Abstract
Stem cell homing is a complex phenomenon that involves multiple steps; thus far, attempts to increase homing efficiency have met with limited success. Spermatogonial stem cells (SSCs) migrate to the niche after microinjection into seminiferous tubules, but the homing efficiency is very low. Here we report that reversible disruption of the blood-testis barrier (BTB) between Sertoli cells enhances the homing efficiency of SSCs. We found that SSCs on a C57BL/6 background are triggered to proliferate in vitro when MHY1485, which stimulates MTORC, were added to culture medium. However, the cultured cells did not produce offspring by direct injection into the seminiferous tubules. When acyline, a gonadotropin-releasing hormone (GnRH) analogue, was administered into infertile recipients, SSC colonization increased by ~5-fold and the recipients sired offspring. In contrast, both untreated individuals and recipients that received leuprolide, another GnRH analogue, remained infertile. Acyline not only decreased CLDN5 expression but also impaired the BTB, suggesting that increased colonization was caused by efficient SSC migration through the BTB. Enhancement of stem cell homing by tight junction protein manipulation constitutes a new approach to improve homing efficiency, and similar strategy may be applicable to other self-renewing tissues.
Keywords: Sertoli cells, Spermatogenesis, Spermatogonia, Tight junction
Stem cells are thought to reside in a special microenvironment called the niche, which provides factors that maintain stem cells in an undifferentiated state. Stem cells have a strong affinity to the niche because transplanted stem cells can migrate to the niche [1]. This phenomenon of stem cell homing has been analyzed most extensively in the hematopoietic system. When hematopoietic stem cells (HSCs) are transplanted into the peripheral blood of animals that underwent radiation or chemical treatments, they can find and actively migrate into empty niches in the bone marrow and regenerate hematopoiesis [2]. A number of studies have revealed molecules involved in stem cell homing, including chemokines and adhesion molecules, the coordinated action of which is required for successful homing. Stem cells respond to subtle differences in the chemokine concentration and migrate through the multiple cell types by modulating the expression of various types of adhesion molecules. However, enhancement of colonization has not been easy due to complexity of homing machinery, and is therefore one of the major goals of stem cell manipulation.
The spermatogenic system is another self-renewing system for which a similar functional stem cell transplantation assay is available. Spermatogonial stem cells (SSCs) comprise a small percentage of the cells in the testis [3]. They are thought to be a part of Asingle (As) spermatogonia, the frequency of which in the testis is only 0.02 to 0.03% [4, 5]. Although it was considered that SSCs do not have migratory activity, development of a spermatogonial transplantation technique revealed that these cells do have homing activity [6]. When dissociated testis cells are transplanted into the seminiferous tubules of infertile mouse testes, donor SSCs attach to the Sertoli cells, migrate through the blood-testis barrier (BTB) between Sertoli cells, and settle and proliferate on the basement membrane. This directed migration from the adluminal compartment of the seminiferous tubules to the basal compartment is striking because germ cells migrate from the basement compartment to the adluminal compartment under physiological conditions. Therefore, it is not surprising that spermatogonial transplantation is an inefficient process and the fertility of recipient mice is very low. It has been estimated that only 5–10% of transplanted SSCs colonize the seminiferous tubules [7, 8], and only one of 122 offspring was found to be derived from donor SSCs in initial experiments [9]. This low homing efficiency has prevented the application of SSCs to animal transgenesis or treatment of infertility.
To improve the homing efficiency, several attempts to enhance SSC colonization have been made. The first successful improvement was achieved by administering leuprolide, a gonadotropin-releasing hormone (GnRH) analogue, to busulfan-treated animals [10]. Leuprolide was originally used to preserve fertility after radiation exposure by decreasing the testosterone concentration [11,12,13]. Suppression of intratesticular testosterone by leuprolide stimulates the recovery of spermatogenesis after irradiation in rats [14]. It was speculated that the enhanced recovery is due to hormonal modulation of Sertoli cells, which in turn alters the paracrine-regulated differentiation of type A spermatogonia. Although leuprolide was not effective in mice after irradiation-induced recovery, administration of leuprolide increased the number of colonies after spermatogonial transplantation in mice [10]. It also improved the condition of recipient rats, which experienced severe edema after busulfan treatment [15]. However, the effect of leuprolide on recovery of fertility after spermatogonial transplantation has proven to be limited (unpublished observations).
An obvious approach to improve germline transmission is increasing the number of transplanted SSCs. Previous experiments have suggested that ~10% of wild-type SSCs are required for restoration of fertility after chemical or radiation treatment [16]. Therefore, the number of SSCs is one of the most critical factors for restoring fertility. However, because the number of SSCs in the testis cell suspension is very low, it is necessary to achieve significant enrichment before transplantation. For example, it is possible to remove differentiating germ cells by making cryptorchids [17], which increases the proportion of SSCs in the total cell suspension. Transplantation of cryptorchid testis cells successfully produced offspring [17]. However, this technique is time-consuming and applicable only on a C57BL/6 (B6) background because more differentiated germ cells remain in other strains. More recently, we established a method to improve germline transmission of recipients by using germline stem (GS) cells, which are cultured spermatogonia with self-renewal potential [18]. GS cells are derived by culturing testis cells in the presence of glial cell line-derived neurotrophic factor (GDNF) and fibroblast growth factor 2 (FGF2), both of which promote SSC self-renewal. Because SSCs comprise 1–2% of GS cells [19], GS cells are significantly more enriched for SSCs compared to adult testis, and we expected that GS cells would increase the amount of transplanted SSCs and thus improve fertility. Indeed, transplantation of GS cells allowed offspring production in all recipient mice as early as 3 months [20]. This result suggested that transplantation of an increased number of SSCs is an effective approach to restore fertility.
Although it is now possible to use GS cells for fertility studies, this approach is not always feasible because there is significant variation in self-renewal efficiency among different strains. For example, SSCs on a DBA/2 background proliferate more actively than those on a B6 background both in vivo and in vitro [18, 21]. Moreover, culture conditions depend on the batch of bovine serum albumin (BSA) [22], and long-term SSC cultures results in senescence and more differentiating division depending on the strain and culture medium composition [23, 24]. Therefore, differences in self-renewal efficiency hamper studies regarding the fertility of SSCs. Although we recently found that addition of PS48, a 3-phosphoinositide dependent protein kinase 1 (PDPK1) activator, stimulates SSC self-renewal via AKT activation and enhances GS cell derivation more reliably [25], it was not clear in the previous study whether such artificial stimulation of self-renewal division allows offspring production by normal fertilization. Thus, there is clearly a need to develop new methods for improving the germline transmission efficiency of SSCs.
Although it is very difficult or impossible to overcome genetic constraints on self-renewal activity, germline transmission is not simply determined by the donor cell factor but also involves the host environment. In the current study, we established better culture conditions for donor SSCs and also found a method for improving host conditions for restoration of natural fertility by manipulating SSC homing. Of the multiple steps involved in SSC homing, the biggest hurdle appears to be passage through the blood-testis barrier (BTB) [26]. The BTB is comprised of several claudin proteins, such as CLDN3, CLDN5 and CLDN11, which make up a tight junction between Sertoli cells [27]. In this study, we found that acyline [28], another GnRH agonist, improves fertility of GS cells by modulating claudin protein expression and transiently compromises BTB function, thereby enhancing germline transmission.
Materials and Methods
Cell culture
GS cells in a DBA/2 background (DBA-GS) cells were previously described [20]. GS cells were derived from both C57BL/6 Tg14(act-EGFP)OsbY01 (designated green; gift from Dr M Okabe, Osaka University) and B6-TgR(ROSA26)26Sor (ROSA26; Jackson laboratory, ME) pups on a B6 background using PS48 (Wako, Kyoto, Japan), as described previously [25]. MHY-GS cells were established from 5–7-day-old green pups on a B6 background using MHY1485 (2 μM; Calbiochem, San Diego, CA) and Iscove modified Dulbecco’s medium (Invitrogen, Carlsbad, CA), which was supplemented with 10 ng/ml human FGF2, 15 ng/ml rat GDNF (both from PeproTech, London, UK), as previously described [29]. All GS cells were maintained on mitomycin C-treated mouse embryonic fibroblasts.
Animals and spermatogonial transplantation
For busulfan-treatment, 4- to 5-week-old B6 or B6 × DBA F1 (BDF1) mice underwent intraperitoneal injection with busulfan (44 mg/kg; Japan SLC, Shizuoka, Japan). BDF1 mice were used for quantification of SSCs, and both BDF1 and B6 mice were used for fertility restoration experiments. Where indicated, we also used 4- to 6-week-old WBB6F1-W/Wv (W) mice (Japan SLC) for fertility restoration experiments. These mice lack endogenous spermatogenesis and allow offspring production without pretreatment.
Acyline (20 mg/kg; provided by the Contraceptive Development Branch of the National Institute of Child Health and Human Development) was administered subcutaneously on the next day after busulfan treatment, and was additionally administered 2 and 4 weeks after busulfan treatment. Leuprolide treatment was administered via subcutaneous injection [30].
Spermatogonial transplantation was carried out by microinjection into the seminiferous tubules of infertile mice via the efferent duct [31]. Approximately 4 or 10 μl of cell suspension was microinjected into the testes of W and busulfan-treated mice, respectively. Each injection filled 75−85% of the seminiferous tubules. For colchicine (20 μM), cytochalasin D (100 μM, both from Calbiochem), EDTA (20 mM, Wako), or CPE (0.5 mg/ml; a gift from Dr Sachiko Tsukita, Osaka University, Osaka, Japan) treatment, donor cells were incubated with the indicated reagent and microinjected into the seminiferous tubules. For Cldn5 overexpression experiments, approximately 10 μl of lentivirus particles, produced by transient expression of CSII-EF-Cldn5-IRES2-Puro (3 × 108 infectious units/ml), were microinjected into the seminiferous tubules of busulfan-treated B6 seminiferous tubules. CSII-EF-Efyp-IRES2-Puro was used as a control. Between 6 and 8 days after virus injection, 5 × 103 MHY-GS cells were transplanted into each testis. All busulfan-treated recipient mice were used at least 4 weeks after busulfan treatment. The Institutional Animal Care and Use Committee of Kyoto University approved all of the animal experimentation protocols.
Analysis of recipient testes
For the assessment of colony counts, recipients were sacrificed 2 months after transplantation, and donor cell colonization was examined under UV light. Germ cell clusters were defined as colonies when the entire basal surface of the tubule was occupied and the cell clusters were at least 0.1 mm in length [7]. For histological evaluation of the recipient testes, the testes were fixed with 10% neutral-buffered formalin and processed for paraffin sectioning. Two histological sections were made from each recipient testis with an interval of 12 μm between sections. All sections were stained with hematoxylin and eosin.
Immunohistochemistry and lectin immunostaining
Samples were fixed in 4% paraformaldehyde for 2 h, and then embedded in Tissue-Tek OCT compound for cryosectioning. Staining of cryosections was carried out by treating the samples with 0.1% Triton-X in phosphate-buffered saline (PBS). After immersion in blocking buffer (0.1%Tween 20, 1% BSA and 1% goat serum in PBS) for > 1 h, samples were incubated with the indicated primary antibodies or peanut agglutinin (PNA) at 4°C overnight. After three washes with PBS, samples were incubated with the secondary antibody. The antibodies and PNA used in the study are listed in Supplementary Table 1 (online only).
Biotin tracer experiment
For the assessment of the BTB function, biotin tracer experiment by microinjecting ~12 μl of EZ-Link sulfo-NHS-LC-Biotin solution (7.5 mg/ml in PBS/1 mM CaCl2; Thermo Fisher Scientific, Waltham, MA) into interstitial area of acyline-treated testes. Samples were collected 30 min after microinjection and fixed in 4% paraformaldehyde for 2 h for cryosectioning. Samples were then stained with streptavidin (Supplementary Table 1).
Analysis of gene expression
Total RNA was isolated using TRIzol® (Invitrogen), and first-strand cDNA was synthesized using the Verso cDNA Synthesis Kit (Thermo Fisher Scientific) and used for reverse transcription polymerase chain reaction (RT-PCR). For real-time PCR, the StepOnePlusTM Real-Time PCR system and FastStart Universal SYBR Green PCR Master Mix (Roche, Basel, Switzerland) were used according to the manufacturer’s protocol (Applied Biosystems, Warrington, UK). Transcript levels were normalized relative to those of Hprt. PCR conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Each reaction was performed in duplicate. PCR primer sequences are listed in Supplementary Table 2 (online only).
Flow cytometry
GS cells were dissociated using Cell Dissociation Buffer (Invitrogen). The cell staining technique was as previously described [18]. Cells were analyzed with a FACS Calibur system (BD Bioscience, San Jose, CA). The antibodies used in the study are listed in Supplementary Table 1.
Statistical analyses
Significant differences between means for single comparisons were determined by Student’s t-tests. Multiple comparison analyses were carried out using ANOVA followed by Tukey’s honest significant difference test. All values are represented by means ± SEM.
Results
Derivation of GS cells on a B6 background by MHY1485
During our recent attempt to establish GS cells on a B6 background, we found that PS48, a PDPK1 activator, stimulates self-renewal of SSCs in vitro (PS48-GS cells) [25]. Because the fertility of PS48-GS cells was demonstrated by microinsemination in the previous study, it was not clear whether sperm derived from PS48-GS cells is competent with respect to producing offspring by natural mating. We transplanted PS48-GS cells derived from green or ROSA26 mice into the seminiferous tubules of W mice. The recipient mice were mated with wild-type females at least 6 weeks after transplantation. Although we kept the males for more than 7 months, none of them became fertile (Table 1). This was in contrast to our previous experiments using GS cells from DBA mice (DBA-GS cells), which sired progeny as early as 3 months after transplantation in both W and busulfan-treated mice [20]. These results suggested that PS48-GS cells are not competent to produce offspring by natural mating.
Table 1. Restoration of fertility by spermatogonial transplantation.
| Recipient type | Host treatment | Cell type (mouse origin) |
Concentration of transplanted cells |
Testis weight (mg) 1 | Number of fertile recipients (%) | EGFP+ offspring 2 (%) |
|---|---|---|---|---|---|---|
| W | No treatment | PS48-B6 (ROSA) | 108/ml | 14.1 ± 1.0 (2) | 0/2 (0) | 0 (0) |
| W | No treatment | PS48-B6 (ROSA) | 5 × 107/ml | 14.6 ± 0.6 (2) | 0/2 (0) | 0 (0) |
| W | No treatment | PS48-B6 (EGFP) | 3 × 107/ml | 26.4 ± 2.7 (3) | 0/5 (0) | 0 (0) |
| BDF1 | Leuprolide | MHY-B6 (EGFP) | 108/ml | 37.7 ± 40.8 (1) | 0/2 (0) | 0 (0) |
| B6 | Leuprolide | MHY-B6 (EGFP) | 108/ml | 24.0 ± 0.7 (2) | 0/6 (0) | 0 (0) |
| B6 | Acyline | MHY-B6 (EGFP) | 108/ml | 42.7 ± 4.9 (2) | 3/4 (75) | 2 (50%) |
Values are mean ± SEM. Recipient mice were kept for 194 to 634 days after transplantation. 1 In parenthesis, the number of animals that survived the whole experimental period is shown. 2 Recipients that sired EGFP+ offspring.
To improve the fertility of GS cells, we carried out additional screening and eventually found that MHY1485 can stimulate colony formation (Fig. 1A). MHY1485 stimulates MTORC. B6 GS cells, derived by MHY1485 stimulation from green mouse pups (MHY-GS cells), appeared similar to those established by PS48 (Fig. 1A). It was not possible to compare the effect of MHY1485 with B6 GS cells because the cells do not proliferate without this chemical. The doubling time of MHY-GS and DBA-GS cells was 2.0 and 1.7 days, respectively (Fig. 1B). The finding that the doubling time of PS48-GS cells was 3.2 days suggested that MHY1485 stimulates self-renewal division more actively than PS48 [25]. Like PS48, supplementation with MHY1485 could reliably stimulate proliferation of spermatogonia regardless of the batch of BSA, which was a critical impediment in the previous method [22]. MHY1485 did not enhance the proliferation of DBA-GS cells (data not shown).
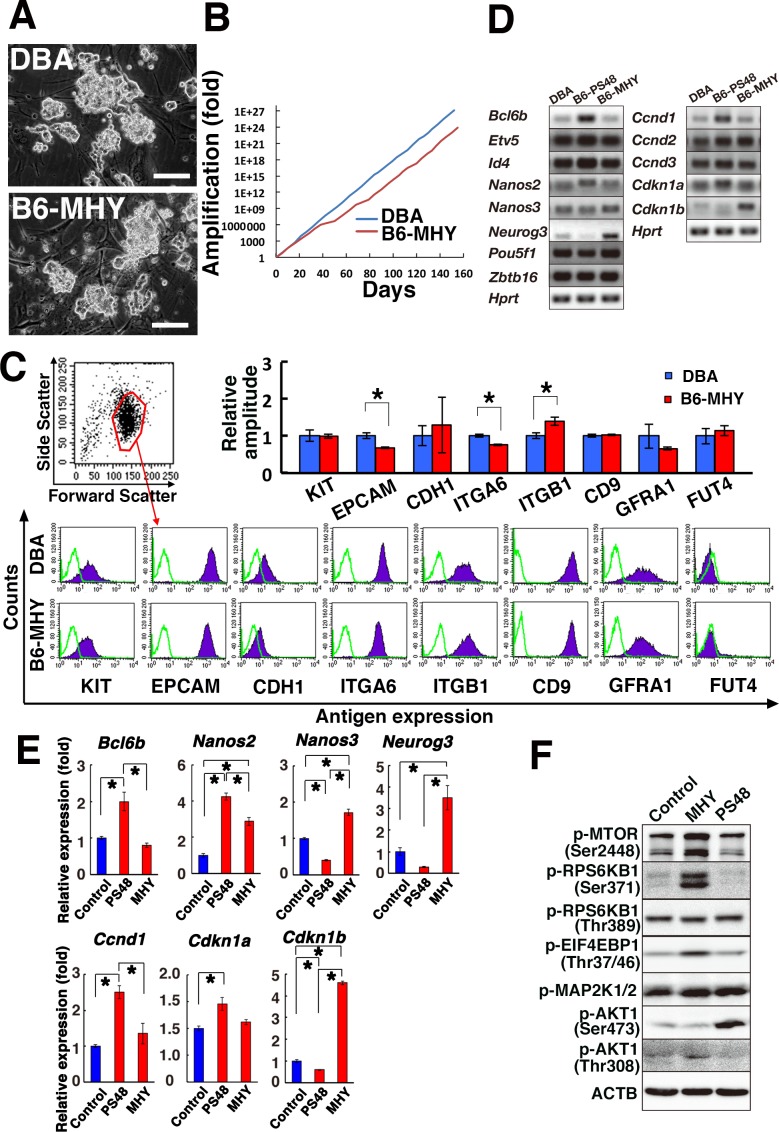
Fig. 1.
Derivation of GS cells by MHY1485 treatment. (A) Appearance of MHY1485-GS cells. (B) Long-term proliferation of MHY1485-GS cells. (C) Flow cytometric analysis of surface markers (n = 4). (D) RT-PCR analysis of SSC makers. (E) Real-time PCR analysis (n = 3). (F) Western blot analysis of MHY1485-GS cells. Bar = 100 μm (A). Asterisk indicates statistical significance (P < 0.05).
To characterize the phenotype of MHY-GS cells, we first examined the cell surface phenotype. Flow cytometric analysis of cell surface markers showed increased expression of ITGB1, but EPCAM and ITGA6 were significantly downregulated in MHY-GS cells compared with DBA-GS cells (Fig. 1C). This was in contrast to PS48-GS cells, which showed downregulation of KIT, ITGB1, and CD9 [25]. Because this suggested a slightly different phenotype of MHY-GS cells, we directly compared the expression of several SSC marker genes between MHY-GS and PS48-GS cells. RT-PCR analysis showed significant changes in the expression of several SSC marker genes (Fig. 1D). Compared with DBA-GS cells, PS48-GS cells showed increased expression of Bcl6b and Nanos2, but Nanos3 and Neurog3 were downregulated. In contrast, MHY-GS cells did not show increased expression of Bcl6b, but Nanos2, Nanos3 and Neurog3 were upregulated. Real-time PCR confirmed these changes (Fig. 1E). Because Nanos2 is expressed only in As and Apaired (Apr) spermatogonia, this result suggested that SSCs are more enriched in PS48-GS cells than in MHY-GS cells.
Because MHY-GS cells proliferated more actively, we also examined the expression of cell cycle-related genes. Of the tested genes, we found that Ccnd1 and Cdkn1a were significantly upregulated in PS48-GS cells, while Cdkn1b was downregulated (Fig. 1D). However, these genes did not show significant changes in MHY-GS cells. Instead, Cdkn1b was significantly increased in MHY-GS cells, whereas no significant changes in Ccnd1 and Cdkn1a expression were found. Because overexpression of Cdkn1b inhibited the proliferation of DBA-GS cells [32], these results suggested that cell cycle regulation in MHY-GS cells is different from that in DBA-GS cells.
To understand the mechanism of MHY1485-mediated self-renewal stimulation, we carried out Western blotting (Fig. 1F). MHY1485 induced increased phosphorylation of MTOR at Ser 2448, which was expected because MHY1485 stimulates MTOR [33]. While MAP2K1/2 phosphorylation levels were comparable to those in DBA-GS cells, phosphorylation of AKT Ser 473 was stronger in PS48-GS cells compared with MHY-GS cells. However, phosphorylation of AKT Thr 308 was increased by MHY1485 treatment. Because phosphorylation of both AKT Ser 473 and Thr 308 further enhances the activation levels of AKT [34], we added both PS48 and MHY1485 to the culture. However, we have not observed the apparent synergistic effects (data not shown). These results suggested that enhanced phosphorylation of AKT Thr 308 is responsible for triggering proliferation of GS cell proliferation on a B6 background.
Phenotypic and functional analysis of MHY1485-stimulated GS cells
To examine the SSC activity of the MHY-GS cells, we carried out spermatogonial transplantation and quantified the concentration of SSCs in these cultures. Cells were transplanted at three different time points to assess the speed of self-renewal division (Table 2). We also used DBA-GS cells as a control. Analysis of recipient testes showed that all donor cell populations contained SSCs (Fig. 2A). The average number of colonies generated by MHY-GS and DBA-GS cells was 381.0 and 228.9, respectively (Fig. 2B). The value was somewhat larger for MHY-GS cells, but there was no statistically significant difference between MHY-GS and DBA-GS cells.
Table 2. Expansion of SSCs during long-term culture.
| Days to transplant 1 | Passage | Colonies/105 (n) | Increase in total cell number (fold) 2 | Increase in SSC number (fold) 3 | |
|---|---|---|---|---|---|
| DBA | 0 | 0 | 275.0 ± 52.0 (4) | 1 | 1 |
| 43 | 8 | 275.0 ± 75.0 (4) | 5.022 × 107 | 5.022 × 107 | |
| 91 | 18 | 225.0 ± 76.1 (6) | 1.736 × 1016 | 1.420 × 1016 | |
| 140 | 30 | 160.0 ± 43.0 (5) | 5.030 × 1024 | 2.92 × 1024 | |
| B6-MHY | 0 | 0 | 58.3 ± 37.5 (6) | 1 | 1 |
| 43 | 8 | 300.0 ± 167.1 (4) | 6.010 × 106 | 3.090 × 107 | |
| 91 | 16 | 558.3 ± 126.8 (6) | 4.947 × 1013 | 4.737 × 1014 | |
| 140 | 28 | 620.0 ± 131.0 (5) | 3.806 × 1021 | 4.048 × 1022 | |
GS cells established from Green mice were cultured on MEFs. In each experiment, 2.0–8.0 × 103 cells were microinjected into the seminiferous tubules of infertile recipient testes. The results are presented as means ± SEMs. 1 The number of days from initiation of culture to transplantation. 2, 3 The increase in the total cell (2) or SSC number (3) from the initial transplantation.
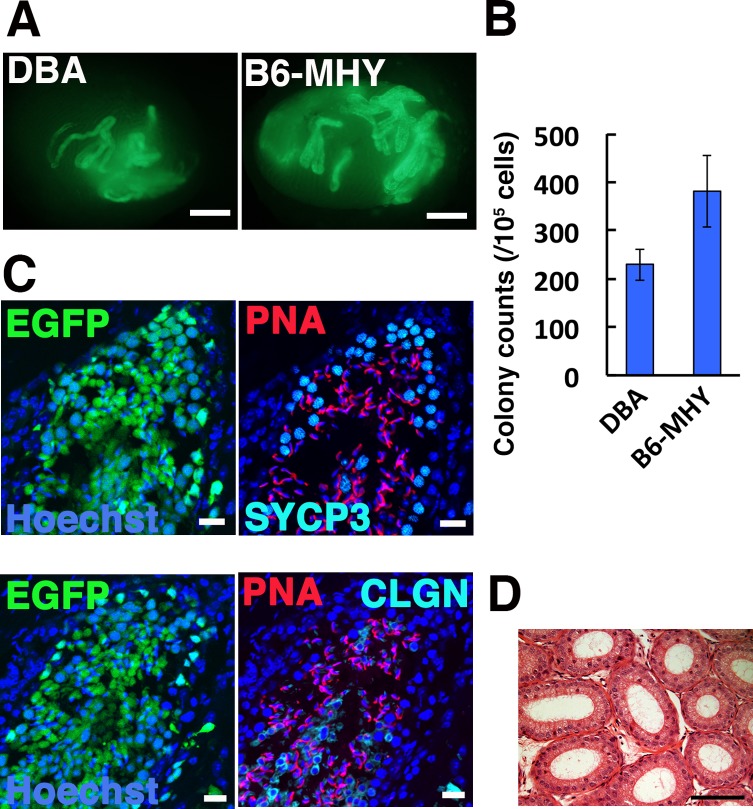
Fig. 2.
Functional analysis of MHY-GS cells by spermatogonial transplantation. (A) Macroscopic appearance of a recipient testis transplanted with MHY-GS cells. Green fluorescence indicates colonies originating from transplanted GS cells. (B) Colony counts (n = 19–21). (C) Immunostaining of recipient testis for SYCP3, CLGN, and PNA. (D) Histological appearance of epididymis of leuprolide-treated mouse. Bar = 1 mm (A), 20 μm (C), 100 μm (D). Counterstain: Hoechst 33342 (C), H & E (D).
Because these GS cell lines appear to have sufficient SSC activities, we transplanted these cells into infertile mice to produce donor-derived offspring. Both busulfan-treated B6 and BDF1 mice were used as recipients. At least three recipient mice were transplanted for each donor cell type. To improve donor cell colonization, we used leuprolide because previous studies showed that it had beneficial effects on spermatogonial transplantation [10]. The recipient males were then housed with three to four wild-type females 4–5 weeks after transplantation. However, none of the animals sired offspring even at 6 months after transplantation (Table 1).
We collected testes and epididymides from these mice and examined the colonization levels. The macroscopic appearance showed significant levels of colonization. Moreover, histological analysis confirmed normal appearing spermatogenesis, but no spermatozoa were found in epididymal tubules (Fig, 2C, D). Therefore, spermatogenic cells produced by the transplanted cells appear to have undergone normal differentiation, but failure to produce offspring suggested that the amount of spermatozoa was not sufficient for fertility recovery by leuprolide treatment.
Improvement of SSC colonization by acyline treatment
Because we failed to produce offspring with MHY-GS cells by simple microinjection into the seminiferous tubules, we sought to improve colonization by conditioning the recipient environments. We employed several approaches focusing on the BTB because the BTB is considered the major impediment to SSC colonization [35]. We first tried to modify the cytoskeleton using cytochalasin D and colchicine, which disrupts actin and the microtubule-based cytoskeleton, respectively. In particular, cytochalasin D was previously shown to transiently disrupt the BTB [36]. We microinjected cytochalasin D or colchicine and transplanted donor testis cells from green mice at the same time. However, there were no significant changes in the donor cell colonization levels (Fig. 3A). In the second set of experiments, testis cells from green mice were suspended in EDTA solution and microinjected into the seminiferous tubules. Because EDTA can chelate calcium, we expected that calcium depletion in the seminiferous tubule fluid would modulate cell-to-cell adhesion and open up the BTB. However, the number of colonies generated by EDTA-treated donor cells was significantly decreased (Fig. 3B, C). In the third set of experiments, we tested the effectiveness of a claudin inhibitor toxin, Clostridium perfringens enterotoxin (CPE) [37]. Because several claudins comprise the BTB, we reasoned that inhibition of the claudins by CPE might disrupt the BTB and improve colonization. However, donor cells transplanted into CPE-treated and control recipients showed comparable levels of colonization and the difference was not statistically significant (Fig. 3D).
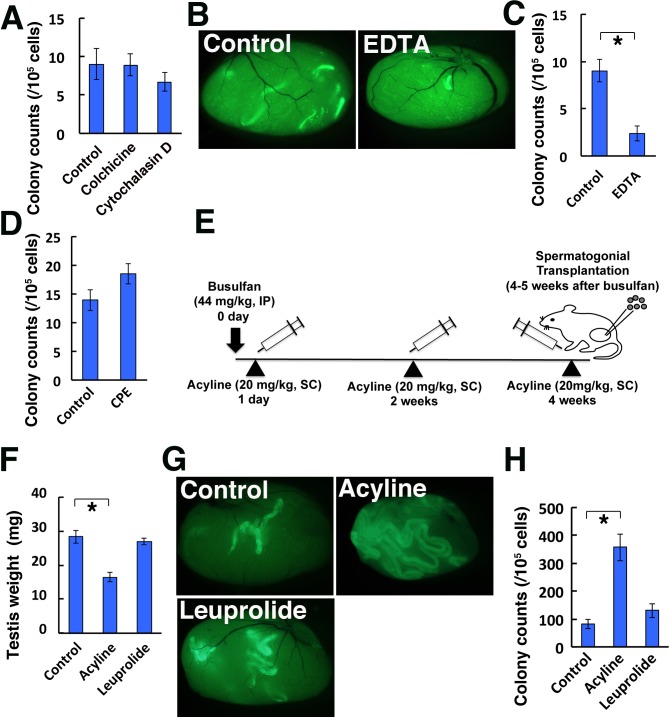
Fig. 3.
Improvement of SSC colonization by acyline. (A) Colony counts in recipient testes after colchicine or cytochalasin D treatment (n = 8–10). (B) Macroscopic appearance of recipient testis that received EDTA. (C) Colony counts in recipient testes after EDTA treatment (n = 8). (D) Colony counts in recipient testis after CPE (n = 6). (E) Experimental scheme of the acyline treatment. (F–H) Testis weight (F; n = 6), macroscopic appearance (G) and colony counts (H; n = 13–18) after leuprolide or acyline treatment. Bar = 1 mm (B, G). IP, intraperitoneal injection, SC, subcutaneous injection. Asterisk indicates statistical significance (P < 0.05).
Finally, we tested the effect of hormones. Acyline is a GnRH agonist [28]. We compared the effect of acyline and leuprolide because they are supposed to have similar effects on GnRH secretion. In this experiment, acyline was administered three times before spermatogonial transplantation (Fig. 3E). In a previous study, leuprolide was administered twice before transplantation to allow achieve a maximal colonization level [30]. When some of the testes were recovered before transplantation, the acyline-treated recipient testes were significantly smaller than leuprolide-treated testes (Fig. 3F). Therefore, we used GS cells for transplantation because they are enriched for SSCs and we thought that we would be able to fill the seminiferous tubules of smaller acyilne-treated testes more efficiently. Spermatogonial transplantation revealed significant enhancement of SSC colonization by acyline treatment (Fig. 3G, H). Although leuprolide also showed a modest increase in colonization levels, the difference was not statistically significant. These results suggested the superiority of acyline over leuprolide in improving donor cell colonization after spermatogonial transplantation.
Analysis of acyline-treated mice
To understand the mechanism of increased colonization in acyline-treated mice, we examined the expression levels of cytokines potentially involved in SSC homing [38,39,40,41]. In this experiment, we used busulfan to mimic the recipient testis environment. Real-time PCR analysis showed a significant increase in Gdnf expression by acyline treatment, while Ccl9 and Cxcl12 did not change significantly (Fig. 4A). In contrast, leuprolide treatment increased both Ccl9 and Cxcl12, while no significant changes were found for Gdnf. However, because Gdnf overexpression did not improve SSC homing in our previous studies [38], Gdnf was unlikely to be involved in increased colonization. Therefore, increased colonization may not have been due to overexpression of chemokines.
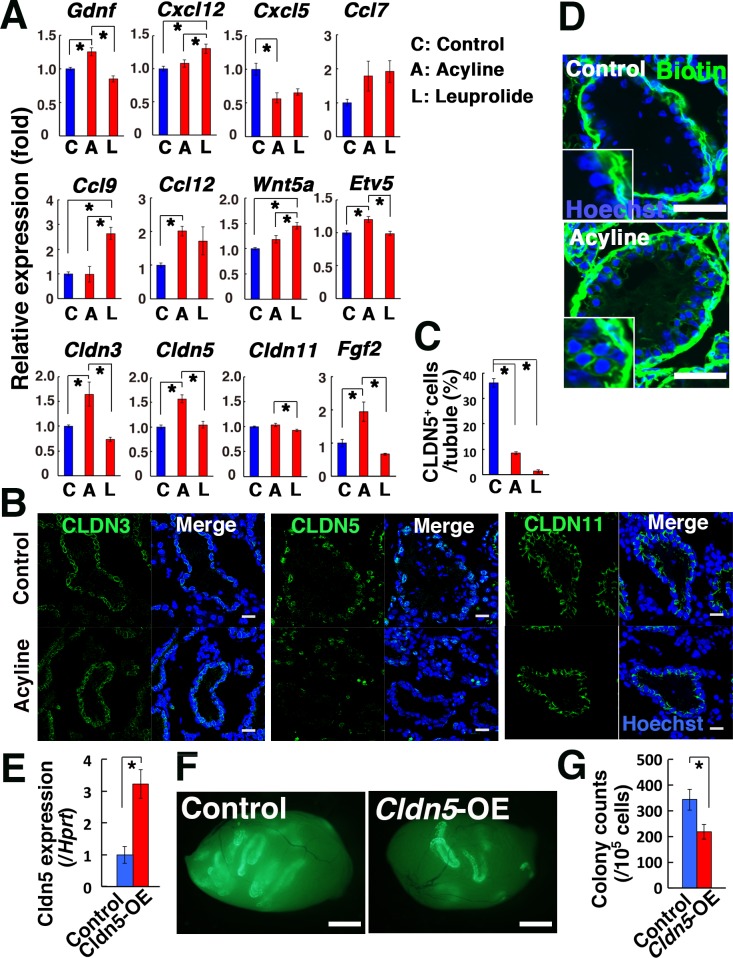
Fig. 4.
Analysis of acyline-treated mouse testes. (A, B) Real-time PCR (A; n = 6) and immunostaining analyses (B) of acyline- and leuprolide-treated testis. Recipient mice were treated with busulfan to remove endogenous spermatogenesis 1 month before analysis. (C) Quantification of tubules with CLDN5+ Sertoli cells. Cells in 10 tubules were counted. (D) Functional assessment of the BTB in acyline-treated mouse testis. Recipient mice were injected interstitially with biotin (green). (E) Real-time PCR analysis of busulfan-treated testes that received lentivirus that overexpress Cldn5 (n = 3). Samples were recovered 2 days after microinjection. (F) Macroscopic appearance of recipient testis that received GS cell transplantation. (G) Colony counts (n = 18 for Cldn5; n = 16 for control). Bar = 20 μm (B), 50 μm (D), 1 mm (F) . Counterstain: Hoechst 33342 (B, D). Asterisk indicates statistical significance (P < 0.05).
We also examined the expression of self-renewal factors. Besides Gdnf, Fgf2 is another critical factor for promoting self-renewal division and it has been used to culture GS cells. Real-time PCR analysis showed significantly increased expression of Fgf2 by acyline treatment (Fig. 4A). Because leuprolide treatment increased Wnt5a expression in our recent study [42], we also checked its expression in acyline-treated mice. However, acyline did not induce Wnt5a expression, which suggested that acyline acts in a different manner from leuprolide. Given the upregulation of Fgf2 and Gdnf by acyline, these results suggested that self-renewal division was stimulated more strongly by acyline than leuprolide during regeneration of spermatogenesis.
Because our previous study suggested that the absence of the BTB enhances SSC colonization [35], we next assessed the expression of tight junction proteins that are thought to be involved in the BTB formation. Real-time PCR analysis showed that acyline upregulated Cldn3 and Cldn5 but did not change the expression level of Cldn11 [43,44] (Fig. 4A). Compared with acyline, leuprolide showed little effect, although it decreased Cldn11 expression. Because these results suggested that acyline enhances tight junction function, we carried out immunohistochemistry of acyline-treated mouse testes to confirm the expression of claudins at the protein level (Fig. 4B). As expected from the real-time PCR analysis, there were no significant changes in the CLDN11 expression pattern according to use of acyline. In addition, although androgens are thought to regulate CLDN3 expression [45], there were no apparent changes in its expression pattern. However, the number of cells expressing CLDN5 was significantly decreased (Fig. 4C). Because CLDN5 expression is downregulated in Etv5 KO mice [46], we examined Etv5 mRNA expression by real-time PCR. Contrary to our expectation, acyline-treated testes showed upregulation of Etv5 (Fig. 4A). Moreover, although Etv5 KO mice showed inflammation in a previous study [46], we found no evidence for the invasion of inflammatory cells in recipient testes (data not shown). These results suggest that downregulation of CLDN5 occurred independently of Etv5.
Because expression of the claudin proteins does not necessarily reflect the function of the BTB, we confirmed the effect of acyline in a functional manner by injecting biotin into the interstitium of the testes (Fig. 4D). As expected from the downregulation of CLDN5, acyline-treated mice showed biotin in the adluminal compartment of the seminiferous tubules, suggesting that acyline compromised BTB function. No such leakage was found in the wild-type control. To further confirm the impact of CLDN5, we overexpressed Cldn5 in the seminiferous tubules to prevent downregulation of CLDN5 expression after acyline treatment. Real-time PCR confirmed increased Cldn5 expression after microinjection of a lentivirus that expresses Cldn5 (Fig. 4E), and GS cells were subsequently transplanted into the seminiferous tubules. Analysis of the recipient testes showed significantly decreased donor cell colonization due to Cldn5 overexpression (Fig. 4F, G). These results indicated that acyline facilitated the colonization of transplanted SSCs by modulating the function of the BTB via CLDN5 downregulation.
Improvement of fertility by acyline treatment
Finally, we used acyline-treated mice to produce offspring. Busulfan-treated mice were administered acyline. Approximately 106 MHY-GS cells were transplanted per testis. Of the four acyline-treated recipient males, three sired offspring as early as 88 days after transplantation (Table 1). This was in contrast to previous experiments, in which no offspring were born regardless of treatment type. Although three males sired offspring, EGFP+ offspring were sired by two recipient mice. In total, 7 of 12 litters contained EGFP+ offspring, and 21.3% (13/61) of the offspring showed EGFP fluorescence under UV light (Fig. 5A), indicating the donor cell origin. Because donor cells were hemizygous for the Egfp transgene, this result suggested that ~40% of the offspring were derived from donor SSCs. Both male and female offspring were born at comparable levels.
Fig. 5.
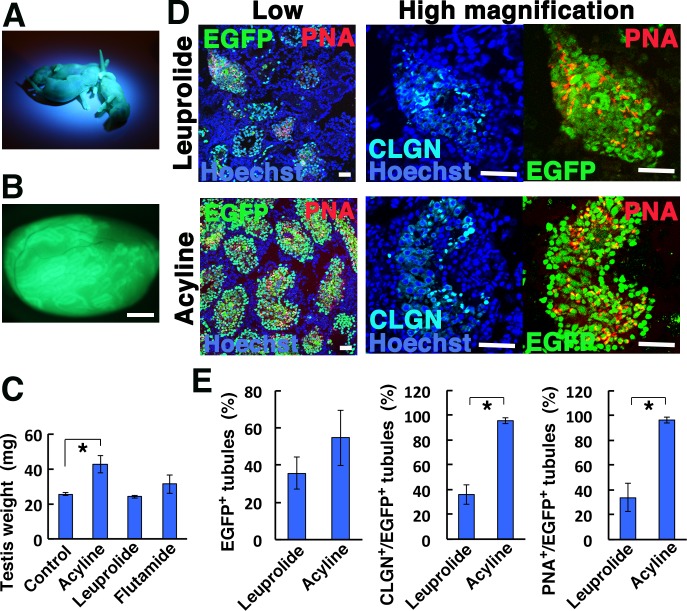
Fertility of recipient mice after acyline treatment. (A) Offspring sired by acyline-treated recipient mice, showing fluorescence under UV light. (B) Macroscopic appearance of a fertile recipient testis transplanted with MHY-GS cells, showing extensive colonization. (C) Testis weight of acyline- and leuprolide-treated testes (n = 4). (D) Immunostaining of recipient testis that underwent acyline or leuprolide treatment. (E) Quantification of tubules with spermatogenesis. At least 271 tubules in four testes were counted. Bar = 1 mm (B), 50 μm (D). Asterisk indicates statistical significance (P < 0.05).
We collected the recipient testes to examine the degree of donor cell colonization (Fig. 5B). The average testicular weight of the fertile mice was significantly higher than that of leuprolide-treated mice (Fig. 5C). Although leuprolide-treated testes contained numerous colonies, the size of the testes was comparable to untransplanted testes, suggesting impairment of germ cell differentiation. Consistent with this idea, although we were not able to find a statistical difference in the level of EGFP+ cell colonization by immunostaining of recipient testes between acyline and leuprolide-treated mice, there were significantly more tubules with CLGN+ and PNA+ spermatogenic cells after acyline treatment (Fig. 5D, E), which suggested more efficient differentiation of donor cells. These results suggested that acyline not only allows increased homing of SSCs but also promotes differentiation of transplanted SSCs.
Discussion
The mechanism of germline transmission has been an enigma for many decades. In males, the amount of sperm is a critical factor for male fertility restoration [16]. It appears to have a close relationship with the number of SSCs, and it is not surprising that the number and quality of sperm are important for the efficiency of germline transmission. However, little is known about the efficiency of fertile sperm production from SSCs. Although addressing this question has proven difficult, spermatogonial transplantation provides a unique opportunity to study this process by not only allowing quantitative assessment of SSCs, but also enabling offspring production. We performed this study to identify factors involved in male germline transmission from SSCs using a spermatogonial transplantation technique.
In the current study, we first focused on donor cells and used GS cells as a donor cell source. Although the usefulness of DBA-GS cells in fertility restoration was demonstrated in our previous study [20], there is significant variation in self-renewal activity among different strains and it was deemed likely that the same approach would not be applicable to all mouse strains. Indeed, we were not able to obtain offspring when we transplanted PS48-GS cells on a B6 background. This is not simply a problem of culture conditions because serial transplantation experiments also showed modest self-renewal division of B6 SSCs compared with DBA SSCs in vivo [21]. Such a genetic limitation is difficult to overcome by in vitro manipulation. After additional screening of cytokines and chemicals, however, we found that GS cells established by MHY1485 stimulation proliferate more actively than those established by PS48. MHY1485 stimulates MTOR [33], which phosphorylates AKT. Because PS48 also stimulates phosphorylation of AKT by activation of PDPK1, these results suggest that activation of AKT activity is critical for deriving GS cells on a B6 background [25]. However, although MHY-GS cells proliferated more actively than PS48-GS cells, none of the recipients could become fertile by simple transplantation of MHY-GS cells. This led us to focus on improving host conditions.
Of the several approaches that we tested, we found that acyline improves donor cell colonization. While leuprolide showed a modest increase in colonization levels, the impact of acyline was more dramatic. Acyline was initially discovered as a new GnRH antagonist that is at least four times more effective at suppression of gonadotropins and testosterone than several previous GnRH antagonists [47]. It also persists longer in vivo: one study in humans showed that the half-life of Na-Glu ([Ac-D2Nal1, D4ClPhe2, D3Pal3, Arg5, DGlu6(AA), DAla10]LHRH), another GnRH antagonist, was 12.8 h, which was approximately half that of acyline [28]. Acyline was co-administered with flutamide, an anti-androgen compound, in a previous study to enhance spermatogonial regeneration [48], but the mechanism of improved spermatogenesis by androgen suppression remains unknown.
Our study suggests that acyline increases the colony number by modulating BTB function. Because single SSCs produce single colonies [49], an increased colony number via acyline suggested that acyline enhances the initial seeding efficiency of SSCs. It has been suggested that transplanted SSCs attach to Sertoli cells, migrate through the BTB, settle on the basement membrane, and migrate toward the niche before undergoing self-renewal division [7]. The biggest hurdle appeared to be passage through the BTB because migration of SSCs to the germline niche from the adluminal lumen to the basal compartment of the seminiferous tubules is a non-physiological process. The importance of the BTB in SSC homing is suggested by the transplantation of SSCs in pup testes, which lack the BTB. Our transplantation experiments showed that SSCs colonize 5–10-fold more in pup testes [35]. Therefore, we concentrated on the analysis of the BTB to understand the mechanism of acyline action.
Several tight junction proteins comprise the BTB, including OCLN, CLDN3, CLDN5, and CLDN11 [26, 27]. Targeted disruption of these proteins results in defective BTB, but the degree of spermatogenic defects varies widely. For example, Ocln KO mice show slow degeneration and have a relatively mild effect [44], whereas Cldn11 KO mice show complete suppression of meiosis [43]. Importantly, some BTB components are hormone sensitive. For example, testosterone regulates Cldn3 expression, although Cldn3 KO mice are fertile [27]. The effect of acyline on the BTB has been examined in previous studies with conflicting observations reported. One study reported that Ocln and Cldn11 expression disappears after acyline treatment in rats [50]. However, another study showed that constant expression of Cldn11 mRNA was not hormonally regulated [51]. Because these experiments were carried out in rats, we analyzed the expression of CLDN11 in acyline-treated mice but did not find abnormalities. Instead, we noted significant downregulation of CLDN5. Although there were no data showing hormonal regulation of CLDN5, injection of biotin tracer into the interstitium of the seminiferous tubules showed leakage into the adluminal cavity, suggesting that acyline modulates BTB function by downregulating CLDN5. Although CLDN5 is thought to be regulated by Etv5 [46], we found increased expression of Etv5 by acyline treatment, suggesting that CLDN5 downregulation occurred independently of Etv5. To our knowledge, this is the first example of enhanced stem cell homing by manipulation of tight junction proteins.
As expected from the increased colonization, offspring were successfully produced by acyline-treated mice that received MHY-GS cells. This result clearly established the usefulness of acyline for germline transmission of transplanted SSCs. Because offspring were born 88 days after transplantation, the speed of fertility recovery was comparable to our recent report using BDF1 recipients transplanted with DBA-GS cells [20], which also occurred within 3 months after transplantation in some cases. In fact, given the relatively slow speed of in vitro proliferation of MHY-GS cells, fertility restoration was more efficient than we originally anticipated. This is probably due to the increased expression of self-renewal factors by acyline treatment. Because both Gdnf and Fgf2 expression was upregulated, the beneficial effect of acyline was not only due to enhanced homing but also increased self-renewal factor stimulation. We speculate that increased numbers of CLGN+ and PNA+ tubules after acyline treatment is caused by the enhanced self-renewal division because the timing of germ cell colony differentiation is greatly influenced by the length of the colony and longer colonies tend to contain more differentiated germ cells [7].
At least two questions arise from the present study. The first concerns the mechanism of CLDN5 suppression. Although several studies implicated CLDN3 in androgen-regulated expression, little is known about the effect of hormonal regulation of CLDN5. Because Cldn5 KO mice die soon after birth [52], it is not currently known if Cldn5 deficiency functionally disturbs the BTB. However, a previous study showed that mice deficient in Etv5 had reduced Cldn5 expression and a defective BTB. Because Etv5 expression was increased by acyline, Etv5 appears to be sensitive to testosterone levels. In addition, both CLDN3 and CLDN5 are primarily expressed in stage VIII seminiferous tubules [46], which is the stage at which preleptotene spermatocytes start to migrate across the BTB. This suggests that SSCs prefer to pass through specific areas of seminiferous tubules, although it is difficult to prove this possibility because no staging is possible in the absence of germ cells after busulfan treatment. The second question concerns the relationship between AKT phosphorylation and self-renewal. MHY1485 activates MTOR, which phosphorylates AKT Ser473. However, phosphorylation was found in AKT Thr308 in MHY-GS cells. Instead, PS48, which is thought to activate PDPK1, induced phosphorylation in AKT Ser473 in PS48-GS cells. Because fresh DBA germ cells show stronger phosphorylation in AKT Thr308 than B6 germ cells [25], AKT Thr308 is likely responsible for enhanced self-renewal of SSCs. However, this pattern of AKT phosphorylation in B6 GS cells suggests that the relationship between self-renewal and AKT phosphorylation is not simple as we originally thought. Although we cannot totally exclude the possibility that chemical activators have additional targets, further studies are required to understand strain differences in self-renewal efficiency.
Germline transmission of GS cells is an important step towards practical application of SSC technology. Because GS cells allow homologous recombination and drug selection of single cell clones [53], they are similar to ES cells in terms of germline modification. However, inefficiency of offspring production has been a critical drawback. Although offspring can be obtained using microinsemination, this technique is apparently not suitable for many animal species. In this sense, GS cell-mediated offspring production by natural mating is attractive because it is not necessary to develop egg culture conditions or embryo transfer methods, which are either very difficult or have not been developed at all for many animal species. SSCs are unique in terms of preservation of genetic information because they are easily frozen using conventional freezing solution for somatic cells. They also preserve genetic diversity because they can undergo meiotic division. Extension of SSC technology to other animal species complements traditional techniques based on oocytes and embryos from females and may overcome problems associated with ES cells or nuclear cloning. Concerted efforts using GS cells and improved host conditioning will achieve this goal, which has important implications for understanding the biology of spermatogenesis, and for animal transgenesis and human infertility treatment.
Supplementary
Acknowledgments
We thank Ms Y Ogata for technical assistance and National Institute of Child Health and Human Development and Contraception Research Branch for providing acyline. Financial support for this research was provided by The Uehara Memorial Foundation, The Takeda Science Foundation, The Naito Foundation, the Japan Science and Technology Agency (PRESTO), and Grants-in-aid for Scientific Research on Innovative Areas “Epigenome Dynamics and Regulation in Germ Cells” (25112003) and “Stem Cell Aging and Disease”(17H05639) from The Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1.Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell 2008; 132: 612–630. [DOI] [PubMed] [Google Scholar]
- 2.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood 2005; 106: 1901–1910. [DOI] [PubMed] [Google Scholar]
- 3.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl 2000; 21: 776–798. [PubMed] [Google Scholar]
- 4.Meistrich ML, van Beek MEAB. Spermatogonial stem cells. In: Desjardins CC, Ewing LL (eds.), Cell and Molecular Biology of the Testis. New York: Oxford University Press; 1993: 266–295. [Google Scholar]
- 5.Tegelenbosch RAJ, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res 1993; 290: 193–200. [DOI] [PubMed] [Google Scholar]
- 6.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA 1994; 91: 11298–11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagano M, Avarbock MR, Brinster RL. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol Reprod 1999; 60: 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagano MC. Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol Reprod 2003; 69: 701–707. [DOI] [PubMed] [Google Scholar]
- 9.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA 1994; 91: 11303–11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Leuprolide, a gonadotropin-releasing hormone agonist, enhances colonization after spermatogonial transplantation into mouse testes. Tissue Cell 1998; 30: 583–588. [DOI] [PubMed] [Google Scholar]
- 11.Karashima T, Zalatnai A, Schally AV. Protective effects of analogs of luteinizing hormone-releasing hormone against chemotherapy-induced testicular damage in rats. Proc Natl Acad Sci USA 1988; 85: 2329–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward JA, Robinson J, Furr BJ, Shalet SM, Morris ID. Protection of spermatogenesis in rats from the cytotoxic procarbazine by the depot formulation of Zoladex, a gonadotropin-releasing hormone agonist. Cancer Res 1990; 50: 568–574. [PubMed] [Google Scholar]
- 13.Kangasniemi M, Wilson G, Huhtaniemi I, Meistrich ML. Protection against procarbazine-induced testicular damage by GnRH-agonist and antiandrogen treatment in the rat. Endocrinology 1995; 136: 3677–3680. [DOI] [PubMed] [Google Scholar]
- 14.Meistrich ML, Kangasniemi M. Hormone treatment after irradiation stimulates recovery of rat spermatogenesis from surviving spermatogonia. J Androl 1997; 18: 80–87. [PubMed] [Google Scholar]
- 15.Ogawa T, Dobrinski I, Brinster RL. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell 1999; 31: 461–472. [DOI] [PubMed] [Google Scholar]
- 16.Meistrich ML. Quantitative correlation between testicular stem cell survival, sperm production, and fertility in the mouse after treatment with different cytotoxic agents. J Androl 1982; 3: 58–68. [Google Scholar]
- 17.Shinohara T, Avarbock MR, Brinster RL. Functional analysis of spermatogonial stem cells in Steel and cryptorchid infertile mouse models. Dev Biol 2000; 220: 401–411. [DOI] [PubMed] [Google Scholar]
- 18.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod 2003; 69: 612–616. [DOI] [PubMed] [Google Scholar]
- 19.Kanatsu-Shinohara M, Shinohara T. Spermatogonial stem cell self-renewal and development. Annu Rev Cell Dev Biol 2013; 29: 163–187. [DOI] [PubMed] [Google Scholar]
- 20.Kanatsu-Shinohara M, Morimoto H, Shinohara T. Fertility of male germline stem cells following transplantation in infertile mouse models. Biol Reprod 2016; 94: 112. [DOI] [PubMed] [Google Scholar]
- 21.Kanatsu-Shinohara M, Ogonuki N, Miki H, Inoue K, Morimoto H, Takashima S, Ogura A, Shinohara T. Genetic influences in mouse spermatogonial stem cell self-renewal. J Reprod Dev 2010; 56: 145–153. [DOI] [PubMed] [Google Scholar]
- 22.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA 2004; 101: 16489–16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helsel AR, Oatley MJ, Oatley JM. Glycolysis-optimized conditions enhance maintenance of regenerative integrity in mouse spermatogonial stem cells during long-term culture. Stem Cell Reports 2017; 8: 1430–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt JA, Abramowitz LK, Kubota H, Wu X, Niu Z, Avarbock MR, Tobias JW, Bartolomei MS, Brinster RL. In vivo and in vitro aging is detrimental to mouse spermatogonial stem cell function. Biol Reprod 2011; 84: 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanatsu-Shinohara M, Tanaka T, Ogonuki N, Ogura A, Morimoto H, Cheng PF, Eisenman RN, Trumpp A, Shinohara T. Myc/Mycn-mediated glycolysis enhances mouse spermatogonial stem cell self-renewal. Genes Dev 2016; 30: 2637–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev 2012; 64: 16–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakraborty P, William Buaas F, Sharma M, Smith BE, Greenlee AR, Eacker SM, Braun RE. Androgen-dependent sertoli cell tight junction remodeling is mediated by multiple tight junction components. Mol Endocrinol 2014; 28: 1055–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbst KL, Anawalt BD, Amory JK, Bremner WJ. Acyline: the first study in humans of a potent, new gonadotropin-releasing hormone antagonist. J Clin Endocrinol Metab 2002; 87: 3215–3220. [DOI] [PubMed] [Google Scholar]
- 29.Kanatsu-Shinohara M, Ogonuki N, Matoba S, Morimoto H, Ogura A, Shinohara T. Improved serum- and feeder-free culture of mouse germline stem cells. Biol Reprod 2014; 91: 88. [DOI] [PubMed] [Google Scholar]
- 30.Dobrinski I, Ogawa T, Avarbock MR, Brinster RL. Effect of the GnRH-agonist leuprolide on colonization of recipient testes by donor spermatogonial stem cells after transplantation in mice. Tissue Cell 2001; 33: 200–207. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa T, Aréchaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol 1997; 41: 111–122. [PubMed] [Google Scholar]
- 32.Kanatsu-Shinohara M, Takashima S, Shinohara T. Transmission distortion by loss of p21 or p27 cyclin-dependent kinase inhibitors following competitive spermatogonial transplantation. Proc Natl Acad Sci USA 2010; 107: 6210–6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi YJ, Park YJ, Park JY, Jeong HO, Kim DH, Ha YM, Kim JM, Song YM, Heo HS, Yu BP, Chun P, Moon HR, Chung HY. Inhibitory effect of mTOR activator MHY1485 on autophagy: suppression of lysosomal fusion. PLoS One 2012; 7: e43418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 1996; 15: 6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 35.Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc Natl Acad Sci USA 2001; 98: 6186–6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber JE, Turner TT, Tung KS, Russell LD. Effects of cytochalasin D on the integrity of the Sertoli cell (blood-testis) barrier. Am J Anat 1988; 182: 130–147. [DOI] [PubMed] [Google Scholar]
- 37.Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, Tsukita S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. J Cell Biol 1999; 147: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanatsu-Shinohara M, Inoue K, Takashima S, Takehashi M, Ogonuki N, Morimoto H, Nagasawa T, Ogura A, Shinohara T. Reconstitution of mouse spermatogonial stem cell niches in culture. Cell Stem Cell 2012; 11: 567–578. [DOI] [PubMed] [Google Scholar]
- 39.Niu Z, Goodyear SM, Avarbock MR, Brinster RL. Chemokine (C-X-C) ligand 12 facilitates trafficking of donor spermatogonial stem cells. Stem Cells Int 2016; 2016: 5796305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon L, Ekman GC, Garcia T, Carnes K, Zhang Z, Murphy T, Murphy KM, Hess RA, Cooke PS, Hofmann MC. ETV5 regulates sertoli cell chemokines involved in mouse stem/progenitor spermatogonia maintenance. Stem Cells 2010; 28: 1882–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang QE, Kim D, Kaucher A, Oatley MJ, Oatley JM. CXCL12-CXCR4 signaling is required for the maintenance of mouse spermatogonial stem cells. J Cell Sci 2013; 126: 1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka T, Kanatsu-Shinohara M, Lei Z, Rao CV, Shinohara T. The luteinizing hormone-testosterone pathway regulates mouse spermatogonial stem cell self-renewal by suppressing WNT5A expression in Sertoli cells. Stem Cell Reports 2016; 7: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, Danias J, Bronstein JM, Kachar B, Lazzarini RA. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 1999; 99: 649–659. [DOI] [PubMed] [Google Scholar]
- 44.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 2000; 11: 4131–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci USA 2005; 102: 16696–16700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrow CM, Tyagi G, Simon L, Carnes K, Murphy KM, Cooke PS, Hofmann MC, Hess RA. Claudin 5 expression in mouse seminiferous epithelium is dependent upon the transcription factor ets variant 5 and contributes to blood-testis barrier function. Biol Reprod 2009; 81: 871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang G, Stalewski J, Galyean R, Dykert J, Schteingart C, Broqua P, Aebi A, Aubert ML, Semple G, Robson P, Akinsanya K, Haigh R, Riviere P, Trojnar J, Junien JL, Rivier JE. GnRH antagonists: a new generation of long acting analogues incorporating p-ureido-phenylalanines at positions 5 and 6. J Med Chem 2001; 44: 453–467. [DOI] [PubMed] [Google Scholar]
- 48.Wang G, Shao SH, Weng CC, Wei C, Meistrich ML. Hormonal suppression restores fertility in irradiated mice from both endogenous and donor-derived stem spermatogonia. Toxicol Sci 2010; 117: 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanatsu-Shinohara M, Inoue K, Miki H, Ogonuki N, Takehashi M, Morimoto T, Ogura A, Shinohara T. Clonal origin of germ cell colonies after spermatogonial transplantation in mice. Biol Reprod 2006; 75: 68–74. [DOI] [PubMed] [Google Scholar]
- 50.McCabe MJ, Tarulli GA, Laven-Law G, Matthiesson KL, Meachem SJ, McLachlan RI, Dinger ME, Stanton PG. Gonadotropin suppression in men leads to a reduction in claudin-11 at the Sertoli cell tight junction. Hum Reprod 2016; 31: 875–886. [DOI] [PubMed] [Google Scholar]
- 51.Zhou W, Bolden-Tiller OU, Shetty G, Shao SH, Weng CC, Pakarinen P, Liu Z, Stivers DN, Meistrich ML. Changes in gene expression in somatic cells of rat testes resulting from hormonal modulation and radiation-induced germ cell depletion. Biol Reprod 2010; 82: 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 2003; 161: 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanatsu-Shinohara M, Ikawa M, Takehashi M, Ogonuki N, Miki H, Inoue K, Kazuki Y, Lee J, Toyokuni S, Oshimura M, Ogura A, Shinohara T. Production of knockout mice by random or targeted mutagenesis in spermatogonial stem cells. Proc Natl Acad Sci USA 2006; 103: 8018–8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.