ABSTRACT
Background
Controversy has emerged about the benefits compared with harms of dairy fat, including concerns over long-term effects. Previous observational studies have assessed self-reported estimates of consumption or a single biomarker measure at baseline, which may lead to suboptimal estimation of true risk.
Objective
The aim of this study was to investigate prospective associations of serial measures of plasma phospholipid fatty acids pentadecanoic (15:0), heptadecanoic (17:0), and trans-palmitoleic (trans-16:1n–7) acids with total mortality, cause-specific mortality, and cardiovascular disease (CVD) risk among older adults.
Design
Among 2907 US adults aged ≥65 y and free of CVD at baseline, circulating fatty acid concentrations were measured serially at baseline, 6 y, and 13 y. Deaths and CVD events were assessed and adjudicated centrally. Prospective associations were assessed by multivariate-adjusted Cox models incorporating time-dependent exposures and covariates.
Results
During 22 y of follow-up, 2428 deaths occurred, including 833 from CVD, 1595 from non-CVD causes, and 1301 incident CVD events. In multivariable models, circulating pentadecanoic, heptadecanoic, and trans-palmitoleic acids were not significantly associated with total mortality, with extreme-quintile HRs of 1.05 for pentadecanoic (95% CI: 0.91, 1.22), 1.07 for heptadecanoic (95% CI: 0.93, 1.23), and 1.05 for trans-palmitoleic (95% CI: 0.91, 1.20) acids. Circulating heptadecanoic acid was associated with lower CVD mortality (extreme-quintile HR: 0.77; 95% CI: 0.61, 0.98), especially stroke mortality, with a 42% lower risk when comparing extreme quintiles of heptadecanoic acid concentrations (HR: 0.58; 95% CI: 0.35, 0.97). In contrast, heptadecanoic acid was associated with a higher risk of non-CVD mortality (HR: 1.27; 95% CI: 1.07, 1.52), which was not clearly related to any single subtype of non-CVD death. No significant associations of pentadecanoic, heptadecanoic, or trans-palmitoleic acids were seen for total incident CVD, coronary heart disease, or stroke.
Conclusions
Long-term exposure to circulating phospholipid pentadecanoic, heptadecanoic, or trans-palmitoleic acids was not significantly associated with total mortality or incident CVD among older adults. High circulating heptadecanoic acid was inversely associated with CVD and stroke mortality and potentially associated with higher risk of non-CVD death.
Keywords: cardiovascular diseases, epidemiology, fatty acids, dairy, mortality
INTRODUCTION
Growing evidence has led to uncertainty about the role of dairy fat in health. Although the major saturated fat palmitic acid increases LDL cholesterol, it also lowers triglyceride-rich lipoproteins, increases HDL cholesterol, and has uncertain effects on apolipoprotein C-III (1, 2). Dairy fat also includes medium-chain triglycerides that have been linked to improved health (1), unsaturated fats, and fat-soluble nutrients, including vitamin D, potassium, phosphorus, and calcium (3). Prospective observational studies have generally shown neutral or beneficial associations between self-reported consumption of some dietary sources of dairy fat, such as cheese or butter, with the risk of cardiovascular disease (CVD) and diabetes (4–8). Self-reported consumption may be limited by errors or reporting bias. In addition, beyond common sources such as cheese or butter, dairy fat may be variably consumed in small amounts across a large range of foods in the food supply, including small amounts of unrecognized or underrecognized milk, cream, cheese, or butter in baked goods, pasta and cream sauces, pizza, fried foods, coffee drinks, and numerous other foods. Thus, self-reported estimates may miss substantial sources of dairy fat in the diet.
These limitations can be reduced by the use of circulating fatty acid measurements, which provide objective measures of fatty acid concentrations in the blood. Circulating pentadecanoic (15:0), heptadecanoic (17:0) and trans-palmitoleic (trans-16:1n–7) acids each correlate with self-reported dairy consumption (r ∼ 0.7 based on 24-h recalls or 7-d food records) (9–11) and are significantly increased in response to dairy or whole-fat dairy consumption in controlled trials (12, 13). These fatty acids are also correlated with each other, even though odd-chain saturated fats (pentadecanoic, heptadecanoic) and the naturally occurring ruminant trans fat (trans-palmitoleic acid) have very different metabolism and chemical structures (Figure 1). Yet, only a handful of studies have evaluated the relation of these biomarkers with specific health outcomes, such as diabetes and CVD, with mixed findings (7, 14–20). No previous studies, to our knowledge, have evaluated all-cause mortality. This is particularly relevant given that higher saturated-fat consumption, in comparison with carbohydrates, increases blood LDL but also increases HDL and decreases triglycerides (21), raising questions with regard to the net effect on total and cause-specific mortality endpoints. In addition, previous studies have evaluated only a single fatty acid measure at baseline, which may not account for potential changes over time. In comparison, serial fatty acid measurements allow estimation of long-term exposure (22), which may be most relevant to chronic disease outcomes. To elucidate potential long-term health effects of dairy fat, we assessed the prospective associations of plasma phospholipid concentrations of pentadecanoic, heptadecanoic, and trans-palmitoleic acids, measured serially at baseline, 6 y, and 13 y, with total and cause-specific mortality and incident CVD in the Cardiovascular Health Study (CHS).
FIGURE 1.
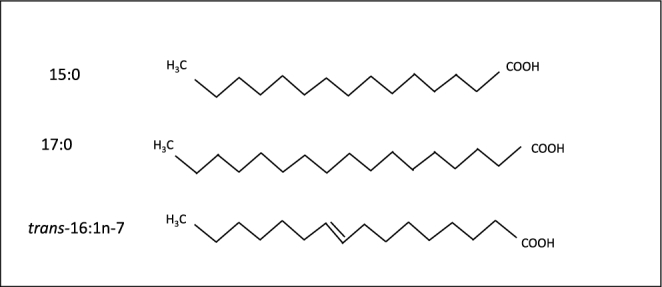
Structure of fatty acids found in dairy fat. Pentadecanoic (15:0) and heptadecanoic (17:0) acids are long-chain SFAs (no carbon-carbon double bonds) containing 15 and 17 carbon atoms respectively. trans-Palmitoleic acid (trans-16:1n–7) is a long-chain MUFA (one carbon-carbon double bond) containing 16 carbon atoms and 1 double bond in the trans configuration. Because mammals cannot synthesize odd-chain or trans-fatty acids in appreciable amounts, circulating concentrations of these fatty acids represent exogenous sources. Among different foods, the strongest correlations are seen for dairy consumption (r ∼ 0.7 based on 24-h recalls or 7-d food records). Biological properties, effects on physiologic risk factors, and relevant molecular pathways of these fatty acids remain mostly unknown.
METHODS
Design and population
The CHS is a population-based, prospective cohort study designed to investigate risk factors for coronary heart disease (CHD) and stroke (primary endpoints) in US older adults (≥65y) (23). In 1989–1990, 5201 noninstitutionalized older adults from 4 US communities (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Allegheny County, Pennsylvania) were randomly recruited from Medicare eligibility lists. From 1992 to 1993, 687 additional black participants were recruited during a supplementary recruitment from the field centers in North Carolina, California, and Pennsylvania using similar methods. Among all eligible adults contacted, 57% were enrolled into the study who were slightly healthier than those who declined to participate. Participants attended annual in-clinic evaluations where repeated phenotypes related to health were obtained through physical examination, diagnostic testing, blood sampling, and questionnaires on health status, medical history, and lifestyle. Information on health status and hospitalizations was also collected via follow-up telephone calls every 6 mo through 2000 and biannually thereafter. All procedures were carried out by trained personnel using standardized procedures. Study protocols were approved by local institutional review boards, and all participants gave written informed consent. Plasma phospholipid fatty acid concentrations were measured in CHS participants with available stored blood samples at the 1992–1993 (n = 3941; 71% of living cohort participants at this visit), 1998–1999 (n = 1400; 48% of living cohort participants), and 2005–2006 (n = 935; 89% of living cohort participants) study visits. In this analysis, we included the 2907 CHS participants who had plasma phospholipid fatty acid measures available in 1992–1993 (the baseline for this analysis) and were free of CVD (Supplemental Figure 1).
Study measures
Fatty acid measurements
Blood was drawn after 12 h of fasting, stored at −70°C, and shipped on dry ice for long-term storage at −80°C. As previously described in detail (24, 25), total lipids were extracted from plasma at the Fred Hutchinson Cancer Research Center Biomarker Laboratory using the methods of Folch et al. (26), and phospholipids were separated from neutral lipids by using one-dimensional, thin-layer chromatography. Fatty acid methyl esters were prepared by direct transesterification by using the methods of Lepage and Roy (27) and separated by using gas chromatography. Quantitative measurements of 45 fatty acids were expressed as a weight percentage of total phospholipid fatty acids measured. Interassay CVs for dairy fatty acids were 4.7% (pentadecanoic), 1.2% (heptadecanoic), and 2.9% (trans-palmitoleic).
Covariates
The CHS assessed repeated measures of major risk factors during study clinic examinations in 1992–1993, 1998–1999, and 2005–2006, including demographic characteristics, medical history, prescription medications, anthropometric measures, blood pressure, and laboratory values, each obtained using standardized equipment and procedures. Leisure activity was assessed by using a modified Minnesota Leisure-Time Activities questionnaire that evaluated the frequency and duration of 15 activities (15). Diet was assessed in 1989–1990 with the use of a validated 99-item food-frequency questionnaire adapted from the National Cancer Institute (28), and again in 1995–1996 with the use of a validated Willett food-frequency questionnaire (29). Food-frequency questionnaires assessed usual intake of various foods including whole-fat dairy products, such as whole milk, cheese, and butter, as well as low-fat dairy milk (1% fat and skim milk), cottage cheese, and yogurt. We used the average of reported intakes from the 2 food-frequency questionnaires to reduce within-person variation and to obtain estimates of long-term intakes of dietary risk factors that could be confounders in the relations of interest such as red meat, fruit, and vegetables.
Ascertainment of endpoints
Participants were followed-up through in-clinic examinations or telephone contacts every 6 mo through 2000 and through biannual telephone contacts thereafter. Vital status follow-up was 100% complete; <1% of all person-time was otherwise missing and censored early. All-cause and cause-specific mortality, as well as all suspected incident (fatal or nonfatal) CHD and stroke events, were assessed and adjudicated by a centralized events committee using available data from interviews, next of kin, death certificates, and medical records including diagnostic tests and consultations. Algorithms and methods for follow-up, confirmation, and classification of deaths, CHD, and stroke have been described (20–22). Total incident CVD comprised myocardial infarction, CHD death, cardiac arrest, other atherosclerotic death, stroke, stroke death, or other CVD death. CHD events comprised myocardial infarction or CHD death. CVD mortality included deaths due to CHD, stroke, other atherosclerotic disease, and other CVD. Non-CVD mortality outcomes included deaths due to cancer, pulmonary diseases, infection, dementia, fractures/trauma, and other causes. The primary outcomes for this analysis were total mortality, cause-specific mortality (CVD, CHD, stroke, and non-CVD mortality), incident CVD, and CVD subtypes (CHD and stroke). In exploratory analysis, we evaluated individual associations with subcategories of non-CVD mortality.
Statistical analysis
We estimated HRs for each outcome with the use of Cox proportional hazards [PROC PHREG procedure for time-dependent variables in SAS (30)] with time-at-risk until death, first event (for incident CVD), or last date of adjudicated follow-up. We found no evidence of violation of the proportional hazards assumption on the basis of Schoenfeld residuals and of Wald tests of time-dependent interactions included in Cox models (i.e., interactions of the predictors and a function of follow-up time). To obtain estimates of long-term exposure with reduced measurement error due to within-person variation over time (22), we used time-varying cumulative average of fatty acid measures: concentrations in 1992–1993 were related to risk from 1992–1998; the average of concentrations in 1992–1993 and 1998–1999 to risk from 1998–2005; and the average of concentrations in 1992–1993, 1998–1999, and 2005–2006 to risk from 2005–2014, with 50% weight assigned to the most recent measurement.
For any missing values of fatty acids, previous values were carried forward. Associations with each circulating fatty acid were evaluated in quintiles as indicator variables and continuously by using the interquintile median range (defined as the difference between the median of the first and fifth quintiles). Potential nonlinear responses were also explored by using restricted cubic splines.
Multivariable analyses were adjusted for age, sex, race, education, and enrollment site at baseline, as well as time-varying risk factors such as smoking status, prevalent diabetes, prevalent hypertension, prevalent atrial fibrillation, physical activity, BMI, waist circumference, and alcohol intake. Final covariates were selected on the basis of biological interest, well-established relations with mortality in older adults, or associations with exposures (25). We imputed missing covariate data (<2% for most lifestyle factors; 8–12% for dietary factors) with the use of single imputation (SAS proc MI) by using multiple demographic and risk variables; results were similar when missing values were excluded. All P values were 2-sided, with P < 0.05 indicating significance. Analyses were performed with the use of Stata, release 12.0 (StataCorp), and SAS, version 9.4 (SAS Institute).
RESULTS
At baseline, mean ± SD age was 74.8 ± 5.2 y, 64% of participants were female, and 87% were white. In unadjusted cross-sectional analysis, quintiles of pentadecanoic, heptadecanoic, and trans-palmitoleic acids had comparable patterns of associations with demographic, clinical, and dietary risk factors. For example, compared with the lowest quintile, participants in the highest quintile of each fatty acid were more likely to be white, have some college education, have diabetes or treated hypertension, and consume less alcohol (Table 1). Mean fatty acid values slightly increased over time (Supplemental Figure 2). Partial Spearman correlations across different fatty acids at any time point ranged from 0.47 to 0.66, consistent with their sharing a common source (Table 2). Repeated within-individual measures of each fatty acid over 3 time points ranged from 0.83 to 0.96.
TABLE 1.
Baseline characteristics of 2907 US adults free of cardiovascular disease at baseline by quintiles of circulating dairy fatty acids in the Cardiovascular Health Study1
| 15:0 | 17:0 | trans-16:1n–7 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| Fatty acids,2 % | 0.11 (0.04,0.12) | 0.15 (0.14,0.16) | 0.20 (0.18,1.41) | 0.31 (0.18,0.35) | 0.40 (0.39,0.42) | 0.48 (0.45,0.74) | 0.11 (0.04,0.12) | 0.15 (0.14,0.16) | 0.20 (0.18,1.41) |
| n | 568 | 572 | 593 | 589 | 587 | 574 | 568 | 572 | 593 |
| Age, y | 73.7 ± 5.0 | 74.9 ± 5.0 | 75.9 ± 5.4 | 73.8 ± 4.7 | 74.8 ± 5.1 | 75.5 ± 5.4 | 73.7 ± 4.6 | 74.7 ± 5.2 | 76 ± 5.4 |
| Race, % white | 73 | 90 | 94 | 88 | 88 | 85 | 84 | 87 | 92 |
| Sex, % male | 36 | 34 | 41 | 27 | 38 | 45 | 27 | 35 | 47 |
| Education, some college degree % | 41 | 46 | 51 | 45 | 47 | 48 | 42 | 49 | 51 |
| Type 2 diabetes, % | 17 | 12 | 15 | 21 | 12 | 15 | 18 | 15 | 12 |
| Treated hypertension, % | 44 | 41 | 38 | 47 | 39 | 37 | 45 | 39 | 37 |
| Physical activity, kcal/wk | 1.1 ± 1.4 | 1.1 ± 1.4 | 1.1 ± 4.2 | 1 ± 1.3 | 1.1 ± 1.5 | 1.1 ± 4.3 | 1 ± 1.2 | 1.2 ± 1.5 | 1.0 ± 4.1 |
| BMI, kg/m2 | 26.9 ± 5 | 26.9 ± 4.4 | 26.1 ± 4.2 | 27.8 ± 4.8 | 26.7 ± 4.5 | 25.5 ± 4.3 | 27.0 ± 5.0 | 26.8 ± 4.6 | 25.9 ± 4.1 |
| Waist circumference, cm | 98 ± 13 | 97 ± 13 | 96 ± 12 | 100 ± 13 | 97 ± 13 | 94 ± 12 | 98 ± 14 | 96 ± 14 | 95 ± 12 |
| Alcohol, servings/wk | 4.1 ± 12 | 1.6 ± 4.0 | 1.3 ± 3.6 | 4.9 ± 12 | 1.5 ± 4.2 | 1.0 ± 2.8 | 3.5 ± 12 | 1.9 ± 4.5 | 1.5 ± 4.0 |
| Intake of whole-fat dairy,3 servings/d | 0.44 ± 0.51 | 0.48 ± 0.46 | 0.70 ± 0.71 | 0.49 ± 0.51 | 0.51 ± 0.52 | 0.66 ± 0.68 | 0.39 ± 0.49 | 0.50 ± 0.47 | 0.72 ± 0.68 |
| Intake of low-fat dairy,3 servings/d | 0.85 ± 0.60 | 0.94 ± 0.64 | 0.98 ± 0.68 | 0.99 ± 0.68 | 0.92 ± 0.63 | 0.95 ± 0.68 | 0.98 ± 0.66 | 0.97 ± 0.69 | 0.93 ± 0.67 |
| Intake of fruit, servings/d | 2.0 ± 1.1 | 2.0 ± 0.9 | 2.1 ± 1.0 | 2.1 ± 1.0 | 2.1 ± 1.0 | 2.1 ± 1.1 | 2.1 ± 1.1 | 2.1 ± 1.0 | 2.0 ± 1.0 |
| Intake of vegetables, servings/d | 3.0 ± 1.7) | 2.9 ± 1.4 | 2.9 ± 1.5 | 3.1 ± 1.5 | 3.0 ± 1.5 | 3.0 ± 1.5 | 3.2 ± 1.6 | 3.0 ± 1.5 | 2.8 ± 1.4 |
| Intake of red meat, servings/d | 0.9 ± 0.6 | 0.8 ± 0.6 | 0.9 ± 0.6 | 0.8 ± 0.6 | 0.8 ± 0.6 | 0.9 ± 0.7 | 0.8 ± 0.6 | 0.8 ± 0.6 | 0.9 ± 0.6 |
| Total circulating trans-fatty acids, % fatty acids | 2.3 ± 0.9 | 2.4 ± 0.7 | 2.1 ± 0.7 | 2.1 ± 0.9 | 2.4 ± 0.8 | 2.1 ± 0.6 | 2.0 ± 0.7 | 2.4 ± 0.8 | 2.3 ± 0.8 |
1Test statistics for univariate models reached significance (P < 0.05) for the following characteristics—15:0: age, race, education, treated hypertension, BMI, whole-fat dairy, low-fat dairy, and total circulating trans-fatty acids; 17:0: age, sex, type 2 diabetes, treated hypertension, BMI, waist circumference, alcohol, whole-fat dairy, vegetables, red meat, and total circulating trans-fatty acids; trans-16:1n–7: age, race, sex, education, type 2 diabetes, treated hypertension, physical activity, BMI, waist circumference, alcohol, whole-fat dairy, vegetables, red meat, and total circulating trans-fatty acids. Q, quintile.
2quintile-specific median(range).
3Whole-fat dairy foods include whole milk, cheese, and butter. Low-fat dairy foods include 2%-fat milk, 1%-fat milk, skim milk, cottage cheese, and yogurt.
TABLE 2.
Correlations between cumulative average plasma circulating fatty acids by time period1
| Percentage of total fatty acids | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 15:0 | 17:0 | trans-16:1n–7 | |||||||
| 1992 | 1998 | 2005 | 1992 | 1998 | 2005 | 1992 | 1998 | 2005 | |
| 15:0 | |||||||||
| 1992 | — | 0.93 | 0.83 | 0.57 | 0.54 | 0.49 | 0.63 | 0.59 | 0.53 |
| 1998 | — | — | 0.96 | 0.53 | 0.56 | 0.54 | 0.59 | 0.63 | 0.59 |
| 2005 | — | — | — | 0.47 | 0.53 | 0.54 | 0.53 | 0.6 | 0.61 |
| 17:0 | |||||||||
| 1992 | — | — | — | — | 0.94 | 0.86 | 0.66 | 0.62 | 0.56 |
| 1998 | — | — | — | — | — | 0.96 | 0.62 | 0.66 | 0.62 |
| 2005 | — | — | — | — | — | — | 0.58 | 0.64 | 0.64 |
| trans-16:1n–7 | |||||||||
| 1992 | — | — | — | — | — | — | — | 0.92 | 0.84 |
| 1998 | — | — | — | — | — | — | — | — | 0.96 |
1Values were estimated by using partial Spearman correlation analysis adjusting for age, sex, and race. n = 2907. All correlations are significant (P < 0.0001).
Total mortality
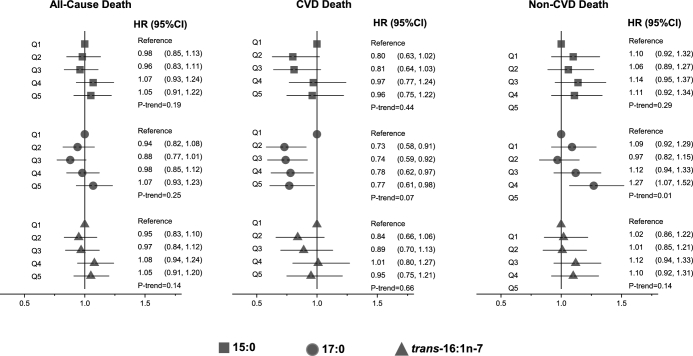
During 22 y (37,498 person-years) of follow-up, 2428 deaths occurred (6.4 deaths per 100 person-years). In multivariate models adjusting for sociodemographic, lifestyle, cardiovascular, and dietary factors, there was no significant association between quintiles of plasma phospholipid pentadecanoic, heptadecanoic, and trans-palmitoleic acids and total mortality (P-trend across quintiles = 0.19, 0.25, and 0.14, respectively; Figure 2, Supplemental Table 1). In continuous analyses, pentadecanoic, but not heptadecanoic or trans-palmitoleic, acid was associated with a higher risk of total mortality, with HRs (95% CIs) per interquintile range of 1.13 (1.04, 1.23), 1.02 (0.91, 1.15), and 1.07 (0.96, 1.19), respectively (Supplemental Table 1). When we explored potential nonlinear associations with the use of cubic spline analysis (31), we found little evidence of nonlinear associations between circulating fatty acids pentadecanoic, heptadecanoic, or trans-palmitoleic acids and risk of all-cause death (P-nonlinearity > 0.05 each).
FIGURE 2.
Risk of total and cause-specific mortality according to quintiles of circulating dairy fatty acids in 2907 older US adults. Values are HRs (95% CIs) estimated by using multivariable Cox proportional hazards models including age (years), sex, race (white or nonwhite), education (less than high school, high school, some college degree or more), enrollment site (4 sites), time-varying smoking status (never, current, or former), alcohol (servings per week), leisure-time physical activity (kilocalories per week), BMI (kg/m2), drug-treated hypertension (yes or no), self-reported general health (excellent, very good, good, or fair/poor), circulating total trans-fatty acids (%), and self-reported consumption of dairy foods, dietary fiber, fruit, vegetables, and red meat. CVD, cardiovascular disease; Q, quintile.
Cardiovascular mortality
When we evaluated cause-specific mortality, plasma phospholipid heptadecanoic acid was associated with lower CVD mortality, with ∼25% lower risk in quintiles 2–5, compared with the lowest quintile (Figure 2, Supplemental Table 1). This inverse association remained significant when evaluated continuously (HR per interquintile range: 0.79; 95% CI: 0.65, 0.97), with little evidence for nonlinearity in spline analyses (P-nonlinearity = 0.22). Assessing CHD and stroke mortality separately, the inverse association appeared strongest for stroke deaths [HR (95% CI): 0.58 (0.35, 0.97) for extreme quintiles and 0.61 (0.40, 0.94) per interquintile range; Supplemental Table 2], with evidence of inverse linear trend across quintiles (P-trend = 0.04; Supplemental Table 2). Pentadecanoic and trans-palmitoleic acids had no significant associations with total CVD, CHD, or stroke mortality; as well as little evidence of nonlinear associations (P- nonlinearity > 0.05 each; Supplemental Tables 1 and 2). All of these relations appeared to be similar in men and women.
Noncardiovascular mortality
In multivariate-adjusted analyses, heptadecanoic acid, but not pentadecanoic or trans-palmitoleic acids, was associated with a higher risk of non-CVD mortality, with an ∼25% higher risk in the highest quintile compared with the lowest (HR: 1.27; 95% CI: 1.07, 1.52; P-trend = 0.01; Figure 2, Supplemental Table 1). Assessed continuously, a 17% higher risk was seen across the interquintile range of heptadecanoic acid (HR: 1.17; 95% CI: 1.01, 1.35), with little evidence for nonlinearity (P-nonlinearity = 0.83). When we explored subcategories of non-CVD mortality, including deaths due to cancer (468 deaths), respiratory diseases (132 deaths), infection (192 deaths), dementia (462 deaths), fractures/trauma (129 deaths), and other causes (212 deaths), no significant associations were evident for any of these subtypes (Supplemental Table 3, Supplemental Figure 3).
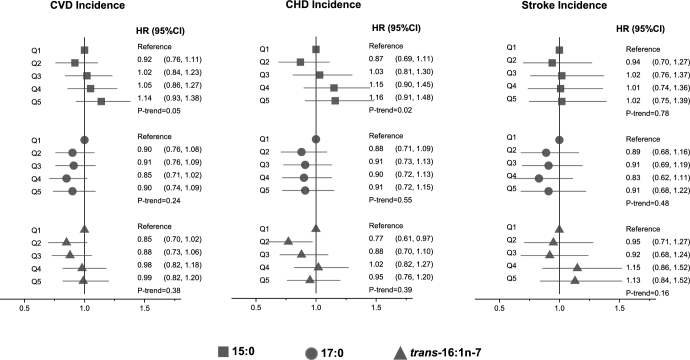
Incident total CVD
When we assessed total incident CVD including fatal and nonfatal events (n = 1301 events), we found no significant associations of circulating phospholipid pentadecanoic, heptadecanoic, or trans-palmitoleic acids with risk (Figure 3, Supplemental Table 4), except that pentadecanoic acid was associated with a trend toward higher CHD incidence across quintiles (P-trend = 0.02), with evidence of nonlinearity in cubic spline analyses (P-nonlinearity < 0.01; Supplemental Figure 4). None of the fatty acids were significantly associated with incident ischemic or hemorrhagic stroke evaluated separately (data not shown). Nonlinear associations with incident CVD were not evident for heptadecanoic or trans-palmitoleic acids (P-nonlinearity >0.05 each). Directions and magnitudes of these findings were generally similar in men and women.
FIGURE 3.
Risk of CVD incidence according to quintiles of circulating dairy fatty acids in 2907 older US adults. Values are HRs (95% CIs) estimated by using multivariable Cox proportional hazards model including age (years), sex, race (white or nonwhite), education (less than high school, high school, some college degree or more), enrollment site (4 sites), time-varying smoking status (never, current, or former), alcohol (servings per week), leisure-time physical activity (kilocalories per week), BMI (kg/m2), drug-treated hypertension (yes or no), self-reported general health (excellent, very good, good, or fair/poor), circulating total trans-fatty acids (%), and self-reported consumption of dairy foods, dietary fiber, fruit, vegetables, and red meat. CHD, coronary heart disease; CVD, cardiovascular disease; Q, quintile.
DISCUSSION
In this prospective study in older US adults, we evaluated, for the first time to our knowledge, the associations of serial measures of circulating fatty acid biomarkers of dairy fat with total mortality, cause-specific mortality, and incident CVD. In multivariable analyses, none of these fatty acids were associated with total mortality. Higher plasma phospholipid heptadecanoic acid was associated with lower CVD mortality, especially stroke mortality, with a 42% lower risk of the latter when comparing participants in extreme quintiles. In contrast, pentadecanoic acid, but not heptadecanoic or trans-palmitoleic acids, was associated with a trend toward a higher risk of total CVD and CHD across quintiles, although this association should be interpreted cautiously because it was not significant within any of the individual quintiles or in analysis using continuous measures. In exploratory analysis, the association between circulating phospholipid pentadecanoic acid and CVD risk, especially CHD risk, appeared to be nonlinear with a U-shaped curve. Finally, heptadecanoic acid was associated with a higher risk of non-CVD mortality, without a clear concentration of risk for any subtype of non-CVD death. These findings expand on previous studies by using repeated circulating fatty acid measures to estimate long-term exposure and examining associations with total and cause-specific mortality and CVD outcomes in a large, prospective cohort of older adults.
Although for decades dairy fat consumption has been hypothesized to be a risk factor for CVD, as well as potentially diabetes, weight gain, and cancer (32–34), little empirical evidence for these effects existed from studies of clinical events. In current years, a growing number of prospective studies have shown generally neutral or protective associations between self-reported dairy foods and dairy fat consumption with the risk of CVD, diabetes, weight gain, and cancers, raising questions about this conventional wisdom (32, 35–40). Dairy fats comprise predominantly SFAs of varying carbon chain lengths with divergent effects on various blood lipids, glucose-insulin homeostasis, and insulin resistance (1, 41), which may partially explain neutral associations. In addition, emerging evidence from randomized controlled trials suggests the differential effects of dairy foods on plasma lipids might be influenced by the presence of milk-fat globule membrane, a tri-layered membrane rich in bioactive phospholipids and proteins enclosing the milk fat (42–44). Consistent with these divergent and complex physiologic effects, our results suggest that overall dairy fat consumption later in life does not significantly influence total mortality but may have associations with specific outcomes.
The lower risk of stroke mortality with higher heptadecanoic acid concentration is consistent with previous large studies evaluating associations between self-reported saturated fat consumption and stroke risk (45–49). For example, recent findings from the Prospective Urban Rural Epidemiology (PURE) Study, including >130,000 diverse participants from 18 countries, showed an RR for total stroke of 0.79 (95% CI: 0.64, 0.98) across quintiles of saturated fat intake (48). Observational studies suggest potential antihypertensive and antithrombotic effects of nutrients in dairy foods, including calcium, vitamin D, phosphorus, and potassium, which could lower stroke risk (50–55). Dairy-specific SFAs also increase HDL cholesterol (1), which could reduce cerebral vascular fragility (45, 56), particular in populations with a relatively low consumption of saturated fat. This potential mechanism is supported by 3 Japanese cohorts in middle-aged adults that reported inverse associations between very low intakes of saturated fat and the risk of hemorrhagic stroke or stroke mortality (45–47), with stronger effects observed in stroke-related lesions in perforating artery regions (45). The lack of significant associations with pentadecanoic acid may be partially due to potential differences in biological processes related to fatty acid absorption (57, 58) or to differing effects of heptadecanoic and pentadecanoic acid on cardiometabolic pathways (59). In the present analysis, we observed stronger associations of heptadecanoic acid with fatal stroke, compared with nonfatal and total stroke, suggesting a possible relation with more severe stroke cases in older adults.
Among 3 previous studies investigating associations of circulating pentadecanoic, heptadecanoic, or trans-palmitoleic acids with stroke, 2 reported no significant associations, whereas one nested case-control study reported inverse associations of plasma phospholipid heptadecanoic, but not pentadecanoic, acid with stroke, with stronger associations in women than in men (60). These previous studies evaluated predominantly middle-aged US or Swedish adults and were limited to a single baseline fatty acid measure. Our findings extend these previous results by using repeated measures to estimate long-term exposure in older adults, in whom the risk of stroke is greatest, and by evaluating total and fatal stroke separately. Our data highlight the need for additional studies to elucidate potential mechanisms underlying the observed relation between dairy fatty acids and stroke risk.
Overall, our findings do not support harmful associations of dairy fat consumption for incident CVD events later in life. These results are supported by cohorts evaluating self-reported whole-fat dairy consumption. In a meta-analysis including ∼76,000 middle-aged participants with ∼5500 CVD events, pooled RRs (95% CIs) per 200 g whole-fat dairy intake/d were 0.93 (0.84, 1.03) for total CVD and 0.99 (0.93, 1.05) for CHD (61). In a meta-analysis of 4 cohorts that used biomarkers and included a total ∼5000 middle-aged adults with ∼2200 CHD events, pooled extreme-tertile RRs (95% CIs) for CHD were 0.94 (0.67, 1.32) for pentadecanoic acid, 0.77 (0.63, 0.93) for heptadecanoic acid, and 0.96 (0.86, 1.08) for trans-palmitoleic acid (7). In light of the previous evidence, the potential higher risk of CHD we observed across quintiles of pentadecanoic acid should be interpreted cautiously, particularly because associations were not seen within individual quintiles or when evaluated continuously. Taken together, the findings from our investigation and previous studies suggest no major effects of dairy fatty acids on CVD risk.
The observed positive association of heptadecanoic acid with a higher risk of non-CVD mortality warrants further investigation. Evidence for effects of dairy fat on cancer mortality, dementia, or inflammatory conditions has been mixed (41, 62–65). In our analysis, higher mortality risk did not appear to be concentrated in any one cause of non-CVD death. Because of the absence of an a priori hypothesis related to non-CVD outcomes, these findings should be interpreted cautiously. Additional studies are needed to further evaluate potential effects of heptadecanoic acid on non-CVD mortality outcomes.
Our study has important strengths. The use of serial biomarker measurements provided objective measures of long-term exposure free from memory recall, while reducing measurement error due to changes in exposure over time. The prospective cohort design minimized recall and selection bias, established temporality, and minimized the possibility of reverse causation. Adjustment for major risk factors at the time of each fatty acid measurement reduced the potential for confounding. The broad, community-based recruitment strategy increased generalizability.
Potential limitations deserve consideration. Although the use of biomarkers has several advantages, circulating fatty acid measures may reflect both dietary intake and metabolism and do not distinguish between different food sources of dairy fat. In addition, 3 serial fatty acid measures included in our analysis may not fully capture potential changes in dairy fat intake over 22 y of follow up. Evidence from animal studies suggests that pentadecanoic acid and heptadecanoic acid may be endogenously produced by elongation of propionic acid (3:0) and heptanoic acid (7:0) (58, 66), or by α-oxidation of stearic acid (18:0) (67). Also, we cannot exclude the possibility of residual confounding, particularly by other components in dairy foods, such protein, lactose, or minerals, or by unmeasured or imprecisely measured confounders. Although events were centrally adjudicated, some deaths may have been misclassified, which would probably be random with respect to fatty acid measures and may lead to potential attenuation of true relations. CHS data were not initially generated to address the aims of the present analysis. The CHS included older men and women, and results may not be generalizable to younger populations.
In summary, our prospective investigation of older adults suggests no major associations between long-term exposure to circulating dairy fatty acids and total mortality, with a potential protective association of heptadecanoic acid with CVD and stroke mortality and a potential higher risk for heptadecanoic acid and non-CVD death.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—MCdOO and DM: designed the research (project conception, development of overall research plan, and study oversight) and wrote the manuscript; MCdOO, DM, RL, IBK, XS, and DSS: conducted the research (hands-on conduct of the experiments and data collection); MCdOO: analyzed data or performed statistical analysis; MCdOO, DM, RL, IBK, XS, and DSS: revised the manuscript and had primary responsibility for the final content; and all authors: read and approved the final manuscript. DM reports ad hoc honoraria or consulting from Life Sciences Research Organization, Astra Zeneca, Boston Heart Diagnostics, Global Organization for EPA and DHA Omega–3, Royal DSM, Nutrition Impact, Haas Avocado Board, and Pollock Communications; scientific advisory board of Omada Health and Elysium Health; and chapter royalties from UpToDate. DM also reports patents US8889739 and US9987243 to Tufts University (unlicensed), listing DM as a co-inventor, for use of trans-palmitoleic acid to prevent and treat insulin resistance, type 2 diabetes, and related conditions, as well as reduce metabolic risk factors. The remaining authors had no conflicts of interest to disclose.
Notes
Supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086 and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by R01AG023629 from the National Institute on Aging. MCdOO was supported by NIH/NHLBI R01HL085710 and NIH/NHLBI 3R01HL085710-07S1; DM was supported by NIH/NHLBI R01HL085710, NIH/NHLBI R01HL115189, NIH/NHLBI R01HL130735, and NIH/NHLBI R01HL135920; the Bill & Melinda Gates Foundation (OPP1099505); and the Federal Emergency Management Agency (EMW-2014-FP-00612).
Supplemental Figures 1–4 and Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used:
- CHS
Cardiovascular Health Study
- CHD
coronary heart disease
- CVD
cardiovascular disease.
REFERENCES
- 1. Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J of Clin Nutr 2003;77(5):1146–55. [DOI] [PubMed] [Google Scholar]
- 2. Sanders TA. Fat and fatty acid intake and metabolic effects in the human body. Ann Nutr Metab 2009;55(1–3):162–72. [DOI] [PubMed] [Google Scholar]
- 3. de Oliveira Otto MC, Mozaffarian D, Kromhout D, Bertoni AG, Sibley CT, Jacobs DR Jr, Nettleton JA. Dietary intakes of saturated fat by food source and incident cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2012;96:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen M, Sun Q, Giovannucci E, Mozaffarian D, Manson JE, Willett WC, Hu FB. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med 2014;12:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qin LQ, Xu JY, Han SF, Zhang ZL, Zhao YY, Szeto IM. Dairy consumption and risk of cardiovascular disease: an updated meta-analysis of prospective cohort studies. Asia Pac J Clin Nutr 2015;24(1):90–100. [DOI] [PubMed] [Google Scholar]
- 6. Pimpin L, Wu JH, Haskelberg H, Del Gobbo L, Mozaffarian D. Is butter back? A systematic review and meta-analysis of butter consumption and risk of cardiovascular disease, diabetes, and total mortality. PloS One 2016;11(6):e0158118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG et al.. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med 2014;160(6):398–406. [DOI] [PubMed] [Google Scholar]
- 8. de Oliveira Otto MC, Mozaffarian D, Kromhout D, Bertoni AG, Sibley CT, Jacobs DR Jr., Nettleton JA. Dietary intake of saturated fat by food source and incident cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2012;96(2):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolk A, Furuheim M, Vessby B. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J Nutr 2001;131(3):828–33. [DOI] [PubMed] [Google Scholar]
- 10. Brevik A, Veierod MB, Drevon CA, Andersen LF. Evaluation of the odd fatty acids 15:0 and 17:0 in serum and adipose tissue as markers of intake of milk and dairy fat. Eur J Clin Nutr 2005;59(12):1417–22. [DOI] [PubMed] [Google Scholar]
- 11. Wolk A, Vessby B, Ljung H, Barrefors P. Evaluation of a biological marker of dairy fat intake. Am J Clin Nutr 1998;68(2):291–5. [DOI] [PubMed] [Google Scholar]
- 12. Abdullah MM, Cyr A, Lepine MC, Labonte ME, Couture P, Jones PJ, Lamarche B. Recommended dairy product intake modulates circulating fatty acid profile in healthy adults: a multi-centre cross-over study. Br J Nutr 2015;113(3):435–44. [DOI] [PubMed] [Google Scholar]
- 13. Golley RK, Hendrie GA. Evaluation of the relative concentration of serum fatty acids C14:0, C15:0 and C17:0 as markers of children's dairy fat intake. Ann Nutr Metab 2014;65(4):310–6. [DOI] [PubMed] [Google Scholar]
- 14. Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, Siscovick DS, Nettleton JA. trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2013;97(4):854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med 2010;153(12):790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB, Crowe FL, Huerta JM, Guevara M, Beulens JW et al.. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol 2014;2(10):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun Q, Ma J, Campos H, Hu FB. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr 2007;86(4):929–37. [DOI] [PubMed] [Google Scholar]
- 18. Warensjo E, Jansson JH, Cederholm T, Boman K, Eliasson M, Hallmans G, Johansson I, Sjogren P. Biomarkers of milk fat and the risk of myocardial infarction in men and women: a prospective, matched case-control study. Am J Clin Nutr 2010;92(1):194–202. [DOI] [PubMed] [Google Scholar]
- 19. Aslibekyan S, Campos H, Baylin A. Biomarkers of dairy intake and the risk of heart disease. Nutr Metab Cardiovasc Dis 2012;22(12):1039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khaw KT, Friesen MD, Riboli E, Luben R, Wareham N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: the EPIC-Norfolk prospective study. PLoS Med 2012;9(7):e1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77(5):1146–55. [DOI] [PubMed] [Google Scholar]
- 22. Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149(6):531–40. [DOI] [PubMed] [Google Scholar]
- 23. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1(3):263–76. [DOI] [PubMed] [Google Scholar]
- 24. Mozaffarian D, Lemaitre RN, King IB, Song X, Spiegelman D, Sacks FM, Rimm EB, Siscovick DS. Circulating long-chain omega-3 fatty acids and incidence of congestive heart failure in older adults: the Cardiovascular Health Study: a cohort study. Ann Intern Med 2011;155(3):160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mozaffarian D, Lemaitre RN, King IB, Song X, Huang H, Sacks FM, Rimm EB, Wang M, Siscovick DS. Plasma phospholipid long-chain omega-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann Intern Med 2013;158(7):515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226(1):497–509. [PubMed] [Google Scholar]
- 27. Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 1986;27(1):114–20. [PubMed] [Google Scholar]
- 28. Kumanyika S, Tell GS, Shemanski L, Polak J, Savage PJ. Eating patterns of community-dwelling older adults: the Cardiovascular Health Study. Ann Epidemiol 1994;4(5):404–15. [DOI] [PubMed] [Google Scholar]
- 29. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 30. Allison PD. Survival analysis using SAS: a practical guide. 2nd ed Cary (NC): SAS Institute, Inc.;2010. [Google Scholar]
- 31. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8(5):551–61. [DOI] [PubMed] [Google Scholar]
- 32. USDA; US Department of Health and Human Services. Dietary guidelines for Americans, 2010. 7th ed Washington (DC): US Government Printing Office;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moorman PG, Terry PD. Consumption of dairy products and the risk of breast cancer: a review of the literature. Am J Clin Nutr 2004;80(1):5–14. [DOI] [PubMed] [Google Scholar]
- 34. FAO. Milk and dairy products in human nutrition. In: Muehlhoff E., Bennett A., McMahon D., editors. (ESN) ND, editor. Rome (Italy); FAO; 2013. [Google Scholar]
- 35. O'Sullivan TA, Hafekost K, Mitrou F, Lawrence D. Food sources of saturated fat and the association with mortality: a meta-analysis. Am J Public Health 2013;103(9):e31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwingshackl L, Schwedhelm C, Hoffmann G, Lampousi AM, Knuppel S, Iqbal K, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr 2017;105(6):1462–73. [DOI] [PubMed] [Google Scholar]
- 37. Tong X, Chen GC, Zhang Z, Wei YL, Xu JY, Qin LQ. Cheese consumption and risk of all-cause mortality: a meta-analysis of prospective studies. Nutrients 2017;9(1):63, doi: 10.3390/nu9010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soedamah-Muthu SS, Ding EL, Al-Delaimy WK, Hu FB, Engberink MF, Willett WC, Geleijnse JM. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr 2011;93(1):158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gijsbers L, Ding EL, Malik VS, de Goede J, Geleijnse JM, Soedamah-Muthu SS. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am J Clin Nutr 2016;103(4):1111–24. [DOI] [PubMed] [Google Scholar]
- 40. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364(25):2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids 2010;45(10):893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosqvist F, Smedman A, Lindmark-Mansson H, Paulsson M, Petrus P, Straniero S, Rudling M, Dahlman I, Riserus U. Potential role of milk fat globule membrane in modulating plasma lipoproteins, gene expression, and cholesterol metabolism in humans: a randomized study. Am J Clin Nutr 2015;102(1):20–30. [DOI] [PubMed] [Google Scholar]
- 43. Rogers TS, Demmer E, Rivera N, Gertz ER, German JB, Smilowitz JT, Zivkovic AM, Van Loan MD. The role of a dairy fraction rich in milk fat globule membrane in the suppression of postprandial inflammatory markers and bone turnover in obese and overweight adults: an exploratory study. Nutr Metab 2017;14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Demmer E, Van Loan MD, Rivera N, Rogers TS, Gertz ER, German JB, Smilowitz JT, Zivkovic AM. Addition of a dairy fraction rich in milk fat globule membrane to a high-saturated fat meal reduces the postprandial insulinaemic and inflammatory response in overweight and obese adults. J Nutr Sci 2016;5:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamagishi K, Iso H, Kokubo Y, Saito I, Yatsuya H, Ishihara J, Inoue M, Tsugane S. Dietary intake of saturated fatty acids and incident stroke and coronary heart disease in Japanese communities: the JPHC study. Eur Heart J 2013;34(16):1225–32. [DOI] [PubMed] [Google Scholar]
- 46. Yamagishi K, Iso H, Yatsuya H, Tanabe N, Date C, Kikuchi S, Yamamoto A, Inaba Y, Tamakoshi A. Dietary intake of saturated fatty acids and mortality from cardiovascular disease in Japanese: the Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC) Study. Am J Clin Nutr 2010;92(4):759–65. [DOI] [PubMed] [Google Scholar]
- 47. Iso H, Sato S, Kitamura A, Naito Y, Shimamoto T, Komachi Y. Fat and protein intakes and risk of intraparenchymal hemorrhage among middle-aged Japanese. Am J Epidemiol 2003;157(1):32–9. [DOI] [PubMed] [Google Scholar]
- 48. Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, Iqbal R, Kumar R, Wentzel-Viljoen E, Rosengren A et al.. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390(10107):2050–62. [DOI] [PubMed] [Google Scholar]
- 49. de Goede J, Soedamah-Muthu SS, Pan A, Gijsbers L, Geleijnse JM. Dairy consumption and risk of stroke: a systematic review and updated dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc 2016;5(5):e002787 doi: 10.1161/JAHA.115.002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alonso A, Nettleton, Ix JA, de Boer JH, Folsom IH, Bidulescu AR, Kestenbaum A, Chambless BR, Jacobs LE, DR Jr. Dietary phosphorus, blood pressure, and incidence of hypertension in the Atherosclerosis Risk in Communities Study and the Multi-Ethnic Study of Atherosclerosis. Hypertension 2010;55(3):776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sacks FM, Willett WC, Smith A, Brown LE, Rosner B, Moore TJ. Effect on blood pressure of potassium, calcium, and magnesium in women with low habitual intake. Hypertension 1998;31(1):131–8. [DOI] [PubMed] [Google Scholar]
- 52. Geleijnse JM, Kok FJ, Grobbee DE. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. J Hum Hypertens 2003;17(7):471–80. [DOI] [PubMed] [Google Scholar]
- 53. Phelan M, Kerins D. The potential role of milk-derived peptides in cardiovascular disease. Food Funct 2011;2(3–4):153–67. [DOI] [PubMed] [Google Scholar]
- 54. Massey LK. Dairy food consumption, blood pressure and stroke. J Nutr 2001;131(7):1875–8. [DOI] [PubMed] [Google Scholar]
- 55. Alvarez-Leon EE, Roman-Vinas B, Serra-Majem L. Dairy products and health: a review of the epidemiological evidence. Br J Nutr 2006;96(Suppl 1):S94–9. [DOI] [PubMed] [Google Scholar]
- 56. Yamagishi K, Iso H, Tsugane S. Saturated fat intake and cardiovascular disease in Japanese population. J Atheroscler Thrombs 2015;22(5):435–9. [DOI] [PubMed] [Google Scholar]
- 57. Jenkins B, West JA, Koulman A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic acid (c15:0) and heptadecanoic acid (c17:0) in health and disease. Molecules 2015;20(2):2425–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pfeuffer M, Jaudszus A. Pentadecanoic and heptadecanoic acids: multifaceted odd-chain fatty acids. Adv Nutr 2016;7(4):730–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zheng JS, Sharp SJ, Imamura F, Koulman A, Schulze MB, Ye Z, Griffin J, Guevara M, Huerta JM, Kroger J et al.. Association between plasma phospholipid saturated fatty acids and metabolic markers of lipid, hepatic, inflammation and glycaemic pathways in eight European countries: a cross-sectional analysis in the EPIC-InterAct study. BMC Med 2017;15(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Warensjo E, Smedman A, Stegmayr B, Hallmans G, Weinehall L, Vessby B, Johansson I. Stroke and plasma markers of milk fat intake—a prospective nested case-control study. Nutr J 2009;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, Soedamah-Muthu SS. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol 2017;32(4):269–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sieri S, Krogh V, Ferrari P, Berrino F, Pala V, Thiebaut AC, Tjonneland A, Olsen A, Overvad K, Jakobsen MU et al.. Dietary fat and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 2008;88(5):1304–12. [DOI] [PubMed] [Google Scholar]
- 63. Qiu W, Lu H, Qi Y, Wang X. Dietary fat intake and ovarian cancer risk: a meta-analysis of epidemiological studies. Oncotarget 2016;7(24):37390–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen H, O'Reilly E, McCullough ML, Rodriguez C, Schwarzschild MA, Calle EE, Thun MJ, Ascherio A. Consumption of dairy products and risk of Parkinson's disease. Am J Epidemiol 2007;165(9):998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington (DC): American Institute for Cancer Research;2007. [Google Scholar]
- 66. Weitkunat K, Schumann S, Petzke KJ, Blaut M, Loh G, Klaus S. Effects of dietary inulin on bacterial growth, short-chain fatty acid production and hepatic lipid metabolism in gnotobiotic mice. J Nutr Biochem 2015;26(9):929–37. [DOI] [PubMed] [Google Scholar]
- 67. Jenkins BJ, Seyssel K, Chiu S, Pan PH, Lin SY, Stanley E, Ament Z, West JA, Summerhill K, Griffin JL et al.. Odd chain fatty acids; new insights of the relationship between the gut microbiota, dietary intake, biosynthesis and glucose intolerance. Sci Rep 2017;7:44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.