused them to fail within minutes, whereas the Hb-301 cuvettes withstood those conditions for 3 wk (573). The largest source of error with this technique is the use of improperly collected capillary samples. This issue is discussed in the preanalytical factors section.
Hematocrit
The Hct does not offer new or additional information about anemia beyond Hb and unless the value is needed as a measure of packed cells to normalize other blood-based measurements, measuring the Hct in the field is probably not worth the effort. Field assays are often unreliable because of difficulties encountered in calibrating portable centrifuges. Automated cell counter analyzers provide calculated Hct results as part of the complete blood count profile (Table 9).
Other red blood cell parameters
The MCV, MCH, and RDW are also part of the complete blood count profile obtained from automated cell counter analyzers, but are no longer commonly used in the diagnosis of iron deficiency. As indicated above, measurement of reticulocyte indexes (CHr and Ret He) require specific models of hematology analyzers and are generally not used in field studies.
Biochemical indicators
Erythrocyte protoporphyrin
Porphyrin compounds fluoresce in the red portion of the spectrum when excited by light at a wavelength corresponding to their Soret absorption maximum. ZPP can be measured directly by either reflective fluorescence in a hematofluorometer or after extraction of the zinc moiety using ethyl acetate and hydrochloric acid. The free erythrocyte protoporphyrin (FEP) is measured by conventional fluorometry at an excitation/emission wavelength of 405/620 nm (Table 9). Both FEP and ZPP should be interchangeable with the term “erythrocyte protoporphyrin” (EP). The 1996 National Committee for Clinical Laboratory Standardization (NCCLS) EP testing guideline (574) and an article by John Beard (575) discussed these two analytical approaches in detail as well as the variety of reporting units and equations used to convert units. The main advantage of the chemical extraction method is that it does not require freshly collected blood. However, the procedure is complex and requires chemicals that pose safety hazards. These may be some of the reasons why it is no longer widely used.
The simpler hematofluorometer method has been employed more often in the recent past, particularly to screen for elevated EP values as a result of iron deficiency or lead poisoning in children. This method has several advantages for field studies: the instrument provides a direct estimate of the ZPP/H, only a drop of capillary or venous blood is required, the volume of the sample need not be measured, and neither processing nor anticoagulation is required. The excitation light at 415 nm is focused on the bottom of a drop of blood on a horizontal glass slide at an angle of 37° to the vertical. The light emitted from the sample at 596 nm is collected below the sample and is proportional to the ZPP/H. Calibration standards are supplied by the manufacturer. While this direct approach is attractive for studies in low-resource settings, two factors limit its use. Most importantly, the instrument requires frequent recalibration to ensure appropriate alignment of the filters and mirrors if it is transported under unfavorable conditions. It has to be shipped back to the manufacturer for this to be done. A secondary concern is interference by non-porphyrin fluorescent compounds, such as bilirubin, riboflavin, and certain medications, that may be present in the plasma. The accuracy of the estimate can be improved by using saline-washed erythrocytes. Continued problems with calibration, sources of bias, and discrepancies in results between the major manufacturers of these instruments have complicated the use and interpretation of ZPP/H assays.
Serum iron, TIBC, and transferrin saturation
The serum iron pool represents the amount of ferric iron (Fe3+) that is in transit through the circulation bound to transferrin. Three assay results are customarily reported together, namely the serum iron (SI), the transferrin concentration, usually reported as the quantity of iron that can be bound to transferrin (TIBC), and the TSAT (= SI × 100/TIBC) (575). The utility of SI and TSAT as screening tools for iron deficiency is, however, limited by the circadian variation, the confounding effects of infectious diseases, and many other clinical disorders.
Analytical methods for SI are either based on colorimetric principles (manual assay or using automated analyzer) or direct assay by atomic absorption spectrophotometry (100, 576) (Tables 10 and 11) (577–579). The former approach is available in most clinical laboratories, whereas the latter approach is mostly available in research laboratories. Both approaches are well-established procedures that have been available for decades. In 1998 the NCCLS approved standards for the determination of serum iron, TIBC, and TSAT and provided a detailed description of the colorimetric manual laboratory procedures (580). While rigorous elimination of iron contamination has been a critical concern for the manual colorimetric assay, this has not been a problem with the use of automated analyzers.
The concentration of serum transferrin can be measured either directly by immunologic methods or by the use of the TIBC as a proxy measure of transferrin (100, 576). The TIBC assay is identical to the SI assay, but applies an additional step (saturation of iron-binding sites of the transferrin molecule with excess iron) followed by the removal of the unbound iron. Most clinical analyzers actually measure the unsaturated iron binding capacity (UIBC) because it is more easily automated; the TIBC concentration is then calculated by summing SI and UIBC concentrations. This works well for the detection of iron depletion when SI concentrations are low and UIBC concentrations are high; however, in the presence of iron overload the measurement of very low UIBC concentrations may result in relatively high method imprecision. In an interlaboratory comparison study, Blanck et al. (581) found no significant differences in SI, TIBC, or UIBC results among methods [and low within-method variation for SI (CV <3%)] regardless of the chromogen used to form the color complex with iron (ferine or ferrozine) and whether the automated methods were corrected for copper and protein.
Serum ferritin
The measurement of SF is a well-established routine procedure carried out by clinical laboratories (Tables 10 and 11). To allow for high sample throughput, most laboratories use fully automated immunoassays for which several manufacturers produce commercial kits for various analyzer platforms. If a laboratory does not have access to a clinical analyzer, a manual ELISA can be used (492, 582). Commercial kits from several manufacturers are available for this type of assay. While procedures for the analysis of ferritin in plasma or serum (583, 584) spotted onto filter paper have been published, they have been only rarely utilized. Procedures for the storage and transport of dried plasma or serum spots are more convenient than those for frozen samples; however, a centrifuge (and electrical power) is still needed. While ferritin can be measured in dried blood spots, its usefulness is diminished by the release of much higher ferritin concentrations from hemolyzed erythrocytes (585). Worwood (490) discusses potential pitfalls in the analysis of SF, including different isoferritin forms, the “high-dose hook” effect, interference by non-ferritin proteins, and antibodies to some animal proteins. None of them seem to cause noteworthy issues with current assays. Generally, ferritin assays compare reasonably well across methods, with a CV of 10–15% (586); however, an improvement in comparability would be desirable. Agreement among laboratories performing the same method is good (CV <10%) (586).
Serum transferrin receptor
Immunoradiometric assays and ELISAs were initially developed for the measurement of sTfR. They were later followed by latex-enhanced immunoassays (nephelometry and turbidimetry) and more recently by fluoroimmunoassays and immunofluorometric assays (530) (Tables 10 and 11). While microplate ELISA assays have been employed for >20 y, commercial kits that can be used on fully automated clinical analyzers have only been available more recently (587, 588). The usefulness of commercial assays has been severely limited by several factors. The various assays express results in different units (mg/L and nmol/L). There is poor agreement between different kits. An international reference standard has been available since 2010 but its use has been limited due to commutability questions. The poor comparability across assays (589) is likely to be due to manufacturers using different calibrators (TfR isolated from human placenta, not complexed to transferrin; sTfR extracted from serum either in free form or complexed with transferrin), different antibodies (monoclonal or polyclonal), and even different reporting units (mg/L and nmol/L) (530). Standards derived from placental TfR have produced higher TfR assay values than standards isolated from serum (590). This lack of commutability, and the relatively high reagent cost, are some of the reasons why sTfR measurements have not been widely adopted in clinical practice. Methods for sTfR measurement using dried blood spots have been reported (585, 591).
Measuring several iron status indicators in one assay
Erhardt et al. (592) have developed an inexpensive and sensitive sandwich ELISA assay that allows the combined measurement of ferritin, sTfR, CRP, and retinol binding protein in ∼50 µL of serum or plasma. It is a convenient option for evaluating iron and vitamin A status and, at the same time, accounting for the effect of infection or inflammation. The current assay also includes the measurement of AGP, a sensitive acute-phase reactant that captures the response to inflammation over a longer time period (4–5 d) compared to CRP (1–2 d). This assay has been successfully applied to numerous micronutrient surveys in low-resource settings where the specimen volume was insufficient to conduct several conventional assays. The assay has the following shortcomings: 1) the interassay variability is generally higher than that of commercial fully automated assays, necessitating the use of a daily “adjustment factor” derived from the analysis of a material with a known value; 2) the assay has not yet been reliably transferred to laboratories in low-resource settings; and 3) some of the antibodies used in the assay are no longer commercially available. However, given that this assay is a desirable and promising platform for future applications, the CDC is evaluating modifications of the assay to incorporate currently commercially available antibodies and improve its ruggedness in the hopes that it will allow successful technology transfer to low-resource settings. PATH in collaboration with Quansys Biosciences have recently developed a commercially available multiplex ELISA assay for simultaneous quantification of iron (serum or plasma ferritin and sTfR), vitamin A, and inflammation status markers (593). The reagent costs for this assay are lower than purchasing separate commercial kits for each indicator and processing time and specimen volume are reduced. However, the performance of this assay requires the following further improvements: 1) to the calibration system by using purified or recombinant antigen cocktails instead of a commercially available control that does not provide optimal concentration ranges for each indicator; 2) to the precision—currently the interassay CV for ferritin is 9.3–14.1% and for sTfR 7.3 –13.5%; and 3) to the correlation of this assay with conventional ELISA assays—satisfactory correlation was obtained for ferritin (r = 0.951), but the correlation was too low for sTfR (r = 0.606). Furthermore, this assay will have to be compared to established methods for which the performance with reference materials is known and acceptable.
Interpretation of data and cutoff values (Tables 12 and Table 13)
TABLE 12.
Cutoff values for erythrocyte protoporphyrin, serum ferritin, and transferrin saturation by stages of iron status and by population group (218)
| Population group | ||
|---|---|---|
| <5 y of age | ≥5 y of age | |
| Erythrocyte protoporphyrin | ||
| Iron overload | Normal | Normal |
| Normal iron status | Normal | Normal |
| Iron depletion | Normal | Normal |
| Iron deficiency with or without anemia | >70 g/dL RBC | >80 µg/dL RBC |
| >2.6 µg/g hemoglobin | >3.0 µg/g hemoglobin | |
| >61 mmol/mol heme | >70 mmol/mol heme | |
| Serum ferritin, µg/L | ||
| Severe risk of iron overload | No cutoff | >200 (adult males) |
| >150 (adult females) | ||
| Depleted iron stores in the presence of infection | <30 | No cutoff |
| Depleted iron stores | <12 | <15 |
| Transferrin saturation, % | ||
| Iron overload | >60–70 | |
| Iron deficiency anemia | <16 | |
TABLE 13.
Relationships between biomarkers and iron status
| Iron status | Sustainable bone marrow iron | Serum ferritin | Transferrin saturation | Erythrocyte protoporphyrin | Serum transferrin receptor | Hemoglobin |
|---|---|---|---|---|---|---|
| Iron-deficiency anemia | Absent | Low | Low | High | High | Low |
| Iron-deficient erythropoiesis | Absent | Low | Low | High | High | Normal |
| Iron depletion | Absent | Low | Normal | Normal | Normal | Normal |
| Normal iron status | Normal | Normal | Normal | Normal | Normal | Normal |
| Iron overload | Normal or increased | High | High | Normal | Normal | Normal |
There are three stages in the development of iron-deficiency anemia: iron depletion, where the amount of storage iron is reduced (low SF); iron-deficient erythropoiesis, which represents restricted iron supply to the bone marrow and thereby mild tissue deficiency (transferrin saturation is reduced, sTfR and EP are increased); and finally IDA, where iron-containing functional compounds including Hb are underproduced (low Hb). In situations of chronic disease, anemia can develop without an initial iron deficiency, so-called ACD. In the presence of iron overload, indicators of storage and transport iron are elevated (high SF and transferrin saturation).
A recent WHO Vitamin and Mineral Nutrition Information System (VMNIS) document provides a summary of cutoff values for Hb for the diagnosis of anemia and assessment of severity by population group (236). This document also provides adjustments for altitude and smokers. The older WHO guide for program managers provides cutoff values for Hct for the same population groups as well as for EP and ferritin for children <5 y of age and persons ≥5 y of age and for TSAT (218). Because of large assay differences in the measurement of sTfR (589), the manufacturer's assay-dependent cutoff values have been used. The Ramco enzyme immunoassay, the first commercially available sTfR kit assay, reported a normal range in healthy volunteers of 2.9–8.3 mg/L (594). Mei et al. (595) derived cutoff values (97.5th percentile in a defined healthy reference population) for two vulnerable US population groups using data from NHANES 2003–2010 as 6.0 mg/L for children 1–5 y and 5.3 mg/L for nonpregnant women 15–49 y. The NHANES data were produced using the Roche Tina-quant sTfR assay, which yields values that are on average 30% lower than those from the Ramco assay (587).
Since the inception of NHANES in 1971, special focus was dedicated to monitoring the iron status of the US population (596). Each NHANES has included a battery of hematologic and biochemical indicators of iron status (597). Since NHANES II (1976–80), models that employed multiple biochemical iron status indicators have been used to define iron deficiency in the population (598). In 1980, the ferritin model, also known as the three-indicator model, was developed and applied to NHANES III (1988–94) as well as to the first few years of the continuous NHANES survey beginning in 1999. In this model, participants who had two out of three abnormal iron status indicators (SF, transferrin saturation, and erythrocyte protoporphyrin) were categorized as iron deficient. Reference data for various hematological and biochemical iron status indicators measured in persons 1 y and older during NHANES III have been reported as part of a National Center for Health Statistics Series 11 report (599). Reference data for biochemical indicators measured in persons ≥1 y from NHANES 1999–2002 were included in the CDC's National Report on Biochemical Indicators of Diet and Nutrition in the US Population 1999–2002 [the First Nutrition Report (429)]. Starting in 2003, NHANES limited the population of interest to children (1–5 y) and women of childbearing age (12–49 y). Furthermore, the measurement of serum sTfR was introduced, which allows the evaluation of iron status by the body iron model (248). Reference data for ferritin, sTfR, and body iron for children and women of childbearing age were reported as part of the CDC's Second Nutrition Report (600).
While SF is the most sensitive index of iron status as long as residual iron stores are available, this biomarker does not reflect the severity of the depletion as it progresses; sTfR concentrations, on the other hand, continue to rise with increasing functional iron deficiency. Because of the reciprocal changes in ferritin and sTfR, the ratio of sTfR/SF is a valuable measure of the extent of iron deficiency (517). At least three different approaches have been used to calculate this ratio. With each approach, cutoff values to interpret the ratio are different. Furthermore, the ratios are derived from different sTfR assays and there is confusion in the scientific community as to what assay data can be used with which ratio.
sTfR index
The sTfR index is calculated as the ratio of sTfR/log SF and was introduced by Punnonen et al. (601) as a parameter for the identification of persons with depleted iron stores. A subsequent review article presented cutoff values for the sTfR index (mg/L) to distinguish between ACD (<1) and IDA (>2) or both conditions (>2) (602). A recent prospective multicenter clinical trial compared the diagnostic accuracy of sTfR and the sTfR index for differential diagnosis of ACD and IDA using the automated Access Beckman Coulter instrument (540). The authors found that the sTfR index was superior to sTfR and use of all three parameters in combination more than doubled the detection of IDA from 41% (SF alone) to 92% (ferritin, sTfR, and sTfR index). The cutoff value for IDA or a combination of IDA and ACD was >1.03 mg/L. The authors claim that the sTfR index has higher sensitivity/specificity than the sTfR/ferritin ratio. The interpretation of the sTfR index is assay dependent. The proposed cutoff values for the sTfR index derived from the Access Beckman Coulter instrument can only be used by other methods if those methods generate comparable sTfR and ferritin results to results generated by the Access Beckman Coulter instrument.
Body iron index
The logarithm of the ratio of sTfR/SF is linearly related to body iron stores expressed as mg per kg body weight, as derived from a phlebotomy study (14 healthy adults aged 24–46 y, 6 men and 8 women) in which iron status and iron deficit were assessed. The formula for this relationship [using both sTfR (Ramco-related assay) and SF in µg/L] is as follows: body iron (mg/kg) = –[log(sTfR/SF) – 2.8229]/0.1207 (248). The investigators who developed this methodology coined the term “body iron” and it has gained widespread acceptance in the literature (517). However, for many readers it is confusing because “body iron” is not a measure of the quantity of iron in the individual's body. It provides a quantitative estimate of the size of the body iron store when iron is present in the store (values >0 mg/kg) or the size of the functional iron deficit that would need to be corrected before iron could again be accumulated in the store in an individual who is iron deficient (values ≤0 mg/kg). Some investigators have proposed that the term “body iron index” might be more appropriate. The I-EP has decided to use this term. Other terms, such as “total body iron” and “total body iron stores,” have also been frequently used in the literature and TBI is often used as an abbreviation.
Advantages of the body iron index estimate include the following. It is conceptually easy to interpret. The results provide a distribution of quantitative estimates for individuals in a population sample and do not depend on dichotomous assignments based on cutoff values. It is possible to estimate the quantitative impact and bioavailability of a fortification or supplementation intervention based on the change in body iron stores and the cumulative consumption of supplemental or fortification iron. Finally, the method has been adopted for NHANES surveys that will provide a useful database for the comparative evaluation of future surveys. The major limitation is that the body iron stores equation derived from the phlebotomy study has only been validated using the in-house ELISA sTfR assay developed by Flowers et al. (516), which is putatively equivalent to the Ramco sTfR assay. To enable the use of body iron index in NHANES, the CDC established the relationship between the Roche assay used in NHANES and the Flowers assay in a method comparison study: Flowers sTfR = 1.5 × Roche sTfR + 0.35 mg/L (587). The CDC also showed that the Roche and Ramco sTfR assays compare similarly to the Roche and Flowers assays, which indirectly demonstrates the equivalence of the Flowers and Ramco sTfR assays (587). Data from other sTfR assays that produce different results from either the Ramco or the Roche assay cannot be directly used on the body iron index equation. It is generally assumed that a body iron index value of 0 should be the criterion for defining iron deficiency. However, consideration should be given to revising this definition for the following reasons:
— An optimal iron supply may depend on the presence of a small amount of storage iron, i.e., the immediate return of iron derived from hemoglobin processing in macrophages may not be quantitatively complete.
— Cogswell et al. (247) used NHANES 2003–2006 data to compare the estimated prevalence of iron deficiency in US women aged 12–49 y based on calculated body iron index with that derived for the ferritin model. The estimated prevalences of iron deficiency based on the ferritin model were 15.6% and 15.7% for women aged 12–19 and 20–49 y respectively. However, only 9.3% and 9.2% of women respectively had body iron index values <0. Had they employed a higher cutoff, say 2 mg/kg, the prevalence values for the body iron index and ferritin models would have been comparable. Examination of their figure describing the relationship between anemia prevalence and the distribution of body iron index values provides some support for this suggestion. Approximately 20% of women with a body iron index of 0 are anemic. However, anemia prevalence approaches zero at body iron index values >∼2 mg/kg, suggesting the iron deficiency is the most important cause of anemia in this population and that a small iron store is necessary for optimal erythropoiesis.
“Simple” ratio of sTfR to ferritin
In a phlebotomy study, Skikne et al. (517) also calculated the ratio of TfR to ferritin (µg/µg). The ratio increased from <100 in those with ample iron stores to >2000 in those with significant functional iron deficiency. A rise >500 occurred when stores were fully depleted (iron stores of 0 mg/kg). This approach has been adopted by several investigators (245, 603, 604). The advantage of this measure is that the calculation is simpler than for body iron. As with the other two measures, the interpretation of this ratio is again assay dependent because the cutoff value for depleted stores has been established with the Ramco assay.
Laboratory infrastructure
While two of the iron status indicators, Hb and ZPP, can be measured quickly and inexpensively in the field, the fieldwork can be expedited and errors minimized if a venous whole blood sample is collected during the day, stored on cold packs in a shipper, and all the laboratory work is conducted at the end of the day in a centralized laboratory using a minimum number of instruments and fewer analysts. While one team can centrifuge the red-top tubes to obtain serum for biochemical measurements, the other team can conduct Hb and ZPP measurements as required. Sixty samples collected during the day by three or four teams can be processed in 2–3 h with two well-trained and organized assistants. It is therefore important to have at least one back-up HemoCue instrument for every three field teams and one back-up hematofluorometer, should the primary machine fail or not work properly.
All other biomarkers require at the minimum a midlevel laboratory infrastructure that guarantees uninterrupted electrical power supply for a freezer, refrigerator, and the operation of analytical instrumentation, including a water purification system that provides deionized water. Protection of specimens from direct sunlight and artificial light is highly recommended for EP testing. Manual ELISA assays require several pieces of instrumentation, but they are comparatively less expensive than a clinical analyzer. They comprise a microplate reader; microplate washer (recommended); a vortexer; a balance accurate to at least two, preferably three decimal points (0.001 g); and various adjustable air displacement pipettes including an eight-channel pipettor and a repeater pipettor. The throughput of the ELISA assay can be greatly enhanced by the use of a liquid handler to automate the various pipetting steps including the dilution of serum samples. A barcode scanner can speed up sample log-in and avoid transcription errors.
Automated analyzers are relatively expensive and most analyzers operate on a closed-channel basis, allowing only reagents from one particular manufacturer to be used. Furthermore, the laboratory is limited to conducting regular simple instrument maintenance, while a certified service engineer takes care of repairs and more complex maintenance, often as part of an annual service agreement. Calibrators and reagents are typically purchased from the manufacturer in a ready-to-use form. They require minimal handling.
Quality assessment
Overview
Quality assessment (QA) helps to ensure accurate and high-quality laboratory results through full staff participation by avoiding mistakes, ensuring consistent performance and data integrity, and offering opportunities for training. The basic components of a QA system include the following: 1) internal quality control (QC) through the use of bench and blind QC samples; 2) external QA via participation in proficiency testing (PT) programs; 3) equipment monitoring and maintenance; 4) documentation of policies and procedures; 5) proper staff training; and 6) laboratory audits. However, methods must be validated (for accuracy, precision, sensitivity, and ruggedness) and verified periodically (verification of assay calibration, verification of pipette and instrument accuracy) before the quality and consistency can be monitored. For a more detailed description of each QA system component, an example of a minimum QA system for a low-resource setting as well as instructions for the preparation, characterization and use of QC materials, the reader is referred to the Survey Toolkit for Nutritional Assessment, Laboratory and Field section, Quality Control and Quality Assurance subsection, developed by the CDC and hosted by the Micronutrient Initiative (570).
While commercial kits typically supply QC materials and manufacturers will require information on how the assay is performing with the commercial QC material if involved in troubleshooting, the user should keep in mind that frequent lot changes on the commercial QC material may prevent an assessment of assay shifts over time. The only way to know whether an assay fluctuates or shifts over time is to analyze well-characterized materials in every assay or at least periodically. In-house preparation of large batches of QC pools has two advantages. It is more cost efficient and it facilitates close monitoring of assay performance. It is advisable to prepare two (normal and abnormal) or three (low, medium, and high) levels of QC pools, characterize them over the course of 20 individual analytical runs to establish target values and assay-associated variability, and then include them in every analytical run together with the unknown samples to judge whether the run is within the pre-established control limits.
Participation in PT programs is recommended for good laboratory practice to allow external verification of results. Such participation is a requirement for the laboratory to be in compliance with certain laboratory certifications. However, PT programs have their limitations. Most PT programs use method means to evaluate laboratories, making it difficult to identify methods with unsatisfactory performance or even monitor method shifts over time due to the lack of a stable reference point. Because PT programs require large sample volumes, must test a range of concentrations, and because it is difficult and expensive to distribute actual pooled human serum on a regular basis, PT samples are often modified (e.g., adding preservatives or other additives, supplementing materials with nonnative forms of analyte, using animal plasma or outdated human plasma from blood banks), potentially changing their behavior in the assay compared to fresh-frozen samples. This may lead to commutability problems with PT materials (605). Noncommutability is when an assay responds differently to processed samples compared to native, nonprocessed samples. As a result, information gained from the PT program may not be used to adjust assays.
Biomarker-specific issues
The availability of an accepted ICSH reference method for Hb measurements (569) and corresponding ICSH reference material since the 1970s (606) made it possible to calibrate most hemoglobinometers and automated blood cell counters throughout the world. The reference material is available through the UK National Institute of Biological Standards and Control (NIBSC) as the WHO International Standard 98/708 and through the European Community Bureau of Reference as CRM 522 (Table 11). The ICSH also recommended a reference method and a simpler “surrogate reference” method for the measurement of Hct or packed cell volume, both of which are fully traceable to the ICSH reference Hb method.
While no formal standardization programs exist for SF or iron/TIBC, most methods for these biomarkers produce reasonably comparable results and the assays display generally good accuracy, precision, and linearity. This is likely to be due to the inclusion of these analytes in many PT programs, some of which are available from State Department of Health PT programs (e.g., New York State Department of Health Wadsworth Center), and the availability of international reference materials for many years (Table 11). Thorpe (607) has reviewed the development, role, and availability of international biological reference materials for the diagnosis of iron deficiency and anemia. The author discusses the difficulties in standardizing immuno-based methods due to the heterogeneity of the antibodies used in the assays, the technical difficulties in producing a standard preparation that is identical to the circulating serum form, and the absence of physicochemical reference methods to establish “true” concentrations. These issues were addressed in a study conducted by Blackmore et al. (586) to assess the traceability of various ferritin assays to the three WHO international standards developed over the years. While four out of five methods recovered all three international standards within acceptable limits (100% ± 10%), one method significantly over-recovered each of the internal standards (124–155%), despite giving SF results that were comparable to the other methods for five native serum samples. This may be explained by the antibodies employed in each of the assays having different epitope specificities or properties.
No reference material has been available for sTfR assays until recently. Several years ago the WHO commissioned the production of a reference standard and because of the difficulties in purifying sufficient quantities of sTfR, efforts were focused on employing recombinant sTfR, which has a slightly shorter molecular structure than serum sTfR. This WHO Reference Reagent (07/202) was evaluated by five manufacturers of commercial kits. Although the dose-response plots demonstrated acceptable parallelism with commercial in-house standards and serum samples, there was poor agreement with the measured values for sTfR in the kits, even between kits expressing sTfR concentrations in the same units. In 2009, the NIBSC made available this WHO Reference Reagent with a value assigned based on a theoretical extinction coefficient and the molecular weight (21.74 mg/L or 303 nmol/L) (589). If the commutability of this material can be confirmed in an ongoing commutability study, it could be used by manufacturers or researchers as a calibrator for immunoassays. While a full standardization of manufacturer assays may take several years, intermittent steps of assay harmonization through a set of well-characterized reference samples may allow users to “calibrate” their assays to a common basis and therefore produce comparable results across laboratories, instruments, and assays.
Preanalytical factors
Overview
Because each method, analyte, and laboratory may have specific sample handling requirements, it is best to discuss details with the laboratory that will perform the analyses at the time of study planning. In general, the iron status biomarkers are quite stable. If the blood sample cannot be centrifuged within 1–2 h of collection, it should be kept cold and protected from light, but freezing should be avoided to prevent hemolysis (vacutainers should not be in direct contact with frozen cold packs). Frozen serum samples should be shipped on dry ice to avoid thawing. For long-term storage, serum samples should be kept frozen at ≤−40°C.
Biomarker-specific issues
Preanalytical factors influencing whole-blood-based indicators of iron status are summarized in Table 14 (290, 608–611). Because the within-person biological variation of Hb and Hct is very low (<3%), one sample is considered sufficient to estimate the biomarker concentration with 95% confidence and 20% accuracy (608). As Hb measurements only require small sample volumes, the use of capillary blood samples is common. However, this can lead to inaccurate or variable results if the capillary sample is not collected properly (e.g. “milking” the finger; use of the first drop of blood). Using 33 paired venous and capillary blood samples collected into a microtainer, Whitehead et al. (573) found no significant differences in mean Hb concentrations. Pooled capillary blood produced comparable results to the second and third, but not the fourth drop of blood (3.3% lower) (573). Others have also reported that pooled capillary blood samples can be reliably used, whereas single drop measurements, as recommended by the manufacturer, may result in slightly different results (609). Delays in Hb measurement (Hb-201 HemoCue® model) for up to 3 d did not affect values (≤2%) if the venous or capillary blood was kept cold (610). However, blood could only be stored for 1 d at 20–23°C prior to Hb measurement with the Hb-301 model (573).
TABLE 14.
Preanalytical factors influencing whole-blood-based indicators of iron status
| Variables | Hemoglobin | Hematocrit | Erythrocyte protoporphyrin |
|---|---|---|---|
| Subject | |||
| Fasting | Not required | Not required | Not required |
| Biological variation | |||
| Within-person | 2.8% (608) | 1.8% (608) | 9.8% (611) |
| Between-person | 32.9% (611) | ||
| Number of samples required1 | 1 (608) | 1 (608) | |
| Sample collection | |||
| Venous vs. capillary blood | Comparable, particularly if pooled capillary sample from microtainer is used (609, 610) | Generally venous blood is used | Comparable (288); partially clotted samples may not give accurate results |
| Avoid “milking” of finger | |||
| Influence of anticoagulants | Anticoagulants other than EDTA are not customary | Anticoagulants other than EDTA are not customary | Anticoagulants other than EDTA are not customary |
| Sample processing | |||
| General requirements | Use freshly collected EDTA whole blood | Use freshly collected EDTA whole blood | Use freshly collected EDTA whole blood; protect sample from light; avoid hemolysis if measured by hematofluorometer; avoid contamination with fluorescent interferences |
| Measure hematocrit to correct for packed cells | |||
| Delayed processing | Whole blood can be kept refrigerated for a few days if measurement cannot be done immediately (avoid freezing to keep RBC intact) (610) | Whole blood can be kept refrigerated for a few days if measurement cannot be done immediately (avoid freezing to keep RBC intact) (610) | Whole blood can be kept refrigerated for a few days or it can be frozen if fluorometric extraction assay is used |
| Sample storage | |||
| Storage stability | Not applicable | Not applicable | Stable for up to 10 d if protected from light and refrigerated; stable for years at ≤–20⁰C |
| Freeze-thaw stability | Not applicable | Not applicable | Excellent stability |
1Number of samples required to estimate biomarker concentrations with 95% confidence and 20% accuracy.
The three main serum-based iron status indicators have different preanalytical requirements, particularly when it comes to variables related to the subject (Table 15) (240, 608, 611–614). Data from several thousand US adults participating in NHANES 2003–06 showed no difference in SF, sTfR, or body iron index whether samples were collected from fasted (≥8 h after the last meal) or nonfasted (<3 h after the last meal) individuals (615). On the other hand, fasting is usually recommended for the measurement of serum iron, particularly in the clinical setting. Moreover day-to-day (∼30%) and diurnal variations (10–20%) within a person are quite large (575, 611). Fasting may be less important in population studies and in fact NHANES has not used fasted serum samples to measure serum iron. Dale et al. (616) have examined diurnal variation of serum iron, iron-binding capacity, and ferritin concentrations and found that although significant differences among mean values for the collection times were noted, no consistent diurnal variation was seen and the between-day variation was similar to the within-day variation. The authors concluded that the practice of restricting iron specimen collection to a specific time of day does not improve the reliability of the test result.
TABLE 15.
Preanalytical factors influencing serum-based indicators of iron status
| Variables | Serum iron/TIBC1 | Serum ferritin | Serum soluble transferrin receptor |
|---|---|---|---|
| Subject | |||
| Fasting | Recommended (in clinical setting) due to diurnal variation | Not required | Not required |
| Biological variation | |||
| Within-person | 31.9%/9.0% for general US population (611); 26.1%/3.8% for elderly women (608) | 20.8% for general US population (611); ∼13% for males, ∼26% for young women, ∼18% on average (240); 9.7% for elderly women (608) | 11.8% for young adults (240); 10.9% for elderly women (608) |
| Between-person | 41.9%/16.1% for general US population (611) | 89.8% for general US population (611) | |
| Number of of samples required2 | 7/1 for elderly women (608) | 3 for males, 4 for young women (240); 1 for elderly women (608) | 1 for males, 1 or 2 for young women (240); 2 for elderly women (608) |
| Sample collection | |||
| Venous vs. capillary blood | Capillary blood generally does not provide sufficient sample volume | Comparable, but higher variability with capillary samples (240) | Comparable, but higher variability with venous samples (240) |
| Influence of anticoagulants | Serum preferred over plasma (might contain fibrinogen clots) | Serum preferred over plasma (might contain fibrinogen clots); EDTA plasma produces comparable results (240) | Serum preferred over plasma (might contain fibrinogen clots); EDTA plasma produces comparable results (240) |
| Sample processing | |||
| General requirements | Prompt processing and freezing of serum desirable | Prompt processing and freezing of serum desirable | Prompt processing and freezing of serum desirable |
| Delayed processing | Prepare serum within 1 d, but no later than within 2–3 d of blood collection; keep blood refrigerated | Prepare serum within 1 d, but no later than within 2–3 d of blood collection; keep blood refrigerated | Prepare serum within 1 d, but no later than within 2–3 d of blood collection; keep blood refrigerated |
| Storage of unprocessed whole blood at room temperature <96 h is acceptable [∼3% increase, but not significant (612)] | Storage of unprocessed whole blood at elevated temperature (37°C) is unacceptable [∼5% increase after 1 d (613)] | ||
| Storage of unprocessed whole blood at elevated temperature (32–37°C) is acceptable if ≤6 h (614), but unacceptable if ≥1 d (∼10% increase) (613) | |||
| Sample storage | |||
| Storage stability | Stable for days if refrigerated; stable for years at ≤–20°C | Stable for up to 14 d if refrigerated (613); stable for years at ≤–20°C | Stable for up to 14 d if refrigerated (613); stable for years at ≤–20°C |
| Freeze-thaw stability | Stable for at least 3 cycles (613) | Stable for at least 3 cycles (613) | Stable for at least 3 cycles (613) |
1TIBC, total iron-binding capacity.
2Number of samples required to estimate biomarker concentrations with 95% confidence and 20% accuracy.
Serum sTfR has a relatively small within-person variation (CVw ∼12%). One sample is generally considered sufficient to estimate the biomarker concentration with 95% confidence and 20% accuracy (240). The CVw for SF is largely dependent on the population group (∼10–15% for males or elderly women, ∼25% for young women) (240, 608). A higher number of samples has therefore been recommended for SF [3 for males, 4 for young women (240)] and serum iron [7 for elderly women (608)] to account for the larger CVw for these two analytes. However, pragmatic considerations frequently limit the number of samples that can be collected in population-based surveys and intervention studies.
It has been recommended that the method imprecision should be less than one-half of the CVw. Although this can be achieved with currently available methods for SF and iron, it is only achievable for sTfR with fully automated assays on clinical analyzers. Manually conducted ELISA assays typically have a method CV of ∼10% (492, 581, 587). Serum-based iron status indicators generally have good storage stability and can also withstand some delays in whole-blood processing as long as the sample is refrigerated (612, 613).
One preanalytical variable that has large influence on iron status indicators in general and SF (being a positive APP) in particular is the confounding effect of infection and inflammation. Using NHANES 2003–06 data from women aged 20–49 y, Haynes et al. (615) found that SF concentrations were 24% higher in women with elevated CRP concentrations (≥5 mg/L). On the other hand sTfR concentrations were only slightly higher (4%). Body iron index also showed a positive association with inflammation, but the difference in body iron index between women with and without inflammation was smaller than for ferritin (12%).
Future directions and new biomarkers
Throughout this report the I-EP has identified critical research gaps and directions for research. These are highlighted in Text Box 38. The I-EP also wants to acknowledge the potential of several new biomarkers.
Text Box 38.
Future directions and research priorities
• Improved understanding of the relationship between iron status and physiology as impacted by both life stage and factors such as inflammation and infection.
• Improved understanding of the relationship between maternal iron status during pregnancy and birth outcome including cognitive and motor development in the infant. To address this need the I-EP highlighted the following areas:
-Assessment tools to distinguish between physiological response and nutritional need.
-Need to match currently available biomarkers of iron status with the time frames for the development of iron-dependent neural systems for which functional tests are available, e.g. myelination, monoamine-driven behaviors.
-Need for bioindicators reflecting relevant functional outcomes (e.g., neurological, cognitive, and behavioral development) that can be used along with appropriate iron biomarkers.
• Advance our understanding of the effect of genotype on risk of iron deficiency.
• Enhanced understanding of the nature and health implications of nutrient-nutrient interactions (iron-zinc, vitamin A etc.).
• Better understanding of the specific role and how best to assess iron status in various infectious diseases (malaria, HIV, TB, diarrheal disease) and noncommunicable diseases (e.g., cancer).
• Approaches for the assessment of iron overload:
-Giving consideration to the context of inflammation and life stage as the interpretation of currently designated upper limits of the most commonly used biomarker (SF) is impacted by both, particularly in older men and postmenopausal women.
-Better tools to assess iron overload for population screening.
-Because iron overload resulting solely from excessive iron consumption is rare in the absence of an identifiable hepcidin or ferroportin abnormality, an alternative approach to address risk of iron overload was proposed by the I-EP and needs to be evaluated. It is based on determining the prevalence of one or more iron-loading genotypes that affect the hepcidin/ferroportin axis in the population under consideration either directly or because of their effect on erythropoietic rate.
Research carried out over the past half century has provided a clear understanding of the relationships between uncomplicated nutritional iron deficiency and anemia. Iron status can be determined with a high degree of accuracy and biomarkers that are suitable for field use are affordable and freely available. However, this approach is less satisfactory in populations where anemia is caused by an infection or an infection and iron deficiency combined. Furthermore, the concepts developed for predicting the risk for iron-deficiency anemia have not been shown to be predictive of some other putative critical functional consequences of iron deficiency, particularly pregnancy outcome and cognitive, motor and emotional development in infancy. There is clearly a need to search for alternative approaches as well as other biomarkers. The measurement of plasma (serum) hepcidin is the most promising option at the present time (617).
Novel red blood cell indexes
Modern hematology analyzers provide a variety of red blood cell parameters that are of potential use, including the following: 1) the proportion of hypochromic red blood cells; 2) the ratio of microcytic to hypochromic red blood cells; 3) the immature reticulocyte fraction; 4) the reticulocyte mean hemoglobin content (as CHr, Ret-He, RHE, or RHCc by different manufacturers); and 5) the mean reticulocyte volume (as MCVr, MCVR, MVR, or MRV by different manufacturers).
None of these potential biomarkers have been studied systematically for the evaluation of nutritional anemia. Moreover, their use is complicated by the lack of standardization, in part because of the different techniques used by the various manufacturers (509). They have, however, gained acceptance for the management of anemia and iron status in patients with chronic kidney disease (512). In addition, the measurement of reticulocyte hemoglobin concentration has been included in the American Academy of Pediatrics guidelines for the evaluation of anemia in childhood (267).
Hepcidin
As described elsewhere, hepcidin plays a central role in controlling iron absorption and systemic iron supply to all cells. The functional form of the peptide can be measured in both plasma and urine. Hepcidin synthesis rises in response to increasing liver iron stores and serum iron concentration. It is therefore an important indicator of iron status.
Similar to SF, levels are also increased by inflammation. A second factor may confound the relationship with iron status. Hepcidin is suppressed in the presence of increased erythropoietic activity, putatively by a recently identified protein that is produced by erythroblasts, erythroferrone (124). Plasma hepcidin levels are also influenced by many other factors (618).
The response of hepcidin to increased erythropoiesis is an important concern with potentially significant public health implications. Jones et al. (435) reported suppressed hepcidin levels and increased iron accumulation in 62 of 69 Sri Lankan patients with HbE β-thalassemia with a moderate or severe phenotype. Sri Lankan school children with β-thalassemia trait also had mildly decreased hepcidin levels. As a consequence, the potential exists for public health interventions (e.g., iron fortification of food, iron supplementation) intended to improve iron status particularly in such groups as women, infants, and school-aged children, to increase risk of iron overload in populations with high prevalence of HbE β-thalassemia (the most common severe thalassemia syndrome in Asia). More information is needed to determine whether programs that rely on hepcidin levels to monitor iron status will both provide a more reliable assessment of iron status than currently available biomarkers (SF) and avoid the risk of contributing to iron overload in populations where thalassemia syndromes and thalassemia carrier status are prevalent.
Hepcidin has been measured in various settings. Plasma concentrations during the first trimester of pregnancy are within the references range for nonpregnant women (226, 255, 619). During the second trimester serum hepcidin concentrations fall to very low levels and remain low in the third trimester (172, 226, 255). The mechanisms underlying hepcidin suppression during pregnancy are unknown, but they do not seem to be related to maternal iron status (256, 620, 621).
Cord blood hepcidin levels may be predictive of anemia and malaria in Tanzanian children (622) and Pasricha et al. (623) have suggested that hepcidin levels may prove to be a valuable tool for identifying IDA in African children. Finally they may be useful in detecting the rare genetic disorder that leads to iron deficiency which is unresponsive to oral iron (IRIDA) (624).
The application of hepcidin assays to the evaluation of nutritional iron status is further complicated by differences in methodology and the lack of assay commutability and standardization, making comparisons between studies difficult. Reference intervals are method dependent. However, considerable progress has recently been made toward the development of a reference material that will improve the equivalences between different hepcidin assay procedures (625).
Based on the current status of our understanding of hepcidin biology and assessment, the I-EP concluded that it is unclear whether hepcidin assays provide any advantage over SF and the other currently available methods for assessing nutritional iron status.
Supplementary Material
Acknowledgements
We thank Jenica K Abram for her perseverance and support in preparing and coordinating the successful preparation of this manuscript for submission. We also thank Ramkripa Raghavan for her assistance in coordinating the early stages of the BOND Iron Expert Panel review process. This review is the result of deliberations and contributions of the BOND Iron Expert Panel (SNL: Chair, CMP, MKG, GB, SF-T, RFH, HJM). Each panel member contributed significant sections of the manuscript with major contributions from CMP, MKG, SF-T. SNL and DJR oversaw the compilation and revisions of the review with input of all panel members throughout the process. SNL and DJR edited all sections of the manuscript and had primary responsibility for the final content. All authors read and approved the final manuscript.
Notes
Published in a supplement to The Journal of Nutrition. The Biomarkers of Nutrition for Development (BOND) project was developed by the nutrition program staff of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the NIH within the US Department of Health and Human Services (DHHS). The initial 6 nutrients selected, iodine, vitamin A, iron, zinc, folate, and vitamin B-12, were chosen for their high public health importance. Expert panels on each nutrient were constituted and charged with developing comprehensive reviews for publication in the BOND series. The BOND program received its core funding from the Bill & Melinda Gates Foundation, PepsiCo, the Division of Nutrition Research Coordination (DNRC, NIH), the Office of Dietary Supplements (ODS, NIH), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, NIH). The Supplement Coordinators for this supplement were Daniel J Raiten (NICHD, NIH) and Jenica Abram, Academy of Nutrition and Dietetics (AND). Supplement Coordinators disclosures: no conflicts of interest. Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of The Journal of Nutrition.
Funding for the BOND project was provided, in part, by the Bill and Melinda Gates Foundation, the PepsiCo, the Office of Dietary Supplements (ODS), National Institutes of Health (NIH), the Division of Nutrition Research Coordination (DNRC), NIH and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH. The iron review was written in response to an invitation from NICHD, NIH within the US Department of Health and Human Services (DHHS). The content represents the views of the Iron Expert Panel (I-EP) and other invited contributors and does not necessarily reflect the official position of the NICHD, the NIH, the CDC/the Agency for Toxic Substances and Disease Registry, or the DHHS. In addition, individual members of the EP may not endorse all statements in this report. The original EP consisted of SL (chair), CMP, MG, GB, SFT, RFH, HJM, and DJR. The BOND project thanks the European Recommendations Aligned (EURRECA) program, the Micronutrient Genomics Project (MGP), the WHO and the CDC for their partnership.
Author disclosures: SL, CMP, MKG, GB, SF-T, RFH, HJM, and DJR, no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ACD, anemia of chronic disease; ACT, α1-antichymotrypsin; AGP, α1-acid glycoprotein; AOAC, Association of Official Analytical Chemists; APP, acute-phase protein; APR, acute-phase response; BMP, bone morphogenetic protein; BOND, Biomarkers of Nutrition for Development; BRINDA, biomarkers reflecting inflammation and nutrition determinants of anemia; CHr, reticulocyte hemoglobin content; CNPase, cyclic nucleotide 3-phophohydrolase; CRP, C-reactive protein; DCYTB, duodenal cytochrome b; DMT-1, divalent metal transporter 1; DR, dietary record; EFSA, European Food Safety Authority; EP, erythrocyte protoporphyrin; ERFE, erythroferrone; EuroFIR, European Food Information Resource; EURRECA, European Recommendations Aligned; FAO, Food and Agriculture Organization of the United Nations; FEP, free erythrocyte protoporphyrin; FPN, ferroportin; GAIN, Global Alliance for Improved Nutrition; HAMP, gene encoding hepcidin; HbE, hemoglobin E; Hct, hematocrit; HFE, human hemochromatosis; HIF-1, hypoxia inducible factor 1; HIF2α, hypoxia inducible factor 2α; HJV, hemojuvelin; ICSH, International Council for Standardization in Haematology; IDA, iron-deficiency anemia; I-EP, Iron Expert Panel; INSPIRE, Inflammation and Nutrition Science for Program/Policy and Interpretation for Research Evidence; IRIDA, iron-refractory iron-deficiency anemia; IRE, iron-responsive element; IRP, iron-regulatory protein; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; mTOR, mechanistic (mammalian) target of rapamycin; NaFeEDTA, sodium iron ethylene diamine tetraacetic acid; NRAMP2, natural resistance-associated macrophage protein 2; PSC, preschool children; PT, proficiency testing; QA, quality assessment; QC, quality control; RAS/RAF MAPK, rapidly accelerated fibrosarcoma mitogen-activated protein kinase; RDW, red blood cell distribution width; Ret He, reticulocyte hemoglobin equivalent; RNI, recommended nutrient intake; SF, serum ferritin; sHJV, soluble hemojuvelin; sTfR, serum (soluble, plasma) transferrin receptor; SMAD, sons of mothers against decapentaplegic; TB, tuberculosis; TfR, transferrin receptor; TIBC, total iron-binding capacity; TSAT, percentage transferrin saturation; T3, triiodothyronine; T4, thyroxine; TTSP, type-two transmembrane serine protease; UIBC, unsaturated iron binding capacity; WRA, women of reproductive age; ZPP, zinc protoporphyrin; ZPP/H, zinc protoporphyrin/heme ratio.
References
- 1. Raiten DJ, Namasté S, Brabin B, Combs G, L'Abbe MR, Wasantwisut E, Darnton-Hill I. Executive summary–Biomarkers of Nutrition for Development: building a consensus. Am J Clin Nutr 2011;94: S633–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rohner F, Zimmermann M, Jooste P, Pandav C, Caldwell K, Raghavan R, Raiten DJ. Biomarkers of Nutrition for Development–iodine review. J Nutr 2014;144: S1322–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF, Mills JL, Pfeiffer CM, Fazili Z, Zhang M, Ueland PM. et al. Biomarkers of Nutrition for Development—folate review. J Nutr 2015;145: S1636–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. King JC, Brown KH, Gibson RS, Krebs NF, Lowe NM, Siekmann JH, Raiten DJ. Biomarkers of Nutrition for Development (BOND)—zinc review. J Nutr 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of Nutrition for Development (BOND)—vitamin A review. J Nutr 2016;146: S1816–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beutler E. History of iron in medicine. Blood Cells Mol Dis 2002;29:297–308. [DOI] [PubMed] [Google Scholar]
- 7. Sheftel AD, Mason AB, Ponka P. The long history of iron in the universe and in health and disease. Biochim Biophys Acta 2012;1820:161–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neuroth ML, Lee CO. A history of Blaud's pills. J Am Pharm Assoc1941;30:60–3. [Google Scholar]
- 9. Trousseau A. Lectures LXXXVII. True and false chlorosis. London: New Sydenham Society (Series); 1872. [Google Scholar]
- 10. Trousseau A, Pidoux H. Treatise on therapeutics. New York: William Wood & Company; 1880. [Google Scholar]
- 11. Carey G. The chemistry of human life. Los Angeles, CA, 1919. pp 9. [Google Scholar]
- 12. Heath CW, Strauss MB, Castle WB. Quantitative aspects of iron deficiency in hypochromic anemia: the parenteral administration of iron. J Clin Invest 1932;11:1293–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Welch S. Transferrin: the iron carrier. Boca Raton, FL: CRC Press; [Google Scholar]
- 14. Laufberger V. Sur la cristallisation de la ferritine. Soc Chim Biol 1937;19:1575–82. [Google Scholar]
- 15. Widdowson EM, McCance RA. The absorption and excretion of iron before, during and after a period of very high intake. Biochem J 1937;31:2029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hahn PF, Bale WF, Ross JF, Balfour WM, Whipple GH. Radioactive iron absorption by gastro-intestinal tract: influence of anemia, anoxia, and antecedent feeding distribution in growing dogs. J Exp Med 1943;78:169–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith MD, Pannacciulli IM. Absorption of inorganic iron from graded doses: its significance in relation to iron absorption tests and mucosal block theory. Br J Haematol 1958;4:428–34. [DOI] [PubMed] [Google Scholar]
- 18. Conrad ME, Crosby WH. Intestinal mucosal mechanisms controlling iron absorption. Blood. 1963;22:406–15. [PubMed] [Google Scholar]
- 19. Gunshin H, Mackenzie B, Berger U V, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997;388:482–8. [DOI] [PubMed] [Google Scholar]
- 20. Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A. et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–81. [DOI] [PubMed] [Google Scholar]
- 21. Park CH, Valore E V, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–10. [DOI] [PubMed] [Google Scholar]
- 22. Crichton RR. Iron metabolism: from molecular mechanisms to clinical consequences. Chichester: John Wiley; 2009. [Google Scholar]
- 23. Micronutrient Genomics Project [Internet] [cited 2013 Aug 8]. Available from: http://www.micronutrientgenomics.org
- 24. Institute NHGR [Internet] [cited 2013 Aug 16]. Available from: http://www.genome.gov.
- 25. van Ommen B El-Sohemy A, Hesketh J, Kaput J, Fenech M, Evelo CT, McArdle HJ, Bouwman J, Lietz G, Mathers JC. et al. The Micronutrient Genomics Project: a community-driven knowledge base for micronutrient research. Genes Nutr 2010;5:285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr 2010;91: S1461–7. [DOI] [PubMed] [Google Scholar]
- 27. Scientific opinion on dietary reference values for iron. EFSA J 2015;13:4254. [Google Scholar]
- 28. McCance RA, Edgecombe CN, Widdowson EM. Phytic acid and iron absorption. Lancet 1943;242:126–8. [Google Scholar]
- 29. Moore C V, Dubach R, Minnich V, Roberts HK. Absorption of ferrous and ferric radioactive iron by human subjects and by dogs. J Clin Invest 1944;23:755–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moore C V, Dubach R. Observations on the absorption of iron from foods tagged with radioiron. Trans Assoc Am Physicians 1951;64:245–56. [PubMed] [Google Scholar]
- 31. Dubach R, Moore C V, Minnich V. Studies in iron transportation and metabolism; utilization of intravenously injected radioactive iron for hemoglobin synthesis, and an evaluation of the radioactive iron method for studying iron absorption. J Lab Clin Med 1946;31:1201–22. [PubMed] [Google Scholar]
- 32. Layrisse M, Cook JD, Martinez C, Roche M, Kuhn IN, Walker RB, Finch CA. Food iron absorption: a comparison of vegetable and animal foods. Blood. 1969;33:430–43. [PubMed] [Google Scholar]
- 33. Cook JD, Layrisse M, Martinez-Torres C, Walker R, Monsen E, Finch CA. Food iron absorption measured by an extrinsic tag. J Clin Invest. 1972;51:805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Björn-Rasmussen E, Hallberg L, Walker RB. Food iron absorption in man. I. Isotopic exchange between food iron and inorganic iron salt added to food: studies on maize, wheat, and eggs. Am J Clin Nutr 1972;25:317–23. [DOI] [PubMed] [Google Scholar]
- 35. Harvey PW, Dexter PB, Darnton-Hill I. The impact of consuming iron from non-food sources on iron status in developing countries. Public Health Nutr 2000;3:375–83. [DOI] [PubMed] [Google Scholar]
- 36. Hallberg L, Björn-Rasmussen E. Determination of iron absorption from whole diet. A new two-pool model using two radioiron isotopes given as haem and non-haem iron. Scand J Haematol 1972;9:193–7. [DOI] [PubMed] [Google Scholar]
- 37. Davidsson L. Minerals and trace elements in infant nutrition. Acta Paediatr 1994;83:S38–42. [DOI] [PubMed] [Google Scholar]
- 38. Lynch SR, Cook JD. Interaction of vitamin C and iron. Ann N Y Acad Sci 1980;355:32–44. [DOI] [PubMed] [Google Scholar]
- 39. Reddy MB, Hurrell RF, Cook JD. Estimation of nonheme-iron bioavailability from meal composition. Am J Clin Nutr 2000;71:937–43. [DOI] [PubMed] [Google Scholar]
- 40. Cook JD, Monsen ER. Food iron absorption. I. Use of semisynthetic diet to study absorption of nonheme iron. Am J Clin Nutr 1975;28:1289–95. [DOI] [PubMed] [Google Scholar]
- 41. Cook JD, Monsen ER. Food iron absorption in human subjects. III. Comparison of the effect of animal proteins on nonheme iron absorption. Am J Clin Nutr 1976;29:859–67. [DOI] [PubMed] [Google Scholar]
- 42. Hallberg L, Rossander L, Skånberg AB. Phytates and the inhibitory effect of bran on iron absorption in man. Am J Clin Nutr. 1987;45:988–96. [DOI] [PubMed] [Google Scholar]
- 43. Hurrell RF, Juillerat MA, Reddy MB, Lynch SR, Dassenko SA, Cook JD. Soy protein, phytate, and iron absorption in humans. Am J Clin Nutr 1992;56:573–8. [DOI] [PubMed] [Google Scholar]
- 44. Gillooly M, Bothwell TH, Charlton RW, Torrance JD, Bezwoda WR, MacPhail AP, Derman DP, Novelli L, Morrall P, Mayet F. Factors affecting the absorption of iron from cereals. Br J Nutr 1984;51:37–46. [DOI] [PubMed] [Google Scholar]
- 45. Disler PB, Lynch SR, Charlton RW, Torrance JD, Bothwell TH, Walker RB, Mayet F. The effect of tea on iron absorption. Gut 1975;16:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lynch SR. The effect of calcium on iron absorption. Nutr Res Rev 2000;13:141–58. [DOI] [PubMed] [Google Scholar]
- 47. Institute of Medicine Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academy Press; 2001. [PubMed] [Google Scholar]
- 48. Hurrell R. The mineral fortification of foods. Leatherhead: Leatherhead International Ltd; 1999. pp. 54–93. [Google Scholar]
- 49. Hoppler M, Egli I, Petry N, Gille D, Zeder C, Walczyk T, Blair MW, Hurrell RF. Iron speciation in beans (Phaseolus vulgaris) biofortified by common breeding. J Food Sci 2014;79:C1629–C1634. [DOI] [PubMed] [Google Scholar]
- 50. Hoppler M, Schönbächler A, Meile L, Hurrell RF, Walczyk T. Ferritin-iron is released during boiling and in vitro gastric digestion. J Nutr 2008;138:878–84. [DOI] [PubMed] [Google Scholar]
- 51. Lönnerdal B, Bryant A, Liu X, Theil EC. Iron absorption from soybean ferritin in nonanemic women. Am J Clin Nutr 2006;83:103–7. [DOI] [PubMed] [Google Scholar]
- 52. Petry N, Egli I, Zeder C, Walczyk T, Hurrell R. Polyphenols and phytic acid contribute to the low iron bioavailability from common beans in young women. J Nutr 2010;140:1977–82. [DOI] [PubMed] [Google Scholar]
- 53. Tuntawiroon M, Sritongkul N, Rossander-Hultén L, Pleehachinda R, Suwanik R, Brune M, Hallberg L. Rice and iron absorption in man. Eur J Clin Nutr 1990;44:489–97. [PubMed] [Google Scholar]
- 54. Petry N, Boy E, Wirth JP, Hurrell RF. Review: The potential of the common bean (Phaseolus vulgaris) as a vehicle for iron biofortification. Nutrients 2015;7:1144–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lynch SR, Bothwell T, Sustain Task Force on Iron Powders A comparison of physical properties, screening procedures and a human efficacy trial for predicting the bioavailability of commercial elemental iron powders used for food fortification. Int J Vitam Nutr Res 2007;77:107–24. [DOI] [PubMed] [Google Scholar]
- 56. Hallberg L. Wheat fiber, phytates and iron absorption. Scand J Gastroenterol 1987;129:S73–9. [DOI] [PubMed] [Google Scholar]
- 57. Hurrell RF. Phytic acid degradation as a means of improving iron absorption. Int J Vitam Nutr Res 2004;74:445–52. [DOI] [PubMed] [Google Scholar]
- 58. Hurrell RF. Fortification: overcoming technical and practical barriers. J Nutr 2002;132: S806–12. [DOI] [PubMed] [Google Scholar]
- 59. Hurrell R, Ranum P, de Pee S, Biebinger R, Hulthen L, Johnson Q, Lynch S. Revised recommendations for iron fortification of wheat flour and an evaluation of the expected impact of current national wheat flour fortification programs. Food Nutr Bull 2010;31:S7–21. [DOI] [PubMed] [Google Scholar]
- 60. Hurrell RF, Reddy M, Cook JD. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br J Nutr 1999;81:289–95. [PubMed] [Google Scholar]
- 61. Gillooly M, Bothwell TH, Torrance JD, MacPhail AP, Derman DP, Bezwoda WR, Mills W, Charlton RW, Mayet F. The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. Br J Nutr 1983;49:331–42. [DOI] [PubMed] [Google Scholar]
- 62. Petry N, Egli I, Campion B, Nielsen E, Hurrell R. Genetic reduction of phytate in common bean (Phaseolus vulgaris L.) seeds increases iron absorption in young women. J Nutr 2013;143:1219–24. [DOI] [PubMed] [Google Scholar]
- 63. Cercamondi CI, Egli IM, Zeder C, Hurrell RF. Sodium iron EDTA and ascorbic acid, but not polyphenol oxidase treatment, counteract the strong inhibitory effect of polyphenols from brown sorghum on the absorption of fortification iron in young women. Br J Nutr 2014;111:481–9. [DOI] [PubMed] [Google Scholar]
- 64. Hallberg L, Brune M, Erlandsson M, Sandberg AS, Rossander-Hultén L. Calcium: effect of different amounts on nonheme- and heme-iron absorption in humans. Am J Clin Nutr 1991;53:112–9. [DOI] [PubMed] [Google Scholar]
- 65. Galan P, Cherouvrier F, Preziosi P, Hercberg S. Effects of the increasing consumption of dairy products upon iron absorption. Eur J Clin Nutr 1991;45:553–9. [PubMed] [Google Scholar]
- 66. Walczyk T, Muthayya S, Wegmüller R, Thankachan P, Sierksma A, Frenken LGJ, Thomas T, Kurpad A, Hurrell RF. Inhibition of iron absorption by calcium is modest in an iron-fortified, casein- and whey-based drink in Indian children and is easily compensated for by addition of ascorbic acid. J Nutr 2014;144:1703–9. [DOI] [PubMed] [Google Scholar]
- 67. Lynch SR, Dassenko SA, Cook JD, Juillerat MA, Hurrell RF. Inhibitory effect of a soybean-protein–related moiety on iron absorption in humans. Am J Clin Nutr 1994;60:567–72. [DOI] [PubMed] [Google Scholar]
- 68. Hurrell RF, Lynch SR, Trinidad TP, Dassenko SA, Cook JD. Iron absorption in humans as influenced by bovine milk proteins. Am J Clin Nutr 1989;49:546–52. [DOI] [PubMed] [Google Scholar]
- 69. Ballot D, Baynes RD, Bothwell TH, Gillooly M, MacFarlane BJ, MacPhail AP, Lyons G, Derman DP, Bezwoda WR, Torrance JD. The effects of fruit juices and fruits on the absorption of iron from a rice meal. Br J Nutr 1987;57:331–43. [DOI] [PubMed] [Google Scholar]
- 70. Conrad ME, Schade SG. Ascorbic acid chelates in iron absorption: a role for hydrochloric acid and bile. Gastroenterology 1968;55:35–45. [PubMed] [Google Scholar]
- 71. Hurrell R. Prospects for improving the iron fortification of foods. Nutritional Anaemias. Vevey: Raven Press, Ltd; 1992. pp. 193–208. [Google Scholar]
- 72. Troesch B, Egli I, Zeder C, Hurrell RF, de Pee S, Zimmermann MB. Optimization of a phytase-containing micronutrient powder with low amounts of highly bioavailable iron for in-home fortification of complementary foods. Am J Clin Nutr. 2009;89:539–44. [DOI] [PubMed] [Google Scholar]
- 73. Lynch SR, Hurrell RF, Dassenko SA, Cook JD. The effect of dietary proteins on iron bioavailability in man. Adv Exp Med Biol 1989;249:117–32. [DOI] [PubMed] [Google Scholar]
- 74. Taylor PG, Martínez-Torres C, Romano EL, Layrisse M. The effect of cysteine-containing peptides released during meat digestion on iron absorption in humans. Am J Clin Nutr 1986;43:68–71. [DOI] [PubMed] [Google Scholar]
- 75. Storcksdieck genannt Bonsmann S. Storcksdieck S, Bonsmann G, Hurrell RF. Iron-binding properties, amino acid composition, and structure of muscle tissue peptides from in vitro digestion of different meat sources. J Food Sci 2007;72:S19–29. [DOI] [PubMed] [Google Scholar]
- 76. Hurrell R, Egli I. Optimizing the bioavailability of iron compounds for food fortification. In: Kraemer K, Zimmermann M, Nutritional Anemia. Basel, Switzerland: Sight and Life Press; 2007. pp. 77–90. [Google Scholar]
- 77. Zimmermann M, Hurrell R. Nutritional iron deficiency. Lancet 2007;370:511–20. [DOI] [PubMed] [Google Scholar]
- 78. World Health Organization, Food and Agriculture Organization of the United Nations Guidelines on food fortification with micronutrients. Allen L, De Benoist B, Dary O, Hurrell R, Geneva: World Health Organization; 2006. [Google Scholar]
- 79. Hertrampf E, Olivares M. Iron amino acid chelates. Int J Vitam Nutr Res 2004;74:435–43. [DOI] [PubMed] [Google Scholar]
- 80. Duque X, Martinez H, Vilchis-Gil J, Mendoza E, Flores-Hernández S, Morán S, Navarro F, Roque-Evangelista V, Serrano A, Mera RM. Effect of supplementation with ferrous sulfate or iron bis-glycinate chelate on ferritin concentration in Mexican schoolchildren: a randomized controlled trial. Nutr J 2014;13:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Troesch B, van Stuijvenberg ME, van Stujivenberg ME, Smuts CM, Kruger HS, Biebinger R, Hurrell RF, Baumgartner J, Zimmermann MB. A micronutrient powder with low doses of highly absorbable iron and zinc reduces iron and zinc deficiency and improves weight-for-age Z-scores in South African children. J Nutr 2011;141:237–42. [DOI] [PubMed] [Google Scholar]
- 82. World Health Organization Recommendations on wheat and maize flour fortification meeting report: interim consensus statement. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 83. MacPhail AP, Patel RC, Bothwell TH, Lamparelli RD. EDTA and the absorption of iron from food. Am J Clin Nutr 1994;59:644–8. [DOI] [PubMed] [Google Scholar]
- 84. Hurrell R, Hess S. Micronutrient deficiencies during the weaning period and the first years of life. In: Pettifor J, , Zlotkin S, Nestlé nutrition workshop series pediatric program, Vol 54 São Paulo: Nestec Ltd; 2004. [Google Scholar]
- 85. Fishman SM, Christian P, West KP. The role of vitamins in the prevention and control of anaemia. Public Health Nutr 2000;3:125–50. [DOI] [PubMed] [Google Scholar]
- 86. Roodenburg A, West C, Beguin Y, Van Dijk J Van Eijk H, Marx J, Beynen A. Indicators of erythrocyte formation and degradation in rats with either vitamin A or iron deficiency. J Nutr Biochem 2000;11:223–30. [DOI] [PubMed] [Google Scholar]
- 87. Means RT. The anaemia of infection. Baillière's Best Pract Res Clin Haematol 2000;13:151–62. [DOI] [PubMed] [Google Scholar]
- 88. García-Casal MN, Layrisse M, Solano L, Barón MA, Arguello F, Llovera D, Ramírez J, Leets I, Tropper E. Vitamin A and beta-carotene can improve nonheme iron absorption from rice, wheat and corn by humans. J Nutr 1998;128:646–50. [DOI] [PubMed] [Google Scholar]
- 89. Walczyk T, Davidsson L, Rossander-Hulthen L, Hallberg L, Hurrell RF. No enhancing effect of vitamin A on iron absorption in humans. Am J Clin Nutr 2003;77:144–9. [DOI] [PubMed] [Google Scholar]
- 90. Davidsson L, Adou P, Zeder C, Walczyk T, Hurrell R. The effect of retinyl palmitate added to iron-fortified maize porridge on erythrocyte incorporation of iron in African children with vitamin A deficiency. Br J Nutr 2003;90:337–43. [DOI] [PubMed] [Google Scholar]
- 91. Zimmermann M, Adou P, Torresani T, Zeder C, Hurrell R. Iron supplementation in goitrous, iron-deficient children improves their response to oral iodized oil. Eur J Endocrinol 2000;142:217–23. [DOI] [PubMed] [Google Scholar]
- 92. Zimmermann MB. Iron status influences the efficacy of iodine prophylaxis in goitrous children in Côte d'Ivoire. Int J Vitam Nutr Res 2002;72:19–25. [DOI] [PubMed] [Google Scholar]
- 93. Hess SY, Zimmermann MB, Arnold M, Langhans W, Hurrell RF. Iron deficiency anemia reduces thyroid peroxidase activity in rats. J Nutr 2002;132:1951–5. [DOI] [PubMed] [Google Scholar]
- 94. Sandström B. Micronutrient interactions: effects on absorption and bioavailability. Br J Nutr. 2001;85(Suppl 2):S181–5. [PubMed] [Google Scholar]
- 95. Lim KHC, Riddell LJ, Nowson CA, Booth AO, Szymlek-Gay EA. Iron and zinc nutrition in the economically-developed world: a review. Nutrients. 2013;5:3184–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fischer Walker C, Kordas K, Stoltzfus RJ, Black RE. Interactive effects of iron and zinc on biochemical and functional outcomes in supplementation trials. Am J Clin Nutr 2005;82:5–12. [DOI] [PubMed] [Google Scholar]
- 97. Institute of Medicine (US) Subcommittee on Interpretation and Uses of Dietary Reference Intakes, Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. DRI Dietary Reference Intakes: applications in dietary assessment. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 98. Finch CA, Huebers H. Perspectives in iron metabolism. N Engl J Med 1982;306:1520–8. [DOI] [PubMed] [Google Scholar]
- 99. Graham RM, Chua ACG, Trinder D. Plasma iron and iron delivery to the tissues. In: Anderson GJ, McLaren GD, Iron physiology and pathophysiology in humans. New York: Humana Press; 2012. p. 117–39. [Google Scholar]
- 100. Bothwell T, Charlton R, Cook J, Finch C. Iron metabolism in man. Oxford: Blackwell Scientific Publications; 1979. [Google Scholar]
- 101. Anderson G, McLaren D. Iron physiology and pathophysiology in humans. New York: Humana Press; 2012. [Google Scholar]
- 102. Hamilton LD, Gubler CJ, Cartwright GE, Wintrobe MM. Diurnal variation in the plasma iron level of man. Proc Soc Exp Biol Med 1950;75:65–8. [DOI] [PubMed] [Google Scholar]
- 103. Finch CA, Sturgeon P. Erythrokinetics in Cooley's anemia. Blood 1957;12:64–73. [PubMed] [Google Scholar]
- 104. Donovan A, Lima C, Pinkus J, Pinkus G, Zon L, Robine S, Andrews N. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 2005;1:191–200. [DOI] [PubMed] [Google Scholar]
- 105. Hentze M, Muckenthaler M, Galy B, Camaschella C. Two to tango: regulation of mammalian iron metabolism. Cell 2010;9:24–38. [DOI] [PubMed] [Google Scholar]
- 106. Marques L, Auriac A, Willemetz A, Banha J, Silva B, Canonne-Hergaux F, Costa L. Immune cells and hepatocytes express glycosylphosphatidylinositol-anchored ceruloplasmin at their cell surface. Blood Cells Mol Dis 2012;15:110–20. [DOI] [PubMed] [Google Scholar]
- 107. Chen H, Attieh ZK, Syed BA, Kuo Y-M, Stevens V, Fuqua BK, Andersen HS, Naylor CE, Evans RW, Gambling L. et al. Identification of zyklopen, a new member of the vertebrate multicopper ferroxidase family, and characterization in rodents and human cells. J Nutr 2010;140:1728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cook JD, Marsaglia G, Eschbach JW, Funk DD, Finch CA. Ferrokinetics: a biologic model for plasma iron exchange in man. J Clin Invest 1970;49:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Fillet G, Cook JD, Finch CA. Storage iron kinetics. VII. A biologic model for reticuloendothelial iron transport. J Clin Invest 1974;53:1527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fillet G, Beguin Y, Baldelli L. Model of reticuloendothelial iron metabolism in humans: abnormal behavior in idiopathic hemochromatosis and in inflammation. Blood 1989;74:844–51. [PubMed] [Google Scholar]
- 111. Kroot JJC, Tjalsma H, Fleming RE, Swinkels DW. Hepcidin in human iron disorders: diagnostic implications. Clin Chem 2011;57:1650–69. [DOI] [PubMed] [Google Scholar]
- 112. Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004;306:2090–3. [DOI] [PubMed] [Google Scholar]
- 113. Drakesmith H, Nemeth E, Ganz T. Ironing out ferroportin Cell Metab. 2015;22:777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ehrenkranz RA, Gettner PA, Nelli CM, Sherwonit EA, Williams JE, Pearson HA, Ting BT, Janghorbani M. Iron absorption and incorporation into red blood cells by very low birth weight infants: studies with the stable isotope 58Fe. J Pediatr Gastroenterol Nutr 1992;15:270–8. [DOI] [PubMed] [Google Scholar]
- 115. McDonald MC, Abrams SA, Schanler RJ. Iron absorption and red blood cell incorporation in premature infants fed an iron-fortified infant formula. Pediatr Res 1998;44:507–11. [DOI] [PubMed] [Google Scholar]
- 116. Widness JA, Lombard KA, Ziegler EE, Serfass RE, Carlson SJ, Johnson KJ, Miller JE. Erythrocyte incorporation and absorption of 58Fe in premature infants treated with erythropoietin. Pediatr Res 1997;41:416–23. [DOI] [PubMed] [Google Scholar]
- 117. Lee S, Guillet R, Cooper EM, Westerman M, Orlando M, Kent T, Pressman E, O'Brien KO. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr Res 2016;79:42–8. [DOI] [PubMed] [Google Scholar]
- 118. Peslova G, Petrak J, Kuzelova K, Hrdy I, Halada P, Kuchel PW, Soe-Lin S, Ponka P, Sutak R, Becker E. et al. Hepcidin, the hormone of iron metabolism, is bound specifically to alpha-2-macroglobulin in blood. Blood 2009;113:6225–36. [DOI] [PubMed] [Google Scholar]
- 119. Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol 2009;122:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Preza GC, Pinon R, Ganz T, Nemeth E, Rastogi A. Cellular catabolism of the iron-regulatory peptide hormone hepcidin. PLoS One 2013;8:e58934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol 2015;15:500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr 2006;26:323–42. [DOI] [PubMed] [Google Scholar]
- 123. Finch C. Regulators of iron balance in humans. Blood 1994;84:1697–702. [PubMed] [Google Scholar]
- 124. Kautz L, Jung G, Valore E V, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet 2014;46:678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, Koeffler HP. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem 1999;274:20826–32. [DOI] [PubMed] [Google Scholar]
- 126. Zhang A-S, Enns CA. Molecular mechanisms of normal iron homeostasis. Hematology Am Soc Hematol Educ Program 2009;207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Nai A, Lidonnici MR, Rausa M, Mandelli G, Pagani A, Silvestri L, Ferrari G, Camaschella C. The second transferrin receptor regulates red blood cell production in mice. Blood 2015;125:1170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Raiten DJ, Sakr Ashour FA, Ross AC, Meydani SN, Dawson HD, Stephensen CB, Brabin BJ, Suchdev PS, van Ommen B. Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE). J Nutr 2015;145:1039S–1108S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Andrews NC. Anemia of inflammation: the cytokine-hepcidin link. J Clin Invest 2004;113:1251–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Cartwright GE, Wintrobe MM. The anemia of infection. XVII. A review. Adv Intern Med 1952;5:165–226. [PubMed] [Google Scholar]
- 131. Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta 2012;1823:1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Mleczko-Sanecka K, Roche F, da Silva AR, Call D, D'Alessio F, Ragab A, Lapinski PE, Ummanni R, Korf U, Oakes C. et al. Unbiased RNAi screen for hepcidin regulators links hepcidin suppression to proliferative Ras/RAF and nutrient-dependent mTOR signaling. Blood 2014;123:1574–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ponka P, Sheftel AD, Zhang A-S. Iron targeting to mitochondria in erythroid cells. Biochem Soc Trans 2002;30:735–8. [DOI] [PubMed] [Google Scholar]
- 134. Richardson DR, Lane DJR, Becker EM, Huang ML-H, Whitnall M, Suryo Rahmanto Y, Sheftel AD, Ponka P. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc Natl Acad Sci U S A 2010;107:10775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Philpott CC, Ryu M-S. Special delivery: distributing iron in the cytosol of mammalian cells. Front Pharmacol 2014;5:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Frey AG, Nandal A, Park JH, Smith PM, Yabe T, Ryu M-S, Ghosh MC, Lee J, Rouault TA, Park MH. et al. Iron chaperones PCBP1 and PCBP2 mediate the metallation of the dinuclear iron enzyme deoxyhypusine hydroxylase. Proc Natl Acad Sci U S A 2014;111:8031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ponka P, Beaumont C, Richardson DR. Function and regulation of transferrin and ferritin. Semin Hematol 1998;35:35–54. [PubMed] [Google Scholar]
- 138. Forejtnikovà H, Vieillevoye M, Zermati Y, Lambert M, Pellegrino RM, Guihard S, Gaudry M, Camaschella C, Lacombe C, Roetto A. et al. Transferrin receptor 2 is a component of the erythropoietin receptor complex and is required for efficient erythropoiesis. Blood 2010;116:5357–67. [DOI] [PubMed] [Google Scholar]
- 139. Bullock GC, Delehanty LL, Talbot A-L, Gonias SL, Tong W-H, Rouault TA, Dewar B, Macdonald JM, Chruma JJ, Goldfarb AN. Iron control of erythroid development by a novel aconitase-associated regulatory pathway. Blood 2010;116:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zhang D-L, Senecal T, Ghosh MC, Ollivierre-Wilson H, Tu T, Rouault TA. Hepcidin regulates ferroportin expression and intracellular iron homeostasis of erythroblasts. Blood 2011;118:2868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhang D-L, Hughes RM, Ollivierre-Wilson H, Ghosh MC, Rouault TA. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab 2009;9:461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Beaumont C, Delaby C. Recycling iron in normal and pathological States. Semin Hematol 2009;46:328–38. [DOI] [PubMed] [Google Scholar]
- 143. Smith A. Iron salvage pathways. In: Anderson GJ, McLaren GD, Iron physiology and pathophysiology in humans. Totowa, NJ: Humana Press; 2012. pp. 141–71. [Google Scholar]
- 144. Green R, Charlton R, Seftel H, Bothwell T, Mayet F, Adams B, Finch C, Layrisse M. Body iron excretion in man: a collaborative study. Am J Med 1968;45:336–53. [DOI] [PubMed] [Google Scholar]
- 145. McKie AT, Simpson RJ. Intestinal iron absorption. In: Anderson GJ, McLaren GD, Iron physiology and pathophysiology in humans. Totowa, NJ: Humana Press; 2012. [Google Scholar]
- 146. Theil EC, Burton JW, Beard JL. A sustainable solution for dietary iron deficiency through plant biotechnology and breeding to increase seed ferritin control. Eur J Clin Nutr 1997;51(Suppl 4):S28–31. [PubMed] [Google Scholar]
- 147. Hilty FM, Arnold M, Hilbe M, Teleki A, Knijnenburg JTN, Ehrensperger F, Hurrell RF, Pratsinis SE, Langhans W, Zimmermann MB. Iron from nanocompounds containing iron and zinc is highly bioavailable in rats without tissue accumulation. Nat Nanotechnol 2010;5:374–80. [DOI] [PubMed] [Google Scholar]
- 148. Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest 2005;115:1258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A. et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001;291:1755–9. [DOI] [PubMed] [Google Scholar]
- 150. Mims MP, Guan Y, Pospisilova D, Priwitzerova M, Indrak K, Ponka P, Divoky V, Prchal JT. Identification of a human mutation of DMT1 in a patient with microcytic anemia and iron overload. Blood 2005;105:1337–42. [DOI] [PubMed] [Google Scholar]
- 151. Cable JW, Cable EE, Bonkovsky HL. Induction of heme oxygenase in intestinal epithelial cells: studies in Caco-2 cell cultures. Mol Cell Biochem 1993;129:93–8. [DOI] [PubMed] [Google Scholar]
- 152. Chen H, Huang G, Su T, Gao H, Attieh ZK, McKie AT, Anderson GJ, Vulpe CD. Decreased hephaestin activity in the intestine of copper-deficient mice causes systemic iron deficiency. J Nutr 2006;136:1236–41. [DOI] [PubMed] [Google Scholar]
- 153. Cherukuri S, Potla R, Sarkar J, Nurko S, Harris ZL, Fox PL. Unexpected role of ceruloplasmin in intestinal iron absorption. Cell Metab 2005;2:309–19. [DOI] [PubMed] [Google Scholar]
- 154. Pietrangelo A. Genetics, genetic testing, and management of hemochromatosis: 15 years since hepcidin. Gastroenterology 2015;149:1240–51.e4. [DOI] [PubMed] [Google Scholar]
- 155. Mena NP, Esparza A, Tapia V, Valdés P, Núñez MT. Hepcidin inhibits apical iron uptake in intestinal cells. Am J Physiol Gastrointest Liver Physiol 2008;294:G192–8. [DOI] [PubMed] [Google Scholar]
- 156. Brasse-Lagnel C, Karim Z, Letteron P, Bekri S, Bado A, Beaumont C. Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology. 2011;140:1261–71.e1. [DOI] [PubMed] [Google Scholar]
- 157. Osterloh KR, Simpson RJ, Snape S, Peters TJ. Intestinal iron absorption and mucosal transferrin in rats subjected to hypoxia. Blut 1987;55:421–31. [DOI] [PubMed] [Google Scholar]
- 158. Shah YM, Matsubara T, Ito S, Yim S-H, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab 2009;9:152–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest 2009;119:1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Simpson RJ, McKie AT. Iron and oxygen sensing: a tale of 2 interacting elements? Metallomics 2015;7:223–31. [DOI] [PubMed] [Google Scholar]
- 161. McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F. et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell 2000;5:299–309. [DOI] [PubMed] [Google Scholar]
- 162. Gunshin H, Allerson CR, Polycarpou-Schwarz M, Rofts A, Rogers JT, Kishi F, Hentze MW, Rouault TA, Andrews NC, Hediger MA. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett 2001;509:309–16. [DOI] [PubMed] [Google Scholar]
- 163. Fuqua BK, Vulpe CD, Anderson GJ. Intestinal iron absorption. J Trace Elem Med Biol 2012;26:115–9. [DOI] [PubMed] [Google Scholar]
- 164. Leong W-I, Bowlus CL, Tallkvist J, Lönnerdal B. DMT1 and FPN1 expression during infancy: developmental regulation of iron absorption. Am J Physiol Gastrointest Liver Physiol 2003;285:G1153–61. [DOI] [PubMed] [Google Scholar]
- 165. Carter AM, Mess AM. Conservation of placentation during the tertiary radiation of mammals in South America. J Morphol 2013;274:557–69. [DOI] [PubMed] [Google Scholar]
- 166. Petry CD, Wobken JD, McKay H, Eaton MA, Seybold VS, Johnson DE, Georgieff MK. Placental transferrin receptor in diabetic pregnancies with increased fetal iron demand. Am J Physiol 1994;267:E507–14. [DOI] [PubMed] [Google Scholar]
- 167. McArdle HJ, Douglas AJ, Morgan EH. Transferrin binding by microvillar vesicles isolated from rat placenta. Placenta 1984;5:131–8. [DOI] [PubMed] [Google Scholar]
- 168. McArdle HJ, Tysoe J. Effect of nicotine on transferrin binding and iron uptake by cultured rat placenta. J Cell Physiol 1988;134:509–13. [DOI] [PubMed] [Google Scholar]
- 169. Wang C-Y, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B, Knutson MD. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J Biol Chem 2012;287:34032–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Unger EL, Hurst AR, Georgieff MK, Schallert T, Rao R, Connor JR, Kaciroti N, Lozoff B, Felt B. Behavior and monoamine deficits in prenatal and perinatal iron deficiency are not corrected by early postnatal moderate-iron or high-iron diets in rats. J Nutr 2012;142:2040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Gambling L, Czopek A, Andersen HS, Holtrop G, Srai SKS, Krejpcio Z, McArdle HJ. Fetal iron status regulates maternal iron metabolism during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol 2009;296:R1063–70. [DOI] [PubMed] [Google Scholar]
- 172. Finkenstedt A, Widschwendter A, Brasse-Lagnel CG, Theurl I, Hubalek M, Dieplinger H, Tselepis C, Ward DG, Vogel W, Zoller H. Hepcidin is correlated to soluble hemojuvelin but not to increased GDF15 during pregnancy. Blood Cells Mol Dis 2012;48:233–7. [DOI] [PubMed] [Google Scholar]
- 173. Andersen HS, Gambling L, Holtrop G, McArdle HJ. Maternal iron deficiency identifies critical windows for growth and cardiovascular development in the rat postimplantation embryo. J Nutr 2006;136:1171–7. [DOI] [PubMed] [Google Scholar]
- 174. Gambling L, Andersen HS, Czopek A, Wojciak R, Krejpcio Z, McArdle HJ. Effect of timing of iron supplementation on maternal and neonatal growth and iron status of iron-deficient pregnant rats. J Physiol 2004;561:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. McArdle HJ, Lang C, Hayes H, Gambling L. Role of the placenta in regulation of fetal iron status. Nutr Rev. 2011;69(Suppl 1):S17–22. [DOI] [PubMed] [Google Scholar]
- 176. Cao C, O'Brien KO. Pregnancy and iron homeostasis: an update. Nutr Rev 2013;71:35–51. [DOI] [PubMed] [Google Scholar]
- 177. O'Brien KO, Zavaleta N, Abrams SA, Caulfield LE. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr 2003;77:924–30. [DOI] [PubMed] [Google Scholar]
- 178. Cornock R, Gambling L, Langley-Evans SC, McArdle HJ, McMullen S. The effect of feeding a low iron diet prior to and during gestation on fetal and maternal iron homeostasis in two strains of rat. Reprod Biol Endocrinol 2013;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Fretham SJB, Carlson ES, Wobken J, Tran P V, Petryk A, Georgieff MK. Temporal manipulation of transferrin-receptor-1-dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment. Hippocampus 2012;22:1691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69(Suppl 1):S43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 2006;64:S34-43-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Dallman PR. Biochemical basis for the manifestations of iron deficiency. Annu Rev Nutr 1986;6:13–40. [DOI] [PubMed] [Google Scholar]
- 183. Morath DJ, Mayer-Pröschel M. Iron deficiency during embryogenesis and consequences for oligodendrocyte generation in vivo. Dev Neurosci 2002;24:197–207. [DOI] [PubMed] [Google Scholar]
- 184. Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia 1996;17:83–93. [DOI] [PubMed] [Google Scholar]
- 185. Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr 2003;133:3215–21. [DOI] [PubMed] [Google Scholar]
- 186. Youdim MB, Green AR, Bloomfield MR, Mitchell BD, Heal DJ, Grahame-Smith DG. The effects of iron deficiency on brain biogenic monoamine biochemistry and function in rats. Neuropharmacology 1980;19:259–67. [DOI] [PubMed] [Google Scholar]
- 187. Clardy SL, Wang X, Zhao W, Liu W, Chase GA, Beard JL, True Felt B, Connor JR. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm 2006;S173–96. [DOI] [PubMed] [Google Scholar]
- 188. Carlson ES, Stead JDH, Neal CR, Petryk A, Georgieff MK. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus. 2007;17:679–91. [DOI] [PubMed] [Google Scholar]
- 189. Brunette KE, Tran P V, Wobken JD, Carlson ES, Georgieff MK. Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev Neurosci 2010;32:238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Jorgenson LA, Sun M, O'Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus 2005;15:1094–102. [DOI] [PubMed] [Google Scholar]
- 191. Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr 1996;126:693–701. [DOI] [PubMed] [Google Scholar]
- 192. Beard J. Iron deficiency alters brain development and functioning. J Nutr. 2003;133:1468S–72S. [DOI] [PubMed] [Google Scholar]
- 193. Burdo J, Connor J. Iron transport in the central nervous system. In: Templeton D, editor Cellular and molecular mechanisms of iron transport. New York: Marcel Dekker; 2001. pp. 487–502. [Google Scholar]
- 194. Leitner D, Connor J. Functional roles of transferrin in the brain. Biochim Biophys Acta 2012;1820:393–402. [DOI] [PubMed] [Google Scholar]
- 195. Zheng W, Monnot A. Regulation of brain iron and copper homeostasis by brain barrier systems: implication in neurodegenerative diseases. Pharmacol Ther 2012;133:177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Todorich B, Zhang X, Slagle-Webb B, Seaman WE, Connor JR. Tim-2 is the receptor for H-ferritin on oligodendrocytes. J Neurochem 2008;107:1495–505. [DOI] [PubMed] [Google Scholar]
- 197. Rouault TA. Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat Rev Neurosci 2013;14:551–64. [DOI] [PubMed] [Google Scholar]
- 198. Siddappa AJM, Rao RB, Wobken JD, Leibold EA, Connor JR, Georgieff MK. Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. J Neurosci Res 2002;68:761–75. [DOI] [PubMed] [Google Scholar]
- 199. Pokorný J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. I. Development of dendritic arborisation in pyramidal neurons. Brain Res Bull 1981;7:113–20. [DOI] [PubMed] [Google Scholar]
- 200. Tran P V, Carlson ES, Fretham SJB, Georgieff MK. Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. J Nutr 2008;138:2495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Fretham SJB, Carlson ES, Georgieff MK. The role of iron in learning and memory. Adv Nutr 2011;2:112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Thompson RA, Nelson CA. Developmental science and the media. Early brain development. Am Psychol 2001;56:5–15. [DOI] [PubMed] [Google Scholar]
- 203. Kretchmer N, Beard JL, Carlson S. The role of nutrition in the development of normal cognition. Am J Clin Nutr 1996;63: S997–1001. [DOI] [PubMed] [Google Scholar]
- 204. Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev 1979;3:79–83. [DOI] [PubMed] [Google Scholar]
- 205. Beard J, Erikson KM, Jones BC. Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J Nutr 2003;133:1174–9. [DOI] [PubMed] [Google Scholar]
- 206. Beard JL, Unger EL, Bianco LE, Paul T, Rundle SE, Jones BC. Early postnatal iron repletion overcomes lasting effects of gestational iron deficiency in rats. J Nutr 2007;137:1176–82. [DOI] [PubMed] [Google Scholar]
- 207. de Deungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res 2000;48:169–76. [DOI] [PubMed] [Google Scholar]
- 208. Nelson C, Silverstein FS. Acute disruption of cytochrome oxidase activity in brain in a perinatal rat stroke model. Pediatr Res 1994;36:12–9. [DOI] [PubMed] [Google Scholar]
- 209. Carlson ES, Tkac I, Magid R, O'Connor MB, Andrews NC, Schallert T, Gunshin H, Georgieff MK, Petryk A. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr. 2009;139:672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Schmidt AT, Waldow KJ, Grove WM, Salinas JA, Georgieff MK. Dissociating the long-term effects of fetal/neonatal iron deficiency on three types of learning in the rat. Behav Neurosci 2007;121:475–82. [DOI] [PubMed] [Google Scholar]
- 211. Ortiz E, Pasquini JM, Thompson K, Felt B, Butkus G, Beard J, Connor JR. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J Neurosci Res 2004;77:681–9. [DOI] [PubMed] [Google Scholar]
- 212. Heeney MM, Finberg KE. Iron-refractory iron deficiency anemia (IRIDA). Hematol Oncol Clin North Am 2014;28:637–52. [DOI] [PubMed] [Google Scholar]
- 213. Dawson AA, Ogston D, Fullerton HW. Evaluation of diagnostic significance of certain symptoms and physical signs in anaemic patients. Br Med J 1969;3:436–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Beard JL, Durward C. The liabilities of iron deficiency. In: Anderson GJ, McLaren GD, Iron physiology and pathophysiology in humans. Totowa, NJ: Humana Press; 2012. pp. 283–302. [Google Scholar]
- 215. Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TP. et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014;123:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr 2009;12:444–54. [DOI] [PubMed] [Google Scholar]
- 217. Centers for Disease Control and Prevention FastStats - Anemia [Internet]. 2016[cited 2016 Aug 15]. Available from:http://www.cdc.gov/nchs/fastats/anemia.htm.
- 218. WHO, UNICEF, UNU Iron deficiency anaemia: assessment, prevention and control: a guide for programme managers. Geneva: World Health Organization; 2001. [Google Scholar]
- 219. Zucconi M, Ferini-Strambi L. Epidemiology and clinical findings of restless legs syndrome. Sleep Med 2004;5:293–9. [DOI] [PubMed] [Google Scholar]
- 220. Sun ER, Chen CA, Ho G, Earley CJ, Allen RP. Iron and the restless legs syndrome. Sleep 1998;21:371–7. [PubMed] [Google Scholar]
- 221. Beaton GH. Iron needs during pregnancy: do we need to rethink our targets? Am J Clin Nutr 2000;72:S265–71. [DOI] [PubMed] [Google Scholar]
- 222. Rush D. Nutrition and maternal mortality in the developing world. Am J Clin Nut. 2000;72:S212–40. [DOI] [PubMed] [Google Scholar]
- 223. Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72:S257–64. [DOI] [PubMed] [Google Scholar]
- 224. Milman N, Paszkowski T, Cetin I, Castelo-Branco C. Supplementation during pregnancy: beliefs and science. Gynecol Endocrinol 2016;32:509–16. [DOI] [PubMed] [Google Scholar]
- 225. Svanberg B, Arvidsson B, Norrby A, Rybo G, Sölvell L. Absorption of supplemental iron during pregnancy - a longitudinal study with repeated bone-marrow studies and absorption measurements. Acta Obstet Gynecol Scand 1975;48:S87–108. [DOI] [PubMed] [Google Scholar]
- 226. van Santen S, Kroot JJC, Zijderveld G, Wiegerinck ET, Spaanderman MEA, Swinkels DW. The iron regulatory hormone hepcidin is decreased in pregnancy: a prospective longitudinal study. Clin Chem Lab Med 2013;51:1395–401. [DOI] [PubMed] [Google Scholar]
- 227. Kuzawa CW, Adair LS, Borja J, McDade TW. C-reactive protein by pregnancy and lactational status among Filipino young adult women. Am J Hum Biol 25:131–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Lee S, Guillet R, Cooper EM, Westerman M, Orlando M, Pressman E, O'Brien KO. Maternal inflammation at delivery affects assessment of maternal iron status. J Nutr 2014;144:1524–32. [DOI] [PubMed] [Google Scholar]
- 229. Mei Z, Li H, Serdula MK, Flores-Ayala RC, Wang L, Liu J-M, Grummer-Strawn LM. C-reactive protein increases with gestational age during pregnancy among Chinese women. Am J Hum Biol 2016;28:574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Cao C, Pressman EK, Cooper EM, Guillet R, Westerman M, O'Brien KO. Prepregnancy body mass index and gestational weight gain have no negative impact on maternal or neonatal iron status. Reprod Sci 2016;23:613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: is hepcidin the link? J Perinatol 2013;33:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Fenton V, Cavill I, Fisher J. Iron stores in pregnancy. Br J Haematol 1977;37:145–9. [PubMed] [Google Scholar]
- 233. Hallberg L, Hultén L. Iron requirements, iron balance and iron deficiency in menstruating and pregnant women. In: Hallberg L, Asp N-G, Iron nutrition in health and disease. London: George Libbey; 1996. pp. 165–82. [Google Scholar]
- 234. Centers for Disease Control Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep 1998;47:1–29. [PubMed] [Google Scholar]
- 235. Recommendations to prevent and control iron deficiency in the United States Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep 1998;47:1–29. [PubMed] [Google Scholar]
- 236. World Health Organization Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva: WHO; 2011. [Google Scholar]
- 237. New S, Wirth M. Anaemia, pregnancy, and maternal mortality: the problem with globally standardised haemoglobin cutoffs. BJOG 2015;122:166–9. [DOI] [PubMed] [Google Scholar]
- 238. Viteri FE, Berger J. Importance of pre-pregnancy and pregnancy iron status: can long-term weekly preventive iron and folic acid supplementation achieve desirable and safe status? Nutr Rev 2005;63:S65–76. [DOI] [PubMed] [Google Scholar]
- 239. Aranda N, Ribot B, Garcia E, Viteri FE, Arija V. Pre-pregnancy iron reserves, iron supplementation during pregnancy, and birth weight. Early Hum Dev 2011;87:791–7. [DOI] [PubMed] [Google Scholar]
- 240. Cooper MJ, Zlotkin SH. Day-to-day variation of transferrin receptor and ferritin in healthy men and women. Am J Clin Nutr 1996;64:738–42. [DOI] [PubMed] [Google Scholar]
- 241. Milman N, Byg K-E, Bergholt T, Eriksen L, Hvas A-M. Body iron and individual iron prophylaxis in pregnancy–should the iron dose be adjusted according to serum ferritin? Ann Hematol 2006;85:567–73. [DOI] [PubMed] [Google Scholar]
- 242. Peyrin-Biroulet L, Williet N, Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr 2015;102:1585–94. [DOI] [PubMed] [Google Scholar]
- 243. Carriaga MT, Skikne BS, Finley B, Cutler B, Cook JD. Serum transferrin receptor for the detection of iron deficiency in pregnancy. Am J Clin Nutr 1991;54:1077–81. [DOI] [PubMed] [Google Scholar]
- 244. Choi JW, Im MW, Pai SH. Serum transferrin receptor concentrations during normal pregnancy. Clin Chem 2000;46:725–7. [PubMed] [Google Scholar]
- 245. Akesson A, Bjellerup P, Berglund M, Bremme K, Vahter M. Soluble transferrin receptor: longitudinal assessment from pregnancy to postlactation. Obstet Gynecol 2002;99:260–6. [DOI] [PubMed] [Google Scholar]
- 246. Walsh T, O'Broin SD, Cooley S, Donnelly J, Kennedy J, Harrison RF, McMahon C, Geary M. Laboratory assessment of iron status in pregnancy. Clin Chem Lab Med 2011;49:1225–30. [DOI] [PubMed] [Google Scholar]
- 247. Cogswell ME, Looker AC, Pfeiffer CM, Cook JD, Lacher DA, Beard JL, Lynch SR, Grummer-Strawn LM. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003–2006. Am J Clin Nutr 2009;89:1334–42. [DOI] [PubMed] [Google Scholar]
- 248. Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood 2003;101:3359–64. [DOI] [PubMed] [Google Scholar]
- 249. Mei Z, Cogswell ME, Looker AC, Pfeiffer CM, Cusick SE, Lacher DA, Grummer-Strawn LM. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am J Clin Nutr 2011;93:1312–20. [DOI] [PubMed] [Google Scholar]
- 250. Wheeler S. Assessment and interpretation of micronutrient status during pregnancy. Proc Nutr Soc 2008;67:437–50. [DOI] [PubMed] [Google Scholar]
- 251. Schifman RB, Thomasson JE, Evers JM. Red blood cell zinc protoporphyrin testing for iron-deficiency anemia in pregnancy. Am J Obstet Gynecol 1987;157:304–7. [DOI] [PubMed] [Google Scholar]
- 252. Romslo I, Haram K, Sagen N, Augensen K. Iron requirement in normal pregnancy as assessed by serum ferritin, serum transferrin saturation and erythrocyte protoporphyrin determinations. Br J Obstet Gynaecol 1983;90:101–7. [DOI] [PubMed] [Google Scholar]
- 253. Harthoorn-Lasthuizen EJ, Lindemans J, Langenhuijsen MM. Erythrocyte zinc protoporphyrin testing in pregnancy. Acta Obstet Gynecol Scand 2000;79:660–6. [PubMed] [Google Scholar]
- 254. Milman N, Agger AO, Nielsen OJ. Iron supplementation during pregnancy. Effect on iron status markers, serum erythropoietin and human placental lactogen. A placebo controlled study in 207 Danish women. Dan Med Bull 1991;38:471–6. [PubMed] [Google Scholar]
- 255. Koenig MD, Tussing-Humphreys L, Day J, Cadwell B, Nemeth E. Hepcidin and iron homeostasis during pregnancy. Nutrients 2014;6:3062–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Hedengran KK, Nelson D, Andersen MR, Stender S, Szecsi PB. Hepcidin levels are low during pregnancy and increase around delivery in women without iron deficiency - a prospective cohort study. J Matern Fetal Neonatal Med 2016;29:1506–8. [DOI] [PubMed] [Google Scholar]
- 257. Gyarmati B, Szabó E, Szalay B, Czuczy N, Toldi G, Cseh A, Vásárhelyi B, Takáts Z. Serum maternal hepcidin levels 3 days after delivery are higher compared to those measured at parturition. J Obstet Gynaecol Res 2011;37:1620–4. [DOI] [PubMed] [Google Scholar]
- 258. Shao J, Lou J, Rao R, Georgieff MK, Kaciroti N, Felt BT, Zhao Z-Y, Lozoff B. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr 2012;142:2004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Zeng L, Dibley MJ, Cheng Y, Dang S, Chang S, Kong L, Yan H. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ 2008;337:a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Dibley MJ, Titaley CR, d'Este C, Agho K. Iron and folic acid supplements in pregnancy improve child survival in Indonesia. Am J Clin Nutr 2012;95:220–30. [DOI] [PubMed] [Google Scholar]
- 261. Lozoff B, Georgieff M. Iron deficiency and brain development. Semin Pediatr Neurol 2006;13:158–65. [DOI] [PubMed] [Google Scholar]
- 262. Lozoff B, Clark K, Jing Y, Armony-Sivan R, Angelilli M, Jacobson S. Dose-response relations between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr 2008;152:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Georgieff M. Iron. Neonatal nutrition and metabolism. 2nd ed Cambridge: Cambridge University Press; 2006. pp. 291–8. [Google Scholar]
- 264. Georgieff MK, Schmidt RL, Mills MM, Radmer WJ, Widness JA. Fetal iron and cytochrome c status after intrauterine hypoxemia and erythropoietin administration. Am J Physiol. 1992;262:R485–91. [DOI] [PubMed] [Google Scholar]
- 265. Rao R, Georgieff M. Microminerals. Nutritional requirements of the premature infant. Cincinnati, OH: Digipub; 2005. pp. 277–310. [Google Scholar]
- 266. Rao R. The nutritionally deprived fetus and newborn infant. International Review of Child Neurology Series: Acquired brain injury in the fetus & newborn. London: Mac Keith Press; 2012. pp. 277–287. [Google Scholar]
- 267. Baker RD, Greer FR, Committee on Nutrition American Academy of Pediatrics Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics. 2010;126:1040–50. [DOI] [PubMed] [Google Scholar]
- 268. Lukowski AF, Koss M, Burden MJ, Jonides J, Nelson CA, Kaciroti N, Jimenez E, Lozoff B. Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci. 2010;13:54–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Fugelstad A, Rao R, Georgieff M. The role of nutrition in cognitive development. Handbook in developmental cognitive neuroscience. Cambridge, MA: MIT Press; 2008. pp. 623–41. [Google Scholar]
- 270. Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131:S649–66; discussion S666–68. [DOI] [PubMed] [Google Scholar]
- 271. Walker S, Wachs T, Gardner J, Lozoff B, Wasserman G, Pollitt E, Carter J, International Child Development Steering Group Child development: risk factors for adverse outcomes in developing countries. Lancet 2007;369:145–57. [DOI] [PubMed] [Google Scholar]
- 272. Walker S, Wachs T, Grantham-McGregor S, Black M, Nelson C, Huffman S, Baker-Henningham H, Chang S, Hamadani J, Lozoff B. et al. Inequality in early childhood: risk and protective factors for early child development. Lancet 2011;378:1325–38. [DOI] [PubMed] [Google Scholar]
- 273. Schmidt MK, Muslimatun S, West CE, Schultink W, Hautvast JGAJ. Mental and psychomotor development in Indonesian infants of mothers supplemented with vitamin A in addition to iron during pregnancy. Br J Nutr 2004;91:279–86. [DOI] [PubMed] [Google Scholar]
- 274. Prado EL, Alcock KJ, Muadz H, Ullman MT, Shankar AH, SUMMIT Study Group Maternal multiple micronutrient supplements and child cognition: a randomized trial in Indonesia. Pediatrics 2012;130:e536–46. [DOI] [PubMed] [Google Scholar]
- 275. Georgieff MK, Landon MB, Mills MM, Hedlund BE, Faassen AE, Schmidt RL, Ophoven JJ, Widness JA. Abnormal iron distribution in infants of diabetic mothers: spectrum and maternal antecedents. J Pediatr 1990;117:455–61. [DOI] [PubMed] [Google Scholar]
- 276. Petry CD, Eaton MA, Wobken JD, Mills MM, Johnson DE, Georgieff MK. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. J Pediatr 1992;121:109–14. [DOI] [PubMed] [Google Scholar]
- 277. Chockalingam UM, Murphy E, Ophoven JC, Weisdorf SA, Georgieff MK. Cord transferrin and ferritin values in newborn infants at risk for prenatal uteroplacental insufficiency and chronic hypoxia. J Pediatr 1987;111:283–6. [DOI] [PubMed] [Google Scholar]
- 278. Sweet DG, Savage G, Tubman TR, Lappin TR, Halliday HL. Study of maternal influences on fetal iron status at term using cord blood transferrin receptors. Arch Dis Child Fetal Neonatal Ed 2001;84:F40–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology 2007;92:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Georgieff M, Berry S, Wobken J, Leibold E. Increased placental iron regulatory protein-1 expression in diabetic pregnancies complicated by fetal iron deficiency. Placenta 1999;20:87–93. [DOI] [PubMed] [Google Scholar]
- 281. Nold J, Georgieff M. Infants of diabetic mothers. Pediatr Clin North Am 2004;51:619–37. [DOI] [PubMed] [Google Scholar]
- 282. Georgieff MK, Petry CD, Wobken JD, Oyer CE. Liver and brain iron deficiency in newborn infants with bilateral renal agenesis (Potter's syndrome). Pediatr Pathol Lab Med 16:509–19. [DOI] [PubMed] [Google Scholar]
- 283. Saarinen UM, Siimes MA. Iron absorption from breast milk, cow's milk, and iron-supplemented formula: an opportunistic use of changes in total body iron determined by hemoglobin, ferritin, and body weight in 132 infants. Pediatr Res 1979;13:143–7. [DOI] [PubMed] [Google Scholar]
- 284. Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier R-A. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res 2004;55:1034–41. [DOI] [PubMed] [Google Scholar]
- 285. Youdim MB, Yehuda S. The neurochemical basis of cognitive deficits induced by brain iron deficiency: involvement of dopamine-opiate system. Cell Mol Biol (Noisy-le-grand). 2000;46:491–500. [PubMed] [Google Scholar]
- 286. de Ungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res 2000;48:169–76. [DOI] [PubMed] [Google Scholar]
- 287. Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci 2003;25:412–20. [DOI] [PubMed] [Google Scholar]
- 288. Ward KL, Tkac I, Jing Y, Felt B, Beard J, Connor J, Schallert T, Georgieff MK, Rao R. Gestational and lactational iron deficiency alters the developing striatal metabolome and associated behaviors in young rats. J Nutr 2007;137:1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Fretham SJB, Carlson ES, Georgieff MK. Neuronal-specific iron deficiency dysregulates mammalian target of rapamycin signaling during hippocampal development in nonanemic genetic mouse models. J Nutr 2013;143:260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. World Health Organization, Centers for Disease Control and Prevention Assessing the iron status of populations. Geneva: World Health Organization; 2007. [Google Scholar]
- 291. Felt B, Beard J, Schallert T, Shao J, Aldridge J, Connor J, Georgieff M, Lozoff B. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res 2006;171:261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Tran P V, Fretham SJB, Carlson ES, Georgieff MK. Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats. Pediatr Res 2009;65:493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Gewirtz J, Hamilton K, Babu M, Wobken J, Georgieff M. Effects of gestational iron deficiency on fear conditioning in juvenile and adult rats. Brain Res 2008;27:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Beard JL, Murray-Kolb LE, Rosales FJ, Solomons NW, Angelilli ML. Interpretation of serum ferritin concentrations as indicators of total-body iron stores in survey populations: the role of biomarkers for the acute phase response. Am J Clin Nutr 2006;84:1498–505. [DOI] [PubMed] [Google Scholar]
- 295. Raiten D, Combs GJ. Directions in nutritional assessment-biomarkers and bio-indicators: providing clarity in the face of complexity. Sight Life 2015;29:39–44. [Google Scholar]
- 296. Tamura T, Goldenberg R, Hou J, Johnston K, Cliver S, Ramey S, Nelson K. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr 2002;140:165–70. [DOI] [PubMed] [Google Scholar]
- 297. Wachs TD, Pollitt E, Cueto S, Jacoby E, Creed-Kanashiro H. Relation of neonatal iron status to individual variability in neonatal temperament. Dev Psychobiol 2005;46:141–53. [DOI] [PubMed] [Google Scholar]
- 298. Amin S, Orlando M, Eddins A, MacDonald M, Monczynski C, Wang H. In utero iron status and auditory neural maturation in premature infants as evaluated by auditory brainstem response. J Pediatr 2010;156:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Chang S, Zeng L, Brouwer ID, Kok FJ, Yan H. Effect of iron deficiency anemia in pregnancy on child mental development in rural China. Pediatrics 2013;131:e755–63. [DOI] [PubMed] [Google Scholar]
- 300. Christian P, Murray-Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, Leclerq SC, Tielsch JM. Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA 2010;304:2716–23. [DOI] [PubMed] [Google Scholar]
- 301. Li Q, Yan H, Zeng L, Cheng Y, Liang W, Dang S, Wang Q, Tsuji I. Effects of maternal multimicronutrient supplementation on the mental development of infants in rural western China: follow-up evaluation of a double-blind, randomized, controlled trial. Pediatrics 2009;123:e685–92. [DOI] [PubMed] [Google Scholar]
- 302. Murray-Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, LeClerq SC, Morgan ME, Tielsch JM, Christian P. Preschool micronutrient supplementation effects on intellectual and motor function in school-aged Nepalese children. Arch Pediatr Adolesc Med 2012;166:404–10. [DOI] [PubMed] [Google Scholar]
- 303. Nelson C. The ontogeny of human memory: a cognitive neuroscience perspective. Dev Psychol 1995;31:723–38. [Google Scholar]
- 304. Deregnier R, Nelson C, Thomas K, Wewerka S, Georgieff M. Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. J Pediatr 2000;137:777–84. [DOI] [PubMed] [Google Scholar]
- 305. Riggins T, Miller NC, Bauer PJ, Georgieff MK, Nelson CA. Consequences of low neonatal iron status due to maternal diabetes mellitus on explicit memory performance in childhood. Dev Neuropsychol 2009;34:762–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Mayes L. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicol Teratol 2002;24:385–95. [DOI] [PubMed] [Google Scholar]
- 307. Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: delayed maturation of auditory brainstem responses. Am J Clin Nutr 1998;68:683–90. [DOI] [PubMed] [Google Scholar]
- 308. Gupta P, Perrine C, Mei Z, Scanlon K. Iron, anemia, and iron deficiency anemia among young children in the United States. Nutrients 2016;8:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Georgieff M, Wewerka S, Nelson C, Deregnier R. Iron status at 9 months of infants with low iron stores at birth. J Pediatr 2002;141:405–9. [DOI] [PubMed] [Google Scholar]
- 310. Georgieff M. Iron Deficiency. In: American Academy of Pediatrics , Kleinman RE, Greer FR, editors. Pediatric nutrition. 7th ed Elk Grove, IL: AAP Press; 2013. pp. 299–312. [Google Scholar]
- 311. Ben-Shachar D, Ashkenazi R, Youdim MB. Long-term consequence of early iron-deficiency on dopaminergic neurotransmission in rats. Int J Dev Neurosci 1986;4:81–8. [DOI] [PubMed] [Google Scholar]
- 312. Youdim MB, Ben-Shachar D, Yehuda S. Putative biological mechanisms of the effect of iron deficiency on brain biochemistry and behavior. Am J Clin Nutr 1989;50:607-15-7. [DOI] [PubMed] [Google Scholar]
- 313. Nelson C, Erikson K, Piñero DJ, Beard JL. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J Nutr 1997;127:2282–8. [DOI] [PubMed] [Google Scholar]
- 314. Erikson K, Jones B, Hess E, Zhang Q, Beard J. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav 2001;69:409–18. [DOI] [PubMed] [Google Scholar]
- 315. Beard J, Erikson K, Jones B. Neurobehavioral analysis of developmental iron deficiency in rats. Behav Brain Res. 2002;134:517–24. [DOI] [PubMed] [Google Scholar]
- 316. Golub M, Hogrefe C, Germann S, Capitanio J, Lozoff B. Behavioral consequences of developmental iron deficiency in infant rhesus monkeys. Neurotoxicol Teratol 2006;28:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Golub MS, Hogrefe CE, Germann SL. Iron deprivation during fetal development changes the behavior of juvenile rhesus monkeys. J Nutr 2007;137:979–84. [DOI] [PubMed] [Google Scholar]
- 318. Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. Iron deficiency anemia and affective response in rhesus monkey infants. Dev Psychobiol 2009;51:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci 2005;8:805–12. [DOI] [PubMed] [Google Scholar]
- 320. Lozoff B. Perinatal iron deficiency and the developing brain. Pediatr Res 2000;48:137–9. [DOI] [PubMed] [Google Scholar]
- 321. Angulo-Kinzler R, Peirano P, Lin E, Algarin C, Garrido M, Lozoff B. Twenty-four-hour motor activity in human infants with and without iron deficiency anemia. Early Hum Dev 2002;70:85–101. [DOI] [PubMed] [Google Scholar]
- 322. Badaracco ME, Siri MVR, Pasquini JM. Oligodendrogenesis: the role of iron. Biofactors 2010;36:98–102. [DOI] [PubMed] [Google Scholar]
- 323. Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia 2009;57:467–78. [DOI] [PubMed] [Google Scholar]
- 324. Beard JL, Wiesinger JA, Connor JR. Pre- and postweaning iron deficiency alters myelination in Sprague-Dawley rats. Dev Neurosci 2003;25:308–15. [DOI] [PubMed] [Google Scholar]
- 325. Algarín C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res 2003;53:217–23. [DOI] [PubMed] [Google Scholar]
- 326. Akman M, Cebeci D, Okur V, Angin H, Abali O, Akman AC. The effects of iron deficiency on infants’ developmental test performance. Acta Paediatr 2004;93:1391–6. [PubMed] [Google Scholar]
- 327. Hasanbegović E, Sabanović S. Effects of iron therapy on motor and mental development of infants and small children suffering from iron deficiency anaemia. Med Arh 2004;58:227–9. [PubMed] [Google Scholar]
- 328. Idjradinata P, Pollitt E. Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet 1993;341:1–4. [DOI] [PubMed] [Google Scholar]
- 329. Lozoff B, Brittenham GM, Wolf AW, McClish DK, Kuhnert PM, Jimenez E, Jimenez R, Mora LA, Gomez I, Krauskoph D. Iron deficiency anemia and iron therapy effects on infant developmental test performance. Pediatrics 1987;79:981–95. [PubMed] [Google Scholar]
- 330. Lozoff B, Wolf A, Jimenez E. Iron-deficiency anemia and infant development: effects of extended oral iron therapy. J Pediatr 1996;129:382–9. [DOI] [PubMed] [Google Scholar]
- 331. Walter T, De Andraca I, Chadud P, Perales CG. Iron deficiency anemia: adverse effects on infant psychomotor development. Pediatrics 1989;84:7–17. [PubMed] [Google Scholar]
- 332. Lozoff B, Armony-Sivan R, Kaciroti N, Jing Y, Golub M, Jacobson SW. Eye-blinking rates are slower in infants with iron-deficiency anemia than in nonanemic iron-deficient or iron-sufficient infants. J Nutr 2010;140:1057–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Burden MJ, Westerlund AJ, Armony-Sivan R, Nelson CA, Jacobson SW, Lozoff B, Angelilli ML, Jacobson JL. An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics 2007;120:e336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Carter RC, Jacobson JL, Burden MJ, Armony-Sivan R, Dodge NC, Angelilli ML, Lozoff B, Jacobson SW. Iron deficiency anemia and cognitive function in infancy. Pediatrics 2010;126:e427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Monga M, Walia V, Gandhi A, Chandra J, Sharma S. Effect of iron deficiency anemia on visual evoked potential of growing children. Brain Dev. 2010;32:213–6. [DOI] [PubMed] [Google Scholar]
- 336. Georgieff MK, Innis SM. Controversial nutrients that potentially affect preterm neurodevelopment: essential fatty acids and iron. Pediatr Res. 2005;57:R99–103. [DOI] [PubMed] [Google Scholar]
- 337. Morley R, Abbott R, Fairweather-Tait S, MacFadyen U, Stephenson T, Lucas A. Iron fortified follow on formula from 9 to 18 months improves iron status but not development or growth: a randomised trial. Arch Dis Child 1999;81:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Williams J, Wolff A, Daly A, MacDonald A, Aukett A, Booth IW. Iron supplemented formula milk related to reduction in psychomotor decline in infants from inner city areas: randomised study. BMJ 1999;318:693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Black M, Sazawal S, Black R, Al E. Micronutrient supplementation leads to improved development and exploratory behavior among infants born small for gestational age. Pediatr Res 2002;51:2565. [Google Scholar]
- 340. Black MM, Baqui AH, Zaman K, Ake Persson L, El Arifeen S, Le K, McNary SW, Parveen M, Hamadani JD, Black RE. Iron and zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. Am J Clin Nutr 2004;80:903–10. [DOI] [PubMed] [Google Scholar]
- 341. Lind T, Lönnerdal B, Stenlund H, Gamayanti IL, Ismail D, Seswandhana R, Persson L-A. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: effects on growth and development. Am J Clin Nutr 2004;80:729–36. [DOI] [PubMed] [Google Scholar]
- 342. Stoltzfus RJ, Kvalsvig JD, Chwaya HM, Montresor A, Albonico M, Tielsch JM, Savioli L, Pollitt E. Effects of iron supplementation and anthelmintic treatment on motor and language development of preschool children in Zanzibar: double blind, placebo controlled study. BMJ 2001;323:1389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics 2003;112:846–54. [PubMed] [Google Scholar]
- 344. Wang B, Zhan S, Gong T, Lee L. Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia. Lee L, editor Cochrane Database Syst Rev. Chichester: John Wiley; 2013; CD001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Siimes MA, Addiego JE, Dallman PR. Ferritin in serum: diagnosis of iron deficiency and iron overload in infants and children. Blood 1974;43:581–90. [PubMed] [Google Scholar]
- 346. Beard J, deRegnier R-A, Shaw M, Rao R, Georgieff M. Diagnosis of Iron Deficiency in Infants. Lab Med 2007;38:103–8. [Google Scholar]
- 347. Sweet DG, Savage GA, Tubman R, Lappin TR, Halliday HL. Cord blood transferrin receptors to assess fetal iron status. Arch Dis Child Fetal Neonatal Ed 2001;85:F46–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Juul SE, Zerzan JC, Strandjord TP, Woodrum DE. Zinc protoporphyrin/heme as an indicator of iron status in NICU patients. J Pediatr 2003;142:273–8. [DOI] [PubMed] [Google Scholar]
- 349. Zamora TG, Guiang SF, Widness JA, Georgieff MK. Iron is prioritized to red blood cells over the brain in phlebotomized anemic newborn lambs. Pediatr Res 2016;79:922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Wallin DJ, Tkac I, Stucker S, Ennis KM, Sola-Visner M, Rao R, Georgieff MK. Phlebotomy-induced anemia alters hippocampal neurochemistry in neonatal mice. Pediatr Res 2015;77:765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Matak P, Matak A, Moustafa S, Aryal DK, Benner EJ, Wetsel W, Andrews NC. Disrupted iron homeostasis causes dopaminergic neurodegeneration in mice. Proc Natl Acad Sci U S A 2016;113:3428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Lozoff B, Smith JB, Kaciroti N, Clark KM, Guevara S, Jimenez E. Functional significance of early-life iron deficiency: outcomes at 25 years. J Pediatr 2013;163:1260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics 29:1433–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Rao R, Ennis K, Oz G, Lubach GR, Georgieff MK, Coe CL. Metabolomic analysis of cerebrospinal fluid indicates iron deficiency compromises cerebral energy metabolism in the infant monkey. Neurochem Res 2013;38:573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Patton SM, Coe CL, Lubach GR, Connor JR. Quantitative proteomic analyses of cerebrospinal fluid using iTRAQ in a primate model of iron deficiency anemia. Dev Neurosci 2012;34:354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Deinard AS, List A, Lindgren B, Hunt J V, Chang PN. Cognitive deficits in iron-deficient and iron-deficient anemic children. J Pediatr 1986;108:681–9. [DOI] [PubMed] [Google Scholar]
- 357. Amin SB, Orlando M, Wang H. Latent iron deficiency in utero is associated with abnormal auditory neural myelination in ≥ 35 weeks gestational age infants. J Pediatr 2013;163:1267–71. [DOI] [PubMed] [Google Scholar]
- 358. Geng F, Mai X, Zhan J, Xu L, Zhao Z, Georgieff M, Shao J, Lozoff B. Impact of fetal-neonatal iron deficiency on recognition memory at 2 months of age. J Pediatr 2015;167:1226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Haas JD, Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131:S676–88; discussion S688–90. [DOI] [PubMed] [Google Scholar]
- 360. McClung JP, Murray-Kolb LE. Iron nutrition and premenopausal women: effects of poor iron status on physical and neuropsychological performance. Annu Rev Nutr 2013;33:271–88. [DOI] [PubMed] [Google Scholar]
- 361. Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol 1982;242:E418–27. [DOI] [PubMed] [Google Scholar]
- 362. Davies S, Henthorn JS, Win AA, Brozovic M. Effect of blood transfusion on iron status in sickle cell anaemia. Clin Lab Haematol 1984;6:17–22. [DOI] [PubMed] [Google Scholar]
- 363. Sharieff W, Horton SE, Zlotkin S. Economic gains of a home fortification program: evaluation of 'Sprinkles' from the provider's perspective. Can J Public Health 97:20–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Horton S, Ross J. The economics of iron deficiency. Food Policy 2003;28:51–75. [Google Scholar]
- 365. Horton S. The economics of food fortification. J Nutr 2006;136:1068–71. [DOI] [PubMed] [Google Scholar]
- 366. Pasricha S-R, Low M, Thompson J, Farrell A, De-Regil L-M. Iron supplementation benefits physical performance in women of reproductive age: a systematic review and meta-analysis. J Nutr 2014;144:906–14. [DOI] [PubMed] [Google Scholar]
- 367. Brutsaert TD, Hernandez-Cordero S, Rivera J, Viola T, Hughes G, Haas JD. Iron supplementation improves progressive fatigue resistance during dynamic knee extensor exercise in iron-depleted, nonanemic women. Am J Clin Nutr 2003;77:441–8. [DOI] [PubMed] [Google Scholar]
- 368. Brownlie T, Utermohlen V, Hinton PS, Giordano C, Haas JD. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. Am J Clin Nutr 2002;75:734–42. [DOI] [PubMed] [Google Scholar]
- 369. Brownlie T, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr 2004;79:437–43. [DOI] [PubMed] [Google Scholar]
- 370. DellaValle DM, Haas JD. Impact of iron depletion without anemia on performance in trained endurance athletes at the beginning of a training season: a study of female collegiate rowers. Int J Sport Nutr Exerc Metab 2011;21:501–6. [DOI] [PubMed] [Google Scholar]
- 371. DellaValle DM, Haas JD. Iron supplementation improves energetic efficiency in iron-depleted female rowers. Med Sci Sports Exerc 2014;46:1204–15. [DOI] [PubMed] [Google Scholar]
- 372. Zimmermann MB. The influence of iron status on iodine utilization and thyroid function. Annu Rev Nutr 2006;26:367–89. [DOI] [PubMed] [Google Scholar]
- 373. Beard JL, Borel MJ, Derr J. Impaired thermoregulation and thyroid function in iron-deficiency anemia. Am J Clin Nutr 1990;52:813–9. [DOI] [PubMed] [Google Scholar]
- 374. Beard J, Finch CA, Green WL. Interactions of iron deficiency, anemia, and thyroid hormone levels in response of rats to cold exposure. Life Sci 1982;30:691–7. [DOI] [PubMed] [Google Scholar]
- 375. Beard JL, Brigham DE, Kelley SK, Green MH. Plasma thyroid hormone kinetics are altered in iron-deficient rats. J Nutr 1998;128:1401–8. [DOI] [PubMed] [Google Scholar]
- 376. Brigham DE, Beard JL. Effect of thyroid hormone replacement in iron-deficient rats. Am J Physiol 1995;269:R1140–7. [DOI] [PubMed] [Google Scholar]
- 377. Surks MI. Effect of thyrotropin on thyroidal iodine metabolism during hypoxia. Am J Physiol. 1969;216:436–9. [DOI] [PubMed] [Google Scholar]
- 378. Zimmermann M, Adou P, Torresani T, Zeder C, Hurrell R. Persistence of goiter despite oral iodine supplementation in goitrous children with iron deficiency anemia in Côte d'Ivoire. Am J Clin Nutr. 2000;71:88–93. [DOI] [PubMed] [Google Scholar]
- 379. Zimmermann MB, Zeder C, Chaouki N, Saad A, Torresani T, Hurrell RF. Dual fortification of salt with iodine and microencapsulated iron: a randomized, double-blind, controlled trial in Moroccan schoolchildren. Am J Clin Nutr 2003;77:425–32. [DOI] [PubMed] [Google Scholar]
- 380. Center for Strategic & International Studies Infectious Diseases | The CSIS Global Health Policy Center [Internet]. 2016. Available from: http://www.smartglobalhealth.org/issues/entry/infectious-diseases.
- 381. World Health Organization Global Health Observatory (GHO) data: Monitoring health for the SDGs [Internet]. World Health Organization; 2016[cited 2016 Nov 22]. Available from:http://www.who.int/gho/en/. [Google Scholar]
- 382. Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK. et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 2006;367:133–43. [DOI] [PubMed] [Google Scholar]
- 383. Raiten DJ, Ashour FA. Iron: current landscape and efforts to address a complex issue in a complex world. J Pediatr 2015;167:S3–7. [DOI] [PubMed] [Google Scholar]
- 384. Raiten DJ, Neufeld LM, De-Regil L-M, Pasricha S-R, Darnton-Hill I, Hurrell R, Murray-Kolb LE, Nair KM, Wefwafwa T, Kupka R. et al. Integration to implementation and the micronutrient forum: a coordinated approach for global nutrition. Case study application: safety and effectiveness of iron interventions. Adv Nutr. 2016;7:135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Kerkhoff AD, Meintjes G, Opie J, Vogt M, Jhilmeet N, Wood R, Lawn SD. Anaemia in patients with HIV-associated TB: relative contributions of anaemia of chronic disease and iron deficiency. Int J Tuberc Lung Dis 2016;20:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Diouf A, Badiane A, Manga NM, Idohou-Dossou N, Sow PS, Wade S. Daily consumption of ready-to-use peanut-based therapeutic food increased fat free mass, improved anemic status but has no impact on the zinc status of people living with HIV/AIDS: a randomized controlled trial. BMC Public Health 2016;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Widen EM, Bentley ME, Chasela CS, Kayira D, Flax VL, Kourtis AP, Ellington SR, Kacheche Z, Tegha G, Jamieson DJ. et al. Antiretroviral treatment is associated with iron deficiency in HIV-infected malawian women that is mitigated with supplementation, but is not associated with infant iron deficiency during 24 weeks of exclusive breastfeeding. J Acquir Immune Defic Syndr 2015;69:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Petraro P, Duggan C, Spiegelman D, Hertzmark E, Makubi A, Chalamilla G, Siril H, Sando D, Aboud S, Fawzi WW. Determinants of anemia among human immunodeficiency virus-positive adults at care and treatment clinics in dar es salaam, Tanzania. Am J Trop Med Hyg 2016;94:384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS, American Association for the Study of Liver Diseases Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011;54:328–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Pietrangelo A. Hereditary hemochromatosis–a new look at an old disease. N Engl J Med 2004;350:2383–97. [DOI] [PubMed] [Google Scholar]
- 391. Whittaker P, Ali SF, Imam SZ, Dunkel VC. Acute toxicity of carbonyl iron and sodium iron EDTA compared with ferrous sulfate in young rats. Regul Toxicol Pharmacol 2002;36:280–6. [DOI] [PubMed] [Google Scholar]
- 392. Barton JC, Lee PL, West C, Bottomley SS. Iron overload and prolonged ingestion of iron supplements: clinical features and mutation analysis of hemochromatosis-associated genes in four cases. Am J Hematol 2006;81:760–7. [DOI] [PubMed] [Google Scholar]
- 393. Bell H, Berg JP, Undlien DE, Distante S, Raknerud N, Heier HE, Try K, Thomassen Y, Haug E, Raha-Chowdhury R. et al. The clinical expression of hemochromatosis in Oslo, Norway. Excessive oral iron intake may lead to secondary hemochromatosis even in HFE C282Y mutation negative subjects. Scand J Gastroenterol 2000;35:1301–7. [DOI] [PubMed] [Google Scholar]
- 394. European Association For The Study Of The Liver EASL clinical practice guidelines for HFE hemochromatosis. J Hepatol 2010;53:3–22. [DOI] [PubMed] [Google Scholar]
- 395. Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, McLaren CE, Bahlo M, Nisselle AE, Vulpe CD. et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med 2008;358:221–30. [DOI] [PubMed] [Google Scholar]
- 396. Strachan GI. The pathology of chronic cervicitis. Br Med J 1929;2:659–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Gordeuk V, Mukiibi J, Hasstedt SJ, Samowitz W, Edwards CQ, West G, Ndambire S, Emmanual J, Nkanza N, Chapanduka Z. Iron overload in Africa. Interaction between a gene and dietary iron content. N Engl J Med. 1992;326:95–100. [DOI] [PubMed] [Google Scholar]
- 398. Brink B, Disler P, Lynch S, Jacobs P, Charlton R, Bothwell T. Patterns of iron storage in dietary iron overload and idiopathic hemochromatosis. J Lab Clin Med. 1976;88:725–31. [PubMed] [Google Scholar]
- 399. Barton JC, Acton RT, Lee PL, West C. SLC40A1 Q248H allele frequencies and Q248H-associated risk of non-HFE iron overload in persons of sub-Saharan African descent. Blood Cells Mol Dis 39:206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Eaton JW, Qian M. Molecular bases of cellular iron toxicity. Free Radic Biol Med 2002;32:833–40. [DOI] [PubMed] [Google Scholar]
- 401. Pippard MJ. Secondary iron overload. In: Brock JH, Halliday JW, Pippard MJ, Powell LW, Iron metabolism in health and disease. London: W.B. Saunders; 1994. pp. 271–309. [Google Scholar]
- 402. Sullivan JL. Iron and the sex difference in heart disease risk. Lancet 1981;1:1293–4. [DOI] [PubMed] [Google Scholar]
- 403. Salonen JT, Nyyssönen K, Korpela H, Tuomilehto J, Seppänen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation 1992;86:803–11. [DOI] [PubMed] [Google Scholar]
- 404. Parrinello CM, Lutsey PL, Ballantyne CM, Folsom AR, Pankow JS, Selvin E. Six-year change in high-sensitivity C-reactive protein and risk of diabetes, cardiovascular disease, and mortality. Am Heart J 2015;170:380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood 1997;89:739–61. [PubMed] [Google Scholar]
- 406. Ledesma M, Hurtado-Roca Y, Leon M, Giraldo P, Pocovi M, Civeira F, Guallar E, Ordovas JM, Casasnovas JA, Laclaustra M. Association of ferritin elevation and metabolic syndrome in males. Results from the Aragon Workers’ Health Study (AWHS). J Clin Endocrinol Metab 2015;100:2081–9. [DOI] [PubMed] [Google Scholar]
- 407. Chen LY, Chang SD, Sreenivasan GM, Tsang PW, Broady RC, Li CH, Zypchen LN. Dysmetabolic hyperferritinemia is associated with normal transferrin saturation, mild hepatic iron overload, and elevated hepcidin. Ann Hematol 2011;90:139–43. [DOI] [PubMed] [Google Scholar]
- 408. Riva A, Trombini P, Mariani R, Salvioni A, Coletti S, Bonfadini S, Paolini V, Pozzi M, Facchetti R, Bovo G. et al. Revaluation of clinical and histological criteria for diagnosis of dysmetabolic iron overload syndrome. World J Gastroenterol 2008;14:4745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Zimmermann MB, Zeder C, Muthayya S, Winichagoon P, Chaouki N, Aeberli I, Hurrell RF. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int J Obes (Lond) 2008;32:1098–104. [DOI] [PubMed] [Google Scholar]
- 410. Cepeda-Lopez AC, Osendarp SJ, Melse-Boonstra A, Aeberli I, Gonzalez-Salazar F, Feskens E, Villalpando S, Zimmermann MB. Sharply higher rates of iron deficiency in obese Mexican women and children are predicted by obesity-related inflammation rather than by differences in dietary iron intake. Am J Clin Nutr 2011;93:975–83. [DOI] [PubMed] [Google Scholar]
- 411. Cepeda-Lopez AC, Aeberli I, Zimmermann MB. Does obesity increase risk for iron deficiency? A review of the literature and the potential mechanisms. Int J Vitam Nutr Res 2010;80:263–70. [DOI] [PubMed] [Google Scholar]
- 412. Ruivard M, Lainé F, Ganz T, Olbina G, Westerman M, Nemeth E, Rambeau M, Mazur A, Gerbaud L, Tournilhac V. et al. Iron absorption in dysmetabolic iron overload syndrome is decreased and correlates with increased plasma hepcidin. J Hepatol 2009;50:1219–25. [DOI] [PubMed] [Google Scholar]
- 413. Nikonorov AA, Skalnaya MG, Tinkov AA, Skalny AV. Mutual interaction between iron homeostasis and obesity pathogenesis. J Trace Elem Med Biol 2015;30:207–14. [DOI] [PubMed] [Google Scholar]
- 414. Aigner E, Feldman A, Datz C. Obesity as an emerging risk factor for iron deficiency. Nutrients 2014;6:3587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Peffer K, Verbeek ALM, Swinkels DW, Geurts-Moespot AJ, den Heijer M, Atsma F. Donation intensity and metabolic syndrome in active whole-blood donors. Vox Sang 2015;109:25–34. [DOI] [PubMed] [Google Scholar]
- 416. Yeap BB, Divitini ML, Gunton JE, Olynyk JK, Beilby JP, McQuillan B, Hung J, Knuiman MW. Higher ferritin levels, but not serum iron or transferrin saturation, are associated with Type 2 diabetes mellitus in adult men and women free of genetic haemochromatosis. Clin Endocrinol 2015;82:525–32. [DOI] [PubMed] [Google Scholar]
- 417. Ruddell R, Ramm G. Hepatic pathology of iron overload. In: Anderson GJ, McLaren GD, Iron physiology and pathophysiology in humans. Totowa, NJ: Humana Press; 2012. pp. 357–83. [Google Scholar]
- 418. Fonseca-Nunes A, Jakszyn P, Agudo A. Iron and cancer risk–a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol Biomarkers Prev 2014;23:12–31. [DOI] [PubMed] [Google Scholar]
- 419. Drakesmith H, Porto G, de Sousa M. Iron and the immune system. In: Anderson GJ, McLaren GD, Iron physiology and pathophysiology in humans. Totowa, NJ: Humana Press; 2012. [Google Scholar]
- 420. Weinberg ED. Nutritional immunity. Host's attempt to withold iron from microbial invaders. JAMA 1975;231:39–41. [DOI] [PubMed] [Google Scholar]
- 421. Raiten DJ, Sakr Ashour FA, Ross AC, Meydani SN, Dawson HD, Stephensen CB, Brabin BJ, Suchdev PS, van Ommen B, INSPIRE Consultative Group Inflammation and nutritional science for programs/policies and interpretation of research evidence (INSPIRE). J Nutr 2015;145:S1039–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Teawtrakul N, Jetsrisuparb A, Sirijerachai C, Chansung K, Wanitpongpun C. Severe bacterial infections in patients with non-transfusion-dependent thalassemia: prevalence and clinical risk factors. Int J Infect Dis 2015;39:53–6. [DOI] [PubMed] [Google Scholar]
- 423. Gerhard GS, Levin KA, Price Goldstein J, Wojnar MM, Chorney MJ, Belchis DA. Vibrio vulnificus septicemia in a patient with the hemochromatosis HFE C282Y mutation. Arch Pathol Lab Med 2001;125:1107–9. [DOI] [PubMed] [Google Scholar]
- 424. Vadillo M, Corbella X, Pac V, Fernandez-Viladrich P, Pujol R. Multiple liver abscesses due to Yersinia enterocolitica discloses primary hemochromatosis: three cases reports and review. Clin Infect Dis 1994;18:938–41. [DOI] [PubMed] [Google Scholar]
- 425. Galan SR, Kann PH, Gress TM, Michl P. Listeria monocytogenes-induced bacterial peritonitis caused by contaminated cheese in a patient with haemochromatosis. Z Gastroenterol 2011;49:832–5. [DOI] [PubMed] [Google Scholar]
- 426. Corke PJ, McLean AS, Stewart D, Adams S. Overwhelming gram negative septic shock in haemochromatosis. Anaesth Intensive Care 1995;23:346–9. [DOI] [PubMed] [Google Scholar]
- 427. Adams P, Brissot P, Powell LW. EASL international consensus conference on haemochromatosis. J Hepatol 2000;33:485–504. [DOI] [PubMed] [Google Scholar]
- 428. Qaseem A, Aronson M, Fitterman N, Snow V, Weiss KB, Owens DK, Clinical Efficacy Assessment Subcommittee of the American College of Physicians Screening for hereditary hemochromatosis: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2005;143:517–21. [DOI] [PubMed] [Google Scholar]
- 429. U.S. Centers for Disease Control and Prevention National report on biochemical indicators of diet and nutrition in the U.S. population 1999–2002. Atlanta, GA; 2008. [Google Scholar]
- 430. McKinnon EJ, Rossi E, Beilby JP, Trinder D, Olynyk JK. Factors that affect serum levels of ferritin in australian adults and implications for follow-up. Clin Gastroenterol Hepatol 2014;12:101–108.e4. [DOI] [PubMed] [Google Scholar]
- 431. Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, Dawkins FW, Acton RT, Harris EL, Gordeuk VR. et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352:1769–78. [DOI] [PubMed] [Google Scholar]
- 432. Gordeuk VR, Lovato L, Barton J, Vitolins M, McLaren G, Acton R, McLaren C, Harris E, Speechley M, Eckfeldt JH. et al. Dietary iron intake and serum ferritin concentration in 213 patients homozygous for the HFEC282Y hemochromatosis mutation. Can J Gastroenterol 2012;26:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Nemeth E. Hepcidin in beta-thalassemia. Ann N Y Acad Sci 2010;1202:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Pasricha S-R, Frazer DM, Bowden DK, Anderson GJ. Transfusion suppresses erythropoiesis and increases hepcidin in adult patients with β-thalassemia major: a longitudinal study. Blood. 2013;122:124–33. [DOI] [PubMed] [Google Scholar]
- 435. Jones E, Pasricha S-R, Allen A, Evans P, Fisher CA, Wray K, Premawardhena A, Bandara D, Perera A, Webster C. et al. Hepcidin is suppressed by erythropoiesis in hemoglobin E β-thalassemia and β-thalassemia trait. Blood 2015;125:873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. Zimmermann MB, Fucharoen S, Winichagoon P, Sirankapracha P, Zeder C, Gowachirapant S, Judprasong K, Tanno T, Miller JL, Hurrell RF. Iron metabolism in heterozygotes for hemoglobin E (HbE), alpha-thalassemia 1, or beta-thalassemia and in compound heterozygotes for HbE/beta-thalassemia. Am J Clin Nutr 2008;88:1026–31. [DOI] [PubMed] [Google Scholar]
- 437. Roy CN, Andrews NC. Anemia of inflammation: the hepcidin link. Curr Opin Hematol 2005;12:107–11. [DOI] [PubMed] [Google Scholar]
- 438. Sugimoto R, Fujita N, Tomosugi N, Hara N, Miyachi H, Tanaka H, Takeo M, Nakagawa N, Iwasa M, Kobayashi Y. et al. Impaired regulation of serum hepcidin during phlebotomy in patients with chronic hepatitis C. Hepatol Res 2009;39:619–24. [DOI] [PubMed] [Google Scholar]
- 439. Liu H, Le TT, Dong H, Keith R, Nelson D, Liu C. Iron regulator hepcidin exhibits antiviral activity against hepatitis C virus. PLoS One 2012;7:e46631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Hörl WH, Schmidt A. Low hepcidin triggers hepatic iron accumulation in patients with hepatitis C. Nephrol Dial Transplant 2014;29:1141–4. [DOI] [PubMed] [Google Scholar]
- 441. Yano M, Hayashi H, Wakusawa S, Sanae F, Takikawa T, Shiono Y, Arao M, Ukai K, Ito H, Watanabe K. et al. Long term effects of phlebotomy on biochemical and histological parameters of chronic hepatitis C. Am J Gastroenterol 2002;97:133–7. [DOI] [PubMed] [Google Scholar]
- 442. Tsai NC, Zuckerman E, Han SH, Goad K, Redeker AG, Fong TL. Effect of iron depletion on long-term response to interferon-alpha in patients with chronic hepatitis C who previously did not respond to interferon therapy. Am J Gastroenterol 1997;92:1831–4. [PubMed] [Google Scholar]
- 443. Hunt JR, Zito CA, Johnson LK. Body iron excretion by healthy men and women. Am J Clin Nutr 2009;89:1792–8. [DOI] [PubMed] [Google Scholar]
- 444. Fomon SJ, Nelson SE, Serfass RE, Ziegler EE. Absorption and loss of iron in toddlers are highly correlated. J Nutr 2005;135:771–7. [DOI] [PubMed] [Google Scholar]
- 445. Harvey LJ, Berti C, Casgrain A, Cetin I, Collings R, Gurinovic M, Hermoso M, Hooper L, Hurst R, Koletzko B. et al. EURRECA—estimating iron requirements for deriving dietary reference values. Crit Rev Food Sci Nutr 2013;53:1064–76. [DOI] [PubMed] [Google Scholar]
- 446. Liu T, Wilson NP, Craig CB, Tamura T, Soong S, Sauberlich HE, Cole P, Butterworth CE. Evaluation of three nutritional assessment methods in a group of women. Epidemiology 1992;3:496–502. [DOI] [PubMed] [Google Scholar]
- 447. Decarli A, Franceschi S, Ferraroni M, Gnagnarella P, Parpinel M, La Vecchia C, Negri E, Salvini S, Falcini F, Giacosa A. Validation of a food-frequency questionnaire to assess dietary intakes in cancer studies in Italy. Results for specific nutrients. Ann Epidemiol 1996;6:110–8. [DOI] [PubMed] [Google Scholar]
- 448. Fayet F, Flood V, Petocz P, Samman S. Relative and biomarker-based validity of a food frequency questionnaire that measures the intakes of vitamin B(12), folate, iron, and zinc in young women. Nutr Res 2011;31:14–20. [DOI] [PubMed] [Google Scholar]
- 449. Bonifacj C, Gerber M, Scali J, Daures JP. Comparison of dietary assessment methods in a southern French population: use of weighed records, estimated-diet records and a food-frequency questionnaire. Eur J Clin Nutr 1997;51:217–31. [DOI] [PubMed] [Google Scholar]
- 450. Heath AL, Skeaff CM, Gibson RS. The relative validity of a computerized food frequency questionnaire for estimating intake of dietary iron and its absorption modifiers. Eur J Clin Nutr 2000;54:592–9. [DOI] [PubMed] [Google Scholar]
- 451. Heath A-LM, Roe MA, Oyston SL, Fairweather-Tait SJ. Meal-based intake assessment tool: relative validity when determining dietary intake of Fe and Zn and selected absorption modifiers in UK men. Br J Nutr 2005;93:403–16. [DOI] [PubMed] [Google Scholar]
- 452. Pfrimer K, Sartorelli D, Rosa F, Mendes Resende C, Viera D, Rabito E, Scagliusi F, Moriguti E, Monteiro J, Ferriolli E. Calibration of the food list and portion sizes of a food frequency questionnaire applied to free-living elderly people. Nutrition 2013;29:760–4. [DOI] [PubMed] [Google Scholar]
- 453. Food Standards Agency Calculation and collation of typical food portion sizes for adults aged 19–64 and older people aged 65 and over.
- 454. United States Department of Agriculture Food and Nutrition Service Products and Services [Internet]. Available from:http://www.ars.usda.gov/Services/docs.htm?docid=120892013).
- 455. Merten C, Ferrari P, Bakker M, Boss A, Hearty A, Leclercq C, Lindtner O, Tlustos C, Verger P, Volatier JL. et al. Methodological characteristics of the national dietary surveys carried out in the European Union as included in the European Food Safety Authority (EFSA) Comprehensive European Food Consumption Database. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2011;28:975–95. [DOI] [PubMed] [Google Scholar]
- 456. Foodrisk Nutrition and Food Intake Databases [Internet]. [cited 2013 Sep 9]. Available from: http://foodrisk.org/databases/nutrition/
- 457. Eurofir European Food Information Resource AISBL [Internet]. Available from:http://www.eurofir.org/
- 458. United States Department of Agriculture Food and nutrient database for dietary studies. Agricultural Research Service; 2008. [Google Scholar]
- 459. Food Standards NUTTAB 2010 [Internet]. [cited 2013 Sep 9]. Available from: http://www.foodstandards.gov.au/science/monitoringnutrients/nutrientables/pages/default.aspx
- 460. Roe MA, Heath A-LM, Oyston SL, Macrow C, Hoogewerff JA, Foxall R, Dainty JR, Majsak-Newman G, Willis G, Fairweather-Tait SJ. Iron absorption in male C282Y heterozygotes. Am J Clin Nutr 2005;81:814–21. [DOI] [PubMed] [Google Scholar]
- 461. Fairweather-Tait S, Lynch S, Hotz C, Hurrell R, Abrahamse L, Beebe S, Bering S, Bukhave K, Glahn R, Hambidge M. et al. The usefulness of in vitro models to predict the bioavailability of iron and zinc: a consensus statement from the HarvestPlus expert consultation. Int J Vitam Nutr Res 2005;75:371–4. [DOI] [PubMed] [Google Scholar]
- 462. Miller DD, Schricker BR, Rasmussen RR, Van Campen D. An in vitro method for estimation of iron availability from meals. Am J Clin Nutr 1981;34:2248–56. [DOI] [PubMed] [Google Scholar]
- 463. Glahn RP, Lee OA, Yeung A, Goldman MI, Miller DD. Caco-2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion/Caco-2 cell culture model. J Nutr 1998;128:1555–61. [DOI] [PubMed] [Google Scholar]
- 464. Pla GW, Fritz JC. Collaborative study of the hemoglobin repletion test in chicks and rats for measuring availability of iron. J Assoc Off Anal Chem 1971;54:13–7. [PubMed] [Google Scholar]
- 465. Fritz JC, Pla GW, Harrison BN, Clark GA, Smith EA. Measurement of the bioavailability of iron, using the rat hemoglobin repletion test. J Assoc Off Anal Chem 1978;61:709–14. [PubMed] [Google Scholar]
- 466. Williams S. Official methods of analysis of the AOAC. 14th ed AOAC International; 1984. [Google Scholar]
- 467. Forbes AL, Arnaud MJ, Chichester CO, Cook JD, Harrison BN, Hurrell RF, Kahn SG, Morris ER, Tanner JT, Whittaker P. Comparison of in vitro, animal, and clinical determinations of iron bioavailability: International Nutritional Anemia Consultative Group Task Force report on iron bioavailability. Am J Clin Nutr 1989;49:225–38. [DOI] [PubMed] [Google Scholar]
- 468. Reddy MB, Cook JD. Assessment of dietary determinants of nonheme-iron absorption in humans and rats. Am J Clin Nutr 1991;54:723–8. [DOI] [PubMed] [Google Scholar]
- 469. Monsen ER, Hallberg L, Layrisse M, Hegsted DM, Cook JD, Mertz W, Finch CA. Estimation of available dietary iron. Am J Clin Nutr 1978;31:134–41. [DOI] [PubMed] [Google Scholar]
- 470. Hallberg L, Hulthén L. Prediction of dietary iron absorption: an algorithm for calculating absorption and bioavailability of dietary iron. Am J Clin Nutr 2000;71:1147–60. [DOI] [PubMed] [Google Scholar]
- 471. Institute of Medicine (US) Panel on Micronutrients Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academies Press; 2001. [PubMed] [Google Scholar]
- 472. Magnusson B, Björn-Rassmussen E, Hallberg L, Rossander L. Iron absorption in relation to iron status. Model proposed to express results to food iron absorption measurements. Scand J Haematol 1981;27:201–8. [PubMed] [Google Scholar]
- 473. Armah SM, Carriquiry A, Sullivan D, Cook JD, Reddy MB. A complete diet-based algorithm for predicting nonheme iron absorption in adults. J Nutr 2013;143:1136–40. [DOI] [PubMed] [Google Scholar]
- 474. Beard JL, Murray-Kolb LE, Haas JD, Lawrence F. Iron absorption prediction equations lack agreement and underestimate iron absorption. J Nutr 2007;137:1741–6. [DOI] [PubMed] [Google Scholar]
- 475. Hoppe M, Hulthén L, Hallberg L. Serum iron concentration as a tool to measure relative iron absorption from elemental iron powders in man. Scand J Clin Lab Invest 2003;63:489–96. [DOI] [PubMed] [Google Scholar]
- 476. Sarria B, Dainty JR, Fox TE, Fairweather-Tait SJ. Estimation of iron absorption in humans using compartmental modelling. Eur J Clin Nutr 2005;59:142–4. [DOI] [PubMed] [Google Scholar]
- 477. Davidsson L, Tanumihardjo S. New frontiers in science and technology: nuclear techniques in nutrition. Am J Clin Nutr 2011;94:691S–5S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478. Cook JD, Dassenko SA, Lynch SR. Assessment of the role of nonheme-iron availability in iron balance. Am J Clin Nutr 1991;54:717–22. [DOI] [PubMed] [Google Scholar]
- 479. Hallberg L, Hulthén L, Garby L. Iron stores in man in relation to diet and iron requirements. Eur J Clin Nutr 1998;52:623–31. [DOI] [PubMed] [Google Scholar]
- 480. Van Thuy P, Berger J, Davidsson L, Khan NC, Lam NT, Cook JD, Hurrell RF, Khoi HH. Regular consumption of NaFeEDTA-fortified fish sauce improves iron status and reduces the prevalence of anemia in anemic Vietnamese women. Am J Clin Nutr 2003;78:284–90. [DOI] [PubMed] [Google Scholar]
- 481. Dainty JR, Berry R, Lynch SR, Harvey LJ, Fairweather-Tait SJ. Estimation of dietary iron bioavailability from food iron intake and iron status. PLoS One. 2014;9:e111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Fairweather-Tait SJ, Jennings A, Harvey LJ, Berry R, Walton J, Dainty JR. Modeling tool for calculating dietary iron bioavailability in iron-sufficient adults. Am J Clin Nutr 2017;ajcn147389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483. Frazer DM, Anderson GJ. Is there a better way to set population iron recommendations? Am J Clin Nutr 2017;ajcn158188. [DOI] [PubMed] [Google Scholar]
- 484. Cook J. Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol 2005;18:319–32. [DOI] [PubMed] [Google Scholar]
- 485. Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1996;1275:161–203. [DOI] [PubMed] [Google Scholar]
- 486. Arosio P, Adelman TG, Drysdale JW. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem 1978;253:4451–8. [PubMed] [Google Scholar]
- 487. Drysdale JW, Munro HN. Regulation of synthesis and turnover of ferritin in rat liver. J Biol Chem 1966;241:3630–7. [PubMed] [Google Scholar]
- 488. Zimmermann MB. Methods to assess iron and iodine status. Br J Nutr 2008;99(Suppl 3):S2–9. [DOI] [PubMed] [Google Scholar]
- 489. Mei Z, Cogswell ME, Parvanta I, Lynch S, Beard JL, Stoltzfus RJ, Grummer-Strawn LM. Hemoglobin and ferritin are currently the most efficient indicators of population response to iron interventions: an analysis of nine randomized controlled trials. J Nutr 2005;135:1974–80. [DOI] [PubMed] [Google Scholar]
- 490. Worwood M. Indicators of the iron status of populations: ferritin. Assessing the iron status of populations: report of a joint World Health Organization/Centers for Disease Control and Prevention technical consultation on the assessment of iron status at the population level. Geneva; 2007. [Google Scholar]
- 491. ten Kate J, Wolthuis A, Westerhuis B, van Deursen C. The iron content of serum ferritin: physiological importance and diagnostic value. Eur J Clin Chem Clin Biochem 1997;35:53–6. [DOI] [PubMed] [Google Scholar]
- 492. Worwood M. The measurement of ferritin. Advanced laboratory methods in haematology. London: Arnold; 2002. pp. 241–67. [Google Scholar]
- 493. Addison GM, Beamish MR, Hales CN, Hodgkins M, Jacobs A, Llewellin P. An immunoradiometric assay for ferritin in the serum of normal subjects and patients with iron deficiency and iron overload. J Clin Pathol 1972;25:326–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494. Miles L, Lipschitz D, Bieber C, Cook J. Measurement of serum ferritin by a 2-site immunoradiometric assay. Anal Biochem 1974;61:209–24. [DOI] [PubMed] [Google Scholar]
- 495. Worwood M. Ferritin in human tissues and serum. Clin Haematol 1982;11:275–307. [PubMed] [Google Scholar]
- 496. Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol 1973;26:770–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497. Hallberg L, Bengtsson C, Lapidus L, Lindstedt G, Lundberg PA, Hultén L. Screening for iron deficiency: an analysis based on bone-marrow examinations and serum ferritin determinations in a population sample of women. Br J Haematol 1993;85:787–98. [DOI] [PubMed] [Google Scholar]
- 498. Bainton DF, Finch CA. The diagnosis of iron deficiency anemia. Am J Med 1964;37:62–70. [DOI] [PubMed] [Google Scholar]
- 499. Cook JD, Finch CA, Smith NJ. Evaluation of the iron status of a population. Blood 1976;48:449–55. [PubMed] [Google Scholar]
- 500. Labbe RF, Rettmer RL. Zinc protoporphyrin: a product of iron-deficient erythropoiesis. Semin Hematol 1989;26:40–6. [PubMed] [Google Scholar]
- 501. Labbé RF, Vreman HJ, Stevenson DK. Zinc protoporphyrin: a metabolite with a mission. Clin Chem 1999;45:2060–72. [PubMed] [Google Scholar]
- 502. Hastka J, Lasserre JJ, Schwarzbeck A, Reiter A, Hehlmann R. Laboratory tests of iron status: correlation or common sense? Clin Chem 1996;42:718–24. [PubMed] [Google Scholar]
- 503. Hirsch RE, Pulakhandam UR, Billett HH, Nagel RL. Blood zinc protoporphyrin is elevated only in sickle cell patients with low fetal hemoglobin. Am J Hematol 1991;36:147–9. [DOI] [PubMed] [Google Scholar]
- 504. Tillyer ML, Tillyer CR. Zinc protoporphyrin assays in patients with alpha and beta thalassaemia trait. J Clin Pathol 1994;47:205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505. Harthoorn-Lasthuizen EJ, Lindemans J, Langenhuijsen MM. Combined use of erythrocyte zinc protoporphyrin and mean corpuscular volume in differentiation of thalassemia from iron deficiency anemia. Eur J Haematol 1998;60:245–51. [DOI] [PubMed] [Google Scholar]
- 506. Marks GS. Exposure to toxic agents: the heme biosynthetic pathway and hemoproteins as indicator. Crit Rev Toxicol 1985;15:151–79. [DOI] [PubMed] [Google Scholar]
- 507. Winzerling JJ, Kling PJ. Iron-deficient erythropoiesis in premature infants measured by blood zinc protoporphyrin/heme. J Pediatr 2001;139:134–6. [DOI] [PubMed] [Google Scholar]
- 508. Thomas DW, Hinchliffe RF, Briggs C, Macdougall IC, Littlewood T, Cavill I, British Committee for Standards in Haematology Guideline for the laboratory diagnosis of functional iron deficiency. Br J Haematol 2013;161:639–48. [DOI] [PubMed] [Google Scholar]
- 509. Buttarello M. Laboratory diagnosis of anemia: are the old and new red cell parameters useful in classification and treatment, how? Int J Lab Hematol 2016;38(Suppl 1):123–32. [DOI] [PubMed] [Google Scholar]
- 510. American Academy of Family Physicians American Family Physician [Internet]. 2017[cited 2017 Jun 27]. Available from: http://www.aafp.org/journals/afp.html?cmpid=_van_188.
- 511. American Academy of Pediatrics AAP.org [Internet]. 2017[cited 2017 Jun 27]. Available from: https://www.aap.org/en-us/Pages/Default.aspx.
- 512. Ratcliffe LEK, Thomas W, Glen J, Padhi S, Pordes BAJ, Wonderling D, Connell R, Stephens S, Mikhail AI, Fogarty DG. et al. Diagnosis and management of iron deficiency in CKD: a summary of the NICE guideline recommendations and their rationale. Am J Kidney Dis 2016;67:548–58. [DOI] [PubMed] [Google Scholar]
- 513. Kohgo Y, Nishisato T, Kondo H, Tsushima N, Niitsu Y, Urushizaki I. Circulating transferrin receptor in human serum. Br J Haematol 1986;64:277–81. [DOI] [PubMed] [Google Scholar]
- 514. Shih YJ, Baynes RD, Hudson BG, Flowers CH, Skikne BS, Cook JD. Serum transferrin receptor is a truncated form of tissue receptor. J Biol Chem 1990;265:19077–81. [PubMed] [Google Scholar]
- 515. Kohgo Y, Niitsu Y, Kondo H, Kato J, Tsushima N, Sasaki K, Hirayama M, Numata T, Nishisato T, Urushizaki I. Serum transferrin receptor as a new index of erythropoiesis. Blood 1987;70:1955–8. [PubMed] [Google Scholar]
- 516. Flowers CH, Skikne BS, Covell AM, Cook JD. The clinical measurement of serum transferrin receptor. J Lab Clin Med 1989;114:368–77. [PubMed] [Google Scholar]
- 517. Skikne BS, Flowers CH, Cook JD. Serum transferrin receptor: a quantitative measure of tissue iron deficiency. Blood 1990;75:1870–6. [PubMed] [Google Scholar]
- 518. Olivares M, Walter T, Cook JD, Hertrampf E, Pizarro F. Usefulness of serum transferrin receptor and serum ferritin in diagnosis of iron deficiency in infancy. Am J Clin Nutr 2000;72:1191–5. [DOI] [PubMed] [Google Scholar]
- 519. Chang J, Bird R, Clague A, Carter A. Clinical utility of serum soluble transferrin receptor levels and comparison with bone marrow iron stores as an index for iron-deficient erythropoiesis in a heterogeneous group of patients. Pathology 2007;39:349–53. [DOI] [PubMed] [Google Scholar]
- 520. Koulaouzidis A, Said E, Cottier R, Saeed AA. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis 2009;18:345–52. [PubMed] [Google Scholar]
- 521. Phiri KS, Calis JCJ, Kachala D, Borgstein E, Waluza J, Bates I, Brabin B, van Hensbroek MB. Improved method for assessing iron stores in the bone marrow. J Clin Pathol 2009;62:685–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522. Huebers HA, Beguin Y, Pootrakul P, Einspahr D, Finch CA. Intact transferrin receptors in human plasma and their relation to erythropoiesis. Blood 1990;75:102–7. [PubMed] [Google Scholar]
- 523. Beguin Y, Clemons GK, Pootrakul P, Fillet G. Quantitative assessment of erythropoiesis and functional classification of anemia based on measurements of serum transferrin receptor and erythropoietin. Blood 1993;81:1067–76. [PubMed] [Google Scholar]
- 524. Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta 2003;329:9–22. [DOI] [PubMed] [Google Scholar]
- 525. Belza A, Ersbøll AK, Henriksen M, Thilsted SH, Tetens I. Day-to-day variation in iron-status measures in young iron-deplete women. Br J Nutr 2005;94:551–6. [DOI] [PubMed] [Google Scholar]
- 526. Ziegler EE, Nelson SE, Jeter JM. Iron supplementation of breastfed infants from an early age. Am J Clin Nutr 2009;89:525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527. Kling PJ, Roberts RA, Widness JA. Plasma transferrin receptor levels and indices of erythropoiesis and iron status in healthy term infants. J Pediatr Hematol Oncol 20:309–14. [DOI] [PubMed] [Google Scholar]
- 528. Ziegler EE, Nelson SE, Jeter JM. Iron stores of breastfed infants during the first year of life. Nutrients 2014;6:2023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529. Pynaert I, De Bacquer D, Matthys C, Delanghe J, Temmerman M, De Backer G, De Henauw S. Determinants of ferritin and soluble transferrin receptors as iron status parameters in young adult women. Public Health Nutr 2009;12:1775–82. [DOI] [PubMed] [Google Scholar]
- 530. Speeckaert MM, Speeckaert R, Delanghe JR. Biological and clinical aspects of soluble transferrin receptor. Crit Rev Clin Lab Sci 2010;47:213–28. [DOI] [PubMed] [Google Scholar]
- 531. Casabellata G, Di Santolo M, Banfi G, Stel G, Gonano F, Cauci S. Evaluation of iron deficiency in young women in relation to oral contraceptive use. Contraception 2007;76:200–7. [DOI] [PubMed] [Google Scholar]
- 532. Woolf K, St Thomas MM, Hahn N, Vaughan LA, Carlson AG, Hinton P. Iron status in highly active and sedentary young women. Int J Sport Nutr Exerc Metab 2009;19:519–35. [DOI] [PubMed] [Google Scholar]
- 533. Yanoff LB, Menzie CM, Denkinger B, Sebring NG, McHugh T, Remaley AT, Yanovski JA. Inflammation and iron deficiency in the hypoferremia of obesity. Int J Obes (Lond) 2007;31:1412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534. Tussing-Humphreys LM, Nemeth E, Fantuzzi G, Freels S, Guzman G, Holterman A-XL, Braunschweig C. Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity (Silver Spring) 2010;18:1449–56. [DOI] [PubMed] [Google Scholar]
- 535. Cheng HL, Bryant C, Cook R, O'Connor H, Rooney K, Steinbeck K. The relationship between obesity and hypoferraemia in adults: a systematic review. Obes Rev 2012;13:150–61. [DOI] [PubMed] [Google Scholar]
- 536. Fernández-Real JM, Moreno JM, López-Bermejo A, Chico B, Vendrell J, Ricart W. Circulating soluble transferrin receptor according to glucose tolerance status and insulin sensitivity. Diabetes Care 2007;30:604–8. [DOI] [PubMed] [Google Scholar]
- 537. Khatami S, Dehnabeh SR, Mostafavi E, Kamalzadeh N, Yaghmaei P, Saeedi P, Shariat F, Bagheriyan H, Zeinali S, Akbari MT. Evaluation and comparison of soluble transferrin receptor in thalassemia carriers and iron deficient patients. Hemoglobin 2013;37:387–95. [DOI] [PubMed] [Google Scholar]
- 538. Ong KH, Tan HL, Tam LP, Hawkins RCW, Kuperan P. Accuracy of serum transferrin receptor levels in the diagnosis of iron deficiency among hospital patients in a population with a high prevalence of thalassaemia trait. Int J Lab Hematol 2008;30:487–93. [DOI] [PubMed] [Google Scholar]
- 539. Knox-Macaulay H, Gravell D, Elender F. Serum transferrin receptor status of healthy adult Arabs. Ann Clin Lab Sci 2007;37:57–62. [PubMed] [Google Scholar]
- 540. Skikne BS, Punnonen K, Caldron PH, Bennett MT, Rehu M, Gasior GH, Chamberlin JS, Sullivan LA, Bray KR, Southwick PC. Improved differential diagnosis of anemia of chronic disease and iron deficiency anemia: a prospective multicenter evaluation of soluble transferrin receptor and the sTfR/log ferritin index. Am J Hematol. 2011;86:923–7. [DOI] [PubMed] [Google Scholar]
- 541. Calis JCJ, Phiri KS, Faragher EB, Brabin BJ, Bates I, Cuevas LE, de Haan RJ, Phiri AI, Malange P, Khoka M. et al. Severe anemia in Malawian children. N Engl J Med 2008;358:888–99. [DOI] [PubMed] [Google Scholar]
- 542. Suominen P, Möttönen T, Rajamäki A, Irjala K. Single values of serum transferrin receptor and transferrin receptor ferritin index can be used to detect true and functional iron deficiency in rheumatoid arthritis patients with anemia. Arthritis Rheum 2000;43:1016–20. [DOI] [PubMed] [Google Scholar]
- 543. England JM, Bain BJ, Fraser PM. Differentiation of iron deficiency from thalassaemia trait. Lancet 1973;1:1514. [DOI] [PubMed] [Google Scholar]
- 544. Green R, King R. A new red cell discriminant incorporating volume dispersion for differentiating iron deficiency anemia from thalassemia minor. Blood Cells 1989;15:481-91-5. [PubMed] [Google Scholar]
- 545. Lorenz L, Peter A, Poets CF, Franz AR. A review of cord blood concentrations of iron status parameters to define reference ranges for preterm infants. Neonatology 2013;104:194–202. [DOI] [PubMed] [Google Scholar]
- 546. Yip R, Johnson C, Dallman PR. Age-related changes in laboratory values used in the diagnosis of anemia and iron deficiency. Am J Clin Nutr 1984;39:427–36. [DOI] [PubMed] [Google Scholar]
- 547. Righetti AA, Wegmüller R, Glinz D, Ouattara M, Adiossan LG, N'Goran EK, Utzinger J, Hurrell RF. Effects of inflammation and Plasmodium falciparum infection on soluble transferrin receptor and plasma ferritin concentration in different age groups: a prospective longitudinal study in Côte d'Ivoire. Am J Clin Nutr 2013;97:1364–74. [DOI] [PubMed] [Google Scholar]
- 548. Hanif E, Ayyub M, Anwar M, Ali W, Bashir M. Evaluation of serum transferrin receptor concentration in diagnosing and differentiating iron deficiency anaemia from anaemia of chronic disorders. J Pak Med Assoc 2005;55:13–6. [PubMed] [Google Scholar]
- 549. Margetic S, Topic E, Ruzic DF, Kvaternik M. Soluble transferrin receptor and transferrin receptor-ferritin index in iron deficiency anemia and anemia in rheumatoid arthritis. Clin Chem Lab Med 2005;43:326–31. [DOI] [PubMed] [Google Scholar]
- 550. Jain S, Narayan S, Chandra J, Sharma S, Jain S, Malhan P. Evaluation of serum transferrin receptor and sTfR ferritin indices in diagnosing and differentiating iron deficiency anemia from anemia of chronic disease. Indian J Pediatr 2010;77:179–83. [DOI] [PubMed] [Google Scholar]
- 551. Punnonen K, Irjala K, Rajamäki A. Iron-deficiency anemia is associated with high concentrations of transferrin receptor in serum. Clin Chem 1994;40:774–6. [PubMed] [Google Scholar]
- 552. Stoltzfus RJ, Chwaya HM, Montresor A, Albonico M, Savioli L, Tielsch JM. Malaria, hookworms and recent fever are related to anemia and iron status indicators in 0- to 5-y old Zanzibari children and these relationships change with age. J Nutr 2000;130:1724–33. [DOI] [PubMed] [Google Scholar]
- 553. Feelders RA, Vreugdenhil G, Eggermont AM, Kuiper-Kramer PA, van Eijk HG, Swaak AJ. Regulation of iron metabolism in the acute-phase response: interferon gamma and tumour necrosis factor alpha induce hypoferraemia, ferritin production and a decrease in circulating transferrin receptors in cancer patients. Eur J Clin Invest 1998;28:520–7. [DOI] [PubMed] [Google Scholar]
- 554. Northrop-Clewes C. The interpretation of indicators of iron status during an acute phase response. Assessing the iron status of populations: report of a Joint World Health Organization/Centers for Disease Control and Prevention technical consultation on the assessment of iron status at the population level. Geneva; 2007. [Google Scholar]
- 555. Baynes R, Bezwoda W, Bothwell T, Khan Q, Mansoor N. The non-immune inflammatory response: serial changes in plasma iron, iron-binding capacity, lactoferrin, ferritin and C-reactive protein. Scand J Clin Lab Invest 1986;46:695–704. [DOI] [PubMed] [Google Scholar]
- 556. World Health Organization WHO | C-reactive protein concentrations as a marker of inflammation or infection for interpreting biomarkers of micronutrient status. World Health Organization; 2015. [Google Scholar]
- 557. Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr 2010;92:546–55. [DOI] [PubMed] [Google Scholar]
- 558. Engle-Stone R, Nankap M, Ndjebayi AO, Erhardt JG, Brown KH. Plasma ferritin and soluble transferrin receptor concentrations and body iron stores identify similar risk factors for iron deficiency but result in different estimates of the national prevalence of iron deficiency and iron-deficiency anemia among women. J Nutr 2013;143:369–77. [DOI] [PubMed] [Google Scholar]
- 559. Glinz D, Hurrell RF, Righetti AA, Zeder C, Adiossan LG, Tjalsma H, Utzinger J, Zimmermann MB, N'Goran EK, Wegmüller R. In Ivorian school-age children, infection with hookworm does not reduce dietary iron absorption or systemic iron utilization, whereas afebrile Plasmodium falciparum infection reduces iron absorption by half. Am J Clin Nutr 2015;101:462–70. [DOI] [PubMed] [Google Scholar]
- 560. Suchdev PS, Namaste SML, Aaron GJ, Raiten DJ, Brown KH, Flores-Ayala R, Working Group BRINDA. Overview of the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Adv Nutr 2016;7:349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561. Namaste SM, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, Mei Z, Rawat R, Williams AM, Raiten DJ. et al. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr 2017;106:359S–371S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562. Mei Z, Namaste SM, Serdula M, Suchdev PS, Rohner F, Flores-Ayala R, Addo OY, Raiten DJ. Adjusting total body iron for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr 2017;106:383S–389S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563. Rohner F, Namaste SM, Larson LM, Addo OY, Mei Z, Suchdev PS, Williams AM, Sakr Ashour FA, Rawat R, Raiten DJ. et al. Adjusting soluble transferrin receptor concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:372S–382S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564. Merrill RD, Burke RM, Northrop-Clewes CA, Rayco-Solon P, Flores-Ayala R, Namaste SM, Serdula MK, Suchdev PS. Factors associated with inflammation in preschool children and women of reproductive age: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:348S–358S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565. Stoltzfus RJ, Klemm R. Research, policy, and programmatic considerations from the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:428S–434S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566. Gale E, Torrance J, Bothwell T. The quantitative estimation of total iron stores in human bone marrow. J Clin Invest 1963;42:1076–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567. Barron BA, Hoyer JD, Tefferi A. A bone marrow report of absent stainable iron is not diagnostic of iron deficiency. Ann Hematol 2001;80:166–9. [DOI] [PubMed] [Google Scholar]
- 568. Henderson PA, Hillman RS. Characteristics of iron dextran utilization in man. Blood 1969;34:357–75. [PubMed] [Google Scholar]
- 569. National Committee for Clinical Laboratory Standards H15-A3: reference and selected procedures for the quantitative determination of hemoglobin in blood. 3rd ed 2000. [Google Scholar]
- 570. US Centers for Disease Control and Prevention Nutrition Survey Toolkit [Internet]. 2016[cited 2017 Jun 27]. Available from: http://surveytoolkit.micronutrient.org/.
- 571. Burger SE, Pierre-Louis JN. How to assess iron deficiency anemia and use the hemocue? New York; 2002. [Google Scholar]
- 572. Mandava U, Drammeh BS, Zhang M, Schleicher RL, Pfeiffer CM. Three models of HemoCue photometers: comparison of venous blood hemoglobin concentrations and influence of heat on cuvettes. FASEB J; 2010;24:538.8. [Google Scholar]
- 573. Whitehead RD, Zhang M, Sternberg MR, Schleicher RL, Drammeh B, Mapango C, Pfeiffer CM. Effects of preanalytical factors on hemoglobin measurement: a comparison of two HemoCue? point-of-care analyzers. Clin Biochem 2017;50:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574. National Committee for Clinical Laboratory Standards C42-A erythrocyte protoporphyrin testing; Approved Guideline 1996.
- 575. Beard J. Indicators of the iron status of populations: free erythrocyte protoporphyrin and zinc protoporphyrin; serum and plasma iron, total iron binding capacity and transferrin saturation; and serum transferrin receptor. Assessing the iron status of populations. Geneva: World Health Organization; 2007. [Google Scholar]
- 576. International Nutritional Anemia Consultative Group Measurements of iron status. ILSI Press; 1985. [Google Scholar]
- 577. NIBSC. National Institute for Biological Standards and Control Biological reference materials [Internet]. [cited 2013 Sep 4]. Available from:http://www.nibsc.org/products/biological_reference_materials.aspx
- 578. National Institute of Standards and Technology Standard reference materials | NIST [Internet]. [cited 2016 Oct 10]. Available from: https://www.nist.gov/srm
- 579. Pfeiffer C, Schleicher R, Caldwell K. Biochemical indices. In: Caballero B, Allen L, Prentice A, Encyclopedia of human nutrition. 3rd ed.New York: Academic Press; 2013. [Google Scholar]
- 580. National Committee for Clinical Laboratory Standards C61-A determination of serum iron, total iron-binding capacity and percent transferrin saturation; approved standard. 1998.
- 581. Blanck HM, Pfeiffer CM, Caudill SP, Reyes M, Gunter EW, Imperatore G, van Assendelft OW, Strider S, Dearth T. Serum iron and iron-binding capacity: a round-robin interlaboratory comparison study. Clin Chem 2003;49:1672–5. [DOI] [PubMed] [Google Scholar]
- 582. Worwood M, Thorpe SJ, Heath A, Flowers CH, Cook JD. Stable lyophilized reagents for the serum ferritin assay. Clin Lab Haematol 1991;13:297–305. [DOI] [PubMed] [Google Scholar]
- 583. Flowers CH, Cook JD. Dried plasma spot measurements of ferritin and transferrin receptor for assessing iron status. Clin Chem 1999;45:1826–32. [PubMed] [Google Scholar]
- 584. Ahluwalia N, de Silva A, Atukorala S, Weaver V, Molls R. Ferritin concentrations in dried serum spots from capillary and venous blood in children in Sri Lanka: a validation study. Am J Clin Nutr 2002;75:289–94. [DOI] [PubMed] [Google Scholar]
- 585. Cook JD, Flowers CH, Skikne BS. An assessment of dried blood-spot technology for identifying iron deficiency. Blood 1998;92:1807–13. [PubMed] [Google Scholar]
- 586. Blackmore S, Hamilton M, Lee A, Worwood M, Brierley M, Heath A, Thorpe SJ. Automated immunoassay methods for ferritin: recovery studies to assess traceability to an international standard. Clin Chem Lab Med 2008;46:1450–7. [DOI] [PubMed] [Google Scholar]
- 587. Pfeiffer CM, Cook JD, Mei Z, Cogswell ME, Looker AC, Lacher DA. Evaluation of an automated soluble transferrin receptor (sTfR) assay on the Roche Hitachi analyzer and its comparison to two ELISA assays. Clin Chim Acta 2007;382:112–6. [DOI] [PubMed] [Google Scholar]
- 588. Abellan R, Ventura R, Pichini S, Sarda MP, Remacha AF, Pascual JA, Palmi I, Bacosi A, Pacifici R, Zuccaro P. et al. Evaluation of immunoassays for the measurement of soluble transferrin receptor as an indirect biomarker of recombinant human erythropoietin misuse in sport. J Immunol Methods 2004;295:89–99. [DOI] [PubMed] [Google Scholar]
- 589. Thorpe SJ, Heath A, Sharp G, Cook J, Ellis R, Worwood M. A WHO reference reagent for the serum transferrin receptor (sTfR): international collaborative study to evaluate a recombinant soluble transferrin receptor preparation. Clin Chem Lab Med 2010;48:815–20. [DOI] [PubMed] [Google Scholar]
- 590. Kogan AE, Filatov VL, Kara AN, Levina AA, Katrukha AG. Comparison of soluble and placental transferrin receptors as standards for the determination of soluble transferrin receptor in humans. Int J Lab Hematol 2007;29:335–40. [DOI] [PubMed] [Google Scholar]
- 591. McDade TW, Shell-Duncan B. Whole blood collected on filter paper provides a minimally invasive method for assessing human transferrin receptor level. J Nutr 2002;132:3760–3. [DOI] [PubMed] [Google Scholar]
- 592. Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr 2004;134:3127–32. [DOI] [PubMed] [Google Scholar]
- 593. Brindle E, Stevens D, Crudder C, Levin CE, Garrett D, Lyman C, Boyle DS. A multiplex immunoassay method for simultaneous quantification of iron, vitamin A and inflammation status markers. PLoS One. 2014;9:e115164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594. Ramco Laboratories Human transferrin receptor enzyme immunoassay [Internet]. 2006. Available from: http://ramcolab.com/page13.html
- 595. Mei Z, Pfeiffer CM, Looker AC, Flores-Ayala RC, Lacher DA, Mirel LB, Grummer-Strawn LM. Serum soluble transferrin receptor concentrations in US preschool children and non-pregnant women of childbearing age from the National Health and Nutrition Examination Survey 2003–2010. Clin Chim Acta 2012;413:1479–84. [DOI] [PubMed] [Google Scholar]
- 596. Pfeiffer C, Looker A. Laboratory methodologies for indicators of iron status: strengths, limitations and analytical challenges. Am J Clin Nutr 2017; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597. Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA 1997;277:973–6. [DOI] [PubMed] [Google Scholar]
- 598. Pilch S, Senti F. Assessment of iron nutritional status of the U.S. population based on data collected in the Second National Health and Nutrition Examination Survey, 1976–1980. Bethesda; 1984.
- 599. Hollowell JG, van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer C, Centers for Disease Control and Prevention, National Center for Health Statistics Hematological and iron-related analytes–reference data for persons aged 1 year and over: United States, 1988–94. Vital Health Stat 11. 2005;1–156. [PubMed] [Google Scholar]
- 600. U.S. Centers for Disease Control and Prevention Second national report on biochemical indicators of diet and nutrition in the U.S. population 2012. Atlanta, GA: CDC; 2012. [Google Scholar]
- 601. Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood 1997;89:1052–7. [PubMed] [Google Scholar]
- 602. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005;352:1011–23. [DOI] [PubMed] [Google Scholar]
- 603. van den Broek NR, Letsky EA, White SA, Shenkin A. Iron status in pregnant women: which measurements are valid? Br J Haematol 1998;103:817–24. [DOI] [PubMed] [Google Scholar]
- 604. Ronnenberg AG, Wood RJ, Wang X, Xing H, Chen C, Chen D, Guang W, Huang A, Wang L, Xu X. Preconception hemoglobin and ferritin concentrations are associated with pregnancy outcome in a prospective cohort of Chinese women. J Nutr 2004;134:2586–91. [DOI] [PubMed] [Google Scholar]
- 605. Bock JL, Endres DB, Elin RJ, Wang E, Rosenzweig B, Klee GG. Comparison of fresh frozen serum to traditional proficiency testing material in a College of American Pathologists survey for ferritin, folate, and vitamin B12. Arch Pathol Lab Med 2005;129:323–7. [DOI] [PubMed] [Google Scholar]
- 606. Davis BH, Jungerius B, International Council for the Standardization of Haematology (ICSH) International Council for Standardization in Haematology technical report 1–2009: new reference material for haemiglobincyanide for use in standardization of blood haemoglobin measurements. Int J Lab Hematol 2010;32:139–41. [DOI] [PubMed] [Google Scholar]
- 607. Thorpe SJ. The development and role of international biological reference materials in the diagnosis of anaemia. Biologicals 2010;38:449–58. [DOI] [PubMed] [Google Scholar]
- 608. Ahluwalia N, Lammi-Keefe CJ, Haley NR, Beard JL. Day-to-day variation in iron-status indexes in elderly women. Am J Clin Nutr 1993;57:414–9. [DOI] [PubMed] [Google Scholar]
- 609. Conway AM, Hinchliffe RF, Earland J, Anderson LM. Measurement of haemoglobin using single drops of skin puncture blood: is precision acceptable? J Clin Pathol 1998;51:248–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610. Drammeh BS, Mandava U, Zhang M, Schleicher RL, Pfeiffer CM. Capillary vs venous blood: delayed measurement of hemoglobin concentrations and hematocrit. FASEB J 2010;24:538.7. [Google Scholar]
- 611. Lacher DA, Hughes JP, Carroll MD. Biological variation of laboratory analytes based on the 1999–2002 National Health and Nutrition Examination Survey. Natl Health Stat Report 2010;1–7. [PubMed] [Google Scholar]
- 612. van Eijsden M, van der Wal MF, Hornstra G, Bonsel GJ. Can whole-blood samples be stored over 24 hours without compromising stability of C-reactive protein, retinol, ferritin, folic acid, and fatty acids in epidemiologic research? Clin Chem 2005;51:230–2. [DOI] [PubMed] [Google Scholar]
- 613. Drammeh BS, Schleicher RL, Pfeiffer CM, Jain RB, Zhang M, Nguyen PH. Effects of delayed sample processing and freezing on serum concentrations of selected nutritional indicators. Clin Chem 2008;54:1883–91. [DOI] [PubMed] [Google Scholar]
- 614. Zhang DJ, Elswick RK, Miller WG, Bailey JL. Effect of serum-clot contact time on clinical chemistry laboratory results. Clin Chem 1998;44:1325–33. [PubMed] [Google Scholar]
- 615. Haynes BMH, Pfeiffer CM, Sternberg MR, Schleicher RL. Selected physiologic variables are weakly to moderately associated with 29 biomarkers of diet and nutrition, NHANES 2003–2006. J Nutr 2013;143:S1001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616. Dale JC, Burritt MF, Zinsmeister AR. Diurnal variation of serum iron, iron-binding capacity, transferrin saturation, and ferritin levels. Am J Clin Pathol 2002;117:802–8. [DOI] [PubMed] [Google Scholar]
- 617. Kemna EHJM, Tjalsma H, Willems HL, Swinkels DW. Hepcidin: from discovery to differential diagnosis. Haematologica 2008;93:90–7. [DOI] [PubMed] [Google Scholar]
- 618. Girelli D, Nemeth E, Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood 2016;127:2809–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619. Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, Klaver SM, Kroot JJ, van Tienoven D, Wetzels JFM, Kiemeney LALM, Sweep FC, den Heijer M. et al. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood 2011;117:e218–25. [DOI] [PubMed] [Google Scholar]
- 620. Rehu M, Punnonen K, Ostland V, Heinonen S, Westerman M, Pulkki K, Sankilampi U. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur J Haematol 2010;85:345–52. [DOI] [PubMed] [Google Scholar]
- 621. Simavli S, Derbent AU, Uysal S, Turhan NÖ. Hepcidin, iron status, and inflammation variables among healthy pregnant women in the Turkish population. J Matern Fetal Neonatal Med 2014;27:75–9. [DOI] [PubMed] [Google Scholar]
- 622. Brickley EB, Spottiswoode N, Kabyemela E, Morrison R, Kurtis JD, Wood AM, Drakesmith H, Fried M, Duffy PE. Cord blood hepcidin: cross-sectional correlates and associations with anemia, malaria, and mortality in a tanzanian birth cohort study. Am J Trop Med Hyg 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623. Pasricha S-R, Atkinson SH, Armitage AE, Khandwala S, Veenemans J, Cox SE, Eddowes LA, Hayes T, Doherty CP, Demir AY. et al. Expression of the iron hormone hepcidin distinguishes different types of anemia in African children. Sci Transl Med 2014;6:235re3. [DOI] [PubMed] [Google Scholar]
- 624. Drakesmith H. Next-generation biomarkers for iron status. Nestlé Nutr Inst Work Ser 2016;84:59–69. [DOI] [PubMed] [Google Scholar]
- 625. van der Vorm LN, Hendriks JCM, Laarakkers CM, Klaver S, Armitage AE, Bamberg A, Geurts-Moespot AJ, Girelli D, Herkert M, Itkonen O. et al. Toward worldwide hepcidin assay harmonization: identification of a commutable secondary reference material. Clin Chem 2016;62:993–1001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.