Abstract
Objective—
Evening, but not morning administration of low dose aspirin (ASA) has been reported to lower blood pressure (BP) in hypertensive patients. The present study was designed to determine whether this phenomenon could be replicated in mice, and if so, whether a time-dependent effect of ASA on BP was due to alteration of circadian clock function.
Approach and Results—
We recapitulated the protective effect of ASA (50µg/d for 7 days) at Zeitgeber Time (ZT) 0 (active-to-rest transit), but not at ZT12, on a high salt diet-induced increase of blood pressure. However, the time of ASA administration did not influence expression of canonical clock genes or their acetylation. We used mouse Bmal1 and Per2-luciferase reporters expressed in U2OS cells to determine the real-time effect of ASA on circadian function but found that the oscillation of bioluminescence was unaltered. Timing of ASA administration also failed to alter urinary prostaglandin metabolites or catecholamines, or the acetylation of its cyclooxygenase-1 (COX-1) target in platelets.
Conclusions—
The time dependent hypotensive effect of aspirin in humans has been recapitulated in hypertensive mice. However, this does not appear to reflect a direct impact of ASA on circadian clocks or on acetylation of platelet COX-1.
Graphical abstract
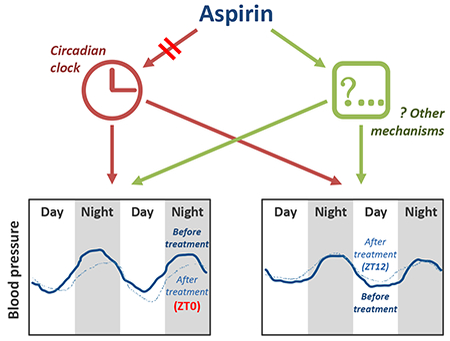
The time dependent hypotensive effect of aspirin does not appear to reflect a direct impact on circadian clocks
Introduction
Aspirin (ASA) is most often used at low doses (~100mg/d) in the secondary prevention of myocardial infarction and stroke1, 2, an effect explained sufficiently by its inhibition of formation of thromboxane (Tx)A2, the predominant product of cyclooxygenase (COX)-1 in platelets3. Higher doses of aspirin are necessary to inhibit COX-2 and thereby relieve pain and inflammation. Deletion of COX-2 accelerates thrombogenesis and elevates blood pressure4. Interestingly, knockdown of COX-1, that simulates the pharmacological effect of low dose ASA in mice, not only delays thrombogenesis, but also restrains the elevation of blood pressure caused by COX-2 inhibition5.
In humans, several controlled clinical trials have reported that low dose aspirin reduces blood pressure in hypertensive populations, but only when given in the evening6,9, raising the possibility that this effect may contribute to its cardiovascular benefit in hypertensive populations6. However, most patients continue to take aspirin in the morning. This may reflect caution emanating from a failure to replicate these findings that mostly derive from the work of one group of investigators10 and a failure to identify a mechanism that might explain this phenomenon. Recently, administration of low dose (150mg/day) aspirin in the evening was reported to reduce the incidence of pre-eclampsia in a susceptible population11, but the study did not include a control group given aspirin in the morning.
Here we wished to determine if we could recapitulate this phenomenon in mice, better to address potential mechanism. Given the broad ranging impact of the molecular clock on drug targets and drug disposition12,13 and the influence of the clock on blood pressure14 and its regulatory mechanisms15, 16 we wondered if this might explain an antihypertensive effect of aspirin conditioned by the time of dosing. As ASA can acetylate proteins beyond platelet COX-117 and because that the function of some core clock proteins can be modulated by acetylation18,19, we hypothesized that a direct impact of ASA on the circadian clock might explain its time-dependent effects on blood pressure.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Experimental design for BP recording
12-week-old male C57BL/6 mice acclimatized to a 12/12-h light/dark cycle for 1 week before radio-telemetry implantation. Although our studies were confined to male mice, the clinical reports of time dependent hypotensive effects of low dose aspirin are evident in both genders. The basal blood pressure (BP) and heart rate (HR) recordings began 7 days after this operation. Mice were subjected to a high salt diet (HSD, 8% NaCl) for 2 weeks to evoke a hypertensive response, better to clarify the phenotype, then divided randomly into two groups and treated with ASA (50μg/d in 100µl of water) by gavage at Zeitgeber Time (ZT) 0 or ZT12 for 7 consecutive days followed by BP recording. Urine was collected for 24 hours using metabolic cages after each BP recording. At the end of the experiment, mice were euthanized by CO2 inhalation for tissue sample collections (Fig. 1). Samples were stored at −80℃ until use.
Figure 1.
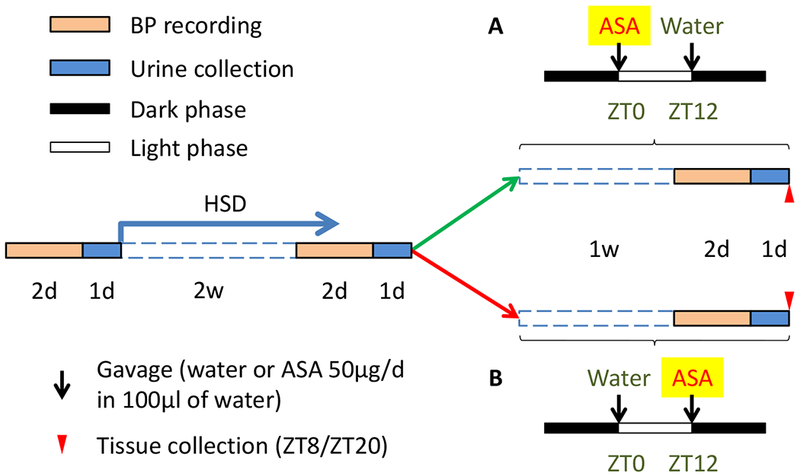
Study design for BP recording. Basal BP of 14-week-old mice was recorded using radiotelemetry followed by 2-week HSD treatment. BP was recorded after HSD and mice were then treated with ASA at ZT0 or ZT12 for 7 consecutive days followed by BP recording. Urine was collected after each BP recording. At the end of the experiment, mice were euthanized for tissue collection.
A separate set of HSD and aspirin treated mice were used for analysis of gene expression assay. Tissue samples were collected at ZT8 and ZT20 after 2, 4, or 8-day treatment of aspirin. In addition, another set of mice treated with HSD for 4 weeks was used, but for in vivo experiments only, including measurements of BP, HR, and locomotor activity. All procedures were approved by the University of Pennsylvania IACUC and the Animal Center at Dalian Medical University, Dalian, China.
Telemetry recordings
Under general anesthesia, PA-C10 telemetry probes (Data Sciences, St. Paul, MN) were implanted in the carotid artery as previously described14. Telemetry recordings began 7 days after surgery. Continuous BP and HR were monitored in unrestricted animals with the use of the Dataquest IV system (Data Sciences).
Urinary prostaglandin (PG) metabolites and catecholamines
Systemic production of PGD2, PGE2, PGI2, and TxA2 was determined by mass spectrometric quantitation of their major urinary metabolites: PGD-M, PGE-M, PGI-M, and Tx-M, respectively as previously described20. Epinephrine (EPI), norepinephrine (NE), and dopamine (DOP) were measured using Elisa (17-TCTHU-E03.1, Alpco Immunoassays) following the manufacturer’s instruction. Data were corrected for urinary creatinine.
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA from aorta, heart, kidney and adrenal gland was extracted using RNeasy kit (QIAGEN) following the manufacturer’s instructions. The concentration of RNA was measured by nanodrop (Thermo Scientific). 0.5μg of each sample was applied reverse transcription with reagents obtained from Applied Biosystem. Taqman universal PCR Master Mix and probes (Applied Biosystems) for 18s rRNA, COXs, and core clock genes, Bmal1, Clock, Npas2, Reverbα, Per1, Per2, Cry1, Cry2, were used for real-time PCR.
Immunoprecipitation and western blot
MOVAS (a mouse aorta smooth muscle cell line) and SVEC4-10 cells (a mouse vascular endothelial cell line) were obtained from American Type Culture Collection (CRL-2797 and −2181, respectively). Primary culture of mouse aorta smooth muscle cells (VSMC) was described previously21. At 80% confluence, the cells, grown in a 12-well plate, were treated with 1mM aspirin for 24 hours, and then lysed in cell extraction buffer (Cat. No. FNN0011, Invitrogen). The cell lysates were applied for immunoprecipitation (IP) using rabbit antibodies (bmal1, sc-48790, Santa Cruz; Ac-Lysine, 9814, Cell Signaling Technology) and Dynabeads Protein G (100.03D, Invitrogen) following manufacturer’s instruction. The precipitated proteins were eluted using NuPAGE LDS sample buffer (NP0007, Invitrogen) containing a reducing agent (NP0004, Invitrogen) at 70℃. The eluted samples were then resolved by SDS-PAGE gel, transferred to PVDF membranes (LC2002, Invitrogen) and analyzed by using antibodies against bmal1, Ac-lysine, and p53 (sc-126, Santa Cruz). As negative controls, IP was performed in the absence of antibody or in the presence of rabbit IgG.
Quantification of COX-1 acetylation by aspirin
Mice were treated with 50μg ASA by gavage at ZT0 or ZT12. Blood samples were collected 0.5 or 24 hours after treatment and platelets were isolated for measurement of COX-1 acetylation by isotope dilution mass spectrometry in multiple reaction monitoring (MRM) mode as previously described22. Briefly, mouse COX-1 specific sequence (MGAPFSLK) and its acetylated form [MGAPFS(+ace)LK] were measured by their unique transitions on a TSQ Vantage triple stage quadrupole mass spectrometer (Thermo Scientific) interfaced with a Nano-ACQUITY UPLC system (Waters). Quantification information was extracted from the peak areas of the transitions using Xcalibur Quan Browser (Thermo Scientific). COX-1 acetylation was calculated using the ratio of peak area of the acetylated peptide divided by total peak area of non-modified peptide and modified peptide.
Clock gene oscillation by Bioluminescence
A clonal line of human osteosarcoma U2OS cells, stably expressing Bmal1-Luc or Per2-Luc reporters was generously provided by John Hogenesch (Cincinnati Children’s Hospital Medical Center). Cells were grown in the DMEM medium supplemented with 10% FBS, 0.3 mg/ml L-glutamine, 100 units/ml penicillin, and 100 g/ml streptomycin. For the luminescence recording, the cells were seeded in 3.5cm dishes in culture medium with addition of 0.1 mM luciferin and 0.1µM Dexamethasone. The luminescence was recorded by an automated robotic system (GNF Systems) equipped with a CCD imager (ViewLux, PerkinElmer).
Statistical Analysis
Statistical analysis was performed using Graph Pad Prism 7. All statistical tests were two-sided. Kruskal–Wallis one-way ANOVA and a subsequent Dunn’s multiple comparisons test was used when a single variable was compared between two or more groups, two-way ANOVA with Sidak multiple comparisons when two variables were compared between groups, and three-way ANOVA with Tukey’s multiple comparisons test when three variables exist as recommended by the software. The cutoff for significance was P < 0.05 (*). **P <0.01, ***P < 0.001. In all figures with error bars unless specified, the graphs depict means ± SEM.
Results
Administration of aspirin at the active/inactive transition has a protective effect on HSD-induced increase of blood pressure.
Two weeks on the HSD increased systolic BP (SBP) from 101.2 ± 0.9 to 110.6±1.9mmHg (P<0.05). Mice were then randomly allocated to aspirin (50µg/d) by gavage once per day for 7 consecutive days at times equivalent to awakening (ZT12) or bedtime (ZT0) while maintained on the HSD. The HSD continued to increase SBP in the group given aspirin at ZT12 (110.5±4.1 vs. 116.1±5.4mmHg, p=0.09, Fig. 2A) with a difference that was more pronounced during the light phase (104.6±4.1 vs. 112.4±5.1mmHg, p<0.05, Fig. 2B). However, when we administered aspirin at ZT0, the rise in SBP was absent (110.8±1.2 vs. 108.9±2.9mmHg). This effect was not accompanied by changes of body weight (Fig 2C). After four weeks on the HSD, ASA treatment at ZT0 lowered SBP in both light and dark phases (Fig. 3), and mean arterial pressure (MAP), HR, and locomotor activity in the light phase, while no obvious change was found in those dosed at ZT12 (table 1). Thus, we recapitulated the time dependent hypotensive effect of aspirin in HSD treated mice.
Figure 2.
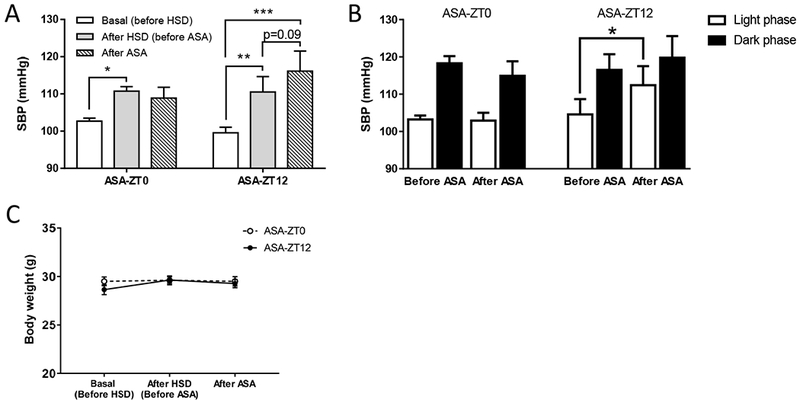
A, ASA (50μg/d for 7 days) administered at ZT0, but not ZT12, prevented a further increase of systolic blood pressure induced by 2-w HSD (2-way ANOVA; *, p<0.05; **; p<0.01; n=6-7). B, The increase of SBP in ASA-ZT12 group was more pronounced during the light phase. (2-way ANOVA; *, p<0.05; n=6–7). C, There is no statistical difference in body weight between the ASA-ZT0 and ASA-ZT12 dosing groups (2-way ANOVA; n=10).
Figure 3.
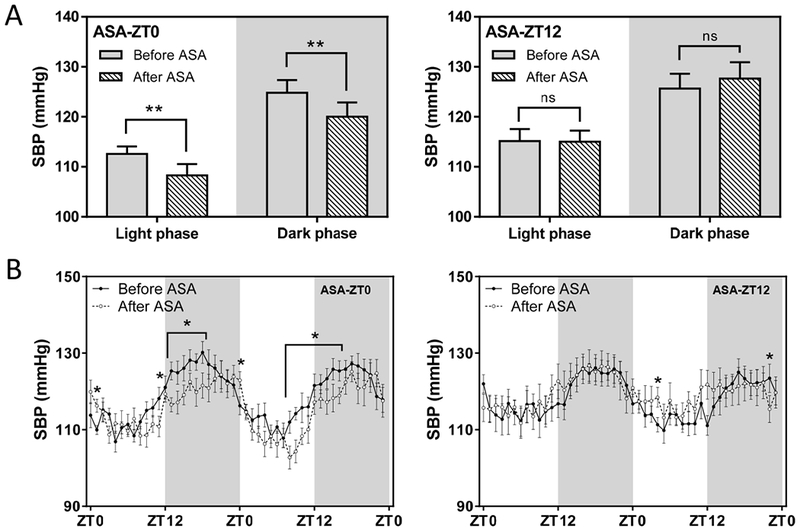
A, ASA (50μg/d for 7 days) administered at ZT0, but not ZT12, lowers high blood pressure induced by 4-w HSD in both light and dark (grey background) phases (2-way ANOVA; *, p<0.05; ns, no statistical difference; n=7–8). B, SBP in A depicted in two consecutive days showed that ASA administered at ZT0 lowers BP at multiple time points across both light and dark phases. (2-way ANOVA; *, p<0.05; n=7–8)
Table 1.
BP, HR, pulse pressure (PP) and activity parameter averages (±SEM) as measured via radiotelemetry on mice fed with HSD for 4-w before ASA treatment. MAP: mean arterial pressure, DBP: diastolic blood pressure. 2-way ANOVA;
| ASA-ZT0 | ASA-ZT12 | |||
|---|---|---|---|---|
| Day | Night | Day | Night | |
| Before ASA treatment | ||||
| SBP (mmHg) | 112.6±1.52 | 124.8±2.54 | 115.1±2.41 | 125.7±2.96 |
| MAP (mmHg) | 99.1±1.13 | 110.7±2.17 | 101.3±2.14 | 111.3±2.55 |
| DBP (mmHg) | 85.1±1.23 | 96.2±2.12 | 87.4±2.61 | 96.6±2.62 |
| PP (mmHg) | 27.4±1.39 | 28.5±1.54 | 28.0±1.77 | 29.0±1.75 |
| HR (bpm) | 470.7±4.70 | 505.5±4.82 | 463.9±9.88 | 531.0±9.94 |
| Activity (arbitrary counts) | 3.0±0.40 | 9.1±2.25 | 2.7±0.57 | 9.3±1.79 |
| After ASA treatment | ||||
| SBP (mmHg) | 108.3±2.24** | 120.0±2.88* | 115.0±2.24 | 127.6±3.26 |
| MAP (mmHg) | 96.3±1.38** | 107.9±1.92 | 100.7±2.06 | 112.9±2.79 |
| DBP (mmHg) | 84.2±0.75 | 95.6±1.31 | 85.9±2.49 | 97.8±2.74 |
| PP (mmHg) | 24.1±1.83 | 24.4±2.04 | 29.2±1.57 | 29.9±1.68 |
| HR (bpm) | 446.0±7.74** | 503.5±10.64 | 487.3±8.47 | 535.4±8.10 |
| Activity (arbitrary counts) | 1.7±0.28** | 6.4±1.03 | 2.1±0.37 | 6.8±0.72 |
P<0.01,
P<0.05, after ASA treatment vs. before ASA treatment.
No time dependent effect of aspirin on the expression of circadian genes in mouse heart, aorta, kidney or adrenal gland.
At the end of the in vivo experiments, we collected several organs relevant to BP regulation including heart, aorta, kidney and adrenal gland at ZT8 and ZT20 when both Bmal1 and its reciprocal core clock partner, Rev-erbα, show differential gene expression12. We found no evidence that time of aspirin dosing differentially impacted expression of the canonical clock genes, Bmal1, Clock, Npas2, Rev-erbα, Per1, Per2, Cry1, or Cry2 at either ZT8 or ZT20 at the end of the study (Fig. 4). To address the possibility that there might have been a reversible impact on clock genes at an earlier stage in the dosing period, we performed another experiment in which mice were sacrificed for tissue collection at multiple time points. As shown in Figure I (in the online-only Data Supplement), among all comparisons between ASA-ZT0 and ASA-ZT12, only Per2 in the heart collected on day-4 showed a statistical difference, noted at ZT8.
Figure 4.
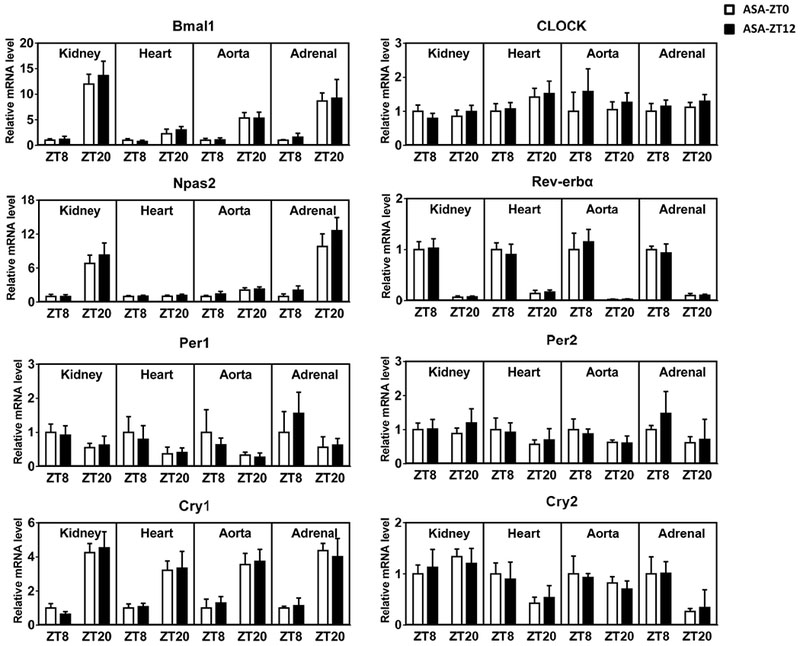
mRNA levels of core clock genes in the kidney, heart, aorta, and adrenal gland were revealed by qRT-PCR. All data were normalized to 18s rRNA. There is no obvious difference between ASA-ZT0 and ASA-ZT12 groups in any examined tissues collected either at ZT8 or ZT20 (2-way ANOVA; n=5–6).
Aspirin failed to influence directly the molecular clock.
We found that P53 was acetylated or its acetylation was increased by ASA in MOVAS and SVEC4-10 cells. Acetylation of Bmal1 was also detected, however, the pattern was not affected by ASA (Fig. 5A). None of the other clock proteins including Clock, Per1, Per2, Cry1 and Cry2 were found acetylated by ASA treatment. Acetylation of platelet COX-1, the mechanism by which aspirin prevents thrombosis, was unaltered by time of drug administration (Fig. 5B). Using Bmal1-Luc and Per2-Luc reporter expressing U2OS cell lines, ASA (0.1mM, 0.5mM, and 2mM), failed to alter the period, amplitude or phases of the oscillating bioluminescence rhythms (Fig. 6).
Figure 5.
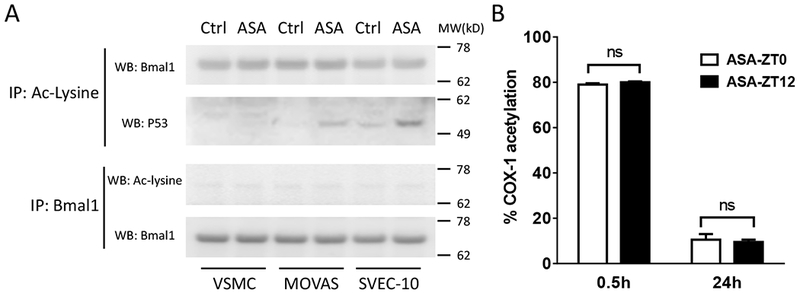
A, Acetylation pattern of P53 (as a positive control) and Bmal1 in ASA treated blood vessel cells was sought using immunoprecipitation (IP) and Western blotting (WB). MW: molecular weight; B, Mouse platelet COX-1 acetylation did not differ at 0.5 or 24 hours after ASA treatment at ZT0 or ZT12 (2-way ANOVA; ns, no statistical difference; n=4).
Figure 6.
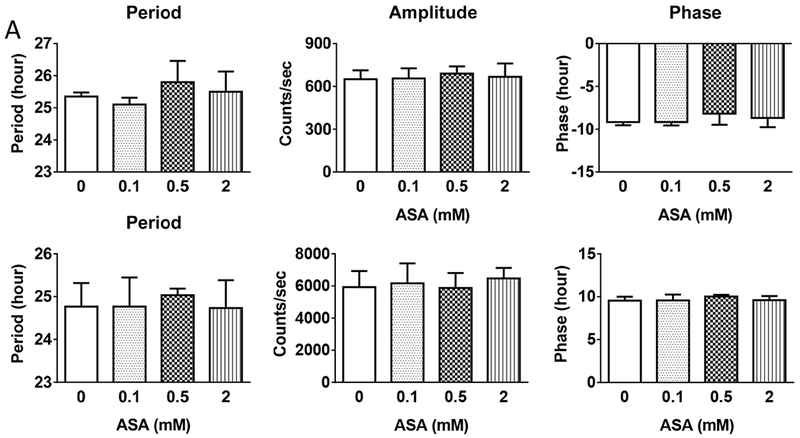
ASA at multiple concentrations did not alter the oscillation of circadian clocks in Bmal1 (A) or Per2 (B)-luciferase reporter expressed U2OS cells. Similar results were obtained from an independent replication of experiment (1-way ANOVA; n=4).
No time dependent effect of aspirin on urinary prostaglandin metabolites or catecholamines.
Aspirin administered at both ZT0 and ZT12 decreased prostaglandin biosynthesis as reflected by the urinary metabolites, PGD-M, PGE-M, PGI-M, and Tx-M. However, this impact was unaltered by the time of dosing (Fig. 7A). There was also no time dependent difference in EPI or NE when measured at the end of the study. DOP was lower on aspirin, but there was no difference between ASA-ZT0 and ASA-ZT12 (Fig. 7B).
Figure 7.
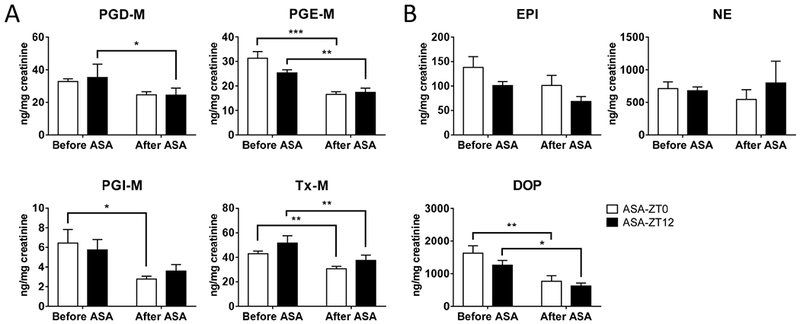
Urinary PG metabolites (A) and catecholamines (B) were measured using mass spectrometry and Elisa, respectively, (2-way ANOVA; *, p<0.05; **; p<0.01; ***, p<0.001; n=9–11). Data were normalized to creatinine.
Discussion
It has long been known that blood pressure (BP) and heart rate (HR) are subject to circadian rhythmicity; indeed, the failure of blood pressure to fall at night (“non-dipping”) has been associated with poor cardiovascular clinical outcomes23. These time-dependent effects are not just consequences of the sleep/wakefulness cycle, but are believed to be attributable to intrinsic properties of the cardiovascular system that fluctuate under the control of the molecular clock during the course of the day24, 25. During the last two decades, Hermida and colleagues have reported several randomized trials in hypertensive populations in which low dose aspirin (100mg) administered at night but not in the morning lowered BP in both genders8, 26–31. Recently, in a multicenter, double-blind, placebo-controlled trial, Rolnik et al found that treatment with 150mg aspirin at night in women at risk reduced the incidence of preterm preeclampsia11, however they did not control for time of drug administration. Despite these observations, there have been reasons for skepticism. Firstly, some groups have failed to reproduce the fundamental finding10, 32. Secondly, there is no mechanistic explanation for the phenomenon.
In the current study, we first recapitulated the time-dependent effect of aspirin on human blood pressure in mice. As in humans, ASA lowered blood pressure in hypertensive mice when administered at the transition between activity and rest. Ironically, replication of this phenotype in a model system lends credence to the veracity of the phenomenon in humans. Given the prevalence of hypertension and the effectiveness and commonality of aspirin consumption for the secondary prevention of cardiovascular disease, a population-based shift to evening dosing might have a considerable effect on cardiovascular outcomes. Interestingly, there was no apparent baroreflex mediated increase in heart rate coincident with the ASA mediated time dependent reduction in BP 28, 30. Here both HR and activity were decreased by aspirin treatment at ZT0 and this may have contributed to the BP lowering effect in mice. Here we saw no impact of ASA on urinary EPI and NE. Although urinary DOP was reduced on ASA, there was no time dependent effect apparent. In future studies we intend to elucidate further the potential contribution of autonomic function as well as addressing other signaling pathways that may underlie these observations.
Indeed, this model affords a more tractable opportunity to address mechanism. The current studies suggest that it does not involve a direct effect of aspirin on the core molecular clock. The time of ASA administration does not obviously influence expression of a variety of clock genes in heart, aorta, kidney or adrenal gland, tissues of direct relevance to blood pressure regulation and cardiovascular homeostasis. Aspirin prevents thrombotic events by acetylating platelet COX-1 but is recognized potentially to acetylate additional proteins33. Although some clock proteins can be modulated by acetylation34, ASA did not alter the acetylation patterns of Bmal1 in cultured mouse vascular smooth muscle cells or endothelial cells in vitro. None of the other clock proteins that we studied, including Clock, Per1, Per2, Cry1 and Cry2 were acetylated by ASA treatment. Besides, platelet COX-1 acetylation did not differ at 0.5 or 24 hours after ASA treatment at ZT0 or ZT12. Furthermore, ASA did not alter the oscillation of bioluminescence in U2OS cells expressing Bmal1 and Per2-luciferase reporters. Finally, although rhythmic histone acetylation underlies transcription in the mammalian circadian clock35, it is not acetylated by aspirin36. These results fail to support the hypothesis that the time dependent effect of ASA is attributable to a direct action on circadian clock.
Aspirin prevents thrombotic events like myocardial infarction by cumulatively acetylating platelet COX-1 dependent TxA2 formation when administered at low doses that have minimal impact on COX-2 derived prostaglandins like PGI23. Here the biochemical profile of the dose of aspirin we selected (suppression of urinary metabolites of PGs of the E, D and I series along with Tx) more closely resembles that of anti-inflammatory doses of aspirin in humans. None of the trials of time dependent aspirin administration in humans have used such higher doses although our recapitulation of the functional phenotype (and the failure to implicate platelet COX-1) suggests that the phenomenon may be independent of aspirin dose.
Our findings appear to contrast with the attenuation of the fall in blood pressure during the rest phase in mice globally deficient in COX-137. This may reflect differences in genetic background and/or variability in tissue distribution of aspirin as well as oscillation in its targets that at this dose would involve both COX-1 and COX-2.
In summary, the time dependent impact of aspirin on blood pressure in hypertensive patients was recapitulated in mice made hypertensive by a HSD. This does not result from a direct effect of aspirin on core clock gene expression or oscillation. Recapitulation of this time dependent effect in mice lends weight to the clinical observation and the argument to advise evening dosing with low dose aspirin by hypertensive patients who take it for cardio-protection.
Supplementary Material
Highlights.
Time dependent hypotensive effect of aspirin in hypertensive patients was recapitulated in mice.
This effect does not result from a direct impact of aspirin on core clock genes.
This effect does not reflect a direct impact of aspirin on acetylation of COX-1 or excretion of urinary prostaglandins and catecholamines.
Acknowledgments
None.
Sources of Funding
Supported by a grant from the National Institutes of Health (1U54 HL117798) and grants from the National Natural Science Foundation of China (81400750, 81670242 & 81570643). Dr. FitzGerald is the McNeil Professor in Translational Medicine and Therapeutics.
Nonstandard Abbreviations and Acronyms
- ASA
aspirin
- BP
blood pressure
- COX
cyclooxygenase
- DOP
dopamine
- EPI
epinephrine
- HR
heart rate
- HSD
high salt diet
- NE
norepinephrine
- PG
prostaglandin
- ZT
Zeitgeber Time
Footnotes
Disclosures
None.
References
- 1.Baigent C, Collins R, Appleby P, Parish S, Sleight P, Peto R. Isis-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The isis-2 (second international study of infarct survival) collaborative group. BMJ. 1998;316:1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis HD Jr, Davis JW, Archibald DG, Steinke WE, Smitherman TC, Doherty JE 3rd, Schnaper HW, LeWinter MM, Linares E, Pouget JM, Sabharwal SC, Chesler E, DeMots H Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a veterans administration cooperative study. N Engl J Med. 1983;309:396–403. [DOI] [PubMed] [Google Scholar]
- 3.Fitzgerald DJ, Fitzgerald GA. Historical lessons in translational medicine: Cyclooxygenase inhibition and p2y12 antagonism. Circ Res. 2013;112:174–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu Y, Ricciotti E, Scalia R, Tang SY, Grant G, Yu Z, Landesberg G, Crichton I, Wu W, Pure E, Funk CD, FitzGerald GA. Vascular cox-2 modulates blood pressure and thrombosis in mice. Sci Transl Med. 2012;4:132ra154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng Y, Wang M, Yu Y, Lawson J, Funk CD, Fitzgerald GA. Cyclooxygenases, microsomal prostaglandin e synthase-1, and cardiovascular function. J Clin Invest. 2006;116:1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the hypertension optimal treatment (hot) randomised trial. Hot study group. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 7.Hermida RC, Ayala DE, Iglesias M. Administration time-dependent influence of aspirin on blood pressure in pregnant women. Hypertension. 2003;41:651–656. [DOI] [PubMed] [Google Scholar]
- 8.Hermida RC, Ayala DE, Iglesias M, Mojon A, Silva I, Ucieda R, Fernandez JR. Time-dependent effects of low-dose aspirin administration on blood pressure in pregnant women. Hypertension. 1997;30:589–595. [DOI] [PubMed] [Google Scholar]
- 9.Hermida RC, Fernandez JR, Ayala DE, Mojon A, Iglesias M. Influence of aspirin usage on blood pressure: Dose and administration-time dependencies. Chronobiol Int. 1997;14:619–637. [DOI] [PubMed] [Google Scholar]
- 10.Dimitrov Y, Baguet JP, Hottelart C, Marboeuf P, Tartiere JM, Ducher M, Fauvel JP. Is there a bp benefit of changing the time of aspirin administration in treated hypertensive patients? Eur J Prev Cardiol. 2012;19:706–711. [DOI] [PubMed] [Google Scholar]
- 11.Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–622. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111:16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paschos GK, Baggs JE, Hogenesch JB, FitzGerald GA. The role of clock genes in pharmacology. Annu Rev Pharmacol Toxicol. 2010;50:187–214. [DOI] [PubMed] [Google Scholar]
- 14.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cugini P, Letizia C, Scavo D. The circadian rhythmicity of serum angiotensin converting enzyme: Its phasic relation with the circadian cycle of plasma renin and aldosterone. Chronobiologia. 1988;15:229–231. [PubMed] [Google Scholar]
- 16.Hossmann V, Fitzgerald GA, Dollery CT. Circadian rhythm of baroreflex reactivity and adrenergic vascular response. Cardiovasc Res. 1980;14:125–129. [DOI] [PubMed] [Google Scholar]
- 17.Alfonso LF, Srivenugopal KS, Bhat GJ. Does aspirin acetylate multiple cellular proteins? (review). Mol Med Rep. 2009;2:533–537. [DOI] [PubMed] [Google Scholar]
- 18.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. Sirt1 regulates circadian clock gene expression through per2 deacetylation. Cell. 2008;134:317–328. [DOI] [PubMed] [Google Scholar]
- 19.Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. Clock-mediated acetylation of bmal1 controls circadian function. Nature. 2007;450:1086–1090. [DOI] [PubMed] [Google Scholar]
- 20.Song WL, Lawson JA, Wang M, Zou H, FitzGerald GA. Noninvasive assessment of the role of cyclooxygenases in cardiovascular health: A detailed hplc/ms/ms method. Methods Enzymol. 2007;433:51–72. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Yang G, Xu X, Grant G, Lawson JA, Bohlooly YM, Fitzgerald GA. Cell selective cardiovascular biology of microsomal prostaglandin e synthase-1. Circulation. 2013;127:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Fries S, Li R, Lawson JA, Propert KJ, Diamond SL, Blair IA, FitzGerald GA, Grosser T. Differential impairment of aspirin-dependent platelet cyclooxygenase acetylation by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci U S A. 2014;111:16830–16835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor KS, Heneghan CJ, Stevens RJ, Adams EC, Nunan D, Ward A. Heterogeneity of prognostic studies of 24-hour blood pressure variability: Systematic review and meta-analysis. PLoS One. 2015;10:e0126375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paschos GK, Fitzgerald GA. Circadian clocks and vascular function. Circ Res. 2010;106:833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durgan DJ, Young ME. The cardiomyocyte circadian clock: Emerging roles in health and disease. Circ Res. 2010;106:647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Ambulatory blood pressure control with bedtime aspirin administration in subjects with prehypertension. Am J Hypertens. 2009;22:896–903. [DOI] [PubMed] [Google Scholar]
- 27.Hermida RC, Ayala DE, Calvo C, Lopez JE. Aspirin administered at bedtime, but not on awakening, has an effect on ambulatory blood pressure in hypertensive patients. J Am Coll Cardiol. 2005;46:975–983. [DOI] [PubMed] [Google Scholar]
- 28.Hermida RC, Ayala DE, Calvo C, Lopez JE, Mojon A, Rodriguez M, Fernandez JR. Differing administration time-dependent effects of aspirin on blood pressure in dipper and non-dipper hypertensives. Hypertension. 2005;46:1060–1068. [DOI] [PubMed] [Google Scholar]
- 29.Hermida RC, Ayala DE, Calvo C, Lopez JE, Fernandez JR, Mojon A, Dominguez MJ, Covelo M. [administration-time dependent effects of acetyl-salicylic acid on blood pressure in patients with mild essential hypertension]. Med Clin (Barc). 2003;120:686–692. [DOI] [PubMed] [Google Scholar]
- 30.Hermida RC, Ayala DE, Calvo C, Lopez JE, Fernandez JR, Mojon A, Dominguez MJ, Covelo M. Administration time-dependent effects of aspirin on blood pressure in untreated hypertensive patients. Hypertension. 2003;41:1259–1267. [DOI] [PubMed] [Google Scholar]
- 31.Hermida RC, Ayala DE, Fernandez JR, Mojon A, Alonso I, Silva I, Ucieda R, Codesido J, Iglesias M. Administration time-dependent effects of aspirin in women at differing risk for preeclampsia. Hypertension. 1999;34:1016–1023. [DOI] [PubMed] [Google Scholar]
- 32.Bonten TN, Snoep JD, Assendelft WJ, Zwaginga JJ, Eikenboom J, Huisman MV, Rosendaal FR, van der Bom JG. Time-dependent effects of aspirin on blood pressure and morning platelet reactivity: A randomized cross-over trial. Hypertension. 2015;65:743–750. [DOI] [PubMed] [Google Scholar]
- 33.Alfonso LF, Srivenugopal KS, Bhat GJ. Does aspirin acetylate multiple cellular proteins? Molecular Medicine REPORTS. 2009 [DOI] [PubMed] [Google Scholar]
- 34.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The nad+-dependent deacetylase sirt1 modulates clock-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature. 2003;421:177–182. [DOI] [PubMed] [Google Scholar]
- 36.Sonnemann J, Huls I, Sigler M, Palani CD, Hong le TT, Volker U, Kroemer HK, Beck JF. Histone deacetylase inhibitors and aspirin interact synergistically to induce cell death in ovarian cancer cells. Oncol Rep. 2008;20:219–224. [PubMed] [Google Scholar]
- 37.Kawada N, Solis G, Ivey N, Connors S, Dennehy K, Modlinger P, Hamel R, Kawada JT, Imai E, Langenbach R, Welch WJ, Wilcox CS. Cyclooxygenase-1-deficient mice have high sleep-to-wake blood pressure ratios and renal vasoconstriction. Hypertension. 2005;45:1131–1138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


