Abstract
Clinical studies indicate that salt-sensitive hypertension is more prevalent in women than men. However, animal models of salt-sensitivity have primarily focused on the mechanisms of salt-sensitivity in male animals, therefore, elucidation of these mechanisms in female animal models are needed. We have previously shown that female Balb/C mice have higher aldosterone synthase expression and aldosterone production than males. We hypothesized that female Balb/C mice develop salt-sensitive increases in blood pressure (BP). Seven day feeding of a 4% NaCl high-salt diet (HSD) increased BP in female mice without altering BP in males. Females on a HSD displayed no apparent increases in sodium retention as assessed by 24-hour urine collection, sodium balance measure and saline loading excretion analysis. Females on HSD exhibited lower renin-angiotensin system activity (plasma angiotensin II, plasma renin activity and angiotensin converting enzyme activity) compared to males but developed a salt-induced elevation in adrenal aldosterone synthase expression and retained higher aldosterone levels than males on high-salt. This resulted in a higher aldosterone/plasma renin activity ratio in females compared to males on high-salt feeding. Adrenal mRNA expression of angiotensinogen and leptin receptor were increased in female mice on HSD. HSD impaired endothelium-dependent relaxation in female mice only. Mineralocorticoid receptor inhibition (eplerenone) restored BP and endothelial function in HSD females. Collectively, these data indicate that Balb/C mice develop sex-discrepant salt-sensitive hypertension likely via aldosterone-mineralocorticoid receptor-mediated mechanisms involving impaired endothelium-dependent relaxation in females only. This study presents the first model of spontaneous sex-specific salt-sensitivity which mimics the human pathology.
Keywords: Hypertension, salt, renin-angiotensin system, aldosterone, mineralocorticoid receptor, endothelial function, sex differences
Summary
Balb/C mice are salt-sensitive in a sex-specific manner. This salt-sensitivity is characterized by elevated aldosterone levels during high salt diet feeding, in contrast to male mice fed a high salt diet, and is reliant on mineralocorticoid receptor activation.
Introduction
Hypertension rates continue to remain around one-third of the adult population in the US1. Dietary factors are known to play a significant role in the control of blood pressure (BP) 2–5 and the link between dietary salt and hypertension has led to public health initiatives aimed at lowering population consumption of salt to reduce BP 6. Some have estimated that up to 50% of essential hypertension cases are related to salt-sensitivity 2, 4. Salt-sensitivity, defined as a BP response to changes in dietary salt 6, is used to delineate individuals at increased risk for hypertension and other cardiovascular diseases with the consumption of a high salt diet. However, despite decades of research the mechanisms for the development of salt-sensitivity remain poorly characterized.
The prevalence of salt-sensitive hypertension is sex-discrepant in humans. Clinical reports indicate that women are more likely to be salt-sensitive than men, and have a more profound BP response to changes in dietary sodium than males7–9 which has led to the clinical dogma that a higher percentage of hypertensive women are salt-sensitive than the percentage of hypertensive men7–9. Despite this discrepancy in the human population, the majority of studies of salt-sensitivity in animal models have been restricted to male animals. Background strains such as the C57BL6 mouse and Sprague-Dawley rat have not been reported to develop salt-sensitive hypertension spontaneously in males or females10, 11. Therefore, transgenic models have traditionally been utilized to examine mechanisms of salt-sensitivity. However, in contrast to clinical reports of a higher salt-sensitivity prevalence in women, animal models of salt sensitive hypertension, such as the Dahl salt-sensitive rat 12, the angiotensin II (ANGII)+high salt 13 and the DOCA+high salt model 14, 15, demonstrate an opposing sex-discrepancy, in that the males of these models have a more pronounced change in BP in response to elevations in dietary salt. While the mechanisms for the sex-discrepancies in BP responses to dietary salt have been investigated in these models 12–14, 16, it remains that these animal models lack a propensity for salt-sensitivity in females, which is a needed model to study mechanisms of salt-sensitivity in women. Therefore, novel animal models to be utilized for the study of the mechanisms of salt-sensitivity that are specific to women are greatly needed.
Our lab has published that female mice are particularly susceptible to mineralocorticoid receptor (MR) activation and aldosterone-mediated hypertension mechanisms 17, 18, studies that were predominantly performed in Balb/C mice who demonstrate a sex-specific heightened aldosterone level in females. A heightened sensitivity to aldosterone and MR activation is implicated in salt-sensitivity in several animal models of hypertension 19, 20. Therefore, we hypothesized in the current study that female Balb/C mice develop elevations in BP and endothelial dysfunction in response to a high salt diet, which is mediated by aldosterone-MR activation.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animal Model
Male and female Balb/C mice (Jackson Labs, Bar Harbor, ME, Catalog #000651) at 10 weeks of age were utilized for all studies. The Institutional Animal Care and Use Committee of Augusta University approved all protocols. Mice were implanted with indwelling telemeters (carotid catheter) (DSI© Model #PA-C10, New Brighton, MN) under isoflurane anesthesia as previously described 17, 18. Following a 7-day recovery period baseline BP measurements were consciously recorded for 7 days while mice were on normal salt diet (NS) (0.2% NaCl, Teklad #2918, Envigo, United Kingdom). Male and female mice were then placed on a 4% NaCl high-salt diet (HS, Teklad #92034, Envigo) for 7-day BP recording. A second group of female mice repeated this protocol followed by an additional 7 days recording on HS with the added supplementation of the MR antagonist eplerenone (oral daily supplement, 200mg/kg/day). Uterine weights were compared from female mice at sacrifice to determine that estrous cycles were similar across all feeding periods.
A detailed description of the methods used is available in the online-only Data Supplement.
Statistics
All data was expressed as MEAN±S.E.M. In non-repeated variables, differences among means was verified by t-test with normality verified by Kolmogrov-Smirnov test. In absence of normality, Mann-Whitney test was used. Parametric and non-parametric data with multiple means utilized one-way ANOVA with Kruskal-Willis test. Repeated variable measures with multiple data sets utilized two-way ANOVA. Tukey post-hoc test followed all ANOVA. A P value of <0.05 was considered significant. All analysis were performed in Graphpad® Prism analysis software (La Jolla, CA).
Results
High salt diet does not change body or organ weight in male and female Balb/C mice
Body and organ weights were measured following NS or HS feeding for seven days to assess potential physiological changes to dietary sodium loading (Supplemental Table S1). Kidney weight was lower, and adrenal weight higher, in female compared to male mice. Kidney weight of male mice remained higher than those of females on HS and adrenal glands also remained larger in HS females compared to HS males. No change was observed in body, heart, kidney nor adrenal weight in male or female mice in response to HS. Leptin levels were unaltered across groups irrespective of sex or HS diet.
High salt diet increased blood pressure only in female Balb/C mice
To assess the sex-specificity of HS diet-induced BP responses in male and female Balb/C mice BP measurements were collected for 7 days for both NS and HS feeding in conscious mice. Averages for day (6:00–18:00) and night (18:00–6:00) BP values were calculated from cycling light/dark recordings on radiotelemetry. Both male and female mice displayed a circadian rhythm of BP on both NS and HS (Figure 1A–F). Male and female mice on NS did not differ in mean arterial (MAP) or systolic (SBP) blood pressures (Figure 1A–D), however, female mice on NS exhibited a higher daytime diastolic blood pressure (DBP) compared to males on NS (Figure 1E). HS feeding did not induce a change in BP in male mice (Figure 1A–F). In contrast, HS feeding induced a significant increase in both day and night MAP, SBP and DBP in female mice compared to female NS (Figure 1A–F). Day and night MAP, SBP and MAP were also significantly higher in female mice on HS compared to males on HS (Figure 1A–F), indicating a sex-specific salt-sensitive increase in BP in female mice alone.
Figure 1. High Salt Diet Increases Blood Pressure in Female Mice Only.

Average day (6:00–18:00) and night (18:00–6:00) blood pressure values over 7 days in male and female mice on NS and HS diets measured as mean arterial pressure (MAP) (A–B) systolic blood pressure (SBP) (C–D) and diastolic blood pressure (DBP) (E–F). *P<0.05. N=5.
Salt-sensitive hypertension in female mice is not associated with an increase in sodium retention
To investigate the potential role for exacerbated renal sodium retention in the development of salt-sensitive hypertension in female mice we assessed sodium and potassium urinary excretion and sodium plasma levels in response to HS feeding. We observed no sex-specific changes in urinary sodium excretion between male and female mice on NS diets (Figure 2A). However, HS feeding induced an increase in sodium excretion in both male and female mice (Figure 2A). Furthermore, female mice on HS excreted more sodium on HS than male mice on HS (Figure 2A). No difference in potassium excretion were observed between male and female mice on NS, however, potassium excretion increased in female mice on HS with no change observed in male mice on HS (Figure 2B). To determine acute renal sodium handling in male and female mice on NS and HS in response to a sodium bolus, a saline load experiment was performed as previously described21. No sex differences in acute renal sodium handling were detected in male versus female mice on either NS or HS in response to a sodium load (Supplemental Figure S1 A, B). Glomerulosclerosis scoring of renal cortex histology samples from males and females on HS indicated no development of advanced glomerulosclerotic fibrosis or hypertrophy in response to HS feeding (Supplemental Figure S1 C, D). In addition, albuminuria, an index of glomerular permeability, was unaltered in male or female mice with NS or HS feeding (Supplemental Figure S1 E). Urinary sodium/potassium ratio, an additional index of renal injury, of male and female mice was increased by high salt diet but not changed between sexes (Figure 2C). Neither sex, dietary sodium nor eplerenone treatment affected plasma levels of sodium (Figure 2D). And finally, we determined that sodium retention was not altered between male and female mice on either NS, HS or HS+eplerenone, although the percentage of sodium intake excreted was increased by HS feeding in both male and female mice (Figure 2E). Collectively, these data indicate that elevated BP in female mice on HS diet is not associated with a sex-specific increase in sodium retention.
Figure 2. High Salt Diet Does Not Induce Salt retention in Female Mice.
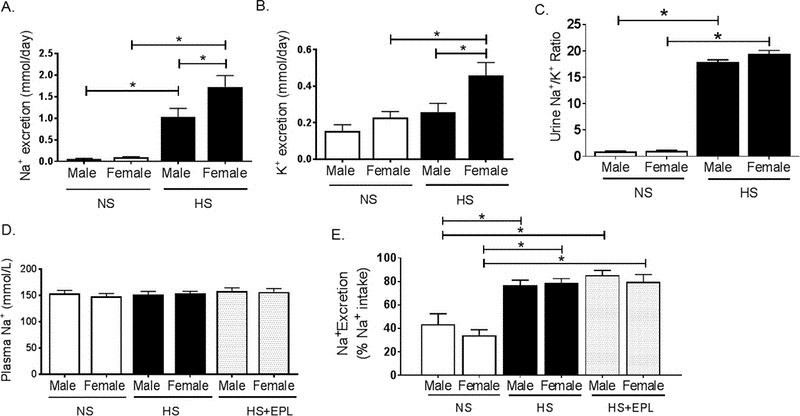
(A) Urinary sodium and (B) potassium excretion and (C) urinary sodium/potassium ratio in male and female mice on NS and HS diets. (D) Plasma sodium in male and female mice on NS, HS and HS+Eplerenone. (E) Sodium excretion per day expressed as a percentage of measured sodium intake per day in metabolic cage collection (24 hours) in male and female mice on gel diets: NS, HS and HS+Eplerenone. *P<0.05. N=6.
Plasma aldosterone/renin activity ratio and adrenal aldosterone synthase expression is increased in female mice on high salt diet
We sought to determine if changes in dietary sodium in male and female mice resulted in dysregulation in the renin-angiotensin aldosterone system. Plasma levels of angiotensin II (ANGII) and angiotensin converting enzyme 1 (ACE) activity were measured by mass spectrometry and plasma renin activity (PRA) calculated from addition of angiotensinogen I and II levels22. No significant baseline differences were observed in ANGII (Figure 3A) or PRA levels (Figure 3B) between male and female mice on a NS diet. Female mice exhibited significantly lower ACE activity (Figure 3C) than males on NS. ANGII, ACE and PRA were unchanged in male mice in response to HS diet, however, these levels were significantly lower in female mice on HS compared to HS males (Figure 3A–C). No baseline differences in plasma aldosterone level were observed in NS male and female mice (Figure 3D), however, HS feeding significantly reduced aldosterone levels in male mice but failed to suppress aldosterone production in female mice and, in addition, female mice on HS feeding had a significantly increased aldosterone plasma level than male mice on HS. Eplerenone treatment significantly increased plasma aldosterone levels in male and female mice on HS diet, but no sex difference in aldosterone level was observed in HS eplerenone male and female mice Figure 3D). Plasma aldosterone/PRA ratio is considered an index of salt-sensitivity in humans23. Female mice had an increased aldosterone/PRA ratio compared to males on NS (Figure 3E). HS feeding significantly suppressed aldosterone/PRA ratio in both male and female mice (Figure 3E), however, female mice on HS retained an aldosterone/PRA significantly higher than that of males on HS (Figure 3E). Adrenal aldosterone synthase (CYP11B2) protein expression was unaltered in male mice on HS compared to NS males, but significantly increased in female mice on HS both compared to female NS mice and male HS mice (Figure 3F). qRT-PCR quantification of indices of intra-adrenal renin-angiotensin system activation indicated no significant change in adrenal renin mRNA expression levels between male and female mice on NS, HS or HS+eplerenone feeding (Figure 4A). In contrast, angiotensinogen mRNA expression was increased in female mice on HS compared to male mice on HS, a sex-discrepant expression that was ablated with eplerenone treatment in male and female mice on HS (Figure 4B). Furthermore, adrenal leptin receptor expression was increased similarly in female mice on HS diet compared to both NS female and HS males, which was restored to NS levels with eplerenone treatment in HS mice (Figure 4C).
Figure 3. High Salt Diet suppresses Aldosterone Level in Male, but not Female, Mice.
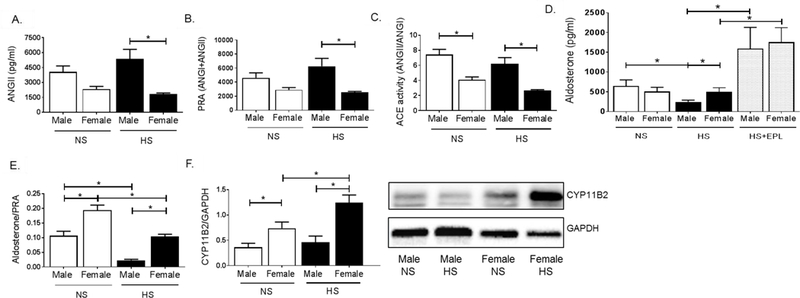
Plasma levels of (A) angiotensin II (ANGII), (B) plasma renin activity (PRA), (C) angiotensin converting enzyme (ACE) activity, (D) aldosterone, (E) aldosterone/plasma renin activity ratio. (F) Western-Blot quantification and representative image for CYP11B2 expression in male and female mice on NS, HS and HS+Eplerenone. *P<0.05. N=4 for mass spectrometry measurements, N=7–18 for aldosterone ELISA, N=5–6 for western blotting.
Figure 4. High Salt Diet Increases Adrenal Angiotensinogen and Leptin Receptor Expression in Female Mice.
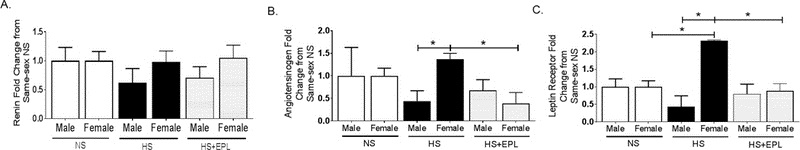
Adrenal mRNA expression of (A) renin, (B) angiotensinogen and (C) leptin receptor in male and female mice on NS, HS or HS+Eplerenone. *P<0.05. N=3.
Eplerenone ablates increases in blood pressure in response to high salt diet in female mice
To assess the functional relevance of a sustained elevation in plasma aldosterone to the salt-sensitive hypertension we observed in female mice, we sought to determine whether MR antagonism would prevent salt-sensitive hypertension in female mice. Eplerenone (EPLR) was administered to mice in conjunction with HS for 7 days in male and female Balb/C mice. Consistent circadian BP fluctuations were observed in HS and HS+EPLR male and female mice (Figure 5A–F). EPLR treatment did not alter either MAP, SBP or DBP in male mice on HS diet, however, EPLR successfully decreased both day and night MAP, SBP and DBP in female mice on HS (Figure 5A–F). Furthermore, EPLR treatment in HS females significantly decreased day and night DBP in HS fed females compared to levels in HS fed males (Figure 5E, F). Therefore, BP elevations in response to HS feeding in females are ablated by MR antagonism.
Figure 5. Mineralocorticoid Receptor Blockade Restores Blood Pressure in Female Mice on a High Salt Diet.
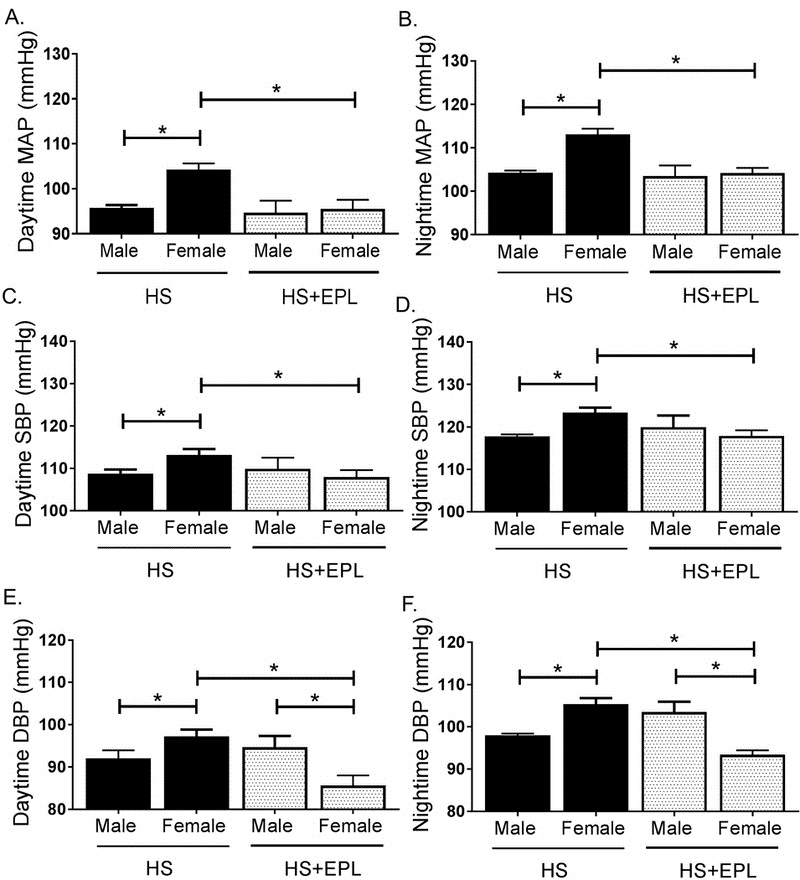
Blood pressure in male and female mice on HSD and HSD+eplerenone. Average day and night (12 hours each) blood pressures over 7 days in male and female mice on HS and HS+eplerenone (EPL) measured as (A–B) mean arterial pressure (MAP), (C–D) systolic blood pressure (SBP) and (E–F) diastolic blood pressure (DBP). *P<0.05. N=4-10.
HS feeding impairs endothelial function in female mice which is prevented by eplerenone treatment
Kurtz et al. suggest that mechanisms of salt-sensitive hypertension primarily derive from vascular dysfunction and are characterized by increases in systemic vascular resistance, and endothelial dysfunction is a noted contributor to systemic vascular resistance 24. To determine if increases in BP induced by HS feeding in female mice was linked to endothelial impairment we assessed endothelial function by relaxation responses to acetylcholine in aorta. HS feeding did not induce endothelial dysfunction in male mice (Figure 6A), however, HS feeding significantly blunted acetylcholine-induced relaxation in female mice compared to NS female mice (Figure 6B). Endothelial-independent responses, as assessed by relaxation responses to sodium nitroprusside, were unaltered in both male and female mice in response to a HS (Supplemental Figure S3 A, B). No differences were observed in constriction responses in male or female mice to the α-agonist phenylephrine (Supplemental Figure S3 C, D). Eplerenone treatment in HS diet-fed male mice did not alter endothelial function (Figure 6C), however, eplerenone significantly attenuated endothelial dysfunction in female mice on HS diet (Figure 6D). Eplerenone treatment did not affect endothelial-independent responses to sodium nitroprusside in male or female mice on HS diet (Supplemental Figure S4 A,B). However, eplerenone treatment significantly reduced constriction responses to phenylephrine in both male and female mice (Supplemental Figure S4 C,D). We confirmed that local vascular mineralocorticoid receptor activation was aldosterone-dependent via assessing 11βHSD2 mRNA expression in male and female aortas. We observed that 11βHSD2 expression increased in female mice on HS compared to males on HS (Supplemental Fig S5). Therefore, cortisol-induced activation of vascular mineralocorticoid receptors is unlikely increased in female mice on HS diet.
Figure 6. High Salt Diet Impairs Endothelial Function in Female Mice Which Is Restored by Eplerenone Treatment.
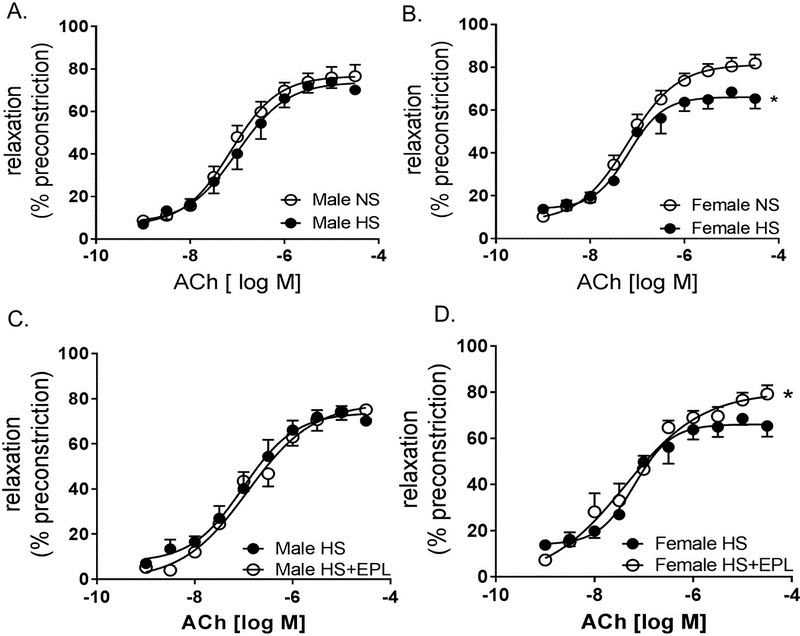
Vascular reactivity in male and female mice on NS, HS and HS+eplerenone (EPL). Aortic ring relaxation responses to acetylcholine (ACH) (endothelium-dependent relaxation) in male (A) and female (B) mice on NS and HS and (C) male and (D) female mice on HS and HS+EPL expressed as a percentage of preconstriction with serotonin. *P<0.05 vs Female NS, N=5-10.
Discussion
In this report, we have demonstrated several novel findings: 1) increases in dietary salt elevates BP and impairs endothelial function in female Balb/C mice only 2) female Balb/C mice fail to decrease aldosterone production in response to HS diet and 3) MR blockade with eplerenone lowers BP and restores endothelial function in female Balb/C mice fed a HS diet. These data indicate that salt-sensitive increases in BP in Balb/C mice are sex-specific, characterized by high plasma aldosterone levels and responsive to MR inhibition.
Several clinical studies have indicated that salt-sensitivity is highly prevalent in women and that women may have more pronounced BP responses to changes in dietary salt compared to men 7–9. Notably, the Intersalt study, a study conducted with more than 10,000 patients from 52 populations from all over the world and including half pre-menopausal women, demonstrated that the index of risk for salt-sensitive BP increases is higher in women than men 8. However, there is at present a discrepancy in clinical and animal studies with regard to the effects of salt on BP in animal models of salt sensitivity that has resulted in a dogma that male rodents are more salt-sensitive than females, and therefore, the study of mechanisms of salt-sensitive hypertension in females is very limited compared to males 25. The development of a female-predominant salt-sensitive phenotype in Balb/C mice has the potential to fill the gap in the literature of female sex-specific salt sensitivity mechanisms for hypertension, both as a wild-type strain exhibiting spontaneous salt-sensitive hypertension and potentially as a background strain for future transgenic salt-sensitive models.
A previous study has observed that female FVB/N mice also develop a greater increase in BP in response to a HS diet compared to male counterparts 26, indicating that the BP elevation in Balb/C mice may not be an anomaly, but rather, a representation of a genetic predisposition for salt sensitivity in these strains of female mice that parallels the human population. Notably, data in the C57BL/6 mouse indicates that no such salt-sensitive hypertension persists in females of that genetic background 11. Therefore, strain and genetic specificity is particularly important for salt-sensitivity studies in females. Further studies are needed to evaluate the genetic variations among these and other strains that do or do not predispose female mice to salt-sensitive changes in BP. Of important note is the potential role of sex hormones and/or chromosomes in the predisposition to BP increases and endothelial dysfunction in response to HS in female mice in the current study. We analyzed uterine weights to determine whether female mice on different diets or eplerenone treatment differed in phase of estrous at sacrifice and did not observe a change between groups. This likely suggests that BP elevation and endothelial dysfunction are not the result of a decrease in estrogen levels. However, additional experiments in in gonadectomized animals as well as in the 4-core genotype mouse model are required to accurately determine whether sex-hormones or chromosomes predispose to salt sensitivity.
In healthy individuals, aldosterone levels are characteristically decreased by an increase in dietary sodium, however, dysregulation of the aldosterone-MR axis is a central player in the pathogenesis of salt-sensitive hypertension in many animal models and in humans27. We observed in our study that aldosterone levels are not suppressed in female Balb/C mice in response to HS feeding. Taken alone, the increase in aldosterone levels in female mice on HS compared to HS fed males indicates an overactive systemic mineralocorticoid receptor activation in females in response to dietary salt. In addition, plasma aldosterone/PRA ratio was increased in Balb/C female mice on HS compared to males on HS, albeit a significant suppression in the ratio was observed in both sexes. An increase in aldosterone/PRA ratio is significantly correlated with BP responses to salt intake, and may be a promising diagnostic tool for the determination of salt-sensitivity in patients 23, 28. The difference in aldosterone/PRA ratio was attributable to a lower PRA in female mice compared to males as well as a failure to suppress aldosterone production in response to HS compared to males on HS. These data indicate that Balb/C mice have a sex-specific down-regulation of PRA with HS that is restricted to female mice, thereby indicating that inappropriate PRA and renin-angiotensin system activation is likely not implicated in salt-sensitive BP in female mice. This mechanism for salt-sensitivity has been proposed in other rodent strains 29, indicating that inappropriate aldosterone production is an important mechanism in salt sensitive BP increases in Balb/C female mice.
Sodium retention, as assessed both by 24-hour urinary sodium excretion and urinary sodium excretion in response to an acute sodium bolus, is not increased in female HS mice compared to males. This is in contrast to studies in other models in which sodium retention is characteristically increased in response to a HS diet in accordance with elevations in BP 12–15. It is important to note, that metabolic cage techniques are the best available technique to measure electrolyte intake/excretion in mice, however, the technique presents many variables consistent with stress of the animals in the cage environment, the contamination of urine with food contents, etc. However, the assessment of sodium excretion in two methods, excretion/day and acute sodium loading, both indicated that sodium handling was apparently not altered in female mice with HS feeding. Furthermore, glomerulosclerosis scoring and albuminuria indicated no progression of kidney injury which may have suggested altered renal mechanisms in female mice on HS diet. We also determined that sodium retention was not altered by sex in NS, HS and HS eplerenone feeding periods between male and female mice, indicating no sex-specific sodium retention in our study. Potassium excretion in female mice also increased in female mice on HS diet, which may be an indication of a renal potassium-excretion response to an elevated renal aldosterone activity in these mice, although further studies are needed to evaluate the activation of renal MR in response to HS in female mice.
Adrenal CYP11B2 activity is the major source of aldosterone production in both male and female mice, and many studies have demonstrated that adrenal CYP11B2 expression is regulated by changes in dietary sodium and is decreased in both mice and rats in response to a HS diet 30, 31. We did not observe a reduction in CYP11B2 protein expression in male mice in response to a HS diet, however, expression was not increased. In contrast, female mice depicted a significant upregulation of adrenal CYP11B2 expression in response to HS. Overexpression of CYP11B2 has been shown to induce low-renin salt-sensitive hypertension30. As would be expected with a failure to suppress adrenal CYP11B2 expression, aldosterone plasma levels were not suppressed in female Balb/C mice in response to HS diet, in contrast to male mice in whom a suppression was indicated. Increases in adrenal CYP11B2 expression are not explained by an increased renin-angiotensin system activation as demonstrated by our plasma measurement of ANGII and PRA. Interestingly, in both male and female mice on HS, adrenal CYP11B2 protein levels did not correlate with circulating aldosterone levels in our study, in that male mice suppressed aldosterone level without a suppression of adrenal CYPP11B2 expression and female mice did not change aldosterone plasma levels in response to HS feeding despite an increase in adrenal CYP11B2 expression. These data indicate that CYP11B2 is a less efficient enzyme to produce aldosterone in response to high salt feeding in male and female Balb/C mice. As this is the first study, to our knowledge, to assess adrenal CYP11B2 and aldosterone levels in Balb/C mice we are limited in proposing an explanation for this phenomenon. We did observe that adrenal renin mRNA expression was unchanged with diet or eplerenone treatment in male and female mice regardless of diet or dietary salt feeding. However, our data indicate that adrenal angiotensinogen mRNA expression of female mice on HS is higher than that of male mice on HS. Furthermore, adrenal angiotensinogen levels were decreased in eplerenone treated female mice on HS compared to HS females, indicating a reduction of high salt diet-induced adrenal stimuli for angiotensinogen expression in females on eplerenone treatment. Peters et al. has described the regulation of the local renin angiotensin aldosterone system in adrenal glands 32, however, the sex specificity of responses of the adrenal renin angiotensin system to dietary sodium is, as yet, unexplored. Therefore, we may conclude that increased adrenal angiotensinogen production in female mice on high salt diet led to an increase in angiotensin type I receptor activation via increased local angiotensin II production, however, further experimentation is warranted to fully elucidate the relationship of high salt diet effects on angiotensinogen-stimulated angiotensin II production and resulting aldosterone synthesis. We have previously shown that leptin is a novel regulator of CYP11B2 expression17, and we show in this study that although leptin levels were unaltered in the circulation, leptin receptor expression in the adrenal gland of female mice only was increased with HS feeding, indicating that local leptin receptor activation may also lead to an increase in CYP11B2 expression.
We have previously demonstrated that obesity-associated hypertension mechanisms in female Balb/C mice involve mineralocorticoid receptor-mediated mechanisms18. Coupled with our present data indicating that adrenal CYP11B2 expression is increased and aldosterone levels are not decreased in female mice in response to a HS diet, we proposed that BP increases in female mice in response to dietary salt may be mediated by MR activation. Eplerenone treatment in female mice on HS ablated BP increases while having no effect in males. The BP lowering effects of eplerenone, in either sex, are commonly attributed to its role in sodium retention. However, our previous studies, along with others 33, 34, have indicated that MR-mediated BP mechanisms in female mice likely involve vascular mechanisms, as obesity-associated endothelial dysfunction is prevented by MR inhibition in female mice17, 18. Our data are in correlation with a recent study that indicates that females are more predisposed to aldosterone production than males 35. A further newly emerging topic in the mineralocorticoid receptor field is the regulation of endothelial function via endothelial mineralocorticoid receptors. As our study indicated that sodium intake and excretion balance was not sex discrepant nor affected by eplerenone in HS male and female mice this brings the discussion of the potential of endothelial mineralocorticoid receptor activation in our current study to the forefront as a potential contributing mechanism. Importantly, studies have demonstrated that the role of the endothelial mineralocorticoid receptor to regulate endothelial function in female mice is more pronounced than males in models of obesity 36, therefore, we may speculate that sustained activation of endothelial mineralocorticoid receptors in HS females contributed to the development of endothelial impairment in the current study. Our data demonstrating that eplerenone restores both BP and endothelial function in female mice on HS feeding, while having no effect on males, further indicates a potential role for the endothelial mineralocorticoid receptor in the salt sensitive phenotype we present in Balb/C mice. Furthermore, we determined that 11βHDS2 expression in the vasculature of our female mice is not suppressed, ergo it is unlikely that local cortisol activation of mineralocorticoid receptors was increased in female mice. Thus, we presume that vascular endothelial mineralocorticoid receptor activation in high salt diet fed female mice is aldosterone-mediated. Future directions include the investigation of the regulation of endothelial mineralocorticoid receptor activity in response to elevations in dietary sodium in male and female Balb/C mice.
We observed in the present study that endothelial dysfunction induced by HS diet in female mice, which did not present in males, is restored by eplerenone treatment. Endothelial dysfunction is well-noted for its role in increasing the risk for hypertension and others have demonstrated that a HS-diet increases the propensity to endothelial impairment 37. This notion is reiterated by the hypothesis of Kurtz et al 24 who suggests it is this propensity for HS-induced impairment in vascular function rather than renal sodium handling that predisposes salt-sensitivity. In our study, the development of endothelial dysfunction in female mice in absence of apparent changes in renal sodium retention indicate that impaired vasodilation is the more dominant BP control mechanism mediating hypertension in female mice on HS diet. Further, it is therefore more likely that the ablation of salt-sensitive BP increases observed in female mice in response to eplerenone is attributable to its beneficial effects on endothelial function rather than alterations in renal sodium handling. These results, in particular, highlight an important role for endothelial dysfunction in the control of BP in response to high salt feeding that is sex-specifically heightened in female Balb/C mice. Although our results are unable to distinguish whether increases in BP were a causation factor of the development of endothelial impairment, or vice versa rather, clinical data supports the notion that females are more likely to develop endothelial dysfunction than males 38. Endothelial dysfunction and salt sensitivity are associated presentations in the clinical population 39. Our study, therefore, indicates that female Balb/C mice are more sensitive to endothelial impairment due to elevated sodium intake compared to males at the 7-day time point studied, which likely predisposed the increases in BP observed in females on high salt diet. These data beg the future directions of determining if longer periods of high salt feeding eventually induce endothelial damage and elevated BP in male mice and, further, examination of the underlying endothelial impairment mechanisms (i.e. reactive oxygen species, reduction in nitric oxide bioavailability) that may have developed earlier in females than males in response to high salt feeding.
The data presented here in Balb/C mice in fact correlate well with clinical studies in patients of African ancestry, which show a strong association of plasma aldosterone/PRA to BP 40, 41. These studies prompt the suggestion of MR antagonists as principal hypertension therapy for these patients. What remains at present very poorly investigated is whether a sex-difference in the clinical population exists. The Balb/C mouse may, therefore, prove a model of salt-sensitive hypertension particularly for women of African American ancestry, whom are more likely to be salt-sensitive than men of African ancestry 42 or women of Caucasian background 43.
In summary, the data indicate that Balb/C mice develop sex-specific salt-sensitive elevations in BP that are associated with an inability to suppress aldosterone production, and further, that that elevation in BP and development of endothelial dysfunction in female mice on HS diet is responsive to MR inhibition. These data parallel data previously observed by our group and others, which indicates that the aldosterone-MR system is of particular importance in female mice for the control of BP in pathological states such as obesity-associated hypertension, and in this report salt-sensitive hypertension 17, 18. Collectively, these data indicate that the evaluation of the regulation of aldosterone production and activity is a promising BP target in females
Perspectives
Sex-discrepant mechanisms of hypertension continue to emerge in a variety of pathologies, including salt-sensitive hypertension. While the development of hypertension in the current study is modest by the standards of some other models, both the American Heart Association and the American College of Cardiology have recently acknowledged that a BP 10 mmHg above normal ranges is a significant risk factor as reflected by the rebranding of this measure as “hypertensive”. Given the current availability and long-term safety evaluations of MR antagonists, the implication of these pharmaceutical agents as promising for hypertension of hypertensive women is highly clinically relevant. In addition, the further development of the Balb/C female mouse as a potential model of MR-mediated hypertension mechanisms, among others, has high potential to impact further investigations in the field.
Supplementary Material
Novelty and Significance.
What is new?
Female Balb/C mice develop salt-sensitive hypertension and endothelial dysfunction
Female mice on high salt diet do not suppress aldosterone as male mice do
Salt-sensitive hypertension and endothelial dysfunction is ablated by mineralocorticoid receptor antagonism in female mice
What is relevant?
Aldosterone-mineralocorticoid receptor mediated mechanisms may play a role in this predisposition to salt sensitivity in women
Eplerenone may be a vital therapeutic target for hypertension in salt sensitive women
Acknowledgements
The authors would wish to thank Dr. Marko Poglitsch of Attoquant Diagnostics in Vienna, Austria for his assistance with plasma mass spectrometry analysis.
Sources of Funding
This work was supported by NIH 1R01HL130301-01 and AHA 16IRG27770047 to EbdC and AHA 17POST33660678 and NIH 5F32HL136191-02 to JLF
Footnotes
Conflict of Interest
The authors have no conflicts of interest.
References
- 1.Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension prevalence and control among adults: United states, 2015–2016. NCHS Data Brief 2017:1–8 [PubMed] [Google Scholar]
- 2.Felder RA, White MJ, Williams SM, Jose PA. Diagnostic tools for hypertension and salt sensitivity testing. Curr Opin Nephrol Hypertens 2013;22:65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang CL, Kuo E. Mechanisms of disease: Wnk-ing at the mechanism of salt-sensitive hypertension. Nat Clin Pract Nephrol 2007;3:623–630 [DOI] [PubMed] [Google Scholar]
- 4.Iatrino R, Manunta P, Zagato L. Salt sensitivity: Challenging and controversial phenotype of primary hypertension. Curr Hypertens Rep 2016;18:70. [DOI] [PubMed] [Google Scholar]
- 5.Van Vliet BN, Montani JP. The time course of salt-induced hypertension, and why it matters. Int J Obes (Lond) 2008;32 Suppl 6:S35–47 [DOI] [PubMed] [Google Scholar]
- 6.Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL, American Heart Association P, Public Education Committee of the Council on H, Council on Functional G, Translational B, Stroke C. Salt sensitivity of blood pressure: A scientific statement from the american heart association. Hypertension 2016;68:e7–e46 [DOI] [PubMed] [Google Scholar]
- 7.Chen J Sodium sensitivity of blood pressure in chinese populations. Curr Hypertens Rep 2010;12:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott P, Dyer A, Stamler R. The intersalt study: Results for 24 hour sodium and potassium, by age and sex. Intersalt co-operative research group. J Hum Hypertens 1989;3:323–330 [PubMed] [Google Scholar]
- 9.Michikawa T, Nishiwaki Y, Okamura T, Asakura K, Nakano M, Takebayashi T. The taste of salt measured by a simple test and blood pressure in japanese women and men. Hypertens Res 2009;32:399–403 [DOI] [PubMed] [Google Scholar]
- 10.Kittikulsuth W, Looney SW, Pollock DM. Endothelin et(b) receptors contribute to sex differences in blood pressure elevation in angiotensin ii hypertensive rats on a high-salt diet. Clin Exp Pharmacol Physiol 2013;40:362–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staehr M, Hansen PB, Madsen K, Vanhoutte PM, Nusing RM, Jensen BL. Deletion of cyclooxygenase-2 in the mouse increases arterial blood pressure with no impairment in renal no production in response to chronic high salt intake. Am J Physiol Regul Integr Comp Physiol 2013;304:R899–907 [DOI] [PubMed] [Google Scholar]
- 12.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension 2000;35:484–489 [DOI] [PubMed] [Google Scholar]
- 13.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin ii when the endogenous renin-angiotensin system is blocked. Hypertension 2008;51:1170–1176 [DOI] [PubMed] [Google Scholar]
- 14.Giachini FR, Sullivan JC, Lima VV, Carneiro FS, Fortes ZB, Pollock DM, Carvalho MH, Webb RC, Tostes RC. Extracellular signal-regulated kinase 1/2 activation, via downregulation of mitogen-activated protein kinase phosphatase 1, mediates sex differences in desoxycorticosterone acetate-salt hypertension vascular reactivity. Hypertension 2010;55:172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouchi Y, Share L, Crofton JT, Iitake K, Brooks DP. Sex difference in the development of deoxycorticosterone-salt hypertension in the rat. Hypertension 1987;9:172–177 [DOI] [PubMed] [Google Scholar]
- 16.Brinson KN, Rafikova O, Sullivan JC. Female sex hormones protect against salt-sensitive hypertension but not essential hypertension. Am J Physiol Regul Integr Comp Physiol 2014;307:R149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, Belin de Chantemele EJ. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 2015;132:2134–2145 [DOI] [PubMed] [Google Scholar]
- 18.Huby AC, Otvos L Jr., Belin de Chantemele EJ. Leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in obese female mice. Hypertension 2016;67:1020–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita T Mechanism of salt-sensitive hypertension: Focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol 2014;25:1148–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, Kawarazaki W, Takeuchi M, Ayuzawa N, Miyoshi J, Takai Y, Ishikawa A, Shimosawa T, Ando K, Nagase M, Fujita T. Rac1 gtpase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest 2011;121:3233–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL, McDonough AA. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol 2017;28:3504–3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basu R, Poglitsch M, Yogasundaram H, Thomas J, Rowe BH, Oudit GY. Roles of angiotensin peptides and recombinant human ace2 in heart failure. J Am Coll Cardiol 2017;69:805–819 [DOI] [PubMed] [Google Scholar]
- 23.Satoh M, Kikuya M, Hosaka M, Asayama K, Inoue R, Metoki H, Tsubota-Utsugi M, Hara A, Hirose T, Obara T, Mori T, Totsune K, Hoshi H, Mano N, Imai Y, Ohkubo T. Association of aldosterone-to-renin ratio with hypertension differs by sodium intake: The ohasama study. Am J Hypertens 2015;28:208–215 [DOI] [PubMed] [Google Scholar]
- 24.Morris RC Jr., Schmidlin O, Sebastian A, Tanaka M, Kurtz TW. Vasodysfunction that involves renal vasodysfunction, not abnormally increased renal retention of sodium, accounts for the initiation of salt-induced hypertension. Circulation 2016;133:881–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ 2012;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirabito KM, Hilliard LM, Kett MM, Brown RD, Booth SC, Widdop RE, Moritz KM, Evans RG, Denton KM. Sex- and age-related differences in the chronic pressure-natriuresis relationship: Role of the angiotensin type 2 receptor. Am J Physiol Renal Physiol 2014;307:F901–907 [DOI] [PubMed] [Google Scholar]
- 27.Kawarazaki W, Fujita T. Aberrant rac1-mineralocorticoid receptor pathways in salt-sensitive hypertension. Clin Exp Pharmacol Physiol 2013;40:929–936 [DOI] [PubMed] [Google Scholar]
- 28.Satoh M, Kikuya M, Ohkubo T, Imai Y. Role of angiotensinogen and relative aldosterone excess in salt-sensitive hypertension. Hypertension 2012;59:e57; author reply e58 [DOI] [PubMed] [Google Scholar]
- 29.Drenjancevic-Peric I, Jelakovic B, Lombard JH, Kunert MP, Kibel A, Gros M. High-salt diet and hypertension: Focus on the renin-angiotensin system. Kidney Blood Press Res 2011;34:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makhanova N, Hagaman J, Kim HS, Smithies O. Salt-sensitive blood pressure in mice with increased expression of aldosterone synthase. Hypertension 2008;51:134–140 [DOI] [PubMed] [Google Scholar]
- 31.Ye P, Kenyon CJ, MacKenzie SM, Seckl JR, Fraser R, Connell JM, Davies E. Regulation of aldosterone synthase gene expression in the rat adrenal gland and central nervous system by sodium and angiotensin ii. Endocrinology 2003;144:3321–3328 [DOI] [PubMed] [Google Scholar]
- 32.Peters J Local renin-angiotensin systems in the adrenal gland. Peptides 2012;34:427–432 [DOI] [PubMed] [Google Scholar]
- 33.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low-dose mineralocorticoid receptor blockade prevents western diet-induced arterial stiffening in female mice. Hypertension 2015;66:99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia G, Habibi J, DeMarco VG, Martinez-Lemus LA, Ma L, Whaley-Connell AT, Aroor AR, Domeier TL, Zhu Y, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial mineralocorticoid receptor deletion prevents diet-induced cardiac diastolic dysfunction in females. Hypertension 2015;66:1159–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shukri MZ, Tan JW, Manosroi W, Pojoga LH, Rivera A, Williams JS, Seely EW, Adler GK, Jaffe IZ, Karas RH, Williams GH, Romero JR. Biological sex modulates the adrenal and blood pressure responses to angiotensin ii. Hypertension 2018;71:1083–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davel AP, Lu Q, Moss ME, Rao S, Anwar IJ, DuPont JJ, Jaffe IZ. Sex-specific mechanisms of resistance vessel endothelial dysfunction induced by cardiometabolic risk factors. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A 2007;104:16281–1628617911245 [Google Scholar]
- 38.Suboc TM, Dharmashankar K, Wang J, Ying R, Couillard A, Tanner MJ, Widlansky ME. Moderate obesity and endothelial dysfunction in humans: Influence of gender and systemic inflammation. Physiol Rep 2013;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bragulat E, de la Sierra A, Antonio MT, Coca A. Endothelial dysfunction in salt-sensitive essential hypertension. Hypertension 2001;37:444–448 [DOI] [PubMed] [Google Scholar]
- 40.Huan Y, Deloach S, Keith SW, Goodfriend TL, Falkner B. Aldosterone and aldosterone: Renin ratio associations with insulin resistance and blood pressure in african americans. J Am Soc Hypertens 2012;6:56–65 [DOI] [PubMed] [Google Scholar]
- 41.Scott L, Woodiwiss AJ, Maseko MJ, Veliotes DG, Majane OH, Paiker J, Sareli P, Norton GR. Aldosterone-to-renin ratio and the relationship between urinary salt excretion and blood pressure in a community of african ancestry. Am J Hypertens 2011;24:951–957 [DOI] [PubMed] [Google Scholar]
- 42.Joseph JJ, Echouffo-Tcheugui JB, Kalyani RR, Yeh HC, Bertoni AG, Effoe VS, Casanova R, Sims M, Correa A, Wu WC, Wand GS, Golden SH. Aldosterone, renin, and diabetes mellitus in african americans: The jackson heart study. J Clin Endocrinol Metab 2016;101:1770–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright JT Jr, Rahman M, Scarpa A, Fatholahi M, Griffin V, Jean-Baptiste R, Islam M, Eissa M, White S, Douglas JG. Determinants of salt sensitivity in black and white normotensive and hypertensive women. Hypertension 2003;42:1087–1092 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


