Abstract
Background and aims:
GlycA is a relatively new biomarker for inflammation as well as cardiometabolic disease risk. However, the effect of exercise on GlycA is largely unknown. Therefore, the purpose of this study was to examine the effects of regular exercise on the inflammatory marker GlycA across seven studies and 14 exercise interventions.
Methods:
Nuclear magnetic resonance spectroscopy, specifically signal amplitudes originating from the N-acetyl methyl group protons of the N-acetylglucosamine residues on the glycan branches of glycoproteins, was used to quantify GlycA concentrations. GlycA was measured before and after completion of an exercise intervention in 1568 individuals across seven studies and 14 exercise interventions. Random effects inverse variance weighting models were used to pool effects across interventions.
Results:
Combined analysis of unadjusted data showed that regular exercise significantly (p=2×10−6) reduced plasma GlycA (−8.26±1.8 μmol/L). This reduction remained significant (−9.12±1.9 μmol/L, p=1.22×10−6) following adjustment for age, sex, race, baseline BMI, and baseline GlycA. Changes in GlycA were correlated with changes in traditional inflammatory markers C-reactive protein, interleukin-6, and fibrinogen, however, these correlations were relatively weak (range r: 0.21-0.38, p<0.0001).
Conclusions:
Regular exercise significantly reduced plasma GlycA across 14 different exercise interventions despite differences in exercise programs and study populations. The current study provides a greater understanding of the use of exercise as a potential therapy for the reduction of systemic inflammation. Further research is needed to understand the mechanisms behind the exercise-related reductions in GlycA.
Keywords: Exercise training, NMR spectroscopy, Inflammation
INTRODUCTION
Inflammation is an important clinical measure because of its association with increased risk of cardiovascular disease (CVD) and mortality.1,2 While C-reactive protein (CRP), fibrinogen, and interleukin-6 (IL-6) are the primary clinical markers of systemic inflammation, CRP is the favored marker because of its strong relationship with CVD.3 However CRP has large within-person variability, which makes risk-stratification based on CRP levels difficult.4 GlycA, a relatively new biomarker for systemic inflammation, is measured via nuclear magnetic resonance (NMR) spectroscopy and reflects the glycosylation states of several acute phase proteins,4 which increase during the inflammatory response to a variety of stimuli.5 Furthermore, GlycA appears to be an independent marker of inflammation, as it is only moderately correlated with CRP, fibrinogen, and IL-6.4 In over 5000 participants from the MESA study, GlycA positively correlated with CRP (r=0.56), fibrinogen (r=0.46), and IL-6 (r=0.35) (all p<0.0001).4 Similar to these traditional inflammatory markers, greater GlycA concentrations are associated with increased risk of type 2 diabetes mellitus, CVD, and all-cause mortality.6-8 GlycA is also higher in subjects with metabolic syndrome and positively correlated with body mass index (BMI) and insulin resistance.9-12 Additionally, GlycA has reduced within-person variability (CV=4.3%) compared to CRP (CV=29.2%)4 and thus may be a more reliable measurement of inflammation for clinical practice. The quantification of GlycA is inexpensive and a high throughput process, therefore GlycA may also represent a more cost and time efficient marker of inflammation, especially in large samples.
While habitual physical activity and exercise decrease markers of systemic inflammation in a dose-response fashion,13,14-16 to date only one study has examined the relationship of regular exercise with plasma GlycA. In overweight individuals with prediabetes, a 6-month intervention of endurance exercise alone or exercise combined with diet significantly reduced GlycA levels by 2%.17 Given the limited knowledge on exercise and GlycA, the purpose of the present study was to examine the effects of regular endurance exercise on plasma GlycA across seven exercise training studies with 14 different exercise interventions. We hypothesized that regular exercise would decrease plasma levels of GlycA across varying endurance exercise interventions.
MATERIALS AND METHODS
The effects of standardized, regular endurance exercise on plasma GlycA concentrations were examined across seven exercise training studies and 14 independent exercise interventions: APOE18, DREW19, HERITAGE20, GERS21, STRRIDE I22, STRRIDE II23, and STRRIDE PD.24 The following studies had only one exercise training group (i.e., prescribed only one dose of exercise): APOE (~13-15 kilocalories per kilogram of body weight per week [KKW]) 18, HERITAGE (~12-14 KKW)20, and GERS (~10-12 KKW).21 The DREW study was a randomized control trial with three different exercise training groups that expended 4, 8, or 12 KKW at 50% VO2peak for six months.19 STRRIDE I consisted of a control group and three exercise groups: MILD (14 KKW), low-amount, moderate-intensity exercise (40-55% VO2peak); MOD (14 KKW), low amount, vigorous-intensity (65-80% VO2peak); and HIGH (23 KKW), high-amount, vigorous-intensity (65-80% VO2peak).22 Similarly, STRRIDE II consisted of three exercise groups: MOD (14 KKW), low amount, vigorous-intensity (65-80% VO2peak); MOD plus resistance training (RT), same as MOD plus RT three days/wk, 3 sets/day, 8-12 repetitions/set; and HIGH (23 KKW), high-amount, vigorous-intensity (65-80% VO2peak).23 Finally, STRRIDE-PD participants were randomized to one of four groups: low-amount and moderate intensity (10 KKW at 50% VO2peak), high amount and moderate intensity (16 KKW at 50% VO2peak), high amount and vigorous intensity (16 KKW at 75% VO2peak), and Clinical Lifestyle intervention: low amount moderate intensity intervention plus diet (with weight loss goal of 7%).24 The sample, study design, and exercise training protocol of each study have been described elsewhere and are summarized in the Supplemental Methods.
Measurements
Blood draws.
Within each study, all blood draws were taken in the fasted state at both baseline and after completion of exercise training (within 16-72 hr of last exercise session). All measures described below were performed on both baseline and post-training samples in all studies.
GlycA measurements.
GlycA was assessed by NMR spectroscopy at LipoScience, Inc (now LabCorp, Morrisville, NC) as previously described.4 Briefly, NMR signal amplitudes originating from the N-acetyl methyl group protons of the N-acetylglucosamine residues on the glycan branches of glycoproteins were used to quantify GlycA concentrations.
Inflammatory markers.
CRP was measured in all studies except APOE, STRRIDE II, and STRRIDE-PD. IL-6 was measured in DREW and STRRIDE I and II, while fibrinogen and tumor necrosis factor alpha (TNF-α) were measured in DREW only. All inflammatory markers were measured using standard methods.
Lipoprotein measurements.
A standard lipid panel was measured using standard techniques in all studies. Data for lipoprotein subfractions quantified using Liposcience’s LipoProfile-3 algorithm were also available in all studies.25 Specifically, data for total, large, medium, and small very low-density lipoprotein (VLDL-P) and high-density lipoprotein particles (HDL-P), total, large, and small LDL particles (LDL-P), and intermediate-density lipoprotein particles (IDL-P), as well as weighted average VLDL-P, LDL-P, and HDL-P sizes were available before and after exercise training. Additionally, small and medium HDL-P concentrations were combined into one category and analyzed together (small-medium HDL-P).
Statistical analysis.
Only two studies (DREW and STRRIDE I) included control groups, thus our primary analyses focused on the exercise groups only. Each group was analyzed independently because of differences in exercise prescription and training programs. The two MOD and the two HIGH programs from STRRIDE I and II were identical, thus these exercise groups were combined for meta-analysis. Therefore, a total of 14 exercise programs were examined in the combined analysis. Correlations between variables were assessed via Pearson correlation coefficients in SAS 9.4 (Cary, NC) by combining all exercise groups within a study (e.g. DREW correlations were done by grouping the 4, 8, and 12 KKW groups together).
Combined analysis.
The effect of regular exercise on plasma GlycA was examined via combined analysis using meta-analytic techniques. The included studies were part of an existing collaboration on lipoprotein responses to exercise training.25 All of the studies met the following criteria: adult participants (≥17 years) with available pre- and post-training NMR GlycA data, sample size ≥50, and the intervention was supervised, standardized, and lasted at least 12 weeks. Combined analysis on individual level data from each study was performed using Comprehensive Meta-Analysis (v2.2, Englewood, NJ). Unadjusted and adjusted (for age, sex, race, baseline BMI, baseline GlycA) random effects inverse variance weighting models were used to pool effects across interventions. We performed secondary analyses that included combined analysis of the DREW and STRRIDE I studies, as both included control groups. The analysis was performed in the same manner as described above, with the exception that net change in GlycA from baseline to post-training was calculated as the difference of the mean changes between each exercise group and their respective control group.
RESULTS
Overall, 1568 (57% Female, 66% White) participants were included in the final combined analysis. Baseline characteristics including mean values for the standard lipid panel and other cardiovascular risk factors across the seven studies are shown in Table 1. The training response of these variables can be found in Supplemental Table 1.
Table 1.
Baseline characteristics of the combined exercise groups within each study, given as means (standard deviation).
| HERITAGE (n=700) |
DREW (n=292) |
GERS (n=78) |
APOE (n=103) |
STRRIDE I (n=132) |
STRRIDE II (n=102) |
STRRIDE PD (n=161) |
|
|---|---|---|---|---|---|---|---|
| Age (years) | 35.1 (13.7) | 57.3 (6.5) | 58.0 (5.8) | 39.1 (10.9) | 52.3 (6.0) | 48.5 (9.6) | 59.3 (7.6) |
| BMI (kg/m2) | 26.4 (5.3) | 31.8 (3.7) | 27.9 (4.1) | 27.6 (4.9) | 29.9 (2.9) | 30.5 (3.3) | 30.9 (5.7) |
| VO2 max (L/min) | 2.3 (0.7) | 1.3 (0.3) | 2.0 (0.5) | 2.6 (0.8) | 2.5 (0.7) | 2.5 (0.6) | 2.2 (0.6) |
| HDL-C (mg/dL) | 40.7 (10.6) | 57.3 (14.3) | 46.1 (13.4) | 50.5 (15.9) | 53.1 (16.8) | 51.0 (13.2) | 44.2 (14.9) |
| LDL-C (mg/dL) | 113.9 (30.9) | 117.7 (26.5) | 130.9 (28.1) | 131.5 (31.8) | 144.9 (31.2) | 144.1 (21.5) | 111.8 (26.1) |
| TC (mg/dL) | 170.8 (35.9) | 200.7 (30.1) | 209.9 (32.4) | 204.5 (37.8) | 233.1 (28.8) | 224.7 (28.1) | 182.8 (31.7) |
| TG (mg/dL) | 112.5 (67.5) | 129.4 (63.4) | 163.7 (86.8) | 137.9 (91.0) | 168.66 (104.3) | 149.9 (73.1) | 133.2 (71.2) |
| CRP (mg/dL) | 2.7 (4.8) | 5.5 (5.3) | 3.2 (3.3) | N/A | 3.4 (3.1) | N/A | N/A |
| SBP (mmHg) | 118.7 (11.9) | 138.8 (13.0) | 129.6 (16.6) | N/A | 130.1 (14.4) | 118.9 (13.6) | N/A |
| DBP (mmHg) | 68.3 (8.9) | 80.8 (8.9) | 83.3 (10.8) | N/A | 84.3 (8.0) | 78.2 (8.7) | N/A |
| Glucose (mg/dL) | 91.6 (11.2) | 94.6 (8.7) | 91.1 (9.2) | N/A | 94.5 (9.7) | 94.6 (11.8) | 105.6 (9.8) |
| Waist (cm) | 90.2 (14.8) | 100.6 (11.6) | 90.9 (13.0) | 89.6 (14.8) | 95.7 (10.3) | 96.9 (9.5) | 99.0 (8.6) |
| Percent fat | 27.6 (10.2) | 28.3 (4.5) | 35.7 (9.3) | 22.7 (6.2) | N/A | N/A | 40.9 (7.8) |
| GlycA (μmol/L) | 324.7 (56.5) | 364.9 (47.8) | 405.718 (64.9) | 422.5 (70.6) | 325.2 (314.0) | 362.6 (65.4) | 347.1 (49.5) |
BMI: body mass index, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, TC: total cholesterol, TG: triglycerides, CRP: C-reactive protein, SBP: systolic blood pressure, DBP: diastolic blood pressure, N/A: data not available.
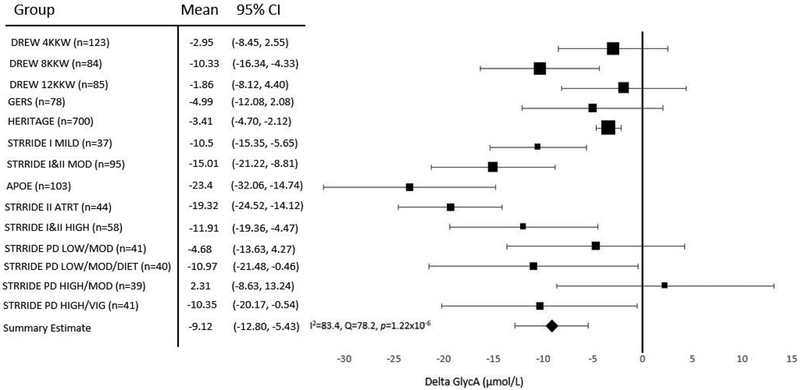
On average, GlycA decreased with regular exercise in 13 of 14 exercise groups, with the adjusted exercise-induced reductions being statistically significant in nine of the 14 groups (Figure 1). Overall, unadjusted combined analysis found GlycA significantly (p=2.38×10−6) decreased by 8.26±1.8 μmol/L (1.79±0.5%) following exercise training (Supplemental Figure 1). The exercise-induced decrease in GlycA remained significant (9.12±1.9 μmol/L or 1.76±0.5%) in combined analysis that adjusted for age, sex, race, baseline BMI, and baseline GlycA levels (Figure 1). In secondary analyses in DREW and STRRIDE I that compared changes in GlycA between the exercise and control groups, there were no differences in mean GlycA change between the groups (adjusted mean change compared to control group: −5.13 ±3.84 μmol/L, p=0.18). GlycA levels declined in the control groups within STRRIDE I (−1.34 μmol/L, p=0.82) and DREW (−2.29 μmol/L, p=0.52), however, neither reduction was statistically significant.
Figure 1. Adjusted meta-analysis results for change in GlycA following various exercise interventions.
Adjusted mean change with 95% confidence intervals (CI) are shown. Mean values were adjusted for age, sex, race, baseline BMI, and baseline GlycA. Summary estimate = −9.12 μmol/L, I2=83.4, Q=78.2, p=1.22×10−6. Size of the marker for the mean value of each exercise group represents the relative weight of the study in the combined analysis. KKW, kilocalories per kilogram of body weight per week.
GlycA positively correlated with CRP at baseline in all five studies with the measure (range: r=0.29–0.51, p<0.0001-0.046). At baseline, GlycA was positively correlated with IL-6 in the combined exercise groups of DREW (r=0.22, p=0.0002) and STRRIDE I & II (r=0.32, p=0.009) and with fibrinogen (r=0.41, p<0.0001) in DREW. Following training, CRP decreased in two out of four studies that measured it (−0.03 mg/dL in DREW and −0.1 mg/dL in GERS), but these decreases were not statistically significant. There were no significant changes in IL-6, fibrinogen, or TNF-α following training. Exercise-induced changes in GlycA were weakly, positively correlated with changes in CRP in HERITAGE, DREW, and STRRIDE I (range: r=0.22-0.38, p<0.0001-0.009). Changes in GlycA were also weakly correlated with changes in both IL-6 (r=0.21, p=0.0004) and fibrinogen (r=0.36, p<0.0001) in the DREW study only.
GlycA levels were also correlated with other CVD risk factors. Baseline BMI and GlycA levels were positively correlated in four of the seven studies (range: r=0.14–0.44, p<0.0001-0.04), but change in BMI and change in GlycA were only significantly correlated in the HERITAGE and STRRIDE-PD studies (r=0.11, p=0.003 and r=0.19, p=0.02, respectively) (Table 2). At baseline, GlycA was weakly correlated with total HDL-P concentration in three of the seven studies: HERITAGE (r=0.28, p<0.0001), DREW (r=0.14, p=0.02), and STRRIDE II (r=0.29, p<0.0001). Exercise-induced changes in GlycA and total HDL-P concentration were significantly correlated in each of these three studies as well as STRRIDE I, with the largest correlation coming from the HERITAGE study (r=0.34, p<0.0001) (Table 2). At baseline, GlycA and small-medium HDL-P were significantly correlated in HERITAGE, DREW, and STRRIDE I & II (range: r=0.19–0.33, p<0.0001-0.0006). Changes in GlycA and changes in small-medium HDL-P following training were significantly correlated in the same four studies and STRRIDE-PD (range r=0.17-0.36, p<0.0001). Changes in GlycA and total LDL-P were significantly correlated in HERITAGE, GERS, and STRRIDE I (range: r=0.26-0.34, p<0.0001) (Table 2).
Table 2.
Correlation between change in GlycA (μmol/L) and concomitant change in select cardiometabolic traits in the seven different studies.
| HERITAGE | DREW | GERS | APOE | STRRIDE I | STRRIDE II | STRRIDE PD | |
|---|---|---|---|---|---|---|---|
| Age (years) | NS | NS | NS | NS | NS | NS | NS |
| BMI (kg/m2) | 0.11 | NS | NS | NS | NS | NS | 0.19 |
| VO2 max (L/min) | NS | NS | NS | NS | 0.18 | NS | NS |
| Total LDL-P (nmol/L) | 0.26 | NS | 0.34 | NS | 0.26 | NS | NS |
| Small LDL-P (nmol/L) | 0.22 | 0.15 | NS | NS | 0.28 | NS | NS |
| Total HDL-P (μmol/L) | 0.34 | 0.20 | NS | NS | 0.26 | 0.33 | NS |
| Small-medium HDL-P (μmol/L) | 0.36 | 0.25 | NS | NS | 0.29 | 0.25 | 0.17 |
| HDL-C (mg/dL) | NS | NS | NS | NS | NS | 0.31 | NS |
| LDL-C (mg/dL) | NS | NS | NS | NS | NS | NS | NS |
| Insulin (pmol/L) | 0.08 | 0.2 | NS | NS | NS | NS | NS |
| CRP (mg/dL) | 0.22 | 0.38 | NS | NS | 0.25 | NS | NS |
| IL-6 (pg/mL) | NS | 0.21 | NS | NS | NS | NS | NS |
| Fibrinogen (mg/dL) | NS | 0.36 | NS | NS | NS | NS | NS |
BMI: body mass index, LDL-P: low-density lipoprotein particles, HDL-P: high-density lipoprotein particles, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, CRP: C-reactive protein, IL-6: interleukin-6
All correlations listed were p<0.05. NS, not significant (p>0.05). In studies with multiple exercise groups, the exercise groups were combined for the correlation analyses.
DISCUSSION
Combined analysis using meta-analytic techniques showed that regular exercise decreased plasma GlycA levels in previously sedentary adults across various exercise interventions. These findings remained significant after taking into account differences in study populations (i.e., age, sex, race, BMI, and baseline GlycA levels). There was no clear pattern of dose-response across the groups ranging in weekly energy expenditure from 4 KKW to 23 KKW, as groups from both the low and high exercise doses showed significant mean reductions in GlycA. However, it should be noted that when compared to the limited control group data available, there was no significant effect of exercise on GlycA. Compared to more traditional inflammatory markers, exercise associated changes in plasma GlycA levels were correlated with changes in CRP, IL-6, and fibrinogen, but the relationships were not particularly strong indicating that GlycA levels may be partially independent.
Chronic systemic inflammation is linked to CVD risk and the pathogenesis of several chronic diseases.3,26-28 Therefore strategies to lower chronic inflammation are of great public health importance. Regular endurance exercise represents one potential strategy, as it has been shown to decrease the risk of chronic disease and may improve inflammatory profiles. However, to date the effects of exercise on inflammation have been inconclusive. The effect of regular endurance exercise on traditional inflammatory markers such as CRP is still largely unclear,29 but large observational cohort studies have shown inverse relationships between inflammatory markers and physical activity or physical fitness.30 The current analysis showed that on average long-term endurance-based exercise interventions reduce plasma GlycA levels and therefore likely reduce systemic inflammation. The novelty and partial independence of GlycA from other traditional inflammatory markers may explain why the present results differ from previous exercise training studies of inflammatory markers. Thus, improvements in GlycA levels with exercise may represent a potential novel pathway contributing to the decreased risk of chronic disease associated with regular exercise.
Elevated concentrations of GlycA are associated with increased mortality and incidence of diabetes, arthritis, CVD, and colorectal cancer.6,31-33 Additionally, several studies31,32 have shown that even small differences in plasma GlycA are associated with large differences in disease risk. A prospective study examining GlycA and colorectal cancer risk showed that compared to plasma GlycA quartile 1 (≤ 326 μmol/L), quartile 2 (327-369 μmol/L) was associated with an approximately 20% increase in colorectal cancer incidence, and for colorectal incidence and mortality the hazard ratio per standard deviation of GlycA was 1.26.32 GlycA levels are also associated with atherosclerosis, as each increasing quartile of GlycA is associated with 48% increased odds of having coronary artery calcium, a marker of atherosclerosis.31 These results demonstrate the clear adverse associations of elevated GlycA concentrations on health outcomes, and therefore strategies for reducing plasma GlycA even slightly may have potential clinical relevance. The observed mean changes in GlycA were rather small (−9.12 μmol/L or about −2%), however each cohort started at differing baseline concentrations of GlycA, thus the reduction will have differing clinical significance based on the starting point. Furthermore, there was large inter-individual variation in GlycA responsiveness to exercise training across the studies (range: −315 to +189 μmol/L). Thus, these results suggest that although regular exercise is effective in reducing plasma GlycA levels in most individuals, these reductions may only be clinically relevant in certain individuals.
The molecular mechanisms responsible for the exercise related reductions in GlycA are still not understood. One potential mechanism for the exercise-mediated decrease in plasma GlycA is through an interaction with lipoproteins. A previous study found an interaction between GlycA and small and medium HDL subclasses, with greater GlycA concentrations mitigating the reduced mortality risk associated with smaller HDL subclasses.34 One of the beneficial actions of HDL that likely contributes to this protective effect is the inhibition of inflammatory endothelial cell adhesion molecule expression.35 By inhibiting these molecules, HDL reduces systemic inflammation. Given that GlycA is a marker of systemic inflammation, there may be an interaction between plasma GlycA levels and HDL. The current study showed that changes in plasma GlycA were significantly correlated with changes in the concentration of total HDL-P, small-medium HDL-P, and even total LDL-P. Most notably, changes in GlycA were significantly correlated with changes in small-medium HDL-P in five of the seven studies, which were made up of 14 different exercise interventions. The correlations observed were mostly weak, therefore it is difficult to conclude that GlycA and lipoprotein subfractions are changing in concert with regular exercise, however the current study may provide some evidence of such an interaction.
Combined analysis comparing the exercise and control groups in DREW and STRRIDE I showed no significant effect of exercise compared to controls on plasma GlycA (p=0.18). The lack of a significant effect of regular endurance exercise compared to control may be due to the lack of power, as only the DREW and STRRIDE I studies included control groups. Additionally, while combined analysis did not show a dose-dependent relationship between endurance exercise training and changes in plasma GlycA, DREW had the lowest intensity exercise program (50% VO2peak). The lower intensity of DREW, along with the uniqueness of the study population (post-menopausal, overweight/obese women with elevated blood pressure) may have played a role in the lack of a training effect compared to controls. Finally, in both control groups we saw nominal reductions in GlycA levels. While not significant, the reductions in GlycA in the control groups suggests that the participants may have changed some behaviors, despite not actively being in an intervention group. These reductions may have blunted the ability to detect differences in change in GlycA between the exercise and control groups after the intervention in these studies. Further randomized controlled trials are needed to clarify the effects of regular endurance exercise on plasma GlycA.
Our study benefitted from a large, diverse sample size and the fact that plasma GlycA was measured using the same methods in the same laboratory across all studies, which minimized the potential for measurement error and variability. The current study was limited by the lack of control groups in almost every study. Therefore, changes in GlycA could only be compared to an individual’s baseline levels. We were also unable to clearly elucidate the driver of the change in GlycA, since the signal measures more than one acute phase protein and the current study did not measure individual acute phase protein responses.
The current study demonstrates that regular endurance exercise may reduce plasma GlycA levels and therefore may contribute to reductions in systemic inflammation. However, the lack of a significant effect of exercise on plasma GlycA compared to controls highlights the need for further research on the effects of exercise on this novel biomarker of inflammation. The current study provides insight into the beneficial effects of exercise and in particular the use of exercise as a potential therapy for the reduction of systemic inflammation.
Supplementary Material
Highlights.
Regular endurance exercise significantly reduces the inflammatory marker GlycA
Regular endurance exercise does not reduce other traditional inflammatory markers
Changes in GlycA are weakly correlated with changes in CRP, IL-6, and fibrinogen
FINANCIAL SUPPORT
This work was supported by multiple grants from the NIH. The exercise training studies were funded by multiple R01s: HL66262 and the Life Fitness Company (DREW); AG17474 and AG15389 (GERS); HL45670, HL47323, HL47317, HL47327, HL47321 (HERITAGE); HL57354 (STRRIDE I and II); DK081559 (STRRIDE-PD). In STRRIDE-PD LipoScience, Inc. (LabCorp, Inc.) kindly funded the GlycA analyses through a funded granting mechanism. MAS was supported in part by U54 GM104940 from the NIGMS, which funds the Louisiana Clinical and Translational Science Center. CB and MAS were partially supported by the NIGMS COBRE center grant 8P20 GM-1033528. ASL is partially supported by the Henry L. Taylor Professorship in Exercise Science and Health Enhancement. CB is partially supported by the John W. Barton, Sr. Endowed Chair in Genetics and Nutrition. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
REFERENCES
- 1.Inflammation Libby P. and cardiovascular disease mechanisms. Am J Clin Nutr 2006;83:456S–60S. [DOI] [PubMed] [Google Scholar]
- 2.Reuben DB, Cheh AI, Harris TB, et al. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc 2002;50:638–44. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–43. [DOI] [PubMed] [Google Scholar]
- 4.Otvos JD, Shalaurova I, Wolak-Dinsmore J, et al. GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clin Chem 2015;61:714–23. [DOI] [PubMed] [Google Scholar]
- 5.Gabay C, Kushner I. Mechanisms of disease: Acute-phase proteins and other systemic responses to inflammation. New Engl J Med 1999;340:448–54. [DOI] [PubMed] [Google Scholar]
- 6.Akinkuolie AO, Pradhan AD, Buring JE, Ridker PM, Mora S. Novel protein glycan side-chain biomarker and risk of incident type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol 2015;35:1544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akinkuolie AO, Buring JE, Ridker PM, Mora S. A novel protein glycan biomarker and future cardiovascular disease events. J Am Heart Assoc 2014;3:e001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawler PR, Akinkuolie AO, Chandler PD, et al. Circulating N-Linked Glycoprotein Acetyls and Longitudinal Mortality Risk. Circ Res 2016;118:1106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorenzo C, Festa A, Hanley AJ, Rewers MJ, Escalante A, Haffner SM. Novel Protein Glycan-Derived Markers of Systemic Inflammation and C-Reactive Protein in Relation to Glycemia, Insulin Resistance, and Insulin Secretion. Diabetes Care 2017;40:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dullaart RPF, Gruppen EG, Connelly MA, Lefrandt JD. GlycA, a pro-inflammatory glycoprotein biomarker, is related to lower bilirubin in metabolic syndrome. Eur J Clin Invest 2016;46:47-. [DOI] [PubMed] [Google Scholar]
- 11.Dullaart RPF, Gruppen EG, Connelly MA, Otvos JD, Lefrandt JD. GlycA, a biomarker of inflammatory glycoproteins, is more closely related to the leptin/adiponectin ratio than to glucose tolerance status. Clin Biochem 2015;48:811–4. [DOI] [PubMed] [Google Scholar]
- 12.Gruppen EG, Connelly MA, Otvos JD, Bakker SJL, Dullaart RPF. A novel protein glycan biomarker and LCAT activity in metabolic syndrome. Eur J Clin Invest 2015;45:850–9. [DOI] [PubMed] [Google Scholar]
- 13.Palmefors H, DuttaRoy S, Rundqvist B, Borjesson M. The effect of physical activity or exercise on key biomarkers in atherosclerosis--a systematic review. Atherosclerosis 2014;235:150–61. [DOI] [PubMed] [Google Scholar]
- 14.Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among US adults. Epidemiology 2002;13:561–8. [DOI] [PubMed] [Google Scholar]
- 15.Gardner AW, Parker DE, Montgomery PS, Blevins SM. Step-Monitored Home Exercise Improves Ambulation, Vascular Function, and Inflammation in Symptomatic Patients With Peripheral Artery Disease: A Randomized Controlled Trial. Journal of the American Heart Association 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You TJ, Arsenis NC, Disanzo BL, LaMonte MJ. Effects of Exercise Training on Chronic Inflammation in Obesity Current Evidence and Potential Mechanisms. Sports Med 2013;43:243–56. [DOI] [PubMed] [Google Scholar]
- 17.Bartlett DB, Slentz CA, Connelly MA, et al. Association of the Composite Inflammatory Biomarker GlycA, with Exercise-Induced Changes in Body Habitus in Men and Women with Prediabetes. Oxid Med Cell Longev 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seip RL, Otvos J, Bilbie C, et al. The effect of apolipoprotein E genotype on serum lipoprotein particle response to exercise. Atherosclerosis 2006;188:126–33. [DOI] [PubMed] [Google Scholar]
- 19.Morss GM, Jordan AN, Skinner JS, et al. Dose Response to Exercise in Women aged 45-75 yr (DREW): design and rationale. Med Sci Sports Exerc 2004;36:336–44. [DOI] [PubMed] [Google Scholar]
- 20.Bouchard C, Leon AS, Rao DC, Skinner JS, Wilmore JH, Gagnon J. The HERITAGE family study. Aims, design, and measurement protocol. Med Sci Sports Exerc 1995;27:721–9. [PubMed] [Google Scholar]
- 21.Halverstadt A, Phares DA, Wilund KR, Goldberg AP, Hagberg JM. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metabolism 2007;56:444–50. [DOI] [PubMed] [Google Scholar]
- 22.Kraus WE, Torgan CE, Duscha BD, et al. Studies of a targeted risk reduction intervention through defined exercise (STRRIDE). Med Sci Sports Exerc 2001;33:1774–84. [DOI] [PubMed] [Google Scholar]
- 23.Bateman LA, Slentz CA, Willis LH, et al. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise - STRRIDE-AT/RT). Am J Cardiol 2011;108:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slentz CA, Bateman LA, Willis LH, et al. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: a randomised controlled trial. Diabetologia 2016;59:2088–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarzynski MA, Burton J, Rankinen T, et al. The effects of exercise on the lipoprotein subclass profile: A meta-analysis of 10 interventions. Atherosclerosis 2015;243:364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. New Engl J Med 1997;336:973–9. [DOI] [PubMed] [Google Scholar]
- 27.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol 2015;12:230–43. [DOI] [PubMed] [Google Scholar]
- 28.Bending D, Zaccone P, Cooke A. Inflammation and type one diabetes. Int Immunol 2012;24:339–46. [DOI] [PubMed] [Google Scholar]
- 29.Kelley GA, Kelley KS. Effects of aerobic exercise on C-reactive protein, body composition, and maximum oxygen consumption in adults: a meta-analysis of randomized controlled trials. Metabolism 2006;55:1500–7. [DOI] [PubMed] [Google Scholar]
- 30.Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta 2010;411:785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ormseth MJ, Chung CP, Oeser AM, et al. Utility of a novel inflammatory marker, GlycA, for assessment of rheumatoid arthritis disease activity and coronary atherosclerosis. Arthritis Research & Therapy 2015;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandler PD, Akinkuolie AO, Tobias DK, et al. Association of N-Linked Glycoprotein Acetyls and Colorectal Cancer Incidence and Mortality. Plos One 2016;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gruppen EG, Riphagen IJ, Connelly MA, Otvos JD, Bakker SJL, Dullaart RPF. GlycA, a Pro-Inflammatory Glycoprotein Biomarker, and Incident Cardiovascular Disease: Relationship with C-Reactive Protein and Renal Function. Plos One 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGarrah RW, Kelly JP, Craig DM, et al. A Novel Protein Glycan-Derived Inflammation Biomarker Independently Predicts Cardiovascular Disease and Modifies the Association of HDL Subclasses with Mortality. Clinical Chemistry 2017;63:288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ansell BJ, Fonarow GC, Fogelman AM. The paradox of dysfunctional high-density lipoprotein. Curr Opin Lipidol 2007;18:427–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.