Abstract
Context
The mechanisms underpinning intrauterine growth restriction (IUGR), as a result of placental insufficiency, remain poorly understood, no specific treatment is available, and clinically useful biomarkers for early detection are lacking.
Objective
We hypothesized that human IUGR is associated with inhibition of mechanistic target of rapamycin (mTOR) and activation of amino acid response (AAR) signaling, increased protein kinase casein kinase-2 (CK2) activity, and increased insulin-like growth factor-binding protein 1 (IGFBP-1) expression and phosphorylation in decidua and that maternal plasma IGFBP-1 hyperphosphorylation in the first trimester predicts later development of IUGR.
Design, Setting, and Participants
Decidua [n = 16 appropriate-for-gestational age (AGA); n = 16 IUGR] and maternal plasma (n = 13 AGA; n = 13 IUGR) were collected at delivery from two different cohorts. In addition, maternal plasma was obtained in the late first trimester from a third cohort of women (n = 7) who later delivered an AGA or IUGR infant.
Main Outcome Measures
Total IGFBP-1 expression and phosphorylation (Ser101/Ser119/Ser169), mTOR, AAR, and CK2 activity in decidua and IGFBP-1 concentration and phosphorylation in maternal plasma.
Results
We show that decidual IGFBP-1 expression and phosphorylation are increased, mTOR is markedly inhibited, and AAR and CK2 are activated in IUGR. Moreover, IGFBP-1 hyperphosphorylation in first-trimester maternal plasma is associated with the development of IUGR.
Conclusions
These data are consistent with the possibility that the decidua functions as a nutrient sensor linking limited oxygen and nutrient availability to increased IGFBP-1 phosphorylation, possibly mediated by mTOR and AAR signaling. IGFBP-1 hyperphosphorylation in first-trimester maternal plasma may serve as a predictive IUGR biomarker, allowing early intervention.
IUGR is associated with inhibition of mTOR, activation of AAR and CK2, and IGFBP-1 hyperphosphorylation in the decidua and increased IGFBP-1 phosphorylation in first-trimester maternal plasma.
Intrauterine growth restriction (IUGR) is a common pregnancy complication that increases the risk for perinatal complications (1) and predisposes to metabolic and cardiovascular disease later in life (2–4). A lack of normal increase in utero-placental blood flow, as a result of inadequate trophoblast invasion, resulting in “placental insufficiency,” is believed to be the most common cause of IUGR (5) in developed countries (6). However, the molecular mechanisms underpinning the development of IUGR remain poorly understood, no specific treatment is available, and clinically useful biomarkers for early detection are lacking.
Insulin-like growth factor (IGF)-binding protein 1 (IGFBP-1) is the predominant IGFBP that determines IGF-I bioavailability during pregnancy (7). Phosphorylation of IGFBP-1 at three serine residues (Ser101, Ser119, and Ser169) is known to increase markedly its affinity for binding IGF-I, thus affecting the ability of IGF-I to interact with the IGF receptor (8). In the fetus, the liver is the primary source of circulating IGFBP-1 (9). Human IUGR is associated with increased IGFBP-1 levels and IGFBP-1 hyperphosphorylation in umbilical cord plasma (10, 11). Placental insufficiency is believed to result in decreased fetal oxygen and nutrient availability, ultimately leading to IUGR.
Based on our previous studies in cultured HepG2 cells and primary fetal baboon hepatocytes, as well as liver tissue from IUGR fetal baboons, we have identified inhibition of mechanistic target of rapamycin (mTOR) signaling and activation of the amino acid response (AAR) pathway as the molecular mechanisms linking hypoxia and decreased amino availability to increased fetal liver IGFBP-1 secretion and phosphorylation (11–13). Moreover, we demonstrated that casein kinase-2 (CK2) constitutes the key kinase, regulated by mTOR and AAR, responsible for IGFBP-1 serine phosphorylation (11, 14, 15).
The decidua synthesizes and secretes IGFBP-1 locally in the maternal-fetal interface, where it regulates the actions of IGF-I, which is known to promote trophoblast invasion and placental function (16). The decidua is also believed to be the primary source of IGFBP-1 in the maternal circulation (17–19) in pregnancy, thereby regulating the bioavailability of maternal IGF-I, a well-established, positive regulator of placental function and growth (20, 21). A lack of normal increase in utero-placental blood flow in IUGR may limit oxygen and nutrient delivery to the decidua (5). With the use of cultured human immortalized endometrial stromal cells (HIESCs), decidualized in vitro, we recently demonstrated that hypoxia and leucine deprivation markedly increased IGFBP-1 phosphorylation and decreased IGF-I bioactivity (22). These observations are consistent with the possibility that IGFBP-1 phosphorylation constitutes a link between decreased decidual oxygen and nutrient availability and reduced fetal growth, mediated by diminished IGF-I bioavailability, resulting in inhibition of trophoblast invasion and placental function.
The molecular mechanisms regulating decidual IGFBP-1 secretion and phosphorylation are largely unknown, and it remains to be established if IGFBP-1 in the maternal circulation shows increased phosphorylation in IUGR. Prenatal identification of babies who are IUGR significantly improves their perinatal outcomes compared with diagnosis at birth (23). In light of these findings, the identification of biomarkers for IUGR in early pregnancy could improve the clinical management of these patients by allowing early intervention, preventing some of the perinatal complications associated with this condition (24, 25). However, recent systematic reviews and meta-analyses suggest that none of the currently available biomarkers predict IUGR with sufficient sensitivity to be used in routine clinical practice (26–28).
In the current study, we tested the hypothesis that human IUGR is associated with inhibition of mTOR, activation of AAR signaling, increased CK2 activity, and increased IGFBP-1 levels and phosphorylation in decidua and that maternal plasma IGFBP-1 hyperphosphorylation in the first trimester predicts later development of IUGR. To address this hypothesis, in this observational study at an academic center, we obtained decidua and maternal plasma at delivery from two different cohorts and maternal plasma in the late first trimester from a third cohort of women who later delivered an appropriate-for-gestational age (AGA)/IUGR infant.
Materials and Methods
Study subjects
Pregnant women were recruited at the London Health Sciences Centre (LHSC; London ON, Canada) after obtaining signed, informed consent under the approval of the Western University Research Ethics Board (#518). Women delivering a singleton AGA infant after an uneventful pregnancy or an IUGR infant by vaginal or caesarean section were included for collection of maternal plasma and placenta at delivery from two separate cohorts. The Neonatal Intensive Care Unit database at LHSC was used to obtain infant and maternal data. Exclusion criteria included pregnancies complicated by smoking, drug abuse, malnutrition, diabetes, thyroid disorders, chronic hypertensive disorders, pre-eclampsia, premature rupture of membranes, placental abruption, fetal congenital and genetic abnormalities, and chorioamnionitis (29, 30).
IUGR was defined as a birth weight less than the fifth percentile using sex-specific growth charts (31). All IUGR cases included in the study, except for one (fifth percentile), were less than the third percentile. Given the conservative birth weight cut-off for inclusion in the IUGR groups and because many of these fetuses had abnormal uterine or umbilical Doppler, the number of constitutionally small infants was likely to be low. Control subjects were pregnant women free of obstetrical complications and delivering an infant with a birth weight AGA between the 10th and 90th percentiles for corresponding gestational age (GA), using sex-specific growth charts. GA was determined by the last menstrual period and confirmed by first-trimester ultrasound crown–rump length.
Maternal plasma
Maternal blood samples were collected in EDTA-coated tubes at the time of delivery. Blood was centrifuged at 3500 g for 15 minutes at 4°C, and the supernatant containing plasma was saved for subsequent analysis at −80°C. In addition, maternal plasma samples were obtained in the first trimester from a group of women in the Baby Blanket Cohort, a biorepository of 1128 women who presented for prenatal care at or before 23 weeks’ gestation and planned to deliver their baby at the University of Colorado Hospital (Aurora, CO) between 2011 and 2013 (32). This prospective cohort study was approved by the Colorado Multiple Institutional Review Board. Plasma samples were aliquoted and stored at −80°C in a Biorepository at the University of Colorado Hospital. Details of the cohort have been previously described (33). We selected four women from this cohort who delivered an IUGR infant (birth weight less than or equal to the fourth percentile) at term (≥37 + 0 weeks of gestation) without other pregnancy complications and from which a maternal blood sample collected at 7 to 9 weeks of gestation was available. Subsequently, we selected study subjects (n = 3) who delivered closest in time (before or after) to the IUGR case that met the following criteria: AGA (birth weight 10th to 90th percentile), term uncomplicated delivery, same infant sex, and an available maternal blood sample collected at 7 to 9 weeks of gestation.
Tissue collection
Placentas were collected at LHSC immediately after delivery. Based on a review of the pathological reports (undertaken at LHSC), all of the placentas were negative for signs of infection and also contained no major infarcts. The decidua was identified as the superficial layer on the maternal-facing side of the placenta. The decidual samples (2 to 3 g) from AGA and IUGR (n = 16 each) were excised from intact placenta, snap frozen in liquid nitrogen, and kept in −80°C for long-term storage. Dissected frozen tissues pieces of the decidua (∼0.2 g each) were homogenized at 4°C in lysis buffer containing protease and phosphatase inhibitors (Cell Signaling Technology, Danvers, MA; Sigma-Aldrich, St. Louis, MO). Subsequently, the homogenates were centrifuged, and the clear supernatant was stored at −80°C. Protein content in the plasma and supernatants from placental extracts was measured by the Bradford reagent (BioRad Laboratories, Burlington, ON, Canada) using BSA as standard. Tissue extracts were frozen in liquid nitrogen and stored at −80°C.
Preparation of tissue sections
Tissue sections from placentas collected at LHSC were prepared for immunofluorescence and proximity ligation assay (PLA). Full-thickness blocks were obtained from placentas collected immediately after delivery. All tissues were sectioned serially (5 µm; Biotron Integrated Microscopy Laboratory, University of Western Ontario, London, ON, Canada) and mounted on pretreated slides (Superfrost Plus; Fisher Scientific, Fairlawn, NJ). The selection of regions within each block of the placenta was based on the criteria that ensured incorporation of all levels/regions from the chorionic plate to basal plate. GA-matched AGA and IUGR placenta tissues were placed side by side on a slide. A stratified random sampling procedure was used. Tissue on the maternal-facing side of the placenta was selected to ensure the inclusion of basal plate decidual (BPD) tissue, as confirmed based on morphology assessed by hemotoxylin and eosin staining.
Western blotting
Western blotting was used to determine total and phosphorylated IGFBP-1 in decidua and plasma samples. Equal aliquots of decidual tissue extracts (50 µg total protein) or plasma (10 µL) were run on SDS-PAGE, and the proteins were transferred to the nitrocellulose membrane by electrophoresis. The membranes were blocked with 5% BSA or 5% nonfat skim milk for 1 hour at room temperature. For total IGFBP-1, the membranes were probed with anti-human IGFBP-1 monoclonal antibody (mAb; 6303; 1:10,000; Medix Biochemica, Kauniainen, Finland). For phosphorylated IGFBP-1, custom-made polyclonal antibodies (anti-phospho-IGFBP-1 Ser101, Ser119, and Ser169; 1:1000; generated at YenZyme Antibodies LLC, San Francisco, CA), which we have extensively validated (8, 11), were used.
The activity of mTOR complex 1 and 2 (mTORC1 and mTORC2) was determined using Western blot and phospho-4E-binding protein 1 [4EBP1; (Thr70)] and -protein kinase B [Akt; (Ser473)] antibodies, respectively. The blots were stripped and reprobed using total 4EBP1 and total Akt, followed by stripping and reprobing using β-actin (1:3000) for normalization. Likewise, the activity of AAR signaling was assessed using antibodies targeting phospho-eukaryotic translation initiation factor 2A [eIF2α; (Ser51)] and -general control nonderepressible 2 [GCN2; (Thr898)] and activating transcription factor 4 (ATF4), followed by stripping and reprobing with total eIF2α, total GCN2, and finally, β-actin to control for uneven loading and transfer. The corresponding bands were detected using the Clarity Western ECL Chemiluminescence substrate reagent, and the images were captured using the Quantity One-VersaDoc (BioRad Laboratories) imaging system; the band intensities were determined using densitometry and Image Laboratory (Beta 3) software (BioRad Laboratories). The AGA group was arbitrarily assigned a value of 100 to facilitate comparisons between groups. Primary antibodies were all from Cell Signaling Technology and used at a dilution of 1:1000. Peroxidase-labeled goat anti-mouse or goat anti-rabbit was used as secondary antibodies (1:10,000; BioRad Laboratories).
Determination of CK2 activity
The CK2 substrate peptide RRRDDDSDDD (100 μM) was used to measure CK2 activity, as described previously (14, 15, 22, 34).
Dual immunofluorescence
Fixed sections of placental tissues from GA-matched IUGR and AGA pregnancies on the same slides were deparaffinized in xylene, rehydrated through a graded ethanol series, and then washed three times in PBS. Tissue samples were then encircled with a hydrophobic pen (Dako, Agilent Technologies, Santa Clara, CA), and all subsequent incubations were done in a humidified chamber at room temperature. The tissues were treated using Biocare Background Sniper (Biocare Medical, Concord, CA) for 10 minutes to reduce nonspecific binding. After a PBS wash, the tissues were incubated with paired primary antibodies (mAb IGFBP-1 6303 + rabbit polyclonal CK2β). The dilutions for the primary antibodies were 1:2500 for IGFBP-1 and 1:1000 for CK2β. The tissues were then washed 3 × 5 minutes each with PBS. Combinations of secondary antibodies, anti-rabbit Alexa 568 (pseudo red, IGFBP-1), and anti-mouse Alexa 660 (pseudo green, CK2, 1:400) were added to the tissues for 1 hour and subsequently washed in PBS (3 × 5 minutes). The tissues were then counterstained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; 1:200 dilution; Life Technologies, Burlington, ON, Canada) for 2 minutes. Subsequently, coverslips were mounted onto the microscope slides (Fisher Scientific) with 60 µL Prolong Diamond Mounting Media (Life Technologies) and dried overnight before imaging. The intensity of fluorescence was measured by Image-Pro Premier software (Media Cybernetics, Rockville, MD).
PLA
Fixed sections of placental tissues from GA-matched IUGR and AGA pregnancies on the same slide were used. After 3 × 5 minutes of PBS washes, tissues were treated with Duolink blocking solution (Sigma-Aldrich) for 30 minutes at 37°C and then probed with a specific combination of primary antibodies for 1 hour at room temperature [mouse IGFBP-1 mAb 6303 (1:2500) and rabbit polyclonal CK2β (1:1000)]. The PLA reaction was performed using PLA secondary probes diluted 1:5 in Duolink antibody diluent (Sigma-Aldrich) and incubated for 1 hour. The PLA secondary probes used were Duolink® PLA® Anti-Rabbit Plus and Anti-Mouse Minus. Subsequent ligation and amplification were performed using reagents and the manufacturer’s instructions and as we described in detail earlier (15). Coverslips were mounted (Sigma-Aldrich) in Duolink mounting media containing DAPI counterstain.
Image standardization and acquisition
Images from dual-stained placenta sections or PLA were captured using an AxioImager Z1 Epifluorescent Microscope and Zen Pro software (Carl Zeiss Canada Ltd., Toronto, ON, Canada). The fluorescent lamp was preheated for 15 minutes before imaging to stabilize lamp intensity. Camera settings were then adjusted to avoid saturation and autofluorescent background and consistently applied at the same settings for all imaging. A constant exposure time was selected using a reference tissue field while checking for fluorescence saturation to avoid background immunofluorescence. After we fixed all acquisition parameters, we then captured representative images that allowed coverage of large areas for each placenta, which subsequently provided an adequately large number of cells/image to represent our results and minimize bias. At 20× magnification, regions were randomly selected per slide for each sample. For PLA imaging, z-stack images were captured and converted to extended depth-of-focus tagged image file format images to allow visualization of all PLA spots. We visually compared the relative dual immunofluorescence and PLA signals in AGA and IUGR placental tissues.
Immunoprecipitation
IGFBP-1 from maternal plasma from IUGR and AGA pregnancies (50 to 100 µL) was enriched using immunoprecipitation (IP) with mAb 6303. After elution using 6× sample buffer containing dithiothreitol, the samples were tested on a one-dimensional immunoblot using either anti-human IGFBP-1 polyclonal antibody or anti-phospho-IGFBP-1 Ser101, Ser119 and Ser169 antibodies. After IP, the samples were washed and either analyzed by Western blot or digested in solution, as described below for multiple reaction monitoring-mass spectrometry (MRM-MS) analysis.
MRM-MS analyses of IGFBP-1 phosphopeptides in maternal plasma
In-solution digestion of the IP samples of IGFBP-1 from maternal plasma at delivery from IUGR and GA-matched AGA were performed, as previously described (13). In brief, the protein sample was first digested using endoproteinase Asp-N incubated overnight at 37°C (Roche Diagnostics, Laval, QC, Canada) and followed by a digestion using trypsin (Roche Diagnostics) overnight at 37°C following the manufacturer’s instruction. IGFBP-1 digests were subsequently analyzed by liquid chromatography-tandem mass spectrometry with a triple-quadruple mass spectrometer (4000 QTrap; AB Sciex, Concord, ON, Canada) using methods that we previously optimized for the detection of site-specific IGFBP-1 phosphorylation (13, 14). A NanoAcquity Ultra Performance Liquid Chromatography system (Waters, Milford, MA), equipped with a C18 analytical column (1.7 µm, 75 µm × 200 mm), was used to separate the peptides at a flow rate of 300 nL/min and operating pressure of 8000 psi. Digested IGFBP-1 peptides were electrosprayed into the mass spectrometer, which monitored 98 transitions per sample with a dwelling time of 50 ms/transition. Relative changes in IGFBP-1 phosphorylation were determined by the total peak height of combined transitions. An internal IGFBP-1 peptide (NH2-ALPGEQQPLHALTR-COOH) was used to normalize all phospho-IGFBP-1 data.
Data presentation and statistics
Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA). Data are presented as means ± SEM. Unpaired t test was used to compare IUGR with AGA, and P < 0.05 was considered significant.
Results
Characteristics of study subjects
Clinical information for study subjects (n = 16 each for AGA and IUGR) who donated decidual tissues at delivery is provided in Table 1. Maternal age, maternal height, and GA did not differ between the two groups. By design, birth weights of IUGR infants were 30% (P = 0.023) lower than birth weights of AGA infants. The placental weights were also significantly decreased (−34%, P = 0.001) in the IUGR group compared with AGA. Blood gas analyses showed a significant reduction in umbilical artery Po2 in the IUGR group, consistent with fetal hypoxemia, as a result of placental insufficiency.
Table 1.
Clinical Characteristics of Study Subjects (Decidual Tissues at Delivery)
| AGA | IUGR | P | |
|---|---|---|---|
| Subjects, n | 16 | 16 | |
| Maternal age, y | 29.8 ± 1.5 | 25.7 ± 1.5 | ns |
| Maternal height, cm | 163.9 ± 2.1 | 158.7 ± 2.0 | ns |
| GA, wk | 35.6 ± 0. 8 | 33.8 ± 1.1 | ns |
| Delivery mode, vaginal/C-section | 13/3 | 6/10 | |
| Placental weight, g | 571 ± 47 | 381 ± 23 | 0.0011 |
| Fetal sex | |||
| Male | 10 | 11 | |
| Female | 6 | 5 | |
| Birth weight, g | 2468 ± 28 | 1738 ± 13 | 0.0230 |
| Birth weight percentile, % | 48.00 ± 6.60 | 1.75 ± 0.23 | 0.0001 |
| Apgar score, 5 min | 8.68 ± 1.60 | 8.56 ± 0.85 | ns |
| Umbilical blood gases | |||
| Arterial | |||
| Po2, Torr | 19.8 ± 2.10 | 13.3 ± 0.81 | 0.0370 |
| Pco2, Torr | 49.0 ± 2.0 | 53.8 ± 1.7 | ns |
| Venous | |||
| Po2, Torr | 30.2 ± 2.6 | 24.7 ± 1.4 | ns |
| Pco2, Torr | 41.9 ± 1.9 | 43.2 ± 1.2 | ns |
Means ± SEM. Unpaired t test, statistical significance at P < 0.0.
Abbreviations: C-section, caesarean section; ns, not significant.
Selected clinical data for participants in the AGA and IUGR groups (n = 13 each) who had blood sampling at delivery are presented in Table 2. As was the case for the decidual sampling, maternal age, height, and GA did not differ between IUGR and AGA groups. Birth weights (−38%, P = 0.001) and placental weights (−32%, P = 0.01) were significantly lower in the IUGR compared with the AGA group. Both umbilical arterial and venous Po2 were significantly lower in the IUGR group, indicating fetal hypoxemia. Moreover, umbilical venous Pco2 was significantly elevated in the IUGR compared with the AGA group.
Table 2.
Clinical Characteristic of Study Subjects (Plasma Samples at Delivery)
| AGA | IUGR | P | |
|---|---|---|---|
| Subjects, n | 13 | 13 | |
| Maternal age, y | 26.7 ± 1.3 | 28.7 ± 1.6 | ns |
| Maternal height, cm | 166.2 ± 2.0 | 160.2 ± 2.2 | ns |
| GA, wk | 35.3 ± 1.3 | 34.3 ± 1.4 | ns |
| Delivery mode, vaginal/C-section | 11/2 | 5/8 | |
| Placental weight, g | 547 ± 43 | 371 ± 40 | 0.0100 |
| Fetal sex | |||
| Male | 6 | 8 | |
| Female | 7 | 5 | |
| Birth weight, g | 2651 ± 21 | 1549 ± 21 | 0.0011 |
| Birth weight percentile, % | 44.50 ± 7.8 | 1.85 ± 0.4 | 0.0001 |
| Apgar score, 5 min | 9.00 ± 0.00 | 8.30 ± 0.36 | 0.0540 |
| Umbilical blood gases | |||
| Arterial | |||
| Po2, Torr | 16.8 ± 1.1 | 14.1 ± 1.8 | 0.0168 |
| Pco2, Torr | 52.1 ± 1.4 | 53.2 ± 1.9 | ns |
| Venous | |||
| Po2, Torr | 29.0 ± 1.6 | 22.4 ± 1.9 | 0.0037 |
| Pco2, Torr | 39.5 ± 1.1 | 45.0 ± 1.9 | 0.0004 |
Means ± SEM. Unpaired t test, statistical significance at P < 0.05.
In addition, clinical data for women in the Baby Blanket Cohort, who delivered an IUGR or AGA baby at term and from which first-trimester plasma samples were used, are presented in Table 3. All IUGR infants in this sample had a birth weight less than or equal to the fourth percentile and had significantly lower ponderal indices than the AGA controls (P = 0.0013), strongly suggesting asymmetric IUGR associated with placental insufficiency.
Table 3.
Clinical Characteristic of Study Subjects (Plasma Samples in the First Trimester)
| AGA | IUGR | P | |
|---|---|---|---|
| Subjects, n | 3 | 4 | |
| Maternal age, y | 28.7 ± 1.5 | 28.0 ± 5.7 | ns |
| Maternal height, cm | 162.6 ± 4.1 | 157.5 ± 2.1 | ns |
| GA at delivery, wk | 39.0 ± 0.35 | 38.4 ± 0.82 | ns |
| GA at sampling, d | 58.0 ± 9.6 | 59.5 ± 3.7 | ns |
| Delivery mode, vaginal/C-section | 1/2 | 4/0 | |
| Ponderal index | 3.05 ± 0.05 | 2.20 ± 0.27 | 0.0013 |
| Fetal sex | |||
| Male | 3 | 4 | |
| Female | 0 | 0 | |
| Birth weight, g | 3460 ± 20 | 2300 ± 81 | 0.0001 |
| Birth weight percentile, % | 67.4 ± 22.0 | 2.50 ± 1.3 | 0.0070 |
| Apgar score, 5 min | 9.34 ± 0.578 | 8.25 ± 1.50 | ns |
Means ± SEM. Unpaired t test, statistical significance at P < 0.05.
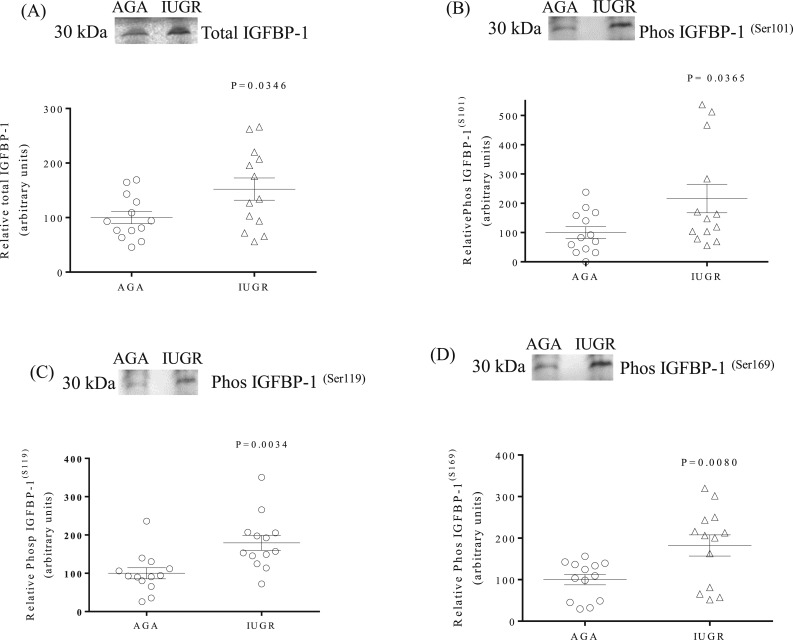
Increased expression and phosphorylation of IGFBP-1 in IUGR decidual tissues
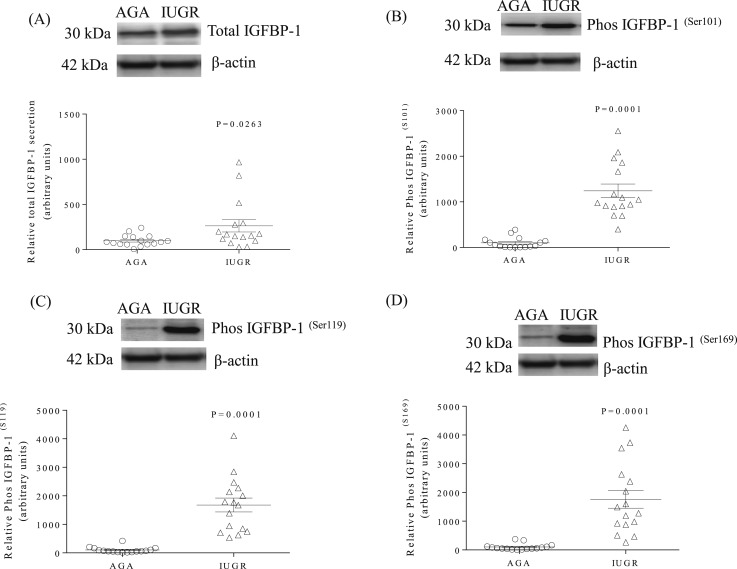
Western blots were used to determine the protein expression of total IGFBP-1 and the phosphorylation of IGFBP-1 at three specific serine sites (Ser101, Ser119, and Ser169). Figure 1(A) demonstrates that total IGFBP-1 expression was higher (+164%, P = 0.026) in IUGR than in AGA decidua. With the use of custom-made, site-specific IGFBP-1 antibodies, we show a marked increase in the phosphorylation of Ser101 [+1142%, P = 0.0001; Fig. 1(B)], Ser119 [+1579%, P = 0.0001; Fig. 1(C)], and Ser169 [+1661%, P = 0.0001; Fig. 1(D)] in IUGR compared with AGA decidual samples.
Figure 1.
IGFBP-1 expression and phosphorylation are increased in IUGR decidual tissue. (A) A representative Western blot of IGFBP-1 expression in decidual tissue from IUGR and AGA groups. (B–D) Representative Western blots of phosphorylated IGFBP-1 at Ser101, Ser119, and Ser169 sites in decidual tissue from AGA and IUGR groups. β-Actin is the loading control. The scatter plots summarize the Western blot data. Values are given as means ± SEM; n = 16 in each group; P < 0.05 vs control; unpaired Student t test.
Inhibition of decidual mTORC1 and mTORC2 signaling in IUGR
We determined the activity mTORC1 and mTORC2 signaling pathways using phosphorylated mTORC1 and mTORC2 targets as well-established functional readouts. Phosphorylation of 4EBP1 at Thr70 (mTORC1 readout) was significantly reduced in IUGR (−85%, P = 0.0001) vs AGA samples. Moreover, phosphorylation of Akt at Ser473 (mTORC2 readout) was also significantly reduced in IUGR (−62%, P = 0.006) vs AGA samples.
Activation of decidual AAR signaling in IUGR
Western blot was used to explore the activity of the decidual AAR pathway in IUGR samples by measuring peIF2α (phosphorylation at Ser51), GCN2 phosphorylation at Thr898, and the expression of ATF4 as functional readouts of the AAR pathway. Phosphorylation of eIF2α was markedly increased in IUGR decidua (+202%, P = 0.0001) vs AGA samples. Likewise, the phosphorylation of GCN2 was increased in IUGR decidua (+210%, P = 0.0001). Total ATF4 expression was also significantly higher in IUGR (+211%, P = 0.0001) compared with AGA decidua.
Increased decidual CK2 expression and activity in IUGR
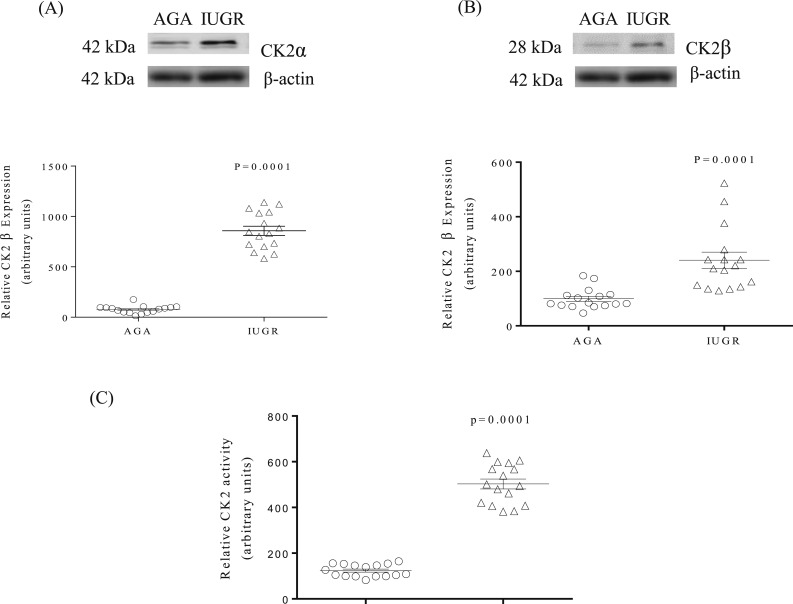
Western blot was used to determine protein expression of CK2α and -β subunits in IUGR and AGA decidua. The data greater in IUGR (+141%, P = 0.001) vs AGA decidua. Furthermore, endogenous CK2 activity, determined in the same decidua extracts collected from AGA and IUGR pregnancies using the CK2 substrate peptide RRRDDDSDDD (35), demonstrated that IUGR was associated with a markedly increased decidual CK2 activity in IUGR (+379%, P = 0.0001) vs AGA samples [Fig. 2(C)].
Figure 2.
CK2 expression and activity are increased in IUGR decidual tissues. (A) and (B) Representative Western blots of CK2α, and CK2β expression in decidual tissue of AGA and IUGR groups. β-Actin is the loading control. The scatter plots summarize the Western blot data. (C) Representative CK2 activity in decidual tissue of AGA and IUGR groups. The kinase activity assays were performed in triplicates using equal protein of whole decidua tissue from AGA and IUGR groups. The scatterplot summarizes the CK2 activity data. Values are given as means ± SEM; n = 16 in each group; P < 0.05 vs control; unpaired Student t test.
Cellular colocalization of CK2 with IGFBP-1 in IUGR decidual tissues
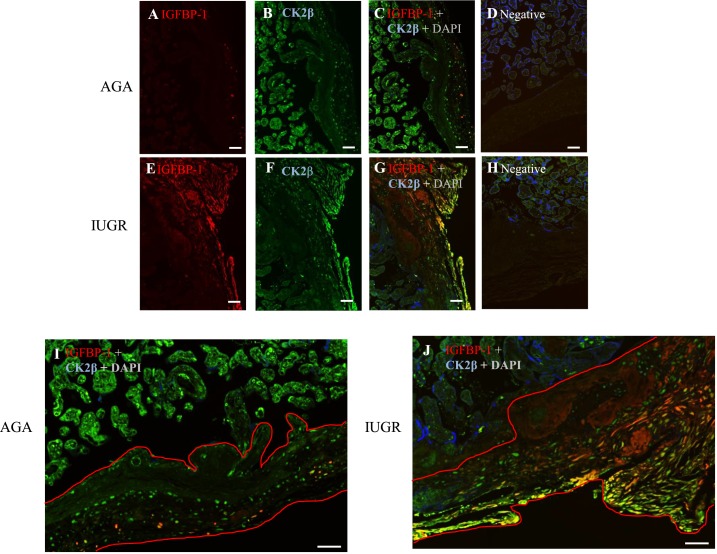
To provide support for a link between an increase in CK2 expression and IGFBP-1 hyperphosphorylation, we performed confocal microscopy with dual immunofluorescence images of placental tissue using IGFBP-1 mAb 6303 (pseudo red) and rabbit polyclonal CK2β (pseudo green) antibodies [Fig. 3(A)–3(H)]. We observed that IGFBP-1 was predominantly present in the decidual region [right side; Fig. 3(A) and 3(E)], whereas CK2 appeared to be widely expressed also in cells outside the decidua, including the trophoblast and endothelial cells of chorionic villi [left side of the image; Fig. 3(B) and 3(F)]. Importantly, dual staining shows a clear, yellow overlap mainly in IUGR [Fig. 3(G)], suggesting that IGFBP-1 and CK2β were colocalized [Fig. 3(C) and 3(G)]. Negative control in the absence of primary antibodies depicted no nonspecific staining [Fig. 3(D) and 3(H)]. High-resolution scans of the decidual region [Fig. 3(I) and 3(J)] showed strong colocalization of IGFBP-1 and CK2β observed by a clear, yellow overlap, mainly in IUGR within the decidual region compared with AGA samples, as depicted in [Fig. 3(C) and 3(G)]. This finding is consistent with immunoblotting using decidual homogenates, demonstrating both increased IGFBP-1 [Fig 1(A)] and CK2 expression [Fig. 2(A) and 2(B)] in IUGR tissue, providing further evidence that CK2 can phosphorylate decidual IGFBP-1.
Figure 3.
Decidual IGFBP-1 and CK2 colocalization is increased in IUGR, as demonstrated by immunofluorescence. (A–D) Representative images of fixed placental tissue from AGA [(A–D)] vs IUGR [(E–H)] placenta, stained with total IGFBP-1 [Alexa 568, red (A) and (E)], CK2β [Alexa 660, green (B) and (F)], and DAPI [blue (D) and (H)]. A full-thickness placenta from AGA with total IGFBP-1 shows some red signal in the decidual region (right), whereas in (E), from the GA-matched IUGR group, the red signal is strong in the decidua (right side of tissue). (B) Full-thickness placenta from AGA with CK2β channels shows the green signal in chorionic villous (to left) and decidual regions (to right); (F) from the GA-matched IUGR group. (C) and (G) Multichannel images show colocalized signal in yellow-orange, mainly in decidual regions (right) with a stronger signal in IUGR (original scale bars, 20 µM). (I) and (J) Zoomed and cropped images showing chorionic villous (upper) and decidual regions (lower and right), shown in red outlines in maternal decidua (original scale bars, 2000 µM). (D–H) Matching no primary antibody negative controls.
Proximity of IGFBP-1 and CK2β proteins in IUGR decidua
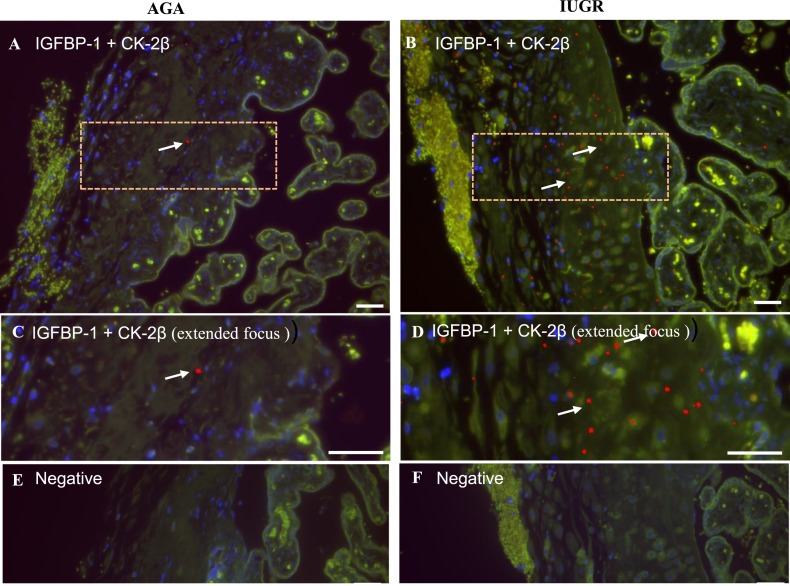
To explore further CK2 and IGFBP-1 interaction in the AGA and IUGR decidua, we used PLA. z-Stack extended-focus PLA images [Fig. 4(C) and 4(D)] were analyzed for positive PLA interactions using Image-Pro Premier software. In the multichannel images, positive interactions appear as pure red [enhanced for visibility, shown with dotted box; Fig. 4(A) and 4(B)], whereas the green tissue autofluorescence channel helps differentiate the bright PLA-positive spots from the normal background autofluorescence present in placental tissue samples. PLA spots (red), representing close proximity between IGFBP-1 and CK2β, were present in both IUGR and AGA [Fig. 4(A) and 4(B)] tissues but were highly increased in number in IUGR cases compared with AGA, shown more clearly in Fig. 4(C) and 4(D). No positive PLA interactions were noted in the absence of primary antibodies [Fig. 4(E) and 4(F)].
Figure 4.
Decidual IGFBP-1 and CK2 colocalization is increased in IUGR, as demonstrated by PLA. (A–E) PLA signals demonstrating the potential interaction of CK2β with total IGFBP-1 in the BPD region of placenta from the AGA group [(A), (C), and (E)] and the IUGR [(B), (D), and (F)] group. A positive PLA signal appears as red punctate spots denoted by white arrows, and DAPI nuclear counterstain (blue) with tissue autofluorescence (pseudo green) is used to show tissue morphology. Pink, dashed, outlined regions in (A) and (B) are shown zoomed in, in (C) and (D). Negative controls are shown in (E) and (F). Original scale bars, 50 µM.
IGFBP-1 is elevated and highly phosphorylated in maternal plasma at delivery in IUGR
The level of total IGFBP-1 and IGFBP-1 phosphorylation in maternal plasma at delivery from IUGR and AGA pregnancies was examined by Western blot. The data in Fig. 5(A) show that total IGFBP-1 in maternal plasma was elevated in IUGR pregnancies at delivery (+52%, P = 0.035) compared with AGA pregnancies. Relative IGFBP-1 phosphorylation levels in maternal plasma are shown in Fig. 5(B)–5(D). Compared with AGA pregnancies, maternal IGFBP-1 phosphorylation at Ser101 [+116%, P = 0.036; Fig. 5(B)], Ser119 [+80%, P = 0.003; Fig. 5(C)], and Ser169 [+82%, P = 0.008; Fig. 5(D)] was increased at delivery in pregnancies complicated by IUGR.
Figure 5.
Increased maternal plasma IGFBP-1 abundance and phosphorylation at delivery in IUGR. (A) A representative Western blot of total IGFBP-1 in maternal plasma from the IUGR and GA-matched AGA group collected at delivery. (B–D) IGFBP-1 phosphorylated at Ser101, Ser119, and Ser169 sites, respectively, in maternal plasma from the IUGR and GA-matched AGA group. The scatter plots summarize the Western blot data. Values are given as means ± SEM; n = 13 in each group; P < 0.05 vs control; unpaired t test.
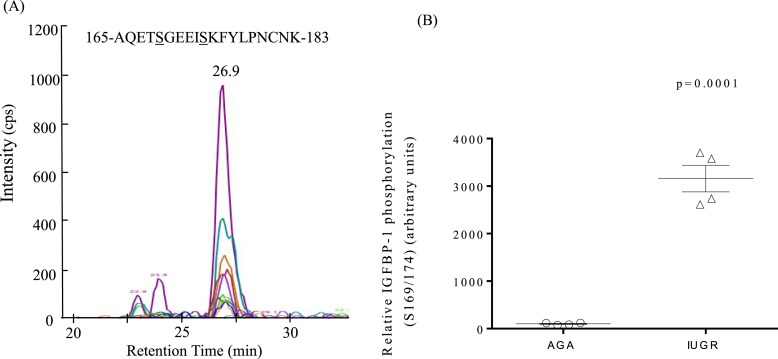
pSer174 is a site that is hyperphosphorylated in combination with pSer169 in IUGR pregnancies
We used targeted MRM-MS (36) as an independent quantitative approach to identify any IGFBP-1 sites in maternal plasma that may be phosphorylated in IUGR. For MRM-MS analysis, we used maternal plasma collected from IUGR and GA-matched AGA, both collected at delivery at 27-week gestation pregnancy [Fig. 6(A) and 6(B)]. The extracted ion chromatographs are shown with the transitions used to validate the phosphorylation at Ser169 + newly identified Ser174 in a doubly phosphorylated peptide (n = 4 technical replicates). Each individual peak represents unique peptide fragment ions (peptide transitions) that were specific to the phosphorylated Ser169 + Ser174 sites on the peptide. A histogram showing relative phosphorylation of IGFBP-1 in AGA and IUGR pregnancies revealed that the doubly phosphorylated peptide (pSer169 + pSer174) was eluted at an earlier retention time of 26.9 minutes [Fig. 6(A)], indicating the high acidity of the peptide as a result of markedly higher phosphorylation. IGFBP-1 in plasma was prominently dually phosphorylated at Ser169 and Ser174 [pSer169 + Ser174; Fig. 6(B)].
Figure 6.
Maternal plasma IGFBP-1 phosphorylation in AGA and IUGR studied by MRM-MS analysis. (A) Depiction of the extracted ion chromatographs showing the transitions used to validate the phosphorylation at Ser169 + Ser 174 in doubly phosphorylated peptides (4000 QTrap linear ion trap mass spectrometer; AB Sciex) in maternal plasma. (B) The scatter plot showing relative IGFBP-1 phosphorylation at Ser169 + Ser174 combined in a dually phosphorylated peptide, as determined from total peak intensities of transition ions from MRM-MS using plasma samples from IUGR and the GA-matched AGA group collected at delivery from 27-week gestation pregnancy. The data are a representative of four pooled technical replicates.
Interestingly, MRM-MS-based quantitative analysis showed that the phosphorylation level of the doubly phosphorylated peptide was significantly elevated (+3058%, P = 0.0001) in the IUGR sample [Fig. 6(B)] relative to GA-matched AGA. The markedly higher level of dual phosphorylation at Ser169 + Ser174, detected by MRM-MS, could not be detected using immunoblot data, mainly as a result of the lack of availability of a specific antibody targeting Ser174 or the dual phosphopeptide (Ser169 + 174). Overall, with the performance of MRM-MS, we discovered a site pSer174 and dual IGFBP-1 phosphorylation, where pSer174, in combination with pSer169, was hyperphosphorylated uniquely in IUGR. These data provide the basis to explore further the possibility of dual phosphorylation at Ser169 + 174 in prediction of IUGR.
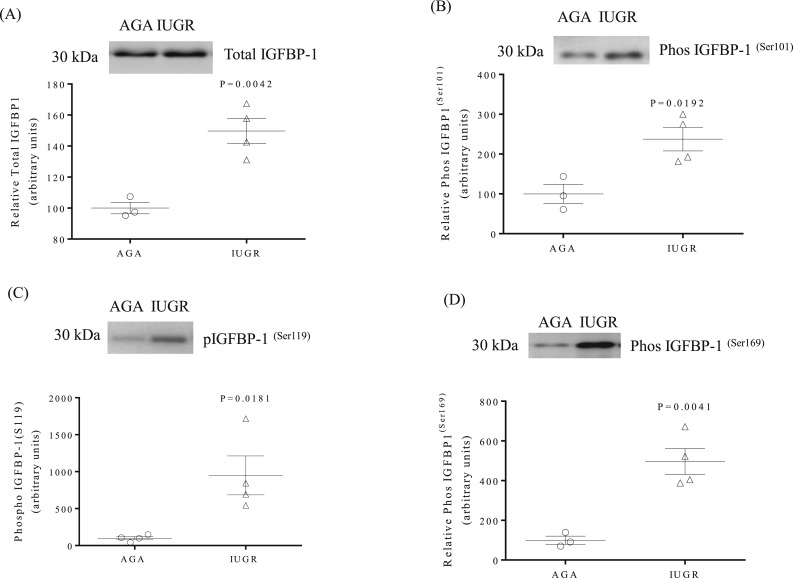
Plasma IGFBP-1 is elevated and highly phosphorylated in the first trimester in women delivering IUGR infants at term
We examined maternal plasma collected from women in the late first trimester who later delivered an IUGR or an AGA infant at term. Total IGFBP-1 [+50%, P = 0.004; Fig. 7(A)] and IGFBP-1 phosphorylation at Ser101 [+137%, P = 0.019; Fig. 7(B)], Ser119 [+ 849%, P = 0.018; Fig. 7(C)], and Ser169 [+ 397%, P = 0.004; Fig. 7(D)] was markedly elevated in first-trimester plasma of mothers who later delivered IUGR infants compared with women delivering AGA infants.
Figure 7.
IGFBP-1 phosphorylation is increased in maternal plasma collected in the first trimester from mothers who later delivered an IUGR infant. Plasma samples were collected from women at 7 to 9 wks of gestation, who later delivered an IUGR or AGA infant. (A) A representative Western blot of total IGFBP-1 in maternal plasma. (B–D) Representative Western blots of IGFBP-1 phosphorylated at Ser101, Ser119, and Ser169 in maternal plasma. The scatter plots summarize the Western blot data. Values are given as means ± SEM; n = 3 in the AGA group and n = 4 in the IUGR group; P < 0.05 vs control; unpaired t test.
Discussion
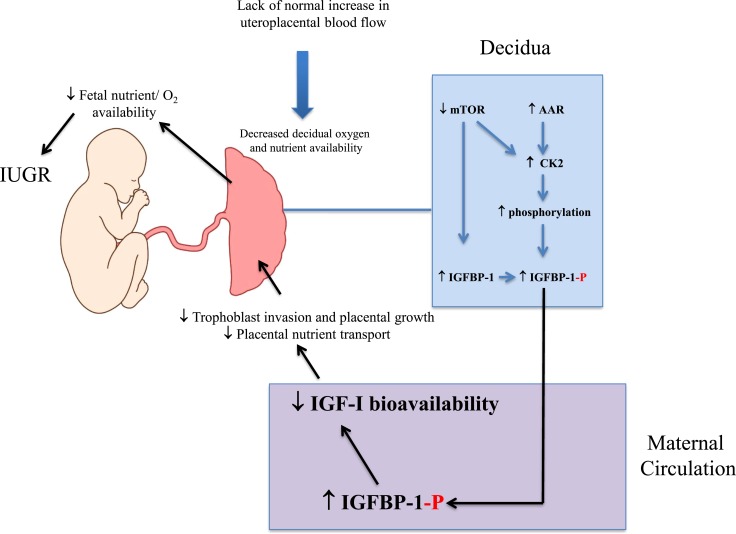
We show that IGFBP-1 is hyperphosphorylated at specific serine residues in the IUGR decidua. We have previously reported that inhibition of mTOR, activation of AAR signaling, and increased CK2 activity are mechanistically linked to increased IGFBP-1 phosphorylation in the fetal liver (11–14). Our current finding of mTOR inhibition, AAR, and CK2 activation in the IUGR decidua is consistent with our previous findings and therefore, suggest that the mechanisms underpinning decidual IGFBP-1 hyperphosphorylation in IUGR are similar. Moreover, we show that maternal plasma IGFBP-1 is hyperphosphorylated at delivery in pregnancies complicated by IUGR. Importantly, maternal plasma IGFBP-1 is hyperphosphorylated at specific serine residues in first trimester in women who later delivered IUGR infants, consistent with the possibility that hyperphosphorylation of maternal IGFBP-1 may serve as an early biomarker of IUGR. Increased phosphorylation of IGFBP-1 at specific serine residues limits IGF-I bioavailability (8) necessary for trophoblast invasion, placental function, and fetal growth (16, 20). Our data provide strong support for our hypothesis that the decidua functions as a nutrient sensor across gestation-linking limited oxygen and nutrient availability to increased secretion and phosphorylation of IGFBP-1, representing a mechanism for regulating fetal growth (Fig. 8).
Figure 8.
Decidual nutrient sensing. A model linking decreased nutrient and oxygen availability in the decidua in early pregnancy to the development of IUGR. -P, phosphorylation.
Early decidual formation requires progesterone stimulation, which promotes stromal differentiation and inhibits decidual apoptosis, resulting in upregulation of IGFBP-1 expression (19, 37). Decidua-derived IGFBP-1 controls invasion and proliferation of trophoblast cells during placentation by sequestering IGFs, and abundance of IGFBP-1 at the maternal-fetal interface therefore inhibits trophoblast invasion (16, 38). The decidua secretes IGFBP-1 in both non- and phosphorylated forms during pregnancy (17), further fine tuning its control over IGF-I bioavailability via paracrine modulation (39). Steroid hormones appear to play a key role in the control of IGF-I action at the maternal-fetal interface through changes of decidual IGFBP-1 phosphorylation and/or proteolysis (40, 41).
With the use of multiple immunoassays (42–46), a few (47–49) but not all studies (50–52) show that IUGR is associated with increased maternal circulating IGFBP-1. Here, we report that maternal plasma IGFBP-1 at delivery is increased in pregnancies complicated by IUGR, confirming some of the previous reports of an inverse association between IGFBP-1 concentrations in maternal plasma and birth weight (47–49). Phosphorylation of maternal IGFBP-1 in relation to fetal growth has also been studied previously (46, 50, 53–55) but with mixed results. As a result of variable antibody recognition of IGFBP-1 isoforms, these studies used two separate immunoassays consisting of two different antibodies to determine the ratios of non- or low- to highly phosphorylated IGFBP-1 in each sample rather than direct quantitation of nonphosphorylated or phosphorylated IGFBP-1 isoforms (46, 50, 53–56). In the current study, we used highly specific, prevalidated, custom-made IGFBP-1 phosphosite-specific antibodies, which provided compelling evidence that IGFBP-1 phosphorylation at specific sites (Ser101, Ser119, and Ser169) in maternal plasma is associated with impaired fetal growth.
We used targeted MRM-MS as an independent quantitative approach to identify additional known or potential IGFBP-1 phosphorylation sites for which antibodies are not yet available. MRM-MS analysis indicated markedly increased phosphorylation at a site Ser174, which was hyperphosphorylated together with Ser169 in maternal plasma in IUGR. The significance of dually phosphorylated IGFBP-1 sites in this study remains to be fully established. However, we have previously demonstrated increased dual IGFBP-1 phosphorylation at Ser174 and Ser169 in HepG2 cells in response to inhibition of mTOR signaling or following hypoxia and that hyperphosphorylation of these two sites may increase IGF-I binding affinity (13). Thus, it is possible that the increased dual (Ser174/Ser169) IGFBP-1 phosphorylation in plasma of mothers carrying an IUGR fetus may further decrease IGF-I bioavailability in the maternal compartment.
Based on our current findings, we suggest that increased IGFBP-1 phosphorylation in IUGR decidual cells occurs via activation of protein kinase CK2, which is a ubiquitous kinase known to phosphorylate multiple substrates (57–60). We used immunofluorescence to assess CK2 and IGFBP-1 expression and their coexpression in the AGA and IUGR decidua. Although CK2 expression was observed in several cell types of the placenta, CK2 expression, specifically in BPD, was greater in the IUGR placenta compared with AGA. Furthermore, with the use of a PLA, we show that the interaction of decidual IGFBP-1/CK2 protein was also much more pronounced in IUGR, consistent with the increased IGFBP-1 phosphorylation in the IUGR decidua. An important role for CK2 in mediating IGFBP-1 phosphorylation at Ser101, Ser119, and Ser169 is supported by previous mechanistic studies in HepG2 cells (14) and in decidualized HIESCs (22) using MRM-MS and CK2 activity assays.
Placental mTOR signaling has been reported to be inhibited in human IUGR (61). The current study demonstrates that an increase in IGFBP-1 phosphorylation is associated with inhibition of mTOR and activation of AAR signaling and increased CK2 activity and expression in the decidua in human IUGR. We have previously reported a link between mTOR inhibition and increased CK2 activity in cultured HepG2 cells and primary baboon fetal hepatocytes (11). Moreover, mTOR regulates fetal IGF-I bioavailability in response to hypoxia by modulating IGFBP-1 phosphorylation in HepG2 cells (13). IGFBP-1 and CK2 are colocalized in HepG2 cells in situ, and hypoxia and rapamycin treatment led to markedly amplified PLA signals for IGFBP-1 and CK2 in HepG2 cells, which was corroborated by stable isotope labeling with MRM-MS analysis (15). Collectively, these observations are consistent with the possibility that the IGFBP-1 hyperphosphorylation in the IUGR decidua is mechanistically linked to the observed inhibition of mTOR, activation of AAR, and increased CK2.
It is reasonable to assume some degree of hypoxia and limited nutrient availability in the decidua in placental insufficiency, and we recently reported that IGFBP-1 in in vitro-decidualized HIESC is hyperphosphorylated in response to hypoxia and/or nutrient deprivation (22). As fetal development and growth are critically dependent on IGF bioavailability at the maternal-fetal interface and in the maternal circulation, regulation of decidual IGFBP-1 phosphorylation by mTOR and AAR signaling and CK2 activity may constitute an important molecular link between placental insufficiency and reduced fetal growth (Fig. 8).
The finding of pronounced, increased phosphorylation of IGFBP-1 in the maternal circulation in first trimester in women who later delivered IUGR infants is consistent with the possibility that decidual nutrient sensing links limitation in nutrient and oxygen supply to IGFBP-1 hyperphosphorylation already early in pregnancy. This also suggests that these decidual responses precede the development of IUGR. Coinciding with uterine spiral artery remodeling, there is an increase in placental Po2 between weeks 8 and 12 of gestation, believed to represent the establishment of an unimpeded uteroplacental blood flow into the intervillous space (62), which likely also contributes to increased oxygenation and nutrition in the decidua in late first trimester. Therefore, of interest, we observed marked hyperphosphorylation of maternal circulating IGFBP-1, believed to originate from the decidua, in mothers later delivering an IUGR infant using samples obtained before the full establishment of maternal placental blood flow. It may be speculated that these findings are consistent with the possibility that some degree of oxygen and nutrient deprivation exists in the decidua of IUGR pregnancies already before intervillous blood flow is fully established.
The identification of biomarkers for IUGR in early pregnancy could improve the clinical management of these patients by allowing early intervention, preventing some of the perinatal complications associated with this condition. None of the currently available biomarkers predict IUGR with sufficient sensitivity to be used in routine clinical practice (26–28). Our findings suggest the possibility that maternal IGFBP-1 phosphorylation at Ser101, Ser119, and Ser169, individually or dually at Ser174 and Ser169 or in combination, constitutes a sensitive early pregnancy biomarker for IUGR.
In conclusion, our data suggest that the decidua links limited oxygen and nutrient availability to increased secretion and phosphorylation of IGFBP-1, mediated by mTOR and AAR signaling, resulting in decreased IGF-I availability and reduced fetal growth. Hyperphosphorylation of maternal IGFBP-1 may serve as an early biomarker of IUGR.
Acknowledgments
We thank Prof. David W. Litchfield (Department of Biochemistry, University of Western Ontario) for his generous support in providing the necessary reagents and the facility for conducting studies with protein kinase CK2 and Biotron Integrated Microscopy, Western University, in aiding dual immunofluorescent and PLA image acquisition and analyses.
Financial Support: This work was supported by grants from the National Institutes of Health (R01HD089980; to M.B.G. and T.J.). This study was also supported, in part, by Lawson Research Grant Funds (to M.B.G.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 4EBP1
4E-binding protein 1
- AAR
amino acid response
- AGA
appropriate-for-gestational age
- Akt
protein kinase B
- ATF4
activating transcription factor 4
- BPD
basal plate decidua
- CK2
casein kinase-2
- DAPI
4′,6-diamidino-2-phenylindole, dihydrochloride
- eIF2α
eukaryotic translation initiation factor 2A
- GA
gestational age
- GCN2
general control nonderepressible 2
- HIESC
human immortalized endometrial stromal cell
- IGF-I
insulin-like growth factor-1
- IGFBP-1
insulin-like growth factor-binding protein 1
- IP
immunoprecipitation
- IUGR
intrauterine growth restriction
- LHSC
London Health Sciences Centre
- mAb
monoclonal antibody
- MRM-MS
multiple reaction monitoring-mass spectrometry
- mTOR
mechanistic target of rapamycin
- mTORC
mechanistic target of rapamycin complex
- PLA
proximity ligation assay
References
- 1. Pallotto EK, Kilbride HW. Perinatal outcome and later implications of intrauterine growth restriction. Clin Obstet Gynecol. 2006;49(2):257–269. [DOI] [PubMed] [Google Scholar]
- 2. Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–1736. [DOI] [PubMed] [Google Scholar]
- 3. Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341(8850):938–941. [DOI] [PubMed] [Google Scholar]
- 4. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krishna U, Bhalerao S. Placental insufficiency and fetal growth restriction. J Obstet Gynaecol India. 2011;61(5):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gagnon R. Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S99–S107. [DOI] [PubMed] [Google Scholar]
- 7. Hills FA, English J, Chard T. Circulating levels of IGF-I and IGF-binding protein-1 throughout pregnancy: relation to birthweight and maternal weight. J Endocrinol. 1996;148(2):303–309. [DOI] [PubMed] [Google Scholar]
- 8. Abu Shehab M, Iosef C, Wildgruber R, Sardana G, Gupta MB. Phosphorylation of IGFBP-1 at discrete sites elicits variable effects on IGF-I receptor autophosphorylation. Endocrinology. 2013;154(3):1130–1143. [DOI] [PubMed] [Google Scholar]
- 9. Han VK, Matsell DG, Delhanty PJ, Hill DJ, Shimasaki S, Nygard K. IGF-binding protein mRNAs in the human fetus: tissue and cellular distribution of developmental expression. Horm Res. 1996;45(3-5):160–166. [DOI] [PubMed] [Google Scholar]
- 10. Wang HS, Lim J, English J, Irvine L, Chard T. The concentration of insulin-like growth factor-I and insulin-like growth factor-binding protein-1 in human umbilical cord serum at delivery: relation to fetal weight. J Endocrinol. 1991;129(3):459–464. [DOI] [PubMed] [Google Scholar]
- 11. Abu Shehab M, Damerill I, Shen T, Rosario FJ, Nijland M, Nathanielsz PW, Kamat A, Jansson T, Gupta MB. Liver mTOR controls IGF-I bioavailability by regulation of protein kinase CK2 and IGFBP-1 phosphorylation in fetal growth restriction. Endocrinology. 2014;155(4):1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malkani N, Jansson T, Gupta MB. IGFBP-1 hyperphosphorylation in response to leucine deprivation is mediated by the AAR pathway. Mol Cell Endocrinol. 2015;412:182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Damerill I, Biggar KK, Abu Shehab M, Li SS, Jansson T, Gupta MB. Hypoxia increases IGFBP-1 phosphorylation mediated by mTOR inhibition. Mol Endocrinol. 2016;30(2):201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malkani N, Biggar K, Shehab MA, Li SS, Jansson T, Gupta MB. Increased IGFBP-1 phosphorylation in response to leucine deprivation is mediated by CK2 and PKC. Mol Cell Endocrinol. 2016;425:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singal SS, Nygard K, Dhruv MR, Biggar K, Shehab MA, Li SS, Jansson T, Gupta MB. Co-localization of insulin-like growth factor binding protein-1, casein kinase-2β, and mechanistic target of rapamycin in human hepatocellular carcinoma cells as Demonstrated by dual immunofluorescence and in situ proximity ligation assay. Am J Pathol. 2018;188(1):111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lacey H, Haigh T, Westwood M, Aplin JD. Mesenchymally-derived insulin-like growth factor 1 provides a paracrine stimulus for trophoblast migration. BMC Dev Biol. 2002;2(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martina NA, Kim E, Chitkara U, Wathen NC, Chard T, Giudice LC. Gestational age-dependent expression of insulin-like growth factor-binding protein-1 (IGFBP-1) phosphoisoforms in human extraembryonic cavities, maternal serum, and decidua suggests decidua as the primary source of IGFBP-1 in these fluids during early pregnancy. J Clin Endocrinol Metab. 1997;82(6):1894–1898. [DOI] [PubMed] [Google Scholar]
- 18. Fang Q, Wang YX, Zhou Y. Insulin-like growth factor binding protein 1 and human embryonic development during 6–10 gestational weeks. Chin Med J (Engl). 2004;117(4):488–491. [PubMed] [Google Scholar]
- 19. Fowler DJ, Nicolaides KH, Miell JP. Insulin-like growth factor binding protein-1 (IGFBP-1): a multifunctional role in the human female reproductive tract. Hum Reprod Update. 2000;6(5):495–504. [DOI] [PubMed] [Google Scholar]
- 20. Sferruzzi-Perri AN, Owens JA, Standen P, Taylor RL, Robinson JS, Roberts CT. Early pregnancy maternal endocrine insulin-like growth factor I programs the placenta for increased functional capacity throughout gestation. Endocrinology. 2007;148(9):4362–4370. [DOI] [PubMed] [Google Scholar]
- 21. Sferruzzi-Perri AN, Owens JA, Standen P, Taylor RL, Heinemann GK, Robinson JS, Roberts CT. Early treatment of the pregnant guinea pig with IGFs promotes placental transport and nutrient partitioning near term. Am J Physiol Endocrinol Metab. 2007;292(3):E668–E676. [DOI] [PubMed] [Google Scholar]
- 22. Shehab MA, Biggar K, Singal SS, Nygard K, Shun-Cheng Li S, Jansson T, Gupta MB. Exposure of decidualized HIESC to low oxygen tension and leucine deprivation results in increased IGFBP-1 phosphorylation and reduced IGF-I bioactivity. Mol Cell Endocrinol. 2017;452:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lindqvist PG, Molin J. Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound Obstet Gynecol. 2005;25(3):258–264. [DOI] [PubMed] [Google Scholar]
- 24. Nicolaides KH. A model for a new pyramid of prenatal care based on the 11 to 13 weeks’ assessment. Prenat Diagn. 2011;31(1):3–6. [DOI] [PubMed] [Google Scholar]
- 25. Lausman A, McCarthy FP, Walker M, Kingdom J. Screening, diagnosis, and management of intrauterine growth restriction. J Obstet Gynaecol Can. 2012;34(1):17–28. [DOI] [PubMed] [Google Scholar]
- 26. Sovio U, White IR, Dacey A, Pasupathy D, Smith GCS. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet. 2015;386(10008):2089–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poljak B, Agarwal U, Jackson R, Alfirevic Z, Sharp A. Diagnostic accuracy of individual antenatal tools for prediction of small-for-gestational age at birth. Ultrasound Obstet Gynecol. 2017;49(4):493–499. [DOI] [PubMed] [Google Scholar]
- 28. Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for predicting intrauterine growth restriction: a systematic review and meta-analysis. BJOG. 2013;120(6):681–694. [DOI] [PubMed] [Google Scholar]
- 29. Seferovic MD, Gupta MB. Increased umbilical cord PAI-1 levels in placental insufficiency are associated with fetal hypoxia and angiogenesis. Dis Markers. 2016;2016:7124186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seferovic MD, Chen S, Pinto DM, Gupta MB. Altered liver secretion of vascular regulatory proteins in hypoxic pregnancies stimulate angiogenesis in vitro. J Proteome Res. 2011;10(4):1495–1504. [DOI] [PubMed] [Google Scholar]
- 31. Zhang J, Merialdi M, Platt LD, Kramer MS. Defining normal and abnormal fetal growth: promises and challenges. Am J Obstet Gynecol. 2010;202(6):522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lynch AM, Wagner BD, Deterding RR, Giclas PC, Gibbs RS, Janoff EN, Holers VM, Santoro NF. The relationship of circulating proteins in early pregnancy with preterm birth. Am J Obstet Gynecol. 2016;214(4):517.e1–517.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lynch AM, Hart JE, Agwu OC, Fisher BM, West NA, Gibbs RS. Association of extremes of prepregnancy BMI with the clinical presentations of preterm birth. Am J Obstet Gynecol. 2014;210(5):428.e1–428.e9. [DOI] [PubMed] [Google Scholar]
- 34. Vilk G, Saulnier RB, St Pierre R, Litchfield DW. Inducible expression of protein kinase CK2 in mammalian cells. Evidence for functional specialization of CK2 isoforms. J Biol Chem. 1999;274(20):14406–14414. [DOI] [PubMed] [Google Scholar]
- 35. Litchfield DW, Dobrowolska G, Krebs EG. Regulation of casein kinase II by growth factors: a reevaluation. Cell Mol Biol Res. 1994;40(5-6):373–381. [PubMed] [Google Scholar]
- 36. Unwin RD, Griffiths JR, Leverentz MK, Grallert A, Hagan IM, Whetton AD. Multiple reaction monitoring to identify sites of protein phosphorylation with high sensitivity. Mol Cell Proteomics. 2005;4(8):1134–1144. [DOI] [PubMed] [Google Scholar]
- 37. Kim JJ, Buzzio OL, Li S, Lu Z. Role of FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: interaction with progesterone receptor. Biol Reprod. 2005;73(4):833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Irwin JC, Giudice LC. Insulin-like growth factor binding protein-1 binds to placental cytotrophoblast alpha5beta1 integrin and inhibits cytotrophoblast invasion into decidualized endometrial stromal cultures. Growth Horm IGF Res. 1998;8(1):21–31. [DOI] [PubMed] [Google Scholar]
- 39. Gibson JM, Aplin JD, White A, Westwood M. Regulation of IGF bioavailability in pregnancy. Mol Hum Reprod. 2001;7(1):79–87. [DOI] [PubMed] [Google Scholar]
- 40. Kabir-Salmani M, Shimizu Y, Sakai K, Iwashita M. Posttranslational modifications of decidual IGFBP-1 by steroid hormones in vitro. Mol Hum Reprod. 2005;11(9):667–671. [DOI] [PubMed] [Google Scholar]
- 41. Westwood M, Gibson JM, Williams AC, Clayton PE, Hamberg O, Flyvbjerg A, White A. Hormonal regulation of circulating insulin-like growth factor-binding protein-1 phosphorylation status. J Clin Endocrinol Metab. 1995;80(12):3520–3527. [DOI] [PubMed] [Google Scholar]
- 42. Rutanen EM, Bohn H, Seppälä M. Radioimmunoassay of placental protein 12: levels in amniotic fluid, cord blood, and serum of healthy adults, pregnant women, and patients with trophoblastic disease. Am J Obstet Gynecol. 1982;144(4):460–463. [DOI] [PubMed] [Google Scholar]
- 43. Lee PD, Conover CA, Powell DR. Regulation and function of insulin-like growth factor-binding protein-1. Proc Soc Exp Biol Med. 1993;204(1):4–29. [DOI] [PubMed] [Google Scholar]
- 44. Rutanen EM, Pekonen F. Assays for IGF binding proteins. Acta Endocrinol (Copenh). 1991;124(Suppl 2):70–73. [PubMed] [Google Scholar]
- 45. Khosravi MJ, Diamandi A, Mistry J. Immunoassay of insulin-like growth factor binding protein-1. Clin Chem. 1997;43(3):523–532. [PubMed] [Google Scholar]
- 46. Westwood M, Gibson JM, Davies AJ, Young RJ, White A. The phosphorylation pattern of insulin-like growth factor-binding protein-1 in normal plasma is different from that in amniotic fluid and changes during pregnancy. J Clin Endocrinol Metab. 1994;79(6):1735–1741. [DOI] [PubMed] [Google Scholar]
- 47. Holmes R, Montemagno R, Jones J, Preece M, Rodeck C, Soothill P. Fetal and maternal plasma insulin-like growth factors and binding proteins in pregnancies with appropriate or retarded fetal growth. Early Hum Dev. 1997;49(1):7–17. [DOI] [PubMed] [Google Scholar]
- 48. Hills FA, Crawford R, Harding S, Farkas A, Chard T. The effects of labor on maternal and fetal levels of insulin-like growth factor binding protein-1. Am J Obstet Gynecol. 1994;171(5):1292–1295. [DOI] [PubMed] [Google Scholar]
- 49. Olausson H, Lof M, Brismar K, Lewitt M, Forsum E, Sohlstrom A. Longitudinal study of the maternal insulin-like growth factor system before, during and after pregnancy in relation to fetal and infant weight. Horm Res. 2008;69(2):99–106. [DOI] [PubMed] [Google Scholar]
- 50. Bhatia S, Faessen GH, Carland G, Balise RL, Gargosky SE, Druzin M, El-Sayed Y, Wilson DM, Giudice LC. A longitudinal analysis of maternal serum insulin-like growth factor I (IGF-I) and total and nonphosphorylated IGF-binding protein-1 in human pregnancies complicated by intrauterine growth restriction. J Clin Endocrinol Metab. 2002;87(4):1864–1870. [DOI] [PubMed] [Google Scholar]
- 51. Qiu C, Vadachkoria S, Meryman L, Frederick IO, Williams MA. Maternal plasma concentrations of IGF-1, IGFBP-1, and C-peptide in early pregnancy and subsequent risk of gestational diabetes mellitus. Am J Obstet Gynecol. 2005;193(5):1691–1697. [DOI] [PubMed] [Google Scholar]
- 52. Sifakis S, Akolekar R, Kappou D, Mantas N, Nicolaides KH. Maternal serum placental growth hormone at 11-13 weeks' gestation in pregnancies delivering small for gestational age neonates. J Matern Fetal Neonatal Med. 2012;25(9):1796–1799. [DOI] [PubMed] [Google Scholar]
- 53. Iwashita M, Sakai K, Kudo Y, Takeda Y. Phosphoisoforms of insulin-like growth factor binding protein-1 in appropriate-for-gestational-age and small-for-gestational-age fetuses. Growth Horm IGF Res. 1998;8(6):487–493. [DOI] [PubMed] [Google Scholar]
- 54. Gibson JM, Westwood M, Lauszus FF, Klebe JG, Flyvbjerg A, White A. Phosphorylated insulin-like growth factor binding protein 1 is increased in pregnant diabetic subjects. Diabetes. 1999;48(2):321–326. [DOI] [PubMed] [Google Scholar]
- 55. Fowler D, Albaiges G, Lees C, Jones J, Nicolaides K, Miell J. The role of insulin-like growth factor binding protein-1 phosphoisoforms in pregnancies with impaired placental function identified by doppler ultrasound. Hum Reprod. 1999;14(11):2881–2885. [DOI] [PubMed] [Google Scholar]
- 56. Loukovaara M, Leinonen P, Teramo K, Nurminen E, Andersson S, Rutanen EM. Effect of maternal diabetes on phosphorylation of insulin-like growth factor binding protein-1 in cord serum. Diabet Med. 2005;22(4):434–439. [DOI] [PubMed] [Google Scholar]
- 57. Hanif IM, Hanif IM, Shazib MA, Ahmad KA, Pervaiz S. Casein kinase II: an attractive target for anti-cancer drug design. Int J Biochem Cell Biol. 2010;42(10):1602–1605. [DOI] [PubMed] [Google Scholar]
- 58. Montenarh M. Cellular regulators of protein kinase CK2. Cell Tissue Res. 2010;342(2):139–146. [DOI] [PubMed] [Google Scholar]
- 59. Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369(Pt 1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pinna LA, Meggio F. Protein kinase CK2 (“casein kinase-2”) and its implication in cell division and proliferation. Prog Cell Cycle Res. 1997;3:77–97. [DOI] [PubMed] [Google Scholar]
- 61. Roos S, Jansson N, Palmberg I, Säljö K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol. 2007;582(Pt 1):449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157(6):2111–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]