SUMMARY
There is emerging evidence that circadian misalignment may alter energy expenditure, leading to obesity risk among those with irregular schedules[1–5]. It has been reported that energy expenditure is affected by the timing of sleep, exercise, and meals[6]. However, it is unclear whether the circadian system also modulates energy expenditure, independent of behavioral state and food intake. Here, we used a forced desynchrony protocol to examine whether fasted resting energy expenditure (REE) varies with circadian phase in 7 participants. This protocol allowed us to uncouple sleep-wake and activity-related effects from the endogenous circadian rhythm, demonstrating that REE varies by circadian phase. REE is lowest at circadian phase ~0°, corresponding to the endogenous core body temperature (CBT) nadir in the late biological night, and highest at circadian phase ~180° in the biological afternoon/evening. Furthermore, we found that respiratory quotient (RQ), reflecting macronutrient utilization, also varies by circadian phase. RQ is lowest at circadian phase ~240° and highest at circadian phase ~60°, which corresponds to biological morning. This is the first characterization of a circadian profile in fasted resting energy expenditure and fasted respiratory quotient (with rhythmic profiles in both carbohydrate and lipid oxidation), decoupled from effects of activity, sleep-wake cycle, and diet in humans. The rhythm in energy expenditure and macronutrient metabolism may contribute to greater weight gain in shift workers and others with irregular schedules.
Keywords: circadian phase, resting energy expenditure, respiratory quotient, carbohydrate oxidation, lipid oxidation
Summary
Zitting et al. demonstrate that resting energy expenditure varies with circadian phase and is lowest in the late biological night. This may contribute to weight gain in people with irregular sleep schedules, and highlights the importance of controlling for circadian phase and sleep-wake behavior when assessing energy expenditure.
RESULTS AND DISCUSSION
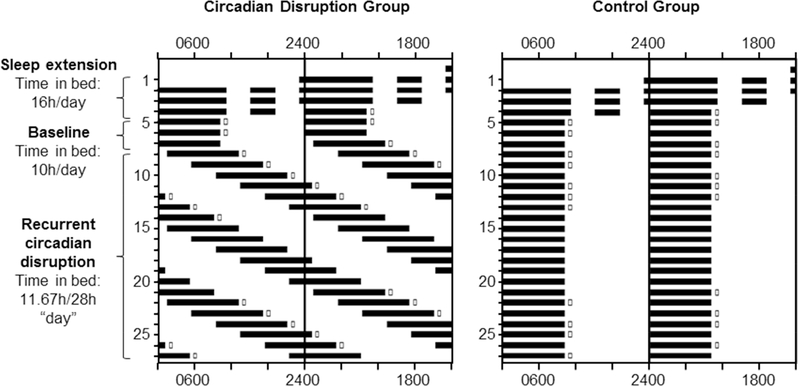
The potential influence of circadian timing on energy expenditure and macronutrient metabolism/utilization independent of behavioral state and food intake has not been investigated in humans. Here, we investigated the influence of circadian timing on resting energy expenditure (REE) and respiratory quotient (RQ) across three weeks of controlled diet and activity within a 37-day inpatient research protocol. Participants experienced either three weeks of recurrent circadian disruption (RCD) on a 28-hour rest-activity schedule (see Figure 1; circadian disruption group; n=7) or three weeks on a regular 24-hour schedule (Figure 1; control group; n=6).
Figure 1. Experimental Protocol.
Participants completed a 37-day laboratory protocol, beginning with 3 days of sleep extension with 16 hours/day time in bed, then a baseline segment of 3 days with 8–10 hours/day time in bed. In the Circadian Disruption group (left panel), sleep opportunities (dark bars) were then spread across the circadian cycle on a 28-hour forced desynchrony protocol, with 11.67 hours time in bed and 16.33 hours of wake for 3 weeks. Open boxes indicate when RMR was measured via calorimetry. In both groups days 28–37 consisted of a recovery/realignment segment (not depicted). Participants in the control group (right panel) had identical sleep extension and baseline segments, but underwent 3 weeks of sleep/wake identical to the baseline schedule with 8–10 hours time in bed each day. See also Table S1.
Circadian Variation of Fasting Resting Energy Expenditure
There is now emerging evidence that an irregular sleep-wake and fasting-feeding cycle, common in people working night or rotating shifts, can lead to disrupted circadian timing, which in turn may alter energy balance and lead to increased obesity risk[2, 4, 5, 7]. The positive energy balance associated with night work (which leads to weight gain and obesity over time) may be cause by increased energy intake (Bonham et al. 2016), changes in the timing/frequency of food intake[8–11], or decreased energy expenditure[1, 12].
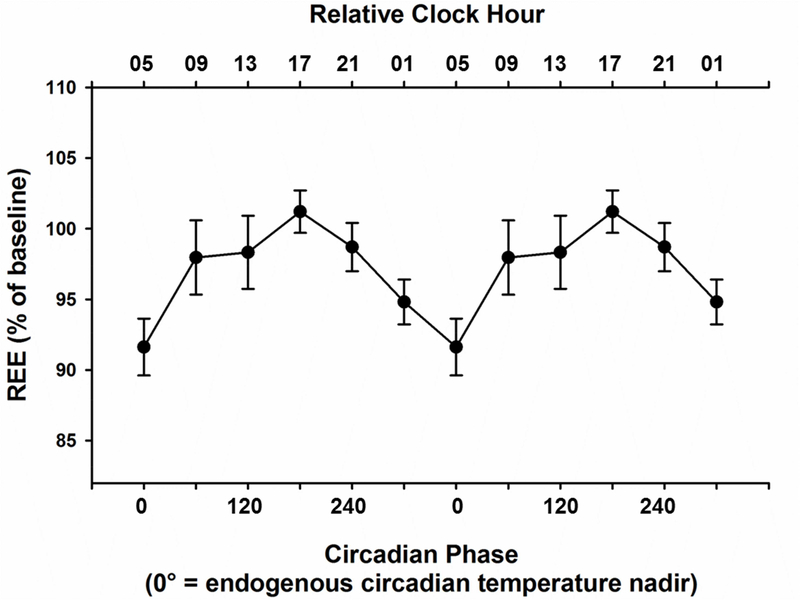
Resting energy expenditure (REE), also known as resting metabolic rate (RMR), is the largest component of daily energy expenditure and a major determinant of changes in weight[13]. REE accounts for 60–70% of all calories burned at rest each day to support basic physiological functions such as ventilation, circulation, temperature regulation, and brain activity. We show that fasted REE, decoupled from behavioral state and food intake, varies with circadian phase (Figure 2., Linear Mixed Model: n=7, F=10.76, p<0.0001), in a protocol that kept caloric intake, time-in-bed sleep opportunity, and exercise levels consistent across all phases, and over the duration of the study. REE is lowest at what we have defined as circadian phase 0°, corresponding to the nadir of the endogenous circadian rhythm of core body temperature in the late biological night, and is highest about 12 hours later at circadian phase ~180°, corresponding to the biological afternoon/evening (Cosinor Model: amplitude=55.2 kcal/day; acrophase=160.8°; H0: p<0.001). Thus, while awake and resting, the human body burns the fewest calories during the late biological night and the most calories during the biological afternoon/evening.
Figure 2. Circadian Variation of Fasting Resting Energy Expenditure.
Fasting levels of Resting Energy Expenditure (REE) are plotted with respect to the circadian phase at which they were recorded. Fasting REE is lowest at circadian phase 0, which corresponds to the endogenous core body temperature nadir (late biological night), and highest approximately 12 hours later at circadian phase 180 corresponding to biological afternoon/evening. Data are double-plotted and represented as mean ± SEM. For reference, a relative clock hour time scale illustrating the approximate time of day is shown across the upper axis, with 05:00 referenced to the endogenous circadian temperature nadir in this group of older individuals. See also Figure S1 and Figure S3.
Previous studies exploring diurnal changes in energy expenditure in humans[14–20] and animals[21–27] have reported mixed results. However, in attempting to evaluate the diurnal/circadian variation of REE, previous studies likely encountered confounding meal effects (e.g., breakfast measurement obtained after an overnight fast, whereas lunch and dinner measurements were obtained after prior meals). In the current study, each fasted recording during the three-week forced desynchrony protocol was performed shortly after a scheduled wake time, following an overnight fast of at least 12 hours. The caloric and macronutrient content of the meals consumed throughout the day after the fasted recording were similar, with calories varying by <5% from day-to-day within each participant.
We found no significant difference in REE values between the first and third weeks in the RCD group (Supplemental Information; Figure S1), suggesting that three weeks of recurrent circadian disruption does not change the overall REE or affect the circadian modulation of REE. There was also no significant difference in REE values between the first and third weeks in the control group (Supplemental Information; Figure S3), in which participants lived on a regular 24-hour schedule. These findings are in contrast to the results from our previous study, when we found that three weeks of sleep restriction combined with recurrent circadian disruption led to an 8% drop in RMR[28]. Sleep restriction alone either decreases fasting energy expenditure[28–30] or leads to no change, when measured at a consistent time of day[31–33]. Because we observed no change with circadian disruption alone (while minimizing sleep restriction) in the current study, the 8% reduction observed in our previous study[28] was likely due to sleep restriction or the combined effects of sleep restriction and circadian disruption.
Interestingly, while there was a significant effect on REE of day-to-day variability in body weight in both groups when tested independently (RCD: n=7, p=0.004; control group: n=6, p=0.009), this effect disappeared in the final statistical model for the RCD group (but not for the control group), suggesting that circadian timing is a stronger determinant of REE than small changes in weight. In our previous study, which included a recent history of sleep restriction combined with circadian disruption, changes in bodyweight were unrelated to changes in RMR[28]. This difference may be due to fewer RMR recordings (3 vs. >10) over the course of the previous study compared to the current study. In the present study baseline REE value predicted the REE during the three weeks of circadian disruption in the RCD group only (n=7; F=36.16, p<0.0001).
Circadian Variation of Fasting Respiratory Quotient, Carbohydrate, and Lipid Oxidation
The mean respiratory quotient (RQ), which is the ratio between carbon dioxide production and oxygen consumption, reflects the amount of energy derived from carbohydrates as opposed to lipids, and tends to be higher in individuals who are obese or who have Type 2 diabetes[13, 34–36] [37]. RQ is around 0.8 for a mixed diet, and typically varies between 0.7 (100% fatty acid oxidation, LO) and 1 (100% carbohydrate oxidation, CHO), depending on the proportion of substrates being metabolized.
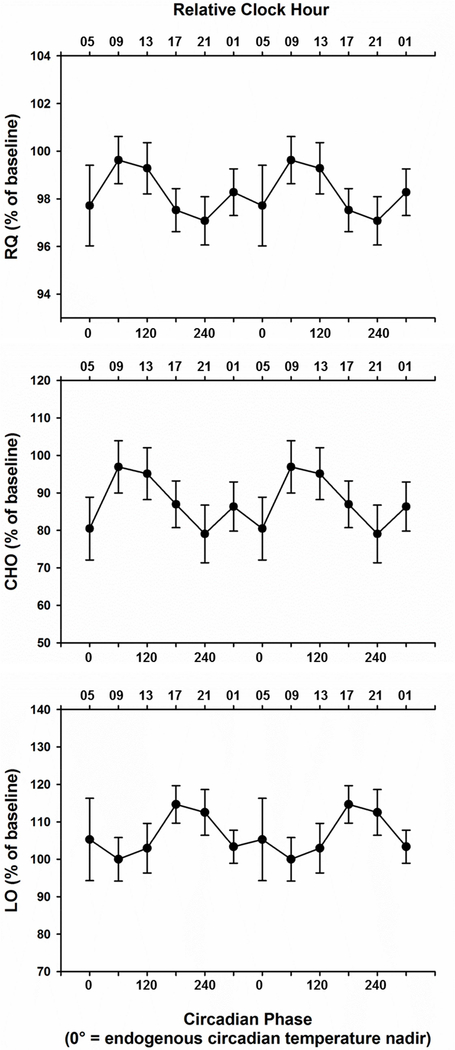
We show that, similar to REE, fasted RQ also varies by circadian phase (Figure 3, Linear Mixed Model: n=7, p=0.036). While mean RQ measured in the biological morning at baseline was 0.84 in both the RCD and control groups (Supplemental Information; Table S1), it varied by circadian phase, and was 2.5% lower in the biological evening compared to the biological morning in the RCD group (Cosinor Model: amplitude=0.012; acrophase=80.4°; H0: p=0.019). Fasting carbohydrate oxidation (CHO) and lipid oxidation (LO) also varied by circadian phase; CHO was highest during the biological morning and lowest during the biological evening (Cosinor Model: amplitude=0.012; acrophase=99.6°; H0: p=0.012), whereas LO was highest during the biological evening and lowest during the biological morning (Cosinor Model: amplitude=0.004; acrophase=226.2°; H0: p=0.019). There was also a significant effect of sex on RQ in the RCD group (n=7; F=14.31, p<0.001).
Figure 3. Circadian Variation of Fasting Respiratory Quotient, Carbohydrate, and Lipid Oxidation.
Top panel. Fasting Respiratory Quotient (RQ), the ratio of oxygen consumption and carbon dioxide production, is plotted with respect to the circadian phase at which it was measured. RQ is lowest at circadian phase 240° (corresponding to late biological evening) and highest at circadian phase 60°, which corresponds to biological morning. Changes in RQ level reflect macronutrient metabolism; higher RQ favors carbohydrate oxidation whereas lower RQ favors lipid oxidation. Middle panel. Fasting Carbohydrate oxidation (CHO) is plotted with respect to the circadian phase at which it was assessed. CHO is highest at circadian phase 60° (corresponding to biological morning) and lowest at circadian phase 240° (corresponding to late biological evening. Bottom panel. Lipid Oxidation (LO) is plotted with respect to the circadian phase at which it was measured. LO is highest at circadian phase 180°, and lowest at circadian phase 60°. Data in all three panels are double-plotted and represented as mean ± SEM. For reference, a relative clock hour time scale illustrating the approximate time of day is shown on the upper axis, with 05:00 referenced to the endogenous circadian temperature nadir in this group of older individuals. See also Figure S2 and Figure S3.
Duration of exposure to recurrent circadian disruption had no significant influence on the effect of circadian phase on RQ (Supplemental Information; Figure S2). This circadian variation in RQ and macronutrient utilization suggests that the body favors carbohydrate oxidation in the biological morning and lipid oxidation in the biological evening. We did not observe a significant difference in RQ between the first and third weeks in the RCD group (Supplemental Information; Figure S2) or in the control group (Supplemental Information; Figure S3), nor did we observe a sex difference in RQ in the control group. However, given that our power was limited due to small sample size, further studies will be needed to investigate variables that did not show significant differences. Our observations of circadian rhythms in fasting RQ, CHO, and LO are similar to those reported by Morris et al.[38]. While they did not find an overall effect of circadian phase on fasted RQ, when their analysis was confined to the acute effect of circadian misalignment Morris et al. observed a 4% decrease in fasted RQ in the biological evening compared to the morning (p=0.006) and a 26% increase in fasted LO in the biological evening compared to the morning (p=0.006), both of which are consistent with our current findings.
REE had a robust circadian rhythm with little variability in the timing of the peak between individuals (Circular Variance=0.264), suggesting that it parallels changes in core body temperature[39, 40]. In contrast, the circadian pattern in RQ was less consistent, showing a larger spread in the timing of the peak between individuals (Circular Variance=0.461). The circadian pattern of CHO was robust (Circular Variance=0.282) and tended to follow the pattern in RQ, whereas the pattern for LO showed larger inter-individual variability in the timing of peaks (Circular Variance=0.750). In summary, we found that REE varies with circadian phase and is highest during the biological day and lowest during the late biological night. This rhythm was robust; three weeks of circadian disruption had no measurable influence on the circadian variation of REE. Furthermore, we found that RQ, CHO, and LO also vary with circadian phase, with inter-individual variability in circadian patterns. These results may have important implications for understanding weight gain and obesity among night shift workers. They may also have relevance to non-shift workers; there is evidence that many individuals keep irregular schedules, including a large portion who have social jet lag. When taken together with evidence that many US adults eat throughout their entire wake episodes[41], the circadian variation in metabolic functions we have observed may impact weight gain more broadly. Future studies are required to investigate the contribution of circadian misalignment-induced changes in energy expenditure to weight gain and adverse metabolic consequences in night shift workers and in the general public.
Our observations also highlight the importance of controlling for the effect of circadian phase (misalignment) when carrying out recordings of energy expenditure in clinical and research settings. Per clinical guidelines and recommendations, REE should be measured in controlled conditions, including resting posture, after an 8-hour sleep, post-12-hour fast, in a thermo-neutral room, with dimmed lighting and quiet ambient conditions (e.g.[42–44]). However, those guidelines do not consider the biological time at which REE should be assessed. The difference of ~129 kcal/day (amplitude of the fitted curve 110 kcal/day) we found between the circadian peak and trough in REE is similar to or greater in magnitude to that caused by acute sleep deprivation [one night of sleep deprivation lowered REE by 103 kcal/day[29], and chronic sleep restriction (five nights of 4 hours time-in-bed lowered REE by 31–42 kcals/day[30, 33]]. Given the magnitude of REE change due to circadian phase alone, it is of utmost importance for research studies using calorimetry as a key outcome to assess energy expenditure to control for the circadian time at which calorimetry recordings are made. Circadian phase timing is influenced by many factors, including recent sleep-wake schedule (e.g. timing and duration of sleep as well as regularity of schedule), light exposure history, travel across time zones, history of night work or rotating shift work, age, sex, and diurnal preference of the participant. At present, there are no reliable methods to approximate circadian phase in individuals whose schedules are irregular, and no single point biomarkers of circadian timing. Researchers can minimize differences in circadian timing by ensuring all participants are on very regular sleep-wake schedules with the same amount of adequate sleep each night for a week prior to assessment, and carrying out calorimetry relative to the individual’s habitual schedule, or to assess circadian time using 24-hour melatonin profiles. Alternatively, researchers should consider using methods such as doubly labelled water, which provide an integrated measure of energy expenditure around the clock, rather than at one discrete phase, when phase cannot be estimated.
Limitations: In addition to the small number of participants, a potential limitation of our study is that the identity of the clocks driving circadian changes in energy expenditure and macronutrient metabolisms is not known, although evidence suggests that they are driven by the hypothalamic master circadian pacemaker.[21]
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jeanne Duffy (jduffy@research.bwh.harvard.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
10 participants completed the study protocol, 7 in the Recurrent Circadian Disruption (RCD) group and 6 in the Control group. One female participant completed the study three times and one male participant completed the study twice. No participant completed the study more than once in the same condition. Thus, we have data from 13 separate trials (mean ± sd, 57.6 + 7.24 years, range 38–69 yrs; 5 female, all post-menopausal). There is evidence that high-fat diet disrupts circadian rhythms and exacerbates the adverse effects of circadian disruption on metabolism [22, 45]. To study the interaction between circadian disruption and macronutrient composition on metabolism, we gave a subset of participants in each experimental condition a high-fat diet during the study (RCD group n=3, Control group n=2). However, there was no significant effect of diet on REE or RQ in the circadian disruption group or in the control group so we combined the diet groups for our analyses. See Supplemental Information Table S1 for details.
Ethical Approval
The Partners Health Care Institutional Review Board reviewed and approved this study (#2014P000243). The study conduct adhered to the ethical principles outlined in the Declaration of Helsinki and each participant provided written informed consent.
METHOD DETAILS
Recruitment and Screening
Healthy adult participants were recruited using online and newspaper advertisements, recruitment letters, and flyers. Participants completed a screening consisting of medical history evaluation, physical examination, electrocardiogram, clinical blood tests, and urinalysis to rule out medical disorders; an all-night at-home polysomnogram to rule out sleep disorders; psychological questionnaires (MMPI, Beck Depression Inventory) and a semi-structured clinical psychological interview to rule out psychological disorders. Exclusion criteria included current or chronic medical conditions, regular use of prescription or over-the-counter medication, BMI>32; current or past psychiatric or psychological disorders; smoking, excessive caffeine consumption; regular night shift work within the last 3 years, travel across more than one time zone within 3 months, significant sleep complaints, and habitual sleep duration shorter than 6.5 hours or longer than 9 hours.
Pre-Study Conditions
Participants maintained a self-selected regular sleep-wake schedule with a 10-hour nighttime sleep opportunity for at least 21 days prior to the inpatient study to ensure they were well-rested before the study began. Compliance was verified by wrist actigraphy, sleep diaries, and time-stamped voicemails at bed time and wake time. Participants were instructed not to use drugs (prescription, over-the-counter, recreational), alcohol, nicotine, or caffeine during this pre-study segment, and had a urine toxicology screen upon study admission to verify their compliance.
Inpatient Study Conditions
Participants were admitted to the Intensive Physiological Monitoring Unit of the Center for Clinical Investigation at Brigham & Women’s Hospital for the 37-day inpatient portion of the study, during which time they lived in an individual study room free of time cues. Light levels were maintained at ~90 lux during scheduled wake episodes and all lights were turned off throughout scheduled sleep opportunities. On three occasions during the study, the participant had a constant posture (CP) procedure, during which they were restricted to bed for the entire wake episode and the room lighting was maintained at a dim (~4 lux) level. To ensure all participants were well-rested before the intervention segment of the study began, the first three inpatient days included a 12-hour nighttime sleep opportunity as well as a 4-hour daytime nap opportunity (sleep extension segment of the study). Days five through seven (referred to as baseline) included an 8- or 10-hour nighttime sleep opportunity (see Figure 1 for details). For participants in the Control group, these baseline conditions were maintained for the remainder of the study (days 8–37). In the RCD group, the next 21 days were under forced desynchrony (FD) conditions consisting of eighteen, 28-hour “days” with 16.33-hour wake episodes and 11.67-hour sleep opportunities, corresponding to 10 hours sleep opportunity / 24 hours. Core body temperature (CBT) was recorded continuously throughout the RCD segment of the study with a rectal thermistor (Measurement Specialties, Inc., Dayton, OH) and was used to estimate circadian phase.
Study Diet
Participants were provided controlled meals throughout the inpatient study consisting of 5860% carbohydrates, 15–17% protein, and 25–27 % fat, with 800–1000 mg of calcium, 100 mEq (+/− 20%) of potassium, and 150 mEq (+/− 20%) of sodium. The 24-hour kilocalorie (kcal) target for each participant was calculated using the Mifflin-St. Joer equation with an activity factor 1.3, and was distributed evenly across breakfast, lunch, and dinner (33% ±30 kcals/meal). During the forced desynchrony segment in the RCD group, participants were given a snack after dinner which contained an additional 16.7% of the 24-hour kcal target to account for the 28-hour day. Participants who received a high-fat diet were provided meals containing 30–40% carbohydrates, 15–20% protein, and 45–50% fat, with 800–1250mg of calcium, 100 mEq (+/− 30%) of potassium, and 150 mEq (+/− 30%) of sodium. The Mifflin-St. Joer equation with an activity factor of 1.6 was used to calculate the 24-hour kcal target for these participants, and kcals were distributed across breakfast, lunch, dinner (25% +/− 5% daily target per meal) and 2 separate snacks (after lunch and after dinner, 12.5% +/− 5% daily kcal target per snack). If a participant’s REE measurements on the baseline days varied by more than 100 kcals from expected values, the kcal target per 24 hours was adjusted to match the measured REE value (See Supplemental Table S1 for individuals with adjusted intervention kcal targets).
Indirect Calorimetry measurements
Fasting indirect calorimetry measurements were performed within ~2 hours of scheduled wake times (VMAX Encore Metabolic Cart, CareFusion, CA). Baseline recordings were taken on study days 5 and 6 across all groups. Additional recordings were taken on most wake episodes during weeks 1 and 3 of the intervention phase. See Figure 1 for details. All participants were in bed and resting for at least 20 minutes before the start of each recording. REE was calculated on a minute-by-minute basis in kcals/day from gas expiration. Measurements from each recording were averaged to estimate REE in kilocalories per day (kcals/day) and RQ (dimensionless number), which is the amount of expired carbon dioxide (VCO2) per the amount of oxygen consumed (VO2). Carbohydrate oxidation and lipid oxidation rates were calculated as grams per minute (g/min) according to the formulae of Frayn[46], assuming negligible protein oxidation. Recordings lasted between 14 and 25 minutes. The first five minutes and the last minute of each recording were excluded from analysis[47]. Only data points with an associated fraction of exhaled carbon dioxide (FECO2) between 0.6–0.9% were included in the analysis[48]. Weight was measured each study day except on the constant posture days and the most recent weight was used for each recording.
Circadian Phase
Core body temperature (CBT) data were edited to remove sensor slips and removals. Intrinsic circadian period of the CBT data from the FD segment of the protocol was estimated for each participant in the RCD group using non-orthogonal spectral analysis (NOSA) [49]. NOSA takes into account the 28-hour periodicity in the data resulting from the imposed rest-activity schedule and searches for an unknown periodicity in the circadian range (search period 20–30 hours). From this estimate, a circadian phase (from 0 to 359°) was assigned to each minute of the study, with 0° corresponding to the minimum of the waveform fit to the entire temperature data series (endogenous circadian temperature nadir). For visualization purposes and to assess the effect of circadian phase on REE, RQ, CHO, and LO, data were averaged within each participant and across participants and binned into six 60-degree (~4-hour) circadian phase bins (0°, 60°, 120°, 180°, 240°, and 300°).
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses were carried out using SAS version 9.4 (SAS Institute, Cary, NC). The primary outcome variables REE and RQ were analyzed using Linear Mixed Models. Model diagnostics did not show a significant violation of the model assumptions for either outcome variable. Variables including age (young vs. older), gender (male vs. female), diet (regular diet vs. high-fat diet), weight (lbs), week (week 1 vs. week 3), baseline REE/RQ value, and circadian phase (RCD group only; 0°, 60°, 120°, 180°, 240°, and 300°) were treated as fixed effects, with a random intercept statement incorporated into the models to allow for means to vary between participants. We first tested each fixed effect independently and only included significant variables in the final multivariable model for each data set. Finally, a cosinor model (period of 24 h) was fitted to the REE and RQ data and to the data from the two secondary outcome variables CHO and LO, averaged across weeks 1 and 3, to estimate amplitude (one-half peak-to-trough variation) and acrophase (peak time) and to test the null hypothesis that the amplitude of the fitted curve is zero, i.e. no rhythm detected[50]. To quantify the spread in the distribution of peak values, a cosinor model was fitted to each individual’s data and the peak values were used to calculate circular variance [51]. Circular variance ranges from 0 to 1, with higher values indicating a larger spread of the data. All results are reported as mean ± SEM and expressed as percentage of the baseline value in the figures. The critical significance level was set to α=0.05 for all tests and only significant results are reported.
DATA AND SOFTWARE AVAILABILITY
Execution of a materials transfer agreement is required by our institution for transfer of data.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and Algorithms | ||
| NOSA (non-orthogonal spectral analysis) | [49] | N/A |
| Vmax Encore 29N | CareFusion | http://www.carefusion.com/ |
| Other | ||
| Rectal thermistor | Measurement Specialties, Inc. Dayton OH | Product# 861526TP |
Supplementary Material
Resting energy expenditure varies with circadian phase in humans.
Respiratory quotient varies with circadian phase in humans.
Resting energy expenditure is lowest in the late biological night.
Circadian variation in energy expenditure exceeds that caused by sleep deprivation.
ACKNOWLEDGEMENTS
We thank the research volunteers for their participation, Brigham and Women’s Hospital Center for Clinical Investigation (CCI) dietary and technical staff and the Division of Sleep and Circadian Disorders Chronobiology Core (Arick Wong, John Slingerland, Michael Harris, Julia Boudreau, Alec Rader, John Wise, Divya Mohan, Audra Murphy) for their assistance with data collection. Funding: This study was supported by a grant from the National Institute on Aging (P01 AG009975) and was conducted at the Brigham and Women’s Hospital Center for Clinical Investigation, part of Harvard Catalyst (Harvard Clinical and Translational Science Center) supported by NIH Award UL1 TR001102 and financial contributions from the Brigham and Women’s Hospital and from Harvard University and its affiliated academic health care centers. KMZ was supported by a fellowship from the Finnish Cultural Foundation. NV was supported by T32HL007901 and F32AG051325. RKY was supported by T32HL007901. CMI was supported by R01 AG044416.
Footnotes
DECLARATION OF INTERESTS
KMZ, NV, RKY, CMI, JEM, WW, JSW, and JFD have nothing to disclose. OBM has received subcontracts to Penn State from Mobile Sleep Technologies (National Science Foundation #1622766, National Institutes of Health R43AG056250). C.A.C. has received consulting fees from or served as a paid member of scientific advisory boards for: Bose Corporation; Boston Celtics; Boston Red Sox; Cephalon, Inc.; Columbia River Bar Pilots; Ganésco Inc.; Institute of Digital Media and Child Development; Jazz Pharmaceuticals; Klarman Family Foundation; Koninklijke Philips Electronics, N.V.; Merck & Co. Inc.; Novartis; Purdue Pharma; Quest Diagnostics, Inc.; Samsung Electronics; Sleep Multimedia, Inc.; Teva Pharmaceuticals; Vanda Pharmaceuticals; Washington State Board of Pilotage Commissioners and Zurich Insurance Company, Ltd. CAC has also received education/research support from Cephalon Inc., Jazz Pharmaceuticals, Mary Ann & Stanley Snider via Combined Jewish Philanthropies, National Football League Charities, Optum, Philips Respironics, ResMed Foundation, San Francisco Bar Pilots, Schneider Inc., Simmons, Sysco and Vanda Pharmaceuticals, Inc. The Sleep and Health Education Program of the Harvard Medical School Division of Sleep Medicine (which CAC directs) has received Educational Grant funding from Cephalon, Inc., Jazz Pharmaceuticals, Takeda Pharmaceuticals, Teva Pharmaceuticals Industries Ltd., Sanofi-Aventis, Inc., Sepracor, Inc. and Wake Up Narcolepsy. CAC is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, CAC has also served as an expert on various legal and technical cases related to sleep and/or circadian rhythms including those involving the following commercial entities: Bombardier, Inc.; Complete General Construction Company; Continental Airlines; FedEx; Greyhound; HG Energy LLC; Purdue Pharma, L.P.; South Carolina Central Railroad CO; Stric-Lan Companies LLC; Texas Premier Resource LLC; and United Parcel Service (UPS). CAC owns or owned an equity interest in Somnus Therapeutics, Inc., and Vanda Pharmaceuticals. He received royalties from the New England Journal of Medicine, McGraw Hill, Houghton Mifflin Harcourt, and Philips Respironics, Inc. for the Actiwatch-2 and Actiwatch-Spectrum devices. CAC’s interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.McHill AW, Melanson EL, Higgins J, Connick E, Moehlman TM, Stothard ER, and Wright KP Jr. (2014). Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proceedings of the National Academy of Sciences of the United States of America 111, 17302–17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Lorenzo L, De Pergola G, Zocchetti C, L’Abbate N, Basso A, Pannacciulli N, Cignarelli M, Giorgino R, and Soleo L (2003). Effect of shift work on body mass index: results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int J Obes.Relat Metab Disord 27, 1353–1358. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Depner CM, Perreault L, Markwald RR, Smith MR, McHill AW, Higgins J, Melanson EL, and Wright KP Jr. (2015). Morning Circadian Misalignment during Short Sleep Duration Impacts Insulin Sensitivity. Current biology : CB 25, 3004–3010. [DOI] [PubMed] [Google Scholar]
- 4.Antunes LC, Levandovski R, Dantas G, Caumo W, and Hidalgo MP (2010). Obesity and shift work: chronobiological aspects. Nutrition Research Reviews 23, 155–168. [DOI] [PubMed] [Google Scholar]
- 5.Markwald R, and Wright KP (2012). Principles and Practice of Sleep Medicine In Sleep Loss and Obesity: Intersecting Epidemics, Shiromani PJ, ed. (Springer Science + Business Media, LLC; ), pp. 101–118. [Google Scholar]
- 6.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, and Wright KP Jr. (2013). Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proceedings of the National Academy of Sciences of the United States of America 110, 5695–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arble DM, Bass J, Behn CD, Butler MP, Challet E, Czeisler C, Depner CM, Elmquist J, Franken P, Grandner MA, et al. (2015). Impact of Sleep and Circadian Disruption on Energy Balance and Diabetes: A Summary of Workshop Discussions. Sleep 38, 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, and Scheer FA (2013). Timing of food intake predicts weight loss effectiveness. International journal of obesity 37, 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LK, Garaulet M, Scheer FA, and Klerman EB (2017). Later circadian timing of food intake is associated with increased body fat. The American journal of clinical nutrition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FA, et al. (2014). Meal frequency and timing in health and disease. Proceedings of the National Academy of Sciences of the United States of America 111, 16647–16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arble DM, Bass J, Laposky AD, Vitaterna MH, and Turek FW (2009). Circadian timing of food intake contributes to weight gain. Obesity. (Silver.Spring) 17, 2100–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bushnell PT, Colombi A, Caruso CC, and Tak S (2010). Work schedules and health behavior outcomes at a large manufacturer. Industrial Health 48, 395–405. [DOI] [PubMed] [Google Scholar]
- 13.Marra M, Scalfi L, Covino A, Esposito-Del Puente A, and Contaldo F (1998). Fasting respiratory quotient as a predictor of weight changes in non-obese women. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 22, 601–603. [DOI] [PubMed] [Google Scholar]
- 14.Bo S, Fadda M, Castiglione A, Ciccone G, De Francesco A, Fedele D, Guggino A, Parasiliti Caprino M, Ferrara S, Vezio Boggio M, et al. (2015). Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. International journal of obesity 39, 1689–1695. [DOI] [PubMed] [Google Scholar]
- 15.Kräuchi K, and Wirz-Justice A (1994). Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. American Journal of Physiology 267, R819–R829. [DOI] [PubMed] [Google Scholar]
- 16.Weststrate JA, Weys PJ, Poortvliet EJ, Deurenberg P, and Hautvast JG (1989). Diurnal variation in postabsorptive resting metabolic rate and diet-induced thermogenesis. The American journal of clinical nutrition 50, 908–914. [DOI] [PubMed] [Google Scholar]
- 17.Romon M, Edme JL, Boulenguez C, Lescroart JL, and Frimat P (1993). Circadian variation of diet-induced thermogenesis. The American journal of clinical nutrition 57, 476–480. [DOI] [PubMed] [Google Scholar]
- 18.Miles CW, Wong NP, Rumpler WV, and Conway J (1993). Effect of circadian variation in energy expenditure, within-subject variation and weight reduction on thermic effect of food. Eur J Clin Nutr 47, 274–284. [PubMed] [Google Scholar]
- 19.Leuck M, Levandovski R, Harb A, Quiles C, and Hidalgo MP (2014). Circadian rhythm of energy expenditure and oxygen consumption. JPEN J Parenter Enteral Nutr 38, 263–268. [DOI] [PubMed] [Google Scholar]
- 20.Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, and Scheer FA (2015). The Human Circadian System Has a Dominating Role in Causing the Morning/Evening Difference in Diet-Induced Thermogenesis. Obesity 23, 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coomans CP, van den Berg SA, Lucassen EA, Houben T, Pronk AC, van der Spek RD, Kalsbeek A, Biermasz NR, Willems van Dijk K, Romijn JA, et al. (2013). The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes 62, 1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. (2005). Obesity and Metabolic Syndrome in Circadian Clock Mutant Mice. Science 308, 1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutter J, Reick M, and McKnight SL (2002). Metabolism and the control of circadian rhythms. Annu. Rev Biochem 71, 307–331. [DOI] [PubMed] [Google Scholar]
- 24.Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, and Panda S (2009). Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proceedings of the National Academy of Sciences of the United States of America 106, 21453–21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Klinken JB, van den Berg SA, Havekes LM, and Willems Van Dijk K (2012). Estimation of activity related energy expenditure and resting metabolic rate in freely moving mice from indirect calorimetry data. PLoS ONE 7, e36162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reznick J, Preston E, Wilks DL, Beale SM, Turner N, and Cooney GJ (2013). Altered feeding differentially regulates circadian rhythms and energy metabolism in liver and muscle of rats. Biochimica et Biophysica Acta 1832, 228–238. [DOI] [PubMed] [Google Scholar]
- 27.Orozco-Solis R, Aguilar-Arnal L, Murakami M, Peruquetti R, Ramadori G, Coppari R, and Sassone-Corsi P (2016). The circadian clock in the ventromedial hypothalamus controls cyclic energy expenditure. Cell Metab 23, 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, and Shea SA (2012). Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Science Translational Medicine 4, 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benedict C, Hallschmid M, Lassen A, Mahnke C, Schultes B, Schioth HB, Born J, and Lange T (2011). Acute sleep deprivation reduces energy expenditure in healthy men. The American Journal of Clinical Nutrition 93, 1229–1236. [DOI] [PubMed] [Google Scholar]
- 30.Spaeth AM, Dinges DF, and Goel N (2015). Resting metabolic rate varies by race and by sleep duration. Obesity 23, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, and Adler GK (2010). Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 59, 2126–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nedeltcheva AV, Kessler L, Imperial J, and Penev PD (2009). Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab 94, 3242–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A, and Jones PJ (2011). Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. The American journal of clinical nutrition 94, 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellis AC, Hyatt TC, Hunter GR, and Gower BA (2010). Respiratory quotient predicts fat mass gain in premenopausal women. Obesity 18, 2255–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, Swinburn BA, Knowler WC, Bogardus C, and Ravussin E (1990). Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol 259, E650–657. [DOI] [PubMed] [Google Scholar]
- 36.Kelley DE, and Mandarino LJ (1990). Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. The Journal of clinical investigation 86, 1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weyer C, Pratley RE, Salbe AD, Bogardus C, Ravussin E, and Tataranni PA (2000). Energy expenditure, fat oxidation, and body weight regulation: a study of metabolic adaptation to long-term weight change. The Journal of clinical endocrinology and metabolism 85, 1087–1094. [DOI] [PubMed] [Google Scholar]
- 38.Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, and Scheer FA (2015). Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proceedings of the National Academy of Sciences of the United States of America 112, E2225–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aschoff J, and Heise A (1972). Thermal conductance in man: Its dependence on time of day and on ambient temperature. In Advances in Climatic Physiology, Itoh S, Ogata K and Yoshimura H, eds. (Tokyo: Igaku, Shoin Limited; ), pp. 334–348. [Google Scholar]
- 40.Krauchi K (2002). How is the circadian rhythm of core body temperature regulated? Clin Auton Res 12, 147–149. [DOI] [PubMed] [Google Scholar]
- 41.Gill S, and Panda S (2015). A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 22, 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Psota T, and Chen KY (2013). Measuring energy expenditure in clinical populations: rewards and challenges. Eur J Clin Nutr 67, 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benedict FG, and Crofts EE (1925). The Fixity of Basal Metabolism. Proceedings of the National Academy of Sciences of the United States of America 11, 585–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wooley JA, and Sax HC (2003). Indirect calorimetry: applications to practice. Nutr Clin Pract 18, 434–439. [DOI] [PubMed] [Google Scholar]
- 45.Dyar KA, Lutter D, Artati A, Ceglia NJ, Liu Y, Armenta D, Jastroch M, Schneider S, de Mateo S, Cervantes M, et al. (2018). Atlas of circadian metabolism reveals system-wide coordination and communication between clocks. Cell 174, 1571–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frayn KN (1983). Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol 55, 628–634. [DOI] [PubMed] [Google Scholar]
- 47.Fullmer S, Benson-Davies S, Earthman CP, Frankenfield DC, Gradwell E, Lee PS, Piemonte T, and Trabulsi J (2015). Evidence analysis library review of best practices for performing indirect calorimetry in healthy and non-critically ill individuals. J Acad Nutr Diet 115, 1417–1446 e1412. [DOI] [PubMed] [Google Scholar]
- 48.Schadewaldt P, Nowotny B, Strassburger K, Kotzka J, and Roden M (2013). Indirect calorimetry in humans: a postcalorimetric evaluation procedure for correction of metabolic monitor variability. The American journal of clinical nutrition 97, 763–773. [DOI] [PubMed] [Google Scholar]
- 49.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, et al. (1999). Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284, 2177–2181. [DOI] [PubMed] [Google Scholar]
- 50.Nelson W, Tong YL, Lee J, and Halberg F (1979). Methods for cosinor-rhythmometry. Chronobiologia 6, 305–323. [PubMed] [Google Scholar]
- 51.Chua EC, Shui G, Lee IT, Lau P, Tan LC, Yeo SC, Lam BD, Bulchand S, Summers SA, Puvanendran K, et al. (2013). Extensive diversity in circadian regulation of plasma lipids and evidence for different circadian metabolic phenotypes in humans. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.