Significance
Urban environments are expanding worldwide, impacting the populations of many organisms. Understanding how and why species are affected by urbanization is thus an important goal. We examined the role of direct competitive interactions among species on the response of bird species to urbanization. We found evidence that urban-adapted, subordinate species were less widespread in cities than closely related dominant species, but only when dominant and subordinate species live together, which suggests that direct competitive interactions reduce the ability of subordinate species to persist in cities. This result depended on the level of economic development of the country, suggesting that economic development may heighten the effects of competition on subordinate species, thereby reducing species diversity in cities.
Keywords: urbanization, interference competition, behavioral dominance, urban adaptation, economic development
Abstract
Urbanization represents an extreme transformation of more natural systems. Populations of most species decline or disappear with urbanization, and yet some species persist and even thrive in cities. What determines which species persist or thrive in urban habitats? Direct competitive interactions among species can influence their distributions and resource use, particularly along gradients of environmental challenge. Given the challenges of urbanization, similar interactions may be important for determining which species persist or thrive in cities; however, their role remains poorly understood. Here, we use a global dataset to test among three alternative hypotheses for how direct competitive interactions and behavioral dominance may influence the breeding occurrence of birds in cities. We find evidence to support the competitive interference hypothesis: behaviorally dominant species were more widespread in urban habitats than closely related subordinate species, but only in taxa that thrive in urban environments (hereafter, urban adapted), and only when dominant and subordinate species overlapped their geographic ranges. This result was evident across diverse phylogenetic groups but varied significantly with a country’s level of economic development. Urban-adapted, dominant species were more widespread than closely related subordinate species in cities in developed, but not developing, countries; countries in economic transition showed an intermediate pattern. Our results provide evidence that competitive interactions broadly influence species responses to urbanization, and that these interactions have asymmetric effects on subordinate species that otherwise could be widespread in urban environments. Results further suggest that economic development might accentuate the consequences of competitive interactions, thereby reducing local diversity in cities.
More than one-half of the people on Earth now live in a city (1); the rapid conversion of relatively natural habitats to urban habitats and the desire to mitigate the subsequent loss of species has fueled the question: How can some species persist—and occasionally flourish—in the face of urbanization, while most do not? Some previous studies have found urban species to have larger brains, broader environmental tolerance, altered endocrine responses, and increased behavioral flexibility, all differences that are thought to be adaptive (2–6). The influence of competitive interactions among species on the differential response of species to urbanization has received less attention, despite recent work suggesting that direct competitive and aggressive interactions (interference competition) among species may have an underappreciated importance in nature (7–9), and evidence suggesting that competitive ability may covary with boldness and environmental tolerance (10, 11)—both traits thought to be important for adaptation to urban environments (3, 6, 12).
Competitive interactions among pairs of species are usually asymmetric, with one consistently dominant species and one consistently subordinate species (13, 14). In animals, these interactions often involve direct aggression among species (7–9), with dominant species aggressively displacing subordinates from shared resources such as food, roost sites, breeding sites, or habitat (7, 13, 14). Many other traits covary with behavioral dominance (11, 15), and thus we might expect competitive interactions to influence the fate of species confronting urbanization either directly (e.g., through competitive exclusion from a resource) or indirectly through other covarying traits (e.g., boldness or aggression) that are adaptive in urban environments (6, 12). The influence of behavioral dominance on the occurrence or distribution of species in cities, however, is likely to depend on the degree to which urban habitats represent an opportunity for species, or, more commonly, a challenge. We propose three alternative hypotheses to describe how behavioral dominance might directly or indirectly influence the occurrence and distribution of species in cities.
H1, Subordinate Tolerance Hypothesis
Urban habitats are novel, challenging environments for most organisms because they represent a recent and dramatic shift from historical habitats within which species have evolved (16, 17). For example, urban environments have greater human disturbance, high-density predator populations (e.g., mesopredators), increased artificial light, higher levels of pollution and toxins, and fewer areas of relatively natural habitat that provide the resources that most species require (18, 19). When confronted with these challenges, most species decline in abundance with increasing urbanization, and many species do not persist in cities (20, 21). Among the species that do persist in cities, we might expect subordinate species to prevail. Subordinate species are often excluded from preferred resources and habitats and, thus, may be better adapted to challenging conditions than dominant species [e.g., low resource, high predation, or disturbed environments (10)]. These adaptations might allow subordinates to persist in the face of the challenges associated with urbanization when dominant species cannot. Subordinate species may also be relegated to urban habitat by dominant species that are unlikely to preferentially occupy areas of low-quality habitat, such as cities.
H2, Competitive Interference Hypothesis
Despite the challenges presented by urban environments, some organisms thrive in cities (refs. 20 and 21; hereafter, referred to as urban adapted). These species take advantage of the many resources available in urban habitats, including excesses of human food waste (22), the fruits and flowers of ornamental plants (21), and the protection that cities provide from other species that cannot persist in urban environments, such as large predators (23). When closely related species are able to take advantage of urban resources in this way (i.e., cities provide more opportunities than challenges), we may expect behaviorally dominant species to monopolize urban habitats (10, 12, 24), excluding or reducing the abundance of ecologically similar, subordinate species that might otherwise prosper in cities. These competitive interactions would reduce the occurrence of subordinate species in cities, but only where dominant species co-occur (sympatry). Additionally, historical interactions between urban-adapted dominant and subordinate species may have led to the ecological divergence of subordinate populations or species in sympatry (15) (through evolution or ecological sorting) that reduces their ability to prosper in urban environments. Such historical competitive interactions could lead to patterns similar to those caused by ongoing competitive exclusion, where urban-adapted dominants thrive in urban habitats while closely related subordinates do not.
H3, Dominant Advantage Hypothesis
Another alternative exists: The challenges of cities may favor specific traits possessed by behaviorally dominant, but not subordinate, species. The aggression involved in behavioral dominance is closely linked to other traits such as boldness and tolerance of disturbance—traits that may provide important fitness benefits to the challenges of city life (11, 12). If these adaptations predispose dominant species to tolerate challenges of urban environments (e.g., human disturbance) or allow them to take advantage of the novel resources that urban environments provide (e.g., unconventional foods or breeding sites), then we might expect behaviorally dominant species to prevail in cities (12). In such cases, adaptations that covary with dominance might help dominant species to overcome specific challenges of cities and create resource opportunities unavailable to subordinate species (12).
Tests of Hypotheses
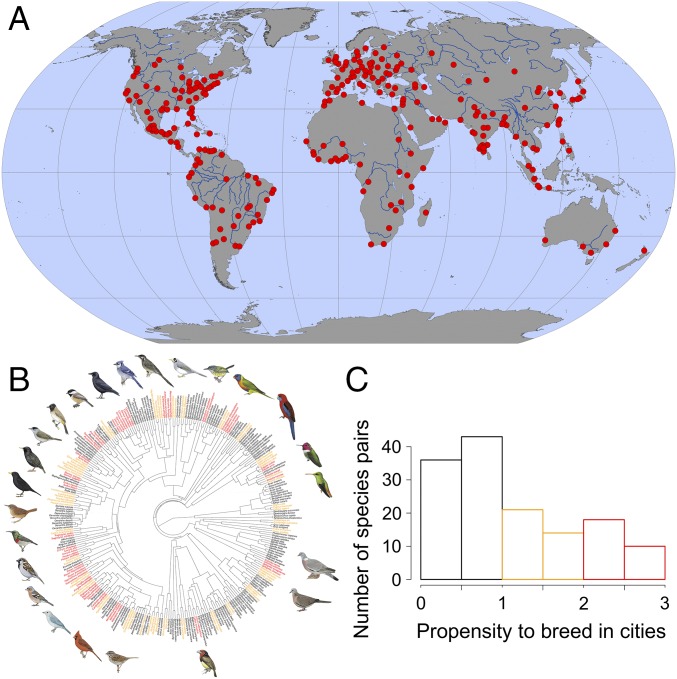
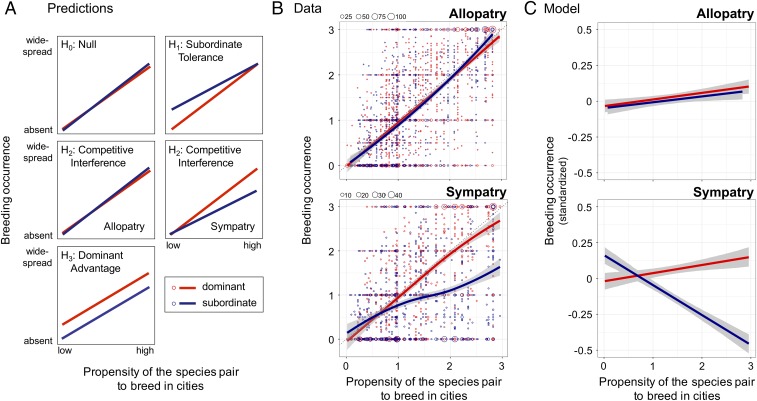
Here, we test among these alternative hypotheses to determine how behavioral dominance might directly or indirectly influence the occurrence of species in urban environments. To do this, we surveyed the breeding birds of large cities from around the world (Fig. 1A and SI Appendix), focusing on species for which dominance relationships among closely related species (congeners) had been documented in previously published work. Once we assembled our list of focal species (Fig. 1B and SI Appendix), we contacted birders and ornithologists from around the world who knew the breeding birds of large cities and asked them to characterize the breeding occurrence of each focal species in urban habitats within the city as follows: absent, a local breeder in just a few locations within the city, between a local and widespread breeder, or a widespread breeder across the city. We used these scores to estimate the propensity for species pairs (i.e., a dominant species paired with its subordinate congener) to breed in cities (Fig. 1C; one value for each species pair), which we use as an index of the degree to which cities represent an opportunity or a challenge—a critical component of the diagnostic predictions of the hypotheses that we tested (Fig. 2A). We used this index, coupled with survey data, to test our null and three alternative hypotheses that predict different patterns of breeding occurrence for dominant and subordinate species within a species pair as a function of their shared propensity to breed in cities (Fig. 2A). Our analysis calculated mean breeding occurrence values across observers for each species in each city, weighted by the observer’s self-reported ability, and thus our response variable ranged continuously between 0 (absent) and 3 (widespread; Fig. 2B).
Fig. 1.
Sampling distributions. (A) Geographic distribution of the cities (red dots) for which we obtained bird breeding occurrence data (n = 260 cities). (B) Phylogenetic distribution of focal species (n = 296 species, representing 142 phylogenetically independent comparisons of dominant and subordinate congeners from 66 taxonomic families of birds). Colors correspond to different propensities of species pairs to breed in cities in C, with red indicating higher propensities (values ≥2), illustrated with examples. Bird illustrations reproduced with permission from del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E (eds) (2018) Handbook of the Birds of the World Alive (Lynx Edicions, Barcelona) (retrieved from www.hbw.com/ on January 2018). (C) Distribution of focal species pairs relative to their propensities for breeding in cities. The propensity to breed in cities was calculated for each paired dominant and subordinate species (one value for each pair) as the maximum breeding occurrence within a species pair for each city, averaged across all focal cities that overlapped their breeding ranges. Thus, a species pair that barely persists in cities would have a score close to 0, while a pair that is widespread in cities would have a score close to 3. Species pairs with a value of 0 (absent from all focal cities across their breeding distributions) were omitted from this study.
Fig. 2.
Predictions and test of hypotheses. (A) Predictions. Three alternative hypotheses and the null predict distinct patterns of breeding occurrence of birds in urban habitats. Lines show predicted patterns for dominant (red) and subordinate (blue) species. For H2, we distinguished between cities that occurred within (sympatry) or outside of (allopatry) areas of range overlap. (B) Data. In allopatry, breeding occurrence values of dominant and subordinate congeners were similar; in sympatry, dominant species were more widespread than subordinate congeners when species pairs had a high propensity to breed in cities. Each point in the figures represents the breeding occurrence of one species in one city (allopatry, n = 3,425; sympatry, n = 2,193); point size reflects the number of overlapping points (see legend at Top Left of graphs). Solid lines (red, dominants; blue, subordinates) are loess splines (span = 1.5) with 95% confidence limits shown in gray. Breeding occurrence values are means for each species in each city (averaged across observers, weighted by observer ability), and range from 0 (absent from urban habitats) to 3 (widespread breeder in urban habitats). Propensity to breed in cities was calculated for each paired dominant and subordinate species as the maximum breeding occurrence within a species pair for each city, averaged across all focal cities that overlapped their breeding ranges (one value per species pair; same value for sympatry and allopatry). (C) Model results. In allopatry, breeding occurrence values of dominant and subordinate congeners did not differ [Bayesian generalized linear mixed model (MCMCglmm), difference in slopes, PMCMC = 0.80]. In sympatry, dominant species were more widespread than subordinate congeners when species pairs had a high propensity to breed in cities (MCMCglmm, difference in slopes, PMCMC < 0.0001). Solid lines (red, dominants; blue, subordinates) are model-predicted values with 95% confidence limits in gray. Slopes in C are flattened relative to slopes in B because statistical models in C incorporated standardized breeding occurrence values (y axes) = [breeding occurrence value − mean(breeding occurrence for the species pair)]/[2 × SD(breeding occurrence for the species pair)]. See B for definition of propensity to breed in cities (x axis).
The processes that structure species assemblages, such as competition, are often thought to be context dependent, varying across geographic locales, species assemblages, and taxonomic groups (25, 26). Upon finding evidence consistent with the competitive interference hypothesis, we tested whether our main result reflected a general pattern across evolutionary lineages of birds around the world (SI Appendix, Tables S1 and S2). We found striking variation in our main effects across continents, with Europe, North America, and Australia showing evidence consistent with, and Africa, South America, and Asia showing evidence inconsistent with, the competitive interference hypothesis. Continent identity is unlikely to influence the outcome of species interactions itself; thus, we tested the predictions of all plausible alternative hypotheses that we could think of to explain the continental variation that we had found. In total, we tested 12 alternative hypotheses that could potentially explain this geographic variation, including variation in latitude, climate, economic development, human population, phylogeny, and sampling (SI Appendix, Tables S3 and S4).
Results
We found patterns consistent with both the competitive interference hypothesis and the subordinate tolerance hypothesis acting through a mechanism of dominance interactions; our results were inconsistent with the dominant advantage and null hypotheses (Fig. 2). Dominant and subordinate species were equally likely to occur in cities where their ranges did not overlap [Fig. 2 B and C, allopatry; Bayesian generalized linear mixed model (MCMCglmm), difference in slopes (subordinates relative to dominants) in allopatry, estimate = −0.0087; 95% CI: −0.075, +0.057; PMCMC = 0.80], suggesting that dominant and subordinate species do not consistently differ in their ability to persist or thrive in urban environments when the focal species do not have an opportunity to interact. In cities where their ranges did overlap (sympatry), however, dominant species were more widespread as breeders in urban habitats than closely related subordinate species, but only in species pairs that had a high propensity to breed in urban environments [Fig. 2 B and C, sympatry; MCMCglmm, difference in slopes (subordinates relative to dominants) in sympatry, estimate = −0.42; 95% CI: −0.50, −0.33; PMCMC < 0.0001; SI Appendix, Table S1; subordinate occurrence relative to dominant at highest level of breeding propensity, estimate (sympatry) = −0.60; 95% CI: −0.69, −0.51; PMCMC < 0.0001]. The reduced prevalence of urban-adapted subordinate species when sympatric with dominants was evident when we reanalyzed our data as bivariate (present versus absent) (SI Appendix, Fig. S1) and with all zero occurrence values removed (SI Appendix, Fig. S2), and persisted after controlling for spatial autocorrelation [SI Appendix, Table S5; generalized least-squares model (gls); subordinate occurrence relative to dominant at highest level of breeding propensity, estimate (sympatry) = −0.43; 95% CI: −0.57, −0.29; P < 0.0001]. In cities where their ranges overlapped, subordinate species were more widespread in urban habitats than closely related dominant species in species pairs that had a very low propensity to breed in urban environments (Fig. 2C, sympatry; MCMCglmm; subordinate occurrence relative to dominant at lowest level of breeding propensity, estimate = +0.18; 95% CI: +0.094, +0.26; PMCMC < 0.0001); this pattern was weaker after controlling for spatial autocorrelation (gls; subordinates relative to dominants at lowest level of breeding propensity, estimate = +0.11; 95% CI: −0.0068, +0.23; P = 0.06).
Phylogeny.
Incorporating phylogeny into our analysis yielded similar results (SI Appendix, Table S1). Dominant and subordinate species were equally likely to occur in cities where their ranges did not overlap [MCMCglmm; difference in slopes (subordinates relative to dominants) in allopatry, estimate = −0.010; 95% CI: −0.075, +0.057; PMCMC = 0.77]. In sympatry, however, urban-adapted dominant species were more widespread as breeders in urban habitats than closely related subordinate species [MCMCglmm, difference in slopes (subordinates relative to dominants) in sympatry, estimate = −0.42; 95% CI: −0.50, −0.34; PMCMC < 0.0001; SI Appendix, Table S1; subordinate occurrence relative to dominant at highest level of breeding propensity (sympatry), estimate = −0.60; 95% CI: −0.69, −0.51; PMCMC < 0.0001]. For urban-challenged species pairs (i.e., species pairs that occupied few cities within their geographic ranges), subordinate species were again more widespread in sympatry than closely related dominant species [MCMCglmm, subordinate occurrence relative to dominant at lowest level of breeding propensity (sympatry), estimate = +0.18; 95% CI: +0.094, +0.26; PMCMC < 0.0001]. The similarity of results between models that incorporated phylogeny and those that did not suggests that our main results were not driven by differences in breeding occurrences in one or a few lineages, but instead reflect a broader pattern across the evolutionary lineages in our study. Nonetheless, variation among phylogenetic groups was significant in our models [MCMCglmm, effect of phylogeny (random effect), estimate = +0.00081; 95% CI: +0.00015, +0.0019].
Geography.
Incorporating continent into our statistical models revealed significant variation in our main result across continents (SI Appendix, Table S2). The relationships between the propensity for species pairs to breed in cities and dominance were qualitatively similar across Australia, Europe, and North America (SI Appendix, Figs. S3 and S4), where subordinate breeding occurrence values were lower in sympatry for species pairs with higher propensities to breed in cities (SI Appendix, Fig. S4 and Table S2). In contrast, dominant and subordinate species showed no striking differences in breeding occurrence across Africa, Asia, or South America in sympatry or allopatry (SI Appendix, Figs. S3 and S4 and Table S2). For urban-challenged species, only Europe showed differences between subordinate and dominant occurrence when species were sympatric, with urban-challenged subordinate species more widespread than closely related dominant species (nonoverlapping 95% confidence limits; SI Appendix, Fig. S4B).
Alternative Hypotheses to Explain Geographic Variation.
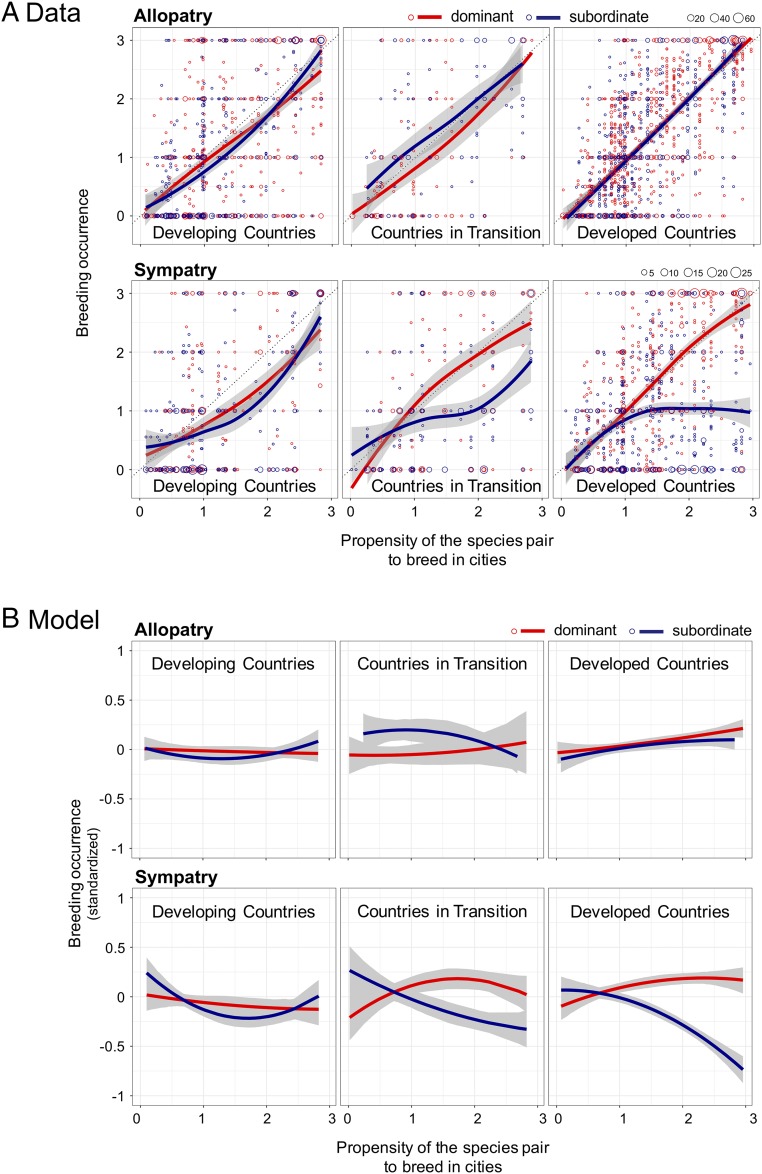
Of the 12 alternative hypotheses that we tested, variation in a country’s level of economic development (27) was the only factor that improved model fit over continent (SI Appendix, Tables S3 and S4) and remained important overall, and in an interaction with our main effects, after controlling for spatial autocorrelation (SI Appendix, Table S5). Dominant, urban-adapted species were more widespread than their subordinate congeners in sympatry, but not allopatry, when cities were in economically developed countries [Fig. 3 and SI Appendix, Table S4; MCMCglmm, difference in linear slopes (subordinates relative to dominants) in sympatry, estimate = −22.01; 95% CI: −26.19, −17.61; PMCMC < 0.0001; allopatry, estimate = −1.04; 95% CI: −4.62, +2.45; PMCMC = 0.56]; the pattern was absent from cities in economically developing countries [Fig. 3 and SI Appendix, Table S4; MCMCglmm, difference in linear slopes (subordinates relative to dominants) in sympatry, estimate = −0.40; 95% CI: −6.18, +5.47; PMCMC = 0.89; allopatry, estimate = +3.23; 95% CI: −0.64, +7.12; PMCMC = 0.10]. Cities in countries with economies in transition between developing and developed showed an intermediate pattern between the two [Fig. 3 and SI Appendix, Table S4; MCMCglmm, difference in linear slopes (subordinates relative to dominants) in sympatry, estimate = −14.78; 95% CI: −22.00, −7.63; PMCMC < 0.0001; allopatry, estimate = −9.42; 95% CI: −18.89, −0.002; PMCMC = 0.051].
Fig. 3.
Variation in the urban-breeding occurrence of dominant and subordinate bird species across levels of economic development. (A) Data. Top shows data for allopatry (i.e., cities where dominant and subordinate congeners did not overlap their breeding ranges); Bottom shows data for sympatry (i.e., cities where dominant and subordinate congeners overlapped breeding ranges). Economic development categorization follows country-level designations of the United Nations from 2014 [economically developed countries, countries in transition, and economically developing countries (27)]. Solid lines (red, dominant species; blue, subordinate species) are loess splines (span = 1.5) with 95% confidence limits shown in gray. Point size reflects the number of overlapping points (see legend at Top Right of graphs). Sample sizes: allopatry, developed, n = 1,803; in transition, n = 303; developing, n = 1,329; sympatry, developed, n = 1,184; in transition, n = 370; developing, n = 639. See Fig. 2 caption for definitions of axes. (B) Model results. In allopatry, slopes for dominant and subordinate species did not differ in developed, in transition, or developing countries (MCMCglmm, difference in linear slopes, PMCMC > 0.05; see SI Appendix, Table S4 for quadratic terms). In sympatry, linear slopes for dominant and subordinate species differed in developed (PMCMC < 0.0001) and in transition countries (PMCMC < 0.0001), but not developing countries (PMCMC > 0.10). Solid lines (red, dominant species; blue, subordinate species) are model predicted values with 95% confidence limits shown in gray. See A for definition of axes and additional details. Slopes in B are flattened relative to slopes in A because statistical models in B incorporated standardized breeding occurrence values (y axes) = [breeding occurrence value − mean(breeding occurrence for the species pair)]/[2 × SD(breeding occurrence for the species pair)].
Discussion
We tested the predictions of three alternative hypotheses and a null for how behavioral dominance and interference competition might directly or indirectly influence the response of bird species to urbanization (Fig. 2A). We found evidence consistent with the competitive interference hypothesis, where behaviorally dominant bird species were more widespread than closely related subordinate species, but only when the species overlapped their geographic ranges, and only when the species pairs were prevalent in urban environments (urban adapted) (Fig. 2). These patterns could result from direct competitive exclusion, from divergent evolution of sympatric, subordinate species that reduces their ability to occupy urban habitats, or both.
The reduced occurrence of urban-adapted, subordinate species in sympatry (Fig. 2) is consistent with a mechanism of asymmetric, interference competition, and inconsistent with alternative hypotheses for interactions among species. Exploitative competition and interactions mediated by shared predators and parasites are all indirect interactions that do not provide a consistent advantage to dominant over subordinate species, and thus do not predict subordinates to become less common in sympatry. Indeed, subordinate species can outcompete dominant species in exploitative competition for food [European tits, Paridae (28)], while the costs of indirect interactions through predation can impact dominant and subordinate species similarly [wood warblers, Parulidae (29)]. In both of these examples, and others (14, 30), however, direct (interference) competition between the species favored the behavioral dominant (28, 29).
Experiments would have been an ideal way to provide direct evidence for a causal role of competition, but repeated experiments across species and cities were not possible in this study. Our approach instead used comparative analyses that allowed spatial and phylogenetic breadth (Fig. 1) that experiments could not achieve. The results are compelling because subordinates are less common in urban habitats only when they co-occur with closely related dominants in sympatry—a pattern that provides strong evidence that interactions between the species underlie the difference in occurrences between dominant and subordinate congeners. These patterns are also repeated across diverse groups and geographic locations; other factors that might correlate with sympatry in one species pair (e.g., habitat differences) are unlikely to explain the repeated patterns across such a disparate and diverse array of species and cities.
Experimental evidence from a diverse array of species, however, has documented socially dominant species impacting the distribution of subordinate species in nature, which is the population-level parameter of direct relevance to our study. For example, removal experiments have shown that dominant species exclude subordinates from preferred habitats (i.e., the population of subordinates in those habitats declines or becomes zero when the dominant is present) [e.g., chipmunks (31), crayfish (32), fish (33, 34), salamanders (35, 36)]. No studies have extended similar experimental work to birds in urban habitats; however, intensive studies of Old World sparrows (Passer) suggest that behaviorally dominant house sparrows (Passer domesticus) limit population expansion of Eurasian tree sparrows (Passer montanus) into suburban and urban habitats by restricting their access to nest sites through aggression (37–39). An alternative mechanism, whereby dominant species might restrict subordinate species from preferred habitats outside of cities, with a secondary effect on their distribution within cities (e.g., reducing the ability of subordinate species to colonize urban habitats), could also contribute to the patterns that we found.
Subordinate Tolerance Hypothesis.
The subordinate tolerance hypothesis suggests that subordinate species are better adapted to challenging conditions than dominant species, including challenging conditions associated with urbanization. This hypothesis predicts that subordinate species will be more widespread in urban habitats than dominant species for urban-challenged species pairs, regardless of allopatry or sympatry (Fig. 2A)—predictions that were inconsistent with our main results (Fig. 2 B and C). An alternative mechanism for this hypothesis suggests that dominant species may actively relegate closely related subordinate species to less-preferred urban habitats. This mechanism predicts subordinate species to be more widespread in urban habitats than closely related dominant species, but only when the species are sympatric—a prediction consistent with our model results in sympatry (Fig. 2C). These results were geographically variable (SI Appendix, Fig. S4), and weak after controlling for spatial autocorrelation, leaving us unsure if these differences reflect a general pattern. Nonetheless, given our data, we cannot presently reject the subordinate tolerance hypothesis acting through direct competitive interactions among species with a low propensity to breed in cities.
Economic Development.
The ability of economic development to explain variation in our main effects, and the intermediate pattern evident in countries in transition between developing and developed economies (Fig. 3), suggests that economic development directly or indirectly influences the outcome of dominance interactions among urban-adapted species when they live in sympatry. This result is important because it suggests that economic development—a goal of most countries (27)—exacerbates the consequences of competition among closely related species of birds, reducing the breeding occurrence and diversity of subordinate species in cities (SI Appendix, Figs. S1 and S2).
Several factors that vary with economic development could influence the interactions between dominant and subordinate species in cities. Economically developed cities are characterized by spatial and temporal clumping of resources, such as excess food and waste that is highly managed and concentrated in dumpsters, landfills, and waste treatment facilities (40). This resource clumping may facilitate the monopolizing of preferred resources by dominant species at the expense of subordinate species (41). A second possibility is that the reduced control of resources (including habitat) in cities in developing countries (42) may create greater resource breadth or structural complexity that provide resource opportunities for subordinate species that dominant species avoid, allowing subordinates to persist in urban environments when closely related dominants are present. A third, and nonexclusive, possibility is that mortality rates (e.g., from predation, disease) could be higher for birds in cities in developing nations, reducing population sizes of dominant species and thwarting competitive exclusion of subordinate species from urban habitats (43). The variation in our main effects with economic development might also reflect a correlation with other causal factors that we have not yet identified.
Conclusions.
We found evidence that direct, competitive interactions broadly influence the occurrence of urban-adapted species in cities. This result supports recent evidence that interspecific aggression and direct (interference) competition play an important role in resource partitioning and community structure across diverse species (7), and species responses to habitat degradation are mediated by competitive interactions (44). Our results also suggest that the effects of competitive interactions are asymmetric, with subordinate, urban-adapted species excluded from urban habitats either proximately (e.g., through aggression) or through divergent evolution or ecological sorting in sympatric populations. Finally, if the patterns with economic development are causal, then our results further suggest that economic development can intensify the consequences of species interactions, reducing the occurrence of subordinate species in cities.
Materials and Methods
Selection of Cities.
We accessed human population size data for the world’s cities from the United Nations (data.un.org/) for the year 2015 (or most recently available data; accessed June 2016) and identified world cities with a population size greater than 750,000 people. We used this arbitrary cutoff to identify urban centers that would challenge many species of birds and that would also provide broad coverage globally with multiple cities across the ranges of most of our focal species. For countries that were omitted from the United Nations dataset (e.g., Syria, Madagascar, Morocco), we referenced data online for cities and population sizes (www.geonames.org/) and added cities to our dataset (uploaded to Dryad) if their reported population exceeded 750,000 people.
To increase the spatial independence of data, we removed cities with a centroid latitude and longitude within 100 km of another city with a larger human population. We first measured the distances between the centroids of all cities with more than 750,000 people using the earth.dist function in the R package fossil (45). For cities within 100 km of each other, we retained the city with the larger population size and removed the smaller city or cities from further analysis. After applying these filters, we had a final list of 492 focal cities.
Selection of Species.
We systematically compiled a dataset composed of all of the species pairs for which we could find dominance data for closely related species of birds within the same genus (following the taxonomy of ref. 46). This compilation involved an exhaustive search of recent reviews (14, 15, 30), regional ornithological references, and other published observations [see SI Appendix and dataset (doi: 10.5061/dryad.t85bf04) for details]. Behaviorally dominant species were defined as those that won the majority of aggressive contests with subordinate species [where majority was defined by a significant (P < 0.05) binomial test for asymmetry], or those that were described as behaviorally dominant over the subordinate species in the literature and where data were not accessible for statistical testing (15). Aggressive interactions included chases, supplantings, displacements, kleptoparasitism, and physical fights (following ref. 15) and were usually associated with a contest over a specific resource (e.g., food). We provide a full list of our focal species and their dominance relationships in SI Appendix, and references for dominance relationships in our dataset.
For our final dataset, we selected only the youngest, phylogenetically independent species pairs for which we had dominance data, provided that one of them was recorded breeding in urban habitats in one of our focal cities (i.e., the breeding occurrence of one of the species was >0). Species pairs where neither species was recorded as breeding in urban habitats in our focal cities were excluded from the analysis. We selected only the youngest species pairs because these are more likely to share ecological traits through recent common ancestry (47), and are thus more likely to interact ecologically (48) and to share adaptations that influence their propensity to breed in urban habitats (2, 49, 50). The youngest species pairs are also less likely to differ in other traits, improving our ability to isolate the effects of dominance from other unrelated differences among the species that could influence their propensity to occur in cities (30). For all comparisons, each subordinate species was more closely related to its dominant species than to any other dominant–subordinate species pair in our dataset (following the most recently available phylogenies for each clade), meaning that each dominant and subordinate species pair represented an evolutionarily independent comparison. We provide specific references that we used to determine phylogenetic relationships in the dataset.
Most congeneric species pairs of birds lack data on dominance relationships, and thus our dataset misses many of the world’s birds. Such omissions are common in any comparative study where focal data are rare. The species pairs included in our dataset, however, are diverse (Fig. 1B) and should not be biased with respect to the hypotheses that we tested because the dominance relationships among species were studied and reported for reasons unrelated to urbanization and compiled from a diverse array of sources.
Breeding Range Overlap with Cities.
We reviewed the breeding ranges for all of our focal species of birds (see reference list in SI Appendix) and intersected these range maps with the geographic locations of the 492 cities identified above. We then removed any dominant–subordinate species pairs from further analysis if the geographic breeding ranges of both species did not overlap at least one of our focal cities. For the remaining species, we identified the cities that fell within their geographic breeding ranges, allowing us to design a separate survey for each city, which we used to query birdwatchers and ornithologists as to whether or not these species breed in urban environments of the cities within their breeding ranges. Cities that were overlapped by both dominant and subordinate species within a species pair were designated as “sympatric” for that species pair; cities overlapped by only the dominant or subordinate species within a species pair were designated as “allopatric.”
Surveys of Cities.
We used SurveyMonkey (https://www.surveymonkey.com/) to create 492 individual surveys, one for each of our focal cities. For each city, we designed a survey that asked whether the focal species with breeding ranges that overlapped the city bred every year within urban habitat within that city. We defined urban habitats as including urban parks and ponds, and industrial, commercial, residential, and suburban areas, in addition to the downtown core, but not natural areas within the city (for example, wildlife preserves, conservation areas, or isolated patches of natural habitat) (following ref. 3). The urban habitats included in our study differ greatly from each other in terms of structure, vegetation, disturbance, and other characteristics. Our interest, however, was focused on the general challenges of urbanization, and thus we included the diverse array of human-altered habitats collectively associated with urbanization. Including this diversity of urban habitats might have increased variation in the patterns of occurrence across species in our study; however, our results still show broad repeatable patterns (Fig. 2).
We recruited prospective survey respondents who would be most knowledgeable about the breeding occurrence of birds in each city. We achieved this by sending emails to ornithologists, naturalists, and birders based in focal cities (e.g., editors of breeding bird atlases, ornithologists working in museums, academics studying urbanization effects on birds, professional birding guides, and active birders living in focal cities), and by posting on listservs (e.g., NEOORN for the Neotropics) and social media (e.g., Facebook) used by active and informed groups (e.g., professional ornithologists across the Neotropics). By targeting survey respondents, we aimed to improve the quality of our breeding occurrence data.
Each survey asked respondents to categorize each species’ breeding occurrence in urban habitat as follows: (i) widespread across the city, (ii) local, found only in very few locations, (iii) somewhere in between local and widespread, or (iv) absent. Respondents could also select “not sure” if they did not know the species’ breeding occurrence in the city. We asked respondents to record the historical breeding status of species that have undergone a recent major decline [e.g., Asian vultures (51)]. At the end of the survey, we asked participants to rate their knowledge of the breeding birds of the focal city on a scale of 1 (“I know the city’s birds a little bit”) to 5 (“I know the city’s birds very well), with 3 being intermediate (“I know the city’s birds moderately well”). In rare cases where the respondent did not score their knowledge of the breeding birds of the focal city, we assigned the minimum score of 1 to their response. All surveys were anonymous; however, an optional section at the end of the survey allowed respondents to provide comments and contact information. We provide an example survey for Toronto, Ontario, Canada, that illustrates the specific format and questions as presented to respondents (SI Appendix, Fig. S5).
Calculated Variables.
We recoded breeding occurrence survey responses as follows: absent = 0; local, found only in very few locations = 1; somewhere in between local and widespread = 2; widespread across the city = 3. We then calculated one breeding occurrence score per species per city by averaging responses across observers for each species within each city, weighted by their self-reported ability (1–5) as follows: [sum(breeding occurrence score × ability score)]/[sum(ability scores)]. The resulting values ranged continuously from 0 to 3 and were then used in plotting and statistical analyses testing among the predictions of our alternative hypotheses.
To characterize the propensity of species pairs to breed within cities, we calculated one urban-breeding propensity score for each phylogenetically independent species pair as follows: the maximum breeding occurrence value within a species pair for each city, averaged across all focal cities that overlapped their breeding ranges. Our focal hypotheses generate predictions that vary with this urban-breeding propensity score, which provides an estimate of the degree to which cities present species with challenges or opportunities.
Phylogeny.
We obtained a phylogeny for our focal species from birdtree.org (52). We first downloaded 1,000 phylogenetic trees for our focal species from the “Hackett all species” set, and then selected the maximum clade credibility tree from these using TreeAnnotator in the program BEAST [version 1.8.1; ref. 53]. We imported our final maximum clade credibility tree into R using the R package ape (54).
Geographic Variation.
We tested for geographic variation in our results by assigning each of our focal cities to a continent (Africa, Asia, Australia, Europe, North America, South America). For the purposes of our analysis, New Zealand was included with Australia, and Central America and the Caribbean were included with North America.
Climate, Population, Economic Development, and Sampling Data for Cities.
After finding significant variation among continents, we tested 12 hypotheses that could potentially underlie this geographic variation.
-
i)
Latitude (decimal degrees). We hypothesized that latitude could explain geographic variation in our main result because latitude is thought to correlate with many factors, such as intensity of biotic selective pressures (55), that could impact when and how dominant and subordinate species interact. We obtained latitude for each of our focal cities from various internet sources, using absolute latitude for each city in the analyses.
-
ii)
Mean annual temperature (degrees Celsius). We hypothesized that relatively stronger or more effective biotic selective pressures at warmer temperatures might promote ecological divergence among closely related dominant and subordinate species (56), allowing them to coexist in warmer cities. We obtained average monthly temperatures for the terrestrial earth (1970–2000; from ref. 57; worldclim.org/version2, 10-min resolution). We imported these data into ArcGIS 10.1 (ESRI) and spatially joined our focal cities to the climate data using the join function. We then took the average temperature across the 12 mo for each city, and used these average annual temperature values in analyses.
-
iii)
Annual net primary productivity (NPP) (grams of carbon per square meter per year). We hypothesized that more available energy might allow closely related dominant and subordinate species to coexist within cities (58). As an estimate of available energy, we used global estimates of NPP (from ref. 59; 0.5° latitude and longitude resolution; data.ceda.ac.uk/badc/islscp2/data/carbon/model_npp_xdeg/), representing values averaged across 17 different global models. We imported these data into ArcGIS 10.1 and spatially joined our focal cities to the NPP data using the join function, providing an annual NPP value for each city for use in analyses.
-
iv)
Economic development. We hypothesized that changes in the abundance and distribution of resources associated with economic development (40, 42) might influence the interactions between, and patterns of coexistence of, dominant and subordinate species in cities. We obtained categorizations of economic development from the United Nations (27) for all of our focal countries, where economic development was categorized as follows: developing economies, economies in transition, and developed economies. We used these designations as categorical predictors in analyses.
-
v)
Human population size of cities. We hypothesized that larger cities (more people) might alter the interactions between, and patterns of coexistence of, dominant and subordinate species in cities. We obtained population size data for our focal cities as described above (Selection of Cities).
-
vi)
Phylogeny/taxonomy. We hypothesized that evolutionary radiations of birds on some continents might be less likely to differ in their breeding occurrence in cities because of shared evolutionary histories. We tested this idea using both a phylogeny of our focal species (included as a random factor in statistical models), and by incorporating taxonomic order as a fixed factor in statistical models. Details of the phylogenetic data are provided above (Phylogeny). Taxonomic orders followed the International Ornithologists’ Union classification (46).
-
vii)
Number of observers. We hypothesized that cities with more respondents might have more accurate estimates of breeding occurrence, and differences in accuracy among cities could explain geographic variation in our main result. Thus, we recorded the number of different observers that completed our survey for each focal city for use in analyses.
-
viii)
Ability of observers. We hypothesized that cities with respondents that knew the breeding birds better might have provided more accurate estimates of breeding occurrence, and that differences in observer ability among cities could explain geographic variation in our main result. Survey respondents rated their knowledge of the breeding birds of each focal city on a scale of 1 (“I know the city’s birds a little bit”) to 5 (“I know the city’s birds very well), with 3 being intermediate (“I know the city’s birds moderately well”). We calculated the average value across respondents for each city for use in analyses.
-
ix)
Number of species per city. We hypothesized that cities with a larger sample of focal species might be more likely to show differences in breeding occurrence between dominant and subordinate species, and variation in sample size of species across cities could explain geographic variation in our main result. For each city, we counted the number of species for which we obtained breeding occurrence data used in our analysis and used these values in analyses.
-
x)
Number of urban-adapted species that occur in sympatry. Our main pattern involved differences in breeding occurrence among urban-adapted species in sympatry. The tendency for closely related species to occur in sympatry varies geographically (60), and thus we hypothesized that variation in the number of sympatric, urban-adapted species could explain the geographic variation in our main result. For each city, we counted the number of species for which we obtained breeding occurrence data that were used in our analysis, and that were both sympatric with the other member of their species pair, and had high values of urban adaptation (above a value of 1.743, based on initial breakpoint analyses that suggested a breaking point in relationships between urban-breeding propensity and breeding occurrence as a function of dominance in sympatry at that value).
-
xi)
Whether or not the continent where the focal city is found includes the centroid of the focal species range. We hypothesized that the ability of species to occupy cities, or the interactions among dominant and subordinate species, might vary between areas central versus peripheral to the breeding ranges of a species, and that this variation on a continental scale could explain geographic variation in our main effects. We thus calculated the area-weighted mean latitude and longitude (centroid) of the breeding ranges of our focal species, using range maps (61) and the calculate geometry function in ArcGIS 10.1 (ESRI). Once the centroids were calculated, we recorded which continent held the centroid for each focal species. In cases where the centroid fell within an ocean, we recorded the geographically closest continent to the centroid. We then coded each record as “inside” if the focal record was within the same continent that held the centroid of the species’ breeding range, or “outside” if the focal record was from a different continent than the species’ centroid.
-
xii)
Whether or not the continent where the focal city is found includes the location where dominance data were gathered for the focal species pair. We hypothesized that the interactions among dominant and subordinate species characterized by our dominance data could change at increasing distances, and at a continental scale, thus explaining geographic variation in our main result (however, for evidence of little geographic variation in dominance relationships, see ref. 14). We thus recorded the continent(s) from which dominance data were collected, and then coded each record as “same” if the record came from the same continent(s) that provided dominance data, or “different” if the record came from a different continent.
Statistical Analyses.
We conducted all of our statistical analyses and data plotting in R (62). We provide the R code that we used for our analyses and figures, along with the datasets, in the data repository Dryad.
We tested the predictions of alternative hypotheses for how competitive dominance might influence urban-breeding occurrence as a function of urban adaptation using mixed-effects models, with breeding occurrence data (one value for each species in each city that overlapped its breeding range) as the response variable, and relative dominance, propensity of the species pair to breed in cities, and sympatry as predictors in a saturated model (i.e., all interactions among predictors were included), and the phylogenetically independent species pair as a random factor. We rescaled breeding occurrence data within each species pair, as follows: zbreed.occur = [breeding occurrence value − mean(breeding occurrence for the species pair)]/[2 × SD(breeding occurrence for the species pair)]. This rescaling resulted in each species pair having a mean zbreed.occur value of 0 and SD of 0.5. We also rescaled the urban-breeding propensity scores across the dataset using the rescale function in the R package arm (63), which subtracts the mean and then divides by 2 SDs. We did not use this function to rescale our response variable because we wanted to rescale values within each species pair, rather than across all of the data, thereby removing the correlation between breeding occurrence and urban-breeding propensity scores.
Linear mixed-effects models did not fit our data well; thus, we moved to Bayesian generalized linear mixed models (MCMCglmms) to test the predictions of our hypotheses. We ran saturated MCMCglmms in the R package MCMCglmm (64, 65). We specified uninformative priors for the fixed effects so that all variance parameters were estimated (64). We specified priors where V = 1 and nu = 0.002 for both R and G structures, following Hadfield (64, 65). We used a Gaussian distribution and ran simulations for 50,000 iterations, with a burnin of 10,000 iterations and a thinning interval of 1. We did not thin our simulations, following recent recommendations (66). We ran each model three times and tested for convergence across the three runs using Gelman and Rubin’s convergence diagnostic [gelman.diag function in the R package coda (67)], where the upper limits should be close to 1 (67). We also examined trace plots to ensure that there were no trends across runs, effective sample sizes to ensure that all values exceeded 200, Geweke’s convergence diagnostics [geweke.diag function in the R package coda (67)] that compare the first 10% with the last 50% of the Markov chain and should generally show absolute values <1.96, and density plots to ensure symmetry and approximate normality of the posterior distributions of parameters (following refs. 64, 65, 68, and 69). Our simulations generally showed evidence of good convergence, effective sample sizes, and posterior distributions (see SI Appendix, Figs. S6–S9 for trace and density plots and SI Appendix, Table S6 for diagnostic results for our main models).
We used a Gaussian, rather than ordinal, distribution for our models because the most appropriate analysis of our data required us to (i) calculate the mean breeding occurrence values across observers for each species in each city, (ii) weight breeding occurrence scores used to calculate these means by the self-reported ability of each observer, and (iii) remove the correlation between urban-breeding propensity and breeding occurrence by rescaling breeding occurrence values (i.e., subtracting the mean, dividing by 2 SDs) within independent dominant–subordinate species pairs. By calculating standardized, weighted means as our response variable, we could no longer use models with ordinal distributions. Nonetheless, rerunning our models using an ordinal distribution [either on raw values (n = 14,681 observations) or on rounded means (n = 5,618 data points)] yielded similar results, even though these models did not fit our data as well (trace plots showed repeated fluctuations or trends).
Initial plots of rescaled data suggested that the relationship between urban-breeding propensity and breeding occurrence in sympatry might be nonlinear or show a breakpoint. Thus, we ran three additional saturated MCMCglmm models as before with the same model checks, one with a second-order polynomial (orthogonal) for urban-breeding propensity, and the other with two different breakpoints. We identified the best point for breaks using breakpoint analyses restricted to subordinates in sympatry, then dominants in sympatry. The best-performing model [lowest deviance information criterion value (DIC)] was the first model without polynomials or breakpoints.
One of our central hypotheses (H2) predicts a three-way interaction between predictor variables (i.e., the effects of dominance on breeding occurrence depends on both urban-breeding propensity and sympatry; Fig. 2A), and thus we reran our best-performing MCMCglmm model with the three-way interaction term removed. We ran and checked the models as before and assessed the importance of the three-way interaction using differences in DIC, in addition to the model estimates.
Our calculations of urban-breeding propensity scores used the maximum breeding occurrence values for each species pair for each city where the species were sympatric. This approach could potentially create a bias between allopatry and sympatry because allopatric values (i.e., breeding occurrence values from cities that only occur in one of the species’ breeding distributions) within a species pair always contribute to the calculations used to derive urban-breeding propensity scores, while only one-half of the sympatric values within a species pair contribute (only the higher of the two values per city that occurs in both of the species’ breeding distributions). We tested whether this potential bias impacted our results by calculating an alternative urban-breeding propensity score as the mean breeding occurrence value within a species pair across all focal cities that overlapped their breeding ranges. We created plots of this mean urban-breeding propensity score (SI Appendix, Fig. S10) and reran our best-performing model with this mean score substituted for the maximum values used in our main models. We ran and checked the models as before.
The bounded nature of the urban-breeding propensity scores meant that for species to have extreme values (close to 0 or 3), most breeding occurrence values must also be 0 or 3, respectively, leading to a potential for convergence of relationships at the extremes. Such convergence was apparent, especially when calculating urban-breeding propensity values using means and when examining presence–absence. Incorporating polynomial expressions for urban-breeding propensity scores helped to address this issue in our statistical models, but our main analysis did not show strong patterns of convergence (Fig. 2B).
Our main analysis discovered significantly lower breeding occurrence values for urban-adapted subordinate species in sympatry (Fig. 2). We wanted to know whether this result reflected the increased absence of urban-adapted subordinate species from cities in sympatry, whether urban-adapted subordinate species persisted in cities in sympatry but were less widespread, or both. To test for changes in presence versus absence, we reclassified raw breeding occurrence values as either present (value > 0) or absent (value = 0). We then ran a MCMCglmm model with the same predictors and random factor as before, but with a “categorical” (bivariate) distribution. We adjusted the prior to match a bivariate response variable with two levels (presence, absence). We ran the model with the same specifications (iterations, burnin, etc.) and model checks as in previous models.
To test whether urban-adapted subordinate species persisted in cities in sympatry but were less widespread, we created a new dataset that excluded all records where species were absent (i.e., raw breeding occurrence = 0). We then ran models with the same predictors, random factor, specifications, and model checks as our main model.
Upon finding evidence for lower breeding occurrence values for urban-adapted subordinate species in sympatry, we wanted to know whether this result was caused by one or a few lineages in our dataset. To address this question, we ran analyses with (i) the full phylogeny of our focal species as a random factor, and (ii) taxonomic order (n = 19) as a fixed effect in a saturated model. Incorporating the phylogeny allowed us to account for phylogenetic variation in the standardized breeding occurrence values across our dataset. Incorporating taxonomic order and its interactions with our main effects allowed us to test whether our main effects varied across taxonomic orders. We could not test for variation in our results at the level of taxonomic family or below because we had 66 families in our dataset with 46 families represented by only one or two comparisons.
We incorporated phylogeny as a random factor into our previous saturated MCMCglmm model, with rescaled breeding occurrence as the response variable, and dominance, rescaled urban-breeding propensity score, and sympatry as predictors. Initial runs showed low values for the effective sample size for phylogeny (less than 200), so we ran simulations for 150,000 iterations with a burnin of 10,000 iterations. We used a thinning interval of 10 to reduce the amount of data used for model checking and plotting. We tested whether accounting for phylogenetic variation in breeding occurrence influenced our main result by running two models and comparing model performance using DIC: one saturated model, and the other with the three-way interaction term removed (both models with phylogeny as a random effect).
We tested the effect of taxonomic order on our main result by adding taxonomic order as a predictor variable into a saturated model with our main effects (dominance, urban-breeding propensity, sympatry). Initial runs with phylogeny as a random effect performed poorly, with low effective sample and poor convergence for the random factor; thus, we ran these models with species pair as the random factor. Following the run of the saturated model, we ran the model again with the four-way interaction (dominance: urban-breeding propensity: sympatry: taxonomic order) removed and compared model performance between the two models using DIC. If variation in our main result (dominance: urban-breeding propensity: sympatry) across taxonomic orders was important, then we predicted that removing the four-way interaction should reduce model performance. We kept the same specifications (iterations, burnin, etc.) as in initial models. For all phylogenetic and taxonomic models, we tested for convergence, adequate sample sizes, and normal and symmetrical posterior distributions as described above for our first MCMCglmm model.
We tested for geographic variation in our main effects by adding continent as a predictor variable into a saturated model with the main effects (dominance, urban-breeding propensity, sympatry). We ran the saturated model, followed by the same model with the four-way interaction removed, and compared model performance between the two models using DIC. If variation in our main result across continents was important, then we predicted that removing the four-way interaction should reduce model performance. We used the same model specifications and checks as the initial MCMCglmm models.
We found significant variation in our main result across continents, leading us to ask why. We tested the predictions of all possible alternative hypotheses that we could think of to explain continental variation. These alternative hypotheses involved differences in (i) latitude (absolute values), (ii) temperature (average annual), (iii) available energy (estimated by models of NPP), (iv) economic development, (v) human population size of cities, (vi) phylogeny/taxonomy, (vii) number of observers reporting, (viii) ability of observers reporting, (ix) number of species per city, (x) number of urban-adapted species occurring in sympatry per city, (xi) whether or not the focal continent included the centroid of the focal species range, and (xii) whether or not the focal continent included the location where dominance data were gathered for the focal species pair. For all hypotheses, we predicted that if the candidate factor caused the continental variation that we found, then it should perform better than continent when included as a predictor variable in a saturated model (with dominance, urban-breeding propensity, sympatry as the other predictors). We also predicted that the candidate factor should interact with our main effects (as continent did), such that the inclusion of a four-way interaction term with the candidate factor significantly improved the model. To test these predictions, we ran two models for each candidate factor: one with the candidate factor, dominance, urban-breeding propensity (either linear, or with a polynomial—whichever had the lowest DIC score), and sympatry as predictor variables, and a second identical to the first but with the four-way interaction removed. We compared model performance between these two models and the model without the candidate factor (i.e., only dominance, urban-breeding propensity, and sympatry included) using DIC, with a lower score indicating the model that performed better. For the phylogeny test, we also reran the original models with continent as a predictor, but added a full phylogeny as a random effect, comparing the saturated model (continent, dominance, urban-breeding propensity, sympatry) to the same model with the four-way interaction removed. We used DIC to compare saturated models with each candidate factor to the saturated model that included continent to test the prediction that the model including the causal factor would perform better than the model that included continent.
Additional Analyses.
We performed additional analyses to ensure that our results were robust to alternative statistical approaches. These analyses included testing for spatial autocorrelation in our residuals, reanalyzing our data using generalized least-squares models that incorporated spatial autocorrelation, retesting our main results using the subset of species pairs for which quantitative dominance data were accessible, and testing for variation in our main results across level of economic development using presence–absence data that might be less prone to cultural bias (SI Appendix).
Supplementary Material
Acknowledgments
Thanks to Alan Cohen, Haley Kenyon, Ádám Lendvai, and Bob Montgomerie for feedback on the study and manuscript. Special thanks to the hundreds of birders and ornithologists around the world who helped with data collection (please see SI Appendix for more extensive acknowledgments), especially Carlos Bosque, Rob Dobbs, Michel Gosselin, Mike Jennings, Jeff Marks, Chloé Montreuil-Spencer, Javier Salgado Ortiz, Kathryn Stewart, Fernando Straube, Haruka Wada, Masaru Wada, and Jongmin Yoon. Thanks to Lynx Edicions for permission to reprint their illustrations. Funding for this work came from the Natural Sciences and Engineering Council of Canada, Discovery Grant program (to P.R.M. and F.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.R. is a guest editor invited by the Editorial Board.
Data deposition: The data and R code reported in this paper have been deposited in the Dryad Digital Repository database, datadryad.org/ (doi: 10.5061/dryad.t85bf04).
See Commentary on page 12331.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1809317115/-/DCSupplemental.
References
- 1.United Nations . World Urbanization Prospects: The 2014 Revision. United Nations; New York: 2015. [Google Scholar]
- 2.Sol D, González-Lagos C, Moreira D, Maspons J, Lapiedra O. Urbanisation tolerance and the loss of avian diversity. Ecol Lett. 2014;17:942–950. doi: 10.1111/ele.12297. [DOI] [PubMed] [Google Scholar]
- 3.Bonier F, Martin PR, Wingfield JC. Urban birds have broader environmental tolerance. Biol Lett. 2007;3:670–673. doi: 10.1098/rsbl.2007.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maklakov AA, Immler S, Gonzalez-Voyer A, Rönn J, Kolm N. Brains and the city: Big-brained passerine birds succeed in urban environments. Biol Lett. 2011;7:730–732. doi: 10.1098/rsbl.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonier F. Hormones in the city: Endocrine ecology of urban birds. Horm Behav. 2012;61:763–772. doi: 10.1016/j.yhbeh.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Lowry H, Lill A, Wong BB. Behavioural responses of wildlife to urban environments. Biol Rev Camb Philos Soc. 2013;88:537–549. doi: 10.1111/brv.12012. [DOI] [PubMed] [Google Scholar]
- 7.Peiman KS, Robinson BW. Ecology and evolution of resource-related heterospecific aggression. Q Rev Biol. 2010;85:133–158. doi: 10.1086/652374. [DOI] [PubMed] [Google Scholar]
- 8.Grether GF, et al. The evolutionary consequences of interspecific aggression. Ann N Y Acad Sci. 2013;1289:48–68. doi: 10.1111/nyas.12082. [DOI] [PubMed] [Google Scholar]
- 9.Grether GF, Peiman KS, Tobias JA, Robinson BW. Causes and consequences of behavioral interference between species. Trends Ecol Evol. 2017;32:760–772. doi: 10.1016/j.tree.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Martin PR. Trade-offs and biological diversity: Integrative answers to ecological questions. In: Martin LB, Ghalambor CK, Woods HA, editors. Integrative Organismal Biology. Wiley; New York: 2014. pp. 291–308. [Google Scholar]
- 11.Sih A, Bell A, Johnson JC. Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol Evol. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Sol D, Lapiedra O, González-Lagos C. Behavioural adjustments for a life in the city. Anim Behav. 2013;85:1101–1112. [Google Scholar]
- 13.Morse DH. Niche breadth and social dominance. Am Nat. 1974;108:818–830. [Google Scholar]
- 14.Martin PR, Freshwater C, Ghalambor CK. The outcomes of most aggressive interactions among closely related bird species are asymmetric. PeerJ. 2017;5:e2847. doi: 10.7717/peerj.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freshwater C, Ghalambor CK, Martin PR. Repeated patterns of trait divergence between closely related dominant and subordinate bird species. Ecology. 2014;95:2334–2345. doi: 10.1890/13-2016.1. [DOI] [PubMed] [Google Scholar]
- 16.Alberti M, et al. Global urban signatures of phenotypic change in animal and plant populations. Proc Natl Acad Sci USA. 2017;114:8951–8956. doi: 10.1073/pnas.1606034114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson MTJ, Munshi-South J. Evolution of life in urban environments. Science. 2017;358:eaam8327. doi: 10.1126/science.aam8327. [DOI] [PubMed] [Google Scholar]
- 18.Grimm NB, et al. Global change and the ecology of cities. Science. 2008;319:756–760. doi: 10.1126/science.1150195. [DOI] [PubMed] [Google Scholar]
- 19.Alberti M. Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol Evol. 2015;30:114–126. doi: 10.1016/j.tree.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Blair RB. Land use and avian species diversity along an urban gradient. Ecol Appl. 1996;6:506–519. [Google Scholar]
- 21.McKinney ML. Urbanization as a major cause of biotic homogenization. Biol Conserv. 2006;127:247–260. [Google Scholar]
- 22.Penick CA, Savage AM, Dunn RR. Stable isotopes reveal links between human food inputs and urban ant diets. Proc Biol Sci. 2015;282:20142608. doi: 10.1098/rspb.2014.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crooks KR, Soulé ME. Mesopredator release and avifaunal extinctions in a fragmented system. Nature. 1999;400:563–566. [Google Scholar]
- 24.Sol D, Bartomeus I, Griffin AS. The paradox of invasion in birds: Competitive superiority or ecological opportunism? Oecologia. 2012;169:553–564. doi: 10.1007/s00442-011-2203-x. [DOI] [PubMed] [Google Scholar]
- 25.Lawton JH. Are there general laws in ecology? Oikos. 1999;84:177–192. [Google Scholar]
- 26.Simberloff D. Community ecology: Is it time to move on? (An American Society of Naturalists presidential address) Am Nat. 2004;163:787–799. doi: 10.1086/420777. [DOI] [PubMed] [Google Scholar]
- 27.United Nations . World Economic Situation and Prospects. United Nations; New York: 2014. [Google Scholar]
- 28.Dhondt AA. Interspecific Competition in Birds. Oxford Univ Press; Oxford: 2012. [Google Scholar]
- 29.Martin PR, Martin TE. Ecological and fitness consequences of species coexistence: A removal experiment with wood warblers. Ecology. 2001;82:189–206. [Google Scholar]
- 30.Martin PR, Ghalambor CK. When David beats Goliath: The advantage of large size in interspecific aggressive contests declines over evolutionary time. PLoS One. 2014;9:e108741. doi: 10.1371/journal.pone.0108741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chappell MA. Behavioral factors in the altitudinal zonation of chipmunks (Eutamias) Ecology. 1978;59:565–579. [Google Scholar]
- 32.Bovbjerg H. Ecological isolation and competitive exclusion in two crayfish (Orconectes virillis and Orconectes immunis) Ecology. 1970;51:225–236. [Google Scholar]
- 33.Hixon MA. Competitive interactions between California reef fishes of the genus Embiotica. Ecology. 1980;61:918–931. [Google Scholar]
- 34.Larson RJ. Competition, habitat selection, and the bathymetric segregation of two rockfish (Sebastes) species. Ecol Monogr. 1980;50:221–239. [Google Scholar]
- 35.Jaeger RG. Competitive exclusion as a factor influencing the distributions of two species of terrestrial salamanders. Ecology. 1971;52:632–637. doi: 10.2307/1934151. [DOI] [PubMed] [Google Scholar]
- 36.Griffis MR, Jaeger RG. Competition leads to an extinction-prone species of salamander: Interspecific territoriality in a metapopulation. Ecology. 1998;79:2494–2502. [Google Scholar]
- 37.Pinowski J. Die Auswahl des Brutbiotops beim Feldsperling (Passer m. montanus L.) Ekol Pol. 1967;15:1–30. [Google Scholar]
- 38.Anderson TR. Population studies of European sparrows in North America. Occ Pap Mus Nat Hist Univ Kansas. 1978;70:1–58. [Google Scholar]
- 39.Cordero PJ, Rodriguez-Teijeiro JD. Spatial segregation and interaction between house sparrows and tree sparrows (Passer spp.) in relation to nest site. Ekol Pol. 1990;38:443–452. [Google Scholar]
- 40.Bozek CK, Prange S, Gehrt SD. The influence of anthropogenic resources on multi-scale habitat selection by raccoons. Urban Ecosyst. 2007;10:413–425. [Google Scholar]
- 41.Maher CR, Lott DF. A review of ecological determinants of territoriality within vertebrate species. Am Midl Nat. 2000;143:1–29. [Google Scholar]
- 42.Cohen B. Urbanization in developing countries: Current trends, future projections, and key challenges for sustainability. Technol Soc. 2006;28:63–80. [Google Scholar]
- 43.Paine RT. Food web complexity and species diversity. Am Nat. 1966;100:65–75. [Google Scholar]
- 44.Bregman TP, et al. Species interactions regulate the collapse of biodiversity and ecosystem function in tropical forest fragments. Ecology. 2015;96:2692–2704. doi: 10.1890/14-1731.1. [DOI] [PubMed] [Google Scholar]
- 45.Vavrek MJ. 2012. fossil: Palaeoecological and Palaeogeographical Analysis Tools. R Package, Version 0.3.7 (R Foundation for Statistical Computing, Vienna)
- 46.Gill F, Donsker D. 2016 IOC World Bird List (Version 6.2). Available at www.worldbirdnames.org/. Accessed June 8, 2016.
- 47.Wiens JJ, et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett. 2010;13:1310–1324. doi: 10.1111/j.1461-0248.2010.01515.x. [DOI] [PubMed] [Google Scholar]
- 48.Violle C, Nemergut DR, Pu Z, Jiang L. Phylogenetic limiting similarity and competitive exclusion. Ecol Lett. 2011;14:782–787. doi: 10.1111/j.1461-0248.2011.01644.x. [DOI] [PubMed] [Google Scholar]
- 49.Knapp S, Kühn I, Schweiger O, Klotz S. Challenging urban species diversity: Contrasting phylogenetic patterns across plant functional groups in Germany. Ecol Lett. 2008;11:1054–1064. doi: 10.1111/j.1461-0248.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- 50.Helmus MR, et al. Communities contain closely related species during ecosystem disturbance. Ecol Lett. 2010;13:162–174. doi: 10.1111/j.1461-0248.2009.01411.x. [DOI] [PubMed] [Google Scholar]
- 51.Prakash V, et al. Catastrophic collapse of Indian white-backed Gyps bengalensis and long-billed Gyps indicus vulture populations. Biol Conserv. 2003;109:381–390. [Google Scholar]
- 52.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. The global diversity of birds in space and time. Nature. 2012;491:444–448. doi: 10.1038/nature11631. [DOI] [PubMed] [Google Scholar]
- 53.Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paradis E, et al. 2016. ape: Analyses of Phylogenetics and Evolution. R Package, Version 3.5 (R Foundation for Statistical Computing, Vienna)
- 55.Schemske DW, Mittelbach GG, Cornell HV, Sobel JM, Roy K. Is there a latitudinal gradient in the importance of biotic interactions? Annu Rev Ecol Evol Syst. 2009;40:245–269. [Google Scholar]
- 56.Bothwell E, Montgomerie R, Lougheed SC, Martin PR. Closely related species of birds differ more in body size when their ranges overlap—in warm, but not cool, climates. Evolution. 2015;69:1701–1712. doi: 10.1111/evo.12706. [DOI] [PubMed] [Google Scholar]
- 57.Fick SE, Hijmans RJ. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int J Climatol. 2017;37:4302–4315. [Google Scholar]
- 58.Pigot AL, Tobias JA, Jetz W. Energetic constraints on species coexistence in birds. PLoS Biol. 2016;14:e1002407. doi: 10.1371/journal.pbio.1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cramer W, et al. Comparing global models of terrestrial net primary productivity (NPP): Overview and key results. Glob Change Biol. 1999;5:1–15. [Google Scholar]
- 60.Martin PR, Montgomerie R, Lougheed SC. Rapid sympatry explains greater color pattern divergence in high latitude birds. Evolution. 2010;64:336–347. doi: 10.1111/j.1558-5646.2009.00831.x. [DOI] [PubMed] [Google Scholar]
- 61.BirdLife International and NatureServe 2011. Bird Species Distribution Maps of the World (BirdLife International, Cambridge, UK; NatureServe, Arlington, VA)
- 62.R Core Team 2016 R: A Language and Environment for Statistical Computing, Version 3.3.1 (R Foundation for Statistical Computing, Vienna). Available at https://www.R-project.org/. Accessed October 14, 2016.
- 63.Gelman A, et al. 2016. arm: Data Analysis Using Regression and Multilevel/Hierarchical Models. R Package, Version 1.9-3 (R Foundation for Statistical Computing, Vienna)
- 64.Hadfield JD. MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R package. J Stat Softw. 2010;33:1–22. [Google Scholar]
- 65.Hadfield J. 2016. MCMCglmm: MCMC Generalised Linear Mixed Models. R Package, Version 2.23 (R Foundation for Statistical Computing, Vienna)
- 66.Link WA, Eaton MJ. On thinning of chains in MCMC. Methods Ecol Evol. 2012;3:112–115. [Google Scholar]
- 67.Plummer M, et al. 2015. coda: Output Analysis and Diagnostics for MCMC. R Package, Version 0.18-1 (R Foundation for Statistical Computing, Vienna)
- 68.Bolker B, Brooks M, Gardner B, Lennert C, Minami M. 2012 Owls example: A zero-inflated, generalized linear mixed model for count data. Available at https://groups.nceas.ucsb.edu/non-linear-modeling/projects/owls/WRITEUP/owls.pdf. Accessed August 2, 2017.
- 69.Horváthová T, Nakagawa S, Uller T. Strategic female reproductive investment in response to male attractiveness in birds. Proc Biol Sci. 2012;279:163–170. doi: 10.1098/rspb.2011.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.