Abstract
A multi-residue method for the determination of the occurrence and prevalence levels of selected veterinary pharmaceutical residues in surface water was developed on a high performance liquid chromatography coupled to ultraviolet-visible (HPLC-UV) detector, and tested with the intent of profiling their distribution. The limit of detection (LOD) and limit of quantitation (LOQ) achieved for the selected pharmaceuticals; acetaminophen, diclofenac, salicylic acid, tetracycline, chloramphenicol, ciprofloxacin, bisphenol–A, 17β–estradiol, estriol, and ivermectin ranged between 0.06–3.45 μg L−1 and 0.17–10.35 μg L−1 respectively. Other International Conference on Harmonization (ICH) parameters for validation of analytical procedures were also evaluated and discussed. Pharmaceutical residues were recovered from surface water samples collected from around livestock farms in Cape Town, South Africa by solid phase extraction (SPE), and thereafter separated and quantified using a validated method on a HPLC-UV-detector. Most frequently detected residues were: acetaminophen (56%), diclofenac (53), tetracycline (72%), 17β–estradiol (73%); chloramphenicol (68%), and salicylic acid (67%), with significantly high (p > 0.05) spatial variability in the concentration distributions of the pharmaceuticals in the surface waters.
Keywords: Analytical chemistry, Environmental science
1. Introduction
There has been a substantial increase in the dependence and use of pharmaceutical products such as antibiotics, steroid hormones and many other classes of drugs in the agricultural industry in contemporary times. This is mainly due to their animal health benefits, as well as other economic advantages derivable from potentiated and unintended drug use; such as growth enhancement induction in farm animals (Steinfield et al., 2006; Allen et al., 2013). Substantial proportions of the administered pharmaceuticals are excreted in animal faeces and urine, either in the form in which they are administered or as metabolites (Nema and Ludwig, 2010; Zhou et al., 2013). Farm wash water and animal litters may therefore contain residues of active pharmaceuticals ingredients, administered for therapeutic/prophylaxis purposes, or as additive in livestock animal feed rations (Daughton and Ternes, 1999; Boxall et al., 2004a).
Redistribution and trans-relocation of residues of pharmaceutical may also occur during mass transport/mobilization. Waterways are a major means of mass transport of veterinary pharmaceutical residues and outfalls (Boxall et al. 2004a, 2004b; Jorgensen and Halling-Sorensen, 2000). Excess water may run into adjoining surface water bodies from drain sewers or from wastewater storage lagoons of animal production facilities, once water volume rises above the holding capacities of the lagoons. Arable farmland soils nutrient enhancement treatment, using animal farm litters/wastes as manure can also lead to soil pollution, especially where farm litter used as soil manure is far more than needed for soil nourishment and nutrient regeneration (Chastain, 1995; Pinheiro et al., 2013). Excess waste litter may therefore run-off into nearby surface water and ground water systems, from soils at maximum mineral stabilization/saturation point (Pinheiro et al., 2013).
Some active pharmaceutical ingredients such as “hormones can remain functional in manure up to 270 days after excretion” (http://www.sustainabletable.org/issues/waterpollution/index_pf.html). While there have been many documented cases of hormones discovered miles downstream of livestock farms (Kolpin et al., 2002), vertical mass (subsurface) movement of soil water contaminated by dissolved pharmaceutical residues, may also result in ground water pollution (Maron et al., 2013).
The pollution of water from industrial farms and other sources pose a serious threat to fish and other aquatic ecosystems components, as well as humans who depend on such water bodies (USEPA 2002a; USEPA 2002b; Stoecker et al., 2006; Ginebreda et al., 2010; Pérez-Carrera et al., 2010; Kosma et al., 2014). For example, exposure of aquatic organisms to steroid hormones, antibiotics etc. have been reported to result in endocrine disruption; manifest in consequences such as the compromise of fish reproductive processes leading to feminization of fish; imposex (imposition of sex organs) in gastropods and some other lower aquatic vertebrates. Cases of bacteria resistance to antibiotics have also been identified (Stoecker et al., 2006; Lee-Ventola, 2015).
Analysis of emerging contaminants including residues of active pharmaceutical ingredients in environmental samples is associated with a number of issues. This include their very low occurrence concentration, matrix interferences, and the lack of quick and reliable method of extraction. Carmona and Pico (2018) reported the various modifications and use of Solid Phase Extraction (SPE), as the most common method of recovery of pharmaceuticals from water matrices. Other methods such as Accelerated Solvent Extraction (ACE), Solid Phase Microextraction (SPME), Liquid-liquid extraction (LLE) can be modified to extract APIs from aqueous, biological and solid samples due to matrix complexity (Shen et al., 2012; Li et al., 2016; Lorenzo et al., 2016; Juhascik and Jenkins, 2009; Jelic et al 2009). Separation and detection is often achieve using High Performance Liquid Chromatography coupled to Mass Spectrometry. Mass spectrometry detection is the most robust and widely applicable, however it is not readily available due to cost implication. Hence there is need to develop rapid and reliable methods using other detection types, although this possess a challenge arising from limitations of instrument's detector sensitivity.
Data concerning the occurrence and characterization of African waters is scanty and scarce. This is because many African Countries are yet to appreciate the enormous potential consequences of the presence of pharmaceutical residues in unintended matrices, as well as the dearth of simple and reliable analytical technique for the recovery and determination of trace and ultra-trace levels of active pharmaceutical ingredients (APIs) in different environmental matrices. In this study a new method for the determination of some pharmaceuticals using high performance liquid chromatography coupled to ultraviolet-visible (HPLC-UV) detector was developed, validated and used to quantify the occurrence levels of selected pharmaceuticals in selected surface water system around some livestock farms in Cape Town and Stellenbosch environments, Western Cape.
2. Materials and methods
2.1. Standards and reagents
Acetaminophen, diclofenac, salicylic acid, tetracycline, chloramphenicol, ciprofloxacin, bisphenol–A, 17β–estradiol, estriol, and ivermectin, were purchased from Sigma Aldrich, Bellefonte, PA, (USA). HPLC grade methanol, ethanol, acetonitrile, acetone and other reagents including formic acid, ethyl acetate were also purchased from Sigma Aldrich Germany. Milli-Q water (Synergy Ultrapure Water System, Millipore, France) was used for all solution preparations.
2.2. Stock solution and calibration standards
Stock solutions (100 mg L−1) of 10 pharmaceuticals; acetaminophen, diclofenac, salicylic acid, tetracycline, chloramphenicol, ciprofloxacin, bisphenol–A, 17β–estradiol, estriol, and ivermectin were prepared on a weight basis, by dissolving 0.01 g neat crystals of each of them in 100 mL methanol. The stock solutions were stored in amber coloured bottles at −20 °C, and used within 48 hr. Tetracycline was further wrapped in aluminium foil before storage as it has been reported to have tendency to photo-degradation. Thereafter, working calibration solutions (100–500 μg L−1) of the each of the standards were prepared by serial dilution and used for instrument optimization and calibration.
2.3. Sampling and sampling sites
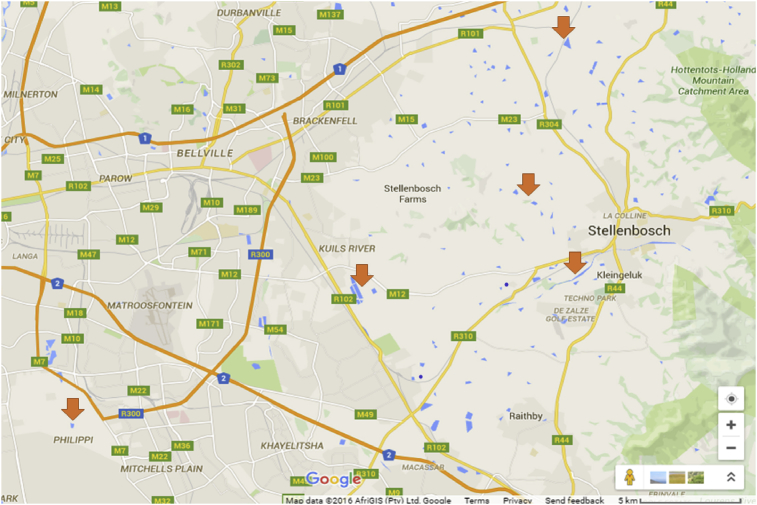
Agriculture is one of the major mainstay occupations in the Western Cape of South Africa. Livestock farms consisting of both informal and industrial arrays of poultries, piggeries, diaries and feedlot cattle farms are practiced within the surroundings of City of Cape Town. Samples were collected in five Sampling Stations in Phillipi (informal farms, consisting of free range sheep/cattle pasture) and a cattle Kraal (SS -34.028518 – SS -34.046683; E 18.557171 – E 18.551356), farm area (SS -33.994118 – SS -34.012491; E 18.665036 – E 18.672502) and Stellenbosch (industrial farms, consisting of a poultry farm, a piggery and a free range sheep grazing field, geo-reference points, SS -33.948049 – SS -33.951467; E 18.824056 – E 18.839457; SS -33.895339 – SS -33.906905; E 18.809398 – E 18.821759; and SS -33.873789 – SS -33.882277; E 18.831282 – E 18.839689) (Fig. 1).
Fig. 1.
Map of Western Cape showing the Stellenbosch Farmland and the informal animal farms in the peripheral of Cape Town (Source - Adapted from Google maps).
Water samples were randomly collected at different points from streams/rivers traversing proximally by some industrial livestock farms, and from some open ponds within the vicinity of the farms, suspect to be potential sources of release of pharmaceutical into the water system vis-a-viz the discharge of farm wash wastewater and farm litters, during autumn, winter, spring and summer.
2.4. Sample preparation
2.4.1. Recovery of pharmaceutical residues (analytes) from water samples
Solid Phase Extraction (SPE) technique was developed for the extraction of pharmaceutical drugs residues from water samples. The columns of hydrophilic-lipophilic balance (HLB) and polymeric reverse phase (PRP) solid phase extraction cartridges (200 mg/6 mL) were condition conditioned by running 3 ml MeOH through the column and a flow rate of 1 mL/min, followed by 5 mL, 30% MeOH at 1 mL min−1 and then 5 mL milliQ water at same flow rate. Thereafter, 500 mL of the pre-filtered water samples were loaded on the conditioned column and eluted at a flow rate of 1 mL/min. The analytes were recovered from each of the columns in MeOH. The MeOH extracts were concentrated under nitrogen stream, and reconstituted in 1 mL, 50% MeOH with MilliQ water.
2.4.2. Instrument parameter for HPLC-UV separation and detection of pharmaceutical residues
The chromatographic separations were performed on “Waters (2695)” high performance liquid chromatography (HPLC) consisting of an auto sampler (Waters 2707), a binary pump (Waters 1525), a thermostat fitted oven and a UV-Vis detector (Waters 2487). 10 μL of each standard was automatically injected to a 150 mm × 4.60 mm, 5 μm particles reverse phase Luna C18 column (Phenomenex), kept isothermal at a column temperature of 25 °C.
Separation was achieved by gradient elution in an elution solvent (mobile phase A) made up of de-ionized water (acidified/modified to pH 3.0, with formic acid) and methanol (MeOH) (mobile phase B) at a flow rate of 1.0 mL min−1. The initial elution phase matrix composition was set at 10 % MeOH, with the solvent composition gradient linearly raised to 100 % MeOH (10 %–100 % MeOH) over streaming time of 35 min. The wavelengths of the dual-wavelength UV-Vis detector was set at 280 nm.
2.4.3. Recovery studies
The efficiency of the solid phase extraction procedure, used for the recovery of residues of pharmaceuticals from the water samples was evaluated. This was done by fortifying some water samples with known concentrations of the pharmaceuticals spiked in triplicates (low, medium and high). The fortified samples were allowed to equilibrate for about 18 hr., after which the pharmaceuticals were recovered from the fortified water using solid phase extraction. Each of the water samples were analyzed in triplicate of each of the spiked levels.
The efficiency of the SPE columns in the extraction of drug residues from aqueous media was evaluated by spiking milliQ water in triplicate concentrations (2, 5 and 10 ppm) of selected pharmaceutical standards (tetracycline, 17β-estradiol, acetaminophen, and ivermectin). These were subsequently recovered from milliQ water using the HLB and PRP cartridges.
3. Results and discussion
3.1. Method validation and instrument performance
The analytical method for the detection and quantitation of pharmaceutical residues was validated in accordance to the International Conference on Harmonization (ICH) procedure (ICH, 2009). The selectivity, linearity and linear range of application, limit of detection (LOD) and limit of quantitation (LOQ), accuracy, precision, and recovery for the simultaneous detection of the selected pharmaceuticals and their residues in aqueous matrices were evaluated.
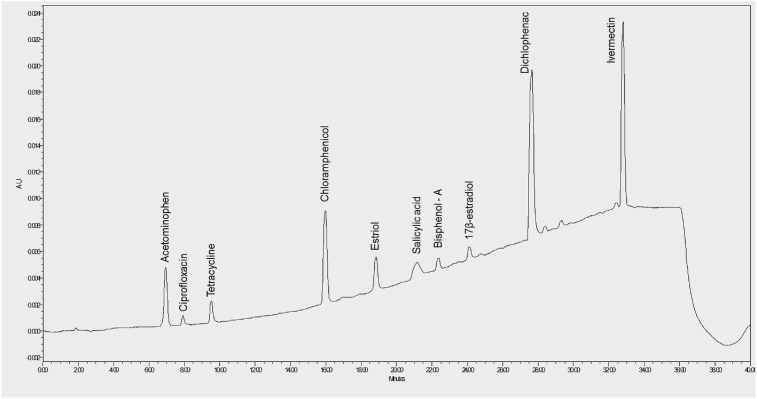
The elution chromatogram for the UV detection at wavelength of 280 nm is presented in Fig. 2.
Fig. 2.
Chromatogram showing the peaks of mixed standards detected at 280 nm wavelength.
3.1.1. Selectivity
The selectivity of the method was evaluated by defining the retention time (tr) of the individual peak of each pharmaceutical. The retention time and optimum elution composition of the mobile phase for the separation of the standards-in-mixed matrix are presented in Table 1 below. Thereafter, the interaction/association of the peaks in the chromatogram of the mixed analytes standards (n = 5) were checked for peak resolution. The co-elution index (Δtr/range)i of the individual peak of the pharmaceuticals in the chromatogram of the multiple analytes ranged between 0.03 (3.82%) – 0.25 (24.85%) units.
Table 1.
Retention time and mobile phase composition for the simultaneous elution of 10 standards.
| Pharmaceutical standards | Flow (mL/min) | Retention time (min) | % A H2O (Acidified with Glacial acetic acid) | % B (MeOH) |
|---|---|---|---|---|
| Acetaminophen | 1.00 | 6.937 | 70 | 30 |
| Ciprofloxacin | 1.00 | 7.925 | 66 | 34 |
| Tetracycline | 1.00 | 9.532 | 61 | 39 |
| Chloramphenicol | 1.00 | 15.959 | 42 | 58 |
| Estriol | 1.00 | 18.841 | 34 | 66 |
| Salicylic acid | 1.00 | 21.121 | 26 | 74 |
| Bisphenol A | 1.00 | 22.363 | 23 | 77 |
| Estradiol | 1.00 | 24.133 | 18 | 82 |
| Diclofenac | 1.00 | 27.665 | 7 | 93 |
| Ivermectin | 1.00 | 32.756 | 7 | 93 |
3.1.2. Limit of detection (LOD) and limit of quantitation (LOQ)
The response of the instrument detector to the injection of equimolar concentration of the cocktail of the different pharmaceutical standards varied. As a consequence, the working range for the calibration standards of the pharmaceutical standards were variable, with those pharmaceuticals with strong instrument response i.e. signals (large peak area or peak height) calibrated on within low concentration range, and those with weak signal response calibrated within higher concentration range of working calibration standards.
The instrument detection limits (LOD) and limit of quantifications (LOQ) for each of the analytes was calculated. The errors associated with the calibrations are random and normally distributed, hence the quantities LOD (y = 3Sblank + yblank) and LOQ (3LOD/slope) for each of the pharmaceutical standards was determined. The limit of detection (LOD, μg L−1) and limit of quantitation (LOQ, μg L−1) for the calibrated tested pharmaceuticals are presented in Table 2.
Table 2.
Results for the calibration of standards used in the development of analytical method.
| Standard | Retention Time (min) | Calibration range (ppm) | Regression equation | Co-efficient of regression (R2) | LOD (μg L−1) | LOQ (μg L−1) |
|---|---|---|---|---|---|---|
| Acetaminophen | 6.937 | 0.1–500 | y = 34.389x + 4.5179 | 0.9999 | 0.4873 | 1.4618 |
| Ciprofloxacin | 7.925 | 0.1–50 | y = 30.883x + 14395 | 0.9680 | 0.2129 | 0.6389 |
| Tetracycline | 9.532 | 0.5–50 | y = 18.005x + 18.713 | 0.9999 | 3.4509 | 10.3528 |
| Chloramphenicol | 15.959 | 0.1–50 | y = 183.56x + 6241.9 | 0.9992 | 0.0559 | 0.1678 |
| Estriol | 18.841 | 0.5–100 | y = 16.172x + 21.772 | 0.9984 | 1.4400 | 4.3100 |
| Salicylic acid | 21.121 | 0.1–50 | y = 25.593x + 1720.5 | 0.9996 | 1.3760 | 4.1279 |
| Bisphenol – A | 22.363 | 0.5–500 | y = 7.9113x + 5967.2 | 0.9136 | 2.2700 | 6.81 |
| 17β – Estradiol | 24.133 | 0.5–100 | y = 37.894x + 836.41 | 0.9999 | 0.7690 | 2.3100 |
| Diclofenac | 27.665 | 0.1–50 | y = 94.663x + 5226.8 | 0.9995 | 0.8545 | 2.5636 |
| Ivermectin | 32.799 | 0.5–500 | y = 14.225x + 20005 | 0.9999 | 1.7400 | 5.2300 |
3.1.3. Linearity and linear range
Instrument responses for the calibrations of working standards of the investigated pharmaceuticals are presented in Table 2. The coefficient of correlation (γ) (linearity) between the instrument response (peak area) and the increasing concentration of the standards was determined by evaluating the relationship between the signal responses of instrument response (peak area) to increasing concentration of the different concentrations of the working calibration standards for each of the analytes.
There was linear relationship between instrument count for peak areas, with gradient concentration increases for the different standards; acetaminophen, R2 = 0.9998; diclofenac, R2 = 0.9995; salicylic acid, R2 = 0.9996; tetracycline, R2 = 0.9999, chloramphenicol, R2 = 0.9992; ciprofloxacin, R2 = 0.9680; bisphenol–A, R2 = 0.9136; 17β– estradiol, R2 = 0.9999, estriol, R2 = 0.9984 and ivermectin, R2 = 0.9999.
The RSD of each of the calibration standard (n = −5) generally varied between 0.06 and 3.10 % with accuracy value ranged from 90 – 96% for solvent calibration curve and 0.18 and 5 63 % with accuracy value ranging from 87.90 – 93. 75 % for standards-in-mix matrix calibration curves.
3.1.4. Intra-day and inter-day precision
Intra-day precision was investigated by conducting series of runs (n = 5) on standards-in-mix matrices at an interval of 90 min. For the inter-day precision, series of runs (n = 5) was carried out on the standards-in-mix matrices over five days period. Results showed intra-day accuracy of 86.34–94.24% (RSD <10 %) and inter-day accuracy of 79.25–90.56% (RSD <15%)
3.1.5. The matrix interferences of the mixed pharmaceutical compounds
The UV spectra and the individual peak purity of the pharmaceutical standard solutions and that in the fortified matrices were tested using Breeze Software on the HPLC-UV. The result showed that, the analyte peaks had purity levels of higher than 95% and independent. Therefore, the peaks are independent of the matrix in which they are contained.
Comparative assessment of the slopes of the calibration curves obtained for the selected analyte standards in pure solvent and standards-in-matrix showed that the signals of the measured analytes are not influenced by matrix interferences. The differences in the parallel characteristics of the slopes of the pure analyte standards and the fortified matrices in solvent and standards-in-matrix were parallel and not significantly different, with a co-efficient of variation in the order of 0.93–4.88 % (2.75%; n = 20).
3.1.6. Recovery studies
The recovery of analytes from aqueous matrices was established by the estimation of the percentage analytes recovered from the fortified samples at low, medium and high concentrations. The recovery obtained for the selected target analytes were ranged 76–97% (RSD <7%) in pure MilliQ water, and 69–88% (RSD <15%) in water sample. Although there were few cases where the observed recoveries were significantly distinct, direct correlation between matrix influence and dependence on concentration could not be established.
The efficiency of the SPE columns in the extraction of drug residues from aqueous media was evaluated by spiking milliQ water in triplicate concentrations (2, 5 and 10 ppm) of selected pharmaceutical standards (tetracycline, 17β-estradiol, acetaminophen, and ivermectin (Table 3)). These were subsequently recovered from milliQ water using the HLB and PRP cartridges.
Table 3.
Results of recovery of analytes from spiked milliQ water.
| HLB % recovery |
PRP % recovery |
|||||
|---|---|---|---|---|---|---|
| 2 (mg L−1) | 5 (mg L−1) | 10 (mg L−1) | 2 (mg L−1) | 5 (mg L−1) | 10 (mg L−1) | |
| 17 β-Estradiol | 76.62 ± 2.14 | 85.47 ± 5.45 | 81.76 ± 4.78 | 80.38 ± 2.67 | 72.82 ± 2.82 | 78.54 ± 4.13 |
| Acetaminophen | 81.49 ± 5.09 | 78.29 ± 2.98 | 94.34 ± 3.60 | 72.65 ± 4.68 | 76.79 ± 1.46 | 84.23 ± 4.29 |
| Tetracycline | 92.15 ± 4.63 | 88.35 ± 2.71 | 89.19 ± 5.16 | 74.29 ± 2.95 | 89. 53 ± 5.69 | 73.94 ± 5.96 |
| Ivermectin | 80.27 ± 3.65 | 84.26 ± 4.10 | 84.89 ± 1.24 | 76.65 ± 5.01 | 74.98 ± 3.54 | 75.46 ± 3.25 |
| Mean ± SD recovery | 82.79 ± 3.88 | 84.09 ± 3.81 | 87.55 ± 3.70 | 75.99 ± 3.83 | 78.53 ± 3.38 | 78.04 ± 4.41 |
The efficiency of recoveries of the selected pharmaceutical without any form of pretreatment varied for HLB and PRP cartridges. Analytes recovery using HLB and PRP SPE columns were ranged between 83 and 88% and 76 and 79% respectively.
3.2. Concentration of pharmaceutical residues in water samples
The large amount of water required for uses, such as drinking, cleaning, animal processing etc., in livestock agriculture (especially in animal farms), suggests the possibility of indiscriminate and unsustainable discharge of pharmaceutical residues contaminated wastewater into adjoining aquatic and land resources. The assessment of selected surface water system revealed the occurrence of residues of some veterinary pharmaceuticals in water samples collected from the different sampling stations, while others did not occur at detectable concentrations. The concentration levels of the investigated pharmaceutical residues were variable within and between sampling stations (Table 4).
Table 4.
Concentrations (μg L−1) of selected pharmaceutical residues in surface water collected from some livestock farms environ.
| Name | Sampling Station 1 |
Sampling Station 2 |
Sampling Station 3 |
Sampling Station 4 |
Sampling Station 5 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pool 1 Conc. | Pool 2 Conc. | Pool 1 Conc. | Pool 2 Conc. | Pool 1 Conc. | Pool 2 Conc. | Pool 1 Conc. | Pool 2 Conc. | Pool 1 Conc. | Pool 2 Conc. | |
| Acetaminophen | <0.48 | 0.52 | 0.70 | 0.61 | <0.48 | <0.48 | 0.93 | <0.48 | 1.054 | 0.72 |
| Bisphenol A | <2.27 | <2.27 | <2.27 | <2.27 | <2.27 | <2.27 | <2.27 | <2.27 | <2.27 | <2.27 |
| Chloramphenicol | 0.353 | 0.76 | 3.10 | 3.39 | 2.17 | 1.95 | 0.78 | 1.30 | 2.06 | 2.48 |
| Ciprofloxacin | <0.21 | <0.21 | <0.21 | <0.21 | <0.21 | <0.21 | <0.21 | <0.21 | <0.21 | 0.21 |
| Diclofenac | 1.38 | 1.26 | 0.86 | 1.25 | 3.67 | 2.08 | 1.88 | 1.13 | 2.05 | 1.59 |
| 17β-Estradiol | 0.98 | 2.39 | 1.073 | 0.873 | 2.784 | 5.64 | 10.94 | 3.96 | 7.45 | 5.97 |
| Estriol | <1.44 | <1.44 | <1.44 | <1.44 | <1.44 | <1.44 | 1.46 | 1.47 | 1.48 | 1.48 |
| Ivermectin | <1.74 | <1.74 | 1.79 | 1.85 | 1.97 | <1.74 | <1.74 | <1.74 | <1.74 | <1.74 |
| Tetracycline | <3.45 | 3.55 | 4.57 | 3.55 | <3.45 | <3.45 | 4.88 | 3.95 | 4.19 | 3.80 |
| Salicylic acid | 3.33 | 3.14 | 4.95 | 5.72 | 7.83 | 4.09 | 11.64 | 19.50 | 1.37 | 1.37 |
The result showed that the prevalent pharmaceutical residues commonly associated with animal waste sources, and constituting contaminants in the water samples include acetaminophen, 17β-estradiol, tetracycline and salicylic acid. Other veterinary drug residues may be present though at levels below the current detection limit. The concentration of salicylic acid (1.37–19.50 μg L−1) was the highest in respect of the investigated pharmaceuticals. This was followed by 17β-estradiol (0.87–10.94 μg L−1), tetracycline (<3.45–4.88 μg L−1), diclofenac (<0.86–3.67), chloramphenicol (0.35–3.39), and acetaminophen (<0.48–1.05 μg L−1), while the other pharmaceuticals were below detection limit. The levels detected exclude the concentrations of the metabolites of the tested APIs, but rather an estimate of the recoverable APIs. This is because study could not apply the Total Residue Approach (TRA) since the succession phases of the metabolites of the parent compounds were not part of the assay, as they are yet to be identified.
Most frequently detected residues were acetaminophen (56%), diclofenac (53), tetracycline (72%), 17β–estradiol (73%); chloramphenicol (68%), and salicylic acid (67%), with concentrations ranged, <0.48–1.054; 0.86–3.67; <3.45–4.88; 0.87–10.94; 0.35–3.39 and 1.37–19.5 μg L−1 respectively. Spatial variability in the concentration distributions of the pharmaceuticals in the surface waters was significantly high (p < 0.05). There was significantly high (p < 0.05) spatial variability in the concentration distributions of the pharmaceuticals in the most of the surface water tested. The variable concentrations in the different surface waters may be animal specific, and this is associated with peculiar drugs commonly administered to the in-breeds types.
The observed concentrations though slightly lower, are consistent with those reported by Heberer (2002) for diclofenac and salicylic acid. The occurrence of antibiotics and painkillers such as tetracycline and salicylic acid respectively, is consistent with administration data on commonly prescribed drugs in animal husbandry. Furthermore, antibiotics and hormonal drugs are added to animal feed as a constant in-feeds to prevent infection, as well as speed up animal growth. Other regulated drugs including β-lactams - penicillin (for gram negative infections), sulfonamides, phosphomycin and potentiated drugs may be detected in different environmental matrices.
Although the effect of various residues of active pharmaceutical substances on plants, animals and microbial in the environment is not clearly known (Murdoch, 2015). However, acute and chronic consequences (such endocrine disruption, potential indirect effects on wider ecosystems) of low levels of exposure to these substances have been reported (Murdoch, 2015). According to Christensen (2003), Campagnolo et al. (2002), Webb et al. (2003) and Jones et al. (2005), the development of sensitization, allergic response and resistant pathogens due to the likelihood of the presence of antibiotics is of most concern. While, human health risk associated with exposure to low concentrations of pharmaceuticals in surface and drinking water is extremely low (Christensen, 2003; Webb et al., 2003; Schulman et al., 2002), in vitro studies revealed that exposure of fish to concentrations as low as 1–10 ng L−1 of 17β-estradiol and 0.1 ng L−1 of 17α-ethylnestradiol in aquatic environment can induce feminization in male of some wild fish species (Heberer, 2002; Routledge et al., 1998; Purdom et al., 1994).
4. Conclusion
The detection and quantitation of the selected pharmaceuticals can be achieved at concentrations greater than the allowed LODs (0.06–3.45 μg L−1) and LOQs (0.17–10.35 μg L−1) achieved for the selected pharmaceuticals. Residues of some veterinary pharmaceuticals were simultaneously detected in the surface water collected from around the investigated livestock agricultural farms. Acetaminophen, diclofenac, 17β –estradiol, tetracycline and salicylic acid were present at at variable concentration levels, while estriol, ciprofloxacin, and bisphenol-A were not present at detectable concentration. Most of the selected detected veterinary pharmaceuticals occurred in nearly in all the sampling stations except one from river water. There was also a high spatial variation in the concentration levels of the different tested veterinary pharmaceutical residue levels at different points in each of the five sampling stations.
Findings showed that livestock agricultural practices might be a major source of pollution of near and downstream columns of fresh surface water catchments in adjoining distance to many livestock farms. Also the application of livestock farm wastes litters as farm manure may result in the redistribution of veterinary pharmaceuticals in soils, and with a potential for translocation into plants. This may pose a potential exposure risk of the residues of the active veterinary ingredients and their metabolites to edaphic features, terrestrial/aquatic wildlife and humans who depend on such contaminated waters and or whose natural habitat is contaminated with veterinary pharmaceuticals.
Declarations
Author contribution statement
Olalekan S. Fatoki: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Beatrice O. Opeolu, Bettina Genthe, Olatunde S. Olatunji: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Water Research Commission (WRC K4/2500) of the People's Republic of South Africa.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Allen H.K., Levine U.Y., Looft T., Bandrick M., Casey T.A. Treatment, promotion, commotion: antibiotic alternatives in food-producing animals. Trends Microbiol. 2013;21(3):114–119. doi: 10.1016/j.tim.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Boxall A.B.A., Fogg L.A., Kay P., Blackwell P.A., Pemberton E.J., Croxford A. Veterinary medicines in the environment. Rev. Environ. Contam. Toxicol. 2004;180:1–91. doi: 10.1007/0-387-21729-0_1. [DOI] [PubMed] [Google Scholar]
- Boxall A.B.A., Sinclair C.J., Fenner K., Kolpin D.W., Maund S. When synthetic chemicals degrade in the environment. Environ. Sci. Technol. 2004;38(19):369A–375A. doi: 10.1021/es040624v. [DOI] [PubMed] [Google Scholar]
- Campagnolo E.R., Johnson K.R., Karpati A., Rubin C.S., Kolpin D.W., Meyer M.T., Esteban J.E., Currier R.W., Smith K., Thu K.M., McGeehin M. Antimicrobial residues in animal waste and water resources proximal to large-scale swine and poultry feeding operations. Sci. Total Environ. 2002;299:89–95. doi: 10.1016/s0048-9697(02)00233-4. [DOI] [PubMed] [Google Scholar]
- Carmona E., Pico Y. The use of chromatographic methods coupled to mass spectrometry for the study of emerging pollutants in the environment. Crit. Rev. Anal. Chem. 2018;48(4):305–316. doi: 10.1080/10408347.2018.1430555. [DOI] [PubMed] [Google Scholar]
- Chastain J.P. Minnesota/Wisconsin Engineering Notes, Winter 1995; 1995. Pollution Potential of Livestock Manure. [Google Scholar]
- Christensen F.M. Pharmaceuticals in the environment – a human risk? Regul. Toxicol. Pharmacol. 2003;28:212–221. doi: 10.1006/rtph.1998.1253. [DOI] [PubMed] [Google Scholar]
- Daughton C.G., Ternes T.A. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ. Health Perspect. 1999;6(107 Suppl):907–938. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginebreda A., Muñoz I., de Alda M.L., Brix R., López-Doval J., Barceló D. Environmental risk assessment of pharmaceuticals in rivers: relationships between hazard indexes and aquatic macroinvertebrate diversity indexes in the Llobregat River (NE Spain) Environ. Int. 2010;36(2):153–162. doi: 10.1016/j.envint.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Heberer T. Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J. Hydrol. 2002;266:175–189. [PubMed] [Google Scholar]
- ICH (International Conference on Harmonization) 2009. Validation of Analytical Procedures: Text and Methodology, Q2R1.http//www.ich.org/LOB/media/MEDIA417.pdf Accessed January 2016. [Google Scholar]
- Jelic A., Petrovic M., Barcelo D. Multi-residue method for trace level determination of pharmaceuticals in solid samples using pressurized liquid extraction followed by liquid chromatography/quadrupole-linear ion trap mass spectrometry. Talanta. 2009;80(1):363–371. doi: 10.1016/j.talanta.2009.06.077. [DOI] [PubMed] [Google Scholar]
- Jones O.A., Lester J.N., Voulvoulis N. Pharmaceuticals: a threat to drinking water? Trends Biotechnol. 2005;23(4):163–167. doi: 10.1016/j.tibtech.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Jorgensen S.E., Halling-Sorensen B. Drugs in the environment. Chemosphere. 2000;40:691–699. doi: 10.1016/s0045-6535(99)00438-5. [DOI] [PubMed] [Google Scholar]
- Juhascik M.P., Jenkins A.J. Comparison of liquid/liquid and solid-phase extraction for alkaline drugs. J. Chromatogr. Sci. 2009;47:553–557. doi: 10.1093/chromsci/47.7.553. Epub 2009/09/24. [DOI] [PubMed] [Google Scholar]
- Kolpin D.W., Furlong E.T., Meyer M.T., Thurman E.M., Zaugg S.D., Barber L.B., Buxton H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. Streams, 1999−2000: a national reconnaissance. Environ. Sci. Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kosma C.I., Lambropoulou D.A., Albanis T.A. Investigation of PPCPs in wastewater treatment plants in Greece: occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014;466:421–438. doi: 10.1016/j.scitotenv.2013.07.044. [DOI] [PubMed] [Google Scholar]
- Lee Ventola C. The antibiotic resistance crisis Part 1: causes and threats. Pharm. Therapeut. 2015;40(4):277–283. [PMC free article] [PubMed] [Google Scholar]
- Li G., Yang F., Liu M., Su X., Zhao M., Zhao L. Development and application of a UPLC-MS/MS method for simultaneous determination of fenofibric acid and berberine in rat plasma: application to the drug–drug pharmacokinetic interaction study of fenofibrate combined with berberine after oral administration in rats. Biomed. Chromatogr. 2016;30(7):1075–1082. doi: 10.1002/bmc.3652. [DOI] [PubMed] [Google Scholar]
- Lorenzo M., Farre M., Blasco C., Onghena M., Pico Y., Barcelo D. Perfluoroalkyl substances in breast milk, infant formula and baby food from Valencian community (Spain) Environ. Nanotechnol. Monit. Manag. 2016;6(Suppl. C):108–115. [Google Scholar]
- Maron D.F., Smith T.J., Nachman K.E. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Glob. Health. 2013;9:48–52. doi: 10.1186/1744-8603-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch K. National Toxic Network; 2015. Pharmaceutical Pollution in the Environment: Issues for Australia, New Zealand and Pacific Island Countries.www.ntn.org.au [Google Scholar]
- Nema S., Ludwig J.D. 2010. Pharmaceutical Dosage Forms – Parenteral Medications, Third Edition., Volume 1: Formulation and Packaging. Biopharmaceutics of NCEs and NBEs, pp. 41 paragraph 2 Informal Healthcare, UK. (Edited) (First published in 1984) [Google Scholar]
- Pérez-Carrera E., Hansen M., Leon V.M., Bjorklund E., Krogh K.A., Halling-Sørensen B., González-Mazo E. Multi-residue method for the determination of 32 human and veterinary pharmaceuticals in soil and sediment by pressurized-liquid extraction and LC-MS/MS. Anal. Bioanal. Chem. 2010;398(3):1173–1184. doi: 10.1007/s00216-010-3862-x. [DOI] [PubMed] [Google Scholar]
- Pinheiro A., Albano R.M.R., Alves T.C., Kaufmann V., da Silva M.R. Veterinary antibiotics and hormones in water from application of pig slurry to soil. Agric. Water Manag. 2013;129:1–8. ISSN 2318-0331. [Google Scholar]
- Purdom C.E., Hardiman P.A., Bye V.J., Eno N.C., Tyler C.R., Sumpter J.P. Estrogenic effects of effluents from sewage treatment works. Chem. Ecol. 1994;8:275–285. [Google Scholar]
- Routledge E.J., Sheahan D., Desbrow C., Sumpter J.P., Waldock M. Identification of estrogenic chemicals in STP effluent 2. In vivo responses in trout and roach. Environ. Sci. Technol. 1998;32:1559–1565. [Google Scholar]
- Schulman L.J., Sargent E.V., Naumann B.D., Faria E.C., Dolan D.G., Wargo J.P. A human health risk assessment of pharmaceuticals in the aquatic environment. Human Ecol. Risk Assessment. 2002;8:657–680. [Google Scholar]
- Shen J.Y., Chang M.S., Yang S.H., Wu G.J. Simultaneous determination of triclosan, triclocarban, and transformation products of triclocarban in aqueous samples using solid-phase micro-extraction-HPLC-MS/MS. J. Separ. Sci. 2012;35(19):2544–2552. doi: 10.1002/jssc.201200181. [DOI] [PubMed] [Google Scholar]
- Steinfield H., Gerber P., Wassernaar T., Castel V., Rosales M., de Haan C. Food and Agricultural Organization of the United Nations; Rome, Italy: 2006. Livestock Long Shadow – Environmental Issues and Options. 390 pp. [Google Scholar]
- Stoecker D.K., Long A., Suttles S.E., Sanford L.P. Effect of small-scale shear on grazing and growth of the dinoflagellate Pfiesteria piscicida. Harmful Algae. 2006;5(4):407–418. [Google Scholar]
- U.S. EPA (Environmental Protection Agency) National Water Quality Inventory: Report 2000a (EPA-841-R-02-001) 2002. Rivers and streams. [Google Scholar]
- U.S. EPA Environmental Protection Agency . National Water Quality Inventory 2002b: 13–14: Report 2000b. U.S. EPA; 2002. Lakes, reservoirs, and ponds; p. 22. August 2002. [Google Scholar]
- Webb S., Ternes T., Gibert M., Olejniczak K. Indirect human exposure to pharmaceuticals via drinking water. Toxicol. Lett. 2003;142:157–167. doi: 10.1016/s0378-4274(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Zhou L.J., Ying G.G., Liu S., Zhang R.Q., Lai H.J., Chen Z.F., Pan C.G. Excretion masses and environmental occurrence of antibiotics in typical swine and dairy cattle farms in China. Sci. Total Environ. 2013;444:183–195. doi: 10.1016/j.scitotenv.2012.11.087. [DOI] [PubMed] [Google Scholar]