Abstract
Predicting the uncertainties of the ever-changing environment provides a competitive advantage for animals. The need to anticipate food sources has provided a strong evolutionary drive for synchronizing behavioral and internal processes with daily circadian cycles. When food is restricted to a few hours per day, rodents exhibit increased wakefulness and foraging behavior preceding the arrival of food. Interestingly, while the master clock located in the suprachiasmatic nucleus entrains daily rhythms to the light cycle, it is not necessary for this food anticipated activity. This suggests the existence of a food-entrained oscillator located elsewhere. Based on the role of nigrostriatal dopamine in reward processing, motor function, working memory, and internal timekeeping, we propose a working model by which food entrained dopamine oscillations in the dorsal striatum can enable animals maintained on a restricted feeding schedule to anticipate food arrival. Finally, we summarize how metabolic signals in the gut are conveyed to the nigrostriatal pathway to suggest possible insight into potential input mechanisms for food anticipatory activity.
Keywords: anticipation, nigrostriatal, dopamine, food-entrained oscillator, circadian, restricted feeding, dorsal striatum, food anticipatory activity
Introduction
“It is not the strongest of the species that survives, nor the most intelligent, but the one most responsive to change.”
Charles Darwin, 1809
Predicting the uncertainties of the ever-changing environment provides a competitive advantage for animals. Anticipating environmental changes such as daily and seasonal variations in food availability can optimize metabolic benefit while minimizing the risk of predation. As a result, there has been a strong evolutionary drive leading to the development of biological clocks that enable internal timekeeping by synchronization to the 24-hour rotation of the earth. Nearly all cells and tissues in the body act as circadian clocks that help coordinate physiological, biological, and behavioral processes with their changing environment. In the case of feeding, physiological processes are engaged in preparation for digestion of an anticipated meal, while simultaneously increasing the motivation to forage and prepare for food.1 These clocks are largely entrained by light, under the control of the master circadian oscillator located in the suprachiasmatic nucleus (SCN) of the hypothalamus.2 However, at least in a lab setting, when food availability is restricted, animals will increase activity levels outside of normal wakeful hours in response to a predicted food source. Therefore, both light and food are capable of entraining circadian rhythms.
Role of the SCN
The clock in the SCN is thought to be the master biological clock, entrained by light from the eyes via the retinohypothalamic circuit.3,4 It synchronizes internal biochemical processes to changing cycles of day and night to set temporal regulation of vital homeostatic functions.2,5–9 Therefore, the SCN clock performs the important function of setting internal rhythms according to predictable external environmental changes, and prevents competing behavioral (ie. wake/sleep) or cellular events occurring simultaneously. At the molecular level, circadian oscillation in the mammalian SCN is controlled by an interlocking feedback loop.10 First, the transcription factors BMAL1 and CLOCK heterodimerize and bind to the e-box sequence in the promoter region of Cryptochrome (CRY) and Period (PER) genes to activate transcription. CLOCK and BMAL1 transcription is in turn inhibited by CRY and PER protein translation in a cycle oscillating at ~24 hours.11 The second half of the interlocking feedback loop involves the transcription factor Rev-Erbα and retinoid-related orphan receptor (ROR), which modulate BMAL1 transcription.12 Depending on the organ, 3–16% of all protein coding gene are under the transcriptional regulation of clock proteins,13 highlighting the importance of circadian oscillation in internal biochemistry and its impact on subsequent behaviors, including feeding.
Disruption of circadian rhythms through lifestyle modifications in human subjects or clock-gene mutations in animal models results in a clear association with obesity and diabetes. Acute sleep disruptions are sufficient to reduce glucose tolerance,14 increase appetite and increase preference for palatable calorie-dense foods.15 In addition, sleep restriction reduces circulating leptin levels while increasing ghrelin levels, resulting in an endocrine state favoring overconsumption of food.16 Clinical studies investigating the role of chronic sleep disruption in individuals that suffer from insomnia or perform shift work show a significantly increased risk of metabolic disease.17–19 Similarly, knockout mice with global loss of CLOCK or PER2 genes have disrupted circadian feeding patterns with increased susceptibility for diet-induced weight gain.20,21
Food Anticipatory Activity
Under fed ad-libitum conditions, the SCN appears to be the dominant oscillator controlling food intake in rodents, with most food consumed in the active (dark) phase of the light cycle. However, when animals are entrained for 3–4 days to a regular daily mealtime at an interval of ~24 hours, they exhibit food anticipatory activity (FAA),22–26 characterized by increased locomotor activity and food-seeking behaviors beginning 1–3 hours prior to mealtime.26 FAA arises regardless of whether the entrained mealtime occurs during the light or dark phase,22,27 although it is most obvious in animals entrained to a mealtime during the inactive (light) phase because of the dramatic phase shift in activity and physiological signals. This suggests a yet undefined feeding entrained oscillator (FEO) that is separate from and takes priority over the SCN clock under conditions where food is only available during regular, finite intervals. When returned to ad-libitum feeding, the SCN reasserts circadian rhythmicity and rodents revert to primarily nocturnal food consumption. Subsequently, if animals are food restricted again, even days or weeks later, FAA immediately reasserts at the previously entrained mealtime after a single exposure.28,29 Further supporting the distinction between the SCN clock and the FEO, animals exposed to heavy water for long periods of time lose circadian rhythmicity due to deuterium-induced increase in the time constant, which prevents light entrainment, but retain FAA at an entrained mealtime.30 Additionally, animals with SCN ablation or lacking the transcription factor BMAL1 can still be entrained to a regular mealtime.22,26,31,32 A similar expression of FAA has been observed in response to daily feeding of a palatable meal in SCN-ablated or mildly food restricted hamsters33 and mildly food restricted rats.34,35
In summary, there are two pacemakers that normally act in concert to regulate daily rhythms of physiology and behavior. The master clock in the suprachiasmatic nucleus entrains daily rhythms to the light cycles, while a second food entrained oscillator promotes food anticipatory activity. The two can become dissociated when bouts of food become regularly available out of synchrony with the normal light-entrained wake cycles. Given that circadian food anticipatory rhythms persist in SCN-ablated rats and mice, it follows that a FEO must be located elsewhere.
DMH neurons as FEO
The source of the FEO in rodents remains unclear. Systematic lesioning studies have been unsuccessful thus far in identifying a single brain region that is responsible for FEO, leading to the idea that the FEO may be made up of a network of neuronal connections in different areas of the brain. Lesion studies have been undertaken throughout the hypothalamus, most notably the dorsomedial hypothalamic nuclei (DMH), due to its direct connections to and from the SCN,36,37 and its role in SCN-mediated circadian food intake, energy homeostasis, and arousal.38–40 Furthermore, the DMH exhibits food-entrained oscillations in clock genes, depending on the timing of the meal.41–45 Rodent studies ablating the DMH, all or in part, have demonstrated conflicting effects on FAA. One study in rats that ablated cell bodies in the DMH but spared passing fibers showed a correlation between lesion size and attenuation of FAA.38 However, several others observed intact FAA following total DMH ablation.24,39,43,46 In mice with fully ablated mediobasal hypothalamic regions including the DMH, ventromedial hypothalamus, and arcuate nucleus, there was a total loss of FAA expression in response to a restricted light phase meal.47 The lack of consistent results suggests that while the DMH may play a role in FAA, it may not be the primary arbiter of FEO. One explanation which might reconcile some of these results is the finding that the ventral portion of the DMH is most critical for FAA expression, because it contains GABAergic projections to the SCN, which are hypothesized to suppress normal circadian rhythms in favor of FAA when the oscillations of the FEO and SCN are in conflict.48 Thus, it is plausible that there exists a subpopulation of neurons in the DMH responsible for FEO function.
Midbrain dopamine neurons as FEO
There is growing evidence that dopaminergic neurons in the midbrain play a role in FAA. There are two important clusters of dopaminergic neurons in the midbrain, localized in the substantia nigra and the ventral tegmental area that are critical for reward-based decision making. These form two distinct circuits: 1) mesolimbic projections connecting the dopaminergic ventral tegmental area neurons to the nucleus accumbens and olfactory tubercle located in the ventral striatum, and 2) the nigrostriatal projections connecting the dopaminergic substantia nigra pars compacta neurons to the caudate nucleus and putamen located in the dorsal striatum. Dopamine signaling through dopamine receptors (D1 and D2) in these regions are typically associated with reward processing,49 motor function,50 and working memory.51 Therefore, the possibility that these circuits may be involved in an activity-based, motivated behavior that relies on remembering past events, such as FAA, makes intuitive sense. This is supported empirically by the fact that dopamine agonists and antagonists have opposing effects on anticipatory rhythms. FAA can be shifted by a single peripheral injection of a D2 receptor agonist,52 and can be attenuated by intraperitoneal injection of D1 and D2 antagonists.53
Importantly, these two dopaminergic circuits are recruited in response to a range of reward stimuli. Palatable calorie dense foods are potent rewards that release DA in both the dorsal and ventral striatum,54 and in humans the rise in dorsal dopamine increases proportionally to the pleasure associated with consumption of food.55 The stimuli that activate these two circuits were recently dissociated in an elegant set of animal experiments.56 Dopamine release in the dorsal and ventral striatum were measured while animals licked a non-nutritive sweetener that triggered intragastric infusion of either the same solution or a caloric sugar. Using this paradigm a rise in ventral dopamine occurred in response to licking irrespective of the intragastric solution, while dorsal dopamine was only elevated in response to intragastric caloric sugar,57 suggesting that dopamine release in the ventral striatum is responsive to gustatory signals while the nigrostriatal dopamine circuit is recruited by metabolic signals. In support of this concept, irrespective of intragastric caloric value, licking a bitter solution prevents release of dopamine in the ventral striatum; conversely, dorsal striatum dopamine is released when paired with intragastric infusion of a caloric – but not a non-nutritive, or non-metabolizable – solution.56 The preference for aversive, but nutritive solutions was abolished when D1 neurons in the dorsal striatum were selectively ablated; conversely, optogenetic stimulation of D1 neurons in the dorsal striatum paired to licking, increased licking for both sweet and bitter solutions.56 This indicates that D1 neurons in the dorsal striatum are recruited by nutritive substances to produce motivated behavior. It is interesting to note, as discussed below in more detail, that FAA also requires nutritive signals and forms independently of gustatory signals,24 suggesting a prominent role for dorsal striatal dopamine release in the formation of FAA.
Rhythmic dopamine release has been reported in the dorsal striatum,58 peaking in the middle of the dark phase when rodents are most active, and falling during the light phase as activity levels drop.59,60 Importantly, dopamine fluctuations in the dorsal striatum parallel the daily rhythm of clock gene PER2.61–63 Depletion of dopamine by lesioning of dopaminergic projections to the dorsal striatum suppresses PER2 oscillations, which is rescued by dopamine agonists.64 Dopamine regulation of clock genes in the dorsal striatum appears to be mediated by D1-receptor signaling since D1, but not D2, knockout mice have blunted PER2 expression rhythms in the dorsal striatum.65 Neither the rhythmic release of dopamine, nor dopamine-dependent oscillation of PER2, require light entrainment and prevail in SCN-lesioned animals.64 In summary, dorsal striatal fluctuations can be dissociated from light entrainment.
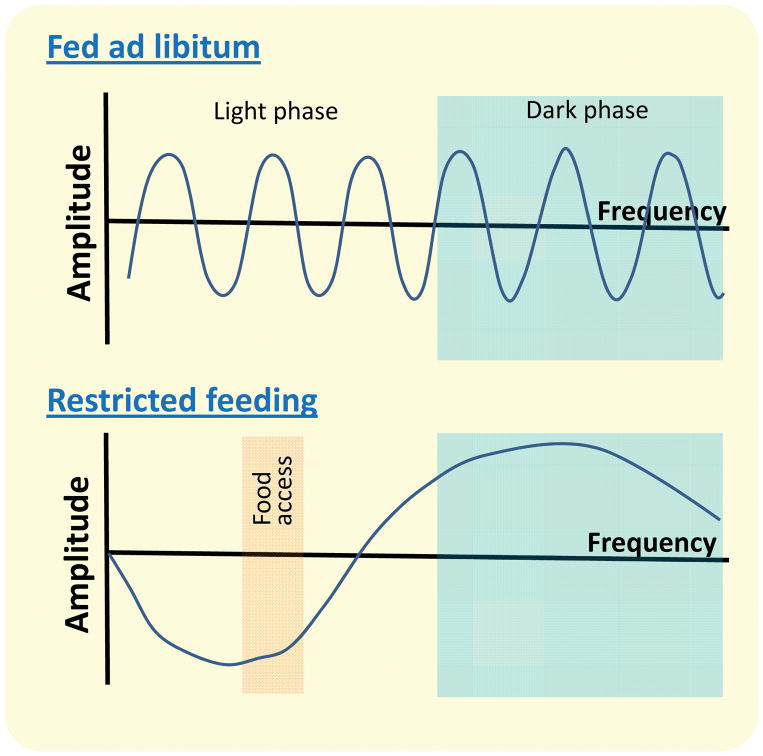
It has been reasoned that the FEO must be entrainable by food, but continue to oscillate at the same frequency in the absence of food, since FAA persists for days in restricted animals even in the absence of food at the anticipated time.24 Dorsal striatal dopamine fluctuations may achieve this by controlling the length of ultradian rhythms.66 Ultradian rhythms are short recurrent cycles that repeat multiple times throughout the day, typically lasting 4h.67 These oscillations persist in the absence of the suprachiasmatic nucleus,66 suggesting that they do not require light entrainment. Preventing dopamine reuptake in the dorsal striatum lengthens ultradian rhythm to 12 hours.66 Conversely, methamphetamine administration, a stimulant that provokes a large release of dopamine in the dorsal striatum, and chemogenetic activation of dopamine neurons projecting to the dorsal striatum both prolong ultradian rhythms, with methamphetamine prolonging cycles up to 100 hours.66
Phasic dopamine has a well-characterized role in reward prediction errors and therefore is an important signal for integrating temporal information about the time delay for a reward.68,69 Exogenous stimulation of dopamine signaling reduces the ability of rodents to perform timing behavior.70,71 Interestingly, optically or chemogenetically overriding dopaminergic neuronal activity in the dorsal striatum alters time estimation,72 linking dorsal striatal dopamine with internal timekeeping. The argument used against an interval timing mechanism is that animals fail to develop food anticipatory activity when daily meal onsets diverge excessively from 24h.73 However, dopamine induced interval timing have an intrinsic rhythmicity that cycles around 24h when entrained by a meal (Figure 1), and would therefore likely fail to generate anticipatory activity if the subsequent arrival of food occurs outside of the window of time when dopamine levels are lowest. Altogether these data support the concept that dorsal striatum dopamine is released in response to a nutritive meal, capable of being dissociated from canonical circadian rhythm, while providing a sense of time linked to reward that can be reinforced to ensure that the arrival of food is appropriately anticipated. Dopamine release in the dorsal striatum therefore exhibits all the hallmarks of a food-entrainable oscillator.
Figure 1. Hypothetical dorsal striatum dopamine oscillations depending on food availability.
A) Phasic dopamine in fed ad libitum conditions with equal amplitude and frequency throughout the day. B) In animals with restricted access to food, dopamine levels rise after consumption of caloric intake in light phase meal 56. We predict based on evidence that rewards prolong dopamine oscillations 66 that phasic dopamine continues but with reduced amplitude, reaching a nadir prior to a meal.
Pathways required for food entrained anticipatory activity
There is remarkable overlap between feeding signals that recruit dorsal striatum circuits and those that can function as zeitgebers to produce FAA. As described above, dorsal striatum dopamine is not released in response to gustatory signals. Similarly, anticipatory activity does not require a sense of taste or smell since it forms normally in anosmic rats74 and following lesions to the olfactory bulb.75 Likewise, the magnitude of the phase delays of anticipatory activity is influenced by caloric intake,76 while the volume of food has no effect on dopamine release77 and is insufficient to promote FAA.76 Fats and sugars are capable of producing food anticipation in rats,78 and are sufficient alone to cause dopamine efflux in the dorsal striatum.77,79 Just as described above for dopamine release, non-nutritive substances are insufficient to produce FAA, irrespective of quantity, taste or texture.80 Both nigrostriatal-dependent behaviors81 and FAA80 can occur in ad libitum fed animals in response to a palatable nutritive snack, but are most effective after partial food restriction.
Given that the neural and molecular substrates that control FAA remain poorly defined, could mechanistic insight be derived from examining the pathways that recruit dorsal striatum circuitry in response to feeding? Given that fats and sugars are potent activators of dopamine release in the dorsal striatum, and promote FAA, we hypothesize that neural and humoral pathways activated in response to fat and sugar ingestion that release dopamine in the dorsal striatum may also be required to produce FAA. Therefore, we first review fat and sugar sensing in the gut, before discussing evidence for these pathways in dorsal striatum dopamine release and assess the role of these mechanisms for the development of FAA. Importantly, this is not meant to be an exhaustive review of lipid and carbohydrate signaling (for more detailed overview see82 but rather to provide a guide to putative signals and pathways that might initiate FAA. We then examine the evidence for these mechanisms in dopamine release and FAA formation.
Nutrient sensing pathways
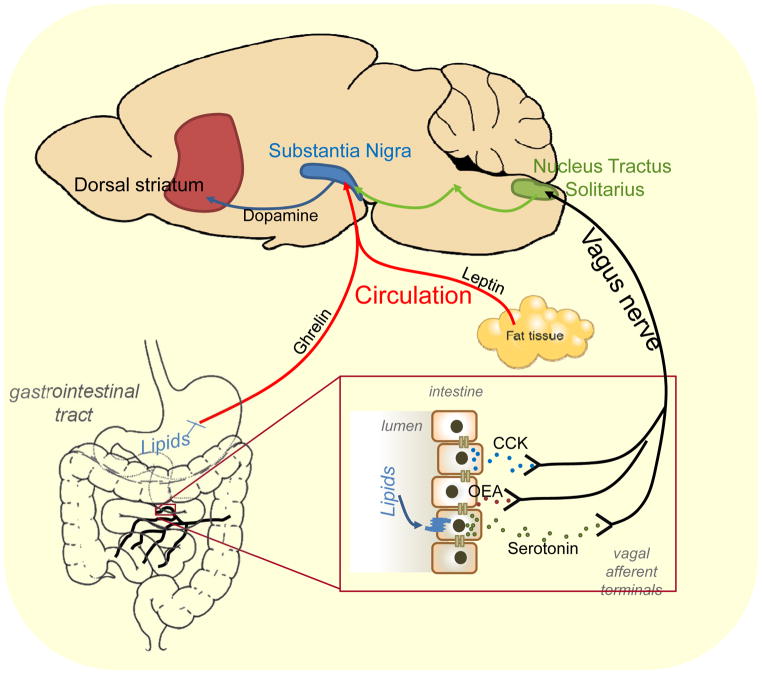
The sight, smell, or taste of palatable foods, particularly those high in fat and sugars, are potent rewards54 that promote food consumption - even in the absence of need. In addition, the gut acts as a surveillance system, sensing the presence or absence of nutrients in the lumen.83 Enterocytes, specialized enteroendocrine cells, microbiota and afferent terminals in the gut all include the necessary machinery to sense the types or volume of nutrients ingested. This offers a highly complex and redundant mechanisms to sense the full range of ingested nutrients. This information is conveyed to the brain by three different pathways: humoral, vagal or spinal routes. Here we briefly review the pathways recruited specifically in response to dietary sugar (figure 2) and fats (figure 3).
Figure 2.
Carbohydrate mediated signaling pathways capable of activating dopaminergic nigrostriatal circuit.
Figure 3.
Lipid mediated signaling pathways capable of activating dopaminergic nigrostriatal circuit.
In the absence of nutrients, the gastrointestinal hormone ghrelin is released from specialized enteroendocrine X/A cells in the stomach. In response to a meal, gastric distension alone is not sufficient to inhibit ghrelin release. Instead the ingestion of either fats or carbohydrates individually are sufficient to inhibit ghrelin synthesis,84 in a process that requires gastric emptying.85 The role of ghrelin in feeding remains unclear. Although originally hypothesized to predict the arrival of food, recent studies indicate that activation of circulating ghrelin requires acylation by the enzyme ghrelin O-acyl-transferase (GOAT) in response to dietary fats.86 Irrespective, ghrelin is a circulating factor that is directly modulated by fats and sugar intake (figure 2 and 3) and may play a role in FAA. Conversely gastric distension is a process that is insensitive to nutritive value87. Mechanosensitive vagal afferent fibers of the stomach are modulated by gastrointestinal hormones and nutrients,88 but the functional significance of this modulation remains to be determined.
Intestinal lipid infusions rapidly and potently reduce food intake in a number of different mammalian species.89 Fats are predominantly digested in the duodenum, with long and medium chain fatty acid products sensed by several de-orphanized g-protein coupled receptors on enteroendocrine cells throughout the length of the small intestine. These receptors include GPR40 (renamed FFAR1), which is responsive to medium-long chain fatty acids,90 GPR41 (renamed FFAR3) and GPR43 (renamed FFAR2), responsive to short chain fatty acids, and GPR120 (renamed O3FAR1), responsive to omega-3 fatty acids.91 This dietary fat sensing results in the release of gastrointestinal satiety peptides including cholecystokinin (CCK)92 and serotonin93 from the duodenum, and GLP-194 from the small intestine. Antagonists of these hormones prevent lipid induced satiation95–97 via a vagal-dependent pathway.93,98,99 Paracrine signaling between enteroendocrine cells and vagal afferent nerves innervating the gut are thought to be the main mechanism of fat signaling to the brain, although there is also preliminary evidence for direct synaptic signaling between enteroendocrine cells and extrinsic afferent nerves.100 In addition, lipids regulate the biosynthesis of the endogenous fatty acid signaling molecule oleoylethanolamide (OEA) in the duodenum and jejunum.101 Exogenous OEA acting through the peroxisome proliferator-activated receptor-α (PPAR-α) suppresses food intake.77,102,103 The mechanism for OEA induced satiation is questionable103 but may possibly involve a mechanism requiring intact sensory vagal fibers.77,102 Finally, excess fats are stored for future use in adipocytes and the level of fat storage in adipose tissue is communicated to the brain by release of the adipokine leptin. Therefore, dietary lipids directly release a number of gastrointestinal hormones, but information is predominantly communicated to the brain by a vagal afferent route, or more indirectly by leptin.
Carbohydrates trigger the release of gastric leptin from chief cells of the stomach.84 Gastric leptin levels fluctuate in response to a meal and make up 20% of circulating leptin.104 In the small intestine, carbohydrates are sensed by enteroendocrine cells using similar machinery to that found in the oral cavity to detect sweetness, a heterodimer of two G-protein coupled receptors, T1R2 and T1R3.105 Sweet sensing results in secretion of glucagon-like peptide-1 (GLP-1), peptide YY (PYY), and gastric inhibitory polypeptide (GIP) from enteroendocrine cells.106 These incretins perform both functions of regulating insulin release from pancreatic B-cells,106 and the feeling of fullness by activating vagal pathways,98,107 although at least PYY may enter the circulation and directly activate Y2 receptor expressing neurons in the brain.108,109 Glucose can also be sensed by vagal and spinal afferent terminals in the portal vein wall, following uptake by sodium glucose transporters.110,111 The importance of this pathway is supported by studies showing that infusion of glucose into the portal vein is sufficient to cause meal termination.110,111 Thus, carbohydrates activate hormones that can communicate to the brain directly via the circulation (e.g. PYY) or indirectly by a vagal afferent pathway (e.g. GLP-1), or activate a poorly defined afferent pathway from the portal system.
Conditioned reward recruitment of nigrostriatal dopaminergic system and FAA
Rats can form normal FAA in response to a meal in the absence of sight or taste, therefore these senses are not necessary. However, it remains unclear if in animals capable of forming conditioned preferences (i.e.. not blind or anosmic) these cues are sufficient for FAA.24 Given that dopamine release in the dorsal striatum is required for conditioned learning,112 it is plausible that once a conditioned preference is formed to the food cues (ie. smells, sounds), that these would be sufficient to maintain normal FAA in the absence of food (unconditioned stimulus).
Vagus nerve control of nigrostriatal dopaminergic system and FAA
Dopamine release occurs within minutes of gastric infusion of fat,77 or sugar,79 suggesting the engagement of a neural pathway. Vagotomy and capsaicin, that inhibit vagal signaling, prevent dorsal striatal dopamine release, at least in response to post-ingestive dietary fats in mice.77 Unpublished data from our lab suggests that stimulation of the vagus nerve is sufficient for dopamine release in the dorsal striatum. The consensus in the field is that the vagus nerve is not involved in regulating FAA.24,113–115 This is based on a study showing anticipatory activity persists after vagotomy.116 However, bilateral vagotomy did increase the duration of FAA,116 which could be interpreted as an impaired estimation of meal time caused by reduced accuracy of timekeeping. In addition, the animals were maintained on a previously learned time schedule after bilateral vagotomy.116 Therefore, while learned FAA can persist after bilateral vagotomy, it remains unknown whether anticipatory activity would track a phase shift in the absence of vagal signaling. Finally, It should also be noted that carbohydrates may activate a nigrostriatal circuit, at least in part via a non-vagal pathway,117 therefore vagotomy may be insufficient alone to prevent FAA in response to a mixed meal. In sum, it may be premature to altogether rule out a role for the vagus nerve as a pathway for conveying food entrainment information to the brain.
Interestingly, vagal afferent neurons express clock genes that oscillate throughout the day.118 These clock gene rhythms are maintained when animals are kept in constant darkness for 3 days,118 suggesting that they may not be entrained by light. However, the animals were only maintained in constant darkness for 3 days in this study, therefore it remains possible that vagal clock genes are under the control of dampened, but persisting, clock gene oscillations in the SCN.119 Importantly, clock gene rhythms in vagal afferent neurons persist in food restricted animals,118 so while vagal clock oscillation may be entrained by food, they are self-sustained. Despite these intriguing findings, the role of clock genes in vagal afferent function remains unclear. Vagal afferent mechanosensitivity correlates with fluctuations in clock genes,118 however the two may not be directly linked since chronic consumption of high fat diet inhibits mechanosensitivity in obese mice without any impact on clock genes rhythm.120 It would be particularly interesting in the context of FAA to determine the role of clock genes in vagal sensing of nutrients and gastrointestinal hormones.
In diet-induced obesity, dopamine release in the dorsal striatum, and dopaminergic-dependent behavior are reduced in response to dietary fat.77 Similarly FAA is abolished in diet-induced obese rats,121 and in obese-prone mice fed a chow diet.122 The mechanisms by which obesity disrupts FAA are not clear. SCN-lesions result in a small but significant increase in body weight and intake during the light phase,123 and circadian activity is disrupted in diet-induced obesity,124 thus diet-induced obesity may disrupt the master clock in the SCN. Examining FAA in SCN-lesioned obese animals would provide valuable insight. Alternatively, leptin resistance in obesity, characterized by reduced leptin signaling,125 may disrupt FAA. Genetically obese Zucker rats that lack functional leptin receptors,126 and ob/ob mice that lack leptin,127 both have increased duration of FAA compared to their wild type counterparts. Leptin replacement therapy in ob/ob mice abolishes FAA,127 but leptin replacement also causes weight loss.128 Therefore, whether changes in FAA are mediated by obesity or loss of leptin signaling remains unclear. An untested possibility is that FAA is disrupted in diet-induced obesity as a result of blunted vagal signaling. In obesity, the excitability of vagal afferent neurons is decreased,129 sensitivity to meal related signals (i.e. gastric distension)129,130 or gastrointestinal hormones129,131 is diminished, and activity of post-synaptic neurons in the caudal hindbrain are reduced in response to a meal.132,133 In support of a role for the vagus nerve in obesity induced loss of FAA, dorsal striatum dopamine release is inhibited in response to chronic dietary fat.77 Conversely, exogenous administration of OEA peripherally restores dopamine signaling via a vagal-dependent mechanism in obese mice.77
Neuroendocrine control of nigrostriatal dopaminergic system and FAA
As discussed in previous reviews,73,134 circulating gastrointestinal hormones could be involved in synchronizing food availability with foraging when access is restricted. The evidence for circulating hormones regulated by food intake in FAA is inconclusive. A full complement of gastrointestinal hormone receptors are expressed on dopamine neurons in the substantia nigra, suggesting that they are capable of receiving signals about metabolic state from circulating factors. In this section we therefore discuss the role of hormones released from the gut in response to dietary fats and sugars in FAA and any evidence that they can activate a nigrostriatal pathway.
Ghrelin
Ghrelin is a key gastrointestinal hormone capable of increasing food intake,135 and is therefore an ideal candidate for signaling anticipation of food. The eating-stimulatory effect of exogenous ghrelin does not require intact abdominal vagal afferents,136 suggesting that it may act via a humoral route. In an FAA paradigm, rats with restricted short access to food during the light phase have elevated plasma ghrelin levels 2h prior, peaking 30 minutes prior to food becoming available.137,138 Plasma ghrelin levels in non-restricted rats that anticipate a palatable snack are also elevated at time of eating compared with chow-fed control rats.139 Importantly, central injection of exogenous ghrelin increased, whereas ghrelin receptor antagonist decreased, the anticipation to the palatable snack,139 suggesting that central ghrelin is important for food anticipation. Studies from global knockout mice lacking ghrelin or ghrelin receptor have shown mixed results ranging from no effect, to attenuated FAA, to complete loss of FAA.73 The fact that ghrelin receptor is widely expressed in a number of neuronal subpopulations associated with appetitive140 or satiety141 function, makes it difficult to interpret these results.
There is evidence that ghrelin can directly activate a nigrostriatal dopaminergic pathway. In slice preparations, ghrelin receptors (GHSR) are expressed in the substantia nigra pars compacta, and ghrelin increases the firing rate of these dopaminergic neurons resulting in increased dopamine release in the dorsal striatum in vitro.142 Peripheral administration of ghrelin results in increased dopamine turnover in the dorsal striatum, and increased impulsivity,143 a behavior that requires an intact dorsal striatum.144 A direct role of nigrostriatal ghrelin signaling in FAA, by means of conditional ghrelin receptor knockdown, still needs to be tested. However, ghrelin has been implicated in activating a mesolimbic dopaminergic pathway to promote feeding.145 It has been suggested that mesolimbic circuit activation would prevent inhibition of downstream nigrostriatal circuits required for feeding,56 and therefore ghrelin may at least indirectly impact nigrostriatal feeding control.
Leptin
Leptin has been implicated in FAA. Leptin levels rise in response to a meal,146 and in rats maintained on a restricted feeding schedule leptin levels increase in response to the meal.147 Genetic animal models lacking leptin signaling have increased activity on a restricted schedule.126,127 It is difficult to determine from these experiments whether leptin itself, or the resultant obesity, affects anticipatory activity; however, there is evidence in lean animals that systemic administration of leptin reduces pre-meal activity when placed on a food restricted schedule.148 Whether gastric leptin, adipose leptin, or both are required remains unclear, but increases in circulating leptin after a meal are most likely a reflection of gastric leptin.
Leptin receptors are widely distributed throughout the brain and periphery, and therefore could impact the gut-striatal system at a number of different sites including the vagus nerve,149 the nucleus tractus solitarius (the site of vagal afferent termination),150 or the substantia nigra directly. Leptin receptor mRNA has been identified in the substantia nigra151 and protein immunoreactivity extensively colocalizes with dopamine neurons in the lateral and medial substantia nigra pars compacta.152 Leptin-receptor deficient Zucker rats show decreased D2 receptor binding in the striatum.57,153 In human patients with congenital leptin deficiency, leptin replacement reduces neural activity in the dorsal striatum.154 However, whether decreased dopamine in the dorsal striatum is an effect of leptin or obesity remains unclear, and the role of nigrostriatal leptin signaling on food intake still needs to be clarified.
Insulin
Insulin levels increase after a meal in proportion to circulating glucose levels. Under a restricted feeding schedule insulin concentrations are lowest prior to meal time and peak immediately prior to the meal.155 A role for insulin in FAA is not clear since pharmacological ablation of insulin producing beta cells in mice does not prevent induction of food anticipatory activity.156,157
Insulin receptor extensively colocalizes with dopamine neurons in the lateral and medial substantia nigra pars compacta.152 Insulin increases firing of dopamine neurons in midbrain slice preparations and this is abolished in slices from mutant mice selectively lacking insulin receptor on dopamine neurons.158 Insulin promotes dopamine release predominantly in the ventral striatum with only a small increase in the dorsal striatum,159 although it is interesting that insulin sensitivity increased in slices from food restricted rats.159 Daily food intake in mice lacking insulin receptor in midbrain dopaminergic neurons is increased compared to controls,158 suggesting that insulin acts directly on dopamine neurons to inhibit feeding. It is interesting to note that insulin injection into the ventral tegmental area (VTA) decreases FAA in mice on a scheduled feeding regimen in which food was restricted to the light phase;160 however, the specific role of insulin on nigrostriatal dopamine induced feeding behavior has not been studied.
PYY
The role of PYY in FAA has not yet been studied. However, there is evidence that PYY can act on the nigrostriatal pathway. PYY receptor binding sites can be found in the substantia nigra pars compacta161 and in vitro stimulation of brain slices with PYY3-36 increases dopamine production.162 Peripheral administration of exogenous PYY3-36, at levels similar to those reached in response to a meal, increases blood-oxygen level dependent (BOLD) activity in the dorsal striatum of human subjects,109 and c-Fos expression in the dorsal striatum of rats.163 Therefore, PYY can recruit the dorsal striatal dopaminergic pathway, however whether this occurs physiologically in response to a meal remains unclear.
Conclusion
The acquisition of food is a necessity for survival. Therefore, there must be a delicate balance between eating and performing other important tasks. In this context the idea that a food can be both satiating and rewarding at the same time is critical. Although rewards are often associated with enhanced motivation they can also be used as a predictive cue for learning about the appropriate value of the stimulus. Here we suggest that satiation, reward conditioning, and anticipatory activity all occur simultaneously in response to the same stimulus. Satiation allows the animal to engage in other activities, conditioned reward ensures that the animal will preferentially consume caloric nutrients when available, and anticipatory activity ensures that the animal is able to predict the time and place of food.
Given the importance of finding food, the existence of a separate oscillator that can be dissociated from circadian cycles to promote food acquisition would provide an evolutionary advantage. The site of the food-entrainable oscillator remains unclear, however dorsal striatum dopamine release exhibits all the necessary hallmarks of a secondary food-entrainable oscillator: 1) phasic dopamine release is entrained by nutritive foods, 2) this can occur in the absence of the SCN, 3) dopamine release reinforces the arrival of reward, 4) it is involved in timekeeping, and 5) the metabolic signals that promote food anticipation greatly overlap with those that release dopamine in the dorsal striatum. Therefore we propose that nutrient reward acts as a zeitgeber. It recalibrates dorsal striatum dopamine release to predict the arrival of rewarding food even outside of normal circadian cues, by increasing the ultradian cycle from 4h to 24h. As the dopamine levels drop this increases the activity of the animal and the anticipation for food arrival. Future work reversibly inhibiting the nigrostriatal dopamine system under a food restricted schedule will be required to confirm the role of dorsal striatum, rather than ventral striatum, dopamine in FAA.
The entrainment mechanisms by which nutritive information is communicated to promote FAA when food is restricted to one daily mealtime remain poorly understood. By examining the pathways through which metabolic signals are relayed to the dorsal striatum, we suggest a putative role for ghrelin, PYY, insulin, leptin and the vagus nerve as mechanisms of food anticipation. Each signal on its own may be sufficient to synchronize dopamine oscillation with food arrival, but may not be required because of the inherent redundancy in signaling pathways. Given the importance of accurately predicting food availability for survival, compensatory overlapping signaling mechanisms would confer an evolutionary advantage.
Importantly, if FAA is under the control of dopamine oscillations, the timing of the signal in relation to the meal may not be a critical component. A drop in phasic dopamine levels in the dorsal striatum, entrained to peak just before, during or after the start of a meal, could be sufficient to increase motivation to search for the anticipated arrival of future food. In support of this concept, under ad libitum conditions, dopamine levels are maintained by animals eating each time dorsal striatum dopamine levels drop.56 Oscillations generated during food restricted schedule could therefore continue in the presence of ad libitum food, but only elicit anticipatory motor activity when a nadir in dopamine oscillations is reached after a sufficient period of food restriction (Figure 1). Importantly, uncertainty caused by dysregulation of dopamine signaling may provide a critical mechanistic link between the gut and the brain in stress,164 and neurodevelopmental disorders such as autism.104 Therefore, further study of this system in the context of anticipation is warranted and may provide novel mechanistic insight for improving treatments for many pathological conditions.
Acknowledgments
This work was funded by a NIH grants R00-DK-094871 and R01-DK-116004 (GL).
Abbreviations
- BOLD
Blood oxygen level dependent
- CCK
Cholecystokinin
- CRY
Cryptochrome gene
- DA
Dopamine
- DMH
Dorsomedial hypothalamic nuclei
- FAA
Food anticipatory activity
- FEO
Feeding entrained oscillator
- GHSR
ghrelin receptors
- GIP
gastric inhibitory polypeptide
- GLP-1
glucagon-like peptide-1
- OEA
oleoylethanolamide
- PER
Period gene
- PYY
peptide YY
- ROR
Retinoid-related orphan receptor
- SCN
Suprachiastic Nucleus
- VTA
ventral tegmental area
Footnotes
Conflict of Interest
There is no conflict of interest for any of the authors
References
- 1.Power ML, Schulkin J. Anticipatory physiological regulation in feeding biology: cephalic phase responses. Appetite. 2008;50(2–3):194–206. doi: 10.1016/j.appet.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendrickson AE, Wagoner N, Cowan WM. An autoradiographic and electron microscopic study of retino-hypothalamic connections. Z Zellforsch Mikrosk Anat. 1972;135(1):1–26. doi: 10.1007/BF00307084. [DOI] [PubMed] [Google Scholar]
- 4.Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. The Journal of Comparative Neurology. 1972;146(1):1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- 5.Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(11):5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 7.Sawaki Y, Nihonmatsu I, Kawamura H. Transplantation of the neonatal suprachiasmatic nuclei into rats with complete bilateral suprachiasmatic lesions. Neurosci Res. 1984;1(1):67–72. doi: 10.1016/0168-0102(84)90031-2. [DOI] [PubMed] [Google Scholar]
- 8.Sollars PJ, Kimble DP, Pickard GE. Restoration of circadian behavior by anterior hypothalamic heterografts. J Neurosci. 1995;15(3 Pt 2):2109–2122. doi: 10.1523/JNEUROSCI.15-03-02109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sujino M, Masumoto KH, Yamaguchi S, van der Horst GT, Okamura H, Inouye ST. Suprachiasmatic nucleus grafts restore circadian behavioral rhythms of genetically arrhythmic mice. Curr Biol. 2003;13(8):664–668. doi: 10.1016/s0960-9822(03)00222-7. [DOI] [PubMed] [Google Scholar]
- 10.Bechtold DA, Loudon ASI. Hypothalamic clocks and rhythms in feeding behaviour. Trends in Neurosciences. 36(2):74–82. doi: 10.1016/j.tins.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Koike N, Yoo SH, Huang HC, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338(6105):349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho H, Zhao X, Hatori M, et al. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature. 2012;485(7396):123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111(45):16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donga E, van Dijk M, van Dijk JG, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95(6):2963–2968. doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- 15.Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nat Commun. 2013;4:2259. doi: 10.1038/ncomms3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89(11):5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 17.Hairston KG, Bryer-Ash M, Norris JM, Haffner S, Bowden DW, Wagenknecht LE. Sleep duration and five-year abdominal fat accumulation in a minority cohort: the IRAS family study. Sleep. 2010;33(3):289–295. doi: 10.1093/sleep/33.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8(12):e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5(5):253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang S, Liu A, Weidenhammer A, et al. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150(5):2153–2160. doi: 10.1210/en.2008-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulos Z, Rosenwasser AM, Terman M. Feeding schedules and the circadian organization of behavior in the rat. Behav Brain Res. 1980;1(1):39–65. doi: 10.1016/0166-4328(80)90045-5. [DOI] [PubMed] [Google Scholar]
- 23.Challet E, Mendoza J, Dardente H, Pevet P. Neurogenetics of food anticipation. Eur J Neurosci. 2009;30(9):1676–1687. doi: 10.1111/j.1460-9568.2009.06962.x. [DOI] [PubMed] [Google Scholar]
- 24.Mistlberger RE. Circadian food-anticipatory activity: Formal models and physiological mechanisms. Neuroscience & Biobehavioral Reviews. 1994;18(2):171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 25.Mistlberger RE. Food-anticipatory circadian rhythms: concepts and methods. The European journal of neuroscience. 2009;30(9):1718–1729. doi: 10.1111/j.1460-9568.2009.06965.x. [DOI] [PubMed] [Google Scholar]
- 26.Mistlberger RE. Neurobiology of food anticipatory circadian rhythms. Physiology & Behavior. 2011;104(4):535–545. doi: 10.1016/j.physbeh.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Boulos Z, Terman M. Food availability and daily biological rhythms. Neurosci Biobehav Rev. 1980;4(2):119–131. doi: 10.1016/0149-7634(80)90010-x. [DOI] [PubMed] [Google Scholar]
- 28.Coleman GJ, Harper S, Clarke JD, Armstrong S. Evidence for a separate meal-associated oscillator in the rat. Physiol Behav. 1982;29(1):107–115. doi: 10.1016/0031-9384(82)90373-0. [DOI] [PubMed] [Google Scholar]
- 29.Rosenwasser AM, Pelchat RJ, Adler NT. Memory for feeding time: possible dependence on coupled circadian oscillators. Physiol Behav. 1984;32(1):25–30. doi: 10.1016/0031-9384(84)90064-7. [DOI] [PubMed] [Google Scholar]
- 30.Mistlberger RE, Marchant EG, Kippin TE. Food-entrained circadian rhythms in rats are insensitive to deuterium oxide. Brain Res. 2001;919(2):283–291. doi: 10.1016/s0006-8993(01)03042-6. [DOI] [PubMed] [Google Scholar]
- 31.Marchant EG. Anticipation and entrainment to feeding time in intact and SCN-ablated C57BL/6j mice. Brain research. 1997;765(2):273. doi: 10.1016/s0006-8993(97)00571-4. [DOI] [PubMed] [Google Scholar]
- 32.Stephan FK. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behavioral and neural biology. 1979;25(4):545. doi: 10.1016/s0163-1047(79)90332-7. [DOI] [PubMed] [Google Scholar]
- 33.Abe H, Rusak B. Anticipatory activity and entrainment of circadian rhythms in Syrian hamsters exposed to restricted palatable diets. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1992;263(1):R116–R124. doi: 10.1152/ajpregu.1992.263.1.R116. [DOI] [PubMed] [Google Scholar]
- 34.Pecoraro N, Gomez F, Laugero K, Dallman MF. Brief access to sucrose engages food-entrainable rhythms in food-deprived rats. Behav Neurosci. 2002;116(5):757–776. [PubMed] [Google Scholar]
- 35.Waddington Lamont E, Harbour VL, Barry-Shaw J, et al. Restricted access to food, but not sucrose, saccharine, or salt, synchronizes the expression of Period2 protein in the limbic forebrain. Neuroscience. 2007;144(2):402–411. doi: 10.1016/j.neuroscience.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 36.Canteras NS, Ribeiro-Barbosa ER, Goto M, Cipolla-Neto J, Swanson LW. The retinohypothalamic tract: comparison of axonal projection patterns from four major targets. Brain Res Rev. 2011;65(2):150–183. doi: 10.1016/j.brainresrev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258(2):204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- 38.Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9(3):398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- 39.Landry GJ, Yamakawa GR, Mistlberger RE. Robust food anticipatory circadian rhythms in rats with complete ablation of the thalamic paraventricular nucleus. Brain Res. 2007;1141:108–118. doi: 10.1016/j.brainres.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 40.Rodgers RJ, Ishii Y, Halford JCG, Blundell JE. Orexins and appetite regulation. Neuropeptides. 2002;36(5):303–325. doi: 10.1016/s0143-4179(02)00085-9. [DOI] [PubMed] [Google Scholar]
- 41.Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320(5879):1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci U S A. 2006;103(32):12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moriya T, Aida R, Kudo T, et al. The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. Eur J Neurosci. 2009;29(7):1447–1460. doi: 10.1111/j.1460-9568.2009.06697.x. [DOI] [PubMed] [Google Scholar]
- 44.Verwey M, Khoja Z, Stewart J, Amir S. Differential regulation of the expression of Period2 protein in the limbic forebrain and dorsomedial hypothalamus by daily limited access to highly palatable food in food-deprived and free-fed rats. Neuroscience. 2007;147(2):277–285. doi: 10.1016/j.neuroscience.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 45.Verwey M, Khoja Z, Stewart J, Amir S. Region-specific modulation of PER2 expression in the limbic forebrain and hypothalamus by nighttime restricted feeding in rats. Neurosci Lett. 2008;440(1):54–58. doi: 10.1016/j.neulet.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 46.Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290(6):R1527–1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- 47.Tahara Y, Hirao A, Moriya T, Kudo T, Shibata S. Effects of medial hypothalamic lesions on feeding-induced entrainment of locomotor activity and liver Per2 expression in Per2::luc mice. J Biol Rhythms. 2010;25(1):9–18. doi: 10.1177/0748730409352782. [DOI] [PubMed] [Google Scholar]
- 48.Acosta-Galvan G, Yi CX, van der Vliet J, et al. Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior. Proc Natl Acad Sci U S A. 2011;108(14):5813–5818. doi: 10.1073/pnas.1015551108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27(31):8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318(14):876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 51.Battig K, Rosvold HE, Mishkin M. Comparison of the effects of frontal and caudate lesions on delayed response and alternation in monkeys. J Comp Physiol Psychol. 1960;53:400–404. doi: 10.1037/h0047392. [DOI] [PubMed] [Google Scholar]
- 52.Smit AN, Patton DF, Michalik M, Opiol H, Mistlberger RE. Dopaminergic regulation of circadian food anticipatory activity rhythms in the rat. PLoS One. 2013;8(11):e82381. doi: 10.1371/journal.pone.0082381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu YY, Liu TY, Qu WM, Hong ZY, Urade Y, Huang ZL. Dopamine is involved in food-anticipatory activity in mice. J Biol Rhythms. 2012;27(5):398–409. doi: 10.1177/0748730412455913. [DOI] [PubMed] [Google Scholar]
- 54.Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2(8):e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19(4):1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 56.Tellez LA, Han W, Zhang X, et al. Separate circuitries encode the hedonic and nutritional values of sugar. Nat Neurosci. 2016;19(3):465–470. doi: 10.1038/nn.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamdi A, Porter J, Prasad C. Decreased striatal D2 dopamine receptors in obese Zucker rats: changes during aging. Brain Res. 1992;589(2):338–340. doi: 10.1016/0006-8993(92)91296-q. [DOI] [PubMed] [Google Scholar]
- 58.Ferris MJ, Espana RA, Locke JL, et al. Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc Natl Acad Sci U S A. 2014;111(26):E2751–2759. doi: 10.1073/pnas.1407935111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Castaneda TR, de Prado BM, Prieto D, Mora F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res. 2004;36(3):177–185. doi: 10.1046/j.1600-079x.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 60.Paulson PE, Robinson TE. Relationship between circadian changes in spontaneous motor activity and dorsal versus ventral striatal dopamine neurotransmission assessed with on-line microdialysis. Behav Neurosci. 1994;108(3):624–635. doi: 10.1037//0735-7044.108.3.624. [DOI] [PubMed] [Google Scholar]
- 61.Masubuchi S, Honma S, Abe H, et al. Clock genes outside the suprachiasmatic nucleus involved in manifestation of locomotor activity rhythm in rats. Eur J Neurosci. 2000;12(12):4206–4214. [PubMed] [Google Scholar]
- 62.Uz T, Akhisaroglu M, Ahmed R, Manev H. The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology. 2003;28(12):2117–2123. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- 63.Verwey M, Amir S. Variable restricted feeding disrupts the daily oscillations of Period2 expression in the limbic forebrain and dorsal striatum in rats. J Mol Neurosci. 2012;46(2):258–264. doi: 10.1007/s12031-011-9529-z. [DOI] [PubMed] [Google Scholar]
- 64.Hood S, Cassidy P, Cossette MP, et al. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci. 2010;30(42):14046–14058. doi: 10.1523/JNEUROSCI.2128-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gallardo CM, Darvas M, Oviatt M, et al. Dopamine receptor 1 neurons in the dorsal striatum regulate food anticipatory circadian activity rhythms in mice. Elife. 2014;3:e03781. doi: 10.7554/eLife.03781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blum ID, Zhu L, Moquin L, et al. A highly tunable dopaminergic oscillator generates ultradian rhythms of behavioral arousal. Elife. 2014:3. doi: 10.7554/eLife.05105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brodsky VY. Circahoralian (ultradian) metabolic rhythms. Biochemistry (Mosc) 2014;79(6):483–495. doi: 10.1134/S0006297914060017. [DOI] [PubMed] [Google Scholar]
- 68.Fiorillo CD, Newsome WT, Schultz W. The temporal precision of reward prediction in dopamine neurons. Nat Neurosci. 2008;11(8):966–973. doi: 10.1038/nn.2159. [DOI] [PubMed] [Google Scholar]
- 69.Kobayashi S, Schultz W. Influence of reward delays on responses of dopamine neurons. J Neurosci. 2008;28(31):7837–7846. doi: 10.1523/JNEUROSCI.1600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Drew MR, Simpson EH, Kellendonk C, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27(29):7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maricq AV, Church RM. The differential effects of haloperidol and methamphetamine on time estimation in the rat. Psychopharmacology (Berl) 1983;79(1):10–15. doi: 10.1007/BF00433008. [DOI] [PubMed] [Google Scholar]
- 72.Soares S, Atallah BV, Paton JJ. Midbrain dopamine neurons control judgment of time. Science. 2016;354(6317):1273–1277. doi: 10.1126/science.aah5234. [DOI] [PubMed] [Google Scholar]
- 73.Patton DF, Mistlberger RE. Circadian adaptations to meal timing: neuroendocrine mechanisms. Front Neurosci. 2013;7:185. doi: 10.3389/fnins.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coleman GJ, Hay M. Anticipatory wheel-running in behaviorally anosmic rats. Physiol Behav. 1990;47(6):1145–1151. doi: 10.1016/0031-9384(90)90365-b. [DOI] [PubMed] [Google Scholar]
- 75.Davidson AJ, Aragona BJ, Werner RM, Schroeder E, Smith JC, Stephan FK. Food-anticipatory activity persists after olfactory bulb ablation in the rat. Physiol Behav. 2001;72(1–2):231–235. doi: 10.1016/s0031-9384(00)00417-0. [DOI] [PubMed] [Google Scholar]
- 76.Stephan FK. Calories affect zeitgeber properties of the feeding entrained circadian oscillator. Physiol Behav. 1997;62(5):995–1002. doi: 10.1016/s0031-9384(97)00204-7. [DOI] [PubMed] [Google Scholar]
- 77.Tellez LA, Medina S, Han W, et al. A gut lipid messenger links excess dietary fat to dopamine deficiency. Science. 2013;341(6147):800–802. doi: 10.1126/science.1239275. [DOI] [PubMed] [Google Scholar]
- 78.Mistlberger RE, Houpt TA, Moore-Ede MC. Food-anticipatory rhythms under 24-hour schedules of limited access to single macronutrients. J Biol Rhythms. 1990;5(1):35–46. doi: 10.1177/074873049000500104. [DOI] [PubMed] [Google Scholar]
- 79.Han W, Tellez LA, Niu J, et al. Striatal Dopamine Links Gastrointestinal Rerouting to Altered Sweet Appetite. Cell Metab. 2016;23(1):103–112. doi: 10.1016/j.cmet.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mistlberger R, Rusak B. Palatable daily meals entrain anticipatory activity rhythms in free-feeding rats: dependence on meal size and nutrient content. Physiol Behav. 1987;41(3):219–226. doi: 10.1016/0031-9384(87)90356-8. [DOI] [PubMed] [Google Scholar]
- 81.Yiin YM, Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric nutrient infusions in food restricted and free-feeding rats. Physiol Behav. 2005;84(2):217–231. doi: 10.1016/j.physbeh.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 82.Gribble FM, Reimann F. Signalling in the gut endocrine axis. Physiol Behav. 2017;176:183–188. doi: 10.1016/j.physbeh.2017.02.039. [DOI] [PubMed] [Google Scholar]
- 83.de Lartigue G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J Physiol. 2016;594(20):5791–5815. doi: 10.1113/JP271538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sanchez J, Oliver P, Palou A, Pico C. The inhibition of gastric ghrelin production by food intake in rats is dependent on the type of macronutrient. Endocrinology. 2004;145(11):5049–5055. doi: 10.1210/en.2004-0493. [DOI] [PubMed] [Google Scholar]
- 85.Williams DL, Cummings DE, Grill HJ, Kaplan JM. Meal-related ghrelin suppression requires postgastric feedback. Endocrinology. 2003;144(7):2765–2767. doi: 10.1210/en.2003-0381. [DOI] [PubMed] [Google Scholar]
- 86.Kirchner H, Gutierrez JA, Solenberg PJ, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15(7):741–745. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mathis C, Moran TH, Schwartz GJ. Load-sensitive rat gastric vagal afferents encode volume but not gastric nutrients. Am J Physiol. 1998;274(2 Pt 2):R280–286. doi: 10.1152/ajpregu.1998.274.2.R280. [DOI] [PubMed] [Google Scholar]
- 88.Page AJ, Kentish SJ. Plasticity of gastrointestinal vagal afferent satiety signals. Neurogastroenterol Motil. 2017;29(5) doi: 10.1111/nmo.12973. [DOI] [PubMed] [Google Scholar]
- 89.Matzinger D, Degen L, Drewe J, et al. The role of long chain fatty acids in regulating food intake and cholecystokinin release in humans. Gut. 2000;46(5):688–693. doi: 10.1136/gut.46.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Briscoe CP, Tadayyon M, Andrews JL, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278(13):11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- 91.Miyamoto J, Hasegawa S, Kasubuchi M, Ichimura A, Nakajima A, Kimura I. Nutritional Signaling via Free Fatty Acid Receptors. Int J Mol Sci. 2016;17(4):450. doi: 10.3390/ijms17040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Douglas BR, Woutersen RA, Jansen JB, de Jong AJ, Lamers CB. The influence of different nutrients on plasma cholecystokinin levels in the rat. Experientia. 1988;44(1):21–23. doi: 10.1007/BF01960229. [DOI] [PubMed] [Google Scholar]
- 93.Zhu JX, Zhu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530(Pt 3):431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138(1):159–166. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- 95.Duca FA, Katebzadeh S, Covasa M. Impaired GLP-1 signaling contributes to reduced sensitivity to duodenal nutrients in obesity-prone rats during high-fat feeding. Obesity (Silver Spring) 2015;23(11):2260–2268. doi: 10.1002/oby.21231. [DOI] [PubMed] [Google Scholar]
- 96.Matzinger D, Gutzwiller JP, Drewe J, et al. Inhibition of food intake in response to intestinal lipid is mediated by cholecystokinin in humans. Am J Physiol. 1999;277(6 Pt 2):R1718–1724. doi: 10.1152/ajpregu.1999.277.6.R1718. [DOI] [PubMed] [Google Scholar]
- 97.Savastano DM, Hayes MR, Covasa M. Serotonin-type 3 receptors mediate intestinal lipid-induced satiation and Fos-like immunoreactivity in the dorsal hindbrain. Am J Physiol Regul Integr Comp Physiol. 2007;292(3):R1063–1070. doi: 10.1152/ajpregu.00699.2006. [DOI] [PubMed] [Google Scholar]
- 98.Krieger JP, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ. Knockdown of GLP-1 Receptors in Vagal Afferents Affects Normal Food Intake and Glycemia. Diabetes. 2016;65(1):34–43. doi: 10.2337/db15-0973. [DOI] [PubMed] [Google Scholar]
- 99.Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol. 1985;249(5 Pt 2):R638–641. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]
- 100.Bohorquez DV, Shahid RA, Erdmann A, et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. The Journal of clinical investigation. 2015;125(2):782–786. doi: 10.1172/JCI78361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwartz GJ, Fu J, Astarita G, et al. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008;8(4):281–288. doi: 10.1016/j.cmet.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodriguez de Fonseca F, Navarro M, Gomez R, et al. An anorexic lipid mediator regulated by feeding. Nature. 2001;414(6860):209–212. doi: 10.1038/35102582. [DOI] [PubMed] [Google Scholar]
- 103.Fedele S, Arnold M, Krieger JP, et al. Oleoylethanolamide-induced anorexia in rats is associated with locomotor impairment. Physiol Rep. 2018;6(3) doi: 10.14814/phy2.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sinha P, Kjelgaard MM, Gandhi TK, et al. Autism as a disorder of prediction. Proc Natl Acad Sci U S A. 2014;111(42):15220–15225. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Laffitte A, Neiers F, Briand L. Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr Opin Clin Nutr Metab Care. 2014;17(4):379–385. doi: 10.1097/MCO.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beglinger C, Degen L. Gastrointestinal satiety signals in humans--physiologic roles for GLP-1 and PYY? Physiol Behav. 2006;89(4):460–464. doi: 10.1016/j.physbeh.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 107.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell. 2016;166(1):209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418(6898):650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 109.Batterham RL, ffytche DH, Rosenthal JM, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450(7166):106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 110.Mithieux G. Metabolic effects of portal vein sensing. Diabetes Obes Metab. 2014;16(Suppl 1):56–60. doi: 10.1111/dom.12338. [DOI] [PubMed] [Google Scholar]
- 111.Tordoff MG, Friedman MI. Hepatic control of feeding: effect of glucose, fructose, and mannitol infusion. Am J Physiol. 1988;254(6 Pt 2):R969–976. doi: 10.1152/ajpregu.1988.254.6.R969. [DOI] [PubMed] [Google Scholar]
- 112.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27(8):468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 113.Blum ID, Lamont EW, Abizaid A. Competing clocks: metabolic status moderates signals from the master circadian pacemaker. Neurosci Biobehav Rev. 2012;36(1):254–270. doi: 10.1016/j.neubiorev.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 114.Carneiro BT, Araujo JF. Food entrainment: major and recent findings. Front Behav Neurosci. 2012;6:83. doi: 10.3389/fnbeh.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Webb IC, Antle MC, Mistlberger RE. Regulation of circadian rhythms in mammals by behavioral arousal. Behav Neurosci. 2014;128(3):304–325. doi: 10.1037/a0035885. [DOI] [PubMed] [Google Scholar]
- 116.Comperatore CA, Stephan FK. Effects of vagotomy on entrainment of activity rhythms to food access. Physiol Behav. 1990;47(4):671–678. doi: 10.1016/0031-9384(90)90076-g. [DOI] [PubMed] [Google Scholar]
- 117.Sclafani A, Lucas F. Abdominal vagotomy does not block carbohydrate-conditioned flavor preferences in rats. Physiol Behav. 1996;60(2):447–453. doi: 10.1016/s0031-9384(96)80018-7. [DOI] [PubMed] [Google Scholar]
- 118.Kentish SJ, Frisby CL, Kennaway DJ, Wittert GA, Page AJ. Circadian variation in gastric vagal afferent mechanosensitivity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(49):19238–19242. doi: 10.1523/JNEUROSCI.3846-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Coomans CP, van den Berg SA, Houben T, et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. Faseb j. 2013;27(4):1721–1732. doi: 10.1096/fj.12-210898. [DOI] [PubMed] [Google Scholar]
- 120.Kentish SJ, Vincent AD, Kennaway DJ, Wittert GA, Page AJ. High-Fat Diet-Induced Obesity Ablates Gastric Vagal Afferent Circadian Rhythms. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016;36(11):3199–3207. doi: 10.1523/JNEUROSCI.2710-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Persons JE, Stephan FK, Bays ME. Diet-induced obesity attenuates anticipation of food access in rats. Physiol Behav. 1993;54(1):55–64. doi: 10.1016/0031-9384(93)90043-f. [DOI] [PubMed] [Google Scholar]
- 122.Luna-Illades C, Morales T, Miranda-Anaya M. Decreased food anticipatory activity of obese mice relates to hypothalamic c-Fos expression. Physiol Behav. 2017;179:9–15. doi: 10.1016/j.physbeh.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 123.Coomans CP, van den Berg SA, Lucassen EA, et al. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes. 2013;62(4):1102–1108. doi: 10.2337/db12-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kohsaka A, Laposky AD, Ramsey KM, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 125.Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21(11):643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mistlberger RE, Marchant EG. Enhanced food-anticipatory circadian rhythms in the genetically obese Zucker rat. Physiol Behav. 1999;66(2):329–335. doi: 10.1016/s0031-9384(98)00311-4. [DOI] [PubMed] [Google Scholar]
- 127.Ribeiro AC, Ceccarini G, Dupre C, Friedman JM, Pfaff DW, Mark AL. Contrasting effects of leptin on food anticipatory and total locomotor activity. PLoS One. 2011;6(8):e23364. doi: 10.1371/journal.pone.0023364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Levenson AE, Haas ME, Miao J, et al. Effect of Leptin Replacement on PCSK9 in ob/ob Mice and Female Lipodystrophic Patients. Endocrinology. 2016;157(4):1421–1429. doi: 10.1210/en.2015-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. The Journal of physiology. 2011;589(Pt 11):2857–2870. doi: 10.1113/jphysiol.2010.204594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kentish S, Li H, Philp LK, et al. Diet-induced adaptation of vagal afferent function. J Physiol. 2012;590(1):209–221. doi: 10.1113/jphysiol.2011.222158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS One. 2012;7(3):e32967. doi: 10.1371/journal.pone.0032967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Covasa M, Grahn J, Ritter RC. Reduced hindbrain and enteric neuronal response to intestinal oleate in rats maintained on high-fat diet. Auton Neurosci. 2000;84(1–2):8–18. doi: 10.1016/S1566-0702(00)00176-4. [DOI] [PubMed] [Google Scholar]
- 133.Covasa M, Grahn J, Ritter RC. High fat maintenance diet attenuates hindbrain neuronal response to CCK. Regul Pept. 2000;86(1–3):83–88. doi: 10.1016/s0167-0115(99)00084-1. [DOI] [PubMed] [Google Scholar]
- 134.Carneiro BT, Araujo JF. The food-entrainable oscillator: a network of interconnected brain structures entrained by humoral signals? Chronobiol Int. 2009;26(7):1273–1289. doi: 10.3109/07420520903404480. [DOI] [PubMed] [Google Scholar]
- 135.Mansouri A, Langhans W. Enterocyte-afferent nerve interactions in dietary fat sensing. Diabetes Obes Metab. 2014;16(Suppl 1):61–67. doi: 10.1111/dom.12339. [DOI] [PubMed] [Google Scholar]
- 136.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26(43):11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Jr, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1071–1079. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- 138.Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147(1):23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- 139.Merkestein M, Brans MA, Luijendijk MC, et al. Ghrelin mediates anticipation to a palatable meal in rats. Obesity (Silver Spring) 2012;20(5):963–971. doi: 10.1038/oby.2011.389. [DOI] [PubMed] [Google Scholar]
- 140.Liu S, Borgland SL. Regulation of the mesolimbic dopamine circuit by feeding peptides. Neuroscience. 2015;289:19–42. doi: 10.1016/j.neuroscience.2014.12.046. [DOI] [PubMed] [Google Scholar]
- 141.Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73(2):317–332. doi: 10.1016/j.neuron.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Andrews ZB, Erion D, Beiler R, et al. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J Neurosci. 2009;29(45):14057–14065. doi: 10.1523/JNEUROSCI.3890-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Anderberg RH, Hansson C, Fenander M, et al. The Stomach-Derived Hormone Ghrelin Increases Impulsive Behavior. Neuropsychopharmacology. 2016;41(5):1199–1209. doi: 10.1038/npp.2015.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci. 2003;117(6):1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- 145.Abizaid A, Liu ZW, Andrews ZB, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116(12):3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ahren B. Diurnal variation in circulating leptin is dependent on gender, food intake and circulating insulin in mice. Acta Physiol Scand. 2000;169(4):325–331. doi: 10.1046/j.1365-201x.2000.00746.x. [DOI] [PubMed] [Google Scholar]
- 147.Martinez-Merlos MT, Angeles-Castellanos M, Diaz-Munoz M, Aguilar-Roblero R, Mendoza J, Escobar C. Dissociation between adipose tissue signals, behavior and the food-entrained oscillator. J Endocrinol. 2004;181(1):53–63. doi: 10.1677/joe.0.1810053. [DOI] [PubMed] [Google Scholar]
- 148.Verhagen LA, Egecioglu E, Luijendijk MC, Hillebrand JJ, Adan RA, Dickson SL. Acute and chronic suppression of the central ghrelin signaling system reveals a role in food anticipatory activity. Eur Neuropsychopharmacol. 2011;21(5):384–392. doi: 10.1016/j.euroneuro.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 149.de Lartigue G, Ronveaux CC, Raybould HE. Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Mol Metab. 2014;3(6):595–607. doi: 10.1016/j.molmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hayes MR, Skibicka KP, Leichner TM, et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11(1):77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395(4):535–547. [PubMed] [Google Scholar]
- 152.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964(1):107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- 153.Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62(1):50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- 154.Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317(5843):1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vahl TP, Drazen DL, Seeley RJ, D’Alessio DA, Woods SC. Meal-anticipatory glucagon-like peptide-1 secretion in rats. Endocrinology. 2010;151(2):569–575. doi: 10.1210/en.2009-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Davidson AJ, Stokkan KA, Yamazaki S, Menaker M. Food-anticipatory activity and liver per1-luc activity in diabetic transgenic rats. Physiol Behav. 2002;76(1):21–26. doi: 10.1016/s0031-9384(02)00680-7. [DOI] [PubMed] [Google Scholar]
- 157.Oishi K, Kasamatsu M, Ishida N. Gene- and tissue-specific alterations of circadian clock gene expression in streptozotocin-induced diabetic mice under restricted feeding. Biochem Biophys Res Commun. 2004;317(2):330–334. doi: 10.1016/j.bbrc.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 158.Konner AC, Hess S, Tovar S, et al. Role for insulin signaling in catecholaminergic neurons in control of energy homeostasis. Cell Metab. 2011;13(6):720–728. doi: 10.1016/j.cmet.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 159.Stouffer MA, Woods CA, Patel JC, et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun. 2015;6:8543. doi: 10.1038/ncomms9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Labouebe G, Liu S, Dias C, et al. Insulin induces long-term depression of ventral tegmental area dopamine neurons via endocannabinoids. Nat Neurosci. 2013;16(3):300–308. doi: 10.1038/nn.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lynch DR, Walker MW, Miller RJ, Snyder SH. Neuropeptide Y receptor binding sites in rat brain: differential autoradiographic localizations with 125I-peptide YY and 125I-neuropeptide Y imply receptor heterogeneity. J Neurosci. 1989;9(8):2607–2619. doi: 10.1523/JNEUROSCI.09-08-02607.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Adewale AS, Macarthur H, Westfall TC. Neuropeptide Y-induced enhancement of the evoked release of newly synthesized dopamine in rat striatum: mediation by Y2 receptors. Neuropharmacology. 2007;52(6):1396–1402. doi: 10.1016/j.neuropharm.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 163.Stadlbauer U, Arnold M, Weber E, Langhans W. Possible mechanisms of circulating PYY-induced satiation in male rats. Endocrinology. 2013;154(1):193–204. doi: 10.1210/en.2012-1956. [DOI] [PubMed] [Google Scholar]
- 164.de Berker AO, Rutledge RB, Mathys C, et al. Computations of uncertainty mediate acute stress responses in humans. Nat Commun. 2016;7:10996. doi: 10.1038/ncomms10996. [DOI] [PMC free article] [PubMed] [Google Scholar]