Abstract
Elevated glycolysis remains a universal and primary character of cancer metabolism, which deeply depends on dysregulated metabolic enzymes. Lactate dehydrogenase A (LDHA) facilitates glycolytic process by converting pyruvate to lactate. Numerous researches demonstrate LDHA has an aberrantly high expression in multiple cancers, which is associated with malignant progression. In this review, we summarized LDHA function in cancer research. First, we gave an introduction of structure, location, and basic function of LDHA. Following, we discussed the transcription and activation mode of LDHA. Further, we focused on the function of LDHA in cancer bio‐characteristics. Later, we discussed the clinical practice of LDHA in cancer prevention and treatment. What we discussed gives a precise insight into LDHA especially in cancer research, which will contribute to exploring cancer pathogenesis and its handling measures.
Keywords: cancer, glycolysis, lactate, LDHA, metabolism
1. INTRODUCTION
Cancer cells possess distinct metabolism from non‐transformed cells, providing sufficient biomaterials and energy for infinite proliferation. Reprogrammed metabolism also contributes to metastasis, resisted cell death, and other malignant characters. Accelerated glycolysis constitutes an important aspect of cancer metabolism as reported by Warburg in 1956.1 High glycolytic activities supply precursors for biomolecules in cellular structure and processes. Importantly, lactate production in glycolysis contributes largely to malignant progression, like replenishing NAD+ for glycolysis, lowering pH for invasion, and triggering immune escape. Lactate dehydrogenase A (LDHA) converts pyruvate to lactate, and aberrant expression and activation of LDHA have been found closely related to diverse cancers.2, 3, 4 Therefore, LDHA has been regarded as a promising target for cancer prevention and treatment.
2. STRUCTURE, LOCATION, AND FUNCTION OF LDHA
Encoded by the LDHA gene, LDHA usually exists as tetramer (LDH‐5). LDHA contains 332 amino acids, forming a bilobal structure. The larger Rossmann domain provides sites for cofactors binding, while the smaller is for substrates.5, 6
The main function of LDHA is to convert pyruvate to lactate, and transform NADH to NAD+. When substrate and cofactor combine LDHA, activated site in the extended groove between two domains will be enclosed in rid of dissociated solvent. Subsequently, Arg 105 in activated circles will grip adhered pyruvate, while the hydrogen anion will transfer from nicotinamide ring of NADH to oxygen atom in carbonyl of pyruvate.6, 7
LDHA is mainly located in the cytoplasm, but LDHA has also been found in the mitochondria and nucleus.8, 9, 10 Outside the nucleus, LDHA play a critical role in glycolysis, while in the nucleus, LDHA function as a single‐stranded DNA‐binding protein (SSB), likely participating in DNA duplication and transcription.
3. REGULATION MODES OF LDHA
In many cancers, LDHA has a high expression profile and activated status, attributed to diverse mechanisms involving almost every step of gene expression regulation (Figure 1).
Figure 1.
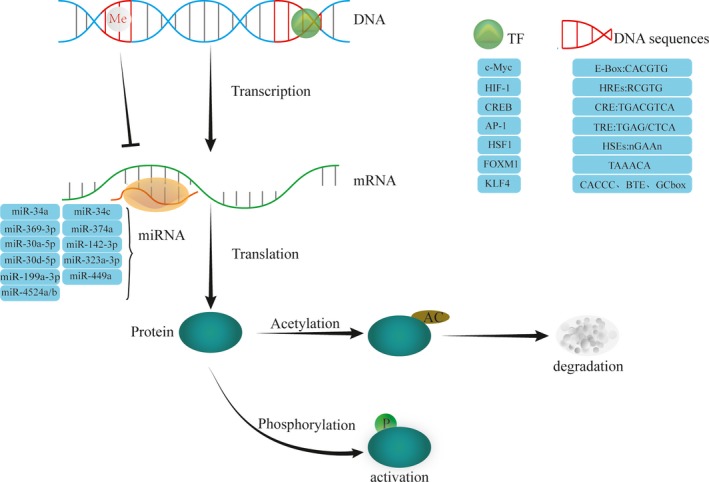
Regulation modes of LDHA. LDHA can be regulated in almost every step of gene expression. Methylation modification could repress LDHA transcription; various transcription factors can function at respective elements in LDHA promoter to activate or curb LDHA transcription; many kinds of microRNAs can bind to mRNA of LDHA to hinder its translation or induce degradation; phosphorylation can activate LDHA, while acetylation triggers its degradation by proteasome. AP‐1, activator protein‐1; CREB, cAMP response element‐binding protein; FOXM1, forkhead box protein M1; HIF‐1, hypoxia‐inducible factor‐1; HSF1, heat‐shock factor 1; KLF4, Kruppel‐like factor 4; TF, transcription factor
3.1. Epigenetic modification
LDHA gene is located in the shorter arm of chromosome 11, a hot spot for methylation. Many researches have proved that DNA methylation in the promoter inhibited LDHA expression. Chesnelong et al found glioma with mutant isocitrate dehydrogenase (IDH) had a low‐level LDHA compared with IDH wild‐type, and the molecular underpinning might be that mutant IDH causes higher methylation in LDHA promoter.11 Besides, Maekawa et al reported retinoblastoma cell line NCC‐RbC‐51 hardly express LDHA, which, however, would be restored by demethylating agent 5‐aza‐2′‐deoxycytidine.12 These all suggest that methylation modification plays a vital part in LDHA activation mechanisms.
3.2. Transcriptional regulation
Promoter region of LDHA contains multiple elements for diverse transcription factors binding; thus, LDHA could be regulated by numerous transcription factors.
3.2.1. c‐Myc
Transcription factors c‐Myc functions as an essential oncogene in numerous cancers.13 c‐Myc could associate with myc‐associated factor X (MAX) to form heterodimer, which binds to E‐box (5′‐CACGTG‐3′) in the promoter of LDHA, thus transactivating LDHA expression.14 In pancreatic cancer, c‐Myc expression was positively related to LDHA, and knockdown of c‐Myc could inhibit LDHA expression, impairing lactate production and glucose consumption.15 Intriguingly, Zhang et al16 found that inhibition of LDHA increased c‐Myc expression, suggesting that LDHA had a negative feedback on c‐Myc expression.
3.2.2. HIF‐1
Hypoxia‐inducible factor‐1 (HIF‐1) is a heterodimer containing α and β subunits. When in hypoxia, stabilized HIF‐1α enters into the nucleus to combine HIF‐1β, and then, such complex binds to hypoxia‐responsive elements (HRE) to transactivate targeted genes. In non–small‐cell lung cancer (NSCLC), Koukourakis et al17 proved that LDHA was positively related to HIF‐1α. Later, Semenza et al18 found HRE existed in the promoter region of LDHA and demonstrated that HIF‐1 could occupy HRE (5′‐RCGTG‐3′) to transactivate LDHA expression. In addition, c‐Myc and HIF‐1 could collaborate to activate LDHA transcription in various cancer cells.19
3.2.3. CREB
Once phosphorylated by the activated cAMP‐PKA signaling pathway, the transcription factor cAMP response element–binding protein (CREB) could engage the cAMP response element (CRE) containing 5′‐TGACGTCA‐3′ sequence, thereby initiating transcription of target genes. The promoter region of LDHA gene also has a highly conserved CRE sequence; thus, LDHA could be regulated by CREB as well.20
3.2.4. AP‐1
12‐O‐Tetradecanoylphorbol‐13‐acetate (TPA) could activate protein kinase C (PKC) signaling pathway, consequently phosphorylating transcription factor activator protein‐1 (AP‐1), which binds to the TPA‐responsive element (TRE) to trigger target gene expression. The promoter region of LDHA gene has a TRE (5′‐TGAG/CTCA‐3′); thus, LDHA could be transcriptionally regulated by AP‐1.21, 22
3.2.5. HSF1
Heat‐shock factor 1 (HSF1), a common transcription factor, regulates heat‐shock protein (HSP) to restore protein homeostasis. In cellular stress, HSF1 forms a transcriptionally active trimer; meanwhile, HSF1 exporting into the cytoplasm is inhibited, and aggregated HSF1 in the nucleus could bind to the heat‐shock elements (HSEs) (nGAAn pentamer inverted repeats) of the heat‐shock protein gene. HSF1 can also function as a transcription factor to regulate the expression of LDHA. Researchers showed that ErbB2 could increase HSF1, which is enriched in promoter region of LDHA, enhancing LDHA expression.23
3.2.6. FOXM1
Forkhead box protein M1 (FOXM1) belongs to Forkhead transcriptional superfamily, recognizing 5′‐TAAACA‐3′ tandem repeat sequences in the promoter region to mediate the transcription of targeted genes like those involved in cell cycle progression.24 FOXM1 has been reported to regulate LDHA expression as well. Cui et al25 found FOXM1 level was positively associated with LDHA in pancreatic cancer. Further, Jiang et al26 demonstrated FOXM1 bound to the promoter of LDHA and promoted its transcription in gastric cancer.
3.2.7. KLF4
Kruppel‐like factor 4 (KLF4) is a zinc‐finger transcription factor, mainly expressed in terminal differentiation of epithelial cells.27, 28 KLF4 transferred into the nucleus exerts transcriptional regulation by binding to the GC box, 5′‐CACCC‐3′ sequence, or basic transcription element (BTE) of the promoter region within target genes.29, 30 KLF4 can also regulate the expression of LDHA. Shi et al31 found KLF4 was negatively related with LDHA level, and KLF4 bound to −371 to −367 bp or −1310 to −1306 bp promoter region of LDHA.
3.3. Posttranscriptional regulation
MicroRNA (miRNA), a kind of small non‐coding RNA, could inhibit translation or promote degradation of targets via combining to the specific region of 3′‐untranslated region (3′‐UTR) in targeted mRNA.32, 33 Until now, several miRNAs like miR‐34a, miR‐34c, miR‐369‐3p, miR‐374a, miR‐30a‐5p, miR‐142‐3p, miR‐30d‐5p, miR‐323a‐3p, miR‑199a‑3p, miR‐449a, and miR‐4524a/b have been found to target the mRNA of LDHA.34, 35, 36, 37, 38, 39, 40, 41, 42, 43 In a recent study on colorectal cancer, miR‐34a, miR‐34c, miR‐369‐3p, miR‐374a, and miR‐4524a/b were established to directly inhibit LDHA.34 Kaller et al32 proved miR‐34a targeting of LDHA via luciferase reporter assay. In advanced colon cancer, miR‐34a was significantly down‐regulated in 5‐fluorouracil‐resistant cancer tissues, while the expression of LDHA was abnormally increased. The expression of LDHA was positively correlated with 5‐fluorouracil resistance; LDHA could be suppressed by introduction of miR‐34a, which could restore the sensitivity of advanced colon cancer to 5‐fluorouracil.44 Besides, miR‐34a could indirectly inhibit LDHA expression by regulating cytokines.45 Thus, these miRNAs play a significant part in negative regulation of LDHA via posttranscriptional modification.
3.4. Posttranslational modification
LDHA could also be modulated by posttranslational modification as exemplified by phosphorylation and acetylation in specific amino acids.
Phosphorylation significantly increased enzymatic activity of LDHA, which is associated with cancer progression. In breast cancer, Jin et al46 revealed that Y10 phosphorylation elicited LDHA activation, promoting cancer cell invasion and enhancing anoikis resistance. Also in colorectal cancer, Ji et al47 found that human coilin–interacting nuclear ATPase protein (hCINAP) expression was positively correlated with the level of Y10 phosphorylated LDHA. The molecular mechanism is that hCINAP binds to the C‐terminal region of LDHA and depends on its own adenylate kinase activity to promote phosphorylation of LDHA catalyzed by fibroblast growth factor receptor 1 (FGFR1) at Y10.47 Furthermore, Fan et al48 found that direct phosphorylation of LDHA at Y10 and Y83 significantly enhanced LDHA tetramer formation and cofactor binding, resulting in a significant increase in lactate dehydrogenase activity.
In addition, lysine acetyl also participates in the regulation of LDHA activity. In human pancreatic cancer, a decrease in acetylated levels of LDHA K5 leads to activation of LDHA enzyme activity and inhibition of LDHA degradation.49 Zhao et al50 showed that in pancreatic cancer LDHA could be deacetylated at K5 by the deacetylase sirtuin 2 (SIRT2). In addition, they found that acetylation on LDHA K5 leads to degradation of LDHA, the underlying mechanism is that the K5‐acetylated LDHA is recognized by the heat‐shock cognate protein 70 (HSC70) and delivered to lysosomes for degradation.50
4. LDHA IN CANCER BIOLOGY
In cancers, enhanced LDHA promotes diverse malignant bio‐characteristics. (Figure 2).
Figure 2.
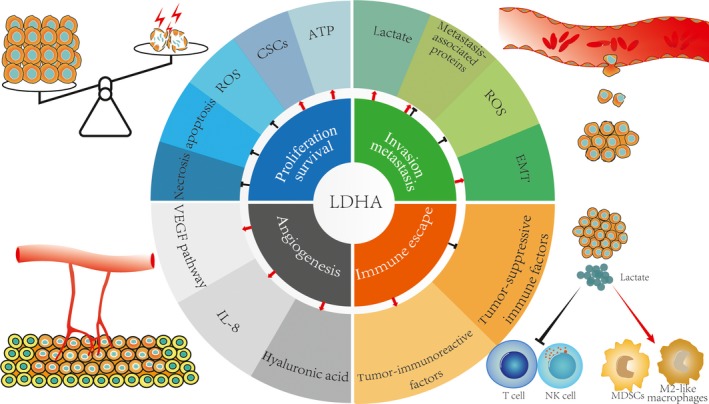
LDHA plays critical roles in hallmarks of cancer. LDHA is closely related to malignant bio‐characteristics of cancer via various mechanisms. LDHA can promote cancer cell proliferation and maintain cell survival; LDHA helps enhance cancer cell invasion and metastasis; LDHA can also trigger angiogenesis; LDHA can assist cancer cells in immune escape. ATP, adenosine triphosphate; CSCs, cancer stem cells; EMT, epithelial‐to‐mesenchymal; IL‐8, interleukin‐8; MDSCs, myeloid‐derived suppressor cells; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor
4.1. Proliferation and survival
Unlimited proliferation and resisted cell death are both hallmarks of cancer, and LDHA contributes greatly to cancer proliferation and survival. Yao et al51 found that LDHA expression was up‐regulated in clinical samples of esophageal squamous cell carcinoma, and LDHA knockdown could inhibit cell growth and migration in vitro and impair tumorigenesis in vivo. Miao et al's52 study on human hepatocellular carcinoma (HCC) also showed that LDHA‐knockdown cells underwent apoptosis. Small molecule inhibitor of human LDHA effectively inhibited tumor growth in human B‐lymphoma and pancreatic cancer xenograft models.3 A study by Dorneburg et al53 on neuroblastoma illustrated that knockout of LDHA suppressed tumor growth and tumorigenicity. Additionally, in K‐RAS‐induced NSCLC mouse model, Xie et al54 demonstrated that LDHA deletion could intensely reduce tumorigenicity.
There have been several explanations for the molecular mechanisms underlying the division and survival of cells with hyperactivity of LDHA. First, LDHA can supply sufficient energy for cancer cells. Fantin et al4 found that under normoxic conditions, the proliferation rate of the cells decreased after downregulating LDHA, and under hypoxic conditions (0.5% oxygen), the growth of tumor cells with LDHA deficiency was also seriously impaired. By measuring adenosine triphosphate (ATP) levels in LDHA‐defective tumor cells under normoxic and hypoxic conditions, they found that tumor cells with reduced LDHA activity could not maintain high ATP levels, which probably contributed to the retardation of cell proliferation under normoxic or hypoxic conditions. Such energy providing efficiency relies on metabolic phenotype of cancer. Cancer cells dependent on glutamine decomposition and fatty acid synthesis are not affected by LDHA inhibitors, because these cells are more dependent on mitochondrial function to produce ATP once the production of lactic acid is inhibited. However, cell lines dependent on the pentose phosphate pathway and glycolysis are dramatically affected by LDHA inhibitors.55, 56
Second, LDHA may be involved in promoting cancer stem cells (CSCs) phenotype. Zhang et al16 found that LDHA was significantly associated with octamer‐binding transcription factor 4 (Oct‐4), which functions critically in self‐renewal of embryonic stem cells. They found that knockdown of LDHA could reduce Oct‐4 expression and tumorigenicity in vitro and in vivo.16
Additionally, LDHA indirectly promotes tumor survival by protecting the tumor from reactive oxygen species (ROS) damage. Mitochondrial ROS is usually elevated because inhibition of LDHA forces cancer cells to produce ATP through oxidative phosphorylation.3, 4, 57 Studies using in vitro and in vivo xenograft mouse models found that treatment of LDHA‐inhibited cells with N‐acetyl‐L‐cysteine (NAC) prevented or partially prevented ROS‐induced apoptosis.2 Accordingly, Sheng et al2 proposed a model of LDHA‐induced apoptosis in human hepatoma cells: LDHA inhibition induced the production of ROS and released cytoplasmic Ca2+, which decreased mitochondrial inner membrane potential (ΔΨm), activated caspase‐9 and caspase‐3, and eventually induced apoptosis.
Moreover, LDHA may also directly inhibit apoptosis. Zhuang et al's58 immunohistochemical study of melanoma revealed that LDHA was strongly correlated with the expression of the antiapoptotic proteins myeloid cell leukemia‐1 (Mcl‐1) and B‐cell lymphoma‐extra large (Bcl‐XL). LDHA knockdown increased poly (ADP‐ribose) polymerase (PARP) cleavage and decreased X‐linked inhibitor of apoptosis protein (XIAP), B‐cell lymphoma 2 (Bcl‐2), and Bcl‐XL expression, leading to decreased the tumorigenicity of a pancreatic cell line, BxPC‐3, in a xenograft model.59 In xenografts of breast cancer cell lines, LDHA knockout was also found to have elevated the levels of Bcl‐2‐associated X protein (Bax), cleaved PARP, cleaved caspase‐9, cytoplasmic cytochrome C, and superoxide anion, while Bcl‐2 expression and mitochondrial membrane potential were reduced.60
Finally, overexpression of LDHA can also promote tumor growth by preventing necrosis in hypoxic environment. Lewis et al61 pointed out that tumors overexpressing LDHA and Rcl displayed little necrotic region compared to tumors overexpressing Rcl and vascular endothelial growth factor (VEGF). This indicates that elevated LDHA expression protected central tumor cells from hypoxia‐induced necrosis.61
4.2. Invasion and metastasis
Invasion and migration are prominent characteristics for malignant progression. LDHA significantly affects the invasion and migration of malignant cells. In Miao et al's52 study, LDHA‐inhibited hepatoma cells exhibited approximately half reduction in motility compared to control cells. Koukourakis et al62 found a positive correlation between LDHA expression and distant metastasis of colorectal cancer. A study by Xie et al63 on hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndromes reported that fumarase (FH)‐deficient cells had a strong invasive potential, while inhibition of LDHA activity inhibits the mobility of cells.
The most common mechanism by which LDHA regulates cell migration and invasion is ascribed to lactate output. Lactic acid levels are related to the incidence of distant metastases.64, 65 High concentrations of lactic acid are associated with high rates of early distant metastases in cancer.66 Exogenous lactic acid can increase tumor cell motility and random migration of different cancer cell lines.67 The effect of lactic acid on metastasis mainly includes activation of matrix metalloproteinases (MMPs) and cathepsin by acidic microenvironment, up‐regulation of VEGF, HIF‐1α, and transforming growth factor‐β2 (TGF‐β2), as well as direct enhancement of cell migrative ability.66, 68, 69, 70, 71
Second, LDHA can regulate metastasis‐associated proteins. Sheng et al2 showed that knockdown of LDHA could reduce the expression of focal adhesion kinase (FAK), matrix metalloproteinase‐2 (MMP‐2) and VEGF, and increase the expression of E‐cadherin. These results suggest that LDHA may affect tumor migration and invasion through the regulation of key players in these cellular processes, for example, inducing degradation of extracellular matrix via stimulating MMP‐2 production, promoting metastatic vasculogenesis through up‐regulation of VEGF, and inhibiting cell adhesion by inhibiting E‐cadherin.
Third, LDHA regulation of invasion and metastasis also involves the production of ROS. In melanoma, Arseneault et al72 found that LDHA inhibition resulted in mitochondrial ROS accumulation, which may alter the structure of tropomyosin via oxidation. Thus, altered tropomyosin could impair its ability to promote actin remodeling, leading to decreased melanoma migration. However, after treatment with antioxidants NAC, migration inhibition and cytoskeletal defects caused by LDHA knockdown could be partially rescued.72
Furthermore, activating epithelial‐to‐mesenchymal transition (EMT) also underlies LDHA contribution to cancer metastasis.73 In bladder cancer, Jiang et al73 found that knockdown of LDHA prevented invasion of cancer cells, accompanied by decreased Snail, N‐cadherin, fibronectin, and vimentin expression but increased E‐cadherin expression.
4.3. Angiogenesis
Angiogenesis is also a core character of malignancy, which is mediated primarily by extracellular factors such as VEGF and interleukin‐8 (IL‐8). Diverse studies have shown that LDHA could regulate tumor angiogenesis. Koukourakis et al found that LDHA expression was positively associated with activation of the VEGF signaling pathway.62, 74, 75, 76 In studies on endometrial cancer, it was confirmed that LDHA expression was significantly related with the activation of vascular endothelial growth factor receptor 2 (VEGFR2) phosphorylation.74 High expression of LDHA was also strongly associated with VEGF expression in non‐small‐cell lung cancer.17 Activated VEGF/VEGFR2 signaling could remarkably promote angiogenesis. At the same time, clinical trials have shown that cancer patients with enhanced LDHA expression could significantly benefit from anti‐angiogenic therapy, suggesting that LDHA may accelerate tumor progression by facilitating angiogenesis.76, 77
Regulation of angiogenesis by LDHA is mainly dependent on the production of lactic acid. Acidification in the microenvironment promotes the production of IL‐8 and VEGF.78 Beckert et al79 confirmed that lactic acid stimulated endothelial cells to produce VEGF and induced neovascularization. In a wound‐healing mouse model, subcutaneously sustained local lactate release could promote angiogenesis and accelerate wound healing.80 Vegran et al81 also found that uptake of lactic acid by vascular endothelial cells triggered the phosphorylation/degradation of IκBα, activated nuclear factor‐kappa B (NF‐κB), promoted IL‐8 expression, and subsequently accelerated angiogenesis and tumor growth. In addition, lactic acid can also promote angiogenesis by increasing the production of hyaluronic acid.82, 83
4.4. Immune escape
Cancer possesses a flexible strategy to escape from immune surveillance. Highly expressed LDHA mediates tumor immune escape by inhibiting immune killing and promoting immunosuppression. The expression of LDHA in human melanoma is negatively correlated with the T‐cell activation markers such as granzyme K (GZMK) and CD25.84 The number of cytotoxic effector cells in tumors with low expression of LDHA was high compared to those with abundant LDHA.84 Husain et al85 observed a decrease in the number of myeloid‐derived suppressor cells (MDSCs) in the spleen of tumor‐bearing mice after depletion of LDHA. Additionally, Seth et al86 found in K‐Ras‐induced cancer mouse model that LDHA deletion diminished PD‐L1+ tumor cells but elevated CD3+ and CD8+ T cells, suggesting significant immune activation in these mice.
The main mechanism of LDHA suppression of immunity is the increase in lactate output, which could impair function of tumor‐suppressive immune factors. First, the acidic microenvironment caused by lactate inhibits the production of interferon‐γ (IFN‐γ) by immunocompetent cells. Brand et al84 found that high expression of LDHA in melanoma resulted in accumulation of lactic acid, and the acidic environment downregulated nuclear factor of activated T cells (NFAT) in T and NK cells, which in turn led to a decrease in IFN‐γ production and attenuated the function of tumor‐infiltrating T cells and NK cells, triggering tumor immunosuppression. Acidic conditions can also inhibit the activity of calcineurin, which in turn disturbs nuclear translocation of NFAT, and impair antitumor immunity.87 Lactic acid also inhibits the glycolytic function of glyceraldehyde phosphate dehydrogenase (GAPDH), allowing it to bind to IFN‐γ mRNA, thus preventing the translation of IFN‐γ.88, 89 In addition, it has also been reported that lactic acid could directly cause apoptosis of T cells and NK cells.84
The accumulation of lactic acid upregulates the levels of tumor‐immunoreactive factors. Shime et al showed that lactic acid promoted the expression and secretion of interleukin‐23 (IL‐23), which impeded infiltration of CD8+ T cells in tumor microenvironment.90, 91 Colegio et al showed that lactic acid upregulated HIF‐1α to facilitate the development of M2‐like macrophages, a subset of macrophages known to promote cancer progression.92, 93 Besides, MDSCs could prohibit function of T cells and NK cells to suppress immune response.94 LDHA‐induced lactate accumulation could also increase the number of MDSCs in immune escape of tumors.85
5. LDHA AS A BIOMARKER AND THERPEUTIC TARGET FOR CANCER
5.1. LDHA as a biomarker for cancer diagnosis and prognosis
Lactate dehydrogenase (LDH) is present in cells. When cells are damaged, they are released into the bloodstream. Therefore, LDH levels in the blood are usually used as indicators of tissue damage. Serum LDH levels also have momentous significance in cancer diagnosis due to tissue destruction caused by tumor growth.52 In general, serum LDH is usually elevated in hematopoietic malignancies such as Hodgkin's lymphoma (HL) and non‐Hodgkin's lymphoma (NHL).95, 96, 97, 98 Colgan et al99 found that surgical removal of the primary tumor resulted in a dramatic decrease in serum LDH levels within first week after surgery. Tumor metastasis can lead to elevated LDH levels, suggesting that LDH may be a potential diagnostic marker for cancer.
Additionally, LDH could serve as an indicator of the prognosis of malignancy. LDHA is a strong predictor of survival in patients with aggressive lymphoma as one of the risk factors in the International Prognostic Index (IPI). Jin et al100 performed a retrospective analysis of the relationship between pre‐ and post‐treatment serum LDH levels and the clinical response and survival outcomes of patients with metastatic nasopharyngeal carcinoma. The results showed that patients with elevated pre‐treatment serum LDH levels had a worse survival rate compared with those displaying normal pre‐treatment serum LDH. Compared with those with normal post‐treatment serum LDH levels, the survival rate of patients with elevated serum LDH was also significantly lowered.100 Due to no clinical differences in the specific subtypes of LDH, the separate role of serum LDHA in diagnosis and prognostic prediction still needs further investigation.
In addition to serum LDH, LDHA in tissues can often be used as a biological indicator of diagnostic and malignant characteristics of tumors. Kayser et al101 found that almost 90% of NSCLC patients were positive for LDHA, while all non‐tumor lung tissues showed negative LDHA expression. In addition, the staining intensity of LDHA was found to be highly correlated with the histological type and lymph node metastasis of lung cancer, indicating that the tissue level of LDHA had prognostic value in NSCLC.101 Up‐regulation of LDHA levels in tumor tissues can be observed in pancreatic cancer and esophageal squamous cell carcinoma (ESCC), which was associated with metastasis, tumor stage, tumor recurrence, and patient survival.25, 51
However, LDHA expression in cancer tissues is not completely consistent with serum LDH levels, which may indicate that tumor LDHA expression and serum LDH levels are two independent predictors of tumors.102 Koukourakis et al62 found that patients with low levels of LDHA in tumor tissues often had low levels of serum LDH, but only 29% of patients with high LDHA expression had elevated serum LDH levels, and 71% of patients with high LDHA expression levels showed normal serum levels. Dong et al102 also found that the expression level of LDHA in triple‐negative breast cancer tissues was not completely consistent with the serum LDH level. Koukourakis et al62 proposed that the level of serum LDH in normal individuals was substantially different, masking the increase in LDHA caused by tumorigenesis.62 Thus, serum LDH and tissue LDHA levels can complement each other and exert a combined role in diagnosis of cancer.76
5.2. LDHA as a target for cancer treatment
As is shown above, numerous tumors exhibit high LDHA expression, which contributes to malignant bio‐characteristics.103, 104, 105 Silencing LDHA expression in tumor models inhibits cell proliferation, migration, and tumor growth.51, 59, 72, 106 However, it is rarely harmful to normal cells.57, 107 Additionally, individuals lacking LDHA subunits only develop muscle rigidity and sudden myoglobinuria after strenuous exercise.108, 109 So, high anticancer efficacy along with safe therapeutic window enables LDHA to be a potential antitumor target. Based on the functional mechanisms, LDHA inhibitors could be divided into the following categories (Table 1).
Table 1.
Diverse drugs that target LDHA
| Mechanism of action | LDHA inhibitor | Cancer | Clinical trials | Drawbacks | References |
|---|---|---|---|---|---|
| Pyruvate‐competitive | Oxamate | Gastric cancer; cervical cancer | No | Limited membrane permeability | 110, 111 |
| NADH‐competitive | Gossypol | Melanoma; lung cancer; breast cancer; cervical cancer; leukemia; glioma; adrenal cancer | Yes | Non‐specific toxicity | 112, 113, 114, 115, 116, 117, 118 |
| FX11 | B‐lymphoma; pancreatic cancer | No | Highly reactive catechol portion of this molecule | 3, 5, 120 | |
| Quinoline 3‐sulfonamides | Hepatocellular carcinoma | No | Low clearance in the body and incompatible with oral bioavailability | 55 | |
| Pyruvate and NADH competitive | NHI | Pancreatic ductal adenocarcinoma; cervical cancer | Unknown | Unknown | 9, 120, 121 |
| Binding the free enzyme | Galloflavin | Breast cancer; hepatocellular carcinoma | Unknown | Unknown | 122, 123, 124 |
5.2.1. Pyruvate‐competitive LDHA inhibitor
As an analogue of pyruvate, oxamate inhibits LDHA via competition with substrates, and its effectiveness has been extensively validated in vitro. In a study on gastric cancer, the human gastric cancer cell lines SGC7901 and BGC823 had higher LDH activity and more lactic acid levels than the immortalized gastric mucosa epithelial cell line GES‐1. Oxamate significantly inhibited the proliferation and lactic acid production of SGC‐7901 and BGC‐823 cells in a dose‐ and time‐dependent manner, but showed less effect on GES‐1 cells.110 Similarly, after treatment with oxamate, the growth and lactic acid production of HeLa cells were also inhibited.111 Unfortunately, due to limited cell membrane permeability, the effective dose of oxamate in cultured cancer cells is too high for in vivo administration.
5.2.2. NADH‐competitive LDHA inhibitors
Gossypol, a natural phenol derived from cotton plants, inhibits LDHA by competing with NADH.112 Gossypol showed dose‐dependent cytotoxic activity in diverse cancer cells, including melanoma (SK‐mel‐19, SK‐mel‐28), small‐cell lung cancer (H69), breast cancer (Walker), cervical cancer (Sihas), myelogenous leukemia (K562), and glioma (HS683, U373, U87, and U138).113, 114 Meanwhile, gossypol shows satisfactory anticancer efficiency in vivo. Coyle et al114 used a nude mouse xenograft model to test the cytotoxicity of Gossypol on BRW (a cell line established from a patient with a primitive neuroectodermal tumor). After oral administration of gossypol by gavage, the average tumor weight was reduced by more than 50%.114 Furthermore, several clinical trials have demonstrated promising tumoricidal potential of gossypol. In metastatic adrenal cancer, Flack et al115 found that after oral administration of Gossypol, the tumor volumes of patients was reduced by half, while the only serious side effect was intestinal obstruction. Likely, oral administration of Gossypol also benefits malignant glioma patients who had a relapse after radiotherapy.116 In a phase I/II clinical study, 20 women with refractory metastatic breast cancer received oral treatment with Gossypol. The results showed that Gossypol treatment was safe and effective in decreasing serum tumor markers.117
Nevertheless, gossypol could interact with different cellular components involved in several biological functions, resulting in non‐specific toxicity of this compound. So further drug‐making attempts have produced frustrating results.112, 118 Therefore, many gossypol analogs are being designed and under evaluation for their safety and anticancer efficacy.
FX11 (11f; [3‐dihydroxy6‐methyl‐7‐(phenylmethyl)‐4‐propylnaphthalene‐1‐carboxylic acid]) is also a competitive LDHA inhibitor through competing with NADH. The human B‐lymphoma P493 cell line treated with FX11 showed abundant cell death. FX11 also inhibits tumor progression and induces oxidative stress and necrosis in human lymphoma and pancreatic cancer xenograft models.3
However, further studies questioned the effectiveness of FX11 since some of the observed effects may not be attributed to LDH inhibition, but rather to the reactive nature of the catechol groups of the molecule.5, 119 Therefore, although FX11 exhibits certain therapeutic potential, the highly reactive catechol portion of this molecule makes it unsuitable as a candidate drug for further development.
Quinoline 3‐sulfonamides are NADH‐competitive LDH inhibitors with higher selectivity for LDHA than lactate dehydrogenase B (LDHB). After treatment with quinoline 3‐sulfonamides, hepatocellular carcinoma Snu398 cells showed increased oxygen consumption, inhibited lactic acid production and cell proliferation, and promoted apoptosis. However, due to low clearance in the body and incompatible with oral bioavailability, it cannot be used in vivo.55
5.2.3. Pyruvate and NADH‐competitive LDHA inhibitors
N‐hydroxyindoles (NHI), a distinguished class of LDH inhibitors that compete with substrates (pyruvate) and cofactors (NADH), are more specific for LDHA than LDHB. Cellular assays have confirmed that they can impede cancer cell proliferation. For example, when treated with NHI compounds, pancreatic ductal adenocarcinoma cells exhibited decreased growth and invasiveness especially under hypoxic conditions. NHI could inhibit cervical cancer HeLa cells as well.120 In addition, NHI compounds can synergize with gemcitabine to exert antitumor effects.121 Further exploration of biological properties of NHI will help better assess its therapeutic potential for various cancers.9
5.2.4. Free enzyme‐binding inhibitor
Galloflavin (GF), a synthetic drug with good cell permeability, inhibits LDHA by preferentially binding to free enzymes without competing with substrates or cofactors.122 It is harmless to mitochondrial respiration, and showed minimal effects on normal cellular metabolism. In breast cancer cell lines, GF inhibits cancer cell proliferation by blocking glycolysis and ATP production, and induces apoptosis.123 GF inhibits aerobic glycolysis and trigger cell death in hepatocellular carcinoma cell line PLC/PRF/5.122 GF is less cytotoxic to normal cells such as human lymphocytes and lymphoblasts.123 A recent study found that GF interferes with LDHA/ssDNA interactions and blocks RNA synthesis in vitro, suggesting the complicated mechanism underlying its toxicity to malignant cells.124
5.2.5. Other unknown mechanisms
Genetech Corporation synthesizes another LDHA inhibitor, GNE‐140, a piperidine derivative, which has been shown to effectively inhibit the proliferation of MiaPaCa‐2 pancreatic cancer cells. In addition, a natural extract with the ability to inhibit LDHA, named gall, has also been found to be very effective in cancer treatment.125
Apart from being applied alone, studies have shown that LDHA inhibitors in combination with other agents exhibited dramatic therapeutic capacity. Miskimins et al126 found that oxamate and phenformin synergistically exerted anticancer effects by simultaneously inhibiting complex I in mitochondria and LDH in cytosol. By combining with the NAD+synthetic inhibitor FK866, FX11 induces lymphoma regression remarkably.3 In pancreatic ductal adenocarcinoma cell lines, the combination of NHI‐1 and NHI‐2 with gemcitabine enhances the anti‐proliferative and anti‐invasive activity of chemotherapeutic drugs both under normoxic or hypoxic conditions.121 In addition, NHI‐2 and a redox‐dependent bioreductive anticancer prodrug, EO9, synergistically induce the death of p53 wild‐type cancer cells.127
6. CONCLUSIONS AND PERSPECTIVE
The unique metabolic pattern of tumor cells significantly promotes its malignant biological characteristics, and abnormally regulated metabolic enzymes are the basis of its metabolic reprogramming. In many malignancies, LDHA may be abnormally overexpressed due to activation of LDHA upstream regulatory mechanisms by cancer‐driving mutations. Up‐regulated LDHA promotes the malignant progression of tumors by increasing lactic acid production, accelerating glycolysis, modulating reactive oxygen species production, and regulating numerous cancer‐related proteins. At the same time, clinical trials using LDHA as a target for diagnosis and treatment have also yielded encouraging results.
However, there are still many problems that remain to be resolved. First of all, it is unclear whether and how LDHA itself or its downstream metabolites affects the expression of cancer‐related genes. Secondly, taking into account the high heterogeneity of tumors, LDHA's biological effects and its roles in the diagnosis and therapy of tumors of different tissue origins, different pathological types, and different molecular subtypes need to be further evaluated. Finally, in addition to sensitizing traditional cytotoxic chemotherapeutics, LDHA inhibitors need to be further explored when used in conjunction with molecular targeted drugs and immunotherapy.
CONFLICT OF INTEREST
The authors have no conflict of interests to declare.
Informed consent: Informed consent was obtained from all individual participants included in the study.
ACKNOWLEDGMENTS
We thank the staff at the Department of Thoracic Surgery, Tangdu Hospital, Fourth Military Medical University, Xi'an, China, and the State Key Laboratory of Cancer Biology, Department of Biochemistry and Molecular Biology, Fourth Military Medical University, Xi'an, China, for support.
Feng Y, Xiong Y, Qiao T, Li X Jia L, Han Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018;7:6124–6136. 10.1002/cam4.1820
Feng, Xiong and Qiao equally contributed to this study.
Funding information
This study was funded by the National Natural Science Foundation of China (81772462).
Contributor Information
Lintao Jia, Email: jialth@fmmu.edu.cn.
Yong Han, Email: hanyong_td@163.com.
REFERENCES
- 1. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309‐314. [DOI] [PubMed] [Google Scholar]
- 2. Sheng SL, Liu JJ, Dai YH, Sun XG, Xiong XP, Huang G. Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J. 2012;279(20):3898‐3910. [DOI] [PubMed] [Google Scholar]
- 3. Le A, Cooper CR, Gouw AM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA. 2010;107(5):2037‐2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fantin VR, St‐Pierre J, Leder P. Attenuation of LDH‐A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9(6):425‐434. [DOI] [PubMed] [Google Scholar]
- 5. Kohlmann A, Zech SG, Li F, et al. Fragment growing and linking lead to novel nanomolar lactate dehydrogenase inhibitors. J Med Chem. 2013;56(3):1023‐1040. [DOI] [PubMed] [Google Scholar]
- 6. Kolappan S, Shen DL, Mosi R, et al. Structures of lactate dehydrogenase A (LDHA) in apo, ternary and inhibitor‐bound forms. Acta Crystallogr D Biol Crystallogr. 2015;71(2):185‐195. [DOI] [PubMed] [Google Scholar]
- 7. Read JA, Winter VJ, Eszes CM, Sessions RB, Brady RL. Structural basis for altered activity of M‐ and H‐isozyme forms of human lactate dehydrogenase. Proteins. 2001;43(2):175‐185. [DOI] [PubMed] [Google Scholar]
- 8. Reddy MA, Shukla SD. Nuclear activation and translocation of mitogen‐activated protein kinases modulated by ethanol in embryonic liver cells. Biochim Biophys Acta. 2000;1497(2):271‐278. [DOI] [PubMed] [Google Scholar]
- 9. Fiume L, Manerba M, Vettraino M, Di Stefano G. Inhibition of lactate dehydrogenase activity as an approach to cancer therapy. Future Med Chem. 2014;6(4):429‐445. [DOI] [PubMed] [Google Scholar]
- 10. Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci USA. 1999;96(3):1129‐1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chesnelong C, Chaumeil MM, Blough MD, et al. Lactate dehydrogenase A silencing in IDH mutant gliomas. Neuro Oncol. 2014;16(5):686‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maekawa M, Inomata M, Sasaki MS, et al. Electrophoretic variant of a lactate dehydrogenase isoenzyme and selective promoter methylation of the LDHA gene in a human retinoblastoma cell line. Clin Chem. 2002;48(11):1938‐1945. [PubMed] [Google Scholar]
- 13. Augoff K, Hryniewicz‐Jankowska A, Tabola R. Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer. Cancer Lett. 2015;358(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 14. Shim H, Dolde C, Lewis BC, et al. c‐Myc transactivation of LDH‐A: implications for tumor metabolism and growth. Proc Natl Acad Sci USA. 1997;94(13):6658‐6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He TL, Zhang YJ, Jiang H, Li XH, Zhu H, Zheng KL. The c‐Myc‐LDHA axis positively regulates aerobic glycolysis and promotes tumor progression in pancreatic cancer. Med Oncol. 2015;32(7):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Y, Zhang X, Wang X, et al. Inhibition of LDH‐A by lentivirus‐mediated small interfering RNA suppresses intestinal‐type gastric cancer tumorigenicity through the downregulation of Oct4. Cancer Lett. 2012;321(1):45‐54. [DOI] [PubMed] [Google Scholar]
- 17. Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Lactate dehydrogenase‐5 (LDH‐5) overexpression in non‐small‐cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89(5):877‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Semenza GL, Jiang BH, Leung SW, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia‐inducible factor 1. J Biol Chem. 1996;271(51):32529‐32537. [DOI] [PubMed] [Google Scholar]
- 19. Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8(1):51‐56. [DOI] [PubMed] [Google Scholar]
- 20. Short ML, Huang D, Milkowski DM, et al. Analysis of the rat lactate dehydrogenase A subunit gene promoter/regulatory region. Biochem J. 1994;304(Pt 2):391‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang D, Jungmann RA. Transcriptional regulation of the lactate dehydrogenase A subunit gene by the phorbol ester 12‐O‐tetradecanoylphorbol‐13‐acetate. Mol Cell Endocrinol. 1995;108(1–2):87‐94. [DOI] [PubMed] [Google Scholar]
- 22. Angel P, Imagawa M, Chiu R, et al. Phorbol ester‐inducible genes contain a common cis element recognized by a TPA‐modulated trans‐acting factor. Cell. 1987;49(6):729‐739. [DOI] [PubMed] [Google Scholar]
- 23. Zhao YH, Zhou M, Liu H, et al. Upregulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene. 2009;28(42):3689‐3701. [DOI] [PubMed] [Google Scholar]
- 24. Littler DR, Alvarez‐Fernandez M, Stein A, et al. Structure of the FoxM1 DNA‐recognition domain bound to a promoter sequence. Nucleic Acids Res. 2010;38(13):4527‐4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cui J, Shi M, Xie D, et al. FOXM1 promotes the warburg effect and pancreatic cancer progression via transactivation of LDHA expression. Clin Cancer Res. 2014;20(10):2595‐2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jiang W, Zhou F, Li N, Li Q, Wang L. FOXM1‐LDHA signaling promoted gastric cancer glycolytic phenotype and progression. Int J Clin Exp Pathol. 2015;8(6):6756‐6763. [PMC free article] [PubMed] [Google Scholar]
- 27. Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22(4):356‐360. [DOI] [PubMed] [Google Scholar]
- 28. Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut‐enriched Kruppel‐like factor expressed during growth arrest. J Biol Chem. 1996;271(33):20009‐20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shields JM, Yang VW. Identification of the DNA sequence that interacts with the gut‐enriched Kruppel‐like factor. Nucleic Acids Res. 1998;26(3):796‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rowland BD, Peeper DS. KLF4, p21 and context‐dependent opposing forces in cancer. Nat Rev Cancer. 2006;6(1):11‐23. [DOI] [PubMed] [Google Scholar]
- 31. Shi M, Cui J, Du J, et al. A novel KLF4/LDHA signaling pathway regulates aerobic glycolysis in and progression of pancreatic cancer. Clin Cancer Res. 2014;20(16):4370‐4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaller M, Liffers ST, Oeljeklaus S, et al. Genome‐wide characterization of miR‐34a induced changes in protein and mRNA expression by a combined pulsed SILAC and microarray analysis. Mol Cell Proteomics. 2011;10(8):M111.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pullen TJ, da Silva XG, Kelsey G, Rutter GA. miR‐29a and miR‐29b contribute to pancreatic beta‐cell‐specific silencing of monocarboxylate transporter 1 (Mct1). Mol Cell Biol. 2011;31(15):3182‐3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang J, Wang H, Liu AF, Fang CG, Hao JG, Wang ZH. Lactate dehydrogenase A negatively regulated by miRNAs promotes aerobic glycolysis and is increased in colorectal cancer. Oncotarget. 2015;6(23):19456‐19468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xiao X, Huang X, Ye F, et al. The miR‐34a‐LDHA axis regulates glucose metabolism and tumor growth in breast cancer. Sci Rep. 2016;6:21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ping W, Senyan H, Li G, Yan C, Long L. Increased lactate in gastric cancer tumor‐infiltrating lymphocytes is related to impaired T cell function due to miR‐34a deregulated lactate dehydrogenase A. Cell Physiol Biochem. 2018;49(2):828‐836. [DOI] [PubMed] [Google Scholar]
- 37. Li X, Lu P, Li B, et al. Sensitization of hepatocellular carcinoma cells to irradiation by miR34a through targeting lactate dehydrogenaseA. Mol Med Rep. 2016;13(4):3661‐3667. [DOI] [PubMed] [Google Scholar]
- 38. Li L, Kang L, Zhao W, et al. miR‐30a‐5p suppresses breast tumor growth and metastasis through inhibition of LDHA‐mediated Warburg effect. Cancer Lett. 2017;400:89‐98. [DOI] [PubMed] [Google Scholar]
- 39. Hua S, Liu C, Liu L, Wu D. miR‐142‐3p inhibits aerobic glycolysis and cell proliferation in hepatocellular carcinoma via targeting LDHA. Biochem Biophys Res Commun. 2018;496(3):947‐954. [DOI] [PubMed] [Google Scholar]
- 40. He Y, Chen X, Yu Y, et al. LDHA is a direct target of miR‐30d‐5p and contributes to aggressive progression of gallbladder carcinoma. Mol Carcinog. 2018;57(6):772‐783. [DOI] [PubMed] [Google Scholar]
- 41. Chen H, Gao S, Cheng C. MiR‐323a‐3p suppressed the glycolysis of osteosarcoma via targeting LDHA. Hum Cell. 2018;31:300‐309. [DOI] [PubMed] [Google Scholar]
- 42. Zhou S, Min Z, Sun K, et al. miR199a3p/Sp1/LDHA axis controls aerobic glycolysis in testicular tumor cells. Int J Mol Med. 2018;42(4):2163‐2174. [DOI] [PubMed] [Google Scholar]
- 43. Li L, Liu H, Du L, et al. MiR‐449a suppresses LDHA‐mediated glycolysis to enhance the sensitivity of non‐small cell lung cancer cells to ionizing radiation. Oncol Res. 2018;26(4):547‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li X, Zhao H, Zhou X, Song L. Inhibition of lactate dehydrogenase A by microRNA‐34a resensitizes colon cancer cells to 5‐fluorouracil. Mol Med Rep. 2015;11(1):577‐582. [DOI] [PubMed] [Google Scholar]
- 45. Zhang DG, Zheng JN, Pei DS. P53/microRNA‐34‐induced metabolic regulation: new opportunities in anticancer therapy. Mol Cancer. 2014;13:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jin L, Chun J, Pan C, et al. Phosphorylation‐mediated activation of LDHA promotes cancer cell invasion and tumour metastasis. Oncogene. 2017;36(27):3797‐3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ji Y, Yang C, Tang Z, et al. Adenylate kinase hCINAP determines self‐renewal of colorectal cancer stem cells by facilitating LDHA phosphorylation. Nat Commun. 2017;8:15308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fan J, Hitosugi T, Chung TW, et al. Tyrosine phosphorylation of lactate dehydrogenase A is important for NADH/NAD(+) redox homeostasis in cancer cells. Mol Cell Biol. 2011;31(24):4938‐4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang W, Wang Z, Lei QY. Acetylation control of metabolic enzymes in cancer: an updated version. Acta Biochim Biophys Sin (Shanghai). 2014;46(3):204‐213. [DOI] [PubMed] [Google Scholar]
- 50. Zhao D, Zou SW, Liu Y, et al. Lysine‐5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer. Cancer Cell. 2013;23(4):464‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yao F, Zhao T, Zhong C, Zhu J, Zhao H. LDHA is necessary for the tumorigenicity of esophageal squamous cell carcinoma. Tumour Biol. 2013;34(1):25‐31. [DOI] [PubMed] [Google Scholar]
- 52. Miao P, Sheng S, Sun X, Liu J, Huang G. Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life. 2013;65(11):904‐910. [DOI] [PubMed] [Google Scholar]
- 53. Dorneburg C, Fischer M, Barth T, et al. LDHA in neuroblastoma is associated with poor outcome and its depletion decreases neuroblastoma growth independent of aerobic glycolysis. Clin Cancer Res. 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 54. Xie H, Hanai J, Ren JG, et al. Targeting lactate dehydrogenase–a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor‐initiating cells. Cell Metab. 2014;19(5):795‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Billiard J, Dennison JB, Briand J, et al. Quinoline 3‐sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 2013;1(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Valvona CJ, Fillmore HL, Nunn PB, Pilkington GJ. The regulation and function of lactate dehydrogenase A: therapeutic potential in brain tumor. Brain Pathol. 2016;26(1):3‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jeong DW, Cho IT, Kim TS, Bae GW, Kim IH, Kim IY. Effects of lactate dehydrogenase suppression and glycerol‐3‐phosphate dehydrogenase overexpression on cellular metabolism. Mol Cell Biochem. 2006;284(1–2):1‐8. [DOI] [PubMed] [Google Scholar]
- 58. Zhuang L, Scolyer RA, Murali R, et al. Lactate dehydrogenase 5 expression in melanoma increases with disease progression and is associated with expression of Bcl‐XL and Mcl‐1, but not Bcl‐2 proteins. Modern Pathol. 2010;23(1):45–53. [DOI] [PubMed] [Google Scholar]
- 59. Rong Y, Wu W, Ni X, et al. Lactate dehydrogenase A is overexpressed in pancreatic cancer and promotes the growth of pancreatic cancer cells. Tumour Biol. 2013;34(3):1523–1530. [DOI] [PubMed] [Google Scholar]
- 60. Wang ZY, Loo TY, Shen JG, et al. LDH‐A silencing suppresses breast cancer tumorigenicity through induction of oxidative stress mediated mitochondrial pathway apoptosis. Breast Cancer Res Treat. 2012;131(3):791–800. [DOI] [PubMed] [Google Scholar]
- 61. Lewis BC, Prescott JE, Campbell SE, Shim H, Orlowski RZ, Dang CV. Tumor induction by the c‐Myc target genes rcl and lactate dehydrogenase A. Cancer Res. 2000;60(21):6178–6183. [PubMed] [Google Scholar]
- 62. Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL, Tumour Angiogenesis Research G . Lactate dehydrogenase 5 expression in operable colorectal cancer: strong association with survival and activated vascular endothelial growth factor pathway–a report of the Tumour Angiogenesis Research Group. J Clin Oncol. 2006;24(26):4301–4308. [DOI] [PubMed] [Google Scholar]
- 63. Xie H, Valera VA, Merino MJ, et al. LDH‐A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol Cancer Ther. 2009;8(3):626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Walenta S, Wetterling M, Lehrke M, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60(4):916–921. [PubMed] [Google Scholar]
- 65. Brizel DM, Schroeder T, Scher RL, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head‐and‐neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(2):349–353. [DOI] [PubMed] [Google Scholar]
- 66. Walenta S, Mueller‐Klieser WF. Lactate: mirror and motor of tumor malignancy. Semin Radiat Oncol. 2004;14(3):267–274. [DOI] [PubMed] [Google Scholar]
- 67. Goetze K, Walenta S, Ksiazkiewicz M, Kunz‐Schughart LA, Mueller‐Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011;39(2):453–463. [DOI] [PubMed] [Google Scholar]
- 68. Baumann F, Leukel P, Doerfelt A, et al. Lactate promotes glioma migration by TGF‐beta2‐dependent regulation of matrix metalloproteinase‐2. Neuro Oncol. 2009;11(4):368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kato Y, Lambert CA, Colige AC, et al. Acidic extracellular pH induces matrix metalloproteinase‐9 expression in mouse metastatic melanoma cells through the phospholipase D‐mitogen‐activated protein kinase signaling. J Biol Chem. 2005;280(12):10938–10944. [DOI] [PubMed] [Google Scholar]
- 70. Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006;66(13):6699–6707. [DOI] [PubMed] [Google Scholar]
- 71. Swietach P, Vaughan‐Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26(2):299–310. [DOI] [PubMed] [Google Scholar]
- 72. Arseneault R, Chien A, Newington JT, Rappon T, Harris R, Cumming RC. Attenuation of LDHA expression in cancer cells leads to redox‐dependent alterations in cytoskeletal structure and cell migration. Cancer Lett. 2013;338(2):255–266. [DOI] [PubMed] [Google Scholar]
- 73. Jiang F, Ma S, Xue Y, Hou J, Zhang Y. LDH‐A promotes malignant progression via activation of epithelial‐to‐mesenchymal transition and conferring stemness in muscle‐invasive bladder cancer. Biochem Biophys Res Commun. 2016;469(4):985–992. [DOI] [PubMed] [Google Scholar]
- 74. Giatromanolaki A, Sivridis E, Gatter KC, Turley H, Harris AL, Koukourakis MI. Lactate dehydrogenase 5 (LDH‐5) expression in endometrial cancer relates to the activated VEGF/VEGFR2(KDR) pathway and prognosis. Gynecol Oncol. 2006;103(3):912–918. [DOI] [PubMed] [Google Scholar]
- 75. Kolev Y, Uetake H, Takagi Y, Sugihara K. Lactate dehydrogenase‐5 (LDH‐5) expression in human gastric cancer: association with hypoxia‐inducible factor (HIF‐1alpha) pathway, angiogenic factors production and poor prognosis. Ann Surg Oncol. 2008;15(8):2336–2344. [DOI] [PubMed] [Google Scholar]
- 76. Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (vatalanib) antiangiogenic therapy. Clin Cancer Res. 2011;17(14):4892–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cetin B, Afsar B, Deger SM, et al. Association between hemoglobin, calcium, and lactate dehydrogenase variability and mortality among metastatic renal cell carcinoma. Int Urol Nephrol. 2014;46(6):1081–1087. [DOI] [PubMed] [Google Scholar]
- 78. Shi Q, Le X, Wang B, et al. Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene. 2001;20(28):3751–3756. [DOI] [PubMed] [Google Scholar]
- 79. Beckert S, Farrahi F, Aslam RS, et al. Lactate stimulates endothelial cell migration. Wound Repair Regen. 2006;14(3):321–324. [DOI] [PubMed] [Google Scholar]
- 80. Porporato PE, Payen VL, De Saedeleer CJ, et al. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis. 2012;15(4):581–592. [DOI] [PubMed] [Google Scholar]
- 81. Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF‐kappaB/IL‐8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71(7):2550–2560. [DOI] [PubMed] [Google Scholar]
- 82. Formby B, Stern R. Lactate‐sensitive response elements in genes involved in hyaluronan catabolism. Biochem Biophys Res Commun. 2003;305(1):203–208. [DOI] [PubMed] [Google Scholar]
- 83. Stern R, Shuster S, Neudecker BA, Formby B. Lactate stimulates fibroblast expression of hyaluronan and CD44: the Warburg effect revisited. Exp Cell Res. 2002;276(1):24–31. [DOI] [PubMed] [Google Scholar]
- 84. Brand A, Singer K, Koehl GE, et al. LDHA‐associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24(5):657–671. [DOI] [PubMed] [Google Scholar]
- 85. Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor‐derived lactate modifies antitumor immune response: effect on myeloid‐derived suppressor cells and NK cells. J Immunol. 2013;191(3):1486–1495. [DOI] [PubMed] [Google Scholar]
- 86. Seth P, Csizmadia E, Hedblom A, et al. Deletion of lactate dehydrogenase‐A in myeloid cells triggers antitumor immunity. Cancer Res. 2017;77(13):3632–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hisamitsu T, Nakamura TY, Wakabayashi S. Na(+)/H(+) exchanger 1 directly binds to calcineurin A and activates downstream NFAT signaling, leading to cardiomyocyte hypertrophy. Mol Cell Biol. 2012;32(16):3265–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Haas R, Smith J, Rocher‐Ros V, et al. Lactate regulates metabolic and pro‐inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 2015;13(7):e1002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chang CH, Curtis JD, Maggi LB Jr, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shime H, Yabu M, Akazawa T, et al. Tumor‐secreted lactic acid promotes IL‐23/IL‐17 proinflammatory pathway. J Immunol. 2008;180(11):7175–7183. [DOI] [PubMed] [Google Scholar]
- 91. Langowski JL, Zhang X, Wu L, et al. IL‐23 promotes tumour incidence and growth. Nature. 2006;442(7101):461–465. [DOI] [PubMed] [Google Scholar]
- 92. Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour‐associated macrophages by tumour‐derived lactic acid. Nature. 2014;513(7519):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro‐environment in tumor progression: the role of tumor‐associated macrophages. Crit Rev Oncol Hematol. 2008;66(1):1–9. [DOI] [PubMed] [Google Scholar]
- 94. Ma J, Xu H, Wang S. Immunosuppressive role of myeloid‐derived suppressor cells and therapeutic targeting in lung cancer. J Immunol Res. 2018;2018:6319649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ferraris AM, Giuntini P, Gaetani GF. Serum lactic dehydrogenase as a prognostic tool for non‐Hodgkin lymphomas. Blood. 1979;54(4):928–932. [PubMed] [Google Scholar]
- 96. Garcia R, Hernandez JM, Caballero MD, et al. Serum lactate dehydrogenase level as a prognostic factor in Hodgkin's disease. Br J Cancer. 1993;68(6):1227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gkotzamanidou M, Kastritis E, Gavriatopoulou MR, et al. Increased serum lactate dehydrogenase should be included among the variables that define very‐high‐risk multiple myeloma. Clin Lymphoma Myeloma Leuk. 2011;11(5):409–413. [DOI] [PubMed] [Google Scholar]
- 98. Lu R, Jiang M, Chen Z, et al. Lactate dehydrogenase 5 expression in Non‐Hodgkin lymphoma is associated with the induced hypoxia regulated protein and poor prognosis. PLoS One. 2013;8(9):e74853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Colgan SM, Mukherjee S, Major P. Hypoxia‐induced lactate dehydrogenase expression and tumor angiogenesis. Clin Colorectal Cancer. 2007;6(6):442–446. [DOI] [PubMed] [Google Scholar]
- 100. Jin Y, Ye X, Shao L, et al. Serum lactic dehydrogenase strongly predicts survival in metastatic nasopharyngeal carcinoma treated with palliative chemotherapy. Eur J Cancer. 2013;49(7):1619–1626. [DOI] [PubMed] [Google Scholar]
- 101. Kayser G, Kassem A, Sienel W, et al. Lactate‐dehydrogenase 5 is overexpressed in non‐small cell lung cancer and correlates with the expression of the transketolase‐like protein 1. Diagn Pathol. 2010;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dong T, Liu Z, Xuan Q, Wang Z, Ma W, Zhang Q. Tumor LDH‐A expression and serum LDH status are two metabolic predictors for triple negative breast cancer brain metastasis. Sci Rep. 2017;7(1):6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Levine AJ, Puzio‐Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330(6009):1340–1344. [DOI] [PubMed] [Google Scholar]
- 104. DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11–20. [DOI] [PubMed] [Google Scholar]
- 105. Buchakjian MR, Kornbluth S. The engine driving the ship: metabolic steering of cell proliferation and death. Nat Rev Mol Biol. 2010;11(10):715–727. [DOI] [PubMed] [Google Scholar]
- 106. Langhammer S, Najjar M, Hess‐Stumpp H, Thierauch KH. LDH‐A influences hypoxia‐inducible factor 1alpha (HIF1 alpha) and is critical for growth of HT29 colon carcinoma cells in vivo. Target Oncol. 2011;6(3):155–162. [DOI] [PubMed] [Google Scholar]
- 107. Kim SH, Lee GM. Down‐regulation of lactate dehydrogenase‐A by siRNAs for reduced lactic acid formation of Chinese hamster ovary cells producing thrombopoietin. Appl Microbiology and Biotechnology. 2007;74(1):152–159. [DOI] [PubMed] [Google Scholar]
- 108. Miyajima H, Takahashi Y, Suzuki M, Shimizu T, Kaneko E. Molecular characterization of gene expression in human lactate dehydrogenase‐A deficiency. Neurology. 1993;43(7):1414–1419. [DOI] [PubMed] [Google Scholar]
- 109. Kanno T, Sudo K, Maekawa M, Nishimura Y, Ukita M, Fukutake K. Lactate dehydrogenase M‐subunit deficiency: a new type of hereditary exertional myopathy. Clin Chim Acta. 1988;173(1):89–98. [DOI] [PubMed] [Google Scholar]
- 110. Zhao Z, Han F, Yang S, Wu J, Zhan W. Oxamate‐mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: involvement of the Akt–mTOR signaling pathway. Cancer Lett. 2015;358(1):17–26. [DOI] [PubMed] [Google Scholar]
- 111. Papaconstantinou J, Colowick SP. The role of glycolysis in the growth of tumor cells. II. The effect of oxamic acid on the growth of HeLa cells in tissue culture. J Biol Chem. 1961;236:285–288. [PubMed] [Google Scholar]
- 112. Rani R, Kumar V. Recent update on human lactate dehydrogenase enzyme 5 (hLDH5) inhibitors: a promising approach for cancer chemotherapy. J Med Chem. 2016;59(2):487–496. [DOI] [PubMed] [Google Scholar]
- 113. Shelley MD, Hartley L, Fish RG, et al. Stereo‐specific cytotoxic effects of gossypol enantiomers and gossypolone in tumour cell lines. Cancer Lett. 1999;135(2):171–180. [DOI] [PubMed] [Google Scholar]
- 114. Coyle T, Levante S, Shetler M, Winfield J. In vitro and in vivo cytotoxicity of gossypol against central nervous system tumor cell lines. J Neurooncol. 1994;19(1):25–35. [DOI] [PubMed] [Google Scholar]
- 115. Flack MR, Pyle RG, Mullen NM, et al. Oral gossypol in the treatment of metastatic adrenal cancer. J Clin Endocrinol Metab. 1993;76(4):1019–1024. [DOI] [PubMed] [Google Scholar]
- 116. Bushunow P, Reidenberg MM, Wasenko J, et al. Gossypol treatment of recurrent adult malignant gliomas. J Neurooncol. 1999;43(1):79–86. [DOI] [PubMed] [Google Scholar]
- 117. Van Poznak C, Seidman AD, Reidenberg MM, et al. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial. Breast Cancer Res Treat. 2001;66(3):239–248. [DOI] [PubMed] [Google Scholar]
- 118. Granchi C, Paterni I, Rani R, Minutolo F. Small‐molecule inhibitors of human LDH5. Future Med Chem. 2013;5(16):1967–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lee CY, Moon YS, Yuan JH, Chen AF. Enzyme inactivation and inhibition by gossypol. Mol Cell Biochem. 1982;47(2):65–70. [DOI] [PubMed] [Google Scholar]
- 120. Granchi C, Roy S, Giacomelli C, et al. Discovery of N‐hydroxyindole‐based inhibitors of human lactate dehydrogenase isoform A (LDH‐A) as starvation agents against cancer cells. J Med Chem. 2011;54(6):1599–1612. [DOI] [PubMed] [Google Scholar]
- 121. Maftouh M, Avan A, Sciarrillo R, et al. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer. 2014;110(1):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Manerba M, Vettraino M, Fiume L, et al. Galloflavin (CAS 568–80‐9): a novel inhibitor of lactate dehydrogenase. ChemMedChem. 2012;7(2):311–317. [DOI] [PubMed] [Google Scholar]
- 123. Farabegoli F, Vettraino M, Manerba M, Fiume L, Roberti M, Di Stefano G. Galloflavin, a new lactate dehydrogenase inhibitor, induces the death of human breast cancer cells with different glycolytic attitude by affecting distinct signaling pathways. Eur J Pharm Sci. 2012;47(4):729–738. [DOI] [PubMed] [Google Scholar]
- 124. Fiume L, Vettraino M, Carnicelli D, Arfilli V, Di Stefano G, Brigotti M. Galloflavin prevents the binding of lactate dehydrogenase A to single stranded DNA and inhibits RNA synthesis in cultured cells. Biochem Biophys Res Commun. 2013;430(2):466–469. [DOI] [PubMed] [Google Scholar]
- 125. Deiab S, Mazzio E, Messeha S, Mack N, Soliman KF. High‐throughput screening to identify plant derived human LDH‐A inhibitors. European J Med Plants. 2013;3(4):603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Miskimins WK, Ahn HJ, Kim JY, Ryu S, Jung YS, Choi JY. Synergistic anti‐cancer effect of phenformin and oxamate. PLoS One. 2014;9(1):e85576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Allison SJ, Knight JR, Granchi C, et al. Identification of LDH‐A as a therapeutic target for cancer cell killing via (i) p53/NAD(H)‐dependent and (ii) p53‐independent pathways. Oncogenesis. 2014;3:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]


