Abstract
Aims
Heart failure (HF) produces left atrial (LA)-selective fibrosis and promotes atrial fibrillation. HF also causes adrenergic activation, which contributes to remodelling via a variety of signalling molecules, including the exchange protein activated by cAMP (Epac). Here, we evaluate the effects of Epac1-signalling on LA fibroblast (FB) function and its potential role in HF-related atrial remodelling.
Methods and results
HF was induced in adult male mongrel dogs by ventricular tachypacing (VTP). Epac1-expression decreased in LA-FBs within 12 h (−3.9-fold) of VTP onset. The selective Epac activator, 8-pCPT (50 µM) reduced, whereas the Epac blocker ESI-09 (1 µM) enhanced, collagen expression in LA-FBs. Norepinephrine (1 µM) decreased Epac1-expression, an effect blocked by prazosin, and increased FB collagen production. The β-adrenoceptor (AR) agonist isoproterenol increased Epac1 expression, an effect antagonized by ICI (β2-AR-blocker), but not by CGP (β1-AR-blocker). β-AR-activation with isoproterenol decreased collagen expression, an effect mimicked by the β2-AR-agonist salbutamol and blocked by the Epac1-antagonist ESI-09. Transforming growth factor-β1, known to be activated in HF, suppressed Epac1 expression, an effect blocked by the Smad3-inhibitor SIS3. To evaluate effects on atrial fibrosis in vivo, mice subjected to myocardial infarction (MI) received the Epac-activator Sp-8-pCPT or vehicle for 2 weeks post-MI; Sp-8-pCPT diminished LA fibrosis and attenuated cardiac dysfunction.
Conclusions
HF reduces LA-FB Epac1 expression. Adrenergic activation has complex effects on FBs, with α-AR-activation suppressing Epac1-expression and increasing collagen expression, and β2-AR-activation having opposite effects. Epac1-activation reduces cardiac dysfunction and LA fibrosis post-MI. Thus, Epac1 signalling may be a novel target for the prevention of profibrillatory cardiac remodelling.
Keywords: Remodelling, Atrial fibrillation, Adrenergic signalling, Atrial fibrosis, Fibroblast regulation
Introduction
Fibroblasts (FBs) contribute to normal cardiac properties and play a critical role in cardiac remodelling during pathological conditions like congestive heart failure (CHF).1,2 Under pathological stimuli, atrial FBs show greater profibrotic responses than ventricular in terms of extracellular matrix (ECM) production, FB-proliferation, and myofibroblast-differentiation.3 HF produces atrial-selective interstitial fibrosis, which contributes to the substrate for atrial fibrillation (AF).4,5 The beta-adrenergic receptor (β-AR) signalling pathway is an important regulator of cardiac function.6 In cardiac FBs, the β2-AR predominates,7 and contributes to the regulation of collagen production.8 Signalling through norepinephrine,9,10 angiotensin-II11 and transforming growth factor (TGF)-β112 influence FB function in CHF.
Exchange protein directly activated by cAMP (Epac) is a PKA-independent signalling molecule activated by adrenergic stimulation.13,14 Two isoforms have been identified, Epac1 and Epac2. Epac1 is expressed ubiquitously, particularly in cardiac cells,13 including fibroblasts.15 Previous studies have shown that Epac mediates cardiac β-adrenergic actions16,17 and decreases collagen mRNA expression in rat cardiac fibroblasts.15 Epac1-expression decreases after myocardial infarction (MI), with reduced Epac1-suppression of fibroblast collagen production potentially contributing to ventricular fibrosis.15 It is unknown whether Epac1 expression is altered under atrial profibrotic conditions and the adrenergic regulation of Epac1 in atrial fibroblasts has not been studied. Here, we aimed to clarify: (i) the changes in Epac1-expression in freshly isolated atrial and ventricular FBs from CHF-dogs; (ii) the effects of Epac1-stimulation and inhibition on FB collagen synthesis; (iii) the adrenergic regulation of Epac1-mediated control of left-atrial (LA) FB collagen production; and (iv) the effects of Epac1-stimulation on LA fibrosis post-MI in mice.
Methods
CHF model
Animal-handling procedures were approved by the Animals Research Ethics Committee of the Montreal Heart Institute and followed National Institute of Health Guidelines. CHF was induced in 47 adult male mongrel dogs (22–36 kg) by ventricular tachypacing (VTP) at 240 b.p.m. as previously described18 for 12 h (n = 8), 24 h (n = 9), 1 week (n = 9) and 2 weeks (n = 9), and the results compared to results in control dogs (n = 12). Dogs were anaesthetized with diazepam (0.25 mg/kg IV)/ketamine (5.0 mg/kg IV)/halothane (1% to 2% IP) for pacemaking lead implantation. After 24 h for post-operative recovery, ventricles were paced at 240 b.p.m. For the terminal study, dogs were anaesthetized with morphine (2 mg/kg, SC) and α-chloralose (120 mg/kg IV). The heart was excised by left thoracotomy and placed in Tyrode solution containing 2 mM Ca2+ for cell isolation.
Fibroblast isolation
The left circumflex coronary artery was cannulated and perfused with Tyrode’s solution containing 2 mmol/L Ca2+ for 15 min, followed by Ca2+-free Tyrode’s solution for 10 min and Ca2+-free Tyrode’s solution containing collagenase type-II (150 U/mL) and 0.1% albumin for 1 h. The perfusate was kept at 37°C and equilibrated with 100% O2. LA and left ventricular19 tissues were harvested by gentle trituration and centrifuged at 800 rpm (5 min) to separate cardiomyocytes from fibroblasts. The supernatant was collected and centrifuged at 2000 rpm for 10 min to pellet fibroblasts.3 Freshly isolated FBs were snap-frozen and stored at −80°C for subsequent biochemical studies [real-time polymerase chain reaction (RT-PCR) and immunoblot].
Cell-culture and drug treatment
LA-FBs were collected in Dulbecco's Modified Eagle Medium (DMEM, Invitrogen) supplemented with 10% foetal bovine serum and 1% penicillin/streptomycin. Cells were plated and cultured for 5–7 days to reach confluence and maintained in 5% CO2/95%-humidified air at 37°C. FBs were trypsinized and plated at 100 000 cells/well in 12-well plates. After 24 h for cell-adherence, FBs were maintained in serum-free medium for 24 h before treatment. Cultured FBs were incubated with various drugs (Table 1) for 48 h. In some experiments, FBs were pre-treated with a Smad3 inhibitor (SIS3, 3 μmol/L) for 1 h before adding TGFβ1 (10 ng/mL) for a 48-h period.20 Culture medium was collected to study protein secretion, and treated cells were collected for RNA extraction.
Table 1.
List of drugs, sources, and concentrations used to treat LA-FBs
| Drugs | Sources | Concentration |
|---|---|---|
| 8-pCPT-2ʹ-O-Me-cAMP (8-pCPT) | Sigma | 50 μmol/L |
| ESI-09 | EMD millipore | 1 μmol/L |
| Prazosin-hydrochloride (PZ) | Sigma | 10 μmol/L |
| Phenylephrine-hydrochloride (PE) | Sigma | 10 μmol/L |
| Norepinephrine (+)-bitartrate salt monohydrate (NE) | Sigma | 1 μmol/L |
| Isoproterenol (ISO) | Sigma | 50 μmol/L |
| (±)-1-[2,3-(Dihydro-7-methyl-1H-inden-4-yl) oxy]-3-[(1-methylethyl) amino]-2-butanol hydrochloride (ICI-118551) | EMD millipore | 2 μmol/L |
| 1-[2-((3-Carbamoyl-4-hydroxy) phenoxy) ethylamino]-3-[4- (1-methyl-4-trifluoromethyl-2-imidazolyl) phenoxy]-2-propanol dihydrochloride (CGP-20712A) | Sigma | 2 μmol/L |
| 8-Bromoadenosine 3ʹ,5ʹ-cyclic monophosphate (8-Br-cAMP) | EMD millipore | 100 μmol/L |
| Salbutamol | Sigma | 10 μmol/L |
Mouse MI-model
Sixty male C57BL/6 mice (8–10 weeks, 20–25 g, Charles River, Saint-Constant, Quebec) were divided into five groups: (i) sham (n = 10); (ii) MI+corn oil (n = 10); (iii) MI+ESI-09 (n = 14); (iv) MI+ 0.9% saline (n = 9); and (v) MI+Sp-8-pCPT (n = 17). All procedures were performed in accordance with the National Institute of Health Guidelines and were approved by the Animals Research Ethics Committee of the Montreal Heart Institute. Animals were intubated and anaesthetized with 1–2% isoflurane mixed with 1 L of oxygen per minute. MI was induced by left anterior descending coronary artery (LAD) ligation with a 10-0 prolene suture. ESI-09 dissolved in ethanol (5 mg/mL) was diluted in corn oil (1:1) and ethanol removed. ESI-09 was administered daily (IP injection) for 5 days, 20 mg/kg/day, beginning 24 h post-MI.21 The sp-8-pCPT-2’-O-Me-cAMPS Na+-salt (Sp-8-pCPT, Axxora) dissolved in 0.9% saline was delivered as a continuous infusion via osmotic minipump (1.6 µg/day, Alzet 2002; Cupertino, CA, USA) for 2 weeks. For in vivo studies we used Sp-8-pCPT, a hydrolysis-resistant form of 8-pCPT with high lipophilicity, allowing for good membrane permeability and potent Epac-stimulation. Osmotic pumps were implanted subcutaneously 24 h post-MI. ECGs and echocardiograms were obtained at baseline and 2 weeks post-MI. Right-atrial (RA) tissues were snap-frozen in liquid-N2 for biochemical analysis. LA and ventricular tissues were preserved in 10%-buffered formalin and embedded in paraffin to determine infarct size and collagen deposition (Masson’s Trichrome stain). Fibrous tissue was quantified (omitting perivascular areas) with Image-Pro 7.0 software. Infarct size was quantified microscopically on transverse sections.
RT-PCR
Total RNA was extracted with Nucleospin® RNA-kits (Macherey-Nagel). Lysis buffer was added directly to FB pellets or cultured cells. The RNA pellet was resuspended in RNA-free water and concentration determined by nanodrop. One microgram of RNA was used for cDNA-synthesis (high-capacity cDNA Archive kit, Applied Biosystems). RT-PCR was performed with TaqMan probes and primers (Applied Biosystems Foster City, CA): COL1A1 (Assay ID: cf02623126_m1), COL3A1 (Assay ID: cf02631369_m1), HPRT1 (Assay ID: Cf02626258_m1), B2M (Assay ID: cf02659077_m1) or custom-made SYBR Green primers: RAPGEF3 (Epac1, Forward primer: 5ʹGATGTGGAAGCAAGACCAT; Reverse primer: 5ʹATCACCGTATACCGGTTCCT), B-actin (Forward primer: 5ʹCAAAAGCCACCCCGTTTCT; Reverse primer: 5ʹTTCTCTTTCCCTCCCCTGTGT). Gene-expression levels were normalized to the geometric average of reference-genes.
Collagen protein quantification
Culture media were collected for collagen secretion assay by immunoblot. Forty microliters of culture medium mixed with 10 μL of 5× loading buffer were heated at 95°C for 5 min. Proteins were separated by electrophoresis on 4–20% precast gels (Mini-PROTEAN® TGXTM, Bio-Rad Laboratories, Inc.) at 120 V (80 min). Gels were transferred to nitrocellulose membranes at 70 V for 75 min in a transfer buffer with 0.1% SDS. Membranes were blocked with 3% BSA in Tris-buffered saline pH-7.4 containing 0.1% Tween-20 (TBST) overnight, followed by 1-h incubation with primary rabbit anti-mouse collagen type-I antibody (1:25 000, MD Bioproducts) at room temperature. After membranes were washed with high-salt solution followed by TBST, they were incubated with an anti-rabbit secondary conjugated to horseradish peroxidase for 1 h (1:10 000, Jackson) at room temperature.
Protein-extraction and immunoblot
Cytoplasmic proteins were extracted with an extraction buffer containing (mmol/L): 20 Tris base, 5 EDTA, 1.3 EGTA, 150 NaCl, 20 NaF, 0.2 Na3VO4, 20 glycerol-2-phosphate, 0.1 AEBSF; 25 μg/mL leupeptin, 1 μmol/L microcystin; pH adjusted to 7.34 with HCl. 1% triton X-100, 10 μg/mL aprotinin and 1 μg/mL pepstatin were added in the buffer. Protein-pellets were homogenized with insulin syringes. Homogenized proteins were incubated on ice for 30 min and triturated every 10 min. After centrifugation at 3, 000 rpm at 4°C (10 min), supernatant protein-concentration was quantified (Bradford assay). Protein-samples were loaded at 30 μg/lane, electrophoretically separated on 4–20% precast gels (Mini-PROTEAN® TGXTM, Bio-rad) and transferred to polyvinylidenedifluoride (PVDF) membranes (90 V; 75 min). Membranes were blocked with 5% non-fat dry milk in phosphate-buffered saline (pH 7.4) containing 0.1% Tween-20 (PBST) for 1 h, followed by incubation with primary antibodies [rabbit anti-RAPGEF3 (401-415, Anti-EPAC1, 1:1000, Sigma-Aldrich) and mouse anti-GAPDH (1:10 000)] in 1% non-fat dry milk in PBST at 4°C overnight. Membranes were washed and incubated with horseradish peroxidase-conjugated goat anti-rabbit or donkey anti-mouse (1:5000, Jackson Immunolabs) secondary antibodies at room temperature for 1 h. Signals were detected with Enhanced Chemiluminescence (ECL, GE Healthcare) and quantified with Bio-Rad Quantity One software. Results are expressed relative to GAPDH-bands on the same samples.
Confocal imaging
Freshly isolated LA-FBs from CTL and 2-week VTP dogs were plated in a FluoroDish Cell Culture Dish (FD35-100, World Precision Instruments) and cultured overnight in DMEM supplemented with 10%-fetal bovine serum and 1% penicillin/streptomycin. Atrial tissues from CTL and 2-week VTP dogs were preserved in 10%-buffered formalin and embedded in paraffin. Primary-cultured FBs were treated for 48 h in a FluoroDish. Plated FBs were rinsed with PBS (pH 7.4) and fixed for 10 min with precooled 1:1 acetone/methanol at -20°C. After rinsing 3 times with PBS, cells were blocked for 1 h with 5%-BSA in PBS at room temperature, followed by incubation overnight at 4°C with primary rabbit anti-RAPGEF3 (401–415, Anti-Epac1, 1:500, Sigma-Aldrich) and goat anti-vimentin (1:500, Santa Cruz) antibodies. After three washes with PBS, cells were incubated with secondary antibodies, donkey anti-goat IgG-Alexa Fluor 555, donkey anti-rabbit IgG-Alexa Fluor 488 (1:500, Invitrogen), and TO-PRO-3 iodide (1:1000, Invitrogen). Fluorescent images were obtained with an Olympus Fluoview FV1000 inverted confocal microscope. Signals were analysed with Image-J.
Data analysis
All data are expressed as mean ± SEM. Statistics were analysed with GraphPad Prism 7. Group-comparisons were performed with unpaired Student’s t-tests when only two groups were compared, one-way ANOVA followed by Dunnett’s multiple-comparison test for comparison to a single control or one-way ANOVA followed by Bonferroni correction for multiple-comparison analyses. Comparisons involving two independent variables were performed with two-way ANOVA followed by the Tukey’s test. P < 0.05 was considered statistically significant.
Results
Epac1 is strongly expressed in atrial fibroblasts and decreased in CHF
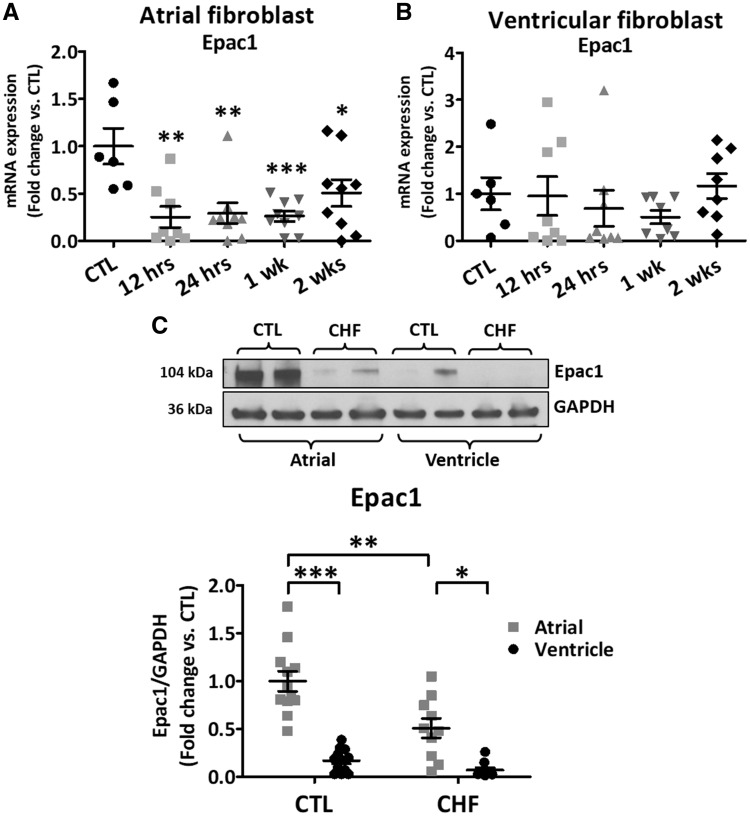
Epac1 mRNA-expression decreased rapidly with VTP in LA-FBs (3.9-fold within 12 h of VTP-onset) and remained decreased throughout VTP (Figure 1A), but did not change significantly in LV FBs (Figure 1B). Epac1-protein is much more strongly expressed in LA-FBs compared to LV (by 5.9-fold, P < 0.0001, Figure 1C) and significantly decreased in CHF LA (−2.0-fold, P < 0.001) but not LV FBs. Epac1 immunofluorescent staining was performed on LA-FBs freshly isolated from CTL and 2-week VTP dogs were cultured overnight to allow cell-adhesion to the surface. CHF cells were larger but showed less intense Epac1 staining (Supplementary material online, Figure S1A). CHF significantly increased LA-FB cell-area (by an average of 178%, Supplementary material online, Figure S1B), and decreased Epac1 expression by 61% vs. control (Supplementary material online, Figure S1C). Epac1 expression was confirmed via atrial tissue immunochemistry; Epac1 was strongly expressed in CTL and its expression decreased in CHF (Supplementary material online, Figure S2A).
Figure 1.
Epac1 expression during VTP time course. (A, B) Epac1 mRNA expression in isolated atrial and ventricular canine fibroblasts (FBs), respectively, during VTP [*P < 0.05, **P < 0.01, ***P < 0.001 vs. control (CTL), one-way ANOVA followed by Dunnett’s multiple comparison test]. (C) Freshly isolated left-atrial and ventricular fibroblasts were obtained from CTL and 2-week VTP (CHF) dogs. Top Immunoblots for Epac1 protein-expression. Bottom Epac1 protein-expression relative to GAPDH (*P < 0.01, **P < 0.001, ***P < 0.0001, two-way ANOVA followed by the Tukey’s test). Each dot represents an individual animal.
Epac1 regulates collagen expression in canine fibroblasts
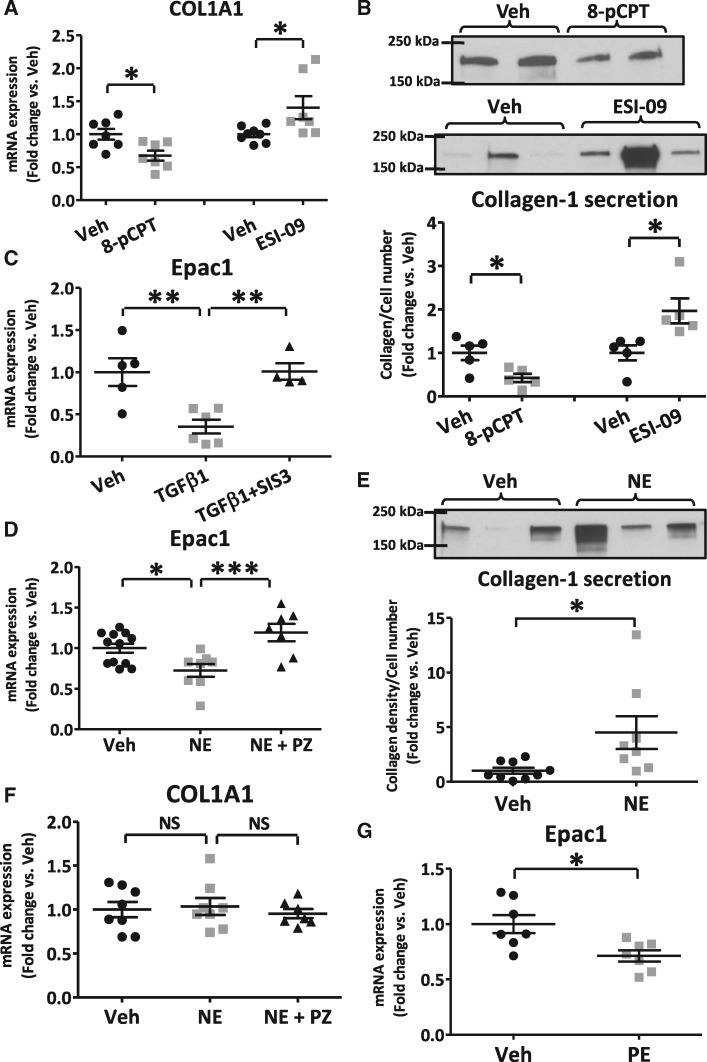
To study the effect of Epac1 on collagen expression in LA-FBs, a selective Epac1 activator, 8-pCPT (50 μmol/L) or Epac-blocker, ESI-09 (1 μmol/L) was applied to primary-cultured LA-FBs for 48 h. The Epac-activator 8-pCPT decreased collagen1A1 (COL1A1) mRNA significantly, whereas the Epac-inhibitor ESI-09 increased COL1A1 mRNA levels, by 32% and 40% respectively vs. vehicle-treated FBs (Figure 2A). Collagen-I protein expression was significantly decreased, by 58%, after 8-pCPT exposure and increased, by 97%, with ESI-09 (Figures 2B).
Figure 2.
Epac1-manipulation in vitro with and without profibrotic stimuli in isolated atrial fibroblasts. (A) Epac1-activation via 8-pCPT (50-μmol/L) significantly decreased collagen1A1 (COL1A1) and Epac1 inhibition with ESI-09 (1-μmol/L) upregulated COL1A1 mRNA-expression. (B) Representative immunoblots (top) and quantification (bottom) for collagen-I secretion. Immunoblots were performed on culture-supernatant after 48-h treatment with 8-pCPT or ESI-09. (C) Incubation with 10 ng/mL TGFβ1 decreased Epac1-expression in atrial fibroblasts, an effect blocked by pre-treatment with the Smad3-inhibitor SIS3 for 1 h before the addition of TGFβ1 for 48 h. (D) Norepinephrine (NE) significantly decreased Epac1-expression. Concomitant treatment with prazosin (PZ) and NE prevented NE-induced Epac1-reduction. (E) NE significantly increased collagen-I protein-expression. Immunoblots are shown (top), with corresponding mean data (bottom). (F) NE and concomitant treatment with PZ did not change COL1A1 mRNA-expression. (G) Phenylephrine (PE) decreased Epac1-expression. Each dot represents an individual independent experiment (*P < 0.05, **P < 0.01, ***P < 0.001; A, B, E, and G, unpaired t-test; C, D, and F, one-way ANOVA followed by Bonferroni correction).
Profibrotic stimuli decrease Epac1 expression in fibroblasts
TGFβ1 is a an important profibrotic mediator in CHF.4,22,23 TGFβ1 (10 ng/mL) significantly reduced Epac1 expression in LA-FBs by 2.8-fold (Figure 2C). This effect was prevented by the Smad3 inhibitor SIS3, indicating Smad3 involvement in downstream signalling of the TGFβ1-effect on Epac1-expression. Norepinephrine (NE) increases collagen gene-expression upon in vivo intravenous injection in rats.24 We therefore assessed the effect of NE on Epac1-expression. NE suppressed Epac1 mRNA-expression (28% reduction, P < 0.05, Figure 2D) (1 μmol/L), while increasing collagen-I protein expression (4.5-fold, Figure 2E). NE-exposure did not change the COL1A1-mRNA level (Figure 2F), indicating post-transcriptional regulation. Concomitant treatment of LA-FBs with NE and prazosin (PZ), an α-adrenergic receptor-blocker, prevented the NE-induced decrease in Epac1-expression (Figure 2D), but did not change the level of COL1A1 mRNA (Figure 2F). The α-AR-agonist phenylephrine (PE) also reduced Epac1-expression in LA-FBs (Figure 2G), confirming the α-adrenergically mediated effect.
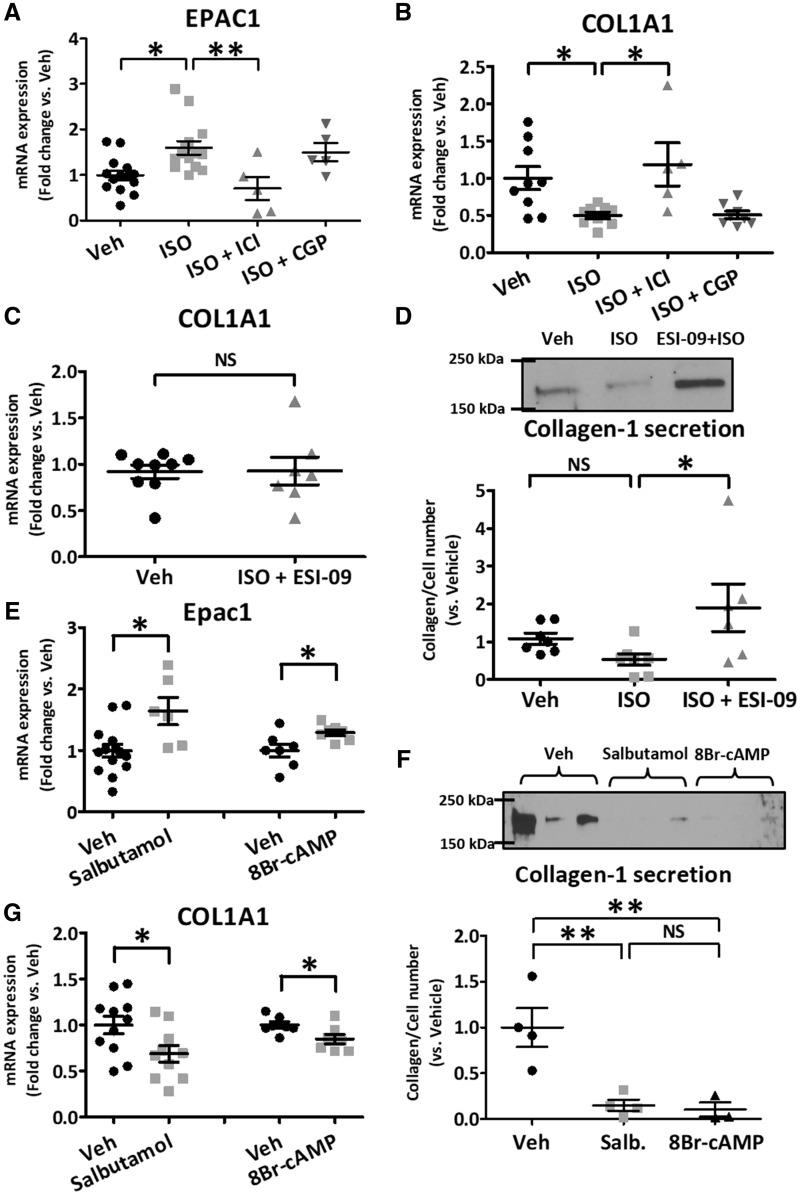
Isoproterenol regulates collagen expression via β2-adrenergic receptors in fibroblasts
To evaluate the β-AR regulation of Epac1 and associated effects on collagen expression, we studied responses to isoproterenol and other β-AR activators or blockers in primary-cultured LA-FBs. Isoproterenol significantly increased Epac1 mRNA-expression (Figure 3A), an effect antagonized by the β2-AR antagonist ICI-118551 (2 µmol/L). Concomitant treatment of LA-FBs with isoproterenol and the β1-AR antagonist CGP-20712A (2-µmol/L) did not alter the isoproterenol-induced increase in Epac1-expression. Collagen expression significantly decreased after β-AR-activation with isoproterenol (Figure 3B), an effect blocked by ICI-118551 but not CGP-20712A. To evaluate the role of Epac1 in isoproterenol-regulation of collagen expression, we studied the effect of the Epac1-blocker ESI-09. We found that even though isoproterenol significantly reduced COL1A1 mRNA in Figure 3B, concomitant treatment with isoproterenol and ESI-09 had no effect on COL1A1 mRNA (Figure 3C). The isoproterenol effect to decrease collagen protein expression was antagonized by ESI-09 (Figure 3D), indicating that this action is mediated via Epac.
Figure 3.
Activation of β-adrenergic receptors and cAMP modulate Epac1 and collagen expression in isolated atrial fibroblasts. (A) Isoproterenol (ISO, 10-µmol/L) increased Epac1 expression, an effect antagonized by ICI-118551 (β2-AR antagonist, 2 µmol/L), but not by CGP-20712A (β1-AR antagonist, 2 µmol/L). (B) ISO decreased collagen1A1 (COL1A1)-expression, an effect blocked by ICI-118551 but not by CGP-20712A. (C) Concomitant treatment with ISO and an Epac1 blocker (ESI-09) did not change collagen-gene expression vs. vehicle (Veh). (D) Immunoblots (top) and quantification for collagen-I protein-secretion (bottom) after treatment with ISO and ISO + ESI-09. (E) β2-AR stimulation with salbutamol (10 µmol/L) and cAMP-signalling activation with 8-bromo-cAMP (8Br-cAMP, 100 μmol/L) increased Epac1 mRNA-expression. (F) Immunoblots (top) and quantification (bottom) of collagen-I protein-secretion after treatment with salbutamol (Salb.) or 8Br-cAMP. (G) Salbutamol and 8Br-cAMP reduced COL1A1 mRNA-expression. (*P < 0.05, **P < 0.01; A, B, D, and F, one-way ANOVA followed by Bonferroni correction; C, E, and G, unpaired t-test). Each dot represents an independent experiment.
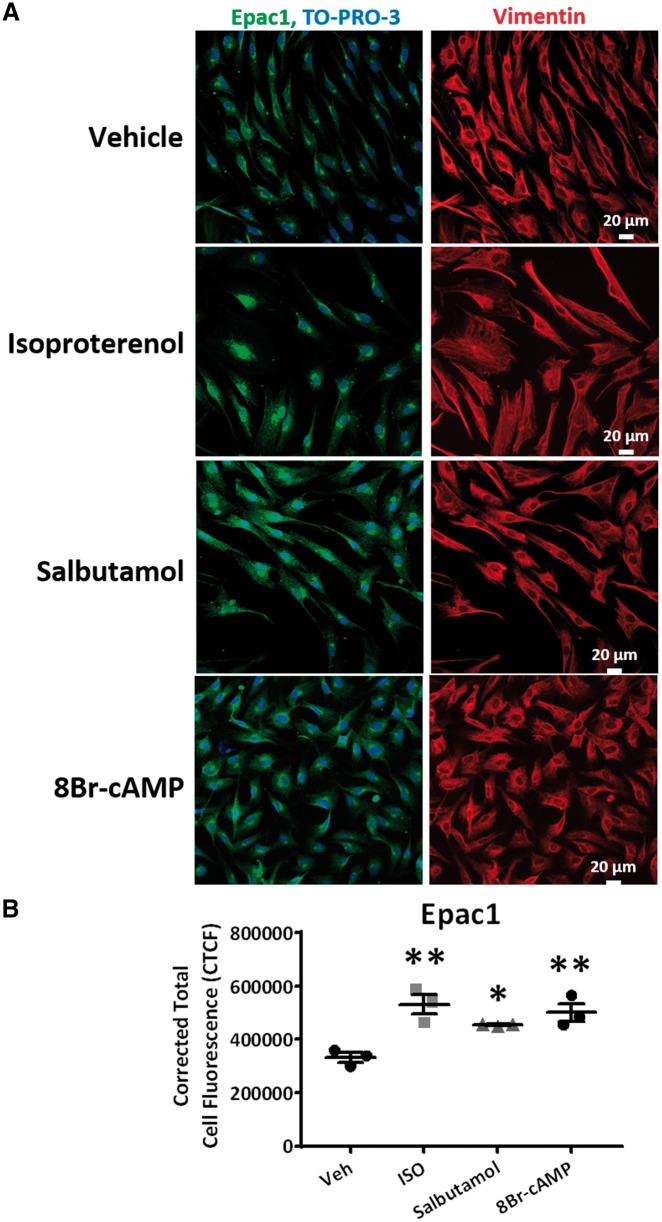
β2-AR-activation by salbutamol and cAMP-signalling enhancement with 8Br-cAMP mimicked the effects of isoproterenol to increase Epac1-expression (Figure 3E) and decrease collagen-secretion (Figure 3F). Furthermore, salbutamol and 8Br-cAMP substantially decreased collagen mRNA-expression (Figure 3G). The effects of salbutamol were blocked by ESI-09 (Supplementary material online, Figure S3A,B). The increase in Epac1-expression upon activation of β2-ARs with the agonists isoproterenol and salbutamol, and in response to enhanced cAMP-signalling with 8Br-cAMP, was confirmed via immunohistochemistry and confocal imaging (Figure 4). These results indicate that isoproterenol’s effect to reduce collagen expression is mediated through β2-AR-mediated enhancement of Epac expression and signalling in LA-FBs.
Figure 4.
Immunostaining for Epac1 expression after activation of β-adrenergic receptors and cAMP in isolated atrial fibroblasts. (A) Expression of Epac1 (green) merged with a nuclear stain, TO-PRO-3 (blue), in atrial fibroblasts. Vimentin (red) was used as a fibroblast stain. (B) Analysis of corrected total cell fluorescence (CTCF) in vehicle (Veh), isoproterenol (ISO), salbutamol, or 8Br-cAMP treatments (*P < 0.05, **P < 0.01 vs. Veh, one-way ANOVA followed by Dunnett’s multiple comparison test). Each dot represents the mean data for a single independent experiment (n = 3 animals) calculated by averaging the results from 8 to 26 cells per treatment.
Epac1-stimulation decreases LA fibrosis and improves cardiac remodelling in MI mice
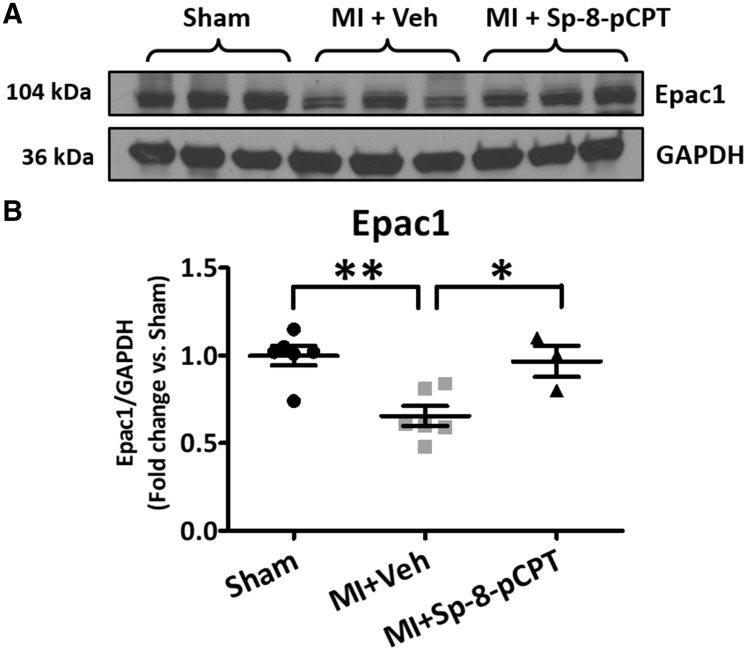
The heart-weight to body-weight (HW/BW) ratio averaged 5.2 ± 0.2 mg/kg in sham-mice and was significantly increased in the MI group (to 6.8 ± 0.5 mg/kg, P < 0.01). To study the effect of Epac1 regulation in vivo in MI-mice, an Epac1 inhibitor (ESI-09) or activator (Sp-8-pCPT) was administered with parallel corn oil-vehicle or saline-vehicle administration, respectively. Survival was quantitatively lower in the ESI-09 group (Supplementary material online, Figure S4A) and higher in the Sp-8-pCPT group (Supplementary material online, Figure S4B) compared to their respective vehicle-controls; however, none of the differences were statistically significant. There were no significant differences between the two vehicle groups (Supplementary material online, Figures S4C,D and S5), and the vehicle groups were combined into a single comparator control group for the drug-treatment responses. The administration of Sp-8-pCPT post-MI prevented the MI-induced increase in the HW/BW ratio (mean value in the Sp-8-pCPT group 5.2 ± 0.1 mg/kg, P < 0.001 vs. MI + vehicle); whereas ESI-09 left HW/BW unaltered compared to MI + vehicle (mean value 7.1 ± 0.4 mg/kg, P = NS vs. MI + vehicle). Atrial Epac1 protein-bands were less intense in MI-vehicle rats; this difference was reversed by Sp-8-pCPT (Figure 5A) but not ESI-09 (Supplementary material online, Figure S6). Overall, MI-alone significantly decreased Epac1 expression, by 34% (Figure 5B). The activation of Epac1 with Sp-8-pCPT reversed the MI-induced down-regulation of Epac1.
Figure 5.
In vivo Epac1 manipulation in myocardial infarction mouse-model. (A) Immunoblots for Epac1 protein expression were obtained in right atrium at 2 weeks post-myocardial infarction (MI) in sham, MI + vehicle (Veh) and MI + Sp-8-pCPT groups. (B) Quantification for Epac1 protein expression. MI significantly decreased Epac1 protein expression, compared to sham-operated group. Sp-8-pCPT prevented Epac1 downregulation with MI (*P < 0.05, **P < 0.01, one-way ANOVA followed by Bonferroni correction). Each dot represents an individual animal.
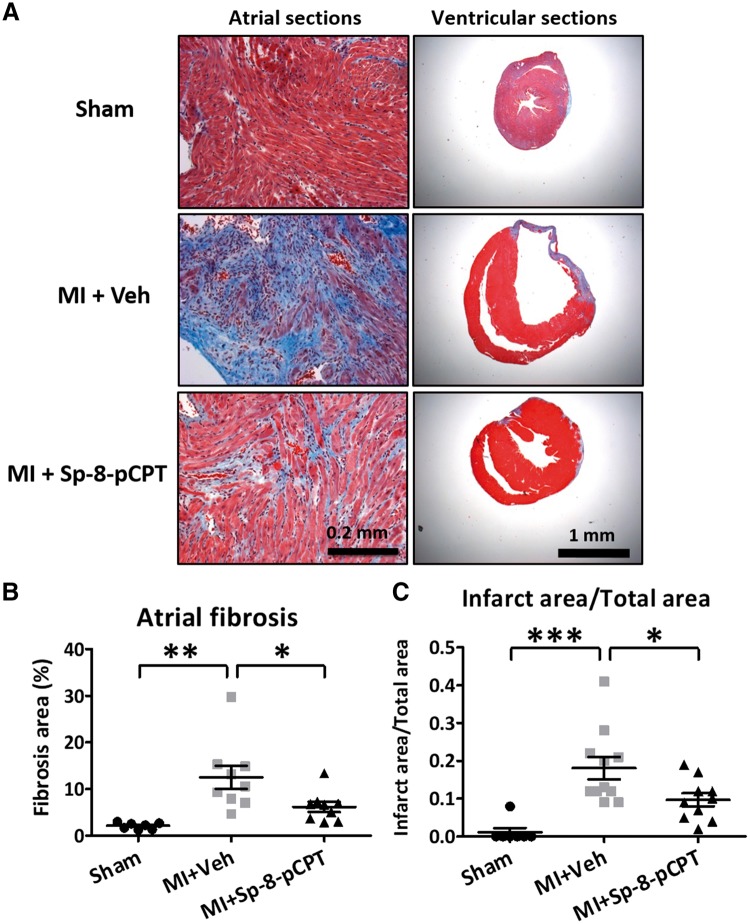
Examples of Masson trichrome-stained LA and ventricle samples are shown in Figure 6A. LA fibrosis was increased five-fold in MI + vehicle mice (P < 0.01) vs. shams (Figure 6B). This effect was attenuated in the Sp-8-pCPT-group, but not significantly affected by ESI-09 (Supplementary material online, Figure S7A,B). An average of about 18% of the ventricle was infarcted in MI-vehicle mice (Figure 6C); infarct-area was significantly reduced by Sp-8-pCPT. ESI-09 did not significantly alter infarct-size (Supplementary material online, Figure S7A,C).
Figure 6.
Histological examination of the collagen content by Masson’s Trichrome staining from left atrium and ventricle in myocardial infarction mouse study. (A) Heart sections from left atrium and ventricle in sham, 2 weeks post-myocardial infarction (MI), MI + vehicle (Veh), and MI + Sp-8-pCPT groups. Representative images obtained with a 20× objective lens for atrium and 4× objective lens for transversely ventricle sections. (B) Quantification of atrial fibrosis as a percentage of total cross-sectional area, excluding blood vessels. (C) The proportion of infarct area in the left ventricle to the total area as a function of cross-sectional area. (*P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA followed by Bonferroni correction). Each dot represents an individual animal.
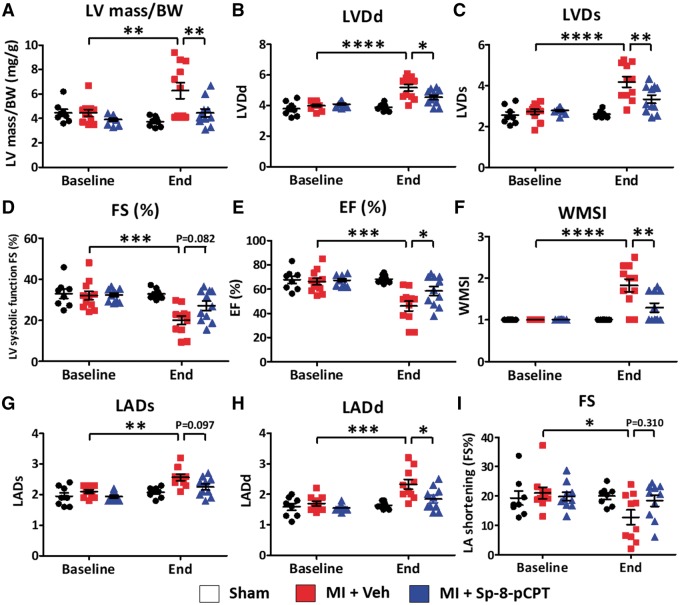
Sp-8-pCPT improved post-MI induced remodelling and dysfunction, decreasing LV mass to body weight ratio (Figure 7A), reducing LV dilation (Figures S7B,C), improving LV systolic function (Figure 7D–F), and virtually normalizing LA properties (Figure 7G–I). MI-mice treated with ESI-09 did not have different echocardiographic outcomes vs. MI + vehicle (Supplementary material online, Figure S8).
Figure 7.
Effect of Sp-8-pCPT on cardiac function. Echocardiography was performed at baseline and 2 weeks post-myocardial infarction (MI). (A) Ratio of left ventricular mass to body weight (BW) was increased 2 weeks after MI; this increase was prevented by Sp-8-pCPT. (B–H) Sp-8-pCPT prevented MI-induced impairment in LV systolic function, LV dilation, left-atrial dilation, and wall-motion abnormalities. (I) Sp-8-pCPT did not change LA shortening. (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, two-way ANOVA followed by Tukey’s test, n = 7–11 mice/group.) Each dot represents an individual animal. LVDd, left ventricular dimension at end-diastole; LVDs, left ventricular dimension at end-systole; FS, fractional shortening; EF, ejection fraction; WMSI, wall motion score index; LADs, left atrial dimension at the end of ventricular systole; LADd left atrium dimension at the end of ventricular diastole.
Discussion
Here, we have investigated for the first time the changes in Epac1-expression in freshly isolated atrial fibroblasts during the evolution of an atrial fibrotic substrate for AF in dogs. We evaluated the regulation of Epac1-expression in atrial fibroblasts and its role in the adrenergic control of collagen secretion, and assessed the results of Epac1 activation and inhibition in an in vivo model of LV-dysfunction associated with atrial fibrosis. A summary of our findings regarding Epac regulation in LA-FBs is shown in Supplementary material online, Figure S9. Epac1 is under complex control by the adrenergic nervous system, with β2-AR-activation enhancing Epac1-expression and reducing LA-FB collagen secretion via cAMP-signalling and α-AR-activation suppressing Epac1-expression and collagen secretion respectively. Similarly, TGFβ suppresses Epac1-expression through Smad3-signalling, enhancing LA-FB collagen-secretion. LA-FB (but not LV-FB) Epac1-expression is rapidly downregulated, and remains decreased throughout the development of CHF, in a well-established dog model exhibiting marked LA-selective fibrosis.5
These results point to an antifibrotic function of Epac1, with its downregulation playing a potential role in the development of profibrillatory atrial fibrosis in our dog model. In an in vivo mouse model, restoration of Epac1-expression with an Epac1-activator attenuated LA fibrosis, while revealing for the first time an infarct-size limiting and cardiac function-preserving effect of Epac1-activation post-MI.
Control of cardiac fibroblast-function by Epac1-regulation
Consistent with our findings, prior work shows that β2-ARs are the predominant β-AR subtype in cardiac FBs25–27 and that β2-ARs are involved in controlling proliferation,26 autophagy,28 and cytokine/growth-factor secretion of cardiac FBs.29
Increased intracellular cAMP-levels prevent FB-to-myofibroblast transformation and produce antifibrotic effects, including inhibition of ECM-synthesis, attenuation of FB-proliferation, and fibrosis.30–32 The action of cAMP was classically attributed to protein-kinase A (PKA)33; however, there is increasing awareness of the role of Epac as a downstream mediator of cAMP-effects.34–36 Studies with rat ventricular FBs reveal that both PKA and Epac inhibit collagen and DNA synthesis, but have opposing actions on fibroblast migration.15,37,38
Our work is the first of which we are aware to analyse the regulation of Epac1 in atrial FBs. Our results regarding β-adrenergic activation are compatible with prior results in ventricular FBs. Recent work suggests that α-AR stimulation activates cardiac FBs through a Ca2+/calcineurin pathway.39 Our studies point to Epac1-suppression as a potential mediator of α-adrenergic fibroblast activation.
Since enhanced sympathetic tone is a cardinal feature of CHF, our results are relevant to the control of atrial fibrotic remodelling in CHF.
Epac1 regulation in cardiac remodelling and AF
Fibrotic remodelling plays a key role in AF-pathophysiology.35 Understanding the molecular control mechanisms that govern FB-function and atrial fibrosis is important for the development of new therapeutic tools and approaches. This study is the first to provide evidence for Epac1 as a significant determinant of atrial FB function and as a factor in atrial fibrosis. We found that Epac1-signalling inhibits LA-FB collagen-secretion, and that inhibiting Epac-1 signalling promotes collagen production. A strength of our study was the extensive use of freshly isolated FBs, since cell-culture can alter FB properties and obscure changes caused by remodelling.36 Atrial FB Epac1-expression was downregulated in two animal models featuring atrial fibrosis. Enhancement of Epac signalling via sustained release of an Epac-selective agonist suppressed atrial fibrogenesis in a mouse model of LV dysfunction.
Epac1-stimulation produced strong cardioprotective effects against adverse LV remodelling post-MI. MI-area was reduced (Figure 6) and many deleterious functional changes were attenuated (Figure 7). To our knowledge, this is the first such observation. Prior studies have pointed towards cardioprotective potential of Epac. A phosphodiesterase-4 inhibitor protected against nitrous oxide-mediated apoptosis in H9c2 cells and neonatal rat cardiomyocytes, in part by enhancing cAMP-mediated Epac/Akt-dependent signalling.38 Mice overexpressing a PKA inhibitory peptide were protected against isoproterenol-induced fibrosis, apoptosis, and cardiac dysfunction, suggesting cardioprotective effects of the other (Epac) pathway.40 In cultured adult feline cardiomyocytes, PKA-inhibition unmasked a protective effect of β-AR-activation against cell-death caused by elevated extracellular Ca2+-concentration mediated via Epac-dependent signalling.40 In a study by Okumura et al.,41 global Epac1 knockout mice showed a smaller magnitude of fibrosis compared to wild-type mice following aortic banding. Additionally, there was a smaller increase in the number of apoptotic cells in the Epac1 knockout group with pressure overload. Cardiac fibrosis and apoptosis were similarly reduced following chronic isoproterenol infusion in Epac1 knockout mice vs. wild-type mice. Laurent et al.42 similarly showed reduced fibrosis in Epac1 knockout mice with chronic isoproterenol infusion. Autophagy was reduced in isoproterenol-treated Epac1 knockout mice, leading to less cardiomyocyte loss. In both studies the reduction in fibrosis was likely due to decreased replacement fibrosis following cardiomyocyte cell death. These results contrast with our finding of significant cardioprotective effects of Epac1-activation following myocardial infarction. Further studies with specific knockdown of Epac1 in fibroblasts vs. cardiomyocytes are required to elucidate the cell-type specific role of Epac1 in the heart.
Our findings point to the complexity of Epac-mediated effects in cardiac pathology. In earlier work, we noted that increased Epac1-expression in guinea pig ventricular cardiomyocytes decreases slow delayed-rectifier K+ current, creating a potentially arrhythmogenic substrate.43 Similarly, although PKA-activation has direct effects to reduce isolated FB collagen-secretion,15,33 its dominant in vivo action is to promote replacement fibrosis by causing cardiomyocyte death and LV-dysfunction.39 Our demonstration of strong cardioprotective effects of Epac-activation post-MI emphasizes the importance of further work to better understand the mechanisms of Epac-mediated cardioprotection and their potential translational value.
Previous studies have principally examined the effects of Epac1 signalling on cardiomyocytes. Accumulating evidence shows that Epac is involved in Ca2+ handling, as well as K+ channel and Na+ channel function.44,45 Epac activation induces SR Ca2+ leak via Ca2+/calmodulin kinase-II (CaMKII) and increases susceptibility to DADs.44 Epac 1 stimulation decreases IKs and prolongs action potential duration, increasing susceptibility to early after depolarizations.43 Additionally, enhancement of late Na+-current via CAMKII also plays a role in the development of arrhythmias.46 More recent studies have shown that mitochondrial Epac1 is protective against ischaemia-induced myocardial death, decreasing infarct size, and cardiomyocyte apoptosis.47 Further studies with cell-type specific knockout or conditional knockout mice are required to elucidate the role of Epac in cardiomyocytes vs. fibroblasts. Our study suggests that restoration of Epac1 expression post-MI has an anti-fibrotic and anti-remodelling effect.
Potential limitations
We performed in vivo studies in a mouse MI-model to assess the effect of Epac1 activation and inhibition on atrial remodelling. Because of the small size of the animals and the miniscule amount of atrial tissue available, we used all of the LA-tissue samples for the analysis of atrial fibrosis, and the right-atrial for Epac1 immunoblots; we did not have remaining tissue with which to analyse associated changes in signalling.
While Epac-activation with 8pCPT produced cardioprotective effects, we did not observe deleterious effects of Epac-inhibition with ESI-09. A number of factors may have contributed to this negative outcome. Epac-expression was significantly downregulated in MI- further inhibition might thus produce smaller changes in cardioprotection-indices that are below the level of detection. The increased mortality with ESI-09 (Supplementary material online, Figure S4A) might have led to the selective death of mice with larger MIs, biasing the results by allowing only mice with smaller initial infarctions to survive. Since mice with large MIs and poor LV-function are much more likely to die, an intervention that increases MI-size and worsens LV-function may manifest its effects by worsening mortality rather than showing deleterious effects on MI-size/LV-function in surviving animals. Many more animals than studied here would be needed to directly address this possibility in a critical way.
Another potential limitation of our study is the use of pharmacological activators and inhibitors to elucidate the role of Epac1 and Epac2 in cardiac fibrosis. Yokoyama et al.15 have demonstrated that Epac1 and not Epac2 is preferentially reduced in response to profibrotic stimuli such as TGF-beta. Additionally, Epac1 is more abundantly expressed in fibroblasts than Epac2. In their study Epac1 overexpression protects against fibrosis induced by TGF-beta1. A whole-body Epac1 knockout mouse model showed protection against fibrosis in a chronic isoproterenol perfusion and chronic pressure overload model (not Epac2 KO41). Given the higher expression of Epac1 in fibroblasts and the importance of Epac1 signalling in fibrosis (based on previous studies), non-selective activation of Epac via 8-pCPT should not affect our overall understanding of this pathway. However, specific overexpression of Epac1 and Epac2 in atrial fibroblasts could further strengthen our understanding of the role of Epac in cardiac fibrosis. CE3F4, a selective antagonist of Epac1, produced a similar increase in collagen 1 in atrial fibroblasts in a dose dependent manner (Supplementary material online, Figure S3D). Future studies will benefit from specific siRNA knockdown of Epac1 and Epac2 in atrial fibroblasts.
Profibrotic stimuli differentially affect Epac1 expression in fibroblasts vs. myofibroblasts.48 Epac1 expression was less reduced in HF LA-FBs at 2 weeks than at earlier time-points; this may reflect a greater degree of myofibroblast differentiation at the later time-point. The detailed mechanisms underlying adrenergic regulation of Epac1 expression would be an interesting area to examine; however, this would require detailed analyses of downstream signalling pathways for both beta- and alpha-adrenergic systems, which while interesting is out of the scope of the present study. We found that Epac inhibitors and agonists change Epac1 expression in the same direction as the change in effect. This regulation of Epac expression in the same direction as that of functional change should provide positive feedback and is an interesting biological response that merits further study but is out of the scope of the present manuscript.
Finally, while the cardioprotective effects of Epac-1 stimulation post-MI that we noted are interesting, novel and potentially important, they limit our ability to draw conclusions about the mechanisms through which Epac-activation reduced LA fibrosis in vivo. While the results are compatible with the predicted inhibition of LA-FB collagen production by enhanced Epac-signalling, a significant proportion of the antifibrotic effect may have been due to attenuation of MI-induced LV dysfunction. Further studies in genetic models targeting Epac activation and suppression selectively to atria vs. ventricles, and to cardiomyocytes vs. FBs, are needed to clarify these issues.
Conclusions
Here, we characterized the regulation of LA-FB function by Epac1, finding that Epac1-enhancement suppresses profibrotic properties while Epac1-suppression increases collagen secretion. Epac1 expression-changes in response to adrenergic mediators and TGFβ likely contribute to the FB-mediated fibrosis-modulating effects of these biologically important agents. LA-FB Epac1-expression is downregulated in dogs with atrial fibrotic remodelling, potentially contributing to fibrosis development. In mice with acute MIs, enhancing Epac-signalling prevents LA fibrosis and shows cardioprotective actions. Additional work in genetic models with tissue and cell-type selective Epac-modulation is needed to clarify the mechanisms and translational potential of antifibrotic and cardioprotective Epac effects.
Supplementary Material
Acknowledgements
We thank Jiening Xiao, Nathalie L’Heureux, and Chantal St-Cyr for technical assistance and suggestions and Jennifer Bacchi for secretarial support.
Conflict of interest: none declared.
Funding
This work was supported by the Canadian Institutes of Health Research [Grant Number MOP6957 to S.N.]; Heart and Stroke Foundation of Canada [Grant Number G-15-0008895 to S.N.]; Thailand Research Fund-Royal Golden Jubilee PhD Program [To S.S. and N.C.]; National Science and Technology Development Agency Thailand [NSTDA Research Chair Grant to N.C.] and Fonds de recherche du Québec – Santé [Post-Doctoral Fellowship to A.T].
Footnotes
Time for primary review: 31 days
References
- 1. Baudino TA, Carver W, Giles W, Borg TK.. Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol 2006;291:H1015–H1026. [DOI] [PubMed] [Google Scholar]
- 2. Camelliti P, Borg TK, Kohl P.. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res 2005;65:40–51. [DOI] [PubMed] [Google Scholar]
- 3. Burstein B, Libby E, Calderone A, Nattel S.. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation 2008;117:1630–1641. [DOI] [PubMed] [Google Scholar]
- 4. Burstein B, Nattel S.. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol 2008;51:802–809. [DOI] [PubMed] [Google Scholar]
- 5. Hanna N, Cardin S, Leung TK, Nattel S.. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res 2004;63:236–244. [DOI] [PubMed] [Google Scholar]
- 6. Lymperopoulos A, Rengo G, Koch WJ.. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res 2013;113:739–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meszaros JG, Gonzalez AM, Endo-Mochizuki Y, Villegas S, Villarreal F, Brunton LL.. Identification of G protein-coupled signaling pathways in cardiac fibroblasts: cross talk between G(q) and G(s). Am J Physiol Cell Physiol 2000;278:C154–C162. [DOI] [PubMed] [Google Scholar]
- 8. Ostrom RS, Naugle JE, Hase M, Gregorian C, Swaney JS, Insel PA, Brunton LL, Meszaros JG.. Angiotensin II enhances adenylyl cyclase signaling via Ca2+/calmodulin. Gq-Gs cross-talk regulates collagen production in cardiac fibroblasts. J Biol Chem 2003;278:24461–24468. [DOI] [PubMed] [Google Scholar]
- 9. Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T.. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984;311:819–823. [DOI] [PubMed] [Google Scholar]
- 10. Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J.. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 2009;54:1747–1762. [DOI] [PubMed] [Google Scholar]
- 11. Brilla CG, Zhou G, Matsubara L, Weber KT.. Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol 1994;26:809–820. [DOI] [PubMed] [Google Scholar]
- 12. Petrov VV, Fagard RH, Lijnen PJ.. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension 2002;39:258–263. [DOI] [PubMed] [Google Scholar]
- 13. Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM.. A family of cAMP-binding proteins that directly activate Rap1. Science 1998;282:2275–2279. [DOI] [PubMed] [Google Scholar]
- 14. de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL.. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998;396:474–477. [DOI] [PubMed] [Google Scholar]
- 15. Yokoyama U, Patel HH, Lai NC, Aroonsakool N, Roth DM, Insel PA.. The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proc Natl Acad Sci USA 2008;105:6386–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci 2006;31:680–686. [DOI] [PubMed] [Google Scholar]
- 17. Metrich M, Lucas A, Gastineau M, Samuel JL, Heymes C, Morel E, Lezoualc'h F.. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res 2008;102:959–965. [DOI] [PubMed] [Google Scholar]
- 18. Dawson K, Wakili R, Ordog B, Clauss S, Chen Y, Iwasaki Y, Voigt N, Qi XY, Sinner MF, Dobrev D, Kaab S, Nattel S.. MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation. Circulation 2013;127:1466–1475. [DOI] [PubMed] [Google Scholar]
- 19. Yang Y, Yang F, Wu X, Lv X, Li J.. EPAC activation inhibits acetaldehyde-induced activation and proliferation of hepatic stellate cell via Rap1. Can J Physiol Pharmacol 2016;94:498–507. [DOI] [PubMed] [Google Scholar]
- 20. Jinnin M, Ihn H, Tamaki K.. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol 2006;69:597–607. [DOI] [PubMed] [Google Scholar]
- 21. Singhmar P, Huo X, Eijkelkamp N, Berciano SR, Baameur F, Mei FC, Zhu Y, Cheng X, Hawke D, Mayor F Jr, Murga C, Heijnen CJ, Kavelaars A.. Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1. Proc Natl Acad Sci USA 2016;113:3036–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lim H, Zhu YZ.. Role of transforming growth factor-beta in the progression of heart failure. Cell Mol Life Sci 2006;63:2584–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Border WA, Noble NA.. Fibrosis linked to TGF-beta in yet another disease. J Clin Invest 1995;96:655–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhambi B, Eghbali M.. Effect of norepinephrine on myocardial collagen gene expression and response of cardiac fibroblasts after norepinephrine treatment. Am J Pathol 1991;139:1131–1142. [PMC free article] [PubMed] [Google Scholar]
- 25. Gustafsson AB, Brunton LL.. beta-adrenergic stimulation of rat cardiac fibroblasts enhances induction of nitric-oxide synthase by interleukin-1beta via message stabilization. Mol Pharmacol 2000;58:1470–1478. [DOI] [PubMed] [Google Scholar]
- 26. Turner NA, Porter KE, Smith WH, White HL, Ball SG, Balmforth AJ.. Chronic beta2-adrenergic receptor stimulation increases proliferation of human cardiac fibroblasts via an autocrine mechanism. Cardiovasc Res 2003;57:784–792. [DOI] [PubMed] [Google Scholar]
- 27. Yin F, Lu ZZ, Han QD, Zhang YY.. Expression of beta2-adrenergic receptor and its effect on the proliferation of neonatal rat cardiac fibroblasts. Sheng Li Xue Bao 2003;55:251–254. [PubMed] [Google Scholar]
- 28. Aránguiz-Urroz P, Canales J, Copaja M, Troncoso R, Vicencio JM, Carrillo C, Lara H, Lavandero S, Díaz-Araya G.. Beta(2)-adrenergic receptor regulates cardiac fibroblast autophagy and collagen degradation. Biochim Biophys Acta 2011;1812:23–31. [DOI] [PubMed] [Google Scholar]
- 29. Porter KE, Turner NA.. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther 2009;123:255–278. [DOI] [PubMed] [Google Scholar]
- 30. Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA.. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci USA 2005;102:437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dubey RK, Gillespie DG, Mi Z, Jackson EK.. Cardiac fibroblasts express the cAMP-adenosine pathway. Hypertension 2000;36:337–342. [DOI] [PubMed] [Google Scholar]
- 32. Lu D, Aroonsakool N, Yokoyama U, Patel HH, Insel PA.. Increase in cellular cyclic AMP concentrations reverses the profibrogenic phenotype of cardiac myofibroblasts: a novel therapeutic approach for cardiac fibrosis. Mol Pharmacol 2013;84:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Insel PA, Bourne HR, Coffino P, Tomkins GM.. Cyclic AMP-dependent protein kinase: pivotal role in regulation of enzyme induction and growth. Science 1975;190:896–898. [DOI] [PubMed] [Google Scholar]
- 34. Gloerich M, Bos JL.. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 2010;50:355–375. [DOI] [PubMed] [Google Scholar]
- 35. Grandoch M, Roscioni SS, Schmidt M.. The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br J Pharmacol 2010;159:265–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Breckler M, Berthouze M, Laurent AC, Crozatier B, Morel E, Lezoualc'h F.. Rap-linked cAMP signaling Epac proteins: compartmentation, functioning and disease implications. Cell Signal 2011;23:1257–1266. [DOI] [PubMed] [Google Scholar]
- 37. Villarreal F, Epperson SA, Ramirez-Sanchez I, Yamazaki KG, Brunton LL.. Regulation of cardiac fibroblast collagen synthesis by adenosine: roles for EPAC and PI3K. Am J Physiol, Cell Physiol 2009;296:C1178–C1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Insel PA, Murray F, Yokoyama U, Romano S, Yun H, Brown L, Snead A, Lu D, Aroonsakool N.. cAMP and Epac in the regulation of tissue fibrosis. Br J Pharmacol 2012;166:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tian CJ, Pang X.. Ca2+-calcineurin signaling is involved in norepinephrine-induced cardiac fibroblasts activation. Int J Clin Exp Pathol 2015;8:5210–5216. [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang X, Szeto C, Gao E, Tang M, Jin J, Fu Q, Makarewich C, Ai X, Li Y, Tang A, Wang J, Gao H, Wang F, Ge XJ, Kunapuli SP, Zhou L, Zeng C, Xiang KY, Chen X.. Cardiotoxic and cardioprotective features of chronic beta-adrenergic signaling. Circ Res 2013;112:498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Okumura S, Fujita T, Cai W, Jin M, Namekata I, Mototani Y, Jin H, Ohnuki Y, Tsuneoka Y, Kurotani R, Suita K, Kawakami Y, Hamaguchi S, Abe T, Kiyonari H, Tsunematsu T, Bai Y, Suzuki S, Hidaka Y, Umemura M, Ichikawa Y, Yokoyama U, Sato M, Ishikawa F, Izumi-Nakaseko H, Adachi-Akahane S, Tanaka H, Ishikawa Y.. Epac1-dependent phospholamban phosphorylation mediates the cardiac response to stresses. J Clin Invest 2014;124:2785–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laurent AC, Bisserier M, Lucas A, Tortosa F, Roumieux M, De Regibus A, Swiader A, Sainte-Marie Y, Heymes C, Vindis C, Lezoualc'h F.. Exchange protein directly activated by cAMP 1 promotes autophagy during cardiomyocyte hypertrophy. Cardiovasc Res 2015;105:55–64. [DOI] [PubMed] [Google Scholar]
- 43. Aflaki M, Qi XY, Xiao L, Ordog B, Tadevosyan A, Luo X, Maguy A, Shi Y, Tardif JC, Nattel S.. Exchange protein directly activated by cAMP mediates slow delayed-rectifier current remodeling by sustained beta-adrenergic activation in guinea pig hearts. Circ Res 2014;114:993–1003. [DOI] [PubMed] [Google Scholar]
- 44. Fujita T, Umemura M, Yokoyama U, Okumura S, Ishikawa Y.. The role of EPAC in the heart. Cell Mol Life Sci 2017;74:591–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lezoualc'h F, Fazal L, Laudette M, Conte C.. Cyclic AMP sensor EPAC proteins and their role in cardiovascular function and disease. Circ Res 2016;118:881–897. [DOI] [PubMed] [Google Scholar]
- 46. Dybkova N, Wagner S, Backs J, Hund TJ, Mohler PJ, Sowa T, Nikolaev VO, Maier LS.. Tubulin polymerization disrupts cardiac beta-adrenergic regulation of late INa. Cardiovasc Res 2014;103:168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fazal L, Laudette M, Paula-Gomes S, Pons S, Conte C, Tortosa F, Sicard P, Sainte-Marie Y, Bisserier M, Lairez O, Lucas A, Roy J, Ghaleh B, Fauconnier J, Mialet-Perez J, Lezoualc’h F.. Multifunctional mitochondrial Epac1 controls myocardial cell death. Circ Res 2017;120:645–657. [DOI] [PubMed] [Google Scholar]
- 48. Olmedo I, Munoz C, Guzman N, Catalan M, Vivar R, Ayala P, Humeres C, Aranguiz P, Garcia L, Velarde V, Diaz-Araya G.. EPAC expression and function in cardiac fibroblasts and myofibroblasts. Toxicol Appl Pharmacol 2013;272:414–422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.