Abstract
Background
Severe hypertriglyceridemia (SHTG, TG ≥5·65 mmol/L), a disease, usually resulting from a combination of genetic and environmental factors, may increase the risk of acute pancreatitis (AP). However, previous genetic analysis has been limited by lacking of related observation of gene to AP.
Methods
The expanding genetic sequencing including 15 TG-related genes (LPL, LMF1, APOC2, GPIHBP1, GCKR, ANGPTL3, APOB, APOA1-A4-C3-A5, TRIB1, CETP, APOE, and LIPI) was performed within 103 patients who were diagnosed with primary SHTG and 46 age- and sex-matched normal controls.
Findings
Rare variants were found in 46 patients and 12 controls. The detection rate of rare variants in SHTG group increased by 19·5% via intensive genetic analysis. Presence of rare variants in LPL, APOA5, five LPL molecular regulating genes and all the sequenced genes were found to be associated with SHTG (p < 0·05). Of noted, patients with history of AP presented higher frequency of rare variants in LPL gene and all the LPL molecular regulating genes (27·8% vs.4·7% and 50·0% vs. 20·0%). The risk scores for SHTG determined by common TG-associated variants were increased in subgroups according to the extent of SHTG when they were compared with that of controls. Finally, patients without rare variants within SHTG group also presented higher risk scores than control group (p < 0·05).
Interpretation
Expanding genetic analysis had a higher detection rate of rare variants in patients with SHTG. Rare variants in LPL and its molecular regulating genes could increase the risk of AP among Chinese patients with SHTG.
Fund
This work was partially supported by the Capital Health Development Fund (201614035) and CAMS. Major Collaborative Innovation Project (2016-I2M-1-011) awarded to Dr. Jian-Jun Li, MD, PhD.
Keywords: Hypertriglyceridemia, Genetic analysis, Acute pancreatitis
Research in context.
Evidence before this study
Severe hypertriglyceridemia, a disease, usually caused by both genetic and environmental risk factors, may increase the risk of acute pancreatitis (AP). Previous genetic studies on patients with severe hypertriglyceridemia (SHTG) were unsatisfied due to limited LPL molecular regulating genes (LPL, APOA5, APOC2, GPIHBP1, and LMF1) and lacking of evaluations concerning genetic background to AP.
Added value of this study
To test the hypothesis that the presence of pathogenic variant may increase the risk of AP, we performed an expanding next generation sequencing analysis in 15 TG-related genes, and found that extensive analysis could increase the detection rate of rare variants in SHTG patients and rare variants in LPL and its molecular regulating genes could increase the risk of AP among Chinese patients with SHTG.
Implications of all the available evidence
Expanding genetic analysis had a higher detection rate of rare variants in patients with SHTG. Detecting rare variants in LPL and its molecular regulating genes might be a tool in estimating risk of AP among Chinese patients with SHTG.
Alt-text: Unlabelled Box
1. Introduction
Hypertriglyceridemia (HTG) has been established as a common lipid disorder in association with many comorbidities including acute pancreatitis (AP) [[1], [2], [3]]. Severe hypertriglyceridemia [SHTG, fasting plasma triglyceride (TG) ≥5·65 mmol/L or 500 mg/dL] in adults is an especially unfavorable state with a high risk of fatal complications, which is worthy of further investigation [4]. However, the causal studies on SHTG have been relatively lagging because of its complexity, which was mainly dependent on the genetic and environmental interactions [5]. Additionally, the measurement of plasma TG has unavoidable intra-individual biological variation and is easily affected by drugs and diet, which increase the difficulty in the diagnosis of SHTG [6,7]. Furthermore, the presence of SHTG is also correlated with a series of the secondary causes, such as extreme obesity, uncontrolled diabetes, severe liver/renal insufficiency, thyroid disease, chemotherapy et al. However, in some patients, their SHTG cannot clinically controlled even if the secondary causes were eliminated, and the genetic features may be an explanation for such patients.
It has been demonstrated that Lipoprotein lipase (LPL) is a critical enzyme in determining plasma triglyceride levels and the defect in LPL gene may result in SHTG [8,9]. Moreover, Apoprotein(apo)A-V, apoC-II, glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 (GPIHBP1), and lipase maturation factor 1 (LMF1) are also co-factors involved in the activation, transportation or maturation of LPL [[10], [11], [12], [13], [14]]. Recently, several studies have reported that defects in LPL, APOA5, APOC2, GPIHBP1, and LMF1 are closely associated with type 1 hyperlipoproteinaemia [15,16]. Most patients in this phenotype have monogenic feature but only account for a minority of SHTG cases. In fact, the majority of patients with SHTG usually present polygenic phenotypes and carry a complex burden of rare and common variants in a broader spectrum of genes [17]. For example, APOA5/C4/A3/A1 gene cluster has been reported to be associated with 38% of genetic variance of triglyceride [18]. Moreover, previous studies have demonstrated that significant accumulation of rare variants in the APOA5/C4/A3/A1 cluster exists in patients with SHTG [19]. Additionally, other genes including APOB, glucokinase regulatory protein (GCKR), angiopoietin-like protein 3 (ANGPTL3), tribbles-1(TRIB1), cholesteryl ester transfer protein (CETP), APOE, and LIPI are also identified to be essential for triglyceride-modulating but their attributions on the presence of SHTG cases have less been determined [17,20,21]. Thus, to find the susceptibility variants, we performed next generation sequencing (NGS) on promoters, exons, and exon–intron boundaries in 15 TG-related genes (LPL, LMF1, APOC2, GPIHBP1, GCKR, ANGPTL3, APOB, APOA1-A4-C3-A5, TRIB1, CETP, APOE, and LIPI) among 103 patients and 46 age- and sex matched normal controls (TG < 1.7 mmol/L). We also conducted a survey of common single nucleotide polymorphisms (SNPs) for the associations with triglyceride levels. Finally, we tested the hypothesis that the presence of pathogenic variant may increase the risk of AP in patients with SHTG.
2. Methods
2.1. Study design and population
Our study complied with the Declaration of Helsinki and was approved by the hospital's ethical review board (Fu Wai Hospital & National Center for Cardiovascular Diseases, Beijing, China). Informed written consents were obtained from all patients enrolled in this study.
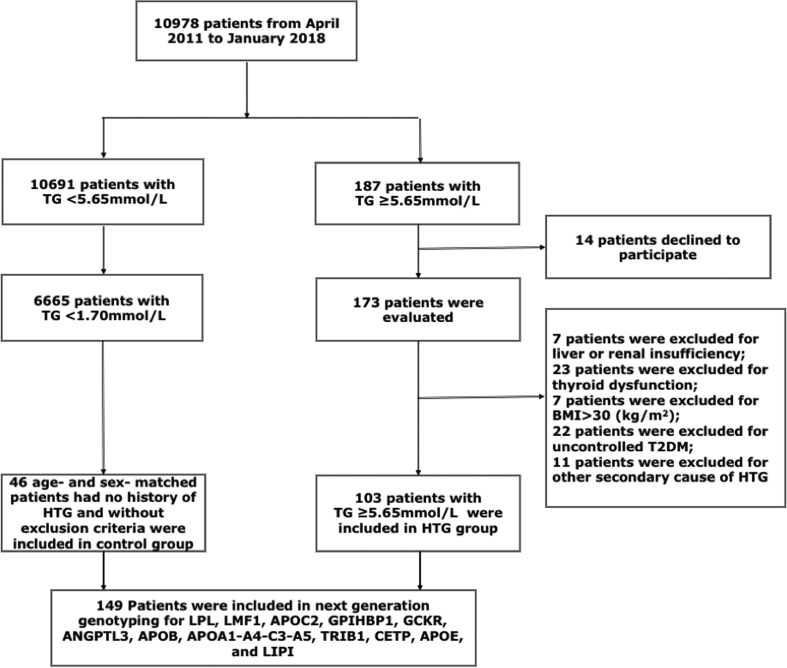
The sequencing cohort included 103 SHTG individuals and 46 controls of Chinese Han population. As is shown in Fig. 1, From April 2011 to January 2018, 10,908 patients attended the division of dyslipidemia of Fu Wai Hospital. 187 (1·7%) patients with SHTG were unrelated subjects who had fasting plasma TG ≥5·65 mmol/L at least twice from a single medical center. TG levels were measured when they were in normal diet (without alcohol intake). Patients with liver or renal insufficiency, thyroid dysfunction, BMI > 30 kg/m2, and uncontrolled type 2 diabetes mellitus (T2DM) were excluded. Patients with other secondary causes of HTG such as chemotherapy, hormonal drugs, and alcohol abusers were also ruled out. Controls were recruited from individuals who had no history of HTG, were without exclusion criteria and with TG <1·7 mmol/L. Finally, 46 controls were each matched with up to 3 HTG cases based on age within 5 years and sex.
Fig. 1.
Flowchart of the study.
Clinical data of each individual who entered the study were collected by experienced physicians and nurses. Coronary artery disease was defined as the presence of coronary stenosis≥50% at least one major artery segment assessed by two experienced physicians according to coronary angiography. Hypertension (HT) was recognized as systolic blood pressure (SBP) ≥140 and/or diastolic blood pressure (DBP) ≥90 mmHg for at least three separate measurement or currently using anti-hypertension drugs. Diabetes mellitus (DM) was diagnosed as fasting glucose ≥7·0 mmol/L or random glucose ≥11·0 mmol/L or hypoglycemic treatments. Family history of SHTG was identified as TG ≥5·65 mmol/L in at least 1 related family members. Alcohol consumption was assessed according to the definition by National Institute on Alcohol Abuse and Alcoholism [22].
2.2. Laboratory examination
Blood samples were collected from cubital vein into EDTA-containing tubes for biochemical measurements after overnight fast. Concentrations of Total cholesterol (TC), TG, high density lipoprotein cholesterol (HDL-C) were measured using automatic biochemistry analyzer (Hitachi 7150, Japan) using enzymatic assay.
2.3. Genetic sequencing
The blood samples for DNA extraction were well preserved at −80 °C after centrifugation for 10 min at 3500 rpm, 4 °C. The genomic DNA was isolated form peripheral blood leukocytes using standard extraction protocols. Each DNA sample is quantied by agarose gel electrophoresis and Nanodrop (Thermo). The amplified DNA was captured with a Hypertriglyceridemia Gene Panel using biotinylated oligo-probes (MyGenostics GenCap Enrichment technologies). The probes were designed according to 15 TG-modulating related genes covering the coding exons, intron-exon boundaries and promoters, exons, exon–intron boundaries and 3′/5′ untranslated region (UTR) of in 15 TG-related genes [LPL (NM_000237), LMF1 (NM_000237), APOC2 (NM_000483), GPIHBP1 (NM_178172), GCKR (NM_001486), ANGPTL3 (NM_014495), APOA1 (NM_000039), APOA4 (NM_000482), APOC3 (NM_000040), APOA5 (NM_052968), TRIB1 (NM_001282985), CETP (NM_000078), APOE (NM_000041), and LIPI (NM_198996)]. The capture experiment was conducted according to manufacturer's protocol. In brief, 1 mg DNA library was mixed with Buffer BL and GenCap probe (MyGenostics, Beijing), heated at 95uC for 7 min and 65uC for 2 min on a PCR machine; 23 mL of the 65uC prewarmed Buffer HY (MyGenostics, Beijing) was then added to the mix, and the mixture was hold at 65uC with PCR lid heat on for 22 h for hybridization. 50 mL MyOne beads (Life Technology) was washed in 500 mL 1Xbinding buffer for 3 times and resuspended in 80 mL 1Xbinding buffer. Sixty-four ml 2Xbinding buffer was added to the hybrid mix, and transferred to the tube with 80 mL MyOne beads. The mix was rotated for 1 h on a rotator. The beads were then washed with WB1 buffer at room temperature for 15 min once and WB3 buffer at 65uC for 15 min three times. The bound DNA was then eluted with Buffer Elute. The eluted DNA was finally amplified for 15 cycles using the following program: 98uC for 30 s (1 cycle); 98uC for 25 s, 65uC for 30 s, 72uC for 30 s (15 cycles) and 72uC for 5 min (1 cycle). The PCR product was purified using SPRI beads (Beckman Coulter) according to manufacturer's protocol. The enrichment libraries were sequenced on Illumina Solexa HiSeq 2000 sequencer for paired read 100 bp.
2.4. Bioinformatics analysis
After HiSeq 2000 sequencing, high-quality reads were retrieved from raw reads by filtering out the low quality reads and adaptor sequences using the Solexa QA package and the cutadapt program (http://code.google.com/p/cutadapt/), respectively. The clean read equences were then aligned to the human genome reference sequence (hg19) using SOAP aligner program. The reads to the reference genome using BWA and identified the insertions or deletions (InDels) using the GATK program (http://www.broadinstitute.org/gsa/wiki/index.php/Home_Page). The identified SNPs and InDels were annotated using the Exome-assistant program (http://122.228.158.106/exomeassistant). Magic Viewer was used to view the short read alignment and validate the candidate SNPs and InDels. Annotated variants were first filtered and defined as uncommon variants according to minor allele frequencies (MAF) <1% in the general population of 1KG, Exome Aggregation Consortium (ExAC), Exome Sequencing Project 6500 (ESP6500) and Inhouse databases. PolyPhen-2, Sorting Tolerant From Intolerant (SIFT), Mutation Taster and were used to determine pathogenicity. A variant was determined to be pathogenic or likely pathogenic if above mentioned system or ACMG criteria provided a deleterious prediction.
2.5. SNP selection and risk score
We preliminary selected SNPs which were previously reported to be associated with hypertriglyceridemia and tested the dose effect of the risk alleles (Supplemental Table S1). The risk score was calculated on the 9 SNPs that were significantly associated with the presence of SHTG. Each individual was given different score as 0, 1 and 2 for number of risk alleles. The cumulative number of risk alleles was calculated for each patient.
2.6. Statistical analysis
The values were expressed as the mean ± SD, number (percentage) for the categorical variables. The differences of clinical characteristics between groups were analyzed using Student t-test, Mann–Whitney U test, χ2-tests, or Fisher's exact test where appropriate. A p-value <0·05 was considered statistically significant. The statistical analysis was performed with SPSS version 21·0 software (SPSS Inc., Chicago, IL, USA).
3. Result
3.1. Baseline characters
Data for the 103 patients with SHTG and the 46 control subjects were shown in Table 1. The levels TG and TC were higher and the levels of HDL-C were lower (P < 0·05) in SHTG subjects than in controls. No other significant differences were found between two groups.
Table 1.
Baseline characteristics.
| Variables | Severe HTG n = 103 | Control N = 46 | P value |
|---|---|---|---|
| Age, years | 50·1 ± 10·0 | 52·3 ± 9·8 | 0·191 |
| Sex, n (%) | 79(76·7) | 33(71·7) | 0·517 |
| HT, n (%) | 53(51·5) | 27(58·7) | 0·415 |
| DM, n (%) | 22(19·6) | 9(21·8) | 0·760 |
| CAD, n(%) | 57(55·3) | 30(65·2) | 0·258 |
| BMI, kg/m2 | 25·2 ± 2·9 | 26·3 ± 2·6 | 0·227 |
| Pancreatitis,n (%) | 18(17·5) | 0 | 0·001 |
| Smoke,n (%) | 60(58·3) | 23(50·0) | 0.349 |
| Alcohol,n (%) | 45(37·0) | 17(43·7) | 0.441 |
| TGadmission (mmol/L) | 9·6 ± 3·9 | 1·2 ± 0·3 | <0·001 |
| TC (mmol/L) | 5·97 ± 1·93 | 4·20 ± 0·89 | <0·001 |
| HDL-C (mmol/L) | 0·80 ± 0·22 | 1·14 ± 0·32 | <0·001 |
Data were expressed as mean ± SD or n (%). HT, hypertension; DM: diabetes mellitus; TG, triglyceride; BMI, body mass index; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol.
3.2. Carrier frequencies for rare variants found in the study
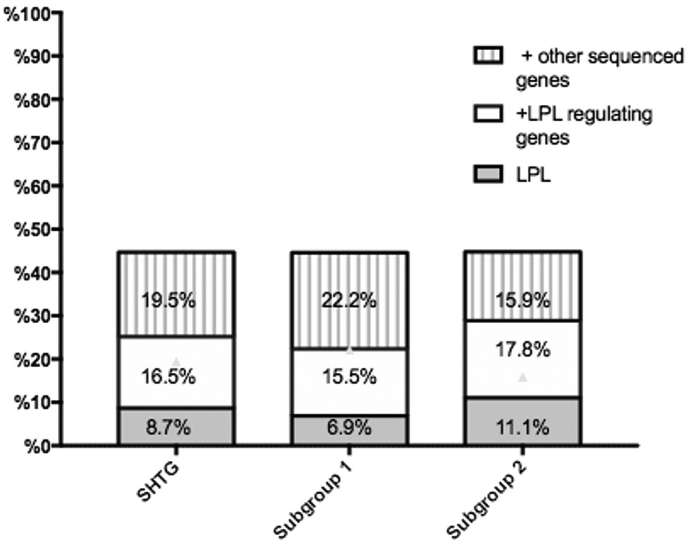
We found 46 patients and 12 controls with rare pathogenic/potentially pathogenic variants (9 in LPL, 7 in APOA5, 13 in LMF1, 4 in GPIHBP1, 10 in GCKR,1 in CETP, 3 in TRIB1, 5 in APOE, 22 in APOB, 3 in APOA4, 2 in APOA1, and 2 in ANGPTL3) after the exclusion of variants that did not produce amino acid substitution (synonymous variants). The details of rare variants were shown in Supplemental Table S2. Types and gene distributions of those rare variants were listed in Table 2. The results indicated that the rare variants in LPL or APOA5 genes were strongly associated with SHTG (8·7% vs. 0% and 6·8% vs. 0%, p < 0·05 respectively). LPL molecular regulating genes cumulatively existed in 25.2% of the SHTG group but did in 8·7% in controls. And also, patients with SHTG had higher frequencies of all the sequenced genes compared to controls. Moreover, it showed significantly higher frequencies of the rare variants in patients with TG ≥ 11·30 mmol/L (subgroup 2). As shown in Fig. 2, the detection rate of the rare variants in SHTG group, subgroup1 and subgroup 2 increased by 19·5%, 22·2%, and 15·9% respectively, which was higher than that in merely sequencing LPL molecular regulating genes analysis.
Table 2.
Carrier frequencies for rare variants found in the study.
| Control n = 46 | Severe HTG n = 103 | Subgroup 1 n = 58 | Subgroup 2 n = 45 | |
|---|---|---|---|---|
| LPL | 0(0) | 9(8·7)⁎ | 4(6·9)+ | 5(11·1)# |
| LMF1 | 2(4·3) | 11(10·7) | 6(10·3) | 5(11·1) |
| GPIHBP1 | 2(4·3) | 2(1·9) | 1(1·7) | 1(2·2) |
| APOA5 | 0(0) | 7(6·8)⁎ | 3(5·2) | 4(8·9)# |
| GCKR | 2(4·3) | 8(7·8) | 4(6·9) | 4(8·9) |
| CETP | 0 | 1(1·0) | 1(1·7) | 0 |
| TRIB1 | 0 | 3(2·9) | 2(3·4) | 1(2·2) |
| APOE | 1(2·2) | 4(3·9) | 1(1·7) | 3(6·7) |
| APOB | 7(15·2) | 15(14·6) | 9(15·5) | 6(13·3) |
| APOA4 | 1(2·2) | 2(1·9) | 1(1.7) | 1(2·2) |
| APOA1 | 0 | 2(1·9) | 0 | 2(4·4) |
| ANGPTL3 | 0 | 2(1·9) | 1(1·7) | 1(2·2) |
| LPL molecular regulating variant | 4(8·7) | 26(25·2)⁎ | 13(22·4) | 13(28·9)# |
| APOA1/C3/A4/A5 variant | 1(2·2) | 10(9·7) | 3(5·2) | 7(15·6) |
| ≥1 variant in all sequenced genes | 12(26·1) | 46(44·7)⁎ | 26(44·8)+ | 20(44·4) |
p < 0.05 for statically significant difference between control group and Severe HTG group.
p < 0.05 for statically significant difference between control group and subgroup 1 (5·65 ≤ TG < 11·30 mmol/L).
p < 0.05 for statically significant difference between control group and subgroup 2(TG ≥ 11·30 mmol/L).
Fig. 2.
Variance of detection rate with different sequenced genes. LPL regulating genes refer to APOA5, APOC2, GPIHBP1 and LMF1.
3.3. Clinical and genetic features in patients with and without acute pancreatitis
When SHTG patients were classified according to previous history of AP, no major clinical differences emerged for gender, TGadmission, BMI, and alcohol consumption. Nevertheless, patients with history of AP showed clinical features as higher levels of maximal TG and higher percentage of family history of HTG. Of noted, genetic features presented higher frequency of the rare variants in LPL gene as well as all the LPL molecular regulating genes (LPL, APOA5, LMF1, GPIHBP1, APOC2, 27·8% vs. 4·7% and 50·0% vs. 20·0%, p < 0·05 respectively, Table 3). Moreover, it had no difference in TG admission in different LPL genetic status (Supplemental Table S3).
Table 3.
Clinical and genetic features in patients with and without acute pancreatitis.
| Variables | With AP |
Without AP |
P value |
|---|---|---|---|
| n = 18 | n = 85 | ||
| Age, years | 45·0 ± 11·4 | 51·3 ± 9·6 | 0·130 |
| Sex, n(%) | 12(66·7) | 66(77·6) | 0·324 |
| TGadmission (mmol/L) | 10·9 ± 3·8 | 9·4 ± 4.0 | 0·147 |
| TGmax (mmol/L) | 16·6 ± 6·7 | 11·3 ± 5·7 | 0·005 |
| Subgroup 1 | 8(44·4) | 50(8·8) | 0·264 |
| Subgroup 2 | 10(55·6) | 35(41·1) | |
| BMI (kg/m2) | 25·4 ± 3·0 | 26.2 ± 2·6 | 0·265 |
| Alcohol, n (%) | |||
| Abstainers | 7(38·9) | 46(55·3) | 0·651 |
| Moderate drinker | 11(61·1) | 39(44·7) | |
| Family history of HTG, n(%) | 9(50·0) | 17(20·0) | 0·018 |
| LPL variation | 5(27·8) | 4(4·7) | 0·007 |
| ≥1 variant in LPL related genes | 9(50·0) | 17(20·0) | 0·018 |
| ≥1 variant in APOA1/C3/A4/A5 gene cluster | 3(16.7) | 7(8.2) | 0.272 |
| ≥1 variant in all sequenced genes | 11(61·1) | 35(41·2) | 0·122 |
TG, triglyceride; HTG: hypertriglyceridemia; BMI, body mass index; AP: acute pancreatitis.
3.4. Associations between genetic risk score with plasma triglyceride levels
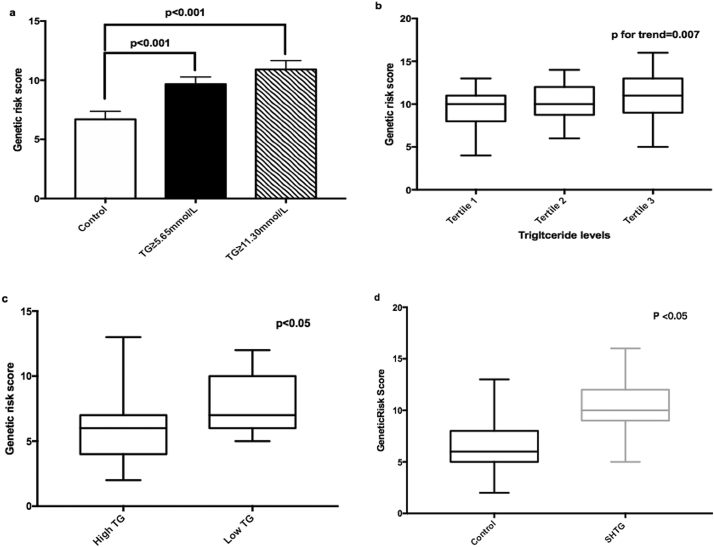
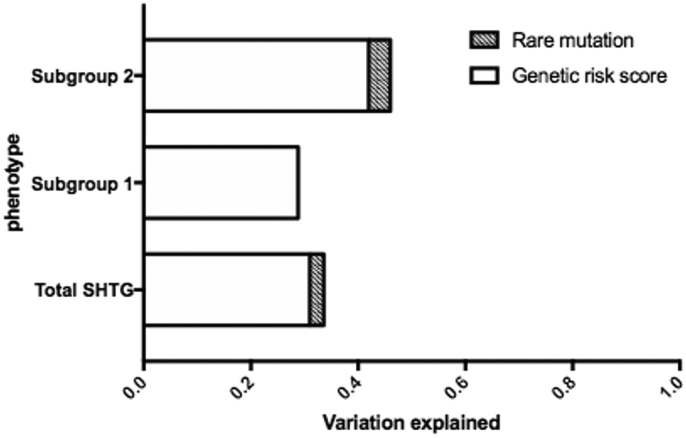
As presented in Fig. 3a, the risk scores for SHTG, determined by common TG-associated variants, were increased in the subgroup 1 and subgroup 2 compared with that in controls (p < 0·05). We also separately evaluated genetic risk scores (GRS) in both control and SHTG groups (Fig. 3b and c). Data suggested that the GRS was significantly increased according to the tertiles of TG in SHTG group (p for trend = 0·013). Patients with relative higher TG levels (classified with the median) had higher GRS than that with normal controls (p < 0·05, Fig. 3c). Patients without rare variants within SHTG group also presented higher GRS than that within control group (p < 0·05 Fig. 3d). Furthermore, as shown in Fig. 4, we compared the variation attributable to clinical and genetic variables in different subgroups. Among SHTG patients, the total proportion of variations explained by the analytical model was 33·6% (common variants explained 31·0% and rare variants explained 2·6%). In subgroup analysis, common and rare variants explained 42·0% and 4·0% of variation in subgroup 2 while only common variants explained 28·8% of variation in subgroup 1.
Fig. 3.
Associations between genetic risk score with plasma triglyceride levels. a. Comparison of genetic risk score between controls and SHTG subgroup(Subgroup 1 and Subgroup 2, the differences between groups were tested by Mann–Whitney U test); b. Genetic risk score according to TG levels within SHTG group (P for trend across tertiles of TG was examined by a generalized linear model); c. Genetic risk score according to TG levels within control group (The differences between groups were tested by Mann–Whitney U test); d. Comparison of genetic risk score between controls and SHTG patients without rare variants (The differences between groups were tested by Mann–Whitney U test).
Fig. 4.
Comparison of variation attributable genetic variables in different severe hypertriglyceridemia subgroups.
4. Discussion
SHTG has become a major challenge in China due to its high prevalence in populations, its complexity in causes, fewer strategies in treatment, and high risk in AP development. Until recently, the most of genetic studies on patients with SHTG mainly focused on LPL molecular regulating genes, namely LPL, APOA5, APOC2, GPHLBP1 and LMF1, which might be in neglect of many other genetic causes [[23], [24], [25], [26]]. In the current study, we performed NGS in 15 reported TG-regulating genes in a cohort of 103 Chinese patients with primary SHTG and 46 age- and sex- matched controls. Overall, we found that pathogenic rare variants were detected in 58 subjects (3·0%). Among these subjects, 46 patients were within SHTG group while 12 individuals were within control cases. The major findings of the present study were: 1) Presence of rare variants in LPL, APOA5, LPL molecular regulating genes and all the sequenced genes were associated with SHTG; 2) Extensive analysis of 15 genes increased the detection rate of the rare variants in SHTG group by 19·5% compared to the analysis of merely sequencing LPL molecular regulating genes; 3) Patients with history of AP presented higher frequency of the rare variants in LPL gene and all the LPL molecular regulating genes; 4) The GRS of some common variants were increased in subgroups according to the extent of SHTG.
It has been demonstrated that SHTG can be induced by both primary or secondary causes. A previous study on 215 Japanese patients with SHTG (TG > 1000 mg/dL) suggested that 74% of them were induced by secondary causes including diabetes, alcohol intake, and drug causes [27]. Before analyzing the genetic basis, therefore, we excluded 39·9% (70 of 173) patients who were clinically considered as secondary causes. Subsequently, for other 103 patients, the 15 genes were sequenced, some of which had been analyzed in previous studies. For example, a study including 110 nondiabetic SHTG patients of European geographic ancestry indicated that 6·4%, 0·9%, and 3·6% of patients had rare variants in LPL, APOA5, and APOC2, respectively [23]. Khovidhunkit and his colleagues reported that in a cohort of Thai patients, 13% vs. 0% and 2% vs. 0% of patients in SHTG (TG > 10 mmol/L) vs. control group (TG < 1·7 mmol/L) had rare variants in LPL and APOA5 while no rare variants in APOC2 was detected [24]. Other studies showed LPL frequency ranging from 1·5% to 36% [19,25,28]. In our study, the detection rate of rare variants in LPL was relatively higher than that in other genes and such variants were found only in SHTG group. However, regarding other genes, rare variants in LMF1, GCKR and APOB accounted for 10·7%, 7·8%, and 14·6% of SHTG subjects in our study, which was similar to those of previous studies.
Recent studies have revealed that other candidate genes except for LPL, APOA5, APOC2, GPHLBP1, and LMF1 are also involved in the manifestation of SHTG in some individuals [20,21]. The association of rare variants in APOB with SHTG has previously been reported in several studies [17]. Gene APOA5 is located on a well-known gene cluster at chromosome 11, namely APOA5/A4/C3/A1, and the accumulating evidence has shown that single nucleotide polymorphisms (SNPs) in the APOA5/A4/C3/A1 gene cluster are associated with high triglyceride levels [19]. Furthermore, in genome-wide association studies, the possible association of polymorphisms in GCKR gene with hypertriglyceridemia has been analyzed [29]. Besides, Santoro et al. has demonstrated that GCKR has a facilitating effect for the manifestation of HTG in young obese individuals [30]. Additionally, TRIB1 is another polygenic determinant of SHTG whose protein facilitates the proteasome dependent protein degradation [31]. Moreover, ANGPTL3 and APOE genotype may also explain additional variation in certain HTG phenotype. In our genetic analysis, detection rate of rare variants in SHTG group, subgroup 1 and subgroup 2 increased by 19·5%, 22·2%, and 15·9%, respectively, which may suggest the necessity for the expanding sequencing.
As is well known, SHTG has many fatal complications among which AP is the most common one. However, the genetic features of patients with SHTG-induced AP is not adequately studied. Recently, the study by Khovidhunkit et al. in Thai individuals reported that among 13 patients who had history of AP, 4 had a heterozygous p.Gly185Cys common variant in APOA5, 3 only had rare variants in LPL (p.Ala98Thr, p.Leu279Val, and p.Asp308Glyfs*3), and the other 6 had no identifiable variants contributing to SHTG [24]. In their study, accurate relation of genetic profile to AP could not identified due to the fact that only limited number of genes were sequenced. Another study performed by Zhu et al. among Chinese patients in emergency department sequenced APOA5, APOC2, APOC3, APOE, BLK, LPL, GPIHBP1, and LMF1 in 11 patients with SHTG and suggested that 6 rare variants in LPL molecular regulating genes (APOA5, GPIHBP1, LMF1) and 1 rare variant in APOE were found in patients with AP. Similarly, their study was also limited by small sample size besides their study had no controls [32]. More importantly, in these studies, the high frequency of LPL molecular regulating genes in SHTG patients was visible but not statistically provable. Interestingly, in our study, patients with history of AP presented statistically higher frequency of rare variants in LPL gene and all the LPL molecular regulating genes (27·8% vs.4·7% and 50·0% vs. 20·0% p < 0·05 respectively), indicating that rare variants in LPL and its molecular regulating genes could increase the risk of AP among Chinese patients with SHTG.
Besides, the present study showed that the risk scores for SHTG determined by common TG-associated variants were increased in subgroups according to the extent of SHTG, which was in coincidence with previous study. The data also indicated that only a small portion of TG variance could be explained by rare variants. Interestingly, it appeared that TG variance in patients with less extent SHTG (5·65 ≤ TG < 11·30 mmol/L) could be explained by only common variants, which might affect the future sequencing strategy.
Although our study might add new information regarding the relation of genetic analysis to SHTG as well as AP, the study also had several limitations. Firstly, the sample size of the present study might be not large enough to reflect the whole patterns of genetic and clinical phenotypes in patients with SHTG. Secondly, the AP history was recognized by patients' oral account and medical records, some patients with mild symptoms might be underdiagnosed. Finally, pathogenic analysis of genes was mainly based on risk prediction software, which might limit its accuracy of the analysis. Hence, a large sample size study and further functional testing may be needed to confirm our findings.
In conclusion, expanding genetic analysis had a higher detection rate of rare variants in patients with SHTG. Detecting rare variants in LPL and its molecular regulating genes might be a tool in estimating risk of AP among Chinese patients with SHTG.
Funding sources
This work was partially supported by the Capital Health Development Fund (201614035) and CAMS Major Collaborative Innovation Project (2016-I2M-1-011) awarded to Dr. Jian-Jun Li, MD, PhD. The study sponsors did not participate in the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the paper for publication.
Conflicts of interest
We declare that we have no conflict of interest.
Authors' contributions
Dr. Jing-Lu Jin completed the project, analyzed the data, and wrote the manuscript. Dr. Jian-Jun Li designed the study, interpreted the data and contributed to critically revising the manuscript. Dr. Di Sun, Dr. Ye Xuan-Cao and Dr. Hui-Wen Zhang contributed to data collection. Drs Wu, Zhu and Guo contributed to recruitment of patients and clinical diagnosis of disease and data collection. Drs Gao and Dong, and Ms. Liu and Dong contributed to the collections of clinical data and procedure of laboratory examination. All authors have approved the final article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.11.001.
Appendix A. Supplementary data
Supplementary material
References
- 1.Miller M., Stone N.J., Ballantyne C. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 2.Fortson M.R., Freedman S.N., Webster P.D. Clinical assessment of hyperlipidemic pancreatitis. Am J Gastroenterol. 1995;90:2134–2139. [PubMed] [Google Scholar]
- 3.Murad M.H., Hazem A., Coto-Yglesias F. The association of hypertriglyceridemia with cardiovascular events and pancreatitis: A systematic review and meta-analysis. BMC Endocr Disord. 2012;12:2. doi: 10.1186/1472-6823-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson T.A., Ito M.K., Maki K.C. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1—full report. J Clin Lipidol. 2015;9:129–169. doi: 10.1016/j.jacl.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Hegele R.A. Plasma lipoproteins: Genetic influences and clinical implications. Nat Rev Genet. 2009;10:109–121. doi: 10.1038/nrg2481. [DOI] [PubMed] [Google Scholar]
- 6.Kissebah A.H., Harrigan P., Wynn V. Mechanism of hypertriglyceridaemia associated with contraceptive steroids. Horm Metab Res. 1973;5:184–190. doi: 10.1055/s-0028-1093969. [DOI] [PubMed] [Google Scholar]
- 7.Haitas B., Disler L.J., Joffe B.I., Seftel H.C. Massive hypertriglyceridemia associated with atenolol. Am J Med. 1988;85:586–587. doi: 10.1016/0002-9343(88)90664-x. [DOI] [PubMed] [Google Scholar]
- 8.Emmerich J., Beg O.U., Peterson J., Previato L., Brunzell J.D., Brewer H.B., Jr. Human lipoprotein lipase. Analysis of the catalytic triad by site-directed mutagenesis of Ser-132, Asp-156, and His-241. J Biol Chem. 1992;267:4161e5. [PubMed] [Google Scholar]
- 9.Rodrigues R., Artieda M., Tejedor D., Martínez A. Pathogenic classification of LPL gene variants reported to be associated with LPL deficiency. J Clin Lipidol. 2016;10:394e409. doi: 10.1016/j.jacl.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Peterfy M. Lipase maturation factor 1: A lipase chaperone involved in lipid metabolism. Biochim Biophys Acta. 1821;2012:790–794. doi: 10.1016/j.bbalip.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies B.S., Beigneux A.P., Barnes R.H., 2nd GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 2010;12:42–52. doi: 10.1016/j.cmet.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsson S.K., Heeren J., Olivecrona G., Merkel M. Apolipoprotein A-V: A potent triglyceride reducer. Atherosclerosis. 2011;219:15–21. doi: 10.1016/j.atherosclerosis.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Santamarina-Fojo S. The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am. 1998;27:551–567. doi: 10.1016/s0889-8529(05)70025-6. [viii] [DOI] [PubMed] [Google Scholar]
- 14.Priore Oliva C., Carubbi F., Schaap F.G., Bertolini S., Calandra S. Hypertriglyceridaemiaand low plasma HDL in a patient with apolipoprotein A-V deficiency due to a novel mutation in the APOA5 gene. J Intern Med. 2008;263:450–458. doi: 10.1111/j.1365-2796.2007.01912.x. [DOI] [PubMed] [Google Scholar]
- 15.Johansen C.T., Hegele R.A. Genetic bases of hypertriglyceridemic phenotypes. Curr Opin Lipidol. 2011;22:247–253. doi: 10.1097/MOL.0b013e3283471972. [DOI] [PubMed] [Google Scholar]
- 16.Chokshi N., Blumenschein S.D., Ahmad Z., Garg A. Genotypephenotype relationships in patients with type I hyperlipoproteinemia. J Clin Lipidol. 2014;8:287–295. doi: 10.1016/j.jacl.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Johansen C.T., Wang J., Lanktree M.B. An increased burden of common and rare lipid-associated risk alleles contributes to the phenotypic spectrum of hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2011;31(8):1916–1926. doi: 10.1161/ATVBAHA.111.226365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talmud P.J., Drenos F., Shah S. Gene-centric association signals for lipids and apolipoproteins identified via the HumanCVD BeadChip. Am J Hum Genet. 2009;85:628–642. doi: 10.1016/j.ajhg.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui G., Li Z., Li R. A functional variant in APOA5/A4/C3/A1 gene cluster contributes to elevated triglycerides and severity of CAD by interfering with microRNA 3201 binding efficiency. J Am Coll Cardiol. 2014;64(3):267–277. doi: 10.1016/j.jacc.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 20.De C.I., Cenarro A., Tejedor M.T. Common variants contribute to hypertriglyceridemia without differences between familial combined hyperlipidemia and isolated hypertriglyceridemia. Circ Cardiovasc Genet. 2014;7(6):814–821. doi: 10.1161/CIRCGENETICS.114.000522. [DOI] [PubMed] [Google Scholar]
- 21.Teslovich T.M., Musunuru K., Smith A.V., Edmondson A.C., Stylianou I.M., Koseki M. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute of Alcohol Abuse and Alcoholism . U.S. Department of Health and Human Services, National Institutes of Health; Washington, DC: 1995. The physicians' guide to helping patients with alcohol problems. [Google Scholar]
- 23.Surendran R.P., Visser M.E., Heemelaar S. Mutations in LPL, APOC2, APOA5, GPIHBP1 and LMF1 in patients with severe hypertriglyceridaemia. J Intern Med. 2012;272(2):185–196. doi: 10.1111/j.1365-2796.2012.02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khovidhunkit W., Charoen S., Kiateprungvej A. Rare and common variants in LPL and APOA5 in Thai subjects with severe hypertriglyceridemia: A resequencing approach. J Clin Lipidol. 2016;10(3):505–511. doi: 10.1016/j.jacl.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Wang J., Cao H., Ban M.R. Resequencing genomic DNA of patients with severe hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2007;27(11):2450–2455. doi: 10.1161/ATVBAHA.107.150680. [DOI] [PubMed] [Google Scholar]
- 26.De C.I., Civeira F., Pueyo M.J. Rare genetic variants with large effect on triglycerides in subjects with a clinical diagnosis of familial vs nonfamilial hypertriglyceridemia. J Clin Lipidol. 2016;10(4):790–797. doi: 10.1016/j.jacl.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Tada H., Kawashiri M.A., Nakahashi T. Clinical characteristics of Japanese patients with severe hypertriglyceridemia. J Clin Lipidol. 2015;9(4):519–524. doi: 10.1016/j.jacl.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Wright W.T., Young I.S., Nicholls D.P., Graham C.A. Genetic screening of the LPL gene in hypertriglyceridaemic patients. Atherosclerosis. 2008;199:187–192. doi: 10.1016/j.atherosclerosis.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 29.Willer C.J., Sanna S., Jackson A.U. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40(2):161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santoro N., Zhang C.K., Zhao H., Pakstis A.J. Variant in the glucokinase regulatory protein (GCKR) gene is associated with fatty liver in obese children and adolescents. Hepatology. 2018;55(3):781–789. doi: 10.1002/hep.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tai E.S., Sim X.L., Ong T.H., Wong T.Y. Polymorphisms at newly identified lipid-associated loci are associated with blood lipids and cardiovascular disease in an Asian Malay population. J Lipid Res. 2009;50(3):514–520. doi: 10.1194/jlr.M800456-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Chen W.J., Sun X.F., Zhang R.X. hypertriglyceridemic acute pancreatitis in an emergency department: The typical clinical features and genetic variants. J Dig Dis. 2017;18(6):359–368. doi: 10.1111/1751-2980.12490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material