ABSTRACT
Apical constriction regulates epithelial morphogenesis during embryonic development, but how this process is controlled is not understood completely. Here, we identify a Rho guanine nucleotide exchange factor (GEF) gene plekhg5 as an essential regulator of apical constriction of bottle cells during Xenopus gastrulation. plekhg5 is expressed in the blastopore lip and its expression is sufficient to induce ectopic bottle cells in epithelia of different germ layers in a Rho-dependent manner. This activity is not shared by arhgef3, which encodes another organizer-specific RhoGEF. Plekhg5 protein is localized in the apical cell cortex via its pleckstrin homology domain, and the GEF activity enhances its apical recruitment. Plekhg5 induces apical actomyosin accumulation and cell elongation. Knockdown of plekhg5 inhibits activin-induced bottle cell formation and endogenous blastopore lip formation in gastrulating frog embryos. Apical accumulation of actomyosin, apical constriction and bottle cell formation fail to occur in these embryos. Taken together, our data indicate that transcriptional regulation of plekhg5 expression at the blastopore lip determines bottle cell morphology via local polarized activation of Rho by Plekhg5, which stimulates apical actomyosin activity to induce apical constriction.
KEY WORDS: Apical constriction, Bottle cell, Gastrulation, RhoGEF, Actin, Myosin, Xenopus
Highlighted Article: The RhoGEF plekhg5 is identified as a blastopore lip-specific gene that, through the organization of actomyosin activity, induces apical constriction, which is required for bottle cell formation during Xenopus gastrulation.
INTRODUCTION
Apical constriction refers to an active reduction of cell apical surface area that then causes further cell shape changes, such as elongation of cells along the apical-basal axis and/or expansion of the basolateral cell compartment. Apical constriction can drive bending of epithelial cell sheets, generate lumens and tubes, facilitate cell ingression and tissue invagination, and promote epithelial-to-mesenchymal transition (EMT). Apical constriction is thus a central mechanism that underlies epithelial morphogenesis during multiple developmental contexts, such as gastrulation, neural tube closure and sensory organ formation (reviewed by Sawyer et al., 2010). In adults, apical constriction is also used in distinct conditions, such as wound healing. The reiterative usage of apical constriction in various tissue contexts highlights the importance of understanding the cellular and the molecular mechanisms that control this fundamental process.
One common theme that is emerging from studies of apical constriction in different tissue contexts in a wide range of animal models is that polarized positioning and activation of the actomyosin cytoskeleton within the constricting cells is crucial (reviewed by Martin and Goldstein, 2014). Both F-actin assembly and myosin accumulation and activation occur preferentially in the apical cell cortex before apical constriction, and the contractile forces generated by this apical actomyosin decrease apical cell surface area (Martin et al., 2009; Ebrahim et al., 2012; Mason et al., 2013). The polarized assembly of the cytoskeleton network near the apical cell membrane must be tightly controlled, both temporally and spatially, for the coordinated individual cell shape changes that drive global tissue morphogenesis to occur (Martin and Goldstein, 2014). Members of the Rho family of small GTPases have often been implicated in such precise control of actomyosin dynamics during apical constriction.
The main members of the Rho family of GTPases include RhoA, Rac1 and Cdc42. All of them are involved in the regulation of actomyosin cytoskeleton, though they exert differential effects on the structure and the dynamics of the actomyosin cytoskeleton. In mammalian cells, RhoA preferentially controls stress fiber and focal adhesion formation, whereas Rac1 and Cdc42 are associated mainly with lamellipodia and filopodia protrusions, respectively (Hall, 1998). The Rho proteins switch between a GTP-bound active state and a GDP-bound inactive state. The conversion between the two states is regulated by guanine nucleotide exchange factors (GEFs), which catalyze the exchange of GDP for GTP to activate Rho proteins, and GTPase-activating proteins (GAPs), which enhance the low intrinsic GTPase activity of Rho members to inactivate them. GEFs and GAPs respond to various intra- and inter-cellular signals to control diverse functions of Rho proteins, such as in cell division, differentiation and movements (Hodge and Ridley, 2016). Rho members, as well as their GEF and GAP regulators, have been shown to regulate apical constriction. During Drosophila gastrulation, a Rho-specific GEF, DRhoGEF2, is enriched apically in invaginating ventral furrow cells, regulates apical myosin II accumulation and F-actin assembly, and is required for RhoA-dependent cell shape changes and normal tissue invagination (Barrett et al., 1997; Hacker and Perrimon, 1998; Nikolaidou and Barrett, 2004; Barmich et al., 2005). A requirement for RhoA-dependent apical constriction has also been described during gastrulation of sea urchin and ascidian, though the upstream Rho regulators have not been reported in these species (Beane et al., 2006; Sherrard et al., 2010). In contrast, Cdc42, but not Rho, appears to be crucial during Caenorhabditis elegans endodermal internalization at gastrulation. Cell contact-induced recruitment of a Cdc42-specific GAP, PAC-1, results in inactivation of Cdc42 at the basolateral cell membrane, leaving active Cdc42 only at the contact-free apical surface. This stimulates the activity of the Cdc42 effector myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK)-1 apically to phosphorylate and activate myosin II for apical constriction of endodermal cells (Lee and Goldstein, 2003; Anderson et al., 2008; Chan and Nance, 2013; Marston et al., 2016). Thus, apical constriction can be driven by different upstream regulators that converge on the regulation of the apical actomyosin cytoskeleton. Unlike in invertebrates, the GEFs and GAPs used during gastrulation of vertebrate embryos have not been described in detail.
During Xenopus gastrulation, a group of surface cells undergo apical constriction and basolateral elongation and expansion to form bottle-shaped cells. The cortical melanosomes become concentrated as the apical cell surface shrinks, marking the bottle cells with dark pigmentation. The bottle cells first appear on the dorsal side (known as the dorsal lip) and subsequently spread laterally and ventrally to encompass the entire blastopore (blastopore lip). Mesodermal and endodermal tissues involute through the blastopore and thereby internalize. The formation, morphology and function of the bottle cells were described using scanning electron microscopy and time-lapse video microscopy studies decades ago (Keller, 1981; Hardin and Keller, 1988), and the molecular machinery that is involved in this process is currently being uncovered. It has been shown that both actin and microtubule cytoskeletons regulate bottle cell formation, and endocytosis is required to remove apical cell membrane for efficient apical constriction (Lee and Harland, 2007, 2010). Upstream regulators of bottle cell formation include the activin/nodal signaling pathway, which can induce ectopic bottle cells that are associated with ectopic mesendoderm in the animal region (Kurth and Hausen, 2000). The components in the Wnt planar cell polarity pathway and the apical-basal polarity protein Lethal-giant-larvae (Lgl) have also been implicated in regulating bottle cell formation (Choi and Sokol, 2009; Ossipova et al., 2015). However, all these factors are expressed more broadly than at the blastopore lip. It is thus unclear how positioning of the bottle cells is regulated in gastrulating embryos and whether and which Rho GEFs or GAPs participate in controlling the apical constriction of bottle cells.
In this study, we report the identification of a RhoGEF, plekhg5, as a blastopore lip-specific gene during Xenopus gastrulation. Plekhg5 protein is apically localized in epithelial cells and can organize apical actomyosin assembly. plekhg5 induces ectopic blastopore lip-like morphology in a Rho-dependent fashion in epithelial cells, and its gene product is required for bottle cell formation in Xenopus embryos. Our studies therefore reveal that expression of a tissue-specific RhoGEF is both necessary and sufficient to induce apical constriction, which is required for bottle cell formation during Xenopus gastrulation.
RESULTS
plekhg5 is expressed in cells at the blastopore lip during Xenopus gastrulation
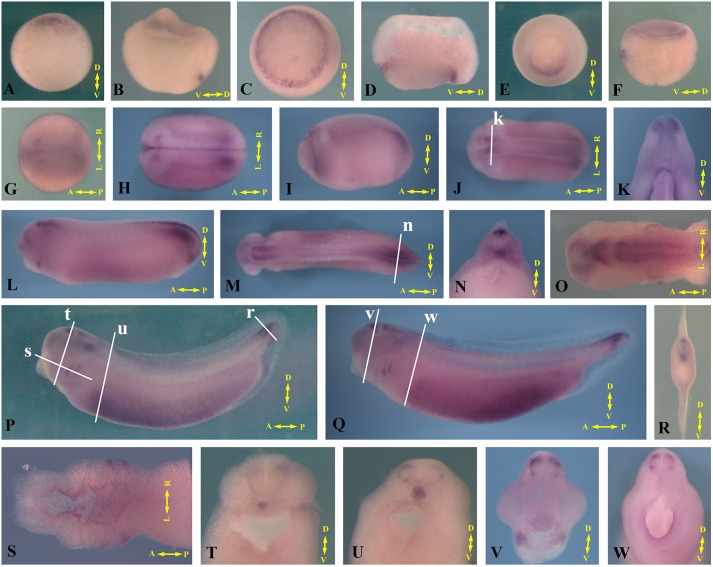
In a previous RNA-seq study of differentially expressed genes in distinct tissues of Xenopus gastrulae, we identified plekhg5 as a RhoGEF that is enriched in the organizer of early Xenopus embryos (Popov et al., 2017). Whole-mount in situ hybridization (ISH) revealed that plekhg5 RNA is first detected in early gastrula embryos in the dorsal lip region. Its expression then spreads to encompass the entire blastopore lip during mid-gastrulation and is downregulated once cells involute inside the embryos and re-spread at late gastrula stages (Fig. 1A-F). Bisected embryos showed that plekhg5 expression is limited to the surface cells at the blastopore lip (Fig. 1B,D,F). At the neurula and early tailbud stages, plekhg5 RNA is seen in the head at the hindbrain level and the tail regions (Fig. 1G-K). This pattern of expression persists through the late tailbud stages, with additional expression apparent in the otic placodes and the pharyngeal pouches (Fig. 1L,M). Both the notochord and the dorsal neural tube in the tail region contain plekhg5 transcripts (Fig. 1N). As development proceeds, plekhg5 expression in the hindbrain region is seen as two distinct domains, with the anterior chevron-shaped domain reminiscent of the rhombic lip structure that contributes to the future cerebellum (Fig. 1O). In tadpoles, plekhg5 expression remains in the hindbrain region, pharyngeal pouches and the tip of the tails (Fig. 1P,Q). In addition, ventral mesodermal cells show increasing plekhg5 expression from tailbud stages onward (Fig. 1L,P,Q). Bisected embryos reveal notochordal and dorsal neural staining of plekhg5 transcripts at the tail (Fig. 1R), whereas more anterior regions have transient expression of plekhg5 in the notochord that disappears at slightly later stages (Fig. 1T-W). Neural crest cells migrating toward and in the dorsal root ganglia also contain plekhg5 (Fig. 1T-W). Furthermore, embryos bisected along the horizontal plane show specific plekhg5 signals at the tips of the protruding pharyngeal pouches and in the epithelial cells lining the pharyngeal cavity (Fig. 1S). The dynamic expression of plekhg5 in tissues that are undergoing morphogenesis and in migrating cells suggests that this gene may regulate epithelial bending and other morphogenetic processes during early Xenopus development.
Fig. 1.
Dynamic expression of plekhg5 in early Xenopus embryos. (A-F) plekhg5 is expressed in the blastopore lip during gastrulation. Vegetal view (A,C,E) and side view of bisected embryos (B,D,F) are shown. (G-O) During neurula (G,H) and tailbud (I-O) stages, plekhg5 is expressed in the tail, hindbrain, otic and olfactory placodes, and pharyngeal pouch. Sections of the embryos reveal plekhg5 transcripts in the dorsal neural tube and the notochord. (P,Q) Expression of plekhg5 at the tadpole stages is detected in the hindbrain, otic vesicles, tail, pharyngeal pouch and the ventral-lateral mesoderm. (R-W) Sections of the tadpole embryos show expression of plekhg5 in the notochord, transiently in the trunk but persisting in the tail, the migrating neural crest cells along the ventral route and in the dorsal root ganglia, the dorsal neural tube in the tail, the tips of the outgrowing pharyngeal pouches, and the lining of the foregut. White lines in J,M,P,Q show the position of the sections in the panels indicted by the letters. The embryonic axes are labeled in each panel: D-V, dorsal-ventral; A-P, anterior-posterior; L-R, left-right.
Ectopic expression of plekhg5 induces blastopore lip-like morphology in a Rho-dependent manner
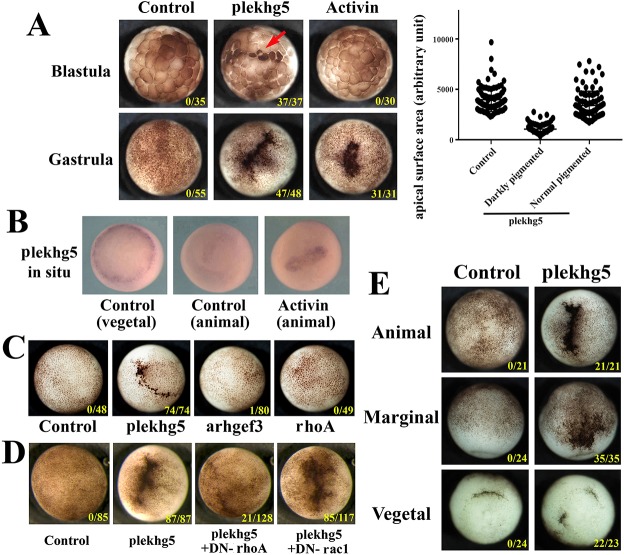
To examine the function of plekhg5, we first injected plekhg5 RNA into the animal region of two-cell-stage embryos. Cells with concentrated pigmentation and reduced apices were observed at early blastula stages, with apical cell surface areas of darkly pigmented cells reducing to about one-third that seen in neighboring cells or cells in control embryos (Fig. 2A, Fig. S1). When compared with the growth factor activin, which has been shown to induce ectopic bottle cells in the animal region (Kurth and Hausen, 2000), we observed that activin treatment induced ectopic blastopore lip at early gastrula, but not blastula, stages (Fig. 2A and Movie 1). This suggested that activin might induce ectopic plekhg5 expression when inducing ectopic blastopore lip, an idea that was supported by our ISH results (Fig. 2B). As Rho signaling has been implicated in bottle cell formation in Xenopus (Ossipova et al., 2015), we examined whether overexpression of rhoA was sufficient to induce ectopic blastopore lip. The injection of tenfold higher doses of rhoA RNA than of plekhg5 RNA (1 ng versus 0.1 ng) did not result in ectopic bottle cell formation (Fig. 2C). In addition, arhgef3, another RhoGEF that is enriched in the early organizer of Xenopus embryos (Hufton et al., 2006), did not induce ectopic blastopore lip in the animal region (Fig. 2C). To confirm that Rho signaling is required by plekhg5 to induce ectopic blastopore lip, we co-injected plekhg5 with a dominant negative rhoA construct (DN-rhoA, or rhoA-T19N). DN-rhoA blocked bottle cell induction by plekhg5, whereas DN-rac1 (rac1-T17N) was inefficient in doing so (Fig. 2D, Fig. S2). Furthermore, neither DN-Rab11 nor Vangl2-MO, which have been shown to regulate bottle cell formation in Xenopus (Ossipova et al., 2015), blocked plekhg5 (Fig. S3). Induction of the blastopore lip-like morphology by plekhg5 was not limited to the animal region, as marginal or vegetal injection of the RNA also induced darkly pigmented cells in those regions (Fig. 2E). ISH of the mesodermal markers brachyury (bra) and goosecoid (gsc) revealed that, although activin-dependent ectopic bottle cell induction was associated with expression of these markers, neither gene was turned on by plekhg5 (Fig. S4). In addition, cells involuted through the ectopic lip induced by activin, whereas plekhg5 caused bending of the ectodermal sheet toward the darkly pigmented cells without efficient invagination (Movie 1). Taken together, the results demonstrate that plekhg5 directly induces cell morphological changes in a Rho-dependent manner without invoking cell fate changes, and that the activity of plekhg5 cannot be attributed simply to general Rho activation in a cell, but may rely on localized regulation of subcellular Rho signaling.
Fig. 2.
plekhg5 induces ectopic blastopore lip-like morphology in early Xenopus embryos in a Rho-dependent manner. (A) plekhg5 expression induces apical cell constriction in ectodermal cells at early blastula stages, whereas activin induces ectopic blastopore lip at the gastrula stages. The apical surface areas of cells at the blastula stages in control and the plekhg5-expressing embryos are measured and compared. The scatter plot shows a typical experiment. plekhg5 significantly reduces apical cell surfaces to about one-third that seen in control cells, with the average surface areas of 3978, 1050 and 3409 (arbitrary units) for control, apically constricted, and normal pigmented cells, respectively. Student's t-test gives a P-value of 3.5E-31 in this experiment. Red arrow indicates apically constricted cells at the blastula stages. (B) Activin induces expression of plekhg5 in the ectoderm when it induces an ectopic blastopore lip. (C) Unlike plekhg5, neither arhgef3 nor general expression of rhoA induces ectopic blastopore lip morphology in the ectoderm. (D) Dominant-negative rhoA, but not rac1, blocks ectopic blastopore lip induction by plekhg5. (E) plekhg5 induces ectopic blastopore lip morphology when injected either in the animal, the marginal zone or the vegetal regions. The doses of RNAs used are 100 pg of plekhg5, 5 pg of activin, 200 pg arhgef3, 0.5-1 ng of rhoA, DN-rhoA and DN-rac1. Numbers in each image indicate embryos exhibiting the ectopic blastopore lip-like morphology over the total number of embryos. All the experiments are repeated at least three times.
plekhg5 promotes elongation of the superficial epithelial cells
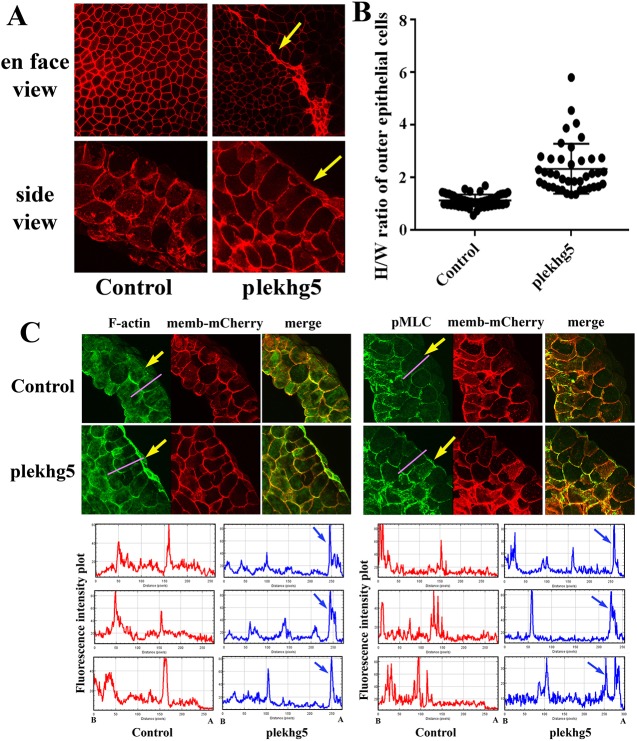
To analyze how plekhg5 expression modulates cell shape, we co-injected RNAs of plekhg5 and membrane-targeted mCherry fluorescent protein in the animal region of early embryos. Cell morphology was examined at early gastrula stages using both en face and side views from confocal microscopy. In control embryos, cells displayed similar sizes from the en face view and showed cuboidal shape from the side view (Fig. 3A). In plekhg5-expressing embryos, cells with dark pigmentation showed enhanced mCherry signals and a mixture of many smaller apices intermingled with several large ones, implying that overexpression of plekhg5 might create a mechanical competition between neighboring cells, in which the tension that is generated by apically constricting cells with reduced apical areas stretches adjacent cells, leading to enlarged apical surfaces. The side view of the cells revealed that the outer epithelial cells had an elongated morphology, many without basal expansion, whereas the deeper cells retained the round shape (Fig. 3A). Measurement of the ratio of apical-basal cell height over apical cell width of the superficial epithelial cells showed that in the plekhg5-expressing embryos, the ratio had a significant increase of 81% over that in control embryos (Fig. 3B). In addition, ectopic plekhg5 expression prevented radial cell intercalation in the animal region so that multi-layered inner cells were observed without the ectodermal thinning that was seen in the control embryos (Fig. S5). The data indicate that plekhg5 acts differentially in epithelial and mesenchymal cells and that plekhg5 regulates apical constriction and apical-basal cell elongation in superficial epithelial cells.
Fig. 3.
plekhg5 induces cell elongation and apical actomyosin accumulation in outer epithelial cells. (A) En face view of early gastrula embryos shows reduced cell surfaces in plekhg5-expressing cells (arrows) compared with those in control embryos. Side view of the bisected embryos shows elongation of superficial epithelial cells from plekhg5-injected embryos. (B) H/W ratio analysis shows that plekhg5-expressing outer epithelial cells have a significant increase in H/W ratio from 1.2 in control cells to 2.2 in plekhg5-expressing cells. Student's t-test gives P=8.3E-21. (C) plekhg5 stimulates apical accumulation of both F-actin and pMLC. The membrane-mCherry signal is used to label the injected cells. Arrows indicate the apical F-actin and pMLC signals. Fluorescence intensity is measured using ImageJ along the axis indicated by the pink line across the animal regions. The plots for three different biological samples are shown with the apical (A) and basal (B) direction labeled at the bottom. Blue arrows point to the apical enhancement of F-actin and pMLC signals.
plekhg5 stimulates apical actomyosin accumulation in outer epithelial cells
Apical constriction is often the result of polarized localization and activation of the actomyosin contractile machinery. To examine how the expression of plekhg5 regulates the actomyosin cytoskeleton, we stained the bisected embryos or dissected animal caps with Alexa Fluor 488-conjugated phalloidin to visualize F-actin and performed fluorescence immunocytochemistry using an anti-phosphorylated myosin regulatory light chain (pMLC) antibody to detect activated myosin (Fig. 3C, Fig. S6). In control embryos, F-actin was seen mainly at the cell junctions, whereas in plekhg5-expressing embryos F-actin was strongly enriched underneath the apical cell membrane in outer epithelial cells, but showed a cell contact-associated distribution in the inner cells that was similar to that seen in control embryos (Fig. 3C, Fig. S6). Similarly, a pMLC signal was detected mainly at the cell borders of the superficial cells, but it showed an apical enrichment in plekhg5-expressing embryos (Fig. 3C, Fig. S6). The data therefore demonstrate that plekhg5 facilitates polarized actomyosin accumulation in the apical cell cortex in superficial epithelial cells.
Plekhg5 is localized apically in superficial epithelial cells
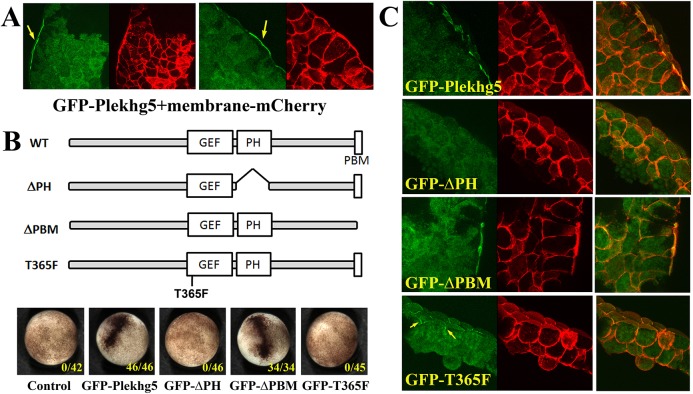
The polarized enrichment of the apical actomyosin cytoskeleton suggests that Plekhg5 protein may be localized in a polarized fashion in epithelial cells. To test this, we inspected Plekhg5 protein distribution using a green fluorescent protein (GFP)-tagged Plekhg5 that preserved its ability to induce ectopic blastopore lip in Xenopus embryos (Fig. 4). When expressed in the ectoderm, GFP-Plekhg5 was detected at high levels near the apical cell surface of the outer epithelial cells but was diffuse in cells of the deeper layers (Fig. 4A).
Fig. 4.
Plekhg5 is apically localized in the superficial epithelial cells. (A) GFP-tagged Plekhg5 protein is detected at the apical cell cortex in the superficial epithelial cells (arrows), but is diffuse in deeper ectodermal cells. (B) Plekhg5 contains a PH domain and a PBM in addition to the GEF domain. Analyses of the deletion mutants that lack one of these domains reveal that removal of the PH domain, but not the PBM motif, abolishes the ability of the protein to induce ectopic blastopore lip. In addition, a point mutation that alters the conserved threonine 365 residue in the GEF domain into phenylalanine also results in non-functional Plekhg5. Numbers in each image indicate embryos exhibiting the ectopic blastopore lip-like morphology over the total number of embryos. (C) Deletion of the PH domain, but not the PBM, results in loss of apical accumulation of the proteins. The T365F GEF mutant protein can be recruited to the cell junctions in epithelial cells (arrows), but is not enriched at the apical cell cortex.
Plekhg5 contains a pleckstrin homology (PH) domain and a PDZ-binding motif (PBM), in addition to the GEF domain (Fig. 4B). To test the role of these domains in Plekhg5 localization, we made two deletion mutants that removed the PH and the PBM domains, respectively. Functional studies showed that deletion of the PH domain, but not the PBM motif, rendered the mutant protein incapable of inducing an ectopic blastopore lip (Fig. 4B). Consistent with the functionality, deletion of the PBM motif did not alter the apical enrichment of the mutant protein, but deletion of the PH domain led to the loss of apical accumulation of Plekhg5 (Fig. 4C). Western blot analysis demonstrated that all the proteins were expressed at similar levels (Fig. S7). To see whether GEF activity is required for apical localization of the protein, we also made a point mutation that altered the conserved threonine at the amino acid position 365 in the GEF domain to phenylalanine (T365F; Aghazadeh et al., 1998; Liu et al., 1998). This GEF mutant could not induce ectopic blastopore lip, but was recruited to the cell junctions in the superficial epithelial cells (Fig. 4A,C). However, unlike the wild-type Plekhg5, the T365F mutant was not enriched underneath the apical cell membrane (Fig. 4C). The results establish that apical accumulation of Plekhg5 in the outer epithelial cells requires both the PH domain and GEF activity.
plekhg5 is required for blastopore lip formation
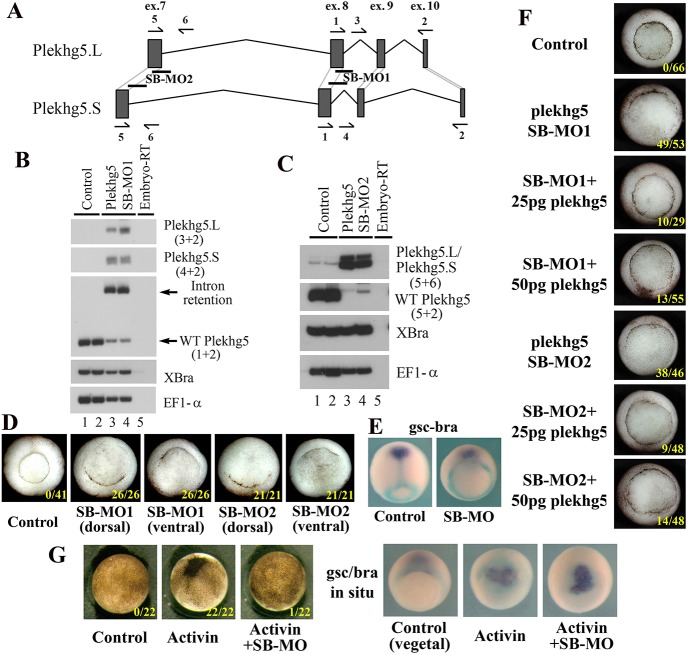
To examine the endogenous function of Plekhg5 during blastopore lip formation, we designed two splicing-blocking (SB) antisense morpholino oligos (MOs). SB-MO1 targeted the 3′ junction of exon 8 and the following intron, whereas SB-MO2 spanned the 3′-end of exon 7 and the adjacent intron (Fig. 5A). These SB-MOs blocked the splicing donor sites that were conserved between both L and S alloalleles in Xenopus laevis, leading to intron retention and premature translational termination. The resulting truncated protein lacked the GEF domain and was expected to be non-functional. RT-PCR analysis of plekhg5 RNA transcripts from the morphant embryos at gastrula stages showed that both SB-MOs worked efficiently to block RNA splicing of both L and S alloalleles (Fig. 5B,C). When injected into the marginal zone of early frog embryos, the MOs blocked formation of the blastopore lip. Depending on the site of MO injection, the blastopore lip from the dorsal, lateral or ventral regions could be affected (Fig. 5D, Fig. S8A). The inhibition of the blastopore lip was not due to altered mesodermal specification, as both the prechordal marker gsc and the trunk mesodermal marker bra were expressed in the morphant embryos, though the movements of the tissues that expressed these markers were impaired (Fig. 5E). The defects in blastopore lip formation in the morphant embryos were largely rescued when the SB-MOs were co-injected with low doses of full length plekhg5 RNA (Fig. 5F, Fig. S9), which demonstrated that the morphant phenotype was specific to the knockdown of the plekhg5 gene.
Fig. 5.
plekhg5 is required for endogenous blastopore lip formation. (A) Schematic of the genomic regions of the L and the S alloalleles of plekhg5 that are targeted by the SB MOs. The positions of the primers used for RT-PCR analysis of splicing efficiency are shown. (B,C) Both SB-MO1 and SB-MO2 efficiently block splicing of both L and S alloalleles, as indicated by the presence of intron-retention products in plekhg5 morphant embryos. The primer pairs used in the PCR reactions are indicated in parentheses. (D) plekhg5 SB MOs prevent formation of the blastopore lip at the sites of its injection. (E) plekhg5 SB MOs do not alter mesodermal cell fates, though the movements of the prechordal tissue (gsc-expressing, purple) and the trunk mesoderm (bra-expressing, cyan) are affected. (F) The blastopore lip defects induced by the SB MOs (25 ng) can be rescued with low doses of co-expressed plekhg5 RNA (25-50 pg). (G) plekhg5 SB MOs (25 ng) block ectopic blastopore lip induction by activin (5 pg) without affecting activin-dependent mesodermal induction. Numbers in each image indicate embryos exhibiting the ectopic blastopore lip defects or ectopic blastopore lips over the total number of embryos.
Knockdown of plekhg5 prevents ectopic induction of the blastopore lip by activin
As activin induced ectopic bottle cells in the animal region with concurrent induction of plekhg5 (Fig. 2), we addressed whether plekhg5 was essential for activin-dependent ectopic blastopore lip formation. Indeed, we observed that upon co-expression with plekhg5-MO, activin could no longer induce ectopic bottle cells even though it induced mesodermal markers efficiently (Fig. 5G, Fig. S8B). The results indicate that plekhg5 is not required for mesodermal fate specification by activin, but its protein product is obligatory in activin-induced blastopore lip formation. As both SB-MOs produced similar phenotypes in all our assays (Fig. 5, Fig. S8), we focused on SB-MO1 (referred to as SB-MO) in all our following experiments.
plekhg5 regulates morphology and apical actomyosin enrichment in bottle cells during blastopore lip formation
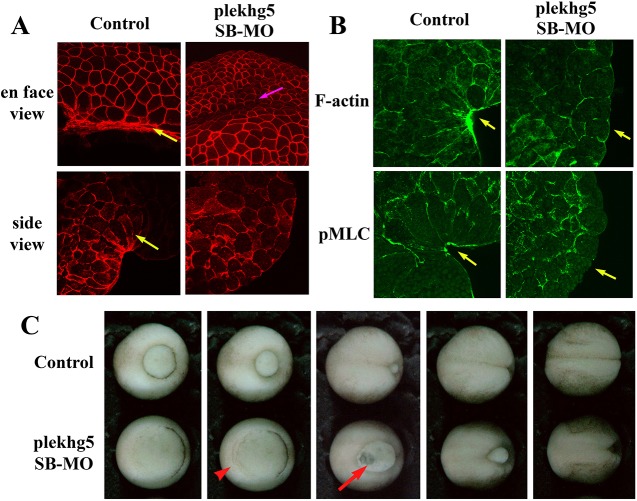
Bottle cells of Xenopus gastrulae assume a distinct morphology of a narrow cell apex, an elongated cell body and the expansion of the basolateral cell compartment. To see how plekhg5 regulates bottle cell shape, we examined cell morphology using the surface view, which revealed both isotropic shrinkage of cell areas and the fusiform-like narrowing of the cell apex in control cells, and the side view, which showed a flask-shaped cell contour of these cells (Fig. 6A). In plekhg5 morphant embryos, the constriction of cell apices was not seen from the surface view and the cells took on a cuboidal or a columnar shape when viewed from the sagittal plane (Fig. 6A). Despite this, there were signs of gastrulation movements in the absence of bottle cell constriction (Fig. 6A). Phalloidin staining of the bisected embryos showed an enrichment of F-actin near the apical membrane of the bottle cells in control embryos (Fig. 6B), whereas in plekhg5 morphant embryos, apical F-actin was detected but no concentrated F-actin signal enrichment was observed (Fig. 6B). Activation of myosin, marked by a pMLC signal, was seen to be enriched around the apical cell membrane in the bottle cells in control embryos, but no apical pMLC signal was detected in the plekhg5 morphant embryos. Instead, pMLC was distributed around the basolateral membrane in the epithelial cells (Fig. 6B). The results demonstrate that plekhg5 facilitates apical assembly of the actomyosin cytoskeleton in bottle cells to promote efficient apical constriction during blastopore lip formation.
Fig. 6.
plekhg5 regulates apical actomyosin cytoskeleton in bottle cells and gastrulation movements. (A) En face and side views of control bottle cells show reduced cell surfaces and wedge-shaped morphology in gastrula embryos, respectively (yellow arrows). However, in plekhg5 morphant embryos, cells do not show great shrinkage of surface areas and only cuboidal epithelial cell shapes are seen from the side view. Despite this, internalization of surface cells appears to happen at imprecise positions in the morphant embryos, as shown by formation of a surface groove (pink arrow). (B) Both F-actin and pMLC are enriched in the apical cell cortex of the bottle cells in bisected control embryos, but no such enrichment is observed in plekhg5 morphant embryos. Yellow arrows indicate apical signals. (C) Gastrulation movements proceed in the absence of the bottle cells, as seen by accumulation of cells in the marginal region from epiboly (red arrowhead) and thinning of the vegetal mass due to rotational movements of the large endodermal cells upward and laterally (red arrow). The blastopore eventually closes in most morphant embryos, but is delayed, when control siblings reach the neurula stages. Selected still frames from a time-lapse video of gastrulating control and plekhg5 morphant embryos are shown.
Gastrulation movements proceed with imprecision in the absence of the blastopore lip
As indicated by the groove formed in the plekhg5 morphant embryos (Fig. 6A), cells might retain the ability to internalize in the absence of apical constriction of the bottle cells. To further analyze gastrulation movements in the absence of the blastopore lip, we performed time-lapse video microscopy to track tissue movements in wild-type and plekhg5 morphant embryos (Fig. 6C, Movies 2-4). Knockdown of plekhg5 did not affect epiboly, as animal cells continued to move down and accumulated in several layers above the marginal zone (Fig. 6C). Vegetal endodermal cell rotation also appeared to proceed normally, as thinning of the vegetal mass, which was more pronounced on the dorsal side, was observed (Fig. 6C). Cell invagination and involution eventually occurred at a delayed time when the sibling embryos entered the neurula stages, and convergent extension might have provided the key driving force for blastopore formation and closure in the absence of the bottle cells (Movies 2,3). Although blastopore closure was seen in embryos with minimal blastopore lip at the mid-gastrula stages with the injection of the plekhg5 SB-MO in all blastomeres of four-cell-stage embryos (Movie 3), we did observe aberrant cell movements and failure in blastopore closure in ∼20% of the embryos (Fig. S9, Movie 4), implying that cell movements in the absence of the bottle cells were less precise and prone to errors. Our data thus demonstrate that several morphogenetic movements can occur in the absence of the plekhg5-dependent formation of the bottle cells to close the blastopore during Xenopus gastrulation. However, the delay in blastopore closure and the relaxation in movement precision is associated with developmental defects in the tadpoles (Fig. S9).
DISCUSSION
Apical constriction is an important cellular process that regulates cell shape changes during multiple developmental processes in diverse animal species. One crucial driving force in initiating and promoting apical constriction is the activation of the actomyosin contractile machinery specifically at the apical cell domain. This step is often controlled temporally and spatially to ensure that changes in individual cell morphology coordinate with global tissue morphogenesis patterns. Different animals employ distinct strategies to regulate apical actomyosin, with most strategies converging at the level of modulating Rho family of small GTPase activity. Understanding the function of tissue-specific regulators of Rho proteins during apical constriction can therefore provide insight into the cellular and molecular mechanisms that regulate this fundamental cell process. In this study, we report that the RhoGEF gene plekhg5 plays an essential role in controlling apical constriction of bottle cells during Xenopus gastrulation.
Expression of plekhg5 in cells undergoing apical constriction
One major question regarding apical constriction is what factor(s) specify a particular group of cells to undertake cell shape changes in particular embryonic regions at particular developmental stages. Studies from different animal models indicate that cell fate determination factors often regulate cell apical constriction. In C. elegans, transcription factors that are responsible for endodermal and mesodermal cell lineages are required for waves of sequential internalization of the corresponding cells. Ectopic formation of endodermal or mesodermal cells is sufficient to induce ectopic apical constriction of these cells at the relevant times (Nance and Priess, 2002; Nance et al., 2005; Lee et al., 2006; Rohrschneider and Nance, 2009; Harrell and Goldstein, 2011). Similarly, Drosophila mesodermal determination transcription factors Snail and Twist control apical constriction of ventral furrow cells via their downstream targets, such as folded gastrulation (fog) and T48, which regulate actomyosin (Leptin and Grunewald, 1990; Martin et al., 2009; Sawyer et al., 2010; Manning and Rogers, 2014). In Xenopus, nodal signaling specifies mesodermal and endodermal cell fates in a dose-dependent manner, and ectopic expression of nodal family ligands in the animal region induces ectopic bottle cell formation in conjunction with mesendodermal markers. Both morphological features and cell cycle control of these ectopic bottle cells are indistinguishable from those at the endogenous positions (Kurth and Hausen, 2000; Kurth, 2005). Xenopus bottle cell formation is thus also linked to cell fate determination, and nodal signaling can function to connect embryonic patterning and morphogenesis. However, the fact that the bottle cells are present specifically in a narrow ring around the blastopore suggests that nodal downstream factors are likely engaged in positive and negative feedback control to precisely position the bottle cells within a narrow domain. Based on the expression pattern and the function of plekhg5, we speculate that nodal controls bottle cell formation via transcriptional regulation of plekhg5. Once plekhg5 is turned on, it is sufficient to induce apical constriction in all epithelial tissues regardless of cell fate. However, in cells of epidermal fate, plekhg5-induced apical constriction often presents without concurrent basal expansion, implying that mesendodermal fate may be important for basal protrusion and expansion in bottle cells. The specific expression of plekhg5 may also contribute to distinct cell behaviors after internalization of mesendodermal cells in diverse amphibian species. Bottle cells form in epithelia of both endodermal and mesodermal fate in variable amounts in different species of amphibian, and their timing of undergoing apical constriction, and whether and when they undergo EMT and ingression to form deep mesenchymal mesodermal cells or re-spread to form an epithelial endodermal sheet, also varies according to species (Shook et al., 2002, 2004; Shook and Keller, 2008a,b). plekhg5 may be the key component in the regulation of apical constriction across different nodal-induced tissue fates. Understanding how plekhg5 expression is controlled therefore becomes essential in comprehending how bottle cells are positioned in gastrulating embryos. Sequence analysis of plekhg5 promoter and putative enhancer regions reveals multiple transcription factor binding motifs, including those of Smad, Sox proteins and T-box transcription factors. Genome-wide ChIP-seq studies indeed show that Smad2/3 and Foxh1, the transcriptional effectors of nodal signaling, can bind to the plekhg5 enhancer directly (Chiu et al., 2014). Further detailed dissection of the functional DNA elements and their binding factors that are involved in plekhg5 expression will be a promising avenue to investigate bottle cell induction at gastrulation. It is interesting to note here that the transcriptional regulation that determines cells undergoing apical constriction is not limited to bottle cells at gastrulation. Shroom3, an actin binding protein that is necessary and sufficient for apical constriction of neural hinge cells during neural tube closure, also appears to be regulated at the transcriptional level in the neural plate (Haigo et al., 2003; Hildebrand, 2005; Lee et al., 2007; 2009). It will be interesting to examine in the future whether transcriptional control of specific actomyosin regulators is a general theme in inducing cell apical constriction in other contexts, such as in the developing gut or during lens morphogenesis (Chung et al., 2010; Plageman et al., 2010). At later stages, dynamic plekhg5 expression is also observed in tissues undergoing epithelial morphogenesis, such as the forming otic vesicles and the tip of the protruding pharyngeal pouches. Plekhg5 may thus regulate additional apical constriction events during organogenesis. In addition, expression of plekhg5 in discrete migratory and mesenchymal cell populations suggests that it may play roles in controlling cell morphology and directional movements during late embryogenesis.
Apical localization of the Plekhg5 protein
Apical actomyosin activation is a common theme for cell shape changes in gastrulating embryos, but different animals use distinct mechanisms to achieve this effect. In Drosophila, two Twist target genes, encoding the transmembrane protein T48 and the secreted factor Fog, regulate apical localization of the PDZ-domain-containing DRhoGEF2 in a partially redundant fashion (Kolsch et al., 2007). The Twist-T48-Fog pathway does not affect DRhoGEF2 that is associated with cell junctions, but is required for the medioapical accumulation of DRhoGEF2 to regulate apical actomyosin dynamics through the Rho1/RhoA and Rok/Rho-dependent protein kinase (ROCK) signaling pathway during ventral furrow formation (Sawyer et al., 2010; Manning and Rogers, 2014; Mason et al., 2016). In C. elegans, apical activation of actomyosin relies on polarized localization of a Cdc42 GAP protein PAC-1 via cell-cell contact-mediated recruitment of PAC-1 to the basolateral domain, leaving active Cdc42 at the contact-free apical surface to stimulate MRCK-1 activity (Lee and Goldstein, 2003; Anderson et al., 2008; Chan and Nance, 2013; Marston et al., 2016). Our studies reveal a similarity to Drosophila development, in that Xenopus also utilizes an apically localized RhoGEF, Plekhg5, to organize a polarized actomyosin cytoskeleton at the cell apex. However, unlike fly DRhoGEF2, Plekhg5 does not contain a PDZ domain, and no vertebrate T48 or Fog homologs exist. Apical recruitment of Plekhg5 therefore relies on a different mechanism. Plekhg5 contains both PH and PBM domains in addition to the GEF motif, and the PBM domain of Plekhg5 homologs has been shown to bind the multiple PDZ-domain-containing factor MUPP1 (Mpdz) and its family member Patj in mammalian cells, zebrafish and C. elegans (Estevez et al., 2008; Ernkvist et al., 2009; Lin et al., 2012). As Patj is an apically localized tight junction protein in the Crumbs protein complex (Tepass, 2012), it is conceivable that Plekhg5 is recruited to the apical surface via its interaction with Patj. However, our structure-function analysis reveals that the PBM domain is dispensable for Plekhg5 localization and function, suggesting that other factors are involved in recruiting Plekhg5. Removal of the PH domain abolishes Plekhg5 apical positioning, which indicates a crucial role of the PH motif in Plekhg5 localization. As PH domains can interact with phospholipids in addition to other proteins (Krahn and Wodarz, 2012), it is possible that binding to the apical membrane lipid phosphotidylinositide 4,5 phosphate helps to recruit Plekhg5 to the apical compartment. This ability is not shared among all PH-containing RhoGEFs, as another organizer-enriched RhoGEF, Arhgef3, localizes mainly in the cell nucleus and cannot induce apical constriction when ectopically expressed (Fig. 2, Fig. S10). The PH domain has also been shown to regulate GEF activities independently of its membrane association (Bi et al., 2001; Baumeister et al., 2006). As the GEF mutant protein can localize to the apical junction but is not enriched in the apical cell cortex (Fig. 4), it is possible that compromise of the GEF function contributes to the loss of apical enrichment of Plekhg5 in both PH deletion and T365F mutants. Taken together, our data suggest the model that Plekhg5 is recruited to the cell junction independently of PBM or the GEF activity, but its enrichment at the apical cortex requires the PH domain and the intact GEF function. Further investigation is needed to test this model and identify the protein(s) and/or the lipid components that interact with Plekhg5 directly.
Rho and its downstream signaling in apical constriction
Localized activation of Rho GTPases controls polarized distribution and activation of actomyosin during apical constriction. Depending on the member of the Rho family GTPases that is activated, different effectors are involved to control actomyosin activity. For example, MRCK is activated downstream of Cdc42 in C. elegans to phosphorylate myosin regulatory light chain and induce cytoskeleton contraction to drive apical constriction (Anderson et al., 2008; Marston et al., 2016). In Drosophila, Rho1/RhoA signaling acts downstream of DRhoGEF2 to control activities of ROCK and Diaphanous (Dia) during cell invagination (Barrett et al., 1997; Hacker and Perrimon, 1998; Mason et al., 2013). Our experiments show that blocking RhoA but not Rac1 prevents blastopore lip induction (Fig. 2), a result consistent with Plekhg5 being a Rho-specific GEF (Marx et al., 2005). Though it is conceivable that ROCK and Dia act downstream of plekhg5/rhoA to regulate actomyosin in Xenopus, application of the ROCK inhibitor Y-27632 is ineffective in blocking blastopore lip formation (Lee and Harland, 2007; Fig. S11). It is unclear whether this is because of insufficient penetration of the inhibitor into the cells or because of the employment of other downstream effectors, such as Citron kinase (Thumkeo et al., 2013), in bottle cell formation. Further investigation of the roles of ROCK, Dia, and Citron kinase will be informative to identify plekhg5/rhoA effectors that mediate their function on actomyosin contraction and apical constriction in Xenopus.
Gastrulation movements in the absence of the bottle cells
Though the appearance of the bottle cells is a striking external indication of gastrulation movements in Xenopus, bottle cells per se do not appear to be absolutely required. Both surgical removal of these cells (Hardin and Keller, 1988) and the prevention of bottle cell formation in plekhg5 morphant embryos can result in complete, albeit delayed, blastopore closure. In the absence of the bottle cells, vegetal rotation – the amoeboid migration movements of the yolky endodermal and mesendodermal cells upward and laterally against the blastocoel walls (Winklbauer and Schurfeld, 1999; Wen and Winklbauer, 2017) – proceeds normally, so that a clear area of cells, which reflects the thinning of the endoderm, often forms on the dorsal vegetal side. Epiboly movements of the animal cells also occur normally as in the control embryos. Mesodermal cell involution appears to be delayed and may happen at more variable positions in the marginal zone, so that the gsc-expressing domain is positioned at variable distances from the blastopore at late gastrulation (Figs 5 and 6). The eventual blastopore closure appears to be driven mainly by convergent extension movements during neurulation, as body elongation helps to push the surface tissues toward the blastopore to facilitate mesendodermal internalization. Blastopore closure appears to proceed with somewhat variable speeds among morphant embryos, with a small portion failing, especially those expressing the SB-MO2 (Fig. S9). The data suggest that, although formation of the blastopore lip is not obligatory for gastrulation movements, it may facilitate coordination of different cell movements and ensure the robustness and reproducibility of gastrulation. The compensation for lack of apical constriction during gastrulation has also been observed in other animals (Llimargas and Casanova, 2010). In sea urchin, laser ablation of the bottle cells that surround the vegetal plate delays but does not abolish the invagination of the vegetal plate (Nakajima and Burke, 1996; Kimberly and Hardin, 1998). In C. elegans, endodermal cells partially internalize into the embryos in the absence of an apical actomyosin network (Nance et al., 2003). It is thus apparent that multiple mechanisms are involved in gastrulation morphogenesis and they work in a partially redundant manner to enable the correct placement of endodermal and mesodermal cells inside the embryos. Apical constriction-mediated cell shape changes help to orchestrate a robust cell movement program for reproducible embryonic patterning and development.
plekhg5 in other tissue contexts
Apical constriction is used reiteratively in multiple developmental contexts. One well studied process is neural tube closure, with the apical constriction of hingepoint cells in the neural plate as a crucial step (Suzuki et al., 2012; Wallingford et al., 2013). ISH of plekhg5 does not reveal a prominent signal in the hingepoint cells, and plekhg5 morphant embryos do not show obvious neural tube closure defects. This indicates that plekhg5 may not participate in neural tube closure. Instead, another RhoGEF, GEF-H1/Arhgef2, has been shown to regulate the apical constriction of neural cells in Xenopus (Itoh et al., 2014). Multiple other factors also participate in the control of apical constriction of hingepoint cells in Xenopus neural plate (reviewed by Suzuki et al., 2012). Although plekhg5 is not involved in neural tube closure, its expression in several other places, such as in the otic vesicle and the cells at the turning points of the protruding pharyngeal pouches, imply that it may regulate apical constriction during organogenesis. In mammalian cell culture and in zebrafish, plekhg5 homologs are also shown to regulate directional migration of cancer and endothelial cells and vasculature formation (Liu and Horowitz, 2006; Garnaas et al., 2008; Ernkvist et al., 2009; Dachsel et al., 2013). This suggests that in migrating cells, plekhg5 may interact with other partners for localized activation of Rho and actomyosin to provide a positional cue for directional movement. Further studies will reveal how plekhg5 controls context-dependent polarization of actomyosin to influence different cell behaviors.
MATERIALS AND METHODS
Obtaining embryos and microinjection
Xenopus laevis frogs were used throughout the study according to the institutional IACUC protocol 09658 at the University of Alabama at Birmingham. Female frogs were primed with 800 units/frog of human chorionic gonadotropin hormone (Sigma-Alrich) the night before use. Embryos were obtained by in vitro fertilization, dejellied with 2% cysteine solution and micro-injected with RNAs or antisense MOs. The animal regions of both blastomeres of two-cell-stage embryos or the marginal zone regions of the two dorsal or two ventral cells of four-cell-stage embryos were injected, as indicated in the text. For vegetal injection, plekhg5 RNA was injected into one vegetal blastomere at stages 6 to 7 to circumvent transportation of the injected RNA into the marginal area by cytoplasmic streaming (Danilchik and Denegre, 1991).
Plasmids and antisense morpholino oligonucleotides (MOs)
The plekhg5 coding sequence was PCR-amplified from gastrula stage cDNA, with the N- and the C-terminal primer sequences: Plekhg5-N(NotI): 5′-AGAAGCGGCCGCACCATGGTATGTCATCATGCAGACTG-3′ and Plekhg5-C(XhoI): 5′-CCGCTCGAGTTACACCTCTGAAGCCGTTAATGTAG-3′. The coding sequence was inserted between the NotI and XhoI sites of the pCS105 vector. GFP-tagged plekhg5 was constructed by inserting the ligation product of NheI/SalI fragment of pEGFP-C3 and SalI/AscI fragment of plekhg5 into the XbaI/AscI sites of the pCS105 vector. The plekhg5 mutants were made using a PCR-based method with the primers: plekhg5-PH-del-for: CACACACAATTGGCACAGAATCTCTTGCAAAGAACGAG; plekhg5-PH-del-rev: TGTGTGCAATTGTGTATCTTCAGGAGATGTTCCAATC; plekhg5-DPBM-C(XhoI): CCGCTCGAGTTATGAAGCCGTTAATGTAGAGTT. All the plasmids were linearized with the AscI enzyme before being transcribed with the SP6 RNA polymerase and 100-200 pg of plekhg5 or its mutant RNAs were used for injection. The sequences of plekhg5 SB MOs are: SB-MO1: 5′-ACAAATTACCTCAGGAACCTCAATG-3′ and SB-MO2: 5′-AGGCAAATATCTTACCCTTCCAAA-3′, both targeting the exon-intron junctional sequences for intron retention. Injections of 20-50 ng MOs were used in the experiments.
RT-PCR
To assay for the efficiency of plekhg5 SB MOs, several primer pairs were designed. The sequences of the primers 1 to 6 (Fig. 5A) are: primer 1 (exon 8, forward): 5′-CAAGTTGCATTCATACAGTATGTTTG-3′; primer 2 (exon 10, reverse): 5′-TCCGGACTCTTGTAGATTCAACAG-3′; primer 3 (intron 8 of plekhg5.L, forward): 5′-GAACAGATTTAGGATTGATAGGTCAG-3′; primer 4 (intron 8 of plekhg5.S, forward): 5′-GAACATATTTAGAATTGATAAGTCAG-3′; primer 5 (exon 7, forward): 5′-GACGCAAGTATTCCGGTACAAGATC-3′; primer 6 (intron 7, reverse): 5′-GGCAATTTTAGCAGTTTGTATAGAAA-3′. The expected sizes of the PCR products are: primers 1+2 (no intron): 277 bp; primers 3+2 (plekhg5.L intron retention with SB-MO1): 508 bp; primers 4+2 (plekhg5.S intron retention with SB-MO1): 385 bp; primers 5+2 (no intron): 441/447 bp (L/S alloalleles); primers 5+6 (intron retention with SB-MO2): ∼270 bp (S alloallele is not annotated clearly).
In situ hybridization
ISH was performed as described by Harland (1991). For plekhg5 in situ, the C-terminal fragment of the coding sequence was used as the probe. The embryos were bisected before or after staining to reveal internal signals.
F-actin staining and immunofluorescence
For F-actin staining, embryos or explants were fixed in MEMFA (0.1 M MOPS, pH 7.4, 2 mM EGTA, 1 mM MgSO4, 3.7% formaldehyde) for 30 min, washed 3× with PBS and stained with 5 units/ml Alexa Fluor 488-conjugated phalloidin (Invitrogen) in PBS with 0.1% Tween 20 for 3 h at room temperature or overnight at 4°C. For immunocytochemistry of pMLC, we adopted the protocol described in Lee and Harland (2007). Anti-phospho-Ser20 myosin light chain rabbit antibody (Abcam, ab2480, 1:500) and Alexa Fluor 488-conjugated secondary antibody (Life Technologies, A-11070, 1:200) were used.
Imaging
For stereo imaging of embryonic phenotypes and ISH, Zeiss M2Bio and Nikon AZ100 microscopes were used. For time-lapse movies, embryos were positioned to the correct orientations (animal or vegetal side up) using modeling clay, and 6-8 h time-lapse imaging was performed with 3 min intervals. For fluorescence microscopy, an Olympus Fluoview 2000 upright confocal microscope was used. Some of the embryos were bisected across the dorsal-ventral midline before imaging. Most of the images were taken using a 20× (NA0.95) lens. Maximum projections of z-stack images were used for the figures.
Morphometrical and statistical analysis
The surface areas of blastula-stage embryos and the height-to-width (H/W) ratio of the outer epithelial animal cells were measured using ImageJ software. For apical cell areas at the blastula stages, a total of 362 cells from 31 control embryos, 347 darkly pigmented cells from 32 plekhg5-injected embryos and 350 normal pigmented cells from 32 plekhg5-injected embryos from four independent experiments were examined. For H/W ratio, a total of 153 cells from 28 control embryos and 129 cells from 26 plekhg5-injected embryos from three independent experiments were measured. Scatter plots of individual datasets were performed using GraphPad Prism7 software. Student's t-test was used to assess the statistical significance in differences between control and plekhg5-injected samples.
Supplementary Material
Acknowledgements
We are grateful to Dr Jianbo Wang for allowing us to use his Olympus Fluoview confocal microscope.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: R.K., C.C.; Methodology: I.K.P., H.J.R., P.S., R.K., C.C.; Formal analysis: I.K.P., R.K., C.C.; Investigation: I.K.P., C.C.; Writing - original draft: I.K.P., C.C.; Writing - review & editing: I.K.P., H.J.R., P.S., R.K., C.C.; Supervision: C.C.; Funding acquisition: R.K., P.S., C.C.
Funding
The study is supported by grants from the National Institute of General Medical Sciences (R01GM098566 to C.C. and R.K.; R01GM099108 to P.S.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.168922.supplemental
References
- Aghazadeh B., Zhu K., Kubiseski T. J., Liu G. A., Pawson T., Zheng Y. and Rosen M. K. (1998). Structure and mutagenesis of the Dbl homology domain. Nat. Struct. Biol. 5, 1098-1107. 10.1038/4209 [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Gill J. S., Cinalli R. M. and Nance J. (2008). Polarization of the C elegans embryo by RhoGAP-mediated exclusion of PAR-6 from cell contacts. Science 320, 1771-1774. 10.1126/science.1156063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmich M. J., Rogers S. and Häcker U. (2005). DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo. J. Cell Biol. 168, 575-585. 10.1083/jcb.200407124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K., Leptin M. and Settleman J. (1997). The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 91, 905-915. 10.1016/S0092-8674(00)80482-1 [DOI] [PubMed] [Google Scholar]
- Baumeister M. A., Rossman K. L., Sondek J. and Lemmon M. A. (2006). The Dbs PH domain contributes independently to membrane targeting and regulation of guanine nucleotide-exchange activity. Biochem. J. 400, 563-572. 10.1042/BJ20061020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beane W. S., Gross J. M. and McClay D. R. (2006). RhoA regulates initiation of invagination, but not convergent extension, during sea urchin gastrulation. Dev. Biol. 292, 213-225. 10.1016/j.ydbio.2005.12.031 [DOI] [PubMed] [Google Scholar]
- Bi F., Debreceni B., Zhu K., Salani B., Eva A. and Zheng Y. (2001). Autoinhibition mechanism of proto-Dbl. Mol. Cell. Biol. 21, 1463-1474. 10.1128/MCB.21.5.1463-1474.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E. and Nance J. (2013). Mechanisms of CDC-42 activation during contact-induced cell polarization. J. Cell Sci. 126, 1692-1702. 10.1242/jcs.124594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W. T., Le R. C., Blitz I. L., Fish M. B., Li Y., Biesinger J., Xie X. and Cho K. W. Y. (2014). Genome-wide view of TGFβ/Foxh1 regulation of the early mesendoderm program. Development 141, 4537-4547. 10.1242/dev.107227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.-C. and Sokol S. Y. (2009). The involvement of lethal giant larvae and Wnt signaling in bottle cell formation in Xenopus embryos. Dev. Biol. 336, 68-75. 10.1016/j.ydbio.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M.-I., Nascone-Yoder N. M., Grover S. A., Drysdale T. A. and Wallingford J. B. (2010). Direct activation of Shroom3 transcription by Pitx proteins drives epithelial morphogenesis in the developing gut. Development 137, 1339-1349. 10.1242/dev.044610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dachsel J. C., Ngok S. P., Lewis-Tuffin L. J., Kourtidis A., Geyer R., Johnson L., Feathers R. and Anastasiadis P. (2013). The Rho guanine nucleotide exchange factor Syx regulates the balance of Dia and ROCK activities to promote polarized-cancer-cell migration. Mol. Cell. Biol. 33, 4909-4918. 10.1128/MCB.00565-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilchik M. V. and Denegre J. M. (1991). Deep cytoplasmic rearrangements during early development in Xenopus laevis. Development 111, 845-856. [DOI] [PubMed] [Google Scholar]
- Ebrahim S., Fujita T., Millis B. A., Kozin E., Ma X., Kawamoto S., Baird M. A., Davidson M., Yonemura S., Hisa Y. et al. (2012). NMII forms a contractile transcellular sarcomeric network to regulate apical cell junctions and tissue geometry. Curr. Biol. 23, 731-736. 10.1016/j.cub.2013.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernkvist M., Persson N. L., Audebert S., Lecine P., Sinha I., Liu M., Schlueter M., Horowitz A., Aase K., Weide T. et al. (2009). The Amot/Patj/Syx signaling complex spatially controls RhoA GFPase activity in migrating endothelial cells. Blood 113, 244-253. 10.1182/blood-2008-04-153874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez M. A., Henderson J. A., Ahn D., Zhu X.-R., Poschmann G., Lubbert H., Marx R. and Baraban J. M. (2008). The neuronal RhoA GEF, Tech, interacts with the synaptic multi-PDZ-domain-containing protein, MUPP1. J. Neurochem. 106, 1287-1297. 10.1111/j.1471-4159.2008.05472.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnaas M. K., Moodie K. L., Liu M., Samant G. V., Li K., Marx R., Baraban J. M., Horowitz A. and Ramchandran R. (2008). Syx, a RhoA guanine exchange factor, is essential for angiogenesis in vivo. Circ. Res. 103, 710-716. 10.1161/CIRCRESAHA.108.181388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker U. and Perrimon N. (1998). DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 12, 274-284. 10.1101/gad.12.2.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo S. L., Hildebrand J. D., Harland R. M. and Wallingford J. B. (2003). Shrrom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr. Biol. 13, 2125-2137. 10.1016/j.cub.2003.11.054 [DOI] [PubMed] [Google Scholar]
- Hall A. (1998). Rho GTPases and the actin cytoskeleton. Science 279, 509-514. 10.1126/science.279.5350.509 [DOI] [PubMed] [Google Scholar]
- Hardin J. and Keller R. (1988). The behaviour and function of bottle cells during gastrulation of Xenopus laevis. Development 103, 211-230. [DOI] [PubMed] [Google Scholar]
- Harland R. M. (1991). In situ hybridization: an improved whole-mount method for Xenopus embryos. Meth. Cell Biol. 36, 685-695. 10.1016/S0091-679X(08)60307-6 [DOI] [PubMed] [Google Scholar]
- Harrell J. R. and Goldstein B. (2011). Internalization of multiple cells during C. elegans gastrulation depends on common cytoskeletal mechanisms but different cell polarity and cell fate regulators. Dev. Biol. 350, 1-12. 10.1016/j.ydbio.2010.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand J. D. (2005). Shroom regulates epithelial cell shape via the apical positioning of an actomyosin network. J. Cell Sci. 118, 5191-5203. 10.1242/jcs.02626 [DOI] [PubMed] [Google Scholar]
- Hodge R. G. and Ridley A. J. (2016). Regulation of Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 17, 496-510. 10.1038/nrm.2016.67 [DOI] [PubMed] [Google Scholar]
- Hufton A. L., Vinayagam A., Suhai S. and Baker J. C. (2006). Genomic analysis of Xenopus organizer function. BMC Dev. Biol. 6, 27 10.1186/1471-213X-6-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Ossipova O. and Sokol S. Y. (2014). GEF-H1 functions in apical constriction and cell intercalations and is essential for vertebrate neural tube closure. J. Cell Sci. 127, 2542-2553. 10.1242/jcs.146811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. E. (1981). An experimental analysis of the role of bottle cells and the deep marginal zone in gastrulation of Xenopus laevis. J. Exp. Zool. 216, 81-101. 10.1002/jez.1402160109 [DOI] [PubMed] [Google Scholar]
- Kimberly E. L. and Hardin J. (1998). Bottle cells are required for the initiation of primary invagination in the sea urchin embryo. Dev. Biol. 204, 235-250. 10.1006/dbio.1998.9075 [DOI] [PubMed] [Google Scholar]
- Kolsch V., Seher T., Fernandez-Ballester G. J., Serrano L. and Leptin M. (2007). Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science 315, 384-386. 10.1126/science.1134833 [DOI] [PubMed] [Google Scholar]
- Krahn M. P. and Wodarz A. (2012). Phosphoinositide lipids and cell polarity: linking the plasma membrane to the cytocortex. Essays Biochem. 53, 15-27. 10.1042/bse0530015 [DOI] [PubMed] [Google Scholar]
- Kurth T. (2005). A cell cycle arrest is necessary for bottle cell formation in the early Xenopus gastrula: integrating cell shape change, local mitotic control and mesodermal patterning. Mech. Dev. 122, 1251-1265. 10.1016/j.mod.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Kurth T. and Hausen P. (2000). Bottle cell formation in relation to mesodermal patterning in the Xenopus embryo. Mech. Dev. 97, 117-131. 10.1016/S0925-4773(00)00428-7 [DOI] [PubMed] [Google Scholar]
- Lee J.-Y. and Goldstein B. (2003). Mechanisms of cell positioning during C. elegans gastrulation. Development 130, 307-320. 10.1242/dev.00211 [DOI] [PubMed] [Google Scholar]
- Lee J.-Y. and Harland R. M. (2007). Actomyosin contractility and microtubules drive apical constriction in Xenopus bottle cells. Dev. Biol. 311, 40-52. 10.1016/j.ydbio.2007.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-Y. and Harland R. M. (2010). Endocytosis is required for efficient apical constriction during Xenopus gastrulation. Curr. Biol. 20, 253-258. 10.1016/j.cub.2009.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Marston D. J., Walston T., Hardin J., Halberstadt A. and Goldstein B. (2006). Wnt/Frizzled signaling controls C. elegans gastrulation by activating actomyosin contractility. Curr. Biol. 16, 1986-1997. 10.1016/j.cub.2006.08.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Scherr H. M. and Wallingford J. B. (2007). Shroom family proteins regulate γ-tubulin distribution and microtubule architecture during epithelial cell shape change. Development 134, 1431-1441. 10.1242/dev.02828 [DOI] [PubMed] [Google Scholar]
- Lee C., Le M.-P. and Wallingford J. B. (2009). The Shroom family proteins play broad roles in the morphogenesis of thickened epithelial sheets. Dev. Dyn. 238, 1480-1491. 10.1002/dvdy.21942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptin M. and Grunewald B. (1990). Cell shape changes during gastrulation in Drosophila. Development 110, 73-84. [DOI] [PubMed] [Google Scholar]
- Lin L., Tran T., Hu S., Cramer T., Komuniecki R. and Steven R. M. (2012). RGEF-2 is an essential Rho-1 specific RhoGEF that binds to the multi-PDZ domain scaffold protein MPZ-1 in Caenorhabditis elegans. PLoS ONE 7, e31499 10.1371/journal.pone.0031499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. and Horowitz A. (2006). A PDZ-binding motif as a critical determinant of Rho guanine exchange factor function and cell phenotype. Mol. Biol. Cell 17, 1880-1887. 10.1091/mbc.e06-01-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wang H., Eberstadt M., Schnuchel A., Olejniczak E. T., Meadows R. P., Schkeryantz J. M., Janowick D. A., Harlan J. E., Harris E. A. S. et al. (1998). NMR structure and mutagenesis of the N-terminal Dbl homology domain of the nucleotide exchange factor Trio. Cell 95, 269-277. 10.1016/S0092-8674(00)81757-2 [DOI] [PubMed] [Google Scholar]
- Llimargas M. and Casanova J. (2010). Apical constriction and invagination: a very self-reliant couple. Dev. Biol. 344, 4-6. 10.1016/j.ydbio.2010.05.498 [DOI] [PubMed] [Google Scholar]
- Manning A. J. and Rogers S. L. (2014). The Fog signaling pathways: insights into signaling in morphogenesis. Dev. Biol. 394, 6-14. 10.1016/j.ydbio.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston D. J., Higgins C. D., Peters K. A., Cupp T. D., Dickinson D. J., Pani A. M., Moore R. P., Cox A. H., Keihart D. P. and Goldstein B. (2016). MRCK-1 drives apical constriction in C. elegans by linking developmental patterning to force generation. Curr. Biol. 26, 2079-2089. 10.1016/j.cub.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. C. and Goldstein B. (2014). Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development 141, 1987-1998. 10.1242/dev.102228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. C., Kaschube M. and Wieschaus E. F. (2009). Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495-499. 10.1038/nature07522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx R., Henderson J., Wang J. and Baraban J. M. (2005). Tech: a RhoA GEF selectively expressed in hippocampal and cortical neurons. J. Neurochem. 92, 850-858. 10.1111/j.1471-4159.2004.02930.x [DOI] [PubMed] [Google Scholar]
- Mason F. M., Tworoger M. and Martin A. C. (2013). Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction. Nat. Cell Biol. 15, 926-936. 10.1038/ncb2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason F. M., Xie S., Vasquez C. G., Tworoger M. and Martin A. C. (2016). RhoA GTPase inhibition organizes contraction during epithelial morphogenesis. J. Cell Biol. 214, 603-617. 10.1083/jcb.201603077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y. and Burke R. D. (1996). The initial phase of gastrulation in sea urchins is accompanied by the formation of bottle cells. Dev. Biol. 179, 436-446. 10.1006/dbio.1996.0273 [DOI] [PubMed] [Google Scholar]
- Nance J. and Priess J. R. (2002). Cell polarity and gastrulation in C. elegans. Development 129, 387-397. [DOI] [PubMed] [Google Scholar]
- Nance J., Munro E. M. and Priess J. R. (2003). C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development 130, 5339-5350. 10.1242/dev.00735 [DOI] [PubMed] [Google Scholar]
- Nance J., Lee J. Y. and Goldstain B. (2005). Gastrulation in C. elegans. WormBook : the online review of C. elegans biology. 10.1895/wormbook.1.23.1 [DOI] [Google Scholar]
- Nikolaidou K. and Barrett K. (2004). A Rho GTPase signaling pathway is used reiteratively in epithelial folding and potentially selects the outcome of Rho activin. Curr. Biol. 14, 1822-1826. 10.1016/j.cub.2004.09.080 [DOI] [PubMed] [Google Scholar]
- Ossipova O., Chuykin I., Chu C. W. and Sokol S. Y. (2015). Vangl2 cooperates with Rab11 and Myosin V to regulate apical constriction during vertebrate gastrulation. Development 142, 99-107. 10.1242/dev.111161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plageman T. F. Jr., Chung M. I., Lou M., Smith A. N., Hildebrand J. D., Wallingford J. B. and Lang R. A. (2010). Pax6-dependent Shroom3 expression regulates apical constriction during lens placode invagination. Development 137, 405-415. 10.1242/dev.045369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov I. K., Kwon T., Crossman D. K., Crowley M. R., Wallingford J. B. and Chang C. (2017). Identification of new regulators of embryonic patterning and morphogenesis in Xenopus gastrulae by RNA sequencing. Dev. Biol. 426, 429-441. 10.1016/j.ydbio.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider M. R. and Nance J. (2009). Polarity and cell fate specification in the control of Caenorhabditis elegans gastrulation. Dev. Dyn. 238, 789-796. 10.1002/dvdy.21893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer J. M., Harrell J. R., Shemer G., Sullivan-Brown J., Rho-Johnson M. and Goldstein B. (2010). Apical constriction: a cell shape change that can drive morphogenesis. Dev. Biol. 341, 5-19. 10.1016/j.ydbio.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard K., Robin F., Lemaire P. and Munro E. (2010). Sequential activation of apical and basolateral contractility drives ascidian endoderm invagination. Curr. Biol. 20, 1499-1510. 10.1016/j.cub.2010.06.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook D. R. and Keller R. (2008a). Epithelial type, ingression, blastopore architecture and the evolution of chordate mesoderm morphogenesis. J. Exp. Zool. 310B, 85-110. 10.1002/jez.b.21198 [DOI] [PubMed] [Google Scholar]
- Shook D. R. and Keller R. (2008b). Morphogenic machines evolve more rapidly than the signals that pattern them: lessons from amphibians. J. Exp. Zool. 310B, 111-135. 10.1002/jez.b.21204 [DOI] [PubMed] [Google Scholar]
- Shook D. R., Majer C. and Keller R. (2002). Urodeles remove mesoderm from the superficial layer by subduction through a bilateral primitive streak. Dev. Biol. 248, 220-239. 10.1006/dbio.2002.0718 [DOI] [PubMed] [Google Scholar]
- Shook D. R., Majer C. and Keller R. (2004). Pattern and morphogenesis of presumptive superficial mesoderm in two closely related species, Xenopus laevis, and Xenopus tropicalis. Dev. Biol. 270, 163-185. 10.1016/j.ydbio.2004.02.021 [DOI] [PubMed] [Google Scholar]
- Suzuki M., Morita H. and Ueno N. (2012). Molecular mechanisms of cell shape changes that contribute to vertebrate neural tube closure. Develop. Growth Differ. 54, 266-276. 10.1111/j.1440-169X.2012.01346.x [DOI] [PubMed] [Google Scholar]
- Tepass U. (2012). The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu. Rev. Cell Dev. Biol. 28, 655-685. 10.1146/annurev-cellbio-092910-154033 [DOI] [PubMed] [Google Scholar]
- Thumkeo D., Watanabe S. and Narumiya S. (2013). Physiological roles of Rho and Rho effectors in mammals. Eur. J. Cell Biol. 92, 303-315. 10.1016/j.ejcb.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Wallingford J. B., Niswander L. A., Shaw G. M. and Finnell R. H. (2013). The continuing challenge of understanding, preventing, and treating neural tube defects. Science 339, 1222002 10.1126/science.1222002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J. W. H. and Winklbauer R. (2017). Ingression-type cell migration drives vegetal endodermal internalisation in the Xenopus gastrula. eLife 6, e27190 10.7554/eLife.27190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklbauer R. and Schurfeld M. (1999). Vegetal rotation, a new gastrulation movement involved in the internalization of the mesoderm and endoderm in Xenopus. Development 126, 3703-3713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.