Abstract
Background:
TNM staging in thyroid carcinoma is important for assessing prognosis. However, patients with stage III or IV disease have overall survival of 90%.. The change of 55 years age cut off will create stage migration, and many patients will be down staged.
Methods:
We reviewed our database of 3,650 patients to analyze the impact of the new AJCC staging system. There were 994 males (27%) and 2,656 females (73%). The median age was 46. Patients were staged using both 7th and 8th editions, with cut off of 55 years and new definitions of T3 and T4, and nodal staging.
Results:
Of 3,650 patients, 1,057 (29%) were downstaged. 104 (10%) from stage IV to I, 109 (10%) from stage IV to stage II, and 68 (6%) to stage III. 218 (21%) were downstaged from stage III to I, and 347 (33%) from stage III to stage II. 211 (20%) were downstaged from stage II to I. The overall disease-specific and relapse-free survival was analyzed and showed better stratification with new staging system.
Conclusions:
The new staging system reflects more appropriately the biology of thyroid cancer and will have significant impact on the management of thyroid cancer.
Keywords: Thyroid cancer, stage migration, AJCC staging system, prognostic factors
INTRODUCTION
The incidence of thyroid cancer is rapidly rising. Over the last 25 years, the annual incidence of thyroid cancer has almost quadrupled in the United States, increasing from approximately 8,000 patients to 54,000 patients1. Interestingly, the majority of the rise is directly related to micro carcinomas. However, the mortality in thyroid cancer has remained relatively stable over the past 20 years2. Thyroid cancer continues to be a unique human neoplasm, where selection of therapy and the outcomes are dependent on prognostic factors and risk group analysis, which are very critical in the evaluation and management of thyroid cancer and include: age, histology, extrathyroidal extension, size of the tumor, and distant metastases. These prognostic factors are repeatedly shown to be the same in separate data sets reported from the Mayo Clinic3, Lahey Clinic4, European Organisation for Research and Treatment of Cancer (EORTC)5, and Memorial Sloan Kettering Cancer Center (MSK)6. Each one of these institutions analyzed their large number of patients with thyroid cancer and defined various prognostic factors, which have helped us to group patients into low, intermediate, and high-risk groups. The 10 year disease-specific survival (DSS) in the low risk group is over 99%, in the intermediate risk group it is 96%−97%, and in the high-risk group, it drops to 78%6.
STAGING OF THYROID CANCER
Staging is very important in evaluation of thyroid cancer. There are a variety of staging systems. However, all around the world, the American Joint Committee on Cancer (AJCC) tumor, node, and metastasis (TNM) system has become very popular and is essentially an initial clinical staging system, labeled as cTNM. There are a variety of other staging systems, including pathological staging (pTNM), recurrence staging (rTNM), etc. The goal of a staging system is to define the prognostic groups, and develop treatment philosophies based on stage groupings. Traditionally, 4 stages have been described in all human cancers. These staging systems are used to compare data and develop treatment philosophies. Most of the time, the stage I and stage II cancers are treated with unimodal treatment, since they are considered to be early cancers with excellent outcome. However, stage III and stage IV cancers have a drop in survival by almost 40%–50%, frequently requiring multimodal therapy. In individual tumor types, the staging system is different.
The staging system for thyroid cancer is unique. It is interesting that age has been considered as an important prognostic factor; this is the only human cancer where age at diagnosis is an independent prognostic factor. In the previous staging systems, the age of 45 was used as a cut-off. There was data available from EORTC5, Mayo Clinic3, Lahey Clinic4, and MSK6, which used the age of 45 as a cut-off, into low and high risk groups, along with other factors. There was no stage III or stage IV for patients below the age of 45, since the mortality was low in this group. Over a period of time, data from all around the world has accumulated, and Nixon et al. reviewed the data from MSK and validated it in a multi institutional study, defining age 55 as a better cutoff than age 457. These data led to the change to age 55 in the 8th edition staging for thyroid cancer. More recently, there have been further analyses done which show that age as a continuum may be a more appropriate prognostic variable8. However, at the present time, it is not possible to design a staging system, which employs age as a continuous variable; therefore, using the age cut of 55 years appears to be more appropriate for the new staging system.
MATERIALS AND METHODS
With institutional IRB approval, we retrospectively reviewed our large data base, which was prospectively collected, to analyze the impact of the new staging system on stage groupings. Our database included 3,650 patients, with detailed information on prognostic factors and treatment outcomes. There were 994 males (27%) and 2,656 females (73%). The median age was 46 years (age range 4–94 years). The interquartile age range was from 25–58 years. We staged these patients based on both the 7th and 8th edition to determine the major reclassification changes with the use of 55 years as a new cut-off age and new definitions of T3 and T4 primary tumors and nodal staging. The changes in staging system are defined in Table 1. We had robust data with detailed follow up of these patients over the past 30 years. Some of the prognostic factors and risk groups were published before by our institution6. We were interested in reviewing our staging groups and analyzed the percentage of downstaging, as per the 8th edition.
Table 1.
Major changes between 7th and 8th edition of staging systems
|
RESULTS
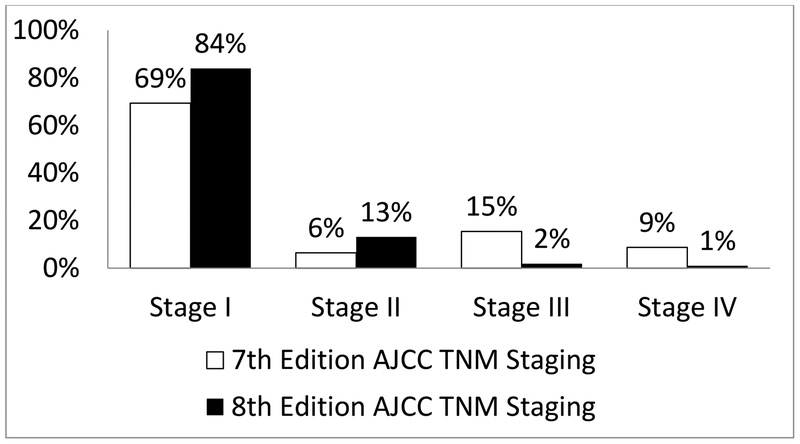
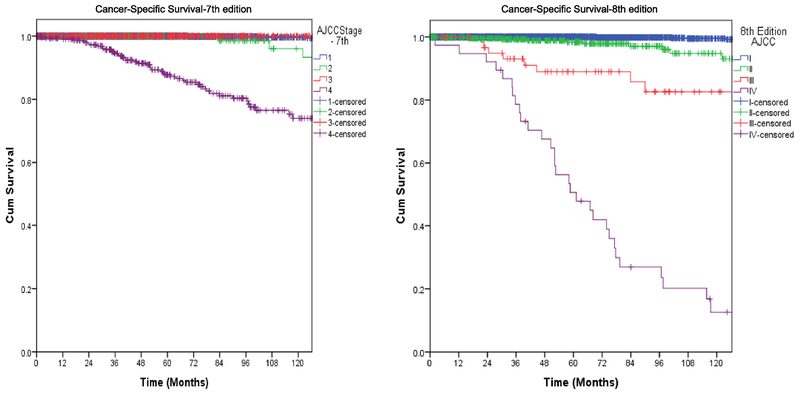
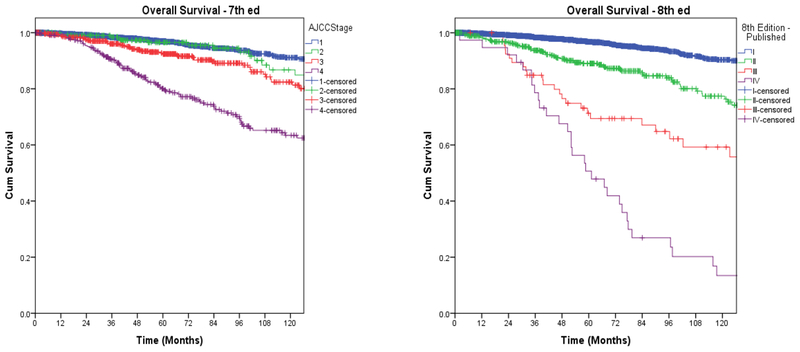
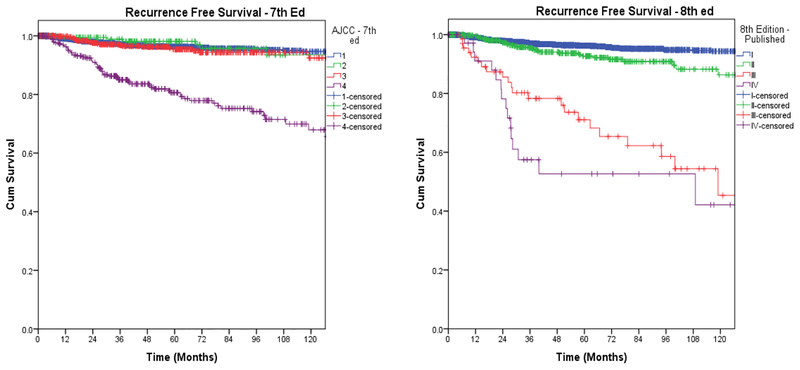
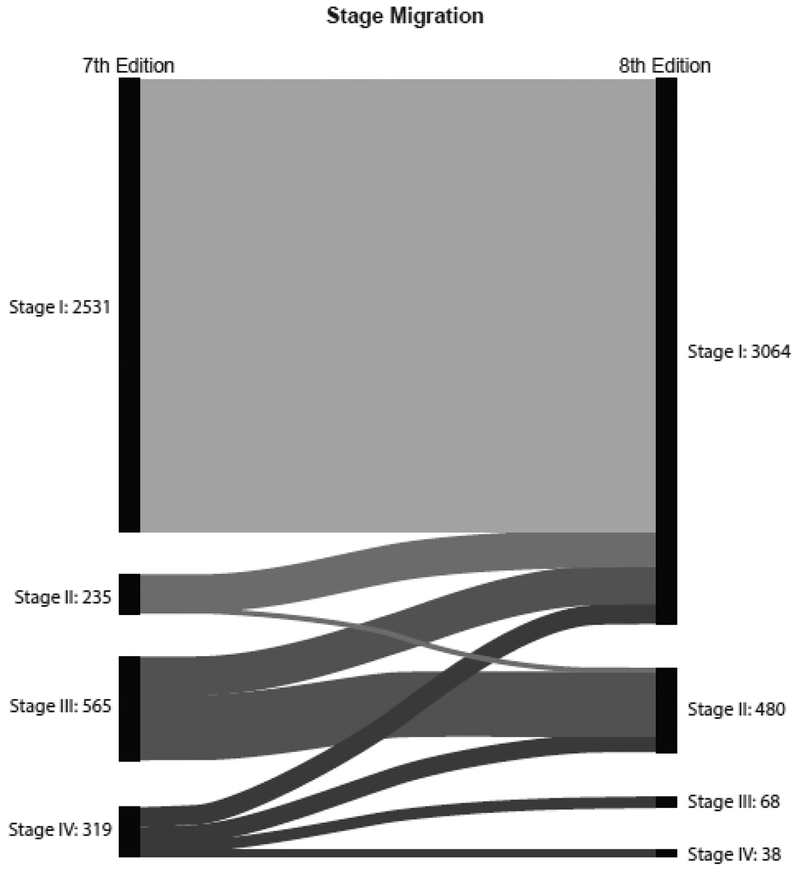
Of 3,650 patients, 1,057 (29%) were downstaged. Of 281 patients with stage IV disease, 104 (10%) were downstaged to stage I, 109 (10%) to stage II, and 68 (6%) from stage IV to stage III. Of 565 patients with stage III disease, 218 (21%) were downstaged to stage I, and 347 (37%) to stage II. 211 (20%) patients with stage II disease were downstaged to stage I. Overall, there was downstaging of 29% of patients (Figure 1 and Table 2). We also wanted to study whether this will have a direct impact on their long-term survival, and on the need for and the results of adjuvant therapy. The overall disease-specific and relapse-free survival was analyzed by both staging systems. Kaplan Meier plots for cancer specific survival (Figure 2), overall survival (Figure 3), and recurrence free survival (Figure 4) showed a more appropriate correlation and stratification with the 8th edition staging system. The data reveals that the new staging system more accurately reelected the biology of the disease with a better spread of survival curves between stage I, II, III and IV, unlike the previous staging system, which lumped stage I, II and III together, with the survival difference shown in only stage IV. Figure 5 demonstrates the stage migration in our data, based on alluvial flow diagram.
Fig. 1.
Stage grouping based on 7th and 8th editions of thyroid cancer staging.
Table 2.
Stage migration; AJCC stage (8th edition).
| I | II | III | IV | Total |
| 2531 | 0 | 0 | 0 | 2531 |
| 211 | 24 | 0 | 0 | 235 |
| 218 | 347 | 0 | 0 | 565 |
| 104 | 109 | 68 | 38 | 319 |
| 3064 | 480 | 68 | 38 | 3650 |
- 1057/3650 (29%) of patients were downstaged
- 104/1057 patients (10%) went from Stage 4 to 1
- 109/1057 patients (10%) went from stage 4 to 2
- 68/1057 patients (6%) went from stage 4 to 3
- 218/1057 patients (21%) went from stage 3 to 1
- 347/1057 patients (33%) went from Stage 3 to 2
- 211/1057 patients (20%) went from Stage 2 to 1
Fig. 2.
Cancer-specific survival based on 7th and 8th editions.
Fig. 3.
Overall survival based on 7th and 8th editions.
Fig. 4.
Recurrence-free survival based on 7th and 8th editions.
Fig. 5.
Alluvial flow diagram based on stage migration in the 7th and 8th editions.
DISCUSSION
The decisions about extent of thyroidectomy and adjuvant treatment are primarily based on the risk group stratification. Recently, the American Thyroid Association (ATA) has also emphasized the need for risk group stratification based on the tumor and patient factors. However, they have emphasized stratification for the risk of recurrence rather than long-term survival9.
The phenomenon of multifocal microscopic thyroid cancer is well known, but it has little impact on outcomes. Similarly, occult microcarcinomas are present in 6%–10% of the population in the United States, with these individuals living unimpacted with their microcarcinoma not becoming clinically evident during life. The other important factor is the presence of nodal metastasis, which has no major impact on long-term outcomes in patients who fall in the low risk category. The only negative impact of nodal metastases is observed in the high risk group of patients. These are usually older patients with large and poorly differentiated cancers.10, 11 This is a unique biological phenomenon of thyroid cancer, which is very important in the understanding and management of thyroid cancer.
Staging of cancer is important to evaluate the extent of the disease and overall prognosis and decisions regarding treatment selection. It also helps us to standardize the evaluation of cancer and compare results from different parts of the world. The history of staging system dates back to the early part of the last century from attempts by Steinthal from Germany and Halsted from the United States12. Pierre Denoix reported on the TNM factors to develop a staging system in 1945. These were adopted by the UICC in 1954, and subsequently, the AJCC and UICC worked together to develop the staging system for cancers. The first edition of staging system was published in 1977, and it was well received around the world. Clinicians and cancer registrars used this staging system routinely. The clinical staging system was defined by TNM factors. In general, patients were divided into 4 groups: stage I, II, III, and IV. The overall survival declined from stage I to stage IV. The staging system for thyroid cancer has also evolved over the past 50 years, with several changes noted in the 6th, 7th and the most recent edition. The 8th edition of the staging more accurately reflects the biology of well differentiated thyroid cancer. The staging manual was published in October 2016 and was implemented in January 2018.
In the past, age 45 was initially used as a stage grouping cut-off. This was revised to age 55, largely based on the international collaborative study reported by Nixon et al., indicating that age 55 is a better cut-off. This was endorsed by many other institutions around the world7. More recent studies by Adama and Nixon have reported that thyroid specific mortality steadily increases with increasing age proving that age as a continuum is more appropriate prognostic variable10, 13. However, the TNM staging system only allows for categorical variables; therefore age of 55 years has been deemed to be the most appropriate age cut off at present. It is possible this may change in future staging systems with the use of nomograms.
The T staging was also revised. Since microscopic extrathyroidal extension had no impact on outcomes, it is no longer used to upstage the tumor to T3. According to the new revisions, T3a is a tumor larger than 4cm, and T3b is a tumor with gross extrathyroidal extension involving the strap muscles or perithyroid soft tissues. The definition for T4 remains unchanged13. The N staging was also revised. N1 disease does not upstage the tumor to stage 3, and level VII nodes [N1B] are not considered to be stage 4.
Since its publication, there have been several studies revisiting the staging of thyroid cancer and updating the individual institutional data. Three major publications were published: 2 from Korea and a study from the National Cancer Database (NCDB), all of which showed downstaging of thyroid cancer in approximately 27%–30% of patients14,15,16. The previous 7th edition staging system showed no major survival difference between stage I, II and III. The new 8th edition staging system shows a more appropriate difference between all 4 stages; however, stage I and stage II are still almost parallel to each other. The nodal metastasis and the stage grouping are also important. In 7th edition staging system, presence of nodal metastasis in patients above the age of 45 was considered stage III. Biologically, this was not stage III, as most of the patients had cancers that behaved as stage I or stage II. This downstaging will help individualize the adjuvant treatment and overall discussion regarding prognosis in patients with thyroid cancer.
The analysis of our large database has shown the new staging system to be more appropriate and biologically sound with outcome differences between stage I–II and III–IV. This will help us understand the overall prognosis of patients with thyroid cancer and more importantly make critical decisions about adjuvant treatment, such as radioactive iodine (RAI). Kim et al. from South Korea retrospectively analyzed 3,176 patients with differentiated thyroid cancer from 1996 to 200516. Upon reclassification, 37.6% of the patients were downstaged. As a result of this, stage I and II tumors increased from 61.9% to 81.1% and from 1.7% to 16%, respectively. Stages III and IVB decreased from 27.6% to 2.3% and 0.8% to 0.5%, respectively.
Pontius et al. compared the 7th and 8th edition staging on outcomes from the database Surveillance, Epidemiology, and End Results (SEER) and NCDB14. They reported that 23% of the patients were downstaged from the 7th to 8th edition in SEER, and 24% in NCDB. They concluded that the 8th edition staging was superior for predicting survival.
Mijin Kim et al. from Assan Medical Center in South Korea reported application of both staging systems to 1,613 patients with differentiated thyroid cancer16. Their median follow-up was 11 years. With the application of the 8th edition staging system, 63% of T3 patients were downgraded to T1/T2. 38% were downstaged according to the 8th edition. They reported 10 year DSS in TNM/7 stages: I, II, III, and IV as 99.7%, 98.2%, 98.8%, and 83.2%, respectively. As per 8th edition, they reported DSS in stages I, II, III, and IV as 99.6%, 95.4%, 72.3%, and 46.6%, respectively. They concluded applying the 8th edition of the TNM staging system could improve the accuracy for predicting DSS in patients with differentiated thyroid cancer. Nixon et al. reviewed databases from 10 institutions, with a total of 9,484 patients13. They reported using age 45 as cutoff, 10 year DSS, rates for stages I to IV as 99.7%, 97.3%, 96.6%, and 76.3%, respectively. Using age 55 as the cutoff, they reported 10 year DSS as 99.5%, 94.7%, 94.1%, and 67.6%, for stages I to IV, respectively. This change resulted in 12% of patients being downstaged, and the downstaged group had a 10 year DSS of 97.6%. They concluded that the 55 year age cutoff would improve the statistical validity of the model, and such a change would be clinically relevant for a large number of patients worldwide by preventing over-staging of patients with low risk disease, while providing a more realistic estimation of prognosis for high risk patients. It is interesting that the new staging system downstages many of the tumors, unlike those reported by Feinstein et al. as Will Rogers phenomenon in stage migration in lung cancer17.
Shaha commented as an editorial on Kim et al.’s paper as paradigm shifts in staging of thyroid cancer18. He concluded that the 8th edition was based on the biology of thyroid cancer, and also commented on the new ATA guidelines as a major advance in the evaluation and management of thyroid cancer19. The new staging system is quite effective in stage grouping and relates to the overall prognosis. We are sure this new staging system will be most appreciated around the world.
Acknowledgment
The authors would like to express their sincere appreciations to: Jessica Massler for her editorial assistance, and Sue Weil Kasas for alluvial flow diagram.
Funding: This work was supported by the National Institutes of Health by the P30 Cancer Support Grant (CCSG) (P30 CA008748).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at 39th Annual Meeting of the American Association of Endocrine Surgeons in Durham, North Carolina, May 6–8, 2018
Conflict of Interest Statement
None declared.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. 2017;317(13):1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, and Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114(6):1057–1058. [PubMed] [Google Scholar]
- 4.Cady B. Hayes Martin Lecture. Our AMES is true: how an old concept still hits the mark: or, risk group assignment points the arrow to rational therapy selection in differentiated thyroid cancer. Am J Surg. 1997;174(5):462–468. [DOI] [PubMed] [Google Scholar]
- 5.Byar DP, Green SB, Dor P, Williams ED, Colon J, van Gilse HA, et al. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. thyroid cancer cooperative group. Eur J Cancer. 1979;15(8):1033–1041. [DOI] [PubMed] [Google Scholar]
- 6.Shaha A. Treatment of thyroid cancer based on risk groups. J Surg Oncol. 2006;94(8):683–691. [DOI] [PubMed] [Google Scholar]
- 7.Nixon IJ, Wang LY, Migliacci JC, Eskander A, Campbell MJ, Aniss A, et al. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid. 2016;26(3):373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adam MA, Thomas S, Hyslop T, Scheri RP, Roman SA, Sosa JA. Exploring the relationship between patient age and cancer-specific survival in papillary thyroid cancer: rethinking current staging systems. J Clin Oncol. 2016;34(36):4415–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haugen BR, Sawka AM, Alexander EK, Bible KC, Caturegli P, Doherty GM, et al. American Thyroid Association Guidelines on the management of thyroid nodules and Differentiated Thyroid Cancer Task Force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid. 2017;27(4):481–483. [DOI] [PubMed] [Google Scholar]
- 10.Adam MA, Pura J, Goffredo P, Dinan MA, Reed SD, Scheri RP, et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid carcinoma. J Clin Oncol. 2015;33(21):2370–2375. [DOI] [PubMed] [Google Scholar]
- 11.McNamara WF, Wang LY, Palmer FL, Nixon IJ, Shah JP, Patel SG, et al. Pattern of neck recurrence after lateral neck dissection for cervical metastases in papillary thyroid cancer. Surgery. 2016;159(6):1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK. Thyroid In: Amin MB, Edge SB, Greene FL, Bryd DR, et al. AJCC Cancer Staging Manual. 8th ed. Irving, TX: Springer International Publishing; 2016. [Google Scholar]
- 13.Nixon IJ, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, et al. Defining a valid age cutoff in staging of well-differentiated thyroid cancer. Ann Surg Oncol. 2016;23(2):410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim TH, Kim YN, Kim HI, Park SY, Choe JH, Kim JH, et al. Prognostic value of the eighth edition AJCC TNM classification for differentiated thyroid carcinoma. Oral Oncol. 2017;71:81–86. [DOI] [PubMed] [Google Scholar]
- 15.Pontius LN, Oyekunle TO, Thomas SM, Stang MT, Scheri RP, Roman SA, et al. Projecting survival in papillary thyroid cancer: A comparison of the seventh and eighth editions of the American Joint Commission on Cancer/Union for International Cancer Control staging systems in two contemporary national patient cohorts. Thyroid. 2017;27(11):1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim M, Kim WG, Oh HS, Park S, Kwon H, Song DE, et al. Comparison of the seventh and eighth editions of the American Joint Committee on Cancer/Union for International Cancer Control Tumor-Node Metastasis Staging system for differentiated thyroid cancer. Thyroid. 2017;27(9):1149–1155. [DOI] [PubMed] [Google Scholar]
- 17.Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312(25):1604–1608. [DOI] [PubMed] [Google Scholar]
- 18.Shaha AR, Ferlito A, Rinaldo A. Thyroid cancer: a unique neoplasm. Acta Otolaryngol. 2002;122(3):343–347. [DOI] [PubMed] [Google Scholar]
- 19.Shaha AR. Paradigm shifts in staging of thyroid cancer. Oral Oncol. 2017;72:188–189. [DOI] [PubMed] [Google Scholar]