Abstract
Background:
Patients with upper gastrointestinal bleeding (UGIB) often require urgent or emergent esophagogastroduodenoscopy (EGD) and are at risk of complications such as aspiration of gastric content or blood. The role of prophylactic endotracheal intubation (PEI) in the absence of usual respiratory status-related indications is not well established.
Methods:
We searched Medline, EMBASE, Cochrane Library's Central Register of Controlled Trials (CENTRAL) and SCOPUS from inception through July 2017 without date or language of publication restriction. We included studies that compared PEI with usual care (UC) in patients with acute UGIB, and reported any of the following outcomes: aspiration, pneumonia, mortality and length of stay. We excluded studies in which majority of included patients required intubation due to respiratory failure or decreased level of consciousness. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence for each outcome.
Results:
We did not identify any randomized trials on this topic. We included 10 observational studies (n = 6068). We were not able to perform any adjusted analyses. PEI was associated with a significant increase in aspiration (OR 3.85, 95% CI, 1.46, 10.25; P = 0.01; I2= 56%; low-quality evidence), pneumonia (OR 4.17, 95% CI, 1.82, 9.57; P = 0.0007; I2=52%; low-quality evidence) and hospital length of stay (mean difference 0.86 days, 95% CI 0.13, 1.59; P = 0.02; I2= 0; low-quality evidence), without clear effect on mortality (OR 1.92, 95% CI, 0.71, 5.23; P = 0.2; I2= 95%; very low-quality evidence).
Conclusions:
Low- to very low-quality evidence from observational studies suggests that PEI in the setting of UGIB may be associated with higher rates of respiratory complications and, less likely, with increased mortality. Although the results are alarming, the lack of higher quality evidence calls for randomized trials to inform practice.
Keywords: Endoscopy, systematic review, meta-analysis, prophylactic endotracheal intubation, upper gastrointestinal bleeding
INTRODUCTION
Upper gastrointestinal bleeding (UGIB) can result in significant morbidity and mortality. The mainstay treatment is endoscopic therapy whenever possible. As opposed to elective esophagogastroduodenoscopies (EGD), EGDs performed in emergency or critical care setting, especially in the presence of significant hematemesis, can be associated with significant cardiac and respiratory compromise.[1] Therefore, it is not uncommon to perform prophylactic endotracheal intubation (PEI) in such patients to prevent aspiration or to assure that a agitated or confused patient is not actively resisting the procedure.
While it is possible that endotracheal intubation is beneficial for patients with UGIB and concomitantly decreased level of consciousness, agitation or hypoxia, the value of endotracheal intubation in patients with large hematemesis and no other indication for intubation is less clear. The recent European guidelines issued a weak recommendation to perform endotracheal intubation in patients with encephalopathy or agitation,[2] while other guidelines did not address this issue.[3,4,5] The issue of performing PEI in patients without the above-mentioned characteristics was not addressed. A survey conducted over a decade ago demonstrated a considerable variation in the believes and practices of gastroenterologists with regards to endotracheal intubation in the presence of UGIB.[6] Due to the complexity of this topic and the lack of clear guidance, we undertook a systematic review to determine the effect of prophylactic intubation on patient-important outcomes in the context of UGIB.
METHODS
Study selection
Studies were eligible if (1) the study design was a randomized controlled trial (RCT) or, if not available, an observational design; (2) the study included patients with UGIB requiring emergent esophagogastroduodenoscopy (EGD); (3) patients underwent PEI (intubation done preemptively to protect the airways in the absence of other indications for intubation) and the control group included patient who did not undergo endotracheal intubation; (4) the study reported any of the following outcomes: aspiration (as defined by authors of those studies), pneumonia (as defined by authors of those studies), mortality and hospital length of stay.
Search strategy
We searched Medline, EMBASE, Cochrane Library's Central Register of Controlled Trials (CENTRAL) and SCOPUS from inception through July 2017. Our search strategy is detailed in Supplementary Appendix I [online only]. We did not apply any language or date of publication restrictions. Two reviewers, in duplicate, screened the titles and abstracts for potentially eligible articles. The reviewers then assessed the full text of the articles for final eligibility. We also screened references of relevant articles to identify additional studies not captured in database searches. Disagreement between reviewers was resolved by consensus and a third reviewer was consulted in cases it was not achieved.
Data extraction
Two reviewers independently extracted data from eligible studies using standard data abstractions forms. We resolved disagreements by discussion and consensus.
Risk of bias assessment
Two reviewers independently assessed the risk of bias. We used the Newcastle-Ottawa Scale (NOS) to assess the risk of bias for non-randomized studies.[7] Using this scale, studies are judged based on the following three domains: selection of the study groups [maximum 4 stars (points)]; comparability of the groups (maximum 2 points) and ascertainment of the outcome of interest (maximum 3 points), yielding a maximum possible score of 9 Supplementary Appendix II, online only].
Statistical analysis
We used Revman software (Review Manager, version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) for data analysis. We used a random-effects model, as described by Dersimonian and Laird,[8] to pool weighted effects of estimates across all studies. Study weights were estimated using the inverse variance method. We calculated pooled odds ratios (OR) and mean differences (MD) for dichotomous and continuous outcomes, respectively, with corresponding 95% confidence intervals (CI). Statistical heterogeneity was assessed using Chi-square and I2 statistics,[9] with significant heterogeneity defined as P < 0.10 or I2> 50%. We planned to conduct a meta-analysis of adjusted effect estimates, if reported, to generate pooled adjusted OR with 95% CI.
Subgroup analysis
We performed one subgroup analysis by type of bleeding (variceal versus other) hypothesizing that variceal bleeding is associated with larger benefit from intubation.
Sensitivity analysis
We performed sensitivity analysis excluding studies published in abstract form only,[10,11,12] and excluding the abstract by Lee et al.,[12] as the data overlapped with their full-text publication on a later date.[13] Finally, we performed a post hoc analysis excluding the study by Rudolph et al.[14] due to lack of clarity in the reporting outcomes of the study groups.
Publication bias
We planned to inspect funnel plots and to use Egger's test to assess for publication bias for outcomes that included ≥10 studies.[15]
Quality of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to assess the quality of evidence for each outcome.[16]
RESULTS
Characteristics of included studies
Our initial search identified a total of 601 citations. After eliminating duplicates, 500 citations remained, of which 489 were non-relevant. Eleven[1,10,11,12,13,14,17,18,19,20,21] articles were retrieved for full-text assessment. Of those, we excluded an abstract[20] that was subsequently published as a full text [Figure 1]. We did not identify any randomized trials. A total of 10[1,10,11,12,13,14,17,18,19,21] retrospective observational studies (7 full-text articles[1,13,14,17,18,19,21] and 3 abstracts[10,11,12]) enrolling 6068 patients met our eligibility criteria. Two studies exclusively enrolled patients with variceal bleeding.[17,21] Characteristics of included studies are presented in Table 1.
Figure 1.
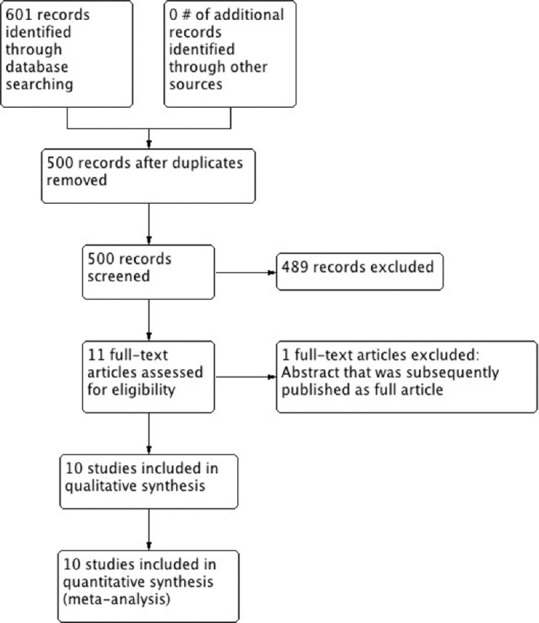
Study flow diagram
Table 1.
Characteristics of included studies
| Author | Design | Population | Interventions | Definition of aspiration | Definition of pneumonia |
|---|---|---|---|---|---|
| Lipper,[1] USA (n = 30) | Case series | ICU admission for active and severe UGIB Age: NR Males: 50% | PEI (n = 6) Usual care (n = 24) Both groups: endoscopy within 12 hours of admission | Direct observation by authors during EGD | New infiltrate on CXR and any one of the following: Fever Leukocytosis |
| Koch,[17] USA (n = 62) | Retrospective cohort | Active esophageal varices bleeding or varices with high-risk stigmata and blood in the stomach Age (mean): 48.7 years Males: 71% Child–Pugh score (mean): 8.6 Encephalopathy (Grade I): 23% | PEI (n = 42) Usual care (n = 20) Both groups: endoscopy within 12 hours of admission | Clinical diagnosis of aspiration by the primary team | Aspiration pneumonia: New pulmonary infiltrates on the post-EGD CXR, or Clinical diagnosis of aspiration by the primary team |
| Rehman,[19] USA (n = 98) | Retrospective case-control | Medical ICU admitted for UGIB with cirrhosis, hematemesis or shock. Age (median): 65 years Males: 62% | PEI (n = 49) Usual care: (n = 49) | Witnessed or suspected abnormal entry of secretions, fluid or particles into lower respiratory airways within 48 hours after EGD | New infiltrate CXR with any two of the following within 48 hours after EGD: Fever Leukocytosis Purulent sputum |
| Perisetti,[10] (Abstract) USA (n = 138) | Retrospective | Admitted to ICU with UGIB Age (mean): 63.5 years Males: NR | PEI (n = 69) Usual care: (n = 69) | NR | NR |
| Lohse,[18] Denmark (n = 3580) | Retrospective database | Nationwide registry of patients with peptic ulcer bleeding undergoing emergency EGD under anesthesia care. Age (mean): 75 years Males: 54% | PEI (n = 2101) Usual care: (n = 1479) | NR | NR |
| Abdulsamad,[11] (Abstract) USA (n = 1474) | Retrospective cohort | UGIB defined as hematemesis, coffee ground emesis or melena who underwent EGD | PEI (n = 264) Usual care (n = 1219) | NR | NR |
| Lee,[12] (Abstract) USA (n = 156) | Retrospective cohort | EGD in ICU for UGIB defined as one of: Hematemesis patient Melena hypovolemic shock with/without cirrhosis Age: NR Males: NR | PEI (n = 78) Usual care (n = 78) | NR | Within 48 hours post-EGD but no definition provided |
| Hayat,[13] USA (n = 200) | Retrospective cohort | EGD in ICU for UGIB defined as one of the following: Hematemesis patient Melena hypovolemic shock (SBP <90 mm Hg and HR >100 beats/min requiring either fluids or vasopressor agents) with/without cirrhosis Age (mean): 59.3 years Males: 63.5% | PEI (n =100) Usual care (n = 100) | NR | New focal infiltrates on CXR with any two of the following: Fever Leukocytosis Productive cough |
| Tang,[21] USA (n = 110) | Retrospective cohort | Medical ICU patients with cirrhosis and hematemesis with EGD findings of active variceal bleeding or blood in stomach plus presence of varices with high-risk stigmata Age (mean): 55 years Males: 67.6% | PEI (n = 65) Usual care (n = 45) | NR | New infiltrate on CXR plus any two the following findings within 48 hours after EGD: Fever (temperature >100.8°F) Leukocytosis (WBC >10,000/mm3) Purulent sputum |
| Rudolph,[14] USA (n = 220) | Retrospective before and after | Admitted to ICU with UGIB in 1988 and 1992 | PEI (n = 21) No intubation (n = 161) | Witnessed aspiration or new infiltrate on CXR | Not an outcome |
PEI – Prophylactic endotracheal intubation; CXR – Chest X-ray; EGD – Esophagogastroduodenoscopy; HR – Heart rate; ICU – Intensive care unit; NR – Not reported; SBP – Systolic blood pressure; UGIB – Upper gastrointestinal bleeding; WBC – White blood cells
Risk of bias assessment
Two reviewers assessed the risk of bias using NOS, and its assessments are presented in Table 2.
Table 2.
Risk of bias assessment
| Study | Selection | Comparability | Outcome |
|---|---|---|---|
| Lipper et al.[1] | ✵✵✵✵ | ✵ | ✵✵✵ |
| Rudolph et al.[14] | ✵✵✵ | ✵ | ✵✵ |
| Koch et al.[17] | ✵✵✵✵ | ✵✵ | ✵✵✵ |
| Rehman et al.[19] | ✵✵✵✵ | ✵✵ | ✵✵✵ |
| Perisetti et al.[10] | ✵✵✵ | ✵ | ✵✵ |
| Lohse et al.[18] | ✵✵✵✵ | ✵✵ | ✵✵✵ |
| Abdulsamad et al.[11] | ✵✵✵ | ✵ | ✵✵✵ |
| Lee et al.[12] | ✵✵✵✵ | ✵ | ✵✵✵ |
| Hayat et al.[13] | ✵✵✵✵ | ✵✵ | ✵✵✵ |
| Tang et al.[21] | ✵✵✵✵ | ✵✵ | ✵✵✵ |
Main outcomes
Aspiration
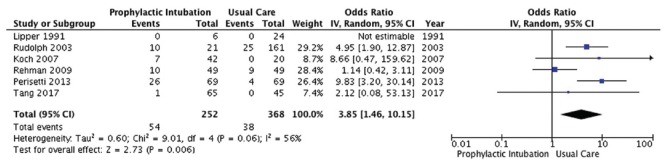
Six studies[1,10,14,17,19,21] enrolling 620 patients reported on incidence of aspiration [Figure 2]. Conventional analysis showed that PEI was associated with a significant increase in probability of aspiration (OR 3.85, 95% CI, 1.46, 10.25; P = 0.01; I2 = 56%; low-quality evidence).
Figure 2.
Aspiration outcome
Pneumonia
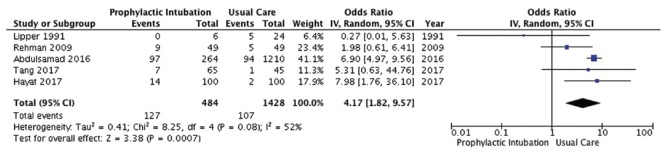
Five studies[1,11,13,19,21] enrolling 1912 patients reported on incidence of pneumonia [Figure 3]. PEI was associated with a significant increase in probability of developing pneumonia (OR 4.17, 95% CI, 1.82, 9.57; P = 0.0007; I2 =52%; low-quality evidence).
Figure 3.
Pneumonia outcome
Mortality
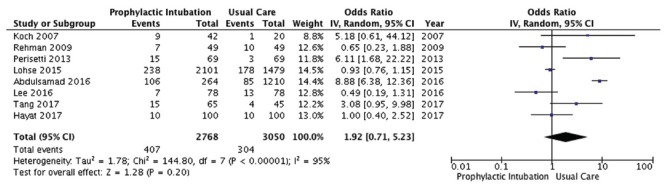
Eight studies[10,11,12,13,17,18,19,21] enrolling 5818 patients reported on mortality [Figure 4]. PEI did not affect mortality to a statistically significant degree (OR 1.92, 95% CI, 0.71, 5.23; P = 0.2; I2 =95%; very low-quality evidence).
Figure 4.
Mortality outcome
Hospital length of stay
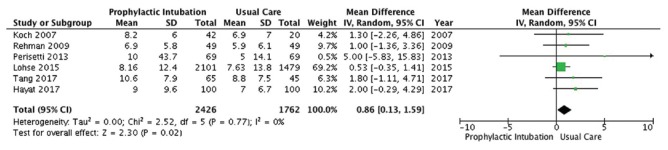
Six studies[10,13,17,18,19,21] enrolling 4188 patients reported on length of stay in hospital [Figure 5]. PEI was associated with a small but statistically significant increase in length of stay (MD 0.86 days, 95% CI 0.13, 1.59; P = 0.02; I2 = 0; low-quality evidence).
Figure 5.
Hospital length of stay outcome
Subgroup analysis
We conducted one subgroup analysis by type of bleeding; two studies (n = 172) included only patients with variceal bleeding.[17,21] We did not detect any significant subgroup differences across all outcomes. Details of the results of subgroup analysis are presented in Supplementary Figures I (762.6KB, tif) –IV (496.1KB, tif) ] [online only].
Subgroup analysis by bleeding type for aspiration outcome
Subgroup analysis by bleeding type for pneumonia outcome
Subgroup analysis by bleeding type for mortality outcome
Subgroup analysis by bleeding type for hospital length of stay outcome
Sensitivity analysis
Sensitivity analysis, excluding three studies published in the abstract form (n = 1768),[10,11,12] yielded similar results for pneumonia, mortality and length of stay outcomes. However, for aspiration outcome, the results were no longer statistically significant (OR 4.39, 95% CI 0.75, 25.66; P = 0.1; I2 = 77%). Our second sensitivity analysis, excluding the Lee et al. abstract, did not significantly alter the effect on mortality (OR 2.3, 95% CI 0.79, 6.99; P = 0.12; I2 = 96). We present the details of sensitivity analyses in Supplementary Figures V (303.3KB, tif) –X (303.3KB, tif) ] [online only].
Sensitivity analysis excluding studies published in abstract form only for aspiration outcome
Sensitivity analysis excluding studies published in abstract form only for pneumonia outcome
Sensitivity analysis excluding studies published in abstract form only for mortality outcome
Sensitivity analysis excluding studies published in abstract form only for LOS outcome
Sensitivity analysis excluding Lee et al for mortality outcome
Sensitivity analysis excluding Rudolph et al for aspiration outcome
Publication bias
Fewer than 10 studies were included for individual outcomes; therefore, we were not able to assess for publication bias.
Quality of evidence
The quality of evidence using the GRADE system ranged between very low to low across study outcomes, mainly due to observational nature of data and the lack of adjustment for important confounders (risk of bias), and also due to inconsistency and imprecision. The large intervention effect was offset by these limitations. The details of quality assessment are presented in Table 3.
Table 3.
Quality of evidence
| Quality assessment | No. of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Prophylactic endotracheal intubation | No intubation | Relative (95% CI) | Absolute (95% CI) | ||
| Mortality | ||||||||||||
| 8 | Observational studies | Seriousa | Very seriousb | Not serious | Not seriousc | None | 407/2768 (14.7%) | 304/3050 (10.0%) | OR 1.92 (0.71-5.23) | 76 more per 1000 (from 27 fewer to 267 more) | ⨁◯◯◯ Very Low | Critical |
| Pneumonia | ||||||||||||
| 5 | Observational studies | Seriousa | Seriousd | Not serious | Not seriouse | Very strong association | 127/484 (26.2%) | 107/1428 (7.5%) | OR 4.17 (1.82-9.57) | 178 more per 1000 (from 54 more to 362 more) | ⨁⨁◯◯ Low | Critical |
| Aspiration | ||||||||||||
| 6 | Observational studies | Seriousa | Seriousf | Not serious | Not seriousg | Very strong association | 54/252 (21.4%) | 38/368 (10.3%) | OR 3.58 (1.46-10.25) | 189 more per 1000 (from 41 more to 438 more) | ⨁⨁◯◯ Low | Critical |
| Hospital length of stay (days) | ||||||||||||
| 6 | Observational studies | Seriousa | Not serious | Not serious | Not serioush | None | 2426 | 1762 | - | MD 0.86 days more (0.13 more to 1.59 more) | ⨁◯◯◯ Very Low | Important |
CI – Confidence interval; OR – Odds ratio; MD – Mean difference; a – We rated down the quality of evidence by one level for risk of bias as non-adjusted estimates were used; therefore, we are uncertain if the observed treatment effect is a result of a confounder or a true effect; b – We rated down the quality of evidence by two levels for inconsistency, the I2=95%; c – Although the confidence interval included significant benefit and harm, we did not rate down the quality of evidence for imprecision; d – We rated down the quality of evidence by one level for inconsistency, the I2=57%; e – Although the CI was wide including small and large harm, we did not rate down the quality of evidence for imprecision; f – We rated down the quality of evidence for inconsistency, I2=64%; g – Although the confidence interval included both small and substantial harm, we did not rate down the quality of evidence for imprecision; h – Although the confidence interval included small and moderate harm, we did not rate down the quality of evidence for imprecision
DISCUSSION
In this systematic review, we identified 10 observational studies (6068 patients) that reported the effect of endotracheal intubation on clinical outcomes of patients with UGIB undergoing endoscopy. Low-quality evidence suggest that PEI is associated with a higher probability of developing pneumonia and aspiration, longer stay in the hospital, and less likely and statistically non-significant impact on mortality.
A recent meta-analysis of four observational studies (n = 367) showed a significant increase in pneumonia within 48 hours of endoscopy in a group of patients undergoing PEI, without affecting the risks of death or aspiration.[22] Our meta-analysis included more studies and patients (10, n = 6068), potentially improving the precision of our findings. We did not apply any restrictions on date or language of publication. In addition, we used the GRADE approach to assess the quality of the evidence, and adhered to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) reporting guidelines.[23]
Although the results of this meta-analysis are intriguing, it needs to be interpreted with great caution. Observational studies tend to be at risk of yielding biased results, study groups differ often in prognosis (i.e. confounders). Even when adjustment for important variables is possible, it may not be enough to yield reliable results. In our meta-analysis, we used only un-adjusted (crude) values, as almost all studies did not report adjusted estimates. This is an important limitation of the results, as it is challenging to determine whether the observed effects are true or confounded. It appears intuitive that the more unstable the patient is (i.e., with more bleeding and vomiting, hypoxic, agitated, non-cooperative, aspirating or judged at higher risk of aspiration), the more likely intubation is performed. Because of the observational nature of studies, lack of adjustment for the severity of clinical situation as well as additional inconsistency among study results and imprecision of estimates, the quality of the results is judged as very low to low. This markedly limits our confidence that the observed effects are true. Therefore, over-interpretation of the results should be avoided and we believe that these results, although alarming, should be considered as hypothesis generating. At the same time, these results should alert clinicians to the fact that PEI may be associated with harm, and that decision-making should take into consideration this possibility. The information we have found, including lack of higher quality data, also indicates the need for a proper randomized trial to be performed in this population of patients.
CONCLUSION
Low to very low- quality evidence suggest that PEI may be associated with higher risk of respiratory complications. Future randomized trials or, if not possible, prospectively matched cohort studies are needed to confirm or dispute these findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
APPENDIX I
Database(s): Embase 1974 to 2017 July 07, OVID Medline Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present
Search Strategy:
| # | Searches | Results |
|---|---|---|
| 1 | endotracheal intubation.mp. or exp Intubation, Intratracheal/ | 84661 |
| 2 | Intubation, Intratracheal/or tracheal intubation.mp. or Airway Management/ | 91542 |
| 3 | airway protection.mp. | 1863 |
| 4 | exp Gastrointestinal Hemorrhage/or exp "Esophageal and Gastric Varices"/or upper gastrointestinal bleed$.mp. | 159044 |
| 5 | gastrointestinal bleeding.mp. | 39897 |
| 6 | exp Hematemesis/ | 10361 |
| 7 | gastrointestinal bleeding.mp. | 39897 |
| 8 | 1 or 2 or 3 | 101741 |
| 9 | 4 or 5 or 6 or 7 | 170084 |
| 10 | 8 and 9 | 499 |
Search strategy for Cochrane Library's Central Register of Controlled Trials (CENTRAL)
Date Run: 13/07/17 18:16:25.978
Description:
ID Search Hits
#1 MeSH descriptor: [Gastrointestinal Hemorrhage] this term only 1473
#2 "gastrointestinal bleeding" or "gastrointestinal hemorrhage" or "esophageal varices" or "varices" 4808
#3 "endotracheal intubation" or "tracheal intubation" 5143
#4 MeSH descriptor: [Airway Management] explode all trees 9051
#5 #1 or #2 4808
#6 #3 or #4 12227
#7 #5 and #6 in Trials 38
Search strategy for SCOPUS
(("endotracheal intubation" OR "tracheal intubation" OR "intratracheal intubation") AND TITLE-ABSKEY ("gastrointestinal hemorrhage" OR "gastrointestinal bleeding" OR "GI bleeding" OR "hematemesis" OR "variceal" OR "varices") AND TITLE-ABS-KEY ("airway protection" OR "prophylactic" OR "prophylaxis"))
Number of results: 64
APPENDIX II
NEWCASTLE - OTTAWA QUALITY ASSESSMENT SCALE COHORT STUDIES
Note: A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability
Selection
-
1)
Representativeness of the exposed cohort
-
a) Truly representative of the average _______________ (describe) in the community ◻b) Somewhat representative of the average ______________ in the community ◻c) Selected group of users eg nurses, volunteers ◻d) No description of the derivation of the cohort ◻
-
-
2)
Selection of the non exposed cohort
-
a) Drawn from the same community as the exposed cohort ◻b) Drawn from a different source ◻c) No description of the derivation of the non exposed cohort ◻
-
-
3)
Ascertainment of exposure
-
a) Secure record (eg surgical records) ◻b) Structured interview ◻c) Written self report ◻d) No description ◻
-
-
4)
Demonstration that outcome of interest was not present at start of study
-
a) Yes ◻b) No ◻
-
Comparability
-
1)
Comparability of cohorts on the basis of the design or analysis
-
a) Study controls for _____________ (select the most important factor) ◻b) Study controls for any additional factor (This criteria could be modified to indicate specific control for a second important factor.) ◻
-
Outcome
-
1)
Assessment of outcome
-
a) Independent blind assessment ◻b) Record linkage ◻c) Self report ◻d) No description ◻
-
-
2)
Was follow-up long enough for outcomes to occur
-
a) Yes (select an adequate follow up period for outcome of interest) ◻b) No ◻
-
-
3)
Adequacy of follow up of cohorts
-
a) Complete follow up - all subjects accounted for ◻b) Subjects lost to follow up unlikely to introduce bias - small number lost - > ____ % (select an adequate %) follow up, or description provided of those lost) ◻c) Follow up rate < ____% (select an adequate %) and no description of those lost ◻d) No statement ◻
-
Wells, G. A, Shea, B., O'Connel, D. et al. The Newcastle-Ottawa scale (NOS) for assessing the quailty of nonrandomised studies in meta-analyses. http://www ohri ca/programs/clinical_epidemiology/oxford htm 2009 Feb 1.
REFERENCES
- 1.Lipper B, Simon D, Cerrone F. Pulmonary aspiration during emergency endoscopy in patients with upper gastrointestinal hemorrhage. Crit Care Med. 1991;19:330–3. doi: 10.1097/00003246-199103000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1–a46. doi: 10.1055/s-0034-1393172. [DOI] [PubMed] [Google Scholar]
- 3.Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101–13. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD Practice Guidelines Committee of American Association for Study of Liver D, Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086–102. doi: 10.1111/j.1572-0241.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- 5.Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345–60. doi: 10.1038/ajg.2011.480. quiz 361. [DOI] [PubMed] [Google Scholar]
- 6.Waye JD. Intubation and sedation in patients who have emergency upper GI endoscopy for GI bleeding. Gastrointest Endosc. 2000;51:768–71. doi: 10.1016/s0016-5107(00)70104-0. [DOI] [PubMed] [Google Scholar]
- 7.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] 2008. [Last accessed on 2017 Jul 10]. Available from: www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 8.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 10.Perisetti A, Khan H, Sahmoun A, Newman W, Meidinger R. Role of prophylactic pre-esophagogastroduodenoscopy (EGD) endotracheal intubation (ETI) in upper gastrointestinal bleed (UGIB): A retrospective study. Am J Gastroenterol. 2013;108:S15–6. [Google Scholar]
- 11.Abdulsamad M, Kamireddy C, Karki N, Sakam S, Kumar K, Ebiem O, et al. Should we intubate the patient first? Outcomes of prophylactic endotracheal intubation for upper gastrointestinal bleeding. Am J Gastroenterol. 2016;111:S1283. [Google Scholar]
- 12.Lee PJ, Hayat U, Ullah H, Lopez R, Vargo JJ. Prophylactic endotracheal intubation in critically ill patients with upper gastrointestinal bleeding is associated with higher cardiopulmonary unplanned events. Gastrointest Endosc. 2016;85:AB201. doi: 10.1016/j.gie.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Hayat U, Lee PJ, Ullah H, Sarvepalli S, Lopez R, Vargo JJ. Association of prophylactic endotracheal intubation in critically ill patients with upper GI bleeding and cardiopulmonary unplanned events. Gastrointest Endosc. 2017 doi: 10.1016/j.gie.2016.12.008. DOI: http://dx.doi.org/10.1016/j.gie.2016.12.008 [In Press] [DOI] [PubMed] [Google Scholar]
- 14.Rudolph SJ, Landsverk BK, Freeman ML. Endotracheal intubation for airway protection during endoscopy for severe upper GI hemorrhage. Gastrointest Endosc. 2003;57:58–61. doi: 10.1067/mge.2003.46. [DOI] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch DG, Arguedas MR, Fallon MB. Risk of aspiration pneumonia in suspected variceal hemorrhage: The value of prophylactic endotracheal intubation prior to endoscopy. Dig Dis Sci. 2007;52:2225–8. doi: 10.1007/s10620-006-9616-0. [DOI] [PubMed] [Google Scholar]
- 18.Lohse N, Lundstrøm LH, Vestergaard TR, Risom M, Rosenstock SJ, Foss NB, et al. Anaesthesia care with and without tracheal intubation during emergency endoscopy for peptic ulcer bleeding: A population-based cohort study. Br J Anaesth. 2015;114:901–8. doi: 10.1093/bja/aev100. [DOI] [PubMed] [Google Scholar]
- 19.Rehman A, Iscimen R, Yilmaz M, Khan H, Belsher J, Gomez JF, et al. Prophylactic endotracheal intubation in critically ill patients undergoing endoscopy for upper GI hemorrhage. Gastrointest Endosc. 2009;69:e55–9. doi: 10.1016/j.gie.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang YM, Wang Y, Wang WW. Elective endotracheal intubation prior to emergent EGD in patients with suspected variceal hemorrhage: An evaluation of outcome and complications. Gastrointest Endosc. 2014;79:AB515–6. [Google Scholar]
- 21.Tang YM, Wang WZ. Prophylactic endotracheal intubation prior to urgent endoscopy in patients with suspected variceal hemorrhage: An evaluation of outcomes and complications. J Gastroenterol Hepatol Res. 2017;6:2324–8. [Google Scholar]
- 22.Almashhrawi AA, Rahman R, Jersak ST, Asombang AW, Hinds AM, Hammad HT, et al. Prophylactic tracheal intubation for upper GI bleeding: A meta-analysis. World J Metaanal. 2015;3:4–10. doi: 10.13105/wjma.v3.i1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting.Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subgroup analysis by bleeding type for aspiration outcome
Subgroup analysis by bleeding type for pneumonia outcome
Subgroup analysis by bleeding type for mortality outcome
Subgroup analysis by bleeding type for hospital length of stay outcome
Sensitivity analysis excluding studies published in abstract form only for aspiration outcome
Sensitivity analysis excluding studies published in abstract form only for pneumonia outcome
Sensitivity analysis excluding studies published in abstract form only for mortality outcome
Sensitivity analysis excluding studies published in abstract form only for LOS outcome
Sensitivity analysis excluding Lee et al for mortality outcome
Sensitivity analysis excluding Rudolph et al for aspiration outcome