Abstract
Weaned piglets are vulnerable to nutritional, physiological, and psychological stressors, leading to abrupt taxonomic and functional shifts in the intestinal microbiome. In this study, an integrated approach combination of 16S rDNA gene sequencing and the mass spectrometry-based metabolomics techniques was used to investigate the effects of weaning stress on intestinal microbial composition and its metabolic profiles of piglets. Three litters of suckling piglets with same parity were chosen. The samples of colonic contents were collected from each selected piglets (weaned day, 3 days after weaned) for microbial and metabolomics analysis. The results showed that Lachnospiraceae, Negativicutes, Selenomonadales, Campylobacterales and other 15 species increased after weaning, while Porphyromonadaceace, Alloprevotella, Barnesiella and Oscillibacter decreased. Based on the function profiles prediction and metabolomic analysis, five key metabolic pathways including Phenylalanine metabolism, Citrate cycle (TCA cycle), Glycolysis or Gluconeogenesis, Propanoate metabolism, Nicotinate and nicotinamide metabolism might be the relevant pathways involved in weaning stress-induced gut microbiota dysbiosis. Taken together, these results indicated that weaning stress not only changed microbial composition and function but altered the microbial metabolic profiles in the intestine, which might provide a new insight in alleviating weaning stress and facilitating disease prevention during the period of weaning in piglets.
Introduction
Enormous numbers and diverse microbiota population inhabit the gastrointestinal tract in human beings and animals, being responsible for gut maturation, pathogens resistance and immune modulation. Shaped by co-evolution more than millions of years, a symbiotic relationship has formed between gut microbiota and host. Gut microbiota play a critical role in nutrient metabolism and in return occupy the nutrient-rich environment1. Increasing evidence showed that the dysbiosis of gut microbiota was intimately associated with many diseases, including inflammatory bowel disease, cardiovascular diseases, allergies, diabetes and obesity2,3. Thus, improvement in host health requires a better understanding of intestinal environment especially the gut microbiota. Gut microbiota remains relatively stable once established. However, many factors such as host genetics, environment, diet, immunological pressures and antibiotics can cause dramatic changes in the microbial community4. Owing to the similarities to human beings in anatomy and nutritional physiology, pigs often served as a biomedical model of human5,6. Investigation of gut microbiota in pigs not only benefits to construct a healthy gut of the animal but also provide important evidence for human.
The pig gut microbiota shows dynamic composition and diversity which shifts over time and along the whole gastrointestinal tract7. Colonization was started at birth and shaped by consumption of the sow’s milk, building a milk-oriented microbiome8. Therefore, the suckling period offers a special window of gut microbiota modifications. Weaning is usually conducted at around 3–4 weeks in the modern swine industry, and piglets are fed with solid diets instead of liquid milk. It is a sudden, complex and highly stressful event in pig’s life. Weaning piglets are usually vulnerable to nutritional, physiological, and psychological stressors, leading to alterations of intestinal morphology, physiological function, and a shift in intestinal microbiome (e.g., increased potential pathogens and diarrhea)9–11. And the disruption of gut microbiota is regarded as one of the major factors leading to post-weaning diarrhea12. Previous study showed the temporal change of gut architecture and function after weaned could be divided two periods: an acute change (about 5 days) occurs immediately after weaned and an adaptive and maturity phase13. Thus 1–5days after weaned are considered to be a period in which piglets suffered the most severe weaning stress.
Recently, the association among gut microbiota, metabolites and host physiology has gained increasing attention. Microbial metabolites are indispensable to maintain the majority of the biological effects of gut microbiota. The extraction, synthesis and absorption of metabolites could be mediated by intestinal commensal bacteria14. Microbiota could exploit ingested dietary components, such as carbohydrates, proteins, and lipids to produce metabolites. These metabolites are mainly the short-chained fatty acids (SCFAs), as well as branched-chain fatty acids. Besides, there are some other metabolites produced by microbiota, including choline metabolites, bile acid metabolites, indole and phenolic derivatives. These metabolites have been appreciated for their beneficial effects on intestinal barrier, immune regulation and inflammation15. Therefore, it is of particular significance to probe the metabolic changes associated with weaning-perturbed gut microbiota. The present work was designed to investigate the effects of weaning stress on gut microbiome and its metabolite profiles in piglets via an integrated approach combining 16S rDNA gene sequencing and GC-TOF/MS (Gas Chromatography Tandem Time-of-Flight Mass Spectrometry) method.
Results
Gut Microbiota Diversity and Structure of Nursing and Weaned Piglets
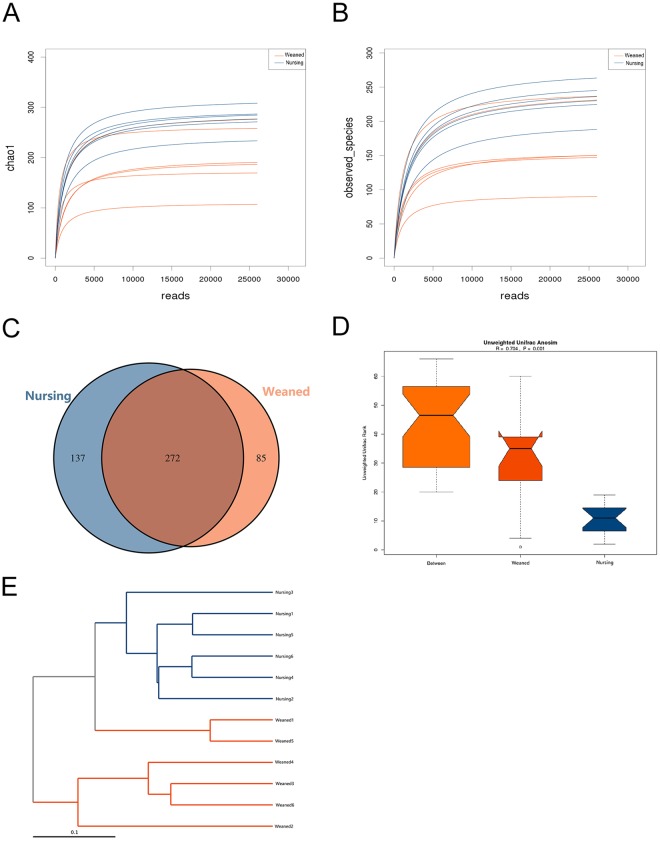
An average of 35595 high quality sequences per sample was obtained from 12 colonic content samples (Supplementary Table S1). The rarefaction Curves tended to be flat as shown in Fig. 1A,B, which demonstrated that almost all the bacterial species were detected in the colonic microbiota. Further, these sequences in the colonic contents of nursing and weaned piglets were assigned to 1,157 and 1,584 OTUs (operational taxonomic units) based on a 97% sequence similarity (Supplementary Table S2), respectively. Venn diagram reflected the number of OTUs that were common in groups as well as within the groups. This analysis showed there were 137 and 85 unique OTUs in weaned piglets and nursing piglets, respectively (Fig. 1C). The indexes of Chao1, observed species, PD-whole-tree, Shannon and Simpson were calculated to estimate alpha diversity. Shannon and Simpson indices, which reflect the species diversity, showed no significant difference in two groups. While, there were significant decrease in richness indices (Chao 1 and observed species) in weaned piglets compared to nursing piglets (Table 1). Unweifhted Unifrac Anosim and unweifhted Unifrac cluster tree based on UPGMA were used to analyze beta diversity (that is, diversity between individuals). Figure 1E showed a strong difference in the community composition of gut microbiota between two groups.
Figure 1.
The overall structure of gut microbiota in nursing and weaned groups. (A), (B): Bacterial rarefaction curves based on Chao 1 and observed species index were used to assess the depth of coverage for each sample. (C) Venn diagram for bacterial OTUs compositions in two groups. (D) Similarity Analysis (Anosim Analysis) based on Unifrac algorithm. (E) Multiple sample similarity phylogenetic tree was built by Unweighted pair group method with arithmetic mean. Each sample was distinguished by different colors of lines. Blue and red represent nursing group and weaned group, respectively.
Table 1.
Richness and diversity of indices of colonic microbiota in nursing and weaned groups.
| Items | Richness indices | Diversity indices | |||
|---|---|---|---|---|---|
| Chao 1 | Observed species | PD_whole_tree | shannon | simpson | |
| nursing | 306.86 | 255.33 | 22.2 | 5.24 | 0.94 |
| weaned | 223.06 | 185.33 | 15.33 | 4.76 | 0.91 |
| p value | 0.03 | 0.03 | 8.66E−03 | 0.24 | 0.24 |
The coverage percentage, richness estimators (Chao1, Observed species), and diversity indices (PD_whole_tree, Shannon and Simpson) were calculated using the QIIME program. PD_whole_tree: (phylogenetic distance) whole tree.
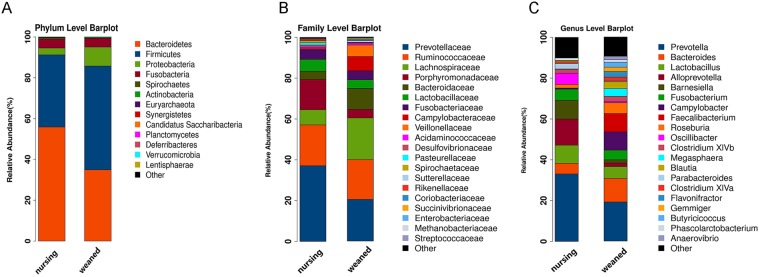
Four predominant phyla (Bacteroidetes, Firmicutes, Proteobacteria, Fusobacteria), which consisted of over 1% of total sequences on average, were found in colon as shown in Fig. 2A. There was no difference detected in dominant bacterial phyla between two groups (Supplementary Table S3). At family level, Prevotellaceae was predominant, followed by Ruminococcaceae, Lachnospiraceae, Porphyromonadaceae, and Bacteroidaceae (Fig. 2B and Supplementary Table S4). At genus level, Precotella, Bacteroides, Lactobacillus and Alloprevotella were the predominant genera (Fig. 2C and Supplementary Table S5).
Figure 2.
Gut microbial community structure in nursing and weaned groups. (A) Microbial community bar plot by phylum. (B) Microbial community bar plot by family. (C) Microbial community bar plot by genus. Each bar represents the average relative abundance of each bacterial taxon within a group. The top 20 abundant taxa are shown.
Differences in Microbiome between Nursing and Weaned Piglets
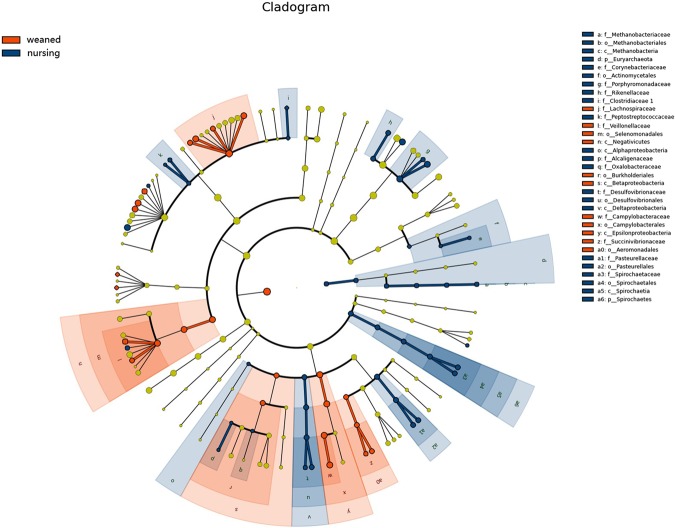
Figure 3 showed the microbiota with significant differences between nursing and weaned piglets. Lachnospiraceae, Negativicutes, Selenomonadales, Campylobacterales and other 15 species increased in weaned piglets, while Porphyromonadaceace, Alloprevotella, Barnesiella and Oscillibacter decreased.
Figure 3.
Cladogram. Bacterial taxa significantly differentiated between Nursing and Weaned group identified by linear discriminant analysis coupled with effect size (LEfSe) using the default parameters.
Predicted Function of Gut Microbiota in Nursing and Weaned Piglets
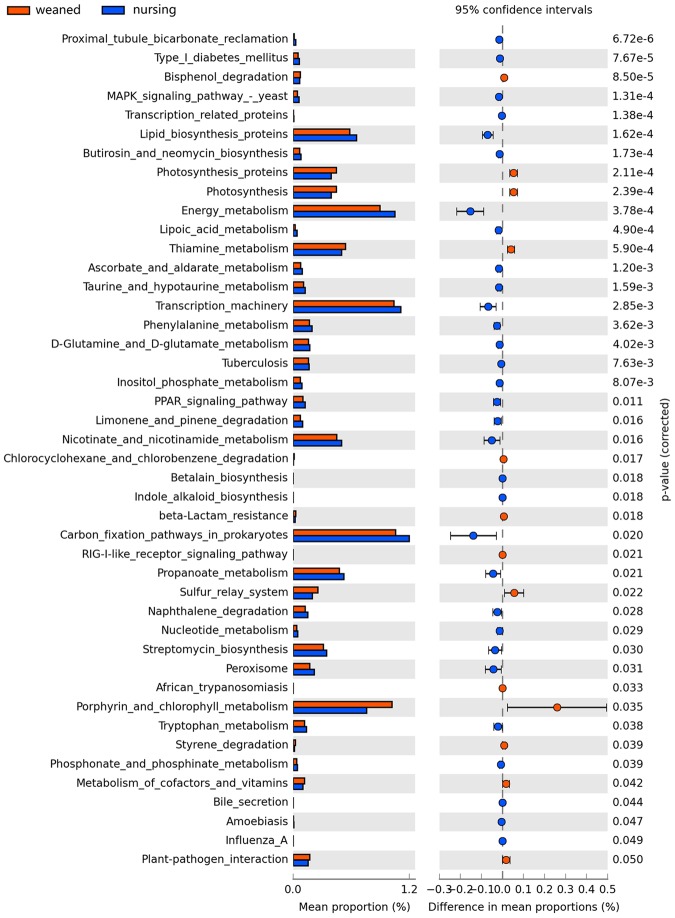
PICRUSt analysis was performed to predict the potential functions of gut microbiota. In comparison two groups, nursing piglets had higher enrichment of the pathways in Lipid biosynthesis proteins, Energy metabolism, Transcription machinery, Nicotinate and nicotinamide metabolism, Carbon fixation pathways in prokaryote, Propanoate metabolism, naphthalene degradation, Streptomycin biosynthesis and peroxisome. While other pathways such as Photosythesis proteins, Thiamine metabolism, Sulfur relay system were overrepresented in weaned piglets (Fig. 4).
Figure 4.
Predicted microbial function in the colon lumen of nursing and weaned groups. The third level of KEGG pathway was showed in the extended error bar. Blue and red represent nursing group and weaned group, respectively.
Metabolic Differences of Gut Microbiota between Nursing and Weaned Piglets
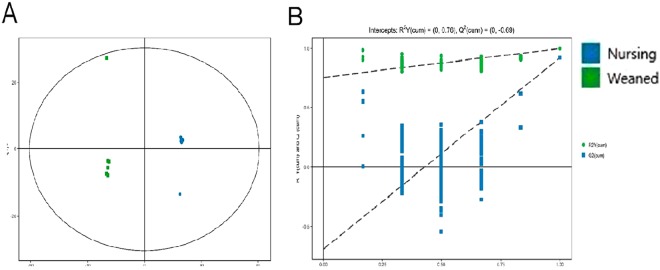
Untargeted metabolomics analysis was performed to evaluate the metabolic differences of gut microbiota in two groups. An OPLS-DA method was performed to better understand the different metabolic patterns. Figure 5A showed significantly separated clusters between two groups. The values for R2X and Q2 and the results of permutation tests indicated that the samples were of reasonable quality (Fig. 5B). In total, relative levels of 433 biomarker metabolites differed significantly between nursing and weaned piglets. There was a clear distribution in metabolite between two groups (Supplementary Fig. 1). Figure 6 showed the variety of metabolic profiles in gut microbiome, with 239 increased and 194 decreased molecular features based on a VIP > 1 in 95% jack-knifed confidence intervals, respectively. And a total of 83 biomarker metabolites were filtered according to the standard with similarity >600, VIP > 1 and p < 0.05. These metabolites, including lipids, amino acids, carbohydrates, benzene, organic acids, amines and others, involved in multiple biochemical processes in the colon (Table 2).
Figure 5.
PCA 3D OPLS-DA score plots and corresponding validation plots of OPLS-DA derived from the GC-TOF/MS metabolite profiles in the colon lumen of nursing and weaned piglets. (A) OPLS-DA score plots showed significantly separated clusters in nursing and weaned group. (B) Permutation test of OPLS-DA model of nursing and weaned group. Blue and red represent nursing group and weaned group, respectively.
Figure 6.
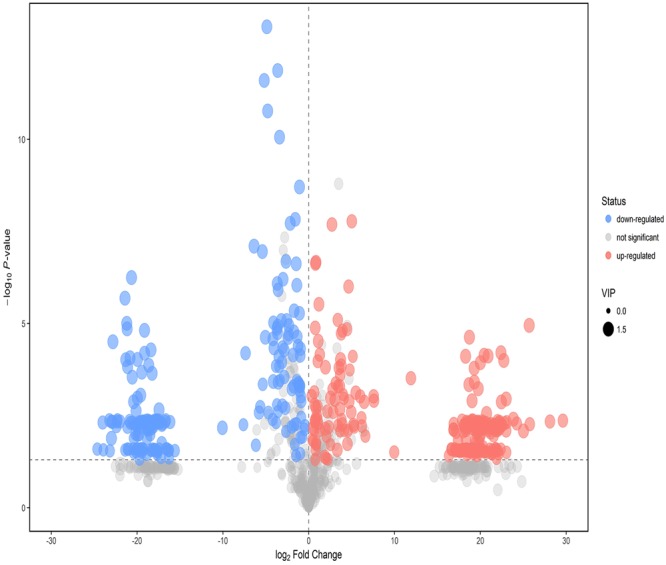
Volcano plot of nursing and weaned group. Each point represents a kind of metabolite, the scatter color represents the final screening result. Significant up-regulated metabolites are indicated in red, significantly down-regulated metabolites are indicated in blue, and non-significant differences in metabolites are grey.
Table 2.
Identified metabolites for discriminating between nursing and weaned groups based on the untargeted metabolomics study.
| Metabolic | Similarity | RTa (min) | MEAN -Weaned | MEAN -Nursing | VIP | t-testb |
|---|---|---|---|---|---|---|
| Lipids | ||||||
| heptadecanoic acid | 828 | 20.337 | 5.57818E-11 | 0.000117 | 1.09225 | * |
| Sphingosine | 904 | 23.217 | 0.000373 | 0.004645 | 1.50233 | *** |
| phytosphingosine | 872 | 24.471 | 0.000206 | 4.89435E-11 | 1.31316 | ** |
| alpha-Tocopherol | 861 | 28.163 | 8.80993E-05 | 0.000391779 | 1.50347 | *** |
| Cholesterol | 899 | 28.352 | 0.002760734 | 4.89435E-11 | 1.31090 | ** |
| Cholestan-3beta-ol | 778 | 28.426 | 0.000120339 | 0.001103108 | 1.53157 | *** |
| Coprostan-3-one | 767 | 28.529 | 5.57818E-11 | 8.6931E-05 | 1.54168 | *** |
| Stigmasterol | 722 | 29.197 | 0.000277268 | 4.89435E-11 | 1.31269 | ** |
| L-Allothreonine | 929 | 12.201 | 0.000465693 | 0.008418029 | 1.53677 | *** |
| 2-Methylglutaric Acid | 866 | 12.596 | 5.57818E-11 | 0.000126571 | 1.54227 | *** |
| Capric Acid | 742 | 13.163 | 5.57818E-11 | 1.7529E-05 | 1.54125 | *** |
| lauric acid | 708 | 15.517 | 0.001545153 | 0.002149401 | 1.11408 | ** |
| Myristic acid | 850 | 17.694 | 0.000486967 | 0.040605731 | 1.54161 | *** |
| pentadecanoic acid | 759 | 18.671 | 0.002088078 | 0.017616468 | 1.41982 | ** |
| palmitoleic acid | 935 | 19.428 | 0.001754687 | 0.020718886 | 1.44486 | *** |
| stearic acid | 893 | 21.495 | 0.040239752 | 4.89435E-11 | 1.30941 | ** |
| Arachidic acid | 934 | 23.100 | 0.004225461 | 0.051756593 | 1.52418 | *** |
| Behenic acid | 951 | 24.606 | 0.002671156 | 0.033936219 | 1.51484 | *** |
| Lignoceric acid | 937 | 26.038 | 0.001859809 | 0.062046719 | 1.53010 | *** |
| Cerotinic acid | 902 | 27.387 | 0.000111121 | 0.001223183 | 1.50911 | *** |
| Carbohydrates | ||||||
| Lyxose | 828 | 15.414 | 0.001186306 | 9.8097E-05 | 1.51917 | *** |
| xylose | 953 | 15.589 | 0.031706877 | 0.002424436 | 1.51325 | *** |
| 6-deoxy-D-glucose | 807 | 16.220 | 0.000764127 | 0.000445482 | 1.34719 | ** |
| fructose | 900 | 17.856 | 0.000335583 | 0.000993779 | 1.49148 | *** |
| Sedoheptulose | 628 | 18.461 | 0.000268555 | 4.89435E-11 | 1.09879 | * |
| D-galacturonic acid | 864 | 18.579 | 0.000413546 | 4.89435E-11 | 1.31565 | ** |
| lactose | 708 | 25.021 | 0.00031404 | 1.14153E-05 | 1.01325 | *** |
| trehalose | 839 | 25.328 | 0.00033928 | 4.89435E-11 | 1.54237 | *** |
| maltose | 775 | 25.296 | 0.000274583 | 0.000162243 | 1.03989 | * |
| Organic acids | ||||||
| Pyruvic acid | 921 | 7.430 | 6.32753E-05 | 0.000116271 | 1.25376 | ** |
| glycolic acid | 930 | 7.829 | 0.007297916 | 0.000592752 | 1.45508 | ** |
| 3-Hydroxypropionic acid | 843 | 8.826 | 0.00325326 | 8.7607E-05 | 1.39673 | ** |
| glutaric acid | 708 | 12.458 | 0.001529942 | 0.000342019 | 1.28988 | ** |
| oxoproline | 950 | 13.968 | 0.04241735 | 0.082504831 | 1.38890 | *** |
| Amino acids | ||||||
| valine | 810 | 8.041 | 0.001631261 | 0.000197131 | 1.46254 | ** |
| Maleimide | 927 | 8.098 | 0.00013127 | 9.90848E-05 | 1.04632 | ** |
| sarcosine | 733 | 8.732 | 5.97859E-05 | 0.000118821 | 1.08313 | * |
| serine | 907 | 10.480 | 5.57818E-11 | 0.000131568 | 1.54214 | *** |
| Isoleucine | 841 | 10.923 | 0.000249148 | 8.0456E-06 | 1.35077 | ** |
| proline | 960 | 11.050 | 0.002575346 | 0.012998171 | 1.50201 | *** |
| glycine | 957 | 11.163 | 0.002625406 | 0.016532029 | 1.47505 | *** |
| beta-Alanine | 945 | 12.773 | 0.001489759 | 9.94769E-05 | 1.43201 | ** |
| aspartic acid | 956 | 13.927 | 0.00684775 | 0.015713239 | 1.25782 | *** |
| 4-aminobutyric acid | 844 | 14.067 | 0.007020642 | 0.000608468 | 1.51846 | *** |
| D-alanyl-D-alanine | 755 | 14.881 | 0.000863753 | 0.000171924 | 1.33635 | ** |
| asparagine | 865 | 15.681 | 5.57818E-11 | 0.000313104 | 1.30777 | ** |
| alpha-Aminoadipic acid | 806 | 16.208 | 0.000124128 | 4.9774E-06 | 1.35393 | *** |
| glutamine | 692 | 16.834 | 5.57818E-11 | 0.00042769 | 1.30790 | ** |
| ornithine | 940 | 17.323 | 0.001909938 | 0.00740552 | 1.48481 | *** |
| citrulline | 806 | 17.389 | 0.000271639 | 0.000529457 | 1.19390 | * |
| histidine | 833 | 18.369 | 5.57818E-11 | 0.00052798 | 1.09632 | * |
| tyrosine | 933 | 18.574 | 0.004176509 | 0.014777744 | 1.50781 | *** |
| Benzene | ||||||
| m-Cresol | 681 | 8.987 | 2.3733E-05 | 0.000171394 | 1.44746 | *** |
| Catechol | 627 | 11.302 | 5.97445E-06 | 4.89435E-11 | 1.31768 | ** |
| phenylethylamine | 627 | 11.302 | 5.97445E-06 | 4.89435E-11 | 1.31768 | ** |
| Phenyllactate | 787 | 14.703 | 0.002295753 | 3.14665E-05 | 1.24267 | ** |
| gentisic acid | 611 | 16.852 | 3.101E-05 | 4.89435E-11 | 1.54228 | *** |
| tyramine | 846 | 18.329 | 0.014177717 | 4.89435E-11 | 1.30991 | ** |
| noradrenaline | 705 | 20.732 | 8.42385E-05 | 4.89435E-11 | 1.54199 | ** |
| Amines | ||||||
| Ethanolamine | 882 | 10.600 | 0.001545522 | 0.00042396 | 1.35263 | * |
| 1,3-diaminopropane | 928 | 15.449 | 0.001651697 | 0.000436322 | 1.25857 | ** |
| putrescine | 920 | 16.513 | 0.163908181 | 0.02821584 | 1.50530 | ** |
| spermine | 799 | 25.154 | 4.66359E-05 | 4.89435E-11 | 1.31451 | ** |
| Organoheterocyclic compounds | ||||||
| 2-Furoic Acid | 604 | 8.695 | 2.60681E-05 | 4.89435E-11 | 1.54240 | ** |
| 5-aminovaleric acid lactam | 843 | 8.834 | 0.00394493 | 0.000152673 | 1.51907 | ** |
| nicotinic acid | 976 | 11.021 | 0.000714674 | 0.001438858 | 1.34719 | *** |
| Orotic acid | 635 | 16.509 | 5.57818E-11 | 7.6404E-05 | 1.08298 | * |
| Others | ||||||
| N-Methyl-DL-alanine | 906 | 9.238 | 0.009567796 | 0.000546294 | 1.13615 | ** |
| Bis(2-hydroxypropyl)amine | 622 | 13.296 | 5.57818E-11 | 3.53733E-05 | 1.28973 | ** |
| Digitoxose | 727 | 15.012 | 5.57818E-11 | 5.96126E-05 | 1.09183 | ** |
| Cyclohexane-1,2-dione | 793 | 15.685 | 2.64145E-05 | 4.89435E-11 | 1.11298 | * |
| fucose | 958 | 16.319 | 0.005013265 | 0.010654332 | 1.46744 | *** |
| Methyl-beta-D-galactopyranoside | 650 | 17.678 | 0.000220863 | 0.001169638 | 1.46330 | *** |
| fructose | 840 | 17.954 | 0.000134698 | 0.000284574 | 1.51000 | *** |
| Methyl Palmitoleate | 740 | 18.258 | 4.88292E-05 | 0.000690577 | 1.16641 | *** |
| 8-Aminocaprylic acid | 734 | 18.511 | 0.002636825 | 4.89435E-11 | 1.54253 | *** |
| Gly-Pro | 867 | 18.984 | 8.3268E-05 | 0.000492377 | 1.51841 | *** |
| xanthine | 913 | 19.307 | 0.000738454 | 4.89435E-11 | 1.54224 | ** |
| N-Acetyl-D-galactosamine | 920 | 19.958 | 0.000980973 | 0.001963837 | 1.39968 | *** |
| 5-Methoxytryptamine | 649 | 23.263 | 0.000471539 | 0.000281012 | 1.07002 | ** |
| DL-dihydrosphingosine | 909 | 23.535 | 0.000341501 | 0.00271986 | 1.46487 | *** |
| adenosine | 640 | 24.531 | 3.20881E-05 | 6.79893E-05 | 1.32913 | *** |
| Monoolein | 662 | 25.420 | 2.73317E-05 | 7.12348E-06 | 1.48583 | *** |
aRentention time.
bStudent t-test of the relative levels of compounds detected in weaned and nursing piglets(n = 6). *p < 0.05, **p < 0.01 and ***p < 0.001, respectively.
Key metabolic pathways analysis
The commercial databases including KEGG (http://www.genome.jp/kegg/) and MetaboAnalyst (http://www.metaboanalyst.ca/) was applied to search for the pathways of metabolites. The result showed that beta-Alanine metabolism, Arginine and proline metabolism, Alanine, aspartate and glutamate metabolism, Nitrogen metabolism and other pathways were altered (Table 3). Among them, five metabolic pathways including Phenylalanine metabolism, Citrate cycle (TCA cycle), Glycolysis or gluconeogenesis, Propanoate metabolism and Nicotinate and nicotinamide metabolism were also found in the functional prediction of gut microbiota as shown in Fig. 4.
Table 3.
Pathway analysis for nursing group and weaned group.
| Pathway | Totala | Hitsb | Raw pc | -ln(p)d | Holm adjuste | FDRf | Impactg |
|---|---|---|---|---|---|---|---|
| Beta-Alanine metabolism | 17 | 6 | 0.0012 | 6.7056 | 0.0992 | 0.0992 | 0.6667 |
| Arginine and proline metabolism | 44 | 9 | 0.0055 | 5.2020 | 0.4404 | 0.1825 | 0.3289 |
| Alanine, aspartate and glutamate metabolism | 23 | 6 | 0.0068 | 4.9967 | 0.5340 | 0.1825 | 0.4633 |
| Nitrogen metabolism | 9 | 3 | 0.0277 | 3.5847 | 1 | 0.5618 | 0 |
| Valine, leucine and isoleucine biosynthesis | 11 | 3 | 0.0486 | 3.0246 | 1 | 0.6558 | 0.6667 |
| D-Glutamine and D-glutamate metabolism | 5 | 2 | 0.0520 | 2.9559 | 1 | 0.6558 | 0 |
| Aminoacyl-tRNA biosynthesis | 64 | 9 | 0.0567 | 2.8704 | 1 | 0.6558 | 0 |
| Glycine, serine and threonine metabolism | 32 | 5 | 0.0991 | 2.3117 | 1 | 0.9605 | 0.3528 |
| Pantothenate and CoA biosynthesis | 15 | 3 | 0.1067 | 2.2375 | 1 | 0.9605 | 0.0408 |
| Phenylalanine metabolism | 9 | 2 | 0.1526 | 1.8796 | 1 | 1 | 0.2222 |
| Butanoate metabolism | 20 | 3 | 0.2024 | 1.5974 | 1 | 1 | 0.0290 |
| Ubiquinone and other terpenoid-quinone biosynthesis | 3 | 1 | 0.2173 | 1.5265 | 1 | 1 | 0 |
| Tyrosine metabolism | 42 | 5 | 0.2280 | 1.4783 | 1 | 1 | 0.0532 |
| Histidine metabolism | 14 | 2 | 0.3012 | 1.1999 | 1 | 1 | 0.2662 |
| Pyrimidine metabolism | 37 | 4 | 0.3286 | 1.1129 | 1 | 1 | 0.0634 |
| Pentose and glucuronate interconversions | 15 | 2 | 0.3311 | 1.1052 | 1 | 1 | 0 |
| Glutathione metabolism | 26 | 3 | 0.3336 | 1.0978 | 1 | 1 | 0.0057 |
| Cyanoamino acid metabolism | 6 | 1 | 0.3877 | 0.9475 | 1 | 1 | 0 |
| Biosynthesis of unsaturated fatty acids | 42 | 4 | 0.4210 | 0.8651 | 1 | 1 | 0 |
| Pentose phosphate pathway | 19 | 2 | 0.4461 | 0.8072 | 1 | 1 | 0.1209 |
| Propanoate metabolism | 20 | 2 | 0.4732 | 0.7483 | 1 | 1 | 0 |
| Citrate cycle (TCA cycle) | 20 | 2 | 0.4732 | 0.7483 | 1 | 1 | 0.1398 |
| Sphingolipid metabolism | 21 | 2 | 0.4995 | 0.6942 | 1 | 1 | 0.0526 |
| Methane metabolism | 9 | 1 | 0.5213 | 0.6515 | 1 | 1 | 0 |
| Vitamin B6 metabolism | 9 | 1 | 0.5213 | 0.6515 | 1 | 1 | 0 |
| Valine, leucine and isoleucine degradation | 38 | 3 | 0.5838 | 0.5383 | 1 | 1 | 0 |
| Riboflavin metabolism | 11 | 1 | 0.5938 | 0.5212 | 1 | 1 | 0 |
| Steroid hormone biosynthesis | 67 | 5 | 0.6158 | 0.4849 | 1 | 1 | 0.0541 |
| Nicotinate and nicotinamide metabolism | 13 | 1 | 0.6555 | 0.4223 | 1 | 1 | 0 |
| Primary bile acid biosynthesis | 46 | 3 | 0.7143 | 0.3365 | 1 | 1 | 0.0667 |
| Purine metabolism | 68 | 4 | 0.7967 | 0.2274 | 1 | 1 | 0.0269 |
| Lysine degradation | 20 | 1 | 0.8068 | 0.2147 | 1 | 1 | 0 |
| Pyruvate metabolism | 22 | 1 | 0.8363 | 0.1788 | 1 | 1 | 0.1875 |
| Starch and sucrose metabolism | 23 | 1 | 0.8493 | 0.1633 | 1 | 1 | 0 |
| Porphyrin and chlorophyll metabolism | 25 | 1 | 0.8724 | 0.1365 | 1 | 1 | 0 |
| Galactose metabolism | 26 | 1 | 0.8826 | 0.1250 | 1 | 1 | 0.0161 |
| Glycolysis or Gluconeogenesis | 26 | 1 | 0.8826 | 0.1250 | 1 | 1 | 0.0989 |
| Cysteine and methionine metabolism | 28 | 1 | 0.9006 | 0.1047 | 1 | 1 | 0.0210 |
| Glycerophospholipid metabolism | 29 | 1 | 0.9085 | 0.0960 | 1 | 1 | 0 |
| Steroid biosynthesis | 35 | 1 | 0.9446 | 0.0570 | 1 | 1 | 0.0539 |
| Amino sugar and nucleotide sugar metabolism | 37 | 1 | 0.9532 | 0.0480 | 1 | 1 | 0 |
| Fatty acid metabolism | 39 | 1 | 0.9604 | 0.0404 | 1 | 1 | 0 |
aThe number of metabolites in the pathway.
bThe number of differential metabolites that hit the pathway.
cThe P value of Metabolic pathway enrichment analysis.
dThe P value of Multiple Hypothesis Test Corrected by Holm-Bonferroni Method.
eThe P values of Multiple Hypothesis Tests for Corrected via the false discovery rate (FDR) Method.
fThe Impact value of metabolic pathway topology analysis.
Discussion
Weaning is a sudden, complex and highly stressful event in piglets’ life, accumulating evidence indicated that piglets have an abrupt taxonomic and functional shift in the intestinal microbiota after weaned8,12. However, the effects of weaning stress on microbial metabolites in piglets are still an unsolved issue. In this study, piglets at weaned day (nursing group) and 3 days after weaned (weaned group) were chose to investigate the effects of weaning stress on the composition of gut microbiota and its metabolites in the colon. The results showed that the diversity of gut microbiota in weaned piglets was similar with nursing piglets, while the richness decreased. These results were analogous with previous study, which found a continuously decreased alpha diversity until 11 days after weaned16. However, other studies presented opposite phenomenon. Those showed a continuous increase in alpha diversity of gut microbiota during weaning transition17,18. The reason might be that our studies focused on the gut microbiota during the early period after weaning, whereas their studies investigated the gut microbiota during the period from weaning to adulthood in pigs. It seems that alpha diversity of gut bacterial community undergoes decreases first during the early period after weaning, and then increases from weaning to adulthood in pigs on the whole.
In line with previous studies, Bacteroidetes and Firmicutes were the two most dominant phyla in the intestine of piglets, regardless of weaning16,19. Proteobacteria and Fusobacteria were the followed abundant phylum. There was no difference in bacterial dominant phyla in the colon between nursing and weaned piglets. And Candidatus Saccharibacteria, Planctomycetes, Verrucomicrobia and Lentisphaerae even cannot be detected in the gut of weaned piglets, suggesting that these bacterial species may not adapt to the intestinal tract environment after weaning. What’s more, the results showed that several bacterial species had a remarkable difference between nursing and weaned piglets. For example, Alloprevotella and Oscillospira were decreased, while Campylobacterales, Campylobacteraceae and Campylobacter were increased in weaned piglets. Alloprevotella mainly produce succinate and acetate, which could improve the gut barrier and exhibit anti-inflammatory function20. Oscillospira species are butyrate producers and can use host glycans as a source of energy21. Study found that the presence of Oscillospira is reduced in diseases that involve inflammation22. Campylobacterales was one of opportunistic pathogens, causing a life-threatening gastrointestinal disease23. And Campylobacteraceae and Campylobacter were also increased after weaning. The increase of this bacterial species in weaned piglets may be one of the major reasons of post-weaning diarrhea. These results indicated that unhealthy alterations in the composition of gut microbiota triggered by weaning stress.
Gut microbiota participate in many functional metabolic pathways through which nutrient digestion and absorption, lipid metabolism, and hormone biosynthes are influenced24. To investigate how the functional capacity of the gut microbiota changed after weaning in piglets, Functional metagenomics prediction approach was used to analyze the KEGG pathways compositions in microbial populations. The results showed that many metabolic pathways such as lipid biosynthesis proteins, energy metabolism and phenylalanine metabolism were changed during the period of weaning. Gut microbiota could also directly alter its metabolic capacity through microbial products, affecting intestinal function. Butyrate, a kind of metabolite, produced by gut microbiome could regulate energy metabolism and autophagy in the mammalian colon25. So GC-TOF/MS method was applied in the current study to quantitatively measure small molecular metabolites in biological samples and identify relevant Pathways. Based on PICRUSt and metabolomics analysis, we found that five metabolic pathways including Phenylalanine metabolism, Citrate cycle (TCA cycle), Glycolysis or Gluconeogenesis, Propanoate metabolism and Nicotinate and nicotinamide metabolism were the key relevant metabolic pathways involved in weaning stress-induced gut microbiota dysbiosis.
Previous study indicated that alterations in the microbiota composition elicited by nutrition are associated with neurotransmitter concentrations26. Nutrition can influence hormonal, neurotransmitter, and signalling pathways, which in turn modulate several brain processes impacting both appetite and mood27. Phenylethylamine, as a neuromodulator or neurotransmitter, participates in the phenylalanine metabolic pathway and plays an important role in mood regulation28. In the present study, the phenylethylamine level decreased in weaned piglets, which indicated piglets had a bad mood caused by stress. And a low level of phenylethylamine could not modulate the mood well, which may cause a decreased feed intake and weight gain of piglets. In addition, changed neurotransmitter noradrenaline and 4-aminobutyric acid level were also found in the present work. Gut microbiota could influence the immune system via neurotransmitters, and proinflammatory cytokines released through microbiota-induced immune response can in turn have a direct influence on the secretion of neurotransmitters such as norepinephrine29. Compelling studies have linked weaning to an upregulation of expression of inflammatory cytokines in the intestine of piglets10,30. Thus the regulatory mechanism and potential role of “microbiota-brain-gut” axis that play during weaning period in piglets are warranted to further investigation.
During a short period after weaning, small intestine length, microvilli height, crypt depth, and barrier function of piglets were changed. What’s more, digestive enzyme activity and absorptive capacity of small intestine were reduced, resulting in a low absorption capacity of amino acids and carbohydrates31. In the present study, decreased essential amino acids such as valine and some carbohydrate in weaned piglets supported this view. TCA cycle is responsible for the oxidative degradation of sugars, fats, and amino acids and could link different types of nutrients together. Pyruvic acid, as an important intermediate involved in the Citrate cycle (TCA cycle), was decreased in the weaned piglets. The reduction in energy metabolism may be part of the gut adaptation process. Microorganisms may engage energy for adaptation mechanisms to ensure necessary physiological functions32.
Notably, pathways related to short chain fatty acid (SCFA) metabolism such as propanoate metabolism were enrichment in piglets. Functional metagenomics prediction analysis using PICRUSt revealed that genes involved in Propanoate metabolism were more abundant in nursing piglets. And the bacteria Oscillospira, known to be as a butyrate producer, decreased in the gut microbiota of weaning piglets, which might indicate the decreased SCFA production after weaning. SCFAs are major anions in the gut and could be absorbed rapidly by colonic epithelial cells. Growing evidence proved that these small molecules could protect the host against colonic diseases, improve the gut barrier function and exhibit anti-inflammatory effects33,34. In addition, SCFA-mediated beneficial metabolic effects on health can be mediated by induction of intestinal gluconeogenesis35. A recent study showed that SCFAs could also improve the feed efficiency of swine36. Thus the reduction in Propanoate metabolism of weaned piglets in the current study might be related to a low growth performance and unhealthy gut environment after weaning.
Administration of niacin (nicotinic acid and nicotinamide) has been shown to beneficially effect the host-microbiome interaction in a mouse model37. Nicotinamide adenine dinucleotide (NAD+) is the central cofactor of metabolism, mediating ATP generation, reactive oxygen species (ROS) detoxification, biosynthetic processes, DNA repair, and nutritionally sensitive gene regulation38. In the present study, decreased nicotinic acid was found in weaning piglets, which indicated an adverse influence on piglets trigged by stress.
Conclusion
In conclusion, our current work demonstrated that weaning stress not only disturbed the gut microbiota but also altered the metabolome profiles in the colon. After weaning, some benefit bacterial species such as Alloprevotella and Oscillibacter decreased in weaned piglets, while some opportunistic pathogens such as Campylobacterales, Campylobacteraceae and Campylobacter increased. In addition, based on PICRUSt and metabolomics analysis, five key metabolic pathways including Phenylalanine metabolism, Citrate cycle (TCA cycle), Glycolysis or Gluconeogenesis, Propanoate metabolism, Nicotinate and nicotinamide metabolism were identified as the relevant pathways associate with weaning stress-induced gut microbiota dysbiosis. These results may provide a new insight in alleviating weaning stress of piglets and facilitating disease prevention during weaning by manipulating host-microbiome metabolites interactions. And future study might focus on the potential functions of neurotransmitters in gut microbiome, neurogenesis, behavior and immune of piglets suffering weaning stress.
Materials and Methods
Animals and Sample Collection
All methods in this study were performed in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institutional Animal Care and Use Committee of Zhejiang University, and animals used in this experiment were approved by the principles of the Zhejiang University Animal Care and Use Committee (NO. 2012-0178). Three litters of suckling piglets (21d, Duroc × Landrace × Yorkshire) with same parity were chosen from a commercial pig farm located in Ningbo city, Zhejiang province, China. Two barrows from each litter were randomly selected and humanely euthanized. The samples of colonic content were collected and transferred into sterile conical tubes. Then remaining piglets in the same trial were separated from sows and transferred into the same pen. These weaned piglets were fed a corn/soybean- based diet and had free access to water and feed. All nutrients reached or exceeded NRC (2012) recommendations for piglets. Three days after weaned, two barrows from each litter were selected and humanely euthanized. The samples of colonic content were collected into sterile conical tubes. All collected colonic samples were immediately frozen in liquid nitrogen and stored at −80 °C.
DNA extraction and 16S rDNA gene amplicon sequencing
Total Microbial genomic DNA extraction was performed from colonic lumen using E.Z.N.A.® Stool DNA Kits (Omega Bio-tek, Norcross, US) according to the instructions of the manufacturer. The method of microbial community analysis was performed according to previous study39. Bacterial univers alprimers (341 F: ACTCCTACGGGAGGCAGCAG, 806 R: GGACTACHVGGGTWTCTAAT) were used for the amplification of the V3–V4 region of the bacterial 16S rDNA gene and subsequent pyrosequencing of the PCR products. Amplicons were confirmed by electrophoresis on a 2% agarose gel, purified with the AxyPrep DNA kit (AXYGEN, Tewksbury, MA, US), quantified by Qubit 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, US) to pool into even concentration. Amplicon libraries were sequenced on Illumina Miseq PE250 platform (Illumina, San Diego, US).
Sequencing data analysis
The paired-end reads were obtained 2 × 250 bp using MiSeq platform. In order to obtain high quality sequences, tags with length of <220 nt, average quality score of <20, and tags containing >3 ambiguous bases were removed by PANDAseq. Data was de-chimeric and clustered using Usearch software, a standard clustering of 97% similarity was applied to obtain OTU (Operational Taxonomic Units). And the taxonomy-based analysis to the OTUs was performed by RDP algorithm using GreenGene database (http://greengenes.lbl.gov). QIIME was used for analyzing alpha diversity, which included calculation of the observed species, Chao 1, Shannon, and Simpson indices. And principal coordinate analysis (PCoA), Anosim Analysis, and unweighted pair group method with arithmetic mean (UPGMA) were performed using QIIME for beta-diversity analysis. Linear discriminant analysis coupled with effect size (LEfSe) was applied to identify the bacterial taxa differentially represented between groups at genus or higher taxonomy levels40.
Metagenome prediction based on 16S rDNA gene data
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt), which makes up for the shortage of 16S rDNA gene studies, was used for predicting the gene family abundances of bacterial populations based on the precalculated GreenGenes (v13.5) database. In brief, the analysis process is divided into two steps: gene content inference and metagenome inference. The software STAMP was conducted to detect the differentially abundant Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways in two groups with false discovery rate correction.
The rank sum test was used to identify species with significant differences between groups. Two-sided Welch’s t-test and Benjamini-Hochberg FDR correction were performed in two-group functional prediction analysis. P < 0.05 was considered statistically significant. And the prediction accuracy of PICRUSt was evaluated by the Nearest Sequenced Taxon Index (NSTI), with lower value indicating a higher accuracy of prediction.
Sample preparation for GC-TOF/MS analysis
Briefly, approximately 1.0 g colonic contents were mixed with 3 ml 4 °C H2O, followed by centrifugation at 3,200 × g for 15 minutes. 50 μl of the supernatant was taken and added with 200 μl of methanol, which contains 12.5 μg/ml of [13C2] myristic-acid. The mixture was maintained at 4°C for 1 h and subsequently centrifuged at 20,000 g for 10 minutes. Then the supernatant fraction was transferred to a glass vial and dried in a vacuum concentrator. And added methoxyamine salt reagent (methoxyamine hydrochloride, dissolved in pyridine 20 mg/mL), incubated in an oven at 80 °C for 30 min, then derivatized using N, O Bis (trimethylsilyl) trifluoroacetamide (BSTFA), incubated for 1 hour at 70 °C. The final mixture was strongly vortexed for 1 min and then subjected to detection.
GC-TOF/MS analysis
The derivatized samples were analyzed using an Agilent 7890 GC system equipped with a Pegasus 4D TOFMS (LECO, St. Joseph, MI) with a DB-5MS capillary column (30 m × 250 μm inner diameter, 0.25 μm film thickness coated with 95% dimethylpolysiloxane cross-linked with 5% diphenyl under the following conditions: initial temperature was kept at 80 °C for 0.2 min, increased to 180 °C at a rate of 10 °C/min, to 240 °C at a rate of 5 °C/min, and further to 290 °C at a rate of 20 °C/min; the column was then maintained for 11 min. One μL of sample solution was injected with helium as the carrier gas at a flow rate of 1 mL/minute. The temperatures of transfer line, and ion source were 245 °C, and 220 °C, respectively. The MS data were acquired with a mass-to-charge ratio (m/z) range of 20−600 in a full-scan mode.
GC-TOF/MS data acquisition and processing
The raw peaks extraction, data baselines filtering and calibration, peak alignment, deconvolution analysis, peak identification, and integration of the peak area were operated using the Chroma TOF4.3X software (LECO) and LECO-Fiehn Rtx5 database. The peak identification was tested using the RI (retention time index) method. The missing values of the original data were filled by half of the minimum value via a numerical simulation method. Noise removal was performed based on an interquartile range to filter data, then, data were normalized by area normalization methods. The SIMCA14.1 software package (Umetrics, Umea, Sweden) was used for multivariate variable pattern recognition analysis: principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA), and orthogonal partial least-squares discriminant analysis (OPLS-DA). PCA was used to show the internal structure of the data and display the similarity and difference. OPLS-DA was applied to obtain a higher level of group separation and better explain the variables. To evaluate the predictive ability and fitting level of the model, the parameters R2Y and Q2 were applied. The metabolites responsible for differentiating two groups were filtered with the following requirements: variable importance in the projection (VIP) >1 and P-values of 0.05 (threshold) with 95% Hotelling’s T-squared ellipse.
Data was analyzed by SPSS 20.0 using Student’s t-test to profile metabolite differences between two groups. The commercial databases including KEGG (http://www.genome.jp/kegg/) and MetaboAnalyst (http://www.metaboanalyst.ca/) was utilized to search for the pathways of metabolites. We can further screen pathways and find the key pathways with the highest correlation with metabolites through comprehensive analysis of the pathways where the differential metabolites are located (including enrichment analysis and topological analysis).
Electronic supplementary material
Author Contributions
X.H. conceived the experiment(s), Y.L., S.C. and S.G. conducted the experiment(s), Y.L., X.J. and X.M. analysed the results. All authors reviewed the manuscript.
Data Availability
All data generated or analysed during this study are included in this article and its Supplementary Information files.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-33649-8.
References
- 1.Lora VH, Andrew J. Macpherson. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nature Reviews Immunology. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 2.Lee W, Hase K. Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 2014;10:416–424. doi: 10.1038/nchembio.1535. [DOI] [PubMed] [Google Scholar]
- 3.Qin J, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 4.Leamy LJ, et al. Host genetics and diet, but not immunoglobulin A expression, converge to shape compositional features of the gut microbiome in an advanced intercross population of mice. Genome Biol. 2014;15:811. doi: 10.1186/s13059-014-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinritz SN, Mosenthin R, Weiss E. Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutr Res Rev. 2013;26:191–209. doi: 10.1017/S0954422413000152. [DOI] [PubMed] [Google Scholar]
- 6.Kim HB, Isaacson RE. The pig gut microbial diversity: Understanding the pig gut microbial ecology through the next generation high throughput sequencing. Vet Microbiol. 2015;12(177):242–251. doi: 10.1016/j.vetmic.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Isaacson. R, et al. The intestinal microbiome of the pig. Anim. Health Res. Rev. 2012;13:100–109. doi: 10.1017/S1466252312000084. [DOI] [PubMed] [Google Scholar]
- 8.Frese SA, et al. Diet shapes the gut microbiome of pigs during nursing and weaning [J] Microbiome. 2015;3:28. doi: 10.1186/s40168-015-0091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meale SJ, et al. Weaning age influences the severity of gastrointestinal microbiome shifts in dairy calves. Sci Rep. 2017;7:198. doi: 10.1038/s41598-017-00223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stokes CR. The development and role of microbial-host interactions in gut mucosal immune development. Journal of animal science and biotechnology. 2017;8:12. doi: 10.1186/s40104-016-0138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H, et al. Effects of weaning on intestinal upper villus epithelial cells of piglets. PLoS One. 2016;11:e0150216. doi: 10.1371/journal.pone.0150216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GRESSE R, et al. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends in microbiology. 2017;25:851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Lucile, M* et al. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning [J]. British Journal of Nutrition 97, 45–57 (2007). [DOI] [PubMed]
- 14.Joanna K-C, et al. Is there a relationship between intestinal microbiota, dietary compounds, and obesity? Trends in Food Science & Technology. 2017;70:105–113. doi: 10.1016/j.tifs.2017.10.010. [DOI] [Google Scholar]
- 15.Kumari M, Kozyrskyj AL. Gut microbial metabolism defines host metabolism: An emerging perspective in obesity and allergic inflammation. Obesity Reviews. 2017;18:18–31. doi: 10.1111/obr.12484. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, et al. Gradual changes of gut microbiota in weaned miniature piglets. Front. Microbiol. 2016;7:1727. doi: 10.3389/fmicb.2016.01727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niu Q, et al. Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Sci. Rep. 2015;5:9938. doi: 10.1038/srep09938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao, W. et al. The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PLoS One. (2015). [DOI] [PMC free article] [PubMed]
- 19.Kim HB, et al. Microbial shifts in the swine distal gut in response to the treatment With antimicrobial growth promoter, tylosin. Proc. Natl. Acad. Sci. USA. 2012;109:15485–15490. doi: 10.1073/pnas.1205147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Downes J, Dewhirst FE, Tanner AC, Wade WG. Description of Alloprevotella rava gen. nov., sp. nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae Moore et al. 1994 as Alloprevotella tannerae gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2013;63:1214–1218. doi: 10.1099/ijs.0.041376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konikoff T, Gophna U. Oscillospira: a central, enigmatic component of the human gut microbiota. TrendsMicrobiol. 2016;24:523–524. doi: 10.1016/j.tim.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Gophna U, Konikoff T, Nielsen HB. Oscillospira and related bacteria – from metagenomic species to metabolic features. Environ. Microbiol. 2017;19:835–841. doi: 10.1111/1462-2920.13658. [DOI] [PubMed] [Google Scholar]
- 23.Xie Y, He Y, Irwin PL, Jin T, Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011;77:2325–2331. doi: 10.1128/AEM.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke G, et al. Minireview: gut microbiota: the neglected endocrine organ. Mol. Endocrinol. 2014;28:1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donohoe DR, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrepf A, Markon K, Lutgendorf SK. From childhood trauma to elevated C-reactive protein in adulthood: the role of anxiety and emotional eating. Psychosom Med. 2014;76:327–336. doi: 10.1097/PSY.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang UE, Beglinger C, Schweinfurth N, Walter M, Borgwardt S. Nutritional aspects of depression. Cellular Physiology and Biochemistry. 2015;37:1029–1043. doi: 10.1159/000430229. [DOI] [PubMed] [Google Scholar]
- 28.Berry MD. The Potential of Trace Amines and Their Receptors for Treating Neurological and Psychiatric Diseases [J] Rev Recent Clin Trials. 2007;2:3–19. doi: 10.2174/157488707779318107. [DOI] [PubMed] [Google Scholar]
- 29.Maestroni GJ. Dendritic cell migration controlled by α1b-adrenergic receptors. The Journal of Immunology. 2000;165:6743–6747. doi: 10.4049/jimmunol.165.12.6743. [DOI] [PubMed] [Google Scholar]
- 30.Wang DF, et al. Effects of Piper sarmentosum extract on the growth performance, antioxidant capability and immune response in weaned piglets. Journal of animal physiology and animal nutrition. 2017;101:105–112. doi: 10.1111/jpn.12517. [DOI] [PubMed] [Google Scholar]
- 31.Xiao YP, et al. Response to weaning and dietary L-glutamine supplementation: metabolomic analysis in piglets by gas chromatography/mass spectrometry. Journal of Zhejiang University Science B. 2012;13:567–578. doi: 10.1631/jzus.B1100343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murínová S, Dercová K. Response mechanisms of bacterial degraders to environmental contaminants on the level of cell walls and cytoplasmic membrane. Int. J. Microbiol. 2014;2014:873081. doi: 10.1155/2014/873081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuda S, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 34.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activationof AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Vadder F, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Quan J, et al. A global comparison of the microbiome compositions of three gut locations in commercial pigs with extreme feed conversion ratios. Scientific reports. 2018;8:4536. doi: 10.1038/s41598-018-22692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hashimoto T, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem. Sci. 2007;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Ba Q, et al. Gender-Dependent Effects of Cadmium Exposure in Early Life on Gut Microbiota and Fat Accumulation in Mice. Environ Health Perspect. 2016;125:437. doi: 10.1289/EHP360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biology12(6), R60, 10.1186/gb-2011-12-6-r60 (2011). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this article and its Supplementary Information files.