Abstract
Zika virus (ZIKV) is a major public health concern due to the risk of congenital Zika syndrome in developing fetuses and Guillain-Barre syndrome in adults. Currently, there are no approved vaccines available to protect against infection. Adenoviruses are safe and highly immunogenic vaccine vectors capable of inducing lasting humoral and cellular immune responses. Here, we developed two Adenovirus (Ad) vectored Zika virus vaccines by inserting a ZIKV prM-E gene expression cassette into human Ad types 4 (Ad4-prM-E) and 5 (Ad5-prM-E). Immune correlates indicate that Ad5-prM-E vaccination induces both an anti-ZIKV antibody and T-cell responses whereas Ad4-prM-E vaccination only induces a T-cell response. In a highly lethal challenge in an interferon α/β receptor knockout mice, 80% of Ad5 vaccinated animals and 33% of Ad4 vaccinated animals survived a lethal ZIKV challenge, whereas no animals in the sham vaccinated group survived. In an infection model utilizing immunocompetent C57BL/6 mice that were immunized and then treated with a blocking anti-IFNAR-1 antibody immediately before ZIKV challenge, 100% of Ad4-prM-E and Ad5-prM-E vaccinated mice survived. This indicates that Ad4-prM-E vaccination is protective without the development of detectable anti-ZIKV antibodies. The protection seen in these highly lethal mouse models demonstrate the efficacy of Ad vectored vaccines for use against ZIKV.
Introduction
Zika virus (ZIKV) is a mosquito-borne, positive-stranded RNA virus that belongs to the genus Flavivirus in the Flaviviridae family1. This genus also contains other important human pathogens, such as Dengue, West Nile, Japanese encephalitis, and yellow fever viruses. ZIKV was first discovered in the Zika forest of Uganda in 1947 and believed to cause only asymptomatic or mild infection in humans2. However, the 2015 outbreak of ZIKV in Brazil showed higher than historical rates of congenital birth defects in fetuses and babies born to ZIKV-infected mothers along with an increase in the rate of Guillain-Barre syndrome in adults3–6. The World Health Organization (WHO) declared ZIKV a global public health emergency in February 2016 after an estimated 500,000–1,500,000 suspected cases of ZIKV infection with more than 4,300 cases of microcephaly were reported7. This state of emergency has since been lifted, although the importance of ZIKV research and vaccine development remains a global priority8.
Although there are no licensed vaccines available to prevent ZIKV infection, much progress has been made since 2015. Many vaccine platforms have been explored including traditional platforms such as live-attenuated9–11, inactivated12–14, and subunit vaccines15–17. Other platforms include the expression of ZIKV structural and non-structural genes in DNA12,14,18–21, mRNA11,22–24, or viral vectors25–29. The precursor-membrane (prM) and envelope (E) protein of ZIKV have been the primary structural antigens used in many recombinant vaccines and have shown promising results11,12,14,18–28. Importantly, it has been shown that expression of the prM and E ZIKV proteins leads to the assembly of ZIKV virus-like particles (VLPs) in vitro30, which could be beneficial in a gene-delivery based vaccine platform, as VLPs can induce highly neutralizing antibodies that recognize conformational epitopes on the viral envelope (E) protein.
Adenovirus viral vectors have been shown to induce both strong humoral and cellular immunity31,32. Adenovirus type 5 (Ad5) has been the most extensively used vector due to the robust immune response it elicits. However, pre-existing immunity to Ad5 has been reported to be as high as 82.5% in Brazil and 70% in the United States33. With high pre-existing immunity to Ad5, other Adenovirus types have been explored. A epidemiological study of military recruits in the United States, showed that only 33% had pre-existing immunity to human Adenovirus type 4 (Ad4)34. Ad4 has been shown to be effective as a viral vector in vaccination against influenza, either alone or in combination with Ad535,36.
In this study, we developed both human Ad5 and Ad4 expressing the ZIKV full-length prM-E genes as a candidate ZIKV vaccine. Ad5-prM-E elicited both a strong antibody and T-cell response, whereas Ad4-prM-E did not induce any detectable anti-ZIKV antibodies but still induced a strong T-cell response. In a lethal ZIKV challenge model using an Ifnar−/− mice, Ad5-prM-E provided superior protection to Ad4-prM-E vaccination. However, both Ad vectors protected 100% of mice in a challenge model using anti-Ifnar1 blocking antibody. These data support the use of Ad vectors as a platform for ZIKV vaccine development.
Results
Construction of replication-defective Adenoviral-vectored vaccines
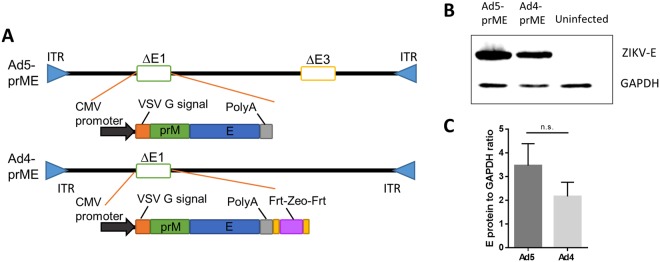
The full length prM-E genes of Zika virus (ZIKV) strain PRVABC59 (Puerto Rico, 2015) were cloned into the E1 region of Ad type 5 (Ad5-prM-E) or Ad type 4 (Ad4-prM-E) to create a replication-defective vector (Fig. 1A). The Ad5-prM-E vector is also E3 deleted to increase cloning capacity. In addition, the Zeocin resistance gene flanked by Frt regions (which was used for effective screening of recombinant clone) was left in the Ad4 vector for simplicity. However, this gene can be easily removed using FLP recombinase for future studies. These differences should not affect in vivo immunogenicity as both of these vectors are replication defective. ZIKV-E protein expression was confirmed in both Ad vaccines by western blotting (Fig. 1B). 293 cells were infected at on MOI of 1 for 48 hours and cell lysate was collected. No significant difference in ZIKV-E protein expression was detected. Data was collected from three separate biological replicates (Fig. 1C).
Figure 1.
Construction and characterization of Adenovirus-vectored vaccine. (A) The ZIKV strain PRVABC59 prM-E region with a VSV G signal peptide under the control of a CMV promoter was cloned into the Ad5 and Ad4 genomes in the E1 region. Ad5 was also deleted for the E3 region. (B) A representative western blot comparing the levels of ZIKV-E protein expression from Ad4-prM-E and Ad5-prM-E infected 293 cells infected at an MOI of 1. GAPDH is used as a cellular control. (C) The ratio of ZIKV-E protein to GAPDH of western blots from C. The western blot data represents three separate biological replicates (n.s. not significant, two-tailed t test). Data are expressed as the mean with standard error (SEM).
Antibody response to vaccination
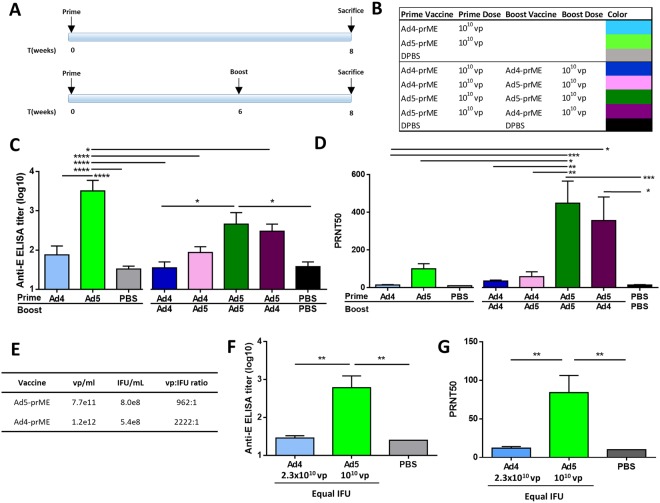
To examine the humoral immunity induced by vaccination, groups of C57BL/6 mice were immunized with a single dose of 1010 virus particles (vp) of Ad vaccine (Ad5-prM-E or Ad4-prM-E) by the intramuscular route (i.m.) on day 0 (Fig. 2A,B). PBS was used as a vaccination negative control. All vaccinated mice were sacrificed on week 8 for immune response analysis. Mice that received single priming dose of Ad5-prM-E showed significantly higher levels of anti-ZIKV E protein antibodies as measured by ELISA (Fig. 2C) compared to the control PBS-treated mice. However, Ad4-prM-E vaccinated mice did not show detectable levels of specific anti-ZIKV-E protein antibodies or neutralizing antibodies (Fig. 2C,D).
Figure 2.
Antibody response from Adenovirus vaccination. (A–D) C57BL/6 mice (n = 5) were immunized with either Ad5-prM-E, Ad4-prM-E, or sham vaccine according to the timeline in (A) and at the indicated dose via the i.m. route (B). ZIKV E protein specific antibodies were measured using an ELISA in (C) and neutralization titer (PRNT50) was determined by plaque reduction assay in (D) (*p < 0.05; **p < 0.01, ***p < 0.001, ****p < 0.0001; one-way ANOVA with Bonferroni multiple comparisons). To ensure that the undetectable antibody response to Ad4-prM-E vaccination was not sue to differences in infectivity of Ad vaccine virus stocks, the virus particle (vp) to infectious unit (IFU) ratio was determined (E). C57BL/6 mice (n = 5) were then vaccinated with an equal IFU dose and the antibody response measured via ELISA (F) and plaque reduction assay (G) **p < 0.01; one-way ANOVA with Bonferroni multiple comparisons). Data are expressed as the mean with standard error (SEM).
To investigate if an Ad prime/boost vaccination strategy would increase immune responses, C57BL/6 mice were vaccinated at day 0 with 1010 vp of Ad vaccine, boosted with 1010 vp of the homologous or heterologous Ad vaccine on week 6, and sacrificed on week 8 (Fig. 2A,B). Prime/boost strategies utilizing Ad5-prM-E as the prime showed significantly higher levels of neutralizing antibodies than control mice (Fig. 2C,D). In contrast, the Ad4-prM-E primed mice showed no boosting effect at all.
Ad4-prM-E vaccination does not induce detectable antibodies
To ensure that the lack of antibody response during Ad4-prM-E vaccination was not due to infectious differences in the Ad vaccine virus stocks, C57BL/6 mice were vaccinated based on infectious units (IFU). The IFU per mL was calculated for both Ad5-prM-E and Ad4-prM-E vaccine stocks. An analysis of virus particle (vp) to IFU showed a 2.3-fold difference between Ad5-prM-E and Ad4-prM-E (Fig. 2E). Mice were then vaccinated with 1010 vp of Ad5-prM-E or 2.3 × 1010 vp of Ad4-prM-E, resulting in a vaccination dose with equal IFU. In order to evaluate the response to vaccination near the predicated peak immune response, mice were euthanized 2 weeks later for immune correlate analysis. Again, Ad4-prM-E vaccination did not result in any detectable anti-ZIKV antibodies as measured by ELISA (Fig. 2F) or plaque reduction neutralization test (Fig. 2G). This data indicates that the lack of antibody development in Ad4-prM-E vaccinated mice is not due to differences in vaccine stock infectivity.
T-cell responses after vaccination
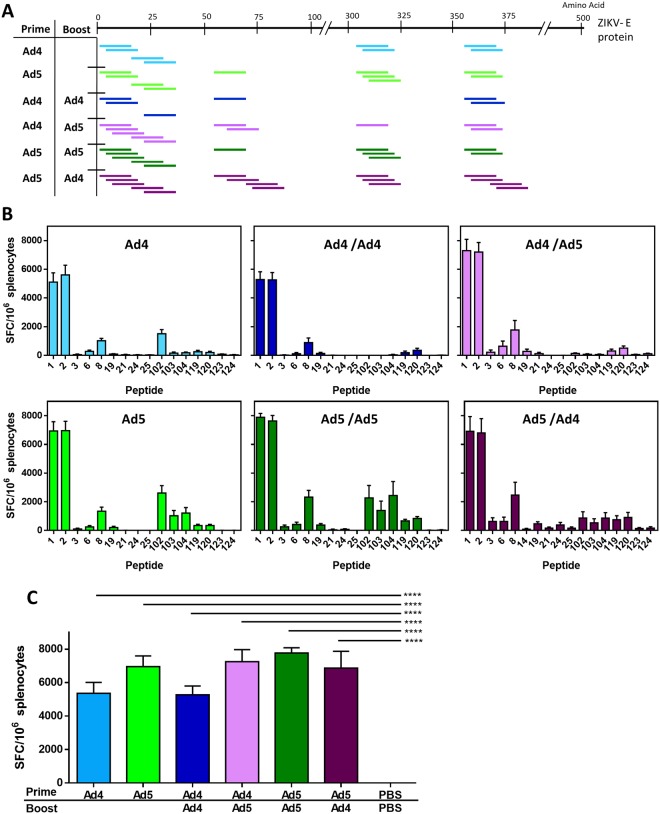
The T-cell response against the tested vaccine strategies and ZIKV infection was examined using an IFN-γ enzyme-linked immunospot assay (ELISPOT). Splenocytes were isolated and incubated with 15-mer peptides with 13 amino acid overlap spanning the entire E protein of ZIKV strain PRVABC59. Of the 164 tested peptides, 16 peptides were positive for a response (Fig. 3; Table. S1). A response was considered positive if it was 4 times the response of the PBS sham vaccinated mice (Fig. 3A). Both Ad5-prM-E and Ad4-prM-E vaccination induced a significant T-cell response against ZIKV E protein peptides (Fig. 3B). Peptide 1 and 2 induced the strongest T-cell response in all Adenovirus vaccinated animals (Fig. 3C) and encompassed the previously identified immunodominant CD8+ T-cell epitope E4–12 (position number E294–302 on the polypeptide)37,38. A strong T-cell response was generated against this epitope after a single shot of either Ad4-prM-E or Ad5-prM-E and was similar in intensity to the T-cell response seen after being boosted with either the homologous or heterologous Ad vaccine. Therefore, the prime/boost strategy and Adenovirus serotype-switching strategy did not significantly increase the T cell responses as judged by IFN-γ ELISPOT.
Figure 3.
T-cell epitope mapping in response to vaccination. (A,B) Splenocytes from the vaccinated C57BL/6 mice (n = 5) were harvested. T-cell epitopes against ZIKV-E protein were mapped using a 164 peptide array of 15-mers with 13 amino acid overlap spanning the entire ZIKV-E protein. Peptides were considered positive if the response was 4 times the sham vaccinated mice. Positive peptides for each vaccination group are indicated in (A). The level of response seen against each positive peptide are reported as spot-forming cells (SFC) per million splenocytes (B). Peptides 1 and 2 encompass a previously described CD8+ immunodominant T-cell epitope37. The T-cell response against this epitope is shown as spot-forming cells (SFC) per million (C) (****p < 0.0001; one-way ANOVA with Bonferroni multiple comparisons). Data are expressed as the mean with standard error (SEM).
Vaccine protection to lethal ZIKV infection in mice
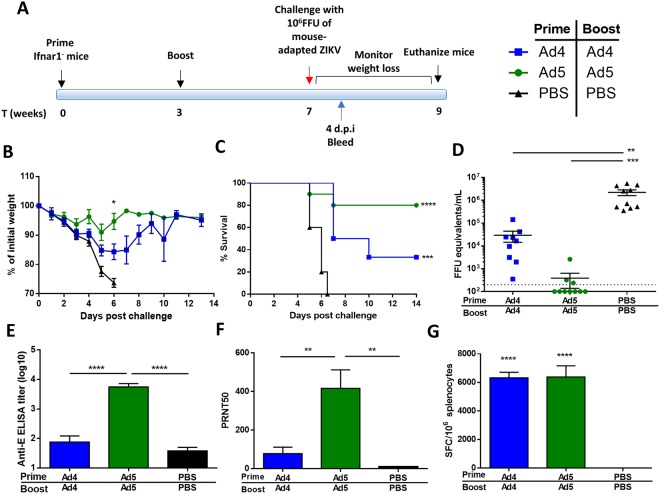
Initially, protection was examined in Ifnar1−/−mice on a C57BL/6 background, which are vulnerable to ZIKV infection39. Since no significant differences were seen in immune correlates between vaccination based on virus particles or infectious units (Fig. 2), challenge studies were carried out with 1010 vp per mouse. Ifnar1−/− mice were primed via i.m. injection at day 0 with 1010 vp Ad4-prM-E or Ad5-prM-E, or PBS as a sham control. Animals were boosted with the same dose of vaccine at week 3 and challenged at week 7 with 106 FFU of mouse-adapted ZIKV strain 4152540 (Fig. 4A). A comparison of the genetic differences between the PRVABC59 vaccination strain and the Dakar 41525 challenge strain can be seen in Supplementary Fig. 1. Studies have identified a single ZIKV serotype indicating that one ZIKV vaccine antigen should protect against all ZIKV strains13,41. After challenge, mice were monitored and euthanized when a weight loss of 25% or more was detected. Ad5-prM-E primed and boosted mice showed an 80% survival rate and Ad4-prM-E primed/boost mice had a 33% survival rate whereas all of the sham vaccination animals succumbed by day 7 (Fig. 4B,C; Fig. S2A). ZIKV viremia in mice peaks between day 3–4 post42,43, so animals were bled 4 days after infection to determine viremia using RT-qPCR (Fig. 4D). Ad5-prM-E animals showed a highly significant decrease in viremia (p < 0.001). Ad4-prM-E vaccination also decreased viremia compared to those in sham vaccinated mice. In addition, a plaque assay was performed to determine infectious ZIKV levels in sera. Both Ad4-prM-E and Ad5-prM-E vaccination significantly reduced infectious plaque forming units in the sera (Fig. S2B). To ensure that the response to vaccination in this immunocompromised mouse is intact, immune correlate analyses were performed after priming and boosting of Ifnar1−/− mice. ELISA (Fig. 4E), PRNT50 (Fig. 4F), and ELISPOT (Fig. 4G) confirmed a vaccine-induced immune response that was similar to wild type C57BL/6 mice.
Figure 4.
Vaccine protection against challenge in Ifnar1−/− mice. (A–D) Ifnar1−/− mice were immunized with 1010 vp of Ad4-prM-E (n = 9), Ad5-prM-E (n = 10), or sham PBS (n = 10) via the i.m. route according to the timeline in (A). Two experimental replicates were performed. Mice were boosted with the same vaccine and dose at week 3 and then challenged with 106 FFU of mouse-adapted ZIKV strain 41519 at week 7. Weight loss was monitored (B) and mice were sacrificed when a 25% weight loss was reached (C). Asterisks indicate significance in weight compared to PBS sham vaccinated mice as determined by two-way ANOVA (p < 0.05). Survival data was analyzed using a log rank test (***p < 0.001, ****p < 0.0001). Blood was sampled at 4 days post infection (d.p.i) to determine the viral load in the sera using RT-qPCR (D) (**p < 0.01; ****p < 0.0001; one-way ANOVA). To determine immune correlates in Ifnar1−/− mice, groups (n = 5) were immunized with 1010 vp of the indicated Ad vaccine and sacrificed 2 weeks later. Sera was used to determine anti-ZIKV IgG (E) and neutralizing antibodies (F). In addition, an ELISPOT was performed to determine spot-forming cells per million splenocytes against the immunodominant E4–12 epitope (G). ELISA, PRNT50, and ELISPOT are analyzed with one-way ANOVA (**p < 0.01, ****p < 0.0001). Data are expressed as the mean with standard error (SEM).
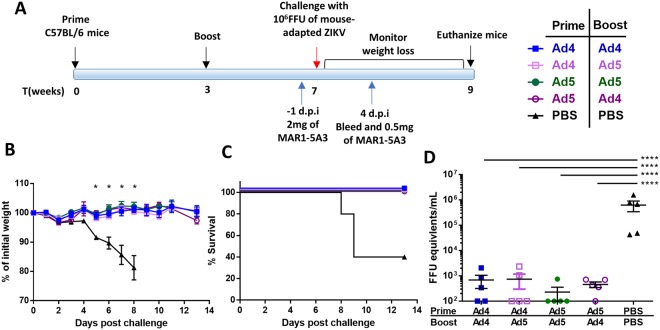
Due to the immunocompromised nature of the Ifnar1−/− mice, we tested a second challenge model that utilized wild type C57BL/6 mice and a blocking anti-Ifnar1 antibody (MAR1-5A3) administered after vaccination but before challenge, which makes naïve mice susceptible to lethal ZIKV infection43. Groups of C57BL/6 mice were immunized via the i.m. route with 1010 vp of Ad4-prM-E or Ad5-prM-E, or PBS (Fig. 5A). Mice were boosted at week 3 with 1010 vp of Adenoviral vector, either heterologous or homologous to the prime Adenovirus type. One day prior to challenge, mice were administered 2 mg of anti-Ifnar1 mAb via i.p. injection. An additional 0.5 mg of mAb was administered on day 4 post infection to ensure lethal challenge. At week 7, all mice were challenged with 106 FFU of mouse adapted ZIKV Dakar strain 41525 and monitored for weight loss (Fig. 5B). All Adenoviral vaccinated mice survived challenge, regardless of prime/boost strategy (Fig. 5C). In comparison, all sham vaccinated mice showed significant weight loss by day 5 (Fig. 5B). All Adenovirus vaccinated animals showed decreased viremia compared to sham vaccinated mice by RT-qPCR (Fig. 5D) and plaque assay (Fig. S2C).
Figure 5.
Vaccine protection against challenge in C57BL/6 mice in anti-Ifnar1 mAb-treated mice. (A–D) C57BL/6 mice (n = 5) were immunized with 1010 vp of Adenovirus vectored vaccine or sham PBS via i.m. injection according to the timeline in (A). Mice were boosted with at week 3 with the indicated vaccine. At week 7, mice were challenged with 106 FFU of mouse-adapted ZIKV strain 41525. One day prior to infection, 2 mg of mouse anti-Ifnar1 was administered via i.p. injection to create a lethal challenge model. Another 0.5 mg of antibody was administered on 4 days post infection (d.p.i). Weight loss was monitored (B) and mice were sacrificed at 25% weight loss (C). Asterisks indicate significant in weight compared to PBS sham vaccinated mice as determined by two-way ANOVA (p < 0.05). Blood was sampled at 4 d.p.i to determine the viral load in the sera using qPCR (D) (****p < 0.0001; one-way ANOVA). Data are expressed as the mean with standard error (SEM).
Discussion
Adenoviruses have been widely used as vaccine vectors due to their ability to induce humoral and cellular immune responses44–46. Replication-defective Adenovirus vectors have been shown to be safe and well tolerated in clinical trials32,47,48. Here we developed replication-defective human Ad5 and Ad4 vectored ZIKV vaccines and characterized their immune responses and protection against ZIKV infection. Ad5-prM-E vaccination induced a strong anti-ZIKV humoral immune response. However, Ad4-prM-E vaccination induced no detectable anti-ZIKV antibodies, even with vaccination with an equal IFU dose to the Ad5-prM-E vaccination or in a prime-boost strategy.
Vaccination by either Ad5-prM-E and Ad4-prM-E resulted in the development of a T-cell immune response. This is important because CD8+ T-cells have been shown to protect against ZIKV infection37,38,49. Notably, both Ad vectored vaccines elicited T-cell responses against the immunodominant epitope E4–12. The ability of Ad4-prME vaccination to induce significant T-cell response but no detectable antibody response could demonstrate preferential development of cellular over humoral immune response in Ad4 vectored vaccination. Ad has a self adjuvanting property, and the Ad4 and Ad5 vectors may initiate very different cytokine profiles that are highly Th1 biased in Ad4 immunized mice but more Th1/Th2 balanced in the Ad5 immunized mice50. A phase I clinical trial utilizing Ad4 as an oral, replication-competent vector for H5 influenza vaccination found similar results. Participants vaccinated with an Ad4-H5 vector developed robust T-cell responses but failed to seroconvert even after 3 vaccinations (11% in vaccinated vs 7% in placebo groups)51. This could indicate that the immune profile of a strong T cell response with limited antibody development that we see with our Ad4-prM-E vaccination is not limited to mouse models or the ZIKV antigen, but further studies are needed to fully evaluate this.
In the anti-Ifnar1 blockade challenge model, both Ad5-prM-E and Ad4-prM-E vaccinated mice protected against weight loss and death. On day 4 post infection, vaccination significantly reduced levels of ZIKV RNA and infectious virus in serum. Even in the highly lethal Ifnar1−/− mouse challenge model, the Ad vectored vaccines were partially protective. The immunocompromised state of the mice along with the challenge dose made this challenge highly stringent. However, as ZIKV infection is not lethal in adult human infection, further studies need to be performed to determine if the partial protection in this highly lethal challenge study translates to protective efficacy in human vaccination.
In conclusion, we constructed two replication-defective Adenovirus (Ad) vectored vaccine candidates by inserting the ZIKV structural prM-E genes into the E1 region of human Ad type 4 and Ad type 5. Both vectors have shown promise by protecting against ZIKV infection in a lethal antibody blockade challenge model. However, immune correlates indicate that Ad4-prM-E vaccination established a different immune profile than Ad5-prM-E vaccination. The ability of Ad4-prM-E vaccination to induce a strong T-cell response with undetectable levels of antibody, while Ad5-prM-E vaccination induces both strong antibody and T-cell responses, warrants further investigation. A comprehensive cytokine/chemokine and cellular profile analysis may elucidate these mechanisms. Due to the potential for antibody dependent enhancement (ADE) of Dengue virus from ZIKV antibodies22,52,53, the lack of antibody development in Ad4-prM-E vaccination could be explored as a potentially safer vaccine platform. Our study highlights the significant differences in immune profiles between these two Ad vectors and supports further investigation of the use of Ad vectors as a platform for ZIKV vaccine development.
Materials and Methods
Ethics statement
B6(Cg)-Ifnar1tm1.2Ees/J Ifnar1−/− mice were purchased from Jackson Laboratories and a breeding colony was established. Female C57BL/6 J mice ages 6–8 weeks were purchased from Jackson Laboratory. Mice were housed in Life Sciences Annex building on the University of Nebraska – Lincoln (UNL) campus under the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) guidelines. The protocols were approved by the UNL Institutional Animal Care and Use Committee (IACUC) (Project ID 1448: Viral Vectored Flavivirus Vaccines). All animal experiments were carried out according to the provisions of the Animal Welfare Act, PHS Animal Welfare Policy, the principles of the NIH Guide for the Care and Use of Laboratory Animals, and the policies and procedures of UNL. All immunizations and bleeds were performed under ketamine and xylazine induced anesthesia.
Viruses
Zika virus (ZIKV) strain PRVABC59 (ZIKV-PR; Puerto Rico, 2015) was provided by the Biodefense and Emerging Infections Research Resources Repository (BEI Resources). ZIKV strain Dakar 41525 was adapted for mice by passage in Rag1−/− mice40. Viral stocks of ZIKV strain PRVABC59 were grown on C6/36 cells and collected after 7 days. Viral stocks of mouse-adapted ZIKV strain Dakar 41525 were grown on Vero cells and harvested after 3 days. Supernatant was collected and clarified by centrifugation at 350 g for 10 min. Aliquots were stored at −80 °C. The virus was quantified by plaque assay or focus forming assay (FFA)39.
Recombinant adenovirus type 5 plasmid construction
The full prM-E gene from ZIKV strain PRVABC59 was codon-optimized for human gene expression, and the VSV G signal sequence was added to the N-terminus of the protein. The DNA was synthesized by GenScript and kindly provided by Dr. Asit Pattnaik and cloned in a pCI-Neo mammalian expression vector. Recombinant Adenovirus 5 was created using the AdEasy Adenoviral Vector System (Agilent). Briefly, to introduce unique restriction enzyme sites for downstream cloning, the prM-E region was amplified by PCR from the pCI-Neo DNA plasmid vaccine using the Q5 High-Fidelity 2X Master Mix (NEB). It was cloned into pCR-Blunt using the Zero Blunt TOPO PCR Cloning Kit (Invitrogen) for sequence confirmation. After confirmation, the gene was cloned into the pShuttle-CMV supplied in the AdEasy kit using T4 DNA ligase (NEB). This shuttle contains both a CMV promoter and a SV40 PolyA signal. The pShuttle-CMV-prM-E was linearized and transformed into BJ5183 electrocompetent cells with the pAdEasy-1 vector (Adenovirus type 5) to undergo homologous recombination. This recombination inserts the prM-E expression cassette into the E1 region of the Adenovirus type 5 (Ad5) genome. In addition, this vector is also deleted for the E3 region. Recombination was confirmed by restriction digest and a successful recombinant was transformed into XL1 cells for midiprep using the Qiagen Plasmid Midi Kit (Qiagen).
Recombinant adenovirus type 4 plasmid construction
The Adenovirus type 4 genome was cloned and a shuttle plasmid to replace the E1 region was created as previously described35. Briefly, the complete Adenovirus type 4 (pAd4) genome was cloned into a single low-copy plasmid, and a shuttle plasmid (pAd4-recomb-cloner) to replace the E1 region of Ad4 was created using overlapping PCR products. The CMV-prM-E-PolyA region from the pShuttle-CMV-prM-E plasmid was amplified using the Platinum PCR SuperMix High Fidelity (Invitrogen) and fused to an Frt-Zeo-Frt fragment using overlapping PCR. This fragment was then cloned into pCR8/Gw/TOPO for sequence confirmation (Invitrogen). After sequence confirmation, the shuttle was linearized and transformed with the pAd4 plasmid into BJ5183 electrocompetent cells for homologous recombination. Recombinants were screened on kanamycin and zeocin and confirmed with restriction digest. A successful recombinant was then transformed into XL1 cells, and plasmid DNA was purified using the Qiagen Plasmid Midi Kit (Qiagen).
Recombinant adenovirus virus rescue and purification
The recombinant Adenovirus 5 and 4 genomes with the ZIKV prM-E insert were linearized and buffer exchanged using a Strataprep PCR purification kit (Agilent Technologies). The linearized recombinant gDNA was transfected into 293 cells using the PolyFect Transfection Reagent (Qiagen). After virus rescue was observed via plaque formation, cells were harvested and virus was released by 3 freeze-thaw cycles. Virus was amplified by sequential passages in 293 cells until a final amplification using a Corning 10-cell stack (~6300 cm2). The virus was purified by 2 sequential CsCl ultracentrifuge gradients, desalted using Econo-Pac 10DG Desalting Columns (Bio-Rad), and stored at −80 °C in Ad-tris buffer (20 mM Tris-HCl, 100 mM NaCl2, 1 mM MgCl2•6H2O, 10% glycerol).
Recombinant adenovirus quantification
After CsCl purification and desalting, the virus particle quantity was determined on a NanoDrop Lite spectrophotometer (Thermo Fisher) with an OD260. The infectious unit titer was determined using the QuickTiter Adenovirus Titer Immunoassay Kit (Cell Biolabs, Inc.). Briefly, 293 cells were infected with serial dilutions of Adenovirus stock in triplicate. After incubation for 48 hours, cells were fixed with cold methanol, blocked with PBS with 1% BSA, and then incubated with anti-hexon antibody. Cell were washed, incubated with secondary HRP-conjugated antibody, and developed with DAB. Positive cells were stained brown and counted to determine the infectious units per mL.
Western blotting
For confirmation of protein expression from the recombinant Adenoviruses, confluent 293 cells were infected with Ad5-prM-E or Ad4-prM-E at either 500 vp/cell or an MOI of 1. They were incubated at 37 °C and 5% CO2 and harvest at 48 hours. Cells were denatured using Laemmli buffer plus 2-mercaptoethanol and boiled at 100 °C for 10 minutes. The sample was then passed through a QIAshredder (Qiagen). Sample was loaded onto a 12.5% SDS-PAGE gel and separated using electrophoresis. Protein was transferred to a nitrocellulose membrane and blocked for 30 minutes with 5% milk in TBST. The membrane was incubated in mouse anti-ZIKV E protein antibody (mAb-0302156; BioFront Technologies) at 1:5000 and mouse anti-GAPDH (sc-47724; Santa Cruz Biotechnology, Inc.) at 1:2000 in TBST 1% milk overnight at 4 °C. After 3 washes in TBST, the membrane was incubated with goat anti-mouse-HRP conjugated antibody (Millipore Sigma) at 1:2000 in TBST 1% milk for 1 hour at room temperature. After incubation, the membrane was washed and developed with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific).
ZIKV plaque assay
Cell supernatants were serially diluted in DMEM 2% FBS and added to confluent Vero cells. After incubation with rocking at 37 °C and 5% CO2 for 1 h, cells were washed once with PBS and 1 mL of agar/DMEM overlay was added. Plates were incubated for 4 days at 37 °C and 5% CO2. After 4 days, cells were fixed with 10% formaldehyde, the agar plugs removed, and the cell monolayer was stained using crystal violet. Plaques were counted and plaque forming unit (PFU) per mL were calculated.
ZIKV foci forming unit assay
Virus stocks were titrated by focus forming assay (FFA) on Vero cells as previously described39 and stored in aliquots at −80 °C.
Mouse challenge studies
Female Ifnar1−/− mice (8-9 weeks old) were immunized intramuscularly at day 0 with 1010 vp of Ad4-prM-E (n = 9), Ad5-prM-E (n = 10), or control PBS (n = 10). Two experimental replicates were performed however, after day 4, three mice in the Ad4-prM-E group were lost to unrelated causes due to anesthesia complications. Female C57BL/6 mice were immunized intramuscularly at day 0 with 1010 vp of the indicated Ad vaccine or control PBS. All mice were boosted at week 3 with 1010 vp of indicated Adenovirus or sham vaccine and then challenged at week 7 with 106 FFU of mouse-adapted ZIKV-Dakar 41525 via the intraperitoneal (i.p.) route. C57BL/6 mice were administered 2 mg of anti-mouse Ifnar1 mAb (MAR1-5A3, BioXcell) via the i.p. route one day before ZIKV infection and given 0.5 mg more at day 4 post infection. All mice were monitored daily for weight loss and mice were euthanized at 25% weight loss.
Antibody and T-cell assays
Female C57BL/6 mice were immunized with 1010 vp of indicated Adenovirus or control PBS. All immunizations were performed intramuscularly with a 27-gauge needle into both quadriceps in two 25 µl injections. At week 6, prime/boost animals were boosted with 1010 vp of indicated Adenovirus. At week 8, all animals were terminally bled via a cardiac puncture and spleens were harvested. Sera was isolated from whole blood with a BD Microtainer Blood Collection Tube (Becton Dickinson) and used for further ELISA and neutralization tests. Splenocytes were harvested for T cell immune assays.
ELISA
Immunolon 4 HBX microtiter 96-well strips (VWR) were coated with 150 ng of recombinant ZIKV E protein (Cat #:MBS596088; MyBioSource) in bicarbonate/carbonate coating buffer overnight at 4 °C. The plates were blocked with 2.0% BSA in PBS for 2 hours at room temperature (RT). Sera was diluted in 1.0% BSA in PBS and incubated for 2 hours at RT. The plates were washed 6X with PBST and incubated with goat anti-mouse-HRP antibody (1:2000; Thermo Fisher) in 1.0% BSA in PBS for 1 hour at RT. After washing 4X with PBST and 2X with PBS, the plate was developed with 1-Step Ultra TMB-ELISA (Thermo Fisher), and the reaction was stopped with 2 M sulfuric acid. The OD450 was detected using a SpectraMax i3x Multi-Mode microplate reader (Molecular Devices), and the endpoint titer was determined as signal that was two times background values.
ELISPOT
The T-cell epitopes were mapped using a peptide array of the ZIKV strain PRVABC59 E protein from BEI resources (Catalog No. NR-50553). This 164-peptide array spans the entire E region and consists of 15-mers with 12 amino acid overlap. Potential immunogenic peptides were identified using a matrix of peptides pools, and the epitopes were confirmed using individual peptides. Splenocytes were isolated from mice using a 40 μm Nylon cell strainer (BD Labware). Red blood cells were lysed using ACK lysis buffer and the splenocytes were resuspended in cRPMI at a concentration of 106 splenocytes/mL. Ninety-six well polyvinylidene difluoride-backed plates (MultiScreen-IP, Millipore) coated with 50 μl of anti-mouse IFN-γ mAb AN18 (5 µg/ml; Mabtech) overnight at 4 °C. Plates were washed and blocked with RPMI at 37 °C for 1 hour. Equal volumes (50 µL) of the single-cell suspension splenocytes and peptide (5ug/mL) were added to the wells in duplicate. Plates were incubated overnight at 37 °C with 5% CO2. The plates were washed 6X with PBS and incubated with 100 μl of biotinylated anti-mouse IFN-γ mAb (1:1000 dilution; Mabtech) diluted in PBS with 1.0% FBS for 1 hour at RT. Plates were washed 6X with PBS and incubated with 100 µl of streptavidin-alkaline phosphatase conjugate (1:1000 dilution; Mabtech) diluted in PBS 1.0% FBS. After 1 hour at RT, the plates were washed 6x with PBS. To develop, 100 µl of BCIP/NBT (Plus) alkaline phosphatase substrate (Thermo Fisher) was added to each well and development was stopped by washing several times in dH2O. The plates were air dried and spots were counted using an automated ELISpot plate reader (AID iSpot Reader Spectrum). Results are expressed as spot-forming cells (SFC) per 106 splenocytes.
qPCR for viral load quantification
Challenged mice were bled from the submandibular vein at day 4 post infection and sera was isolated. RNA from sera was extracted using the PureLink Viral RNA/DNA Mini Kit according to manufacturer’s instructions (Invitrogen). Real time RT-qPCR was performed using the Luna Universal Probe One-Step RT-qPCR Kit (NEB). It was run on a QuantStudio 3 Real-Time PCR System (Applied Biosystems) using the Luna kit cycling conditions (55 °C for 10 min, 95 °C for 1 min, and 40 cycles of 95 °C for 10 s and 60 °C for 1 min). Results were compared to a standard curve created using ZIKV RNA extracted from a known quantity of infectious virus. Unamplified samples were set at the limit of detection (100 FFU equivalent/mL). The following primer probe set was used: 1183 F: 50-CCACCAATGTTCTCTTGCAGACATATTG-30; 1268 R: 50-TTCGGACAGCCGTTGTCCAACACAAG-30; and probes (1213 F): 5′FAM/AGCCTACCTTGACAAGCAGTC/BHQ1–3′22.
Plaque reduction neutralization assay
Mouse sera was heat-inactivated at 56 °C for 30 min. Sera then was diluted 2-fold in DMEM 2% FBS and incubated with 50 PFU of ZIKV strain PRVABC59 for 2 hours at 37 °C and 5% CO2. This was then added to a 24-well plate of confluent Vero cells. The plaque assay was continued as described above.
Statistical analysis
GraphPad Prism software was used to analyze all data. Data are expressed as the mean with standard error (SEM). Survival curves were analyzed using the log rank test and weight loss was analyzed using two-way ANOVA multiple comparison test. ELISA, PRNT50, T-cell data, and viral burden were analyzed using one-way ANOVA with Bonferroni multiple comparisons. A p value < 0.05 was considered statistically significant (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).
Electronic supplementary material
Acknowledgements
We would like to acknowledge Amy Lingel for help in growing the ZIKV PRVABC59 strain. We thank the Biodefense and Emerging Infectious Disease (BEI) Repository for reagents used in this study. We would also like to thank the World Reference Center for Emerging Viruses and Arboviruses (University of Texas Medical Branch at Galveston) for the original ZIKV 41525 strain. This research was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award 1 T32 AI125207 from the National Institute of Allergy and Infectious Diseases and by NIH NIAID grant R01 AI097241 and R01 AI073755.
Author Contributions
E.A.W., M.S.D. and B.L.B. designed and planned the study. B.L.B., B.N.C., and E.A.W. performed the experiments. M.J.G. and M.S.D. provided protocols and essential virus reagents. B.L.B., B.N.C., and E.A.W. analyzed the data, created figures, and wrote the manuscript. All authors reviewed and approved the final manuscript.
Competing Interests
M.S.D. is a consultant for Inbios and is on the Scientific Advisory Board of Moderna.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35755-z.
References
- 1.Fields, B. N., Knipe, D. M. & Howley, P. M. Fields virology. (Wolters Kluwer Health/Lippincott Williams & Wilkins 2013).
- 2.Koppolu V, Shantha Raju T. Zika virus outbreak: a review of neurological complications, diagnosis, and treatment options. Journal of NeuroVirology. 2018 doi: 10.1007/s13365-018-0614-8. [DOI] [PubMed] [Google Scholar]
- 3.Parra B, et al. Guillain–Barré Syndrome Associated with Zika Virus Infection in Colombia. New England Journal of Medicine. 2016;375:1513–1523. doi: 10.1056/NEJMoa1605564. [DOI] [PubMed] [Google Scholar]
- 4.Garcez PP, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352:816. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- 5.Shao Q, et al. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development. 2016;143:4127–4136. doi: 10.1242/dev.143768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirsch AJ, et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nature communications. 2018;9:263. doi: 10.1038/s41467-017-02499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. New England Journal of Medicine. 2016;374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 8.Organization, W. H. 2018 Annual review of diseases prioritized under the Research and Development Blueprint. WHO Research and Development Blueprint (2018).
- 9.Shan C, et al. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nature Medicine. 2017;23:763–767. doi: 10.1038/nm.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shan, C. et al. A single-dose live-attenuated vaccine prevents Zika virus pregnancy transmission and testis damage. Nature Communications8, 10.1038/s41467-017-00737-8 (2017). [DOI] [PMC free article] [PubMed]
- 11.Richner JM, et al. Vaccine Mediated Protection Against Zika Virus-Induced Congenital Disease. Cell. 2017;170:273–283. e212. doi: 10.1016/j.cell.2017.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbink P, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353:1129–1132. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumathy K, et al. Protective efficacy of Zika vaccine in AG129 mouse model. Scientific Reports. 2017;7:46375. doi: 10.1038/srep46375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larocca RA, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536:474–478. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han JF, et al. Immunization with truncated envelope protein of Zika virus induces protective immune response in mice. Sci Rep. 2017;7:10047. doi: 10.1038/s41598-017-10595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M, Dent M, Lai H, Sun H, Chen Q. Immunization of Zika virus envelope protein domain III induces specific and neutralizing immune responses against Zika virus. Vaccine. 2017;35:4287–4294. doi: 10.1016/j.vaccine.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang, M., Sun, H., Lai, H., Hurtado, J. & Chen, Q. Plant-produced Zika virus envelope protein elicits neutralizing immune responses that correlate with protective immunity against Zika virus in mice. Plant Biotechnol J, 10.1111/pbi.12796 (2017). [DOI] [PMC free article] [PubMed]
- 18.Yi, G. et al. A DNA Vaccine Protects Human Immune Cells against Zika Virus Infection in Humanized Mice. EBioMedicine, 10.1016/j.ebiom.2017.10.006. [DOI] [PMC free article] [PubMed]
- 19.Muthumani K, et al. In vivo protection against ZIKV infection and pathogenesis through passive antibody transfer and active immunisation with a prMEnv DNA vaccine. npj Vaccines. 2016;1:16021. doi: 10.1038/npjvaccines.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowd KA, et al. Rapid Development of a DNA Vaccine for Zika Virus. Science (New York, N.Y.) 2016;354:237–240. doi: 10.1126/science.aai9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaudinski, M. R. et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. The Lancet, 10.1016/S0140-6736(17)33105-7. [DOI] [PMC free article] [PubMed]
- 22.Richner JM, et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell. 2017;168:1114–1125.e1110. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chahal JS, et al. An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8 + T cell responses in a mouse model. Sci Rep. 2017;7:252. doi: 10.1038/s41598-017-00193-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardi, N. et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature advance online publication, (2017). [DOI] [PMC free article] [PubMed]
- 25.Betancourt D, de Queiroz NMGP, Xia T, Ahn J, Barber GN. Cutting Edge: Innate Immune Augmenting Vesicular Stomatitis Virus Expressing Zika Virus Proteins Confers Protective Immunity. The Journal of Immunology. 2017;198:3023–3028. doi: 10.4049/jimmunol.1602180. [DOI] [PubMed] [Google Scholar]
- 26.Abbink P, et al. Durability and correlates of vaccine protection against Zika virus in rhesus monkeys. Science translational medicine. 2017;9:eaao4163. doi: 10.1126/scitranslmed.aao4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim E, et al. Preventative Vaccines for Zika Virus Outbreak: Preliminary Evaluation. EBioMedicine. 2016;13:315–320. doi: 10.1016/j.ebiom.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu, K. et.al. Recombinant Chimpanzee Adenovirus Vaccine AdC7-M/E Protects against Zika Virus Infection and Testis Damage (2018). [DOI] [PMC free article] [PubMed]
- 29.Brault AC, et al. A Zika Vaccine Targeting NS1 Protein Protects Immunocompetent Adult Mice in a Lethal Challenge Model. Scientific Reports. 2017;7:14769. doi: 10.1038/s41598-017-15039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvo MA, Kingstad-Bakke B, Salas-Quinchucua C, Camacho E, Osorio JE. Zika virus like particles elicit protective antibodies in mice. PLOS Neglected Tropical Diseases. 2018;12:e0006210. doi: 10.1371/journal.pntd.0006210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, Zhou D. Adenoviral vector-based strategies against infectious disease and cancer. Human Vaccines & Immunotherapeutics. 2016;12:2064–2074. doi: 10.1080/21645515.2016.1165908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wold WSM, Toth K. Adenovirus Vectors for Gene Therapy, Vaccination and Cancer Gene Therapy. Current gene therapy. 2013;13:421–433. doi: 10.2174/1566523213666131125095046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mast TC, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: Correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28:950–957. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 34.Ludwig SL, et al. Prevalence of antibodies to adenovirus serotypes 4 and 7 among unimmunized US Army trainees: results of a retrospective nationwide seroprevalence survey. The Journal of Infectious Diseases. 1998;178:1776–1778. doi: 10.1086/314498. [DOI] [PubMed] [Google Scholar]
- 35.Weaver EA. Vaccines within vaccines: The use of adenovirus types 4 and 7 as influenza vaccine vectors. Human Vaccines & Immunotherapeutics. 2014;10:544–556. doi: 10.4161/hv.27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lingel A, Bullard BL, Weaver EA. Efficacy of an Adenoviral Vectored Multivalent Centralized InfluenzaVaccine. Scientific Reports. 2017;7:14912. doi: 10.1038/s41598-017-14891-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elong Ngono A, et al. Mapping and Role of the CD8 + T Cell Response During Primary Zika Virus Infection in Mice. Cell Host Microbe. 2017;21:35–46. doi: 10.1016/j.chom.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang, H. et al. CD8 + T Cell Immune Response in Immunocompetent Mice during Zika Virus Infection. J Virol91, 10.1128/JVI.00900-17 (2017). [DOI] [PMC free article] [PubMed]
- 39.Lazear HM, et al. A Mouse Model of Zika Virus Pathogenesis. Cell Host & Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorman MJ, et al. An Immunocompetent Mouse Model of Zika Virus Infection. Cell Host & Microbe. 2018;23:672–685.e676. doi: 10.1016/j.chom.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dowd KA, et al. Broadly Neutralizing Activity of Zika Virus-Immune Sera Identifies a Single Viral Serotype. Cell reports. 2016;16:1485–1491. doi: 10.1016/j.celrep.2016.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aliota MT, et al. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Neglected Tropical Diseases. 2016;10:e0004682. doi: 10.1371/journal.pntd.0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith DR, et al. Neuropathogenesis of Zika Virus in a Highly Susceptible Immunocompetent Mouse Model after Antibody Blockade of Type IInterferon. PLoS Neglected Tropical Diseases. 2017;11:e0005296. doi: 10.1371/journal.pntd.0005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lasaro MO, Ertl HC. New insights on adenovirus as vaccine vectors. Molecular Therapy. 2009;17:1333–1339. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tatsis N, Ertl HCJ. Adenoviruses as vaccine vectors. Molecular Therapy. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holst, P. J. & Thomsen, A. R. In Viral Gene Therapy (ed. Ke Xu) Ch. 08 (InTech 2011).
- 47.Keefer MC, et al. A Phase I Double Blind, Placebo-Controlled, Randomized Study of a Multigenic HIV-1 Adenovirus Subtype 35 Vector Vaccine in Healthy Uninfected Adults. PLoS ONE. 2012;7:e41936. doi: 10.1371/journal.pone.0041936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baden LR, et al. Induction of HIV-1–Specific Mucosal Immune Responses Following Intramuscular Recombinant Adenovirus Serotype 26 HIV-1 Vaccination of Humans. The Journal of Infectious Diseases. 2015;211:518–528. doi: 10.1093/infdis/jiu485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winkler CW, et al. Adaptive Immune Responses to Zika Virus Are Important for Controlling Virus Infection and Preventing Infection in Brain and Testes. J Immunol. 2017;198:3526–3535. doi: 10.4049/jimmunol.1601949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molinier-Frenkel V, et al. Adenovirus Hexon Protein Is a Potent Adjuvant for Activation of a Cellular Immune Response. Journal of Virology. 2002;76:127–135. doi: 10.1128/JVI.76.1.127-135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gurwith M, et al. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study. The Lancet Infectious Diseases. 2013;13:238–259. doi: 10.1016/S1473-3099(12)70345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stettler K, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 53.George J, et al. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Scientific Reports. 2017;7:10498. doi: 10.1038/s41598-017-10901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.