Abstract
Objective:
Find genetic contributions to glaucoma in African Americans.
Design:
Cross-sectional, case-control study.
Participants:
1875 POAG cases and 1709 controls, self-identified as African Descent (AD), from the African Descent and Glaucoma Evaluation Study (ADAGESIII) and Wake Forest School of Medicine.
Methods:
MegaChip genotypes were imputed to Thousand Genomes data. Association of SNPs with POAG and advanced POAG was tested by linear mixed model correcting for relatedness and population stratification. Genetic risk scores were tested by Receiver Operator Characteristics (ROC-AUC).
Main Outcome:
POAG defined by visual field loss without other non-ocular conditions (N=1875). Advanced POAG was defined by age-based mean deviation of visual field (N=946).
Results:
18,281,920 SNPs met imputation quality r2>0.7 and minor allele frequency>0.005. Association of a novel locus, ENO4, was observed for advanced POAG (rs185815146 beta 0.36, SE 0.065, p<3×10−8). For POAG, an AD signal was observed at 9p21 ED POAG signal (rs79721419; p<6.5×10−5) independent of the previously observed 9p21 ED signal (rs2383204; p<2.3×10−5) by conditional analyses. An association with POAG in FNDC3B (rs111698934, p<3.9×10−5) was observed, not in LD with the previously reported ED SNP. Additional previously identified loci associated with POAG in AD were: 8q22, AFAP1, TMCO1. An AUC of 0.62 was observed with an unweighted genetic risk score composed of 11 SNPs in candidate genes. Two additional risk scores were studied by using a penalized matrix decomposition with cross-validation; risk scores of 50 and 400 SNPs were identified with ROC of AUC=0.74 and AUC=0.94, respectively.
Conclusions:
A novel association with advanced POAG in the ENO4 locus was putatively identified in subjects of African descent. In addition to this finding, this GWAS in AD POAG subjects contributes to POAG genetics by identification of novel signals in prior loci (9p21), as well as advancing the fine-mapping of regions due to shorter average linkage disequilibrium (FNDC3B). While not useful without confirmation and clinical trials, the use of genetic risk scores demonstrated that considerable AD-specific genetic information remains in these data.
Precis
Genome-wide association study of POAG in subjects of African descent shows both similarities and differences with loci previously identified in large-scale studies of POAG in subjects of European Descent.
INTRODUCTION
The African Descent and Glaucoma Evaluation Study (ADAGES) was designed to advance understanding of the greater susceptibility and higher rates of blindness due to primary open angle glaucoma (POAG) in individuals of sub-Saharan African continental descent (AD) such as US African Americans.1-3 AD POAG patients tend to have worse visual field (VF) damage and progression of disease. For example, among glaucoma patients with elevated intra-ocular pressure (IOP), AD POAG patients were 5.2 times more likely to develop VF loss than individuals of European continental descent (ED).4 Such examples of racial differences point to a genetic contribution to the pathophysiology of POAG.
This genetic component to POAG is consistent with accumulating evidence.5 Rare forms of POAG affecting children and young adults are usually inherited as Mendelian disorders with either recessive or dominant inheritance of a single gene.5 Linkage studies of POAG families have identified a role for the myocilin gene (MYOC; dbGENE 4653; OMIM 601652) in POAG.6, 7 In contrast, common forms of POAG affecting adults over the age of 50 are inherited as non-Mendelian or complex traits. Several genome-wide association studies (GWAS) and meta-analyses for POAG have now been completed, mostly in ED populations. A strong and consistent finding across multiple ethnic groups is the association of POAG with the region including the cyclin dependent kinase inhibitor 2B region (CDKN2B; dbGENE 1030) and its antisense RNA CDKN2B-AS (dbGENE 100048912) at the 9p21 locus.8 This association has been observed, for example, in Australian,9 European,10 Chinese,11 Japanese, 12, 13 other Pacific Rim countries,10 US Caucasian,14, 15 Barbados,16 and African-American17 populations. This region was also among the first observed associations with coronary artery disease and type 2 diabetes,18, 19 and accumulating evidence points to disruption of tissue-specific TGF-beta regulation in aortic smooth muscle cells.20, 21 Additional POAG genes reaching genome-wide significance include: AFAP1 (dbGENE 60312), ATXN2 (dbGENE 6311),22 CAV1/CAV2 (dbGENE 857, 858),23 FOXC1/GMDS (dbGENE 2296,2762),22, 24 SIX6 (dbGENE 4990),25 TGFBR3 (dbGENE 7048),10 TMCO1 (dbGENE 54499),9 and TXNRD2 (dbGENE 10587).22 Recently, SNP rs76481776 in the microRNA (miRNA) gene MIR182 (dbGENE 406958) has associated with POAG by the NEI Glaucoma Human Genetic Collaboration Heritable Overall Operational Database (NEIGHBORHOOD) consortium.26 Functional analyses suggested that MIR182 was expressed in trabecular meshwork cells and was higher in the aqueous humor from glaucoma patients when compared with controls.
In this paper we present a GWAS of AD glaucoma patients from the ADAGESIII Contribution of Genotype to Glaucoma Phenotype in African Americans cohort.27 We then compare our results with loci previously identified in both early onset and in adult glaucoma cases in ED populations.
METHODS
Subjects
The African Descent and Glaucoma Evaluation Study (ADAGESIII) has been described in our earlier publication.27 “African Descent” and “AD” in this paper are defined as “Descent from sub-Saharan Africa.” Subjects in this report consist of POAG cases (n=2014) and controls (n=234) ascertained by the eye clinics of this consortium and additional “convenience controls” (n=2049) ascertained at Wake Forest School of Medicine. All subjects were self-identified for African Descent (AD). Advanced POAG was defined by age-based mean deviation: −10dB and age > 60 yr, or −8dB and age >50 yr, or −6dB and age > 40 yr (n=946). This study was approved by the institution review boards of the clinical sites, the University of California San Diego, Wake Forest School of Medicine, and Los Angeles Biomedical Research Institute.
Generation of Genomic Data
Genotyping.
DNA was isolated from either whole blood or saliva from subjects ascertained by ADAGES and at Wake Forest School of Medicine (WFSM). These samples were genotyped using the Illumina MegaChip (Ilumina, San Diego, CA) in the LA Biomed Genomics Center and the Center for Genomics and Personalized Medicine Research at WFSM, respectively. Output files (“idat files”) from both LA Biomed and WFSM were merged and genotypes were called across both studies as a single project using the Illumina software (GenomeStudio 2.0).28, 29 This “common calling” provided genotype data for 2049 WFSM controls, 2014 ADAGES POAG cases, and 234 ADAGES controls. Subjects were removed for failed genotyping, unresolvable gender discrepancies, being outliers by principal component analyses, and by relatedness with . Genotyping rates were >99% per subject and > 99% per single nucleotide polymorphism (SNP). The final dataset consisted of 870,067 autosomal SNPs with a minor allele frequency (MAF) >0.005 in 1875 POAG cases and 1709 controls.
AD Estimate.
Genotype data for Chromosomes 1-22 were pruned for linkage disequilibrium (PLINK, at r2<0.2) and the amount of AD for each subject was estimated by an unsupervised analysis using Admixture 1.3.0 with K=3 for sub-Saharan African, European, and Native American continental ancestries.30, 31 “K,” or the number of populations expected in the analysis, was set at 3 because ADAGESIII recruits AD POAG cases from San Diego, New York, Alabama, Georgia, and Texas, and while research subjects self-identify as AD, they may also have an additional and different Native American contributions.
Imputation.
SNP data were uploaded to the Michigan Imputation Server for imputation to the 1kGP phase 3 dataset.32 SNP data with imputation quality score > 0.70 and minor allele frequency (MAF) > 0.005 were then tested for discrepancies between allele frequencies in the ADAGES and WFSM controls and removed if a Hardy-Weinberg test was significant (p-value < 0.05). A total of 18,281,920 autosomal SNPs were tested for association.
Genetic Analyses
Association with POAG and advanced POAG.
The association of a SNP with POAG or advanced POAG was tested using a fast, variance components, mixed model approach (EMMAX).33, 34 The use of a mixed model association test with correction by a genetic relationship matrix provided superior correction for population stratification and genetic relatedness.35, 36 Covariates were Principal Components 1-3. Subjects over 8 standard deviations from the center of each component were removed (smartPCA). Since the glaucoma patients are recruited at tertiary referral centers, we did not observe an association with age and sex with these two phenotypes and so these were not included as covariates.
Candidate Gene Study.
Both rare and common previously identified genetic variants were selected as candidate genes for study;5, 37, 38 the list included variants contributing to early onset POAG and factors with genome-wide significance for POAG identified by GWAS. We tested 35,865 SNPs for association in this part of the study; these SNPs are located between the start and end of transcription in the following 25 loci: 8q2214, 9p217, 8, 14, ABCA111, 24, ABO39, AFAP140, ARHGEF1241, ATOH742, ATXN26, 22, CAV123, 43, CYP1B1 (OMIM#231300), FNDC3B10, GAS722, FOXC1/GMDS15, 22, LTBP2 (OMIM#613086)13, MIR18226, MYOC (OMIM*601652)7, OPTN (OMIM#137760), PMM211, SIX1/SIX625, TBK1 (OMIM#177700), TGFBR310, TMOC19, and TXNRD222. Association with POAG and advanced POAG was tested as above. The list of loci and SNPs tested is in Supplemental Table 1.
Rationale for Genetic Risk Scores.
In addition to being composed of highly significant GWAS SNPs only, genetic risk scores may also be composed of moderately significant GWAS SNPs since it is well-known that: (1) GwAs results contain a mix of “true positive” and “false positive” loci, and (2) “true positive” loci will contribute to the ability of the score to discriminate phenotype or disease and “false positive” loci will contribute “noise”.44-46 We tested two types of score: for Genetic Risk Score #1, SNPs were selected from the well-identified loci associated in this study (11 SNPs, see Table 2); for Genetic Risk Score #2 SNPs were selected from all SNPs showing some association with AD POAG (~12,900 SNPs with an association p-value less than 0.001). Since all ~12,900 SNPs together did not produce a usable genetic risk score (see below), in order to enrich for “true positives” over “false positives,” or “signal” over “noise,” a regularized (also known as a penalized) method was applied. Furthermore, regularized methods work well when the number of SNPs is much greater than the number of subjects, as in this study and are robust to correlation between SNPs.47 The following should be noted: first, this analysis is not the same as using SNPs with the highest effect sizes in the model because the use of regularization tests the SNPs together, not individually; and second, the original development of receiver operating characteristics was in the context of “signal” over “noise” and so is appropriate for this context for selecting “true positives” from “false positives.”
Table 2.
Combined logistic regression of 11 SNPs and POAG
| Estimate | Standard Error | p value | ||
|---|---|---|---|---|
| Intercept | −0.833 | 0.334 | 0.0126 | |
| African continental ancestry | 0.432 | 0.250 | 0.0838 | |
| rs10529326 | TMCO1 | 0.364 | 0.073 | 5.4×10−7 |
| rs2432663 | CYP1B1 | 0.476 | 0.119 | 6.3×10−5 |
| rs199612704 | FNDC3B | 0.818 | 0.163 | 4.9×10−7 |
| rs4328916 | AFAP1 | 0.197 | 0.048 | 4.3×10−5 |
| rs7009228 | 8q22 | 0.259 | 0.051 | 5.1×10−7 |
| rs2383204 | 9p21#1 | 0.232 | 0.051 | 4.9×10−6 |
| rs79721419 | 9p21#2 | 0.616 | 0.151 | 4.5×10−4 |
| rs551169 | ABO | 0.196 | 0.046 | 1.7×10−5 |
| rs8080535 | GAS7 | 0.159 | 0.047 | 0.00071 |
| rs235902 | MYOC | 0.174 | 0.067 | 0.0092 |
| rs1332985 | PMM2 | 0.104 | 0.053 | 0.050 |
The genotypes of those SNPs in Table 1 with negative effect sizes were re-coded to the other allele so that the number of alleles for each SNP was additive for increased POAG risk. All SNPs were then combined into a single logistic regression model. AD for each subject was estimated using Admixture 1.3.0 with K=3 on a subset of the genotyping data pruned for linkage disequilibrium. The dataset for this table, with genotypes coded to be additive, was then used to create Genetic Risk Score #1. Note that both 9p21 SNPs, rs2383204 and rs79721419, remained in this model, further supporting their statistical independence.
Genetic Risk Score #1.
We selected SNPs for this score (a) associated with POAG or advanced POAG from ED and AD SNPs with p-value < 0.001, imputation r2<0.7, maf>0.005, and one per locus (Table 2) were used to calculate genetic risk score #1 for POAG. First, the genotypes of SNPs with negative effect sizes were re-coded to the other allele so that the number of “risk” alleles for each SNP was additive for increased POAG risk, i.e. an “unweighted score.” Table 2 shows how these 11 SNPs perform together in a single logistic regression model. These allele counts were then added for each subject in order to provide the genetic risk score. The utility of this score for prediction of POAG within this dataset was then tested by estimating the area under the receiver-operator characteristic (AUC; ROC).48 The R package “ROCR” was used for calculating ROC and plotting AUC.44 (http://rocr.bioinf.mpi-sb.mpg.de)
Genetic Risk Scores #2 and #3.
All SNPs associated with POAG or advanced POAG with p-values <0.001 were used to calculate Genetic Risk Score #2 and #3 for POAG. Genotypes of the SNPs in this subset with negative effect sizes (betas) were re-coded to the other allele so that the number of “risk” alleles for each SNP was additive for increased POAG risk. These allele counts were then added for each subject in order to provide the genetic risk score, i.e. “unweighted” risk score. Since the number of SNPs greatly exceeded the number of subjects, a penalized logistic regression was employed with 10-fold cross-validation to find the SNP subset associated with increased POAG risk (R package GLMNET).47, 49 This approach combined a maximum likelihood method to test significance with coordinate descent to choose the penalty.50 The greater the penalty, the fewer SNPs that remain in the model. This analysis was performed with two different penalties: one (Genetic Risk Score #2) that would provide 40-50 SNPs in the score, the second (Genetic Risk Score #3) that would provide 400-500 SNPs in the score. The utility of both Genetic Score #2 and #3 was then tested by receiver operating characteristics as for Genetic Risk Score #1.
RESULTS
Inflation due to population stratification and cryptic relatedness was minimal for SNPs with minor allele frequencies (MAF) greater than 0.005 (Supplemental Figures 1 & 2; λs for POAG was 1.017 and for advanced POAG 1.022). The final genotype dataset consisted of ~18.3 million SNPs with imputation scores > 0.7 and MAF > 0.005 in controls. After quality control of sample data, there were 1875 AD POAG cases (of which 946 had advanced POAG) and 1709 AD “convenience” controls.
Putative AD Novel Locus
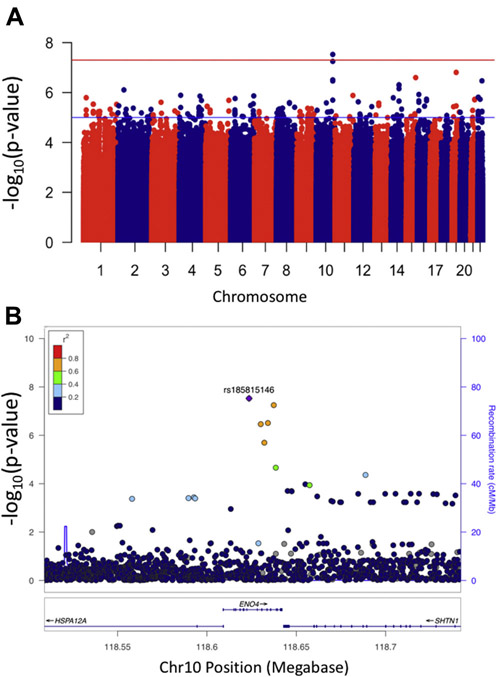
An association of advanced POAG with the ENO4 locus was observed at SNP rs185815146 (Fig. 1A, “Manhattan” plot; Fig. 1B, “LocusZoom” plot). The effect allele frequency (“A”) in cases was 0.029 and in controls 0.007, the effect size (beta) = 0.36 with standard error = 0.065, p-value = 3.0×10−8. In Thousand Genomes Project data (1kGP), this SNP has only been observed in the African and African American populations (AD; dbSNP). The hg19 coordinates of this SNP are at Chr10:118,623,580 and the ENO4 locus is located between the HSPA12A and SHTN1 (aka KIAA1598) loci. No SNP was associated with POAG at the genome-wide significance level (Supplementary Fig. 3).
Figure 1. Genome-wide association with advanced POAG.
A) Manhattan plot showing association with advanced POAG in AD subjects. Plot generated using R package “qqman”.55 Red line indicates p-value of 5×10−8; point above red line is rs185815146. B) LocusZoom plot of ENO4 locus. Plot generated using the LocusZoom “standalone” program. Color of points shows linkage disequilibrium (LD) between rs185815146 and the other SNPs as found in the 1kGP data subset of sub-Saharan Africans (AFR). No LD between rs185815146 and the other SNPs was observed using the “EUR” subset.
Previously Identified Loci
The association results were further examined within the regions of loci previously reported to be associated with POAG (Table 1 and Supplementary Table 1).
Table 1.
Candidate Genes Associated with POAG and with Advanced POAG in AD Subjects
| Phenotype | Locus | SNP | Chr | Position (hg19) |
“Other” Allele |
Effect Allele |
Effect Size |
Standard Error |
P value | Reference Example |
|---|---|---|---|---|---|---|---|---|---|---|
| POAG | TGFBR3 | rs11466588 | 1 | 92,293,416 | C | A | 0.190 | 0.056 | 6.5×10−4 | 10 |
| POAG | TMCO1 | rs10529326 | 1 | 165,719,351 | ATCTT | A | −0.077 | 0.017 | 1.2×10−5 | 9 |
| POAG | MYOC | rs12129856 | 1 | 171,580,024 | A | G | 0.051 | 0.015 | 6.6×10−4 | OMIM *601652 |
| POAG | CYP1B1 | rs2432663 | 2 | 38,351,642 | T | C | 0.096 | 0.027 | 4.5×10−4 | OMIM #231300 |
| POAG | FNDC3B | rs199612704 | 3 | 172,033,011 | G | C | 0.164 | 0.040 | 3.9×10−5 | 10, 39 |
| POAG | AFAP1 | rs4328916 | 4 | 7,8235861 | TATAC | T | 0.044 | 0.011 | 5.9×10−5 | 24 |
| POAG | GMDS | rs182635870 | 6 | 2,345,598 | G | T | 0.481 | 0.126 | 1.4×10−4 | 24 |
| POAG | 8q22 | rs7009228 | 8 | 105,721,414 | A | G | −0.051 | 0.012 | 2.0×10−5 | 14 |
| POAG | 9p21 | rs2383204 | 9 | 22,055,048 | G | A | 0.048 | 0.011 | 2.3×10−5 | 9, 24 |
| POAG | 9p21 | rs79721419 | 9 | 22,200,956 | A | G | 0.141 | 0.035 | 6.6×10−5 | this study |
| POAG | ABO | rs551169 | 9 | 136,197,286 | A | G | 0.047 | 0.011 | 1.1×10−5 | 39 |
| advanced | PMM2 | rs13332985 | 16 | 8,908,857 | A | G | −0.049 | 0.013 | 4.1×10−4 | 11 |
| advanced | GAS7 | rs8080535 | 17 | 9,968,097 | G | A | −0.044 | 0.011 | 1.3×10−4 | 54 |
Sample size was 1875 cases and 1709 controls for POAG and 946 cases and 1709 controls for advanced POAG. For this table the “best phenotype,” the phenotype with the greatest significance of association, is reported.
Correction for multiple testing is 0.05 / (2 phenotypes times 25 candidate genes) = 0.001. Uncorrected p-values are given.
SNPs listed have p value < 0.001, MAF > 0.005, and imputation quality > 0.70, one result per locus shown.
The following 25 loci were tested: 8q22 14, 9p21 7, 8, 14 ABCA1 11, 24, ABO 39, AFAP1 40, ARHGEF12 41, ATOH7 42, ATXN2 6, 22, CAV1 23, 43, CYP1B1 (OMIM#231300), FNDC3B 10, GAS7 22, FOXC1/GMDS 15, 22, LTBP2 (OMIM#613086)13, MIR182 26, MYOC (OMIM*601652) 7, OPTN (OMIM#137760), PMM2 11 SIX1/SIX6 25, TBK1 (OMIM#177700), TGFBR3 10, TMCO1 9, TXNRD2 22.
9p21/CDKN2B.
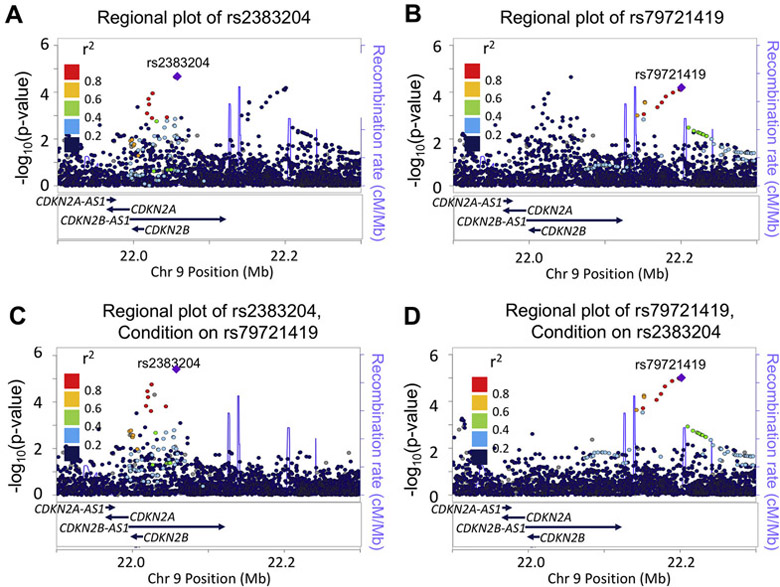
The first observed pattern of association was that of an additional, independent AD signal in a previously identified locus for POAG. The most significant and consistent association with POAG observed in previous GWAS studies has been the 9p21 locus.8 This 9p21 association was confirmed with AD POAG (rs2383204, 9p21 Signal #1; Table 1 and Fig. 2A). This SNP is in LD with the previously reported ED SNPs rs106319242 and rs4977756.9 A second association signal at SNP rs79721419 was observed (9p21 Signal #2; Table 1 and Fig. 2B). There was no LD between Signal #1, rs2383204, and Signal #2, rs79721419, and a region of high recombination rate in the 1kGP AFR subset separates them; this subset includes AD subjects from the Southwest US (ASW). The independence of these two association signals was tested by conditional analyses; the association at rs2383204 remained when conditioning on rs79721519 (Fig. 2C), and the association at rs79721419 remained when conditioning on rs2383204 (Fig. 2D). Since the rs2383204 signal has been observed in European populations, this independence supported the concept that the rs2383204 signal is of European continental origin and the rs79721419 was of African continental origin.
Figure 2. The 9p21 Locus and AD POAG.
Association results from the ~5300 SNPs between 21.9 Megabases (Mb) and 22.4 Mb on Chromosome 9. Linkage disequilibrium values (LD; colors) are from the AFR subset of the 1kGP data; this subset includes African-Americans from the Southwestern U.S. (ASW) and were calculated using the LocusZoom “Standalone” program.56 Blue lines indicate the recombination rate in centimorgans/million basepairs (cM/Mb) in the AMR data subset. (A) Association results with POAG; colors represent LD with rs2383204. (B) The same association results with POAG; colors represent LD with rs79721419. (C) Association with POAG conditioned on rs79721419; colors represent LD with rs2383204. Two previously reported SNPs were also in LD with rs2383204 and associated with POAG: rs1063192 (estimate, 0.052, p=0.013) and rs518394 (estimate −0.056, p=0.0063). (D) Association with POAG, conditioned on rs2383204; colors represent LD with rs79721419. The same two previously reported SNPs, rs1063192 and rs518394 were not significant. In addition, no statistical interaction was observed between rs2383204 and rs79721419 in a general linear model with POAG as the outcome variable (Table 2).
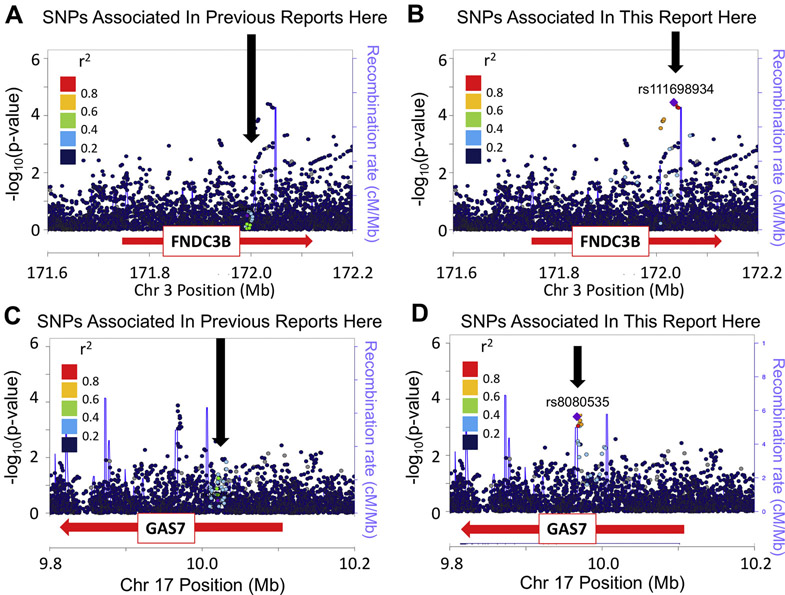
FNDC3B with POAG; GAS7 with advanced POAG.
Two loci showed the second pattern: (1) a SNP associated with either POAG or advanced POAG, (2) no LD with previously reported ED SNPs, and (3) separated from the previously reported ED SNP(s) by a region of high recombination rate in the 1kGP AFR subset. SNP rs199612704 in the FNDC3B locus was associated with POAG (Table 1); this SNP was not in LD with the previously reported ED SNPs rs12897 or rs4894796, and is separated by a region of high recombination (Fig. 3A and B). Similarly, SNP rs8080535 in the GAS7 locus was associated with advanced POAG (Supplementary Table 1); this SNP is not in LD with the previously reported ED SNPs rs9897123, rs9913911, or rs121502840, and was separated by a region of high recombination (Fig. 3C&D).22, 39, 51 These observations support the concept that SNPs contributing to POAG and advanced POAG are present in AD subjects, but additional to the SNPs reported for ED.
Figure 3. Two Similar Results: Association of FNDC3B with POAG and GAS7 with Advanced POAG.
LocusZoom Plots produced using the LocusZoom “Standalone” version. AFR LD values are in color. Blue lines indicate the recombination rate in AFR (cM/Mb). (A) FNDC3B gene and POAG. LD with rs12897, previously reported to be associated with POAG but not associated here in African-Americans. (B) FNDC3B gene and POAG. The same association results with the colors representing LD, with rs11698934. (C) GAS7 gene and severity. LD with rs9913911, previously reported to be associated with POAG but not associated here in African-Americans. (D) GAS7 gene and severity. The same association results with the colors representing LD, with rs8080535.
8q22, AFAP1.
A third pattern was observed: an associated SNP not in LD with previously reported SNPs or separated by a region of high recombination, “same locus different SNP.” SNP rs7009228 in the 8q22 locus was associated with POAG (Table 1) but was not in LD with the previously reported ED SNPs rs1521444, rs284489, or rs284495.14 SNP rs4328916 in the AFAP1 locus was associated with POAG but was not in LD with ED SNP rs4619890.24
TMCO1.
The fourth pattern observed was that of a SNP associated with POAG and is in LD with previously reported ED SNPs. SNP rs10529326 in the TMCO1 locus is in LD with ED SNPs rs4656461 and rs7518099.9 We observed an association with this last SNP and POAG.
Genetic Risk Score for POAG
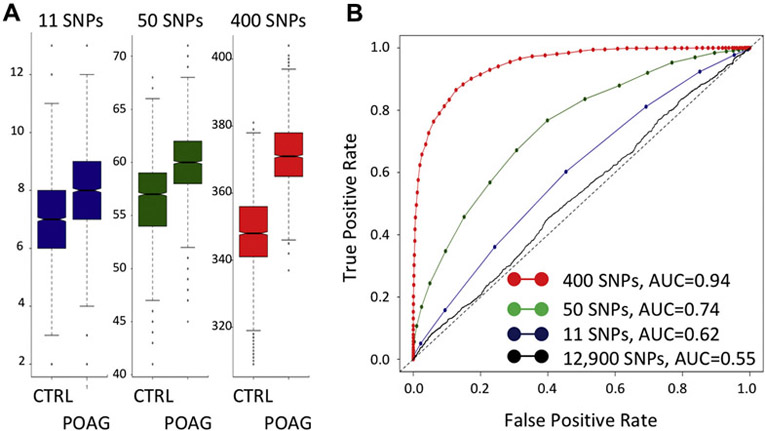
Genetic Risk Score #1. First, 11 loci associated with POAG were selected (Table 2) from ED and AD SNPs associated with POAG (Table 2). We included top SNPs with African-specific signals for this model.Genotypes were first recoded to the opposite strand if the effect estimate (beta) was negative and then allele counts were added. In this way, a higher score will be associated with greater risk for POAG. This produced an unweighted score that varied between 2 and 15 (Fig. 4A, 11 SNPs). Next, this score was then tested for its ability to predict POAG using receiver operation characteristics (ROC), and an area under the curve (AUC) value of 0.62 was observed (Fig. 4B, blue solid line). For comparison, the null result of AUC=0.5 is included in Fig.4 (Fig. 4B, dotted black line).
Figure 4. Test of Genetic Risk Scores by Receiver Operating Characteristics.
Genetic risk scores are described were created by adding allele counts across selected SNPs for each subject; a SNP with a negative estimate (beta) was recoded to the other allele so that genotype counts would be additive for POAG risk. SNPs for Genetic Risk Score #1 were the 11 significantly associated SNPs in Table 1 (B, blue line). SNPs for Genetic Risk Score #2 were 50 SNPs selected using a penalized logistic regression and cross-validation procedure (B, green line). SNPs for Genetic Risk Score #3 were 400 SNPs selected by the same penalized logistic regression and cross-validation procedure (B, red line). For comparison, AUC=0.5 is the dotted black line, and AUC from all ~12,900 SNPs is the solid black line. CTRL, controls; POAG, cases.
Genetic Risk Score #2 & #3.
First, genotypes for ~12,900 SNPs (all SNPs with p-value < 0.001) were recoded to the opposite strand if the effect estimate (beta) was negative. As above, this provides an unweighted, greater score value with greater POAG risk. Next, in order to improve “signal” over “noise”, a regularized 10-fold cross-validation procedure was applied to find a subset of the ~12,900 SNPs that together have the highest ability to predict POAG.50. For Genetic Risk Score #2, the penalty was selected to result in 40-50 SNPs for the score, and for #3, the penalty was selected to result in 400-500 SNPs for the score (Fig. 4A for both scores). Then, the ability of these scores to predict POAG was tested further by calculating ROC; the AUC for Genetic Risk Score #2 was 0.74 (Fig. 4B, green solid line) and for #3 was 0.94 (Fig. 4B, red solid line). For comparison, the solid black line shows the AUC for all ~12,900 SNPs (equal to 0.55).
DISCUSSION
The results of our GWAS of POAG and advanced POAG in AD subjects suggested that a novel locus, ENO4, is associated with advanced POAG in AD, that novel SNPs in the already identified loci, 9p21 and FNDC3B are associated with POAG, and that novel SNPs in the already identified locus GAS7 are associated with advanced POAG.
Novel Loci
ENO4 & advanced POAG.
A putative novel finding was the association of advanced POAG with the enolase 4 gene (ENO4; dbGENE 387712; Fig. 1); this locus is located on Chromosome 10 at position 118,623,580 (SNP rs185815146; “A” allele; genome build hg19). SNP rs185815146 has been observed only in the AFR sub-set of the 1kGP data. This finding should be considered preliminary pending further collaborations to enlarge the sample size and to confirm results across African Descent datasets. One of the weaknesses of our study is that we do not have a confirmation dataset to confirm this finding at this time. However, we propose this locus for POAG of subjects with African Descent because (a) the minor allele is found only in African populations, (b) Expression of ENO4 and the flanking genes HSPA12A and SHTN1 has been observed in POAG-related tissues, e.g. the optic nerve head and the trabecular meshwork (Ocular Tissue Database; https://genome.uiowa.edu/otdb),53 (c) using the advanced POAG sample size, our study has 0.69 power to detect an effect size of 2.5 at MAF 0.005, at genomewide significance, assuming there are 100 loci associated with POAG and greater than 0.8 at effect size 2.2 and MAF 0.01 for the 100 loci model and at effect size 2.4 and MAF 0.015 for a 25 locus model. The role of metabolism in the pathophysiology of POAG is suggested by the fact that the enolase 4 enzyme (4.2.1.11) participates in glucose metabolism as a proximal step to phosphoenol pyruvate (KEGG).
Utility of Studying Multiple Ethnic Groups
Our findings reinforce the rationale for performing GWAS in multiple US populations (summarized in Table 3): (a) potential to find new genes, (b) find population-specific associations that may shed additional light on the role of that locus in disease development, (c) find recombination events that may allow finer mapping of an association due to the shorter linkage disequilibrium in populations of African descent, and (d) provide a “natural mix” of disease loci from both European descent and African descent genomic segments to allow study of the related genes in different combinations.
Table 3.
Summary of Findings
| Gene | SNP in this report | Phenotype | Relationship(s) to previously reported SNPs for POAG |
|---|---|---|---|
| TGFBR3 | rs11466588 | POAG | No LD with prior rs2810903. |
| TMCO1 | rs10529326 | POAG | In LD with prior rs4656461 and prior rs7518099.9 |
| MYOC | rs12129856 | POAG | No association observed with previously published SNPs. SNP is located in adjacent gene, PRRC2C. (Fig. 4) |
| CYP1B1 | rs2432663 | POAG | rs2432663 is located between CYP1B1 and CYP1B1-AS1. |
| FNDC3B | rs199612704 | POAG | No LD with prior rs12897; separated by region of high recombination (Fig. 3A&B). |
| AFAP1 | rs4328916 | POAG | No LD with prior rs4619890.24 |
| 8q22 | rs7009228 | POAG | No LD with previously reported rs1521444, rs284489, or rs284495.14 Closer to LRP12 gene. |
| 9p21 | rs2383204, rs79721419 |
POAG | Two independent signals identified. Signal #1 at rs2383204 is in linkage disequilibrium with previously published SNPS rs1063192 42 and rs4977756.9 Signal #2 at rs79721419 is approximately 150kb away from #1 and separated by a region of high recombination (Fig. 2) |
| ENO4 | rs185815146 | Advanced POAG |
Putative novel locus for AD POAG |
| ABO | rs551169 | POAG | No LD with prior rs8176743; located in ABO promoter.39 |
| PMM2 | rs13332985 | Advanced POAG |
No LD with prior rs3785176 or rs8057024.11 |
| GAS7 | rs8080535 | Advanced POAG |
No LD with prior rs12150284; separated by region of high recombination (Fig. 3C&D).54 |
Note: Linkage disequilibrium (LD) between SNPs was assessed using the “AMR” subset of the 1kGP data. This set will contain genomic segments from African, Amerindian, European ancestry. “Prior” indicates SNPs reported to be genome-wide significant in a genome-wide association study.
Genetic Risk Score
For Genetic Risk Score #1, the sum of the allele counts across the 11 SNPs from the candidate gene study (Tables 1 and 2) resulted in a genetic risk score with an area under the curve (AUC) value of 0.62 (Fig. 4). For Genetic Risk Scores #2 & #3, allele counts across the all of the ~12,900 SNPs with p-values less than 0.001 resulted in a score with an AUC of 0.55 (Fig. 4B, solid black line). The fact that this score is lower than the AUC for score #1 for suggests that noise is being introduced from SNPs in the ~12,900 that do not contribute to the determination of POAG risk. Thus, in order to find the combination of SNPs within the 12,900 that together are associated with greater POAG risk, a regularized matrix decomposition method was applied. Regularization methods are recommended for the type of “big data” problem represented here where the number of features (~12,900 SNPs) is much greater than the number of subjects (1875 POAG cases and 1709 controls).49 The penalty was selected to result in 40-50 SNPs (Genetic Risk Score #2) and 400-500 SNPs (Genetic Risk Score #3) contributing to the risk score. 10-fold cross-validation at each penalty value is used to test the ability to predict POAG. The AUC value for Genetic Risk Score #2 was 0.74 and for #3 was 0.94. We emphasize for this report: the requisite confirmation studies and prospective clinical trials required to design a valid genetic test for AD POAG risk have not been completed and so this genetic risk score is not suitable for clinical prediction at this time. However, the AUC difference between the AUC values of Genetic Risk Score #1 and Genetic Risk Score #2 and #3 clearly indicates that there are additional SNPs that contribute to AD POAG risk even though these did not meet the multiple testing criteria required for either a candidate gene study or a genome-wide study. The identification of additional loci will be possible as meta-analyses are conducted by worldwide collaborations and their overall contribution to POAG risk will be elucidated by the methods used here.
In conclusion, this candidate gene study of POAG and advanced POAG in AD subjects has fulfilled some of its promise for the genetic study of POAG, revealing additional SNPS in already identified loci, narrowing of regions by the different linkage disequilibrium structure of AD possible, and supporting the concept that further loci are waiting to be revealed.
Supplementary Material
Supplementary Figure 1. “Q-Q” plot for POAG.
“Q-Q” plot for POAG association results, separated by minor allele frequency as shown. Plot generated by EMMAX software. λ = 1.017.
Supplementary Figure 2. “Q-Q” plot for advanced POAG.
“Q-Q” plot for advanced POAG association results, separated by minor allele frequency as shown. Plot generated by EMMAX software. λ = 1.022.
Supplementary Figure 3. “Manhattan” plot for POAG.
Manhattan plot for POAG association results. Plot generated using R package “qqman.”
Acknowledgments
Financial Support
National Eye Institute: EY023704, P30EY022589, EY110008, EY019869, EY021818 National Institutes of Health: R01 DK087914, R01 DK066358, R01 DK053591, U01 DK105556, R01 HL56266, R01 DK070941, DRC DK063491, and CTSI UL1TR001881
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
KDT: none
XG: none
LMZ: Carl Zeiss Meditec, Heidelberg Engineering, Optovue Inc.,Topcon Medical System Inc. (financial support)
JML: Alcon, Allergan, Bausch & Lomb, Carl Zeiss Meditec, Heidelberg Engineering, Reichert, Valeant Pharmaceuticals (Consultant); Bausch & Lomb, Carl Zeiss Meditec, Heidelberg Engineering, National Eye Institute, Optovue, Reichert, Topcon
CAG: National Eye Institute, EyeSight Foundation of Alabama, Research to Prevent Blindness, Carl Zeiss Meditec, Heidelberg Engineering, SOLX (financial support)
RMF, HD, YH, BCS, JFP, LAA-A, SCP, CT, JC, RB, PAS, GAC, DB, RR, NPB, LSB, GD: none FAM: Alcon, Allergan, Bauch + Lomb, Carl Zeiss Meditec, Heidelberg Engineering, Merck, Reichert, Sensimed and Topcon (financial support); Alcon, Allergan, Carl Zeiss Meditec, National Eye Institute, and Reichert (research support); Allergan, Carl Zeiss Meditec, Novartis (consultant)
SKD, JD, CDL, NDP, BIF, DWB, MCYN, YDIC, RA, JIR: none
RNW: Aerie Pharma, Allergan, Eyenovia, Unity Bio (Consultant); Bausch & Lomb, Centervue, Heidelberg Engineering, Carl Zeiss Meditec, Genentech, Optovue, Topcon (Financial support)
References
- 1.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol 2009;127(9): 1136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girkin CA, Sample PA, Liebmann JM, et al. African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol 2010;128(5):541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Racette L, Liebmann JM, Girkin CA, et al. African Descent and Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Arch Ophthalmol 2010;128(5):551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khachatryan N, Medeiros FA, Sharpsten L, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): predictors of visual field damage in glaucoma suspects. Am J Ophthalmol 2015;159(4):777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang R, Wiggs JL. Common and rare genetic risk factors for glaucoma. Cold Spring Harb Perspect Med 2014;4(12):a017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allingham RR, Wiggs JL, De La Paz MA, et al. Gln368STOP myocilin mutation in families with late-onset primary open-angle glaucoma. Invest Ophthalmol Vis Sci 1998;39(12):2288–95. [PubMed] [Google Scholar]
- 7.Wiggs JL, Allingham RR, Vollrath D, et al. Prevalence of mutations in TIGR/Myocilin in patients with adult and juvenile primary open-angle glaucoma. Am J Hum Genet 1998;63(5): 1549–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng SK, Casson RJ, Burdon KP, Craig JE. Chromosome 9p21 primary open-angle glaucoma susceptibility locus: a review. Clin Exp Ophthalmol 2014;42(1):25–32. [DOI] [PubMed] [Google Scholar]
- 9.Burdon KP, Macgregor S, Hewitt AW, et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat Genet 2011;43(6):574–8. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Allingham RR, Nakano M, et al. A common variant near TGFBR3 is associated with primary open angle glaucoma. Hum Mol Genet 2015;24(13):3880–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Lin Y, Vithana EN, et al. Common variants near ABCA1 and in PMM2 are associated with primary open-angle glaucoma. Nat Genet 2014;46(10): 1115–9. [DOI] [PubMed] [Google Scholar]
- 12.Takamoto M, Kaburaki T, Mabuchi A, et al. Common variants on chromosome 9p21 are associated with normal tension glaucoma. PLoS One 2012;7(7):e40107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osman W, Low SK, Takahashi A, et al. A genome-wide association study in the Japanese population confirms 9p21 and 14q23 as susceptibility loci for primary open angle glaucoma. Hum Mol Genet 2012;21(12):2836–42. [DOI] [PubMed] [Google Scholar]
- 14.Wiggs JL, Yaspan BL, Hauser MA, et al. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS Genet 2012;8(4):e1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasquale LR, Loomis SJ, Kang JH, et al. CDKN2B-AS1 genotype-glaucoma feature correlations in primary open-angle glaucoma patients from the United States. Am J Ophthalmol 2013;155(2):342–53 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao D, Jiao X, Liu X, et al. CDKN2B polymorphism is associated with primary open-angle glaucoma (POAG) in the Afro-Caribbean population of Barbados, West Indies. PLoS One 2012;7(6):e39278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Hauser MA, Akafo SK, et al. Investigation of known genetic risk factors for primary open angle glaucoma in two populations of African ancestry. Invest Ophthalmol Vis Sci 2013;54(9):6248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diabetes Genetics Initiative of Broad Institute of H, Mit LU, Novartis Institutes of BioMedical R, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007;316(5829):1331–6. [DOI] [PubMed] [Google Scholar]
- 19.Samani NJ, Erdmann J, Hall AS, et al. Genomewide association analysis of coronary artery disease. N Engl J Med 2007;357(5):443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen HH, Almontashiri NA, Antoine D, Stewart AF. Functional genomics of the 9p21.3 locus for atherosclerosis: clarity or confusion? Curr Cardiol Rep 2014;16(7):502. [DOI] [PubMed] [Google Scholar]
- 21.Almontashiri NA, Antoine D, Zhou X, et al. 9p21.3 Coronary Artery Disease Risk Variants Disrupt TEAD Transcription Factor-Dependent Transforming Growth Factor beta Regulation of p16 Expression in Human Aortic Smooth Muscle Cells. Circulation 2015;132(21):1969–78. [DOI] [PubMed] [Google Scholar]
- 22.Bailey JN, Loomis SJ, Kang JH, et al. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat Genet 2016;48(2):189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loomis SJ, Kang JH, Weinreb RN, et al. Association of CAV1/CAV2 genomic variants with primary open-angle glaucoma overall and by gender and pattern of visual field loss. Ophthalmology 2014;121(2):508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gharahkhani P, Burdon KP, Fogarty R, et al. Common variants near ABCA1, AFAP1 and GMDS confer risk of primary open-angle glaucoma. Nat Genet 2014;46(10):1120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carnes MU, Liu YP, Allingham RR, et al. Discovery and functional annotation of SIX6 variants in primary open-angle glaucoma. PLoS Genet 2014;10(5):e1004372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Bailey JC, Helwa I, et al. A Common Variant in MIR182 Is Associated With Primary Open-Angle Glaucoma in the NEIGHBORHOOD Consortium. Invest Ophthalmol Vis Sci 2016;57(10):3974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zangwill LM, Ayyagari R, Liebmann JM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES) III: Contribution of genotype to glaucoma phenotype in African-Americans: Study design and baseline data Ophthalmology; 2018;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunderson KL, Steemers FJ, Lee G, et al. A genome-wide scalable SNP genotyping assay using microarray technology. Nat Genet 2005;37(5):549–54. [DOI] [PubMed] [Google Scholar]
- 29.Gunderson KL, Kuhn KM, Steemers FJ, et al. Whole-genome genotyping of haplotype tag single nucleotide polymorphisms. Pharmacogenomics 2006;7(4):641–8. [DOI] [PubMed] [Google Scholar]
- 30.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res 2009;19(9):1655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander DH, Lange K. Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinformatics 2011;12:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das S, Forer L, Schonherr S, et al. Next-generation genotype imputation service and methods. Nat Genet 2016;48(10): 1284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang HM, Sul JH, Service SK, et al. Variance component model to account for sample structure in genome-wide association studies. Nat Genet 2010;42(4):348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price AL, Zaitlen NA, Reich D, Patterson N. New approaches to population stratification in genome-wide association studies. Nat Rev Genet 2010;11(7):459–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conomos MP, Laurie CA, Stilp AM, et al. Genetic Diversity and Association Studies in US Hispanic/Latino Populations: Applications in the Hispanic Community Health Study/Study of Latinos. Am J Hum Genet 2016;98(1):165–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen H, Wang C, Conomos MP, et al. Control for Population Structure and Relatedness for Binary Traits in Genetic Association Studies via Logistic Mixed Models. Am J Hum Genet 2016;98(4):653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiggs JL, Pasquale LR. Genetics of glaucoma. Hum Mol Genet 2017;26(R1):R21–R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis CJ, Hedberg-Buenz A, DeLuca AP, et al. Primary congenital and developmental glaucomas. Hum Mol Genet 2017;26(R1):R28–R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hysi PG, Cheng CY, Springelkamp H, et al. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat Genet 2014;46(10):1126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook JP, Morris AP. Multi-ethnic genome-wide association study identifies novel locus for type 2 diabetes susceptibility. Eur J Hum Genet 2016;24(8): 1175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Springelkamp H, Iglesias AI, Cuellar-Partida G, et al. ARHGEF12 influences the risk of glaucoma by increasing intraocular pressure. Hum Mol Genet 2015;24(9):2689–99. [DOI] [PubMed] [Google Scholar]
- 42.Ramdas WD, van Koolwijk LM, Lemij HG, et al. Common genetic variants associated with open-angle glaucoma. Hum Mol Genet 2011;20(12):2464–71. [DOI] [PubMed] [Google Scholar]
- 43.Thorleifsson G, Walters GB, Hewitt AW, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet 2010;42(10):906–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haritunians T, Taylor KD, Targan SR, et al. Genetic predictors of medically refractory ulcerative colitis. Inflamm Bowel Dis 2010;16(11):1830–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lubitz SA, Yin X, Lin HJ, et al. Genetic Risk Prediction of Atrial Fibrillation. Circulation 2017;135(14):1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooke Bailey JN, Igo RP. Genetic Risk Scores. Current Protocols in Human Genetics 2016;91(9):1.29.1–1..9. [DOI] [PubMed] [Google Scholar]
- 47.Lange K, Papp JC, Sinsheimer JS, Sobel EM. Next Generation Statistical Genetics: Modeling, Penalization, and Optimization in High-Dimensional Data. Annu Rev Stat Appl 2014;1(1):279–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Power Dudbridge F. and predictive accuracy of polygenic risk scores. PLoS Genet 2013;9(3):e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning : data mining, inference, and prediction, 2nd ed. New York, NY: Springer, 2009; xxii, 745 p. [Google Scholar]
- 50.Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw 2010;33(1): 1–22. [PMC free article] [PubMed] [Google Scholar]
- 51.Verma SS, Cooke Bailey JN, Lucas A, et al. Epistatic Gene-Based Interaction Analyses for Glaucoma in eMERGE and NEIGHBOR Consortium. PLoS Genet 2016;12(9):e1006186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nag A, Lu H, Arno M, et al. Evaluation of the Myocilin Mutation Gln368Stop Demonstrates Reduced Penetrance for Glaucoma in European Populations. Ophthalmology 2017;124(4):547–53. [DOI] [PubMed] [Google Scholar]
- 53.Wagner AH, Anand VN, Wang WH, et al. Exon-level expression profiling of ocular tissues. Exp Eye Res 2013;111:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Koolwijk LM, Ramdas WD, Ikram MK, et al. Common genetic determinants of intraocular pressure and primary open-angle glaucoma. PLoS Genet 2012;8(5):e1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turner SD. qqman: An R package for visualizing GWAS results using Q-Q and manhattan plots. biorXiv, 2014. [Google Scholar]
- 56.Pruim RJ, Welch RP, Sanna S, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 2010;26(18):2336–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. “Q-Q” plot for POAG.
“Q-Q” plot for POAG association results, separated by minor allele frequency as shown. Plot generated by EMMAX software. λ = 1.017.
Supplementary Figure 2. “Q-Q” plot for advanced POAG.
“Q-Q” plot for advanced POAG association results, separated by minor allele frequency as shown. Plot generated by EMMAX software. λ = 1.022.
Supplementary Figure 3. “Manhattan” plot for POAG.
Manhattan plot for POAG association results. Plot generated using R package “qqman.”