Keywords: nerve regeneration, depressive disorder, cat stress, cat odor stress, behavioral evaluation, open field test, elevated plus maze test, dark-avoidance test, 5-hydroxytryptamine, 5-hydroxyindoleacetic acid, hippocampal neurogenesis, neural regeneration
Abstract
Stress has been suggested to disturb the 5-hydroxytryptamine system and decrease neurogenesis, which contribute to the development of depression. Few studies have investigated the effect of predator stress, a type of psychological stress, on depression and hippocampal neurogenesis in adult mice; we therefore investigated this in the present study. A total of 35 adult male Kunming mice were allocated to a cat stress group, cat odor stress group, cat stress + fluoxetine group, cat odor stress + fluoxetine group, or a control group (no stress/treatment). After 12 days of cat stress or cat odor stress, behavioral correlates of depression were measured using the open field test, elevated plus maze test, and dark-avoidance test. The concentrations of hippocampal 5-hydroxytryptamine and 5-hydroxyindoleacetic acid were measured using high-performance liquid chromatography-electrochemical detection. Neurogenesis was also analyzed using a bromodeoxyuridine and doublecortin double-immunostaining method. Cat stress and cat odor stress induced depression-like behaviors; this effect was stronger in the cat stress model. Furthermore, compared with the control group, cat stress mice exhibited lower 5-hydroxytryptamine concentrations, higher 5-hydroxyindoleacetic acid concentrations, and significantly fewer bromodeoxyuridine+/doublecortin+-labeled cells in the dentate gyrus, which was indicative of less neurogenesis. The changes observed in the cat stress group were not seen in the cat stress + fluoxetine group, which suggests that the effects of predator stress on depression and neurogenesis were reversed by fluoxetine. Taken together, our results indicate that depression-like behaviors induced by predator stress are associated with the inhibition of hippocampal neurogenesis.
Chinese Library Classification No. R459.9; R364
Introduction
Depression is a major psychiatric disorder (Narrow et al., 2002) and associated with genetic, environmental, and psychological factors. Several stress paradigms, including unpredictable chronic mild stress (UCMS), social defeat stress (SDS), and predator stress, have been established to study the influence of psychological stress on depression. The effect of UCMS on depression is unclear, with inconsistent findings of previous studies (Cryan and Mombereau, 2004; Mineur et al., 2006). Similarly, large individual differences in response to SDS in rodents have been reported, whereby some mice were resilient to the effects of SDS and still displayed normal social interactions (Meduri et al., 2013). It is necessary to explore a more suitable stress model for investigating the relationship between stress and depression.
Predator stress, including predator odor stress and living predator stress, has been used as a potent stress stimulus (Apfelbach et al., 2005). Burgado et al. (2014) reported that a chronic predatory stress paradigm (15-day exposure of mice to a rat) causes depressive behaviors in adult mice. However, the natural predator of mice is the cat. The effect of cat stress and cat odor stress on depression in mice and rats has been studied in previous work (Saboory et al., 2011; Wright et al., 2013). However, models that have used cats as predators have primarily investigated the effect of predator stress on adolescent mice (Saboory et al., 2011; Toledo and Sandi, 2011) or the effect of juvenile stress in adult mice (Tsoory et al., 2007; Wright et al., 2013; Post et al., 2014). Evidence for the direct effect of cat stress or cat odor stress on depression in adult mice is limited. Models of predator stress, such as cat odor stress or cat stress, have the potential to be powerful paradigms to induce and measure the effects of physiological stress in adult mice, and to better define the mechanisms underlying predator stress.
Hippocampal neurogenesis happens throughout life (Mirescu and Gould, 2006; Li et al., 2017; Tawarayama, 2018). During neurogenesis in the hippocampus, newly born cells move to the granule cell layer of the dentate gyrus (Tashiro et al., 2006). According to two studies, hippocampal volume is decreased in patients with depression and increases in patients that have been treated with antidepressants (Kempermann, 2002; Kempermann and Kronenberg, 2003). In rodents, the impairment of hippocampal neurogenesis has been linked to depression (Reif et al., 2006; Airan et al., 2007).
Hippocampal neurogenesis is regulated by 5-hydroxytryptamine (5-HT); removal of 5-HT neurons in rats has been found to reduce neurogenesis, and recovery of neurogenesis observed after 5-HT re-innervation (Brezun and Daszuta, 2000). Fluoxetine, a selective 5-HT reuptake inhibitor, is an antidepressant that can decrease stress-induced depressive-like behaviors and increase adult hippocampal neurogenesis (David et al., 2009; Mahar et al., 2014). In this study, we compared the impacts of two models of predator stress, cat stress and cat odor stress, on depression, and investigated the mechanism underlying predator stress in adult mice using fluoxetine as a positive control.
Materials and Methods
Animals and treatment
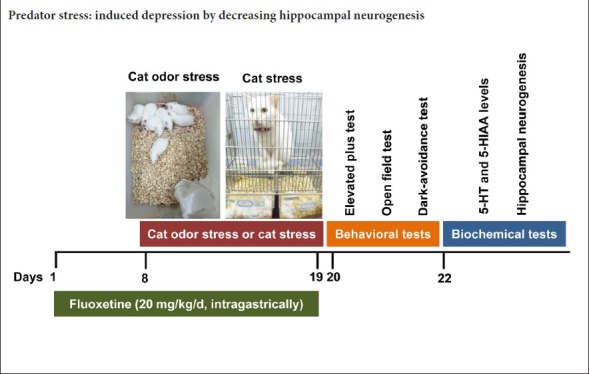
A total of 35 male Kunming mice aged 6–8 weeks old and weighing 18–22 g were purchased from the Animal Center, Guangdong Medical Laboratory, China (license No. SCXK (Yue) 2013-0002). Mice were fed in a specific-pathogen-free animal room at 23 ± 1°C with a 12-hour light dark cycle with lights on from 8:00 to 20:00. Chow and tap water were given to mice every day. One laboratory cat was used for all groups, and it was the same cat every time. All animal care and experimental procedures were approved by the Animal Care and Use Committee of Jinan University, China (approval No. 20120806003) and were in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals.
The mice were equally and randomly allocated to a control group (n = 7), cat odor stress group (n = 7), cat stress group (n = 7), cat odor stress + fluoxetine group (n = 7), and cat stress + fluoxetine group (n = 7). The control group was given water as a vehicle control through oral administration every day and was not exposed to any stress. The cat odor stress and cat stress groups were given water as a vehicle control through oral administration every day, and were exposed to cat odor stress and cat stress, respectively. The cat odor stress + fluoxetine and cat stress + fluoxetine groups were given fluoxetine (20 mg/kg/d; Lilai Suzhou Pharmaceutical Co., Ltd., Suzhou, China) through intragastic administration for 19 consecutive days (7 days before cat odor stress or cat stress, and 12 days during cat odor stress or cat stress) (Dulawa et al., 2004; Guo et al., 2004; Cryan et al., 2005).
The cat odor stress paradigm was performed according to previous studies (Takahashi et al., 2005; Kendig et al., 2011) with some modifications. A cloth was put on an adult female cat’s bed overnight and rubbed on its body the next day to obtain cat odor. On the day of cat odor stress, the mice were exposed to the cloth filled with cat odor for 15 minutes. A non-odor exposure group (the control group) was exposed to the same set up but with a clean cloth. This operation was repeated for 12 days.
The model of cat stress was induced according to a previous study with some modifications (Fleshner et al., 2004). Mice were placed in a ventilated Plexiglas holding chamber and exposed to an adult female cat with sight, smell, and sound stress effects but without physical contact. This exposure lasted 15 minutes and was implemented for 12 consecutive days.
The effects of cat stress and cat odor stress on depressive-like behaviors were measured using the open field test, elevated plus maze test, and dark-avoidance test, which have been extensively applied to measure depressive-like behaviors (David et al., 2009; Duric et al., 2010; Snyder et al., 2011).
The elevated plus maze test
The elevated plus maze test is used to measure anxiety-like behavior in rodents, and has thus been used to measure the efficacy of anti-anxiety medication. Decreased activity in the open arm (time spent and/or number of entries) is considered to reflect anxiety-like behavior (Walf et al., 2007). On day 20, the mice were placed in the center of a plus maze (Jiliang Software Technology Co., Ltd., Shanghai, China) with two open arms and two closed arms. The behavior of mice was recorded and analyzed using an Elevated Plus Maze Video Analysis System (Dig-Behv EPM, Jiliang Software Technology Co., Ltd., Shanghai, China) for 5 minutes. The number of entries and time spent in the closed or open arms was recorded. The time spent in the open arm (%) and the open arm entry number (%) were calculated using the following formulas (Hogg, 1996; Cryan et al., 2005; Manduca et al., 2015; Seidenbecher et al., 2016):
Percentage of time spent in open arm = (time spent in open arm/total time spent in open arm and closed arm) × 100%;
Percentage of open arm entries = (entry number to open arm/total entry number to open arm and closed arm) × 100%.
Open field test
The open field test, developed by Hall and Ballachey (1932), was designed to measure locomotor activity and willingness to explore in rodents. The open field apparatus is a box with a width of 25 cm, a length of 25 cm, and a height of 31 cm. The open field is divided into a central area and a peripheral region area. The central area is defined as that a center with half of area of the whole. On day 21, animals were transferred individually to the open field apparatus, and the central distance (the total distance mice moved in the central area of the open field, which is taken as a measure of locomotor activity) and central time (the total time spent in central area of the open field) were recorded for 5 minutes and analyzed by a Locomotion Activity Video Analysis System (Dig-Behv LA, Jiliang Software Technology Co., Ltd.). The frequency of rearing (defined as standing on both hind paws in a vertical upright position without touching the wall) was manually counted. Less activity in the open field is considered as a symptom of depression.
Dark-avoidance test
The dark-avoidance test was used to observe the behavior of mice in response to stressors, according to the innate aversion to light and spontaneous exploratory behavior. A decrease in spontaneous locomotion is considered as depressive-like behavior (Bourin et al., 2003). The dark-avoidance test was conducted on day 22 in an apparatus that consisted of two equally sized dark/light compartments. The dark compartment was composed of black plastic walls, roof, and floor, while the light compartment was illuminated with a 700 lx white light and consisted of a white plastic floor and three transparent Plexiglas walls. These two compartments were connected by a doorway, which allowed the mice to move between them. The latency to enter, the number of entries, and the total time spent in the dark compartment were recorded and analyzed using a Step Through Video Analysis System (DigBehv-ST, Jiliang Software Technology Co., Ltd.) for 5 minutes (Steenbergen et al., 2011).
5-HT and 5-HIAA levels in the hippocampus
To confirm whether the cat stress-induced depressive-like behaviors were associated with changes in neurogenesis and 5-HT levels, biochemical tests were performed after the behavioral tests (Kung et al., 2010). First, we detected 5-HT and 5-HIAA levels in the hippocampus using high-performance liquid chromatography-electrochemical detection (HPLC-ECD). On day 23, mice of each group were anesthetized using diethyl ether (Shangdong Yuwang Industrial Co., Ltd., Yucheng, China) and the hippocampus was dissected, placed on the ice, and weighed. Subsequently, the hippocampus (approximately 30 mg) was treated with 3% perchloric acid (Damao Chemical Regent Factory, Tianjin, China) in PBS solution at a ratio of 1:3 (w/v). Tissues were homogenized for 30 seconds in an ice-cold condition and centrifuged at 13,200 × g at 4°C for 15 minutes. Hippocampus samples were obtained by filtering supernatant using a 0.2 μm filter attached with a 1 mL syringe, and analyzed using HPLC-ECD (ESA Biosciences, Chelmsford, MA, USA) by injecting 20 μL of the sample. 5-HT and 5-HIAA were separated on a reverse-phased column (NacalaiTesque, Kyoto, Japan) using a flow rate of 0.8 mL/minute. The 5-HT and 5-HIAA standards were purchased from Sigma (St. Louis, MO, USA). A series of concentrations of 5-HT and 5-HIAA (50, 100, 200, 400, and 800 ng/L) were diluted by standards. The corresponding peak areas of different concentrations of 5-HT and 5-HIAA were obtained by HPLC-ECD, and then a standard curve was obtained. Subsequently, the peak area in different groups of mice was also obtained by HPLC-ECD, and the concentrations of 5-HT and 5-HIAA in different groups were calculated according to the standard curve.
Bromodeoxyuridine/doublecortin double immunostaining
On day 23, mice of each group were intraperitoneally administrated with bromodeoxyuridine (BrdU) (75 mg/kg; Abcam, Cambridge, UK) every 2 hours for 12 hours. Two hours after the last injection of BrdU, the mice were anesthetized with diethyl ether. Blood in the brains was flushed out using normal saline through a heart perfusion, and the brains were fixed using 4% paraformaldehyde (Guangzhou Chemical Reagent Factory, Guangzhou, China), also using heart perfusion. Subsequently, the fixed brains were extracted and continued to fix in a fresh 4% paraformaldehyde for 1 week. The fixed brains were paraffin-embedded and sectioned.
After the denaturation of DNA and antigen retrieval, sections were incubated with a mixture of primary antibody BrdU (1:1400; a marker of newly generated cells) and doublecortin (DCX) (1:400; a marker of immature neurons) (Abcam, Cambridge, UK) at 4°C overnight. The next day, sections were incubated with goat anti-mouse IgG-Alexa 488 (1:600) and goat anti-rabbit IgG-PE (1:400) (Invitrogen, CA, USA) in the dark for 2 hours. DNA in the nucleus was stained by DAPI dye (1:1298) (Boyotime Institute of Biotechnology, Haimen, China) for 5 minutes. Finally, sections were mounted using anti-fade mounting medium, and then stored at 4°C in the dark. A section incubated without primary antibodies was considered as a negative control (Additional Figure 1 (177KB, pdf) ).
Negative control in double immunostaining of BrdU/DCX in dentate gyrus.
Analysis of BrdU+/DCX+-labeled cells
After double immunostaining for BrdU/DCX, immunofluorescent images were obtained using an inverted fluorescence microscope (LX51; Olympus, Tokyo, Japan) equipped with a charge-coupled-device camera (DP70; Olympus) and captured in the suitable time of exposure in different fluorescent light channels. The exposure time of each image was constant in each fluorescent light channel. Cell counting was performed according to previous methods (Van Praag et al., 1999; Chen et al., 2000; Shioda et al., 2008; Lu et al., 2015). We traced the areas of dentate gyrus in the section using a stereo investigator software (MBF, Biosciences, Williston, VT, USA), and the approximate volume of dentate gyrus was obtained according the fractionator method. The number of BrdU+/DCX+-labeled cells was counted in every 12th section of the dentate gyrus. The total number of BrdU+/DCX+-labeled cells was analyzed by summing the traced area of sections in the dentate gyrus and multiplying by the distance between each section sample. Cell counts were performed in a blinded manner from six mice per condition.
Statistical analysis
Data are expressed as the mean ± SEM, and were analyzed using one-way analysis of variance and Tukey’s post hoc test with GraphPad Prism 6 software (GraphPad Software, La Jolla, CA, USA). P-values < 0.05 were considered as statistically different (Guo et al., 2018; Jiang et al., 2018; Yang et al., 2018; Zhang et al., 2018).
Results
Cat stress causes more obvious depressive-like behaviors than cat odor stress
Compared with the control group, the cat odor stress group showed a significant decrease of total distance (P < 0.05), central distance (P < 0.05), in the open field test. The cat stress group also exhibited a significant decrease in rearing (P < 0.01), total distance (P < 0.001), central distance (P < 0.001), and central times (P < 0.001) compared with the control group (Figure 1). These decreases induced by cat stress were significantly reversed by fluoxetine treatment (P < 0.05).
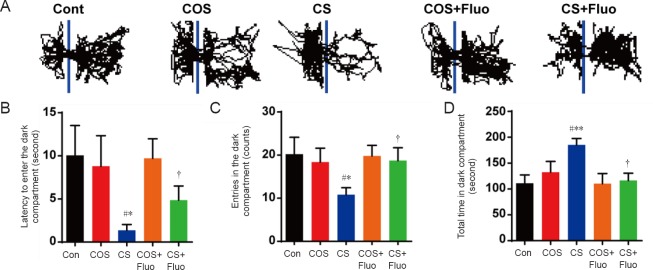
Figure 1.
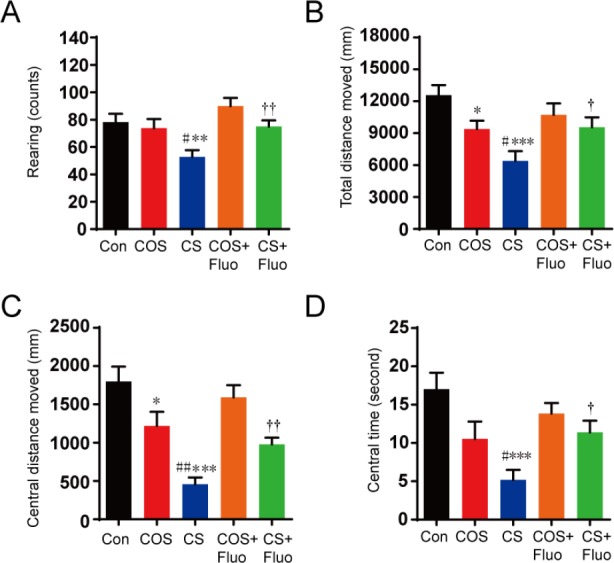
Effect of cat odor stress and cat stress on behavior in the open field test.
The effect of cat odor stress and cat stress on (A) the number of rearing, (B) the total distance moved, (C) the central distance moved, and (D) the central time in open field test, which was implemented on day 21. Data are expressed as the mean ± SEM (n = 7 per group; one-way analysis of variance and Tukey’s post hoc tests). *P < 0.05, **P < 0.01, ***P < 0.001, vs. Con; ##P < 0.01, vs. COS; †P < 0.05, ††P < 0.01, vs. CS. Con: Control; COS: cat odor stress; COS + Fluo: cat odor stress + fluoxetine; CS: cat stress; CS + Fluo: cat stress + fluoxetine.
In the elevated plus maze, mice in the cat stress group spent significantly less time (P < 0.001) and made significantly fewer entries (P < 0.001) into the open arm than the control group, while the cat odor stress group showed no significant differences in the time spent in the open arm or the number of entries into the open arm compared with the control group (Figure 2). As expected, cat stress + fluoxetine group also showed a significantly longer time spent in the open arm (P < 0.05) and more open arm entries (P < 0.01) than the cat stress group.
Figure 2.
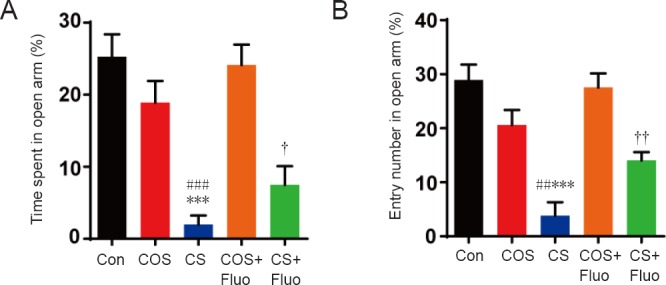
Effect of cat odor stress and cat stress on behavior in the elevated plus maze test.
The effect of cat odor stress and cat stress on (A) the time spent in open arm (%) and (B) the number of entries into the open arm in the elevated plus maze test, which was implemented on day 20. Data are expressed as the mean ± SEM (n = 7 per group; one-way analysis of variance and Tukey’s post hoc tests). ***P < 0.001, vs. Con; ##P < 0.01, ###P < 0.001, vs. COS; †P < 0.05, ††P < 0.01, vs. CS. Con: Control; COS: cat odor stress; COS + Fluo: cat odor stress + fluoxetine; CS: cat stress; CS + Fluo: cat stress + fluoxetine.
In the dark-avoidance test, the cat stress group had a significantly lower number of entries into the dark compartment (P < 0.05), a shorter latency to enter the dark compartment (P < 0.05), and spent more total time in the dark compartment (P < 0.01) compared with the control group (Figure 3). However, there were no obvious changes in the cat odor stress group compared with the control group. Depressive/anxiety-like behaviors in the cat stress group were not shown in with fluoxetine treatment (P < 0.05).
Figure 3.
Effect of cat odor stress and cat stress on behavior in the dark-avoidance test.
The effect of cat odor stress and cat stress on (A) movement (the representative trace of an individual mouse), (B) the latency to enter the dark compartment, (C) the number of entries into the dark compartment, and (D) the total time spent in the dark compartment in dark-avoidance test, which was implemented on day 22. Data are expressed as the mean ± SEM (n = 7 per group; one-way analysis of variance and Tukey’s post hoc tests). *P < 0.05, **P < 0.01, vs. Con; #P < 0.05 vs. COS; †P < 0.05, vs. CS. Con: Control; COS: cat odor stress; COS + Fluo: cat odor stress + fluoxetine; CS: cat stress; CS + Fluo: cat stress + fluoxetine.
The cat stress group also showed significant changes (P < 0.05) in the open field test, elevated plus maze test, and dark-avoidance test compared with the cat odor stress group.
Cat stress disturbs hippocampal 5-HT and 5-HIAA levels
As shown in Figure 4, cat stress significantly decreased 5-HT levels (P < 0.01), but increased 5-HIAA levels (P < 0.01) in the hippocampus of mice compared with the control group. The cat stress + fluoxetine group showed significantly restored 5-HT (P < 0.05) and 5-HIAA (P < 0.05) levels compared with cat stress group.
Figure 4.
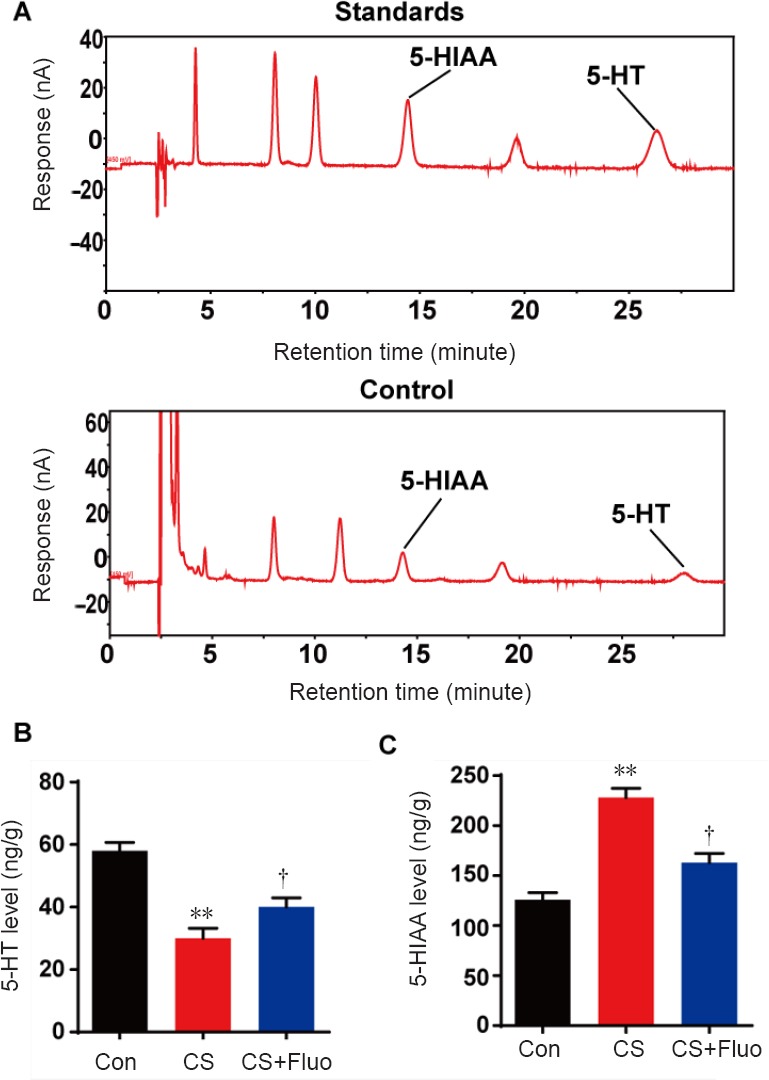
Effect of cat stress on 5-HT and 5-HIAA levels in the hippocampus of mice on day 23.
(A) The levels of 5-HT and 5-HIAA in the standards and control. (B, C) The hippocampal 5-HT and 5-HIAA levels in control, cat stress, and cat stress + fluoxetine mice. Data are expressed as the mean ± SEM (n = 7 per group; one-way analysis of variance and Tukey’s post hoc tests). **P < 0.01, vs. Con; †P < 0.05, vs. CS. Con: Control; CS: cat stress; CS + Fluo: cat stress + fluoxetine; 5-HIAA: 5-hydroxyindoleacetic acid; 5-HT: 5-hydroxytryptamine.
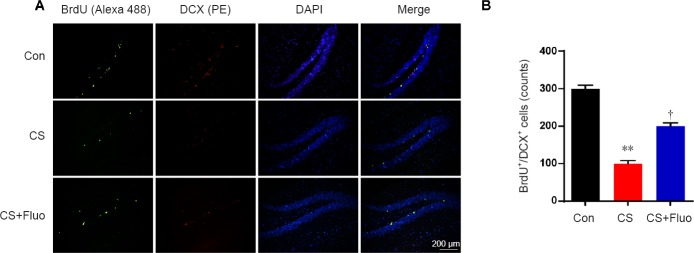
Cat stress inhibits neurogenesis in the dentate gyrus of the hippocampus
Subsequently, double immunostaining for BrdU and DCX was used to evaluate the proliferative effect of cat stress on hippocampal neuronal cells. The number of BrdU+/DCX+-labeled cells in the dentate gyrus was significantly lower in the cat stress group compared with the control group (P < 0.01) (Figure 5). The cat stress + fluoxetine group showed significantly recovered neurogenesis compared with cat stress group (P < 0.05).
Figure 5.
Effects of cat stress on neuronal cell proliferation in the dentate gyrus.
(A) Microscopy images of sections of the dentate gyrus stained for BrdU. The fluorescent signals for Alexa 488 labeling (green) and PE (red) were used to detect BrdU and DCX, respectively. DAPI (blue) was used to stain nucleus DNA, so that the dentate gyrus of the hippocampus could be seen clearly. All micrographs are at the same magnification. Scale bar: 200 μm. (B) The number of BrdU and DCX co-expressing cells in the dentate gyrus was quantitatively analyzed. Data are expressed as the mean ± SEM (n =7 per group; analysis of variance and Tukey’s post hoc tests). **P < 0.01, vs. Con; †P < 0.05, vs. CS. BrdU: 5-bromodeoxyuridine; Con: control; CS: cat stress; CS + Fluo: cat stress + fluoxetine; DAPI: 4′,6-diamidino-2-phenylindole; DCX: doublecortin.
Discussion
Previous findings concerning UCMS and the negative effect of social defeat stress have been inconsistent. Only predator stress has been revealed to directly influence depression in adolescence and to indirectly influence depression in adulthood after juvenile exposure (Tsoory et al., 2007; Saboory et al., 2011; Toledo and Sandi, 2011; Wright et al., 2013; Post et al., 2014). Evidence for the direct effect of predatory stress on adult mice is limited. Therefore, we compared the influence of two kinds of predatory stresses, namely, cat odor stress and cat stress, on depression in adult mice. We also investigated the mechanism of predator stress in adult mice using fluoxetine as a positive control. We found that both kinds of stress induced depressive-like behaviors in adult mice, but cat stress showed more obvious changes in these depressive-like behaviors tests than cat odor stress. Furthermore, cat stress-induced depression disturbed the 5-HT system and was associated with a decrease of hippocampal neurogenesis in adult mice.
The influence of cat stress on depressive-like behavior was more evident than that of cat odor stress. This might be because cat stress elicits fear not only from smell but also from the sight and sound of the predator; this more realistically imitates real stress in human life. We propose that cat odor stress elicited an innate fear response through imagination of the natural predator. However, we cannot exclude the possibility that the odor dissipated over time in the cat odor stress model, which may slightly affect the comparison between cat odor stress and cat stress. This could indicate that cat stress is a more robust, effective, and suitable paradigm to induce depressive-like behaviors in mice. However, the direct mechanism of cat stress on depression in adult mice is still unclear, and requires further discussion in follow-up studies.
Related studies have reported that 5-HT, a key modulatory neurotransmitter, and 5-HIAA levels are associated with depression caused by SDS, UCMS, and chronic corticosterone administration (Hong et al., 1999; Mahar et al., 2014; Sachs et al., 2015). We found that stress reduced 5-HT neurotransmission and increased its metabolite (5-HIAA), and that these effects were attenuated by the administration of fluoxetine, a selective 5-HT reuptake inhibitor. Even in normal mice, fluoxetine treatment can increase 5-HT levels in the hippocampus (Imoto et al., 2015) and counteract depression-like behaviors in the elevated plus maze and open field test (Silva and Brandao, 2000; Dulawa et al., 2004; Drapier et al., 2007). Consistent with these reports, we found that fluoxetine also decreased cat stress-induced depressive-like behaviors and that this was accompanied by a decrease of 5-HT levels and an increase of 5-HIAA levels in adult mice. This suggests that depressive-like behaviors induced by cat stress in adult mice are also associated with the disruption of the 5-HT system and that fluoxetine treatment can counteract these effects.
Numerous studies have shown that several kinds of stress, such as SDS and UCMS, can induce the release of corticosteroids to the brain via the hypothalamic-pituitary-adrenal axis, and strongly downregulate hippocampal neurogenesis, which has been considered as a new target for treating depression (Lemaire et al., 2000; Wainwright and Galea, 2013). The decrease of BrdU+/DCX+ cell represents a decrease of hippocampal neurogenesis. Compared to the reported stress on mice, the result of BrdU/DCX staining in the present study showed that cat stress was also decreased the number of BrdU+/DCX+ cells, thereby inhibiting hippocampal neurogenesis. In recent years, the relationship between 5-HT and hippocampal neurogenesis has received more attention. Hippocampal neurogenesis has been shown to be regulated by 5-HT (Brezun and Daszuta, 2000). Removal of 5-HT neurons in rats reduced neurogenesis, which was recovered by 5-HT re-innervation (Brezun and Daszuta, 2000). The stimulation of specific 5-HT receptors, such as 5-HT1A, 5-HT2B, or the suppression of 5-HT transporter expression has been found to improve hippocampal neurogenesis (Mahar et al., 2014). It has also been reported that fluoxetine regulates hippocampal plasticity via 5-HT receptors and induces immaturity of the dentate granule cells (Kobayashi et al., 2010). In our study, cat stress caused a decrease in 5-HT levels and hippocampal neurogenesis in adult mice, and this was recovered by fluoxetine. In line with previous studies, these results suggest that cat stress-induced depressive-like behaviors in adult mice are associated with a decrease of 5-HT and hippocampal neurogenesis. However, to determine whether the depression induced by cat stress in adult mice relates to the regulation of cAMP-response element binding protein/brain-derived neurotrophic factor/Tyrosine kinase B signals, further investigations into the mechanisms of cat stress-induced depression in adult mice are required.
Overall, we found that both cat stress and cat odor stress models induced depression in adult mice compared to a control group. On the basis of this result, the effect of these two models on depression was also compared. Fluoxetine was used as a positive control and its effect helped us to explain the mechanism of cat stress on depression in adult mice. We found that cat stress had an obvious influence on depression, and our results indicate that this is caused by disturbed hippocampal neurogenesis. These findings provide evidence that the cat stress paradigm is a suitable predator stress model with which to study the influence of psychological stress on depressive-like disorders and adult hippocampal neurogenesis, as well as evaluating the potential anti-depressive effects of drugs.
Additional file:
Additional Figure 1: Negative control in double immunostaining of BrdU/DCX in the dentate gyrus.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81473115 (to RRH), 81622050 (to RRH); the Natural Science Foundation of Guangdong Province of China for Distinguished Young Scholars, No. 2017A030306004 (to YFL); the Youth Top-Notch Talent Support Program of Guangdong Province of China, No. 2016TQ03R586 (to YFL). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: All animal care and experimental procedures were approved by the Animal Care and Use Committee of Jinan University, China (approval No. 20120806003). All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editor: ZM; S-Editors: Wang J, Li CH; L-Editors: Cason N, de Souza M, Qiu Y, Song LP; T-Editor: Liu XL
Funding: This study was supported by the National Natural Science Foundation of China, No. 81473115 (to RRH), 81622050 (to RRH); the Natural Science Foundation of Guangdong Province of China for Distinguished Young Scholars, No. 2017A030306004 (to YFL); the Youth Top-Notch Talent Support Program of Guangdong Province of China, No. 2016TQ03R586 (to YFL).
References
- 1.Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 2.Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29:1123–1144. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 4.Brezun JM, Daszuta A. Serotonin may stimulate granule cell proliferation in the adult hippocampus, as observed in rats grafted with foetal raphe neurons. Eur J Neurosci. 2000;12:391–396. doi: 10.1046/j.1460-9568.2000.00932.x. [DOI] [PubMed] [Google Scholar]
- 5.Burgado J, Harrell CS, Eacret D, Reddy R, Barnum CJ, Tansey MG, Miller AH, Wang H, Neigh GN. Two weeks of predatory stress induces anxiety-like behavior with co-morbid depressive-like behavior in adult male mice. Behav Brain Res. 2014;275:120–125. doi: 10.1016/j.bbr.2014.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- 7.Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 8.David DJ, Samuels BA, Rainer Q, Wang J-W, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP. Neurogenesis-dependent and-independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drapier D, Bentue-Ferrer D, Laviolle B, Millet B, Allain H, Bourin M, Reymann J-M. Effects of acute fluoxetine, paroxetine and desipramine on rats tested on the elevated plus-maze. Behav Brain Res. 2007;176:202–209. doi: 10.1016/j.bbr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Dulawa SC, Hollick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- 11.Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 12.Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, Newton SS, Duman RS. A negative regulator of MAP kinase causes depressive behavior. Nat Med. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleshner M, Campisi J, Amiri L, Diamond DM. Cat exposure induces both intra-and extracellular Hsp72: the role of adrenal hormones. Psychoneuroendocrinology. 2004;29:1142–1152. doi: 10.1016/j.psyneuen.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Gould E, Tanapat P, McEwen BS, Flügge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo X, Ma Z, Du B, Li T, Li W, Xu L, He J, Kang L. Dop1 enhances conspecific olfactory attraction by inhibiting miR-9a maturation in locusts. Nat Commun 9 1193. 2018 doi: 10.1038/s41467-018-03437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo YS, Kong LD, Wang YM, Huang ZQ. Antidepressant evaluation of polysaccharides from a Chinese herbal medicine Banxia-houpu decoction. Phytother Res. 2004;18:204–207. doi: 10.1002/ptr.1394. [DOI] [PubMed] [Google Scholar]
- 17.Hall C, Ballachey EL. A study of the rat's behavior in a field: a contribution to method in comparative psychology. Los Angeles: University of California Publications in Psychology, USA; 1932. [Google Scholar]
- 18.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 19.Hong CJ, Tsai SJ, Cheng CY, Liao WY, Song HL, Lai HC. Association analysis of the 5-HT6 receptor polymorphism (C267T) in mood disorders. Am J Med Genet. 1999;88:601–602. [PubMed] [Google Scholar]
- 20.Imoto Y, Kira T, Sukeno M, Nishitani N, Nagayasu K, Nakagawa T, Kaneko S, Kobayashi K, Segi-Nishida E. Role of the 5-HT 4 receptor in chronic fluoxetine treatment-induced neurogenic activity and granule cell dematuration in the dentate gyrus. Mol Brain. 2015;8:29. doi: 10.1186/s13041-015-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang XW, Zhang BQ, Qiao L, Liu L, Wang XW, Yu WH. Acetyl-11-keto-β-boswellic acid extracted from Boswellia serrata promotes Schwann cell proliferation and sciatic nerve function recovery. Neural Regen Res. 2018;13:484. doi: 10.4103/1673-5374.228732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kempermann G. Regulation of adult hippocampal neurogenesis-implications for novel theories of major depression. Bipolar Disord. 2002;4:17–33. doi: 10.1034/j.1399-5618.2002.40101.x. [DOI] [PubMed] [Google Scholar]
- 23.Kempermann G, Kronenberg G. Depressed new neurons? Adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry. 2003;54:499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- 24.Kendig MD, Bowen MT, Kemp AH, McGregor IS. Predatory threat induces huddling in adolescent rats and residual changes in early adulthood suggestive of increased resilience. Behav Brain Res. 2011;225:405–414. doi: 10.1016/j.bbr.2011.07.058. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi K, Ikeda Y, Sakai A, Yamasaki N, Haneda E, Miyakawa T, Suzuki H. Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc Natl Acad Sci U S A. 2010;107:8434–8439. doi: 10.1073/pnas.0912690107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kung JC, Chen TC, Shyu BC, Hsiao S, Huang AC. Anxiety-and depressive-like responses and c-fos activity in preproenkephalin knockout mice: oversensitivity hypothesis of enkephalin deficit-induced posttraumatic stress disorder. J Biomed Sci. 2010;17:29. doi: 10.1186/1423-0127-17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemaire V, Koehl M, Le Moal M, Abrous D. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li JP, Lu W, Yang L, Xie MX, He X, Pan AH. Neurons in the hippocampus of chemobrain versus non-chemotherapy brain. Zhongguo Zuzhi Gongcheng Yanjiu. 2017;21:4523–4528. [Google Scholar]
- 29.Lu KT, Huang TC, Wang JY, You YS, Chou JL, Chan MW, Wo PY, Amstislavskaya TG, Tikhonova MA, Yang YL. NKCC1 mediates traumatic brain injury-induced hippocampal neurogenesis through CREB phosphorylation and HIF-1αexpression. Pflugers Arch. 2015;467:1651–1661. doi: 10.1007/s00424-014-1588-x. [DOI] [PubMed] [Google Scholar]
- 30.Mahar I, Bambico FR, Mechawar N, Nobrega JN. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev. 2014;38:173–192. doi: 10.1016/j.neubiorev.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Manduca A, Morena M, Campolongo P, Servadio M, Palmery M, Trabace L, Hill MN, Vanderschuren LJMJ, Cuomo V, Trezza V. Distinct roles of the endocannabinoids anandamide and 2-arachidonoylglycerol in social behavior and emotionality at different developmental ages in rats. Eur Neuropsychopharmacol. 2015;25:1362–1374. doi: 10.1016/j.euroneuro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 32.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 33.Meduri JD, Farnbauch LA, Jasnow AM. Paradoxical enhancement of fear expression and extinction deficits in mice resilient to social defeat. Behav Brain Res. 2013;256:580–590. doi: 10.1016/j.bbr.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 35.Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- 36.Narrow WE, Rae DS, Robins LN, Regier DA. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’estimates. Arch Gen Psychiatry. 2002;59:115–123. doi: 10.1001/archpsyc.59.2.115. [DOI] [PubMed] [Google Scholar]
- 37.Post RJ, Dahlborg KM, O’Loughlin LE, Bloom CM. Effects of juvenile exposure to predator odor on adolescent and adult anxiety and pain nociception. Physiol Behav. 2014;131:57–61. doi: 10.1016/j.physbeh.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch K. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- 39.Saboory E, Ahmadzadeh R, Roshan-Milani S. Prenatal exposure to restraint or predator stresses attenuates field excitatory postsynaptic potentials in infant rats. Int J Dev Neurosci. 2011;29:827–831. doi: 10.1016/j.ijdevneu.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Sachs BD, Ni JR, Caron MG. Brain 5-HT deficiency increases stress vulnerability and impairs antidepressant responses following psychosocial stress. Proc Natl Acad Sci U S A. 2015;112:2557–2562. doi: 10.1073/pnas.1416866112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seidenbecher T, Remmes J, Daldrup T, Lesting J, Pape HC. Distinct state anxiety after predictable and unpredictable fear training in mice. Behav Brain Res. 2016;304:20–23. doi: 10.1016/j.bbr.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Shioda N, Han F, Morioka M, Fukunaga K. Bis (1-oxy-2-pyridinethiolato) oxovanadium (IV) enhances neurogenesis via phosphatidylinositol 3-kinase/Akt and extracellular signal regulated kinase activation in the hippocampal subgranular zone after mouse focal cerebral ischemia. Neuroscience. 2008;155:876–887. doi: 10.1016/j.neuroscience.2008.05.056. [DOI] [PubMed] [Google Scholar]
- 43.Silva RCB, Brandao ML. Acute and chronic effects of gepirone and fluoxetine in rats tested in the elevated plus-maze: An ethological analysis. Pharmacol Biochem Behav. 2000;65:209–216. doi: 10.1016/s0091-3057(99)00193-8. [DOI] [PubMed] [Google Scholar]
- 44.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steenbergen PJ, Richardson MK, Champagne DL. Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: a pharmacological study. Behav Brain Res. 2011;222:15–25. doi: 10.1016/j.bbr.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev. 2005;29:1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 48.Tawarayama H. Novel function of the chemorepellent draxin as a regulator for hippocampal neurogenesis. Neural Regen Res. 2018;13:799–800. doi: 10.4103/1673-5374.232465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toledo M, Sandi C. Stress during adolescence increases novelty seeking and risk-taking behavior in male and female rats. Front Behav Neurosci. 2011;5:17. doi: 10.3389/fnbeh.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsoory M, Cohen H, Richter-Levin G. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur Neuropsychopharmacol. 2007;17:245–256. doi: 10.1016/j.euroneuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 51.Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 52.Wainwright SR, Galea LA. The neural plasticity theory of depression: assessing the roles of adult neurogenesis and PSA-NCAM within the hippocampus. Neural Plast 2013. 2013:805497. doi: 10.1155/2013/805497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright LD, Muir KE, Perrot TS. Stress responses of adolescent male and female rats exposed repeatedly to cat odor stimuli, and long-term enhancement of adult defensive behaviors. Dev Psychobiol. 2013;55:551–567. doi: 10.1002/dev.21060. [DOI] [PubMed] [Google Scholar]
- 55.Yang S, Liu L, Cao C, Song N, Wang Y, Ma S, Zhang Q, Yu N, Ding X, Yang F, Tian S, Zhang K, Sun T, Yang J, Yao Z, Wu S, Shi L. USP52 acts as a deubiquitinase and promotes histone chaperone ASF1A stabilization. Nat Commun. 2018;9:1285. doi: 10.1038/s41467-018-03588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang XH, Feng ZH, Wang XY. The ROCK pathway inhibitor Y-27632 mitigates hypoxia and oxidative stress-induced injury to retinal Müller cells. Neural Regen Res. 2018;13:549. doi: 10.4103/1673-5374.228761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Negative control in double immunostaining of BrdU/DCX in dentate gyrus.