Abstract
Rationale and Objective:
Cardiovascular disease is common and overall graft survival suboptimal among kidney transplant recipients. While albuminuria is a known risk factor for adverse outcomes among persons with native chronic kidney disease, the relationship of albuminuria with cardiovascular and kidney outcomes in transplant recipients is uncertain.
Study Design:
Post-hoc, longitudinal cohort analysis of the Folic Acid for Vascular Outcomes Reduction in Transplantation (FAVORIT) Trial
Setting and Participants:
Stable kidney transplant recipients with elevated homocysteine level from 30 sites in the United States, Canada and Brazil
Predictor:
Urine albumin-creatinine ratio (ACR) at randomization.
Outcomes:
Allograft failure, cardiovascular disease (CVD), and all-cause death.
Analytical Approach:
Multivariable Cox models adjusted for age, sex, race, randomized treatment allocation, country, systolic and diastolic blood pressure, history of CVD, diabetes and hypertension, smoking, cholesterol, body mass index, estimated glomerular filtration rate (eGFR), donor type, transplant vintage, medications, and immunosuppression.
Results:
Among 3,511 participants with complete data, median ACR was 24 (Q1-Q3, 9–98) mg/g, mean eGFR 49 ± 18 (standard deviation) ml/min/1.73m2, mean age 52 ± 9 years, and median graft vintage 4.1 (Q1-Q3, 1.7–7.4) years. There were 1017 (29%) with ACR <10 mg/g, 912 (26%) with 10–29 mg/g, 1,134 (32%) with 30–299 mg/g, and 448 (13%) with ACR ≥300 mg/g. Over approximately 4 years, 282 allograft failure events, 497 CVD events, and 407 deaths occurred. Event rates were higher at both lower eGFR and higher ACR. ACR of 30–299 and ≥300 mg/g relative to ACR <10 mg/g were independently associated with graft failure [HRs of 3.40 (95% CI, 2.19–5.30) and 9.96 (95% CI, 6.35–15.62), respectively], CVD events [HRs of 1.25 (95% CI, 0.96–1.61) and 1.55 (95% CI, 1.13–2.11), respectively], and all-cause death [HRs of 1.65 (95% CI, 1.23–2.21) and 2.07 (95% CI, 1.46–2.94), respectively].
Limitations:
No data on rejection; single ACR assessment
Conclusion:
In a large population of stable kidney transplant recipients, elevated baseline ACR is independently associated with allograft failure, CVD, and death. Future studies are needed to evaluate whether reducing albuminuria improves these outcomes.
Keywords: albuminuria, kidney failure, allograft failure, death, cardiovascular disease (CVD), kidney transplant outcomes, urinary albumin-creatinine ratio (UACR), graft survival, renal transplantation, end-stage renal disease (ESRD), protein excretion, biomarker
Introduction
Despite substantial decreases in rates of acute rejection of kidney transplants, improvements in long term graft survival have not kept pace (1, 2). Accordingly, improving patients’ long term outcomes by preventing late allograft loss and adverse patient events remains a clinical priority (3). While immunologic factors are important, patient clinical factors may also be associated with adverse patient and graft outcomes (4). Specifically, the association of allograft function measures, including albuminuria, with transplant recipient outcomes including cardiovascular disease, mortality, and kidney outcomes, remains less certain.
In diseases of the native kidneys, lower estimated glomerular filtration rate (GFR) and greater albuminuria are risk factors for subsequent kidney failure, cardiovascular disease, and death (5, 6). Additional risk factors that associated with primary kidney failure include cardiovascular disease, diabetes, hypertension, obesity, hypercholesterolemia, and smoking (7), many of which also are associated with albuminuria (8). Albuminuria in kidney transplant recipients (KTRs) may reflect different pathogenesis than in patients with native kidney disease. First, protein excretion from the native kidneys may muddle the prognostic significance of ACR (9). Second, although even low levels of albuminuria are a strong risk factor in native CKD as albuminuria likely reflects systemic vascular disease (10), the transplanted kidney has not been exposed to a life-time of risk factors of the host, such as diabetes and hypertension, suggesting either more rapid kidney injury associated with these risk factors than is typical, recurrence of a primary kidney disease, or use of medications such as mammalian target of rapamycin (mTOR) inhibitors that may specifically predispose to proteinuria (11–13). Third, both albuminuria and lower GFR in KTRs may reflect both donor and immunologic factors. Many of these risk factors are potentially modifiable; accordingly, a better understanding of the association among albuminuria, modifiable risk factors and kidney and patient outcomes in KTRs could suggest treatment targets for future clinical trials to prevent cardiovascular disease, death and late allograft loss (11).
The Folic Acid for Vascular Outcomes Reduction in Transplantation (FAVORIT) Trial was a large randomized controlled trial designed to test whether high dose folic and vitamins B6 and B12 reduced CVD events in over 4000 kidney transplant recipients, with systematic and detailed ascertainment of CVD risk factors, CVD events, and kidney disease events (14–16). In prior work, we showed that an estimated GFR below 45 ml/min/1.73m2 was associated with an increased risk of cardiovascular disease, suggesting that reduced kidney function itself rather than preexisting comorbidity may lead to CVD (17). Additionally, we have shown associations between markers of kidney tubular injury (18) and of fibrosis (19) with cardiovascular outcomes, suggesting that systemic risk can also be captured by markers of kidney damage from the allograft. Thus, whether or not albuminuria in KTRs captures systemic kidney, cardiovascular and mortality risk is of particular interest, especially considering that albuminuria is more easily and commonly measured than many other biomarkers.
Methods
This is a post hoc analysis of data from the FAVORIT trial (NCT00064753), a multicenter double-blind randomized controlled clinical trial conducted in the United States, Canada and Brazil that evaluated whether lowering homocysteine levels with vitamin therapy reduced the rate of CVD outcomes in first time prevalent KTRs (14, 15). There was no benefit associated with the high dose treatment arm, thus permitting the two treatment groups to be combined for analyses as a single cohort (14, 15). The FAVORIT trial protocol was approved by the human subjects research entity with oversight at each center, and patients provided written informed consent prior to trial participation. The data coordinating center at the University of North Carolina tracked institutional review board approvals for all sites. The current research was conducted under a Data Use Agreement between Tufts Medical Center and the University of North Carolina.
Population
Kidney transplant recipients were eligible for the study if they were at least 6 months post-transplantation, had elevated total serum homocysteine levels (≥ 11 µmol/L for women; and 12 µmol/L for men), and had stable kidney function, initially defined by an estimated creatinine clearance ≥ 30mL/min in men and ≥ 25 mL/min in women (14). From August 2002 through January 2007, 4,110 participants were randomized to receive either a standard multivitamin with high doses of folic acid (5 mg), vitamin B6 (pyridoxine; 50 mg) and vitamin B12 (cyanocobalamin; 1 mg) or a multivitamin containing low doses of vitamin B6 (1.4 mg) and vitamin B12 (2 µg) without folic acid. Since the high dose vitamin intervention did not reduce the risk of kidney failure, CVD, or all-cause death in comparison to the low dose vitamin (16), data from the two treatment groups were combined with a term retained for randomization allocation in all analyses. Details of baseline characteristics of study participants have been described previously (15).
Study Variables
Characteristics assessed at enrollment include kidney measures (albuminuria and eGFR), patient characteristics, and transplant factors. Urine creatinine and albumin were measured by modified Jaffe kinetic reaction and immunoturbidimetric method, respectively (Olympus AU400 analyzer); the albumin-creatinine ratio (ACR) was log transformed for use in modeling. Serum creatinine was measured using frozen sera from the baseline visit at the FAVORIT central lab using an IDMS calibrated assay; GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation (20), which has been validated for KTRs (21). Other covariates included baseline demographics, country, smoking status, lipid profile, blood pressure, diabetes, and medication use. Baseline CVD was defined as the presence of prior myocardial infarction, coronary artery revascularization, stroke, carotid arterial revascularization, abdominal or thoracic aortic aneurysm repair, and/or lower extremity arterial revascularization. Diabetes mellitus was defined as use of insulin or oral hypoglycemic medications or patient history. Smoking status was classified as current, former, or never by patient report. Seated blood pressure was measured twice at 5–10 minute intervals during each clinic visit, with the average value used for analyses. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Total cholesterol, high density lipoprotein (HDL) cholesterol and triglycerides were measured at baseline. Low-density lipoprotein (LDL) cholesterol was estimated using the Friedewald equation at triglyceride levels below 400 mg/dL and measured in the 234 participants with triglyceride levels above 400 mg/dL (22). Transplant characteristics include donor type defined as living versus non-living donor, time since transplantation, and immunosuppression regimen.
Outcomes
The primary outcomes for this analysis were time to allograft failure (defined as initiation of dialysis) and time to a CVD event (defined by CVD death, myocardial infarction, resuscitated sudden death, stroke, coronary revascularization or peripheral, carotid, aortic or renal artery procedures). Secondary outcomes included all-cause mortality and a composite of allograft failure and all-cause mortality. The first four components of the CVD outcome were centrally reviewed and adjudicated by the FAVORIT Clinical Endpoints Committee; the remaining outcomes were identified through medical records.
Statistical Analyses
Baseline characteristics were compared across clinically relevant ACR categories (23) using one-way ANOVA for normally distributed variables or Kruskal-Wallis for non-normally distributed variables and chi-square tests for categorical variables. Kaplan-Meier survival curves were constructed by strata of baseline ACR and the log-rank test was used to compare these survival curves. Poisson regression was used to calculate adjusted event rates. Cox proportional-hazards regression was used to examine the association between ACR and time to primary and secondary study outcomes in unadjusted and adjusted models, and restricted cubic splines were constructed to display multivariable adjusted continuous associations between ACR and outcomes. The proportional hazards assumption was examined by regressing the scaled Schoenfeld residuals against follow-up time. Reported hazard ratios show the association of each two-fold higher ACR with outcomes.
Parsimonious models were a priori adjusted for age, sex, race, country, study treatment assignment, aspirin use, statin use, transplant graft vintage, donor type, and calcineurin inhibitor and sirolimus use. Extended multivariable models were a priori further adjusted for estimated GFR, history of CVD, history of diabetes, smoking status, systolic BP, diastolic BP, BMI, HDL cholesterol, LDL cholesterol, and triglyceride levels, and use of an ACE inhibitor or angiotensin receptor blocker. GFR was modeled using 2-slopes with an inflection at 45 ml/min/1.73m2 while diastolic BP was modeled using 2-slopes with inflection at 70 mm Hg (17, 24). Interactions between ACR and eGFR and between ACR and systolic BP were assessed a priori (25). Additional tested interactions included eGFR, diabetes and CVD. Sensitivity analyses used the Fine and Gray method to account for competing risks of death for outcomes where death was not included.
Analyses were performed using SAS 9.4 and R language (version 3.3.1, R Foundation for Statistical Computing, Vienna Austria). Authors H.T. and D.E.W. had full access to the data and take responsibility for the accuracy of data analyses.
Results
Baseline Characteristics
Among 4110 enrolled participants from 30 transplant centers in the United States (n=27), Canada (n=2), and Brazil (n=1), 599 participants were excluded from analyses for missing outcome data, resulting in a final population of 3,511 participants (Figure 1). Selected characteristics of included participants did not differ substantially from those excluded, with the exception that more participants with missing data were from sites located in the United States (Table S1).
Figure 1.
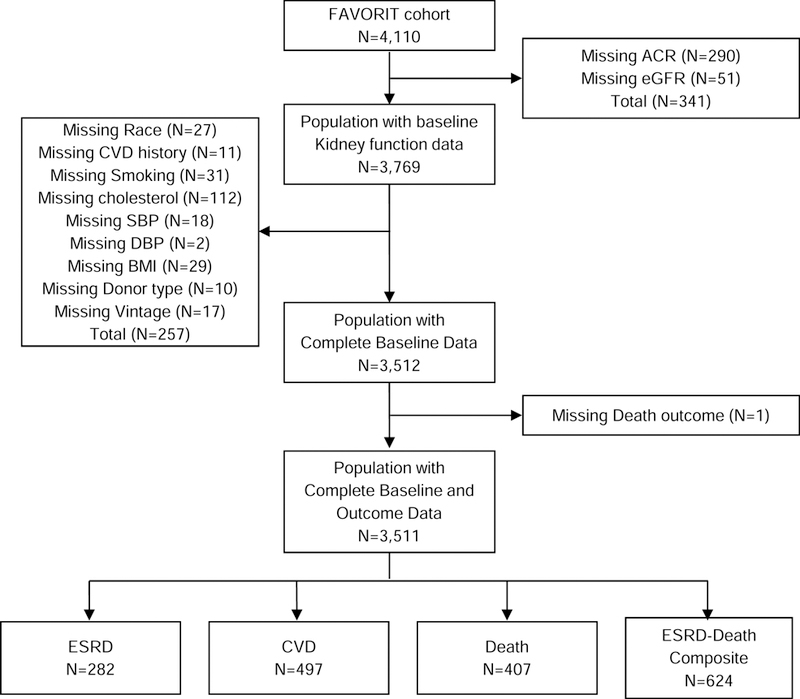
Derivation of the study population
At baseline, mean age was 52 ± 9 (standard deviation) years; 17% were black, 37% were women, median graft vintage was 4.1 (1.7–7.4) years, 43% had a living donor kidney, and 20% had prior CVD. Median ACR was 24.1 (Q1-Q3, 8.5–97.8) mg/g; 1017 (29%) had ACR below 10 mg/g, 912 (26%) had ACR of 10 to 29 mg/g, 1134 (32%) 30 to 299 mg/g, and 448 (13%) 300 mg/g or higher. Mean eGFR was 48.9 ± 17.5 ml/min/1.73m2. Participants with higher urine ACR had longer time since kidney transplantation (graft vintage), and were more likely of African American race, to have deceased donor transplants, prior CVD, diabetes, higher systolic and diastolic BP, and lower eGFR (Table 1).
Table 1.
Baseline characteristics of participants stratified by Urine ACR category
| Urine ACR Category | ||||||
|---|---|---|---|---|---|---|
| Total (N=3511) |
<10 mg/g (n=1017; 29%) |
10-<30 mg/g (n=912; 26%) |
30-<300 mg/g (n=1134; 32%) |
>=300 mg/g (n=448; 13%) |
Trend p | |
| Age (y) | 51.8 ± 9.4 | 51.2 ± 9.0 | 52.6 ± 9.5 | 52.0 ± 9.5 | 50.9 ± 9.6 | 0.8 |
| Women | 1309 (37%) | 389 (38%) | 341 (37%) | 398 (35%) | 181 (40%) | 0.8 |
| Race | <0.001 | |||||
| White | 2679 (76%) | 811 (80%) | 721 (79%) | 830 (73%) | 317 (71%) | |
| Black | 609 (17%) | 140 (14%) | 134 (15%) | 231 (20%) | 104 (23%) | |
| Other | 223 (6%) | 66 (6%) | 57 (6%) | 73 (6%) | 27 (6%) | |
| Treatment group | 0.4 | |||||
| High dose vitamin | 1755 (50%) | 505 (50%) | 450 (49%) | 564 (50%) | 236 (53%) | |
| Low dose vitamin | 1756 (50%) | 512 (50%) | 462 (51%) | 570 (50%) | 212 (47%) | |
| Location | 0.02 | |||||
| United States | 2508 (71%) | 758 (75%) | 657 (72%) | 773 (68%) | 320 (71%) | |
| Canada | 394 (11%) | 94 (9%) | 103 (11%) | 140 (12%) | 57 (13%) | |
| Brazil | 609 (17%) | 165 (16%) | 152 (17%) | 221 (19%) | 71 (16%) | |
| Graft vintage (y) | 4.1 (1.7–7.4) | 3.6 (1.6–6.9) | 3.3 (1.3–6.8) | 4.4 (1.9–8.0) | 5.3 (2.4–9.2) | <0.001 |
| Living donor kidney | 1497 (43%) | 473 (47%) | 395 (43%) | 458 (40%) | 171 (38%) | <0.001 |
| Medications | ||||||
| Cyclosporine | 1774 (51%) | 542 (53%) | 467 (51%) | 569 (50%) | 196 (44%) | 0.002 |
| Tacrolimus | 1334 (38%) | 410 (40%) | 361 (40%) | 403 (36%) | 160 (36%) | 0.02 |
| Any CNI | 3101 (88%) | 948 (93%) | 826 (91%) | 971 (86%) | 356 (79%) | <0.001 |
| Sirolimus | 293 (8%) | 35 (3%) | 72 (8%) | 124 (11%) | 62 (14%) | <0.001 |
| ACEi/ARB | 1557 (44%) | 428 (42%) | 382 (42%) | 505 (45%) | 242 (54%) | <0.001 |
| Statin | 1833 (52%) | 510 (50%) | 496 (54%) | 597 (53%) | 230 (51%) | 0.6 |
| Aspirin | 1460 (42%) | 441 (43%) | 409 (45%) | 446 (39%) | 164 (37%) | 0.003 |
| Medical History | ||||||
| CVD | 703 (20%) | 164 (16%) | 189 (21%) | 247 (22%) | 103 (23%) | <0.001 |
| Diabetes Mellitus | 1403 (40%) | 374 (37%) | 360 (39%) | 481 (42%) | 188 (42%) | 0.01 |
| Hypertension | 3228 (92%) | 892 (88%) | 833 (91%) | 1071 (94%) | 432 (96%) | <0.001 |
| Smoking | 0.03 | |||||
| Never | 1703 (49%) | 494 (49%) | 444 (49%) | 534 (47%) | 231 (52%) | |
| Former | 1422 (41%) | 422 (41%) | 380 (42%) | 465 (41%) | 155 (35%) | |
| Current | 386 (11%) | 101 (10%) | 88 (10%) | 135 (12%) | 62 (14%) | |
| Examination Findings | ||||||
| SBP (mm Hg) | 136.2 ± 19.8 | 130.4 ± 17.0 | 134.4 ± 18.5 | 139.4 ± 20.9 | 144.9 ± 21.2 | <0.001 |
| DBP (mm Hg) | 78.8 ± 12.4 | 76.6 ± 11.3 | 77.5 ± 11.8 | 80.4 ± 12.9 | 82.6 ± 13.3 | <0.001 |
| BMI (kg/m2) | 29.2 ± 6.2 | 29.3 ± 6.3 | 29.0 ± 6.1 | 29.1 ± 6.1 | 29.4 ± 6.2 | 0.6 |
| Laboratory Results | ||||||
| Total cholesterol, mg/dL |
185.0 ± 44.2 | 181.4 ± 38.7 | 181.4 ± 40.9 | 185.7 ± 45.5 | 199.1 ± 54.7 | <.001 |
| HDL cholesterol, mg/dL |
46.2 ± 13.9 | 46.4 ± 13.2 | 46.2 ± 14.2 | 46.0 ± 14.1 | 46.6 ± 14.8 | 0.4 |
| LDL cholesterol, mg/dL |
101.3 ± 34.6 | 99.9 ± 31.8 | 98.3 ± 32.0 | 101.9 ± 35.8 | 109.1 ± 41.1 | 0.001 |
| Triglycerides | 164 (113–238) | 157 (106–224) | 163 (112–235) | 167 (114–239) | 184 (124–266) | <0.001 |
| Scr, mg/dL | 1.7 ± 0.6 | 1.5 ± 0.5 | 1.6 ± 0.5 | 1.7 ± 0.6 | 1.9 ± 0.7 | <0.001 |
| eGFR, mL/min/1.73 m2 |
48.9 ± 17.5 | 52.7 ± 17.0 | 50.1 ± 16.9 | 47.0 ± 17.7 | 42.7 ± 17.3 | <0.001 |
| ACR (mg/g) | 24.1 (8.5–97.8) | 5.4 (3.6–7.6) | 16.8 (12.8–22.4) | 75.2 (44.4– 136.2) |
682.3 (408.8– 1347.3) |
|
Values for categorical variables are given as count (percentage); for continuous variables as mean ± standard deviation or median (25th –75th percentile). ACR, albumin-creatinine ratio; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; DBP, diastolic blood pressure; SBP, systolic blood pressure; BMI, body mass index; HDL, high density lipoprotein; LDL, low density lipoprotein; CVD, cardiovascular disease; Scr, serum creatinine.
Allograft Failure
Over mean follow-up of 3.9 ± 1.6 years, there were 282 allograft failure events. Figure 2 presents adjusted event rates for eGFR and ACR strata. In unadjusted models and in parsimonious models, each two-fold higher ACR was associated with a 47% (HR, 1.47; 95% CI, 1.40–1.54) and 48% (HR, 1.48; 95% CI, 1.41–1.56) higher risk for allograft failure, respectively. In the extended adjusted model, each two-fold higher ACR was associated with a 43% (HR, 1.43; 95% CI, 1.36–1.51) higher risk of allograft failure (Figure S1). Risk by ACR strata is shown in Table 2 and Figure S2, demonstrating a statistically significant increased risk of allograft failure among those with ACR 30 to <300 mg/g and 300+ mg/g [HRs of 3.40 (95% CI, 2.19–5.30) and 9.96 (95% CI, 6.35–15.62), respectively, compared with ACR <10 mg/g].
Figure 2. Adjusted event rates per 1000 patient years by eGFR and urine albumin-creatinine ratio strata.
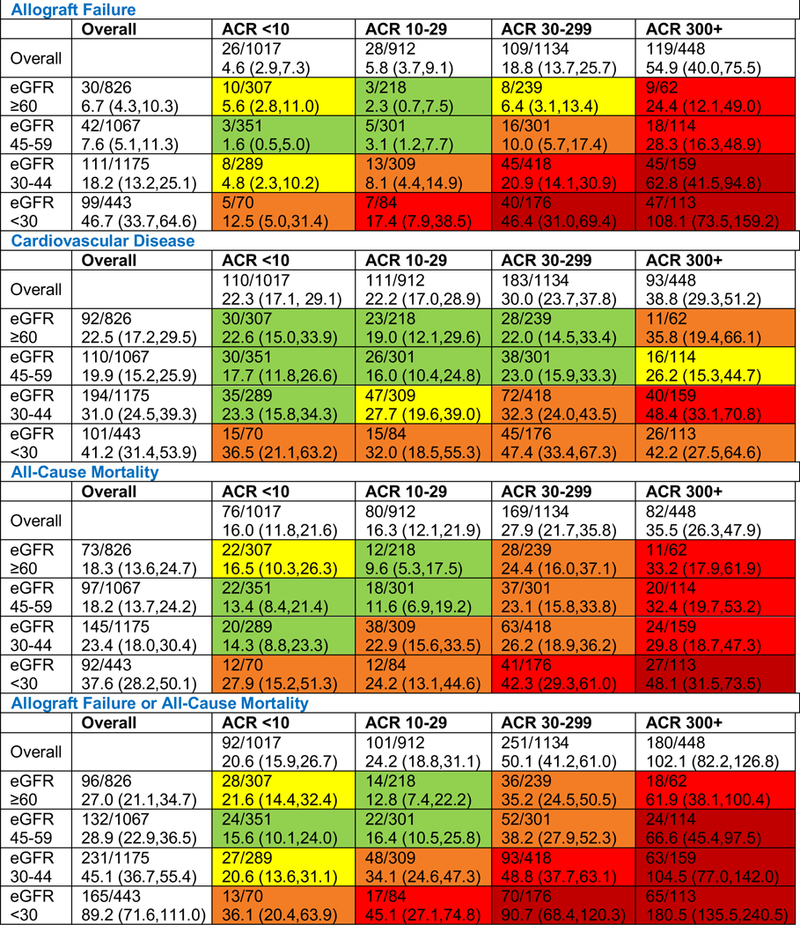
Rates are presented per 1,000 person-years of clinical outcomes calculated using Poisson regression. Data are presented as the number of individuals with an event/number in the eGFR/ACR stratum followed by the adjusted rate (95% confidence interval). For CVD, mortality, and the composite of allograft failure and mortality outcomes, as compared to the lowest group, green shading indicates a 1 to 1.5 fold increased event rate; yellow a 1.5–2 fold increased rate; orange a 2–3 fold increased rate; red a 3–5 fold increased rate, and dark red a 5+ fold increase. For allograft failure, green shading indicates a 1–2 fold increased rate; yellow 2–5; orange, 5–10; red, 10–20 and dark red, 20+. For the composite of allograft failure and death, green shading indicates a 1–2 fold increased rate; yellow 2–5; orange, 5–10; red, 10–20 and dark red, 20+. Models adjust for age, sex, race, study allocation, country, graft vintage, donor type, calcineurin inhibitor use, sirolimus use, diabetes, history of cardiovascular disease, smoking status, systolic blood pressure, diastolic blood pressure, body mass index, HDL cholesterol, LDL cholesterol, triglycerides, angiotensin converting enzyme inhibitor or angiotensin receptor blocker use, aspirin use, and statin use. ACR, albumin-creatinine ratio in mg/g; eGFR, estimated glomerular filtration rate in mL/min/1.73m2.
Table 2.
Albuminuria strata and risk of cardiovascular disease, mortality and dialysis outcomes
| Urine ACR Category | Continuous: Per ACR doubling* |
||||
|---|---|---|---|---|---|
| < 10 mg/g (n=1017) |
10-<30 mg/g (n=912) |
30-<300 mg/g (n=1134) |
>=300 mg/g (n=448) |
||
| Allograft Failure | |||||
| Unadjusted | 1.00 (ref) | 1.27 (0.74, 2.16) | 4.46 (2.91, 6.83) |
15.00 (9.81, 22.94) |
1.47 (1.40, 1.54) |
| Parsimonious Adjusted |
1.00 (ref) | 1.32 (0.77, 2.25) | 4.63 (3.02, 7.10) |
14.89 (9.66, 22.96) |
1.48 (1.41, 1.56) |
| Extended Adjusted | 1.00 (ref) | 1.24 (0.73, 2.11) | 3.40 (2.19, 5.30) |
9.96 (6.35, 15.62) |
1.43 (1.36, 1.51) |
| Cardiovascular Disease | |||||
| Unadjusted | 1.00 (ref) | 1.16 (0.89, 1.52) | 1.65 (1.30, 2.09) |
2.29 (1.74, 3.02) | 1.11 (1.08, 1.15) |
| Parsimonious Adjusted |
1.00 (ref) | 1.09 (0.84, 1.43) | 1.68 (1.32, 2.15) |
2.44 (1.83, 3.25) | 1.12 (1.09, 1.17) |
| Extended Adjusted | 1.00 (ref) | 0.99 (0.75, 1.29) | 1.25 (0.96, 1.61) |
1.55 (1.13, 2.11) | 1.06 (1.02, 1.10) |
| All-Cause Mortality | |||||
| Unadjusted | 1.00 (ref) | 1.23 (0.90, 1.68) | 2.25 (1.71, 2.95) |
2.98 (2.18, 4.09) | 1.18 (1.14, 1.23) |
| Parsimonious Adjusted |
1.00 (ref) | 1.09 (0.79, 1.49) | 2.05 (1.56, 2.71) |
2.80 (2.02, 3.89) | 1.18 (1.13, 1.22) |
| Extended Adjusted | 1.00 (ref) | 1.02 (0.74, 1.39) | 1.65 (1.23, 2.21) |
2.07 (1.46, 2.94) | 1.13 (1.09, 1.18) |
| Allograft Failure or All-Cause Mortality | |||||
| Unadjusted | 1.00 (ref) | 1.28 (0.97, 1.70) | 2.84 (2.24, 3.61) |
6.25 (4.84, 8.07) | 1.30 (1.27, 1.34) |
| Parsimonious Adjusted |
1.00 (ref) | 1.23 (0.93, 1.64) | 2.76 (2.17, 3.53) |
6.04 (4.64, 7.87) | 1.30 (1.26, 1.34) |
| Extended Adjusted | 1.00 (ref) | 1.17 (0.88, 1.56) | 2.20 (1.71, 2.83) |
4.55 (3.46, 5.99) | 1.26 (1.22, 1.31) |
ACR of <10 mg/mg is considered normal; 10-<30 mg/mg is considered high-normal (mildly increased); 30-<300 mg/g is moderately increased; >=300 mg/g is severely increased
Rates are unadjusted. Models present the hazard ratio (95% confidence interval). The parsimonious model is adjusted for age, sex, race, study allocation, country, graft vintage, donor type, calcineurin inhibitor use and sirolimus use, aspirin use, and statin use, while the extended model is adjusted for age, sex, race, study allocation, country, graft vintage, donor type, calcineurin inhibitor use, sirolimus use, diabetes, history of cardiovascular disease, smoking status, systolic blood pressure, diastolic blood pressure, body mass index, HDL cholesterol, LDL cholesterol, triglycerides, angiotensin converting enzyme inhibitor or angiotensin receptor blocker use, aspirin use, statin use, and estimated GFR. PY, person-year.
based on data from participants in all ACR categories
Cardiovascular Outcomes
Over mean follow-up of 3.8 ± 1.7 years, there were 497 CVD events. Figure 2 presents adjusted event rates for eGFR and ACR strata. Event rates were higher at higher levels of albuminuria (Figure 3). In unadjusted and parsimonious models, each two-fold higher ACR was associated with an 11% (HR, 1.11; 95% CI, 1.08–1.15) and 12% (HR, 1.12; 95% CI, 1.09–1.17) higher risk for CVD outcomes, respectively. In the extended adjusted model, each two-fold higher ACR was associated with a 6% (HR, 1.06; 95% CI, 1.02–1.10) higher risk for CVD outcomes (Figure S1). Risk by ACR strata is shown in Table 2 and Figure S2; compared with ACR < 10 mg/g, in those with ACR 30 to <300 mg/g the CVD risk was nominally elevated but this was not statistically significant (HR, 1.25; 95% CI, 0.96–1.61), and among those with ACR 300+ mg/g, the increased risk of CVD was statistically significant (HR, 1.55; 95% CI, 1.13–2.11).
Figure 3.
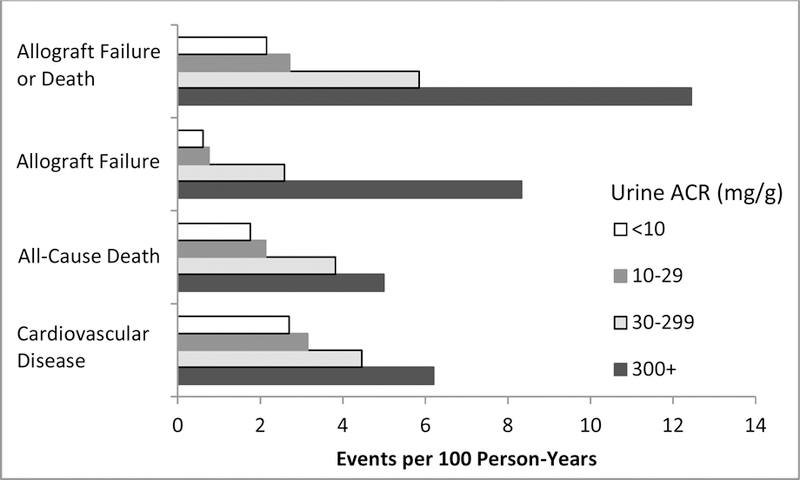
Event rates stratified by urine ACR level
Mortality Outcomes
Over mean follow-up of 4.0 ± 1.5 years, there were 407 deaths; Figure 2 presents adjusted event rates for eGFR and ACR strata. In unadjusted models and in parsimonious models, each two-fold higher ACR was associated with an 18% higher risk for all-cause mortality outcomes (HRs of 1.18 (95% CI, 1.14–1.23) and 1.18 (95% CI, 1.13–1.22), respectively). In the extended adjusted model, each two-fold higher ACR was associated with a 13% (HR, 1.13; 95% CI, 1.09–1.18) higher risk of all-cause mortality (Figure S1). Risk by ACR strata is shown in Table 2 and Figure S2, demonstrating a statistically significant increased risk of death among those with ACR 30 to <300 mg/g and 300+ mg/g [HRs of 1.65 (95% CI, 1.23–2.21) and 2.07 (95% CI, 1.46–2.94), respectively, compared with ACR <10 mg/g].
Composite Outcome
Over mean follow-up of 3.9 years, there were 624 episodes of either allograft failure or all-cause mortality. Figure 2 presents adjusted event rates for eGFR and ACR strata. In unadjusted models and in parsimonious models, each two-fold higher ACR was associated with a 30% higher risk of the composite outcome (HRs of 1.30 (95% CI, 1.27–1.34) and 1.30 (95% CI, 1.26–1.34), respectively). In the extended adjusted model, each two-fold higher ACR was associated with a 26% (HR, 1.26; 95% CI, 1.22–1.31) higher risk of the allograft failure-mortality composite (Figure S1). Risk by ACR strata is shown in Table 2 and Figure S2, demonstrating a statistically significant increased risk of the allograft failure-mortality composite among those with urine ACR 30 to <300 mg/g and 300+ mg/g [HRs of 2.20 (95% CI, 1.71–2.83) and 4.55 (95% CI, 3.46–5.99), respectively, compared with ACR <10 mg/g].
Secondary and Sensitivity Analyses
Competing risks models had similar results to primary analyses (Table S2). The interaction between urine ACR and systolic blood pressure was not significant for any outcome; additionally, the interactions between urine ACR and eGFR stratified at 45 ml/min/1.73m2and between ACR and history of cardiovascular disease were not significant for any outcome the presence of albuminuria was of greater import for composite outcomes among those without diabetes (Figure S3). There was a significant interaction between baseline ACR and diabetes, such that the presence of albuminuria was of greater import for composite outcomes among those without diabetes (Figure S3).
Discussion:
In a large, well-characterized cohort of stable KTRs, higher levels of ACR were associated with higher risk of graft failure, CVD, and death independent of allograft function, traditional CVD risk factors, and limited transplant characteristics. Although these associations were strongest for graft failure, the presence of elevated urine ACR was strongly associated with both all-cause mortality and CVD events. These findings expand on prior work through inclusion of a large, multicenter, multinational population with detailed comorbidity assessment, central laboratory measures, and consensus outcomes ascertainment with a focus on CVD. These findings are especially interesting considering the parallel associations of ACR with CVD and mortality events in native kidneys and the relatively limited explorations to date of the association between albuminuria and CVD among kidney transplant recipients (6, 26).
Viewed in conjunction with other studies, these results demonstrate that kidney damage not only is associated with risk of CKD progression but also with cardiovascular and mortality outcomes, potentially suggesting that comorbid conditions associated with development and progression of albuminuria may concurrently impact cardiovascular risk. The magnitude of the associations of ACR with outcomes in the current study exceeded that seen previously with tubular injury markers in a subset of the FAVORIT Trial (19, 27). ACR is more easily measured than many other urine biomarkers of kidney injury, has a stronger and more consistent association with outcomes, and, in the general population, can be used to assess the potential effectiveness of treatments, such as renin-angiotensin-aldosterone system blockade (28).
Our findings are notable considering that increased CVD and mortality risk was seen even at moderately elevated levels of albuminuria (30–300 mg/g) at all levels of kidney function, suggesting that even mild damage identified in the allograft reflects systemic cardiovascular and mortality risk in the recipient and that, regardless of the etiology of albuminuria, its presence is clinically important. In other words, ACR may capture a systemic microvascular and vascular disease that indicates cardiovascular and mortality risk beyond kidney function, with the association in KTRs similar in magnitude to that seen for albuminuria and outcomes in native kidney disease (29). Alternatively, inflammation or other disease processes contributing to cardiovascular disease may also induce microvascular damage in the transplanted kidney, accelerating kidney disease and albuminuria (7, 30).
The associations of kidney function assessed by eGFR and long-term graft function have been well described, including in the FAVORIT Trial (17, 31). Previous studies also have evaluated the association of ACR with outcomes in KTRs (32–38). A study by Nauta et al found that ACR predicted graft loss in a single-center study of 606 KTRs with 42 graft failure events (39). More recently, a longitudinal cohort study of 1490 KTRs at a single European center showed that proteinuria measured by 24-hour urine collection was independently associated with graft failure, independent of kidney pathology (40). Similarly, a recent Canadian study evaluating 900 KTRs between 2002 and 2011, using administrative data to assess outcomes, found that the KDIGO staging used in native CKD had graded associations with outcomes of graft loss and mortality in transplant recipients (41). Overall, these studies focused on graft loss and mortality using various measures of graft function. Our study adds significantly to this literature as the FAVORIT study performed careful prospective adjudication of CVD events, and we showed that a simple measure of ACR was strongly associated not only with allograft failure but also with CVD events and mortality after multivariate adjustment, including adjustment for kidney function.
Among KTRs chronic allograft rejection, recurrent or de novo glomerulonephritis and transplant glomerulopathy are leading causes of proteinuria (40,42). The majority of cases of transplant glomerulopathy are secondary to chronic antibody-mediated rejection, suggesting that immune-mediated injury may be a more important cause of proteinuria than previously thought (3, 43–47), with medication non-adherence a major cause of graft failure from rejection (46). Accordingly, the current study is important as available therapeutic interventions may be considered to reduce ACR in KTRs, including RAAS blockade, immunosuppression strategies, and strategies to improve medication adherence (48, 49), although existing data, including a recent, 4-year randomized trial evaluating the effect of ramipril on clinical outcomes in 213 KTRs with 200 mg or more of proteinuria per day, do not show a significant beneficial effect with ramipril as compared to placebo despite modestly lower proteinuria in ramipril recipients (50). Further study is needed to determine how to incorporate albuminuria assessment into risk assessment and to evaluate whether it has utility in guiding non-immunologic therapies. The optimal choice of immunosuppression to mitigate ACR and improve cardiovascular outcomes is also of great interest (51, 52). Finally, use of ACR to guide decisions about cardiovascular testing and imaging is another potential application of these findings.
Our study has several strengths. FAVORIT study participants are well characterized, with adjudicated cardiovascular events as the main outcome of the parent study. The FAVORIT trial is large and represents stable KTRs at least six months post transplantation from multiple centers across US, Canada, and Brazil. This study also has limitations. We used ACR measured at one time point only from spot urine samples collected at baseline; however, this would likely bias to a null finding (53). Similarly, we used kidney function data from baseline only. While including both ACR and eGFR as time varying terms could further elucidate the associations among kidney markers and kidney and CVD outcomes, absence of measures at one year in many participants would render conclusions uncertain. Kidney biopsy pathologic data, pre-transplantation dialysis vintage, HLA status, and anti-HLA antibody status are not available. As acute and chronic rejection can contribute to albuminuria and cardiovascular risk (44), this is an important consideration; notably, enrollment in the FAVORIT trial required participants to be stable and confounding by presence of acute rejection at enrollment is unlikely to have markedly influenced our results while future acute rejection episodes are likely to bias to the null finding unless prevalent albuminuria is a marker of future rejection. Recruitment focused on prevalent KTRs also likely minimized the possibility that albuminuria reflected disease in functioning native kidneys. Additionally, we do not have data on whether participants died of kidney failure without initiating dialysis; accordingly, we included a composite outcome that shows consistent associations between albuminuria and events. Finally, as with any observational study, we can neither exclude the possibility of residual confounding nor determine causality.
In conclusion, among stable KTRs in the FAVORIT trial, we found that ACR is independently and strongly associated with graft failure, CVD events and mortality. The association of ACR with outcomes in KTRs is similar to that seen between ACR and outcomes in the general population. As ACR is easily obtained in clinical practice, its uniform use in KTRs may be important in helping to assess risk of adverse outcomes, including cardiovascular disease outcomes. Future studies should further evaluate methods to reduce ACR as an intervention to improve outcomes in KTRs as well as mechanisms by which ACR and kidney injury lead to systemic disease and poor outcomes.
Supplementary Material
Acknowledgements:
We would like to acknowledge the kidney transplant recipients who participated in FAVORIT and the clinical sites that made the trial possible.
Support: The FAVORITE trial was supported by NIH grants U01 DK061700 and T32 DK007777. The National Institutes of Health oversaw study procedures and JWK, who is an NIH employee, was involved in writing this report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Other Disclosures: JWK is an employee of the US government. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Disclaimer:
Prior Presentation: Early results of these analyses were presented at 2014 Kidney Week.
Peer Review: Received October 30, 2017. Evaluated by 3 external peer reviewers, with direct editorial input from a Statistics/Methods Editor and an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form May 23, 2018.
The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
References
- 1.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant 2011;11(3):450–62. [DOI] [PubMed] [Google Scholar]
- 2.Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant 2004;4(8):1289–95. [DOI] [PubMed] [Google Scholar]
- 3.Stegall MD, Park WD, Dean PG, Cosio FG. Improving long-term renal allograft survival via a road less traveled by. Am J Transplant 2011;11(7):1382–7. [DOI] [PubMed] [Google Scholar]
- 4.Porrini E, Delgado P, Bigo C, et al. Impact of metabolic syndrome on graft function and survival after cadaveric renal transplantation. Am J Kidney Dis 2006;48(1):134–42. [DOI] [PubMed] [Google Scholar]
- 5.Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375(9731):2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin AP, Whaley-Connell AT, Li S, et al. The synergistic relationship between estimated GFR and microalbuminuria in predicting long-term progression to ESRD or death in patients with diabetes: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2013;61(4 Suppl 2):S12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsayed EF, Tighiouart H, Griffith J, et al. Cardiovascular disease and subsequent kidney disease. Arch Intern Med 2007;167(11):1130–6. [DOI] [PubMed] [Google Scholar]
- 8.Chang TI, Li S, Chen SC, et al. Risk factors for ESRD in individuals with preserved estimated GFR with and without albuminuria: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2013;61(4 Suppl 2):S4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myslak M, Amer H, Morales P, et al. Interpreting post-transplant proteinuria in patients with proteinuria pre-transplant. Am J Transplant 2006;6(7):1660–5. [DOI] [PubMed] [Google Scholar]
- 10.de Zeeuw D, Parving HH, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol 2006;17(8):2100–5. [DOI] [PubMed] [Google Scholar]
- 11.Halimi JM. Low-grade proteinuria and microalbuminuria in renal transplantation. Transplantation 2013;96(2):121–30. [DOI] [PubMed] [Google Scholar]
- 12.Wiseman AC, McCague K, Kim Y, Geissler F, Cooper M. The effect of everolimus versus mycophenolate upon proteinuria following kidney transplant and relationship to graft outcomes. Am J Transplant 2013;13(2):442–9. [DOI] [PubMed] [Google Scholar]
- 13.Franz S, Regeniter A, Hopfer H, Mihatsch M, Dickenmann M. Tubular toxicity in sirolimus- and cyclosporine-based transplant immunosuppression strategies: an ancillary study from a randomized controlled trial. Am J Kidney Dis 2010;55(2):335–43. [DOI] [PubMed] [Google Scholar]
- 14.Bostom AG, Carpenter MA, Kusek JW, et al. Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. Am Heart J 2006;152(3):448 e1–7. [DOI] [PubMed] [Google Scholar]
- 15.Bostom AG, Carpenter MA, Hunsicker L, et al. Baseline characteristics of participants in the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial. Am J Kidney Dis 2009;53(1):121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bostom AG, Carpenter MA, Kusek JW, et al. Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients: primary results from the folic Acid for vascular outcome reduction in transplantation trial. Circulation 2011;123(16):1763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiner DE, Carpenter MA, Levey AS, et al. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: the FAVORIT trial. Am J Transplant 2012;12(9):2437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bansal N, Carpenter MA, Weiner DE, et al. Urine Injury Biomarkers and Risk of Adverse Outcomes in Recipients of Prevalent Kidney Transplants: The Folic Acid for Vascular Outcome Reduction in Transplantation Trial. J Am Soc Nephrol 2016; 27(7): 2109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park M, Katz R, Shlipak MG, et al. Urinary Markers of Fibrosis and Risk of Cardiovascular Events and Death in Kidney Transplant Recipients: The FAVORIT Trial. Am J Transplant 2017; 17(10): 2640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis 2014;63(5):820–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaffi K, Uhlig K, Perrone RD, et al. Performance of Creatinine-Based GFR Estimating Equations in Solid-Organ Transplant Recipients. Am J Kidney Dis 2014;63(6):1007–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18(6):499–502. [PubMed] [Google Scholar]
- 23.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter, Suppl 2013; 3: 1–150. [Google Scholar]
- 24.Carpenter MA, John A, Weir MR, et al. BP, cardiovascular disease, and death in the Folic Acid for Vascular Outcome Reduction in Transplantation trial. J Am Soc Nephrol 2014;25(7):1554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003;139(4):244–52. [DOI] [PubMed] [Google Scholar]
- 26.Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2015;3(7):514–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ix JH, Katz R, Bansal N, et al. Urine Fibrosis Markers and Risk of Allograft Failure in Kidney Transplant Recipients: A Case-Cohort Ancillary Study of the FAVORIT Trial. Am J Kidney Dis 2017;69(3):410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrahim HN, Jackson S, Connaire J, et al. Angiotensin II blockade in kidney transplant recipients. J Am Soc Nephrol 2013;24(2):320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011;79(12):1331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta J, Mitra N, Kanetsky PA, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol 2012;7(12):1938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int 2002;62(1):311–8. [DOI] [PubMed] [Google Scholar]
- 32.Hohage H, Kleyer U, Bruckner D, August C, Zidek W, Spieker C. Influence of proteinuria on long-term transplant survival in kidney transplant recipients. Nephron 1997;75(2):160–5. [DOI] [PubMed] [Google Scholar]
- 33.Halimi JM, Laouad I, Buchler M, et al. Early low-grade proteinuria: causes, short-term evolution and long-term consequences in renal transplantation. Am J Transplant 2005;5(9):2281–8. [DOI] [PubMed] [Google Scholar]
- 34.Roodnat JI, Mulder PG, Rischen-Vos J, et al. Proteinuria after renal transplantation affects not only graft survival but also patient survival. Transplantation 2001;72(3):438–44. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Fresnedo G, Plaza JJ, Sanchez-Plumed J, Sanz-Guajardo A, Palomar-Fontanet R, Arias M. Proteinuria: a new marker of long-term graft and patient survival in kidney transplantation. Nephrol Dial Transplant 2004;19 Suppl 3:iii47–51. [DOI] [PubMed] [Google Scholar]
- 36.Fellstrom B, Holdaas H, Jardine AG, et al. Risk factors for reaching renal endpoints in the assessment of Lescol in renal transplantation (ALERT) trial. Transplantation 2005;79(2):205–12. [DOI] [PubMed] [Google Scholar]
- 37.Rosenkranz AR, Mayer G. Proteinuria in the transplanted patient. Nephrol Dial Transplant 2000;15(9):1290–2. [DOI] [PubMed] [Google Scholar]
- 38.Reichel H, Zeier M, Ritz E. Proteinuria after renal transplantation: pathogenesis and management. Nephrol Dial Transplant 2004;19(2):301–5. [DOI] [PubMed] [Google Scholar]
- 39.Nauta FL, Bakker SJL, van Oeveren W, et al. Albuminuria, Proteinuria, and Novel Urine Biomarkers as Predictors of Long-term Allograft Outcomes in Kidney Transplant Recipients. American Journal of Kidney Diseases 2011;57(5):733–43. [DOI] [PubMed] [Google Scholar]
- 40.Naesens M, Lerut E, Emonds MP, et al. Proteinuria as a Noninvasive Marker for Renal Allograft Histology and Failure: An Observational Cohort Study. J Am Soc Nephrol 2016;27(1):281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam NN, Tonelli M, Lentine KL, et al. Albuminuria and posttransplant chronic kidney disease stage predict transplant outcomes. Kidney Int 2017; 92(2): 470–8. [DOI] [PubMed] [Google Scholar]
- 42.Peddi VR, Dean DE, Hariharan S, Cavallo T, Schroeder TJ, First MR. Proteinuria following renal transplantation: correlation with histopathology and outcome. Transplant Proc 1997;29(1–2):101–3. [DOI] [PubMed] [Google Scholar]
- 43.Cosio FG, Gloor JM, Sethi S, Stegall MD. Transplant glomerulopathy. Am J Transplant 2008;8(3):492–6. [DOI] [PubMed] [Google Scholar]
- 44.de Mattos AM, Prather J, Olyaei AJ, et al. Cardiovascular events following renal transplantation: role of traditional and transplant-specific risk factors. Kidney Int 2006;70(4):757–64. [DOI] [PubMed] [Google Scholar]
- 45.Ducloux D, Kazory A, Chalopin JM. Predicting coronary heart disease in renal transplant recipients: a prospective study. Kidney Int 2004;66(1):441–7. [DOI] [PubMed] [Google Scholar]
- 46.Sellares J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 2012;12(2):388–99. [DOI] [PubMed] [Google Scholar]
- 47.Sis B, Campbell PM, Mueller T, et al. Transplant glomerulopathy, late antibody-mediated rejection and the ABCD tetrad in kidney allograft biopsies for cause. Am J Transplant 2007;7(7):1743–52. [DOI] [PubMed] [Google Scholar]
- 48.Hiremath S, Fergusson DA, Fergusson N, Bennett A, Knoll GA. Renin-Angiotensin System Blockade and Long-term Clinical Outcomes in Kidney Transplant Recipients: A Meta-analysis of Randomized Controlled Trials. Am J Kidney Dis 2017;69(1):78–86. [DOI] [PubMed] [Google Scholar]
- 49.Xie X, Liu Y, Perkovic V, Li X, Ninomiya T, Hou W, et al. Renin-Angiotensin System Inhibitors and Kidney and Cardiovascular Outcomes in Patients With CKD: A Bayesian Network Meta-analysis of Randomized Clinical Trials. Am J Kidney Dis 2016;67(5):728–41. [DOI] [PubMed] [Google Scholar]
- 50.Knoll GA, Fergusson D, Chasse M, et al. Ramipril versus placebo in kidney transplant patients with proteinuria: a multicentre, double-blind, randomised controlled trial. Lancet Diabetes Endocrinol 2016;4(4):318–26. [DOI] [PubMed] [Google Scholar]
- 51.Vanrenterghem YF, Claes K, Montagnino G, et al. Risk factors for cardiovascular events after successful renal transplantation. Transplantation 2008;85(2):209–16. [DOI] [PubMed] [Google Scholar]
- 52.Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med 2016;374(4):333–43. [DOI] [PubMed] [Google Scholar]
- 53.Naresh CN, Hayen A, Weening A, Craig JC, Chadban SJ. Day-to-day variability in spot urine albumin-creatinine ratio. Am J Kidney Dis 2013;62(6):1095–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


