Abstract
Arginine vasopressin (AVP) made by hypothalamic neurons is released into the circulation to stimulate water resorption by the kidneys and restore water balance after blood loss. Patients who lack this antidiuretic hormone suffer from central diabetes insipidus. We observed that many of these patients were anemic and asked whether AVP might play a role in red blood cell (RBC) production. We found that all three AVP receptors are expressed in human and mouse hematopoietic stem and progenitor cells. The AVPR1B appears to play the most important role in regulating erythropoiesis in both human and mouse cells. AVP increases phosphorylation of signal transducer and activator of transcription 5, as erythropoietin (EPO) does. After sublethal irradiation, AVP-deficient Brattleboro rats showed delayed recovery of RBC numbers compared to control rats. In mouse models of anemia (induced by bleeding, irradiation, or increased destruction of circulating RBCs), AVP increased the number of circulating RBCs independently of EPO. In these models, AVP appears to jump-start peripheral blood cell replenishment until EPO can take over. We suggest that specific AVPR1B agonists might be used to induce fast RBC production after bleeding, drug toxicity, or chemotherapy.
INTRODUCTION
After hemorrhage, there is a compensatory increase in red blood cell (RBC) production (erythropoiesis). Erythropoietin (EPO), a hormone made by specialized cells in the kidney, drives this via effects on survival, proliferation, and differentiation of erythroid progenitor cells (1). However, it does not act very rapidly. Three to 4 days pass before immature RBCs (reticulocytes) are seen in the blood after EPO administration (1).
Hypovolemia or hyperosmolality are strong stimuli for both synthesis and release of arginine vasopressin (AVP) from the posterior pituitary (2–4). Blood loss resulting in hypovolemia and hypotension is immediately followed by AVP release into the circulation (2). In dogs, AVP concentrations in plasma are 40 times greater than normal shortly after the onset of experimental hemorrhagic shock and gradually decline thereafter (5). In humans, hemorrhage may cause a 50- to 100-fold increase in circulating AVP concentrations (3) paralleled by increases in plasma concentrations of EPO, catecholamines, cortisol, aldosterone, and renin/angiotensin (6). In the first 48 hours after a severe hemorrhage, two peaks of mitotic activity (at 4 and 18 hours) are observed in the bone marrow of rats (7). The first of these is absent in hypophysectomized rats and in rats that have congenital diabetes insipidus [rats that lack AVP (7)]. We noticed that many people who suffer from central diabetes insipidus (CDI) are also anemic and hypothesized that vasopressin might directly stimulate erythropoiesis. Here, we show that hematopoietic stem and progenitor cells (HSPCs), especially the more mature species, have vasopressin receptors and rapidly respond to vasopressin by proliferating and differentiating. Thus, AVP appears to speed the proliferation/differentiation of bone marrow erythroid precursors in anemia and release RBCs from the bone marrow to jump-start replenishment of blood cells until EPO can take effect.
RESULTS
Patients with CDI have anemia
We analyzed the records of patients in the National Institutes of Health (NIH) clinical database [Biomedical Translational Research Information System (BTRIS)] who had well-documented CDI [defined as having a consistent diagnosis, sometimes over years, and being successfully treated with desmopressin (dDAVP or 1-deamino-8-D-AVP)]. The BTRIS database spans two decades, and many of the patients have been followed at the NIH Clinical Center for long periods of time. Some of the patients had primary CDI; more commonly, they developed it after surgery for pituitary tumors. In some cases, we were unable to determine whether the CDI was primary (inherited) or secondary.
Among the 92 patients with CDI, 45 were male (1 to 65 years of age), and 47 were female (4 to 60 years of age). Eighty-seven percent of the males and 51% of the females were always or intermittently anemic. Figure 1A shows the patients’ average hematocrit (Hct), hemoglobin (Hgb), and RBC distribution width (RDW) values (see table S1 for more RBC indices). In the United States, only 1.5 to 6% of males and 4.4 to 12.2% of females in these age ranges are anemic (8). We compared the rates of anemia in the CDI patients to the maximum percentages seen in the “normal” population—6% in males and 12.2% in females, testing the hypothesis that the proportions in the CDI population and the U.S. population at large are the same, using a two-sample binominal test of proportions, rejecting the null hypothesis if our Z score is >1.96 (α = 0.05, two-sided). For both males and females, our Z scores were >>>1.96, giving P < 0.001.
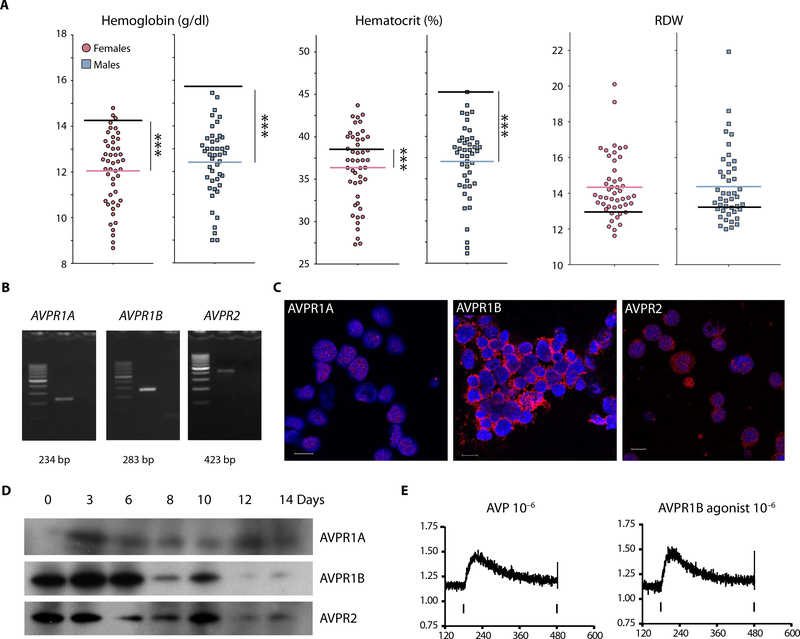
Fig. 1. Anemia in patients with central diabetes insipidus.
(A) Ninety-two patients who had central diabetes insipidus were identified at the National Institutes of Health Clinical Center: 45 women (red circles) and 47 men (blue squares). Their hemoglobin, hematocrit, and red blood cell distribution width (RDW) values were averaged for all the measurements made and are depicted by a single, thin red or blue line on the graph. The black line on each graph represents the average values in healthy subjects. The hematocrit and hemoglobin values of these patients are significantly (***P < 0.0001) different from the general U.S. population. (B) Reverse transcription polymerase chain reaction (RT-PCR)-based detection of AVPR1A, AVPR1B, and AVPR2 mRNAs in human CD34+ cells. Lane 1,100-base pair (bp) ladder;lane 2, RT-PCR product;lane3, no template. (C) Human CD34+cells on day 3 of two-phase liquid cultures (immunocytochemistry). All three arginine vasopressin (AVP) receptors are present. AVPR1B appears to be much more abundant than AVPR1A and AVPR2. Scale bars, 10 mm. (D) AVPR1A, AVPR1B, and AVPR2 receptor proteins during erythroid differentiation in two-phase liquid culture (Western blotting). (E) AVP and d(Cha4)-AVP (an AVPR1B agonist) increase intracellular Ca2+ in human hematopoietic stem and progenitor cells (HSPCs).
Thus, we concluded the following:
Sixty percent of the patients with CDI were anemic despite the fact that they were being treated with dDAVP.
During their anemic periods, the patients’ Hct, Hgb, and RBC numbers are low. Their mean corpuscular volume values were within normal limits (table S1). Their serum sodium concentrations were normal (table S1), indicating that the anemia did not result from dDAVP-induced hemodilution.
Many of the anemic patients had abnormally large RDWs, just as patients with myelodysplasia do (9). Although the doses of dDAVP used to treat CDI were sufficient to restore water homeostasis, it is possible that they were not enough to drive RBC production efficiently in the patients who were anemic.
All three AVP receptors are expressed in human HSPCs and respond to AVP and AVPR1B stimulation by increasing intracellular calcium
Using a microarray-based method (10), we previously found that hematopoietic progenitors (human CD34+/CD38- and CD34+/CD38+ cells) make mRNAs that encode AVP receptors. We looked for vasopressin receptor mRNAs in a population of HSPCs (11, 12) using reverse transcription polymerase chain reaction (RT-PCR). As shown in Fig. 1B, mRNAs encoding all three AVP receptors were detected in human HSPCs. Immunocytochemical (Fig. 1C) and Western blot (Fig. 1D) analyses of AVP receptors during erythroid differentiation in a two-phase liquid culture showed that all AVP receptors are expressed. AVPR1B and AVPR2 receptors appeared to decrease during terminal differentiation of erythroid cells.
To identify which AVP receptors regulate hematopoiesis in HSPCs, we first asked whether AVP drives an increase in intracellular calcium. As shown in Fig. 1E, both AVP and d(Cha4)-AVP, a human-specific AVPR1B agonist, increase calcium in the cytoplasm of human CD34+ cells. [Phe2,Ile3,Orn8]VP, a specific AVPR1A receptor agonist, had no effect on intracellular calcium at physiological dose (fig. S1A). AVP also increased cyclic adenosine 3’,5’-monophosphate (cAMP) in human CD34+ progenitors harvested from two donors (fig. S1B). These results suggested that AVPR1B and/or AVPR2 could potentially affect hematopoiesis.
AVP drives the proliferation of hematopoietic progenitors via AVP1B receptors
To determine whether AVP1B drives hematopoiesis, we looked at the proliferation of human CD34+ cells stimulated with AVP or the AVPR1B-specific agonist d(Cha4)-AVP in the presence or absence of the AVPR1B antagonist, SSR 149415. A 5-bromo-2’-deoxyuridine (BrdU) proliferation assay showed that both AVP and d(Cha4)-AVP increased the proliferation of cultured human HSPCs (Fig. 2A). These increases in proliferation were completely blocked by the AVPR1B antagonist. These results suggested that the AVP1B receptor plays a key role in promoting human HSPC proliferation. Because giving a peripherally active AVPR1B agonist should be relatively free from side effects (13), we focused on this receptor in subsequent experiments
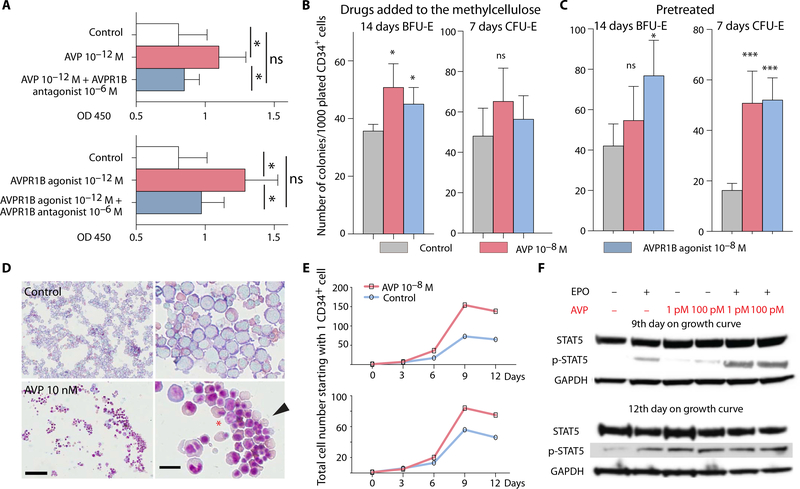
Fig. 2. Erythroid differentiation of human CD34+ cells promoted by AVP.
(A) Both AVP and AVPR1B agonist cause HSPCs (CD34+) to proliferate in vitro. This effect was blocked by a specific AVPR1B antagonist. Data and error bars are means + SEM;ns, not significant. *P < 0.05. OD 450, optical density at 450 nm. (B) Colony-forming assays were conducted to determine burst-forming unit-erythroid (BFU-E) and colony-forming unit-erythroid (CFU-E) colony numbers when CD34+ cells were exposed to AVP or a specific AVPR1B agonist added to the methylcellulose. AVP and the AVPR1B agonist significantly increased BFU-E colonies but not CFU-E. n = 3 independent donors and two replicates each. Data and error bars are means + SEM. *P < 0.05. (C) Colony-forming assays were conducted after 48 hours of treatment with AVP or AVPR1B agonist. Both AVP and AVPR1B agonist significantly increased CFU-E, but only the AVPR1B agonist increased BFU-E colonies. n = 2 independent donors and two replicates each. Data and error bars are means + SEM. *P < 0.05, ***P < 0.001. (D) May-Grünwald/Giemsa staining of cells from BFU-E colonies. Cells were harvested by Cytospin after growing for 10 days in control medium or medium with 10 μM AVP. Most of the cells in the control colonies are basophilic erythroblasts. Addition of AVP seems to accelerate differentiation; many reticulocytes can be seen, indicated by the arrowhead. The asterisk is placed between two erythrocytes that are in the process of enucleation to become reticulocytes. Scale bars, 100 μm (low magnification;left) and 10 μm (high magnification; right). (E) Human CD34+ cells were cultured with or without AVP. The cells were counted every 3 days. Each graph represents one donor. (F) Western blots of p-STAT5 and signal transducer and activator of transcription 5 (STAT5). Human CD34+ cells were challenged with erythropoietin (EPO), AVP, or EPO + AVP on days 9 and 12. On day 9, the effect of EPO + AVP on STAT5 phosphorylation was markedly greater than the effects of EPO or AVP alone. On day 12, the late-stage erythroid cells were more responsive to AVP than EPO, and the two agents no longer appeared to act in concert. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
AVP increases expansion of erythroid elements in cultures of human CD34+ cells
We assessed the effects of AVP on human hematopoietic progenitor cells with a methylcellulose colony assay in two ways. In one condition, freshly isolated HSPCs were placed into methylcellulose that contained AVP or the AVPR1B agonist. In the other, HSPCs were pretreated with AVP or the AVPR1B agonist before being placed in methylcellulose that contained no AVP or agonist. As shown in Fig. 2B, when AVP and the AVPR1B agonist d(Cha4)-AVP were added to the medium, they increased burst-forming unit-erythroid (BFU-E; an early erythroid progenitor) but not colony-forming unit-erythroid (CFU-E; a late erythroid progenitor) numbers. Norepinephrine was used as a positive control for BFU-E effects (fig. S2). In the other condition, HSPCs were pretreated with AVP or the AVPR1B agonist for 48 hours in liquid culture and then plated, but no AVP or agonist was added in the methylcellulose medium. AVP and d(Cha4)-AVP increased CFU-E, but only d(Cha4)-AVP affected BFU-E (Fig. 2C). These results suggest that short exposure to AVP and d(Cha4)-AVP stimulates CFU-E and that AVP and the AVPR1B agonist accelerate erythroid progenitor cell differentiation.
We have also observed that cells isolated (using Cytospin) from selected BFU-E colonies grown in methylcellulose were composed of a homogeneous collection of mostly basophilic erythroblasts, whereas those from colonies grown in the presence of AVP consisted of more mature RBCs, including many reticulocytes that have already expelled their nuclei (Fig. 2D). Furthermore, colonies grown in the presence of AVP and AVPR1B agonist (fig. S3) were larger in size than those grown in control medium.
To see whether AVP and the AVPR1B agonist could accelerate the production of terminally differentiated erythroid cells, we induced human CD34+ cells to form erythroid cells in a two-phase liquid culture (14) for 12 days with AVP. We looked at the growth characteristics in the presence and absence of AVP. Single human CD34+ cells from two healthy donors proliferated faster in the presence of AVP starting at day 6, and the difference between AVP-treated and untreated cell proliferation was further increased after 9 and 12 days in culture (Fig. 2E). Forty to 50% more erythroid cells were observed in the presence of AVP than in control cultures, suggesting that AVP can increase erythroid differentiation.
To understand the signaling pathway by which AVP promotes erythroid differentiation, we looked at the phosphorylation of signal transducer and activator of transcription 5 (STAT5) because EPO-driven proliferation and survival of erythroid precursors have been shown to depend on STAT5 phosphorylation (15–17). Western blot analyses (Fig. 2F) and quantification (fig. S4) after 9 days in two-phase erythroid cultures showed that EPO activated STAT5 and that AVP treatment further increased EPO-stimulated p-STAT5 production. At this time, AVP alone had little or no effect on p-STAT5. After 12 days of the two-phase culture, AVP alone increased p-STAT5 as much as EPO did, but the combination of EPO and AVP was neither additive nor synergistic. Overall, these data suggest that AVP can regulate erythroid precursor formation and accelerate terminal erythroid differentiation.
Brattleboro (AVP-deficient) rats show delayed recovery from anemia
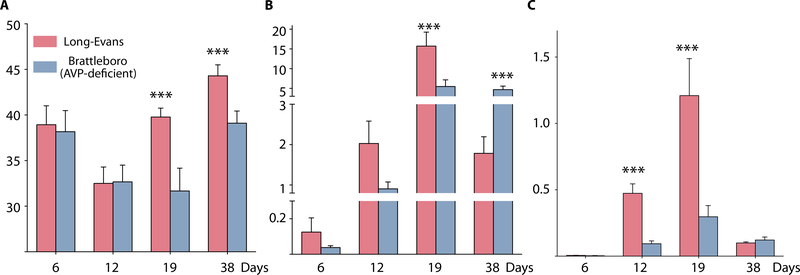
To study the role of AVP in anemia, we first used Brattleboro (BB) rats. These animals cannot synthesize AVP and have diabetes insipidus. BB rats are homozygous for a frameshift mutation in the coding region of the AVP gene. This mutation arose spontaneously in a population of Long-Evans (LE) rats that can be used as controls (18). We exposed the BB and LE rats to sublethal irradiation to destroy most of their hematopoietic stem cells (HSCs) and then monitored their Hct and reticulocyte counts over time. As shown, by day 19, LE rats had higher Hcts (Fig. 3A) and reticulocyte counts (Fig. 3B) than BB rats. Furthermore, the LE rats had more high-intensity reticulocytes (the youngest reticulocytes in circulation) than the BB rats (Fig. 3C), suggesting that AVP promotes the release of immature erythrocyte precursors from the bone marrow. These results suggest that AVP is required for stress erythropoiesis.
Fig. 3. Recovery of AVP-deficient rats from anemia induced by sublethal irradiation.
AVP-deficient Brattleboro and control Long-Evans rats were subjected to sublethal irradiation. Hematocrit (A), corrected reticulocyte percentage (calculated by multiplying the reticulocyte percentage by the ratio of measured hematocrit and normal hematocrit) (B), and high-intensity reticulocyte percentage (C) were measured on the days indicated. The recovery of Brattleboro rats was significantly slower than the recovery of control Long-Evans rats. Data and error bars are means + SD. Brattleboro (n = 6), ***P < 0.001;Long-Evans (n = 9), ***P < 0.001.
AVP receptors are expressed in mouse HSCs and terminally differentiating erythroid cells, and AVP increases steady-state erythropoiesis
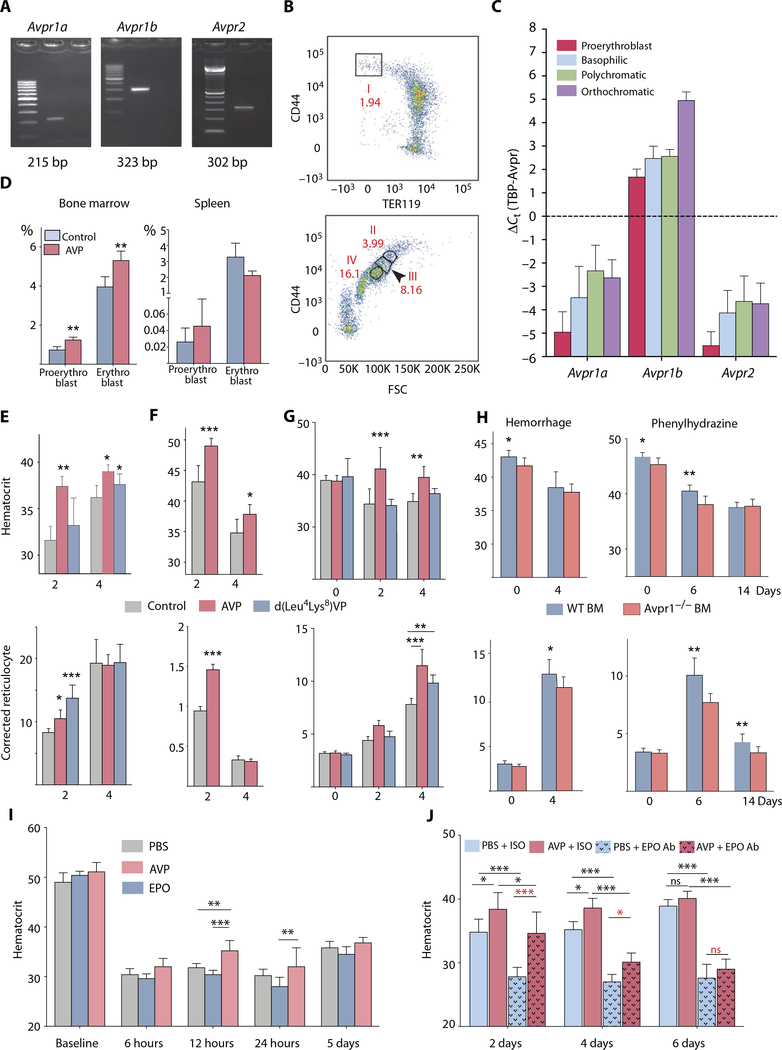
To determine whether AVP has effects on mouse HSCs similar to those that we observed in human cells, we began by confirming by RT-PCR that all three AVP receptors are expressed in mouse HSCs (Fig. 4A). We sorted the developing erythroid populations from mouse bone marrow using CD44 and Ter119 (Fig. 4B) (19) using fluorescence-activated cell sorting (FACS). All three receptor mRNAs were detected in the four erythroid subpopulations studied (Fig. 4C). The Avprlb transcript seemed to be the most abundant, and it gradually increased as the cells developed. The Avprlb mRNA expression was highest in orthochromatic erythroblasts. Thus, AVP receptors are expressed in mouse HSCs and terminally differentiating erythroid cells.
Fig. 4. The effects of AVP or d(Leu4Lys8)VP, a specific AVPR1B agonist, on anemic rodents.
(A) Avprla, Avprlb, and Avpr2 receptor expression in fluorescence-activated cell-sorted mouse bone marrow (BM) lin-Sca-1+c-kit+ (LSK) cells. Lane 1,100-bp marker; lane 2, RT-PCR product; lane 3, no template. (B) Flow cytometry sorting of mouse bone marrow erythroid lineage cells using CD44 and TER119 markers into four populations. Population I, proerythroblast; population II, basophilic; population III, polychromatic; population IV, orthochromatic. Numbers represent the percentage of total TER119-positive cells. n = 4 mice, C57BLL6. (C) Gene expression changes of Avprla, Avprlb, and Avpr2 receptors in four different populations of bone marrow erythroid lineage cells. Error bars represent SEM; n = 4 mice, C57BL/6. TBP, TATA box-binding protein. (D) C57BL/6 mice were injected with AVP (100 μg/kg), and after 16 hours, the erythroid progenitors in the bone marrow and spleen were analyzed with flow cytometry. Data and error bars are means + SD; n = 5 mice per group; **P < 0.01. (E) C57BL/6 mice were hemorrhaged and injected once with AVP (100 μg/kg) or AVPR1B agonist (100 μg/kg). Hematocrit and corrected reticulocytes were measured on the days indicated. After hemorrhage, both AVP and d(Leu4Lys8)VP (AVPR1B agonist) significantly improved recovery times. Data and error bars are means + SD; n = 6 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001. (F) C57BL/6 mice received whole-body irradiation (440 centigrays; two times within 12 hours) and were injected with AVP (100 μg/kg). Hematocrit and corrected reticulocytes were measured on the days indicated. After irradiation of the bone marrow, AVP administration resulted in a significantly higher hematocrit and reticulocyte values. Data and error bars are means + SD; n = 6 mice per group. *P < 0.05, ***P < 0.001. (G) C57BL/6 mice were splenectomized and allowed to recover for 1 month. The mice were subjected to hemorrhage and immediately injected with AVP (100 mg/kg) or AVPR1B agonist (100 μg/kg). Hematocrit and corrected reticulocytes were measured on the days indicated. In splenectomized mice, AVP still promoted recovery from anemia. Data and error bars are means + SD; n = 6 mice per group. **P < 0.01; ***P < 0.001. (H) Mice were sublethally irradiated and then given either wild-type (WT) or Avpr7b-deficient bone marrow. Two months later, they had 10 to 20% of their blood withdrawn or were treated with phenylhydrazine. Four days after hemorrhage, there was a significant increase in blood-corrected reticulocyte counts in animals given wild-type versus receptor-deficient bone marrow. This was not reflected in an increase in hematocrit. Six to 14 days after the phenylhydrazine treatment, corrected reticulocyte counts were significantly elevated in mice given wild-type bone marrow versus receptor-deficient bone marrow. On day 6, the hematocrit of the wild-type mice was also higher. Data and error bars are means + SD; n = 6 mice per group. *P < 0.05, **P < 0.01. (I) C57BL/6 mice were hemorrhaged (this sample was considered as the baseline) and injected with phosphate-buffered saline (PBS) or EPO (50 U/kg) or AVP (100 mg/kg). Hematocrits were measured at the time points indicated. AVP treatment significantly increased hematocrit by 12 hours compared to EPO or vehicle (PBS) treatment. Error bars represent SD; n = 6 mice per group. **P < 0.01, ***P < 0.001. (J) C57BL/6 mice were hemorrhaged and injected with either 100 μg of EPO-neutralizing antibody (Ab) or its isotype (ISO) control, with or without AVP (100 μg/kg). Hematocrits were measured at the time points indicated. Data and error bars are means + SD; n = 5 mice per group. *P < 0.05, ***P < 0.001.
To see whether AVP can affect steady-state erythropoiesis, we injected the peptide into mice (100 mg/kg body weight) and examined cells in the bone marrow and spleen by FACS 16 hours later. As shown in Fig. 4D, AVP increases the number of proerythroblasts and erythroblasts in the bone marrow but not in the spleen.
AVP speeds up the recovery of anemic mice
We tested the ability of AVP and d(Leu4Lys8)VP, an AVPR1B agonist that is specific for the rodent receptor, to stimulate RBC production in mice after hemorrhage or irradiation. After hemorrhage, the mice were treated with AVP, the AVPR1B agonist, or vehicle. By day 2, both Hct and reticulocyte numbers increased in AVP-treated mice versus vehicle-treated animals; only reticulocytes increased in AVPR1B agonist-treated mice (Fig. 4E).
Mice exposed to sublethal irradiation lose HSCs and progenitors (20). When such animals were treated with AVP for 2 days, increases in their Hcts and corrected reticulocyte numbers (Fig. 4F) were seen in AVP versus vehicle-treated mice.
In mice, the spleen has been implicated in extramedullary regeneration of blood cells after anemia (21). To see whether the effects of AVP on RBC numbers could result from release of splenic as opposed to bone marrow blood cells into the circulation, we splenectomized mice. After they recovered, we subjected them to hemorrhage. The AVP-induced increase in Hct in splenectomized mice was similar to that seen in non-splenectomized animals, suggesting that the spleen did not play a major role in quickly replenishing RBCs in mice in response to AVP (Fig. 4G). On the basis of the results summarized above, it appears that AVP plays an important role in stress-induced erythropoiesis.
The effect of AVP in mouse models of anemia is mediated by the AVP1b receptor
To learn about the effects of Avp1b receptor, specifically on bone marrow cells, we irradiated age- and gender-matched mice and transplanted the mice with either wild-type or Avprlb-deficient mouse bone marrow. After the mice recovered, anemia was induced by hemorrhage or phenylhydrazine (PHZ), an agent that lyses RBCs. In both anemia models, mice with wild-type bone marrow were less severely affected than those with Avprlb-deficient bone marrow (Fig. 4H). We conclude that the AVPR1B receptors are the principal mediators of AVP’s effects on RBC production in mice. This parallels the effects we see in human cells in vitro.
The effect of AVP on Hct is independent of EPO
To determine whether the effect of AVP on RBC production could be caused by EPO release, we designed two experiments. First, we injected wild-type mice with EPO or AVP immediately after hemorrhage and looked at Hct values at different times afterward. The effect of AVP on Hct seemed to begin within 6 hours; the increase was statistically significant (P < 0.001) 12 hours after blood loss, long before an effect of EPO was observed. Differences between the effects of EPO and AVP disappeared 5 days after hemorrhage (Fig. 4I).
In a second experiment, we injected mice with AVP or phosphate-buffered saline after hemorrhage and gave them an EPO-neutralizing antibody to eliminate the effect of endogenous EPO. Even after EPO neutralization, AVP increased Hct values (Fig. 4J), whereas without AVP injection, the Hct values dropped because the endogenously released EPO was no longer available to respond to blood loss. As time elapsed, the difference between Hct values of AVP-injected and control mice (which only have endogenous EPO) gradually disappeared. These experiments show that AVP acts faster than EPO and that it can function acutely independent of EPO, but later, EPO seems to take over.
DISCUSSION
Hemorrhage results in a loss of cells and plasma. In response to this, AVP, also known as the antidiuretic hormone, is released into the circulation by magnocellular cells of the hypothalamo-hypophyseal system (22). This hormone stimulates water retention by the kidneys, preventing further volume loss (22), but it has not been suggested to have a direct effect on the recovery of erythrocytes in the blood. Instead, stimulation of erythrocyte production is attributed to EPO, which is released by specialized interstitial fibroblasts in the kidney. EPO stimulates the division and differentiation of several consecutive cell populations in the bone marrow, and it takes 3 to 5 days for EPO to increase the number of reticulocytes and mature RBCs in the periphery (1, 23). Because HSCs and precursors have receptors for AVP, we wondered whether the hormone might help speed up RBC production. Several decades ago, Hunt et al. reported that there are two peaks of mitotic activity (at 4 and 18 hours) in the bone marrow of rats in the first 48 hours after a severe hemorrhage (7). The first of these is absent in hypophysectomized rats or BB rats, animals that lack AVP due to a mutation in its precursor (7). This supported the notion that AVP might, directly or indirectly, affect the proliferation of cells in the bone marrow.
The data in our study indicate that AVP can drive HSPC proliferation and differentiation. The concentration of AVP in human serum is normally 0.3 × 10−12 to 2 × 10−12 M (24). After blood loss during surgery, serum AVP concentrations increase to 0.5 × 10−10 to 1.0 × 10−10 M (25), concentrations that were optimal for the proliferative effect observed in vitro.
Exposing cultured human CD34+ progenitors to AVP or d(Cha4)-AVP (a selective human-specific AVPR1B receptor agonist) increased intracellular Ca2+, but no effect was seen when a specific AVPR1A agonist was tested. In addition to increasing calcium, AVP also increased intracellular cAMP. Although this is likely to have been caused by AVPR2 receptor activation, it is possible that AVPR1B receptors were involved (26). The data above suggest that the AVPR1B receptor may be the main mediator of the proliferative effect of AVP.
The slow recovery of RBC numbers in irradiated BB versus wildtype (LE) rats suggests that AVP has a physiological role in stress erythropoiesis. The involvement of AVP1B receptors in the process is supported by our observation that transplanted bone marrow cells lacking such receptors did not promote recovery of RBCs as effectively as the bone marrow derived from wild-type donors after lethal irradiation of the host mice. We studied three different models of anemia. After hemorrhage, peripheral blood cells and volume are lost; after PHZ treatment, RBCs are lysed, but there is no volume loss; and after irradiation, peripheral cells are not affected, but the bone marrow is injured. In all three of the models studied, AVP induced a rapid increase in reticulocytes and Hct. Young (high-intensity) reticulocytes appeared to enter the bloodstream especially quickly.
In principle, AVP could act indirectly by increasing EPO. However, this seems unlikely because exogenously administered AVP acts more quickly than EPO does, within 12 hours of hemorrhage. EPO needs 3 to 5 days to increase peripheral blood counts (1). Furthermore, we found that neutralization of EPO did not eliminate the fast action of AVP. On the other hand, we could demonstrate that AVP and EPO act together on STAT5 phosphorylation by early erythroid progenitors in vitro but that AVP induces this effect alone in late-stage erythroid progenitors. These results suggest that AVP acts independently ofEPO on late-stage RBC precursors. It was just as effective in splenectomized animals as it is in normal mice. Thus, the actions ofAVP cannot be attributed to release of blood cell reserves in the spleen.
On the basis of our results, we suggest that AVP has multiple roles in animals that have lost blood and that it acts in concert with EPO (fig. S5). AVP activates AVPR2 receptors in the kidney (27), stimulating water resorption to compensate for volume loss. It helps to stop bleeding by releasing von Willebrand factor from endothelial cells (28). Simultaneously, it jump-starts hematopoiesis contributing to the replacement of lost erythrocytes. In addition to inducing proliferation and likely speeding up differentiation of blood cell precursors, AVP also seems to be able to stimulate the movement of large numbers of immature reticulocytes from the bone marrow into the circulation. These cells appear larger and contain more reticulum (cytoplasmic reticular network of ribosomal RNA) than normal reticulocytes. Finally, STAT5 phosphorylation by AVP at the late stages of erythropoiesis might reduce apoptosis of more mature erythroid cells in the bone marrow after hemorrhage, because the anti-apoptotic gene Bcl-xL is induced by p-STAT5 (15). AVP has anti-apoptotic effects in a variety of mammalian cells, including renal tubular and endothelial cells (29). This anti-apoptotic effect might contribute to the sudden elevation of Hct and circulating reticulocytes.
Currently, EPO is the only agent that is used clinically to stimulate erythropoiesis, but there are patients who do not respond to EPO or who cannot take the drug because it stimulates tumor growth. AVP appears to be an EPO-independent, fast-acting agent that increases RBC numbers after anemia. It jump-starts hematopoiesis while EPO induces the production of early progenitors and therefore takes effect later. Support for the idea that vasopressin may play an important role in regulating RBC production in humans comes from our observation that patients with CDI (vasopressin deficiency) appear to suffer from anemia much more commonly than people in the general population do. A shortcoming of the information that we extracted from the NIH BTRIS database is that the patients’ diagnoses are not always well annotated. For example, some of the patients have inherited CDI, some developed it after they underwent surgery to remove pituitary tumors, and in other cases it was not clear whether the problem was primary or secondary. It will be important to determine whether anemia is a feature of both forms of CDI.
The NIH patients with CDI were all given dDAVP, a mixed AVPR1B/ AVPR2 agonist, to help with their water imbalance, but the dose given may not have completely compensated for their lack of AVP with respect to blood cell production; the treatment may have resulted in desensitization of the AVPR1B receptor; or in postsurgical patients, deficiencies in pituitary hormones other than AVP may have added to the deficit.
A specific AVPR1B agonist maybe useful to induce RBC production in severe anemia due to bleeding, toxic effects of drugs, or chemotherapy without affecting AVPR2 receptor-mediated water homeostasis. This hypothesis is consistent with our animal data and should be tested in the clinic.
MATERIALS AND METHODS
Study design
After we discovered that NIH patients with CDI were commonly anemic, we designed a series of studies of cultured human and mouse RBC progenitors. Our goal was to learn whether vasopressin could stimulate erythropoiesis in vitro, which receptor(s) mediated this, whether it was EPO-dependent, and how it occurred. Having answered these questions, we embarked on whole animal experiments to test the hypothesis that vasopressin and a vasopressin 1B receptor agonist with high specificity in rodents might have beneficial effects on recovery from anemia induced by hemorrhage, irradiation, or RBC lysis.
We did not use power analysis to predetermine sample size. In vitro experiments were performed at least twice in duplicates or triplicates. In vivo experiments were done using six to eight mice per group at the start. Because homozygous BB rats were difficult to breed in large numbers and have littermate controls, the group sizes were sometimes as low as four rats per group by the end of the experiments. These experiments were not repeated because of difficulty in acquiring enough rats. Rare statistical outliers were determined using the Grubbs’ test according to GraphPad (www.graphpad.com/quickcalcs/Grubbs1.cfm) and were excluded from further analysis (one mouse from the sublethal irradiation, one from the PHZ experiments, and one LE rat from the sublethal irradiation). Mice in the in vivo experiments were randomized within cages, and the investigator sampling the blood was blinded to the treatment.
Patients
Archival data were obtained through the NIH BTRIS, Limited Dataset application under agreement no. BTRIS_2016_1110_Mezey_E_NIDCR (table S1). This patient study was retrospective.
Rodents
Animal housing and maintenance were in full compliance with NIH criteria for care and use of laboratory animals. The Animal Care and Use Committee of National Institute of Dental and Craniofacial Research (NIDCR), NIH, approved all of the rodent studies [ASP (Animal Study Protocol) #13–706].
To create the rat anemia model, young adult (100 to 120 g) male LE rats and BB rats (lacking AVP due to a natural mutation in LE animals) were purchased from MMRRC/RRRC (mutant mouse resource and research center/rat resource and research center) from the University of Missouri (www.rrrc.us). Two-month-old homozygous BB rats and control LE rats were exposed to sublethal irradiation, and blood samples were taken from the orbital plexus 2,4,6,12,19, and 38 days afterward.
In mouse anemia models, 8- to 12-week-old age- and gender-matched C57BL/6 mice were used. For the hemorrhage model, 25% of the circulating blood volume (based on Animal Research Advisory Committee Guidelines for survival of mice and rats after blood loss, Office of Animal Care and Use, NIH) was drained from the retro-orbital plexus. For the PHZ model, PHZ (50 mg/kg body weight) was administered intraperitoneally on days 0 and 1. For sublethal irradiation, mice were irradiated (4.5 grays) and transplanted the next day with 2 million bone marrow cells from wild-type or Avprlb knockout mice (30), depending on the experiment, via the tail vein. The animals were treated with trimethoprim-sulfamethoxazole for 7 days before irradiation and for an additional 4 weeks after irradiation. After hemorrhage, PHZ, or irradiation, AVP [Arg8]-vasopressin (AnaSpec) along with o-phenantroline (20 mg/kg body weight; Sigma-Aldrich) or d(Leu4Lys8)VP (a gift from M. Manning), a vasopressin Avprlb agonist (100 mg/kg body weight), was administered intraperitoneally. For EPO studies, epoetin alfa (50 U/kg body weight; Epogen) or EPO-neutralizing antibody (100 mg per mice; mouse EPO monoclonal antibody, rat IgG2A, MAB 9591, R&D Systems) or rat IgG2A isotype control, MAB006, R&D Systems) was administered intraperitoneally to mice immediately after hemorrhage. Blood samples were taken on days 2,4, and, in some cases, 6 to determine Hct and corrected reticulocyte values. The reticulocyte ratio in peripheral blood was determined using the Retic-Count flow kit (BD Biosciences) according to the manufacturer’s instructions. Hct values were measured with an automated analyzer and micro-Hct tubes. The reticulocyte index was calculated as follows: reticulocyte ratio % = total gated cells × Hct/45.
To perform splenectomies, mice were anesthetized with isoflurane, and a 2-cm dorsal midline skin incision was made with its caudal terminus at the level of the 13th rib. The spleen was exposed by opening the left abdominal wall 1.5 to 2.5 cm from midline. With blunt forceps, the organ (with accompanying blood vessels and pancreatic tissue) was pulled through the incision. The blood vessels were ligated, and the spleen was removed. The pancreas was replaced, the muscle was approximated, and the skin incision was closed with wound clips. Sham splenectomies were performed identically, except that no ligation took place and that the spleen was replaced intact.
Human CD34+ cells
Human peripheral blood CD34+ cells were isolated from healthy donors by apheresis after 5 days of treatment with filgrastim (10 μg/kg; Amgen). Cells were sorted using anti-CD34 magnetic beads (Miltenyi Biotec) and subsequently frozen and stored at −150°C (National Heart, Lung, and Blood Institute-sponsored protocol: 02-H-0160, NCT00033774; www.clinicaltrials.gov ). Human CD34+ cells were also isolated from peripheral blood buffy coats from healthy volunteers at the NIH Clinical Center Department of Transfusion Medicine. Informed consent was obtained from the donors according to the Declaration of Helsinki.
Two-phase liquid culture of CD34+ cells was done as previously described with modifications (14). Cells were cultured for 6 days in phase 1 medium containing StemSpan (STEMCELL Technologies) medium with 10% fetal bovine serum (FBS), stem cell factor (SCF; 100 μg/ml), interleukin-3 (IL-3; 10 ng/ml), EPO (0.5 U/ml), 10−5 M β-mercaptoethanol, penicillin (100 U/ml), and streptomycin (100 mg/ml) (37°C and 5% CO2). In phase 2, cells were cultured in StemSpan medium with 30% FBS, 10−5 M ß-mercaptoethanol, SCF (10 μg/ml), EPO (2 U/ml), and the above antibiotics for 8 days. Cells were counted at the indicated time points for growth curve analysis.
For the BrdU cell proliferation assay, human CD34+ cells were plated on a 96-well plate in StemSpan medium with penicillin and streptomycin, supplemented with thrombopoietin (TPO; 100 ng/ml), Fms-related tyrosine kinase 3 ligand (FLT3L; 50 ng/ml), IL-3 (50 ng/ml), and SCF (100 ng/ml) (PeproTech). Cells were treated with 10−8 to 10−14 M AVP (Phoenix Pharmaceuticals). After 24 hours, BrdU (Sigma-Aldrich) at a 1:1000 concentration was added to the cultures, and BrdU incorporation was measured using the Cell Proliferation ELISA BrdU kit (Roche) after another 24 hours of incubation.
Methylcellulose assay
CD34+ cells from healthy donors were plated into StemMACS HSC-CFU basic medium (Miltenyi Biotec) containing hematopoietic factors [granulocyte-macrophage CSF (5 ng/ml), IL-3 (5 ng/ml), SCF (50 ng/ ml), EPO 2.5 (U/ml)] for BFU-E assays and counted on day 14. For CFU-E assays, cells were grown in StemMACS HSC-CFU basic medium with EPO (3 U/ml) and counted on day 7. AVP or a human-specific AVPR1B receptor agonist d(Cha4)-AVP (Tocris) was added to the medium at a concentration of 10−8 M. For pretreatment experiments, CD34+ cells were stimulated with AVP or AVPR1B receptor agonist in StemSpan medium supplemented with TPO (100 ng/ml), FLT3L (50 ng/ml), IL-3 (50 ng/ml), and SCF (100 ng/ml) for 48 hours. After 48 hours, the cells were seeded in StemMACS HSC-CFU basic medium with cytokines as described above but without AVP or AVPR1B receptor agonist. Colonies were counted using a STEMvision automated CFU colony counter (STEMCELL Technologies).
Cytospin preparation
Two BFU-E colonies were picked in cytobuffer [Hanks’ balanced salt solution (Gibco, Thermo Fisher Scientific), with 0.5% bovine serum albumin (United States Biological) and 2 mM EDTA], washed, and dissociated to single-cell suspension. Cytospin cell samples were prepared using Shandon 3 and SuperFrost slides. The cells were fixed and stained with May-Grünwald solution (Sigma-Aldrich) for 15 min, rinsed in 40 mM tris buffer (pH 7.4) for 2 min, and stained with Giemsa solution (Sigma-Aldrich) for 40 min. The cells were imaged using ScanScope.
Immunocytochemistry
Human CD34+ cells from two-phase liquid cultures were harvested on day 3. The cells were isolated (Cytospin) as described above and fixed with 4% formaldehyde. The antibodies were diluted (1:1000), and the slides were incubated overnight at 4°C. The antibodies used were AVPR1A (SC30025, Santa Cruz Biotechnology), AVPR1B (LS-A266, LifeSpan), and AVPR2 (A272, LifeSpan). After several washes, the anti-rabbit Fab2 conjugated to Alexa Fluor 594 secondary antibody was applied at a dilution of 1:1000 for 1 hour at room temperature. The slides were then washed and examined under a Leica SP8 confocal microscope. Control slides were treated similarly, except that no specific primary antibody was applied.
RT-PCR and real-time PCR
Total RNA was isolated from human CD34+ cells and sorted mouse bone marrow lineage-negative, Sca-1-positive, and c-kit-positive (LSK) cells using the Stratagene Absolutely RNA Microprep kit. The deoxyribonuclease-treated total RNA was reverse-transcribed using oligo(dT) primers and Moloney murine leukemia virus reverse transcriptase according to the manufacturer’s instructions (Promega). RT-PCR conditions were as follows: 50°C for 30 min of reverse transcription (only for the one-step RT-PCR), 95°Cfor 15 min of initial activation of the Taq polymerase and denaturation of the reverse transcriptase, and then 40 cycles of 94°C for 15 s of denaturation, various primer-specific temperatures (tables S2 and S3) for 30 s of annealing, and 72°C for 30 s of extension. PCR products were run on 2% agarose gels and visualized with ethidium bromide. Quantitative PCR was done on the sorted population for all AVP receptors using SYBR Green Master Mix (Applied Biosystems). Relative expression was determined using a ΔCt method and was normalized to Mus musculus TATA box-binding protein, Tbp, as a housekeeping gene (table S4).
Western blotting
For AVP receptor expression, protein samples were collected at the indicated time points, and protein extraction was done using radioimmunoprecipitation assay (RIPA) buffer. Equal amounts of protein (20 mg) were loaded in all lanes, and all three vasopressin receptors were examined using the following antibodies: AVPR1A (H00000552-m07A, Abnova), AVPR1B (LS-A266, LifeSpan), and AVPR2 (A272, LifeSpan). The blots were incubated at 4°C overnight at a dilution of 1:1000, and the corresponding secondary antibody was incubated for 1 hour at room temperature at a dilution of 1:50,000. Goat anti-rabbit immunoglobulin G-horseradish peroxidase (IgG-HRP) and goat antimouse IgG-HRP were from SouthernBiotech, and the rabbit anti-goat IgG-HRP was purchased from KPL. Millipore Immobilon Western Chemiluminescent HRP Substrate was used for detection. The AVPR1A band was only visible after a 60-min exposure; the AVPR1B and AVPR2 bands were visible after a 1-min exposure time.
For STAT5 analysis, on days 9 and 12 of differentiation, the cells were incubated for 80 min in growth medium containing no EPO and in the presence of 10−10 or 10−12 M AVP or no AVP. EPO was added back to the medium (1 U/ml), and after 10 min, cells were collected for the preparation of protein lysates. Untreated cells that were incubated in the absence of AVP and EPO for the whole 90 min were used as control. Cells were lysed in RIPA buffer, and proteins were resolved on the basis of size in a NuPAGE electrophoresis system (Life Technologies). The primary antibodies used were anti-STAT5 (catalog number 9363) and anti-p-STAT5 (catalog number 9359) from Cell Signaling and anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (ab9485) from Abcam.
Flow cytometry
Mouse erythroid lineage cells were FACS-sorted as previously published (19) using CD44 and Ter119. Briefly, 4-month-old C57BL/6 mouse bone marrow was extracted, and CD45+ cells were depleted. CD45- cells were sorted on the basis of CD44 and Ter119 into four populations. For mouse HSCs, LSK cells were sorted. Rat anti-mouse lineage antibodies (anti-CD4, CD8, B220, Ter119, Gr-1, and CD11b) were used for lineage depletion (see table S5). Lineage-negative cells were stained with c-kit phycoerythrin/Cy5, and Sca-1-fluorescein isothiocyanate antibodies and LSK cells were sorted on a DAKO Cytomation MoFlo cell sorter.
Intracellular Ca2+ and cAMP measurements
Intracellular Ca2+ concentration was assayed as described previously (31). Human CD34+ cells were loaded using Fura-2, and cytoplasmic Ca2+ measurements were performed in cell suspensions using a fluorescence spectrophotometer (DeltaScan, Photon Technology International). Ratiometric measurements were recorded at 340-/380-nm excitation and 500-nm emission, and the ratios were plotted against time. Ionomycin (10 pM) was added at the end of each run to determine the maximum fluorescence ratio (31). For cAMP measurements, the cAMP femto 2 kit (catalog number 62AM5PEB, Cisbio) was used according to the manufacturer’s instructions.
In addition to the AVPR1B agonist, we also used a human-specific AVPR1A agonist (Phe2, Ile3, and Orn8) and AVP (American Peptide Company). The measurements were done on a Wallac Victor2 1420 multilabel counter (PerkinElmer).
Statistical analyses
The statistical analysis of the in vivo experiments was performed by Social and Scientific Systems Inc. (www.s-3.com), and the in vitro data were analyzed by the authors. Student’s t test (for two groups) or analysis of variance (ANOVA) was used with appropriate corrections. Data were evaluated with GraphPad Prism 5.0 software, and a P value of <0.05 was accepted as statistically significant. Data are means ± SD. The number of samples used is indicated in the figure legends or as described above for each experiment. For analysis of the human (CDI versus normal population) data, SAS version 9.3 software was used.
Supplementary Material
Table S1. RBC indices for patients (provided as an Excel file).
Fig. S1. AVP agonists affect intracellular calcium, and AVP increases cAMP ¡n human progen¡tors.
Fig. S2. Norepinephrine and AVP induce an increase in the number of BFU-E colonies.
Fig. S3. AVP and AVPR1B agonist [d(Cha4)-AVP] increase BFU-E formation.
Fig. S4. Band intensities from Western blots were analyzed by ImageJ.
Fig. S5. Response to anemia is coordinated by AVP and EPO.
Table S2. Human RT-PCR primers.
Table S3. Mouse RT-PCR primers.
Table S4. Mouse real-time PCR primers.
Table S5. Antibodies used for flow cytometry.
Acknowledgments:
WethankT. Balla and M. Korzeniowski for help with intracellular Ca2+ measurements; E. Shepard, S. Williams, and A. Parmelee for excellent technical assistance; and M. Manning for providing the rat-specific AVPR1B agonist. We are grateful for the expert help of T.Kilts and the National Eye Institute and NIDCR animal facilities. Wethank H. Gainer for comments and support during the study. The invaluable assistance of A. Beri (NIH, Clincal Center, and BTRIS) in identifying patients and helping analyze details in the NIH database is greatly appreciated. We want to acknowledge the excellent work of D. Fetterer and H. Maibach (Social and Scientific Systems Inc., www.s-3.com) in processing the biostatistical analysis of the in vivo experiments.
Funding: This research was supported bythe Division of Intramural Research program of NIDCR (ZIA DE000714– 07 and ZIA DE000676–19 CSDB), NIH, Department of Health and Human Services. H.-J.L. and W.S.Y. were supported bythe National Institute of Mental Health Intramural Research Program (Z01-MH-002498). HJ.-L. wasalso supported by the National Research Foundation of Korea (MSIP,2008–0062282).
Footnotes
Competing interests: É.M., B.M., K.N., and M.K. are inventors on a patent application (US 20160220631 A1) held by the U.S. Department of Health and Human Services that covers “Methods of modulating erythropoiesis with arginine vasopressin receptor 1b molecules.” All other authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Jelkmann W, Physiology and pharmacology of erythropoietin. Transfus. Med. Hemother. 40, 302–309 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginsburg M, Heller H, Antidiuretic activity in blood obtained from various parts of the cardiovascular system. J. Endocrinol. 9, 274–282 (1953). [DOI] [PubMed] [Google Scholar]
- 3.Share L, Vasopressin, its bioassay and the physiological control of its release. Am. J. Med. 42, 701–712 (1967). [DOI] [PubMed] [Google Scholar]
- 4.Weitzman RE, Glatz TH, Fisher DA, The effect of hemorrhage and hypertonic saline upon plasma oxytocin and arginine vasopressin in conscious dogs. Endocrinology 103, 2154–2160 (1978). [DOI] [PubMed] [Google Scholar]
- 5.Errington ML, Rocha M Silva e Jr., Vasopressin clearance and secretion during haemorrhage in normal dogs and in dogs with experimental diabetes insipidus. J. Physiol. 227, 395–418 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velasquez MT, Menitove JE, Skelton MM, Cowley AW Jr., Hormonal responses and blood pressure maintenance in normal and hypertensive subjects during acute blood loss. Hypertension 9, 423–428 (1987). [DOI] [PubMed] [Google Scholar]
- 7.Hunt NH, Perris AD, Sandford PA, Role of vasopressin in the mitotic response of rat bone marrow cells to haemorrhage. J. Endocrinol. 72, 5–16 (1977). [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC, Prevalence of anemia in persons 65 years and older in the United States: Evidence for a high rate of unexplained anemia. Blood 104, 2263–2268 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Kaferle J, Strzoda CE, Evaluation of macrocytosis. Am. Fam. Physician 79, 203–208 (2009). [PubMed] [Google Scholar]
- 10.Hansen A, Chen Y, Inman JM, Phan QN, Qi Z-Q, Xiang CC, Palkovits M, Cherman N, Kuznetsov SA, Robey PG, Mezey E, Brownstein MJ, Sensitive and specific method for detecting G protein-coupled receptor mRNAs. Nat. Methods 4,35–37 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G, Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V^, V^, V2 and OT receptors: Research tools and potential therapeutic agents. Prog. Brain Res. 170, 473–512 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Roper JA, O’Carroll A-M, Young III WS, Lolait SJ, The vasopressin Avpr1b receptor: Molecular and pharmacological studies. Stress 14, 98–115 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serradeil-Le Gal C, Derick S, Brossard G, Manning M, Simiand J, Gaillard R, Griebel G, Guillon G, Functional and pharmacological characterization of the first specific agonist and antagonist for the V1b receptor in mammals. Stress 6, 199–206 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Li J, Hale J, Bhagia P, Xue F, Chen L, Jaffray J, Yan H, Lane J, Gallagher PG, Mohandas N, Liu J, An X, Isolation and transcriptome analyses of human erythroid progenitors: BFU-E and CFU-E. Blood 124, 3636–3645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koulnis M, Porpiglia E, Hidalgo D, Socolovsky M, Erythropoiesis: From molecular pathways to system properties, in A Systems Biology Approach to Blood, Corey SJ, Ed. (Springer Science, 2014), pp. 37–58. [DOI] [PubMed] [Google Scholar]
- 16.Socolovsky M, Molecular insights into stress erythropoiesis. Curr. Opin. Hematol. 14, 215–224 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Socolovsky M, Nam H.-s., Fleming MD, Haase VH, Brugnara C, Lodish HF, Ineffective erythropoiesis in Stat5a-/−5b−/− mice due to decreased survival of early erythroblasts. Blood 98, 3261–3273 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Kim JK, Summer SN, Wood WM, Brown JL, Schrier RW, Arginine vasopressin secretion with mutants of wild-type and Brattleboro rats AVP gene. J. Am. Soc. Nephrol. 8, 1863–1869 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Zhang J, Ginzburg Y, Li H, Xue F, De Franceschi L, Chasis JA, Mohandas N, An X, Quantitative analysis of murine terminal erythroid differentiation in vivo: Novel method to study normal and disordered erythropoiesis. Blood 121, e43–e49 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gridley DS, Pecaut MJ, Dutta-Roy R, Nelson GA, Dose and dose rate effects ofwhole-body proton irradiation on leukocyte populations and lymphoid organs: Part I. Immunol. Lett. 80, 55–66 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Paulson RF, Shi L, Wu D-C, Stress erythropoiesis: New signals and new stress progenitor cells. Curr. Opin. Hematol. 18, 139–145 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knepper MA, Kwon T-H, Nielsen S, Molecular physiology of water balance. N. Engl. J. Med. 372, 1349–1358 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An X, Mohandas N, Erythroblastic islands, terminal erythroid differentiation and reticulocyte maturation. Int. J. Hematol. 93, 139–143 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Wun T, Paglieroni T, Lachant NA, Physiologic concentrations of arginine vasopressin activate human platelets in vitro. Br. J. Haematol. 92, 968–972 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Grant PJ, Davies JA, Tate GM, Boothby M, Prentice CRM, Effects of physiological concentrations of vasopressin on haemostatic function in man. Clin. Sci. (Lond.) 69, 471–476 (1985). [DOI] [PubMed] [Google Scholar]
- 26.Orcel H, Albizu L, Perkovska S, Durroux T, Mendre C, Ansanay H, Mouillac B, Rabié A, Differential coupling of the vasopressin V1b receptor through compartmentalization within the plasma membrane. Mol. Pharmacol. 75, 637–647 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Jard S, Vasopressin receptors. A historical survey. Adv. Exp. Med. Biol. 449, 1–13 (1998). [PubMed] [Google Scholar]
- 28.Kaufmann JE, Oksche A, Wollheim CB, Günther G, Rosenthal W, Vischer UM, Vasopressin-induced von Willebrand factor secretion from endothelial cells involves V2 receptors and cAMP. J. Clin. Invest. 106, 107–116 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller RL, Sandoval PC, Pisitkun T, Knepper MA, Hoffert JD, Vasopressin inhibits apoptosis in renal collecting duct cells. Am. J. Physiol. Renal Physiol. 304, F177–F188 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wersinger SR, Ginns EI, O’Carroll A-M, Lolait SJ, Young WS III, Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol. Psychiatry 7, 975–984 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Ely JA, Ambroz C, Baukal AJ, Christensen SB, Balla T, Catt KJ, Relationship between agonist- and thapsigargin-sensitive calcium pools in adrenal glomerulosa cells. Thapsigargin-induced Ca2+ mobilization and entry. J. Biol. Chem. 266, 18635–18641 (1991). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. RBC indices for patients (provided as an Excel file).
Fig. S1. AVP agonists affect intracellular calcium, and AVP increases cAMP ¡n human progen¡tors.
Fig. S2. Norepinephrine and AVP induce an increase in the number of BFU-E colonies.
Fig. S3. AVP and AVPR1B agonist [d(Cha4)-AVP] increase BFU-E formation.
Fig. S4. Band intensities from Western blots were analyzed by ImageJ.
Fig. S5. Response to anemia is coordinated by AVP and EPO.
Table S2. Human RT-PCR primers.
Table S3. Mouse RT-PCR primers.
Table S4. Mouse real-time PCR primers.
Table S5. Antibodies used for flow cytometry.