Abstract
Objective
Non-alcoholic fatty liver disease (NAFLD) risk begins in utero in offspring of obese mothers. A critical unmet need in this field is to understand the pathways and biomarkers underlying fetal hepatic lipotoxicity and whether maternal dietary intervention during pregnancy is an effective countermeasure.
Methods
We utilized a well-established non-human primate model of chronic, maternal, Western-style diet induced obesity (OB-WSD) compared with mothers on a healthy control diet (CON) or a subset of OB-WSD mothers switched to the CON diet (diet reversal; OB-DR) prior to and for the duration of the next pregnancy. Fetuses were studied in the early 3rd trimester.
Results
Fetuses from OB-WSD mothers had higher circulating triglycerides (TGs) and lower arterial oxygenation suggesting hypoxemia, compared with fetuses from CON and OB-DR mothers. Hepatic TG content, oxidative stress (TBARs), and de novo lipogenic genes were increased in fetuses from OB-WSD compared with CON mothers. Fetuses from OB-DR mothers had lower lipogenic gene expression and TBARs yet persistently higher TGs. Metabolomic profiling of fetal liver and serum (umbilical artery) revealed distinct separation of CON and OB-WSD groups, and an intermediate phenotype in fetuses from OB-DR mothers. Pathway analysis identified decreased tricarboxylic acid cycle intermediates, increased amino acid (AA) metabolism and byproducts, and increased gluconeogenesis, suggesting an increased reliance on AA metabolism to meet energy needs in the liver of fetuses from OB-WSD mothers. Components in collagen synthesis, including serum protein 5-hydroxylysine and hepatic lysine and proline, were positively correlated with hepatic TGs and TBARs, suggesting early signs of fibrosis in livers from the OB-WSD group. Importantly, hepatic gluconeogenic and arginine related intermediates and serum levels of lactate, pyruvate, several AAs, and nucleotide intermediates were normalized in the OB-DR group. However, hepatic levels of CDP-choline and total ceramide levels remained high in fetuses from OB-DR mothers.
Conclusions
Our data provide new metabolic evidence that, in addition to fetal hepatic steatosis, maternal WSD creates fetal hypoxemia and increases utilization of AAs for energy production and early activation of gluconeogenic pathways in the fetal liver. When combined with hyperlipidemia and limited antioxidant activity, the fetus suffers from hepatic oxidative stress and altered intracellular metabolism which can be improved with maternal diet intervention. Our data reinforce the concept that multiple “first hits” occur in the fetus prior to development of obesity and demonstrate new biomarkers with potential clinical implications for monitoring NAFLD risk in offspring.
Keywords: Liver, Fetus, Steatosis, Hypoxemia, Fetal programming, Metabolomic
Abbreviations: AA, amino acid; AUC, area under the curve; CON, control; DAG, diacylglyceride; DNL, de novo lipogenesis; IV-GTT, intravenous glucose tolerance test; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NEFA, non-esterified fatty acid; NHP, non-human primate; OB-DR, diet reversal group; OB-WSD, Western-style diet induced obesity group; PC, phosphatidylcholine; PLS-DA, partial least squares discriminant analysis; TBARs, thiobarbituric acid reactive substances; TCA, tricarboxylic acid cycle; TG, triglyceride; VIP, variable importance in projection; VLDL, very low-density lipoprotein; WSD, Western-style diet
Graphical abstract
Highlights
-
•
Maternal WSD increases fetal hypoxemia and utilization of AAs for gluconeogenesis.
-
•
Maternal WSD increases fetal oxidative stress and precursors to liver fibrosis.
-
•
Carnosine and l-proline uniquely correlated with fetal TG and oxidative stress.
-
•
Fetal TGs were correlated with fetal arterial oxygen saturation.
-
•
Diet reversal in obese WSD mothers prevents fetal hypoxemia and oxidative stress.
1. Introduction
An overwhelming body of evidence supports the concept that an adverse maternal environment affects the development of the fetus and infant, thereby increasing the risk profile for disease later in life. A striking example of developmental programming is the impact of exposure to maternal obesity on the susceptibility to non-alcoholic fatty liver disease (NAFLD) in childhood and progression to non-alcoholic steatohepatitis (NASH) across the lifespan [1]. NAFLD affects over 35% of obese children in North America [2] and half have already progressed to the more severe NASH at time of diagnosis [3]. Human studies have shown increased intrahepatocellular lipid storage in infants born to mothers with obesity [4], [5]. These offspring are at a higher risk of progressing toward obesity, NAFLD, cardiovascular disease, and hepatic carcinoma later in life, regardless of gender [6], [7], [8]. The critical importance of the in utero environment to accelerated pediatric NAFLD progression is further illustrated in a cross-sectional study of 538 children with biopsy-proven NAFLD that showed that those born with higher or lower birthweight had 2-fold greater incidence of advanced liver disease [9] compared with normal-weight children, even after adjusting for body mass index. Numerous studies in rodent models [10], [11], [12], [13], [14] and our non-human primate (NHP) model [15], [16], [17], [18], [19] support a role for gestational exposures in the persistence of NAFLD later in life. While these studies demonstrate that NAFLD risk begins in utero, the mechanisms and primary drivers of this disorder during fetal life are poorly understood.
The fetal overnutrition hypothesis suggests that exposure to excess maternal fuels, including glucose, lipids, and/or amino acids, contribute to increased fetal growth and adiposity [20], [21]. These fuels, which are in greater abundance in maternal obesity [22], [23], can contribute to NAFLD risk beginning in utero [1], [24], but the metabolic pathways and mechanisms remain unclear. The fetus develops in a low oxygen environment and has a limited capacity for lipid oxidation until birth [25], [26], [27], [28], [29]. The fetal liver has fewer mitochondria, low activity of carnitine palmitoyl-CoA transferase 1, and little activation of gluconeogenesis [30], [31] compared with adult liver, supporting a distinction between fetal and adult hepatic metabolism. Thus, metabolic fuel overload in utero might interrupt normal development of metabolic pathways that accelerate NAFLD risk in the next generation [32], [33]. In our NHP model of maternal diet induced obesity, the offspring demonstrate increased lipid accumulation, oxidative stress, and apoptosis in the fetal liver [15], [34]. This phenotype persists through 1 year of age, even after implementing a healthy diet post-weaning [16], demonstrating that maternal diet has long lasting effects.
Studies have yet to investigate whether dietary interventions during pregnancy in obese women can mitigate the adverse effects on fetal hepatic metabolic programming. Further, these alterations in the fetus are infeasible to study in humans. While rodent models have the advantage of genetic manipulation, they have a much shorter gestation period and multiple fetuses, restricting their utility in studies of fetal metabolism. Sheep models have been used to study fetal metabolism; however, their capacity for modeling human fetal lipid metabolism is limited. Studies in our NHP model take advantage of the fact that we can test the effect of reversal of maternal diet in chronically obese, Western-style diet (WSD)-fed mothers prior to a subsequent pregnancy on systemic and hepatic metabolism in the fetus. Here, we focused on a combination of targeted metabolomic and lipidomic assays, limited transcriptional analysis, and fetal blood gas measures to test the hypothesis that dysregulated fetal fuel metabolism due to maternal WSD causes liver injury in utero. Further, we tested whether switching obese females to a healthy diet prior to pregnancy can mitigate detrimental effects on the biochemical pathways that contribute to NAFLD risk in the fetal offspring.
2. Methods
2.1. Maternal chronic WSD induced obesity model
Adult female Japanese macaques were fed a WSD (36.6% calories from fat and 5.5% fructose and 8.8% sucrose) for 2–9 years prior to conception and throughout pregnancy to produce WSD-induced obese (OB-WSD) mothers or maintained on a control diet (CON mothers; 14.6% calories from fat and 2.8% sucrose and 0.2% fructose) as described [35]. Maternal body composition was measured by dual-energy X-ray absorptiometry (DEXA; Hologic QDR Discovery A; Hologic, Inc., Bedford, MA) before pregnancy. Maternal body weight and plasma insulin, glucose, and triglyceride (TG) measurements were collected and intravenous glucose tolerance tests (IV-GTT) were performed before pregnancy and during the 3rd trimester of pregnancy [15], [36], [37]. The data in this manuscript include fetuses from CON mothers with a pre-pregnancy body fat percentage <25.0 (n = 21) and OB-WSD mothers with pre-pregnancy body fat percentage >25.0 (n = 39) studied between 2008 and 2014 [38]. All mothers were phenotyped before pregnancy and in the 3rd trimester, and all fetuses had liver TG assays and growth measurements, as shown in Table 1. Other measurements were performed on subsets of CON and OB-WSD fetuses as indicated in the figure legends. All animal procedures were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Oregon National Primate Research Center (ONPRC). The ONPRC abides by the Animal Welfare Act and Regulations enforced by the United States Department of Agriculture.
Table 1.
Maternal and fetal characteristics.
| CON | OB-WSD | OB-DR | ANOVA | |
|---|---|---|---|---|
| Maternal age (years) | 9.1 ± 0.5a | 10.3 ± 0.4b | 14.5 ± 0.2c | <0.001 |
| Years on WSD | NA | 4.6 ± 0.3a | 9.1 ± 0.0b | <0.001 |
| Maternal, pre-pregnancy | ||||
| Body weight (kg) | 8.9 ± 0.3a | 13.4 ± 0.4b | 14.6 ± 0.6b | <0.001 |
| Body fat (%) | 15.0 ± 1.1a | 35.6 ± 1.1b | 40.3 ± 1.9b | <0.001 |
| Insulin (mU/L) | 15.8 ± 2.3b | 40.1 ± 4.5b | 36.7 ± 8.1a,b | <0.005 |
| Glucose (mg/dL) | 54.5 ± 3.1 | 52.8 ± 1.9 | 49.3 ± 3.3 | 0.70 |
| GTT insulin AUC | 4017.2 ± 681.0a | 9456.8 ± 878.3b | 7652.3 ± 2059.2b | <0.005 |
| GTT glucose AUC | 9241.9 ± 298.1 | 10362.9 ± 348.8 | 11411.5 ± 899.1 | 0.07 |
| Maternal, pregnancy, 3rd trimester | ||||
| Body weight (kg) | 9.9 ± 0.3a | 13.4 ± 0.5b | 12.4 ± 0.6b | <0.001 |
| Insulin (mU/L) | 15.8 ± 2.6 | 55.9 ± 14.3 | 31.1 ± 7.1 | 0.11 |
| Glucose (mg/dL) | 42.7 ± 1.9 | 42.2 ± 1.2 | 37.0 ± 4.2 | 0.34 |
| GTT insulin AUC | 5030.3 ± 762.2a | 18054.2 ± 3074.9b | 6983.4 ± 2414.2a,b | <0.005 |
| GTT glucose AUC | 7255.7 ± 239.5 | 7486.0 ± 232.4 | 7216.4 ± 428.9 | 0.77 |
| Fetal characteristics at c-section | ||||
| Fetal age (days) | 130.0 ± 0.2 | 130.8 ± 0.4 | 130.8 ± 0.6 | 0.30 |
| Fetal sex (female:male) | 11:10 | 16:23 | 1:4 | |
| Fetal weight (g) | 340.2 ± 6.0 | 348.5 ± 7.4 | 367.2 ± 11.5 | 0.38 |
| Fetal liver weight (g) | 9.5 ± 0.4a | 10.2 ± 0.2a,b | 11.1 ± 0.5b | 0.05 |
| Fetal liver:body weight (%) | 2.78 ± 0.09 | 2.95 ± 0.05 | 3.04 ± 0.14 | 0.13 |
| Placenta weight (g) | 112.7 ± 3.3 | 106.7 ± 2.7 | 109.9 ± 5.3 | 0.37 |
| Insulin, plasma (mU/L)A | 5.2 ± 0.8 | 8.0 ± 2.8 | 7.1 ± 1.2 | 0.75 |
| Umbilical arteryB | ||||
| Triglycerides (mg/mL) | 0.0795 ± 0.0112a | 0.1787 ± 0.0332b | 0.0887 ± 0.0145a | <0.05 |
| NEFA (units) | 0.0765 ± 0.0131 | 0.0822 ± 0.0087 | 0.0843 ± 0.0139 | 0.91 |
| Glucose (mg/dL) | 30.5 ± 2.2 | 36.1 ± 1.7 | 32.4 ± 1.8 | 0.14 |
| Lactate (mM) | 2.32 ± 0.78 | 3.30 ± 0.51 | 2.26 ± 0.54 | 0.42 |
| pH | 7.29 ± 0.03 | 7.20 ± 0.02 | 7.26 ± 0.02 | 0.06 |
| sO2 (%) | 15.0 ± 1.8a | 8.2 ± 1.0b | 11.8 ± 1.7a.b | <0.005 |
| pO2 (mm Hg) | 14.8 ± 0.9a | 10.7 ± 0.7b | 12.8 ± 1.2a.b | <0.01 |
| O2 content (mM) | 1.15 ± 0.12a | 0.65 ± 0.07b | 0.91 ± 0.13a.b | <0.005 |
| pCO2 (mm Hg) | 58.5 ± 3.9 | 72.2 ± 4.0 | 60.2 ± 2.2 | 0.07 |
| CO2 content (mM) | 28.9 ± 0.5 | 29.7 ± 0.6 | 29.0 ± 1.0 | 0.71 |
| Hematocrit (%) | 36.5 ± 0.9 | 37.9 ± 0.7 | 36.0 ± 1.3 | 0.34 |
| Hemoglobin (mg/dL) | 12.4 ± 0.3 | 12.9 ± 0.2 | 12.2 ± 0.4 | 0.34 |
A Measured in fetal blood collected from post-euthanasia cardiac exsanguination.
B Measured in umbilical artery blood collected at cesarean section before cord was severed.
Data represented as mean ± SEM; total number of fetuses studied: n = 21 (CON), 39 (OB-WSD), 5 (OB-DR); umbilical artery analyses n = 8 (CON; 5 female, 3 male), 20 (OB-WSD; 6 female, 14 male), 5 (OB-DR; 1 female, 4 male). ANOVA with fixed effect for maternal diet is shown and when significant (P < 0.05), individual post-test comparisons are indicated. Different letters represent differences between groups (P < 0.05).
AUC, area under the curve; GTT, glucose tolerance test; NA, not applicable; NEFA, non-esterified fatty acids; WSD, Western-style diet.
2.2. Maternal diet reversal model
A cohort of obese mothers fed a chronic WSD for 9 years (n = 5) were switched as a group to the CON diet prior to the fall breeding season, allowed to breed naturally, and maintained on the CON diet throughout pregnancy. The average days to conception after diet reversal ranged from 27 to 96 days (mean = 65 days). These mothers (OB-DR, diet reversal) were studied as described above before and during pregnancy. Additionally, body weight was measured frequently in OB-DR mothers once DR intervention was started during pre-conception and pregnancy (see Fig. S1).
2.3. Fetal measurements and sample collection
Three groups of fetuses were studied from CON-fed mothers (CON; n = 21), obese WSD-fed mothers (OB-WSD; n = 39), and diet reversal mothers (OB-DR; n = 5). At ∼130 days in gestation (gestation period is 165 days), fetuses were delivered by cesarean section while mothers were under anesthesia. Before cutting the umbilical cord, umbilical artery blood samples were drawn from a section of the cord that was clamped on each end. Samples were immediately analyzed for pH, blood gasses, glucose, and lactate (EC8 and CG4 i-STAT test cartridges, Abbott, Abbott Park, IL), and serum was stored at −80 °C for later analyses. Fetal weights were measured, and liver tissue samples were flash-frozen in liquid nitrogen and stored at −80 °C for subsequent analyses.
2.4. Fetal liver tissue analyses
Liver TG content was measured following lipid extraction and averaged in samples from the right and left lobes of the liver from all fetuses in the study (Infinity Triglyceride Reagent, Thermo Scientific, Waltham, MA) [15], [16]. Fetal liver thiobarbituric acid reactive substances (TBARs) content was measured in protein lysate samples prepared from the left lobe of the fetal liver and measured calorimetrically (#700870, Cayman Chemical) and expressed relative to protein content. The citrate synthase activity assay was performed using 30 mg homogenized liver tissue (left lobe) as described [39] with minor modifications to analyze on a microplate reader. RNA was extracted from fetal liver samples and gene expression measured for major de novo lipogenic (DNL) genes (FAS, ACC1, SREBP1C, and SREBP2) and lipid oxidation genes listed in supplementary results using real time PCR (LightCycler 480, Roche) as described [16]. Results were normalized to 18s rRNA. TBARs, CS activity, and gene expression were measured in representative subsets of fetuses as indicated in figure legends.
2.5. Hepatic diacylglycerol and ceramide species profiling
Hepatic diacylglycerol (DAG) and ceramide species were analyzed in a subset of representative fetuses by an Agilent 1100 HPLC connected to an API 2000 triple quadrupole mass spectrometer as previously described [40]. The 1,3- and 1,2-DAG isomers were separated chromatographically using a Hilic 2.1 micron, 3 × 100 mm column. Concentration was determined by comparing ratios of unknowns to di-C15:0 DAG and C12:0 ceramide and compared to standard curves representing the majority of DAG and ceramide species run with each sample set. DAG and ceramide data were normalized to protein content. Multivariate analysis was performed in MetaboAnalyst 4.0 [41] with all DAG and ceramide species and data were log-transformed.
2.6. Targeted serum and liver metabolite analysis with UHPLC-MS
Umbilical artery serum and fetal liver tissue samples were used from a subset of representative fetuses in targeted metabolomic profiling at the University of Colorado Metabolomic Core [42], [43]. Briefly, serum (10 μL) and liver tissue (25 mg) samples were extracted in ice-cold lysis/extraction buffer (methanol:acetonitrile:water 5:3:2). Analyses were performed using a Vanquish UHPLC system coupled online to a Q Exactive mass spectrometer (Thermo). Samples were resolved over a Kinetex C18 column (2.1 × 150 mm, 1.7 μm; Phenomenex, Torrance, CA) at 25 °C using a 3 min isocratic condition of 5% acetonitrile, 95% water, and 0.1% formic acid flowing at 250 μL/min, or using a 9 min gradient at 400 μL/min from 5 to 95% B (A: water/0.1% formic acid; B: acetonitrile/0.1% formic acid) [44], [45]. Mass spectrometry analysis and data elaboration were performed as described [45]. Metabolite assignments were performed using MAVEN [46]. In addition, liver tissue concentrations of acylcarnitines and bile acids were measured, and quantifications were made relative to internal standards [38]. Metabolites were excluded if more than half the samples contained a zero value, and metabolites where less than half the samples contained a zero value were replaced with the half minimum value for the metabolite. Peak intensity values for serum metabolites or liver metabolites (including acylcarnitine and bile acid species) were analyzed with MetaboAnalyst 4.0 [41]. Sample normalization was performed by normalization by sum with data autoscaling for liver and by normalization by median with data range scaling for serum.
Multivariate principal component analysis was performed using partial least squares discriminant analysis (PLS-DA) with all 3 groups. A second PLS-DA was performed with only the CON and OB-WSD groups. The 25 metabolites with the highest variable importance in projection (VIP) scores (rank order) from this PLS-DA with just CON and OB-WSD groups were identified and data for these 25 features from all samples in all 3 groups in liver or serum were loaded into a clustered heatmap. Significance was declared with P < 0.05 and a cutoff of P < 0.15 was used to declare potential differences for hypothesis generation. To account for over-fitting and false positives with PLS-DA, metabolites from the top 25 rank list with P > 0.15 (by t-test) in the CON vs OB-WSD comparison were excluded from pathway and further analysis (indicated in Tables S2–S5). For pathway enrichment analysis, the remaining VIP ranked metabolites plus additional metabolites with a significant raw P-value by t-test (P < 0.05) between CON and OB-WSD (indicated in Tables S2–S5) were used to comprise the compound input list. These additions added 12 metabolites in the liver set and 13 in the serum set. These metabolites were added so that a larger input list could be used for pathway identification. For pathway analysis, Human Metabolome Database IDs were assigned to each metabolite in the Pathway Analysis module in MetaboAnalyst 4.0 and an FDR <0.10 was used.
To assess the effect in the OB-DR group, univariate comparisons were made relative to CON and OB-WSD by raw P-value by t-test using P < 0.10 to declare differences between groups. Metabolites and acylcarnitines (for liver) were considered to be: 1) normalized in OB-DR group when similar to CON (P > 0.10 in OB-DR vs CON) and different from OB-WSD (P < 0.10 in OB-DR vs OB-WSD); 2) not normalized in OB-DR group and different from CON (P < 0.10 in OB-DR vs CON); or 3) intermediate in OB-DR when not different from CON (P > 0.10 in OB-DR vs CON) or OB-WSD (P > 0.10 in OB-DR vs OB-WSD). Due to the relatively small sample size within each group and inclusion of a larger number of features for more thorough pathway approximation, less stringent statistical cut-offs (P < 0.15 or P > 0.15, used as described earlier) were utilized to assess group differences.
2.7. Statistical analysis
Data were analyzed by ANOVA with fixed effect of maternal diet (CON, OB-WSD, OB-DR) using SAS (PROC MIXED). Fetal sex was tested as a fixed effect in the model and remained in the model when significant (P < 0.05). Individual post-test comparisons were made when the overall ANOVA P-value was significant (P < 0.05) and for metabolites of interest as described in section 2.6. Models accounted for the inequality of variances, when indicated, between maternal diet groups.
3. Results
3.1. Switching from a chronic OB-WSD to CON diet prior to pregnancy produces weight loss and improves insulin sensitivity in OB-DR mothers
To determine the metabolic effects of switching to a healthy diet in NHP mothers with chronic obesity, we studied obese mothers who had been on a chronic WSD for ∼9 years and then switched to a healthy CON diet prior to conception and remained on this CON diet through their subsequent pregnancy (OB-DR group). Pre-pregnancy measurements were made on these OB-DR females just prior to the diet switching and in the groups of CON and OB-WSD females. Before pregnancy, OB-WSD females and those assigned to the OB-DR group had increased obesity and insulin resistance as evidenced by increased body weight, percent body fat, fasting plasma insulin concentrations, and insulin area under the curve (AUC) during an IV-GTT compared with the group of CON mothers (Table 1). However, none of the mothers developed gestational diabetes as glucose concentrations and glucose AUC during the IV-GTT were similar. During the 3rd trimester of pregnancy following diet switching, OB-DR mothers still weighed more than CON mothers but had a net 15% reduction in body weight compared with their pre-pregnancy body weight, and their fasting insulin concentrations and insulin AUC were improved relative to OB-WSD mothers (Table 1). All OB-DR mothers rapidly lost weight over the first ∼75 days during the pre-conception period (Fig. S1A). As a group, there was a net loss of 2.2 kg prior to conception (Fig. S1B). During pregnancy, however, 4 out of 5 mothers had a net weight gain, while 1 mother continued to lose weight (Fig. S1B). Thus, switching to a CON diet prior to a subsequent pregnancy produced weight loss in OB-DR mothers and prevented the large increase in insulin resistance in the 3rd trimester of pregnancy observed in OB-WSD mothers. Fetal body weight, liver weight, and plasma (mixed arterial and venous fetal sample) insulin concentrations in the early 3rd trimester were similar between groups (Table 1).
3.2. Fetal umbilical arterial blood gas and serum nutrient analysis
To characterize the effect of maternal obesity and maternal diet reversal on the fetal circulation, we measured umbilical (fetal) arterial serum TGs, non-esterified fatty acids (NEFAs), glucose, and lactate concentrations and blood gas levels in fetuses from CON, OB-WSD, and OB-DR mothers (Table 1). Fetal serum TG concentrations were higher in fetuses from OB-WSD mothers versus fetuses from CON and OB-DR mothers, with no differences in NEFA concentrations. Fetuses from OB-WSD mothers had lower O2 saturation, pO2, and O2 content and higher pCO2 compared with fetuses from CON mothers, suggesting that fetuses from OB-WSD mothers had some degree of hypoxemia. Arterial oxygenation in fetuses from OB-DR mothers was not different compared with fetuses from CON mothers, suggesting that hypoxemia was partially restored with maternal diet reversal. No differences in glucose, lactate, hematocrit, or hemoglobin concentrations were observed between groups. We found no significant differences between male and female fetuses within the groups (data not shown).
3.3. Increased hepatic steatosis in fetuses from OB-WSD mothers was not fully ameliorated in fetuses from OB-DR mothers
Fetal hepatic TGs were significantly increased in fetuses from OB-WSD mothers and were intermediate in fetuses from OB-DR mothers relative to the CON and OB-WSD groups (Figure 1A). This is consistent with the partial improvement in hepatic steatosis noted previously in fetuses from diet reversal mothers [15]. The variability in hepatic TGs in the OB-DR group was not explained by the differences in net maternal body weight change nor the duration of diet switching prior to conception (Figs. S1C and D). Further, while maternal age was higher in the OB-DR group, mothers of similar age are included in the OB-WSD group, and no relationship between maternal age and fetal hepatic TGs was observed (data not shown).
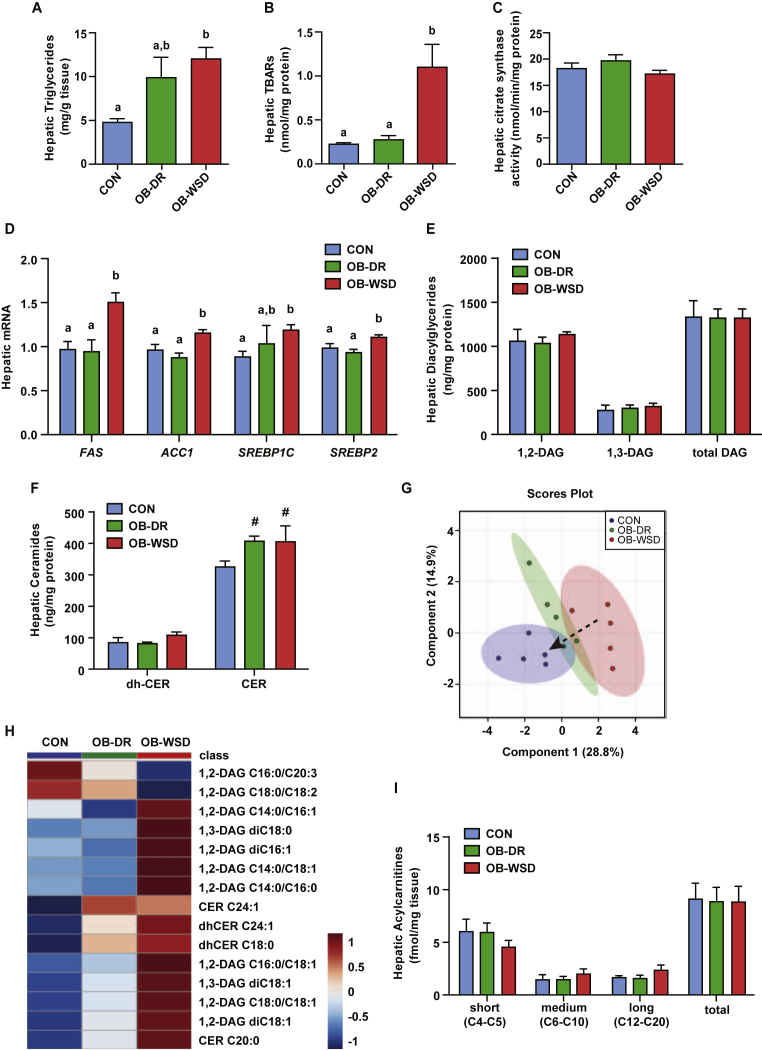
Figure 1.
Effect of maternal OB-WSD and OB-DR on fetal hepatic lipid accumulation, oxidative stress, lipogenic gene expression, and lipid profiles. (A) Liver triglyceride concentrations measured in fetuses from CON (blue bars), OB-DR (green bars), and OB-WSD (red bars) mothers. Bars with different letters are statistically different from each other. n = 21 CON (11 female, 10 male), 5 OB-DR (1 female, 4 male), and 28 OB-WSD (16 female, 22 male). (B) Lipid peroxidation measured by TBARs as marker of oxidative stress in fetal livers. CON, n = 5 (3 female, 2 male); OB-DR, n = 5 (1 female, 4 male); OB-WSD, n = 7 (2 female, 5 male) (C) Hepatic citrate synthase activity. n = 4 CON (2 female, 2 male), 5 OB-DR (1 female, 4 male), 13 OB-WSD (4 female, 9 male). (D) Expression of lipogenic genes in CON, OB-DR, and OB-WSD fetal livers. n = 13 CON (6 female, 7 male), 5 OB-DR (1 female, 4 male), 27 OB-WSD (12 female, 15 male). Bars with different letters represent significant differences between maternal diet groups (P < 0.05). (E–F) Fetal liver samples from CON, OB-DR, and OB-WSD maternal diet groups were subjected to targeted assays for diacylglyceride (DAG) and ceramide (CER) concentrations. The sum of 1,2-DAGs, 1,3-DAGs, and all DAGs (E) and the sum of dihydroceramides and non-dihydroceramides (F) are shown. CON, n = 5 (3 female, 2 male); OB-DR, n = 5 (1 female, 4 male); OB-WSD, n = 5 (2 female, 3 male). #P < 0.10 vs CON by Student's t-test. A-F) Data are represented as mean ± SEM. (G) PLS-DA was used to identify lipid with changes in abundance that defined separation of samples between the maternal diet groups; 2-dimensional blot is shown. Dashed arrow indicates shift in lipid profile with OB-DR. (H) Heat map of the 15 lipids with the highest variable importance in projection (VIP) scores. Each square is representative of the mean levels of that lipid. Row values are normalized for each lipid and quantitative changes are color coded from blue (low) to red (high). Lipid names are shown on right y-axis. (I) Fetal liver acylcarnitines showing sums of short, medium, and long chain, and all acylcarnitines. y-axis values x 1000. Data are represented as mean ± SEM. CON, n = 5 (4 female, 1 male); OB-DR, n = 5 (1 female, 4 male); OB-WSD, n = 5 (2 female, 3 male).
We next sought to determine if oxidative stress, mitochondrial activity, and DNL gene expression were altered with maternal diet. Fetuses from OB-WSD mothers had a >3-fold increase in hepatic TBAR concentrations, a marker of lipid peroxidation and oxidative stress, and no change in hepatic citrate synthase enzyme activity, a marker of mitochondrial content (Figure 1B,C). No differences between male and female fetuses on hepatic TGs, TBARs, or citrate synthase activity were observed (Figs. S2A–C). We measured expression of DNL genes and found 5–15% lower expression levels of FAS, ACC1, and SREBP2 in male versus female fetuses, regardless of maternal diet group (Fig. S2D). In terms of maternal diet, fetuses from OB-WSD mothers had increased hepatic expression of DNL genes including FAS, ACC1, SREBP1C, and SREBP2 (Figure 1D) [15], [18], [36]. No differences in hepatic expression of genes involved in lipid oxidation were found (CPT1A, ACOX1, HADHA, MCAD, and LCAD; Fig. S3). Importantly, hepatic TBAR levels and lipogenic gene expression were normalized in fetal livers from OB-DR mothers and were similar to the CON group. Thus, despite the normalization of DNL gene expression, oxidative stress, and normal mitochondrial content, the maternal diet improvements were unable to completely restore hepatic TG accumulation in fetuses from OB-DR mothers.
3.4. Hepatic lipid profile
Given the role of diet on lipotoxic lipids and stress signaling on development of steatosis and insulin resistance [47], we assessed the profile DAGs and ceramides in the fetal liver (Figure 1E,F; Table S1). In the fetal liver, ∼80% of DAGs were the 1,2-isomer and 20% were the 1,3-isomer. Total hepatic concentrations of 1,2-DAGs, 1,3-DAGs, and the sum of all DAG species were not different between groups (Figure 1E). Fetal hepatic concentrations of the sum of dihydroceramides, precursor molecules in ceramide synthesis, were not different between groups, yet total hepatic concentrations of ceramides showed trending increases in fetuses from OB-WSD and OB-DR mothers (P < 0.10) compared with CON (Figure 1F). The individual DAG and ceramide species (Table S1) were analyzed with PLS-DA. The PLS-DA showed tight clustering of samples within maternal diet groups and a distinct separation between CON and OB-WSD groups with a marked shift in the OB-DR group away from the OB-WSD group and toward the CON group (Figure 1G). The 15 lipids with the highest VIP scores from the PLS-DA were loaded into a clustered heat map (Figure 1H). Outcomes in the fetal liver from OB-WSD mothers included decreased C16:0/C20:3 and C18:0/C18:2, both 1,2-DAGs; increased C14:0/C16:1, di-C16:1, C14:0/C18:1, C14:0/C16:0, C16:0/C18:1, C18:0/C18:1, and di-C18:1, all 1,2-DAGs; and increased di-C18:0 and di-C18:1, both 1,3-DAGs. In the ceramide profile, the fetal liver from OB-WSD mothers had increased levels of C24:1, dihydro-C24:1, dihydro-C18:0, and C20:0 ceramides. Importantly, hepatic DAG and ceramide profiles in the fetuses from OB-DR mothers were similar to the CON group (P > 0.05, Table S1) with the exception of C24:1 ceramide which remained increased at the level of the OB-WSD group. Thus, dysregulation of hepatic ceramide but not DAG metabolism might underlie the persistent steatosis in fetuses from OB-DR mothers. This is important because hepatic ceramides are increased during the progression from NAFLD to NASH and might underlie mitochondrial dysfunction [48], [49].
To assess hepatic substrate and incomplete lipid oxidation, we measured acylcarnitine profiles in the fetal liver. The sum of all acylcarnitines, as well as the groups of short, medium, and long chain acylcarnitines, were similar in livers of fetuses from CON, OB-DR, and OB-WSD mothers (Figure 1I). This data, combined with no differences in citrate synthase activity or expression of lipid oxidation genes, suggests that fetuses from OB-WSD mothers maintained overall hepatic lipid oxidation capacity, although specific lipid intermediates increased or decreased based on differences upstream for specific substrate preference and pathway flux, as discussed later.
3.5. Effects of maternal obesity and diet reversal on fetal arterial and liver metabolites
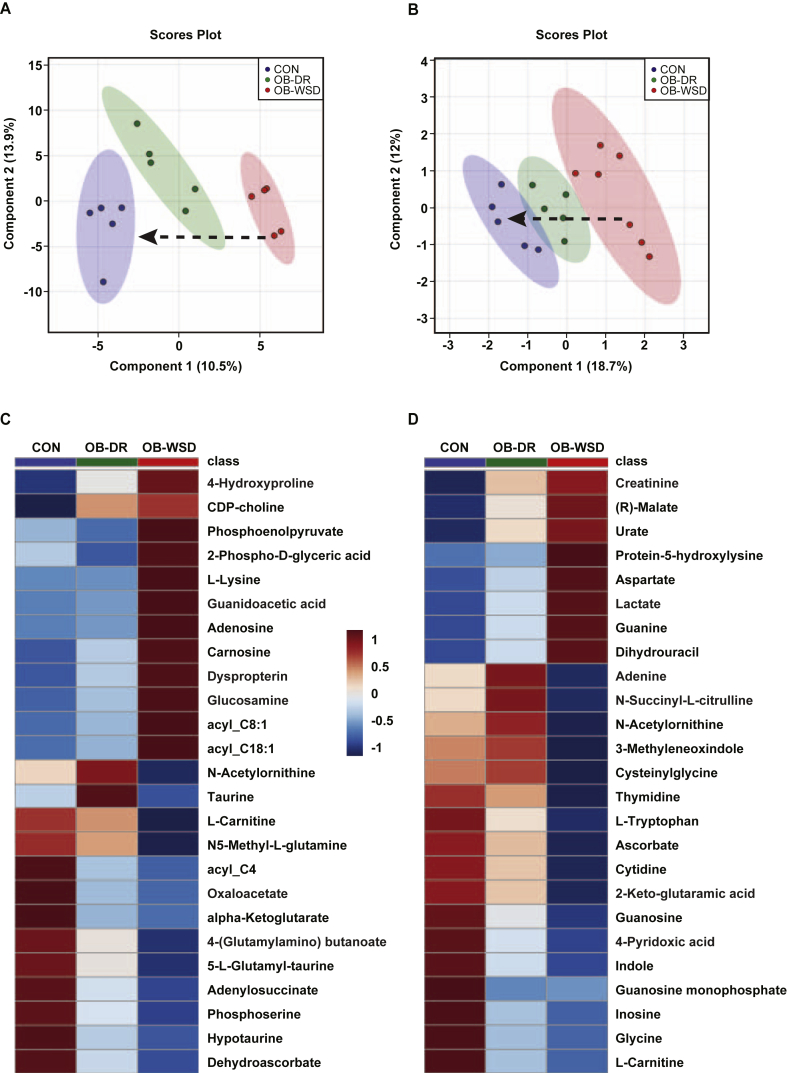
To isolate the effects of maternal obesity and diet reversal on fetal systemic and hepatic metabolites, we used targeted metabolomic screens in fetal liver and umbilical (fetal) artery serum samples. In liver, this included 166 metabolites plus panels for 17 acylcarnitines and 9 bile acids (Tables S2, S3, and S4, respectively). In serum, 118 metabolites were detected (Table S5). PLS-DA showed a tight clustering pattern of samples within each maternal diet group and distinct separation between CON and OB-WSD samples with a shift in the OB-DR group toward the CON group indicating an improvement in the metabolome, but incomplete normalization in both the liver (Figure 2A) and serum (Figure 2B). A separate PLS-DA with only the CON and OB-WSD liver (Fig. S4A) or serum (Fig. S4B) samples was used to identify features with the highest VIP scores to focus on metabolites most affected by maternal diet. The relative levels of the top 25 features in the liver or serum in all samples from CON, OB-DR, and OB-WSD fetuses were loaded into a clustered heat map (Figure 2C,D and Figs. S5A and B for individual samples). An intermediate phenotype in both the liver and serum was observed in the OB-DR group with several metabolites normalizing to CON levels yet many remaining different from the CON group, as further described in sections 3.8, 3.9.
Figure 2.
Effect of maternal OB-WSD and OB-DR on fetal liver and serum metabolome. Fetal liver and arterial serum from CON, OB-WSD, and OB-DR maternal diet groups were subjected to global metabolomics profiling. PLS-DA was used to identify metabolites with changes in abundance that defined separation of samples between the maternal diet groups in liver (A) and serum (B). 2-dimensional blot is shown. Dashed arrow illustrates a shift in the fetal metabolome with OB-DR. CON, n = 5 (4 female, 1 male); OB-DR, n = 5 (1 female, 4 male); OB-WSD, n = 5 (2 female, 3 male) for liver. CON, n = 5 (3 female, 2 male); OB-DR, n = 5 (1 female, 4 male); OB-WSD, n = 7 (2 female, 5 male) for serum. Heat map of the 25 metabolites with the highest variable importance in projection (VIP) scores from liver (C) and serum (D). Each square is representative of the mean levels of that metabolite. Row values are normalized for each metabolite and quantitative changes are color coded from blue (low) to red (high). Metabolite names are shown on right y-axis.
3.6. Pathways dysregulated in fetuses from OB-WSD mothers: liver
To approximate the pathways affected in the fetal liver by exposure to maternal obesity, we performed pathway enrichment analysis. Feature input lists were created that included top metabolites from the VIP analysis and additional metabolites that were differentially expressed based on univariate comparisons between CON and OB-WSD groups in fetal liver (as described in section 2.6; Tables S2–S4). The pathways enriched in the OB-WSD fetal liver were associated with arginine and proline metabolism, taurine and hypotaurine metabolism, tricarboxylic acid (TCA) cycle, alanine, aspartate, and glutamate metabolism, glycolysis/gluconeogenesis, and pyruvate metabolism (Table 2). In the OB-WSD fetal liver, the enrichment was driven by increased levels of the key gluconeogenic intermediates (phosphoenolpyruvate, 2-phospho-d-glyceric acid, and fructose 1,6-bisphosphate) and increased levels of intermediates in arginine and proline metabolism (ornithine, l-proline, guanidoacetic acid, 4-hydroxyproline, and creatinine). Increased proline and 4-hydroxyproline might be linked with early signs of hepatic fibrosis as these metabolites are involved in extracellular matrix formation and cross-linking [50]. Hepatic levels of alpha-ketoglutarate (P = 0.04) and oxaloacetate (P = 0.01), both mitochondrial TCA alpha-keto acid derivatives, were decreased of fetuses from OB-WSD mothers (Table S2). This suggests increased cataplerosis and use of TCA cycle intermediates for gluconeogenesis in the fetal liver in the OB-WSD group.
Table 2.
Pathway enrichment analysis in liver and serum between fetuses from CON and OB-WSD mothers.
| Metabolite Set | Hits | FDR | Up in OB-WSD | Down in OB-WSD |
|---|---|---|---|---|
| Liver | ||||
| Arginine and proline metabolism | 7 | 0.0029 | Ornithine; l-Proline; Guanidoacetic acid; 4-Hydroxyproline; Creatinine | N-Acetylornithine; 4-(Glutamylamino) butanoate |
| Taurine and hypotaurine metabolism | 3 | 0.0507 | Acetylphosphate | Hypotaurine; 5-L-Glutamyl-taurine |
| Citrate cycle (TCA cycle) | 3 | 0.0507 | Phosphoenolpyruvate | alpha-Ketoglutarate; Oxaloacetate |
| Alanine, aspartate and glutamate metabolism | 3 | 0.0652 | Adenylosuccinate; alpha-Ketoglutarate; Oxaloacetate | |
| Glycolysis or Gluconeogenesis | 4 | 0.0993 | Phosphoenolpyruvate; 2-Phospho-d-glyceric acid; Fructose 1,6-bisphosphate | Oxaloacetate |
| Pyruvate metabolism | 3 | 0.0993 | Phosphoenolpyruvate; Acetylphosphate | Oxaloacetate |
| Serum | ||||
| beta-Alanine metabolism | 5 | 0.0007 | Aspartate; Spermidine; Dihydrouracil; Uracil; Carnosine | |
| Arginine and proline metabolism | 7 | 0.0007 | Aspartate; Glutamate; Spermidine; Pyruvate; Creatinine; Putrescine | N-Acetylornithine |
| Glutathione metabolism | 5 | 0.0015 | Glutamate; Spermidine; Putrescine | Cysteinylglycine; Ascorbate |
| Pantothenate and CoA biosynthesis | 4 | 0.0042 | Dihydrouracil; Pyruvate; Aspartate; Uracil | |
| Purine metabolism | 6 | 0.0080 | Guanine, Urate | Guanosine; Glycine; Inosine; Guanosine monophosphate |
| Alanine, aspartate and glutamate metabolism | 3 | 0.0323 | Aspartate; Pyruvate; Glutamate | |
| Pyrimidine metabolism | 4 | 0.0521 | Dihydrouracil; Uracil | Cytidine; Thymidine |
Pathways and inclusive metabolite sets with FDR < 0.10 are listed.
FDR, false discovery rate; TCA, tricarboxylic acid.
3.7. Pathways dysregulated in fetuses from OB-WSD mothers: arterial serum
As with the fetal liver, pathway enrichment analysis was performed in arterial fetal serum from CON, OB-DR, and OB-WSD groups. In the serum of fetuses from OB-WSD mothers, the pathways affected included beta-alanine metabolism, arginine and proline metabolism, glutathione metabolism, pantothenate and CoA biosynthesis, purine metabolism, alanine, aspartate, and glutamate metabolism, and pyrimidine metabolism (Table 2). Increased levels of aspartate, spermidine, dihydrouracil, uracil, carnosine, creatinine, and putrescine were associated with overlapping roles in glutathione, beta-alanine, arginine/proline, and pantothenate/CoA synthesis. Increased urate (uric acid) is a marker of purine nucleoside catabolism. This is further supported by decreased guanosine (nucleoside) in favor of increased guanine (base), and decreased inosine (nucleoside). For the pyrimidines, there is a similar pattern of increased catabolism based on decreased cytidine and thymidine (nucleosides = nucleotide + ribose sugar) and increased uracil (base only). Increased levels of several AA in the serum of fetuses from OB-WSD mothers might reflect differences in umbilical supply or shifts in substrate preference by the fetus, either in the liver or other organs.
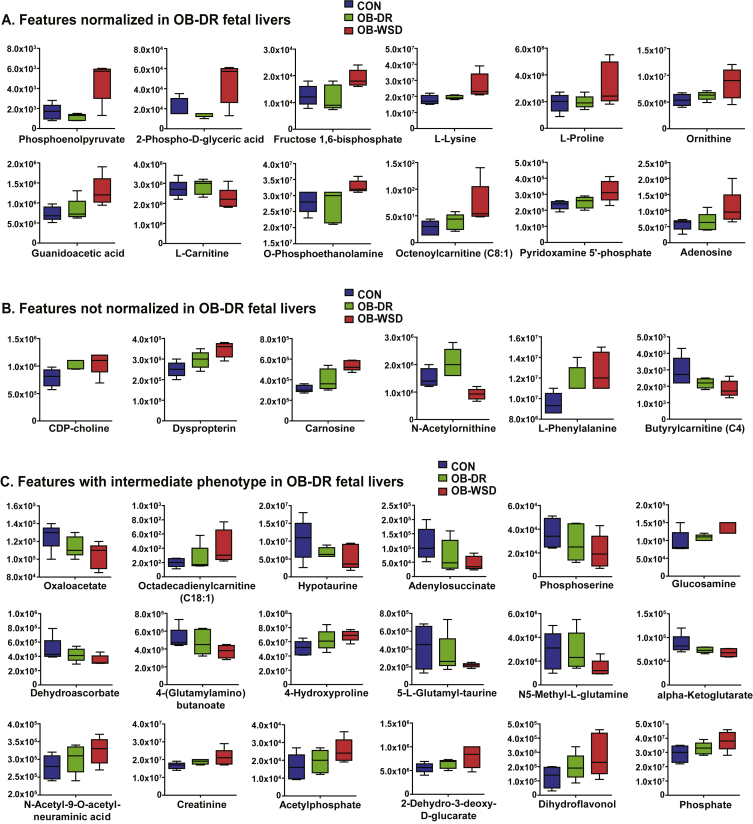
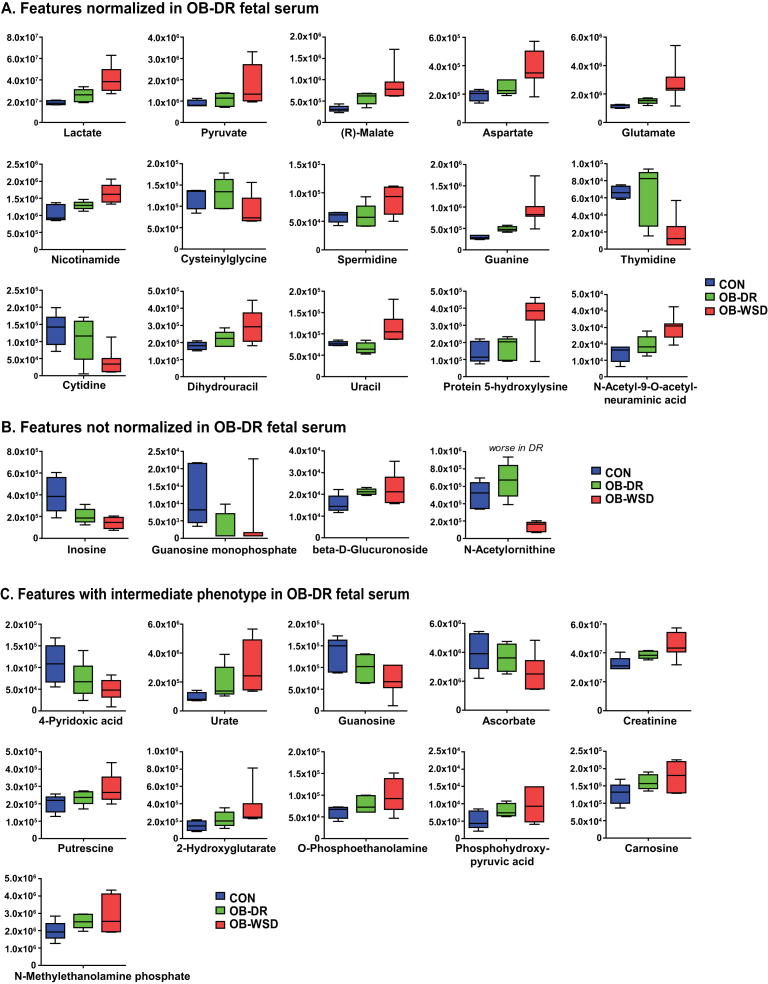
3.8. Metabolites improved in fetal liver with maternal diet reversal
To determine the efficacy of maternal diet reversal, the abundance of the top features that were different between CON and OB-WSD groups and submitted for pathway analysis were individually evaluated in the liver of fetuses from OB-DR mothers (Tables S2–S4). Liver metabolites that were normalized in fetuses from OB-DR mothers included gluconeogenic intermediates (phosphoenolpyruvate, 2-phospho-d-glyceric acid, and fructose 1,6-bisphosphate), l-lysine, l-proline, ornithine, guanidinoacetic acid, l-carnitine, O-phosphoethanolamine, octenoylcarnitine (C8:1), pyridoxamine 5′-phosphate, and adenosine (Figure 3A). Several hepatic metabolites were not normalized in the OB-DR group and include CDP-choline, 6-pyruvoyltetrahydropterin (dyspropterin), carnosine, N-acetylornithine, l-phenylalanine, and butyrylcarnitine (C4) (Figure 3B). Interestingly, hepatic levels of N-acetylornithine and adenosine monophosphate were highest in fetuses from OB-DR mothers compared with CON and OB-WSD groups (Table S2). The remaining metabolites had partial improvement in the livers of fetuses from OB-DR mothers with an intermediate abundance compared with the CON and OB-WSD groups (Figure 3C).
Figure 3.
Effect of diet reversal on metabolites in the liver of fetuses from OB-DR mothers. The top features with either the highest variable importance in projection (VIP) scores or significant difference by t-test between CON and OB-WSD groups are shown here and analyzed by univariate analysis with all 3 groups: CON, OB-DR, and OB-WSD and grouped as indicated. (A) Metabolites that are normalized in OB-DR and are similar to CON (P > 0.10, OB-DR vs CON) yet different from OB-WSD (P < 0.10, OB-DR vs OB-WSD). (B) Metabolites that are not normalized in OB-DR and different from CON (P < 0.10, OB-DR vs CON). (C) Metabolites with an intermediate phenotype in OB-DR that are not different from CON (P > 0.10, OB-DR vs CON) and OB-WSD (P > 0.10, OB-DR vs OB-WSD). Box and whisker plots with 5–95% interval. CON, n = 5 (4 female, 1 male); OB-DR, n = 5 (1 female, 4 male); OB-WSD, n = 5 (2 female, 3 male).
3.9. Metabolites improved in fetal serum with maternal diet reversal
In serum, the top features that were different between CON and OB-WSD groups were individually evaluated in fetuses from OB-DR mothers (Table S5). The abundance of lactate, pyruvate, malate, aspartate, glutamate, and nicotinamide were all decreased (relative to OB-WSD) and normalized in fetuses from OB-DR mothers (relative to CON) (Figure 4A). In addition, glutathione metabolites (cysteinylglycine and spermidine), purine and pyrimidine metabolites (guanine, thymidine, cytidine, dihydrouracil, and uracil), protein 5-hydroxylysine, and N-acetyl-9-O-acetylneuraminic acid were all normalized in the serum of fetuses from OB-DR mothers and similar to the abundance in serum from the CON group (Figure 4A). Despite normalization of several nucleotide metabolites, levels of inosine and guanosine monophosphate, both purine metabolites, remained decreased and levels of beta-d-glucuronoside remained increased in fetuses from OB-DR mothers (Figure 4B). Interestingly, serum levels of N-acetylornithine were increased in the OB-DR group relative to the OB-WSD group but to levels greater than observed in fetuses in the CON group (Figure 4B). The abundance of the remaining features that were different in the serum between fetuses from CON and OB-WSD mothers remained intermediate in fetuses from OB-DR mothers (Figure 4C).
Figure 4.
Effect of diet reversal on metabolites in the umbilical artery serum of fetuses from OB-DR mothers. The top features with either the highest variable importance in projection (VIP) scores or significant difference by t-test between CON and OB-WSD groups are shown here and analyzed by univariate analysis with all 3 groups: CON, OB-DR, and OB-WSD and grouped as indicated. (A) Metabolites that are normalized in OB-DR and are similar to CON (P > 0.10, OB-DR vs CON) yet different from OB-WSD (P < 0.10, OB-DR vs OB-WSD). (B) Metabolites that are not normalized in OB-DR and different from CON (P < 0.10, OB-DR vs CON). (C) Metabolites with an intermediate phenotype in OB-DR that are not different between CON (P > 0.10, OB-DR vs CON) and OB-WSD (P > 0.10, OB-DR vs OB-WSD). Box and whisker plots with 5–95% interval. CON, n = 5 (3 female, 2 male); OB-DR, n = 5 (1 female, 4 male); OB-WSD, n = 7 (2 female, 5 male).
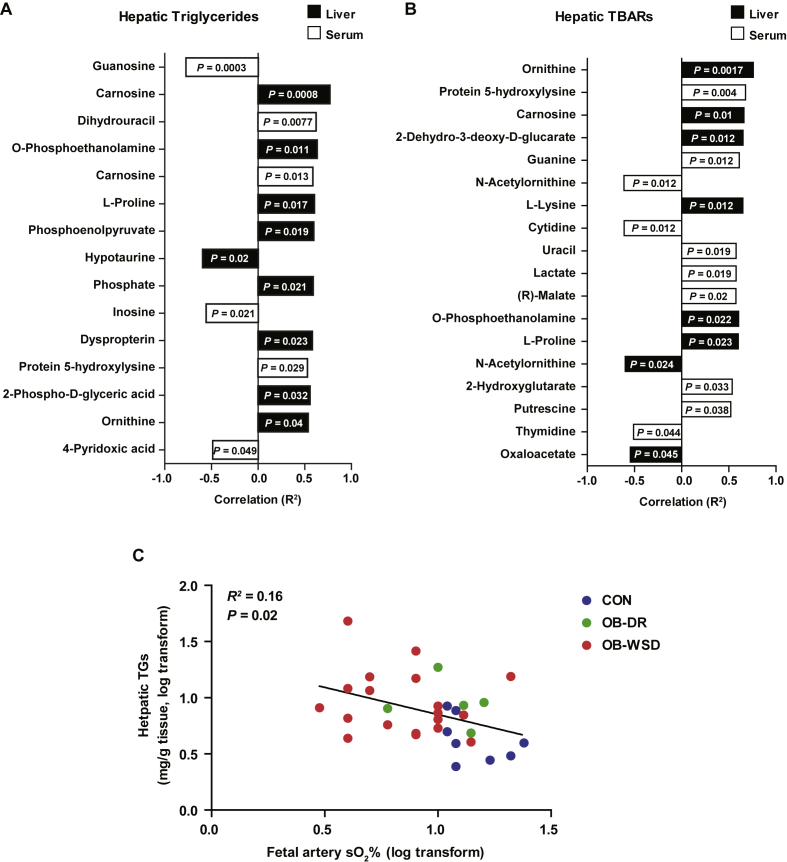
3.10. Hepatic metabolites correlated with fetal hepatic steatosis and oxidative stress
Given the incomplete normalization of hepatic steatosis in fetuses from OB-DR mothers, we sought to determine which metabolites were associated with steatosis and oxidative stress. Hepatic carnosine levels had the highest correlation with increased hepatic TG concentrations across all fetuses from CON, OB-WSD, and OB-DR mothers (Figure 5A). Hepatic levels of O-phosphoethanolamine, l-proline, phosphoenolpyruvate, hypotaurine, phosphate, dyspropterin, 2-phospho-d-glyceric acid, and ornithine were also correlated with hepatic TG content. Of the serum metabolites, guanosine, dihydrouracil, carnosine, inosine, protein 5-hydroxylysine, and 4-pyridoxic acid were correlated with hepatic TG concentrations (Figure 5A). We also tested which metabolites were associated with oxidative stress in the fetal liver and serum. Hepatic levels of ornithine, carnosine, 2-dehydro-3-deoxy-d-glucarate, l-lysine, O-phosphoethanolamine, l-proline, N-acetylornithine, and oxaloacetate and serum levels of protein 5-hydroxylysine, guanine, N-acetylornithine, cytidine, uracil, lactate, malate, 2-hydroxyglutarate, putrescine, and thymidine were correlated with hepatic TBARs (Figure 5B). Interestingly, only metabolite levels of hepatic carnosine, O-phosphoethanolamine, ornithine, and l-proline were associated with both fetal hepatic TG accumulation and oxidative stress. Hepatic TGs were correlated with fetal arterial oxygen saturation (Figure 5C), suggesting a link with fetal hypoxemia.
Figure 5.
Metabolites significantly correlated with fetal hepatic steatosis. The top features in liver (black bars) and serum (white bars) with significant correlation (P < 0.05) with fetal hepatic steatosis (triglycerides) (A) and TBARs (B). The correlation (R2) and direction (positive or negative) of the relationship is indicated on horizontal axis. Rank along vertical axis from top to bottom is by P-value significance. (C) Relationship between fetal hepatic TGs and fetal arterial oxygen saturation (sO2) in 32 fetuses, as indicated in CON, OB-DR, and OB-WSD groups. n = 8 CON (5 female, 3 male), 5 OB-DR (1 female, 4 male), and 19 OB-WSD (6 female, 13 male).
4. Discussion
4.1. Summary of key findings
Maternal obesity is widespread and linked to NAFLD risk in the offspring; however, the underlying molecular mechanisms and metabolic pathways driving hepatic dysfunction distinct to the fetus of an obese mother remain poorly understood. Initial studies from our laboratory and our colleagues demonstrated that fetuses from NHP mothers on a WSD have increased hepatic TG accumulation, oxidative stress, and lipogenic and gluconeogenic gene expression [15], [16], [17], [18], [19]. Here, we report that early 3rd trimester fetuses from OB-WSD mothers demonstrate, in addition to steatosis, hypoxemia, induction of unique intracellular metabolic pathways that create oxidative stress, activation of hepatic gluconeogenesis, and upregulation of metabolites that are precursors to liver injury. Surprisingly, our results also demonstrate incomplete normalization in the liver and serum metabolomes in fetuses from OB-DR mothers. These data indicate that the fetal metabolic responses are still poised toward an early steatotic phenotype despite the obese mother's consumption of a healthy CON diet and that the fetal liver is uniquely vulnerable to dysregulated fuel metabolism in maternal obesity.
4.2. Unique mechanisms for hepatic steatosis, gluconeogenesis, and oxidative stress in fetuses from OB-WSD mothers
The pathologic mechanisms for childhood NAFLD are complex and multifactorial, but some have centered on mitochondrial dysfunction that might actually precede, if not coincide with, hepatic lipid accumulation and insulin resistance [51]. Decreases in mitochondrial content and activity have been reported in the livers of post-weaned offspring of rodent dams fed a high-fat diet [52], [53]. However, no studies have investigated whether impaired mitochondrial function exists in the liver of a fetus from an obese mother, despite numerous studies in the postnatal period in humans and animal models [54], [55], [56], [57]. In the fetus from OB-WSD mothers, we found no differences in hepatic citrate synthase activity, lipid oxidation gene expression, or total levels of acylcarnitines supporting a minimal effect of maternal WSD on mitochondrial content and/or decreased oxidative capacity in the fetal liver, despite a 2-fold increase in TGs. These data expand our previous findings that, despite fuel overload in utero, increased hepatic TG accumulation in fetuses from OB-WSD mothers is not associated with impaired liver mitochondrial function, leaving open the possibility that mitochondria worsen over time postnatally.
In adults with NAFLD, Lambert et al. showed that hepatic DNL is increased, independent of contributions from dietary fat and plasma free fatty acids to hepatic lipids [58]. Notably, in the human fetus, the capacity for DNL is limited as there is a maternal supply of lipids which are used for fat accretion in the fetus [59], [60]. In the livers of fetuses from OB-WSD mothers, we found upregulation of genes in the DNL pathway suggesting that WSD triggers a perturbation in these genes in development [16]. In the context of our observations, the increased fetal lipid and sugar-derived substrates (TGs, pyruvate, lactate, glutamate, citrate, malate, and succinate) in serum of fetuses from OB-WSD mothers provide common metabolic fuels for increased hepatic TG deposition as well as increased gluconeogenesis.
Anaplerotic processes use AAs to replenish TCA cycle intermediates and are coupled with cataplerotic processes that utilize TCA intermediates during gluconeogenesis to make glucose. In the liver of fetuses from OB-WSD mothers, we found that levels of alpha-ketoglutarate and oxaloacetate were decreased compared with CON suggesting increased cataplerosis and use of these TCA intermediates for increased gluconeogenesis (Figure 6). Importantly, accelerated anaplerosis/cataplerosis in the liver is also linked with hepatic oxidative stress and inflammation [61], [62], [63]. Fetuses from OB-WSD mothers demonstrated a metabolite profile with altered energy metabolites and oxidative stress, as indicated by increased hepatic TBAR levels. Serum from these fetuses had increased urate, dihydrouracil, uracil, nicotinamide, guanine, and creatinine levels and decreased thymidine, cytidine, guanosine, guanosine monophosphate, and inosine levels compared with CON, suggestive of impaired nucleotide and energy metabolites. In liver, the level of adenylosuccinate, a product of aspartate and inosine monophosphate, was decreased in OB-WSD compared with CON, supporting purine catabolism resulting in increased levels of adenosine base (Figure 6).
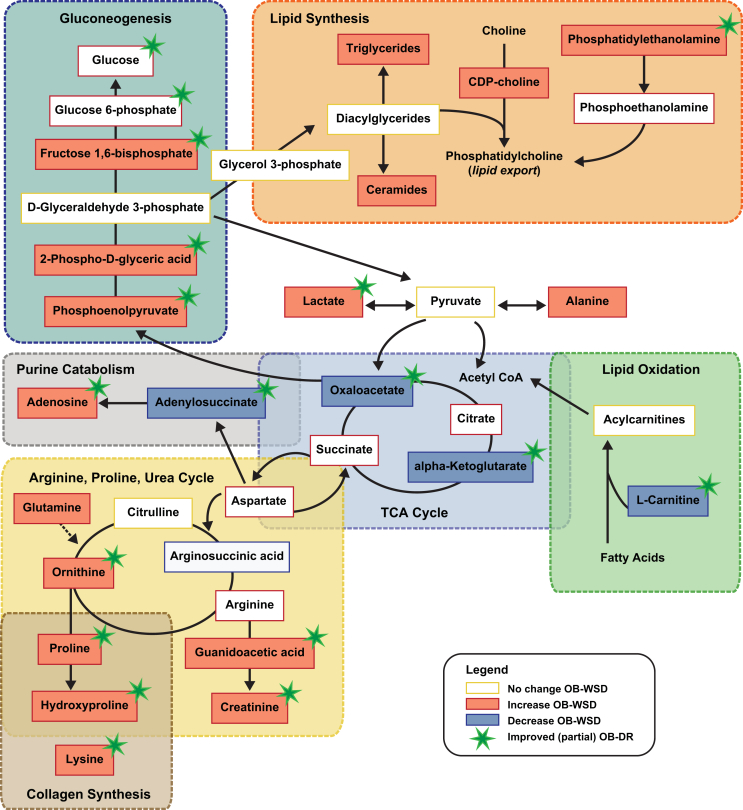
Figure 6.
Summary and integration of pathways affected in liver of fetuses from OB-WSD mothers and corrected with maternal diet reversal in fetuses from OB-DR mothers. Metabolites that increased (solid orange) or decreased (solid blue) in the liver of fetuses from OB-WSD mothers (P < 0.15 in CON vs OB-WSD) are indicated. Metabolites are linked in pathways with a shaded box and dashed outline with indicating overlap when pathways have metabolites in common. Directional arrows are generalized and simplified for illustrative purposes. Metabolites that improved with maternal diet reversal in fetuses from OB-DR mothers are indicated with a green star. Metabolites that had numeric increase or decrease (P > 0.15) are indicated with orange or blue outline (no fill). Metabolites measured but not different have yellow outline. Metabolites that were not measured have no outline. TCA, tricarboxylic acid.
Fetuses from OB-WSD mothers showed limited antioxidant activity, as demonstrated by decreased serum levels of ascorbate and cysteinylglycine and decreased hepatic dehydroascorbate levels, all components in glutathione metabolism. Increased serum levels of glutamate, spermidine, and putrescine suggest a potential shift in the utilization of these precursors away from glutathione synthesis and toward beta-alanine metabolism. In line with this, levels of carnosine, a product of beta-alanine metabolism [64], were increased in both liver and serum of fetuses from OB-WSD mothers and were correlated with hepatic TGs and TBARs. Thus, in the setting of excess fuel and reduced antioxidant capacity, increases in hepatic oxidative stress in the fetus of the OB-WSD mother were found, which likely contribute to fetal apoptosis noted in our model prior to the development of obesity [65].
4.3. Maternal preconception diet reversal in obese mothers normalizes DNL and gluconeogenic capacity in the fetal liver
One of the main findings in the present study was the demonstration that in fetuses from OB-DR mothers, plasma TG concentrations in fetal umbilical artery serum were normalized, as were several measures of hepatic lipogenic activity and gluconeogenic pathways (Figure s 1 and Figure 6). This includes a reduction in expression of hepatic lipogenic genes FAS and SREBP1C, which are increased in fetuses from OB-WSD mothers [18]. Normalization of these genes in the fetus is important because increased lipogenic activation is present in liver tissue and isolated hepatocytes from OB-WSD offspring at 1 year of age, despite switching to a chow diet post-weaning [16]. Moreover, fetuses from OB-DR mothers showed normalization of the hepatic DAG isomer profile relative to fetuses from CON mothers. Importantly, with maternal diet reversal, hepatic levels of phosphoenolpyruvate, 2-phospho-d-glyceric acid, fructose 1,6-bisphosphate, oxaloacetate, and alpha-ketoglutarate were all improved (or intermediate) in the OB-DR group, indicating a reduction in the early activation of gluconeogenic flux as a result of modifying the maternal diet.
4.4. Hypoxemia and hepatic oxidative stress are ameliorated in fetuses from OB-DR mothers
Diet reversal was also beneficial in ameliorating fetal hepatic oxidative stress. The serum abundance of thymidine, lactate, guanine, aspartate, cytidine, malate, urate, uracil, dihydrouracil, and guanosine were improved in fetuses from OB-DR mothers, suggesting improved nucleotide and energy metabolism and increased antioxidant mechanisms. Notably, serum levels of inosine and guanosine monophosphate remained decreased and hepatic levels of adenosine monophosphate were highest in fetuses from OB-DR mothers compared with fetuses from CON and OB-WSD mothers, which might indicate low energy status and stress in the liver or reflect negative energy balance in the OB-DR mothers experiencing rapid weight loss. Hypoxemia, based on lower fetal pO2, was partially improved in the fetus from OB-DR mothers. In mice, genetic activation of the hypoxia inducible factor (HIF) pathways results in hepatic steatosis [66], [67]. Hypoxia and an aberrant oxygen gradient across the liver can induce hepatic steatosis [68], is associated with NAFLD and the progression of liver disease [69], [70], and has been reported to mediate fibrosis in NAFLD [71], [72], [73]. Importantly, we also found that serum levels of protein 5-hydroxylysine and hepatic levels of proline and lysine (Figure 6), components in collagen synthesis [50], were positively correlated with hepatic TGs and TBARs, suggesting that the early biochemical pathways to fibrosis are already present in the livers of fetuses from OB-WSD mothers. The abundance of these metabolites was reduced in fetuses from OB-DR mothers compared with fetuses from OB-WSD mothers, as was hypoxemia. Acute hypoxia has been shown to induce hepatic glucose output [74], [75] and chronic anemic hypoxemia increases Pck1 expression [76] in fetal sheep. Moreover, Pck1 expression is increased in neonatal and postnatal offspring in a mouse model of maternal obesity and WSD exposure [77]. Thus, hypoxemia in fetuses from obese mothers and prevention by diet reversal might be a key driver of both the activation of gluconeogenesis and early signs of fibrosis in the liver.
4.5. Unique biomarkers in the fetus for NAFLD risk following intrauterine exposure to maternal obesity
Hepatic TGs, which are packaged into very low-density lipoprotein (VLDL) particles and exported, or stored within the cytosol of the hepatocyte, were partially normalized in fetuses from OB-DR mothers. Our analysis of the fetal arterial serum and liver metabolome in fetuses from OB-WSD and OB-DR mothers showed metabolites supporting glycerophospholipid biosynthesis with increased levels of O-phosphoethanolamine and CDP-choline. Phosphatidylcholine (PC) synthesis is required for VLDL secretion and lipid export from liver and, when reduced, TGs accumulate in the liver [78]. The rate of PC synthesis is determined by the amount(s) of CDP-choline and/or DAG co-substrate available from upstream enzymatic activities [79], [80]. Therefore, the high hepatic CDP-choline levels noted in fetuses from OB-WSD and OB-DR mothers, together with the normalization of DAG levels, suggest that there is a bottleneck in the pathway for PC biogenesis. Whether this causes decreased VLDL secretion and thus leads to TG accumulation requires further investigation. The CDP–choline cycle not only provides PC but also might play a role as a biomarker indicating reduced PC synthesis. We also found that total ceramide levels were increased in the liver of fetuses from OB-WSD mothers and remained higher in fetuses from OB-DR mothers. Although the source of increased hepatic ceramide content was not addressed, PC consumes ceramide for the synthesis of sphingomyelin [81], suggesting that the increased ceramide content reflects the limitation in sphingomyelin synthesis due to low PC availability (Figure 6). The exact role each pathway contributes to the assembly and retention of hepatic TGs is not clear; however, the CDP-choline cycle is integrated into a larger metabolic network and interruption of the cycle might have a global impact on lipid-related metabolites in other pathways. CDP-choline can also protect against hypoxia and oxidative stress [82]. Thus, increased CDP-choline levels might represent a new biomarker to explain the retention of TGs in the fetal liver.
Carnosine, a powerful antioxidant, remained increased in the liver of fetuses from OB-DR mothers and carnosine levels were highly correlated with hepatic steatosis and oxidative stress. Carnosine is an endogenous dipeptide with antioxidant properties, which acts as a scavenger for reactive oxygen species and toxic aldehydes and suppresses lipid and protein oxidation [64], [83]. While the connection between carnosine and steatosis remains unclear, we speculate that carnosine is increased in an attempt to combat oxidative stress, which is greater in fetuses from OB-WSD mothers. Another important pathway for NAFLD risk is uric acid. Increased uric acid is associated with NASH in children [84] and progression to NAFLD [85]. We found a reduction in nucleotide substrates for uric acid synthesis and normalization in uracil levels in fetuses from OB-DR mothers. Uric acid levels rise during fetal hypoxemia and during metabolic stress [86]. Thus, decreased uric acid and improved TCA metabolism intermediates might be biomarkers for mitigating oxidative stress and improving energy metabolism in fetuses from OB-DR mothers.
4.6. Conclusions and future directions
Overall, the biochemical pathways revealed by our metabolomic analysis suggest that dysregulated energy metabolism in the fetus results in distinct plasma and liver metabolic signatures leading to multiple “first hits” in defense against fuel overload from an OB-WSD mother. The OB-DR mother remains obese but is consuming a normal diet and thus presumably has normal nutrient supply to the fetus. However, the phenotype of the fetus from the OB-DR mother is only partially normalized, suggesting that maternal obesity interrupts normal development of metabolic pathways and is itself a risk factor for NAFLD. The fuel overload that characterizes the OB-WSD mother, combined with fetal hypoxemia, favors increased fetal liver AA utilization over glucose for supporting gluconeogenesis and lipid synthesis and deposition without a block in lipid oxidation. When combined with lower levels of serum antioxidants, hyperlipidemia and hypoxemia render the fetal liver highly susceptible to oxidative stress that primes the fetal liver of an obese mother to early NAFLD and susceptibility to NASH. Importantly, our maternal diet intervention demonstrates that switching to a healthy CON diet in obese mothers before conception decreased fetal hypoxemia, lipogenesis, hyperlipidemia and markers of oxidative stress and partially improved the fetal serum and hepatic metabolome and liver TG levels. This suggests that interventions aimed at controlling diet during pregnancy in mothers with obesity has major positive effects on fetal liver health.
We are now facing major challenges in tackling the alarming increase and rising epidemic of NAFLD around the world. The considerable risk of NAFLD progression to advanced liver disease (NASH), particularly in children, combined with the lack of pharmacological approaches and suitable biomarkers for the progression of disease are important roadblocks for understanding NAFLD. The pathogenesis of NASH is incompletely understood, but generally occurs in individuals with preexisting NAFLD or uncomplicated steatosis. Thus, in addition to oxidative stress and steatosis, early exposure to maternal WSD changes metabolomic pathways favoring fibrosis, apoptosis, and inflammation that predisposes these offspring to an accelerated disease phenotype upon exposure to a “second hit” postnatally [16]. Follow-up of these NHP offspring to 3 years of age in another cohort is currently underway to determine whether postnatal diet revisions can halt their progression to NASH and whether these biomarkers identified in the fetus have predictive power for the evolution of NAFLD risk.
Acknowledgements
We thank Dr. Kevin Grove for helpful oversight of these studies. This study was supported by the National Institutes of Health grants R24-DK090964 (J.E.F.), P30-DK048520 (Colorado Nutrition Obesity Research Center, J.E.F.) and R01-DK108910 (S.R.W.).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2018.09.008.
Conflicts of interest
J.E.F. is a consultant to the scientific advisory board of Janssen Pharmaceuticals. All other authors declare no conflict of interest.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wesolowski S.R., El Kasmi K.C., Jonscher K.R., Friedman J.E. Developmental origins of NAFLD: a womb with a clue. Nature Reviews Gastroenterology & Hepatology. 2017;14(2):81–96. doi: 10.1038/nrgastro.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson E.L., Howe L.D., Jones H.E., Higgins J.P., Lawlor D.A., Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PloS One. 2015;10(10):e0140908. doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal N.P., Schwimmer J.B. The progression and natural history of pediatric nonalcoholic fatty liver disease. Clinics in Liver Disease. 2016;20(2):325–338. doi: 10.1016/j.cld.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modi N., Murgasova D., Ruager-Martin R., Thomas E.L., Hyde M.J., Gale C. The influence of maternal body mass index on infant adiposity and hepatic lipid content. Pediatric Research. 2011;70(3):287–291. doi: 10.1203/PDR.0b013e318225f9b1. [DOI] [PubMed] [Google Scholar]
- 5.Brumbaugh D.E., Tearse P., Cree-Green M., Fenton L.Z., Brown M., Scherzinger A. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. The Journal of Pediatrics. 2013;162(5):930–936. doi: 10.1016/j.jpeds.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francque S.M., van der Graaff D., Kwanten W.J. Non-alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. Journal of Hepatology. 2016;65(2):425–443. doi: 10.1016/j.jhep.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Hagström H., Stål P., Hultcrantz R., Hemmingsson T., Andreasson A. Overweight in late adolescence predicts development of severe liver disease later in life: a 39 years follow-up study. Journal of Hepatology. 2016;65(2):363–368. doi: 10.1016/j.jhep.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Houghton L.C., Ester W.A., Lumey L.H., Michels K.B., Wei Y., Cohn B.A. Maternal weight gain in excess of pregnancy guidelines is related to daughters being overweight 40 years later. American Journal of Obstetrics and Gynecology. 2016;215(2) doi: 10.1016/j.ajog.2016.02.034. 246 e241-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newton K.P., Feldman H.S., Chambers C.D., Wilson L., Behling C., Clark J.M. Low and high birth weights are risk factors for nonalcoholic fatty liver disease in children. The Journal of Pediatrics. 2017;187:141–146. doi: 10.1016/j.jpeds.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce K.D., Cagampang F.R., Argenton M., Zhang J., Ethirajan P.L., Burdge G.C. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50(6):1796–1808. doi: 10.1002/hep.23205. [DOI] [PubMed] [Google Scholar]
- 11.Mouralidarane A., Soeda J., Visconti-Pugmire C., Samuelsson A.M., Pombo J., Maragkoudaki X. Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice. Hepatology. 2013;58(1):128–138. doi: 10.1002/hep.26248. [DOI] [PubMed] [Google Scholar]
- 12.Oben J.A., Mouralidarane A., Samuelsson A.M., Matthews P.J., Morgan M.L., McKee C. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. Journal of Hepatology. 2010;52(6):913–920. doi: 10.1016/j.jhep.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 13.Jonscher K.R., Stewart M.S., Alfonso-Garcia A., deFelice B.C., Wang X.X., Luo Y. Early PQQ supplementation has persistent long-term protective effects on developmental programming of hepatic lipotoxicity and inflammation in obese mice. Federation of American Societies for Experimental Biology Journal. 2017;31(4):1434–1448. doi: 10.1096/fj.201600906R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seki Y., Suzuki M., Guo X., Glenn A.S., Vuguin P.M., Fiallo A. In utero exposure to a high-fat diet programs hepatic hypermethylation and gene dysregulation and development of metabolic syndrome in male mice. Endocrinology. 2017;158(9):2860–2872. doi: 10.1210/en.2017-00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCurdy C.E., Bishop J.M., Williams S.M., Grayson B.E., Smith M.S., Friedman J.E. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. Journal of Clinical Investigation. 2009;119(2):323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorn S.R., Baquero K.C., Newsom S.A., El Kasmi K.C., Bergman B.C., Shulman G.I. Early life exposure to maternal insulin resistance has persistent effects on hepatic NAFLD in juvenile nonhuman primates. Diabetes. 2014;63(8):2702–2713. doi: 10.2337/db14-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aagaard-Tillery K.M., Grove K., Bishop J., Ke X., Fu Q., McKnight R. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. Journal of Molecular Endocrinology. 2008;41(2):91–102. doi: 10.1677/JME-08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suter M.A., Chen A., Burdine M.S., Choudhury M., Harris R.A., Lane R.H. A maternal high-fat diet modulates fetal SIRT1 histone and protein deacetylase activity in nonhuman primates. Federation of American Societies for Experimental Biology Journal. 2012;26(12):5106–5114. doi: 10.1096/fj.12-212878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suter M.A., Sangi-Haghpeykar H., Showalter L., Shope C., Hu M., Brown K. Maternal high-fat diet modulates the fetal thyroid axis and thyroid gene expression in a nonhuman primate model. Molecular Endocrinology. 2012;26(12):2071–2080. doi: 10.1210/me.2012-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker P.R., II, Patinkin Z., Shapiro A.L.B., de la Houssaye B.A., Woontner M., Boyle K.E. Maternal obesity and increased neonatal adiposity are associated with altered infant mesenchymal stem cell metabolism. JCI Insight. 2017;2(21):e94200. doi: 10.1172/jci.insight.94200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker P.R., II, Patinkin Z.W., Shapiro A.L.B., de la Houssaye B.A., Janssen R.C., Vanderlinden L.A. Altered gene expression and metabolism in fetal umbilical cord mesenchymal stem cells correspond with differences in 5-month-old infant adiposity gain. Scientific Reports. 2017;7(1):18095. doi: 10.1038/s41598-017-17588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandler V., Reisetter A.C., Bain J.R., Muehlbauer M.J., Nodzenski M., Stevens R.D. Associations of maternal BMI and insulin resistance with the maternal metabolome and newborn outcomes. Diabetologia. 2017;60(3):518–530. doi: 10.1007/s00125-016-4182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacob S., Nodzenski M., Reisetter A.C., Bain J.R., Muehlbauer M.J., Stevens R.D. Targeted metabolomics demonstrates distinct and overlapping maternal metabolites associated with BMI, glucose, and insulin sensitivity during pregnancy across four ancestry groups. Diabetes Care. 2017;40(7):911–919. doi: 10.2337/dc16-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catalano P.M., Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. British Medical Journal. 2017;356:j1. doi: 10.1136/bmj.j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Battaglia F.C. Amino acid oxidation and urea production rates in fetal life. Biology of the Neonate. 1995;67(3):149–153. doi: 10.1159/000244156. [DOI] [PubMed] [Google Scholar]
- 26.Herrera E., Amusquivar E. Lipid metabolism in the fetus and the newborn. Diabetes/Metabolism Research and Reviews. 2000;16(3):202–210. doi: 10.1002/1520-7560(200005/06)16:3<202::aid-dmrr116>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Battaglia F.C., Meschia G. Principal substrates of fetal metabolism. Physiological Reviews. 1978;58(2):499–527. doi: 10.1152/physrev.1978.58.2.499. [DOI] [PubMed] [Google Scholar]
- 28.Yoshioka T., Takehara Y., Shimatani M., Abe K., Utsumi K. Lipid peroxidation and antioxidants in rat liver during development. Tohoku Journal of Experimental Medicine. 1982;137(4):391–400. doi: 10.1620/tjem.137.391. [DOI] [PubMed] [Google Scholar]
- 29.Pegorier J.P., Prip-Buus C., Duee P.H., Girard J. Hormonal control of fatty acid oxidation during the neonatal period. Diabetes & Metabolism. 1992;18:156–160. [PubMed] [Google Scholar]
- 30.Kim S.R., Kubo T., Kuroda Y., Hojyo M., Matsuo T., Miyajima A. Comparative metabolome analysis of cultured fetal and adult hepatocytes in humans. Journal of Toxicological Sciences. 2014;39(5):717–723. doi: 10.2131/jts.39.717. [DOI] [PubMed] [Google Scholar]
- 31.Kalhan S., Parimi P. Gluconeogenesis in the fetus and neonate. Seminars in Perinatology. 2000;24(2):94–106. doi: 10.1053/sp.2000.6360. [DOI] [PubMed] [Google Scholar]
- 32.Samuel V.T., Shulman G.I. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. Journal of Clinical Investigation. 2016;126(1):12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rui L. Energy metabolism in the liver. Comprehensive Physiology. 2014;4(1):177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grant W.F., Nicol L.E., Thorn S.R., Grove K.L., Friedman J.E., Marks D.L. Perinatal exposure to a high-fat diet is associated with reduced hepatic sympathetic innervation in one-year old male Japanese macaques. PloS One. 2012;7(10):e48119. doi: 10.1371/journal.pone.0048119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson J.R., Valleau J.C., Barling A.N., Franco J.G., DeCapo M., Bagley J.L. Exposure to a high-fat diet during early development programs behavior and impairs the central serotonergic system in juvenile non-human primates. Frontiers in Endocrinology. 2017;8:164. doi: 10.3389/fendo.2017.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts V.H., Pound L.D., Thorn S.R., Gillingham M.B., Thornburg K.L., Friedman J.E. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. Federation of American Societies for Experimental Biology Journal. 2014;28(6):2466–2477. doi: 10.1096/fj.13-245472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salati J.A., Roberts V.H.J., Schabel M.C., Lo J.O., Kroenke C.D., Lewandowski K.S. Maternal high-fat diet reversal improves placental hemodynamics in a nonhuman primate model of diet-induced obesity. International Journal of Obesity. 2018 doi: 10.1038/s41366-018-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCurdy C.E., Schenk S., Hetrick B., Houck J., Drew B.G., Kaye S. Maternal obesity reduces oxidative capacity in fetal skeletal muscle of Japanese macaques. JCI Insight. 2016;1(16):e86612. doi: 10.1172/jci.insight.86612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frazier A.E., Thorburn D.R. Biochemical analyses of the electron transport chain complexes by spectrophotometry. Methods in Molecular Biology. 2012;837:49–62. doi: 10.1007/978-1-61779-504-6_4. [DOI] [PubMed] [Google Scholar]
- 40.Perreault L., Newsom S.A., Strauss A., Kerege A., Kahn D.E., Harrison K.A. Intracellular localization of diacylglycerols and sphingolipids influences insulin sensitivity and mitochondrial function in human skeletal muscle. JCI Insight. 2018;3(3):e96805. doi: 10.1172/jci.insight.96805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong J., Soufan O., Li C., Caraus I., Li S., Bourque G. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Research. 2018;46(W1):W486–W494. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D'Alessandro A., Moore H.B., Moore E.E., Wither M., Nemkov T., Gonzalez E. Early hemorrhage triggers metabolic responses that build up during prolonged shock. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2015;308(12):R1034–R1044. doi: 10.1152/ajpregu.00030.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Alessandro A., Slaughter A.L., Peltz E.D., Moore E.E., Silliman C.C., Wither M. Trauma/hemorrhagic shock instigates aberrant metabolic flux through glycolytic pathways, as revealed by preliminary (13)C-glucose labeling metabolomics. Journal of Translational Medicine. 2015;13:253. doi: 10.1186/s12967-015-0612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nemkov T., Hansen K.C., D'Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Communications in Mass Spectrometry. 2017;31(8):663–673. doi: 10.1002/rcm.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D'Alessandro A., Nemkov T., Yoshida T., Bordbar A., Palsson B.O., Hansen K.C. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion. 2017;57(2):325–336. doi: 10.1111/trf.13892. [DOI] [PubMed] [Google Scholar]
- 46.Clasquin M.F., Melamud E., Rabinowitz J.D. LC-MS data processing with MAVEN: a metabolomic analysis and visualization engine. Current Protocols in Bioinformatics. 2012;37:14. doi: 10.1002/0471250953.bi1411s37. 11.11-14.11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersen M.C., Shulman G.I. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends in Pharmacological Sciences. 2017;38(7):649–665. doi: 10.1016/j.tips.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pagadala M., Kasumov T., McCullough A.J., Zein N.N., Kirwan J.P. Role of ceramides in nonalcoholic fatty liver disease. Trends in Endocrinology and Metabolism. 2012;23(8):365–371. doi: 10.1016/j.tem.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raichur S., Wang S.T., Chan P.W., Li Y., Ching J., Chaurasia B. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metabolism. 2014;20(4):687–695. doi: 10.1016/j.cmet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Barbul A. Proline precursors to sustain mammalian collagen synthesis. Journal of Nutrition. 2008;138(10):2021s–2024s. doi: 10.1093/jn/138.10.2021S. [DOI] [PubMed] [Google Scholar]
- 51.Baker P.R., 2nd, Friedman J.E. Mitochondrial role in the neonatal predisposition to developing nonalcoholic fatty liver disease. Journal of Clinical Investigation. 2018;128(9):3692–3703. doi: 10.1172/JCI120846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borengasser S.J., Faske J., Kang P., Blackburn M.L., Badger T.M., Shankar K. In utero exposure to prepregnancy maternal obesity and postweaning high-fat diet impair regulators of mitochondrial dynamics in rat placenta and offspring. Physiological Genomics. 2014;46(23):841–850. doi: 10.1152/physiolgenomics.00059.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alfaradhi M.Z., Fernandez-Twinn D.S., Martin-Gronert M.S., Musial B., Fowden A., Ozanne S.E. Oxidative stress and altered lipid homeostasis in the programming of offspring fatty liver by maternal obesity. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2014;307(1):R26–R34. doi: 10.1152/ajpregu.00049.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jelenik T., Kaul K., Séquaris G., Flögel U., Phielix E., Kotzka J. Mechanisms of insulin resistance in primary and secondary nonalcoholic fatty liver. Diabetes. 2017;66(8):2241–2253. doi: 10.2337/db16-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monsénégo J., Mansouri A., Akkaoui M., Lenoir V., Esnous C., Fauveau V. Enhancing liver mitochondrial fatty acid oxidation capacity in obese mice improves insulin sensitivity independently of hepatic steatosis. Journal of Hepatology. 2012;56(3):632–639. doi: 10.1016/j.jhep.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 56.Sunny N.E., Parks E.J., Browning J.D., Burgess S.C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metabolism. 2011;14(6):804–810. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rector R.S., Thyfault J.P., Uptergrove G.M., Morris E.M., Naples S.P., Borengasser S.J. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. Journal of Hepatology. 2010;52(5):727–736. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lambert J.E., Ramos-Roman M.A., Browning J.D., Parks E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(3):726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haggarty P., Allstaff S., Hoad G., Ashton J., Abramovich D.R. Placental nutrient transfer capacity and fetal growth. Placenta. 2002;23(1):86–92. doi: 10.1053/plac.2001.0743. [DOI] [PubMed] [Google Scholar]
- 60.Herrera E., Amusquivar E., López-Soldado I., Ortega H. Maternal lipid metabolism and placental lipid transfer. Hormone Research. 2006;65(Suppl 3):59–64. doi: 10.1159/000091507. [DOI] [PubMed] [Google Scholar]
- 61.Begriche K., Massart J., Robin M.A., Bonnet F., Fromenty B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology. 2013;58(4):1497–1507. doi: 10.1002/hep.26226. [DOI] [PubMed] [Google Scholar]
- 62.Satapati S., Kucejova B., Duarte J.A., Fletcher J.A., Reynolds L., Sunny N.E. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. Journal of Clinical Investigation. 2015;125(12):4447–4462. doi: 10.1172/JCI82204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Satapati S., Sunny N.E., Kucejova B., Fu X., He T.T., Méndez-Lucas A. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. Journal of Lipid Research. 2012;53(6):1080–1092. doi: 10.1194/jlr.M023382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boldyrev A.A., Aldini G., Derave W. Physiology and pathophysiology of carnosine. Physiological Reviews. 2013;93(4):1803–1845. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- 65.Grant W.F., Gillingham M.B., Batra A.K., Fewkes N.M., Comstock S.M., Takahashi D. Maternal high fat diet is associated with decreased plasma n-3 fatty acids and fetal hepatic apoptosis in nonhuman primates. PloS One. 2011;6(2):e17261. doi: 10.1371/journal.pone.0017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim W.Y., Safran M., Buckley M.R., Ebert B.L., Glickman J., Bosenberg M. Failure to prolyl hydroxylate hypoxia-inducible factor alpha phenocopies VHL inactivation in vivo. The EMBO Journal. 2006;25(19):4650–4662. doi: 10.1038/sj.emboj.7601300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taniguchi C.M., Finger E.C., Krieg A.J., Wu C., Diep A.N., Lagory E.L. Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes. Nature Medicine. 2013;19(10):1325–1330. doi: 10.1038/nm.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jungermann K., Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31(2):255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- 69.Mantena S.K., Vaughn D.P., Andringa K.K., Eccleston H.B., King A.L., Abrams G.A. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochemical Journal. 2009;417(1):183–193. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ju C., Colgan S.P., Eltzschig H.K. Hypoxia-inducible factors as molecular targets for liver diseases. Journal of Molecular Medicine (Berlin, Germany) 2016;94(6):613–627. doi: 10.1007/s00109-016-1408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qu A., Taylor M., Xue X., Matsubara T., Metzger D., Chambon P. Hypoxia-inducible transcription factor 2α promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology. 2011;54(2):472–483. doi: 10.1002/hep.24400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Copple B.L., Bustamante J.J., Welch T.P., Kim N.D., Moon J.O. Hypoxia-inducible factor-dependent production of profibrotic mediators by hypoxic hepatocytes. Liver International. 2009;29(7):1010–1021. doi: 10.1111/j.1478-3231.2009.02015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mesarwi O.A., Shin M.K., Bevans-Fonti S., Schlesinger C., Shaw J., Polotsky V.Y. Hepatocyte hypoxia inducible factor-1 mediates the development of liver fibrosis in a mouse model of nonalcoholic fatty liver disease. PloS One. 2016;11(12):e0168572. doi: 10.1371/journal.pone.0168572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bristow J., Rudolph A.M., Itskovitz J., Barnes R. Hepatic oxygen and glucose metabolism in the fetal lamb. Response to hypoxia. Journal of Clinical Investigation. 1983;71(5):1047–1061. doi: 10.1172/JCI110855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rudolph C.D., Roman C., Rudolph A.M. Effect of acute umbilical cord compression on hepatic carbohydrate metabolism in the fetal lamb. Pediatric Research. 1989;25(3):228–233. doi: 10.1203/00006450-198903000-00002. [DOI] [PubMed] [Google Scholar]
- 76.Culpepper C., Wesolowski S.R., Benjamin J., Bruce J.L., Brown L.D., Jonker S.S. Chronic anemic hypoxemia increases plasma glucagon and hepatic PCK1 mRNA in late-gestation fetal sheep. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2016;311(1):R200–R208. doi: 10.1152/ajpregu.00037.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roepke T.A., Yasrebi A., Villalobos A., Krumm E.A., Yang J.A., Mamounis K.J. Loss of ERα partially reverses the effects of maternal high-fat diet on energy homeostasis in female mice. Scientific Reports. 2017;7(1):6381. doi: 10.1038/s41598-017-06560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yao Z.M., Vance D.E. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. Journal of Biological Chemistry. 1988;263(6):2998–3004. [PubMed] [Google Scholar]
- 79.Sriburi R., Bommiasamy H., Buldak G.L., Robbins G.R., Frank M., Jackowski S. Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis. Journal of Biological Chemistry. 2007;282(10):7024–7034. doi: 10.1074/jbc.M609490200. [DOI] [PubMed] [Google Scholar]
- 80.Wright M.M., Henneberry A.L., Lagace T.A., Ridgway N.D., McMaster C.R. Uncoupling farnesol-induced apoptosis from its inhibition of phosphatidylcholine synthesis. Journal of Biological Chemistry. 2001;276(27):25254–25261. doi: 10.1074/jbc.M011552200. [DOI] [PubMed] [Google Scholar]
- 81.Morales A., Lee H., Goñi F.M., Kolesnick R., Fernandez-Checa J.C. Sphingolipids and cell death. Apoptosis. 2007;12(5):923–939. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 82.Zazueta C., Buelna-Chontal M., Macias-López A., Román-Anguiano N.G., González-Pacheco H., Pavón N. Cytidine-5'-diphosphocholine protects the liver from ischemia/reperfusion injury preserving mitochondrial function and reducing oxidative stress. Liver Transplantation. 2018;24(8):1070–1083. doi: 10.1002/lt.25179. [DOI] [PubMed] [Google Scholar]
- 83.Hipkiss A.R. Carnosine, diabetes and Alzheimer's disease. Expert Review of Neurotherapeutics. 2009;9(5):583–585. doi: 10.1586/ern.09.32. [DOI] [PubMed] [Google Scholar]
- 84.Mosca A., Nobili V., De Vito R., Crudele A., Scorletti E., Villani A. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. Journal of Hepatology. 2017;66(5):1031–1036. doi: 10.1016/j.jhep.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 85.Lombardi R., Pisano G., Fargion S. Role of serum uric acid and ferritin in the development and progression of NAFLD. International Journal of Molecular Sciences. 2016;17(4):548. doi: 10.3390/ijms17040548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kane A.D., Hansell J.A., Herrera E.A., Allison B.J., Niu Y., Brain K.L. Xanthine oxidase and the fetal cardiovascular defence to hypoxia in late gestation ovine pregnancy. The Journal of Physiology. 2014;592(3):475–489. doi: 10.1113/jphysiol.2013.264275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.