Abstract
Background
The burden of Tuberculosis (TB) has not been comprehensively evaluated over the last 25 years in Ethiopia. In this study, we used the 2016 Global Burden of Diseases, Injuries and Risk Factors (GBD) data to analyze the incidence, prevalence and mortality rates of tuberculosis (TB) in Ethiopia over the last 26 years.
Methods
The GBD 2016 is a mathematical modeling using different data source for Ethiopia such as verbal autopsy (VA), prevalence surveys and annual case notifications. Age and sex specific causes of death for TB were estimated using the Cause of Death Ensemble Modeling (CODEm). We used the available data such as annual notifications and prevalence surveys as an input to estimate incidence and prevalence rates respectively using DisMod-MR 2.1, a Bayesian meta-regression tool.
Results
In 2016, we estimated 219,186 (95%UI: 182,977–265,292) new, 151,602 (95% UI: 126,054–180,976) prevalent TB cases and 48,910(95% UI: 40,310–58,195) TB deaths. The age-standardized TB incidence rate decreased from 201.6/100,000 to 88.5/100,000 (with a total decline of 56%) between 1990 to 2016. Similarly, the age-standardized TB mortality rate declined from 393.8/100,000 to 100/100,000 between 1990 and 2016(with a total decline of 75%).
Conclusions
Ethiopia has achieved the 50% reduction of most of the Millennium Development Goals (MDGs) targets related to TB. However, the decline of TB incidence and prevalence rates has been comparatively slow. The country should strengthen the TB case detection and treatment programs at community level to achieve its targets during the Sustainable Development Program (SDGs)-era.
Keywords: Tuberculosis, Burden, Ethiopia
Introduction
The United Nations General Assembly endorsed the historical resolution of the Millennium Development Goals (MDG) in 2000. The Tuberculosis (TB) specific target of the MDG was to halt and began to reverse the incidence of TB by 2015(1,2). Despite remarkable progress during the MDG era globally, TB ranks to be the top killer infectious diseases worldwide. In 2016, there were 6.3 million new TB cases and 1.3 million TB deaths (3).
In Ethiopia, TB is still a major public health problem (1–3). The country is still among the 22 high TB burden countries with high number of missed and infectious TB cases in the community (1–3). The prevalence and incidence of TB in Ethiopia in 2014 were 211 and 214 per 100,000 populations respectively (4). Increasing the trends of multidrug resistance TB (2% among new cases in 2006 vs. 4.5% among new cases in 2016) is a serious public health challenge for the country (4,5). TB is among the top ten causes of admission and deaths in adults in Ethiopia (6). In line with the Sustainable Development Goals (SDGs), Ethiopia recently launched a five years' ambitious Health Sector Transformation Plan (HSTP) to address major diseases of public health importance including TB (6). Information revolution is one of the core agenda items of the HSTP to inform decision makers for timely action. However, Ethiopia still does not have a strong health management information system to capture the burden of TB and track the progresses of TB interventions. Because of weak health information system and very few national surveys, the burden of TB was not comprehensively assessed in Ethiopia over the last 3 decades. In the last few years, there were few TB studies with limited geographic areas (5) that may not provide national representative information. In this article, we used the Global Burden of Disease Study (GBD) 2016 data (7–11) to assess the mortality, incidence, prevalence and Disability-adjusted Life Years Lost (DALY) rates of TB over the last 26 years. The rationale of using the GBD data include: i) GBD modeling strategy provides nationally representative estimates which cannot be obtained through small scale studies or surveys; ii) GBD uses standard modeling approach globally and our finding (Ethiopian performance) can be compared with others who are using GBD; iii) The study provides evidence on the achievement of Ethiopia on the MDG targets and the findings will also serve as a baseline for future tracking of TB targets. The performance of Ethiopia cannot be measured by data from small scale surveys; iv) Lastly, the study also helps decision makers to allocate resources based on the burden of TB.
Methods
Ethiopian population (approximately 100 million) is the second largest in Africa with diverse population mix and unique cultural heritage (6). The GBD 2016 data were used to identify the trends in the incidence, prevalence and mortality rates of TB. The GBD 2016 utilizes available sources of data and rigorous analysis to estimate trends in the burden of TB for 195 countries and territories. The detailed methods used to estimate the TB burden have been published elsewhere (11–13), and we provided a detailed description of the modeling in the following section.
Data sources: This study used a mathematical modeling using the GBD data. The GBD 2016 used a different data sources for Ethiopia (http://ghdx.healthdata.org/). The key sources of data for TB include verbal autopsy (VA) studies, national and subnational tuberculosis prevalence surveys that are published, published population-based tuberculin surveys, case notifications and cohort studies reporting on the risk of developing active TB disease as a function of induration size. We excluded unpublished data and hospital records.
Data analysis and modeling : Causes of death by age groups, sex and year for TB were estimated using the Cause of Death Ensemble modeling (CODEm). A detailed description of CODEm is reported elsewhere (14–17). In brief, CODEm tests a wide range of models such as mixed effects linear models and spatial-temporal Gaussian process regression (ST-GPR) models using various combinations of covariates and constructs an ensemble model based on the performance of the different models (15). All models are assessed using out-of-sample predictive validity tests and an ensemble of models that perform best is selected. Non-fatal TB modeling prevalence and incidence rates of TB were estimated using a Bayesian Regression model using the input data such as prevalence surveys and case notifications (http://ghdx.healthdata.org/gbd-2016/data-input-sources). The detailed methodology is described elsewhere (11). First, we estimated the risk-weighted prevalence of latent TB infection (LTBI) using data from population-based tuberculin surveys and cohort studies examining the risk of developing active TB as a function of induration size (11). Next, we divided the inputs on prevalence (from TB prevalence surveys), incidence (estimated based on a mortality-to-incidence ratio approach) and cause specific mortality estimates by the risk-weighted LTBI prevalence to model TB among those at risk. We used DisMod-MR 2.1, the GBD Bayesian meta-regression tool to generate internally consistent estimates of incidence, prevalence and mortality. We then multiplied the DisMod-MR 2.1 outputs by the risk-weighted prevalence of LTBI to get population-level estimates of incidence and prevalence (11).
Quality assurance: VA data were corrected for garbage coding based on the GBD algorithm (15). Garbage coding is a bias due to the assignment of causes of death that are not underlying causes of death (14). We included published articles and surveys. Unpublished data or reports were excluded.
Ethical approval: The study used secondary data from the GBD 2016 study, and permission was obtained from Institute of Health Metrics and Evaluation at Washington University in the United States to utilize the data. The GBD 2016 data can be caccessed at the GBD website (http://vizhub.healthdata.org/gbd-compare/).
Results
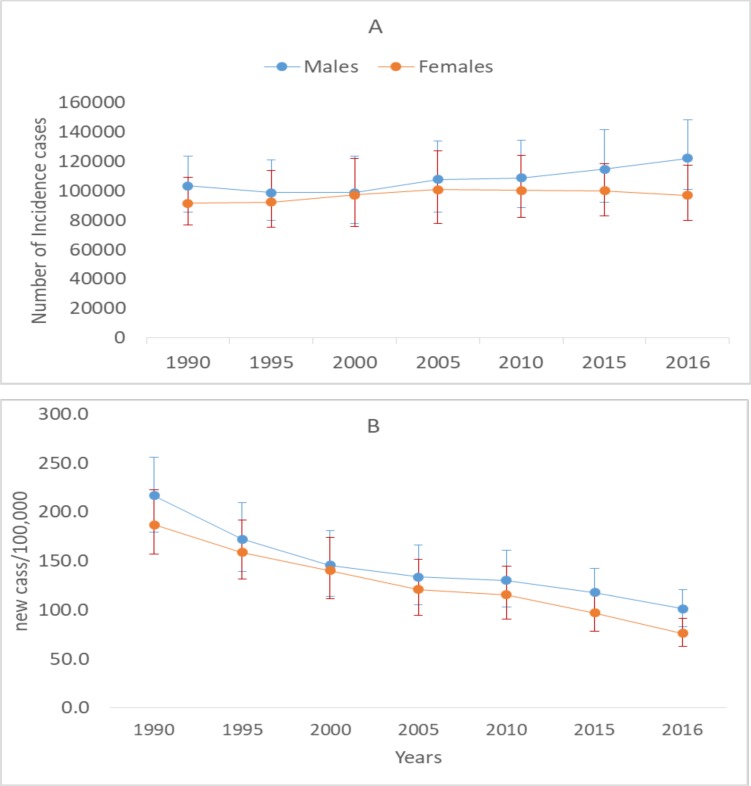
An estimated 219,186 (95%UI: 182,977–265,292) incident of TB cases occurred in Ethiopia in 2016. The age-standardised TB incidence rate decreased from 201.6/100,000 to 88.5/100,000 (with a total decline of 56%) between 1990 to 2016. Males had higher TB incidence rate than females during the last 26 years (P-value for trend<0.05). Annualized rate of change (ARC) for the age-standardised incidence rate was −3.2%. The ARC between males and females was not statistically significant (P=0.06) (Figure 1).
Figure 1.
TB incidence cases (A) and TB incidence rate (B) by sex in Ethiopia in 2016
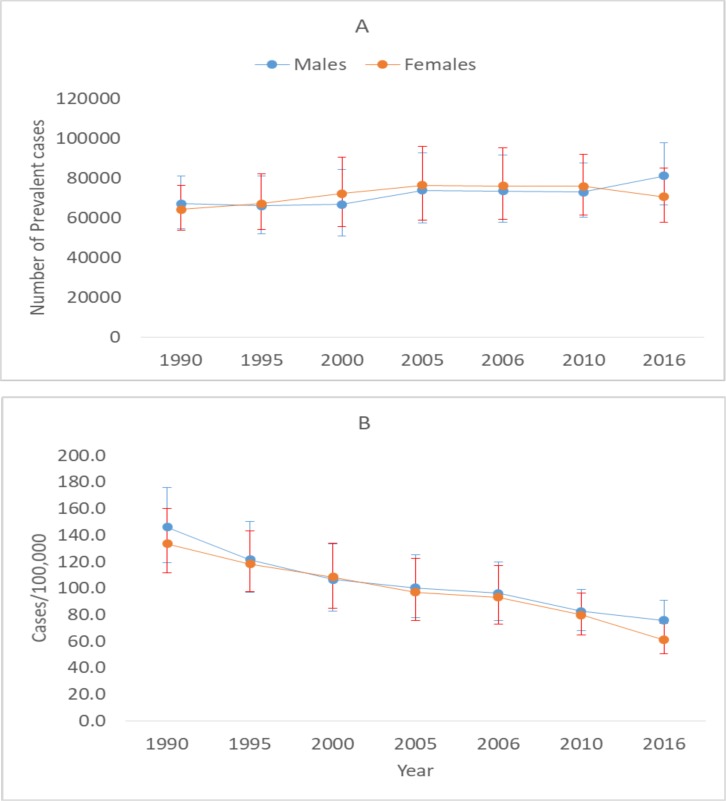
In 2016, about 151,602 (95% UI: 126054–180,976) prevalent TB cases were estimated to occur in Ethiopia. The annualized rate of decline for the age-standardised TB prevalence rate was nearly 3% during the last 26 years. The age standardized TB prevalence rate declined by 51% (from 139.4/100,000 to 68.2/100,000) between 1990 to 2016 (Figure 2).
Figure 2.
Number of TB prevalent cases (A) and TB prevalence rate (B) between 1990 and 2016
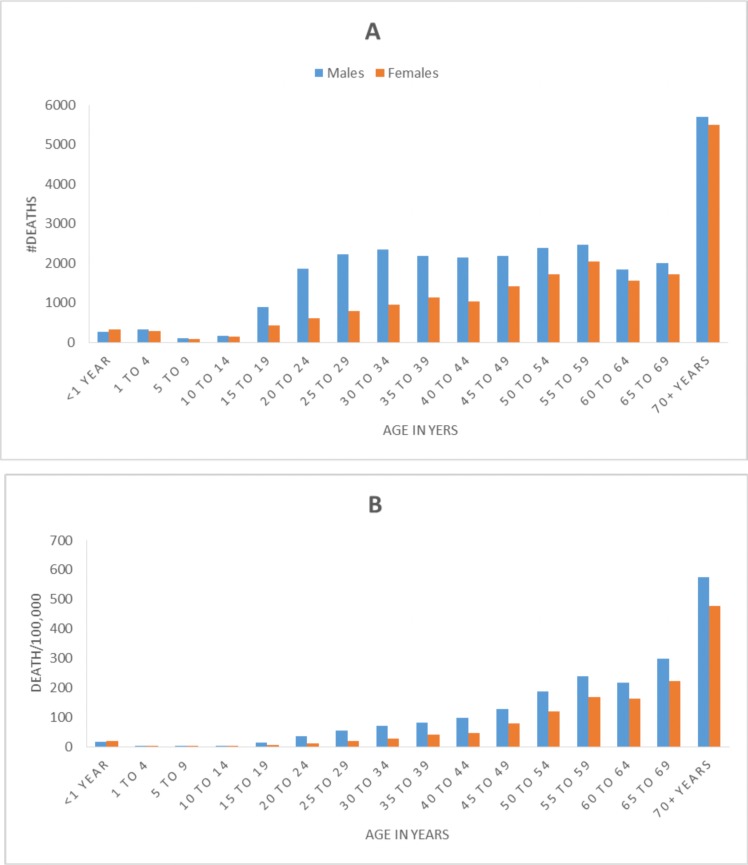
An estimated 48,910(95% UI: 40,310–58,195) TB deaths occurred in 2016. The age-standardised TB mortality rate declined from 393.8/100,000 to 100/100,000 between 1990 and 2016 (with a total decline of 75%) (Table 1). TB deaths increased as age increased, and males had higher TB mortality rate than females (Figure 3).
Table 1.
Number of deaths and mortality rate of Tuberculosis in Ethiopia, between 1990 and 2016
| Year | Number of deaths |
95%UI | Rate/100,000 | 95%UI | ||
| 1990 | 91150 | 62040 | 107222 | 393.8 | 265.1 | 472.7 |
| 1995 | 84245 | 65653 | 96138 | 311.1 | 240.4 | 359.9 |
| 2000 | 74563 | 63272 | 84064 | 242.6 | 205.4 | 275.0 |
| 2005 | 70921 | 62195 | 79623 | 201.2 | 177.4 | 225.6 |
| 2010 | 59597 | 52167 | 69144 | 145.7 | 128.1 | 167.4 |
| 2015 | 49875 | 41423 | 59434 | 105.3 | 89.0 | 124.7 |
| 2016 | 48910 | 40310 | 58195 | 100.0 | 83.4 | 118.5 |
Figure 3.
Number of TB deaths (A) and TB mortality rate (B) by age group in 2016
An estimated of 1.8 million (95%UI: 1.5 million–2.2 million) TB related DALY were registered in Ethiopia in 2016. Age-standardised TB DALY rate declined from 11411/100,000 in 1990 to 2771.9/100,000 in 2016 with a total reduction of 76%. ARC for TB DALY rate was −5.4% (Table 2).
Table 2.
Number of DALY and DALY rates due to Tuberculosis between 1990 and 2016
| Year | Number of DALY | 95%UI | DALY rate/100,000 |
95%UI | ||
| 1990 | 3573109 | 2454050 | 4191207 | 11411.0 | 7893.6 | 13400.5 |
| 1995 | 3277927 | 2587075 | 3759007 | 8936.5 | 7040.2 | 10222.8 |
| 2000 | 2916341 | 2462535 | 3300836 | 6968.6 | 5921.4 | 7842.8 |
| 2005 | 2795015 | 2455346 | 3155602 | 5724.0 | 5003.2 | 6437.8 |
| 2010 | 2296405 | 1982065 | 2646342 | 4095.5 | 3569.1 | 4774.8 |
| 2015 | 1886205 | 1574282 | 2238669 | 2924.5 | 2429.2 | 3452.9 |
| 2016 | 1842618 | 1521283 | 2209096 | 2771.9 | 2286.5 | 3312.6 |
Discussion
Ethiopia has reduced the TB mortality rate by 75% and the TB incidence rate by more than 50% during 1990 and 2016. This is in line with the MDG targets of halving mortality rate of TB by 2015 and efforts to reverse the incidence of these diseases (1,2).
Other reports also show that Ethiopia has achieved most of the MDG targets related to TB (18–20). Assefa and colleagues reported that TB incidence rate and TB mortality rate e declined by 54% and by 72% respectively during the MDG era (20). The World Health Organization (WHO) report indicates that the performance of Ethiopia in reducing TB mortality and incidence rates has been remarkable over the last decade. Since 2010, Ethiopia has reduced the TB mortality rate by more than 6% annually and the incidence rate of TB by more than 4% annually (3). The WHO estimates of TB prevalence and incidence rates were higher than the GBD estimates particularly in the early 1990s. However, the WHO estimates are lower than the GBD estimates over the years particularly after 2010. The GBD estimation of prevalence was derived from local or national prevalence survey (10,15). However, the WHO estimated TB prevalence as a product of incidence and duration of the diseases in the absence of national TB prevalence survey (15,21). Estimation of TB incidence rate by WHO was based on case-notification and expert judgment (21).
There are very limited studies in Ethiopia that assessed the burden of TB comprehensively during the MDG era. A study conducted in Oromia region in 2016 shows that incidence and prevalence rates of TB were 214/100,000 and 109/100,000 respectively (22). The prevalence of bacteriologically confirmed TB at the national level in 2011 was 277/100,000 (23). On the other hand, Berhe and colleagues reported a prevalence rate of bacteriologically confirmed TB of 352/100,000 in Tigray in 2012 (24). The GBD TB prevalence estimates are lower than the estimates of the aforementioned studies (12,13). On the other hand, our prevalence estimates are higher than the estimate reported by Deribew et al in Jimma (108/100,000) (25) and Yimer et al in Gondar (80/100,000) (26).
The performance of Ethiopia in reducing the burden of TB and reversing these epidemics is remarkable particularly since 2010 (15). Several factors could have helped Ethiopia to achieve the MDG targets. First, the commitment and leadership of the health sector leaders in Ethiopia could have helped Ethiopia to achieve most of the MDG targets (27). Second, the Health Extension Programs (HEP) in Ethiopia would have created an opportunity to improve access to care and treatment for TB suspects. Some reports show that the HEP has been instrumental to make health services accessible to the poor (19, 27–29). It also improves TB case detection rates at community level (30). Implementation of the Directly Observed Treatment for TB (DOTS) over the last two decades could have also significant impact on the burden of TB in Ethiopia. Third, the contribution of development partners to fight TB (PEPFAR) has been also vital in fighting TB in Ethiopia. The government has used flexible modalities of One-plan, One-budget and One-Report concept and effectively utilized the funding to scale up interventions towards TB (31). Donors' funding could decline during the coming years and Ethiopia should strengthen the low-cost and high impact HEP-based interventions to sustain progress (6,32).
Despite a remarkable progress, Ethiopia has to overcome several challenges to achieve the End TB Targets of reducing TB mortality by 90% and TB incidence rate by 80% by 2030 (33). TB/HIV co-infection and its impact on stigma and mental health problems could also be a big hurdle for the HIV/AIDS control program (34,35). In the rural part of the country, stigma and prejudices are still widespread which needs tailored intervention (36). Continuous community mobilization, stigma reduction, and care and support services have been vital for increasing utilization of ART services and improving retention in care that helps to reduce early mortality due to HIV/AIDS in Ethiopia (37).
On the other hand, very low TB case detection rate and presence of infectious TB cases at the community without treatment poses serious problems on the TB control program. Despite the implementation of the directly-observed treatment, short-course for TB (DOTS) since 2000(38), the TB case detection rate of 36%, remains very low in the country (39). So, decentralization of TB diagnostic and treatment services down to the lower health facilities and involvement of health extension workers in TB control program to revitalize and improve case detection system in the country is acceptable (28,40).
On the other hand, the burden of TB is highly variable by region and population groups, which requires proper planning. Some regions have higher TB prevalence that needs to be addressed to bring equity (24,41). The TB epidemic is also concentrated in some vulnerable populations particularly in prison inmates (42,43). The increasing trends of multi-drug resistant TB (MDR-TB), which requires expensive and more toxic drugs, remain a serious challenge ahead in the coming years (44).
This study is based on the GBD 2016 that uses comprehensive data sources and rigorous analysis. However, the study has some limitations. First, the use of verbal autopsy (VA) data in morality estimation may introduce misclassification bias. Use of published articles could introduce publication bias since unfavorable findings may not be published.
In conclusion, Ethiopia has achieved most of the MDG targets related to TB. However, the decline in TB incidence and prevalence rates has been slow. The country thus should strengthen TB case detection and treatment programs at community level through the HEP to reduce the burden of these diseases during the SDG-era.
Acknowledgments
We are grateful for the GBD team at the University of Washington and the Institute of Health Metrics and Evaluation (IHME) for the support in providing the data.
References
- 1.WHO, author. From MDG to SDG. World Health Organization(WHO); 2015. [November 28, 2017]. ( http://apps.who.int/iris/bitstream/10665/200009/1/9789241565110_eng.pdf?ua=1) [Google Scholar]
- 2.UN, author. The Millennium Development Goals Report. United Nations; 2015. http://www.un.org/millenniumgoals/2015_MDG_Report/pdf/MDG%202015%20rev%20(July%201).pdf. [Google Scholar]
- 3.WHO, author. Global Tuberculosis Report 2017. Geneva: World Health Organization (WHO); 2017. [Google Scholar]
- 4.WHO, author. Ethiopia Tuberculosis Progress in 2014. World Health Organization(WHO); 2015. http://www.afro.who.int/en/ethiopia/country-programmes/topics/4481-tuberculosis.html. [Google Scholar]
- 5.Eshetie S, Gizachew M, Dagnew M, Kumera G, Woldie H, Ambaw F, et al. Multidrug resistant tuberculosis in Ethiopian settings and its association with previous history of antituberculosis treatment: a systematic review and meta-analysis. BMC infectious diseases. 2017;17(1):219. doi: 10.1186/s12879-017-2323-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FMOH, author. Health Sector Transformation Plan 2015/16–2019/20. Federal Democratic Republic of Ethiopia; 2015. [Google Scholar]
- 7.GBD, author. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: a systematic analysis for the Global Burden of Disease Study(GBD) 2015. Lancet. 2016;388(10053):1725. doi: 10.1016/S0140-6736(16)31575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GBD, author. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease(GBD) Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Wolock TM, Carter A, Nguyen G, Kyu HH, Gakidou E, et al. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. The lancet HIV. 2016;3(8):e361–e387. doi: 10.1016/S2352-3018(16)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GBD collaborators, author. The burden of tuberculosis from 1990 to 2016: results from the Global 1 Burden of Diseases, Injuries, and Risk Factors (GBD) 2016 Study (Submitted to the Lancet)
- 12.GBD, author. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GBD, author. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foreman KJ, Lozano R, Lopez AD, Murray CJ. Modeling causes of death: an integrated approach using CODEm. Population health metrics. 2012;10:1. doi: 10.1186/1478-7954-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray CJ, Ezzati M, Flaxman AD, Lim S, Lozano R, Michaud C, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380(9859):2063–2066. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 18.NPC, UN, author. Millennium Development Goals Report 2014. Assessment of Ethiopia's Progress towards the MDGs. National Planning Commission(NPC) and the United Nations(UN) in Ethiopia; 2015. [Google Scholar]
- 19.Accorsi S, Bilal NK, Farese P, Racalbuto V. Countdown to 2015: comparing progress towards the achievement of the health Millennium Development Goals in Ethiopia and other sub-Saharan African countries. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2010;104(5):336–342. doi: 10.1016/j.trstmh.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Assefa Y, Damme WV, Williams OD, Hill PS. Successes and challenges of the millennium development goals in Ethiopia: lessons for the sustainable development goals. BMJ global health. 2017;2(2):e000318. doi: 10.1136/bmjgh-2017-000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glaziou P, Sismanidis C, Zignol M, Floyd K. Methods used by WHO to estimate the global burden of TB disease. Global TB Programme. Geneva, Switzerland: World Health Organization; 2016. http://www.who.int/tb/publications/global_report/gtbr2016_online_technical_appendix_global_disease_burden_estimation.pdf. [Google Scholar]
- 22.Hamusse S, Demissie M, Teshome D, Hassen MS, Lindtjorn B. Prevalence and Incidence of Smear-Positive Pulmonary Tuberculosis in the Hetosa District of Arsi Zone, Oromia Regional State of Central Ethiopia. BMC infectious diseases. 2017;17(1):214. doi: 10.1186/s12879-017-2321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kebede AH, Alebachew Z, Tsegaye F, Lemma E, Abebe A, Agonafir M, et al. The first population-based national tuberculosis prevalence survey in Ethiopia, 2010–2011. The International journal of Tuberculosis and Lung Disease. 2014;18(6):635. doi: 10.5588/ijtld.13.0417. [DOI] [PubMed] [Google Scholar]
- 24.Berhe G, Enqueselassie F, Hailu E, Mekonnen W, Teklu T, Gebretsadik A, et al. Population-based prevalence survey of tuberculosis in the Tigray region of Ethiopia. BMC infectious diseases. 2013;13:448. doi: 10.1186/1471-2334-13-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deribew A, Abebe G, Apers L, Abdissa A, Deribe F, Woldemichael K, et al. Prevalence of pulmonary TB and spoligotype pattern of Mycobacterium tuberculosis among TB suspects in a rural community in Southwest Ethiopia. BMC infectious diseases. 2012;12:54. doi: 10.1186/1471-2334-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yimer S, Holm-Hansen C, Yimaldu T, Bjune G. Evaluating an active case-finding strategy to identify smear-positive tuberculosis in rural Ethiopia. The International Journal of Tuberculosis and Lung Disease. 2009;13(11):1399–1404. [PubMed] [Google Scholar]
- 27.Wakabi W. Extension workers drive Ethiopia's primary health care. Lancet. 2008;372(9642):880. doi: 10.1016/s0140-6736(08)61381-1. [DOI] [PubMed] [Google Scholar]
- 28.Yassin MA, Datiko DG, Tulloch O, Markos P, Aschalew M, Shargie EB, et al. Innovative community-based approaches doubled tuberculosis case notification and improve treatment outcome in Southern Ethiopia. PloS One. 2013;8(5):e63174. doi: 10.1371/journal.pone.0063174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gebrehiwot TG, San Sebastian M, Edin K, Goicolea I. The Health Extension Program and Its Association with Change in Utilization of Selected Maternal Health Services in Tigray Region, Ethiopia: A Segmented Linear Regression Analysis. PloS One. 2015;10(7):e0131195. doi: 10.1371/journal.pone.0131195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Datiko DG, Yassin MA, Tulloch O, Asnake G, Tesema T, Jamal H, et al. Exploring providers' perspectives of a community based TB approach in Southern Ethiopia: implication for community based approaches. BMC health services research. 2015;15:501. doi: 10.1186/s12913-015-1149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waddington C, Alebachew A, Chabot J. Roadmap for enhancing the implementation of One Plan, One Budget and One Report in Ethiopia. Addis Ababa: Ministry of Health; 2012. [Google Scholar]
- 32.Teklehaimanot HD, Teklehaimanot A. Human resource development for a community-based health extension program: a case study from Ethiopia. Human resources for health. 2013;11:39. doi: 10.1186/1478-4491-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO, author. Implementing the End TB strategy: The essentials. Geneva: World Health organization(WHO); 2015. WHO/HTM/TB/2015.31. [Google Scholar]
- 34.Deribew A, Hailemichael Y, Tesfaye M, Desalegn D, Wogi A, Daba S. The synergy between TB and HIV co-infection on perceived stigma in Ethiopia. BMC research notes. 2010;3:249. doi: 10.1186/1756-0500-3-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deribew A, Negussu N, Kassahun W, Apers L, Colebunders R. Uptake of provider-initiated counselling and testing among tuberculosis suspects, Ethiopia. The International Journal of Tuberculosis and Lung Disease. 2010;14(11):1442–1446. [PubMed] [Google Scholar]
- 36.Deribew A, Abebe G, Apers L, Jira C, Tesfaye M, Shifa J, et al. Prejudice and misconceptions about tuberculosis and HIV in rural and urban communities in Ethiopia: a challenge for the TB/HIV control program. BMC Public Health. 2010;10:400. doi: 10.1186/1471-2458-10-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Assefa Y, Alebachew A, Lera M, Lynen L, Wouters E, Van Damme W. Scaling up antiretroviral treatment and improving patient retention in care: lessons from Ethiopia, 2005–2013. Globalization and health. 2014;10:43. doi: 10.1186/1744-8603-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sisay S, Mengistu B, Erku W, Woldeyohannes D. Directly Observed Treatment Short-course (DOTS) for tuberculosis control program in Gambella Regional State, Ethiopia: ten years experience. BMC research notes. 2014;7:44. doi: 10.1186/1756-0500-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.FMOH, author. Tuberculosis, Leprosy and TB/HIV Prevention and Control Programme Manual. 5th Edition. Addis Ababa: Federal Ministry of Health of Ethiopia (FMOH); 2012. [Google Scholar]
- 40.Datiko DG, Lindtjorn B. Health extension workers improve tuberculosis case detection and treatment success in southern Ethiopia: a community randomized trial. PloS One. 2009;4(5):e5443. doi: 10.1371/journal.pone.0005443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steenkamp L, Goosen A, Venter D, Beeforth M. Food insecurity among students living with HIV: Strengthening safety nets at the Nelson Mandela Metropolitan University, South Africa. PloS One. 2016;13(1):106–112. doi: 10.1080/17290376.2016.1218791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali S, Haileamlak A, Wieser A, Pritsch M, Heinrich N, Loscher T, et al. Prevalence of Pulmonary Tuberculosis among Prison Inmates in Ethiopia, a Cross-Sectional Study. PLoS One. 2015;10(12):e0144040. doi: 10.1371/journal.pone.0144040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biadglegne F, Rodloff AC, Sack U. A first insight into high prevalence of undiagnosed smearnegative pulmonary tuberculosis in Northern Ethiopian prisons: implications for greater investment and quality control. PLoS One. 2014;9(9):e106869. doi: 10.1371/journal.pone.0106869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shenoi S, Heysell S, Moll A, Friedland G. Multidrug-resistant and extensively drug-resistant tuberculosis: consequences for the global HIV community. Current Opinion in Infectious Diseases. 2009;22(1):11–17. doi: 10.1097/QCO.0b013e3283210020. [DOI] [PMC free article] [PubMed] [Google Scholar]