Abstract
Introduction:
To evaluate the body of evidence on the predictive value of preoperative cognitive impairment on in-hospital, short-term, and midterm postoperative outcomes for elderly patients undergoing total knee arthroplasty (TKA).
Significance:
With an aging population, an increasing percentage of the U.S. patient population will be living with cognitive impairment. There is currently no systematic review that assesses postoperative outcomes of patients with mild cognitive impairment (MCI) or preexisting diagnosis of dementia while undergoing elective primary TKA.
Results:
A database search between January 1, 1997, and November 1, 2017 in EMBASE, MEDLINE, and PubMed was conducted to identify articles that compared postoperative outcomes after TKA between patients aged 60 years with and without cognitive impairment. Cognitive impairment included preexisting diagnosis of dementia or MCI identified during preoperative assessment. Eligible articles were selected using dual reviewer and third-party arbitrator. The quality of the studies was evaluated using the Newcastle-Ottawa Scale. The strength of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation approach. A total of 6163 abstracts were screened. Only 11 full text articles met inclusion criteria, including 1 case–control, 5 prospective cohort, and 5 retrospective cohort studies. Two studies were of poor quality. Overall, there is moderate strength of evidence for increased risk of postoperative delirium, increased length of stay, and discharge to health-care facility among patients with preoperative MCI or preexisting dementia. The body of evidence is weak for other outcomes of interest including mortality, functionality and complications while in-hospital and in the short- and midterm.
Conclusion:
This review highlights the need for additional good quality studies to provide more information about MCI and dementia as risk factors in primary TKA.
Keywords: dementia, cognitive impairment, total knee arthroplasty, postoperative, outcomes, systematic review
Introduction
Cognitive impairment has been shown to be strongly associated with frailty and to independently contribute to poor postoperative outcomes.1,2 However, cognitive impairment is not a standard or routine part of preoperative risk stratification of the surgical patient. A recent prospective cohort study showed that patients undergoing knee or hip replacement who had a Mini-Cog examination score of less than or equal to 2 during their preoperative assessment were more likely to be discharged to a health-care facility, develop delirium, and have longer hospital stays compared to patients with normal preoperative Mini-Cog scores.3 A Mini-Cog examination is one of several tools available to screen for possible cognitive impairment. Despite numerous studies demonstrating associations between preexisting cognitive impairment and poor surgical outcomes, preoperative evaluation typically is limited to assessing cardiac, pulmonary, renal, and hepatic organ systems.4
Older adults undergo more than half of the surgeries in the United States.5 Based on the 2010 US Census, the population of individuals aged 65 years and older is projected to double from 43.1 million in 2012 to 83.7 million in 2050.6 About 2% to 10% of dementia cases occur before the age of 65 years, with the prevalence doubling every 5 years thereafter.7 With an overall aging population, a greater percentage of the US patient population will be living with cognitive impairment. In a recent study, as many as 23% of patients randomly screened at a preadmission evaluation center had mild cognitive impairment (MCI) using the Clock-in-the-Box test or Mini-Cog.8 Various theories attempt to explain how both patient and procedural factors contribute to the increased surgical risk of poor preoperative outcomes for patients with cognitive impairment. Studies suggest that patients with MCI are more likely to have poor adherence to postoperative regimens, develop delirium, be unable to verbalize pain or communicate symptoms, and have a higher risk of aspiration from dysphagia.9-12 Other studies have pointed to procedural factors; intraoperative transfusions, perioperative hyper- or hypoglycemia, and hypothermia may be deliriogenic and aggravate subclinical Alzheimer disease.13,14
There is currently no systematic review or meta-analysis that has assessed postoperative outcomes of patients with likely MCI or a preexisting diagnosis of dementia while undergoing elective primary total knee arthroplasty (TKA). Therefore, we conducted a systematic review of prospective, retrospective, and other observational studies that compare the postoperative outcomes of patients with and without preoperative cognitive impairment after undergoing elective primary TKA requiring general or neuraxial anesthesia. The aims were to assess the impact of a spectrum of preoperative cognitive impairment on postoperative recovery and to evaluate the strength of the existing body of evidence. One hypothesis is that patients with preoperative cognitive impairment will have a more complicated in-hospital course, including higher risk of delirium, higher rates of mortality and complications, likelier discharge to a health-care facility, and lengthier hospital stays. Another hypothesis is that these patients will have higher rates of complications and mortality in the short term (within 30 days of surgery), higher rates of mortality and complications, and decreased functionality in the midterm (1 month to 2 years after surgery). Finally, this systematic review analyzes the body of literature to determine the strength of current knowledge and proposes areas for future research.
Methods
Protocol and Registration
The protocol for this systematic review was designed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We enlisted the assistance of a research librarian and statistician in developing the protocol at Countway Library of Medicine, Boston, Massachusetts. The protocol was registered with International prospective register of systematic reviews (PROSPERO) (registration number: CRD42017072154. Web site: http://www.crd.york.ac.uk/PROSPERO).
We performed a literature search in several databases, including PubMed, Embase, and MEDLINE on November 5, 2017, for literature published between January 1, 1997, and November 1, 2017. Database-specific search was completed using the following search term list: Alzheimer, dementia, cognition, cognitive defects, cognitive deficits, cognitive disorders, cognitive dysfunction, cognitive function, cognitive impairment, cognitive status, memory defects, memory deficits, memory disorders, memory dysfunction, memory impairment, or mental function; and anesthesia, surgery, surgical procedure, or operation; and outcomes, outcome assessment, prognosis, or surgical outcomes; not children or pediatrics.
We applied filters to narrow search results to human species, English-only, and by study type to exclude conference abstracts, case reports, comments, editorials, essays, letters, meta-analyses, reviews, and unpublished studies. Duplicates from the databases were removed. Any additional articles that were later identified through reviewing the references of the included articles were added.
Inclusion/Exclusion Criteria
We included studies of patients with stated cognitive impairment who received an elective primary TKA. Cognitive impairment is defined by previously diagnosed dementia or through preoperative cognitive assessment. Included studies focused on adult patients aged ≥60 years, had a comparator group of patients who did not have cognitive impairment, were peer reviewed, and had a study design of a prospective cohort, retrospective cohort, or case–control study.
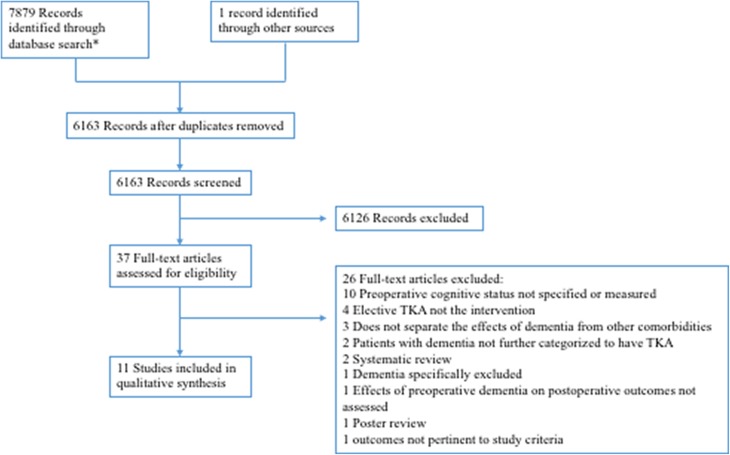
We excluded studies that lacked a comparator group of patients without cognitive impairment, excluded patients with cognitive impairment, did not specify patients’ cognitive status, did not have a TKA as the surgical intervention, and did not study the outcomes of interest. We also excluded studies that were not in English, case reports, oral presentations, posters, opinion articles, and abstracts. Other reasons for exclusion are listed in Figure 1.
Figure 1.
Flowchart for literature search and screening. *Articles were identified from PubMed (n = 4242) and Embase/MEDLINE (n = 3637).
We were interested in in-hospital outcomes (delirium, mortality, complications, discharge disposition, and length of stay [LoS]), short-term outcomes (mortality and complications within 30 days of operation), and midterm outcomes (mortality, complications, and functionality between 1 month and 2 years of operation).
Study Selection and Data Extraction
The studies were selected through a 2-step process. In the first phase, 2 reviewers (BLE and JME) independently ran the search criteria in the databases and reviewed the titles and abstracts to determine eligibility. In the second phase, one reviewer (BLE) obtained full-text articles from the initial screen and independently determined whether articles were to be included. A second reviewer (RDU) assessed the validity of reasons for the excluded articles. Data were extracted from the final set of studies. Extracted data included author, publication year, location, study design, setting, population, size, intervention, method of cognitive assessment, criteria for cognitive impairment, outcome measures, and results.
Data Analysis
We performed a qualitative analysis of the included studies. Given the paucity of studies and heterogeneity of the patient population, definition of cognitive impairment, and outcome measures, we were not able to perform a meta-analysis. In our qualitative analysis, we summarized the study characteristics, measures used to assess cognitive impairment, and the associations found between preoperative cognitive impairment and in-hospital, short-term, and midterm postoperative outcomes. We then assessed the strength of evidence for each outcome measure based on the number and quality of the studies and consistency of results.
Quality Assessment
The quality of the observational studies was assessed using the Newcastle-Ottawa Scale, which is a quality evaluation tool for nonrandomized studies that evaluates studies based on the domains of selection, comparability, and outcome.15 Any study scoring 3 or 4 stars in the selection domain, 1 or 2 stars in the comparability domain, and 2 or 3 stars in outcome/exposure domain was considered “good quality.” A study scoring 2 stars in the selection domain, 1 or 2 stars in the comparability domain, and 2 or 3 stars in the outcome/exposure domain was “fair quality.” Otherwise, the study was “poor quality.” We then used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach in assessing the strength of the body of evidence based on the number and quality of studies and consistency of results.
We attempted to minimize study selection bias by having predefined inclusion and exclusion criteria, dual review, a third-party arbitrator, and documentation of reasons for excluding studies. No studies were excluded based on how cognitive impairment was defined, study quality, or size.
Results
Literature Search
A total of 6163 unique articles were identified and screened. Of the 37 full-text articles that were reviewed, 25 articles were excluded for reasons listed in Figure 1. Eleven articles fulfilled selection criteria and were included in this review.
Study Characteristics
Individual study characteristics are listed in Table 1. All 11 studies assessed for preoperative cognitive impairment and evaluated its effects on postoperative outcomes. Seven of the studies were conducted in the United States. The remaining included 2 from Finland and 1 each from Italy and Taiwan. All were published during or after 2010. Study design included 1 case–control study, 6 prospective cohort studies, and 4 retrospective cohort studies. The studies involved a total of 9 487 108 patients undergoing primary elective TKA. Six studies involved patients undergoing either total hip arthroplasty (THA) or TKA, 2 were spine/THA/TKA, and 3 were TKA-only.
Table 1.
Characteristics of Included Studies.
| First Author, Year (Location) | Design, Setting | Population | n (TKA; Total) | Intervention | Cognitive Assessment | Criteria for Cognitive Impairment | Associations |
|---|---|---|---|---|---|---|---|
| Guerini, 2010 (Italy)16 | Case–control, single site | ≥65 years, MMSE > 15 | 88; 222 | TKA/THA | MMSE | None | Lower MMSE scores were strongly linked to mortality at 12 months |
| Jankowski, 2011 (United States)17 | Prospective cohort, single site | ≥65 years, excluded MMSE < 23 | 209; 418 | TKA/THA/revision | MMSE, AMNART, AVLT, SCWT, COWAT | None | Low AVLT scores, but not MMSE or AMNART, strongly predicted POD |
| Liang, 2015 (Taiwan)18 | Prospective cohort, single site | ≥60 years | 200; 461 | TKA/THA/spine | MMSE | MMSE <24/30 | MMSE <24 independently predicts POD, longer LoS, nursing home admission, mortality at 1- and 3-month follow-up, and functional decline at 3, 6, and 12 months |
| Tow, 2016 (United States)19 | Prospective cohort, single site | ≥60 years, excluded dementia or MMSE <24 | 98; 142 | TKA/THA/spine | CAS, MMSE | MMSE <27/30 | MMSE >27 and participation in greater number of cognitive activities were independently associated with decreased risk for POD |
| Puustinen, 2016 (Finland)20 | Prospective cohort, single site | ≥74 years | 28; 52 | TKA/THA | MoCA, MMSE, Mini-Cog, Clock-Drawing Test | MoCA <25/30; MMSE <24/30 | Low MoCA and MMSE scores were possibly associated with POD, discharge to health-care facility, and decreased functionality at 3 months |
| Culley, 2017 (United States)8 | Prospective cohort, single site | ≥65 years, excluded dementia | 123; 211 | TKA/THA | Mini-Cog | Mini-Cog ≤2 | Low Mini-Cog score independently associated with POD, discharge to place other than home, and longer LoS. No association between low Mini-Cog scores and in-hospital adverse events, or 30-day emergency visit |
| Memtsoudis, 2010 (United States)21 | Retrospective cohort, multisite | ≥60 years, excluded dementia or MMSE <24 | 3 830 234; 6 901 324 | TKA/THA/revision | Preexisting dementia | Preexisting | Preexisting dementia possibly associated with in-hospital death |
| Bozic, 2012 (United States)22 | Retrospective cohort, multisite | ≥65 years | 83 011; 83 011 | TKA | Preexisting dementia | Preexisting | Preexisting dementia possibly independently associated with 90-day postoperative mortality |
| Bozic, 2014 (United States)23 | Retrospective cohort, multisite | ≥65 years | 117 903; 117 903 | TKA | Preexisting dementia | Preexisting | Preexisting dementia possibly not associated with revision TKA in a year |
| Buller, 2014 (United States)24 | Retrospective cohort, multisite | ≥18 years | 5 455 047; 8 379 490 | TKA/THA | Preexisting dementia | Preexisting | Preexisting dementia possibly independently associated with in-hospital adverse events and nonroutine discharge. Possible association with in-hospital mortality and longer LoS |
| Vekama, 2015 (Finland)25 | Prospective cohort, single site | ≥75 years | 167; 167 | TKA | Preexisting dementia | Preexisting | No association between dementia and ADL 12 months postoperatively |
Abbreviations: ADL, activities of daily living; AMNART, American National Adult Reading Test; AVLT, Auditory Verbal Learning Test; CAS, Cognitive Activity Scale; COWAT, Controlled Word Association Test; LoS, length of stay; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; POD, postoperative delirium; SCWT, Stroop Color-Word Test; TKA, total knee arthroplasty; THA, total hip arthroplasty.
Measures of Cognitive Impairment
Five studies assessed for preoperative cognitive impairment based on preexisting dementia diagnosis and the remaining 6 used various combinations of cognitive evaluation tools, which are specified in Table 1. Among these 6 studies, all but one study included Mini-Mental State Examination (MMSE) in their assessment tool box. Three utilized MMSE in combination with other assessment tools. For example, Jankowski et al utilized American National Adult Reading Test (AMNART), Auditory Verbal Learning Test (AVLT), Stroop Color-Word Test (SCWT), and Controlled Word Association Test (COWAT), while Puustinen et al also utilized Mini-Cog and Clock-Drawing Test.17,20 On the other hand, Culley et al relied solely on the Mini-Cog for assessment.3 Four of the 6 studies that utilized cognitive evaluation tools had specific cutoff values for cognitive impairment, which are specified in Table 1. The other 2 studies analyzed cognitive impairment as a linear variable.
Association of Cognitive Impairment With In-Hospital Outcomes
Five studies investigated the impact of preoperative cognitive impairment on postoperative delirium (POD). All 5 used prospective cohort, single-site study design with a combined total of 658 patients who underwent TKA. All but one were good quality based on the Newcastle-Ottawa Scale and had consistent results demonstrating that poor preoperative performance on cognitive function tests predicted POD. However, these studies excluded patients who had diagnoses of dementia or had a low MMSE. Therefore, the conclusions of these studies apply to patients who have a normal or nearly normal MMSE but have subtle cognitive impairment that may not be apparent without performing time-consuming cognitive testing. Using univariate analysis, Jankowski et al showed that lower AVLT, SCWT, and COWAT scores, rather than MMSE and AMNART scores, predicted development of POD.17 Preoperative functional ability, assessed by activities of daily living (ADL) and instrumental ADL (IADL), were also lower in those who developed POD. In a multivariate analysis controlling for age, AVLT, a verbal learning and memory test, best predicted POD (odds ratio [OR] = 0.58, 95% confidence interval [CI] = 0.41-0.82, P = .002). Similarly, Liang et al used multivariate analysis that controlled for age, gender, risk of malnutrition, blood transfusion, and surgery type to show that MMSE <24 independently predicted POD (OR = 3.181, 95% CI = 1.410-7.173).18 Other predictors of POD included age ≥75, male gender, Charlson comorbidity index ≥2, risk of malnutrition, blood transfusion, and spine surgery. In addition, the Tow et al’s study found that when controlling for age, sex, Charlson comorbidity index, and literacy, a greater number of regular cognitive activities was associated with lower incidence of POD (OR = 0.92 for an increase in 1 activity per week, 95% CI = 0.86-0.98, P = .006).19 Higher MMSE score of >27 was also independently associated with lower risk for POD (OR = 0.67, 95% CI = 0.52-0.85, P = .001). Additionally, Puustinen et al showed that preoperative Montreal Cognitive Assessment (MoCA) <25 or MMSE <24 scores were associated with POD.20 However, this study did not demonstrate representativeness of the selected cohort and did not use multivariate analysis to control for comorbidities. Finally, Culley et al demonstrated that a Mini-Cog ≤2 predicted development of POD (OR = 4.52, 95% CI = 1.30-15.68) when controlling for age.3
Two studies investigated the impact of preoperative cognitive impairment on postoperative in-hospital mortality. All were large, retrospective, population-based, multisite cohort studies. All were good quality studies according to the Newcastle-Ottawa Scale. However, neither evaluated for MCI as they relied on preexisting diagnosis of dementia, and the results were generally consistent. Buller et al found that patients with dementia were at risk of in-hospital mortality.24 No multivariate analysis was conducted by Buller et al to control for demographics or comorbidities. Lastly, in a study that controlled for age, sex, hospital size, procedure type, and payment source, Memtsoudis et al found that a preexisting diagnosis of dementia (OR = 7), along with renal disease (OR = 7) and cerebrovascular disease (OR = 4.5), was associated with in-hospital death.21
Two studies investigated the impact of preoperative cognitive impairment on postoperative in-hospital complications. One was a large, retrospective, population-based, multisite cohort study; one was a prospective cohort, single-site study. Both were good quality studies according to the Newcastle-Ottawa Scale. However, even though both performed multivariate analysis, the results were inconsistent between the studies. For example, Culley et al demonstrated that a Mini-Cog of ≤2 was associated with adverse cardiac events via age-adjusted univariate but not multivariate analysis.3 Other complications occurred too infrequently to be analyzed. On the contrary, Buller et al, in a retrospective cohort, multisite study demonstrated that patients with all preexisting diagnosis of psychiatric comorbidities including dementia had a higher odds of adverse events (OR = 1.056, 95% CI = 1.019-1.095, P < .001) when controlling for age and other comorbidities.24 Adverse events such as postoperative bleeding, acute postoperative anemia, transfusion of blood, pulmonary embolism, and myocardial infarction were more common among patients with dementia (all P <.001).
Four studies investigated the impact of preoperative cognitive impairment on discharge disposition. Three of these studies were prospective, single-site studies, and 1 was a large, retrospective, population-based, multisite cohort study. Two of the 3 prospective studies were good quality studies based on the Newcastle-Ottawa Scale. These 2 studies had a combined TKA patient population of 323. The results of all 4 studies consistently suggested that patients with MCI were at higher risk of discharge to a health-care facility. For example, Puustinen et al used univariate analysis to show that preoperative MoCA <25, MMSE <24, and Mini-Cog ≤2 predicted the need for discharge to a health-care facility other than home.20 Similarly, Culley et al demonstrated in a multivariable analysis adjusting for age that a Mini-Cog ≤2 predicted discharge to a place other than home (OR = 3.88, 95% CI = 1.58-9.55).3 Additionally, Buller et al, using multivariable logistic regression analysis controlling for age and other comorbidities, demonstrated that patients with all psychiatric comorbidities, including dementia, had higher odds of nonroutine discharge (OR = 4.523, 95% CI = 4.367-4.684, P < .001).24 Finally, Liang et al’s risk model, which included MMSE <24, age ≥75, male gender, Charlson comorbidity index ≥2, risk of malnutrition, blood transfusion, and spine surgery, predicted nursing home admission.18
Three studies investigated the impact of preoperative cognitive impairment on hospital LoS. The study designs varied: 2 prospective cohort single-site, and 1 large population-based, retrospective, multisite cohort study. All were good quality studies based on the Newcastle-Ottawa Scale, and the results consistently showed that patients with cognitive impairment had higher risk for lengthier hospital stays. The prospective cohort studies had a total of 323 participants undergoing TKA. Liang et al’s risk model, as previously described, suggested that MMSE <24 predicted lengthier hospital stays.18 Furthermore, Culley’s multivariable model demonstrated that a Mini-Cog ≤2 predicted longer hospital stay (hazard ratio [HR] = 0.63, 95% CI = 0.42-0.95).3 Finally, using univariate analysis, Buller suggested an association between a preexisting dementia diagnosis and longer hospital stay.24
Association of Cognitive Impairment With Short-Term Outcomes
Two studies investigated the impact of preoperative cognitive impairment on mortality within 30 days and one of these studies also investigated the impact of preoperative cognitive impairment on complications within 30 days. The prospective cohort studies had a total of 323 patients with TKA. All were good quality studies according to the Newcastle-Ottawa Scale. However, results among the studies were inconsistent. For example, Culley et al found that a Mini-Cog ≤2 did not predict an emergency department visit within 30 days, while 30-day mortality occurred too infrequently to be analyzed.3 On the other hand, Liang et al’s risk model, described previously, suggested that MMSE <24 predicted mortality at 30-day follow-up.18
Association of Cognitive Impairment With Midterm Outcomes
Three studies investigated the impact of preoperative cognitive impairment on mortality between 2 months and 2 years postoperatively. All were good quality studies according to the Newcastle-Ottawa Scale. Aside from the large retrospective study, the studies included a total of 288 patients with TKA. All found that patients with dementia were at higher risk of mortality within the first year postoperatively. In a case–control study, Guerini et al found that patients who died at 12 months postoperatively were older and had lower average MMSE scores.16 Liang et al’s risk model, described previously, suggested that MMSE <24 predicted mortality at 90 days postoperatively.18 Bozic et al’s retrospective cohort study showed that when controlling for other comorbidities, patients with preexisting dementia were at increased risk for 90-day postoperative mortality (HR = 1.83, 95% CI = 1.10-3.07, P = .0206).22 However, this study did not assess the effects of MCI.
Three studies investigated the impact of preoperative cognitive impairment on functionality between 2 months and 2 years postoperatively. The findings from these studies were inconsistent. A total of 395 patients with TKA were included. In Vekama et al’s study, of 167 patients, only 5 had preexisting dementia.25 The study found that the effect of dementia on ADL performance 1 year postoperatively was not significant. However, ADL survey results were self-reported. Liang et al’s risk model, described previously, suggested that MMSE <24 predicted functional decline at 3, 6, and 12 months.18 Puustinen et al showed that preoperative MoCA scores <25 predicted decreased IADLs 3 months after surgery by an average of 0.48 points but did not predict changes in ADLs or low Knee Society Score.20 However, this study did not demonstrate representativeness of selected cohort and did not use multivariate analysis to control for demographics and comorbidities.
Only one study investigated the impact of preoperative cognitive impairment on complications between 2 months and 2 years postoperatively. This study was a good quality large retrospective cohort study. Bozic et al found that dementia was not a risk factor for revision TKA within 12 months, even when controlling for other comorbidities.23 However, only 0.9% of the study participants had dementia, and it is possible, although not explicitly stated by the authors, that the study was underpowered to detect a meaningful statistical association.
Quality of Included Studies and Risk for Bias
The grading scheme by domain for each article is specified in Table 2. Ten of the 12 articles were good quality, while 2 were poor quality. One of the poor quality articles had high risk of bias in comparability because the study performed only univariate analysis (Puustinen et al). The other study of poor quality had self-reported outcomes, contributing to high risk of bias in the outcomes domain (Vekama et al). The good quality articles had variable risk of bias in selection, comparability, and outcome.
Table 2.
Newcastle-Ottawa Scale Assessment of Study Quality.
| First Author, Year | Selection | Comparability | Outcome | Total | Quality |
|---|---|---|---|---|---|
| Guerinia, 201016 | 4 | 1 | 3 | 8 | Good |
| Jankowski, 201117 | 4 | 2 | 3 | 9 | Good |
| Vekama, 201525 | 3 | 2 | 1 | 6 | Poor |
| Liang, 201517 | 4 | 2 | 2 | 8 | Good |
| Tow, 201619 | 4 | 2 | 3 | 9 | Good |
| Puustinen, 201620,18 | 2 | 0 | 3 | 5 | Poor |
| Culley, 20178 | 3 | 2 | 3 | 8 | Good |
| Memtsoudis, 201021 | 4 | 2 | 3 | 9 | Good |
| Bozic, 201222 | 4 | 2 | 3 | 9 | Good |
| Bozic, 201423 | 4 | 2 | 3 | 9 | Good |
| Buller, 201424 | 4 | 2 | 3 | 9 | Good |
Strength of Evidence
The strength of evidence for each outcome measure is specified in Table 3. Using the GRADE criteria, which is based on the number and quality of studies and consistency of results, the body of evidence for the effect of preoperative cognitive impairment on in-hospital outcomes such as POD, disposition, and LoS is of moderate quality. The body of evidence regarding in-hospital mortality, in-hospital complications, and short-term and midterm outcomes is of low quality.
Table 3.
Outcome Measures Assessed in Studies Meeting Inclusion Criteria and the Strength of the Body of Evidence for Each Outcome Studied.
| First Author, Year | In-Hospital | Short-Term (Within 30 days) | Midterm (1 Month to 2 Years) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| POD | Mortality | Complications | Discharge Disposition | Length of Stay | Mortality | Complications | Mortality | Functionality | Complications | |
| Guerinia, 201016 | X | |||||||||
| Jankowski, 201117 | X | |||||||||
| Vekama, 201525 | X | |||||||||
| Liang, 201518 | X | X | X | X | X | X | ||||
| Tow, 201619 | X | |||||||||
| Puustinen, 201620 | X | X | X | |||||||
| Culley, 20178 | X | X | X | X | X | X | ||||
| Memtsoudis, 201021 | X | |||||||||
| Bozic, 201222 | X | |||||||||
| Bozic, 201423 | X | |||||||||
| Buller, 201424 | X | X | X | X | ||||||
| Summary of evidencea | (4) 2b, (1) 4; consistent results | (2) 2b; inconsistent results | (2) 2b; inconsistent results | (3) 2b, (1) 4; consistent results | (3) 2b, consistent results | (2) 2b; inconsistent results | (1) 2b; inconsistent results | (1) 3b, (2) 2b; consistent results | (2) 4, (1) 2b; inconsistent results | (1) 2b |
| Quality of body of evidenceb | Moderate | Low | Low | Moderate | Moderate | Low | Low | Low | Low | Low |
a Level of evidence based on Oxford Centre for Evidence-based Medicine’s “Levels of Evidence.”
b Grading based on the Cincinnati Children’s Hospital Medical Center Evidence Collaboration’s “Grading the Body of Evidence.”
Discussion
Based on a total of 11 studies identified using our search criteria, this systematic review demonstrates that there is moderate quality evidence that preoperative cognitive impairment predisposes patients undergoing elective primary TKA to worse in-hospital outcomes, increasing their risk for delirium, lengthier hospital stays, and discharge to a health-care facility. There is low-quality evidence that preoperative cognitive impairment increases the risk for in-hospital complications and mortality or worsens short-term outcomes (mortality, complications) within 30 days postoperatively and midterm outcomes (mortality, functionality) within 1 month to 2 years postoperatively.
This is the first systematic review to specifically assess preoperative cognitive impairment as a prognostic factor for postoperative outcomes among patients with TKA aged ≥60 years. Previously, a systematic review by Bin Abd Razak in 2015 assessed the risk factors for POD in patients undergoing hip arthroplasty and concluded that independent predictors included age, history of psychiatric illness, decreased functional status, and verbal memory.26 Several features distinguish our systematic review from Bin Abd Razak’s. Rather than searching for all risk factors for delirium, we examined studies that specifically assessed for preoperative cognition and that compared outcomes between patients with and without cognitive impairment. We also included in our review 4 articles on POD published after 2015 and examined other outcomes besides delirium. We also focused specifically on knee replacement surgery because significant differences distinguish the population receiving THA from those receiving TKA.27-29 Patients receiving TKA are generally older and have more severe osteoarthritis, greater number of comorbidities, yet better presurgical functionality. Other systematic reviews more often evaluate orthopedic surgery along with nonorthopedic surgeries. For example, one systematic review aimed to identify preoperative risk factors that predict postoperative complications in elderly patients.30 The review found that functional dependence, frailty, history of falls, weight loss ≥10%, American Society of Anesthesiologists Physical Status classification score ≥2, and cognitive impairment predicted discharge destination. Cognitive impairment also predicted lengthier hospital stays and postoperative mortality. However, the review did not specifically include articles on patients undergoing TKA or assess for cognitive function.
The US population will have an increasing percentage of adults older than 65 years, and this population is expected to double from 43.1 million in 2012 to 83.7 million in 2050.6 Between 2000 and 2006, the rate of TKA among Medicare enrollees increased by 58% from 145 242 in 2000 to 248 267 in 2006.31 It has been established that preexisting dementia is prevalent among this aging population. Alzheimer disease is estimated to be as prevalent as 1 in 9 individuals aged 65 years or older.32 The number of patients with Alzheimer disease will triple by 2050.33 It is also the fifth leading cause of death in individuals over 65 years.34 However, these statistics do not account for those with MCI. In the included studies of this systematic review, patients with MCI were overall as prevalent as 24%, as shown in a study by Culley.3 Mild cognitive impairment and preexisting dementia altogether may constitute an even greater percentage of patients undergoing primary TKA.
This systematic review found a moderate quality body of evidence suggesting that MCI or preexisting dementia is a prognostic factor for POD, lengthier hospital stay, and discharge to health-care facility after primary TKA. Dementia predisposes patients to POD due to decreased cognitive reserve.35 However, this systematic review suggests that MCI may also predispose patients to POD. We also found that patients with MCI have a lengthier hospital stay and higher risk for discharge to rehabilitation. Possible reasons for these include a higher risk of aspiration from dysphagia and inability to verbalize pain or communicate symptoms.10-12
Most of the studies examined suggest that preoperative knowledge of dementia or MCI cannot prevent postoperative complications, increased LoS, or risk for POD. However, the knowledge of the patient’s cognitive status preoperatively can still help the physician and patient’s family plan to prevent these complications postoperatively. It may help facilitate shared decision-making about the patient’s plan of care and help better inform patients and their families about a higher risk for complications. For example, team-based approaches in the postoperative setting with geriatric consultation and comanagement have reduced complications in elderly patients, thereby reducing readmission rates and cost of care.36-40 Once a cognitive impairment is suspected or diagnosed, preoperative physical and cognitive exercises have also been used to decrease the risk of delirium.40,41 Knowledge of postoperative outcomes is an integral part of discharge planning, including anticipating if patients will require discharge to a rehabilitation facility, discharge home with special services, or have more frequent follow-up in the clinic as patients with MCI may have a decreased ability to care for wounds, manage medications, and participate adequately with physical rehabilitation.42
For those with more advanced forms of dementia, realistic discussions of outcomes after surgery are a vital part of informed consent. The existing literature shows that patients with limited life expectancy are often unwilling to undergo procedures that carry a significant risk of impairing their functional status.43 The increased mortality observed after TKA in patients with preoperative dementia may reflect a risk commensurate with the difference in population norms, and unfortunately, the studies analyzed here do not evaluate that effect. Increased risk of the postoperative course must be weighed against the baseline outcomes of the dementia itself. One study showed that approximately one-fourth of all patients with a diagnosis of advanced dementia will die within 6 months; moreover, half will die within 18 months.44 With a median survival rate of 1.3 years in the study sample, this leaves life expectancy in advanced dementia approximately the same as that of other terminal illnesses, such as metastatic breast cancer.45 However, only 18% of all individuals who were listed as the patient’s health-care proxy reported receiving prognostic information from a physician.44
In summary, by understanding the risks and benefits of surgery in an individual with MCI or dementia among elderly patients, we can optimize resource allocation in the care of these individuals postoperatively and to encourage shared decision-making with patients and their families regarding postdischarge planning. Further studies are needed to strengthen the current body of evidence to identify the types of in-hospital complications encountered by patients with MCI and to determine what method of cognitive assessment is best used in the preoperative setting. Further studies on cognitive function as a prognostic factor after primary TKA are also needed to help us better understand short-term and midterm outcomes within this population.
Strengths and Limitations
Our systematic review has several strengths. First, we developed a specific protocol with dual reviewers, clear inclusion and exclusion criteria, and good documentation of reasons for exclusion. Second, our search terms included a broad range of terms relating to cognitive impairment that ensures the inclusion of all relevant published literature.
There are several limitations of our systematic review. We included English-only studies, so we may have inadvertently excluded good quality studies published in other languages. We also have restricted our search to 3 databases, so we may have missed studies not published in Embase, MEDLINE, or PubMed. We also excluded posters and abstracts, so there are data not included in this review. There may also be unpublished data that did not find a significant prognostic effect of cognitive impairment. Another limitation is that 4 of the studies refer to large database studies, and half of them report on the same population. There is also the common problem associated with database studies, specifically the inconsistencies with accuracy and rigorousness of International Classification of Diseases (ICD)-9 diagnoses which could lead to an overestimation or underestimation of the actual effect of dementia on the outcomes studied.
Conclusion
We identified a small number of both good and poor quality studies that evaluated preoperative cognitive impairment as a prognostic factor for postoperative in-hospital, short-term, and midterm outcomes after primary TKA. The overall body of evidence is moderate for increased risk of POD, increased LoS, and discharge to health-care facility in patients with MCI or preexisting dementia. The body of evidence is weak for other outcomes of interest. This review highlights the need for good quality studies to provide more information about MCI and dementia as a risk factor in primary TKA.
Footnotes
Authors’ Note: Betty M. Luan Erfe and Richard D. Urman are now affiliated to Department of Medicine, Internal Medicine Program, Queens Hospital Center, NY, USA. J. Mark Erfe is now affiliated to Division of Cardiac Surgery, Bluhm Cardiovascular Institute of Northwestern Medicine, IL, USA.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Kulmala J, Nykänen I, Mänty M, Hartikainen S. Association between frailty and dementia: a population-based study. Gerontology. 2014;60(1):16–21. [DOI] [PubMed] [Google Scholar]
- 2. Robinson TN, Wu DS, Pointer LF, Dunn CL, Moss M. Preoperative cognitive dysfunction is related to adverse postoperative outcomes in the elderly. J Am Coll Surg. 2012;215(1):12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Culley DJ, Flaherty D, Fahey MC, Rudolph JL Javedan H, Huang CC. Poor performance on a preoperative cognitive screening test predicts postoperative complications in older orthopedic surgical patients. Perioperative medicine. Anesthesiology. 2017;127(5):765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laine C, Williams SV, Wilson JF. In the clinic. Preoperative evaluation. Ann Intern Med. 2009;151(1):ITC1–ITC15. [DOI] [PubMed] [Google Scholar]
- 5. Durso SC. Geriatric Review Syllabus—A Core Curriculum in Geriatric Medicine. In: Medina-Walpole A, Pcala JT, eds. New York, NY: American Geriatrics Society; 2006. [Google Scholar]
- 6. US Census. An Aging Nation: The Older Population in the United States. 2014. https://www.census.gov/prod/2014pubs/p25-1140.pdf. Accessed November 23, 2018.
- 7. World Health Organization. Dementia: a public health priority. 2012. http://www.who.int/mental_health/publications/dementia_report_2012/en/. Accessed November 23, 2018.
- 8. Culley DJ, Flaherty D, Reddy S, et al. Preoperative cognitive stratification of older elective surgical patients: a cross-sectional study. Anesth Analg. 2016;123(1):186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zaal IJ, Devlin JW, Peelen LM, Slooter AJ. A systematic review of risk factors for delirium in the ICU. Crit Care Med. 2015;43(1):40–47. [DOI] [PubMed] [Google Scholar]
- 10. Ninoa GD, Adversia M, Boaz G, et al. Peri-operative risk management in patients with Alzheimer’s disease. J Alzheimers Dis. 2010;22(suppl 3):S121–S127. [DOI] [PubMed] [Google Scholar]
- 11. Hu CJ, Liao CC, Chang CC, Wu CH, Chen TL. Postoperative adverse outcomes in surgical patients with dementia: a retrospective cohort study. World J Surg. 2012;36(9):2051–2058. [DOI] [PubMed] [Google Scholar]
- 12. Horner J, Alberts MJ, Dawson DV, Cook GM. Swallowing in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 1994;8(3):177–189. [DOI] [PubMed] [Google Scholar]
- 13. Ansaloni L, Catena F, Chattat R, et al. Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br J Surg. 2010;97(2):273–280. [DOI] [PubMed] [Google Scholar]
- 14. Baranov D, Bickler PE, Crosby GJ, et al. First international workshop on anesthetics and Alzheimer’s disease consensus statement: first international workshop on anesthetics and Alzheimer’s disease. Anesth Analg. 2009;108(5);1627–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newcastle-Ottawa Quality Assessment Scale Case Control Studies. Ottawa Hospital; http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf. Accessed November 23, 2018. [Google Scholar]
- 16. Guerini F, Morghen S, Lucchi E, Bellelli G, Trabucchi M. Depressive symptoms and one year mortality among elderly patients discharged from a rehabilitation ward after orthopaedic surgery of the lower limbs. Behav Neurol. 2010;23(3):117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jankowski CJ, Trenerry MR, Cook DJ, et al. Cognitive and functional predictors and sequelae of postoperative delirium in elderly patients undergoing elective joint arthroplasty. Anesth Analg. 2011;112(5):1186–1193. [DOI] [PubMed] [Google Scholar]
- 18. Liang CK, Chu CL, Chou MY, et al. Developing a prediction model for post-operative delirium and long-term outcomes among older patients receiving elective orthopedic surgery: a prospective cohort study in Taiwan. Rejuvenation Res. 2015;18(4):347–355. [DOI] [PubMed] [Google Scholar]
- 19. Tow A, Holtzer R, Wang C, et al. Cognitive reserve and postoperative delirium in older adults. J Am Geriatr Soc. 2016;64(6):1341–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puustinen J, Luostarinen L, Luostarinen M, et al. The use of MoCA and other cognitive tests in evaluation of cognitive impairment in elderly patients undergoing arthroplasty. Geriatr Orthop Surg Rehabil. 2016;7(4):183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Memtsoudis SG, Della Valle AG, Besculides MC, Esposito M, Koulouvaris P, Salvati EA. Risk factors for perioperative mortality after lower extremity arthroplasty: a population-based study of 6,901,324 patient discharges. J Arthroplasty. 2010;25(1):19–26. [DOI] [PubMed] [Google Scholar]
- 22. Bozic KJ, Lau E, Kurtz S, Ong K, Berry DJ. Patient-related risk factors for postoperative mortality and periprosthetic joint infection in medicare patients undergoing TKA. Clin Orthop Relat Res. 2012;470(1):130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary TKA in Medicare patients. Clin Orthop Relat Res. 2014;472(1):232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buller LT, Best MJ, Klika AK, Barsoum WK. The influence of psychiatric comorbidity on perioperative outcomes following primary total hip and knee arthroplasty; a 17-year analysis of the national hospital discharge survey database. J Arthroplasty. 2015;30(2):165–170. [DOI] [PubMed] [Google Scholar]
- 25. Vekama L, Puolakka T, Honkasalo M, Huhtala H, Moilanen T, Jämsen E. Functional gain following knee replacement in patients aged 75 and older: a prospective follow-up study. Aging Clin Exp Res. 2015;27(6):865–876. [DOI] [PubMed] [Google Scholar]
- 26. Bin Abd Razak HR, Yung WY. Postoperative delirium in patients undergoing total joint arthroplasty: a systematic review. J Arthroplasty. 2015;30(8):1414–1417. [DOI] [PubMed] [Google Scholar]
- 27. Neuprez A, Neuprez AH, Kurth W, Gillet P, Bruyère O, Reginster JY. Profile of osteoarthritic patients undergoing hip or knee arthroplasty, a step toward a definition of the “need for surgery”. Aging Clin Exp Res. 2018;30(4):315–321. [DOI] [PubMed] [Google Scholar]
- 28. Dabare C, Le Marshall K, Leung A, Page CJ, Choong PF, Lim KK. Differences in presentation, progression and rates of arthroplasty between hip and knee osteoarthritis: observations from an osteoarthritis cohort study—a clear role for conservative management. Int J Rheum Dis. 2017;20(10):1350–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tilbury C, Holtslag MJ, Tordoir RL, et al. Outcome of total hip arthroplasty, but not of total knee arthroplasty, is related to the preoperative radiographic severity of osteoarthritis. A prospective cohort study of 573 patients. Acta Orthop. 2016;87(1):67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watt JA, Tricco A, Talbot-Hamon C, Grudniewicz A, Sinclair D, Straus S. Preoperative risk factors predict risk of delirium and other postoperative complications among elderly patients undergoing elective surgery: a systematic review. J Gen Intern Med. 2016;31:S356–S357. [Google Scholar]
- 31. Centers for Disease Control and Prevention. Racial disparities in total knee replacement among Medicare enrollees—United States, 2000-2006. 2009. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5806a1.htm. Accessed November 23, 2018. [PubMed]
- 32. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thies W, Bleiler L. 2011. Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7(2):208–244. [DOI] [PubMed] [Google Scholar]
- 34. Alzheimer’s Association. 2013. Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2013. https://www.alz.org/alzheimers-dementia/facts-figures. Accessed November 23, 2018. [DOI] [PubMed]
- 35. Silverstein JH, Deiner SG. Perioperative delirium and its relationship to dementia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338–1344. [DOI] [PubMed] [Google Scholar]
- 37. Flood KL, Maclennan PA, McGrew D, Green D, Dodd C, Brown CJ. Effects of an acute care for elders unit on costs and 30-day readmissions. JAMA Intern Med. 2013;173(11):981–987. [DOI] [PubMed] [Google Scholar]
- 38. Ellis G, Whitehead MA, Robinson D, O’Neill D, Langhorne P. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. Br Med J. 2011;343:d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Partridge JS, Harari D, Martin FC, Dhesi JK. The impact of pre-operative comprehensive geriatric assessment on postoperative outcomes in older patients undergoing scheduled surgery: a systematic review. Anaesthesia. 2014;69(Suppl 1):8–16. [DOI] [PubMed] [Google Scholar]
- 40. Stammers AN, Kehler DS, Afilalo J, et al. Protocol for the PREHAB study—preoperative rehabilitation for reduction of hospitalization after coronary bypass and valvular surgery: a randomised controlled trial. BMJ Open. 2015;5(3):e007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Humeidan ML, Otey A, Zuleta-Alarcon A, Mavarez-Martinez A, Stoicea N, Bergese S. Perioperative cognitive protection–cognitive exercise and cognitive reserve (The Neurobics Trial): a single-blind randomized trial. Clin Ther. 2015;37(12):2641–2650. [DOI] [PubMed] [Google Scholar]
- 42. Robinson TN, Wallace JI, Wu DS, Wiktor A, Pointer LF, Pfister SM. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg. 2011;213(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061–1066. [DOI] [PubMed] [Google Scholar]
- 44. Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fabio E, Biganzoli L. Breast cancer In: Glare PC, Christakis NA, eds. Prognosis in Advanced Cancer. Oxford, England: Oxford University Press; 2008:123–132. [Google Scholar]