Infection is a dynamic biological process underpinned by a complex interplay between the pathogen and the host. Microbes from all domains of life, including bacteria, viruses, fungi, and protozoan parasites, have the capacity to cause infection.
KEYWORDS: AIM2, NAIP, NLRC4, NLRP1, NLRP3, Pyrin, caspase-1, caspase-11, gasdermin, pyroptosis
SUMMARY
Infection is a dynamic biological process underpinned by a complex interplay between the pathogen and the host. Microbes from all domains of life, including bacteria, viruses, fungi, and protozoan parasites, have the capacity to cause infection. Infection is sensed by the host, which often leads to activation of the inflammasome, a cytosolic macromolecular signaling platform that mediates the release of the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18 and cleavage of the pore-forming protein gasdermin D, leading to pyroptosis. Host-mediated sensing of the infection occurs when pathogens inject or carry pathogen-associated molecular patterns (PAMPs) into the cytoplasm or induce damage that causes cytosolic liberation of danger-associated molecular patterns (DAMPs) in the host cell. Recognition of PAMPs and DAMPs by inflammasome sensors, including NLRP1, NLRP3, NLRC4, NAIP, AIM2, and Pyrin, initiates a cascade of events that culminate in inflammation and cell death. However, pathogens can deploy virulence factors capable of minimizing or evading host detection. This review presents a comprehensive overview of the mechanisms of microbe-induced activation of the inflammasome and the functional consequences of inflammasome activation in infectious diseases. We also explore the microbial strategies used in the evasion of inflammasome sensing at the host-microbe interaction interface.
INTRODUCTION
Bacteria, fungi, viruses, and protozoa are capable of causing infection, potentially leading to death of the host. The host immune system acts as a guardian and defends the body from challenge by pathogens. Both innate and adaptive immune systems contribute to the killing and clearance of invading microbes. Pattern recognition receptors (PRRs) of the innate immune system initiate sensing of pathogens and danger signals by recognizing pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs), respectively. Activation of PRRs induces a cascade of inflammatory host responses that, in most cases, rapidly resolve the infection (1). However, inflammation is a double-edged sword and can result in the development of autoimmunity, inflammatory diseases, and cancer.
PRRs evoke diverse antimicrobial activities by initiating activation of the transcription factor NF-κB, mitogen-activated protein kinase (MAPK), and interferon (IFN) signaling pathways, leading to the transcription of hundreds of genes that collectively induce an antipathogen state in the cell. PRRs include the family members Toll-like receptors (TLRs), C-type lectin receptors (CLRs), RIG-I-like receptors (RLRs), NOD-like receptors (NLRs), AIM2-like receptors (ALRs), and cytoplasmic DNA and RNA sensors (2–4). Both membrane-bound and cytoplasmic sensors function synergistically to mount an effective antimicrobial response against invading microbes. TLRs and CLRs are membrane-bound receptors which detect PAMPs and DAMPs on the cell surface and within endosomes. ALRs, NLRs, RLRs, and cytoplasmic DNA and RNA sensors recognize PAMPs and DAMPs that have reached the cytoplasm of the cell, which is achieved via either infection by pathogens or damage to organelles of the host cell leading to the release of endogenous DAMPs.
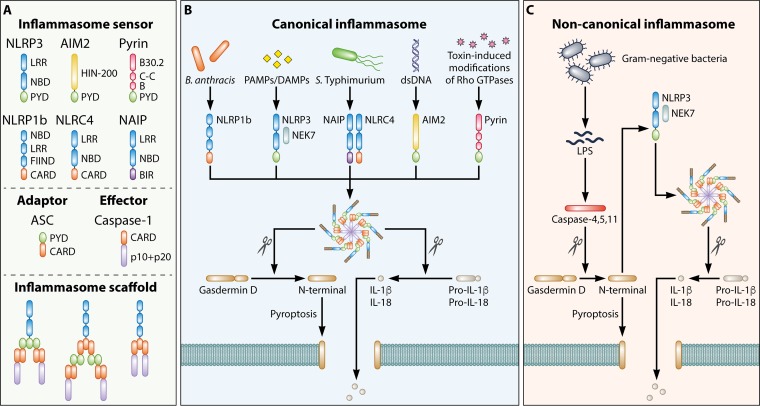
Certain NLRs and ALRs assemble inflammasome complexes in response to PAMPs and DAMPs (5). The inflammasome is a multiprotein complex which regulates activation of the cysteine protease caspase-1, leading to proteolytic processing and secretion of the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18. In addition, this multimeric complex induces an inflammatory form of cell death called pyroptosis (6). An inflammasome complex comprises one or more sensors (NLRs, ALRs, or Pyrin), the adaptor protein apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC), and caspase-1 (7, 8) (Fig. 1A). On stimulation with PAMPs or DAMPs, an inflammasome sensor is activated and associates with ASC, thereby leading to oligomerization of ASC and formation of a filamentous scaffold (9, 10). ASC filaments interact with inactive procaspase-1 monomers to facilitate proximity-induced activation of caspase-1 (9, 10). This sensor–ASC–caspase-1 scaffold can readily be visualized endogenously as a cytoplasmic speck of 0.8 to 1 μm in diameter (11–14). Furthermore, inflammasome specks act as DAMPs following their release by pyroptotic cells, resulting in amplification of inflammation (15, 16). Caspase-1 activated within the inflammasome complex executes pyroptosis by inducing cleavage of the propyroptotic factor gasdermin D (17–19), yielding a cleaved N-terminal fragment of gasdermin D that oligomerizes and forms pores on the host cell membrane (20–24) (Fig. 1B). The consequences of this event are cell swelling, lytic cell death, and liberation of cytoplasmic contents, including biologically active IL-1β and IL-18. Evidence suggests that the pores formed by gasdermin D allow passive release of IL-1β and DAMPs of less than 10 to 16 nm in diameter from the cytoplasm of macrophages to the extracellular space, even prior to lysis and death of the host cell (25–27). Self-cleavage of caspase-1 at the caspase activation and recruitment domain (CARD) linker region releases caspase-1 from the inflammasome complex, resulting in termination of caspase-1 activity (28).
FIG 1.
Architecture of inflammasome complexes. (A) Formation of an inflammasome complex is initiated by an inflammasome sensor. Inflammasome sensors carry a pyrin domain (PYD) and/or a caspase activation and recruitment domain (CARD). They may also carry a leucine-rich-repeat domain (LRR), a nucleotide-binding domain (NBD), a HIN-200 domain, a B30.2 domain, a coiled-coil domain (C-C), a B-box domain (B), a function-to-find domain (FIIND), or a baculovirus inhibitor of apoptosis repeat (BIR). Other inflammasome components include the inflammasome adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) and the effector protein caspase-1. A PYD-containing inflammasome sensor interacts with the PYD of ASC, allowing the CARD of ASC to interact with the CARD of caspase-1. A CARD-containing inflammasome sensor can interact with the CARD of ASC, whereby the PYD of ASC interacts with the PYD of an additional ASC. The CARD of ASC then interacts with the CARD of caspase-1. Alternatively, a CARD-containing inflammasome sensor may directly interact with caspase-1 via their respective CARDs. (B) Canonical inflammasome complexes are activated by a range of pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs). Caspase-1 cleaves the pore-forming factor gasdermin D, whereby the N-terminal domain of gasdermin D forms pores in the host cell membrane. Caspase-1 also cleaves the proinflammatory cytokines pro-IL-1β and pro-IL-18, generating biologically active versions of these cytokines for release through the membrane pores generated by gasdermin D. The pores formed by gasdermin D also lead to lytic cell death via pyroptosis. (C) The noncanonical inflammasome is a pathway specifically activated by Gram-negative bacteria. In this pathway, lipopolysaccharides (LPS) are introduced into the cytoplasm during infection and sensed by human caspase-4 and caspase-5 and mouse caspase-11. These inflammatory caspases can also cleave gasdermin D, in a manner similar to that by caspase-1, leading to the induction of pyroptosis. The N-terminal domain of gasdermin D also induces activation of the NLRP3 inflammasome and the associated proteolytic cleavage of pro-IL-1β and pro-IL-18.
Pathogens have evolved various immune evasion mechanisms. To counteract these evasion strategies, both extracellular and intracellular surveillance systems must overlap and operate in unity to provide efficient recognition of the pathogen. This review focuses on the diverse cytosolic innate immune recognition pathways of microorganisms, with a focus on inflammasome sensors and the respective families of PAMPs that they recognize. We also shed light on the downstream effector functions of the inflammasome that drive protection against infectious diseases.
GENERAL OVERVIEW OF INFLAMMASOME SENSING AND SIGNALING
Several cytoplasmic innate immune sensors form inflammasome complexes in response to PAMPs and DAMPs, including AIM2, NAIP, NLRC4, NLRP1, NLRP3, Pyrin, and caspase-11 (Fig. 1); further, mouse NLRP6, NLRP9b, and NLRP12 and human NLRP2, NLRP7, and IFN-inducible protein 16 (IFI16) have also been proposed to activate caspase-1 (5), although the ability of these sensors to form bona fide inflammasome complexes has remained uncertain (29). Inflammasome sensors initiate a generally proinflammatory signaling cascade upon activation; however, the molecular mechanisms regulating the activation of each sensor and the composition of each inflammasome complex are distinct.
Inflammasome sensors differ based on whether they are activated by direct interaction with a ligand or by indirect sensing of broader cellular perturbations (29). AIM2, NAIP, and caspase-11 directly bind to their cognate ligands, whereas NLRP1, NLRP3, and Pyrin respond to cellular perturbations (Fig. 1B). AIM2 binds exclusively to cytoplasmic double-stranded DNA (dsDNA), independent of the sequence of the dsDNA (30–33). Similarly, NAIP proteins directly bind flagellin and components of the type III secretion systems (T3SS) of Gram-negative bacteria, such as Salmonella enterica serovar Typhimurium, leading to activation of the NLRC4 inflammasome (discussed below) (34, 35). Mouse caspase-11 and the two human analogs caspase-4 and caspase-5 are all sensors of cytoplasmic lipopolysaccharide (LPS) (36–39) (Fig. 1C). Direct interaction between the lipid A portion of LPS and the CARD of caspase-11 catalyzes oligomerization of caspase-11 and activation of the noncanonical NLRP3 inflammasome (36, 40–44). While caspase-11 cannot directly cleave pro-IL-1β and pro-IL-18, it can induce pyroptosis in the absence of caspase-1 by cleaving gasdermin D (40). Further, LPS-induced activation of caspase-11 leads to potassium efflux (45) or caspase-11-dependent cleavage of the membrane channel pannexin-1, followed by an ATP release that subsequently activates the purinergic receptor P2X7R and pyroptosis (46).
NLRP3 responds to cellular perturbations emanating from stimulation with PAMPs and DAMPs rather than through binding to an activating ligand (Fig. 1B). These cellular cues include lysosomal disruption and subsequent leakage of cathepsin B (47), potassium efflux via P2X7R (48–51), formation of pores on the cell membrane (52), translocation of cardiolipin from the inner to the outer mitochondrial membrane (53), production of reactive oxygen species (ROS) (54, 55), oxidized mitochondrial DNA (56), calcium influx and a reduction in cellular cyclic AMP (57–59), and alteration of the cell volume (60). While several molecular mechanisms involving homeostatic disruption have been proposed, a single unifying signal leading to NLRP3 inflammasome activation has yet to emerge.
Similar to NLRP3, NLRP1 and Pyrin respond to cellular perturbations rather than to direct binding to a specific ligand. The mechanism of NLRP1 activation is cleavage dependent; human NLRP1 and mouse NLRP1b are activated by any protein capable of inducing N-terminal proteolytic cleavage of NLRP1 (61–67), demonstrating that these inflammasome sensors are not specific to any single ligand. Activation of Pyrin is more complex. In homeostasis, Pyrin is phosphorylated on two serine residues by the RhoA effector serine-threonine kinases PKN1 and PKN2, promoting interaction between Pyrin and the regulatory proteins 14-3-3ε and 14-3-3τ (68–70). The interaction between Pyrin and 14-3-3 regulatory proteins holds Pyrin in an inactive state to prevent activation of the Pyrin inflammasome in unstimulated cells (70, 71). Rho-inactivating toxins and T3SS effectors produced by many bacteria inhibit the action of PKN1 and PKN2, thereby relieving phosphorylation of Pyrin and promoting activation of the Pyrin inflammasome (71–76) (Fig. 1B).
The requirement of the inflammasome adaptor protein ASC for the formation of an inflammasome complex also differs among inflammasome sensors (Fig. 1A). ASC is a bipartite protein comprised of a pyrin domain (PYD) and a CARD. AIM2, NLRP3, and Pyrin carry a PYD but not a CARD and must therefore bind ASC in order to recruit the CARD-bearing procaspase-1. In contrast, NLRP1 and NLRC4 carry a CARD and can therefore directly recruit procaspase-1 in the absence of ASC (77–80). However, optimal secretion of IL-1β is often achieved only when ASC is present in these inflammasome complexes (12, 13, 81), indicating the universal importance of ASC in inflammasome signaling.
Secretion of IL-1β and IL-18 and induction of pyroptosis have instrumental roles in eliciting, magnifying, and perpetuating inflammation and, in most cases, reducing the overall pathogen burden. Both cytokines serve as a bridge between the innate and adaptive immune systems: IL-1β is a potent promoter of inflammation, immune cell extravasation, and vasodilation with additional capabilities in modulating adaptive immunity (82), while IL-18 triggers local inflammation as well as IFN-γ production in natural killer (NK) cells, CD4+ TH1 cells, and CD8+ cytotoxic T cells and augments the development of CD4+ TH2 cells (83). In addition to promoting the release of cytokines and DAMPs, another overarching function of pyroptosis is to expel an infected cell from the tissue (84, 85) or to expel pathogens from infected macrophages (86, 87). In contrast, neutrophils release IL-1β without undergoing pyroptosis (88), reflecting the different roles of immune cells in clearing pathogens. Thus, the inflammasome facilitates disruption of the replicative niche exploited by intracellular pathogens and exposes them to potentially less favorable conditions (discussed below).
BACTERIA
A plethora of bacteria engage with and shape the development of the host immune system. Reflecting the coevolution of bacteria and the host immune system, host cells encode innate immune sensors capable of detecting and exploiting the conservation of key bacterial PAMPs (Fig. 2). In this section, we discuss several groups of bacterial PAMPs that are recognized by their respective inflammasome sensors and highlight some of the strategies employed by bacteria to block inflammasome activation.
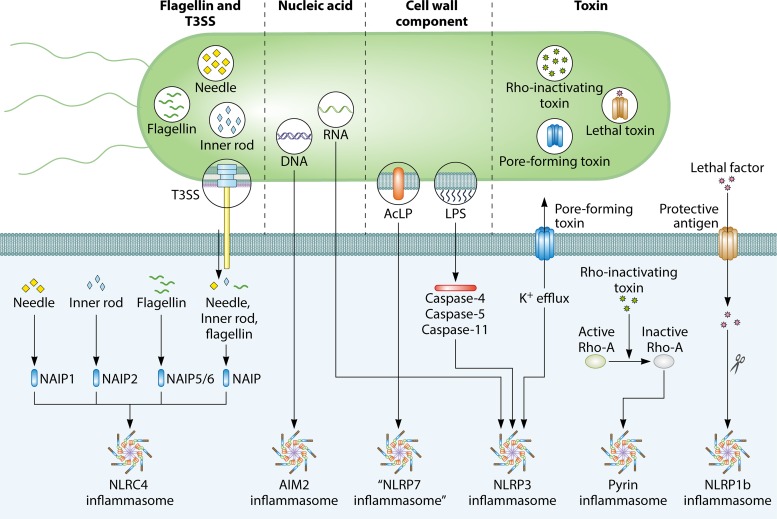
FIG 2.
Major bacterial activators of the inflammasome. Four major groups of bacterial components trigger activation of the inflammasome. Flagellin and components of the type III secretion system (T3SS) can be injected into the cytoplasm of the host cell via the T3SS. The T3SS needle and inner rod proteins are sensed by mouse NAIP1 and NAIP2, respectively, whereas flagellin is sensed by mouse NAIP5 or NAIP6. The needle and inner rod proteins and flagellin are all sensed by human NAIP. Ligand-bound NAIPs recruit NLRC4 to the same complex to drive activation of the NLRC4 inflammasome. The bacterial nucleic acid molecules DNA and RNA can activate the AIM2 and NLRP3 inflammasomes, respectively. RNA-DNA hybrids derived from bacteria (not shown) can also activate the NLRP3 inflammasome. The cell wall components acylated lipopeptides (AcLP) might activate a putative NLRP7 inflammasome in human macrophages. Lipopolysaccharides (LPS) from the cell walls of Gram-negative bacteria activate human caspase-4 and caspase-5 and mouse caspase-11. These inflammatory caspases oligomerize and drive activation of the NLRP3 inflammasome. Pore-forming toxins produced by bacteria induce K+ efflux, a physiological aberration sensed by the NLRP3 inflammasome. Rho-inactivating toxins inactivate the host GTPase RhoA, which relieves inhibition of Pyrin, leading to activation of the Pyrin inflammasome. The protective antigen of lethal toxin generates pores on the host cell membrane, allowing lethal factor to enter the cytoplasm and mediate cleavage of NLRP1b. Cleaved NLRP1b induces formation of the NLRP1b inflammasome.
LPS
The cell wall of bacteria functions to maintain bacterial cell integrity and resist osmotic stress. LPS, a key component of the outer membrane of Gram-negative bacteria, is composed of a lipid A portion, a core oligosaccharide, and an O antigen consisting of repeating glycan subunits. LPSs from many Gram-negative bacteria are recognized by human caspase-4, human caspase-5, or mouse caspase-11 (37–43, 89–91) (Table 1). Caspase-11 directly binds to the penta- or hexa-acylated lipid A portion of cytoplasmic LPS via its CARD, inducing self-oligomerization and activation (36, 44). Some Gram-negative bacteria, including Porphyromonas gingivalis (92), Yersinia pestis (93), Francisella tularensis (44), and Rhodobacter sphaeroides (36), have altered lipid A structures that cannot activate mouse caspase-11. In contrast, human caspase-4 is able to recognize underacylated LPS from F. tularensis subsp. novicida and Bacteroides vulgatus in macrophages, in addition to detecting penta- or hexa-acylated lipid A (94). This interspecies difference in sensing of distinct LPS moieties may reflect differences in the CARDs of caspase-4 and caspase-11, which share 51% sequence identity (94). Whether caspase-5, which shares only 39% sequence identity with caspase-11 in the CARD (94), can sense a different repertoire of LPS variants or other PAMPs remains to be determined. These inflammatory caspases subsequently trigger activation of the NLRP3 inflammasome upon sensing LPS, giving rise to the nomenclature of the noncanonical NLRP3 inflammasome pathway (Fig. 1C) (40).
TABLE 1.
Gram-negative bacteria produce PAMPs and DAMPs during infection that activate inflammasomes and other sensors
| Bacterium | Inflammasome sensor(s) [activator(s)] [reference(s)] |
|---|---|
| Acholeplasma laidlawii | NLRP3 (unknown activator) (119), NLRP7 (acylated lipopeptide) (119), NLRP12 (unknown activator) (119) |
| Acinetobacter baumannii | NLRP3 (ROS, cathepsin release, K+ efflux, P2X7R) (114, 347), caspase-11 (LPS) (112, 114) |
| Aeromonas hydrophila | NLRP3 (aerolysin, hemolysin, multifunctional repeat-in-toxin) (348) |
| Aeromonas trota | NLRP3 (aerolysin) (52), NLRC4 (unknown activator) (52) |
| Aeromonas veronii | NLRC4 (T3SS) (349), NLRP3 (aerolysin, T3SS) (349) |
| Aggregatibacter actinomycetemcomitans | NLRP3 (cytolethal distending toxin) (350) |
| Bordetella pertussis | NLRP3 (adenylate cyclase toxin, CyaA) (351), Pyrin (pertussis toxin) (74) |
| Brucella abortus | NLRP3 (mitochondrial ROS) (352, 353), AIM2 (dsDNA) (352–354) |
| Burkholderia cenocepacia | NLRP3 (T2SS, T4SS, T6SS) (355), mouse Pyrin (T6SS dependent) (72), human Pyrin (T6SS effector TecA) (73, 76) |
| Burkholderia pseudomallei | NLRC4 (T3SS rod BsaK, flagellin) (123, 356), NLRP3 (unknown activator) (356), caspase-11 (LPS) (42) |
| Burkholderia thailandensis | NAIP2-NLRC4 (T3SS rod BsaK) (35), human NAIP-NLRC4 (T3SS needle BsaL and rod BsaK) (35, 133), caspase-11 (LPS) (42) |
| Campylobacter jejuni | NLRP3 (unknown activator) (155) |
| Chlamydia pneumoniae | NLRP3 (mitochondrial dysfunction) (357, 358) |
| Chlamydia trachomatis | NLRP3 (LPS, mitochondrial ROS) (103, 359), AIM2 (dsDNA) (103, 359) |
| Chlamydia muridarum | Caspase-11 (LPS) (103), AIM2 (dsDNA) (103) |
| Chromobacterium violaceum | Human NAIP-NLRC4 (T3SS needle CprI) (35) |
| Citrobacter rodentium | NLRC4 (unknown activator) (360, 361), NLRP3 (T3SS independent) (360, 362), caspase-11 (LPS) (40, 104) |
| Escherichia coli | NLRC4 (T3SS rod EprJ and EscI) (123), NLRP3 (RNA-DNA hybrids, RNA) (177, 180), NLRP6 (unknown activator) (363), human NAIP-NLRC4 (T3SS needle EprI) (35), caspase-11 (LPS) (40, 104) |
| Francisella tularensis | AIM2 (dsDNA) (100, 157–160, 162–165, 171, 364), NLRP3 (IgG-opsonized, inactivated F. tularensis LVS) (365–367), caspase-4 (LPS) (94) |
| Fusobacterium nucleatum | NLRP3 (mitochondrial ROS) (92, 368) |
| Helicobacter pylori | NLRP3 (ROS signaling, lysosomal destabilization, K+ efflux, VacA) (369–373) |
| Histophilus somni | Pyrin (IbpA toxin) (72) |
| Klebsiella pneumoniae | NLRC4 (unknown activator) (374), NLRP3 (unknown activator) (375), caspase-11 (LPS) (113) |
| Legionella pneumophila | AIM2 (DNA) (173), NAIP5 and NAIP6-NLRC4 (flagellin) (35, 126, 139, 376, 377), caspase-4 (LPS) (37), caspase-11 (LPS) (91) |
| Neisseria gonorrhoeae | NLRP3 (lipooligosaccharide) (378) |
| Photorhabdus luminescens | NAIP5 and NAIP6-NLRC4 (flagellin) (35) |
| Porphyromonas gingivalis | NLRP3 (ATP, K+ efflux, and cathepsin B) (379, 380), caspase-11 (LPS via OMVs) (381), AIM2 (dsDNA) (379) |
| Pseudomonas aeruginosa | NAIP5 and NAIP6-NLRC4 (flagellin) (35, 123, 382, 383), NAIP2-NLRC4 (T3SS rod PscI) (35, 123, 384), NLRP3 (exolysin toxin ExlA) (385), human NAIP-NLRC4 (T3SS rod PscI and needle PscF) (132), caspase-11 (LPS) (104) |
| Salmonella enterica serovar Typhimurium | Human NAIP-NLRC4 (T3SS needle PrgI and rod PrgJ, flagellin) (35, 127, 130, 133), mouse NAIP1-NLRC4 (T3SS needle) (131), mouse NAIP2-NLRC4 (T3SS rod PrgJ) (34, 35, 123, 186), mouse NAIP5 or NAIP6-NLRC4 (flagellin) (34, 35, 138), caspase-4 and caspase-5 (LPS) (38, 85), caspase-11 (LPS) (13, 14) |
| Serratia marcescens | NLRP3 (toxin ShlA) |
| Shigella flexneri | NAIP2-NLRC4 (T3SS rod MxiI) (123, 129), NLRP3 (unknown activator, Shiga toxin) (386, 387), NLRP1b (metabolic stress) (388), human NAIP-NLRC4 (T3SS needle MxiH and rod MxiI) (35, 130, 133) |
| Tannerella forsythia | NLRP3–caspase-11 (LPS via OMVs) (381), AIM2 (dsDNA) (381) |
| Treponema denticola | NLRP3 (surface protein Td92, caspase-11 via LPS in OMVs) (381, 389), AIM2 (dsDNA) (381) |
| Treponema pallidum | NLRP3? (TpF1-induced ATP release) (390) |
| Vibrio cholerae | NLRP3 (cholera toxin B, El Tor hemolysin HlyA, cytotoxin MARTXVc) (40, 184), caspase-11 (LPS) (40) |
| Vibrio parahaemolyticus | Pyrin (toxin VopS) (72) |
| Vibrio vulnificus | NLRP3 (hemolysin/cytolysin VvhA, cytotoxin MARTXVv) (184) |
| Yersinia spp. | NAIP5 and NAIP6-NLRC4 (flagellin) (35, 93), NLRP3 (T3SS effectors YopB and YopD) (93, 391–393), NLRP12 (T3SS) (394), Pyrin (YopE and YopT) (395, 396) |
The entry of LPS into the cytoplasm is an important event that leads to activation of the noncanonical NLRP3 inflammasome. Several mechanisms have been discovered to explain how LPS might reach the cytoplasm of the host cell for sensing by caspase-11. Naturally cytoplasmic pathogens, such as Burkholderia thailandensis and Burkholderia pseudomallei, introduce LPS into the cytoplasm as they escape the pathogen-containing vacuole, thereby driving rapid and robust caspase-11 activation (42). However, vacuole-restricted bacteria, such as Escherichia coli, Citrobacter rodentium, and Vibrio cholerae, can also activate the noncanonical NLRP3 inflammasome (14, 40, 90, 95, 96), implying the existence of alternative routes by which LPS can enter the cytoplasm. In the case of vacuole-restricted bacteria, type I IFN signaling, which is normally induced in response to infection, has an important role in the liberation of LPS in the cytoplasm. Type I IFNs upregulate the expression of host IFN-inducible GTPases, including guanylate-binding proteins (GBPs) and immunity-related GTPases (IRGs) (97). In mouse macrophages, GBPs colocalize with pathogen-containing vacuoles, where they facilitate lysis of the vacuolar membrane such that the bacteria and their associated LPS are released into the cytoplasm for detection by caspase-11 (98, 99). GBPs can also direct IRGB10 to the cell membrane of cytoplasm-exposed Gram-negative bacteria to induce bacteriolysis (100), thereby increasing the amount of free LPS, but also DNA (discussed further below), in the cytoplasm. Bioinformatic analysis of IRG family members identified an amphipathic helix on the C terminus of IRGB10 (100) that is a putative transmembrane region with antimicrobial potential. It is possible that the transmembrane region of IRGB10 may allow insertion into and destabilization of bacterial membranes. In mouse embryonic fibroblasts, pathogen-containing vacuoles carrying Chlamydia trachomatis are decorated with ubiquitin in an IRG-dependent manner (101). This ubiquitination facilitates GBP recruitment to the membrane and subsequent lysis of the pathogen-containing vacuole (101). Therefore, lysis of pathogen-containing vacuoles represents an important mechanism by which LPS from vacuole-restricted bacteria can enter the cytoplasm (102). However, it is noteworthy that in the case of the related bacterium Chlamydia muridarum, recruitment of GBPs to the pathogen-containing vacuole or the bacterial cell wall is not necessary for activation of the inflammasome (103), suggesting a further route by which ligands from this bacterium can be introduced into the cytoplasm.
Another mechanism by which LPS can be introduced into the cytoplasm in the absence of cytoplasmic invasion by bacteria is through outer membrane vesicles (OMVs). LPS encapsulated within OMVs, which are shed by Gram-negative bacteria (such as E. coli), can be endocytosed via a clathrin-mediated process (104). This process is followed by escape of the LPS from the endocytic compartment into the cytoplasm of mouse macrophages to induce caspase-11 activation (104). It has also been demonstrated that GBPs encoded on chromosome 3 or GBP2 is involved (105). Mechanistically, isoprenylated GBPs were found to associate with the surface of OMVs or with transfected LPS (106), suggesting that LPS is the PAMP which targets GBPs to bacterial membranes and OMVs. However, the exact mechanism by which GBPs facilitate release of LPS for sensing by caspase-11 remains poorly defined. It is possible that GBPs induce the rupture of OMVs to release LPS into the cytoplasm, perhaps by recruiting additional membrane-disrupting proteins, such as IRGB10 (100). Alternatively, GBPs bound to OMVs may directly facilitate binding of caspase-11 to LPS embedded in the OMV membrane.
Sensing of LPS via caspase-11 and activation of the noncanonical NLRP3 inflammasome can lead to a protective or detrimental outcome. For example, injection of LPS into mice induces lethality (18, 40, 41, 44, 107–110). In this case, caspase-11- and gasdermin D-mediated tissue damage and pyroptosis of endothelial cells in response to LPS have been proposed to be responsible for driving the lethality of endotoxemia (18, 40, 44, 95, 111). In contrast, caspase-11 provides host protection in vivo in response to infection by Gram-negative bacterial pathogens, including S. Typhimurium, enteropathogenic E. coli, B. thailandensis, B. pseudomallei, Legionella pneumophila, Acinetobacter baumannii, and Klebsiella pneumoniae (42, 85, 89, 112–114). The increased susceptibility of mice lacking caspase-11 or gasdermin D to these bacteria can likely be attributed to defective activation of pyroptosis and an increased bacterial burden. Indeed, pyroptosis can induce extrusion of infected cells from the epithelium (85). In addition, pyroptosis can release intracellular bacteria into the extracellular milieu to promote phagocytosis and killing by neutrophils (86, 115–117) or can shed infected cells for removal by the host (84, 85). These different experimental systems provide insights into the outcome of caspase-11 activation by Gram-negative bacterial pathogens.
In humans, extracellular LPS has also been reported to trigger an alternative NLRP3 inflammasome pathway in monocytes (118). This pathway is propagated by TLR4–TRIF–RIPK1–FADD–caspase-8 signaling upstream of NLRP3 but differs from both the canonical and noncanonical NLRP3 inflammasomes in that activation of this pathway does not lead to pyroptosis or ASC speck formation and is not dependent on potassium efflux (118). Furthermore, the alternative NLRP3 inflammasome pathway appears to be species and cell type specific, as it is absent in mice and in human macrophages and dendritic cells (118). This TLR4-mediated sensing of extracellular LPS may initiate an early warning system for infection by secreting IL-1β without the cell undergoing pyroptosis. In this way, LPS, one of the strongest bacterium-derived activators of the immune system and inflammasomes, can trigger activation of inflammasome pathways without entering the cytoplasm of human monocytes.
Other Cell Wall Components
There is some evidence that bacterial cell wall components other than LPS, including lipopeptides and muramyl dipeptide, activate the inflammasome (Tables 1 to 3). Both Gram-negative and Gram-positive bacteria have acylated lipopeptides associated with their cell walls, which activate NLRP7 in human macrophages (119). Activation of NLRP7 has been reported to result in ASC-dependent caspase-1 activation and restriction of the replication of Staphylococcus aureus and Listeria monocytogenes (119); however, human macrophages lacking NLRP7 retain some inflammasome activation in response to bacterial infection (119). NLRP3 knockdown also results in decreased IL-1β secretion upon S. aureus and L. monocytogenes infection (119), suggesting that NLRP7 may function redundantly with NLRP3 for inflammasome activation in these infections. Further studies are required to more fully elucidate the relative contributions of NLRP7 and NLRP3 to inflammasome activation in these instances.
TABLE 2.
Gram-positive bacteria produce PAMPs and DAMPS during infection that activate inflammasomes and other sensors
| Bacterium | Inflammasome sensor(s) [activator(s)] [reference(s)] |
|---|---|
| Bacillus anthracis | NLRC4 (unknown activator) (397), NOD2 and NLRP1 (muramyl dipeptide) (122), NLRP1 (lethal toxin) (61, 62, 190) |
| Bacillus amyloliquefaciens | NLRP3 (amylosin toxin) (398) |
| Clostridium difficile | NLRP3 (toxins TcdA and TcdB) (399), Pyrin (toxins TcdA and TcdB) (71, 72, 75) |
| Clostridium botulinum | Pyrin (C3 toxin) (72) |
| Lactobacillus casei | NLRP3 (cathepsin B release triggered by cell wall fragments) (120) |
| Listeria monocytogenes | AIM2 (dsDNA) (154, 400–403), NLRC4 (flagellin) (137, 400), NLRP3 (listeriolysin O?) (154, 187, 400), NLRP7 (acylated lipopeptide?) (119), NLRP1b (metabolic stress) (388) |
| Propionibacterium acnes | NLRP3 (mitochondrial ROS, ATP, K+ efflux) (404–407) |
| Staphylococcus aureus | NLRP3 (α-, β-, and γ-hemolysins) (119, 185, 408), NLRP7 (acylated lipopeptide?) (119), AIM2 (dsDNA) (409), NLRC4 (cathepsin B release via alpha-toxin) (410) |
| Streptococcus agalactiae | NLRP3 (RNA, β-hemolysin) (178) |
| Streptococcus pyogenes | NLRP3 (streptolysin O, ADP-ribosyltransferase toxin, surface protein M1) (121, 411–413) |
| Streptococcus pneumoniae | AIM2 (dsDNA) (414, 415), NLRP3 (pneumolysin) (414, 416–420) |
TABLE 3.
Gram-variable bacteria produce PAMPs and DAMPs during infection that activate inflammasomes and other sensors
| Bacterium | Inflammasome sensor(s) [activator(s)] [reference(s)] |
|---|---|
| Mycobacterium bovis | AIM2 (dsDNA) (421) |
| Mycobacterium marinum | NLRP3 (Esx-1, type VII secretion system) (422) |
| Mycobacterium tuberculosis | AIM2 (dsDNA) (423), NLRP3 (ESAT-6, dsRNA) (424, 425) |
| Mycoplasma pneumoniae | NLRP3 (ADP-ribosylation by CARDS toxin) (191, 192) |
| Mycoplasma spp. | NLRP7 (acylated lipopeptide) (119), NLRP3 (cathepsin B, K+ efflux, ROS) (426, 427) |
Cell wall fragments derived from Lactobacillus casei elicit NLRP3 inflammasome activation in mouse vascular endothelial cells (120). Mechanistically, cell wall fragments increase lysosomal permeability and thereby facilitate release of cathepsin B into the cytoplasm to activate NLRP3 (120). The cell surface protein M1 of Streptococcus pyogenes can likewise activate the NLRP3 inflammasome upon clathrin-mediated endocytosis, in a manner dependent on potassium efflux (121). A further study suggests that the muramyl dipeptide of Bacillus anthracis is detected by its cognate cytosolic sensor, NOD2, which has been reported to recruit NLRP1 to mediate caspase-1 activation and secretion of IL-1β (122). However, the mechanistic relationship, if any, between NOD1 and NLRP1 activation remains unclear. Previous studies have shown that NLRP1 is activated by protease-specific cleavage of the N-terminal domain or the function-to-find domain; therefore, NOD2 binding to muramyl dipeptide potentially has some role in triggering this cleavage event. However, it is unlikely that muramyl dipeptide would induce direct cleavage of NLRP1. Taken together, these studies highlight the potential of cell wall components to trigger activation of multiple inflammasomes.
Flagellin and Secretion Systems
Motile bacteria frequently encode specialized organelles, called flagella, which facilitate bacterial movement and attachment. Repeating subunits of the monomeric flagellin protein are the main constituents of bacterial flagella (123). Many Gram-negative bacteria utilize a related but specialized apparatus, called a secretion system, to inject virulence factors into the host cytoplasm. While the virulence factors injected depend on the bacterial species, the secretion system apparatus itself is relatively conserved (124, 125).
Many flagellated bacteria trigger activation of the inflammasome via NAIP and NLRC4 (Tables 1 and 2). Cytoplasmic flagellin monomers are initially sensed by murine NAIP5 or NAIP6 (34, 35, 126) and the single human NAIP (127). A stretch of 35 amino acids from the C terminus of flagellin is required to trigger activation of the NLRC4 inflammasome (126). Mutating the inflammasome-activating flagellin residues abolishes motility in L. pneumophila (126), indicating that these residues are critical for flagellin function. Furthermore, structural analysis suggests that upon binding NAIP5, flagellin adopts a conformation similar to its structure in flagellar filaments (128), implying that mutating residues important for NAIP recognition would also disrupt formation of flagellar filaments.
Elements that constitute the T3SS apparatus can also be recognized directly by inflammasome sensors (Fig. 2). The T3SS rod proteins of many Gram-negative bacteria are sensed by the murine NAIP2 protein (34, 35, 123, 129) (Table 1); likewise, the T3SS needle proteins of many Gram-negative bacteria are sensed by NAIP1 in mice and by NAIP in humans (35, 130, 131) (Table 1). A further study has indicated that both the inner rod (PscI) and needle (PscF) T3SS proteins of Pseudomonas aeruginosa are sensed by human NAIP in macrophages and peripheral blood mononuclear cells (PBMCs) (132). An additional study broadened the role of human NAIP even further, with evidence that NAIP can recognize the T3SS inner rod proteins of S. Typhimurium, Shigella flexneri, and Burkholderia spp., in addition to flagellin and T3SS needle proteins from these bacteria (133). These studies have therefore shown the single human NAIP protein to be a versatile sensor capable of interacting with multiple PAMPs.
Further studies have mapped the nucleotide-binding domain (NBD) of mouse NAIP2, -5, and -6 (134), as well as the LRR domain of NAIP5 (128, 135), as the region responsible for ligand specificity. The evolutionary and functional basis for the different specificities of murine and human NAIPs remains a mystery. Rodent NAIP paralogs are likely to have evolved from expansion or duplication of a single progenitor, with NAIP1, -2, and -3 representing one family and NAIP4, -5, -6, and -7 representing another (136). Furthermore, murine NAIPs have been reported to undergo extensive recombination within regions conferring ligand specificity (134), which may facilitate positive diversifying selection to combat the evolution of bacterial ligands. However, why mice encode several NAIP proteins with specificity for distinct bacterial ligands while humans encode a single NAIP capable of recognizing the same set of ligands remains uncertain.
Flagellin must be introduced into the cytoplasm for recognition by cytoplasmic NAIP proteins to occur. Certain bacteria, such as L. monocytogenes, invade the cytoplasm shortly after phagocytosis in order to replicate and thereby directly introduce flagellin and other PAMPs into the cytoplasm (137). However, other bacteria remain in a pathogen-containing vacuole, and their flagellin is therefore sequestered and not exposed to the cytoplasm. In these instances, the T3SS or T4SS apparatus can pierce the pathogen-containing vacuole membrane or host cell membrane to deliver virulence factors and effector proteins, some of which may include flagellin subunits, into the cytoplasm (124, 125). For example, infection with the vacuole-restricted bacteria S. Typhimurium and L. pneumophila triggers activation of the NLRC4 inflammasome, a process which requires a functional T3SS and T4SS, respectively (138, 139). During this process, flagellin subunits can be secreted into the host cytoplasm through these secretion systems (13, 138, 140).
Activated NAIPs subsequently bind NLRC4 to initiate inflammasome formation (Fig. 2). Mechanistically, an association between the ligand and NAIP induces NLRC4 binding, causing a conformational change in bound NLRC4 that exposes an oligomerization surface to which additional inactive NLRC4 subunits are recruited to initiate inflammasome formation (128, 141–143). Activation of the NAIP-NLRC4 inflammasome can lead to the recruitment of NLRP3 to the same inflammasome complex (14), which may require phosphorylation of NLRC4 at residue S533 (144–146). A further study has shown that the leucine-rich repeat-containing kinase LRRK2 complexes with NLRC4 in response to S. Typhimurium infection, leading to phosphorylation of NLRC4 at residue S533 and to activation of the NLRC4 inflammasome (147).
The ability of NAIP-NLRC4 to recognize flagellin and T3SS apparatus proteins provides host protection against many Gram-negative bacterial infections (13, 14, 148). NLRC4 is highly expressed in mouse enterocytes and intestinal immune cells (149). Studies focusing on S. Typhimurium infection of the intestinal epithelium have suggested the mechanism by which NLRC4 and pyroptosis mediate the host defense against bacterial infection. Specifically, S. Typhimurium-infected wild-type (WT) enterocytes are ejected from the intestinal epithelium via NLRC4-driven pyroptosis (84). This expulsion mechanism is accompanied by the release of eicosanoids and IL-18 and is dependent on NAIP-NLRC4, caspase-1, and caspase-8 (150, 151). Consistent with this observation, caspase-8 is recruited to the NAIP-NLRC4 inflammasome in response to S. Typhimurium infection (152); however, it is robustly activated only with the absence or inhibition of gasdermin D or caspase-1 (19, 153).
Phase variation, downregulation, and concealment of ligands are important mechanisms used by bacteria to avoid or minimize detection by the NAIP-NLRC4 inflammasome. For instance, S. Typhimurium switches expression of T3SS proteins from SPI-1 to SPI-2 upon reaching the pathogen-containing vacuole, facilitating the secretion of effector proteins that promote survival in the vacuole; importantly, the SPI-2 rod protein SsaI is not recognized by NAIP (123), further contributing to pathogen survival. Further, a strain of S. Typhimurium that constitutively expresses flagellin is rapidly cleared in mice by the inflammasome pathway (86), suggesting that regulating flagellin expression is critical for bacterial survival. Similarly, L. monocytogenes flagellin expression is suppressed at 37°C (154), potentially allowing evasion of NLRC4 inflammasome detection in the gastrointestinal tract. Interestingly, the flagellins of Campylobacter jejuni and Helicobacter pylori fail to activate the NAIP-NLRC4 inflammasome (145, 155), most likely due to differences in flagellin sequence composition compared to that of flagellins produced by NLRC4-activating bacteria. These flagellins also fail to activate TLR5 (156), allowing these bacteria to evade detection by both known sensors of flagellin. Further, a T3SS-secreted effector protein of pathogenic Yersinia spp., YopK, can bind to its own T3SS translocon (93), thereby masking and preventing recognition of the T3SS by NAIPs. The prevalence of evasion mechanisms directed against the NAIP-NLRC4 inflammasome highlights the importance of this signaling axis in clearing Gram-negative bacterial infections.
Bacterial DNA
Both microbes and mammalian cells use the same basic biomolecules to encode genetic information. Mammalian cells have evolved mechanisms to distinguish self nucleic acids from nonself nucleic acids. DNA of host cells is normally sequestered inside the nucleus and mitochondria; such spatial separation allows cytosolic DNA sensors to respond only to PAMP- or DAMP-associated DNA in the cytoplasmic space (Fig. 2).
The inflammasome-forming DNA sensor AIM2 responds to infections by a range of Gram-negative, Gram-positive, and Gram-variable bacteria (Tables 1 to 3). The mechanism by which DNA, normally sequestered within the cell membrane and cell wall of bacteria, becomes exposed in the cytoplasm for inflammasome sensing during infection of a host cell has begun to be unraveled by use of the intracellular Gram-negative bacterium Francisella novicida as a model. Upon infection of the host cell, F. novicida is contained within a pathogen-containing vacuole; however, in order to replicate, F. novicida escapes from the pathogen-containing vacuole via a T6SS and invades the cytoplasm (157). Initial studies found that mutant strains of F. novicida that are unable to escape the pathogen-containing vacuole fail to activate the AIM2 inflammasome in macrophages and dendritic cells (158, 159). Furthermore, mutant strains of F. novicida that are prone to intracellular lysis exhibit an increased ability to trigger activation of the AIM2 inflammasome in macrophages (160). This finding suggests that bacteriolysis is an important mechanism in the liberation of bacterial DNA for AIM2 sensing.
Further studies have provided insights into the mechanisms by which F. novicida is lysed during infection. Type I IFN signaling is induced during infection with F. novicida and is required for inflammasome activation (161). This signaling cascade leads to the upregulation of IFN-inducible proteins, including GBPs and IRGs, by the transcription factor IRF1 (162). Evidence indicates that GBP members GBP2 and GBP5 target cytoplasmic F. novicida and recruit the IRG member IRGB10 to the bacterial cell membrane (100), where they instigate bacteriolysis to induce the release of bacterial DNA into the host cytoplasm for sensing by the AIM2 inflammasome (100, 162, 163). Consistent with the role of the AIM2 inflammasome in antibacterial host defense, mice lacking AIM2, GBPs, or IRGB10 are highly susceptible to infection by F. novicida and have an impaired ability to induce activation of the inflammasome (158, 162–167).
Cytoplasmic DNA sensing in certain human myeloid cells has been proposed to employ an alternative pathway that is independent of AIM2 (168). Upon F. novicida infection, cGAS and STING induce lysosomal cell death, potassium efflux, and subsequent activation of the NLRP3 inflammasome in an AIM2-independent manner (168). However, why AIM2 is not functional in human monocytes was not addressed in that study. Indeed, AIM2 is the major DNA-sensing inflammasome pathway in THP-1 cells, monocyte-derived dendritic cells, and keratinocytes in humans and in myeloid cells in mice (169, 170), suggesting that this alternative DNA-sensing inflammasome pathway is cell type rather than species specific. Further, this alternative pathway may have some capability to compensate the AIM2 inflammasome pathway in human monocytes when it is inhibited by certain virulence factors.
Several bacteria strategically prevent DNA release and activation of the AIM2 inflammasome to ensure survival and dissemination in the infected host. F. novicida encodes a lipid II flippase, MviN, and a CRISPR-Cas system to enhance the integrity of the bacterial envelope and to resist bacteriolysis and subsequent release of DNA (171, 172). Similarly, L. pneumophila produces an effector protein, SdhA, that helps to maintain membrane integrity of the pathogen-containing vacuole (173). In the absence of SdhA, more L. pneumophila DNA is released into the host cytoplasm, activating the AIM2 inflammasome and pyroptosis within 5 h of infection of human macrophages (173). It is likely that other cytosolic bacteria have similar strategies to limit the amount of free bacterial DNA in the cytoplasm. For example, S. flexneri encodes an E3 ubiquitin ligase, IpaH9.8, which mediates ubiquitination and degradation of mouse and human GBPs (174–176). Given the importance of GBPs in mediating bacteriolysis, IpaH9.8 potentially prevents GBP-induced binding to bacteria and subsequent bacteriolysis and DNA release. Additional searches for similar cytosolic bacterial virulence factors that promote degradation of GBPs and IRGs or DNA sensors would further unveil the complexity of the microbial DNA sensing pathway.
Bacterial RNA and Other Bacterial Nucleic Acids
Bacterial RNA species activate multiple cytoplasmic RNA sensors, including the inflammasome sensor NLRP3 and the noninflammasome RLRs and non-RLR helicases. Purified total RNAs derived from both Gram-positive and Gram-negative bacteria trigger activation of the NLRP3 inflammasome in mouse macrophages (177, 178). In addition, both mRNA and RNA-DNA hybrids of E. coli activate NLRP3 in mouse macrophages and dendritic cells (179, 180). Further studies indicate that bacterial mRNA, tRNA, and rRNA are all capable of activating NLRP3 in human macrophages (181). It has been proposed that bacterial RNA might serve as an important “vita-PAMP” to signify the presence of viable microbes to host sensors (179). mRNA from bacteria, but not that from eukaryotic cells, activates the NLRP3 inflammasome, and 3′ polyadenylation of bacterial mRNA abolishes its inflammasome-activating activity (179), suggesting that the 3′ end of bacterial mRNA might be important for NLRP3 engagement and that polyadenylation of host RNA might be sufficient to suppress immune recognition of self RNA. However, the mechanism by which NLRP3 senses and is activated by bacterial RNA remains poorly defined.
Two hypotheses have been postulated to describe how bacterial RNA species might activate NLRP3. First, bacterial RNA and RNA-DNA hybrids colocalize with NLRP3 and caspase-1 inflammasome specks in mouse macrophages infected with E. coli (180), providing some evidence that NLRP3 may interact with bacterial RNA. However, whether this finding reflects a direct interaction between NLRP3 and bacterial RNA or the requirement of an RNA-binding mediator has yet to be shown conclusively. Second, the RNA helicase DHX33 may function as an upstream sensor of bacterial mRNA and subsequently interact with and activate NLRP3 (182, 183). Further studies are therefore required to elucidate whether cytoplasmic bacterial RNA activates NLRP3 directly, through an additional sensor, such as DHX33, or via a mechanism involving nonspecific homeostatic disruption. It would also be interesting to investigate how bacterial RNA molecules gain access to the cytoplasm to more fully elucidate the molecular mechanisms governing the host response to bacterial RNA.
Toxins
Bacteria encode an arsenal of toxins that modify the function, metabolism, and physiology of the host cell to favor bacterial infection. However, the host immune system can sense some bacterial toxins and activate the inflammasome in response to bacterial infection. Inflammasome activation by bacterial toxins is generally achieved through the pore-forming and/or enzymatic activity of the toxin (Fig. 2).
Many bacterial toxins function by forming pores in the host cell membrane. Certain pore-forming toxins produced by both Gram-positive and Gram-negative bacteria are indirectly sensed by the NLRP3 inflammasome (Tables 1 and 2). Mechanistically, toxin-induced pore formation in the host cell membrane can cause potassium efflux (51), which in turn activates the NLRP3 inflammasome (Fig. 2). Indeed, potassium efflux has been proposed as a common upstream activator of the NLRP3 inflammasome in response to bacterial toxins (51). However, the presence of other bacterial PAMPs in concert with potassium efflux appears to be important for NLRP3 inflammasome activation by some pore-forming toxins. For instance, the Gram-negative pathogenic bacteria V. cholerae and Vibrio vulnificus (184) and the Gram-positive bacterium S. aureus (185) produce hemolysin toxins that induce potassium efflux in macrophages. Culture supernatants derived from V. cholerae can trigger potassium efflux and activation of caspase-1 via the NLRP3 inflammasome in macrophages; however, purified hemolysin induces substantially less caspase-1 activation (184). It is possible that other bacterial PAMPs, such as peptidoglycan, may provide a priming signal (signal 1) to upregulate inflammasome components, while toxin-induced potassium efflux acts as the inflammasome-activating signal (signal 2). In this way, hemolysins may function in concert with other bacterial virulence factors to maximally activate the NLRP3 inflammasome.
Introducing pores into the pathogen-containing vacuole membrane can also permit bacterial escape, and subsequent release of PAMPs, into the cytoplasm for innate immune sensing. For instance, the L. monocytogenes toxin listeriolysin O is required for bacterial invasion of the cytoplasm from the pathogen-containing vacuole (186). A mutant strain of L. monocytogenes lacking listeriolysin O fails to induce IL-1β secretion in macrophages (186), likely due to inflammasome-activating PAMPs remaining sequestered in the pathogen-containing vacuole. A further study implicated listeriolysin O-mediated rupture of phagosomes and release of cathepsin B as the main mechanism by which NLRP3 is activated upon L. monocytogenes infection of human PBMCs (187). Similarly, Streptococcus agalactiae produces a β-hemolysin toxin which forms pores in the pathogen-containing vacuole and presumably releases RNA and lysosomal components into the cytoplasm for sensing by the NLRP3 inflammasome (178).
The lethal toxin produced by B. anthracis performs both pore-forming and enzymatic activities that lead to activation of the NLRP1 inflammasome. The lethal toxin is a two-component toxin comprising a pore-forming protective antigen and an enzymatically active lethal factor (188). The protective antigen facilitates lethal factor entry into the host cell cytoplasm, where the lethal factor cleaves mouse NLRP1b in the N-terminal region, leading to formation and activation of the NLRP1b inflammasome (61–64, 66). Mice carrying a cleavage-resistant variant of NLRP1b are unable to sense B. anthracis infection and succumb to infection faster than WT mice do (189, 190). Proteolytic cleavage therefore represents one mechanism by which the inflammasome can be activated by bacterial toxins.
Posttranslational modifications induced by enzymatically active toxins represent an additional mechanism by which the inflammasome can be activated. For instance, Mycoplasma pneumoniae encodes an ADP-ribosylating and vacuolating toxin, designated community-acquired respiratory distress syndrome (CARDS) toxin (191), which activates the NLRP3 inflammasome by direct ADP-ribosylation of NLRP3 (191). Mice lacking NLRP3 and infected with M. pneumoniae are unable to produce IL-1β and exhibit delayed bacterial clearance during acute pulmonary infection (192), clearly indicating a role for the NLRP3 inflammasome in bacterial clearance; however, whether activation of the NLRP3 inflammasome by the CARDS toxin directly relates to the observed in vivo protection from M. pneumoniae infection remains unclear. It would therefore be prudent to generate a mutant M. pneumoniae strain lacking the CARDS toxin to more clearly define the contribution of this toxin to bacterial clearance in vivo.
Posttranslational modification and inactivation of Rho family GTPases by enzymatically active bacterial toxins can also indirectly activate the Pyrin inflammasome. Pyrin is normally phosphorylated by host RhoA serine-threonine kinases PKN1 and PKN2, promoting Pyrin inhibition by 14-3-3 proteins (68–70). Bacterial toxins posttranslationally modify Rho family GTPases in the I-switch region and inhibit RhoA-dependent kinase activity, thereby relieving inhibition of Pyrin and licensing activation of the Pyrin inflammasome (68, 71, 72). Specifically, the Clostridium difficile cytotoxins TcdA and TcdB monoglucosylate RhoA, the Clostridium botulinum toxin C3 ADP-ribosylates RhoA, and the Vibrio parahaemolyticus toxin VopS and the Histophilus somni toxin IbpA adenylate RhoA (71, 72, 74, 75). These studies highlight that diverse posttranslational modifications induced by bacterial toxins can be sensed by multiple inflammasome sensors.
VIRUSES
Viruses enter the host cell to hijack the machinery necessary for viral replication. Viral PAMPs can trigger innate immune responses either on the host cell surface, within the endosome, or in the cytoplasm. Emerging evidence also suggests that innate immune detection might occur in the nucleus. Inflammasome sensors are activated in response to both DNA and RNA viruses, ensuring efficient immunosurveillance and elimination of different families of viruses. In this section, we discuss the molecular mechanisms regulating virus-induced activation of the inflammasome.
DNA Viruses
DNA viruses replicate in either the cytoplasm or the nucleus, and the majority activate the inflammasome through innate immune recognition of viral DNA (Table 4). Cytoplasmic DNA is sensed by the inflammasome sensor AIM2; however, not all DNA viruses activate AIM2 (discussed further below). Murine cytomegalovirus (MCMV) and vaccinia virus trigger activation of the AIM2 inflammasome in mouse bone marrow-derived macrophages (BMDMs) or bone marrow-derived dendritic cells (BMDCs) (31, 162, 164), suggesting that their viral DNA activates the AIM2 inflammasome (Fig. 3). In addition, mice lacking AIM2 and infected with MCMV have lower levels of circulating IL-18 and higher viral titers after 36 h than those of infected WT mice (164). Similar to its murine counterpart, human cytomegalovirus (HCMV) also activates the AIM2 inflammasome in human THP-1 monocytic cells (193). Unlike the case in bacterium-induced activation of the AIM2 inflammasome, type I IFN signaling and IFN-inducible proteins are not required to facilitate sensing of MCMV by AIM2 (100, 162). Interestingly, HCMV expresses the tegument protein pUL83, which interacts with and inhibits AIM2 (194) and the related ALR member IFI16 (195–197), indicating the importance of DNA inflammasome sensors for detection of this viral pathogen.
TABLE 4.
DNA viruses produce PAMPs and DAMPs during infection that activate inflammasomes and other sensors
| Virus (type of genome) | Inflammasome sensor(s) [activator(s)] [reference(s)] |
|---|---|
| Adenovirus (dsDNA) | NLRP3 (cathepsin B, ROS) (208, 210, 212, 213) |
| Bovine herpesvirus 1 (dsDNA) | IFI16 (viral DNA) (428), NLRP3 (unknown activator) (428) |
| Epstein-Barr virus (dsDNA) | AIM2 (viral DNA) (200, 204), IFI16 (viral DNA) (429) |
| Hepatitis B virus (circular, partially dsDNA) | AIM2 (viral DNA) (198, 203, 430, 431) |
| Herpes simplex virus (dsDNA) | NLRP3 (ROS) (201, 205–207), IFI16 (viral DNA) (205, 215) |
| Human bocavirus (ssDNA) | NLRP3 (viral noncoding RNA) (432) |
| Human cytomegalovirus (dsDNA) | AIM2 (viral DNA) (193) |
| Human papillomavirus 16 (dsDNA) | AIM2 (viral DNA) (199) |
| Kaposi's sarcoma-associated herpesvirus (dsDNA) | IFI16 (viral DNA) (433, 434) |
| Modified vaccinia virus Ankara (dsDNA) | AIM2 (viral DNA) (201), NLRP3 (unknown activator) (168, 209) |
| Mouse cytomegalovirus (dsDNA) | AIM2 (viral DNA) (162, 164) |
| Vaccinia virus (dsDNA) | AIM2 (viral DNA) (31, 164) |
| Varicella-zoster virus (dsDNA) | NLRP3 (unknown activator) (211) |
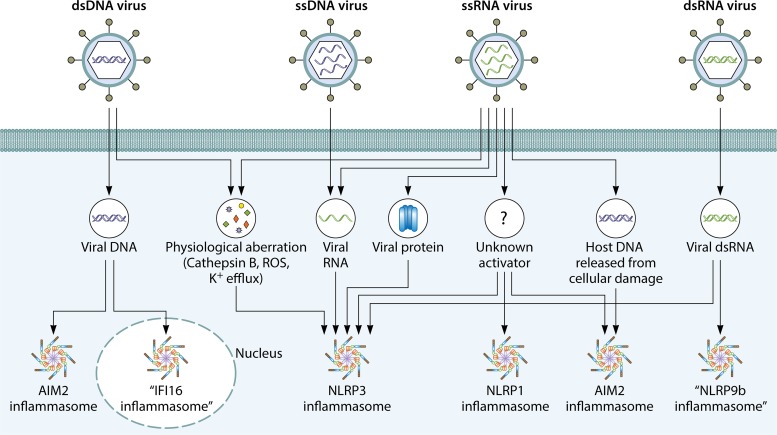
FIG 3.
Viral activators of the inflammasome. DNA and RNA viruses carry viral PAMPs and induce physiological aberrations and host cell damage that can trigger activation of the inflammasome. DNA from double-stranded DNA (dsDNA) viruses can enter the cytoplasm and the nucleus to engage activation of the AIM2 inflammasome and the putative IFI16 inflammasome, respectively. Both DNA and RNA viruses can cause physiological aberrations in the form of cathepsin B release, production of ROS, and potassium efflux, which are signaling cues leading to activation of the NLRP3 inflammasome. RNA and viral proteins of single-stranded RNA (ssRNA) viruses and replication intermediaries of a single-stranded DNA (ssDNA) virus have been shown to trigger activation of the NLRP3 inflammasome. Host DNA release from virus-induced damage can lead to activation of the AIM2 inflammasome. There is some evidence to suggest that an ssRNA virus might activate the NLRP1 inflammasome. Double-stranded RNA (dsRNA) from a dsRNA virus has been shown to activate a putative NLRP9b inflammasome in mouse enterocytes.
Other DNA viruses, including hepatitis B virus (HBV), human papillomavirus 16 (HPV16), Epstein-Barr virus (EBV), and modified vaccinia virus Ankara (MVA), can activate the AIM2 inflammasome (198–201) (Table 4). HBV infection induces AIM2-mediated caspase-1 activation or IL-1β and IL-18 secretion in human glomerular mesangial cells, hepatocytes, and PBMCs (198, 202, 203). Similar observations have been made in human keratinocytes infected with HPV (199) and human THP-1 monocytes infected with EBV (200). Transfected EBV DNA induces IL-1β secretion in the human tumor cell line HK1 via the AIM2 inflammasome (204). Despite these observations, the molecular mechanism underpinning activation of the AIM2 inflammasome by DNA viruses has not completely emerged. The putative activator of AIM2 in these cases is presumably the genomic DNA and/or replication intermediaries of these viruses. However, biochemical and imaging studies are required to confirm whether viral DNA binds to AIM2 in the cytoplasm and/or the nucleus during viral infection.
Furthermore, not all DNA viruses activate the AIM2 inflammasome (Table 4). The DNA viruses adenovirus, herpes simplex virus 1 (HSV-1), MVA, varicella-zoster virus (VZV), and human bocavirus 1 all preferentially activate the NLRP3 inflammasome over AIM2 (164, 205–211). The main activator of the NLRP3 inflammasome induced by DNA viruses is unknown. However, adenovirus infection leads to disruption of lysosomal membranes, which results in the release of cathepsin B and activation of NLRP3 (210, 212, 213). In addition, adenovirus infection induces the production of ROS in THP-1 cells, which also contributes to NLRP3 inflammasome activation (208, 210, 213). Similarly, HSV-1 infection induces expression of NLRP3, cleavage of caspase-1, and maturation of IL-1β in human foreskin fibroblasts and human THP-1 cells (205, 208). Secretion of IL-1β in response to HSV-1 was also found to be independent of AIM2 in mouse thioglycolate-elicited macrophages (164). Together these examples highlight that although DNA viruses possess DNA, some preferentially activate the NLRP3 inflammasome over AIM2.
The reasons underlying why certain DNA viruses do not engage activation of the AIM2 inflammasome are unknown. Given that AIM2 binds DNA in a sequence-independent manner (169), it is unlikely that DNA sequences from these viruses are not recognized by AIM2. A more plausible explanation is that these viruses encode inhibitory proteins similar to the host-derived AIM2-inhibiting PYD-containing proteins that can directly bind and inhibit AIM2 (169, 214). Viral DNA binding proteins may also prevent access of AIM2 to the DNA and thereby prevent inflammasome activation. Alternatively, it is possible that rapid shuttling of viral DNA into the host nucleus evades detection by AIM2 in the cytoplasm. In the latter case, other nuclear DNA sensors might be able to compensate the innate immune recognition of the virus. Indeed, the DNA of HSV-1 has been shown to activate IFI16 in the nucleus (Fig. 3) (205, 215). It is also possible that, as shown for human monocytes, the cGAS-STING axis may drive NLRP3 inflammasome activation in response to cytosolic DNA (168). This would support findings of past studies suggesting that DNA viruses activate the NLRP3 inflammasome rather than the AIM2 inflammasome in human monocyte-derived cells but cannot explain why AIM2 is not activated in other cell types (168, 205, 208, 210, 211). Taken together, the data show that DNA viruses can activate either the AIM2 or NLRP3 inflammasome (Fig. 3); however, the precise activators and regulatory mechanisms leading to activation of these inflammasomes are still not entirely clear.
RNA Viruses
RNA viruses can harbor either a single- or double-stranded RNA genome. An increasing number of RNA viruses have been found to activate the inflammasome (Table 5), including those which pose major threats to public health. Below, we focus our discussions primarily on influenza virus and human immunodeficiency virus (HIV) and on how infection by each of these viruses leads to activation of the inflammasome (Fig. 3).
TABLE 5.
RNA viruses produce PAMPs and DAMPs during infection that activate inflammasomes and other sensors
| Virus (type of genome) | Inflammasome sensor(s) [activator(s)] [reference(s)] |
|---|---|
| Chikungunya virus (positive-sense ssRNA) | AIM2 (unknown activator) (435) |
| Dengue virus (positive-sense ssRNA) | NLRP3 (K+ efflux, cathepsin B) (436) |
| Encephalomyocarditis virus (positive-sense ssRNA) | NLRP3 (viroporin 2B) (261, 437) |
| Enterovirus type 71 (positive-sense ssRNA) | NLRP3 (3D protein, viroporin 2B) (253, 254, 261), AIM2 (unknown activator) (255) |
| Hepatitis C virus (positive-sense ssRNA) | NLRP3 (viral RNA, protein P7) (239, 257–259, 438, 439) |
| Human immunodeficiency virus (positive-sense ssRNA) | NLRP3 (protein R, Tat protein) (239, 240, 243, 244) |
| Influenza A virus (negative-sense ssRNA) | NLRP3 (viral RNA, PB1-F2 peptide, M2) (217–223, 225, 232), AIM2 (host cell dsDNA) (233, 234) |
| Japanese encephalitis virus (positive-sense ssRNA) | NLRP3 (ROS, K+ efflux) (440) |
| Lymphocytic choriomeningitis virus (negative-sense ssRNA) | NLRP1 (unknown activator) (441) |
| Measles virus (negative-sense ssRNA) | NLRP3 (unknown activator) (442) |
| Newcastle disease virus (negative-sense ssRNA) | NLRP3 (unknown activator) (443) |
| Parainfluenza virus type 3 (negative-sense ssRNA) | NLRP3 (K+ efflux) (444) |
| Poliovirus (positive-sense ssRNA) | NLRP3 (viroporin 2B) (261) |
| Porcine reproductive and respiratory syndrome virus (positive-sense ssRNA) | NLRP3 (unknown activator) (445, 446) |
| Rabies virus (negative-sense ssRNA) | NLRP3 (unknown activator) (447) |
| Respiratory syncytial virus (negative-sense ssRNA) | NLRP3 (SH viroporin, viral RNA) (182, 260, 448, 449) |
| Reovirus (dsRNA) | NLRP3 (viral RNA) (182) |
| Rhinovirus (positive-sense ssRNA) | NLRP3 and NLRC5 (ion channel protein 2B) (262) |
| Rotavirus (dsRNA) | NLRP9b (dsRNA) (263) |
| Sendai virus (negative-sense ssRNA) | NLRP3 (viral RNA) (222, 450) |
| Vesicular stomatitis virus (negative-sense ssRNA) | NLRP3 (viral RNA) (225, 437, 451) |
| West Nile virus (positive-sense ssRNA) | NLRP3 (unknown activator) (452), AIM2 (unknown activator) (435) |
| Zika virus (positive-sense ssRNA) | NLRP3 (oxidative stress, NS5 protein) (256, 453, 454), AIM2 (unknown activator) (455) |
Influenza A virus.
Influenza A virus is an important human pathogen that has caused multiple pandemics in human history. The virus is subtyped based on its surface glycoproteins hemagglutinin (H) and neuraminidase (N) (216). There is strong evidence to demonstrate that multiple subtypes of influenza A virus trigger activation of the NLRP3 inflammasome in macrophages and dendritic cells (217–224). Several mechanisms have been proposed to explain how influenza virus infection leads to NLRP3 activation. Both RNA and protein components of influenza virus trigger activation of the NLRP3 inflammasome (Fig. 3) (219–221, 223, 225). Transfection of RNA derived from the A/H7N9 strain activates the NLRP3 inflammasome in BMDCs (219, 225), implying that viral RNA is sufficient to activate the NLRP3 inflammasome. However, the viral peptide PB1-F2, derived from strains A/H7N9 and A/H1N1, can also activate NLRP3 in BMDMs (220, 221). Further studies have identified additional mechanisms by which influenza virus can activate the NLRP3 inflammasome. For instance, influenza virus strain A/H3N2 encodes an ion channel, M2, causing ion imbalances during infection of mouse BMDMs and BMDCs and thereby activates NLRP3 (223). Mice lacking NLRP3 and administered the viral peptide PB1-F2 of strain A/H7N9 produce less IL-1β in the lungs than that in WT mice (221), suggesting that this viral peptide mediates activation of the NLRP3 inflammasome in vivo. Together these observations show that it is possible that both viral RNA and peptides are the PAMPs responsible for activating the NLRP3 inflammasome during influenza virus infection.
Studies have also suggested that an RNA-protein complex comprised of the influenza A viral RNA and viral proteins NP and PB1 is recognized by the innate immune sensor ZBP1 (also known as DAI or DLM-1) (226–228). Recognition of this RNA-protein complex by ZBP1 drives activation of the NLRP3 inflammasome and multiple forms of programmed cell death in a manner dependent on the kinases RIPK1 and RIPK3 and on caspase-8 (226). How the ZBP1–RIPK1–RIPK3–caspase-8 scaffold mediates activation of the NLRP3 inflammasome is unknown. Nevertheless, these studies suggest that activation of the NLRP3 inflammasome by influenza virus might require an upstream sensor that recognizes viral PAMPs. Together these studies indicate that in the context of infection of a host, multiple pathogen detection pathways and mechanisms of inflammasome activation are likely to be utilized.
Given the importance of NLRP3 in sensing infection, influenza virus encodes a nonstructural protein, NS1, that is capable of binding to and inhibiting activation of the NLRP3 inflammasome (229, 230). Specifically, mutating residues in the RNA- and TRIM25-binding domains of NS1 prevented immunoprecipitation of NS1 with NLRP3 and increased secretion of IL-1β relative to that with WT NS1 (229), indicating that these regions are important for mediating NLRP3 binding and inhibition.
While the NLRP3 inflammasome responds to influenza virus infection, it remains unclear whether this pathway confers protection against or susceptibility to the virus in vivo. A study has shown that mice lacking NLRP3 and infected with influenza virus strain A/H1N1 (also known as A/PR/8/34) harbor higher viral titers in their lungs and have a higher mortality rate than those of WT mice (217, 218). Contrastingly, another study found that mice lacking NLRP3 and infected with the highly pathogenic influenza virus strain A/H7N9 have increased survival and develop less pulmonary inflammation than that in WT mice (219). These differing results may be explained, in part, by studies which report that temporal inhibition of NLRP3 by use of the small-molecule compound MCC950 influences the survival rate of mice infected with the influenza virus strains A/WSN/1933 (231) and A/PR/8/34 (232). The latter study found that delayed treatment with MCC950 increases survival of mice, whereas treatment with MCC950 as early as 1 day after infection results in lethality (232). These results partially reconcile the earlier conflicting results and suggest that the NLRP3 inflammasome may contribute to antiviral host defense at the early stages of infection while driving overt inflammation and immunopathology at later stages.
Conflicting results also exist regarding the role of AIM2 in influenza virus infection. Influenza A virus infection of lung epithelial cells can cause substantial damage, resulting in the release of host DNA that can activate the AIM2 inflammasome (233, 234). Whether AIM2 inflammasome activation benefits the host during influenza virus infection remains uncertain. While a study reported that mice lacking AIM2 and infected with influenza virus strain A/H1N1 have less lung injury and a higher survival rate than those of infected WT mice (233), another study found that mice lacking AIM2 are more susceptible than infected WT mice to infection with A/H1N1 (234). These conflicting results may be due to differences in the source of the A/H1N1 virus or the infection dose used or to subtle differences in mouse genetic background, microbiota, or the vivarium used to house these animals (235). However, the exciting finding that both influenza virus-derived PAMPs and the DAMPs generated as a result of infection can trigger activation of two inflammasome complexes, NLRP3 and AIM2, clearly highlights the functional overlap between inflammasome sensors in host defense against this clinically important virus.
HIV.
Since the discovery and initial isolation of HIV 30 years ago, approximately 75 million people have been infected, and more than 37 million currently live with the infection worldwide (236). Polymorphisms in the genes encoding NLRP3 and IL-1β are associated with susceptibility to HIV infection (237, 238). HIV infection triggers activation of the NLRP3 inflammasome in a variety of cell types, including human monocytes (237, 239–241), human and rodent microglia (242–244), and human podocytes (245). While the mechanism of NLRP3 inflammasome activation by HIV has yet to be fully defined, evidence suggests that ROS, cathepsin B, and virus-induced potassium efflux from the host cell might all contribute to activation of NLRP3 (240, 242, 245). Furthermore, transfection of HIV-derived single-stranded RNA (ssRNA) is sufficient to activate caspase-1 and induce secretion of IL-1β (240), implying that viral RNA may activate the NLRP3 inflammasome. There is also some evidence to suggest that the HIV proteins R and Tat can activate NLRP3 in human and rodent microglia, respectively (243, 244). However, further biochemical and mechanistic studies are required to more fully assess which viral PAMPs activate the NLRP3 inflammasome and whether direct or indirect sensing of PAMPs is involved.
Further studies have suggested that HIV infection can trigger pyroptosis in a manner that is independent of the NLRP3 inflammasome in tissue-derived CD4+ T cells but not in blood-derived CD4+ T cells (246–249). In this scenario, the incomplete reverse transcripts of HIV are recognized by IFI16 in CD4+ T cells of the lymphoid tissue, which subsequently drives the infected cells toward pyroptosis (246, 248, 249). The levels of IFI16 expression in tissue- and blood-derived CD4+ T cells might provide an explanation for this observation; indeed, qPCR analysis revealed that tissue-derived CD4+ T cells have three times the level of IFI16 transcripts in blood-derived CD4+ T cells (246). Further studies revealed that the virological synapse—an intercellular junction between an infected cell and an uninfected cell that is generated by HIV—mediates viral transmission and pyroptosis between neighboring lymphoid CD4+ T cells, ultimately accelerating the demise of the CD4+ T cell population and the progression to AIDS (248–250).
Although pyroptosis of CD4+ T cells is detrimental to the host, there is some evidence that anti-HIV therapies are linked to inflammasome activation. For example, the nucleoside analog reverse transcriptase inhibitor abacavir, which is used as part of anti-HIV therapies, induces mitochondrial damage and subsequent activation of the NLRP3 inflammasome in human monocytes (251). Similarly, nelfinavir, an inhibitor of the HIV aspartyl protease, is used in the treatment of HIV infection and potentially activates the AIM2 inflammasome owing to the ability of the drug to rupture the nuclear envelope (252). In this way, activation of inflammasome pathways by anti-HIV therapies potentially contributes to their anti-HIV effects, although further research is needed to substantiate this hypothesis. Indeed, it has been suggested that inflammasome activation by abacavir contributes to severe delayed-type drug hypersensitivity (251), an off-target effect sometimes experienced by patients. The inflammasome pathway therefore may be beneficial in the detection of HIV infection but appears to also have detrimental outcomes in causing CD4+ T cell pyroptosis and some of the side effects associated with anti-HIV therapies.
Other RNA viruses.
Other RNA viruses, including enterovirus type 71 (EV71), hepatitis C virus (HCV), Japanese encephalitis virus (JEV), respiratory syncytial virus (RSV), rhinovirus, encephalomyocarditis virus (EMCV), measles virus, parainfluenza virus type 3, vesicular stomatitis virus (VSV), Newcastle disease virus (NDV), rabies virus, Sendai virus, dengue virus, and Zika virus, have been shown to trigger activation of the NLRP3 inflammasome (Table 5). EV71 has been reported to be recognized by both the NLRP3 and AIM2 inflammasomes, depending on the cell types studied (253–255). The RNA-dependent RNA polymerase (RdRp) 3D protein of EV71 has been suggested to directly bind and activate the NLRP3 inflammasome in human THP-1 cells, rhabdomyosarcoma cells, and HEK293T cells (254). In a similar manner, the RdRp NS5 protein produced by Zika virus directly binds and activates NLRP3 (256), implying that NLRP3 recognition of viral RdRp proteins may facilitate inflammasome activation in response to ssRNA viruses more generally. It will be interesting to assess whether RdRp proteins expressed by other ssRNA viral pathogens can likewise bind to and activate NLRP3. While EV71 infection also appears to induce activation of the AIM2 inflammasome in neuronal cells (255), the activator in this case is unknown. The EV71 proteases 2A and 3C specifically cleave NLRP3 and prevent it from forming an inflammasome complex in infected cells (253).
Viral RNA and the viral ion channel proteins of RNA viruses have been suggested to activate the inflammasome. For example, the viral RNA of reovirus (182), the viral RNA and viral protein viroporin P7 of HCV (257–259), the viral RNA and viroporin SH of RSV (182, 226, 260), and the viroporin 2B proteins of EMCV, EV71, poliovirus, and rhinovirus (261, 262) all result in activation of the NLRP3 inflammasome. A study reported that the RNA of rotavirus triggers a poorly characterized NLR, NLRP9b, in intestinal organoids (Fig. 3) (263). The rotavirus RNA interacts with the host RNA helicase DHX9, which then binds NLRP9b and licenses activation of the inflammasome (263). Moreover, mice lacking NLRP9b and infected with rotavirus have an increased viral load in enterocytes, increased fecal antigen shedding, the occurrence of diarrhea, and more severe pathology than that in WT mice (263). The discovery of a new putative inflammasome sensor in enterocytes argues that many more sensors might have inflammasome-forming capacity and are expressed in a cell-type-specific manner (264). It is possible that distinct inflammasome complexes are formed in different cell types in response to different viral factors generated by the same virus. An exciting avenue of research would be to investigate how these viral factors are liberated and presented to inflammasome sensors in immune and nonimmune cells to provide an integrated antiviral response in the host.
FUNGI
Fungi are eukaryotic organisms that represent some of the most important human pathogens. Evidence suggests that fungal DNA, spores, and cell wall-associated polysaccharides are recognized by inflammasome sensors (265–269) (Table 6). Aspergillus fumigatus is a major human pathogen which causes opportunistic infections and mortality in immunocompromised individuals. A. fumigatus produces an abundance of spores, known as conidia (270), which are able to activate multiple inflammasomes (265, 271). Germinating conidia induce activation of both the AIM2 and NLRP3 inflammasomes in BMDCs (265). Indeed, mice lacking both AIM2 and NLRP3 and infected with A. fumigatus suffer more damage and hemorrhage, harbor a higher fungal load, and produce less IL-1β and IL-18 in the lungs than those in WT mice or mice lacking either AIM2 or NLRP3 (265). Mechanistically, A. fumigatus infection induces potassium efflux and ROS production in mouse BMDCs and human THP-1 cells, which triggers activation of the NLRP3 inflammasome (265, 271). In addition, NLRP3 activation requires phagocytosis of A. fumigatus conidia by the host cell (265, 271, 272). However, the mechanism by which fungal DNA enters the cytoplasm for sensing by AIM2 remains unknown. It is possible that, similar to observations made in the context of bacterial infection (100, 162), fungal conidia phagocytosed by the host cells are subsequently attacked by IFN-inducible proteins, liberating fungal DNA into the cytoplasm. Mice lacking caspase-11 are also more susceptible than WT mice to infection by A. fumigatus (110). Although caspase-11 is a cytoplasmic sensor of bacterial LPS, caspase-11 has been shown to recognize the host-derived danger signal oxidized phospholipids (273), which may be liberated in the lungs over the course of A. fumigatus infection in vivo. However, the role of caspase-11 in sensing oxidized phospholipids is controversial and may vary depending on cell type (274); further studies are required to ascertain the function of caspase-11 in A. fumigatus infection.
TABLE 6.
Fungi produce PAMPs and DAMPs during infection that activate inflammasomes and other sensors
| Fungus | Inflammasome sensor(s) [activator(s)] [reference(s)] |
|---|---|
| Aspergillus fumigatus | NLRP3 (K+ efflux, ROS) (265, 271, 272), AIM2 (presumably DNA) (265, 271, 272), caspase-11 (unknown activator) (110) |
| Candida albicans | NLRP3 (K+ efflux, ROS) (275–277, 282), NLRC4 (unknown activator) (282) |
| Candida parapsilosis | NLRP3 (K+ efflux) (276) |
| Cryptococcus neoformans | NLRP3 (K+ efflux, ROS) (284, 285) |
| Histoplasma capsulatum | NLRP3 (K+ efflux, cathepsin B) (289) |
| Malassezia furfur | NLRP3 (K+ efflux) (456) |
| Microsporum canis | NLRP3 (K+ efflux, ROS, cathepsin B) (286) |
| Paracoccidioides brasiliensis | NLRP3 (K+ efflux, ROS, cathepsin B) (288, 457) |
| Saccharomyces cerevisiae | NLRP3 (zymosan and mannan) (266) |
| Sporothrix schenckii | NLRP3 (unknown activator) (458) |
| Trichophyton schoenleinii | NLRP3 (K+ efflux, ROS) (287) |
The other major fungal pathogen, Candida albicans, has also been shown to activate the inflammasome. C. albicans infection of LPS-primed human and mouse monocytes or mouse peritoneal exudate cells activates the NLRP3 inflammasome (268, 275–280), with evidence of potassium efflux occurring in response to infection (276). Similarly, mice lacking NLRP3 are hypersusceptible to C. albicans infection and carry substantially higher fungal burdens in the kidneys than those in WT mice at 3 to 7 days postinfection (275, 281). The NLRC4 inflammasome also confers host protection in the oral cavity of mice in response to infection with C. albicans (282). Mice lacking NLRC4 have an elevated oral fungal burden and substantially increased susceptibility to the dissemination of the fungus compared to those of WT controls (282). The mechanism underpinning NLRC4-mediated protection is unknown; however, the protective effect of NLRC4 stems from the stromal compartments (282). Other than the bona fide NLRC4 activators bacterial flagellin and T3SS proteins, mitochondrial DNA has also been proposed to activate NLRC4 (283). Therefore, it is plausible that damage-induced release of mitochondrial DNA might activate the NLRC4 inflammasome to drive a protective response during C. albicans infection.
The cell wall components zymosan and mannan, derived from the yeast Saccharomyces cerevisiae, are able to activate the NLRP3 inflammasome in mouse BMDMs and BMDCs treated with ATP (266), suggesting that these cell wall components may serve as a priming signal (signal 1) via activation of CLRs rather than as inflammasome-activating agents (signal 2). Cell wall components of certain fungi often provide a protective mask to evade recognition by inflammasome sensors. Cryptococcus neoformans is an encapsulated human pathogen which can cause disease in both immunocompetent and immunocompromised hosts. This fungal pathogen activates the NLRP3 inflammasome in human and mouse macrophages only when it lacks the polysaccharide capsule (284, 285), strongly suggesting that encapsulation is a potential mechanism by which this fungus evades detection by the inflammasome.
Other fungi can also trigger activation of the NLRP3 inflammasome (Table 6). Similar to Candida species, the majority of these fungi induce potassium efflux and ROS production that culminate in activation of the NLRP3 inflammasome in infected host cells (286–289). Taken together, the data show that fungal infection appears to predominantly trigger activation of the NLRP3 inflammasome, with some fungal species eliciting an innate immune response via the AIM2 and NLRC4 inflammasomes.
PROTOZOA
Protozoa are unicellular eukaryotic organisms which have the potential to cause life-threatening conditions in higher animals, especially in tropical regions (290). The clinically relevant protozoa include the genera Plasmodium, Leishmania, Trypanosoma, Toxoplasma, and Entamoeba. In this section, we discuss emerging evidence of immunomodulatory effects of inflammasomes during protozoal infection (291).
Plasmodium spp.
Plasmodium spp. are the causative agents of malaria, a debilitating disease which places a heavy burden on public health, especially in Africa and South Asia. The WHO estimated that 212 million cases of malaria were reported in 2015 worldwide, resulting in 429,000 deaths (292). Malaria in humans is mainly caused by five species of the genus Plasmodium: Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi. Polymorphisms in the genes encoding NLRP1 and IL-1β are associated with more severe P. vivax infection, whereas a polymorphism in the gene encoding IL-18 is associated with protection against P. vivax-induced anemia (293). Further, patients infected with Plasmodium have elevated levels of proinflammatory cytokines, including tumor necrosis factor (TNF) and IL-1β (294); the level of IL-1β in patients correlates with disease severity, particularly for cerebral malaria (295).
The replication cycle of Plasmodium spp. exists in two phases: the reproductive phase, which occurs in a vector (female mosquitoes of the Anopheles genus), and the infectious phase, which occurs in a mammalian host (296). During the infectious phase of its life cycle, the parasite digests host-derived hemoglobin in the blood. This process generates free heme, which is toxic to the parasite. Plasmodium spp. detoxify and convert free heme into a pathogen-derived metabolite, hemozoin, which is a major activator of the inflammasome.
Hemozoin and hemozoin-DNA complexes can both induce activation of the inflammasome. Hemozoin itself triggers activation of the NLRP3 inflammasome in mouse BMDMs and BMDCs and in the human monocytic THP-1 cell line (297–300). Hemozoin-mediated activation of NLRP3 in mouse BMDMs requires the tyrosine kinases SYK and LYN (299). In addition to hemozoin, the DAMP heme can also activate NLRP3 in mouse macrophages, in a manner dependent on SYK, NADPH oxidase 2, mitochondrial ROS, and potassium efflux but independent of heme internalization, lysosomal damage, ATP release, the purinergic receptor P2X7R, and cell death (301).
Hemozoin might also transport plasmodial DNA into the lysosomal compartment, where the hemozoin-plasmodium-DNA complex interacts with the DNA-sensing receptor TLR9 (302). Indeed, the hemozoin-plasmodium-DNA complex triggers TLR9 translocation from the endoplasmic reticulum to the phagolysosome (303), where hemozoin-induced phagolysosomal instability triggers activation of NLRP3 (303). Further, the hemozoin-plasmodium-DNA complex induces neutrophil infiltration in mice, in a manner dependent on AIM2 and NLRP3 (303). Upon dissociation from hemozoin, plasmodial DNA can activate AIM2 (303). In humans, immunocomplexes containing both human and plasmodial DNAs are found at high levels in the blood of patients infected with P. falciparum or P. vivax (294). These immunocomplexes induce colocalization of NLRP3 and AIM2 in the inflammasome speck and activation of caspase-1 in human monocytes (294). In addition, NLRP3 and NLRP12 complexes have been observed in PBMCs of patients infected with P. vivax (304). These studies collectively demonstrate that pathogen-derived metabolites and DAMPs generated during Plasmodium infection can activate inflammasome responses in humans and mice.
Although studies have indicated the importance of inflammasomes in sensing Plasmodium infection, inflammasome-mediated responses can result in overt inflammation in the host (297, 301, 303). For instance, WT mice injected with hemozoin develop acute peritonitis, whereas mice lacking NLRP3, caspase-1, and IL-1 receptor (IL-1R) do not (297). Likewise, hemozoin was also found to mediate hemolysis-induced lethality in mice via activation of the NLRP3 inflammasome and production of IL-1β (301). These studies suggest that hemozoin-induced activation of the NLRP3 inflammasome pathway is a potent driver of acute inflammation in vivo, often to the detriment of the host.
More comprehensive studies are required to investigate the synergy between inflammasome complexes in the context of Plasmodium infection. For example, the relevance of NLRP1 and NLRP12 to Plasmodium infection is of particular interest. While no known ligand has been associated with NLRP12, NLRP1 is activated by any protease that can induce cleavage at the N-terminal domain of NLRP1 (61–67). These findings raise the question of whether certain Plasmodium spp. encode an NLRP1-targeting protease. Additional studies of the inflammasome by use of human cells and tissues would allow better extrapolation of the data for the design of therapy to treat patients affected by malaria.
Leishmania spp.
Twenty species of Leishmania have been reported to cause infection in humans, resulting in an estimated 1.5 million new cases of leishmaniasis worldwide every year (305, 306). Upon receiving a bite from an infected Phlebotomus sand fly, a motile and extracellular form of Leishmania called a promastigote is introduced into the host; promastigotes can be phagocytosed by macrophages, neutrophils, and dendritic cells (307). Genomic profiling has revealed that genes encoding NLRP3, AIM2, caspase-1, and IL-1β are highly expressed in human lesions caused by Leishmania braziliensis infection (308). Further, increased production of IL-1β by monocytes and of serum IL-1β has been observed in patients with Leishmania mexicana infection (309).
Infections caused by Leishmania amazonensis, L. braziliensis, and Leishmania infantum chagasi can induce activation of the NLRP3 inflammasome and restriction of parasite replication in both mouse macrophages and mice (310). Multiple species of Leishmania activate the NLRP3 inflammasome via potassium channels and cathepsin release (310) (Table 7). In response to L. amazonensis infection, IL-1β signaling upregulates nitric oxide (NO) synthase and thereby induces NO production and subsequent host protection (310). Infection caused by Leishmania major also induces activation of the NLRP3 inflammasome in mouse macrophages and mice (310–313); however, inflammasome signaling is dispensable for the development of lesions or restriction of parasite burdens in mice (310).
TABLE 7.
Protozoa produce PAMPs and DAMPs during infection that activate inflammasomes and other sensors
| Protozoan | Inflammasome sensor(s) [activator(s)] [reference(s)] |
|---|---|
| Entamoeba histolytica | NLRP3 (ATP release by P2X7R, K+ efflux) (340, 341) |
| Leishmania spp., including L. major, L. amazonensis, L. braziliensis, and L. infantum chagasi | NLRP3 (K+ efflux, cathepsin B) (310–313) |
| Leishmania infantum | NLRP3 (unknown activator) (459) |
| Plasmodium spp., including P. falciparum and P. berghei | NLRP3 (hemozoin, ROS, K+ efflux, lysosome rupture) (297–301, 303), AIM2 (plasmodial DNA) (303), NLRP12 (unknown activator) (304) |
| Toxoplasma gondii | NLRP1 (unknown activator) (320, 324, 325), NLRP3 (unknown activator) (323, 460) |
| Tritrichomonas musculis | ASC-dependent inflammasome (unknown activator) (461) |
| Trypanosoma cruzi | NLRP3 (K+ efflux, ROS, cathepsin B, lysosomal rupture) (337, 338) |
Two other studies found that NLRP3 accentuates nonhealing L. major infections in mice on either the BALB/c or C57/BL6 background (311, 313). The first study found that, in BALB/c mice, IL-18 production as a result of NLRP3 inflammasome activation promotes TH2 responses as signified by elevated IL-4 production (311). Furthermore, IL-18 production somehow inhibits TH1 responses as signified by reduced IFN-γ production (311). The IL-18-mediated TH2 response increases susceptibility to L. major infection, and neutralization of IL-18 in mice provides resistance to L. major infection (311). Another group found that when C57BL/6 mice are infected with the nonhealing L. major Seidman strain, the ensuing NLRP3-dependent response drives IL-1β production and severe immunopathology (313). Mechanistically, secretion of IL-1β leads to an influx of neutrophils at the site of infection and promotes the development of skin lesions (313). Although these studies collectively demonstrate that Leishmania spp. can induce activation of the NLRP3 inflammasome, the resultant outcomes in the host are very different. While the NLRP3 inflammasome and IL-1β provide protection against L. amazonensis, L. braziliensis, and L. infantum chagasi (310), NLRP3 contributes to driving host susceptibility in the case of L. major infection (310, 311, 313). The discrepancies observed in these studies may be multifactorial. First, the use of different Leishmania strains introduces species-specific differences among the studies. Second, the studies utilized different infection doses of L. major: specifically, one study infected C57BL/6 mice with 1 × 106 stationary-phase L. major promastigotes (310), whereas another infected C57BL/6 mice with 1,000 metacyclic promastigotes of L. major (313). Another possibility is the use of mouse strains on different genetic backgrounds: C57BL/6 mice tend to be relatively resistant to leishmaniasis compared to BALB/c mice, and the genetic background can dictate whether IL-18 drives a TH1 or TH2 response (314, 315). Further, L. major and L. mexicana encode a metalloprotease, GP63, which cleaves human NLRP3 at six putative sites and mouse NLRP3 at three putative sites, resulting in inhibition of the NLRP3 inflammasome (312). These virulence factors conferring evasion of the host immune surveillance might also be present in specific species or strains, suggesting that a more extensive investigation that controls for the aforementioned host and parasite factors is required to further assess the physiological role of inflammasomes in leishmaniasis.
A further study introduced an extra layer of complexity, showing that the gut microbiota of infected sand flies contributes to inflammasome signaling in Leishmania donovani infection (316). L. donovani and gut microbes induced activation of the NLRP3 inflammasome and production of IL-1β to levels higher than those induced by L. donovani infection alone (316). The authors suggested that the gut microbes provided a priming signal (signal 1), whereas the L. donovani infection provided the NLRP3 activation signal (signal 2) (316). This study therefore highlights an involvement of gut microbe-derived PAMPs in the complex interplay between inflammasome signaling and Leishmania infection (316).
Toxoplasma gondii
The obligate intracellular parasite Toxoplasma gondii is the causative agent of toxoplasmosis, which is transmitted to humans through ingestion of contaminated food, water, or dust, through vertical transmission in utero, and through contact with infected transplants (317, 318). T. gondii induces transcriptional upregulation of various inflammasome components, including NLRP1, NLRP3, NLRC4, AIM2, and NAIP, in human THP-1 macrophages (319). Genetic analysis of patients with congenital toxoplasmosis further revealed that polymorphisms in the gene encoding NLRP1 are associated with an increased risk of toxoplasmosis (320). Knockdown of NLRP1 by RNA interference in the human monocytic leukemia MonoMac6 cell line results in an increased parasitic burden in response to T. gondii infection (320). However, it was reported that these cells have reduced viability in response to T. gondii infection (320), which is unexpected given that knockdown of NLRP1 would lead to a reduced level of pyroptosis. However, it is possible that other cell death pathways, such as the pyronecrosis observed in mouse macrophages infected with T. gondii (321), compensate for the lack of pyroptosis. Further genetic evidence revealed that short hairpin RNA (shRNA)-mediated inhibition of ASC and caspase-1 leads to a substantial reduction in IL-1β production in primary human monocytes and THP-1 cells infected with T. gondii (322).
Both NLRP1b and NLRP3 have been found to provide protection against T. gondii infection in mice or rats (323–325); however, the activators triggering either type of inflammasome in response to T. gondii infection are unknown. It is thought that the granule protein GRA15, which is secreted by the parasite during invasion of monocytes, provides a priming signal for the NLRP3 inflammasome by inducing NF-κB-mediated upregulation of IL-1β (322, 326). While the NLRP3 inflammasome-activating signal has not been identified, it may be a secreted parasitic PAMP or a DAMP generated by dying neighboring cells (322). In this context, GBPs appear to have a role in releasing PAMPs from the Toxoplasma-containing vacuole into the cytoplasm for inflammasome sensing in certain cell types (101, 327–330). For example, GBP1 is recruited to the T. gondii-containing vacuole in human mesenchymal stromal cells (331) but not in human lung epithelial A549 cells (332). In addition, given that certain proteases can cleave and activate NLRP1 (61, 63, 65), future studies could investigate the protease(s) encoded by T. gondii that is capable of cleaving and activating NLRP1. The molecular pathway involved in NLRP3 inflammasome activation by T. gondii and how NLRP3 synergizes with NLRP1 to mediate the host defense against this parasite remain to be investigated.
Trypanosoma spp.
Trypanosomiasis is a parasitic disease caused by members of the Trypanosoma genus, which are transmitted to humans by a bite from an infected tsetse fly and infect 10 million people worldwide (333–335). The NLRP3 inflammasome confers host protection during Trypanosoma cruzi infection (336, 337); mice lacking NLRP3, ASC, and caspase-1 and infected with T. cruzi have an elevated parasite burden, mortality rate, and heart inflammation compared to those of WT mice (336, 337). Activation of the NLRP3 inflammasome in response to T. cruzi infection appears to be multifactorial, given that ROS production, potassium efflux, cathepsin B release, and lysosomal damage have all been implicated in NLRP3 activation in BMDMs (337, 338). Furthermore, splenocytes derived from mice lacking NLRP3 or caspase-1 exhibit defects in NO production (336), suggesting a possible interplay between the inflammasome and NO pathways in mediating host protection against T. cruzi infection. It is also possible that GBPs and IRGs disrupt the membrane of the trypanosome-containing vacuole and thereby release pathogens and/or PAMPs into the host cytoplasm, as has been observed for T. gondii (97). However, further biochemical and imaging studies are required to gain insight into the mechanisms by which T. gondii and/or its PAMPs are sensed by the NLRP3 inflammasome. Given the availability of mice lacking either IL-1β, IL-18, or gasdermin D, future studies can also focus on revealing the relative contributions of IL-1β, IL-18, and pyroptosis to the host defense against Trypanosoma infection.
Entamoeba histolytica
Entamoeba histolytica is the causative agent of amebiasis, which affects more than 100 million people and causes 100,000 deaths worldwide every year (339). Cell surface interactions between E. histolytica and macrophages trigger NLRP3 inflammasome activation. Mechanistically, the E. histolytica surface protease EhCP5 activates macrophage α5β1 integrin, subsequently leading to the release of ATP through the pannexin-1 channel (340). This extracellular burst of ATP opens the potassium channel P2X7R, leading to potassium efflux and activation of the NLRP3 inflammasome (341). Interestingly, activation of α5β1 integrin has also been proposed to drive the recruitment of NLRP3 from the cytoplasm to regions of the macrophage membrane that are in association with the parasite (340); however, the significance of this recruitment has yet to be investigated fully. EhCP5 was also found to cleave and inactivate IL-1β and IL-18 (342, 343), which might be an important strategy used by the parasite to reduce inflammasome responses. In addition, E. histolytica produces a monocyte locomotion inhibitory factor which decreases expression of the gene encoding IL-1β in mouse macrophages (344). Further, two cysteine proteases of E. histolytica, EhCP1 and EhCP4, degrade immune components, including pro-IL-18, the complement component C3, and immunoglobulin G (IgG) (345, 346), contributing to the pathogenesis of E. histolytica infection. These studies demonstrate that E. histolytica encodes multiple virulence factors to counteract the effects of the inflammasome and other innate immune components, highlighting the importance of these host defense systems against E. histolytica infection. It would be worthwhile to investigate potential cross talk between inflammasome complexes, other innate immune components, and adaptive immune cells during infection by this pathogenic parasite.
CONCLUSIONS AND FUTURE PERSPECTIVES
Less than 2 decades into its discovery, the inflammasome has become one of the centerpieces of immunology and has continued to inspire research into pattern recognition and innate immunity. A central feature of the inflammasome is its ability to respond to pathogens and danger signals from within the host cytoplasm and to emanate a rapid inflammatory and cell death response. Inflammasomes can recognize the basic architecture and cellular composition of microbes, including nucleic acids and virulence factors, but also can recognize cellular perturbations caused by the infection process. In most cases, the inflammasome induces a protective response, either by induction of inflammation and cellular recruitment via IL-1β and IL-18 or by physical destruction or removal of the infected cell via pyroptosis. Emerging evidence suggests that gasdermin D pores can damage the bacterial cell membrane, but the role of gasdermin D in nonbacterial infections remains largely unknown. In addition, the relative contributions of gasdermin D and cytokine production to the overall inflammasome response against infectious disease has not been investigated thoroughly. This intriguing research area will be explored further with the availability of mice lacking gasdermin D, IL-1β, or IL-18.
Another important issue to address is the mechanisms by which microbial ligands are presented to inflammasome sensors. While mechanistic details have emerged describing the liberation of ligands from bacteria, how ligands from other pathogens are released remains a mystery. Further, how do inflammasomes cooperate with one another in response to pathogens that are capable of activating multiple inflammasome sensors? Recruitment of more than one inflammasome sensor to a single multimeric complex has been observed in BMDMs. Does this phenomenon also occur in a physiological setting? Furthermore, how does the inflammasome regulate other cell-autonomous and immunological processes, including the production of inflammatory lipids and antimicrobial mediators, autophagy, cell migration, and adaptive immunity? Of all inflammasome complexes, the NLRP3 inflammasome is functionally the most versatile, in that it can respond to a plethora of microbial triggers and danger signals. Importantly, it is still unclear how NLRP3 is able to respond to such diverse stimuli or whether a unifying signal triggering activation of the NLRP3 inflammasome exists. Perhaps the compositional makeup of the NLRP3 inflammasome can differ to allow it to respond to structurally diverse activators. Finding answers to these scientific queries will require cross-disciplinary and collaborative approaches.
Uncontrolled inflammasome activity is detrimental to the host, leading to tissue damage and even death. Experimental therapies that are able to attenuate inflammasome hyperactivation have been successful in the treatment of endotoxemia and autoinflammatory disorders, suggesting that immunomodulators of the inflammasome are a versatile armamentarium that may be applied more widely in clinics. For example, inhibition of the inflammasome may be beneficial for curtailing overt inflammatory responses in sepsis that often lead to rapid lethality. In contrast, heightening the activity of the inflammasome may lead to enhanced immunity against certain infectious agents, as evidenced by the mechanistic actions of certain antiviral therapies. Further exploration of small-molecule activators and inhibitors of the inflammasome would provide new options for targeting specific inflammasomes in the treatment of diseases.
ACKNOWLEDGMENTS
A.M. is supported by a scholarship from The John Curtin School of Medical Research, The Australian National University. S.M.M. is supported by the Australian National University Futures Award, The Gretel and Gordon Bootes Medical Research Foundation, the National Health and Medical Research Council of Australia (under project grants APP1141504 and APP1146864), and the R. G. Menzies Early Career Fellowship (grant APP1091544).
We acknowledge researchers whose work was not cited or was cited through reviews owing to space limitations.
We have no competing interests.
REFERENCES
- 1.Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Man SM, Kanneganti TD. 2016. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol 16:7–21. doi: 10.1038/nri.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medzhitov R. 2007. Recognition of microorganisms and activation of the immune response. Nature 449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 4.O'Neill LA, Golenbock D, Bowie AG. 2013. The history of Toll-like receptors—redefining innate immunity. Nat Rev Immunol 13:453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 5.Mathur A, Hayward JA, Man SM. 2018. Molecular mechanisms of inflammasome signaling. J Leukoc Biol 103:233–257. doi: 10.1189/jlb.3MR0617-250R. [DOI] [PubMed] [Google Scholar]
- 6.Vande Walle L, Lamkanfi M. 2016. Pyroptosis. Curr Biol 26:R568–R572. doi: 10.1016/j.cub.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Rathinam VA, Fitzgerald KA. 2016. Inflammasome complexes: emerging mechanisms and effector functions. Cell 165:792–800. doi: 10.1016/j.cell.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broz P, Dixit VM. 2016. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 9.Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schroder GF, Fitzgerald KA, Wu H, Egelman EH. 2014. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai X, Chen J, Xu H, Liu S, Jiang QX, Halfmann R, Chen ZJ. 2014. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell 156:1207–1222. doi: 10.1016/j.cell.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. 2007. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. 2010. Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8:471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. 2010. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med 207:1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Man SM, Hopkins LJ, Nugent E, Cox S, Gluck IM, Tourlomousis P, Wright JA, Cicuta P, Monie TP, Bryant CE. 2014. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc Natl Acad Sci U S A 111:7403–7408. doi: 10.1073/pnas.1402911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baroja-Mazo A, Martin-Sanchez F, Gomez AI, Martinez CM, Amores-Iniesta J, Compan V, Barbera-Cremades M, Yague J, Ruiz-Ortiz E, Anton J, Bujan S, Couillin I, Brough D, Arostegui JI, Pelegrin P. 2014. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol 15:738–748. doi: 10.1038/ni.2919. [DOI] [PubMed] [Google Scholar]
- 16.Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR, Al-Amoudi A, Mangan MS, Zimmer S, Monks BG, Fricke M, Schmidt RE, Espevik T, Jones B, Jarnicki AG, Hansbro PM, Busto P, Marshak-Rothstein A, Hornemann S, Aguzzi A, Kastenmuller W, Latz E. 2014. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol 15:727–737. doi: 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. 2015. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 18.Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. 2015. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 19.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. 2015. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res 25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. 2016. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 21.Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, Dueber EC. 2016. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A 113:7858–7863. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. 2016. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sborgi L, Ruhl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Muller DJ, Broz P, Hiller S. 2016. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J 35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, He WT, Hu L, Li J, Fang Y, Wang X, Xu X, Wang Z, Huang K, Han J. 2016. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res 26:1007–1020. doi: 10.1038/cr.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC. 2018. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48:35.e6–44.e6. doi: 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conos SA, Lawlor KE, Vaux DL, Vince JE, Lindqvist LM. 2016. Cell death is not essential for caspase-1-mediated interleukin-1beta activation and secretion. Cell Death Differ 23:1827–1838. doi: 10.1038/cdd.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heilig R, Dick MS, Sborgi L, Meunier E, Hiller S, Broz P. 2018. The Gasdermin-D pore acts as a conduit for IL-1β secretion in mice. Eur J Immunol 48:584–592. doi: 10.1002/eji.201747404. [DOI] [PubMed] [Google Scholar]
- 28.Boucher D, Monteleone M, Coll RC, Chen KW, Ross CM, Teo JL, Gomez GA, Holley CL, Bierschenk D, Stacey KJ, Yap AS, Bezbradica JS, Schroder K. 2018. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J Exp Med 215:827–840. doi: 10.1084/jem.20172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Man SM, Kanneganti TD. 2015. Regulation of inflammasome activation. Immunol Rev 265:6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. 2009. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol 10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 33.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, Hume DA, Stacey KJ. 2009. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 34.Kofoed EM, Vance RE. 2011. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 36.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. 2014. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 37.Casson CN, Yu J, Reyes VM, Taschuk FO, Yadav A, Copenhaver AM, Nguyen HT, Collman RG, Shin S. 2015. Human caspase-4 mediates noncanonical inflammasome activation against Gram-negative bacterial pathogens. Proc Natl Acad Sci U S A 112:6688–6693. doi: 10.1073/pnas.1421699112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker PJ, Boucher D, Bierschenk D, Tebartz C, Whitney PG, D'Silva DB, Tanzer MC, Monteleone M, Robertson AA, Cooper MA, Alvarez-Diaz S, Herold MJ, Bedoui S, Schroder K, Masters SL. 2015. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur J Immunol 45:2918–2926. doi: 10.1002/eji.201545655. [DOI] [PubMed] [Google Scholar]
- 39.Schmid-Burgk JL, Gaidt MM, Schmidt T, Ebert TS, Bartok E, Hornung V. 2015. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur J Immunol 45:2911–2917. doi: 10.1002/eji.201545523. [DOI] [PubMed] [Google Scholar]
- 40.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. 2011. Non-canonical inflammasome activation targets caspase-11. Nature 479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 41.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, Dixit VM. 2013. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 42.Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, Miao EA. 2013. Caspase-11 protects against bacteria that escape the vacuole. Science 339:975–978. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casson CN, Copenhaver AM, Zwack EE, Nguyen HT, Strowig T, Javdan B, Bradley WP, Fung TC, Flavell RA, Brodsky IE, Shin S. 2013. Caspase-11 activation in response to bacterial secretion systems that access the host cytosol. PLoS Pathog 9:e1003400. doi: 10.1371/journal.ppat.1003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. 2013. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruhl S, Broz P. 2015. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol 45:2927–2936. doi: 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]
- 46.Yang D, He Y, Munoz-Planillo R, Liu Q, Nunez G. 2015. Caspase-11 requires the Pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity 43:923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. 2008. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perregaux D, Gabel CA. 1994. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem 269:15195–15203. [PubMed] [Google Scholar]
- 49.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. 2007. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 50.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. 2007. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem 282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 51.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. 2013. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. 2006. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 53.Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, Eisenbarth SC, Nauseef WM, Cassel SL, Sutterwala FS. 2013. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39:311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou R, Yazdi AS, Menu P, Tschopp J. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature 469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 55.Gross CJ, Mishra R, Schneider KS, Medard G, Wettmarshausen J, Dittlein DC, Shi H, Gorka O, Koenig PA, Fromm S, Magnani G, Cikovic T, Hartjes L, Smollich J, Robertson AA, Cooper MA, Schmidt-Supprian M, Schuster M, Schroder K, Broz P, Traidl-Hoffmann C, Beutler B, Kuster B, Ruland J, Schneider S, Perocchi F, Gross O. 2016. K+ efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity 45:761–773. doi: 10.1016/j.immuni.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. 2012. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T. 2012. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A 109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. 2012. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rossol M, Pierer M, Raulien N, Quandt D, Meusch U, Rothe K, Schubert K, Schoneberg T, Schaefer M, Krugel U, Smajilovic S, Brauner-Osborne H, Baerwald C, Wagner U. 2012. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun 3:1329. doi: 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Compan V, Baroja-Mazo A, Lopez-Castejon G, Gomez AI, Martinez CM, Angosto D, Montero MT, Herranz AS, Bazan E, Reimers D, Mulero V, Pelegrin P. 2012. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity 37:487–500. doi: 10.1016/j.immuni.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 61.Boyden ED, Dietrich WF. 2006. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet 38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 62.Levinsohn JL, Newman ZL, Hellmich KA, Fattah R, Getz MA, Liu S, Sastalla I, Leppla SH, Moayeri M. 2012. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog 8:e1002638. doi: 10.1371/journal.ppat.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hellmich KA, Levinsohn JL, Fattah R, Newman ZL, Maier N, Sastalla I, Liu S, Leppla SH, Moayeri M. 2012. Anthrax lethal factor cleaves mouse nlrp1b in both toxin-sensitive and toxin-resistant macrophages. PLoS One 7:e49741. doi: 10.1371/journal.pone.0049741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chavarria-Smith J, Vance RE. 2013. Direct proteolytic cleavage of NLRP1B is necessary and sufficient for inflammasome activation by anthrax lethal factor. PLoS Pathog 9:e1003452. doi: 10.1371/journal.ppat.1003452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chavarria-Smith J, Mitchell PS, Ho AM, Daugherty MD, Vance RE. 2016. Functional and evolutionary analyses identify proteolysis as a general mechanism for NLRP1 inflammasome activation. PLoS Pathog 12:e1006052. doi: 10.1371/journal.ppat.1006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frew BC, Joag VR, Mogridge J. 2012. Proteolytic processing of Nlrp1b is required for inflammasome activity. PLoS Pathog 8:e1002659. doi: 10.1371/journal.ppat.1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finger JN, Lich JD, Dare LC, Cook MN, Brown KK, Duraiswami C, Bertin J, Gough PJ. 2012. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J Biol Chem 287:25030–25037. doi: 10.1074/jbc.M112.378323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao W, Yang J, Liu W, Wang Y, Shao F. 2016. Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc Natl Acad Sci U S A 113:E4857–E4866. doi: 10.1073/pnas.1601700113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeru I, Papin S, L'Hoste S, Duquesnoy P, Cazeneuve C, Camonis J, Amselem S. 2005. Interaction of pyrin with 14.3.3 in an isoform-specific and phosphorylation-dependent manner regulates its translocation to the nucleus. Arthritis Rheum 52:1848–1857. doi: 10.1002/art.21050. [DOI] [PubMed] [Google Scholar]
- 70.Park YH, Wood G, Kastner DL, Chae JJ. 2016. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol 17:914–921. doi: 10.1038/ni.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Masters SL, Lagou V, Jeru I, Baker PJ, Van Eyck L, Parry DA, Lawless D, De Nardo D, Garcia-Perez JE, Dagley LF, Holley CL, Dooley J, Moghaddas F, Pasciuto E, Jeandel PY, Sciot R, Lyras D, Webb AI, Nicholson SE, De Somer L, van Nieuwenhove E, Ruuth-Praz J, Copin B, Cochet E, Medlej-Hashim M, Megarbane A, Schroder K, Savic S, Goris A, Amselem S, Wouters C, Liston A. 2016. Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Sci Transl Med 8:332ra345. doi: 10.1126/scitranslmed.aaf1471. [DOI] [PubMed] [Google Scholar]
- 72.Xu H, Yang J, Gao W, Li L, Li P, Zhang L, Gong YN, Peng X, Xi JJ, Chen S, Wang F, Shao F. 2014. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 513:237–241. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 73.Gavrilin MA, Abdelaziz DH, Mostafa M, Abdulrahman BA, Grandhi J, Akhter A, Abu Khweek A, Aubert DF, Valvano MA, Wewers MD, Amer AO. 2012. Activation of the Pyrin inflammasome by intracellular Burkholderia cenocepacia. J Immunol 188:3469–3477. doi: 10.4049/jimmunol.1102272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dumas A, Amiable N, de Rivero Vaccari JP, Chae JJ, Keane RW, Lacroix S, Vallieres L. 2014. The inflammasome pyrin contributes to pertussis toxin-induced IL-1beta synthesis, neutrophil intravascular crawling and autoimmune encephalomyelitis. PLoS Pathog 10:e1004150. doi: 10.1371/journal.ppat.1004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Gorp H, Saavedra PH, de Vasconcelos NM, Van Opdenbosch N, Vande Walle L, Matusiak M, Prencipe G, Insalaco A, Van Hauwermeiren F, Demon D, Bogaert DJ, Dullaers M, De Baere E, Hochepied T, Dehoorne J, Vermaelen KY, Haerynck F, De Benedetti F, Lamkanfi M. 2016. Familial Mediterranean fever mutations lift the obligatory requirement for microtubules in Pyrin inflammasome activation. Proc Natl Acad Sci U S A 113:14384–14389. doi: 10.1073/pnas.1613156113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aubert DF, Xu H, Yang J, Shi X, Gao W, Li L, Bisaro F, Chen S, Valvano MA, Shao F. 2016. A Burkholderia type VI effector deamidates Rho GTPases to activate the Pyrin inflammasome and trigger inflammation. Cell Host Microbe 19:664–674. doi: 10.1016/j.chom.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 77.Van Opdenbosch N, Gurung P, Vande Walle L, Fossoul A, Kanneganti TD, Lamkanfi M. 2014. Activation of the NLRP1b inflammasome independently of ASC-mediated caspase-1 autoproteolysis and speck formation. Nat Commun 5:3209. doi: 10.1038/ncomms4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guey B, Bodnar M, Manie SN, Tardivel A, Petrilli V. 2014. Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function. Proc Natl Acad Sci U S A 111:17254–17259. doi: 10.1073/pnas.1415756111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, Alnemri ES. 2001. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem 276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 80.Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C, Nunez G. 2007. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog 3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. 2004. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 82.Joosten LA, Netea MG, Dinarello CA. 2013. Interleukin-1beta in innate inflammation, autophagy and immunity. Semin Immunol 25:416–424. doi: 10.1016/j.smim.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 83.Dinarello CA, Novick D, Kim S, Kaplanski G. 2013. Interleukin-18 and IL-18 binding protein. Front Immunol 4:289. doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sellin ME, Muller AA, Felmy B, Dolowschiak T, Diard M, Tardivel A, Maslowski KM, Hardt WD. 2014. Epithelium-intrinsic NAIP/NLRC4 inflammasome drives infected enterocyte expulsion to restrict Salmonella replication in the intestinal mucosa. Cell Host Microbe 16:237–248. doi: 10.1016/j.chom.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 85.Knodler LA, Crowley SM, Sham HP, Yang H, Wrande M, Ma C, Ernst RK, Steele-Mortimer O, Celli J, Vallance BA. 2014. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 16:249–256. doi: 10.1016/j.chom.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. 2010. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Man SM, Karki R, Kanneganti TD. 2017. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen KW, Gross CJ, Sotomayor FV, Stacey KJ, Tschopp J, Sweet MJ, Schroder K. 2014. The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge. Cell Rep 8:570–582. doi: 10.1016/j.celrep.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 89.Akhter A, Caution K, Abu Khweek A, Tazi M, Abdulrahman BA, Abdelaziz DH, Voss OH, Doseff AI, Hassan H, Azad AK, Schlesinger LS, Wewers MD, Gavrilin MA, Amer AO. 2012. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity 37:35–47. doi: 10.1016/j.immuni.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. 2012. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490:288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Case CL, Kohler LJ, Lima JB, Strowig T, de Zoete MR, Flavell RA, Zamboni DS, Roy CR. 2013. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc Natl Acad Sci U S A 110:1851–1856. doi: 10.1073/pnas.1211521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taxman DJ, Swanson KV, Broglie PM, Wen H, Holley-Guthrie E, Huang MT, Callaway JB, Eitas TK, Duncan JA, Ting JP. 2012. Porphyromonas gingivalis mediates inflammasome repression in polymicrobial cultures through a novel mechanism involving reduced endocytosis. J Biol Chem 287:32791–32799. doi: 10.1074/jbc.M112.401737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brodsky IE, Palm NW, Sadanand S, Ryndak MB, Sutterwala FS, Flavell RA, Bliska JB, Medzhitov R. 2010. A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe 7:376–387. doi: 10.1016/j.chom.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lagrange B, Benaoudia S, Wallet P, Magnotti F, Provost A, Michal F, Martin A, Di Lorenzo F, Py BF, Molinaro A, Henry T. 2018. Human caspase-4 detects tetra-acylated LPS and cytosolic Francisella and functions differently from murine caspase-11. Nat Commun 9:242. doi: 10.1038/s41467-017-02682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. 2012. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gurung P, Malireddi RK, Anand PK, Demon D, Vande Walle L, Liu Z, Vogel P, Lamkanfi M, Kanneganti TD. 2012. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-beta (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J Biol Chem 287:34474–34483. doi: 10.1074/jbc.M112.401406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ngo CC, Man SM. 2017. Mechanisms and functions of guanylate-binding proteins and related interferon-inducible GTPases: roles in intracellular lysis of pathogens. Cell Microbiol 19:e12791. doi: 10.1111/cmi.12791. [DOI] [PubMed] [Google Scholar]
- 98.Meunier E, Dick MS, Dreier RF, Schurmann N, Kenzelmann Broz D, Warming S, Roose-Girma M, Bumann D, Kayagaki N, Takeda K, Yamamoto M, Broz P. 2014. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 509:366–370. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- 99.Pilla DM, Hagar JA, Haldar AK, Mason AK, Degrandi D, Pfeffer K, Ernst RK, Yamamoto M, Miao EA, Coers J. 2014. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc Natl Acad Sci U S A 111:6046–6051. doi: 10.1073/pnas.1321700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Man SM, Karki R, Sasai M, Place DE, Kesavardhana S, Temirov J, Frase S, Zhu Q, Malireddi RK, Kuriakose T, Peters JL, Neale G, Brown SA, Yamamoto M, Kanneganti TD. 2016. IRGB10 liberates bacterial ligands for sensing by the AIM2 and caspase-11-NLRP3 inflammasomes. Cell 167:382.e317–396.e317. doi: 10.1016/j.cell.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haldar AK, Foltz C, Finethy R, Piro AS, Feeley EM, Pilla-Moffett DM, Komatsu M, Frickel EM, Coers J. 2015. Ubiquitin systems mark pathogen-containing vacuoles as targets for host defense by guanylate binding proteins. Proc Natl Acad Sci U S A 112:E5628–E5637. doi: 10.1073/pnas.1515966112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Man SM, Place DE, Kuriakose T, Kanneganti TD. 2017. Interferon-inducible guanylate-binding proteins at the interface of cell-autonomous immunity and inflammasome activation. J Leukoc Biol 101:143–150. doi: 10.1189/jlb.4MR0516-223R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Finethy R, Jorgensen I, Haldar AK, de Zoete MR, Strowig T, Flavell RA, Yamamoto M, Nagarajan UM, Miao EA, Coers J. 2015. Guanylate binding proteins enable rapid activation of canonical and noncanonical inflammasomes in Chlamydia-infected macrophages. Infect Immun 83:4740–4749. doi: 10.1128/IAI.00856-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vanaja SK, Russo AJ, Behl B, Banerjee I, Yankova M, Deshmukh SD, Rathinam VAK. 2016. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 165:1106–1119. doi: 10.1016/j.cell.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Finethy R, Luoma S, Orench-Rivera N, Feeley EM, Haldar AK, Yamamoto M, Kanneganti TD, Kuehn MJ, Coers J. 2017. Inflammasome activation by bacterial outer membrane vesicles requires guanylate binding proteins. mBio 8:e01188-. doi: 10.1128/mBio.01188-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Santos JC, Dick MS, Lagrange B, Degrandi D, Pfeffer K, Yamamoto M, Meunier E, Pelczar P, Henry T, Broz P. 2018. LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. EMBO J 37:e98089. doi: 10.15252/embj.201798089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sarkar A, Hall MW, Exline M, Hart J, Knatz N, Gatson NT, Wewers MD. 2006. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1beta and interleukin-18. Am J Respir Crit Care Med 174:1003–1010. doi: 10.1164/rccm.200604-546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. 1998. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92:501–509. doi: 10.1016/S0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- 109.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, Towne E, Tracey D, Wardwell S, Wei F-Y, Wong W, Kamen R, Seshadri T. 1995. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 110.Man SM, Karki R, Briard B, Burton A, Gingras S, Pelletier S, Kanneganti TD. 2017. Differential roles of caspase-1 and caspase-11 in infection and inflammation. Sci Rep 7:45126. doi: 10.1038/srep45126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheng KT, Xiong S, Ye Z, Hong Z, Di A, Tsang KM, Gao X, An S, Mittal M, Vogel SM, Miao EA, Rehman J, Malik AB. 2017. Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J Clin Invest 127:4124–4135. doi: 10.1172/JCI94495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang W, Shao Y, Li S, Xin N, Ma T, Zhao C, Song M. 2017. Caspase-11 plays a protective role in pulmonary Acinetobacter baumannii infection. Infect Immun 85:e00350-. doi: 10.1128/IAI.00350-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang J, Shao Y, Wang W, Li S, Xin N, Xie F, Zhao C. 2017. Caspase-11 deficiency impairs neutrophil recruitment and bacterial clearance in the early stage of pulmonary Klebsiella pneumoniae infection. Int J Med Microbiol 307:490–496. doi: 10.1016/j.ijmm.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 114.Dikshit N, Kale SD, Khameneh HJ, Balamuralidhar V, Tang CY, Kumar P, Lim TP, Tan TT, Kwa AL, Mortellaro A, Sukumaran B. 2018. NLRP3 inflammasome pathway has a critical role in the host immunity against clinically relevant Acinetobacter baumannii pulmonary infection. Mucosal Immunol 11:257–272. doi: 10.1038/mi.2017.50. [DOI] [PubMed] [Google Scholar]
- 115.Jorgensen I, Rayamajhi M, Miao EA. 2017. Programmed cell death as a defence against infection. Nat Rev Immunol 17:151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jorgensen I, Zhang Y, Krantz BA, Miao EA. 2016. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med 213:2113–2128. doi: 10.1084/jem.20151613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jorgensen I, Lopez JP, Laufer SA, Miao EA. 2016. IL-1beta, IL-18, and eicosanoids promote neutrophil recruitment to pore-induced intracellular traps following pyroptosis. Eur J Immunol 46:2761–2766. doi: 10.1002/eji.201646647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, Robertson AA, Cooper MA, Graf T, Hornung V. 2016. Human monocytes engage an alternative inflammasome pathway. Immunity 44:833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 119.Khare S, Dorfleutner A, Bryan NB, Yun C, Radian AD, de Almeida L, Rojanasakul Y, Stehlik C. 2012. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity 36:464–476. doi: 10.1016/j.immuni.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen Y, Li X, Boini KM, Pitzer AL, Gulbins E, Zhang Y, Li PL. 2015. Endothelial Nlrp3 inflammasome activation associated with lysosomal destabilization during coronary arteritis. Biochim Biophys Acta 1853:396–408. doi: 10.1016/j.bbamcr.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Valderrama JA, Riestra AM, Gao NJ, LaRock CN, Gupta N, Ali SR, Hoffman HM, Ghosh P, Nizet V. 2017. Group A streptococcal M protein activates the NLRP3 inflammasome. Nat Microbiol 2:1425–1434. doi: 10.1038/s41564-017-0005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, Nizet V, Dixit VM, Karin M. 2008. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A 105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. 2010. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A 107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Galan JE, Lara-Tejero M, Marlovits TC, Wagner S. 2014. Bacterial type III secretion systems: specialized nanomachines for protein delivery into target cells. Annu Rev Microbiol 68:415–438. doi: 10.1146/annurev-micro-092412-155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Du J, Reeves AZ, Klein JA, Twedt DJ, Knodler LA, Lesser CF. 2016. The type III secretion system apparatus determines the intracellular niche of bacterial pathogens. Proc Natl Acad Sci U S A 113:4794–4799. doi: 10.1073/pnas.1520699113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun YH, Cado D, Dietrich WF, Monack DM, Tsolis RM, Vance RE. 2008. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol 9:1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kortmann J, Brubaker SW, Monack DM. 2015. Cutting edge: inflammasome activation in primary human macrophages is dependent on flagellin. J Immunol 195:815–819. doi: 10.4049/jimmunol.1403100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tenthorey JL, Haloupek N, Lopez-Blanco JR, Grob P, Adamson E, Hartenian E, Lind NA, Bourgeois NM, Chacon P, Nogales E, Vance RE. 2017. The structural basis of flagellin detection by NAIP5: a strategy to limit pathogen immune evasion. Science 358:888–893. doi: 10.1126/science.aao1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suzuki S, Franchi L, He Y, Munoz-Planillo R, Mimuro H, Suzuki T, Sasakawa C, Nunez G. 2014. Shigella type III secretion protein MxiI is recognized by Naip2 to induce Nlrc4 inflammasome activation independently of Pkcdelta. PLoS Pathog 10:e1003926. doi: 10.1371/journal.ppat.1003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang J, Zhao Y, Shi J, Shao F. 2013. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc Natl Acad Sci U S A 110:14408–14413. doi: 10.1073/pnas.1306376110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rayamajhi M, Zak DE, Chavarria-Smith J, Vance RE, Miao EA. 2013. Cutting edge: mouse NAIP1 detects the type III secretion system needle protein. J Immunol 191:3986–3989. doi: 10.4049/jimmunol.1301549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grandjean T, Boucher A, Thepaut M, Monlezun L, Guery B, Faudry E, Kipnis E, Dessein R. 2017. The human NAIP-NLRC4-inflammasome senses the Pseudomonas aeruginosa T3SS inner-rod protein. Int Immunol 29:377–384. doi: 10.1093/intimm/dxx047. [DOI] [PubMed] [Google Scholar]
- 133.Reyes Ruiz VM, Ramirez J, Naseer N, Palacio NM, Siddarthan IJ, Yan BM, Boyer MA, Pensinger DA, Sauer JD, Shin S. 2017. Broad detection of bacterial type III secretion system and flagellin proteins by the human NAIP/NLRC4 inflammasome. Proc Natl Acad Sci U S A 114:13242–13247. doi: 10.1073/pnas.1710433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tenthorey JL, Kofoed EM, Daugherty MD, Malik HS, Vance RE. 2014. Molecular basis for specific recognition of bacterial ligands by NAIP/NLRC4 inflammasomes. Mol Cell 54:17–29. doi: 10.1016/j.molcel.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang J, Zhao Y, Li P, Yang Y, Zhang E, Zhong M, Li Y, Zhou D, Cao Y, Lu M, Shao F, Yan H. 2018. Sequence determinants of specific pattern-recognition of bacterial ligands by the NAIP-NLRC4 inflammasome. Cell Discov 4:22. doi: 10.1038/s41421-018-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Endrizzi MG, Hadinoto V, Growney JD, Miller W, Dietrich WF. 2000. Genomic sequence analysis of the mouse Naip gene array. Genome Res 10:1095–1102. doi: 10.1101/gr.10.8.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Warren SE, Mao DP, Rodriguez AE, Miao EA, Aderem A. 2008. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol 180:7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. 2006. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol 7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 139.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Nunez G. 2006. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem 281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 140.Sun YH, Rolan HG, Tsolis RM. 2007. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J Biol Chem 282:33897–33901. doi: 10.1074/jbc.C700181200. [DOI] [PubMed] [Google Scholar]
- 141.Halff EF, Diebolder CA, Versteeg M, Schouten A, Brondijk TH, Huizinga EG. 2012. Formation and structure of a NAIP5-NLRC4 inflammasome induced by direct interactions with conserved N- and C-terminal regions of flagellin. J Biol Chem 287:38460–38472. doi: 10.1074/jbc.M112.393512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hu Z, Zhou Q, Zhang C, Fan S, Cheng W, Zhao Y, Shao F, Wang HW, Sui SF, Chai J. 2015. Structural and biochemical basis for induced self-propagation of NLRC4. Science 350:399–404. doi: 10.1126/science.aac5489. [DOI] [PubMed] [Google Scholar]
- 143.Zhang L, Chen S, Ruan J, Wu J, Tong AB, Yin Q, Li Y, David L, Lu A, Wang WL, Marks C, Ouyang Q, Zhang X, Mao Y, Wu H. 2015. Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science 350:404–409. doi: 10.1126/science.aac5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qu Y, Misaghi S, Newton K, Maltzman A, Izrael-Tomasevic A, Arnott D, Dixit VM. 2016. NLRP3 recruitment by NLRC4 during Salmonella infection. J Exp Med 213:877–885. doi: 10.1084/jem.20132234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Matusiak M, Van Opdenbosch N, Vande Walle L, Sirard JC, Kanneganti TD, Lamkanfi M. 2015. Flagellin-induced NLRC4 phosphorylation primes the inflammasome for activation by NAIP5. Proc Natl Acad Sci U S A 112:1541–1546. doi: 10.1073/pnas.1417945112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Qu Y, Misaghi S, Izrael-Tomasevic A, Newton K, Gilmour LL, Lamkanfi M, Louie S, Kayagaki N, Liu J, Komuves L, Cupp JE, Arnott D, Monack D, Dixit VM. 2012. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature 490:539–542. doi: 10.1038/nature11429. [DOI] [PubMed] [Google Scholar]
- 147.Liu W, Liu X, Li Y, Zhao J, Liu Z, Hu Z, Wang Y, Yao Y, Miller AW, Su B, Cookson MR, Li X, Kang Z. 2017. LRRK2 promotes the activation of NLRC4 inflammasome during Salmonella Typhimurium infection. J Exp Med 214:3051–3066. doi: 10.1084/jem.20170014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ, Flavell RA, Galan JE. 2006. Role of the caspase-1 inflammasome in Salmonella Typhimurium pathogenesis. J Exp Med 203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Man SM. Inflammasomes in the gastrointestinal tract: infection, cancer and gut microbiota homeostasis. Nat Rev Gastroenterol Hepatol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, van Rooijen N, Brown CR, Krantz BA, Leppla SH, Gronert K, Vance RE. 2012. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490:107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rauch I, Deets KA, Ji DX, von Moltke J, Tenthorey JL, Lee AY, Philip NH, Ayres JS, Brodsky IE, Gronert K, Vance RE. 2017. NAIP-NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of caspase-1 and -8. Immunity 46:649–659. doi: 10.1016/j.immuni.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Man SM, Tourlomousis P, Hopkins L, Monie TP, Fitzgerald KA, Bryant CE. 2013. Salmonella infection induces recruitment of caspase-8 to the inflammasome to modulate IL-1beta production. J Immunol 191:5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mascarenhas DPA, Cerqueira DM, Pereira MSF, Castanheira FVS, Fernandes TD, Manin GZ, Cunha LD, Zamboni DS. 2017. Inhibition of caspase-1 or gasdermin-D enable caspase-8 activation in the Naip5/NLRC4/ASC inflammasome. PLoS Pathog 13:e1006502. doi: 10.1371/journal.ppat.1006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim S, Bauernfeind F, Ablasser A, Hartmann G, Fitzgerald KA, Latz E, Hornung V. 2010. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur J Immunol 40:1545–1551. doi: 10.1002/eji.201040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bouwman LI, de Zoete MR, Bleumink-Pluym NM, Flavell RA, van Putten JP. 2014. Inflammasome activation by Campylobacter jejuni. J Immunol 193:4548–4557. doi: 10.4049/jimmunol.1400648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, Logan SM, Aderem A. 2005. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci U S A 102:9247–9252. doi: 10.1073/pnas.0502040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brodmann M, Dreier RF, Broz P, Basler M. 2017. Francisella requires dynamic type VI secretion system and ClpB to deliver effectors for phagosomal escape. Nat Commun 8:15853. doi: 10.1038/ncomms15853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O'Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, Dixit VM, Monack DM. 2010. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A 107:9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Belhocine K, Monack DM. 2012. Francisella infection triggers activation of the AIM2 inflammasome in murine dendritic cells. Cell Microbiol 14:71–80. doi: 10.1111/j.1462-5822.2011.01700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Peng K, Broz P, Jones J, Joubert LM, Monack D. 2011. Elevated AIM2-mediated pyroptosis triggered by hypercytotoxic Francisella mutant strains is attributed to increased intracellular bacteriolysis. Cell Microbiol 13:1586–1600. doi: 10.1111/j.1462-5822.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. 2007. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med 204:987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Man SM, Karki R, Malireddi RK, Neale G, Vogel P, Yamamoto M, Lamkanfi M, Kanneganti TD. 2015. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol 16:467–475. doi: 10.1038/ni.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Meunier E, Wallet P, Dreier RF, Costanzo S, Anton L, Ruhl S, Dussurgey S, Dick MS, Kistner A, Rigard M, Degrandi D, Pfeffer K, Yamamoto M, Henry T, Broz P. 2015. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat Immunol 16:476–484. doi: 10.1038/ni.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. 2010. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, Eisenlohr L, Landel CP, Alnemri ES. 2010. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol 11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wallet P, Benaoudia S, Mosnier A, Lagrange B, Martin A, Lindgren H, Golovliov I, Michal F, Basso P, Djebali S, Provost A, Allatif O, Meunier E, Broz P, Yamamoto M, Py BF, Faudry E, Sjostedt A, Henry T. 2017. IFN-gamma extends the immune functions of guanylate binding proteins to inflammasome-independent antibacterial activities during Francisella novicida infection. PLoS Pathog 13:e1006630. doi: 10.1371/journal.ppat.1006630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhu Q, Man SM, Karki R, Malireddi RKS, Kanneganti TD. 2018. Detrimental type I interferon signaling dominates protective AIM2 inflammasome responses during Francisella novicida infection. Cell Rep 22:3168–3174. doi: 10.1016/j.celrep.2018.02.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gaidt MM, Ebert TS, Chauhan D, Ramshorn K, Pinci F, Zuber S, O'Duill F, Schmid-Burgk JL, Hoss F, Buhmann R, Wittmann G, Latz E, Subklewe M, Hornung V. 2017. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell 171:1110–1124. doi: 10.1016/j.cell.2017.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Man SM, Karki R, Kanneganti TD. 2016. AIM2 inflammasome in infection, cancer, and autoimmunity: role in DNA sensing, inflammation, and innate immunity. Eur J Immunol 46:269–280. doi: 10.1002/eji.201545839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Eichholz K, Bru T, Tran TT, Fernandes P, Welles H, Mennechet FJ, Manel N, Alves P, Perreau M, Kremer EJ. 2016. Immune-complexed adenovirus induce AIM2-mediated pyroptosis in human dendritic cells. PLoS Pathog 12:e1005871. doi: 10.1371/journal.ppat.1005871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ulland TK, Buchan BW, Ketterer MR, Fernandes-Alnemri T, Meyerholz DK, Apicella MA, Alnemri ES, Jones BD, Nauseef WM, Sutterwala FS. 2010. Cutting edge: mutation of Francisella tularensis mviN leads to increased macrophage absent in melanoma 2 inflammasome activation and a loss of virulence. J Immunol 185:2670–2674. doi: 10.4049/jimmunol.1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sampson TR, Napier BA, Schroeder MR, Louwen R, Zhao J, Chin CY, Ratner HK, Llewellyn AC, Jones CL, Laroui H, Merlin D, Zhou P, Endtz HP, Weiss DS. 2014. A CRISPR-Cas system enhances envelope integrity mediating antibiotic resistance and inflammasome evasion. Proc Natl Acad Sci U S A 111:11163–11168. doi: 10.1073/pnas.1323025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ge J, Gong YN, Xu Y, Shao F. 2012. Preventing bacterial DNA release and absent in melanoma 2 inflammasome activation by a Legionella effector functioning in membrane trafficking. Proc Natl Acad Sci U S A 109:6193–6198. doi: 10.1073/pnas.1117490109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li P, Jiang W, Yu Q, Liu W, Zhou P, Li J, Xu J, Xu B, Wang F, Shao F. 2017. Ubiquitination and degradation of GBPs by a Shigella effector to suppress host defence. Nature 551:378–383. doi: 10.1038/nature24467. [DOI] [PubMed] [Google Scholar]
- 175.Wandel MP, Pathe C, Werner EI, Ellison CJ, Boyle KB, von der Malsburg A, Rohde J, Randow F. 2017. GBPs inhibit motility of Shigella flexneri but are targeted for degradation by the bacterial ubiquitin ligase IpaH9.8. Cell Host Microbe 22:507.e505–518.e505. doi: 10.1016/j.chom.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Piro AS, Hernandez D, Luoma S, Feeley EM, Finethy R, Yirga A, Frickel EM, Lesser CF, Coers J. 2017. Detection of cytosolic Shigella flexneri via a C-terminal triple-arginine motif of GBP1 inhibits actin-based motility. mBio 8:e01979-. doi: 10.1128/mBio.01979-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Nunez G. 2006. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 178.Gupta R, Ghosh S, Monks B, DeOliveira RB, Tzeng TC, Kalantari P, Nandy A, Bhattacharjee B, Chan J, Ferreira F, Rathinam V, Sharma S, Lien E, Silverman N, Fitzgerald K, Firon A, Trieu-Cuot P, Henneke P, Golenbock DT. 2014. RNA and beta-hemolysin of group B Streptococcus induce interleukin-1beta (IL-1beta) by activating NLRP3 inflammasomes in mouse macrophages. J Biol Chem 289:13701–13705. doi: 10.1074/jbc.C114.548982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sander LE, Davis MJ, Boekschoten MV, Amsen D, Dascher CC, Ryffel B, Swanson JA, Muller M, Blander JM. 2011. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature 474:385–389. doi: 10.1038/nature10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kailasan Vanaja S, Rathinam VA, Atianand MK, Kalantari P, Skehan B, Fitzgerald KA, Leong JM. 2014. Bacterial RNA:DNA hybrids are activators of the NLRP3 inflammasome. Proc Natl Acad Sci U S A 111:7765–7770. doi: 10.1073/pnas.1400075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sha W, Mitoma H, Hanabuchi S, Bao M, Weng L, Sugimoto N, Liu Y, Zhang Z, Zhong J, Sun B, Liu YJ. 2014. Human NLRP3 inflammasome senses multiple types of bacterial RNAs. Proc Natl Acad Sci U S A 111:16059–16064. doi: 10.1073/pnas.1412487111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mitoma H, Hanabuchi S, Kim T, Bao M, Zhang Z, Sugimoto N, Liu YJ. 2013. The DHX33 RNA helicase senses cytosolic RNA and activates the NLRP3 inflammasome. Immunity 39:123–135. doi: 10.1016/j.immuni.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Weng L, Mitoma H, Trichot C, Bao M, Liu Y, Zhang Z, Liu YJ. 2014. The E3 ubiquitin ligase tripartite motif 33 is essential for cytosolic RNA-induced NLRP3 inflammasome activation. J Immunol 193:3676–3682. doi: 10.4049/jimmunol.1401448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Toma C, Higa N, Koizumi Y, Nakasone N, Ogura Y, McCoy AJ, Franchi L, Uematsu S, Sagara J, Taniguchi S, Tsutsui H, Akira S, Tschopp J, Nunez G, Suzuki T. 2010. Pathogenic Vibrio activate NLRP3 inflammasome via cytotoxins and TLR/nucleotide-binding oligomerization domain-mediated NF-kappa B signaling. J Immunol 184:5287–5297. doi: 10.4049/jimmunol.0903536. [DOI] [PubMed] [Google Scholar]
- 185.Munoz-Planillo R, Franchi L, Miller LS, Nunez G. 2009. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J Immunol 183:3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in Salmonella-infected macrophages. Nat Immunol 7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 187.Meixenberger K, Pache F, Eitel J, Schmeck B, Hippenstiel S, Slevogt H, N′Guessan P, Witzenrath M, Netea MG, Chakraborty T, Suttorp N, Opitz B. 2010. Listeria monocytogenes-infected human peripheral blood mononuclear cells produce IL-1beta, depending on listeriolysin O and NLRP3. J Immunol 184:922–930. doi: 10.4049/jimmunol.0901346. [DOI] [PubMed] [Google Scholar]
- 188.Liu S, Moayeri M, Leppla SH. 2014. Anthrax lethal and edema toxins in anthrax pathogenesis. Trends Microbiol 22:317–325. doi: 10.1016/j.tim.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Terra JK, Cote CK, France B, Jenkins AL, Bozue JA, Welkos SL, LeVine SM, Bradley KA. 2010. Cutting edge: resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J Immunol 184:17–20. doi: 10.4049/jimmunol.0903114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Moayeri M, Crown D, Newman ZL, Okugawa S, Eckhaus M, Cataisson C, Liu S, Sastalla I, Leppla SH. 2010. Inflammasome sensor Nlrp1b-dependent resistance to anthrax is mediated by caspase-1, IL-1 signaling and neutrophil recruitment. PLoS Pathog 6:e1001222. doi: 10.1371/journal.ppat.1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bose S, Segovia JA, Somarajan SR, Chang TH, Kannan TR, Baseman JB. 2014. ADP-ribosylation of NLRP3 by Mycoplasma pneumoniae CARDS toxin regulates inflammasome activity. mBio 5:e02186-. doi: 10.1128/mBio.02186-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Segovia JA, Chang TH, Winter VT, Coalson JJ, Cagle MP, Pandranki L, Bose S, Baseman JB, Kannan TR. 2017. NLRP3 is a critical regulator of inflammation and innate immune cell response during Mycoplasma pneumoniae infection. Infect Immun 86:e00548-. doi: 10.1128/IAI.00548-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Huang Y, Liu L, Ma D, Liao Y, Lu Y, Huang H, Qin W, Liu X, Fang F. 2017. Human cytomegalovirus triggers the assembly of AIM2 inflammasome in THP-1-derived macrophages. J Med Virol 89:2188–2195. doi: 10.1002/jmv.24846. [DOI] [PubMed] [Google Scholar]
- 194.Huang Y, Ma D, Huang H, Lu Y, Liao Y, Liu L, Liu X, Fang F. 2017. Interaction between HCMV pUL83 and human AIM2 disrupts the activation of the AIM2 inflammasome. Virol J 14:34. doi: 10.1186/s12985-016-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li T, Chen J, Cristea IM. 2013. Human cytomegalovirus tegument protein pUL83 inhibits IFI16-mediated DNA sensing for immune evasion. Cell Host Microbe 14:591–599. doi: 10.1016/j.chom.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Biolatti M, Dell'Oste V, Pautasso S, von Einem J, Marschall M, Plachter B, Gariglio M, De Andrea M, Landolfo S. 2016. Regulatory interaction between the cellular restriction factor IFI16 and viral pp65 (pUL83) modulates viral gene expression and IFI16 protein stability. J Virol 90:8238–8250. doi: 10.1128/JVI.00923-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cristea IM, Moorman NJ, Terhune SS, Cuevas CD, O'Keefe ES, Rout MP, Chait BT, Shenk T. 2010. Human cytomegalovirus pUL83 stimulates activity of the viral immediate-early promoter through its interaction with the cellular IFI16 protein. J Virol 84:7803–7814. doi: 10.1128/JVI.00139-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhen J, Zhang L, Pan J, Ma S, Yu X, Li X, Chen S, Du W. 2014. AIM2 mediates inflammation-associated renal damage in hepatitis B virus-associated glomerulonephritis by regulating caspase-1, IL-1beta, and IL-18. Mediators Inflamm 2014:190860. doi: 10.1155/2014/190860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Reinholz M, Kawakami Y, Salzer S, Kreuter A, Dombrowski Y, Koglin S, Kresse S, Ruzicka T, Schauber J. 2013. HPV16 activates the AIM2 inflammasome in keratinocytes. Arch Dermatol Res 305:723–732. doi: 10.1007/s00403-013-1375-0. [DOI] [PubMed] [Google Scholar]
- 200.Torii Y, Kawada JI, Murata T, Yoshiyama H, Kimura H, Ito Y. 2017. Epstein-Barr virus infection-induced inflammasome activation in human monocytes. PLoS One 12:e0175053. doi: 10.1371/journal.pone.0175053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Strittmatter GE, Sand J, Sauter M, Seyffert M, Steigerwald R, Fraefel C, Smola S, French LE, Beer HD. 2016. IFN-γ primes keratinocytes for HSV-1-induced inflammasome activation. J Invest Dermatol 136:610–620. doi: 10.1016/j.jid.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 202.Pan X, Xu H, Zheng C, Li M, Zou X, Cao H, Xu Q. 2016. Human hepatocytes express absent in melanoma 2 and respond to hepatitis B virus with interleukin-18 expression. Virus Genes 52:445–452. doi: 10.1007/s11262-016-1327-9. [DOI] [PubMed] [Google Scholar]
- 203.Wu DL, Xu GH, Lu SM, Ma BL, Miao NZ, Liu XB, Cheng YP, Feng JH, Liu ZG, Feng D, Na L, Li WQ, Zhao YR. 2013. Correlation of AIM2 expression in peripheral blood mononuclear cells from humans with acute and chronic hepatitis B. Hum Immunol 74:514–521. doi: 10.1016/j.humimm.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 204.Chen LC, Wang LJ, Tsang NM, Ojcius DM, Chen CC, Ouyang CN, Hsueh C, Liang Y, Chang KP, Chen CC, Chang YS. 2012. Tumour inflammasome-derived IL-1beta recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma. EMBO Mol Med 4:1276–1293. doi: 10.1002/emmm.201201569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Johnson KE, Chikoti L, Chandran B. 2013. Herpes simplex virus 1 infection induces activation and subsequent inhibition of the IFI16 and NLRP3 inflammasomes. J Virol 87:5005–5018. doi: 10.1128/JVI.00082-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gimenez F, Bhela S, Dogra P, Harvey L, Varanasi SK, Jaggi U, Rouse BT. 2016. The inflammasome NLRP3 plays a protective role against a viral immunopathological lesion. J Leukoc Biol 99:647–657. doi: 10.1189/jlb.3HI0715-321R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang SL, Zhao G, Zhu W, Dong XM, Liu T, Li YY, Song WG, Wang YQ. 2015. Herpes simplex virus-1 infection or simian virus 40-mediated immortalization of corneal cells causes permanent translocation of NLRP3 to the nuclei. Int J Ophthalmol 8:46–51. doi: 10.3980/j.issn.2222-3959.2015.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. 2008. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 209.Delaloye J, Roger T, Steiner-Tardivel QG, Le Roy D, Knaup Reymond M, Akira S, Petrilli V, Gomez CE, Perdiguero B, Tschopp J, Pantaleo G, Esteban M, Calandra T. 2009. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog 5:e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 210.Barlan AU, Griffin TM, McGuire KA, Wiethoff CM. 2011. Adenovirus membrane penetration activates the NLRP3 inflammasome. J Virol 85:146–155. doi: 10.1128/JVI.01265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nour AM, Reichelt M, Ku CC, Ho MY, Heineman TC, Arvin AM. 2011. Varicella-zoster virus infection triggers formation of an interleukin-1beta (IL-1beta)-processing inflammasome complex. J Biol Chem 286:17921–17933. doi: 10.1074/jbc.M110.210575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Teigler JE, Kagan JC, Barouch DH. 2014. Late endosomal trafficking of alternative serotype adenovirus vaccine vectors augments antiviral innate immunity. J Virol 88:10354–10363. doi: 10.1128/JVI.00936-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Barlan AU, Danthi P, Wiethoff CM. 2011. Lysosomal localization and mechanism of membrane penetration influence nonenveloped virus activation of the NLRP3 inflammasome. Virology 412:306–314. doi: 10.1016/j.virol.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Maruzuru Y, Ichinohe T, Sato R, Miyake K, Okano T, Suzuki T, Koshiba T, Koyanagi N, Tsuda S, Watanabe M, Arii J, Kato A, Kawaguchi Y. 2018. Herpes simplex virus 1 VP22 inhibits AIM2-dependent inflammasome activation to enable efficient viral replication. Cell Host Microbe 23:254.e257–265.e257. doi: 10.1016/j.chom.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 215.Ansari MA, Dutta S, Veettil MV, Dutta D, Iqbal J, Kumar B, Roy A, Chikoti L, Singh VV, Chandran B. 2015. Herpesvirus genome recognition induced acetylation of nuclear IFI16 is essential for its cytoplasmic translocation, inflammasome and IFN-beta responses. PLoS Pathog 11:e1005019. doi: 10.1371/journal.ppat.1005019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Horimoto T, Kawaoka Y. 2005. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol 3:591–600. doi: 10.1038/nrmicro1208. [DOI] [PubMed] [Google Scholar]
- 217.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. 2009. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Thomas PG, Dash P, Aldridge JR Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. 2009. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ren R, Wu S, Cai J, Yang Y, Ren X, Feng Y, Chen L, Qin B, Xu C, Yang H, Song Z, Tian D, Hu Y, Zhou X, Meng G. 2017. The H7N9 influenza A virus infection results in lethal inflammation in the mammalian host via the NLRP3-caspase-1 inflammasome. Sci Rep 7:7625. doi: 10.1038/s41598-017-07384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.McAuley JL, Tate MD, MacKenzie-Kludas CJ, Pinar A, Zeng W, Stutz A, Latz E, Brown LE, Mansell A. 2013. Activation of the NLRP3 inflammasome by IAV virulence protein PB1-F2 contributes to severe pathophysiology and disease. PLoS Pathog 9:e1003392. doi: 10.1371/journal.ppat.1003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pinar A, Dowling JK, Bitto NJ, Robertson AA, Latz E, Stewart CR, Drummond GR, Cooper MA, McAuley JL, Tate MD, Mansell A. 2017. PB1-F2 peptide derived from avian influenza A virus H7N9 induces inflammation via activation of the NLRP3 inflammasome. J Biol Chem 292:826–836. doi: 10.1074/jbc.M116.756379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Nunez G. 2006. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem 281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 223.Ichinohe T, Pang IK, Iwasaki A. 2010. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol 11:404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kuriakose T, Kanneganti TD. 2017. Regulation and functions of NLRP3 inflammasome during influenza virus infection. Mol Immunol 86:56–64. doi: 10.1016/j.molimm.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chakrabarti A, Banerjee S, Franchi L, Loo YM, Gale M Jr, Nunez G, Silverman RH. 2015. RNase L activates the NLRP3 inflammasome during viral infections. Cell Host Microbe 17:466–477. doi: 10.1016/j.chom.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kuriakose T, Man SM, Malireddi RK, Karki R, Kesavardhana S, Place DE, Neale G, Vogel P, Kanneganti TD. 2016. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol 1:aag2045. doi: 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Thapa RJ, Ingram JP, Ragan KB, Nogusa S, Boyd DF, Benitez AA, Sridharan H, Kosoff R, Shubina M, Landsteiner VJ, Andrake M, Vogel P, Sigal LJ, ten Oever BR, Thomas PG, Upton JW, Balachandran S. 2016. DAI senses influenza A virus genomic RNA and activates RIPK3-dependent cell death. Cell Host Microbe 20:674–681. doi: 10.1016/j.chom.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kesavardhana S, Kuriakose T, Guy CS, Samir P, Malireddi RKS, Mishra A, Kanneganti TD. 2017. ZBP1/DAI ubiquitination and sensing of influenza vRNPs activate programmed cell death. J Exp Med 214:2217–2229. doi: 10.1084/jem.20170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Moriyama M, Chen IY, Kawaguchi A, Koshiba T, Nagata K, Takeyama H, Hasegawa H, Ichinohe T. 2016. The RNA- and TRIM25-binding domains of influenza virus NS1 protein are essential for suppression of NLRP3 inflammasome-mediated interleukin-1beta secretion. J Virol 90:4105–4114. doi: 10.1128/JVI.00120-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cheong WC, Kang HR, Yoon H, Kang SJ, Ting JP, Song MJ. 2015. Influenza A virus NS1 protein inhibits the NLRP3 inflammasome. PLoS One 10:e0126456. doi: 10.1371/journal.pone.0126456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Coates BM, Staricha KL, Ravindran N, Koch CM, Cheng Y, Davis JM, Shumaker DK, Ridge KM. 2017. Inhibition of the NOD-like receptor protein 3 inflammasome is protective in juvenile influenza A virus infection. Front Immunol 8:782. doi: 10.3389/fimmu.2017.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tate MD, Ong JD, Dowling JK, McAuley JL, Robertson AB, Latz E, Drummond GR, Cooper MA, Hertzog PJ, Mansell A. 2016. Reassessing the role of the NLRP3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Sci Rep 6:27912. doi: 10.1038/srep27912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang H, Luo J, Alcorn JF, Chen K, Fan S, Pilewski J, Liu A, Chen W, Kolls JK, Wang J. 2017. AIM2 inflammasome is critical for influenza-induced lung injury and mortality. J Immunol 198:4383–4393. doi: 10.4049/jimmunol.1600714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Schattgen SA, Gao G, Kurt-Jones EA, Fitzgerald KA. 2016. Cutting edge: DNA in the lung microenvironment during influenza virus infection tempers inflammation by engaging the DNA sensor AIM2. J Immunol 196:29–33. doi: 10.4049/jimmunol.1501048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Man SM, Karki R, Kanneganti TD. 2016. DNA-sensing inflammasomes: regulation of bacterial host defense and the gut microbiota. Pathog Dis 74:ftw028. doi: 10.1093/femspd/ftw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Deeks SG, Overbaugh J, Phillips A, Buchbinder S. 2015. HIV infection. Nat Rev Dis Primers 1:15035. doi: 10.1038/nrdp.2015.35. [DOI] [PubMed] [Google Scholar]
- 237.Pontillo A, Brandao LA, Guimaraes RL, Segat L, Athanasakis E, Crovella S. 2010. A 3′UTR SNP in NLRP3 gene is associated with susceptibility to HIV-1 infection. J Acquir Immune Defic Syndr 54:236–240. doi: 10.1097/QAI.0b013e3181dd17d4. [DOI] [PubMed] [Google Scholar]
- 238.Pontillo A, Oshiro TM, Girardelli M, Kamada AJ, Crovella S, Duarte AJ. 2012. Polymorphisms in inflammasome' genes and susceptibility to HIV-1 infection. J Acquir Immune Defic Syndr 59:121–125. doi: 10.1097/QAI.0b013e3182392ebe. [DOI] [PubMed] [Google Scholar]
- 239.Chattergoon MA, Latanich R, Quinn J, Winter ME, Buckheit RW III, Blankson JN, Pardoll D, Cox AL. 2014. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon. PLoS Pathog 10:e1004082. doi: 10.1371/journal.ppat.1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Guo H, Gao J, Taxman DJ, Ting JP, Su L. 2014. HIV-1 infection induces interleukin-1beta production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J Biol Chem 289:21716–21726. doi: 10.1074/jbc.M114.566620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hernandez JC, Latz E, Urcuqui-Inchima S. 2014. HIV-1 induces the first signal to activate the NLRP3 inflammasome in monocyte-derived macrophages. Intervirology 57:36–42. doi: 10.1159/000353902. [DOI] [PubMed] [Google Scholar]
- 242.Walsh JG, Reinke SN, Mamik MK, McKenzie BA, Maingat F, Branton WG, Broadhurst DI, Power C. 2014. Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS. Retrovirology 11:35. doi: 10.1186/1742-4690-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mamik MK, Hui E, Branton WG, McKenzie BA, Chisholm J, Cohen EA, Power C. 2017. HIV-1 viral protein R activates NLRP3 inflammasome in microglia: implications for HIV-1 associated neuroinflammation. J Neuroimmune Pharmacol 12:233–248. doi: 10.1007/s11481-016-9708-3. [DOI] [PubMed] [Google Scholar]
- 244.Chivero ET, Guo ML, Periyasamy P, Liao K, Callen SE, Buch S. 2017. HIV-1 Tat primes and activates microglial NLRP3 inflammasome-mediated neuroinflammation. J Neurosci 37:3599–3609. doi: 10.1523/JNEUROSCI.3045-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Haque S, Lan X, Wen H, Lederman R, Chawla A, Attia M, Bongu RP, Husain M, Mikulak J, Saleem MA, Popik W, Malhotra A, Chander PN, Singhal PC. 2016. HIV promotes NLRP3 inflammasome complex activation in murine HIV-associated nephropathy. Am J Pathol 186:347–358. doi: 10.1016/j.ajpath.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Munoz-Arias I, Doitsh G, Yang Z, Sowinski S, Ruelas D, Greene WC. 2015. Blood-derived CD4 T cells naturally resist pyroptosis during abortive HIV-1 infection. Cell Host Microbe 18:463–470. doi: 10.1016/j.chom.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, Greene WC. 2014. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, Greene WC. 2014. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 343:428–432. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Galloway NL, Doitsh G, Monroe KM, Yang Z, Munoz-Arias I, Levy DN, Greene WC. 2015. Cell-to-cell transmission of HIV-1 is required to trigger pyroptotic death of lymphoid-tissue-derived CD4 T cells. Cell Rep 12:1555–1563. doi: 10.1016/j.celrep.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hubner W, McNerney GP, Chen P, Dale BM, Gordon RE, Chuang FY, Li XD, Asmuth DM, Huser T, Chen BK. 2009. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science 323:1743–1747. doi: 10.1126/science.1167525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Toksoy A, Sennefelder H, Adam C, Hofmann S, Trautmann A, Goebeler M, Schmidt M. 2017. Potent NLRP3 inflammasome activation by the HIV reverse transcriptase inhibitor abacavir. J Biol Chem 292:2805–2814. doi: 10.1074/jbc.M116.749473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Di Micco A, Frera G, Lugrin J, Jamilloux Y, Hsu ET, Tardivel A, De Gassart A, Zaffalon L, Bujisic B, Siegert S, Quadroni M, Broz P, Henry T, Hrycyna CA, Martinon F. 2016. AIM2 inflammasome is activated by pharmacological disruption of nuclear envelope integrity. Proc Natl Acad Sci U S A 113:E4671–E4680. doi: 10.1073/pnas.1602419113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Wang H, Lei X, Xiao X, Yang C, Lu W, Huang Z, Leng Q, Jin Q, He B, Meng G, Wang J. 2015. Reciprocal regulation between enterovirus 71 and the NLRP3 inflammasome. Cell Rep 12:42–48. doi: 10.1016/j.celrep.2015.05.047. [DOI] [PubMed] [Google Scholar]
- 254.Wang W, Xiao F, Wan P, Pan P, Zhang Y, Liu F, Wu K, Liu Y, Wu J. 2017. EV71 3D protein binds with NLRP3 and enhances the assembly of inflammasome complex. PLoS Pathog 13:e1006123. doi: 10.1371/journal.ppat.1006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yogarajah T, Ong KC, Perera D, Wong KT. 2017. AIM2 inflammasome-mediated pyroptosis in enterovirus A71-infected neuronal cells restricts viral replication. Sci Rep 7:5845. doi: 10.1038/s41598-017-05589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wang W, Li G, De W, Luo Z, Pan P, Tian M, Wang Y, Xiao F, Li A, Wu K, Liu X, Rao L, Liu F, Liu Y, Wu J. 2018. Zika virus infection induces host inflammatory responses by facilitating NLRP3 inflammasome assembly and interleukin-1beta secretion. Nat Commun 9:106. doi: 10.1038/s41467-017-02645-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, Delker DA, Jo J, Bertoletti A, Hagedorn CH, Gale M Jr. 2013. IL-1beta production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog 9:e1003330. doi: 10.1371/journal.ppat.1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Chen W, Xu Y, Li H, Tao W, Xiang Y, Huang B, Niu J, Zhong J, Meng G. 2014. HCV genomic RNA activates the NLRP3 inflammasome in human myeloid cells. PLoS One 9:e84953. doi: 10.1371/journal.pone.0084953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Farag NS, Breitinger U, El-Azizi M, Breitinger HG. 2017. The p7 viroporin of the hepatitis C virus contributes to liver inflammation by stimulating production of interleukin-1beta. Biochim Biophys Acta 1863:712–720. doi: 10.1016/j.bbadis.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 260.Triantafilou K, Kar S, Vakakis E, Kotecha S, Triantafilou M. 2013. Human respiratory syncytial virus viroporin SH: a viral recognition pathway used by the host to signal inflammasome activation. Thorax 68:66–75. doi: 10.1136/thoraxjnl-2012-202182. [DOI] [PubMed] [Google Scholar]
- 261.Ito M, Yanagi Y, Ichinohe T. 2012. Encephalomyocarditis virus viroporin 2B activates NLRP3 inflammasome. PLoS Pathog 8:e1002857. doi: 10.1371/journal.ppat.1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Triantafilou K, Kar S, van Kuppeveld FJ, Triantafilou M. 2013. Rhinovirus-induced calcium flux triggers NLRP3 and NLRC5 activation in bronchial cells. Am J Respir Cell Mol Biol 49:923–934. doi: 10.1165/rcmb.2013-0032OC. [DOI] [PubMed] [Google Scholar]
- 263.Zhu S, Ding S, Wang P, Wei Z, Pan W, Palm NW, Yang Y, Yu H, Li HB, Wang G, Lei X, de Zoete MR, Zhao J, Zheng Y, Chen H, Zhao Y, Jurado KA, Feng N, Shan L, Kluger Y, Lu J, Abraham C, Fikrig E, Greenberg HB, Flavell RA. 2017. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature 546:667–670. doi: 10.1038/nature22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ngo C, Man SM. 2017. NLRP9b: a novel RNA-sensing inflammasome complex. Cell Res 27:1302–1303. doi: 10.1038/cr.2017.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Karki R, Man SM, Malireddi RK, Gurung P, Vogel P, Lamkanfi M, Kanneganti TD. 2015. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe 17:357–368. doi: 10.1016/j.chom.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lamkanfi M, Malireddi RK, Kanneganti TD. 2009. Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem 284:20574–20581. doi: 10.1074/jbc.M109.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kankkunen P, Teirila L, Rintahaka J, Alenius H, Wolff H, Matikainen S. 2010. (1,3)-Beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J Immunol 184:6335–6342. doi: 10.4049/jimmunol.0903019. [DOI] [PubMed] [Google Scholar]
- 268.Kumar H, Kumagai Y, Tsuchida T, Koenig PA, Satoh T, Guo Z, Jang MH, Saitoh T, Akira S, Kawai T. 2009. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta-glucan. J Immunol 183:8061–8067. doi: 10.4049/jimmunol.0902477. [DOI] [PubMed] [Google Scholar]
- 269.Huang Y, Hua M, Cui X. 2018. Fungal β-glucan activates the NLRP3 inflammasome in human bronchial epithelial cells through ROS production. Inflammation 41:164–173. doi: 10.1007/s10753-017-0674-6. [DOI] [PubMed] [Google Scholar]
- 270.Botterel F, Gross K, Ibrahim-Granet O, Khoufache K, Escabasse V, Coste A, Cordonnier C, Escudier E, Bretagne S. 2008. Phagocytosis of Aspergillus fumigatus conidia by primary nasal epithelial cells in vitro. BMC Microbiol 8:97. doi: 10.1186/1471-2180-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Said-Sadier N, Padilla E, Langsley G, Ojcius DM. 2010. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One 5:e10008. doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Iannitti RG, Napolioni V, Oikonomou V, De Luca A, Galosi C, Pariano M, Massi-Benedetti C, Borghi M, Puccetti M, Lucidi V, Colombo C, Fiscarelli E, Lass-Florl C, Majo F, Cariani L, Russo M, Porcaro L, Ricciotti G, Ellemunter H, Ratclif L, De Benedictis FM, Talesa VN, Dinarello CA, van de Veerdonk FL, Romani L. 2016. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat Commun 7:10791. doi: 10.1038/ncomms10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J, Donado CA, Shao F, Wu H, Springstead JR, Kagan JC. 2016. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 352:1232–1236. doi: 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Chu LH, Indramohan M, Ratsimandresy RA, Gangopadhyay A, Morris EP, Monack DM, Dorfleutner A, Stehlik C. 2018. The oxidized phospholipid oxPAPC protects from septic shock by targeting the non-canonical inflammasome in macrophages. Nat Commun 9:996. doi: 10.1038/s41467-018-03409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. 2009. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Toth A, Zajta E, Csonka K, Vagvolgyi C, Netea MG, Gacser A. 2017. Specific pathways mediating inflammasome activation by Candida parapsilosis. Sci Rep 7:43129. doi: 10.1038/srep43129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wellington M, Koselny K, Sutterwala FS, Krysan DJ. 2014. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot Cell 13:329–340. doi: 10.1128/EC.00336-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Vylkova S, Lorenz MC. 2017. Phagosomal neutralization by the fungal pathogen Candida albicans induces macrophage pyroptosis. Infect Immun 85:e00832-. doi: 10.1128/IAI.00832-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. 2009. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol 183:3578–3581. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Uwamahoro N, Verma-Gaur J, Shen HH, Qu Y, Lewis R, Lu J, Bambery K, Masters SL, Vince JE, Naderer T, Traven A. 2014. The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. mBio 5:e00003-. doi: 10.1128/mBio.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. 2009. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 282.Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, Fitzgerald KA, Hise AG. 2011. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog 7:e1002379. doi: 10.1371/journal.ppat.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Jabir MS, Hopkins L, Ritchie ND, Ullah I, Bayes HK, Li D, Tourlomousis P, Lupton A, Puleston D, Simon AK, Bryant C, Evans TJ. 2015. Mitochondrial damage contributes to Pseudomonas aeruginosa activation of the inflammasome and is downregulated by autophagy. Autophagy 11:166–182. doi: 10.4161/15548627.2014.981915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Guo C, Chen M, Fa Z, Lu A, Fang W, Sun B, Chen C, Liao W, Meng G. 2014. Acapsular Cryptococcus neoformans activates the NLRP3 inflammasome. Microbes Infect 16:845–854. doi: 10.1016/j.micinf.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 285.Chen M, Xing Y, Lu A, Fang W, Sun B, Chen C, Liao W, Meng G. 2015. Internalized Cryptococcus neoformans activates the canonical caspase-1 and the noncanonical caspase-8 inflammasomes. J Immunol 195:4962–4972. doi: 10.4049/jimmunol.1500865. [DOI] [PubMed] [Google Scholar]
- 286.Mao L, Zhang L, Li H, Chen W, Wang H, Wu S, Guo C, Lu A, Yang G, An L, Abliz P, Meng G. 2014. Pathogenic fungus Microsporum canis activates the NLRP3 inflammasome. Infect Immun 82:882–892. doi: 10.1128/IAI.01097-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Li H, Wu S, Mao L, Lei G, Zhang L, Lu A, An L, Yang G, Abliz P, Meng G. 2013. Human pathogenic fungus Trichophyton schoenleinii activates the NLRP3 inflammasome. Protein Cell 4:529–538. doi: 10.1007/s13238-013-2127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Tavares AH, Magalhaes KG, Almeida RD, Correa R, Burgel PH, Bocca AL. 2013. NLRP3 inflammasome activation by Paracoccidioides brasiliensis. PLoS Negl Trop Dis 7:e2595. doi: 10.1371/journal.pntd.0002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Chang TH, Huang JH, Lin HC, Chen WY, Lee YH, Hsu LC, Netea MG, Ting JP, Wu-Hsieh BA. 2017. Dectin-2 is a primary receptor for NLRP3 inflammasome activation in dendritic cell response to Histoplasma capsulatum. PLoS Pathog 13:e1006485. doi: 10.1371/journal.ppat.1006485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Fletcher SM, Stark D, Harkness J, Ellis J. 2012. Enteric protozoa in the developed world: a public health perspective. Clin Microbiol Rev 25:420–449. doi: 10.1128/CMR.05038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zamboni DS, Lima-Junior DS. 2015. Inflammasomes in host response to protozoan parasites. Immunol Rev 265:156–171. doi: 10.1111/imr.12291. [DOI] [PubMed] [Google Scholar]
- 292.Molina-Cruz A, Zilversmit MM, Neafsey DE, Hartl DL, Barillas-Mury C. 2016. Mosquito vectors and the globalization of Plasmodium falciparum malaria. Annu Rev Genet 50:447–465. doi: 10.1146/annurev-genet-120215-035211. [DOI] [PubMed] [Google Scholar]
- 293.Santos ML, Reis EC, Bricher PN, Sousa TN, Brito CF, Lacerda MV, Fontes CJ, Carvalho LH, Pontillo A. 2016. Contribution of inflammasome genetics in Plasmodium vivax malaria. Infect Genet Evol 40:162–166. doi: 10.1016/j.meegid.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 294.Hirako IC, Gallego-Marin C, Ataide MA, Andrade WA, Gravina H, Rocha BC, de Oliveira RB, Pereira DB, Vinetz J, Diamond B, Ram S, Golenbock DT, Gazzinelli RT. 2015. DNA-containing immunocomplexes promote inflammasome assembly and release of pyrogenic cytokines by CD14+ CD16+ CD64high CD32low inflammatory monocytes from malaria patients. mBio 6:e01605-. doi: 10.1128/mBio.01605-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Prakash D, Fesel C, Jain R, Cazenave PA, Mishra GC, Pied S. 2006. Clusters of cytokines determine malaria severity in Plasmodium falciparum-infected patients from endemic areas of central India. J Infect Dis 194:198–207. doi: 10.1086/504720. [DOI] [PubMed] [Google Scholar]
- 296.Kwiatkowski D, Nowak M. 1991. Periodic and chaotic host-parasite interactions in human malaria. Proc Natl Acad Sci U S A 88:5111–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, Suva ML, Stehle JC, Kopf M, Stamenkovic I, Corradin G, Tschopp J. 2009. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One 4:e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Griffith JW, Sun T, McIntosh MT, Bucala R. 2009. Pure hemozoin is inflammatory in vivo and activates the NALP3 inflammasome via release of uric acid. J Immunol 183:5208–5220. doi: 10.4049/jimmunol.0713552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Shio MT, Eisenbarth SC, Savaria M, Vinet AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A, Flavell RA, Olivier M. 2009. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog 5:e1000559. doi: 10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Reimer T, Shaw MH, Franchi L, Coban C, Ishii KJ, Akira S, Horii T, Rodriguez A, Nunez G. 2010. Experimental cerebral malaria progresses independently of the Nlrp3 inflammasome. Eur J Immunol 40:764–769. doi: 10.1002/eji.200939996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Dutra FF, Alves LS, Rodrigues D, Fernandez PL, de Oliveira RB, Golenbock DT, Zamboni DS, Bozza MT. 2014. Hemolysis-induced lethality involves inflammasome activation by heme. Proc Natl Acad Sci U S A 111:E4110–E4118. doi: 10.1073/pnas.1405023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, Visintin A, Halmen KA, Lamphier M, Olivier M, Bartholomeu DC, Gazzinelli RT, Golenbock DT. 2007. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A 104:1919–1924. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Kalantari P, DeOliveira RB, Chan J, Corbett Y, Rathinam V, Stutz A, Latz E, Gazzinelli RT, Golenbock DT, Fitzgerald KA. 2014. Dual engagement of the NLRP3 and AIM2 inflammasomes by Plasmodium-derived hemozoin and DNA during malaria. Cell Rep 6:196–210. doi: 10.1016/j.celrep.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Ataide MA, Andrade WA, Zamboni DS, Wang D, Souza MC, Franklin BS, Elian S, Martins FS, Pereira D, Reed G, Fitzgerald KA, Golenbock DT, Gazzinelli RT. 2014. Malaria-induced NLRP12/NLRP3-dependent caspase-1 activation mediates inflammation and hypersensitivity to bacterial superinfection. PLoS Pathog 10:e1003885. doi: 10.1371/journal.ppat.1003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M. 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kedzierski L. 2010. Leishmaniasis vaccine: where are we today? J Glob Infect Dis 2:177–185. doi: 10.4103/0974-777X.62881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kaye P, Scott P. 2011. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol 9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 308.Novais FO, Carvalho LP, Passos S, Roos DS, Carvalho EM, Scott P, Beiting DP. 2015. Genomic profiling of human Leishmania braziliensis lesions identifies transcriptional modules associated with cutaneous immunopathology. J Invest Dermatol 135:94–101. doi: 10.1038/jid.2014.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Fernandez-Figueroa EA, Rangel-Escareno C, Espinosa-Mateos V, Carrillo-Sanchez K, Salaiza-Suazo N, Carrada-Figueroa G, March-Mifsut S, Becker I. 2012. Disease severity in patients infected with Leishmania mexicana relates to IL-1beta. PLoS Negl Trop Dis 6:e1533. doi: 10.1371/journal.pntd.0001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Lima-Junior DS, Costa DL, Carregaro V, Cunha LD, Silva AL, Mineo TW, Gutierrez FR, Bellio M, Bortoluci KR, Flavell RA, Bozza MT, Silva JS, Zamboni DS. 2013. Inflammasome-derived IL-1beta production induces nitric oxide-mediated resistance to Leishmania. Nat Med 19:909–915. doi: 10.1038/nm.3221. [DOI] [PubMed] [Google Scholar]
- 311.Gurung P, Karki R, Vogel P, Watanabe M, Bix M, Lamkanfi M, Kanneganti TD. 2015. An NLRP3 inflammasome-triggered Th2-biased adaptive immune response promotes leishmaniasis. J Clin Invest 125:1329–1338. doi: 10.1172/JCI79526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Shio MT, Christian JG, Jung JY, Chang KP, Olivier M. 2015. PKC/ROS-mediated NLRP3 inflammasome activation is attenuated by Leishmania zinc-metalloprotease during infection. PLoS Negl Trop Dis 9:e0003868. doi: 10.1371/journal.pntd.0003868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Charmoy M, Hurrell BP, Romano A, Lee SH, Ribeiro-Gomes F, Riteau N, Mayer-Barber K, Tacchini-Cottier F, Sacks DL. 2016. The Nlrp3 inflammasome, IL-1beta, and neutrophil recruitment are required for susceptibility to a nonhealing strain of Leishmania major in C57BL/6 mice. Eur J Immunol 46:897–911. doi: 10.1002/eji.201546015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Wei XQ, Niedbala W, Xu D, Luo ZX, Pollock KG, Brewer JM. 2004. Host genetic background determines whether IL-18 deficiency results in increased susceptibility or resistance to murine Leishmania major infection. Immunol Lett 94:35–37. doi: 10.1016/j.imlet.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 315.Xu D, Trajkovic V, Hunter D, Leung BP, Schulz K, Gracie JA, McInnes IB, Liew FY. 2000. IL-18 induces the differentiation of Th1 or Th2 cells depending upon cytokine milieu and genetic background. Eur J Immunol 30:3147–3156. doi:. [DOI] [PubMed] [Google Scholar]
- 316.Dey R, Joshi AB, Oliveira F, Pereira L, Guimaraes-Costa AB, Serafim TD, de Castro W, Coutinho-Abreu IV, Bhattacharya P, Townsend S, Aslan H, Perkins A, Karmakar S, Ismail N, Karetnick M, Meneses C, Duncan R, Nakhasi HL, Valenzuela JG, Kamhawi S. 2018. Gut microbes egested during bites of infected sand flies augment severity of leishmaniasis via inflammasome-derived IL-1beta. Cell Host Microbe 23:134.e136–143.e136. doi: 10.1016/j.chom.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Robert-Gangneux F, Darde ML. 2012. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 25:264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 319.Chu JQ, Shi G, Fan YM, Choi IW, Cha GH, Zhou Y, Lee YH, Quan JH. 2016. Production of IL-1beta and inflammasome with up-regulated expressions of NOD-like receptor related genes in Toxoplasma gondii-infected THP-1 macrophages. Korean J Parasitol 54:711–717. doi: 10.3347/kjp.2016.54.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Witola WH, Mui E, Hargrave A, Liu S, Hypolite M, Montpetit A, Cavailles P, Bisanz C, Cesbron-Delauw MF, Fournie GJ, McLeod R. 2011. NALP1 influences susceptibility to human congenital toxoplasmosis, proinflammatory cytokine response, and fate of Toxoplasma gondii-infected monocytic cells. Infect Immun 79:756–766. doi: 10.1128/IAI.00898-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Zhao YO, Khaminets A, Hunn JP, Howard JC. 2009. Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNgamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog 5:e1000288. doi: 10.1371/journal.ppat.1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Gov L, Karimzadeh A, Ueno N, Lodoen MB. 2013. Human innate immunity to Toxoplasma gondii is mediated by host caspase-1 and ASC and parasite GRA15. mBio 4:e00255-. doi: 10.1128/mBio.00255-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Gorfu G, Cirelli KM, Melo MB, Mayer-Barber K, Crown D, Koller BH, Masters S, Sher A, Leppla SH, Moayeri M, Saeij JP, Grigg ME. 2014. Dual role for inflammasome sensors NLRP1 and NLRP3 in murine resistance to Toxoplasma gondii. mBio 5:e01117-. doi: 10.1128/mBio.01117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ewald SE, Chavarria-Smith J, Boothroyd JC. 2014. NLRP1 is an inflammasome sensor for Toxoplasma gondii. Infect Immun 82:460–468. doi: 10.1128/IAI.01170-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Cirelli KM, Gorfu G, Hassan MA, Printz M, Crown D, Leppla SH, Grigg ME, Saeij JP, Moayeri M. 2014. Inflammasome sensor NLRP1 controls rat macrophage susceptibility to Toxoplasma gondii. PLoS Pathog 10:e1003927. doi: 10.1371/journal.ppat.1003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KD, Saeij JP. 2011. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med 208:195–212. doi: 10.1084/jem.20100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Degrandi D, Kravets E, Konermann C, Beuter-Gunia C, Klumpers V, Lahme S, Wischmann E, Mausberg AK, Beer-Hammer S, Pfeffer K. 2013. Murine guanylate binding protein 2 (mGBP2) controls Toxoplasma gondii replication. Proc Natl Acad Sci U S A 110:294–299. doi: 10.1073/pnas.1205635110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Kravets E, Degrandi D, Ma Q, Peulen TO, Klumpers V, Felekyan S, Kuhnemuth R, Weidtkamp-Peters S, Seidel CA, Pfeffer K. 2016. Guanylate binding proteins directly attack Toxoplasma gondii via supramolecular complexes. Elife 5:e11479. doi: 10.7554/eLife.11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Selleck EM, Fentress SJ, Beatty WL, Degrandi D, Pfeffer K, Virgin HW IV, Macmicking JD, Sibley LD. 2013. Guanylate-binding protein 1 (Gbp1) contributes to cell-autonomous immunity against Toxoplasma gondii. PLoS Pathog 9:e1003320. doi: 10.1371/journal.ppat.1003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Yamamoto M, Okuyama M, Ma JS, Kimura T, Kamiyama N, Saiga H, Ohshima J, Sasai M, Kayama H, Okamoto T, Huang DC, Soldati-Favre D, Horie K, Takeda J, Takeda K. 2012. A cluster of interferon-gamma-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity 37:302–313. doi: 10.1016/j.immuni.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 331.Qin A, Lai DH, Liu Q, Huang W, Wu YP, Chen X, Yan S, Xia H, Hide G, Lun ZR, Ayala FJ, Xiang AP. 2017. Guanylate-binding protein 1 (GBP1) contributes to the immunity of human mesenchymal stromal cells against Toxoplasma gondii. Proc Natl Acad Sci U S A 114:1365–1370. doi: 10.1073/pnas.1619665114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Johnston AC, Piro A, Clough B, Siew M, Virreira Winter S, Coers J, Frickel EM. 2016. Human GBP1 does not localize to pathogen vacuoles but restricts Toxoplasma gondii. Cell Microbiol 18:1056–1064. doi: 10.1111/cmi.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. 2008. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis 2:e300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Lescure FX, Le Loup G, Freilij H, Develoux M, Paris L, Brutus L, Pialoux G. 2010. Chagas disease: changes in knowledge and management. Lancet Infect Dis 10:556–570. doi: 10.1016/S1473-3099(10)70098-0. [DOI] [PubMed] [Google Scholar]
- 335.Tanowitz HB, Machado FS, Jelicks LA, Shirani J, de Carvalho AC, Spray DC, Factor SM, Kirchhoff LV, Weiss LM. 2009. Perspectives on Trypanosoma cruzi-induced heart disease (Chagas disease). Prog Cardiovasc Dis 51:524–539. doi: 10.1016/j.pcad.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Goncalves VM, Matteucci KC, Buzzo CL, Miollo BH, Ferrante D, Torrecilhas AC, Rodrigues MM, Alvarez JM, Bortoluci KR. 2013. NLRP3 controls Trypanosoma cruzi infection through a caspase-1-dependent IL-1R-independent NO production. PLoS Negl Trop Dis 7:e2469. doi: 10.1371/journal.pntd.0002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Silva GK, Costa RS, Silveira TN, Caetano BC, Horta CV, Gutierrez FR, Guedes PM, Andrade WA, De Niz M, Gazzinelli RT, Zamboni DS, Silva JS. 2013. Apoptosis-associated speck-like protein containing a caspase recruitment domain inflammasomes mediate IL-1beta response and host resistance to Trypanosoma cruzi infection. J Immunol 191:3373–3383. doi: 10.4049/jimmunol.1203293. [DOI] [PubMed] [Google Scholar]
- 338.Dey N, Sinha M, Gupta S, Gonzalez MN, Fang R, Endsley JJ, Luxon BA, Garg NJ. 2014. Caspase-1/ASC inflammasome-mediated activation of IL-1beta-ROS-NF-kappaB pathway for control of Trypanosoma cruzi replication and survival is dispensable in NLRP3−/− macrophages. PLoS One 9:e111539. doi: 10.1371/journal.pone.0111539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Stanley SL., Jr 2003. Amoebiasis. Lancet 361:1025–1034. doi: 10.1016/S0140-6736(03)12830-9. [DOI] [PubMed] [Google Scholar]
- 340.Mortimer L, Moreau F, Cornick S, Chadee K. 2015. The NLRP3 inflammasome is a pathogen sensor for invasive Entamoeba histolytica via activation of alpha5beta1 integrin at the macrophage-amebae intercellular junction. PLoS Pathog 11:e1004887. doi: 10.1371/journal.ppat.1004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Marie C, Verkerke HP, Theodorescu D, Petri WA. 2015. A whole-genome RNAi screen uncovers a novel role for human potassium channels in cell killing by the parasite Entamoeba histolytica. Sci Rep 5:13613. doi: 10.1038/srep13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Que X, Kim SH, Sajid M, Eckmann L, Dinarello CA, McKerrow JH, Reed SL. 2003. A surface amebic cysteine proteinase inactivates interleukin-18. Infect Immun 71:1274–1280. doi: 10.1128/IAI.71.3.1274-1280.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Zhang Z, Wang L, Seydel KB, Li E, Ankri S, Mirelman D, Stanley SL Jr. 2000. Entamoeba histolytica cysteine proteinases with interleukin-1 beta converting enzyme (ICE) activity cause intestinal inflammation and tissue damage in amoebiasis. Mol Microbiol 37:542–548. [DOI] [PubMed] [Google Scholar]
- 344.Velazquez JR, Garibay-Martinez L, Martinez-Tejada P, Leal YA. 2012. An amebic anti-inflammatory peptide down-regulates ex vivo IL-1beta expression in patients with rheumatoid arthritis. Rheumatol Clin 8:315–320. doi: 10.1016/j.reuma.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 345.Melendez-Lopez SG, Herdman S, Hirata K, Choi MH, Choe Y, Craik C, Caffrey CR, Hansell E, Chavez-Munguia B, Chen YT, Roush WR, McKerrow J, Eckmann L, Guo J, Stanley SL Jr, Reed SL. 2007. Use of recombinant Entamoeba histolytica cysteine proteinase 1 to identify a potent inhibitor of amebic invasion in a human colonic model. Eukaryot Cell 6:1130–1136. doi: 10.1128/EC.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.He C, Nora GP, Schneider EL, Kerr ID, Hansell E, Hirata K, Gonzalez D, Sajid M, Boyd SE, Hruz P, Cobo ER, Le C, Liu WT, Eckmann L, Dorrestein PC, Houpt ER, Brinen LS, Craik CS, Roush WR, McKerrow J, Reed SL. 2010. A novel Entamoeba histolytica cysteine proteinase, EhCP4, is key for invasive amebiasis and a therapeutic target. J Biol Chem 285:18516–18527. doi: 10.1074/jbc.M109.086181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Kang MJ, Jo SG, Kim DJ, Park JH. 2017. NLRP3 inflammasome mediates interleukin-1beta production in immune cells in response to Acinetobacter baumannii and contributes to pulmonary inflammation in mice. Immunology 150:495–505. doi: 10.1111/imm.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.McCoy AJ, Koizumi Y, Toma C, Higa N, Dixit V, Taniguchi S, Tschopp J, Suzuki T. 2010. Cytotoxins of the human pathogen Aeromonas hydrophila trigger, via the NLRP3 inflammasome, caspase-1 activation in macrophages. Eur J Immunol 40:2797–2803. doi: 10.1002/eji.201040490. [DOI] [PubMed] [Google Scholar]
- 349.McCoy AJ, Koizumi Y, Higa N, Suzuki T. 2010. Differential regulation of caspase-1 activation via NLRP3/NLRC4 inflammasomes mediated by aerolysin and type III secretion system during Aeromonas veronii infection. J Immunol 185:7077–7084. doi: 10.4049/jimmunol.1002165. [DOI] [PubMed] [Google Scholar]
- 350.Shenker BJ, Ojcius DM, Walker LP, Zekavat A, Scuron MD, Boesze-Battaglia K. 2015. Aggregatibacter actinomycetemcomitans cytolethal distending toxin activates the NLRP3 inflammasome in human macrophages, leading to the release of proinflammatory cytokines. Infect Immun 83:1487–1496. doi: 10.1128/IAI.03132-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Dunne A, Ross PJ, Pospisilova E, Masin J, Meaney A, Sutton CE, Iwakura Y, Tschopp J, Sebo P, Mills KH. 2010. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J Immunol 185:1711–1719. doi: 10.4049/jimmunol.1000105. [DOI] [PubMed] [Google Scholar]
- 352.Gomes MT, Campos PC, Oliveira FS, Corsetti PP, Bortoluci KR, Cunha LD, Zamboni DS, Oliveira SC. 2013. Critical role of ASC inflammasomes and bacterial type IV secretion system in caspase-1 activation and host innate resistance to Brucella abortus infection. J Immunol 190:3629–3638. doi: 10.4049/jimmunol.1202817. [DOI] [PubMed] [Google Scholar]
- 353.Miraglia MC, Costa Franco MM, Rodriguez AM, Bellozi PM, Ferrari CC, Farias MI, Dennis VA, Barrionuevo P, de Oliveira AC, Pitossi F, Kim KS, Delpino MV, Oliveira SC, Giambartolomei GH. 2016. Glial cell-elicited activation of brain microvasculature in response to Brucella abortus infection requires ASC inflammasome-dependent IL-1beta production. J Immunol 196:3794–3805. doi: 10.4049/jimmunol.1500908. [DOI] [PubMed] [Google Scholar]
- 354.Costa Franco MM, Marim F, Guimaraes ES, Assis NRG, Cerqueira DM, Alves-Silva J, Harms J, Splitter G, Smith J, Kanneganti TD, de Queiroz N, Gutman D, Barber GN, Oliveira SC. 2018. Brucella abortus triggers a cGAS-independent STING pathway to induce host protection that involves guanylate-binding proteins and inflammasome activation. J Immunol 200:607–622. doi: 10.4049/jimmunol.1700725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Rosales-Reyes R, Aubert DF, Tolman JS, Amer AO, Valvano MA. 2012. Burkholderia cenocepacia type VI secretion system mediates escape of type II secreted proteins into the cytoplasm of infected macrophages. PLoS One 7:e41726. doi: 10.1371/journal.pone.0041726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Ceballos-Olvera I, Sahoo M, Miller MA, Del Barrio L, Re F. 2011. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1beta is deleterious. PLoS Pathog 7:e1002452. doi: 10.1371/journal.ppat.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. 2010. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J Immunol 184:5743–5754. doi: 10.4049/jimmunol.0903937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Shimada K, Crother TR, Karlin J, Chen S, Chiba N, Ramanujan VK, Vergnes L, Ojcius DM, Arditi M. 2011. Caspase-1 dependent IL-1beta secretion is critical for host defense in a mouse model of Chlamydia pneumoniae lung infection. PLoS One 6:e21477. doi: 10.1371/journal.pone.0021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Webster SJ, Brode S, Ellis L, Fitzmaurice TJ, Elder MJ, Gekara NO, Tourlomousis P, Bryant C, Clare S, Chee R, Gaston HJS, Goodall JC. 2017. Detection of a microbial metabolite by STING regulates inflammasome activation in response to Chlamydia trachomatis infection. PLoS Pathog 13:e1006383. doi: 10.1371/journal.ppat.1006383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Liu Z, Zaki MH, Vogel P, Gurung P, Finlay BB, Deng W, Lamkanfi M, Kanneganti TD. 2012. Role of inflammasomes in host defense against Citrobacter rodentium infection. J Biol Chem 287:16955–16964. doi: 10.1074/jbc.M112.358705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Nordlander S, Pott J, Maloy KJ. 2014. NLRC4 expression in intestinal epithelial cells mediates protection against an enteric pathogen. Mucosal Immunol 7:775–785. doi: 10.1038/mi.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Song-Zhao GX, Srinivasan N, Pott J, Baban D, Frankel G, Maloy KJ. 2014. Nlrp3 activation in the intestinal epithelium protects against a mucosal pathogen. Mucosal Immunol 7:763–774. doi: 10.1038/mi.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Anand PK, Malireddi RK, Lukens JR, Vogel P, Bertin J, Lamkanfi M, Kanneganti TD. 2012. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488:389–393. doi: 10.1038/nature11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, Weiss DS, Henry T. 2012. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ 19:1709–1721. doi: 10.1038/cdd.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Duffy EB, Periasamy S, Hunt D, Drake JR, Harton JA. 2016. FcgammaR mediates TLR2- and Syk-dependent NLRP3 inflammasome activation by inactivated Francisella tularensis LVS immune complexes. J Leukoc Biol 100:1335–1347. doi: 10.1189/jlb.2A1215-555RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Atianand MK, Duffy EB, Shah A, Kar S, Malik M, Harton JA. 2011. Francisella tularensis reveals a disparity between human and mouse NLRP3 inflammasome activation. J Biol Chem 286:39033–39042. doi: 10.1074/jbc.M111.244079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Periasamy S, Le HT, Duffy EB, Chin H, Harton JA. 2016. Inflammasome-independent NLRP3 restriction of a protective early neutrophil response to pulmonary tularemia. PLoS Pathog 12:e1006059. doi: 10.1371/journal.ppat.1006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Hung SC, Huang PR, Almeida-da-Silva CLC, Atanasova KR, Yilmaz O, Ojcius DM. 2017. NLRX1 modulates differentially NLRP3 inflammasome activation and NF-κB signaling during Fusobacterium nucleatum infection. Microbes Infect 2017:S1286-4579(17)30158-2. doi: 10.1016/j.micinf.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Koch KN, Hartung ML, Urban S, Kyburz A, Bahlmann AS, Lind J, Backert S, Taube C, Muller A. 2015. Helicobacter urease-induced activation of the TLR2/NLRP3/IL-18 axis protects against asthma. J Clin Invest 125:3297–3302. doi: 10.1172/JCI79337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Perez-Figueroa E, Torres J, Sanchez-Zauco N, Contreras-Ramos A, Alvarez-Arellano L, Maldonado-Bernal C. 2016. Activation of NLRP3 inflammasome in human neutrophils by Helicobacter pylori infection. Innate Immun 22:103–112. doi: 10.1177/1753425915619475. [DOI] [PubMed] [Google Scholar]
- 371.Li X, Liu S, Luo J, Liu A, Tang S, Liu S, Yu M, Zhang Y. 2015. Helicobacter pylori induces IL-1beta and IL-18 production in human monocytic cell line through activation of NLRP3 inflammasome via ROS signaling pathway. Pathog Dis 73:ftu024. doi: 10.1093/femspd/ftu024. [DOI] [PubMed] [Google Scholar]
- 372.Semper RP, Mejias-Luque R, Gross C, Anderl F, Muller A, Vieth M, Busch DH, Prazeres da Costa C, Ruland J, Gross O, Gerhard M. 2014. Helicobacter pylori-induced IL-1beta secretion in innate immune cells is regulated by the NLRP3 inflammasome and requires the cag pathogenicity island. J Immunol 193:3566–3576. doi: 10.4049/jimmunol.1400362. [DOI] [PubMed] [Google Scholar]
- 373.Kim DJ, Park JH, Franchi L, Backert S, Nunez G. 2013. The Cag pathogenicity island and interaction between TLR2/NOD2 and NLRP3 regulate IL-1beta production in Helicobacter pylori infected dendritic cells. Eur J Immunol 43:2650–2658. doi: 10.1002/eji.201243281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Cai S, Batra S, Wakamatsu N, Pacher P, Jeyaseelan S. 2012. NLRC4 inflammasome-mediated production of IL-1beta modulates mucosal immunity in the lung against Gram-negative bacterial infection. J Immunol 188:5623–5635. doi: 10.4049/jimmunol.1200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, Duncan JA, Ting JP. 2009. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol 183:2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, Swanson MS. 2006. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med 203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. 2006. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog 2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Duncan JA, Gao X, Huang MT, O'Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, Ting JP. 2009. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol 182:6460–6469. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Park E, Na HS, Song YR, Shin SY, Kim YM, Chung J. 2014. Activation of NLRP3 and AIM2 inflammasomes by Porphyromonas gingivalis infection. Infect Immun 82:112–123. doi: 10.1128/IAI.00862-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Huang MT, Taxman DJ, Holley-Guthrie EA, Moore CB, Willingham SB, Madden V, Parsons RK, Featherstone GL, Arnold RR, O'Connor BP, Ting JP. 2009. Critical role of apoptotic speck protein containing a caspase recruitment domain (ASC) and NLRP3 in causing necrosis and ASC speck formation induced by Porphyromonas gingivalis in human cells. J Immunol 182:2395–2404. doi: 10.4049/jimmunol.0800909. [DOI] [PubMed] [Google Scholar]
- 381.Cecil JD, O'Brien-Simpson NM, Lenzo JC, Holden JA, Singleton W, Perez-Gonzalez A, Mansell A, Reynolds EC. 2017. Outer membrane vesicles prime and activate macrophage inflammasomes and cytokine secretion in vitro and in vivo. Front Immunol 8:1017. doi: 10.3389/fimmu.2017.01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Nunez G. 2007. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol 37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 383.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. 2008. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci U S A 105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. 2007. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med 204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Basso P, Wallet P, Elsen S, Soleilhac E, Henry T, Faudry E, Attree I. 2017. Multiple Pseudomonas species secrete exolysin-like toxins and provoke caspase-1-dependent macrophage death. Environ Microbiol 19:4045–4064. doi: 10.1111/1462-2920.13841. [DOI] [PubMed] [Google Scholar]
- 386.Willingham SB, Bergstralh DT, O'Connor W, Morrison AC, Taxman DJ, Duncan JA, Barnoy S, Venkatesan MM, Flavell RA, Deshmukh M, Hoffman HM, Ting JP. 2007. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2:147–159. doi: 10.1016/j.chom.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Lee MS, Kwon H, Lee EY, Kim DJ, Park JH, Tesh VL, Oh TK, Kim MH. 2015. Shiga toxins activate the NLRP3 inflammasome pathway to promote both production of the proinflammatory cytokine interleukin-1beta and apoptotic cell death. Infect Immun 84:172–186. doi: 10.1128/IAI.01095-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Neiman-Zenevich J, Stuart S, Abdel-Nour M, Girardin SE, Mogridge J. 2017. Listeria monocytogenes and Shigella flexneri activate the NLRP1B inflammasome. Infect Immun 85:e00338-. doi: 10.1128/IAI.00338-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Jun HK, Lee SH, Lee HR, Choi BK. 2012. Integrin alpha5beta1 activates the NLRP3 inflammasome by direct interaction with a bacterial surface protein. Immunity 36:755–768. doi: 10.1016/j.immuni.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 390.Babolin C, Amedei A, Ozolins D, Zilevica A, D'Elios MM, de Bernard M. 2011. TpF1 from Treponema pallidum activates inflammasome and promotes the development of regulatory T cells. J Immunol 187:1377–1384. doi: 10.4049/jimmunol.1100615. [DOI] [PubMed] [Google Scholar]
- 391.Zwack EE, Snyder AG, Wynosky-Dolfi MA, Ruthel G, Philip NH, Marketon MM, Francis MS, Bliska JB, Brodsky IE. 2015. Inflammasome activation in response to the Yersinia type III secretion system requires hyperinjection of translocon proteins YopB and YopD. mBio 6:e02095-. doi: 10.1128/mBio.02095-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Zwack EE, Feeley EM, Burton AR, Hu B, Yamamoto M, Kanneganti TD, Bliska JB, Coers J, Brodsky IE. 2017. Guanylate binding proteins regulate inflammasome activation in response to hyperinjected Yersinia translocon components. Infect Immun 85:e00778-. doi: 10.1128/IAI.00778-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Kwuan L, Adams W, Auerbuch V. 2013. Impact of host membrane pore formation by the Yersinia pseudotuberculosis type III secretion system on the macrophage innate immune response. Infect Immun 81:905–914. doi: 10.1128/IAI.01014-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, Aune MH, Conlon JE, Burbage JJ, Proulx MK, Liu Q, Reed G, Mecsas JC, Iwakura Y, Bertin J, Goguen JD, Fitzgerald KA, Lien E. 2012. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity 37:96–107. doi: 10.1016/j.immuni.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Chung LK, Park YH, Zheng Y, Brodsky IE, Hearing P, Kastner DL, Chae JJ, Bliska JB. 2016. The Yersinia virulence factor YopM hijacks host kinases to inhibit type III effector-triggered activation of the Pyrin inflammasome. Cell Host Microbe 20:296–306. doi: 10.1016/j.chom.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Ratner D, Orning MP, Proulx MK, Wang D, Gavrilin MA, Wewers MD, Alnemri ES, Johnson PF, Lee B, Mecsas J, Kayagaki N, Goguen JD, Lien E. 2016. The Yersinia pestis effector YopM inhibits Pyrin inflammasome activation. PLoS Pathog 12:e1006035. doi: 10.1371/journal.ppat.1006035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Ali SR, Timmer AM, Bilgrami S, Park EJ, Eckmann L, Nizet V, Karin M. 2011. Anthrax toxin induces macrophage death by p38 MAPK inhibition but leads to inflammasome activation via ATP leakage. Immunity 35:34–44. doi: 10.1016/j.immuni.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Rasimus-Sahari S, Teplova VV, Andersson MA, Mikkola R, Kankkunen P, Matikainen S, Gahmberg CG, Andersson LC, Salkinoja-Salonen M. 2015. The peptide toxin amylosin of Bacillus amyloliquefaciens from moisture-damaged buildings is immunotoxic, induces potassium efflux from mammalian cells, and has antimicrobial activity. Appl Environ Microbiol 81:2939–2949. doi: 10.1128/AEM.03430-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Ng J, Hirota SA, Gross O, Li Y, Ulke-Lemee A, Potentier MS, Schenck LP, Vilaysane A, Seamone ME, Feng H, Armstrong GD, Tschopp J, Macdonald JA, Muruve DA, Beck PL. 2010. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology 139:542–552, 552.e1–552.e3. doi: 10.1053/j.gastro.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 400.Wu J, Fernandes-Alnemri T, Alnemri ES. 2010. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J Clin Immunol 30:693–702. doi: 10.1007/s10875-010-9425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Sauer JD, Witte CE, Zemansky J, Hanson B, Lauer P, Portnoy DA. 2010. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe 7:412–419. doi: 10.1016/j.chom.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Warren SE, Armstrong A, Hamilton MK, Mao DP, Leaf IA, Miao EA, Aderem A. 2010. Cutting edge: cytosolic bacterial DNA activates the inflammasome via Aim2. J Immunol 185:818–821. doi: 10.4049/jimmunol.1000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Tsuchiya K, Hara H, Kawamura I, Nomura T, Yamamoto T, Daim S, Dewamitta SR, Shen Y, Fang R, Mitsuyama M. 2010. Involvement of absent in melanoma 2 in inflammasome activation in macrophages infected with Listeria monocytogenes. J Immunol 185:1186–1195. doi: 10.4049/jimmunol.1001058. [DOI] [PubMed] [Google Scholar]
- 404.Guo M, An F, Yu H, Wei X, Hong M, Lu Y. 2017. Comparative effects of schisandrin A, B, and C on Propionibacterium acnes-induced, NLRP3 inflammasome activation-mediated IL-1beta secretion and pyroptosis. Biomed Pharmacother 96:129–136. doi: 10.1016/j.biopha.2017.09.097. [DOI] [PubMed] [Google Scholar]
- 405.Qin M, Pirouz A, Kim MH, Krutzik SR, Garban HJ, Kim J. 2014. Propionibacterium acnes induces IL-1beta secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol 134:381–388. doi: 10.1038/jid.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Sahdo B, Sarndahl E, Elgh F, Soderquist B. 2013. Propionibacterium acnes activates caspase-1 in human neutrophils. APMIS 121:652–663. doi: 10.1111/apm.12035. [DOI] [PubMed] [Google Scholar]
- 407.Kistowska M, Gehrke S, Jankovic D, Kerl K, Fettelschoss A, Feldmeyer L, Fenini G, Kolios A, Navarini A, Ganceviciene R, Schauber J, Contassot E, French LE. 2014. IL-1beta drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J Invest Dermatol 134:677–685. doi: 10.1038/jid.2013.438. [DOI] [PubMed] [Google Scholar]
- 408.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 409.Hanamsagar R, Aldrich A, Kielian T. 2014. Critical role for the AIM2 inflammasome during acute CNS bacterial infection. J Neurochem 129:704–711. doi: 10.1111/jnc.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Ma J, Gulbins E, Edwards MJ, Caldwell CC, Fraunholz M, Becker KA. 2017. Staphylococcus aureus alpha-toxin induces inflammatory cytokines via lysosomal acid sphingomyelinase and ceramides. Cell Physiol Biochem 43:2170–2184. doi: 10.1159/000484296. [DOI] [PubMed] [Google Scholar]
- 411.Harder J, Franchi L, Munoz-Planillo R, Park JH, Reimer T, Nunez G. 2009. Activation of the Nlrp3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-kappa B activation but proceeds independently of TLR signaling and P2X7 receptor. J Immunol 183:5823–5829. doi: 10.4049/jimmunol.0900444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Latvala S, Makela SM, Miettinen M, Charpentier E, Julkunen I. 2014. Dynamin inhibition interferes with inflammasome activation and cytokine gene expression in Streptococcus pyogenes-infected human macrophages. Clin Exp Immunol 178:320–333. doi: 10.1111/cei.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Lin AE, Beasley FC, Keller N, Hollands A, Urbano R, Troemel ER, Hoffman HM, Nizet V. 2015. A group A Streptococcus ADP-ribosyltransferase toxin stimulates a protective interleukin 1β-dependent macrophage immune response. mBio 6:e00133. doi: 10.1128/mBio.00133-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Fang R, Tsuchiya K, Kawamura I, Shen Y, Hara H, Sakai S, Yamamoto T, Fernandes-Alnemri T, Yang R, Hernandez-Cuellar E, Dewamitta SR, Xu Y, Qu H, Alnemri ES, Mitsuyama M. 2011. Critical roles of ASC inflammasomes in caspase-1 activation and host innate resistance to Streptococcus pneumoniae infection. J Immunol 187:4890–4899. doi: 10.4049/jimmunol.1100381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Fang R, Hara H, Sakai S, Hernandez-Cuellar E, Mitsuyama M, Kawamura I, Tsuchiya K. 2014. Type I interferon signaling regulates activation of the absent in melanoma 2 inflammasome during Streptococcus pneumoniae infection. Infect Immun 82:2310–2317. doi: 10.1128/IAI.01572-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.McNeela EA, Burke A, Neill DR, Baxter C, Fernandes VE, Ferreira D, Smeaton S, El-Rachkidy R, McLoughlin RM, Mori A, Moran B, Fitzgerald KA, Tschopp J, Petrilli V, Andrew PW, Kadioglu A, Lavelle EC. 2010. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog 6:e1001191. doi: 10.1371/journal.ppat.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.van Lieshout MHP, de Vos AF, Dessing MC, de Porto A, de Boer OJ, de Beer R, Terpstra S, Florquin S, Van't Veer C, van der Poll T. 2018. ASC and NLRP3 impair host defense during lethal pneumonia caused by serotype 3 Streptococcus pneumoniae in mice. Eur J Immunol 48:66–79. doi: 10.1002/eji.201646554. [DOI] [PubMed] [Google Scholar]
- 418.Kim JY, Paton JC, Briles DE, Rhee DK, Pyo S. 2015. Streptococcus pneumoniae induces pyroptosis through the regulation of autophagy in murine microglia. Oncotarget 6:44161–44178. doi: 10.18632/oncotarget.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Karmakar M, Katsnelson M, Malak HA, Greene NG, Howell SJ, Hise AG, Camilli A, Kadioglu A, Dubyak GR, Pearlman E. 2015. Neutrophil IL-1beta processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J Immunol 194:1763–1775. doi: 10.4049/jimmunol.1401624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Witzenrath M, Pache F, Lorenz D, Koppe U, Gutbier B, Tabeling C, Reppe K, Meixenberger K, Dorhoi A, Ma J, Holmes A, Trendelenburg G, Heimesaat MM, Bereswill S, van der Linden M, Tschopp J, Mitchell TJ, Suttorp N, Opitz B. 2011. The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia. J Immunol 187:434–440. doi: 10.4049/jimmunol.1003143. [DOI] [PubMed] [Google Scholar]
- 421.Yang Y, Zhou X, Kouadir M, Shi F, Ding T, Liu C, Liu J, Wang M, Yang L, Yin X, Zhao D. 2013. The AIM2 inflammasome is involved in macrophage activation during infection with virulent Mycobacterium bovis strain. J Infect Dis 208:1849–1858. doi: 10.1093/infdis/jit347. [DOI] [PubMed] [Google Scholar]
- 422.Carlsson F, Kim J, Dumitru C, Barck KH, Carano RA, Sun M, Diehl L, Brown EJ. 2010. Host-detrimental role of Esx-1-mediated inflammasome activation in mycobacterial infection. PLoS Pathog 6:e1000895. doi: 10.1371/journal.ppat.1000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Saiga H, Kitada S, Shimada Y, Kamiyama N, Okuyama M, Makino M, Yamamoto M, Takeda K. 2012. Critical role of AIM2 in Mycobacterium tuberculosis infection. Int Immunol 24:637–644. doi: 10.1093/intimm/dxs062. [DOI] [PubMed] [Google Scholar]
- 424.Mishra BB, Moura-Alves P, Sonawane A, Hacohen N, Griffiths G, Moita LF, Anes E. 2010. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol 12:1046–1063. doi: 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 425.Basu S, Fowler B, Kerur N, Arnvig KB, Rao NA. 2018. NLRP3 inflammasome activation by mycobacterial ESAT-6 and dsRNA in intraocular tuberculosis. Microb Pathog 114:219–224. doi: 10.1016/j.micpath.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 426.Sugiyama M, Saeki A, Hasebe A, Kamesaki R, Yoshida Y, Kitagawa Y, Suzuki T, Shibata K. 2016. Activation of inflammasomes in dendritic cells and macrophages by Mycoplasma salivarium. Mol Oral Microbiol 31:259–269. doi: 10.1111/omi.12117. [DOI] [PubMed] [Google Scholar]
- 427.Xu Y, Li H, Chen W, Yao X, Xing Y, Wang X, Zhong J, Meng G. 2013. Mycoplasma hyorhinis activates the NLRP3 inflammasome and promotes migration and invasion of gastric cancer cells. PLoS One 8:e77955. doi: 10.1371/journal.pone.0077955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Wang J, Alexander J, Wiebe M, Jones C. 2014. Bovine herpesvirus 1 productive infection stimulates inflammasome formation and caspase 1 activity. Virus Res 185:72–76. doi: 10.1016/j.virusres.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Ansari MA, Singh VV, Dutta S, Veettil MV, Dutta D, Chikoti L, Lu J, Everly D, Chandran B. 2013. Constitutive interferon-inducible protein 16-inflammasome activation during Epstein-Barr virus latency I, II, and III in B and epithelial cells. J Virol 87:8606–8623. doi: 10.1128/JVI.00805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Du W, Zhen J, Zheng Z, Ma S, Chen S. 2013. Expression of AIM2 is high and correlated with inflammation in hepatitis B virus associated glomerulonephritis. J Inflamm (Lond) 10:37. doi: 10.1186/1476-9255-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Han Y, Chen Z, Hou R, Yan D, Liu C, Chen S, Li X, Du W. 2015. Expression of AIM2 is correlated with increased inflammation in chronic hepatitis B patients. Virol J 12:129. doi: 10.1186/s12985-015-0360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Deng X, Zou W, Xiong M, Wang Z, Engelhardt JF, Ye SQ, Yan Z, Qiu J. 2017. Human parvovirus infection of human airway epithelia induces pyroptotic cell death by inhibiting apoptosis. J Virol 91:e01533-. doi: 10.1128/JVI.01533-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, Otageri P, Chandran B. 2011. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe 9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Singh VV, Kerur N, Bottero V, Dutta S, Chakraborty S, Ansari MA, Paudel N, Chikoti L, Chandran B. 2013. Kaposi's sarcoma-associated herpesvirus latency in endothelial and B cells activates gamma interferon-inducible protein 16-mediated inflammasomes. J Virol 87:4417–4431. doi: 10.1128/JVI.03282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Ekchariyawat P, Hamel R, Bernard E, Wichit S, Surasombatpattana P, Talignani L, Thomas F, Choumet V, Yssel H, Despres P, Briant L, Misse D. 2015. Inflammasome signaling pathways exert antiviral effect against Chikungunya virus in human dermal fibroblasts. Infect Genet Evol 32:401–408. doi: 10.1016/j.meegid.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 436.Wu MF, Chen ST, Yang AH, Lin WW, Lin YL, Chen NJ, Tsai IS, Li L, Hsieh SL. 2013. CLEC5A is critical for dengue virus-induced inflammasome activation in human macrophages. Blood 121:95–106. doi: 10.1182/blood-2012-05-430090. [DOI] [PubMed] [Google Scholar]
- 437.Rajan JV, Rodriguez D, Miao EA, Aderem A. 2011. The NLRP3 inflammasome detects encephalomyocarditis virus and vesicular stomatitis virus infection. J Virol 85:4167–4172. doi: 10.1128/JVI.01687-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Burdette D, Haskett A, Presser L, McRae S, Iqbal J, Waris G. 2012. Hepatitis C virus activates interleukin-1beta via caspase-1-inflammasome complex. J Gen Virol 93:235–246. doi: 10.1099/vir.0.034033-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 439.McRae S, Iqbal J, Sarkar-Dutta M, Lane S, Nagaraj A, Ali N, Waris G. 2016. The hepatitis C virus-induced NLRP3 inflammasome activates the sterol regulatory element-binding protein (SREBP) and regulates lipid metabolism. J Biol Chem 291:3254–3267. doi: 10.1074/jbc.M115.694059. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 440.Kaushik DK, Gupta M, Kumawat KL, Basu A. 2012. NLRP3 inflammasome: key mediator of neuroinflammation in murine Japanese encephalitis. PLoS One 7:e32270. doi: 10.1371/journal.pone.0032270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Masters SL, Gerlic M, Metcalf D, Preston S, Pellegrini M, O'Donnell JA, McArthur K, Baldwin TM, Chevrier S, Nowell CJ, Cengia LH, Henley KJ, Collinge JE, Kastner DL, Feigenbaum L, Hilton DJ, Alexander WS, Kile BT, Croker BA. 2012. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity 37:1009–1023. doi: 10.1016/j.immuni.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Komune N, Ichinohe T, Ito M, Yanagi Y. 2011. Measles virus V protein inhibits NLRP3 inflammasome-mediated interleukin-1beta secretion. J Virol 85:13019–13026. doi: 10.1128/JVI.05942-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Wang B, Zhu J, Li D, Wang Y, Zhan Y, Tan L, Qiu X, Sun Y, Song C, Meng C, Ying L, Xiang M, Meng G, Ding C. 2016. Newcastle disease virus infection induces activation of the NLRP3 inflammasome. Virology 496:90–96. doi: 10.1016/j.virol.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 444.Shil NK, Pokharel SM, Banerjee AK, Hoffman M, Bose S. 2018. Inflammasome antagonism by human parainfluenza virus type 3 C protein. J Virol 92:e01776-. doi: 10.1128/JVI.01776-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Wang C, Shi X, Zhang X, Wang A, Wang L, Chen J, Deng R, Zhang G. 2015. The endoribonuclease activity essential for the nonstructural protein 11 of porcine reproductive and respiratory syndrome virus to inhibit NLRP3 inflammasome-mediated IL-1beta induction. DNA Cell Biol 34:728–735. doi: 10.1089/dna.2015.2929. [DOI] [PubMed] [Google Scholar]
- 446.Bi J, Song S, Fang L, Wang D, Jing H, Gao L, Cai Y, Luo R, Chen H, Xiao S. 2014. Porcine reproductive and respiratory syndrome virus induces IL-1beta production depending on TLR4/MyD88 pathway and NLRP3 inflammasome in primary porcine alveolar macrophages. Mediators Inflamm 2014:403515. doi: 10.1155/2014/403515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Lawrence TM, Hudacek AW, de Zoete MR, Flavell RA, Schnell MJ. 2013. Rabies virus is recognized by the NLRP3 inflammasome and activates interleukin-1beta release in murine dendritic cells. J Virol 87:5848–5857. doi: 10.1128/JVI.00203-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Segovia J, Sabbah A, Mgbemena V, Tsai SY, Chang TH, Berton MT, Morris IR, Allen IC, Ting JP, Bose S. 2012. TLR2/MyD88/NF-kappaB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One 7:e29695. doi: 10.1371/journal.pone.0029695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Harker JA, Godlee A, Wahlsten JL, Lee DC, Thorne LG, Sawant D, Tregoning JS, Caspi RR, Bukreyev A, Collins PL, Openshaw PJ. 2010. Interleukin 18 coexpression during respiratory syncytial virus infection results in enhanced disease mediated by natural killer cells. J Virol 84:4073–4082. doi: 10.1128/JVI.02014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Park S, Juliana C, Hong S, Datta P, Hwang I, Fernandes-Alnemri T, Yu JW, Alnemri ES. 2013. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J Immunol 191:4358–4366. doi: 10.4049/jimmunol.1301170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Wang X, Jiang W, Yan Y, Gong T, Han J, Tian Z, Zhou R. 2014. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat Immunol 15:1126–1133. doi: 10.1038/ni.3015. [DOI] [PubMed] [Google Scholar]
- 452.Ramos HJ, Lanteri MC, Blahnik G, Negash A, Suthar MS, Brassil MM, Sodhi K, Treuting PM, Busch MP, Norris PJ, Gale M Jr. 2012. IL-1beta signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog 8:e1003039. doi: 10.1371/journal.ppat.1003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Khaiboullina SF, Uppal T, Sarkar R, Gorzalski A, St Jeor S, Verma SC. 2017. ZIKV infection regulates inflammasomes pathway for replication in monocytes. Sci Rep 7:16050. doi: 10.1038/s41598-017-16072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Tricarico PM, Caracciolo I, Crovella S, D'Agaro P. 2017. Zika virus induces inflammasome activation in the glial cell line U87-MG. Biochem Biophys Res Commun 492:597–602. doi: 10.1016/j.bbrc.2017.01.158. [DOI] [PubMed] [Google Scholar]
- 455.Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Despres P, Amara A, Yssel H, Misse D. 2015. Biology of Zika virus infection in human skin cells. J Virol 89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Kistowska M, Fenini G, Jankovic D, Feldmeyer L, Kerl K, Bosshard P, Contassot E, French LE. 2014. Malassezia yeasts activate the NLRP3 inflammasome in antigen-presenting cells via Syk-kinase signalling. Exp Dermatol 23:884–889. doi: 10.1111/exd.12552. [DOI] [PubMed] [Google Scholar]
- 457.Ketelut-Carneiro N, Silva GK, Rocha FA, Milanezi CM, Cavalcanti-Neto FF, Zamboni DS, Silva JS. 2015. IL-18 triggered by the Nlrp3 inflammasome induces host innate resistance in a pulmonary model of fungal infection. J Immunol 194:4507–4517. doi: 10.4049/jimmunol.1402321. [DOI] [PubMed] [Google Scholar]
- 458.Goncalves AC, Ferreira LS, Manente FA, de Faria C, Polesi MC, de Andrade CR, Zamboni DS, Carlos IZ. 2017. The NLRP3 inflammasome contributes to host protection during Sporothrix schenckii infection. Immunology 151:154–166. doi: 10.1111/imm.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Esch KJ, Schaut RG, Lamb IM, Clay G, Morais Lima AL, do Nascimento PR, Whitley EM, Jeronimo SM, Sutterwala FS, Haynes JS, Petersen CA. 2015. Activation of autophagy and nucleotide-binding domain leucine-rich repeat-containing-like receptor family, pyrin domain-containing 3 inflammasome during Leishmania infantum-associated glomerulonephritis. Am J Pathol 185:2105–2117. doi: 10.1016/j.ajpath.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Wang S, Wang Z, Gu Y, Li Z, Li Z, Wei F, Liu Q. 2016. Toxoplasma gondii mitogen-activated protein kinases are associated with inflammasome activation in infected mice. Microbes Infect 18:696–700. doi: 10.1016/j.micinf.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 461.Chudnovskiy A, Mortha A, Kana V, Kennard A, Ramirez JD, Rahman A, Remark R, Mogno I, Ng R, Gnjatic S, Amir ED, Solovyov A, Greenbaum B, Clemente J, Faith J, Belkaid Y, Grigg ME, Merad M. 2016. Host-protozoan interactions protect from mucosal infections through activation of the inflammasome. Cell 167:444.e414–456.e414. doi: 10.1016/j.cell.2016.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]