Abstract
Members of Mas related G-protein coupled receptors (Mrgpr) are known to mediate itch. To date, several compounds have been shown to activate these receptors, including chloroquine, a common antimalarial drug, and peptides of the RF-amide family. However, specific ligands for these receptors are still lacking and there is a need for novel compounds that can be used to modulate the receptors in order to understand the cellular and molecular mechanism in which they mediate itch. Some cone snail venoms were previously shown to induce itch in mice. Here, we show that the venom of Conus textile induces itch through activation of itch-sensing sensory neurons, marked by their sensitivity to chloroquine. Two RF-amide peptides, CNF-Tx1 and CNF-Tx2, were identified in a C. textile venom gland transcriptome. These belong to the conorfamide family of peptides which includes previously described peptides from the venoms of Conus victoriae (CNF-Vc1) and Conus spurius (CNF-Sr1 and CNF-Sr2). We show that CNF-Vc1 and CNF-Sr1 activate MrgprC11 whereas CNF-Vc1 and CNF-Tx2 activate the human MrgprX1 (hMrgprX1). The peptides CNF-Tx1 and CNF-Sr2 do not activate MrgprC11 or hMrgprX1. Intradermal injection of CNF-Vc1 and CNF-Tx2 into the cheek of a transgenic mouse expressing hMrgprX1 instead of endogenous mouse Mrgprs resulted in itch-related scratching thus demonstrating the in vivo activity of these peptides. Using truncated analogues of CNF-Vc1, we identified amino acids at positions 7 – 18 as important for activity against hMrgprX1. The conopeptides reported here are tools that can be used to advance our understanding of the cellular and molecular mechanism of itch mediated by Mrgprs.
Keywords: Conopeptide, Conorfamide, RF-amide, hMrgprX1, MrgprC11, itch
1. Introduction
Itch is defined as an unpleasant sensation that causes the reflex to scratch (1–3), and can be acute, like an itch caused by an insect bite, or chronic, as in the case of itching from atopic dermatitis (4, 5). Severe chronic itch can greatly reduce quality of life (3, 6, 7). The neurological processes of itch detection can be separated into two classes: histamine-dependent itch that is induced by the activation of histamine receptors and histamine-independent itch that does not require the activation of histamine receptors (8, 9).
Mas-related G-protein coupled receptors (Mrgpr) are a family of G protein coupled receptors, with many members linked to the detection of histamine-independent itch (10). For example MrgprC11 was shown to mediate itch in mice (11, 12) whereas the activation of human MrgprX1 (hMrgprX1) was shown to induce itch in humans (11). To date several Mrgpr ligands have been identified including chloroquine, and members of the RF-amide peptide family that are characterized by RF(Y)G or RF(Y)-amide at the C-terminus (13). However, the paucity of selective Mrgpr ligands and the fact that the Mrgrpr ligand binding domain structure has not yet been definitively resolved necessitate the discovery of structurally diverse Mrgpr ligands with varying degrees of specificity and potency. These Mrgpr ligands will be useful in our understanding of the cellular and molecular mechanism of itch mediated by this receptor family.
In our effort to identify new ligands for Mrgprs, we searched the venom from several species of cone snails. Cone snails are venomous marine animals found in tropical waters around the world. There are ~750 cone snail species (14), which can be classified into three major groups on the basis of their prey: the fish hunters (piscivores), the mollusk hunters (molluscivores) and the worm hunters (vermivores) (15–17). All cone snail species use venom for various biotic interactions, including prey capture (18, 19). The venom is primarily composed of small peptidic compounds, known as conotoxins or conopeptides, with a diverse array of pharmacological targets (20–22). Cone snail venom has proven to be a valuable source of bioactive peptides with mammalian targets. Conopeptides that target voltage-gated ion channels, ligand-gated ion channels, GPCR’s and transporters have already been described (22). Previous studies have shown evidence indicating that components of cone snail venom could potentially activate itch receptors. Conopeptides purified from the venoms of Conus geographus and Conus textile produce effects in mice suggestive of itch (23, 24), however, the identity and mechanism of action of the itch-inducing components are yet to be investigated.
Here we show that (i) the venom of the mollusk-hunter C. textile is capable of inducing itch in mice (ii) its venom, and that of a related species C. victoriae, contains a class of RF-amide peptides (conorfamides), which (iii) cause itch in mice via agonism of hMrgprX1 in a humanized transgenic mouse expressing hMrgprX1 instead of mouse Mrgprs in primary sensory neurons.
2. Materials and Methods
2.1. Mouse behavior
Protocols for experiments involving the use of live animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Washington University in St. Louis. Male mice 8 – 10 weeks old were used for all behavioral experiments. Mice were acclimated to the handling during cheek injection and the testing environment for three days prior to the experiment. Injection was not performed during acclimatization. During the experiment, a 20 µL solution containing 70 µg of venom extract or 20 µL solution containing 10 nmol of the peptide was injected intradermally into the mouse cheek. The mouse behavior was videotaped for 30 min and the number of scratching bouts on the injected cheek over the 30 min period was counted. Different sets of mice were used for testing each venom or peptide.
2.2. Cone snail venom extractions
Conopeptides from C. geographus (23) and C. textile (24) were previously shown to elicit itch in mice, thus these snails were selected for this study. C. geographus is a fish hunter while C. textile is a mollusk hunter. These snails represent two of the three major Conus feeding group. Conus miles, a worm hunter was chosen to represent this group. Cone snails were collected from the Philippines. Venom from C. geographus, C. miles and C. textile were extracted from dissected venom glands by manual extrusion. Crude venom extracts were lyophilized and stored at −20 °C until use.
2.3. C. textile venom gland transcriptome
A single live adult specimen of C. textile was collected from Oahu, Hawaii; the venom gland was dissected and stored in RNA-later at −80 °C. RNA extraction and sequencing of a C. textile venom gland transcriptome was performed as described previously (25).
Using the hmmbuild tool from the HMMER 3.0 package, a profile-Hidden Markov Model (pHMM) was built based on the prepropeptide sequence of CNF-Vc1, an RF-amide previously identified in the venom of C. victoriae. The hmmsearch tool was then applied to the C. textile venom gland transcriptome database of translated open reading frames. Each identified transcript was then manually examined for assembly fidelity using the Map-to-Reference tool of Geneious, version 8.1.7 (26).
2.4. Cell culture and transfection
KNRK cells plated on glass coverslips were grown in Dulbecco’s modified Eagle’s media (DMEM; Sigma, St. Louis, MO) containing 10% (v/v) FBS, 100 units/mL penicillin, 100 µg/mL streptomycin, and 2 mM L-glutamine at 37 °C an d 5% CO2. One day before the experiment, the cells were plated on poly-D-lysine coated coverslips and transfected with human MrgprX1 (hMrgprX1) or MrgprC11 using lipofectamine 2000 (Invitrogen, Carlsbad, CA) and following the manufacturer’s recommended protocol. Briefly, 2.5 µL of lipofectamine 2000 and a solution containing 1 µg of hMrgprX1 or MrgprC11 plasmid were separately diluted with FBS-free DMEM to a total volume of 50 µL and allowed to stand for 5 min. After 5 min, the lipofectamine and plasmid solutions were mixed and the lipofectamine-plasmid complex was formed for 30 minutes at room temperature. After 30 min, the lipofectamine-plasmid complex was added to the cells in a 3.5 cm culture dish containing 2 mL of growth media. The transfection reaction proceeded overnight in a humidified incubator at 37 °C and 95% CO 2.
2.5. Dorsal root ganglion (DRG) neuron isolation and culture
Protocols for experiments involving the use of live animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Washington University in St. Louis. DRG neurons from wild type (WT) C57/B6J mice were dissociated, plated on poly-D-lysine coated coverslips and cultured in DMEM/Nutrient mixture F-12 Ham (Sigma, St. Louis, MO) containing 15 mM HEPES, 15 mM NaHCO3, 10% (v/v) FBS, 100 units/mL penicillin, 100 µg/mL streptomycin, 25 µg/µL NGF and 50 µg/µL GDNF. Neurons were grown overnight in a humidified incubator at 37 °C and 5% CO 2.
2.6. Calcium imaging
KNRK cells on glass coverslips, transfected with either MrgprC11 or hMrgprX1 were washed 3 times with calcium imaging buffer (CIB) (130 mM NaCl, 3 mM KCl, 2.5 mM CaCl2, 0.6 mM MgCl2, 10 mM HEPES, 10 mM glucose, 1.2 mM NaHCO3, pH 7.4). The cells were then incubated for 30 min in a solution of CIB containing 500 ng/mL FURA-2 AM (Invitrogen, Carlsbad, Ca). After 30 min, the cells were washed 3 times with CIB and allowed to recover for 10 min before starting the calcium imaging experiments. Calcium imaging experiments were performed by first applying 200 µL of 2 µM BAM 8–22, an agonist of MrgprC11 and hMrgprX1 to identify the cells expressing the receptor. The cells were then washed with CIB for 5 minutes. After 5 minutes of washing, the peptide (10 µM final concentration) was then applied to determine whether it activates MrgprC11 or hMrgprX1. Experiments for the concentration-dependent activation of hMrgprX1 were performed by first applying the peptides. The cells were washed with CIB for 5 min and then 2 µM BAM 8–22 was applied to identify cells that express hMrgprX1. The percentage of cells activated by peptide against cells activated by BAM 8–22 was determined for each peptide concentration.
Calcium imaging of DRG neurons proceeded as described for KNRK cells transfected with either MrgprC11or hMrgprX1. A 200 µL solution of 1 mM chloroquine (CQ) was applied to identify the MrgprA3 population. The cells were then washed for 5 min after which, 50 µL of 3.3 µg/µL solution of crude venom was applied. The percentage of CQ+ cells activated by venom against the total number of venom+ cells was recorded.
2.7. Peptide Synthesis
Synthesis of CNF-Vc1, CNF-Sr1 and CNF-Sr2 has been previously described (27–29). Here we describe the synthesis of CVF-Vc1(7–18), CNF-Vc1(15–18), CNF-Tx1 and CNF-Tx2. Peptides were synthesized using an Apex 396 automated peptide synthesizer (AAPPTec; Louisville, KY) applying standard solid-phase Fmoc (9-fluorenylmethyloxy carbonyl) protocols. Peptides was constructed on preloaded resins as follows: CNF-Vc1(15–18) and CNF-Vc1(7–18) on Fmoc-Phe-Wang resin (substitution: 0.28 mmol/g; Peptides International, Louisville, KY), CNF-Tx1 on Fmoc-Tyr(tBu)-Wang resin (substitution: 0.3 mmol/g; Peptides International), CNF-Tx2 on Fmoc-Ile-Wang resin (substitution: 0.34 mmol/g; Peptides International). All standard amino acids were purchased from AAPPTec. Side-chain protection for the amino acids was as follows: Asp, t-butyl ester (OtBu); His and Asn, trityl (Trt); Lys and Trp, tert-butyloxycarbonyl (Boc); Ser and Tyr, tert-butyl ether (tBu); Arg, N-alpha-Fmoc-N-g-(2,2,4,6,7pentamethyldihydrobenzofuran-5-sulfonyl) (Pbf). Tenfold excess of standard amino acids was used except for Fmoc-Gla(OtBu)2, for which threefold excess was used. The coupling activation was achieved with one equivalent of 0.4 M benzotriazol-1-yloxytripyrrolidinophosphonium hexafluorophosphate (PyBOP) and two equivalents of 2 M N,N-diisopropylethyl amine (DIPEA) in N-methyl-2 pyrrolidone (NMP) as the solvent. Each coupling reaction was conducted for 60 min except for the special amino acids for which the reaction was conducted for 90 min. Fmoc deprotection was carried out for 20 min with 20% piperidine in dimethylformamide (DMF).
2.8. Peptide cleavage and purification.
Peptides were cleaved from 24–54 mg of resin by 2.5–3.5 h treatment with 1 mL of Reagent K (TFA/water/ phenol/ thioanisole/1,2-ethanedithiol 82.5/5/5/5/2.5 by volume). Next, the cleavage mixture was filtered and precipitated with 10 mL of cold methyl-tert-butyl ether (MTBE). The crude peptide was then precipitated by centrifugation at 7,000 x g for 6 min and washed once with 10 mL cold MTBE. The crude peptides were purified by reversed-phase (RP) HPLC using a semi-preparative C18 Vydac column (218TP510, 250 ×10 mm, 5-µm particle size) eluted with a linear gradient ranging from 10% to 40% solvent B in 30 min at a flow rate 4 mL/min for CNF-Vc1(15–18), 15% to 45% solvent B in 30 min for CNF-Vc1(7–18), 20% to 50% solvent B in 30 min for CNF-Tx1 and CNF-Tx2. The HPLC solvents were 0.1% (vol/vol) TFA in water (solvent A) and 0.1% TFA (vol/vol) in 90% aqueous acetonitrile (vol/vol) (solvent B). The eluent was monitored by measuring absorbance at 220/280 nm. Purity of the peptide was assessed by analytical C18 Vydac RP-HPLC (218TP54, 250 ×4.6 mm, 5-µm particle size) using the same gradient as described for the semi-prep purification but with a flow rate of 1 mL/min. The peptides were quantified by UV absorbance at 280 nm, using an extinction coefficient (ε) value of 1490 M−1·cm−1 for CNF-Tx1, 5500 M−1·cm−1 for CNF-Tx2, CNF-Vc1(7–18), CNF-Vc1(15–18) was quantified using analytical HPLC, where the area under the peak of CNF-Vc1(15–18) was compared to an area under the peak of a peptide standard. The identity of the peptides was confirmed by MALDI mass spectrometry analysis at the Mass Spectrometry and Proteomics Core Facility at the University of Utah to be as follows: CNF-Vc1(7–18) calculated [MH]+ = 1420.77, determined: [MH]+ = 1420.77; CNF-Vc1(15–18) calculated [MH]+ = 567.34, determined: [MH]+ = 567.34; CNF-Tx1 calculated [MH]+ = 2236.11, determined: [MH]+ = 2236.12; CNF-Tx2 calculated [MH]+ = 2078.16.
3. Results
3.1. Venom from C. textile elicits itch and activates pruriceptive neurons in the mouse dorsal root ganglion (DRG)
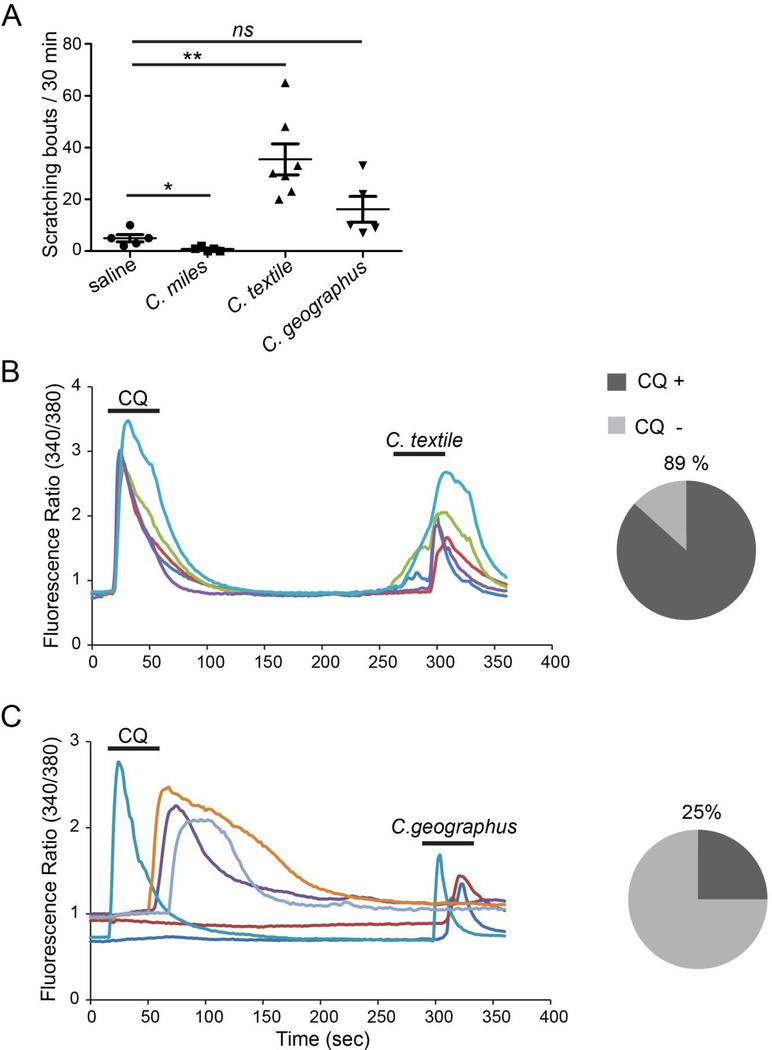
We investigated the venoms of Conus geographus, Conus textile and Conus miles for pruritogenic (itch-inducing) activity. These species were selected for this study because they represent the three major Conus feeding group. Moreover, conopeptides from C. geographus and C. textile were previously shown to induce itch-related scratching in mice (23, 24). Injection of C. textile venom induced scratching in mice, whereas C. geographus did not elicit responses significantly different from saline injected control mice (Fig 1A). Interestingly venom from C. miles induces less scratching when compared with control mice (Fig 1A).
Figure 1.
A) The itch-inducing effects of 20 µL intradermal injection of crude venom extracts (20 µg/µL) from three cone snail species into the cheek of WT (C57/B6J) mice. Each dot represents an individual mouse. Data are presented as mean ± SEM. *, p<0.05**, p<0.005; two tailed unpaired t-test, ns: not significant. The p value for each crude venom compared against the control are: C. miles, p = 0.0187; C. textile, p = 0.0019; and C. geographus, p = 0.0610. B) 89% of the DRG neurons activated by the C. textile venom are CQ sensitive whereas the remaining 11% are non-responsive to chloroquine. The left Ca2+ imaging traces show the representative responses of chloroquine-sensitive neurons to C. textile venom. The pie chart shows the fraction of venom-sensitive neurons that respond to chloroquine C) 25% of DRG neurons activated by venom from C. geographus are CQ sensitive while a majority (75%) did not respond to chloroquine. The left panel shows a representative calcium imaging trace while the right panel shows the percentage of venom-sensitive neurons that respond to chloroquine.
Since the C. textile venom was pruritogenic, we further tested whether this venom activates a population of itch-sensing neurons (pruriceptors) characterized by their sensitivity to the anti-malaria drug chloroquine (CQ). Chloroquine is an agonist of the itch receptor MrgprA3 (10, 30). Further, the CQ sensitive population also expresses MrgprC11 (10, 31), another receptor that mediates itch. The venom from C. geographus was similarly tested to determine whether the behavioral results can be validated by calcium imaging experiments. Calcium imaging experiments showed that 89% of sensory neurons activated by the C. textile venom are also CQ sensitive whereas only 25% of neurons activated by the C. geographus venom are CQ sensitive (Fig 1 B-C). The remainder of neurons; 11% for C. textile and 75% for C. geographus are insensitive to chloroquine. These results mirror our behavioral data showing that C. textile venom is pruritogenic whereas C. geographus venom is not pruritogenic under our experimental conditions. These results suggest that the itching behavior observed when C. textile venom extracts were injected into the mouse cheek is at least partially due to the Mrgpr mediated activity of the CQ sensitive sensory neurons.
3.2. Conorfamides from mollusk hunting cone snails activate hMrgprX1 in vitro and elicits scratching in vivo
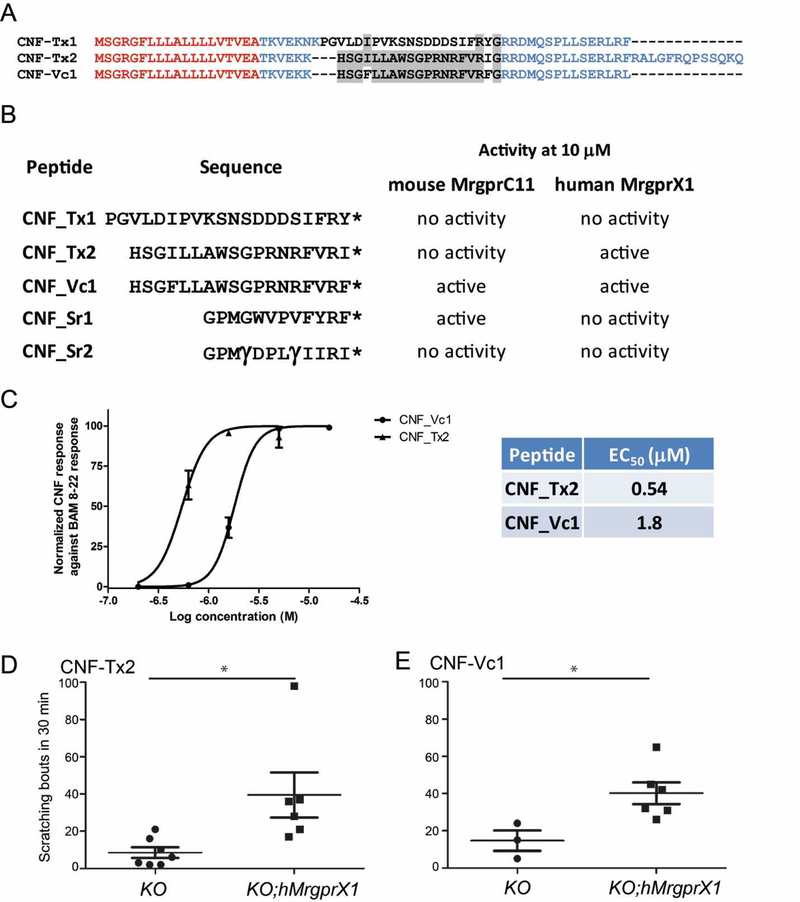
Most of the known peptides that activate MrgprC11 and human MrgprX1 (hMrgprX1) are characterized by a conserved RF(Y)-amide or RF(Y)G motif at the Cterminus (13, 32). An RF-amide peptide, CNF-Vc1, was previously described in the venom of C. victoriae (27), a species closely related to C. textile (33). A search of the C. textile venom gland transcriptome, using a profile-Hidden Markov Model (pHMM) based on CNF-Vc1, revealed two sequences with high sequence similarities (Fig 2 AB): CNF-Tx1 that terminates in RY-amide and CNF-Tx2 with an RI-amide at the C-terminus. A single C.textile specimen was sufficient to achieve our objective of discovering novel RF-amide peptides hence we did not extend our search to other C. textile specimens. Two peptides previously identified from the venom of C. spurius, CNF-Sr1 and CNF-Sr2, also share the RF(Y/I)-amide at the C-terminus (28, 29), but share low sequence similarity with CNF-Vc1, CNF-Tx1 and CNF-Tx2 (Figure 2B).
Figure 2.
A) Sequence alignment of the precursor structure of CNF-Tx1, CNF-Tx2 and CNF-Vc1 (27). Sequences in red denote the signal sequence those in blue are the propeptide sequence and sequences in black are the toxin sequence. Note the conserved C-terminal RF(I)-amide motif. Conserved amino acids between toxin sequences are highlighted in grey. B) Peptides that were tested for activity against MrgprC11 and hMrgprX1. The activity of each peptide against MrgprC11 and hMrgprX1 at 10 µM is indicated. * indicates a C-terminal amide; γ indicates γ-carboxyglutamate. C) Concentration-dependent activation of hMrgprX1 by CNF-Tx2 and CNF-Vc1. Each data point is the average of at least 3 experiments. Error bars represents SEM. D) Cheek injection of CNF-Tx2 (10 nM) and E) CNF-Vc1 (10 nM) into hMrgprX1;Mrgpr-cluster∆−/− (KO;hMrgprX1) mouse induces significant itch-related scratching versus control Mrgprcluster∆−/− (KO). Different sets of mice were used for the CNF-Tx2 and CNF-Vc1 experiments. Each dot represents data from an individual mouse. Data are presented as mean ± SEM. *, P<0.05; two tailed unpaired t-test. The p value for each peptide compared against the control are: CNF-Tx2, p = 0.0217; CNF-Vc1, p = 0.0489
Because the crude venom of C. textile was active on CQ+ sensory neurons which express Mrgprs, we tested these peptides for activity against MrgprC11 and its functional orthologue in human, hMrgprX1. We performed calcium imaging experiments using either MrgprC11 or hMrgprX1 expressed heterelogously in KNRK cells. We found that three of these peptides at 10 µM, were able to activate Mrgprs: CNF-Tx2 activates hMrgprX1; CNF-Sr1 activates MrgprC11; and CNF-Vc1 activates both MrgprC11 and hMrgprX1. CNF-Tx1 and CNF-Sr2 did not activate MrgprC11 or hMrgprX1 at the concentration used in these experiments (Fig. 2B).
hMrgprX1 mediates histamine-independent itch in humans (10, 32, 34), therefore we further investigated the activity of CNF-Vc1 and CNF-Tx2 against this receptor. Experiments revealed that both conopeptides activate the receptor in a concentration dependent manner and that CNF-Tx2 with an RI-amide at the C-terminus is more potent, with an EC50 of 0.54 µM against hMrgprX1, versus 1.8 µM for CNF-Vc1 (Fig 2C). We next tested the itch-inducing effects of CNF-Tx2 and CNF-Vc1 in vivo. For these mouse behavioral experiments, we used a humanized hMrgprX1;Mrgpr-cluster∆−/− transgenic mouse, in which hMrgprX1, instead of mouse Mrgprs, is expressed in primary sensory neurons (10). Intradermal cheek injection of a 20 µL solution containing 10 nmol CNF-Tx2 or CNF-Vc1 induces significant scratching in hMrgprX1;Mrgpr-cluster∆−/− mice versus the control Mrgpr-cluster∆−/− mice (Fig 2 D-E), indicating that both CNF-Tx2 and CNF-Vc1 are able to induce itch sensations in vivo through the activation of hMrgprX1.
3.3. Structure activity relationship (SAR) studies identified structural features important for agonist potency towards hMrgprX1
The conopeptides CNF-Sr1 and CNF-Vc1 activate MrgprC11 while CNF-Vc1 and CNF-Tx2 activate hMrgprX1. The conopeptides CNF-Sr1 and CNF-Vc1 contain the Cterminal RF(Y)-amide motif characteristic of the currently known peptidic ligands of MrgprC11 and hMrgprX1 (13, 35). The conopeptide CNF-Tx2 is highly similar to CNFVc1, but terminates in RI-amide. Interestingly, CNF-Tx2 is more potent than CNF-Vc1 in activating hMrgprX1. The high degree of similarity between CNF-Tx2 and CNF-Vc1 allowed for identification of structural features important for hMrgprX1 activity. Sequence comparison between CNF-Vc1 and CNF-Tx2 showed that these peptides differ in only two sites. The first is a phenylalanine (Phe) to isoleucine (Ile) substitution at position 4 and the second is the same substitution at position 18 (Fig 2A-2B). Thus, these substitutions affect the potency of the peptides against hMrgprX1.
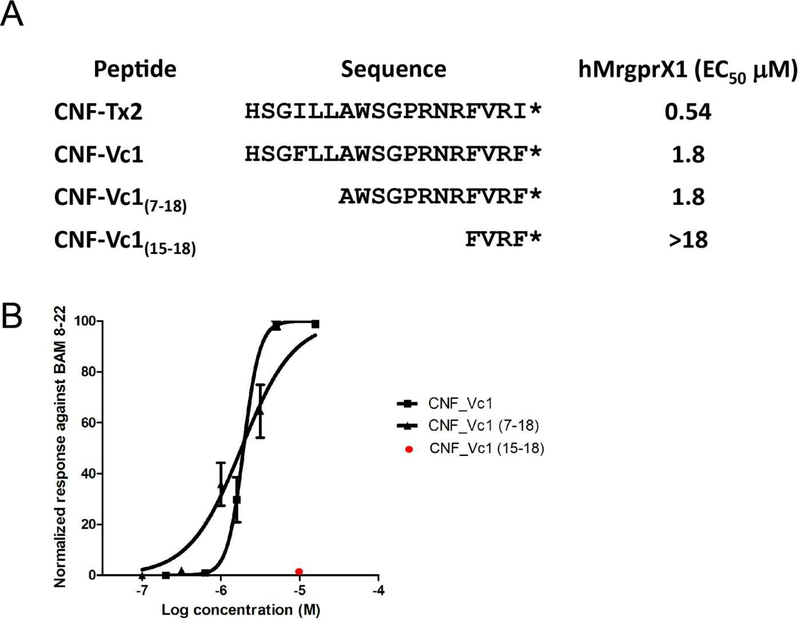
To identify which Phe to Ile substitution affected potency and to identify other structural features important for activity, we synthesized two N-terminal truncated analogues of CNF-Vc1. C-terminal truncated analogues were not tested since previous studies have shown that this region is required for activity against Mrgpr’s (13). The first analogue CNF-Vc1(7–18), lacks the first six amino acids of CNF-Vc1, whereas the second analogue CNF-Vc1(15–18), lacks the first 14 amino acids of CNF-Vc1(Fig 3A).
Figure 3.
A) Activation of hMrgprX1 by CNF-Tx2, CNF-Vc1 and CNF-Vc1 analogues. The EC50 values for the peptides or analogues are indicated. * indicates a C-terminal amide B). Dose-dependent activation of hMrgprX1 by CNF-Vc1 and CNF-Vc1(7–18) each data point is the average of at least 3 experiments. CNF-Vc1(15–18) (red) does not activate the receptor at 10 µM. Error bars represent SEM.
Tests showed that CNF-Vc1(7–18) at 10 µM can activate hMrprX1 (Fig 3A). Concentration-response studies showed that CNF-Vc1(7–18) has an hMrgprX1 EC50 of 1.8 µM similar to full-length CNF-Vc1 (Fig 3 A-B). This indicates that the first six amino acids in the CNF-Vc1 sequence are not crucial for the activity against the receptor. Moreover, this result also suggests that the substitution of Phe with Ile at position 4 does not affect potency whereas the same substitution at the C-terminal is the major determinant for the increased potency of CNF-Tx2.
The second truncated peptide CNF-Vc1(15–18), in which the first 14 amino acids were deleted, failed to activate hMrgprX1 at concentrations up to 18 µM. (Fig 3 A-B); indicating that additional amino acids at positions seven to fourteen in the CNF-Vc1 sequence are necessary for activity against hMrgprX1. Since CNF-Vc1 and CNF-Tx2 are 100% similar in this region, the same conclusion can be made for the activity CNFTx2 against hMrgprX1.
4. Discussion
In this paper, we report the discovery of conopeptide agonists of Mrgprs. The peptides examined in this paper were identified from the venom of the mollusk-hunting cone snails Conus textile and Conus victoriae as well as the worm hunter, Conus spurius. These peptides belong to the class of conopeptides known as conorfamides. Here we show that CNF-Sr1, CNF-Vc1 and CNF-Tx2, activate Mrgprs. Importantly, two of these peptides; CNF-Vc1 and CNF-Tx2 activate the human MrgprX1 (hMrgprX1) in vitro and in vivo.
The EC50 values of CNF-Tx2 and CNF-Vc1 against hMrgprX1 heterologously expressed in KNRK cells are 0.54 µM and 1.8 µM respectively. These EC50 values are comparable to, and in some cases higher than the EC50 values of previously described ligands of hMrgprX1 (10, 32, 35, 36). Compared with hMrgprX1 agonist chloroquine, the anti-malaria drug that causes severe itching via the activation of hMrgprX1 (10), CNFTx2 and CNF-Vc1 are six hundred times and two hundred times more potent respectively. The number of scratching bouts resulting from the intradermal cheek injection of 10 nmol CNF-Tx2 and 10 nmol CNF-Vc1 is comparable to the number of scratching bouts resulting from the intradermal injection of an equivalent dose of histamine (37).
The very high degree of similarity between CNF-Tx2 and CNF-Vc1 allowed for the evaluation of the effects of amino acid substitution at positions 4 and 18 on the potency of the conopeptides to activate hMrgprX1. A comparison of the EC50 of both CNF-Tx2 and CNF-Vc1 (Fig 2C) showed that CNF-Tx2 is at least threefold more potent in activating hMrgprX1. Thus, the substitution of phenylalanines (Phe) in CNF-Vc1 with isoleucines (Ile) in CNF-Tx2 appears to be responsible for the increased potency of CNF-Tx2. The truncated peptide CNF-Vc1(7–18) which lacks the first six amino acids of CNF-Vc1, including the Phe at position four, allowed us to determine which Phe to Ile substitution is the major determinant for the increased potency of CNF-Tx2. Our results showed that the deletion of the first six amino acids, which includes the Phe at position four, did not affect the EC50 of CNF-Vc1(7–18) when compared with the native CNF-Vc1, hence the C-terminal Phe to Ile substitution affects potency.
Amino acids between positions 7–15 of CNF-Vc1 are crucial for activity against hMrgprX1 since CNF-Vc1(15–18) significantly lost its potency in activating hMrgprX1 (Fig 3 A-B).This region is positively charged owing to the presence of arginines. Interestingly peptides that don’t activate hMrgprX1 are negatively charged (e.g. CNF-Tx1 and CNFSr2) or uncharged (CNF-Sr1) in this region. Thus it is possible that the presence of arginine or other positively charged amino acid between position 7 and 15 in the sequence could be a factor important for the activity of the peptides against hMrgprX1. It should be noted that CNF-Tx2 is structurally different from currently known Mrgpr agonist as it terminates in RI-amide. This suggests that the replacement of an aromatic residue (Phe or Tyr) with an aliphatic residue (Ile) does not compromise receptor binding and activity.
Known Conus RF-amides are biologically active in mice. For example, CNF-Sr1 and CNF-Sr2 elicit hyperactivity when injected intracranially (IC) (28, 29), whereas CNFVc1 renders mice unable to move (27). The C. spurius RF-amide CNF-Sr3 was shown to inhibit the shaker K+ channel (38) while the RPRF-amide from C. textile targets acid-sensing ion channel 3 (ASIC3) and potentiates muscle pain (39). Together these activities suggest a diversity of Conus RF-amide targets in mice.
Application of CNF-Vc1 into a culture of DRG neurons activate a broad class of neuronal population and when injected intracranially results in hypoactive behavior (27). These results indicate that CNF-Vc1 and possibly the closely related CNF-Tx2 could potentially target other receptors and/or ion channels in the mouse nervous system. In the skin however, it can be argued that these peptides preferentially target hMrgprX1 since itching is the predominant behavior observed when these peptides are injected intradermally.
It is interesting to note that Mrgprs are only expressed in tetrapods (40), hence this receptor family is not expressed in snails, the prey of C. victoriae and C. textile or in worms which are the prey of C. spurius. Thus, the activity of these peptides against Mrgprs is an example of an epiphenomenon where the activation of Mrgpr’s by these peptides is a secondary activity in addition to the intended activity of these peptides in the snails prey or competitor. RF-amide peptides in mollusks participate in physiological processes such as reproduction, development and survival (38, 39). It is possible that the Conus RF-amide peptides reported here could perturb any of the aforementioned physiological processes.
In summary, we have described peptides that activate the Mrgprs from cone snails. Two of these peptides CNF-Tx2 and CNF-Vc1 activate hMrgprX1 while CNF-Vc1 and CNF-Sr1 also activate MrgprC11. Structural features identified to be important for activity against hMrgprx1, can be exploited in the design of ligands with specific pharmacological properties against the receptor. Importantly, these peptides are tools that can be used to modulate Mrgpr, allowing for the detailed understanding of the cellular and molecular mechanism of itch mediated by these receptors.
Supplementary Material
Highlights.
Crude venom from C. textile activates a population of sensory neurons dedicated to sensing itch.
RF-amide peptides that activate itch receptors were identified from C. textile, C. victoriae and C.
spurius.
CNF-Tx2, CNF-Vc1 activate the human itch receptor hMrgprX1 while CNF-Sr1 and CNF-Vc1 activate the mouse itch receptor MrgprC11.
CNF-Tx2 and CNF-Vc1 induce scratching in vivo in a humanized mouse expressing hMrgprX1.
Structural features potentially important for activity against hMrgprx1 were identified.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (R01EY024704 and 1R01AI125743) and a Pew Scholar Award to Q.L. and by the NIH Grants PO1 GM48677 to B.M.O. We thank Chanxiong Guo for reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Tominaga M & Takamori K (2013) An update on peripheral mechanisms and treatment of itch. Biol Pharm Bull 36(8):1241–1247. [DOI] [PubMed] [Google Scholar]
- 2.Wilson S & Bautista D (2013) Itching for relief. Nat Neurosci 16(7):775–777. [DOI] [PubMed] [Google Scholar]
- 3.Yosipovitch G, Greaves MW, & Schmelz M (2003) Itch. The Lancet 361(9358):690–694. [DOI] [PubMed] [Google Scholar]
- 4.Ikoma A, Steinhoff M, Stander S, Yosipovitch G, & Schmelz M (2006) The neurobiology of itch. Nat Rev Neurosci 7(7):535–547. [DOI] [PubMed] [Google Scholar]
- 5.Paus R, Schmelz M, Biro T, & Steinhoff M (2006) Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest 116(5):1174–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr CW, Veledar E, & Chen SC (2014) Factors mediating the impact of chronic pruritus on quality of life. JAMA Dermatol 150(6):613–620. [DOI] [PubMed] [Google Scholar]
- 7.Kini SP , et al. (2011) The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol 147(10):1153–1156. [DOI] [PubMed] [Google Scholar]
- 8.Roberson DP , et al. (2013) Activity-dependent silencing reveals functionally distinct itchgenerating sensory neurons. Nat Neurosci 16(7):910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akiyama T, Tominaga M, Takamori K, Carstens MI, & Carstens E (2014) Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch. Pain 155(1):80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Q , et al. (2009) Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139(7):1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNeil B & Dong X (2014) Mrgprs as Itch Receptor Itch: Mechanisms and Treatment, eds Carstens E & Akiyama T (CRC Press/Taylor & Francis, Boca Raton (FL)). [PubMed] [Google Scholar]
- 12.Liu Q , et al. (2011) The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Science Signalling 4(181):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han SK , et al. (2002) Orphan G protein-coupled receptors MrgA1 and MrgC11 are distinctively activated by RF-amide-related peptides through the Gαq 11 pathway. Proc Natl Acad Sci U S A 99(23):14740–14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rockel D, Korn W, & Kohn AJ (1995) Manual of the Living Conidae (Verlag Christa Hemmen, Wiesbaden, Germany: ). [Google Scholar]
- 15.McIntosh JM & Jones RM (2001) Cone venom--from accidental stings to deliberate injection. Toxicon 39(10):1447–1451. [DOI] [PubMed] [Google Scholar]
- 16.Kohn AJ (1959) The Ecology of Conus in Hawaii. Ecology Monograph 29:47–90. [Google Scholar]
- 17.Olivera BM (1997) E.E. Just Lecture, 1996. Conus venom peptides, receptor and ion channel targets, and drug design: 50 million years of neuropharmacology. Molecular biology of the cell 8(11):2101–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favreau P & Stocklin R (2009) Marine snail venom: use and trends in receptor and channel pharmacology. Curr Opinion in Pharmacology (9):594–601. [DOI] [PubMed] [Google Scholar]
- 19.Olivera BM (2002) Conus Venom Peptides: Reflections from the Biology of Clades and Species. Annual Review of Ecology and Systematics 33(1):25–47. [Google Scholar]
- 20.Olivera BM , et al. (1985) Peptide neurotoxins from fish-hunting cone snails. Science 230:13381343. [DOI] [PubMed] [Google Scholar]
- 21.Teichert RW, Olivera BM, McIntosh JM, Bulaj G, & Horvath MP (2015) The Molecular Diversity of Conoidean Venom Peptides and their Targets: From Basic Research to Therapeutic Applications Venom to Drugs: Venom as a Source for the Development of Human Therapeutics . , ed King GF (RSC Publishing, London: ), Vol RSC Drug Discovery, pp 163–203. [Google Scholar]
- 22.Terlau H & Olivera BM (2004) Conus venoms a rich source of novel ion channel-targeted peptides. Physiol Rev 84:41–68. [DOI] [PubMed] [Google Scholar]
- 23.Cruz LJ , et al. (1987) Invertebrate vasopresin oxytocin homologs characterization of peptides from Conus geographus and Conus striatus venom. J Biol Chem 262(33):15821–15824. [PubMed] [Google Scholar]
- 24.Olivera BM , et al. (1990) Diversity of Conus neuropeptides. Science 249(4966):257–263. [DOI] [PubMed] [Google Scholar]
- 25.Robinson SD , et al. (2017) The Venom Repertoire of Conus gloriamaris (Chemnitz, 1777), the Glory of the Sea. Mar Drugs 15(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kearse M , et al. (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12):1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson SD , et al. (2015) Discovery by proteogenomics and characterization of an RF-amide neuropeptide from cone snail venom. J Proteomics 114:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maillo M , et al. (2002) Conorfamide, a Conus venom peptide belonging to the RFamide family of neuropeptides. Toxicon 40(4):401–407. [DOI] [PubMed] [Google Scholar]
- 29.Aguilar MB , et al. (2008) Conorfamide-Sr2, a gamma-carboxyglutamate-containing FMRFamiderelated peptide from the venom of Conus spurius with activity in mice and mollusks. Peptides 29(2):186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han L , et al. (2013) A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 16(2):174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zylka MJ, Dong X, & Southwell AL (2003) Atypical expansion in mice of the sensory neuronspecific Mrg G protein-coupled receptor family. Proc Natl Acad Sci U S A 100(17):10043–10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lembo PM , et al. (2002) Proenkephalin A gene products activate a new family of sensory neuron--specific GPCRs. Nat Neurosci 5(3):201–209. [DOI] [PubMed] [Google Scholar]
- 33.Puillandre N , et al. (2014) Molecular phylogeny and evolution of the cone snails (Gastropoda, Conoidea). Mol Phylogenet Evol 78:290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sikand P, Dong X, & LaMotte RH (2011) BAM8–22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J Neurosci 31(20):7563–7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karhu T , et al. (2017) Isolation of new ligands for orphan receptor MRGPRX1-hemorphins LVVH7 and VV-H7. Peptides 96:61–66. [DOI] [PubMed] [Google Scholar]
- 36.Tatemoto K , et al. (2006) Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun 349(4):1322–1328. [DOI] [PubMed] [Google Scholar]
- 37.Shimada SG & LaMotte RH (2008) Behavioral differentiation between itch and pain in mouse. Pain 139(3):681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campos-Lira E , et al. (2017) Conorfamide-Sr3, a structurally novel specific inhibitor of the Shaker K(+) channel. Toxicon 138:53–58. [DOI] [PubMed] [Google Scholar]
- 39.Reimers C , et al. (2017) Identification of a cono-RFamide from the venom of Conus textile that targets ASIC3 and enhances muscle pain. Proc Natl Acad Sci U S A 114(17):E3507-e3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bader M, Alenina N, Andrade-Navarro MA, & Santos RA (2014) MAS and its related G proteincoupled receptors, Mrgprs. Pharmacol Rev 66(4):1080–1105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.