Abstract
Research on visual rivalry has demonstrated that consecutive dominance durations are serially dependent, implying that the underlying competition mechanism is not driven by some random process but includes a memory component. Here we asked whether serial dependence is also observed in continuous flash suppression (CFS). We addressed this question by analyzing a large dataset of time series of suppression durations obtained in a series of so-called “breaking CFS” experiments in which the duration of the period is measured until a suppressed target breaks through the CFS mask. Across experimental manipulations, stimuli, and observers, we found that (i) the distribution of breakthrough rates was fit less well by a gamma distribution than in conventional visual rivalry paradigms, (ii) the suppression duration on a previous trial influenced the suppression duration on a later trial up to as long as a lag of eight trials, and (iii) the mechanism underlying these serial correlations was predominantly monocular. We conclude that the underlying competition mechanism of CFS also includes a memory component that is primarily, but not necessarily exclusively, monocular in nature. We suggest that the temporal dependency structure of suppression durations in CFS is akin to those observed in binocular rivalry, which might imply that both phenomena tap into similar rather than distinct mechanisms.
Keywords: psychophysics, perception, awareness
Introduction
A main challenge for the visual system is to create a stable perceptual world from a noisy stream of sensory input. One way in which perceptual continuity can be achieved is by having the current percept not only influenced by the current input, but also by the input from the recent past. This would necessarily entail a certain degree of serial dependence in time series pertaining to visual perception. Indeed, recent studies have indicated that perceived orientation, numerosity, or face identity are influenced by stimuli presented in the recent past (Cicchini et al., 2014; Fischer and Whitney, 2014; Liberman et al., 2014).
The question of serial (in)dependence in time series has also been addressed in studies on visual rivalry where perception alternates between competing interpretations of the sensory input in a seemingly random fashion (Blake and Logothetis, 2002; Alais, 2012; Alais and Blake, 2015). Early studies on visual rivalry reported that consecutive percept durations did not show any relationship, and if they did, the correlation was judged to be too small to be meaningful (Fox and Herrmann, 1967; Blake et al., 1971; Borsellino et al., 1972; Walker, 1975; Lehky, 1995; Logothetis et al., 1996). More recently, however, a number of studies have rejected the independence between successive dominance durations in visual rivalry (Mamassian and Goutcher, 2005; van Ee, 2005, 2009; Pastukhov and Braun, 2011). Small, but consistently significant serial correlations (most pronounced at lag 1) have been reported for both binocular rivalry as well as for the ambiguously rotating sphere (van Ee, 2009). The finding that consecutive percept durations are serially dependent is important because it provides a footprint of the neural alternation mechanism (van Ee, 2009). That is, it shows that the underlying dynamics are not completely random but include a memory component (as revealed by the serial correlations).
In this article, we focus on continuous flash suppression (CFS) (Tsuchiya and Koch, 2005; Tsuchiya et al., 2006), an interocular suppression paradigm in which discrepant images are presented to corresponding retinal locations of both eyes. In CFS, the input to one of the eyes is continuously updated at a rate of about 100 ms (i.e. ∼10 Hz) yielding prolonged and stable suppression of the stimulus presented to the other eye. Although it is still debated whether CFS is simply just a stronger form of binocular rivalry or involves distinct mechanisms (Tsuchiya and Koch, 2005; Tsuchiya et al., 2006; Kaunitz et al., 2014; Moors et al., 2014), the main factors involved in visual rivalry in general, cross-inhibition and self-adaptation, presumably also come into play during CFS (Shimaoka and Kaneko, 2011). As serial correlations could provide a footprint of the underlying alternation mechanism (van Ee, 2009), a first goal of this article was to analyze the pattern of serial correlations in CFS by capitalizing on a large dataset (n = 393 sessions) consisting of suppression durations obtained in several different so-called breaking CFS (b-CFS) experiments (Jiang et al., 2007; Stein et al., 2011). b-CFS refers to a paradigm in which CFS has been implemented to study unconscious visual processing (note that the validity of using b-CFS to infer unconscious visual processing has been questioned (Stein et al., 2011; Stein and Sterzer, 2014), yet a discussion of this issue is beyond the scope of this article). In a typical b-CFS study, the CFS mask and a target stimulus of interest are presented to different eyes on each trial. The target stimulus will initially be suppressed from visual awareness but will eventually, after several seconds, “break through” the CFS mask (i.e. become detectable). In these experiments, suppression duration is the main dependent variable and is used as a measure to assess whether different classes of stimuli break suppression differentially. For example, the classic study by Jiang et al. (2007) showed that mean suppression durations for upright faces are shorter than those for inverted faces.
A second goal of this article pertains to a long-standing debate in the literature on binocular rivalry with respect to the nature and site of interocular suppression. That is, does binocular rivalry suppression entail inhibitory interactions between neurons at a monocular level (Verhoeff, 1935; Levelt, 1965; Blake, 1989) or does competition also occur at levels upstream in the visual cortex, involving competition between binocular neurons (Walker, 1978; Logothetis et al., 1996)? Although this debate has been settled more or less by proposing a hybrid view of binocular rivalry in which rivalry is proposed to happen at multiple stages in the visual hierarchy, both at the monocular and binocular level (Blake and Logothetis, 2002; Tong et al., 2006), the nature of our dataset enabled us to shed some more light on this issue. That is, our dataset contains two different types of b-CFS experiments, one in which the eye to which the CFS mask was presented was determined randomly on each trial (“variable eye presentation”) and the other in which the eye to which the CFS mask is presented was kept fixed throughout the experiment (“fixed eye presentation”). This enabled us to test the extent to which potential serial correlations in CFS are driven by monocular rather than binocular mechanisms. Furthermore, it is currently still debated whether differences in suppression times are mostly driven by low-level rather than high-level mechanisms (Lupyan and Ward, 2013; Gayet et al., 2014; Hesselmann and Moors, 2015; Pinto et al., 2015). The extent to which potential serial correlations are predominantly relying on monocular or binocular mechanisms could also shed some light on this discussion.
In the remainder of this article, we start by describing the dataset that was used for the analysis. In the first part of the analysis, we summarize the dataset through a classical analysis of fitting a gamma distribution to the breakthrough rate distribution. In the second part, we report on serial correlations of suppression durations observed across experiments with different observers, target stimuli, and CFS masks.
Materials and Methods
The dataset
Our dataset consists of 24 different experiments ran in four separate studies (see Tables 1 and 2). Three of these studies (16 experiments) have already been published (Stein et al., 2012, 2014; Heyman and Moors, 2014). We refer to these studies for the methodological and procedural details of each experiment. All reported studies were conducted in line with the ethical principles regarding research with human participants as specified in The Code of Ethics of the World Medical Association (Declaration of Helsinki). The study was approved by the respective local ethics committees (Ethical Committee of the Faculty of Psychology and Educational Sciences (EC FPPW) of the University of Leuven, and the Charité ethics committee), and all participants gave written informed consent before starting the experiment. Note that Experiment 2 reported in Heyman and Moors (2014) consisted of a test–retest design in which the same experiment was run on the same set of subjects on two consecutive days. Given that these sessions were run on separate days, these two experimental sessions are regarded as two experiments in our analysis. Note, however, that including only one of the sessions rather than both did not change the results. The remaining experiments comprise hitherto unpublished data. All unpublished PM experiments involved presenting illusory shape stimuli in a typical b-CFS design (for a partial report of these data, we refer to Moors et al., 2013). TS16 comprises an unpublished control experiment belonging to the set of experiments reported in Stein et al. (2014). TS4 and TS5 refer to unpublished datasets in the context of Stein et al. (2012). The number of trials in all these experiments ranged between 192 and 768. We aimed at including only experiments that contained ∼200 trials at least since this yields ∼90% power to detect a correlation of ∼0.2 (van Ee, 2009).
Table 1.
Description of dataset for “fixed eye” CFS experiments
| Experiment | Participants | Number of trials | Stimulus fade-in time (s) | Mask fade-out onset (s) | Mask fade-out time (s) | Max trial duration (s) | Mask type | Published as |
|---|---|---|---|---|---|---|---|---|
| PM1 | 19 | 300 | 2 | / | / | / | 100 squares between 1° and 2° | Moors, van Crombruggen, Wagemans et al. (2013) |
| PM2 | 20 | 192 | 2 | / | / | / | 144 geometrical shapes | Moors et al. (2013) |
| PM3 | 20 | 288 | 2 | / | / | / | 144 geometrical shapes | Unpublished |
| PM4 | 18 | 308 | 2 | / | / | / | 200 squares between 0.2° and 1.2° | Heyman and Moors (2014) |
| PM5 | 31 | 460 | 2 | / | / | / | 200 squares between 0.2° and 1.2° | Heyman and Moors (2014) |
| PM6 | 31 | 460 | 2 | / | / | / | 200 squares between 0.2° and 1.2° | Heyman & Moors (2014) |
| PM7 | 21 | 288 | 2 | / | / | / | 144 geometrical shapes | Unpublished |
| PM8 | 20 | 288 | 2 | / | / | / | 48 geometrical shapes | Unpublished |
| TS9 | 12 | 576 | 1.1 | 1.1 | 4 | 7 | Circles between 0.3° and 1.4° | Stein, Seymour, Hebart, & Sterzer (2014)—Exp 1a |
| TS10 | 16 | 384 | 1.1 | 1.1 | 4 | 7 | Circles between 0.3° and 1.4° | Stein et al. (2014)—Exp 1b |
| TS11 | 16 | 384 | 1.1 | 1.1 | 7 | 10 | Circles between 0.3° and 1.4° | Stein et al. (2014)—Exp 1c |
| TS12 | 12 | 384 | 1.1 | 1.1 | 4 | 7 | Circles between 0.3° and 1.4° | Stein et al. (2014)—Control Exp 1a |
| TS13 | 14 | 768a | 1.1 | 1.1 | 4 | 7 | Circles between 0.3° and 1.4° | Stein et al. (2014)— Control Exp 1b |
| TS14 | 12 | 256b | 1.1 | 1.1 | 7 | 10 | Circles between 0.3° and 1.4° | Stein et al. (2014)—Control Exp 2 |
| TS15 | 12 | 256 | 1.11 | 1.1 | 7 | 10 | Circles between 0.3° and 1.4° | Stein et al. (2014)—Control Exp 3 |
| TS16 | 16 | 384c | 1.1 | 1.1 | 7 | 10 | Circles between 0.3° and 1.4° | Unpublished |
a The experiment crashed for 1 participant and only 524 trials were recorded for this participant.
b The experiment crashed for 2 participants and only 252 and 240 trials were recorded for these participants.
c The experiment crashed for 1 participant and only 260 trials were recorded for this participant.
Table 2.
Description of dataset for “variable eye” CFS experiments
| Experiment | Participants | Number of trials | Stimulus fade-in time (s) | Mask fade-out onset (s) | Mask fade-out time (s) | Maximum trial duration (s) | Mask type | Published as |
|---|---|---|---|---|---|---|---|---|
| TS1 | 12 | 288 | 1 | 1.1 | 7 | 10 | Circles between 0.4° and 1.8° | Stein et al. (2012)—Exp 2 |
| TS2 | 11 | 320 | 1 | 1.1 | 7 | 10 | Circles between 0.4° and 1.8° | Stein et al. (2012)—Exp 4 |
| TS3 | 10 | 200 | 1 | 1.1 | 7 | 10 | Circles between 0.4° and 1.8° | Stein et al. (2012)—Exp 5 |
| TS4 | 10 | 320 | 1 | 1.1 | 7 | 10 | / | Unpublished |
| TS5 | 21 | 240 | 1 | 1.1 | 7 | 10 | / | Unpublished |
| TS6 | 13 | 192 | 1 | 1 | 7 | 10 | Circles between 0.4° and 1.8° | Stein et al. (2011)—Exp 1 |
| TS7 | 13 | 192 | 1 | 1 | 7 | 10 | Circles between 0.4° and 1.8° | Stein et al. (2011)—Exp 2 |
| TS8 | 13 | 192 | 1 | 1 | 7 | 10 | Circles between 0.4° and 1.8° | Stein et al. (2011)—Exp 3 |
As mentioned in the Introduction, our dataset contains two different types of b-CFS experiments, depending on whether the CFS mask was presented in the same eye throughout the experiment or randomly to one of both eyes on each trial. We refer to these experiments as fixed eye (n = 290) and variable eye experiments (n = 103), respectively. A summary of some experimental details for both datasets can be found in Tables 1 and 2.
All experiments consisted of the typical b-CFS design. A CFS mask (with varying properties, see Tables) was presented to the dominant or non-dominant eye or variably to one of both eyes and the suppressed stimulus was presented to the other eye and gradually increased in contrast. Additionally, in all TS experiments the CFS mask was gradually decreased in contrast throughout a trial, to ensure sufficient breakthroughs for all participants (Yang et al., 2007) (none of the PM experiments relied on this procedure). The specifics of this mask fade-out procedure are reported in Tables 1 and 2. It is important to note that this mask fade-out procedure has important implications for the resulting suppression durations. Given that the mask invariably disappears after a fixed presentation time, the suppression duration distribution is necessarily censored at this point. Upon breakthrough, participants always had to perform a localization task (i.e. indicate whether the stimulus was presented above or below fixation or whether it was presented left or right of fixation, or in which quadrant the stimulus was shown) and the time it took participants to make the localization response was recorded as the suppression duration. Because blocking rather than randomizing experimental conditions could artificially induce serial correlations, only experiments in which all experimental conditions were randomized across trials were included in the dataset.
Data analysis
The first part of the analysis consisted of cleaning the data in two steps to ensure no correlations would be observed that could be attributable to either of the following two factors. First, we excluded for each observer the first 5 trials of each session (i.e. akin to the removal of the first 30 seconds of each trial in van Ee, 2009). Second, we corrected the data for drift (i.e. suppression times tend to become shorter, on average, over the course of the experiment). Because the drift was potentially nonlinear, we performed a local regression (LOESS) on the dataset, with trial number as the predictor of suppression duration. The smoothing parameter was automatically selected based on the bias-corrected Akaike information criterion (Hurvich et al., 1998).
As highlighted in the description of the dataset, all TS experiments relied on a mask fade-out procedure, in which the CFS mask contrast was gradually ramped down over the course of a trial. Because this introduces an artificial cutoff in the suppression duration distributions (i.e. when the CFS mask disappears, participants will always see the stimulus in the following second or so), we removed all trials in which the recorded suppression duration was higher than the time point at which the CFS mask disappeared. If this led to a removal of more than 10% of trials, we removed this participant from the dataset. We used this cutoff to ensure that potential serial correlations could not be induced by responses to stimuli in the absence of a CFS mask. This procedure led to a removal of 12 and 24 participants for the fixed and variable eye experiments, respectively. Please note, however, that the overall pattern of serial correlations (especially with respect to the early lags) does not change when these participants are included (see Supplementary Data for a figure including these participants, as well as excluding the cleaning steps).
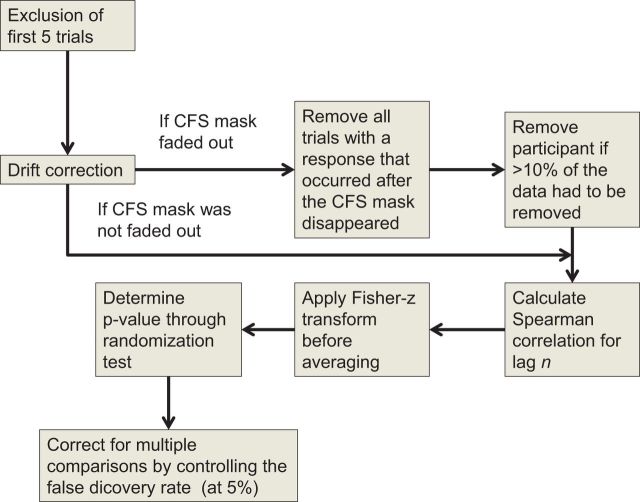
Because the suppression durations follow a non-normal distribution, we calculated Spearman rank correlations at the various lags, where lag n refers to the nth trial before the current trial. The maximum lag that was considered was lag 10 because this proved to be the lag up to which the data were most reliable for most participants. A Fisher-z transformation was applied to the Spearman correlations before averaging them (due to the range of trials in the experiments included, a weighted average was used, where the weight was the number of trials used in the experiment). After averaging, the resulting correlation was back-transformed using the inverse transformation. The significance of the average Spearman correlations was assessed using one-sample randomization tests in Fisher-z space. Since this analysis essentially involves comparing 10 different p-values against zero, the significance of the p-values was determined by controlling the false discovery rate (FDR, at 5%), using the method introduced by Benjamini and Hochberg (1995). A summary of the analysis pipeline is visualized in Fig. 1. Additionally, to compare both experiment types, we used polynomial mixed-effect regression modeling with random intercepts and random slopes for participants (without correlations between random effects). Polynomial regression was used to account for the nonlinear relationship between lag and the observed Spearman correlation (infra). Drop-in-deviance tests were used to compare different statistical models.
Figure 1.
An overview of the analysis pipeline.
Results
Suppression duration variability across observers
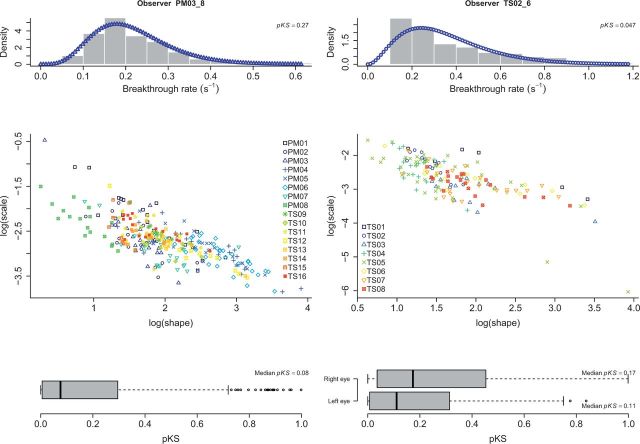
We first provide a description of the dataset in terms of a classic analysis of suppression durations. Because Brascamp et al. (2005) showed better fits for alternation rates rather than percept duration for a range of visual rivalry stimuli, we fitted a gamma distribution to the distribution of breakthrough rates rather than suppression durations (i.e. breakthrough rate is 1 per suppression duration). Figure 2 depicts the distributions of two different observers overlaid with the best fitting gamma distributions (see Supplementary Figs S1, S2, and S3 for an overview of all observers, split up by eye for the variable eye experiments, for the fixed and variable eye experiments, respectively). For both experiment types, scatter plots of the shape and scale parameters of the individual observer fits are depicted. Note that there is considerable interindividual variability in the estimates of the shape and scale parameters, reflecting the variability in breakthrough rates across observers. Furthermore, it should be noted that the tight negative relationship between shape and scale parameters in log–log space is to be expected due to the parameterization of the gamma distribution (Borsellino et al., 1972; Brouwer and van Ee, 2006; van Ee et al., 2006; Wagenmakers and Brown, 2007). The boxplots summarize the goodness of fit quantified through the probability pKS obtained from the Kolmogorov–Smirnov test, which involves the largest overall deviation between the empirical and fitted cumulative distribution (Brascamp et al., 2005, referred to as the D statistic). pKS ranges between 0 and 1 where higher values indicate good fit. Because estimated parameters were used for the Kolmogorov–Smirnov test, the associated pKS value is no longer valid (Durbin, 1973). Therefore, we used a Monte Carlo procedure to compute pKS. In this procedure, we generated new datasets for each participant based on the estimated parameters and computed the D statistic for each simulated dataset. For each participant, we repeated this procedure 10,000 times and computed pKS as the proportion of simulated D values more extreme than the D value observed in the data.
Figure 2.
Distribution analysis of breakthrough rate. (left) Fixed eye experiments. The histogram depicts the distribution of one typical observer overlaid with the best fitting gamma distribution. The middle left figure depicts the shape and scale estimates for all observers in log-log space. Goodness of fit was quantified through the pKS statistic, relying on the Kolmogorov-Smirnov test. A summary is depicted in the bottom left boxplot (high values indicate good fit). (right) Variable eye experiments.
As is apparent from Fig. 2, the median pKS value is equal to 0.08 (0.17 and 0.11 for the variable eye experiments, right and left eye respectively), indicating that the fit quality is generally low for the breakthrough rate distributions, especially compared to Brascamp et al. Indeed, for both the fixed and variable eye experiments, the upper limit of the interquartile range of the pKS never exceeds the lower limit of the interquartile range reported in Brascamp et al. This discrepancy between the quality of fits generally observed in visual rivalry and the ones observed here is further touched upon in the discussion.
Aggregated serial correlation data
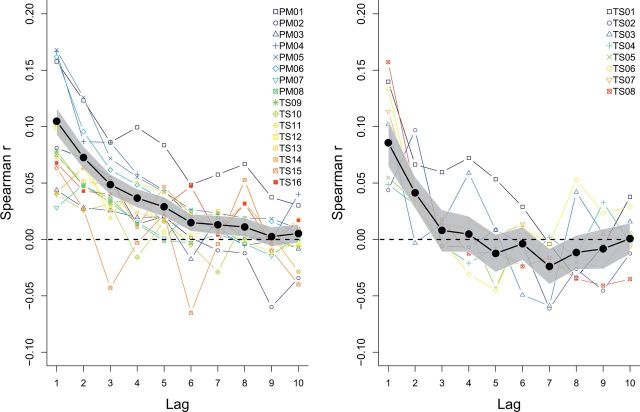
Figure 3 depicts the Spearman rank correlation coefficients up to lag 10 aggregated across all observers using a weighted average (weighed by the number of trials used in the experiment) (black line; shaded gray area indicates the bootstrapped 95% confidence interval), for both datasets. To assess the variability across experiments, the aggregated data are also plotted for each experiment separately (colored lines). For the fixed eye experiments, the correlation at lag 1 is positive and significantly different from zero (r = 0.10). Furthermore, rather than immediately dropping to zero, the serial correlations gradually decay to zero until they are no longer significant from lag 9 onwards. In contrast, for the variable eye experiments, a markedly different pattern arises. The correlation at lag 1 is slightly lower (r = 0.09) and quickly drops to zero from lag 3 onwards. This difference between experiment types is confirmed by a polynomial mixed effects regression analysis. A model including main effects of lag (polynomials up to the order of three) and experiment type and their interaction was preferred over a model only including the main effects (drop-in-deviance test, χ(3) = 9.4079, p = 0.024).
Figure 3.
Aggregated serial correlation data for the fixed (left) and variable (right) eye experiments. Mean Spearman rank correlations across all observers as a function of lag. The gray, shaded area indicates the 95% bootstrapped confidence intervals. The colored lines depict the mean Spearman rank correlations for each experiment separately.
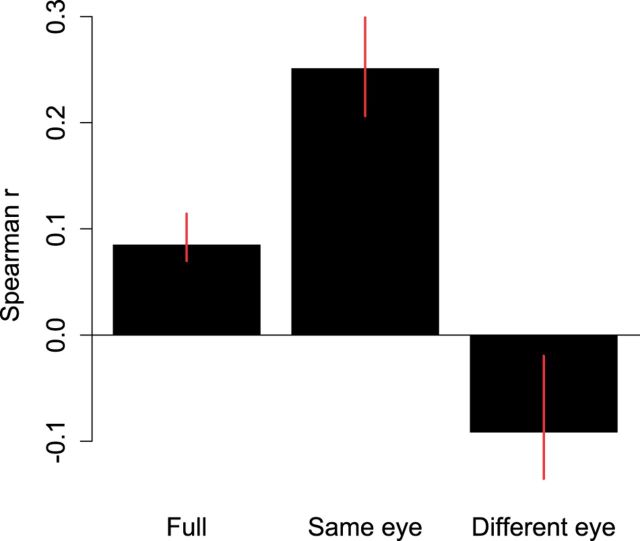
The variable eye experiments contain both trials in which the CFS mask is kept constant on trial n + 1 as well as switched to the previously suppressed eye. Thus, we were interested in examining the influence of swapping eyes across trials for these experiments. Therefore, we split the data for each observer into a dataset in which for all trials the CFS mask was presented to the same or different eye on the previous trial (i.e. lag 1). We restricted our analyses to lag 1 only to ensure that we still had sufficient data. Figure 4 depicts the results of this analysis. As is apparent from this figure, the major contribution to the positive lag 1 correlation observed for the variable eye experiments stems from the trials in which the CFS mask is presented to the same eye as in the previous trial. Moreover, rather than being positive, the lag 1 correlation for the swap trials is low and negative, presumably due to the participants’ eye dominance.
Figure 4.
Lag 1 correlation analysis for the variable eye experiments. The bar plot depicts the lag 1 Spearman correlations for the full dataset (as depicted in Figure 3), and the dataset split up in trials in which the CFS mask was always presented to the same or different eye on the previous trial. The same eye trials contribute significantly to the positive lag 1 correlations. Error bars denote 95% confidence intervals.
Control data
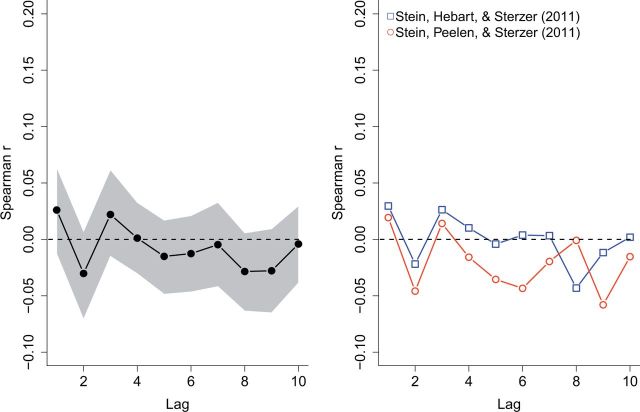
To ensure that our results were specific to CFS and not any reaction-time-based process in general, we subjected the data of the control experiments reported in Stein et al. (2011) (Experiment 1) and Stein et al. (2011) to the same analysis as we did above. Their particular experiments tested whether upright faces (or face-like stimuli) would break suppression faster than inverted faces in a control condition in which the target and mask stimulus were both presented in both eyes. Because the inclusion criteria for these experiments were quite stringent (same fade-in time and onset as in the CFS condition, CFS and control condition blocked rather than mixed), these experiments contained less trials than those in the main dataset (n = 120). Figure 5 depicts the aggregated serial correlation data for these two experiments. As is apparent from this figure, no consistent serial correlation pattern was observed. Indeed, a mixed-effects regression model with a main effect of lag was not preferred over one not including the main effect (χ(1) = 2.42, p = 0.1197)
Figure 5.
Serial correlations for the control dataset. For the binocular control condition (i.e. the CFS mask and the target stimulus are both presented to both eyes), there is no consistent decay in serial correlations.
Discussion
The goal of this study was to analyze whether successive suppression durations obtained in a b-CFS experiment show serial dependence, as this is considered to be a marker of the underlying neural alternation mechanism. When serial correlations are observed, this indicates that the underlying mechanism is not random and includes a memory component (van Ee, 2009). We have performed a serial correlation analysis on a large dataset of b-CFS sessions. In line with previous reports on binocular and perceptual rivalry, we observed small, but significant serial correlations in the suppression durations obtained in several, different b-CFS experiments. It should be noted that none of these experiments was originally designed to test the temporal dependency structure of suppression durations in a b-CFS paradigm. Therefore, one might argue that the results we obtained are merely due to methodological peculiarities inherent to some of the experiments we included. In contrast, we consider the diversity of our dataset as a strength and are encouraged by the fact that similar temporal dependency patterns (taking into account sampling variability) are observed across observers, stimuli, and laboratories.
The observations reported in this study further substantiate a growing literature on serial dependence in visual rivalry and furthermore provides insight into the temporal dynamics of interocular suppression induced through CFS. It has been debated whether CFS relies on distinct mechanisms or operates similar to binocular rivalry (Tsuchiya and Koch, 2005; Tsuchiya et al., 2006,Kaunitz et al., 2014; Moors et al., 2014). Our results suggest that the temporal dependency structure of suppression durations in CFS is akin to those observed in binocular rivalry which might imply that both phenomena tap into similar rather than distinct mechanisms. Interestingly, our distributional analysis of breakthrough rates indicated that fitting a gamma distribution to breakthrough rates yielded considerably worse fits (as quantified through the probability pKS) compared to what has been observed in other studies (Brascamp et al., 2005). This should not be too surprising, however, given that CFS is known to substantially increase the proportion of long suppression durations. On top of the positively skewed distribution that is generally observed, this aspect introduces a long and thick tail in the distribution that is not well captured by a gamma distribution. Moreover, the mask fade-out procedure that was employed in some of the experiments introduced, for some observers, a second peak in the distribution when the CFS mask reached a low contrast. Nevertheless, it should be noted that the overall low fit quality was also observed in the experiments that did not rely on this procedure (i.e. all PM experiments, see Table 1). When comparing both experiment types, however, the variable eye experiments yielded somewhat better fits compared to the fixed-type experiments. This might be explained by the fact that these experiments all relied on a mask fade-out procedure, which might have facilitated breakthroughs for some observers and yielded a better fit compared to the absence of a mask fade-out procedure (despite the censoring of the breakthrough rate distribution). In sum, it remains to be investigated whether the distribution of suppression durations or breakthrough rates can be captured by a single distribution or rather that a mixture of different distributions is more suitable to take into account the very long suppression durations observed in a typical b-CFS experiment.
Previous studies have generally shown evidence for serial dependence in dominance durations, mostly restricted to lag 1. Interestingly, we observed a gradual decay of serial correlations in the fixed eye experiments. In the variable eye experiments, the pattern of serial correlations was more in line with a previous study from visual rivalry (van Ee, 2009) in that they were most pronounced at lags 1 and 2 and fell off quickly to zero for longer lags. The same study simulated significant serial correlations at lag 1 using a computational model of visual rivalry (Noest et al., 2007) to which white noise was added at the slow timescale of percept adaptation (van Ee, 2009). The divergence between the observed serial correlation patterns in our fixed and variable eye experiments can also be interpreted in the light of these simulation results. That is, in the fixed eye experiments in which the CFS mask is continuously presented to the same eye, we observed gradually decreasing serial correlations for increasing lags. If the adaptation state of neurons involved in representing the CFS mask is responsible for the serial correlations in suppression durations, one would expect a longer-lasting influence for conditions in which the perceptual dominance of the CFS mask is caused by the continuous presentation to the same eye.
Those simulations, using white noise added at the slow timescale of percept adaptation (van Ee, 2009), did not allow for the reproduction of serial correlations at higher lags. To intuit how higher order serial correlations (beyond lag 1) can be obtained, the phenomenon of perceptual stabilization for an ambiguous stimulus upon intermittent presentation might be relevant. That is, if an ambiguous stimulus is intermittently presented, it is observed that its perception stabilizes upon repeated presentation (i.e. the percept at repetition n–1 transfers to repetition n) (Leopold et al., 2002). This phenomenon is generally explained by a short-lived perceptual memory mechanism that favors the most recent percept. A number of studies have shown, however, that perceptual stabilization also involves a longer term memory mechanism based on the relative proportion of dominance of one or the other stimulus (Brascamp et al., 2008; de Jong et al., 2012). It is possible to model the higher order serial dependencies by extending a conventional model in visual rivalry (Noest et al., 2007) to include adaptation at multiple time-scales (Brascamp et al., 2008). More recent work concluded that previously perceived interpretations dominate at the onset of ambiguous sensory information, whereas alternative interpretations dominate prolonged viewing (de Jong et al., 2012). At first instance ambiguous information seems to be judged using familiar percepts, while re-evaluation later on allows for alternative interpretations. Thus, the observed serial dependency structure might be modeled by including adaptation dynamics at multiple time scales (including noise at each of these levels). On a speculative note, the higher order serial dependency structure observed across all observers might be due to the fact that, during a single trial, CFS invokes mechanisms similar to perceptual stabilization. The continuous updating of the contents of the CFS mask may be thought of as intermittently presenting a stream of visual stimuli that might stabilize the current percept (the CFS mask) and therefore prolong dominance durations compared to regular binocular rivalry.
A second goal of our study pertained to the nature and site of the underlying mechanisms generating the serial dependency in suppression durations. That is, the nature of our dataset (fixed versus variable eye experiments) enabled us to test the relative influence of presenting the CFS mask continuously to the same eye rather than randomly swapping it throughout the experiment. As the analysis of the aggregated data indicated, the pattern of serial correlations diverges between the types of experiments considered, fixed versus variable eye presentation of the CFS masks. At first sight this would seem to suggest that both monocular and binocular mechanisms are at play in generating these serial correlations. Furthermore, given the discrepancy between both datasets at lags beyond 2, monocular mechanisms would be primarily responsible for the serial correlations observed at those lags. An additional analysis of the variable eye experiments indicated that the serial correlations observed in these experiments seem to be mostly driven by the trials in which the CFS mask is not switched to the other eye across consecutive trials. Thus, our data suggest that the mechanisms responsible for generating the observed temporal dynamics in CFS are primarily, but not necessarily exclusively, monocular in nature. This observation is well in line with other CFS studies highlighting that adaptation, perceptual learning, and stimulus-reward learning are primarily monocular (Seitz et al., 2009; Stein and Sterzer, 2011; Mastropasqua et al., 2015).
In this respect, it is interesting to note that Logothetis et al. (1996) also observed a lag 1 correlation of ∼0.1 for stimulus rivalry. Stimulus rivalry refers to the observation that rapidly and repetitively swapping the rivalling stimuli between the eyes does not substantially change the rivalry dynamics, indicating that rivalry would not be purely eye-based. However, Lee and Blake (1999) have shown that stimulus rivalry is limited to a certain combination of spatiotemporal parameters. Otherwise, eye rivalry dominates. Furthermore, recent studies showed that monocular channels contribute to stimulus rivalry (Brascamp et al., 2013) and that individual differences in the temporal dynamics of conventional binocular rivalry and stimulus rivalry are tightly linked (Patel et al., 2014), suggesting that both forms of rivalry might rely on similar mechanisms. In combination with our data, this might indicate that serial correlations in stimulus rivalry also have a monocular basis.
Conclusion
In this article, we asked whether consecutive suppression durations obtained across several different breaking CFS experiments are serially dependent, which would provide evidence for the underlying mechanism being nonrandom and having a memory component. Our serial correlation analysis indicated a gradual decay of serial correlations at the aggregate level for experiments in which the eye to which the CFS mask was presented was kept constant across trials. Thus, we conclude that the underlying competition mechanism of CFS includes a memory component. A different pattern emerged in the experiments in which the eye to which the CFS mask was presented was randomly determined on each trial. Here, serial correlations decayed more rapidly to zero beyond lag 2. However, these correlations at early lags were shown to be due to trials in which the CFS mask was not switched across consecutive trials. This indicates that the observed serial correlations are predominantly driven by a process that is monocular in nature. A control analysis confirmed that the serial correlations were not due to any generic reaction-time-based process. These findings further substantiate the literature on serial dependence in visual rivalry and furthermore shed light on the similarities and differences between the underlying dynamics in these breaking CFS experiments and those observed in binocular rivalry. We suggest that the temporal dependency structure of suppression durations in CFS is akin to those observed in binocular rivalry, which might imply that both phenomena tap into similar rather than distinct mechanisms.
Supplementary Data
Supplementary data is available at Neuroscience of Consciousness Journal online.
Data Availability Statement
All data reported in this paper is available upon request from the corresponding author.
Additional Information
The authors declare no competing financial interests.
Supplementary Material
Acknowledgments
This work was supported by a Methusalem Grant (METH/08/02 and METH/14/02) awarded to Johan Wagemans, a doctoral fellowship from the Research Foundation—Flanders (FWO) awarded to Pieter Moors, and a German Research Foundation grant (grant STE 2239/1-1) awarded to Timo Stein. Raymond van Ee was supported by FWO and the EU Horizon 2020 program (HealthPac). The research leading to these results has received funding from the People Programme (Marie Curie Actions) of the European Union”s Seventh Framework Programme (FP7/2007–2013) under REA grant agreement number 329363.
References
- Alais D. Binocular rivalry: competition and inhibition in visual perception. Wiley Interdiscip Rev Cogn Sci 2012, 3(1): 87–103. 10.1002/wcs.151. [DOI] [PubMed] [Google Scholar]
- Alais D, Blake R. Binocular rivalry and perceptual ambiguity. In: Wagemans J. (ed.), Oxford Handbook of Perceptual Organization 2015. Oxford, UK: Oxford University Press. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995; 57(1): 289–300. [Google Scholar]
- Blake R. A neural theory of binocular rivalry. Psychol Rev 1989; 96(1): 145–67. [DOI] [PubMed] [Google Scholar]
- Blake R, Fox R, McIntyre C. Stochastic properties of stabilized-image binocular rivalry alternations. J Exp Psychol 1971; 88(3): 327–32. [DOI] [PubMed] [Google Scholar]
- Blake R, Logothetis NK. Visual competition. Nat Rev Neurosci 2002; 3(1): 13–21. 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- Borsellino A, De Marco A, Allazetta A, et al. Reversal time distribution in the perception of visual ambiguous stimuli. Kybernetik 1972; 10(3): 139–44. 10.1007/BF00290512. [DOI] [PubMed] [Google Scholar]
- Brascamp JW, Knapen THJ., Kanai R, et al. Multi-timescale perceptual history resolves visual ambiguity. PLoS ONE 2008; 3(1): e1497 10.1371/journal.pone.0001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brascamp JW, Sohn H, Lee S-H, et al. A monocular contribution to stimulus rivalry. Proc Natl Acad Sci USA 2013; 110(21): 8337–44. 10.1073/pnas.1305393110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brascamp JW, van Ee R, Pestman WR, et al. Distributions of alternation rates in various forms of bistable perception. J Vis 2005; 5(4): 1 10.1167/5.4.1. [DOI] [PubMed] [Google Scholar]
- Brouwer GJ, van Ee R. Endogenous influences on perceptual bistability depend on exogenous stimulus characteristics. Vis Res 2006; 46(20): 3393–402. 10.1016/j.visres.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Cicchini GM, Anobile G, Burr DC. Compressive mapping of number to space reflects dynamic encoding mechanisms, not static logarithmic transform. Proc Natl Acad Sci USA 2014; 111(21): 7867–72. 10.1073/pnas.1402785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong MC, Knapen T, van Ee R. Opposite influence of perceptual memory on initial and prolonged perception of sensory ambiguity. PLoS ONE 2012; 7(1): e30595 10.1371/journal.pone.0030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin J. Distribution Theory for Tests Based on the Sample Distribution Function 1973. (Vol. 9). Philadelphia, PA: Siam. [Google Scholar]
- Fischer J, Whitney D. Serial dependence in visual perception. Nat Neurosci 2014; 17(5), 738–743. 10.1038/nn.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R, Herrmann J. Stochastic properties of binocular rivalry alternations. Percept Psychophys 1967; 2(9), 432–36. 10.3758/BF03208783. [DOI] [Google Scholar]
- Gayet S, Van Der Stigchel S, Paffen C. Breaking continuous flash suppression: competing for consciousness on the pre-semantic battlefield. Front Psychol 2014; 5: 460 10.3389/fpsyg.2014.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann G, Moors P. Definitely maybe: can unconscious processes perform the same functions as conscious processes? Front Psychol 2015; 6: 584. 10.3389/fpsyg.2015.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman T, Moors P. Frequent words do not break continuous flash suppression differently from infrequent or nonexistent words: implications for semantic processing of words in the absence of awareness. PLoS ONE 2014; 9(8): e104719 10.1371/journal.pone.0104719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurvich CM, Simonoff JS, Tsai CL. Smoothing parameter selection in nonparametric regression using an improved akaike information criterion. J Roy Stat Soc B 1998; 60: 271–93. [Google Scholar]
- Jiang Y, Costello P, He S. Processing of invisible stimuli: advantage of upright faces and recognizable words in overcoming interocular suppression. Psychol Sci 2007; 18(4): 349–55. 10.1111/j.1467-9280.2007.01902.x. [DOI] [PubMed] [Google Scholar]
- Kaunitz LN, Fracasso A, Skujevskis M, et al. Waves of visibility: probing the depth of inter-ocular suppression with transient and sustained targets. Front Psychol 2014; 5: 804 10.3389/fpsyg.2014.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Blake R. Rival ideas about binocular rivalry. Vis Res 1999; 39(8): 1447–54. [DOI] [PubMed] [Google Scholar]
- Lehky SR. Binocular rivalry is not chaotic. Proc Roy Soc B 1995; 259(1354): 71–6. 10.1098/rspb.1995.0011. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Wilke M, Maier A, et al. Stable perception of visually ambiguous patterns. Nat Neurosci 2002; 5(6): 605–9. 10.1038/nn0602-851. [DOI] [PubMed] [Google Scholar]
- Levelt WJM. On Binocular Rivalry 1965. Assen, The Netherlands: Van Gorcum. [Google Scholar]
- Liberman A, Fischer J, Whitney D. Serial dependence in the perception of faces. Curr Biol; 24(21), 2569– 74 10.1016/j.cub.2014.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Leopold DA, Sheinberg DL. What is rivalling during binocular rivalry? Nature 1996; 380(6575): 621–4. 10.1038/380621a0 [DOI] [PubMed] [Google Scholar]
- Lupyan G, Ward EJ. Language can boost otherwise unseen objects into visual awareness. Proc Natl Acad Sci 2013. 10.1073/pnas.1303312110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamassian P, Goutcher R. Temporal dynamics in bistable perception. J Vis 2005; 5(4): 361–75. http://doi.org/10:1167/5.4.7 [DOI] [PubMed] [Google Scholar]
- Mastropasqua T, Tse PU, Turatto M. Learning of monocular information facilitates breakthrough to awareness during interocular suppression. Atten Percept Psychophys 2015; 77: 790–803. 10.3758/s13414-015-0839-z. [DOI] [PubMed] [Google Scholar]
- Moors P, van Crombruggen S, Wagemans J, et al. Perceptual grouping without awareness: collinear contour facilitation or surface filling-in? Perception 2013; 42: 114. [Google Scholar]
- Moors P, Wagemans J, de-Wit L. Moving stimuli are less effectively masked using traditional continuous flash suppression (CFS) compared to a moving mondrian mask (MMM): a test case for feature-selective suppression and retinotopic adaptation. PLoS ONE 2014; 9(5): e98298 10.1371/journal.pone.0098298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noest AJ, van Ee R, Nijs MM, et al. Percept-choice sequences driven by interrupted ambiguous stimuli: a low-level neural model. J Vis 2007; 7(8): 10 10.1167/7.8.10. [DOI] [PubMed] [Google Scholar]
- Pastukhov A, Braun J. Cumulative history quantifies the role of neural adaptation in multistable perception. J Vis 2011; 11(10): 12 10.1167/11.10.12. [DOI] [PubMed] [Google Scholar]
- Patel V, Stuit S, Blake R. Individual differences in the temporal dynamics of binocular rivalry and stimulus rivalry. Psychon Bull Rev 2014. 10.3758/s13423-014-0695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto Y, van Gaal S, de Lange FP, et al. Expectations accelerate entry of visual stimuli into awareness. J Vis 2015; 15(8): 13 10.1167/15.8.13. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Kim D, Watanabe T. Rewards evoke learning of unconsciously processed visual stimuli in adult humans. Neuron 2009; 61(5): 700–7. 10.1016/j.neuron.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka D, Kaneko K. Dynamical systems modeling of continuous flash suppression. Vis Res 2011; 51(6): 521–8. 10.1016/j.visres.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Stein T, Hebart MN, Sterzer P. Breaking continuous flash suppression: a new measure of unconscious processing during interocular suppression? Front Hum Neurosci 2011; 5:167 10.3389/fnhum.2011.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein T, Peelen MV, Sterzer P. Adults “awareness of faces follows newborns” looking preferences. PLoS ONE 2011; 6(12): e29361 10.1371/journal.pone.0029361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein T, Seymour K, Hebart MN, et al. Rapid fear detection relies on high spatial frequencies. Psychol Sci 2014; 25(2): 566–74. 10.1177/0956797613512509. [DOI] [PubMed] [Google Scholar]
- Stein T, Sterzer P. High-level face shape adaptation depends on visual awareness: evidence from continuous flash suppression. J Vis 2011; 11(8): 1–14. 10.1167/11.8.5. [DOI] [PubMed] [Google Scholar]
- Stein T, Sterzer P. Unconscious processing under interocular suppression: getting the right measure. Frontiers in Psychology 2014; 5: 387 http://doi.org/doi: 10.3389/fpsyg.2014.00387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein T, Sterzer P, Peelen MV. Privileged detection of conspecifics: evidence from inversion effects during continuous flash suppression. Cognition 2012; 125(1): 64–79. 10.1016/j.cognition.2012.06.005 [DOI] [PubMed] [Google Scholar]
- Tong F, Meng M, Blake R. Neural bases of binocular rivalry. Trends Cogn Sci 2006; 10(11): 502–11. 10.1016/j.tics.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Koch C. Continuous flash suppression reduces negative afterimages. Nat Neurosci 2005; 8(8): 1096–101. 10.1038/nn1500 [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Koch C, Gilroy LA, et al. Depth of interocular suppression associated with continuous flash suppression, flash suppression, and binocular rivalry. J Vis 2006;6(10): 1068–78. 10.1167/6.10.6 [DOI] [PubMed] [Google Scholar]
- Van Ee R. Dynamics of perceptual bi-stability for stereoscopic slant rivalry and a comparison with grating, house-face, and Necker cube rivalry. Vis Res 2005; 45(1): 29–40. 10.1016/j.visres.2004.07.039 [DOI] [PubMed] [Google Scholar]
- Van Ee R. Stochastic variations in sensory awareness are driven by noisy neuronal adaptation: evidence from serial correlations in perceptual bistability. J Opt Soc Am A Opt Image Sci Vis 2009; 26(12): 2612–22. [DOI] [PubMed] [Google Scholar]
- Van Ee R, Noest AJ, Brascamp JW, et al. Attentional control over either of the two competing percepts of ambiguous stimuli revealed by a two-parameter analysis: means do not make the difference. Vis Res 2006; 46(19): 3129–41. 10.1016/j.visres.2006.03.017 [DOI] [PubMed] [Google Scholar]
- Verhoeff FH. A new theory of binocular vision. Arch Ophthalmol 1935; 13: 151–75. 10.1001/archopht.1935.00840020011001. [DOI] [Google Scholar]
- Wagenmakers E-J, Brown S. On the linear relation between the mean and the standard deviation of a response time distribution. Psychol Rev 2007; 114(3): 830–41. 10.1037/0033-295X.114.3.830. [DOI] [PubMed] [Google Scholar]
- Walker P. Stochastic properties of binocular rivalry alternations. Percept Psychophy 1975; 18(6): 467–73. 10.3758/BF03204122. [DOI] [Google Scholar]
- Walker P. Binocular rivalry: central or peripheral selective processes? Psychol Bull 1978; 85(2): 376–89. 10.1037/0033-2909.85.2.376. [DOI] [Google Scholar]
- Yang E, Zald DH, Blake R. Fearful expressions gain preferential access to awareness during continuous flash suppression. Emotion 2007; 7(4): 882–6. 10.1037/1528-3542.7.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper is available upon request from the corresponding author.