Abstract
Background
An increasing number of psychoradiology studies that use tract-based spatial statistics (TBSS) of diffusion tensor imaging have reported abnormalities of white matter in patients with bipolar disorder; however, robust conclusions have proven elusive, especially considering some important clinical and demographic factors. In the present study, we performed a quantitative meta-analysis of TBSS studies to elucidate the most consistent white-matter abnormalities in patients with bipolar disorder.
Methods
We conducted a systematic search up to May 2017 for all TBSS studies comparing fractional anisotropy (FA) between patients with bipolar disorder and healthy controls. We performed anisotropic effect size–signed differential mapping meta-analysis.
Results
We identified a total of 22 data sets including 556 patients with bipolar disorder and 623 healthy controls. We found significant FA reductions in the genu and body of the corpus callosum in patients with bipolar disorder relative to healthy controls. No regions of increased FA were reported. In subgroup analyses, the FA reduction in the genu of the corpus callosum retained significance in patients with bipolar disorder type I, and the FA reduction in the body of the corpus callosum retained significance in euthymic patients with bipolar disorder. Meta-regression analysis revealed that the percentage of female patients was negatively correlated with reduced FA in the body of the corpus callosum.
Limitations
Data acquisition, patient characteristics and clinical variables in the included studies were heterogeneous. The small number of diffusion tensor imaging studies using TBSS in patients with bipolar disorder type II, as well as the lack of other clinical information, hindered the application of subgroup meta-analyses.
Conclusion
Our study consistently identified decreased FA in the genu and body of the corpus callosum, suggesting that interhemispheric communication may be the connectivity most affected in patients with bipolar disorder.
Introduction
Bipolar disorder (BD) is a serious chronic disease characterized by the co-occurrence of manic and depressive symptoms, and it has a worldwide prevalence of approximately 1%.1 Two main forms are distinguished in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5): BD type I (BD I) is the classic manic–depressive disorder; a diagnosis of BD type II (BD II) requires at least 1 episode of major depression and 1 hypomanic episode.2 Bipolar disorder leads to severe impairments in work role function and a high lifetime suicide risk; thus, there is a pressing need for a better understanding of its neural basis. Magnetic resonance imaging has been a useful way to pursue this, and numerous cerebral morphological and functional abnormalities have been reported in patients with BD.3
In addition to structural and functional alterations in multiple brain regions, aberrant connectivity between regions seems to be crucial to the pathogenesis of BD.4,5 Diffusion tensor imaging (DTI), an important psychoradiology technique (https://radiopaedia.org/articles/psychoradiology), can probe the white-matter tracts that form the infrastructure of that connectivity. It maps the diffusion of water molecules, and the calculated quantity of fractional anisotropy (FA) contains information about the directionality and coherence of neuronal fibre tracts.6,7
In studies of patients with BD, however, various regions showing both FA increases and decreases have been reported: for example in the prefrontal white matter, temporal white matter and cingulum bundle.8–10 Performing a meta-analysis is a powerful way to integrate the results from many studies in an unbiased way.11 Therefore, using voxel-based meta-analysis to detect common abnormalities in patients with BD from multiple original studies is of particular importance. To our knowledge, there have been 3 published voxel-wise whole-brain meta-analyses of pertinent DTI studies on BD.12–14 However, these reviews had some technical limitations. First, all 3 studies integrated both voxel-based analysis (VBA) and tract-based spatial statistics (TBSS) techniques to compare white-matter abnormalities between patients with BD and healthy controls. Although VBA and TBSS both explore whole-brain white-matter alterations, they have inherent methodological differences that make combined meta-analysis problematic. Voxel-based analysis is relatively straightforward and involves spatial normalization of high-resolution images from all studied participants to the same stereotactic space.15 The TBSS technique was specifically developed to analyze diffusion data, and it restricts analysis to the centre of major white-matter tracts by projecting each participant’s FA data onto the mean skeleton, minimizing the misalignment problems that can arise from regular VBA16 and arguably making TBSS a more accurate technique for detecting white-matter abnormalities. Second, given the limited number of reported DTI studies, these previous meta-analyses were unable to consider the potentially different neural alterations in patients with BD I and BD II, or the influence of the mood state (manic, depressed or euthymic) during MRI scanning. Some of the inconsistencies in reported white-matter findings may be due to different selection of patient subtypes. For example, Versace and colleagues17 reported FA increases in the left uncinate fasciculus, left optic radiation and right anterothalamic radiation, and FA decreases in the right uncinate fasciculus in patients with BD I compared with healthy controls. Including patients with both BD I and BD II, Haller and colleagues18 reported FA decreases in the ventral part of the corpus callosum compared with healthy controls. Ambrosi and colleagues19 reported lower FA in the right inferior longitudinal fasciculus in patients with BD II compared with patients with BD I. These different results may reflect different neural pathophysiologies in BD I and BD II, so it is important to identify their separate neurobiological markers. Now that more TBSS studies have reported results taking BD subtypes into consideration, the time is ripe to conduct an updated TBSS meta-analysis to explore how these factors influence white-matter microstructural abnormalities in patients with BD.
The first aim of this study was to conduct a meta-analysis to identify the most prominent and replicable white-matter microstructural abnormalities associated with BD. We used anisotropic effect size–signed differential mapping (AES-SDM), a recently developed meta-analytic technique with the potential to quantify the reproducibility of neuroimaging findings.20 This technique enables the results from individual studies to be weighted and controlled for multiple moderators, including demographics, clinical information and imaging factors. It has the additional advantage that all information from contributing studies is used in the same map, including positive, negative and null results. The AES-SDM technique has been successfully applied in other neuropsychiatric studies using TBSS, including major depressive disorder and attention-deficit/hyperactivity disorder.21,22 The second aim was to perform subgroup meta-analyses based on important clinical and demographic factors of BD. The third aim was to perform a multiple meta-regression analysis to explore the potential effects of demographic and clinical characteristics on white-matter microstructural differences, taking advantage of AES-SDM’s ability to control for moderators.
Methods
Inclusion of studies
We conducted our meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.23 We searched PubMed, Web of Knowledge, MEDLINE, PsycINFO, ERIC, CINAHL, Google Scholar and Embase using the following keywords to identify relevant articles published up to May 2017: DTI < OR > diffusion tensor imaging < OR > TBSS < OR > tract-based spatial statistics < OR > fractional anisotropy; and bipolar disorder < OR > bipolar depression < OR > mania. We also manually checked the reference lists of the retrieved articles for additional relevant studies.
The inclusion criteria for the study selections were as follows: studies that compared whole-brain FA alteration between patients with BD (diagnosed according to DSM-IV criteria) and healthy controls; studies that used the TBSS approach for DTI data analysis; studies that reported Montreal Neurological Institute or Talairach coordinates of the whole brain contrast of FA; studies that were published in English in a peer-reviewed journal; and studies that used significance thresholds for data that were either corrected for multiple comparisons or uncorrected with spatial extent thresholds.
The exclusion criteria were as follows: studies that were case reports or reviews; studies that provided 3-dimensional coordinates in stereotactic space (Montreal Neurological Institute or Talairach) were unavailable; studies that reported fewer than 10 participants in either the BD or healthy control groups; studies in which the patients were explicitly recruited to have multiple combined axis I diagnoses; and the presence of sample overlap with other included studies of larger sample sizes.
Quality assessment and data extraction
Two authors (C.Y. and L.L.) independently searched the literature, assessed the quality of the retrieved articles and extracted and cross-checked the data. In cases of disagreement, another author mediated until consensus was obtained. We assessed study quality using a 12-point checklist (see Appendix 1, Table S1, available at jpn.ca/170221-a1) adapted from previous meta-analyses24 that considered not only the demographic and clinical characteristics of individual study participants (items 1 to 4), but also the specific imaging methodology (items 5 to 10) and the consistency of the conclusions and the results (items 11 and 12). Each item was given a score of 1, 0.5 or 0 to indicate whether the criteria were fully met, partially met or unfulfilled, respectively.
We extracted the following data from each selected study: the characteristics of the participants and their illness (sample size, mean age of participants, sex, age at onset, illness duration, symptom severity, diagnosis type, drug status, mood states and comorbidities); magnetic resonance methodology (magnetic field strength, acquisition voxel size, number of diffusion directions and type of analysis); statistical methodology (statistical threshold and correction methods for multiple comparisons); and 3-dimensional coordinates (for voxel-level quantitative meta-analyses).
Voxel-wise meta-analysis: AES-SDM
For the voxel-wise meta-analysis of the selected studies to detect FA differences in white matter between patients with BD and healthy controls, we used AES-SDM (www.sdmproject.com).20 The AES-SDM technique uses effect sizes to combine reported peak coordinates that are extracted from databases with statistical parametric maps, and it recreates original maps of the effect size of FA difference in white matter between patients and controls. We performed the analysis as described in the AES-SDM tutorial and related publications.25 We used MRIcron software (www.mricro.com/mricron/) to visualize AES-SDM maps overlaid on 3 subgroup analyses onto a high-resolution brain template generated by the International Consortium for Brain Mapping.
The AES-SDM methods are briefly summarized here but have been described in detail elsewhere.26,27 First, from each data set we extracted the peak coordinates of all white-matter differences at the whole-brain level, and the t values or z-scores of the regions. We converted z-scores for significant clusters straightforwardly to t statistics using an online converter. Studies reporting no group differences were also included. To avoid any potential bias toward liberally thresholded regions, we excluded the peaks that did not appear statistically significant at the whole brain level.26 Second, we recreated the peak coordinates for each study using a standard Montreal Neurological Institute map of the effect size of the group differences in FA by means of an anisotropic Gaussian kernel. We used a relatively wide full width at half maximum (20 mm) and DTI fractional anisotropy (TBSS) templates to control false-positive results. Findings from studies that reported no group difference were also recreated with effect-size maps, the difference being that all voxels in the effect-size group were estimated to have a null effect size. Third, we conducted standard meta-analysis to create a mean map via voxel-wise calculation of the random-effects mean of the study maps, taking into account sample size, intrastudy variance and between-study heterogeneity. The analytical parameters of the AES-SDM were as follows: voxel threshold p = 0.005, peak height threshold Z = 1.00 and cluster size threshold = 100 voxels.
Jackknife sensitivity analysis and subgroup analysis
To assess the robustness of the findings, we conducted a systematic whole-brain voxel-based jackknife analysis, in which we iteratively repeated the analysis, excluding 1 data set at a time to establish the extent to which the results could be replicated. If a brain region remained significant in all or most of the combinations of studies, we considered the finding to be highly replicable.26
When the sample size was sufficient, we conducted sensitivity subgroup analyses to test the robustness of the statistically significant findings by excluding studies with potential confounds on a one-off basis. We performed subgroup analyses of patients with BD I or BD II; with psychotic or nonpsychotic symptoms; during euthymic, depressed or mania status; and of adult or pediatric patients compared with healthy controls separately if sufficient data sets were available. We also conducted a subgroup meta-analysis of studies with corrected results. We conducted jackknife sensitivity analysis for each subgroup result.
Meta-regression analysis
We performed meta-regression analyses using age, percentage of female patients, symptom severity (Hamilton Depression Rating Scale28), age at onset and illness duration in each study as the independent variables. We could not study non-drug therapy status because of a lack of reported data. We could not explore symptom severity as rated by the Young Mania Rating Scale29 by meta-regression because there were only 6 data sets. The results were weighted by the square root of the sample size. To minimize the reporting of spurious relationships, we selected a more conservative threshold of p = 0.0005 as used in previous studies,20 requiring abnormalities to be detected both in the slope and in one of the extremes of the regressor, and discarding findings in regions other than those detected in the main analyses. We displayed the main output for each variable in a map of the regression slope. Finally, we visually inspected regression plots to discard fittings that were obviously driven by too few studies.26
Analysis of heterogeneity and publication bias
Heterogeneity refers to between-study variations. We conducted a between-study heterogeneity analysis of individual clusters using a random-effects model with Q statistics, with thresholds of p = 0.005, peak Z = 1.00 and a cluster extent of 10 voxels. Areas showing significant heterogeneity that also overlapped with the main findings were explored using meta-regression analyses to understand the sources of between-study variability. We also assessed publication bias by testing funnel plots using the Egger test via STATA (www.stata.cn), in which any result showing p < 0.05 was regarded as having significant publication bias.
Fibre tracking
We used DTIquery software (http://graphics.stanford.edu/projects/dti/) and an atlas of human white-matter anatomy30 to help identify the most probable white-matter tracts passing through the clusters of voxels that showed significant FA group difference. We used the sample data of a healthy 35-year-old male provided by the DTIquery software. We mapped the white-matter tracts using streamline tracking techniques and filtered them by tract length and a box-shaped region of interest centred on the coordinates of significant clusters.
Results
Included studies and sample characteristics
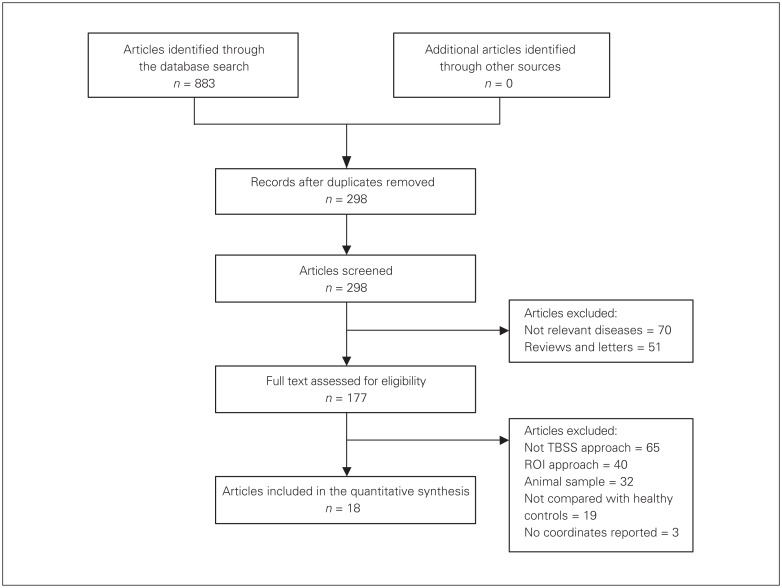
Systematic search of the databases yielded 883 English research papers. Of these, 18 whole-brain TBSS studies with 22 data sets met our inclusion criteria. Figure 1 shows the flow diagram of selection of studies. The included studies compared FA differences in white matter between 556 patients with BD (mean age 38.2 yr) and 623 healthy controls (mean age 39.3 yr). Four studies reported subgroup comparisons: 1 study31 compared drug-free patients with BD and patients treated with lithium alone with the same healthy control group; another study32 compared patients with BD (with and without a history of suicide attempts) with the same healthy control group; and 2 studies19,33 compared subgroups of patients with BD (BD I and BD II) with the same healthy control group. For these 4 studies, we treated each subgroup comparison as an independent data set.
Fig. 1.
Flow diagram for the identification and exclusion of studies. ROI = region of interest; TBSS = tract-based spatial statistics.
The demographic and clinical characteristics of the samples are reported in Table 1. Of the 556 patients, 57 (10.3%) were in a manic state at the time of scanning, 162 (29.1%) were in a depressive state, and 232 (41.7%) were euthymic; the mood states of 105 (18.9%) were not reported. The patients included 411 with BD I, 87 with BD II and 58 whose BD subtype was not mentioned. A total of 165 patients (29.7%) were reported to have comorbidities, which included substance abuse, panic disorder, anxiety disorder, posttraumatic stress disorder, obsessive–compulsive disorder and attention-deficit/hyperactivity disorder, among others. Of the 18 DTI studies, 2 studies43,44 involved 36 adolescents with BD.
Table 1.
Demographic and clinical characteristics of the participants in the 18 studies (22 data sets) included in the meta-analysis
| Study | Participants, n (females) | Age, yr | Age at onset, yr | Illness duration, yr | Severity (scale type) | Diagnosis | Statistical threshold | Drug states | Mood states | Comorbidity* | Quality scores | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| BD | HC | BD | HC | ||||||||||
| Ambrosi et al., BD I19 | 25 (12) | 50 (24) | 48.6 | 48.3 | NA | 20.3 | 9.2 (HAMD) | BD I | p < 0.008 (multiple comparison) | 10 AD, 16 AP, 11 AE, 16 Li, 6 BDZ | NA | NA | 11.5 |
| Ambrosi et al., BD II19 | 25 (12) | 50 (24) | 48.3 | 48.3 | NA | 23.9 | 14.5 (HAMD) | BD II | p < 0.008 (multiple comparison) | 6 AD, 12 AP, 14 AE, 10 Li, 13 BDZ | NA | NA | 11.5 |
| Versace et al.17 | 31 (20) | 25 (14) | 35.9 | 29.5 | 25.4 | 12.3 | RBD 2.1, DBD 15.2 (HAMD) | BD I | p < 0.05 (multiple comparison) | 5 Li, 12 MS, 8 AP, 7 AD, 7 BDZ | 17 euthymic, 14 depressed | NA | 11.5 |
| Kumar et al.34 | 22 (7) | 41 (11) | 34.7 | 33.2 | NA | 10.7 | 8.57 (SSPI) | BD | pTFCE < 0.05 | 55 on medications, including AP and MS | NA | NA | 11 |
| Delaloye et al.35 | 15 (NA) | 15 (NA) | 67.9 | 68.3 | 34.1 | 33.8 | 1.00 (YMRS) | BD | pTFCE < 0.05 | 10 MS, 3 AD, 6 BDZ, 4 NL | Euthymic | NA | 10.5 |
| Haller et al.18 | 19 (14) | 47 (22) | 68.5 | 69.8 | 39.4 | 29.2 | 0.95 (YMRS) | BD I BD II |
pTFCE < 0.05 | 15 MS, 5 AP, 7 BDZ, 5 NL, 3 UM | Euthymic | NA | 11.5 |
| Benedetti et al., FREE31† | 26 (17) | 21 (10) | 45.1 | 39.9 | 31.6 | 13.5 | 20.1 (HAMD) | BD I | pTFCE < 0.05 | 26 UM | Depressed | NA | 11 |
| Benedetti et al., TREAT31† | 14 (11) | 21 (10) | 47.8 | 39.9 | 31.1 | 17.6 | 19.1 (HAMD) | BD I | pTFCE < 0.05 | 14 Li | Depressed | NA | 11 |
| Chan et al.36 | 16 (4) | 16 (4) | 36.9 | 37.3 | NA | 0.2 | 3.8 (YMRS) | BD I | p < 0.001 (uncorrected) | 6 Li, 7 MS (sodium valproate), 12 AP | Euthymic | NA | 11 |
| Lagopoulos et al.37 | 58 (41) | 40 (24) | 23.0 | 24.1 | 15.4 | 7.5 | 13.4 (HAMD) | BD I, BD II, spectrum | pFWE < 0.05 | 25 AD, 23 MS, 37 AP, 8 UM | Depressed | NA | 11.5 |
| Mahon et al., SU32† | 14 (5) | 15 (7) | 33.3 | 33.7 | 20.8 | 12.5 | 6.8 (HAMD) | BD I | pFWE < 0.05 | 10 MS, 6 AP, 3 UM | NA | Yes | 10.5 |
| Mahon et al., NSU32† | 15 (6) | 15 (7) | 36.5 | 33.7 | 24.8 | 11.7 | 5.4 (HAMD) | BD I | pFWE < 0.05 | 14 AP, 14 MS | NA | Yes | 10.5 |
| Sprooten et al.38 | 64 (46) | 46 (31) | 31.7 | 30.1 | 18.0 | 10.0 | 2.0 (HAMD) | BD I | pFWE < 0.05 | 34 MS, 28 AD, 23 AP, 12 Li, 11 UM | Euthymic | Yes | 12 |
| Versace et al.39 | 15 (14) | 24 (15) | 36.3 | 27.7 | 21.6 | 14.7 | 14.9 (HAMD) | BD I | pTFCE < 0.05 | 10 MS, 10 AP, 8 AP, 6 BDZ | Depressed | NA | 11.5 |
| Wessa et al.40 | 22 (11) | 21 (9) | 45.4 | 43.0 | 23.0 | 22.0 | 0.1 (HAMD) | BD I, BD II | pFDR < 0.05 | 10 Li, 11 AC, 6 AD, 5 AP, 4 UM | Euthymic | Yes | 10.5 |
| Liu et al., BD I33 | 14 (7) | 21 (13) | 35.6 | 38.3 | NA | 7.3 | 6.7 (HAMD) | BD I | p < 0.001 (uncorrected) | 2 Li, 12 AC, 12 AD, 1 AP | 7 manic, 7 depressed | NA | 11 |
| Liu et al., BD II33 | 13 (11) | 21 (13) | 35.1 | 38.3 | NA | 9.4 | 9.5 (HAMD) | BD II | p < 0.001 (uncorrected) | 10 AC, 10 AD, 1 AP | 5 manic, 8 depressed | NA | 11 |
| Magioncalda et al.41 | 61 (43) | 42 (27) | 44.6 | 44.3 | 25.1 | 19.6 | 6.9 manic, 21.5 depressed, 3.6 euthymic (HAMD) | BD I | pTFCE < 0.01 | 52 MS, 22 AD, 35 AP, 21 BDZ, 2 UM | 21 manic, 20 depressed, 20 euthymic | NA | 11.6 |
| Oertel-Knochel et al.42 | 30 (14) | 32 (16) | 39.2 | 39.2 | NA | 10.2 | NA | BD I | pTFCE < 0.01 | 24 MS, 12 AD, 13 NL, 5 AL | Euthymic | NA | 10.5 |
| Gao et al.43 | 18 (12) | 18 (12) | 15.1 | 14.1 | 13.8 | 1.3 | 33.4 (YMRS) | BD I, BD II | pTFCE < 0.05 | 2 UM, 8 VP, 7 Li, 13 AP, 3 AD | Manic | Yes | 11 |
| Teixeira et al.44 | 18 (6) | 20 (6) | 12.3 | 12.7 | 9.5 | 2.8 | 8.7 (YMRS) | BD I, BD II, BD NOS‡ | pTFCE < 0.05 | All UM | 6 manic, 8 euthymic, 4 mixed | Yes | 11 |
| Haarman et al.45 | 21 (12) | 22 (11) | 44.7 | 38.2 | 19.5 | 25.2 | 0.2 (YMRS) | BD I | pTFCE < 0.05 | Medicated | Euthymic | No | 11.5 |
AC = anticonvulsant; AD = antidepressant medication; AE = antiepileptic medication; AL = anxiolytics; AP = antipsychotic medication; BD = bipolar disorder; BDZ = benzodiazepine; DBP = depressed with bipolar disorder; FDR = false discovery rate; FWE = family-wise error corrected; HAMD = Hamilton Depression Rating Scale; HC = healthy controls; Li = lithium; MS = mood-stabilizing medication; NA = not available; NL = neuroleptics; NOS = not otherwise specified; RBD = remitted with bipolar disorder; SSPI = Signs and Symptoms in Psychotic Illness scale; TFCE = threshold-free cluster enhancement; UM = unmedicated; VP = valproate; YMRS = Young Mania Rating Scale.
Comorbidity:
• Mahon et al., SU: substance abuse or dependence (n = 3), anorexia nervosa (n = 1), panic disorder with (n = 1) and without (n = 1) a history of agoraphobia, anxiety disorder NOS (n = 2), posttraumatic stress disorder (n = 2), generalized anxiety disorder (n = 1), specific phobia (n = 1) and bulimia nervosa (n = 1).
• Mahon et al., NSU: substance abuse or dependence (n = 5), obsessive–compulsive disorder (n = 1) and agoraphobia without a history of panic disorder (n = 1).
• Sprooten et al.: anxiety disorder (n = 30), alcohol use disorder (n = 33), substance use disorder (n = 29), nicotine dependence, current (n = 21).
• Gao et al.: anxiety (n = 1), attention-deficit hyperactivity disorder (n = 1), obsessive–compulsive disorder (n = 2).
• Teixeira et al.: attention-deficit/hyperactivity disorder (n = 9), oppositional defiant disorder (n = 5), generalized anxiety disorder (n = 3), conduct disorder (n = 2), simple phobia (n = 2), posttraumatic stress disorder (n = 2), panic disorder (n = 1), separation anxiety disorder (n = 1), obsessive–compulsive disorder (n = 1) and Tourette syndrome (n = 1).
• Wessa et al.: panic disorder (n = 1).
FREE indicates that the study includes a subgroup of drug-free patients with BD. TREAT indicates that the study includes a subgroup of lithium-treated patients with BD. SU indicates that the study includes subgroup of patients with a previous suicide attempt. NSU indicates that the study includes subgroup of patients without a previous suicide attempt.
Children and adolescents were diagnosed as BD NOS if they presented with clear manic or hypomanic episodes with elation and/or grandiosity but lacked the duration needed to be classified as BD I or BD II.
Pooled meta-analysis of all included studies
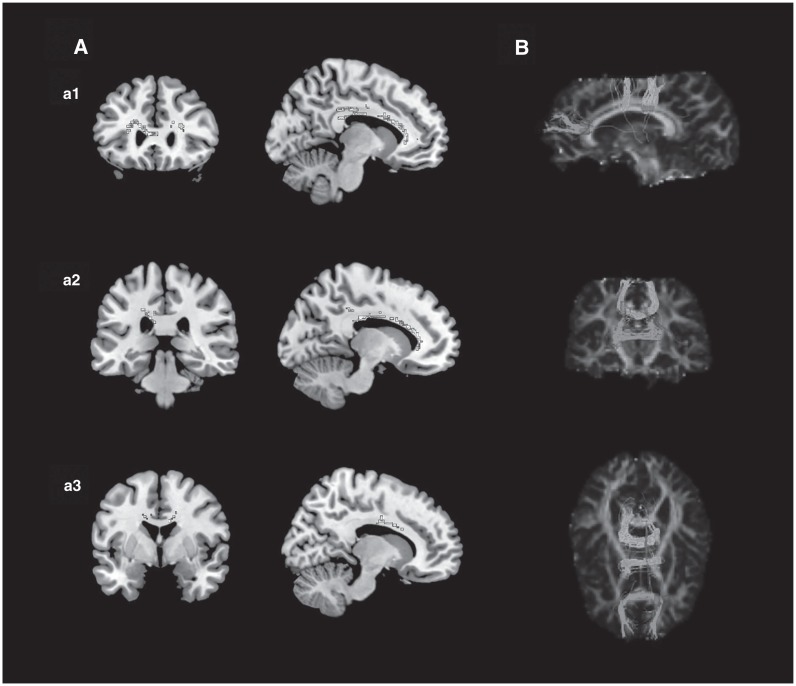
The pooled meta-analysis of the 22 data sets with 71 clusters revealed decreased FA in the genu of the corpus callosum and 2 clusters in the body of the corpus callosum in all patients with BD compared with healthy controls (Fig. 2 and Table 2). No regions with increased FA were found. The white-matter tracts running through these 3 regions provide interhemispheric connections to the prefrontal, orbitofrontal, inferior temporal and superior parietal cortices (Fig. 3). These results showed no significant between-study heterogeneity. Extraction of funnel plots revealed no publication bias. The Egger test showed no significant publication bias in the genu (Egger p = 0.62) or in the anterior (Egger p = 0.99) or posterior (Egger p = 0.23) parts of the body of the corpus callosum.
Fig. 2.
Results of pooled meta-analysis. Panel A shows the coronal and sagittal view showing decreased fractional anisotropy in patients with bipolar disorder versus healthy controls in the genu of corpus callosum (a1), the posterior of the body of corpus callosum (a2) and the anterior of the body of corpus callosum (a3). Panel B shows the most probable white-matter tracts passing through 3 clusters of voxels (−10, 28, 16; −18, −32, 32; and 16, −6, 36) in 3-dimensional images using DTIquery.
Table 2.
Clusters of fractional anisotropy reductions in patients with bipolar disorder relative to healthy controls
| Maximum | Cluster | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Region | MNI coordinates x, y, z |
SDM Z value |
p value | Number of voxels | Breakdown (number of voxels) |
| Genu of corpus callosum | −10, 28, 16 | −2.209 | ~0 | 388 | Corpus callosum (321) Left striatum (21) Left anterior thalamic projections (20) Left inferior fronto-occipital fasciculus (15) Left cingulum (11) |
| Body of corpus callosum | −18, −32, 32 | −2.470 | ~0 | 180 | Corpus callosum (125) Left cingulum (43) Left superior longitudinal fasciculus (12) |
| Body of corpus callosum | 16, −6, 36 | −1.303 | <0.001 | 127 | Corpus callosum (112) Right anterior thalamic projections (15) |
MNI = Montreal Neurological Institute; SDM = signed differential mapping.
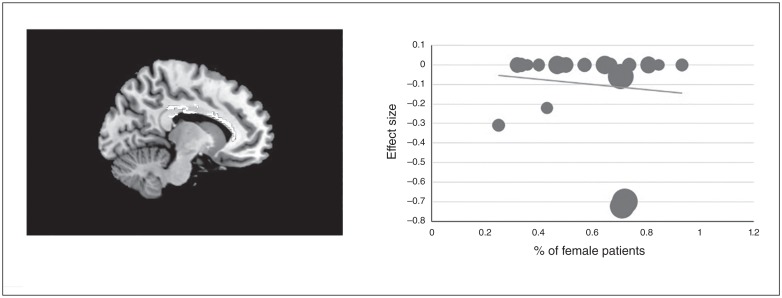
Fig. 3.
Results of meta-regression analysis illustrated a negative correlation between the percentage of female patients studied and fractional anisotropy in the body of corpus callosum. We extracted AES-SDM values to create a meta-regression plot. Each study is presented as a dot, and larger dots indicate larger sample sizes. The regression line (meta-regression signed differential mapping slope) is presented as a straight line. AES-SDM = anisotropic effect size–signed differential mapping.
As shown in Table 3, the whole-brain jackknife sensitivity analysis of the pooled meta-analysis found that all 3 clusters were highly replicable. The most robust cluster was found in the left genu of the corpus callosum, which was preserved throughout all 22 combinations of the data sets. The other 2 results in the body of the corpus callosum remained significant in all but 2 and 7 combinations of the data sets, respectively.
Table 3.
Results of jackknife analysis and subgroup analysis from 18 studies (22 data sets) included in the meta-analysis*
| Analysis | Region of FA reduction: genu of corpus callosum | Region of FA reduction: body of corpus callosum | Region of FA reduction: body of corpus callosum |
|---|---|---|---|
| Jackknife sensitivity analysis | |||
| Excluding Wessa et al.40 | Yes | Yes | Yes |
| Excluding Versace et al.17 | Yes | Yes | Yes |
| Excluding Chan et al.36 | Yes | Yes | Yes |
| Excluding Benedetti et al., FREE31 | Yes | Yes | No |
| Excluding Benedetti et al., TREAT31 | Yes | Yes | Yes |
| Excluding Haller et al.18 | Yes | Yes | Yes |
| Excluding Delaloye et al.35 | Yes | Yes | No |
| Excluding Mahon et al., SU32 | Yes | Yes | Yes |
| Excluding Mahon et al., NSU32 | Yes | Yes | No |
| Excluding Gao et al.43 | Yes | No | No |
| Excluding Sprooten et al.38 | Yes | Yes | Yes |
| Excluding Oertel-Knochel et al.42 | Yes | Yes | Yes |
| Excluding Lagopoulos et al.37 | Yes | Yes | No |
| Excluding Teixeira et al.44 | Yes | Yes | Yes |
| Excluding Ambrosi et al., BD I19 | Yes | Yes | Yes |
| Excluding Ambrosi et al., BD II19 | Yes | No | No |
| Excluding Magioncalda et al.41 | Yes | Yes | No |
| Excluding Versace et al.39 | Yes | Yes | Yes |
| Excluding Kumar et al.34 | Yes | Yes | Yes |
| Excluding Liu et al., BD I33 | Yes | Yes | Yes |
| Excluding Liu et al., BD II33 | Yes | Yes | Yes |
| Excluding Haarman et al.45 | Yes | Yes | Yes |
| Subgroup analysis | |||
| Studies with adult BD | Yes | Yes | No |
| Studies with BD I | Yes | No | No |
| Studies with euthymic-state BD | No | Yes | No |
BD = bipolar disorder; FA = fractional anisotropy.
“Yes” indicates that the specific region of FA reduction was significant in the specific jackknife analysis and subgroup analysis; “No” indicates that the specific region of FA reduction was not significant in specific analysis.
Subgroup meta-analysis
The subgroup meta-analysis of the studies of patients with BD I included 13 data sets that compared 346 patients with BD I to 350 healthy controls. The FA in patients with BD I was decreased in the genu of the corpus callosum relative to that of healthy controls (Table 3). We were unable to conduct subgroup meta-analyses of the studies of patients with BD II compared with either healthy controls or patients with BD I because of the limited number of data sets.
The subgroup meta-analysis of euthymic patients with BD included 7 data sets that compared 187 euthymic patients with BD to 199 healthy controls. Relative to controls, patients in the euthymic state exhibited decreased FA in the body of the corpus callosum (Table 3). We were unable to conduct subgroup meta-analyses of manic or depressed patients compared with healthy controls because of the limited number of data sets.
The subgroup meta-analysis of adult patients with BD (age > 18 yr) included 20 data sets that compared 520 adult patients with BD to 585 healthy controls. The adult patients had decreased FA in the genu and the body of the corpus callosum, sharing 2 clusters with the results of the pooled meta-analysis (Table 3). We were unable to conduct subgroup meta-analysis of adolescent patients because of a lack of data sets.
The subgroup meta-analysis of the studies with corrected results included 19 data sets that compared 513 patients with BD and 565 healthy controls. The results were consistent with the pooled meta-analysis (Appendix 1, Table S2), showing few effects of the uncorrected results on the overall conclusions.
Only 5 included studies34,37,38,43,44 reported white-matter alterations in patients with BD and psychosis. One study included only BD patients with psychosis, and the other 4 reported only the percentage of patients with psychosis and did no subgroup analysis. The limited number of studies prevented the detection of white-matter abnormalities when comparing patients with BD with or without psychosis.
The subgroup jackknife sensitivity analyses of bipolar subtypes, mood states and adult participants, as well as the corrected studies, found the meta-analysis results to be highly replicable when focusing only on the BD I, euthymic, adult and corrected subgroups.
Meta-regression analysis
The results of the meta-regression analysis showed that the percentage of female patients with BD driven by 21 data sets was negatively associated with FA reduction in the body of the corpus callosum (Fig. 3). However, the results should be interpreted with some caution, because they were seemingly driven by 2 outlier studies with very high site effects.37,38 We detected no effect of Hamilton Depression Rating Scale score, mean age, age at onset or illness duration on white-matter alterations.
Discussion
The current study pooled the largest number of TBSS studies to date in patients with BD to conduct a quantitative meta-analysis. To the best of our knowledge, no similar work has been reported, especially regarding the clinical and demographic effects of BD subtypes, psychotic features, mood states, age and sex. Voxel-wise meta-analysis using AES-SDM revealed that patients with BD have decreased FA in the genu of the corpus callosum and in 2 clusters in the body of the corpus callosum; these results were robust under jackknife analysis. We found a significant negative association between the percentage of female patients and the FA in the body of the corpus callosum. Subgroup analyses of the BD I studies, euthymic studies and adult studies reproduced the significant findings of decreased FA in the genu and the body of the corpus callosum. These results suggest that abnormalities in white-matter tracts may be involved in the pathological mechanisms of BD.
The corpus callosum is the largest white-matter bundle that connects the bilateral cerebral hemispheres, integrating emotional, cognitive, motor and sensory functions.46–48 We found decreased FA in the genu and body of the corpus callosum in patients with BD compared with healthy controls, a result that was consistent with those of 2 other DTI studies using a region-of-interest method focusing on the corpus callosum.49,50 This brain region has been increasingly implicated in patients with BD: for example, a multicentre structural MRI study reported a decrease in the mid-sagittal area of the body of the corpus callosum in patients with BD compared with healthy controls.51 It is tempting to speculate that impaired interhemispheric communication is important in the pathophysiology of BD. The recent meta-analysis by Wise and colleagues14 reported that patients with BD showed decreased FA in the left cingulum, the left genu of the corpus callosum and the right anterior superior longitudinal fasciculus compared with healthy controls. Our findings are not completely consistent with those of previous meta-analyses, because we found no significant abnormalities in any tracts other than the corpus callosum. There are several possible reasons for this. First, previous meta-analyses included both TBSS and VBA studies, whereas we only included TBSS studies, because there is reason to believe that these can more accurately identify white-matter abnormalities.16 Second, we were able to include many more TBSS studies than the previous meta-analyses, increasing the precision of effect-size estimates. Third, the clinical samples in our included studies differed in detail from those in previous meta-analyses.
The white-matter fibres passing through the genu of the corpus callosum connect the bilateral prefrontal cortices, which are known to play a role in decision-making, attention, reward-processing and emotion regulation.52,53 Previous structural studies found decreased volume in both the ventral and dorsal prefrontal cortex in patients with BD,54,55 and several functional studies have observed decreased dorsal and ventral prefrontal activity in patients with BD during language tasks and executive-related tasks.56,57 Additionally, several studies have reported that the ventral prefrontal cortex is implicated in the “top–down” regulation of emotional processing in patients with BD.58,59 The FA reduction in the genu of the corpus callosum in the present study suggests impaired prefrontal interhemispheric connectivity, perhaps leading to neurocognitive deficits in processing speed and working memory,60 for example, and to emotional dysregulation in patients with BD. Our subgroup analysis of patients with BD I reproduced the finding of decreased FA in the genu of corpus callosum compared with healthy controls, which was consistent with the findings of some previous DTI studies.61,62 There are clinical differences between BD I and BD II. In DSM-5, BD I is the classic manic–depressive disorder, while BD II features depressive and hypomanic episodes; furthermore, the clinical manifestations and treatments of BD I and BD II are different.63,64 The FA reduction in the genu of the corpus callosum reflected a disconnection of the paralimbic system, which plays a central role in emotional regulation,3,65 and might lead to manic-type behaviours such as inappropriate euphoria, excessive psychomotor behaviour, hypersexuality and paranoia, consistent with the clinical characteristics of patients with BD I.65
The pathobiological interpretation of FA reduction is complex, because it can be influenced by many factors, such as regional myelination levels, intra- and extracellular volume, the degree of intra-voxel fibre crossing, axonal density and average axonal diameter.66 Previous studies have suggested that abnormalities in the corpus callosum that are detected early in pediatric patients with BD may be due to altered myelination during neurodevelopment.67 Meanwhile, several studies have reported a reduction of myelin-producing oligodendrocytes in the prefrontal cortex in patients with BD.68–70 The genu of the corpus callosum and the prefrontal cortex are both late-myelinating areas and are therefore more vulnerable to damage than the early-myelinating splenium.71–73 We speculate that the FA reduction in the genu of the corpus callosum may be related to a reduction of myelination and may result in a pathobiological process that directly slows the transfer of interhemispheric information in patients with BD.47 To help confirm this hypothesis, further studies with more DTI indices, such as axial diffusivity and radial diffusivity, would be useful, because there is evidence from animal studies that axial diffusivity is primarily an axonal marker and radial diffusivity is primarily a myelin marker.74–76
Our finding of decreased FA in the body of the corpus callosum in patients with BD compared with healthy controls was consistent with several previous studies.77–79 The body of the corpus callosum connects several areas, including the lateral primary motor cortex, supplementary motor areas (SMA), the primary sensory cortex and the parietal cortex.80 Previous functional studies have shown that the rostral part of the SMA (the pre-SMA) is involved in memory storage, learning, transition, and motor and speech control, and the pre-SMA connects to the cingulated gyrus, which is part of the limbic system related to cognitive and emotional regulation, while the caudal part of the SMA, the SMA proper and the primary motor area are responsible for motor function.81 Together, the SMA may be a transitional region of the limbic and motor system, which plays an important role in the translation of emotion into motor actions.81 Although emotional dysfunction is one of the main symptoms of BD, previous studies have shown that patients with BD also have motor impairments such as psychomotor retardation,82 agitation, 83 attentional deficits and impairments in fine-motor skills.84–87 We therefore suggest that the FA decrease in the body of the corpus callosum might be related to motor and emotional dysfunction in patients with BD. Interestingly, our subgroup analyses of euthymic patients with BD and adult patients with BD replicated the FA decrease in the body of the corpus callosum. This finding was consistent with 1 previous study, which found that euthymic patients with BD had decreased FA in the body of the corpus callosum compared with unaffected siblings.73 Several studies have reported that brain structural and functional abnormalities are related to current mood states in patients with BD.88,89 Our results may indicate a persistent callosal dysconnectivity in euthymic patients with BD, which is consistent with the observation that cognitive impairment and executive dysfunction still exist in euthymic patients with BD.90,91
Meta-regression analysis found that FA in the body of the corpus callosum (decreased overall in patients with BD compared with healthy controls) was negatively associated with the percentage of female patients. This result may indicate that female patients have lower FA values in the body of the corpus callosum than male patients. Sex differences have been observed in the clinical characteristics of BD. Female patients with BD may be more likely to have comorbidities and depressive symptoms, which may result in high suicide risk and impaired occupational function.2 The sex differences of FA in the body of the corpus callosum may be related to differences in the clinical aspects of BD. A series of morphological studies reported a smaller corpus callosum in female patients than in male patients with BD.92–94 However, 1 study reported no significant difference in the integrity of the body of the corpus callosum when comparing male with female patients with BD.67 These inconsistencies may have resulted from different demographic characteristics, data acquisition methods and other confounding factors in studies of patients with BD.
Medication exposure is an important potential confounder, and understanding the effect of medications on white-matter abnormalities in patients with BD is critical for the interpretation of results. Because only 1 primary study enrolled unmedicated patients with BD, we could not exclude the confounding influences of medication. Several imaging studies have evaluated the effect of psychotropic medications on white-matter changes in patients with BD. One structural MRI study reported larger bilateral temporal lobe white-matter volumes in patients with BD who were taking antipsychotic medications than in patients who were not taking them.95 Among patients with BD, longer duration of lithium treatment is associated with increased FA, suggesting that this medication might enhance white-matter integrity.96 In another study, decreased FA was found in participants treated with lithium, but not in those who were unmedicated; however, there was no significant difference when directly comparing lithium-using and non-lithium-using patients.31 It has been reported that lithium and other mood stabilizers can improve white-matter integrity and promote myelination by acting on neuroglial signalling pathways and increasing neurotrophic factors.97,98 Further studies designed to detect the effect of medication exposure on white-matter changes in patients with BD are needed.
Limitations
First, the participants varied in terms of sociodemographic and clinical characteristics. Although we performed subgroup analyses of patients with BD I, euthymic patients with BD, and adult patients with BD, the limited data sets precluded comprehensive subgroup analyses (in particular of the important contrast between BD I and BD II) and meta-regression analyses. Further research is needed investigating the contribution of other clinical characteristics, such as age of onset, comorbidities and disorder severity. Second, the heterogeneity of MRI image acquisition — including voxel size, field intensity of the magnetic resonance system, diffusion direction and slice thickness — may influence the accuracy of the results of our meta-analysis. Third, the main meta-analysis included primary studies, all but 1 of which enrolled medicated patients, so the confounding influences of medication could not be excluded. Fourth, the meta-regression finding that the percentage of female patients with BD was negatively related to the FA in the body of the corpus callosum was driven by 2 outlying studies. Fifth, it has been reported that more than 50% of patients with BD will experience psychotic symptoms in their lifetime, and it is difficult to accurately distinguish BD from other psychiatric disorders in patients with psychotic symptoms.99 However, data limitations precluded a subgroup analysis comparing patients with BD with or without psychosis. Because 5 of our primary studies included patients with BD and psychosis, we could not exclude the effects of psychosis features on our results. Future studies comparing patients who have BD with and without psychosis are needed to elucidate this.
Conclusion
We qualitatively and quantitatively reviewed a large number of published TBSS studies in patients with BD. Meta-analysis of FA findings found that the most robust and replicable white-matter differences were in the genu and body of the corpus callosum. These white-matter tracts connect the bilateral frontal, temporal and parietal cortices, indicating the importance of disrupted interhemispheric communication in the pathophysiology of BD. Subgroup analyses found white-matter structural differences in patients with BD I, and in adult and euthymic patients. Furthermore, the results of the meta-regression analysis shed light on the structural underpinnings of the sex differences in the clinical manifestations of patients with BD. In future studies, attention should be given to differences in clinical type, mood state, and demographics to better define neural mechanisms in patients with BD. Differentiating mood- and type-related white-matter abnormalities is important for elucidating the core pathophysiology of BD and will give better insight into the nature of the disease. The present study also adds to the development of psychoradiology, the branch of radiology that applies clinical imaging to psychiatry and psychology.100–103
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant nos. 81621003, 81220108013, 81761128023, 81227002 and 81030027), the National Key Technologies R&D Program (program no. 2012BAI01B03) and the Program for Changjiang Scholars and Innovative Research Team (PCSIRT, grant no. IRT16R52) at the University of China. Q. Gong acknowledges support from his Changjiang Scholar Professorship Award of China (award no. T2014190) and American CMB Distinguished Professorship Award (award no. F510000/G16916411), administered by the Institute of International Education, in the United States.
Footnotes
Competing interests: None declared.
Contributors: C. Yang, L. Li, X. Hu and Q. Gong designed the study. C. Yang and L. Li acquired and analyzed the data, which X. Hu, Q. Luo, W. Kuang, S. Lui, X. Huang, J. Dai, M. He, G.J. Kemp and J.A. Sweeney also analyzed. C. Yang, L. Li, X. Hu, Q. Luo, W. Kuang and J. Dai wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Merikangas KR, Chakravarti A, Moldin SO, et al. Future of genetics of mood disorders research. Biol Psychiatry. 2002;52:457–77. doi: 10.1016/s0006-3223(02)01471-3. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. American Psychiatric Association; Washington (DC): 2013. DSM-5 Task Force. [Google Scholar]
- 3.Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–16. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 4.Brambilla P, Bellani M, Yeh PH, et al. White matter connectivity in bipolar disorder. Int Rev Psychiatry. 2009;21:380–6. doi: 10.1080/09540260902962172. [DOI] [PubMed] [Google Scholar]
- 5.Sarrazin S, Poupon C, Linke J, et al. A multicenter tractography study of deep white matter tracts in bipolar I disorder: psychotic features and interhemispheric disconnectivity. JAMA Psychiatry. 2014;71:388–96. doi: 10.1001/jamapsychiatry.2013.4513. [DOI] [PubMed] [Google Scholar]
- 6.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- 7.Repple J, Meinert S, Grotegerd D, et al. A voxel-based diffusion tensor imaging study in unipolar and bipolar depression. Bipolar Disord. 2017;19:23–31. doi: 10.1111/bdi.12465. [DOI] [PubMed] [Google Scholar]
- 8.Ha TH, Her JY, Kim JH, et al. Similarities and differences of white matter connectivity and water diffusivity in bipolar I and II disorder. Neurosci Lett. 2011;505:150–4. doi: 10.1016/j.neulet.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Bruno S, Cercignani M, Ron MA. White matter abnormalities in bipolar disorder: a voxel-based diffusion tensor imaging study. Bipolar Disord. 2008;10:460–8. doi: 10.1111/j.1399-5618.2007.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahapatra A, Khandelwal SK, Sharan P, et al. Diffusion tensor imaging tractography study in bipolar disorder patients compared to first-degree relatives and healthy controls. Psychiatry Clin Neurosci. 2017;71:706–15. doi: 10.1111/pcn.12530. [DOI] [PubMed] [Google Scholar]
- 11.Müller VI, Cieslik EC, Laird AR, et al. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev. 2018;84:151–61. doi: 10.1016/j.neubiorev.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nortje G, Stein DJ, Radua J, et al. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J Affect Disord. 2013;150:192–200. doi: 10.1016/j.jad.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Vederine FE, Wessa M, Leboyer M, et al. A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1820–6. doi: 10.1016/j.pnpbp.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Wise T, Radua J, Nortje G, et al. Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol Psychiatry. 2016;79:293–302. doi: 10.1016/j.biopsych.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 16.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Versace A, Almeida JR, Hassel S, et al. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry. 2008;65:1041–52. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haller S, Xekardaki A, Delaloye C, et al. Combined analysis of grey matter voxel-based morphometry and white matter tract-based spatial statistics in late-life bipolar disorder. J Psychiatry Neurosci. 2011;36:391–401. doi: 10.1503/jpn.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambrosi E, Chiapponi C, Sani G, et al. White matter microstructural characteristics in bipolar I and bipolar II disorder: a diffusion tensor imaging study. J Affect Disord. 2016;189:176–83. doi: 10.1016/j.jad.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 20.Radua J, Rubia K, Canales-Rodriguez EJ, et al. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front Psychiatry. 2014;5:13. doi: 10.3389/fpsyt.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen G, Hu X, Li L, et al. Disorganization of white matter architecture in major depressive disorder: a meta-analysis of diffusion tensor imaging with tract-based spatial statistics. Sci Rep. 2016;6:21825. doi: 10.1038/srep21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Hu X, Ouyang L, et al. A systematic review and meta-analysis of tract-based spatial statistics studies regarding attention- deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2016;68:838–47. doi: 10.1016/j.neubiorev.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du M, Liu J, Chen Z, et al. Brain grey matter volume alterations in late-life depression. J Psychiatry Neurosci. 2014;39:397–406. doi: 10.1503/jpn.130275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim L, Radua J, Rubia K. Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. Am J Psychiatry. 2014;171:854–63. doi: 10.1176/appi.ajp.2014.13101427. [DOI] [PubMed] [Google Scholar]
- 26.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 27.Radua J, Via E, Catani M, et al. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol Med. 2011;41:1539–50. doi: 10.1017/S0033291710002187. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 30.Wakana S, Jiang H, Nagae-Poetscher LM, et al. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 31.Benedetti F, Yeh PH, Bellani M, et al. Disruption of white matter integrity in bipolar depression as a possible structural marker of illness. Biol Psychiatry. 2011;69:309–17. doi: 10.1016/j.biopsych.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 32.Mahon K, Burdick KE, Wu J, et al. Relationship between suicidality and impulsivity in bipolar I disorder: a diffusion tensor imaging study. Bipolar Disord. 2012;14:80–9. doi: 10.1111/j.1399-5618.2012.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu JX, Chen YS, Hsieh JC, et al. Differences in white matter abnormalities between bipolar I and II disorders. J Affect Disord. 2010;127:309–15. doi: 10.1016/j.jad.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Kumar J, Iwabuchi S, Oowise S, et al. Shared white-matter dysconnectivity in schizophrenia and bipolar disorder with psychosis. Psychol Med. 2015;45:759–70. doi: 10.1017/S0033291714001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delaloye C, Moy G, de Bilbao F, et al. Longitudinal analysis of cognitive performances and structural brain changes in late-life bipolar disorder. Int J Geriatr Psychiatry. 2011;26:1309–18. doi: 10.1002/gps.2683. [DOI] [PubMed] [Google Scholar]
- 36.Chan WY, Yang GL, Chia MY, et al. Cortical and subcortical white matter abnormalities in adults with remitted first-episode mania revealed by tract-based spatial statistics. Bipolar Disord. 2010;12:383–9. doi: 10.1111/j.1399-5618.2010.00829.x. [DOI] [PubMed] [Google Scholar]
- 37.Lagopoulos J, Hermens DF, Hatton SN, et al. Microstructural white matter changes in the corpus callosum of young people with bipolar disorder: a diffusion tensor imaging study. PLoS One. 2013;8:e59108. doi: 10.1371/journal.pone.0059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sprooten E, Brumbaugh MS, Knowles EE, et al. Reduced white matter integrity in sibling pairs discordant for bipolar disorder. Am J Psychiatry. 2013;170:1317–25. doi: 10.1176/appi.ajp.2013.12111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Versace A, Almeida JR, Quevedo K, et al. Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biol Psychiatry. 2010;68:560–7. doi: 10.1016/j.biopsych.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wessa M, Houenou J, Leboyer M, et al. Microstructural white matter changes in euthymic bipolar patients: a whole-brain diffusion tensor imaging study. Bipolar Disord. 2009;11:504–14. doi: 10.1111/j.1399-5618.2009.00718.x. [DOI] [PubMed] [Google Scholar]
- 41.Magioncalda P, Martino M, Conio B, et al. Patterns of microstructural white matter abnormalities and their impact on cognitive dysfunction in the various phases of type I bipolar disorder. J Affect Disord. 2016;193:39–50. doi: 10.1016/j.jad.2015.12.050. [DOI] [PubMed] [Google Scholar]
- 42.Oertel-Knochel V, Reinke B, Alves G, et al. Frontal white matter alterations are associated with executive cognitive function in euthymic bipolar patients. J Affect Disord. 2014;155:223–33. doi: 10.1016/j.jad.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Gao W, Jiao Q, Qi R, et al. Combined analyses of gray matter voxel-based morphometry and white matter tract-based spatial statistics in pediatric bipolar mania. J Affect Disord. 2013;150:70–6. doi: 10.1016/j.jad.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 44.Teixeira AM, Kleinman A, Zanetti M, et al. Preserved white matter in unmedicated pediatric bipolar disorder. Neurosci Lett. 2014;579:41–5. doi: 10.1016/j.neulet.2014.06.061. [DOI] [PubMed] [Google Scholar]
- 45.Haarman BCM, Riemersma-Van der Lek RF, Burger H, et al. Diffusion tensor imaging in euthymic bipolar disorder: a tract-based spatial statistics study. J Affect Disord. 2016;203:281–91. doi: 10.1016/j.jad.2016.05.040. [DOI] [PubMed] [Google Scholar]
- 46.Bellani M, Yeh PH, Tansella M, et al. DTI studies of corpus callosum in bipolar disorder. Biochem Soc Trans. 2009;37:1096–8. doi: 10.1042/BST0371096. [DOI] [PubMed] [Google Scholar]
- 47.Brambilla P, Nicoletti M, Sassi RB, et al. Corpus callosum signal intensity in patients with bipolar and unipolar disorder. J Neurol Neurosurg Psychiatry. 2004;75:221–5. [PMC free article] [PubMed] [Google Scholar]
- 48.Giedd JN, Blumenthal J, Jeffries NO, et al. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:571–88. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Kale Edmiston E, Chen K, et al. A comparative diffusion tensor imaging study of corpus callosum subregion integrity in bipolar disorder and schizophrenia. Psychiatry Res. 2014;221:58–62. doi: 10.1016/j.pscychresns.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Wang F, Kalmar JH, Edmiston E, et al. Abnormal corpus callosum integrity in bipolar disorder: a diffusion tensor imaging study. Biol Psychiatry. 2008;64:730–3. doi: 10.1016/j.biopsych.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarrazin S, d’Albis MA, McDonald C, et al. Corpus callosum area in patients with bipolar disorder with and without psychotic features: an international multicentre study. J Psychiatry Neurosci. 2015;40:352–9. doi: 10.1503/jpn.140262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neubert FX, Mars RB, Sallet J, et al. Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proc Natl Acad Sci U S A. 2015;112:E2695–704. doi: 10.1073/pnas.1410767112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volman I, Roelofs K, Koch S, et al. Anterior prefrontal cortex inhibition impairs control over social emotional actions. Curr Biol. 2011;21:1766–70. doi: 10.1016/j.cub.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 54.Haldane M, Frangou S. New insights help define the pathophysiology of bipolar affective disorder: neuroimaging and neuropathology findings. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:943–60. doi: 10.1016/j.pnpbp.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 55.Wang F, Kalmar JH, He Y, et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66:516–21. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Curtis VA, Thompson JM, Seal ML, et al. The nature of abnormal language processing in euthymic bipolar I disorder: evidence for a relationship between task demand and prefrontal function. Bipolar Disord. 2007;9:358–69. doi: 10.1111/j.1399-5618.2007.00422.x. [DOI] [PubMed] [Google Scholar]
- 57.Monks PJ, Thompson JM, Bullmore ET, et al. A functional MRI study of working memory task in euthymic bipolar disorder: evidence for task-specific dysfunction. Bipolar Disord. 2004;6:550–64. doi: 10.1111/j.1399-5618.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 58.Chepenik LG, Raffo M, Hampson M, et al. Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry Res. 2010;182:207–10. doi: 10.1016/j.pscychresns.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foland LC, Altshuler LL, Bookheimer SY, et al. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ajilore O, Vizueta N, Walshaw P, et al. Connectome signatures of neurocognitive abnormalities in euthymic bipolar I disorder. J Psychiatr Res. 2015;68:37–44. doi: 10.1016/j.jpsychires.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chaddock CA, Barker GJ, Marshall N, et al. White matter microstructural impairments and genetic liability to familial bipolar I disorder. Br J Psychiatry. 2009;194:527–34. doi: 10.1192/bjp.bp.107.047498. [DOI] [PubMed] [Google Scholar]
- 62.Emsell L, Leemans A, Langan C, et al. Limbic and callosal white matter changes in euthymic bipolar I disorder: an advanced diffusion magnetic resonance imaging tractography study. Biol Psychiatry. 2013;73:194–201. doi: 10.1016/j.biopsych.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 63.Ayuso-Gutiérrez JL, Ramos-Brieva JA. The course of manic-depressive illness. A comparative study of bipolar I and bipolar II patients. J Affect Disord. 1982;4:9–14. doi: 10.1016/0165-0327(82)90013-1. [DOI] [PubMed] [Google Scholar]
- 64.Grande I, Berk M, Birmaher B, et al. Bipolar disorder. Lancet. 2016;387:1561–72. doi: 10.1016/S0140-6736(15)00241-X. [DOI] [PubMed] [Google Scholar]
- 65.Blond BN, Fredericks CA, Blumberg HP. Functional neuroanatomy of bipolar disorder: structure, function, and connectivity in an amygdala-anterior paralimbic neural system. Bipolar Disord. 2012;14:340–55. doi: 10.1111/j.1399-5618.2012.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beaulieu C. The basis of anisotropic water diffusion in the nervous system — a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 67.Caetano SC, Silveira CM, Kaur S, et al. Abnormal corpus callosum myelination in pediatric bipolar patients. J Affect Disord. 2008;108:297–301. doi: 10.1016/j.jad.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uranova NA, Vostrikov VM, Orlovskaya DD, et al. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–75. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 69.Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr Res. 2007;94:273–80. doi: 10.1016/j.schres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 70.Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–5. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu PH, Lee GJ, Raven EP, et al. Age-related slowing in cognitive processing speed is associated with myelin integrity in a very healthy elderly sample. J Clin Exp Neuropsychol. 2011;33:1059–68. doi: 10.1080/13803395.2011.595397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kochunov P, Thompson PM, Lancaster JL, et al. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage. 2007;35:478–87. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 73.Saricicek A, Zorlu N, Yalin N, et al. Abnormal white matter integrity as a structural endophenotype for bipolar disorder. Psychol Med. 2016;46:1547–58. doi: 10.1017/S0033291716000180. [DOI] [PubMed] [Google Scholar]
- 74.Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 75.Song SK, Sun SW, Ju WK, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–22. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 76.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–40. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 77.Lloyd AJ, Ali HE, Nesbitt D, et al. Corpus callosum changes in euthymic bipolar affective disorder. Br J Psychiatry. 2014;204:129–36. doi: 10.1192/bjp.bp.112.123687. [DOI] [PubMed] [Google Scholar]
- 78.Walterfang M, Wood AG, Barton S, et al. Corpus callosum size and shape alterations in individuals with bipolar disorder and their first-degree relatives. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1050–7. doi: 10.1016/j.pnpbp.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 79.Cyprien F, de Champfleur NM, Deverdun J, et al. Corpus callosum integrity is affected by mood disorders and also by the suicide attempt history: a diffusion tensor imaging study. J Affect Disord. 2016;206:115–24. doi: 10.1016/j.jad.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 80.Kim JE, Oh JS, Sung JJ, et al. Diffusion tensor tractography analysis of the corpus callosum fibers in amyotrophic lateral sclerosis. J Clin Neurol. 2014;10:249–56. doi: 10.3988/jcn.2014.10.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bozkurt B, Yagmurlu K, Middlebrooks EH, et al. Microsurgical and tractographic anatomy of the supplementary motor area complex in humans. World Neurosurg. 2016;95:99–107. doi: 10.1016/j.wneu.2016.07.072. [DOI] [PubMed] [Google Scholar]
- 82.Buyukdura JS, McClintock SM, Croarkin PE. Psychomotor retardation in depression: biological underpinnings, measurement, and treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:395–409. doi: 10.1016/j.pnpbp.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alderfer BS, Allen MH. Treatment of agitation in bipolar disorder across the life cycle. J Clin Psychiatry. 2003;64(suppl 4):3–9. [PubMed] [Google Scholar]
- 84.McClure EB, Treland JE, Snow J, et al. Memory and learning in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:461–9. doi: 10.1097/01.chi.0000156660.30953.91. [DOI] [PubMed] [Google Scholar]
- 85.Wilder-Willis KE, Sax KW, Rosenberg HL, et al. Persistent attentional dysfunction in remitted bipolar disorder. Bipolar Disord. 2001;3:58–62. doi: 10.1034/j.1399-5618.2001.030202.x. [DOI] [PubMed] [Google Scholar]
- 86.Singh MK, Chang KD, Mazaika P, et al. Neural correlates of response inhibition in pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2010;20:15–24. doi: 10.1089/cap.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Larson ER, Shear PK, Krikorian R, et al. Working memory and inhibitory control among manic and euthymic patients with bipolar disorder. J Int Neuropsychol Soc. 2005;11:163–72. doi: 10.1017/s1355617705050228. [DOI] [PubMed] [Google Scholar]
- 88.Caligiuri MP, Brown GG, Meloy MJ, et al. An fMRI study of affective state and medication on cortical and subcortical brain regions during motor performance in bipolar disorder. Psychiatry Res. 2003;123:171–82. doi: 10.1016/s0925-4927(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 89.Zanetti MV, Jackowski MP, Versace A, et al. State-dependent microstructural white matter changes in bipolar I depression. Eur Arch Psychiatry Clin Neurosci. 2009;259:316–28. doi: 10.1007/s00406-009-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cavanagh JT, Van Beck M, Muir W, et al. Case-control study of neurocognitive function in euthymic patients with bipolar disorder: an association with mania. Br J Psychiatry. 2002;180:320–6. doi: 10.1192/bjp.180.4.320. [DOI] [PubMed] [Google Scholar]
- 91.Martinez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004;161:262–70. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- 92.Luders E, Narr KL, Zaidel E, et al. Gender effects on callosal thickness in scaled and unscaled space. Neuroreport. 2006;17:1103–6. doi: 10.1097/01.wnr.0000227987.77304.cc. [DOI] [PubMed] [Google Scholar]
- 93.Luders E, Toga AW, Thompson PM. Why size matters: differences in brain volume account for apparent sex differences in callosal anatomy: the sexual dimorphism of the corpus callosum. Neuroimage. 2014;84:820–4. doi: 10.1016/j.neuroimage.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Newman SD. Differences in cognitive ability and apparent sex differences in corpus callosum size. Psychol Res. 2016;80:853–9. doi: 10.1007/s00426-015-0688-3. [DOI] [PubMed] [Google Scholar]
- 95.Jones LD, Payne ME, Messer DF, et al. Temporal lobe volume in bipolar disorder: relationship with diagnosis and antipsychotic medication use. J Affect Disord. 2009;114:50–7. doi: 10.1016/j.jad.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gildengers AG, Butters MA, Aizenstein HJ, et al. Longer lithium exposure is associated with better white matter integrity in older adults with bipolar disorder. Bipolar Disord. 2015;17:248–56. doi: 10.1111/bdi.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McQuillin A, Rizig M, Gurling HM. A microarray gene expression study of the molecular pharmacology of lithium carbonate on mouse brain mRNA to understand the neurobiology of mood stabilization and treatment of bipolar affective disorder. Pharmacogenet Genomics. 2007;17:605–17. doi: 10.1097/FPC.0b013e328011b5b2. [DOI] [PubMed] [Google Scholar]
- 98.Marlinge E, Bellivier F, Houenou J. White matter alterations in bipolar disorder: potential for drug discovery and development. Bipolar Disord. 2014;16:97–112. doi: 10.1111/bdi.12135. [DOI] [PubMed] [Google Scholar]
- 99.Dunayevich E, Keck PE., Jr Prevalence and description of psychotic features in bipolar mania. Curr Psychiatry Rep. 2000;2:286–90. doi: 10.1007/s11920-000-0069-4. [DOI] [PubMed] [Google Scholar]
- 100.Lui S, Zhou XJ, Sweeney JA, et al. Psychoradiology: the frontier of neuroimaging in psychiatry. Radiology. 2016;281:357–72. doi: 10.1148/radiol.2016152149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kressel HY. Setting sail. Radiology. 2017;282:4–6. doi: 10.1148/radiol.2016162471. [DOI] [PubMed] [Google Scholar]
- 102.Sun H, Chen Y, Huang Q, et al. Psychoradiologic utility of MR imaging for diagnosis of attention deficit hyperactivity disorder: a radiomics analysis. Radiology. 2018;287(2):620–30. doi: 10.1148/radiol.2017170226. [DOI] [PubMed] [Google Scholar]
- 103.Port J. Diagnosis of attention deficit hyperactivity disorder by using MR imaging and radiomics: a potential tool for clinicians. Radiology. 2018;287(2):631–2. doi: 10.1148/radiol.2018172804. [DOI] [PubMed] [Google Scholar]