Summary
Mutations of the Recombinase Activating Genes (RAG) in humans underlie a broad spectrum of clinical and immunological phenotypes that reflect different degrees of impairment of T and B cell development and alterations of mechanisms of central and peripheral tolerance. Recent studies have shown that this phenotypic heterogeneity correlates, albeit imperfectly, with different levels of recombination activity of the mutant RAG proteins. Furthermore, studies in patients and in newly developed animal models carrying hypomorphic RAG mutations have disclosed various mechanisms underlying immune dysregulation in this condition. Careful annotation of clinical outcome and immune reconstitution in RAG-deficient patients who have received hematopoietic stem cell transplantation has shown that progress has been made in the treatment of this disease, but new approaches remain to be tested to improve stem cell engraftment and durable immune reconstitution. Finally, initial attempts have been made to treat RAG deficiency with gene therapy.
Keywords: Recombinase Activating Genes, immunodeficiency, autoimmunity, Omenn syndrome, immunological tolerance
Introduction
Originally defined as inborn errors characterized by increased susceptibility to infections, primary immune deficiencies (PID) have been progressively recognized to include a broader spectrum of clinical phenotypes (1). In particular, autoimmunity and exaggerated inflammatory responses are often observed in patients with PID, and in some cases may even represent the predominant manifestation. Recognition of such expanded phenotypic spectrum has been also facilitated by advances in molecular genetics, in particular development of targeted sequencing panels and whole exome/whole genome sequencing (WES, WGS). These approaches have permitted to appreciate that different mutations in the same gene may even underlie distinct clinical phenotypes. Furthermore, careful biochemical analysis has revealed that such phenotypic heterogeneity may also be contributed by different mechanisms of disease, including loss-of-function (LOF), haploinsufficiency, dominant negative, and gain-of-function (GOF) effects. In this regard, the study of human diseases (and PID in particular) has rapidly overcome some intrinsic limitations of animal models, that for many decades have largely been based on gene disruption.
Mutations of the Recombinase Activating Genes 1 and 2 (RAG1, RAG2) represent an interesting example in which different mutations may cause distinct clinical and immunological phenotypes, with some overlap. At variance with other PID gene defects, in which a similar phenomenon may reflect the existence of LOF and GOF alleles (2), RAG mutations underlie a continuum of degree of catalytic function mediated by the RAG complex. This translates in a variable degree of impairment of T and B cell development, affecting composition and diversity of the immune repertoire and immune homeostasis. Consequently, RAG mutations have been associated with severe combined immunodeficiency with absence of T and B cells (T− B− SCID), Omenn syndrome (OS) with presence of oligoclonal and activated T cells infiltrating and damaging target tissues, atypical SCID (AS) with residual presence of oligoclonal T (and occasionally, B) cells, and combined immunodeficiency associated with granulomas and/or autoimmunity (CID-G/AI) (3). Overall, recognition of this broad spectrum of clinical and immunological phenotypes underpins the critical role of the RAG complex in immune homeostasis. Here, we will discuss recent advances in the mechanisms underlying the pathophysiology of the immune dysregulation associated with hypomorphic RAG mutations in patients and in animal models.
Molecular and biochemical structure of human RAG1 and RAG2
The generation of an extensive repertoire of immunoglobulin and T cell receptor (TCR) molecules in developing lymphocytes is ensured by the combinatorial association of dispersed variable (V), diversity (D) and joining (J) gene segments through the V(D)J recombination process (4). Recombination signal sequences (RSSs), flanking each of the V, D and J gene segments containing conserved consensus nonamer and heptamer elements separated by a degenerate spacer of either 12 or 23 nucleotides are recognized by the Recombination activating gene proteins, RAG1 and RAG2 (5). Expression of RAG genes is tightly regulated and occurs at early stages of T and B cell differentiation. The RAG proteins form a heterotetramer with two subunits each of RAG1 and RAG2, that recognizes and binds to a pair of RSSs, introducing a DNA double strand break at the junction with the coding gene segment. Efficient recombination occurs only when the RAGs bind one 12RSS and one 23RSS (the so called “12/23 rule”). However, in the rearrangement of T-cell receptor beta and delta loci, joining of V and J gene segments bordered by the 23 and 12 RSS does not occur, and an intervening D segment has to be joined to J before a V segment can be joined to the rearranged DJ product (the so-called “beyond 12/23 restriction”) (6, 7).
The human RAG1 and RAG2 genes are located in a tail to tail configuration on chromosome 11p13 and are separated by only 8 kb (8). Both the genomic organization of the RAG genes and the amino acid composition of the RAG proteins are highly conserved throughout evolution. Furthermore, the observation that RAG proteins share similarities with various transposases and can mediate transposition (9, 10), supports the hypothesis that RAG recombinase originates from a common transponsable element that entered the genome of a common ancestor of all jawed vertebrate. Consistent with this hypothesis, the ProtoRAG transposon superfamily has been recently identified in the genome of the basal chordate amphioux (11–13). Multiple levels of regulation of RAG gene expression have been hypothesized to occur because of the on-off fluctuation observed during lymphocyte development. Furthermore, expression of the RAG proteins is also regulated at the post-translational level. In vitro and in vivo data indicate the existence of cis-regulatory elements in the RAG locus, and an additional regulatory mechanism has been described to mediate the regulated degradation of the RAG2 protein via phosphorylation at threonine 490 (T490) and targeting to the ubiquitin-proteosomal pathway (8).
Structural studies have recently demonstrated the architecture of the core RAG heterotetramer. Binding of a RAG1/RAG2 heterotetramer together with the high mobility complex groups (HMGB1 or HMGB2) to one RSS and synapsis with a partner RSS results in introduction of a nick on one DNA strand between the heptamer and the flanking coding element during the G0/G1 phase of the cell cycle, with generation of cleavage paired complex. Subsequently, a transesterification reaction occurs, with formation of sealed hairpin coding ends and RSS-containing blunted signals ends, to which the RAG heterotetramer remains bound in a cleaved signal complex. Upon ARTEMIS activation by the DNA-dependent protein kinase catalytic subunit (DNA-PKcs), opening of the hairpins occurs, and both coding and signal ends are processed by proteins of the non-homologous end joining (NHEJ) pathway, leading to the joining of the two coding ends and formation of a circular DNA product containing the signal ends (14, 15). During the joining process, asymmetrical opening of the hairpin by ARTEMIS may allow incorporation of palindromic sequences (P-nucleotides), and terminal deoxynucleotidyl transferase (TdT) may introduce additional nucleotides (N-nucleotides) in the coding sequence.
Structure of the human RAG 1 and RAG2 proteins
The RAG1 and RAG2 protein sequences are not related to each other. The human RAG1 protein is composed of 1043 amino acids. The N-terminal region of the protein has been implicated in non-specific interactions and involved in ubiquitylation-dependent regulatory processes (7). It contains a series of basic motifs, involved in RAG1 subnuclear localization, a C3HC4 RING finger domain followed by a zinc finger motif that coordinates zinc ions and has histone H3 ubiquitin ligase activity. Nuclear magnetic resonance and crystallography studies have suggested that these motifs are located in functionally relevant structural domains (16, 17), and this hypothesis has been confirmed by in vitro analysis of recombination activity of mutant RAG proteins (18).
The catalytic core of human RAG1 (amino acids 387–1011) is structurally modular and includes several domains: a nonamer binding domain (NBD, amino acids 394–460), a dimerization and DNA-binding domain (DDBD, amino acids 461–517), pre-RNase H (preR, amino acids 518–590), catalytic RNase H (RNH, amino acids 591–721), zinc-binding domain (amino acids 722–965) including two distinct regions with canonical cysteine (ZnC2) and histidine zinc-binding (ZnH2) residues, and the carboxy-terminal domain (CTD, amino acids 966–1008). Structural and biochemical evidence suggest that additional regions along the central and C-terminal regions serve as DNA-binding surfaces for the RSS heptamer and coding flank, respectively (7).
In the past, mutations found in patients with various immune phenotype have been instrumental for the evaluation of the effects of different codon changes. Extensive mutagenesis studies have been reported. In particular, mutations in the highly conserved catalytic residues (D600, D708, E962 DDE motif) revealed their critical role in catalysis (19–21) and additional observations have highlighted the effect on hairpin formation in case of mutations occurring close to amino acid D600 (22)
The RAG2 protein is composed of 527 amino acids. The RAG2 biochemically competent “core” is included in the region spanning amino acids 1–383 or 1–387 (7), although recent evidence indicates amino acids 1–351 as the minimal RAG2 region required for catalytic function of the RAG complex (23). Secondary structure prediction and mutagenesis studies have suggested that the core domain of RAG2 adopts a six-bladed beta-propeller fold structure with the second beta-strand of each repeat highly conserved both within and between kelch repeat-containing proteins (24, 25). This core region is essential for DNA cleavage activity and enhances RSS binding and interaction with RAG1. Extensive site-directed mutagenesis studies within each of the six kelch motifs, revealed the critical role of both hydrophobic and glycine-rich regions within the second beta-strand for RAG1-RAG2 interaction and recombination signal recognition and cleavage (24). Furthermore, residues 350–383 are responsible for demethylation of the endogenous Igκ locus in pre-B cells through a RAG1-independent process (26). The C-terminus “noncore” region (amino acids 384–527) contains multiple regulatory motifs and is involved both in chromatin accessibility and in the inhibitory regulation of RAG2 expression. A highly conserved acidic “hinge” region plays a critical role in post-cleavage DNA ends stabilization and DNA repair pathway choice (27). Importantly, a noncanonical plant homeodomain (PHD) (amino acids 414–487) is involved in chromatin interaction by binding to histone 3 carrying a trimethylated lysine 4 (H3K4me3), an epigenetic marker of active transcription start sites (25, 28, 29). Binding of the PHD to H3K4me3 promotes recombination activity of the RAG complex. Phosphorylation of residue T490 by the cyclin A-CDK2 complex controls the proper expression of the RAG2 before G1 to S phase transition in the cell cycle (30). The phosphorylated RAG2 protein is polyubiquitylated by the (S-phase kinase associated protein (SKP2)-SKp1-CUL1-F box) SCF complex and targeted for proteasomal degradation (31). The critical role of this motif has been further confirmed by the generation of mouse models carrying deletion in the RAG2 C-terminal domain. Destabilization of the post-cleavage RAG complex and increased aberrant recombination product including chromosomal translocations involving VDJ loci were observed in mutants lacking the C-terminal residues 352–527 (27, 32–34). Furthermore, p53-deficient animals carrying a core RAG2 deletion have increased NHEJ activity associated with genomic instability and accelerated lymphomagenesis (34, 35)
The crystal and cryo-electron microscopy structures of RAG1/RAG2 core proteins have revealed a Y shaped structure (23, 36), with the RAG1 NBD dimer forming the stem of the Y, while additional regions along the central and C terminal region of RAG1 act as DNA-binding surfaces for the RSS heptamer and coding flank. The three catalytic residues (D603, D711 and E965) reside in the RNH domain and together with the carboxy-terminal domain form the crevice of the Y structure (37). The RAG2 core region folds in a six-bladed β-propeller associates with the RAG1 domains downstream of the DDBD, creating the arms of the Y structure.
These studies have been instrumental to illuminate the structural effect of various amino acid changes identified in patients with RAG mutations and may guide in the interpretation of genotype-phenotype correlation in this disease. However, important questions that remain to be addressed include how RAG activity is regulated by histones and how different amino acid substitutions occurring at the same codon result in distinct immunological phenotypes.
Spectrum of clinical manifestations caused by RAG gene defects
Defects in RAG genes cause a large spectrum of clinical phenotypes in humans (3). Following the demonstration that disruption of Rag1 and Rag2 genes in mice abolishes V(D)J recombination and causes an early and severe block in T and B cell development (38, 39), search for mutations in the orthologue human genes was attempted, focusing on infants affected with severe combined immune deficiency with absence of B lymphocytes (B− SCID). The first description of null mutations in the RAG genes causing B− SCID was reported by Klaus Schwarz in 1996 (40). Transient transfection assays in human fibroblast 293 cells co-transfected with suitable V(D)J recombination substrates showed that plasmids expressing the mutant RAG proteins were virtually devoid of recombination activity. Importantly, in this study RAG mutations were found only in six of 14 patients with B− SCID tested. It was subsequently demonstrated that fibroblast cell lines obtained from RAG-mutated patients show normal cellular radiosensitivity, whereas another group of patients with B− SCID with increased cellular radiosensitivity carry mutations in genes encoding various components of the NHEJ pathway (41).
Patients with null mutations in the RAG genes have typical clinical manifestations of SCID, and are at increased risk of severe infections since early in life, leading to death in the absence of hematopoietic cell transplantation (HCT). In most cases, these patients present with T− B− NK+ SCID, reflecting the notion that normal function of the RAG proteins is required for T and B cell development, but is dispensable for NK cell differentiation. However, a proportion of patients may present with a variable number of T cells of maternal origin. Maternal T cell engraftment is frequent in SCID babies, and may have variable clinical consequences, with lack of specific symptoms in some patients, whereas others may have clinical signs of graft-versus-host disease that most frequently involve the skin and the liver (42). Importantly, T cells of maternal origin have an activated (CD45R0+) phenotype and are typically oligoclonal.
In 1991, five years before RAG gene defects were discovered in humans, Geneviève de Saint Basile reported a family with two siblings presenting with T− B− SCID and with Omenn syndrome (OS), respectively (43). OS was originally described in 1965 by Gilbert Omenn in a patient with immunodeficiency and generalized erythroderma due to skin infiltration of eosinophils, lymphocytes and histocytes (44, 45). This disease is also characterized by eosinophilia, as well as by lymphadenopathy, hepatosplenomegaly, diarrhea, and alopecia, reflecting infiltration of various target tissues by autologous, oligoclonal and activated T cells (43, 46). In most cases, no circulating B cells are present, and immunoglobulin serum levels are very low, but IgE serum levels are typically elevated. The autologous origin of the T lymphocytes distinguishes OS from the Omenn-like manifestations of maternal T cell engraftment that may occur in patients with SCID. The occurrence of T− B− SCID and OS in two siblings from the same family led our group and the group of Alain Fischer to postulate that they might represent allelic diseases. Indeed, in 1998, we reported biallelic RAG mutations in seven unrelated infants with OS (45). Importantly, the majority of the mutations were missense, and in each patient at least of the two alleles retained partial recombination activity in vitro. An apparent exception is represented by N-terminal frameshift RAG1 mutations, that are often associated with an OS phenotype, even when present as homozygous trait. In 2000, we showed that these mutations result in internal methionine usage, with expression of N-terminal truncated protein with partial recombination activity (47). Together, these data established for the first time that hypomorphic RAG mutations may cause a phenotype other than T− B− SCID, and yet confirmed that robust levels of recombination activity are necessary to allow production of a polyclonal repertoire of T and B cells and preserve key mechanisms of immune homeostasis. In 2001, the group of Jean-Pierre de Villartay in Paris made another important discovery that validated the original observation made by de Saint Basile in 1991 (43), demonstrating that identical RAG mutations may cause either T− B− SCID or OS (48). The authors proposed that an additional factor (besides the RAG gene defect) may be required in certain circumstances for the development of the OS phenotype. They further speculated that such factor might be represented by a particular antigen that is present in patients with OS, but not in those with T− B− SCID, and that may cause activation and expansion of antigen-specific T cell clonotypes. They also hypothesized that this antigen should be present in the skin and the gut, because these are the two major sites of T cell infiltration in patients with OS. This hypothesis was also supported by our own finding that TCR CDR3 sequences from patients with OS are enriched for certain amino acids at specific positions (46), possibly suggesting antigen-driven selection. Alternatively, environmental factors may also play a role in triggering clonotypic T cell expansions and manifestations of OS in certain patients. Indeed, evolution of SCID to OS phenotype has been reported after exposure to parainfluenza infection (49). However, other mechanisms may also contribute to determine the OS phenotype, as discussed later in this review.
To further broaden the clinical and immunological phenotype of RAG deficiency, a series of patients have been reported in whom the presence of activated, oligoclonal, and autologous T cells is not associated with typical features of OS (50). This condition is often referred to as “atypical SCID” (AS) or “leaky SCID”. A distinct form of AS associated with expansion of γδ T cells has been reported in RAG-deficient patients upon cytomegalovirus (CMV) infection (51, 52). Interestingly, circulating B cells are often detectable in these patients, and the clinical phenotype of this condition may include Epstein-Barr virus (EBV)-driven lymphoproliferative disease and autoimmune cytopenias, indicating that the leakiness of the gene defect is not sufficient to preserve immune homeostasis.
In 2008, Catharina Schuetz reported three patients in whom RAG mutations were associated with an atypical, late onset phenotype characterized by extensive granulomatous disease involving the skin, internal organs and mucous membranes. All three patients developed serious complications after viral infections, including EBV-driven lymphoma (53). They had low but detectable number of circulating T and B cells; immunoglobulin serum levels were low, but specific antibody responses were preserved, at least to some vaccine antigens. When the recombination activity of the mutant RAG alleles was tested in vitro using transfection assays with a suitable recombination substrate, values between 5% and 30% of normal control were observed. This figure is significantly higher than what reported for mutations associated with OS and even with for most cases of AS. Since then, several other patients have been described in which hypomorphic RAG mutations associate with granulomatous disease and autoimmune manifestations, either alone or combined (54–59). Therefore, we proposed that this phenotype be defined as “combined immunodeficiency with granuloma and/or autoimmunity” (CID-G/AI) (60). In a series of 68 patients with CID-G/AI included in a recent review, we observed that autoimmune cytopenias were particularly common, being found in 53% of the patients (3). However, severe vasculitis and organ-specific autoimmunity affecting gut, liver, thyroid, kidney, and muscles. Recurrent respiratory tract infections leading to bronchiectasis, and chronic and recurrent viral infections due to herpesviruses and human papillomavirus have been frequently reported in patients with CID-G/AI. Fatal progressive multifocal leukoencephalopathy caused by to JC virus infection has been seen in one patient (59). Interestingly, presence of the rubella virus vaccine strain has been demonstrated in the granulomas of some of these patients (61). The immunological phenotype of RAG deficiency manifesting as CID-G/AI is variable. T cells are usually present in lower number, and the proportion of naïve T cells is also reduced; however, in vitro T cell proliferation to mitogens is often normal or modestly reduced. Circulating B cells are present in variable number; immunoglobulin serum levels are often normal but may also be low or high, and specific antibody responses may be variably affected (3). Finally, these patients produce a broad spectrum of autoantibodies; neutralizing anti-cytokine antibodies directed against interferon-α and IFN-ω have been reported especially in patients with a history of varicella zoster virus infection (60, 62).
Moreover, patients with hypomorphic RAG mutations may initially be given other diagnoses, including idiopathic CD4+ lymphopenia (63), common variable immunodeficiency (64, 65), IgA deficiency (66), selective deficiency of polysaccharide specific antibodies (67), hyper-IgM syndrome (68) and autoimmune lymphoproliferative syndrome (69). Although at least in some of these cases the clinical and laboratory data were clearly suggestive of a combined immunodeficiency, the fact remains that these clinical examples have taught us how diverse is the clinical and immunological phenotype associated with hypomorphic RAG mutations.
Finally, because CID-G/AI and other milder clinical phenotypes have been described only in recent years, the relative proportion of the various clinical phenotypes of human RAG deficiency remains unknown. Universal newborn screening for SCID permits the identification of patients with typical and atypical SCID, including OS, making it possible to assess the frequency with which RAG deficiency in humans manifests with either typical or atypical SCID. Data from the Primary Immune Deficiency Treatment Consortium (PIDTC) indicate that in the period 2010–2018, 49 cases of RAG deficiency have been identified in North America (70). Of these 49 infants, 21 presented with typical SCID and 28 with OS or AS. In the same study, RAG deficiency was found to account for 11.5% of all cases of typical SCID, and as many as 41.8% of cases of atypical SCID. These data emphasize the notion that hypomorphic RAG mutations, far from representing the exception, are in fact more common in RAG deficiency and are responsible for leaky phenotypes. Whether newborn screening can also capture patients who are going to develop a CID-G/AI phenotype is unknown.
Genotype-phenotype correlation in RAG deficiency
When it became apparent that RAG mutations in humans may associate with a diverse spectrum of clinical and immunological phenotypes, we and others investigated whether such phenotypic heterogeneity is supported by different levels of RAG protein expression and function. Since the RAG proteins are only expressed at early stages of lymphoid development, assessing their activity is challenging. Not infrequently, novel RAG gene variants have been attributed a disease-causing role in patients with SCID or other severe phenotypes, in the absence of any formal evidence of pathogenicity. More recently, public databases (ExAC, GnomAD, 1000 Genomes, and others) of genomic variants have become available. However, the simple documentation of a given RAG variant in such databases does not rule out its pathogenicity, unless the frequency reported in the general population is >0.01. Eventually, functional tests are needed. Because the RAG proteins are not expressed in peripheral lymphocytes, for many years tests to assess recombination activity of RAG variants have been based on transfection assays in which fibroblasts or 293T cells were co-transfected with plasmids expressing wild-type or mutant RAG1 and RAG2 and suitable recombination substrates in which recognition of RSS by the RAG complex would allow DNA recombination and expression of antibiotic resistance genes (40) or alternatively generation of coding and signal joint recombination products that can be identified by polymerase chain reaction amplification (71). Although these assays are both sensitive and specific, they explore RAG function on extrachromosomal substrates, i.e. in a non-physiologic setting, and may not be ideal to test the activity of mutations affecting nuclear translocation of the RAG proteins. To circumvent this problem, we have used a different approach in which Abelson-virus transformed Rag1−/− (or Rag2−/−) mouse pro-B cells engineered to express an inverted green fluorescent protein (GFP) cassette flanked by RSS and an Em-bcl2 transgene are transduced with retroviral vectors expressing either wild-type or mutant human RAG1 (hRAG1) or hRAG2. Upon treatment of cells with imatinib to maintain cells in G0-G1 and enhance RAG expression, flow-cytometric assessment of GFP is used to assess recombination activity of the RAG mutant (72, 73). Furthermore, this assay also permits to analyze the efficiency of V(D)J recombination at the endogenous Ighc locus. More recently, we have further modified the flow cytometry-based assay by using bicistronic vectors that allow simultaneous expression of two RAG variants at 1:1 ratio (73). Because the RAG complex is a heterotetramer, use of bicistronic vectors is especially important to assess the net effect of compound heterozygous missense mutations.
Overall, these assays have demonstrated that naturally occurring missense RAG mutations present a wide range of recombination activity, with robust, albeit not absolute correlation with the clinical and immunological phenotype (3). They have also helped demonstrate that some variants that had been reported in patients with SCID/OS, support wild-type levels of recombination activity and therefore are presumably not disease-causing, as shown for the RAG2 N474S variant (73, 74).
Another evidence in favor of genotype-phenotype correlation in RAG deficiency comes from studies of the diversity of T and B cell repertoire based on and high throughput sequencing of rearrangement products at TCR and immunoglobulin loci. Analysis of Vβ family expression by flow cytometry, and spectratyping to analyze complementarity-determining region 3 (CDR3) length distribution, have revealed a restricted usage of V-J gene segments, and skewed distribution of TCRβ-CDR3length with predominance of shorter CDR3 regions in OS patients (75), whereas less pronounced oligoclonality has been reported in patients with AS (51, 52), and significant diversity in patients with CID-G/AI (53). More recently, high throughput sequencing techniques have permitted a more detailed and extensive characterization of T and B cell repertoire. In particular, we have observed progressive reduction of diversity and increased clonality when moving from patients with less severe phenotype (CID-G/AI) to those with AS and OS (76, 77). Although patients with OS have a markedly restricted T cell repertoire, they do not share the same rearrangements. Furthermore, within the same patient distinct TCR clonotypes are identified in tissue-infiltrating T cells from different organs (46), suggesting clonal proliferation in response to tissue-specific antigens. Furthermore, somatic mutations restoring the RAG1 reading frame and resulting in missense mutations, have been reported to cause a phenotypic switch from T− B− SCID to OS in a patient (78), whereas the occurrence of a true somatic reversion on one allele was not sufficient to normalize T cell development in another infant, but resulted again in an OS phenotype, presumably because the event occurred at a stage in T cell development where only a limited pool of T cell progenitors capable of performing V(D)J recombination efficiently was generated (79).
Nonetheless, genotype-phenotype correlation in RAG deficiency is not absolute. In particular, the group of Mirjam van der Burg has reported a group of 22 patients with RAG deficiency, resulting in the same N-terminal truncation of the RAG1 protein, who manifested a variable clinical phenotype ranged from SCID to OS and CID (80). Consistent with the critical role of RAG proteins in V(D)J recombination, analysis of the bone marrow from patients with SCID and OS has revealed a block at the pre-B-I stage, confirming previous observations (81, 82), whereas those presenting with CID have a reduced, but detectable number of pre-B-II and even immature B cells. Moreover, similar abnormalities of B and T cell repertoire were observed in all patients (80). While this study highlights the imprecise correlation between genotype and phenotype in RAG deficiency, it should be noted that N-terminal truncating mutations of RAG1 support very modest levels of recombination activity, and more significant differences of repertoire diversity and composition may be observed when comparing patients with a broader range of recombination activity.
The study of T cell repertoire has also provided novel insights into the mechanisms by which hypomorphic RAG mutations may affect T cell diversity even prior to positive selection, i.e. by affecting composition of the pre-immune repertoire. During positive thymocytes selection, the TCRα (TRA) locus undergoes multiple waves of V-J recombination that progressively associate more upstream TRAV segments to more downstream TRAJ elements (83). Hypomorphic RAG mutations are predicted to compromise sequential V(D)J rearrangements. The group of Jean-Pierre de Villartay has recently provided important evidence in support of this hypothesis (84). By using high throughput sequencing and multiplex PCR to analyze the relative frequency of 8 TRAV genes (and their partner TRAJ elements) corresponding to proximal, middle or distal location along the TRA locus, they observed a biased usage of proximal TRAV/TRAJ elements in patients with RAG deficiency or other defects of V(D)J recombination. Furthermore, the few rearrangements involving distal TRAV elements were often including proximal TRAJ segments, a combination that is largely underrepresented in healthy controls. This observation of impaired sequential rearrangements at the TRA locus has been translated into a flow cytometry-based assay that may help in the diagnosis of RAG deficiency and other defects of V(D)J recombination. In particular, using a monoclonal antibody directed against TCR-Vα7.2, the most distal TRAV gene segment, the de Villartay laboratory has demonstrated that patients with CID-G/AI, CID and OS have a markedly reduced proportion or even complete absence of T lymphocytes expressing this gene (84), which marks mucosal-associated invariant T (MAIT) lymphocytes. MAIT cells are activated by microbial compounds derived from bacterial riboflavin biosynthesis (85). It is possible that the markedly reduced number of MAIT cells may contribute to the increased susceptibility to bacterial infections observed in patients with RAG deficiency.
Finally, high-throughput sequencing of T and B cell repertoire may also provide insights into mechanisms of immune dysregulation associated with RAG deficiency. In this regard, we have shown that peripheral B cells from patients with CID-G/AI showed increased usage of IGHV3–9, IGHV4–31 and IGHV3–23 (76), which are variable regions expressed by autoreactive B cells in patients with cancer and autoimmunity (86). B cell self-reactivity has been associated with variations in the hydrophobicity profile of the IGH-CDR3 region (87), that normally contains a discrete proportion (~15%) of tyrosine residues. We have observed a significant reduction in the frequency of tyrosine in the IGH-CDR3 region of peripheral B cells from patients with RAG defects, irrespective of their clinical phenotype, and we have documented that this abnormality is caused by decreased usage of the IGHJ6 gene segment (77), confirming recent observations from the van der Burg laboratory (80). Moreover, an increased proportion of class-switched immunoglobulin heavy chain transcripts (especially of IGHE transcripts) and a higher rate of somatic hypermutation were observed in the periphery of OS patients (77). These findings are in line with the presence of immunoglobulin-secreting cells in lymph nodes of these patients (88), and indicate that in vivo activation of B cells occurs in OS.
Furthermore, by applying high throughput sequencing of the T cell repertoire to sorted T cell subsets from patients with hypomorphic RAG mutations and controls, we have found that both in patients with OS and in those with AS or CID-G/AI, the diversity of Treg repertoire was 100-fold reduced, and that of conventional CD4+ cells (Tconv) was 10-fold reduced as compared to equivalent subpopulations from healthy controls (76). This result is consistent with our that RAG mutations are associated with impaired thymic generation of Treg cells both in patients and in mice (54, 89–91). Of note, a high degree of repertoire overlap was found between Treg and Tconv cells in OS patients, further corroborating the hypothesis that circulating CD4+ Foxp3+ cells from these patients are not “bona fide” Treg cells, but rather activated T cells, as also indicated by defective suppression function (76, 92). Finally, analysis of amino acid usage at positions P6-P7 of the TCRβ CDR3 region of sorted Tconv cells revealed increase usage of amino acids associated with self-reactivity (93), suggesting that RAG deficiency may also affect negative selection.
Mouse models of null and hypomorphic Rag mutations
Generation of mouse models have been instrumental to unveil the in vivo effects of RAG mutations (Table 1). Functional disruption of either Rag1 or Rag2 genes by homologous recombination leads to a lymphoid arrest at a stage prior to the recombination of the antigen receptor loci, corresponding to double negative 3 (DN3) and pre-B cells along T and B cell development, respectively (38, 39, 94). Accordingly, these mice show absence of peripheral T and B lymphocytes.
Table 1.
Features of mouse models of RAG deficiency
| Mouse model | Phenotype | T cells | B cells | Ab Response and BAFF levels | Equivalent human phenotype | Reference |
|---|---|---|---|---|---|---|
| Core Rag2 knock in | Not described | Low number of T cells Block at DN3 stage Reduced % of γδ+ cells |
Low B cell count. Reduction of pre-B and IgM+ B cells |
No description | 95 | |
| Core Rag2 mouse | Not described | Low number of T cells. Block at DN3 stage Reduced % of γδ+ cells |
Low B cell count Reduction of pre-B and IgM+ B cells |
No description | 96 | |
| Rag1R972Q/R972Q (MM) | Red skin but no cellular infiltrates Hepatosplenomegaly Eosinophilia |
Reduced, oligoclonal, activated T cells Increased % of memory T cells |
Low B cell counts Elevated IgM, IgG, IgE. Enlarged germinal centers |
Normal response to TD- and TI-Ag | AS | 99 |
| Rag1S723C/S723C (mut/mut) | No alopecia No erythroderma No splenomegaly No hepatomegaly No eosinophilia |
Reduced, oligoclonal, activated T cells Reduced % of γδ+ cells Reduced NKT cells |
Low B cell count Low IgA, normal to elevated IgE |
Defective response to TD- and TI-Ag Anti-ssDNA and anti-chromatin antibodies Elevated BAFF levels |
AS | 100–102 |
| Rag1R972W/R972W | Occasional splenomegaly T cell infiltrates in various organs |
Very low T cell count, with oligoclonal, activated T cells | Very low B cell count Decreased % of Igλ+ B cells Elevated IgE Very few B cell follicles |
Defective response to TD- and TI-Ag Very high serum BAFF |
AS | 91 |
| Rag1F971L/R971L | No signs of OS | Very low T cell count, but presence of a sizeable % of naïve T cells Increased % of γδ+ cells |
Very low B cell count Decreased % of Igλ+ B cells Few B cell follicles |
Defective response to TD-Ag, but normal response to TI-Ag High serum BAFF levels |
CID | 91 |
| Rag1R972Q/R972Q | No signs of OS | Low T cell count, but presence of a sizeable % of naïve T cells Increased % of γδ+ cells |
Low B cell count Decreased % of Igλ+ B cells Elevated IgM Presence of B cell follicles |
Defective response to TD-Ag, but normal response to TI-Ag Modestly increased serum BAFF levels Increased proportion of ABC cells High levels of IgM autoAb |
CID-G/AI | 91 |
| Rag2R229Q/R229Q | Erythroderma Alopecia Hepatosplenomegaly Eosinophilia Colitis |
Very low T cell count, with oligoclonal, activated T cells No NKT cells |
Barely detectable B cells. Low IgG, low IgM, elevated IgE Barely detectable B cell follicles |
Defective response to TD- and TI-Ag High serum BAFF levels Increased IgM and IgG high-affinity autoAb |
OS | 90 |
Ab: antibody; ABC: Age-associated B cells; AS: atypical SCID; BAFF: B cell activating factor; BM: bone marrow; CID: combined immune deficiency; CID-G/AI: combined immune deficiency with granulomas and/or autoimmunity; DN3: double-negative 3; NKT: natural killer T cells; OS: Omenn syndrome; SCID: severe combined immune deficiency; TD: T-dependent; TI: T-independent
To define the specific role played by the different domains of the RAG proteins, additional mouse models have been generated. In particular, a Rag2 mutant lacking the C-terminal domain of the protein and carrying only the core region of Rag2 (and for this reason named “core-RAG2 knock-in” mouse) showed a partial block in lymphocyte development, with severe impairment of V(D)J recombination at the Igh and Trb loci, that affected proximal and distal V gene segments equivalently (95, 96).
However, these models do not recapitulate the broad spectrum of clinical phenotypes seen in patients, and in particular the immune dysregulation that is often associated with hypomorphic RAG mutations. To this end, we generated a knock-in mouse model carrying a homozygous missense mutation in the Rag2 gene (90) resulting in the same amino acid change (R229Q) previously described in a patient with OS (45). A careful characterization of the Rag2R229Q/R229Q mouse indicated that this model fully recapitulates the human OS phenotype, including presence of oligoclonal and activated T cells, and virtual absence of circulating B cells in the presence of elevated serum IgE. Importantly, some mutant mice develop severe erythrodermia with presence of abundant T cell and eosinophil infiltrates in the derma. Cellular infiltrates are often seen also in other organs of these mutant mice, including the gut, liver and lung (90, 97). Despite the severe B cell lymphopenia, high affinity antibodies directed against self-antigens are present in the serum of Rag2R229Q/R229Q mutant mice, reflecting important immune dysregulation and contributing to tissue organ damage (88, 98).
At the same time when we described Rag2R229Q/R229Q mice, the Marrack laboratory reported a spontaneous mouse mutant (named mutant mouse, MM), carrying a homozygous point mutation (R972Q) in the Rag1 gene (99). Compound heterozygosity for the equivalent mutation (R975Q) in human RAG1, in association with the R396L mutation, had been previously reported by our group in another patient with OS (50). This mutant mouse was also described as a model of OS. However, although it had several features of OS (low number of T and B cells, presence of an oligoclonal T cell repertoire, predominance of peripheral activated/memory T cells that responded poorly to mitogens, and peripheral eosinophilia), it lacked the prominent T cell infiltrates in the skin and other tissues that characterize the human disease (99). Furthermore, MM mice showed high levels of IgM, IgG and IgE, and were able to mount normal antibody responses to T-dependent and independent antigens. Intriguingly, germinal centers were detected in high number in MM mice in the absence of immunization.
In parallel, Rag1S723C/S723C mice were generated by JoAnn Sekiguchi (100) and further characterized by our group (101, 102). In this particular case, the decision to generate this mutant was not dictated by attempting to model in mice the effects of a naturally occurring human mutation, but rather to test in vivo the consequences of a mutation that results in production of a RAG1 protein that is proficient for DNA cleavage, but defective in postcleavage complex formation and end joining in vitro. Rag1S723C/S723C mice showed an incomplete block in T cell development, with accumulation of DN3 cells in the thymus, and a more severe block in B cell development at the pro-B stage, with few pre-B cells and absence of immature and mature B cells in the bone marrow (100). In spite of this, Rag1S723C/S723C mice were shown to produce high amounts of low-affinity self-reactive antibodies, associated with accumulation of plasma cells in extrafollicular areas (101).
While the Rag mutant mice reported above may represent valuable models of OS and AS, none of them recapitulates the human CID-G/AI phenotype. In order to generate such a model, we used CRISPR-based gene editing to introduce in the coding flank-sensitive region of RAG1 CTD mutations that are equivalent to those identified in patients with CID-G/AI. In particular, we generated three mouse models, homozygous for F971L R972Q and R972W amino acid changes (91). One of these models (the Rag1R972Q mouse) carries the same mutation as the MM mouse model reported above. Based on structural modeling, this mutation and the F971L mutation were predicted to be less severe than the R972W mutation. These different mutants represent a suitable platform to test the hypothesis that mutations in the coding flank-sensitive region of RAG1 alter selection of V, D, and J genes targeted to recombination, perturbing T and B cell repertoire prior to positive selection.
Both T and B cell development were severely compromised in Rag1R972W mice, but less so in Rag1F971L and especially in Rag1R972Q mice. Moreover, while the number of naïve T cells in the spleen was decreased in all three models, a significant proportion of naïve T cells were present in both Rag1F971L and Rag1R972Q mice. The number of B cell follicles in the spleen correlated with the degree of B cell lymphopenia and was more preserved in Rag1R972Q mice. Both Rag1F971L and Rag1R972Q mice had normal serum IgG levels; elevated IgM were present in Rag1R972Q mice, and Rag1R972W mice were the only ones to show elevated serum IgE. The antibody response to T-dependent antigens was defective in all three models, but both Rag1F971L and Rag1R972Q mice had normal antibody levels in response to the T-independent antigen TNP-Ficoll. Importantly, IgM autoantibodies were present in the serum of all three mutant strains, and were more abundant in Rag1R972Q mice. Altogether, these data suggest that the Rag1R972Q and Rag1F971L mice are models of leaky forms of RAG deficiency, and Rag1R972Q mice in particular may represent a model of CID-G/AI. While no granulomatous lesions are observed in these mice under specific paghogen free conditions, it is possible that development of granulomas may require challenge with pathogens.
Overall, mouse models carrying hypomorphic Rag mutations represent a useful tool to address some of mechanisms of immune dysregulation left unsolved in human pathology.
Abnormalities of central and peripheral T cell tolerance
Clinical manifestations in patients carrying hypomorphic RAG mutations clearly indicate immune dysregulation as a cardinal feature of OS, AS and CID-G/AI. Studies in patients and in suitable animal models have helped define that the mechanisms underlying such immune dysregulation are both complex and broad. In particular, there is extensive evidence that both central and peripheral mechanisms of T cell tolerance are disrupted (Fig. 1).
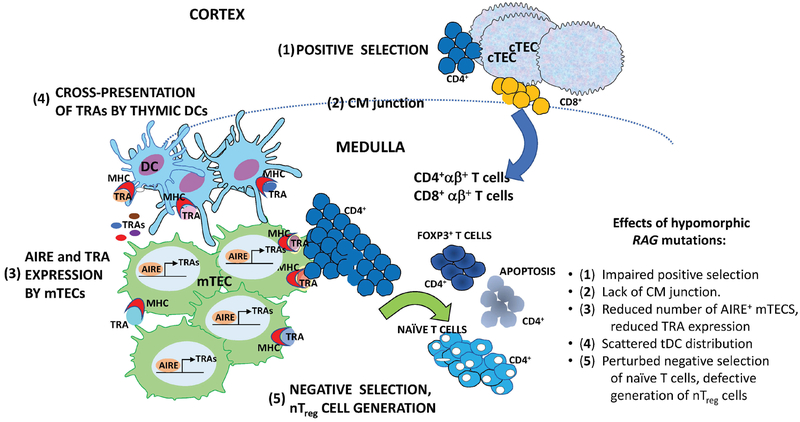
Figure 1. Schematic representation of positive and negative selection in the thymus.
Five steps of T cell maturation and thymocyte selection are shown, which can be affected in patients and animals with hypomorphic RAG mutations. 1) Cortical thymic epithelial cells (cTEC) expressing MHC-peptide ligands mediate positive selection of developing thymocytes. 2) Normal thymic architecture is characterized by demarcation of cortical and medullary areas, with clear evidence of the cortico-medullary junction (shown as dashed dine) where thymic dendritic cells (tDCs) are mainly distributed. Positively selected CD4+ αβ+ and CD8 +αβ+ T cells migrate from the cortex to the medulla, where 3) mature medullary thymic epithelial cells (mTECs) express tissue restricted antigens (TRA) under the control of the transcriptional activator AIRE. CD4+ thymocytes recognizing with high affinity TRAs in the complex with MHC class II molecules on the surface of mTECs or 4) cross-presented by tDCs undergo 5) negative selection or diversion to become FOXP3+ natural regulatory T cells. Hypomorphic RAG mutations may interfere with each of these steps as indicated on the right.
During T cell development, thymocytes undergo positive and negative selection (103, 104). This process requires a well-organized thymic architecture, that permits interaction of thymocytes with cortical and medullary thymic epithelial cells (cTECs, mTECs) and other thymic stromal cells. In particular, positively selected thymocytes migrate to the thymic medulla, where they interact with mTECs, a subset of which express the transcriptional co-activator AutoImmune Regulator (AIRE), which dives expression of tissue-restricted antigens (TRAs) (104, 105). These TRAs, in association with MHC molecules, are presented by mTECs to single-positive T cells. As a result of this interaction, thymocytes expressing a high-affinity TCR for self-peptides are negatively selected in the medulla. Resident conventional DC (cDC), preferentially located within the medulla, also contribute to self-antigen presentation, whilst migratory cDCs, scattered between the medulla and the cortex, have the dual function to transport peripheral acquired self-antigens and to process blood-born self-antigens (106). More recently, another transcription factor, Fezf2, has been also proposed to regulate expression of a large number of TRAs (104, 107). Interaction between TECs and thymic DCs has been implicated to mediate differentiation of self-reactive thymocytes into natural Treg (nTreg) cells (108).
In turn, developing thymocytes express a number of molecules that support the differentiation and maturation of TECs, including RANKL, CD40L, and lymphotoxin (109). Analysis of thymic tissue from RAG-deficient patients has shown severe abnormalities, with loss of cortico-medullary demarcation (CMD) and lack of Hassall’s corpuscles (89, 110). A severe loss of mature mTECs, identified by the expression of Claudin-4, Ulex Europaeus Agglutinin 1 (UEA-1) binding and AIRE, was found in the thymus of OS patients (89), and correlated with dramatic reduction of AIRE-dependent TRA expression and severe depletion of FOXP3+ cells (111). Similar defects have been also observed in the thymus of a patient with CID-G/AI (54). Interestingly, we have reported that patients with CID-G/AI have an increased frequency of neutralizing anti-IFN-α and anti-IFN-ω antibodies (60), which is a distinctive feature of Autoimmune PolyEndocrinopathy-Candidiasis and Ectodermal Dystrophy (APECED), a monogenic autoimmune disease caused by AIRE gene mutations (112). To further clarify this issue, more detailed investigations were performed in the murine counterparts focusing on the effect of the partial VDJ process on the epithelial component maturation (113).
Similar abnormalities have been reported in the thymus of Rag1S723C and Rag2 R229Q mice, with poor CMD, virtual lack of expression of UEA-1 ligand and claudin-4, abundance of immature TEC progenitors, severe reduction of CK8− CK5+ mTECs and markedly reduced expression of AIRE and AIRE-dependent TRAs (90, 102, 113). Furthermore, the number of both cDC and plasmacytoid DC (pDC) and generation of thymic Treg cells were severely reduced in both Rag1S723C and Rag2R229Q mice (114, 115). The thymic medulla was also very small in Rag1R972Q, Rag1F971L and Rag1R972W mice, although CMD was partially preserved (91). As ultimate proof of the importance of lymphostromal cross-talk for TEC maturation, we tested in vivo the effect of pre-TCR signaling on the epithelial compartment. To this end, we transplanted a high number of Rag2R229Q bone marrow progenitor cells into Rag2−/− recipients previously conditioned with anti-CD3ε mAb, which mimics pre-TCR signaling (97). Treated mice displayed improved AIRE expression and appearance of CMD. Importantly, thymic amelioration correlated with significant reduction in peripheral immunopathology as documented by reduction in peripheral infiltrates and a decrease in serum IgE levels as compared to Rag2−/− mice transplanted with Rag2R229Q bone marrow cells without pre-treatment with anti-CD3ε mAb. These findings demonstrate that improvement of the thymic microenvironment may prevent the development of severe inflammation and tissue infiltration by self-reactive T cells (97).
High throughput sequencing of the Trb repertoire in DN4 thymocytes from Rag1R972Q and Rag1F971L mice has revealed skewed usage of V and J genes, with a pattern that was consistent between the two models (91). Similarly, these mutant mice presented skewed usage of IghV and IghJ genes in pro-B cells. These data identify abnormal selection of TCR and Ig genes targeted to V(D)J recombination as a novel mechanism by which hypomorphic RAG mutations may impinge on T and B cell repertoire composition already prior to selection, with possible implications on immune dysregulation.
Treg abnormalities are also an important factor that may contribute to autoimmune manifestations in RAG deficiency. Reduced number of thymic Treg, and a restricted repertoire of peripheral Treg cells, have been reported both in patients (76, 89, 110) and in Rag mutant mice (91, 102, 113). Moreover, we have shown that Treg from patients with OS, AS and CID-G/AI have reduced suppressive activity (76, 92). Although Treg function was apparently preserved in Rag1R972Q and Rag1F971L mice (91), the restricted repertoire of these cells may translate in decreased suppressive function in response to specific antigens.
Finally, an additional important immunoregulatory player is represented by invariant natural killer T (iNKT) cells, whose role is associated with protection against autoimmunity. NKT cell development is dependent on V(D)J recombination and was found to be defective in the Rag2R229Q mice. Consistent with this, we demonstrated severe reduction or absence of iNKT cells also in patients with OS (116)
Dysregulation of humoral immunity associated with hypomorphic RAG mutations
A puzzling clinical feature observed in patients and mouse models with hypomorphic RAG mutations is the presence of a broad range of autoantibodies despite a low number B cells and variable levels of immunoglobulins (51, 52, 60, 101). These findings suggest alterations in the mechanisms of B cell tolerance, which operate at several stages of B cell development both in the bone marrow and in the periphery (Fig. 2) (117).
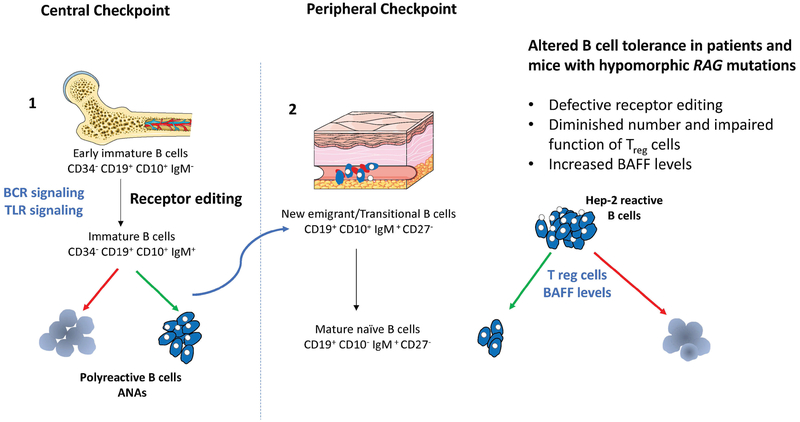
Figure 2. Schematic representation of B cell tolerance checkpoints.
Left panel: The majority of newly generated B cells in bone marrow express self-reactive antibodies including polyreactive specificities. At the first checkpoint, counterselection of highly polyreactive and self-reactive antibodies positive B cells relies on three mechanisms: 1) receptor editing, which involves RAG re-expression and depends on BCR cross-linking by self-antigens; 2) deletion of cells recognizing with high affinity self-antigens; and, 3) anergy, by which B cells become unresponsive to antigen. BCR synergizes with TLR signaling for the selection of autoreactive clones.
Right panel: Despite these mechanisms, some self-reactive immature B cells are released from the bone marrow and reach the periphery where they undergo a second checkpoint before maturing into naïve B cells. The number of self-reactive B cells, dramatically drops during this transition., as shown by the reduction in the proportion of B cells expressing immunoglobulins that recognize Hep-2 cells. Both Treg cells and levels of BAFF are involved in the maintenance of peripheral tolerance. In particular, high levels of BAFF are present in B cell lymphopenic hosts, and promote the survival of transitional B cells, including those expressing self-reactive specificities. Hypomorphic RAG mutations may interfere with both central and peripheral B cell tolerance as indicated on the right.
A large proportion of immature B cells in the bone marrow express a BCR that recognizes self-antigens. Autoreactive B cells generated by random V(D)J recombination are eliminated at two distinct checkpoints, first in the bone marrow and then in the periphery (118). Receptor editing, which requires re-expression of the RAG genes, is the major central B tolerance mechanism by which most autoreactive B cells expressing polyreactive antibodies are removed in the bone marrow at a stage between early immature and immature B cells (117). Secondary recombination events, first at the IGK and then at the IGL locus, provide attempts to edit autoreactive antibodies by substituting light chains until BCR autoreactivity is either abolished or diminished to levels that allow B cell development to proceed. Usage of more upstream V and more downstream J gene usage is a molecular signature for secondary recombination events. A second checkpoint of B cell tolerance takes place in the periphery at the transition between transitional B and mature naive B cells. Because receptor editing does not remove all self-reactive B cells, ~5–10% of transitional B cells express self-reactive specificities, which are largely purged from the naïve B cell compartment. These two populations are also characterized by different levels of expression of B cell activating factor receptor (BAFF-R), which serves as a survival signal, so that naïve cells (that have higher levels of BAFF-R) have an advantage when BAFF levels are limiting. B cell lymphopenia and inflammation are associated with increased BAFF levels, thereby promoting survival of transitional B cells and enriching the peripheral B cell pool of self-reactive B cells. Studies in patients and mice with hypomorphic RAG mutations indicate perturbation of both central and peripheral mechanisms of B cell tolerance. In particular, reduced use of the downstream IGKJ5 gene (a marker of defective gene editing), and elevated BAFF levels have been reported in RAG-mutated patients, along with increased presence of antinuclear antibodies in the serum (80, 101).
These abnormalities of checkpoints of B cell tolerance are also associated with an aberrant distribution and function of B cells at various stages of development. In particular, in spite of very low frequencies of circulating B cells, histological analysis of lymph node biopsies from OS patients unexpectedly revealed few Blimp1+ CD138+ terminally differentiated B cells indicating for the first-time the presence of B cells within the tissues (88). Furthermore, although 92% of bone marrow B cells from these patients are arrested at the pre-B cell stage and mature (CD19+SmIgM+ SmIgD+) B cells are drastically reduced (less than 0.06% vs. 24% in healthy donors), ELISpot analysis showed the presence of immunoglobulin secreting cells in the bone marrow of OS patients (88). These unexpected findings in patients prompted us to investigate in greater detail the B cell compartment in Rag2 and Rag1 hypomorphic mice. We demonstrated that Rag2R229Q mice manifest an expansion of immunoglobulin-secreting cells (ISC) in secondary lymphoid organs, in spite of the severe block of B cell development in the bone marrow (88). Analysis of the Igh repertoire showed one dominant set of clonally related cells (CRC) in each of the mutant mice analyzed, indicating the occurrence of clonal expansions (88). Importantly, we observed that plasma cell differentiation in Rag2R229Q mice is sustained by increased expression of Blimp1 and Xbp1, and that reduced expression of Bcl-6 and c-Myc sustain differentiation of plasma blasts to ISC (88). Furthermore, we observed that Rag2R229Q mice have an increased proportion of CD4+ cells expressing ICOS, CXCR5 and PD-1, and increased serum levels of IL-21 (88). These data suggest that the T cell compartment of these mice is enriched in T follicular helper (TFH) cells which may promote plasma blast differentiation. Whether a similar pattern is also present in patients with OS is currently unknown.
T cell-mediated inflammation may also contribute to promote B cell survival and activation. Unexpectedly, while data from the literature suggest that T cells from OS patients have a predominant TH2 profile (119, 120), Rag2R229Q CD4+ T cells display increased mRNA levels for the TH1-specific transcription factor T-bet, and their serum contains high levels of IFN-γ (88). Interestingly, in vivo T cell depletion by anti-CD4 mAb in Rag2R229Q mice resulted in a complete normalization of serum IgE and decrease in the concentration of cytokines sustaining B cell activation. Finally, similarly to what observed in patients, Rag2R229Q mice have elevated serum BAFF levels. Abundant infiltrates composed of B220+ IgM+ cells were documented in various organs, including the kidney with consequent proteinuria, and administration of anti-BAFF-R antibody to Rag2R229Q mice dramatically decreased cellular infiltration in all organs (86), supporting the important role of BAFF in sustaining B cell-driven autoimmunity.
Similar results were demonstrated in the Rag1S723C mouse model of AS (101). Despite the severe block in B cell development, this mouse shows high serum levels of IgG1, IgG2a and IgE, with an oligoclonal profile. The splenic B cell compartment was skewed to ISC, with high levels of expression of Xbp1 and Iμ-Cγ1 switch transcripts. Receptor editing was impaired, as demonstrated by reduced levels of Vκ-RS rearrangement and markedly decreased proportion of Igλ+ B cells, and BAFF serum levels were elevated. These abnormalities mice were associated with production of autoantibodies to single-stranded DNA and chromatin, and lymphoid infiltrates were present in various organs, although they were less prominent than in Rag2R229Q mice and did not cause proteinuria (101). Finally, production of polyreactive antibodies has been documented in Rag1R972Q mice, associated with an increased proportion of CD11c+ T-bet+ splenic age-associated B cells (91), that have been reported to contribute to autoantibody production (121). Similarlyto the other hypomorphic Rag mutant mouse models, also Rag1R972Q mice have a low proportion of splenic Igλ+ B cells and elevated BAFF serum levels (91).
Collectively, studies in mice have provided robust evidence that hypomorphic RAG mutations cause perturbation in both central and peripheral B cell tolerance. The differences observed among these models, with production of high-affinity autoantibodies and more prominent lymphoid infiltrates in Rag2R229Q mice versus presence of low-affinity autoantibodies and less severe organ damage in Rag1 mutant mice, mimics the phenotypic heterogeneity seen in patients and possibly reflects the variable contribution of various factors in determining the severity of immune dysregulation. It is possible that environmental factors may also exacerbate the effects of impaired immune tolerance, triggering autoimmunity. In this regard, mouse models may help investigate the impact of antigen exposure in driving tissue inflammation and immune dysregulation associated with hypomorphic RAG mutations (115, 122).
Gut Immune dysregulation and Rag hypomorphic mutations
Inflammation and tissue infiltration affect the permeability of tissue barriers, particularly skin and gut, altering host-microbial homeostasis and thus affecting immune responses (123, 124). Patients with hypomorphic RAG mutations often present with cutaneous and gastrointestinal clinical manifestations, reflecting immune dysregulation in these compartments. Similar to OS patients, a significant proportion of Rag2R229Q mice develop spontaneous signs of intestinal disease, with infiltration of the lamina propria by activated T cells expressing high levels of CCR9 (a gut homing receptor) and of Th1 and Th17 cytokines (115). These mice also show significant accumulation of Treg cells in the gut lamina propria (likely as an attempt to maintain immune tolerance), despite the low number of these cells in peripheral lymphoid tissues. However, transfer experiments have shown that Rag2R229Q Treg cells are dysfunctional and unable to prevent colitis. In addition, immunodeficient animals transplanted with Rag2R229Q CD4+ T cells develop colitis, confirming the pathogenic role of Tconv cells in this model (115). Besides T cells, also B cells may contribute to the immune dysregulation in mucosal tissues. Production of secretory IgA is one of the most prominent effects of intestinal colonization. Dramatic reduction of intestinal high affinity IgA associated with development of dysbiosis in AID deficient mice (125–127). We have observed that IgA deficiency in the gut of Rag2R229Q mice is associated with disruption of the gut barrier, resulting in increased gut permeability, and enhanced absorption of lipopolysaccharide (LPS) (115). Furthermore, consistent with the role of secretory IgA in restricting bacterial access to the epithelium and modulating bacterial flora, Rag2R229Q mice showed a dramatic reduction in microbiota richness and diversity, and enrichment of several genera within the Proteobacteria phylum that have been previously reported to associate with chronic inflammatory conditions (128). Prolonged administration of antibiotics (metronidazole, vancomycin, ampicillin) in Rag2R229Q mice caused dramatic reduction of CCR9+ TH1/TH17 cell infiltrates, a decline of IFN-γ and TNF-α in the serum, and normalization of serum IgE. Final fecal transfer experiments showed that wild-type mice recolonized with fecal materials obtained from Rag2R229Q mice developed increased frequencies of inflammatory cells enriched in TH1/TH17 cells in the gut lamina propria and in the spleen. By contrast, transfer of feces from wild-type mice into Rag2 mutants ameliorated gut inflammation (115).
Collectively, these findings demonstrate that in conditions associated with reduced V(D)J recombination activity, oligoclonal T cells become activated and release cytokines that sustain inflammation and promote immunoglobulin secretion at the same time. Lack of IgA results in a defect of the gut barrier, with consequent transendothelial passage of microbial agents and self-antigens that are presented to T cells in peripheral lymphoid organs, further increasing their activated immune phenotype, worsening inflammation and tissue damage at distant sites. These findings highlight the contributive role of dysbiosis in sustaining immune dysregulation in the presence of hypomorphic RAG mutations. In parallel, these results suggest that microbiota modulation (e.g., with gut decontamination, fecal transfer, or possibly with pharmacological interventions aimed at promoting intestinal microbiota diversity) may dampen the inflammation and immune dysregulation of this condition.
Treatment of RAG deficiency: From hematopoietic cell transplantation toward gene therapy
Severe forms of RAG deficiency, including T− B− SCID, OS, and AS are associated with increased susceptibility to serious infections, and are inevitably fatal early in life unless immune reconstitution is attained with hematopoietic cell transplantation (HCT). In a series of 76 SCID/OS/AS patients with RAG deficiency who received HCT at three major centers between 1985 and 2009, overall survival was >80% when the transplant was performed from HLA-identical donors without myeloablative conditioning, and the vast majority of these patients did not develop severe or recurrent infections after transplant (129). By contrast, for patients who received haploidentical HCT without myeloablative conditioning, a very high rate (75%) of graft failure was observed, so that repeat HCT had to be frequently used, and none of the patients who engrafted in the absence of myeloablative conditioning reconstituted B cell immunity. Immune reconstitution was more frequently achieved when chemotherapy was used as part of the conditioning regimen for haploidentical transplantation, however survival in this group of patients was significantly lower (~60%) (129). Data from the Primary Immune Deficiency Treatment Consortium (PIDTC) give a different picture, with an overall survival of ~80% after transplantation from donors other than matched siblings; however, also this study confirmed that in the absence of myeloablative or reduced intensity conditioning, RAG-deficient patients experienced a high rate of graft failure and poor T and B cell immune reconstitution (130). Natural killer (NK) cells may contribute to the increased rate of graft rejection following unconditioned haploidentical HCT for RAG deficiency. Cell fate mapping studies have shown that RAG expression may initiate in common lymphoid progenitor cells that give rise to T, B, and NK cells (131–133). Expression of RAG at this stage selects cells with higher DNA repair capacity. Studies in Rag−/− mice have shown that their NK cells have an activated and mature phenotype, have decreased cellular fitness associated with enhanced cytotoxic function (134). We have found that also NK cells from RAG-mutated patients, irrespective of their clinical phenotype, have enhanced expression of perforin and increased degranulation capacity, but at variance with murine counterparts display an immature phenotype (135). In any case, the increased cytotoxic activity of NK cells from RAG-mutated patients may contribute to the higher rate of graft rejection. For this reason, inclusion of serotherapy depleting NK cells in the conditioning regimen of HCT for RAG deficiency may be beneficial.
Besides donor type, other factors that are associated with superior outcome after HCT include young age (<3.5 months of life) and lack of infections at the time of transplantation (130, 136). These data emphasize the importance of newborn screening in order to achieve early detection and prompt referral to transplant for RAG-deficient patients. Finally, while initial studies had suggested that patients with OS/AS may have a worse outcome than those with T− B− SCID (137, 138), a similar and improved survival rate has been observed in both group of patients in a recent PIDTC study (130). Overall, these data indicate that HCT can be curative in severe forms of RAG deficiency; however, for patients who lack matched donors, graft failure and poor immune reconstitution represent significant problems when no myeloablative conditioning is used, and on the other hand use of myeloablative chemotherapy exposes to potentially severe adverse effects.
In patients with RAG deficiency, genetically-defective early lymphoid progenitor cells are present and occupy bone marrow and thymus niches. In the absence of conditioning, lack of durable and robust hematopoietic stem cell engraftment, puts the donor-derived lymphoid progenitor cells that engraft at competition with endogenous, genetically defective lymphoid progenitors, and this may significantly limit the capacity to attain T and B cell immune reconstitution. This may be even more of a problem in patients with CID-G/AI, where such competition would also extend to more mature T and B cell subsets. Limited data are available on the outcome of HCT in this group of patients (3). Out of 26 patients reported in the literature who have received HCT because of CID-G/AI, eighteen (69.2%) were reported to be alive with a median follow-up of 9 months. Because many of these patients may be referred to transplant later in life, when organ damage may already be present, novel and safer forms of conditioning regimen should be considered that would facilitate stem cell engraftment, especially if they were also able to deplete the endogenous gene-mutated lymphoid cells. Immunotoxins may represent an attractive possibility (139).
An alternative approach to overcome the obstacles with HCT is represented by gene therapy. Selective advantage of gene-corrected hematopoietic stem cells to overcome the block of T and B cells that occur in the absence of RAG activity represents the rationale for developing such a strategy. In recent years, lentiviral vectors have become the strategy of choice to deliver the transgene of interest, and allow its expression under the control of suitable promoters. In the case of RAG deficiency, the observation that endogenous RAG gene expression is tightly regulated during cell cycle and during lymphoid development, may expose to the risk that ectopic or dysregulated gene expression could lead to immune dysregulation or leukemia (140). With this caveat, several groups have examined the safety and efficacy of lentivirus-mediated gene therapy for RAG deficiency using animal models.
Results with lentivirus vectors expressing hRAG1 in Rag1−/− mice have been controversial. In one study, use of codon-optimized human RAG1 cDNA sequence (coRAG1) improved levels of transgene expression, but the efficiency of T and B cell reconstitution was variable, depending on the nature of the promoter used (141). Furthermore, while this study demonstrated that gene therapy-treated mice developed a polyclonal T cell repertoire, had good levels of serum IgM, IgG and IgA, and were able to mount antigen-specific antibody responses, a parallel study using the same vectors revealed that gene therapy-treated Rag1−/− mice developed severe signs of inflammation, with cellular infiltrates in the skin, lung, liver, kidney, and presence of circulating anti-double strand DNA (142). All these signs resemble OS, and support the hypothesis that suboptimal expression of RAG1 may lead to partial reconstitution of T and B cell development and sustain immune dysregulation (143). Moreover, environmental factors could also play a role in triggering breakage of immune tolerance, especially when immune reconstitution is not complete. Overall, these data significant concerns on the clinical use of vectors that allow suboptimal levels and deregulated pattern of gene expression.
To overcome this problem, several groups are working at the development of novel vectors and at the optimization of transduction protocols to achieve adequate levels of RAG1 expression with a low vector copy number (VCN) per cell. An alternative approach could be based on the use of engineered nucleases, such as Zinc Finger Nucleases (ZFNs), TALENs and CRISPR-Cas9, that might permit targeted integration of the hRAG1 transgene at the endogenous locus (144, 145).
More promising preclinical results have been obtained with gene therapy for RAG2 deficiency. When Rag2−/− mice were transplanted with Rag2−/− lineage-negative bone marrow cells transduced with a lentiviral vector expressing codon-optimized hRAG2 (coRAG2) under the control of ubiquitous chromatin opening element (UCOE), good levels of both T and B cell reconstitution were observed at a VCN of 1 (146). In particular, gene therapy-treated mice showed normalization of thymic morphology with appearance of AIRE+ mature mTECs. In the periphery, polyclonality of the T cell repertoire, normalization of T cell subsets and of immunoglobulin levels, and production of antibodies to T-dependent and T-independent antigens were detected. Based on these results, we have recently tested use of the UCOE-RAG2co lentiviral vector in the Rag2R229Q mouse model of OS, where selective advantage for gene-transduced cells is expected to be less prominent because of the presence of residual presence of endogenous T and B cells (98). As compared to Rag2R229Q mice who had received Rag2R229Q bone marrow cells, gene therapy-treated mice showed significant improvement of T and B cell counts, although these remained lower than what observed in Rag2R229Q mice transplanted with wild-type bone marrow cells. Furthermore, gene therapy-treated mice also showed dramatic improvement of thymic architecture with presence of AIRE+ mTECs, associated with normalization of the proportion of naïve T cells and polyclonal T cell repertoire in the periphery. Analysis of the B cell compartment showed partial immune reconstitution, with lower than normal B cell counts both in the bone marrow and in the periphery. However, the block at pro-B cell stage in the bone marrow was overcome, normal maturation of follicular B cells was observed in the spleen, associated with reduction of BAFF and normalization of immunoglobulin levels in the serum. Finally, gene therapy-treated mice produced specific antibodies to T-dependent and T-independent antigens. This improvement of immune function was accompanied by normalization of autoantibody production and mitigation of immune infiltrates in the skin (98). Overall, these data suggest that gene therapy for RAG2 deficiency may be feasible also for patients carrying hypomorphic RAG2 mutations associated with autoimmune and inflammatory manifestations. However, further modifications of the lentiviral platform, along with careful long-term monitoring of the efficacy and safety of gene therapy in knock-out and hypomorphic mouse models, are mandatory before moving to clinical trials. Finally, with gene therapy there is a theoretical possibility that the endogenous mutant allele might interfere with the wild-type RAG cDNA introduced with the lentiviral vector. To mitigate, at least in part, this concern is the observation that individuals who are heterozygous for RAG missense mutations are healthy.
Conclusions
The study of patients with RAG mutations has shown that robust levels of recombination activity are essential to allow development of a diversified repertoire of T and B cells, and to enable proper activation of mechanisms of central and peripheral tolerance. The abnormalities observed in patients and in animal models with hypomorphic RAG mutations that sustain reduced levels of recombination activity are not unique to this condition. Indeed, a similar spectrum of phenotypic manifestations may be expected also for other genetic defects affecting the NHEJ pathway or that decrease the strength of TCR signaling (147). Animal models of RAG deficiency have been instrumental to better define the mechanisms of immune dysregulation associated with this condition, and have revealed an important role of lymphostromal cross-talk in the thymus, and of interaction with the microbiota in mucosal tissues in maintain mechanisms of immune homeostasis. Despite these advances, there is a need to better define the natural history and response to treatment, in particular for patients with CID-G/AI. In this regard, international registries may serve an important function. Finally, although significant progress has been made in the treatment of RAG deficiency, additional work remains to be done to overcome the high rate of graft rejection and poor immune reconstitution that has been frequently observed after HCT for this disease. The recent development of several mouse models with hypomorphic Rag mutation may help test novel forms of conditioning regimen as well as safety and efficacy of novel approaches to gene therapy for this condition.
Acknowledgements
This work was supported by Fondazione Telethon (SR-Tiget E2 core grant to AV), National Program of CNR Aging Project (to A.V), and by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (to L.D.N.).
Footnotes
The authors declare no conflicts of interest.
References
- 1.Picard C, Gaspar BH, Al-Herz W, et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J Clin Immunol 2018;38:96–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jhamnani RD, Rosenzweig SD. An update on gain-of-function mutations in primary immunodeficiency diseases. Curr Opin Allergy Clin Immunol 2017;17:391–397. [DOI] [PubMed] [Google Scholar]
- 3.Delmonte OM, Schuetz C, Notarangelo LD. RAG Deficiency: Two Genes, Many Diseases. J Clin Immunol 2018; July 25 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tonegawa S Somatic generation of antibody diversity. Nature 1983;302:575–581. [DOI] [PubMed] [Google Scholar]
- 5.Fugmann SD, Lee AI, Shockett PE, Villey IJ, Schatz DG The RAG proteins and V(D)J recombination: complexes, ends, and transposition. Annu Rev Immunol 2000;18:495–527. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee JK, Schatz DG. Synapsis alters RAG-mediated nicking at Tcrb recombination signal sequences: implications for the “beyond 12/23” rule. Mol Cell Biol 2014;34:2566–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng G, Schatz DG. Regulation and Evolution of the RAG Recombinase. Adv Immunol 2015;128:1–39. [DOI] [PubMed] [Google Scholar]
- 8.Kuo TC, Schlissel MS. Mechanisms controlling expression of the RAG locus during lymphocyte development. Curr Opin Immunol 2009;21:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lescale C, Deriano L. The RAG recombinase: Beyond breaking. Mech Ageing Dev 2017;165:3–9. [DOI] [PubMed] [Google Scholar]
- 10.Schatz DG, Spanopoulou E. Biochemistry of V(D)J recombination. Curr Top Microbiol Immunol 2005;290:49–85. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal A, Eastman QM, Schatz DG. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature 1998;394:744–751. [DOI] [PubMed] [Google Scholar]
- 12.Yasuda K, Richez C, Maciaszek JW, et al. Murine dendritic cell type I IFN production induced by human IgG-RNA immune complexes is IFN regulatory factor (IRF)5 and IRF7 dependent and is required for IL-6 production. J Immunol 2007;178:6876–6885. [DOI] [PubMed] [Google Scholar]
- 13.Carmona LM, Schatz DG. New insights into the evolutionary origins of the recombination-activating gene proteins and V(D)J recombination. FEBS J 2017;284:1590–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schatz DG, Ji Y. Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol 2011;11:251–263. [DOI] [PubMed] [Google Scholar]
- 15.Schatz DG, Swanson PC. V(D)J recombination: mechanisms of initiation. Annu Rev Genet 2011;45:167–202. [DOI] [PubMed] [Google Scholar]
- 16.Cortes P, Ye ZS, Baltimore D. RAG-1 interacts with the repeated amino acid motif of the human homologue of the yeast protein SRP1. Proc Natl Acad Sci U S A. 1994;91:7633–7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng Z, Liu H, Liu X. RAG1-mediated ubiquitylation of histone H3 is required for chromosomal V(D)J recombination. Cell Research 2015;25:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Notarangelo LD, Kim MS, Walter JE, Lee YN. Human RAG mutations: biochemistry and clinical implications. Nat Rev Immunol 2016;16:234–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fugmann SD, Villey IJ, Ptaszek LM, Schatz DG. Identification of two catalytic residues in RAG1 that define a single active site within the RAG1/RAG2 protein complex. Molecular Cell 2000;5:97–107. [DOI] [PubMed] [Google Scholar]
- 20.Kim DR, Dai Y, Mundy CL, Yang W, Oettinger MA. Mutations of acidic residues in RAG1 define the active site of the V(D)J recombinase. Genes Dev 1999;13:3070–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landree MA, Wibbenmeyer JA, Roth DB. Mutational analysis of RAG1 and RAG2 identifies three catalytic amino acids in RAG1 critical for both cleavage steps of V(D)J recombination. Genes Dev 1999;13:3059–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kale SB, Landree MA, Roth DB. Conditional RAG-1 mutants block the hairpin formation step of V(D)J recombination. Mol Cell Biol 2001;21:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MS, Lapkouski M, Yang W, Gellert M. Crystal structure of the V(D)J recombinase RAG1-RAG2. Nature 2015;518:507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez CA, Ptaszek LM, Villa A, et al. Mutations in conserved regions of the predicted RAG2 kelch repeats block initiation of V(D)J recombination and result in primary immunodeficiencies. Mol Cell Biol 2000;20:5653–5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Callebaut I, Mornon JP. The V(D)J recombination activating protein RAG2 consists of a six-bladed propeller and a PHD fingerlike domain, as revealed by sequence analysis. Cell Mol Life Sci 1998;54:880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C, Dong Y, Zhao X, et al. RAG2 involves the Igkappa locus demethylation during B cell development. Mol Immunol 2017;88:125–134. [DOI] [PubMed] [Google Scholar]
- 27.Coussens MA, Wendland RL, Deriano L, Lindsay CR, Arnal SM, Roth DB. RAG2’s acidic hinge restricts repair-pathway choice and promotes genomic stability. Cell Rep 2013;4:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews AG, Kuo AJ, Ramon-Maiques S, et al. RAG2 PHD finger couples histone H3 lysine 4 trimethylation with V(D)J recombination. Nature 2007;450:1106–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Subrahmanyam R, Chakraborty T, Sen R, Desiderio S. A plant homeodomain in RAG-2 that binds Hypermethylated lysine 4 of histone H3 is necessary for efficient antigen-receptor-gene rearrangement. Immunity 2007;27:561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Reynolds TL, Shan X, Desiderio S. Coupling of V(D)J recombination to the cell cycle suppresses genomic instability and lymphoid tumorigenesis. Immunity 2011;34:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang H, Chang FC, Ross AE, et al. Ubiquitylation of RAG-2 by Skp2-SCF links destruction of the V(D)J recombinase to the cell cycle. Molecular Cell 2005;18:699–709. [DOI] [PubMed] [Google Scholar]
- 32.Corneo B, Wendland RL, Deriano L, et al. Rag mutations reveal robust alternative end joining. Nature 2007;449:483–486. [DOI] [PubMed] [Google Scholar]
- 33.Curry JD, Schlissel MS. RAG2’s non-core domain contributes to the ordered regulation of V(D)J recombination. Nucleic Acids Res 2008;36:5750–5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deriano L, Chaumeil J, Coussens M, et al. The RAG2 C terminus suppresses genomic instability and lymphomagenesis. Nature 2011;471:119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mijuskovic M, Chou YF, Gigi V, et al. Off-Target V(D)J Recombination Drives Lymphomagenesis and Is Escalated by Loss of the Rag2 C Terminus. Cell Rep 2015;12:1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ru H, Chambers MG, Fu TM, Tong AB, Liao M, Wu H. Molecular Mechanism of V(D)J Recombination from Synaptic RAG1-RAG2 Complex Structures. Cell 2015;163:1138–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim MS, Chuenchor W, Chen X, et al. Cracking the DNA Code for V(D)J Recombination. Molecular Cell 2018;70:358–370 e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 1992;68:869–877. [DOI] [PubMed] [Google Scholar]
- 39.Shinkai Y, Rathbun G, Lam KP, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 1992;68:855–867. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz K, Gauss GH, Ludwig L, et al. RAG mutations in human B cell-negative SCID. Science 1996;274:97–99. [DOI] [PubMed] [Google Scholar]
- 41.Gennery AR, Cant AJ, Jeggo PA. Immunodeficiency associated with DNA repair defects. Clin Exp Immunol 2000;121:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller SM, Ege M, Pottharst A, Schulz AS, Schwarz K, Friedrich W. Transplacentally acquired maternal T lymphocytes in severe combined immunodeficiency: a study of 121 patients. Blood 2001;98:1847–1851. [DOI] [PubMed] [Google Scholar]
- 43.de Saint-Basile G, Le Deist F, de Villartay JP, et al. Restricted heterogeneity of T lymphocytes in combined immunodeficiency with hypereosinophilia (Omenn’s syndrome). J Clin Invest 1991;87:1352–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omenn GS. Familial Reticuloendotheliosis with Eosinophilia. N Engl J Med.1965;273:427–432. [DOI] [PubMed] [Google Scholar]
- 45.Villa A, Santagata S, Bozzi F, et al. Partial V(D)J recombination activity leads to Omenn syndrome. Cell 1998;93:885–896. [DOI] [PubMed] [Google Scholar]
- 46.Signorini S, Imberti L, Pirovano S, et al. Intrathymic restriction and peripheral expansion of the T-cell repertoire in Omenn syndrome. Blood 1999;94:3468–3478. [PubMed] [Google Scholar]
- 47.Santagata S, Gomez CA, Sobacchi C, et al. N-terminal RAG1 frameshift mutations in Omenn’s syndrome: internal methionine usage leads to partial V(D)J recombination activity and reveals a fundamental role in vivo for the N-terminal domains. Proc Natl Acad Sci U S A. 2000;97:14572–14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corneo B, Moshous D, Gungor T, et al. Identical mutations in RAG1 or RAG2 genes leading to defective V(D)J recombinase activity can cause either T-B-severe combined immune deficiency or Omenn syndrome. Blood 2001;97:2772–2776. [DOI] [PubMed] [Google Scholar]
- 49.Dalal I, Tabori U, Bielorai B, et al. Evolution of a T-B- SCID into an Omenn syndrome phenotype following parainfluenza 3 virus infection. Clin Immunol 2005;115:70–73. [DOI] [PubMed] [Google Scholar]
- 50.Villa A, Sobacchi C, Notarangelo LD, Lim A, Al-Mpusaet al. V(D)J recombination defects in lymphocytes due to RAG mutations: severe immunodeficiency with a spectrum of clinical presentations. Blood 2001;97:81–88. [DOI] [PubMed] [Google Scholar]
- 51.de Villartay JP, Lim A, Al-Mousa H, et al. A novel immunodeficiency associated with hypomorphic RAG1 mutations and CMV infection. J Clin Invest 2005;115:3291–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ehl S, Schwarz K, Enders A, Huck K, Gudowius S, et al. A variant of SCID with specific immune responses and predominance of gamma delta T cells. J Clin Invest 2005;115:3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuetz C, Huck K, Gudowius S, et al. An immunodeficiency disease with RAG mutations and granulomas. N Engl J Med 2008;358:2030–2038. [DOI] [PubMed] [Google Scholar]
- 54.De Ravin SS, Cowen EW, Zarember KA, et al. Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood 2010;116:1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharapova SO, Migas A, Guryanova I, et al. Late-onset combined immune deficiency associated to skin granuloma due to heterozygous compound mutations in RAG1 gene in a 14 years old male. Hum Immunol 2013;74:18–22. [DOI] [PubMed] [Google Scholar]
- 56.Avila EM, Uzel G, Hsu A, et al. Highly variable clinical phenotypes of hypomorphic RAG1 mutations. Pediatrics 2010;126:e1248–1252. [DOI] [PubMed] [Google Scholar]
- 57.Reiff A, Bassuk AG, Church JA, Campbell E, Bing X, Ferguson PJ. Exome sequencing reveals RAG1 mutations in a child with autoimmunity and sterile chronic multifocal osteomyelitis evolving into disseminated granulomatous disease. J Clin Immunol 2013;33:1289–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henderson LA, Frugoni F, Hopkins G, et al. Expanding the spectrum of recombination-activating gene 1 deficiency: a family with early-onset autoimmunity. J Allergy Clin Immunol 2013;132:969–971 e961–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schroder C, Baerlecken NT, Pannicke U, et al. Evaluation of RAG1 mutations in an adult with combined immunodeficiency and progressive multifocal leukoencephalopathy. Clin Immunol 2017;179:1–7. [DOI] [PubMed] [Google Scholar]
- 60.Walter JE, Rosen LB, Csomos K, et al. Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J Clin Invest 2016;126:4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neven B, Perot P, Bruneau J, et al. Cutaneous and Visceral Chronic Granulomatous Disease Triggered by a Rubella Virus Vaccine Strain in Children With Primary Immunodeficiencies. Clin Infect Dis 2017;64:83–86. [DOI] [PubMed] [Google Scholar]
- 62.Goda V, Malik A, Kalmar T, et al. Partial RAG deficiency in a patient with varicella infection, autoimmune cytopenia, and anticytokine antibodies. Journal All Clin Immunol Pract 2018; February 2 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuijpers TW, Ijspeert H, van Leeuwen EM, et al. Idiopathic CD4+ T lymphopenia without autoimmunity or granulomatous disease in the slipstream of RAG mutations. Blood 2011;117:5892–5896. [DOI] [PubMed] [Google Scholar]
- 64.Buchbinder D, Wang N, Aghamohammadi A, Baker R, Lee YN, et al. Identification of Patients with RAG Mutations Previously Diagnosed with Common Variable Immunodeficiency Disorders. J Clin Immunol 2015;35:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abolhassani H,Wang N, Aghamohammadi A, et al. A hypomorphic recombination-activating gene 1 (RAG1) mutation resulting in a phenotype resembling common variable immunodeficiency. J Allergy Clin Immunol 2014;134:1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kato T, Crestani E, Kamae C, et al. RAG1 deficiency may present clinically as selective IgA deficiency. J Clin Immunol 2015;35:280–288. [DOI] [PubMed] [Google Scholar]
- 67.Geier CB, Piller A, Linder A, Sauerwein KM, Eibl MM, Wolf HM. Leaky RAG Deficiency in Adult Patients with Impaired Antibody Production against Bacterial Polysaccharide Antigens. PLoS One 2015;10:e0133220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chou J, Hanna-Wakim R, Tirosh I, et al. A novel homozygous mutation in recombination activating gene 2 in 2 relatives with different clinical phenotypes: Omenn syndrome and hyper-IgM syndrome. J Allergy Clin Immunol 2012;130:1414–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Westermann-Clark E, Grossi A, Fioredda F, et al. RAG deficiency with ALPS features successfully treated with TCRalphabeta/CD19 cell depleted haploidentical stem cell transplant. Clin Immunol 2018;187:102–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dvorak CC, Haddad E, Buckley RH et al. The Genetic Landscape of SCID in the Current Era. J Allergy Clin Immunol 2018. [Google Scholar]
- 71.Weis-Garcia F, Besmer E, Sawchuk DJ, et al. V(D)J recombination: in vitro coding joint formation. Mol Cell Biol 1997;17:6379–6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee YN, Frugoni F, Dobbs K, et al. A systematic analysis of recombination activity and genotype-phenotype correlation in human recombination-activating gene 1 deficiency. J Allergy Clin Immunol 2014;133:1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tirosh I, Yamazaki Y, Frugoni F, et al. Recombination activity of human recombination-activating gene 2 (RAG2) mutations and correlation with clinical phenotype. J Allergy Clin Immunol 2018; June 16 [Epub ehead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Couedel C, Roman C, Jones A, Vezzoni P, Villa A, Cortes P. Analysis of mutations from SCID and Omenn syndrome patients reveals the central role of the Rag2 PHD domain in regulating V(D)J recombination. J Clin Invest 2010;120:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu X, Almeida JR, Darko S, et al. Human syndromes of immunodeficiency and dysregulation are characterized by distinct defects in T-cell receptor repertoire development. J Allergy Clin Immunol 2014;133:1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rowe JH, Stadinski BD, Henderson LA, et al. Abnormalities of T-cell receptor repertoire in CD4+ regulatory and conventional T cells in patients with RAG mutations: Implications for autoimmunity. J Allergy Clin Immunol 2017;140:1739–1743 e1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee YN, Frugoni F, Dobbs K, et al. Characterization of T and B cell repertoire diversity in patients with RAG deficiency. Science Immunology 2016;1(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wada T, Toma T, Okamoto H, et al. Oligoclonal expansion of T lymphocytes with multiple second-site mutations leads to Omenn syndrome in a patient with RAG1-deficient severe combined immunodeficiency. Blood 2005;106:2099–2101. [DOI] [PubMed] [Google Scholar]
- 79.Crestani E, Choo S, Frugoni F, et al. RAG1 reversion mosaicism in a patient with Omenn syndrome. J Clin Immunol 2014;34:551–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ijspeert H, , Driessen GJ, Moorhouse MJ, et al. Similar recombination-activating gene (RAG) mutations result in similar immunobiological effects but in different clinical phenotypes. J Allergy Clin Immunol 2014;133:1124–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Noordzij JG, Verkaik NS, Hartwig NG, de Groot R, van Gent DC, van Dongen JJ. N-terminal truncated human RAG1 proteins can direct T-cell receptor but not immunoglobulin gene rearrangements. Blood 2000;96:203–209. [PubMed] [Google Scholar]
- 82.Noordzij JG, de Bruin-Versteeg S, Verkaik NS, et al. The immunophenotypic and immunogenotypic B-cell differentiation arrest in bone marrow of RAG-deficient SCID patients corresponds to residual recombination activities of mutated RAG proteins. Blood 2002;100:2145–2152. [PubMed] [Google Scholar]
- 83.Brandle D, Muller C, Rulicke T, Hengartner H, Pircher H. Engagement of the T-cell receptor during positive selection in the thymus down-regulates RAG-1 expression. Proc Natl Acad Sci U S A. 1992;89:9529–9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berland A, Rosain J, Kaltenbach S, et al. PROMIDISalpha: A T-cell receptor alpha signature associated with immunodeficiencies caused by V(D)J recombination defects. J Allergy Clin Immunol. 2018; June 12 [Eub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 85.Chandra S, Kronenberg M. Activation and Function of iNKT and MAIT Cells. Adv Immunol 2015;127:145. [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Bu D, Zhu X. Immunoglobulin variable region gene analysis to the autoantibody-secreting B cells from tumors in association with paraneoplastic autoimmune multiorgan syndrome. International Journal of Dermatology 2007;46:1146–1154. [DOI] [PubMed] [Google Scholar]
- 87.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science 2003;301:1374–1377. [DOI] [PubMed] [Google Scholar]
- 88.Cassani B, Poliani PL, Marrella V, et al. Homeostatic expansion of autoreactive immunoglobulin-secreting cells in the Rag2 mouse model of Omenn syndrome. J Exp Med 2010;207:1525–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Poliani PL, Facchetti F, Ravanini M, et al. Early defects in human T-cell development severely affect distribution and maturation of thymic stromal cells: possible implications for the pathophysiology of Omenn syndrome. Blood 2009;114:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marrella V, Poliani PL, Casati A, et al. A hypomorphic R229Q Rag2 mouse mutant recapitulates human Omenn syndrome. J Clin Invest 2007;117:1260–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ott de Bruin LM, Bosticardo M, Barbieri A, et al. Hypomorphic Rag1 mutations alter the preimmune repertoire at early stages of lymphoid development. Blood 2018;132:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cassani B, Poliani PL, Moratto D, et al. Defect of regulatory T cells in patients with Omenn syndrome. J Allergy Clin Immunol 2010;125:209–216. [DOI] [PubMed] [Google Scholar]
- 93.Stadinski BD, Shekhar K, Gomez-Tourino I, et al. Hydrophobic CDR3 residues promote the development of self-reactive T cells. Nat Immunol 2016;17:946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diamond RA, Ward SB, Owada-Makabe K, Wang H, Rothenberg EV. Different developmental arrest points in RAG-2−/− and SCID thymocytes on two genetic backgrounds: developmental choices and cell death mechanisms before TCR gene rearrangement. J Immunol 1997;158:4052–4064. [PubMed] [Google Scholar]
- 95.Liang HE, Hsu LY, Cado D, Cowell LG, Kelsoe G, Schlissel MS. The “dispensable” portion of RAG2 is necessary for efficient V-to-DJ rearrangement during B and T cell development. Immunity 2002;17:639–651. [DOI] [PubMed] [Google Scholar]
- 96.Akamatsu Y, Monroe R, Dudley DD, et al. Deletion of the RAG2 C terminus leads to impaired lymphoid development in mice. Proc Natl Acad Sci U S A. 2003;100:1209–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marrella V, Poliani PL, Fontana E, et al. Anti-CD3epsilon mAb improves thymic architecture and prevents autoimmune manifestations in a mouse model of Omenn syndrome: therapeutic implications. Blood 2012;120:1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Capo V, Castiello MC, Fontana E, et al. Efficacy of lentivirus-mediated gene therapy in an Omenn syndrome recombination-activating gene 2 mouse model is not hindered by inflammation and immune dysregulation. J Allergy Clin Immunol.2017; December 11 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khiong K, Murakami M, Kitabayashi C, et al. Homeostatically proliferating CD4 T cells are involved in the pathogenesis of an Omenn syndrome murine model. J Clin Invest 2007;117:1270–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Giblin W, Chatterji M, Westfield G, et al. Leaky severe combined immunodeficiency and aberrant DNA rearrangements due to a hypomorphic RAG1 mutation. Blood 2009;113:2965–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walter JE, Rucci F, Patrizi L, et al. Expansion of immunoglobulin-secreting cells and defects in B cell tolerance in Rag-dependent immunodeficiency. J Exp Med 2010;207:1541–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rucci F, Poliani PL, Caraffi S, et al. Abnormalities of thymic stroma may contribute to immune dysregulation in murine models of leaky severe combined immunodeficiency. Front Immunol 2011;2(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell 1987;49:273–280. [DOI] [PubMed] [Google Scholar]
- 104.Takaba H, Takayanagi H. The Mechanisms of T Cell Selection in the Thymus. Trends Immunol 2017;38:805–816. [DOI] [PubMed] [Google Scholar]
- 105.Cheng M, Anderson MS. Thymic tolerance as a key brake on autoimmunity. Nat Immunol 2018; June 20 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat Rev Immunol 2014;14:377–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takaba H, Morishita Y, Tomofuji Y, et al. Fezf2 Orchestrates a Thymic Program of Self-Antigen Expression for Immune Tolerance. Cell 2015;163:975–987. [DOI] [PubMed] [Google Scholar]
- 108.Watanabe N, Wang YH, Lee HK, et al. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 2005;436:1181–1185. [DOI] [PubMed] [Google Scholar]
- 109.Abramson J, Anderson G. Thymic Epithelial Cells. Annu Rev Immunol 2017;35:85–118. [DOI] [PubMed] [Google Scholar]
- 110.Poliani PL, Vermi W, Facchetti F. Thymus microenvironment in human primary immunodeficiency diseases. Curr Opin Allergy Clin Immunol 2009;9:489–495. [DOI] [PubMed] [Google Scholar]
- 111.Cavadini P, Vermi W, Facchetti F, et al. AIRE deficiency in thymus of 2 patients with Omenn syndrome. J Clin Invest 2005;115:728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Perniola R Twenty Years of AIRE. Front Immunol 2018;9:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marrella V, Poliani PL, Notarangelo LD, Grassi F, Villa A. Rag defects and thymic stroma: lessons from animal models. Front Immunol 2014;5:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maina V, Marrella V, Mantero S, et al. Hypomorphic mutation in the RAG2 gene affects dendritic cell distribution and migration. J Leukoc Biol 2013;94:1221–1230. [DOI] [PubMed] [Google Scholar]
- 115.Rigoni R, Fontana E, Guglielmetti S, et al. Intestinal microbiota sustains inflammation and autoimmunity induced by hypomorphic RAG defects. J Exp Med 2016;213:355–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Matangkasombut P, Pichavant M, Saez DE, et al. Lack of iNKT cells in patients with combined immune deficiency due to hypomorphic RAG mutations. Blood 2008;111:271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meffre E The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Ann N Y Acad Sci 2011;1246:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol 2008;20:632–638. [DOI] [PubMed] [Google Scholar]
- 119.Schandene L, Ferster A, Mascart-Lemone F, et al. T helper type 2-like cells and therapeutic effects of interferon-gamma in combined immunodeficiency with hypereosinophilia (Omenn’s syndrome). Eur J Immunol 1993;23:56–60. [DOI] [PubMed] [Google Scholar]
- 120.Chilosi M, Facchetti F, Notarangelo LD, et al. CD30 cell expression and abnormal soluble CD30 serum accumulation in Omenn’s syndrome: evidence for a T helper 2-mediated condition. Eur J Immunol 1996;26:329–334. [DOI] [PubMed] [Google Scholar]
- 121.Rubtsov AV, Rubtsova K, Fischer A, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood 2011;118:1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rigoni R, Grassi F, Villa A, Cassani B. RAGs and BUGS: An alliance for autoimmunity. Gut Microbes 2016;7:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 2013;13:321–335. [DOI] [PubMed] [Google Scholar]
- 124.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science 2012;336:1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pabst O New concepts in the generation and functions of IgA. Nat Rev Immunol 2012;12:821–832. [DOI] [PubMed] [Google Scholar]
- 126.Wei M, Shinkura R, Doi Y, Maruya M, Fagarasan S, Honjo T. Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nat Immunol 2011;12:264–270. [DOI] [PubMed] [Google Scholar]
- 127.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 2002;298:1424–1427. [DOI] [PubMed] [Google Scholar]
- 128.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol 2012;9:599–608. [DOI] [PubMed] [Google Scholar]
- 129.Schuetz C, Neven B, Dvorak CC, et al. SCID patients with ARTEMIS vs RAG deficiencies following HCT: increased risk of late toxicity in ARTEMIS-deficient SCID. Blood 2014;123:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Haddad E, Logan BR, Griffith LM et al. SCID genotype and 6-month post-transplant CD4 count predict survival and immune recovery: a PIDTC Retrospective Study. Blood 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity 2002;17:117–130. [DOI] [PubMed] [Google Scholar]
- 132.Borghesi L, Hsu LY, Miller JP, et al. B lineage-specific regulation of V(D)J recombinase activity is established in common lymphoid progenitors. J Exp Med 2004;199:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pilbeam K, Basse P, Brossay L, et al. The ontogeny and fate of NK cells marked by permanent DNA rearrangements. J Immunol 2008;180:1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Karo JM, Schatz DG, Sun JC. The RAG recombinase dictates functional heterogeneity and cellular fitness in natural killer cells. Cell 2014;159:94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dobbs K, Tabellini G, Calzoni E, et al. Natural Killer Cells from Patients with Recombinase-Activating Gene and Non-Homologous End Joining Gene Defects Comprise a Higher Frequency of CD56bright NKG2A+++ Cells, and Yet Display Increased Degranulation and Higher Perforin Content. Front Immunol 2017;8:798 Erratum in: Fron Immunol 2017;8:1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pai SY, Logan BR, Griffith LM, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med 2014;371:434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Antoine C, Muller S, Cant A, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet 2003;361:553–560. [DOI] [PubMed] [Google Scholar]
- 138.Gennery AR, Slatter MA, Grandin L, et al. Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol 2010;126:602–610 e601–611. [DOI] [PubMed] [Google Scholar]
- 139.Logan AC, Weissman IL, Shizuru JA. The road to purified hematopoietic stem cell transplants is paved with antibodies. Curr Opin Immunol 2012;24:640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lagresle-Peyrou C, Yates F, Malassis-Seris M, et al. Long-term immune reconstitution in RAG-1-deficient mice treated by retroviral gene therapy: a balance between efficiency and toxicity. Blood 2006;107:63–72. [DOI] [PubMed] [Google Scholar]
- 141.Pike-Overzet K, Rodijk M, Ng YY, et al. Correction of murine Rag1 deficiency by self-inactivating lentiviral vector-mediated gene transfer. Leukemia 2011;25:1471–1483. [DOI] [PubMed] [Google Scholar]
- 142.van Til NP, Sarwari R, Visser TP, et al. Recombination-activating gene 1 (Rag1)-deficient mice with severe combined immunodeficiency treated with lentiviral gene therapy demonstrate autoimmune Omenn-like syndrome. J Allergy Clin Immunol 2014;133:1116–1123. [DOI] [PubMed] [Google Scholar]
- 143.Pike-Overzet K, Baum C, Bredius RG, et al. Successful RAG1-SCID gene therapy depends on the level of RAG1 expression. J Allergy Clin Immunol 2014;134:242–243. [DOI] [PubMed] [Google Scholar]
- 144.Genovese P, Schiroli G, Escobar G, et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature 2014;510:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science 2018;359. [DOI] [PubMed] [Google Scholar]
- 146.van Til NP, de Boer H, Mashamba N, et al. Correction of murine Rag2 severe combined immunodeficiency by lentiviral gene therapy using a codon-optimized RAG2 therapeutic transgene. Mol Ther 2012;20:1968–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Notarangelo LD. Functional T cell immunodeficiencies (with T cells present). Annu Rev Immunol 2013;31:195–225. [DOI] [PubMed] [Google Scholar]