Abstract
The effect of single nucleotide variants (SNVs) in coding and noncoding regions is of great interest in genetics. Although many computational methods aim to elucidate the effects of SNVs on cellular mechanisms, it is not straightforward to comprehensively cover different molecular effects. To address this, we compiled and benchmarked sequence and structure‐based variant effect predictors and we computed the impact of nearly all possible amino acid and nucleotide variants in the reference genomes of Homo sapiens, Saccharomyces cerevisiae and Escherichia coli. Studied mechanisms include protein stability, interaction interfaces, post‐translational modifications and transcription factor binding sites. We apply this resource to the study of natural and disease coding variants. We also show how variant effects can be aggregated to generate protein complex burden scores that uncover protein complex to phenotype associations based on a set of newly generated growth profiles of 93 sequenced S. cerevisiae strains in 43 conditions. This resource is available through mutfunc (www.mutfunc.com), a tool by which users can query precomputed predictions by providing amino acid or nucleotide‐level variants.
Keywords: burden score, genetic variants, genotype‐to‐phenotype, model organisms, resource
Subject Categories: Chromatin, Epigenetics, Genomics & Functional Genomics; Computational Biology; Methods & Resources
Introduction
One of the key challenges of biology is to understand how genetic variation drives changes in phenotypes. Genome‐wide association studies (GWASs) have made progress in identifying causal genetic loci, and over the past decade, a large number of associations have been made between genetic variation and phenotypic traits including disease risk (Welter et al, 2014). However, GWASs are typically limited in their ability to identify the causal variant at the associated locus and further limited by the ability to explain the underlying mechanism that may be influenced by candidate causal variants. This missing mechanistic layer severely limits our understanding of how variants cause phenotypic variability.
Variants occurring in coding and noncoding regions can influence a diversity of molecular functions. For instance, noncoding variants can affect chromatin accessibility (Kumasaka et al, 2016), splice sites (Xiong et al, 2015) and epigenetic modifications (Rintisch et al, 2014). Coding variants can affect post‐translational modification (PTM) sites (Reimand et al, 2015; Wagih et al, 2015), protein folding and stability (Lorch et al, 2000), protein interaction interfaces (Engin et al, 2016) and subcellular localization (Björses et al, 2000), and introduce premature stop codons. Understanding the disrupted biological mechanisms underlying genetic variation is key to many applications in genetics such as genetically engineering organisms, assessing drug efficacy and drug discovery (Labaudinière, 2002; Lutz, 2010; Nelson et al, 2016).
The ability to predict the degree to which genetic variation would alter such mechanisms offers a time and cost‐effective alternative over experimental approaches to prioritize variants of interest and to facilitate the understanding of the mechanisms underlying causal variants. A multitude of in silico predictors aimed at predicting such effects has been proposed (Schymkowitz et al, 2005; Kumar et al, 2009; Adzhubei et al, 2010; Wagih et al, 2015), yet they often require significant computational power, expertise and time to be used. Furthermore, each of the currently available tools does not comprehensively provide predicted effects across different molecular mechanisms (i.e. disruption of stability, interfaces, TF binding).
Accordingly, we have compiled and benchmarked commonly used sequence and structure‐based predictors of mutational consequences and predicted the effect of nearly all possible variants in the reference genomes of Homo sapiens, Saccharomyces cerevisiae and Escherichia coli. The impact of variants was measured in the context of conserved protein regions, protein stability, protein–protein interaction (PPI) interfaces, PTMs, kinase–substrate interactions, short linear motifs (SLiMs), start and stop codons, and transcription factor (TF) binding sites (TFBSs). This resource is available through the mutfunc resource (http://mutfunc.com/), which allows for prioritization of variants while providing insight into the altered mechanisms.
To demonstrate the utility of mutfunc, we assessed variants of uncertain clinical significance (VUSs) in H. sapiens. We further applied mutfunc to publically available variants for yeast S. cerevisiae strains to generate protein complex burden scores. We then phenotyped 93 sequenced S. cerevisiae strains in 43 conditions and utilized burden scores to associate protein complexes to phenotypes. This yielded associations that would not be possible through traditional variant‐based GWAS approaches. mutfunc is a computational resource that will facilitate the study of the mechanistic impacts of genetic variation.
Results
Functional genomic regions display evolutionary constraint across yeast and human individuals
In order to set up the variant effect prediction approaches, we first derived, for E. coli, S. cerevisiae and H. sapiens, molecular information such as experimental and homology‐based protein structural models for individual proteins and protein interfaces, TF binding sites, protein kinase targets sites, post‐translational modification sites and linear motif regions (Materials and Methods). Structural models were used to identify interface residues and residues with different surface accessibility. Given that functionally relevant regions of the genome are under evolutionary constraint, we took the opportunity to use this large collection of functional regions to test whether these tend to be depleted of natural variants. For yeast, 896,772 natural variants and their allele frequencies were compiled from 405 yeast strains (Bergström et al, 2014; Strope et al, 2015; Gallone et al, 2016; Zhu et al, 2016), of which 478,857 were coding variants. For human, over 3.2M coding variants from over 65,000 individuals were obtained from the ExAC consortium (Lek et al, 2016).
Natural variants were mapped to 9,837 protein structures and homology models (n = 6,737 human, n = 3,100 yeast), and the residues were binned according to relative surface accessibility (RSA). Similarly, 9,883 structures (n = 7,693 human, n = 2,190 yeast) for protein interaction pairs were obtained from Interactome3D and the difference in surface accessibility (∆RSA) between the unbound and bound complex was determined to identify interface residues, corresponding to those with the highest ∆RSA (Materials and Methods). The number of variants per position of each bin of RSA and ∆RSA was compared to counts observed in random positions in the protein, permuted 1,000 times. Fewer variants were found in buried regions and interface regions when compared to exposed regions in both yeast and human (Fig 1A P < 1.28 × 10−34 and B P < 2.28 × 10−33). To study variation at 296,147 and 26,560 human and yeast PTM sites, the variant counts over random expectation were calculated for a window of ±5 residues flanking the PTM positions. The level of constraint was different across PTM types (Fig 1C) with ubiquitylation showing the lowest level of constraint. Interestingly, the level of constraint for PTMs increases with the number of other neighbouring PTMs present in a 10 amino acid window (Fig 1D) suggesting that the clustering of PTMs may have important biological functions such as cross‐talk regulation (Beltrao et al, 2013).
Figure 1. Population‐level sequence constraint in genome functional elements.
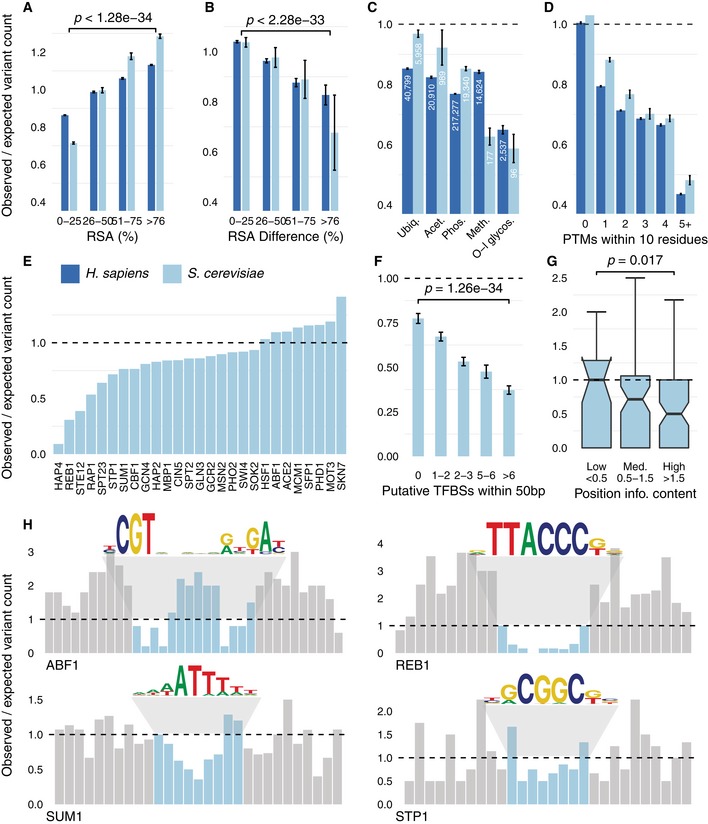
- Regions buried within a protein structure with a low RSA typically exhibit higher evolutionary constraint.
- Similarly, regions buried within interaction interfaces exhibit a high ∆RSA and demonstrate stronger sequence constraints.
- Sequence constraint on PTMs, where numbers reflect the number of PTM sites for each modification.
- PTMs with a higher number of neighbouring PTMs show stronger constraint.
- Variability in constraint among bindings sites for TFs with at least 40 sites.
- TFBSs that coexist with other binding sites are under stronger constraint.
- Position‐specific constraint shows that positions of higher relevance for binding in TFs with at least 20 sites are under stronger constraint. Notches represent the 95% CI in the median, box limits the IQR and upper whiskers the 75th percentile. The horizontal line represents the null expectation of no difference between observed and expected, same as in all other panels of this figure.
- Four examples where the bar plots reflect the position‐specific constraint in (blue) and around (grey) the binding site, along with sequence logos for the binding specificities.
It has been shown that TF binding sites tend to have lower‐than‐expected variation across populations in particular for crucial specificity‐determining positions (Spivakov et al, 2012). We therefore tested if similar observations are found at our putative TF binding sites for S. cerevisiae that were predicted using a combination of TF specificity models, TF knockout gene expression studies and TF ChIP‐seq or ChIP‐chip data (Materials and Methods). A total of 4,523 potential binding sites were identified across 93 TFs of S. cerevisiae. We computed the ratio between the variant counts within the predicted binding sites to that of random genomic sites of the same length and within the same ChIP regions. By combining the analysis across all putative binding sites of each TF, we observed that binding sites for some TFs are generally more constrained than others (Fig 1E). Those with higher levels of constraint include HAP4, a global regulator of respiratory genes and general transcriptional regulators such as REB1 and RAP1. At the level of individual TF binding sites, we observed that those found within clusters of binding sites tended to show higher levels of constraints than isolated sites (Fig 1F). Additionally, the TF binding positions for each TF were stratified according to their importance for binding as measured by the position‐specific information content (IC) of the TF specificity position weight matrices. In accordance with expectation, positions with high IC, which correspond to positions that are important for binding, tend to have fewer variants than less important positions (Fig 1G). Position‐specific constraint for individual TFs highlights this difference between high and low IC positions (Fig 1H).
Overall, these results provide an overview of how population‐level variation differs across diverse set of genome functional elements and recapitulates findings from analysis of specific types of functional elements (Spivakov et al, 2012; de Beer et al, 2013; Reimand et al, 2015). Additionally, it suggests that our collection of functional elements (e.g. structures, interfaces, PTMs and TF binding sites) shows evolutionary constraints and therefore can be used further for the establishment of the variant effect prediction pipeline.
A comprehensive resource of mechanistic effects of single nucleotide variants
We sought to better understand the mechanistic impact of point mutations affecting the above described functional elements. To do this, a set of commonly used predictors were used to assess the impact of every possible single amino acid or nucleotide substitution across H. sapiens, S. cerevisiae and E. coli, where applicable. We performed a large‐scale computational estimation of the impact of variants on conserved protein regions, protein stability, protein interaction interfaces, kinase–substrate phosphorylation and other PTMs, linear motifs, TFBSs and start and stop codons (illustrated in Fig 2A, Materials and Methods). These results were deposited in the mutfunc resource, which offers a quick and interactive way by which users can gain predicted mechanistic insight for variants of interest. Although the algorithms used have been previously described, this resource allows to easily query all predictors in a unified and consistent interface.
Figure 2. The mutfunc resource and benchmarking of underlying variant effect predictors.
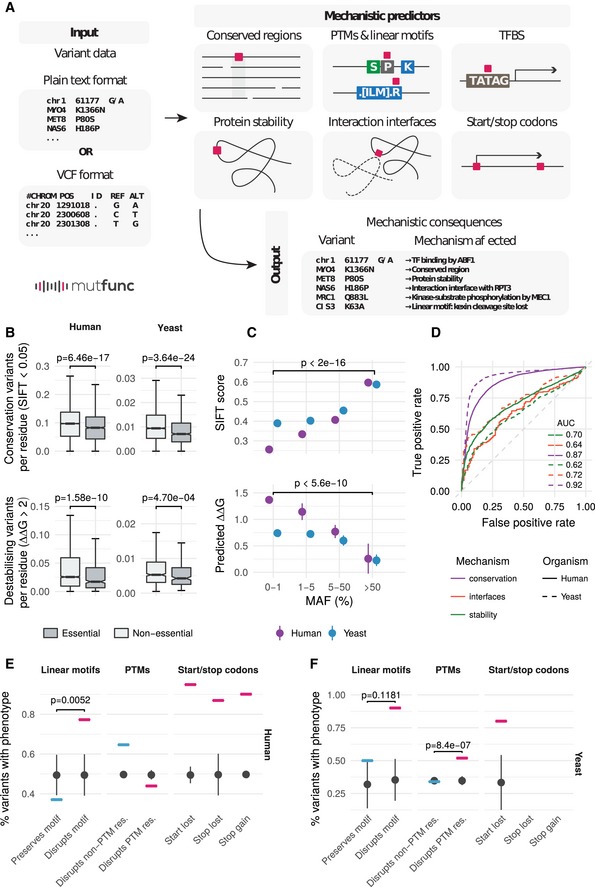
-
AThe mutfunc interface provides an intuitive, user‐friendly way by which users can query the resource using DNA or protein substitutions provided in plain text format or the variant call format (VCF). The impact of variants across different mechanisms is provided with information on impact strength in downloadable format and/or protein structural views.
-
BThe fraction of variants predicted to affect a conserved or structural important residues for essential and nonessential genes. For yeast SIFT, the number of essential/non‐essential genes are 3,967 and 906, respectively. For yeast foldx the numbers are 925 and 281. For human sift the numbers are 15,542 and 1,575. For human foldx the numbers are 3,702 and 499.
-
CMean SIFT scores and predicted ∆∆G values for human and yeast variants within different MAF bins. Error bars represent the standard error, and P‐values are calculated based on a one‐sided Wilcoxon test.
-
DPathogenic and benign variants were obtained for human (from ClinVar) and yeast (curated) as described in the Materials and Methods section. These were used to benchmark the capacity of different predictors to discriminate between known pathogenic and benign variants.
-
E, FThe proportion of pathogenic versus benign variants that disrupt or not different functional annotations (SLiMs, PTMs or stop gains/losses) in human (E) and yeast (F). Number of replicates is 100 (i.e. random samples).
To measure the impact on conserved regions, we constructed 29,027 multiple sequence alignments for proteins of the three organisms (n = 19,497 H. sapiens, n = 5,498 S. cerevisiae, n = 4,032 E. coli) and used the SIFT algorithm (Ng & Henikoff, 2003) to assess the impact of all possible 291.7M protein coding variants (n = 212.2M H. sapiens, n = 53.4M yeast, n = 26.1M E. coli). To measure the impact on protein stability, the FoldX algorithm (Schymkowitz et al, 2005) was applied to 11,771 structures (including homology models) across the three organisms (Materials and Methods and Fig EV1) and precomputed effects of 55.9M protein coding substitutions (n = 42.7M H. sapiens, n = 5.3M S. cerevisiae, n = 8.1M E. coli). We identified interface residues in 10,675 structures of binary PPIs from Interactome3D across the three organisms and similarly applied FoldX to compute the effects of 11.2M possible interface mutations on binding stability (n = 7.2M H. sapiens, n = 2.3M S. cerevisiae, n = 1.6M E. coli). To identify variants that could impact kinase–substrate sites, we used MIMP (Wagih et al, 2015) to predict the impact of all possible 541,161 variants (n = 485,736 H. sapiens, n = 55,425 S. cerevisiae) falling within ±5 residues of a known kinase–substrate phosphorylation site (phosphosite) on a kinase's specificity. Specificities for 56 kinases in H. sapiens and 46 kinases in S. cerevisiae were considered. Kinase–phosphosite relationships for E. coli are not well established and cannot be scored in the same way. For all other PTMs such as methylation, ubiquitination and acetylation for which we do not have explicit flanking sequence specificity models, a variant was considered damaging if it directly altered the modified site. This resulted in a total of 6.3M possible variants that could alter such PTM sites across the three organisms (n = 5.8M H. sapiens, n = 537,434 S. cerevisiae, n = 9,177 E. coli). For linear motif information, not available for E. coli, we gathered 1,668 experimentally identified linear motifs (n = 1,525 H. sapiens, n = 143 S. cerevisiae), along with their derived regular expression pattern from the ELM database (Dinkel et al, 2012) and computed the impact of all possible 226,920 variants (n = 205,120 H. sapiens, n = 21,800 S. cerevisiae) on binding patterns. Finally, for TFBSs, for organisms without well‐defined functional TFBSs (H. sapiens and S. cerevisiae), we defined putative TF‐gene regulatory network using TF‐knockdown expression data and/or ChIP‐seq/ChIP‐chip (Materials and Methods). We then used PWMs to identify putative binding sites, and predict the impact (Materials and Methods) of all possible 3.6M variant substitutions (n = 3.3M H. sapiens, n = 236,382 yeast, n = 46,768 E. coli) on specificities of 217 TFs (n = 72 H. sapiens, n = 104 S. cerevisiae, n = 41 E. coli).
Figure EV1. Impact of structural models on structure‐based variant effect prediction.
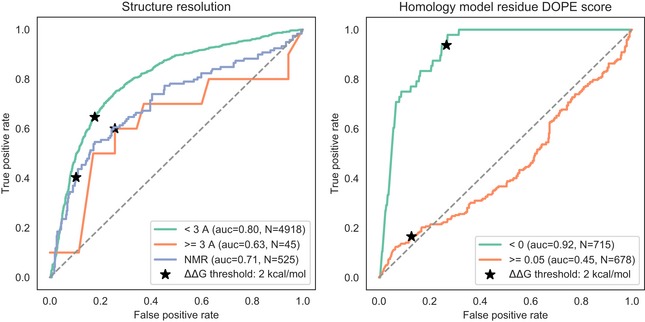
We obtained from the ProTherm database variants with experimentally determined impact on stability and classified them as destabilizing if ΔΔG > 2 and not destabilizing otherwise. FoldX‐based predictions were tested on their capacity to discriminate between these two classes of variants using different types of experimental models and regions within homology models with different predicted quality.
These precomputed variant effect predictions constitute a resource that can be used in diverse ways. In the next sections, we benchmark this resource and illustrate some of its possible applications.
Functionally important positions are enriched in predicted deleterious variants
In order to benchmark the variant effect predictions that underlie the mutfunc resource, we first asked whether essential genes would harbour fewer natural variants that are predicted to be deleterious. Essential genes in yeast (Giaever & Nislow, 2014) and human (Blomen et al, 2015) consistently demonstrated significantly lower frequencies of variants predicted to affect conserved sites (SIFT score < 0.05, P = 6.46 × 10−17 human, P = 3.64 × 10−24 yeast, Fig 2B) and protein stability (∆∆G pred > 2, P = 1.58 × 10−10 human, P = 4.7 × 10−4 yeast, Fig 2B). Variants of higher allele frequency in the population are expected to be less impactful, and in accordance with this, we observed an increase in deleterious scores, as predicted from SIFT and FoldX, for variants of lower allele frequencies (Fig 2C). In addition to allele frequencies, we analysed mutations that are known to be deleterious. For H. sapiens, we used 34,600 variants annotated to be pathogenic (n = 17,167) or benign (n = 17,433) from the ClinVar (Landrum et al, 2014). For S. cerevisiae, we used 8,083 variants consolidate by Jelier et al (2011) as either tolerated (n = 5,271) or affecting function (n = 2,812; Materials and Methods). The different predictors consistently discriminated tolerated from pathogenic variants as measured by the area under the receiver operating characteristic curve (AUC). SIFT performed the best at discriminating pathogenic variants from benign (AUC H. sapiens = 0.87, S. cerevisiae = 0.92), followed by FoldX interfaces (AUC H. sapiens = 0.64, S. cerevisiae = 0.72) and FoldX stability (AUC H. sapiens = 0.70, S. cerevisiae = 0.62, Fig 2D).
For other heuristic‐based predictors such as SLiMs, PTMs or stop gains/losses, we compared the proportion of pathogenic versus benign variants that disrupt or not the annotation. Despite the low number of pathogenic variants overlapping with these features, we observed an enrichment of pathogenic variants for mutations that disrupt such features (Fig 2E and F). The only exceptions were for PTM‐disrupting variants in human and for linear motif‐disrupting variants in yeast. In contrast, there were significant differences for the enrichment of pathogenic variants disrupting human linear motifs (P = 5.23 × 10−3) and yeast PTM sites (P = 8.44 × 10−7). For some of annotations, the lack of statistical significance may be due to the small number of testable variants.
The results here demonstrate that the predictors used in mutfunc are generally capable of enriching for variants of functional significance. The resource can be used to prioritize variants according to the degree of pathogenicity as well as provide molecular mechanisms affected.
Predicting mechanistic impacts of variants of uncertain significance
Variants that have been identified through disease‐related genetic testing but are yet to be deemed benign or pathogenic are termed variants of uncertain significance (VUS). The interpretation of such variants is a common challenge in genetics, one that is often aided by computational predictors. A total of 64,692 variants labelled with “uncertain significance” were collected from ClinVar (Landrum et al, 2014). VUSs were annotated using mutfunc and 21,584 variants were predicted impactful by at least one of the mechanistic predictors, not including SIFT (n = 7,547 stability, n = 751 interfaces, n = 139 linear motifs, 2,372 PTMs, 57 kinase binding). From these, we focused on variants predicted to impact the structural integrity of proteins (stability and interaction interfaces) since they hold the highest coverage.
Of the VUSs predicted to interfere with interface or protein stability, we retained those in which (i) the protein also harbours a known pathogenic variant with the same predicted structural impact, and (ii) both the pathogenic variant and VUSs are identified in patients with the same disease. This allows us to connect a variant of uncertain significance with a pathogenic variant by the fact that they occur in patients of the same disease and are predicted to have the same molecular consequence at the protein level. We demonstrate a few examples of VUSs that are predicted to alter binding (Fig 3A–C) or structural stability (Fig 3D and E). For instance, primary hyperoxaluria is a disease caused primarily by mutations in GRHPR, a glyoxylate and hydroxypyruvate reductase (Cramer et al, 1999; Cregeen et al, 2003), and its enzymatic activity requires homodimerization (Booth et al, 2006). For this enzyme, the variants R302H and E113K have been implicated in primary hyperoxaluria, are annotated to be pathogenic in ClinVar (Landrum et al, 2014) and are predicted here to impact on binding stability (Fig 3A, ΔΔG > 2.15). We can reason that other variants in patients of the same disease impacting on GRHPR homodimerization are therefore also likely to have the same phenotypic outcome. For example, the variant R171H is predicted to impact a conserved region as well as the homodimerization stability (Fig 3A, ΔΔG = 2.19, s < 0.018) and found in primary hyperoxaluria patients. Although R171H is of uncertain significance, our analysis strongly suggests that it is very likely to have the same phenotypic consequences and act via the same molecular mechanism as R302H and E113K. Similarly compelling examples are found for other proteins such as fumarate hydratase (Fig 3B) and lamin (Fig 3C).
Figure 3. Analysis of variants of uncertain clinical significance using mutfunc.
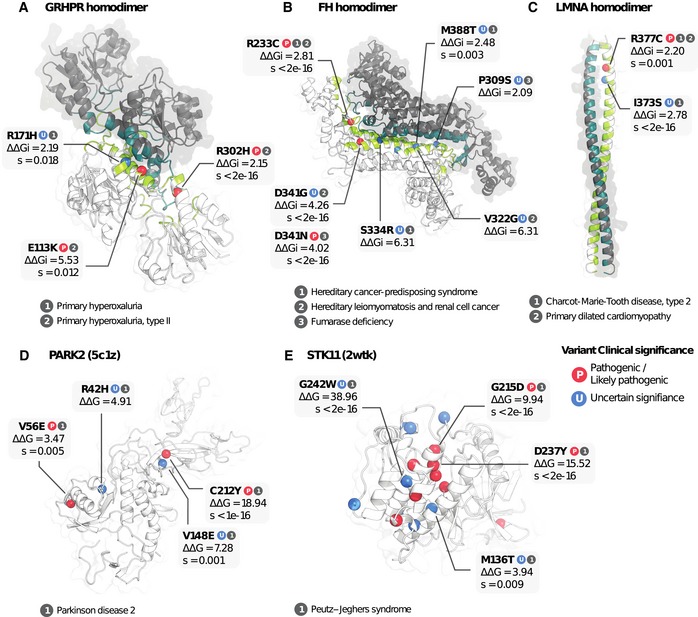
-
A–CThree examples of interaction interfaces containing variants predicted to impact binding stability. Subunits of the interaction complex are coloured in dark grey and white, and respective interface residues in dark green and green.
-
D, ETwo examples of variants predicted to impact protein stability. Pathogenic variants are labelled “P” in red, and VUSs “U” in blue.
Similar to interface variants, we analysed variants that destabilize the protein structure. We identified 1,182 VUSs predicted to alter stability in proteins containing pathogenic variants also predicted to be destabilizing. For instance, the ubiquitin ligase PARK2, implicated in Parkinson's disease, contains two variants (V56E and C232Y) annotated to be pathogenic in ClinVar (Landrum et al, 2014) that we predict to impact on its stability. For this protein, two other variants of uncertain significance (R42H, V148E) were found in Parkinson's disease patients and also predicted to destabilize the protein (ΔΔG > 4.7, Fig 3D). Therefore, we would suggest that the R42H and V148E variants are likely to cause the same phenotypes as the V56E and C232Y variants. In the tumour suppressor serine/threonine‐protein kinase STK11, pathogenic and VUS identified in Peutz–Jeghers syndrome patients can be similarly linked (Fig 3E).
The analysis here demonstrates how mutfunc could be applied to systematically prioritize pathogenic variants through altered mechanisms that may be the molecular cause of the phenotype and the combination of algorithms that cover different molecular mechanisms.
S. cerevisiae strain genomic differences are a significant but weak predictor of phenotypic similarity
We sought to illustrate the use of mutfunc for genotype‐to‐phenotype association analysis. Using S. cerevisiae as a case study, we first phenotyped growth for a panel of 166 strains in 43 conditions (Materials and Methods). Colony sizes for strains were quantified, normalized and scored relative to all strains in a condition to produce a phenotypic measure defined as the S‐score (Collins et al, 2006). Positive and negative values indicate higher or lower than expected growth for a given strain and a specific condition (Materials and Methods). S‐scores for biological replicates demonstrated a high degree of concordance (r = 0.91, P < 2.22 × 10−16, Fig 4A) suggesting a high degree of confidence in phenotypic measurements. S‐scores for each strain and growth condition are provided in Dataset EV1.
Figure 4. Phenotypic screening of 166 yeast strains.
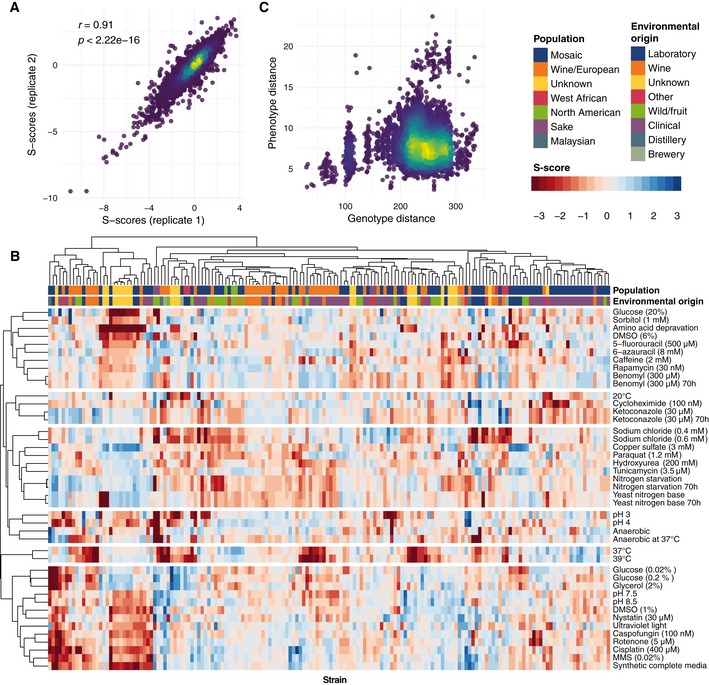
- Concordance between replicate s‐score measurements.
- Heatmap of s‐scores showing hierarchical clustering of both strains and conditions reveals clusters of phenotypically similar strains and conditions.
- Comparison of pairwise genotype and phenotype distances between 93 sequenced strains shows little observable correlation.
Hierarchical clustering of growth phenotypes revealed known clusters of related stressors (Fig 4B). Clusters of similar phenotypic profiles included, for example, UV light, cisplatin and MMS, which are all DNA‐damaging agents (mean Pearson's r = 0.51); nystatin and caspofungin (Kathiravan et al, 2012), known to interfere with the cell wall (r = 0.49); and caffeine and rapamycin (Reinke et al, 2006), both known to inhibit TOR signalling (r = 0.41). Furthermore, strains belonging to the same population structure (Strope et al, 2015) or environmental origin often showed similar phenotypic profiles (Fig 4B). Genome sequences were available for 93 of the 166 profiled strains and used to calculate pairwise genomic similarity as the euclidean distance of the vector of SNPs. As expected, genetic similarity is significantly correlated with phenotypic similarity (Fig 4C, r = 0.12, P < 0.0001) but alone explains a small amount of the phenotypic diversity. This is not unexpected since most genetic variation is neutral and distantly related strains accumulate variation that may not have an impact on the phenotypes tested. While strains having very similar genomes tend to have very similar phenotypes, strains with more divergent genotypes can show either a similar or very different phenotypic profiles (Fig 4C).
Gene and complex disruption scores for genotype‐to‐phenotype associations
Given that most variants are expected to be neutral, we used the predictions collected in mutfunc to interpret the observed variants in each strain at the gene level by computing a total gene burden or disruption score using the mechanistic predictions for conservation (SIFT), protein stability (FoldX) and protein truncating variants (PTVs, including start loss, nonstop and nonsense variants; Fig 5A). Scores produced by predictors are standardized to reflect the likelihood they are deleterious (Fig 5B, Materials and Methods). This allows for effects of rare variants to be combined across different protein positions and predictors into a single probability that the gene is affected (P AF score or burden score; Jelier et al, 2011; Galardini et al, 2017).
Figure 5. Gene and protein complex‐level aggregation of variant effects for phenotype association analysis.
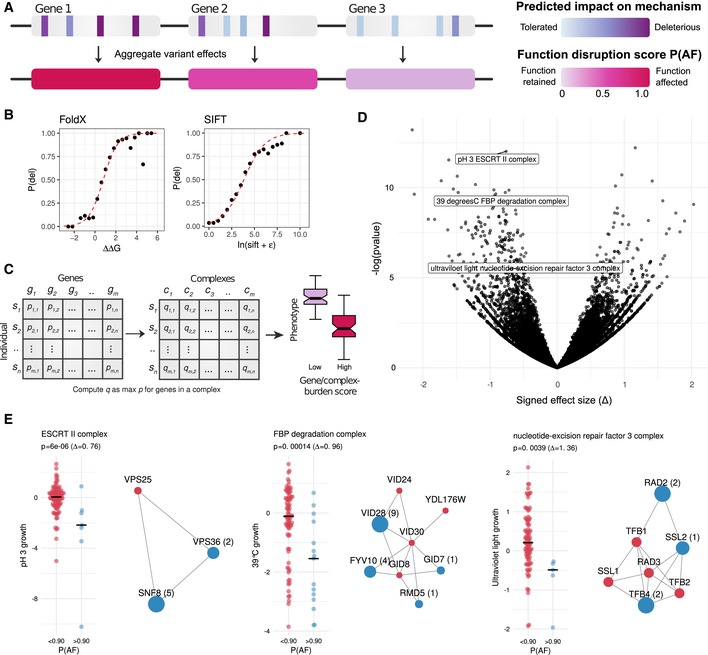
- Diagram demonstrating the aggregation of variant impact. Each variant is first assigned a probability of deleteriousness, which are aggregated at the gene level using the maximum impact.
- The probability of deleteriousness for FoldX and SIFT was computed by assessing the proportion of deleterious variants in gold‐standard data for FoldX and SIFT. A logistic regression model (red line) is fit to compute subsequent probabilities. Protein complex‐level burden scores were taken to be the maximal burden for any complex member.
- Gene and complex burden scores for each strain, gene/complex‐phenotype associations were carried out.
- Volcano plot with gene–complex associations highlighting the effect size and P‐value of selected examples.
- S‐score growth distributions for strains having a low (P AF < 90, red) or high (P AF > 90, blue) burden scores for three selected complexes. The protein subunits of each complex are shown with affected subunits in blue with the number of strains in which the subunit is predicted to be impaired in parenthesis. Subunits in red are not predicted to be impaired in any strain.
Using the gene‐level disruption scores, we performed phenotype association analysis. Scores were binned based on high (P AF > 0.90) or low (P AF < 0.90) burden (Fig 5C). Associations were carried out for 1,446 genes (with at least three strains containing a P AF > 0.90) against growth phenotypes across 43 conditions (Materials and Methods). We identified 872 statistically significant gene–phenotype associations at P < 1 × 10−3 and FDR < 10%, with 82% (717/872) being negative. A negative association here indicates that the disruption of the gene is linked to decreased growth, while a positive association would suggest that the disruption is associated with a better than expected growth. Under the assumption that gene function is conserved across strains of S. cerevisiae, we expected these associations to be enriched in genes that cause a condition‐specific phenotype when knocked out. Such association between gene KOs and condition‐specific growth phenotypes exists for the laboratory strain as part of extensive published chemical genetic studies. We found KO chemical genetic data for 35 of the 43 conditions tested. Of the significant negative associations, only 9% (65/717) are validated by the chemical genetic data. The validation rate increases for higher effect sizes (Fig EV2A) to 15% (55/367) and 28% (20/71) for at Δ > 1 and at Δ > 1.8, respectively. However, based on permutation testing only the enrichment found at large effect sizes (Δ > 1.8) was significant (P = 0.04, Fig EV2A).
Figure EV2. Gene and protein complex‐level phenotype association analysis show significant but modest enrichment in prior knowledge of gene KO growth phenotypes.
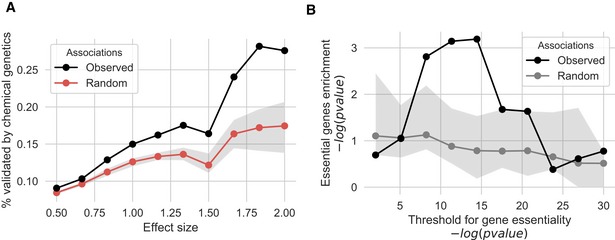
- The fraction of gene–phenotype associations that is validated by chemical genetic information derived from gene‐deletion experiments. Shaded area reports the interquartile range over 100 iterations. The significance of the observed overlap was tested using permutation testing.
- Associations between protein complexes and conditions were benchmarked by calculating the enrichment of previously known gene‐condition associations from gene‐deletion studies. Shaded area reports the interquartile range over 100 iterations. An enrichment was observed for some cut‐offs for the gene‐deletion condition‐dependent essentiality but only found to be better than random expectation for stringent cut‐off.
We next reasoned that protein complex members often act as coherent functional units and that the dysfunction of complex subunits often elicits similar phenotypic outcomes (Collins et al, 2007). Therefore, we aggregated the gene‐level scores to identify complexes that were potentially defective in a given strain with the assumption that the complex was defective if at least one subunit was predicted to be impaired (P AF < 0.90). We performed protein complex‐level associations focusing on 263 complexes predicted to be defective in more than two strains (Fig 5D). A total of 106 significant complex–phenotype associations were identified (P < 1 × 10−3, FDR < 10%), 80 (75%) of which had a high effect size (∆ > 1). The 80 associations involved 31 conditions and were preferentially negative associations (65 of 80, 81%). As we did for the gene‐condition associations, we benchmarked the complex‐condition associations and found a significant enrichment in KO chemical genetic data (Fig EV2B) that was significantly higher than observed based on random permutation testing (Fig EV2B, P = 0.04). This enrichment is observed only for stringent cut‐offs for defining gene‐deletion phenotypes from the KO chemical genetic studies (Hillenmeyer et al, 2008).
Some examples illustrate how the analysis at the protein complex level may increase power for the identification of associations (Fig 5E). For example, we found validated associations between the ESCRT II complex and growth in low pH (Xu et al, 2004). We have also found an association between high heat and the GID FBP degradation complex that contains several subunits (VID24, GID8 and VID28) that cause increased sensitivity to heat when deleted (as annotated in www.yeastgenome.org). In addition, we recover also the well‐established requirement for the nucleotide excision repair complex for growth under UV light (Prakash et al, 1993). From these three validated examples, only one subunit of the ESCRT II complex (SNF8) shows a significant gene‐level burden association with the respective condition (low pH). The other two complexes would not have been associated based on gene‐level burden scores likely due to insufficient recurrency of mutation at the gene level.
This association analysis indicates that there is value in combining effects of rare variants at the protein and protein complex level to perform association studies. Although the current study is limited due to the relative small number of strains studied, it illustrates how mutfunc can be applied to the study of diverse set of problems.
Discussion
The mutfunc resource makes use of established variant predictors to precompute millions of variant effects across the reference genomes of H. sapiens, S. cerevisiae and E. coli. This resource is not a new variant effect predictor nor an attempt to create an integrated score. The predictors used and their performance have been previously described, but the large computational effort and the accompanying web service (mutfunc.com) constitute a resource that facilitates their use. Within mutfunc, conservation effects hold the highest coverage, (H. sapiens 98.6%, S. cerevisiae 87.9% and 96.1% E. coli) followed by stability (H. sapiens 18.9%, S. cerevisiae 7.9% and 30.1% E. coli) and interfaces (H. sapiens 2.20%, S. cerevisiae 2.84% and 4.45% E. coli). Other mechanisms like PTMs and TFBSs are likely to have lower coverage, but it is unclear at the moment what would constitute 100% coverage for these features. As additional data become available, mutfunc will be updated to improve coverage and future work could expand the set of mechanisms studied such as drug or small‐molecule binding sites, RNA‐binding interfaces, among others. The effects of variants on molecular and cellular phenotypes are increasingly being probed directly by large‐scale mutagenesis experiments (Fowler & Fields, 2014; Weile et al, 2017), which will likely result in improved variant effect prediction algorithms (Gray et al, 2018). The curation of such experimentally determined effects and the improved algorithms can be integrated in future iterations of mutfunc.
A strength of mutfunc lies in its large set of precomputed SNV effects allowing for genome‐wide variants to be rapidly queried. However, within such a framework, combinatorial and potential epistatic effects cannot be precomputed due to a large number of possible combinations. Similarly, many other types of genetic variation such as copy number variations and indels (Chuzhanova et al, 2003; Beroukhim et al, 2010) have not be considered in mutfunc due to their complex structure. Lastly, many organisms in which genetic variation is commonly studied are not included in mutfunc. These include Mus musculus, Drosophila melanogaster and Arabidopsis thaliana, which contain an abundance of data and could be added in the future.
Understanding how disrupted cellular mechanisms propagate to changes in phenotypes is critical for variant interpretation. We show here how different variants can be integrated using effect predictors and protein complex annotations to perform genotype‐to‐phenotype associations for full genome sequences. In addition, we and others have also shown how prior knowledge of gene function and variant effect predictions can be used to predict growth differences of different strains of S. cerevisiae (Jelier et al, 2011) and E. coli (Galardini et al, 2017). These analyses illustrate ways to calculate gene burden scores across different effect predictors. We found a significant but limited overlap between the gene‐condition associations derived here with those found in gene KO studies in the reference laboratory strain. This small overlap could be due to a number of reasons including errors in variant effect predictions; limited sample size for the associations (i.e. 93 strains); and epistatic interactions of variants and different protocols for fitness measurements. The effects of a genetic variation in vivo can be complex and depend on both genetic and environmental factors (Burga et al, 2011; Wray et al, 2013; Perez et al, 2017). Several studies have shown that many variants annotated as disease‐causing or predicted as deleterious have been identified in healthy humans (Xue et al, 2012). In addition to these potential causes of error, it is assumed here that the loss of function of a given gene will have the same phenotypic consequence across individuals of the same species. The extent by which this assumption is true remains to be tested.
Despite the limitations discussed, given the growing number of efforts to sequence exome and genomes for panels of individuals, the incorporation of variant prioritization by different approaches into association analyses will become more prevalent. The mutfunc resource can provide such variant effect predictions with mechanistic annotations for three species. We illustrate how this resource can be applied in different scenarios, and given the architecture used, these analyses can be easily incorporated into large‐scale full genome or exome sequencing efforts.
Materials and Methods
Genetic variant data collection
A total of 896,772 genetic variants occurring in for 405 haploid and diploid S. cerevisiae strains were collected from four studies (Bergström et al, 2014; Strope et al, 2015; Gallone et al, 2016; Zhu et al, 2016). All but one study by Strope et al provided processed variant calls in VCF format. Variants were called for the Strope et al study using the following pipeline. Raw reads were obtained from the ENA resource (Leinonen et al, 2011). Adapter sequences were removed using cutadapt v1.8.1, and reads were mapped to the S. cerevisiae genome version 64 using BWA‐MEM v0.7.8 (https://arxiv.org/abs/1303.3997). Duplicate reads were discarded using Picard v1.96 (https://github.com/broadinstitute/picard), and reads were realigned using the GATK IndelRealigner v3.3 (McKenna et al, 2010). Base alignment qualities were computed using SAMtools v1.2 (Li et al, 2009), and variants were called using FreeBayes v0.9.21‐15‐g8a06a0b and the following parameters –no‐complex, –genotype‐qualities, –ploidy 1 and –theta 0.006. The VCF was filtered for calls with QUAL > 30, GQ > 30 and DP > 4. VCF for individual S. cerevisiae strains was combined, and coding variants were called using the predictCoding function of the VariantAnnotation R package (Obenchain et al, 2014).
A total of 3,198,692 coding variants in H. sapiens for over 65,000 individuals were collected from the ExAC consortium along with corresponding adjusted allele frequencies. Ensembl transcript positions were mapped to UniProt by performing Needleman–Wunsch global alignment of translated Ensembl transcript sequences against the UniProt sequence using the pairwiseAlignment function in the Biostrings R package. The mapping between Ensembl transcript IDs (v81) and UniProt accessions was obtained from the biomaRt R package (Smedley et al, 2015). In the case that multiple alleles mapped to the sample single amino acid substitution, the one with the highest adjusted allele frequency was retained.
A total of 139,167 variants were obtained from ClinVar. Only variants that did not match one of the following clinical significance terms were removed: “Benign”, “Benign/Likely benign”, “Likely benign”, “Likely pathogenic”, “Pathogenic/Likely pathogenic” and “Pathogenic”. Variants with a review status of “no assertion criteria provided” were also removed, as those reflect variants that have been assigned clinical significance without any particular criteria. The final filtered set contained 39,597 variants. Of these variants, 44% were classified as pathogenic or likely pathogenic. For S. cerevisiae, a total of 8,083 manually curated variants were obtained from Jelier et al (2011), 34.5% (2,812) of which were labelled as deleterious. Variants were collected from a combination of the UniProt database (Apweiler et al, 2004), Protein Mutant Database (Kawabata et al, 1999), Saccharomyces Genome Database (Cherry et al, 2012) and mutations that are identified in essential genes (Liti et al, 2009). These variants were used for the benchmarking of the variant effect predictors in Fig 2.
Essential genes
A total of 2,501 essential genes identified using gene trapping technology in two haploid H. sapiens cell lines KBM7 and HAP1 were obtained from Blomen et al (2015). These were further filtered for genes that were essential in both cell lines, for a total of 1,734 genes. A total of 1,156 essential genes in S. cerevisiae were obtained from the Saccharomyces Genome Deletion Project (Giaever et al, 2002).
Predicting impact on protein stability and protein interaction interfaces
Experimentally determined structures were obtained from the Protein Data Bank (PDB). Large structures that did not have a corresponding PDB file were downloaded in mmCIF format and converted to PDBs using the PyMOL Python library v1.2r3pre (pymol.org). Mapping of coordinates from PDB to UniProt residues was derived from the SIFTS database (Velankar et al, 2013). Structures with a resolution above three angstroms were discarded, and a single representative structure maximizing the coverage of the protein was retained. Homology modelling was carried out for proteins with no experimentally determined structures using ModPipe version 2.2.0 (Pieper et al, 2009) and the following parameters: –hits_mode 1110 and –score_by_tsvmod OFF. For each protein, we excluded models with a ModPipe Protein Quality Score lower than 1.1 and then kept the model with the highest normalized DOPE score. Finally, we excluded residues with a residue‐level DOPE score (rDOPE) greater than 0 as stability predictions for such residues are error prone (Fig EV1). Experimental and homology modelled structures for protein interactions were obtained from the Interactome3D database (Mosca et al, 2012). Relative solvent accessibility (RSA) for all residue atoms was computed using NACCESS for proteins individually, and in the interaction complex. Interface residues were defined as those with any change in RSA. All other calculation of RSA was carried out using FreeSASA v1.1 (Mitternacht, 2016).
The impact of variant on stability was computed using FoldX v.4.0 (Schymkowitz et al, 2005). All structures were first split by chain into individual PDB files and repaired using the RepairPDB command, with default parameters. The Pssm command is then used to predict ΔG with numberOfRuns=5. This performs the mutation multiple times with variable rotamer configurations, to ensure the algorithm achieves convergence. The average ΔG of all runs is computed, and the ΔΔG is computed as the difference between the wildtype and mutant. The impact of variants on interaction interfaces is measured similarly, with the exception of structures being provided in binary interaction, rather than individual chains.
Predicting the impact of variants on PTMs and linear motifs
For S. cerevisiae, a total of 20,056 phosphosites and 2,219 kinase–substrate associations were obtained from the PhosphoGRID database (Sadowski et al, 2013). A total of 1,070 of other PTM sites were obtained from the dbPTM database (Lee et al, 2006). For H. sapiens, all PTM data, including that of phosphorylation and kinase–substrate associations, were obtained from PhosphoSitePlus (Hornbeck et al, 2012), for a total of 296,147 sites. For E. coli, a total of 483 PTM sites were obtained from dbPTM (Lee et al, 2006). Linear motif data for S. cerevisiae and H. sapiens, including annotated linear motif binding sites and regular expression patterns, were obtained from the ELM database (Dinkel et al, 2016).
Impact of variants on phosphosites and flanking regions was measured using the MIMP algorithm (Wagih et al, 2015), with default parameters. For other PTMs, a variant was predicted to be impactful if it resulted in the change of the modified residue. For linear motifs, a variant was predicted to be impactful if it causes a loss of match for associated regular expression pattern.
Predicting the functional impact of variants using conservation
All protein alignments were built against UniRef50 (Suzek et al, 2015), using the seqs_chosen_via_median_info.csh script in SIFT 5.1.1 (Ng & Henikoff, 2003). The siftr R package (https://github.com/omarwagih/siftr), an implementation of the SIFT algorithm, was used to generate SIFT scores with parameters ic_thresh=3.25 and residue_thresh=2.
Transcription factor binding sites
A total of 177 S. cerevisiae TFs binding models were collected in form of a position frequency matrices (PFMs) from JASPAR (Sandelin et al, 2004) and converted to position weight matrices (PWMs) using the TFBSTools R package (Tan & Lenhard, 2016). PWMs were trimmed to eliminate consecutive stretches of low information content (< 0.2) on either terminus. To identify genes likely regulated by a particular TF, a combination of TF knockout expression and ChIP‐chip experiments was used, as similarly described in Gonçalves et al (2017). Genome‐wide gene expression profiles for 837 gene‐knockout strains were obtained from three studies (Chua et al, 2006; Hu et al, 2007; Kemmeren et al, 2014), 148 of which were a known TF with a defined PWM. Studies provided either a Z‐score or P‐value for each gene as a measure of over or under‐expression, relative to the distribution of values for all genes. Two‐tailed P‐values were computed from Z‐scores when a P‐value was not provided. In cases where TF knockout was repeated between studies, the lowest P‐value for each gene was used. ChIP‐chip tracks for 355 TFs were collected from four studies (Harbison et al, 2004; Tachibana et al, 2005; Rhee & Pugh, 2011; Venters et al, 2011) via the Saccharomyces genome database. Of the 355 of the TFs, 144 (56%) had a defined PWM. Potential binding sites were then only searched for in TF‐gene pairs with a P‐value below 0.01 and the corresponding ChIP‐chip region upstream of the regulated gene. A normalized log score of 0.80 was used as the cut‐off for defining putative binding sites. Similarly, for H. sapiens, 454 TF PWMs were generated from JASPAR PFMs. ENCODE clustered ChIP‐seq data were obtained for 161 TFs, of which 72 had a PWM. Only those regions were scored against the corresponding PWM. For E. coli, a total of 1,905 TF‐matching sequences across 84 TFs were obtained from RegulonDB (Gama‐Castro et al, 2016) and used to construct PWMs. A total of 2,416 experimentally identified TFBS were obtained for 79/84 TFs from RegulonDB. These sites were used as putative binding sites for downstream variant predictions.
Potential target sequences were scored against the PWM using the log‐scoring scheme defined in Wasserman and Sandelin (2004) and normalized to the best and worst matching sequence to the PWM. The resulting score lies between 0 and 1, where 1 signifies strong predicted binding by the factor, whereas 0 signifies predicted lack of binding. Potential binding sites were scored in the presence (Swt) and absence (Smt) of a variant. Three separate metrics are used to quantify the change in binding between the reference and alternate allele. The first one is simply the difference in the normalized log score, Swt − Smt, where a large positive value indicates loss of binding. The second is the difference in binding percentile. Here, random oligonucleotides are used to generate a negative distribution of log normalized scores for each TF. The percentile of each wildtype pwt and mutant scores pmt is computed from this distribution, and the difference, pwt − pmt, is used to quantify the magnitude of impact. The last is the difference in the relative information content. This can be thought of as the difference of letter height in a sequence logo. Given that the wildtype and mutant bases have relative frequencies of fwt and fmt, respectively, and a position has an IC value of γ, then this is computed as (fwt · γ) − (fmt · γ). This value ranges from 0 to 2, where 0 indicates little to no impact on a critical base, and 2 indicates a strong one.
Implementation of mutfunc
Described predictors were used to precompute effects for all amino acid and nucleotide substitutions. The mutfunc web server at http://mutfunc.com uses the Java and Scala‐based Play Framework v1.3.7 backend (http://www.playframework.org) along with a MySQL database. The front end utilizes a modified version of the Twitter Bootstrap UI library (http://getbootstrap.com/). Visualization tools used include a modified version of the neXtProt feature viewer v0.1.52 (https://github.com/calipho-sib/feature-viewer) for interactive visualization of protein sequence features, WebGL protein viewer v1.1 for interactive visualization of protein structures v1.8.1 (https://github.com/biasmv/pv) and a modified version of the JSAV v.1.10 library (https://github.com/AndrewCRMartin/JSAV) for visualization of multiple sequence alignments.
Chemical genetic screening
The screening was carried out in 1,536 format on synthetic complete media with the addition of the appropriate chemical at a specific concentration. The Singer RoToR (Singer Instruments, UK) was used to replicate screening plates in 1,536 format. Agar plates were pinned onto the conditioned media and allowed to grow for 48 or 72 h at 30°C (unless specified otherwise). Each experiment was replicated once for quality control. After incubation, plates were imaged and colony sizes were extracted using IRIS version v0.9.7 (Kritikos et al, 2017) with the “Colony growth” profile, which extracts colony size, circularity and opacity from each colony in each plate. Individual strains were scored using the E‐MAP software, which transforms colony sizes into s‐scores (Collins et al, 2006). In brief, a surface correction algorithm is applied to each plate, the outer frame effect is corrected by bringing the two outermost rows and columns to the plate middle median. All the plates are then normalized to the overall median, followed by a variance correction and finally the s‐score calculation. The resulting s‐scores are quantile normalized in each condition separately, and final s‐scores from both replicates are averaged.
Calculating gene and complex disruption scores
Scores produced by different predictors were standardized in order to reflect the likelihood of identifying a deleterious mutation (P del). For SIFT, a curated gold‐standard set of 8,083 variants in 1,346 yeast genes with known tolerated or deleterious effects were obtained from Jelier et al (2011). The negative natural logarithm of the SIFT score was binned by 0.5, and for each bin, the proportion of deleterious variants was computed. A binomial logistic regression was fit to the proportion values and used to compute subsequent P del values for subsequent SIFT scores. For FoldX, 964 gold‐standard mutations across 34 experimentally identified proteins structures with both experimentally quantified ∆∆G values and FoldX‐predicted ∆∆G values were obtained from Guerois et al (2002). A variant was labelled destabilizing if ∆∆G was > 1. Mutations were binned by predicted ∆∆G at intervals of 0.4, and for each bin, the proportion of destabilizing variants was computed. A binomial logistic regression model was similarly fit to the data and used to compute subsequent P del for FoldX‐predicted ∆∆G values. For variants disrupting start or stop codons, we assigned P del value of 1. Since nonsense variants occurring closer to the C‐terminal of a protein are less likely to impact function, we only assign P del value of 1 for nonsense variants occurring in the first 50% of the protein; otherwise, a value of 0 was used. Gene burden scores are then computed as the variant with the maximum P del score and described the predicted likelihood that a protein has an affected function (P AF). Similarly, for protein complexes the maximum P del score for any complex subunit was selected to reflect the protein complex P AF score. Variants with a MAF > 20% were considered unlikely to be deleterious given their high frequency in the population and were discarded prior to the burden score analysis.
Genotype‐to‐phenotype association analysis
The associations were carried out using the MatrixEQTL R package (Shabalin, 2012) with the modelLINEAR mode. The significance of the association was measured using a t‐statistic. For the associations, genes and complex binarized P AF scores were used as genotypes where a P AF score above or below 0.9 is given a value 1 and 0, respectively, and growth phenotypes are used in lieu of gene expression. A P‐value threshold of 0.001 was used for all associations, and multiple testing correction was carried out using the false discovery method. Effect size was computed using Glass's ∆. For the case (P) and control (n) group, differences in the mean were computed relative to the standard deviation of one of the groups. Given the mean (μi) and standard deviation (σi) for a given group i, this is computed as ∆i = (μP − μn)/σi. For robustness, this was computed in both direction and the final effect size, ∆, is reported as the minimum absolute value of effect sizes in both directions.
Data availability
The precomputed impact of single nucleotide variants in human, yeast and E. coli is available through the mutfunc web interface (www.mutfunc.com). Bug reports and feature requests can be submitted through the project's bug tracker at https://github.com/evocellnet/mutfunc-webserver.
Author contributions
OW designed, developed and implemented the mutfunc resource. OW, MG and DM contributed to data analysis. BB designed and performed the S. cerevisiae strain growth experiments. AT and PB oversaw the project. OW, MG and PB wrote the manuscript, and all authors revised it.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Dataset EV1
Review Process File
Mol Syst Biol. (2018) 14: e8430
See also: G Slodkowicz & M Madan Babu (December 2018)
References
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR (2010) A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apweiler R, Bairoch A, Wu CH, Barker WC, Boeckmann B, Ferro S, Gasteiger E, Huang H, Lopez R, Magrane M, Martin MJ, Natale DA, O'Donovan C, Redaschi N, Yeh L‐SL (2004) UniProt: the Universal Protein knowledgebase. Nucleic Acids Res 32: D115–D119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer TAP, Laskowski RA, Parks SL, Sipos B, Goldman N, Thornton JM (2013) Amino acid changes in disease‐associated variants differ radically from variants observed in the 1000 genomes project dataset. PLoS Comput Biol 9: e1003382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrao P, Bork P, Krogan NJ, van Noort V (2013) Evolution and functional cross‐talk of protein post‐translational modifications. Mol Syst Biol 9: 714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström A, Simpson JT, Salinas F, Barré B, Parts L, Zia A, Nguyen Ba AN, Moses AM, Louis EJ, Mustonen V, Warringer J, Durbin R, Liti G (2014) A high‐definition view of functional genetic variation from natural yeast genomes. Mol Biol Evol 31: 872–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, Mc Henry KT, Pinchback RM, Ligon AH, Cho Y‐J, Haery L, Greulich H, Reich M, Winckler W, Lawrence MS, Weir BA et al (2010) The landscape of somatic copy‐number alteration across human cancers. Nature 463: 899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björses P, Halonen M, Palvimo JJ, Kolmer M, Aaltonen J, Ellonen P, Perheentupa J, Ulmanen I, Peltonen L (2000) Mutations in the AIRE gene: effects on subcellular location and transactivation function of the autoimmune polyendocrinopathy‐candidiasis‐ectodermal dystrophy protein. Am J Hum Genet 66: 378–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomen VA, Májek P, Jae LT, Bigenzahn JW, Nieuwenhuis J, Staring J, Sacco R, van Diemen FR, Olk N, Stukalov A, Marceau C, Janssen H, Carette JE, Bennett KL, Colinge J, Superti‐Furga G, Brummelkamp TR (2015) Gene essentiality and synthetic lethality in haploid human cells. Science 350: 1092–1096 [DOI] [PubMed] [Google Scholar]
- Booth MPS, Conners R, Rumsby G, Brady RL (2006) Structural basis of substrate specificity in human glyoxylate reductase/hydroxypyruvate reductase. J Mol Biol 360: 178–189 [DOI] [PubMed] [Google Scholar]
- Burga A, Casanueva MO, Lehner B (2011) Predicting mutation outcome from early stochastic variation in genetic interaction partners. Nature 480: 250–253 [DOI] [PubMed] [Google Scholar]
- Cherry JM, Hong EL, Amundsen C, Balakrishnan R, Binkley G, Chan ET, Christie KR, Costanzo MC, Dwight SS, Engel SR, Fisk DG, Hirschman JE, Hitz BC, Karra K, Krieger CJ, Miyasato SR, Nash RS, Park J, Skrzypek MS, Simison M et al (2012) Saccharomyces Genome Database: the genomics resource of budding yeast. Nucleic Acids Res 40: D700–D705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua G, Morris QD, Sopko R, Robinson MD, Ryan O, Chan ET, Frey BJ, Andrews BJ, Boone C, Hughes TR (2006) Identifying transcription factor functions and targets by phenotypic activation. Proc Natl Acad Sci USA 103: 12045–12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuzhanova NA, Anassis EJ, Ball EV, Krawczak M, Cooper DN (2003) Meta‐analysis of indels causing human genetic disease: mechanisms of mutagenesis and the role of local DNA sequence complexity. Hum Mutat 21: 28–44 [DOI] [PubMed] [Google Scholar]
- Collins SR, Schuldiner M, Krogan NJ, Weissman JS (2006) A strategy for extracting and analyzing large‐scale quantitative epistatic interaction data. Genome Biol 7: R63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SR, Miller KM, Maas NL, Roguev A, Fillingham J, Chu CS, Schuldiner M, Gebbia M, Recht J, Shales M, Ding H, Xu H, Han J, Ingvarsdottir K, Cheng B, Andrews B, Boone C, Berger SL, Hieter P, Zhang Z et al (2007) Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature 446: 806–810 [DOI] [PubMed] [Google Scholar]
- Cramer SD, Ferree PM, Lin K, Milliner DS, Holmes RP (1999) The gene encoding hydroxypyruvate reductase (GRHPR) is mutated in patients with primary hyperoxaluria type II. Hum Mol Genet 8: 2063–2069 [DOI] [PubMed] [Google Scholar]
- Cregeen DP, Williams EL, Hulton S, Rumsby G (2003) Molecular analysis of the glyoxylate reductase (GRHPR) gene and description of mutations underlying primary hyperoxaluria type 2. Hum Mutat 22: 497 [DOI] [PubMed] [Google Scholar]
- Dinkel H, Michael S, Weatheritt RJ, Davey NE, Van Roey K, Altenberg B, Toedt G, Uyar B, Seiler M, Budd A, Jödicke L, Dammert MA, Schroeter C, Hammer M, Schmidt T, Jehl P, McGuigan C, Dymecka M, Chica C, Luck K et al (2012) ELM–the database of eukaryotic linear motifs. Nucleic Acids Res 40: D242–D251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkel H, Van Roey K, Michael S, Kumar M, Uyar B, Altenberg B, Milchevskaya V, Schneider M, Kühn H, Behrendt A, Dahl SL, Damerell V, Diebel S, Kalman S, Klein S, Knudsen AC, Mäder C, Merrill S, Staudt A, Thiel V et al (2016) ELM 2016–data update and new functionality of the eukaryotic linear motif resource. Nucleic Acids Res 44: D294–D300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin HB, Kreisberg JF, Carter H (2016) Structure‐based analysis reveals cancer missense mutations target protein interaction interfaces. PLoS One 11: e0152929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler DM, Fields S (2014) Deep mutational scanning: a new style of protein science. Nat Methods 11: 801–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galardini M, Koumoutsi A, Herrera‐Dominguez L, Cordero Varela JA, Telzerow A, Wagih O, Wartel M, Clermont O, Denamur E, Typas A, Beltrao P (2017) Phenotype inference in an Escherichia coli strain panel. Elife 6: e31035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallone B, Steensels J, Prahl T, Soriaga L, Saels V, Herrera‐Malaver B, Merlevede A, Roncoroni M, Voordeckers K, Miraglia L, Teiling C, Steffy B, Taylor M, Schwartz A, Richardson T, White C, Baele G, Maere S, Verstrepen KJ (2016) Domestication and divergence of Saccharomyces cerevisiae beer yeasts. Cell 166: 1397–1410 e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama‐Castro S, Salgado H, Santos‐Zavaleta A, Ledezma‐Tejeida D, Muñiz‐Rascado L, García‐Sotelo JS, Alquicira‐Hernández K, Martínez‐Flores I, Pannier L, Castro‐Mondragón JA, Medina‐Rivera A, Solano‐Lira H, Bonavides‐Martínez C, Pérez‐Rueda E, Alquicira‐Hernández S, Porrón‐Sotelo L, López‐Fuentes A, Hernández‐Koutoucheva A, Del Moral‐Chávez V, Rinaldi F et al (2016) RegulonDB version 9.0: high‐level integration of gene regulation, coexpression, motif clustering and beyond. Nucleic Acids Res 44: D133–D143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G, Chu AM, Ni L, Connelly C, Riles L, Véronneau S, Dow S, Lucau‐Danila A, Anderson K, André B, Arkin AP, Astromoff A, El‐Bakkoury M, Bangham R, Benito R, Brachat S, Campanaro S, Curtiss M, Davis K, Deutschbauer A et al (2002) Functional profiling of the Saccharomyces cerevisiae genome. Nature 418: 387–391 [DOI] [PubMed] [Google Scholar]
- Giaever G, Nislow C (2014) The yeast deletion collection: a decade of functional genomics. Genetics 197: 451–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves E, Raguz Nakic Z, Zampieri M, Wagih O, Ochoa D, Sauer U, Beltrao P, Saez‐Rodriguez J (2017) Systematic analysis of transcriptional and post‐transcriptional regulation of metabolism in yeast. PLoS Comput Biol 13: e1005297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray VE, Hause RJ, Luebeck J, Shendure J, Fowler DM (2018) Quantitative missense variant effect prediction using large‐scale mutagenesis data. Cell Syst 6: 116–124 e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerois R, Nielsen JE, Serrano L (2002) Predicting changes in the stability of proteins and protein complexes: a study of more than 1000 mutations. J Mol Biol 320: 369–387 [DOI] [PubMed] [Google Scholar]
- Harbison CT, Gordon DB, Lee TI, Rinaldi NJ, Macisaac KD, Danford TW, Hannett NM, Tagne J‐B, Reynolds DB, Yoo J, Jennings EG, Zeitlinger J, Pokholok DK, Kellis M, Rolfe PA, Takusagawa KT, Lander ES, Gifford DK, Fraenkel E, Young RA (2004) Transcriptional regulatory code of a eukaryotic genome. Nature 431: 99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillenmeyer ME, Fung E, Wildenhain J, Pierce SE, Hoon S, Lee W, Proctor M, St Onge RP, Tyers M, Koller D, Altman RB, Davis RW, Nislow C, Giaever G (2008) The chemical genomic portrait of yeast: uncovering a phenotype for all genes. Science 320: 362–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, Latham V, Sullivan M (2012) PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post‐translational modifications in man and mouse. Nucleic Acids Res 40: D261–D270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Killion PJ, Iyer VR (2007) Genetic reconstruction of a functional transcriptional regulatory network. Nat Genet 39: 683–687 [DOI] [PubMed] [Google Scholar]
- Jelier R, Semple JI, Garcia‐Verdugo R, Lehner B (2011) Predicting phenotypic variation in yeast from individual genome sequences. Nat Genet 43: 1270–1274 [DOI] [PubMed] [Google Scholar]
- Kathiravan MK, Salake AB, Chothe AS, Dudhe PB, Watode RP, Mukta MS, Gadhwe S (2012) The biology and chemistry of antifungal agents: a review. Bioorg Med Chem 20: 5678–5698 [DOI] [PubMed] [Google Scholar]
- Kawabata T, Ota M, Nishikawa K (1999) The protein mutant database. Nucleic Acids Res 27: 355–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmeren P, Sameith K, van de Pasch LAL, Benschop JJ, Lenstra TL, Margaritis T, O'Duibhir E, Apweiler E, van Wageningen S, Ko CW, van Heesch S, Kashani MM, Ampatziadis‐Michailidis G, Brok MO, Brabers NACH, Miles AJ, Bouwmeester D, van Hooff SR, van Bakel H, Sluiters E et al (2014) Large‐scale genetic perturbations reveal regulatory networks and an abundance of gene‐specific repressors. Cell 157: 740–752 [DOI] [PubMed] [Google Scholar]
- Kritikos G, Banzhaf M, Herrera‐Dominguez L, Koumoutsi A, Wartel M, Zietek M, Typas A (2017) A tool named Iris for versatile high‐throughput phenotyping in microorganisms. Nat Microbiol 2: 17014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Henikoff S, Ng PC (2009) Predicting the effects of coding non‐synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4: 1073–1081 [DOI] [PubMed] [Google Scholar]
- Kumasaka N, Knights AJ, Gaffney DJ (2016) Fine‐mapping cellular QTLs with RASQUAL and ATAC‐seq. Nat Genet 48: 206–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labaudinière R (2002) The increasing importance of genetic variation in drug discovery and development. Curr Opin Mol Ther 4: 559–564 [PubMed] [Google Scholar]
- Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, Maglott DR (2014) ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res 42: D980–D985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T‐Y, Huang H‐D, Hung J‐H, Huang H‐Y, Yang Y‐S, Wang T‐H (2006) dbPTM: an information repository of protein post‐translational modification. Nucleic Acids Res 34: D622–D627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen R, Akhtar R, Birney E, Bower L, Cerdeno‐Tárraga A, Cheng Y, Cleland I, Faruque N, Goodgame N, Gibson R, Hoad G, Jang M, Pakseresht N, Plaister S, Radhakrishnan R, Reddy K, Sobhany S, Ten Hoopen P, Vaughan R, Zalunin V et al (2011) The European nucleotide archive. Nucleic Acids Res 39: D28–D31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O'Donnell‐Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce‐Hoffman E, Berghout J, Cooper DN et al (2016) Analysis of protein‐coding genetic variation in 60,706 humans. Nature 536: 285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G, Carter DM, Moses AM, Warringer J, Parts L, James SA, Davey RP, Roberts IN, Burt A, Koufopanou V, Tsai IJ, Bergman CM, Bensasson D, O'Kelly MJT, Oudenaarden AV, Barton DBH, Bailes E, Nguyen AN, Jones M, Quail MA et al (2009) Population genomics of domestic and wild yeasts. Nature 458: 337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch M, Mason JM, Sessions RB, Clarke AR (2000) Effects of mutations on the thermodynamics of a protein folding reaction: implications for the mechanism of formation of the intermediate and transition states. Biochemistry 39: 3480–3485 [DOI] [PubMed] [Google Scholar]
- Lutz S (2010) Beyond directed evolution–semi‐rational protein engineering and design. Curr Opin Biotechnol 21: 734–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next‐generation DNA sequencing data. Genome Res 20: 1297–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitternacht S (2016) FreeSASA: an open source C library for solvent accessible surface area calculations. F1000Res 5: 189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca R, Céol A, Aloy P (2012) Interactome3D: adding structural details to protein networks. Nat Methods 10: 47–53 [DOI] [PubMed] [Google Scholar]
- Nelson MR, Johnson T, Warren L, Hughes AR, Chissoe SL, Xu C‐F, Waterworth DM (2016) The genetics of drug efficacy: opportunities and challenges. Nat Rev Genet 17: 197–206 [DOI] [PubMed] [Google Scholar]
- Ng PC, Henikoff S (2003) SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res 31: 3812–3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenchain V, Lawrence M, Carey V, Gogarten S, Shannon P, Morgan M (2014) VariantAnnotation: a Bioconductor package for exploration and annotation of genetic variants. Bioinformatics 30: 2076–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MF, Francesconi M, Hidalgo‐Carcedo C, Lehner B (2017) Maternal age generates phenotypic variation in Caenorhabditis elegans . Nature 552: 106–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper U, Eswar N, Webb BM, Eramian D, Kelly L, Barkan DT, Carter H, Mankoo P, Karchin R, Marti‐Renom MA, Davis FP, Sali A (2009) MODBASE, a database of annotated comparative protein structure models and associated resources. Nucleic Acids Res 37: D347–D354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S, Sung P, Prakash L (1993) DNA repair genes and proteins of Saccharomyces cerevisiae . Annu Rev Genet 27: 33–70 [DOI] [PubMed] [Google Scholar]
- Reimand J, Wagih O, Bader GD (2015) Evolutionary constraint and disease associations of post‐translational modification sites in human genomes. PLoS Genet 11: e1004919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke A, Chen JC‐Y, Aronova S, Powers T (2006) Caffeine targets TOR complex I and provides evidence for a regulatory link between the FRB and kinase domains of Tor1p. J Biol Chem 281: 31616–31626 [DOI] [PubMed] [Google Scholar]
- Rhee HS, Pugh BF (2011) Comprehensive genome‐wide protein‐DNA interactions detected at single‐nucleotide resolution. Cell 147: 1408–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintisch C, Heinig M, Bauerfeind A, Schafer S, Mieth C, Patone G, Hummel O, Chen W, Cook S, Cuppen E, Colomé‐Tatché M, Johannes F, Jansen RC, Neil H, Werner M, Pravenec M, Vingron M, Hubner N (2014) Natural variation of histone modification and its impact on gene expression in the rat genome. Genome Res 24: 942–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski I, Breitkreutz B‐J, Stark C, Su T‐C, Dahabieh M, Raithatha S, Bernhard W, Oughtred R, Dolinski K, Barreto K, Tyers M (2013) The PhosphoGRID Saccharomyces cerevisiae protein phosphorylation site database: version 2.0 update. Database 2013: bat026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelin A, Alkema W, Engström P, Wasserman WW, Lenhard B (2004) JASPAR: an open‐access database for eukaryotic transcription factor binding profiles. Nucleic Acids Res 32: D91–D94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schymkowitz J, Borg J, Stricher F, Nys R, Rousseau F, Serrano L (2005) The FoldX web server: an online force field. Nucleic Acids Res 33: W382–W388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalin AA (2012) Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics 28: 1353–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedley D, Haider S, Durinck S, Pandini L, Provero P, Allen J, Arnaiz O, Awedh MH, Baldock R, Barbiera G, Bardou P, Beck T, Blake A, Bonierbale M, Brookes AJ, Bucci G, Buetti I, Burge S, Cabau C, Carlson JW et al (2015) The BioMart community portal: an innovative alternative to large, centralized data repositories. Nucleic Acids Res 43: W589–W598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivakov M, Akhtar J, Kheradpour P, Beal K, Girardot C, Koscielny G, Herrero J, Kellis M, Furlong EEM, Birney E (2012) Analysis of variation at transcription factor binding sites in Drosophila and humans. Genome Biol 13: R49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strope PK, Skelly DA, Kozmin SG, Mahadevan G, Stone EA, Magwene PM, Dietrich FS, McCusker JH (2015) The 100‐genomes strains, an S. cerevisiae resource that illuminates its natural phenotypic and genotypic variation and emergence as an opportunistic pathogen. Genome Res 25: 762–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzek BE, Wang Y, Huang H, McGarvey PB, Wu CH, UniProt Consortium (2015) UniRef clusters: a comprehensive and scalable alternative for improving sequence similarity searches. Bioinformatics 31: 926–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana C, Yoo JY, Tagne J‐B, Kacherovsky N, Lee TI, Young ET (2005) Combined global localization analysis and transcriptome data identify genes that are directly coregulated by Adr1 and Cat8. Mol Cell Biol 25: 2138–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G, Lenhard B (2016) TFBSTools: an R/bioconductor package for transcription factor binding site analysis. Bioinformatics 32: 1555–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velankar S, Dana JM, Jacobsen J, van Ginkel G, Gane PJ, Luo J, Oldfield TJ, O'Donovan C, Martin M‐J, Kleywegt GJ (2013) SIFTS: structure integration with function, taxonomy and sequences resource. Nucleic Acids Res 41: D483–D489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venters BJ, Wachi S, Mavrich TN, Andersen BE, Jena P, Sinnamon AJ, Jain P, Rolleri NS, Jiang C, Hemeryck‐Walsh C, Pugh BF (2011) A comprehensive genomic binding map of gene and chromatin regulatory proteins in Saccharomyces . Mol Cell 41: 480–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagih O, Reimand J, Bader GD (2015) MIMP: predicting the impact of mutations on kinase‐substrate phosphorylation. Nat Methods 12: 531–533 [DOI] [PubMed] [Google Scholar]
- Wasserman WW, Sandelin A (2004) Applied bioinformatics for the identification of regulatory elements. Nat Rev Genet 5: 276–287 [DOI] [PubMed] [Google Scholar]
- Weile J, Sun S, Cote AG, Knapp J, Verby M, Mellor JC, Wu Y, Pons C, Wong C, van Lieshout N, Yang F, Tasan M, Tan G, Yang S, Fowler DM, Nussbaum R, Bloom JD, Vidal M, Hill DE, Aloy P et al (2017) A framework for exhaustively mapping functional missense variants. Mol Syst Biol 13: 957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, Klemm A, Flicek P, Manolio T, Hindorff L, Parkinson H (2014) The NHGRI GWAS Catalog, a curated resource of SNP‐trait associations. Nucleic Acids Res 42: D1001–D1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Yang J, Hayes BJ, Price AL, Goddard ME, Visscher PM (2013) Pitfalls of predicting complex traits from SNPs. Nat Rev Genet 14: 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong HY, Alipanahi B, Lee LJ, Bretschneider H, Merico D, Yuen RKC, Hua Y, Gueroussov S, Najafabadi HS, Hughes TR, Morris Q, Barash Y, Krainer AR, Jojic N, Scherer SW, Blencowe BJ, Frey BJ (2015) RNA splicing. The human splicing code reveals new insights into the genetic determinants of disease. Science 347: 1254806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Smith FJ Jr, Subaran R, Mitchell AP (2004) Multivesicular body‐ESCRT components function in pH response regulation in Saccharomyces cerevisiae and Candida albicans . Mol Biol Cell 15: 5528–5537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Chen Y, Ayub Q, Huang N, Ball EV, Mort M, Phillips AD, Shaw K, Stenson PD, Cooper DN, Tyler‐Smith C, 1000 Genomes Project Consortium (2012) Deleterious‐ and disease‐allele prevalence in healthy individuals: insights from current predictions, mutation databases, and population‐scale resequencing. Am J Hum Genet 91: 1022–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YO, Sherlock G, Petrov DA (2016) Whole genome analysis of 132 clinical Saccharomyces cerevisiae strains reveals extensive ploidy variation. G3 (Bethesda) 6: 2421–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Dataset EV1
Review Process File
Data Availability Statement
The precomputed impact of single nucleotide variants in human, yeast and E. coli is available through the mutfunc web interface (www.mutfunc.com). Bug reports and feature requests can be submitted through the project's bug tracker at https://github.com/evocellnet/mutfunc-webserver.


