Abstract
In Japan the highest use of veterinary antimicrobials is in pig production. To obtain useful information to achieve the best approach to reducing this use, we analyzed the association between the level of on-farm biosecurity and animal welfare with the level of antimicrobial use as recorded on prescriptions on 38 pig farms under contract to veterinarians of the Japanese Association of Swine Veterinarians. To determine the level of welfare we recorded the risk of pre- and post-weaning deaths and the floor space available per fattening pig (m2/head). Multivariable linear regression analysis was performed, using biosecurity scores and animal welfare indicators as independent variables and the amount of antimicrobial usage as dependent variables. The results showed that the higher scores for the site condition (location) and external biosecurity scores of the sub-categories ‘farm contractors’ were strongly associated with the lower use of oral antimicrobials (P<0.05). This suggests that in order to reduce the usage of antimicrobials for herd treatments, farmers should consider the location when building a new farm or pig house and strengthen the entrance requirements for high risk visitors. Regression analysis for the respective antimicrobials showed that the site condition, the biosecurity scores of the sub-categories ‘farm contractors’, ‘pen layouts’ (e.g. independence of pens and sites), ‘pig flows’ (e.g. the completeness of all-in/ all-out system) and an animal welfare indicator (i.e. post-weaning mortality risk) were significantly associated with the use of one or more antimicrobials (P<0.05).
Keywords: animal welfare, antimicrobial usage, biosecurity, multivariable linear regression, pig production
Antimicrobial resistance is a growing global public health threat worldwide. Currently 700,000 people die of resistant infections every year. If no proactive solutions are taken to slow down the rise of drug resistance, then by 2050, some 10 million lives per year could be at risk from drug resistant infections [13]. In response to a request by the United Nations World Health Organization (WHO), the Japanese government adopted an action plan against antimicrobial resistance in April 2016. This action plan sets strategic objectives to lower the proportion of tetracycline resistant Escherichia coli and to maintain the proportion of E. coli resistant to the 3rd generation cephalosporin and fluoroquinolones in food producing animals at the same level as in other G7 countries by 2020 [20]. The bacterial resistance arises through complex mechanisms, normally through mutation and selection, or by acquiring from other bacteria the genetic information that encodes resistance [5]. Therefore, reducing the selection pressure by reducing antimicrobial usage is considered one of the important strategies to decrease the resistance rate [5]. As in European countries, more than half of the veterinary antimicrobial sales in Japan are for use in pigs [2, 4, 11, 22]. Therefore, reducing the use of antimicrobials and promotion of a more prudent use in pig production is important to achieve the action plan objectives.
Four main measures are taken to reduce the risks from infectious diseases in pig farming: biosecurity measures to prevent disease introduction and spread within herds; vaccination against infectious diseases; animal welfare measures to remove disease causing stressors; and metaphylactic and prophylactic treatment using antimicrobials, although prophylactic treatment is totally prohibited and metaphylactic treatment is only allowed under certain conditions in Japan [7, 18, 19]. These measures are taken complementarily to achieve the best disease management and thus business performance.
This study aims to analyze the relationship between the level of on-farm biosecurity, animal welfare indicators and annual usage of antimicrobials (in grams of active substance divided by the number of pigs shipped for slaughter) and to estimate the effect of each factor in order to advise the best approach to reduce the usage of antimicrobials. The results will be utilized to promote feasible and sustainable disease prevention without resorting to the use of antimicrobials in pig farming.
MATERIALS AND METHODS
Selection of pig farms for the analysis
A total of 121 pig farms that were under contract to the members of the Japanese Association of Swine Veterinarians (JASV) were subjected to the measurement of antimicrobial usage in 2015. Of these farms, 38 farms that participated in a biosecurity questionnaire survey in 2017 and in PigInfo in 2015 were selected for the analysis. PigInfo is a benchmarking system for pig farmers’ productivity, developed jointly by the JASV and National Agriculture and Food Research Organization (https://www.piginfo.jp/setsumei001.html). Although data on the biosecurity level was not collected in the same year as data of antimicrobial usage and data from PigInfo, we nevertheless used these data for this cross-sectional study, assuming that farmers would not significantly alter their site location or the ways of implementing biosecurity measures during these years. This is a reasonable assumption, considering that no severe outbreaks of infectious disease such as porcine reproductive and respiratory syndrome (PRRS) and porcine epidemic diarrhea was reported on these farms during these years. [personal communication with JASV].
Data collection on antimicrobial usage
Based on prescription records of JASV veterinarians, data on the annual usage of antimicrobials on the 38 pig farms were collected during the period of 1 January-31 December 2015. To calculate the amount in grams of active substance used we used a 7-digit ID coding system developed by Matsuda et al. based on the WHO ATCvet classification (https://www.whocc.no/atcvet/) [10]. In this coding system, we used a 7-digit code as a unique identifier for each antimicrobial package size, dosage and formulation of the antimicrobial presentation. A unique 7-digit code is given to each approved antimicrobial product for use in pigs in Japan by antimicrobial class (13 classes) and sub-class (42 sub-classes) and by administration route (oral and injection). The annual total amount of antimicrobials used on a farm in grams of active ingredient was divided by the number of pigs shipped for slaughter from that farm so as to make the usage data comparable between farms:
Annual amount of antimicrobial usage in grams of active ingredient per fattening pig inv2015 (g/head)
The data for denominator was obtained from the PigInfo database.
Quantification of on-farm biosecurity level
The on-farm biosecurity level was assessed during the period from 1 July to 31 December 2017 using paper-based questionnaire developed by the authors. This was based on the biosecurity assessment tool ‘BioAsseT’ developed by PRRS-Japan Elimination Team (P-JET), a group of swine veterinarians and other experts who are actively involved in the control of PRRS in Japan (http://site-pjet.com/). The biosecurity questionnaire comprised a number of questions of three main categories and a total of 13 sub-categories: i. site condition; ii. external biosecurity for prevention of disease introduction to herds; and iii. internal biosecurity for prevention of disease spread within herds (see Table1). The site condition was evaluated by the number of other farms surrounding the farm; distance from the nearest neighboring farm; distance from the nearest slaughter house and the situation of the nearest public road. The level of external biosecurity was evaluated with questions on replacement gilts, employees, transport vehicles, manure and carcasses, vermin controls and farm contractors. The level of internal biosecurity was evaluated with questions on pen layouts, pig-flows, cleaning and disinfection, employees, injection needles and delivery stalls (for details of the respective questions used see Supplementary Table 1). A maximum score of 4 was given to a question if the farmer had full implementation of measures relevant to that question. A minimum score of zero was given to a question if the farmer had absolute lack of biosecurity relevant to that question, with an intermediate score being given for partial compliance. The best site condition and the full implementation of external and internal biosecurity measures summed up to maximum scores of 16, 24 and 24, respectively.
Table 1. Categories and sub-categories of biosecurity level evaluation, number of questions used to evaluate these categories and sub-categories, scores allocated to them, and the results from 38 farms.
| Categories | Sub-categories | #Questions | Subtotal scores | Average | SD | Max | Min | 95% confidence interval |
|---|---|---|---|---|---|---|---|---|
| I. Site | i. Site conditions | 4 | 16 | 9.58 | 3.70 | 16.00 | 3.67 | 8.35–10.81 |
| II.External biosecurity | Subtotal | 18 | 24 | 13.08 | 3.59 | 20.73 | 5.61 | 11.88–14.27 |
| ii. Replacement gilts | 3 | 4 | 1.90 | 0.78 | 3.56 | 0.44 | 1.64–2.16 | |
| iii. Employee | 2 | 4 | 2.26 | 1.10 | 4.00 | 0.00 | 1.90–2.63 | |
| iv. Transport vehicles | 4 | 4 | 2.77 | 0.70 | 4.00 | 1.00 | 2.53–3.00 | |
| v. Manure & carcass | 5 | 4 | 2.53 | 0.72 | 4.00 | 1.13 | 2.29–2.77 | |
| vi. Vermin controls | 2 | 4 | 2.23 | 0.88 | 3.33 | 0.00 | 1.94–2.52 | |
| vii. Farm contractors | 2 | 4 | 1.39 | 1.10 | 4.00 | 0.00 | 1.02–1.75 | |
| III. Internal biosecurity | Subtotal | 26 | 24 | 12.38 | 3.66 | 21.48 | 6.31 | 11.16–13.60 |
| viii. Pen layouts | 2 | 4 | 2.05 | 1.07 | 4.00 | 0.00 | 1.69–2.41 | |
| ix. Pig-flows | 6 | 4 | 2.07 | 0.95 | 3.67 | 0.00 | 1.76–2.38 | |
| x. Cleaning & disinfection | 9 | 4 | 2.58 | 0.65 | 3.70 | 1.04 | 2.36–2.8 | |
| xi. Employee | 2 | 4 | 1.09 | 1.04 | 4.00 | 0.00 | 0.74–1.43 | |
| xii. Injection needles | 3 | 4 | 1.45 | 1.2 | 4.00 | 0.00 | 1.05–1.85 | |
| xiii. Delivery stalls | 4 | 4 | 3.14 | 0.7 | 4.00 | 1.00 | 2.91–3.37 | |
| Total | 48 | - | - | - | - | - | ||
Quantification on animal welfare indicators
In the present study, we used the density per fattening pigs (m2/head), as well as pre- and post-weaning mortality risk (%) as animal welfare indicators. This is because lower mortality risk reflect stress-free feeding and housing conditions [3, 16]. This idea is supported by the fact that farmers participating in quality-assurance schemes defined animal welfare in terms of animal health and production-performances [1]. Also, in previous studies, farmers with good production parameters were found to have more positive attitudes toward improvement of animal welfare [6, 8]. The definition formulae for calculating animal welfare indicators in the delivered month are shown in Fig. 1.
Fig. 1.
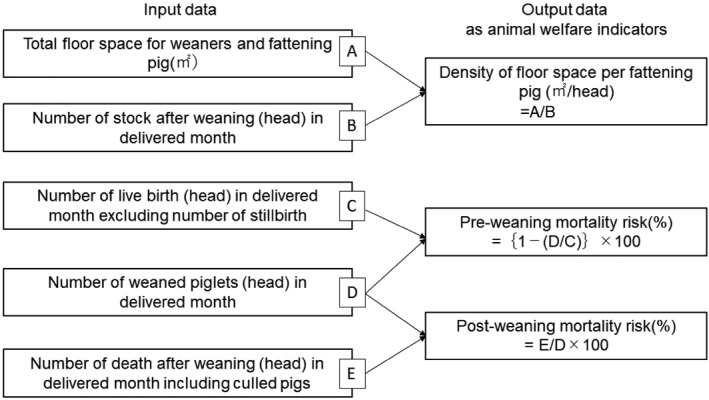
Definition formula for calculating animal welfare indicators using data from PigInfo database.
Statistical analysis
Before subjecting the data for statistical analyses, we performed a log transformation of the antimicrobial usage data to correct for the right skewedness of this variable, after adding a constant value of 0.5 to the original values to adjust for zero values in the data. Then, we performed univariate analysis using biosecurity scores and animal welfare indicators as independent variables and usage of respective antimicrobial as dependent variables. After removing variables that had insignificant association (P>0.05) with the usage of antimicrobials as a result of univariate analysis, multivariable linear regression analyses were performed to identify factors associated with the total usage of oral antimicrobials and usage of respective antimicrobials. The univariate and multivariable linear regression models using biosecurity scores and animal welfare indicators as independent variables and usage of antimicrobials as dependent variables are shown in Table 2. We applied a backward stepwise selection procedure to identify a model with highest predictability using AIC values. All the statistical analyses were performed using SPSS statistics version 24 (IBM). The associations in the multivariable regression with P<0.05 were considered significant. The power of the regression models was calculated using R pwr package (R Core Team) with a significance level of 0.05.
Table 2. Results of univariable and multivariable general linear regression models (n=38).
| Independent variables | Dependent variables, Standardized coefficients (β) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOG total oral antimicrobial usage | LOG doxycycline usage | LOG amphenicols usage | LOG penicillins usage | LOG macrolides usage | LOG fluoroquinolones usage | ||||||||
| Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | ||
| Site external biosecurity | 1. Site conditions | −0.439c) | −0.342b) | −0.441c) | −0.472c) | −0.504c) | −0.441c) | −0.347b) | −0.252a) | ||||
| 2. Replacement gilts | −0.142 | −0.178 | −0.336b) | −0.108 | −0.188 | −0.194 | |||||||
| 3. Employee | −0.359b) | −0.163 | −0.385b) | −0.125 | −0.38b) | −0.182 | |||||||
| 4. Transport vehicles | −0.334b) | 0.043a) | −0.177 | −0.302a) | −0.369b) | −0.205 | |||||||
| 5. Manure & carcass | −0.268a) | −0.392b) | −0.306a) | −0.255a) | −0.356b) | −0.274a) | |||||||
| 6. Vermin controls | −0.398b) | −0.331b) | −0.355b) | −0.272a) | −0.302b) | −0.102 | |||||||
| 7. Farm contractors | −0.438c) | −0.342b) | −0.184 | −0.449c) | −0.257a) | −0.496c) | −0.33b) | −0.272a) | |||||
| Internal biosecurity | 8. Pen layouts | −0.336b) | −0.456b) | −0.654c) | −0.372b) | −0.393b) | −0.507c) | −0.352b) | −0.369a) | ||||
| 9. Pig flows | −0.25a) | −0.423c) | −0.36b) | −0.542c) | −0.284b) | −0.396b) | −0.306b) | −0.368b) | −0.558c) | −0.558c) | |||
| 10. Cleaning & disinfection | −0.102 | −0.148 | −0.247a) | 0.12 | −0.199 | −0.187 | |||||||
| 11. Employee | 0.032 | 0.178 | −0.087 | 0.078 | −0.196 | 0.099 | |||||||
| 12. Injection needles | −0.185 | −0.02 | −0.19 | −0.208 | −0.178 | −0.172 | |||||||
| 13. Delivery stalls | 0.004 | 0.035 | 0.01 | −0.108 | 0.109 | −0.147 | |||||||
| Animal welfare indicators | 14. Density of fattening pig | 0.084 | −0.014 | −0.048 | −0.294a) | 0.123 | −0.129 | ||||||
| 15. Pre-weaning mortality risk | 0.187a) | 0.33b) | 0.367b) | 0.097 | 0.282a) | 0.129 | |||||||
| 16. Post-weaning mortality risk | 0.15 | 0.501c) | 0.45c) | 0.516c) | 0.344c) | 0.247a) | 0.256a) | 0.079 | |||||
| Adjusted R2 | 0.26 | 0.342 | 0.537 | 0.306 | 0.304 | 0.292 | |||||||
| Power of test | 0.943 | 0.989 | 1 | 0.976 | 0.975 | 0.971 | |||||||
LOG: log transformed. Values with a–c indicate that these variables are significantly associated with antimicrobial usage with P-values <0.2, <0.05, and <0.01 respectively. Independent variables in boldface indicate that they are significantly associated with antimicrobial usage in two or more multivariable models. Adjucted R2 values in boldface indicate multivariable models that have more than two independent variables significantly associated with the antimicrobial usage.
RESULTS
Antimicrobial usage
The average number of slaughter pigs shipped from these 38 farms was 16,936 head (Standard Deviation (SD)=25,620) indicating a high variability between farms. The average usage of total antimicrobials in grams of active ingredient per fattening pig in 2015 was 25.62 g/head (SD=22.35). Most (97%) of the antimicrobials were used mostly for herd treatment and thus used orally. The most used antimicrobials were tetracyclines (12.28 g/head) representing 47.92% of the total, followed by macrolides (13.26%, 3.40 g/head), penicillins (10.54%, 2.70 g/head) and sulfonamides (9.05%, 2.32 g/head). Within the tetracyclines, the most used subclass was oxytetracycline (7.35 g/head), representing 59.86% of the total tetracyclines used, followed by doxycycline (31.93%, 3.92 g/head) and chlortetracycline (8.21%, 1.01 g/head). In terms of critically important antimicrobials, less than 50% of the farms used 3rd generation cephalosporin and colistin. See Table 3 and Fig. 2 for more detailed information.
Table 3. Descriptive statistics of antimicrobial usage amount in grams of active ingredient per fattening pig and the animal welfare indicators in 38 farms.
| Unit | Average | SD | Minimum | 25 percentile | Median | 75 percentile | Maximum | ||
|---|---|---|---|---|---|---|---|---|---|
| Number of slaughter pigs shipped | heads | 16,936.0 | 25,963.93 | 1,226.0 | 4,375.3 | 7,412.0 | 16,016.5 | 115,839.0 | |
| Total antimicrobial | g/head | 25.62 | 22.65 | 0.05 | 7.44 | 18.68 | 44.04 | 78.10 | |
| Total oral antimicrobial | g/head | 24.87 | 22.54 | 0.00 | 7.19 | 18.46 | 43.43 | 77.75 | |
| Total injectable antimicrobial | g/head | 0.75 | 1.07 | 0.00 | 0.21 | 0.39 | 0.77 | 4.65 | |
| Tetracyclines | g/head | 12.28 | 14.71 | 0.00 | 0.03 | 4.86 | 18.73 | 51.68 | |
| Oxytetracycline | g/head | 7.35 | 13.42 | 0.00 | 0.00 | 0.00 | 7.64 | 51.68 | |
| Chlortetracycline | g/head | 1.01 | 3.86 | 0.00 | 0.00 | 0.00 | 0.00 | 17.14 | |
| Doxycycline | g/head | 3.92 | 10.08 | 0.00 | 0.00 | 0.00 | 0.57 | 45.20 | |
| Amphenicols | g/head | 0.94 | 1.72 | 0.00 | 0.00 | 0.12 | 0.90 | 6.18 | |
| Penicillins | g/head | 2.70 | 3.07 | 0.00 | 0.59 | 1.35 | 3.72 | 11.71 | |
| Cephalosporins | g/head | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 | 0.01 | 0.06 | |
| Sulfonamides | g/head | 2.32 | 4.10 | 0.00 | 0.00 | 0.00 | 2.48 | 16.20 | |
| Trimethoprim | g/head | 1.68 | 3.39 | 0.00 | 0.00 | 0.00 | 2.09 | 16.20 | |
| Macrolides | g/head | 3.40 | 3.96 | 0.00 | 0.01 | 2.09 | 5.60 | 16.29 | |
| Lincomycin | g/head | 0.33 | 0.82 | 0.00 | 0.00 | 0.00 | 0.04 | 3.06 | |
| Aminoglycosides | g/head | 0.52 | 1.29 | 0.00 | 0.00 | 0.16 | 0.46 | 7.69 | |
| Fluoroquinolones | g/head | 0.03 | 0.05 | 0.00 | 0.00 | 0.01 | 0.04 | 0.16 | |
| Polymyxin (Colistin) | g/head | 0.32 | 0.67 | 0.00 | 0.00 | 0.00 | 0.01 | 2.25 | |
| Density of fattening pig | m2/head | 1.40 | 0.79 | 0.50 | 0.99 | 1.26 | 1.62 | 5.18 | |
| Pre-weaning mortality risk | % | 11.36 | 3.49 | 4.88 | 9.09 | 11.36 | 13.58 | 20.54 | |
| Post-weaning mortality risk | % | 6.71 | 3.64 | 0.93 | 4.13 | 6.49 | 7.57 | 17.21 | |
Fig. 2.
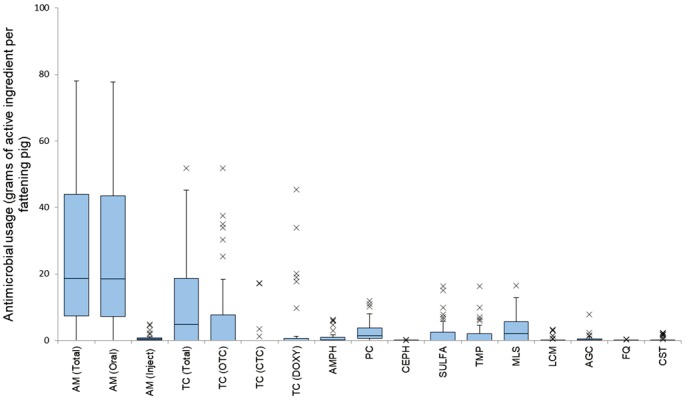
Box-and-whisker plot of total usage of all, oral and injectable antimicrobials and usage of each antimicrobial in grams of active ingredient per fattening pig shipped for slaughter. The crosses stand for range out of ± 1.5 × interquartile range (IQR). AM (Total): Total of all antimicrobials; AM (Oral): Total of oral antimicrobials; AM (Inject): Total of injectable antimicrobials; TC (Total): All tetracyclines; TC (OTC): Oxytetracycline; TC (CTC): Chlortetracycline; TC (DOXY): Doxycycline; AMPH: Amphenicols; PC: Penicillins; CEPH: Cephalosporins; SULFA: Sulfonamides; TMP: Trimethoprim; MLS: Macrolides; LCM: Lincomycin; AGC: Aminoglycosides; FQ: Fluoroquinolones; CST: Colistin.
On-farm biosecurity level
The average scores of the site condition and external and internal biosecurity were 9.85/16 (SD=3.70), 13.08/24 (SD=3.59) and 12.38/24 (SD=3.66), respectively. The average scores by sub-category are shown in Table 1. The scores of respective biosecurity measures were generally normally distributed.
Animal welfare indicators
The average surface space per fattening pig was 1.40 m2/head (SD=0.78, 95% confidence interval (CL): 1.11–1.68). The average pre- and post-weaning mortality risk was 11.36% (SD=3.44, 95%CL:10.21–12.50) and 6.71% (SD=3.59, 95%CL: 5.51–7.90), respectively.
Factors affecting antimicrobial usage amount
As a result of the stepwise selection procedure, following regression models were identified as the ones with highest predictability. The power of these regression models valued between 0.943 and 1.000. There was no model identified as significant for other antimicrobial agent:
| logYtotal=4.466−0.133Xsite condition−0.443Xfarm contractors |
| logYDOXY=−0.043+17.312Xpw mortality−0.529Xpig−flow |
| logYAMPH=0.508−0.279Xpen layouts+7.730Xpw mortality−0.244Xpig−flow |
| logYPC=2.330−0.101Xsite condition−0.277Xpig−flow |
| logYMLS=2.029−0.377Xpen layouts−0.344Xfarm contractors |
| logYFQ=−0.531−0.050Xpig−flow |
where Ytotal is the total usage of antimicrobial; YDOXY, YPC, YMLS and YFQ are the usage of doxycycline, penicilins, macrolides and fluoroquinolones respectively; and Xsite condition Xfarm contractors, Xpw mortality, Xpig-flow, Xpen layouts are the scores for site condition, farm contractors, post-weaning mortality, pig-flow and pen layouts, respectively.
These results showed that better site conditions and higher external biosecurity scores in the sub-categories of ‘farm contractors’ were strongly associated with lower oral usage of antimicrobials (P<0.05, Table 2). The adjusted coefficient of determination (R2) of the model was low at 26.0%. Neither internal biosecurity nor animal welfare indicators showed significant association with the total oral usage of antimicrobials.
The regression model using usage of tetracyclines as a dependent variable did not show significant relationship with any independent variables, thus sub-classes from the tetracycline class (oxytetracycline, chlortetracycline and doxycycline) were subjected to analysis separately. Both regression models using the use of doxycycline and amphenicols as dependent variables resulted in higher adjusted coefficient of determination value (R2>0.3) with animal welfare indicators being a factor with a significant association with the use of these antimicrobials. The common factors affecting both doxycycline and amphenicols usage in ascending order of importance were ‘post-weaning mortality risk’ and ‘pig flows’, one of the sub-categories of internal biosecurity (all at P<0.05).
DISCUSSION
The antimicrobial usage in 2015 from 38 farms generally showed right skewed distribution. This result implies that subjecting the higher antimicrobial users to intensive reduction measures might enable a successful reduction of antimicrobial usage. It was not possible to make comparison of farm level antimicrobial usage between Japan and other countries because of the different indicators used for antimicrobial usage quantification [12, 15]. The most used oxytetracycline within tetracyclines did not show any significant association with any of the independent variables, while usage of the second most used doxycycline had significant association with some of the independent variables in the regression analysis. This result may have reflected the difference of prescription patterns of these antimicrobials: oxytetracycline was used in more than half of the participating farms in certain quantities while doxycycline was used in less than 30% with variance.
The external biosecurity score was higher and was regarded as more important than the internal biosecurity score. This tendency that farmers paid more attention to external biosecurity than internal biosecurity was also observed in a study conducted among European farmers [15]. In terms of site conditions, pig farms isolated from other farms and located in areas with low pig density used lower amount of antimicrobials for oral use. Regarding external and internal biosecurity levels, farms that required shower-in/out with a fumigation system against contractors who previously visited other farms used lower amount of oral antimicrobials. These results suggest that in order to reduce the use of antimicrobials for herd treatment, farmers should consider the location when building a new farm or pig house and preferentially reinforce the entrance requirements against high risk visitors. These can protect the farm from the introduction of disease-causing agents. A similar result in respect to entrance requirements was observed on horse farms which had strict entrance requirements for surgical practitioners, which used less antimicrobial for infectious disease treatment than those with no entrance requirements [21].
Farms with lower post-weaning mortality risk with complete control of pig flow used less doxycycline and amphenicols. Since both antimicrobials are often used to treat against porcine pleuropneumonia [17], better practice in preventing respiratory diseases may lead to lower usage of these antimicrobials.
Farms that apply all-in and all-out system at all stages used lower antimicrobials that are often used for treatment of pneumonia and edema disease in piglets (e.g. doxycycline, amphenicols, penicillins and fluoroquinolones [17]). This indicates the effectiveness of all-in and all-out system in cleaning and disinfection of the premises contaminated with the causative agents of these diseases during the empty period. A previous study conducted in 4 European countries also pointed out the importance of longer periods between batches that enables a better separation between age groups and provides sufficient time for cleaning and disinfection to lower the risk of transmission of pathogens [14].
In generalizing the results of this study using the data of pig farms contracted with members of JASV and antimicrobial usage data based on their prescription, there are some potential biases that we should keep in mind. Because the JASV member veterinarians are actively involved in an attempt to reduce the antimicrobial use, the farmers in this study might have a higher level of awareness on antimicrobial usage and biosecurity measures than the representative pig farmers in Japan. Also there might be information bias because prescription amounts might not reflect the amount of antimicrobials actually used. Further studies are needed to validate how much the prescribed amount differs from the amount actually used.
In this study, we attempted to evaluate the association between on-farm biosecurity level, animal welfare indicators and usage of antimicrobials using the latest available data. The results highlighted that lower usage of antimicrobials is associated with better site condition, higher biosecurity score and lower mortality risk. The negative association between biosecurity level and the antimicrobial usage is also revealed in the similar previous study conducted in European countries [9, 13].
We used the floor space per fattening pig and pre- and post-weaning mortality risk as animal welfare indicators as these are the only data obtainable from the PigINFO database that are related to animal welfare. The animal welfare standards are generally subjected to sows since they have been kept the longest period in farms. Further consideration is needed to develop indicators that are capable of evaluating the animal welfare level in sows on Japanese pig farms more accurately. Also, a cost benefit analysis of biosecurity and animal welfare measures as an alternative to antimicrobials might be useful in identifying cost-effective interventions to reduce the use of antimicrobials.
In this cross-sectional study, data of 38 farms were used to identify factors that are associated with the use of antimicrobials. With this sample size, the statistical power was strong enough to identify six regression models with significant association, but might not have been sufficient to identify the real effect of variables in other models that were not found significant in this study. To verify this, further studies are needed with a larger sample size. Nevertheless, policy makers, veterinarians and farmers should benefit from this study to reduce the antimicrobial usage on pig farms, and thus reduce the risk of development of antimicrobial resistance.
Supplementary Material
Acknowledgments
The authors are grateful to veterinarians who are the members of JASV and pig producers under their contract for being cooperative in collecting data for this study. They also thank P-JET and PigINFO members for sharing assessment tools and productivity data with us. They also thank Dr. Ray Bradley for carefully reading the manuscript and providing them with useful information and suggestions. This work was supported by grants for studying and research on meat production from the Ito Foundation.
REFERENCES
- 1.Bock B. B., van Huik M. M.2007. Animal welfare: the attitudes and behaviour of European pig farmers. Br. Food J. 109: 931–944. doi: 10.1108/00070700710835732 [DOI] [Google Scholar]
- 2.Bos M. E., Taverne F. J., van Geijlswijk I. M., Mouton J. W., Mevius D. J., Heederik D. J., Netherlands Veterinary Medicines Authority SDa2013. Consumption of antimicrobials in pigs, veal calves, and broilers in the Netherlands: quantitative results of nationwide collection of data in 2011. PLoS One 8: e77525. doi: 10.1371/journal.pone.0077525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farm Animal Welfare Committee2012. Farm Animal Welfare: Health and Disease. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/324616/FAWC_report_on_farm_animal_welfare_-_health_and_disease.pdf [accessed May 10, 2018].
- 4.Filippitzi M. E., Callens B., Pardon B., Persoons D., Dewulf J.2014. Antimicrobial use in pigs, broilers and veal calves in Belgium. Vlaams Diergen. Tijds. 83: 215–224. [Google Scholar]
- 5.Holmes A. H., Moore L. S. P., Sundsfjord A., Steinbakk M., Regmi S., Karkey A., Guerin P. J., Piddock L. J. V.2016. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 387: 176–187. doi: 10.1016/S0140-6736(15)00473-0 [DOI] [PubMed] [Google Scholar]
- 6.Jääskeläinen T., Kauppinen T., Vesala K. M., Valros A.2014. Relationships between pig welfare, productivity and farmer disposition. Anim. Welf. 23: 435–443. doi: 10.7120/09627286.23.4.435 [DOI] [Google Scholar]
- 7.Japan Pharmaceutical and Medical Device Act. 2016. Amendment. http://elaws.e-gov.go.jp/search/elawsSearch/elaws_search/lsg0500/detail?openerCode=1&lawId=335AC0000000145_20160401_427AC0000000050 [accessed on August 10, 2018].
- 8.Kauppinen T., Vesala K. M., Valros A.2012. Farmers attitude toward improvement of animal welfare is correlated with piglet production parameters. Livest. Sci. 143: 142–150. doi: 10.1016/j.livsci.2011.09.011 [DOI] [Google Scholar]
- 9.Laanen M., Persoons D., Ribbens S., de Jong E., Callens B., Strubbe M., Maes D., Dewulf J.2013. Relationship between biosecurity and production/antimicrobial treatment characteristics in pig herds. Vet. J. 198: 508–512. doi: 10.1016/j.tvjl.2013.08.029 [DOI] [PubMed] [Google Scholar]
- 10.Matsuda M., Ito M., Sugiura K.2018. The first attempt to create an antimicrobial agent identification code list approved for use in pigs to measure the antimicrobial use in pig farms. J. Vet. Med. Ass. 71: 15–17. [Google Scholar]
- 11.Matsuda M., Kwan N., Kawanishi M., Koike Y., Sugiura K.2017. The evaluation of veterinary antimicrobial use in the food-producing animals in Japan. J. Animal Hyg. 42: 191–197. [Google Scholar]
- 12.Merle R., Robanus M., Hegger-Gravenhorst C., Mollenhauer Y., Hajek P., Käsbohrer A., Honscha W., Kreienbrock L.2014. Feasibility study of veterinary antibiotic consumption in Germany--comparison of ADDs and UDDs by animal production type, antimicrobial class and indication. BMC Vet. Res. 10: 7. doi: 10.1186/1746-6148-10-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’neill J.2016. Tackling drug-resistant infections globally: Final report and recommendations. The review on antimicrobial resistance.
- 14.Postma M., Backhans A., Collineau L., Loesken S., Sjölund M., Belloc C., Emanuelson U., Grosse Beilage E., Stärk K. D. C., Dewulf J., MINAPIG consortium2016. The biosecurity status and its associations with production and management characteristics in farrow-to-finish pig herds. Animal 10: 478–489. doi: 10.1017/S1751731115002487 [DOI] [PubMed] [Google Scholar]
- 15.Postma M., Backhans A., Collineau L., Loesken S., Sjölund M., Belloc C., Emanuelson U., Grosse Beilage E., Nielsen E. O., Stärk K. D. C., Dewulf J., MINAPIG consortium2016. Evaluation of the relationship between the biosecurity status, production parameters, herd characteristics and antimicrobial usage in farrow-to-finish pig production in four EU countries. Porcine Health Manag. 2: 9. doi: 10.1186/s40813-016-0028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott P. R.2013. The challenges to improve farm animal welfare in the United Kingdom by reducing disease incidence with greater veterinary involvement on farm. Animals (Basel) 3: 629–646. doi: 10.3390/ani3030629 [DOI] [Google Scholar]
- 17.The Japanese Society of Antimicrobials for Animals2013. Manual for Veterinary Antimicrobials, 2nd ed. p.75. Interzoo, Tokyo. [Google Scholar]
- 18.The Ministry of Agriculture Forestry and Fisheries. 2013. Guideline for the use of antimicrobial substance in livestock mutual aid. http://www.maff.go.jp/j/keiei/nogyohoken/kokuzi_tsuchi/attach/pdf/index-102.pdf [accessed on April 4, 2018].
- 19.The Ministry of Agriculture Forestry and Fisheries. 2013. Promoting responsible and prudent use of veterinary antimicrobials. http://www.maff.go.jp/j/syouan/tikusui/yakuzi/pdf/vet_panf_prudent_use.pdf [accessed on August 10, 2018].
- 20.The Ministry of Agriculture Forestry and Fisheries. 2016. National action plan on antimicrobial resistance (AMR) 2016–2020. http://www.maff.go.jp/j/syouan/tikusui/yakuzi/pdf/yakuzai_honbun.pdf [accessed on May 10, 2018].
- 21.Traub-Dargatz J., Kopral C., Wagner B.2012. Relationship of biosecuriy practices with the use of antibiotics for the treatment of infectious disease on U.S. equine operations. Prev. Vet. Med. 104: 107–113. doi: 10.1016/j.prevetmed.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 22.Van Boeckel T. P., Brower C., Gilbert M., Grenfell B. T., Levin S. A., Robinson T. P., Teillant A., Laxminarayan R.2015. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. U.S.A. 112: 5649–5654. doi: 10.1073/pnas.1503141112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


