Abstract
Speciation mechanisms in marine organisms have attracted great interest because of the apparent lack of substantial barriers to genetic exchange in marine ecosystems. Marine mussels of the Mytilus edulis species complex provide a good model to study mechanisms underlying species formation. They hybridise extensively at many localities and both pre- and postzygotic isolating mechanisms may be operating. Mussels have external fertilisation and sperm cells should show specific adaptations for survival and successful fertilisation. Sperm thus represent key targets in investigations of the molecular mechanisms underlying reproductive isolation. We undertook a deep transcriptome sequencing (RNA-seq) of mature male gonads and a 2DE/MS-based proteome analysis of sperm from Mytilus edulis and M. galloprovincialis raised in a common environment. We provide evidence of extensive expression differences between the two mussel species, and general agreement between the transcriptomic and proteomic results in the direction of expression differences between species. Differential expression is marked for mitochondrial genes and for those involved in spermatogenesis, sperm motility, sperm-egg interactions, the acrosome reaction, sperm capacitation, ATP reserves and ROS production. Proteins and their corresponding genes might thus be good targets in further genomic analysis of reproductive barriers between these closely related species.
Keywords: Sperm, gonad, external fertilisation, marine invertebrates, reproductive isolation, speciation, proteomics, transcriptomics
Graphic Abstract

1. Introduction
The study of the mechanisms that lead to the formation of new species is of special interest in marine ecosystems due to the lack of obvious barriers to gene flow, and is especially relevant in organisms with a prolonged period of larval dispersion [1]. Many marine species release gametes into seawater, so fertilization occurs externally. Because of this, research on speciation in marine systems has focused on the evolution of gamete recognition systems because of their potential as prezygotic reproductive isolation mechanisms [2–4]. The role of postzygotic mechanisms has been less studied and is controversial [5] despite their potential relevance to maintain the integrity of species [6]. It seems obvious that gametes are key cell targets in investigations of the molecular mechanisms underlying reproductive isolation. Molecular studies on gametes are however quite scarce and largely restricted to a few model organisms. The molecular basis of fertilisation including the sperm-egg recognition system is still a poorly understood, yet basic, biological process [7–8]. In marine invertebrates such studies have focused on sea urchins, starfish, clams, oysters, abalones, sea snails and worms [8–9]. The use of a greater diversity of species has recently been advocated as a good way to shed light on diverse questions that remain open in reproductive biology [10], including the molecular basis of species-specificity gamete interactions during fertilisation.
Sperm are highly differentiated cells with marked genetic, cellular and functional differences from other cell types, reflecting important roles in fertilization, embryonic development, and heredity [11]. The sperm cell has also been put forward as an ideal candidate for proteomic analyses [12], mainly because it is thought to be transcriptionally inert (but see [13]). So far only a few proteomics studies have focussed on sperm cells, mostly in widely studied model organisms (see [4, 14]). The ascidian Ciona intestinales [15], the red abalone Haliotis rufescens [16], the Pacific oyster Crassostrea gigas [17], the king scallop Pecten maximus [18] and the marine mussels Mytilus edulis [19–20] and M. galloprovincialis [21], are the only marine organisms, all of them external fertilisers, currently in the sperm cell proteomic literature. Furthermore to the best of our knowledge, there are no comparative quantitative proteomic studies of sperm of closely related species, with the exception of an analysis of different ungulate and rodent species [14, 22]. A comparative research strategy involving proteomics should contribute towards elucidating the molecular basis underlying reproductive isolation mechanisms and the evolutionary forces involved, as well as to obtaining a better understanding of basic functional aspects of sperm biology at the molecular level.
Marine mussels from the Mytilus edulis complex are represented by three closely related species (Mytilus edulis, M. galloprovincialis and M. trossulus) that are able to hybridise at some rocky shore areas where their distributions overlap [23]. Hence, mussels represent a good model to address evolutionary hypotheses and study mechanisms underlying the formation of new species. On European coasts, M. edulis has a more northerly and M. galloprovincialis a more southerly distribution, while M. trossulus is mainly restricted to the Baltic Sea area. There are many localities where hybridisation and variable levels of genome introgression occur between the species. Research on Mytilus spp. has also attracted attention because of the important mussel aquaculture industry. Marine mussels are external fertilisers with a prolonged planktonic larval stage facilitating dispersal over great distances [24]. In order to preserve their genome integrity, despite extensive hybridisation, different reproductive mechanisms are likely to be operating both at the pre- and postzygotic level, though their relative contribution and underlying molecular mechanisms are not yet well understood. Cross-species fertilisation in Mytilus might be prevented to some degree by molecular incompatibilities resulting from the rapid evolution of reproductive proteins. Evidence for positive selection on M7 and M3 sperm lysin protein was provided for sympatric and allopatric populations of Mytilus spp. [25–28]. However prezygotic barriers might not be strong enough to prevent introgression due to extensive hybrid zones and wide variation in the genomic introgression rates observed in natural populations [29]. Weaknesses of prezygotic barriers are also suggested by contrasting results from interspecific crosses under laboratory conditions between Mytilus spp. [30–36].
The arrival of high-throughput genomics and proteomics techniques is allowing the expansion of classical evolutionary studies over large protein datasets [37]. Despite this advance, less attention is still paid in evolutionary ecology studies to the proteome as compared to the transcriptome or genome, even though the proteome is closer to the molecular phenotype, and thus a more direct target for natural selection [38–40]. The choice of reproductive tissues or gametes as the main focus of research helps to bridge the gap between reproductive phenotypes and underlying molecular mechanisms [37, 41]. A 2-DE based proteomic study using a somatic tissue, the foot, from two sympatric Mytilus species (M. edulis and M. galloprovincialis) and their hybrids showed differences in the protein expression patterns of hybrids when compared with the two parental species, providing evidence compatible with Dobzhansky-Muller incompatibilities (DMI) between both parental genomes in hybrids [42]. Thus postzygotic isolation factors may also have played a role in limiting the degree of introgression among genomes of Mytilus spp. New studies using high throughput genomics and proteomics on gametes should provide a significantly better understanding of the molecular mechanisms underlying reproductive isolation and evolution of Mytilus spp.
A good strategy when working with less well studied organisms to significantly boost the number and quality of protein identifications obtained through mass spectrometry analysis is to generate a customised protein database, for example through the translation of tissue and species-specific transcriptome datasets available in public databases or obtained from in-house experiments [37]. An additional resource for mussels is a recently published M. galloprovincialis genome [43]. However the availability of protein databases derived from transcriptomes provides a useful and complementary tool because of known limitations in the prediction and annotation of genes and posttranscriptional variants [44]. Moreover the combined use of transcriptomic and proteomic data specifically in non-model organisms has been advocated as one of the most useful proteogenomic approaches [45–46], because of its high and proven potential for synergy between the two approaches.
In this study we undertook a deep transcriptome sequencing (RNA-seq) of mature male gonads obtained from Mytilus edulis and M. galloprovincialis individuals acclimatised for several weeks to common laboratory conditions after collection from their native localities. The results from this study contribute to, 1) providing a tissue Mytilusspecific protein database to enhance protein identifications in follow-up proteomic analyses, and 2) providing a preliminary list of candidate gene products with potential involvement in sperm biology, fertilisation and reproductive isolation mechanisms in the two Mytilus species. A second complementary analysis based on a 2-DE+MS/MS proteomic approach, with the use of different customised protein databases, including one derived from our transcriptome data, to enhance protein identification, was carried out directly on sperm samples. This was to assess whether sperm samples from the same two Mytilus species and populations, that were acclimatised to common laboratory conditions for several months, presented proteomic differences which would be a consequence of underlying genetic differences between the populations and species. The level of concordance of differential expression results between transcriptome and proteome data is evaluated, while the functional consequence of the observed variation is discussed from an evolutionary perspective in relation to sperm biology, and the potential role of the variation in fertilisation and reproductive isolation.
2. Materials and Methods
Extended versions of Material and Methods for RNA-seq and proteomic analysis are provided in Ref. [47] and File S1 respectively.
2.1. Transcriptome (RNA-seq) analysis of mature male gonad tissues from two Mytilus spp.
2.1.1. Sampling and histological analysis
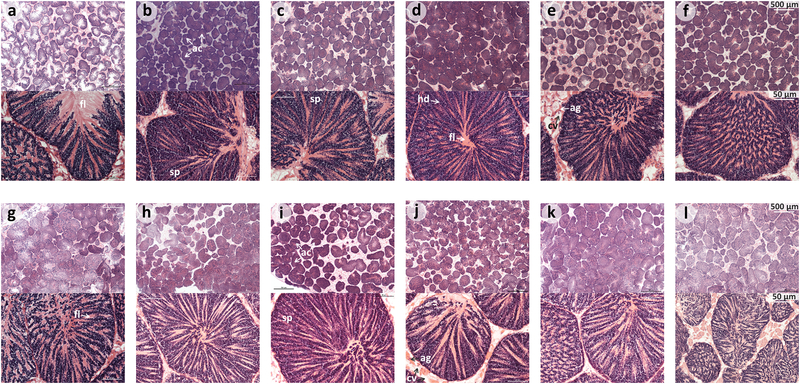
Mussels from Mytilus edulis and Mytilus galloprovincialis species were collected from rocky shores in Swansea (South Wales, UK) and Ria de Vigo (North-West Spain) respectively during the end of January of 2012, transported to aquarium facilities in the marine station at the University of Vigo (ECIMAT), and kept there in seawater under the same conditions for at least 2 months. This design ensured that all analysed individuals shared the same environmental conditions, and that gene expression differences between species were not therefore the results of differences in the immediate environment [48]. After 2 months, mussels from each species were processed individually. From each mussel, one piece of gonad tissue was immediately snap frozen and preserved in liquid nitrogen for further RNA-seq analysis, while a second piece of the same tissue was used for a histological test to assess the sex and reproductive stage of the mussel. For this purpose gonad tissues were fixed in Davidson´s solution and embedded in paraffin. Paraffin blocks were sectioned at 5μm with a microtome. Tissue sections were deparaffinised, stained with Harris´ hematoxylin and eosin, and examined by light microscopy for a histological study. Finally, 6 individual samples from each Mytilus species corresponding to reproductively mature male individuals were chosen for RNA extraction (Figure 1).
Figure 1:
Histological tests of mature male gonads of the six Mytilus edulis (a-f) and six M. galloprovincialis (g-l) mussels selected to make each pool for RNA-seq analysis. There are two different zoom views (see 500 and 50 μm scale respectively, above and below) shown for each histology test and individual mussel. Ac: male gonadal follicles with spermatozoa (sp), where heads (hd) and flagella (fl) can be seen and differentiated. Adipogranular (ag) and vesicular connective tissue (cv) cells can be found between the spermatic acini.
2.1.2. RNA extraction, mRNA library and Illumina paired-end sequencing
RNA extraction was carried out using a protocol based on the Qiagen RNeasy® Mini kit (Qiagen, Valencia, CA, USA) with tissue homogenization in QIAshredder columns (Qiagen). The quantification of RNA samples was carried out using a NanoDrop 1000 Spectrophotometer (Thermo scientific, DE, USA), and the RNA quality was assessed in an Agilent 2100 bioanalyzer (Agilent Technologies, CA, USA). Total RNA extracts from these selected samples were used to make two pools of 6 individuals each, one pool for each of the two Mytilus species. 700 ng of RNA per individual sample was used, so each pool contained 4.2 μg of total RNA. mRNA libraries were generated using the Illumina Truseq Small RNA Preparation kit (Illumina, CA, USA) according to Illumina's TruSeq Small RNA Sample Preparation Guide v2 (low sample protocol). Agarose gel-based selection was carried out to obtain libraries with fragments close to 500 bp in length, and their quality was assessed through Bioanalyzer profiles using a high sensitivity DNA chip. Finally, libraries were quantified, by using quantitative PCR with specific primers complementary to the library adapters and KAPA SYBR FAST Universal qPCR Kit (Kapa Biosystems, MA, USA), and diluted to 12 pM before sequencing. Each library, corresponding to each of the two pools, was analysed in a full line of the flow cell from an Illumina HiScanSQ instrument (Illumina) and using TruSeq SBS v3 chemistry (Illumina) to generate 2 × 100 bases long paired-end reads. After sequencing, data were acquired and analysed by using the Genome Analyzer Sequencing Control Software (SCS 2.6) and Real Time Analyser (RTA 1.6) software from Illumina. A total of 124,102,082 and 111,865,458 raw reads were obtained from the Mytilus edulis and Mytilus galloprovincialis pooled samples respectively. Raw data were deposited into SRA-NCBI database (BioProject ID: PRJNA451093). The quality control and filtering of nucleotide sequences was carried out as explained in Ref. [47], yielding 187,829,361 confident reads that were used for de novo assembly and generation of a consensus transcriptome.
2.1.3. De novo transcriptome assembly and functional annotation
Due to absence of a complete Mytilus spp. genome sequence (but see a recently published low-coverage M. galloprovincialis genome in [43]), it was necessary to follow a de novo assembly approach in order to build a consensus transcriptome from mature male gonad from both Mytilus spp. Thus, reads from both Mytilus species were assembled to generate a set of contigs (herein isotigs). The full set of isotigs should represent the majority of transcribed genes in this specific tissue in either one or both Mytilus species. This approach allowed the comparison of the expression levels from the different isotigs between samples of the two species. De novo transcriptome assembly was carried out by using Velvet followed by Oases software [49–50]. Oases uses the preliminary assembly made by Velvet to complete the assembling of reads into isotigs. Finally, it clusters the isotigs into small groups called loci (synonymous with the term isogroups, also used in the literature), representing the consensus transcriptome of the samples under study. These are not genetic loci, but rather a collection of similar sequences (isotigs), which might include different splice variants, alleles and partial assemblies of longer transcripts. Hence, it might be said that there are different isotigs for each locus (consensus transcript). Nevertheless, many loci contain only one isotig, though some others may contain hundreds of isotigs. The generated consensus transcriptome was annotated against a non-redundant UniProtKB/SwissProt sequence database using the program BlastX [51]. For comparative purposes the annotation was repeated against the published genome of another marine bivalve the Pacific oyster Crassostrea gigas [52], against all EST sequences available in NCBI from “Mytilus”[organism], and against two protein databases with sequences retrieved from NCBI either for "Mytilus"[Organism] or "Mollusca"[Organism] using a threshold evalue of 1×10−3. Functional annotation based on Gene Ontology (GO) terms was performed using the tool Blast2GO [53]. An enrichment analysis of GO terms was carried out for those transcripts that showed significant differences between samples of the two Mytilus spp. (see below) using Fisher's exact test with a FDR=5% (see Ref. [47] for further details on method). This might provide some clues about the differences at functional level present in mature male gonad tissue of the two Mytilus spp.
2.1.4. Differential expression analyses
In the present study, differential gene expression analysis from mature male gonad tissue (pooled samples) between Mytilus edulis and M. galloprovincialis was carried using the RNA-seq data at isotig level. In circumstances where one biological replicate is available for each treatment group, methods based on the Negative Binomial (NB) distribution [54] can be used to make inferences about differential expression between the Mytilus species and identify isotigs with higher effect-size. These changes could be supported in complementary studies, for instance by proteomic analysis with an appropriate biological replication (see section 2.2). The pooling approach met the requirements to fulfil one of the main objectives of the current study. This is to generate a tissue-specific Mytilus protein database from a high coverage reference transcriptome of both species in order to increase the success of protein identifications in proteomic analysis on sperm cells (see section 2.2). RSEM [55] combined with EBSeq [56] software were used to calculate differential expression (p<0.05, FDR=5%). This pipeline is appropriate in situations where a reference genome is not available, enabling accurate transcript quantification after transcriptomic de novo assembly [55], while controlling the false discovery rate (FDR) [57]. Functional annotation and an enrichment analysis for those differentially expressed transcripts was carried out as explained in the above section 2.1.3 and Ref. [47].
2.2. Proteomic analyses of sperm samples from two Mytilus spp.
2.2.1. Sampling of mussels and sperm sample collection
Mussels from Mytilus edulis and Mytilus galloprovincialis species were collected from rocky shores in Swansea (South Wales, UK) and Ria de Vigo (North-West Spain) respectively at different times within the spawning period (end of January and April) in 2012, transported and kept under as far as possible the same laboratory conditions for at least 2 months, in order to minimize the differences between mussel species due to immediate environmental effects (see [48]). After 2 months, mussels were periodically induced to spawn following a thermal shock procedure (see detail in File S1). Sperm samples released into filtered/UV-treated seawater in individual bottles were collected, filtered twice (300 μm and 41 μm sieves), and centrifuged for 10 min at 24400 g, 10ºC. After discarding the supernatant, the pellet containing sperm was resuspended in 150 μl of a 10% glycerol solution, snap frozen in liquid nitrogen, and finally preserved at -80ºC until further analysis. In parallel, a drop of seawater for each sample containing sperm cells was examined under the microscope in order to check that the sperm presented good morphology, high motility and density, otherwise the sample was discarded for any further analysis.
2.2.2. Protein extraction and 2-DE electrophoresis
Proteins were extracted from sperm samples of the two Mytilus spp. (10 biological replicates for each Mytilus spp. Two of them were run twice) in 0.3–0.5 ml of lysis buffer (7M urea, 2M thiourea, 4% CHAPS, 1% DTT and 1% carrier ampholytes 3–10) aided by sonication on ice (Branson Digital Sonifier 250, CT, USA). After centrifugation for 30 min at 21,000g, at 10°C, the supernatant was stored at -80ºC until electrophoresis. Protein concentration was measured with the Bradford method [58]. Approximately 200 μg of total protein was used for 2-DE. The first dimension electrophoresis was carried out with immobilized pH gradient strips (pH 5–8/17cm, BioRad) in a horizontal electrophoresis apparatus Protean IF System (BioRad) after strip equilibration. The second dimension of gel electrophoresis was carried out in 12.5 % polyacrylamide gels using an EttanDaltsix electrophoresis system (GE Healthcare, Little Chalfont, UK) at 20ºC, 15W/gel, and ~ 6h. Protein spots were visualized using SYPRO-Ruby (Molecular Probes, OR, USA), following the protocol described in [48]. Stained gels were scanned with a Pharox FX Plus molecular imager (BioRad), and 2DE gel images saved in TIFF file format. The SameSpots vs.4.1 (Nonlinear Dynamics Ltd, Newcastle upon Tyne, UK) software was used for 2-DE gel image and protein spot detection analysis (including background subtraction and normalisation) following the same procedure described in [59]. Normalised protein spot volumes for each 2-DE gel were saved in csv file format for further statistical analyses.
2.2.3. Statistical analyses of 2-DE gels
Normalised spot volumes were transformed to a logarithmic scale to fit normality and homoscedasticity assumptions of parametric tests [42]. Spearman's correlation coefficient and coefficient of variation (CV) calculations were carried out using the whole protein spot dataset from technical replicates, aiming to assess the experimental reproducibility. Analysis of variance (one-way ANOVA) using the log normalised volume of each protein spot (dependent variable) was carried out to test for significant differences in protein expression patterns in sperms cells of the two Mytilus spp., where biological replicates were used to provide the error variance in the analysis. Different corrections to account for the multiple hypothesis testing problem were calculated by using the SGoF+ software v.3.8 [60], thus following the procedure and rationale discussed in Ref. [61]. Heat map analysis was used to group protein spots and individual samples according to their similarity in expression pattern. The heat map and hierarchical clustering analyses were conducted with the R package gplots [62], using Euclidean distance and the complete linkage method. Chi-square contingency tests were used to compare distributions of ontology terms for the protein spot identification and RNA-seq results, with significance levels determined by bootstrapping using FORTRAN programs written for this purpose and which allow for test of significance of individual rows in contingency tables.
2.2.4. Mass spectrometry analysis and protein identification
The protein spots of interest were visualized on a blue-light DarkReader (Clare Chemical Research, CO, USA), excised and processed following the protocol described in Ref. [48]. Resulting peptides were analyzed in an Orbitrap Elite mass spectrometer coupled to a Proxeon EASY-nLC 1000 UHPLC system (Thermo Fisher, San Jose CA). Peptide separation was performed on RP columns (EASY-Spray column, 50 cm × 75 μm ID, PepMap C18, 2 μm particles, 100 Å pore size, Thermo Scientific) using a 120 min linear gradient from 5 to 25 % of acetonitrile at a flow rate of 300 nL/min. For ionization, the spray voltage used was 1.95 kV, the capillary temperature was 260ºC and the Orbitrap set at 120,000 resolution. A positive mode from 400 to 1,700 amu (1 μscan), 15 data dependent CID MS/MS scans using an isolation window of 2 amu and a normalized collision energy of 35%, with a dynamic exclusion for 80s after the fragmentation event, were used for peptide analysis. Singly charged ions were excluded from MS/MS analysis. MS/MS spectra were searched using PEAKS Studio v.7.0 program (Bioinformatics Solutions Inc., Waterloo, ON, Canada) against three customized protein databases. Databases were made from the tissue and Mytilusspecific RNA-seq data provided in this study, EST sequences available in NCBI for four Mytilus species retrieved using “Mytilus”[organism] as search term, and protein sequences deposited in NCBInr for “Mollusca” [organism] (see further detail in File S1). Positive protein identifications (FDR <1%) were only accepted when at least two matched and one unique peptide sequences were obtained. BlastX analyses against a non-redundant (nr) protein sequence database of all organisms were carried out in order to ascertain the final protein identities of translated EST and RNA-seq sequences using default parameters and a threshold e-value of 1×10−6.
3. Results
3.1. Transcriptome (RNA-seq) analysis of mature male gonad tissues from Mytilus edulis and M. galloprovincialis
3.1.1. De novo assembly and Blast analyses of the consensus transcriptome from both Mytilus spp.
RNA-seq analyses of the two pooled samples from mature male gonad tissues, one from Mytilus edulis and one from M. galloprovincialis, produced more of 200 million 100bp paired-end reads. After filtering steps, more than 187 million reads remained valid to be used for de novo assembly, hence the generation of a consensus transcriptome for both Mytilus spp. (Table 1). De novo assembly produced a total of 97,425 isotigs, grouped in 49,713 loci (see Files S1-S2 in Ref. [47]). Thus a consensus transcriptome for mature male gonads of the two Mytilus species was obtained. This provides a reference transcriptome to which individual reads from each pooled sample could be mapped in differential expression analysis. Moreover it provides a tissue and Mytilus-specific database that, once translated to six-reading frames, can be used for protein identification in the proteomic studies carried out on sperm samples (see section 3.2.2). The mean (median), maximum and N50 length of isotigs is 706 (434), 13,604 and 1,071 nucleotides, respectively (Table 1). The estimated size calculated for the consensus transcriptome of both Mytilus spp. is 35.1 Mb. The redundancy level found for the transcriptome assembly was low (1.5% of loci). Results from Blast analysis against different databases (see Materials and Methods, and Figure 4 in Ref. [47]) are summarised in Table 1. A total of 13,498 sequences (27.2% of total loci) were successfully identified against a non-redundant UniProtKB/SwissProt database. This moderate to low similarity with the database may be due to potential novel genes (or variants) in these two species, whose full genomes had not been sequenced at the time of elaborating this paper. This is supported by the following results. When Blast analysis was carried out against the published and annotated oyster (C. gigas) genome [52], another marine bivalve mollusc, the number of positive identifications rose to 18,279 transcripts (36.8%). The relatively modest increase in identifications may be due to the long divergence time between Mytilus and C. gigas even though they belong to the same phylum and class. This percentage is in line with the identification success (17,529 transcripts, 35.3%) and database coverage (% of sequences from NCBI database giving positive match against our transcriptome) obtained from Blast analysis against protein sequences from Molluscs retrieved from NCBI (Table 1). Despite the low number of protein sequences for Mytilus spp. available in protein databases, the Blast analysis showed, as expected, a level of coverage for a protein sequence database (Mytilus[organism], NCBInr) of 81.3%. A similar result, a database coverage of 82.7%, was obtained after Blast analyses against all EST sequences available in NCBI for Mytilus[organism] that were translated to proteins by using the six-reading frames. Although the redundancy level of these EST sequences is high, the number of sequences is high so it is not surprising to see that a positive match/identification was reached for 31,428 (63.2%) of loci from our consensus transcriptome.
Table 1:
Summary results from RNA-seq data and annotation through Blast analysis against different databases: 1) all protein sequences available in SwissProt (UniProtKB/SwissProt), 2) the Pacific oyster Crassostrea gigas genome (Oyster_Genome), 3) all EST sequences available in NCBI from “Mytilus”, 4) protein sequences retrieved from NCBI for "Mytilus" (NCBI_MytProt), and 5) protein sequences retrieved from NCBI for "Mollusca" (NCBI_MolluscaProt). See further details in materials and methods.
| Number of reads (raw / filtered) | 235,967,540 / 187,829,361 |
| Number of Isotigs | 97,425 |
| Number of Loci | 49,713 |
| Maximum sequence length (bp) | 13,604 |
| Mean / Median sequence length (bp) | 706 / 434 |
| N50 length (bp) | 1,071 |
| Number of Loci identified following: | |
| BlastX (UniProtKB/SwissProt) | 13,498 (27.1% of total loci)* |
| tBlastX (Oyster_Genome) | 18,279 (36.8%) |
| tBlastX (NCBI_MytESTs) | 31,428 (63.2%); database coverage [56,253 of total 67,990 MytEST sequences (82.7%)] |
| BlastX (NCBI_MytProt) | 2,234 (4.5%); database coverage [5,153 of total 6338 MytProt sequences (81.3%)] |
| BlastX (NCBI_MolluscaProt) | 17,529 (35.3%); database coverage [70,317 of total 190,951 MolluscaProt sequences (36.8%)] |
13,283 loci were functionally annotated using Blast2GO, including InterProScan.
3.1.2. Functional annotation of the consensus transcriptome from both Mytilus spp.
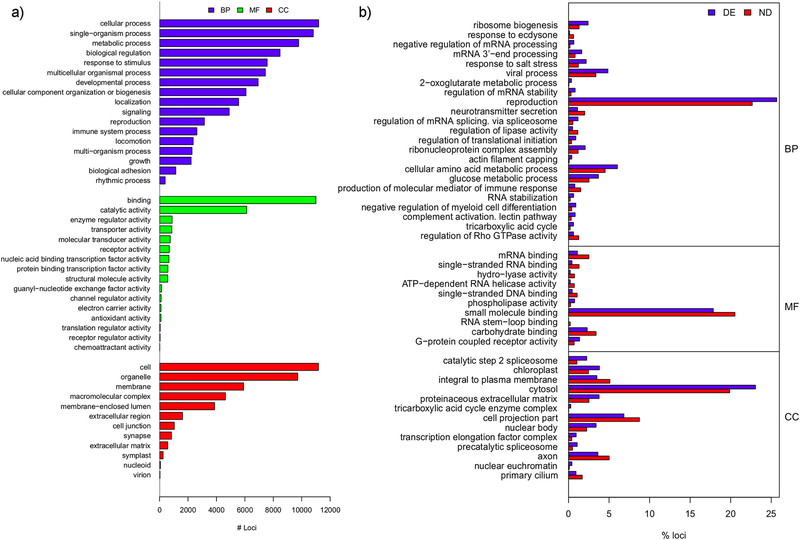
From functional analysis using Blast2GO, 12,156 loci were successfully annotated for GO terms (File S3 in Ref. [47]). The annotation was improved after InterProScan analysis, raising the number of successful annotations to 13,283 loci (File S4 in Ref. [47]). This might be interesting because functional information, e.g. a peptide signal sequence from the differential expressed sequences between Mytilus spp., is still reported despite the inability to get a confident gene/transcript identity during BlastX analysis. The distribution of GO-terms for the full annotated transcriptome at different levels, molecular function (MF), biological process (BP) and cellular component (CC) categories, is displayed in Figure 2a. It is reassuring to see that “reproduction” term is represented in BP category. The dominance of “binding”, a general term related to the non-covalent union or interaction of different molecules, in MF is also interesting because when checking MF terms for the more specific tree hierarchy level 3 (Figure 5 in Ref. [47]), the highest representation is for protein binding, a term related to interactions among proteins or protein complexes. This category should include sperm proteins involved in sperm-egg interaction. Finally it is interesting to highlight in category CC, in both Figure 2a and Figure 5 in Ref. [47], the high representation for terms related to membrane proteins that potentially include those that might be involved in the sperm-egg recognition mechanisms.
Figure 2:
a) Distribution of Level 2 GO terms of loci annotated in three ontological categories: biological process (BP), molecular function (MF) and cellular component (CC). Note that only those GO terms with annotations in at least 100 and 10 loci, for BP and MF respectively are shown. b) Enrichment analysis results for GO terms in differentially expressed loci between mature male gonads of the two Mytilus spp. according to Fisher's exact test (FDR<0.05). DE: differentially expressed, ND: not differentially expressed set of loci defined after RSEM analysis. Length of bars represents the percentage of loci annotated for each term in the DE (blue bars) and ND (red bars) sets. A blue longer than red bar indicates that that GO term is overrepresented in the differentially expressed loci. GO terms are grouped by their ontological category (BP, MF, CC), and within category, GO terms are displayed sorted by increasing p-values.
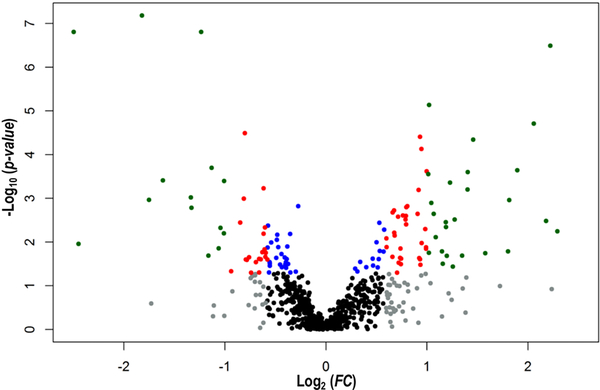
Figure 5:
Volcano plot made with the 727 sperm protein spots analysed by 2DE. Log2 of the ratio of average expression values between Swansea and Vigo populations (FC) plotted against log10 of p-values derived from the one-way ANOVA analysis. Note that positive and negative Log2 (FC) values mean higher expression on average in samples from Vigo (M. galloprovincialis) and Swansea (M. edulis), respectively. Grey (FC>1.5) and black (up to 1.5 FC) represent non-significant protein spots (p>0.05), while colour represents protein spots significant after one-way ANOVA (p≤0.05); blue, <1.5 FC; red, between 1.5 and 2.0 FC; green, >2.0 FC.
3.1.3. Differential expression analysis between Mytilus edulis and M. galloprovincialis.
A total of 27,233 isotigs (28% of the 97,425 occurring in the transcript assembly) are differentially expressed between pooled samples of the two Mytilus spp. at FDR 5%, of which 20,997 (21.6%) are significant at FDR 1%. This corresponds to 14,737 loci (29.6% of 49,713 loci in the transcript assembly) which are significant (in that they have at least one significant isotig) at FDR 5% of which 11,335 (22.8%) are significant at FDR 1%. Files S5 and S6 in Ref. [47] contain expression and statistical values from this analysis. File S7 in Ref. [47] contains the annotation based on BlastX (see section above 2.1.3) for all transcripts (loci) where a significant differential expression result was found. A total of 4338 (4223 at FDR 1%) differentially expressed loci were successfully annotated after Blast2GO including InterProScan 5.0 [63] analysis. The most relevant result of the GO term enrichment analysis in relation to this study is an overrepresentation of the BP term “reproduction” (Figure 2b). These loci form the main analytical focus in this paper. To pursue this, we chose those functional annotated loci (a total of 309 of the 4338 in total that are differentially expressed) that code for proteins specifically related to fertilisation and sperm biology processes. From these, 61 loci corresponding to 50 different proteins are shortlisted based on the prediction that they have signal peptide or transmembrane domains by using SignalP 4.1 [64] and TMHMM 2.0 [65] servers, available in CGS Technical University of Denmark, respectively, and complemented with results from InterProScan 5.0 analysis described above (Table 2). These types of domains indicate that protein can be either secreted (e.g., present in the sperm acrosomal content) or located in the sperm plasma membrane respectively, hence with high potential to play a role in the sperm-egg recognition system or gamete fusion [16]. We thus wish to specifically focus on these as good candidates for more detailed consideration and perhaps future study. These candidate loci (Table 2) code for proteins that are mainly involved in different steps of spermatogenesis (Cdyl2, Ggnbp2, Nphp1, Rarb, Irs, Iap2, Tmbim6, eif4g2, CtsB, CtsL, CtsL2, Prdm9, Suv39h2), sperm motility (Dnal1, Ropn1, Ift172, Slc26, Slc6a5, Slc9c1), binding of sperm to the egg vitelline coat (Cct2, Cct3, Cct4, Cct5, Cct6a, Cct7, Cct8, Psma2, Ubc8, Pc1, Hya, Spag1, Thbs1, Zan, vitelline coat lysins M3 and M6), acrosome reaction and sperm capacitation (Cdc42, Spa17, CtsB). For each of the above candidate genes (loci), in some cases, isotigs within a locus varied in the nature and extent of differential expression between the two Mytilus species, see final two columns in Table 2. The expression differences could have resulted from simple allele differences between the mussels making up the pools, or more complex alternative splicing events producing different protein isoforms in the two species. It also might be the result of differential regulation of expression of the same protein isoform in the mature male gonad of the two different Mytilus species. It is important to note that allele differences can have two main different effects at the molecular phenotype level, either changing the mRNA/protein sequence or acting as expression modifiers. The latter effect can be associated with changes in non-coding usually cis-regulatory regions, though getting direct evidence for this is rather difficult [66].
Table 2:
Transcripts (loci) showing significant differences (FDR 1% at isotig level) in expression of mature male gonad tissue between Mytilus edulis (mussels from Swansea, E) and M. galloprovincialis (mussels from Vigo, G), with GO or protein name terms associated with the search term string “SPERM*” OR “FERT*” and a prediction that they have a signal peptide (SP) or a transmembrane (TM) domain in their sequences, this later information coming from SignalP 4.1, TMHMM 2.0 and InterProScan 5.0 analysis. Transcripts were functionally annotated using Blast2GO against UniProt-SwissProt database [all organisms], but protein names below are derived by checking against the nrNCBI[Mollusca] protein database. The numbers of significant isotigs from each locus (FDR 1%) with higher expression levels in M. edulis compared to M. galloprovincialis (E<G) and vice-versa (G>E) are also displayed.
| Transcript # | Gene name | Protein name (nrNCBI [Mollusca]) | Function | SP, TM | N. Isotigs E>G | N. Isotigs G>E |
|---|---|---|---|---|---|---|
| Locus_2854 | Iap2 | Apoptosis 2 inhibitor [C. gigas] | Spermatogenesis, acrosome reaction | TM | 1 | 2 |
| Locus_3972 | Tmbim6 | Bax inhibitor-1 protein [M. galloprovincialis] | Spermatogenesis, acrosome reaction | TM | 3 | 2 |
| Locus_9050 | Bre-4 | Beta-1,4-N-acetylgalactosaminyltransferase bre-4 [C. gigas] | Sperm-egg interaction | TM | 0 | 1 |
| Locus_1384 | CtsB | Cathepsin B [C. ariakensis] | Spermatogenesis, acrosome reaction | SP, TM | 2 | 2 |
| Locus_175 | CtsL | Cathepsin L [C. gigas] | Spermatogenesis, acrosome reaction | SP, TM | 3 | 2 |
| Locus_2547 | 0 | 2 | ||||
| Locus_587 | CtsL2 | Cathepsin L2 cysteine protease [P. fucata] | Spermatogenesis, acrosome reaction | TM | 1 | 1 |
| Locus_6135 | Cdc42 | Cell division cycle 42 [Mytilus sp. ZED-2008] | Sperm capacitation, acrosome reaction | TM | 1 | 0 |
| Locus_24960 | Cht3 | Chitinase-3 [H. cumingii] | Sperm-egg interaction | TM | 0 | 3 |
| Locus_6902 | Cdyl2 | Chromodomain Y-like protein 2 [C. gigas] | Spermatogenesis | TM | 0 | 1 |
| Locus_1290 | Cng | Cyclic nucleotide-gated channel rod photoreceptor sub. alpha [C. gigas] | Spermatogenesis | TM | 0 | 1 |
| Locus_1433 | Dnal1 | Dynein light chain 1, axonemal, partial [C. gigas] | Sperm motility | TM | 0 | 1 |
| Locus_2552 | Eif4g2 | Eukaryotic translation initiation factor 4 gamma 2 [C. gigas] | Spermatogenesis | SP, TM | 1 | 2 |
| Locus_5126 | Ggnbp2 | Gametogenetin-binding protein 2 [C. gigas] | Spermatogenesis | TM | 1 | 3 |
| Locus_134 | Hsp90 | Heat shock protein 90 [M. galloprovincialis] | Spermatogenesis | TM | 1 | 1 |
| Locus_22899 | Prdm9 | Histone-lysine N-methyltransferase PRDM9 [C. gigas] | Spermatogenesis | TM | 0 | 1 |
| Locus_18746 | Suv39h2 | Histone-lysine N-methyltransferase SUV39H2 [C. gigas] | Spermatogenesis | TM | 1 | 1 |
| Locus_6027 | Hya | Hyaluronidase [C. gigas] | Sperm-egg interaction | SP, TM | 1 | 0 |
| Locus_1259 | Irs | Insulin-related peptide receptor [P. fucata] | Spermatogenesis | SP, TM | 1 | 6 |
| Locus_12988 | 1 | 1 | ||||
| Locus_5663 | Ift172 | Intraflagellar transport protein 172 homolog, predicted [A.
californica] |
Sperm motility | TM | 2 | 2 |
| Locus_2244 | Imp2 | Mitochondrial inner membrane protease subunit 2 [C. gigas] | Spermatogenesis | TM | 1 | 2 |
| Locus_10336 | Nphp1 | Nephrocystin-1 [C. gigas] | Spermatogenesis | SP, TM | 0 | 2 |
| Locus_9945 | Pmca | Plasma membrane calcium ATPase [P. fucata] | Sperm motility | TM | 2 | 4 |
| Locus_1143 | Phb | Prohibitin [O. tankahkeei] | Spermatogenesis | TM | 1 | 1 |
| Locus_1157 | Phb2 | Prohibitin-2-like, predicted [A. californica] | Spermatogenesis | TM | 0 | 1 |
| Locus_19017 | Pc1 | Prohormone convertase 1 [H. diversicolor sup.] | Sperm-egg interaction, sperm capacitation, sperm motility | SP, TM | 0 | 2 |
| Locus_2686 | Psma2 | Proteasome subunit alpha type-2 [C. gigas] | Sperm capacitation, acrosome reaction | TM | 0 | 1 |
| Locus_29609 | Rarb | Retinoic acid receptor beta [C. gigas] | Spermatogenesis | SP, TM | 0 | 3 |
| Locus_29136 | Ropn1 | Ropporin-1-like protein [C. gigas] | Spermatogenesis, sperm motility | TM | 0 | 1 |
| Locus_815 | Sqstm1 | Sequestosome-1 [C. gigas] | Spermatogenesis | TM | 0 | 1 |
| Locus_9081 | Slc6a5 | Sodium- and chloride-dependent glycine transporter 2 [C. gigas] | Sperm motility | TM | 1 | 2 |
| Locus_3269 | Slc9c1 | Sodium/hydrogen exchanger 10 [C. gigas] | Spermatogenesis, sperm motility | TM | 2 | 2 |
| Locus_29004 | Spatc1 | Speriolin [C. gigas] | Spermatogenesis | TM | 1 | 5 |
| Locus_13213 | Spa17 | Sperm surface protein Sp17 [C. gigas] | Spermatogenesis, sperm-egg interaction, sperm capacitation, acrosome reaction | TM | 0 | 1 |
| Locus_12286 | Spag1 | Sperm-associated antigen 1 [C. gigas] | Sperm-egg interaction | TM | 1 | 1 |
| Locus_1176 | Srsf4 | Splicing factor, arginine/serine-rich 4 [C. gigas] | Spermatogenesis | SP, TM | 1 | 1 |
| Locus_10277 | 0 | 1 | ||||
| Locus_18976 | Samd7 | Sterile alpha motif domain-containing protein 7 [C. gigas] | Spermatogenesis | TM | 1 | 1 |
| Locus_1959 | Slc26 | Sulfate transporter-like, predicted [A. californica] | Sperm motility | TM | 2 | 2 |
| Locus_4801 | Cct2 | T-complex protein 1 (TCP-1) subunit beta [C. gigas] | Sperm-egg interaction | TM | 1 | 0 |
| Locus_586 | Cct4 | T-complex protein 1 (TCP-1) subunit delta [C. gigas] | Sperm-egg interaction | TM | 0 | 2 |
| Locus_1374 | Cct5 | T-complex protein 1 (TCP-1) subunit epsilon [C. gigas] | Sperm-egg interaction | - | 4 | 3 |
| Locus_24738 | Cct7 | T-complex protein 1 (TCP-1) subunit eta [C. gigas] | Sperm-egg interaction | TM | 0 | 1 |
| Locus_22131 | Cct3 | T-complex protein 1 (TCP-1) subunit gamma [C. gigas] | Sperm-egg interaction | TM | 0 | 2 |
| Locus_25048 | 2 | 2 | ||||
| Locus_36832 | 0 | 1 | ||||
| Locus_20775 | Cct8 | T-complex protein 1 (TCP-1) subunit theta [C. gigas] | Sperm-egg interaction | TM | 2 | 1 |
| Locus_188 | Cct6a | T-complex protein 1 (TCP-1) subunit zeta [C. gigas] | Sperm-egg interaction | - | 1 | 0 |
| Locus_8047 | Thbs1 | Thrombospondin-1 [C. gigas] | Sperm-egg interaction | SP, TM | 0 | 2 |
| Locus_29534 | 0 | 1 | ||||
| Locus_17402 | Ubc8 | Ubiquitin-conjugating enzyme E2–24 kDa [C. gigas] | Spermatogenesis | TM | 1 | 0 |
| Locus_39229 | M3 | vitelline coat lysin M3 [M. edulis] | Sperm-egg interaction | SP | 1 | 1 |
| Locus_25485 | 0 | 1 | ||||
| Locus_24 | M6 | vitelline coat lysin M6 [M. edulis] | Sperm-egg interaction | SP | 1 | 2 |
| Locus_30388 | 0 | 2 | ||||
| Locus_3846 | Zfr | Zinc finger RNA-binding protein [C. gigas] | Spermatogenesis | TM | 1 | 0 |
| Locus_1040 | Zan | Zonadhesin [C. gigas] | Sperm-egg interaction | TM | 1 | 0 |
| Locus_1240 | 1 | 2 | ||||
| Locus_1570 | 1 | 1 | ||||
| Locus_2570 | 0 | 1 |
3.2. Proteomic analysis of sperm cells from Mytilus edulis and M. galloprovincialis
3.2.1. Two-dimensional electrophoresis (2DE) and differential expression analyses
After applying the quality filter based on comparisons made for each 2DE gel against a pre-defined “gold standard 2D gel”, a tool implemented in SameSpots software, two out of ten 2DE gels of sperm samples analysed from the Swansea population (M. edulis) were removed from further analysis, while all 2DE gel samples from Vigo population (M. galloprovincialis) successfully passed this pre-defined filter (File S2). The analysis of the 2DE gel images produced a final dataset of 727 protein spots (File S3). Results from the reproducibility experiment, where two sperm samples one from each species were analysed twice, permitted the comparison of technical and biological variation. For each of the 727 spots the CV of spot volume was calculated over 10 biological replicates for M. galloprovincialis and over 8 biological replicates for M. edulis. The technical variation was measured for each species from the sample of two technical replicates for each species. The spot-specific CV values averaged over both spots and species are 41.2 ± 0.29 (SE) and 19.0 ± 0.34 for biological and technical variation respectively. Because of the small number of technical replicates, nonparametric tests were further used to gauge the significance of this difference. Thus of the 727 spots, 638 and 611 had higher CV for biological than technical replication in M. galloprovincialis and M. edulis respectively. χ2 tests against a 1:1 expectation were made where the null hypothesis is that higher CV is equally likely for biological and technical replicates. The expected frequencies in each category are thus 363.5:363.5. The χ2 value is highly significant in each species, even a ratio of 408:319 would be significant at p<0.001. Even if spot volume values are not independent for some pairs or groups of spots, this test is highly suggestive of significantly greater CV for biological than technical replicates. In a further test the Spearman correlation was computed over spots between technical replicates within each species. The values are 0.953 and 0.927 for M. galloprovincialis and M. edulis respectively. The corresponding correlation values between biological replicates vary between 0.767 and 0.895 for M. galloprovincialis and 0.780 and 0.896 for M. edulis. Both tests confirm that spot volumes are much more different between biological than technical replicates providing clear evidence of biological signal within each species.
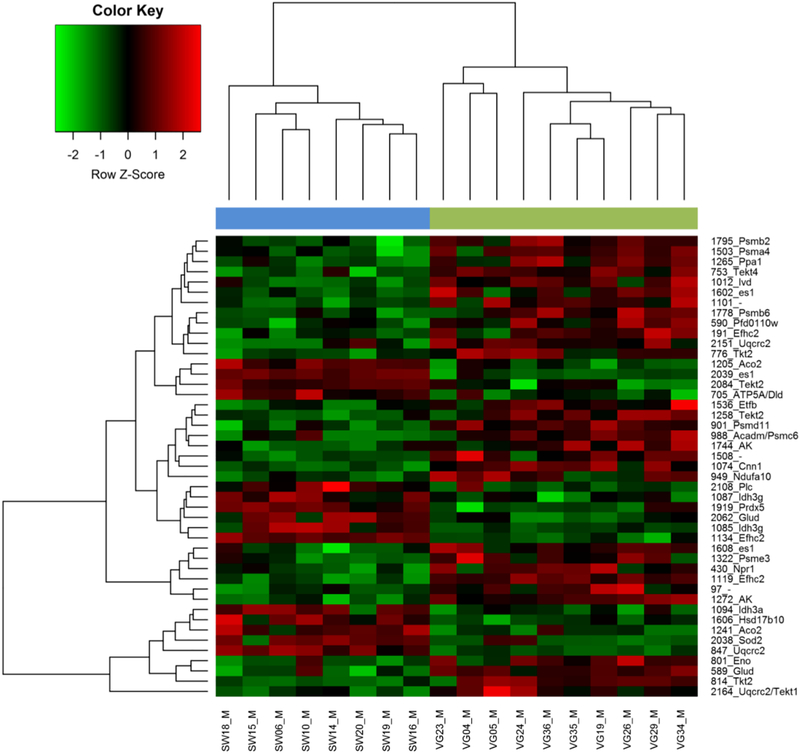
One-way ANOVA (“Species”; fixed factor) for each spot resulted in 17.6% of the protein spots showing significant differences (a priori p<0.05) in their expression levels between mussel populations from the two Mytilus species. After applying several correction methods to control for the type I error using a procedure we have advocated previously [61] (see File S3), most of these spots remained significant, especially when more powerful correction methods were used (e.g., 125 and 123 spots after applying the SGoF+ and SFisher correction respectively). Reassuringly, the q-values indicate a low expected false positive rate for the 128 significant spots (q=0.208), while fixing a q-value at 5% level provides 45 significant spots (Figure 3 and File S3). A heat map including the expression data for the 45 significant spots (q<0.05) shows samples for each population in one of two different clusters without any exceptional individuals (Figure 4). The same pattern is observed when the 128 a priori significant spots (p<0.05) are used (File S4). A Volcano plot (Figure 5) shows important size-effects in either Mytilus spp. directions. For example, there are significant differences (p<0.05) in expression associated with higher than 1.5 and 2.0 fold differences in 57 and 26 spots respectively comparing M. galloprovincialis with M. edulis, with higher expression in M. galloprovincialis, while 32 and 14 spots follow the same pattern but with opposite fold change direction with higher expression in M. edulis.
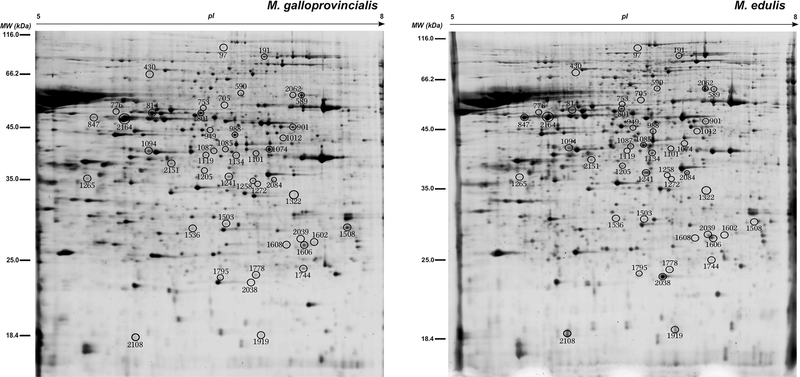
Figure 3:
2DE gels showing sperm proteome from a representative Mytilus galloprovincialis and M. edulis mussel respectively. 45 spots that showed significant differences between the two Mytilus populations and species (q≤0.05) and were identified (all except one) by MS (see Table 3) are numbered and encircled.
Figure 4:
Hierarchical clustering and heat map made using log normalised expression data for the 45 protein spots of sperm samples that showed significant differences in expression level (q≤0.05) between the two Mytilus species and populations (SW: Swansea, VG: Vigo) and were identified (all except one) by MS (see Figure 3). Each column and row contains information for an individual mussel and protein spot respectively. The numbers on the right are the protein spot numbers to each of which isattached an abbreviation that corresponds to gene name that code for the identified protein (see Table 3). Note that for two identified protein spots (1101 and 1508) there are no gene name abbreviations available. Cells are coloured according to z-scores, showing up-regulation (red) or down-regulation (green) of protein spot volumes in the individual mussels compared with average expression values calculated from all mussel samples.
3.2.2. Protein identification by mass spectrometry (MS)
From a total of 45 candidate protein spots (q<0.05; see Figure 3), all except one were successfully identified after the analysis of mass spectrometry data against different customised databases used in this study (Table 3 and File S5). Spots 1101 and 1508 were annotated against protein sequences generated from our RNA-seq dataset, though blast analysis of these RNA sequences against the NCBI protein database did not provide any significant match. It is important to note that in three analysed spots two different proteins were identified with very high confidence, PSMs and scores. These are spot 2164 (Uqcrc2 and Tekt1), spot 705 (Atp5a and Dld) and spot 988 (Acadm and Psmc6). An explanation for this result is that the “protein-pairs” identified for these spots present similar MW and pI, hence 2-DE analysis was not able to resolve them and they were sampled together when the spots were excised.
Table 3:
Identification by MS/MS of 44 out of 45 protein spots (see Fig. 3) from sperm that showed significant differences (q<0.05) between the two analysed species and populations of mussels (M. galloprovincialis from Vigo vs M. edulis from Swansea). Gene, the name of the gene (official gene symbol, retrieved from UniProt) that code for the protein sequence described in “Protein id” column. FC, fold change, defined as the unstandardized effect size with higher expression in either M. galloprovincialis (G) or M. edulis (E) mussel species. The databases from which an identification of the protein spots was obtained are given in the Database column: EST, expression sequence tags from Mytilus spp. available in Genbank, RNA, sequences obtained in the current study and, NCBI, protein sequences from Mollusca available in NCBI (see Materials and Methods).
| Spot | Gene | Protein id | FC | Database | Cellular location | Molecular Function |
|---|---|---|---|---|---|---|
| 1205 | Aco2 | Aconitate hydratase | 1.7 E | RNA, NCBI | Mitochondrion | Tricarboxylic acid cycle, sperm capacitation, motility |
| 1241 | 2.0 E | RNA, NCBI | ||||
| 1272 | Ak | Arginine kinase | 2.0 G | RNA, EST, NCBI | Cytoplasm | Phosphorylation, motility |
| 1744 | 2.1 G | RNA, EST, NCBI | ||||
| 705a | Atp5a | ATP synthase subunit alpha | 1.8 E | RNA, EST, NCBI | Mitochondrion | Respiratory electron transport chain, motility |
| 430 | Npr1 | Atrial natriuretic peptide receptor 1 | 1.7 G | RNA, NCBI | Membrane | Hormone binding, capacitation, chemotaxis |
| 1074 | Cnn1 | Calponin protein | 4.2 G | RNA, EST, NCBI | Cytoskeleton | Actin binding, motility |
| 2151 | Uqcrc2 | Cytochrome b-c1 complex subunit 2 | 1.9 G | RNA, EST | Mitochondrion | Respiratory electron transport chain, motility |
| 2164a | 1.6 G | RNA, EST | ||||
| 847 | 5.6 E | RNA, EST | ||||
| 705b | Dld | Dihydrolipoyl dehydrogenase | 1.8 E | RNA, EST, NCBI | Mitochondrion | Capacitation, acrosome reaction, motility |
| 1119 | Efhc2 | EF-hand domain-containing family member C2 | 4.7 G | RNA, EST, NCBI | Ubiquitous | Calcium binding, acrosome reaction, motility |
| 1134 | 2.4 E | RNA, EST, NCBI | ||||
| 191 | 2.3 G | RNA, EST, NCBI | ||||
| 1536 | Etfb | Electron transfer flavoprotein subunit beta | 1.4 G | RNA, EST, NCBI | Mitochondrion | Respiratory electron transport chain, motility |
| 801 | Eno | Enolase | 1.6 G | RNA, NCBI | Cytoplasm | Glycolysis, motility |
| 1608 | es1 | es1 protein | 2.1 G | RNA, EST | Mitochondrion | Unknown |
| 2039 | 3.5 E | RNA, EST, NCBI | ||||
| 1602 | 2.4 G | RNA, EST | ||||
| 589 | Glud | Glutamate dehydrogenase | 2.0 G | RNA, EST, NCBI | Mitochondrion | Oxidoreductase |
| 2062 | 2.5 E | RNA, EST, NCBI | ||||
| 1265 | Ppa1 | Inorganic pyrophosphatase | 2.6 G | RNA, EST, NCBI | Cytoplasm | Hydrolase, motility, sperm capacitation, acrosome reaction |
| 1094 | Idh3a | Isocitrate dehydrogenase [NAD] subunit alpha | 1.2 E | RNA, EST, NCBI | Mitochondrion | Tricarboxylic acid cycle, motility |
| 1085 | Idh3g | Isocitrate dehydrogenase [NAD] subunit gamma | 3.1 E | RNA, EST, NCBI | Mitochondrion | Tricarboxylic acid cycle, motility |
| 1087 | 1.5 E | RNA, EST | ||||
| 1012 | Ivd | Isovaleryl-CoA dehydrogenase | 2.6 G | RNA, EST, NCBI | Mitochondrion | Aminoacid degradation |
| 988a | Acadm | Medium-chain specific acyl-CoA dehydrogenase | 1.7 G | RNA, EST, NCBI | Mitochondrion | Beta-oxidation |
| 2038 | Sod2 | Mitochondrial manganese superoxide dismutase | 3.4 E | RNA, EST, NCBI | Mitochondrion | Antioxidant, oxidoreductase |
| 949 | Ndufa10 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 10 | 4.5 G | RNA, EST | Mitochondrion | Respiratory electron transport chain, motility |
| 2108 | Plc | Perlucin | 1.8 E | RNA | Extracellular region | Shell formation |
| 1919 | Prdx5 | Peroxiredoxin-5 | 2.2 E | EST, NCBI | Mitochondrion | Antioxidant, peroxidase, sperm-egg interaction |
| 1322 | Psme3 | Proteasome activator complex subunit 3 | 1.7 G | RNA, EST | Cytoplasm, nucleus | Proteolysis, sperm capacitation, acrosome reaction |
| 1503 | Psma4 | Proteasome subunit alpha type-4 | 1.9 G | RNA, EST, NCBI | Cytoplasm, nucleus | Proteolysis, sperm capacitation, acrosome reaction |
| 1795 | Psmb2 | Proteasome subunit beta type-2 | 2.0 G | RNA, EST, NCBI | Cytoplasm, nucleus | Proteolysis, sperm capacitation, acrosome reaction |
| 1778 | Psmb6 | Proteasome subunit beta type-6 | 1.6 G | EST, NCBI | Cytoplasm, nucleus | Proteolysis, sperm capacitation, acrosome reaction |
| 590 | Pfd0110w | Reticulocyte-binding protein PFD0110w isoform X3 | 1.7 G | RNA, EST | Membrane | Cell-cell adhesion |
| 2164b | Tekt1 | Tektin-1 | 1.6 G | EST, NCBI | Cytoskeleton | Motility |
| 1258 | Tekt2 | Tektin-2 | 1.7 G | RNA, EST, NCBI | Cytoskeleton | Motility |
| 2084 | 2.5 E | RNA, EST, NCBI | ||||
| 776 | 2.3 G | RNA, EST, NCBI | ||||
| 814 | 2.7 G | RNA, EST, NCBI | ||||
| 753 | Tekt4 | Tektin-4 | 1.9 G | RNA, EST, NCBI | Cytoskeleton | Motility |
| 1508 | -- | Uncharacterized protein LOC105318227 | 3.5 G | RNA, EST | -- | -- |
| 1101 | -- | Uncharacterized protein ZK1073.1 isoform X2 | 3.7 G | RNA, EST | -- | -- |
| 988b | Psmc6 | 26S protease regulatory subunit 10B | 1.7 G | EST, NCBI | Cytoplasm, nucleus | Proteolysis, sperm capacitation, acrosome reaction |
| 901 | Psmd11 | 26S proteasome non-ATPase regulatory subunit 11 | 1.9 G | RNA, EST, NCBI | Cytoplasm, nucleus | Proteolysis, sperm capacitation, acrosome reaction |
| 1606 | Hsd17b10 | 3-hydroxyacyl-CoA dehydrogenase type-2 | 1.5 E | RNA, EST, NCBI | Mitochondrion | Beta-oxidation, oxidoreductase |
| 97# | 1.7 G |
: due to technical problems this protein spot was not identified by MS. Note that three spots (705, 988 and 2164) were identified as two different proteins (a-b).
There are several spots showing differences in MW and pI (Figure 3) that were identified as the same protein (see Table 3). One possible explanation for this is that these originate by different post-transcriptional or post-translational modifications (PTMs). The correct interpretation of these candidate “multi-spot” proteins is important from a functional viewpoint to prevent misleading conclusions (see Box 2 in [38]). For example, in protein isoforms of Aco2 (spots 1205 and 1241) and Idh3g (spots 1085 and 1087) a concordant pattern of up-regulation in M. edulis was observed, whereas protein isoforms for Uqcrc2 (spots 847, 2164a and 2151), Efhc2 (spots 1119, 1134 and 191), es1 (spots 1608, 2039 and 1602), and Glud (spots 589 and 2062) showed a discordant pattern (see Table 3, Figures 3 and 4). Phosphorylation is one of the well-known PTMs that usually implies modification in the pI of phosphorylated protein but little MW change [67]. An advantage of using 2-DE for proteome separation compared to gel-free (shotgun) proteomic approaches is that it provides the possibility of assessing the effects of differential post-translational modifications and different isoform expression between samples [68–70]. The observation of spots resolved in close proximity in the 2-DE gel such as Idh3g (spots 1085 and 1087), Uqcrc (spots 847 and 2164), Glud (spots 589 and 2062), and Tekt2 (spots 814 and 776) is also compatible with differential phosphorylation events in the sperm of the two Mytilus spp., and could be verified by further phosphoproteomic analysis [71].
The list of protein identifications from excised spots contained many proteins potentially involved in sperm function. There are proteins involved in cell energy production, hence potentially affecting sperm motility, such as different members of the electron transport chain (ETC) protein complex (Nadufa10, Uqcrc2, Atp5a) or in close relation to ETC (Etfb), while Ppa1, Idh3g, Idh3a, Eno and Ak are other identified enzymes that also contribute to maintain the energetic cellular resources. An interesting observation is that about half of identified proteins are located in mitochondria (Table 3), so playing a role in cellular energy homeostasis either through ETC or different metabolic pathways. Proteins that contribute to flagellum structure could play a role in sperm motility, like Tekt1, Tekt2, Tekt4, and Cnn1. There are also proteins involved in sperm capacitation, for example Aco2, Dld, and Npr1. The identifications include also different catalytic and regulatory subunits of the proteasome (Psmb2, Psma4, Psmb6, Psmc6, Psmd11, and Psme3). There is a group of identified proteins with a less obvious sperm-specific function role (Acadm, Pfd0110w, Ivd, Efhc2, Glud, Hsd17b10, Prdx5, Sod2, Plc, and an es1 protein).
3.3. Proteomic and transcriptomic differential expression results: in good agreement?
Although gene expression studies based on transcriptomic analysis have relied on mRNA abundance as a good proxy for corresponding protein abundance, results from a number of studies have questioned the validity of this assumption [72]. Substantial posttranscriptional and posttranslational modifications are expected and this can also affect the correlation between protein and transcript levels for many but not all gene products [73]. In this study we have tested the general level of agreement in the direction of the differential expression between proteomics (identified protein spots in Table 3) and transcriptomics data (see Files S5-S6 in Ref. [47]). The data are summarised in File S6 where for both protein and mRNA-seq data E and G are used as abbreviations for M. edulis and M. galloprovincialis. Worksheet Table S6 of this file lists the protein spots which show differential expression between the two species, and for which of the two species the expression is higher. Then in addition for each spot the number of mRNA isotigs showing differential expression (E>G and G>E) are given in separate columns.
For those protein spots showing higher M. edulis protein expression the total number of isotigs over all spots with E>G and G>E are 14 and 26 respectively: with higher M. galloprovincialis expression the numbers are 8 and 52. A χ2 heterogeneity test reveals that the overall preponderance of isotigs with G>E is significant (pooled χ2 = 31.360 df=1 p=0.000) and that the ratios 14:26 and 8:52 are different (heterogeneity χ2= 4.507 df=1 p=0.034) (File S6, worksheet Test). Thus spots which show G>E have a tendency towards an excess of isotigs also showing G>E. The data in Table S6 has also been used to directly correlate the fold change values for the proteomics data and for the RNA-seq. The data and plot is given in File S6 worksheet 2Dplot. There is a positive correlation which though weak (Spearman’s Rho = 0.126, p=0.210) is nevertheless consistent with the above χ2 analysis in showing some general correspondence between the two types of data. Expectation of a positive correlation would depend on assumption of generalised up or down regulation for the protein in question. However in general there is not good correspondence between proteomics and transcriptomics data with cellular concentrations of proteins not correlating highly with the abundance of their RNAs [72–73]. This may be related to a number of factors including variation in protein turnover rate, variation in the extent and nature of posttrancriptional and posttranslational modification and measurement error.
Given that many isotigs in the overall dataset do not show differential expression, it is of interest to know whether a protein spot with E>G (or G>E) has at least one isotig with differential expression in the same direction. The number of spots showing such agreement can be contrasted with the number of spots for which all isotigs show differential expression but in the opposite direction to that shown by the protein spot. The numbers in these two categories are 28:4 over all spots (χ2 = 18.000 df=1 p<0.001, for test against 1:1 expectation, see File S6 worksheet Table S6 for further details) and 20:4 when counting for protein identities, that is spots for the same protein are counted once only (χ2= 10.667 df=1 p=0.001). These significant results provide additional evidence for concordance between the two types of expression data. In addition to spots with isotigs showing differential expression, 15 protein spots (32% of the total number of spots) do not have any isotigs showing differential expression (File S6 worksheet Table S6, total spots with “0” in column K). It is important to highlight that four of these protein spots were identified as different proteasome subunits with higher expression in M. galloprovincialis sperm (File S6 worksheet Table S6, column D).
For the two categories of proteins with expression E>G and G>E, the distribution of number of spots for different ontology terms was determined. This is carried out for two ontology classifications, Cellular Location and Molecular Function, which are derived from the classifications shown in Figure 2. The resulting distributions with further analysis are given in File S6 worksheet Test. The ontology terms having greatest frequency overall are Mitochondrion (43%) and Cytoplasm (20%) for Cellular Location, and Motility (29%), Capacitation (12%) and Acrosome reaction (12%) for Molecular Function. The results of χ2 contingency tests in which the ontology distributions are compared between E>G and G>E indicate a significant effect overall for both Cellular Location (p=0.002) and Molecular Function (p=0.027). Individual ontology terms which contribute most to the overall effect are Mitochondrion (p=0.000, higher number of spots for E>G), Cytoplasm (p=0.020, higher for G>E), Proteolysis (p=0.083, higher for G>E), and Tricarboxylic acid cycle (p=0.005, higher for E>G). So while there is a correspondence overall for Cellular Location between the highest frequency terms and those differing in frequency most markedly between species, this is not observed for Molecular Function.
3.4. Customised tissue and species-specific protein databases enhance protein identifications
While identifying peptides from MS data together with the corresponding proteins in model organisms is quite straightforward, the situation becomes more challenging when working with non-model organisms because the availability of genomic and protein sequences in the latter is scarce. However there are different alternatives to overcome this limitation (see [37, 45–46]). For example, the generation of customised protein databases obtained from tissue and species-specific transcriptome datasets (RNA-seq) or from expression sequence tags (ESTs) deposited and available through NCBI. Also de novo interpretation of MS/MS spectra can provide complementary results when combined with the use of customised protein databases, specifically in providing information about unknown mutations and PTMs, this latter being also valid for model organisms.
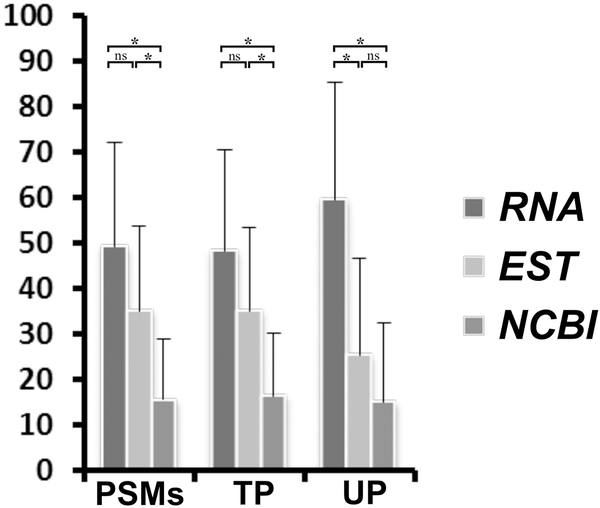
In order to assess whether the use of customised protein sequence databases has improved the quality and quantity of protein identifications in the current study on two Mytilus spp., we compared the number of peptide spectrum matches (PSMs), total (TP) and unique peptides (UP) obtained in the identification of 44 protein spots from sperm samples (see section 3.2.2) using 3 different customised databases (see section 2.2.4). Graph displayed in Figure 6, made from data available in File S5, shows that using a protein database made from our consensus tissue and species-specific transcriptome data provide on average across 44 spots better results in terms of a significantly higher number of PSMs (Kruskal-Wallis test; H=25.27, df=2, p<0.0001), TP (H=24.29, df=2, p<0.0001) and UP (H=34.48, df=2, p<0.0001) when compared with the other two protein databases. When these results are inspected in a pair-wise comparison basis, after applying Dunn post-hoc test for multiple comparisons, it is worth noting that the customised Mytilus-ESTs-based protein database also presented good results for PSMs and TP, but with a significantly lower number of UP, when compared with the RNAseq-based protein database (see Figure 6). It is also clear that the results of these two customised Mytilus specific protein databases are significantly better than those obtained after using a NCBI[Mollusca]-based protein database, except the pairwise comparison between Mytilus-ESTs-based and NCBI[Mollusca]-based protein databases for UP (see Figure 6). The lower number for UP can be explained by high redundancy found in EST databases. The confirmation that EST sequences from Mytilus spp. are generally shorter than protein sequences derived from our RNA-seq project can be easily reached from inspection of matched protein sequences from each database used in the protein spot identifications (see File S5). File S5 also provides useful information about potential PTMs and mutations, ascertained with the PEAKS program through de novo interpretation of MS/MS spectra, present in the sequences of proteins to which the different spots were identified.
Figure 6:
Comparative results of protein spot identifications by MS using different customised protein databases (see Materials and Methods). Bars represent the total number of peptide spectrum matches (PSMs), total peptides (TP) and unique peptides (UP), expressed as percentage, obtained against each of the three protein databases made from: 1) RNA-seq data from the current study (RNA), 2) EST sequences available in NCBI from Mytilus[organism] (EST), and 3) protein sequences available in NCBI for Mollusca[organism] (NCBI). *: p<0.001, ns: not significant, for Kruskal-Wallis and post-hoc pairwise tests (after Dunn correction to account for multiple comparisons) between the different protein databases either for the total number of PSMs, TP or UP.
4. Discussion
4.1. Transcriptomic differences in mature male gonad between two Mytilus spp. shed light on proteins with potential involvement in reproductive isolation
Results from transcriptomic experiments using next-generation sequencing technology (RNA-seq) with a focus on different biological questions have been reported for M. edulis (e.g. [74], in a study of gene regulation during early development) and M. galloprovincialis (e.g. [75], to compare transcript expression profiles in four different tissues). However there has not been any attempt to deep sequence the mature male gonad transcriptome and compare transcriptomic data in these two Mytilus species. The current RNA-seq analysis provides evidence of high variation in the mature male gonad transcriptome, with 22.8% of analysed loci differing (at FDR 1%) between M. galloprovincialis and M. edulis samples. In a high number of instances the differential expression was detected at isotig level within each consensus transcript (locus), with contrasting results among different isotigs within loci, both in terms of effect-size and direction of the expression level between the two Mytilus spp. (see Table 2). The RNA transcripts showing different expression in Table 2 are both derived from sperm and have sperm associated GO terms with their protein names. We would thus expect many of these transcripts to be expressed as proteins for specific functioning in this tissue. However in general it cannot be assumed that all isotigs showing differential expression are translated into proteins [76], and it may be that a single transcript is dominant in terms of protein expression [77]. The statistical correspondence in the direction of expression between species for isotigs and protein spots (χ2 heterogeneity test in File S6, Table S6) give further evidence that some of the isotigs are translated into protein even if it is not possible to pinpoint exactly which isotigs are translated and which are not.
Samples from both species shared a common laboratory environment for at least two months. This design often referred to as a common garden experiment (e.g. [78]), aims to demonstrate that observed phenotypic differences are mainly attributable to speciesspecific (genetic) rather than sampling-site environmental differences, and is becoming important for studying adaption in genomic studies [79]). Although acclimation to the same laboratory conditions should help to minimise the effects of local environmental differences between the original sampling sites, some of these environmental effects may be retained permanently even after acclimation for several weeks [80]. When the aim is to compare allopatric population of different species, genetic and local environmental differences may always be confounded, but the long period of acclimation used in the current study (at least 2 months) should have maximised genetically based, as compared with environmentally based, transcriptome differences between the species. Variation between gonadal development stages in transcript abundance have been reported in M. galloprovincialis [81]. However in the present study mussels at the same stage of development, according to histological tests, were used in the two species.
From the list of genes which show significant expression differences between M. edulis and M. galloprovincialis at the mRNA level, there are several that produce proteins with functional roles in sperm biology and fertilization (Table 2). Most of these proteins are thus good candidates for evolutionary study due to their potential role in reproductive isolation mechanisms and ultimately in the formation of new species, and are discussed below.
4.1.1. T-complex protein 1 (TCP-1) and ubiquitin-proteasome system (UPS) might be involved in intraspecific gamete preference and reproductive isolation in Mytilus spp.
One of the most important results is the concerted differential expression between the two Mytilus spp. for seven out of eight subunits of the T-complex protein 1 (TCP-1). A chaperonin-containing T-complex protein 1 was found in the periacrosomal region of human and mouse sperm heads with an involvement in mediating sperm-ZP interaction [82–83]. Evidence was found to support the view that TCP-1 and the ubiquitinproteasome system (UPS) might by concerted action be involved in gamete interaction [82–83]. Hence TCP-1 and UPS are good targets for further investigation in relation to involvement in prezygotic reproductive mechanisms that could be operating between Mytilus spp. It is possible that differences in the expression level or in the sequence of TCP-1 and UPS related proteins can lead to a preference for intraspecific rather than interspecific fertilisations in Mytilus spp. UPS is involved in the process where protein substrates are labelled with different ubiquitins to be later recognised by the 26S proteasome complex machinery for protein substrate degradation playing important roles during sperm capacitation, the acrosome reaction and sperm-egg interactions (reviewed in [84]). Two candidate differentially expressed transcripts found in our study (Table 2) relate to the ubiquitin-proteasome system (UPS). These are the ubiquitinconjugating enzyme (UBC) E2–24 kDa (Ubc8) and the proteasome subunit alpha type-2 (Psma2). Testis-specific isoforms of the first protein were found in the ascidian Ciona intestinalis and rat spermatozoa and a mutant mouse for this enzyme showed alterations in sperm as well as a reduced sperm number and motility [84]. Inactivation of an ubiquitin-conjugating enzyme in Drosophila causes male infertility due to abnormal levels of spermatogenesis [85]. It was demonstrated in ascidians, sea urchins and mammals that ubiquitin-conjugating enzymes regulate the penetration of spermatozoa into the vitelline coat (VC) of the egg and degrade the ubiquitinated sperm receptors on the VC (zona pellucida-ZP, in mammals) of eggs during fertilisation, contributing to the avoidance of polyspermy, with some roles also during sperm capacitation and regulation of acrosomal exocytosis (reviewed in [84, 86]. In relation to the second protein (Psma2), sperm proteasomes are released extracellularly as part of the acrosomal content during fertilisation. Together with an intracellular UPS inside the fertilised egg, it seems that animal fertilisation is also dependant of an extracellular UPS driven by the acrosomal exocytosis of different enzymes/proteins, and this mechanism seems to be quite evolutionarily conserved in the animal kingdom with small differences in ascidians compared with sea urchins and mammals. Its functional importance in fertilisation has been empirically confirmed, suggesting that UPS proteins are a good target for controlling fertilisation, and hence reproduction, in different organisms [84]. Proteasome subunit alpha was also identified among those proteins with higher expression in Mytilus edulis sperm [20].
4.1.2. Other candidate sperm-specific gene products linked to acrosome reaction, sperm-egg interaction and rapid evolution
The presence of a beta-n-acetylhexosaminidase (Bre-4) among the candidate proteins is interesting because glycosidic enzymes were observed in the sperm acrosome content and found to be necessary for penetration of the ZP during fertilisation in some mammals, as well as acting as important sperm receptors for the extracellular matrix of the oocyte in ascidians [87–88]. The sperm surface protein SP17 (Spa17) is of interest because it might be involved in spermatogenesis, sperm capacitation, the acrosomal reaction and sperm-egg interactions during fertilisation [89]. Evidence of high Spa17 protein expression was obtained in Mytilus edulis sperm [20], and in the current study one isoform shows differential expression. Sperm proteins with testis-specific expression have been found to evolve more rapidly on average than proteins expressed in testis alone and in non-reproductive tissues. This is probably due to functional constraints associated with housekeeping tasks of this latter-type of protein (see [90]). The relative contribution of neutral and naturally selected genetic variation has been a long debated and investigated issue during the last 50 years in evolutionary biology [91]. In this context, SP17 was found to evolve rapidly by positive selection in several mammalian species [92]. Similarly zonadhesin protein (Zan) was found to evolve rapidly in primate species [93]. It is a large sperm-specific protein localised in the sperm head within the acrosomal matrix with multiple domains involved in the speciesspecific recognition of ZP in eggs during fertilisation in mammals (reviewed in [94]). The acrosome content is quite variable between mammals and marine invertebrates. In sea urchins and abalones, bindin and lysin sperm acrosomal proteins are rapidly evolving species-specific proteins that recognise the vitelline coat of the egg (corresponding to ZP in mammals) during fertilisation, while evolution of zonadhesin is also driven by positive selection and involved in the same function in mammals, despite these three proteins being evolutionarily unrelated (reviewed in [2, 94]). The protein structure of zonadhesin is quite conserved despite high aminoacid divergence across different species. A precursor form of zonadhesin protein is produced during spermatogenesis and quickly processed to produce 3 polypeptides of 300, 105 and 45 kDa respectively in pig spermatozoa [94]. We provide evidence of four different Zan loci and a total of seven isotigs with differential expression between the Mytilus spp., so making this gene a target of interest in further studies of reproductive isolation in Mytilus species. Evidence has been actually reported for positive selection acting on the M7 lysin gene in some sympatric and allopatric Mytilus populations [25–27, but see 28] and the M3 lysin gene [95]. M3 and M7, together with the less studied M6 lysin, are non-orthologous highly abundant acrosomal proteins responsible for dissolving the egg vitelline envelope during fertilisation [96], so are thought to play an important role in the gamete recognition process. Interestingly in our study we found evidence of differential expression for a total of eight different isotigs of M3 and M6 lysins, but no differential expression of M7 lysin.
4.1.3. Prdm9 and Suv39h2 gene products are promising targets to study postzygotic reproductive isolation mechanisms and sex differences in Mytilus spp.
Finally two other candidate gene products displayed in Table 2, Prdm9 and Suv39h2, can be highlighted. When two populations that have evolved allopatrically come into secondary contact, gamete compatibility may still occur and hybrid individuals produced as observed for Mytilus spp. However hybrids can be sterile or have reduced fitness due to epistatic interactions of alleles from the two diverged genomes. This phenomenon known as Dobzhansky-Muller incompatibility (DMI) can lead to the formation of new species. Only a very few genes responsible for such low hybrid fitness have been discovered so far (see [97]). Prdm9, which shows differential expression for one isotig, is also known as Meisetz, is a histone H3 methyltransferase, and is expressed in mouse testis and ovaries [98]. This gene activates other essential genes for meiosis by means of specific-histone methylation. Sterile hybrid male mice had small testes, spermatogenic arrest and lacked sperm, the same phenotype as observed in null-Prdm9 mutant mice [98]. The cause of sterility seems to be DMI generated by epistatic interaction between Prdm9 and other genes located on chromosome × (see [97]). In view of the discoveries in the mouse, we suggest that Prdm9 deserves further attention in evolutionary studies on Mytilus spp where reproductive isolation is incomplete. On the other hand, Suv39h2, differentially expressed here in two isotigs, is another histone H3 methyltransferase, and was found to be specifically expressed in mouse adult testes but not ovaries [99] and specifically accumulates with chromatin of the sex chromosomes silencing their expression during early meiosis. Possibly this protein could be useful for the development of a sex specific marker in Mytilus. This is currently lacking in Mytilus spp for which there is currently no evidence of sex chromosome dimorphism. For example, Suv39h2 as a target protein in immunofluorescence analysis for detecting differences between males and females.
4.2. Sperm proteome differences between Mytilus edulis and M. galloprovincialis
In line with the RNA-seq results, proteomic analysis on sperm samples from individuals from M. edulis and M. galloprovincialis provide evidence of high proteome differences between the species, occurring in 17.6% of protein spots analysed (q=0.208). All mussels were kept under common laboratory conditions for at least 2 months and thus had a long period to acclimate prior to the collection of sperm for proteomics analysis. Following the reasoning given above in the discussion of the transcriptome results, proteome differences between the species can therefore be attributed entirely or in large part to genetic differences between the species. A reassuring result is that from a similar proteomic experiment on sperm samples of individual mussels from a hybrid population at Croyde (UK) with sympatric M. edulis and M. galloprovincialis, species-specific proteomic patterns were also observed [100], strengthening the evidence that speciesspecific proteomic differences between mussels raised under similar conditions are genetically based. Although differential expression may be associated with the processes of protein synthesis, post-translational modification, and protein degradation, all may result in variation in protein abundance and have functional implications [101]. From the list of 44 protein spots (q=0.05) with differential expression and identified by MS, there are a number of proteins with key functional roles in sperm biology and fertilization (Table 3) that make them good targets (hereafter candidates) for potential involvement in reproductive isolation mechanisms. A feature of the results shown in Table 3 is that different spots for the same protein may differ in the species in which they show higher expression. Some proteins given in Table 3 which are of particular interest are highlighted and discussed below.
4.2.1. Mitochondrial proteins linked to energy production and antioxidant enzymes are up-regulated in M. edulis
Alterations in ETC-related proteins, and hence in cellular energetic production, have been linked to lack of sperm motility and, hence fertility, in some mammals [102–103], so any observed differences between the two Mytilus species could be the result of their following different adaptive strategies relating to sperm motility. From the list of identified proteins showing differential expression (Table 3), NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 10 (Ndufa10), Cytochrome b-c1 complex subunit 2 (Uqcrc2), and ATP synthase subunit alpha (Atp5a) are nuclear encoded and from the different complexes of the respiratory electron transport chain (ETC) in mitochondria. Remarkably, the list of protein identifications (Table 3) reveals that nearly half of the identified proteins develop their functions and are located in mitochondria. A similar result was observed for highly expressed proteins in the sperm of Mytilus edulis [20]. Proteins from these ETC-associated complexes might be implicated in postzygotic isolating mechanisms due to coevolution of nuclear and mitochondrial genomes to ensure appropriate functional interactions between the nuclear and mitochondrial coded protein subunits of these complexes [104–105]. Marine mussels of Mytilus spp. as well as other bivalves present an unusual mtDNA inheritance mechanism (termed doublyuniparental inheritance, DUI) in which distinct mtDNA genomes are passed through the male and female lines of descent and which is coupled to sex determination in these species [106–109] with opportunity for selection to act directly on mtDNA coded sperm proteins. Negative epistatic interactions between nuclear and mitochondrial genomes in hybrids could contribute to the maintenance of species integrity, consistent with observations of DUI disruption in crosses between these two Mytilus species [110].
Other identified differentially expressed mitochondrial proteins relate to energy metabolism. These include isocitrate dehydrogenase (Idh3a and Idh3g), aconitate hydratase (Aco2) and dihydrolipoyl dehydrogenase (Dld). Idh3 was identified as having the highest expression levels in a previous proteomics study of Mytilus edulis sperm [20]. Low expression levels of Aco2 were reported in human sperm with reduced motility [111], and higher levels during mice sperm capacitation [112]. Deficiency of Dld mature protein was associated with low sperm motility in humans [113], while enzymes of this complex were also related to sperm capacitation and the acrosome reaction in the hamster and humans [114–115]. The higher expression of such proteins in might result in higher ATP production and a fitness advantage under certain ecological and environmental conditions (see section 4.3). However production of ATP through oxidative phosphorylation (OXPHOS) may produce high reactive oxygen species (ROS) in sperm leading to mitochondrial mutations [116] and evolution of a trade-off between higher OXPHOS and higher activity of antioxidant enzymes to neutralise high ROS production. Related to this is the observation in the present study that the differentially expressed antioxidant enzymes peroxiredoxin-5 (PRDX5) and manganese superoxide dismutase (SOD2) were associated with abnormal sperm and infertility in several mammals [117–118]. SOD activity may have detrimental effects on human sperm motility [119], and PRDX5 might play a role in sperm-egg interaction through the induction of signalling events by means of redox reactions after ZP binding [120].
4.2.2. Up-regulation of rapid energy supply and alternative production pathways in M. galloprovincialis
It is of interest that different species, for different cellular types, could have evolved different strategies and molecular pathways for energy production [121] driven by different ecological or environmental pressures. For example, glutamate dehydrogenase (Glud) converts glutamate to α-ketoglutarate potentially enhancing the activity of the TCA cycle in which α-ketoglutarate is an intermediate. Two spots closely located in the 2-DE map, were identified as Glud. These could be isoforms resulting from different posttranscriptional and posttranslational modifications (e.g. phosphorylation) implying functional changes [122] in sperm of both Mytilus spp.
ATP production through the glycolytic pathway in the sperm is compartmentalised in the principal piece of the flagellum, and this ATP source may be important in the sperm motility process known as hyperactivation [123]. The glycolytic enzyme enolase (Eno) was also differentially expressed. Disruption of expression of this enzyme sperm causes sperm structural defects and male infertility in the mouse [124]. In general glycolytic ATP is produced faster but less efficiently than ATP from aerobic pathways. Thus a trade-off between speed and amount of ATP production in sperm cells might also be of functional significance in sperm.
Phosphagen kinases are involved in intracellular energy transport and temporal buffering of ATP levels, specifically in flagellated cells, and hence probably play a role in sustained sperm motility [125]. The enzyme also influences sperm tail length and flagellar bending [126–127] and sperm-specific isoforms have been reported in various invertebrates [20, 125]. One of these enzymes, arginine kinase (Ak) was differentially expressed here in two spots. Phosphagen molecules also regulate intracellular inorganic phosphate levels [128] and play an important role in sperm motility, capacitation, the acrosome reaction and sperm-egg fusion [129]. Inorganic pyrophosphate (PPi) is degraded by pyrophosphatase 1 (Ppa1) for which one differentially expressed spot was identified. PPi enhances sperm proteasome activity, of key importance for the spermegg interaction during fertilization [129]. Interestingly several differentially expressed spots related to the proteasome complex have been identified in the present study (see section 4.2.3).
4.2.3. Up-regulation of sperm proteasome activity in M. galloprovincialis: contrasting transcriptomic and proteomic results
Six protein spots were identified as different structural (alpha), catalytic (beta) and regulatory subunits of the proteasome complex (Psma4, Psmb2, Psmb6, Psme3, Psmc6 and Psmd11). The important role of the ubiquitin-proteasome system (UPS) during fertilization, including sperm capacitation, acrosome reaction and sperm-ZP binding, has been considered in section 4.1. It is notable that all these had higher expression in M. galloprovincialis (Table 3 and File S6) suggesting that this species could have evolved specific regulatory mechanisms that increase the abundance of these proteins in sperm cells. Interestingly several proteasome subunit alpha components were also identified in M. edulis eggs and linked to the molecular mechanism underlying doublyuniparental inheritance (DUI, see section 4.2.1) of mtDNA in Mytilus spp. [59, 130]. Sperm mitochondria are labelled through ubiquitination during spermatogenesis [131] and thus marked for elimination by the proteasome complex in the fertilised oocyte. Three of the differentially expressed transcripts (Table 2) are two prohibitins (Phb and Phb2) and sequestosome-1 (Sqstm1). Prohibitins play a role in mtDNA inheritance [132], and are targets for ubiquitination in sperm mitochondria [133] while Sqstm1 has been linked to sperm mitophagy in mammals [134]. Thus there may be a link between the observed species-specific expression differences of these proteins in this study and disruption in DUI reported in inter-specific crosses [110], and of relevance to Dobzhansky-Muller incompatibilities (DMI) in hybrids between these species.
4.2.4. Higher expression of tektins suggests high motility sperm in M. galloprovincialis
Another interesting functional group of proteins showing differential expression are tektins. Six spots were identified as three different tektin proteins (Tekt1, Tekt2 and Tekt4) (Table 3). Of these, five had higher expression in M. galloprovincialis. Tektins are cytoskeletal proteins of the sperm flagellum and involved in sperm motility and flagellar bending. Differences in expression between normal and low motility sperm in humans were reported for Tekt1 and Tekt2, and Tekt4 was found to be essential for proper coordinated beating of the flagellum and for fertility [135–139]. Tektin expression occurs in the sperm acrosomal region perhaps indicating some specific role during fertilisation (see [135]) and has been implicated in flagellar bending and motility patterns [135, 140].
4.2.5. Other identified proteins with sperm-specific functional links
Three different spots with differential expression were identified as the protein EF-hand domain-containing family member C2-like (Efhc2). Sperm proteins with EF-hand domains play a key role in activation of the oocyte during fertilisation in mammals [141], and can also be involved in the acrosome reaction in invertebrates [9] and motility regulation of sperm [142–143]. Three protein spots identified as ES1 also showed differential expression. There is little functional information on this protein though it has been related to differential sperm motility in humans [111]. Other proteins showing differential expression are 3-hydroxyacyl-CoA dehydrogenase type-2 (Hsd17b10), potentially involved in the regulation of steroid hormones in reproduction and reported in several molluscs [144], and atrial natriuretic peptide receptor (Npr1) which acts on capacitation, chemotaxis and chemokinesis [145–146] and thus might potentially play a role in species-specific sperm-egg recognition in Mytilus spp. driven by chemotaxis signals released from eggs.
4.3. Rapid evolution and sperm function trade-offs may explain species-specific proteome differences
4.3.1. Selective pressures and adaptation in sperm
In external fertilisers such as mussels, sperm are expected to be under a variety of selective pressures relating to the different biological strategies for fertilisation and the ecological and environmental challenges they experience. Mussel settlements are patchy along rocky shores, and population density may vary considerably on a geographic or seasonal basis. Even though there may be synchronous spawning of eggs and sperm, the impact of varying gamete density and the role of sperm limitation is unclear [147–148]. If sperm density is too low then the probability of successful fertilisation may be low: on the other hand if sperm density is too high polyspermy may occur also resulting in incomplete fertilisation [149]. With sexual conflict, competition between sperm to achieve successful fertilisation may be accompanied by selection for eggs that block fertilization to prevent polyspermy. This can lead to rapid co-evolution of proteins in eggs and sperm in the context of sexual conflict. The rapid evolution of sperm proteins has been observed in many animal groups from mammals to different marine invertebrates such as sea urchins, abalones, turban snails, oysters, sea stars and mussels [9, 150–151]. In a comparison of sperm proteins between M. galloprovincialis and M. edulis the highest non-synonymous to synonymous substitutions rates were observed for proteins involved in fertilisation [21]. Sperm limitation should exert strong selection for adaptations increasing the chance of successful fertilisation in marine organisms with external fertilisation [152–153]. These include spawning synchrony, high levels of sperm production, chemotaxis over short distances, and sperm longevity. There is evidence that sperm energetics, for example higher ATP production may enhance sperm performance through an increase in swimming speed [154] and increase the chance of fertilization. But given finite energy resources to allocate to sperm properties and function, trade-offs between sperm traits are expected. For example trade-offs between sperm velocity and longevity occur both within and between species [154–155]. However there are numerous complicating factors such as the ability of sperm to maintain flagellar beats with low ATP and high inorganic phosphate levels, or the use of alternative pathways for energy production [121, 156] despite oxidative phosphorylation and glycolysis in the sperm midpiece being the major source of ATP production [123].
4.3.2. Sperm proteins upregulated in M. edulis and M. galloprovincialis
In the present study many proteins connected with sperm function which are upregulated in M. edulis or M. galloprovincialis (Table 3) have been identified and their properties discussed above (see section 4.2). As contrasting scenarios, selective pressures in the native environments of the two species could be somewhat similar or quite different. In the former scenario suppose that selection favoured upregulation of proteins improving motility to enhance fertilisation success. This could be achieved by upregulating different genes of the same protein in the two species. For example proteins from different Tektin-2 spots are upregulated in M. edulis and M. galloprovincialis (Table 3). This differential effect could be achieved by selection or drift increasing the frequency of different locus specific expression modifiers in the two species. Alternatively different proteins potentially affecting motility could be differentially upregulated in the two species. For example, isocitrate dehydrogenase is upregulated in M. edulis and arginine kinase is upregulated in M. galloprovincialis (Table 3). In the latter scenario where selection pressures differ between species, proteins for quite different traits may obviously be upregulated in the two species.
A summary of the proteins of Table 3 matched with sperm functional traits is given in File S7. Column I marks the particular 4.2 sub-sections in which proteins were flagged as having predominantly higher expression in M. edulis (4.2.1) or M. galloprovincialis (4.2.2, 4.2.3, 4.2.4). Column G assigns functional trait terms to the proteins and the count and % frequency distributions for these terms are given in Figure 7. These distributions give at least an approximate guide to which sperm traits are upregulated in the two species. In both species proteins relating to motility are important in this regard. After this, proteins relating to ATP reserves and perhaps ROS production are important in M. edulis whereas proteins relating to the acrosome reaction, capacitation, and spermegg interaction might be highlighted in M. galloprovincialis. On this basis it is possible to hypothesise that motility is important in both species but particularly M. edulis, whereas in M. galloprovincialis proteins relating to sperm maturation and the fertilization process should be highlighted.
Figure 7:
Summary of counts and percentages of sperm trait and functional terms for proteins having higher expression in M. edulis and M. galloprovincialis. The data is derived from Table 3 and from File S7, worksheet Table S7 where it is further elaborated (see captions of Tables S6-S7). Columns 2–5 give the counts and % values of sperm trait terms assigned to proteins having higher expression in M. edulis and M. galloprovincialis. Red and green fill indicate higher and lower % values in each row. Columns 6 and 7 indicate the number of occurrences of terms according to a tentative hypothesis on perceived benefit of higher expression to the species at the head of the columns (in red font) or perceived disadvantage (green font). Black font indicates that a conclusion in relation to benefit or disadvantage could not easily be made.
The potential biological consequences of these sperm traits are elaborated in File S7 in column H. A notable feature is that upregulation of many proteins in Table 3 can be hypothesised to result in a functional advantage for sperm. In this circumstance red font is used in columns G and H. For example in M. edulis, aconitate hydratase has higher expression than in M. galloprovincialis and this higher expression could be interpreted as a functional benefit in terms of faster swimming speed or endurance as well as improved maturation of sperm. By contrast the higher expression of es1 protein in M. edulis affecting the sperm trait motility might be hypothesised to reduce motility, a functional disadvantage, on the basis that lower motility was observed in human sperm with higher levels of this protein. This is represented by green text font in File S7 columns G and H. Where it is more difficult to arrive at a functional benefit or disadvantage, black font is used. The counts of the number of spots in which the sperm trait terms can be flagged with red, green or black font are also given in the final two columns of Figure 7. There is a clear preponderance of protein spots in which higher expression can be hypothesised to be a functional benefit in terms of sperm performance in the species in which this higher expression occurs, the functional benefits being largely in sperm motility and related traits and the fertilization process.
In both species, the higher expression of proteins associated with various aspects of sperm function are consistent with positive natural selection towards improved function and fitness of sperm. Closely related hybridising species such as M. edulis and M. galloprovincialis might be expected to show few or many differences in expression as a result of selection pressures arising from ecological forces. The wide range of differentially expressed proteins observed in the current study is consistent with evidence from the mouse where a diverse set of 81 different protein genes, including 23 sperm membrane proteins all gave evidence of positive selection [157], and where proteins involved in sperm-egg interactions in particular show accelerated evolution [151]. Such a large number of genes involved in sperm function could underline that there may be a high selection intensity acting on sperm. This may also provide multiple opportunities for disrupting sperm function. For example it has been reported that in sea urchins as few as 10 amino acid changes in the protein bindin are needed for complete gamete incompatibility [158], so limited changes occurring at different loci might have similar effects.
4.3.3. Differential expression: implications for hybridization of M. edulis and M. galloprovincialis
The observation of protein expression differences for many different genes connected with sperm function has implications for models of hybridization and introgression between the species. An earlier proteomic study of a hybrid zone between M. edulis and M. galloprovincialis using somatic tissue found evidence of high gene expression variation amongst hybrids consistent with segregation at expression modifier loci as introgression proceeds [42]. Such segregation of modifiers at many sperm function related genes differing in protein expression between the species could result lowered expression or general disruption of expression of these genes depending on dominance relationships at and epistatic interaction between the modifier loci. This could contribute to lowered fertility of hybrids or lowered fitness of larvae as has been observed experimentally between different Mytilus spp. [33, 35]. It might also contribute to the observed disruption of doubly uniparental inheritance (DUI) in crosses between these two species [159] or other pair of Mytilus spp. [160–162].
4.3.4. Possible influences of environmental variation on sperm function
M. edulis evolved in the North Atlantic whereas M. galloprovincialis evolved in the Mediterranean [101, 162–163]. The most prominent environmental factors that might have exerted selective influences in the past are first temperature and then salinity which are both higher in the Mediterranean. These environmental differences persist in the contrast between Vigo and Swansea today, with seawater temperature about 4°C higher at Vigo during the spawning season. There is evidence that changes in seawater temperature may affect sperm function. Thus in M. galloprovincialis higher temperature is associated with lower fertilization rates on average [164] and sperm motility and linearity of swimming patterns are affected by temperature and its interaction with pH [165]. This may have fitness consequences as swimming speed has also been associated with higher fertilisation rates [166]. In some circumstances, for example when chemoattractants are not present, non-linear swimming patterns may be advantageous to maximise the chance of fertilisation [147, 167–168]. Other environmental factors may be important for successful fertilisation for example viscosity which is a function of temperature and salinity [169]. Factors such as seawater specific gravity and turbulence may also be important in determining the chance of successful fertilisation [170–171].
4.3.5. Selective pressures and interpretation of present results
The historical and current environmental factors affecting M. edulis and M. galloprovincialis could have generated different selective forces to cause divergence in sperm phenotype. This could include modification of functional trade-offs between traits such as swimming speed and endurance [172]. Differential selection modifying sperm phenotype are expected to cause differences in gene expression which could be reflected in the observed differences in protein expression as observed in the present study (Table 3, Figure 7 and File S7). Higher temperature and salinity in the evolution of M. galloprovincialis might relate to another factor, oxygen solubility which is lower at higher temperature and salinity. Stress from reduced oxygen could impact negatively on ATP production impacting on energy dependent biological processes such as motility, swimming speed and endurance in M. galloprovincialis from Vigo. In the present study however it appears that motility related proteins are relatively upregulated in M. edulis whereas proteins involved in sperm maturation and fertilisation are upregulated in M. galloprovincialis (Figure 7 and File S7).
4.3.6. Future studies integrating proteomics and experimental work on sperm
Clearly relating proteomics data and biochemical interpretations to environmental factors and to variation between species in sperm functional traits is a complex task for the future. Measuring intra and interspecific variation in sperm functional traits is in itself not an easy task [154]. Currently we are not aware of any direct comparative study of some sperm functional traits, like speed, longevity and movement pattern, between M. edulis and M. galloprovincialis. An experimental design in which sperm from M. edulis and M. galloprovincialis are spawned and their performance in motility and endurance as well as fertilisation success assessed, at a range of temperature and salinity conditions would be informative. This could be combined with further proteomics studies applied to sperm from individual mussels from these experiments. The sperm phenotype is highly plastic and evidence already exists for genotype-by-environmental interaction effects on sperm function [172]. An experimental design such as the one described above should allow detecting main effects and interactions involving species differences, reflecting genetic adaptation, contemporary environmental variation and underlying gene expression data. Such approaches could be further extended to the study of hybrid populations of the two species.
5. Concluding remarks
In order to achieve fertilization a sperm must come into contact with an egg and interact with it appropriately. Proteins mediate the interactions between sperm and egg at each step of the fertilisation process, and there is growing evidence that multiple protein complexes might be involved in concert during gamete interaction [82–83]. Species differences in these proteins are proposed as one of the key factors that lead to speciesspecific fertilisation and reproductive isolation. When prezygotic barriers fail, interspecies hybrids can occur. When this happens, postzygotic barriers play an important role in preservation of species integrity. We provide evidence of extensive variation in the mature male gonad transcriptome and sperm proteome in two mussel species, M. edulis and M. galloprovincialis. From the transcriptome analysis, we provide a preliminary list of proteins with sperm-specific functions. These functions are related to sperm-egg interaction, the acrosome reaction, spermatogenesis and motility. From the proteome analysis, we provide evidence of an overrepresentation of mitochondrial proteins among those candidate protein spots identified by MS, as well as contrasting differential expression in isoforms of many proteins. The use of customised speciesspecific protein databases significantly enhance both the quantity and quality of protein identifications, with the use of RNA-seq derived protein databases showing superior results to other customised databases analysed in this study. Our results provide evidence of agreement between the transcriptomic and proteomic results in the direction of expression differences between species. Our results highlight that some candidate sperm proteins, specifically those relating to sperm motility, ATP reserves, and ROS production in M. edulis and proteins relating to sperm motility, the acrosome reaction, capacitation and sperm-egg interaction in M. galloprovincialis might be good targets in further genomic analysis of reproductive barriers between closely related species.
Supplementary Material
Highlights.
Mytilus spp. are valuable in reproductive isolation and speciation studies.
Gametes are key cell targets in investigations of speciation mechanisms.
Mytilus spp. show proteome and transcriptome differences in male gonads and sperm.
Identified proteins are involved in sperm motility and sperm-egg interactions.
Joint proteomic and RNA-seq analysis provide candidate proteins for evolution studies.
Significance.
Model systems for the study of fertilization include marine invertebrates with external fertilisation, such as abalones, sea urchins and mussels, because of the ease with which large quantities of gametes released into seawater can be collected after induced spawning. Unlike abalones and sea urchins, hybridisation has been reported between mussels of different Mytilus spp., which thus makes them very appealing for the study of reproductive isolation at both pre- and post-zygotic levels. There is a lack of empirical proteomic studies on sperm samples comparing different Mytilus species, which could help to advance this study. A comparative analysis of sperm proteomes across different taxa may provide important insights into the fundamental molecular processes and mechanisms involved in reproductive isolation. It might also contribute to a better understanding of sperm function and of the adaptive evolution of sperm proteins in different taxa. There is now growing evidence from genomics studies that multiple protein complexes and many individual proteins might have important functions in sperm biology and the fertilisation process. From an applied perspective, the identification of sperm-specific proteins could also contribute to the improved understanding of fertility problems and as targets for fertility control.
6. Acknowledgements
The authors are very grateful to Nerea González-Lavín for technical assistance during sample processing, the staff of CIM-UVIGO for technical support in the marine station facilities, colleagues from the biochemistry area for sharing equipment and providing useful advice during histological test interpretation, Susana Catarino and David Arteta (Progenika Biopharma) for assistance during RNA-seq analysis, Manuel Marcos (Proteomics service, CACTI, University of Vigo) and José M. Gallardo (IIM-CSIC, Vigo) for assistance and helpful advice in MS/MS analysis. This work was funded by the Spanish “Ministerio de Economía y Competitividad” (codes BFU2011–22599 and AGL2014–52062-R)”, Fondos Feder and Xunta de Galicia (“Grupos de Referencia Competitiva” ED431C 2016–037), and NIH grant HD076862. M.R. Romero was supported by a predoctoral fellowship from Xunta de Galicia (Campus do Mar).
Footnotes
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- [1].Palumbi SR. Genetic divergence, reproductive isolation, and marine speciation. Annual Review of Ecology and Systematics 1994, 25: 547–572. [Google Scholar]
- [2].Swanson WJ, Vacquier VD. The rapid evolution of reproductive proteins. Nature Reviews Genetics 2002, 3: 137–144. [DOI] [PubMed] [Google Scholar]
- [3].Lessios HA. Speciation genes in free-spawning marine invertebrates. Integrative and Comparative Biology 2011, 51: 456–465 [DOI] [PubMed] [Google Scholar]
- [4].McDonough CE, Whittington E, Pitnick S, Dorus S. Proteomics of reproductive systems: towards a molecular understanding of postmating, prezygotic reproductive barriers. Journal of Proteomics 2016, 135: 26–37. [DOI] [PubMed] [Google Scholar]
- [5].Corbett-Detig RB, Zhou J, Clark AG, Hartl DL, Ayroles JF. Genetic incompatibilities are widespread within species. Nature 2013, 504: 135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Orr HA, Presgraves DC. Speciation by postzygotic isolation: forces, genes and molecules. BioEssays 2000, 22: 1085–1094. [DOI] [PubMed] [Google Scholar]
- [7].Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014, 508: 483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wilburn DB, Swanson WJ. From molecules to mating: Rapid evolution and biochemical studies of reproductive proteins. Journal of Proteomics 2016, 135: 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vacquier VD, Swanson WJ. Selection in the rapid evolution of gamete recognition proteins in marine invertebrates. Cold Spring Harbor Perspectives in Biology 2011, 3: a002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Klinovska K, Sebkova N, Dvorakova-Hortova K. Sperm-egg fusion: a molecular enigma of mammalian reproduction. International Journal of Molecular Sciences 2014, 15: 10652–10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oliva R, de Mateo S, Estanyol JM. Sperm cell proteomics. Proteomics 2009, 9: 1004–1017. [DOI] [PubMed] [Google Scholar]
- [12].Karr TL. Fruit flies and the sperm proteome. Human Molecular Genetics 2007, 16: R124–R133. [DOI] [PubMed] [Google Scholar]
- [13].Gur Y, Breitbart H. Protein synthesis in sperm: dialog between mitochondria and cytoplasm. Molecular and Cellular Endocrinology 2008, 282: 45–55. [DOI] [PubMed] [Google Scholar]
- [14].Bayram HL, Claydon AJ, Brownridge PJ, Hurst JL, Mileham A, Stockley P, Beynon RJ, Hammond DE. Cross-species proteomics in analysis of mammalian sperm proteins. Journal of Proteomics 2016, 135: 38–50. [DOI] [PubMed] [Google Scholar]
- [15].Nakachi M, Nakajima A, Nomura M, Yonezawa K, Ueno K, Endo T, Inaba K. Proteomic profiling reveals compartment-specific, novel functions of ascidian sperm proteins. Molecular reproduction and development 2011, 78: 529–549. [DOI] [PubMed] [Google Scholar]
- [16].Palmer MR, McDowall MH, Stewart L, Ouaddi A, MacCoss MJ, Swanson WJ. Mass spectrometry and next-generation sequencing reveal an abundant and rapidly evolving abalone sperm protein. Molecular reproduction and development 2013, 80: 460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kingtong S, Kellner K, Bernay B, Goux D, Sourdaine P, Berthelin CH. Proteomic identification of protein associated to mature spermatozoa in the Pacific oyster Crassostrea gigas. Journal of Proteomics 2013, 82: 81–91. [DOI] [PubMed] [Google Scholar]
- [18].Boonmee A, Berthelin CH, Kingtong S, Pauletto M, Bernay B, Adeline B, Suquet M, Sourdaine P, Kellner K. Differential protein expression during sperm maturation and capacitation in an hermaphroditic bivalve, Pecten maximus (Linnaeus, 1758). Journal of Molluscan Studies 2016, 82: 575–584. [Google Scholar]
- [19].Bartel M, Hartmann S, Lehmann K, Postel K, Quesada H, Philipp EER, Heilmann K, Micheel B, Stuckas H. Identification of sperm proteins as candidate biomarkers for the analysis of reproductive isolation in Mytilus: a case study for the enkurin locus. Marine Biology 2012, 159: 2195 [Google Scholar]
- [20].Diz AP, Dudley E, Skibinski DOF. Identification and characterization of highly expressed proteins in sperm cells of the marine mussel Mytilus edulis. Proteomics 2012, 12: 1949–1956. [DOI] [PubMed] [Google Scholar]
- [21].Zhang Y, Mu H, Lau SC, Zhang Z, Qiu JW. Sperm proteome of Mytilus galloprovincialis: Insights into the evolution of fertilization proteins in marine mussels. Proteomics 2015, 15: 4175–4179. [DOI] [PubMed] [Google Scholar]
- [22].Vicens A, Borziak K, Karr TL, Roldan ERS, Dorus S. Comparative sperm proteomics in mouse species with divergent mating systems. Molecular Biology and Evolution 2017, 34, 1403–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Koehn RK. The genetics and taxonomy of species in the genus Mytilus. Aquaculture 1991, 94: 125–145. [Google Scholar]
- [24].Ecology Seed R., in: Bayne BL (Ed.). Marine mussels: their ecology and physiology. International Biological Programme 1976, 10: 13–65. [Google Scholar]
- [25].Riginos C, McDonald JH. Positive selection on an acrosomal sperm protein, M7 lysin, in three species of the mussel genus Mytilus. Molecular Biology and Evolution 2003, 20: 200–207. [DOI] [PubMed] [Google Scholar]
- [26].Riginos C, Wang D, Abrams AJ. Geographic variation and positive selection on M7 lysin, an acrosomal sperm protein in mussels (Mytilus spp.). Molecular Biology and Evolution 2006, 23: 1952–1965. [DOI] [PubMed] [Google Scholar]
- [27].Springer SA, Crespi BJ. Adaptive gamete-recognition divergence in a hybridizing Mytilus population. Evolution 2007, 61: 772–783. [DOI] [PubMed] [Google Scholar]
- [28].McCartney MA, Lima TG. Evolutionary consequences of introgression at M7 Lysin, a gamete recognition locus, following secondary contact between blue mussel species. Integrative and Comparative Biology 2011, 51: 474–484. [DOI] [PubMed] [Google Scholar]
- [29].Brannock PM, Hilbish TJ. Hybridization results in high levels of sterility and restricted introgression between invasive and endemic marine blue mussels. Marine Ecology Progress Series 2010, 406: 161–171. [Google Scholar]
- [30].Beaumont AR, Abdul-Matin AKM, Seed R. Early development, survival and growth in pure and hybrid larvae of Mytilus edulis and M. galloprovincialis. Journal of Molluscan Studies 1993, 59: 120–123. [Google Scholar]
- [31].Beaumont AR, Turner G, Wood AR, Skibinski DO. Hybridisations between Mytilus edulis and Mytilus galloprovincialis and performance of pure species and hybrid veliger larvae at different temperatures. Journal of Experimental Marine Biology and Ecology 2004, 302: 177–188. [Google Scholar]
- [32].Bierne N, David P, Boudry P, Bonhomme F. Assortative fertilization and selection at larval stage in the mussels Mytilus edulis and M. galloprovincialis. Evolution 2002, 56: 292–298. [DOI] [PubMed] [Google Scholar]
- [33].Bierne N, Bonhomme F, Boudry P, Szulkin M, David P. Fitness landscapes support the dominance theory of post-zygotic isolation in the mussels Mytilus edulis and M. galloprovincialis. Proceedings of the Royal Society of London B: Biological Sciences 2006, 273: 1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rawson PD, Slaughter C, Yund PO. Patterns of gamete incompatibility between the blue mussels Mytilus edulis and M. trossulus. Marine Biology 2003, 143: 317–325. [Google Scholar]
- [35].Miranda MBB, Innes DJ, Thompson RJ. Incomplete reproductive isolation in the blue mussel (Mytilus edulis and M. trossulus) hybrid zone in the Northwest Atlantic: role of gamete interactions and larval viability. The Biological Bulletin 2010, 218: 266–281. [DOI] [PubMed] [Google Scholar]
- [36].Klibansky LK, McCartney MA. Conspecific sperm precedence is a reproductive barrier between free-spawning marine mussels in the northwest Atlantic Mytilus hybrid zone. PloS One 2014, 9: e108433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Findlay GD, Swanson WJ. Proteomics enhances evolutionary and functional analysis of reproductive proteins. Bioessays 2010, 32: 26–36. [DOI] [PubMed] [Google Scholar]
- [38].Diz AP, Martínez-Fernández M, Rolán-Alvarez E. Proteomics in evolutionary ecology: linking the genotype with the phenotype. Molecular Ecology 2012, 21: 10601080. [DOI] [PubMed] [Google Scholar]
- [39].Baer B, Millar AH. Proteomics in evolutionary ecology. Journal of Proteomics 2016, 135: 4–11. [DOI] [PubMed] [Google Scholar]
- [40].Diz AP, Calvete JJ. Ecological proteomics: is the field ripe for integrating proteomics into evolutionary ecology research?. Journal of Proteomics 2016, 135: 1–3. [DOI] [PubMed] [Google Scholar]
- [41].Palumbi SR. Speciation and the evolution of gamete recognition genes: pattern and process. Heredity 2009, 102: 66–76. [DOI] [PubMed] [Google Scholar]
- [42].Diz AP, Skibinski DOF. Evolution of 2-DE protein patterns in a mussel hybrid zone. Proteomics 2007, 7: 2111–2120. [DOI] [PubMed] [Google Scholar]
- [43].Murgarella M, Puiu D, Novoa B, Figueras A, Posada D, Canchaya C. A first insight into the genome of the filter-feeder mussel Mytilus galloprovincialis. PloS one 2016, 11: e0151561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Findlay GD, MacCoss MJ, Swanson WJ. Proteomic discovery of previously unannotated, rapidly evolving seminal fluid genes in Drosophila. Genome Research 2009, 19: 886–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Armengaud J, Trapp J, Pible O, Geffard O, Chaumot A, Hartmann EM. Non-model organisms, a species endangered by proteogenomics. Journal of Proteomics 2014, 105: 5–18. [DOI] [PubMed] [Google Scholar]
- [46].Nesvizhskii AI. Proteogenomics: concepts, applications and computational strategies. Nature methods 2014, 11: 1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Diz AP, Romero MR, Pérez-Figueroa A, Swanson WJ, Skibinski DOF RNA-seq data from mature male gonads of marine mussels Mytilus edulis and M. galloprovincialis. Data in Brief, 2018) 21, 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Atasaral-Şahin Ş, Romero MR, Cueto R, González-Lavín N, Marcos M, Diz AP. Subtle tissue and sex-dependent proteome variation in mussel (Mytilus galloprovincialis) populations of the Galician coast (NW Spain) raised in a common environment. Proteomics 2015, 15: 3993–4006. [DOI] [PubMed] [Google Scholar]
- [49].Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Research 2008, 18: 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schulz MH, Zerbino DR, Vingron M, Birney E. Oases: robust de novo RNA-seq assembly across the dynamic range of expression levels. Bioinformatics 2012, 28: 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology 1990, 215: 403–410. [DOI] [PubMed] [Google Scholar]
- [52].Zhang G, Fang X, Guo X, Li L, Luo R, Xu F, Yang P, Zhang L, Wang X, Qi H, Xiong Z et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490: 49–54. [DOI] [PubMed] [Google Scholar]
- [53].Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21: 3674–3676. [DOI] [PubMed] [Google Scholar]
- [54].Zhang ZH, Jhaveri DJ, Marshall VM, Bauer DC, Edson J, et al. A comparative study of techniques for differential expression analysis on RNA-Seq data. Plos One 2014, 9: e103207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 2011, 12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Leng N, Dawson JA, Thomson JA, Ruotti V, Rissman AI, Smits BMG, Haag JD, Gould MN, Stewart RM, Kendziorski C. EBSeq: an empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 2013, 29: 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Schurch NJ, Schofield P, Gierliński M, Cole C, Sherstnev A, Singh V, Wrobel N, Gharbi K, Simpson GG, Owen-Hughes T, Blaxter M, Barton GJ. How many biological replicates are needed in an RNA-seq experiment and which differential expression tool should you use? RNA 2016, 22:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ramagli LS, Rodriguez LV. Quantitation of microgram amounts of protein in twodimensional polyacrylamide gel electrophoresis sample buffer. Electrophoresis 1985, 6: 559–563. [Google Scholar]
- [59].Diz AP, Dudley E, Cogswell A, MacDonald BW, Kenchington EL, Zouros E, Skibinski DOF. Proteomic analysis of eggs from Mytilus edulis females differing in mitochondrial DNA transmission mode. Molecular & Cellular Proteomics 2013, 12: 3068–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Carvajal-Rodriguez A, de Uña-Alvarez J. Assessing significance in highthroughput experiments by sequential goodness of fit and q-value estimation. PLoS One 2011, 6: e24700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Diz AP, Carvajal-Rodríguez A, Skibinski DOF. Multiple hypothesis testing in proteomics: a strategy for experimental work. Molecular & Cellular Proteomics 2011, 10: M110–004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Warnes GR, Bolker B, Bonebakker L, Gentleman R, Liaw WHA, Lumley T, Maechler M, Magnusson A, Moeller S, Schwartz M, Venables B. gplots: Various R Programming Tools for Plotting Data 2014. R package version 2.14.2. https://CRAN.Rproject.org/package=gplots [Google Scholar]
- [63].Quevillon E, Silventoinen V, Pillai S, Harte N, Mulder N, Apweiler R, Lopez R. InterProScan: protein domains identifier. Nucleic Acids Research 2005, 33: W116–W120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Petersen TN, Brunak S, Heijne von G, Nielsen H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nature Methods 2011, 8: 785–786. [DOI] [PubMed] [Google Scholar]
- [65].Krogh A, Larsson B, Von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. Journal of Molecular Biology 2001, 305: 567–580. [DOI] [PubMed] [Google Scholar]
- [66].Buckland PR. Allele-specific gene expression differences in humans. Human Molecular Genetics 2004, 13, R255–R260. [DOI] [PubMed] [Google Scholar]
- [67].Yamagata A, Kristensen DB, Takeda Y, Miyamoto Y, Okada K, Inamatsu M, Yoshizato K. Mapping of phosphorylated proteins on two-dimensional polyacrylamide gels using protein phosphatase. Proteomics 2002, 2: 1267–1276. [DOI] [PubMed] [Google Scholar]
- [68].Morandell S, Stasyk T, Grosstessner-Hain K, Roitinger E, Mechtler K, Bonn GK, Huber LA. Phosphoproteomics strategies for the functional analysis of signal transduction. Proteomics 2006, 6: 4047–4056. [DOI] [PubMed] [Google Scholar]
- [69].Rabilloud T, Lelong C. Two-dimensional gel electrophoresis in proteomics: a tutorial. Journal of Proteomics 2011, 74: 1829–1841. [DOI] [PubMed] [Google Scholar]
- [70].Diz AP, Álvarez-Rodríguez M, Romero MR, Rolán-Alvarez E, Galindo J. Limited proteomic response in the marine snail Melarhaphe neritoides after long-term emersion. Current Zoology 2017, 63, 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Thingholm TE, Jensen ON, Larsen MR. Analytical strategies for phosphoproteomics. Proteomics 2009, 9: 1451–1468. [DOI] [PubMed] [Google Scholar]
- [72].Feder ME, Walser JC. The biological limitations of transcriptomics in elucidating stress and stress responses. Journal of Evolutionary Biology 2005, 18: 901–910. [DOI] [PubMed] [Google Scholar]
- [73].Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nature Reviews Genetics 2012, 13: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bassim S, Tanguy A, Genard B, Moraga D, Tremblay R. Identification of Mytilus edulis genetic regulators during early development. Gene 2014, 551: 65–78. [DOI] [PubMed] [Google Scholar]
- [75].Moreira R, Pereiro P, Canchaya C, Posada D, Figueras A, Novoa B. RNA-Seq in Mytilus galloprovincialis: comparative transcriptomics and expression profiles among different tissues. BMC genomics 2015, 16: 728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ezkurdia I, del Pozo A, Frankish A, Rodriguez JM, Harrow J, Ashman K, Valencia A, Tress ML. Comparative proteomics reveals a significant bias toward alternative protein isoforms with conserved structure and function. Molecular Biology and Evolution 2012, 29: 2265–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gonzàlez-Porta M, Frankish A, Rung J, Harrow J, Brazma A. Transcriptome analysis of human tissues and cell lines reveals one dominant transcript per gene. Genome Biology 2013, 14: R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pelini SL, Diamond SE, MacLean H, Ellison AM, Gotelli NJ, Sanders NJ, Dunn RR. Common garden experiments reveal uncommon responses across temperatures, locations, and species of ants. Ecology and Evolution 2012, 2: 3009–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].de Villemereuil P, Gaggiotti OE, Mouterde M, Till-Bottraud I. Common garden experiments in the genomic era: new perspectives and opportunities. Heredity 2016, 116: 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sanford E, Kelly MW. Local adaptation in marine invertebrates. Annual Review of Marine Science 2011, 3: 509–535. [DOI] [PubMed] [Google Scholar]
- [81].Banni M, Negri A, Mignone F, Boussetta H, Viarengo A, Dondero F. Gene expression rhythms in the mussel Mytilus galloprovincialis (Lam.) across an annual cycle. PLoS One 2011, 6: e18904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Dun MD, Smith ND, Baker MA, Lin M, Aitken RJ, Nixon B. The chaperonin containing TCP1 complex (CCT/TRiC) is involved in mediating sperm-oocyte interaction. Journal of Biological Chemistry 2011, 286: 36875–36887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Redgrove KA, Anderson AL, Dun MD, McLaughlin EA, O'Bryan MK, Aitken RJ, Nixon B. Involvement of multimeric protein complexes in mediating the capacitationdependent binding of human spermatozoa to homologous zonae pellucidae. Developmental Biology 2011, 356: 460–474. [DOI] [PubMed] [Google Scholar]
- [84].Sutovsky P Sperm proteasome and fertilization. Reproduction 2011, 142: 1–14. [DOI] [PubMed] [Google Scholar]
- [85].Hershko A, Ciechanover A. The ubiquitin system. Annual Review of Biochemistry 1998, 67: 425–479. [DOI] [PubMed] [Google Scholar]
- [86].Zimmerman S, Sutovsky P. The sperm proteasome during sperm capacitation and fertilization. Journal of Reproductive Immunology 2009, 83: 19–25. [DOI] [PubMed] [Google Scholar]
- [87].Miranda PV, González-Echeverría F, Blaquier JA, Mahuran DJ, Tezón JG. Evidence for the participation of β-hexosaminidase in human sperm–zona pellucida interaction in vitro. Molecular human reproduction 2000, 6: 699–706. [DOI] [PubMed] [Google Scholar]
- [88].Koyanagi R, Honegger TG. Molecular cloning and sequence analysis of an ascidian egg β-N-acetylhexosaminidase with a potential role in fertilization. Development, Growth & Differentiation 2003, 45: 209–218. [DOI] [PubMed] [Google Scholar]
- [89].Chiriva-Internati M, Gagliano N, Donetti E, Costa F, Grizzi F, Franceschini B, Albani E, Levi-Setti PE, Gioia M, Jenkins M, Cobos E, Kast WM. Sperm protein 17 is expressed in the sperm fibrous sheath. Journal of Translational Medicine 2009, 7: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Schumacher J, Rosenkranz D, Herlyn H. Mating systems and protein–protein interactions determine evolutionary rates of primate sperm proteins. Proceedings of the Royal Society of London B: Biological Sciences 2014, 281: 2013–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Enard D, Depaulis F, Crollius HR. Human and non-human primate genomes share hotspots of positive selection. PLoS Genetics 2010, 6: e1000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Swanson WJ, Nielsen R, Yang Q. Pervasive adaptive evolution in mammalian fertilization proteins. Molecular Biology and Evolution 2003, 20: 18–20. [DOI] [PubMed] [Google Scholar]
- [93].Gasper J, Swanson WJ. Molecular population genetics of the gene encoding the human fertilization protein zonadhesin reveals rapid adaptive evolution. The American Journal of Human Genetics 2006, 79: 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Tardif S, Cormier N. Role of zonadhesin during sperm–egg interaction: a speciesspecific acrosomal molecule with multiple functions. Molecular Human Reproduction 2011, 17: 661–668. [DOI] [PubMed] [Google Scholar]
- [95].Lima TG, McCartney MA. Adaptive evolution of M3 lysin—a candidate gamete recognition protein in the Mytilus edulis species complex. Molecular Biology and Evolution 2013, 30: 2688–2698. [DOI] [PubMed] [Google Scholar]
- [96].Takagi T, Nakamura A, Deguchi R, Kyozuka KI. Isolation, characterization, and primary structure of three major proteins obtained from Mytilus edulis sperm. Journal of Biochemistry 1994, 116: 598–605. [DOI] [PubMed] [Google Scholar]
- [97].Flachs P, Bhattacharyya T, Mihola O, Piálek J, Forejt J, Trachtulec Z. Prdm9 incompatibility controls oligospermia and delayed fertility but no selfish transmission in mouse intersubspecific hybrids. PloS One 2014, 9: e95806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Mihola O, Trachtulec Z, Vlcek C, Schimenti JC, Forejt J. A mouse speciation gene encodes a meiotic histone H3 methyltransferase. Science 2009, 323: 373–375. [DOI] [PubMed] [Google Scholar]
- [99].O'Carroll D, Scherthan H, Peters AH, Opravil S, Haynes AR, Laible G, Rea S, Schmid M, Lebersorger A, Jerratsch M, Sattler L, Mattei MG, Denny P, Brown SDM, Schweizer D, Januwein T. Isolation and characterization of Suv39h2, a second histone H3 methyltransferase gene that displays testis-specific expression. Molecular and Cellular Biology 2000, 20: 9423–9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Romero MR. Evolutionary and functional proteomic study of gametes from Mytilus edulis and M. galloprovincialis integrating different -omics technologies (Unpublished doctoral dissertation 2017). University of Vigo, Spain. [Google Scholar]
- [101].Tomanek L Environmental proteomics of the mussel Mytilus: implications for tolerance to stress and change in limits of biogeographic ranges in response to climate change. Integrative and Comparative Biology 2012, 52: 648–664. [DOI] [PubMed] [Google Scholar]
- [102].Ruiz-Pesini E, Diez C, Lapeña AC, Pérez-Martos A, Montoya J, Alvarez E, Arenas J, López-Pérez MJ. Correlation of sperm motility with mitochondrial enzymatic activities. Clinical Chemistry 1998, 44: 1616–1620. [PubMed] [Google Scholar]
- [103].Zhao C, Huo R, Wang FQ, Lin M, Zhou ZM, Sha JH. Identification of several proteins involved in regulation of sperm motility by proteomic analysis. Fertility and Sterility 2007, 87: 436–438. [DOI] [PubMed] [Google Scholar]
- [104].Dowling DK, Friberg U, Lindell J. Evolutionary implications of non-neutral mitochondrial genetic variation. Trends in Ecology & Evolution 2008, 23: 546–54. [DOI] [PubMed] [Google Scholar]
- [105].Skibinski DOF, Ghiselli F, Diz AP, Milani L, Mullins JG. Structure-related differences between cytochrome oxidase I proteins in a stable heteroplasmic mitochondrial system. Genome Biology and Evolution 2017, 9: 3265–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Skibinski DOF, Gallagher C, Beynon CM. Sex-limited mitochondrial-DNA transmission in the marine mussel Mytilus edulis. Genetics 1994, 138: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Skibinski DOF, Gallagher C, Beynon CM. Mitochondrial-DNA inheritance. Nature 1994, 368: 817–818. [DOI] [PubMed] [Google Scholar]
- [108].Zouros E, Ball AO, Saavedra C, Freeman KR. An unusual type of mitochondrialDNA inheritance in the blue mussel Mytilus. Proceedings of the National Academy of Sciences of the USA 1994, 91: 7463–7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zouros E, Ball AO, Saavedra C, Freeman KR. Mitochondrial-DNA inheritance. Nature, 1994, 368: 818. [DOI] [PubMed] [Google Scholar]
- [110].Wood AR, Turner G, Skibinski DOF, Beaumont AR. Disruption of doubly uniparental inheritance of mitochondrial DNA in hybrid mussels (Mytilus edulis×M. galloprovincialis). Heredity 2003, 91: 354–360. [DOI] [PubMed] [Google Scholar]
- [111].Amaral A, Paiva C, Attardo Parrinello C, Estanyol JM, Ballescà JL, RamalhoSantos J, Oliva R. Identification of proteins involved in human sperm motility using high-throughput differential proteomics. Journal of Proteome Research 2014, 13: 5670–5684. [DOI] [PubMed] [Google Scholar]
- [112].Zhao C, Guo XJ, Shi ZH, Wang FQ, Huang XY, Huo R, Zhu H, Wang XR, Liu JY, Zhou ZM, Sha JH. Role of translation by mitochondrial-type ribosomes during sperm capacitation: an analysis based on a proteomic approach. Proteomics 2009, 9: 1385–1399. [DOI] [PubMed] [Google Scholar]
- [113].Martínez-Heredia J, de Mateo S, Vidal-Taboada JM, Ballescà JL, Oliva R. Identification of proteomic differences in asthenozoospermic sperm samples. Human Reproduction 2008, 23: 783–791. [DOI] [PubMed] [Google Scholar]
- [114].Kumar V, Kota V, Shivaji S. Hamster sperm capacitation: role of pyruvate dehydrogenase A and dihydrolipoamide dehydrogenase 1. Biology of Reproduction 2008, 79: 190–199. [DOI] [PubMed] [Google Scholar]
- [115].Siva AB, Panneerdoss S, Sailasree P, Singh DK, Kameshwari DB, Shivaji S. Inhibiting sperm pyruvate dehydrogenase complex and its E3 subunit, dihydrolipoamide dehydrogenase affects fertilization in syrian hamsters. PloS One 2014, 9: e97916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ghiselli F, Breton S, Milani L. Mitochondrial activity in gametes and uniparental inheritance: a comment on ‘What can we infer about the origin of sex in early eukaryotes?’. Philosophical Transactions of the Royal Society B 2018, 373: 2017–0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Ji G, Gu A, Wang Y, Huang C, Hu F, Zhou, Y et al. Genetic variants in antioxidant genes are associated with sperm DNA damage and risk of male infertility in a Chinese population. Free Radical Biology and Medicine 2012, 52: 775–780. [DOI] [PubMed] [Google Scholar]
- [118].Saadi HAS, van Riemsdijk E, Dance AL, Rajamanickam GD, Kastelic JP, Thundathil JC. Proteins associated with critical sperm functions and sperm head shape are differentially expressed in morphologically abnormal bovine sperm induced by scrotal insulation. Journal of Proteomics 2013, 82: 64–80. [DOI] [PubMed] [Google Scholar]
- [119].Kobayashi T, Miyazaki T, Natori M, Nozawa S. Protective role of superoxide dismutase in human sperm motility: superoxide dismutase activity and lipid peroxide in human seminal plasma and spermatozoa. Human Reproduction 1991, 6: 987–991. [DOI] [PubMed] [Google Scholar]
- [120].van Gestel RA, Brewis IA, Ashton PR, Brouwers JF, Gadella BM. Multiple proteins present in purified porcine sperm apical plasma membranes interact with the zona pellucida of the oocyte. Molecular Human Reproduction 2007, 13: 445–454. [DOI] [PubMed] [Google Scholar]
- [121].Müller M, Mentel M, van Hellemond JJ, Henze K, Woehle C, Gould SB, Yu RY, van der Giezen M, Tielens AG, Martin WF. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiology and Molecular Biology Reviews 2012, 76: 444–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Bunik V, Artiukhov A, Aleshin V, Mkrtchyan G. Multiple forms of glutamate dehydrogenase in animals: structural determinants and physiological implications. Biology 2016, 5: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Visconti PE. Sperm bioenergetics in a nutshell. Biology of reproduction 2012, 87: 72–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Nakamura N, Dai Q, Williams J, Goulding EH, Willis WD, Brown PR, Eddy EM. Disruption of a spermatogenic cell-specific mouse enolase 4 (eno4) gene causes sperm structural defects and male infertility. Biology of reproduction 2013, 88: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Strong SJ, Ellington WR. Horseshoe crab sperm contain a unique isoform of arginine kinase that is present in midpiece and flagellum. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology 1993, 267: 563–571. [Google Scholar]
- [126].Tombes RM, Shapiro BM. Energy transport and cell polarity: Relationship of phosphagen kinase activity to sperm function. Journal of Experimental Zoology 1989, 251: 82–90. [DOI] [PubMed] [Google Scholar]
- [127].Tombes RM, Brokaw CJ, Shapiro BM. Creatine kinase-dependent energy transport in sea urchin spermatozoa. Flagellar wave attenuation and theoretical analysis of high energy phosphate diffusion. Biophysical Journal 1987, 52: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ellington WR. Evolution and physiological roles of phosphagen systems. Annual Review of Physiology 2001, 63: 289–325. [DOI] [PubMed] [Google Scholar]
- [129].Yi YJ, Sutovsky M, Kennedy C, Sutovsky P. Identification of the inorganic pyrophosphate metabolizing, ATP substituting pathway in mammalian spermatozoa. PloS One 2012, 7: e34524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Diz AP, Dudley E, MacDonald BW, Piña B, Kenchington EL, Zouros E, Skibinski DOF. Genetic variation underlying protein expression in eggs of the marine mussel Mytilus edulis. Molecular & Cellular Proteomics 2009, 8: 132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, Schatten G. Ubiquitinated sperm mitochondria, selective proteolysis, and the regulation of mitochondrial inheritance in mammalian embryos. Biology of Reproduction 2000, 63: 582–590. [DOI] [PubMed] [Google Scholar]
- [132].Berger KH, Yaffe MP. Prohibitin family members interact genetically with mitochondrial inheritance components in Saccharomyces cerevisiae. Molecular and Cellular Biology 1998, 18: 4043–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sutovsky P, Van Leyen K, McCauley T, Day BN, Sutovsky M. Degradation of paternal mitochondria after fertilization: implications for heteroplasmy, assisted reproductive technologies and mtDNA inheritance. Reproductive Biomedicine Online 2004, 8: 24–33. [DOI] [PubMed] [Google Scholar]
- [134].Song WH, Yi YJ, Sutovsky M, Meyers S, Sutovsky P. Autophagy and ubiquitin– proteasome system contribute to sperm mitophagy after mammalian fertilization. Proceedings of the National Academy of Sciences of the USA 2016, 113: E5261–E5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Roy A, Lin YN, Agno JE, DeMayo FJ, Matzuk MM. Absence of tektin 4 causes asthenozoospermia and subfertility in male mice. The FASEB Journal 2007, 21: 1013–1025. [DOI] [PubMed] [Google Scholar]
- [136].Siva AB, Kameshwari DB, Singh V, Pavani K, Sundaram CS, Rangaraj N, Deenadayal M, Shivaji S. Proteomics-based study on asthenozoospermia: differential expression of proteasome alpha complex. Molecular Human Reproduction 2010, 16: 452–462. [DOI] [PubMed] [Google Scholar]
- [137].Thepparat T, Katawatin S, Vongpralub T, Duangjinda M, Thammasirirak S, Utha A. Separation of bovine spermatozoa proteins using 2D-PAGE revealed the relationship between tektin-4 expression patterns and spermatozoa motility. Theriogenology 2012, 77: 1816–1821. [DOI] [PubMed] [Google Scholar]
- [138].Ashrafzadeh A, Karsani SA, Nathan S. Mammalian sperm fertility related proteins. International Journal of Medical Sciences 2013, 10: 1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Bhilawadikar R, Zaveri K, Mukadam L, Naik S, Kamble K, Modi D, Hinduja I. Levels of Tektin 2 and CatSper 2 in normozoospermic and oligoasthenozoospermic men and its association with motility, fertilization rate, embryo quality and pregnancy rate. Journal of Assisted Reproduction and Genetics 2013, 30: 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Mariappa D, Aladakatti RH, Dasari SK, Sreekumar A, Wolkowicz M, van der Hoorn F, Seshagiri PB. Inhibition of tyrosine phosphorylation of sperm flagellar proteins, outer dense fiber protein-2 and tektin-2, is associated with impaired motility during capacitation of hamster spermatozoa. Molecular Reproduction and Development 2010, 77: 182–193. [DOI] [PubMed] [Google Scholar]
- [141].Nomikos M, Sanders JR, Parthimos D, Buntwal L, Calver BL, Stamatiadis P, Smith A, Clue M, Sideratou Z, Swann K, Lai FA. Essential role of the EF-hand domain in targeting sperm phospholipase Cζ to membrane phosphatidylinositol 4, 5bisphosphate (PIP2). Journal of Biological Chemistry 2015, 290: 29519–29530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Wang D, Hu J, Bobulescu IA, Quill TA, McLeroy P, Moe OW, Garbers DL. A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC). Proceedings of the National Academy of Sciences 2007, 104: 9325–9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Di Sole F, Vadnagara K, Moe OW, Babich V. Calcineurin homologous protein: a multifunctional Ca2+-binding protein family. American Journal of Physiology-Renal Physiology 2012, 303: F165–F179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Fernandes D, Loi B, Porte C. Biosynthesis and metabolism of steroids in molluscs. The Journal of Steroid Biochemistry and Molecular Biology 2011, 127: 189–195. [DOI] [PubMed] [Google Scholar]
- [145].Rotem R, Zamir N, Keynan N, Barkan D, Breitbart H, Naor Z. Atrial natriuretic peptide induces acrosomal exocytosis of human spermatozoa. American Journal of Physiology-Endocrinology and Metabolism 1998, 274: E218–E223. [DOI] [PubMed] [Google Scholar]
- [146].Zhang M, Hong H, Zhou B, Jin S, Wang C, Fu M, Wang S, Xia G. The expression of atrial natriuretic peptide in the oviduct and its functions in pig spermatozoa. Journal of Endocrinology 2006, 189: 493–507. [DOI] [PubMed] [Google Scholar]
- [147].Stewart DT, Jha M, Breton S, Hoeh WR, Blier PU. No effect of sperm interactions or egg homogenate on sperm velocity in the blue mussel, Mytilus edulis (Bivalvia: Mytilidae). Canadian Journal of Zoology 2012, 90: 1291–1296. [Google Scholar]
- [148].Beekman M, Nieuwenhuis B, Ortiz-Barrientos D, Evans JP. Sexual selection in hermaphrodites, sperm and broadcast spawners, plants and fungi. Philosophical Transactions of the Royal Society B 2016, 371: 2015–0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Bode M, Marshall DJ. The quick and the dead? Sperm competition and sexual conflict in sea. Evolution 2007, 61: 2693–2700. [DOI] [PubMed] [Google Scholar]
- [150].Sunday JM, Hart MW. Sea star populations diverge by positive selection at a sperm egg compatibility locus. Ecology and Evolution 2013, 3: 640–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Vicens A, Lüke L, Roldan ERS. Proteins involved in motility and sperm-egg interaction evolve more rapidly in mouse spermatozoa. Plos One 2014, 9:e91302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Yund PO. How severe is sperm limitation in natural populations of marine freespawners?. Trends in Ecology & Evolution 2000, 15: 10–13. [DOI] [PubMed] [Google Scholar]
- [153].Evans JP, Sherman CD. Sexual selection and the evolution of egg-sperm interactions in broadcast-spawning invertebrates. The Biological Bulletin 2013, 224: 166–183. [DOI] [PubMed] [Google Scholar]
- [154].Fitzpatrick JL, Lüpold S. Sexual selection and the evolution of sperm quality. Molecular Human Reproduction 2014, 20: 1180–1189. [DOI] [PubMed] [Google Scholar]
- [155].Levitan DR. Sperm velocity and longevity trade off each other and influence fertilization in the sea urchin Lytechinus variegatus. Proceedings of the Royal Society of London B: Biological Sciences 2000, 267: 531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Kamp G, Büsselmann G, Lauterwein J. Spermatozoa: models for studying regulatory aspects of energy metabolism. Experientia 1996, 52: 487–494. [DOI] [PubMed] [Google Scholar]
- [157].Dorus S, Wasbrough ER, Busby J, Wilkin EC, Karr TL. Sperm proteomics reveals intensified selection on mouse sperm membrane and acrosome genes. Molecular Biology and Evolution 2010, 27: 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zigler KS, McCartney MA, Levitan DR, Lessios HA. Sea urchin bindin divergence predicts gamete compatibility. Evolution 2005, 59: 2399–2404. [PubMed] [Google Scholar]
- [159].Kenchington EL, Hamilton L, Cogswell A, Zouros E. Paternal mtDNA and maleness are co-inherited but not causally linked in Mytilid mussels. PLoS One 2009, 4: e6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Brannock PM, Roberts MA, Hilbish TJ. Ubiquitous heteroplasmy in Mytilus spp. resulting from disruption in doubly uniparental inheritance regulation. Marine Ecology Progress Series 2013, 480: 131–143. [Google Scholar]
- [161].Śmietanka B, Burzyński A. Disruption of doubly uniparental inheritance of mitochondrial DNA associated with hybridization area of European Mytilus edulis and Mytilus trossulus in Norway. Marine Biology 2017, 164: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Skibinski DOF, Beardmore JA, Cross TF. Aspects of the population-genetics of Mytilus (Mytilidae, Mollusca) in the British-Isles. Biological Journal of the Linnean Society 1983, 19: 137–183. [Google Scholar]
- [163].Saarman NP, Kober KM, Simison WB, Pogson GH. Sequence-based analysis of thermal adaptation and protein energy landscapes in an invasive blue mussel (Mytilus galloprovincialis). Genome Biology and Evolution 2017, 9: 2739–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Eads AR, Evans JP, Kennington WJ. Plasticity of fertilization rates under varying temperature in the broadcast spawning mussel, Mytilus galloprovincialis. Ecology and Evolution 2016, 6: 6578–6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Eads AR, Kennington WJ, Evans JP. Interactive effects of ocean warming and acidification on sperm motility and fertilization in the mussel Mytilus galloprovincialis. Marine Ecology Progress Series 2016, 562: 101–111. [Google Scholar]
- [166].Oliver M, Evans JP. Chemically moderated gamete preferences predict offspring fitness in a broadcast spawning invertebrate. Proceedings of the Royal Society of London B: Biological Sciences 2014, 281: 2014–0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Fitzpatrick JL, Simmons LW, Evans JP. Complex patterns of multivariate selection on the ejaculate of a broadcast spawning marine invertebrate. Evolution 2012, 66: 2451–2460. [DOI] [PubMed] [Google Scholar]
- [168].Liu G, Innes D, Thompson RJ. Quantitative analysis of sperm plane circular movement in the blue mussels Mytilus edulis, M. trossulus and their hybrids. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology 2011, 315: 280–290. [DOI] [PubMed] [Google Scholar]
- [169].El-Dessouky HT, Ettouney HM. Fundamentals of salt water desalination. Elsevier, 2002, Pages 525–563. [Google Scholar]
- [170].Ospina-Álvarez A, Palomera I, Parada C. Changes in egg buoyancy during development and its effects on the vertical distribution of anchovy eggs. Fisheries Research 2012, 117: 86–95. [Google Scholar]
- [171].Sundby S, Kristiansen T. The principles of buoyancy in marine fish eggs and their vertical distributions across the world oceans. Plos One 2015, 10: e0138821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Reinhardt K, Dobler R, Abbott J. An ecology of sperm: sperm diversification by natural selection. Annual Review of Ecology, Evolution, and Systematics 2015, 46: 435–459. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.