Abstract
One-carbon metabolism generates the one-carbon units required to synthesize many critical metabolites, including nucleotides. The pathway has cytosolic and mitochondrial branches, and a key step is the entry, through an unknown mechanism, of serine into mitochondria, where it is converted into glycine and formate. In a CRISPR-based genetic screen in human cells for genes of the mitochondrial pathway, we found sideroflexin 1 (SFXN1), a multipass inner mitochondrial membrane protein of unclear function. Like cells missing mitochondrial components of one-carbon metabolism, those null for SFXN1 are defective in glycine and purine synthesis. Cells lacking SFXN1 and one of its four homologs, SFXN3, have more severe defects, including being auxotrophic for glycine. Purified SFXN1 transports serine in vitro. Thus, SFXN1 functions as a mitochondrial serine transporter in one-carbon metabolism.
One-carbon metabolism uses serine to generate the reactive one-carbon donors, such as 5,10-methylene-tetrahydrofolate, required for many basic processes, including nucleotide and lipid synthesis [reviewed in (1–3)]. An interesting aspect of the one-carbon pathway is that although partially redundant isozymes exist in the cytosol and mitochondrial matrix, in most proliferating cells, the pathway primarily flows from the cytosol into mitochondria and back out (Fig. 1A). Cytosolic serine enters the mitochondrial matrix and is converted to glycine and formate, which then exits to the cytosol where it is used to generate the charged folates that serve as one-carbon donors (4) (Fig. 1A). In dividing mammalian cells, the mitochondrial catabolism of serine supplies most of the one-carbon units needed for biosynthesis (5–9), but the cytosolic branch can compensate for its loss (8). Given that the entry of serine into mitochondria is a critical stepin the generation of one-carbon units, it is surprising that the mitochondrial transporter(s) for serine remains unknown (5, 10–12). To seek such a transporter, we designed a CRISPR-Cas9–mediated genetic screen based on the likelihood that loss of mitochondrial serine transport will reduce the proliferation of cells lacking the cytosolic branch of one-carbon metabolism. Moreover, we reasoned that even if there are redundant mechanisms for serine transport, we can sensitize cells to its partial inhibition by lowering cytosolic serine concentrations, which is easily achieved by removing exogenous serine (fig. S1A) (13). Thus, we sought genes required for the optimal proliferation of cells lacking the cytosolic one-carbon pathway when cultured in serine-free media.
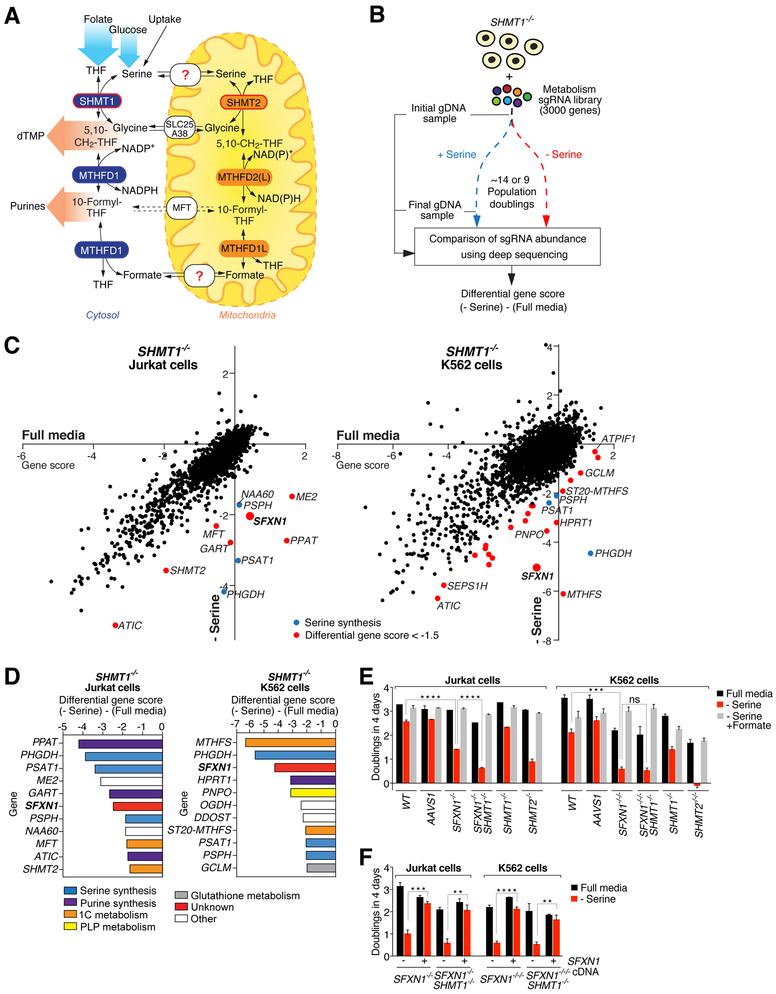
Fig. 1. A genetic screen for components of the one-carbon metabolism pathway yields SFXN1.
(A) Schematic of the one-carbon metabolism pathway. dTMP, deoxythymidine monophosphate; THF, tetrahydrofolate; CH2THF, methyleneTHF. NAD(P)H, nicotinamide adenine dinucleotide (phosphate); SHMT, serine hydroxymethyltransferase; MFT, mitochondrial folate transporter/carrier; MTHFD, methylenetetrahydrofolate dehydrogenase. The dashed arrows indicate that the exact nature of the substrate for MFT is unknown. (B) CRISPRCas9–based screening strategy designed to identify new components of the mitochondrial one-carbon pathway. The cells in the serine-free media were collected after ~9 population doublings because they proliferated more slowly than cells in full media, which were collected after ~14 doublings. For each gene, we calculated its gene score as the mean log2 foldchange in the abundance of the 10 sgRNAs targeting the gene. The differential gene score is the difference in scores in the absence versus presence of serine. sgRNA, single guide RNA; gDNA, genomic DNA. (C) SFXN1 emerges as a hit in both the Jurkat and K562 screens. Gene scores in full media were plotted against those in serine-deficient media. Genes with a differential score of < 0.001, ****P < 0.0001; ns, not significant). AAVS1 indicates control cells that were treated with an sgRNA targeting the AAVS1 locus as described previously (14). SFXN1- and SHMT2-null K562 cells are designated as −/−/− as they are triploid for these genes. (F) Expression of an sgRNA-resistant cDNA for SFXN1 in the SFXN1-null cells restores their proliferation rate in serine-deficient media (mean ± SD; n = 3; **P < 0.01, ****P < 0.0001). Two-tailed t tests were used for comparisons between groups.
A genetic screen for components of the mitochondrial one-carbon metabolism pathway yields SFXN1
To implement such a screening strategy, we first generated human Jurkat leukemic T cells and K562 erythroleukemic cells null for serine hydroxymethyltransferase 1 (SHMT1), an isozyme of mitochondrial SHMT2 and a key component of the cytosolic one-carbon pathway that interconverts serine and glycine (Fig. 1A). We chose Jurkat and K562 cells because they are suitable for screening (14, 15) and have high mitochondrial one-carbon pathway activity (8, 16). We transduced the SHMT1-null cells with a lentiviral single guide RNA (sgRNA) library that targets ~3000 metabolic enzymes, small-molecule transporters, and metabolism-related transcription factors (~10 sgRNAs per gene) and also contains 499 control sgRNAs (15). The transduced cells were cultured in RPMI media with or without serine, and for each gene, we generated a gene score by calculating the mean log2 fold-change in the abundance from the beginning to end of the culture period of all the sgRNAs targeting the gene (15) (Fig. 1B). We also obtained a differential gene score that reflects the relative importance of the gene in the presence or absence of serine. As expected, most genes, as well as the control sgRNAs, had similar scores in cells cultured under both media conditions (Fig. 1C). Multiple classes of genes behaved as predicted. For example, in both cell lines, the three genes in the serine synthesis pathway (PHGDH, PSAT1, PSPH) were required for proliferation in serine-free media, as were components of the purine synthesis pathway, which is downstream of one-carbon metabolism (Fig. 1D and fig. S1B). Established components of the mitochondrial one-carbon pathway, such as SHMT2 and the mitochondrial folate transporter (MFT), scored differentially in Jurkat cells, as did 5,10-methenyltetrahydrofolate synthetase (MTHFS), which returns 5-formyl-tetrahydrofolate to the tetrahydrofolate (THF) cofactor pool (17), in K562 cells. Notably, the only gene of unknown molecular function that scored differentially in both cell lines was the mitochondrial transmembrane protein sideroflexin 1 (SFXN1) (Fig. 1, C and D, and fig. S1, B and C). Sfxn1 was originally identified as the gene mutated in a mouse mutant with anemia and axial skeletal abnormalities and is part of the sideroflexin family of proteins conserved throughout eukaryotes (18,19). In humans, SFXN1 is highly expressed in the blood, liver, and kidney, which are tissues with high one-carbon metabolism activity (fig. S2, A and B). To follow up the screen, we generated Jurkat and K562 cells lacking SFXN1 alone or in combination with SHMT1 (fig. S1D). In the absence of serine, the SFXN1-null Jurkat cells proliferated more slowly than the wild-type or AAVS1- targeted control cells, and the loss of SHMT1 exacerbated the defect, consistent with the screening results (Fig. 1E). The SFXN1-null K562 cells proliferated less well than their Jurkat counterparts, but SHMT1 deletion did not exacerbate the defect, likely because K562 cells express very low levels of SHMT1 to begin with (Fig. 4C). The addition of formate, the product of the mitochondrial one-carbon pathway, completely reversed the slow proliferation of the Jurkat and K562 single- and double-null cells in the serine-free media, directly implicating an insufficient supply of one-carbon units in their defective proliferation (Fig. 1E). Notably, expression of an sgRNAresistant SFXN1 cDNA restored the proliferation rate of the SFXN1-null cells to that of the wildtype cells (Fig. 1F). SHMT2-null cells exhibited similar albeit more profound proliferation defects than the cells lacking SFXN1 (Fig. 1E).
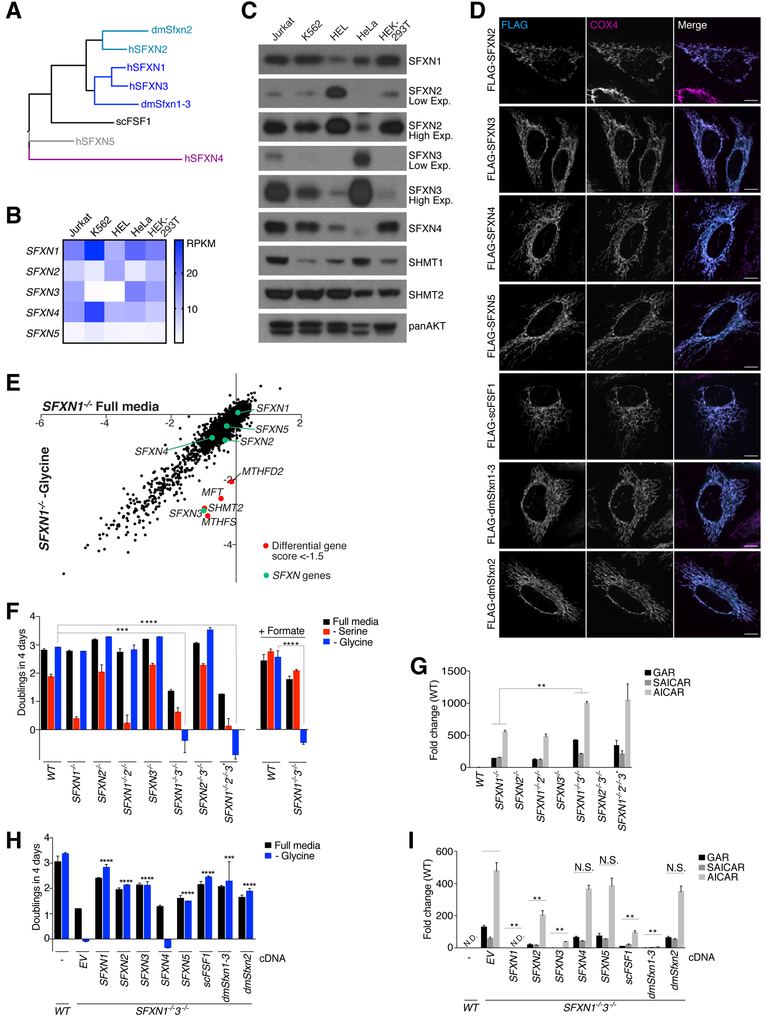
Fig. 4. SFXN3 and fly and yeast sideroflexin homologs can substitute for SFXN1 loss.
(A) Phylogenetic tree of human, Drosophila melanogaster, and Saccharomyces cerevisae sideroflexins. (B) mRNA levels of the five human sideroflexins in commonly used cell lines. RPKM (reads per kilobase million) levels were extracted from the Cancer Cell Line Encyclopedia. (C) Sideroflexin protein levels in commonly used cell lines. Cell lysates were equalized for total protein amounts and analyzed by immunoblotting for the levels of the indicated proteins. (D) FLAG-tagged sideroflexin homologs localize to mitochondria. Wild-type HeLa cells transiently expressing FLAG-sideroflexin homologs were processed for immunofluorescence detection of the FLAG epitope (cyan) and the mitochondrial inner membrane marker COX4 (magenta). The merged image shows the overlap of both channels in white. (E) CRISPR-Cas9–based genetic screen reveals that SFXN3 is required for proliferation in the absence of SFXN1 and glycine. Gene scores in SFXN1-null cells cultured in the presence or absence of glycine were plotted against each other. Except for SFXN3, genes with a differential gene score of < 0.001, ****P < 0.0001). (G) The accumulation of purine synthesis intermediates is exacerbated in cells lacking both SFXN1 and SFXN3 compared to their single-knockout counterparts. The asterisk denotes a cell clone lacking SFXN1 and SFXN2 and with incomplete deletion of SFXN3. Purine intermediates were measured by LC-MS in extracts from the indicated cells (mean ± SD; n = 3; **P < 0.01). Abbreviations as in Fig. 2C. (H) Human, yeast, and Drosophila sideroflexin homologs, with the exception of SFXN4, rescue the glycine auxotrophy of cells lacking both SFXN1 and SFXN3. Single cell–derived doubleknockout Jurkat cells were transduced with an empty vector (EV) or cDNAs of human, yeast, and Drosophila sideroflexin homologs. Asterisks denote statistically significant differences in proliferation in media lacking glycine between the cells expressing the empty vector and the sideroflexin homologs. Mean ± SD; n = 3; ***P < 0.001, ****P < 0.0001). (I) Sideroflexin homologs rescue to varying degrees the purine synthesis defects of cells lacking SFXN1 and SFXN3. Purine intermediates were measured by LC-MS in extracts from wild-type Jurkat cells or the double-knockout Jurkat cells expressing an empty vector (EV) or cDNAs of human, yeast, and Drosophila sideroflexin homologs. Asterisks denote statistically significant differences between the cells expressing the empty vector and the sideroflexin homologs. Values were normalized to the average value of the wild-type samples in (G) because purine synthesis intermediates were not detected in the wild-type samples in this experiment (mean ± SD; n = 3, **P < 0.01; N.D., not detected; N.S., not significant). Two-tailed t tests were used for comparisons between metabolites.
Loss of SFXN1 phenocopies mutants in mitochondrial one-carbon metabolism
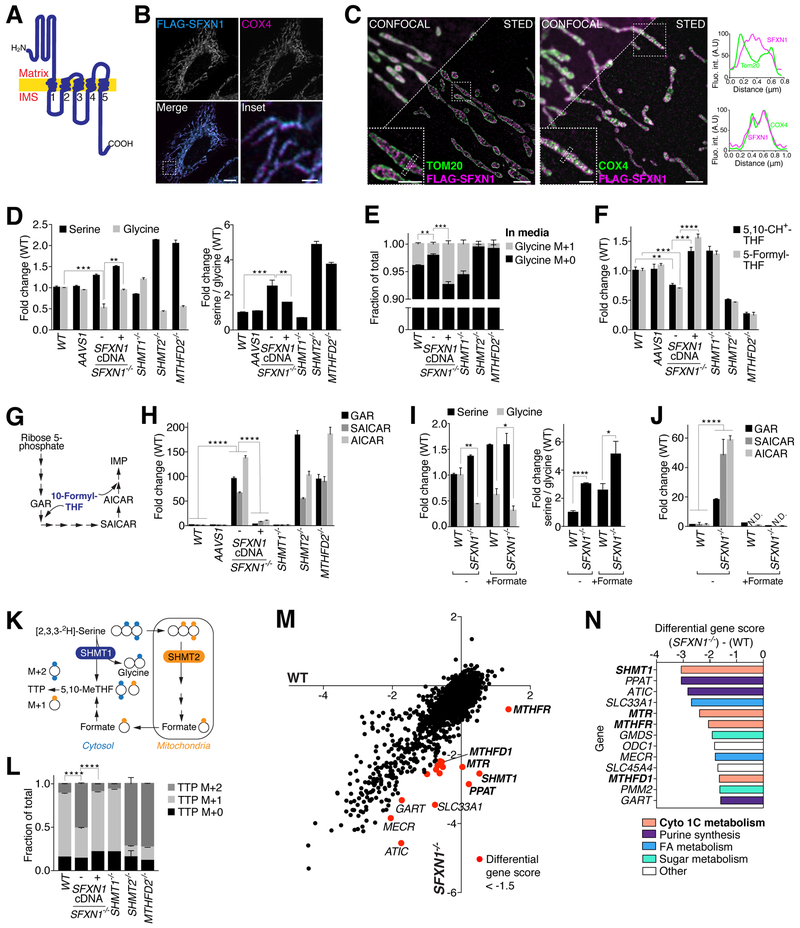
SFXN1 localizes to the inner mitochondrial membrane and is predicted to have five transmembrane domains [(18) and our analysis with Protter (20)], with its N terminus in the matrix and C terminus in the intermembrane space (Fig. 2A) (21). As expected, in HeLa cells, FLAG-tagged SFXN1 colocalized with the inner mitochondrial membrane protein COX4 (Fig. 2B), and endogenous SFXN1 was enriched in mitochondria purified from Jurkat and K562 cells (fig. S5B). Using super-resolution microscopy, we confirmed that SFXN1 localizes to the inner and not outer mitochondrial membrane (Fig. 2C). The outer mitochondrial membrane, which can be marked by Tom20 (Fig. 2C), is permeable to most small metabolites owing to the presence of the VDAC porins. Given these attributes and its emergence from our screen, SFXN1 was an excellent candidate to be a mitochondrial serine transporter. If this were the case, cells lacking SFXN1 should have metabolic defects similar to those of cells missing SHMT2 or MTHFD2, the enzymes needed to convert serine to glycine and formate in mitochondria. Indeed, loss of SFXN1, SHMT2, or MTHFD2, but not SHMT1, caused the depletion of glycine in Jurkat cells, an increase in the serine-to-glycine ratio, and a reduction in the amount of de novo–synthesized glycine secreted into the media as measured in tracing experiments with labeled serine (Fig. 2, D and E, and fig. S3A). The null cells also had lower levels of the charged folate species that we were able to detect (5,10-methenyl-THF and 5-formyl-THF), and this was not secondary to a drop in THF or folate levels (Fig. 2F and fig. S3B). The sgRNAresistant SFXN1 cDNA complemented all the metabolic defects of the SFXN1-null cells. To determine if loss of SFXN1 affects pathways that consume one-carbon donors, we examined purine synthesis, as it uses large amounts of 10-formyl-THF as a cofactor (Fig. 2G). Indeed, the purine synthesis intermediates 5′-phosphoribosylglycinamide (GAR), phosphoribosylaminoimidazolesuccinocarboxamide (SAICAR), and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) accumulated in SFXN1-null cells to similar extents as in cells lacking SHMT2 and MTHFD2 (8) (Fig. 2H and fig. S3C). In this respect, loss of SFXN1 mimicked serine starvation, which in wild-type cells also caused an accumulation of the intermediates (fig. S3D). Consistent with SFXN1 acting upstream of formate production, formate abolished the accumulation of the intermediates in the SFXN1-null cells but did not rescue the defects in glycine levels (Fig. 2, I and J). To monitor the relative contributions of the cytosolic and mitochondrial pathways to the synthesis of one-carbon units in SFXN1-null cells, we used a reported strategy to trace [2,3,3–2 H3]- serine to thymidine triphosphate (TTP) (8, 22). The deuterated serine will give rise to TTP shifted by two mass units (TTP M+2) when catabolized through the cytosolic pathway but only by one mass unit (TTP M+1) if via the mitochondrial pathway (Fig. 2K). In wild-type cells, the M+1 form was the predominant species of newly synthesized TTP, as expected in cells generating most of their one-carbon donors through mitochondria. By contrast, in cells lacking SFXN1, SHMT2, or MTHFD2, most of the newly synthesized TTP was of the M+2 species (Fig. 2L), consistent with SFXN1 being important for the function of the mitochondrial one-carbon pathway. To corroborate this conclusion, we used our metabolism-focused sgRNA library to screen for genes important for the optimal proliferation of SFXN1-null but not wild-type cells. Gratifyingly, among the top hits were many components of the cytosolic one-carbon pathway, including SHMT1, which was the most differentially required gene (Fig. 2, M and N, and fig. S3E). We conclude that SFXN1 is part of the mitochondrial one-carbon pathway and its loss, like that of established components, makes cells more dependent on the cytosolic branch of the pathway.
Fig. 2. Loss of SFXN1 phenocopies mutants in mitochondrial one-carbon metabolism.
(A) Model of the predicted topology of SFXN1 in the mitochondrial inner membrane. Transmembrane helices are indicated by numbers. IMS, intermembrane space. (B) FLAG-tagged SFXN1 localizes to mitochondria. Wild-type HeLa cells transiently expressing FLAG-SFXN1 were processed for immunofluorescence detection of the FLAG epitope (cyan) and the mitochondrial inner membrane marker cytochrome c oxidase subunit 4 (COX4) (magenta). The merged image shows the overlap of both channels in white. Scale bar is 10 mm in the full image and 2 mm in the inset. (C) Super-resolution microscopy confirms SFXN1 localization to the inner membrane of mitochondria. Wild-type HeLa cells transiently expressing FLAG-SFXN1 were processed for immunofluorescence detection of the FLAG epitope (magenta) and the outer mitochondrial membrane marker Tom20 (left panel, green) or the mitochondrial inner membrane marker cytochrome c oxidase subunit 4 (COX4) (right panel, green) and imaged by STED microscopy. Overlap of magenta and green channels is shown in white, and line profiles show fluorescent signals of each channel across mitochondria where marked by the dotted rectangles. Scale bars are 2 mm in the full images and 1 mm in the insets. (D) As in cells lacking known components of the mitochondrial one-carbon pathway, glycine levels are reduced and the cellular serine/glycine ratio is increased in SFXN1-null cells. Serine and glycine levels were measured by gas chromatography–mass spectrometry (GC-MS) in extracts from wild-type Jurkat cells or single-cell–derived control and knockout clones (mean ± SD; n = 3; **P < 0.01, ***P < 0.001). (E) Loss of SFXN1 causes a glycine synthesis defect. GC-MS was used to measure glycine in the culture media of wild-type Jurkat cells or single-cell–derived knockout clones incubated for 12 hours with 2,3,3–2 H3-serine as the only serine source. The glycine M+0 species is the unlabeled species. The glycine M+1 species is derived from 2,3,3–2 H3-serine (mean ± SD; n = 3; **P < 0.01, ***P < 0.001). (F) Levels of charged folate species are decreased in SFXN1-null cells. Metabolites were measured by LC-MS in extracts from wild-type Jurkat cells or single-cell–derived control and knockout clones (mean ± SD; n = 3; **P < 0.01, ***P < 0.001, ****P < 0.0001). 5,10-CH+ -THF, 5,10-methenyl-THF. (G) Schematic of the purine synthesis pathway, indicating steps using one-carbon units in the form of 10-formyl-THF. GAR, 5′-phosphoribosyl-glycinamide. SAICAR, phosphoribosylaminoimidazolesuccinocarboxamide. AICAR, 5-aminoimidazole-4- carboxamide ribonucleotide. IMP, inosine monophosphate. (H) The purine synthesis intermediates GAR, SAICAR, and AICAR accumulate in SFXN1-null cells. Purine synthesis intermediates were measured by LC-MS in extracts from wild-type Jurkat cells or single-cell–derived control and knockout clones (mean ± SD; n = 3; ****P < 0.0001). (I) Addition of 1 mM formate does not rescue glycine levels and serine/glycine ratio of SFXN1- null cells. Serine and glycine levels were measured by LC-MS in extracts from wild-type Jurkat cells or single-cell–derived SFXN1-null cells incubated for 24 hours in the indicated media (mean ± SD; n = 3; *P < 0.05, **P < 0.01). (J) Addition of 1 mM formate reverses the accumulation of purine synthesis intermediates in SFXN1-null cells. Intermediates were measured by LC-MS in extracts from wild-type Jurkat cells or single-cell– derived SFXN1-null cells incubated for 24 hours in the indicated media (mean ± SD; n = 3; ****P < 0.0001; N.D., not detected). (K) Tracing strategy to differentiate contribution of cytosolic and mitochondrial pathways to cytosolic TTP synthesis. Oxidation of 2,3,3–2 H3-serine by SHMT2 and subsequent enzymes in mitochondria gives rise to a singly labeled formate species, and thus singly labeled (one mass unit heavier, M+1) TTP. Oxidation by SHMT1 in the cytosol gives rise to doubly labeled (two mass units heavier, M+2) TTP. The difference between unlabeled (M+0), M+1, and M+2 TTP can be resolved on a high-resolution mass spectrometer. The ratio of M+1 to M+2 is indicative of the contribution of mitochondria- versus cytosol-derived one-carbon units to nucleotide synthesis. Adapted from (8). TTP, thymidine triphosphate. (L) The relative contribution of the cytosolic and mitochondrial one-carbon pathways to TTP synthesis is inverted in SFXN1-null compared to wild-type cells. Wild-type Jurkat or single-cell-derived knockout cells were cultured for 12 hours in media containing 2,3,3–2 H3-serine as the only serine source before harvesting and LC-MS analysis (mean ± SD; n = 3, ****P < 0.0001). (M) Genes of the cytosolic one-carbon pathway are selectively required for the optimal proliferation of SFXN1-null cells. Gene scores in wild-type cells were plotted against those in SFXN1-null cells. Genes with a differential gene score of <−1.5 are shown in red. (N) Serine Hydroxymethyltransferase 1 (SHMT1) was the top hit from the SFXN1 synthetic lethality screen. Genes were ranked according to differential gene score between wild-type and SFXN1-null cells. Cyto 1C metabolism, cytosolic one-carbon metabolism; FA, fatty acid. Two-tailed t tests were used for comparisons between metabolites.
SFXN1 transports serine in vitro
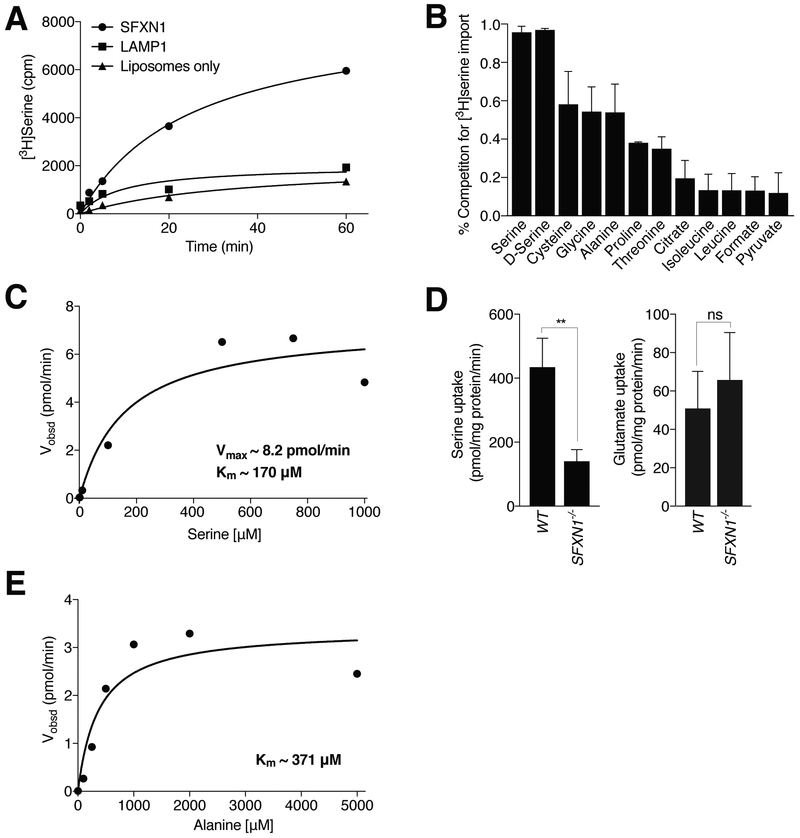
To test if SFXN1 can directly transport serine, we purified the FLAG-tagged protein from mammalian cells (fig. S4A) and reconstituted it into liposomes. Recombinant SFXN1 mediated serine uptake into liposomes (Fig. 3A). Both L- and Dserine competed with the transport of the labeled serine, as did other amino acids, including the structurally related amino acids alanine, cysteine, and glycine, whereas other metabolites did so to negligible extents (Fig. 3B). Neither formate nor citrate competed with serine transport. In vitro, SFXN1 transports serine with a Michaelis constant (Km) of ~170 mM (Fig. 3C), which suggests that SFXN1 can transport serine at the estimated cellular serine concentration of 300 mM. Consistent with these findings, serine uptake by mitochondria isolated from SFXN1-null cells was reduced compared to that by wild-type mitochondria, whereas the uptake of the structurally unrelated amino acid glutamate was unaffected (Fig. 3D). Because alanine, cysteine, and glycine partially competed with serine in the in vitro transport assay, we tested whether SFXN1 can also transport these amino acids. Indeed, SFXN1 transported alanine at the physiologically relevant concentration of 371 mM (Fig. 3E). We could not reliably measure cysteine and glycine transport by SFXN1 and suspect that further optimization of the assay might be necessary to determine whether SFXN1 can also directly transport these amino acids. To begin to assess a potential role for SFXN1 in the metabolism of these amino acids, we incubated cells in media with or without them. Although the presence or absence of alanine or glycine did not affect the proliferation of SFXN1-null cells, they proliferated better than their wild-type counterparts in media with low concentrations of cystine, suggesting that SFXN1 may play a role in intracellular cysteine transport (fig. S4C and fig. S5A) (see Discussion).
Fig. 3. SFXN1 transports serine in vitro.
(A) Time course of radioactive serine uptake into proteoliposomes containing SFXN1. LAMP1-containing or empty liposomes were used as controls. Values are the averages of two replicates. Cpm, counts per minute. (B) Competition of serine uptake after 60 min by different metabolites at 500 mM (mean ± SD; n = 3). (C) Steady-state kinetic analysis of SFXN1-mediated serine transport reveals a Vmax of ~ 8.2 pmol/min and a Km of ~170 mM. Velocity, as shown, was calculated as a function of the serine concentration. Each data point was calculated from three replicate data points. (D) Radioactive serine uptake into mitochondria purified from SFXN1-null cells is reduced compared to that into mitochondria purified from wild-type cells, whereas glutamate uptake is unchanged (mean ± SD; n = 3, **P < 0.01; ns, not significant). (E) Steady-state kinetic analysis of SFXN1-mediated alanine transport reveals a Km of ~371 mM for alanine. Each data point was calculated from three replicate data points
Homologs of SFXN1 can compensate for its loss
Cells lacking components of the mitochondrial one-carbon pathway are auxotrophic for glycine (6, 23–25), which we confirmed using our SHMT2- and MTHFD2-null cells (fig. S5A). Because loss of SFXN1 did not cause glycine auxotrophy, we reasoned that there must be genes that can partially compensate for it. In mammals there are five sideroflexins, with SFXN3 being the closest homolog of SFXN1 (88% protein sequence similarity) (Fig. 4A). Budding yeast has only one sideroflexin (FSF1), while Drosophila melanogaster has two, one most similar to SFXN1 and SFXN3 (Sfxn1–3) and the other to SFXN2 (Sfxn2) (Fig. 4A). The human, fly, and yeast sideroflexins that we examined localized to mitochondria when expressed in HeLa cells (Fig. 4D). Because Jurkat and K562 cells, like other commonly used cell lines, express multiple sideroflexins (Fig. 4, B and C), we hypothesized that one or more family members might partially compensate for the loss of SFXN1, thus explaining why the SFXN1-null cells are not auxotrophic for glycine. To explore this possibility, we used the metabolism-focused sgRNA library to screen for genes required for SFXN1-null Jurkat cells to proliferate in the absence of glycine. In addition to several genes in the mitochondrial (MTHFD2, SHMT2, MFT) and cytosolic (MTHFS) one-carbon pathways, the only other gene to score was SFXN3 (Fig. 4E), suggesting that it has redundant functions with SFXN1. Indeed, like cells lacking SHMT2 or MTHFD2, cells null for both SFXN1 and SFXN3 [SFXN1&3 DKO (double knockout) cells] did not proliferate in the absence of glycine (Fig. 4F). Consistent with these cells having a severe defect in glycine synthesis, the addition of formate to the glycinefree media did not reverse their proliferation defect (Fig. 4F), whereas expression of either SFXN1 or SFXN3 did (fig. S5, D and E). Purine synthesis intermediates accumulated to greater extents in the DKO cells than in those lacking only SFXN1, whereas the single or combined deletions of SFXN3 and SFXN2 did not affect these metabolites (Fig. 4G). Compared to the SFXN1-null and wild-type cells, the DKO cells proliferated slowly even in full media, suggesting that beyond experiencing one-carbon unit stress, these cells have limiting amounts of one-carbon donors and/or glycine. Whereas mitochondrial mass, morphology, and function were not affected in SFXN1-null cells, SFXN1&3 DKO cells did have defects in these parameters (fig. S6). Mitochondrial onecarbon metabolism, as well as serine itself, is needed for mitochondrial protein synthesis and, indeed, the DKO cells had reduced levels of mitochondrially encoded proteins, likely explaining their mitochondrial dysfunction (fig. S6G) (26–29). Despite multiple attempts and the supplementation of full media with formate, we failed to isolate SFXN1&2&3 triple knockout cells, suggesting that such cells are not viable. However, we were able to obtain one cell clone lacking SFXN1 and SFXN2 and containing low levels of SFXN3 as a result of an in-frame deletion (fig. S5C), and these cells were also unable to proliferate in the absence of glycine (Fig. 4F). Because SFXN1 and SFXN3 are among the most highly expressed sideroflexins in Jurkat cells (Fig. 4B), we asked if other homologs can compensate for them if expressed at higher levels. With the exception of SFXN4, overexpression of any of the human sideroflexins reversed the glycine auxotrophy of the Jurkat SFXN1&3 DKO cells, as did heterologous expression of yeast FSF1 and Drosophila Sfxn1–3 and Sfxn2 (Fig. 4H and fig. S5F). However, besides SFXN1 and SFXN3, only SFXN2, FSF1, and Sfxn1–3 (the closest Drosophila homolog of SFXN1) ameliorated, to differing degrees, the defects in purine synthesis (Fig. 4I). These results suggest that serine transport is an evolutionarily conserved feature of the sideroflexins but that their kinetic properties and likely substrate specificities vary so that not all can support the high rate of mitochondrial serine import required to fulfill the demand for one-carbon units of proliferating cells. In vitro transport assays optimized for each sideroflexin will be required to test this idea.
Discussion
Our work reveals SFXN1 as a previously missing component of the one-carbon metabolism pathway that functions as a mitochondrial serine transporter. We propose that SFXN1 and SFXN3 (and perhaps SFXN2) are the main mitochondrial serine transporters in human cells and that Sfxn1–3 also has this function in D. melanogaster. SFXN1 and SFXN3 likely have other physiologically relevant substrates besides serine, such as alanine or cysteine, a notion supported by our in vitro transport results, the finding that cells lacking both have more severe proliferation defects than those missing established components of the mitochondrial one-carbon pathway, and that cells lacking SFXN1 have a proliferation advantage in media containing low cystine, the oxidized dimer of cysteine present in RPMI media and taken up by cells (fig. S4C). A major use of cysteine is cytosolic glutathione synthesis, and it is possible that loss of SFXN1 and thus a reduction in mitochondrial cysteine import should increase its availability for this use, which is known to be limiting for cell proliferation (30). Two reports proposed that SFXN1 can transport citrate in vitro (31, 32), but the physiological relevance of this remains unclear because SLC25A1 is well established as the mitochondrial citrate carrier (33–35) and citrate did not compete with serine in our in vitro assays (Fig. 3B). Moreover, these previous studies used purified endogenous rather than recombinant protein, raising the possibility that the observed activity was due to a copurifying contaminating protein. In addition, we excluded another potential substrate for the sideroflexins, pyridoxine (36), which is a precursor for the pyridoxal 5′-phosphate cofactor of SHMT2, ALAS2, and mitochondrial transaminases, because the levels of pyridoxal-conjugated proteins were unchanged in the mitochondria of SFXN1- or SFXN1&3-null cells (fig. S6H). Furthermore, SLC25A39 is likely responsible for pyridoxal 5′- phosphate transport into mitochondria (37). When overexpressed, SFXN5 only partially complements loss of SFXN1, and we suspect that its main function is not as a serine transporter— similarly to SFXN4, which in our experimental systems cannot substitute for SFXN1. It is interesting that budding yeast only has one sideroflexin (FSF1), perhaps suggesting that is has broader functions than its homologs in other species. Mice with a loss-of-function mutation in Sfxn1 have a sideroblastic-like anemia characterized by iron accumulation in mitochondria (18, 39). Although Sfxn1 has been challenged as the causative gene (39), our work does provide a possible explanation for the phenotype. In humans, mutations that impair the part of the heme synthesis pathway that occurs in mitochondria, which requires glycine, cause sideroblastic anemia (40). Given that mitochondria make glycine from serine, we speculate that in the Sfxn1-mutant mice, an insufficient import of serine into mitochondria results in a decrease in glycine and thus heme synthesis. Iron itself is unlikely to be a direct substrate of SFXN1 as the mitoferrins are reported mitochondrial iron transporters (41). There are multiple sideroflexins, and their expression varies across tissues (fig. S2 A, B). Like other genes of the mitochondrial one-carbon pathway, SFXN1, 2, and 3 expression is likely regulated by the Myc transcription factor, as we found 34, 20, and 14 Myc-binding sites in the promoters of SFXN1, SFXN2, and SFXN3, respectively. SFXN1 is expressed in many cancers, most highly in leukemias and lymphomas (fig. S2C), Thus, SFXN1 and its homologs may turn out to be important nodes for regulating the fate of serine in cells and also play unexplored roles in cancer cell growth.
Methods summary
CRISPR screens performed in human SHMT1- null Jurkat and K562 cells identified SFXN1 as one of the most differentially scoring genes in media with and without serine in both cell lines. The proliferation and metabolism of single-cell– derived SFXN1-null cells generated using CRISPRCas9 was analyzed by using Cell Titer Glo assays and mass spectrometry–based metabolite profiling and compared with mutants of the known one-carbon metabolism genes SHMT1, SHMT2, and MTHFD2. The localization of SFXN1 and its homologs was analyzed by spinning disk confocal and stimulated emission depletion (STED) super-resolution microscopy. In vitro transport assays and uptake assays into isolated mitochondria confirmed SFXN1 as a serine transporter. The redundancy of genes homologous to SFXN1 (the sideroflexins) was analyzed by using a SFXN1 synthetic lethality CRISPR screen, by studying double deletion cells, and in complementation assays.
Supplementary Material
ACKNOWLEDGMENTS
We thank all members of the Sabatini lab for helpful insights, suggestions, and discussion, in particular G. Sienski. We thank T. Kunchok from the Whitehead Institute Metabolite Profiling Facility, P. Thiru from BARC, the Whitehead Institute FACS facility, Genome Technology Core, and MIT MicrobioCenter for technical assistance, and the Vander Heiden laboratory for access to a GC-mass spectrometer. We thank A. Mennone and the Center for Cellular and Molecular Imaging (CCMI) imaging facilities at Yale University, supported by NIH grant S10 OD020142, as well as the W. M. Keck microscopy facility at the Whitehead Institute.
Funding: This work was supported by grants from the NIH to D.M.S (R01 CA103866, R01 CA129105, and R37 AI47389), and by the Department of Defense (W81XWH-07–0448) and a Cancer Research Institute Irvington Fellowship (Y.E.G.). N.K. is an HHMI fellow of the Damon-Runyon Cancer Research Foundation. D.M.S. is an investigator of the Howard Hughes Medical Institute and an ACS Research Professor.
Footnotes
Competing interests: N.K. and D.M.S. are inventors on a patent application filed by the Whitehead Institute relating to work described in this paper. None of the authors have a competing interest. Data and materials availability: All data are available in the manuscript or the supplementary materials. All expression plasmids were deposited at Addgene.
REFERENCES AND NOTES
- 1.Yang M, Vousden KH, Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 16, 650–662 (2016). doi: 10.1038/nrc.2016.81; pmid: 27634448 [DOI] [PubMed] [Google Scholar]
- 2.Locasale JW, Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 (2013). doi: 10.1038/nrc3557; pmid: 23822983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ducker GS, Rabinowitz JD, One-Carbon Metabolism in Health and Disease. Cell Metab. 25, 27–42 (2017). doi: 10.1016/j.cmet.2016.08.009; pmid: 27641100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tibbetts AS, Appling DR, Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr 30, 57–81 (2010). doi: 10.1146/annurev.nutr.012809.104810; pmid: 20645850 [DOI] [PubMed] [Google Scholar]
- 5.Barlowe CK, Appling DR, In vitro evidence for the involvement of mitochondrial folate metabolism in the supply of cytoplasmic one-carbon units. Biofactors 1, 171–176 (1988). pmid: 2475123 [PubMed] [Google Scholar]
- 6.Pfendner W, Pizer LI, The metabolism of serine and glycine in mutant lines of Chinese hamster ovary cells. Arch. Biochem. Biophys 200, 503–512 (1980). doi: 10.1016/0003-9861(80)90382-3; pmid: 6776895 [DOI] [PubMed] [Google Scholar]
- 7.Narkewicz MR, Sauls SD, Tjoa SS, Teng C, Fennessey PV, Evidence for intracellular partitioning of serine and glycine metabolism in Chinese hamster ovary cells. Biochem. J 313, 991–996 (1996). doi: 10.1042/bj3130991; pmid: 8611185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducker GS et al. , Reversal of Cytosolic One-Carbon Flux Compensates for Loss of the Mitochondrial Folate Pathway. Cell Metab. 23, 1140–1153 (2016). doi: 10.1016/j.cmet.2016.04.016; pmid: 27211901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu TF, Rife JP, Schirch V, The role of serine hydroxymethyltransferase isozymes in one-carbon metabolism in MCF-7 cells as determined by (13)C NMR. Arch. Biochem. Biophys 393, 42–50 (2001). doi: 10.1006/abbi.2001.2471; pmid: 11516159 [DOI] [PubMed] [Google Scholar]
- 10.Yu C, Claybrook DL, Huang AH, Transport of glycine, serine, and proline into spinach leaf mitochondria. Arch. Biochem. Biophys 227, 180–187 (1983). doi: 10.1016/0003-9861(83)90361-2; pmid: 6416178 [DOI] [PubMed] [Google Scholar]
- 11.Cybulski RL, Fisher RR, Mitochondrial neutral amino acid transport: Evidence for a carrier mediated mechanism. Biochemistry 16, 5116–5120 (1977). doi: 10.1021/bi00642a026; pmid: 911815 [DOI] [PubMed] [Google Scholar]
- 12.Cybulski RL, Fisher RR, Intramitochondrial localization and proposed metabolic significance of serine transhydroxymethylase. Biochemistry 15, 3183–3187 (1976). doi: 10.1021/bi00660a004; pmid: 952851 [DOI] [PubMed] [Google Scholar]
- 13.Labuschagne CF, van den Broek NJ, Mackay GM, Vousden KH, Maddocks OD, Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Reports 7, 1248–1258 (2014). doi: 10.1016/j.celrep.2014.04.045; pmid: 24813884 [DOI] [PubMed] [Google Scholar]
- 14.Wang T et al. , Identification and characterization of essential genes in the human genome. Science 350, 1096–1101 (2015). doi: 10.1126/science.aac7041; pmid: 26472758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birsoy K et al. , An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell 162, 540–551 (2015). doi: 10.1016/j.cell.2015.07.016; pmid: 26232224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pikman Y et al. , Targeting MTHFD2 in acute myeloid leukemia. J. Exp. Med 213, 1285–1306 (2016). doi: 10.1084/jem.20151574; pmid: 27325891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Field MS, Szebenyi DM, Stover PJ, Regulation of de novo purine biosynthesis by methenyltetrahydrofolate synthetase in neuroblastoma. J. Biol. Chem 281, 4215–4221 (2006). doi: 10.1074/jbc.M510624200; pmid: 16365037 [DOI] [PubMed] [Google Scholar]
- 18.Fleming MD, Campagna DR, Haslett JN, Trenor CC 3rd, Andrews NC, A mutation in a mitochondrial transmembrane protein is responsible for the pleiotropic hematological and skeletal phenotype of flexed-tail (f/f) mice. Genes Dev. 15, 652–657 (2001). doi: 10.1101/gad.873001; pmid: 11274051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miotto G, Tessaro S, Rotta GA, Bonatto D, In silico analyses of Fsf1 sequences, a new group of fungal proteins orthologous to the metazoan sideroblastic anemia-related sideroflexin family. Fungal Genet. Biol 44, 740–753 (2007). doi: 10.1016/j.fgb.2006.12.004; pmid: 17240176 [DOI] [PubMed] [Google Scholar]
- 20.Omasits U, Ahrens CH, Müller S, Wollscheid B, Protter: Interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 30, 884–886 (2014). doi: 10.1093/bioinformatics/btt607; pmid: 24162465 [DOI] [PubMed] [Google Scholar]
- 21.Lee SY et al. , APEX Fingerprinting Reveals the Subcellular Localization of Proteins of Interest. Cell Reports 15, 1837–1847 (2016). doi: 10.1016/j.celrep.2016.04.064; pmid: 27184847 [DOI] [PubMed] [Google Scholar]
- 22.Herbig K et al. , Cytoplasmic serine hydroxymethyltransferase mediates competition between folate-dependent deoxyribonucleotide and S-adenosylmethionine biosyntheses. J. Biol. Chem 277, 38381–38389 (2002). doi: 10.1074/jbc.M205000200; pmid: 12161434 [DOI] [PubMed] [Google Scholar]
- 23.Kao FT, Puck T, Mutagenesis and genetic analysis with Chinese hamster auxotrophic cell markers. Genetics 79 (Suppl), 343–352 (1975). pmid: 1171046 [PubMed] [Google Scholar]
- 24.McBurney MW, Whitmore GF, Isolation and biochemical characterization of folate deficient mutants of Chinese hamster cells. Cell 2, 173–182 (1974). doi: 10.1016/0092-8674(74)90091-9; pmid: 4547236 [DOI] [PubMed] [Google Scholar]
- 25.Patel H, Pietro ED, MacKenzie RE, Mammalian fibroblasts lacking mitochondrial NAD+-dependent methylenetetrahydrofolate dehydrogenase-cyclohydrolase are glycine auxotrophs. J. Biol. Chem 278, 19436–19441 (2003). doi: 10.1074/jbc.M301718200; pmid: 12646567 [DOI] [PubMed] [Google Scholar]
- 26.Bianchetti R, Lucchini G, Crosti P, Tortora P, Dependence of mitochondrial protein synthesis initiation on formylation of the initiator methionyl-tRNAf. J. Biol. Chem 252, 2519–2523 (1977). pmid: 323247 [PubMed] [Google Scholar]
- 27.Takeuchi N et al. , Recognition of tRNAs by Methionyl-tRNA transformylase from mammalian mitochondria. J. Biol. Chem 276, 20064–20068 (2001). doi: 10.1074/jbc.M101007200; pmid: 11274157 [DOI] [PubMed] [Google Scholar]
- 28.Morscher RJ et al. , Mitochondrial translation requires folate-dependent tRNA methylation. Nature 554, 128–132 (2018). doi: 10.1038/nature25460; pmid: 29364879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minton DR et al. , Serine Catabolism by SHMT2 Is Required for Proper Mitochondrial Translation Initiation and Maintenance of Formylmethionyl-tRNAs. Mol. Cell 69, 610–621.e5 (2018). doi: 10.1016/j.molcel.2018.01.024; pmid: 29452640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ducker GS et al. , Human SHMT inhibitors reveal defective glycine import as a targetable metabolic vulnerability of diffuse large B-cell lymphoma. Proc. Natl. Acad. Sci. U.S.A 114, 11404–11409 (2017). doi: 10.1073/pnas.1706617114; pmid: 29073064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyake S et al. , Identification and characterization of a novel mitochondrial tricarboxylate carrier. Biochem. Biophys. Res. Commun 295, 463–468 (2002). doi: 10.1016/S0006-291X(02)00694-0; pmid: 12150972 [DOI] [PubMed] [Google Scholar]
- 32.Azzi A, Glerum M, Koller R, Mertens W, Spycher S, The mitochondrial tricarboxylate carrier. J. Bioenerg. Biomembr 25, 515–524 (1993). doi: 10.1007/BF01108408; pmid: 8132491 [DOI] [PubMed] [Google Scholar]
- 33.Bisaccia F, De Palma A, Palmieri F, Identification and purification of the tricarboxylate carrier from rat liver mitochondria. Biochim. Biophys. Acta 977, 171–176 (1989). doi: 10.1016/S0005-2728(89)80068-4; pmid: 2804096 [DOI] [PubMed] [Google Scholar]
- 34.Kaplan RS, Mayor JA, Wood DO, The mitochondrial tricarboxylate transport protein. cDNA cloning, primary structure, and comparison with other mitochondrial transport proteins. J. Biol. Chem 268, 13682–13690 (1993). pmid: 8514800 [PubMed] [Google Scholar]
- 35.Palmieri F, The mitochondrial transporter family SLC25: Identification, properties and physiopathology. Mol. Aspects Med. 34, 465–484 (2013). doi: 10.1016/j.mam.2012.05.005; pmid: 23266187 [DOI] [PubMed] [Google Scholar]
- 36.Ye X et al. , Isolation and characterization of a novel human putative anemia-related gene homologous to mouse sideroflexin. Biochem. Genet 41, 119–125 (2003). doi: 10.1023/A:1022026001114; pmid: 12670026 [DOI] [PubMed] [Google Scholar]
- 37.Whittaker MM, Penmatsa A, Whittaker JW, The Mtm1p carrier and pyridoxal 5′-phosphate cofactor trafficking in yeast mitochondria. Arch. Biochem. Biophys 568, 64–70 (2015). doi: 10.1016/j.abb.2015.01.021; pmid: 25637770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grüneberg H, The anaemia of flexed-tailed mice (Mus musculus L.) II. Siderocytes. J. Genet 44, 246–271 (1942). doi: 10.1007/BF02982831 [DOI] [Google Scholar]
- 39.Lenox LE, Perry JM, Paulson RF, BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood 105, 2741–2748 (2005). doi: 10.1182/blood-2004-02-0703; pmid: 15591122 [DOI] [PubMed] [Google Scholar]
- 40.Fleming MD, Congenital sideroblastic anemias: Iron and heme lost in mitochondrial translation. Hematology 2011, 525–531 (2011). doi: 10.1182/asheducation-2011.1.525; pmid: 22160084 [DOI] [PubMed] [Google Scholar]
- 41.Paradkar PN, Zumbrennen KB, Paw BH, Ward DM, Kaplan J, Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol. Cell. Biol 29, 1007–1016 (2009). doi: 10.1128/MCB.01685-08; pmid: 19075006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.