Abstract
Introduction:
Arteries, veins, and lymphatic vessels are distinguished by structural differences that correspond to their different functions. Each of these vessels is also defined by specific molecular markers that persist throughout adult life; these markers are some of the molecular determinants that control the differentiation of embryonic undifferentiated cells into arteries, veins, or lymphatics.
Methods:
Review of experimental literature.
Results:
The Eph-B4 receptor and its ligand, ephrin-B2, are critical molecular determinants of vessel identity, arising on endothelial cells early in embryonic development. Eph-B4 and ephrin-B2 continue to be expressed on adult vessels and mark vessel identity. However, following vascular surgery, vessel identity can change and is marked by altered Eph-B4 and ephrin-B2 expression. Vein grafts show loss of venous identity, with less Eph-B4 expression.
Arteriovenous fistulae show gain of dual arterial-venous identity, with both Eph-B4 and ephrin-B2 expression, and manipulation of Eph-B4 improves arteriovenous fistula patency. Patches used to close arteries and veins exhibit context-dependent gain of identity, that is patches in the arterial environment gain arterial identity whereas patches in the venous environment gain venous identity; these results show the importance of the host infiltrating cells in determining vascular identity after vascular surgery.
Conclusions:
Changes in vessel molecular identity correspond to structural changes following vascular surgery that depend on the host post-surgical environment. Regulation of vascular identity and the underlying molecular mechanisms may allow new therapeutic approaches to improve vascular surgical procedures.
Keywords: identity, vasculogenesis, angiogenesis, arteriogenesis, Eph-B4, ephrin-B2
The structural differences that define vessels as arteries, veins, or lymphatics, are well established.1 The molecular identity of arteries, veins, and lymphatics is determined as the circulatory system develops during embryogenesis. Until recently, vessel identity was thought to arise in response to hemodynamic factors generated by the developing heart as it begins to establish pulsatile blood flow; however, molecular determinants of vessel identity are present prior to the first heart beats,2 and therefore molecular markers of vessel identity are now thought to be not just passive markers but determinants of vascular identity.3,4 In mice, venous-specific extracellular receptors, signaling proteins, and transcription factors are found exclusively on venous endothelium beginning as early as embryologic day 9 (E9.0), whereas arterial-specific markers have been described as early as day E8.5.5,6 Lymphatic vessels, which bud from veins, express lymphatic markers by E9.0.7
Vessel identity is established during embryogenesis and the molecular markers are retained on vessels throughout adult life; however, expression of molecular markers changes after some procedures performed by vascular surgeons such as venous bypass, arteriovenous fistula (AVF) creation, or patch angioplasty.8–13 In addition, some vascular pathologies, such as arteriovenous malformations (AVM) or Kaposi’s sarcoma, are also characterized by mutations or aberrant expression of vascular markers of identity.14,15 This review focuses on molecular markers of vessel identity and their importance in human vascular biology and pathology.
Vascular development: A brief overview
Vasculogenesis
During early embryogenesis, blood vessels are derived from mesoderm in the two-step process of vasculogenesis. At approximately embryologic day E6.5 (in mice), the mesoderm differentiates into hemangioblasts that give rise to two different cell populations: hematopoietic precursor cells and endothelial precursor cells.16,17 The endothelial precursor cells then associate with each other to form blood islands that further coalesce into capillary-like vessels as well as the dorsal aorta by E9.0 (Fig 1A).18–21 The critical factor initiating vasculogenesis is vascular endothelial growth factor (VEGF);2 by day E7.0, the VEGF receptor 2 (VEGFR-2) is found exclusively on endothelial cell precursors lining the blood islands.22,23 The main product of vasculogenesis is the primitive capillary plexus that further remodels as the embryo grows.16 Vasculogenesis occurs only during embryogenesis and not during normal adult life. After the primitive capillary network forms during vasculogenesis, vessel differentiation and reorganization into networks of arteries, veins, and lymphatic vessels is necessary for embryonic viability. Further development of the mature circulatory system requires angiogenesis.
Figure 1:
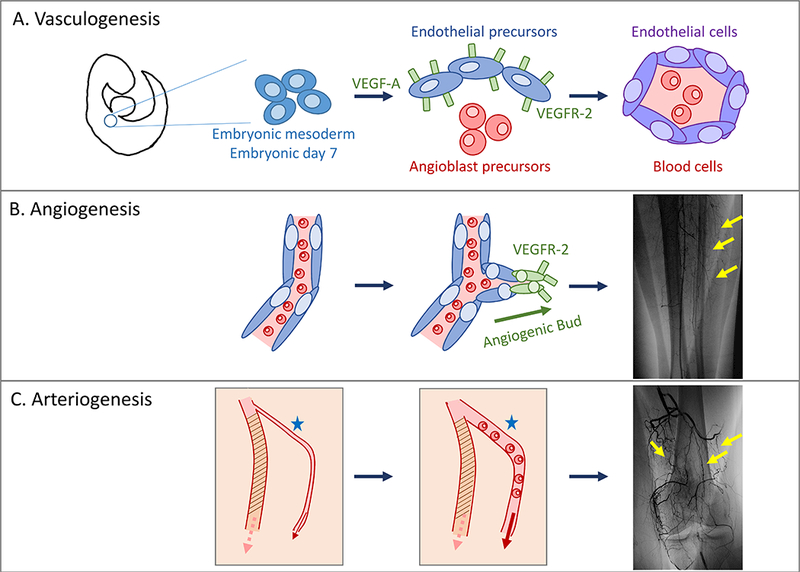
Vasculogenesis, angiogenesis, and arteriogenesis. A. Vasculogenesis is the process by which embryonic mesoderm differentiates into endothelial cell and angioblast precursors to form the primitive capillary plexus. B. Angiogenesis describes the growth of new vessels from preexisting vessels; vessels bud towards angiogenic signals including VEGF. This process occurs during embryogenesis as well as later in life. C. Arteriogenesis is the process of vessel dilation and remodeling. In patients suffering chronic ischemia, arteriogenesis occurs in existing collateral vessels and does not involve the growth of new vessels.
Angiogenesis
As the embryo grows, tissues rapidly increase in volume and outgrow their blood supply. Therefore, the primitive capillary network must remodel and expand to meet oxygen demand using a sprouting process called angiogenesis. Angiogenesis occurs during embryogenesis but, unlike vasculogenesis, angiogenesis continues throughout adult life.24
Sprouting of new vessels occurs in response to signals released from oxygen-deprived tissues. Hypoxia-inducible factor-1α (HIF-1α) induces sprouting of new capillaries from existing vessels (Fig 1B).16 VEGF directly stimulates sprouting by interacting with VEGFR-2 on the cells, stimulating the tip cells, that is the leading sprouting vessel;25,26 tip cells are derived from endothelial cells in existing vessels and grow into the surrounding tissue to form a web-like capillary plexus that is then pruned.27,28,24 The molecular characterization of sprouting is not completely understood. For example, the tip endothelial cells that form both venous- and arterial-derived capillaries may both originate from veins.27 Arterial sprouts are more responsive to VEGF than venous sprouts; however, venous endothelial cells proliferate more rapidly than arterial endothelial cells within the sprouting capillaries.27,28 VEGF also mediates intussusception, that is splitting of a vessel, during angiogenesis, effectively increasing the area of a capillary bed and its oxygen diffusing capacity.29
Arteriogenesis
Arteriogenesis is the dilation and remodeling of pre-existing small arteries or capillaries into collateral vessels capable of supporting increased blood flow.30,31 Arteriogenesis is fundamentally different than angiogenesis and vasculogenesis; whereas vasculogenesis and angiogenesis are the processes by which new vessels are formed, arteriogenesis is the remodeling of existing vessels. During embryogenesis, arteriogenesis requires differentiation of the mesoderm that surrounds existing arteries to participate in the establishment of a larger vessel.32,33 The vessel media is then restructured in a process requiring inflammation;32 smooth muscle cells (SMC) secrete factors such as transforming growth factor-β (TGF-β) and platelet-derived growth factor-B (PDGF-B) to establish a thicker media.32 In adults, arteriogenesis occurs when collateral vessels are recruited to increase blood flow to chronically ischemic tissues (Fig 1C). Like angiogenesis, the process of arteriogenesis occurs throughout adult life.
Vascular identity
Ephs and ephrins
The erythropoietin-producing hepatocellular (Eph) receptor family is the largest family of receptor tyrosine kinases in humans.34 Receptor tyrosine kinases are cell surface molecules that initiate intracellular signaling cascades following stimulation by ligands such as the growth factors VEGF or TGF-β.35 Eph receptors are classified into 2 groups, Eph-A and Eph-B, depending upon the ligands that bind to them; the 5 Eph-B receptors (Eph-B1–4 and 6) are stimulated by Ephrin-B transmembrane ligands, whereas the 9 Eph-A receptors (Eph-A1–8 and 10) are stimulated by Ephrin-A glycosylphosphatidylinositol-anchored ligands.36 The Ephrin ligands and Eph receptors are frequently found within the nervous system, but also serve as the canonical markers of vessel identity.37
Eph-B4 is of particular interest to vascular biology since it is located preferentially on venous endothelium, beginning by day E9.0 in mice (Table I).5,6,38 Additionally, the Eph-B4 receptor only binds a single ligand, ephrin-B2, which is somewhat unusual among Eph receptors that frequently bind multiple ephrin ligands.39 The ligand ephrin-B2 is found preferentially on arterial endothelium (Table I).5,6 It appears slightly earlier than Eph-B4 in embryogenesis, at E8.5 in mice.5 Throughout adult life, ephrin-B2 and Eph-B4 remain segregated to arterial or venous endothelium, respectively.11
Table I:
Molecular markers of vessel identity arise early in embryogenesis.
| Vessel | Marker | Embryologic day* | Function |
|---|---|---|---|
| Vein | BRG1 | 9.0 | Transcriptional Activator |
| COUP-TFII | 9.0 | Transcription Factor | |
| Eph-B4 | 9.0 | Membrane Receptor | |
| Artery | VEGF-R2 | 7.0 | Membrane Receptor |
| Notch | 7.5 | Transcription Factor | |
| Ephrin-B2 | 8.5 | Membrane Receptor | |
| Lymphatic | Sox18 | 9.0 | Transcription Factor |
| Prox1 | 9.5 | Transcription Factor | |
| VEGF-R3 | 8.5 | Membrane Receptor |
All embryologic times are derived from studies in mice and correspond to the embryologic day the marker is first detected.
Activation of Ephrin-Eph signaling is particularly interesting in molecular biology and may be a consequence of Ephrin location on the cell surface rather than being a soluble small molecule. Ephrin-Eph signaling can be bi-directional; in addition to the typical forward signaling through the Eph receptor, there can also be reverse signaling though the Ephrin-B ligand, thus generating signals into two different neighboring cells (Fig 2).40 Generation of signals in two different directions is crucial for vascular development during embryogenesis and is necessary for establishment of both arterial and venous fate.41 Furthermore, Eph-B4 is located in cell surface regions called caveolae, suggesting another function as a sensor for mechanical forces.42 Eph receptors are also activated in clusters, rather than as single receptors;43–45 Eph receptor density can affect its function, with activation in the context of high Eph receptor density inducing cells to move away from each other, while activation in lower density promotes cell adhesion.46
Figure 2:
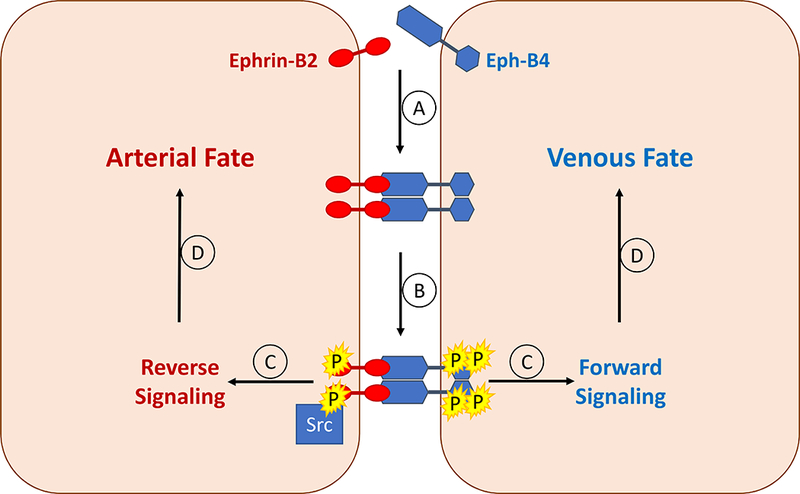
Bidirectional signaling in undifferentiated endothelial cells results in arterial or venous fate during embryonic development. A. Ephrin-B2 binds to Eph-B4 on a neighboring cell, resulting in clustering of receptors. B. The Eph-B4 receptors autophosphorylate each other. Similarly the ephrin-B2 receptors are phosphorylated by the Src kinase. C. Activated Eph-B4 and ephrin-B2 signaling within each cell; in the Eph-B4-containing cell, this results in forward signaling, whereas in the ephrin-B2-containing cell this results in reverse signaling. D. Determination of arterial or venous fate by activated forward (venous) or reverse (arterial) signaling.
Markers of Vascular Identity
Veins
COUP-TFII
The default pathway for vessel identity is venous differentiation: in the absence of a signal, a vessel will become a vein (Fig 3). The chicken ovalbumin UP-transcription factor II (COUP-TFII) is a transcription factor expressed only in venous endothelial cells, beginning at E8.5 in mice.47,48 COUP-TFII induces EphB4 expression in veins.48 COUP-TFII also suppresses the arterial phenotype; knock-out mutants of COUP-TFII express arterial markers on venous endothelium.48 Suppression of arterial markers like ephrin-B2 occurs through direct inhibition of Notch by COUP-TFII.49 COUP-TFII also stimulates expression of the anti-atherogenic gene tissue plasminogen activator, while decreasing expression of pro-atherogenic genes such as plasminogen activator inhibitor type 1, thrombospondin, and PDGF-β.50 Expression of COUP-TFII is regulated by the brahma-related gene 1 (BRG1), which is an ATPase integrated within an ATP-dependent chromatin remodeling complex.51 BRG1 binds to COUP-TFII promoter regions to induce DNA remodeling, allowing for the transcriptional machinery to reach the COUP-TFII gene; however, COUP-TFII expression is likely also regulated by other unknown factors.48
Figure 3:
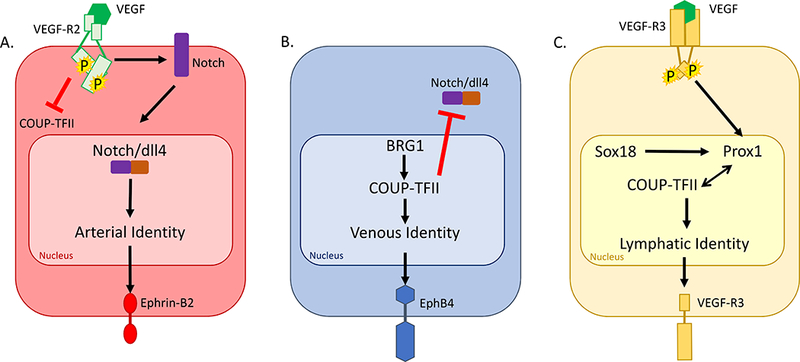
Cell signaling determines the molecular determinants of endothelial cell identity. A. Binding of VEGF to the VEGFR-2 receptor induces Notch activation; the cytoplasmic domain of Notch is cleaved and interacts with dll4, and together they translocate to the nucleus. Activation of arterial gene expression results in Ephrin-B2 expression on the endothelial cell surface. B. Transcription of COUP-TFII is induced in part by BRG1. COUP-TFII is a transcription factor that results in Eph-B4 expression on the endothelial cell surface. C. Binding of VEGF to the VEGFR-3 receptor results in upregulation of Prox1. Prox1 forms a heterodimer with COUP-TFII to induce lymphatic identity with VEGFR-3 expression on the endothelial cell surface.
Eph-B4
Eph-B4 is the principal marker of venous identity. Endothelial cells destined to become veins can be identified as early as day E9.0 by their expression of Eph-B4. Eph-B4 remains on the venous endothelium throughout adult life (Table II).9,52,53 Eph-B4 forward signaling is stimulated by its ligand ephrin-B2, resulting in autophosphorylation of Eph-B4 intracellular tyrosines and recruitment of additional signaling molecules, thereby transmitting a signal into the cell.37,40 Eph-B4 activation increases expression of adhesion molecules such as integrin in cancer cells, allowing invasion of surrounding tissues.54 Since knockdown of Eph-B4 increases expression of myosin VI and matrix metalloproteinase 12 (MMP12),54 and increased plasma levels of MMP12 is associated with increased incidence of large artery atherosclerosis and stroke,55 it is interesting to speculate that Eph-B4 maintenance of venous phenotype may be associated with less atherosclerosis and associated disease progression in veins compared to arteries.
Table II:
Expression of Eph-B4 or ephrin-B2 changes depending on the vascular environment.
| Eph-B4 | Ephrin-B2 | |
|---|---|---|
| Artery | + | +++ |
| Vein | +++ | + |
| Vein graft | + | + |
| Arteriovenous fistula | +++ | +++ |
| Arterial patch | - | +++ |
| Venous patch | +++ | - |
| Venous patch, AVF | +++ | +++ |
Arteries
VEGF-R2
The growth factor VEGF and one of its receptors, VEGFR-2, are crucial for initiation of vasculogenesis, as described above. In addition, VEGF-A, an isoform of VEGF, is also closely linked to determination of arterial identity, since VEGF-A activation of VEGFR-2 increases expression of ephrin-B2 in embryonic stem cells (Fig 3).56 VEGF-A suppresses venous identity; in adult venous endothelial cells, VEGF-A inhibits the expression of Eph-B4 and stimulates dll4 expression without upregulating ephrin-B2.57 Activated VEGFR2 stimulates Notch and also suppresses expression of COUP-TFII.58
Notch
Notch is a highly-conserved family of proteins that are crucial signaling molecules throughout embryogenesis. Vertebrates express 4 Notch receptors (Notch-1–4) and 5 Notch ligands (delta-like ligand (Dll)-1, −3, and −4, and Jagged-1 and −2).59 The Notch receptor is a transmembrane protein that is cleaved after binding a ligand, allowing the Notch receptor intracellular domain to translocate into the nucleus.59 Mutations in Notch receptors, ligands, or target genes result in vascular abnormalities.60 These abnormalities are likely due to failure of proper interactions between endothelial cells and smooth muscle cells within the developing vasculature, since activation of Notch in endothelial cells favors maturation of the vessel over endothelial cell proliferation.61 Notch proteins are crucial to many diverse processes throughout the body, beginning in early embryogenesis and continuing throughout adult life.59 Interestingly, Dll-4, Notch-1, and Notch-4 are solely expressed in arteries, and not in veins.62 Dll4 activation of Notch induces expression of ephrin-B2 while suppressing COUP-TFII-mediated expression of Eph-B4.57,58,63 Localization of SMC to the arterial wall is mediated by endothelial Jagged-1.64 VEGF-dependent angiogenic sprouting occurs via Dll-4,25,65,66 and activation of dll4 and Notch in sprouts stabilizes the sprout to allow it to mature into a stable artery.67
Ephrin-B2
Ephrin-B2 is first expressed in mouse embryos at day E8.5 (Table I). Its expression alone is not sufficient for proper establishment of the circulatory system; rather, bi-directional signaling through both ephrin-B2- and Eph-B4-expressing cells is necessary for proper vascular development. This reciprocal relationship between ephrin-B2 and EphB4 suggests that they might regulate opposite functions in the vasculature.68 Ephrin-B2 mediates the clathrin-mediated endocytosis of VEGF receptors, promoting VEGFR-2 signaling.68 Conversely, Ephrin-B2 antagonizes PDGF endocytosis, promoting maturation of SMCs in arterial walls.69 The balance of these and other signals may affect the stability of the arterial phenotype in the vessel.
Lymphatics
Prox1
Lymphatic-fated endothelial cells originate in the venous endothelium. Venous endothelial cells transition to lymphatic fate as a result of Prox1 expression; Prox1 is a transcription factor that binds with COUP-TFII and thus may alter venous signaling to induce lymphatic identity (Fig 3).70,71 Prox1 is first detected in mice at day E9.5 within the cardinal vein (Table I).72–74 Prox1 itself is regulated by the transcription factor Sox18; at day E9.0 in mice, expression of Sox18 is noted within the endothelial cells of the cardinal vein, and by day E10.5 Sox18-positive cells have migrated to lymphatic sacs.7
VEGF-R3
VEGF receptor 3 (VEGF-R3) is initially located in veins and mesenchyme by day E8.5 (in mice); however, by days E14.5–16.5, VEGF-R3 is segregated exclusively to the lymphatic vessels (Table I).75 VEGF-C is the isoform that preferentially binds VEGF-R3, and it is coexpressed with VEGF-R3 during sprouting of lymphatic vessels in the embryo.76,77 Unlike arteries and veins, the basement membrane of lymphatic vessels must be fenestrated to facilitate fluid absorption in the tissues; as such, the specificity of VEGF-R3 and VEGF-C during lymphangiogenesis may be an important mechanism of lymphatic structure and function.78 Neuropilin-2 promotes lymphangiogenesis by acting as a co-receptor for VEGFR-3 and regulates its signaling.79
LYVE-1
The lymphatic vessel endothelial hyaluronic acid receptor-1 (LYVE-1) is also specific to lymphatic endothelium. Its role in lymphatic vessels is to bind hyaluronic acid and thus promote migration of leukocytes.80 Therefore, in addition to structural changes, the molecular determinants of lymphatic vessels are necessary for proper function of the immune system.
Clinical applications
After vascular surgery, some markers of identity appear to be plastic, that is vessel identity can change in adult vascular cells. For example, venous identity changes after placement of a vein into the arterial or fistula environment (Table II);10,13,81,82 interestingly, these changes in the vein do not occur as originally suggested that vein grafts “arterialize”.83,84 Furthermore, some identity markers are differentially expressed in pathologic conditions such as chronic ischemia or cancer.42 It is currently unknown whether promoting or preventing these changes may improve outcomes of vascular procedures, or might be important targets for pharmacotherapy, in human patients with vascular disease. However, it has been recently shown that human saphenous veins have functional Eph-B4 receptors and that stimulating them prevents neointimal hyperplasia in vitro.11 In addition, stimulation of Eph-B4 increases the patency of AVF in a mouse model.10 These results suggest that regulation of vascular identity may promote patency of vein grafts and AVF in human patients and may be worthy of future clinical trials.
Vein grafts
Veins surgically placed into the arterial environment, such as a saphenous vein graft, lose expression of Eph-B4 (Table II).82 Loss of Eph-B4 appears to require VEGF-A expression and is mediated by upregulation of dll4, a Notch ligand.82,85 However, although dll4 is upregulated, ephrin-B2 does not appear on the endothelium of venous grafts.82 Vein grafts therefore lose their markers of venous identity without acquiring arterial identity, that is vein grafts do not “arterialize” when analyzed on a molecular basis.83,84
While some diameter expansion is beneficial for vein graft patency, excessive wall thickening, that is neointimal hyperplasia, contributes to vein graft stenosis and failure. Interestingly, stimulation of Eph-B4 by administration of its activated ligand ephrin-B2 results in continued Eph-B4 expression and prevents neointimal hyperplasia, suggesting a new strategy to promote vein graft patency.11,53 Stimulation of Eph-B4 also promotes phosphorylation of endothelial NO synthase (eNOS), allowing venous dilation.52 However, dll4 activation may induce inflammation and smooth muscle cell proliferation,85 emphasizing the importance of understanding how Eph-B4 functions within the vein graft.
Arteriovenous Fistula
Autogenous AVF are currently the preferred method of hemodialysis access, but have poor patency and low maturation.86,87 In AVF, the venous limb gains expression of ephrin-B2 (Table II), as well as ECM proteins such as collagen and fibronectin, and these proteins result in outward remodeling of the vein wall.88 Poor maturation may be due to inadequate outward remodeling and/or unregulated wall thickening within the vein.89,90 After surgical creation of an AVF, the venous limb of the AVF gains expression of ephrin-B2 and retains expression of Eph-B4.10 Thus AVF gain dual arterial-venous identity within the fistula environment, a distinctly different identity than the vein graft. However, treatment of the AVF with Ephrin-B2 does prevent excessive wall thickening and promotes AVF patency, and suggests that treatment of human AVF with agents that promote retention of venous identity may improve AVF patency.10
Patch angioplasty
Pericardial patches are frequently used in vascular surgery to close both arteries and veins. Synthetic patches are necessarily acellular, and patches derived from biological tissues are typically treated to remove cellular antigens and reduce antigenicity;91 therefore, prior to implantation, patches do not have vascular identity. However, after surgical implantation there is an influx of host cells that contribute to neointima and healing.13,92 Interestingly, patches express the arterial marker Ephrin-B2 in arterial environments, whereas patches express the venous marker Eph-B4 in venous environments (Table II),13,81 that is, pericardial patches gain the identity of the vessel in which they are placed (“context-dependent gain of identity”). Venous patches additionally exposed to arterial flow also gain arterial identity, that is they gain dual arterial-venous identity, similar to arteriovenous fistulae (Table II).10,12 These changes in identity occur in synthetic patches, suggesting that host cells infiltrating into the vascular patch determine vessel identity in these models.12 It is interesting to speculate that manipulation of vascular patch identity may be possible, and if so, this may be a strategy to inhibit local development of neointimal hyperplasia.
Revascularization of ischemic tissues
The molecular determinants of vascular identity also regulate angiogenesis, and thus have been assessed for therapeutic angiogenesis for patients with peripheral arterial disease.93 Despite initial optimism, larger double-blind trials showed no significant clinical benefit.94,95 The RAVE trial used a replication-deficient adenovirus to deliver a VEGF transgene by injection into the thigh at both low- and high-doses. After 12 weeks, however, there were no significant differences in peak walking time, the main endpoint, between placebo, low dose, and high dose groups.96 The Genzyme-funded HIF-1α trial delivered the HIF-1α gene to patients with claudication or control patients but detected no significant differences in walking time, claudication onset time, or quality of life.97 The TALISMAN 201 trial used a plasmid to deliver fibroblast growth factor to patients with ulcers not suitable for revascularization but showed no effect on ulcer healing.98 However, methods to upregulate expression of endogenous VEGF-A by giving patients activators of transcription are under investigation.99 Other therapeutic approaches that may involve vascular identity include promoting arteriogenesis or delivery of stem cell therapy.100,101
Summary
Protein markers specific to veins, arteries, or lymphatics define vessel identity throughout life. During embryogenesis these markers determine the differential development of these vessels; veins are determined by expression of COUP-TFII, arteries by VEGFR-2, Notch, and dll4, and lymphatics by Prox1. Expression of vascular identity persists in adult vessels; veins predominantly express EphB4, and arteries predominantly express ephrin-B2. These markers are altered after some vascular surgical procedures, suggesting potential therapeutic targets that could promote procedural patency and prevent patient morbidity.
Acknowledgements
This work was supported by the National Institutes of Health R01-HL128406 [A.D.], the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development Program Merit Review Award I01-BX002336 [A.D.], a Sarnoff Cardiovascular Foundation Fellowship [K.W.], a Society for Vascular Surgery Student Research Fellowship [K.W.], as well as with the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT.
We appreciate the generous help and support of Bogdan Yatsula, Hualong Bai, Tun Wang, Shirley Liu, Shun Ono, Ocean Setia, Lara Lopes, and Xiangjiang Guo.
Footnotes
Author contributions
Conception and design: KW, HH, TI, AD
Analysis and interpretation: KW, HH, TI, AD
Data collection: KW, HH, TI
Writing the article: KW, AD
Critical revision of the article: KW, HH, TI, AD
Final approval of the article: KW, HH, TI, AD
Agreement to be accountable: KW, HH, TI, AD
Obtained funding: AD, KW
Overall responsibility: AD
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.delà Paz NG, D’Amore PA. Arterial versus venous endothelial cells. Cell Tissue Res. 2009;335(1):5–16. doi: 10.1007/s00441-008-0706-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438(7070):937–945. doi: 10.1038/nature04479 [DOI] [PubMed] [Google Scholar]
- 3.Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95(5):1671–1679. [PubMed] [Google Scholar]
- 4.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–693. doi: 10.1038/nm0603-685 [DOI] [PubMed] [Google Scholar]
- 5.Wang HU, Chen Z-F, Anderson DJ. Molecular Distinction and Angiogenic Interaction between Embryonic Arteries and Veins Revealed by ephrin-B2 and Its Receptor Eph-B4. Cell. 1998;93(5):741–753. doi: 10.1016/S0092-8674(00)81436-1 [DOI] [PubMed] [Google Scholar]
- 6.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale N, Deutsch U, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13(3):295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.François M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456(7222):643–647. doi: 10.1038/nature07391 [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Collins MJ, Foster TR, Bai H, Hashimoto T, Santana J, et al. Eph-B4 mediates vein graft adaptation by regulation of endothelial nitric oxide synthase. J Vasc Surg. 2017;65(1):179–189. doi: 10.1016/j.jvs.2015.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Model LS, Hall MR, Wong DJ, Muto A, Kondo Y, Ziegler K, et al. Arterial shear stress reduces eph-b4 expression in adult human veins. Yale J Biol Med. 2014;87(3):359–371. [PMC free article] [PubMed] [Google Scholar]
- 10.Protack CD, Foster TR, Hashimoto T, Yamamoto K, Lee M, Kraehling J, et al. Eph-B4 regulates adaptive venous remodeling to improve arteriovenous fistula patency. Sci Rep. 2017;7(1):15386. doi: 10.1038/s41598-017-13071-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong DJ, Lu DY, Protack CD, Kuwahara G, Bai H, Sadaghianloo N, et al. Ephrin type-B receptor 4 activation reduces neointimal hyperplasia in human saphenous vein in vitro. J Vasc Surg. 2016;63(3):795–804. doi: 10.1016/j.jvs.2014.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai H, Hu H, Guo J, Ige M, Wang T, Isaji T, et al. Polyester vascular patches acquire arterial or venous identity depending on their environment. J Biomed Mater Res A. September 2017. doi: 10.1002/jbm.a.36193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Jadlowiec C, Guo Y, Protack C, Ziegler K, Lv W, et al. Pericardial Patch Angioplasty Heals via an Ephrin-B2 and CD34 Positive Cell Mediated Mechanism. PLoS ONE. 2012;7(6). doi: 10.1371/journal.pone.0038844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wetzel-Strong SE, Detter MR, Marchuk DA. The pathobiology of vascular malformations: insights from human and model organism genetics. J Pathol. 2017;241(2):281–293. doi: 10.1002/path.4844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masood R, Xia G, Smith DL, Scalia P, Still J, Tulpule A, et al. Ephrin B2 expression in Kaposi sarcoma is induced by human herpesvirus type 8: phenotype switch from venous to arterial endothelium. Blood. 2005;105(3):1310–1318. doi: 10.1182/blood-2004-03-0933 [DOI] [PubMed] [Google Scholar]
- 16.Patel-Hett S, D’Amore PA. Signal Transduction in Vasculogenesis and Developmental Angiogenesis. Int J Dev Biol. 2011;55(0):353–363. doi: 10.1387/ijdb.103213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson AJ, Zon LI. Turning mesoderm into blood: The formation of hematopoietic stem cells during embryogenesis. Curr Top Dev Biol. 2000;50:45–60. doi: 10.1016/S0070-2153(00)50003-9 [DOI] [PubMed] [Google Scholar]
- 18.Arboleda-Velasquez J, D’Amore P. Chapter 10: Vasculogenesis and Angiogenesis In: Cellular and Molecular Pathobiology of Cardiovascular Disease. [Google Scholar]
- 19.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445 [DOI] [PubMed] [Google Scholar]
- 20.Lindskog H, Kim YH, Jelin EB, Kong Y, Guevara-Gallardo S, Kim T, et al. Molecular identification of venous progenitors in the dorsal aorta reveals an aortic origin for the cardinal vein in mammals. Dev Camb Engl. 2014; 141(5):1120–1128. doi: 10.1242/dev.101808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pardanaud L, Dieterlen-Lievre F. Emergence of endothelial and hemopoietic cells in the avian embryo. Anat Embryol (Berl). 1993;187(2):107–114. [DOI] [PubMed] [Google Scholar]
- 22.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu X, Breitman M, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376(6535):62–66. doi: 10.1038/376062a0 [DOI] [PubMed] [Google Scholar]
- 23.Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17(10):611–625. doi: 10.1038/nrm.2016.87 [DOI] [PubMed] [Google Scholar]
- 24.DiPietro LA. Angiogenesis and wound repair: when enough is enough. J Leukoc Biol. 2016;100(5):979–984. doi: 10.1189/jlb.4MR0316-102R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–1177. doi: 10.1083/jcb.200302047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22(5):617–625. doi: 10.1016/j.ceb.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 27.Xu C, Hasan SS, Schmidt I, Rocha S, Pitulescu M, Bussmann J, et al. Arteries are formed by vein-derived endothelial tip cells. Nat Commun. 2014;5:5758. doi: 10.1038/ncomms6758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blebea J, Vu J- H, Assadnia S, McLaughlin PJ, Atnip RG, Zagon IS. Differential effects of vascular growth factors on arterial and venous angiogenesis. J Vasc Surg. 2002;35(3):532–538. doi: 10.1067/mva.2002.120042 [DOI] [PubMed] [Google Scholar]
- 29.Gianni-Barrera R, Bartolomeo M, Vollmar B, Djonov V, Banfi A. Split for the cure: VEGF, PDGF-BB and intussusception in therapeutic angiogenesis. Biochem Soc Trans. 2014;42(6):1637–1642. doi: 10.1042/BST20140234 [DOI] [PubMed] [Google Scholar]
- 30.Grundmann S, Piek JJ, Pasterkamp G, Hoefer IE. Arteriogenesis: basic mechanisms and therapeutic stimulation. Eur J Clin Invest. 2007;37(10):755–766. doi: 10.1111/j.1365-2362.2007.01861.x [DOI] [PubMed] [Google Scholar]
- 31.Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirc N Y N 1994. 2003;10(1):83–97. doi: 10.1038/sj.mn.7800173 [DOI] [PubMed] [Google Scholar]
- 32.Carmeliet P Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389. [DOI] [PubMed] [Google Scholar]
- 33.Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32(4):687–698. doi: 10.1016/S0008-6363(96)00063-6 [DOI] [PubMed] [Google Scholar]
- 34.Bennett BD, Zeigler FC, Gu Q, Fendly B, Goddard AD, Gillet N, et al. Molecular cloning of a ligand for the EPH-related receptor protein-tyrosine kinase Htk. Proc Natl Acad Sci U S A. 1995;92(6):1866–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlessinger Bae* JH and J. Asymmetric Tyrosine Kinase Arrangements in Activation or Autophosphorylation of Receptor Tyrosine Kinases. Mol Cells. 2010;29(5):443–448. doi: 10.1007/s10059-010-0080-5 [DOI] [PubMed] [Google Scholar]
- 36.Himanen JP. Ectodomain structures of Eph receptors. Semin Cell Dev Biol. 2012;23(1):35– 42. doi: 10.1016/j.semcdb.2011.10.025 [DOI] [PubMed] [Google Scholar]
- 37.Lisabeth EM, Falivelli G, Pasquale EB. Eph Receptor Signaling and Ephrins. Cold Spring Harb Perspect Biol. 2013;5(9). doi: 10.1101/cshperspect.a009159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4(3):403–414. [DOI] [PubMed] [Google Scholar]
- 39.Dai D, Huang Q, Nussinov R, Ma B. Promiscuous and specific recognition among Ephrins and Eph receptors. Biochim Biophys Acta. 2014;1844(10):1729–1740. doi: 10.1016/j.bbapap.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murai KK, Pasquale EB. ‘Eph9ective signaling: forward, reverse and crosstalk. J Cell Sci. 2003;116(14):2823–2832. doi: 10.1242/jcs.00625 [DOI] [PubMed] [Google Scholar]
- 41.Pasquale EB. Eph-Ephrin Bidirectional Signaling in Physiology and Disease. Cell. 2008;133(1):38–52. doi: 10.1016/j.cell.2008.03.011 [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto T, Tsuneki M, Foster TR, Santana JM, Bai H, Wang M, et al. Membrane-mediated regulation of vascular identity. Birth Defects Res Part C Embryo Today Rev. 2016;108(1):65–84. doi: 10.1002/bdrc.21123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, et al. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266(5186):816–819. [DOI] [PubMed] [Google Scholar]
- 44.Wimmer-Kleikamp SH, Janes PW, Squire A, Bastiaens PIH, Lackmann M. Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J Cell Biol. 2004;164(5):661–666. doi: 10.1083/jcb.200312001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nikolov DB, Xu K, Himanen JP. Eph/ephrin recognition and the role of Eph/ephrin clusters in signaling initiation. Biochim Biophys Acta. 2013;1834(10):2160–2165. doi: 10.1016/j.bbapap.2013.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poliakov A, Cotrina ML, Pasini A, Wilkinson DG. Regulation of EphB2 activation and cell repulsion by feedback control of the MAPK pathway. J Cell Biol. 2008;183(5):933–947. doi: 10.1083/jcb.200807151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13(8):1037–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.You L-R, Lin F-J, Lee CT, DeMayo FJ, Tsai M-J, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435(7038):98–104. doi: 10.1038/nature03511 [DOI] [PubMed] [Google Scholar]
- 49.Chen X, Qin J, Cheng C-M, Tsai M-J, Tsai SY. COUP-TFII Is a Major Regulator of Cell Cycle and Notch Signaling Pathways. Mol Endocrinol. 2012;26(8):1268–1277. doi: 10.1210/me.2011-1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui X, Lu YW, Lee V, Kim D, Dorsey T, Wang Q, et al. Venous Endothelial Marker COUP-TFII Regulates the Distinct Pathologic Potentials of Adult Arteries and Veins. Sci Rep. 2015;5:srep16193. doi: 10.1038/srep16193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davis RB, Curtis CD, Griffin CT. BRG1 promotes COUP-TFII expression and venous specification during embryonic vascular development. Dev Camb Engl. 2013;140(6): 1272–1281. doi: 10.1242/dev.087379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Miura N, Bonelli A, Mole P, Carlesso N, Olson D, et al. Receptor tyrosine kinase, EphB4 (HTK), accelerates differentiation of select human hematopoietic cells. Blood. 2002;99(8):2740–2747. doi: 10.1182/blood.V99.8.2740 [DOI] [PubMed] [Google Scholar]
- 53.Muto A, Yi T, Harrison KD, Davalos A, Fancher T, Ziegler K, et al. Eph-B4 prevents venous adaptive remodeling in the adult arterial environment. J Exp Med. 2011;208(3):561–575. doi: 10.1084/jem.20101854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mertens-Walker I, Fernandini BC, Maharaj MS, Rockstroh A, Nelson C, Herington A, et al. The tumour-promoting receptor tyrosine kinase, EphB4, regulates expression of Integrin-β8 in prostate cancer cells. BMC Cancer. 2015;15. doi: 10.1186/s12885-015-1164-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahdessian H, Perisic Matic L, Lengquist M, Gertow K, Sennblad B, Baldassarre D, et al. Integrative studies implicate matrix metalloproteinase-12 as a culprit gene for large-artery atherosclerotic stroke. J Intern Med. July 2017. doi: 10.1111/joim.12655 [DOI] [PubMed] [Google Scholar]
- 56.Masumura T, Yamamoto K, Shimizu N, Obi S, Ando J. Shear stress increases expression of the arterial endothelial marker ephrinB2 in murine ES cells via the VEGF-Notch signaling pathways. Arterioscler Thromb Vasc Biol. 2009;29(12):2125–2131. doi: 10.1161/ATVBAHA.109.193185 [DOI] [PubMed] [Google Scholar]
- 57.Yang C, Guo Y, Jadlowiec CC, Li X, Lv W, Model L, et al. Vascular endothelial growth factor-A inhibits EphB4 and stimulates delta-like ligand 4 expression in adult endothelial cells. J Surg Res. 2013;183(1):478–486. doi: 10.1016/j.jss.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang J, Yoo J, Lee S, Tang W, Aguilar B, Ramu S, et al. An exquisite cross-control mechanism among endothelial cell fate regulators directs the plasticity and heterogeneity of lymphatic endothelial cells. Blood. 2010;116(1):140–150. doi: 10.1182/blood-2009-11-252270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siebel C, Lendahl U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol Rev. 2017;97(4):1235–1294. doi: 10.1152/physrev.00005.2017 [DOI] [PubMed] [Google Scholar]
- 60.Iso T, Hamamori Y, Kedes L. Notch Signaling in Vascular Development. Arterioscler Thromb Vasc Biol. 2003;23(4):543–553. doi: 10.1161/01.ATV.0000060892.81529.8F [DOI] [PubMed] [Google Scholar]
- 61.Tian D-Y, Jin X-R, Zeng X, Wang Y. Notch Signaling in Endothelial Cells: Is It the Therapeutic Target for Vascular Neointimal Hyperplasia? Int J Mol Sci. 2017;18(8). doi: 10.3390/ijms18081615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev. 2001;108(1):161–164. doi: 10.1016/S0925-4773(01)00469-5 [DOI] [PubMed] [Google Scholar]
- 63.Swift MR, Pham VN, Castranova D, Bell K, Poole RJ, Weinstein BM. SoxF factors and Notch regulate nr2f2 gene expression during venous differentiation in zebrafish. Dev Biol. 2014;390(2): 116–125. doi: 10.1016/j.ydbio.2014.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scheppke L, Murphy EA, Zarpellon A, Hofmann J, Merkulova A, Shields D, et al. Notch promotes vascular maturation by inducing integrin-mediated smooth muscle cell adhesion to the endothelial basement membrane. Blood. 2012;119(9):2149–2158. doi: 10.1182/blood-2011-04-348706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104(9):3219–3224. doi: 10.1073/pnas.0611206104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suchting S, Freitas C, Noble F le, Benedito R, Breant C, Duarte A, et al. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci. 2007;104(9):3225–3230. doi: 10.1073/pnas.0611177104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geudens I, Gerhardt H. Coordinating cell behaviour during blood vessel formation. Development. 2011;138(21):4569–4583. doi: 10.1242/dev.062323 [DOI] [PubMed] [Google Scholar]
- 68.Pitulescu ME, Adams RH. Regulation of signaling interactions and receptor endocytosis in growing blood vessels. Cell Adhes Migr. 2014;8(4):366–377. doi: 10.4161/19336918.2014.970010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakayama A, Nakayama M, Turner CJ, Hoing S, Lepore JJ, Adams RH. Ephrin-B2 controls PDGFRβ internalization and signaling. Genes Dev. 2013;27(23):2576–2589. doi: 10.1101/gad.224089.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aranguren XL, Beerens M, Coppiello G, Wiese C, Vandersmissen I, Nigro A, et al. COUP-TFII orchestrates venous and lymphatic endothelial identity by homo- or hetero-dimerisation with PROX1. J Cell Sci. 2013;126(5): 1164–1175. doi: 10.1242/jcs.116293 [DOI] [PubMed] [Google Scholar]
- 71.Lee S, Kang J, Yoo J, Ganesan SK, Cook SC, Aguilar B, et al. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood. 2009;113(8): 1856–1859. doi: 10.1182/blood-2008-03-145789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Srinivasan RS, Dillard ME, Lagutin OV, Lin F, Tsai S, Tsai M, et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21(19):2422–2432. doi: 10.1101/gad.1588407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang Y, Garcia-Verdugo JM, Soriano-Navarro M, Srinivasan RS, Scallan JP, Sing MK, et al. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood. 2012;120(11):2340–2348. doi: 10.1182/blood-2012-05-428607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wigle JT, Oliver G. Prox1 Function Is Required for the Development of the Murine Lymphatic System. Cell. 1999;98(6):769–778. doi: 10.1016/S0092-8674(00)81511-1 [DOI] [PubMed] [Google Scholar]
- 75.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92(8):3566–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282(5390):946–949. [DOI] [PubMed] [Google Scholar]
- 77.Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Dev Camb Engl. 1996;122(12):3829–3837. [DOI] [PubMed] [Google Scholar]
- 78.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1(3):219–227. doi: 10.1016/S1535-6108(02)00051-X [DOI] [PubMed] [Google Scholar]
- 79.Deng Y, Zhang X, Simons M. Molecular controls of lymphatic VEGFR3 signaling. Arterioscler Thromb Vasc Biol. 2015;35(2):421–429. doi: 10.1161/ATVBAHA.114.304881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jackson DG, Prevo R, Clasper S, Banerji S. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22(6):317–321. doi: 10.1016/S1471-4906(01)01936-6 [DOI] [PubMed] [Google Scholar]
- 81.Bai H, Wang M, Foster TR, Hu H, He H, Hashimoto T, et al. Pericardial patch venoplasty heals via attraction of venous progenitor cells. Physiol Rep. 2016;4(12). doi: 10.14814/phy2.12841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kudo FA, Muto A, Maloney SP, Pimiento JM, Bergaya S, Fitzgerald TN, et al. Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler Thromb Vasc Biol. 2007;27(7):1562–1571. doi: 10.1161/ATVBAHA.107.143032 [DOI] [PubMed] [Google Scholar]
- 83.Kunlin J Long vein transplantation in treatment of ischemia caused by arteritis. Rev Chir. 1951;70(7–8):206–235. [PubMed] [Google Scholar]
- 84.May A, DeWeese J, Rob C. Arterialized in Situ Saphenous Vein. Arch Surg. 1965;91:743–750. [DOI] [PubMed] [Google Scholar]
- 85.Koga J-I, Nakano T, Dahlman JE, Figueiredo J, Zhang H, Decano J, et al. Macrophage Notch Ligand Delta-Like 4 Promotes Vein Graft Lesion Development: Implications for the Treatment of Vein Graft Failure. Arterioscler Thromb Vasc Biol. 2015;35(11):2343–2353. doi: 10.1161/ATVBAHA.115.305516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Santoro D, Benedetto F, Mondello P, Pipito N, Barilla D, Spinelli F, et al. Vascular access for hemodialysis: current perspectives. Int J Nephrol Renov Dis. 2014;7:281–294. doi: 10.2147/IJNRD.S46643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA. 2008;299(18):2164–2171. doi: 10.1001/jama.299.18.2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hall MR, Yamamoto K, Protack CD, Tsuneki M, Kuwahara G, Assi R, et al. Temporal regulation of venous extracellular matrix components during arteriovenous fistula maturation. J Vasc Access. 2015;16(2):93–106. doi: 10.5301/jva.5000290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu DY, Chen EY, Wong DJ, Yamamoto K, Protack CD, Williams WT, et al. Vein graft adaptation and fistula maturation in the arterial environment. J Surg Res. 2014;188(1):162– 173. doi: 10.1016/j.jss.2014.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hu H, Patel S, Hanisch JJ, Santana JM, Hashimoto T, Bai H, et al. Future research directions to improve fistula maturation and reduce access failure. Semin Vasc Surg. 2016;29(4):153–171. doi: 10.1053/j.semvascsurg.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Umashankar PR, Arun T, Kumari TV. Short duration gluteraldehyde cross linking of decellularized bovine pericardium improves biological response. J Biomed Mater Res A. 2011;97(3):311–320. doi: 10.1002/jbm.a.33061 [DOI] [PubMed] [Google Scholar]
- 92.Yen Chang, Huang-Chien Liang, Hao-Ji Wei, Chih-Ping Chu, Hsing-Wen Sung. Tissue regeneration patterns in acellular bovine pericardia implanted in a canine model as a vascular patch. J Biomed Mater Res A. 2004;69A(2):323–333. doi: 10.1002/jbm.a.30003 [DOI] [PubMed] [Google Scholar]
- 93.Collinson DJ, Donnelly R. Therapeutic Angiogenesis in Peripheral Arterial Disease: Can Biotechnology Produce an Effective Collateral Circulation? Eur J Vasc Endovasc Surg. 2004;28(1):9–23. doi: 10.1016/j.ejvs.2004.03.021 [DOI] [PubMed] [Google Scholar]
- 94.Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet Lond Engl. 1996;348(9024):370–374. [DOI] [PubMed] [Google Scholar]
- 95.Kusumanto YH, van Weel V, Mulder NH, Smit AJ, van den Dungen JJ, Hooymans JM, et al. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: a double-blind randomized trial. Hum Gene Ther. 2006;17(6):683–691. doi: 10.1089/hum.2006.17.683 [DOI] [PubMed] [Google Scholar]
- 96.Rajagopalan S, Mohler ER, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108(16):1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29 [DOI] [PubMed] [Google Scholar]
- 97.Creager MA, Olin JW, Belch JJF, Moneta GL, Henry TD, Rajagopalan S, et al. Effect of hypoxia-inducible factor-1alpha gene therapy on walking performance in patients with intermittent claudication. Circulation. 2011;124(16):1765–1773. doi: 10.1161/CIRCULATIONAHA.110.009407 [DOI] [PubMed] [Google Scholar]
- 98.Nikol S, Baumgartner I, Van Belle E, Diehm C, Visona A, Capogrossi MC, et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol Ther J Am Soc Gene Ther. 2008;16(5):972–978. doi: 10.1038/mt.2008.33 [DOI] [PubMed] [Google Scholar]
- 99.Giacca M, Zacchigna S. VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond. Gene Ther. 2012;19(6):622–629. doi: 10.1038/gt.2012.17 [DOI] [PubMed] [Google Scholar]
- 100.Guo J, Guo L, Cui S, Tong Z, Dardik A, Gu Y. Autologous bone marrow-derived mononuclear cell therapy in Chinese patients with critical limb ischemia due to thromboangiitis obliterans: 10-year results. Stem Cell Res Ther. 2018;9(1):43. doi: 10.1186/s13287-018-0784-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liang TW, Jester A, Motaganahalli RL, Wilson MG, G’Sell P, Akingba GA, et al. Autologous bone marrow mononuclear cell therapy for critical limb ischemia is effective and durable. J Vasc Surg. 2016;63(6):1541–1545. doi: 10.1016/j.jvs.2016.01.022 [DOI] [PubMed] [Google Scholar]


