Abstract
Although short-term exposure to ambient ozone (O3) can cause poor respiratory health outcomes, the shape of the concentration-response (C-R) between O3 and respiratory morbidity has not been widely investigated. We estimated the effect of daily O3 on emergency department (ED) visits for selected respiratory outcomes in 5 U.S. cities under various model assumptions and assessed model fit. Population-weighted average 8-hr maximum O3 concentrations were estimated in each city. Individual-level data on ED visits were obtained from hospitals or hospital associations. Poisson log-linear models were used to estimate city-specific associations between the daily number of respiratory ED visits and 3- day moving average O3 levels controlling for long-term trends and meteorology. Linear, linear-threshold, quadratic, cubic, categorical, and cubic spline O3 C-R models were considered. Using linear C-R models, O3 was significantly and positively associated with respiratory ED visits in each city with rate ratios of 1.02-1.07 per 25 ppb. Models suggested that O3-ED C-R shapes were linear until O3 concentrations of roughly 60 ppb at which point risk continued to increase linearly in some cities for certain outcomes while risk flattened in others. Assessing C-R shape is necessary to identify the most appropriate form of the exposure for each given study setting.
Keywords: ozone, respiratory health, concentration-response, linearity, threshold
Introduction
Short-term exposure to ambient ozone (O3), a strong oxidant, has been shown to be consistently associated with poor respiratory health outcomes (1, 2). The U.S. Environmental Protection Agency’s most recent Integrated Science Assessment for O3 reported that the overall evidence supported a causal relationship between ambient O3 exposures and increased respiratory morbidity (3). However, the shape of the O3 and respiratory morbidity concentration-response (C-R) function has not been systematically evaluated.
Past studies that have examined the C-R function for acute O3 health effects have focused on the association between O3 and daily all-cause mortality (4–11). Most of these mortality studies described a linear C-R relationship, although some suggested non-linear C-R functions with J-shapes or other curvilinear shapes with flattened slopes at high O3 levels. However, the O3 C-R function for mortality may differ from that for morbidity for several reasons. First, it is possible that exposure magnitudes needed to induce health effects vary by the specific outcome type. For example, higher O3 levels may be necessary to cause death while lower O3 levels may be sufficient to cause substantial morbidity. Second, the adverse effect of O3 on the respiratory tract might be stronger than the adverse effects of O3 on other anatomical systems that might contribute to all-cause mortality. Third, mortality data are more likely to represent outcomes in older populations compared to morbidity data, which can represent outcomes in people of all ages (12). Children are particularly susceptible to O3-induced respiratory distresses like asthma but are less likely to die (13); and, due to underdeveloped respiratory and immune systems, children may be more susceptible to health effects at low O3 levels compared to adults (14). The ability to assess air pollution health effects across the life span might be particularly important, because age can be related to both personal exposure to ambient O3 and susceptibility to specific types of disease. Children might spend more time outside than adults and their exposure is likely greater than adults at the same ambient concentrations due to breathing more air per unit of body weight than adults (15). Children are also disproportionately affected by asthma while chronic obstructive pulmonary disease (COPD) symptoms typically do not appear until at least 40 years of age (16). Both differences in personal exposure and differences in specific disease types could lead to differences in O3-morbidity C-R functions by age.
A linear relationship between the log of daily respiratory event counts and daily concentrations of O3 (and other pollutants) is often assumed. Because a linear C-R function assumes a constant slope, the regression parameter represents the average change in the log event count per unit change in O3 concentration, which makes it easy to interpret. The parameter estimate can be interpreted as the average relative risk of the outcome per unit change in O3 concentration, which is assumed to be constant across all O3 concentrations. In many cases, the assumption of linearity is made for ease of interpretation of a single parameter estimate summarizing the overall effects. However, if the C-R relationship is truly nonlinear but modeled as linear, the relative risk for a given change in O3 concentration may be over- or under-estimated, depending on the absolute O3 concentration. Inaccurate estimates may have intervention and policy implications (17). Evaluating whether to model a potentially non-linear C-R relationship as linear ideally requires an examination as to the extent of the error this would lead to in the estimates at various O3 levels.
As part of the Emory University-Georgia Tech Southeastern Center for Air Pollution and Epidemiology (SCAPE), we conducted a multi-city study of air pollution and acute morbidity (18–20). Here, we use data from this study to evaluate the O3 C-R function shapes for acute respiratory morbidity outcomes. Specifically, we 1) estimated the effect of daily O3 on the number of respiratory emergency department (ED) visits in five U.S. cities under various model assumptions, for respiratory ED visits overall and for ED visits for specific types of respiratory disease and 2) examined the consistency of the results across the five cities, for all ages combined and for children and adults separately. Each city’s data spanned a recent multi-year period and encompassed a broad range of O3 levels.
Materials and Methods
The study used data for five U.S. metropolitan areas: 20-county Atlanta, Georgia (2002-2008); 7- county Birmingham, Alabama (2002-2008); 12-county Dallas-Fort Worth, Texas (2006-2008); 3-county Pittsburgh, Pennsylvania (2002-2008); and 16-county St. Louis Missouri-Illinois (2002-2007). The estimated population of the five metropolitan areas using county-level data from the 2010 U.S. Census ranged from 1.1 million people in Birmingham to 6.3 million people in Dallas.
Year-round estimated daily 8-hour maximum O3 concentrations in parts per billion (ppb) were obtained by fusing observational data from available network monitors with pollutant concentration simulations from the Community Multi-Scale Air Quality (CMAQ) emissions-based chemical transport model at 12x12 km grids over each city. These daily fields were then spatially averaged in each metropolitan area using population weighting (21–23). The temporal resolution of CMAQ O3 concentrations was the same as the monitors: both were hourly observations used to calculate daily 8-hour maximums. Daily temperature and dew-point data were obtained from the National Climatic Data Center for measurements made at automated surface observing stations at the major airport in each city.
Individual-level data on ED visits were obtained directly from hospitals or from hospital associations for each city (18–20). Data were not available from any Veterans Administration hospitals nor from the one children’s hospital in Birmingham. International Classification of Diseases, 9th revision [ICD-9] codes representing the primary reason for the ED visit were used to identify ED visits for specific respiratory health outcomes, including asthma [493, 786.07], upper respiratory tract infection (URI) [460-466, 477], COPD [491-492, 496], and a combined respiratory disease (RD) group that included visits for asthma, URI, COPD, plus pneumonia [480-486], and bronchiolitis [466.1, 466.11, 466.19]. Patient-level data were aggregated by day to obtain daily counts of ED visits for each outcome for each city for all ages as well as for children (those ≤18 years at time of ED visit) and adults (those >18 years at time of ED visit) separately. The data were used in accordance with our data use agreements with the hospitals and hospital associations. The Emory University Institutional Review Board approved this study.
Poisson log-linear models were used to estimate associations between 3-day moving average (of lag days 0-2) O3 concentrations and ED visits for each respiratory outcome in each city, using various functions of concentrations and controlling for long-term trends and meteorology. Standard errors were scaled to account for Poisson overdispersion. Models controlled for flexible time trends specified using cubic splines with monthly knots; and indicator variables for day of week, federal holidays, individual hospital’s participation, and season. Models also included cubic terms for same-day (lag 0) maximum temperature, 2-day moving average of minimum temperature (lags 1-2), and 3-day moving average of mean dew point temperature (lags 0-2). The models also included terms for the interaction between season and the lag 0 maximum temperature cubic polynomial terms, and terms for the interaction between the season and weekday/holiday terms. The temporal metric for O3 concentration (3-day moving average of lag days 0-2) and control variables were chosen a priori and based on previous research (18). The model can be represented in the form:
where Yt represents the daily count of ED visits on day t, APt denotes the level of 3-day moving average O3 (of lag days 0-2) on day t, and f(time-varying covariates)t represent the control variables.
To examine the O3 C-R shape for each outcome in each city, six different approaches for modeling O3 concentrations were considered: 1) linear C-R models, in which O3 concentration was included in the model as a continuous linear variable; 2) linear-threshold C-R models, in which O3 was modeled as having no effect at concentrations less than or equal to a threshold and a linear effect at concentrations greater than the threshold. The threshold was determined for each city and outcome by the O3 level that maximized the log likelihood of the model. Specifically, for each city and outcome, thresholds ranging from the minimum observed ozone concentration for that city through 50 ppb by 1 ppb increments were considered in individual models and the O3 concentration from the model with the largest log likelihood was chosen as the threshold for that city and outcome; 3) quadratic C-R models, in which both linear and quadratic terms for O3 concentration were included; 4) cubic C-R models, in which linear, quadratic, and cubic terms for O3 concentration were included; 5) categorical C-R models, in which the effects of quintile-based categories of O3 concentration (25 to <35, 35 to <45, 45 to <60, and ≥60 ppb) relative to the lowest quintile of O3 (<25 ppb) were determined; and 6) cubic spline C-R models, in which O3 was modeled as a cubic spline with knots at 30 and 50 ppb, corresponding to the approximate 25th and 75th O3 percentiles across cities. Cubic spline C-R models included cubic, quadratic, and linear terms for O3 concentration as well as terms that allowed the cubic term to vary at the knot points.
The Akaike Information Criteria (AIC) value was obtained for each model and was used to compare model fit between models with various O3 effect specifications, with the lowest AIC representing the best model fit for a given respiratory outcome in a given city. Rate ratios (RRs) and 95% confidence intervals (CIs) from linear and threshold models were calculated for each city and outcome per 25 ppb increase in O3 concentration; 25 ppb represents the average interquartile range of O3 levels among the 5 cities. Chi-square tests of heterogeneity were conducted to determine whether RRs from the linear models varied significantly across cities for any respiratory outcome (24). RRs and confidence intervals from the categorical and cubic spline models were plotted and AICs were used to compare results among outcomes, approaches, and cities. The referent O3 level in the cubic spline plots was 20 ppb which roughly corresponded to the 10% percentile for each city.
Secondary analyses included stratifying analyses by age group (children ≤18 years vs. adults >18 years) among four cities: age-specific models were not examined for Birmingham because data were not available from the one children’s hospital in that city. Age-specific models also did not examine COPD as an outcome because nearly all ED visits for COPD occurred among adults. Preliminary analyses examined 3 age groups: ≤18 years, 19-64 years, and ≥65 years. Because there were fewer respiratory-related ED visits among adults ≥65 years compared to other ages (e.g. in Atlanta, 55% of asthma ED visits were among children, 40% among adults 19-64 years, and 5% among adults ≥65 years) and because general C-R shapes were similar for both the 19-64 and ≥65 year age groups, the two adult categories were combined. As O3 concentrations are impacted by ambient nitrogen dioxide (NO2) and temperature, sensitivity analyses also examined the effect of O3 in cubic spline models that additionally adjusted for estimated daily 1-hour maximum NO2 concentrations as a 3-day moving average. Finally, sensitivity analyses testing the robustness of the temporal metric for O3 and meteorological control variables were run for the RD and asthma models. These models examined different lag structures for O3 (same day, lagged 1 day, lagged 2 days, and lagged 3 days) and temperature. To further test for residual confounding, a model designed to show no association examined the association between tomorrow’s O3 levels (O3 lag negative-1 day) and same day respiratory morbidity while also controlling for O3 3-day moving average. This check is based on the understanding that if covariate control is adequate, there should be no association between future O3 levels and the outcome (25).
We used these data in accordance with our data use agreements with the hospitals and hospital associations from which the data were received. The study was approved by the Emory University Institutional Review Board which granted exemption from informed consent requirements, given the minimal risk nature of the study and the infeasibility of obtaining informed consent from individual patients for the large number of ED visit and hospitalization records examined in this study.
Results
The five cities each contributed 1096 to 2557 days to the analysis (Table 1). There were wide-ranging O3 levels across days: nearly 20% of days had O3 concentrations <25 ppb and 11% of days had O3 concentrations >60 ppb (Table 1). Of the five cities, Pittsburgh had the widest range of O3 levels (3.9 ppb to 106.3 ppb). O3 levels varied by season with higher O3 concentrations occurring on summer days and lower concentrations on winter days (Supplemental Table 1). Counts of respiratory disease ED visits ranged from 68 visits per day in Birmingham to 448 visits per day in Dallas (Table 2). On average, 56% of respiratory disease ED visits were due to URI (range=42-61% across cities), 19% of visits were due to asthma (range=16-25% across cities), and 6% of visits were due to COPD (range=5-10% across cities). Half of ED visits for asthma occurred among children 18 years and younger (52%) while nearly all COPD visits occurred among adults (99%) (Table 2).
Table 1.
Summary statistics of daily 8-hour maximum ambient ozone concentrations by city
| City | Period [# of days] | O3 concentration (ppb) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Min | 10th % | 25th % | 75th % | 90th % | Max | IQR | ||
| Atlanta | 2002-2008 [2 557] | 42.2 | 41.3 | 9.2 | 22.8 | 28.2 | 54.4 | 63.5 | 96.5 | 26.2 |
| Birmingham | 2002-2008 [2 557] | 41.4 | 40.9 | 9.4 | 24.8 | 29.8 | 51.2 | 59.4 | 83.5 | 21.4 |
| Dallas | 2006-2008 [1 096] | 41.8 | 40.6 | 9.2 | 26.9 | 31.8 | 50.3 | 59.3 | 80.2 | 18.5 |
| Pittsburgh | 2002-2008 [2 557] | 37.5 | 35.9 | 3.9 | 16.8 | 22.8 | 50.6 | 59.9 | 106.3 | 27.8 |
| St. Louis | 2002-2007 [2 004] | 38.5 | 37.1 | 6.5 | 19.0 | 24.9 | 50.1 | 59.6 | 89.6 | 25.2 |
Abbreviations: IQR, interquartile range
Table 2.
Summary of emergency department visit data for respiratory outcomes by city and age group
| City | Period [# of days] | Total # of ED visits [Mean # of ED visits per day] | |||
|---|---|---|---|---|---|
| RD | Asthma | URI | COPD | ||
| ALL AGES | |||||
| Atlanta | 2002-2008 [2 557] | 1 022 735 [400.0] | 213 126 [83.4] | 590 474 [230.9] | 51 290 [20.1] |
| Birmingham | 2002-2008 [2 557] | 173 317 [67.8] | 27 113 [10.6] | 105 761 [41.4] | 15 866 [6.2] |
| Dallas | 2006-2008 [1 096] | 490 826 [447.8] | 82 798 [75.5] | 282 866 [258.1] | 24 614 [22.5] |
| Pittsburgh | 2002-2008 [2 557] | 264 558 [103.5] | 64 828 [25.4] | 111 811 [43.7] | 27 183 [10.6] |
| St. Louis | 2002-2007 [2 004] | 572 494 [285.7] | 102 460 [51.1] | 331 837 [165.6] | 31 361 [15.6] |
| CHILDREN ≤ 18 YEARS | |||||
| Atlanta | 2002-2008 [2 557] | 539 273 [210.9] | 117 645 [46.0] | 337 060 [131.8] | 259 [0.10] |
| Birmingham | 2002-2008 [2 557] | 43 424 [17.0] | 4 806 [1.9] | 35 030 [13.7] | 39 [0.02] |
| Dallas | 2006-2008 [1 096] | 272 523 [248.7] | 46 687 [42.6] | 170 144 [155.2] | 41 [0.04] |
| Pittsburgh | 2002-2008 [2 557] | 109 417 [42.8] | 29 881 [11.7] | 57 484 [22.5] | 44 [0.02] |
| St. Louis | 2002-2007 [2 004] | 254 743 [127.1] | 55 677 [27.8] | 155 357 [77.5] | 123 [0.06] |
| ADULTS >18 YEARS | |||||
| Atlanta | 2002-2008 [2 557] | 483 256 [189.0] | 95 448 [37.3] | 253 301 [99.1] | 51 017 [20.0] |
| Birmingham | 2002-2008 [2 557] | 124 238 [48.6] | 21 058 [8.2] | 66 885 [26.2] | 15 539 [6.1] |
| Dallas | 2006-2008 [1 096] | 218 271 [199.2] | 36 106 [32.9] | 112 699 [102.8] | 24 573 [22.4] |
| Pittsburgh | 2002-2008 [2 557] | 155 141 [60.7] | 34 947 [13.7] | 54 327 [21.2] | 27 139 [10.6] |
| St. Louis | 2002-2007 [2 004] | 317 750 [158.6] | 46 783 [23.3] | 176 480 [88.1] | 31 238 [15.6] |
Abbreviations: RD, combined respiratory disease group; URI, upper respiratory tract infection; COPD, chronic obstructive pulmonary disease
We first compared RRs for a 25 ppb increase in O3 from models for each health outcome in each city that assumed a linear C-R function for O3 (Table 3). In linear C-R models, O3 was positively associated with ED visits for all outcomes in each city (with the exception of the association between O3 and COPD in Birmingham), with no statistically significant heterogeneity in associations across cities. Specifically, for RD ED visits, associations were statistically significant in each city with rate ratios of 1.02-1.05 per 25 ppb increase in O3 (Table 3). Additionally, associations were statistically significant for asthma ED visits in four cities and for URI ED visits in three cities. For COPD ED visits, associations were generally positive but not significant, likely due in part to lower power resulting from smaller daily ED visit counts for COPD. O3 had a stronger detrimental effect on ED visits for asthma compared to URIs in St. Louis (RR=1.07 for asthma vs. 1.01 for URI, p-value for difference=0.012).
Table 3.
City-specific rate ratios and 95% confidence intervals per 25 ppb increment in 8-hr maximum ambient O3 from linear C-R models for four respiratory ED outcomes
| City | RD | Asthma | URI | COPD | ||||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | P-value | RR (95% CI) | P-value | RR (95% CI) | P-value | RR (95% CI) | P-value | |
| Atlanta | 1.03 (1.01-1.05) | <0.01 | 1.04 (1.02-1.07) | <0.01 | 1.03 (1.01-1.05) | <0.01 | 1.00 (0.96-1.04) | 0.963 |
| Birmingham | 1.03 (1.00-1.06) | 0.023 | 1.04 (0.98-1.11) | 0.151 | 1.03 (0.99-1.06) | 0.114 | 0.99 (0.92-1.07) | 0.844 |
| Dallas | 1.05 (1.02-1.07) | <0.01 | 1.04 (1.00-1.08) | 0.047 | 1.06 (1.03-1.09) | <0.01 | 1.05 (0.99-1.11) | 0.110 |
| Pittsburgh | 1.03 (1.01-1.05) | <0.01 | 1.04 (1.01-1.08) | 0.023 | 1.03 (1.00-1.06) | 0.072 | 1.03 (0.98-1.09) | 0.246 |
| St. Louis | 1.02 (1.01-1.04) | 0.011 | 1.07 (1.03-1.11) | <0.01 | 1.01 (0.99-1.04) | 0.269 | 1.04 (0.98-1.10) | 0.177 |
| P-value testing whether city RRs differ3 | 0.748 | 0.765 | 0.184 | 0.564 | ||||
P-values from chi-square tests of city heterogeneity
Results of linear-threshold C-R models were similar to results of linear C-R models (Table 4). Above the identified threshold, O3 was positively associated with ED visits for all outcomes in each city, with RRs per 25 ppb increase in O3 concentrations above the threshold (Table 4) that were similar to the RRs per 25 ppb increase in O3 concentrations in linear C-R models (Table 3). O3 threshold analyses tended to either show a threshold at the lowest observed O3 level in the city or alternatively, showed thresholds around 25 ppb. For example, for the overall RD outcome, thresholds of 10 ppb were identified in Birmingham and Dallas which effectively demonstrated no threshold since the lowest observed O3 levels in each city were 9.4 and 9.2 ppb, respectively. However, evidence of thresholds of 24, 24, and 26 ppb were identified in Atlanta, Pittsburgh, and St. Louis despite similar minimum observed O3 levels as Birmingham and Dallas. A threshold of 40 ppb was identified in St. Louis for the COPD outcome.
Table 4.
City-specific thresholds and above threshold rate ratios and 95% confidence intervals per 25 ppb increment in 8-hr maximum ambient O3 from linear-threshold C-R models for four respiratory ED outcomes3
| City | RD | Asthma | URI | COPD | ||||
|---|---|---|---|---|---|---|---|---|
| Threshold (ppb) | RR (95% CI) | Threshold (ppb) | RR (95% CI) | Threshold (ppb) | RR (95% CI) | Threshold (ppb) | RR (95% CI) | |
| Atlanta | 24 | 1.04 (1.02-1.05) | 24 | 1.05 (1.03-1.08) | 25 | 1.04 (1.02-1.06) | 23 | 1.00 (0.95-1.04) |
| Birmingham | 10 | 1.03 (1.00-1.06) | 31 | 1.05 (0.99-1.12) | 25 | 1.04 (1.00-1.07) | 27 | 0.98 (0.91-1.06) |
| Dallas | 10 | 1.05 (1.02-1.07) | 10 | 1.04 (1.00-1.08) | 10 | 1.06 (1.03-1.09) | 10 | 1.05 (0.99-1.11) |
| Pittsburgh | 24 | 1.05 (1.02-1.07) | 23 | 1.07 (1.02-1.11) | 24 | 1.04 (1.01-1.08) | 26 | 1.06 (1.00-1.12) |
| St. Louis | 26 | 1.03 (1.01-1.05) | 27 | 1.08 (1.04-1.12) | 21 | 1.02 (0.99-1.04) | 40 | 1.05 (1.00-1.11) |
Thresholds determined by O3 level that maximized the log likelihood of the model
In categorical models for each city, there was generally an increasing risk of an RD, asthma, or URI ED visit with increasing O3 level relative to the reference level of <25 ppb (Figure 1). Rate ratios comparing days with ≥60 ppb O3 to days with O3 ≤25 ppb in the various cities ranged from 1.03-1.08 for RD, 1.04-1.11 for asthma, and 1.02-1.09 for URI. However, for COPD ED visits, risk did not generally increase by O3 level in three cities and corresponding rate ratios were not significant in any city at any O3 level.
Figure 1.
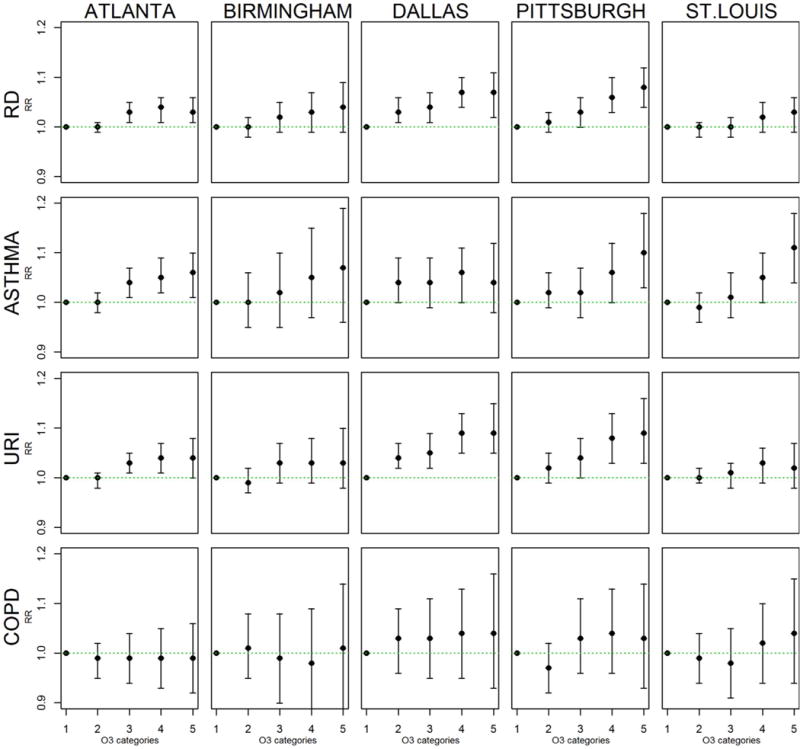
Rate ratios and 95% confidence intervals from categorical C-R models. O3 categories are <25 ppb (reference), 25 to <35 ppb, 35 to <45 ppb, 45 to <60 ppb, and ≥60 ppb.
Regression coefficients for quadratic terms in quadratic models were generally not significant (p-values >0.05 in 16 out of 20 quadratic city-specific respiratory outcome models) nor were coefficients for cubic terms in cubic models (p-values>0.05 in 15 of 20 models). Because cubic spline models did not impose a distribution assumption, rate ratios from cubic spline models were plotted to aid in visualizing the C-R function shape (Figure 2). The cubic spline curves were largely consistent with results from the categorical models in Figure 1, although provided more detail on specific shapes. RR plots from cubic spline models typically illustrated the increasing risk of respiratory ED visits with increasing O3 although plots from all 5 cities showed a flattening of RD ED risk at higher O3 levels (i.e. >60 ppb) (Figure 2).
Figure 2.
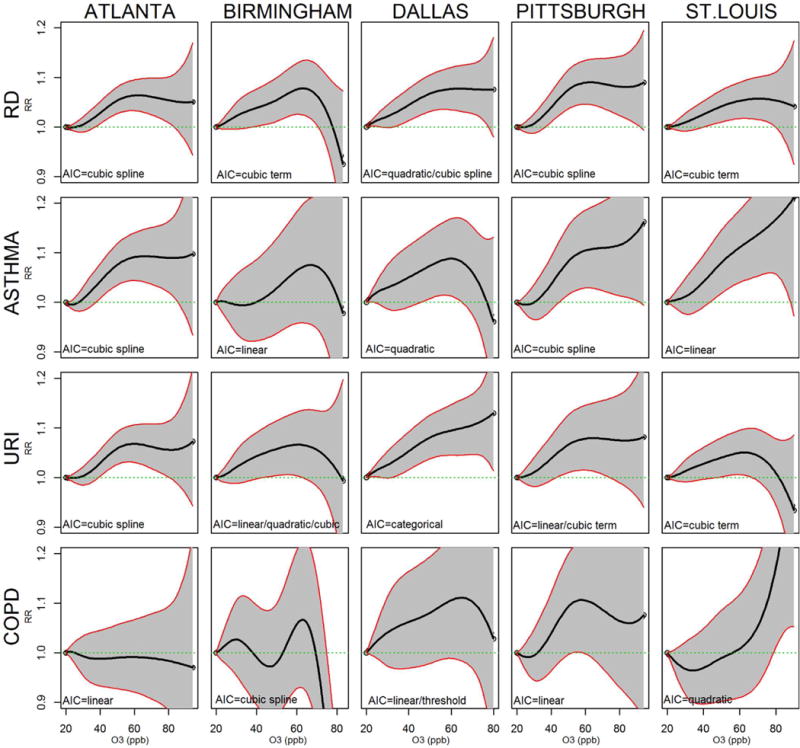
Rate ratios and 95% confidence intervals from cubic spline C-R models. Rate ratios use O3 of 20 ppb as reference.a The best fitting model across all six O3 modeling approaches, as determined by AIC, is also listed on each figure for comparison purposes.
a20 ppb and 60 ppb correspond to 10th and 90th O3 percent
To compare model fit among the approaches for each city-outcome combination, AICs are presented in Table 5, with the lowest AIC for each city-outcome presented in bold. The approach yielding the best fit for O3 varied by city and by outcome. Among the 5 cities and 4 respiratory outcomes, linear models fit best or equally as well as any other model approaches in n=7 instances, cubic spline models in another n=7, cubic models in n=3, quadratic in n=2, and categorical in n=1 instance. Although there was some suggestion of O3 thresholds (e.g. of 24-26 ppb for RD) in some cities (Table 4), linear C-R models fit as well as or better than threshold C-R models as determined by AICs (Table 5). Linear C-R models also fit better than categorical C-R models in all cases except for RD and URI outcomes in Dallas. Comparing the linear fit to the fit of approaches with flexible terms resulted in mixed findings. Cubic spline or cubic C-R models described the O3-RD shape better than the linear approach in all five cities (cubic spline in 3 cities and cubic in 2 cities). However, best fitting models for the more specific respiratory outcomes varied by city. For example, for asthma, linear C-R models fit best in 2 cities, cubic spline C-R models fit best in another 2 cities, and a quadratic C-R model fit best in 1 city. Best fitting C-R models also varied by outcome within the same city. For example, a linear C-R model best described the O3-asthma relationship in St. Louis while a cubic C-R model best described the St. Louis O3-URI relationship. Cubic spline plots generally reflected the C-R function identified by the lowest AIC (Figure 1). Cubic spline plot shapes generally did not differ substantially between city as evidenced by overlapping confidence interval bands.
Table 5.
Comparing AICs from six approaches for modeling O3 concentrations*
| City | Linear | Threshold | Categorical | Quadratic | Cubic | Cubic Spline |
|---|---|---|---|---|---|---|
| RD | ||||||
| Atlanta | + 39 | + 43 | + 42 | + 39 | + 9 | 28206 |
| Birmingham | + 4 | + 20 | + 26 | + 2 | 19115 | + 1 |
| Dallas | + 12 | + 12 | + 4 | 11713 | +2 | 11713 |
| Pittsburgh | + 17 | + 24 | + 24 | + 18 | + 6 | 20368 |
| St. Louis | + 2 | + 19 | + 28 | + 4 | 19466 | + 4 |
| ASTHMA | ||||||
| Atlanta | + 24 | + 27 | + 35 | + 26 | + 9 | 20018 |
| Birmingham | 13503 | + 8 | + 14 | + 1 | + 3 | + 5 |
| Dallas | + 7 | + 7 | + 10 | 8434 | + 1 | + 1 |
| Pittsburgh | + 3 | + 16 | + 20 | + 3 | + 1 | 16044 |
| St. Louis | 14664 | + 23 | + 26 | + 2 | + 4 | + 7 |
| URI | ||||||
| Atlanta | + 24 | + 30 | + 25 | + 23 | + 8 | 25595 |
| Birmingham | 17674 | + 14 | + 17 | 17674 | 17674 | + 3 |
| Dallas | + 5 | + 5 | 10536 | + 2 | + 4 | + 5 |
| Pittsburgh | 17869 | + 10 | + 9 | + 2 | 17869 | + 2 |
| St. Louis | + 10 | + 30 | + 33 | + 10 | 17804 | + 3 |
| COPD | ||||||
| Atlanta | 15112 | + 11 | + 17 | + 2 | + 4 | + 7 |
| Birmingham | + 12 | + 20 | + 24 | + 13 | + 11 | 11969 |
| Dallas | 6734 | 6734 | + 8 | + 1 | + 3 | + 6 |
| Pittsburgh | 13334 | + 7 | + 11 | + 2 | + 1 | + 2 |
| St. Louis | + 8 | + 16 | + 23 | 11482 | + 2 | + 5 |
for each city-outcome combination, the AIC from the modeling approach yielding the lowest AIC is presented in bold, and differences from the lowest AIC are presented for the other modeling approaches
In secondary analyses, we assessed whether C-R curves varied by age for all cities (except Birmingham due to data missing from the children’s hospital). Smaller ED counts created more uncertainty around all estimates in age-specific analyses. However, results still suggested that C-R shapes for several outcomes may have differed between children and adults. The risk of a respiratory-related ED visit at lower O3 levels (<40 ppb) was often greater for children compared to adults as represented by a steeper slope at low O3 levels in children-specific cubic spline RR plots compared to adult-specific plots (Supplemental Figure 1). Differences in C-R shapes between children and adults were most visible for asthma ED visits. The risk of an asthma ED visit at lower O3 levels was greater for children compared to adults in all 4 cities. The data additionally suggested that children may be more impacted by very low O3 levels (<30 ppb) compared to adults. For example, the risk of a respiratory-related ED visit in Atlanta and St. Louis began at 20 ppb among children but the same risk did not occur in adults until O3 levels of 30 or 40 ppb. The same phenomenon was evident for asthma in Dallas, Pittsburgh, and St. Louis and for URI in Atlanta and Pittsburgh.
Sensitivity analyses that examined results from models that additionally adjusted for NO2 found that the C-R shape in each city for each outcome was nearly identical to the shapes generated in the models that did not adjust for NO2. Sensitivity analyses that considered various temporal definitions for O3 and meterological measures demonstrated a robust model: associations and C-R shapes were similar when O3 was modeled as either same day, lag 1, lag 2, lag 3, or as a moving average of 3 days. Models that considered O3 lagged -1 day, showed no O3-morbidity associations with future O3 levels, as expected.
Discussion
We found positive significant relationships between O3 and respiratory health outcomes across five U.S. cities. This finding was consistent across all C-R modeling assumptions. Linear and semiparametric models most often fit best. Depending on the specific city and respiratory morbidity outcome examined, we found either no threshold or thresholds of roughly 25 ppb which correspond to typical O3 background levels in the U.S. (26). Results suggested that although risk of respiratory-related ED visits typically increased with increasing O3 levels, the risk among children aged 18 years and younger was higher compared to adults at low O3 levels (<40 ppb).
For the RD outcome which encompassed ED visits for asthma, URI, COPD, pneumonia, and bronchiolitis, semi-parametric models best represented the relationship in all five cities. These semiparametric RD models described a linearly increasing RD risk with increasing O3 up until about concentrations of 60 ppb at which point risk “flattened” out at O3 levels above 60 ppb (e.g. suggesting that O3 levels of 80 ppb confer the same RD risk as 60 ppb). However, in our study, each city had only approximately 10% of days on which O3 concentration was above 60 ppb and this likely led to uncertain results at high O3 concentrations; confidence intervals were wide at O3 concentrations above 60 ppb making it unclear whether this plateau in risk at high O3 levels truly occurs. Moreover, for the RD outcome specifically, the observed plateauing of risk at higher O3 concentrations may have simply been a reflection of this outcome being a combination of specific respiratory sub-outcomes with differing C-R shapes. For example, in Dallas, the C-R shape for URI was linear through O3 concentrations of 90 ppb while the C-R shape for asthma suggested a decreasing risk beginning at O3 concentrations of 60 ppb. When combined into the overall Dallas-specific RD outcome, risk naturally averaged (or “flattened”) out at O3 concentrations ≥60 ppb.
Considering the specific outcomes of asthma and URI, the observed leveling off of risk at high O3 levels was not consistent across cities. Southern cities (Atlanta, Birmingham, and Dallas) all showed a flattened asthma ED risk at O3 concentrations ≥60 ppb, but asthma ED risk in Pittsburgh and St. Louis continued to increase through O3 concentrations of 90 ppb. If flattening of risk at high O3 levels was due to a biologic reason, we would expect similar patterns across cities for the same outcome. This was not the case here suggesting the importance of other factors. For example, this could reflect different population behavior patterns in relation to exposure to ambient O3 across cities. People in the southern cities may be less likely to go outside on days with high O3 concentrations due either to awareness of air pollution through smog alerts or because of associated high temperatures; days with high O3 concentrations tend to also be days with high temperature in the summer (although not exclusively), people may have naturally chosen to decrease their time outside on those days resulting in an overall less exposed population to high O3 concentrations. In addition, residents of Pittsburgh and St. Louis may have less access to air conditioning compared to their southern counterparts and thus more exposure to O3 through open windows on hot summer days (https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk). Previous research has shown that increased prevalence of air conditioning is associated with a decreased effect of PM25 (27, 28). Finally, it is possible that there was more exposure measurement error at certain parts of the O3 distribution (e.g. above 60 ppb) and/or alternatively, that O3 in some cities was measured more accurately than in other cities. These scenarios could cause more uncertainty (i.e. wider confidence intervals) in the C-R functions which could result in different C-R shapes across cities.
Given that C-R patterns across cities for URI did not match those for asthma (e.g. linear shape observed for O3-URI in Dallas, despite high air conditioning prevalence in Dallas) suggests complexity in factors that dictate C-R shape. For example, differences in C-R shapes may vary within city across outcomes if different pollution mixtures exist at different ambient O3 levels with some mixtures being more likely to cause poor asthma outcomes as opposed to URI, COPD, or pneumonia outcomes. Thus overall heterogeneity in O3 C-R functions across cities may be caused by a complex array of factors, such as O3 exposure ranges, behaviors, or other pollutant compositions. Population susceptibility may also play a role. As just one assessment of susceptibility, we examined C-R functions across cities by age. Steeper slopes at O3 concentrations below 60 ppb reflect overall stronger associations for children compared to adults that we have previously observed for asthma (18); the linear C-R curves observed in overall analyses for asthma in St. Louis were driven by children. Mixed results on C-R shape observed here suggest the need for assessment of the C-R function specific to the study region, population, and outcome of interest. Similar to the uncertainty we found when assessing the effects of O3 concentrations above 60 ppb, associations at very low O3 concentrations were challenging to interpret. Each city experienced only 10% of days on which O3 concentrations were below 20 ppb which limited our ability to assess whether a threshold existed at very low O3 concentrations (i.e. down to zero). Because short-term exposure to O3 can impact the respiratory system by causing declines in pulmonary function and increases in inflammatory response, it is biologically plausible that there is no threshold (29). However, the shape of the C-R function for the respiratory outcomes we examined may or may not be linear at low O3 levels. It is possible that an increased risk of having a respiratory-related ED visit or hospitalization may occur at the very lowest O3 levels but that the steepness of the risk increases around 8-hour maximum O3 concentrations of 20 ppb as the severity of the health outcome may be driven by the specific concentration and exposure duration experienced. Our results are specific only to the ranges of ozone levels that the 5 cities experienced during the time periods. It would be speculative to infer the dose-response for ozone values both above and below the ozone levels we observed.
The shapes of the cubic spline plots generally represented the C-R function identified as the best fitting model by the AIC. Both cubic spline plots and AICs provide useful information that can be used to determine the shape of the C-R function and make modeling decisions. Although cubic and cubic spline functions best modeled the O3-RD relationship in all five cities as determined by the lowest AIC, choosing to model the relationships as linear - at least until O3 concentrations of 60 ppb - is not unreasonable given the shape of the cubic spline plots. The effect of O3 on health outcomes is often assumed to be linear and linear models may give similar results as semi-parametric models for particular O3 levels but determining what the specific levels are for a certain location and health outcome requires investigating the shape of the C-R function early on in the analysis. AICs and plots based on cubic spline models may help assess this. Cubic splines do not impose distribution assumptions and may give better estimates of measures of association at particular pollutant concentrations (9, 30–35).
There are several limitations to this analysis. First, while AICs are commonly used to determine best shape (32, 36), it is unclear whether a small difference in AICs between models translates to a meaningful difference in practice. Second, there were differences in power across O3 concentration ranges which likely resulted in uncertainty in the C-R function shape at higher O3 levels (>60 ppb) in some cities for certain outcomes. Third, imprecision in the measurement of O3 could affect C-R function assessment (37). We used O3 estimates from monitors that were then fused with spatial concentration estimates and based on population weights to reduce the impact of exposure measurement error (38). Fourth, metropolitan-level O3 estimates were used rather than a finer spatial resolution (like county level). However, because O3 is a secondary pollutant shown to have little spatial variability, results likely are not significantly impacted by aggregating counts over the geographical area (21, 39, 40).
We assessed the shape of the O3 and respiratory morbidity relationship measured by ED visit counts in five U.S. cities using several C-R modeling assumptions. O3 was positively associated with respiratory ED visits overall as well as with ED visits for asthma and URI in all models. C-R functions were typically linear up until O3 concentrations of 60 ppb at which point some cities and outcomes suggested a flattening out of risk at O3 concentrations ≥60 ppb while others suggested a continually linear relationship through O3 concentrations of 90 ppb. Confidence intervals at ozone concentrations ≥60 ppb were wide. Assessing C-R shape is necessary to identify the most appropriate form of the exposure to be used in the model for the specific city, population, and outcome of interest.
Supplementary Material
Acknowledgments
This publication is based in part upon information obtained through the Georgia Hospital Association, the Missouri Hospital Association, the Dallas Fort Worth Hospital Council Foundation Information and Quality Services Center’s collaborative hospital data initiative, and individual hospitals and hospital systems in Birmingham and Pittsburgh. We are grateful for the support of all participating hospitals. This publication was developed under Assistance Agreement No. EPA834799 awarded by the U.S. Environmental Protection Agency (USEPA) to Emory University and Georgia Institute of Technology as well as by funding from the Electric Power Research Institute (EPRI, 10002467). Research reported in this publication was also supported by grants to Emory University from the USEPA (R82921301), the National Institute of Environmental Health Sciences (R01ES11294), and EPRI (EP-P27723/C13172, EP-P4353/C2124, EP-P34975/C15892, EP-P45572/C19698, EP-P25912/C12525). This publication has not been formally reviewed by USEPA or NIH. The views expressed in this document are solely those of the authors and do not necessarily reflect those of either Agency. USEPA does not endorse any products or commercial services mentioned in this publication.
Footnotes
Conflicts of interest: All authors report no conflicts of interest including no competing financial interests
Supplementary information is available at the Journal of Exposure Science and Environmental Epidemiology’s website.
References
- 1.Ji M, Cohan DS, Bell ML. Meta-analysis of the Association between Short-Term Exposure to Ambient Ozone and Respiratory Hospital Admissions. Environmental research letters: ERL [Web site] 2011;6(2) doi: 10.1088/1748-9326/6/2/024006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality, and air pollution study. Epidemiology (Cambridge, Mass) 2005;16(4):436–45. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.EPA US. Integrated Science Assessment for Ozone and Related Photochemical Oxidants. 2013 [Google Scholar]
- 4.Atkinson RW, Yu D, Armstrong BG, Pattenden S, Wilkinson P, Doherty RM, et al. Concentration- response function for ozone and daily mortality: results from five urban and five rural U.K. populations. Environmental health perspectives. 2012;120(10):1411–7. doi: 10.1289/ehp.1104108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae S, Lim YH, Kashima S, Yorifuji T, Honda Y, Kim H, et al. Non-Linear Concentration-Response Relationships between Ambient Ozone and Daily Mortality. PloS one. 2015;10(6):e0129423. doi: 10.1371/journal.pone.0129423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell ML, Peng RD, Dominici F. The exposure-response curve for ozone and risk of mortality and the adequacy of current ozone regulations. Environmental health perspectives. 2006;114(4):532–6. doi: 10.1289/ehp.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gryparis A, Forsberg B, Katsouyanni K, Analitis A, Touloumi G, Schwartz J, et al. Acute effects of ozone on mortality from the “air pollution and health: a European approach” project. American journal of respiratory and critical care medicine. 2004;170(10):1080–7. doi: 10.1164/rccm.200403-333OC. [DOI] [PubMed] [Google Scholar]
- 8.Hoek G, Schwartz JD, Groot B, Eilers P. Effects of ambient particulate matter and ozone on daily mortality in Rotterdam, The Netherlands. Archives of environmental health. 1997;52(6):455–63. doi: 10.1080/00039899709602224. [DOI] [PubMed] [Google Scholar]
- 9.Kim SY, Lee JT, Hong YC, Ahn KJ, Kim H. Determining the threshold effect of ozone on daily mortality: an analysis of ozone and mortality in Seoul, Korea, 1995-1999. Environmental research. 2004;94(2):113–9. doi: 10.1016/j.envres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Moolgavkar SH. Air pollution and daily mortality in two U.S. counties: season-specific analyses and exposure-response relationships. Inhalation toxicology. 2003;15(9):877–907. doi: 10.1080/08958370390215767. [DOI] [PubMed] [Google Scholar]
- 11.Touloumi G, Katsouyanni K, Zmirou D, Schwartz J, Spix C, de Leon AP, et al. Short-term effects of ambient oxidant exposure on mortality: a combined analysis within the APHEA project. Air Pollution and Health: a European Approach. American journal of epidemiology. 1997;146(2):177–85. doi: 10.1093/oxfordjournals.aje.a009249. [DOI] [PubMed] [Google Scholar]
- 12.Medina-Ramon M, Schwartz J. Who is more vulnerable to die from ozone air pollution? Epidemiology (Cambridge, Mass) 2008;19(5):672–9. doi: 10.1097/EDE.0b013e3181773476. [DOI] [PubMed] [Google Scholar]
- 13.National Center for Environmental Health CfDCaP, editor. CDC US. National Asthma Mortality by Age. 2014. [Google Scholar]
- 14.Vancza EM, Galdanes K, Gunnison A, Hatch G, Gordon T. Age, strain, and gender as factors for increased sensitivity of the mouse lung to inhaled ozone. Toxicological sciences: an official journal of the Society of Toxicology. 2009;107(2):535–43. doi: 10.1093/toxsci/kfn253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bateson TF, Schwartz J. Children’s response to air pollutants. Journal of toxicology and environmental health Part A. 2008;71(3):238–43. doi: 10.1080/15287390701598234. [DOI] [PubMed] [Google Scholar]
- 16.Rudd RA, Moorman JE. Asthma incidence: data from the National Health Interview Survey, 1980-1996. The Journal of asthma: official journal of the Association for the Care of Asthma. 2007;44(1):65–70. doi: 10.1080/02770900601125896. [DOI] [PubMed] [Google Scholar]
- 17.Levy JI. Issues and uncertainties in estimating the health benefits of air pollution control. Journal of toxicology and environmental health Part A. 2003;66(16–19):1865–71. doi: 10.1080/15287390306423. [DOI] [PubMed] [Google Scholar]
- 18.Alhanti BA, Chang HH, Winquist A, Mulholland JA, Darrow LA, Sarnat SE. Ambient air pollution and emergency department visits for asthma: a multi-city assessment of effect modification by age. J Expo Sci Environ Epidemiol. 2016;26(2):180–8. doi: 10.1038/jes.2015.57. [DOI] [PubMed] [Google Scholar]
- 19.Krall JR, Mulholland JA, Russell AG, Balachandran S, Winquist A, Tolbert PE, et al. Associations between Source-Specific Fine Particulate Matter and Emergency Department Visits for Respiratory Disease in Four U.S. Cities. Environ Health Perspect. 2017;125(1):97–103. doi: 10.1289/EHP271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Lenick C, Chang HH, Kramer MR, Winquist A, Mulholland JA, Friberg MD, et al. Ozone and childhood respiratory disease in three US cities: evaluation of effect measure modification by neighborhood socioeconomic status using a Bayesian hierarchical approach. Environmental health: a global access science source. 2017;16(1):36. doi: 10.1186/s12940-017-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friberg MD, Kahn RA, Holmes HA, Chang HH, Sarnat SE, Tolbert PE, et al. Daily ambient air pollution metrics for five cities: Evaluation of data-fusion-based estimates and uncertainties. Atmospheric Environment. 2017;158:36–50. [Google Scholar]
- 22.Friberg MD, Zhai X, Holmes HA, Chang HH, Strickland MJ, Sarnat SE, et al. Method for Fusing Observational Data and Chemical Transport Model Simulations To Estimate Spatiotemporally Resolved Ambient Air Pollution. Environmental science & technology. 2016;50(7):3695–705. doi: 10.1021/acs.est.5b05134. [DOI] [PubMed] [Google Scholar]
- 23.Ivy D, Mulholland JA, Russell AG. Development of ambient air quality population-weighted metrics for use in time-series health studies. Journal of the Air & Waste Management Association (1995) 2008;58(5):711–20. doi: 10.3155/1047-3289.58.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothman KJGS. Introduction to Stratified Analysis, Testing Homogeneity. In: Wilkins LWa., editor. Modern Epidemiology, Second Edition. 1998. p. 275. [Google Scholar]
- 25.Flanders WD, Klein M, Darrow LA, Strickland MJ, Sarnat SE, Sarnat JA, et al. A method for detection of residual confounding in time-series and other observational studies. Epidemiology (Cambridge, Mass) 2011;22(1):59–67. doi: 10.1097/EDE.0b013e3181fdcabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiore A, Jacob DJ, Liu H, Yantosca RM, Fairlie TD, Li Q. Variability in surface ozone background over the United States: Implications for air quality policy. Journal of Geophysical Research: Atmospheres. 2003;108(D24):n/a–n/a. [Google Scholar]
- 27.Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. Journal of exposure science & environmental epidemiology. 2007;17(3):279–87. doi: 10.1038/sj.jes.7500530. [DOI] [PubMed] [Google Scholar]
- 28.Bell ML, Ebisu K, Peng RD, Dominici F. Adverse health effects of particulate air pollution: modification by air conditioning. Epidemiology (Cambridge, Mass) 2009;20(5):682–6. doi: 10.1097/EDE.0b013e3181aba749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lippmann M. Effects of ozone on respiratory function and structure. Annual review of public health. 1989;10:49–67. doi: 10.1146/annurev.pu.10.050189.000405. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong B. Models for the relationship between ambient temperature and daily mortality. Epidemiology (Cambridge, Mass) 2006:624–31. doi: 10.1097/01.ede.0000239732.50999.8f. 2006/10/10 ed. [DOI] [PubMed] [Google Scholar]
- 31.Cakmak S, Burnett RT, Krewski D. Methods for detecting and estimating population threshold concentrations for air pollution-related mortality with exposure measurement error. Risk analysis: an official publication of the Society for Risk Analysis. 1999;19(3):487–96. doi: 10.1023/a:1007008914354. [DOI] [PubMed] [Google Scholar]
- 32.Daniels MJ, Dominici F, Zeger SL, Samet JM. The National Morbidity, Mortality, and Air Pollution Study. Part III: PM10 concentration-response curves and thresholds for the 20 largest US cities. Research report (Health Effects Institute) 2004;(94 Pt 3):1–21. discussion 3-30. [PubMed] [Google Scholar]
- 33.Dominici F, Sheppard L, Clyde M. Health Effects of Air Pollution: A Statistical Review. International Statistical Review. 2003;71(2):243–76. [Google Scholar]
- 34.May S, Bigelow C. Modeling nonlinear dose-response relationships in epidemiologic studies: statistical approaches and practical challenges. Dose-response: a publication of International Hormesis Society. 2005;3(4):474–90. doi: 10.2203/dose-response.003.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samoli E, Touloumi G, Zanobetti A, Le Tertre A, Schindler C, Atkinson R, et al. Investigating the dose-response relation between air pollution and total mortality in the APHEA-2 multicity project. Occupational and environmental medicine. 2003;60(12):977–82. doi: 10.1136/oem.60.12.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lepeule J, Laden F, Dockery D, Schwartz J. Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environmental health perspectives. 2012;120(7):965–70. doi: 10.1289/ehp.1104660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environmental health perspectives. 2000;108(5):419–26. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strickland MJ, Gass KM, Goldman GT, Mulholland JA. Effects of ambient air pollution measurement error on health effect estimates in time-series studies: a simulation-based analysis. Journal of exposure science & environmental epidemiology. 2015;25(2):160–6. doi: 10.1038/jes.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarnat SE, Klein M, Sarnat JA, Flanders WD, Waller LA, Mulholland JA, et al. An examination of exposure measurement error from air pollutant spatial variability in time-series studies. Journal of exposure science & environmental epidemiology. 2010;20(2):135–46. doi: 10.1038/jes.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarnat SE, Sarnat JA, Mulholland J, Isakov V, Ozkaynak H, Chang HH, et al. Application of alternative spatiotemporal metrics of ambient air pollution exposure in a time-series epidemiological study in Atlanta. Journal of exposure science & environmental epidemiology. 2013;23(6):593–605. doi: 10.1038/jes.2013.41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


