Abstract
Objective
Naringenin, a citrus flavonoid, prevents diet-induced weight gain and improves glucose and lipid metabolism in rodents. There is evidence that naringenin activates brown fat and increases energy expenditure in mice, but little is known about its effects in humans. Our goal was to examine the effects of naringenin on energy expenditure in adipose tissue.
Methods
Human white adipocyte cultures (hADSC), and subcutaneous abdominal adipose tissue (pWAT) were treated with naringenin for 7–14 days. Expression (qRT-PCR, immunoblotting) of candidate genes involved in thermogenesis and glucose metabolism was measured. Oxygen consumption rate (OCR) was measured in hADSC using a Seahorse Flux analyzer.
Results
In hADSC, naringenin increased expression of the genes associated with thermogenesis and fat oxidation including uncoupling protein 1, adipose triglyceride lipase, and key factors associated with insulin sensitivity including glucose transporter 4, adiponectin, and carbohydrate response element binding protein (p<0.01). Similar responses were observed in pWAT. Basal, ATP-linked, maximal, and reserve OCR increased in the naringenin-treated hADSC (p<0.01).
Conclusions
Naringenin increases energy expenditure in hADSC and stimulates expression of key enzymes involved in thermogenesis and insulin sensitivity in hADSC and pWAT. Naringenin may promote conversion of human white adipose tissue to a brown/beige phenotype.
Keywords: Naringenin, Thermogenesis, Insulin Sensitivity, Human Adipocytes, Human Adipose Tissue
Introduction
Browning of white adipose tissue has garnered interest in research on obesity because it engenders adaptive thermogenesis, a process mediated by mitochondrial uncoupling protein 1 (UCP1). When activated, UCP1 short circuits the electrochemical gradient that drives ATP synthesis. Downregulation of the proton gradient stimulates respiratory chain activity and provokes heat production through combustion of available substrates (1). This type of metabolically active and energy dissipating tissue offers a potential solution to counteract excessive fat accumulation, especially since brown adipose tissue is typically low in humans and inversely correlates with body mass index (2). The browning of adipose tissue may also modulate systemic insulin action through non-thermogenic mechanisms such as the secretion of insulin sensitizing factors from adipose tissue (1). Thus, the therapeutic potential of brown fat transcends obesity and may be considered for other metabolic abnormalities such as insulin resistance.
Carbohydrate response element binding protein (ChREBP) is a transcription factor that is known to be expressed as α and β alternatively spliced isoforms. In humans, expression of both isoforms in white adipose tissue correlates with insulin sensitivity; (3, 4, 5) but, obesity is associated with a specific reduction in ChREBPβ expression (3, 5). Overexpression of ChREBP in mice fed a western diet enhances systemic insulin sensitivity, reduces fat mass and increases expression of the genes associated with thermogenesis suggesting a role for ChREBP in whole body insulin sensitivity and brown adipose tissue function (6). To date, the only compound known to stimulate ChREBP expression (in mouse adipose tissue) is the incretin mimetic liraglutide (7).
Strategies to enhance adipose tissue glucose uptake and restore depleted levels of ChREBP expression can be particularly beneficial to individuals with obesity and insulin resistance. Naringenin a citrus flavonoid, reduces diet-induced weight gain, increases energy expenditure, and improves glucose and lipid metabolism in animal models (8, 9, 10, 11, 12). Activation of adenosine monophosphate-activated kinase (AMPK), regulation of insulin signaling pathways and lipid metabolism, relief from oxidative stress in pancreatic beta cells, and increases in brown fat thermogenic gene expression are among the mechanisms that have been implicated (8, 9, 10, 11). The effects of naringenin on energy expenditure and expression of the genes involved in glucose metabolism in human adipose tissue have not been investigated.
The literature is replete with the beneficial effects of naringenin in obesity, cardiovascular disease, and cancer (13). However, grapefruit has been shown to increase the bioavailability of drugs administered orally. Several compounds in grapefruit including the flavonoids (naringin and naringenin), furanocoumarins and sesquiterpens have been implicated (14). Naringenin being polyphenolic and high in electrons, can theoretically inhibit cytochrome P450 enzymes and enhance the bioavailability of medications including statins. Although inhibition of the cytochrome P450 enzyme system has been demonstrated in vitro and in rodent models, the results of in vivo studies in humans suggest that naringenin is not the main inhibitory compound in grapefruit (15, 16). The most potent inhibitor of CYP3A4, the main enzyme in the cytochrome P450 family that is involved in drug metabolism is bergapten, a furanocoumarin derivative (17).
We investigated the effects of naringenin on energy expenditure in human adipocytes, and primary human white adipose tissue. Preliminary studies demonstrated that exposure of human adipocytes to naringenin upregulated the mRNA expression of UCP1 and GLUT4. We therefore hypothesized that naringenin acts in white adipose tissue to promote conversion of human white adipocytes to the beige/brown phenotype.
Materials and Methods
Materials
Hyclone™ Dulbecco’s Modified Eagle Medium/Ham’s F12 (DMEM/F12 1:1) and Gibco™ fetal bovine serum (FBS) were purchased from ThermoFisher Scientific (Waltham, MA), Rosiglitazone was purchased from AK Scientific (Union City, CA). All other reagents used in the medium were purchased from Sigma-Aldrich (St. Louis, MO). Naringenin (purity ≥ 98%) was purchased from Cayman Chemicals (Ann Arbor, MI). Protease and phosphatase were purchased from Cell Signaling Technology (Danvers, MA), RIPA buffer from Sigma, TGX protein gels from BIO-RAD (Hercules, CA). Seahorse Assay Medium and sodium pyruvate were purchased from Agilent (Santa Clara, CA). carbonyl cyanide-4-(trifluoromethoxy) phenyhydrazone (FCCP), Oligomycin, and Antimycin A were purchased from Sigma-Aldrich.
Cell Culture (Adipocytes)
Human adipose-derived stem cells from overweight and obese female donors were purchased from LaCell, LLC (New Orleans, Louisiana). Preadipocytes were seeded in DMEM/F12 1:1 supplemented with 10% FBS and 1% antibiotic (penicillin/streptomycin/amphotericin). One day after reaching confluence, cells were differentiated (hADSC) in media containing 70% DMEM and 30% DMEM/F12 1:1 supplemented with 3% FBS, 1% antibiotic, 1μM dexamethasone, 33uM biotin, 0.1μM insulin, 20μM pantothenate, 5μM rosiglitazone and 500μM 3-isobutylmethylxanthine. After induction in the differentiation media (4 or 5 days), cells were maintained in medium composed of 70% DMEM and 30% DMEM/F12 1:1 supplemented with 3% FBS, 1μM dexamethasone, 33μM biotin, 0.1μM insulin, and 20μM pantothenate (Day 0). Cells were kept at 37°C in a humidified atmosphere of 95% air and 5% CO2. Treatments (naringenin 8μM based on pharmacokinetic study done in humans (18)) started from Day 3, were administered every two days over a period of 6 days, and cells were harvested the following day (Day 10). Dexamethasone was removed from the maintenance medium during treatment to promote browning of adipocytes, (19) and FBS was inactivated by heating for 30 minutes at 56°C. Following harvest, Trizol reagent was added to lyse adipocytes and the contents of each well were homogenized. RNeasy Mini Kit (Qiagen, Germantown, MD) was used to isolate RNA following manufacturer’s protocol.
Ex Vivo Adipose Tissue Explants
Abdominal subcutaneous adipose tissue (pWAT) procured from four females, subjects 1, 2, 3, and 4 undergoing elective panniculectomies was mechanically minced into 0.5–1mm diameter segments and 500μl was suspended in 3ml adipocyte maintenance medium for each replicate (Day 0). Treatments (naringenin 8μM) started from Day 3, were administered to subjects 1, 2, and 3 every two days over a period of 14 days or were started at Day 10 and treated over 7 days. All pWAT samples including the untreated controls were harvested on the same day. In a separate experiment pWAT from subjects 1, 2, and 4 were treated in a similar fashion with GW 7647 (0.3μM). At the end of the treatment period, adipocytes were isolated from pWAT after standard collagenase digestion and RNA was extracted with Trizol. Subject characteristics are provided in Table 2.
Table 2.
Characteristics of donors of adipose tissue used in the explant experiments
| Subject | Age | BMI | Race |
|---|---|---|---|
| 1 | 33 | 29.2 | Caucasian |
| 2 | 45 | 25.8 | Caucasian |
| 3 | 39 | 29.3 | African American |
| 4 | 35 | 26.1 | African American |
Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
The RNA was quantified using Invitrogen Qubit RNA broad range assay kit and read using Qubit Fluorometer (ThermoFisher Scientific). Reverse transcriptase and PCR was conducted in one reaction with the reverse PCR primer priming cDNA synthesis using SuperScript® III Platinum One-Step Quantitative RT-PCR System with Rox from Invitrogen (ThermoFisher Scientific). Amplicons were designed to span an intron-exon junctions to avoid amplification of genomic sequences. Primer and probe oligonucleotide sets were purchased from Integrated DNA Technologies (Coralville, IOWA) and are provided in Table 1. RPL13A (Taqman® ID Hs04194366_g1, Thermofisher Scientific, Waltham, MA.) was used as a housekeeping gene. The RT-PCR assay for each sample was conducted in duplicate using the protocol for Applied Biosystems Instruments (7900 HT), with the exception that an extension temperature of 61°C was used in the assay to measure ChREBPβ.
Table 1.
sPrimer and probe sequences for qPCR
| Gene | Primer and Probe | Sequences | NCBI Reference Sequence |
|---|---|---|---|
| Adiponectin | Forward | CTGGTGAGAAGGGTGAGAAAG | NM_004797.2 |
| Reverse | CTCCTTTCCTGCCTTGGATT | ||
| Probe | TGGTCCTAAGGGAGACATCGGTGA | ||
| ATGL | Forward | CGTGTACTGTGGGCTCATC | NM_020376.3 |
| Reverse | GGACACTGTGATGGTGTTCTTA | ||
| Probe | ATGGTGGCATTTCAGACAACCTGC | ||
| CPT-1β | Forward | TACCATGGGTGGATGTTTGAG | NM_013261.3 |
| Reverse | GTCTGGAAGCTGTAGAGCATAG | ||
| Probe | TCTGGGCTATGTGTATCCGCCTTCTA | ||
| ChREBP | Forward | TCT GGA CAC AGC CGT CT | NM_032951 |
| Reverse | GCT TGG GGT CTT CAG GAA | ||
| Probe | ATCAGAACTCAGGAAGGCGCTGG | ||
| ChREBPβ | Forward | GAGCGGATTCCAGGTGAG | BG616809.1 |
| Reverse | TTGTTCAGGCGGATCTTGT | ||
| Probe | TCATCAGTGGCAAGCTGGTGTCTC | ||
| GLUT4 | Forward | GTATCATCTCTCAGTGGCTTGG | NM_001042 |
| Reverse | ATAGGAGGCAGCAGCATTG | ||
| Probe | AAAGGGCCATGCTGGTCAACAATG | ||
| PGC-1α | Forward | CACCAAACCCACAGAGAACA | NM_00145134.1 |
| Reverse | GGGTCAGAGGAAGAGATAAAGTTG | ||
| Probe | AAAGAAGTCCCACACACAGTCGCA | ||
| PGC-1β | Forward | GAGGCGCTTTGAAGTGTTTG | NM_001172699 |
| Reverse | GAACACCGGTAGGTGATGAAG | ||
| Probe | CAGCACCTCGCACTCCTCAATCTC | ||
| PRDM16 | Forward | TCGGAAATCAGAAACTTTATTGCC | NM_199454 |
| Reverse | TCCTGCTTCTCACTGGCTA | ||
| Probe | TCTCTGTTCGCGTTGATGCTTGGT | ||
| SIRT1 | Forward | GTTTCATGATAGCAAGCGGTTC | NM_001142498 |
| Reverse | GTCATGGTTCCTTTGCAACAG | ||
| Probe | CTCGATGTCCTAGGTGCCCAGC | ||
| UCP1 | Forward | GAGGAGTGGCAGTATTCATTGG | NM_021833 |
| Reverse | CCGTGTAGCGAGGTTTGATT | ||
| Probe | TTCAAGCACAGAGCCATCTCCACG |
National Center for Biotechnology Information (NCBI), Adipose triglyceride lipase (ATGL), Carnitine palmitoyltransferase 1B (CPT-1B), carbohydrate response element binding protein (alpha+beta, ChReBP), ChREBP beta (ChREBPβ), glucose transporter type 4 (GLUT4), peroxisome proliferator-activated receptor gamma coactivator 1-alpha/beta(PGC-1α/β), PR domain containing protein (PRDM16), sirtuin 1 (SIRT1), uncoupling protein 1 (UCP1)
Western Blots
Cells from three donors treated with naringenin at 10μM for six days or untreated controls were lysed in RIPA buffer containing a cocktail of protease and phosphatase. TGX SDS-PAGE gels (Any Kd™, BioRad) were used to separate 50μg of solubilized protein per sample. Following transfer, nitrocellulose membranes were probed overnight at 4°C with primary antibodies against UCP1 (#MAB6158, R&D Systems), GLUT4 (#sc-53566, Santa Cruz), ChREBP (#sc515922, Santa Cruz), AMPK (#2535S, Cell Signaling Technology, CST), p-AMPK (T-172, #2532S, CST), and β-Actin (A5316, Sigma). HRP-linked anti-rabbit (12–348, Sigma) and anti-mouse (AP130P, Sigma) were used to detect specific antibody-antigen complexes. Proteins were visualized by chemiluminescence (Western Lightning Plus-ECL, PerkinElmer, Waltham, MA). Densitometric analyses were performed using Image J software (National Institutes of Health) ) and the relative expression of the target protein versus β-actin was calculated.
Mitochondrial Bioenergetics
To study mitochondrial bioenergetics in human white adiopocytes, a SeaHorse XF24 Extracellular Flux Analyzer (SeaHorse Bioscience, Billerica, MA) was used. The human adipose-derived stem cells were seeded onto the SeaHorse XF24 V7 plate. The protocol for evaluating the bioenergetics parameters of the adipocytes is previously described (20). Briefly, sequential injections of oligomycin (2 μM) to inhibit ATP synthase, carbonyl cyanide-4(trifluoromethoxy) phenyhydrazone (FCCP, 2 μM), to uncouple electron transport and allow for maximum electron flux through the electron transport chain, and antimycin A (1.5 μg/mL), to inhibit complex III were used to determine oxygen consumption rate (OCR). Basal OCR is measured before any injections. The difference between basal OCR and respiration that results when Oligomycin is injected reflects ATP-linked OCR. Following FCCP injection, maximal OCR is measured, and the difference between this value and basal OCR reflects the reserve capacity. Non-mitochondrial OCR is measured after injecting Antimycin A and the difference between this value and OCR that results from inhibition of ATP synthase by Oligomycin is the basal proton leak.
Cells were treated every other day over five days with naringenin 8 μM, rosiglitazone 0.1 μM, GW 7647 0.3 μM or were untreated controls. Prior to the assay on Day 6, 625 μl/well serum-free SeaHorse XF assay media, containing 5mM pyruvate was loaded onto the plates. Plates were equilibrated in a non-CO2 incubator at 37 ºC for 20 minutes before obtaining the bioenergetics profiles. Experiments included four plates (4–8 replicates) with naringenin, rosiglitazone and control as the treatments, and two plates also included GW 7647 as a treatment.
Statistical Analysis
A general linear model was used to perform analysis of variance (ANOVA). The primary outcomes which were differences from the control were analyzed after Welch’s test of homogeneity of variances. The assumption of normality was assessed using the Shapiro-Wilks test. For data that were not normally distributed, statistical significance was verified by logarithm or square root transformations or by non-parametric analysis (Wilcoxon rank-sum test). For the seahorse data, a general linear model was used to perform ANOVA to assess the statistical significance of differences among the four treatments (naringenin, rosiglitazone, GW 7647 and control) after accounting for statistical variability in the respective outcomes that is attributable to plates. Six separate models were analyzed with each of the six outcomes (basal, ATP-linked, maximal, reserve, proton-linked and non-mitochondrial OCR) taken in turn as the dependent variable and as a linear function of models containing the explanatory variables (treatment, plates nested within treatments and wells nested within plates). The magnitude of the component of outcome variance attributable to plates was tested against the alternative of being significantly different from zero and, if there was strong evidence in favor of the alternative, the mean square for plates within treatments was used as a denominator in testing for treatment differences. If there was not strong evidence in favor of a significant component of variability attributable to plates, the mean square for wells within plates was used as a denominator in testing for treatment differences. Statistical significance of specific pairwise differences between treatments was tested by formulating specific contrasts among the four treatments. Significance was set at p < 0.05. Outcomes are summarized as means ± SE. All analyses were performed using SAS 9.4 (SAS Institute, Cary NC).
Results
Naringenin Promotes Thermogenic Gene expression in Human Adipocytes
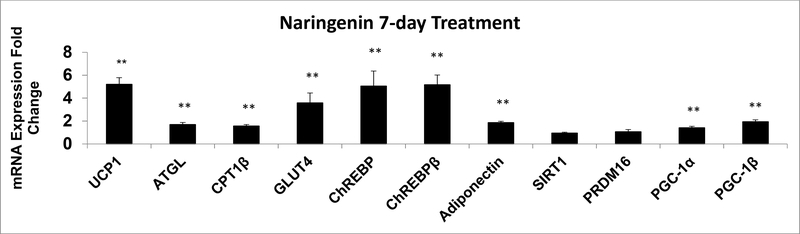
As shown in Figure 1, naringenin treatment increased mRNA levels of UCP1,PGC-1α and PGC-1β. PGCs can mediate PPARγ-dependent transcriptional responses and are involved in mitochondrial biogenesis and uncoupling. PGC-1α and PGC-1β can exhibit different regulation patterns (21). Although the induction of PGC-1α was small (40% over control), the regulation was statistically significant (p = 0.013).
Figure 1. qRT-PCR in Human Adipocytes Treated with Naringenin.
qRT-PCR assays for mRNA expression conducted in duplicates following naringenin treatment of hADSC for seven days compared to untreated hADSC (Control): uncoupling protein 1 (UCP1, n = 26), adipose triglyceride lipase (ATGL, n = 12), carnitine palmitoyltransferase 1β (n = 16), glucose transporter type 4 (GLUT4, n = 16), carbohydrate response element binding protein α + β (ChREBP, n = 12), ChREBP beta isoform (ChREBPβ, n = 12), adiponectin (n = 12), sirtuin 1(SIRT1, n = 4), PR domain containing 16 (PRDM 16, n = 12), peroxisome proliferator-activated receptor gamma coactivator-1alpha/beta (PGC-1α/β, n = 12). The results are presented as mean ± SE, **p < 0.01 compared to respective controls. Each experiment was conducted with four samples except for measurement of UCP1 mRNA that had four or six samples.
Fat mobilization in adipose tissue is effected by ATGL (22). We observed that ATGL mRNA increased with naringenin treatment. Expression of CPT1β, which transports fatty acids into the mitochondria, was increased by 55% over control but this regulation was statistically significant (p <0.01). The mRNA expression of genes associated with insulin sensitivity GLUT4, ChREBP (which reflects α + β), ChREBPβ and adiponectin increased in the naringenin treated adipocytes (p <0.01). Although SIRT1 activity increases the thermogenic capacity of white adipose tissue by mediating PRDM16 driven activation of thermogenesis (23), we did not observe any changes in the mRNA expression of SIRT1 or PRDM16 (Figure 1).
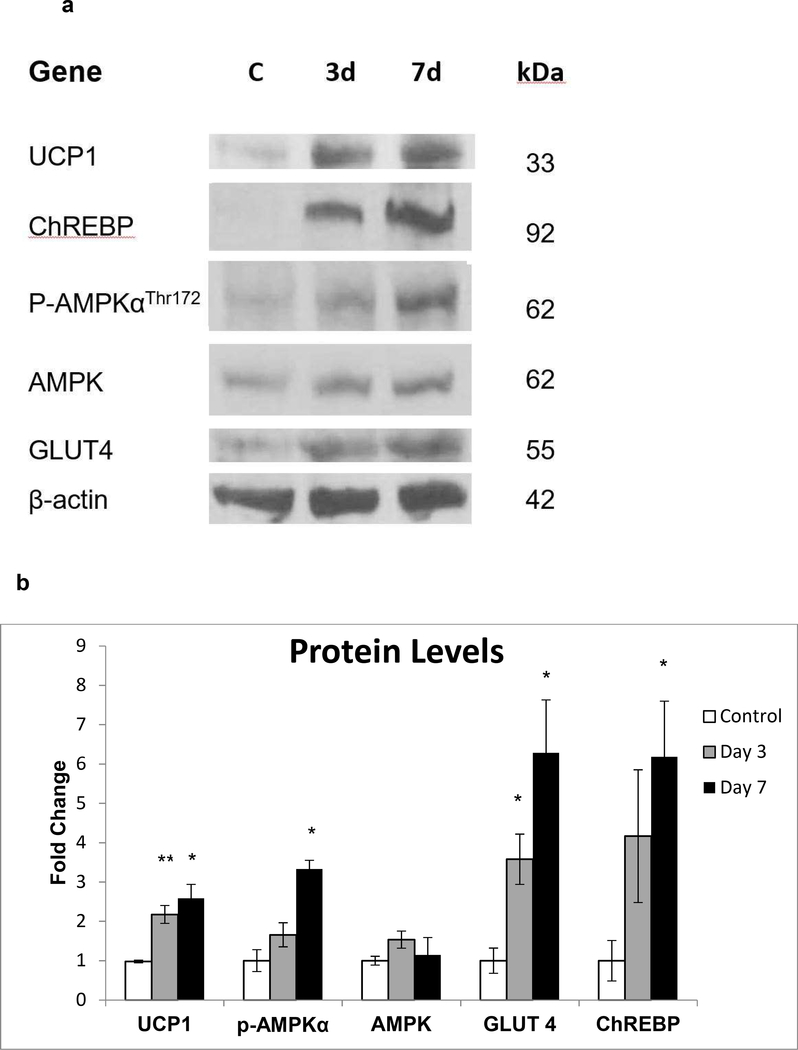
Chronic AMPK activation is involved in the remodeling of adipose tissue through upregulation of pathways that favor energy dissipation (24). In hADSC, naringenin treatment increased protein expression of UCP1 (3 day: p < 0.01, 7 day: p = 0.01), GLUT4 (3 day and 7 day: p = 0.02), ChREBP (7 day: p = 0.02) and phosphorylation of AMPK (7 day: p = 0.02) (Figure 2).
Figure 2. Immunoblotting in Human Adipocytes Treated with Naringenin.
Human adipocytes from three donors were treated with naringenin for three or seven days. (a) Cellular protein was extracted and Western blot analysis was used to detect protein levels of UCP1, GLUT4, ChREBP, and phosphorylation levels of AMP-activated protein kinase (AMPK) in the untreated control (C) after three days (3d) and seven days (7d) of naringenin treatment. (b) Densitometric analysis
In Primary Adipose Tissue the Response to Naringenin Varies Among Subjects
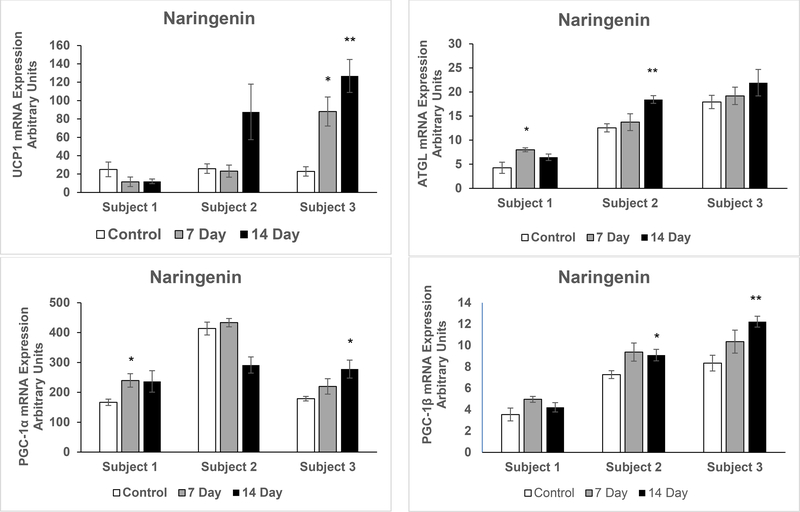
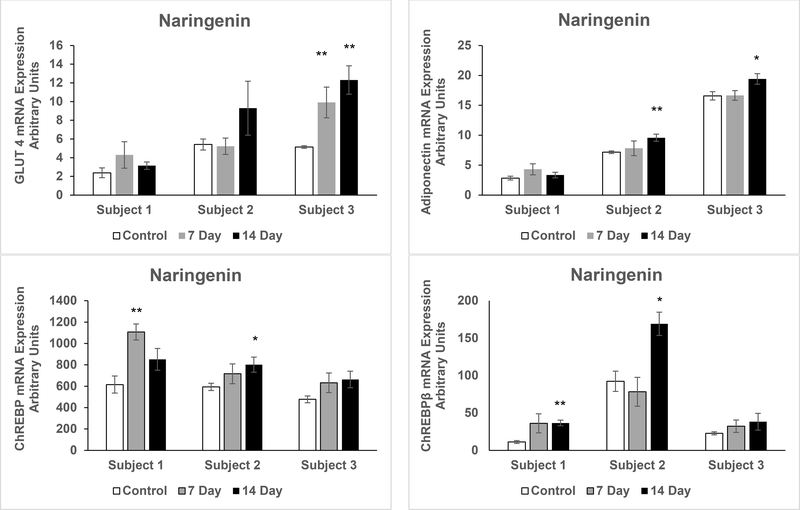
To more closely model the response of adult white adipose tissue in vivo, pWAT was maintained in organ culture with exposure to vehicle or naringenin for seven or 14 days. There were marked differences in the response to naringenin treatment in the adipose tissues from the three different subjects. In Subject 1, mRNA expression of UCP1 did not increase. In Subject 2, there was a substantial increase in UCP1 but the variability among the samples precluded attainment of statistical significance. However, in Subject 3 the increase in UCP1 expression was statistically significant at both seven and 14 days (p = 0.008 and p = 0.001 respectively) of naringenin treatment. The expression of GLUT4 followed a similar pattern (p = 0.03 and p = 0.003 at seven and 14 days respectively). Although ChREBP is expressed in brown adipose tissue, induction of UCP1 was not accompanied by increased expression of ChREBPβ in subject 3. ChREBPβ expression increased in subjects 1 and 2 (p = 0.002 and p = 0.013 respectively) at 14 days of naringenin treatment. In pWAT of subjects 1 and 3 PGC-1α increased at days 7 and 14 respectively (p =0.04 and p = 0.02) whereas PGC-1β increased at 14 days in subjects 2 and 3 (p = 0.03 and p = 0.005 respectively). In pWAT of subjects 1 and 2 ATGL increased at days 7 and 14 respectively (p =0.02 and p = 0.003) and adiponectin increased at 14 days in pWAT of subjects 2 and 3 (p = 0.008 and p = 0.005 respectively (Figure 3).
Figure 3. qRT-PCR in Primary Human Adipose Tissue Treated with Naringenin.
qRT-PCR assays for mRNA expression conducted in duplicates following naringenin treatment of quadruplicate pWAT samples, from three overweight female donors, for seven and 14 days compared to untreated pWAT (Control): a) uncoupling protein 1 (UCP1) b) adipose triglyceride lipase (ATGL) c) peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) d) PGC-1 beta (PGC-1β) e) glucose transporter type 4 (GLUT4) e) Adiponectin f) carbohydrate response element binding protein α + β (ChREBP) g) ChREBP beta isoform (ChREBPβ), **p < 0.01. The results are presented as mean ± SE, *p < 0.05 compared to respective control.
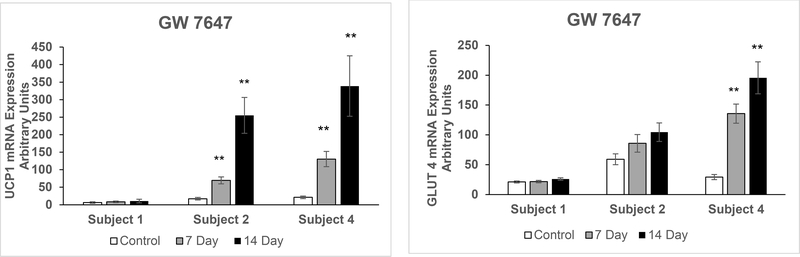
In pWAT from three subjects (including subjects 1, 2, and 4) we examined the mRNA expression of UCP1 and GLUT4 following treatment with the pharmacologic PPARα agonist GW 7647. Similar to the naringenin treatment, in pWAT from the subject 1 there was no increase in the mRNA expression of UCP1 and GLUT4, whereas in pWAT from subject 2 there was a significant increase in UCP1 (p = 0.003 and p = 0.004 at 7 and 14 days respectively) and substantial increase in GLUT4, but this regulation was not statistically significant (Figure 4).
Figure 4. qRT-PCR in Primary Human Adipose Tissue Treated with GW 7647.
qRT-PCR assays for mRNA expression conducted in duplicates following GW 7647 (pharmacologic PPARα agonist) treatment of quadruplicate pWAT samples, from three overweight female donors, for seven and 14 days compared to untreated pWAT (Control): a) uncoupling protein 1 (UCP1) b) glucose transporter type 4 (GLUT4). The results are presented as mean ± SE, **p < 0.01 compared to respective control.
Naringenin Increases Energy Expenditure
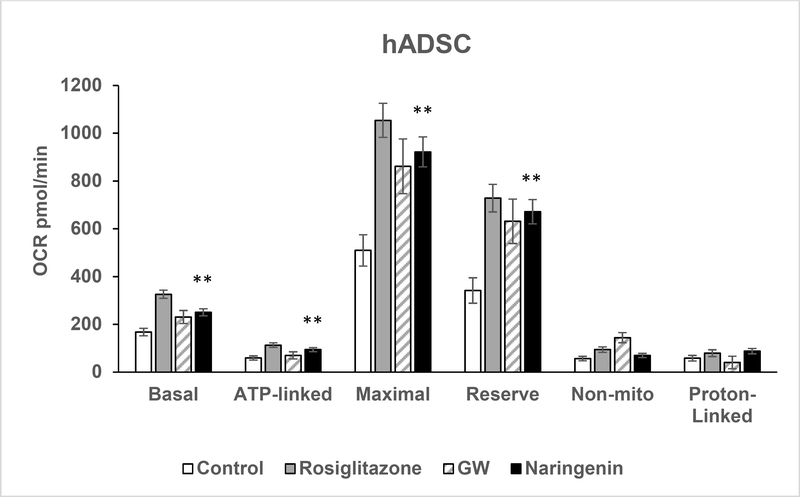
As shown in Figure 5, naringenin increased basal, ATP-linked, maximal, and reserve OCR in intact adipocytes compared to controls (p < 0.01). Basal proton linked OCR and nonmitochondrial OCR were not significantly different between vehicle and treatment groups. ATPlinked, maximal, reserve and proton-linked respiration in human adipocytes exposed to naringenin was similar to the positive controls, rosiglitazone and GW 7647 (p > 0.05).
Figure 5. Oxygen Consumption Rate in Human Adipocytes Treated with Naringenin.
Oxygen consumption rate (OCR) was measured in mature human adipocytes that were grown in four SeaHorse XF24 V7 plates. Cells were treated for six days with naringenin, rosiglitazone, GW 7647 or were untreated controls. Measurements include basal OCR, ATP⁰ linked OCR after inhibition of ATP synthase by oligomycin, maximal respiratory rate and reserve capacity after artificial uncoupling by FCCP, non-mitochondrial respiration after inhibition of complex III by antimycin A, and proton-linked OCR. Basal, ATP-linked, maximal and reserve OCR were significantly different between the naringenin-treated cells and the untreated controls. ATP-linked, maximal, reserve, and proton-linked OCR were not significantly different (p > 0.05) between naringenin and the positive controls rosiglitazone and GW 7647. The results are presented as mean ± SE, **p < 0.01 compared to control.
Discussion
Pharmacologic browning of fat correlates with resistance to high-fat diet induced obesity and insulin resistance. In our study we demonstrated an increase in the mRNA and protein expression of UCP1, GLUT4, and ChREBP, the genes involved in thermogenesis and insulin sensitivity, when hADSC were treated with naringenin at concentrations that are physiologically attainable in humans (18). Naringenin also stimulated mRNA expression of PGC-1 α and β (nuclear receptor co-activators involved in thermogenesis), ATGL and CPT1β (key enzymes necessary for fat oxidation), and adiponectin (insulin-sensitizing adipokine), in addition to increasing OCR.
In pWAT maintained ex vivo, the response was varied among the adipose tissue obtained from three overweight female subjects. UCP1 and GLUT4 expression significantly increased in one of the three subjects which was accompanied by an increase in the expression of PGC-1α and PGC-1β. However, expression of ChREBP and ChREBPβ increased in pWAT of the subjects that did not manifest an increase in UCP1 and GLUT4. Interestingly, the lack of any change in UCP1 and GLUT4 expression in response to naringenin treatment in pWAT of subject 1 was also seen when this pWAT was treated with GW 7647, a potent pharmacologic PPARα agonist. In human adipocytes PPARα upregulates the expression of the genes involved in lipid metabolism and increases maximal OCR (25). Since naringenin activates PPARα (10), it is likely that PPARα plays an important role in the thermogenic effect of naringenin but some humans may not be responders to this pathway. However, the notion bears investigation with larger sample sizes.
Browning of human adipocytes is induced by pharmacologic PPARα and γ agonists, GW 7647 and rosiglitazone respectively, through activation of a comprehensive gene program that leads to increased mitochondrial function and energy consumption (19, 26, 27). Naringenin is an activator of PPARα and γ (10). In mice, naringenin has been shown to increase energy expenditure, upregulate UCP1 in brown fat, and promote the induction of fat oxidation (12, 28). Our in vitro studies in human adipocytes suggest that naringenin may induce a browning of human white adipose tissue through an upregulation of UCP1 (Figures 1 – 3) and an increase in OCR (Figure 5). Additionally, the expression of the PPARγ co-activators PGC1α and β were stimulated in human adipocytes and pWAT. Both members of the PGC1 family are important for mitochondrial oxidative metabolism, although differences in their interactions with other transcription factors mediate differences in their functional specificity (29).
The relevance of increased energy consumption induced by browning to human physiology may be debatable, but its effects on glucose metabolism have physiologic significance. In humans, approximately 70 ml of brown fat can clear 20–25 g of glucose from circulation in 24 hours (30). Our in vitro studies in human adipocytes clearly demonstrate that naringenin increases expression of ChREBP, ChREBPβ, and GLUT4. In white adipose tissue, the transcriptional targets of ChREBP include the genes involved in adipocyte differentiation, lipid metabolism, and browning (6). Mice with adipose-specific GLUT4 overexpression display improved glucose control, and upregulation of ChREBP expression (4). In humans grouped according to their two-hour blood glucose concentrations, the expression of GLUT4 and ChREBP measured in subcutaneous fat biopsies decreased as blood glucose concentrations rose across the groups (5). Interestingly, expression of adipose ChREBP correlated positively with expression of GLUT4 and insulin sensitivity (5). Although the study was done in obese adolescents, it provides preliminary evidence that in humans, ChREBP, ChREBPβ, and GLUT4 gene expression correlate with physiologic outcomes related to glucose homeostasis. Secretion of adipokines such as adiponectin is another key role of adipose tissue that contributes to whole body insulin sensitivity (31). Thiazolidinediones (TZDs) are PPARγ agonists that increase insulin sensitivity at least in part through an increase in adiponectin secretion (32). In the promoter region of the human adiponectin gene a PPAR-responsive element has been identified and is thought to be the mechanism by which TZDs increase plasma adiponectin. (33) In our study, naringenin treatment increased adiponectin mRNA expression suggesting a role for naringenin in protecting against insulin resistance.
Shortage of cellular energy stores marked by an increase in AMP levels and a decline in ATP levels activates AMPK which then initiates cellular programs that increase catabolism, mostly by enhancing oxidative metabolism and mitochondrial biogenesis (34). Activation of AMPK increases UCP1 and induces an accumulation of brown adipocytes in murine WAT (35). Naringenin promotes glucose homeostasis in animal models through activation of AMPK signaling in the liver, skeletal muscle, and in macrophages differentiated from the human cell line THP-1 (36). In our study, activation of AMPK was stimulated in human adipocytes treated with naringenin (Figure 2). Consistent with the activation of AMPK and increase in catabolism, our studies show that naringenin increases mRNA expression of ATGL in hADSC and pWAT. Catabolism of fatty acids from triglyceride stores is the function of lipolytic enzymes, particularly ATGL which catalyzes the initial step in triglyceride hydrolysis (22).
The distinctive function of brown adipocytes is thermogenic energy expenditure, propelled by the catabolic breakdown of lipids. Therefore, to distinguish between white adipocytes and those that have potentially acquired a brown/beige phenotype, we extended our characterization to the mitochondrial function of naringenin-treated adipocytes by analyzing OCR. Basal OCR and ATP-linked OCR were higher in the naringenin-treated cells compared to the control or untreated cells. To functionally determine browning in hADS, higher substrate oxidation capacity than controls must be demonstrated as would be reflected by substantial increases in maximal respiration rate. Following sequential injection of compounds that modulate mitochondrial function, naringenin increased maximal respiratory capacity and this increase was not different from the positive controls rosiglitazone and GW 7647. However, induction of UCP1 following treatment with naringenin is by far lower than that of rosiglitazone, in the adipocytes (37).Therefore, the increase in energy expenditure may not be completely mediated by UCP1. Proton leak was not significantly different between naringenin and control. UCP1 is not inherently active in the mitochondria and is activated by fatty acids. In intact cells, proton leak represents basal uncoupled respiration. In rodent adipocytes, injecting isoproterenol during the OCR measurement stimulates an adrenergic response and UCP1-mediated proton leak can be determined (38). However, in human adipocytes from obese individuals the response to βadrenergic stimulation by isoproterenol is impaired (39).
Conclusions
Robust data from rodent studies and in vitro models demonstrate that naringenin increases energy expenditure and improves glucose metabolism. Although our study in hADSC and pWAT builds upon this data, it demonstrates that the response to naringenin may differ among human subjects based on their response to pathways that activate energy expenditure. The results warrant further investigation to determine their clinical relevance.
What is known about this subject?
Naringenin, a citrus flavonoid, increases insulin sensitivity and improves glucose and lipid metabolism in animal and in vitro models.
Naringenin activates brown fat and increases energy expenditure in mice.
The improvements in glucose and lipid metabolism have been attributed to the antioxidant effects of naringenin, increase in glucose uptake in muscle, reduction in hepatic glucose production, and lowering of plasma lipids.
What does this study add?
In humans, naringenin is a potential therapeutic agent targeting energy expenditure.
Our study builds upon robust data from rodent studies showing that naringenin improves glucose metabolism, but proposes for the first time adipose tissue as the site of mechanistic action in humans.
Our study demonstrates that the response to naringenin may differ among human subjects based on their response to pathways that activate energy expenditure.
Acknowledgements
This publication was supported in part by the National Center For Complementary & Integrative Health and the Office of Dietary Supplements of the National Institutes of Health under Award Number P50AT002776 which funds the Botanical Dietary Supplements Research Center of Pennington Biomedical Research Center, in part on work that was supported by the National Institutes of Health under Award Number T32 A T004094 and in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health which funds the Louisiana Clinical and Translational Science Center. This work utilized the facilities of the Genomics Core that are supported in part by COBRE (NIH8 1P30GM118430–01) and NORC (P30DK072476) center grants from the National Institutes of Health.
Footnotes
Disclosure: The authors declare no conflict of interest
References:
- 1.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013;19: 1252–1263. [DOI] [PubMed] [Google Scholar]
- 2.Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 2011;96: 192–199. [DOI] [PubMed] [Google Scholar]
- 3.Eissing L, Scherer T, Todter K, Knippschild U, Greve JW, Buurman WA, et al. De novo lipogenesis in human fat and liver is linked to ChREBP-beta and metabolic health. Nat Commun 2013;4: 1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herman MA, Peroni OD, Villoria J, Schon MR, Abumrad NA, Bluher M, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012;484: 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kursawe R, Caprio S, Giannini C, Narayan D, Lin A, D’Adamo E, et al. Decreased transcription of ChREBP-alpha/beta isoforms in abdominal subcutaneous adipose tissue of obese adolescents with prediabetes or early type 2 diabetes: associations with insulin resistance and hyperglycemia. Diabetes 2013;62: 837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuotio-Antar AM, Poungvarin N, Li M, Schupp M, Mohammad M, Gerard S, et al. FABP4-Cre Mediated Expression of Constitutively Active ChREBP Protects Against Obesity, Fatty Liver, and Insulin Resistance. Endocrinology 2015;156: 4020–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decara J, Arrabal S, Beiroa D, Rivera P, Vargas A, Serrano A, et al. Antiobesity efficacy of GLP-1 receptor agonist liraglutide is associated with peripheral tissue-specific modulation of lipid metabolic regulators. Biofactors 2016;42: 600–611. [DOI] [PubMed] [Google Scholar]
- 8.Zygmunt K, Faubert B, MacNeil J, Tsiani E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochemical and biophysical research communications 2010;398: 178–183. [DOI] [PubMed] [Google Scholar]
- 9.Kannappan S, Anuradha CV. Naringenin enhances insulin-stimulated tyrosine phosphorylation and improves the cellular actions of insulin in a dietary model of metabolic syndrome. Eur J Nutr 2010;49: 101–109. [DOI] [PubMed] [Google Scholar]
- 10.Goldwasser J, Cohen PY, Yang E, Balaguer P, Yarmush ML, Nahmias Y. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: role of PPARalpha, PPARgamma and LXRalpha. PLoS One 2010;5: e12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharya S, Oksbjerg N, Young JF, Jeppesen PB. Caffeic acid, naringenin and quercetin enhance glucose-stimulated insulin secretion and glucose sensitivity in INS-1E cells. Diabetes Obes Metab 2014;16: 602–612. [DOI] [PubMed] [Google Scholar]
- 12.Thaiss CA, Itav S, Rothschild D, Meijer M, Levy M, Moresi C, et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016. [DOI] [PubMed] [Google Scholar]
- 13.Mulvihill EE, Burke AC, Huff MW. Citrus Flavonoids as Regulators of Lipoprotein Metabolism and Atherosclerosis. Annu Rev Nutr 2016;36: 275–299. [DOI] [PubMed] [Google Scholar]
- 14.Kiani J, Imam SZ. Medicinal importance of grapefruit juice and its interaction with various drugs. Nutr J 2007;6: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards DJ, Bernier SM. Naringin and naringenin are not the primary CYP3A inhibitors in grapefruit juice. Life Sci 1996;59: 1025–1030. [DOI] [PubMed] [Google Scholar]
- 16.Bailey DG, Kreeft JH, Munoz C, Freeman DJ, Bend JR. Grapefruit juice-felodipine interaction: effect of naringin and 6’,7’-dihydroxybergamottin in humans. Clin Pharmacol Ther 1998;64: 248–256. [DOI] [PubMed] [Google Scholar]
- 17.Ho PC, Saville DJ, Wanwimolruk S. Inhibition of human CYP3A4 activity by grapefruit flavonoids, furanocoumarins and related compounds. J Pharm Pharm Sci 2001;4: 217–227. [PubMed] [Google Scholar]
- 18.Kanaze FI, Bounartzi MI, Georgarakis M, Niopas I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur J Clin Nutr 2007;61: 472–477. [DOI] [PubMed] [Google Scholar]
- 19.Elabd C, Chiellini C, Carmona M, Galitzky J, Cochet O, Petersen R, et al. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells 2009;27: 2753–2760. [DOI] [PubMed] [Google Scholar]
- 20.Dranka BP, Benavides GA, Diers AR, Giordano S, Zelickson BR, Reily C, et al. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic Biol Med 2011;51: 1621–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng T, Sieglaff DH, Zhang A, Lyon CJ, Ayers SD, Cvoro A, et al. A peroxisome proliferator-activated receptor gamma (PPARgamma)/PPARgamma coactivator 1beta autoregulatory loop in adipocyte mitochondrial function. J Biol Chem 2011;286: 3072330731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004;306: 1383–1386. [DOI] [PubMed] [Google Scholar]
- 23.Jokinen R, Pirnes-Karhu S, Pietilainen KH, Pirinen E. Adipose tissue NAD(+)homeostasis, sirtuins and poly(ADP-ribose) polymerases -important players in mitochondrial metabolism and metabolic health. Redox biology 2017;12: 246–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vila-Bedmar R, Lorenzo M, Fernandez-Veledo S. Adenosine 5’-monophosphateactivated protein kinase-mammalian target of rapamycin cross talk regulates brown adipocyte differentiation. Endocrinology 2010;151: 980–992. [DOI] [PubMed] [Google Scholar]
- 25.Barquissau V, Beuzelin D, Pisani DF, Beranger GE, Mairal A, Montagner A, et al. Whiteto-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Mol Metab 2016;5: 352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pisani DF, Djedaini M, Beranger GE, Elabd C, Scheideler M, Ailhaud G, et al. Differentiation of Human Adipose-Derived Stem Cells into “Brite” (Brown-in-White) Adipocytes. Front Endocrinol (Lausanne) 2011;2: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loft A, Forss I, Siersbaek MS, Schmidt SF, Larsen AS, Madsen JG, et al. Browning of human adipocytes requires KLF11 and reprogramming of PPARgamma superenhancers. Genes Dev 2015;29: 7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulvihill EE, Allister EM, Sutherland BG, Telford DE, Sawyez CG, Edwards JY, et al. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes 2009;58: 2198–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 2008;88: 611–638. [DOI] [PubMed] [Google Scholar]
- 30.Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014;63: 4089–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arioglu E, Rother KI, Reitman ML, Premkumar A, Taylor SI. Lipoatrophy syndromes: when ‘too little fat’ is a clinical problem. Pediatr Diabetes 2000;1: 155–168. [DOI] [PubMed] [Google Scholar]
- 32.Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, et al. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology 2002;143: 998–1007. [DOI] [PubMed] [Google Scholar]
- 33.Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 2003;52: 1655–1663. [DOI] [PubMed] [Google Scholar]
- 34.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A 2002;99: 15983–15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384: 766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nyane NA, Tlaila TB, Malefane TG, Ndwandwe DE, Owira PMO. Metformin-like antidiabetic, cardio-protective and non-glycemic effects of naringenin: Molecular and pharmacological insights. Eur J Pharmacol 2017;803: 103–111. [DOI] [PubMed] [Google Scholar]
- 37.Rebello CJ, Greenway FL, Johnson WD, Ribnicky D, Poulev A, Stadler K, et al. Fucoxanthin and Its Metabolite Fucoxanthinol Do Not Induce Browning in Human Adipocytes. J Agric Food Chem 2017;65: 10915–10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Fromme T, Schweizer S, Schottl T, Klingenspor M. Taking control over intracellular fatty acid levels is essential for the analysis of thermogenic function in cultured primary brown and brite/beige adipocytes. EMBO Rep 2014;15: 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yehuda-Shnaidman E, Buehrer B, Pi J, Kumar N, Collins S. Acute stimulation of white adipocyte respiration by PKA-induced lipolysis. Diabetes 2010;59: 2474–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]