Significance
Grooming and cleaning are part of a multibillion dollar industry from carpet cleaning to human hair care to pet grooming. Advancements in this field focus primarily on novel cleaning fluids, with less focus on brush development. This study focuses on the cat, one of nature’s most fastidious groomers. We discover structures on the cat tongue, hollow spines that we call cavo papillae, shared across six species of cats. The papillae wick saliva deep into recesses of the fur, and the flexible base of the papilla permits hairs to be easily removed from the tongue. These multifunctional spines may provide inspiration to soft robotics and biologically inspired technologies for sorting, cleaning, and depositing fluids into fur and arrays of flexible filaments.
Keywords: comb, capillarity, 3D printing, cooling
Abstract
The cat tongue is covered in sharp, rear-facing spines called papillae, the precise function of which is a mystery. In this combined experimental and theoretical study, we use high-speed film, grooming force measurements, and computed tomography (CT) scanning to elucidate the mechanism by which papillae are used to groom fur. We examine the tongues of six species of cats from domestic cat to lion, spanning 30-fold in body weight. The papillae of these cats each feature a hollow cavity at the tip that spontaneously wicks saliva from the mouth and then releases it onto hairs. The unique shape of the cat’s papillae may inspire ways to clean complex hairy surfaces. We demonstrate one such application with the tongue-inspired grooming (TIGR) brush, which incorporates 3D-printed cat papillae into a silicone substrate. The TIGR brush experiences lower grooming forces than a normal hairbrush and is easier to clean.
The family Felidae roamed the Earth for 11 million y before being domesticated 10,000 y ago in Southwest Asia (1). Today, house cats can spend up to 24% of their waking time grooming their fur coat (2). Grooming helps the cat to remove pesky fleas, loose hairs, and excess heat (3–5). In the absence of grooming, excess debris can tangle fur, causing painful tugging of the skin and even infection. Grooming a cat’s fur is challenging due to its two layers: an exposed topcoat for protection and a hidden undercoat of down hairs for warmth (6).
The cat tongue is most recognized for its hundreds of sharp, backward-facing keratin spines called filiform papillae, shown in Fig. 1 A and B. A 1982 study concluded that a cat papilla has the shape of a solid cone (7), an observation that remained undisputed for two decades (8, 9). In our study, we show that the papilla is in fact scoop shaped, enabling it to use surface tension forces to wick saliva. Surface tension is exploited by animals to drink, walk, climb, and jump (10–12). Cats use surface tension to pull up water during lapping (13), while dogs use their tongues like ladles to drink (14).
Fig. 1.
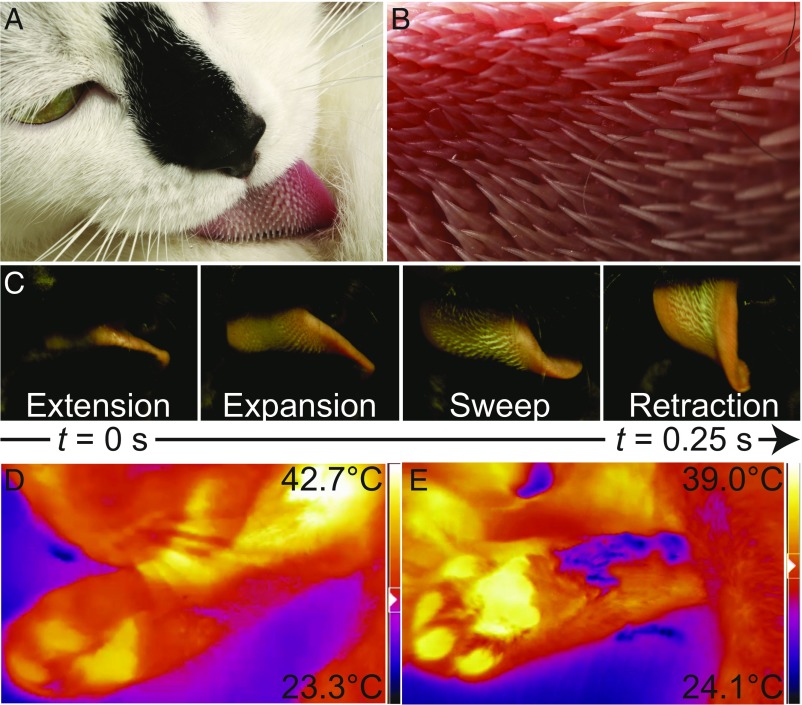
Kinematics of cat grooming. (A) A domestic cat grooms its fur. (B) Close-up view of its tongue showing the anisotropic papillae, which point to the left toward the throat. (C) The four phases of cat grooming. (D and E) Thermal images of a cat grooming its leg. White colors are hottest and dark blue colors are coolest as shown by the legend on the right. During the groom (D), fur is separated by the motion of the tongue, exposing the skin. Heat from the tongue and the skin are indicated by the white color. After the groom (E), evaporation causes a temperature drop of C as shown by the dark purple.
Cats have sweat glands only on their paws (15). Thus, it has long been hypothesized that grooming helps cats thermoregulate. Indeed, many other animals lick themselves to keep cool. Rats do so (16), and kangaroos even possess thin-skinned regions on their elbows (17) that are used especially for this purpose. The dairy industry sprays water on their cows to keep them cool, a common practice used to increase dairy yield (18). It has been estimated that up to one-third of the cat’s evaporative water loss is due to saliva evaporation from the fur (19). In this study, we quantify the saliva deposited and demonstrate its utility in cooling the cat.
Results
Grooming Kinematics and Forces of the Domestic Cat.
Using high-speed videography, we filmed three adult short-hair domestic cats (Felis catus; n = 3) grooming their own fur (Fig. 1A). Additionally, we filmed an adult domestic cat grooming a faux fur surface attached to a force plate. In all instances, the cat’s groom consisted of four phases, depicted in Fig. 1C; these phases include extension of the tongue, lateral expansion and stiffening of the tongue tissue, a sweep of the tongue through the fur, and retraction of the tongue in a U-shaped curl. During expansion, the spines rotate until they are perpendicular to the tongue, as shown in the high-speed film in Movie S1. This allows the papillae to stand erect to increase their contact area with fur.
During grooming, the domestic cat’s tongue traveled a distance of = 63 20 mm at an associated speed of = 220 9 mm/s and a frequency of 1.4 0.6 licks per second. Moreover, the tongue pressed down on fur with 0.13 0.13 N of force. Other species of cat have papillae on their tongues and groom in a similar manner to the domestic cat (Movie S2 and SI Appendix, Table S1). Since we did not find systematic trends in terms of speed, frequency, or lick length for other cat species, we consider the kinematics of the domestic cat to test the mathematical models in the following sections.
Cats Have Hollow Papillae That Wick Saliva.
Fig. 2A shows the tongues of six species of cats, which we collected postmortem. These cats include the domestic cat F. catus, bobcat Lynx rufus, cougar Puma concolor, snow leopard Panthera uncia, tiger Panthera tigris, and lion Panthera leo. A previously reported phylogenetic tree shows that these cat species are distantly related (20).
Fig. 2.
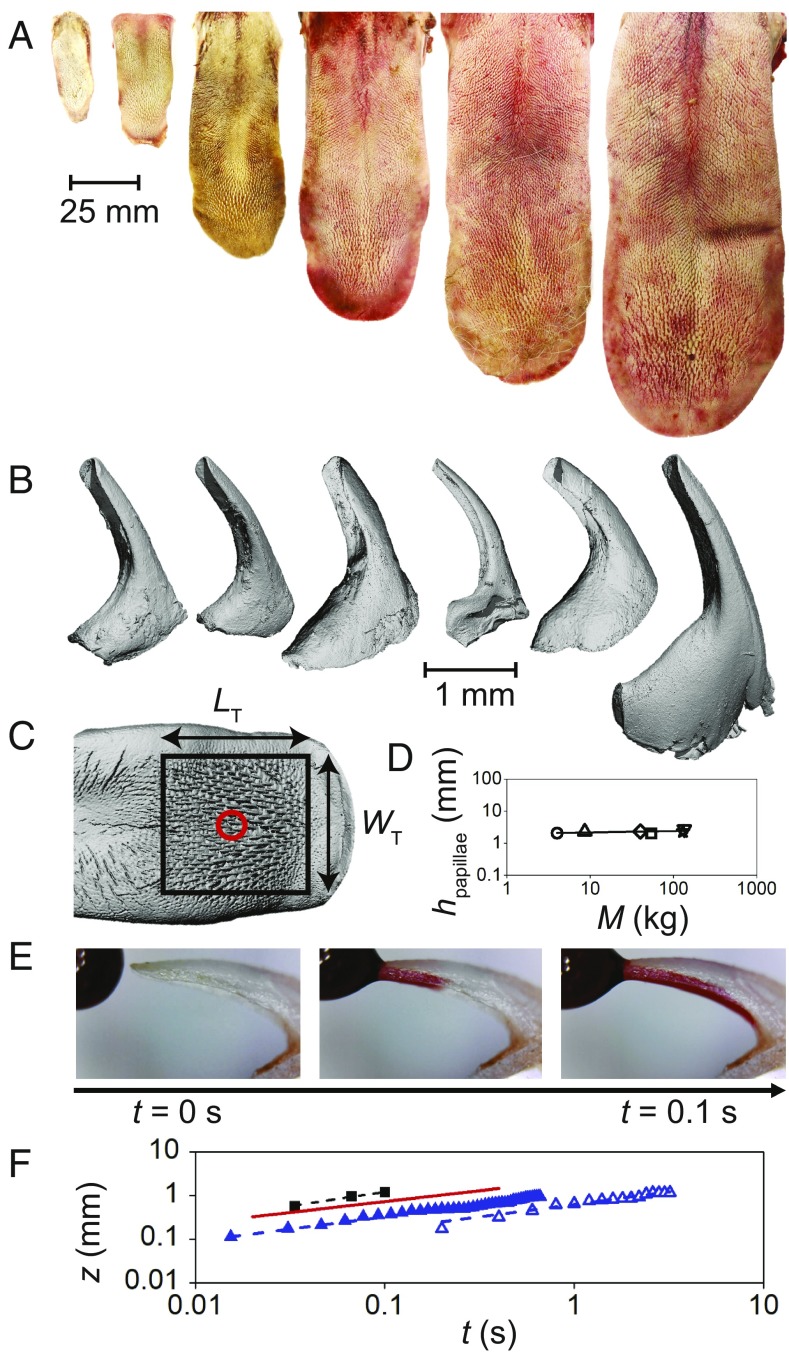
The cat tongue and its wicking papillae. (A and B) From left to right, (A) excised tongues and (B) micro-CT scans of cavo papillae from a domestic cat, bobcat, cougar, snow leopard, tiger, and lion. (C) Micro-CT scan of a domestic cat tongue. The distal region of the tongue contains large, rigid cavo papillae (bounded by the black box). Scanned cavo papillae from B are taken from the area denoted by the red circle. (D) The relationship between cavo papillae height and body mass, where the fit line is , indicating that papillae height is constant. (E) Domestic cat papilla wicking red food dye in under 0.1 s. (F) Time course of red food dye wicking into a cat papilla (black square) and two tiger papillae (solid and open triangles). Washburn’s Law prediction is shown by the red line.
We 3D scanned a domestic cat tongue using micro-computed tomography (micro-CT), identifying two distinct regions of papillae on the tongue. The distal region, demarcated by the black box in Fig. 2C, contains large papillae in sparse density, while the proximal region contains small papillae in high density. Our high-speed footage of grooming shows that only the distal region contacts the fur during grooming. Therefore, from hereon, all references to the papillae will refer to papillae in this distal region. Similarly, tongue length and width will refer not to the entire tongue but to the length and width of the distal region. We report these measurements for six cat species in SI Appendix, Table S2.
Next, we identified the largest cavo papilla from the center of the distal region in all six cat tongues. For the domestic cat tongue, this papilla is shown by the red circle in Fig. 2C. Before papilla removal, papilla height was measured from the tissue surface to the papilla tip. The taller the papillae, the deeper it can penetrate the fur during grooming, as shown in Fig. 3B. Despite the six species of cats spanning over 30-fold in body weight, their tongue papillae have constant height: = 2.3 0.2 mm (n = 6), as shown in Fig. 2D. A constant papillae height is suggestive of the papillae’s key role during grooming.
Fig. 3.
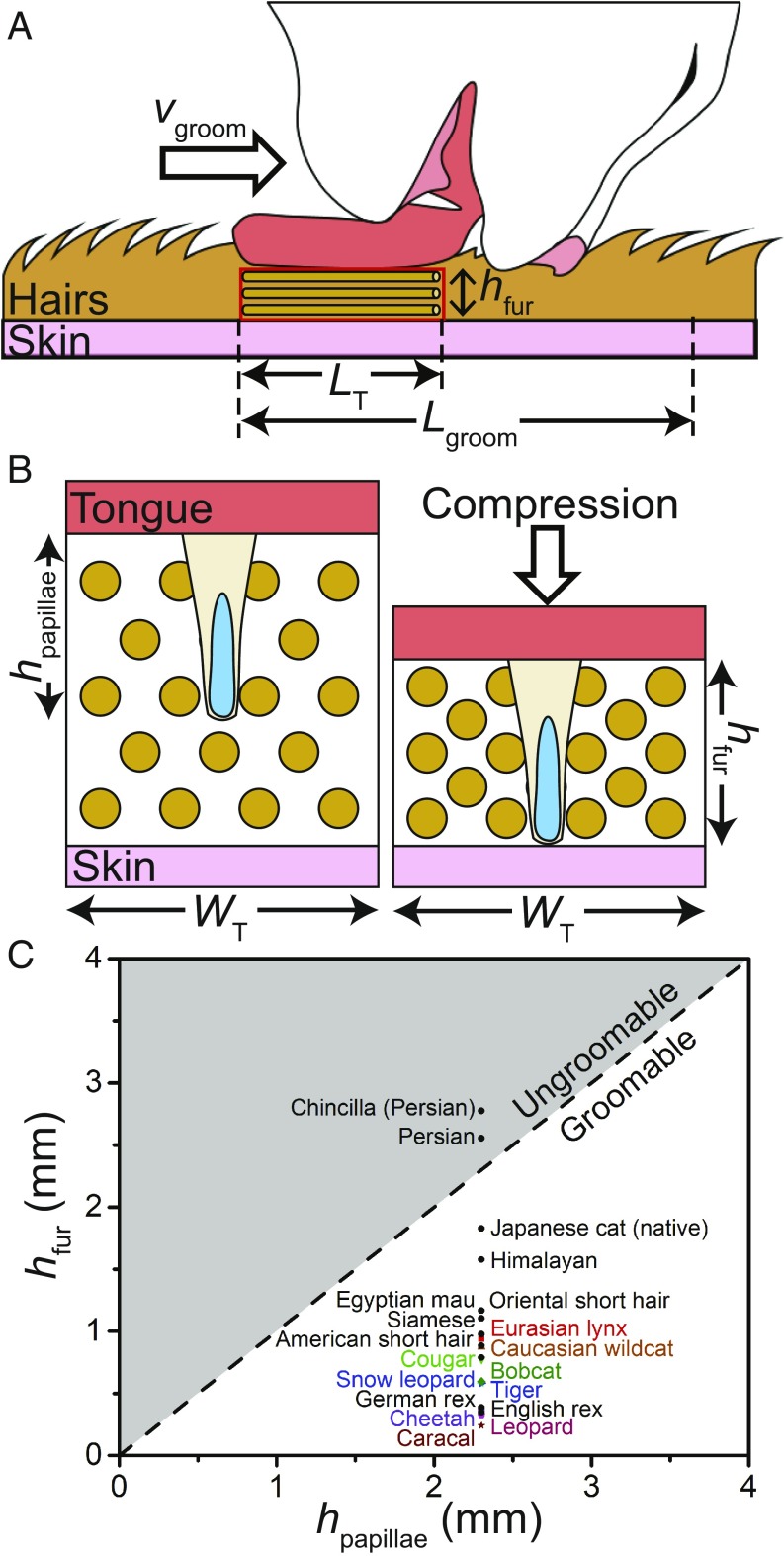
Ease of cat grooming as indicated by fur and papillae geometry. (A) Schematic of cat grooming its own fur. The tongue compresses the fur and then travels to the right. (B) Close-up view of the fur before and after compression. When the tongue pushes down, fur depth and porosity both decrease. Papillae and hairs are exaggerated for clarity. (C) Relationship between the compressed fur height and papillae height . The dashed line, , indicates the separation between groomable and ungroomable cats.
We measured the hardness of a domestic cat papilla and a freshly excised cat tongue from a domestic cat postmortem. The Young’s modulus of a domestic cat papilla is 1.66–1.94 GPa (three tests done on a single cat papilla), similar to human fingernails (21) and five orders of magnitude stiffer than the cat tongue tissue (9.1 3.7 kPa; n = 2). We used these values in selecting materials for our cat tongue mimic as shown in a later section.
We cleaned a papilla from each of the six cat species and scanned them using micro-CT to generate the 3D models shown in Fig. 2B. A papilla’s unique features are made visible by the transparent view of the papilla in SI Appendix, Fig. S4. A cat papilla has two hollow regions: a cavity at the base for tissue attachment and a U-shaped cavity at the tip for wicking saliva. We report the papillae measurements for six cat species in SI Appendix, Table S3.
We conducted wicking experiments by contacting a drop of food coloring with the tip of domestic cat and tiger papillae. The fluid spontaneously rose into the U-shaped cavity in 0.1 s, as shown by the image sequence in Fig. 2E. Fig. 2F shows the time course of the front of this fluid into the papillae, where is the distance from the papillae tip. The rapid rate of fluid rise is consistent with Washburn’s Law for wicking into a half-pipe (22). This wicking acts like a lock and key for the saliva: after it is wicked into the papillae, the fluid is quite stable, even if the papillae is turned upside down. To remove the saliva, the cat simply contacts its tongue with fur, as shown in Fig. 4.
Fig. 4.
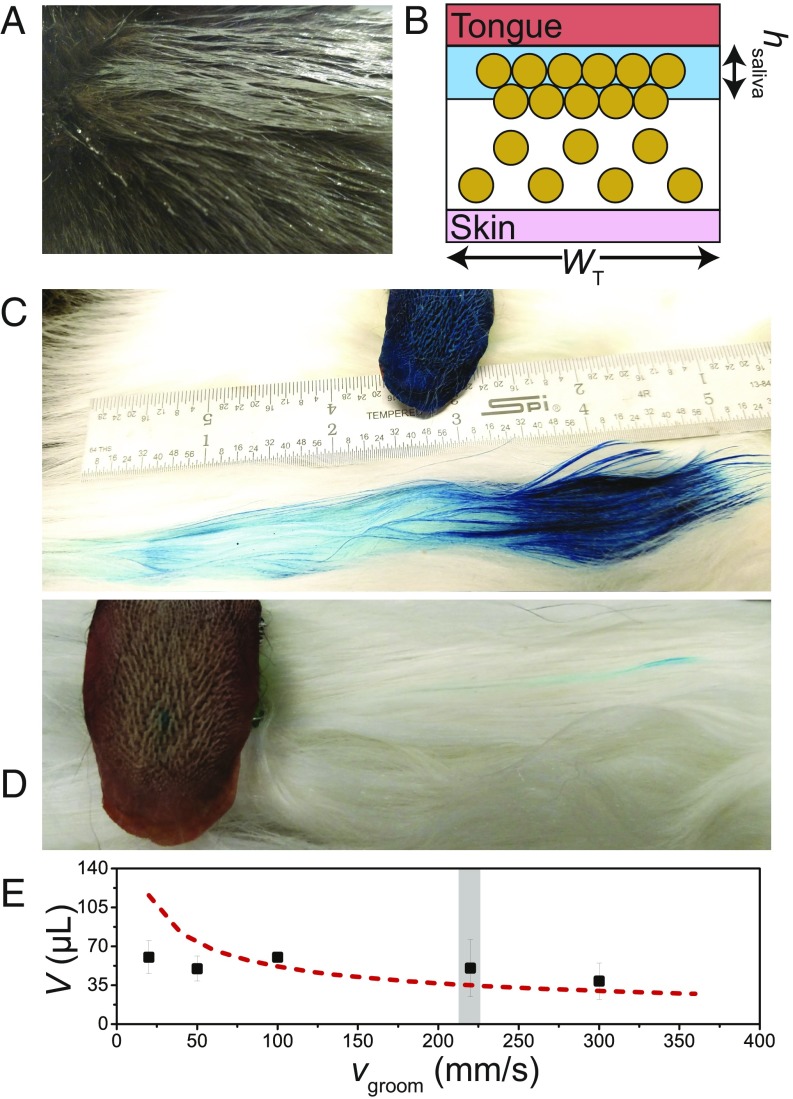
The transfer of saliva from tongue to fur. (A) Cat fur forms clumps when wetted. (B) Schematic of clumped wetted hairs, which have a local reduction in porosity. (C) A single groom of the cat tongue as visualized by blue food dye transferred from the tongue to the fur. The length of groom corresponds to the grooming length in domestic cats. (D) A groom of the cat tongue, this time with blue dye wicked into a single cavo papillae. (E) The relationship between the volume of water transferred and the velocity of the groom. The vertical gray bar indicates the grooming speed of a domestic cat. The dashed line shows the predictions of our mathematical model (see SI Appendix), using a wetted fur porosity of .
While fluid rises quickly in the papilla, the combined fluid in all of the papillae is small compared with that available on the tongue surface. For the domestic cat, each papilla captures 0.014 L of saliva for a total of 4.1 L across 290 papillae or a 10th of an eyedropper drop. We dipped a severed cat tongue in water, allowing excess fluid to drip off, and found that the fluid in the papillae cavities accounts for 5% of total fluid on the top of the tongue. While it is not a large volume, we will show that the papillae penetration into fur allows saliva to reach areas that the tongue surface cannot.
Papillae Height Dictates a Cat’s Groomability.
To characterize fur across cats, we measured by hand the hair radius and hair length of nine species of cats (Materials and Methods). Additionally, fur density and length values were gathered from the literature (23–25), giving us a total of 19 species of cats, the fur properties of which are given in SI Appendix, Table S4. To fully clean their fur coat, cats must distribute saliva to the hair roots. To determine if papillae are long enough to penetrate the fur coat and reach the skin, we consider a cat compressing its own fur, as shown in Fig. 3A. The cat fur coat has two layers: the topcoat and the undercoat. The topcoat consists of thick guard hairs, which are used to protect the undercoat from the environment. Although hidden from sight, the undercoat primarily consists of thin down hairs, which can outnumber guard hairs 24 to 1 and are used for thermoregulation (6). Given their predominance in giving cat fur its shape, we only consider down hairs in our analysis.
We compare cat furs using the fur’s porosity , the fraction of air in a given volume:
| [1] |
where is the volume of air in a given volume of fur . A cat’s uncompressed fur is composed of mostly air, with porosity values approaching 1 (0.97 for domestic cat, 0.98 for tiger, 0.99 for snow leopard). Like a down jacket, this large air fraction provides the cat excellent insulation from the elements. As the tongue presses down on the fur during grooming, air evacuates, decreasing fur porosity as shown in Fig. 3B. The minimum compression height can be calculated considering the geometry of the hairs, as shown in SI Appendix. Our calculations show the American short-hair domestic cat compresses its fur from 37 to 1.2 mm, a tiger compresses its fur from 30 to 0.6 mm, and a Persian domestic cat compresses its fur from 81 to 2.6 mm.
Fig. 3C shows the relationship between compressed fur height and papillae height for 20 cats. For six cats, the papillae height was measured directly; for the remaining 14 cats, a tongue sample was not available, and therefore, we used the average cat papillae height of = 2.3 0.2 mm. Two distinct regimes are evident depending on the height of the compressed fur. If a cat’s papillae can penetrate through its compressed fur and touch the skin (), the cat can successfully groom itself. The caracal, cheetah, and leopard are the most “groomable” cats due to their short, sparse fur. Even the snow leopard, known for its plush fur, can easily groom itself. Its fur is long but sparse, and therefore, its compressed fur has a height of only 0.6 mm, which is easily penetrated by its papillae.
Conversely, if the papillae cannot reach the skin (), much of its fur cannot be accessed, making the cat “ungroomable.” Long-haired domestic breeds, such as Persian domestic cats, are notorious for their matted fur if not cared for properly. According to Veterinary Centers of America (VCA) animal hospitals, Persian cat owners should comb their cat daily and give it baths monthly to redistribute the fur’s natural oils (26). Consistent with these care instructions, two Persian breeds are the only animals to fall into the ungroomable region, as shown in the upper one-half of Fig. 3C. Our measurements were made on the dorsal fur, which is usually longer than hairs on the paws, limbs, and face, all regions that are likely easier to groom. In the next section, we show how saliva from the tongue interacts with the fur.
Papillae Necessary to Reach Deep into Fur.
Next, we determined how much saliva is transferred during the grooming process. We designed and built an automated grooming machine capable of simulating grooming conditions, as detailed in SI Appendix. A cat tongue wetted with food dye was pulled through a sample of the cat’s own fur at various speeds from 20 to 300 mm/s. This experiment generated a dyed path similar to the one shown in Fig. 4C. The length of the lick was that for the domestic cat (6.3 cm), and the path’s width closely matches the width of the tongue. By weighing the tongue after each lick and accounting for the effects of evaporation, we estimated the small amounts of fluid transferred.
Fig. 4E shows the relationship between the speed of the cat tongue and the volume of water transferred. A gray bar marks the domestic cat’s grooming velocity of 220 mm/s. At this speed, the tongue deposits 56.6 25.6 in a single lick, nearly 50% of the fluid on the tongue. The deposited volume is closely predicted by our theory (Eq. S8 in SI Appendix), shown by the dashed red line in Fig. 4E, where we assume that wetted clumped hair has a porosity of . We thus conclude that our mathematical model has captured the essence of saliva release. Our experiments also indicate that the cat grooming is robust, depositing 50–60 of fluid across a 30-fold range in speed. This suggests that grooming can be accomplished by cats both large and small.
The fluid delivered by the tongue’s surface penetrates to only a 0.54-mm depth (as shown by Eq. S6 in SI Appendix). Thus, grooming leaves the majority of the fur untouched if it were not for the papillae. Although the papillae only holds up to 5% of the tongue’s saliva volume, the height of the papillae enables this volume to be spread along the roots of the hairs. Fig. 4D shows the path of dye created by a single papilla. If all of the papillae were dyed, 290 such streaks would appear along the roots of the hairs during each lick. The saliva deposited contains enzymes that can dissolve blood and other contaminants. Moreover, as the saliva evaporates, it directly cools the skin, as we show in the next section.
Saliva Evaporation Can Help Cats Thermoregulate.
An animal’s basal metabolic rate (BMR) reflects the amount of energy expended per unit time when the animal is at rest. This energy is necessarily expended as heat, and to avoid overheating, animals must find ways to transfer this heat to their surroundings. For domestic cats, the BMR is well predicted by an intraspecies allometric scaling (27, 28) of BMR = 293, where is body mass in kilograms and BMR is in kilojoules per day. Based on this scaling, a domestic cat of mass of 2.2 kg must expel heat at a rate of 5.7 W to not overheat.
In an ideal scenario, all saliva held by the papillae would be deposited with each lick. This deposited saliva has the potential to cool the animal at a rate of
| [2] |
where is the amount of saliva deposited per unit time and is the latent heat of vaporization of water at a body temperature. For the average cat body temperature of C, the latent heat of vaporization of water is = 575 cal/g (5). A domestic cat sleeps on average 14 h/d and grooms 24% of its awake time (29); therefore, a cat grooms 2.4 h/d. Based on the measured lick frequency of 1.4 licks per second and maximum total papillae cavity fluid of 4 L, the domestic cat can deposit 48 g of saliva per day with its 290 papillae, where we assume the papillae are fully refilled in the mouth after every lick. If we consider saliva transfer by only the papillae distributing saliva close to the skin surface, we find that cooling rate can reach a maximum of 1.3 W, nearly 25% of the needed heat release. The remaining 75% of heat would be transferred by conduction, convection, and radiation from the hairs, paws, and ears. The tongue tissue also wets hairs for added evaporation. Using a thermal camera, we see that saliva deposited on fur can generate a temperature difference of up to C between skin and topcoat and increase the cooling rate even further (Fig. 1C). In the next section, we consider the mechanical benefits conveyed by the papilla’s flexible attachment to the tongue.
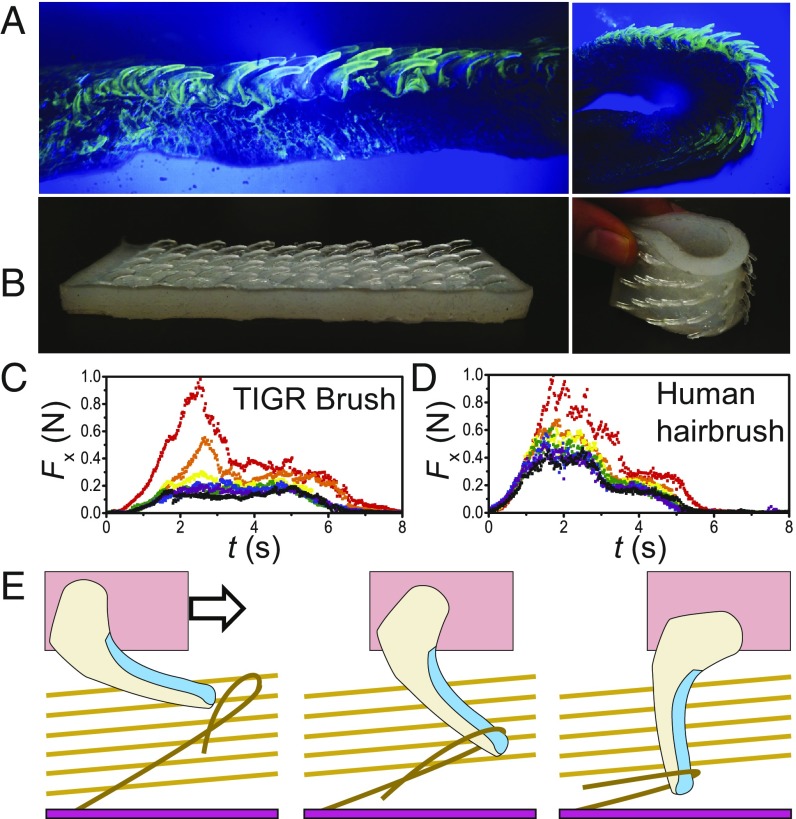
The Tongue-Inspired Grooming Brush.
We designed and built the tongue-inspired grooming (TIGR) brush to measure the forces involved in grooming. Fig. 5A shows a cutaway view of the cat tongue, highlighting the streamlining of the papillae. Fig. 5B shows our device, which was built at 400% scale of the domestic cat tongue by using 3D models of the domestic cat papillae. Note that the flexible substrate allows both the tongue and the brush to conform to curved surfaces.
Fig. 5.
The TIGR brush. (A) Slice of a domestic cat tongue illuminated using UV dye and a black light. (B) A 3D-printed mimic displaying flexibility similar to the cat tongue. (C and D) Artificial grooming data for the 3D-printed mimic (C) and a human hairbrush (D). Each color represents a successive trial in rainbow order (red, orange, yellow, green, blue, purple, black). Force peaks indicate an encountered tangle. (E) Schematic displaying a relaxed, streamlined papilla encountering a tangle (Left), catching on a tangle (Center), and rotating until perpendicular to the tongue (Right).
We pulled the TIGR brush and a human hairbrush through faux nylon fur using our grooming machine. The time course of the forces observed is shown in Fig. 5 C and D, where the colors represent the successive grooms (starting with red and proceeding in order of the colors of the rainbow). During the first three trials of both brushes, grooming force peaked at 0.6–1 N. This is likely due to the brushes encountering tangles within the fur. If a papilla catches on a tangle, it can rotate outward to achieve a height of 9 mm, which increases the applied torque on the tangle (as shown in Fig. 5E). In a real cat tongue, the deformation of the soft tissue causes a papilla to increase exponentially in resistive torque as it rotates outward (30). After just four trials, the cat tongue mimic reached a steady-state grooming force of 0.2 N, less than one-half the steady-state force of the human hairbrush. We surmise that the decreased forces were due to the streamlined posture of the papillae: if a papilla did not encounter a tangle, it remained at a height of 4 mm.
After multiple grooms, both cat and human hairbrushes accumulated hairs. Because the human hairbrush’s bristles are imbedded into a stiff matrix, hairs must be removed with an implement, such as tweezers. The cat tongue brush is much easier to clean, because the papillae are streamlined. We found that a swiping motion along the papillae direction removed nearly all of the trapped fur in a single matted roll, as shown in Movie S4. In cats, hair removal from the tongue might be accomplished by rhythmic motion of the rugullae, the wavy patterns on the roof of the mouth.
Discussion
In this study, we highlighted one function of the papillae, but it is possible that there may be others. Previous studies suggest that papillae shape may help the cat with gripping food during eating (30). The sharp tip may help with tissue deformation and penetration into meat. It may also play a role in stimulating the cat’s own skin during grooming.
As shown in SI Appendix, Table S3, the longest papillae in our study were those of the lion at 2.7 mm or 35% longer than those of a domestic cat. This lion was female, but it is possible that male lions also have longer papillae to groom and wet their manes. In 1871, Charles Darwin (31) first postulated that male lions grow a thick mane around their neck for protection against fights with other males. Since then, it has been shown that the mane acts as a status symbol yet comes with a cost. Male lions endure higher heat stress than the maneless females, especially those with the darkest, most desirable manes (32). Grooming the mane may provide additional cooling effects to the burdened male lions.
Cat owners in the United States are numerous: nearly 35% of households own a cat, despite nearly 6 million Americans being allergic to cats (33). Cat allergies have been linked with the protein Fel d 1, which is highly concentrated within cat saliva, dermis oils, and anal glands. Previous research has shown high concentrations of Fel d 1 in the fur (34, 35). Our study on saliva-filled papillae may shed light on how the protein is spread and how the protein can be selectively cleaned from the cat. Current solutions to ameliorate cat allergies include allergy shots, pills for the pet owner (36), or a daily bath for the cat, painful for both pet and owner (33). Our TIGR brush might be used to distribute cleaning solutions or medications right onto the cat skin, and provide an alternate solution to cat allergies.
Conclusion
The cat tongue is a multifunctional tool, capable of distributing saliva to clean and cool the fur layer. In our study, we found that six species of cats, from domestic cats to lions, possess cavo papillae with hollow cavities that spontaneously wick water. These papillae are necessary to apply saliva to the base of their hairs. Without these papillae, saliva on the tongue’s surface would only wet the top layer of fur, leaving fur underneath untouched. We used theory and experiments with a grooming machine to show how the papillae transfer water to cat fur. While the saliva in the papillae cavities only accounts for 5% of the total fluid on the top of the tongue, saliva deposition near the cat’s skin can provide up to 25% of the needed cooling for a cat’s thermoregulation. Our study culminates with a biologically inspired brush that applies lower force during grooming and is easier to clean than a standard human hairbrush.
Materials and Methods
High-Speed Videography, Kinematics, and Forces During Grooming.
Using a Phantom Miro M110 high-speed camera at 500 frames per second, we filmed an adult short-haired domestic cat grooming its back fur. We also observed seven additional cat species grooming on YouTube videos. Tongue motion in all videos was tracked using Tracker software; kinematic data are tabulated in SI Appendix, Table S1. Additionally, we filmed a domestic cat grooming artificial fur and measured respective grooming forces using an AMTI HE6x6 force plate, the results of which are shown in SI Appendix, Fig. S1.
Tongue and Papilla Micro-CT Visualization.
We measured tongue dimensions by hand for domestic cat, bobcat, cougar, snow leopard, tiger, and lion tongues and report these values in SI Appendix, Table S2. We separately CT scanned an entire domestic cat tongue and cavo papillae from six cat species using a Scanco Micro-CT50 at 45 kVp and 200 A. We measured the cavity width, height, and volume from the 3D scan using Blender software and tabulate the data in SI Appendix, Table S3.
Young’s Modulus of Tissue and Papilla.
Using a TA Instruments ElectroForce 3100 microindentation machine, we measured the Young’s modulus of the underside of the domestic cat tongue using an aluminum flat-ended cylindrical indenter of diameter of 2 mm. The Young’s modulus of a cavo papilla was measured using a Hysitron TriboIndenter.
Measuring Cat Fur Properties.
We measured the diameter and length of down hairs for nine cat species and fur density for two cat species using a portable Andonstar A1 USB microscope, and we tabulate data in SI Appendix, Table S4. Additional fur density and length values were gathered from literature (23–25).
Grooming Machine.
We designed and constructed a “grooming machine” that is able to pull a tongue across a sample of fur and measure respective grooming forces (SI Appendix, Fig. S2). An encoded motor (12V 25D-mm gear motor from Pololu.com), controlled by an Arduino microcontroller, drives a rack and pinion horizontally. The frame was constructed of 80/20 T-slotted aluminum. To measure grooming forces, we used an AMTI HE6x6 force plate, with 2.2-N capacity in the x and y directions and 4.4-N capacity in the z direction (into the plate).
Measurement of Fluid Transferred from Cat Grooming.
Using the grooming machine, we simulated a grooming lick by pulling a severed, wetted cat tongue through a sample of cat fur at grooming speed and grooming force of 0.1 N. We measured the change in weight of the wetted tongue to determine fluid transferred to the fur, accounting for evaporation.
Ethics.
This study was approved by the Office of Research Integrity Assurance and conducted in accordance with all protocols filed under the Georgia Tech Institutional Animal Care and Use Committee. All tongue tissue samples were donated post mortem.
Supplementary Material
Acknowledgments
We thank National Science Foundation Grants DGE-1650044 and PHY-1255127 for funding theoretical work and the Woodruff Faculty Fellowship for funding experimental work.
Footnotes
Conflict of interest statement: The authors have filed a provisional patent of the technology described in the article.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1809544115/-/DCSupplemental.
References
- 1.O’Brien SJ, et al. State of cat genomics. Trends Genet. 2008;24:268–279. doi: 10.1016/j.tig.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaver BV. Feline Behavior. Elsevier Health Sciences; St. Louis: 2003. [Google Scholar]
- 3.Hsu MH, Hsu TC, Wu WJ. Distribution of cat fleas (siphonaptera: Pulicidae) on the cat. J Med Entomol. 2002;39:685–688. doi: 10.1603/0022-2585-39.4.685. [DOI] [PubMed] [Google Scholar]
- 4.Amador GJ, Hu DL. Cleanliness is next to godliness: Mechanisms for staying clean. J Exp Biol. 2015;218:3164–3174. doi: 10.1242/jeb.103937. [DOI] [PubMed] [Google Scholar]
- 5.Gebremedhin KG, Wu B. A model of evaporative cooling of wet skin surface and fur layer. J Therm Biol. 2001;26:537–545. [Google Scholar]
- 6.Miller WH, Griffin CE, Campbell KL, Muller GH. Muller and Kirk’s Small Animal Dermatology. 7th Ed Elsevier Health Sciences; St. Louis: 2013. [Google Scholar]
- 7.Boshel J, Wilborn W, Singh B. Filiform papillae of cat tongue. Cells Tissues Organs. 1982;114:97–105. doi: 10.1159/000145583. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki S. Surface structure and keratinization of the mucosal epithelium of the domestic cat tongue. J Mammalogical Soc Jpn. 1990;15:1–13. [Google Scholar]
- 9.Ojima K. Quantitative and distributive study of the fungiform papillae in the cat tongue in microvascular cast specimens. Ann Anat-Anat Anz. 1998;180:409–414. doi: 10.1016/S0940-9602(98)80101-5. [DOI] [PubMed] [Google Scholar]
- 10.Prakash M, Quéré D, Bush JW. Surface tension transport of prey by feeding shorebirds: The capillary ratchet. Science. 2008;320:931–934. doi: 10.1126/science.1156023. [DOI] [PubMed] [Google Scholar]
- 11.Hu DL, Chan B, Bush JW. The hydrodynamics of water strider locomotion. Nature. 2003;424:663–666. doi: 10.1038/nature01793. [DOI] [PubMed] [Google Scholar]
- 12.Koh JS, et al. Jumping on water: Surface tension–dominated jumping of water striders and robotic insects. Science. 2015;349:517–521. doi: 10.1126/science.aab1637. [DOI] [PubMed] [Google Scholar]
- 13.Reis PM, Jung S, Aristoff JM, Stocker R. How cats lap: Water uptake by felis catus. Science. 2010;330:1231–1234. doi: 10.1126/science.1195421. [DOI] [PubMed] [Google Scholar]
- 14.Gart S, Socha JJ, Vlachos PP, Jung S. Dogs lap using acceleration-driven open pumping. Proc Natl Acad Sci USA. 2015;112:15798–15802. doi: 10.1073/pnas.1514842112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenner WR. Quick Reference to Veterinary Medicine. Wiley-Blackwell; Hoboken, NJ: 1991. [Google Scholar]
- 16.Hainsworth F. Saliva spreading, activity, and body temperature regulation in the rat. Am J Physiol–Legacy Content. 1967;212:1288–1292. doi: 10.1152/ajplegacy.1967.212.6.1288. [DOI] [PubMed] [Google Scholar]
- 17.Dawson TJ, Robertshaw D, Taylor CR. Sweating in the kangaroo: A cooling mechanism during exercise, but not in the heat. Am J Physiol. 1974;227:494–498. doi: 10.1152/ajplegacy.1974.227.2.494. [DOI] [PubMed] [Google Scholar]
- 18.Kimmel E, Arkin H, Broday D, Berman A. A model of evaporative cooling in a wetted hide. J Agric Eng Res. 1991;49:227–241. [Google Scholar]
- 19.Hart B. Feline Practice. Veterinary Practice Publishing Co.; Santa Barbara, CA: 1976. Feline behavior: The role of grooming activity; pp. 14–16. [Google Scholar]
- 20.Sakamoto M, Ruta M. Convergence and divergence in the evolution of cat skulls: Temporal and spatial patterns of morphological diversity. PLoS One. 2012;7:e39752. doi: 10.1371/journal.pone.0039752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farren L, Shayler S, Ennos A. The fracture properties and mechanical design of human fingernails. J Exp Biol. 2004;207:735–741. doi: 10.1242/jeb.00814. [DOI] [PubMed] [Google Scholar]
- 22.Washburn EW. The dynamics of capillary flow. Phys Rev. 1921;17:273–283. [Google Scholar]
- 23.Searle A, Jude A. The rex type of coat in the domestic cat. J Genet. 1956;54:506–512. [Google Scholar]
- 24.Kitchener AC, Van Valkenburgh B, Yamaguchi N. Felid form and function. In: MacDonald DW, Loveridge AJ, editors. Biology Conservation Wild Felids. Oxford Univ Press; Oxford: 2010. pp. 83–106. [Google Scholar]
- 25.Sato H, Matsuda H, Kubota S, Kawano K. Statistical comparison of dog and cat guard hairs using numerical morphology. Forensic Sci Int. 2006;158:94–103. doi: 10.1016/j.forsciint.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 26.VCA Hospitals 2018 Persian. Available at https://vcahospitals.com/know-your-pet/cat-breeds/persian. Accessed October 31, 2018.
- 27.Earle K, Smith P. Digestible energy requirements of adult cats at maintenance. J Nutr. 1991;121:S45–S46. doi: 10.1093/jn/121.suppl_11.S45. [DOI] [PubMed] [Google Scholar]
- 28.White CR, Seymour RS. Mammalian basal metabolic rate is proportional to body mass2/3. Proc Natl Acad Sci USA. 2003;100:4046–4049. doi: 10.1073/pnas.0436428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe S, Izawa M, Kato A, Ropert-Coudert Y, Naito Y. A new technique for monitoring the detailed behaviour of terrestrial animals: A case study with the domestic cat. Appl Anim Behav Sci. 2005;94:117–131. [Google Scholar]
- 30.Noel AC, Hu DL. The tongue as a gripper. J Exp Biol. 2018;221:jeb176289. doi: 10.1242/jeb.176289. [DOI] [PubMed] [Google Scholar]
- 31.Darwin C. The Descent of Man and Selection in Relation to Sex. Murray; London: 1871. [Google Scholar]
- 32.West PM. The lion’s mane. Am Sci. 2005;93:226–235. [Google Scholar]
- 33.Avner DB, Perzanowski MS, Platts-Mills TA, Woodfolk JA. Evaluation of different techniques for washing cats: Quantitation of allergen removed from the cat and the effect on airborne fel d 1. J Allergy Clin Immunol. 1997;100:307–312. doi: 10.1016/s0091-6749(97)70242-2. [DOI] [PubMed] [Google Scholar]
- 34.Carayol N, et al. Fel d 1 production in the cat skin varies according to anatomical sites. Allergy. 2000;55:570–573. doi: 10.1034/j.1398-9995.2000.00588.x. [DOI] [PubMed] [Google Scholar]
- 35.Charpin C, et al. Fel d i allergen distribution in cat Fur and skin. J Allergy Clin Immunol. 1991;88:77–82. doi: 10.1016/0091-6749(91)90303-6. [DOI] [PubMed] [Google Scholar]
- 36.Zhu D, et al. A chimeric human-cat fusion protein blocks cat-induced allergy. Nat Med. 2005;11:446. doi: 10.1038/nm1219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.