Abstract
Obesity and related pathologies such as diabetes and metabolic syndrome are associated with chronic inflammation and cancer. The serum level of xanthine oxidoreductase (XOR) is correlated to obesity-associated metabolic disorders. XOR can play a role in the pathogenesis of both metabolic syndrome and cancer through the inflammatory response and the oxidative stress elicited by the products of its activity. The reactive oxygen and nitrogen species and the uric acid derived from XOR concur to the development of hypertension, dyslipidemia and insulin resistance and participate in both cell transformation and proliferation, as well as in the progression and metastasis process. Despite the availability of different drugs to inhibit in vivo XOR activity, the complexity of XOR inhibition effects should be carefully considered before clinical application, save in the case of symptomatic hyperuricemia.
Abbreviations: COX-2, cyclooxygenase-2; EMT, epithelial-mesenchymal transition; FAD, flavin adenine dinucleotide; HIF-1α, hypoxia-inducible factor-1-alpha; IGF-1, insulin-like growth factor-1; HDL, high-density lipoprotein; IL-6, interleukin-6; MMP, matrix metalloproteases; LDL, low-density lipoprotein; Moco, molybdopterin cofactor; NAD, nicotinamide adenine dinucleotide; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NO, nitric oxide; PPARs, peroxisome proliferator-activated receptors; RNS, reactive nitrogen species; ROS, reactive oxygen species; TGF-β, transforming growth factor-beta; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor; XDH, xanthine dehydrogenase; XO, xanthine oxidase; XOR, xanthine oxidoreductase
Keywords: Cancer risk, Inflammation, Metabolic syndrome, Oncogenesis, Oxidative stress, Xanthine oxidoreductase
Graphical abstract
Highlights
-
•
Metabolic syndrome (MS) increases the risk of cancer development.
-
•
Xanthine oxidoreductase (XOR) plays a role in both MS and cancer.
-
•
Uric acid, ROS and RNS produced by XOR cause inflammation and oxidative stress.
-
•
Inflammation and oxidative stress contribute to the pathogenesis of MS and cancer.
-
•
XOR activity can be pharmacologically controlled.
1. Metabolic syndrome
Metabolic syndrome is a multifactorial pathological condition characterized by the presence of any three of the following five risk factors: hyperglycemia, hypertension, hypertriglyceridemia, low level of high-density lipoprotein (HDL)-cholesterol, and an enlarged waist circumference [1]. Patients with metabolic syndrome manifest a prothrombotic and a proinflammatory state. In addition, they have an elevated risk of developing cardiovascular diseases, type 2 diabetes mellitus and some type of neoplasia. In this review, the association between metabolic syndrome and cancer will be treated in relation to xanthine oxidoreductase (XOR) activity and products.
The metabolic syndrome is worldwide distributed, mostly in relation to ageing and obesity, and is typified by insulin resistance and lipolysis. The pathogenesis of insulin resistance may have a genetic component, which manifests itself during ageing through the reduction of glucose uptake by adipose tissue and muscle, thereby resulting in hyperglycemia. In addition, insulin-resistant adipocytes liberate free fatty acids in blood. Also, obese adipose tissue, especially in visceral obesity induced by a sedentary lifestyle, releases an exceeding amount of free fatty acids into circulation, as a consequence of the altered level of adipokines, namely increased resistin and decreased adiponectin production. This derangement of adipokine balance is responsible not only of lipolysis but also of insulin resistance, as well as increased production of angiotensinogen and inflammatory cytokines, thus altering the metabolism of liver and kidney and inducing the endothelial dysfunction (reviewed in [2]).
The accumulation of free fatty acids in liver generates the hypertriglyceridemia that is associated to a decreased cholesterol content in HDL and contributes to hyperglycemia by favoring the gluconeogenesis. Lipolysis and insulin resistance also reduce the muscle utilization of glucose, thus contributing to hyperglycemia and promoting hyperinsulinemia.
Obese adipocytes can elicit hypertension through several mechanisms: i) by inducing the hyperinsulinemia that causes an increased kidney reabsorption of sodium and urate, ii) by increasing the release of angiotensinogen with consequent up-regulation of renin/angiotensin pathway, iii) by the production of inflammatory cytokines determining endothelial dysfunction and oxidative stress with consequent impaired nitric oxide (NO)-dependent vasodilation (reviewed in [3], [4]).
2. Xanthine oxidoreductase activities and products
XOR belongs to a family of metalloflavoenzymes, which is widely distributed from prokaryotic organisms to humans, and it is possibly derived from a common ancestral XOR-coding gene system (reviewed in [5]). In lower organisms, the enzyme has only dehydrogenase activity, whereas in mammals the enzyme has two interchangeable activities: xanthine dehydrogenase (XDH, EC 1.17.1.4) and xanthine oxidase (XO, EC 1.17.3.2). XDH is the native form, mostly present inside the cell, and can be converted to XO by oxidation of sulfhydryl groups or limited proteolysis. XO is the form prevailing in biological fluids, such as milk and plasma (reviewed in [6]). The transition from XDH to XO includes an intermediate XOR form with both the NAD+-dependent dehydrogenase and the O2-dependent oxidase activities [7]. XOR may produce reactive oxygen species (ROS), in particular hydrogen peroxide and superoxide anion, which, in the presence of transition metals, may generate the highly cytotoxic hydroxyl radical through the Haber-Weiss and Fenton reactions. Thus, the phylogenetic evolution empowered XOR to produce oxidant species that influence the redox equilibrium and are implicated in many biological processes, including inflammation, cell proliferation and neoplastic transformation (reviewed in [8]).
In uricothelic primates, the last two steps of purine catabolism are catalyzed by XOR that has a rate-limiting effect on the recovery of nucleotides by precluding the purine salvage pathway (reviewed in [9]). XOR may also have nicotinamide adenine dinucleotide (NADH) oxidase and nitrate reductase activities, especially in conditions of low pH and hypoxia, thus generating superoxide anion and NO, respectively (reviewed in [10]). In addition, XOR is able to act on a variety of substrates. The broad substrate specificity and different catalytic activities of XOR justify its wide capability of metabolize both endogenous and xenobiotic compounds, such as drugs (reviewed in [11]).
The molecular structure of XOR protein is homodimeric and each monomer has three domains, which are characterized by a different cofactor. The C-terminal domain has a molybdenum-containing molybdopterin cofactor (Moco), the intermediate one has a flavin adenine dinucleotide (FAD) cofactor, and the N-terminal one has two unequal iron-sulfur redox clusters, of approximately 85, 40 and 20 kDa, respectively. During catalysis, the electrons are transferred from Moco to FAD by the ferredoxin centers (reviewed in [5]).
The transcription of human XOR gene is down-regulated in most cell types with the exclusion of the epithelial cells of gastrointestinal tract, kidney, liver and lactating breast. Various cytokines, growth factors, hormones and also low oxygen tension can increase XOR expression (reviewed in [12]). The post-translational modulation may give rise to XOR demolybdo-and/or desulfo-forms, which are inactive in purine oxidative hydroxylation and nitrate reductase activities, because of the Moco site blockage, although the NADH oxidase activity at the FAD site is retained. Similarly, the production of oxidant species at the FAD site is not lost when competitive, such as allopurinol, or non-competitive XOR inhibitors, such as febuxostat, inactivate Moco site (reviewed in [6]).
Through the generation of ROS and NO, XOR activity may contribute to the formation of reactive nitrogen species (RNS), which are essential to the anti-bacterial defenses during phagocytosis. However, XOR-derived ROS and RNS may result harmful in various pathological conditions such as during the ischemia/reperfusion injury (reviewed in [13]). XOR released from dead cells may be delivered into circulation and adhere to the endothelial binding sites of heparin. ROS, RNS and uric acid produced by endothelium-linked XOR contribute to the physiological modulation of arteriolar tone, although, an exceeding amount of them may be responsible of the endothelial dysfunction that is at the basis of atherosclerosis and related cardiovascular diseases (reviewed in [14], [15]).
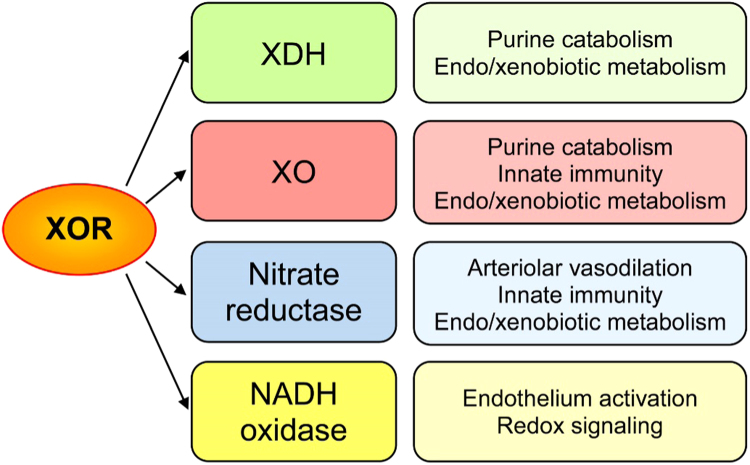
The different XOR catalytic activities and their main functions are summarized in Fig. 1.
Fig. 1.
XOR catalytic activities and their main functions. In all cells, the expression of XOR gene generates XDH with the housekeeping role of purine catabolism. Liver cells utilize XDH, XO and nitrate reductase activities of XOR as well for the metabolism of other endogenous and exogenous substrates, including some drugs. XO derives from a partial oxidation of the enzymatic molecule that, in mammals, has acquired the ability to produce hydrogen peroxide and superoxide ion. ROS generation by XO enables part of the XOR pro-phlogistic and redox signaling functions, which in addition depend on the production of superoxide ion and NO by NADH oxidase and nitrate reductase activities of XOR, respectively, particularly in endothelial cells. ROS and RNS are essentials for the innate immunity and NO have also the role of regulating the arteriolar tone.
3. Involvement of xanthine oxidoreductase in metabolic syndrome
In various animal models, XOR inhibition with either allopurinol or febuxostat reduced hypertension, insulin resistance and production of pro-phlogistic cytokines by macrophages, suggesting that XOR activity is implicated in the pathogenesis of metabolic syndrome [16], [17], [18], [19]. In rat and mouse models of diet-induced obesity, XOR activity was higher in adipose tissue of obese animals than in controls and it has been implicated in the development of metabolic alterations characteristic of metabolic syndrome. The increased XOR activity may favor the purine catabolism with consequent overproduction of uric acid [20] as well as ROS, which may drive to endothelial dysfunction and be responsible of reduced NO-dependent arteriolar dilation [21].
Hyperuricemia is not one of the diagnostic criteria used to identify metabolic syndrome, although it is often associated with this pathological condition [22], as well as with type 2 diabetes (reviewed in [23]) and obesity (reviewed in [24]). In fact, hyperuricemia may contribute to the development of visceral obesity and hypertriglyceridemia, as well as of insulin resistance, hyperinsulinemia and hyperglycemia, thus contributing to the pathogenesis of metabolic syndrome (reviewed in [25]). Moreover, hyperuricemia may induce hypertension by up-regulating the renin/angiotensin pathway, increasing the cyclooxygenase-2 (COX-2) expression, promoting the migration and proliferation of vascular myocytes and reducing the availability of NO. Uric acid and uric acid-derived free radicals have also a pro-inflammatory action by inducing the production of macrophage cytokines and by activating platelets (reviewed in [25]). Because of these pro-hypertensive and pro-phlogistic activities, hyperuricemia represents an independent risk factor with an unfavorable prognostic significance for cardiovascular diseases [26]. Accordingly, the beneficial effect of XOR inhibition has been suggested in patients with cardiovascular diseases (reviewed in [27]).
Many clinical evidences underline the link between XOR activity, obesity and insulin resistance. XOR plasmatic activity was higher in obese than in normal weight children and positively correlated with body mass index, waist circumference and oxidized low-density lipoprotein (LDL), whereas it negatively correlated with HDL-cholesterol and adiponectin [28]. Moreover, in adolescents with severe obesity weight loss caused a decrease of plasma XOR activity, although without affecting uricemia and blood pressure [29]. Furthermore, the serum level of XOR activity was significantly higher in diabetic than in control subjects and directly correlated with body mass index and serum level of glycated hemoglobin [30]. In addition, in healthy volunteers, the plasma level of XOR activity positively correlated with body mass index, insulin resistance and subclinical inflammation, whereas it negatively correlated to adiponectin level [31].
By inhibiting XOR activity with allopurinol, the control of hyperuricemia reduced systemic inflammation and insulin resistance in asymptomatic patients [32]. A positive correlation has been reported between the serum levels of XOR and C-reactive protein, glycemia, insulinemia, insulin resistance index, and triglyceride/HDL-cholesterol ratio in women with polycystic ovary syndrome that is often associated to metabolic syndrome [33]. The plasma level of XOR activity significantly correlated to body mass index, left ventricular ejection fraction and hypertrophy as well as to the level of glycated hemoglobin and aminotransferases in patients with cardiac diseases [34].
Despite the lack of evidence of a direct role of XOR and uric acid in the pathogenesis of visceral obesity, all the above reported results suggest the correlation between the metabolic syndrome and the serum level of XOR and uric acid, at least in some circumstances (as discussed in [25]).
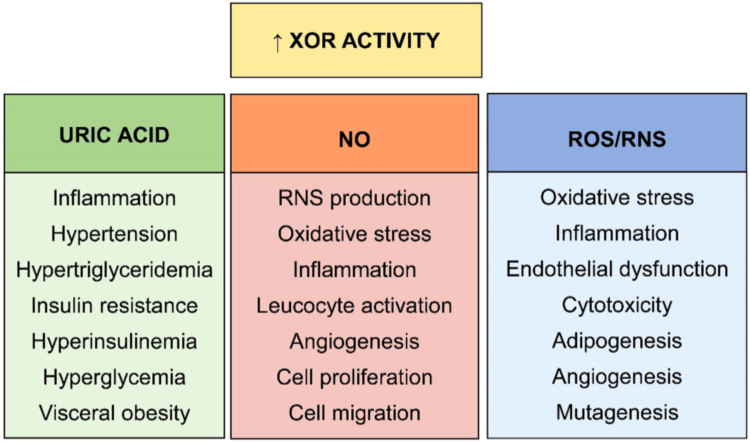
The negative consequences of increased XOR activity and exceeding production of uric acid, ROS/RNS and NO are listed in Fig. 2.
Fig. 2.
Consequences of exceeding XOR activity. Increased XOR activity may have different consequences depending on the products of the reaction. An excessive purine catabolism may result in local accumulation of uric acid and inflammation or even hyperuricemia, which favors hypertension and a series of derangements in lipidic and glycidic metabolism that may include visceral obesity. In the presence of low pH and low oxygen tension, XOR may produce NO and superoxide ion, with consequent generation of RNS. NO promotes oxidative stress, inflammation and leukocyte activation. In addition, its action includes angiogenesis, cell proliferation and migration. A high level of ROS/RNS induces oxidative stress, inflammation and even endothelial dysfunction. They also mediated various effects on cells including a cytotoxic action, adipogenesis, angiogenesis and mutagenesis.
4. Metabolic syndrome and cancer
The association between metabolic syndrome and cancer has been the object of various reviews taking in consideration the researches of the last two decades. Several evidences relate the high incidence of cancer in metabolic syndrome to an over activation of the hypothalamus-pituitary-adrenal axis leading to hypercortisolemia and immune suppression, which may favor tumorigenesis and chronic inflammation (reviewed in [35]).
The main actor in metabolic syndrome physiopathology is the adipose tissue, particularly in visceral deposits. The altered production of adipokines, cytokines and hormones, such as adiponectin, leptin, tumor necrosis factor-alfa, interleukin-6, sex hormones and insulin creates a pro-tumorigenic environment resulting in increased tumor progression and reduced patient survival (reviewed in [36]). An unbalanced production of adipokines can induce insulin resistance and hyperinsulinemia, which promote cellular growth by activating the insulin growth factors pathway, and may lead to reduced apoptosis by inhibiting the Bcl-2-associated death promoter pathway. Adipokines may also be responsible for a chronic inflammatory condition that favors cell proliferation and angiogenesis by activation of transcription factors, such as hypoxia-inducible factor-1-alfa and nuclear factor kappa-light-chain-enhancer of activated B cells, and nuclear receptors of the peroxisome proliferator-activated receptors family (reviewed in [37]).
Hyperglycemia and dyslipidemia may play a role both in tumor initiation and progression through the production of ROS that results in mutagenesis and carcinogenesis. In addition, the hyperinsulinemia consequent to obesity and insulin resistance leads to cell proliferation (reviewed in [38]). Furthermore, visceral adipose tissue is infiltrated with CD8+ T lymphocytes, natural killer cells and macrophages. These cells are responsible for the production of inflammatory cytokines and contribute to the induction of non-alcoholic steatohepatitis together with liver accumulation of free fatty acids released from adipocytes into portal vein. The chronic liver inflammation causes oxidative stress, increased cell death and regeneration leading to cirrhosis and hepatocarcinoma (reviewed in [39]).
In metabolic syndrome, a high amount of estrogens has been often reported, due to aromatase overexpression by adipocytes in visceral obesity, which has been included among the preventable causes of breast and colon cancers. In breast cancer, the altered adiponectin/leptin ratio induces an increased level of systemic and local estrogens, as well as the hyper-production of insulin and insulin-like growth factor-1 and the development of chronic inflammation (reviewed in [40]). Adipose tissue expansion in obesity can produce local hypoxia that contributes to the chronic inflammatory state with macrophages recruitment and tumor necrosis factor alpha hyper-production. Moreover, visceral obesity is associated to leptin overexpression that increases the risk of several malignancies, including colon cancer, by promoting tumor growth and progression (reviewed in [41]).
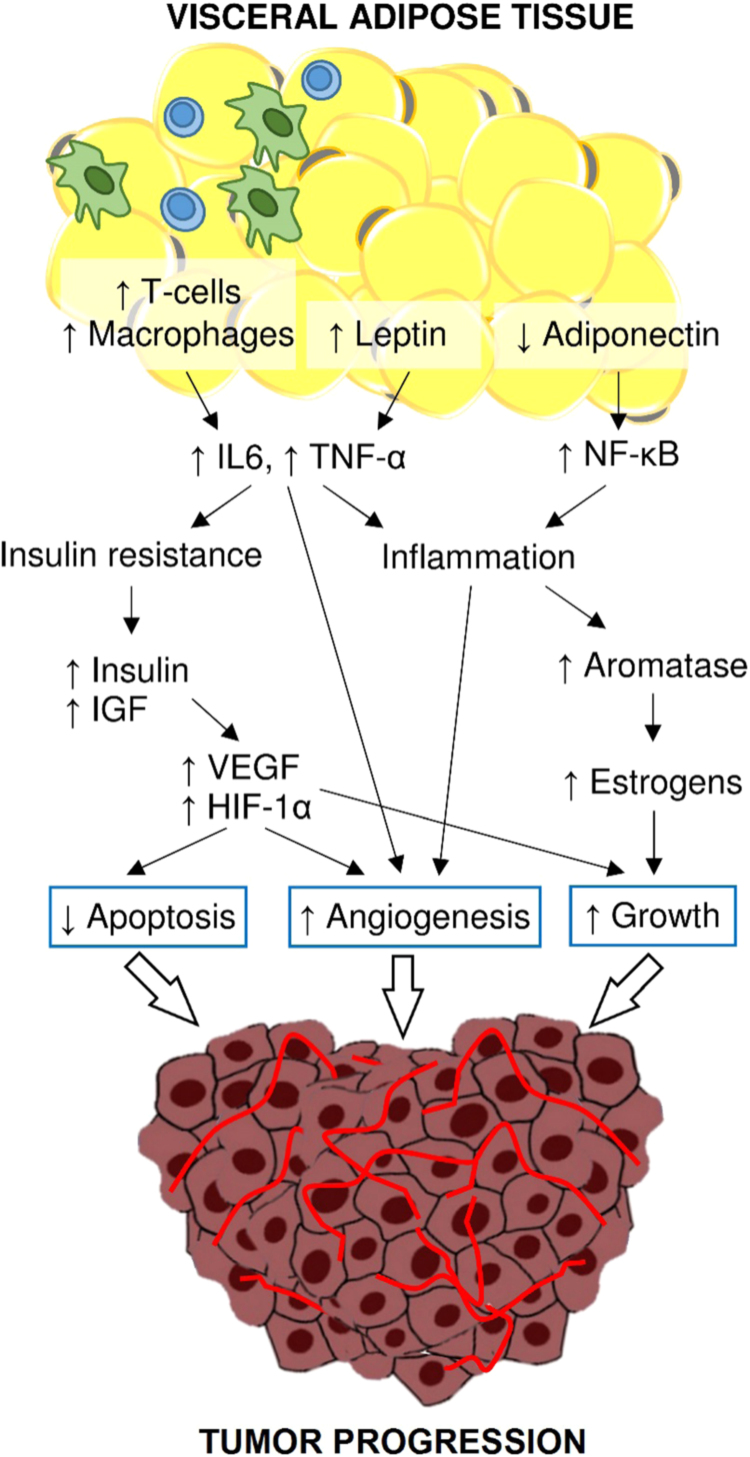
The mechanisms linking metabolic syndrome and cancer are described in Fig. 3.
Fig. 3.
Metabolic syndrome-related cancer risk. The reduced adiponectin availability abolish its anti-inflammatory and anti-proliferative actions due to the inhibition of NF-κB. The increased leptin availability together with T-cell accumulation and macrophage recruitment in adipose tissue contribute to the upregulation of TNF-α and IL-6. These cytokines have a pro-inflammatory action, promote angiogenesis and are associated with insulin resistance. Adipose tissue inflammation is responsible for increased aromatase activity and estrogens production that favor cell proliferation. Insulin resistance favors a hyper-production of insulin and IGF-1, which inhibit apoptosis, support cell proliferation and VEGF release, as well as HIF-1α activation. In turn, HIF-1α assures cell surviving and promotes angiogenesis.
5. Role of xanthine oxidoreductase in cancer risk associated to metabolic syndrome
Through prospective cohort studies and related meta-analyses, metabolic syndrome has been significantly associated with various malignancies, in particular with breast postmenopausal, colorectal, endometrial, pancreas and rectal cancers in woman and with bladder, colorectal and liver cancers in man (reviewed in [42]).
Colon cancer has a multifactorial etiology including dietary factors, genetic predisposition, microbiota alteration, obesity and chronic inflammation, such as inflammatory bowel disease. However, the neoplastic transformation is often induced by oxidative and nitrosative stress, in part derived from XOR activity (reviewed in [43]).
In susceptible subjects, the adverse outcomes of an unhealthy diet that leads to obesity may include increased risk of cancer, in addition to metabolic alterations, according to the common soil hypothesis. This theory is based on the assumption that low-grade inflammation and oxidative stress may derive from the diet, when it is hypercaloric and consists in ultra-processed food, rich of fat and poor in fiber (reviewed in [44]). As a demonstration of the accuracy of this supposition, the introduction of a healthy diet is accompanied by a reduction in both the risk of metabolic syndrome onset and cancer-related mortality (reviewed in [45]).
The well-known radical scavenging action of circulating uric acid brings down neoplastic transformation, thus it has been correlated to a reduced cancer risk, at least when uricemia is in the normal range (reviewed in [12]). However, hyperuricemia is associated to an increased cancer risk and may represent one of the factors linking diabetes, metabolic syndrome and obesity to chronic inflammation and cancer. Hyperuricemia can increase the intracellular concentration of uric acid, which reacting with ROS, NO and RNS can be responsible of oxidant and pro-inflammatory activity with consequent cell transformation. In transformed cells, an excessive intracellular concentration of uric acid promotes the downregulation of XOR expression, thus increasing the purine salvage pathway, in turn supporting cell proliferation. In addition, reduced XOR expression is associated with poor cell differentiation, increased aggressiveness and metastatic ability of breast cancer by inducing the expression of COX-2 and matrix metalloprotease (reviewed in [46]).
As outlined above, XOR expression increases in breast epithelial cells during lactation, whereas aggressive breast cancer is characterized by a downregulation of XOR expression [47]. In premenopausal women, hormonal mechanisms have been postulated to explain the well-known protective effect of breast-feeding against breast cancer [48], although its biology still remain unclear [49]. Thus, the increased expression of XOR in mammary cell during lactation could be hypothesized as one of the reasons for the protection given by breast-feeding against cancer.
Even in other tissues expressing XOR at high level, XOR activity correlates to tumor prevention, because it favors cell differentiation and suppress angiogenesis by increasing the expression of genes with anti-proliferative and anti-cancer activity. Already in the 70's it was demonstrated that XOR was downregulated in hepatocellular cancer whereas it was expressed normally in hyperplastic liver tissue [50], [51]. Accordingly, a decreased XOR expression was reported during the development and progression of cancer, also in breast, gastrointestinal and kidney tissue (reviewed in [12]). XOR downregulation results favorable to cancer cell migration and invasion, because it is associated to an increased expression of genes activating transforming growth factor-β and inducing the epithelial-mesenchymal transition (EMT) [52].
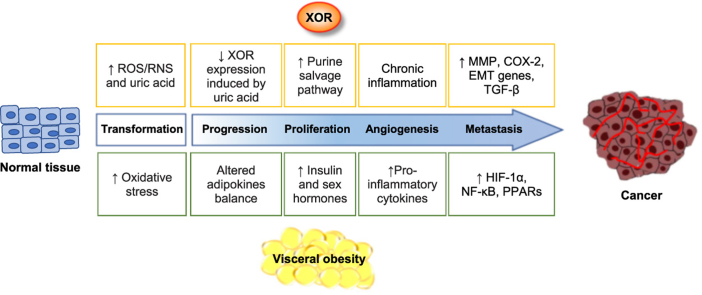
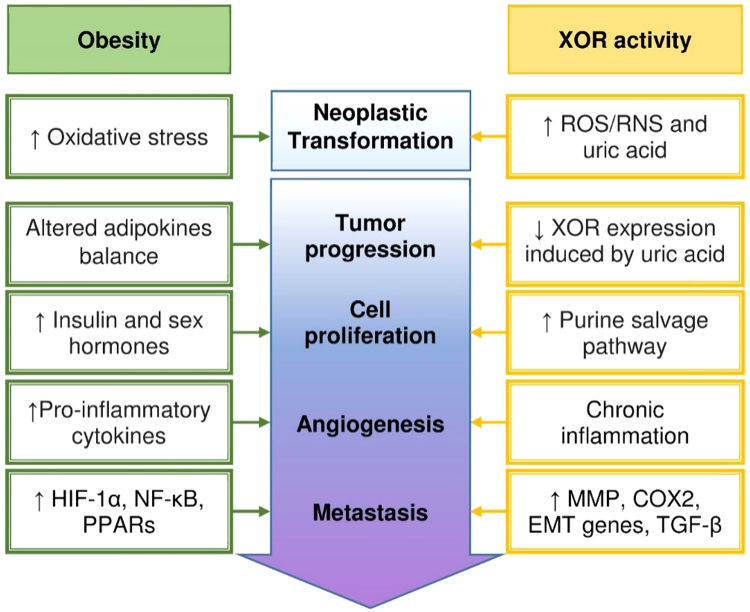
Fig. 4 describes the converging effects of both obesity and XOR activity on tumor initiation and progression, as a result of chronic inflammation and oxidative stress.
Fig. 4.
Tumor initiation and progression induced by obesity and XOR. The initial neoplastic transformation is favored by the oxidative stress that is associated with fatness or can be elicited by oxidant products of XOR activity. Tumor progression toward malignity in visceral obesity is sustained by the altered adipokine balance, resulting in increased insulin and sex hormone production that promote cell proliferation. The latter can be boosted by reduced XOR expression, which can be caused by increased intracellular concentration of uric acid, since XOR activity has a limiting role on the purine salvage pathway that in turn helps the synthesis of nucleic acids. Chronic inflammation is responsible for angiogenesis and can be the consequence of either enhanced production of pro-phlogistic cytokines by the exceeding adipose tissue or the pro-inflammatory action of uric acid and ROS/RNS generated by XOR activity. Eventually, the formation of metastasis is fostered by the activation of HIF-1α and NF-κB and the PPAR family during inflammation as well as by the increased expression of MMP, COX-2, EMT genes and TGF-β as a consequence of XOR downregulation.
6. Conclusions
The XOR catalyzes the last two steps of purine catabolism in uricothelic primates. During phylogenesis, mammalian XOR acquired the ability of producing ROS that influence the redox equilibrium and are implicated in many biological processes, including inflammation, cell proliferation and neoplastic transformation. In addition, XOR activity modulates NO availability, essential for endothelial function and vascular tone, and XOR expression controls the activation of genes involved in adipogenesis, differentiation and EMT. Hence, no surprise that XOR is called into question about both the metabolic syndrome and cancer. In addition, the final product of XOR catalysis is uric acid, which has an anti-oxidant activity in plasma, but may have an intracellular pro-oxidant activity. Hyperuricemia may contribute to the development of hypertension, visceral obesity, hypertriglyceridemia, insulin resistance, hyperinsulinemia and hyperglycemia, thus contributing to the pathogenesis of metabolic syndrome.
The incidence of metabolic syndrome is increasing, not only for the lengthening of life, but possibly also because of the diffusion of industrial food together with the reduction of physical activity, which favor the development of cardiovascular diseases, type 2 diabetes and cancer. Metabolic syndrome and cancer have in common chronic inflammation and oxidative stress, which are constantly associated to metabolic alterations and are a suitable soil for the initiation and progression of neoplasia. XOR and its products can induce both inflammation and oxidative stress, thus justifying XOR implication in the development of either metabolic syndrome or cancer. XOR activity can be pharmacologically inhibited by authorized drugs. However, it must be considered that XOR inhibition could have adverse effects by reducing cell differentiation and favoring EMT, angiogenesis and metastatic process. For this reason, at the moment, the only recommended use of XOR inhibitors concerns symptomatic hyperuricaemia.
Acknowledgements
This work was supported by funds for selected research topics from the Alma Mater Studiorum - University of Bologna and by the Pallotti Legacies for Cancer Research.
Acknowledgments
Role of the funding sources
The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Conflict of interest disclosure statement
Potential conflicts of interest do not exist for any of the authors, including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that could inappropriately influence, or be perceived to influence, their work.
Contributor Information
Maria Giulia Battelli, Email: mariagiulia.battelli@unibo.it.
Massimo Bortolotti, Email: massimo.bortolotti2@unibo.it.
Letizia Polito, Email: letizia.polito@unibo.it.
Andrea Bolognesi, Email: andrea.bolognesi@unibo.it.
References
- 1.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr International diabetes federation task force on epidemiology and prevention, National heart, lung, and blood Institute, American heart association, world heart federation, International atherosclerosis society, International association for the study of obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International diabetes federation task force on epidemiology and prevention; National heart, lung, and blood Institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Fuentes E., Fuentes F., Vilahur G., Badimon L., Palomo I. Mechanisms of chronic state of inflammation as mediators that link obese adipose tissue and metabolic syndrome. Mediat. Inflamm. 2013;2013:136584. doi: 10.1155/2013/136584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckel R.H., Grundy S.M., Zimmet P.Z. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 4.Moller D.E., Kaufman K.D. Metabolic syndrome: a clinical and molecular perspective. Annu. Rev. Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 5.Terao M., Romão M.J., Leimkühler S., Bolis M., Fratelli M., Coelho C., Santos-Silva T., Garattini E. Structure and function of mammalian aldehyde oxidases. Arch. Toxicol. 2016;90:753–780. doi: 10.1007/s00204-016-1683-1. [DOI] [PubMed] [Google Scholar]
- 6.Battelli M.G., Bolognesi A., Polito L. Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi-tasking enzyme. Biochim. Biophys. Acta. 2014;1842:1502–1517. doi: 10.1016/j.bbadis.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Nishino T., Okamoto K., Kawaguchi Y., Matsumura T., Eger B.T., Pai E.F., Nishino T. The C-terminal peptide plays a role in the formation of an intermediate form during the transition between xanthine dehydrogenase and xanthine oxidase. FEBS J. 2015;282:3075–3090. doi: 10.1111/febs.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battelli M.G., Polito L., Bortolotti M., Bolognesi A. Xanthine oxidoreductase-derived reactive species: physiological and pathological effects. Oxid. Med. Cell Longev. 2016;2016:3527579. doi: 10.1155/2016/3527579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camici M., Allegrini S., Tozzi M.G. Interplay between adenylate metabolizing enzymes and AMP-activated protein kinase. FEBS J. 2018;285:3337–3352. doi: 10.1111/febs.14508. [DOI] [PubMed] [Google Scholar]
- 10.Kelley E.E. A new paradigm for XOR-catalyzed reactive species generation in the endothelium. Pharmacol. Rep. 2015;67:669–674. doi: 10.1016/j.pharep.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battelli M.G., Polito L., Bortolotti M., Bolognesi A. Xanthine oxidoreductase in drug metabolism: beyond a role as a detoxifying enzyme. Curr. Med. Chem. 2016;23:4027–4036. doi: 10.2174/0929867323666160725091915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Battelli M.G., Polito L., Bortolotti M., Bolognesi A. Xanthine oxidoreductase in cancer: more than a differentiation marker. Cancer Med. 2016;5:546–557. doi: 10.1002/cam4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granger D.N., Kvietys P.R. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doehner W., Landmesser U. Xanthine oxidase and uric acid in cardiovascular disease: clinical impact and therapeutic options. Semin. Nephrol. 2011;31:433–440. doi: 10.1016/j.semnephrol.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Battelli M.G., Polito L., Bolognesi A. Xanthine oxidoreductase in atherosclerosis pathogenesis: not only oxidative stress. Atherosclerosis. 2014;237:562–567. doi: 10.1016/j.atherosclerosis.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin W., McRae S., Marek G., Wymer D., Pannu V., Baylis C., Johnson R.J., Sautin Y.Y. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60:1258–1269. doi: 10.2337/db10-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nomura J., Busso N., Ives A., Matsui C., Tsujimoto S., Shirakura T., Tamura M., Kobayashi T., So A., Yamanaka Y. Xanthine oxidase inhibition by febuxostat attenuates experimental atherosclerosis in mice. Sci. Rep. 2014;4:4554. doi: 10.1038/srep04554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Bassossy H.M., Shaltout H.A. Allopurinol alleviates hypertension and proteinuria in high fructose, high salt and high fat induced model of metabolic syndrome. Transl. Res. 2015;165:621–630. doi: 10.1016/j.trsl.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Aibibula Z., Ailixiding M., Iwata M., Piao J., Hara Y., Okawa A., Asou Y. Xanthine oxidoreductase activation is implicated in the onset of metabolic arthritis. Biochem. Biophys. Res. Commun. 2016;472:26–32. doi: 10.1016/j.bbrc.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 20.Tsushima Y., Nishizawa H., Tochino Y., Nakatsuji H., Sekimoto R., Nagao H., Shirakura T., Kato K., Imaizumi K., Takahashi H., Tamura M., Maeda N., Funahashi T., Shimomura I. Uric acid secretion from adipose tissue and its increase in obesity. J. Biol. Chem. 2013;288:27138–27149. doi: 10.1074/jbc.M113.485094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erdei N., Tóth A., Pásztor E.T., Papp Z., Edes I., Koller A., Bagi Z. High-fat diet-induced reduction in nitric oxide-dependent arteriolar dilation in rats: role of xanthine oxidase-derived superoxide anion. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H2107–H2115. doi: 10.1152/ajpheart.00389.2006. [DOI] [PubMed] [Google Scholar]
- 22.Gonçalves J.P., Oliveira A., Severo M., Santos A.C., Lopes C. Cross-sectional and longitudinal associations between serum uric acid and metabolic syndrome. Endocrine. 2012;41:450–457. doi: 10.1007/s12020-012-9629-8. [DOI] [PubMed] [Google Scholar]
- 23.Bjornstad P., Lanaspa M.A., Ishimoto T., Kosugi T., Kume S., Jalal D., Maahs D.M., Snell-Bergeon J.K., Johnson R.J., Nakagawa T. Fructose and uric acid in diabetic nephropathy. Diabetologia. 2015;58:1993–2002. doi: 10.1007/s00125-015-3650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King C., Lanaspa M.A., Jensen T., Tolan D.R., Sánchez-Lozada L.G., Johnson R.J. Uric acid as a cause of the metabolic syndrome. Contrib. Nephrol. 2018;192:88–102. doi: 10.1159/000484283. [DOI] [PubMed] [Google Scholar]
- 25.Battelli M.G., Bortolotti M., Polito L., Bolognesi A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim. Biophys. Acta. 2018;1864:2557–2565. doi: 10.1016/j.bbadis.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Storhaug H.M., Norvik J.V., Toft I., Eriksen B.O., Løchen M.L., Zykova S., Solbu M., White S., Chadban S., Jenssen T. Uric acid is a risk factor for ischemic stroke and all-cause mortality in the general population: a gender specific analysis from The Tromsø Study. BMC Cardiovasc. Disord. 2013;13:115. doi: 10.1186/1471-2261-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins P., Dawson J., Lees K.R., McArthur K., Quinn T.J., Walters M.R. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta-analysis. Cardiovasc. Ther. 2012;30:217–226. doi: 10.1111/j.1755-5922.2011.00277.x. [DOI] [PubMed] [Google Scholar]
- 28.Tam H.K., Kelly A.S., Metzig A.M., Steinberger J., Johnson L.A. Xanthine oxidase and cardiovascular risk in obese children. Child Obes. 2014;10:175–180. doi: 10.1089/chi.2013.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam H.K., Kelly A.S., Fox C.K., Nathan B.M., Johnson L.A. Weight loss mediated reduction in xanthine oxidase activity and uric acid clearance in adolescents with severe obesity. Child Obes. 2016;12:286–291. doi: 10.1089/chi.2015.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miric D.J., Kisic B.M., Filipovic-Danic S., Grbic R., Dragojevic I., Miric M.B., Puhalo-Sladoje D. Xanthine oxidase activity in Type 2 diabetes mellitus patients with and without diabetic peripheral neuropathy. J. Diabetes Res. 2016;2016:4370490. doi: 10.1155/2016/4370490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Washio K.W., Kusunoki Y., Murase T., Nakamura T., Osugi K., Ohigashi M., Sukenaga T., Ochi F., Matsuo T., Katsuno T., Moriwaki Y., Yamamoto T., Namba M., Koyama H. Xanthine oxidoreductase activity is correlated with insulin resistance and subclinical inflammation in young humans. Metabolism. 2017;70:51–56. doi: 10.1016/j.metabol.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 32.Takir M., Kostek O., Ozkok A., Elcioglu O.C., Bakan A., Erek A., Mutlu H.H., Telci O., Semerci A., Odabas A.R., Afsar B., Smits G., ALanaspa M., Sharma S., Johnson R.J., Kanbay M. Lowering uric acid with allopurinol improves insulin resistance and systemic inflammation in asymptomatic hyperuricemia. J. Investig. Med. 2015;63:924–929. doi: 10.1097/JIM.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 33.Isık H., Aynıoglu O., Tımur H., Sahbaz A., Harma M., Can M., Guven B., Alptekin H., Kokturk F. Is Xanthine oxidase activity in polycystic ovary syndrome associated with inflammatory and cardiovascular risk factors? Reprod. Immunol. 2016;116:98–103. doi: 10.1016/j.jri.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Fujimura Y., Yamauchi Y., Murase T., Nakamura T., Fujita S.I., Fujisaka T., Ito T., Sohmiya K., Hoshiga M., Ishizaka N. Relationship between plasma xanthine oxidoreductase activity and left ventricular ejection fraction and hypertrophy among cardiac patients. PLoS One. 2017;12:e0182699. doi: 10.1371/journal.pone.0182699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hristova M.G. Neuroendocrine and immune disequilibrium as a probable link between metabolic syndrome and carcinogenesis. Med. Hypotheses. 2018;118:1–5. doi: 10.1016/j.mehy.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 36.Doyle S.L., Donohoe C.L., Lysaght J., Reynolds J.V. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc. Nutr. Soc. 2012;71:181–189. doi: 10.1017/S002966511100320X. [DOI] [PubMed] [Google Scholar]
- 37.Mendonça F.M., de Sousa F.R., Barbosa A.L., Martins S.C., Araújo R.L., Soares R., Abreu C. Metabolic syndrome and risk of cancer: which link? Metabolism. 2015;64:182–189. doi: 10.1016/j.metabol.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 38.O'Neill S., O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 39.Zhao J., Lawless M.W. Stop feeding cancer: pro-inflammatory role of visceral adiposity in liver cancer. Cytokine. 2013;64:626–637. doi: 10.1016/j.cyto.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Argolo D.F., Hudis C.A., Iyengar N.M. The impact of obesity on breast cancer. Curr. Oncol. Rep. 2018;20:47. doi: 10.1007/s11912-018-0688-8. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz B., Yehuda-Shnaidman E. Putative role of adipose tissue in growth and metabolism of colon cancer cells. Front. Oncol. 2014;4:164. doi: 10.3389/fonc.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esposito K., Chiodini P., Colao A., Lenzi A., Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35:2402–2411. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mangerich A., Dedon P.C., Fox J.G., Tannenbaum S.R., Wogan G.N. Chemistry meets biology in colitis-associated carcinogenesis. Free Radic. Res. 2013;47:958–986. doi: 10.3109/10715762.2013.832239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esposito K., Ciardiello F., Giugliano D. Unhealthy diets: a common soil for the association of metabolic syndrome and cancer. Endocrine. 2014;46:39–42. doi: 10.1007/s12020-013-0151-4. [DOI] [PubMed] [Google Scholar]
- 45.Bellastella G., Scappaticcio L., Esposito K., Giugliano D., Maiorino M.I. Metabolic syndrome and cancer: "the common soil hypothesis". Diabetes Res. Clin. Pract. 2018;143:389–397. doi: 10.1016/j.diabres.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 46.Fini M.A., Elias A., Johnson R.J., Wright R.M. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin. Transl. Med. 2012;1:1–16. doi: 10.1186/2001-1326-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linder N., Lundin J., Isola J., Lundin M., Raivio K.O., Joensuu H. Down-regulated xanthine oxidoreductase is a feature of aggressive breast cancer. Clin. Cancer Res. 2005;11:4372–4381. doi: 10.1158/1078-0432.CCR-04-2280. [DOI] [PubMed] [Google Scholar]
- 48.Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360:187–195. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Y., Chen J., Li Q., Huang W., Lan H., Jiang H. Association between breastfeeding and breast cancer risk: evidence from a meta-analysis. Breastfeed. Med. 2015;10:175–182. doi: 10.1089/bfm.2014.0141. (Erratum in: Breastfeed Med 2015;10:288) [DOI] [PubMed] [Google Scholar]
- 50.Prajda N., Weber G. Malignant transformation-linked imbalance: decreased xanthine oxidase activity in hepatomas. FEBS Lett. 1975;59:245–249. doi: 10.1016/0014-5793(75)80385-1. [DOI] [PubMed] [Google Scholar]
- 51.Prajda N., Morris H.P., Weber G. Imbalance of purine metabolism in hepatomas of different growth rates as expressed in behavior of xanthine oxidase (EC 1.2.3.2) Cancer Res. 1976;36:4639–4646. [PubMed] [Google Scholar]
- 52.Chen G.L., Ye T., Chen H.L., Zhao Z.Y., Tang W.Q., Wang L.S., Xia J.L. Xanthine dehydrogenase downregulation promotes TGFβ signaling and cancer stem cell-related gene expression in hepatocellular carcinoma. Oncogenesis. 2017;6:e382. doi: 10.1038/oncsis.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]